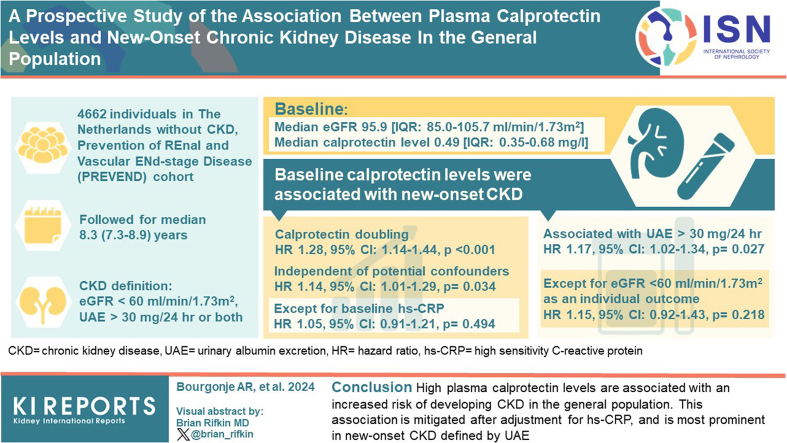
Abstract
Introduction
Systemic inflammation has been associated with chronic kidney disease (CKD). In this study, we aimed to investigate a potential association between the plasma biomarker of inflammation calprotectin and new-onset CKD in a population-based cohort study.
Methods
Individuals without CKD at baseline (n = 4662) who participated in the Prevention of REnal and Vascular ENd-stage Disease (PREVEND) prospective population-based cohort study in the Netherlands were included. Baseline plasma calprotectin levels were assessed in samples that had been stored at −80 °C. Occurrence of new-onset CKD was defined as a composite outcome of an estimated glomerular filtration rate (eGFR) <60 ml/min per 1.73 m2, urinary albumin excretion (UAE) >30 mg/24h, or both.
Results
Baseline median (interquartile range) plasma calprotectin levels were 0.49 (0.35–0.68) mg/l and baseline median eGFR was 95.9 (interquartile range: 85.0–105.7) ml/min per 1.73 m2. After median follow-up of 8.3 (7.8–8.9) years, 467 participants developed new-onset CKD. Baseline plasma calprotectin levels were significantly associated with an increased risk of new-onset CKD (hazard ratio [HR] per doubling 1.28 [95% confidence interval, CI: 1.14–1.44], P < 0.001), independent of potentially confounding factors (HR 1.14 [95% CI: 1.01–1.29], P = 0.034), except for baseline high-sensitive C-reactive protein (hs-CRP) (HR 1.05 [0.91–1.21], P = 0.494). In secondary analyses, the association between plasma calprotectin and occurrence of UAE >30 mg/24h remained significant (HR 1.17 [1.02–1.34], P = 0.027), but not significantly so for the incidence of eGFR <60 ml/min per 1.73 m2 as individual outcome (HR 1.15 [0.92–1.43], P = 0.218).
Conclusion
Higher plasma calprotectin levels are associated with an increased risk of developing CKD in the general population. This association is mitigated after adjustment for hs-CRP, and more pronounced with new-onset CKD defined by UAE.
Keywords: biomarker, calprotectin, chronic kidney disease, epidemiology, inflammation, population science
Graphical abstract
CKD poses a significant global health concern, impacting nearly one billion individuals worldwide.1 CKD is usually accompanied by a high burden of cardiovascular complications and is linked to premature mortality, underscoring the critical importance of early detection and proper staging of CKD. Given the complex and multifactorial nature of CKD, our understanding of the underlying mechanisms remains incomplete, making it crucial to identify biomarkers, such as UAE, that can aid in the early identification and treatment of individuals at risk of developing CKD within the general population.2 Early treatment initiation may have the potential to delay or even prevent the need for dialysis or kidney transplantation, improving the overall prognosis and quality of life for affected individuals.3
Calprotectin, which primarily originates from myeloid cells such as macrophages and neutrophils,4 is actively released during neutrophil degranulation and can function as an endogenous danger signaling molecule (alarmin) or as antimicrobial substance within neutrophil extracellular traps.5,6 Circulating calprotectin is considered an acute-phase protein, and given its indication of systemic inflammation, it might be potentially associated with CKD. Earlier research in population-based cohorts has demonstrated associations between plasma calprotectin levels and the risk of development of cardiovascular diseases, including ischemic cardiovascular manifestations as well as hypertension.7, 8, 9, 10 Importantly, calprotectin levels in fecal samples are clinically utilized as a biomarker for intestinal inflammation in patients with inflammatory bowel disease, and they have also been linked to systemic inflammation in this context.11 Given the role of systemic inflammation in the development of CKD,12, 13, 14 and specifically the involvement of myeloid cells such as neutrophils and macrophages in CKD,15, 16, 17 it is conceivable that circulating levels of calprotectin might be potentially indicative of the early stages of CKD.
In the current study, we set out to explore the relationship between plasma calprotectin levels and the risk of developing new-onset CKD in individuals derived from the general population. By investigating this association, we aimed to gain insight into the potential utility of calprotectin as a predictive biomarker for identifying individuals at risk of CKD in its early stages. Beyond its potential relevance for CKD, calprotectin may also indicate the presence of systemic chronic low-grade inflammation, which contributes to the development of various noncommunicable diseases.
