Abstract
Among many nanoparticle-based delivery platforms, liposomes have been particularly successful with many formulations passed into clinical applications. They are well-established and effective gene and/or drug delivery systems, widely used in cancer therapy including breast cancer. In this review we discuss liposome design with the targeting feature and triggering functions. We also summarise the recent progress (since 2014) in liposome-based therapeutics for breast cancer including chemotherapy and gene therapy. We finally identify some challenges on the liposome technology development for the future clinical translation.
Keywords: Breast cancer, Cancer therapy, Liposomal delivery, Triggerable liposomes
Introduction
Breast cancer is one of the most commonly diagnosed cancers (11.7% of the total cases) and the leading cause of cancer death among women worldwide, according to GLOBOCAN 2020 [1]. The estimated number of new cases of breast carcinomas worldwide is expected to increase to 2.50 million and it is predicted that the breast cancer-related mortality will be 768,646 by 2025. Metastatic progression represents the major risk factor affecting the survival rates [2]. In contrast to the primary tumours that can be surgically operated under standard of care approach, the secondary foci of breast cancer are less approachable, and therefore, chemotherapy and radiotherapy are currently the main treatment methods for metastatic breast cancer [3].
Severe side effects and rapidly developing drug resistance of the tumour cells are the main challenges of the conventional chemotherapy. As most of chemotherapeutic drugs are not selective to cancer cells, one of the most important tasks to improve the effectiveness and tolerability of chemotherapy is selective delivery of the therapeutic agent to cancer tissues with simultaneous minimization of the damage of the healthy organs. Drug resistance of malignant cells is another deficiency of chemotherapy, which is attributed to genetical factors, and first of all, to the heterogeneity of the tumour cellular populations [4], as well as to the effects of the tumour microenvironment and the limited tumour tissue penetrating capability of the drugs [5, 6]. At the same time, molecular studies unveiled that the development and progression of breast cancer is governed by the mutated genes’ expression to a significant extent [7]. In this context, gene therapy is emerging to revolutionize the classic breast cancer treatment paradigm [8]. However, gene therapy is also facing a problem regarding to safe and efficient delivery of therapeutic genes or gene-regulating products into the nucleus of mammalian cells.
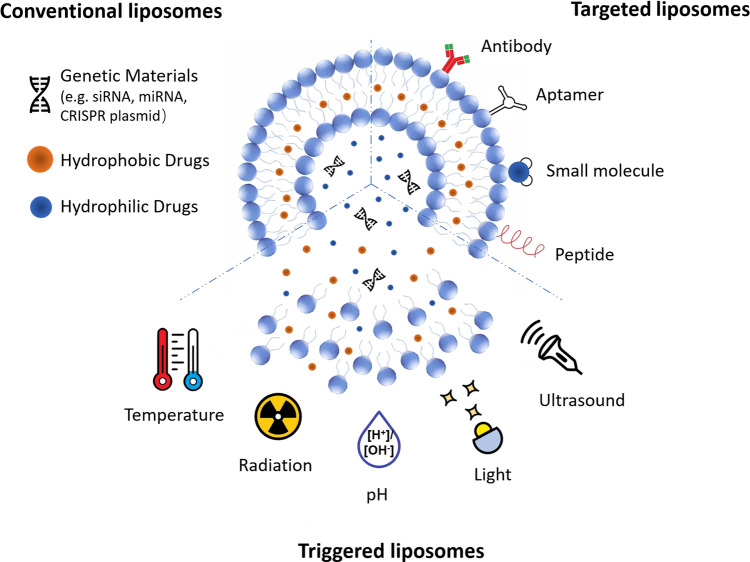
To address this challenge, numerous drug and gene delivery systems have been developed including viral vectors [9–11] and non-viral vectors, such as liposomes [12], polymers [13–15] and inorganic nanomaterials [16]. Viral vectors are the most common gene delivery systems reported in clinical trials, but the safety concerns and limited cargo size are the obstacles hindering their applications [9–11]. To overcome these limitations, nanoparticle-based vectors have been explored [17]. Liposomes are well-established nanomaterials for drug/gene delivery [18, 19]. Among the advantages of liposomes are high loading capacity, convenient preparation and excellent biocompatibility [13–15]. Liposomes are composed of phospholipid molecules which contain hydrophobic tails and hydrophilic heads, forming the amphiphilic vesicle structures in aqueous solutions. Structurally, liposomes are divided into small unilamellar vesicles (~ 100 nm) and large unilamellar vesicles (200–800 nm) with a single bilayer, and multilamellar vesicles (500–5000 nm) containing multiple bilayers [20]. Due to their amphipathic nature in aqueous media, liposomes have the unique capability of entrapment of both hydrophilic and hydrophobic compounds [21]. The hydrophobic drugs can in principle be encapsulated between each bilayer of liposomes, while water-soluble drugs can be efficient loaded in the middle core. The minimization of side effects of anticancer drugs for the patients can be achieved by targeted liposomes [22]. The surface of liposomes can be modified by appropriate ligands to target the specific receptors of breast cancer or its microenvironment to achieve selective delivery. Triggering is another option that allows to control the local dose of the drug and, for example, initiate the drug release at certain time point after accumulation of a required dose, of when the tumour is sensitive [23]. These superior properties make liposomes promising in cancer therapy including breast cancer, compared with other nanoparticle-based delivery systems. Schematic diagram of liposome-based delivery systems with versatile functionalities is shown in Fig. 1.
Fig.1.
Schematic diagram of liposome-based delivery systems with various functions
Liposomal drug loading can be achieved via passive and active strategies [24, 25]. Passive loading employs the procedure in which liposomes are formed concurrently with drug loading, such as the thin lipid film method [26]. During the bilayer formation in aqueous solution, water-soluble substances are passively encapsulated inside the formed vesicles. Although this method is simple, it only allows a low encapsulation efficiency limited by the aqueous solubility of drugs [26]. In contrast, active loading can result in high drug-loading efficiency by changing medium pH to increase aqueous solubility of drugs [27]. In active loading, the loading drugs typically contains an ionizable amine group and liposomes are first prepared with transmembrane pH gradient (an ammonium sulfate gradient), where the pH value of aqueous phases inside and outside the liposomes is different. The pH outside the liposome allows migration of drug dissolved in the external aqueous phase across the lipid bilayer. Once internalised into the liposomes, the drug becomes protonated and subsequently trapped there due to the differing pH, reaching concentration of 250 mM in liposomes [28–31]. Then, this selection criterion may exclude a large number of hydrophobic drugs from the list of candidates for liposomal delivery due to the poor aqueous solubility.
Although the liposomes exhibit superior properties compared with other nanocarriers, they still face another major issue, such as the structural instability. Some unsaturated lipid components from natural sources (egg or soybean phosphatidylcholine) form less stable bilayers that undergo oxidation and/or hydrolysis [32, 33]. This disadvantage may cause leakage of encapsulated payloads and fusion of the damaged liposomes. To avoid the oxidation problem, one could adjust the molar ratio between saturated and unsaturated lipids by increasing the lipid saturation level [34]. Another solution is to add small amounts of antioxidants during the liposome manufacturing steps. To keep the hydrolysis to a minimum, liposome formulations are often lyophilized during the fabrication for longer term storage [35, 36].
In this review, we first discuss breast cancer characteristics and the related drug delivery strategies. Next, we summarise recent achievements in liposome-based drug delivery and gene therapies of breast cancer. A special emphasis is placed on the identification of the key challenges that need to be addressed to improve the utility of liposomes in clinical settings. Finally, we provide our perspectives of further clinical translation of the liposome technology.
Breast cancer characteristics
Breast cancer shares the principal cancer hallmarks with many other cancers [37, 38]. However, this complex disease has some features that differentiate it from other malignant neoplasms. First of all, it develops from the mammary gland cells, which are epithelial cells by embryonic origin and morphology forming ducts and lobules in the healthy organ. According to this, the breast cancer tumours stemming from mammary gland ducts develop ductal carcinoma, and the transformed cells of lobuli form lobular carcinomas [39]. Ductal carcinomas tend to appear as solid tumour masses, sometimes having distorted glandular architecture. The cells of lobular breast carcinomas are most commonly distanced from each other and form files or sheets [39, 40].
Next, breast carcinomas may grow within the borders of the original site within the mammary gland or go beyond them. This defines the small, early stage, carcinomas in situ and invasive carcinomas, respectively. The secondary colonies of breast cancer develop following spreading of metastatic cancer cells to distant organs [41]. Different cancers have different patterns of metastatic spreading (organ tropism of metastases) [42]. This as well makes one of the specific features of breast cancer, which most commonly metastasise to the lungs, bones, liver, and brain [43]. The size of the original tumour, the extent of its invasion and metastatic distribution, including the status if regional lymph nodes and distant organs, defines the stage of the breast cancer by tumour (T), node (N), and metastasis (M) classification [44]. The histological grading system, in parallel, defines the degree of malignant transformation of the mammary gland tissue (in terms of loss of differentiation, nuclear polymorphism and mitosis rate).
Finally, the breast cancer is classified by the intensity of expression of certain molecular markers, such as estrogen receptor (ER), progesterone receptor (PR) and HER2-receptor [45]. This classification links the phenotype of cancer cells with a specific origin (luminal vs. basal cells of the mammary ducts). According to this, there are two luminal subtypes of breast cancer (ER+, PR+, HER2−), triple negative (basal-like) breast cancer (ER−, PR−, HER2−), HER2-enriched type (ER−, PR−, HER2+) and normal-like breast cancer (ER+, PR+, HER2−, with low mitotic rates). This classification is very important for the selection of the treatment strategy of breast cancer. In particular, it indicates that certain subtypes of breast cancer are sensitive to hormones and can be treated with hormone-based targeting [45]. The HER2-enriched breast cancer is an indication for the targeted therapy with the ligands to HER2-receptor [46]. The pharmaceutical treatment options for the triple negative breast cancer are limited due to the absence of the established molecular targets. The molecular subtypes of breast cancer demonstrate different biological behaviour. For example, triple negative breast cancer has the highest invasion potential and anomalously high frequency of hepatic metastases compared to the other subtypes [46]. As a result of combination of the biological features and availability/efficiency of the treatment, the molecular subtypes of breast cancer have different prognosis and survival rates.
The idea for the rational design of the nanoparticle-based drug delivery systems for breast cancer treatment may stem from some features of these tumours. The mammary glands, where the breast cancer originally develops naturally undergoes age-related replacement with adipose and fibrous connective tissue [47–49]. This creates an additional vulnerability of the mammary cells but also brings into light the effects of the tumour microenvironment on the tumour growth and its response on treatment. For example, pro-angiogenic and pro-fibrotic signalling pathways, such as TGF-β1, which also is a key mechanism of epithelial-to-mesenchymal transition (EMT), are commonly upregulated in advanced breast carcinomas [50]. This may result in excessive accumulation of collagenous connective tissue and scar-like hardening of the affected zone. Enhanced angiogenesis, in turn, stimulates overgrowth of blood vessels with abnormal structure, which can result in a notable enhanced permeability and retention effect (EPR) and increased interstitial pressure in the tumour [51]. These factors may affect biodistribution of the nanoscale drug delivery systems (i.e., stimulate accumulation of the nanodrug in the outer parts of the tumour and prevent penetration of the therapeutic agent to the deep part of it). In addition, the interaction between the nanoparticles and breast cancer cells largely depends on the nanoparticle design and modification. For example, the cationic lipid components can be refined to improve liposomes’ cellular uptake capability, thus, increasing therapeutic efficacy on breast cancer [52]. The morphology of the liposome also played an important role in cell-mediated endocytosis. Soft and disordered liposomes exhibit a lower uptake than those with a rigid and ordered lipid membrane [52]. To optimise the therapeutic outcomes, these factors should be taken into account when designing liposomal nanoparticles.
In contrast to many other cancers, the location of the primary breast carcinoma is surgically approachable, and also can be treated with various local applications of physical factors, such as X-rays, ultrasound, light or magnetic fields which could be combined with drug delivery systems. On the other hand, the main danger of breast cancer is not the primary tumours, but the metastatic secondary tumours [53]. Therefore, for the successful eradication of the metastatic breast cancer, the combination of the molecular subtype properties with the challenges of organ-specific microenvironments (e.g., blood–brain barrier for the metastases to the brain) should be considered in the development of the treatment strategy.
Liposome-based drug delivery
Breast cancer treatment by liposome-formulated drugs
Chemotherapy involves the use of anti-cancer drugs and it is a widely used treatment tool. Common chemotherapeutic drugs used for breast cancer include anthracyclines [doxorubicin (DOX), epirubicin (EPR), daunorubicin (DNR)) and taxanes (paclitaxel (PTX), docetaxel (DTX)] [54, 55]. However, they have major shortfalls including unnecessary cytotoxic exposure, systemic toxicity as well as chemoresistance [56]. These limitations became even worse when combining two or more different drugs simultaneously for breast cancer therapy [57]. For example, the co-administration of DOX and PTX exhibited high response rates; however, a major limitation in its clinical use was high levels of cardiotoxicity induced by combinational chemotherapy. Due to their different pharmacokinetics, free PTX interferes with DOX elimination, resulting in high plasma concentrations of the cardiotoxic DOX as well as its highly cardiotoxic metabolite, doxorubicinol (DOXL) [58].
