Introduction
Immune checkpoint therapy (ICT), an emerging transformative cancer treatment, targets inhibitory proteins in the immune system, such as programmed cell death protein 1 (PD-1) and its ligand (PD-L1), and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4). This strategy has remarkably improved disease outcomes for various cancers.1 However, ICT can lead to immune-related adverse events (irAEs), which are characterized by an uncontrolled and dysregulated immune response that affects multiple organs. Although the precise mechanisms underlying ICT-induced autoimmune disease are not fully understood, autoantibody development in response to treatment has been well established.
Renal irAEs are less frequent but potentially severe. Despite the reported incidence of renal irAEs being relatively low, any renal impairment can significantly impact patient outcomes, leading to reduced overall survival and potential ineligibility for future clinical trials.1 Close monitoring of kidney function during ICT is critical to preserve and optimize renal health. Renal irAEs have diverse clinical presentations, including electrolyte disturbances, acute tubulointerstitial nephritis, acute tubular necrosis, vasculitis, glomerulonephritis, and, rarely, sarcoidosis.2,3 Among these, glomerulonephritis, an autoimmune disease affecting the glomeruli, is a rare, serious complication following ICT exposure. Recent studies have shown that glomerulonephritis accounts for a significant proportion of ICT-associated glomerular disease, with pauci-immune glomerulonephritis and renal vasculitis being the most commonly observed subtypes.2
However, the association between preexisting autoantibodies and irAE risk remains inconclusive, particularly regarding renal irAEs. Further investigation into specific autoantibodies, such as antineutrophil cytoplasmic antibodies (ANCAs), before and after ICT exposure may provide valuable insights into predicting irAEs and overall survival in patients with cancer.4,5
Management of glomerulonephritis after ICT involves kidney biopsies for accurate diagnosis and tailored treatment approaches. Corticosteroids are a common treatment approach; however, a more targeted approach is required to prevent immune checkpoint inhibitor (ICI) discontinuation and enhance outcomes. Rituximab is an effective treatment for inducing remission in ICT-induced kidney injury and vasculitis and continued cancer remission.6,7
Because renal irAEs are clinically significant and potentially impact patient outcomes, further research is needed to better understand the pathophysiology of induction of autoimmune disease after ICT. Identifying predictive factors and refining treatment strategies is essential to optimize ICT use and ensure patient safety and treatment efficacy.
Here, we report on an older patient with solitary metastatic renal cell cancer with prior ICT exposure who developed lupus nephritis characterized by the production of autoantibodies. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center (PA16-1016). Informed consent was not required.
Case Presentation
A Caucasian man in his 80s was admitted to the hospital with acute kidney injury, volume overload, ascites, and hyponatremia. He had a history of hypertension, coronary artery disease, hypothyroidism, prostate cancer, and metastatic renal cell carcinoma and had undergone a left radical nephrectomy. Approximately a year later, his renal cell cancer recurred, and he received 3 cycles of pembrolizumab and axitinitib. He initially responded to treatment; however, his disease progressed and subsequently cabozantinib was started later that year.
