Abstract
Background and study aims The effectiveness of colorectal cancer screening programs depends on adherence to surveillance protocols for screening-positive individuals. We evaluated adherence in the Danish population-based screening program and estimated the volume of diagnostic resources required to achieve this adherence.
Patients and methods In this register- and population-based study, we included individuals with a positive fecal immunochemical test (FIT) screening from 2014 to 2017 and followed them until mid-2022. All endoscopic, imaging, and surgical procedures performed at public and private hospitals were identified. Adherence to national protocols was reported in terms of proportions and timeliness. Use of diagnostic and surveillance procedures was estimated during a 4-year post-screening period.
Results Among 82,221 individuals with a positive FIT test, 84% had a baseline colonoscopy within 1 month. After removal of intermediate or high-risk adenomas, 12% and 6%, respectively, did not have any follow-up. Only ~50% had timely surveillance. Approximately 10% to 20%, depending on their referral diagnosis, did not have a second surveillance colonoscopy. In addition, 12% with a negative colonoscopy had a second colonoscopy within 4 years.
Conclusions High adherence to baseline colonoscopy after positive FIT-screening is followed by lower adherence throughout the adenoma surveillance program. Better adherence to the guidelines could potentially improve the effectiveness and efficiency of the screening program.
Keywords: Endoscopy Lower GI Tract, Polyps / adenomas / ..., Colorectal cancer, CRC screening
Introduction
Population-based colorectal cancer (CRC) screening programs using fecal immunochemical tests (FIT) increase diagnosis of early-stage cancers and precursor lesions, i.e. adenomas or serrated polyps 1 and decrease CRC-specific mortality 2 . They have been in place in several high-income countries since 2006 3 . Most European countries offer regional or national biennial FIT screening to men and women from ages 50 to 55 years to ages 70 to 74 years 3 .
Individuals with positive FIT tests are offered a colonoscopy to detect and remove any prevalent lesions. However, even when only adenomas are detected and removed, the risk of CRC remains elevated 4 and these individuals require further surveillance. Thus, the effectiveness and efficiency of screening programs as a whole are highly dependent on appropriateness, and adherence to, the recommended surveillance.
Denmark has the fourth highest incidence of CRC worldwide among individuals aged 50 to 75 years 5 . The FIT-based screening program was implemented in 2014. Even though it was implemented gradually to allow for sufficient colonoscopy capacity, there were reports of capacity constraints during the first period 6 . Other FIT-based screening programs have also experienced both overuse and under-use of colonoscopy services after abnormal screening tests 7 8 9 10 11 12 . Although these data were primarily based on early stages of implementation, they indicate that programs require a thorough understanding of how to efficiently utilize colonoscopy resources and secure timely detection of CRC and its precursor lesions.
We evaluated adherence to the recommended surveillance pathways after a positive FIT test and after screen-detected intermediate- and high-risk adenomas in Denmark. In addition, we estimated the real-life resources employed for the follow-up of screen-detected abnormalities.
Patients and methods
Setting
The Danish CRC screening program utilizes FIT testing. It was gradually implemented between March 1, 2014 and December 31, 2017. During this 4-year prevalence screening, all screening-eligible individuals were randomly invited once. Those who turned 50 or 75 years during this period were invited just before their birthday, resulting in a slight over-representation of 50- and 75-year-olds. Since then, all Danish residents have been invited when they have turned age 50 and thereafter biennially until they have turned age 75 years.
After a positive FIT test, defined as >100 µg Hgb/L (20 µg Hgb/g feces), a full colonoscopy is recommended within 14 days. If this is not possible due to pain or technical difficulties, a computed tomography (CT) colonography is recommended on the same day to avoid additional bowel preparation. If no polyps or cancers are detected, the colorectum is considered “clean.” Participants with clean colons after a full colonoscopy are quarantined from the screening program for 8 years 13 . Detected polyps are removed and individuals are offered colonoscopy surveillance according to the 2012 European guidelines 14 . The guidelines define three adenoma risk levels: 1) low-risk adenomas or a clean CT colonography, discharged to routine biennial FIT screening (or in up to 4 years during the prevalence round); 2) intermediate-risk adenomas, for which the first surveillance colonoscopy is recommended after 3 years; and 3) high-risk adenomas, for which the first surveillance colonoscopy is recommended after 1 year. The recommended second surveillance depends on the results of the first surveillance colonoscopy (Supplementary material, Fig. S1) 13 .
Data sources
Information on FIT tests was retrieved from the National Colorectal Cancer Screening Database 15 and was merged with The Danish Civil Registration System to access vital and resident status information using the unique Danish identification numbers (CPR numbers). Cancer history was retrieved from the Cancer Registry 16 and the National Pathology Register 17 , while information on diagnostic procedures such as colonoscopies, sigmoidoscopies, other endoscopic procedures, imaging procedures, biopsies, polypectomies, and surgeries performed by public hospitals or private specialists was retrieved from the National Patient Register 18 and the National Health Service Register 19 . These registries were merged on an individual level.
