Abstract
This literature review provides an up-to-date exploration of the multifaceted attributes of maitake mushrooms (Grifola frondosa), elucidating their bioactive phytochemicals and diverse health advantages, including their substantial role in supporting human health and potential incorporation into the medicinal industry. Carbohydrates and protein are the major constituents contributing to the dry weight of G. frondosa, taking up around 70–80 % and 13–21 %, respectively, with emerging research linking these constituents to various health benefits. By synthesising current research findings, this review emphasises the substantial role of maitake mushrooms in supporting human health and underscores their potential incorporation into the medicinal industry. To further advance our understanding, future research should delve into the mechanisms underlying their health-promoting effects, with a focus on conducting quantitative studies to elucidate physiological pathways and potential drug interactions. Additionally, exploring their integration into functional foods or nutraceuticals through quantitative assessments of bioavailability and efficacy will be crucial for maximising their therapeutic benefits. This review aims to provide comprehensive insights, catalysing further research and innovation in utilising maitake mushrooms for improved well-being and industry advancement.
Keywords: Maitake mushrooms, Grifola frondose, Anti-Cancer, Anti-microbial, Anti-diabetic, Immunomodulation
1. Introduction
The maitake mushroom (Grifola frondosa), renowned for its substantial size and belonging to the Polyporaceae family, traces its origins to northern Japan [1]. With a distinctly earthy flavour, captivating aroma, and robust, meat-like texture, it has long been cherished in traditional Asian medicine and cuisine [2]. Notably versatile in culinary applications, maitake mushrooms are prized ingredients in sautés, risottos, and as meat substitutes in Western dishes. Both the fruiting bodies and fungal mycelium of this edible polypore fungus, characterized by overlapping caps and a smoky brown hue [3], boast a rich history of health-promoting benefits, firmly rooted in East Asian traditional medicine. Thus, beyond its culinary versatility, maitake mushrooms have emerged as a beacon of novelty and innovation, inspiring chefs and researchers alike to explore their untapped potential.
While historically treasured for their adaptogenic properties, recognised for enhancing vitality and bolstering the immune system, maitake mushrooms beckon attention for their novel applications and potential benefits [4]. Referred to as "hen-of-the-woods" in traditional Japanese and Chinese medicine and symbolising joy in their discovery ("Dancing Mushrooms") [5], these mushrooms hold cultural significance beyond their medicinal properties. In Chinese tradition, they are also known as "hui-shu-hua" or "grey tree flower," owing to their distinctive appearance [6].
Recent years have witnessed a resurgence of interest in maitake mushrooms, particularly in the realms of alternative medicine and functional foods [7]. Cultivation techniques, ranging from traditional log-based methods to innovative sawdust substrates, testify to the mushroom's adaptability and accessibility on a global scale. Within their unassuming caps lies a treasure trove of bioactive compounds—polysaccharides, glucans, triterpenes, and phenolic compounds—each with their own unique promise. These compounds exhibit not only immunomodulatory and anti-inflammatory properties but also novel anticancer and antioxidant potentials amongst others [[8], [9], [10]]. Therefore, throughout this review, the medicinal benefits and future prospects of G.frondosa are highlighted.
2. Methodology
2.1. Literature search strategy
A comprehensive literature search was conducted to identify relevant studies for the review. The search was carried out in electronic databases including PubMed, Scopus, and Google Scholar. Keywords used included "maitake mushrooms," "Grifola frondosa,” "phytochemicals in maitake mushrooms," "health benefits of maitake mushrooms," "anti-cancer and immunomodulating mechanisms of maitake mushrooms," "anti-microbial properties of maitake mushrooms," "anti-diabetic effects of maitake mushrooms," "maitake mushrooms in cosmetics," and related terms.
2.2. Inclusion and exclusion criteria
This review included studies centred on investigating the phytochemical composition of maitake mushrooms. It encompassed research elucidating the diverse health benefits of maitake mushrooms, encompassing their anti-cancer, anti-microbial, anti-diabetic, and immunomodulatory effects as well as their potential for skin care applications. Studies selected for inclusion were sourced from reputable journals and were included if the data was sufficient and relevant.
Studies that primarily focused on mushroom species other than maitake were excluded from this review. Non-English articles lacking comprehensive translations were not included. Studies with insufficient relevant data were excluded.
3. The geographical distribution of maitake mushroom
The maitake mushroom, widely distributed throughout the Northern Hemisphere [11], thrives primarily in temperate woodlands across Asia, Europe, and North America [4]. Revered in Japan for centuries for its culinary and medicinal qualities, maitake mushrooms also grace the forests of France, Italy, and Poland in Europe [12]. However, they are most commonly found in the northeastern and midwestern regions of North America. Typically flourishing at the bases of hardwood trees such as oak, maple, and elm, maitake mushrooms favour damp, shaded environments [2,3].
4. The life cycle of maitake mushroom
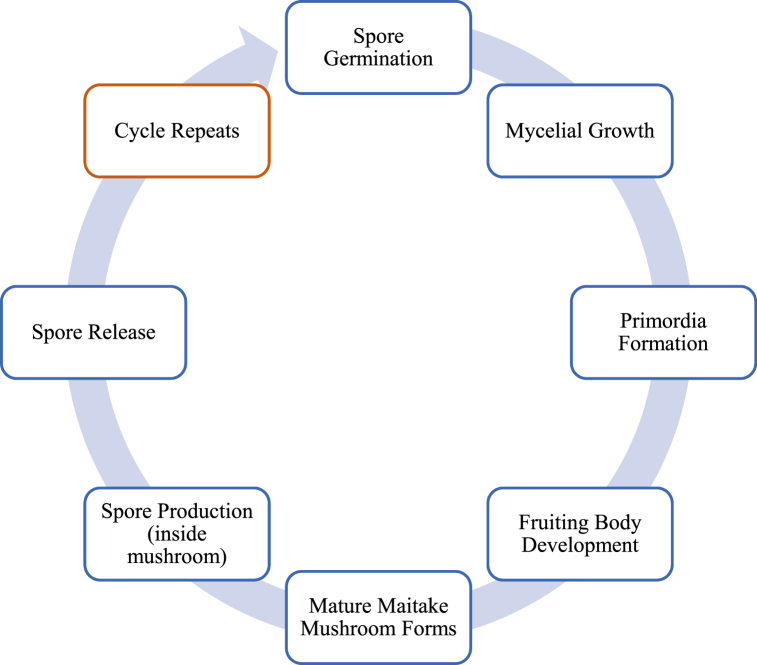
As shown in Fig. 1, the life cycle of the maitake mushroom epitomises the characteristics of basidiomycetes [13], a group of fungi distinguished by the presence of basidia, specialised structures containing spores. This cycle begins with the sexual reproduction of the maitake mushroom, where haploid spores from two compatible mating strains merge. Germination of these haploid spores initiates the life cycle, leading to the formation of hyphae, branching filaments constituting the fungus's vegetative body. As the hyphae grow and proliferate, they form a network known as mycelium, serving as the structural framework for nutrient absorption [4]. Under favourable conditions, maitake mycelia generate primordia, specialised structures signalling the onset of fruiting. With further differentiation, these primordia develop into mature fruiting bodies, commonly recognised as maitake mushrooms. These mushrooms exhibit distinctive features such as overlapping caps and tightly clustered, downward-hanging, fan-shaped fronds, contributing to their characteristic appearance [14]. During the reproductive phase, basidia located inside the pores or gills on the mushroom's underside undergo meiosis, producing haploid basidiospores [15,16]. The primary mode of dispersal for the species involves the release of these basidiospores into the surrounding environment [17].
