Abstract
All living organisms need energy to carry out their essential functions. The importance of energy metabolism is increasingly recognized in human pluripotent stem cells. Energy production is not only essential for cell survival and proliferation, but also critical for pluripotency and cell fate determination. Thus, energy metabolism is an important target in cellular regulation and stem cell applications. In this review, we will discuss key factors that influence energy metabolism and their association with stem cell functions.
Keywords: Glycolysis, Oxidative phosphorylation, pH, Oxygen, Nutrient, Insulin
Introduction
Cells are dynamic systems that rely on continuous production of cellular energy to carry out various biological processes. Energy metabolism involves diverse substrates and many interconnected pathways, which require coordinated regulation to maintain a balanced support for both energy production and cell type-specific functions under different circumstances. In recent years, numerous studies report that energy metabolism undergo dramatic changes during embryogenesis, inflammation, and cancer development [46, 102, 116, 118]. Besides the many studies on cell type-specific metabolic profiles and their functional impacts, the regulatory mechanisms that control cellular metabolic activities are gaining more and more interest. In this article, we will focus on how energy metabolism is regulated in human pluripotent stem cells (hPSCs).
hPSCs include human embryonic stem cells (hESCs) derived from the inner cell mass (ICM) of blastocyst and human induced pluripotent stem cells (hiPSCs) that are reprogrammed from somatic cells [96, 97, 112]. hPSCs can self-renew unlimitedly and have the potential to generate any cell types in the body, which have attracted enormous interest in regenerative medicine. hPSCs are also invaluable models to understand early embryogenesis. Depending on the culture conditions, hPSCs can be categorized into primed and naïve pluripotent stages that correspond to postimplant epiblast and preimplant ICM [41, 57, 97], respectively. Naïve and primed stem cells display key differences in colony morphology, developmental potential, epigenetic landscape and X-chromosome inactivation. Meanwhile, cells in each stage have distinct metabolic profiles [105]. Naïve cells rely on both glycolysis and oxidative phosphorylation for energy production, while cells in primed stage are mostly glycolytic [10, 40, 105]. A shift from bivalent to glycolytic metabolism takes place during the transition from naïve to primed state, and this process is regulated by hypoxia-inducible factor 1α (HIF-1α) [120]. The differences in glucose and glutamine metabolism contribute to distinct epigenetic modifications, which modulate gene expression to influence various cellular functions [116, 118]. For example, naïve cells display a higher α-KG/succinate ratio, which favors demethylation of repressive chromatin markers and enhances pluripotency [11]. Primed hPSCs are the most widely used in basic research and applications, so hPSC studies discussed in this review mainly refer to cells at primed stage unless otherwise mentioned.
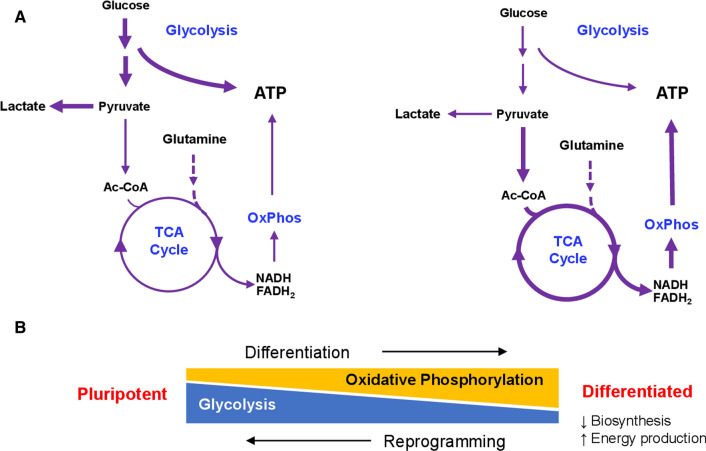
hPSCs grow quickly in vitro, and can double every day in feeder-free conditions [58]. The fast proliferation puts great demand on both energy production and the supply of key building blocks from cellular metabolites. Glycolysis and oxidative phosphorylation are the two main energy metabolism processes in hPSCs (Fig. 1A). Through glycolysis in the cytosol, one glucose molecule can generate two net adenosine triphosphate (ATP), two reduced nicotinamide adenine dinucleotide (NADH) and two pyruvate molecules. ATP can be directly utilized by various biological processes. Meanwhile, pyruvate can either be converted to lactic acid by lactate dehydrogenase, or serve as the starting material for the TCA cycle, in which every two pyruvate molecules produce eight NADH, two FADH2 and two GTP molecules. Energy stored in NADH and FADH2 are then utilized for ATP production in oxidative phosphorylation at the electron transport chain. Electrons are transported from NADH to O2 through a series of carriers while a proton gradient is formed across the mitochondrial inner membrane. Protons are then transported back into the mitochondrial matrix through complex V, forming ATP in the process. The net result is the generation of ~ 30 ATPs from each molecule of glucose. Even though glycolysis is less efficient in ATP generation compared to oxidative phosphorylation, high glycolysis flux is commonly seen in stem cells [40, 104]. Energy production through glycolysis is faster when the supply of glucose is abundant [100]. In addition, high levels of metabolism through glycolysis and the non-oxidative branch of the pentose pathway also provide macromolecular precursors to support the biosynthesis of nucleotides, amino acids and lipids, which is advantageous in highly proliferative cells like hPSCs [61, 101, 104].
Fig. 1.
Energy metabolism in pluripotent and differentiated cells. A Main energy metabolism processes contributing to ATP production in hPSCs (left) and differentiated cells (right). B Metabolic changes associated with differentiation and reprogramming. OxPhos, oxidative phosphorylation
Although glycolysis produces the majority of ATP in hESCs, oxidative phosphorylation is still indispensable. Mitochondrial respiration not only contributes to substantial energy production, but also plays essential roles in fundamental processes such as cell survival, proliferation and differentiation [6, 22, 55, 63, 83, 116, 118]. Suppression of mitochondrial function in mouse and human ESCs reduces ATP content, increases ROS production, and slows down cell proliferation [63]. Excessive mitochondrial respiration has been shown to cause DNA damage and epigenetic changes [113]. A change in redox status occurs during mESC differentiation, and metabolites involved in oxidative metabolism promote differentiation when added into the culture medium [108]. Inhibitors of the electron transport chain, such as complex III inhibitor antimycin A, have been shown to enhance pluripotency and suppress differentiation [76, 103]. These observations suggest that the balance between glycolysis and oxidative phosphorylation is essential for hPSC homeostasis.
When hPSCs exit the self-renewal cycle, the balance of energy metabolism is shifted toward oxidative phosphorylation in somatic cell types (Fig. 1B). In contrast, during reprogramming, cellular metabolism undergoes remodeling toward a glycolytic phenotype [74, 115]. Upregulation of glycolysis was reported to enhance reprogramming, while inhibition of glycolysis reduced reprogramming efficiency. PS48, a chemical activator of pyruvate dehydrogenase kinase (PDK), can promote the expression of glycolytic enzymes, and enhance the efficiency of reprogramming from keratinocytes by about 15-fold [121]. On the other hand, suppression of glycolysis with 2-deoxyglucose (2DG), hexokinase 2 inhibitor 3-bromopyruvic acid (BrPA) or PDK inhibitor dichloroacetate (DCA) reduces the efficiency of nuclear reprogramming [32]. These observations demonstrate that the manipulation of hPSC metabolism can redirect cell fate or improve iPSC derivation, both of which are invaluable for clinical applications. With improved efficiency of reprogramming and differentiation, high-quality cells can be derived for disease modeling, drug screening, toxicity test, and cell therapy.
