Abstract
YAP and TAZ are ubiquitously expressed homologous proteins originally identified as penultimate effectors of the Hippo signaling pathway, which plays a key role in maintaining mammalian tissue/organ size. Presently, it is known that YAP/TAZ also interact with various non-Hippo signaling pathways, and have diverse roles in multiple biological processes, including cell proliferation, tissue regeneration, cell lineage fate determination, tumorigenesis, and mechanosensing. In this review, we first examine the various microenvironmental cues and signaling pathways that regulate YAP/TAZ activation, through the Hippo and non-Hippo signaling pathways. This is followed by a brief summary of the interactions of YAP/TAZ with TEAD1-4 and a diverse array of other non-TEAD transcription factors. Finally, we offer a critical perspective on how increasing knowledge of the regulatory mechanisms of YAP/TAZ signaling might open the door to novel therapeutic applications in the interrelated fields of biomaterials, tissue engineering, regenerative medicine and synthetic biology.
Keywords: Hippo, Mechanisms, Regulation, Signaling pathways, TAZ, YAP
Introduction
YAP and TAZ—homologous transcriptional factors that play key roles in diverse biological processes
YAP (Yes-associated protein, also referred to as YAP1) and its smaller paralog TAZ (transcriptional co-activator with PDZ-binding motif) are transcriptional co-activator proteins that shuttle between the nucleus and cytoplasm, and regulate gene expression through binding with the TEAD (TEA/ATTS domain) family of transcription factors (TEAD1–4) within the cell nucleus [1, 2]. Due to their close structural similarities, YAP and TAZ share overlapping roles in many key biological functions, but there are important differences. The major structural difference is that the smaller TAZ protein (43 kDa) lacks the SH3-BM proline-rich region and one WW domain present in the larger YAP protein (65 kDa) [1]. Two splice isoforms of the YAP protein have been identified YAP1-1 and YAP1-2, which differ by the presence of an additional WW domain (38 amino acids) on YAP1-2, compared with YAP1-1 [1]. YAP and TAZ are ubiquitously co-expressed in most mammalian tissues, though there are some exceptions. For example, YAP expression is absent in the hippocampus and parathyroid gland [3], while the thymus and peripheral blood leukocytes lack TAZ expression [4].
Historically, YAP/TAZ were identified as the penultimate effectors of the Hippo signaling pathway, which plays a crucial role in regulating tissue/organ size [5]. In recent years, many other functions of YAP/TAZ have been found, in development, cell proliferation, tissue regeneration and cell lineage fate determination [6], as well as in mechanosensing and mechanotransduction at cell-ECM/biomaterial interfaces [7]. These functions are all highly relevant to the fields of tissue engineering and regenerative medicine. Furthermore, YAP/TAZ also have roles in other biological processes and disease pathologies, most notably in tissue/organ homeostasis and cancer metastasis [8].
In this review, we will give an overview of the various microenvironmental cues and signaling pathways that regulate YAP/TAZ activity, followed by a brief summary of YAP/TAZ interaction with TEAD and non-TEAD transcription factors. Then, we will present a critical perspective, suggesting how our increasing knowledge of YAP/TAZ signaling might advance therapeutic applications in the fields of biomaterials, tissue engineering, regenerative medicine and synthetic biology.
YAP/TAZ—key mediators of cell interaction with the microenvironment
YAP/TAZ mediate cellular responses to (i) biomechanical cues [9–17], (ii) extracellular ligands, such as growth factors and lipids [18–21], (iii) energy, osmotic and hypoxic stress [22–25], and (iv) inflammation and tissue injury [26–30].
Biomechanical cues refer to the mechanical forces generated upon cell interaction with the extracellular matrix (ECM) or substrata, cell-to-cell contact, and liquid shear forces encountered by cells (best exemplified by the endothelial cells lining blood vessels). The responses to these cues mediated by YAP/TAZ in turn play key roles in orchestrating organ/tissue development and regulating homeostasis, and are therefore of interest in connection with the fabrication of improved biomimetic materials for tissue engineering applications.
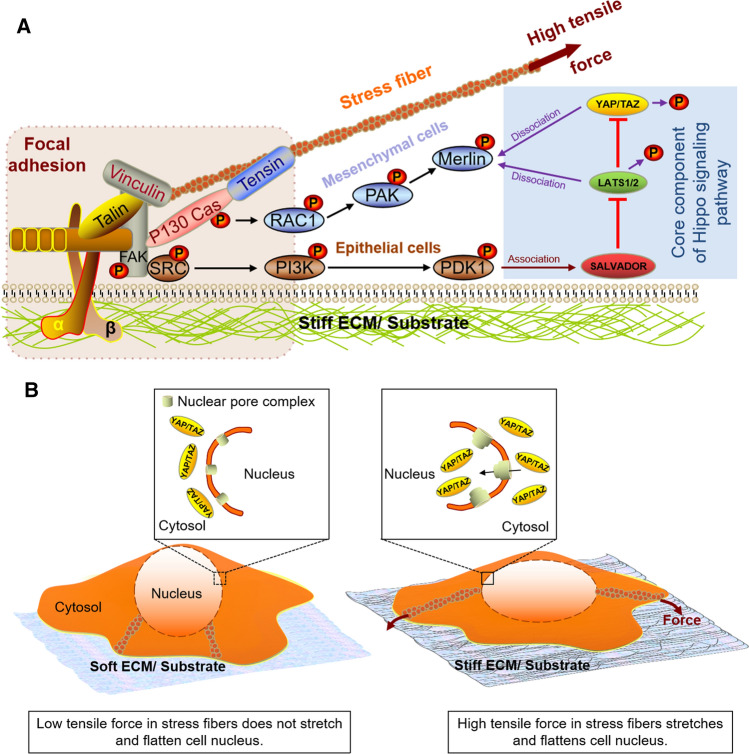
Mechanosensing of ECM/substrata stiffness is mediated primarily by focal adhesions (FAs), which influence cell adhesion, spreading, and remodeling of the actin cytoskeleton via RhoA activity [9–11]. The FAs in turn modulate Hippo-dependent and independent signaling pathways that control cell proliferation and differentiation through YAP/TAZ. Generally, with a stiffer substrate, there is increased cytosol to nuclear translocation of YAP/TAZ (Fig. 2), which can be attributed to increased number of FAs per cell, as well as increased tensile force on the stress fibers connecting the FAs that cause the cell to spread over a larger surface area [10, 11]. In the case of bone marrow-derived mesenchymal stem cells (BMSCs), increased YAP/TAZ activation on a stiff substrate leads to enhancement of osteogenic differentiation [11].
Fig. 2.
The underlying mechanisms by which ECM/substrata stiffness or cell density is transduced by FAs to effect YAP/TAZ activation or inactivation involve both the Hippo-signaling pathway (a), as well as a Hippo-independent mechanism (b). In the Hippo-dependent mechanism (a), high tensile force at the interface between FAs and stress fibers with stiff substrata is detected by β1-integrin, which leads to sequential phosphorylation of FAK (focal adhesion kinase), SRC (steroid receptor coactivator) and/or P130 Cas. In epithelial cells, phosphorylated SRC activates the PI3K–PDK1 signaling pathway. Upon activation, PDK1 associates with the core Hippo pathway-kinase complex through the scaffold protein Salvador, leading to inhibtion of LATS1/2 phosphorylation of YAP, thereby enhancing its nuclear translocation [38]. On the other hand, in mesenchymal cells, the phosphorylated P130 Cas activates the Rac1–PAK-Merlin pathway. In the unphosphorylated state, Merlin normally interacts with both YAP and LATS1/2 via its C-terminal moiety and FERM domain, respectively, facilitating phosphorylation of both proteins. PAK1-mediated Merlin phosphorylation on Ser-518 reduces Merlin’s interactions with both LATS1/2 and YAP1, resulting in YAP dephosphorylation and nuclear translocation [39]. In the Hippo-independent mechanism (b), increased contractile force generated by the actino-myosin stress fibers when cells encounter a stiff substrate, flattens the cell nuclei. This in turn increases the curvature of the lateral part of the nuclear membrane, which enlarges the diameter of the nuclear pore on the cytoplasmic side of the nuclear membrane, while at the same time reducing the pore diameter at the opposite side. Such asymmetric deformation of the nuclear pores in turn favors nuclear import, rather than export, of YAP/TAZ. Additionally, it is also thought that increased curvature of the lateral portion of the nuclear membrane that occurs through cell nuclei flattening via contractile force of the stress fibers, exposes the inner surface of the nuclear pore to the cytosol, which in turn favors import rather than export of YAP/TAZ. This is thought to be mediated by the disorganized meshwork of flexible FG-nups comprised phenylalanine–glycine (FG) repeats on the inner lumen of nuclear pores, which facilitates protein unfolding and subsequent passage through the nuclear pore via repulsive interaction between the FG repeats and proteins
Another biomechanical cue is cell density. With increasing cell density, there is increased cell-to-cell contact, and the cell shape changes from a more flattened to a more rounded geometry, with a consequent reduction in FAs, but an increase in the numbers of tight junctions (TJs) and adherens junctions (AJs) between adjacent cells [12, 13]. This inhibits cytosolic to nuclear translocation of YAP/TAZ through both the Hippo signaling pathway and Hippo-independent mechanisms. Generally, a decrease in YAP/TAZ nuclear-to-cytoplasmic ratio impedes cell proliferation, resulting in the commonly observed phenomenon of contact inhibition of cell proliferation [12, 13].
Cellular response to shear stress is also mediated by YAP/TAZ [14–17]. Studies with a microfluidic perfusion device demonstrated that exposure to shear force enhances YAP translocation to the cell nucleus, which in turn promotes osteogenesis and inhibits adipogenesis in mesenchymal stem cells (MSCs), but initiates dedifferentiation of chondrocytes [14]. In the case of endothelial cells, laminar (unidirectional) shear stress, as is normally encountered in healthy blood vessels, suppresses YAP/TAZ activation, whereas oscillatory (disturbed or turbulent) shear stress, which is associated with choked blood vessels, enhances YAP/TAZ activation [15–17]. These findings have major implications for the pathogenesis of arteriosclerosis [15–17].
The signaling pathways of some cell-surface receptors of extracellular ligands, such as lipids, hormones and growth factors, can also activate YAP/TAZ through interactions with the Hippo signaling pathway (Fig. 1). These ligands include lysophosphatidic acid (LPA), sphingosine 1-phosphate (S1P), glucagon and epinephrine, which act through G protein-coupled receptors (GPCRs) [18, 19], as well as the receptor tyrosine kinases (RTKs) for nerve growth factor (NGF) and epidermal growth factor (EGF) [20, 21].