Fig. 1.
A depicted summary of the maitake mushroom's life cycle.
5. The chemical components of maitake mushroom
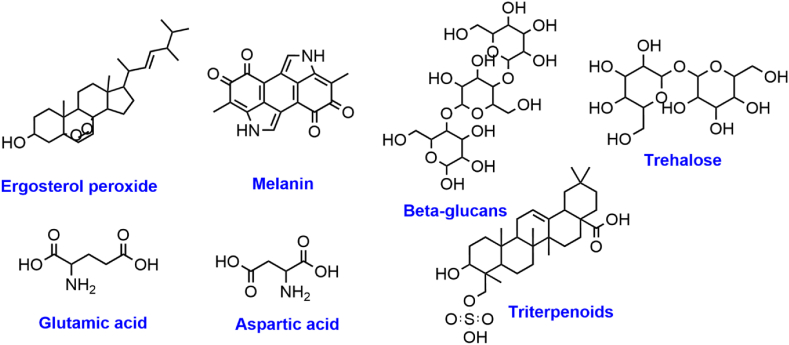
The complex chemical makeup of maitake mushrooms adds to their distinct flavour, fragrance, and health advantages. Some components such as trehalose, glutamic acid, aspartic acid, and 5′-nucleotides provide this fungus with its distinctive flavour [18]. As seen in Fig. 2, numerous bioactive substances, including polysaccharides, β-D-glucans, ergosterol, lactulose, dextrin, oligofructose, triterpenes, and different phenolic compounds, are present in maitake mushrooms contributing to their health benefits [2,19]. Additionally having high nutritional value, maitake also has pharmacological properties, such as anticancer activity linked to its β-D -glucan content. These complex carbohydrates possess immunomodulatory activities, which improve the performance of the immune system and have anti-cancer properties [2].
Fig. 2.
Some prominent bioactive compounds found in the mycelium and the fruiting bodies of Maitake mushrooms. It can be appreciated that these bioactive chemicals in Maitake play a pivotal role in contributing to its various medicinal attributes.
More importantly, maitake synthesises several polysaccharide fractions with anti-inflammatory, anticancer, antiviral, and immunomodulatory properties. Very recently, polysaccharides extracted from this fungus have been found to alter the gut microbiota [20,21], which may also affect immunological homeostasis and result in anticancer effects. Additionally, these polysaccharides may control the makeup of the gut microbiota in the treatment of metabolic diseases and such extracts obtained from this mushroom may act as a probiotic [[21], [22], [23]] Additionally, maitake has proteins and tiny biomolecules with a range of health advantages, such as antioxidant, immune-boosting, anti-diabetic, anti-obesity and other actions [[24], [25], [26]].
Furthermore, when exposed to ultraviolet light, the sterol component ergosterol, which is present in maitake mushrooms, acts as a precursor to producing vitamin D. Triterpenes, a different family of substances found in maitake mushrooms, have anti-inflammatory and anticancer properties, which makes them highly enticing for pharmaceutical study [27,28]. Maitake mushrooms also contain phenolic substances such as flavonoids and phenolic acids, which are known for their antioxidant effects [13]. Numerous health advantages, including cardiovascular protection and anti-ageing effects, have been linked to these substances [29].
Additionally, maitake can degrade lignocellulose by producing different enzymes such as endoglucanases, exogloconases, β-glucosidases, xylanases, lignin peroxidase (LiP), Mn-peroxidase (MnP) and laccases (Lacc) [14]. Thus, Maitake can be used for the biodegradation of different toxic compounds. Maitake has also been shown to have mercury absorption activity [30].
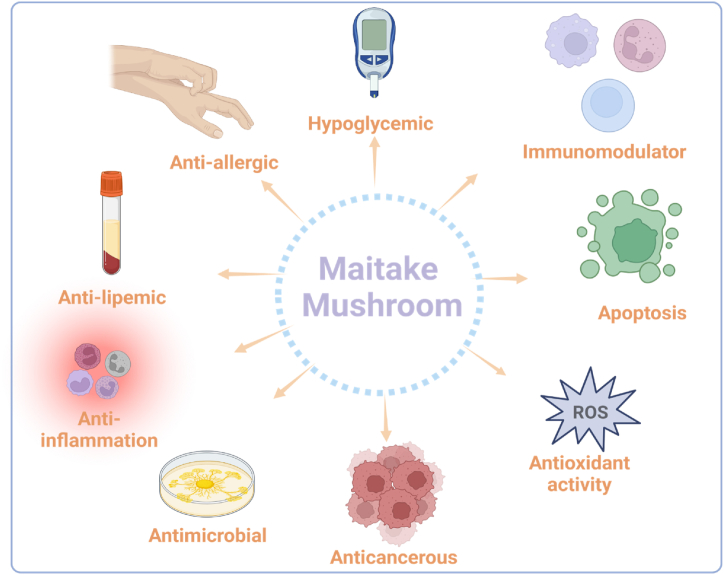
As depicted in Fig. 3, Maitake mushrooms are a significant source of natural bioactive substances with a range of medicinal uses because of their complex chemical makeup [5]. To completely understand the precise mechanisms and advantages connected with the many chemical components contained in this amazing fungus, further scientific research is required [28]. Recently, the albino mutation in G. frondosa was determined to be caused by a single base deletion in the coding region of the tyronisase2 (tyr2) [31]. Because of the lack of undesirable dark brown pigment during processing, these white strains are highly prized for culinary use [31].
Fig. 3.
Utilisation of the extracts of Maitake mushrooms for curing different ailments. As seen in the image below extracts obtained from Maitake mushrooms can aid in managing several diseases such as cancer, inflammation and allergies.
6. Antimicrobial activity of maitake mushroom
Maitake extract has antibacterial activity (against S. epidermis and P.aeruginosa), and antifungal activity furanone from maitake (Pseudallescheria boydii). Ethanol extracts of G. frondosa (rich in organic acids, alkaloids, phenolics, and terpenoids) increased microbiota levels of Oscillibacter, Butyricimonas, Barnesiella, Turicibacter, Methanosphaera, Asaccharobacter, Globicatella, Bifidobacterium, Allobaculum, and Romboutsia [32]. Despite this, the antimicrobial activity of maitake mushrooms is still poorly understood. This is evidenced by the small number of scientific publications on this subject.