More and more studies demonstrate that energy metabolism is associated with gene expression and epigenetic changes, and modulation of metabolic activities can dictate stem cell pluripotency and cell fate determination through signal transduction and epigenetic mechanisms. Glycolysis and TCA cycle metabolites, such as acetyl-CoA, NAD+ and α-KG, can serve as substrates or cofactors for epigenetic enzymes, and are involved in the regulation of pluripotency and differentiation [28]. For instance, acetyl-CoA generated from glycolysis is a substrate for histone acetylation, and manipulation of acetyl-CoA production can affect hESC differentiation [69]. Elevated intracellular α-KG serves as a cofactor for JHDMs and TET DNA demethylases, promotes histone and DNA demethylation, and enhances Nanog expression in mouse ESCs [11]. These interesting findings have been reviewed elsewhere [13, 24, 66, 92, 100]. Given the key roles of metabolic changes in stem cell function, a thorough understanding of factors that can regulate stem cell metabolism will enable us to develop powerful tools in stem cell technology.
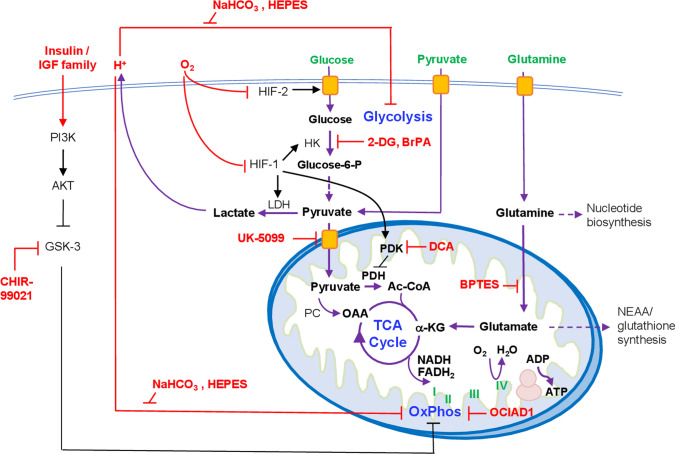
During embryogenesis in vivo, pluripotent cells only exist transiently. hPSCs cultured in vitro are able to remain pluripotent for a prolonged period of time because they are maintained by artificial culture conditions. Factors in the culture system, either provided by the researcher or from the cells themselves, can change the microenvironment and profoundly influence hPSC metabolism. As discussed above, appropriate metabolic states and transitions between them are necessary for stem cell pluripotency, proliferation and differentiation, as well as the reprogramming from somatic cells. Factors that regulate hPSC metabolism are important targets through which stem cell functions can be controlled and improved. Here we will discuss a few main contributing factors that regulate hPSC energy metabolism, including (1) contribution by nutrients; (2) composition of culture components—oxygen and medium pH; and (3) regulation by signal transduction pathways (Fig. 2).
Fig. 2.
Regulation of hPSC metabolism. Major energy substrates in hPSC medium are labeled in green. Regulators of metabolic processes are labeled in red. OxPhos, oxidative phosphorylation. α-KG, α- ketoglutarate. OAA oxaloacetate, PC pyruvate carboxylase, HK hexokinase, LDH lactate dehydrogenase
Nutrients in energy metabolism
Numerous culture media have been successfully developed to sustain hPSC self-renewal, all of which provide glucose, glutamine and pyruvate as energy substrates [57]. Each energy substrate contributes to glycolysis and oxidative phosphorylation in a distinct fashion.
Glucose
Glucose is the starting material for glycolysis, and generally is present in almost all the media for various cell types. Without glucose in the culture medium, there is no glycolysis and hPSCs cannot survive beyond a few days. The cell death is further accelerated when glutamine is simultaneously depleted [98]. Glycolysis is the primary pathway of glucose metabolism in hPSCs. Although glucose metabolism generates pyruvate which can be converted to acetyl-CoA and feed into the TCA cycle, pyruvate is generally not catabolized efficiently through the TCA cycle and oxidative phosphorylation in hPSCs, due to low levels of mitochondrial enzymes for converting citrate to α-KG in the TCA cycle [98]. Instead, pyruvate is mainly converted to lactic acid in hESCs [100]. Not only is glucose not a major contributor to oxidative phosphorylation, but it also suppresses basal oxidative phosphorylation and inhibits pyruvate- and glutamine-based respiration [80]. It suggests that glycolysis and oxidative phosphorylation are intrinsically linked in a dynamic balance in hPSCs.
The functional implications of glucose metabolism in hPSCs have been investigated in recent years. 2-Deoxyglucose (2-DG), a glucose analog, is often used to mimic glucose deprivation and manipulate glycolytic activity. 2-DG enters the cell via glucose transporters, and inhibits glycolysis by suppressing hexokinase [17]. 2-DG treatment at millimolar concentrations inhibits glycolysis by ~ 50% [2, 54]. In hPSCs, 2-DG was reported to lead to decreased expression of pluripotency genes, suggesting loss of pluripotency following manipulation of glycolytic activity. Treatment with BrPA, which inhibits glycolysis at glyceraldehyde 3-phosphate dehydrogenase (GAPDH), had similar effects. Inhibition of glycolysis by 2-DG or BrPA also decreased acetyl-CoA production and consequently impaired histone acetylation, implying the impact of metabolic regulation on epigenetics [69]. Based on the decreased pluripotency marker expression following glycolysis inhibition, glycolysis is considered to be essential for pluripotency. Because metabolites from glycolysis are involved in many other metabolic processes, the interactions of glucose metabolism with these pathways are still to be explored in hPSCs in the near future.
Glutamine
Glutamine is the best studied substrate for oxidative phosphorylation in hPSCs, and it is indispensable for hPSC maintenance [98]. Glutamine contributes to nucleotide synthesis in the cytosol. When transported into the mitochondria, glutamine is converted to glutamate by glutaminase, and further into alpha-ketoglutarate (α-KG) which is the key intermediate metabolite for both lipid synthesis and for NADH/FADH2 production in the TCA cycle. Glutamine-derived α-KG is converted to succinate, and subsequently generates two NADHs and one FADH2 in the latter half of the TCA cycle, which are utilized in oxidative phosphorylation. Glutamate can be transported to the cytosol and contributes to glutathione and amino acid synthesis [110]. In the absence of glucose, the function of glutamine in hPSCs was rescued by dimethyl-α-KG, a cell-permeable form of α-KG, but not by pyruvate, fatty acid, nucleoside, or glutathione, highlighting the importance of glutamine-derived α-KG in hPSC survival [98]. Without glutamine, cellular oxidative phosphorylation is significantly decreased. The glutaminase inhibitor bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide (BPTES) was shown to suppress glutamine-dependent oxidative phosphorylation in hESCs [80]. On the other hand, deprivation of glucose led to increased glutamine oxidation, suggesting the interaction between the metabolism of these two major energy substrates [98].
Other aspects of glutamine metabolism are also important for stem cell functions. In addition to feeding into the TCA cycle, glutamine also contributes to the biosynthesis of nucleotides [56]. Inhibition of glutaminase by 6-diazo-5-oxo-l-norleucine (DON) was reported to suppress the de novo synthesis of nucleotides in hematopoietic stem cells and affect erythroid commitment [71]. Glutamine is also required for maintaining intracellular glutathione levels and suppressing reactive oxygen species. Depletion of glutamine led to the oxidation and degradation of pluripotency marker OCT4, and promoted hESC differentiation [64].