Study population
We included all men and women aged 50 to 75 years with a positive FIT test during the prevalence screening. If an individual had more than one positive FIT test, only the first one was included. Exclusion criteria were: death or emigration within 3 months of an abnormal screening sample, when the surveillance colonoscopy was due after the end of follow-up (30 June 2022), inactive or missing CPR information, not a Danish resident, history of colorectal or anal cancer, history of inflammatory bowel disease and familial adenomatous polyposis (these patients often attend specific surveillance programs), or registrations of “no adenoma surveillance due to severe comorbidity.” The individuals were followed from the abnormal screening sample through baseline and surveillance procedures until death, cancer diagnosis, emigration, or end of follow-up, whichever came first.
Definition of risk stratification based on colonoscopies
During the initial diagnostic period following a positive FIT test (Supplementary material, Fig. S2), some individuals had more than one baseline colonoscopy or an incomplete baseline colonoscopy leading to a CT colonography. Therefore, baseline risk assessment for adenomas was based on all relevant information registered during this period. We considered all examinations within 3 months after baseline colonoscopy as a continuation of the initial exam. The same was applied after surveillance colonoscopies (Supplementary material, Fig. S2).
National guidelines for medical coding require specific risk assessment codes. The risk assessments made by the endoscopist performing the colonoscopy, based on the size and number of adenomas detected, were supported by pathological data on the morphology and degree of dysplasia. In case these codes were not registered within 3 months after baseline colonoscopy, an algorithm based on pathological information was used to risk-stratify as many as possible, based on national guidelines 20 . All visits that included a colonoscopy code or a risk assessment code after the 3-month baseline period were considered surveillance visits. In addition, visits with no risk assessment or colonoscopy code, but with a polypectomy code associated with an “adenoma control code” or “colorectal cancer screening code” were also included as both the first or second surveillance (Supplementary material, Table S1).
Adherence to recommended follow-up
Assessment of adherence to recommended follow-up was made in terms of its timeliness and whether a colonoscopy (or CT-colonoscopy) was performed at baseline (the first colonoscopy after a positive FIT), at first surveillance, or at second surveillance whenever such was recommended.
Adherence to baseline colonoscopy was considered as having been in accordance with the guidelines if a colonoscopy was performed within 1 month after the positive FIT test. “Insufficient technique or delayed” baseline colonoscopy was defined as a colonoscopy performed 1 to 3 months after the positive FIT test or if other endoscopic procedures were performed instead of a colonoscopy (e.g., sigmoidoscopies and proctoscopies). Individuals without endoscopic procedures performed within 3 months were considered as having had no follow-up.
Only individuals with intermediate- and high-risk adenomas detected at baseline colonoscopy were recommended for surveillance. Thereafter, adherence to the second surveillance was only assessed for those with high-risk assessments at the baseline colonoscopy because the available follow-up time did not suffice to fully capture the recommended follow-up protocols after intermediate-risk assessments (Supplementary material, Fig. S1). For both first and second surveillance examinations, those with 3-year surveillance protocols were assessed in terms of timeliness, categorized as “on time” if attending in 2.5 to 3.5 years, “delayed” if attending >3.5 years later, and “early” if attending <2.5 years after the previous examination. Timeliness among those with 1-year surveillance protocols was categorized into “on time” if attending in 9 to 15 months (3–15 months for piecemeal resections because extra colonoscopies may be recommended 20 ), “delayed” if attending later than 15 months, and “early” if attending in <9 months (<3 months for piecemeal resections).
Outcomes and analyses
Adherence to baseline colonoscopy and surveillance exams after screening was described as proportions depending on patient risk assessment. Mutually adjusted generalized linear regression models were used to analyze potential associations between non-adherence and age at screening, sex, and comorbidities 21 22 . The latter were categorized using the Charlson’s Comorbidity Index score for the last 10 years as no, mild, moderate, and severe comorbidities.
Use of post-screening endoscopic, surgical, and diagnostic imaging procedures was presented as proportions of screened individuals with at least one procedure performed during a 4-year period after baseline risk assessment, and as numbers of procedures performed during the same 4-year period.
Sensitivity analysis
We repeated the analyses to investigate the timeliness of follow-up with a definition of an additional 3 and 6 months before follow-up as either “delayed” or too early.
In 2017, the international multicentre European Polyp Surveillance (EPoS) trial randomizing adenoma patients to different follow-up schedules was initiated in the Central Denmark Region 23 . Lacking information about trial participants, we excluded the Central Denmark Region in this period in a separate analysis.
Results
We identified 85,338 screening participants with a positive FIT test, of whom 82,221 were included in our study after exclusions (Supplementary material, Fig. S3). Of those, 46,057 (56%) were men and 28,559 (35%) had one or more comorbidities. Among all screened individuals, the age distribution reflected the invitation strategy during the prevalence round, with slight over-representation of the youngest and oldest individuals ( Table 1 ).