GFAHP, a protein obtained from an extract of G. frondosa fruiting bodies, has anti-herpes simplex virus (HSV) activity. In plaque reduction assay mean IC50 for GFAHP was 4.1 μg/ml and was ten times higher than for acyclovir. The studies found that GFAHP at a concentration of 30 or 60 μg/ml had no effect on the inhibition of HSV-1 attachment but inhibited HSV-1 penetration by 83.7 % and 93.5 %, respectively. Simultaneously, high concentrations of GFAHP (125 and 500 μg/ml) significantly reduced virus production and the severity of HSV-1-induced ocular diseases in animal models [33]. D-fraction obtained from the G. frondosa fruiting bodies showed an effect against the hepatitis B virus (HBV). D-fraction inhibited HBV DNA in HepG2 cells with the IC50 of 0.59 mg/ml. At the same time, it was found that D-fraction can act synergistically with interferon (IFN) and increase its antiviral activity up to 9 times [34]. A β-glucan (MD-fraction) extracted from G. frondosa has a positive effect on HIV patients. 35 patients were administered Maitake tablets containing 250 mg of dried Maitake powder and 5 mg of vitamin C for 12 months. In 20 persons, an increase of CD4+ cell amount was reported and in 10 people was reported a decrease in viral load [35]. Ethanolic extract of G. frondosa has activity against enterovirus EV71 cultured in Vero cells. The extract inhibited EV71 viral replication, VP1 protein expression, and genomic RNA synthesis. The best activity was observed at 200 μg/ml concentration with an inhibition rate of 88.18 % and IC50 of 194.80 μg/ml [36]. Activity against EV71 has also heteropolysaccharide from G. frondosa mycelia at the concentration of 250 μg/ml. It inhibits viral replication, VP1 protein expression, and genomic RNA synthesis [37].
In crystal violet assay, G. frondosa extract has been shown to inhibit biofilm development by methicillin-resistant Staphylococcus aureus. After incubation with G. frondosa extract, the average OD value was 0.21 and was significantly lower than the OD in the control group of 0.42 [38]. In Chinese studies, it was found that the water extract of G. frondosa has some antibacterial activity. Obtained zones of growth inhibition to Bacillus subtilis, Escherichia coli, and S. aureus were 8.7, 10.2, and 12.4 mm, respectively [39]. Giving into consideration our previous studies, these zones can be assumed to indicate moderate and good (>10 mm) antibacterial activity [40,41]. Also, polysaccharides from G. frondosa had an antibacterial effect. In MIC studies first, polysaccharide IPS acted against S. aureus (MIC 2.5 mg/ml), E. coli (5 mg/ml), Listeria monocytogenes (5 mg/ml), and Bacillus megaterium (10 mg/ml). The second compound, intracellular zinc polysaccharide, was found to be more effective against the above bacteria, with MICs <0.625 mg/ml, 1.25 mg/ml, 2.5 mg/ml, and 2.5 mg/ml, respectively [42]. It has been shown that D-fraction obtained from the G. frondosa increases the survivability of Listeria monocytogenes-infected mice. In the control group, all mice died within three days after the bacteria inoculation. After using vancomycin at a dose of 20 mg/kg per day survival rate was higher than 50 %. Administration of the D-fraction at a dose of 10 mg/kg per day improved the survival rate to 60 %. Simultaneously, D-fraction administered at the concentration of 10 mg/kg per day reduced the number of Listeria in the peritoneal cavity by 33 % compared to the control sample. The use of vancomycin reduced the number of bacteria by 63 %, and the combination of vancomycin and D-fraction reduced the number of Listeria by as much as 94 % [43].
A furanone, grifolaone A, isolated from G. frondosa, shows antifungal activity. Potent inhibition of fungal growth was observed against phytopathogens Fusarium oxysporum, Gibberella zeae, and Piricularia oryzae with MIC values of 2.5, 2.5, and 1.25 μg/mL, respectively. For comparison, MICs of carbendazim against the above strains were higher and amounted to 10, 2.5, and 5 μg/mL, respectively. Grifolaone A also had activity against the human pathogen Pseudallescheria boydii, with a MIC value of 0.15 μg/mL. This concentration was lower than to amphotericin B (0.31 μg/mL) [44]. Presented above results are shown in Table 1.
Table 1.
Antimicrobial activity of Grifola frondosa.
| Compound/s | Target microorganisms | Active concentration/s | Reference |
|---|---|---|---|
| GFAHP protein | Herpesvirus 1 (HSV) | mean IC50 4.1 μg/ml; at concentrations 30 and 60 μg/ml inhibited HSV-1 penetration by 83.7 % and 93.5 %, respectively; at concentrations 125 and 500 μg/ml reduced virus production | [33] |
| D-fraction | hepatitis B virus (HBV) | IC50 0.59 mg/ml; synergistic activity with IFN | [34] |
| β-glucan (MD-fraction) | Human immunodeficiency virus (HIV) | Among 35 patients, in 20 was an increase of CD4+ cell amount and in 10 was a decrease in viral load | [35] |
| Extract | Enterovirus EV71 | Inhibition of viral replication, viral VP1 protein expression and genomic RNA synthesis, at the concentration of 200 μg/ml and IC50 194.80 μg/ml | [36] |
| Heteropolysaccharide | Enterovirus EV71 | Inhibition of viral replication, viral VP1 protein expression and genomic RNA synthesis, at the concentration of 250 μg/ml | [37] |
| Extract | Methicillin-resistant Staphylococcus aureus (MRSA) | inhibition of the MRSA biofilm development, decrease of OD to 0.21 in comparison to the control group of 0.42 | [38] |
| Water extract | Bacillus subtilis, Escherichia coli and S. aureus | zones of growth inhibition were 8.7, 10.2 and 12.4 mm, respectively | [39] |
| Polysaccharides | S. aureus, E. coli, Listeria monocytogenes, Bacillus megaterium | MICs for polysaccharide IPS 2.5 mg/ml, 5 mg/ml, 5 mg/ml and 10 mg/ml, respectively; MICs for zinc polysaccharide <0.625 mg/ml, 1.25 mg/ml, 2.5 mg/ml, and 2.5 mg/ml, respectively. | [42] |
| D-fraction | Listeria monocytogenes | D-fraction at a dose 10 mg/kg per day improved the survival rate of Listeria-infected mice to 60 %; reduction of the number of Listeria in the peritoneal cavity by 33 % | [43] |
| Grifolaone A | Fusarium oxysporum, Gibberella zeae, Piricularia oryzae, Pseudallescheria boydii | MIC values of 2.5, 2.5, 0.15 and 1.25 μg/mL, respectively | [44] |
7. Immunomodulatory and anti-cancer activities of maitake mushroom
As well established G. frondosa stands as both an edible delicacy and a cornerstone of traditional medicine, with roots dating back centuries [[45], [46], [47]]. Rich in bioactive compounds such as β-glucans and protein units including D-fraction, X-fractions, grilofan, and SX-fraction, this mushroom exhibits potent anti-proliferative and immunomodulatory effects [48]. Numerous studies highlight its ability to activate key immune cells, notably macrophages, natural killer cells (NK), and cytotoxic T cells, pivotal in immune defence and direct tumour cell destruction [49]. Moreover, its glucans stimulate the production of cytokines such as interleukin-1 and interleukin-2, crucial mediators of immune responses [49].
