Abstract
Alterations in the vascular smooth muscle cells (VSMC) phenotype play a critical role in the pathogenesis of several cardiovascular diseases, including hypertension, atherosclerosis, and restenosis after angioplasty. MicroRNAs (miRNAs) are a class of endogenous noncoding RNAs (approximately 19–25 nucleotides in length) that function as regulators in various physiological and pathophysiological events. Recent studies have suggested that aberrant miRNAs’ expression might underlie VSMC phenotypic transformation, appearing to regulate the phenotypic transformations of VSMCs by targeting specific genes that either participate in the maintenance of the contractile phenotype or contribute to the transformation to alternate phenotypes, and affecting atherosclerosis, hypertension, and coronary artery disease by altering VSMC proliferation, migration, differentiation, inflammation, calcification, oxidative stress, and apoptosis, suggesting an important regulatory role in vascular remodeling for maintaining vascular homeostasis. This review outlines recent progress in the discovery of miRNAs and elucidation of their mechanisms of action and functions in VSMC phenotypic regulation. Importantly, as the literature supports roles for miRNAs in modulating vascular remodeling and for maintaining vascular homeostasis, this area of research will likely provide new insights into clinical diagnosis and prognosis and ultimately facilitate the identification of novel therapeutic targets.
Keywords: miRNA, VSMC, Phenotypic transformation, Contractile phenotype, Synthetic phenotype
Introduction
Blood vessels play a crucial role in maintaining the normal physiological functions of an organism and that regulates vascular remodeling, which alters the vascular configuration. Vascular activities are involved in communication between vascular smooth muscle cells (VSMCs) and other vascular cells. Upon exposure to pathophysiological stimuli, VSMC proliferation and migration are core processes in vascular remodeling and influenced by growth factors and signaling networks [1]. Vascular remodeling is characterized by the aggregation of VSMCs in intima and can change their phenotype in response to environmental cues. VSMC within the walls of blood vessels exhibit marked phenotypic plasticity characterized by transformation between differentiated (contractile) and proliferative (synthetic) phenotypes [2, 3]. Herein, we realize that the word synthetic is outdated, but that they mean by this term all VSMC phenotypes that are non-contractile phenotypes, which VSMC can acquire upon different stimuli during physiological or pathological processes. Such transformations occur in response to both the physiological stimuli and the pathological processes that underlie a number of cardiovascular diseases (CVD), including hypertension, atherosclerosis, restenosis following angioplasty, aneurysm formation, and chronic obstructive pulmonary disease [4–6]. Phenotypic modulation is a prerequisite for the proliferation and migration of VSMC from the tunica media to the tunica intima of the arterial wall. The main manifestations of this process include the transformation of cells from a contractile to a synthetic phenotype, which is characterized by altered proliferation, migration, and synthetic capabilities and by the secretion of large quantities of extracellular matrix (ECM) components [7–10]. Studies have also shown that diabetic and cardiovascular disorders induce phenotypic transformation of VSMC changing from contractile/differentiated to synthetic/proliferative phenotypes and thus contributing to vascular remodeling. During this course, SMC-specific contractile genes including Smooth Muscle α-actin (SMA), Smooth muscle22alpha (SM22α), Smooth muscle-myosin heavy chain (SM-MHC), and Smoothelin-B levels are decreased and the expression levels of SMC-specific synthetic genes, including nonmuscle myosin heavy chain B (SMemb), CCND1 (cyclin D1), and CCND2 (cyclin D2) are increased [11, 12]. Synthetic VSMC display alterations in increased proliferation and migration, and VSMC dedifferentiation impairs proliferative and migratory capacity. Additionally, VSMC undergo the phenotypic changes from contractile to synthetic state possibly enabling the mobilization and proliferation during arteriogenesis, and are capable to differentiate into pericytes to coat around capillaries during angiogenesis, which is a key phenomenon that influences vascular disease with the expansion of new vascular networks from the existing vascular system in a budding or non-budding form. Moreover, the ability of vascular endothelial cell (VEC) to proliferate and to secrete growth factors, such as vascular endothelial-derived growth factor (VEGF) and platelet-derived growth factor (PDGF), is crucial for the new vascular network development and subsequent arterialization via recruitment of mural cells.
MicroRNAs (miRNAs) are endogenous, short (approximately 19–25 base pairs in length) noncoding RNAs that act as negative regulators of eukaryotic gene expression and can inhibit the translation of target mRNA molecules by binding to the 3′ untranslated regions (3′-UTRs) of target gene mRNAs via complementarity [13, 14]. Thus, miRNAs have been shown to play important roles in multiple physiological processes, such as developmental timing, apoptosis, fat metabolism, neuronal development, cell differentiation, hormone secretion, and the occurrence and development of multiple diseases [15–17]. Of direct relevance to this review, the previous studies have demonstrated that the phenotypic transformation of VSMC is accompanied by tissue-specific expression of a number of miRNAs [18–20]. Such miRNAs appear to regulate the phenotypic transformations of VSMC by targeting specific genes that either participate in the maintenance of the contractile phenotype or contribute to the transformation to alternate phenotypes and thereby affect cell proliferation, migration, hypertrophy, and differentiation. Several studies have confirmed that various biological factors, e.g., growth factors and transcription factors, including those that are specific targets of certain miRNAs, can affect the phenotypic transformation of VSMC [21–23]. The additional functional miRNAs pathways involved in miRNA-mediated VSMC phenotypic transformations and the mechanisms regulating gene expression have recently become an active focus of research. This review summarizes miRNAs that have been identified to be involved in the phenotypic transformation of VSMC and recent progress toward understanding the mechanisms and functions underlying this process. Specifically, we emphasize the gene expression regulatory networks through which miRNAs regulate the phenotypic transformation of VSMC and the mechanisms through which miRNAs act on target genes. Furthermore, the molecular regulatory mechanisms through which miRNAs mediate the phenotypic modulation of VSMC and the broad clinical applications of miRNAs in the phenotypic transformation of VSMC, such as novel therapeutic targets and pathways that could be used for the clinical diagnosis and treatment of CVD, are discussed.
miRNAs implicated in VSMC phenotypic transformation
Since the first miRNA (miR-21) regulating VSMC proliferation was identified by Ji and colleagues [24], numerous studies have provided evidence for the role of more than 20 additional functional miRNAs, including miR-1/133, miR-22, miR-221, miR-143/145, miR-195, miR-424/322, and miR-638. It has been demonstrated that the high level of miR-21 expression in mouse carotid artery wall is determined by balloon injury and that miR-21 inhibition reverses the mice vascular remodeling induced by balloon injury [24]. More concretely, miR-21 directly targets phosphatase and tensin homologs (PTEN) and B-cell lymphoma 2 (Bcl-2), thereby regulating the proliferation and apoptosis of VSMC [25–27]. In addition, miR-21 expression was found to be regulated by the bone morphogenetic protein 4 (BMP4), while miR-21 inhibits the proliferation and migration of VSMC and stimulates the contraction by targeting and regulating programmed cell death protein 4 (PDCD4) and cytoplasmic division factor (DOCK) proteins [28–30]. However, in those initial studies, the miR-21-mediated regulation of the phenotypic transformation of VSMC was not investigated in depth. Interestingly, in a similar model, miR-145 was revealed to be highly expressed in VSMC and to control the formation of vascular neointimal lesions by targeting the Kruppel-like factor 5 (KLF5) gene to regulate the phenotypic transformation of VSMC [31]. Notably, as the scope of the study expanded, the functions of more miRNAs were gradually revealed. For example, PDGF-induced high expression of miR-221 significantly promoted VSMC proliferation and migration, partly by downregulating the expression of target genes c-KIT and p27 (kip1) to promote a synthetic phenotype of VSMC [32, 33] These findings demonstrate that miRNAs have important regulatory roles in VSMC phenotype and function, which may provide guidance in treatment of vascular diseases.
Fundamentally, since Dicer is the key enzyme responsible for most miRNA maturation, alteration of Dicer leads to a complete loss of miRNAs. The role of Dicer-dependent miRNAs in VSMC development and function in vivo was investigated using SM22Cre Dicerflox (SMDicer-KO) mice. Dicer deletion was confirmed to increase embryonic lethality with abnormal vascular architecture and loss of VSMC contractile function [34]. Specific inactivation of Dicer significantly caused renal vascular abnormalities and striated fibrosis as well as type B aortic arch artery disruption [35, 36]. These results indicate that Dicer-generated miRNAs are essential for vascular remodeling and normal VSMC development, differentiation, and contractile function. Overall, miRNAs regulate the phenotypic transformation of VSMC and thereby play important regulatory roles in processes such as VSMC differentiation, contractile or synthetic function, and in vivo angiogenesis. Such miRNAs further influence the transformation of VSMC between contractile and synthetic phenotypes by regulating their proliferation, migration, and differentiation, as shown in Table 1. In the following sections, to illustrate how miRNAs contribute to VSMC phenotypic transformation, we explore specific miRNAs that have been reported to exert mechanistic roles in VSMC.
Table 1.
Related miRNAs affect and regulate vascular remodeling for maintaining vascular homeostasis
| miRNAs | Validated targets | Cellular functions | Physiological function and pathological changes | Inducer and regulator | References |
|---|---|---|---|---|---|
| miR-223 | Downregulate PDGFRβ | Promote VSMC differentiation | Increase medial thickening, fragmentation of medial elastic fibers | imatinib | [19, 22] |
| miR-21 | Decrease PTEN | Induce cell proliferation and decrease apoptosis | Promote abdominal aortic aneurysm development | / | [26, 27] |
| miR-124 | Suppress SP1 | Inhibit proliferation and migration | AmeliorateAtherosclerosis | PDGF-BB | [37] |
| miR-29 | Upregulate ECM related proteins | Increase matrix synthesis | Promote the expansion of mouse aorta | Angiotensin II/Fibulin-4 | [38] |
| miR-29a | Downregulate Fbw7/CDC4 | Enhance VSMC proliferation | Promote atherosclerosis | oxLDL | [39] |
| miR-29b | Downregulate MMP2/elastin | Cause extracellular matrix deficiencies | Promote the early aneurysm development | / | [40] |
| miR-26b | Upregulate HMGA2 | Promote cell viability and proliferation | Regulate Stanford type A aortic dissection | / | [41] |
| miR-9 | Upregulate HIF-1α | Promote proliferative phenotype | Improve development of hypoxic pulmonary hypertension | Hypoxia 1α | [42] |
| miR-132 | Upregulate PTEN | Antagonize the differentiation-promoting ability of cilostazol | Influence on the repair of carotid artery in rats with balloon injury | Cilostazol | [43] |
| miR-138 | Decrease SIRT1 | Enhance VSMC proliferation and migration | Improve diabetes blood vessels | High glucose | [44] |
| miR-146a | Decrease KLF4 | Promote VSMC proliferation | Increase neointimal hyperplasia | KLF5 | [45] |
| miR-214 | Upregulate NCKAP1 | Decrease proliferation and migration | Prevent neointimal SMC proliferation after injury | Serum-starved | [46] |
| miR-1260b | Upregulate GDF11 | Promote Proliferation | Improve hypoxia-stimulated environmental stress | Hypoxia | [47] |
| miR-155-5p | Downregulate PKG1 | Impair NO/cGMP-mediated maintenance of contractile phenotype and vasodilation | Improve inflammatory vascular disease | TNFα | [48] |
| miR-135a | Downregulate FOXO1 | Attenuate pro-inflammatory responses | improve diabetes | / | [49] |
| miR-133a | Reduce LDLRAP1, oxLDL | Reduce lipid accumulation | Improve the formation of atherosclerosis | IL-19 | [50] |
| miR-574 | Inhibit ZDHHC14 | Promote cell proliferation and inhibit apoptosis | Improve coronary artery disease | / | [51] |
| miR-31-5p | Increase myocardin | Attenuate contraction gene | Inhibit ablated pathological VSMC phenotypical switch | ALDH2, Max | [52] |
| miR-128 | Downregulate KLF4 | Promote contraction | Prevent endometrial hyperplasia | / | [53] |
| miR-501-5p | Decreases α-SMA, calponin and Smad3 | Promotes VSMC proliferation and migration | Attenuates carotid balloon injury in rats | / | [54] |
| miR-214 | Reduce Smad7 levels and increase Smad3 phosphorylation | Promotes VSMC proliferation and migration | Increase medial thickness and blood vessels areas | Ang II | [55] |
miRNAs contribute to the phenotypic transformation of VSMC
Recent studies have used miRNA microarrays to identify differentially expressed miRNAs in the cardiovascular system and identify functional miRNAs that play key regulatory roles in VSMC phenotypic transformation. It was confirmed that some miRNAs are involved in regulating the proliferation, migration, and differentiation of VSMC and the remodeling of the cytoskeleton [52, 56]. To date, however, only the functions of a few miRNAs have been associated with the phenotypic transformation of VSMC. In this section, the role of miRNAs in the progression of vascular phenotypic transformation is discussed.
miRNAs regulate the transformation of VSMC to the synthetic phenotype
Reversing or inhibiting the transformation from a contractile to a synthetic VSMC phenotype or enhancing the stability of the contractile VSMC phenotype plays a key role in the prognosis of CVD, such as atherosclerosis [57–59]. The significant differential expression of a number of miRNAs in cardiovascular disease suggests that some miRNAs might regulate the transformation of VSMC to the synthetic phenotype. For example, Chan et al. [60] found that PDGF promotes the expression of miR-24, which targets and inhibits tribbles homologue-3 (TRIB3) and thereby disrupts the TRIB3-mediated degradation of ubiquitinated Smad ligase. This disruption leads to increased levels of Surf1 and subsequent reductions in Smad1/2/3 and Ras homologue family member A (RhoA) expression, which results in inhibition of the BMP-induced transformation of VSMC to the contractile phenotype and stabilization of the synthetic phenotype of VSMC. Additionally, PDGF receptor β (PDGFRB) levels can significantly affect VSMC phenotype. It was shown that myocardin reduced VSMC proliferation and migration by downregulating PDGFRB expression, and antagonizing miR-24 and miR-29a restored PDGFRB-induced VSMC migration and showed an augmented neointima formation [61]. Furthermore, miR-26a was significantly increased in PDGF-stimulated cultured VSMC and arteries with neointimal lesion formation by targeting Smad1, a key mediator of BMP pro-contractile signaling, to promote VSMC differentiation for the intervention of proliferative vascular disease [62]. Likewise, miR-31 plays a pivotal role in the synthetic phenotype of VSMC. Specifically, miR-31 levels in VSMC were significantly induced by PDGF, after which miR-31 promoted VSMC proliferation by targeting large tumor suppressor 2 (LATS2) levels and enhancing proliferating cell nuclear antigen (PCNA) expression [63]. Subsequently, miR-31 can also inhibit the expression of cellular repressor of E1A-stimulated genes (CREG) and genes involved in VSMC differentiation to promote their transformation to the synthetic phenotype [64]. In addition, miR-96 expression was regulated by BMP-4, further targeting and repressing the expression of tribbles homolog 3 (TRB3) to promote the transformation of VSMC to an undifferentiated phenotype [65]. These events trigger the transformation of VSMC to the synthetic phenotype.
Besides, Dong and colleagues further revealed that miR-146a is highly expressed in proliferating VSMC and that the knockout of miR-146a can inhibit the proliferation and migration of VSMC and stimulate VSMC apoptosis, which suggests that miR-146a can promote the transformation of VSMC to a synthetic phenotype [66]. Admittedly, inhibition of the contractile phenotype of VSMC in pulmonary hypertension is mediated by miR-214 through inhibition of the MEF2C–MYOCD–leiomodin1 (LMOD1) signaling axis, providing a theoretical basis for the treatment of vascular hyperproliferative diseases [67]. Collectively, these findings have concluded that the mechanisms of miR-24, miR-29a, miR-31, miR-96, miR-214, and multiple other miRNAs, including miR-138, miR-9, and miR-29b [44, 68, 69] contribute to the transformation of VSMC to a synthetic phenotype. Figure 1 shows the network of important miRNAs regulating a synthetic phenotype of VSMC.
