Abstract
Thymically-derived Foxp3+ regulatory T cells (Treg) critically control immunological tolerance. These cells are generated in the medulla through high affinity interactions with medullary thymic epithelial cells (mTEC) expressing the Autoimmune regulator (Aire). Recent advances have revealed that thymic Treg contain not only developing but also recirculating cells from the periphery. Although Aire is implicated in the generation of Foxp3+ Treg, its role in the biology of recirculating Treg remains elusive. Here, we show that Aire regulates the suppressive signature of recirculating Treg independently of the remodeling of the medullary 3D organization throughout life where Treg reside. Accordingly, the adoptive transfer of peripheral Foxp3+ Treg in AireKO recipients led to an impaired suppressive signature upon their entry into the thymus. Furthermore, recirculating Treg from AireKO mice failed to attenuate the severity of multiorgan autoimmunity, demonstrating that their suppressive function is altered. Using bone marrow chimeras, we reveal that mTEC-specific expression of Aire controls the suppressive signature of recirculating Treg. Finally, mature mTEC lacking Aire were inefficient in stimulating peripheral Treg both in polyclonal and antigen-specific co-culture assays. Overall, this study demonstrates that Aire confers to mTEC the ability to restimulate recirculating Treg, unravelling a novel function for this master regulator in Treg biology.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-022-04328-9.
Keywords: Autoimmune regulator, Autoimmunity, Medullary thymic epithelial cells, Foxp3+ regulatory T cells, Thymus
Introduction
CD25+Foxp3+ regulatory T cells (Treg) constitute a distinct subset of CD4+ T cells endowed with suppressive functions. By maintaining immune tolerance, Treg critically prevent the emergence of autoimmune and inflammatory diseases. The vast majority of Treg is produced in the thymic medulla where they arise from two distinct developmental programs involving CD25+Foxp3− (CD25+ TregP) and CD25−Foxp3lo (Foxp3lo TregP) precursors [1–4]. For a long-time, thymic CD25+Foxp3+ Treg were thought to exclusively correspond to developing cells. However, recent studies have shown that circulating CD25+Foxp3+ Treg have the ability to migrate back into the thymus [5–7]. Thus, thymic CD25+Foxp3+ Treg is a heterogeneous population containing both developing and recirculating cells [3]. Interestingly, recirculating Treg show suppressive properties similar to their splenic counterparts [7]. They exhibit an activated and differentiated phenotype and were found to negatively regulate the de novo generation of CD25+Foxp3+ Treg through IL-2 consumption [5].
The recirculation of peripheral Foxp3+ Treg in the thymus is mediated at least by two chemokine receptors, CCR6 and CXCR4 [5, 8]. Interestingly, the entry of peripheral Ccr6−/− Treg into the thymus is impaired, suggesting that CCR6, expressed by effector/memory T cells, is involved in the recirculation of Foxp3+ Treg from the periphery to this organ [8]. Furthermore, the expression of the CCR6 ligand, CCL20, is regulated by the Autoimmune regulator (Aire) [8]. Aire is mainly expressed by a subset of medullary thymic epithelial cells (mTEC) commonly called mTEChi, characterized by a CD80hiMHCIIhi phenotype. Furthermore, upon recirculation into the thymus, a subset of B cells has been described to upregulate Aire [9]. Independent reports have described that CD25+Foxp3+ Treg are reduced in the thymus of AireKO mice [10, 11]. Accordingly, Aire expression was proposed to direct autoreactive CD4+ thymocytes into the Treg cell lineage [12]. Interestingly, in the perinatal life, Aire promotes the generation of a distinct population of Foxp3+ Treg, which persists in adults and prevents the emergence of autoimmunity [13]. In C57BL/6 mice, Aire-deficiency is associated with mild autoimmunity characterized by lymphocyte infiltrations and autoantibody production targeting several organs such as the pancreas, eyes, salivary glands, liver and lungs [14–17]. In human, autosomal recessive mutations in the Aire gene induce a life-threatening pathology called autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy (APECED), also known as autoimmune polyendocrine syndrome-type 1 (APS-1) [18, 19]. Interestingly, numbers, activation and suppressive functions of Foxp3+ Treg are altered in APECED patients [20–22].
Although thymic Foxp3+ Treg have been found to be more heterogeneous than previously thought [3], this emerging population of recirculating peripheral Treg remains poorly described. In particular, the mechanisms that control their suppressive phenotype in the thymus are unknown. In this study, we show that Aire regulates the suppressive properties of recirculating CCR6+ Treg, independently of the remodeling of the 3D organization of the thymic medulla where they reside [5]. High-throughput sequencing revealed that recirculating CCR6+ Treg in the thymus of AireKO mice have impaired expression of several genes associated with Treg suppressive functions and helper T cell polarization. Accordingly, recirculating CCR6+ Treg from AireKO mice failed to attenuate the severity of multiorgan autoimmunity, demonstrating that their suppressive activity is impaired. Moreover, the adoptive transfer of splenic Foxp3+ Treg in AireKO recipients resulted in an impaired suppressive signature upon their recirculation in the thymus. Using bone marrow (BM) chimeras, we demonstrate that Aire expressed by mTEC rather than by thymic B cells is responsible for the suppressive properties of recirculating CCR6+ Treg. Finally, recirculating Treg were found in close contact with Aire+ mTEC and restimulated in an antigen-specific manner. Altogether, our data reveal that Aire confers to mTEC the capacity to control the restimulation of recirculating CCR6+ Treg in the thymus.
Materials and methods
Mice
CD45.1 WT (B6.SJL-PtprcaPepcb/BoyCrl, Stock n°002,014, Charles River), CD45.2 WT (Stock n°000,664, Charles River), CD45.1/2 WT, CD45.2 AireKO [23], Rag2KO [24], CD45.1 Foxp3eGFP mice [25], OTII [26] and Rip-mOVA [27] were on a C57BL/6 J background. Rip-mOVA × OTII mice were backcrossed on a Rag2KO background. All mice were maintained under specific pathogen-free conditions at the Centre d’Immunologie de Marseille–Luminy (CIML, France). Standard food and water were given ad libitum. Males and females were used at the age of 5 days, 9 days, 6 weeks and 1 year. Chimeras were generated between 6 and 10 weeks of age. All experiments were done in accordance with National and European laws for laboratory animal welfare (EEC Council Directive 2010/63/UE) and the Marseille Ethical Committee for Animal experimentation no. 14.
BM chimeras
BM chimeras were generated by injecting intravenously (i.v.) 5.106 BM cells from CD45.1 Foxp3eGFP mice into lethally irradiated CD45.2 AireWT or AireKO recipients (two doses of 500 rads, 8 h apart, X-ray using a RS-2 000 Irradiator; Rad Source Technologies). Similarly, 5.106 BM cells from either CD45.2 AireWT or AireKO mice were injected into lethally irradiated CD45.1/2 WT recipients. Mice were analyzed 6-weeks post-reconstitution.
Foxp3+ Treg adoptive transfer
5.105 cell-sorted splenic congenic CD45.1 CD4+CD25+Foxp3eGFP Treg were i.v injected into sublethally irradiated CD45.2 AireWT or AireKO recipient mice (one dose of 500 rads, X-ray using a RS-2 000 Irradiator; Rad Source Technologies). Mice were analyzed 1-week post-Treg adoptive transfer.
Multiorgan autoimmunity experiments
Rag2KO recipients were injected i.v. with 3.106 CD4+CD25+ Treg-depleted splenocytes purified from CD45.1 WT mice. Four weeks later, 15.104 splenic CD4+CD25hi Treg or 10.104 recirculating thymic CCR6+CD4+CD25+ Treg from AireWT or AireKO mice were adoptively transferred. Mice that did not receive any Treg were used as controls. Peripheral tissues were harvested and analyzed three weeks later.
Cell isolation
Thymic Treg, splenic Treg and Treg-depleted splenocytes were isolated by scratching the thymus or the spleen on a 70-μm mesh. Red blood cells were lysed with RBC lysis buffer (eBioscience). Thymic and splenic Treg cells were pre-enriched by depleting CD8+ and CD19+ cells using biotinylated anti-CD8α (clone 53.6.7; BD Biosciences) and anti-CD19 (clone 1D3; BD Biosciences) antibodies with antibiotin microbeads by AutoMACS using the Deplete program (Miltenyi Biotec). Recirculating thymic Treg were sorted as CCR6+CD4+CD8−CD25+ cells and splenic Treg as CD4+CD25hi cells using a FACSAria III cell sorter (BD Biosciences). Splenocytes were depleted of CD4+CD25+ Treg using a FACSAria III cell sorter (BD Biosciences). Thymus were digested at 37 °C in HBSS medium containing Liberase TM (50 µg/ml; Roche) and DNase I (100 μg/ml; Roche) until complete tissue digestion. Total mTEC (CD45−EpCAM+UEA-1+Ly51−/lo) or mTEChi (CD45−EpCAM+UEA-1+Ly51−/loCD80hi) were pre-enriched by depleting CD45 hematopoietic cells using anti-CD45 magnetic beads (Miltenyi Biotec) by AutoMACS with the DepleteS program and sorted using a FACSAria III cell sorter (BD Biosciences).
In vitro co-culture assays
3.104 cell-sorted splenic CD4+CD25hi Treg from AireWT or AireKO mice were co-cultured for 24 h with 6.103 CD45−EpCAM+UEA-1+Ly51−/lo mTEC from AireWT or AireKO mice in RPMI (ThermoFisher) supplemented with 10% FBS (Sigma Aldrich), L-glutamine (2 mM, ThermoFisher), sodium pyruvate (1 mM, ThermoFisher), 2-mercaptoethanol (2 × 10–5 M, ThermoFisher), penicillin (100 IU/ml, ThermoFisher), streptomycin (100 μg /ml, ThermoFisher) and mouse IL-2 (40 ng/ml, Immunotools). For antigen-specific co-culture assays, 5.103 cell-sorted splenic CD4+CD25hi Treg from Rip-mOVA x OTII x Rag2KO mice were co-cultured for 24 h with 1.103 CD45−EpCAM+Ly51−/loAireeGFP mTEC from Airehet or AireKO mice previously loaded with OVA323-339 peptide (5 µg/ml, Polypeptide group) for 2 h.
