Abstract
Although 5-methylcytosine (m5C) has been identified as a novel and abundant mRNA modification and associated with energy metabolism, its regulation function in adipose tissue and skeletal muscle is still limited. This study aimed at investigating the effect of mRNA m5C on adipogenesis and myogenesis using Jinhua pigs (J), Yorkshire pigs (Y) and their hybrids Yorkshire–Jinhua pigs (YJ). We found that Y grow faster than J and YJ, while fatness-related characteristics observed in Y were lower than those of J and YJ. Besides, total mRNA m5C levels and expression rates of NSUN2 were higher both in backfat layer (BL) and longissimus dorsi muscle (LDM) of Y compared to J and YJ, suggesting that higher mRNA m5C levels positively correlate with lower fat and higher muscle mass. RNA bisulfite sequencing profiling of m5C revealed tissue-specific and dynamic features in pigs. Functionally, hyper-methylated m5C-containing genes were enriched in pathways linked to impaired adipogenesis and enhanced myogenesis. In in vitro, m5C inhibited lipid accumulation and promoted myogenic differentiation. Furthermore, YBX2 and SMO were identified as m5C targets. Mechanistically, YBX2 and SMO mRNAs with m5C modification were recognized and exported into the cytoplasm from the nucleus by ALYREF, thus leading to increased YBX2 and SMO protein expression and thereby inhibiting adipogenesis and promoting myogenesis, respectively. Our work uncovered the critical role of mRNA m5C in regulating adipogenesis and myogenesis via ALYREF-m5C-YBX2 and ALYREF-m5C-SMO manners, providing a potential therapeutic target in the prevention and treatment of obesity, skeletal muscle dysfunction and metabolic disorder diseases.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-022-04474-0.
Keywords: Adipogenesis, Myogenesis, m5C modification, YBX2, SMO, ALYREF
Introduction
Obesity, characterized by excessive deposition of adipose tissue, has emerged as a global public health issue which substantially increases the risk of metabolic diseases, such as type 2 diabetes mellitus, cardiovascular diseases and non-alcoholic fatty liver disease [1]. Adipose tissue is essential as energy storage while skeletal muscle is the energy burning “machinery” [2]. Skeletal muscle can account for ~ 40% of adult human body weight, and impaired myogenesis can cause muscular dystrophies and disrupted muscle regeneration, leading to physical frailty and even severe metabolic disorders [3, 4]. Several studies have demonstrated that adipose tissue is associated with skeletal muscle function and that they play a critical role in the dynamic regulation of metabolic activity and metabolic health [1, 5]. Thus, a deeper understanding of the mechanisms behind adipose and muscle development is both timely and relevant.
Numerous studies have demonstrated that RNA modifications, such as N6-methyladenosine (m6A), could serve as novel epigenetic marker with profound significance in regulating adipogenesis and myogenesis via modulating RNA metabolism [6, 7]. Recently, 5-methylcytosine (m5C) has been identified as another new internal mRNA modification [8]. m5C modification can be reversibly regulated by its methyltransferases (“writers”) and demethylases (“erasers”) [8–10]. Functionally, mRNA m5C modification was reported to play a critical role in the adaptation to high temperature, in ovarian germ line stem cell development, in tumorigenesis and maternal-to-zygotic transition, also known as embryonic genome activation [11–15]. Although the role of mRNA m5C in adipogenesis has been previously reported, the regulatory mechanism of m5C in adipose tissue and muscle development is still limited.
On the basis of their anatomical, physiological and genomic similarities with humans, pigs have served as ideal biomedical model system to study organ development and disease progression for decades before [16]. For example, pigs are increasingly recruited as models for research on obesity and diabetes mellitus [17]. The Jinhua pig (J), a typical fat-type breed characterized by its black head and tail, high backfat layer and body fat ratio, low growth rate and less lean mass, is one of the local, popular breeds in China [18–20]. In contrast, the Yorkshire pigs (Y), as a traditional lean-type breed originating from the United States, have less fat content, higher rapid growth ratio and more muscle mass than J [21, 22]. Here, we used Jinhua pigs, Yorkshire pigs and their hybrids Yorkshire–Jinhua pigs (YJ) to explore the role of mRNA m5C in adipose tissue and skeletal muscle development. RNA bisulfite sequencing (RNA-BisSeq) in tissues (adipose tissue and skeletal muscle) and in vitro studies demonstrated that m5C negatively regulates adipogenesis and positively affects myogenesis. We also performed RNA-BisSeq of primary cells isolated from pig adipose tissue and skeletal muscle, and Y-box-binding protein 2 (YBX2) and smoothened (SMO) were screened out as m5C targets by combining RNA-BisSeq from both tissue and cell data. Mechanistically, YBX2/SMO with higher m5C were recognized and exported to cytoplasm from the nucleus by the Aly/REF export factor (ALYREF), leading to increased YBX2/SMO protein expression, thereby inhibiting adipogenesis and promoting myogenesis. Our findings reveal a regulatory mechanism of mRNA m5C-mediated impaired adipogenesis and advantaged myogenesis in pigs and provide an important resource for studying the function of the mRNA modification during the development of obesity, skeletal muscle dysfunction and diseases caused by metabolic disorders.
Materials and methods
Animals and tissue collection
A total of 4 Jinhua pigs (male), 4 Yorkshire pigs (male) and 4 Yorkshire–Jinhua pigs (male) were humanely killed at the age of 6 months, and adipose tissue (backfat layer, BL) and skeletal muscle samples (longissimus dorsi muscles, LDM) from the three breeds were rapidly removed from each carcass, immediately frozen in liquid nitrogen, and stored at − 80 °C until RNA extraction.
Plasmid construction, siRNA, adenovirus production and cell transfection
For NOP2/Sun RNA methyltransferase 2 (NSUN2) and ALYREF recombinant adenovirus construction, the porcine NSUN2 and ALYREF were cloned into the pDC315-EGFP vector (Hanbio), respectively. shRNA sequences (GenePharma) were cloned into the pDC311-U6-MCMV-EGFP vector (Hanbio). The siRNA (GenePharma) and plasmid transfections were performed using Lipofectamine RNAiMAX (Invitrogen), Lipofectamine 2000 (Invitrogen) or EZ Trans (Life-iLab, China), according to the manufacturers’ instructions. The target sequences for shRNA and siRNA are listed in Table S1.
Cell isolation and cell culture
Porcine subcutaneous fat pre-adipocytes (PFs) and muscle satellite cells (SCs) were isolated from subcutaneous adipose tissue and Longissimus dorsi muscle of 5-day-old Duroc–Landrace–Yorkshire piglets under sterile conditions, respectively [19, 23]. Cells were cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM, Thermo Fisher Scientific, Waltham, MA, USA) or DMEM/F12 medium containing 10% fetal bovine serum (Thermo Fisher Scientific) and 1% penicillin–streptomycin in the cell culture dish (NEST Biotechnology, Wuxi, China). After 2 days post confluence of cells, adipocyte differentiation was induced with adipogenic differentiation medium (containing 0.5 mM IBMX, 1 μM dexamethasone and 10 μg/mL insulin). After 2 days, the medium was replaced with a maintenance medium (containing 10% 10 μg/mL insulin) to allow for adipogenic differentiation. Cell culture and myogenic differentiation of pig SCs were performed as previously described with some modifications [24]. Briefly, when SCs become 90–100% confluent, myogenic differentiation were induced with DMEM/F12 medium containing 2% horse serum and 1% penicillin–streptomycin (Thermo Fisher Scientific). The differentiation medium was changed every 24 h, and myogenic differentiation was induced for 4–6 days.
Quantitative real-time PCR (qPCR) analysis
Total RNA from cells or tissues were extracted using TRIzol reagent (Thermo Fisher Scientific) and reverse-transcribed into cDNA using M-MLV reverse transcriptase (Thermo Fisher Scientific). qPCR was performed using the SYBR Green PCR Master Mix (Sigma-Aldrich, St. Louis, MO, USA) with the ABI Step-One Plus™ Real-Time PCR System (Thermo Fisher Scientific). The data were analyzed following the 2−ΔΔCt method. The primer sequences are presented in Table S2.
Oil red O staining
The differentiated PFs were washed with 1 × PBS twice and fixed in 10% formalin at room temperature for 1 h. Cells were incubated in 60% isopropanol for 5 min at room temperature and then allowed to dry completely. The cells were then stained with the Oil Red O working solution (Solarbio Science and Technology Co., Ltd., Beijing, China) at room temperature for 10 min followed by 3 rinses in 1 × PBS before imaging. For quantification, Oil Red O-stained lipids were eluted in 100% isopropanol for 5 min at room temperature and absorbance was measured at 510 nm.
