Abstract
Obesity-related asthma is associated with high disease burden and poor response to existent asthma therapies, suggesting that it is a distinct asthma phenotype. The proposed mechanisms that contribute to obesity-related asthma include the effects of mechanical load of obesity, adipokine perturbations, and immune dysregulation. Each of these influence airway smooth muscle (ASM) function. Mechanical fat load alters ASM stretch affecting airway wall geometry, ASM contractility, and agonist delivery; weight loss strategies, including medically-induced weight loss, counters these effects. Among the metabolic disturbances, insulin resistance, and free fatty acid receptor activation influence distinct signaling pathways in the ASM downstream of both M2 muscarinic receptor and β2 adrenergic receptor, such as phospholipase C (PLC) and extracellular signal-regulated kinases (ERK) signaling cascade. Medications that decrease insulin resistance and dyslipidemia are associated with lower asthma disease burden. Leptin resistance is best understood to modulate muscarinic receptors via the neural pathways but there are no specific therapies for leptin resistance. From the immune perspective, monocytes and T helper cells are involved in systemic pro-inflammatory profiles driven by obesity, notably associated with elevated levels of interleukin (IL) 6. Clinical trials on Tocilizumab, anti-IL6 antibody, are ongoing for obesity-related asthma. This armamentarium of therapies are distinct from standard asthma medications, and once investigated for their efficacy and safety among children, will serve as novel therapeutic interventions for pediatric obesity-related asthma. Irrespective of the directionality of the association between asthma and obesity, airway-specific mechanistic studies are needed to identify additional novel therapeutic targets for obesity-related asthma.
INTRODUCTION
Obesity is an independent risk factor and disease modifier for asthma in both adults and children [1, 2]. Pediatric obesity increases asthma risk by 20–25% [3]. In keeping with this, asthma prevalence in obese adults is 11.1% as compared to 7.1% in lean adults, with the relationship being the strongest among obese women with asthma prevalence of 14.6% [4].
Substantial epidemiological evidence suggests that obesity-related asthma causes a high disease burden. Compared to lean individuals, obese individuals with asthma experience more exacerbations, higher asthma severity, poorer disease control, more frequent emergency department visits, higher need for mechanical ventilation, and lower quality of life, in the setting of suboptimal response to conventional asthma therapy [5–11]. These features suggests that obesity-related asthma is a distinct phenotype which needs an improved understanding of its underlying mechanisms, which will directly inform development of targeted therapies.
Airway hyperresponsiveness (AHR), a hallmark feature of asthma, defines a predisposition of the airways to narrow excessively in response to stimuli [12]. In clinical trials, AHR is measured by reduction in forced expiratory volume in 1st second (FEV1) in response to challenge with escalating concentrations of bronchial agonist [13]. In experimental and in vivo models, AHR and bronchomotor tone is regulated by airway smooth muscle (ASM) shortening that narrows the airway diameter [14]. The pathogenesis of obesity-related asthma has been attributed to three possible factors, the effects of mechanical load of obesity, metabolic dysregulation, and excess systemic inflammatory responses [15], but the specific molecular mechanisms by which each of these factors contribute to AHR remains poorly understood. In this review article, we discuss our current understanding of these mechanisms, with a focus on their effects on ASM function, which inform existent and future therapeutic approaches for obesity-related asthma.
a. The role of mechanical effect of adipose tissue
AHR, a multifactorial process, comprises of four types of mechanical determinants: mechanical load on ASM, airway wall geometry, ASM contractility, and agonist delivery [13]; all four of these mechanical determinants are altered in obesity-related asthma. The mechanical load against which ASM contracts is influenced by two factors, airway wall stiffness and the outward tethering force exerted by the surrounding parenchyma [16, 17]. In the context of obesity-related asthma, both these features are affected due to excessive accumulation of truncal fat, which compresses the chest wall and elevates the diaphragm. Along these lines, airway wall geometry plays an important role in AHR. The ASM cells are distributed in a variety of angles relative to the airway circumference such that ASM cell contraction causing airway narrowing depends on the relative airway wall stiffness in the radial and axial directions [13]. The angle of ASM distribution is of greater importance when there is airway inflammation because inflammation-induced increase in tissue thickness between the ASM and the lumen causes airway narrowing even with normal extent of ASM shortening. Although airway luminal inflammation has not been reported in obese individuals with asthma, there is evidence of increased deposition of submucosal eosinophils [18] and of links between systemic measures of inflammation with lower lung function in obese individuals with asthma [19, 20], suggesting that altered immune responses may influence airway geometry in obese individuals with asthma. A third determinant involves the factors affecting ASM contractility. Since the cross sectional area of the airway plays a substantial role in baseline ASM tension, one may speculate that reduced cross sectional area, as observed in obesity [21], may increase ASM contractility. Lastly, agonist delivery may also influence AHR in obese individuals with asthma. Mice with allergic airway inflammation demonstrated exaggerated ASM shortening when given intravenous methacholine as compared to aerosolized methacholine, suggesting that delivery of bronchial agonist to ASM via inflamed airways may be impaired due to epithelial hypertrophy and increased mucus [22]. In the setting of obesity, circulating pro-inflammatory mediators may influence AHR by this mechanism, linking immune perturbation with pulmonary function deficits among individuals with obesity-related asthma [19, 20]. Based on these studies, we suggest that alteration in mechanical load on ASM with a potential contribution of circulating pro-inflammatory mediators secreted from adipose tissue leads to reduction in lung volumes, particularly the vital capacity (VC), residual volume (RV) and expiratory reserve volume (ERV) [23].
Therapies for mechanical effect of adipose tissue and their effect on obesity-related asthma
Both adult and pediatric studies have investigated the effects of weight loss on asthma symptoms and AHR [24–26]. While studies on the effects of bariatric surgery on asthma symptoms are mostly from the adult literature [26–29], pediatric interventions focused on dietary modification and physical activity [25]. Irrespective of the approach, weight loss is consistently associated with improvement in asthma control. However, mixed results have been noted on improvement in AHR following weight loss wherein some studies found improvement with dietary interventions [24] or bariatric surgery [26, 28], while others did not [27, 30]. These differences can stem from differences in asthma phenotypes associated with obesity [29] since bariatric surgery improves AHR more among individuals with non-atopic asthma as compared to those with atopic asthma [26]. Moreover, since other obesity-related factors, such as metabolic and immune dysregulation also improve with weight loss, differential change in these factors may also underlie differences in the effect of weight loss on AHR. However, few studies have examined the independent contribution of reduction in mechanical fat load relative to that of improvement in metabolic and immune parameters to improved AHR with weight loss.
b. The role of metabolic dysregulation
i). Insulin resistance
Insulin resistance (IR) is a well-described obesity-mediated metabolic complication that has been linked with obesity-related asthma [20, 31, 32]. In a cross-sectional study in adolescents, the inverse association between obesity and FEV1 and FVC values, was more pronounced among obese children with IR [33]. A prospective study on a community-based cohort of 4827 Korean adult participants over 4 years reported a negative linear association between homeostatic measurement of insulin resistance (HOMA-IR) and annual changes in percent predicted FEV1 and FVC, particularly in subjects above 50 years of age, after adjusting for other risk factors such as smoking, physical activity and BMI [34]. Impaired glucose metabolism, without overt IR, has also been associated with AHR, independent of BMI [35]. Direct evidence of the adverse effects of insulin on lung function was supported by greater than 15% decrease in FEV1 in 1.3% of patients with type 1 diabetes and 5% of patients with type 2 diabetes following administration of inhaled insulin as therapy for diabetes [36].Resolution of FEV1 decline within 6 weeks of discontinuation of inhaled insulin, even after up to 2 years of therapy, supports reversibility of the effect of insulin on lung function.
Insulin signaling is tissue-specific. Kleemann et al. [37] demonstrated that liver and white adipose tissue became insulin resistant after 6 and 12 weeks, respectively, of high-fat diet (HFD) ingestion in murine models, whereas skeletal muscle remained sensitive to insulin even at 12 weeks. Since obesity-induced insulin resistance is associated with compensatory hyperinsulinemia, we speculate that, like muscle, the ASM retains insulin sensitivity and therefore has an augmented response to insulin exposure. Yet, the direct impact of insulin resistance and hyperinsulinemia on lung tissue is poorly understood.
Proskocil et al. reported that a 16-hour exposure to insulin in both obese-prone and obese-resistant murine models potentiated ASM contraction by vagal stimulation, which was blocked by atropine, supporting a role of muscarinic receptors [38]. A 30-min exposure of rat and human tracheal smooth muscle to insulin induced contraction in response to methacholine, suggesting that even short-term exposure to insulin primes ASM for a stronger agonist-induced contraction. Nie et al. [39] validated these findings and further demonstrated that AHR to vagal stimulation was increased in obese-prone animals on HFD as compared to those fed low-fat diet or obese-resistant rats; these effects were independent of body weight and body fat. Suppressing insulin significantly reduced vagally induced bronchoconstriction in rats on HFD, suggesting that hyperinsulinemia, rather than obesity alone, underlie obesity-induced bronchoconstriction via parasympathetic nerves. Similar findings have been reported with bovine ASM [40, 41]. However, a 24-hour insulin treatment of human ASM (HASM) from obese individuals was not associated with increased calcium release in response to carbachol [42]. While differences in duration and dose of insulin exposure may potentially explain these differences, these incongruent results identify a need for further investigation, specifically on HASM. Furthermore, since AHR is a balance between muscarinic and adrenergic receptor activation, Xu et al. investigated insulin-mediated effects on β2 adrenergic receptors (β2AR) and found a novel role for transactivation of a G protein-coupled receptor kinase 2 (GRK2)-dependent β2AR-Gi-ERK1/2 cascade in ASM cells that was associated with reduced cAMP accumulation with impaired ASM relaxation [43]. Together, these studies demonstrate that effects of insulin on AHR are mediated by dysfunction of both M2 muscarinic receptor and β2AR [Figure 1].
Figure 1. Effects of insulin on ASM.