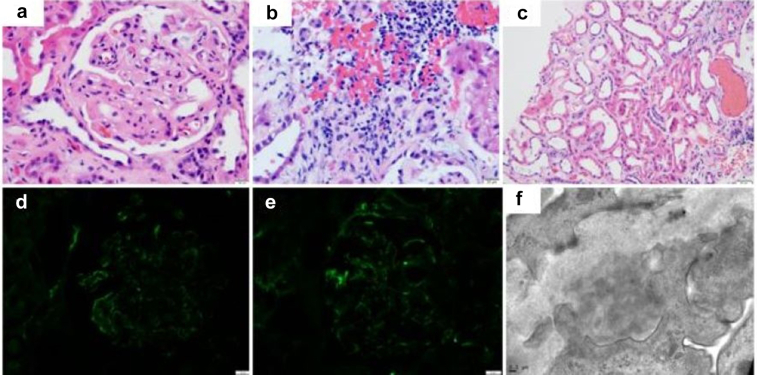
At the time of hospital admission, the patient’s creatinine level was 6.0 mg/dl (baseline: 2.0 mg/dl). He had hematuria, eosinophilia, and proteinuria (4.5 g) and an albumin level of 2.1 mg/dl, which were all negative before he started pembrolizumab and axitinitib. An autoimmune workup revealed an antinuclear antibody level of 1:320, with positive results for anti–double-stranded DNA and antihistone antibodies, and normal complement levels. Notably, the workup findings were also positive for myeloperoxidase antibodies (>8 ng/l). Of note, the patient had been started on hydralazine after initiation of axitinitib due to uncontrolled hypertension a month before admission for acute kidney injury. Renal ultrasonography indicated an 11.8 cm-long kidney with increased echogenicity. Because of continued elevated creatinine levels, a kidney biopsy was performed. The biopsy showed mild glomerulonephritis with segmental endocapillary hypercellularity, and immunofluorescence staining revealed positive for C3, C1q, IgA, IgM, IgG, Kappa, and Lambda referred to as a “full-house” immunofluorescence pattern. It also revealed ultrastructural features consistent with thrombotic microangiopathy–like changes with focal global glomerulosclerosis (6 of 41 glomeruli, approximately 15%), mild and focal acute tubulointerstitial nephritis with diffuse acute tubular injury, interstitial fibrosis and tubular atrophy (approximately 30%), and moderate to severe arteriosclerosis and arteriolosclerosis, consistent with the patient’s history of hypertension (Figure 1).
Figure 1.
Kidney biopsy. (a) Hematoxylin and eosin stain: a glomerulus with segmental sclerosis and mesangial expansion and hypercellularity. (b) Hematoxylin and eosin stain: interstitial nephritis with lymphocytes, plasma cells and a few eosinophils. (c) Hematoxylin and eosin stain: acute tubular injury. (d) Immunofluorescence: mesangial/paramesangial and capillary walls staining for IgG. Similar findings are seen with IgM and IgA. (e) Immunofluorescence: mesangial/paramesangial and capillary walls staining for C1q. Similar findings are seen with C3. (f) Electron microscopy: organized electron-dense deposits with fingerprint pattern.
Because the findings indicated an autoimmune process, the patient was given methylprednisolone 250 mg pulse as an i.v. infusion daily for 3 days and then started on prednisone 60 mg daily. He was also started on hydroxychloroquine 200 mg twice daily. His creatinine level initially improved to 4.53 mg/dl; however, due to increasing azotemia and volume overload, hemodialysis was started. Suspecting autoimmune disease, the patient was treated with a course of rituximab consisting of two 1 g doses given by i.v. infusion 2 weeks apart. Dialysis was discontinued after 4 months. At the time of writing, the patient has a stable glomerular filtration rate of 15 ml/min (Supplementary Figure S1A and B). His proteinuria and hypervolemia have resolved, and he is receiving prednisone 7.5 mg daily, hydroxychloroquine 200 mg twice daily, and minimal diuretics due to his chronic kidney disease.
Discussion
Recently, the increasing use of ICTs in patients with relapsed and refractory cancer has highlighted the irAEs associated with these therapies. As the use of ICT has grown, renal irAE incidence has risen, providing valuable insights into the pathogenic mechanisms underlying ICI-associated nephrotoxicity.8 Initial reports suggested that anti-CTLA-4 agents may be more likely to cause glomerular diseases than PD-1 and PD-L1 inhibitors, but a recent meta-analysis indicated that PD-1 and PD-L1 inhibitors may be more likely than anti-CTLA-4 agents to cause glomerular diseases.2 Here, we report a patient with solitary kidney who experienced lupus nephritis after receiving pembrolizumab, a PD-1 inhibitor, and was effectively treated with rituximab with maintenance off dialysis and stable cancer.