Flow cytometry
Cells were stained with standard procedures using antibodies listed in Table S2. For intracellular staining with anti-Foxp3, anti-GITRL and anti-OX40L antibodies, cells were fixed, permeabilized and stained with the Foxp3 staining kit according to the manufacturer’s instructions (eBioscience). Stained cells were analyzed with FACSCanto II and LSR II (BD Biosciences) and data were analyzed using FlowJo software (Tree Star).
Quantitative RT-PCR
The total RNA was extracted with TRIzol (Invitrogen) and cDNA was synthesized with random oligo dT primers and Superscript II reverse transcriptase (Invitrogen). Quantitative PCR was performed with SYBR Premix Ex Taq Master Mix (Takara) on an ABI 7500 fast real-time PCR system (Applied Biosystem). The results were normalized to actin mRNA expression. A list of primer sequences is provided in Table S3.
RNA-sequencing experiments
5.104 CCR6+ Treg were cell-sorted from the thymus of 6-week-old AireWT and AireKO mice. Two biological replicates were prepared for each condition. The total RNA was extracted using the RNeasy Micro Kit (Qiagen) and treated with DNase I. RNA-seq libraries were prepared using the TruSeq Stranded mRNA kit (Illumina) and sequenced with the Illumina HiSeq 2000 machine to generate datasets of single-end 50 bp reads. The reads were mapped to the mouse reference genome (mm10) using TopHat2 (version 2.0.12) [28], then counted using Cufflinks or Cuffdiff (version 2.2.1) [29, 30] and the mm10 genome GTF gene annotation file. In addition to read counting, Cuffdiff performs between-sample normalization and was used to calculate the differential gene expression and its statistical significance in AireWT vs AireKO Treg by using the default “pooled” dispersion method that applies to experiments having few (≥ 2) biological replicates per condition. Expression levels generated by Cufflinks, as fragments per kilobase of transcript per million mapped reads (FPKM), were processed by the Matrix2png program [31] to generate heatmaps of gene expression levels. The dataset generated in this study are available in the Gene Expression Omnibus (GEO) database under accession number GSE188419. The expression of Tnfsf4 and Tnfsf18 was analyzed in RNA-sequencing dataset from AireKO mTEChi (GSE87133).
Immunofluorescence staining
Thymi were collected from 9-day-, 6-week- and 1-year-old AireWT or AireKO mice and fat tissues removed to avoid any interference with the 3D reconstitution process. Organs were longitudinally included in O.C.T (Sakura Finetek), frozen at -80 °C and cut in 20-µm-thick slices. Thymic sections were fixed with 2% paraformaldehyde, then saturated with 3% BSA and 0.01% Triton X100 in 0.1 M Tris HCl buffer. Sections were next stained for 45 min with rabbit anti-keratin 14 or with anti-Aire Alexa Fluor 488 (5H12; eBioscience), anti-Foxp3 PE (FJK-16 s; eBioscience) and anti-CD73 Alexa Fluor 647 (TY/11.8; BioLegend) in hybridization buffer (1% BSA and 0.02% Triton X100 in 0.1 M Tris–HCl, pH 7.4). Keratin 14 staining was revealed with Cy3-conjugated anti-rabbit (Invitrogen). Sections were counterstained with DAPI (1 µL/ml, BioLegend) and mounted with Mowiol (Calbiochem). For 3D reconstruction, images were acquired with a slide scanner (Panoramic SCAN II; 3D Histech). Confocal microscopy was performed with a LSM 780 confocal microscope (Carl Zeiss Microscopy).
Detection of immune infiltrates and autoantibodies
Peripheral tissues were fixed in buffered 10% formalin solution. 4 µm-thick paraffin-embedded sections were counterstained with hematoxylin and eosin. Autoantibody production was assessed by immunostaining organ sections from Rag2KO mice with the sera (1/80) of analyzed mice. Autoantibodies were revealed with FITC-conjugated goat anti-mouse IgG. Sections were counterstained with DAPI and mounted with Mowiol (Calbiochem). All images were acquired with a slide scanner (Panoramic SCAN II; 3D Histech) and analyzed with ImageJ software (National Institutes of Health) to compute the mean fluorescence intensity of each image.
Thymic 3D reconstitution
For 3D reconstitution, images from the entire thymus of 9-day-, 6-week- and 1-year-old AireWT and AireKO mice were processed with For3D software as previously described [32, 33]. Briefly, images were smoothed by median and Gaussian filtering and medulla volumes were determined using ImageJ and Matlab (The Mathworks) software. Medullary islets identified in the 3D structures were measured individually using ImageJ and were color-coded using Imaris (Bitplane).
Statistical analysis
Statistics were performed with GraphPad Prism 9.1 software. Normal distribution of the data was assessed using d'Agostino–Pearson omnibus normality test. Statistical significance was then assessed using unpaired Student’s t test for two normal distributions, Mann–Whitney test for two non-normal distributions or Kruskal–Wallis test for more than two distributions. *p < 0.05; **p < 0.01; ***p < 0.001, ****p < 0.0001. All bar graphs show mean ± SEM, unless mentioned.
Results
Aire regulates the recirculation of peripheral CD25+Foxp3+ Treg in the thymus, independently of the remodeling of the 3D organization of the medulla throughout life
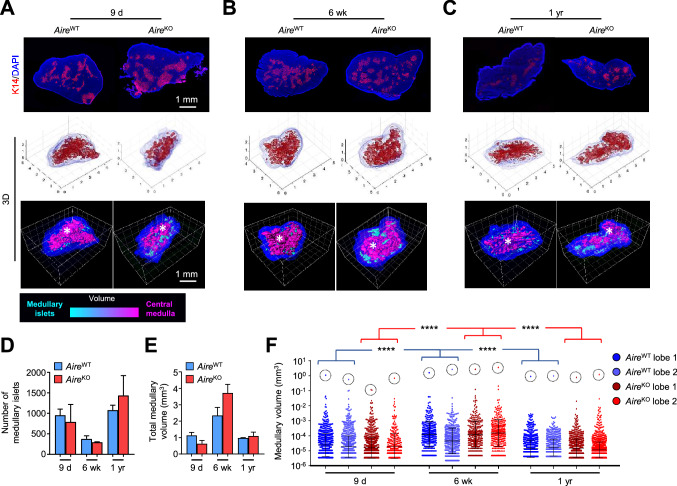
Considering the importance of Aire in the induction of self-tolerance [34], we first assessed whether it could be involved in the topology of the thymic medulla. For this, we compared WT (AireWT mice) with Aire-deficient mice in which the first exon of the Aire locus was replaced by the sequence of enhanced green fluorescent protein (eGFP) (AireKO mice) [23]. Thymic sections of 9-day-, 6-week- and 1-year-old, either from AireWT or AireKO mice, were stained with the keratin-14 mTEC-specific marker [35] and reconstructed with our in-house dedicated “Full organ reconstruction in 3D” (For3D) software [33] (Fig. 1A–C and Movies S1–6). Interestingly, the number of medullary islets in AireWT mice diminished between 9 days and 6 weeks of age while it increased between 6 weeks and 1 year of age (Fig. 1D). Furthermore, the total and individual medullary volumes increased between 9 days and 6 weeks while it decreased between 6 weeks and 1 year (Fig. 1E, F). Nevertheless, the central compartment of ~ 1 mm3 was observed at all ages analyzed (Fig. 1F) These observations reveal that the medulla organization is dynamic throughout life. In comparison to AireWT mice, the thymi of AireKO mice show a similar dynamic medulla organization characterized by normal numbers of medullary islets and total medullary volumes with the presence of a major compartment (Fig. 1A–C, F). These results indicate that the dynamic remodeling of the 3D organization of the thymic medulla throughout life is not regulated by Aire.
Fig. 1.