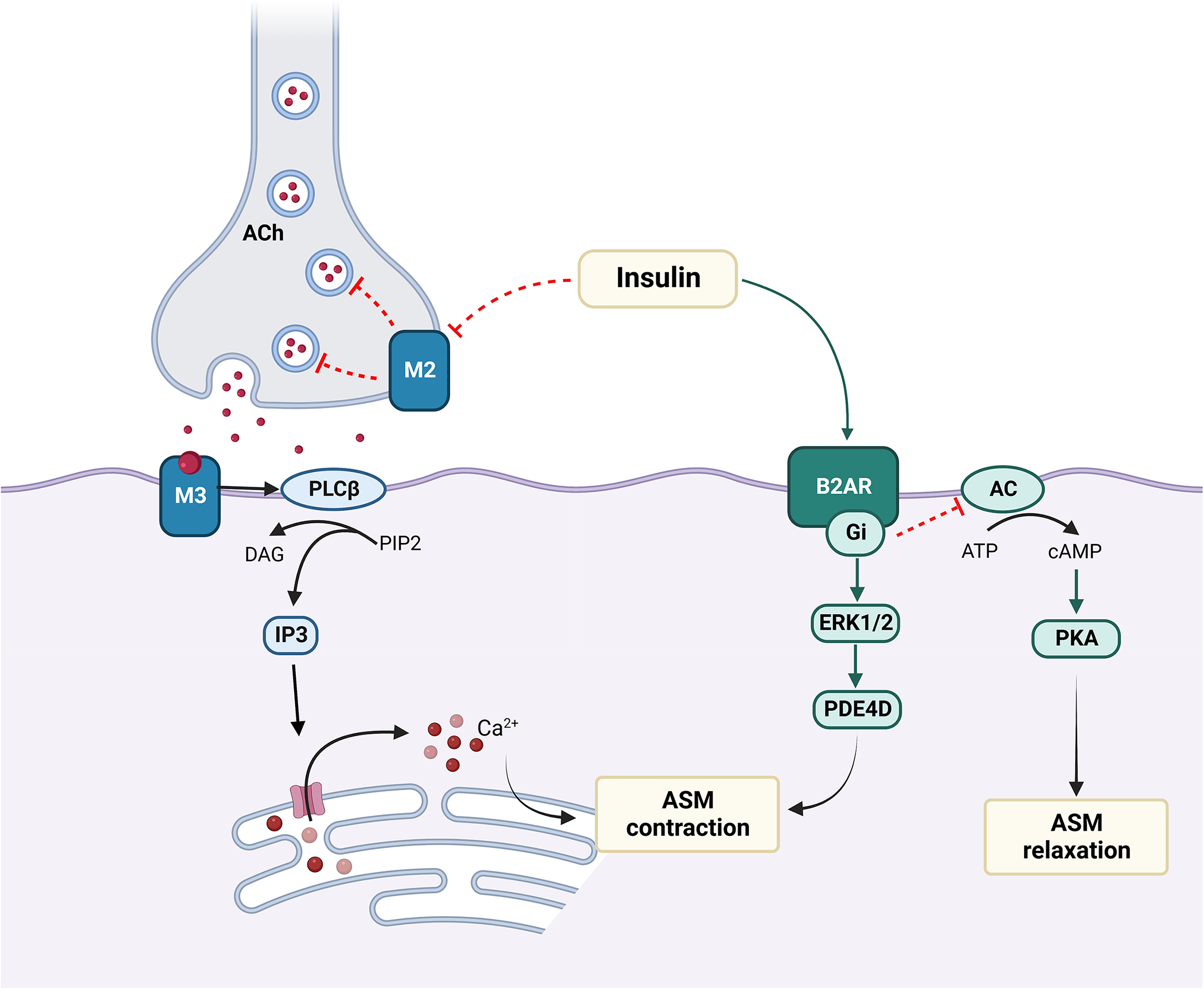
Insulin-mediated dysfunction of autoinhibitory neuronal M2 muscarinic receptor results in vagally induced acetylcholine (Ach) release at neuromuscular junction, which then interacts with M3 muscarinic receptor activating pro-contractile responses in the ASM with IP3 activation and calcium mobilization. Insulin also causes activation of β2AR-Gi-ERK/PDE4D pathway, which induces ASM contraction, while inhibition of β2AR-Gi signaling decreases cAMP accumulation and impairs ASM relaxation.
abbreviations. Ach: Acetyl choline; β2AR: Beta 2 adrenergic receptor; M2: M2 muscarinic receptor; PIP2: phosphatidylinositol (4,5) bisphosphate; PLCβ: Phospholipase C β; DAG: Diacylglycerol; IP3: Inositol trisphosphate; ASM: airway smooth muscle; ERK: extracellular signal-regulated kinase; PDE4D: Phosphodiesterase 4D; AC: adenylyl cyclase; PKA: protein kinase alpha; ATP: adenosine triphosphate; cAMP: cyclic adenosine monophosphate
In addition to AHR, hyperinsulinemia has been associated with increased α-smooth muscle actin and peri-bronchial collagen deposition, with increased levels of β-catenin in lung tissue, suggesting proliferation of ASM cells; the latter was reduced in PI3K-inhibitor treated mice [44]. These observations were validated in HASM cells where treatment with increasing concentrations of insulin for 72 hours was associated with PI3/Akt pathway activation causing an eightfold increase in cell numbers, and deposition of collagen, which is involved in airway remodeling [44] supporting potentially irreversible pro-constrictive and profibrotic effects of prolonged insulin exposure on ASM [Figure 1].
Therapies for insulin resistance and their effect on obesity-related asthma
In keeping with these effects of insulin on ASM, treatment with anti-diabetic medications, including metformin, a biguanide, and glucagon-like peptide-1 receptor (GLP-1R) agonists, are associated with decreased asthma disease burden [45, 46]. However, pioglitazone, a thiazolidinedione, also used as an anti-diabetic medication, does not decrease asthma burden [47]. These discrepant results may be due to differences in mechanisms of action of these anti-diabetic medications. Metformin decreases airway inflammation as well as airway remodeling by modulating 5′-adenosine monophosphate-activated protein kinase α (AMPK-α) activity [48, 49]. In addition, murine studies have reported altered activation of molecules such as insulin receptor substrate (IRS-1) and AKT downstream of insulin through altered glycation and nitration, which is due to enhanced oxidative stress [50]. Based on this, glycation modulation by GLP-1R agonists [51] and decrease in oxidative stress by metformin [52], may explain their association with improved asthma control in individuals with obesity and asthma [53]. Although metformin is a standard therapy for prediabetes and GLP-1R agonist use is increasing in children [54, 55], their effectiveness in childhood obesity-related asthma is not known. Given their effectiveness in decreasing asthma burden in adults and known safety profile in children, we propose that these medication classes may serve as potential novel therapy for childhood obesity-related asthma.
ii). Dyslipidemia and dysregulation of fatty acids
Free fatty acids (FFA), classified by the length of their carbon chains as small-chain, medium-chain or long-chain, are known to influence the pathogenesis of metabolic diseases including obesity, type II diabetes, and atherosclerosis. Medium-chain FFA (MCFAs) and long-chain FFA (LCFAs) are derived from de novo synthesis or through dietary intake [56], while small-chain FFA (SCFAs) are synthesized by gut microbiota through fermentation of undigested carbohydrates in the cecum and colon [57]. LCFAs are chronically elevated in obese individuals due to increased amount of adipose tissue [56]. Several G-protein-coupled receptors (GPCRs) function as specific receptors for FFAs (FFARs) such that LCFAs and MCFAs act as ligands for FFAR1 (GPR40) and FFAR4 (GPR120), whereas SCFAs act as ligands for FFAR2 (GPR43) and FFAR3 (GP141). FFARs expression have been identified on airway structural cells including ASM cells. Therefore, there is a need to define the contribution of FFA and FFARs in the pathogenesis of obesity-related asthma.
FFAR1
FFAR1, is expressed in HASM and bronchial epithelial cells [58], as well as pancreatic β-cells, taste buds, intestine, bone marrow and central nervous system [57], is activated by either LCFAs or MCFAs. FFAR1 activation induces activation of phospholipase C (PLC) and extracellular signal-regulated kinases (ERK) signaling cascade, resulting in increased intracellular calcium [Figure 2].
Figure. 2. Effects of FFAs on ASM.
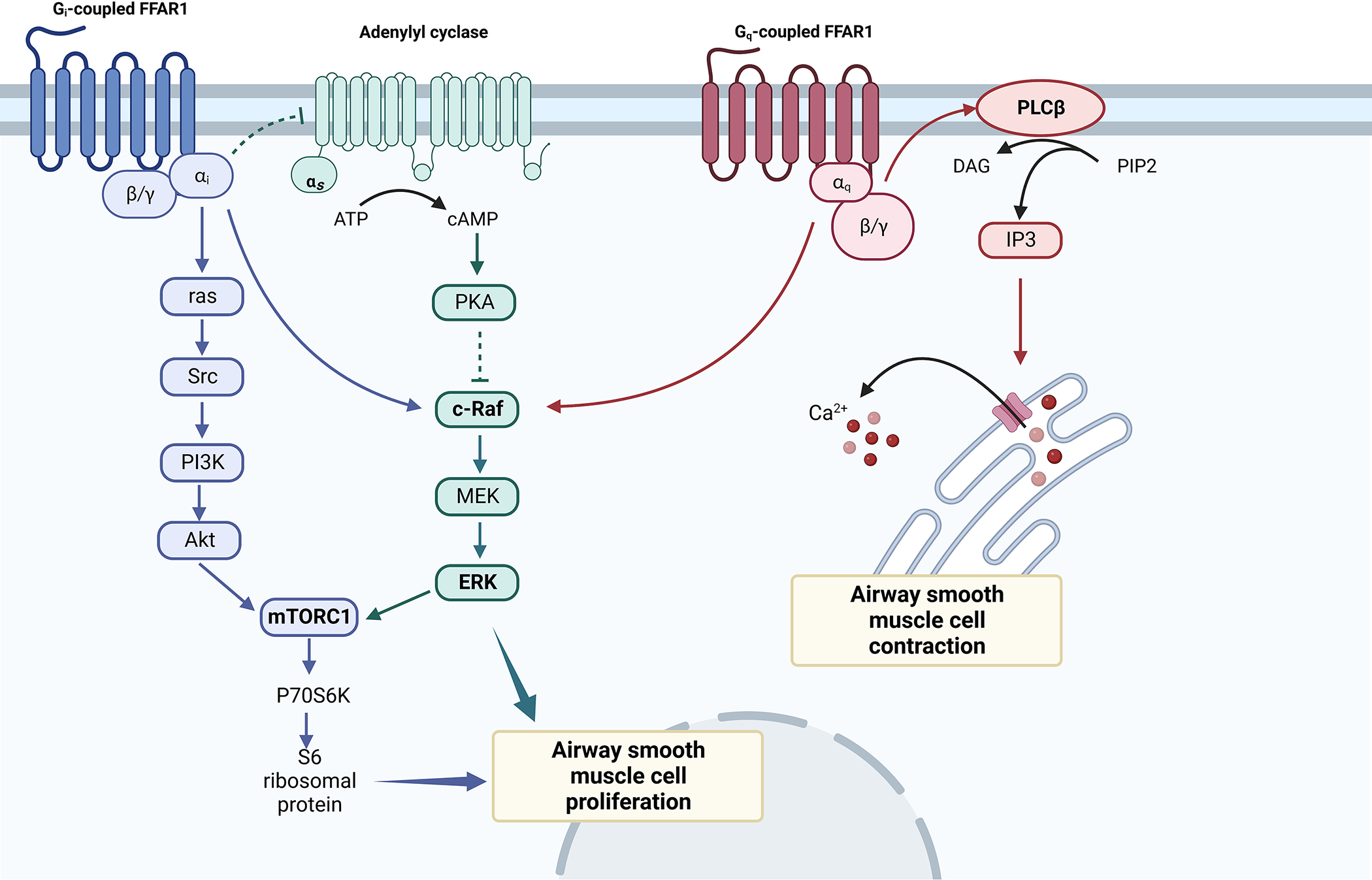
FFAR1 coupling with Gβγ activates PLC/IP3/Ca2+ pathway-mediated ASM contraction (red arrows), while its coupling with Gi receptors decreases cAMP/PKA activity via adenylyl cyclase (AC) inhibition, causing c-raf inhibition. Both Gαi and Gαq-coupled FFAR1 activate c-raf/MEK/ERK signaling pathway, which induces HASM cell proliferation independently or through mTORC1. Gi-coupled FFAR1 activates PI3K/Akt pathways with downstream activation of mTORC1, inducing p70S6K phosphorylation and leading to HASM cell proliferation.