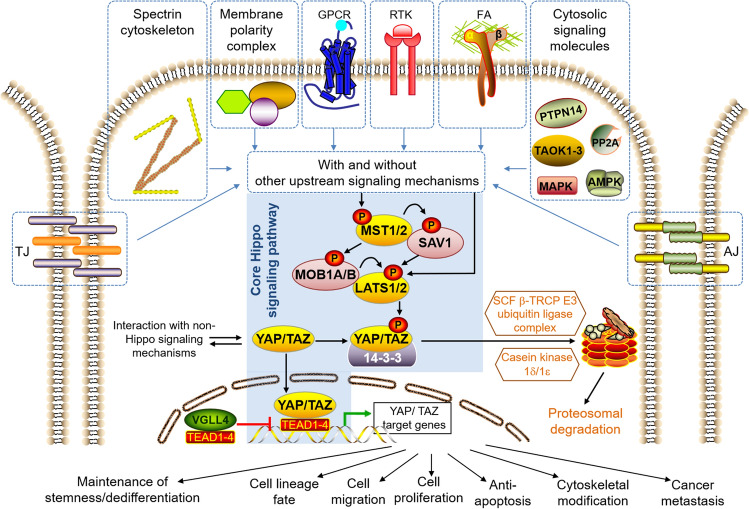
Fig. 1.
The core of the canonical Hippo signaling pathway is a kinase cascade that in mammals comprises the MST1/2 complex (MST1 & MST2, also known as STK4 & STK3, respectively), LATS1/2 complex (LATS1 & LATS2, large tumour supressor kinase 1 & 2, respectively), the adaptor proteins: SAV1 (Salvador 1), MOB1A and MOB1B, the transcriptional co-activators YAP/TAZ, the 14-3-3 protein that binds only to phosphorylated YAP/TAZ, and finally the TEAD transcription factors (TEAD1–TEAD4) that activate the transcription of specific genes upon binding to unphosphorylated YAP/TAZ. The Hippo signaling pathway can be initiated upon phosphorylation of MST1/2 or LATS1/2 by various upstream signaling mechanisms that may involve Focal adhesions (FAs), G protein-coupled receptors (GPCRs), Receptor tyrosine kinases (RTKs), Adherens junctions (AJs), Tight junctions (TJs), Spectrin cytoskeleton, Membrane polarity complexes (i.e. Crumbs, Scribble, aPKC-PAR) and various cytosolic signaling molecules (i.e. PP2A, TAOK1/2/3, MAPK, AMPK, and PTPN14). The activated MST1/2 complex phosphorylates the adaptor proteins SAV1, MOB1A and MOB1B, which in turn assist the MST1/2 complex in recruiting, phosphorylating and activating the LATS1/2 complex. Nevertheless, it must be noted that the LATS1/2 complex is not necessarily activated only by the MST1/2 complex, but can also be phosphorylated and activated by other upstream signaling mechanisms. Upon activation, the LATS1/2 complex phosphorylates YAP/TAZ, which then binds to the 14-3-3 protein. This in turn prevents the phosphorylated YAP/TAZ from being translocated into the cell nuclei, and it is then targeted for proteasomal degradation through further phosphorylation by casein kinase 1δ/1ε and ubiquitination by the SCF β-TRCP E3 ubiquitin ligase. By contrast, when MST1/2 and LATS1/2 are not activated, the unphosphorylated YAP/TAZ remains active and is translocated to the cell nuclei where it binds TEAD transcription factors (TEAD1 to TEAD4) to activate the transcription of specific output genes, which may be involved in cell lineage fate, cell proliferation, cell migration, anti-apoptosis, cancer metastasis, cytoskeletal modification, ‘stemness’ maintenance and dedifferentiation. VGLL4 acts as a competitive inhibitor of YAP/TAZ binding to TEAD, resulting in repression of target gene expression
Cellular responses to microenvironmental stress factors have also been linked to YAP/TAZ via interaction of various cytosolic factors with the Hippo signaling pathway (Fig. 1). Energy stress induced by inhibitors of glucose or ATP metabolism decreases YAP/TAZ activation through the Hippo signaling pathway via AMPK (AMP-activated protein kinase) [22, 23]. It was reported that osmotic stress promotes YAP translocation to cell nuclei via Nemo-like kinase (NLK)-mediated phosphorylation of the Ser128 residue [24], while hypoxic stress activates YAP through the action of SIAH2 ubiquitin E3 on the Hippo signaling pathway [25].
Pro-inflammatory cytokines, such as prostaglandin E2 and tumor necrosis factor alpha (TNF-α), also activate YAP/TAZ, but the underlying mechanisms remain unclear [26, 27]. YAP/TAZ are also activated in response to liver, skin, myocardial and intestinal epithelium injury [28–30], and facilitate healing by enhancing cell proliferation. However, the underlying mechanisms through which YAP/TAZ are activated upon tissue injury are still largely uncharacterized.
Regulatory mechanisms of YAP/TAZ activity
Regulation of YAP/TAZ activity through the canonical Hippo signaling pathway
Core components of the canonical Hippo signaling pathway
The canonical Hippo signaling pathway is highly conserved in different species ranging from fruitflies to mammals [31], and was so named because deletion mutants in Drosophila melanogaster led to loss of control of tissue/organ size, which in turn resulted in a superficial resemblance to the hippopotamus. The core of the canonical Hippo signaling pathway (Fig. 1) is a kinase cascade that in mammals consists of the MST1/2 complex (MST1 and MST2, also known as STK4 and STK3, respectively), LATS1/2 complex (LATS1 and LATS2, large tumor suppressor kinases 1 and 2, respectively), the adaptor proteins SAV1 (Salvador 1), MOB1A and MOB1B, the transcriptional co-activators YAP/TAZ, the 14-3-3 protein that binds only to phosphorylated YAP/TAZ, and finally the TEAD transcription factors (TEAD1–TEAD4) that activate the transcription of specific target genes (Table 1) upon binding to unphosphorylated YAP/TAZ.
Table 1.
Examples of YAP/TAZ effector genes that are activated via TEAD
| Function | Genes activated by YAP/TAZ via TEAD | Key references |
|---|---|---|
| Stemness/Dedifferentiation | C-Myc | Schutte et al. [114] |
| Sox2, Oct4 & Nanog | Lian et al. [115] | |
| Anti-apoptosis/cell survival | CTGF, ANKRD, BCL2 & CYR61 | Wang et al. [116] |
| MCL-1 | Tian et al. [117] | |
| Cell lineage fate decisions/differentiation | Cdx2 | Yagi et al. [118] |
| MSTN & MyoG | Lv et al. [119] | |
| GATA3 | Ralston et al. [120] | |
| Myf5 | Ribas et al. [121] | |
| Cell migration | CDH2, MACF1, ABL2 & TNS3 | Liu et al. [122] |
| Cytoskeleton/morphology | ARHGAP29 | Qiao et al. [123] |
| Cell proliferation/Cell cycle/Tumorigenesis | MCM3, MCM6 & Cdk1 | Ehmer et al. [124] |
| CDC6, CDT1, MCM4 & MCM10 | Shen and Stanger [125] | |
| MCM7 | Lo Sardo et al. [126] | |
| RHAMM | Wang et al. [127] | |
| CTGF | Zhao et al. [128] | |
| Cyr61 | Zhang et al. [129]. | |
| AXL | Xu et al. [130] | |
| EDN1 | Zhang et al. [131] |
The Hippo signaling pathway is initiated upon activation (phosphorylation) of MST1/2 by various upstream signaling factors (Fig. 1). The activated MST1/2 complex phosphorylates the adaptor proteins SAV1, MOB1A and MOB1B, which in turn assist the MST1/2 complex in recruiting, phosphorylating and activating the LATS1/LATS2 complex. It should be noted that the LATS1/LATS2 complex is not necessarily activated only by the MST1/2 complex, but can also be phosphorylated and activated by a whole multitude of cytosolic factors that are implicated in other signaling pathways. Hence, there is a rather high degree of overlap with other signaling pathways.
Upon activation, the LATS1/LATS2 complex phosphorylates YAP/TAZ, which then bind to the 14-3-3 protein. This in turn prevents the phosphorylated YAP/TAZ from being translocated to the cell nucleus, and they are then targeted for proteasomal degradation through further phosphorylation by casein kinase 1δ/1ε and ubiquitination by the SCF β-TRCP E3 ubiquitin ligase complex [32]. In contrast, when MST1/2 and LATS1/2 are not activated, the unphosphorylated YAP/TAZ are translocated to the cell nucleus, where they bind to TEAD transcription factors to activate the transcription of specific target genes (Table 1).
Hence, when the core Hippo signaling pathway is switched ‘ON’, YAP/TAZ are inactivated through phosphorylation, and their translocation to the cell nucleus is blocked. On the other hand, when Hippo signaling is switched ‘OFF’, the non-phosphorylated YAP/TAZ can be translocated to the cell nucleus, where they bind to TEAD transcription factors and activate the transcription of specific target genes (Table 1). Nevertheless, it is important to note that in reality, the Hippo signaling pathway does not behave digitally only in the ‘ON’ or ‘OFF’ mode. Depending on the relative activities of MST1/2, LATS1/2, and other cytosolic factors involved in the activation/deactivation of these two complexes (Fig. 1), YAP/TAZ localization may be partially cytoplasmic or partially nuclear. The situation is further complicated by the existence of competitive inhibitors of YAP/TAZ binding to TEAD, such as VGLL4 [33], which represses target gene expression. Hence, depending on the cell type and biological context, variations in the relative activity of the core Hippo signaling cascade and YAP/TAZ may arise from differing balance in the activity/or quantity of the various regulatory kinases, which can function in a complementary manner and which may substitute each other. This in turn would have critical implications for the practical implementation and data interpretation of YAP/TAZ assays.
YAP/TAZ regulation by focal adhesions through the Hippo signaling pathway
Focal adhesions (FAs) are integrin-containing macromolecular assemblies that form a mechanical linkage between the extracellular matrix and cytoskeleton via intracellular actin bundles (stress fibers) [7, 9]. As a consequence of their structural role, FAs are the primary conduits for cellular sensing of ECM/substrata stiffness [7, 9], in addition to being sensors of cell density [34]. At low cell density, cells tend to be more spread out on the substrata surface and have higher numbers of FAs and associated stress fibers; whereas at high density, cells are more rounded and compact, with fewer FAs and associated stress fibers [34].
Different cell types, such as epithelial and mesenchymal cells, will exhibit different responses to stiff and soft substrates, as well as to high and low cell densities which can be attributed to varying combinations and quantities of TEAD transcription factors present in different cell types that can potentially be activated by YAP/TAZ. Additionally, complex interaction and cross-talk between the core Hippo signaling cascade (Fig. 1) with other signaling pathways in different cell types, will undoubtedly lead to variations in the signaling mechanisms by which ECM/substrata stiffness or cell density is transduced by FAs to modulate YAP/TAZ activity in different cell lineages.