Methods
Study Design and Population
This study was carried out using data from the PREVEND study, a comprehensive prospective population-based cohort study that was initiated in 1997 in Groningen, the Netherlands.18 In short, the initial PREVEND population consisted of 8592 participants (comprising 6000 with urinary albumin concentration >10 mg/l and 2592 with urinary albumin concentration <10 mg/l). Between 2001 and 2003, a second round of study investigations was carried out with the goal of collecting additional data and biomaterials such as blood and urine samples from 6894 participants. This second study round served as the baseline for the current study. After this round, participants visited the outpatient research clinic of the University Medical Center Groningen for a medical examination at ∼3-year intervals. These examinations were divided over 2 outpatient visits separated by 3 weeks, which took place during a third (2003–2006), fourth (2006–2008), and fifth (2009–2011) period of study investigations. The study follow-up period concluded on January 1, 2011. From this cohort, individuals with established CKD at enrollment, as defined by an eGFR <60 ml/min per 1.73 m2 (n = 1082), or with unknown CKD status (n = 337) were excluded. In addition, participants in whom plasma calprotectin levels could not be determined (n = 813) due to either missing samples or insufficient sample volumes were excluded. Consequently, the final sample size for analysis of the current study consisted of n = 4662 participants. Collection of biomedical and laboratory data was conducted at the outpatient research clinic of the University Medical Center Groningen. Ethical approval for the PREVEND study was obtained from the institutional review board of the University Medical Center Groningen (in Dutch: “Medisch Ethische Toetsingscommissie” (METc), IRB number 01/139). All participants provided written informed consent before participating in the study, and the research adhered to the principles outlined in the Declaration of Helsinki (2013). Furthermore, the study reporting followed the guidelines of the EQUATOR (Enhancing the QUAlity and Transparency Of health Research) network, specifically the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).19
Data Collection
At baseline, comprehensive data were collected from participants, encompassing demographic information, medical history (including cardiorenal diseases and diabetes), lifestyle-related factors (such as smoking behavior), and anthropometric measurements (e.g., body height, weight, and waist circumference). Data acquisition was facilitated through questionnaires that participants completed. To obtain blood pressure values, participants assumed a supine position for 8 minutes, and measurements were taken using the Dinamap XL Model 9300 series device (Johnson & Johnson Medical, Tampa, FL). Smoking behavior was categorized as “never,” “former,” or “current” based on self-reported information. Waist circumference was measured on the bare skin at the natural indentation between the 10th rib and the iliac crest. Blood pressure measurements were performed every minute, and for analytic purposes, the average of the last 2 measurements was considered, following previously established procedures.20,21 Information about medication use was primarily self-reported by participants, but it was cross-referenced and supplemented with data from a pharmacy-dispensing registry. This registry provided comprehensive information about drug usage for over 95% of the PREVEND study participants.22,23
Fasting venous plasma and serum samples were collected and centrifuged at 4 °C. Processed samples were stored at −80 °C until further analysis. Urine samples were stored at −20 °C until analysis. Urinary albumin concentration was determined using nephelometry (threshold of 2.3 mg/l and intraassay and interassay coefficients variation of 2.2% and 2.6%, respectively; Dade Behring Diagnostics, Marburg, Germany). Two consecutive 24-hour urine collections were used for UAE measurement, and the average was used for calculations. Serum creatinine was measured with an enzymatic method (Roche Modular Analyzer, Roche Diagnostics, Mannheim, Germany). Similarly, serum cystatin C levels were measured using the Gentian Cystatin C Immunoassay on a modular analyzer (Roche Diagnostics). Calibration standards for cystatin C were employed following the manufacturer's instructions, and the assay adhered to the guidelines outlined by the International Federation of Clinical Chemistry Working Group for Standardization of Serum Cystatin C.24 For measurements of hs-CRP, nephelometry was used (Dade Behring Diagnostics, Marburg, Germany). Total cholesterol was assayed using routine automated methods as described.25 Plasma glucose was measured using dry chemistry (Eastman Kodak, Rochester, NY).
Plasma Calprotectin Measurements
Calprotectin levels were measured in ethylenediaminetetraacetic acid anticoagulated plasma samples with a turbidimetric immunoassay (Gentian AS, Moss, Norway) leveraging a Mindray BS-400 analyzer (Mindray, Shenzhen, China). Total imprecision for all samples and controls with calprotectin concentrations >1 mg/l was <3%. The Gentian Calprotectin Immunoassay for plasma samples (analytical measurement range: 0.2–20 mg/l) is robust in frozen samples over at least 1 freeze and thaw cycle. A previous study that investigated the effect of freezing on the stability of calprotectin could not report reduced stability over 9 freezing cycles in blood samples with concentrations of about 2 to 3 mg/l.26 Another study using purified antigen solution instead of native samples found that levels decreased by 14% after 1 freeze cycle but remained stable for the next 3 cycles, thereby staying within ±20% recovery criteria.27 In conclusion, the results from this study and others cited demonstrate that blood samples (be it serum or plasma) can be frozen for long-term storage and measurement reliability can be assured in those samples for up to 4 cycles of freezing and thawing.
Study Outcomes and Definitions
The primary outcome of this study was the development of new-onset CKD, which was defined as the first occurrence of either an eGFR <60 ml/min per 1.73 m2, UAE >30 mg/24h, or both combined. Secondary outcomes included the first occurrence of an eGFR <60 ml/min per 1.73 m2 and UAE >30 mg/24h treated as individual outcomes. eGFR was calculated using the combined cystatin C-based CKD Epidemiology Collaboration equation.28 Type 2 diabetes was identified if participants met any of the following criteria: fasting glucose concentration ≥7.0 mmol/l, nonfasting glucose level ≥11.1 mmol/l as per the guidelines set by the American Diabetes Association29 or the use of antidiabetic drugs or self-report of a physician diagnosis.
Statistical Analyses
Baseline characteristics of the study population were presented in different formats depending on the nature of the variables. Continuous variables describing demographic, anthropometric, and laboratory characteristics, were expressed as mean ± SD. For skewed variables, medians with interquartile ranges were reported. Normality assessment of the data was conducted through visual inspection of normal probability (Q-Q) plots and histograms. Differences in baseline characteristics across tertiles of plasma calprotectin concentrations were analyzed using one-way analysis of variance for normally distributed variables, Kruskal-Wallis tests for skewed variables, and chi-square tests for categorical variables. To evaluate survival distributions of participants in each tertile of plasma calprotectin levels, Kaplan-Meier survival probabilities were calculated. The log-rank test was used to compare survival curves between the tertiles of plasma calprotectin levels. For time-to-event analyses, the study's starting point was defined as baseline, which corresponded to the time of plasma sample collection. The end point was defined as the date of the last examination attended by participants, the occurrence of CKD, death, or January 1, 2011 (end of follow-up), whichever occurred first. Cox proportional hazards regression analyses were performed to examine associations between plasma calprotectin levels and the risk of new-onset CKD, both as a composite outcome and separately for each individual outcome (eGFR or UAE). Plasma calprotectin levels were 2log-transformed and the results were presented as HRs per doubling in plasma calprotectin level, along with corresponding 95% CIs. The proportionality of hazards assumption was checked to ensure that there were no violations. Subsequently, stratified analyses were conducted to explore potential associations between plasma calprotectin and the risk of new-onset CKD in relevant subgroups. Interaction terms were included in the models to assess whether there were significant effect-modifications in these subgroups (Pinteraction < 0.05). To examine potential nonlinearity of the association between plasma calprotectin and the risk of new-onset CKD, restricted cubic splines with 3 knots were fitted. Nonlinearity was statistically evaluated using likelihood ratio tests, comparing models with linear terms or both linear and cubic spline terms. Data analysis and visualization were performed using SPSS Statistics 28.0 software package (SPSS Inc., Chicago, IL) and R (v.4.0.1, Vienna, Austria). Two-tailed P-values ≤ 0.05 were considered statistically significant.