Table 1 Study population characteristics.
| Included individuals with positive FIT tests | Screened individuals | |
| *Charlson comorbidity index. | ||
| Total | 82,221 (100%) | 1,241,645 (100%) |
| Gender | ||
| Male | 46,057 (56.0) | 573,742 (46.2) |
| Female | 36,164 (44.0) | 667,903 (53.8) |
| Age group | ||
| 49–54 years | 12,942 (15.7) | 314,657 (25.3) |
| 55–59 years | 11,232 (13.7) | 218,917 (17.6) |
| 60–64 years | 14,403 (17.5) | 217,693 (17.5) |
| 65–69 years | 18,207 (22.1) | 229,943 (18.5) |
| 70–75 years | 25,437 (30.9) | 260,419 (21.0) |
| Comorbidity* | ||
| None | 53,662 (65.3) | 921,988 (74.3) |
| Mild | 21,390 (26.0) | 257,566 (20.7) |
| Moderate | 4,976 (6.1) | 44,529 (3.6) |
| Severe | 2,193 (2.7) | 17,562 (1.4) |
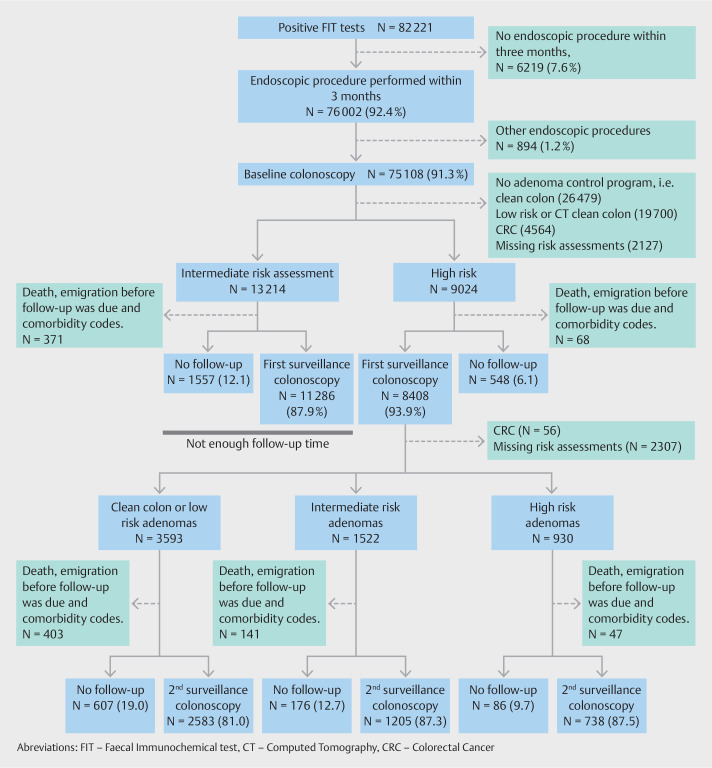
After a positive FIT test, 76,002 individuals (92.4%) had a diagnostic procedure performed within 3 months ( Fig. 1 ). The majority, 69,123 (84.1%), had a baseline colonoscopy performed within 1 month. A small number, 894 (1.2%), had endoscopic procedures other than colonoscopy ( Fig. 1 ). Supplemental Fig. S4 shows that extending the inclusion period to 6 or 12 months would only increase the proportion correctly followed up by approximately 1%. Thus, the majority of individuals who do not have their baseline colonoscopy within 3 months end up not having it at all (Fig. S4).
Fig. 1.
Flowchart of pathways of follow-up in the study population.
The baseline colonoscopy identified 13,214 individuals with intermediate-risk and 9,024 individuals with high-risk adenomas ( Fig. 1 ). Among those, 439 either died or emigrated before their surveillance examination was due or were not considered suitable for surveillance due to comorbidity ( Fig. 1 ). Approximately 90% of intermediate- and high-risk patients had surveillance colonoscopies, but only 53% had them on time ( Table 2 ). Sensitivity analyses extending the “on time” interval by 3 months increased the proportion having surveillance colonoscopy to 60% (data not shown). Extending the interval by 6 months for the 3-year follow-up recommendation and by 6 months for the 1-year follow-up recommendations increased the overall proportions with timely adherence to 64%. This means that 26% of the eligible individuals ended up with either no surveillance colonoscopy or had it delayed by more than 1 year (data not shown).
Table 2 Adherence to baseline, first, and second surveillance colonoscopy.