Under conditions of stiff/fibronectin-rich ECM or low cell density, the high tensile force at the interface between FAs and stress fiber is detected by β1-integrin, leading to sequential phosphorylation of FAK (focal adhesion kinase), SRC (steroid receptor co-activator) and/or P130 Cas via integrin-linked kinase (ILK) [35] (Fig. 2a). These subsequently trigger two distinct parallel signaling pathways that regulate YAP activation: (i) the β1-integrin–FAK–SRC–PI3K–PDK1 signaling pathway that has been reported for epithelial cell types [36–38], and (ii) the β1-integrin-FAK-P130–Cas–Rac1–PAK-Merlin pathway that has been reported for mesenchymal cells [39] (Fig. 2a). Activation of either signaling pathway inhibits LATS1/2 activity [36–39], thereby enhancing the nuclear translocation of YAP/TAZ and subsequent transcriptional activation of specific target genes through TEAD transcription factors (Table 1).
On the other hand, with high cell density or soft ECM/synthetic substrata, cells do not spread much, and have fewer FAs per cell with reduced tensile force within the stress fibers connected to FAs. As a result, the two aforementioned signaling pathways are not activated and there is no inhibition of LATS1/2, leading to increased phosphorylation of YAP/TAZ and sequestration within the cytosol. Currently, it is unclear whether in other cell types, these two aforementioned signaling pathways emanating from FAs can act simultaneously in parallel to modulate YAP/TAZ activity.
YAP/TAZ regulation by adherens junctions (AJs) through the Hippo signaling pathway
AJs are macromolecular assemblies that mediate adhesion between cells, and mechanically link the actin cytoskeletons of adjacent cells [40]. They are primarily composed of proteins of the cadherin and catenin families, including α-catenin and β-catenin, which form mechanical linkages with bundles of actin fibers that connect directly to the cytoskeleton [40]. In addition, proteins that play a role in Hippo signaling bind with AJs. These include Merlin (also known as neurofibromin 2 or NF2), which binds directly to α-catenin [41], and KIBRA (kidney and brain protein) that binds to Merlin [42]. As a result of their structural function in cell-to-cell contact and mechanical linkage of the cytoskeleton, AJs can serve as a means by which cells sense their density and initiate contact inhibition of proliferation [43].
Generally, the higher the cell density, the higher will be the number of AJs between adjacent cells. It is thought that Merlin (NF2) associated with AJs can bind and sequester LATS1/2 at the plasma membrane, which in turn facilitates LATS1/2 phosphorylation and activation by MST1/2 and MAP4Ks [44, 45]. Subsequent phosphorylation of YAP/TAZ by the activated LATS1/2 prevents their translocation to the cell nucleus, thereby leading to inactivation of target genes, some of which are involved in cell proliferation. This accounts for contact inhibition of cell proliferation at high cell densities. It has also been reported that homophilic dimerization of E-cadherin, which takes place during formation of AJs between adjacent epithelial cells, leads to phosphorylation of LATS1/2 and subsequent inhibition of YAP/TAZ translocation into the cell nucleus, thereby resulting in contact inhibition of proliferation [12]. The underlying mechanisms are unclear, but appear to involve NHERF (Na+/H+ exchange regulatory factor), Merlin, Kibra and LATS1/2 [12].
Dutta et al. [46] reported yet another signaling mechanism for contact inhibition of cell proliferation mediated by AJs. At high cell densities, increasing mechanical tension of actin stress fibers at AJs facilitates the recruitment of TRIP6 to AJs by vinculin, which subsequently binds to and phosphorylate LATS1/2, thereby inhibiting YAP/TAZ translocation into the cell nucleus [46].
Hence, increasing numbers of AJs and heightened mechanical tension within their associated actin stress fibers exert an opposite effect in downregulating YAP/TAZ activity, as compared to signaling mechanisms associated with FAs that promote YAP/TAZ activation. This in turn can be attributed to the opposite but complementary roles of AJs and FAs in sensing cell density and effecting contact inhibition of cell proliferation. AJs are localized at the interface between adjacent cells, whereas FAs by contrast, are localized at the interface between cells and ECM/substrata. Consequently, with higher cell density, there is an increasing number of AJs, but decreasing number of FAs per cell, which therefore explains their antagonistic effects on regulating YAP/TAZ activity that effects contact inhibition of proliferation at high cell densities.
YAP/TAZ regulation by tight junctions (TJs) through the Hippo signaling pathway
Tight junctions (TJs), also known as occluding junctions, are the closely associated areas of two adjacent cells where the membranes are joined together via strands of transmembrane proteins, such as claudins and occludins, to form an impermeable barrier. TJs are expressed exclusively by epithelial cells and function as a selective barrier to regulate the permeability of epithelial cell sheets to diffusing molecules. During differentiation and maturation of the epithelial cell, increasing numbers of TJs are formed between adjacent cells, in tandem with increasing cell density within the epithelial sheet [47]. It has been shown that increasing formation of TJs during maturation of the epithelial cell reduces cell proliferation via downregulation of YAP/TAZ activity [13]. Paramasivam et al. [13] identified a tight-junction protein of the angiomotin family, AMOTL2, which serves as an activator of LATS2 in the Hippo signaling cascade, leading to phosphorylation and sequestration of YAP within the cytosol. Subsequently, it was found that AMOTL2 can also bind to MST2, LATS2, and YAP, facilitating the sequestration and activation of Hippo pathway components at TJs. This in turn might lead to inhibition of YAP translocation into the cell nucleus, thereby leading to inhibition of cell proliferation in response to increasing numbers of TJs within the maturing epithelial cell sheet.
YAP/TAZ regulation by the spectrin cytoskeleton through the Hippo signaling pathway
Spectrins are filamentous proteins that are organized into a polygonal meshwork underneath mammalian cell membranes [48]. They play key roles in the formation of cell membrane microdomains, axonal growth, and synapse development [48]. Knockdown of spectrin in mammalian cells abrogates contact inhibition of proliferation through maintenance of YAP translocation into the cell nucleus [49, 50], via deactivation of LATS1/2 [50]. Currently, the underlying mechanisms are still unclear, but is thought to involve the role of spectrin as an actin cross-linking protein, which enables it to modulate YAP/TAZ activity by effecting tension within actin stress fibers [49, 50].
YAP/TAZ regulation by surface receptors of soluble ligands (GPCRs and RTKs) through the Hippo signaling pathway
Yu et al. [19] reported that YAP/TAZ are regulated by soluble ligands of G protein-coupled receptors (GPCRs), which are the targets of about 40% of pharmaceutical drugs on the market [51]. GPCRs are a family of cell-surface receptors characterized by seven transmembrane helical domains. Upon binding to their cognate ligands, intracellular signals are transduced through their heterotrimeric G-proteins (Gα, Gβ & Gγ subunits) [51]. GPCRs can be classified into 4 groups according to their heterotrimeric G-proteins: G12/13, Gq/11, Gi/o and Gs. These groups are associated with different intracellular signaling pathways [51]. GPCRs that activate G12/13, Gq/11, and Gi/o inhibit LATS1/2 phosphorylation, thus leading to increased nuclear translocation of YAP/TAZ [19]. In contrast, GPCRs that activate Gs signaling enhance the phosphorylation of LATS1/2, leading to increased YAP/TAZ sequestration within the cytosol. The underlying mechanisms involved remain unclear, but Yu et al. [19] provided some evidence for the involvement of Rho GTPases and actin cytoskeleton organization in mediating LATS1/2 phosphorylation by GPCRs.
Besides GPCRs, the receptor tyrosine kinase (RTK) family has also been implicated in YAP/TAZ regulation through the Hippo signaling pathway. Yang et al. [20] demonstrated that inhibition of the nerve growth factor (NGF) receptor tyrosine kinase (NTRK1) decreases YAP-driven transcription, proliferation and migration of cancer cells, through increased phosphorylation of LATS1/2. In contrast, Fan et al. [38] showed that activation of the epidermal growth factor receptor (EGFR), which also belongs to the RTK family, instead leads to increased translocation of YAP to the cell nucleus. This is achieved by activation of the PI3K-PDK1 (phosphoinositide 3-kinase-phosphoinositide-dependent kinase-1) signaling pathway by EGFR upon ligand binding, and this in turn inhibits MST1/2 phosphorylation of LATS1/2 [38, 52].
YAP/TAZ regulation by the aPKC-PAR, Crumbs and Scribble polarity complexes through the Hippo signaling pathway
Cell polarity is a distinctive feature of various cell lineages and is crucial for various developmental and physiological functions [53–58]. Membrane protein complexes that play key roles in cell polarity include aPKC-PAR [53–55], Crumbs [56, 57] and Scribble [58].
A number of studies have established that the aPKC-PAR (atypical protein kinase C-partitioning defective) polarity complex regulates YAP/TAZ activity via the Hippo signaling pathway. Archibald et al. [59] studied the mechanism by which elevated aPKC expression leads to malignant transformation in epithelial cells, and proposed a model in which aPKC binding to MST1/2 blocks its ability to phosphorylate LATS1/2. This in turn increases nuclear translocation of YAP, which leads to activation of proliferation-related genes. Zhou et al. [60] found that elevated expression of Par3, a key component of the PAR complex, is involved in prostate cancer metastasis. They proposed that Par3 inhibits phosphorylation of LATS1/2 by sequestrating KIBRA, leading to enhanced nuclear translocation of YAP [60].
The Crumbs polarity complex also regulates YAP/TAZ activity via the Hippo signaling pathway. Szymaniak et al. [57] showed that Crb3, a Crumbs isoform, promotes the interaction between LATS1/2 and YAP at apical cell junctions, which in turn promotes YAP phosphorylation and retention within the cytoplasm. Narimatsu et al. [61] also showed that the Crumbs complex promotes phosphorylation and inactivation of YAP/TAZ, which in turn inhibits TGFβ-induced Smad nuclear accumulation and activity.
Liu et al. [62] reported that the disks large homolog 5 (DLG5) protein facilitates the interaction of Scribble with MST1/2 and LATS1/2 in the core Hippo signaling cascade, which in turn promotes phosphorylation and inactivation of YAP, resulting in inhibition of breast cancer cell proliferation.