abbreviations. ATP: adenosine triphosphate; cAMP: cyclic adenosine monophosphate; PKA: protein kinase alpha; MEK: Mitogen-activated protein kinase kinase; ERK: extracellular signal-regulated kinase; mTORC1: mammalian target of rapamycin complex 1; P70S6K Ribosomal protein S6 kinase beta-1, PI3K: Phosphatidylinositol-3-kinase; PIP2: phosphatidylinositol (4,5) bisphosphate; PLCβ: Phospholipase C β; DAG: Diacylglycerol; IP3: Inositol trisphosphate;
Mizuta et al. [58] investigated the FFAR1-mediated signaling pathways in HASM cells and foun that FFAR1 activation by LCFAs or GW9508 (FFAR1/ FFAR4 synthetic dual agonist) caused a rapid and transient rise of intracellular calcium in HASM cells in a dose-dependent manner, which was suppressed in FFAR1-knockdown HASM cells compared to control. LCFAs and GW9508 induced a sustained increase of acetylcholine-induced contractile tone. While LCFAs additionally attenuated the relaxant effect of isoproterenol, a β2AR agonist, GW9508 did not exert any effect of isoproterenol-induced relaxation. In addition to increased intracellular calcium, LCFAs and GW9508 induced actin reorganization, which is required for smooth muscle contraction. Collectively, the results suggest that LCFAs activate FFAR1 to induce calcium mobilization in HASM cells via the Gq-PLC/IP3 pathway. Investigating the effects of FFRA1 activation on ASM proliferation in human and murine models, Matoba et al. [59] additionally reported that LCFA and GW9508 exposure for 48 hours was associated with ERK and Akt phosphorylation in HASM cells, with downstream mTORC1 activation, which induced ASM proliferation. This effect of FFAR1 activation induced ASM proliferation was inhibited by pretreatment with Gi protein inhibitor (PTX), which abolished both ERK and Akt phosphorylation. Contrasting results were reported by Xu et al. [60], where pre-treatment with TAK875 (another selective FFAR1 agonist) attenuated histamine-induced myosin light chain (MLC) phosphorylation and carbachol-induced MLC phosphorylation, and inhibited ASM shortening in β2AR-desensitized HASM cells, independent of cAMP levels and PI3/Akt activation. In light of the limited literature, these studies suggest that the differences may stem from differences between donors as well as differences in the FFAR1 agonists and more studies are needed to verify the role of SCFAs in obesity-related asthma [Figure 2].
FFAR2, FFAR3, and FFAR4
Less is known about the effects of FFAR2, 3 and 4. Both FFAR2 and FFAR3 are activated by SCFAs such as acetate, propionate, and butyrate. FFAR2 couples to either Gq or Gi proteins while FFAR3 exclusively uses Gi protein signaling [56]. Although FFAR2 and FFAR3 expression has been identified on sinoepithelial cells [61], pulmonary fibroblasts [62], and HASM [63], there is limited data regarding the role of SCFAs and these receptors in airway disease. In murine models, SCFAs have demonstrated a protective effect in allergic airway inflammation mediated by FFAR3 activation [64], and FFAR2-knockout mice showed exacerbated or unresolving inflammation in models of asthma [65].
Mizuta et al [66] studied the effects of FFAR2 and 3 in HASM tone modulation. While there is no expression of FFAR2 in human ASM cells, a 15-min treatment with SCFAs induced FFAR3 activation which provoked transient intracellular calcium increases through Giβγ-PLC-IP3 pathway, but inhibited cAMP production mediated by Gi protein-coupled FFAR3. In addition, SCFAs enhanced acetylcholine-stimulated calcium mobilization and actin reorganization in HASM cells via FFAR3 stimulation.
FFAR4 are expressed in taste buds, immune cells, intestine and adipocytes and exert many physiological functions including energy regulation and immunological homeostasis [57]. mRNA encoding for FFAR4 has been identified in HASM cells [58], however there is limited data regarding its role in ASM regulation. Previously described research by Mizuta [58] demonstrated that FFAR4-knockdown HASM cells was associated with a smaller suppression of calcium mobilization induced by LCFAs compared to FFAR1-knockdown HASM cells. In addition, selective FFAR4 agonist TUG-891 failed to induce stress fiber formation or potentiate acetylcholine-induced ASM contraction [56], further supporting the limited role of FFAR4 in ASM tone modulation or ASM cell proliferation. Gq-coupled FFAR4 activation by its ligands (LCFAs and MCFAs) results in elevation of intracellular calcium and Gq-coupled FFAR4 downstream signaling is purported to involve ERK and IP3 pathways, yet the precise cascade is still under investigation [57].
Therapies for dyslipidemia/FFAs and their effect on obesity-related asthma
The role of SCFAs in causing AHR by modulation of ASM cells, and the effect of statins in lowering FFAs [67], support epidemiological reports of the beneficial effect of statins in the context of asthma [68]. A meta-analysis of RCTs demonstrated that statins improve asthma control test (ACT) scores and were independently associated with reduction of asthma-related ED visits and hospitalizations compared to control patients [68]. However, the meta-analysis did not find an association of statin use with pre- and post-bronchodilator FEV1, and peak expiratory flow (PEF), suggesting that the effect of statins on FFAs may not directly translate into decrease in AHR.
Given the dearth of mechanistic studies of how lipid lowering agents influence asthma disease burden and the better understanding of differences between anti-diabetic agents in their effectiveness in decreasing asthma burden, we speculate that similar differences may exist for lipid lowering agents and suggest that an improved mechanistic understanding of the effect of lipid lowering medications will inform their choice for management of obesity-related asthma. The recent discovery of anti-inflammatory and immunomodulatory properties of statins beyond its cholesterol-lowering function, has resulted in a novel and innovative research avenue relevant to lung diseases. Statins act as 3-hydroxymethyl-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, which block cholesterol biosynthesis pathway in the mevalonate cascade [69]. Depletion of mevalonate ultimately modifies GTPases including RhoA, which plays a role in ASM contractility as well as proliferation [70]. Experimental mouse models have demonstrated that simvastatin attenuates eosinophilic airway inflammation via inhibition of HMG-CoA, however AHR and lung compliance variations were mevalonate- and HMG-CoA-independent [71], suggesting that statins may not directly influence AHR, but may influence it via their immune modulation. Additional mechanistic studies that explain how lipid lowering agents influence asthma disease burden are needed before they can be considered for therapy for childhood obesity-related asthma.
In addition to statins and lipid lowering agents, anti-ERK and Akt therapies have been developed for diseases such as cancer [72]. Once there is verification of the role of these signaling pathways downstream of FFARs, these therapies can also be considered for repurposing for obesity-related asthma.
c). Role of Adipocytokines: Leptin and adiponectin
Adipose tissue is an active endocrine organ, producing hormones such as adiponectin, leptin and resistin [73]. Obesity is associated with higher serum concentrations of leptin and resistin with lower concentrations of adiponectin [73]. Several studies have explored the association of leptin and adiponectin with childhood obesity-related asthma. In a meta-analysis, higher levels of leptin and lower levels of adiponectin were associated with asthma diagnosis in adult and pediatric populations [74]. Recent evidence suggests that adiponectin and leptin interact with the ASM, likely via the Adiponectin and leptin receptors are expressed in airways in animal models and human airways [75]. However, there is insufficient and conflicting data about the mechanistic pathways linking adipocytokines to AHR.
i). Adiponectin
Adiponectin, the most abundant and adipocyte-specific cytokine, manifests anti-inflammatory and antiproliferative properties [76], and levels are inversely associated with obesity and its related complications. Adiponectin function is mediated by several binding proteins including AdipoR1, AdipoR2, T-cadherin, and calreticulin [73]. Expression of both AdipoR1 and AdipoR2 have been identified in human ASM cells [77]. There is a poor correlation between plasma and airway concentrations of adiponectin, and T-cadherin may play an important role in transit of adiponectin from blood to lungs [73].
In murine models of ovalbumin-induced allergic airway inflammation and AHR, Shore et al. [78] found the ovalbumin challenge decreased serum adiponectin levels and adipose tissue adiponectin mRNA expression, and exogenous continuous infusion of adiponectin resulted in marked reduction in methacholine-induced AHR and suppression of Th2-cytokine levels. Consistent with these observations, Medoff et al. [79] reported that adiponectin deficiency amplified allergic airway inflammation in a murine model of chronic asthma. These observations suggest that adiponectin and airway allergic responses are inversely associated.
Despite the evidence of anti-inflammatory effects of adiponectin in allergic airway disease, few studies examined the specific influence of adiponectin on ASM cells, independent of immune pathways. To elucidate the hormone-to-cell interaction, Shi et al. [77] found that adiponectin did not suppress PDGF-enhanced ASM cell proliferation and did not influence VEGF release and MCP-1 secretion. While these studies suggest an effect of adiponectin on the airway immune milieu, additional research is needed on its effect on the ASM function and the interplay between airway immune responses and ASM contractility.
Therapies for adiponectin and their effect on obesity-related asthma
While adiponectin is not available as a therapeutic intervention, several medications, including statins, angiotensin converting enzyme inhibitors, and anti-hyperglycemic agents, including thiazolidinediones (TZDs), such as rosiglitazone and pioglitazone, which function as PPARγ agonists, increase circulating levels of adiponectin [80]. Furthermore, L-cysteine and manganese supplementation increase adiponectin secretion in adipocytes [81], suggesting that dietary modification may influence adiponectin levels. None of these have been specifically investigated but remain options for clinical trials for obese individuals with asthma.
ii). Leptin
Leptin, an adipocytokine primarily functions as a satiety hormone [82] but can also function as a pro-inflammatory protein within the setting of obesity [83, 84], when it contributes to adipose tissue and systemic inflammation. Investigation of the mechanisms by which early-onset obesity and leptin dysregulation evoke asthma-like disease in adult mice had identified that HFD is associated with increase in airway resistance and AHR with leptin/IL-6 activation, which causes STAT3 signaling with increase in inflammation and cell proliferation, which is inhibited by SOSC3 [85]. Since bronchial epithelial cells and ASM cells express the leptin receptor, investigation of the direct effect of leptin on HASM cells from non-asthmatic donors revealed a concentration-dependent inhibition of smooth muscle proliferation, migration towards platelet-derived growth factor (PDGF) and IL-13-induced eotaxin production [86]. These effects were partially abolished by indomethacin, suggesting that the inhibitory effects of leptin are partially mediated by PGE2 secretion.
Arteaga-Solis et al. [87] who investigated the neural effects of leptin on bronchial diameter regulation and its possible mechanisms, found a substantial increase in baseline resistance and AHR in obese leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mouse models as compared to wild type (WT) mice. As validation, a mouse model with a partial gain-of-function mutation in the leptin receptor displayed a decrease of baseline resistance and AHR compared to WT mice. These results suggest that leptin signaling regulates airway diameter independently to its consequences on appetite or energy metabolism and leptin resistance contributes to narrowing of airway lumen. Intriguingly, Arteaga-Solis et al. [87] showed airway inflammation in their obese mouse models was uncoupled from leptin resistance and suggested that lack of leptin signaling decreased airway diameter via neural mechanisms without triggering inflammation.