Bisulfite conversion of RNA, RNA-BisSeq library construction and sequencing
RNA bisulfite conversion was performed as previously described [8]. In brief, 1 μg mRNAs along with 5 ng dihydrofolate reductase (Dhfr) RNA as methylation conversion control were fragmented into ~ 200-nucleotide fragments by RNA Fragmentation Reagent (Thermo Fisher Scientific). The fragmented RNA was re-suspended in 100 μl bisulfite solution (pH 5.1), which is a 100:1 mixture of 40% sodium bisulfite (Sigma-Aldrich) and 600 μM hydroquinone (Sigma-Aldrich) and was subjected to heat incubation at 75 °C for 4 h. The reaction mixture was desalted with Nanoseq with a 3 K omega 500/pk centrifuge (Pall Corporation, New York, USA), and then desulfonated by incubation with an equal volume of 1 M Tris (pH 9.0) at 75 °C for 1 h. After ethanol precipitation, the RNAs were re-suspended in 10 μl RNase-free water and used for library construction. cDNA libraries were constructed using the KAPA Stranded mRNA-Seq Kit (Roche, Shanghai, China). Reverse transcription was carried out using ACT random hexamers and Superscript III Reverse Transcriptase (Thermo Fisher Scientific) according to the manufacturer’s instructions. Sequencing was performed on an Illumina HiSeq2500 instrument with paired end 150 bp read length.
Methylated RNA immunoprecipitation real-time PCR (meRIP-qPCR)
m5C meRIP-qPCR was performed as previously described with some modifications [14]. Briefly, around 2 μg mRNAs were randomly fragmented into 200–300 nt fragments by fragmentation buffer (Thermo Fisher Scientific). The fragmented mRNAs were precipitated with ethanol for following reaction. One-tenth of the fragmented mRNAs were saved as input control. 4 μg anti-m5C antibody (Epigentek, Farmingdale, USA) or IgG antibody (ABclonal Biotechnology Co., Ltd, Wuhan, China) was incubated with 40 μl Dynabeads Protein A (Thermo Fisher Scientific) in 300 μl IP buffer (10 mM Tris–HCl pH 7.5, 150 mM NaCl, 0.05% Triton X (v/v)) for 2 h at 4 °C followed by 3 rinses with 500 μl IP buffer, then the fragmented mRNAs were incubated with the prepared antibody beads at 4 °C overnight in 300 μl IP buffer. The mRNA–antibody beads complexes were washed three times with 500 μl IP buffer and then incubated in 300 μl elution buffer (5 mM Tris–HCl pH 7.5, 1 mM EDTA, 0.05% SDS, and 80 μg proteinase K) for 1 h at 50 °C, following ethanol precipitation. The eluted mRNAs and input control mRNAs were reverse-transcribed with random hexamers, and m5C enrichment was determined by qPCR. The data were analyzed following the 2−ΔΔCt method, and the relative enrichment of m5C in each sample was calculated by normalizing to input. The primers were listed in Table S2.
m5C dot blots
For m5C dot blots, RNAs were denatured at 65 °C for 5 min. Samples were spotted on hybond-N + membrane (GE Healthcare, Chicago, USA). After UV crosslinking, the membrane was washed with PBST buffer, blocked with 5% non-fat milk and incubated with anti-m5C antibody overnight at 4 °C. Then, the secondary antibody was incubated at room temperature for 1 h. The membrane was visualized using chemiluminescence (ECL Plus detection system). Primary antibodies are listed in Table S3.
High-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS)
100–200 ng of mRNAs was digested by nuclease P1 (2U) in 25 μl of buffer containing 10 mM of NH4OAc (pH = 5.3) at 42 °C for 2 h, followed by the addition of NH4HCO3 (1 M, 3 μl, freshly made) and 0.5 U alkaline phosphatase with incubation at 37 °C for 2 h. Then, the sample was diluted to 50 μl and filtered (0.22 μm pore size, 4 mm diameter, Millipore), and 5 μl of the solution was injected into HPLC–MS/MS. Nucleosides were separated by reverse-phase ultra-performance liquid chromatography on a C18 column with on-line MS detection using an Agilent 6410 QQQ triple–quadrupole LC mass spectrometer in positive electrospray ionization mode. The nucleosides were quantified using the nucleoside to base ion mass transitions of 258–126 (m5C) and 244–112 (rC). Quantification was performed in comparison with the standard curve obtained from pure nucleoside standards running on the same batch of samples. The ratio of m5C to C was calculated based on the calibrated concentrations.
ALREF RNA Immunoprecipitation (RIP) assay
Flag-ALYREF overexpressing cell pellets were re-suspended with two volumes of lysis buffer (150 mM KCl, 10 mM HEPES pH 7.6, 2 mM EDTA, 0.5% NP-40, 0.5 mM DTT, 1:100 protease inhibitor cocktail, 400 U/ml RNase inhibitor), and incubated at 4 °C for 30 min while rotating. Then, the lysate was centrifuged at 12,000g for 20 min and mixed with M2 beads (Sigma-Aldrich) containing 600 μl NT2 buffer (200 mM NaCl, 50 mM HEPES pH 7.6, 2 mM EDTA, 0.05% NP-40, 0.5 mM DTT, 200 U/ml RNase inhibitor) and incubated at 4 °C for 4 h with rotation. After washing three times with NT2 buffer, the beads were digested with 200 μl pre-heated (20 min at 50 °C) proteinase K solution (4 mg/ml) for 40 min at 50 °C in rotation at 2000 rpm/min. After centrifugation at top speed for 5 min, the supernatant was transferred and RNAs were extracted with an equal volume of acid–phenol:chloroform, pH 4.5 (Thermo Fisher Scientific). The RNAs were used for qPCR analysis.
Bioinformatic analysis
Raw reads were cleaned using Trimmomatic software [25]. For RNA-seq, the cleaned reads were mapped against the Sus scrofa genome (Sus scrofa11.1) with TopHat2 (version 2.0.13) [26]. The number of reads mapped to each ensemble gene was counted using the HTSeq software. FPKM was computed as the number of reads mapped per kilobase in the exon model per million mapped reads for each gene. mRNAs with FPKM ≥ 1 were regarded as expressing genes. For RNA-BisSeq, the cleaned reads were mapped to Sus scrofa11.1 by meRanTK (version 1.2.0). Only sites with a coverage depth ≥ 30, methylation level ≥ 0.1 and methylated cytosine depth ≥ 5 were considered credible. Only overlapping m5C sites between two replicates were used for the following analyses. The differences in m5C site level that were greater than 5% between two samples were considered increased or decreased m5C site methylations. The m5C sites were annotated by BEDTools’ intersectBed. To acquire the sequence preference proximal to m5C sites, 21 nt sequences centering to each m5C site were extracted with Bedtools, and logo plots were generated with ggseqlogo. Gene ontology (GO) and KEGG pathway analyses were performed using the DAVID bioinformatics database.
Statistical analysis
All bioinformatics-related statistical analyses were performed using the R package for statistical computing. For experimental quantification, the unpaired t test in GraphPad Prism 6 software was applied, and error bars are given based on standard deviation of the mean (SD) (unless stated otherwise). P < 0.05 is considered as statistically significant.
Results
Phenotypes of three pig breeds
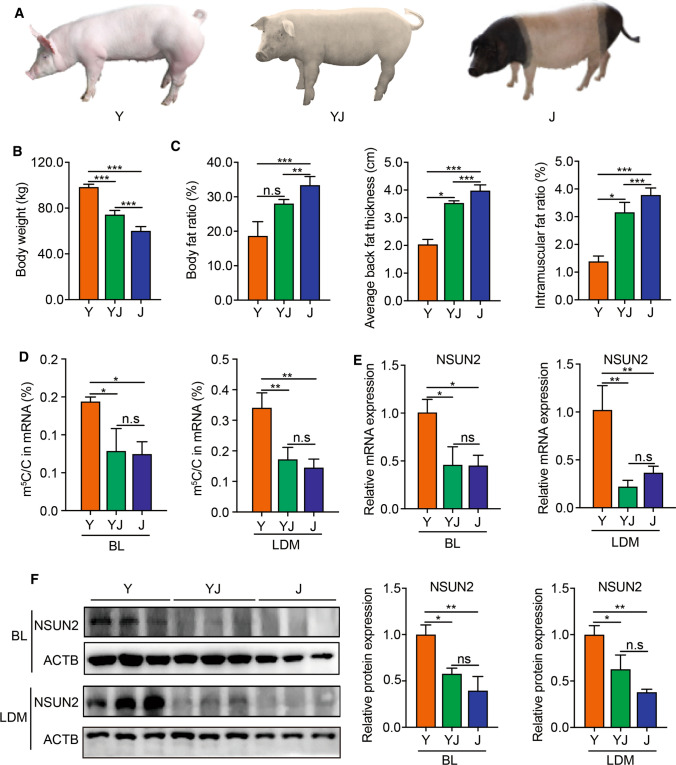
Here, we hybridized Yorkshire and Jinhua pigs and constructed an intermediate-type breed named Yorkshire–Jinhua pigs (YJ) (Fig. 1A). A total of 12 pigs (4 per breed) at an age of 180 days were randomly chosen to measure fatness/growth-related phenotypes. The body weights of Y were significantly higher than J, and YJ were just intermediate between Y and J (Fig. 1B), indicating that Y grow faster than J and YJ. However, the body fat ratio, average back fat thickness and intramuscular fat ratio of Y were significantly lower than those of J. These fatness-related characters of YJ were just between the two breeds of Y and J (Fig. 1C).
Fig. 1.