Alonso et al. (2013) reported that the β-glucan component of maitake's D-fraction can influence the switching on and off of genes expressed in human breast cancer Michigan Cancer Foundation (MCF)-7 cells, leading to apoptosis induction. This process controls the breast cancer phenotype and may contribute to the reversal of malignant characteristics. Additionally, other researchers have suggested that maitake extracts exert a direct apoptotic effect on prostate cells, breast cancer cells [50], and kidney cells [51]. Zhao et al. (2017) also noted that combining D-fraction (0.2 mg/mL) and vitamin C (0.3 mmol/L) resulted in a 70 % reduction in human hepatocarcinoma SMMC-7721 cell viability further solidifying maitake's anti-cancer properties [6].
Furthermore, animal studies have demonstrated the safety of maitake D-fraction proteoglucan treatment, showing no toxic effects while providing health benefits and improvements in various types of cancer. These include colon cancer [52], bladder cancer [53], brain cancer, leukemia, liver cancer, breast cancer, and kidney cancer [54]. Additionally, maitake extractions activate effector cells of both the innate [55] and adaptive [56] immune systems, enhancing the production and release of interleukins and lymphokines.
8. Antidiabetic activity of maitake mushroom
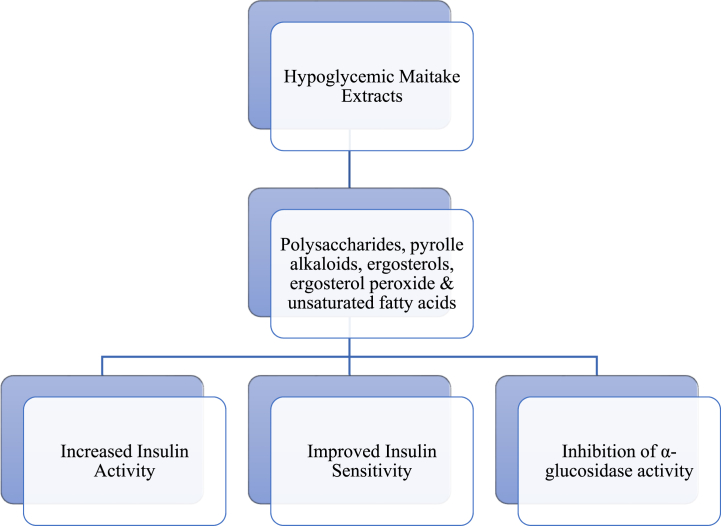
Animal studies demonstrated that maitake extracts have a hypoglycemic activity. As summarised in Fig. 4, the maitake extract content of polysaccharides, pyrrole alkaloids, ergosterols, ergosterol peroxide, and unsaturated fatty acids play significant roles in the hypoglycemic effect through the insulin signal pathway [57,58]. Previous studies reported that the hypoglycemic effect may be due to the facilitated glucose uptake, leading to the stimulation of insulin receptors (IR, IRS-1), and eventually resulting in increased insulin secretion [58]. Xiao et al. (2012) reported that the polysaccharides of Maitake extract improve insulin sensitivity and decrease fasting serum glucose by increasing protein levels of insulin receptors and decreasing protein levels of insulin receptor substrate 1 [57].
Fig. 4.
A summary of the hypoglycemic activity of maitake mushrooms.
Other studies demonstrated that the antidiabetic activity may be due to the inhibition of α-glucosidase activity [59,60], whereas others suggested that this effect is due to the presence of ergosterols and pyrrole alkaloids in the extracts [60,61]. However, one study suggested that the inhibition of the α-glucosidase activity was attributed to the unsaturated fatty acids the oleic acid and linoleic acid [62].
Additionally, Kubo and Nanba (1997) observed that G.frondosa's fruiting body feed significantly reduced serum triglyceride, cholesterol, and phospholipid levels in rats by 30–80 %, while also lowering liver weight by 60–70 % and increasing faecal cholesterol excretion by 1.8 times. Similarly, Fukushima et al. (2001) reported similar outcomes with G. frondosa-fed rats showing lowered serum total cholesterol and VLDL levels, along with increased faecal cholesterol excretion. This further highlights maitake's potential in improving overall health in diabetics since such patients often present with deranged lipid profiles too in conjunction with elevated blood glucose since insulin plays a role in regulating various steps of lipid metabolism.
9. Maitake mushrooms’ potential use in skin products
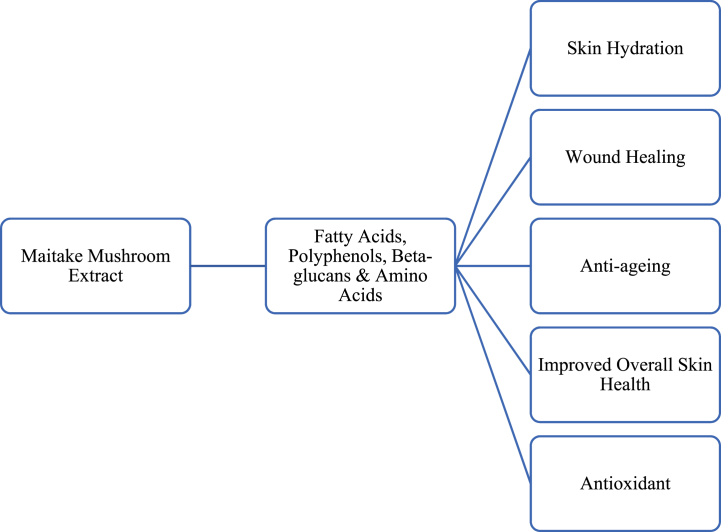
β-Glucans (short β-1,6-branched β-1,3-glucans), found abundantly in fungi, bacteria, yeast, and cereal cell walls, are notable for their diverse health benefits, including anticancer, antioxidative, and anti-inflammatory effects [63,64]. They promote the production of growth factors, collagen biosynthesis, and possess gel-forming properties [65,66]. In skin care, β-glucans are utilised for skin hydration, wound healing, antiaging, and addressing skin burns [67,68]. Studies suggest that β-(1,3)-glucans can mitigate skin cells' oxidative stress and inflammation caused by environmental factors [69]. Maitake extract, rich in β-1,3-glucans, demonstrates antitumorigenesis and anti-carcinogenesis activity [70], while G. frondosa mycelium extracts enhance collagen biosynthesis [68]. Polysaccharide-rich extracts also exhibit hypoglycemic and hypolipidemic activities, further highlighting the therapeutic potential of β-glucans [71]. Additionally, Huang et al., 2014 suggest that maitake polysaccharides combined with chitosan can be a promising material for wound healing. Glucans are also used as thickening and stabilising agents in different industries.