Pyruvate
Pyruvate is the product of glycolysis, and it is also supplemented in most culture media. Pyruvate takes multiple potential routes in energy metabolism. In hPSCs, pyruvate produced from glucose is mainly converted to lactate in the cytosol at the expense of one NADH [13], and it does not have immediate impact on oxidative phosphorylation. In contrast, supplementation of pyruvate in the cell culture medium promoted oxygen consumption and suppressed glycolysis, suggesting exogenous pyruvate contributes significantly to oxidative phosphorylation [94]. In extracellular flux assays, the immediate impact of pyruvate supplementation on oxidative phosphorylation was shown to be stronger than glutamine [80]. Pyruvate can enter the TCA cycle through two routes, either oxidation to acetyl-CoA by pyruvate dehydrogenase, or conversion to oxaloacetate by pyruvate carboxylase to replenish TCA cycle intermediates [100]. The enzymes involved in these steps are likely targets for the manipulation of hPSC oxidative phosphorylation. Pyruvate-associated oxidative phosphorylation can be elevated by inhibition of pyruvate dehydrogenase kinase (PDK), which phosphorylates and inactivates pyruvate dehydrogenase [8, 89, 106]. Oxidative phosphorylation is suppressed by UK-5099 that inhibits pyruvate transportation into mitochondria [42, 67]. Not all pyruvate-derived acetyl-CoA are used in energy production. Instead, part of the acetyl-CoA contributes to lipid synthesis and protein acetylation [100]. These alternative uses are likely due to the compartmentation of pyruvate and associated enzymes in the cell. Pyruvate interacts with other substrates in their metabolism. Pyruvate and glutamine synergistically elevate oxidative phosphorylation, especially in the absence of glucose. Glucose suppresses pyruvate-dependent oxidative phosphorylation to a lower level [80]. The elevation of exogenous pyruvate significantly increases oxidative phosphorylation, while suppressing glycolysis at the same time [94]. It would be interesting to further investigate the interactions among the metabolism of major hPSC energy substrates.
Lipids
Although lipid supplements are often used in hPSC culture, they are not essential for the maintenance of pluripotency [15, 57]. Cellular lipids can be generated by hPSCs through de novo lipogenesis using energy and metabolites from glycolysis and oxidative phosphorylation. As energy substrates, endogenous lipids contribute to a large portion of oxidative phosphorylation. Interestingly, exogenous lipids are not immediately utilized in oxidative phosphorylation [80, 95]. Depending on the specific lipid supplement, the metabolic landscape of hPSCs could be shifted greatly. For example, serum extract AlbuMAX contains diverse lipid species, and it significantly alters the balance of glycolysis and TCA cycle while relieving the metabolic burden of de novo lipogenesis [114]. It is proposed that lipid deprivation and the increased dependence on de novo lipogenesis could change the pluripotency state and induce naïve-like features in hPSCs [23]. Because of the diverse lipids involved, it is still unclear which lipid plays the key role and how it leads to significant metabolic, transcriptional, and epigenetic changes.
Vitamins
When energy substrates are provided, hPSCs rely on specific enzymes to carry out the metabolic processes. Many of these enzymes require various vitamins as cofactors. Water-soluble B family vitamins play particularly important roles in energy metabolism. Vitamins B1 and B3 are involved in glycolysis, while B1, B2, B3, B5 and B7 are important for the TCA cycle and oxidative phosphorylation. Lipid synthesis and metabolism require the actions of B2, B3, B5 and B7; Vitamins B3, B6, B9 and B12 are involved in amino acid metabolism [37]. All of them are usually supplied in the base medium for hPSCs.
Culture environmental components
In addition to all the energy substrates in the medium, hPSCs also require suitable environmental factors to maintain normal energy metabolism, such as pH and oxygen. Culture pH is normally regulated by CO2 infusion and buffering reagents in the medium such as HEPES and sodium bicarbonate (NaHCO3). Most cell cultures are maintained under normoxic conditions (~ 20% oxygen). The oxygen level can be adjusted to fit different purposes, either decreased to mimic the hypoxic physiological environment or elevated to improve expansion of suspension culture [65, 87]. Changes in pH and oxygen level have profound impacts on cellular metabolism.
Biological control of pH and its impact on hESC metabolism
Environmental and intracellular pH has profound impacts on cellular functions, yet has received surprisingly little attention. The ionization status of acidic or basic amino acids in enzymes may change according to pH, leading to conformational changes and resulting in pH sensitivity. Various cellular processes, including membrane transport, cell proliferation, membrane potential, energy metabolism, signal transduction, etc., have been shown to respond to pH variations [30, 36, 48, 62, 84].
It is well known that elevated pH promotes glycolysis and stimulates the production of lactate [44, 79]. Many enzymes in the glycolysis pathway and the TCA cycle have been shown to be pH sensitive [53, 60, 77, 99]. Under acidic pH conditions, both glycolysis and oxidative phosphorylation are suppressed [58]. In contrast, elevation of pH leads to higher energy production through glycolysis and oxidative phosphorylation. Because glycolysis is the main energy producing process in hESCs, more lactic acid is released into the medium as the cell density increases with proliferation. This phenomenon leads to a self-induced proliferation control for hESCs through metabolism. When cell density increases to near confluence, medium acidosis turns off glycolysis, leading to growth arrest or even cell death. When additional sodium bicarbonate is applied to increase medium pH and buffer capacity, it significantly promotes glucose uptake and its consumption through both glycolytic and oxidative metabolism, and increases ATP production, which allows hESC proliferation to higher densities [58]. In addition to the impact on glucose metabolism, medium acidosis also affects CO2 fixation. Many cell culture systems utilize the CO2/HCO3− buffer system, where HCO3− is included in the medium and CO2 is supplied to the incubator. A drop in pH leads to the loss of HCO3− from the medium, which normally serves as the carbon source for the synthesis of oxaloacetate, an essential intermediate in the TCA cycle utilized for biosynthetic pathways [38, 91].
Intracellular acid–base balance is maintained by an intricate system of buffers, membrane transporters and enzymes [12, 84]. Biological buffers, including phosphate groups, amino acid side chains, and HCO3− groups, allow cells to respond rapidly to acute changes in pH. Cells are also equipped with multiple transporters in the plasma membrane, including ATP-driven proton pumps (ATPases), monocarboxylate transporters (MCTs), Na+/Bicarbonate cotransporters (NBCs), sodium-dependent chloride/bicarbonate exchangers (NCBE), Na+/H+ exchangers (NHEs), and Cl−/HCO3− anion exchangers (AEs). V-type H+ ATPases, NHEs, NBCs and NCBEs drive H+ efflux or HCO3− influx to protect cells against acidification, while AEs and NBCs are capable of transporting HCO3− out of the cell to prevent alkalinization. In cell types with high levels of glycolysis, outward transport of lactate along with H+ by MCTs also comprises an important pH regulatory mechanism [43]. During early chicken embryo development, a pH gradient exists in parallel with graded MCT1 expression in the tail bud, and plays important roles in cell fate specification [73].
Cellular pH and metabolism can be manipulated by targeting pH regulators. Knockout of Na+/bicarbonate cotransporter NBCn1 in mice resulted in reduced intracellular pH in endothelial cells [7]. In human pluripotent stem cells, MCT1 is expressed at high levels, and inhibition of MCT1 was shown to reduce glycolytic flux [40]. In cell culture systems, control and regulation of environmental pH are achieved with buffering agents. The most frequently used buffer in cell culture media is the physiological CO2/HCO3− buffer system, which can be used in combination with non-volatile buffers such as HEPES [26, 68]. Given the close relationship between pH control, hypoxia, and energy metabolism, it would be interesting to further investigate the impact of environmental and cellular pH changes on hESC function.