Phenotypes of three pig breeds. A Photos of Yorkshire pigs (Y), Yorkshire × Jinhua pigs (YJ) and Jinhua pigs (J). B Body weights in Y, YJ and J at 180 days. Error bars, means ± S.D., n = 4. C Body fat ratio, average back fat thickness, intramuscular fat ratio in Y, YJ and J at 180 days of age. Error bars, means ± S.D., n = 4. D HPLC–MS/MS quantification of m5C/C in mRNA of backfat layer (BL) and longissimus dorsi muscle (LDM) in Y, YJ and J. Error bars, means ± S.D., n = 3. E qPCR analysis of NSUN2 expression in BL and LDM in Y, YJ and J at 180 days of age. 18S rRNA served as an internal RNA control. Error bars, means ± S.D., n = 3. F Western blotting of NSUN2 expression in BL and LDM in Y, YJ and J at 180 days of age. ACTB was used as a loading control. Error bars, means ± S.D., n = 3. The P values were determined using one-way ANOVA. *P < 0.05 **P < 0.01, ***P < 0.001
m5C has been shown to be closely related to energy metabolism [15]. Therefore, we measured m5C abundance in BL and LDM from three pig breeds by liquid chromatography–tandem mass spectrometry (LC–MS/MS) to explore the potential relationship between m5C and fatness/muscle growth. We observed that the ratio of m5C/C in total mRNA from the BL and LDM of the three breeds ranged from 0.1 to 0.4%, and a statistically significant higher m5C level was found both in BL and LDM of Y compared to J or YJ (Fig. 1D). Since NSUN2 was identified as the main m5C methyltransferase of mRNA in many species including human and mouse [27], we analyzed its mRNA and protein expressions in BL and LDM from the three pig breeds. The results suggest that NSUN2 mRNA and protein expression levels were much higher both in BL and LDM of Y compared to J or YJ (Fig. 1E, F). These results indicate that higher m5C levels in mRNA may be associated with lower fat mass, and higher muscle mass in pigs, due to intrinsic genetic, or epigenetic differences between the pig breeds.
Distribution of mRNA m5C in BL and LDM from three pig breeds
For a comprehensive overview of a transcriptome-wide landscape of m5C profiles at single-base pair resolution, we performed an improved RNA bisulfite sequencing (RNA-BisSeq) analysis on RNA samples purified from adipose tissue (backfat layer, BL) and skeletal muscle (longissimus dorsi muscles, LDM) of three pig breeds as previously described [8]. To ensure the efficiency of bisulfite-mediated C to T conversion, the threshold for global conversion rate (C–T) was set to > 99% using Dhfr control (Table S4). The results were highly reproducible between independent replicates (Fig. S1A–D). Thus, our approach was considered successful for identifying m5C sites in transcriptomes.
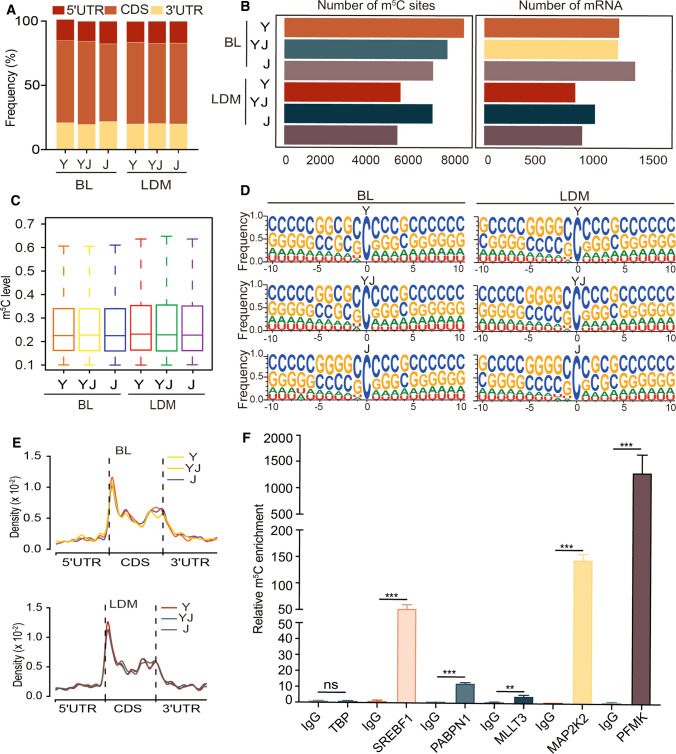
The m5C distribution of mRNA in distinct regions (5′ untranslated region, 5′UTR; coding sequences, CDS; 3′ untranslated region, 3′UTR) showed that the majority of m5C sites was found to be in CDS ranging from 45 to 60% in BL and LDM of Y, YJ, and J (Fig. 2A). A total of 8 384, 7 615, and 6 950 m5C sites within 1 127, 1 118, 1 261 mRNAs were identified in BL from Y, YJ and J, respectively (Fig. 2B, Table S5). A total of 5 430, 6 925, and 5 287 m5C sites within 761, 926, 819 mRNAs were identified in LDM from Y, YJ and J, respectively (Fig. 2B, Table S5). Notably, the median m5C level of mRNA was about 20% in all samples (Fig. 2C). Interestingly, sequence frequency logo analysis demonstrated that the majority of mRNA m5C sites were in CG-rich environments (Fig. 2D). Next, we determined the distribution pattern of m5C sites along mRNA transcripts, and m5C sites showed an enrichment in CDS regions immediately downstream of the translation initiation sites (Fig. 2E). These results are consistent to recently published studies from humans and mice [8], suggesting that highly conserved features not only exist in multiple species, but are observed in pig adipose and muscle tissues. To further validate our results from the RNA-BisSeq analysis, five m5C-modified genes were randomly selected to perform m5C meRIP-qPCR. We observed a significant m5C enrichment of all the five candidates over the IgG control (Fig. 2F).
Fig. 2.
Distribution of mRNA m5C in BL and LDM from three different pig breeds. A Transcriptome-wide distribution of mRNA m5C sites. The bar chart shows the percentages of m5C sites within distinct mRNA regions: the 5’UTR, the CDS and the 3’UTR. B Bar charts showing the numbers of m5C sites (left) and m5C-modified mRNAs (right) in BL and LDM from Y, YJ and J. C Boxplots showing the overall distributions of mRNA m5C levels in BL and LDM from Y, YJ and J. D Sequence frequency logo for the sequences proximal to mRNA m5C sites. E The m5C distributions pattern within mRNA in different regions. F m5C-RIP-qPCR analysis of representative m5C-modified genes. TBP served as an unmodified negative control. Error bars, means ± S.D., n = 3. The P values were determined using Student’s t tests. *P < 0.05 **P < 0.01, ***P < 0.001
Taken together, using the stringent m5C calling parameters and the alternative method, our RNA-BisSeq results allow a reliable picture of the mRNA m5C epi-transcriptome in adipose tissue and skeletal muscles of the three pig breeds.
Hypermethylated m5C in B Land LDM strongly relate to fatness/growth-related phenotypes
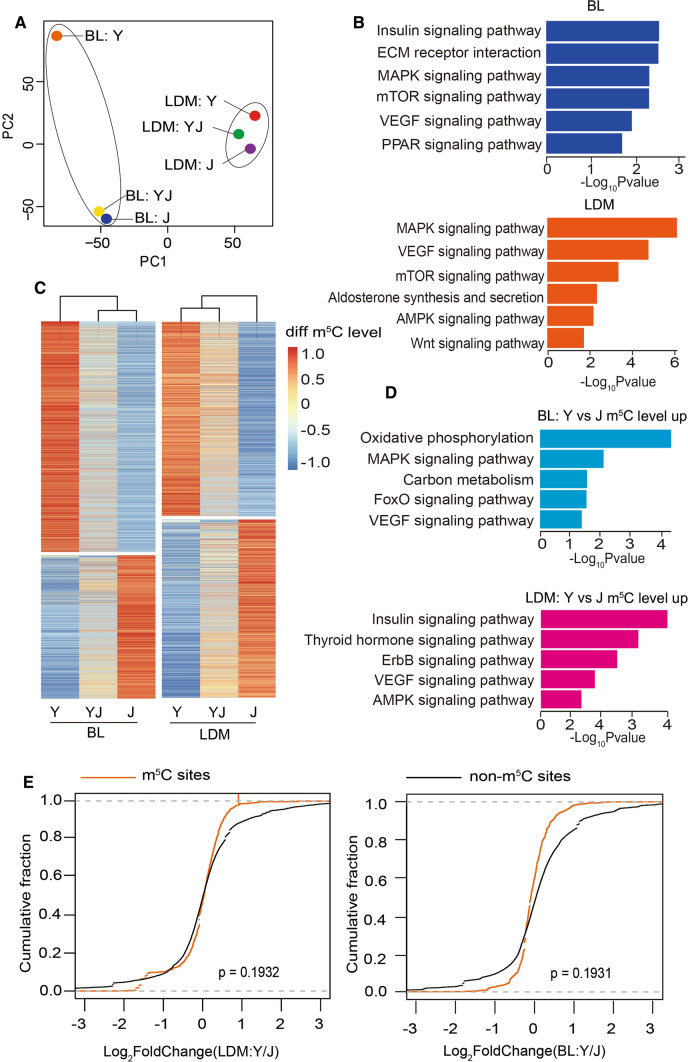
To explore the relationship between m5C and fatness/growth-related phenotypes between breeds, we performed a principal component analysis (PCA) based on all m5C sites identified by RNA-BisSeq. The result suggest that the samples were clustered into the BL and LDM group, indicating the potentially specific roles of m5C in different tissues (Fig. 3A). Next, we combined all m5C-modified genes in BL or LDM from the three breeds and performed KEGG pathway enrichment analysis. The m5C-containing genes in BL were clearly involved in the lipid metabolism-related pathways during adipogenesis, such as insulin signaling, ECM receptor, MAPK signaling, mTOR signaling, VEGF signaling and PPAR (Fig. 3B). In LDM, m5C-containing genes were involved in MAPK signaling, VEGF receptor, and mTOR signaling, Aldosterone synthesis and secretion, AMPK signaling and the Wnt signaling pathway (Fig. 3B), which are closely linked to myogenesis. These results suggest that m5C may play an important role for accounting for phenotype differences in BL or LDM between the three breeds pigs. As significant phenotypic differences are observed between Y and J, the significantly different m5C sites were screened in BL or LDM from Y and J and were subjected to hierarchical cluster analysis. The m5C site levels in BL or LDM showed big differences between Y and J (Fig. 3C). More importantly, the phenotypic parameters of YJ ranked intermediate among the three pig breeds (Fig. 1B, C), and the m5C site levels in BL or LDM from YJ were also intermediate (Fig. 3C), implying that these different m5C sites were strongly related to fatness/growth-related phenotypes.