Selection and Rationale for Confounding Variables: The Directed Acyclic Graph Method
A directed acyclic graph was created as a causal model to identify potentially confounding variables that should be considered when estimating the outcome of interest in the study: new-onset CKD.30,31 Directed acyclic graphs provide a theoretical basis by defining the causal mechanisms hypothesized to underlie the variables of interest (Supplementary Figure S1). In the directed acyclic graph, arrows indicate the hypothesized direct causal effects between variables, whereas the absence of arrows indicates the assumption of no such direct effect. In this study, the primary goal was to investigate the association between plasma calprotectin levels and new-onset CKD. To achieve an unconfounded effect estimate in statistical analyses, a specific set of potentially confounding variables was identified and conditioned for.32, 33, 34, 35, 36 These variables were considered as covariates in the analysis, in addition to age and sex. The selected confounding variables included history of cardiovascular disease, history of diabetes, presence of hypertension, baseline eGFR (reflecting renal function), and hs-CRP (reflecting systemic inflammation). By including these variables in the analysis, we aimed to control for their potential influence on the association between plasma calprotectin levels and the development of new-onset CKD, thereby obtaining a more reliable and unbiased estimate.
Results
Baseline Study Population Characteristics
Baseline characteristics of the study population divided by tertiles of plasma calprotectin levels are presented in Table 1. Median (interquartile range) plasma calprotectin levels were 0.49 (0.35–0.68) mg/l (mean: 0.56 mg/l; full range: 0–13.2 mg/l). Mean (±SD) age of participants was 51.4 (11.4) years and 2514 (53.9%) participants were female. Median baseline eGFR was 95.9 (85.0–105.7) ml/min per 1.73 m2, which was lowest in participants within the highest (T3) tertile of plasma calprotectin levels (P < 0.001) compared to participants within T1 or T2. Median baseline UAE was 7.68 (5.78–11.2) mg/l, which was highest in the highest (T3) tertile of plasma calprotectin levels (P < 0.001). Similarly, participants within the highest (T3) tertile demonstrated the highest rate of new-onset CKD (composite outcome: P = 0.002; based on eGFR <60 ml/min per 1.73 m2: P = 0.026; based on UAE >30 mg/24h: P = 0.012).
Table 1.
Baseline demographic, clinical and biochemical characteristics, and outcomes of the study population, stratified by tertiles of plasma calprotectin levels
| Characteristics | Total | T1 | T2 | T3 | P-value |
|---|---|---|---|---|---|
| ♂: <0.42 μg/l ♀: <0.38 μg/l |
♂: 0.42–0.62 μg/l ♀: 0.38–0.59 μg/l |
♂: >0.62 μg/l ♀: >0.59 μg/l |
|||
| Plasma calprotectin (μg/l) | 0.49 [0.35–0.68] | 0.30 [0.24–0.35] | 0.49 [0.44–0.53] | 0.78 [0.68–0.98] | |
| Demographics | |||||
| Age (yr) | 51.4 ± 11.4 | 50.2 ± 11.3 | 51.4 ± 11.1 | 52.5 ± 11.6 | <0.001 |
| Women, n (%) | 2514 (53.9) | 938 (59.8) | 775 (51.4) | 801 (50.5) | <0.001 |
| Race, n (%) | 0.003 | ||||
| White, n (%) | 4441 (96.0) | 1463 (94.4) | 1448 (96.6) | 1530 (96.8) | |
| Black, n (%) | 42 (0.9) | 15 (1.0) | 10 (0.7) | 17 (1.1) | |
| Asian, n (%) | 94 (2.0) | 47 (3.0) | 28 (1.9) | 19 (1.2) | |
| Other, n (%) | 51 (1.1) | 24 (1.5) | 13 (0.9) | 14 (0.9) | |
| Anthropometrics | |||||
| BMI (kg/m2) | 25.7 [23.4–28.4] | 24.7 [22.6–27.5] | 25.8 [23.6–28.5] | 26.5 [24.2–29.2] | <0.001 |
| Waist circumference (cm) | 90 [81–98] | 86 [78–95] | 91 [82–99] | 92 [84–101] | <0.001 |
| Cardiovascular risk factors | |||||
| SBP (mm Hg) | 120 [111–133] | 117 [108–128] | 122 [112–133] | 124 [113–136] | <0.001 |
| DBP (mm Hg) | 72 [66-78] | 70 [65–76] | 72 [67–78] | 73 [68–79] | <0.001 |
| Smoking | <0.001 | ||||
| Never, n (%) | 1448 (31.5) | 561 (36.3) | 485 (32.5) | 402 (25.7) | |
| Current, n (%) | 1283 (27.9) | 310 (20.0) | 387 (25.9) | 586 (37.5) | |
| Former, n (%) | 1872 (40.7) | 676 (43.7) | 621 (41.6) | 575 (36.8) | |
| History of CVD, n (%) | 123 (2.6) | 32 (2.0) | 44 (2.9) | 47 (3.0) | 0.193 |
| Diabetes, n (%) | 68 (1.5) | 16 (1.0) | 18 (1.2) | 34 (2.2) | 0.018 |
| Hypertension, n (%) | 1168 (25.1) | 317 (20.2) | 376 (24.9) | 475 (30.0) | <0.001 |
| Medication | |||||
| Antihypertensive drugs, n (%) | 626 (13.8) | 174 (11.4) | 192 (13.1) | 260 (16.9) | <0.001 |
| Lipid-lowering drugs, n (%) | 272 (5.8) | 68 (4.3) | 97 (6.4) | 107 (6.8) | 0.007 |
| Glucose-lowering drugs, n (%) | 39 (1.0) | 8 (0.6) | 13 (1.0) | 18 (1.3) | 0.143 |
| Laboratory measurements | |||||
| Total cholesterol (mmol/l) | 5.36 [4.72–6.10] | 5.25 [4.60–6.00] | 5.31 [4.69–6.07] | 5.50 [4.84–6.22] | <0.001 |
| hs-CRP (mg/l) | 1.16 [0.56–2.65] | 0.73 [0.34–1.44] | 1.12 [0.61–2.37] | 2.14 [1.01–4.35] | <0.001 |
| Baseline eGFR (ml/min per 1.73 m2) | 95.9 [85.0–105.7] | 97.8 [86.9–107.4] | 96.1 [85.2–105.5] | 94.1 [82.2–103.5] | <0.001 |
| Baseline UAE (mg/l) | 7.68 [5.78–11.2] | 7.16 [5.67–10.2] | 7.75 [5.76–11.1] | 8.13 [5.96–12.3] | <0.001 |
| Serum creatinine (μmol/l) | 81.1 [72.9–90.4] | 80.1 [71.9–89.3] | 82.1 [72.9–91.4] | 82.1 [73.9–92.4] | <0.001 |
| Urine creatinine (mmol/24h) | 11.9 [9.91–14.5] | 11.6 [9.83–14.2] | 12.0 [10.1–14.7] | 12.1 [9.91–14.7] | <0.001 |
| New-onset CKD after follow-up | |||||
| CKD (eGFR <60 ml/min per 1.73 m2), n (%) | 151 (3.2) | 38 (2.4) | 48 (3.2) | 65 (4.1) | 0.026 |
| CKD (UAE >30 mg/24h), n (%) | 349 (7.5) | 98 (5.3) | 109 (7.2) | 142 (9.0) | 0.012 |
| CKD (combined), n (%) | 467 (10.0) | 132 (8.4) | 144 (9.5) | 191 (12.1) | 0.002 |
BMI, body-mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitive C-reactive protein; SBP, systolic blood pressure; UAE, urinary albumin excretion.
