Abstract
Vessel remodeling is essential for a functional and mature vascular network. According to the difference in endothelial cell (EC) behavior, we classified vessel remodeling into vessel pruning, vessel regression and vessel fusion. Vessel remodeling has been proven in various organs and species, such as the brain vasculature, subintestinal veins (SIVs), and caudal vein (CV) in zebrafish and yolk sac vessels, retina, and hyaloid vessels in mice. ECs and periendothelial cells (such as pericytes and astrocytes) contribute to vessel remodeling. EC junction remodeling and actin cytoskeleton dynamic rearrangement are indispensable for vessel pruning. More importantly, blood flow has a vital role in vessel remodeling. In recent studies, several mechanosensors, such as integrins, platelet endothelial cell adhesion molecule-1 (PECAM-1)/vascular endothelial cell (VE-cadherin)/vascular endothelial growth factor receptor 2 (VEGFR2) complex, and notch1, have been shown to contribute to mechanotransduction and vessel remodeling. In this review, we highlight the current knowledge of vessel remodeling in mouse and zebrafish models. We further underline the contribution of cellular behavior and periendothelial cells to vessel remodeling. Finally, we discuss the mechanosensory complex in ECs and the molecular mechanisms responsible for vessel remodeling.
Keywords: Zebrafish, EC rearrangement, Vessel pruning, Hemodynamics, Wnt signaling
Introduction
The vasculature is the earliest organ formed during embryonic development and provides nutrients for the development of other tissues. The functional vascular network is a closed circulating lumen composed of arteries, veins and capillaries that delivers oxygen, nutrients and hormones to tissues and organs and excretes metabolic wastes. In the early stage of embryonic development, the primary vascular plexus is formed by vasculogenesis and angiogenesis with increased vessel density [1]. This immature plexus is followed by complex vessel remodeling to form a tree-like vascular network through rearranging endothelial cells (ECs) [1]. The process of vasculogenesis, angiogenesis, and vessel remodeling to form a functional vascular network is called “angioadaptation” [2, 3]. At the same time, the vasculature always adjusts its morphology to meet the needs of the body at different developmental stages. In this review, we classified vessel remodeling into vessel pruning, vessel regression and vessel fusion based on the EC’s behaviors. Vessel pruning is defined here as the segment-by-segment reshaping of a vascular bed, characterized by loss of ECs and retention of type IV collagen (Col. IV) positive empty sleeves [4, 5]. The result of vessel pruning is that the break of two previously connected vessels and then merge into the existing adjacent vessels. Vessel regression is defined here as the complete involution of a vascular bed dependent on the programmed apoptosis of ECs [4, 5], such as the pupillary membrane (PM) and hyaloid vessels, which usually contributes to nourishing the development of retinal vascular beds. Vessel fusion is defined as two adjacent vessels that fuse into one, accompanied by rapid dilation of the vessel diameter [6]. In addition, vascular remodeling not only occurs in the process of angiogenesis but also exists in pathological conditions, such as pulmonary hypertension, diabetes and tumors.
In this review, we aim to summarize the vessel remodeling process in vertebrates, the role of ECs, pericytes and astrocytes in vessel remodeling, the effect of hemodynamics on vessel remodeling, and the mechanisms that regulate vessel remodeling.
The way of vessel remodeling
EC programmed apoptosis-mediates vessel regression
Different from vessel pruning, programmed vessel regression, such as the PM and hyaloid vessels of the developing eye, mainly refers to the loss of blood vessels of the entire network. The hyaloid capillary network is a transient vascular network that completely regresses during ocular development and is mediated by macrophage-dependent programmed EC apoptosis [7–10]. Lobov et al. found that the PM, tunica vasculosa lentis and hyaloid vessels are more abundant at P8 in PU.1 mutant mice, which lack macrophages, than in WT mice [7]. Endothelial conditional knockout of Bim (BimFlox/Flox, VE-cadherin-cre, BimEC), a proapoptotic Bcl-2 family member, decreases retinal vascular apoptosis and hyaloid vessel regeneration [11]. Periostin, secreted by intraocular macrophages, promotes hyaloid vessel regression by enhancing the adhesion of macrophages to hyaloid vessels [12]. These results strongly support that macrophages contribute to the regression of PM and hyaloid vessels. During vessel regression in PM, the first apoptotic vessel EC (VEC) is macrophage dependent. Then, this VEC enters the vessel lumen to cause angiostenosis and blood flow stasis, which trigger subsequent apoptosis of the remaining VECs in the affected segments [13, 14]. These results demonstrated that not only are macrophages indispensable for programmed vessel regression, but blood flow also contributes to VEC apoptosis.