Fig. 3.
Hypermethylated m5C in BL and LDM strongly relate to fatness/growth-related phenotypes. A PCA analysis of m5C in BL and LDM from Y and YJ. B Bar plot showing the result of KEGG pathway analysis of m5C-modified mRNAs in BL and LDM (all m5C-modified mRNAs combined from Y, YJ and J). C Heatmap and cluster analysis of differentially m5C-modified sites (|Y-J|> 0.05) in BL and LDM from Y, YJ, and J. D Bar plot showing the result of KEGG pathway analysis of mRNAs with hypermethylation in BL and LDM of Y compared with J. E Cumulative distribution analysis of the expression level changes in mRNAs with or without m5C modification
Next, we explored the underlying function of different m5C sites in BL or LDM. Since total mRNA m5C levels in BL or LDM were significantly higher in Y compared with J (Fig. 1D), genes with higher m5C site levels in Y than J were selected and subjected to KEGG analysis. We observed that hypermethylated genes in BL and LDM were significantly enriched in signaling pathways linked to impaired adipogenesis and enhanced myogenesis (Fig. 3D), indicating that m5C negatively can regulate fat deposition and positively affects muscle differentiation. Besides, hypomethylated genes in BL and LDM were related to pathways, such as metabolic pathways and mTOR signaling (Fig. S1E), indicating that mRNA m5C may play an important role in metabolic regulation. To identify the function of m5C in our study, we performed RNA-seq of BL and LDM in the three breeds (Table S6). When comparing RNA abundance between m5C-modified and non-m5C-modified mRNAs in BL and LDM between Y and J, no significant correlation between mRNA abundance and m5C levels was observed (Fig. 3E), suggesting that m5C may not influenced adipose and muscle development by maintaining mRNAs expression.
Taken together, our hypothesis that m5C contributes to the fatness/growth-related phenotype in pigs, suggests that it has an important mechanistic role in adipogenesis and myogenesis.
m5C inhibits adipogenesis in PFs and promotes myogenesis in SCs
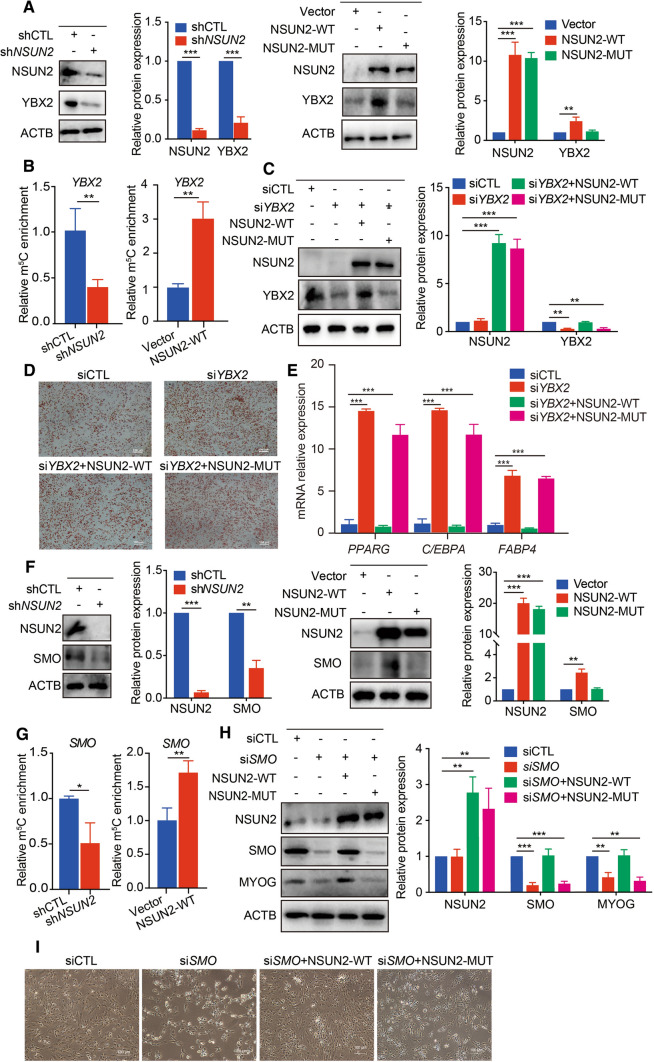
Since NSUN2 was identified as the main mRNA m5C methyltransferase in many species [27], NSUN2 was used to manipulate the m5C level in our study. Expectedly, knockdown of NSUN2 significantly decreased the m5C level (Fig. S2A). Next, we constructed wild-type and mutant NSUN2 (C274A/C324A) adenovirus plasmids (NSUN2-WT, NSUN2-MUT) by changing the m5C methyltransferase activity based on published work [8]. HPLC–MS/MS results show that overexpressing NSUN2-WT could significantly increase mRNA m5C levels, but overexpressing NSUN2-MUT did not affect mRNA m5C levels (Fig. S2B).
To explore the role of m5C in regulating adipogenesis, PFs were isolated from BL. In PFs, decreasing m5C levels by silencing NSUN2 significantly enhanced lipid accumulation and mRNA expression of adipogenic transcription factors including peroxisome proliferator activated receptor gamma (PPARG), fatty acid binding protein 4 (FABP4) and CCAAT/enhancer binding protein alpha (C/EBPA) (Fig. 4A–C). Increasing m5C levels by overexpressing of NSUN2-WT (rather than NSUN2-MUT) impaired PFs adipogenic differentiation and inhibited expression of PPARG, FABP4 and C/EBPA (Fig. 4D–F). Porcine muscle satellite cells (SCs) were isolated from LDM to investigate the role of m5C in regulating myogenesis. In SCs, decreasing m5C levels by silencing NSUN2 impaired protein expression of myosin heavy chain 1 (MYH1) and the mRNA expression of the myogenic differentiation marker gene creatine kinase M-type (CKM) (Fig. 4G–I). Expectedly, only increasing m5C levels by overexpressing NSUN2-WT rather than NSUN2-MUT could promote myogenesis in SCs (Fig. 4J–L), indicating that m5C triggers myogenesis. Thus, m5C blocked adipogenesis and up- regulated myogenesis.
Fig. 4.