Liposomes, as versatile delivery platforms for various drug encapsulation, offer a promising solution to minimise the toxicity issues of chemotherapeutic drugs [59–63]. Franco et al. found that compared to free PTX and DOX, a 1:10 co-encapsulation ratio of PTX and DOX in liposomes was able to improve cardiac toxicity profile by eliminating pharmacokinetic interactions between PTX and both DXR and its metabolite, doxorubicinol in mice bearing the 4T1 breast tumor [58]. A strategy that could possibly stabilise ratiometric drug delivery by encapsulating drug-loaded liposomes in a thermogel matrix was demonstrated elsewhere [64]. It also was observed that the nanohybrid carriers exhibited a sustained local release. This phenomenon could be explained by the diffusion-controlled process, where the encapsulated anthracycline was first released from the liposomes and then diffused through the hydrogel matrix. In vivo studies confirmed that lower cardiotoxicity level from liposome–hydrogel hybrid delivery system was achieved compared to that of liposomal anthracycline without gel encapsulation [64].
The gradually accumulating evidence indicate that the use of liposomal drug delivery systems can help to overcome multidrug resistance (MDR). Liu et al. developed mitochondrial targeting liposomes via the surface modification with dequalinium (DQ), a positively charged chemical that allows to employ negative mitochondrial membrane potential. Two types of drugs, EPR and quinine (QN), were co-loaded in the liposomes [65]. Facilitated by DQ, the unruptured liposomes entered the cells via phagocytosis and were internalized in mitochondria, where QN and EPR upregulated the proapoptotic protein Bax and downregulated the anti-apoptotic protein Mcl-1. This led to the release of cytochrome complex and the activation of caspases 9 and 3, resulting in a cascade of apoptotic reactions in cancer cells. This study minimised the cellular effect on both extrinsic and intrinsic resistance to drugs via the engineered liposome formulations. However, due to the use of multiple drugs within one liposome delivery system, more rigid and comprehensive evaluation procedures are required prior to clinical rollover.
P-glycoprotein (P-gp) that form efflux pumps are particularly responsible for MDR [66]. P-gp is one of the most common ATP binding cassette transporters that overexpressed in breast cancer cells [67]. An interesting nuclear-targeting strategy allowed to minimise MDR in breast cancer using a liposome platform, where aptamer AS1411 (single stranded DNA) was co-encapsulated with DOX. After the cellular internalisation, the aptamer–DOX complex was released from the liposomes and migrated to the nucleus via the aptamer–nucleolin interaction. This nuclear targeting interaction enabled the evasion of DOX efflux by P-gp pumps. As a result, the therapeutic efficacy was enhanced [66]. Although the in vitro results were promising in this study, in vivo evidence needs to be collected for further assessment and validation.
Other strategies to address the P-gp-aided MDR rely on the inhibition of P-gp expression and consequent enhancement of the drug concentration in the cancer cell environment. Liu et al. reported the liposomes co-loaded with tetrandrine (TET) and DNR and functionalised with wheat germ agglutinin (WGA) [68]. WGA promotes cellular uptake via receptor mediated endocytosis by targeting N-acetyl-d-glucosamine and sialic acid on the surface of cells [69]. TET, loaded delivered inside the lipid bilayers, allowed to overcome chemoresistance by suppressing the expression of P-gp. This suppression enabled a greater concentration of DNR in malignant cells. In vitro studies confirmed that the functionalised liposomes effectively accumulated in cancer cells (MCF-7 and MCF-7/ADR), significantly increased the expression of pro-apoptotic proteins (Bax and Bak) and activated caspase 8, 9 and 3 apoptosis pathways. In vivo studies further validated the therapeutic efficacy of such liposomes by comparing their tumour inhibiting capabilities with other treatment conditions, indicating that the liposome-formulated drugs could be a potential strategy in overcoming MDR in MCF-7 breast cancer cells.
Liposomal drug delivery also has the potential to prevent metastatic progression of breast cancer. In the last decade, it has been confirmed that both EMT and vasculogenic mimicry channels (VMC) play a role in the metastasis and chemoresistance of breast cancer [70, 71]. Therefore, the formulation of synergistic liposomes that suppress these mechanisms and induce cancer cell apoptosis is an interesting field of research. EMT is the process, whereby epithelial cells exhibit decreased adhesion and enhanced migration, transforming into mesenchymal cells [72]. To inhibit EMT mechanism in breast cancer, the commonly used drugs for synergistic liposomes include anthracyclines co-delivered with dioscin (DIS) or dihydroartemisin (DHA) [73, 74]. The last two substances are able to suppress EMT by affecting the regulation of key proteins. DIS is responsible for the upregulation of E-cadherin and downregulation of vascular endothelial growth factor (VEGF), matrix metalloproteinase-9 (MMP-9) and vimentin [73]. DHA works in a similar manner, however, is also responsible for downregulation of TGF-β1 and α5β1-integrin [74]. Both liposome-formulated drugs have been tested for metastatic breast cancer in vitro and in vivo, indicating effective anti-tumour capabilities with minimum toxicity [73, 74]. VMC is the formation of vascular channels lacking endothelial cells [75]. Under the hypoxic condition, aggressive breast cancer cells can also form VMC without the involvement of endothelial cells [75]. Synergistic liposomes have been developed to supress VMC process and induce breast cancer cell apoptosis. Drugs used in such liposomes include anthracycline co-encapsulated with celecoxib or honokiol [76, 77]. In vitro studies demonstrated that both liposomal formulations significantly downregulated the expression of key VMC proteins, resulting in a destruction of these channels [76, 77]. In vivo work further exhibited higher anticancer efficacy of the synergistic liposomes on breast cancer metastasis, compared with other treatment conditions [76, 77]. Overall, arming liposomes with synergistic mechanisms could provide a promising strategy in the treatment and prevention of invasive breast cancer.
Breast cancer active targeting by liposomes
Recent research efforts adapted different targeting strategies to reduce nonspecific toxic effects of conventional chemotherapeutic drugs. The term “active targeting liposomes” refers to the liposomes functionalised with targeting reagents that possess a high affinity to molecules overexpressed by the cells of interest. As a result, such delivery systems can selectively deliver therapeutic agents to primary or metastatic tumours, limiting the probability and the potential severity toxic side effects [78]. Furthermore, active targeting strategies are also capable to overcome resistance incurred by conventional drug delivery systems relying on passive cellular uptake of nanocarriers [63]. By utilising targeting ligands, the functionalized liposome undergoes receptor-mediated endocytosis, which results in rapid cellular internalisation. In contrast to passive targeting, where liposomes diffuse through the cell membrane, the targeting feature also enables the complete evasion of P-gp efflux pumps [67].
A wide range of targeting ligands have been explored and tested in in vitro and in vivo breast cancer therapy, including antibodies, aptamers, small molecules, and peptides [74, 77, 79–86]. Antibodies offer sufficient binding affinities and targeting specificity to the antigens overexpressed by the breast cancer cells. However, they encompass high production costs and complex conjugation methodologies [87]. Similarly, nucleic acid strands known as aptamers, demonstrate a relatively high level of binding affinity and target specificity [88]. However, they are susceptible to nucleic degradation over time and may induce potential immunogenicity [89]. Small molecules are inexpensive in scale-up manufacturing and involve simple conjugation with nanocarriers. They also exhibit minimal cytotoxicity and immunogenicity [90]. Some receptors overexpressed by cancer cells, such as the folate receptor utilise small molecules as their targeting ligand [91]. Peptides, with their relatively small molecular size and weight, also offer high binding affinity and specificity, economic cost of production and low immunogenicity [89]. Table 1 presented a list of receptors and corresponding ligands used in targeted liposomes for breast cancer therapy.
Table 1.
Targeted liposomal drug delivery for breast cancer treatments
| Target | Description | Ligand | Cell line | In vivo work | References |
|---|---|---|---|---|---|
| Somatostatin receptor 2 | Overexpressed in breast cancer cells | Somatostatin analogue (SST) | MDA-MB-231 | Yes | [79] |
| Chemokine Receptor (CXCR4) | Overexpressed in solid breast tumours | AMD3100 |
MCF-7 MDA-MB-231 |
Yes | [80, 81] |
| Mucin1 | Associated with metastasis of tumours | Anti-Muc1 Aptamer | MCF-7 | Yes | [82] |
| Integrin avb3 receptor | Overexpressed in breast cancer cells | Arginine8–glycine–aspartic acid (R8GD) | MDA-MB-435S | Yes | [83] |
| CD44 receptor | Overexpressed in breast cancer cells | Hyaluronic acid |
MCF-7 MDA-MB-435S |
Yes | [77] |
| Somatostatin receptors | Overexpressed in breast cancer cells | Octreotide | MDA-MB-435S | Yes | [74] |
| Urokinase plasminogen activator receptor | Present in early breast cancer lesions | Urokinase-type plasminogen activator |
MCF-7 MDA-MB-231 |
Yes | [84] |
| Folate Receptor | Overexpressed in breast cancers cells | Folic acid | 4T1 | Yes | [85] |
| Epidermal Growth Factor Receptor (EGFR) | Overexpressed in breast cancer cells | Anti-EGFR antibody |
MCF-7 MDA-MB-468 |
Yes | [86] |
| Neuropilin 1 | Overexpression in TNBC | Oleyl-peptide |
MCF-7 MDA-MB-468 |
No | [92] |
Breast cancer treatment by triggerable liposome-based drug delivery
On-demand release of encapsulated drugs from liposomes emerged as a recent advancement. Optimisation of this technology via engineering of triggerable liposomes attracts great attention [93–97]. Various triggering modalities were explored for stimulating an immediate drug release from liposomes and classified into internal and external triggers [93, 96, 98]. Internal triggers correspond to the unique physiological characteristics of tumour microenvironment and include pH variation [99–102] and enzyme effects [103]. Heat [15, 104–108], light [109–113], ultrasound [107] and magnetic fields [108] are among the external triggering sources. These triggering modalities have been widely applied to the liposome technology for breast cancer treatment in preclinical applications. While very important, the triggering mechanisms are not the primary focus of this article as they were comprehensively reviewed, discussed and interpreted recently [109–113].
pH-sensitive liposomes
The pH of extracellular space of the tumour tissues is lower relative to normal cells [114], due to lactate production and increased hydrolysis of ATP by cancer cells. Considering this condition, the pH-sensitive liposomes that can maintain stability in normal physiological conditions, while disassemble and release the drugs in an acidic microenvironment were engineered [115]. These liposomes responded to the variation of pH values between normal and cancerous tissues by releasing the therapeutic payload. Jiang et al. reported a pH-sensitive liposome called Trojan horse liposome encapsulating PTX for breast cancer therapy [99]. The liposome introduced a pH-responsive dimethylmaleic amide (DMA) bond into 1,2-distearoyl-sn-glycero-3-phosphorylethanolamine (DSPE) with a linker of lysine to form DLD/PTX-Lips. In weak acidic pH microenvironment, the cleavage of DMA amide transferred the zeta-potential of liposome from negative to positive, which facilitated intercellular uptake and endosomal escape. As a result, more PTX were released from liposome and drug accumulation in tumour sites was subsequently enhanced. In vitro results showed that the DLD/PTX-Lips exhibited much higher cytotoxicity to 4T1 murine breast cancer cells than free PTX with concentrations from 0.01 to 5 μg/mL and conventional liposomes. In vivo anticancer efficacy was assessed in a mouse model bearing with 4T1cells. The tumour inhibition rate of the DLD/PTX-Lips was 57.4%, significantly higher than that of free PTX (25.1%) and conventional liposome (30.4%).
In addition, certain ligands may promote receptor-mediated endocytosis when bounded with pH-sensitive liposomes for targeted delivery [116]. Silva et al. developed a folate-coated and DOX-loaded pH-sensitive liposomes (SpHL-DOX-Fol), where folate ligand was conjugated to the liposome surface [116]. The release of DOX was increased from 21.5% ± 3.9% to 53.6% ± 5.7% when pH decreased from 7.4 to 5.0. The results in 4T1 cell viability showed that liposomes with low concentration of 0.15 μM had higher cytotoxicity than free drug, but no statistical differences were observed. The in vivo antitumour activities of the thermo-sensitive liposomes were conducted in BALB/c mice bearing 4T1 cells, with the better therapeutic outcomes (68% tumour growth reduction) being observed compared to free DOX and liposome-formulated DOX.