The medullary topology is dynamic throughout life, independently of Aire expression. A–C Representative images of thymic sections stained for keratin 14 (red) and counterstained with DAPI (blue) (upper panel). For3D reconstruction of thymic lobes from 9-day- (A), 6-week- (B) and 1-year-old (C) AireWT and AireKO mice, using Matlab (middle panel) and Imaris to depict medullary regions according to their volumes from cyan (smallest) to magenta (largest) (lower panel). Axes are graduated in millimeters (mm). Scale bar, 1 mm. The asterisk denotes the central medulla. D,E Histograms show the number of medullary islets (D) and the total medullary volume (E) derived from two thymic lobes for each condition. F The graph shows the volumes of each medullary islet derived from two thymic lobes for each condition from individual mice measured by For3D. The dashed circle denotes the central medulla of each lobe. Horizontal lines represent the geometric mean and SD. ****p < 0.0001 using Kruskal–Wallis test for (F)
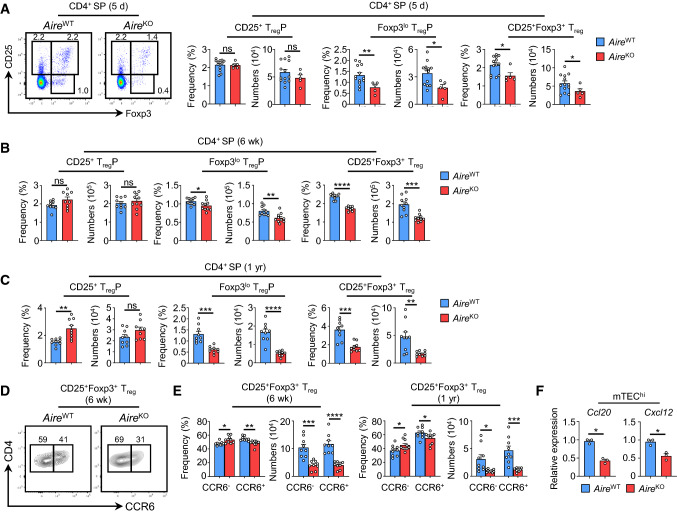
Because medulla formation correlates with Foxp3+ Treg emergence during ontogeny [36], we next analyzed whether Aire deficiency could affect their development in neonates of 5 days of age. In contrast to CD25+ TregP, frequencies and numbers of Foxp3lo TregP and mature CD25+Foxp3+ Treg were diminished in the thymus of 5-day-old AireKO mice as compared to their respective counterparts (Fig. 2A). This decrease was also observed in 6-week- and 1-year-old AireKO mice (Fig. 2B, C). Since mature CD25+Foxp3+ Treg contain both developing and recirculating cells in the adult thymus, we used the key thymus-homing chemokine receptor CCR6 to distinguish developing (CCR6−) and recirculating (CCR6+) mature Treg [6]. Interestingly, both numbers of CCR6− and CCR6+ mature Treg were reduced in AireKO mice compared to AireWT mice at 6 weeks and 1 year of age (Fig. 2D, E). Furthermore, 1-year-old AireWT mice showed a marked reduction in numbers of CCR6− and CCR6+ mature CD25+Foxp3+ Treg as compared to 6-week-old AireWT mice, which reflects the effect of thymic involution on Treg cells in normal conditions. To date, two chemokine receptors, CCR6 and CXCR4 have been implicated in the recirculation of peripheral Treg into the thymus [5, 8]. Strikingly, the expression of their respective ligands, Ccl20 and Cxcl12, was substantially reduced in AireKO mTEChi (Fig. 2F), consistently with the altered recirculation of peripheral Treg in the thymus. Altogether, these results show that Aire controls both the development and recirculation of Foxp3+ Treg throughout life.
Fig. 2.
Developing CCR6− and recirculating CCR6+ Treg are reduced in AireKO mice throughout life. A Flow cytometry profiles, frequencies and numbers of CD25+ TregP, Foxp3lo TregP and CD25+Foxp3+ Treg analyzed in CD4+ SP thymocytes from the thymus of 5-day-old AireWT and AireKO mice. The data are derived from 3 independent experiments (n = 2–4 mice per group and per experiment). B, C Frequencies and numbers of CD25+ TregP, Foxp3lo TregP and CD25+Foxp3+ Treg in the thymus of 6-week- (B) and 1-year- (C) old AireWT and AireKO mice. D, E Flow cytometry profiles (D), frequencies and numbers (E) of CCR6− and CCR6+ cells in CD25+Foxp3+ Treg from 6-week- and 1-year-old AireWT and AireKO mice. The data are derived from at least 3 independent experiments (n = 3–4 mice per group and per experiment). F The expression of Ccl20 and Cxcl12 was measured by qPCR in purified mTEChi (EpCAM+UEA-1+Ly51−/loCD80hi) from 6-week-old AireWT and AireKO mice. Bar graphs show ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 using unpaired Student’s t test (A, B, C, E) and two-tailed Mann–Whitney test for (F)
Thymic CCR6+ Treg from AireKO mice show an impaired effector and suppressive phenotype
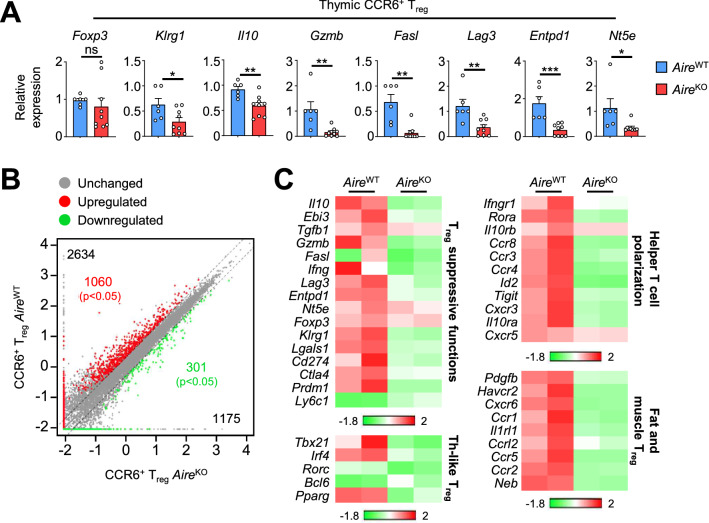
Because Aire controls the recirculation of CCR6+ Treg, we made the hypothesis that it could also control their suppressive properties. To test this hypothesis, we first measured the expression level of several genes associated with Treg suppressive functions in purified CCR6+CD4+CD25+ single-positive (SP) thymocytes that correspond to CD25+Foxp3+ Treg (Fig. S1). Interestingly, whereas Foxp3 level was normal, the expression of Klrg1, a marker of terminally differentiated Treg [37], was reduced in CCR6+ Treg from 6-week-old AireKO mice (Fig. 3A). Accordingly, the expression of genes encoding for the inhibitory cytokine Il10, the cytolytic molecules Gzmb and Fasl, Lag3-associated with dendritic cell modulation, as well as the ectoenzymes Entpd1 (CD39) and Nt5e (CD73), implicated in target cell metabolic disruption, was reduced in CCR6+ Treg of AireKO mice. A similar altered suppressive signature was also observed in CCR6+ Treg purified from the thymus of 1-year-old AireKO mice (Fig. S2).
Fig. 3.
The suppressive signature of recirculating CCR6+ Treg is impaired in the thymus of AireKO mice. A The expression level of Foxp3, Klrg1, Il10, Gzmb, Fasl, Lag3, Entpd1 and Nt5e was measured by qPCR in thymic CCR6+ Treg from 6-week-old AireWT (n = 6) and AireKO (n = 9) mice. Bar graphs show mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001 using two-tailed Mann–Whitney test. B Scatterplot representations of log10 gene expression levels (FPKM) in recirculating CCR6+ Treg from AireWT and AireKO mice. For representation purposes, expression values of genes below 0.01 were assigned to 0.01. Genes with fold difference > 2 and p-adj < 0.05 were considered as up- or down-regulated genes (red and green dots, respectively). RNA-seq was performed on 2 independent biological replicates derived from two mice. C Heatmap of selected genes down-regulated in recirculating CCR6+ AireKO Treg (Fold Change > 2) compared to their CCR6+ AireWT counterparts and involved in Treg suppressive functions, Th–like Treg, helper T cell polarization, fat and muscle Treg. Two biological replicates are shown for each condition
To further determine the impact of Aire on the functional properties of CCR6+ Treg, we analyzed their gene expression profile by high-throughput RNA sequencing (Fig. 3B, C). Genes showing a significant variation (p ≤ 0.05) in gene expression between AireWT and AireKO CCR6+ Treg with a fold change difference > 2 or < 0.5 were considered as up- and down-regulated, respectively. We identified a total of 2 634 upregulated genes reaching significance for 1060 of them (Cuffdiff p < 0.05) in AireWT CCR6+ Treg as compared to their AireKO counterparts (Fig. 3B). Moreover, only 1 175 genes were downregulated with 301 of them reaching significance (Cuffdiff p < 0.05). Thus, the expression of Aire in the thymus upregulates three times more genes than it downregulates in recirculating CCR6+ Treg. In accordance with the altered Treg suppressive signature observed by qPCR (Fig. 3A), we found that Il10, Gzmb, Fasl, Lag3, Entpd1 and Nt5e were downregulated in CCR6+ Treg of AireKO mice (Fig. 3C and Table S1). Furthermore, the expression of Prdm1 (Blimp-1), which characterizes effector Th-like Treg [38] and the terminally differentiated markers Klrg1 and Tigit [37, 39] was reduced in CCR6+ Treg of AireKO mice compared to their AireWT counterparts. Strikingly, CCR6+ Treg of AireKO mice also expressed lower levels of several genes associated with their suppressive signature such as Ctla4 and Lgals1 (galectin-1) implicated respectively in dendritic cell modulation and target cell apoptosis [40, 41]. The expression of Tbx21 and Irf4, encoding for transcription factors associated with Th1- and Th2-like Treg, as well as Pparg and Id2 genes associated with fat-resident effector Treg [42] was also diminished. Accordingly, the expression of the chemokine receptors Cxcr3 of Th1-like, Ccr4 and Ccr8 of Th2-like as well as Ccr1 and Ccr2 of fat-resident Treg [43], implicated in effector Treg migration to the inflammatory site, was reduced in CCR6+ AireKO Treg. Altogether, these results indicate that Aire expression is crucial for the effector and suppressive properties of recirculating CCR6+ Treg in the thymus.