m5C inhibited adipogenesis in PFs and promoted myogenesis in SCs. A Western blot analysis in porcine subcutaneous fat preadipocytes (PFs) transfected with shCTL and shNSUN2 adenovirus. ACTB was used as a protein loading control. The relative protein expression was quantified by densitometry and normalized to ACTB. All data are shown as means ± S.D. B Oil Red O staining analysis in porcine subcutaneous fat preadipocytes (PFs) transfected with shCTL and shNSUN2 adenovirus. Scale bar: 100 μm. C qRT-PCR analysis in porcine subcutaneous fat preadipocytes (PFs) transfected with shCTL and shNSUN2 adenovirus. 18S rRNA served as an internal RNA control. Error bars represent means ± SD; n = 3. D Western blotting analysis in PFs transfected with control (Vector), NSUN2-WT and NSUN2-MUT adenovirus. ACTB was used as a protein loading control. The relative protein expression was quantified by densitometry and normalized to ACTB. All data are shown as means ± S.D. E Oil Red O staining analysis in PFs transfected with control (Vector), NSUN2-WT and NSUN2-MUT adenovirus. Scale bar: 100 μm. F qRT-PCR analysis in PFs transfected with control (Vector), NSUN2-WT and NSUN2-MUT adenovirus. 18S rRNA served as an internal RNA control. Error bars represent means ± SD; n = 3. G Western blotting analysis in porcine muscle satellite cells (SCs) transfected with shCTL or shNSUN2. ACTB was used as a protein loading control. The relative protein expression was quantified by densitometry and normalized to ACTB. All data are shown as means ± S.D. H Phase images analysis in porcine muscle satellite cells (SCs) transfected with shCTL or shNSUN2. Scale bar: 100 μm. I qRT-PCR analysis in porcine muscle satellite cells (SCs) transfected with shCTL or shNSUN2. 18S rRNA served as an internal RNA control. Error bars represent means ± SD; n = 3. J Western blotting analysis in SCs transfected with control (Vector), NSUN2-WT and NSUN2-MUT adenovirus. ACTB was used as a protein loading control. The relative protein expression was quantified by densitometry and normalized to ACTB. All data are shown as means ± S.D. K Phase images analysis in SCs transfected with control (Vector), NSUN2-WT and NSUN2-MUT adenovirus. Scale bar: 100 μm. L qRT-PCR analysis in SCs transfected with control (Vector), NSUN2-WT and NSUN2-MUT adenovirus. 18S rRNA served as an internal RNA control. Error bars represent means ± SD; n = 3. The p values were determined using Student’s t tests. *P < 0.05 **P < 0.01, ***P < 0.001
YBX2 and SMO are the potential m5C targets for the regulation of adipogenesis and myogenesis
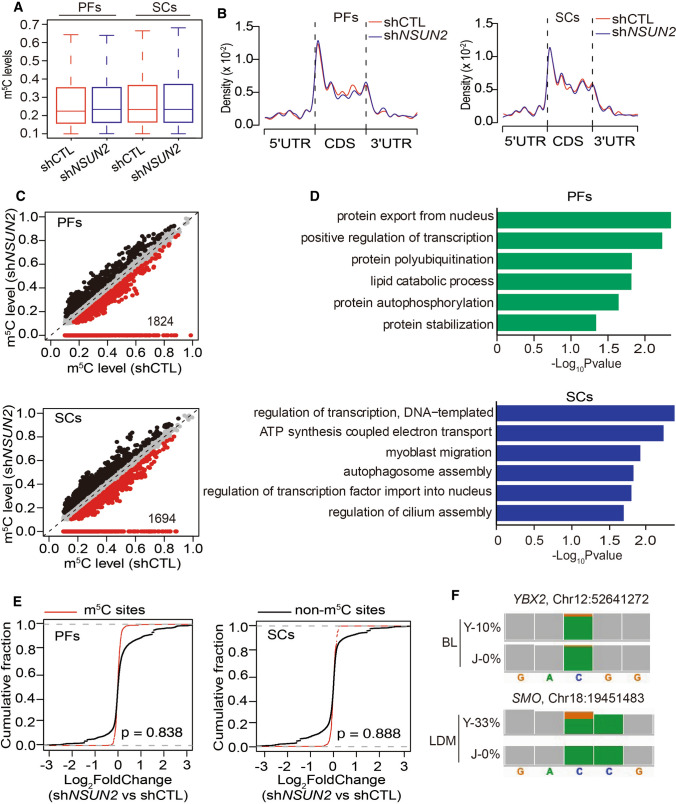
To uncover the underlying mechanism of m5C in adipogenesis and myogenesis, RNA-BisSeq and RNA-seq were conducted in PFs and SCs with or without knocking down of NSUN2. Only candidate m5C sites present in two replicates were used for further analyses (Fig. S3A, B, Table S7, 8). It is worth noting that median m5C levels (Fig. 5A) and distribution patterns of m5C sites in PFs and SCs (Fig. 5B) were similar in BL or LDM (Fig. 2C, E). When we identified 1824 and 1694 m5C sites with methylation levels reduction upon NSUN2 knockdown in PFs and SCs (Fig. 5C), we observed almost the same result as in humans[8], suggesting that knockdown of NSUN2 could significantly reduce the mRNA m5C level in PFs or SCs.
Fig. 5.
YBX2 and SMO are the potential m5C targets for the regulation of adipogenesis and myogenesis. A Boxplots showing the overall distributions of mRNA m5C levels in PFs and SCs with or without NSUN2 knockdown. B Transcriptome-wide distribution pattern of mRNA m5C sites within distinct mRNA regions (5’UTR, CDS and 3’UTR) in PFs and SCs with or without NSUN2 knockdown. C Scatter plot showing m5C levels in PFs and SCs with or without NSUN2 knockdown. For m5C sites in shCTL PFs, sites with increased or reduced methylation levels depending on NSUN2 knockdown highlighted in black or red color. D Bar plot showing the result of GO analysis of mRNAs with differentially expressed m5C sites in PFs and SCs with or without NSUN2 knockdown. E Cumulative distribution analysis of the expression level changes in mRNAs with or without m5C modification in PFs and SCs. F Genome browser view of differentially methylated genes, YBX2 and SMO, in BL or LDM between Y and J by Integrative Genomics Viewer (IGV). The orange boxes indicate methylated cytosine. The green boxes indicate non-methylated cytosine. The proportion of orange in column represents m5C level in YBX2 or SMO
Next, enrichment analysis was performed to illustrate the potential function of m5C in NSUN2-knocked-down cells. In PFs with NSUN2 knockdown, the differentially m5C-modified genes were significantly enriched in lipid catabolic processes which negatively correlated with adipogenesis (Fig. 5D). The differentially m5C-modified genes in NSUN2-knocked-down SCs were linked to myoblast migration which was essential for myogenesis (Fig. 5D). Interestingly, consistent with the tissue findings outlined above (Fig. 3E), depending on silencing of NSUN2, no significant RNA abundance changes were found between m5C-modified and non-m5C-modified mRNAs in PFs and SCs (Fig. 5E, Tables S9, S10), suggesting that m5C indeed regulated adipogenesis and myogenesis not by stabilizing mRNAs. Subsequently, to figure out potential m5C targets for the regulation of adipogenesis and myogenesis, we combined the RNA-BisSeq results from both tissues and cell data. YBX2 and SMO were then ruled out as potential m5C targets for the regulation of adipogenesis and myogenesis. The methylation level of YBX2 and SMO was higher in BL and LDM of Y compared with J (Fig. 5F).
Together, our results suggest that m5C regulation during inhibition of adipogenesis and promotion of myogenesis may occur through controlling lipid catabolism and myoblast migration via YBX2 and SMO.
YBX2 and SMO inhibit adipogenesis and promote myogenesis in m5C-dependent manner
YBX2 belongs to the YBX family which is mainly involved in RNA binding, transcription and translation, and was previously reported to be required for cold-induced brown adipose tissue activation, which strongly relates to lipid catabolism [28]. It is worth noting that the protein levels of YBX2 decreased when NSUN2 was inhibited, and overexpression of NSUN2-WT rather than NSUN2-MUT could promote the expression of YBX2 (Fig. 6A). Besides, meRIP-qPCR results showed that silencing NSUN2 significantly decreased m5C level of YBX2 mRNA, while overexpression of NSUN2 increased the m5C level of YBX2 mRNA (Fig. 6B), suggesting that YBX2 was indeed affected in an m5C-dependent manner. Then, YBX2 was blocked using YBX2 siRNA during adipogenesis. We observed an aggravated lipid accumulation and increased mRNA expression of PPARG, C/EBPA and FABP4 in YBX2 knockdown PFs (Fig. 6C–E and Fig. S4A), which was consistent with the results in NSUN2-silenced PFs (Fig. 4A, B). More importantly, the enhanced adipogenesis in YBX2 knocked-down PFs could be reversed by transfection with NSUN2-WT rather than NSUN2-MUT (Fig. 6C–E and Fig. S4A). These findings demonstrate that m5C inhibited lipid accumulation by targeting YBX2.
Fig. 6.
YBX2 and SMO inhibit adipogenesis and promote myogenesis in m5C-dependent manner. A Western blotting of NSUN2 and YBX2 expression in PFs transfected with shCTL, shNSUN2, vector, NSUN2-WT or NSUN2-MUT adenovirus. ACTB was used as a loading control. The relative protein expression was quantified by densitometry and normalized to ACTB. All data are shown as means ± S.D. B m5C-RIP-qPCR analysis of YBX2 in PFs transfected with shCTL, shNSUN2, Vector or NSUN2-WT adenovirus. Error bars, means ± S.D., n = 3. C Western blotting analysis in control or YBX2 knockdown PFs transfected with NSUN2-WT or NSUN2-MUT adenovirus. ACTB was used as a protein loading control. The relative protein expression was quantified by densitometry and normalized to ACTB. All data are shown as means ± S.D. D Oil Red O staining analysis in control or YBX2 knockdown PFs transfected with NSUN2- WT or NSUN2-MUT adenovirus. Scale bar: 100 μm. E qPCR analysis in control or YBX2 knockdown PFs transfected with NSUN2-WT or NSUN2-MUT adenovirus. 18S rRNA served as an internal RNA control. Error bars represent means ± SD; n = 3. F Western blotting of NSUN2 and SMO expression in SCs transfected with shCTL, shNSUN2, Vector, NSUN2-WT or NUSN2-MUT adenovirus. ACTB was used as a loading control. The relative protein expression was quantified by densitometry and normalized to ACTB. All data are shown as means ± S.D. G m5C-RIP-qPCR analysis of SMO in SCs transfected with shCTL, shNSUN2, Vector or NSUN2-WT adenovirus. Error bars, means ± S.D., n = 3. H Western blotting in control or SMO knockdown SCs transfected with NSUN2-WT or NSUN2-MUT adenovirus. ACTB was used as a protein loading control. The relative protein expression was quantified by densitometry and normalized to ACTB. All data are shown as means ± S.D. I Phase image in control or SMO knockdown SCs transfected with NSUN2-WT or NSUN2-MUT adenovirus. Scale bar: 100 μm. The P values were determined using Student’s t tests. *P < 0.05 **P < 0.01, ***P < 0.001
As a G protein-coupled receptor, SMO reportedly participates in myoblast migration and skeletal muscle development [29]. Deletion of NSUN2 blocked protein expression of SMO in SCs, while only overexpression of NSUN2-WT rather than NSUN2-MUT increased the SMO protein level (Fig. 6F). In addition, meRIP-qPCR showed that SMO mRNA could be dynamically methylated by silencing or overexpressing NSUN2 (Fig. 6G), suggesting that m5C was required for the regulation of SMO expression. To investigate the role of the candidate gene for myogenesis, we knocked down SMO and observed decreased expression of myogenin (MYOG) and a sharp decline in cell number (Fig. 6H, I and Fig. S4B). More importantly, the blocked expression of MYOG and myogenesis in SMO knocked-down SCs could be rescued by upregulating NSUN2-WT rather than NSUN2-MUT (Fig. 6H, I and Fig. S4B). Thus, m5C promoted myogenesis through targeting SMO.