Thermo-sensitive liposomes (TSL)
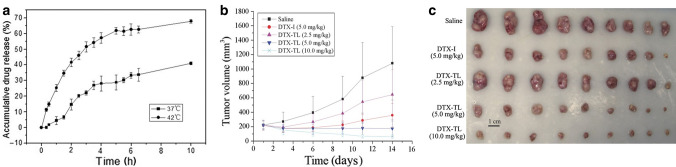
Under normal physiological temperature, the lipid membrane structure of TSL was tightly arranged at the gelatinous state, which protects the encapsulated drug from the diffusion through the membrane. However, when these liposomes were heated to transition temperature (Tm), such as, for example, the Tm of dipalmitoylphosphatidylcholine (DPPC) is 41.5 °C, the lipids underwent a gel-to-liquid phase transition, leading to structure destabilisation and drug release [109, 117, 118]. Various TSL encapsulating anticancer drugs were developed and used for breast cancer treatments [119–122]. Zhang et al. developed a novel thermo-sensitive liposome incorporating DTX (DTX-TL) to improve antitumour effects of the drug [123]. In vitro release studies showed that the drug release at 42 °C was significantly higher than that at 37 °C, indicating the temperature control on drug release (Fig. 2a). For in vivo drug release, the tumour of a mouse model bearing with MCF-7 cells was heated to 42 °C for 30 min using a homemade hyperthermia device connected with a thermostatic circulator. The work displayed that mice treated with TSL exhibited the maximal tumour size reduction compared with the mouse groups treated by other conditions (Fig. 2b, c). TSL can also be engineered to deliver dual drugs via the one platform for enhanced therapeutic efficacy. The co-delivery of tamoxifen and imatinib using TSL was developed by Jose et al. for synergistic breast cancer treatment [119]. More than 80% drugs were released from TSL in 30 min after the temperature was above transition temperature of 39.4 °C. At 40 °C, the growth inhibition of MCF-7 cells treated with this liposome formulation co-encapsulating 5 μM tamoxifen and 3.75 μM imatinib was observed to increase to 86.3 ± 1.5%, compared with the liposomes loaded with the singlet drug at the same concentration (70.6 ± 2.4% for tamoxifen and 43.0 ± 3.3% for imatinib). The enhanced in vitro therapeutic efficacy of the same liposomes in MDA-MB-231 cells was also reported, with the growth inhibition of 66.5 ± 3.9%.
Fig.2.
a Drug release from thermo-sensitive liposomes incorporating DTX (DTX-TL) over time determined at 37 °C and 42 °C, respectively. When temperature was achieved the phase transition temperature (42 °C) of DTX-TL, release of DTX was increased due to thermosensitivity of DTX-TL. b Tumour growth of mice bearing MCF-7 breast carcinoma (n = 9) after the treatment with saline, DTX injection (DTX-I) (5.0 mg/kg) or DTX-TL injection (2.5, 5.0, and 10.0 mg/kg) for every 4 days (a total of four injections). The tumour was then heated at 42 °C for 30 min after the injection. The volume of tumour treated with DTX-TL (10.0 mg/kg) was significantly reduced, suggesting the highest treatment efficacy of the DTX-TL. c Photograph of tumours collected from the mice treated with the same conditions as b,
adapted from ref. [123]
When TSL were used in combination with chemotherapy and thermotherapy, these liposomes demonstrated the dual advantages of temperature-triggered drug release and hyperthermia effect. In addition to chemotherapeutic drug release upon heating, hyperthermia effect is directly cytotoxic to cancer cells at the exposed area, resulting in the improved therapeutic efficacy [124]. Ou et al. developed TSL by utilising gold nanoantennas to generate mild hyperthermia and release DOX from TSL upon illumination by near-infrared laser at 808 nm wavelength. The unique geometry of multibranched gold coated on the surface of the liposomes was utilised to enable the energy transfer from the light to heat, activating the hyperthermia and drug release from TLS simultaneously [120]. In vitro studies revealed the higher toxicity of such TSL towards MDA-MB-231 cells compared to free DOX even at low drug concentration of 0.5 μg/mL (33% vs. 17%). However, this work did not demonstrate the in vivo therapeutic efficacy of the combined treatment via the TSL. To the best of our knowledge, ThermoDOX® was only one thermo-sensitive liposome formulation under Phase I/II clinical trials for cancer therapy [125]. In ThermoDOX®, lysolipids were incorporated into the formulation to lower the liposome phase transition at room temperature, facilitating rapid drug release upon heating. This lysolipid-based liposome formulation containing DOX was developed by Needham et al. and has been invested by Celsion Corp [126]. It was utilised to combine hyperthermia and chemotherapy for treatment of breast patients with chest wall recurrence [127].
Light-sensitive liposomes
External light source is a convenient stimulus employed in the activation of the on-demand release from the liposomes due to its tuneable spectral properties, illumination intensities and times. What’s more, spatial and temporal control of light sources provides an extra flexibility to precisely tune the release of cargo. The mechanism of light-sensitive liposomes can be classified into photophysical effect via molecular absorbers, plasmonic nanoparticles and inorganic nanomaterials and photochemical activation effect including photoisomerization, photocleavage, and photosensitization-induced oxidation [113]. Photosensitization-induced oxidation strategies involves reactive oxygen species (ROS) generation from photosensitisers (PSs) when activated by light illumination at specific wavelengths [128–130]. Singlet oxygen is one type of ROS generated via photosensitiser, which has unpaired electrons and unstable bonds [113]. The unsaturated carbon–carbon bond in lipid chains can be oxidised by singlet oxygen to form hydroperoxides that in turn undergoes decomposition of the lipid bilayers [131].
Based on the triggering mechanism mentioned above, the light-sensitive liposomes could be engineered by incorporating PS into the liposome formulation. Under light illumination, PS was activated to generate singlet oxygen or other ROS, oxidising the lipid components, and causing the destabilisation of the liposome structure.
Verteporfin is a well-known PS that has already been clinically approved for the photodynamic therapy (PDT) of macular degeneration and used for treatment of cancers, such as ophthalmic, small cell lung, dermatological, head and neck, brain, gastroenterological and gynaecological cancers [132]. Sneider et al. designed liposomes loaded with verteporfin for the treatment of triple negative breast cancer (TNBC) [91]. Liposomes were modified with DSPE-PEG2000-folic acid to help the liposomes with cancer targeting capability and enhanced cellular uptake. In vitro studies demonstrated that MDA-MB-231 cells treated with the light-sensitive liposomes at 690 nm light exhibited 33% cell viability. Although this work applied PDT effect to the cancer cells via light-sensitive liposomes, drug release could also be achieved using this triggering mechanism. The light source used in this work has some disadvantages limiting the utility of visible light (380–740 nm) in in vivo therapies. First, limited tissue penetration depth of the visible light does not allow it to sufficiently treat deep tissues; second, light energy in the range of 200–650 nm can be absorbed by many endogenous fluorophores, including epidermis pigments, hemoglobins, and chlorophylls [133]. Compared to shorter wavelengths, the near infrared (NIR, 750–1870 nm) light has the relatively lower absorption of hemoglobin and water, resulting in deeper tissue penetration and making it advantageous for in vivo applications [134]. Yang et al. designed a liposome delivery system that can be triggered by near-infrared light at 808 nm [135]. Lipophilic IR780 was incorporated into the lipid bilayer and hydrophilic chemotherapeutic TPZ was co-loaded into the liposomal core (Lip(IR780&TPZ)). Cell apoptosis analysis showed that the proportion of apoptotic 4T1 cells was about 36.2% after the treatment with Lip(IR780&TPZ) at 808 nm laser irradiation. In vivo studies further demonstrated the tumour size in BALB/c mice bearing 4T1 cellular xenografts treated with Lip(IR780&TPZ) was significantly smaller than that of mouse groups treated with other conditions including free drug, empty liposomes and Lip(IR780&TPZ) without laser irradiation, indicating the enhanced antitumour therapeutic efficacy of light-triggered liposomes for breast cancer.
Ultrasound- and magnetic-sensitive liposomes
Ultrasound waves and magnetic fields were widely explored as an external triggering modality in combination with TSL discussed above [136, 137]. Due to the physical properties of acoustic waves and magnetic fields, local heat can be generated from these two external sources with high intensities, which are more tumour site-specific and non-invasive in practice. In addition, they both exhibited excellent tissue penetration capability [109]. In addition to triggering drug release from TSL, high-intensity focused ultrasound (HIFU) or magnetic fields can kill cancer cells via hyperthermia process. Magnetic resonance guided HIFU combining with ThermoDox® was under phase I clinical study on stage IV HER2-negative breast cancer patients [138].
Another triggering mechanism of ultrasound-sensitive liposomes is based on mechanical cavitation by incorporating the liposomes with microbubbles [139, 140]. Depending on the amplitude and frequency of ultrasound waves as well as the size and properties of microbubbles, stable cavitation or internal cavitation will occur upon the ultrasound triggering. At lower intensities, the microbubbles undergo oscillation (stable cavitation), resulting in local swirling and fluid convection. The corresponding shear stresses in the surrounding fluid can rupture and deform liposomes, leading to the drug release [141]. Low-intensity ultrasound has slight influence on chemical properties and anti-tumour activities of encapsulated drugs [142]. Unlike stable cavitation, internal cavitation under high intensities ultrasound will cause collapses of microbubbles and generate shockwaves which can increase the permeability of membrane [109, 143]. This effect not only induces the drug release from liposomes but also facilitates the cellular uptake of liposomes.
Magnetic-sensitive liposomes can also be used for magnetic resonance imaging (MRI) guided cancer therapy [144]. In general, magnetic nanoparticles (MNPs), such as iron oxides, are encapsulated in liposomes to achieve MRI and drug release simultaneously. The movements of MNPs aligned to external magnetic fields can induce mechanical forces to rupture the liposomes. Furthermore, liposome accumulation at tumour site can also be enhanced by external magnetic field guidance [145]. For examples, Song et al. designed the liposomes co-loaded with magnetic nanocubes and emodin to enhance the chemotherapeutic effect in breast cancers [144]. The in vitro results demonstrated that MCF-7 cell killing effect was increased by 24.1% with the liposome–emodin treatment alone and MRI-mediate tumour target further enhanced the effect of the liposomal chemotherapy by 8.67%. In vivo study confirmed that MRI-guided liposome accumulation within the tumour site in mice bearing 4T1 breast cancer cells was observed and the tumour weight of the treated group was 12 times less than control.
Liposome-based gene therapy for breast cancer
Each breast cancer subtype was associated with gene mutations, causing certain cells in the breast become abnormal. Gene therapy is a promising strategy for treatment of breast cancer subtypes bearing distinct genetic alterations, especially for triple negative breast cancers which cannot be treated by effective targeted therapies due to the loss of receptors [146]. Cationic liposomes are potential gene delivery systems able to naturally complex with the negatively charged DNA [147]. The liposome bilayers can protect complexed nucleic acids against degradation by cell and neutralization by antibodies [148]. In addition, the positive charge of cationic liposomes can facilitate their interaction with the negatively charged cell membrane by endocytosis, resulting in efficient cellular uptake and content release into the cytoplasm [13, 149]. The approach for cancer gene therapy is by encapsulating plasmids [150] and oligonucleotides [151] in cationic liposomes [152]. Notably CRISPR/Cas9 system as the most promising gene-editing technology used for cancer gene therapy will be discussed independently.
Liposome-formulated oligonucleotide therapeutics
Oligonucleotides are short synthetic nucleic acids with the potential to treat or manage a wide range of diseases [153]. These gene agents are capable to modulate expression levels of protein-coding genes by binding to specified sequences within a genome or RNA [154]. Among the various oligonucleotide-based therapies, antisense oligonucleotides (ASOs) and small interfering ribonucleic acids (siRNAs) were the most widely explored and used in research and clinical applications for breast cancer therapy [155–157]. Comprised of a singular RNA strand, ASOs are complementary to messenger RNAs (mRNA) that are responsible for the coding of proteins. As ASOs carry a non-coding RNA (ncRNA) segment, they effectively silence genes of interest by hybridizing to a specific section within mRNA, inhibiting the production of respective proteins. siRNA are artificially synthesized double-stranded RNA molecules. They are widely used for transient silencing of gene of interest, which involves the design and production of a sequence specific to the target mRNA [158]. siRNA cleaves the mRNA through RNA induced silencing protein-complex (RISC)-mediated process [159]. The performance of ASO and siRNA-based therapeutics will pave the way for more clinical trials on cancer therapy. However, there were some challenges for using these agents including their rapid degradation, poor cellular uptake and rapid renal clearance following systemic administration [159, 160]. To overcome these limits and enhance the therapeutic outcomes, many efforts have been made to develop the nanocarriers delivering ASO and siRNA, such as liposomes that have the potential to be an effective vehicle with improved efficacy and safety profiles [161–167].
Sharma et al. developed a cationic liposomal delivery system loaded with ASO to inhibit miRNA-191, an oncogenic miRNA overexpressed on breast cancer tissue attributable to malignant transformation progression [164]. After encapsulating the corresponding antisense oligonucleotide anti-miRNA-191, the in vitro inhibiting efficacy of the liposome delivery platform was tested in MCF-7 and ZR-75-1 breast cancer cell lines. The authors found the liposome-mediated anti-miR-191 delivery exhibited better transfection efficiency of anti-miR-191 in breast cancer cells. Another interesting result obtained from this work indicated that the engineered liposomes alone could inhibit growth of breast cancer cells. Thus, the synergistic effect of stearylamine–liposome in combination with anti-miR-191 displayed elevated levels of cell apoptosis and migration suppression, in addition to elevating chemosensitivity of breast cancer cells to anti-cancer drugs [164].
Another recent work reported synergistic anti-tumour activity of PTX and Polo-like kinase 1 (PLK-1)-targeting siRNA in breast cancer via cationic liposome delivery systems [166]. These liposomes were engineered to co-load PTX and siPLK-1, followed by surface modification with targeting aptamer (AS1411) to further enhance tumour targeting capability. PLK1 mRNA expression level of breast cancer cells (MCF-7) was obviously reduced, with approximately 79% knockdown after the treatment with the liposomes. In addition, tumour growth was significantly inhibited and survival rate of tumour-bearing mice was prolonged after the treatment with such liposomes. Collectively, co-delivery of chemotherapeutic drugs and siRNA via this liposome system may have synergistic anti-breast cancer effect.