Metabolic regulation by oxygen tension
Oxygen is the terminal electron acceptor in metabolic pathways, and serves as the substrate/cofactor for many metabolic enzymes. Environmental oxygen level is a key regulator of hESC metabolism.
Oxygen homeostasis in hESCs is maintained by hypoxia-inducible factors (HIFs), global regulators of hypoxic responses [20, 47, 78]. HIFs are heterodimeric transcription factors consisting of the inducible HIF-α subunit and the constitutively expressed HIF-β (ARNT) subunit. hESCs express three different HIF-α isoforms (HIF-1α, HIF-2α and HIF-3α). Among these, HIF-3α lacks the binding domain for coactivators, and is proposed to work by regulating the expression of other isoforms. HIF-1α is transiently expressed for 48 h under hypoxia, suggesting a role in the initial adaptation to hypoxic conditions. In contrast, upregulation and nuclear translocation of HIF-2α and HIF-3α occur after long-term culture under hypoxia [34].
HIF is one of the central regulators of glycolysis. hESCs cultured under hypoxic conditions were shown to consume less oxygen, utilize more glucose and less pyruvate, and produce more lactate, suggesting elevated levels of glycolysis [33]. Expression of glucose transporters GLUT1 and GLUT3 in hESCs are both upregulated under hypoxic conditions [5, 21, 33], suggesting increased glucose uptake under decreased oxygen tension. Multiple metabolic enzymes have been shown to be regulated by HIF, including pyruvate dehydrogenase kinase [51], lactate dehydrogenase, hexokinase, and several other glycolytic enzymes [50, 81, 85, 86]. The increased glycolysis flux maintains ATP generation, while PDK and LDH activation decreases substrate availability for the TCA cycle and attenuates mitochondrial ROS production to protect cells against oxidative stress under hypoxia [51].
As hESCs possess a unique metabolic program with high glycolytic flux [31, 100], metabolic changes under hypoxia are beneficial for hESC maintenance and generation. Hypoxic culture conditions were reported to enhance the expression of pluripotency markers SOX2, NANOG and POU5F1 in hESCs, mediated by HIF-2α [34]. Spontaneous differentiation of hESCs were reduced when cultured under low oxygen tension, indicating beneficial effects of hypoxia on hESC maintenance [29]. Reprogramming of mouse fibroblasts to induce pluripotent stem cells was also enhanced by hypoxia [111].
In addition to carbohydrate metabolism, hypoxia and HIFs are also extensively involved in lipid metabolism [70], although the functional implications for hESCs are not well understood. In recent years, HIFs have also been reported to participate in epigenetics, exosome release as well as biogenesis of non-coding RNAs [20], and novel mechanisms may be revealed in the near future which underlies the impact of oxygen tension on hESCs.
Mitogenic regulation of hPSC energy metabolism
Given the pivotal role of metabolic homeostasis in hPSCs, the control of substrate and environmental factors are still not sufficient to control the balance of glycolysis and oxidative phosphorylation. Mitogenic regulation is an obvious candidate to coordinate metabolism and stem cell function. The maintenance of hPSCs requires continuous mitogenic stimulation, including FGF2, TGFβ and insulin [15]. All these mitogens have been implied in energy metabolism in somatic cells, but their roles in hPSCs are often difficult to study. The suppression of any of these pathways could lead to cell differentiation, which often complicates the interpretation of metabolic phenotypes. The immediate measurement of metabolic profile upon signal manipulation is essential for people to understand a mitogen’s impact on energy metabolism without the interference from potential cell type changes.
Among all essential mitogens, the insulin/IGF family is the most important factor for hPSC survival and function, and is utilized in various lineage-specific induction protocols, such as neural cell fate induction [14], skin keratinocyte [119] and retinal pigmented epithelium differentiation [9]. IGF signaling also shifts cell fate during mesoderm induction [109]. During hESC maintenance, insulin/IGF is the only factor important for immediate metabolic regulation [80]. Continuous insulin stimulation is essential to sustain oxidative phosphorylation. If insulin stimulation is removed, oxidative phosphorylation level decreases immediately. Insulin promotes oxidative metabolism associated with pyruvate, glutamine and lipid. Oxidative phosphorylation is enhanced by insulin through the PI3K/AKT/GSK3 pathway. Insulin activates PI3K and AKT, which subsequently inhibits GSK3. Inhibition of GSK3 is able to partially rescue oxidative phosphorylation in the absence of insulin. The insulin-dependent oxidative phosphorylation is stem cell specific, and the phenomenon disappears during differentiation. FGF2 and TGFβ removal for two days is sufficient for the loss of insulin-dependent oxidative phosphorylation. It suggests that FGF2 and TGFβ are in play with metabolic regulation but in an indirect manner. Interestingly, insulin does not promote glycolysis, probably due the fact that hPSCs express the insulin-independent glucose transporter GLUT1 [27, 80].
Besides the insulin pathway, other pathways could also play a role in metabolic control in hPSCs. OCIAD1, a mitochondrial protein, was reported to actively regulate oxidative phosphorylation in hPSCs. Reduction of OCIAD1 expression levels led to increased activity of mitochondrial complex I and promoted oxidative phosphorylation, enhancing hPSC differentiation upon induction [90]. OCIAD1 was previously reported to regulate the JAK/STAT signaling pathway and promote STAT3 activation in mESCs [93], and also shown to modulate NOTCH signaling in Drosophila [52]. It is plausible that these pathways may be involved in the regulation of hPSC energy metabolism.
Regulation of metabolism by nuclear factors
In addition to HIFs that were discussed earlier, other nuclear factors may also contribute to the regulation of hESC metabolism. High mobility group A (HMGA) proteins, including HMGA1 and HMGA2, are chromatin factors involved in global chromatin remodeling. Both HMGA1 and HMGA2 are highly expressed in hESCs, and can regulate pluripotency and differentiation by controlling gene expression [18, 75]. The expression level of HMGA1 is high in undifferentiated hESCs and declines during differentiation. Its ectopic expression maintains pluripotency markers, prevents hESC differentiation and enhances reprogramming [88]. At the same time, HMGA1 is also an important regulator of glucose production and metabolism. HMGA1 is essential for the transcription of both INS and INSR gene, and is also a downstream target of insulin action through the PI-3K/AKT pathway [19]. Consistently, Hmga1 knockout mice display glucose intolerance due to down-regulation of insulin receptors [35].
Another group of nuclear factors known to regulate metabolism are the Forkhead box protein O (FOXO) transcription factors, which regulate a diverse array of cellular functions, including DNA repair, cell proliferation, cell death, and metabolism [1, 39]. FOXO1 is a key regulator of glucose production. FOXO1 is downstream of insulin signaling and the PI-3K/AKT pathway, and the phosphorylation by AKT inhibits FOXO1’s nuclear localization and function [72]. Under fasting conditions, FOXO1 is upregulated by the cAMP-PKA pathway to maintain glucose levels [107]. In hESCs, FOXO1 is abundantly expressed, and the expression level decreases upon differentiation, coinciding with the metabolic transition [59]. FOXO1 is essential for maintaining the expression of pluripotency genes, and remains active in hESCs despite its phosphorylation by AKT [117]. The mechanism through which FOXO1 regulates hESC metabolism remains to be explored. HMGA1 was reported to be a positive modulator of FoxO1 [4], potentially enabling the crosstalk between metabolism and pluripotency.