Recirculating CCR6+ Treg from AireKO mice fail to attenuate the severity of multiorgan autoimmunity
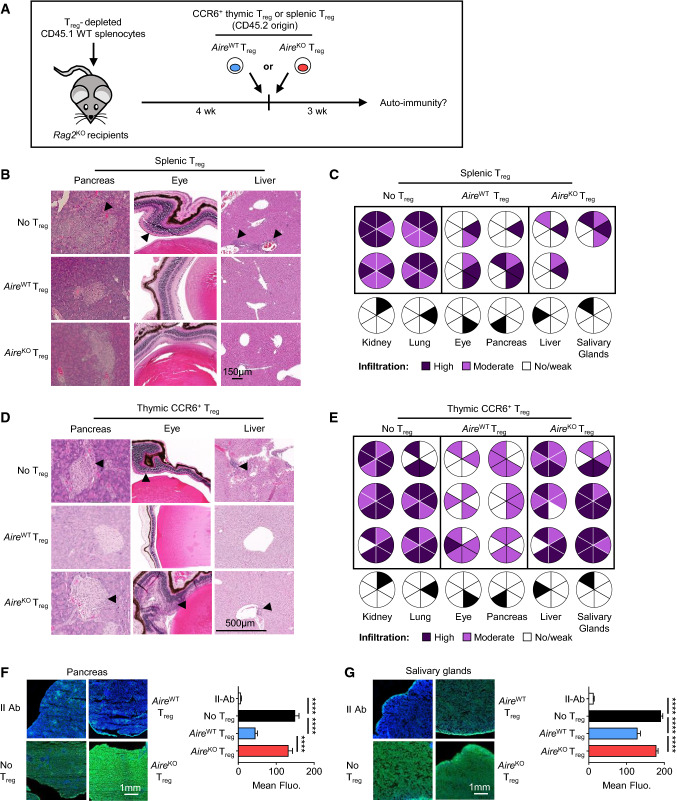
Because numbers and suppressive signature of recirculating CCR6+ Treg were reduced in the thymus of AireKO mice, we analyzed whether these defects would be also observed in the periphery. Blood and splenic CD4+Foxp3+ Treg from 6-week- and 1-year-old AireKO mice showed similar frequencies, numbers and expression levels of suppressive genes as compared to their AireWT counterparts (Fig. S3). We next assessed the ability of peripheral and thymic CCR6+ Treg from AireKO mice to dampen the severity of multiorgan autoimmunity. To this end, CD4+CD25+ Treg-depleted splenocytes from CD45.1 WT mice were transferred into Rag2KO lymphopenic recipients. Four weeks later, splenic Treg or thymic CCR6+ Treg purified from either AireWT or AireKO mice were adoptively transferred into these recipients (Figs. 4A and S4A). Signs of autoimmunity in peripheral tissues were visualized by histology and quantified by flow cytometry three weeks later. Rag2KO mice that did not receive any Treg were used as controls. Accordingly with the normal suppressive signature of splenic Treg from AireKO mice (Fig. S3), Rag2KO mice adoptively transferred with these cells show tissue infiltration levels similar to mice transferred with AireWT splenic Treg (Fig. 4B, C). In marked contrast to mice that received AireWT thymic CCR6+ Treg, mice adoptively transferred with thymic CCR6+ Treg from AireKO mice failed to attenuate T-cell infiltration in several peripheral tissues despite similar numbers of CD45.2 donor Foxp3+ Treg in lymph nodes (Fig. 4D, E and S4B, C). Examination of CD45.1 donor cell infiltration by flow cytometry revealed that 100% of Rag2KO mice that received AireKO thymic CCR6+ Treg showed a high infiltration level in the pancreas and salivary glands, 83% in eyes, 33% in the lung and liver as well as 16% in the kidney (Fig. 4E). In contrast, only 16% of Rag2KO mice transferred with AireWT thymic CCR6+ Treg showed a high infiltration in the liver. Furthermore, flow cytometry analysis showed that thymic CCR6+ Treg of AireKO mice were unable to prevent the infiltration of CD45.1 CD4+ and CD8+ T cells in the pancreas and eyes as well as CD45.1 CD8+ T cells in salivary glands (Fig. S4D). Finally, immunostaining of Rag2KO tissue sections with sera from these mice revealed higher levels of autoantibodies against the pancreas and salivary glands in mice transferred with AireKO thymic CCR6+ Treg than in mice injected with AireWT thymic CCR6+ Treg (Fig. 4F, G). Thus, thymic CCR6+ Treg of AireKO mice failed to attenuate the severity of multiorgan autoimmunity, demonstrating that their suppressive activity was impaired.
Fig. 4.
The adoptive transfer of recirculating CCR6+ Treg of the AireKO thymus fails to protect from multi-organ autoimmunity. A Experimental setup: Rag2KO recipients were adoptively transferred with CD4+CD25+ Treg-depleted CD45.1 WT splenocytes. Four weeks later, they were injected with splenic Treg or thymic CCR6+ Treg derived from AireWT or AireKO mice. Three weeks after Treg adoptive transfer, peripheral tissues were examined for immune infiltration by histology and quantified by flow cytometry. Rag2KO recipients injected only with CD4+CD25+ Treg-depleted CD45.1 WT splenocytes were used as controls. B-E Representative photographies of peripheral tissue sections derived from mice transferred with splenic Treg (B) or thymic CCR6+ Treg (D) and counterstained with hematoxylin/eosin. Diagrams represent organ infiltration levels by CD45.1 donor cells measured by flow cytometry upon splenic Treg (C) or thymic CCR6+ Treg (E) transfer. Infiltration levels were normalized to the infiltration observed in controls. Dark and light violet in diagram represent high and low infiltrations. Each diagram represents one individual mouse. Scale bar, 150 µm for (B), 500 µm for (D). F, G Sera from mice transferred with CCR6+ Treg were tested for the presence of autoantibodies (green) against pancreas (F) and salivary glands (G) of Rag2KO mice. Nuclei were counterstained with DAPI (blue). Secondary antibodies (II Abs) alone were used as controls. Scale bar, 1 mm. Histograms show mean fluorescence intensity for each condition. Data are derived from 2 to 3 independent experiments (n = 3–5 mice per group and per experiment). Bar graphs show mean ± SEM, ****p < 0.0001 using unpaired Student’s t test for (F, G)
Aire expression in the thymic stroma controls the suppressive signature of recirculating CCR6+ Treg
Since Aire expression is not restricted to mTEC but was also found in thymic B cells [9], we then investigated its respective contribution in the stromal and hematopoietic compartments to control the recirculation and suppressive properties of CCR6+ Treg. To determine the role of Aire in hematopoietic cells, we generated BM chimeras by reconstituting lethally irradiated CD45.1/2 WT recipients with either CD45.2 AireWT or AireKO BM cells (Fig. S5A). Six weeks later, AireKO BM chimeras did not show major defects in total CD19+B220+ B cells, neither in the IgD− and IgD+ B cell subsets, both described to express Aire [9] (Fig. S5B, C). Overall, frequencies and numbers of CD25+ TregP, Foxp3lo TregP, CCR6− and CCR6+ mature CD25+Foxp3+ Treg were also normal (Fig. S5D, E). We next cell-sorted CCR6+ Treg from the thymus of these BM chimeras and analyzed their suppressive signature. Recirculating Treg from both chimeras exhibited a similar expression of suppressive genes that was altered in CCR6+ Treg of AireKO mice (Fig. S5F). Thus, Aire expression in thymic B cells is unlikely involved in Treg development, recirculation and suppressive signature.
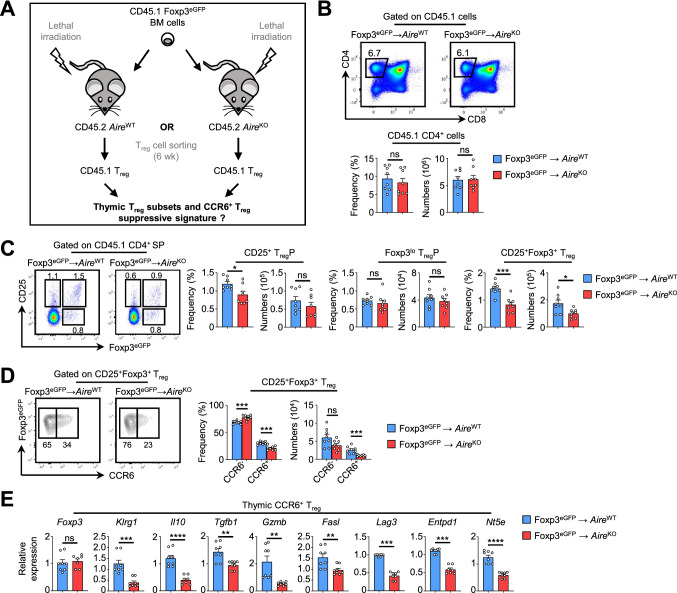
We then analyzed whether Aire expression in stromal cells controls CCR6+ Treg functional suppressive properties. To this end, we generated BM chimeras in which lethally irradiated CD45.2 AireWT or AireKO mice were reconstituted with CD45.1 Foxp3eGFP BM cells (Fig. 5A). Six weeks later, similar numbers of CD4+ SP thymocytes of CD45.1 donor origin were observed in AireWT and AireKO recipients (Fig. 5B). Although numbers of CD25+ TregP and Foxp3lo TregP were also similar in both groups, frequencies and numbers of mature CD25+Foxp3+ Treg were specifically reduced in AireKO chimeras (Fig. 5C). This defect was attributable to diminished frequencies and numbers of CCR6+ Treg (Fig. 5D). Recirculating CCR6+ mature CD25+Foxp3+ Treg of CD45.1 origin were then cell-sorted from the thymus of both chimeras and analyzed for the expression of several genes associated with Treg effector functions. Whereas Foxp3 level was normal, the expression of Klrg1, Il10, Tgfb1, Gzmb, Fasl, Lag3, Entpd1 and Nt5e was reduced in AireKO chimeras as compared to control chimeras (Fig. 5E). Altogether, these results show that whereas Aire in hematopoietic cells is dispensable, its specific expression in stromal cells controls both recirculation and suppressive properties of CCR6+ Treg.
Fig. 5.