To identify the effects of leptin in allergen-induced airway inflammation and AHR in murine models, Shore et al. delivered an exogenous continuous infusion of leptin and found that ovalbumin challenged mice demonstrated a further increase in leptin levels and had augmented response to methacholine challenge [88]. These observations suggest a positive feedback loop between leptin and airway allergic responses and differ from those of Arteaga-Solis et al. who demonstrated no local effect of leptin on regulation of bronchial diameter, and those by Nair et al., who found no effect of leptin on contractile or relaxation responses of bovine tracheal rings [86, 87]. Given lack of local influence of leptin in an organ bath model but constriction in response to continuous infusion, these findings suggest that leptin may indeed act via neural mechanisms to regulate airway diameter. In support of this hypothesis, Arteaga-Solis et al. found that murine models with leptin receptor knockdown neurons had a similar increase in baseline resistance and AHR as obese leptin-deficient (ob/ob) mice. Furthermore, leptin-induced bronchodilation was found to be mediated by inhibition of parasympathetic signaling that acts upon M3 muscarinic receptor in ASM cells [87] since leptin-deficient mice treated with anticholinergic agents, in particular M3R-specific antagonists, had normal baseline resistance and AHR comparable to WT mice [Figure 3].
Figure 3. Effects of leptin on ASM.
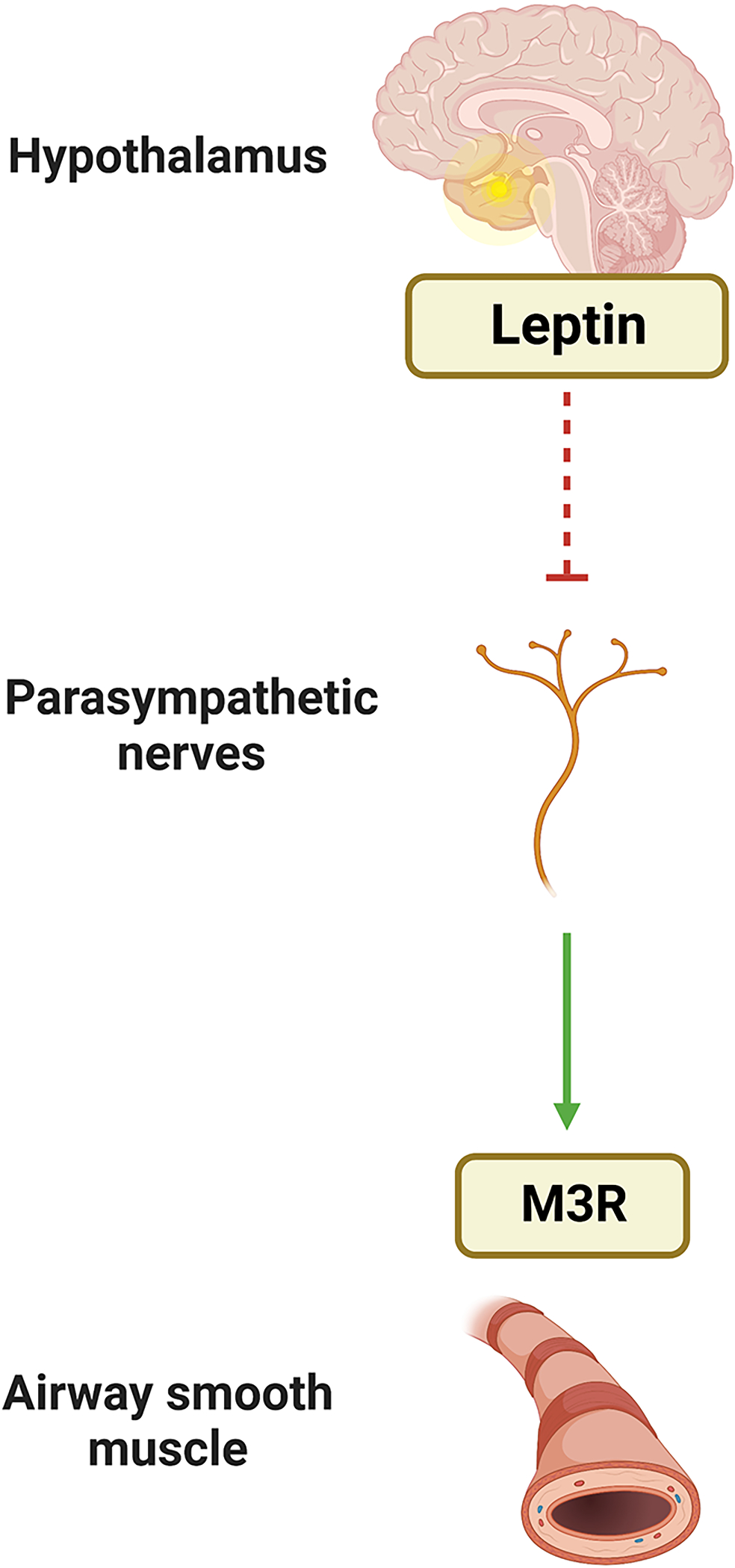
Leptin effects on the airway are mediated by central receptors which inhibit downstream parasympathetic signaling that acts upon M3 muscarinic receptor in ASM cells.
abbreviation: M3R: M3 muscarinic receptor
Therapies for leptin and their effect on obesity-related asthma
Similar to adiponectin, there are few direct therapies for leptin resistance. However, medications such as metformin and GLP1-R agonists decrease leptin resistance [89]. From the perspective of airway therapy, tiotropium, an anticholinergic agent used for asthma management, normalized baseline resistance and AHR in ovalbumin-sensitized leptin-deficient obese mice. These observations suggest that anticholinergic agents may be therapeutic options for bronchoconstriction caused by both leptin-deficient signaling and allergen-induced lung disease and tiotropium has been found to be effective for obesity-related asthma [90, 91].
d). The role of immune dysregulation: T cells and monocytes
Given that inflammation is a common mechanism for asthma and obesity, it has been one of the major areas for mechanistic studies on links between asthma and obesity. The investigations have focused on monocytes and T cells as the two main cell types involved in systemic pro-inflammatory profiles driven by obesity [Figure 4].
Figure 4. Obesity-mediated immune perturbations and their effect on ASM.
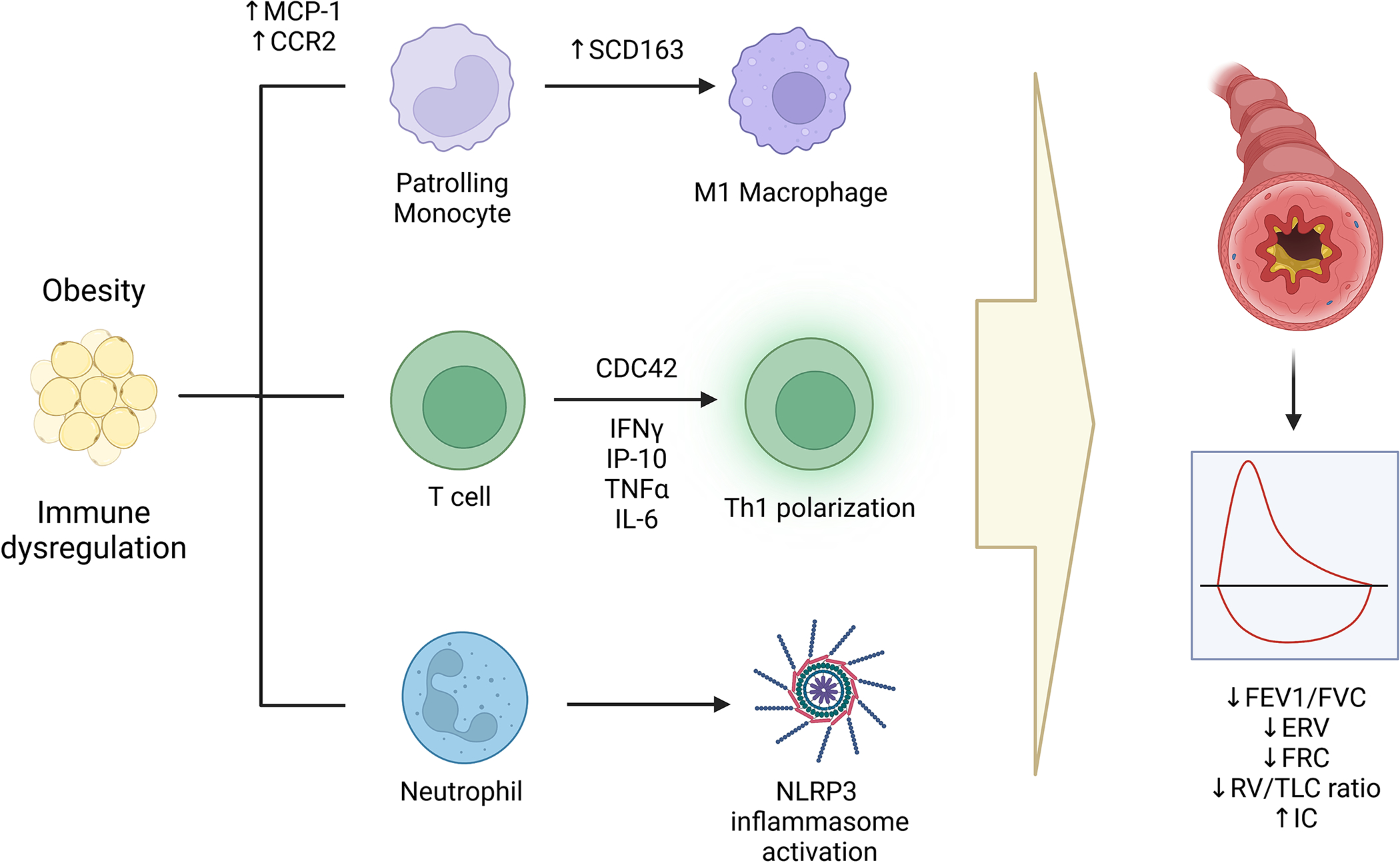
Obesity is associated with activation of monocytes, T helper cells, preferentially of T helper 1 subset and neutrophils. Each of these cell subtypes have been associated with pulmonary function deficits found in obesity-related asthma.
abbreviations. MCP1: Monocyte chemotactic protein 1; CCR2: C-C chemokine receptor type 2; SCD163: soluble cellular differentiation 163; CDC42: cell division cycle 42; IFNγ: interferon gamma; TNFα: tumor necrosis factor alpha; IL-6: Interleukin-6; NLRP3: NOD-, LRR- and pyrin domain-containing protein 3; FVC: Forced vital capacity; FEV1: Forced expiratory volume in 1st second; ERV: Expiratory reserve volume; FRC: Functional residual capacity; TLC: Total lung capacity; RV: Residual volume, IC: Inspiratory capacity.