Besides nuclear gene expression, changes in mitochondria DNA can also have significant impacts on metabolism. Pathogenic mtDNA variations have been reported to alter the process of oxidative phosphorylation and lead to disorders involving multiple organ systems. iPSCs offer an invaluable model system to study mitochondrial disorders and test potential therapeutics [45, 49].
Summary
Although we discuss all the energy metabolism regulators separately, they actually cross talk with each other. Oxygen and pH fluctuations lead to changes in HIF and PI3K/AKT pathway [3, 16], which are directly related to substrate utilization and final energy production. Meanwhile, the amount of substrate available can also regulate signaling pathways, such as the mTOR and AMPK pathway that could affect overall metabolism beyond just energy production. Many current studies imply the association between metabolism and cell fate determination through epigenetics [25, 82]. Given the intermingled relationships between metabolism, signal transduction, nuclear signals and epigenetic modifications, it is possible that more complicated regulatory mechanisms are involved at multiple levels. Further analysis of the regulators of hPSC metabolism and their roles in stem cell function is necessary and may provide new insights into the applications of stem cell technology.
Acknowledgements
We thank the council members of Macau Society for Stem Cell Research (MSSCR) for the constructive discussions.
Author contributions
Both authors conceptualized and drafted the article. WL designed the figures. Both authors have read and agreed to the final version of the manuscript.
Funding
This work was supported by the University of Macau (File No. MYRG2018-00135-FHS and MYRG2019-00147-FHS), and also by the Science and Technology Development Fund, Macau SAR (File No. 0059/2019/A1, 0123/2019/A3 and 0011/2019/AKP).
Availability of data and material
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors consent to publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117(4):421–426. doi: 10.1016/S0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 2.Aft RL, Zhang FW, Gius D. Evaluation of 2-deoxy-d-glucose as a chemotherapeutic agent: mechanism of cell death. Br J Cancer. 2002;87(7):805–812. doi: 10.1038/sj.bjc.6600547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Tejado M, Naranjo-Suarez S, Jimenez C, Carrera AC, Landazuri MO, del Peso L. Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells: protective role in apoptosis. J Biol Chem. 2001;276(25):22368–22374. doi: 10.1074/jbc.M011688200. [DOI] [PubMed] [Google Scholar]
- 4.Arcidiacono B, Chiefari E, Messineo S, Bilotta FL, Pastore I, Corigliano DM, Foti DP, Brunetti A. HMGA1 is a novel transcriptional regulator of the FoxO1 gene. Endocrine. 2018;60(1):56–64. doi: 10.1007/s12020-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arthur SA, Blaydes JP, Houghton FD. Glycolysis regulates human embryonic stem cell self-renewal under hypoxia through HIF-2alpha and the glycolytic sensors CTBPs. Stem Cell Rep. 2019;12(4):728–742. doi: 10.1016/j.stemcr.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birket MJ, Orr AL, Gerencser AA, Madden DT, Vitelli C, Swistowski A, Brand MD, Zeng X. A reduction in ATP demand and mitochondrial activity with neural differentiation of human embryonic stem cells. J Cell Sci. 2011;124(Pt 3):348–358. doi: 10.1242/jcs.072272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boedtkjer E, Praetorius J, Matchkov VV, Stankevicius E, Mogensen S, Fuchtbauer AC, Simonsen U, Fuchtbauer EM, Aalkjaer C. Disruption of Na+, HCO(3)(−) cotransporter NBCn1 (slc4a7) inhibits NO-mediated vasorelaxation, smooth muscle Ca(2)(+) sensitivity, and hypertension development in mice. Circulation. 2011;124(17):1819–1829. doi: 10.1161/CIRCULATIONAHA.110.015974. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11(1):37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Buchholz DE, Pennington BO, Croze RH, Hinman CR, Coffey PJ, Clegg DO. Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Transl Med. 2013;2(5):384–393. doi: 10.5966/sctm.2012-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbognin E, Betto RM, Soriano ME, Smith AG, Martello G. Stat3 promotes mitochondrial transcription and oxidative respiration during maintenance and induction of naive pluripotency. EMBO J. 2016;35(6):618–634. doi: 10.15252/embj.201592629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518(7539):413–416. doi: 10.1038/nature13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11(1):50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 13.Chakrabarty RP, Chandel NS. Mitochondria as signaling organelles control mammalian stem cell fate. Cell Stem Cell. 2021;28(3):394–408. doi: 10.1016/j.stem.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen GK, Gulbranson DR, Hou ZG, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JMC, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8(5):424–U476. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JL, Lucas JE, Schroeder T, Mori S, Wu J, Nevins J, Dewhirst M, West M, Chi JT. The genomic analysis of lactic acidosis and acidosis response in human cancers. PLoS Genet. 2008;4(12):e1000293. doi: 10.1371/journal.pgen.1000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W, Gueron M. The inhibition of bovine heart hexokinase by 2-deoxy-d-glucose-6-phosphate: characterization by 31P NMR and metabolic implications. Biochimie. 1992;74(9–10):867–873. doi: 10.1016/0300-9084(92)90070-U. [DOI] [PubMed] [Google Scholar]
- 18.Chiefari E, Foti DP, Sgarra R, Pegoraro S, Arcidiacono B, Brunetti FS, Greco M, Manfioletti G, Brunetti A. Transcriptional regulation of glucose metabolism: the emerging role of the HMGA1 chromatin factor. Front Endocrinol (Lausanne) 2018;9:357. doi: 10.3389/fendo.2018.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiefari E, Nevolo MT, Arcidiacono B, Maurizio E, Nocera A, Iiritano S, Sgarra R, Possidente K, Palmieri C, Paonessa F, Brunetti G, Manfioletti G, Foti D, Brunetti A. HMGA1 is a novel downstream nuclear target of the insulin receptor signaling pathway. Sci Rep. 2012;2:251. doi: 10.1038/srep00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choudhry H, Harris AL. Advances in hypoxia-inducible factor biology. Cell Metab. 2018;27(2):281–298. doi: 10.1016/j.cmet.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Christensen DR, Calder PC, Houghton FD. GLUT3 and PKM2 regulate OCT4 expression and support the hypoxic culture of human embryonic stem cells. Sci Rep. 2015;5:17500. doi: 10.1038/srep17500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung S, Dzeja PP, Faustino RS, Perez-Terzic C, Behfar A, Terzic A. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S60–67. doi: 10.1038/ncpcardio0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornacchia D, Zhang C, Zimmer B, Chung SY, Fan Y, Soliman MA, Tchieu J, Chambers SM, Shah H, Paull D, Konrad C, Vincendeau M, Noggle SA, Manfredi G, Finley LWS, Cross JR, Betel D, Studer L. Lipid deprivation induces a stable, naive-to-primed intermediate state of pluripotency in human PSCs. Cell Stem Cell. 2019;25(1):120–136 e110. doi: 10.1016/j.stem.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahan P, Lu V, Nguyen RMT, Kennedy SAL, Teitell MA. Metabolism in pluripotency: both driver and passenger? J Biol Chem. 2019;294(14):5420–5429. doi: 10.1074/jbc.TM117.000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai Z, Ramesh V, Locasale JW. The evolving metabolic landscape of chromatin biology and epigenetics. Nat Rev Genet. 2020;21(12):737–753. doi: 10.1038/s41576-020-0270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eagle H. Buffer combinations for mammalian cell culture. Science. 1971;174(4008):500–503. doi: 10.1126/science.174.4008.500. [DOI] [PubMed] [Google Scholar]