Aire deficiency in the thymic stroma impairs the recirculation and the suppressive signature of CCR6+ Treg. A Experimental setup: lethally irradiated CD45.2 AireWT or AireKO recipients were reconstituted with BM cells from CD45.1 Foxp3eGFP mice. Six weeks later, thymic Treg subsets of CD45.1 origin were analyzed by flow cytometry. CCR6+ Treg were cell-sorted to measure the expression levels of genes associated with their suppressive functions. B Flow cytometry profiles, frequencies and numbers of CD4+ SP thymocytes of CD45.1 origin in the thymus of BM chimeric mice. C, D Flow cytometry profiles and numbers of CD25+ TregP, Foxp3lo TregP and total CD25+Foxp3+ cells (C) as well as of CCR6− and CCR6+ cells in CD25+Foxp3+ Treg (D) of CD45.1 origin in the thymus of BM chimeras. Data are derived from 2 independent experiments (n = 4 mice per group and per experiment). E The expression level of Foxp3, Klrg1, Il10, Tgfb1, Gzmb, Fasl, Lag3, Entpd1 and Nt5e was measured by qPCR in purified CCR6+CD25+Foxp3+ Treg from CD45.1 Foxp3eGFP → AireWT (n = 8) and CD45.1 Foxp3eGFP → AireKO (n = 9) chimeras. Data are derived from 2 independent experiments (n = 4–5 mice per group and per experiment). Bar graphs show mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 using unpaired Student’s t test for (B-D) and two-tailed Mann–Whitney test for (E)
Antigen-specific restimulation of recirculating Treg by Aire+ mTEC
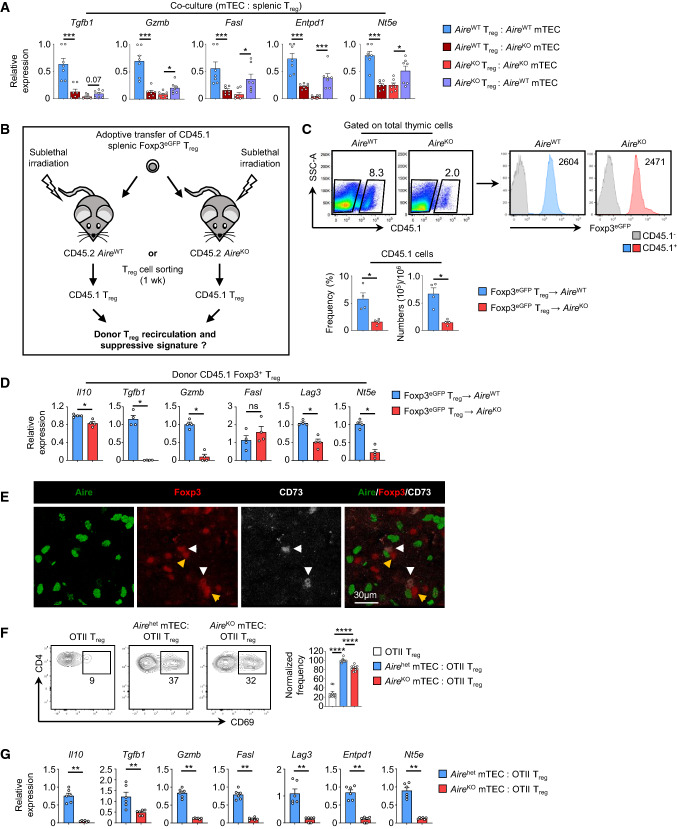
To further decipher the impact of Aire-expressing mTEC in the suppressive signature of recirculating Treg, we used an in vitro co-culture setup of peripheral Treg and mTEC purified from AireWT or AireKO mice. Compared with AireWT splenic Treg co-cultured with AireWT mTEC, the expression of Tgfb1, Gzmb, Fasl, Entpd1 and Nt5e was reduced in AireWT splenic Treg co-cultured with AireKO mTEC (Fig. 6A). Moreover, AireKO splenic Treg co-cultured with AireWT mTEC upregulated the expression of these genes in contrast to AireKO splenic Treg co-cultured with AireKO mTEC. Thus, as compared to their AireWT counterparts, AireKO mTEC failed to enhance suppressive signatures of both AireWT and AireKO splenic Treg.
Fig. 6.
Aire+ mTEC control the activation and suppressive signature of recirculating Treg through antigen-dependent contact. A The expression level of Tgfb1, Gzmb, Fasl, Entpd1 and Nt5e was measured by qPCR in splenic Treg from AireWT or AireKO mice co-cultured with AireWT or AireKO mTEC. Data are derived from 2 independent experiments. B Experimental setup: purified splenic Treg from CD45.1 Foxp3eGFP mice were adoptively transferred i.v. into sublethally irradiated CD45.2 AireWT or AireKO recipients. Adoptively transferred CD45.1 Foxp3eGFP Treg were cell-sorted from the thymus of recipient mice one week later and their suppressive signature was analyzed by qPCR. C Flow cytometry profiles, frequencies and numbers of CD45.1 Foxp3eGFP donor Treg observed in the thymus of CD45.2 AireWT or AireKO recipients. D The expression level of Il10, Tgfb1, Gzmb, Fasl, Lag3 and Nt5e was measured by qPCR in CD45.1 Foxp3eGFP donor Treg transferred into AireWT (n = 4) or AireKO (n = 4) recipients. E Representative images of WT thymic sections stained for Aire (green), Foxp3 (red) and CD73 (white). Yellow and white arrowheads denote developing CD73− and recirculating CD73+ Treg, respectively. Scale bar, 30 µm. F Flow cytometry profiles of CD69 activation of splenic OTII Treg from Rip-mOVA x OTII x Rag2KO mice co-cultured with OVA323-339-loaded Airehet (AireeGFP/WT) or AireKO (AireeGFP/eGFP) mTEChi after 24 h later. The histogram shows the frequencies of CD69+ Treg normalized to the activation of OTII Treg co-cultured with Airehet mTEChi. G The expression level of Il10, Tgfb1, Gzmb, Fasl, Lag3, Entpd1 and Nt5e was measured by qPCR in splenic OTII Treg from Rip-mOVA x OTII x Rag2KO mice co-cultured with OVA323-339-loaded Airehet (AireeGFP/WT; n = 6) or AireKO (AireeGFP/eGFP; n = 6) mTEChi. Data are derived from 2 independent experiments. Bar graphs show mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 using two-tailed Mann–Whitney test for (A, C, D, F, G)
We then analyzed the in vivo effect of the AireKO thymic stroma in regulating the suppressive signature of peripheral WT Treg. To this end, splenic Treg purified from CD45.1 Foxp3eGFP congenic mice were adoptively transferred into either AireWT or AireKO recipients (Fig. 6B). One week later, we observed reduced frequencies and numbers of donor CD45.1 Foxp3eGFP Treg in the thymus of AireKO mice as compared to AireWT mice (Fig. 6C). Moreover, the remaining donor Foxp3eGFP Treg that recirculated back to the thymus exhibited an impaired suppressive signature (Fig. 6D). These results not only confirm that Aire favors the recirculation of peripheral Treg in the thymus but also highlight its key role in enhancing their suppressive properties.
Thus, we made the hypothesis that recirculating Treg could be restimulated by Aire+ mTEC. To test this, WT thymic sections were first stained for Aire, Foxp3 and CD73, the latter being a reliable marker of recirculating Treg [1, 44]. Of note, anti-CCR6 antibody was not used in this experiment because it failed to give any signal in our hands. Interestingly, we observed that both developing CD73− and recirculating CD73+ Treg were found in close proximity to Aire+ mTEC (Fig. 6E). These results suggest that recirculating Treg could be restimulated by establishing antigen-specific contacts with Aire+ mTEC. To test this hypothesis, splenic OTII Treg from Rip-mOVA x OTII mice were co-cultured with OVA323-339-loaded Airehet (AireeGFP/WT) or AireKO (AireeGFP/eGFP) mTEChi (Fig. S6). When compared with unstimulated OVA-specific Treg, AireKO mTEChi were able to activate OTII Treg but to a lesser extent than Airehet mTEChi (Fig. 6F). These results demonstrate that Aire+ mTEC can stimulate peripheral Treg in an antigen-specific manner and this ability is impaired in the absence of Aire. Furthermore, as compared to OTII Treg co-cultured with Airehet mTEChi, the expression of Il10, Tgfb1, Gzmb, Fasl, Lag3, Entpd1 and Nt5e was reduced in OTII Treg co-cultured with AireKO mTEChi (Fig. 6G), indicating that this antigen-specific restimulation is much less efficient. We thus analyzed MHCII expression in mTEC from AireKO mice. In accordance with a previous study [45] and the pro-apoptotic role of Aire [46], we found increased frequencies of MHCII+ mTEC in these mice (Fig. S7A). We then made the hypothesis that costimulatory signals may be implicated in the phenotype observed. Two ligands of the Tumor Necrosis Factor Superfamily (TNFSF) have been described to be constitutively expressed by mTEC and participate in thymic Treg development [47]. Interestingly, we found that mTEChi expressed reduced levels of Tnfsf4 (OX40L, AireWT: 2.32 FPKM vs AireKO: 0.37) and Tnfsf18 (GITRL, AireWT: 2.92 vs AireKO: 0.81) in AireKO mice (Fig. S7B). This result was also confirmed at the protein level by flow cytometry (Fig. S7C). These observations are consistent with the reduced cellularity of Foxp3lo TregP and CD25+Foxp3+ Treg in AireKO mice (Fig. 2A–C). Further investigations are needed to decipher the role of these two TNFSF ligands in the biology of recirculating Treg in the thymus. Altogether, our data reveal that Aire+ mTEC, through antigen-specific restimulation, are responsible for the strong suppressive signature of recirculating Treg.
Discussion
Our study demonstrates that Aire expression by mTEC promotes the suppressive properties of recirculating CCR6+ Treg independently of the dynamic remodeling of the medullary 3D organization, where recirculating Treg reside. We previously described that the medulla of 6-week-old young adult mice is complex with a large central compartment surrounded by hundreds of individual islets [32]. Nevertheless, determining whether this topology varies throughout life and whether Aire is implicated remained open issues. Interestingly, we found that 9-day- and 6-week-old WT mice show ~ 1000 and ~ 400 medullary islets, respectively. Given that individual islets arise from a single progenitor [48], these observations suggest that during thymic development, they grow and fuse together, leading to a reduced number of islets in young adult mice as compared to neonates. In 1-year-old mice, the number of medullary islets increases to reach ~ 1000 islets, as observed in neonates. Considering that the medulla topology is governed by crosstalk with autoreactive CD4+ thymocytes [32, 49–52], the high islet number observed in aged mice could be due to a suboptimal cellular crosstalk due to reduced cellularity of CD4+ thymocytes linked to age-related thymic involution. Although Aire plays multiple roles in T-cell tolerance induction [34], our results show that it does not shape the 3D organization of the thymic medulla. This is consistent with the fact that the clonal deletion of autoreactive CD4+ thymocytes is impaired in AireKO mice [45, 53], which consequently leads to an effective medulla organization.