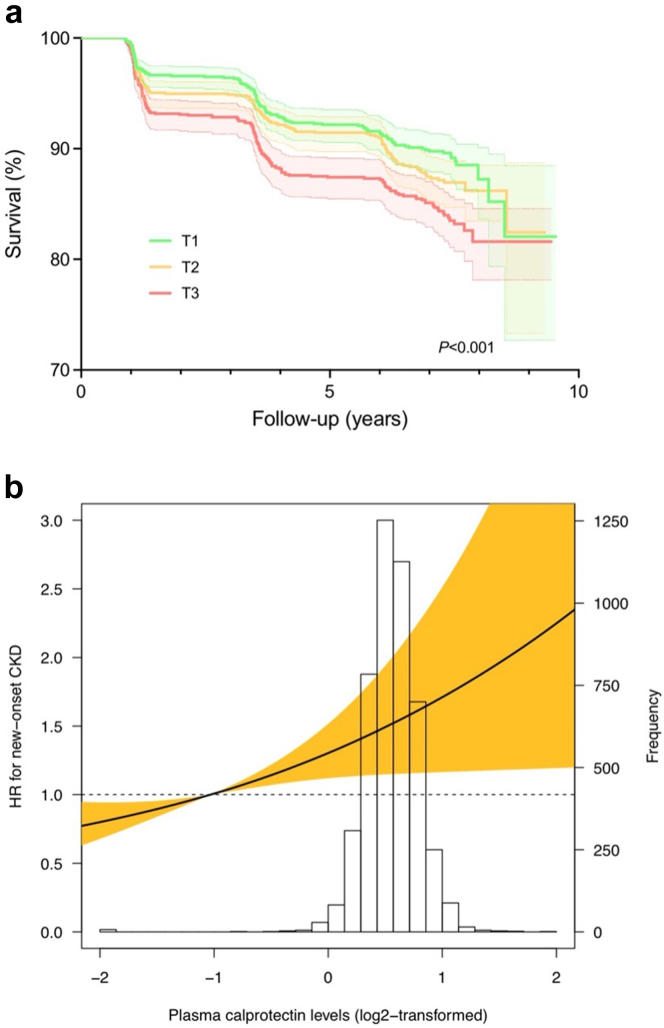
Plasma Calprotectin Levels and the Risk of New-Onset CKD
Over a median follow-up of 8.3 (7.8–8.9) years, a total of 467 participants developed new-onset CKD, among which 151 were based on eGFR (<60 ml/min per 1.73 m2), 349 were based on UAE (30 mg/24h), and 33 were based on both. The highest rate of new-onset CKD occurred in the highest tertile of plasma calprotectin levels (n = 191, 12.1%, P < 0.01). Kaplan-Meier survival analysis demonstrated statistically significant differences in survival distribution for tertiles of plasma calprotectin levels and the risk of new-onset CKD (P < 0.001, log-rank test) (Figure 1a). Cox proportional hazards regression analyses revealed a statistically significant association between plasma calprotectin levels and the risk of new-onset CKD (Table 2, Model 1, HR per doubling of plasma calprotectin levels 1.28 [95% CI: 1.14–1.44], P < 0.001). When adjusting for potentially confounding factors (age, sex, history of cardiovascular disease, history of diabetes, presence of hypertension, and baseline eGFR), this association remained significant (Table 2, Model 4, HR per doubling 1.14 [95% CI: 1.01–1.29], P = 0.034). However, after additional adjustment for hs-CRP, this association lost statistical significance (Model 5, HR per doubling 1.05 [0.91–1.21], P = 0.494). When these analyses were performed for the individual components of the composite outcome (eGFR <60 ml/min per 1.73 m2 and UAE >30 mg/24h), a stronger association was found for eGFR decline as outcome (Supplementary Table 1A, Model 1, HR per doubling 1.44 [1.17–1.77], P < 0.001) when compared to UAE as outcome (Supplementary Table S1B, Model 1, HR per doubling 1.26 [1.10–1.43], P < 0.001). However, after adjusting for potentially confounding variables, the association between plasma calprotectin levels and the risk of new-onset CKD as defined by eGFR <60 ml/min per 1.73 m2 lost statistical significance after adjusting for baseline GFR (Supplementary Table S1A, Model 4, HR per doubling 1.15 [0.92–1.43], P = 0.218), which did not occur for UAE as individual outcome (Supplementary Table S1B, Model 4, HR per doubling 1.17 [1.02–1.34], P = 0.027). This association, however, also lost statistical significance when additionally adjusting for hs-CRP, similar to what occurred to the association with the composite outcome (Model 5, 1.06 [0.90–1.25], P = 0.500). When tertiles of plasma calprotectin levels were used instead of plasma calprotectin levels as continuous variable, the highest tertile (T3) of plasma calprotectin was consistently significantly associated with the risk of new-onset CKD (as composite outcome and individually by UAE >30 mg/24h) after adjustment for confounding variables (Table 2 and Supplementary Table S1B, Model 4, HR per doubling 1.26 [1.01–1.57], P = 0.044 [composite outcome] and 1.33 [1.02–1.72], P = 0.033 [UAE >30 mg/24h]), but significance was abrogated after additional adjustment for hs-CRP (all P > 0.05). No significant deviance from linear association with the risk of new-onset CKD (composite outcome) was observed when restricted cubic splines were fitted (composite: χ2 = 0.08, P = 0.773) (Figure 1b).
Figure 1.
Plasma calprotectin levels associate with an increased risk of new-onset CKD. (a) Kaplan-Meier survival curves for the association between plasma calprotectin levels and the risk of new-onset CKD based on the composite outcome (eGFR, UAE or both) The highest rate of new-onset CKD was observed in the highest tertile (T3) of plasma calprotectin levels. (b) Restricted cubic splines (RCS) were fitted to test for potential nonlinearity of the association between plasma calprotectin levels and the risk of new-onset CKD. Estimated associations were derived from the Cox proportional hazards regression models and RCS with 3 knots (set at the 1st, 50th, and 99th percentiles). A likelihood ratio test for nonlinearity was nonsignificant for this model (χ2 = 0.08, P = 0.773). Shaded areas of the RCS curve represent the 95% confidence intervals. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; UAE, urinary albumin excretion
Table 2.