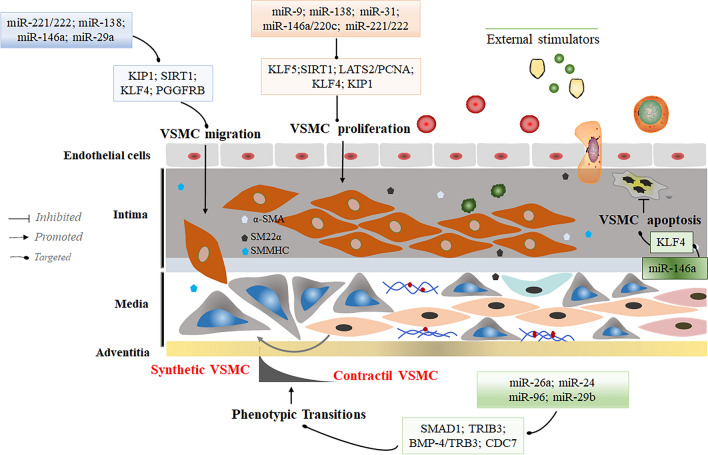
Fig. 1.
MicroRNAs regulate synthetic phenotypic transformations in VSMC. Considerable amounts of miRNA, such as miR-221/222, miR-138, miR-24, and miR-146 through modulation of direct targets or in the presence of external stimuli can promote the conversion of VSMC to a synthetic phenotype. Growth factors, such as α-SMA, SM22α, and SMMHC, were significantly overexpressed during this process. The synthetic phenotype is characterized by increased proliferation and migration to maintain the stability of the VSMC synthetic phenotype against conversion to a contractile phenotype
Recently, Kruppel-like factor 4 (KLF4), an evolutionarily conserved zinc finger-containing transcription factor involved in various cell growth, differentiation, and proliferation, has been proved to cause substantial impacts on regulating CVD [70, 71]. Several miRNAs are closely associated with KLF4 in VSMC regulatory network. For example, miR-146a represses KLF4 expression by targeting its 3′-untranslated region (3′-UTR). Mechanistically, KLF4 competes with KLF5 to bind to and regulate the miR-146a promoter, and that KLF4 and KLF5 exert opposing effects on the miR-146a promoter[45, 72]. MiR-146a and KLF4 form a feedback loop to directly regulate the expression of each other, which provides new insights into the significant reduction of neointimal hyperplasia via targeting miR-146a and KLF4 axial in carotid arteries. Similarly, KLF4 mRNA-binding motif (CACCC) (or the reverse orientation sequence, GGGTG) binds to and regulate the miR-220c promoter, and represses its transcriptional level to reduce VSMC proliferation[73], potentially representing a novel approach to prevent restenosis. These results suggest that some miRNAs that form a negative feedback regulatory mechanism with KLF4 play an important regulatory role in the conversion of VSMC to a synthetic phenotype.
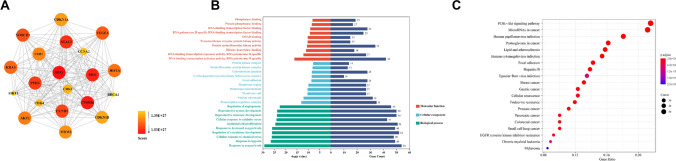
Meanwhile, to explore the linkage between miRNAs subgroups that regulate angiogenic phenotypic target genes, we performed enrichment analysis of known effector proteins and found the closest linkage between TP53, MYC, PTEN, STAT3, CCND1, and CTNNB1 proteins (Fig. 2A). Gene Ontology (GO) analysis revealed that target proteins, mainly represented by protein kinase complexes and serine/threonine protein kinase complexes, can significantly affect the regulation of angiogenesis and vasculature development, further affecting numerous DNA, RNA, and kinase activities, thus regulating the vascular synthesis phenotype (Fig. 2B). In addition, the major signaling pathways clustered in the PI3K–AKT signaling pathway and miRNAs in cancer (Fig. 2C). Interestingly, overexpression of miR-126 prevents vascular restenosis by downregulating PI3K/AKT signaling, thus activating MEK/ERK signaling pathway to enhance vascular remodeling and promoting VSMC migration and proliferation to reduce zone differentiation[74]. In conclusion, miRNAs are important for the regulation of synthetic phenotypes in vascular remodeling in a variety of vascular diseases.
Fig. 2.
Merged enrichment map of target proteins of synthetic phenotype regulated by miRNAs obtained from KEGG and GO. A Linkages between target proteins associated with miRNA-influenced synthesis phenotypes were analyzed by R language; B histograms of three typical enrichment items for molecular function (red), cellular composition (blue), and biological processes (green), and the top 10 GO items in each category are plotted according to p value. C KEGG enrichment analysis network based on the association of target genes; the most significant one is marked in red
miRNAs regulate the transformation of VSMC to the contractile phenotype
Under physiological conditions, the VSMC contractile phenotype is key to the maintenance of normal vascular function, including the regulation of vascular resistance, blood pressure, and contractile ability. Some miRNAs have been demonstrated to be specifically expressed in normal VSMC at high levels and to show differential downregulation after vascular injury, which suggests that these miRNAs might specifically regulate VSMC transformation to the contractile phenotype. For example, miR-1 overexpression induced by myocardin yielded the targeted negative regulation of the serine/threonine protein kinase Pim-1, which subsequently resulted in significant inhibition of Pim-1-mediated VSMC proliferation and indirect promotion of the transformation of VSMC to the contractile phenotype [75]. Moreover, miR-133 can promote the transformation of VSMC to the contractile phenotype via the PDGF/MAPK-ERK1/2/miR-133/Sp-1/KLF4/myocardin signaling pathway [76]. Specifically, exogenous PDGF can inhibit high miR-133 expression, and this inhibition can be alleviated by suppressing the MAPK/ERK1/2 signaling pathway. However, miR-133 overexpression can inhibit specificity protein-1 (Sp-1) expression to induce the downregulation of the transcription factor KLF4 and increase the expression of the downstream molecule myocardin, and these effects inhibit VSMC proliferation and migration and promote VSMC differentiation. Correspondingly, Tang et al. [37] revealed that miR-124 is expressed at low levels in proliferating VSMC, whereas upregulation of miR-124 expression significantly enhances the expression of VSMC differentiation-related protein genes, such as SMA, SM22α, and calponin. Their results further revealed that miR-124 can inhibit the expression of the downstream factor Sp-1 to prevent VSMC proliferation and thus indirectly promote the transformation of VSMC to the contractile phenotype.
Extensive studies have confirmed that miR-145 can be used as a potential marker for VSMC phenotypic transformation. A study has shown that lentivirus delivery micR-145 can significantly increase the area of fibrous cap, reduce the area of necrotic core and increase the content of plaque collagen in the aorta of apolipoprotein E(−/−) mice by reducing KLF4 and increasing the expression of myocardial protein, so as to limit the morphology and cellular composition of atherosclerotic plaque and balance the stability of atherosclerotic plaque and plaque rupture [77, 78]. Interestingly, in a VSMC dedifferentiation model using balloon injury or PDGF induction, Cheng and colleagues found that promoting miR-145 expression reduced VSMC dedifferentiation, while inhibiting miR-145 expression led to VSMC dedifferentiation [79]. Mechanistically, miR-145 restores balloon-injured arterial neointima growth by targeting inhibition of KLF5 and its downstream signaling molecule myocardin. Meanwhile, miR-145 significantly reversed the suppression of atherosclerosis-induced VSMC differentiation marker genes, such as SMα actin, calmodulin, and MYOCD [79, 80]. These new findings may have broad significance for the diagnosis and treatment of a variety of proliferative vascular diseases. Venous grafts are commonly used as autografts in ischemic disease revascularization procedures, but long-term patency is poor, because exposure to arterial blood pressure accelerates VSMC hyperplasia. Japanese researchers reported that transduction of miR-145 in venous grafts attenuated venous intimal hyperplasia by maintaining more SM22α mature VSMC and fewer Ki-67-positive proliferating cells, allowing VSMC to shift from a proliferative to a contractile state [81, 82]. Further atherosclerosis was treated with nanoparticle micelles packed with miR-145, and miR-145 micelles were found to reverse the atherosclerosis-induced decrease in VSMC protective contractile markers, such as cardiac myosin, α-SMA, calmodulin, ACTA2, and MYH11 [83, 84]. Notably, miR-145 has been studied quite extensively in VSMC. Either exogenous overexpression of miR-145 effectively inhibits high glucose-induced VSMC over-proliferation and migration [85]; or induces VSMC initiation and development via CD40 and further relies on Jag-1 activation of Notch receptors leading to CBF1-dependent upregulation of miR-145, increasing differentiation and decreasing proliferation [86, 87]. These demonstrate that miR-145 is highly plastic in resident VSMC and is capable of converting VSMC from a migratory proliferative synthetic phenotype to a protective contractile phenotype, expanding the path for subsequent vasodilator drug development.
Analogically, high expression levels of miR-15b/16 are closely related to the contractile phenotype in VSMC, and overexpression of miR-15b/16 can promote the expression of genes encoding smooth muscle contractile-related proteins and inhibit the proliferation and migration of VSMC [88]. Studies have shown that miR-15b/16 can promote the conversion of VSMC to a contractile phenotype by targeting the downstream gene yes-associated protein 1 (YAP) [89]. Furthermore, Li et al. [90] found that the overexpression of miR-663 increases the expression of VSMC differentiation markers, such as SM-22α and calmodulin, and inhibits PDGF-induced VSMC proliferation and migration. Mechanistically, miR-663 promotes the transformation of VSMC to the differentiated phenotype through the targeted inhibition of JunB and its downstream molecules, such as myosin light chain 9 (MYL-9) and matrix metallopeptidase 9 (MMP-9).
Several studies have detailed other miRNAs and their impact on the VSMC contractile phenotype. For example, miR-424/322 directly targets and regulates Cyclin D1 and calumenin, indirectly targets stromal interaction molecule 1 (STIM1), and inhibits VSMC proliferation and migration, suggesting that miR-424/322 indirectly stimulates the transformation of VSMC to the contractile phenotype [91]. Furthermore, miR-638, which is highly expressed in differentiated VSMC, exhibits extremely low expression in dedifferentiated VSMC and can inhibit the expression of orphan nuclear receptor (NOR1), which leads to reduced expression of the downstream factor cyclin D1 and decreased VSMC proliferation and migration [92]. These events limit the conversion of VSMC to synthetic phenotypes by reducing the proliferation and migration of VSMC. In addition, miR-34c was significantly upregulated in a rat carotid artery injury model and increased the levels of p21, p27, and Bax through targeted suppression of stem cell factor (SCF), leading to the transformation of VSMC to a differentiated phenotype [93]. Furthermore, overexpression of miR-206 inhibited proliferation and migration of human pulmonary artery smooth muscle cells (HPASMC) by downregulating Notch-3 expression and further increased the levels of VSMC differentiation markers α-smooth muscle actin and calmodulin to promote VSMC differentiation, reflecting the importance of miR-206 in the contractile phenotype [94]. Accordingly, VSMC phenotypic transformation can modulate atherosclerosis progression by affecting cell proliferation and migration through the myocardial-miR-206-Cx43 loop [95]. In short, various miRNAs have a non-negligible role in VSMC phenotypic transformation and can provide good predictions for the progression of multiple diseases by facilitating the transformation to a contracted phenotype and serve as diagnostic markers and new targets for therapeutic intervention.
The targets and functions of miR-23b and miR-182 have been extensively characterized in vascular smooth muscle. Specifically, Iaconetti et al. [96] found that miR-23b is downregulated after vascular injury in a rat carotid artery balloon injury model and that overexpression of miR-23b significantly reduces neointimal hyperplasia in balloon-injured arteries. These results further demonstrated that miR-23b can significantly promote the expression of VSMC marker genes, such as ACTA2 and MYH11, and that miR-23b can target the transcription factor Forkhead box O4 (FoxO4) to inhibit downstream MMP-9 expression and TNFα-induced VSMC migration. In addition, miR-23b can inhibit urokinase-type plasminogen activator and Smad3, thus inhibiting cell proliferation and migration mediated by the above two factors [97, 98]. In general, miR-23b can be speculated to be able to positively regulate VSMC transformation to the contractile phenotype. Similarly, miR-182 expression is significantly altered during the phenotypic transformation of VSMC. For example, miR-182 upregulation increases the expression of SMC contractile-specific genes, including α-smooth muscle actin (SMA), SM-22α, and calponin, whereas downregulation of miR-182 expression induces dedifferentiation of VSMC from the contractile to the synthetic phenotype [99]. Furthermore, additional results indicated that miR-182 could target fibroblast growth factor 9 (FGF9) and myeloid-associated differentiation marker (MYADM) by mediating PDGFRβ or TGF-β signaling to prevent SMC dedifferentiation [99–101]. These events all point to the ability of miR-182 to significantly modulate the contractile phenotype of VSMC, providing a promising target for the treatment of vascular diseases. The major miRNA networks regulating the VSMC contractile phenotype are shown in Fig. 3.
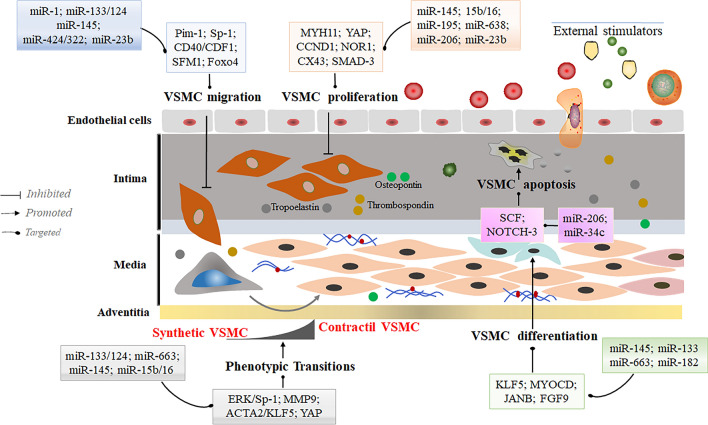
Fig. 3.
MicroRNAs regulate contractile phenotype transformation in VSMC. Inhibition of VSMC proliferation and migration and acceleration of VSMC differentiation are direct evidence of promoted VSMC conversion to a contractile phenotype. Factors, such as miR-1, miR-145, miR-195, miR-124, miR-34c through modulation of direct targets, or external stimulating factors can slow cell proliferation and migration or maintain the contractile phenotype of VSMC by partially affecting apoptosis through miR-206. Moreover, miR-145 and miR-133 could directly improve the differentiation ability of VSMC. In this process, tropoelastin, osteopontin, thrombospondin, and other proteins characteristic of the VSMC contractile phenotype were highly expressed to prevent conversion to the synthetic phenotype
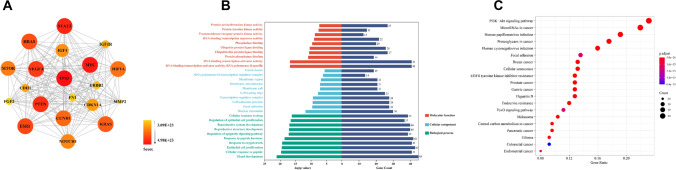
Meanwhile, to explore the linkage between target genes regulating vasoconstriction phenotypic miRNAs, we performed enrichment analysis of known effector proteins and identified the closest linkage between TP53, MYC, VEGRA, PTEN, STAT3, HRAS, and ESR1 proteins (Fig. 4A). GO analysis showed that multiple protein complexes can significantly influence biological processes such as epithelial cell proliferation and glandular development, modulating DNA/RNA polymerase activity and partial protease/phosphatase-binding capacity, thereby regulating the vasoconstriction phenotype (Fig. 4B). In addition, the major signaling pathways clustered in the PI3K–AKT signaling pathway and miRNAs in cancer (Fig. 4C). Fortunately, miR-145 was highly expressed in small arteries of hypertensive patients remodeled by exercise, significantly reduced AKT phosphorylation and IGF-1R mRNA, and contributed to significant upregulation of the VSMC contractile marker calponin, which induced VSMC to maintain a contractile phenotype [102]. In conclusion, the regulation of contractile phenotype in vascular remodeling by miRNAs is not negligible and provides a therapeutic basis for some vascular diseases.