Several studies have shown that mTEC are implicated in Foxp3+ Treg development [54–56]. Nevertheless, the specific role of Aire in thymic Treg heterogeneity throughout life remained to be defined. Interestingly, we found that Aire controls the cellularity of Foxp3lo TregP and mature CD25+Foxp3+ Treg in 5-day-, 6-week- and 1-year-old mice. This is illustrated by decreased numbers of Foxp3lo TregP and CD25+Foxp3+ Treg in AireKO mice, which could not be due to impaired medulla organization since no defect was observed at this level. Given that CD25+ TregP and Foxp3lo TregP show distinct developmental pathways that give rise to CD25+Foxp3+ Treg with non-overlapping regulatory activities [1], our results provide new insights in the role of Aire in the emergence of Foxp3lo TregP. Furthermore, Aire regulates the pool of recirculating CCR6+ Treg throughout life. Interestingly, CCR6+ Treg from AireKO mice expressed reduced levels of several genes associated with their polarization and suppressive functions. Importantly, they express normal levels of Foxp3, indicating that they remain engaged in the Treg cell lineage. In accordance with the defective suppressive signature of CCR6+ Treg from AireKO mice, we found that the adoptive transfer of these cells failed to attenuate the severity of multiorgan autoimmunity. In contrast to their thymic counterparts, splenic Treg of AireKO mice show a protection similar to AireWT splenic Treg, consistently with their normal suppressive signature. Altogether, these results indicate that Aire is crucial for the suppressive functions of recirculating CCR6+ Treg in the thymus.
Although Aire is expressed by a subset of recirculating thymic B cells [9], we found that its absence in hematopoietic cells had no impact neither in Treg development nor in CCR6+ Treg recirculation and suppressive signature. Considering the weak expression of Aire in thymic B cells as compared to mTEC, it is not surprising that Aire deficiency in hematopoietic cells does not control the cellularity and suppressive signature of CCR6+ Treg. In marked contrast, beyond controlling the recirculation of CCR6+ Treg, BM chimeras in AireKO recipients revealed that Aire expression in stromal cells is responsible for their highly suppressive phenotype.
Interestingly, recirculating Foxp3+ Treg were observed in close proximity to Aire+ mTEC, similarly to developing Foxp3+ Treg. The reduced expression of Ccl20 and Cxcl12, likely responsible for the lower amount of recirculating Treg in these mice [5, 8], could contribute to a lesser stimulation of recirculating Treg by mTEC. However, in vitro co-culture experiments suggest that defective chemoattraction is unlikely responsible for the phenotype observed in AireKO mice. Furthermore, in vitro co-culture assays revealed that Aire+ mTEC were capable to activate peripheral Treg in an antigen-specific manner. Our results suggest that peripheral Treg could regulate de novo Treg development not only by competing for IL-2 [5, 57] but also for the cognate self-antigen. In the absence of Aire, in vitro co-culture assays and in vivo adoptive transfer experiments demonstrate that recirculating Treg activation and suppressive signature were altered. Therefore, our results unravel that Aire+ mTEC control the activated and differentiated phenotype of recirculating Treg upon their entry into the thymus. A possible explanation could be that Aire controls co-stimulation signals that are responsible for maintaining the effector phenotype of recirculating Treg. In addition, Aire could also modulate indirectly CCR6+ Treg suppressive properties through other mechanisms such as the medullary positioning of XCR1+ type 1 conventional dendritic cells by controlling the production of the chemokine XCL1 [11]. Moreover, further investigations are required to determine the fate of CCR6+ Treg in the thymic medulla. Three possibilities can be envisaged: CCR6+ Treg (1) become long-term resident cells, (2) migrate back to the periphery or (3) die by apoptosis.
This study ameliorates our understanding on recirculating Treg in the thymus, which remain poorly described to date. In summary, it identifies that Aire controls the suppressive properties of recirculating CCR6+ Treg in the thymus. It also assigns a new role for Aire in conferring to mTEC the aptitude to restimulate recirculating Treg. Thus, this study furthers our understanding on the mechanisms allowing recirculating Treg to fine-tune de novo Treg production. Finally, our results are expected to contribute to a better understanding of Treg deficiencies observed in the human pathology APECED.
Supplementary Information
Below is the link to the electronic supplementary material.
Movie S1. 3D rotation of AireWT thymic lobe of 9-day-old mouse, with medullary compartment colored according to their volume. AireWT thymic lobe (DAPI, blue) of a 9-day-old mouse is rendered in 3D with medullary compartments (Keratin 14, pseudo-colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Movie S2. 3D rotation of AireKO thymic lobe of 9-day-old mouse, with medullary compartment colored according to their volume. AireKO thymic lobe (DAPI, blue) of a 9-day-old mouse is rendered in 3D with medullary compartments (Keratin 14, pseudo-colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Movie S3. 3D rotation of AireWT thymic lobe of 6-week-old mouse, with medullary compartment colored according to their volume. AireWT thymic lobe (DAPI, blue) of a 6-week-old mouse is rendered in 3D with medullary compartments (Keratin 14, pseudo-colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Movie S4. 3D rotation of AireKO thymic lobe of 6-week-old mouse, with medullary compartment colored according to their volume. AireKO thymic lobe (DAPI, blue) of a 6-week-old mouse is rendered in 3D with medullary compartments (Keratin 14, pseudo-colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Movie S5. 3D rotation of AireWT thymic lobe of 1-year-old mouse, with medullary compartment colored according to their volume. AireWT thymic lobe (DAPI, blue) of a 1-year-old mouse is rendered in 3D with medullary compartments (Keratin 14, pseudo-colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Movie S6. 3D rotation of AireKO thymic lobe of 1-year-old mouse, with medullary compartment colored according to their volume. AireKO thymic lobe (DAPI, blue) of a 1-year-old mouse is rendered in 3D with medullary compartments (keratin 14, pseudo colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Fig. S1 Gating strategy used to purify recirculating CCR6+ Treg in the thymus. CD4+CD25+ cells were identified in CCR6+CD4+ T cells and analyzed for Foxp3 expression by flow cytometry.
Fig. S2 The suppressive signature of thymic CCR6+ Treg from 1-year-old AireKO mice is altered. The expression level of Foxp3, Klrg1, Il10, Gzmb, Fasl, Entpd1 and Nt5e was measured by qPCR in thymic CCR6+ Treg from 1-year-old AireWT (n=5-6) and AireKO (n=9) mice. Bar graphs show mean ± SEM, ns>0.05, ***p<0.001, ****p<0.0001 using two-tailed Mann–Whitney test.
Fig. S3 Splenic AireKO Treg show a normal suppressive signature throughout life. A, B Flow cytometry profiles, frequencies and numbers of CD4+Foxp3+ Treg in the blood of 6-week- (A) and 1-year-old (B) AireWT and AireKO mice. C, D Flow cytometry profiles, frequencies and numbers of CD4+Foxp3+ Treg in the spleen of 6-week- (C) and 1-year-old (D) AireWT or AireKO mice. Data are derived from 2 independent experiments (n=2-5 mice per group and per experiment). E, F The expression level of Foxp3, Il10, Tgfb1, Gzmb, Fasl, Lag3, Entpd1 and Nt5e was measured by qPCR in splenic Treg from 6-week- (E) and 1-year- (F) old AireWT (n=4-9 for 6 wk and n=8-13 for 1 yr) and AireKO (n=5-9 for 6 wk and n=8-13 for 1 yr) mice. Bar graphs show mean ± SEM, *p<0.05 and **p<0.01 using unpaired Student’s t test for C, D.
Fig. S4 The adoptive transfer of thymic CCR6+ Treg from AireKO mice fail to attenuate peripheral tissue infiltration. A Gating strategy used to sort CCR6+CD4+CD8-CD25+ cells, corresponding to CCR6+ Treg from the thymus of 6-week-old AireWT and AireKO mice. B Flow cytometry profiles and numbers of CD45.2 donor Treg in inguinal lymph nodes. C Flow cytometry profiles, frequencies and numbers of CD45.1 infiltrating cells in the pancreas, eyes and salivary glands. D Flow cytometry profiles and numbers of CD4+ and CD8+ T cells of CD45.1 origin infiltrating the pancreas, eyes and salivary glands. Data are derived from 3 independent experiments (n=2-5 mice per group and per experiment). Bar graphs show mean ± SEM, *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001 using two-tailed Mann–Whitney test for B or using unpaired Student’s t test for C,D.
Fig. S5 Aire expression in hematopoietic cells does not control the recirculation and suppressive signature of thymic CCR6+ Treg. A Experimental setup: Lethally irradiated CD45.1/2 WT recipients were reconstituted with CD45.2 AireWT or AireKO BM cells. Six weeks later, the recirculation and the suppressive signature of thymic CCR6+ Treg of CD45.2 origin were analyzed by flow cytometry and qPCR, respectively. B,C Flow cytometry profiles, frequencies and numbers of total B220+CD19+ B cells (B) and of IgD- or IgD+ cells in B220+CD19+ B cells (C). D,E Flow cytometry profiles, frequencies and numbers of CD25+ TregP, Foxp3lo TregP and CD25+Foxp3+ Treg (D) as well as CCR6- and CCR6+ cells in total CD25+Foxp3+ Treg (E). F The expression level of Foxp3, Klrg1, Il10, Gzmb, Fasl, Lag3, Entpd1 and Nt5e was measured by qPCR in CCR6+ Treg of CD45.2 origin purified from the thymus of AireWT (n=8) and AireKO (n=8) BM chimeric mice. Data are derived from 2 independent experiments (n=4 mice per group and per experiment). Bar graphs show mean ± SEM, *p<0.05 using two-tailed Mann–Whitney test for B, D.