Cox proportional hazards regression analyses for associations between plasma calprotectin levels and the risk of incident CKD (as composite outcome)
| Models | HR per doubling | T1 | T2 | T3 |
|---|---|---|---|---|
| <0.40 mg/l | 0.40–0.60 mg/l | >0.60 mg/l | ||
| CKD (composite outcome) (n = 467) | ||||
| Model 1 | 1.28 [1.14–1.44], P < 0.001 | 1.00 (reference) | 1.19 [0.94–1.51], P = 0.147 | 1.56 [1.25–1.95], P < 0.001 |
| Model 2 | 1.21 [1.07–1.37], P = 0.002 | 1.00 (reference) | 1.07 [0.85–1.36], P = 0.558 | 1.37 [1.10–1.71], P = 0.006 |
| Model 3 | 1.18 [1.04–1.33], P = 0.009 | 1.00 (reference) | 1.06 [0.83–1.34], P = 0.644 | 1.32 [1.06–1.65], P = 0.015 |
| Model 4 | 1.14 [1.01–1.29], P = 0.034 | 1.00 (reference) | 1.05 [0.83–1.34], P = 0.668 | 1.26 [1.01–1.57], P = 0.044 |
| Model 5 | 1.05 [0.91–1.21], P = 0.494 | 1.00 (reference) | 0.96 [0.74–1.25], P = 0.764 | 1.13 [0.87–1.46], P = 0.355 |
CKD, chronic kidney disease; HR, hazard ratio
Stratified Analyses
Stratified analyses for the association between plasma calprotectin levels and the risk of new-onset CKD were performed, which demonstrated consistently positive associations in almost all analyzed subgroups (Supplementary Table S2). Stratification by the median of baseline UAE and current smoking showed significant effect modification (Pinteraction < 0.001 for both). The corresponding HRs were higher for participants with below-median baseline UAE (<13.5 mg/24h) and for nonsmokers.
Sensitivity Analyses
When participants with eGFR <65 ml/min per 1.73 m2 at baseline (instead of <60 ml/min per 1.73m2) were excluded, the association between plasma calprotectin levels and the risk of new-onset CKD (composite outcome) did not substantially change (HR per doubling 1.16 [1.02–1.32], P = 0.023). However, the association between plasma calprotectin levels and the risk of new-onset CKD when adjusted for potentially confounding variables (Model 4, Table 2) remained similar, albeit lost statistical significance when participants with UAE >25 mg/24h (instead of 30 mg/24h) at baseline were excluded (HR per doubling 1.12 [95% CI: 0.98–1.27], P = 0.096). When extreme outliers of plasma calprotectin levels (<0.5th percentile and >99.5th percentile) were excluded, the association between plasma calprotectin and the risk of new-onset CKD did not materially change (HR per doubling 1.16 [1.02–1.33], P = 0.025). Similarly, when excluding individuals with new-onset CKD within the first year of follow-up, the association between plasma calprotectin and the risk of new-onset CKD remained present (HR per doubling 1.13 [1.00–1.28], P = 0.049).
Discussion
This study demonstrated a significant association between plasma calprotectin levels and an increased risk of new-onset CKD in the general population over a nearly 10-year follow-up period. This association remained statistically significant after adjusting for well-established risk factors for CKD, including age, sex, history of cardiovascular disease, diabetes, hypertension, as well as renal function as represented by baseline eGFR. However, after further adjusting for hs-CRP, being a marker for systemic inflammation, the association lost significance, indicating that plasma calprotectin is associated with new-onset CKD, but not independently of the degree of systemic inflammation. When considering the individual components defining new-onset CKD (eGFR <60 ml/min per 1.73 m2 or UAE >30 mg/24h), the associations with UAE >30 mg/24h remained, while a nonsignificant association with eGFR <60 ml/min per 1.73 m2 was found. However, this difference could also be attributed to a difference in statistical power across both analyses, because the event rate for new-onset CKD was substantially higher when UAE was taken as individual outcome compared with eGFR. Stratified analyses across relevant subgroups consistently demonstrated positive associations between plasma calprotectin levels and the risk of new-onset CKD. Notably, significant interactions were observed for smoking and baseline UAE, indicating that associations were stronger in nonsmokers and in participants with below-median baseline UAE, respectively. Collectively, our results suggest that plasma calprotectin levels are linked to an increased risk of new-onset CKD; this association seems dependent on already existing systemic inflammation at baseline. Calprotectin may potentially serve as a biomarker for preclinical renal damage, aiding in risk stratification in preventive settings with the goal of mitigating the future burden of CKD.
Given that myeloid cells such as neutrophils and macrophages are the primary source of calprotectin, elevated levels of circulating calprotectin may be reflective of myeloid cell infiltration of cardiovascular and renal tissues.37, 38, 39 Previous studies have shown that neutrophils may aid in the formation of atherosclerotic plaques by activating vascular endothelium, disrupting its integrity, promoting apoptosis, and inducing the generation of reactive oxygen species, resulting in oxidative stress and the oxidation of low-density lipoproteins.40, 41, 42 In addition, plasma calprotectin levels may contribute to the activation of the receptor for advanced glycation end products, which in turn triggers a variety of inflammatory and thrombotic cascades, further contributing to atherosclerosis.43 The process of CKD development additionally involves endothelial and perivascular inflammation, vascular fibrosis, calcification, and other morphological changes.44 Although the precise role of calprotectin in the pathogenesis of CKD remains incompletely understood, circulating calprotectin levels likely stimulate the inflammatory response by triggering leukocyte recruitment to tissues and activating neighboring immune cells. Furthermore, their release is accompanied by an overproduction of reactive oxygen species, resulting in oxidative stress, and the secretion of proinflammatory cytokines, the latter of which is also considered proatherogenic and may, in parallel, contribute to CKD development.45 In keeping with this, a previous study from our center found plasma calprotectin to be associated with incident cardiovascular disease in the general population.7 A more recent study employing proteomic analysis has unveiled a strong correlation between elevated circulating levels of calprotectin and an increased susceptibility to cardiovascular complications and mortality in patients with CKD.46 Furthermore, it demonstrated the therapeutic efficacy of targeting calprotectin through the administration of a calprotectin inhibitor, paquinimod, which could prevent vascular calcification in mice with nephrectomy-induced CKD on a high-phosphate diet. These findings were substantiated by analogous observations in apolipoprotein-E deficient mice on a high-phosphate diet, strongly suggesting that inhibiting calprotectin could be a promising approach to avert vascular calcification in high-risk patients with CKD. This might also hold true for other inflammatory conditions associated with the development of calcification, such as atherosclerosis, media calcification, and calciphylaxis.