YAP/TAZ regulation by other cytosolic signaling molecules (PP2A, TAOK1/2/3, MAPK, AMPK, PTPN14) through the Hippo signaling pathway
YAP/TAZ are also regulated by other cytosolic signaling molecules that enable overlap and cross-talk between the Hippo signaling pathway and other signaling pathways. Protein phosphatase 2A (PP2A) is a ubiquitously expressed serine threonine phosphatase that has diverse roles in tumor suppression, apoptosis, cell proliferation and signal transduction. It acts by dephosphorylating various cytosolic signaling molecules, such as Akt, p53, c-Myc and β-catenin. Bae et al. [63] showed that PP2A regulates YAP/TAZ through dephosphorylation of MST1/2, and its activity may be inhibited by SAV1, which is a part of the core Hippo signaling cascade. Thousand and one kinases 1/2/3 (TAOK1/2/3), which have diverse functions in various signaling pathways relating to inflammation, microtubule dynamics, and stress response, also have a role in modulating YAP/TAZ activity. Boggiano et al. [64] showed that TAOK1/2/3 regulate YAP/TAZ activity by facilitating phosphorylation of MST1/2 within the Hippo signaling cascade. MAP kinase kinase kinase kinases (MAP4Ks) are a family of serine threonine kinases that regulate a diverse array of biological processes including cell survival, proliferation, motility and differentiation, and also regulate YAP through the Hippo signaling pathway [65]. Meng et al. [65] showed that several members of the MAP4K family act in parallel to MST1/2 to activate LATS1/2 within the Hippo pathway, leading to increased YAP phosphorylation and sequestration in the cytosol. Hence in this respect, MAP4Ks functionally overlap in part with MST1/2. Adenosine monophosphate kinase (AMPK) is a key regulator of cellular metabolism and a sensor of energy/nutrient stress. Mo et al. [23] showed that in the presence of nutrient starvation or energy stress, AMPK activates LATS1/2, leading to YAP phosphorylation and inhibition of nuclear translocation. They further showed that AMPK can also phosphorylate YAP directly at the Ser 94 residue, which is essential for its binding to TEAD, thus inhibiting target gene activation by blocking YAP-TEAD interaction. A notable example is the inhibition of gene expression of glucose-transporter 3 (GLUT3), which is involved in glucose metabolism [66]. In addition to AJs and TJs, non-receptor tyrosine phosphatase 14 (PTPN14) also mediates contact inhibition of cell proliferation by regulating YAP/TAZ activity through the core Hippo signaling pathway [67, 68]. At high cell densities, PTPN14 is abundantly expressed and forms a complex with KIBRA, which activates the core Hippo signaling cascade to phosphorylate YAP/TAZ via MST1/2 and LATS1/2, and this in turn represses TEAD-specific genes involved in cell proliferation [67, 68].
Regulation of YAP/TAZ activity through Hippo-independent mechanisms
Hippo-independent regulation of YAP/TAZ through stress fibers connected to focal adhesions (FAs)
Besides FA-mediated mechanotransduction through the canonical Hippo signaling pathway (Fig. 1), there is also a Hippo-independent pathway mediated through contractile force generated by actinomyosin stress fibers, which directly connect FAs to the apical surface of the cell nuclei via the LINC (linker of the nucleoskeleton and cytoskeleton) complex of the nuclear envelope [69]. Elosegui-Artola et al. [69] proposed that increased contractile force generated by the actinomyosin stress fibers when cells encounter a stiff substrate flattens the cell nuclei (Fig. 2b). This in turn increases the curvature of the lateral part of the nuclear membrane, which induces asymmetric morphological changes to the openings of nuclear pores on both the cytoplasmic and nuclear side of the membrane. This in turn favors nuclear import, rather than export, of YAP/TAZ (Fig. 2b). It is hypothesized that morphological changes to the nuclear pore exposes the disorganized meshwork of flexible FG-nups comprising phenylalanine–glycine (FG) repeats [70] on the inner lumen of the nuclear pores to the cytosol, facilitating protein unfolding and subsequent passage of YAP/TAZ through the nuclear pore via repulsive interaction between the FG repeats and the protein.
Hippo-independent regulation of YAP/TAZ through adherens junctions (AJs) and tight junctions (TJs)
As previously discussed, AJs mechanically link the cytoskeleton of adjacent cells, and serve as a means by which cells sense and react to their density via the Hippo-signaling pathway through the AJ-associated protein Merlin (also known as neurofibromin 2 or NF2). Although Merlin is closely associated with the Hippo signaling pathway, it is not considered to be a central component of the core signaling Hippo signaling cascade (Fig. 1). Besides the mechanism described in 2.1.3, it was also reported that Merlin can act independently of the core Hippo signaling cascade to repress YAP/TAZ activation [71]. At high cell densities, increased tensile force exerted on the actomyosin-based stress fibers tethered at AJs induces dissociation of Merlin from AJs. Subsequently, the dissociated Merlin is translocated to the cell nucleus, where it directly binds and forms a complex with YAP. This Merlin-YAP complex is translocated from the cell nuclei to the cytosol via the nuclear export signals of Merlin, leading to repression of TEAD-specific genes involved in cell proliferation and thus, contact inhibition of proliferation.
As previously discussed, TJs regulate YAP activity through the Hippo signaling pathway via AMOTL2. On the other hand, Domínguez-Calderó [72] found that TJs can also regulate YAP activity through a Hippo-independent mechanism via the tight-junction protein zona occludens 2 (ZO-2), which binds to and sequesters YAP within the cytosol. Upon ZO-2 gene silencing, hypertropy of renal cells was observed concurrently with increased YAP accumulation within the cell nuclei [72].
Hippo-independent regulation of YAP/TAZ through the Wnt-β-catenin pathway
The regulation of β-catenin through a cytoplasmic destruction complex forms the crux of the Wnt signaling cascade [73]. This β-catenin destruction complex is a multi-protein complex that includes Axin, adenomatous polyposis coli (APC), glycogen synthase kinase-3 (GSK3), and casein kinase 1 (CK1) [73]. When Wnt signaling is inactive, this destruction complex captures not only cytosolic β-catenin [73], but also cytosolic YAP [74], leading ultimately to degradation by the β-TrCP ubiquitin ligase. The activation of Wnt signaling inactivates the destruction complex and allows escape of both β-catenin [73] and YAP [74] from degradation, which in turn enables their translocation and accumulation in the cell nucleus.
Hippo-independent regulation of YAP/TAZ through methylation and phosphorylation
SET7 (also known as SETD7) is a SET-domain-containing lysine methyltransferase that methylates and alters the function of a variety of proteins. Oudhoff et al. [75] demonstrated that SET7 methylates the lysine 494 residue of YAP, which inhibits its translocation to the cell nucleus and results in its sequestration within the cytosol. Knockout of SET7 in mice resulted in a larger progenitor compartment in the intestine, concomitantly with increased expression of YAP target genes.
As previously discussed, PTPN14 forms a complex with KIBRA protein to regulate YAP/TAZ activity via the core Hippo signaling pathway. Liu et al. [76] showed that PTPN14 can also directly phosphorylate and inactivate YAP, through interaction between the WW domain of YAP and the PPxY domain of PTPN14. The presence of a YAP-PTPN14 complex was validated by co-immunoprecipitation [76].
Hippo-independent regulation of YAP/TAZ through the Notch signaling pathway
The Notch signaling pathway is a major signaling pathway with diverse biological functions, which regulates cell-to-cell communications in a juxtacrine manner, via interaction between Notch receptors (Notch 1 to 4 isoforms) and their corresponding ligands (JAG1, JAG2, DLL1, DLL3, and DLL4) expressed on adjacent cells [77]. Notch receptor-ligand binding triggers proteolytic cleavage and subsequent release of the Notch intracellular domain (NICD), which translocates into the cell nucleus, where it binds and activates the transcription factor RBPJ (recombining binding protein suppressor of hairless), as well as the nuclear effector Mastermind-like (MAML), thereby initiating transcription of Notch target genes [77].
Although, it is well-known that there is much cross-talk between YAP/TAZ and the Notch signaling pathway [78], and numerous examples whereby these can work synergistically together to regulate the expression of various genes and hence biological processes [78], there have only been a few studies to date, which have reported upstream regulation of YAP/TAZ activity via the Notch signaling pathway [79–81]. In the study of Li et al. [79] on murine neural stem cells, it was shown by gain- and loss-of-function experiments that the Notch signaling pathway exert positive upstream control of YAP activity, which in turn regulated the proliferation of these cells. Further investigations revealed that the RBPJ transcription factor of the canonical Notch signaling pathway directly control transcription of the YAP1 protein by binding to its promoter sequence [79]. Although, Li et al. [79] observed that RBPJ could also bind to the promoter sequence of TEAD2, this by itself was insufficient to initiate transcription of TEAD2. Very similar results were reported by the study of Slemmons et al. [80] on human rhabdomyosarcoma cells, which also utilized gain- and loss-of-function experiments to demonstrate positive regulation of YAP activity by the Notch signaling pathway. Again, it was demonstrated that RBPJ can bind directly to the YAP promoter and activate its transcription. Additionally, Slemmons et al. [80] showed that the Notch signaling pathway can also facilitate YAP nuclear translocation, but the underlying mechanism of how this occurs is currently unknown, and it is unclear whether this involves the core Hippo signaling cascade. Contradictory results were, however, reported by the study of Lu et al. [81] on murine hepatic stem cells. Although, RBPJ was again shown to bind directly to the YAP promoter, it repressed rather than activated transcription of the YAP protein [81]. This in turn led to Notch signaling promoting the differentiation of hepatic stem cells, while at the same time inhibiting their proliferation [81] by reducing YAP activity. Hence, it is likely that specific upstream regulation of YAP/TAZ activity by Notch signaling may vary according to the particular cell type, being dependent on complex interactions with other signaling processes present in different cell lineages.
Hippo-independent regulation of YAP/TAZ through the TGF-β signaling pathway
Transforming growth factor beta (TGF-β) with five know isoforms (TGF-β1 to 5), together with other members of the TGF-β superfamily, i.e. bone morphogenetic proteins (BMPs), growth differentiation factors (GDFs), activin and inhibin, play key roles in numerous diverse biological processes, such as embryonic development, cell proliferation, differentiation and migration [82]. Nevertheless, the focus here will be solely on the TGF-β signaling pathway [83].
As in the case of the Notch signaling pathway, the TGF-ß signaling pathway is also known to have extensive cross-talk and interactions with YAP/TAZ, in the regulation of numerous genes and biological processes [84, 85]; but again, there are few studies that have reported upstream modulation of YAP/TAZ activity by the TGF-ß signaling pathway. It must be noted that to date, studies have reported that TGF-ß signaling can only exert upstream regulation of TAZ, but not YAP [86, 87], even though it is known that YAP can promote TGF-ß signaling by binding to and facilitating the nuclear translocation of SMAD2/3 [88]. For example, in the study of Miranda et al. [86] on rodent mesenchymal and epithelial cell lines, it was shown that TGF-ß signaling can induce only robust expression of TAZ, but not YAP. Further investigations revealed that the underlying mechanism was independent of SMAD2/3, and involved sequential activation of p38 MAPK and its major downstream target MK2 by TGF-ß signaling, which in turn activates myocardin-related transcription factor (MTRF) that drives the TAZ promoter in a CC(A/T-rich)6GG (CArG) box-dependent manner and induced TAZ protein expression [86]. Additionally, Miranda et al. [86] also identified a positive feedback loop mechanism that amplified TGF-ß-induced TAZ expression. This involved potentiation of Nox4 expression by MTRF, with NOX4 in turn enhancing MTRF activation via phosphorylation, thereby amplifying TGF-ß-induced TAZ expression [86]. The study of Wang et al. [87] on murine hepatic stellae cells uncovered another mechanism of upstream regulation of TAZ activity by the TGF-ß signaling pathway, at the post-translational level. Utilizing co-immunoprecipitation, immunofluorescence, and nuclear fractionation assays, Wang et al. [87] showed that TGF-β1 signaling promoted binding of SMAD2/3 and TAZ to the co-activator p300, leading to formation of a p300/SMAD2/3/TAZ heterocomplex that more readily translocate into the cell nucleus. Hence in this case, the p300 co-activator induced by TGF-β1 signaling, acted as a shuttle for the nuclear transport SMAD2/3 and TAZ, as both proteins lacked nuclear localization signals (NLS). Further investigations by Wang et al. [87] revealed that both the protein scaffolding function and acetyltransferase activity of p300 were essential for its nuclear transport function, and that p300 played no role in YAP nuclear translocation.