- 27.Ebeling P, Koistinen HA, Koivisto VA. Insulin-independent glucose transport regulates insulin sensitivity. FEBS Lett. 1998;436(3):301–303. doi: 10.1016/S0014-5793(98)01149-1. [DOI] [PubMed] [Google Scholar]
- 28.Etchegaray JP, Mostoslavsky R. Interplay between metabolism and epigenetics: a nuclear adaptation to environmental changes. Mol Cell. 2016;62(5):695–711. doi: 10.1016/j.molcel.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci USA. 2005;102(13):4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang Y, Liu Z, Chen Z, Xu X, Xiao M, Yu Y, Zhang Y, Zhang X, Du Y, Jiang C, Zhao Y, Wang Y, Fan B, Terheyden-Keighley D, Liu Y, Shi L, Hui Y, Zhang X, Zhang B, Feng H, Ma L, Zhang Q, Jin G, Yang Y, Xiang B, Liu L, Zhang X. Smad5 acts as an intracellular pH messenger and maintains bioenergetic homeostasis. Cell Res. 2017;27(9):1083–1099. doi: 10.1038/cr.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folmes CD, Dzeja PP, Nelson TJ, Terzic A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. 2012;11(5):596–606. doi: 10.1016/j.stem.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14(2):264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forristal CE, Christensen DR, Chinnery FE, Petruzzelli R, Parry KL, Sanchez-Elsner T, Houghton FD. Environmental oxygen tension regulates the energy metabolism and self-renewal of human embryonic stem cells. PLoS ONE. 2013;8(5):e62507. doi: 10.1371/journal.pone.0062507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139(1):85–97. doi: 10.1530/REP-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foti D, Chiefari E, Fedele M, Iuliano R, Brunetti L, Paonessa F, Manfioletti G, Barbetti F, Brunetti A, Croce CM, Fusco A, Brunetti A. Lack of the architectural factor HMGA1 causes insulin resistance and diabetes in humans and mice. Nat Med. 2005;11(7):765–773. doi: 10.1038/nm1254. [DOI] [PubMed] [Google Scholar]
- 36.Fukamachi T, Ikeda S, Wang X, Saito H, Tagawa M, Kobayashi H. Gene expressions for signal transduction under acidic conditions. Genes (Basel) 2013;4(1):65–85. doi: 10.3390/genes4010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Godoy-Parejo C, Deng C, Zhang Y, Liu W, Chen G. Roles of vitamins in stem cells. Cell Mol Life Sci. 2020;77(9):1771–1791. doi: 10.1007/s00018-019-03352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graves CN, Biggers JD. Carbon dioxide fixation by mouse embryos prior to implantation. Science. 1970;167(3924):1506–1508. doi: 10.1126/science.167.3924.1506. [DOI] [PubMed] [Google Scholar]
- 39.Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27(16):2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 40.Gu W, Gaeta X, Sahakyan A, Chan AB, Hong CS, Kim R, Braas D, Plath K, Lowry WE, Christofk HR. Glycolytic metabolism plays a functional role in regulating human pluripotent stem cell state. Cell Stem Cell. 2016;19(4):476–490. doi: 10.1016/j.stem.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo G, von Meyenn F, Rostovskaya M, Clarke J, Dietmann S, Baker D, Sahakyan A, Myers S, Bertone P, Reik W, Plath K, Smith A. Epigenetic resetting of human pluripotency. Development. 2017;144(15):2748–2763. doi: 10.1242/dev.146811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halestrap AP. The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. Biochem J. 1975;148(1):85–96. doi: 10.1042/bj1480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447(5):619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 44.Halperin ML, Connors HP, Relman AS, Karnovsky ML. Factors that control the effect of pH on glycolysis in leukocytes. J Biol Chem. 1969;244(2):384–390. doi: 10.1016/S0021-9258(18)94442-X. [DOI] [PubMed] [Google Scholar]
- 45.Johannsen DL, Ravussin E. The role of mitochondria in health and disease. Curr Opin Pharmacol. 2009;9(6):780–786. doi: 10.1016/j.coph.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung J, Zeng H, Horng T. Metabolism as a guiding force for immunity. Nat Cell Biol. 2019;21(1):85–93. doi: 10.1038/s41556-018-0217-x. [DOI] [PubMed] [Google Scholar]
- 47.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Kaminskas E. The pH-dependence of sugar-transport and glycolysis in cultured Ehrlich ascites-tumour cells. Biochem J. 1978;174(2):453–459. doi: 10.1042/bj1740453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kargaran PK, Mosqueira D, Kozicz T. Mitochondrial medicine: genetic underpinnings and disease modeling using induced pluripotent stem cell technology. Front Cardiovasc Med. 2020;7:604581. doi: 10.3389/fcvm.2020.604581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kierans SJ, Taylor CT. Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J Physiol. 2021;599(1):23–37. doi: 10.1113/JP280572. [DOI] [PubMed] [Google Scholar]
- 51.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3(3):177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Kulkarni V, Khadilkar RJ, Magadi SS, Inamdar MS. Asrij maintains the stem cell niche and controls differentiation during Drosophila lymph gland hematopoiesis. PLoS ONE. 2011;6(11):e27667. doi: 10.1371/journal.pone.0027667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuwata F, Suzuki N, Otsuka K, Taguchi M, Sasai Y, Wakino H, Ito M, Ebihara S, Suzuki K. Enzymatic regulation of glycolysis and gluconeogenesis in rabbit periodontal ligament under various physiological pH conditions. J Nihon Univ Sch Dent. 1991;33(2):81–90. doi: 10.2334/josnusd1959.33.81. [DOI] [PubMed] [Google Scholar]
- 54.Laszlo J, Humphreys SR, Goldin A. Effects of glucose analogues (2-deoxy-d-glucose, 2-deoxy-d-galactose) on experimental tumors. J Natl Cancer Inst. 1960;24:267–281. [PubMed] [Google Scholar]
- 55.Leese HJ, Barton AM. Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J Reprod Fertil. 1984;72(1):9–13. doi: 10.1530/jrf.0.0720009. [DOI] [PubMed] [Google Scholar]
- 56.Levitzki A, Koshland DE., Jr Cytidine triphosphate synthetase. Covalent intermediates and mechanisms of action. Biochemistry. 1971;10(18):3365–3371. doi: 10.1021/bi00794a008. [DOI] [PubMed] [Google Scholar]
- 57.Liu W, Deng C, Godoy-Parejo C, Zhang Y, Chen G. Developments in cell culture systems for human pluripotent stem cells. World J Stem Cells. 2019;11(11):968–981. doi: 10.4252/wjsc.v11.i11.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu W, Ren Z, Lu K, Song C, Cheung ECW, Zhou Z, Chen G. The suppression of medium acidosis improves the maintenance and differentiation of human pluripotent stem cells at high density in defined cell culture medium. Int J Biol Sci. 2018;14(5):485–496. doi: 10.7150/ijbs.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ludikhuize MC, Rodriguez Colman MJ. Metabolic regulation of stem cells and differentiation: a Forkhead box o transcription factor perspective. Antioxid Redox Signal. 2021;34(13):1004–1024. doi: 10.1089/ars.2020.8126. [DOI] [PubMed] [Google Scholar]