Vessel fusion contributes to vessel dilation
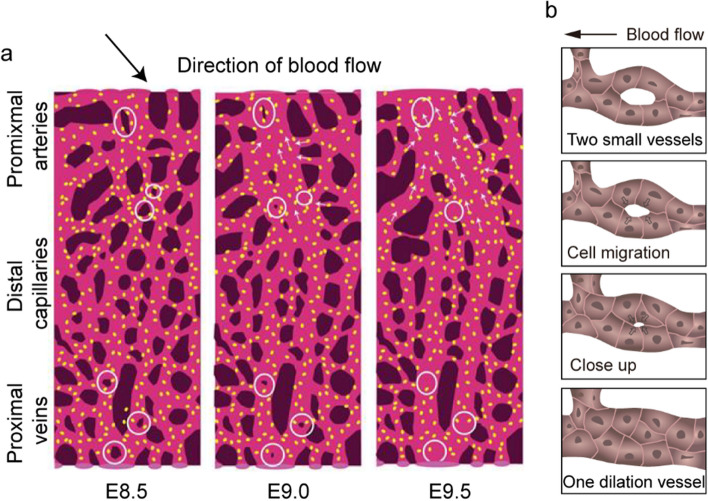
Vessel fusion is an autonomous activity of ECs in which two small vessels fuse into one vessel with a larger diameter [15] (Fig. 1a, b). Vessel fusion plays a vital role in the dilation of vessel diameter [15, 16]. Vessel fusion occurs at the early stage of embryo development, even in the absence of vessel smooth muscle cells (vSMCs) [15]. Vessel fusion between the dorsal aorta and lateral capillary plexus is regulated by vascular endothelial growth factor (VEGF) during Japanese quail embryo development [17]. In vitro models of three-dimensional vascular microtissue or uniluminal vascular spheroids can fuse to form a larger diameter spheroidal structure depending on VEGF and preserve the morphological architecture of the cultured spheroids [18, 19]. Ryan et al. found that vessel fusion was restricted to the highest blood flow near the vitelline artery and vein, resulting in a rapid increase in the vessel diameter of the cultured mouse embryo [6]. The ECs of capillaries with low blood flow migration to larger vessels with higher blood flow also contribute to enlarging the vessel diameter (Fig. 1a) [6]. Knockout of Mlc2a reduced blood flow velocity, which blocked vessel fusion and inhibited the increase in vessel diameter mediated by EC migration against blood flow. In conclusion, vessel fusion can not only simplify the vascular network, but also, more importantly, automatically select the fused vessel segment according to the magnitude of blood flow velocity, which contributes to enlarging the vessel diameter and forming a tree-like structure vascular network.
Fig. 1.
Vessel fusion contributes to engaging the vessel diameter. a Vessel fusion in the cultured mouse embryo. Blood enters the vascular plexus through the vitelline artery to the distal capillaries and is ultimately collected by the vitelline vein at E8.5. Vessel fusion (marked with circles) occurs in the proximal vessels that are exposed to high blood flow, resulting in a rapid change in vessel diameter. Vessel hierarchy is established through vessel fusion by E9.0–9.5, resulting in different flow velocity distributions in arteries, capillaries and veins. b Vessel fusion is mediated by the redistribution of ECs. Figure a adapted from Ryan et al. [6]
EC rearrangement mediates vessel pruning
The primary vasculature is formed by angiogenesis, endothelial sprouting and proliferation and contains a large number of redundant and nonfunctional vessels. These redundant vessels are remodeled into a mature and functional tree-like network, which is characterized by a large number of excess capillaries undergoing physiological regression [20, 21]. In addition, vessel pruning is also accompanied by a reduction in the number of vascular loops.