| Adherence to baseline colonoscopy after a positive FIT test result | ||||
| *Including incomplete colonoscopies initiated within 30 days. †Medium-risk patients are recommended follow-up colonoscopy after 3 years. N=371 were excluded because of death, emigration, or codes for severe comorbidity before the follow-up was due. High-risk patients are recommended follow-up colonoscopy after 1 year. N=69 were excluded (N=22 among those with piecemeal resection). ‡Of those 1,035 (medium risk) and 580 (high-risk) was non-colonoscopy follow-up. §Patients with negative and low-risk assessments at first surveillance after initial high-risk assessment at baseline colonoscopy are recommended to undergo a new colonoscopy in 3 years. N=403 were excluded because of death, emigration, codes for severe comorbidity, or the end of study on June 30, 2022, which was earlier than when follow-up exam was due. Patients with medium-risk assessments at first surveillance after initial high-risk assessments at baseline colonoscopy were recommended to undergo a new colonoscopy in 3 years. N=141 were excluded. Patients with high-risk assessments at first surveillance and baseline colonoscopies were recommended to under a new colonoscopy in 1 year. N=47 were excluded. | ||||
| Full adherence, within the recommended time (<31 days) and with a colonoscopy* | Late follow-up (31–91 days) or procedures other than colonoscopy | No follow-up within 3 months | ||
| Total (N=82,221) | 69,123 (84.1%) | 6,879 (8.4%) | 6,219 (7.6%) | |
| First surveillance for patients with intermediate - and high-risk adenomas at baseline colonoscopy (N=21,799 † ) | ||||
| Medium-risk adenomas at baseline colonoscopy (recommended: 3 years) | ||||
| On time (2.5–3.5 years) | Late (>3.5 years) | Early (<2.5 years) | No 1 st surveillance colonoscopy | |
| Total (N=12,843) | 6,632 (51.6) | 2,888 ‡ (22.5) | 1,766 (13.8) | 1,557 (12.1) |
| High-risk adenomas at baseline colonoscopy (recommended: 1 year) | ||||
| On time, 9–15 months (3–15 months if piecemeal resection) | Late, >15 months | Early, <9 months (1–3 months if piecemeal resection) | No 1 st surveillance colonoscopy | |
| Total (N=8,956) | 4,879 (54.5) | 2,449 ‡ (27.3) | 1,080 (12.1) | 548 (6.1) |
| Of which piecemeal resection (N=2,048) | 1,266 (61.8) | 529 (25.8) | 154 (7.5) | 99 (4.8) |
| Second surveillance for patients with high-risk adenomas at baseline colonoscopy (N=5,454 § ) | ||||
| Negative or low risk at first surveillance colonoscopy | ||||
| On time (2.5–3.5 years) | Late (>3.5 years) | Early (<2.5 years) | No 2 nd surveillance colonoscopy | |
| Total (N=3,190) | 1,083 (34.0) | 832 (26.1) | 668 (20.9) | 607 (19.0) |
| Medium-risk at first surveillance colonoscopy | ||||
| On time (2.5–3.5 years) | Late or other procedures (>3.5 years) | Early (<2.5 years) | No 2 nd surveillance colonoscopy | |
| Total (N=1,381) | 444 (32.2) | 371 (26.9) | 390 (28.2) | 176 (12.7) |
| High-risk at first surveillance colonoscopy | ||||
| On time, 9–15 months (3–15 months if piecemeal resection) | Late, (>15 months) | Early, <9 months (1–3 months if piecemeal resection) | No 2 nd surveillance colonoscopy | |
| Total (N=883) | 466 (52.8) | 228 (25.8) | 103 (11.7) | 86 (9.7) |
Risk assessment codes were not used to the same extent in the surveillance program, because 2,307 (27%) could not be risk-stratified. Among the 6,101 individuals (73%) that we were able to risk-stratify, only about one-third of those with low-risk or intermediate-risk assessment attended for the second surveillance colonoscopy on time ( Table 2 ). The proportion without a second surveillance colonoscopy was 19% among clean-colon or low-risk adenomas and 13% among intermediate-risk adenomas ( Table 2 ). After two high-risk assessments, 53% had timely and 10% had no second surveillance. The sensitivity analyses extending the “on time” period by 3 months increased the proportion with timely second surveillance from 37% to 43%. Extending the 3-year and 1-year follow-up recommendations by 6 months increased the proportion with timely follow-up to 47% (data not shown).
Women, the youngest and oldest age groups, and those with any comorbidity were more likely to not undergo baseline colonoscopy, whereas older age and any comorbidity were associated with deviations from recommendations for the first surveillance exam. Similar patterns were seen for the second surveillance, although they were not statistically significant in case of comorbidity ( Table 3 ).
Table 3 Relative risk of deviations from the recommended baseline colonoscopy and first surveillance colonoscopy, by sex, age at screening, and comorbidity index status at screening.
| All deviations vs. full adherence to baseline colonoscopy RR (95% CI) | All deviations vs. full adherence to first surveillance colonoscopy among intermediate and high-risk assessments at baseline RR (95% CI) | All deviations vs. full adherence to second surveillance colonoscopy among high-risk assessments at baseline RR (95% CI) | |
| NOTE: non-significant interactions between age and comorbidity are present. RR, relative risk; CI, confidence interval. | |||
| Men | ref | ref | ref |
| Women | 1.07 (1.03; 1.10) | 1.02 (0.99; 1.05) | 1.04 (0.99; 1.08) |
| 49–54 years | 1.19 (1.11; 1.27) | 1.03 (0.97; 1.09) | 0.94 (0.86; 1.02) |
| 55–59 years | 1.06 (0.99; 1.14) | 1.03 (0.98; 1.09) | 1.00 (0.93; 1.08) |
| 60–64 years | ref | Ref | Ref |
| 65–69 years | 1.05 (0.98; 1.12) | 1.04 (0.99; 1.08) | 0.97 (0.91; 1.03) |
| 70–75 years | 1.20 (1.13; 1.28) | 1.14 (1.09; 1.18) | 1.08 (1.02; 1.14) |
| No comorbidity | ref | ref | Ref |
| Mild comorbidity | 1.27 (1.16; 1.39) | 1.10 (1.06; 1.13) | 1.04 (0.99; 1.09) |
| Moderate comorbidity | 1.78 (1.54; 2.06) | 1.24 (1.17; 1.31) | 1.07 (0.98; 1.17) |
| Severe comorbidity | 1.65 (1.32; 2.04) | 1.24 (1.14; 1.35) | 1.08 (0.93; 1.24) |
Table 4 shows that among the 3% of individuals who could not be risk-stratified after baseline colonoscopy, almost 27% underwent at least one colonoscopy and 15% underwent polypectomy in the following 4 years ( Table 4 ). Among those with a clean colon, the proportions were 12% and 4%, respectively, while among those with a low-risk baseline colonoscopy, the proportions were 22% and 13%, respectively. These two groups contributed 9,835 colonoscopies and 4,503 polypectomies, or 27% of all colonoscopies and 22% of all polypectomies performed in the study population during this period ( Table 5 ).