- 60.Luna LA, Lesecq Z, White KA, Hoang A, Scott DA, Zagnitko O, Bobkov AA, Barber DL, Schiffer JM, Isom DG, Sohl CD. An acidic residue buried in the dimer interface of isocitrate dehydrogenase 1 (IDH1) helps regulate catalysis and pH sensitivity. Biochem J. 2020;477(16):2999–3018. doi: 10.1042/BCJ20200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 62.Mackenzie CG, Mackenzie JB, Beck P. The effect of pH on growth, protein synthesis, and lipid-rich particles of cultured mammalian cells. J Biophys Biochem Cytol. 1961;9:141–156. doi: 10.1083/jcb.9.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mandal S, Lindgren AG, Srivastava AS, Clark AT, Banerjee U. Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells. 2011;29(3):486–495. doi: 10.1002/stem.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marsboom G, Zhang GF, Pohl-Avila N, Zhang Y, Yuan Y, Kang H, Hao B, Brunengraber H, Malik AB, Rehman J. Glutamine metabolism regulates the pluripotency transcription factor OCT4. Cell Rep. 2016;16(2):323–332. doi: 10.1016/j.celrep.2016.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mas-Bargues C, Sanz-Ros J, Roman-Dominguez A, Ingles M, Gimeno-Mallench L, El Alami M, Vina-Almunia J, Gambini J, Vina J, Borras C. Relevance of oxygen concentration in stem cell culture for regenerative medicine. Int J Mol Sci. 2019;20(5):1195. doi: 10.3390/ijms20051195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mathieu J, Ruohola-Baker H. Metabolic remodeling during the loss and acquisition of pluripotency. Development. 2017;144(4):541–551. doi: 10.1242/dev.128389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCommis KS, Finck BN. Mitochondrial pyruvate transport: a historical perspective and future research directions. Biochem J. 2015;466(3):443–454. doi: 10.1042/BJ20141171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Michl J, Park KC, Swietach P. Evidence-based guidelines for controlling pH in mammalian live-cell culture systems. Commun Biol. 2019;2:144. doi: 10.1038/s42003-019-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moussaieff A, Rouleau M, Kitsberg D, Cohen M, Levy G, Barasch D, Nemirovski A, Shen-Orr S, Laevsky I, Amit M, Bomze D, Elena-Herrmann B, Scherf T, Nissim-Rafinia M, Kempa S, Itskovitz-Eldor J, Meshorer E, Aberdam D, Nahmias Y. Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab. 2015;21(3):392–402. doi: 10.1016/j.cmet.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 70.Mylonis I, Simos G, Paraskeva E. Hypoxia-inducible factors and the regulation of lipid metabolism. Cells. 2019;8(3):214. doi: 10.3390/cells8030214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oburoglu L, Tardito S, Fritz V, de Barros SC, Merida P, Craveiro M, Mamede J, Cretenet G, Mongellaz C, An X, Klysz D, Touhami J, Boyer-Clavel M, Battini JL, Dardalhon V, Zimmermann VS, Mohandas N, Gottlieb E, Sitbon M, Kinet S, Taylor N. Glucose and glutamine metabolism regulate human hematopoietic stem cell lineage specification. Cell Stem Cell. 2014;15(2):169–184. doi: 10.1016/j.stem.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 72.Ochocki JD, Simon MC. Nutrient-sensing pathways and metabolic regulation in stem cells. J Cell Biol. 2013;203(1):23–33. doi: 10.1083/jcb.201303110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oginuma M, Harima Y, Tarazona OA, Diaz-Cuadros M, Michaut A, Ishitani T, Xiong F, Pourquie O. Intracellular pH controls WNT downstream of glycolysis in amniote embryos. Nature. 2020;584(7819):98–101. doi: 10.1038/s41586-020-2428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Panopoulos AD, Yanes O, Ruiz S, Kida YS, Diep D, Tautenhahn R, Herrerias A, Batchelder EM, Plongthongkum N, Lutz M, Berggren WT, Zhang K, Evans RM, Siuzdak G, Izpisua Belmonte JC. The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. 2012;22(1):168–177. doi: 10.1038/cr.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parisi S, Piscitelli S, Passaro F, Russo T. HMGA proteins in stemness and differentiation of embryonic and adult stem cells. Int J Mol Sci. 2020;21(1):362. doi: 10.3390/ijms21010362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pereira SL, Graos M, Rodrigues AS, Anjo SI, Carvalho RA, Oliveira PJ, Arenas E, Ramalho-Santos J. Inhibition of mitochondrial complex III blocks neuronal differentiation and maintains embryonic stem cell pluripotency. PLoS ONE. 2013;8(12):e82095. doi: 10.1371/journal.pone.0082095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quach CH, Jung KH, Lee JH, Park JW, Moon SH, Cho YS, Choe YS, Lee KH. Mild alkalization acutely triggers the warburg effect by enhancing hexokinase activity via voltage-dependent anion channel binding. PLoS ONE. 2016;11(8):e0159529. doi: 10.1371/journal.pone.0159529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ratcliffe PJ. Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J Physiol. 2013;591(8):2027–2042. doi: 10.1113/jphysiol.2013.251470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Relman AS. Metabolic consequences of acid-base disorders. Kidney Int. 1972;1(5):347–359. doi: 10.1038/ki.1972.46. [DOI] [PubMed] [Google Scholar]
- 80.Ren Z, Zhong H, Song C, Deng C, Hsieh HT, Liu W, Chen G. Insulin promotes mitochondrial respiration and survival through PI3K/AKT/GSK3 pathway in human embryonic stem cells. Stem Cell Rep. 2020;15(6):1362–1376. doi: 10.1016/j.stemcr.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Riddle SR, Ahmad A, Ahmad S, Deeb SS, Malkki M, Schneider BK, Allen CB, White CW. Hypoxia induces hexokinase II gene expression in human lung cell line A549. Am J Physiol Lung Cell Mol Physiol. 2000;278(2):L407–416. doi: 10.1152/ajplung.2000.278.2.L407. [DOI] [PubMed] [Google Scholar]
- 82.Ryall JG, Cliff T, Dalton S, Sartorelli V. Metabolic reprogramming of stem cell epigenetics. Cell Stem Cell. 2015;17(6):651–662. doi: 10.1016/j.stem.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schieke SM, Ma M, Cao L, McCoy JP, Jr, Liu C, Hensel NF, Barrett AJ, Boehm M, Finkel T. Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. J Biol Chem. 2008;283(42):28506–28512. doi: 10.1074/jbc.M802763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seifter JL. Body fluid compartments, cell membrane ion transport, electrolyte concentrations, and acid-base balance. Semin Nephrol. 2019;39(4):368–379. doi: 10.1016/j.semnephrol.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 85.Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta. 2011;1813(7):1263–1268. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271(51):32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 87.Serra M, Brito C, Sousa MF, Jensen J, Tostoes R, Clemente J, Strehl R, Hyllner J, Carrondo MJ, Alves PM. Improving expansion of pluripotent human embryonic stem cells in perfused bioreactors through oxygen control. J Biotechnol. 2010;148(4):208–215. doi: 10.1016/j.jbiotec.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 88.Shah SN, Kerr C, Cope L, Zambidis E, Liu C, Hillion J, Belton A, Huso DL, Resar LM. HMGA1 reprograms somatic cells into pluripotent stem cells by inducing stem cell transcriptional networks. PLoS ONE. 2012;7(11):e48533. doi: 10.1371/journal.pone.0048533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shen YC, Ou DL, Hsu C, Lin KL, Chang CY, Lin CY, Liu SH, Cheng AL. Activating oxidative phosphorylation by a pyruvate dehydrogenase kinase inhibitor overcomes sorafenib resistance of hepatocellular carcinoma. Br J Cancer. 2013;108(1):72–81. doi: 10.1038/bjc.2012.