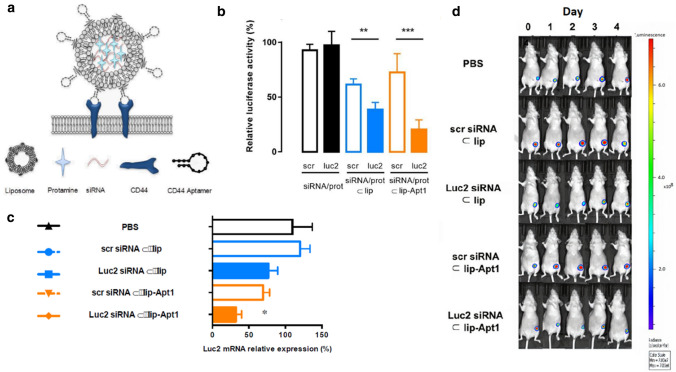
Although cationic liposomes were the most common and well-investigated nanocarriers for ASO and siRNA delivery, they may cause some changes to cells, such as cell shrinking, reduced number of mitoses and vacuolization of the cytoplasm [168]. Therefore, the potential of non-cationic liposomes as gene delivery systems was investigated. Alshaer et al. developed a non-cationic liposomal delivery system loaded with siRNA–protamine (siRNA/prot) complex. Its surface was further modified with the anti-CD44 aptamer (Apt1) to actively target CD44 expressing TNBC cells (Fig. 3a) [167]. CD44, the cell surface glycoprotein, is an appropriate targeting receptor for targeted therapeutics due to its superficial overexpression on tumours. The luciferase (luc2) gene silencing efficacy of this targeted liposomal system was tested in both in vitro (MDA-MB-231-Luc2-eGFP cells) and in vivo settings (TNBC mouse model). The maximal in vitro gene silencing activity was observed in the cells treated with the Apt1 functionalised liposomes (gene expression level of 25.7 ± 15.1%), compared with non-targeted liposomes (47.2 ± 10.6%) (Fig. 3b). The in vivo work further demonstrated the Luc2 mRNA expression level was significantly inhibited by Apt1 functionalised liposomes, compared with PBS control group (Fig. 3c). Furthermore, the observed bioluminescence signal emitted from the tumours exemplifies the tumour inhibiting capability of the siRNA-loaded liposome systems (Fig. 3d).
Fig.3.
a Schematic illustration of anti-CD44 aptamer (Apt-1) conjugated liposomes loaded with siRNA–protamine complex (siRNA/prot). b In vitro Luc2 gene silencing in MDA-MB-231-Luc2-GFP cells after the treatments with scramble siRNA and anti-luc2 siRNA in different forms: free, siRNA–proamine complex (siRNA/prot), loaded in liposomes (siRNA/prot ⊂ lip), and loaded in Apt1-functionalized liposomes (siRNA/prot ⊂ lip-Apt1). Only siRNA/prot ⊂ lip and siRNA/prot ⊂ lip-Apt1 induced specific Luc2 gene expression reduction. Among these two groups, siRNA/prot ⊂ lip-Apt1 exhibited higher inhibiting capability, which may be attributed to its higher cellular uptake compared with non-functionalised liposomes. c Q-Polymerise Chain Reaction (qPCR) results demonstrated the Luc gene silencing effect in a mouse model bearing MDA-MB-231-Luc2-GFP cells treated with PBS, liposomes loaded with scrambled siRNA/prot (scr siRNA ⊂ lip), liposomes loaded with luc2 siRNA/prot (luc2-siRNA/prot ⊂ lip), aptamer-functionalized liposomes loaded with scrambled siRNA/prot (scr siRNA ⊂ lip-Apt1), and aptamer-functionalized liposomes loaded with Luc2 siRNA/prot (Luc2-siRNA ⊂ lip-Apt1). d Bioluminescence signals from tumours of mice treated with different siRNA formulations as c,
adapted from ref. [167]
Alternative gene therapy for breast cancer is using microRNA (miRNA). A miRNA is a short non-coding RNA molecule containing about 20 nucleotides. It was found in plants, animals and some viral cells with the function of regulating gene expression post-transcriptionally [169]. miRNA act as a guide by base-pairing with complementary sequences within mRNA molecules to negatively regulate its expression. This feature is used for silencing specific oncogene by engineered extrinsic miRNA. A number of miRNA formulations have been studied for cancer gene therapy [170, 171]. A functional miRNA liposome was constructed by Yan et al. to treat TNBC by silencing the Slug gene. The 25-nucleotide sense strand of miRNA was encapsulated into DSPE-PEG2000-tLyp-1 peptide-modified functional liposomes. In vitro results showed that Slug gene was silenced and the TGF-β1/Smad pathway was inhibited in TNBC cells, leading to inhibition of invasiveness and growth of TNBC cells. A stronger anticancer effect than functional vinorelbine liposomes was observed in TNBC cancer-bearing mice and nearly complete inhibition of tumour growth was achieved by combining functional miRNA liposomes and functional vinorelbine liposomes [172].
Liposome-formulated CRISPR therapeutics
Over recent years, new genetic editing techniques including zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and clustered regularly interspaced short palindromic repeats (CRISPR) have established themselves as a prominent therapeutic option for various cancers including breast cancer [173–178]. Among these genome editing technologies, CRISPR has emerged as a potential alternative to ZFNs and TALENs due to its preparatory simplicity, high gene editing efficiency and simultaneous multiple loci editing [179, 180]. It became more suitable for preclinical and clinical applications compared to other gene editing technologies. In this approach, a nuclease protein (Cas9) introduces a double-stranded break (DSB) in the target sequence of a DNA molecule, enabling the incorporation of a new sequence into the genome as directed by the guide RNA (gRNA) repair template [181]. So far, CRISPR has been successful in cancer CAR-T immunotherapy to treat primary defects of the immune system, hemoglobinopathies, hemophilia, metabolic disorders, and muscular dystrophy [182–184]. Major advances have recently been made in the clinical applications of CRISPR through the development of therapeutics that can specifically disrupt the expression of disease-relevant genes [185–187]. However, this technology remains at relatively early stages of development and has not been clinically tested for breast cancer yet. This is due to the lack of efficient delivery systems, inadequate transfection efficiency, quick rate of biodegradability and potential off-target effect [188–190]. Viral-based delivery systems have largely been used for CRISPR transfection. However, the major challenge was associated with CRISPR/Cas9-specific immunogenicity induced by viral vectors [191]. As a promising delivery alternative, various non-viral delivery strategies have been explored and developed, including liposome delivery systems [192–195]. Liposome-based CRISPR therapeutics, while few articles were currently reported for breast cancer, appears to have promise in the field of cancer gene therapy [196–198].
Zhang et al. employed a cationic liposomal system to overcome the CRISPR’s inadequate transfection efficiency [199]. The authors constructed a polyethylene–glycol–phospholipid-modified (PLNP) liposome system encapsulating a Cas9/single-guide RNA (sgRNA) plasmid (DNA). To demonstrate the transfection efficiency of such engineered liposomes, the authors selected to knock down polo-like kinase 1 (PLK-1) gene, a master regulator of cancer cell division, using these nanocarriers. In vitro transfection results exhibited higher transfection efficiency of 37.8% in breast cancer cells (MCF-7) treated with PLNP containing CRISPR/sgRNA plasmid, compared to the Lipofectamine2000 (a commercial liposome transfection agent) which demonstrated 3.15% only. This work did not show the in vivo therapeutic effect of this liposome-formulated CRISPR technology in breast cancer. However, the authors claimed the in vivo efficacy of these liposomes in a mouse model bearing melanoma cells (A375).
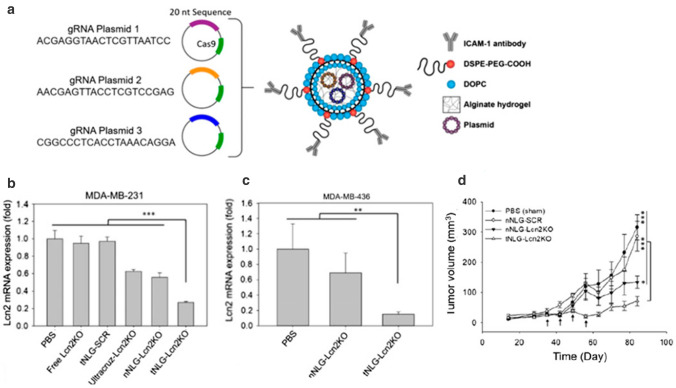
Guo et al. applied a noncationic, tumour-targeting liposome–hydrogel hybrid system to knock out Lipocalin 2 (Lcn2), a breast cancer-promoting gene, through CRISPR-based genome editing [200]. This system encapsulated three CRISPR plasmids encoding a Cas9 nuclease and a guide RNA sequence for identification and disruption of the Lcn2 gene in the genome of targeted human TNBC cells (Fig. 4a). The in vitro genome editing efficiency demonstrated that Lcn2 mRNA expression levels in TNBC cells were largely reduced, with ~ 80% of Lcn2 loss observed in both MDA-MB-231 and MDA-MB-436 cell lines (Fig. 4b, c). In vivo therapeutic efficacy of this liposome system was tested in a mouse model bearing MDA-MB-231 cells. The nanocarrier treated mouse group displayed significant inhibition of tumour growth by 77% in volume, compared with other treatment conditions (Fig. 4d). The results obtained from these two studies indicated liposomes may be considered as a promising delivery formulation for enhanced transfection of CRISPR and subsequently therapeutic effect in breast cancer. It is notable that in these two studies the authors used CRISPR plasmid DNA to achieve gene knockdown effect. However, the major issue associated with plasmid DNA was high levels of unintended gene edits due to the relatively long period that plasmids persist inside cells, which would hamper the clinical translation of CRISPR technology [201].
Fig.4.
a Schematic diagram of the engineered liposome–hydrogel structure and CRISPR Cas9 genetic materials. In vitro Lnc2 gene editing efficacy of liposome-formulated CRISPR in b MDA-MB-231 cells and c MDA-MB-436 cells. Cells were treated with PBS, free Lcn2 CRISPR knockout plasmid (free Lcn2KO), tNLGs encapsulating scrambled CRISPR plasmid (tNLG-SCR), a complex of ultracruz and Lcn2 CRISPR knockout plasmid (Ultracruz-Lcn2KO), a nonspecific liposome–hydrogel encapsulating Lcn2 CRISPR knockout plasmid (nNLGLcn2KO), and a TNBC-specific liposome–hydrogel encapsulating Lcn2 CRISPR knockout plasmid (tNLG-Lcn2KO). d Weekly tumour volume measurement demonstrating tumour progression over 84 days after the different treatments indicated in the figure,
adapted from ref. [200]
Conclusions
The experimental development of liposome delivery systems is progressing at a fast pace, following the demand for the new strategies for breast cancer treatment. However, there is no well-developed understanding or road map on the design of the new liposome formulation for breast cancer. Selection of the targeting and triggering modalities in most publications largely depends on the molecular subtypes of the tumour and the ongoing conventional treatments. Despite the traditional liposome-formulated chemotherapeutic drugs have been widely used in the clinical practice in breast cancer treatment, there are some barriers for the clinical implementation of these new liposome formulations. In the case of the triggerable liposomes, the triggering mechanisms need to be further investigated when designing such liposome formulations. For example, the choice of the phospholipid component for light-triggered liposomes needs to be based on the desired photo-induced mechanisms. If a photochemical pathway, such as photo-oxidative reaction, is applied, unsaturated phospholipids would be used in the formulation. In addition, active ingredients used in the triggerable liposome formulation also need to be optimized to weight up their benefits and risks to the healthy tissues.
From the perspective of the clinical applications, we envision that far-reaching development of the liposome technology will eventually benefit the breast cancer patients. Many studies confirmed that various liposome constructs loaded with drugs can lower the levels of cardiotoxicity, address drug resistance and improve the overall drug release profile. By modifying the liposome surface with targeting ligands, these liposomes additionally offer opportunities for designing site-specific therapy, minimising non-specific effect of traditional chemotherapeutic drugs. The new generation liposomes with triggering features even allows exquisite control of payload release, largely enhancing the therapeutic outcomes for breast cancer patients. We believe that these liposome formulations would expand the range of drug/gene delivery options for the treatments of breast cancer, addressing the critical problems of drug toxicity and limited therapeutic effects.
Acknowledgements
This project was supported by the Sydney Vital, Translational Cancer Research, Sydney, NSW, Australia, through a Cancer Institute NSW competitive grant. The views expressed herein are those of the authors and are not necessarily those of the Cancer Institute NSW.
Author contributions
BY had the idea for the article, performed the literature search and drafted the manuscript, BS and SS performed the literature search and drafted some parts of the manuscript, AG contributed to breast cancer characterisation part, and WD supervised the work and revised the entire manuscript.