Blood monocytes
Circulating monocytes, classified as classical or resting, and resident, and patrolling (most activated) [92], play a key role in obesity-mediated low-grade systemic inflammation, which is sustained by leptin [93]. Obese adipocytes release monocyte chemotactic protein (MCP-1) in response to which patrolling monocytes are recruited to adipose tissue where they differentiate into M1 macrophages [92, 94, 95]. Obese Hispanic and African American adolescents with asthma had evidence of systemic monocyte activation with fewer classical monocytes and more patrolling monocytes as compared to healthy-weight controls [96]. Circulating sCD163, a marker of macrophage activation, was higher in obese Caucasian girls with asthma, particularly in those with an android pattern of fat distribution, and correlated with lower lung function and asthma quality of life [97]. Reduced serum levels of C-C chemokine receptor type 2 (CCR2), which typically decreases with differentiation of classical to patrolling monocytes [98], directly correlated with lung volumes, supporting an direct association between monocyte activation and lung function deficits unique to pediatric obesity-related asthma [96]. These studies suggest that systemic monocyte activation plays a role in the obese asthma phenotype.
Given that monocytes differentiate into macrophages in adipose tissue, murine studies have reported increased proportion and activation of pro-inflammatory airway macrophages in obese mice and those fed HFD [99, 100], which when depleted, were associated with decreased AHR [101]. While there are no pediatric studies on airway macrophages, higher proportion of airway macrophages, poorly responsive to glucocorticoids, have been observed in some [102], but not all studies [100, 103] in obese adults with asthma.
T helper (Th) cell responses
Obese children with asthma have evidence of systemic Th1 polarization [104] that directly correlated with serum leptin and IL-6 levels [96, 105], and patrolling monocytes [96], and inversely correlated with classical monocytes. Circulating markers of Th1 polarization, interferon gamma (IFNγ) and interferon-gamma induced protein (IP-10) inversely correlated with FEV1/FVC ratio [105]. These findings suggest that obesity is driving Th1 systemic inflammation in obese children, which is more robust in those with asthma as compared to obese without asthma [96]. However, there is lack of data on Th cell patterns in the airway among obese children as compared to healthy-weight children with asthma. RNA Seq analysis of Th cells from obese children with asthma [106], has revealed upregulation of several genes in the CDC42 pathway, among which an inverse correlation of transcripts of two genes, CDC42EP4 and DOCK5, with FEV1/FVC ratio, uniquely among obese asthmatic children [107]. CDC42 is a RhoGTPase that plays a key role in several aspects of Th cell physiology, including Th cell differentiation, preferentially to Th1 cells [108], and in cytokine production and exocytosis [109]. These findings support a novel role of CDC42 pathway in the Th1 responses in obesity-related asthma and the CDC42 pathway could serve as a novel therapeutic target.
Role of neutrophils
Th1 polarization is associated with neutrophilic inflammation that is linked to severe asthma [110]. While higher systemic and airway neutrophil counts have been observed in obese adults with asthma who had lower FEV1 relative to healthy-weight individuals [111–113], obese children with asthma had evidence of blood [114] but not of airway neutrophilia [115]. While the biologic mechanisms through which neutrophils contribute to the obese asthma phenotype are not known, a role for NLRP3 inflammasome has been reported in obese asthma [116]. A pattern recognition receptor, NLRP3 has been associated with neutrophilic airway inflammation in severe asthma [117], and found to be increased in the airways of obese individuals with asthma, particularly in response to diet rich in saturated fatty acids [118] [Figure 4].
Therapies for immune dysregulation and their effect on obesity-related asthma
Since IL-6 among other cytokines have been implicated in modulating these immune profiles, IL-6 has provided a focus for potential therapy in both asthma and obesity. In the Severe Asthma Research Program-3 (SARP3) adult cohort, IL-6 was a biomarker of exacerbation-prone asthma, associated with more frequent exacerbations and low lung function [119]. Subjects with high IL-6 also had higher BMI and more frequent comorbid conditions including hypertension and diabetes. These findings were replicated in the SARP-3 pediatric cohort and Asthma Phenotypes in the Inner City (APIC) study where high IL-6 was associated with higher BMI, as well as a higher propensity for exacerbations and pulmonary function impairment [120, 121]. Investigation using airway epithelial samples from the Unbiased Biomarkers in Prediction of Respiratory Disease (U-BIOPRED) cohort found that the subset of participants with high IL-6 epithelial signaling were frequent exacerbators and had blood eosinophilia and submucosal T cell and macrophage infiltration [122]. Experimental studies have shown that IL-1 and TGFβ-stimulated HASM cells produce IL-6 [123], and IL-6 exposure in guinea pig ASM cells promotes ASM hyperplasia and hypertrophy [124] with upregulation of expression of genes involved in airway remodeling, cell proliferation, and immune response in a dose-dependent fashion in HASM cells [125]. These findings, in conjunction with the observation of higher IL-6 levels among those of African American ancestry [126], and in light of existing and emerging anti-IL6 therapies, including tocilizumab, IL-6 is an attractive therapeutic target for obesity-related asthma. A recent case series reported a beneficial effect of tocilizumab in two cases of childhood severe persistent asthma [127], suggesting that it is prime time to investigate its role in ameliorating the disease burden due to childhood obesity-related asthma.
Patrolling monocyte activation and Th1 polarization are also associated with elevated TNF levels. TNF receptor is highly expressed in asthmatic airways [128] which induces CD38, a cell-surface protein expressed in human ASM [129], and its associated cyclic ADP ribose (cADPR) production in ASM to provoke agonist-mediated calcium elevation and ASM contraction. Higher levels of CD38 expression are found in asthmatic ASM compared to healthy ASM cells [130]. Post-transcriptional regulation of TNF-related CD38 expression is mediated by microRNA such as miRNA-708 and miRNA-140–3p via enhancing MAPK and IP3/AKT pathways which modulate ASM contraction and proliferation [131, 132]. On the contrary, miRNA-15b-5p attenuated TNFα-induced ASM cell growth, migration, and ECM deposition by downregulating YAP1 expression [133] while phosphatase and tensin homologue deleted on chromosome ten (PTEN) suppresses TNFα-induced ASM cell proliferation by downregulating CD38/Cyclic AMP response-element binding protein (CREB) axis [134]. Anti-TNF therapy has been investigated for asthma but was found to be too toxic [135, 136]. However, in light of the more routine use of etanercept, anti-TNF receptor therapy in pediatric rheumatologic diseases [133], we speculate that further investigation of the mechanisms by which TNF contributes to obesity-related asthma will directly support its use, specifically for non-allergic pediatric obesity-related asthma.
Atopy and obesity-related asthma
While we have discussed immune therapy in the context of non-allergic obesity-related asthma, it is important to highlight that obesity and atopy are not mutually exclusive in children with asthma. Obesity has been linked with atopic asthma in females [137], and leptin, an adipokine increased in the obese, has been associated with atopic asthma in males [138]. Obesity has also been linked to increased eosinophil activation and chemotaxis in children with asthma [139] and with increased submucosal eosinophilia in obese adults with severe asthma [140]. While baseline eosinophil counts were similar in a clinical trial involving adults on the comparative efficacy of dietary restriction versus exercise-based intervention, the latter was associated with a decrease in sputum eosinophils while the former was associated with reduction in airway neutrophils [25]. In keeping with these differing studies, there are conflicting reports on patterns of fractional exhaled nitric oxide (FeNO), a marker of allergic airway inflammation, in obese children with asthma. While one study found no association of FeNO with asthma in obese children [141], another reported an association of adiposity measures with asthma severity in children with low FeNO but higher asthma severity and poorer disease control among children with high FeNO [142]. These findings suggest that obesity also influences asthma among individuals with allergic sensitization. Moreover, these disparate results support the hypothesis that there are multiple phenotypes of obesity-related asthma, of which two distinct ones are apparent, one where obesity worsens atopic asthma [143], and one where obesity is a risk factor for non-atopic asthma [144].
REVERSE ASSOCIATION BETWEEN OBESITY AND ASTHMA
The discussion above has focused on obesity as the risk factor for asthma. However, we would like to also emphasize that the interrelationship between obesity and asthma is complex and poorly understood. The prenatal origins of both conditions complicate the search for temporality [145], which creates a chicken-or-egg situation. A reverse obesity-asthma association, which refers to asthma as a risk factor for later obesity, is also a contributor to the burden of obesity-related asthma.
In a longitudinal cohort study of kindergarten and first-grade children from Southern California, non-obese children with early-life diagnosis of asthma or wheezing were at higher risk of developing obesity over a 10-year follow-up as compared to children without history of asthma or wheezing [146]. Similar findings were reported in European cohort of children ages 3 to 4-years [147]. These observations are not limited to children and have been found in adults as well, where, participants with active asthma, particularly those with non-atopic disease, longer disease duration or those on oral corticosteroids, were at higher risk of developing obesity as compared to individuals without asthma [148].
A potential hypothesis for the obese-asthma reverse association may be reduced physical activity due to respiratory restrictions due to poor control of asthma. However, no evidence of physical activity levels as mediator in the asthma-obesity association was observed in children and adult studies [146, 148]. Another potential explanation for the obese-asthma reverse association is steroid-induced weight gain [149] mediated by increased lipid uptake and insulin resistance. This hypothesis was validated in an adult cohort which showed higher risk of obesity among individuals with asthma using oral corticosteroids [148]. In contrast, a pediatric study found no association of controller and steroid medication use with obesity, while albuterol use reduced the risk of incident obesity, suggesting a direct benefit of beta agonist therapies on the adipocytes mediated by increasing energy expenditure and enhancing lipolysis [146]. Together, these findings, in conjunction with the causative association between obesity and asthma emphasize the diverse ways in which obesity and asthma may coexist.
Conclusions
Irrespective of the directionality of the association between asthma and obesity, it is evident that obesity-related asthma is associated with high disease burden and is poorly responsive to existent asthma therapies, supporting it to be a distinct asthma phenotype. While early management of obesity or weight gain, and better management of childhood asthma with routine incorporation of physical activity may decrease the burden of disease, further clinical and experimental research is needed to understand the relationship between the two conditions.
In this review, we have summarized the known mechanisms by which obesity-related complications including the burden of mechanical fat load, metabolic abnormalities, adipokine perturbations, and immune dysregulation contribute to obesity-related asthma, focusing on the effects of these perturbations on ASM function, where it has been investigated. There has been much progress in the use of weight management approaches and repurposing of those for metabolic abnormalities, such as insulin resistance and dyslipidemia for obesity-related asthma. Immune profiles also identify a novel area for incorporation of anti-IL6 therapy. We would like to conclude by highlighting several areas of future investigations. These are mechanistic studies that incorporate these varied obesity-related complications together, since it is evident that these complications do not occur in isolation. Airway specific investigations will directly inform development of targeted therapies for the airway. As existent medications for metabolic abnormalities and non-allergic immune responses are being considered for obesity-related asthma, their underlying mechanisms need to be better elucidated. Together, these studies are critical to identify additional therapeutic targets specifically for obesity-related asthma.
Keypoints:
Influence of obesity-mediated complications on airway smooth muscle likely underlies the burden of obesity-related asthma. Therapeutic interventions for these obesity-related complications may serve as novel therapies for obesity-related asthma.
Funding:
National Institutes of Health (NIH) Grant#HL141849
Footnotes
Competing interests: The authors declare no competing interests.
Data Availability:
Data sharing is not applicable to this article as this is a review article and no datasets were generated or analysed during the current study.