Interaction of YAP/TAZ with TEAD transcription factors
As already noted, YAP/TAZ controls the expression of target genes primarily by acting as a co-activator of TEAD transcription factors. There are four isoforms of TEAD in mammals (TEAD1–4), all of which contain a highly conserved TEA DNA-binding domain (DBD) and a YAP-binding domain (YBD), separated by a proline-rich region (PRR) [89]. There is evidence that early mammalian development may be associated with distinct spatio-temporal expression patterns of TEAD1-4 [90]. The various target genes regulated by YAP/TAZ through TEAD1-4 have been linked to a diverse array of biological processes. These can be broadly classified into the following six categories [91]: (i) cell proliferation, cell cycle and tumorigenesis, (ii) cell migration, (iii) stemness/dedifferentiation, (iv) cell lineage fate determination and differentiation, (iv) cytoskeleton and cell morphology, and (vi) anti-apoptosis and cell survival (Table 1). The YAP/TAZ regulation of TEAD1-4-activated target genes appears to be precisely controlled in a tissue- and cell type-specific manner, possibly through expression of specific TEAD isoforms in different tissue/cell types [90]. Epigenetic mechanisms may also be involved, since there is evidence that YAP/TAZ activity can be mediated by various chromatin complexes, including the NCOA6 histone methyltransferase complex and SWI/SNF chromatin remodeling complex [92, 93]. Currently, the precise mechanisms by which YAP/TAZ mediates tissue/cell-type specific activation of target genes via TEAD1-4 remain unclear.
Some nuclear proteins can regulate YAP/TAZ activity by modulating their binding interaction with TEAD transcription factors within the cell nucleus. Two prominent examples are p38 MAPK (mitogen-activated protein kinase) and VGLL4. In the presence of cellular stresses, such as hyperosmolarity, high cell density, and cell detachment, increased shuttling of TEAD from the cell nucleus to the cytosol takes place [94]. Subsequently, it was found that these cellular stresses activate p38 MAPK, which in turn binds to TEAD and drives the translocation of TEAD from the nucleus to the cytosol, thus repressing the expression of YAP/TAZ target genes [94]. This translocation of TEAD does appear to involve p38 MAPK-mediated phosphorylation of TEAD. However, p38 MAPK has no binding interaction with YAP/TAZ [94]. VGLL4 blocks the YAP-TEAD interaction by competing directly with YAP for binding to TEAD [33, 95]. In addition, Lin et al. [96] found that p300-mediated acetylation of VGLL4 disrupts its interaction with TEAD1 in the neonatal heart. This results in increased expression of target genes activated by YAP–TEAD1 interaction, which promotes the growth of heart tissue. On the other hand, disruption of VGLL4 acetylation led to suppression of YAP–TEAD binding interaction by VGLL4, slowing the growth of heart tissue [96].
Interaction of YAP/TAZ with non-TEAD transcription factors
Although TEAD1–4 are the major transcription factors that interact with YAP/TAZ to regulate the expression of specific target genes in the Hippo signaling pathway, it is also known that YAP/TAZ can also bind to and modulate the activity of various other non-TEAD transcription factors. These regulate a diverse array of biological processes, with YAP/TAZ demonstrating different effects on different cell types. Nevertheless, there are some commonalities among different cell types, particularly in the case of major key signaling pathways. For example, it is well-known that YAP/TAZ binding to the transcription factor Smad2/3 mediate cross-talk between the Hippo and TGF-β signaling pathways in diverse cell types ranging from epithelial, mesenchymal, keratinocyte and colon cancer cell lines [88, 97].
Very often, YAP and TAZ interaction with the same transcription factor, modulate different signaling pathways in different cell types, thus eliciting different biological effects, for example, in the case of RUNX2. Brusgard et al. [98] demonstrated that the binding interaction of RUNX2 with TAZ initiated ectodomain shedding of an oncogenic soluble E-cadherin fragment (sE-Cad), which worked synergistically with human epidermal growth factor receptor-2 (HER2/ErbB2) to stimulate the growth of breast cancer cells; whereas, Lin et al. [99] reported that RUNX2 binding interaction with YAP can inhibit osteogenic differentiation of mesenchymal stem cells, and that this inhibition can be overcome through competitive binding of YAP with AP2a.
Many of the non-TEAD transcription factors that have binding interactions with YAP/TAZ are implicated in oncogenesis and cancer metastasis. For example, Tomlinson et al. [100] and Roperch et al. [101] established that apoptosis can be regulated by the binding of YAP/TAZ with the p53-related proteins p63 and p73. Bora-Singhal et al. [102] showed that YAP can bind to and enhance OCT4 activity, which in turn upregulated SOX2 expression to promote self-renewal and vascular mimicry of stem-like cells from non-small cell lung cancer. Kuser-Abali et al. [103] identified YAP as a physiological binding partner and positive regulator of androgen receptors in prostate cancer. Liu et al. [104] showed that the binding of YAP with PRDM4 induced leukocyte-specific integrin β2 (ITGB2) expression in cancer cells, which in turn promoted cell invasion through the endothelium in a similar manner to leukocytes.
Conclusion
Recent years have seen an exponential growth of scientific knowledge on the intricate molecular mechanisms regulating YAP/TAZ and their associated signaling pathways. Despite commonalities in the various upstream and downstream signaling pathways related to YAP/TAZ, it should be noted that the specific biological effects elicited by YAP/TAZ ultimately depend on the specific cell type and lineage in question, simply because different cell types and lineages have variable amounts and proportions of diverse signaling and effector molecules that interact with YAP/TAZ. For example, variable proportions of the TEAD 1 to 4 isoforms in different cell types lead to activation of different subsets of effector genes by YAP/TAZ. Moreover, different cell types and lineages may have differing sensitivity to YAP/TAZ, so that what may be considered ‘low’ YAP/TAZ activity in one particular cell type or lineage, may in fact work as ‘high’ or ‘moderate’ activity in another cell type or lineage.
New insights into the regulatory mechanisms of YAP/TAZ can potentially lead to novel therapeutic applications. First, they may offer clues to solving one of the most difficult and intractable challenges in tissue engineering and regenerative medicine, i.e., to quickly obtain sufficient numbers of tissue-specific adult stem cells or progenitors for transplantation therapy. Due to the key roles of YAP/TAZ in activating the proliferation and self-renewal of various adult stem cell lineages during the regeneration process, and in maintaining the ‘stemness’ of these cells [105, 106], it may be useful to employ high-throughput drug screening technology to screen various natural-product-derived or artificially synthesized small molecules for the capacity to activate proliferation or enhance ‘stemness’ of specific adult stem cell types via upregulation of YAP/TAZ activity (e.g., by utilizing fluorescent reporter genes). Such newly identified small molecule drugs might not only find application to facilitate the expansion of transplantable adult stem/progenitor cells in vitro, but also might be delivered into the human body directly or via controlled release from biomedical implants to aid tissue regeneration via activation and mobilization of the body’s endogenous pool of adult stem cells.
Second, new knowledge on YAP/TAZ should help to improve the biomimetic properties and therapeutic efficacy of implant materials. There are already much data on how the biomechanical properties of substrata, such as stiffness [9–11] and topography (i.e. micro- and nanopatterning) [107–111], can modulate YAP/TAZ activity. Much research has been focused on the role of YAP/TAZ in the mechanosensing of substrata biomechanical properties via both Hippo-dependent and Hippo-independent mechanisms. Increasing knowledge of how YAP/TAZ activity correlates with the healing and regeneration processes at specific tissue/organ sites, may enable us to fine tune the biomechanical properties of implant materials. For example by screening the YAP/TAZ activity of relevant cell lineages cultured on newly-developed biomaterials in vitro.
Third, the rapidly progressing field of synthetic biology may open up intriguing new possibilities for applying our increasing knowledge of YAP/TAZ in hitherto unimagined ways. Synthetic biology is the science of creating and engineering artificial biological systems (often for therapeutic applications) through reassembling biological items with known functions (i.e. genes, proteins) in a systematic and rational manner [112, 113]. Because YAP/TAZ are key mediators of cellular interaction with the microenvironment, including (i) biomechanical cues [9–17], (ii) extracellular ligands, such as growth factors and lipids [18–21], (iii) energy, osmotic and hypoxic stress [22–25], and (iv) inflammation and tissue injury [26–30], it is possible that YAP/TAZ and related effector and signaling molecules could be integrated into complex synthetic gene networks that would activate the transcription of specific therapeutic transgenes in response to changes in YAP/TAZ activity [112, 113]. Alternatively, synthetic gene circuits might be designed to activate YAP/TAZ in response to specific stimuli, such as molecular markers of tissue injury or metabolic stress. We hope that the present review will inspire new advances in these and other areas.
Acknowledgments
This work was supported by the National Key R&D Program of China (Grant No. 2018YFC1105303/04), National Natural Science Foundation of China (Grant Nos. 51772006, 31670993, 51973004, 81991505), Beijing Municipal Science & Technology Commission Projects (Grant No. Z181100002018001), and Peking University Medicine Fund (Grant Nos. PKU2020LCXQ009, BMU2020PYB029).
Footnotes
Boon Chin Heng and Xuehui Zhang contributed equally to this work.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Martin Fussenegger, Email: martin.fussenegger@bsse.ethz.ch.
Xuliang Deng, Email: kqdengxuliang@bjmu.edu.cn.