Fig. 4.
Merged enrichment map of target proteins of contractile phenotype regulated by miRNAs obtained from KEGG and GO. A The linkage between miRNAs affecting contracted phenotype-associated target proteins was Analyzed by R language; B histograms of three typical enrichment items for molecular function (red), cellular composition (blue) and biological processes (green), and the top 10 GO items in each category were plotted according to p value. C KEGG enrichment analysis network based on the association of target genes, the most significant one is marked in red
Taken together, these studies clearly demonstrate that multiple miRNAs have strong regulatory roles in maintaining VSMC phenotypic transformation. However, the exact role of these miRNAs seems to depend on the microenvironment and the cells responsible for disease pathology, and further refinement and expansion of the profound role of miRNAs in the contractile and synthetic phenotypes of VSMC is still needed.
VSMC phenotypic miRNAs involved in the regulation of vascular remodeling and homeostasis
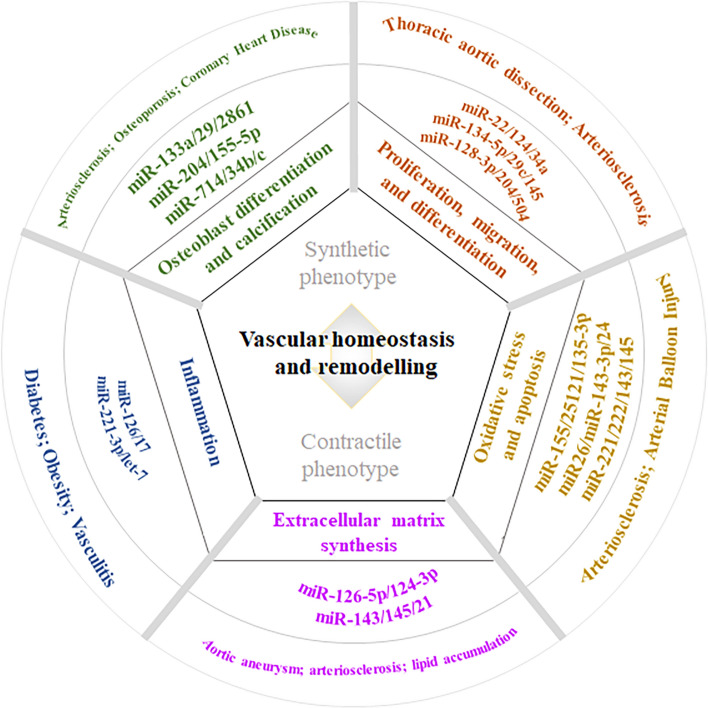
Vascular remodeling and homeostasis are key components of many vascular diseases. Vascular remodeling is both an adaptive physiological process that maintains vascular homeostasis and a key pathological link that is commonly found in a number of vascular pathologies [103, 104]. In this regard, cardiovascular and cerebrovascular diseases, such as coronary heart disease, hypertension, stroke, PAH, and organ injury, pose substantial threats to human health and well-being [105, 106]. The vascular remodeling underlying these disorders results from an imbalance between vascular function and injury repair. As small regulatory transcripts, numerous miRNA species are involved in VSMC phenotypic changes, which are characterized by VSMC proliferation, migration and differentiation, oxidative stress and apoptosis, ECM synthesis, inflammation, osteoblast differentiation, and calcification and contribute to arterial stiffness, hypertension, and coronary heart disease [107–109], thus playing an important regulatory role in vascular remodeling and homeostasis (Fig. 5).
Fig. 5.
miRNAs regulate VSMC phenotypic transformations to influence vascular remodeling and homeostasis by regulating different pathophysiological aspects, thus affecting various cardiovascular disease processes. Vascular remodeling and homeostasis are key in many vascular diseases. miRNAs, as a class of small regulatory transcripts, can modulate the phenotypic transformation of VSMC to repair the balance between vascular function and injury by regulating the proliferation, migration and differentiation, oxidative stress and apoptosis, extracellular matrix (ECM) synthesis, inflammation, and osteoblast differentiation and calcification of VSMVs. Therefore, miRNAs have considerable potential for targeted therapy and prevention in cardiovascular diseases, including thoracic aortic coarctation, atherosclerosis, aortic aneurysm, diabetes, vasculitis, osteoporosis, and coronary artery disease
miRNAs in VSMC proliferation, migration, and differentiation
Recent studies have demonstrated that miRNAs are essential regulators of VSMC regulation and further identified several target genes that are implicated in VSMC proliferation, migration, and differentiation. For example, overexpression of miR-22 significantly increased the expression of VSMC phenotypic marker genes and led to inhibition of the proliferation and migration of VSMC, whereas the opposite effect was observed when endogenous miR-22 was knocked down [107]. Similarly, miR-22 overexpression in damaged vessels significantly reduced the expression of its target gene, eosinophil integration site 1 protein homolog (EVI1), and inhibited neointima formation in wire-injured femoral arteries by reducing VSMC proliferation [107]. In addition, a study by Tang et al. [37] found that overexpressed miR-124 significantly attenuated PDGF-induced human arterial smooth-muscle cell (HASMC) proliferation and phenotypic transformation. Overexpression of Sp1, as the downstream target of miR-124, appeared to reverse the anti-proliferative effect of miR-124 in HASMC, suggesting that miR-124 is a key regulator of HASMC differentiation, proliferation, and phenotypic transformation. In addition, a similar experiment performed by Chen et al. [110] showed that increased miR-34a around the blood vessels significantly reduced the expression level of its target gene Notch1 and thus decreased VSMC proliferation and inhibited neointimal formation in wire-injured femoral arteries. Mimetically, miR-134-5p effectively inhibited PDGF-induced VSMC phenotypic transformation and migration, while ectopic expression of miR-134-5p obviously promoted VSMC differentiation and expression of contractile markers [111]. The study further determined that miR-134-5p could inhibit the phenotypic transformation and thoracic aortic dissection (TAD) of VSMC by targeting signal transducer and activator of transcription 5B (STAT5B) and integrin beta 1 (ITGB1), suggesting that miR-134-5p is a critical regulator of phenotypic transformation and migration in human HVSMC.
Abnormal phenotypic transformation of VSMC is a hallmark of vascular disorders, and evidence shows that miRNAs play key roles in VSMC functions. For example, miR-128-3p, which is considered to be associated with VSMC phenotypic transformation and a new key factor in regulating vascular disease, increases the methylation status of the VSMC gene myosin heavy chain 11 (Myh11) to affect VSMC proliferation, migration, differentiation, and contractility after stress due to stimulation [112]. In addition, miR-145 is a key factor in regulating VSMC plasticity, which can be reduced in vascular disease. It was shown that miR-145 regulates the essential role of VSMC phenotype by inhibiting ubiquitin-like containing PHD and RING finger domains 1 (UHRF1) mRNA translation to reduce proliferation and dedifferentiation [113]. Moreover, miR-145 could inhibit VSMC proliferation, migration, and phenotypic transformation by preventing activation of the PI3K/AKT/mTOR signaling pathway [114]. Overall, abnormalities in the VSMC phenotype and its regulators are factors that may have therapeutic potential in vascular lesions.
Several recent studies have indicated that type 2 diabetes mellitus (T2DM) can influence the proliferation, migration, and phenotypic transformation of VSMC and thus contribute to atherosclerosis and diabetes-induced vascular remodeling [74, 115]. Moreover, during the course of T2DM, the expression level of VSMC-specific miRNAs that are closely related to contractile or synthetic phenotypes will be differentially expressed. For example, using right carotid artery experimental angioplasty and RNA sequencing, Torella et al. [116] revealed that miR-29c and miR-204 were most significantly dysregulated in atherosclerotic plaques from patients with T2DM. miR-29c overexpression and miR-204 inhibition could enhance additive reductions in VSMC proliferation and thus attenuate VSMC phenotypic transformation in DM by targeting epithelial membrane protein 2 (Emp2) and caveolin-1 (Cav1), respectively. Another recent study demonstrated that miR-504 could promote diabetic VSMC dysfunction by targeting signaling adaptor growth factor receptor-bound protein 10 (Grb10) and transcription factor early growth response 2 (EGR2), thereby being involved in the metabolic memory of vascular complications [117]. Likewise, miR-504 Overexpression suppressed contractile genes and increased the migration and proliferation of VSMC from diabetic mice, indicating that miR-504 can promote VSMC dysfunction.
Collectively, these results appear to indicate that several miRNAs have been extensively characterized as key regulators of VSMC proliferation, migration, differentiation, and phenotypic transformation, and play important roles in the pathogenesis of many aspects of vascular disease.
miRNAs in VSMC apoptosis and oxidative stress
VSMC apoptosis plays an important role in vascular remodeling and in the pathophysiology of vascular diseases and atherosclerosis. Apoptosis of VSMC is considered to be triggered by oxidative stress present in the diseased vascular milieu. Identifying the regulatory mechanisms of oxidative stress-induced VSMC apoptosis potentially may reveal novel therapeutic approaches for vascular remodeling and atherosclerotic plaque instability. miRNAs have been experimentally validated to regulate apoptotic factors and oxidative stress, which is beneficial to the dissolution of atherosclerotic plaques, the proliferation of VSMC, and the formation of new intima. For example, Yang et al. [118] found that mammalian sterile 20-like kinase 2 (MST2) regulated miR-155-promoted inflammatory and oxidative stress responses by altering the interaction of MEK with Raf-1 and MST2 in response to vascular injury, indicating that suppression of endogenous miR-155 is a potential therapeutic strategy for vascular injury and remodeling. In addition, loss of VSMC through apoptotic cell death can cause fibrous cap thinning, necrotic core formation, and calcification that may destabilize plaques. miR-25 blocks corticosterone-induced VSMC apoptosis by targeting modulator of apoptosis 1 (MOAP1) and the p70S6k pathway [119]. A study by Peng et al. [120] elucidated the role of miR-26a in VSMC apoptosis and indicated that miR-26a protected VSMC against H2O2-induced injury through activation of the PTEN/AKT/mTOR pathway and may be considered a potential prognostic biomarker and therapeutic target in the treatment of abdominal aortic aneurysm (AAA). Similarly, miR-26 attenuates vascular smooth muscle maturation and indirectly promotes a differentiated smooth muscle phenotype and apoptosis through BMP signaling modulation in endothelial cells by targeting Smad1, which is a BMP signaling effector [121]. These results clearly reveal that miRNAs broadly regulate VSMC apoptosis to influence vascular remodeling and homeostasis, providing a reference for vascular disease treatment.
The cellular metabolism of all aerobic organisms produces reactive oxygen species (ROS), which have been associated with the physiopathology of several diseases and can be neutralized by nonenzymatic defenses and the activity of antioxidant enzyme. Notably, mammals have the ability to scavenge excess free radicals, which depends on the endogenous free radical scavenging system, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH), as well as nuclear factor E2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) cascade signal pathway [122–125]. Antioxidant enzymes can change the reactive oxygen free radicals in the body into substances with low activity, thus weakening their attack on the body [126, 127]. Although evidence for direct targeting of miRNAs to regulate oxidation-related enzymes has not emerged, however, some miRNAs can indirectly alter the activity of various oxidative enzymes to exert in vivo and cellular scavenging of free radicals for maintaining oxidative homeostasis in and around blood vessels [128, 129]. A recent study has shown that miR-135a-5p reversed ox-LDL-induced alterations in SOD and MDA activities in VSMC by increasing XBP1 expression, suggesting a potential therapeutic strategy for miRNAs in atherosclerosis treatment [130]. Moreover, miR-143-3p, which directly targets the inhibition of a disintegrin and metalloproteinase 10 (ADAM10), significantly affected the levels of superoxide dismutase and malondialdehyde, ameliorating the cytotoxicity triggered by angiotensin II in VSMC [131]. In addition, the VSMC isolated from the thoracic aorta of diabetes rats were transfected with miR-24, and it was found that the oxidative stress of VSMC was inhibited and the expression level of Nrf2/HO-1 was increased, and the degree of recovery was related to SOD and GSH-px [132]. The above results suggest that miRNAs regulate oxidative stress levels in VSMC by affecting related oxidative enzymes and proteins. Further exploration of their direct mechanisms and targets may provide more therapeutic prospects for the prevention and treatment of vascular diseases.
Moreover, upregulation of miR-21 and miR-221/222 in the area damaged by experimental arterial balloon injury promotes intimal hyperplasia and induces subsequent stenosis. In contrast, downregulation of miR-221/222 expression significantly inhibits VSMC proliferation and improves local vascular patency after angioplasty [29, 133]. Additional studies have proposed that a miR-143/145 cluster acts as a “switch” that regulates the phenotypic transformation of VSMC during vascular remodeling. Thus, the miR-143/145 cluster is elevated by dedifferentiation of the VSMC phenotype but decreased in lesions and atherosclerotic vessels. miR-143/145 expression during arterial repair postinjury inhibits VSMC proliferation and intimal growth. However, miR-143/145-knockout mice are also resistant to pathological vascular remodeling due to experimental injury, which suggests that the miR-143/145 clusters are both positive and negative regulators of VSMC differentiation and proliferation [81, 87]. Furthermore, their dual targeted vascular stress response mode can maintain the phenotypic plasticity of VSMC and promote appropriate responses to pathological injury. Overall, modulating vascular oxidative stress and affecting vascular apoptosis by altering the levels of miRNAs in vascular cells and blood circulation are a potentially effective means of treating some cardiovascular conditions.
miRNAs in VSMC ECM synthesis
VSMC are surrounded by ECM [134], which is a fundamental component of multicellular organisms that not only provides essential physical scaffolding for cellular constituents but also initiates crucial biochemical and biomechanical cues that are needed for vessel wall stability. Moreover, the ECM is a complex meshwork of both structural and functional proteins assembled in unique tissue-specific architectures that play important roles in guiding VSMC function [135]. Several studies have shown that miRNAs have emerged as key modulators of ECM homeostasis and thereby have been proposed to explore the role of miRNAs deeply in vascular remodeling. For example, Wang et al. [136] demonstrated that VSMC exhibit remarkable plasticity to undergo phenotypic modulation, in which the expression of VSMC markers is markedly attenuated, while conversely, ECM expression is dramatically increased. Myocardin not only activates smooth muscle-specific genes but also regulates ECM expression through induction of transcription of miR-143 during smooth muscle differentiation. Moreover, miR-126-5p promotes contractile transformation of VSMC by targeting VEPH1 and alleviates Ang II-induced abdominal aortic aneurysm in mice [137]. Ang II-induced upregulation of MMP9 and MMP2, two key proteases responsible for ECM degradation, in mouse aortas and human aortic smooth muscle cells (HAOSMC) was also reduced by miR-126-5p overexpression. The results reveal a novel role of miR-126-5p in inhibiting AAA development-associated aortic VSMC dedifferentiation.