Funding
This project was supported by the Sydney Vital, Translational Cancer Research, through a Cancer Institute NSW competitive grant.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021 doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Martin TA, Ye L, Sanders AJ, Lane J, Jiang WG. Madame Curie bioscience database. Austin: Landes Bioscience; 2013. Cancer invasion and metastasis: molecular and cellular perspective. [Google Scholar]
- 3.Al-Mahmood S, Sapiezynski J, Garbuzenko OB, Minko T. Metastatic and triple-negative breast cancer: challenges and treatment options. Drug Deliv Transl Res. 2018;8(5):1483–1507. doi: 10.1007/s13346-018-0551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim ZF, Ma PC. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J Hematol Oncol. 2019;12(1):134. doi: 10.1186/s13045-019-0818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99(19):1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 6.Tannock IF, Lee CM, Tunggal JK, Cowan DS, Egorin MJ. Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clin Cancer Res. 2002;8(3):878–884. [PubMed] [Google Scholar]
- 7.Yang HT, Jaeger M, Walker A, Wei D, Leiker K, Tao WT. Break breast cancer addiction by CRISPR/Cas9 genome editing. J Cancer. 2018;9(2):219–231. doi: 10.7150/jca.22554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E, Group EGW Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii11–vii19. doi: 10.1093/annonc/mds232. [DOI] [PubMed] [Google Scholar]
- 9.Huo WY, Zhao GN, Yin JG, Ouyang X, Wang YN, Yang CH, Wang BJ, Dong PX, Wang ZX, Watari H, Chaum E, Pfeffer LM, Yue JM. Lentiviral CRISPR/Cas9 vector mediated miR-21 gene editing inhibits the epithelial to mesenchymal transition in ovarian cancer cells. J Cancer. 2017;8(1):57–64. doi: 10.7150/jca.16723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li A, Lee CM, Hurley AE, Jarrett KE, De Giorgi M, Lu WQ, Balderrama KS, Doerfler AM, Deshmukh H, Ray A, Bao G, Lagor WR. A self-deleting AAV-CRISPR system for in vivo genome editing. Mol Ther-Meth Clin D. 2019;12:111–122. doi: 10.1016/j.omtm.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maggio I, Zittersteijn HA, Wang Q, Liu J, Janssen JM, Ojeda IT, van der Maarel SM, Lankester AC, Hoeben RC, Goncalves MAFV. Integrating gene delivery and gene-editing technologies by adenoviral vector transfer of optimized CRISPR-Cas9 components. Gene Ther. 2020;27:209–225. doi: 10.1038/s41434-019-0119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zununi Vahed S, Salehi R, Davaran S, Sharifi S. Liposome-based drug co-delivery systems in cancer cells. Mater Sci Eng C Mater Biol Appl. 2017;71:1327–1341. doi: 10.1016/j.msec.2016.11.073. [DOI] [PubMed] [Google Scholar]
- 13.Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, Maeder ML, Joung JK, Chen ZY, Liu DR. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat Biotechnol. 2015;33(1):73–80. doi: 10.1038/nbt.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Zuris JA, Meng FT, Rees H, Sun S, Deng P, Han Y, Gao X, Pouli D, Wu Q, Georgakoudi I, Liu DR, Xu QB. Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles. Proc Natl Acad Sci USA. 2016;113(11):2868–2873. doi: 10.1073/pnas.1520244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen WJ, Deng W, Goldys EM. Light-triggerable liposomes for enhanced endolysosomal escape and gene silencing in PC12 cells. Mol Ther-Nucl Acids. 2017;7:366–377. doi: 10.1016/j.omtn.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma PA, Xiao HH, Li CX, Dai YL, Cheng ZY, Hou ZY, Lin J. Inorganic nanocarriers for platinum drug delivery. Mater Today. 2015;18(10):554–564. doi: 10.1016/j.mattod.2015.05.017. [DOI] [Google Scholar]
- 17.Wang HX, Li M, Lee CM, Chakraborty S, Kim HW, Bao G, Leong KW. CRISPR/Cas9-based genome editing for disease modeling and therapy: challenges and opportunities for nonviral delivery. Chem Rev. 2017;117(15):9874–9906. doi: 10.1021/acs.chemrev.6b00799. [DOI] [PubMed] [Google Scholar]
- 18.Pattni BS, Chupin VV, Torchilin VP. New developments in liposomal drug delivery. Chem Rev. 2015;115(19):10938–10966. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- 19.Zylberberg C, Gaskill K, Pasley S, Matosevic S. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther. 2017;24(8):441–452. doi: 10.1038/gt.2017.41. [DOI] [PubMed] [Google Scholar]
- 20.Chinnagounder Periyasamy P, Leijten JC, Dijkstra PJ, Karperien M, Post JN. Nanomaterials for the local and targeted delivery of osteoarthritis drugs. J Nanomater. 2012;2012:1–13. doi: 10.1155/2012/673968. [DOI] [Google Scholar]
- 21.Olusanya TOB, Ahmad RRH, Ibegbu DM, Smith JR, Elkordy AA. Liposomal drug delivery systems and anticancer drugs. Molecules. 2018;23(4):907. doi: 10.3390/molecules23040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Federman N, Denny CT. Targeting liposomes toward novel pediatric anticancer therapeutics. Pediatr Res. 2010;67(5):514–519. doi: 10.1203/PDR.0b013e3181d601c5. [DOI] [PubMed] [Google Scholar]
- 23.Moussa HG, Martins AM, Husseini GA. Review on triggered liposomal drug delivery with a focus on ultrasound. Curr Cancer Drug Tar. 2015;15(4):282–313. doi: 10.2174/1568009615666150311100610. [DOI] [PubMed] [Google Scholar]
- 24.Pauli G, Tang WL, Li SD. Development and characterization of the solvent-assisted active loading technology (SALT) for liposomal loading of poorly water-soluble compounds. Pharmaceutics. 2019 doi: 10.3390/pharmaceutics11090465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009;30(11):592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Barenholz Y. Relevancy of drug loading to liposomal formulation therapeutic efficacy. J Liposome Res. 2003;13(1):1–8. doi: 10.1081/lpr-120017482. [DOI] [PubMed] [Google Scholar]
- 27.Gubernator J. Active methods of drug loading into liposomes: recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin Drug Deliv. 2011;8(5):565–580. doi: 10.1517/17425247.2011.566552. [DOI] [PubMed] [Google Scholar]
- 28.Lee C, Na K. Anthocyanin-loaded liposomes prepared by the pH-gradient loading method to enhance the anthocyanin stability, antioxidation effect and skin permeability. Macromol Res. 2020;28(3):289–297. doi: 10.1007/s13233-020-8039-7. [DOI] [Google Scholar]
- 29.Abraham SA, Edwards K, Karlsson G, Hudon N, Mayer LD, Bally MB. An evaluation of transmembrane ion gradient-mediated encapsulation of topotecan within liposomes. J Control Release. 2004;96(3):449–461. doi: 10.1016/j.jconrel.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Fritze A, Hens F, Kimpfler A, Schubert R, Peschka-Suss R. Remote loading of doxorubicin into liposomes driven by a transmembrane phosphate gradient. Biochim Biophys Acta. 2006;1758(10):1633–1640. doi: 10.1016/j.bbamem.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim Biophys Acta. 1993;1151(2):201–215. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 32.Samuni AM, Lipman A, Barenholz Y. Damage to liposomal lipids: protection by antioxidants and cholesterol-mediated dehydration. Chem Phys Lipids. 2000;105(2):121–134. doi: 10.1016/s0009-3084(99)00136-x. [DOI] [PubMed] [Google Scholar]
- 33.Anchordoquy TJ, Koe GS. Physical stability of nonviral plasmid-based therapeutics. J Pharm Sci. 2000;89(3):289–296. doi: 10.1002/(SICI)1520-6017(200003)89:3<289::AID-JPS1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 34.Rudolphi-Skorska E, Filek M, Zembala M. The effects of the structure and composition of the hydrophobic parts of phosphatidylcholine-containing systems on phosphatidylcholine oxidation by ozone. J Membr Biol. 2017;250(5):493–505. doi: 10.1007/s00232-017-9976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Payton NM, Wempe MF, Xu Y, Anchordoquy TJ. Long-term storage of lyophilized liposomal formulations. J Pharm Sci. 2014;103(12):3869–3878. doi: 10.1002/jps.24171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crowe LM, Womersley C, Crowe JH, Reid D, Appel L, Rudolph A. Prevention of fusion and leakage in freeze-dried liposomes by carbohydrates. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1986;861:131–140. doi: 10.1016/0005-2736(86)90411-6. [DOI] [Google Scholar]
- 37.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 38.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Makki J. Diversity of breast carcinoma: histological subtypes and clinical relevance. Clin Med Insights Pathol. 2015;8:23–31. doi: 10.4137/CPath.S31563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCart Reed AE, Kutasovic JR, Lakhani SR, Simpson PT. Invasive lobular carcinoma of the breast: morphology, biomarkers and 'omics. Breast Cancer Res. 2015;17:12. doi: 10.1186/s13058-015-0519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sainsbury JR, Anderson TJ, Morgan DA. ABC of breast diseases: breast cancer. BMJ. 2000;321(7263):745–750. doi: 10.1136/bmj.321.7263.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen WJ, Hoffmann AD, Liu HP, Liu X. Organotropism: new insights into molecular mechanisms of breast cancer metastasis. Npj Precis Oncol. 2018;2:1–2. doi: 10.1038/s41698-018-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu X, Kang Y. Organotropism of breast cancer metastasis. J Mammary Gland Biol Neoplasia. 2007;12(2–3):153–162. doi: 10.1007/s10911-007-9047-3. [DOI] [PubMed] [Google Scholar]
- 44.Singletary SE, Connolly JL (2006) Breast cancer staging: working with the sixth edition of the AJCC Cancer Staging Manual. CA Cancer J Clin 56(1):37–47. 10.3322/canjclin.56.1.37(quiz 50–31) [DOI] [PubMed]
- 45.Higgins MJ, Baselga J. Targeted therapies for breast cancer. J Clin Invest. 2011;121(10):3797–3803. doi: 10.1172/JCI57152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jamdade VS, Sethi N, Mundhe NA, Kumar P, Lahkar M, Sinha N. Therapeutic targets of triple-negative breast cancer: a review. Br J Pharmacol. 2015;172(17):4228–4237. doi: 10.1111/bph.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mancuso P, Bouchard B. The impact of aging on adipose function and adipokine synthesis. Front Endocrinol (Lausanne) 2019;10:137. doi: 10.3389/fendo.2019.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sims AH, Howell A, Howell SJ, Clarke RB. Origins of breast cancer subtypes and therapeutic implications. Nat Clin Pract Oncol. 2007;4(9):516–525. doi: 10.1038/ncponc0908. [DOI] [PubMed] [Google Scholar]
- 49.Nie K, Su MY, Chau MK, Chan S, Nguyen H, Tseng T, Huang Y, McLaren CE, Nalcioglu O, Chen JH. Age- and race-dependence of the fibroglandular breast density analyzed on 3D MRI. Med Phys. 2010;37(6):2770–2776. doi: 10.1118/1.3426317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Kruijf EM, Dekker TJA, Hawinkels L, Putter H, Smit V, Kroep JR, Kuppen PJK, van de Velde CJH, Ten Dijke P, Tollenaar R, Mesker WE. The prognostic role of TGF-beta signaling pathway in breast cancer patients. Ann Oncol. 2013;24(2):384–390. doi: 10.1093/annonc/mds333. [DOI] [PubMed] [Google Scholar]
- 51.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–284. doi: 10.1016/S0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 52.Abumanhal-Masarweh H, da Silva D, Poley M, Zinger A, Goldman E, Krinsky N, Kleiner R, Shenbach G, Schroeder JE, Shklover J, Shainsky-Roitman J, Schroeder A. Tailoring the lipid composition of nanoparticles modulates their cellular uptake and affects the viability of triple negative breast cancer cells. J Control Release. 2019;307:331–341. doi: 10.1016/j.jconrel.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen MT, Sun HF, Zhao Y, Fu WY, Yang LP, Gao SP, Li LD, Jiang HL, Jin W. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population-based analysis. Sci Rep. 2017;7(1):9254. doi: 10.1038/s41598-017-10166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31(1):63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willson ML, Burke L, Ferguson T, Ghersi D, Nowak AK, Wilcken N. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev. 2019;9(9):Cd004421. doi: 10.1002/14651858.CD004421.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bazak R, Houri M, El Achy S, Kamel S, Refaat T. Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol. 2015;141(5):769–784. doi: 10.1007/s00432-014-1767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mayer LD, Janoff AS. Optimizing combination chemotherapy by controlling drug ratios. Mol Interv. 2007;7(4):216–223. doi: 10.1124/mi.7.4.8. [DOI] [PubMed] [Google Scholar]