Nagao et al., 2009 showed that ethanol extracts of G. aempfer had the effect of increasing the formation of lipids inside the cells, leading to a higher production of triacylglycerides, and it also triggered the activation of diacylglycerol acyltransferase. Maitake ethanolic extract can aem be used for different skin problems associated with dry skin or xeroderma such as atopic dermatitis [72]. Other studies suggest that G. frondosa polysaccharides have inhibitory effects on melanogenesis and can be used as skin-whitening agents.
Moreover, the G. aempfer extracts have been demonstrated to represent an important source of bioactive compounds with antioxidant activities. In fact, Zhang et al. (2002), demonstrated that fatty acids in G. aempfer inhibited cyclooxygenase (COX)-1 and COX-2 enzyme activities by 98 % and 99 %, respectively, and showed 79 % inhibition of liposome peroxidation, emphasising its significant antioxidant and anti-inflammatory properties [6]. Additionally, polyphenols with known antioxidant activities found in maitake are tannic acids, gallic acids, flavonoids (naringenin, hesperidin, pseudobaptigenin, cyaniding 3-O-xylosylrutinoside) [73] chlorogenic acid, and kaempferol [74]. Sharpe et al., 2021 tested the antioxidant activity of six mushroom species (maitake, Chaga, shiitake, reishi, turkey tail, and lion's mane) and showed that the higher antioxidant activity was found in chaga and maitake. Ziewlewska et al., 2023 showed that maitake extracts have higher polyphenol, flavonoid content, and antioxidant activity compared with reishi and lion's mane extracts. Ji et al., 2019 showed that maitake alcohol-soluble polysaccharides have excellent antioxidant activity. Maitake contains also a high quantity of ergothioneine sulfur-containing amino acid with antioxidant activities. In saying this, maitake's antioxidant properties make it a viable and potential component that can be incorporated into skin care products as seen in Fig. 5.
Fig. 5.
A visual overview of the potential of maitake mushrooms' bioactive compounds in skin products.
Furthermore, terpenoids and sterols are found in maitake extracts. Fatty acids produced by maitake are palmitic, oleic, linoleic acids, ergosterol, and ergosterol peroxide. Amino acids found in maitake are l-Leucine, l-alanine, l- Arginine, l- Aspartic acid, l-Glutamic acid, GABA, glycine, l-histidine, l-Isoleucine, l-leucine, l-lysine, l-methionine, l-Phenylalanine, l-serine, l-threonine, l-tryptophan, l-tyrosine andL-valine [6]. The composition of fatty acids and amino acids in maitake mushrooms holds promise for the development of cosmetic products aimed at maintaining and repairing the skin's hydrolipidic film.
10. Conclusion
Conclusively, this comprehensive review has demonstrated the multifaceted attributes and promising potential of maitake mushrooms across medicinal, therapeutic, nutraceutical, and cosmetic realms. The evidence presented underscores their effectiveness in combatting various health conditions, spanning from cancer and diabetes to immune disorders and skin ailments. Notably, key bioactive components, particularly polysaccharides, play pivotal roles in eliciting diverse health benefits, including antitumor, immunomodulatory, antimicrobial, anti-inflammatory, antidiabetic, lipid metabolism regulation and antioxidative effects.
Looking forward, future research efforts must prioritise the complete understanding of the physiological mechanisms responsible for maitake mushrooms' therapeutic properties. Moreover, conducting robust clinical trials to assess potential side effects and drug interactions in human subjects is imperative. Quantitative evaluations of these parameters will not only deepen our understanding of maitake product safety but also guide healthcare providers and consumers in making informed decisions. Additionally, gauging consumer and medical practitioner acceptance of maitake-based products will be pivotal for their seamless integration into mainstream healthcare practices.
Furthermore, in light of growing concerns about health threats such as food contamination, environmental pollution, and emerging infectious diseases like the COVID-19 virus, leveraging G. frondosa's immunomodulatory and health-promoting functions holds significant promise for safeguarding human health. Thus, in summary, by persistently exploring maitake mushrooms' medicinal and therapeutic potential through quantitative research methodologies and rigorous clinical trials, one can foster widespread acceptance and utilisation, thereby enhancing human health and well-being.
Funding
This research did not receive any specific grant from funding agencies on the public, commercial, or not-for-profit sectors.
Ethics declaration
Review and/or approval by an ethics committee as well as informed consent was not required for this study because this literature review only used existing data from published studies and did not involve any direct experimentation/studies on living beings.
Data availability statement
No data was used for the research described in the article. No data associated in this article has been deposited into a publicly available repository.
CRediT authorship contribution statement
Emma Camilleri: Writing – review & editing, Supervision, Project administration, Conceptualization. Renald Blundell: Supervision, Conceptualization. Bikash Baral: Writing – original draft. Tomasz M. Karpiński: Writing – original draft. Edlira Aruci: Writing – original draft. Omar M. Atrooz: Writing – original draft.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Emma Camilleri, Email: emma.k.camilleri.20@um.edu.mt.
Renald Blundell, Email: renald.blundell@um.edu.mt.
Bikash Baral, Email: bikubaral@yahoo.com.
Tomasz M. Karpiński, Email: tkarpin@ump.edu.pl.
Edlira Aruci, Email: edlira.aruci@wbu.edu.al.
Omar M. Atrooz, Email: omihandd@gmail.com.