Fig. S6 Gating strategy used to purify Aire+ mTEChi. Aire+ mTEChi were identified as EpCAM+Ly51-/loCD80+AireeGFP cells and purified from Airehet (AireeGFP/WT) and AireKO (AireeGFP/eGFP) mice.
Fig. S7 AireKO mTEC express reduced levels of OX40L and GITRL. A Representative flow cytometry profiles of MHCII in mTEC from AireWT and AireKO mice. The histogram shows the frequency of MCHII+ mTEC. Bar graphs show mean ± SEM, **p<0.001 using two-tailed Mann–Whitney. B-C AireKO mTEC express reduced levels of OX40L and GITRL. Expression levels of Tnfsf4 (OX40L) and Tnfsf18 (GITRL) measured by RNA-seq (B) and flow cytometry (C) in AireWT and AireKO mTEChi. Bar graphs show mean ± SEM, **p<0.01 using two-tailed Mann–Whitney test for A.
Table S1. FPKM values of RNA-seq data derived from thymic CCR6+ Treg from AireWT and AireKO mice.
Table S2. List of antibodies used for flow cytometry.
Table S3. List of primers used for RT-qPCR.
Acknowledgements
We are grateful to Georg Holländer (University of Basel, Switzerland) and Bernard Malissen (CIML, Marseille, France) for providing us AireKO and Foxp3eGFP mice, respectively. We thank the CIML flow cytometry, histology, PICSL imaging facility of the CIML (ImagImm) and animal facility platforms for technical support. We thank Cloé Zamit (CIML, France) for help with mouse genotyping.
Author contributions
JC, AB, JCS, LC and MI conducted the experiments, analyzed and interpreted the data. MG and AS analyzed the data. JC, AB, JCS and MI wrote the manuscript. MI initiated, supervised and conceived the study.
Funding
This work was supported by institutional grants from INSERM, CNRS and Aix-Marseille Université. The Immune Tolerance and T-Cell Differentiation laboratory received funding from the ARC Foundation (PJA20171206491 to M.I.), CoPoC-proof of concept (MAT-PI-17326-A-01 to M.I.), a prematuration grant from A*MIDEX, a French “Investissements d'avenir” program (LTalpha-Treg to M.I.) and Agence Nationale de la Recherche (grant ANR-19-CE18-0021–01, RANKLthym to M.I.). We also acknowledge financial support from France Bio Imaging (ANR-10-INBS-04–01) and France Génomique national infrastructure, funded as part of the "Investissements d'Avenir" program managed by the ANR (ANR-10-INBS-0009). J.C. and A.B. were supported by a PhD fellowship from the Ministère de l’Enseignement Supérieur et de la Recherche et de l’Innovation (MESRI).
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. The dataset generated in this study are available in the Gene Expression Omnibus (GEO) database under accession number GSE188419.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments were done in accordance with National and European laws for laboratory animal welfare (EEC Council Directive 2010/63/UE) and the Marseille Ethical Committee for Animal experimentation.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jonathan Charaix, Alexia Borelli have contributed equally to this work.
References
- 1.Owen DL, Mahmud SA, Sjaastad LE, Williams JB, Spanier JA, Simeonov DR, et al. Thymic regulatory T cells arise via two distinct developmental programs. Nat Immunol. 2019;20(2):195–205. doi: 10.1038/s41590-018-0289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28(1):100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santamaria JC, Borelli A, Irla M. Regulatory T cell heterogeneity in the thymus: impact on their functional activities. Front Immunol. 2021;12:643153. doi: 10.3389/fimmu.2021.643153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall D, Sinclair C, Tung S, Seddon B. Differential requirement for IL-2 and IL-15 during bifurcated development of thymic regulatory T cells. J Immunol. 2014;193(11):5525–5533. doi: 10.4049/jimmunol.1402144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiault N, Darrigues J, Adoue V, Gros M, Binet B, Perals C, et al. Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat Immunol. 2015;16:628–634. doi: 10.1038/ni.3150. [DOI] [PubMed] [Google Scholar]
- 6.Cowan JE, McCarthy NI, Anderson G. CCR7 controls thymus recirculation, but not production and emigration, of Foxp3(+) T cells. Cell Rep. 2016;14(5):1041–1048. doi: 10.1016/j.celrep.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang E, Zou T, Leichner TM, Zhang SL, Kambayashi T. Both retention and recirculation contribute to long-lived regulatory T-cell accumulation in the thymus. Eur J Immunol. 2014;44(9):2712–2720. doi: 10.1002/eji.201444529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowan JE, Baik S, McCarthy NI, Parnell SM, White AJ, Jenkinson WE, et al. Aire controls the recirculation of murine Foxp3(+) regulatory T-cells back to the thymus. Eur J Immunol. 2018;48(5):844–854. doi: 10.1002/eji.201747375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamano T, Nedjic J, Hinterberger M, Steinert M, Koser S, Pinto S, et al. Thymic B cells are licensed to present self antigens for central T cell tolerance induction. Immunity. 2015;42:1048–1061. doi: 10.1016/j.immuni.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Aricha R, Feferman T, Scott HS, Souroujon MC, Berrih-Aknin S, Fuchs S. The susceptibility of Aire(-/-) mice to experimental myasthenia gravis involves alterations in regulatory T cells. J Autoimmun. 2011;36(1):16–24. doi: 10.1016/j.jaut.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med. 2011;208(2):383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malchow S, Leventhal DS, Lee V, Nishi S, Socci ND, Savage PA. Aire enforces immune tolerance by directing autoreactive T cells into the regulatory T cell lineage. Immunity. 2016;44(5):1102–1113. doi: 10.1016/j.immuni.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348(6234):589–594. doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298(5597):1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 15.Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202(6):805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuroda N, Mitani T, Takeda N, Ishimaru N, Arakaki R, Hayashi Y, et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005;174(4):1862–1870. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 17.Ramsey C, Winqvist O, Puhakka L, Halonen M, Moro A, Kämpe O, et al. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11(4):397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- 18.Consortium. F-GA An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17(4):399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 19.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17(4):393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 20.Ryan KR, Lawson CA, Lorenzi AR, Arkwright PD, Isaacs JD, Lilic D. CD4+CD25+ T-regulatory cells are decreased in patients with autoimmune polyendocrinopathy candidiasis ectodermal dystrophy. J Allergy Clin Immunol. 2005;116(5):1158–1159. doi: 10.1016/j.jaci.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 21.Kekäläinen E, Tuovinen H, Joensuu J, Gylling M, Franssila R, Pöntynen N, et al. A defect of regulatory T cells in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Immunol. 2007;178(2):1208–1215. doi: 10.4049/jimmunol.178.2.1208. [DOI] [PubMed] [Google Scholar]
- 22.Laakso SM, Laurinolli TT, Rossi LH, Lehtoviita A, Sairanen H, Perheentupa J, et al. Regulatory T cell defect in APECED patients is associated with loss of naive FOXP3(+) precursors and impaired activated population. J Autoimmun. 2010;35(4):351–357. doi: 10.1016/j.jaut.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Sansom SN, Shikama-Dorn N, Zhanybekova S, Nusspaumer G, Macaulay IC, Deadman ME, et al. Population and single-cell genomics reveal the aire dependency, relief from polycomb silencing, and distribution of self-antigen expression in thymic epithelia. Genome Res. 2014;24(12):1918–1931. doi: 10.1101/gr.171645.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68(5):855–867. doi: 10.1016/0092-8674(92)90029-C. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Kissenpfennig A, Mingueneau M, Richelme S, Perrin P, Chevrier S, et al. Th2 lymphoproliferative disorder of LatY136F mutant mice unfolds independently of TCR-MHC engagement and is insensitive to the action of Foxp3+ regulatory T cells. J Immunol. 2008;180(3):1565–1575. doi: 10.4049/jimmunol.180.3.1565. [DOI] [PubMed] [Google Scholar]
- 26.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76(1):34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 27.Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184(3):923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5):511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol. 2013;31(1):46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavlidis P, Noble WS. Matrix2png: a utility for visualizing matrix data. Bioinformatics. 2003;19(2):295–296. doi: 10.1093/bioinformatics/19.2.295. [DOI] [PubMed] [Google Scholar]
- 32.Irla M, Guenot J, Sealy G, Reith W, Imhof BA, Serge A. Three-dimensional visualization of the mouse thymus organization in health and immunodeficiency. J Immunol. 2013;190(2):586–596. doi: 10.4049/jimmunol.1200119. [DOI] [PubMed] [Google Scholar]