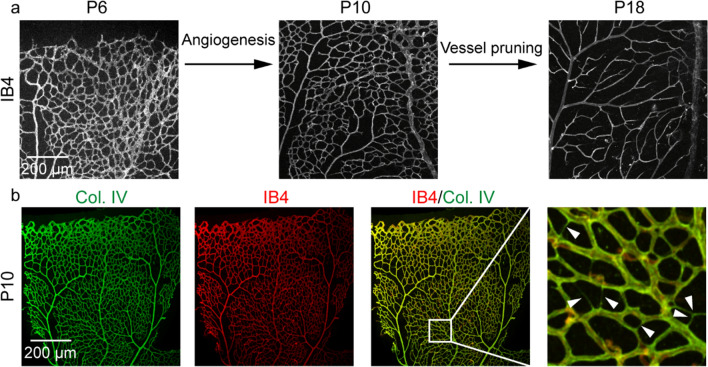
The retinal vasculature develops postnatally in mice, which is a good model to study sprouting angiogenesis and vessel remodeling (Fig. 2). The mature retinal vasculature consists of three distinct vascular plexuses. The primary pluxus with few vessels sprouts from the optic nerve head at postnatal day (P) 1 and then outgrows to reach the periphery at P8 [22], which localizes to the inner retina ganglion cell layer [9]. The veins of the primary pluxus insert into the deeper pluxus at the outer edge of the inner nuclear layer around P7 [22, 23]. The vessel density, segment length and number of branch points peaked at P10 (Fig. 2a). After angiogenesis of the retinal vasculature, vessel pruning is necessary to simplify the primary vascular network (Fig. 2a), which is characterized by an increase in IB4-/Col. IV + antibody staining (Fig. 2b) [24]. At P18, the retinal vasculature was more hierarchical and functional (Fig. 2a). Research has shown that 95% of vessel pruning events in the mouse retina were not related to EC apoptosis but through EC rearrangement [20]. Our group found that Tecr, a very-long-chain fatty acid synthesis, contributes to retinal angiogenesis. Endothelial-specific deletion of Tecr caused vascular defects with decreased vessel density and branch points at P7, but this phenotype disappeared at P5 and P10 [25]. We speculated that a transient effect on vessel density after abolishing Tecr was caused by excessive vessel pruning.
Fig. 2.
Vessel pruning in the mouse retinas. a Fluorescence immunohistochemistry of the mouse retinas using isolectin B4 (IB4, grey) to show the vasculature. The process of angiogenesis and vessel pruning in the mouse retina from P6 to P18. The vessel density reaches a pink at P10. Vessel pruning contributes to decreasing the vessel density from P10 to P18. Scale bar 200 μm. b Fluorescence immunohistochemistry of the mouse retinas using anti-collagen type IV (Col. IV, green) and IB4 (red) to visualize vessel pruning at P10. Arrowheads indicate the segments undergoing vessel pruning, characterized by IB4-/Col. IV + antibody staining. Scale bar 200 μm
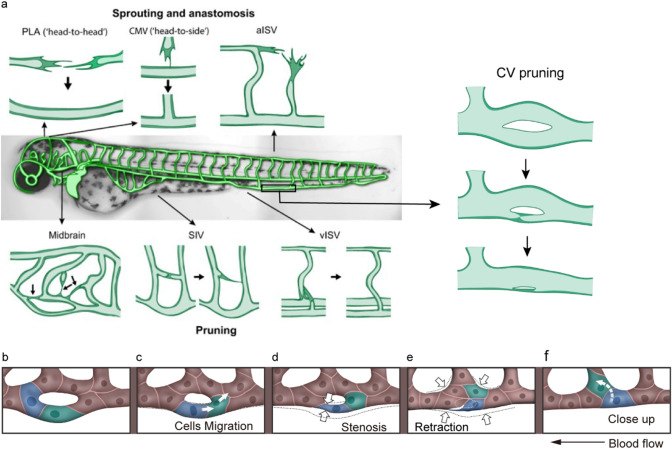
Zebrafish embryos develop in vitro, and their transparent characteristics make them an excellent model for studying vascular development. Researches have shown that the process of vessel pruning is present in zebrafish Cranial Division of the Internal Carotid Artery (CrDI) [26], zebrafish intersegmental vessels (ISVs) [20], zebrafish subintestinal veins (SIVs) [27] and zebrafish caudal veins (CV) (Fig. 3a) [28].The formation of zebrafish ISVs and endothelial lumen involved cell divisions, cell arrangements and dynamic alterations in intercellular junctional complexes by anastomosis (Fig. 3a) [29]. Claudio et al. found that arterial cells disconnected and retracted from the aorta where venous sprouts connected to the ISV during ISV pruning by monitoring the dynamics of ECs with mosaic endothelial expression of membrane-bound eGFP in Tg(kdrl:mCherry-CAAX) embryos (Fig. 3a) [20]. The disconnection of the ISVs involved cell migration but was unrelated to EC apoptosis. The parallel and vertical branches of SIVs in zebrafish are remodeled from the reticular structure with multiple vascular loops, accompanied by an increase in nuclei number(Fig. 3a) [27]. Therefore, SIV pruning is a synergistic effect involving the dynamic migration of cells and the collapse of the lumens. During 1.0–4.0 days postfertilization (dpf) of the zebrafish embryos, midbrain vasculature formed by angiogenesis with a large number of vascular loops and redundant segments. Vessel pruning plays a critical role in the development of midbrain vasculature to reduce the complexity of the vascular network by migrating ECs from pruned segments to adjacent unpruned segments (Fig. 3a) [30]. Furthermore, vessel pruning of zebrafish midbrain vascular networks was preferentially restricted to segments that were either located between two parallel primary vessels (H-type) or were one of the two nearby segments that formed a small local loop (O-type) [30]. zebrafish CV is remodeled from ventral capillaries of the CV plexus (CVP) as a novel animal model to study vessel pruning [28]. We showed that CV formation is accompanied by a decrease in vascular loops from 36 h postfertilization (hpf) to 72 hpf through vessel pruning (Fig. 3a) [28].