References
- 1.Pan Y.-Y., Zeng F., Guo W.-L., Li T.-T., Jia R.-B., Huang Z.-R., et al. Effect of Grifola frondosa 95% ethanol extract on lipid metabolism and gut microbiota composition in high-fat diet-fed rats. Food Funct. 2018;9(12):6268–6278. doi: 10.1039/c8fo01116h. [DOI] [PubMed] [Google Scholar]
- 2.Zhuang C., Wasser S.P. Medicinal value of culinary-medicinal maitake mushroom Grifola frondosa (Dicks.:Fr.) S.F. Gray (aphyllophoromycetideae). Review. Int. J. Med. Mushrooms. 2004;6(4):287–314. [Google Scholar]
- 3.He X., Wang X., Fang J., Chang Y., Ning N., Guo H., et al. Polysaccharides in Grifola frondosa mushroom and their health promoting properties: a review. Int. J. Biol. Macromol. 2017 Aug;101:910–921. doi: 10.1016/j.ijbiomac.2017.03.177. [DOI] [PubMed] [Google Scholar]
- 4.Chen A.W., Stamets P., Cooper R.B., Huang N.L., Han S.-H. Ecology, morphology, and morphogenesis in nature of edible and medicinal mushroom Grifola frondosa (Dicks.: Fr.) S.F.Gray—maitake (aphyllophoromycetideae) Int. J. Med. Mushrooms. 2000;2(3):8. [Google Scholar]
- 5.Mayell M. Maitake extracts and their therapeutic potential. Altern Med Rev. 2001 Feb;6(1):48–60. [PubMed] [Google Scholar]
- 6.Wu J.-Y., Siu K.-C., Geng P. Bioactive ingredients and medicinal values of Grifola frondosa (maitake) Foods. 2021 Jan 5;10(1):95. doi: 10.3390/foods10010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gargano M.L., van Griensven L.J.L.D., Isikhuemhen O.S., Lindequist U., Venturella G., Wasser S.P., et al. Medicinal mushrooms: valuable biological resources of high exploitation potential. Plant Biosyst - An Int J Deal with all Asp Plant Biol. 2017 May 4;151(3):548–565. [Google Scholar]
- 8.Sang W., Kim Hwang J., Lee B., Yun J.W. Submerged production and characterization of Grifola frondosa polysaccharides- A new application to cosmeceuticals. Food Technol. Biotechnol. 2007;45(3):295–305. [Google Scholar]
- 9.Gu Y.-H., Belury M.A. Selective induction of apoptosis in murine skin carcinoma cells (CH72) by an ethanol extract of Lentinula edodes. Cancer Lett. 2005 Mar;220(1):21–28. doi: 10.1016/j.canlet.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 10.Hong L., Weiyu W., Qin W., Shuzhen G., lebin W. Antioxidant and immunomodulatory effects of a α-glucan from fruit body of maitake (Grifola frondosa) Food Agric. Immunol. 2013 Dec;24(4):409–418. [Google Scholar]
- 11.Gargano M.L., Zervakis G.I., Isikhuemhen O.S., Venturella G., Calvo R., Giammanco A., et al. Ecology, phylogeny, and potential nutritional and medicinal value of a rare white “maitake” collected in a mediterranean forest. Diversity. 2020 Jun 8;12(6):230. [Google Scholar]
- 12.Ferraro V., Venturella G., Pecoraro L., Gao W., Gargano M.L. Cultivated mushrooms: importance of a multipurpose crop, with special focus on Italian fungiculture. Plant Biosyst - An Int J Deal with all Asp Plant Biol. 2022 Jan 2;156(1):130–142. [Google Scholar]
- 13.Yeh J.-Y., Hsieh L.-H., Wu K.-T., Tsai C.-F. Antioxidant properties and antioxidant compounds of various extracts from the edible basidiomycete Grifola frondosa (maitake) Molecules. 2011 Apr 15;16(4):3197–3211. doi: 10.3390/molecules16043197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barreto S.M., López M.V., Levin L. Effect of culture parameters on the production of the edible mushroom Grifola frondosa (maitake) in tropical weathers. World J. Microbiol. Biotechnol. 2008 Aug 24;24(8):1361–1366. [Google Scholar]
- 15.Mandal S. Biology, Cultivation and Applications of Mushrooms. Springer Singapore; Singapore: 2022. Beauty, diversity, and potential uses of certain macrofungi; pp. 3–25. [Google Scholar]
- 16.Singh K., Prasad G. Biology and growth characteristics of edible mushroom: agaricus compestris, Agaricus bisporous, Coprinus comatus. J Biotechnol Bioche. 2019;5(1):46–59. [Google Scholar]
- 17.Li Y., Feng Y., Shang Y., Xu H., Xia R., Hou Z., et al. Sexual spores in edible mushroom: bioactive components, discharge mechanisms and effects on fruiting bodies quality. Food Sci. Hum. Wellness. 2023 Nov;12(6):2111–2123. [Google Scholar]
- 18.Huang S.-J., Tsai S.-Y., Lin S.-Y., Liang C.-H., Mau J.-L. Nonvolatile taste components of culinary-medicinal maitake mushroom, Grifola frondosa (Dicks.:Fr.) S.F. Gray. Int. J. Med. Mushrooms. 2011;13(3):265–272. doi: 10.1615/intjmedmushr.v13.i3.60. [DOI] [PubMed] [Google Scholar]
- 19.Jayachandran M., Xiao J., Xu B. A critical review on health promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017 Sep 8;18(9):1934. doi: 10.3390/ijms18091934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips J.M., Ooi S.L., Pak S.C. Health-promoting properties of medicinal mushrooms and their bioactive compounds for the COVID-19 era—an appraisal: do the pro-health claims measure up? Molecules. 2022 Apr 1;27(7):2302. doi: 10.3390/molecules27072302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venturella G., Ferraro V., Cirlincione F., Gargano M.L. Medicinal mushrooms: bioactive compounds, use, and clinical trials. Int. J. Mol. Sci. 2021 Jan 10;22(2):634. doi: 10.3390/ijms22020634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam K.-L., Chi-Keung Cheung P. Non-digestible long chain beta-glucans as novel prebiotics. Bioact Carbohydrates Diet Fibre. 2013 Jul;2(1):45–64. [Google Scholar]
- 23.Nowak R., Nowacka-Jechalke N., Juda M., Malm A. The preliminary study of prebiotic potential of Polish wild mushroom polysaccharides: the stimulation effect on Lactobacillus strains growth. Eur. J. Nutr. 2018 Jun 28;57(4):1511–1521. doi: 10.1007/s00394-017-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishihira J., Sato M., Tanaka A., Okamatsu M., Azuma T., Tsutsumi N., et al. Maitake mushrooms (Grifola frondosa) enhances antibody production in response to influenza vaccination in healthy adult volunteers concurrent with alleviation of common cold symptoms. Funct Foods Heal Dis. 2017 Jul 31;7(7):462. [Google Scholar]
- 25.Aoki H., Hanayama M., Mori K., Sato R. Grifola frondosa (maitake) extract activates PPARδ and improves glucose intolerance in high-fat diet-induced obese mice. Biosci. Biotechnol. Biochem. 2018 Sep 2;82(9):1550–1559. doi: 10.1080/09168451.2018.1480348. [DOI] [PubMed] [Google Scholar]
- 26.Sharpe E., Farragher-Gnadt A.P., Igbanugo M., Huber T., Michelotti J.C., Milenkowic A., et al. Comparison of antioxidant activity and extraction techniques for commercially and laboratory prepared extracts from six mushroom species. J Agric Food Res. 2021 Jun;4 [Google Scholar]