- 58.Franco MS, Roque MC, de Barros ALB, de Oliveira SJ, Cassali GD, Oliveira MC. Investigation of the antitumor activity and toxicity of long-circulating and fusogenic liposomes co-encapsulating paclitaxel and doxorubicin in a murine breast cancer animal model. Biomed Pharmacother. 2019;109:1728–1739. doi: 10.1016/j.biopha.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 59.Zhao M, Ding XF, Shen JY, Zhang XP, Ding XW, Xu B. Use of liposomal doxorubicin for adjuvant chemotherapy of breast cancer in clinical practice. J Zhejiang Univ Sci B. 2017;18(1):15–26. doi: 10.1631/jzus.B1600303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Din FU, Aman W, Ullah I, Qureshi OS, Mustapha O, Shafique S, Zeb A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomed. 2017;12:7291–7309. doi: 10.2147/ijn.S146315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong M, Luo L, Ying X, Lu X, Shen J, Jiang Z, Wang L. Comparable efficacy and less toxicity of pegylated liposomal doxorubicin versus epirubicin for neoadjuvant chemotherapy of breast cancer: a case-control study. Onco Targets Ther. 2018;11:4247–4252. doi: 10.2147/ott.S162003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomed. 2012;7:49–60. doi: 10.2147/ijn.S26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moosavian SA, Bianconi V, Pirro M, Sahebkar A. Challenges and pitfalls in the development of liposomal delivery systems for cancer therapy. Semin Cancer Biol. 2019 doi: 10.1016/j.semcancer.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 64.Cao D, Zhang X, Akabar MD, Luo Y, Wu H, Ke X, Ci T. Liposomal doxorubicin loaded PLGA-PEG-PLGA based thermogel for sustained local drug delivery for the treatment of breast cancer. Artif Cells Nanomed Biotechnol. 2019;47(1):181–191. doi: 10.1080/21691401.2018.1548470. [DOI] [PubMed] [Google Scholar]
- 65.Liu L, Mu LM, Yan Y, Wu JS, Hu YJ, Bu YZ, Zhang JY, Liu R, Li XQ, Lu WL. The use of functional epirubicin liposomes to induce programmed death in refractory breast cancer. Int J Nanomed. 2017;12:4163–4176. doi: 10.2147/IJN.S133194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X, Wu X, Yang H, Li L, Ye Z, Rao Y. A nuclear targeted Dox-aptamer loaded liposome delivery platform for the circumvention of drug resistance in breast cancer. Biomed Pharmacother. 2019;117:109072. doi: 10.1016/j.biopha.2019.109072. [DOI] [PubMed] [Google Scholar]
- 67.Muley H, Fado R, Rodriguez-Rodriguez R, Casals N. Drug uptake-based chemoresistance in breast cancer treatment. Biochem Pharmacol. 2020;177:113959. doi: 10.1016/j.bcp.2020.113959. [DOI] [PubMed] [Google Scholar]
- 68.Liu S, Song XL, Wang YH, Wang XM, Xiao Y, Wang X, Cheng L, Li XT. The efficacy of WGA modified daunorubicin anti-resistant liposomes in treatment of drug-resistant MCF-7 breast cancer. J Drug Target. 2017;25(6):541–553. doi: 10.1080/1061186X.2017.1298602. [DOI] [PubMed] [Google Scholar]
- 69.Liu Y, Xie X, Hou X, Shen J, Shi J, Chen H, He Y, Wang Z, Feng N. Functional oral nanoparticles for delivering silibinin and cryptotanshinone against breast cancer lung metastasis. J Nanobiotechnology. 2020;18(1):83. doi: 10.1186/s12951-020-00638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elzamly S, Badri N, Padilla O, Dwivedi AK, Alvarado LA, Hamilton M, Diab N, Rock C, Elfar A, Teleb M, Sanchez L, Nahleh Z. Epithelial-mesenchymal transition markers in breast cancer and pathological responseafter neoadjuvant chemotherapy. Breast Cancer (Auckl) 2018;12:1178223418788074. doi: 10.1177/1178223418788074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen Y, Quan J, Wang M, Li S, Yang J, Lv M, Chen Z, Zhang L, Zhao X, Yang J. Tumor vasculogenic mimicry formation as an unfavorable prognostic indicator in patients with breast cancer. Oncotarget. 2017;8(34):56408–56416. doi: 10.18632/oncotarget.16919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao XM, Niu FJ, Kong L, Cai FY, Jing M, Fu M, Liu JJ, He SY, Zhang L, Liu XZ, Ju RJ, Li XT. GGP modified daunorubicin plus dioscin liposomes inhibit breast cancer by suppressing epithelial-mesenchymal transition. Drug Dev Ind Pharm. 2020;46(6):916–930. doi: 10.1080/03639045.2020.1763397. [DOI] [PubMed] [Google Scholar]
- 74.Ju RJ, Cheng L, Peng XM, Wang T, Li CQ, Song XL, Liu S, Chao JP, Li XT. Octreotide-modified liposomes containing daunorubicin and dihydroartemisinin for treatment of invasive breast cancer. Artif Cells Nanomed Biotechnol. 2018;46(sup1):616–628. doi: 10.1080/21691401.2018.1433187. [DOI] [PubMed] [Google Scholar]
- 75.Andonegui-Elguera MA, Alfaro-Mora Y, Caceres-Gutierrez R, Caro-Sanchez CHS, Herrera LA, Diaz-Chavez J. An overview of vasculogenic mimicry in breast cancer. Front Oncol. 2020;10:220. doi: 10.3389/fonc.2020.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ju RJ, Li XT, Shi JF, Li XY, Sun MG, Zeng F, Zhou J, Liu L, Zhang CX, Zhao WY, Lu WL. Liposomes, modified with PTD(HIV-1) peptide, containing epirubicin and celecoxib, to target vasculogenic mimicry channels in invasive breast cancer. Biomaterials. 2014;35(26):7610–7621. doi: 10.1016/j.biomaterials.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 77.Ju RJ, Cheng L, Qiu X, Liu S, Song XL, Peng XM, Wang T, Li CQ, Li XT. Hyaluronic acid modified daunorubicin plus honokiol cationic liposomes for the treatment of breast cancer along with the elimination vasculogenic mimicry channels. J Drug Target. 2018;26(9):793–805. doi: 10.1080/1061186X.2018.1428809. [DOI] [PubMed] [Google Scholar]
- 78.Olusanya TOB, Haj Ahmad RR, Ibegbu DM, Smith JR, Elkordy AA. Liposomal drug delivery systems and anticancer drugs. Molecules. 2018 doi: 10.3390/molecules23040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bharti R, Dey G, Banerjee I, Dey KK, Parida S, Kumar BN, Das CK, Pal I, Mukherjee M, Misra M, Pradhan AK, Emdad L, Das SK, Fisher PB, Mandal M. Somatostatin receptor targeted liposomes with Diacerein inhibit IL-6 for breast cancer therapy. Cancer Lett. 2017;388:292–302. doi: 10.1016/j.canlet.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 80.Zevon M, Ganapathy V, Kantamneni H, Mingozzi M, Kim P, Adler D, Sheng Y, Tan MC, Pierce M, Riman RE, Roth CM, Moghe PV. CXCR-4 targeted, short wave infrared (SWIR) emitting nanoprobes for enhanced deep tissue imaging and micrometastatic cancer lesion detection. Small. 2015;11(47):6347–6357. doi: 10.1002/smll.201502202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walenkamp AME, Lapa C, Herrmann K, Wester HJ. CXCR4 ligands: the next big hit? J Nucl Med. 2017;58(Suppl 2):77S–82S. doi: 10.2967/jnumed.116.186874. [DOI] [PubMed] [Google Scholar]
- 82.Kim D-M, Kim M, Park H-B, Kim K-S, Kim D-E. Anti-MUC1/CD44 dual-aptamer-conjugated liposomes for cotargeting breast cancer cells and cancer stem cells. ACS Appl Biol Mater. 2019;2(10):4622–4633. doi: 10.1021/acsabm.9b00705. [DOI] [PubMed] [Google Scholar]
- 83.Fu M, Tang W, Liu JJ, Gong XQ, Kong L, Yao XM, Jing M, Cai FY, Li XT, Ju RJ. Combination of targeted daunorubicin liposomes and targeted emodin liposomes for treatment of invasive breast cancer. J Drug Target. 2020;28(3):245–258. doi: 10.1080/1061186X.2019.1656725. [DOI] [PubMed] [Google Scholar]
- 84.Belfiore L, Saunders DN, Ranson M, Vine KL. N-alkylisatin-loaded liposomes target the urokinase plasminogen activator system in breast cancer. Pharmaceutics. 2020 doi: 10.3390/pharmaceutics12070641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nguyen VD, Min HK, Kim CS, Han J, Park JO, Choi E. Folate receptor-targeted liposomal nanocomplex for effective synergistic photothermal-chemotherapy of breast cancer in vivo. Colloids Surf B Biointerfaces. 2019;173:539–548. doi: 10.1016/j.colsurfb.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 86.Thomas E, Menon JU, Owen J, Skaripa-Koukelli I, Wallington S, Gray M, Mannaris C, Kersemans V, Allen D, Kinchesh P, Smart S, Carlisle R, Vallis KA. Ultrasound-mediated cavitation enhances the delivery of an EGFR-targeting liposomal formulation designed for chemo-radionuclide therapy. Theranostics. 2019;9(19):5595–5609. doi: 10.7150/thno.34669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chapman AP. PEGylated antibodies and antibody fragments for improved therapy: a review. Adv Drug Deliv Rev. 2002;54(4):531–545. doi: 10.1016/s0169-409x(02)00026-1. [DOI] [PubMed] [Google Scholar]
- 88.Vandghanooni S, Eskandani M, Barar J, Omidi Y. Bispecific therapeutic aptamers for targeted therapy of cancer: a review on cellular perspective. J Mol Med (Berl) 2018;96(9):885–902. doi: 10.1007/s00109-018-1669-y. [DOI] [PubMed] [Google Scholar]
- 89.Mendes TF, Kluskens LD, Rodrigues LR. Triple negative breast cancer: nanosolutions for a big challenge. Adv Sci (Weinh) 2015;2(11):1500053. doi: 10.1002/advs.201500053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoo J, Park C, Yi G, Lee D, Koo H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers (Basel) 2019 doi: 10.3390/cancers11050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sneider A, Jadia R, Piel B, VanDyke D, Tsiros C, Rai P. Engineering remotely triggered liposomes to target triple negative breast cancer. Oncomedicine. 2017;2:1–13. doi: 10.7150/oncm.17406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kangarlou S, Ramezanpour S, Balalaie S, Roudbar Mohammadi S, Haririan I. Curcumin-loaded nanoliposomes linked to homing peptides for integrin targeting and neuropilin-1-mediated internalization. Pharm Biol. 2017;55(1):277–285. doi: 10.1080/13880209.2016.1261301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Puri A. Phototriggerable liposomes: current research and future perspectives. Pharmaceutics. 2013;6(1):1–25. doi: 10.3390/pharmaceutics6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huynh E, Zheng G. Porphysome nanotechnology: a paradigm shift in lipid-based supramolecular structures. Nano Today. 2014;9(2):212–222. doi: 10.1016/j.nantod.2014.04.012. [DOI] [Google Scholar]
- 95.Alvarez-Lorenzo C, Concheiro A. Smart drug delivery systems: from fundamentals to the clinic. Chem Commun (Camb) 2014;50(58):7743–7765. doi: 10.1039/c4cc01429d. [DOI] [PubMed] [Google Scholar]
- 96.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 97.Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13(11):813–827. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Massiot J, Rosilio V, Makky A. Photo-triggerable liposomal drug delivery systems: from simple porphyrin insertion in the lipid bilayer towards supramolecular assemblies of lipid-porphyrin conjugates. J Mater Chem B. 2019;7(11):1805–1823. doi: 10.1039/c9tb00015a. [DOI] [PubMed] [Google Scholar]
- 99.Jiang L, He B, Pan D, Luo K, Yi Q, Gu Z. Anti-cancer efficacy of paclitaxel loaded in pH triggered liposomes. J Biomed Nanotechnol. 2016;12(1):79–90. doi: 10.1166/jbn.2016.2123. [DOI] [PubMed] [Google Scholar]
- 100.Yoshizaki Y, Yuba E, Sakaguchi N, Koiwai K, Harada A, Kono K. pH-sensitive polymer-modified liposome-based immunity-inducing system: effects of inclusion of cationic lipid and CpG-DNA. Biomaterials. 2017;141:272–283. doi: 10.1016/j.biomaterials.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 101.Almurshedi AS, Radwan M, Omar S, Alaiya AA, Badran MM, Elsaghire H, Saleem IY, Hutcheon GA. A novel pH-sensitive liposome to trigger delivery of afatinib to cancer cells: impact on lung cancer therapy. J Mol Liq. 2018;259:154–166. doi: 10.1016/j.molliq.2018.03.024. [DOI] [Google Scholar]
- 102.Zhen S, Liu Y, Lu JJ, Tuo XQ, Yang XL, Chen H, Chen W, Li X. Human papillomavirus oncogene manipulation using clustered regularly interspersed short palindromic repeats/Cas9 delivered by pH-sensitive cationic liposomes. Hum Gene Ther. 2020;31:309–324. doi: 10.1089/hum.2019.312. [DOI] [PubMed] [Google Scholar]