Table 4 Number and proportions of individuals attending diagnostic and treatment procedures within 4 years after the baseline colonoscopy, by risk assessment at baseline colonoscopy.
| Number and proportions of individuals with at least one of the following procedures after the initial assessment * | Clean colon N=26,479 | Low risk † N=19,700 | Medium risk N=13,214 | High risk N=9,024 | No risk assessment N=2,127 | Cancer N=4,564 | Total N=75,108 |
| *Not including the period and procedures during the baseline assessment. †Including also clean-colon assessments after computed tomography colonography. ‡After first colonoscopy and not latest date in the diagnostic period (up to 3 months after first colonoscopy). | |||||||
| Colonoscopy, N (%) | 3,260 (12) | 4,350 (22) | 9,387 (71) | 7,609 (84) | 571 (27) | 2.308 (51) | 27,485 (37) |
| Other endoscopic procedures, N (%) | 1,753 (7) | 1,208 (6) | 1,014 (8) | 1,341 (15) | 249 (12) | 1,540 (34) | 7,105 (10) |
| CT colonography, N (%) | 101 (0) | 167 (1) | 159 (1) | 99 (1) | 42 (2) | 24 (1) | 592 (1) |
| Colorectal surgery, N (%) | 274 (1) | 332 (2) | 352 (3) | 576 (6) | 57 (3) | 3,789 ‡ (83) | 5,380 (7) |
| Colorectal biopsies, N (%) | 1,486 (6) | 1,542 (8) | 1,773 (13) | 2,127 (24) | 220 (10) | 1055 (23) | 8,206 (11) |
| Colorectal polypectomy, N (%) | 1,157 (4) | 2,492 (13) | 5,367 (41) | 4,989 (55) | 325 (15) | 1,363 (30) | 15,693 (21) |
Table 5 Number of diagnostic procedures performed in a 4-year period after the baseline colonoscopy, by initial risk assessment/diagnosis (whereby one patient from Table 4 could have several procedures of the same kind).
| Resource use after the baseline colonoscopy and for the proceeding four years, by risk group assessment at baseline colonoscopy (N=75,108)* | Clean colon | Low risk † | Medium risk | High risk | No risk assessment | Cancer |
Total
N (%) |
| *Not including the period and procedures during the baseline assessment. †Including also clean-colon assessments after computed tomography colonography. ‡After first colonoscopy and not latest date in the diagnostic period (up to 3 months after first colonoscopy). §Only counted one per visit. CT, computed tomography. | |||||||
| Number of colonoscopies | 4,086 (11) | 5,749 (16) | 11,199 (30) | 11,382 (31) | 842 (2) | 3,640 (10) | 36,898 (100) |
| Number of other endoscopic procedures | 3,059 (23) | 1,908 (14) | 1,515 (11) | 2,098 (16) | 372 (3) | 4,325 (32) | 13,277 (100) |
| Number of CT colonographies | 110 (17) | 175 (28) | 162 (26) | 103 (16) | 44 (8) | 27 (4) | 621 (100) |
| Number of colorectal surgeries | 321 (5) | 373 (6) | 386 (6) | 640 (10) | 64 (1) | 4367 ‡ (71) | 6,151 (100) |
| Number of biopsies | 1,864 (18) | 1,872 (18) | 2,066 (20) | 2,656 (26) | 298 (3) | 1,462 (14) | 10,218 (100) § |
| Number of polypectomies | 1,394 (7) | 3,109 (15) | 6,269 (31) | 7,236 (35) | 426 (2) | 2,057 (10) | 20,491 (100) § |
The sensitivity analysis excluding 2,643 residents of the Central Denmark Region showed similar results (not reported).
Discussion
Summary of main results
As expected, most individuals with positive FIT tests received a baseline colonoscopy. Nevertheless, adherence to first and second surveillance colonoscopies was substantially lower. Variation in time at which these surveillance colonoscopies took place was substantial. On average, the endoscopic resources to investigate intermediate- and high-risk adenomas were in accordance with the recommended surveillance protocols. However, the expected colonoscopy savings from the 8-year quarantine strategy after normal colonoscopies may not be as large as expected, because this group still is associated with a significant proportion of colonoscopies.