Collagen is the most abundant protein in animals, accounting for more than 30% of the total protein in the human body, is distributed in various organs and tissues, and can be synthesized and secreted by many kinds of cells constituting the framework structures in the ECM [138]. Collagen synthesis in VSMC is very important in atherosclerosis, as it affects plaque stability [139]. A study showed that miR-124-3p inhibited VSMC collagen synthesis and restrained protein levels of type I and type III collagen in aortas and atherosclerotic plaques by directly targeting prolyl 4-hydroxylase subunit alpha 1 (P4HA1), which might decrease atherosclerotic plaque stability [140]. Bekelis et al. [141] indicated that miR-21, miR-143, and miR-145 post-transcriptionally regulated the expression of multiple targets with a function in the ECM, such as collagen (COL1A1, COL5A1, and COL5A2) and MMP-13, which are associated with collagen formation, VSMC phenotypic modification, extracellular matrix remodeling, inflammation signaling, and lipid accumulation. These results indicate that microRNAs, as an important regulator of VSMC function, play a significant regulatory role in the formation and stability of vascular scaffolds, providing a theoretical basis for angiogenesis.
miRNAs in VSMC inflammation
Since VSMC proliferation and endothelial cell (EC) dysfunction are crucial in the pathogenesis of diabetic atherosclerosis and inflammatory symptoms, it is increasingly valuable to explore strategies for their prevention and treatment [142, 143]. Meanwhile, increasing evidence suggests that miRNAs and their target genes may be involved in VSMC inflammatory processes [144, 145]. For example, a study performed by Brennan et al. [146] showed that both PDGF- and TNF-α-induced VSMC and EC activation were associated with reduced let-7 miRNA expression via LIN28b, a negative regulator of let-7 biogenesis. Ectopic overexpression of let-7 in VSMC inhibited inflammatory responses, including proliferation, migration, monocyte adhesion, and nuclear factor-κB activation. The results suggested that restoration of let-7 expression in VSMC could provide a novel target for an anti-inflammatory approach in diabetic vascular disease. Furthermore, another study demonstrated that perivascular adipose tissue (PVAT)-derived extracellular vesicles (EVs) and their encapsulated miR-221-3p could be taken up into VSMC [147]. Transfer and direct overexpression of miR-221-3p dramatically enhanced VSMC proliferation and migration and caused vascular dysfunction by suppressing contractile genes peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1α) in the arterial wall. The results provided an EV-miR-221-3p-mediated mechanism by which PVAT triggers early stage vascular remodeling in the context of obesity-associated inflammation.
Adhesion molecules expressed by activated EC play a key role in regulating leukocyte trafficking to sites of inflammation. Resting endothelial cells normally do not express adhesion molecules, but cytokines activate endothelial cells to express adhesion molecules, such as vascular cell adhesion molecule 1 (VCAM-1), which mediate leukocyte adherence to endothelial cells. For example, Tamia A. Harris et al. [148] found that EC expressed miR-126, which inhibited VCAM-1 expression. Decreasing endogenous miR-126 levels increases leukocyte adherence to EC. These data suggest that miRNAs can regulate adhesion molecule expression and may provide additional control of vascular inflammation. In addition, inflammation and excessive proliferation of VSMC have key roles in various vascular disorders. However, how miRNAs mediate the regulation of VSMC proliferation during inflammation through NF-kappa B (NF-κB) activation remains unknown. Notably, the results from Yang et al. [149] indicated that VSMC proliferation under inflammation is regulated by NF-κB p65/miR-17/RB pathway activation, providing a mechanism for the excessive proliferation of VSMC under inflammation during vascular disorders, which may help identify novel targets for the treatment of vascular diseases.
miRNAs in VSMC osteoblast differentiation and vascular calcification
Vascular calcification is characterized by pathological deposition of calcium and phosphate in the arteries through an active and regulated biological process in which VSMC transform into osteoblast-like cells and is a key pathologic component of vascular diseases, such as atherosclerosis, coronary artery disease, and peripheral vascular disease. Several studies have demonstrated that miRNAs regulate osteoblast differentiation and thus modulate VSMC-mediated arterial calcification (Fig. 6). For example, miR-133a overexpression inhibits VSMC transdifferentiation into osteoblast-like cells by targeting the amounts of bone runt-related transcription factor 2 (Runx2) to inhibit alkaline phosphatase activity, osteocalcin secretion, and mineralized nodule formation [150]. The results suggested that miR-133a is a key negative regulator of the osteogenic differentiation of VSMC. However, another study performed by Zhao et al. [151] revealed that reticulocalbin-2 (RCN2) promoted VSMC osteogenic differentiation and calcification by inducing signal transducer and activator of transcription 3 (STAT3) phosphorylation. Furthermore, inhibition of STAT3 activation promoted miR-155-5p expression in VSMC and inhibited RCN2 expression. Finally, RCN2 overexpression partially offset the miR-155-5p-mediated inhibition of VSMC calcification, acting as a positive feedback loop.
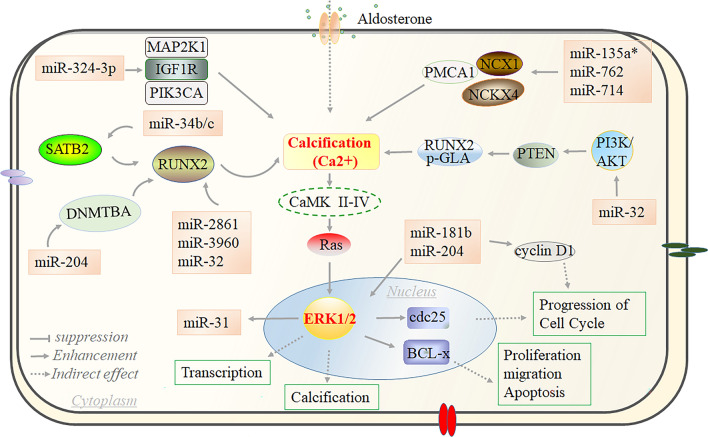
Fig. 6.
miRNAs act on the calcification signaling pathway to regulate VSMC phenotypic transformation. Calcification is one of the key regulatory signals in VSMC phenotypic transformation. Activation of VSMC calcification was promoted by miR-324-3p via enhancement of PI3K3A and MAP2K1 or miR-762/714 via activation of NCKX4 and PMCA1 or miR-32 and miR-34b/c targeting RUNX2. Subsequently, Ca2+ can promote increased ERK1/2 expression through activation of RAS, which further regulates downstream key factors regulating cell proliferation, migration, and transcription. In addition, the key protein ERK1/2 can activate miR-31 or be regulated by miR-181b to affect VSMC performance transformation
It is well known that vascular calcification significantly increases the risk of cardiovascular mortality. Several studies have shown that the use and accumulation of inorganic phosphate (Pi) raises the likelihood of vascular calcification and is one of the important causes of cardiovascular disease prevention and treatment that cannot be ignored. For example, microRNA miR-134-5p enhances inorganic phosphate (Pi)-induced calcium deposition in VSMC by inhibiting the expression of histone deacetylase 5 (HDAC5) and osteoprotegerin (OPG) and increasing Runx2 protein and bone morphogenetic protein 2 (BMP2) mRNA [152]. Similarly, miR-221 and miR-222 mimics caused increased VSMC calcium deposition by inducing significant changes in ectonucleotide phosphodiesterase 1 (Enpp1) and Pit-1 expression, which increased cellular inorganic phosphate and pyrophosphate levels [153]. In addition to these, increased expression of miR-135a*, miR-762, miR-714, and miR-712* in VSMC may be involved in Pi-induced VSMC calcification by disrupting Ca(2+) efflux proteins by regulating the Ca2+ efflux proteins NCX1, PMCA1, and NCKX4 [154, 155]. These results suggest that some miRNAs have the potential to act as markers of vascular calcification, offering the possibility of clinical identification for early calcification in cardiovascular disease.
Arterial calcification is a common macroangiopathy that initiates from a VSMC-regulated process of osteoblastic differentiation. Accumulating evidence has demonstrated that epigenomic regulation by specific miRNAs might play an important role in VSMC calcification. For example, Cui et al. [156] have indicated that miR-204 is an endogenous attenuator of Runx2 in VSMC calcification. Downregulation of miR-204 may contribute to β-glycerophosphate-induced VSMC calcification by regulating Runx2, indicating that miR-204 is a potential novel therapeutic target in medial artery calcification. Interestingly, miR-204 overexpression attenuated osteoblastic differentiation of VSMC by targeting DNA methyltransferases 3A (DNMT3A). Moreover, when DNMT3A was knocked down, the methylation ratio of miR-204 was decreased significantly, and miR-204 expression resulted in abrogation of the effect of high-phosphate concentrations on VSMC calcification [157]. Furthermore, overexpression of miR-125b inhibited β-glycerophosphoric acid-induced osteogenic marker expression and calcification of VSMC, whereas knockdown of miR-125b promoted the phenotypic transformation of VSMC and calcification by regulating Ets1. Additionally, both miR-2861 and miR-3960 levels were increased during β-glycerophosphate-induced osteogenic transdifferentiation of VSMC, and miR-2861 and miR-3960 promoted osteogenic transdifferentiation of VSMC by targeting histone deacetylase 5 and homeobox A2, respectively, resulting in increased Runx2 protein production [158]. The above results suggest that the induction of osteogenic differentiation of VSMC by β-glycerophosphate to cause vascular calcification can be interfered by some miRNAs, thus reducing the possibility of large vessel disease development.
Vascular calcification is an actively regulated process similar to osteogenesis and osteoporosis. Increasing evidence suggests that miRNAs contribute to the development of VSMC calcification. For example, miR-34b/c, which is downregulated during VSMC calcification, exacerbated aldosterone-induced VSMC calcification by regulating the SATB2/Runx2 pathway, and overexpression of miR-34b/c attenuated aldosterone-induced VSMC calcification [159]. Conversely, miR-32 regulates the process of vascular calcification by activating phosphatidylinositol 3-kinase (PI3K) signaling and increasing RUNX2 expression and phosphorylation by targeting the 3′ untranslated region of the phosphatase and tensin homolog (PTEN) in mouse VSMC [160]. Homoplastically, miR-324-3p significantly increased PIK3CA and MAP2K1 expression to promote vascular calcification [161]. In addition, exosomes are stably present in circulation, which protects the functional miRNA from degradation and renders competence for the induction of VSMC calcification [162, 163]. Therefore, by exploring the evidence of circulating miRNA transduction to VSMC, it will facilitate the development of diagnostic, disease progression, and/or miRNA-derived therapeutic approaches in cardiovascular disease research.
To sum up, these findings suggest a potential role of miRNAs, which determine VSMC fate and reduce neointima formation following arterial injury, thereby acting as therapeutic targets for restenosis and vascular remodeling after angioplasty. Increasing discoveries are being made about miRNAs and their targets affecting multiple CVD by regulating VSMC phenotypic transformation (Table 2), and we need to identify specific miRNAs in specific CVD and interfere with them for therapeutic purposes. In addition, deeply systematic and functional analysis of miRNAs in vascular smooth muscles is required before a clear set of restricted patterns of tissue and principles will become more apparent.
Table 2.
Characterization of VSMC phenotypic transformations regulated by miRNAs in vascular remodeling for maintaining vascular homeostasis
| Characteristics affecting phenotypic transformation | ncRNAs | Target gene | Regulation category | Physiological function | Disease type | References |
|---|---|---|---|---|---|---|
| Proliferation, Migration, and Differentiation | miR-124 | Sp1 | Attenuat VSMC proliferation and phenotypic transformation | Reduce aortic dissection | Aortic dissection | [37] |
| miR-128 | KLF4/ Myh11 | Reduce VSMC proliferation and migration | Reduce proliferation and migration, improve differentiation and contractility | Carotid restenosis | [53] | |
| miR-22 | EVI1 | Reduce VSMC proliferation | Inhibit neointima formation in wire-injured femoral arteries | Vascular injury | [107] | |
| miR-34a | Notch1 | Reduce VSMC proliferation and migration | Inhibit neointima formation | Vascular injury | [110] | |
| miR-134-5p | STAT5B/ ITGB1 | Inhibit VSMC phenotypic transformation and migration | Suppress the aorta dilatation and vascular media degeneration | Thoracic aortic dissection | [111] | |
| miR-145 | UHRF1 | Reduce VSMC proliferation and migration | Prevent intimal hyperplasia in mouse carotid artery | Intimal hyperplasia/ restenosis | [113] | |
| miR-504 | Grb10/Egr2 | Enhance VSMC proliferation and migration | Improve VSMC dysfunction | Diabetes mellitus | [117] | |
| miR-93 | Mfn2 | Promote VSMC proliferation and migration | Promote neointimal formation | Atherosclerosis and restenosis | [164] | |
| miR-101 | DOCK4 | Reduce VSMC proliferation and migration | / | / | [165] | |
| 212-5p | PAFAH1B2 | Reduce VSMC proliferation and migration | Promote contraction | Hypertension | [166] | |
| miR-4463 | Bfgf | Reduces VSMC proliferation and intimal hyperplasia | Reverse the injury-induced dedifferentiation | Arterial injury | [167] | |
| Apoptosis and Oxidative stress | MiR-25 | MOAP1 | Inhibit apoptosis | Reduce the formation of unstable plaque | Atherosclerosis | [119] |
| miR-26 | PTEN | Enhance cell viability | Protecte VSMCs against H2O2-induced injury | Abdominal aortic aneurysm | [120] | |
| miR-24 | Nrf2/Ho-1 | Suppress HG-induced oxidative stress | Promotes re-endothelialization in balloon-injured diabetic rats | Balloon Injury | [132] | |
| miR-31-5p | FNDC5 | Attenuate oxidative stress | Promote VSMC migration | Spontaneously hypertensive | [168] | |
| miR-4787-5p | PC1 | Promote apoptosis | Induced polycystic kidney disease | Aortic dissection | [169] | |
| ECM synthesis | miR-143 | Versican | Attenuate ECM versican protein expression | Inhibit migration and proliferation | / | [136] |
| miR-126-5p | VEPH1 | Attenuat aortic dilation and elastin degradation | Restore hAoSMCs differentiation | Abdominal aortic aneurysm | [137] | |
| miR-124-3p | P4HA1 | Decrease type I and type III collagen | Decrease content of plaques | Atherosclerosis | [140] | |
| miR-106a | TIMP-2 | Accelerate ECM degradation | Inhibit cell viability and promote apoptosis | Abdominal aortic aneurysm | [170] | |
| Inflammation | let-7 | Lin28b | Inhibit inflammatory responses | Reduce proliferation, migration and monocyte adhesion | Atherosclerosis | [146] |
| miR-221-3p | PGC-1α | Promote inflammatory macrophage infiltration | Induce fat cell hypertrophy and increase lipogenesis | Adiposity | [147] | |
| miR-17 | RB | Improve inflammatory reaction | Stimulate VSMCsproliferation, enhance G1/S transition | / | [149] | |
| miR-712-5p | / | Promote TNF-α-induced VSMC inflammation | Reduce carotid artery ligation-induced intimal hyperplasia | Proliferative vascular diseases | [171] | |
| miR-376b-3p | KLF15 | Promote inflammatory | Induce expression of inflammatory cytokines IL-1β and TNF-α | Diabetes and hyperglycemia | [172] | |
| miR-378a | PGC-1α | Reduce inflammation | Inhibit free fatty acid-induced cell proliferation and migration | Atherogenesis | [173] | |
| Osteoblast differentiation and Vascular calcification | miR-133a/204 | Runx2 | Inhibit VSMCs osteogenic differentiation | Inhibit calcification | Atherogenesis | [150] |
| miR-155-5p | RCN2 | Reduce SMC osteogenic differentiation and calcification | Inhibit calcification | Peripheral arterial disease | [151] | |
| miR-125b | Ets1 | Inhibit osteogenic markers expression | Inhibit VSMCs calcification | Chronic kidney disease | [157] | |
| miR-2861/3960 | HDAC5/ HOXA2 | Promote VSMCs osteogenic transdifferentiation | Promote vascular calcification | / | [158] | |
| miR-34b/c | SATB2 | Alleviate VSMC calcification | Antagonize the effects of aldosterone | Vascular disease | [159] | |
| miR-32 | PTEN | Promote osteogenic protein expression | Promote vascular calcification | Coronary artery calcification | [160] | |
| miR-204 | DNMT3A | Alleviate VSMCs osteoblastic differentiation | Reduce arterial calcification | Uremia | [174] |
Furthermore, other non-coding RNAs (ncRNAs), including long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs), have been reported to play important roles in the phenotypic transformation of VSMC. Here, we summarized the role of lncRNAs and circRNAs in regulating VSMC phenotypic transformation, as shown in Table 3, which indicates that other ncRNAs also have great potential in the treatment of CVD, including coronary artery disease, hypertension, and aortic coarctation. However, given the limited data reported, this does not provide definitive corroboration and needs to be further explored.