ICT-induced acute interstitial nephritis has been associated with increased urinary tumor necrosis factor-α, which induces human leukocyte antigen and activates antigen-presenting cells and increased dendritic cells in the kidney tissue of patients treated with ICTs. These findings support a loss of tolerance induced by ICT exposure that leads to autoreactive T-cell infiltration.S1 Another study validated these findings, demonstrating that patients exposed to proton pump inhibitors and nonsteroidal antiinflammatory drugs had an increased risk of acute interstitial nephritis after ICT exposure where tolerance to these medications would be lost.S2,S3
Previous work demonstrated that increased T-cell infiltration and expansion can lead to an inflammatory response in ANCA-associated glomerulonephritis and vasculitis. The exhaustion of T cells in vasculitis and other autoimmune diseases prevents disease progression. The expressions of coinhibitory molecules such as PD-1 and CTLA-4 prevent the differentiation of nonexhausted T cells. In the pathogenesis of ANCA-associated vasculitis, CD8+ T cells, which express the CTLA-4 receptor, may activate polymorphonuclear cells, leading to the exposure of proteinase-3 and myeloperoxidase antigens on their surface. These antigens may then react with ANCAs, leading to ANCA-induced disease.S4 Regarding further induction of an autoimmune disease, PD-1 is expressed on B cells, including immunosuppressive B regulatory cells, and has a well-established role in B-cell tolerance.S5 ICT induction of B cells has been shown to specifically correlate with irAEs.S6 In addition, in patients with B-cell depletion who were treated with ICIs for their underlying cancer, B-cell depletion was shown not to impede the ICIs’ antitumor effects.S7
Our case had “full-house” immunofluorescence staining results, with positive results for antinuclear antibodies, double-stranded DNA, and histones; these findings are typical features of lupus nephritis. There have been few case reports of lupus nephritis–like syndrome after ICI exposure; however, in mouse models, disruption of PD-1 has been shown to lead to systemic lupus and glomerulonephritis.S8 Although Pippin et al. indicated that treatment with anti–PD-1 antibodies led to antiaging effects on podocytes in mouse models, continued exposure to anti–PD-1 antibodies may lead to the induction of an autoimmune disease.S9 Furthermore, in mouse models, treatment with a PD-L1–agonist antibody reduced proteinuria and decreased serum levels of anti–double-stranded DNA antibodies and IgG glomerular deposition, with an associated increased survival rate.S10 In the few published case reports of ICT-induced lupus nephritis, stopping ICTs and starting steroids when patients’ proteinuria responded also led to excellent tumor response (Supplementary Table S1).S11–S13 To our knowledge, we are the first to report on a lupus nephritis case with positive histone antibodies, which is highly associated with drug-induced lupus and could have been exacerbated by the patients’ exposure to hydralazine.
Initial guidelines for managing renal irAEs recommended initiating corticosteroid-based therapy without performing a kidney biopsy.S12 Since then, however, increasing evidence has shown that the induction of an autoimmune kidney disease can be present and lead to both poor renal outcomes and poor overall survival.2 A recent case series showed that a kidney biopsy can be used to diagnose vasculitis following ICT exposure, thereby challenging the conventional approach.9
Several case reports have demonstrated that rituximab, a monoclonal anti-CD20 antibody, can potentially induce renal recovery and disease remission, allowing patients to continue ICI therapy despite developing glomerulonephritis.2,9 Rituximab disrupts pathogenic B-lymphocyte interaction with cytotoxic T lymphocytes, reduces chemokine production, and limits endothelial injury without inhibiting the antineoplastic effects of ICIs.S7,S14,S15 One study reported partial to complete renal recovery and remission following rituximab treatment in ICI-induced renal vasculitis.6 However, maintenance rituximab should be avoided after achieving renal remission because suppression of the B cells can reduce the overall antitumor effects of the T cells due to the lack of the B-cell antigen presentation. A recent evaluation of the role of intratumoral B cells confirmed their importance in tumor responses in patients treated with anti–PD-1 or anti–PD-L1 immunotherapy.S16 For patients with profound autoimmune symptoms, plasmapheresis has shown potential benefits in removing autoantibodies, especially in ANCA-associated vasculitis.S17 A summary of learning points can be found in Table 1.
Table 1.
Teaching points
| 1. | Kidney biopsy in patients with suspected ICI kidney toxicity should be first line in diagnosis |
| 2. | Autoimmune induction in the kidney although rare would need to be in the differential of ICI-induced kidney injury to allow a more guided and effective treatment |
| 3. | Rituximab can provide an effective steroid sparing treatment for autoimmune disease induced by checkpoint inhibitors and allow continued tumor response |
ICI, immune checkpoint inhibitor.