Comparison with other studies and implications for practice
The high rate of compliance with baseline colonoscopy, 84%, is similar to that observed in the Dutch screening program, reported as 82% 24 . Several other programs have been less successful. The Catalan FIT-based program, for example, recorded 61% adherence within 60 days 25 . The tendency toward a diminishing adherence at the stage of surveillance has also been seen elsewhere. Our findings of approximately 50% timely adherence to the first and approximately 30% to 50% timely adherence to the second surveillance examination were broadly in line with a meta-analysis in which adherence at either stage was on average 49% 9 . Another meta-analysis focused specifically on any adherence to second surveillance and reported a pooled estimate of 72% 26 . In Denmark, this was approximately 85% overall, with a large variation in timing ( Table 2 ). Nevertheless, these findings do show that the Danish program has managed to achieve somewhat higher adherence compared with other European programs 9 25 . One explanation for this favorable observation could be the national screening implementation strategy with a national steering group that was appointed to monitor outcomes of the program. Furthermore, in the beginning, there were no additional qualifications required for endoscopists examining individuals with positive screening tests. This fact considerably increased available colonoscopy capacity 13 . Finally, all Danish residents have a national personal identification number, which is used for all communication with health services and makes it easy to track and reinvite those who have moved to another region.
Failure to obtain follow-up after a positive FIT test is associated with a higher risk of CRC and CRC-specific mortality 27 . Of the 6,219 individuals (7.8%) in our study who had no follow-up 3 months after a positive FIT test, 70% had no endoscopic procedures performed during the next 4 years (Supplementary material, Fig. S4). Ten percent for whom first surveillance and 15% of for whom second surveillance was recommended no longer engaged with the service ( Table 2 ). As in other screening programs, the proportions with no follow-up varied with the severity of the findings at the previous colonoscopy 9 28 Although this was a register-based study and we were not able to determine the reasons for non-adherence, they could be either patient-driven or due to organizational failures, including capacity constraints. A qualitative study found that the most frequent patient-driven cause was unwillingness to participate in surveillance 29 . Of those who did not attend the first or second surveillance in Denmark, we suspect that 20% to 30% may have been discharged from surveillance due to age, potentially in combination with comorbidity (data not shown). International guidelines recommend ending surveillance after age 75 years, but because that was not explicitly described in the Danish guidelines, there might not have been complete consensus about it among endoscopists.
Colonoscopy use after normal and low-risk assessments
We found that 22% of patients with low-risk assessments at baseline colposcopy had a colonoscopy despite it not being recommended for surveillance. Although this may signal overuse of diagnostic services, we should contrast it with the recent finding that only 72% of these individuals attended for subsequent screening 30 , with a potentially high persistent FIT test positivity rate at that screen 31 . We do not have data to describe the overlap between those with unexpected colonoscopies and those with positive tests at the next screen.
A previous Danish study describing the phase during the gradual implementation of the screening program showed that the yearly average use of colonoscopies was 13.6/1000 among “not yet invited” individuals in the program’s target age range. 32 In comparison, our estimate of 4,086 colonoscopies over a 4-year period after a clean colonoscopy corresponds to on average of 39/1000 per year in this group. Although other countries have reported even higher proportions of colonoscopies after normal and low-risk assessments 7 8 9 10 , this rate is high, given that the risk after a clean high-quality colonoscopy is low compared with the general population 33 . However, the subgroup with additional colonoscopies might still have a higher risk of other colorectal diseases, such as diverticulitis, upper gastrointestinal disease, or hemorrhoids, because they did present with a positive FIT test. Nevertheless, their use of colonoscopy should be monitored carefully. In fact, in a US setting with opportunistic screening with short re-screening intervals, Kruse and colleagues estimated that 88% of colonoscopies performed after a normal screening colonoscopy represented overuse, i.e. they were not due to symptoms 10 . Early colonoscopies may put a strain on colonoscopy services if they are not performed because of symptoms or other conditions. In Denmark, early colonoscopies represented approximately 12% of first and 11% to 28% of second surveillance colonoscopies. A meta-analysis reported that 42% of the time, endoscopists requested colonoscopies within a shorter follow-up interval than recommended 9 . Another study found that the reasons for endoscopists recommending a shorter surveillance interval included individual assessments including poor bowel preparation, fear of missed polyps, and family history of CRC 34 .
Danish guidelines for adenoma surveillance were changed in 2023 to follow the 2020 European Society of Gastrointestinal Endoscopy recommendations. In these guidelines, adenomas at risk are allocated to 3-year surveillance, thus more individuals will be discharged to routine screening after high-quality colonoscopy 35 . In light of our findings, clinicians and healthcare planners should be aware that the expected lower demand for colonoscopy services might not be fully realized if the guidelines are not adhered to. Post-hoc analyses showed that approximately 27% of intermediate-risk adenomas will be allocated to the low-risk group under the new guidelines (polyps <10 mm, no adenomas with high-grade dysplasia, and no sessile lesions with dysplasia), and thus, return to FIT screening in 2 years. In addition, 3-year instead of 1-year surveillance will be recommended to all individuals with high-risk adenomas. While these figures may be quite accurate and most likely will lead to a reduction in colonoscopy demand, quantification of the impact on colonoscopy resources is much more uncertain, because positivity and adherence rates may differ with the new risk stratification.
Strengths and limitations
Danish healthcare registers are valid and very complete 16 17 18 19 . We linked several registers to fully capture the entire screening and surveillance pathways. At least 4.5 years of follow-up was available for all screened individuals with positive FIT tests.