- 27.Pathak S., Martin N., Kim S., Ramesh S., Nadar R.M., DeRuiter J., et al. Mushrooms with Therapeutic Potentials. Springer Nature Singapore; Singapore: 2023. Preventative and curative properties of reishi and maitake mushrooms in cancer; pp. 493–509. [Google Scholar]
- 28.Pinya S., Ferriol P., Tejada S., Sureda A. Nonvitamin and Nonmineral Nutritional Supplements. Elsevier; 2019. Mushrooms reishi (Ganoderma lucidum), shiitake (Lentinela edodes), maitake (Grifola frondosa) pp. 517–526. [Google Scholar]
- 29.Panda S.K., Luyten W. Medicinal mushrooms: clinical perspective and challenges. Drug Discov. Today. 2022 Feb;27(2):636–651. doi: 10.1016/j.drudis.2021.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W., Jiang X., Zhao S., Zheng X., Lan J., Wang H., et al. A polysaccharide-peptide with mercury clearance activity from dried fruiting bodies of maitake mushroom Grifola frondosa. Sci. Rep. 2018 Dec 4;8(1) doi: 10.1038/s41598-018-35945-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawaguchi N., Hayashi M., Chen F.-C., Shimomura N., Yamaguchi T., Aimi T. Genetic analyses of causal genes of albinism (white fruiting body) in Grifola frondosa. J. Wood Sci. 2019 Dec 13;65(1):32. [Google Scholar]
- 32.Li N., Gao X., Pan Y., Liu B., Pang J., Zhao C., et al. Effects of alkaloid-rich extracts obtained from Grifola frondosa on gut microbiota and glucose homeostasis in rats. Food Funct. 2022;13(5):2729–2742. doi: 10.1039/d1fo04062f. [DOI] [PubMed] [Google Scholar]
- 33.Gu C.-Q., Li J.-W., Chao F., Jin M., Wang X.-W., Shen Z.-Q. Isolation, identification and function of a novel anti-HSV-1 protein from Grifola frondosa. Antiviral Res. 2007 Sep;75(3):250–257. doi: 10.1016/j.antiviral.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Gu C.-Q., Li J.-W., Chao F.-H. Inhibition of hepatitis B virus by D-fraction from Grifola frondosa: synergistic effect of combination with interferon-α in HepG2 2.2.15. Antiviral Res. 2006 Nov;72(2):162–165. doi: 10.1016/j.antiviral.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Nanba H., Kodama N., Schar D., Turner D. Effects of Maitake (Grifola frondosa) glucan in HIV-infected patients. Mycoscience. 2000 Aug;41(4):293–295. [Google Scholar]
- 36.Wenyu X., Junqiang H.E., Danlin T., Peizhi P., Xiaoqin J., Bin L.I.U. Active ingredients and antioxidative and anti-EV71 virus effects of Grifola frondosa ethanol extract. Fujian J. Agric. Sci. 2023;38(4):497–505. [Google Scholar]
- 37.Zhao C., Gao L., Wang C., Liu B., Jin Y., Xing Z. Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohydr. Polym. 2016 Jun;144:382–389. doi: 10.1016/j.carbpol.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Fasciana T., Gargano M.L., Serra N., Galia E., Arrigo I., Tricoli M.R., et al. Potential activity of albino Grifola frondosa mushroom extract against biofilm of meticillin-resistant Staphylococcus aureus. J Fungi. 2021 Jul 10;7(7):551. doi: 10.3390/jof7070551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jing Y., Li J., Zhang Y., Zhang R., Zheng Y., Hu B., et al. Structural characterization and biological activities of a novel polysaccharide from Glehnia littoralis and its application in preparation of nano-silver. Int. J. Biol. Macromol. 2021;183:1317–1326. doi: 10.1016/j.ijbiomac.2021.04.178. [DOI] [PubMed] [Google Scholar]
- 40.Karpiński T.M., Adamczak A. Antibacterial activity of ethanolic extracts of some moss species. Herba Pol. 2017 Sep 26;63(3):11–17. [Google Scholar]
- 41.Karpiński T.M., Adamczak A. Fucoxanthin—an antibacterial carotenoid. Antioxidants. 2019 Jul 24;8(8):239. doi: 10.3390/antiox8080239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang C., Gao Z., Hu C., Zhang J., Sun X., Rong C., et al. Antioxidant, antibacterial and anti-aging activities of intracellular zinc polysaccharides from Grifola frondosa SH-05. Int. J. Biol. Macromol. 2017 Feb;95:778–787. doi: 10.1016/j.ijbiomac.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Kodama N., Yamada M., Nanba H. Addition of maitake D-fraction reduces the effective dosage of vancomycin for the treatment of listeria-infected mice. Jpn. J. Pharmacol. 2001;87(4):327–332. doi: 10.1254/jjp.87.327. [DOI] [PubMed] [Google Scholar]
- 44.He X., Du X., Zang X., Dong L., Gu Z., Cao L., et al. Extraction, identification and antimicrobial activity of a new furanone, grifolaone A, from Grifola frondosa. Nat. Prod. Res. 2016 Apr 17;30(8):941–947. doi: 10.1080/14786419.2015.1081197. [DOI] [PubMed] [Google Scholar]
- 45.Lull C., Wichers H.J., Savelkoul H.F.J. Antiinflammatory and immunomodulating properties of fungal metabolites. Mediators Inflamm. 2005;2005(2):63–80. doi: 10.1155/MI.2005.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Illana-Esteban C. El hongo maitake (Grifola frondosa) y su potential terapéutico. Rev. Iberoam. De. Micol. 2008 Sep;25(3):141–144. doi: 10.1016/s1130-1406(08)70033-0. [DOI] [PubMed] [Google Scholar]
- 47.De Silva D.D., Rapior S., Fons F., Bahkali A.H., Hyde K.D. Medicinal mushrooms in supportive cancer therapies: an approach to anti-cancer effects and putative mechanisms of action. Fungal Divers. 2012 Jul 27;55(1):1–35. [Google Scholar]
- 48.Roca-Lema D., Martinez-Iglesias O., Portela CF. de A., Rodríguez-Blanco A., Valladares-Ayerbes M., Díaz-Díaz A., et al. In vitro anti-proliferative and anti-invasive effect of polysaccharide-rich extracts from Trametes Versicolor and Grifola frondosa in colon cancer cells. Int. J. Med. Sci. 2019;16(2):231–240. doi: 10.7150/ijms.28811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masuda Y., Togo T., Mizuno S., Konishi M., Nanba H. Soluble β-glucan from Grifola frondosa induces proliferation and Dectin-1/Syk signaling in resident macrophages via the GM-CSF autocrine pathway. J. Leukoc. Biol. 2012 Apr 1;91(4):547–556. doi: 10.1189/jlb.0711386. [DOI] [PubMed] [Google Scholar]
- 50.Roldan‐Deamicis A., Alonso E., Brie B., Braico D.A., Balogh G.A. Maitake Pro4X has anti‐cancer activity and prevents oncogenesis in <scp>BALB</scp> c mice. Cancer Med. 2016 Sep 11;5(9):2427–2441. doi: 10.1002/cam4.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexander B., Fishman A.I., Eshghi M., Choudhury M., Konno S. Induction of cell death in renal cell carcinoma with combination of D-fraction and vitamin C. Integr. Cancer Ther. 2013 Sep;12(5):442–448. doi: 10.1177/1534735412473643. https://pubmed.ncbi.nlm.nih.gov/23341484/ [Internet] [cited 2024 Apr 2] Available from: [DOI] [PubMed] [Google Scholar]