The role of YBX2 and SMO was mediated by ALYREF in a m5C-dependent manner
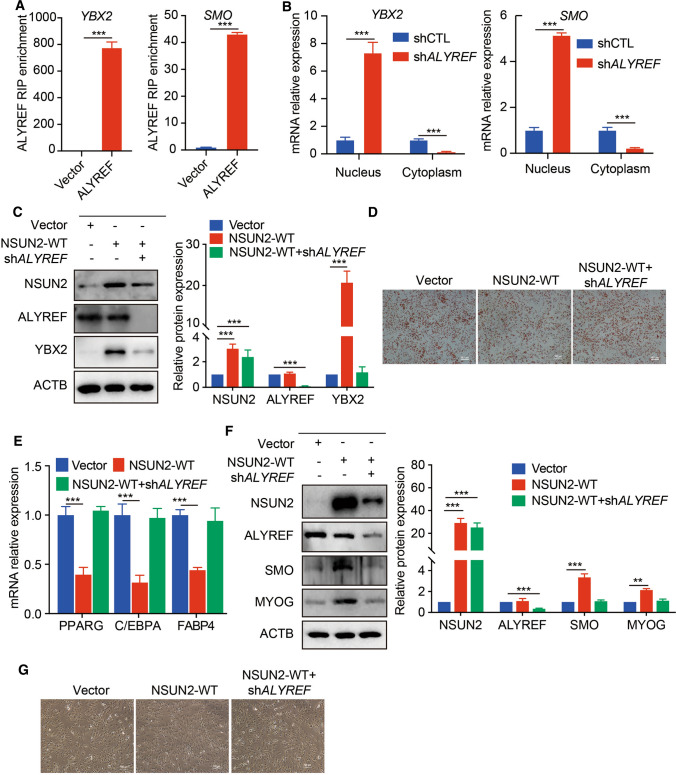
Generally, m5C need to be recognized by its readers, Y-box-binding protein 1 (YBX1) or ALYREF, to exert a specific role for regulating mRNA stability and mRNA export [8, 14]. As the m5C modification did not affect mRNA levels of YBX2 and SMO, we examined whether ALYREF was the potential reader in controlling adipogenesis and myogenesis. To test this hypothesis, we performed RIP-qPCR analysis around the m5C sites and observed a significant enrichment of YBX2 and SMO in ALYREF-flag overexpressing cells (Fig. 7A). ALYREF could directly bind to YBX2 and SMO mRNA by recognizing m5C modification. Next, nucleus and cytoplasm RNAs were isolated to determine YBX2/SMO mRNAs subcellular location mediated by ALYREF. qPCR analysis revealed that the majority of mRNAs of YBX2 or SMO were accumulated in the nucleus when ALYREF was inhibited (Fig. 7B), suggesting that ALYREF served indeed as reader for the binding to YBX2 and SMO to regulate mRNA export. Functionally, increased YBX2 expression, decreased mRNA expression of adipogenic differentiation marker genes and reduced lipid accumulation in NSUN2-WT overexpressing PFs could be rescued by silencing of ALYREF (Fig. 7C–E and Fig. S4C). Knockdown of ALYREF could reverse the augmented expression of SMO and MYOG and reinforcement of myogenesis when NSUN2-WT was overexpressed in SCs (Fig. 7F, G and Fig. S4D). Taken together, m5C inhibited adipogenesis and promoted myogenesis through ALYREF-m5C-YBX2 and ALYREF-m5C-SMO.
Fig. 7.
The role of YBX2 and SMO was mediated by ALYREF in a m5C-dependent manner. A RIP-qPCR analysis of YBX2 in PFs (left) and SCs (right) transfected with vector or ALYREF-Flag adenovirus. Error bars, means ± S.D., n = 3. B qPCR analysis of YBX2 and SMO mRNA in the nucleus or cytoplasm of control and ALYREF knockdown PF and SCs respectively. 18S rRNA served as an internal RNA control. Error bars represent means ± SD; n = 3. C Western blotting analysis in control or NSUN2-WT overexpressing PFs transfected with or without shALYREF adenovirus. ACTB was used as a protein loading control. The relative protein expression was quantified by densitometry and normalized to ACTB. All data are shown as means ± S.D. D Oil Red O staining analysis in control or NSUN2-WT overexpressing PFs transfected with or without shALYREF adenovirus. Scale bar: 100 μm. E qPCR analysis in control or NSUN2-WT overexpressing PFs transfected with or without shALYREF adenovirus. 18S rRNA served as an internal RNA control. Error bars represent means ± SD; n = 3. F Western blotting in control or NSUN2-WT overexpressing SCs transfected with or without shALYREF adenovirus. ACTB was used as a protein loading control. The relative protein expression was quantified by densitometry and normalized to ACTB. All data are shown as means ± S.D. G Phase image in control or NSUN2-WT overexpressing SCs transfected with or without shALYREF adenovirus. Scale bar: 100 μm. The P values were determined using Student’s t tests. *P < 0.05 **P < 0.01, ***P < 0.001
Discussion
Previous studies indicated that m5C is involved in various biological processes, including oxidative stress, adaptation to high temperatures, germ line stem cell development, maternal-to-zygotic transition and tumorigenesis, all by modulating mRNA subcellular localization, stability and translation [8, 11–14, 30, 31]. We found that m5C may play a critical role in tissue-specific functions including lipid metabolism and muscle development. We demonstrated that hypermethylated genes in BL and LDM were linked to reduced adipogenesis and enhanced myogenesis. NSUN2 was identified as the main m5C methyltransferase of mRNA in eukaryotes [8]. NSUN2-mediated m5C could control cell cycle progression via modulating cyclin-dependent kinase 1 (CDK1) and cyclin-dependent kinase inhibitor 1A (CDKN1A) translation in human cells [32, 33]. Moreover, reduction of m5C by depleting NSUN2 caused disturbed neuron differentiation and migration of neuroepithelial progenitors during brain development, resulting in Dubowitz-like syndrome [34]. Interestingly, it was demonstrated that the depletion of transcription factor MSX2 suppressed the transcription of NSUN2 [35]. In this study, we found that NSUN2 mRNA was much higher both in BL and LDM of Y compared to J or YJ, leading us to speculate that there may be some transcription factors that can regulate NSUN2 transcription in different pig breeds. In our study, we demonstrated that knockdown of NSUN2 could significantly reduce the mRNA m5C level in PFs or SCs. In addition to in vitro assays, we performed NSUN2 knockdown or overexpression assays to manipulate the mRNA m5C levels and we found that m5C inhibited adipogenesis and promoted myogenesis.
From the RNA-BisSeq data, we found that hypermethylated genes in BL and LDM were related to impaired adipogenesis and enhanced myogenesis. It was worth noting that the methylation level of YBX2 and SMO was higher in BL and LDM of Y compared with J, which was consistent with the RNA-BisSeq data from tissues, promoting us to explore the function of these two gene in pigs. It was observed that YBX2 is involved in the biological process of tumorigenesis, including human testicular seminoma and ovarian asexual germ cell tumor and oral squamous cell carcinoma [36, 37]. Besides, YBX2 was found to be enriched in BAT to target and maintain PPAR GC1A RNA stability during cold-induced brown fat activation [28]. In our study, we found that NSUN2-mediated m5C inhibition of adipogenesis by increasing YBX2 protein expression. However, whether YBX2 could regulate mitochondrial function and lipolysis via stabilizing PPARGC1A or other mRNAs will have to be answered in future experiments. As key regulator for fate determination of embryonic stem cells, Hedgehog signaling activation was frequently observed in development and tumorigenesis [38, 39]. SMO, the key component of Hedgehog signaling [40], was reported to promote myogenic proliferation and differentiation via the MAPK/ERK and PI3K/Akt pathway [29]. We observed restrained cell proliferation and differentiation of SCs during transfection with SMO siRNA. More importantly, the inhibitory effect in SMO knocked-down SCs could be rescued by overexpressing of NSUN2-WT, suggesting that the m5C-SMO-Hedgehog signaling axis may play a critical regulatory role during myogenesis or muscle development.