Table 3.
Characterization of VSMC phenotypic transformations regulated by lncRNAs and circRNAs in vascular remodeling for maintaining vascular homeostasis
| ncRNAs | Target gene | Regulation category | Physiological function | Disease type | References |
|---|---|---|---|---|---|
| lncRNA-PEBP1P2 | CDK9 | Repress VSMC proliferation, migration, and dedifferentiation | Suppress neointima formation | Coronary heart disease and carotid atherosclerotic | [175] |
| lncRNA-CARMN | Myocardin | Maintain contractile phenotype of VSMC | Suppress neointima formation | Carotid artery injury | [176] |
| lncRNA-PSR | YBX1 | Promote VSMC proliferation and migration | Promote neointima formation | Spontaneous hypertension | [177] |
| lncRNA-MYOSLID | MKL1 | Promote VSMC differentiation and inhibit proliferation | Amplify VSMC differentiation program | / | [178] |
| lncRNA-AK098656 | MYH11/FN1 | IncreaseVSMC proliferation and migration, elevate extracellular matrix proteins | Narrow resistant arteries | Spontaneous hypertension | [179] |
| LncRNA- MRAK048635_P1 | / | Inhibit VSMC proliferation and migration and promote apoptosis | Reduce vascular damage in hypertension | Essential hypertension | [180] |
| lncRNA-NEAT1 | WDR5 | Promote VSMC proliferation and migration | Promote neointima formation | Occlusive vascular diseases | [181] |
| lncRNA-FOXC2_AS1 | Akt/mTOR | Promote VSMC proliferation, migration, and dedifferentiation | Promote neointima formation | / | [182] |
| lncRNA-SENCR | Myocardin | Inhibit VSMC proliferation, migration and synthetic phenotype-related gene expression | Suppress rupture of mice aortic media | Aortic dissection | [183] |
| circRNA-SATB2 | SM22-α/STIM1 | Promote VSMC proliferation and migration | Promote neointima formation | Coronary heart disease | [184] |
| circRNA-ZXDC | ABCC6 | Increase VSMC proliferation and migration activity | Increase MMD vessels intima thickness | Moyamoya disease | [185] |
| circ-COL1A1 | miR-30a-5p/SMAD30 | Promote VSMC proliferation and migration | Promote human saphenous vein tissue growth | Atherosclerosis | [186] |
Outlook
The identification of molecular mechanisms related to the miRNA-mediated phenotypic transformation of VSMC and the rapid progress in relevant research have enabled screening and identification of miRNAs that are differentially expressed during the phenotypic transformation of VSMC. These miRNAs can act as potential markers of the phenotypic transformation of VSMC that can be used for early clinical diagnosis, prognosis, and efficacy evaluations of diseases, such as atherosclerosis and restenosis after angioplasty, as shown in Table 1. Research on miRNAs that regulate vascular remodeling and homeostasis in vascular lesions has become an area of intense research interest. Previous studies have shown that some miRNAs involved in the phenotypic transformation of VSMC play an important role in the regulation of vascular remodeling and homeostasis. Therefore, the key goal of the current research is to conduct a more comprehensive and thorough investigation of the upstream regulatory factors and downstream target factors of miRNAs involved in the phenotypic transformation of VSMC, which would allow a more detailed understanding of the overall regulatory network of miRNA-mediated VSMC phenotypic transformation. Based on the current research results, the present review identifies potential gene expression regulatory pathways of miRNAs involved in the phenotypic transformation of VSMC.
The occurrence and development of cardiovascular disease is caused by the phenotypic transformation of VSMC, which is accompanied by the differential expression of a large number of specific markers, including SM22α, SMA, and CCND1. As a special marker, miR-21 has considerable potential for cardiovascular disease prediction. For example, miR-21 overexpression increased the spread cell area and proliferative capacity of saphenous VSMC and MMP-1 expression while reducing RECK protein, indicating a transformation to the synthetic phenotype [199]. Conversely, low miR-21 expression downregulated the mRNA and protein expression levels of α-SMA and AP-1, further inhibiting VSMC proliferation, invasion, and migration [200]. miR-21 has similar regulatory effects on TSP-1 and ERK 1/2 signaling [201]. Overall, inhibition of miR-21 expression significantly reduced VSMC proliferation and migration, which may provide new therapeutic targets for some human cardiovascular pathologies [202]. Thus, the development of safe and efficient miR-21 inhibitory drugs may be effective for controlling acute cardiovascular events by reducing VSMC proliferation and migration. Interestingly, if VSMC phenotypically transformed miRNA markers can specifically increase or inhibit the expression of VSMC-specific marker proteins in patients, miRNA gene therapy can be applied and intervene in the expression of target VSMC marker proteins in vivo, providing directions for future CVD or even vascular tumor diseases. Accumulating evidence has shown that miRNAs exhibit great potential in the diagnosis and prognosis of cardiovascular diseases with high sensitivity. For example, the high-level expression of miR-208, miR-134, miR-449, miR-21, miR-133a, miR-29b, miR-566, miR-7-1, miR-92a, miR-455-3p, miR-126, miR-636, miR-486, and miR-129 was significantly indicative of the occurrence of acute myocardial infarction [187–191]. Significant correlation with the development of heart failure was shown by detecting the levels of multiple miRNAs, including miR-320a, miR-22, miR-126, miR-132, and miR-103 [192–194]. Meanwhile, miR-126, miR-182, miR-197, and miR-223 were found to predict the ability of future adverse cardiovascular events in the general population and in patients with known CVD [195, 196]. These results suggest that some miRNAs are specific to CVD and may serve as important biomarkers with clinical applications for the diagnosis and therapy of disease activity. In addition, the major drawbacks of miRNA therapeutic regimens are off-target effects in organ systems. Thus, the implementation of local or cell-type-specific delivery mechanisms using nanoparticles on stents is highly desirable for application in miRNA therapeutics. For example, coating miR-145-containing nanoparticles on drug-eluting stents can further prevent restenosis by enabling VSMC growth around the stent [197]. Similarly, anti-miR-21 can reduce restenosis by reducing peri-coated stent intimal neogenesis [198]. Therefore, the specific role, contribution, and therapeutic potential of miRNA drugs have shown great potential to treat cardiovascular diseases.
Vascular remodeling is reportedly a key step in vascular injury repair that is accompanied by the phenotypic transformation of VSMC characterized by their proliferation [203–205]. Based on the discovery of some miRNA markers for VSMC phenotypic transformation, the in vivo expression of some functional miRNAs that regulate VSMC phenotypic transformation can be upregulated or downregulated to improve pathological vascular remodeling. The miR-143/145 cluster is both a positive and negative regulator of VSMC differentiation and antiproliferation [206]. Based on this dual targeted vascular stress response model, specific gene therapy drugs that target miR-145 can be developed to effectively maintain the phenotypic plasticity of VSMC, significantly inhibit reactive remodeling after vascular injury, and achieve effective prevention and treatment of restenosis, aneurysm, and other diseases after angioplasty [207, 208]. In addition, the high expression of miR-221/miR-222 after vascular injury plays a key role in the transformation of VSMC to the contractile phenotype. This finding suggests that clinical drugs developed to inhibit miR-221/miR-222 in vivo can effectively inhibit synthesis and improve pathological vascular remodeling, which would allow the effective prevention and treatment of vascular restenosis after stenting, coronary artery bypass grafting, heart transplantation, and other procedures [32, 33, 209].
However, several limitations complicate the current understanding of miRNA regulation of phenotypic transformations in vascular smooth muscle. For example, a given miRNA can regulate the expression of many different targets, and a single target can be regulated by multiple miRNAs, suggesting that the mechanisms of specific miR-mRNA interactions and their regulatory patterns during the phenotypic transformation of VSMC are elaborate and complex. Moreover, further investigations are necessary to determine whether other epigenetic conditions, such as DNA methylation and histone modifications, can influence the differential expression of VSMC phenotypic miRNAs, thereby promoting or inhibiting pathological smooth muscle remodeling.
The discovery of miRNA markers for the phenotypic transformation of VSMC and the clarification of their gene expression regulatory mechanisms provide a new platform for further investigation of the molecular mechanisms underlying the phenotypic transformation of VSMC. In-depth investigation of the comprehensive miRNA regulatory network of VSMC phenotypic transformation will contribute to the design of future studies investigating the complex molecular mechanisms of miRNA-mediated VSMC phenotypic transformation. Functional studies of miRNA markers involved in the phenotypic transformation of VSMC that could be used for early diagnosis, treatment, and prognosis and efficacy evaluations of CVD and in the development of gene therapies are likely to become future areas of intense clinical research.
Acknowledgements
The authors would like to thank Dr. Michael A. Hill (Dalton Cardiovascular Research Center, University of Missouri-Columbia) for technical assistance with professional English language editing of the manuscript.
Abbreviations
- VSMC
Vascular smooth muscle cells
- CVD
Cardiovascular diseases
- ncRNA
Non-coding RNA
- miRNA
MicroRNAs
- lncRNAs
Long non-coding RNAs
- circRNAs
Circular RNAs
- PTEN
Phosphatase and tensin homologue
- Bcl-2
B-cell lymphoma 2
- KLF4
Kruppel-like factor 4
- BMP-4
Bone morphogenic protein-4
- PDCD4
Programmed cell death protein 4
- DOCK
Dedicator of cytokinesis
- VEGF
Vascular endothelial-derived growth factor
- PDGF
Platelet-derived growth factor
- TRIB3
Tribbles homologue-3
- RhoA
Ras homologue family member A
- MAPK
Mitogen-activated protein kinase
- ERK
Extracellular signal-related kinase
- PCNA
Proliferating cell nuclear antigen
- LATS2
Large tumor suppressor 2
- CREG
Cellular repressor of E1A-stimulated genes
- TRB3
Tribbles homologue 3
- Sp-1
Specificity protein-1
- ELK1
ETS transcription factor ELK1
- ACE
Angiotensin-converting enzyme
- MYL9
Myosin light chain 9
- MMP-9
Matrix metallopeptidase 9
- STIM1
Stromal interaction molecule 1
- NOR1
Orphan nuclear receptor
- SCF
Stem cell factor
- PAH
Pulmonary arterial hypertension
- HPASMC
Human pulmonary arterial smooth muscle cells
- FoxO4
Forkhead box O4
- FGF9
Fibroblast growth factor 9
- MYADM
Myeloid-associated differentiation
- ECM
Extracellular matrix
- EVI1
Eosinophil integration site 1 protein homologue
- HASMC
Human arterial smooth-muscle cells
- STAT5A
Signal transducer and activator of transcription 5A
- ITGB1
Integrin beta 1
- Myh11
Myosin heavy chain 11
- T2DM
Type 2 diabetes mellitus
- Emp2
Epithelial membrane protein 2
- Cav1
Caveolin-1
- Grb10
Growth factor receptor-bound protein 10
- EGR2
Early growth response 2
- MST2
Mammalian sterile 20-like kinase 2
- MOAP1
Modulator of apoptosis 1
- AAA
Abdominal aortic aneurysm
- HAOSMC
Human aortic smooth muscle cells
- P4HA1
Prolyl 4-hydroxylase subunit alpha 1
- PVAT
Perivascular adipose tissue
- EVs
Extracellular vesicles
- EC
Endothelial cell
- VCAM-1
Vascular cell adhesion molecule 1
- NF-κB
NF-kappa B
- RCN2
Reticulocalbin-2
- STAT3
Signal transducer and activator of transcription 3
- DNMT3A
DNA methyltransferases 3A
- SMA
Smooth Muscle α-actin
- SM22α
Smooth muscle22alpha
- SM-MHC
Smooth muscle-myosin heavy chain
Author contribution
GW, YL, DF, and ML conceived the idea, analysis of literature, and writing of the manuscript; GW and FY read the literature and revised the article; XG, YL, and JW read through and corrected the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [Grant no. 81800434], and Grant of Sichuan Province Science and Technology Agency Grant [2022YFS0627].
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gang Wang and Yulin Luo have contributed equally to this work.
Contributor Information
Dan Fang, Email: fangdan198301@163.com.
Mao Luo, Email: luomao20050908@163.com.