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shetty DK, Kalamkar KP, Inamdar MS. OCIAD1 controls electron transport chain complex I activity to regulate energy metabolism in human pluripotent stem cells. Stem Cell Rep. 2018;11(1):128–141. doi: 10.1016/j.stemcr.2018.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shyh-Chang N, Daley GQ, Cantley LC. Stem cell metabolism in tissue development and aging. Development. 2013;140(12):2535–2547. doi: 10.1242/dev.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shyh-Chang N, Ng HH. The metabolic programming of stem cells. Genes Dev. 2017;31(4):336–346. doi: 10.1101/gad.293167.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sinha A, Khadilkar RJ, Vinay KS, Roychowdhury Sinha A, Inamdar MS. Conserved regulation of the Jak/STAT pathway by the endosomal protein asrij maintains stem cell potency. Cell Rep. 2013;4(4):649–658. doi: 10.1016/j.celrep.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song C, Xu F, Ren Z, Zhang Y, Meng Y, Yang Y, Lingadahalli S, Cheung E, Li G, Liu W, Wan J, Zhao Y, Chen G. Elevated exogenous pyruvate potentiates mesodermal differentiation through metabolic modulation and AMPK/mTOR pathway in human embryonic stem cells. Stem Cell Rep. 2019;13(2):338–351. doi: 10.1016/j.stemcr.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sperber H, Mathieu J, Wang Y, Ferreccio A, Hesson J, Xu Z, Fischer KA, Devi A, Detraux D, Gu H, Battle SL, Showalter M, Valensisi C, Bielas JH, Ericson NG, Margaretha L, Robitaille AM, Margineantu D, Fiehn O, Hockenbery D, Blau CA, Raftery D, Margolin AA, Hawkins RD, Moon RT, Ware CB, Ruohola-Baker H. The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nat Cell Biol. 2015;17(12):1523–1535. doi: 10.1038/ncb3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 97.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 98.Tohyama S, Fujita J, Hishiki T, Matsuura T, Hattori F, Ohno R, Kanazawa H, Seki T, Nakajima K, Kishino Y, Okada M, Hirano A, Kuroda T, Yasuda S, Sato Y, Yuasa S, Sano M, Suematsu M, Fukuda K. Glutamine oxidation is indispensable for survival of human pluripotent stem cells. Cell Metab. 2016;23(4):663–674. doi: 10.1016/j.cmet.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 99.Trivedi B, Danforth WH. Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem. 1966;241(17):4110–4112. doi: 10.1016/S0021-9258(18)99819-4. [DOI] [PubMed] [Google Scholar]
- 100.Tsogtbaatar E, Landin C, Minter-Dykhouse K, Folmes CDL. Energy metabolism regulates stem cell pluripotency. Front Cell Dev Biol. 2020;8:87. doi: 10.3389/fcell.2020.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vander Heiden MG, DeBerardinis RJ. Understanding the intersections between metabolism and cancer biology. Cell. 2017;168(4):657–669. doi: 10.1016/j.cell.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Varum S, Momcilovic O, Castro C, Ben-Yehudah A, Ramalho-Santos J, Navara CS. Enhancement of human embryonic stem cell pluripotency through inhibition of the mitochondrial respiratory chain. Stem Cell Res. 2009;3(2–3):142–156. doi: 10.1016/j.scr.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Varum S, Rodrigues AS, Moura MB, Momcilovic O, Easley CAT, Ramalho-Santos J, Van Houten B, Schatten G. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE. 2011;6(6):e20914. doi: 10.1371/journal.pone.0020914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weinberger L, Ayyash M, Novershtern N, Hanna JH. Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nat Rev Mol Cell Biol. 2016;17(3):155–169. doi: 10.1038/nrm.2015.28. [DOI] [PubMed] [Google Scholar]
- 106.Whitehouse S, Cooper RH, Randle PJ. Mechanism of activation of pyruvate dehydrogenase by dichloroacetate and other halogenated carboxylic acids. Biochem J. 1974;141(3):761–774. doi: 10.1042/bj1410761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wondisford AR, Xiong L, Chang E, Meng S, Meyers DJ, Li M, Cole PA, He L. Control of Foxo1 gene expression by co-activator P300. J Biol Chem. 2014;289(7):4326–4333. doi: 10.1074/jbc.M113.540500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yanes O, Clark J, Wong DM, Patti GJ, Sanchez-Ruiz A, Benton HP, Trauger SA, Desponts C, Ding S, Siuzdak G. Metabolic oxidation regulates embryonic stem cell differentiation. Nat Chem Biol. 2010;6(6):411–417. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Y, Ren Z, Xu F, Meng Y, Zhang Y, Ai N, Long Y, Fok HI, Deng C, Zhao X, Huang L, Zhao Q, Wang J, Liu W, Ge W, Chen G. Endogenous IGF signaling directs heterogeneous mesoderm differentiation in human embryonic stem cells. Cell Rep. 2019;29(11):3374–3384 e3375. doi: 10.1016/j.celrep.2019.11.047. [DOI] [PubMed] [Google Scholar]
- 110.Yoo HC, Yu YC, Sung Y, Han JM. Glutamine reliance in cell metabolism. Exp Mol Med. 2020;52(9):1496–1516. doi: 10.1038/s12276-020-00504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5(3):237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 112.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 113.Zhang C, Skamagki M, Liu Z, Ananthanarayanan A, Zhao R, Li H, Kim K. Biological significance of the suppression of oxidative phosphorylation in induced pluripotent stem cells. Cell Rep. 2017;21(8):2058–2065. doi: 10.1016/j.celrep.2017.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang H, Badur MG, Divakaruni AS, Parker SJ, Jager C, Hiller K, Murphy AN, Metallo CM. Distinct metabolic states can support self-renewal and lipogenesis in human pluripotent stem cells under different culture conditions. Cell Rep. 2016;16(6):1536–1547. doi: 10.1016/j.celrep.2016.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang J, Nuebel E, Daley GQ, Koehler CM, Teitell MA. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell. 2012;11(5):589–595. doi: 10.1016/j.stem.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang J, Zhao J, Dahan P, Lu V, Zhang C, Li H, Teitell MA. Metabolism in pluripotent stem cells and early mammalian development. Cell Metab. 2018;27(2):332–338. doi: 10.1016/j.cmet.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 117.Zhang X, Yalcin S, Lee DF, Yeh TY, Lee SM, Su J, Mungamuri SK, Rimmele P, Kennedy M, Sellers R, Landthaler M, Tuschl T, Chi NW, Lemischka I, Keller G, Ghaffari S. FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nat Cell Biol. 2011;13(9):1092–1099. doi: 10.1038/ncb2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Y, Cui P, Li Y, Feng G, Tong M, Guo L, Li T, Liu L, Li W, Zhou Q. Mitochondrially produced ATP affects stem cell pluripotency via Actl6a-mediated histone acetylation. FASEB J. 2018;32(4):1891–1902. doi: 10.1096/fj.201700626RR. [DOI] [PubMed] [Google Scholar]
- 119.Zhong H, Ren Z, Wang X, Miao K, Ni W, Meng Y, Lu L, Wang C, Liu W, Deng CX, Xu RH, Chen G. Stagewise keratinocyte differentiation from human embryonic stem cells by defined signal transduction modulators. Int J Biol Sci. 2020;16(8):1450–1462. doi: 10.7150/ijbs.44414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou W, Choi M, Margineantu D, Margaretha L, Hesson J, Cavanaugh C, Blau CA, Horwitz MS, Hockenbery D, Ware C, Ruohola-Baker H. HIF1alpha induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J. 2012;31(9):2103–2116. doi: 10.1038/emboj.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, Kim J, Zhang K, Ding S. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. 2010;7(6):651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.