References
- 1.Chen YC, Dong GH, Lin KC, Lee YL. Gender difference of childhood overweight and obesity in predicting the risk of incident asthma: a systematic review and meta-analysis. Obes Rev. 2013. Mar;14(3):222–31. [DOI] [PubMed] [Google Scholar]
- 2.Wang E, Wechsler ME, Tran TN, Heaney LG, Jones RC, Menzies-Gow AN, et al. Characterization of Severe Asthma Worldwide: Data From the International Severe Asthma Registry. Chest. 2020. Apr;157(4):790–804. [DOI] [PubMed] [Google Scholar]
- 3.Lang JE, Bunnell HT, Hossain MJ, Wysocki T, Lima JJ, Finkel TH, et al. Being Overweight or Obese and the Development of Asthma. Pediatrics. 2018. Dec;142(6). [DOI] [PubMed] [Google Scholar]
- 4.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018. Apr;141(4):1169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmadizar F, Vijverberg SJ, Arets HG, de Boer A, Lang JE, Kattan M, et al. Childhood obesity in relation to poor asthma control and exacerbation: a meta-analysis. Eur Respir J. 2016. Oct;48(4):1063–73. [DOI] [PubMed] [Google Scholar]
- 6.Belamarich PF, Luder E, Kattan M, Mitchell H, Islam S, Lynn H, et al. Do obese inner-city children with asthma have more symptoms than nonobese children with asthma? Pediatrics. 2000. Dec;106(6):1436–41. [DOI] [PubMed] [Google Scholar]
- 7.Borrell LN, Nguyen EA, Roth LA, Oh SS, Tcheurekdjian H, Sen S, et al. Childhood obesity and asthma control in the GALA II and SAGE II studies. Am J Respir Crit Care Med. 2013. Apr 1;187(7):697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aragona E, El-Magbri E, Wang J, Scheckelhoff T, Scheckelhoff T, Hyacinthe A, et al. Impact of Obesity on Clinical Outcomes in Urban Children Hospitalized for Status Asthmaticus. Hosp Pediatr. 2016. Apr;6(4):211–8. [DOI] [PubMed] [Google Scholar]
- 9.Okubo Y, Nochioka K, Hataya H, Sakakibara H, Terakawa T, Testa M. Burden of Obesity on Pediatric Inpatients with Acute Asthma Exacerbation in the United States. J Allergy Clin Immunol Pract. 2016. Nov - Dec;4(6):1227–31. [DOI] [PubMed] [Google Scholar]
- 10.McGarry ME, Castellanos E, Thakur N, Oh SS, Eng C, Davis A, et al. Obesity and bronchodilator response in black and Hispanic children and adolescents with asthma. Chest. 2015. Jun;147(6):1591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Gent R, van der Ent CK, Rovers MM, Kimpen JL, van Essen-Zandvliet LE, de Meer G. Excessive body weight is associated with additional loss of quality of life in children with asthma. J Allergy Clin Immunol. 2007. Mar;119(3):591–6. [DOI] [PubMed] [Google Scholar]
- 12.Chapman DG, Irvin CG. Mechanisms of airway hyper-responsiveness in asthma: the past, present and yet to come. Clin Exp Allergy. 2015. Apr;45(4):706–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates JH. Physiological Mechanisms of Airway Hyperresponsiveness in Obese Asthma. Am J Respir Cell Mol Biol. 2016. May;54(5):618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauzon AM, Martin JG. Airway hyperresponsiveness; smooth muscle as the principal actor. F1000Res. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rastogi D, Holguin F. Metabolic Dysregulation, Systemic Inflammation, and Pediatric Obesity-related Asthma. Ann Am Thorac Soc. 2017. Nov;14(Supplement_5):S363–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pare PD, McParland BE, Seow CY. Structural basis for exaggerated airway narrowing. Can J Physiol Pharmacol. 2007. Jul;85(7):653–8. [DOI] [PubMed] [Google Scholar]
- 17.Bates JH, Lauzon AM. Parenchymal tethering, airway wall stiffness, and the dynamics of bronchoconstriction. J Appl Physiol (1985). 2007. May;102(5):1912–20. [DOI] [PubMed] [Google Scholar]
- 18.Desai D, Newby C, Symon FA, Haldar P, Shah S, Gupta S, et al. Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with severe asthma. Am J Respir Crit Care Med. 2013. Sep 15;188(6):657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rastogi D, Khan UI, Isasi CR, Coupey SM. Associations of obesity and asthma with functional exercise capacity in urban minority adolescents. Pediatr Pulmonol. 2012. Nov;47(11):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rastogi D, Fraser S, Oh J, Huber AM, Schulman Y, Bhagtani RH, et al. Inflammation, metabolic dysregulation, and pulmonary function among obese urban adolescents with asthma. Am J Respir Crit Care Med. 2015. Jan 15;191(2):149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beuther DA. Obesity and asthma. Clin Chest Med. 2009. Sep;30(3):479–88, viii. [DOI] [PubMed] [Google Scholar]
- 22.Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JH. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol (1985). 2004. Jun;96(6):2019–27. [DOI] [PubMed] [Google Scholar]
- 23.Rutting S, Mahadev S, Tonga KO, Bailey DL, Dame Carroll JR, Farrow CE, et al. Obesity alters the topographical distribution of ventilation and the regional response to bronchoconstriction. J Appl Physiol (1985). 2020. Jan 1;128(1):168–77. [DOI] [PubMed] [Google Scholar]
- 24.Pakhale S, Baron J, Dent R, Vandemheen K, Aaron SD. Effects of weight loss on airway responsiveness in obese adults with asthma: does weight loss lead to reversibility of asthma? Chest. 2015. Jun;147(6):1582–90. [DOI] [PubMed] [Google Scholar]
- 25.Scott HA, Gibson PG, Garg ML, Pretto JJ, Morgan PJ, Callister R, et al. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: a randomized trial. Clin Exp Allergy. 2013;43(1):36–49. [DOI] [PubMed] [Google Scholar]
- 26.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011. Sep;128(3):508–15 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zerah-Lancner F, Boyer L, Rezaiguia-Delclaux S, D’Ortho MP, Drouot X, Guilloteau-Schoennagel I, et al. Airway responsiveness measured by forced oscillation technique in severely obese patients, before and after bariatric surgery. J Asthma. 2011. Oct;48(8):818–23. [DOI] [PubMed] [Google Scholar]
- 28.Boulet LP, Turcotte H, Martin J, Poirier P. Effect of bariatric surgery on airway response and lung function in obese subjects with asthma. Respir Med. 2012. May;106(5):651–60. [DOI] [PubMed] [Google Scholar]
- 29.Chapman DG, Irvin CG, Kaminsky DA, Forgione PM, Bates JH, Dixon AE. Influence of distinct asthma phenotypes on lung function following weight loss in the obese. Respirology. 2014. Nov;19(8):1170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dias-Junior SA, Reis M, de Carvalho-Pinto RM, Stelmach R, Halpern A, Cukier A. Effects of weight loss on asthma control in obese patients with severe asthma. Eur Respir J. 2014. May;43(5):1368–77. [DOI] [PubMed] [Google Scholar]
- 31.Cardet JC, Ash S, Kusa T, Camargo CA Jr, Israel E. Insulin resistance modifies the association between obesity and current asthma in adults. Eur Respir J. 2016. Aug;48(2):403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forno E. Asthma and diabetes: Does treatment with metformin improve asthma? Respirology. 2016. Oct;21(7):1144–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forno E, Han YY, Muzumdar RH, Celedon JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol. 2015. Aug;136(2):304–11 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SH, Kim HS, Min HK, Lee SW. Association between insulin resistance and lung function trajectory over 4 years in South Korea: community-based prospective cohort. BMC Pulm Med. 2021. Apr 1;21(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karampatakis N, Karampatakis T, Galli-Tsinopoulou A, Kotanidou EP, Tsergouli K, Eboriadou-Petikopoulou M, et al. Impaired glucose metabolism and bronchial hyperresponsiveness in obese prepubertal asthmatic children. Pediatr Pulmonol. 2017. Feb;52(2):160–6. [DOI] [PubMed] [Google Scholar]
- 36.McMahon GT, Arky RA. Inhaled insulin for diabetes mellitus. N Engl J Med. 2007. Feb 1;356(5):497–502. [DOI] [PubMed] [Google Scholar]
- 37.Kleemann R, van Erk M, Verschuren L, van den Hoek AM, Koek M, Wielinga PY, et al. Time-resolved and tissue-specific systems analysis of the pathogenesis of insulin resistance. PLoS One. 2010. Jan 21;5(1):e8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Proskocil BJ, Calco GN, Nie Z. Insulin acutely increases agonist-induced airway smooth muscle contraction in humans and rats. Am J Physiol Lung Cell Mol Physiol. 2021. Apr 1;320(4):L545–L56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nie Z, Jacoby DB, Fryer AD. Hyperinsulinemia potentiates airway responsiveness to parasympathetic nerve stimulation in obese rats. Am J Respir Cell Mol Biol. 2014. Aug;51(2):251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaafsma D, McNeill KD, Stelmack GL, Gosens R, Baarsma HA, Dekkers BG, et al. Insulin increases the expression of contractile phenotypic markers in airway smooth muscle. Am J Physiol Cell Physiol. 2007;293(1):C429–39. [DOI] [PubMed] [Google Scholar]
- 41.Gosens R, Nelemans SA, Hiemstra M, Grootte Bromhaar MM, Meurs H, Zaagsma J. Insulin induces a hypercontractile airway smooth muscle phenotype. Eur J Pharmacol. 2003;481(1):125–31. [DOI] [PubMed] [Google Scholar]
- 42.Orfanos S, Jude J, Deeney BT, Cao G, Rastogi D, van Zee M, et al. Obesity increases airway smooth muscle responses to contractile agonists. Am J Physiol Lung Cell Mol Physiol. 2018. Nov 1;315(5):L673–L81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu R, Gopireddy RR, Wu Y, Wu L, Tao X, Shao J, et al. Hyperinsulinemia promotes heterologous desensitization of beta2 adrenergic receptor in airway smooth muscle in obesity. FASEB J. 2020. Mar;34(3):3996–4008. [DOI] [PubMed] [Google Scholar]
- 44.Singh S, Bodas M, Bhatraju NK, Pattnaik B, Gheware A, Parameswaran PK, et al. Hyperinsulinemia adversely affects lung structure and function. Am J Physiol Lung Cell Mol Physiol. 2016. May 1;310(9):L837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu TD, Keet CA, Fawzy A, Segal JB, Brigham EP, McCormack MC. Association of Metformin Initiation and Risk of Asthma Exacerbation. A Claims-based Cohort Study. Ann Am Thorac Soc. 2019. Dec;16(12):1527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foer D, Beeler PE, Cui J, Karlson EW, Bates DW, Cahill KN. Asthma Exacerbations in Patients with Type 2 Diabetes and Asthma on Glucagon-like Peptide-1 Receptor Agonists. Am J Respir Crit Care Med. 2021. Apr 1;203(7):831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dixon AE, Subramanian M, DeSarno M, Black K, Lane L, Holguin F. A pilot randomized controlled trial of pioglitazone for the treatment of poorly controlled asthma in obesity. Respir Res. 2015. Nov 26;16:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park CS, Bang BR, Kwon HS, Moon KA, Kim TB, Lee KY, et al. Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochem Pharmacol. 2012. Dec 15;84(12):1660–70. [DOI] [PubMed] [Google Scholar]