References
- 1.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 2.Basatemur GL, Jørgensen HF, Clarke MCH, et al. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16:727–744. doi: 10.1038/s41569-019-0227-9. [DOI] [PubMed] [Google Scholar]
- 3.Green ID, Liu R, Wong JJL. The expanding role of alternative splicing in vascular smooth muscle cell plasticity. Int J Mol Sci. 2021;22:10213. doi: 10.3390/ijms221910213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miano JM, Fisher EA, Majesky MW. Fate and state of vascular smooth muscle cells in atherosclerosis. Circulation. 2021;143:2110–2116. doi: 10.1161/CIRCULATIONAHA.120.049922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grootaert MOJ, Moulis M, Roth L, et al. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res. 2018;114:622–634. doi: 10.1093/cvr/cvy007. [DOI] [PubMed] [Google Scholar]
- 6.Oller J, Gabandé-Rodríguez E, Ruiz-Rodríguez MJ, et al. Extracellular tuning of mitochondrial respiration leads to aortic aneurysm. Circulation. 2021;143:2091–2109. doi: 10.1161/CIRCULATIONAHA.120.051171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonetti J, Corti A, Lerouge L, et al. Phenotypic modulation of macrophages and vascular smooth muscle cells in atherosclerosis-nitro-redox interconnections. Antioxidants (Basel, Switzerland) 2021;10:516. doi: 10.3390/antiox10040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Su X, Qin Q, et al. New insights into phenotypic switching of VSMCs induced by hyperhomocysteinemia: role of endothelin-1 signaling. Biomed Pharmacother. 2020;123:109758. doi: 10.1016/j.biopha.2019.109758. [DOI] [PubMed] [Google Scholar]
- 9.Durham AL, Speer MY, Scatena M, et al. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res. 2018;114:590–600. doi: 10.1093/cvr/cvy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorokin V, Vickneson K, Kofidis T, et al. Role of vascular smooth muscle cell plasticity and interactions in vessel wall inflammation. Front Immunol. 2020;11:599415. doi: 10.3389/fimmu.2020.599415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabbiani G, Schmid E, Winter S, et al. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci USA. 1981;78:298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babij P, Kelly C, Periasamy M. Characterization of a mammalian smooth muscle myosin heavy-chain gene: complete nucleotide and protein coding sequence and analysis of the 5’ end of the gene. Proc Natl Acad Sci USA. 1991;88:10676–10680. doi: 10.1073/pnas.88.23.10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 14.Ambros V, Lee RC, Lavanway A, et al. MicroRNAs and other tiny endogenous RNAs in C. elegans. Curr Biol. 2003;13:807–818. doi: 10.1016/s0960-9822(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 15.Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234:5451–5465. doi: 10.1002/jcp.27486. [DOI] [PubMed] [Google Scholar]
- 16.Galagali H, Kim JK. The multifaceted roles of microRNAs in differentiation. Curr Opin Cell Biol. 2020;67:118–140. doi: 10.1016/j.ceb.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agbu P, Carthew RW. MicroRNA-mediated regulation of glucose and lipid metabolism. Nat Rev Mol Cell Biol. 2021;22:425–438. doi: 10.1038/s41580-021-00354-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu J, Liu B, Wang Z, et al. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics. 2019;9:6901–6919. doi: 10.7150/thno.37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng Z, Xia L, Fan X, et al. Platelet-derived miR-223 promotes a phenotypic switch in arterial injury repair. J Clin Invest. 2019;129:1372–1386. doi: 10.1172/JCI124508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu F, Zhong J-Y, Lin X, et al. Melatonin alleviates vascular calcification and ageing through exosomal miR-204/miR-211 cluster in a paracrine manner. J Pineal Res. 2020;68:e12631. doi: 10.1111/jpi.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong P, Yu Y, Wang L, et al. circ-Sirt1 controls NF-κB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells. Nucleic Acids Res. 2019;47:3580–3593. doi: 10.1093/nar/gkz141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Wang Y, Zhang L, et al. Reduced platelet miR-223 induction in kawasaki disease leads to severe coronary artery pathology through a miR-223/PDGFRβ vascular smooth muscle cell axis. Circ Res. 2020;127:855–873. doi: 10.1161/CIRCRESAHA.120.316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang G-S, Zheng B, Qin Y, et al. derived miRNAs suppress vascular remodeling through regulating OTUD7B/KLF4/NMHC IIA axis. Theranostics. 2020;10:7787–7811. doi: 10.7150/thno.46911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji R, Cheng Y, Yue J, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 25.Scortegagna M, Lau E, Zhang T, et al. PDK1 and SGK3 contribute to the growth of BRAF-mutant melanomas and are potential therapeutic targets. Cancer Res. 2015;75:1399–1412. doi: 10.1158/0008-5472.CAN-14-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maegdefessel L, Azuma J, Toh R, et al. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci Transl Med. 2012;4:122ra22. doi: 10.1126/scitranslmed.3003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin H, Li DY, Chernogubova E, et al. Local delivery of miR-21 stabilizes fibrous caps in vulnerable atherosclerotic lesions. Mol Ther. 2018;26:1040–1055. doi: 10.1016/j.ymthe.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ajuyah P, Hill M, Ahadi A, et al. MicroRNA (miRNA)-to-miRNA regulation of programmed cell death 4 (PDCD4) Mol Cell Biol. 2019;39:e00086–e119. doi: 10.1128/MCB.00086-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim K, Kim S, Moh SH, Kang H. Kaempferol inhibits vascular smooth muscle cell migration by modulating BMP-mediated miR-21 expression. Mol Cell Biochem. 2015;407:143–149. doi: 10.1007/s11010-015-2464-5. [DOI] [PubMed] [Google Scholar]
- 30.Kang H, Davis-Dusenbery BN, Nguyen PH, et al. Bone morphogenetic protein 4 promotes vascular smooth muscle contractility by activating microRNA-21 (miR-21), which down-regulates expression of family of dedicator of cytokinesis (DOCK) proteins. J Biol Chem. 2012;287:3976–3986. doi: 10.1074/jbc.M111.303156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cordes KR, Sheehy NT, White MP, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis BN, Hilyard AC, Nguyen PH, et al. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem. 2009;284:3728–3738. doi: 10.1074/jbc.M808788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Cheng Y, Zhang S, et al. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albinsson S, Suarez Y, Skoura A, et al. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler Thromb Vasc Biol. 2010;30:1118–1126. doi: 10.1161/ATVBAHA.109.200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nie X, Wang Q, Jiao K. Dicer activity in neural crest cells is essential for craniofacial organogenesis and pharyngeal arch artery morphogenesis. Mech Dev. 2011;128:200–207. doi: 10.1016/j.mod.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sequeira-Lopez MLS, Weatherford ET, Borges GR, et al. The microRNA-processing enzyme dicer maintains juxtaglomerular cells. J Am Soc Nephrol. 2010;21:460–467. doi: 10.1681/ASN.2009090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang Y, Yu S, Liu Y, et al. MicroRNA-124 controls human vascular smooth muscle cell phenotypic switch via Sp1. Am J Physiol Heart Circ Physiol. 2017;313:H641–H649. doi: 10.1152/ajpheart.00660.2016. [DOI] [PubMed] [Google Scholar]
- 38.Boon RA, Seeger T, Heydt S, et al. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circ Res. 2011;109:1115–1119. doi: 10.1161/CIRCRESAHA.111.255737. [DOI] [PubMed] [Google Scholar]
- 39.Zheng B, Zheng C-Y, Zhang Y, et al. Regulatory crosstalk between KLF5, miR-29a and Fbw7/CDC4 cooperatively promotes atherosclerotic development. Biochim Biophys Acta Mol Basis Dis. 2018;1864:374–386. doi: 10.1016/j.bbadis.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 40.Merk DR, Chin JT, Dake BA, et al. miR-29b participates in early aneurysm development in Marfan syndrome. Circ Res. 2012;110:312–324. doi: 10.1161/CIRCRESAHA.111.253740. [DOI] [PubMed] [Google Scholar]
- 41.Yang P, Wu P, Liu X, et al. MiR-26b suppresses the development of Stanford type A aortic dissection by regulating HMGA2 and TGF-β/Smad3 signaling pathway. Ann Thorac Cardiovasc Surg. 2020;26:140–150. doi: 10.5761/atcs.oa.19-00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shan F, Li J, Huang Q-Y. HIF-1 alpha-induced up-regulation of miR-9 contributes to phenotypic modulation in pulmonary artery smooth muscle cells during hypoxia. J Cell Physiol. 2014;229:1511–1520. doi: 10.1002/jcp.24593. [DOI] [PubMed] [Google Scholar]
- 43.Chen W-J, Chen Y-H, Hsu Y-J, et al. MicroRNA-132 targeting PTEN contributes to cilostazol-promoted vascular smooth muscle cell differentiation. Atherosclerosis. 2018;274:1–7. doi: 10.1016/j.atherosclerosis.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Li L, Yun H, Han Y. MiR-138 promotes smooth muscle cells proliferation and migration in db/db mice through down-regulation of SIRT1. Biochem Biophys Res Commun. 2015;463:1159–1164. doi: 10.1016/j.bbrc.2015.06.076. [DOI] [PubMed] [Google Scholar]
- 45.Sun S, Zheng B, Han M, et al. miR-146a and Krüppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Rep. 2011;12:56–62. doi: 10.1038/embor.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Afzal TA, Luong LA, Chen D, et al. NCK Associated protein 1 modulated by miRNA-214 determines vascular smooth muscle cell migration, proliferation, and neointima hyperplasia. J Am Heart Assoc. 2016;5:e004629. doi: 10.1161/JAHA.116.004629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seong M, Kang H. Hypoxia-induced miR-1260b regulates vascular smooth muscle cell proliferation by targeting GDF11. BMB Rep. 2020;53:206–211. doi: 10.5483/BMBRep.2020.53.4.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi S, Park M, Kim J, et al. TNF-α elicits phenotypic and functional alterations of vascular smooth muscle cells by miR-155-5p-dependent down-regulation of cGMP-dependent kinase 1. J Biol Chem. 2018;293:14812–14822. doi: 10.1074/jbc.RA118.004220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu X, Yin D, Zhou B, Li T. MiR-135a promotes inflammatory responses of vascular smooth muscle cells from db/db mice via downregulation of FOXO1. Int Heart J. 2018;59:170–179. doi: 10.1536/ihj.17-040. [DOI] [PubMed] [Google Scholar]
- 50.Gabunia K, Herman AB, Ray M, et al. Induction of MiR133a expression by IL-19 targets LDLRAP1 and reduces oxLDL uptake in VSMC. J Mol Cell Cardiol. 2017;105:38–48. doi: 10.1016/j.yjmcc.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai Z, Lin P, Weng X, et al. MicroRNA-574-5p promotes cell growth of vascular smooth muscle cells in the progression of coronary artery disease. Biomed Pharmacother. 2018;97:162–167. doi: 10.1016/j.biopha.2017.10.062. [DOI] [PubMed] [Google Scholar]
- 52.Yang K, Ren J, Li X, et al. Prevention of aortic dissection and aneurysm via an ALDH2-mediated switch in vascular smooth muscle cell phenotype. Eur Heart J. 2020;41:2442–2453. doi: 10.1093/eurheartj/ehaa352. [DOI] [PubMed] [Google Scholar]
- 53.Farina FM, Hall GF, Serio S, Zani S, Climent M, Salvarani N, Carullo P, Civilini E, Condorelli G, Leonardo Elia MQ, Expand A. Correction to: miR-128-3p Is a novel regulator of vascular smooth muscle cell phenotypic switch and vascular diseases. Circ Res. 2020;127:e141. doi: 10.1161/CIRCRESAHA.120.316489. [DOI] [PubMed] [Google Scholar]
- 54.Gao X-F, Wang Z-M, Chen A-Q, et al. Plasma small extracellular vesicle-carried miRNA-501-5p promotes vascular smooth muscle cell phenotypic modulation-mediated in-stent restenosis. Oxid Med Cell Longev. 2021;2021:6644970. doi: 10.1155/2021/6644970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y, Li H, Xing W, et al. Vascular smooth muscle cell-specific miRNA-214 knockout inhibits angiotensin II-induced hypertension through upregulation of Smad7. FASEB J. 2021;35:e21947. doi: 10.1096/fj.202100766RR. [DOI] [PubMed] [Google Scholar]
- 56.Li R, Jiang Q, Zheng Y. Circ_0002984 induces proliferation, migration and inflammation response of VSMCs induced by ox-LDL through miR-326-3p/VAMP3 axis in atherosclerosis. J Cell Mol Med. 2021;25:8028–8038. doi: 10.1111/jcmm.16734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grootaert MOJ, Finigan A, Figg NL, et al. SIRT6 protects smooth muscle cells from senescence and reduces atherosclerosis. Circ Res. 2021;128:474–491. doi: 10.1161/CIRCRESAHA.120.318353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ouyang S, You J, Zhi C, et al. Ferroptosis: the potential value target in atherosclerosis. Cell Death Dis. 2021;12:782. doi: 10.1038/s41419-021-04054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pi S, Mao L, Chen J, et al. The P2RY12 receptor promotes VSMC-derived foam cell formation by inhibiting autophagy in advanced atherosclerosis. Autophagy. 2021;17:980–1000. doi: 10.1080/15548627.2020.1741202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan MC, Hilyard AC, Wu C, et al. Molecular basis for antagonism between PDGF and the TGFbeta family of signalling pathways by control of miR-24 expression. EMBO J. 2010;29:559–573. doi: 10.1038/emboj.2009.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Talasila A, Yu H, Ackers-Johnson M, et al. Myocardin regulates vascular response to injury through miR-24/-29a and platelet-derived growth factor receptor-β. Arterioscler Thromb Vasc Biol. 2013;33:2355–2365. doi: 10.1161/ATVBAHA.112.301000. [DOI] [PubMed] [Google Scholar]
- 62.Yang X, Dong M, Wen H, et al. MiR-26a contributes to the PDGF-BB-induced phenotypic switch of vascular smooth muscle cells by suppressing Smad1. Oncotarget. 2017;8:75844–75853. doi: 10.18632/oncotarget.17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X, Cheng Y, Chen X, et al. MicroRNA-31 regulated by the extracellular regulated kinase is involved in vascular smooth muscle cell growth via large tumor suppressor homolog 2. J Biol Chem. 2011;286:42371–42380. doi: 10.1074/jbc.M111.261065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J, Yan C-H, Li Y, et al. MicroRNA-31 controls phenotypic modulation of human vascular smooth muscle cells by regulating its target gene cellular repressor of E1A-stimulated genes. Exp Cell Res. 2013;319:1165–1175. doi: 10.1016/j.yexcr.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 65.Kim S, Hata A, Kang H. Down-regulation of miR-96 by bone morphogenetic protein signaling is critical for vascular smooth muscle cell phenotype modulation. J Cell Biochem. 2014;115:889–895. doi: 10.1002/jcb.24730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dong S, Xiong W, Yuan J, et al. MiRNA-146a regulates the maturation and differentiation of vascular smooth muscle cells by targeting NF-κB expression. Mol Med Rep. 2013;8:407–412. doi: 10.3892/mmr.2013.1538. [DOI] [PubMed] [Google Scholar]
- 67.Sahoo S, Meijles DN, Al Ghouleh I, et al. MEF2C-MYOCD and Leiomodin1 suppression by miRNA-214 promotes smooth muscle cell phenotype switching in pulmonary arterial hypertension. PLoS ONE. 2016;11:e0153780. doi: 10.1371/journal.pone.0153780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu X, Ma S-T, Zhou B, Lı T. MiR-9 promotes the phenotypic switch of vascular smooth muscle cells by targeting KLF5. Turkish J Med Sci. 2019;49:928–938. doi: 10.3906/sag-1710-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma Q, Zhang J, Zhang M, et al. MicroRNA-29b targeting of cell division cycle 7-related protein kinase (CDC7) regulated vascular smooth muscle cell (VSMC) proliferation and migration. Ann Transl Med. 2020;8:1496. doi: 10.21037/atm-20-6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang C, Xiao X, Huang L, et al. Role of Kruppel-like factor 4 in atherosclerosis. Clin Chim Acta. 2021;512:135–141. doi: 10.1016/j.cca.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 71.Ghaleb AM, Yang VW. Krüppel-like factor 4 (KLF4): what we currently know. Gene. 2017;611:27–37. doi: 10.1016/j.gene.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao B-J, Wang X-W, Zhu L, et al. MicroRNA-146a sponge therapy suppresses neointimal formation in rat vein grafts. IUBMB Life. 2019;71:125–133. doi: 10.1002/iub.1946. [DOI] [PubMed] [Google Scholar]