Previous studies have shown a clear relationship between circulating calprotectin and hs-CRP, obviously because of macrophage and neutrophil activation in circumstances of systemic inflammation.46, 47, 48 In the current study, the association between plasma calprotectin and the risk of new-onset CKD was dependent on hs-CRP. These findings support the relevance of hs-CRP as a potential confounding factor and also favor the hypothesis of inflammation as a pathogenic driver of cardiovascular and renal disease development.7 In addition, calprotectin may not only be a marker of macrophage and neutrophil activation, it may also be more directly involved in the pathogenesis of CKD via inflammatory responses.49 Like hs-CRP, calprotectin has also been shown to strongly associate with proinflammatory cytokines such as tumor necrosis factor-alpha and interferons.50 These molecules are also involved in the development of vascular damage.45 Interestingly, the association between plasma calprotectin levels and the risk of new-onset CKD was at least as strong when new-onset CKD was defined by UAE >30 mg/24h. This observation corroborates previously reported findings on the association between albuminuria and circulating calprotectin levels.51,52 As such, when we additionally adjusted our Cox proportional hazards regression models for hs-CRP at baseline, statistically significant relationships were abrogated.
In stratified analyses, fairly consistent positive associations were observed between plasma calprotectin levels and the risk of new-onset CKD across most subgroups. Notably, the associations between plasma calprotectin and the risk of new-onset CKD were particularly stronger in individuals who did not smoke and those with below-median baseline UAE. These subgroups showed significant interactions, indicating that certain factors, such as smoking, may influence the relationship between plasma calprotectin levels and CKD risk. The link between tobacco smoke exposure and plasma calprotectin levels has been variably reported in different clinical contexts.51, 52, 53 In the present study, it was observed that the highest tertile of plasma calprotectin levels also contained a higher prevalence of current smokers. The weaker association of plasma calprotectin levels with the risk of new-onset CKD in smoking participants and those with relatively higher baseline UAE could potentially imply that elevated calprotectin levels due to such factors may reduce their variation, possibly impacting the predictive performance regarding new-onset CKD. In addition, the observation that the associations between plasma calprotectin and new-onset CKD lost statistical significance after adjusting for hs-CRP underlines the intimate link between systemic inflammation and the risk of developing CKD. However, it is essential to cautiously interpret the findings from these secondary stratified analyses, because sample sizes in some of the subgroups were particularly smaller and occasionally imbalanced, which may introduce bias. Further research with larger and more balanced sample sizes is needed to validate and better understand the observed interactions and associations.
This study has several relevant strengths. First, the large number of study participants and cases of new-onset CKD within this extensively characterized dataset, concomitant with a relatively long follow-up period of almost 10 years, allowed a comprehensive analysis of plasma calprotectin as prognostic biomarker for the development of CKD. Furthermore, it allowed the adjustment for numerous potential confounding variables in a prospective setting, and the exclusion of individuals with new-onset CKD within the first year after baseline did not materially change the observed association, reducing the risk of reverse causation. However, certain limitations must also be recognized. Because this was a single-center study with predominantly White participants from the north of the Netherlands, the generalizability of the results to other ethnicities or geographic locations may be poor. Second, due to unavailability of sufficient plasma samples for selected participants, they had to be excluded from the analyses. Third, given the observational nature of this study, causal relationships could not be established. Instead, we were only able to document associations between plasma calprotectin levels and risk of new-onset CKD. Despite the well-characterized phenotypes of the study cohort, it is important to recognize that residual confounding factors may still exist. Finally, it is essential to acknowledge that in this study, no additional blood samples were available to explore and compare other inflammatory biomarkers. Utilizing multiomics-based platforms, such as proteomics or metabolomics approaches, could have been a valuable strategy. These advanced techniques could be leveraged to analyze a comprehensive set of molecular components involved in inflammation. By employing such approaches, additional insights into the key integrative inflammatory factors driving the development of CKD could be obtained, potentially leading to the identification of a biomarker signature with higher predictive value. In future research, incorporating multiomics approaches may help unravel the complexities of inflammation and its association with CKD, ultimately contributing to the discovery of more accurate and powerful predictive biomarkers. Overall, though this study presents valuable insights into the potential use of plasma calprotectin as a renal function biomarker, its limitations underscore the need for further research in diverse clinical populations and the inclusion of additional biomarker measurements to enhance our understanding of its clinical utility.
In conclusion, this study demonstrates a significant correlation between plasma calprotectin levels and an increased risk of new-onset CKD in the general population. However, within this cohort of population-based individuals, the relationship between plasma calprotectin and the risk of new-onset CKD appears to depend on the level of systemic inflammation, which may suggest that calprotectin does not provide much additive value on top of existing biomarkers for the future occurrence of CKD. Still, this scenario might differ in patients with already impaired renal function or those with specific kidney diseases. Furthermore, our findings still affirm the relevance of systemic inflammation in CKD development and might indicate calprotectin as a potential etiological factor. Future studies are therefore warranted to explore the mechanistic underpinnings of this association as well as the potential utility of circulating calprotectin levels in distinct clinical populations, for example, those with already impaired kidney function or with specific kidney diseases. These future endeavors will be essential in shedding more light on the potential clinical significance of calprotectin as a predictive biomarker in different contexts of CKD.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors would like to express their gratitude to all participants of the PREVEND cohort study.
The Prevention of REnal and Vascular ENd-stage Disease study has been made possible by grants from the Dutch Kidney Foundation (grant no. E.013).
Data Availability Statement
The data underlying this article are available upon reasonable request to the corresponding author.
Author Contributions
ARB, AEA, RTG, SJLB, and HvG were involved in conceptualization and study design. RTG and SJLB were responsible for funding acquisition and resources. RTG, SJLB, TN, and CH collected all study data. ARB performed data analysis and data visualization. ARB wrote the first draft of the manuscript. All authors contributed to results interpretation, critically reviewed the manuscript, contributed to manuscript revision, and read and approved the final version of the manuscript to be submitted for publication.
Footnotes
Figure S1. Direct acyclic graph (DAG) representing the hypothesized causal relationships that underlie the studied association between plasma calprotectin levels and the risk of new-onset CKD in the general population.
Table S1. Cox proportional hazards regression analyses for associations between plasma calprotectin levels and the risk of incident CKD, based on its individual determinants (eGFR and UAE).
Table S2. Stratified analyses for the association between plasma calprotectin levels and the risk of incident CKD across various subgroups.
STROBE Statement.