- 49.Ma W, Jin Q, Guo H, Han X, Xu L, Lu S, et al. Metformin Ameliorates Inflammation and Airway Remodeling of Experimental Allergic Asthma in Mice by Restoring AMPKalpha Activity. Front Pharmacol. 2022;13:780148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andre DM, Calixto MC, Sollon C, Alexandre EC, Tavares EBG, Naime ACA, et al. High-fat diet-induced obesity impairs insulin signaling in lungs of allergen-challenged mice: Improvement by resveratrol. Sci Rep. 2017. Dec 11;7(1):17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen DV, Linderholm A, Haczku A, Kenyon N. Glucagon-like peptide 1: A potential anti-inflammatory pathway in obesity-related asthma. Pharmacol Ther. 2017. Dec;180:139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren H, Shao Y, Wu C, Ma X, Lv C, Wang Q. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol Cell Endocrinol. 2020. Jan 15;500:110628. [DOI] [PubMed] [Google Scholar]
- 53.Rastogi D Evidence Builds for a Role of Metformin in Asthma Management. Ann Am Thorac Soc. 2019. Dec;16(12):1497–9. [DOI] [PubMed] [Google Scholar]
- 54.Esquivel Zuniga R, DeBoer MD. Prediabetes in Adolescents: Prevalence, Management and Diabetes Prevention Strategies. Diabetes Metab Syndr Obes. 2021;14:4609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bensignor MO, Wolf JM, Rudser KD, Kelly AS, Arslanian S. Glucagon-like peptide-1 receptor agonist prescribing patterns in adolescents with type 2 diabetes. Diabetes Obes Metab. 2022. Jul;24(7):1380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizuta K, Matoba A, Shibata S, Masaki E, Emala CW, Sr. Obesity-induced asthma: Role of free fatty acid receptors. Jpn Dent Sci Rev. 2019. Nov;55(1):103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kimura I, Ichimura A, Ohue-Kitano R, Igarashi M. Free Fatty Acid Receptors in Health and Disease. Physiol Rev. 2020. Jan 1;100(1):171–210. [DOI] [PubMed] [Google Scholar]
- 58.Mizuta K, Zhang Y, Mizuta F, Hoshijima H, Shiga T, Masaki E, et al. Novel identification of the free fatty acid receptor FFAR1 that promotes contraction in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2015. Nov 1;309(9):L970–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matoba A, Matsuyama N, Shibata S, Masaki E, Emala CW, Sr., Mizuta K. The free fatty acid receptor 1 promotes airway smooth muscle cell proliferation through MEK/ERK and PI3K/Akt signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2018. Mar 1;314(3):L333–L48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu S, Schwab A, Karmacharya N, Cao G, Woo J, Kim N, et al. FFAR1 activation attenuates histamine-induced myosin light chain phosphorylation and cortical tension development in human airway smooth muscle cells. Respir Res. 2020. Nov 30;21(1):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imoto Y, Kato A, Takabayashi T, Sakashita M, Norton JE, Suh LA, et al. Short-chain fatty acids induce tissue plasminogen activator in airway epithelial cells via GPR41&43. Clin Exp Allergy. 2018. May;48(5):544–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rutting S, Xenaki D, Lau E, Horvat J, Wood LG, Hansbro PM, et al. Dietary omega-6, but not omega-3, polyunsaturated or saturated fatty acids increase inflammation in primary lung mesenchymal cells. Am J Physiol Lung Cell Mol Physiol. 2018. Jun 1;314(6):L922–L35. [DOI] [PubMed] [Google Scholar]
- 63.Aisenberg WH, Huang J, Zhu W, Rajkumar P, Cruz R, Santhanam L, et al. Defining an olfactory receptor function in airway smooth muscle cells. Sci Rep. 2016. Dec 1;6:38231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014. Feb;20(2):159–66. [DOI] [PubMed] [Google Scholar]
- 65.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009. Oct 29;461(7268):1282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mizuta K, Sasaki H, Zhang Y, Matoba A, Emala CW, Sr. The short-chain free fatty acid receptor FFAR3 is expressed and potentiates contraction in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2020. Jun 1;318(6):L1248–L60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sahebkar A, Simental-Mendia LE, Pedone C, Ferretti G, Nachtigal P, Bo S, et al. Statin therapy and plasma free fatty acids: a systematic review and meta-analysis of controlled clinical trials. Br J Clin Pharmacol. 2016. May;81(5):807–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sunata K, Kabata H, Kuno T, Takagi H, So M, Masaki K, et al. The effect of statins for asthma. A systematic review and meta-analysis. J Asthma. 2022. Apr;59(4):801–10. [DOI] [PubMed] [Google Scholar]
- 69.Tulbah AS. The potential of Atorvastatin for chronic lung diseases therapy. Saudi Pharm J. 2020. Nov;28(11):1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeki AA, Kenyon NJ, Goldkorn T. Statin drugs, metabolic pathways, and asthma: a therapeutic opportunity needing further research. Drug Metab Lett. 2011. Jan;5(1):40–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeki AA, Franzi L, Last J, Kenyon NJ. Simvastatin inhibits airway hyperreactivity: implications for the mevalonate pathway and beyond. Am J Respir Crit Care Med. 2009. Oct 15;180(8):731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moschos SJ, Sullivan RJ, Hwu WJ, Ramanathan RK, Adjei AA, Fong PC, et al. Development of MK-8353, an orally administered ERK1/2 inhibitor, in patients with advanced solid tumors. JCI Insight. 2018. Feb 22;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sood A, Shore SA. Adiponectin, Leptin, and Resistin in Asthma: Basic Mechanisms through Population Studies. J Allergy (Cairo). 2013;2013:785835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang L, Yin Y, Zhang H, Zhong W, Zhang J. Association of asthma diagnosis with leptin and adiponectin: a systematic review and meta-analysis. J Investig Med. 2017. Jan;65(1):57–64. [DOI] [PubMed] [Google Scholar]
- 75.Giesler A, Mukherjee M, Radford K, Janssen L, Nair P. Modulation of human airway smooth muscle biology by human adipocytes. Respir Res. 2018. Feb 27;19(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond). 2006. Mar;110(3):267–78. [DOI] [PubMed] [Google Scholar]
- 77.Shin JH, Kim JH, Lee WY, Shim JY. The expression of adiponectin receptors and the effects of adiponectin and leptin on airway smooth muscle cells. Yonsei Med J. 2008. Oct 31;49(5):804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006. Aug;118(2):389–95. [DOI] [PubMed] [Google Scholar]
- 79.Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, et al. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol. 2009. Oct;41(4):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Phillips SA, Kung JT. Mechanisms of adiponectin regulation and use as a pharmacological target. Curr Opin Pharmacol. 2010. Dec;10(6):676–83. [DOI] [PubMed] [Google Scholar]
- 81.Achari AE, Jain SK. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int J Mol Sci. 2017. Jun 21;18(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bosse Y Endocrine regulation of airway contractility is overlooked. J Endocrinol. 2014. Aug;222(2):R61–73. [DOI] [PubMed] [Google Scholar]
- 83.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995. Nov;1(11):1155–61. [DOI] [PubMed] [Google Scholar]
- 84.Obradovic M, Sudar-Milovanovic E, Soskic S, Essack M, Arya S, Stewart AJ, et al. Leptin and Obesity: Role and Clinical Implication. Front Endocrinol (Lausanne). 2021;12:585887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sarvas JL, Khaper N, Lees SJ. The IL-6 Paradox: Context Dependent Interplay of SOCS3 and AMPK. J Diabetes Metab. 2013. May 24;Suppl 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nair P, Radford K, Fanat A, Janssen LJ, Peters-Golden M, Cox PG. The effects of leptin on airway smooth muscle responses. Am J Respir Cell Mol Biol. 2008. Oct;39(4):475–81. [DOI] [PubMed] [Google Scholar]
- 87.Arteaga-Solis E, Zee T, Emala CW, Vinson C, Wess J, Karsenty G. Inhibition of leptin regulation of parasympathetic signaling as a cause of extreme body weight-associated asthma. Cell Metab. 2013. Jan 8;17(1):35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005. Jan;115(1):103–9. [DOI] [PubMed] [Google Scholar]
- 89.Quarta C, Sanchez-Garrido MA, Tschop MH, Clemmensen C. Renaissance of leptin for obesity therapy. Diabetologia. 2016. May;59(5):920–7. [DOI] [PubMed] [Google Scholar]
- 90.Cheng WC, Liao WC, Wu BR, Chen CY, Shen MF, Chen WC, et al. Clinical predictors of asthmatics in identifying subgroup requiring long-term tiotropium add-on therapy: a real-world study. J Thorac Dis. 2019. Sep;11(9):3785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khurana S, Paggiaro P, Buhl R, Bernstein JA, Haddon J, Unseld A, et al. Tiotropium reduces airflow obstruction in asthma patients, independent of body mass index. J Allergy Clin Immunol Pract. 2019. Sep - Oct;7(7):2425–8 e7. [DOI] [PubMed] [Google Scholar]
- 92.Dalmas E, Clément K, Guerre-Millo M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011;32(7):307–14. [DOI] [PubMed] [Google Scholar]
- 93.Fantuzzi G Adipose tissue, adipokines, and inflammation. J Allergy Clin Immmunol. 2005;115(5):911–19. [DOI] [PubMed] [Google Scholar]
- 94.Ferrante AW Jr. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262(4):408–14. [DOI] [PubMed] [Google Scholar]
- 95.Ferrante AW Jr. The immune cells in adipose tissue. Diabetes Obes Metab. 2013;15 (Suppl. 3):34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rastogi D, Fraser S, Oh J, Huber AM, Schulman Y, Bhagtani RH, et al. Inflammation, Metabolic Dysregulation and Pulmonary Function Among Obese Asthmatic Urban Adolescents. Am J Resp Crit Care Med. 2015;191(2):149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Periyalil HA, Wood LG, Scott HA, Jensen ME, Gibson PG. Macrophage activation, age and sex effects of immunometabolism in obese asthma. Eur Respir J. 2015. Feb;45(2):388–95. [DOI] [PubMed] [Google Scholar]
- 98.Fantuzzi L, Borghi P, Ciolli V, Pavlakis G, Belardelli F, Gessani S. Loss of CCR2 expression and functional response to monocyte chemotactic protein (MCP-1) during the differentiation of human monocytes: role of secreted MCP-1 in the regulation of the chemotactic response. Blood. 1999;94(3):875–83. [PubMed] [Google Scholar]
- 99.Tashiro H, Takahashi K, Sadamatsu H, Kato G, Kurata K, Kimura S, et al. Saturated Fatty Acid Increases Lung Macrophages and Augments House Dust Mite-Induced Airway Inflammation in Mice Fed with High-Fat Diet. Inflammation. 2017. Jun;40(3):1072–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Diaz J, Warren L, Helfner L, Xue X, Chatterjee PK, Gupta M, et al. Obesity shifts house dust mite-induced airway cellular infiltration from eosinophils to macrophages: effects of glucocorticoid treatment. Immunol Res. 2015. Dec;63(1–3):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim JY, Sohn JH, Lee JH, Park JW. Obesity increases airway hyperresponsiveness via the TNF-alpha pathway and treating obesity induces recovery. PLoS One. 2015;10(2):e0116540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Periyalil HA, Wood LG, Wright TA, Karihaloo C, Starkey MR, Miu AS, et al. Obese asthmatics are characterized by altered adipose tissue macrophage activation. Clin Exp Allergy. 2018. Jun;48(6):641–9. [DOI] [PubMed] [Google Scholar]