- 73.Zheng B, Bernier M, Zhang X, et al. miR-200c-SUMOylated KLF4 feedback loop acts as a switch in transcriptional programs that control VSMC proliferation. J Mol Cell Cardiol. 2015;82:201–212. doi: 10.1016/j.yjmcc.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arevalo-Martinez M, Cidad P, Moreno-Estar S, et al. miR-126 contributes to the epigenetic signature of diabetic vascular smooth muscle and enhances antirestenosis effects of Kv1.3 blockers. Mol. Metab. 2021;53:101306. doi: 10.1016/j.molmet.2021.101306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen J, Yin H, Jiang Y, et al. Induction of microRNA-1 by myocardin in smooth muscle cells inhibits cell proliferation. Arterioscler Thromb Vasc Biol. 2011;31:368–375. doi: 10.1161/ATVBAHA.110.218149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Torella D, Iaconetti C, Catalucci D, et al. MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ Res. 2011;109:880–893. doi: 10.1161/CIRCRESAHA.111.240150. [DOI] [PubMed] [Google Scholar]
- 77.Lovren F, Pan Y, Quan A, et al. MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation. 2012;126:S81–S90. doi: 10.1161/CIRCULATIONAHA.111.084186. [DOI] [PubMed] [Google Scholar]
- 78.Shyu K-G, Cheng W-P, Wang B-W. Angiotensin II downregulates MicroRNA-145 to regulate Kruppel-like factor 4 and myocardin expression in human coronary arterial smooth muscle cells under high glucose conditions. Mol Med. 2015;21:616–625. doi: 10.2119/molmed.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng Y, Liu X, Yang J, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y-N, Xie B-D, Sun L, et al. Phenotypic switching of vascular smooth muscle cells in the “normal region” of aorta from atherosclerosis patients is regulated by miR-145. J Cell Mol Med. 2016;20:1049–1061. doi: 10.1111/jcmm.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ohnaka M, Marui A, Yamahara K, et al. Effect of microRNA-145 to prevent vein graft disease in rabbits by regulation of smooth muscle cell phenotype. J Thorac Cardiovasc Surg. 2014;148:676–682. doi: 10.1016/j.jtcvs.2013.11.054. [DOI] [PubMed] [Google Scholar]
- 82.Nishio H, Minatoya K, Masumoto H. A rabbit venous interposition model mimicking revascularization surgery using vein grafts to assess intimal hyperplasia under arterial blood pressure. J Vis Exp. 2020;15:e60931. doi: 10.3791/60931. [DOI] [PubMed] [Google Scholar]
- 83.Chin DD, Poon C, Wang J, et al. miR-145 micelles mitigate atherosclerosis by modulating vascular smooth muscle cell phenotype. Biomaterials. 2021;273:120810. doi: 10.1016/j.biomaterials.2021.120810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nishio H, Masumoto H, Sakamoto K, et al. MicroRNA-145-loaded poly(lactic-co-glycolic acid) nanoparticles attenuate venous intimal hyperplasia in a rabbit model. J Thorac Cardiovasc Surg. 2019;157:2242–2251. doi: 10.1016/j.jtcvs.2018.08.115. [DOI] [PubMed] [Google Scholar]
- 85.Chen M, Zhang Y, Li W, Yang J. MicroRNA-145 alleviates high glucose-induced proliferation and migration of vascular smooth muscle cells through targeting ROCK1. Biomed Pharmacother. 2018;99:81–86. doi: 10.1016/j.biopha.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 86.Li Y, Huang J, Jiang Z, et al. MicroRNA-145 regulates platelet-derived growth factor-induced human aortic vascular smooth muscle cell proliferation and migration by targeting CD40. Am J Transl Res. 2016;8:1813–1825. [PMC free article] [PubMed] [Google Scholar]
- 87.Boucher JM, Peterson SM, Urs S, et al. The miR-143/145 cluster is a novel transcriptional target of Jagged-1/Notch signaling in vascular smooth muscle cells. J Biol Chem. 2011;286:28312–28321. doi: 10.1074/jbc.M111.221945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim S, Kang H. miR-15b induced by platelet-derived growth factor signaling is required for vascular smooth muscle cell proliferation. BMB Rep. 2013;46:550–554. doi: 10.5483/BMBRep.2013.46.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu F, Ahmed ASI, Kang X, et al. MicroRNA-15b/16 attenuates vascular neointima formation by promoting the contractile phenotype of vascular smooth muscle through targeting YAP. Arterioscler Thromb Vasc Biol. 2015;35:2145–2152. doi: 10.1161/ATVBAHA.115.305748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li P, Zhu N, Yi B, et al. MicroRNA-663 regulates human vascular smooth muscle cell phenotypic switch and vascular neointimal formation. Circ Res. 2013;113:1117–1127. doi: 10.1161/CIRCRESAHA.113.301306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Merlet E, Atassi F, Motiani RK, et al. miR-424/322 regulates vascular smooth muscle cell phenotype and neointimal formation in the rat. Cardiovasc Res. 2013;98:458–468. doi: 10.1093/cvr/cvt045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li P, Liu Y, Yi B, et al. MicroRNA-638 is highly expressed in human vascular smooth muscle cells and inhibits PDGF-BB-induced cell proliferation and migration through targeting orphan nuclear receptor NOR1. Cardiovasc Res. 2013;99:185–193. doi: 10.1093/cvr/cvt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Choe N, Kwon J-S, Kim YS, et al. The microRNA miR-34c inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia by targeting stem cell factor. Cell Signal. 2015;27:1056–1065. doi: 10.1016/j.cellsig.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 94.Jalali S, Ramanathan GK, Parthasarathy PT, et al. Mir-206 regulates pulmonary artery smooth muscle cell proliferation and differentiation. PLoS ONE. 2012;7:e46808. doi: 10.1371/journal.pone.0046808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li H, Xiang Y, Fan L-J, et al. Myocardin inhibited the gap protein connexin 43 via promoted miR-206 to regulate vascular smooth muscle cell phenotypic switch. Gene. 2017;616:22–30. doi: 10.1016/j.gene.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 96.Iaconetti C, De Rosa S, Polimeni A, et al. Down-regulation of miR-23b induces phenotypic switching of vascular smooth muscle cells in vitro and in vivo. Cardiovasc Res. 2015;107:522–533. doi: 10.1093/cvr/cvv141. [DOI] [PubMed] [Google Scholar]
- 97.Au Yeung CL, Tsang TY, Yau PL, Kwok TT. Human papillomavirus type 16 E6 induces cervical cancer cell migration through the p53/microRNA-23b/urokinase-type plasminogen activator pathway. Oncogene. 2011;30:2401–2410. doi: 10.1038/onc.2010.613. [DOI] [PubMed] [Google Scholar]
- 98.Liu H, Hao W, Wang X, Su H. miR-23b targets Smad 3 and ameliorates the LPS-inhibited osteogenic differentiation in preosteoblast MC3T3-E1 cells. J Toxicol Sci. 2016;41:185–193. doi: 10.2131/jts.41.185. [DOI] [PubMed] [Google Scholar]
- 99.Dong N, Wang W, Tian J, et al. MicroRNA-182 prevents vascular smooth muscle cell dedifferentiation via FGF9/PDGFRβ signaling. Int J Mol Med. 2017;39:791–798. doi: 10.3892/ijmm.2017.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun L, Bai Y, Zhao R, et al. Oncological miR-182-3p, a novel smooth muscle cell phenotype modulator, evidences from model rats and patients. Arterioscler Thromb Vasc Biol. 2016;36:1386–1397. doi: 10.1161/ATVBAHA.115.307412. [DOI] [PubMed] [Google Scholar]
- 101.Bai Y, Wang J, Chen Y, et al. The miR-182/Myadm axis regulates hypoxia-induced pulmonary hypertension by balancing the BMP- and TGF-β-signalling pathways in an SMC/EC-crosstalk-associated manner. Basic Res Cardiol. 2021;116:53. doi: 10.1007/s00395-021-00892-6. [DOI] [PubMed] [Google Scholar]
- 102.Liao J, Zhang Y, Wu Y, et al. Akt modulation by miR-145 during exercise-induced VSMC phenotypic switching in hypertension. Life Sci. 2018;199:71–79. doi: 10.1016/j.lfs.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 103.Humphrey JD, Schwartz MA. Vascular mechanobiology: homeostasis, adaptation, and disease. Annu Rev Biomed Eng. 2021;23:1–27. doi: 10.1146/annurev-bioeng-092419-060810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peng Y, Wu S, Li Y, Crane JL. Type H blood vessels in bone modeling and remodeling. Theranostics. 2020;10:426–436. doi: 10.7150/thno.34126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brown IAM, Diederich L, Good ME, et al. Vascular smooth muscle remodeling in conductive and resistance arteries in hypertension. Arterioscler Thromb Vasc Biol. 2018;38:1969–1985. doi: 10.1161/ATVBAHA.118.311229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53:1801887. doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang F, Chen Q, He S, et al. miR-22 is a novel mediator of vascular smooth muscle cell phenotypic modulation and neointima formation. Circulation. 2018;137:1824–1841. doi: 10.1161/CIRCULATIONAHA.117.027799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Han Y, Zhang J, Huang S, et al. MicroRNA-223-3p inhibits vascular calcification and the osteogenic switch of vascular smooth muscle cells. J Biol Chem. 2021;296:100483. doi: 10.1016/j.jbc.2021.100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang J, Hu X, Hu X, et al. MicroRNA-520c-3p targeting of RelA/p65 suppresses atherosclerotic plaque formation. Int J Biochem Cell Biol. 2021;131:105873. doi: 10.1016/j.biocel.2020.105873. [DOI] [PubMed] [Google Scholar]
- 110.Chen Q, Yang F, Guo M, et al. miRNA-34a reduces neointima formation through inhibiting smooth muscle cell proliferation and migration. J Mol Cell Cardiol. 2015;89:75–86. doi: 10.1016/j.yjmcc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 111.Wang Y, Dong C-Q, Peng G-Y, et al. MicroRNA-134-5p regulates media degeneration through inhibiting VSMC phenotypic switch and migration in thoracic aortic dissection. Mol Ther Nucleic Acids. 2019;16:284–294. doi: 10.1016/j.omtn.2019.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Farina FM, Hall IF, Serio S, et al. miR-128-3p is a novel regulator of vascular smooth muscle cell phenotypic switch and vascular diseases. Circ Res. 2020;126:e120–e135. doi: 10.1161/CIRCRESAHA.120.316489. [DOI] [PubMed] [Google Scholar]
- 113.Elia L, Kunderfranco P, Carullo P, et al. UHRF1 epigenetically orchestrates smooth muscle cell plasticity in arterial disease. J Clin Invest. 2018;128:2473–2486. doi: 10.1172/JCI96121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang M, Li F, Wang X, et al. MiR-145 alleviates Hcy-induced VSMC proliferation, migration, and phenotypic switch through repression of the PI3K/Akt/mTOR pathway. Histochem Cell Biol. 2020;153:357–366. doi: 10.1007/s00418-020-01847-z. [DOI] [PubMed] [Google Scholar]
- 115.McCallinhart PE, Cho Y, Sun Z, et al. Reduced stiffness and augmented traction force in type 2 diabetic coronary microvascular smooth muscle. Am J Physiol Heart Circ Physiol. 2020;318:H1410–H1419. doi: 10.1152/ajpheart.00542.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Torella D, Iaconetti C, Tarallo R, et al. miRNA regulation of the hyperproliferative phenotype of vascular smooth muscle cells in diabetes. Diabetes. 2018;67:2554–2568. doi: 10.2337/db17-1434. [DOI] [PubMed] [Google Scholar]
- 117.Reddy MA, Das S, Zhuo C, et al. Regulation of vascular smooth muscle cell dysfunction under diabetic conditions by miR-504. Arterioscler Thromb Vasc Biol. 2016;36:864–873. doi: 10.1161/ATVBAHA.115.306770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang Z, Zheng B, Zhang Y, et al. miR-155-dependent regulation of mammalian sterile 20-like kinase 2 (MST2) coordinates inflammation, oxidative stress and proliferation in vascular smooth muscle cells. Biochim Biophys Acta. 2015;1852:1477–1489. doi: 10.1016/j.bbadis.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 119.Zhang B, Zhang G, Wei T, et al. MicroRNA-25 protects smooth muscle cells against corticosterone-induced apoptosis. Oxid Med Cell Longev. 2019;2019:2691514. doi: 10.1155/2019/2691514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peng J, He X, Zhang L, Liu P. MicroRNA-26a protects vascular smooth muscle cells against H2O2-induced injury through activation of the PTEN/AKT/mTOR pathway. Int J Mol Med. 2018;42:1367–1378. doi: 10.3892/ijmm.2018.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Watterston C, Zeng L, Onabadejo A, Childs SJ. MicroRNA26 attenuates vascular smooth muscle maturation via endothelial BMP signalling. PLoS Genet. 2019;15:e1008163. doi: 10.1371/journal.pgen.1008163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc Natl Acad Sci USA. 2018;115:5839–5848. doi: 10.1073/pnas.1804932115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Farzaei MH, Zobeiri M, Parvizi F, et al. Curcumin in liver diseases: a systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients. 2018;10:855. doi: 10.3390/nu10070855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sivandzade F, Prasad S, Bhalerao A, Cucullo L. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21:101059. doi: 10.1016/j.redox.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Q, Liu J, Duan H, et al. Activation of Nrf2/HO-1 signaling: an important molecular mechanism of herbal medicine in the treatment of atherosclerosis the protection of vascular endothelial cells from oxidative stress. J Adv Res. 2021;34:43–63. doi: 10.1016/j.jare.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van der Pol A, van Gilst WH, Voors AA, van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail. 2019;21:425–435. doi: 10.1002/ejhf.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ornatowski W, Lu Q, Yegambaram M, et al. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. 2020;36:101679. doi: 10.1016/j.redox.2020.101679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ebrahimi SO, Reiisi S, Shareef S. miRNAs, oxidative stress, and cancer: a comprehensive and updated review. J Cell Physiol. 2020;235:8812–8825. doi: 10.1002/jcp.29724. [DOI] [PubMed] [Google Scholar]
- 129.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34:575–584. doi: 10.1016/j.cjca.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chang M, Liu G, Wang Y, et al. Long non-coding RNA LINC00299 knockdown inhibits ox-LDL-induced T/G HA-VSMC injury by regulating miR-135a-5p/XBP1 axis in atherosclerosis. Panminerva Med. 2022;64:38–47. doi: 10.23736/S0031-0808.20.03942-7. [DOI] [PubMed] [Google Scholar]
- 131.Liu Y, Zhong Z, Xiao L, et al. Identification of Circ-FNDC3B, an overexpressed circRNA in abdominal aortic aneurysm, as a regulator of vascular smooth muscle cells. Int Heart J. 2021;62:1387–1398. doi: 10.1536/ihj.21-186. [DOI] [PubMed] [Google Scholar]
- 132.Zhang J, Cai W, Fan Z, et al. MicroRNA-24 inhibits the oxidative stress induced by vascular injury by activating the Nrf2/Ho-1 signaling pathway. Atherosclerosis. 2019;290:9–18. doi: 10.1016/j.atherosclerosis.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 133.Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV. Human miR-221/222 in physiological and atherosclerotic vascular remodeling. Biomed Res Int. 2015;2015:354517. doi: 10.1155/2015/354517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Clause KC, Barker TH. Extracellular matrix signaling in morphogenesis and repair. Curr Opin Biotechnol. 2013;24:830–833. doi: 10.1016/j.copbio.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang X, Hu G, Zhou J. Repression of versican expression by microRNA-143. J Biol Chem. 2010;285:23241–23250. doi: 10.1074/jbc.M109.084673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shi X, Ma W, Pan Y, et al. MiR-126-5p promotes contractile switching of aortic smooth muscle cells by targeting VEPH1 and alleviates Ang II-induced abdominal aortic aneurysm in mice. Lab Invest. 2020;100:1564–1574. doi: 10.1038/s41374-020-0454-z. [DOI] [PubMed] [Google Scholar]
- 138.Sorushanova A, Delgado LM, Wu Z, et al. The collagen suprafamily: from biosynthesis to advanced biomaterial development. Adv Mater. 2019;31:e1801651. doi: 10.1002/adma.201801651. [DOI] [PubMed] [Google Scholar]
- 139.Zhang C, Chen D, Maguire EM, et al. Cbx3 inhibits vascular smooth muscle cell proliferation, migration, and neointima formation. Cardiovasc Res. 2018;114:443–455. doi: 10.1093/cvr/cvx236. [DOI] [PubMed] [Google Scholar]
- 140.Chen W, Yu F, Di M, et al. MicroRNA-124-3p inhibits collagen synthesis in atherosclerotic plaques by targeting prolyl 4-hydroxylase subunit alpha-1 (P4HA1) in vascular smooth muscle cells. Atherosclerosis. 2018;277:98–107. doi: 10.1016/j.atherosclerosis.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 141.Bekelis K, Kerley-Hamilton JS, Teegarden A, et al. MicroRNA and gene expression changes in unruptured human cerebral aneurysms. J Neurosurg. 2016;125:1390–1399. doi: 10.3171/2015.11.JNS151841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 143.Xu S, Ilyas I, Little PJ, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. 2021;73:924–967. doi: 10.1124/pharmrev.120.000096. [DOI] [PubMed] [Google Scholar]