Supplementary Material
Figure S1. Direct acyclic graph (DAG) representing the hypothesized causal relationships that underlie the studied association between plasma calprotectin levels and the risk of new-onset CKD in the general population.
Table S1. Cox proportional hazards regression analyses for associations between plasma calprotectin levels and the risk of incident CKD, based on its individual determinants (eGFR and UAE).
Table S2. Stratified analyses for the association between plasma calprotectin levels and the risk of incident CKD across various subgroups.
STROBE Statement.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney disease: improving global outcomes (KDIGO) CKD work group. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 2.Shlipak M.G., Day E.C. Biomarkers for incident CKD: a new framework for interpreting the literature. Nat Rev Nephrol. 2013;9:478–483. doi: 10.1038/nrneph.2013.108. [DOI] [PubMed] [Google Scholar]
- 3.Gansevoort R.T., de Jong P.E. The case for using albuminuria in staging chronic kidney disease. J Am Soc Nephrol. 2009;20:465–468. doi: 10.1681/ASN.2008111212. [DOI] [PubMed] [Google Scholar]
- 4.Foell D., Wittkowski H., Roth J. Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut. 2009;58:859–868. doi: 10.1136/gut.2008.170019. [DOI] [PubMed] [Google Scholar]
- 5.Wang S., Song R., Wang Z., Jing Z., Wang S., Ma J. S100A8/A9 in inflammation. Front Immunol. 2018;9:1298. doi: 10.3389/fimmu.2018.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teng T.S., Ji A.L., Ji X.Y., Li Y.Z. Neutrophils and immunity: from bactericidal action to being conquered. J Immunol Res. 2017;2017 doi: 10.1155/2017/9671604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunutsor S.K., Flores-Guerrero J.L., Kieneker L.M., et al. Plasma calprotectin and risk of cardiovascular disease: findings from the PREVEND prospective cohort study. Atherosclerosis. 2018;275:205–213. doi: 10.1016/j.atherosclerosis.2018.06.817. [DOI] [PubMed] [Google Scholar]
- 8.Bourgonje A.R., Bourgonje M.F., la Bastide-van Gemert S., et al. Plasma calprotectin levels associate with new-onset hypertension in the general population: a prospective cohort study. J Am Heart Assoc. 2024;13 doi: 10.1161/JAHA.123.031458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Løfblad L., Hov G.G., Åsberg A., Videm V. Calprotectin and CRP as biomarkers of cardiovascular disease risk in patients with chronic kidney disease: a follow-up study at 5 and 10 years. Scand J Clin Lab Investig. 2023;83:258–263. doi: 10.1080/00365513.2023.2211779. [DOI] [PubMed] [Google Scholar]
- 10.Altwegg L.A., Neidhart M., Hersberger M., et al. Myeloid-related protein 8/14 complex is released by monocytes and granulocytes at the site of coronary occlusion: a novel, early, and sensitive marker of acute coronary syndromes. Eur Heart J. 2007;28:941–948. doi: 10.1093/eurheartj/ehm078. [DOI] [PubMed] [Google Scholar]
- 11.Bourgonje A.R., von Martels J.Z.H., de Vos P., Faber K.N., Dijkstra G. Increased fecal calprotectin levels in Crohn’s disease correlate with elevated serum Th1- and Th17-associated cytokines. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gansevoort R.T., Correa-Rotter R., Hemmelgarn B.R., et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 13.Zoccali C., Vanholder R., Massy Z.A., et al. The systemic nature of CKD. Nat Rev Nephrol. 2017;13:344–358. doi: 10.1038/nrneph.2017.52. [DOI] [PubMed] [Google Scholar]
- 14.Rapa S.F., Di Iorio B.R., Campiglia P., Heidland A., Marzocco S. Inflammation and oxidative stress in chronic kidney disease-potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int J Mol Sci. 2019;21:263. doi: 10.3390/ijms21010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshitomi R., Nakayama M., Sakoh T., et al. High neutrophil/lymphocyte ratio is associated with poor renal outcomes in Japanese patients with chronic kidney disease. Ren Fail. 2019;41:238–243. doi: 10.1080/0886022X.2019.1595645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liew P.X. Mired in the glomeruli: witnessing live neutrophil recruitment in the kidney. Am J Physiol Cell Physiol. 2021;321:C384–C393. doi: 10.1152/ajpcell.00429.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinzelmann M., Mercer-Jones M.A., Passmore J.C. Neutrophils and renal failure. Am J Kidney Dis. 1999;34:384–399. doi: 10.1016/s0272-6386(99)70375-6. [DOI] [PubMed] [Google Scholar]
- 18.Hillege H.L., Janssen W.M., Bak A.A., et al. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med. 2001;249:519–526. doi: 10.1046/j.1365-2796.2001.00833.x. [DOI] [PubMed] [Google Scholar]
- 19.von Elm E., Altman D.G., Egger M., et al. STROBE initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 20.Post A., Kremer D., Swarte J.C., et al. Plasma creatine concentration is associated with incident hypertension in a cohort enriched for the presence of high urinary albumin concentration: the Prevention of Renal and Vascular endstage Disease study. J Hypertens. 2021;40:229–239. doi: 10.1097/HJH.0000000000002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourgonje A.R., Bourgonje M.F., Post A., et al. Systemic oxidative stress associates with new-onset hypertension in the general population. Free Radic Biol Med. 2022;187:123–131. doi: 10.1016/j.freeradbiomed.2022.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Kappelle P.J.W.H., Gansevoort R.T., Hillege J.L., Wolffenbuttel B.H.R., Dullaart R.P.F., PREVEND study group PREVEND study group. Apolipoprotein B/A-I and total cholesterol/high-density lipoprotein cholesterol ratios both predict cardiovascular events in the general population independently of nonlipid risk factors, albuminuria and C-reactive protein. J Intern Med. 2011;269:232–242. doi: 10.1111/j.1365-2796.2010.02323.x. [DOI] [PubMed] [Google Scholar]
- 23.Borggreve S.E., Hillege H.L., Wolffenbuttel B.H.R., et al. The effect of cholesteryl ester transfer protein -629C->A promoter polymorphism on high-density lipoprotein cholesterol is dependent on serum triglycerides. J Clin Endoc Metab. 2005;90:4198–4204. doi: 10.1210/jc.2005-0182. [DOI] [PubMed] [Google Scholar]
- 24.Grubb A., Blirup-Jensen S., Lindström V., et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010;48:1619–1621. doi: 10.1515/CCLM.2010.318. [DOI] [PubMed] [Google Scholar]