In conclusion, ICTs have revolutionized cancer management, providing durable antitumor responses and improved survival. However, clinicians must remain vigilant for ICT-induced irAEs, including ICI-induced vasculitis and glomerulonephritis, that can lead to significant morbidity. Timely monitoring of serum creatinine levels and prompt initiation of appropriate therapies, such as corticosteroids, plasmapheresis, or rituximab, can lead to renal recovery and disease remission, allowing patients to continue ICT therapy. Further research should focus on the mechanisms and risk factors of ICT-induced autoimmune disease, which can lead to improved management strategies and patient outcomes.
Disclosure
All the authors declared no competing interests.
Patient Consent
The authors declare that they have obtained consent from the patients discussed in the report.
Acknowledgments
Editorial support was provided by Madison Semro, Associate Scientific Editor, and Stephanie Deming, Senior Scientific Editor, in the Research Medical Library at The University of Texas MD Anderson Cancer Center. This study was supported by the National Institutes of Health/National Cancer Institute under award number P30CA016672 (used the Clinical Trials Office and Biostatistics Resource Group).
Author Contributions
Data acquisition was performed by IHH and AA. Figures were drawn by SA. MAS performed the histologic examination of the kidney biopsies and contributed to writing the pathology section of the manuscript. The manuscript was prepared by IHH, SA, MAS, HL, RA, and SG. All the authors contributed to the quality control data, analysis, interpretation of data and writing and final proof of paper. All authors read and approved the final manuscript.
Footnotes
Supplementary References.
Figure S1. (A) Trend of creatinine mg/dl and (B) Trend of albumin and proteinuria over time.
Table S1. Summary of all cases published with lupus like glomerulonephritis after ICI treatment.
Supplementary Material
Supplementary References.
Figure S1. (A) Trend of creatinine mg/dl and (B) Trend of albumin and proteinuria over time.
Table S1. Summary of all cases published with lupus like glomerulonephritis after ICI treatment.
ICI, immune checkpoint inhibitor
References
- 1.Toi Y., Sugawara S., Sugisaka J., et al. Profiling preexisting antibodies in patients treated with anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol. 2019;5:376–383. doi: 10.1001/jamaoncol.2018.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitchlu A., Jhaveri K.D., Wadhwani S., et al. A systematic review of immune checkpoint inhibitor-associated glomerular disease. Kidney Int Rep. 2021;6:66–77. doi: 10.1016/j.ekir.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charkviani M., Herrmann S.M. Immune checkpoint inhibitor-associated sarcoidosis reaction in the kidney: case report. Kidney Med. 2023;5 doi: 10.1016/j.xkme.2023.100626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakakida T., Ishikawa T., Chihara Y., et al. Safety and efficacy of PD-1/PD-L1 blockade in patients with preexisting antinuclear antibodies. Clin Transl Oncol. 2020;22:919–927. doi: 10.1007/s12094-019-02214-8. [DOI] [PubMed] [Google Scholar]
- 5.Barth D.A., Stanzer S., Spiegelberg J., et al. Evaluation of autoantibodies as predictors of treatment response and immune-related adverse events during the treatment with immune checkpoint inhibitors: a prospective longitudinal pan-cancer study. Cancer Med. 2022;11:3074–3083. doi: 10.1002/cam4.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mamlouk O., Lin J.S., Abdelrahim M., et al. Checkpoint inhibitor-related renal vasculitis and use of rituximab. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abudayyeh A. Evaluation and Management of Suspected Immune-Mediated Nephritis. https://www.mdanderson.org/content/dam/mdanderson/documents/for-physicians/algorithms/clinical-management/clin-management-nephritis-web-algorithm.pdf
- 8.Herrmann S.M., Perazella M.A. Immune checkpoint inhibitors and immune-related adverse renal events. Kidney Int Rep. 2020;5:1139–1148. doi: 10.1016/j.ekir.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin J.S., Wang D.Y., Mamlouk O., et al. Immune checkpoint inhibitor associated reactivation of primary membranous nephropathy responsive to rituximab. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.