Similar to m6A, m5C readers, including ALYREF and YBX1, were required to recognize m5C modification in mRNA to exert its regulatory function. There is experimental evidence that ALYREF contributes to promoting mRNA export of m5c-containing mRNA [41]. Different from ALYREF, YBX1, a cytoplasmic mRNA m5C reader, regulates mRNA stability in the fruit fly, human and zebrafish [13, 14]. In our work, we could not confirm a significant difference in RNA abundance between m5C-modified and non-m5C-modified mRNA in vivo and in vitro. Presumably, m5C modification generally had no effect on mRNA stabilization in the development of adipose tissue and skeletal muscle. Rather, ALYREF was identified as m5C reader, regulating adipogenesis and myogenesis by promoting YBX2 and SMO mRNA export. In conclusion, we describe a novel mechanism of mRNA m5C-mediated inhibition of adipogenesis and improved myogenesis. YBX2 and SMO with higher m5C were recognized and exported to cytoplasm from nucleus by ALYREF leading to increased YBX2 and SMO protein expression, thereby inhibiting adipogenesis and promoting myogenesis respectively. Although pigs are very similar to humans, it is necessary to verify the function of mRNA m5C in human tissues. As we known, the RNA m5C machinery is complex, thus further investigation focusing on systematic study of the underlying regulatory network of m5C-modified genes would provide significant opportunity to get insight into the coordination between mRNA m5C metabolism and adipose tissue or skeletal muscle development. Our work provides a valuable resource for deciphering the function of mRNA m5C modification in the emergence of fatness and diseases relating to skeletal muscle dysfunction.
Conclusion
We discovered a novel mechanism of mRNA m5C-mediated inhibition of adipogenesis and advancement of myogenesis. YBX2 and SMO with higher m5C are recognized and exported to the cytoplasm from the nucleus by ALYREF leading to increased YBX2 and SMO protein expression, thereby inhibiting adipogenesis and promoting myogenesis respectively.
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. S1 Transcriptome-wide detection of m5C sites in adipose tissue and skeletal muscle tissue. A Venn diagrams showing the overlap of methylated sites between the two replicates in BL from Y, YJ and J. B Venn diagrams showing the overlap of methylated sites between the two replicates in LDM from Y, YJ and J. C Scatter plots illustrating the methylation levels of the two replicates in BL from Y, YJ and J. Pearson’s correlation coefficients (R) are shown. D Scatter plots illustrating the methylation levels of the two replicates in LDM from Y, YJ and J. Pearson correlation coefficients (R) are shown. E Bar plot showing the results of KEGG pathway analysis of mRNAs with hypomethylated in BL and LDM of Y compared with J (JPEG 1554 KB)
Fig. S2 m5C inhibits adipogenesis in PFs and promotes myogenesis in SCs. A Dot blot was used to detect the m5C modification after knockdown of NSUN2. Methylene blue staining was used as a loading control (n = 3). B HPLC–MS/MS quantification of the m5C/C in mRNA of IPEC-J2 cells transfected with control (Vector), NSUN2-WT and NSUN2-MUT adenovirus. Error bars, means ± S.D., n = 3. The P values were determined using Student’s t tests. *P < 0.05 **P < 0.01, ***P < 0.001. (JPEG 708 KB)
Fig. S3 Knockdown of NSUN2 could significantly reduce the mRNA m5C level. A Scatter plots illustrating the methylation levels of the two replicates in PFs and SCs with or without NSUN2 knockdown. Pearson correlation coefficients (R) are shown. B Venn diagrams showing the overlap of methylated sites between the two replicates in PFs and SCs with or without NSUN2 knockdown. (JPEG 1196 KB)
Fig. S4 The quantitative data of cell number and oil red staining. A Relative lipid accumulation was quantified-based oil red staining results in Fig. 6D. All data are shown as means ± S.D. B Relative cell number was quantified-based phase images in Fig. 6I. All data are shown as means ± S.D. C Relative lipid accumulation was quantified-based oil red staining results in Fig. 7D. All data are shown as means ± S.D. D Relative cell number was quantified-based phase images in Fig. 7G. All data are shown as means ± S.D. The P values were determined using Student’s t tests. *P < 0.05 **P < 0.01, ***P < 0.001. (JPEG 1152 KB)
Acknowledgements
We would like to thank the reviewers for their constructive comments.
Author contributions
YHL, YY, RFW, XL, BTZ, CQH, YJL, YXL, YSC, WC, QJL, QJ, YLZ, ZB, GQG and YXY performed experiments under the supervision of XXW. YHL, CCG and XH performed bioinformatics analysis of sequence data. YHL wrote the manuscript under the supervision of XXW. YX, XJZ, YZW and XXW designed the project and XXW provided the final approval of the manuscript. Teresa G. Valencak reorganized the writing and figure layout, and revised the typo errors and grammar mistakes throughout our manuscript.
Funding
This work is supported by the Natural Science Foundation of Zhejiang Province (LZ22C170002); the National Natural Science Foundation of China (U21A20249); the Fundamental Research Funds for the Central Universities (2019XZZX003-13); the National Key R & D Program (2018YFD0500400); the National Natural Science Foundation of China (31572413); the Natural Science Foundation of Zhejiang Province (LZ17C170001).
Availability of data and material
All data generated or analyzed during this study are included in this article. The datasets used during the current study have been deposited in the Genome Sequence Archive under the accession number CRA005591 linked to the project PRJCA007533.
Code availability
The authors declare that the codes for data analyses from this study are available from the corresponding authors on reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
Sample collection in this study was performed according to the guidelines for the care and use of experimental animals established by the Ministry of Agriculture of China. All procedures were approved by the Committee on Animal Care and Use and Committee on the Ethics of Animal Experiments of Zhejiang University (ZJU2015-458-09).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goodpaster BH, Sparks LM. Metabolic flexibility in health and disease. Cell Metab. 2017;5(25):1027–1036. doi: 10.1016/j.cmet.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ukropec J, Ukropcova B, Kurdiova T, Gasperikova D, Klimes I. Adipose tissue and skeletal muscle plasticity modulates metabolic health. Arch Physiol Biochem. 2008;5(114):357–368. doi: 10.1080/13813450802535812. [DOI] [PubMed] [Google Scholar]
- 3.Tidball JG. Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol. 2017;3(17):165–178. doi: 10.1038/nri.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang S, Yang G, Wu H, Kang L, Xiang J, Zheng P, et al. MicroRNA-193b impairs muscle growth in mouse models of type 2 diabetes by targeting the PDK1/Akt signalling pathway. Diabetologia. 2021 doi: 10.1007/s00125-021-05616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;4(98):2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;1(18):31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu R, Wang X. Epigenetic regulation of adipose tissue expansion and adipogenesis by N(6) -methyladenosine. Obesity Rev. 2020 doi: 10.1111/obr.13124. [DOI] [PubMed] [Google Scholar]
- 8.Yang X, Yang Y, Sun BF, Chen YS, Xu JW, Lai WY, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. 2017;5(27):606–625. doi: 10.1038/cr.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu RJ, Long T, Li J, Li H, Wang ED. Structural basis for substrate binding and catalytic mechanism of a human RNA:m5C methyltransferase NSun6. Nucleic Acids Res. 2017;11(45):6684–6697. doi: 10.1093/nar/gkx473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selmi T, Hussain S, Dietmann S, Heiss M, Borland K, Flad S, et al. Sequence- and structure-specific cytosine-5 mRNA methylation by NSUN6. Nucleic Acids Res. 2021;2(49):1006–1022. doi: 10.1093/nar/gkaa1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Y, Gao CC, Gao Y, Yang Y, Shi B, Yu JL, et al. OsNSUN2-mediated 5-methylcytosine mRNA modification enhances rice adaptation to high temperature. Dev Cell. 2020;3(53):272–286. doi: 10.1016/j.devcel.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Zou F, Tu R, Duan B, Yang Z, Ping Z, Song X, et al. Drosophila YBX1 homolog YPS promotes ovarian germ line stem cell development by preferentially recognizing 5-methylcytosine RNAs. Proc Natl Acad Sci USA. 2020;7(117):3603–3609. doi: 10.1073/pnas.1910862117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Li A, Sun BF, Yang Y, Han YN, Yuan X, et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat Cell Biol. 2019;8(21):978–990. doi: 10.1038/s41556-019-0361-y. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Wang L, Han X, Yang WL, Zhang M, Ma HL, et al. RNA 5-methylcytosine facilitates the maternal-to-zygotic transition by preventing maternal mRNA decay. Mol Cell. 2019;6(75):1188–1202. doi: 10.1016/j.molcel.2019.06.033. [DOI] [PubMed] [Google Scholar]