References
- 1.Webb C, Upadhyay A, Giuntini F, Eggleston I, Furutani-Seiki M, Ishima R, Bagby S. Structural features and ligand binding properties of tandem WW domains from YAP and TAZ, nuclear effectors of the Hippo pathway. Biochemistry. 2011;50(16):3300–3309. doi: 10.1021/bi2001888. [DOI] [PubMed] [Google Scholar]
- 2.Lin KC, Park HW, Guan KL. Regulation of the hippo pathway transcription factor TEAD. Trends Biochem Sci. 2017;42(11):862–872. doi: 10.1016/j.tibs.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanai F, Marignani PA, Sarbassova D, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19:6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 5.Piccolo S, Cordenonsi M, Dupont S. Molecular pathways: YAP and TAZ take center stage in organ growth and tumorigenesis. Clin Cancer Res. 2013;19(18):4925–4930. doi: 10.1158/1078-0432.CCR-12-3172. [DOI] [PubMed] [Google Scholar]
- 6.Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141(8):1614–1626. doi: 10.1242/dev.102376. [DOI] [PubMed] [Google Scholar]
- 7.Dupont S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp Cell Res. 2016;343(1):42–53. doi: 10.1016/j.yexcr.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163(4):811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nardone G, Oliver-De La Cruz J, Vrbsky J, Martini C, Pribyl J, Skládal P, Pešl M, Caluori G, Pagliari S, Martino F, Maceckova Z, Hajduch M, Sanz-Garcia A, Pugno NM, Stokin GB, Forte G. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat Commun. 2017;8:15321. doi: 10.1038/ncomms15321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardo-Pastor C, Rubio-Moscardo F, Vogel-González M, Serra SA, Afthinos A, Mrkonjic S, Destaing O, Abenza JF, Fernández-Fernández JM, Trepat X, Albiges-Rizo C, Konstantopoulos K, Valverde MA. Piezo2 channel regulates RhoA and actin cytoskeleton to promote cell mechanobiological responses. Proc Natl Acad Sci USA. 2018;115(8):1925–1930. doi: 10.1073/pnas.1718177115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 12.Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA. 2011;108(29):11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paramasivam M, Sarkeshik A, Yates JR, 3rd, Fernandes MJ, McCollum D. Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Mol Biol Cell. 2011;22(19):3725–3733. doi: 10.1091/mbc.E11-04-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong W, Tian K, Zheng X, Li L, Zhang W, Wang S, Qin J. Mesenchymal stem cell and chondrocyte fates in a multishear microdevice are regulated by Yes-associated protein. Stem Cells Dev. 2013;22(14):2083–2093. doi: 10.1089/scd.2012.0685. [DOI] [PubMed] [Google Scholar]
- 15.Wang KC, Yeh YT, Nguyen P, Limqueco E, Lopez J, Thorossian S, Guan KL, Li YJ, Chien S. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc Natl Acad Sci USA. 2016;113(41):11525–11530. doi: 10.1073/pnas.1613121113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Luo JY, Li B, Tian XY, Chen LJ, Huang Y, Liu J, Deng D, Lau CW, Wan S, Ai D, Mak KK, Tong KK, Kwan KM, Wang N, Chiu JJ, Zhu Y, Huang Y. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016;540(7634):579–582. doi: 10.1038/nature20602. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima H, Yamamoto K, Agarwala S, Terai K, Fukui H, Fukuhara S, Ando K, Miyazaki T, Yokota Y, Schmelzer E, Belting HG, Affolter M, Lecaudey V, Mochizuki N. Flow-dependent endothelial YAP regulation contributes to vessel maintenance. Dev Cell. 2017;40(6):523–536. doi: 10.1016/j.devcel.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Cai H, Xu Y. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Commun Signal. 2013;11(1):31. doi: 10.1186/1478-811X-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150(4):780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X, Shen H, Buckley B, Chen Y, Yang N, Mussell AL, Chernov M, Kobzik L, Frangou C, Han SX, Zhang J. NTRK1 is a positive regulator of YAP oncogenic function. Oncogene. 2018 doi: 10.1038/s41388-018-0609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Harris RC. Interaction of the EGF receptor and the hippo pathway in the diabetic kidney. J Am Soc Nephrol. 2016;27(6):1689–1700. doi: 10.1681/ASN.2015040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeRan M, Yang J, Shen CH, Peters EC, Fitamant J, Chan P, Hsieh M, Zhu S, Asara JM, Zheng B, Bardeesy N, Liu J, Wu X. Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep. 2014;9(2):495–503. doi: 10.1016/j.celrep.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, Lim DS, Guan KL. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17(4):500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon S, Kim W, Kim S, Kim Y, Song Y, Bilousov O, Kim J, Lee T, Cha B, Kim M, Kim H, Katanaev VL, Jho EH. Phosphorylation by NLK inhibits YAP-14-3-3-interactions and induces its nuclear localization. EMBO Rep. 2017;18(1):61–71. doi: 10.15252/embr.201642683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma B, Chen Y, Chen L, Cheng H, Mu C, Li J, Gao R, Zhou C, Cao L, Liu J, Zhu Y, Chen Q, Wu S. Hypoxia regulates Hippo signalling through the SIAH2 ubiquitin E3 ligase. Nat Cell Biol. 2015;17(1):95–103. doi: 10.1038/ncb3073. [DOI] [PubMed] [Google Scholar]
- 26.Kim HB, Kim M, Park YS, Park I, Kim T, Yang SY, Cho CJ, Hwang D, Jung JH, Markowitz SD, Hwang SW, Yang SK, Lim DS, Myung SJ. Prostaglandin E2 activates YAP and a positive-signaling loop to promote colon regeneration after colitis but also carcinogenesis in mice. Gastroenterology. 2017;152(3):616–630. doi: 10.1053/j.gastro.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi HJ, Kim NE, Kim BM, Seo M, Heo JH. TNF-α-induced YAP/TAZ activity mediates leukocyte-endothelial adhesion by regulating vcam1 expression in endothelial cells. Int J Mol Sci. 2018;19(11):3428. doi: 10.3390/ijms19113428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tharehalli U, Svinarenko M, Kraus JM, Kühlwein SD, Szekely R, Kiesle U, Scheffold A, Barth TFE, Kleger A, Schirmbeck R, Kestler HA, Seufferlein T, Oswald F, Katz SF, Lechel A. YAP activation drives liver regeneration after cholestatic damage induced by Rbpj deletion. Int J Mol Sci. 2018;19(12):3801. doi: 10.3390/ijms19123801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flinn MA, Link BA, O’Meara CC. Upstream regulation of the Hippo-Yap pathway in cardiomyocyte regeneration. Semin Cell Dev Biol. 2019;100:10–11. doi: 10.1016/j.semcdb.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526(7575):715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 31.Kim W, Jho EH. The history and regulatory mechanism of the Hippo pathway. BMB Rep. 2018;51(3):106–118. doi: 10.5483/BMBRep.2018.51.3.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim Y, Jho EH. Regulation of the Hippo signaling pathway by ubiquitin modification. BMB Rep. 2018;51(3):143–150. doi: 10.5483/BMBRep.2018.51.3.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng X, Fang L. VGLL4 is a transcriptional cofactor acting as a novel tumor suppressor via interacting with TEADs. Am J Cancer Res. 2018;8(6):932–943. [PMC free article] [PubMed] [Google Scholar]
- 34.Dobrokhotov O, Samsonov M, Sokabe M, Hirata H. Mechanoregulation and pathology of YAP/TAZ via Hippo and non-Hippo mechanisms. Clin Transl Med. 2018;7(1):23. doi: 10.1186/s40169-018-0202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serrano I, McDonald PC, Lock F, Muller WJ, Dedhar S. Inactivation of the Hippo tumour suppressor pathway by integrin-linked kinase. Nat Commun. 2013;4:2976. doi: 10.1038/ncomms3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim NG, Gumbiner BM. Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J Cell Biol. 2015;210(3):503–515. doi: 10.1083/jcb.201501025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elbediwy A, Vincent-Mistiaen ZI, Spencer-Dene B, Stone RK, Boeing S, Wculek SK, Cordero J, Tan EH, Ridgway R, Brunton VG, Sahai E, Gerhardt H, Behrens A, Malanchi I, Sansom OJ, Thompson BJ. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development. 2016;143(10):1674–1687. doi: 10.1242/dev.133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan R, Kim NG, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci USA. 2013;110(7):2569–2574. doi: 10.1073/pnas.1216462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabra H, Brunner M, Mandati V, Wehrle-Haller B, Lallemand D, Ribba AS, Chevalier G, Guardiola P, Block MR, Bouvard D. β1 integrin-dependent Rac/group I PAK signaling mediates YAP activation of Yes-associated protein 1 (YAP1) via NF2/merlin. J Biol Chem. 2017;292(47):19179–19197. doi: 10.1074/jbc.M117.808063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ladoux B, Nelson WJ, Yan J, Mège RM. The mechanotransduction machinery at work at adherens junctions. Integr Biol (Camb) 2015;7(10):1109–1119. doi: 10.1039/c5ib00070j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gladden AB, Hebert AM, Schneeberger EE, McClatchey AI. The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev Cell. 2010;19(5):727–739. doi: 10.1016/j.devcel.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu J, Zheng Y, Dong J, Klusza S, Deng WM, Pan D. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. 2010;18(2):288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gumbiner BM, Kim NG. The Hippo-YAP signaling pathway and contact inhibition of growth. J Cell Sci. 2014;127(Pt 4):709–717. doi: 10.1242/jcs.140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154(6):1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, Park HW, Mo JS, Lu W, Lu S, Flores F, Yu FX, Halder G, Guan KL. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;5(6):8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dutta S, Mana-Capelli S, Paramasivam M, Dasgupta I, Cirka H, Billiar K, McCollum D. TRIP6 inhibits Hippo signaling in response to tension at adherens junctions. EMBO Rep. 2018;19(2):337–350. doi: 10.15252/embr.201744777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yi C, Troutman S, Fera D, Stemmer-Rachamimov A, Avila JL, Christian N, Persson NL, Shimono A, Speicher DW, Marmorstein R, Holmgren L, Kissil JL. A tight junction-associated Merlin-angiomotin complex mediates Merlin’s regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell. 2011;19(4):527–540. doi: 10.1016/j.ccr.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liem RK. Cytoskeletal integrators: the spectrin superfamily. Cold Spring Harb Perspect Biol. 2016;8(10):a018259. doi: 10.1101/cshperspect.a018259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong KK, Li W, An Y, Duan Y, Li Z, Kang Y, Yan Y. β-Spectrin regulates the hippo signaling pathway and modulates the basal actin network. J Biol Chem. 2015;290(10):6397–6407. doi: 10.1074/jbc.M114.629493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fletcher GC, Elbediwy A, Khanal I, Ribeiro PS, Tapon N, Thompson BJ. The spectrin cytoskeleton regulates the Hippo signalling pathway. EMBO J. 2015;34(7):940–954. doi: 10.15252/embj.201489642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heng BC, Aubel D, Fussenegger M. An overview of the diverse roles of G-protein coupled receptors (GPCRs) in the pathophysiology of various human diseases. Biotechnol Adv. 2013;31(8):1676–1694. doi: 10.1016/j.biotechadv.