Fig. 3.
Anastomosis and vessel pruning events in the zebrafish model. a The process of anastomosis and vessel pruning in zebrafish vasculature. The central panel shows an overview of the zebrafish vascular beds. Sprouting and anastomosis have been studied in the palatocerebral artery (PLA), the communicating vessel (CMV) and the segmental arteries (aISV). Vessel pruning has been studied in the midbrain vasculature, the subintestinal vein (SIV), the segmental veins (vISV) and the caudal vein (CV). Figure a adapted from Charles et al. [34]. The process of zebrafish CV pruning is mediated by EC rearrangement, which includes the stages of selection pruning segment (b), cell migration (c), stenosis (d), retraction (e) and close-up (f). b The diameters of the lower branch and the upper branch are the same at the beginning of vessel pruning. c, d The ECs marked with blue and green migrate against the blood flow, resulting in vessel stenosis at the lower branch. e, f The ECs marked with blue and green migrate into the adjacent vessel, finishing the pruning of the lower branch. The arrow indicates the direction of blood flow. The rearrangement of ECs during vessel pruning is marked with blue and green. The arrows in (c, f) indicate the direction of EC migration. The arrows in (d, e) indicate vessel stenosis. Figure b–f adapted from Wen et al. [28]
Vessel pruning is a process of EC rearrangement, which resembles anastomosis in reverse in morphology [20, 27, 31]. Franco et al. proposed that vessel pruning includes four distinct steps: initial selection, stenosis, retraction and resolution [20, 32]. Our results showed a similar process of zebrafish CV pruning driven by EC rearrangement (Fig. 3b–f) [28]. Tg(kdrl:mCherry; fli1a:nEGFP) and Tg(fli1a:nEGFP);Ki(cdh5-mRFP) transgenic fish were used to explore the relationship between EC migration and vessel stenosis. At the beginning of CV pruning, the diameters of the two branches were almost the same (Fig. 3b). Then, EC nuclei, marked with blue and green at the lower branch, migrated against the blood flow (Fig. 3c), resulting in vessel stenosis (Fig. 3d). After that, the ECs retracted to the neighboring segments from the lower branch (Fig. 3e, f). Finally, the lower branch was pruned (Fig. 3f) [28]. The results demonstrated that the migration of EC nuclei contributes to vessel stenosis. In vessels without perfusion, both vessel stenosis and EC migration to the neighboring branch contribute to vessel pruning [27, 28]. Lowell et al. found that the preservation of vessel segments is determined by cellular decision-making behavior at bifurcations. The pruned cells prefer to choose vessel segments with larger shear stress or more cells [33].