- 25.Corsetti J.P., Gansevoort R.T., Bakker S.J., Sparks C.E., Vart P., Dullaart R.P. Apolipoprotein B attenuates albuminuria-associated cardiovascular disease in prevention of renal and vascular endstage disease (PREVEND) participants. J Am Soc Nephrol. 2014;25:2906–2915. doi: 10.1681/ASN.2013121256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao J., Ulvik A., McCann A., Ueland P.M., Meyer K. Microheterogeneity and preanalytical 520 stability of protein biomarkers of inflammation and renal function. Talanta. 2021;223 doi: 10.1016/j.talanta.2020.121774. [DOI] [PubMed] [Google Scholar]
- 27.Nilsen T., Haugen S.H., Larsson A. Extraction, isolation, and concentration of calprotectin 523 antigen (S100A8/S100A9) from granulocytes. Health Sci Rep. 2018;1:e35. doi: 10.1002/hsr2.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inker L.A., Schmid C.H., Tighiouart H., et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbasi A., Corpeleijn E., Gansevoort R.T., et al. Role of HDL cholesterol and estimates of HDL particle composition in future development of type 2 diabetes in the general population: the PREVEND study. J Clin Endocrinol Metab. 2013;98:E1352–1359. doi: 10.1210/jc.2013-1680. [DOI] [PubMed] [Google Scholar]
- 30.la Bastide-van Gemert S., van den Heuvel E. Exploring causal hypotheses: breaking with long-standing research traditions. Dev Med Child Neurol. 2013;55:975–976. doi: 10.1111/dmcn.12269. [DOI] [PubMed] [Google Scholar]
- 31.Lederer D.J., Bell S.C., Branson R.D., et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16:22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 32.Tzoulaki I., Elliott P., Kontis V., Ezzati M. Worldwide exposures to cardiovascular risk factors and associated health effects: current knowledge and data gaps. Circulation. 2016;133:2314–2333. doi: 10.1161/CIRCULATIONAHA.115.008718. [DOI] [PubMed] [Google Scholar]
- 33.Pencina M.J., D’Agostino R.B., Larson M.G., Massaro J.M., Vasan R.S. Predicting the 30-year risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:3078–3084. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salzano S., Checconi P., Hanschmann E.M., et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc Natl Acad Sci U S A. 2014;111:12157–12162. doi: 10.1073/pnas.1401712111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao W., Srinivasan S.R., Berenson G.S. Persistent elevation of plasma insulin levels is associated with increased cardiovascular risk in children and young adults. The Bogalusa heart study. Circulation. 1996;93:54–59. doi: 10.1161/01.cir.93.1.54. [DOI] [PubMed] [Google Scholar]
- 36.Singh-Manoux A., Shipley M.J., Bell J.A., Canonico M., Elbaz A., Kivimäki M. Association between inflammatory biomarkers and all-cause, cardiovascular and cancer-related mortality. CMAJ. 2017;189:e384–390. doi: 10.1503/cmaj.160313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng W.H., Jian W.X., Li H.L., et al. Increased serum myeloid-related protein 8/14 level is associated with atherosclerosis in type 2 diabetic patients. Cardiovasc Diabetol. 2011;10:41. doi: 10.1186/1475-2840-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Goor H., Ding G., Kees-Folts D., Grond J., Schreiner G.F., Diamond J.R. Macrophages and renal disease. Lab Investig. 1994;71:456–464. [PubMed] [Google Scholar]
- 40.Viemann D., Barczyk K., Vogl T., et al. MRP8/MRP14 impairs endothelial integrity and induces a caspase-dependent and -independent cell death program. Blood. 2007;109:2453–2460. doi: 10.1182/blood-2006-08-040444. [DOI] [PubMed] [Google Scholar]
- 41.Della Bona R., Cardillo M.T., Leo M., et al. Polymorphonuclear neutrophils and instability of the atherosclerotic plaque: a causative role? Inflamm Res. 2013;62:537–550. doi: 10.1007/s00011-013-0617-0. [DOI] [PubMed] [Google Scholar]
- 42.Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res. 2012;110:875–888. doi: 10.1161/CIRCRESAHA.111.257535. [DOI] [PubMed] [Google Scholar]
- 43.Hofmann M.A., Drury S., Fu C., et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 44.Ungvari Z., Tarantini S., Donato A.J., Galvan V., Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber C., Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 46.Amaya-Garrido A., Brunet M., Buffin-Meyer B., et al. Calprotectin is a contributor to and potential therapeutic target for vascular calcification in chronic kidney disease. Sci Transl Med. 2023;15 doi: 10.1126/scitranslmed.abn5939. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen L., Nybo M., Poulsen M.K., Henriksen J.E., Dahl J., Rasmussen L.M. Plasma calprotectin and its association with cardiovascular disease manifestations, obesity and the metabolic syndrome in type 2 diabetes mellitus patients. BMC Cardiovasc Disord. 2014;14:196. doi: 10.1186/1471-2261-14-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mylemans M., Nevejan L., Van Den Bremt S., et al. Circulating calprotectin as biomarker in neutrophil-related inflammation: pre-analytical recommendations and reference values according to sample type. Clin Chim Acta. 2021;517:149–155. doi: 10.1016/j.cca.2021.02.022. [DOI] [PubMed] [Google Scholar]
- 49.Morrow D.A., Wang Y., Croce K., et al. Myeloid-related protein 8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the pravastatin or atorvastatin Evaluation and Infection Therapy: thrombolysis in myocardial infarction (PROVE IT-TIMI 22) trial. Am Heart J. 2008;155:49–55. doi: 10.1016/j.ahj.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cotoi O.S., Dunér P., Ko N., et al. Plasma S100A8/A9 correlates with blood neutrophil counts, traditional risk factors, and cardiovascular disease in middle-aged healthy individuals. Arterioscler Thromb Vasc Biol. 2014;34:202–210. doi: 10.1161/ATVBAHA.113.302432. [DOI] [PubMed] [Google Scholar]
- 51.Oosterwijk M.M., Bakker S.J.L., Nilsen T., Navis G., Laverman G.D. Determinants of increased serum calprotectin in patients with type 2 diabetes mellitus. Int J Mol Sci. 2020;21:8075. doi: 10.3390/ijms21218075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmaderer C., Kemmner S., Burkhardt K., Heemann U., Baumann M. Serum myeloid-related protein 8/14 complex is associated with microalbuminuria in patients with type 2 diabetes. Ther Adv Cardiovasc Dis. 2014;8:80–88. doi: 10.1177/1753944714528270. [DOI] [PubMed] [Google Scholar]
- 53.Cobanoglu N., Dalkan C., Galip N., Tekguc H., Uncu M., Bahceciler N.N. Is calprotectin a marker of tobacco smoke related inflammation?: a pilot study in children. Inhal Toxicol. 2012;24:486–491. doi: 10.3109/08958378.2012.693137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available upon reasonable request to the corresponding author.