- 103.Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, et al. Obesity and Asthma: An Inflammatory Disease of Adipose Tissue Not the Airway. Am J Resp Crit Care Med. 2012;186(7):598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nyambuya TM, Dludla PV, Mxinwa V, Nkambule BB. Obesity-related asthma in children is characterized by T-helper 1 rather than T-helper 2 immune response: A meta-analysis. Ann Allergy Asthma Immunol. 2020;125:425–32. [DOI] [PubMed] [Google Scholar]
- 105.Rastogi D, Canfield S, Andrade A, Hall CB, Isasi CR, Rubinstein A, et al. Obesity-associated asthma in children: A distinct entity. Chest. 2012;141(4):895–905. [DOI] [PubMed] [Google Scholar]
- 106.Melendez J, Grogg M, Zheng Y. Signaling role of Cdc42 in regulating mammalian physiology. J Biol Chem. 2011. Jan 28;286(4):2375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rastogi D, Nico J, Johnson AD, Tobias TA, Jorge Y, Macian F, et al. CDC42-related genes are upregulated in T helper cells from obese asthmatic children. J Allergy Clin Immunol. 2018;141(2):539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Panhuys N TCR Signal Strength Alters T-DC Activation and Interaction Times and Directs the Outcome of Differentiation. Front Immunol. 2016;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chemin K, Bohineust A, Dogniaux S, Tourret M, Guégan S, Miro F, et al. Cytokine secretion by CD4+ T cells at the immunological synapse requires Cdc42-dependent local actin remodeling but not microtubule organizing center polarity. J Immunol. 2012;189(5):2159–68. [DOI] [PubMed] [Google Scholar]
- 110.Uddin M, Nong G, Ward J, Seumois G, Prince LR, Wilson SJ, et al. Prosurvival activity for airway neutrophils in severe asthma. Thorax. 2010;65(8):684–9. [DOI] [PubMed] [Google Scholar]
- 111.Telenga ED, Tideman SW, Kerstjens HA, Hacken NH, Timens W, Postma DS, et al. Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy. 2012. Aug;67(8):1060–8. [DOI] [PubMed] [Google Scholar]
- 112.Scott HA, Gibson PG, Garg ML, Upham JW, Wood LG. Sex hormones and systemic inflammation are modulators of the obese-asthma phenotype. Allergy 2016;71(7):1037–47. [DOI] [PubMed] [Google Scholar]
- 113.Fu JJ, Baines KJ, Wood LG, Gibson PG. Systemic inflammation is associated with differential gene expression and airway neutrophilia in asthma. OMICS. 2013. Apr;17(4):187–99. [DOI] [PubMed] [Google Scholar]
- 114.Rhee H, Love T, Harrington D. Blood Neutrophil Count is Associated with Body Mass Index in Adolescents with Asthma. JSM Allergy Asthma. 2018;3(1). [PMC free article] [PubMed] [Google Scholar]
- 115.Jensen ME, Gibson PG, Collins CE, Wood LG. Airway and systemic inflammation in obese children with asthma. Eur Respir J. 2013. Oct;42(4):1012–9. [DOI] [PubMed] [Google Scholar]
- 116.Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20(1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lachowicz-Scroggins ME, Dunican EM, Charbit AR, Raymond W, Looney MR, Peters MC, et al. Extracellular DNA, Neutrophil Extracellular Traps, and Inflammasome Activation in Severe Asthma. Am J Respir Crit Care Med. 2019. May 1;199(9):1076–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wood LG, Li Q, Scott HA, Rutting S, Berthon BS, Gibson PG, et al. Saturated fatty acids, obesity, and the nucleotide oligomerization domain-like receptor protein 3 (NLRP3) inflammasome in asthmatic patients. J Allergy Clin Immunol. 2019. May 30;143(1):305–15. [DOI] [PubMed] [Google Scholar]
- 119.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016. Jul;4(7):574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Permaul P, Peters MC, Petty CR, Cardet JC, Ly NP, Ramratnam SK, et al. The association of plasma IL-6 with measures of asthma morbidity in a moderate-severe pediatric cohort aged 6–18 years. J Allergy Clin Immunol Pract. 2021. Jul;9(7):2916–9 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jackson DJ, Bacharier LB, Calatroni A, Gill MA, Hu J, Liu AH, et al. Serum IL-6: A biomarker in childhood asthma? J Allergy Clin Immunol. 2020. Jun;145(6):1701–4 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jevnikar Z, Ostling J, Ax E, Calven J, Thorn K, Israelsson E, et al. Epithelial IL-6 trans-signaling defines a new asthma phenotype with increased airway inflammation. J Allergy Clin Immunol. 2019. Feb;143(2):577–90. [DOI] [PubMed] [Google Scholar]
- 123.Elias JA, Wu Y, Zheng T, Panettieri R. Cytokine- and virus-stimulated airway smooth muscle cells produce IL-11 and other IL-6-type cytokines. Am J Physiol. 1997. Sep;273(3 Pt 1):L648–55. [DOI] [PubMed] [Google Scholar]
- 124.De S, Zelazny ET, Souhrada JF, Souhrada M. IL-1 beta and IL-6 induce hyperplasia and hypertrophy of cultured guinea pig airway smooth muscle cells. J Appl Physiol (1985). 1995. Apr;78(4):1555–63. [DOI] [PubMed] [Google Scholar]
- 125.Robinson MB, Deshpande DA, Chou J, Cui W, Smith S, Langefeld C, et al. IL-6 trans-signaling increases expression of airways disease genes in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2015. Jul 15;309(2):L129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.White SR, Laxman B, Naureckas ET, Hogarth DK, Solway J, Sperling AI, et al. Evidence for an IL-6-high asthma phenotype in asthmatic patients of African ancestry. J Allergy Clin Immunol. 2019. Jul;144(1):304–6 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Esty B, Harb H, Bartnikas LM, Charbonnier LM, Massoud AH, Leon-Astudillo C, et al. Treatment of severe persistent asthma with IL-6 receptor blockade. J Allergy Clin Immunol Pract. 2019. May - Jun;7(5):1639–42 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, et al. Interleukin-4, −5, and −6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994. May;10(5):471–80. [DOI] [PubMed] [Google Scholar]
- 129.Guedes AG, Deshpande DA, Dileepan M, Walseth TF, Panettieri RA Jr., Subramanian S, et al. CD38 and airway hyper-responsiveness: studies on human airway smooth muscle cells and mouse models. Can J Physiol Pharmacol. 2015. Feb;93(2):145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jude JA, Solway J, Panettieri RA Jr., Walseth TF, Kannan MS. Differential induction of CD38 expression by TNF-{alpha} in asthmatic airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2010. Dec;299(6):L879–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dileepan M, Jude JA, Rao SP, Walseth TF, Panettieri RA, Subramanian S, et al. MicroRNA-708 regulates CD38 expression through signaling pathways JNK MAP kinase and PTEN/AKT in human airway smooth muscle cells. Respir Res. 2014. Aug 31;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dileepan M, Sarver AE, Rao SP, Panettieri RA, Jr., Subramanian S, Kannan MS. MicroRNA Mediated Chemokine Responses in Human Airway Smooth Muscle Cells. PLoS One. 2016;11(3):e0150842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zeng S, Cui J, Zhang Y, Zheng Z, Meng J, Du J. MicroRNA-15b-5p inhibits tumor necrosis factor alpha-induced proliferation, migration, and extracellular matrix production of airway smooth muscle cells via targeting yes-associated protein 1. Bioengineered. 2022. Mar;13(3):5396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu Y, Lu Y, Zou F, Fan X, Li X, Zhang H, et al. PTEN participates in airway remodeling of asthma by regulating CD38/Ca(2+)/CREB signaling. Aging (Albany NY). 2020. Aug 27;12(16):16326–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Taillé C, Poulet C, Marchand-Adam S, Borie R, Dombret MC, Crestani B, et al. Monoclonal Anti-TNF-α Antibodies for Severe Steroid-Dependent Asthma: A Case Series. Open Respir Med J. 2013;7:21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wenzel SE, Barnes PJ, Bleecker ER, Bousquet J, Busse W, Dahlen SE, et al. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-alpha blockade in severe persistent asthma. Am J Respir Crit Care Med. 2009. Apr 1;179(7):549–58. [DOI] [PubMed] [Google Scholar]
- 137.Huang SL, Shaio GM, Chou P. Association between body mass index and allergy in teenage girls in Taiwan. Clin Exp Allergy. 1999;29(3):323–9. [DOI] [PubMed] [Google Scholar]
- 138.Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J Allergy Clin Immunol. 2004. Aug;114(2):254–9. [DOI] [PubMed] [Google Scholar]
- 139.Grotta MB, Squebola-Cola DM, Toro AA, Ribeiro MA, Mazon SB, Ribeiro JD, et al. Obesity increases eosinophil activity in asthmatic children and adolescents. BMC Pulm Med. 2013. Jun 18;13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Desai D, Newby C, Symon FA, Haldar P, Shah S, Gupta S, et al. Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with severe asthma. Am J Resp Crit Care Med. 2013;188(6):657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Santamaria F, Montella S, De Stefano S, Sperlì F, Barbarano F, Valerio G. Relationship between exhaled nitric oxide and body mass index in children and adolescents. J Allergy Clin Immunol. 2005;116(5):1163–4; author reply 4–5. [DOI] [PubMed] [Google Scholar]
- 142.Han Y-Y, Forno E, Celedon JC. Adiposity, fractional exhaled nitric oxide, and asthma in U.S. children. Am J Resp Crit Care Med. 2014;190(1):32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011. Jun;127(6):1486–93 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dixon AE, Poynter ME. Mechanisms of Asthma in Obesity. Pleiotropic Aspects of Obesity Produce Distinct Asthma Phenotypes. Am J Respir Cell Mol Biol. 2016. May;54(5):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Muc M, Mota-Pinto A, Padez C. Association between obesity and asthma - epidemiology, pathophysiology and clinical profile. Nutr Res Rev. 2016. Dec;29(2):194–201. [DOI] [PubMed] [Google Scholar]
- 146.Chen Z, Salam MT, Alderete TL, Habre R, Bastain TM, Berhane K, et al. Effects of Childhood Asthma on the Development of Obesity among School-aged Children. Am J Respir Crit Care Med. 2017. May 1;195(9):1181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Contreras ZA, Chen Z, Roumeliotaki T, Annesi-Maesano I, Baiz N, von Berg A, et al. Does early onset asthma increase childhood obesity risk? A pooled analysis of 16 European cohorts. Eur Respir J. 2018. Sep;52(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moitra S, Carsin AE, Abramson MJ, Accordini S, Amaral AFS, Anto J, et al. Long-term effect of asthma on the development of obesity among adults: an international cohort study, ECRHS. Thorax. 2022. Apr 27. [DOI] [PubMed] [Google Scholar]
- 149.Jani M, Ogston S, Mukhopadhyay S. Annual increase in body mass index in children with asthma on higher doses of inhaled steroids. J Pediatr. 2005. Oct;147(4):549–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as this is a review article and no datasets were generated or analysed during the current study.