- 52.Masuda Y., Ito K., Konishi M., Nanba H. A polysaccharide extracted from Grifola frondosa enhances the anti-tumor activity of bone marrow-derived dendritic cell-based immunotherapy against murine colon cancer. Cancer Immunol. Immunother. 2010 Oct 20;59(10):1531–1541. doi: 10.1007/s00262-010-0880-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hillelsohn J., Stern M., Iskander M., Choudhury M., Konno S. Chemosensitizing effect of maitake mushroom extract on carmustine cytotoxicity in human bladder cancer cells. Int J Res Med Sci. 2020 Aug 26;8(9):3154. [Google Scholar]
- 54.Konno S. Synergistic potentiation of D-fraction with vitamin C as possible alternative approach for cancer therapy. Int. J. Gen. Med. 2009 May;91 doi: 10.2147/ijgm.s5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alonso E.N., Ferronato M.J., Fermento M.E., Gandini N.A., Romero A.L., Guevara J.A., et al. Antitumoral and antimetastatic activity of Maitake D-Fraction in triple-negative breast cancer cells. Oncotarget. 2018 May 4;9(34):23396–23412. doi: 10.18632/oncotarget.25174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kodama N., Harada N., Nanba H. A polysaccharide, extract from Grifola frondosa, induces Th-1 dominant responses in carcinoma-bearing BALB/c mice. Jpn. J. Pharmacol. 2002;90(4):357–360. doi: 10.1254/jjp.90.357. [DOI] [PubMed] [Google Scholar]
- 57.Lei H., Guo S., Han J., Wang Q., Zhang X., Wu W. Hypoglycemic and hypolipidemic activities of MT-α-glucan and its effect on immune function of diabetic mice. Carbohydr. Polym. 2012 Jun;89(1):245–250. doi: 10.1016/j.carbpol.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Konno S., Alexander Zade. Choudhury. Possible hypoglycemic action of SX-fraction targeting insulin signal transduction pathway. Int. J. Gen. Med. 2013 Mar;181 doi: 10.2147/IJGM.S41891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen K.-P., Su C.-H., Lu T.-M., Lai M.-N., Ng L.-T. Effects of Grifola frondosa non-polar bioactive components on high-fat diet fed and streptozotocin-induced hyperglycemic mice. Pharm. Biol. 2015 May 4;53(5):705–709. doi: 10.3109/13880209.2014.939290. [DOI] [PubMed] [Google Scholar]
- 60.Chen S., Yong T., Xiao C., Su J., Zhang Y., Jiao C., et al. Pyrrole alkaloids and ergosterols from Grifola frondosa exert anti-α-glucosidase and anti-proliferative activities. J. Funct.Foods. 2018 Apr;43:196–205. [Google Scholar]
- 61.Masuda Y., Inoue H., Ohta H., Miyake A., Konishi M., Nanba H. Oral administration of soluble β‐glucans extracted from Grifola frondosa induces systemic antitumor immune response and decreases immunosuppression in tumor‐bearing mice. Int. J. Cancer. 2013 Jul 15;133(1):108–119. doi: 10.1002/ijc.27999. [DOI] [PubMed] [Google Scholar]
- 62.Su C., Lu T., Lai M., Ng L. Inhibitory potential of Grifola frondosa bioactive fractions on α‐amylase and α‐glucosidase for management of hyperglycemia. Biotechnol. Appl. Biochem. 2013 Jul 22;60(4):446–452. doi: 10.1002/bab.1105. [DOI] [PubMed] [Google Scholar]
- 63.Du B., Lin C., Bian Z., Xu B. An insight into anti-inflammatory effects of fungal beta-glucans. Trends Food Sci. Technol. 2015 Jan;41(1):49–59. [Google Scholar]
- 64.Rop O., Mlcek J., Jurikova T. Beta-glucans in higher fungi and their health effects. Nutr. Rev. 2009 Nov;67(11):624–631. doi: 10.1111/j.1753-4887.2009.00230.x. [DOI] [PubMed] [Google Scholar]
- 65.Pillai R., Redmond M., Röding J. Anti‐wrinkle therapy: significant new findings in the non‐invasive cosmetic treatment of skin wrinkles with beta‐glucan. Int. J. Cosmet. Sci. 2005 Oct 13;27(5):292. [Google Scholar]
- 66.Gautier S., Xhauflaire‐Uhoda E., Gonry P., Piérard G.E. Chitin–glucan, a natural cell scaffold for skin moisturization and rejuvenation. Int. J. Cosmet. Sci. 2008 Dec 5;30(6):459–469. doi: 10.1111/j.1468-2494.2008.00470.x. [DOI] [PubMed] [Google Scholar]
- 67.Fusté N.P., Guasch M., Guillen P., Anerillas C., Cemeli T., Pedraza N., et al. Barley β-glucan accelerates wound healing by favoring migration versus proliferation of human dermal fibroblasts. Carbohydr. Polym. 2019 Apr;210:389–398. doi: 10.1016/j.carbpol.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 68.Lee B.C., Bae J.T., Pyo H.B., Choe T.B., Kim S.W., Hwang H.J., et al. Biological activities of the polysaccharides produced from submerged culture of the edible Basidiomycete Grifola frondosa. Enzyme Microb Technol. 2003 Apr;32(5):574–581. [Google Scholar]
- 69.Ozanne H., Toumi H., Roubinet B., Landemarre L., Lespessailles E., Daniellou R., et al. Laminarin effects, a β-(1,3)-glucan, on skin cell inflammation and oxidation. Cosmetics. 2020 Aug 16;7(3):66. [Google Scholar]
- 70.Okuno K., Aoyama T., Oba K., Yokoyama N., Matsuhashi N., Kunieda K., et al. Randomized phase III trial comparing surgery alone to UFT + PSK for stage II rectal cancer (JFMC38 trial) Cancer Chemother. Pharmacol. 2018 Jan 1;81(1):65–71. doi: 10.1007/s00280-017-3466-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo W.-L., Deng J.-C., Pan Y.-Y., Xu J.-X., Hong J.-L., Shi F.-F., et al. Hypoglycemic and hypolipidemic activities of Grifola frondosa polysaccharides and their relationships with the modulation of intestinal microflora in diabetic mice induced by high-fat diet and streptozotocin. Int. J. Biol. Macromol. 2020 Jun;153:1231–1240. doi: 10.1016/j.ijbiomac.2019.10.253. [DOI] [PubMed] [Google Scholar]
- 72.Nagao M., Matsuzaki S., Sato T., Ito A., Takahashi M. Clinical trial of gripin ® cream (maitake mushroom extract cream) for xerosis therapy. J Investig dermatology. 2007 [Google Scholar]
- 73.Deng J., Guo W., Guo J., Li Y., Zhou W., Lv W., et al. Regulatory effects of a Grifola frondosa extract rich in pseudobaptigenin and cyanidin-3-O-xylosylrutinoside on glycolipid metabolism and the gut microbiota in high-fat diet-fed rats. J. Funct.Foods. 2020 Dec;75 [Google Scholar]
- 74.Kim J.-H., Lim S.-R., Jung D.-H., Kim E.-J., Sung J., Kim S.C., et al. Grifola frondosa extract containing bioactive components blocks skin fibroblastic inflammation and cytotoxicity caused by endocrine disrupting chemical, bisphenol A. Nutrients. 2022 Sep 15;14(18):3812. doi: 10.3390/nu14183812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article. No data associated in this article has been deposited into a publicly available repository.