- 103.Meers P. Enzyme-activated targeting of liposomes. Adv Drug Deliv Rev. 2001;53(3):265–272. doi: 10.1016/s0169-409x(01)00205-8. [DOI] [PubMed] [Google Scholar]
- 104.Chen WJ, Deng W, Xu X, Zhao X, Vo JN, Anwer AG, Williams TC, Cui HX, Goldys EM. Photoresponsive endosomal escape enhances gene delivery using liposome-polycation-DNA (LPD) nanovectors. J Mater Chem B. 2018;6(32):5269–5281. doi: 10.1039/C8TB00994E. [DOI] [PubMed] [Google Scholar]
- 105.Fologea D, Salamo G, Henry R, Borrelli MJ, Corry P. X-ray controlled drug release from liposomes. Biophys J. 2016;110(3):200a–200a. doi: 10.1016/j.bpj.2015.11.1115. [DOI] [Google Scholar]
- 106.de Matos MBC, Beztsinna N, Heyder C, Fens M, Mastrobattista E, Schiffelers RM, Leneweit G, Kok RJ. Thermosensitive liposomes for triggered release of cytotoxic proteins. Eur J Pharm Biopharm. 2018;132:211–221. doi: 10.1016/j.ejpb.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 107.Zhang J, Wang S, Deng Z, Li L, Tan G, Liu X, Zheng H, Yan F. Ultrasound-triggered drug delivery for breast tumor therapy through iRGD-targeted paclitaxel-loaded liposome-microbubble complexes. J Biomed Nanotechnol. 2018;14(8):1384–1395. doi: 10.1166/jbn.2018.2594. [DOI] [PubMed] [Google Scholar]
- 108.Vlasova KY, Piroyan A, Le-Deygen IM, Vishwasrao HM, Ramsey JD, Klyachko NL, Golovin YI, Rudakovskaya PG, Kireev II, Kabanov AV, Sokolsky-Papkov M. Magnetic liposome design for drug release systems responsive to super-low frequency alternating current magnetic field (AC MF) J Colloid Interface Sci. 2019;552:689–700. doi: 10.1016/j.jcis.2019.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Ballegooie C, Man A, Win M, Yapp DT. Spatially specific liposomal cancer therapy triggered by clinical external sources of energy. Pharmaceutics. 2019 doi: 10.3390/pharmaceutics11030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zangabad PS, Mirkiani S, Shahsavari S, Masoudi B, Masroor M, Hamed H, Jafari Z, Taghipour YD, Hashemi H, Karimi M, Hamblin MR. Stimulus-responsive liposomes as smart nanoplatforms for drug delivery applications. Nanotechnol Rev. 2018;7(1):95–122. doi: 10.1515/ntrev-2017-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee Y, Thompson DH. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017 doi: 10.1002/wnan.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miranda D, Lovell JF. Mechanisms of light-induced liposome permeabilization. Bioeng Transl Med. 2016;1(3):267–276. doi: 10.1002/btm2.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen W, Goldys EM, Deng W. Light-induced liposomes for cancer therapeutics. Prog Lipid Res. 2020;79:101052. doi: 10.1016/j.plipres.2020.101052. [DOI] [PubMed] [Google Scholar]
- 114.Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56(6):1194–1198. [PubMed] [Google Scholar]
- 115.Karanth H, Murthy RS. pH-sensitive liposomes–principle and application in cancer therapy. J Pharm Pharmacol. 2007;59(4):469–483. doi: 10.1211/jpp.59.4.0001. [DOI] [PubMed] [Google Scholar]
- 116.de Oliveira SJ, Fernandes RS, Ramos Oda CM, Ferreira TH, Machado Botelho AF, Martins Melo M, de Miranda MC, Assis Gomes D, Dantas Cassali G, Townsend DM, Rubello D, Oliveira MC, de Barros ALB. Folate-coated, long-circulating and pH-sensitive liposomes enhance doxorubicin antitumor effect in a breast cancer animal model. Biomed Pharmacother. 2019;118:109323. doi: 10.1016/j.biopha.2019.109323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ruocco MJ, Siminovitch DJ, Griffin RG. Comparative study of the gel phases of ether- and ester-linked phosphatidylcholines. Biochemistry. 1985;24(10):2406–2411. doi: 10.1021/bi00331a003. [DOI] [PubMed] [Google Scholar]
- 118.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202(4374):1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 119.Jose A, Ninave KM, Karnam S, Venuganti VVK. Temperature-sensitive liposomes for co-delivery of tamoxifen and imatinib for synergistic breast cancer treatment. J Liposome Res. 2019;29(2):153–162. doi: 10.1080/08982104.2018.1502315. [DOI] [PubMed] [Google Scholar]
- 120.Ou YC, Webb JA, Faley S, Shae D, Talbert EM, Lin S, Cutright CC, Wilson JT, Bellan LM, Bardhan R. Gold nanoantenna-mediated photothermal drug delivery from thermosensitive liposomes in breast cancer. ACS Omega. 2016;1(2):234–243. doi: 10.1021/acsomega.6b00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rengan AK, Jagtap M, De A, Banerjee R, Srivastava R. Multifunctional gold coated thermo-sensitive liposomes for multimodal imaging and photo-thermal therapy of breast cancer cells. Nanoscale. 2014;6(2):916–923. doi: 10.1039/c3nr04448c. [DOI] [PubMed] [Google Scholar]
- 122.Kheirolomoom A, Lai CY, Tam SM, Mahakian LM, Ingham ES, Watson KD, Ferrara KW. Complete regression of local cancer using temperature-sensitive liposomes combined with ultrasound-mediated hyperthermia. J Control Release. 2013;172(1):266–273. doi: 10.1016/j.jconrel.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang H, Gong W, Wang ZY, Yuan SJ, Xie XY, Yang YF, Yang Y, Wang SS, Yang DX, Xuan ZX, Mei XG. Preparation, characterization, and pharmacodynamics of thermosensitive liposomes containing docetaxel. J Pharm Sci. 2014;103(7):2177–2183. doi: 10.1002/jps.24019. [DOI] [PubMed] [Google Scholar]
- 124.Ta T, Porter TM. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J Control Release. 2013;169(1–2):112–125. doi: 10.1016/j.jconrel.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Borys N (2000) Phase 1/2 study of thermodox with approved hyperthermia in treatment of breast cancer recurrence at the chest wall (DIGNITY). ClinicalTrials gov Identifier: NCT00826085
- 126.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia: characterization and testing in a human tumor xenograft model. Cancer Res. 2000;60(5):1197–1201. [PubMed] [Google Scholar]
- 127.Rugo H, Zagar T, Formenti S, Vujaskovic Z, Muggia F, O'Connor B, Myerson R, Hsu I-C, Borys N, Blackwell K. Abstract P4–15-05: novel targeted therapy for breast cancer chest wall recurrence: Low temperature liposomal doxorubicin and mild local hyperthermia. Philadelphia: AACR; 2013. [Google Scholar]
- 128.Pooler JP. Photooxidation of cell membranes using eosin derivatives that locate in lipid or protein to study the role of diffusible intermediates. Photochem Photobiol. 1989;50(1):55–68. doi: 10.1111/j.1751-1097.1989.tb04129.x. [DOI] [PubMed] [Google Scholar]
- 129.Girotti AW. Photodynamic lipid peroxidation in biological systems. Photochem Photobiol. 1990;51(4):497–509. doi: 10.1111/j.1751-1097.1990.tb01744.x. [DOI] [PubMed] [Google Scholar]
- 130.Robertson CA, Evans DH, Abrahamse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J Photochem Photobiol B. 2009;96(1):1–8. doi: 10.1016/j.jphotobiol.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 131.Tejero I, Gonzalez-Lafont A, Lluch JM, Eriksson LA. Photo-oxidation of lipids by singlet oxygen: a theoretical study. Chem Phys Lett. 2004;398(4–6):336–342. doi: 10.1016/j.cplett.2004.09.093. [DOI] [Google Scholar]
- 132.Huang Z. A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat. 2005;4(3):283–293. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Son J, Yi G, Yoo J, Park C, Koo H, Choi HS. Light-responsive nanomedicine for biophotonic imaging and targeted therapy. Adv Drug Deliv Rev. 2019;138:133–147. doi: 10.1016/j.addr.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hemmer E, Acosta-Mora P, Méndez-Ramos J, Fischer S. Optical nanoprobes for biomedical applications: shining a light on upconverting and near-infrared emitting nanoparticles for imaging, thermal sensing, and photodynamic therapy. J Mater Chem B. 2017;5(23):4365–4392. doi: 10.1039/C7TB00403F. [DOI] [PubMed] [Google Scholar]
- 135.Yang Y, Yang X, Li H, Li C, Ding H, Zhang M, Guo Y, Sun M. Near-infrared light triggered liposomes combining photodynamic and chemotherapy for synergistic breast tumor therapy. Colloids Surf B Biointerfaces. 2019;173:564–570. doi: 10.1016/j.colsurfb.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 136.Alawak M, Abu Dayyih A, Mahmoud G, Tariq I, Duse L, Goergen N, Engelhardt K, Reddy Pinnapireddy S, Jedelska J, Awak M, Konig AM, Brussler J, Bartsch JW, Bakowsky U. ADAM 8 as a novel target for doxorubicin delivery to TNBC cells using magnetic thermosensitive liposomes. Eur J Pharm Biopharm. 2021;158:390–400. doi: 10.1016/j.ejpb.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 137.Amrahli M, Centelles M, Cressey P, Prusevicius M, Gedroyc W, Xu XY, So PW, Wright M, Thanou M. MR-labelled liposomes and focused ultrasound for spatiotemporally controlled drug release in triple negative breast cancers in mice. Nanotheranostics. 2021;5(2):125–142. doi: 10.7150/ntno.52168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.de Maar JS, Suelmann BBM, Braat M, van Diest PJ, Vaessen HHB, Witkamp AJ, Linn SC, Moonen CTW, van der Wall E, Deckers R. Phase I feasibility study of Magnetic Resonance guided High Intensity Focused Ultrasound-induced hyperthermia, Lyso-Thermosensitive Liposomal Doxorubicin and cyclophosphamide in de novo stage IV breast cancer patients: study protocol of the i-GO study. BMJ Open. 2020;10(11):e040162. doi: 10.1136/bmjopen-2020-040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Y, An H, Wang X, Wang P, Qu F, Jiao Y, Zhang K, Liu Q. Ultrasound-triggered release of sinoporphyrin sodium from liposome-microbubble complexes and its enhanced sonodynamic toxicity in breast cancer. Nano Res. 2018;11(2):1038–1056. doi: 10.1007/s12274-017-1719-8. [DOI] [Google Scholar]
- 140.Prabhakar A, Banerjee R. Nanobubble liposome complexes for diagnostic imaging and ultrasound-triggered drug delivery in cancers: a theranostic approach. ACS Omega. 2019;4(13):15567–15580. doi: 10.1021/acsomega.9b01924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Marmottant P, Hilgenfeldt S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature. 2003;423(6936):153–156. doi: 10.1038/nature01613. [DOI] [PubMed] [Google Scholar]
- 142.Schroeder A, Avnir Y, Weisman S, Najajreh Y, Gabizon A, Talmon Y, Kost J, Barenholz Y. Controlling liposomal drug release with low frequency ultrasound: mechanism and feasibility. Langmuir. 2007;23(7):4019–4025. doi: 10.1021/la0631668. [DOI] [PubMed] [Google Scholar]
- 143.Pecha R, Gompf B. Microimplosions: cavitation collapse and shock wave emission on a nanosecond time scale. Phys Rev Lett. 2000;84(6):1328–1330. doi: 10.1103/PhysRevLett.84.1328. [DOI] [PubMed] [Google Scholar]
- 144.Song Y, Sheng Z, Xu Y, Dong L, Xu W, Li F, Wang J, Wu Z, Yang Y, Su Y, Sun X, Ling D, Lu Y. Magnetic liposomal emodin composite with enhanced killing efficiency against breast cancer. Biomater Sci. 2019;7(3):867–875. doi: 10.1039/c8bm01530a. [DOI] [PubMed] [Google Scholar]
- 145.Wang Y, Kohane DS. External triggering and triggered targeting strategies for drug delivery. Nat Rev Mater. 2017;2(6):1–14. doi: 10.1038/natrevmats.2017.20. [DOI] [Google Scholar]
- 146.Bottai G, Truffi M, Corsi F, Santarpia L. Progress in nonviral gene therapy for breast cancer and what comes next? Expert Opin Biol Ther. 2017;17(5):595–611. doi: 10.1080/14712598.2017.1305351. [DOI] [PubMed] [Google Scholar]
- 147.Simoes S, Filipe A, Faneca H, Mano M, Penacho N, Duzgunes N, de Lima MP. Cationic liposomes for gene delivery. Expert Opin Drug Deliv. 2005;2(2):237–254. doi: 10.1517/17425247.2.2.237. [DOI] [PubMed] [Google Scholar]
- 148.Judge AD, Bola G, Lee AC, MacLachlan I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol Ther. 2006;13(3):494–505. doi: 10.1016/j.ymthe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 149.Miller AD. Cationic liposomes for gene therapy. Angew Chem Int Ed. 1998;37(13–14):1768–1785. doi: 10.1002/(SICI)1521-3773(19980803)37:13/14<1768::AID-ANIE1768>3.0.CO;2-4. [DOI] [Google Scholar]