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 52.Xia H, Dai X, Yu H, Zhou S, Fan Z, Wei G, Tang Q, Gong Q, Bi F. EGFR-PI3K-PDK1 pathway regulates YAP signaling in hepatocellular carcinoma: the mechanism and its implications in targeted therapy. Cell Death Dis. 2018;9(3):269. doi: 10.1038/s41419-018-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirate Y, Hirahara S, Inoue K, Kiyonari H, Niwa H, Sasaki H. Par-aPKC-dependent and -independent mechanisms cooperatively control cell polarity, Hippo signaling, and cell positioning in 16-cell stage mouse embryos. Dev Growth Differ. 2015;57(8):544–556. doi: 10.1111/dgd.12235. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T, Ohno S. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol. 2001;152(6):1183–1196. doi: 10.1083/jcb.152.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vorhagen S, Niessen CM. Mammalian aPKC/Par polarity complex mediated regulation of epithelial division orientation and cell fate. Exp Cell Res. 2014;328(2):296–302. doi: 10.1016/j.yexcr.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Yin Y, Sheng J, Hu R, Yang Y, Qing S. The expression and localization of Crb3 in developmental stages of the mice embryos and in different organs of 1-week-old female mice. Reprod Domest Anim. 2014;49(5):824–830. doi: 10.1111/rda.12374. [DOI] [PubMed] [Google Scholar]
- 57.Szymaniak AD, Mahoney JE, Cardoso WV, Varelas X. Crumbs3-mediated polarity directs airway epithelial cell fate through the hippo pathway effector yap. Dev Cell. 2015;34(3):283–296. doi: 10.1016/j.devcel.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yates LL, Schnatwinkel C, Hazelwood L, Chessum L, Paudyal A, Hilton H, Romero MR, Wilde J, Bogani D, Sanderson J, Formstone C, Murdoch JN, Niswander LA, Greenfield A, Dean CH. Scribble is required for normal epithelial cell-cell contacts and lumen morphogenesis in the mammalian lung. Dev Biol. 2013;373(2):267–280. doi: 10.1016/j.ydbio.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Archibald A, Al-Masri M, Liew-Spilger A, McCaffrey L. Atypical protein kinase C induces cell transformation by disrupting Hippo/Yap signaling. Mol Biol Cell. 2015;26(20):3578–3595. doi: 10.1091/mbc.E15-05-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou PJ, Xue W, Peng J, Wang Y, Wei L, Yang Z, Zhu HH, Fang YX, Gao WQ. Elevated expression of Par3 promotes prostate cancer metastasis by forming a Par3/aPKC/KIBRA complex and inactivating the hippo pathway. J Exp Clin Cancer Res. 2017;36(1):139. doi: 10.1186/s13046-017-0609-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narimatsu M, Samavarchi-Tehrani P, Varelas X, Wrana JL. Distinct polarity cues direct Taz/Yap and TGFβ receptor localization to differentially control TGFβ-induced Smad signaling. Dev Cell. 2015;32(5):652–656. doi: 10.1016/j.devcel.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 62.Liu J, Li J, Li P, Wang Y, Liang Z, Jiang Y, Li J, Feng C, Wang R, Chen H, Zhou C, Zhang J, Yang J, Liu P. Loss of DLG5 promotes breast cancer malignancy by inhibiting the Hippo signaling pathway. Sci Rep. 2017;7(7):42125. doi: 10.1038/srep42125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bae SJ, Ni L, Osinski A, Tomchick DR, Brautigam CA, Luo X. SAV1 promotes Hippo kinase activation through antagonizing the PP2A phosphatase STRIPAK. Elife. 2017;6:e30278. doi: 10.7554/eLife.30278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boggiano JC, Vanderzalm PJ, Fehon RG. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev Cell. 2011;21(5):888–895. doi: 10.1016/j.devcel.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, Park HW, Mo JS, Lu W, Lu S, Flores L, Yu FX, Halder G, Guan KL. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, Chen J. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17(4):490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang W, Huang J, Wang X, Yuan J, Li X, Feng L, Park JI, Chen J. PTPN14 is required for the density-dependent control of YAP1. Genes Dev. 2012;26(17):1959–1971. doi: 10.1101/gad.192955.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson KE, Li YW, Yang N, Shen H, Orillion AR, Zhang J. PTPN14 forms a complex with Kibra and LATS1 proteins and negatively regulates the YAP oncogenic function. J Biol Chem. 2014;289(34):23693–23700. doi: 10.1074/jbc.M113.534701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ, Oria R, Kechagia JZ, Rico-Lastres P, Le Roux AL, Shanahan CM, Trepat X, Navajas D, Garcia-Manyes S, Roca-Cusachs P. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell. 2017;171(6):1397–1410. doi: 10.1016/j.cell.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 70.Frey S, Görlich D. A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell. 2007;130:512–523. doi: 10.1016/j.cell.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 71.Furukawa KT, Yamashita K, Sakurai N, Ohno S. The Epithelial Circumferential Actin Belt Regulates YAP/TAZ through Nucleocytoplasmic Shuttling of Merlin. Cell Rep. 2017;20(6):1435–1447. doi: 10.1016/j.celrep.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 72.Domínguez-Calderón A, Ávila-Flores A, Ponce A, López-Bayghen E, Calderón-Salinas JV, Luis Reyes J, Chávez-Munguía B, Segovia J, Angulo C, Ramírez L, Gallego-Gutiérrez H, Alarcón L, Martín-Tapia D, Bautista-García P, González-Mariscal L. ZO-2 silencing induces renal hypertrophy through a cell cycle mechanism and the activation of YAP and the mTOR pathway. Mol Biol Cell. 2016;27(10):1581–1595. doi: 10.1091/mbc.E15-08-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schaefer KN, Bonello TT, Zhang S, Williams CE, Roberts DM, McKay DJ, Peifer M. Supramolecular assembly of the beta-catenin destruction complex and the effect of Wnt signaling on its localization, molecular size, and activity in vivo. PLoS Genet. 2018;14(4):e1007339. doi: 10.1371/journal.pgen.1007339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, Piccolo S. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158(1):157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 75.Oudhoff MJ, Freeman SA, Couzens AL, Antignano F, Kuznetsova E, Min PH, Northrop JP, Lehnertz B, Barsyte-Lovejoy D, Vedadi M, Arrowsmith CH, Nishina H, Gold MR, Rossi FM, Gingras AC, Zaph C. Control of the hippo pathway by Set7-dependent methylation of Yap. Dev Cell. 2013;26(2):188–194. doi: 10.1016/j.devcel.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 76.Liu X, Yang N, Figel SA, Wilson KE, Morrison CD, Gelman IH, Zhang J. PTPN14 interacts with and negatively regulates the oncogenic function of YAP. Oncogene. 2013;32(10):1266–1273. doi: 10.1038/onc.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hozumi K. Distinctive properties of the interactions between Notch and Notch ligands. Dev Growth Differ. 2020;62(1):49–58. doi: 10.1111/dgd.12641. [DOI] [PubMed] [Google Scholar]
- 78.Totaro A, Castellan M, Di Biagio D, Piccolo S. Crosstalk between YAP/TAZ and notch signaling. Trends Cell Biol. 2018;28(7):560–573. doi: 10.1016/j.tcb.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y, Hibbs MA, Gard AL, Shylo NA, Yun K. Genome-wide analysis of N1ICD/RBPJ targets in vivo reveals direct transcriptional regulation of Wnt, SHH, and hippo pathway effectors by Notch1. Stem Cells. 2012;30(4):741–752. doi: 10.1002/stem.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Slemmons KK, Crose LES, Riedel S, Sushnitha M, Belyea B, Linardic CM. A novel Notch-YAP circuit drives stemness and tumorigenesis in embryonal rhabdomyosarcoma. Mol Cancer Res. 2017;15(12):1777–1791. doi: 10.1158/1541-7786.MCR-17-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu J, Zhou Y, Hu T, Zhang H, Shen M, Cheng P, Dai W, Wang F, Chen K, Zhang Y, Wang C, Li J, Zheng Y, Yang J, Zhu R, Wang J, Lu W, Zhang H, Wang J, Xia Y, De Assuncao TM, Jalan-Sakrikar N, Huebert RC, Bin Z, Guo C. Notch signaling coordinates progenitor cell-mediated biliary regeneration following partial hepatectomy. Sci Rep. 2016;6:22754. doi: 10.1038/srep22754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Derynck R, Budi EH. Specificity, versatility, and control of TGF-β family signaling. Sci Signal. 2019;12(570):5183. doi: 10.1126/scisignal.aav5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chaikuad A, Bullock AN. Structural basis of intracellular TGF-β signaling: receptors and smads. Cold Spring Harb Perspect Biol. 2016;8(11):a022111. doi: 10.1101/cshperspect.a022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ben Mimoun S, Mauviel A. Molecular mechanisms underlying TGF-ß/Hippo signaling crosstalks—role of baso-apical epithelial cell polarity. Int J Biochem Cell Biol. 2018;98:75–81. doi: 10.1016/j.biocel.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 85.Saito A, Nagase T. Hippo and TGF-β interplay in the lung field. Am J Physiol Lung Cell Mol Physiol. 2015;309(8):L756–L767. doi: 10.1152/ajplung.00238.2015. [DOI] [PubMed] [Google Scholar]
- 86.Miranda MZ, Bialik JF, Speight P, Dan Q, Yeung T, Szászi K, Pedersen SF, Kapus A. TGF-β1 regulates the expression and transcriptional activity of TAZ protein via a Smad3-independent, myocardin-related transcription factor-mediated mechanism. J Biol Chem. 2017;292(36):14902–14920. doi: 10.1074/jbc.M117.780502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y, Tu K, Liu D, Guo L, Chen Y, Li Q, Maiers JL, Liu Z, Shah VH, Dou C, Tschumperlin D, Voneschen L, Yang R, Kang N. p300 acetyltransferase is a cytoplasm-to-nucleus shuttle for SMAD2/3 and TAZ nuclear transport in transforming growth factor β-stimulated hepatic stellate cells. Hepatology. 2019;70(4):1409–1423. doi: 10.1002/hep.30668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pefani DE, Pankova D, Abraham AG, Grawenda AM, Vlahov N, Scrace S, O’Neill E. TGF-β targets the hippo pathway scaffold RASSF1A to facilitate YAP/SMAD2 nuclear translocation. Mol Cell. 2016;63(1):156–166. doi: 10.1016/j.molcel.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 89.Holden JK, Cunningham CN. Targeting the hippo pathway and cancer through the TEAD family of transcription factors. Cancers (Basel). 2018;10(3):81. doi: 10.3390/cancers10030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yasunami M, Suzuki K, Ohkubo H. A novel family of TEA domain-containing transcription factors with distinct spatiotemporal expression patterns. Biochem Biophys Res Commun. 1996;228(2):365–370. doi: 10.1006/bbrc.1996.1667. [DOI] [PubMed] [Google Scholar]