- 144.Poznyak A, Grechko AV, Poggio P, et al. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. 2020;21:1835. doi: 10.3390/ijms21051835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fodor A, Lazar AL, Buchman C, et al. MicroRNAs: the link between the metabolic syndrome and oncogenesis. Int J Mol Sci. 2021;22:6337. doi: 10.3390/ijms22126337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brennan E, Wang B, McClelland A, et al. Protective effect of let-7 miRNA family in regulating inflammation in diabetes-associated atherosclerosis. Diabetes. 2017;66:2266–2277. doi: 10.2337/db16-1405. [DOI] [PubMed] [Google Scholar]
- 147.Li X, Ballantyne LL, Yu Y, Funk CD. Perivascular adipose tissue-derived extracellular vesicle miR-221-3p mediates vascular remodeling. FASEB J. 2019;33:12704–12722. doi: 10.1096/fj.201901548R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Harris TA, Yamakuchi M, Ferlito M, et al. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang D, Sun C, Zhang J, et al. Proliferation of vascular smooth muscle cells under inflammation is regulated by NF-κB p65/microRNA-17/RB pathway activation. Int J Mol Med. 2018;41:43–50. doi: 10.3892/ijmm.2017.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liao X-B, Zhang Z-Y, Yuan K, et al. MiR-133a modulates osteogenic differentiation of vascular smooth muscle cells. Endocrinology. 2013;154:3344–3352. doi: 10.1210/en.2012-2236. [DOI] [PubMed] [Google Scholar]
- 151.Zhao J, Liu Z, Chang Z. Osteogenic differentiation and calcification of human aortic smooth muscle cells is induced by the RCN2/STAT3/miR-155-5p feedback loop. Vascul Pharmacol. 2021;136:106821. doi: 10.1016/j.vph.2020.106821. [DOI] [PubMed] [Google Scholar]
- 152.Choe N, Shin S, Joung H, et al. The microRNA miR-134-5p induces calcium deposition by inhibiting histone deacetylase 5 in vascular smooth muscle cells. J Cell Mol Med. 2020;24:10542–10550. doi: 10.1111/jcmm.15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mackenzie NCW, Staines KA, Zhu D, et al. miRNA-221 and miRNA-222 synergistically function to promote vascular calcification. Cell Biochem Funct. 2014;32:209–216. doi: 10.1002/cbf.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gui T, Zhou G, Sun Y, et al. MicroRNAs that target Ca(2+) transporters are involved in vascular smooth muscle cell calcification. Lab Invest. 2012;92:1250–1259. doi: 10.1038/labinvest.2012.85. [DOI] [PubMed] [Google Scholar]
- 155.Sudo R, Sato F, Azechi T, Wachi H. MiR-29-mediated elastin down-regulation contributes to inorganic phosphorus-induced osteoblastic differentiation in vascular smooth muscle cells. Genes Cells. 2015;20:1077–1087. doi: 10.1111/gtc.12311. [DOI] [PubMed] [Google Scholar]
- 156.Cui R-R, Li S-J, Liu L-J, et al. MicroRNA-204 regulates vascular smooth muscle cell calcification in vitro and in vivo. Cardiovasc Res. 2012;96:320–329. doi: 10.1093/cvr/cvs258. [DOI] [PubMed] [Google Scholar]
- 157.Wen P, Cao H, Fang L, et al. miR-125b/Ets1 axis regulates transdifferentiation and calcification of vascular smooth muscle cells in a high-phosphate environment. Exp Cell Res. 2014;322:302–312. doi: 10.1016/j.yexcr.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 158.Xia Z-Y, Hu Y, Xie P-L, et al. Runx2/miR-3960/miR-2861 positive feedback loop is responsible for osteogenic transdifferentiation of vascular smooth muscle cells. Biomed Res Int. 2015;2015:624037. doi: 10.1155/2015/624037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hao J, Zhang L, Cong G, et al. MicroRNA-34b/c inhibits aldosterone-induced vascular smooth muscle cell calcification via a SATB2/Runx2 pathway. Cell Tissue Res. 2016;366:733–746. doi: 10.1007/s00441-016-2469-8. [DOI] [PubMed] [Google Scholar]
- 160.Liu J, Xiao X, Shen Y, et al. MicroRNA-32 promotes calcification in vascular smooth muscle cells: implications as a novel marker for coronary artery calcification. PLoS ONE. 2017;12:e0174138. doi: 10.1371/journal.pone.0174138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pan W, Liang J, Tang H, et al. Differentially expressed microRNA profiles in exosomes from vascular smooth muscle cells associated with coronary artery calcification. Int J Biochem Cell Biol. 2020;118:105645. doi: 10.1016/j.biocel.2019.105645. [DOI] [PubMed] [Google Scholar]
- 162.Kapustin AN, Shanahan CM. Emerging roles for vascular smooth muscle cell exosomes in calcification and coagulation. J Physiol. 2016;594:2905–2914. doi: 10.1113/JP271340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Guo Y, Bao S, Guo W, et al. Bone marrow mesenchymal stem cell-derived exosomes alleviate high phosphorus-induced vascular smooth muscle cells calcification by modifying microRNA profiles. Funct Integr Genomics. 2019;19:633–643. doi: 10.1007/s10142-019-00669-0. [DOI] [PubMed] [Google Scholar]
- 164.Feng S, Gao L, Zhang D, et al. MiR-93 regulates vascular smooth muscle cell proliferation, and neointimal formation through targeting Mfn2. Int J Biol Sci. 2019;15:2615–2626. doi: 10.7150/ijbs.36995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Park N, Kang H. BMP-induced MicroRNA-101 expression regulates vascular smooth muscle cell migration. Int J Mol Sci. 2020;21:4764. doi: 10.3390/ijms21134764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim GR, Zhao T, Kee HJ, et al. MicroRNA-212-5p and its target PAFAH1B2 suppress vascular proliferation and contraction via the downregulation of RhoA. PLoS ONE. 2021;16:e0249146. doi: 10.1371/journal.pone.0249146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang X, Li H, Zhang Y, et al. Suppression of miR-4463 promotes phenotypic switching in VSMCs treated with Ox-LDL. Cell Tissue Res. 2021;383:1155–1165. doi: 10.1007/s00441-020-03338-y. [DOI] [PubMed] [Google Scholar]
- 168.Zhou B, Wu L-L, Zheng F, et al. miR-31-5p promotes oxidative stress and vascular smooth muscle cell migration in spontaneously hypertensive rats via inhibiting FNDC5 expression. Biomedicines. 2021;9:1009. doi: 10.3390/biomedicines9081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang L, Wang Z, Zhang R, et al. MiR-4787-5p regulates vascular smooth muscle cell apoptosis by targeting PKD1 and inhibiting the PI3K/Akt/FKHR pathway. J Cardiovasc Pharmacol. 2021;78:288–296. doi: 10.1097/FJC.0000000000001051. [DOI] [PubMed] [Google Scholar]
- 170.Han Z-L, Wang H-Q, Zhang T-S, et al. Up-regulation of exosomal miR-106a may play a significant role in abdominal aortic aneurysm by inducing vascular smooth muscle cell apoptosis and targeting TIMP-2, an inhibitor of metallopeptidases that suppresses extracellular matrix degradation. Eur Rev Med Pharmacol Sci. 2020;24:8087–8095. doi: 10.26355/eurrev_202008_22493. [DOI] [PubMed] [Google Scholar]
- 171.Qin Y, Zheng B, Yang G-S, et al. Tanshinone IIA inhibits VSMC inflammation and proliferation in vivo and in vitro by downregulating miR-712-5p expression. Eur J Pharmacol. 2020;880:173140. doi: 10.1016/j.ejphar.2020.173140. [DOI] [PubMed] [Google Scholar]
- 172.Yang B, Gao X, Sun Y, et al. Dihydroartemisinin alleviates high glucose-induced vascular smooth muscle cells proliferation and inflammation by depressing the miR-376b-3p/KLF15 pathway. Biochem Biophys Res Commun. 2020;530:574–580. doi: 10.1016/j.bbrc.2020.07.095. [DOI] [PubMed] [Google Scholar]
- 173.Chong H, Wei Z, Na M, et al. The PGC-1α/NRF1/miR-378a axis protects vascular smooth muscle cells from FFA-induced proliferation, migration and inflammation in atherosclerosis. Atherosclerosis. 2020;297:136–145. doi: 10.1016/j.atherosclerosis.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 174.Lin X, Xu F, Cui R-R, et al. Arterial calcification is regulated via an miR-204/DNMT3a regulatory circuit both in vitro and in female mice. Endocrinology. 2018;159:2905–2916. doi: 10.1210/en.2018-00320. [DOI] [PubMed] [Google Scholar]
- 175.He X, Lian Z, Yang Y, et al. Long Non-coding RNA PEBP1P2 suppresses proliferative VSMCs phenotypic switching and proliferation in atherosclerosis. Mol Ther Nucleic Acids. 2020;22:84–98. doi: 10.1016/j.omtn.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dong K, Shen J, He X, et al. CARMN is an evolutionarily conserved smooth muscle cell-specific LncRNA that maintains contractile phenotype by binding myocardin. Circulation. 2021;144:1856–1875. doi: 10.1161/CIRCULATIONAHA.121.055949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yu J, Wang W, Yang J, et al. LncRNA PSR regulates vascular remodeling through encoding a novel protein arteridin. Circ Res. 2022;131:768–787. doi: 10.1161/CIRCRESAHA.122.321080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhao J, Zhang W, Lin M, et al. MYOSLID is a novel serum response factor-dependent long noncoding RNA that amplifies the vascular smooth muscle differentiation program. Arterioscler Thromb Vasc Biol. 2016;36:2088–2099. doi: 10.1161/ATVBAHA.116.307879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jin L, Lin X, Yang L, et al. AK098656, a novel vascular smooth muscle cell-dominant long noncoding RNA, promotes hypertension. Hypertens (Dallas, Tex. 1979) 2018;71:262–272. doi: 10.1161/HYPERTENSIONAHA.117.09651. [DOI] [PubMed] [Google Scholar]
- 180.Fang G, Qi J, Huang L, Zhao X (2019) LncRNA MRAK048635_P1 is critical for vascular smooth muscle cell function and phenotypic switching in essential hypertension. Biosci Rep 39:BSR20182229 [DOI] [PMC free article] [PubMed]
- 181.Ahmed ASI, Dong K, Liu J, et al. Long noncoding RNA NEAT1 (nuclear paraspeckle assembly transcript 1) is critical for phenotypic switching of vascular smooth muscle cells. Proc Natl Acad Sci USA. 2018;115:E8660–E8667. doi: 10.1073/pnas.1803725115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lim Y-H, Ryu J, Kook H, Kim Y-K. Identification of long noncoding RNAs involved in differentiation and survival of vascular smooth muscle cells. Mol Ther Nucleic Acids. 2020;22:209–221. doi: 10.1016/j.omtn.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Song Y, Wang T, Mu C, et al. LncRNA SENCR overexpression attenuated the proliferation, migration and phenotypic switching of vascular smooth muscle cells in aortic dissection via the miR-206/myocardin axis. Nutr Metab Cardiovasc Dis. 2022;32:1560–1570. doi: 10.1016/j.numecd.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 184.Mao Y-Y, Wang J-Q, Guo X-X, et al. Circ-SATB2 upregulates STIM1 expression and regulates vascular smooth muscle cell proliferation and differentiation through miR-939. Biochem Biophys Res Commun. 2018;505:119–125. doi: 10.1016/j.bbrc.2018.09.069. [DOI] [PubMed] [Google Scholar]
- 185.Liu Y, Huang Y, Zhang X, et al. CircZXDC promotes vascular smooth muscle cell transdifferentiation via regulating miRNA-125a-3p/ABCC6 in moyamoya disease. Cells. 2022;11:3792. doi: 10.3390/cells11233792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ye M, Ni Q, Wang H, et al. CircRNA circCOL1A1 Acts as a sponge of miR-30a-5p to promote vascular smooth cell phenotype switch through regulation of smad1 expression. Thromb Haemost. 2023;123:097–107. doi: 10.1055/s-0042-1757875. [DOI] [PubMed] [Google Scholar]
- 187.Ji X, Takahashi R, Hiura Y, et al. Plasma miR-208 as a biomarker of myocardial injury. Clin Chem. 2009;55:1944–1949. doi: 10.1373/clinchem.2009.125310. [DOI] [PubMed] [Google Scholar]
- 188.Olivieri F, Antonicelli R, Lorenzi M, et al. Diagnostic potential of circulating miR-499-5p in elderly patients with acute non ST-elevation myocardial infarction. Int J Cardiol. 2013;167:531–536. doi: 10.1016/j.ijcard.2012.01.075. [DOI] [PubMed] [Google Scholar]
- 189.Cheng Y, Tan N, Yang J, et al. A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin Sci (Lond) 2010;119:87–95. doi: 10.1042/CS20090645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Widera C, Gupta SK, Lorenzen JM, et al. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J Mol Cell Cardiol. 2011;51:872–875. doi: 10.1016/j.yjmcc.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 191.Grabmaier U, Clauss S, Gross L, et al. Diagnostic and prognostic value of miR-1 and miR-29b on adverse ventricular remodeling after acute myocardial infarction—the SITAGRAMI-miR analysis. Int J Cardiol. 2017;244:30–36. doi: 10.1016/j.ijcard.2017.06.054. [DOI] [PubMed] [Google Scholar]
- 192.Goren Y, Kushnir M, Zafrir B, et al. Serum levels of microRNAs in patients with heart failure. Eur J Heart Fail. 2012;14:147–154. doi: 10.1093/eurjhf/hfr155. [DOI] [PubMed] [Google Scholar]
- 193.Täubel J, Hauke W, Rump S, et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur Heart J. 2021;42:178–188. doi: 10.1093/eurheartj/ehaa898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schwartz Longacre L, Kloner RA, Arai AE, et al. New horizons in cardioprotection: recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation. 2011;124:1172–1179. doi: 10.1161/CIRCULATIONAHA.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kiechl S, Lorenz E, Reindl M, et al. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 196.Schulte C, Molz S, Appelbaum S, et al. miRNA-197 and miRNA-223 predict cardiovascular death in a cohort of patients with symptomatic coronary artery disease. PLoS ONE. 2015;10:e0145930. doi: 10.1371/journal.pone.0145930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Che H-L, Bae I-H, Lim KS, et al. Novel fabrication of MicroRNA nanoparticle-coated coronary stent for prevention of post-angioplasty restenosis. Korean Circ J. 2016;46:23–32. doi: 10.4070/kcj.2016.46.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang D, Deuse T, Stubbendorff M, et al. Local MicroRNA modulation using a novel Anti-miR-21-eluting stent effectively prevents experimental in-stent restenosis. Arterioscler Thromb Vasc Biol. 2015;35:1945–1953. doi: 10.1161/ATVBAHA.115.305597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Alshanwani AR, Riches-Suman K, O’Regan DJ, et al. MicroRNA-21 drives the switch to a synthetic phenotype in human saphenous vein smooth muscle cells. IUBMB Life. 2018;70:649–657. doi: 10.1002/iub.1751. [DOI] [PubMed] [Google Scholar]
- 200.Li Y, Yan L, Zhang W, et al. MicroRNA-21 inhibits platelet-derived growth factor-induced human aortic vascular smooth muscle cell proliferation and migration through targeting activator protein-1. Am J Transl Res. 2014;6:507–516. [PMC free article] [PubMed] [Google Scholar]
- 201.Stein JJ, Iwuchukwu C, Maier KG, Gahtan V. Thrombospondin-1-induced vascular smooth muscle cell migration and proliferation are functionally dependent on microRNA-21. Surgery. 2014;155:228–233. doi: 10.1016/j.surg.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 202.Wang M, Li W, Chang G-Q, et al. MicroRNA-21 regulates vascular smooth muscle cell function via targeting tropomyosin 1 in arteriosclerosis obliterans of lower extremities. Arterioscler Thromb Vasc Biol. 2011;31:2044–2053. doi: 10.1161/ATVBAHA.111.229559. [DOI] [PubMed] [Google Scholar]
- 203.Michel J-B, Jondeau G, Milewicz DM. From genetics to response to injury: vascular smooth muscle cells in aneurysms and dissections of the ascending aorta. Cardiovasc Res. 2018;114:578–589. doi: 10.1093/cvr/cvy006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Méndez-Barbero N, Gutiérrez-Muñoz C, Blanco-Colio LM. Cellular crosstalk between endothelial and smooth muscle cells in vascular wall remodeling. Int J Mol Sci. 2021;22:7284. doi: 10.3390/ijms22147284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wadey K, Lopes J, Bendeck M, George S. Role of smooth muscle cells in coronary artery bypass grafting failure. Cardiovasc Res. 2018;114:601–610. doi: 10.1093/cvr/cvy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hergenreider E, Heydt S, Tréguer K, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 207.Li B, Fan J, Chen N. A novel regulator of type II diabetes: MicroRNA-143. Trends Endocrinol Metab. 2018;29:380–388. doi: 10.1016/j.tem.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 208.Vacante F, Denby L, Sluimer JC, Baker AH. The function of miR-143, miR-145 and the MiR-143 host gene in cardiovascular development and disease. Vascul Pharmacol. 2019;112:24–30. doi: 10.1016/j.vph.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Coleman CB, Lightell DJ, Moss SC, et al. Elevation of miR-221 and -222 in the internal mammary arteries of diabetic subjects and normalization with metformin. Mol Cell Endocrinol. 2013;374:125–129. doi: 10.1016/j.mce.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.