One of the limitations of our study is that, due to late implementation of the program and the long prevalence round, our estimates may not be fully generalizable to the subsequent screening rounds. Second, while 97% of the population could be risk-stratified at the baseline colonoscopy, it was only possible for approximately 70% at surveillance colonoscopies. Lastly, the COVID-19 pandemic did not disrupt the screening program in Denmark, although it may have led to postponement of some of the surveillance examinations in the spring of 2020. A separate analysis performed for screening in 2014 and 2015 showed that adherence to second surveillance among individuals whose baseline assessment showed them to be high risk but low-risk/normal or intermediate risk at first surveillance was about 10 percentage points better than in the main analysis.
Conclusions
Although adherence to baseline colonoscopy after a positive FIT screening test was high, that trend did not continue throughout the adenoma surveillance program and requires further elucidation. In addition, more research is needed to determine whether colonoscopies after a clean colon and low-risk assessments represent excess use of colonoscopy services. An improvement in these indicators may improve the effectiveness and efficiency of the screening program.
Acknowledgement
We would like to acknowledge the steering group of the Danish Colorectal Cancer Screening database (DTS) and the Danish Clinical Quality Program – National Clinical Registries (RRKP) for the availability and delivery of data for this study.
Footnotes
Conflict of Interest Matejka Rebolj declares the following conflict of interest: The former Public Health England (now: NHS England and Department of Health/Office for Health Improvement and Disparities) provided financing for the epidemiological evaluation of various cervical screening studies; current and former member of various expert groups providing advice to the English Cervical Screening Programme; attended meetings with HPV test manufacturers; fees for attendance at advisory board and other meetings organised by Hologic, shared with employer, including travel cost reimbursement if applicable. The remaining authors have no conflict of interest to declare.
Supplementary Material
References
- 1.Larsen MB, Njor S, Ingeholm P et al. Effectiveness of colorectal cancer screening in detecting earlier-stage disease-a nationwide cohort study in Denmark. Gastroenterology. 2018;155:99–106. doi: 10.1053/j.gastro.2018.03.062. [DOI] [PubMed] [Google Scholar]
- 2.Njor SH, Larsen MB, Søborg B et al. Colorectal cancer mortality after randomized implementation of FIT-based screening – a nationwide cohort study. J Med Screening. 2022;29:241–248. doi: 10.1177/09691413221102212. [DOI] [PubMed] [Google Scholar]
- 3.Ebell MH, Thai TN, Royalty KJ. Cancer screening recommendations: an international comparison of high income countries. Public Health Rev. 2018;39:7–18. doi: 10.1186/s40985-018-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JK, Jensen CD, Levin TR et al. Long-term risk of colorectal cancer and related death after adenoma removal in a large, community-based population. Gastroenterology. 2020;158:884–INF. doi: 10.1053/j.gastro.2019.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferlay J EM, Lam F, Colombet M et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; 2020. https://gco.iarc.fr/today. https://gco.iarc.fr/today.
- 6.Ringgaard A. Dagens Medicin; 2015. Kræftscreening sætter midtjyske hospitaler under pres. [Google Scholar]
- 7.Gessl I, Waldmann E, Britto-Arias M et al. Surveillance colonoscopy in Austria: Are we following the guidelines? Endoscopy. 2018;50:119–127. doi: 10.1055/s-0043-119637. [DOI] [PubMed] [Google Scholar]
- 8.van Heijningen EM, Lansdorp-Vogelaar I, Steyerberg EW et al. Adherence to surveillance guidelines after removal of colorectal adenomas: a large, community-based study. Gut. 2015;64:1584–1592. doi: 10.1136/gutjnl-2013-306453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Djinbachian R, Dubé AJ, Durand M et al. Adherence to post-polypectomy surveillance guidelines: a systematic review and meta-analysis. Endoscopy. 2019;51:673–683. doi: 10.1055/a-0865-2082. [DOI] [PubMed] [Google Scholar]
- 10.Kruse GR, Khan SM, Zaslavsky AM et al. Overuse of colonoscopy for colorectal cancer screening and surveillance. J Gen Internal Med. 2015;30:277–283. doi: 10.1007/s11606-014-3015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoen RE, Pinsky PF, Weissfeld JL et al. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010;138:73–81. doi: 10.1053/j.gastro.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy CC, Sandler RS, Grubber JM et al. Underuse and overuse of colonoscopy for repeat screening and surveillance in the Veterans Health Administration. Clin Gastroenterol Hepatol. 2016;14:436–INF. doi: 10.1016/j.cgh.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundhedsstyrelsen. Anbefalinger vedrørende screening for tyk- & endetarmskræft 2012 [Recommendations regarding colo-rectal cancer screening 2012]. 2012. Contract No.: Report
- 14.Atkin WS, Valori R, Kuipers EJ et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Colonoscopic surveillance following adenoma removal. Endoscopy. 2012;44:Se151–163. doi: 10.1055/s-0032-1309821. [DOI] [PubMed] [Google Scholar]
- 15.Tarmkræftscreeningsdatabase SfD. Årsrapport 2019. Dansk Tarmkræftscreeningsdatabase. RKKP; 2021
- 16.Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39:42–45. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
- 17.Bjerregaard B, Larsen OB. The Danish Pathology Register. Scand J Public Health. 2011;39:72–74. doi: 10.1177/1403494810393563. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M, Schmidt SAJ, Sandegaard JL et al. The Danish National Patient Registry: a review of content, data quality, and research potential.(Report) Clin Epidemiol. 2015;7:449. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahl Andersen J, De Fine Olivarius N, Krasnik A. The Danish National Health Service Register. Scand J Public Health. 2011;39:34–37. doi: 10.1177/1403494810394718. [DOI] [PubMed] [Google Scholar]
- 20.Danish Pathology Association. Screenings – og adenomkontrolprogram for colorectal cancer [in Danish]: Danish Pathology Association; 2014. https://danskpatologi.org/wp-content/uploads/2016/02/Screenings-og-adenomkontrol-program-tyk-og-endtarmskr%C3%A6ft.pdf. https://danskpatologi.org/wp-content/uploads/2016/02/Screenings-og-adenomkontrol-program-tyk-og-endtarmskr%C3%A6ft.pdf.