- 33.Serge A, Bailly AL, Aurrand-Lions M, Imhof BA, Irla M. For3D: full organ reconstruction in 3D, an automatized tool for deciphering the complexity of lymphoid organs. J Immunol Methods. 2015;424:32–42. doi: 10.1016/j.jim.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Perniola R. Twenty Years of AIRE Front Immunol. 2018;9:98. doi: 10.3389/fimmu.2018.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klug DB, Carter C, Crouch E, Roop D, Conti CJ, Richie ER. Interdependence of cortical thymic epithelial cell differentiation and T-lineage commitment. Proc Natl Acad Sci USA. 1998;95(20):11822–11827. doi: 10.1073/pnas.95.20.11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202(7):901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng G, Yuan X, Tsai MS, Podack ER, Yu A, Malek TR. IL-2 receptor signaling is essential for the development of Klrg1+ terminally differentiated T regulatory cells. J Immunol. 2012;189(4):1780–1791. doi: 10.4049/jimmunol.1103768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cretney E, Kallies A, Nutt SL. Differentiation and function of Foxp3(+) effector regulatory T cells. Trends Immunol. 2013;34(2):74–80. doi: 10.1016/j.it.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40(4):569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4(12):1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 41.Garín MI, Chu CC, Golshayan D, Cernuda-Morollón E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109(5):2058–2065. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 42.Frias AB, Jr, Hyzny EJ, Buechel HM, Beppu LY, Xie B, Jurczak MJ, et al. The transcriptional regulator Id2 is critical for adipose-resident regulatory T cell differentiation, survival, and function. J Immunol. 2019;203(3):658–664. doi: 10.4049/jimmunol.1900358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawant DV, Vignali DA. Once a Treg, always a Treg? Immunol Rev. 2014;259(1):173–191. doi: 10.1111/imr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peligero-Cruz C, Givony T, Sebé-Pedrós A, Dobeš J, Kadouri N, Nevo S, et al. IL18 signaling promotes homing of mature Tregs into the thymus. Elife. 2020;9:e58213. doi: 10.7554/eLife.58213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23(2):227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007;204(11):2521–2528. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahmud SA, Manlove LS, Schmitz HM, Xing Y, Wang Y, Owen DL, et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat Immunol. 2014;15(5):473–481. doi: 10.1038/ni.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodewald HR, Paul S, Haller C, Bluethmann H, Blum C. Thymus medulla consisting of epithelial islets each derived from a single progenitor. Nature. 2001;414(6865):763–768. doi: 10.1038/414763a. [DOI] [PubMed] [Google Scholar]
- 49.Irla M, Guerri L, Guenot J, Serge A, Lantz O, Liston A, et al. Antigen recognition by autoreactive cd4(+) thymocytes drives homeostasis of the thymic medulla. PLoS ONE. 2012;7(12):e52591. doi: 10.1371/journal.pone.0052591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopes N, Serge A, Ferrier P, Irla M. Thymic crosstalk coordinates medulla organization and T-cell tolerance induction. Front Immunol. 2015;6:365. doi: 10.3389/fimmu.2015.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopes N, Boucherit N, Santamaria JC, Provin N, Charaix J, Ferrier P, et al. Thymocytes trigger self-antigen-controlling pathways in immature medullary thymic epithelial stages. Elife. 2022;11:e69982. doi: 10.7554/eLife.69982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borelli A, Irla M. Lymphotoxin: from the physiology to the regeneration of the thymic function. Cell Death Differ. 2021;28(8):2305–2314. doi: 10.1038/s41418-021-00834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4(4):350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 54.Cowan JE, Parnell SM, Nakamura K, Caamano JH, Lane PJ, Jenkinson EJ, et al. The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. J Exp Med. 2013;210(4):675–681. doi: 10.1084/jem.20122070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8(4):351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 56.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339(6124):1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weist BM, Kurd N, Boussier J, Chan SW, Robey EA. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat Immunol. 2015;16(6):635–641. doi: 10.1038/ni.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. 3D rotation of AireWT thymic lobe of 9-day-old mouse, with medullary compartment colored according to their volume. AireWT thymic lobe (DAPI, blue) of a 9-day-old mouse is rendered in 3D with medullary compartments (Keratin 14, pseudo-colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Movie S2. 3D rotation of AireKO thymic lobe of 9-day-old mouse, with medullary compartment colored according to their volume. AireKO thymic lobe (DAPI, blue) of a 9-day-old mouse is rendered in 3D with medullary compartments (Keratin 14, pseudo-colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Movie S3. 3D rotation of AireWT thymic lobe of 6-week-old mouse, with medullary compartment colored according to their volume. AireWT thymic lobe (DAPI, blue) of a 6-week-old mouse is rendered in 3D with medullary compartments (Keratin 14, pseudo-colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Movie S4. 3D rotation of AireKO thymic lobe of 6-week-old mouse, with medullary compartment colored according to their volume. AireKO thymic lobe (DAPI, blue) of a 6-week-old mouse is rendered in 3D with medullary compartments (Keratin 14, pseudo-colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Movie S5. 3D rotation of AireWT thymic lobe of 1-year-old mouse, with medullary compartment colored according to their volume. AireWT thymic lobe (DAPI, blue) of a 1-year-old mouse is rendered in 3D with medullary compartments (Keratin 14, pseudo-colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Movie S6. 3D rotation of AireKO thymic lobe of 1-year-old mouse, with medullary compartment colored according to their volume. AireKO thymic lobe (DAPI, blue) of a 1-year-old mouse is rendered in 3D with medullary compartments (keratin 14, pseudo colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Fig. S1 Gating strategy used to purify recirculating CCR6+ Treg in the thymus. CD4+CD25+ cells were identified in CCR6+CD4+ T cells and analyzed for Foxp3 expression by flow cytometry.
Fig. S2 The suppressive signature of thymic CCR6+ Treg from 1-year-old AireKO mice is altered. The expression level of Foxp3, Klrg1, Il10, Gzmb, Fasl, Entpd1 and Nt5e was measured by qPCR in thymic CCR6+ Treg from 1-year-old AireWT (n=5-6) and AireKO (n=9) mice. Bar graphs show mean ± SEM, ns>0.05, ***p<0.001, ****p<0.0001 using two-tailed Mann–Whitney test.
Fig. S3 Splenic AireKO Treg show a normal suppressive signature throughout life. A, B Flow cytometry profiles, frequencies and numbers of CD4+Foxp3+ Treg in the blood of 6-week- (A) and 1-year-old (B) AireWT and AireKO mice. C, D Flow cytometry profiles, frequencies and numbers of CD4+Foxp3+ Treg in the spleen of 6-week- (C) and 1-year-old (D) AireWT or AireKO mice. Data are derived from 2 independent experiments (n=2-5 mice per group and per experiment). E, F The expression level of Foxp3, Il10, Tgfb1, Gzmb, Fasl, Lag3, Entpd1 and Nt5e was measured by qPCR in splenic Treg from 6-week- (E) and 1-year- (F) old AireWT (n=4-9 for 6 wk and n=8-13 for 1 yr) and AireKO (n=5-9 for 6 wk and n=8-13 for 1 yr) mice. Bar graphs show mean ± SEM, *p<0.05 and **p<0.01 using unpaired Student’s t test for C, D.
Fig. S4 The adoptive transfer of thymic CCR6+ Treg from AireKO mice fail to attenuate peripheral tissue infiltration. A Gating strategy used to sort CCR6+CD4+CD8-CD25+ cells, corresponding to CCR6+ Treg from the thymus of 6-week-old AireWT and AireKO mice. B Flow cytometry profiles and numbers of CD45.2 donor Treg in inguinal lymph nodes. C Flow cytometry profiles, frequencies and numbers of CD45.1 infiltrating cells in the pancreas, eyes and salivary glands. D Flow cytometry profiles and numbers of CD4+ and CD8+ T cells of CD45.1 origin infiltrating the pancreas, eyes and salivary glands. Data are derived from 3 independent experiments (n=2-5 mice per group and per experiment). Bar graphs show mean ± SEM, *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001 using two-tailed Mann–Whitney test for B or using unpaired Student’s t test for C,D.
Fig. S5 Aire expression in hematopoietic cells does not control the recirculation and suppressive signature of thymic CCR6+ Treg. A Experimental setup: Lethally irradiated CD45.1/2 WT recipients were reconstituted with CD45.2 AireWT or AireKO BM cells. Six weeks later, the recirculation and the suppressive signature of thymic CCR6+ Treg of CD45.2 origin were analyzed by flow cytometry and qPCR, respectively. B,C Flow cytometry profiles, frequencies and numbers of total B220+CD19+ B cells (B) and of IgD- or IgD+ cells in B220+CD19+ B cells (C). D,E Flow cytometry profiles, frequencies and numbers of CD25+ TregP, Foxp3lo TregP and CD25+Foxp3+ Treg (D) as well as CCR6- and CCR6+ cells in total CD25+Foxp3+ Treg (E). F The expression level of Foxp3, Klrg1, Il10, Gzmb, Fasl, Lag3, Entpd1 and Nt5e was measured by qPCR in CCR6+ Treg of CD45.2 origin purified from the thymus of AireWT (n=8) and AireKO (n=8) BM chimeric mice. Data are derived from 2 independent experiments (n=4 mice per group and per experiment). Bar graphs show mean ± SEM, *p<0.05 using two-tailed Mann–Whitney test for B, D.
Fig. S6 Gating strategy used to purify Aire+ mTEChi. Aire+ mTEChi were identified as EpCAM+Ly51-/loCD80+AireeGFP cells and purified from Airehet (AireeGFP/WT) and AireKO (AireeGFP/eGFP) mice.
Fig. S7 AireKO mTEC express reduced levels of OX40L and GITRL. A Representative flow cytometry profiles of MHCII in mTEC from AireWT and AireKO mice. The histogram shows the frequency of MCHII+ mTEC. Bar graphs show mean ± SEM, **p<0.001 using two-tailed Mann–Whitney. B-C AireKO mTEC express reduced levels of OX40L and GITRL. Expression levels of Tnfsf4 (OX40L) and Tnfsf18 (GITRL) measured by RNA-seq (B) and flow cytometry (C) in AireWT and AireKO mTEChi. Bar graphs show mean ± SEM, **p<0.01 using two-tailed Mann–Whitney test for A.
Table S1. FPKM values of RNA-seq data derived from thymic CCR6+ Treg from AireWT and AireKO mice.
Table S2. List of antibodies used for flow cytometry.
Table S3. List of primers used for RT-qPCR.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files. The dataset generated in this study are available in the Gene Expression Omnibus (GEO) database under accession number GSE188419.
Not applicable.