- 15.Van Haute L, Lee SY, McCann BJ, Powell CA, Bansal D, Vasiliauskaite L, et al. NSUN2 introduces 5-methylcytosines in mammalian mitochondrial tRNAs. Nucleic Acids Res. 2019;16(47):8720–8733. doi: 10.1093/nar/gkz559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moretti A, Fonteyne L, Giesert F, Hoppmann P, Meier AB, Bozoglu T, et al. Somatic gene editing ameliorates skeletal and cardiac muscle failure in pig and human models of Duchenne muscular dystrophy. Nat Med. 2020;2(26):207–214. doi: 10.1038/s41591-019-0738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koopmans SJ, Schuurman T. Considerations on pig models for appetite, metabolic syndrome and obese type 2 diabetes: from food intake to metabolic disease. Eur J Pharmacol. 2015;759:231–239. doi: 10.1016/j.ejphar.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 18.Miao Z, Wang S, Wang Y, Wei P, Khan MA, Zhang J, et al. Comparison of microRNAs in the intramuscular adipose tissue from Jinhua and Landrace pigs. J Cell Biochem. 2019;1(120):192–200. doi: 10.1002/jcb.27298. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Sun B, Jiang Q, Wu R, Cai M, Yao Y, et al. mRNA m(6)A plays opposite role in regulating UCP2 and PNPLA2 protein expression in adipocytes. Int J Obes (Lond) 2018;11(42):1912–1924. doi: 10.1038/s41366-018-0027-z. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Q, Sun B, Liu Q, Cai M, Wu R, Wang F, et al. MTCH2 promotes adipogenesis in intramuscular preadipocytes via an m(6)A-YTHDF1-dependent mechanism. FASEB J. 2019;2(33):2971–2981. doi: 10.1096/fj.201801393RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Xiao J, Liu X, Gao Y, Luo Z, Gu X, et al. Tandem mass tag-labeled quantitative proteomic analysis of tenderloins between Tibetan and Yorkshire pigs. Meat Sci. 2021;172:108343. doi: 10.1016/j.meatsci.2020.108343. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Chen Q, Xu L, Cai J, Zhang J. The potential role of PSMA6 in modulating fat deposition in pigs by promoting preadipocyte proliferation and differentiation. Gene. 2021;769:145228. doi: 10.1016/j.gene.2020.145228. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Zhao W, Li Q, Huang Z, Shi G, Li C. Long Non-coding RNA H19 promotes porcine satellite cell differentiation by interacting with TDP43. Genes (Basel) 2020 doi: 10.3390/genes11030259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding S, Wang F, Liu Y, Li S, Zhou G, Hu P. Characterization and isolation of highly purified porcine satellite cells. Cell Death Discov. 2017;3:17003. doi: 10.1038/cddiscovery.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;15(30):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;4(14):R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YS, Yang WL, Zhao YL, Yang YG. Dynamic transcriptomic m(5) C and its regulatory role in RNA processing. Wiley Interdiscip Rev RNA. 2021;4(12):e1639. doi: 10.1002/wrna.1639. [DOI] [PubMed] [Google Scholar]
- 28.Xu D, Xu S, Kyaw AMM, Lim YC, Chia SY, Chee Siang DT, et al. RNA binding protein Ybx2 regulates RNA stability during cold-induced brown fat activation. Diabetes. 2017;12(66):2987–3000. doi: 10.2337/db17-0655. [DOI] [PubMed] [Google Scholar]
- 29.Madhala-Levy D, Williams VC, Hughes SM, Reshef R, Halevy O. Cooperation between Shh and IGF-I in promoting myogenic proliferation and differentiation via the MAPK/ERK and PI3K/Akt pathways requires Smo activity. J Cell Physiol. 2012;4(227):1455–1464. doi: 10.1002/jcp.22861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.David R, Burgess A, Parker B, Li J, Pulsford K, Sibbritt T, et al. Transcriptome-wide mapping of RNA 5-methylcytosine in Arabidopsis mRNAs and noncoding RNAs. Plant Cell. 2017;3(29):445–460. doi: 10.1105/tpc.16.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amort T, Rieder D, Wille A, Khokhlova-Cubberley D, Riml C, Trixl L, et al. Distinct 5-methylcytosine profiles in poly(A) RNA from mouse embryonic stem cells and brain. Genome Biol. 2017;1(18):1. doi: 10.1186/s13059-016-1139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xing J, Yi J, Cai X, Tang H, Liu Z, Zhang X, et al. NSun2 promotes cell growth via elevating cyclin-dependent kinase 1 translation. Mol Cell Biol. 2015;23(35):4043–4052. doi: 10.1128/MCB.00742-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Li X, Tang H, Jiang B, Dou Y, Gorospe M, et al. NSUN2-mediated m5C methylation and METTL3/METTL14-mediated m6A methylation cooperatively enhance p21 translation. J Cell Biochem. 2017;9(118):2587–2598. doi: 10.1002/jcb.25957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flores JV, Cordero-Espinoza L, Oeztuerk-Winder F, Andersson-Rolf A, Selmi T, Blanco S, et al. Cytosine-5 RNA methylation regulates neural stem cell differentiation and motility. Stem Cell Reports. 2017;1(8):112–124. doi: 10.1016/j.stemcr.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He X, Yang Z, Chu XY, Li YX, Zhu B, Huang YX, et al. ROR2 downregulation activates the MSX2/NSUN2/p21 regulatory axis and promotes dental pulp stem cell senescence. Stem Cells. 2022;3(40):290–302. doi: 10.1093/stmcls/sxab024. [DOI] [PubMed] [Google Scholar]
- 36.Niu X, Yang B, Liu F, Fang Q. LncRNA HOXA11-AS promotes OSCC progression by sponging miR-98-5p to upregulate YBX2 expression. Biomed Pharmacother. 2020;121:109623. doi: 10.1016/j.biopha.2019.109623. [DOI] [PubMed] [Google Scholar]
- 37.Chen F, Liu M, Yu Y, Sun Y, Li J, Hu W, et al. LINC00958 regulated miR-627-5p/YBX2 axis to facilitate cell proliferation and migration in oral squamous cell carcinoma. Cancer Biol Ther. 2019;9(20):1270–1280. doi: 10.1080/15384047.2019.1617571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu X, Xiao S, Zhang M, Yang L, Zhong J, Li B, et al. A novel protein encoded by circular SMO RNA is essential for Hedgehog signaling activation and glioblastoma tumorigenicity. Genome Biol. 2021;1(22):33. doi: 10.1186/s13059-020-02250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao X, Tang JJ, Peng C, Wang Y, Fu L, Qiu ZP, et al. Cholesterol modification of smoothened is required for hedgehog signaling. Mol Cell. 2017;1(66):154–162. doi: 10.1016/j.molcel.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Yang C, Zheng X, Ye K, Sun Y, Lu Y, Fan Q, et al. Retraction notice to: miR-135a inhibits the invasion and migration of Esophageal cancer stem cells through the hedgehog signaling pathway by targeting Smo. Mol Ther Nucleic Acids. 2021;26:1198. doi: 10.1016/j.omtn.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eckwahl M, Xu R, Michalkiewicz J, Zhang W, Patel P, Cai Z, et al. 5-Methylcytosine RNA modifications promote retrovirus replication in an ALYREF reader protein-dependent manner. J Virol. 2020 doi: 10.1128/JVI.00544-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Transcriptome-wide detection of m5C sites in adipose tissue and skeletal muscle tissue. A Venn diagrams showing the overlap of methylated sites between the two replicates in BL from Y, YJ and J. B Venn diagrams showing the overlap of methylated sites between the two replicates in LDM from Y, YJ and J. C Scatter plots illustrating the methylation levels of the two replicates in BL from Y, YJ and J. Pearson’s correlation coefficients (R) are shown. D Scatter plots illustrating the methylation levels of the two replicates in LDM from Y, YJ and J. Pearson correlation coefficients (R) are shown. E Bar plot showing the results of KEGG pathway analysis of mRNAs with hypomethylated in BL and LDM of Y compared with J (JPEG 1554 KB)
Fig. S2 m5C inhibits adipogenesis in PFs and promotes myogenesis in SCs. A Dot blot was used to detect the m5C modification after knockdown of NSUN2. Methylene blue staining was used as a loading control (n = 3). B HPLC–MS/MS quantification of the m5C/C in mRNA of IPEC-J2 cells transfected with control (Vector), NSUN2-WT and NSUN2-MUT adenovirus. Error bars, means ± S.D., n = 3. The P values were determined using Student’s t tests. *P < 0.05 **P < 0.01, ***P < 0.001. (JPEG 708 KB)
Fig. S3 Knockdown of NSUN2 could significantly reduce the mRNA m5C level. A Scatter plots illustrating the methylation levels of the two replicates in PFs and SCs with or without NSUN2 knockdown. Pearson correlation coefficients (R) are shown. B Venn diagrams showing the overlap of methylated sites between the two replicates in PFs and SCs with or without NSUN2 knockdown. (JPEG 1196 KB)
Fig. S4 The quantitative data of cell number and oil red staining. A Relative lipid accumulation was quantified-based oil red staining results in Fig. 6D. All data are shown as means ± S.D. B Relative cell number was quantified-based phase images in Fig. 6I. All data are shown as means ± S.D. C Relative lipid accumulation was quantified-based oil red staining results in Fig. 7D. All data are shown as means ± S.D. D Relative cell number was quantified-based phase images in Fig. 7G. All data are shown as means ± S.D. The P values were determined using Student’s t tests. *P < 0.05 **P < 0.01, ***P < 0.001. (JPEG 1152 KB)
Data Availability Statement
All data generated or analyzed during this study are included in this article. The datasets used during the current study have been deposited in the Genome Sequence Archive under the accession number CRA005591 linked to the project PRJCA007533.
The authors declare that the codes for data analyses from this study are available from the corresponding authors on reasonable request.