- 21.Klabunde CN, Zheng Y, Quinn VP et al. Influence of age and comorbidity on colorectal cancer screening in the elderly. Am J Prevent Med. 2016;51:e67–e75. doi: 10.1016/j.amepre.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomsen MK, Rasmussen M, Njor SH et al. Demographic and comorbidity predictors of adherence to diagnostic colonoscopy in the Danish Colorectal Cancer Screening Program: a nationwide cross-sectional study. (ORIGINAL RESEARCH) Clin Epidemiol. 2018;10:1733. doi: 10.2147/CLEP.S176923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juul FE, Garborg K, Nesbakken E et al. Rates of repeated colonoscopies to clean the colon from low-risk and high-risk adenomas: results from the EPoS trials. Gut. 2023;72:951–957. doi: 10.1136/gutjnl-2022-327696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toes-Zoutendijk E, van Leerdam ME, Dekker E et al. Real-time monitoring of results during first year of Dutch colorectal cancer screening program and optimization by altering fecal immunochemical test cut-off levels. Gastroenterology. 2017;152:767–INF. doi: 10.1053/j.gastro.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 25.Binefa G, Garcia M, Milà N et al. Colorectal cancer screening programme in Spain: Results of key performance indicators after five rounds (2000–2012) Scientific Rep. 2016;6:19532. doi: 10.1038/srep19532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gingold-Belfer R, Leibovitzh H, Boltin D et al. The compliance rate for the second diagnostic evaluation after a positive fecal occult blood test: A systematic review and meta-analysis. United Europ Gastroenterol J. 2019;7:424–448. doi: 10.1177/2050640619828185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doubeni CA, Fedewa SA, Levin TR et al. Modifiable failures in the colorectal cancer screening process and their association with risk of death. Gastroenterology. 2019;156:63–7.4E67. doi: 10.1053/j.gastro.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chubak J, McLerran D, Zheng Y et al. Receipt of colonoscopy following diagnosis of advanced adenomas: an analysis within integrated healthcare delivery systems. Cancer Epidemiol Biomarkers Prev. 2019;28:91–98. doi: 10.1158/1055-9965.EPI-18-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gauci C, Lendzion R, Phan-Thien K-C et al. Patient compliance with surveillance colonoscopy: patient factors and the use of a graded recall system. ANZ Journal of Surgery. 2018;88:311–315. doi: 10.1111/ans.14296. [DOI] [PubMed] [Google Scholar]
- 30.Bülow Therkildsen S, Larsen PT, Njor S. Subsequent participation in organized FIT based screening following screen-derived colonoscopy – A Danish nationwide cohort study. Prev Med Rep. 2023;32:102125. doi: 10.1016/j.pmedr.2023.102125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark GR, Fraser CG, Strachan JA et al. Comparison with first round findings of faecal haemoglobin concentrations and clinical outcomes in the second round of a biennial faecal immunochemical test based colorectal cancer screening programme. J Med Screening. 2022;29:249–254. doi: 10.1177/09691413221110012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsen MB, Njor SH, Jensen TM et al. Potential for prevention: a cohort study of colonoscopies and removal of adenomas in a FIT-based colorectal cancer screening programme. Scand J Gastroenterol. 2019;54:1008–1014. doi: 10.1080/00365521.2019.1647282. [DOI] [PubMed] [Google Scholar]
- 33.Cross AJ, Robbins EC, Pack K et al. Long-term colorectal cancer incidence after adenoma removal and the effects of surveillance on incidence: a multicentre, retrospective, cohort study. Gut. 2020;69:1645–1658. doi: 10.1136/gutjnl-2019-320036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson JC, Baron JA, Ahnen DJ et al. Factors associated with shorter colonoscopy surveillance intervals for patients with low-risk colorectal adenomas and effects on outcome. Gastroenterology. 2017;152:1933–INF. doi: 10.1053/j.gastro.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassan C, Antonelli G, Dumonceau JM et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline – Update 2020. Endoscopy. 2020;52:687–700. doi: 10.1055/a-1185-3109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.