- 91.Moya IM, Halder G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol. 2018 doi: 10.1038/s41580-018-0086-y. [DOI] [PubMed] [Google Scholar]
- 92.Oh H, Slattery M, Ma L, White KP, Mann RS. Irvine KD Yorkie promotes transcription by recruiting a histone methyltransferase complex. Cell Rep. 2014;8(2):449–459. doi: 10.1016/j.celrep.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu Y, Li D, Wang Y, Pei C, Liu S, Zhang L, Yuan Z, Zhang P. Brahma regulates the Hippo pathway activity through forming complex with Yki-Sd and regulating the transcription of Crumbs. Cell Signal. 2015;27(3):606–613. doi: 10.1016/j.cellsig.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 94.Lin KC, Moroishi T, Meng Z, Jeong HS, Plouffe SW, Sekido Y, Han J, Park HW, Guan KL. Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation. Nat Cell Biol. 2017;19(8):996–1002. doi: 10.1038/ncb3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang W, Gao Y, Li P, Shi Z, Guo T, Li F, Han X, Feng Y, Zheng C, Wang Z, Li F, Chen H, Zhou Z, Zhang L, Ji H. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. 2014;24(3):331–343. doi: 10.1038/cr.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin Z, Guo H, Cao Y, Zohrabian S, Zhou P, Ma Q, VanDusen N, Guo Y, Zhang J, Stevens SM, Liang F, Quan Q, van Gorp PR, Li A, Dos Remedios C, He A, Bezzerides VJ, Pu WT. Acetylation of VGLL4 Regulates Hippo-YAP Signaling and Postnatal Cardiac Growth. Dev Cell. 2016;39(4):466–479. doi: 10.1016/j.devcel.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grannas K, Arngården L, Lönn P, Mazurkiewicz M, Blokzijl A, Zieba A, Söderberg O. Crosstalk between Hippo and TGFβ: subcellular Localization of YAP/TAZ/Smad complexes. J Mol Biol. 2015;427(21):3407–3415. doi: 10.1016/j.jmb.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 98.Brusgard JL, Choe M, Chumsri S, Renoud K, MacKerell AD, Jr, Sudol M, Passaniti A. RUNX2 and TAZ-dependent signaling pathways regulate soluble E-Cadherin levels and tumorsphere formation in breast cancer cells. Oncotarget. 2015;6(29):28132–28150. doi: 10.18632/oncotarget.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin X, Yang H, Wang L, Li W, Diao S, Du J, Wang S, Dong R, Li J, Fan Z. AP2a enhanced the osteogenic differentiation of mesenchymal stem cells by inhibiting the formation of YAP/RUNX2 complex and BARX1 transcription. Cell Prolif. 2019;52(1):e12522. doi: 10.1111/cpr.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tomlinson V, Gudmundsdottir K, Luong P, Leung KY, Knebel A, Basu S. JNK phosphorylates Yes-associated protein (YAP) to regulate apoptosis. Cell Death Dis. 2010;1:e29. doi: 10.1038/cddis.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roperch JP, El Ouadrani K, Hendrix A, Emami S, De Wever O, Melino G, Gespach C. Netrin-1 induces apoptosis in human cervical tumor cells via the TAp73alpha tumor suppressor. Cancer Res. 2008;68(20):8231–8239. doi: 10.1158/0008-5472.CAN-08-1483. [DOI] [PubMed] [Google Scholar]
- 102.Bora-Singhal N, Nguyen J, Schaal C, Perumal D, Singh S, Coppola D, Chellappan S. YAP1 regulates OCT4 activity and SOX2 expression to facilitate self-renewal and vascular mimicry of stem-like cells. Stem Cells. 2015;33(6):1705–1718. doi: 10.1002/stem.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kuser-Abali G, Alptekin A, Lewis M, Garraway IP, Cinar B. YAP1 and AR interactions contribute to the switch from androgen-dependent to castration-resistant growth in prostate cancer. Nat Commun. 2015;1(6):8126. doi: 10.1038/ncomms9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu H, Dai X, Cao X, Yan H, Ji X, Zhang H, Shen S, Si Y, Zhang H, Chen J, Li L, Zhao JC, Yu J, Feng XH, Zhao B. PRDM4 mediates YAP-induced cell invasion by activating leukocyte-specific integrin β2 expression. EMBO Rep. 2018;19(6):e45180. doi: 10.15252/embr.201745180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Panciera T, Azzolin L, Fujimura A, Di Biagio D, Frasson C, Bresolin S, Soligo S, Basso G, Bicciato S, Rosato A, Cordenonsi M, Piccolo S. Induction of expandable tissue-specific stem/progenitor cells through transient expression of YAP/TAZ. Cell Stem Cell. 2016;19(6):725–737. doi: 10.1016/j.stem.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ohgushi M, Minaguchi M, Sasai Y. Rho-signaling-directed YAP/TAZ activity underlies the long-term survival and expansion of human embryonic stem cells. Cell Stem Cell. 2015;17(4):448–461. doi: 10.1016/j.stem.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 107.Yang W, Han W, He W, Li J, Wang J, Feng H, Qian Y. Surface topography of hydroxyapatite promotes osteogenic differentiation of human bone marrow mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2016;60:45–53. doi: 10.1016/j.msec.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Y, Gong H, Sun Y, Huang Y, Fan Y. Enhanced osteogenic differentiation of MC3T3-E1 cells on grid-topographic surface and evidence for involvement of YAP mediator. J Biomed Mater Res A. 2016;104(5):1143–1152. doi: 10.1002/jbm.a.35648. [DOI] [PubMed] [Google Scholar]
- 109.Qian W, Gong L, Cui X, Zhang Z, Bajpai A, Liu C, Castillo AB, Teo JCM, Chen W. Nanotopographic regulation of human mesenchymal stem cell osteogenesis. ACS Appl Mater Interfaces. 2017;9(48):41794–41806. doi: 10.1021/acsami.7b16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hwang JH, Lee DH, Byun MR, Kim AR, Kim KM, Park JI, Oh HT, Hwang ES, Lee KB, Hong JH. Nanotopological plate stimulates osteogenic differentiation through TAZ activation. Sci Rep. 2017;7(1):3632. doi: 10.1038/s41598-017-03815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arslan E, Hatip Koc M, Uysal O, Dikecoglu B, Topal AE, Garifullin R, Ozkan AD, Dana A, Hermida-Merino D, Castelletto V, Edwards-Gayle C, Baday S, Hamley I, Tekinay AB, Guler MO. Supramolecular peptide nanofiber morphology affects mechanotransduction of stem cells. Biomacromol. 2017;18(10):3114–3130. doi: 10.1021/acs.biomac.7b00773. [DOI] [PubMed] [Google Scholar]
- 112.Sedlmayer F, Aubel D, Fussenegger M. Synthetic gene circuits for the detection, elimination and prevention of disease. Nat Biomed Eng. 2018;2(6):399–415. doi: 10.1038/s41551-018-0215-0. [DOI] [PubMed] [Google Scholar]
- 113.Tolle F, Stücheli P, Fussenegger M. Genetic circuitry for personalized human cell therapy. Curr Opin Biotechnol. 2019;7(59):31–38. doi: 10.1016/j.copbio.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 114.Schütte U, Bisht S, Heukamp LC, Kebschull M, Florin A, Haarmann J, Hoffmann P, Bendas G, Buettner R, Brossart P, Feldmann G. Hippo signaling mediates proliferation, invasiveness, and metastatic potential of clear cell renal cell carcinoma. Transl Oncol. 2014;7(2):309–321. doi: 10.1016/j.tranon.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel MA, Goldstein LS, Abujarour R, Ding S, Guan KL. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24(11):1106–1118. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang S, Ma K, Chen L, Zhu H, Liang S, Liu M, Xu N (2016) TAZ promotes cell growth and inhibits Celastrol-induced cell apoptosis. Biosci Rep 36(5) [DOI] [PMC free article] [PubMed]
- 117.Tian T, Li A, Lu H, Luo R, Zhang M, Li Z. TAZ promotes temozolomide resistance by upregulating MCL-1 in human glioma cells. Biochem Biophys Res Commun. 2015;463(4):638–643. doi: 10.1016/j.bbrc.2015.05.115. [DOI] [PubMed] [Google Scholar]
- 118.Yagi R, Kohn MJ, Karavanova I, Kaneko KJ, Vullhorst D, DePamphilis ML, Buonanno A. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development. 2007;134(21):3827–3836. doi: 10.1242/dev.010223. [DOI] [PubMed] [Google Scholar]
- 119.Lv XY, Sun W, Su R, Li D, Wang QZ, Musa HH, Chen L, Zhang YF, Wu WZ. Correlation between sheep YAP1 temporal and spatial expression trends and MSTN and MyoG gene expression. Genet Mol Res. 2015;14(2):3244–3256. doi: 10.4238/2015.April.13.3. [DOI] [PubMed] [Google Scholar]
- 120.Ralston A, Cox BJ, Nishioka N, Sasaki H, Chea E, Rugg-Gunn P, Guo G, Robson P, Draper JS, Rossant J. Gata3 regulates trophoblast development downstream of Tead4 and in parallel to Cdx2. Development. 2010;137(3):395–403. doi: 10.1242/dev.038828. [DOI] [PubMed] [Google Scholar]
- 121.Ribas R, Moncaut N, Siligan C, Taylor K, Cross JW, Rigby PW, Carvajal JJ. Members of the TEAD family of transcription factors regulate the expression of Myf5 in ventral somitic compartments. Dev Biol. 2011;355(2):372–380. doi: 10.1016/j.ydbio.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu X, Li H, Rajurkar M, Li Q, Cotton JL, Ou J, Zhu LJ, Goel HL, Mercurio AM, Park JS, Davis RJ, Mao J. Tead and AP1 Coordinate Transcription and Motility. Cell Rep. 2016;14(5):1169–1180. doi: 10.1016/j.celrep.2015.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qiao Y, Chen J, Lim YB, Finch-Edmondson ML, Seshachalam VP, Qin L, Jiang T, Low BC, Singh H, Lim CT, Sudol M. YAP regulates actin dynamics through ARHGAP29 and promotes metastasis. Cell Rep. 2017;19(8):1495–1502. doi: 10.1016/j.celrep.2017.04.075. [DOI] [PubMed] [Google Scholar]
- 124.Ehmer U, Zmoos AF, Auerbach RK, Vaka D, Butte AJ, Kay MA, Sage J. Organ size control is dominant over Rb family inactivation to restrict proliferation in vivo. Cell Rep. 2014;8(2):371–381. doi: 10.1016/j.celrep.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shen Z, Stanger BZ. YAP regulates S-phase entry in endothelial cells. PLoS One. 2015;10(1):e0117522. doi: 10.1371/journal.pone.0117522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lo Sardo F, Forcato M, Sacconi A, Capaci V, Zanconato F, Di Agostino S, Del Sal G, Pandolfi PP, Strano S, Bicciato S, Blandino G. MCM7 and its hosted miR-25, 93 and 106b cluster elicit YAP/TAZ oncogenic activity in lung cancer. Carcinogenesis. 2017;38(1):64–75. doi: 10.1093/carcin/bgw110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Z, Wu Y, Wang H, Zhang Y, Mei L, Fang X, Zhang X, Zhang F, Chen H, Liu Y, Jiang Y, Sun S, Zheng Y, Li N, Huang L. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci U S A. 2014;111(1):E89–E98. doi: 10.1073/pnas.1319190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22(14):1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang H, Pasolli HA, Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci U S A. 2011;108(6):2270–2275. doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu MZ, Chan SW, Liu AM, Wong KF, Fan ST, Chen J, Poon RT, Zender L, Lowe SW, Hong W, Luk JM. AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene. 2011;30(10):1229–1240. doi: 10.1038/onc.2010.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang Y, Xia H, Ge X, Chen Q, Yuan D, Chen Q, Leng W, Chen L. Tang Q1 Bi F. CD44 acts through RhoA to regulate YAP signaling. Cell Signal. 2014;26(11):2504–2513. doi: 10.1016/j.cellsig.2014.07.031. [DOI] [PubMed] [Google Scholar]