- 150.Kaneda Y, Iwai K, Uchida T. Increased expression of DNA cointroduced with nuclear protein in adult rat liver. Science. 1989;243(4889):375–378. doi: 10.1126/science.2911748. [DOI] [PubMed] [Google Scholar]
- 151.Thierry AR, Dritschilo A. Intracellular availability of unmodified, phosphorothioated and liposomally encapsulated oligodeoxynucleotides for antisense activity. Nucleic Acids Res. 1992;20(21):5691–5698. doi: 10.1093/nar/20.21.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Paliwal SR, Paliwal R, Agrawal GP, Vyas SP. Liposomal nanomedicine for breast cancer therapy. Nanomedicine (Lond) 2011;6(6):1085–1100. doi: 10.2217/nnm.11.72. [DOI] [PubMed] [Google Scholar]
- 153.Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov. 2020;19(10):673–694. doi: 10.1038/s41573-020-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wan J, Bauman JA, Graziewicz MA, Sazani P, Kole R. Oligonucleotide therapeutics in cancer. Cancer Treat Res. 2013;158:213–233. doi: 10.1007/978-3-642-31659-3_9. [DOI] [PubMed] [Google Scholar]
- 155.Zuo J, Jiang Y, Zhang E, Chen Y, Liang Z, Zhu J, Zhao Y, Xu H, Liu G, Liu J, Wang W, Zhang S, Zhen Y. Synergistic effects of 7-O-geranylquercetin and siRNAs on the treatment of human breast cancer. Life Sci. 2019;227:145–152. doi: 10.1016/j.lfs.2019.04.047. [DOI] [PubMed] [Google Scholar]
- 156.Crooke ST. Molecular mechanisms of antisense oligonucleotides. Nucleic Acid Ther. 2017;27(2):70–77. doi: 10.1089/nat.2016.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Le BT, Raguraman P, Kosbar TR, Fletcher S, Wilton SD, Veedu RN. Antisense oligonucleotides targeting angiogenic factors as potential cancer therapeutics. Mol Ther Nucleic Acids. 2019;14:142–157. doi: 10.1016/j.omtn.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang J, Lu Z, Wientjes MG, Au JL. Delivery of siRNA therapeutics: barriers and carriers. AAPS J. 2010;12(4):492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ahmadzada T, Reid G, McKenzie DR. Fundamentals of siRNA and miRNA therapeutics and a review of targeted nanoparticle delivery systems in breast cancer. Biophys Rev. 2018;10(1):69–86. doi: 10.1007/s12551-017-0392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Eloy JO, Petrilli R, Raspantini GL, Lee RJ. Targeted liposomes for siRNA delivery to cancer. Curr Pharm Des. 2018;24(23):2664–2672. doi: 10.2174/1381612824666180807121935. [DOI] [PubMed] [Google Scholar]
- 161.Sakurai Y, Hada T, Kato A, Hagino Y, Mizumura W, Harashima H. Effective therapy using a liposomal siRNA that targets the tumor vasculature in a model murine breast cancer with lung metastasis. Mol Ther Oncolytics. 2018;11:102–108. doi: 10.1016/j.omto.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wan Y, Dai W, Nevagi RJ, Toth I, Moyle PM. Multifunctional peptide-lipid nanocomplexes for efficient targeted delivery of DNA and siRNA into breast cancer cells. Acta Biomater. 2017;59:257–268. doi: 10.1016/j.actbio.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 163.Gujrati M, Vaidya AM, Mack M, Snyder D, Malamas A, Lu ZR. Targeted dual pH-sensitive lipid ECO/siRNA self-assembly nanoparticles facilitate in vivo cytosolic sieIF4E delivery and overcome paclitaxel resistance in breast cancer therapy. Adv Healthc Mater. 2016;5(22):2882–2895. doi: 10.1002/adhm.201600677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sharma S, Rajendran V, Kulshreshtha R, Ghosh PC. Enhanced efficacy of anti-miR-191 delivery through stearylamine liposome formulation for the treatment of breast cancer cells. Int J Pharm. 2017;530(1–2):387–400. doi: 10.1016/j.ijpharm.2017.07.079. [DOI] [PubMed] [Google Scholar]
- 165.Tang J, Howard CB, Mahler SM, Thurecht KJ, Huang L, Xu ZP. Enhanced delivery of siRNA to triple negative breast cancer cells in vitro and in vivo through functionalizing lipid-coated calcium phosphate nanoparticles with dual target ligands. Nanoscale. 2018;10(9):4258–4266. doi: 10.1039/c7nr08644j. [DOI] [PubMed] [Google Scholar]
- 166.Yu S, Bi X, Yang L, Wu S, Yu Y, Jiang B, Zhang A, Lan K, Duan S. Co-delivery of paclitaxel and PLK1-targeted siRNA using aptamer-functionalized cationic liposome for synergistic anti-breast cancer effects in vivo. J Biomed Nanotechnol. 2019;15(6):1135–1148. doi: 10.1166/jbn.2019.2751. [DOI] [PubMed] [Google Scholar]
- 167.Alshaer W, Hillaireau H, Vergnaud J, Mura S, Delomenie C, Sauvage F, Ismail S, Fattal E. Aptamer-guided siRNA-loaded nanomedicines for systemic gene silencing in CD-44 expressing murine triple-negative breast cancer model. J Control Release. 2018;271:98–106. doi: 10.1016/j.jconrel.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 168.Kedmi R, Ben-Arie N, Peer D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials. 2010;31(26):6867–6875. doi: 10.1016/j.biomaterials.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 169.Cannell IG, Kong YW, Bushell M. How do microRNAs regulate gene expression? Biochem Soc Trans. 2008;36(Pt 6):1224–1231. doi: 10.1042/BST0361224. [DOI] [PubMed] [Google Scholar]
- 170.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 171.Mathiyalagan P, Sahoo S. Exosomes-based gene therapy for MicroRNA delivery. Methods Mol Biol. 2017;1521:139–152. doi: 10.1007/978-1-4939-6588-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yan Y, Li XQ, Duan JL, Bao CJ, Cui YN, Su ZB, Xu JR, Luo Q, Chen M, Xie Y, Lu WL. Nanosized functional miRNA liposomes and application in the treatment of TNBC by silencing Slug gene. Int J Nanomed. 2019;14:3645–3667. doi: 10.2147/IJN.S207837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen Y, Zhang Y. Application of the CRISPR/Cas9 system to drug resistance in breast cancer. Adv Sci (Weinh) 2018;5(6):1700964. doi: 10.1002/advs.201700964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Huang N, Huang Z, Gao M, Luo Z, Zhou F, Liu L, Xiao Q, Wang X, Feng W. Induction of apoptosis in imatinib sensitive and resistant chronic myeloid leukemia cells by efficient disruption of bcr-abl oncogene with zinc finger nucleases. J Exp Clin Cancer Res. 2018;37(1):62. doi: 10.1186/s13046-018-0732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Puria R, Sahi S, Nain V. HER2+ breast cancer therapy: by CPP-ZFN mediated targeting of mTOR? Technol Cancer Res Treat. 2012;11(2):175–180. doi: 10.7785/tcrt.2012.500247. [DOI] [PubMed] [Google Scholar]
- 176.Wang J, Li T, Zhou M, Hu Z, Zhou X, Zhou S, Wang N, Huang L, Zhao L, Cao Y, Xiao M, Ma D, Zhou P, Shang Z, Zhou J. TALENs-mediated gene disruption of FLT3 in leukemia cells: using genome-editing approach for exploring the molecular basis of gene abnormality. Sci Rep. 2015;5:18454. doi: 10.1038/srep18454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nyquist MD, Li Y, Hwang TH, Manlove LS, Vessella RL, Silverstein KA, Voytas DF, Dehm SM. TALEN-engineered AR gene rearrangements reveal endocrine uncoupling of androgen receptor in prostate cancer. Proc Natl Acad Sci USA. 2013;110(43):17492–17497. doi: 10.1073/pnas.1308587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhan T, Rindtorff N, Betge J, Ebert MP, Boutros M. CRISPR/Cas9 for cancer research and therapy. Semin Cancer Biol. 2019;55:106–119. doi: 10.1016/j.semcancer.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 179.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Savic N, Schwank G. Advances in therapeutic CRISPR/Cas9 genome editing. Transl Res. 2016;168:15–21. doi: 10.1016/j.trsl.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 181.Lino CA, Harper JC, Carney JP, Timlin JA. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. 2018;25(1):1234–1257. doi: 10.1080/10717544.2018.1474964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Maeder ML, Gersbach CA. Genome-editing technologies for gene and cell therapy. Mol Ther. 2016;24(3):430–446. doi: 10.1038/mt.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cornu TI, Mussolino C, Cathomen T. Refining strategies to translate genome editing to the clinic. Nat Med. 2017;23(4):415–423. doi: 10.1038/nm.4313. [DOI] [PubMed] [Google Scholar]
- 184.Xia AL, He QF, Wang JC, Zhu J, Sha YQ, Sun B, Lu XJ. Applications and advances of CRISPR-Cas9 in cancer immunotherapy. J Med Genet. 2019;56(1):4–9. doi: 10.1136/jmedgenet-2018-105422. [DOI] [PubMed] [Google Scholar]
- 185.Mullard A. Novartis secures first CRISPR pharma collaborations. Nat Rev Drug Discov. 2015;14(2):82–82. [Google Scholar]
- 186.Cyranoski D. First trial of CRISPR in people. Nature. 2016;535(7613):476–477. doi: 10.1038/nature.2016.20302. [DOI] [PubMed] [Google Scholar]
- 187.Le Page M. CRISPR shows promise in cancer First trial of this form of gene editing for people with tumours passes safety test. New Sci. 2020;245(3269):12–12. [Google Scholar]
- 188.Guo P, Liu D, Subramanyam K, Wang B, Yang J, Huang J, Auguste DT, Moses MA. Nanoparticle elasticity directs tumor uptake. Nat Commun. 2018;9(1):130. doi: 10.1038/s41467-017-02588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yin H, Song CQ, Dorkin JR, Zhu LJ, Li Y, Wu Q, Park A, Yang J, Suresh S, Bizhanova A, Gupta A, Bolukbasi MF, Walsh S, Bogorad RL, Gao G, Weng Z, Dong Y, Koteliansky V, Wolfe SA, Langer R, Xue W, Anderson DG. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat Biotechnol. 2016;34(3):328–333. doi: 10.1038/nbt.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tao Y, Hou X, Zuo F, Li X, Pang Y, Jiang G. Application of nanoparticle-based siRNA and CRISPR/Cas9 delivery systems in gene-targeted therapy. Nanomedicine (Lond) 2019;14(5):511–514. doi: 10.2217/nnm-2018-0522. [DOI] [PubMed] [Google Scholar]
- 191.Mintz RL, Gao MA, Lo K, Lao YH, Li M, Leong KW. CRISPR technology for breast cancer: diagnostics, modeling, and therapy. Adv Biosyst. 2018 doi: 10.1002/adbi.201800132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li L, Hu S, Chen X. Non-viral delivery systems for CRISPR/Cas9-based genome editing: challenges and opportunities. Biomaterials. 2018;171:207–218. doi: 10.1016/j.biomaterials.2018.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jain V, Kumar H, Anod HV, Chand P, Gupta NV, Dey S, Kesharwani SS. A review of nanotechnology-based approaches for breast cancer and triple-negative breast cancer. J Control Release. 2020;326:628–647. doi: 10.1016/j.jconrel.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 194.Pahle J, Walther W. Vectors and strategies for nonviral cancer gene therapy. Expert Opin Biol Ther. 2016;16(4):443–461. doi: 10.1517/14712598.2016.1134480. [DOI] [PubMed] [Google Scholar]
- 195.Chen F, Alphonse M, Liu Q. Strategies for nonviral nanoparticle-based delivery of CRISPR/Cas9 therapeutics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(3):e1609. doi: 10.1002/wnan.1609. [DOI] [PubMed] [Google Scholar]
- 196.Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L, Tan J. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci (Weinh) 2018;5(4):1700611. doi: 10.1002/advs.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chen Z, Liu F, Chen Y, Liu J, Wang X, Chen AT, Deng G, Zhang H, Liu J, Hong Z, Zhou J. Targeted delivery of CRISPR/Cas9-mediated cancer gene therapy via liposome-templated hydrogel nanoparticles. Adv Funct Mater. 2017 doi: 10.1002/adfm.201703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Eoh J, Gu L. Biomaterials as vectors for the delivery of CRISPR-Cas9. Biomater Sci. 2019;7(4):1240–1261. doi: 10.1039/c8bm01310a. [DOI] [PubMed] [Google Scholar]
- 199.Zhang LM, Wang P, Feng Q, Wang NX, Chen ZT, Huang YY, Zheng WF, Jiang XY. Lipid nanoparticle-mediated efficient delivery of CRISPR/Cas9 for tumor therapy. Npg Asia Mater. 2017;9:e441. doi: 10.1038/am.2017.185. [DOI] [Google Scholar]
- 200.Guo P, Yang J, Huang J, Auguste DT, Moses MA. Therapeutic genome editing of triple-negative breast tumors using a noncationic and deformable nanolipogel. Proc Natl Acad Sci USA. 2019;116(37):18295–18303. doi: 10.1073/pnas.1904697116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Peng R, Lin G, Li J. Potential pitfalls of CRISPR/Cas9-mediated genome editing. FEBS J. 2016;283(7):1218–1231. doi: 10.1111/febs.13586. [DOI] [PubMed] [Google Scholar]