Usually, vessel pruning is irrelevant to EC apoptosis. However, Eva et al. found that CrDI pruning in zebrafish is accompanied by EC death [26]. In contrast to the regression of hyaloid vessels in mice [7], EC death during CrDI pruning is independent of macrophages [26]. During dorsal CrDI pruning, 1–2 of a total of 3–4 ECs undergo apoptosis, and the remaining ECs migrate toward the dorsally located Primordial Midbrain Channel or the ventral CrDI [26]. Zhang et al. reported that EC apoptosis was indeed associated with brain vessel pruning in zebrafish [35]. Microglia, but not macrophages, contribute to the clearance of apoptotic ECs. However, microglia are dispensable for brain vessel pruning. The EC apoptosis-accompanied pruned vessels had two important characteristics: 1) most of these segments were longer than those without apoptosis, and 2) the nuclei of adjacent blood vessels occupied both ends [35]. Although EC apoptosis occurs during vessel pruning, it is not absolutely required for the completion of this process [26, 36]. EC-specific deletion of Caspase-8 or Bak/Bax in mice did not affect vessel pruning, even though it decreased cell death [37, 38]. These results strongly proved that EC apoptosis is not essential to vessel pruning and cannot trigger vessel pruning under physiological conditions.
EC junction/F-actin dynamic rearrangement contribute to vessel remodeling
Vessel pruning is a process of cell rearrangement that involves lumen collapse and cell-to-cell junction remodeling. Lenard et al. characterized vessel pruning as type I and II pruning depending on the perfusion of the pruning branches [27, 39]. Type I pruning is characterized by lumen collapse before cell rearrangement with nonlumenized, multicellular tube with continuous junctional connections. Type II pruning is cell rearrangement resulting in a unicellular tube by cell self-fusion that then collapses, and the bridging cell is incorporated into the major branch [27]. In the mouse retina, lumen disconnections occur in nonperfused vessels with discontinuous vascular endothelial junctions labeled by VE-cadherin or zona occludens proteins (ZO-1) [20]. Usually, the junctions form an isolated ring structure surrounding a patch of apical endothelial membrane with a continuous Col. IV basement membrane, which is similar to morphological anastomosis in reverse [20].
Research has proven that dynamic F-actin rearrangement at EC junctions and assembly of endothelial filopodia are indispensable to angiogenesis sprouting and lumen formation [40, 41]. Actin dynamics interact with both the VE-cadherin/catenin complex and the membrane cytoskeleton to control cell–cell adhesion, cell shape change or cell motion [42]. F-actin or stress fibers aligned parallel to the direction of flow when ECs were exposed to high blood flow in vivo or shear stress in vitro [43]. Once blood flow is reduced, ECs will reorganize the actin skeleton with a decreased number of stress fibers and change their position to the cells’ peripheral band [44]. To dissect the role of EC junctions and F-actin in vessel pruning, we generated the knock-in (KI) zebrafish Ki(cdh5-mRFP) and the transgenic line Tg(Fliep:lifeact-EGFP), in which VE-cadherin is marked with red fluorescence and EC F-actin is marked with green fluorescence. We showed that the dynamic polymerization and depolymerization of VE-cadherin and F-actin were observed during CV pruning. Usually, discontinuous VE-cadherin occurred before disruption of F-actin at the multicellular to unicellular stage and retraction stage. Moreover, deletion of klf6a or tagln2 in zebrafish resulted in abnormal CV pruning caused by disruption of VE-cadherin and F-actin rearrangement [28]. Therefore, it is clear that vessel pruning is a dynamic process of EC junction and F-actin cytoskeleton rearrangement.
The contribution of periendothelial cells to vessel remodeling
The role of periendothelial cells, such as pericytes and astrocytes, in vascular development and maturation is well studied. Their contribution during vessel remodeling is rather unclear. The interaction between pericytes and ECs is one of the important factors affecting vascular morphology and vessel remodeling [45]. As early as 1998, Benjamin et al. found that disruption of pericyte-EC associations leads to excessive vessel pruning in a hyperoxia-induced mouse model [46]. During the regression of hyaloid vessels, apoptosis is observed in ECs and pericytes. Furthermore, the apoptosis of pericytes is more frequent than that of ECs [10]. Bim alleles (BimFlox/Flox) and pericyte pdgfrb-cre (BimPC) mice were used to reduce pericyte apoptosis, which inhibited hyaloid vessel regression and retinal vessel pruning [11]. Suppression of VEGF production leads to an immature vascular network because of reduced pericyte coverage [47]. Pericytes secrete CXC receptor 3 (CXCR3) ligands, which activate the CXCR3 signaling pathway in ECs and promote vessel pruning [48]. Pericyte maturation is regulated by phosphoinositide 3-kinase (PI3K) signaling. Pericyte conditional knockdown of PI3K blocks pericyte proliferation and enhances pericyte maturation, resulting in fewer branch points of retinal vessels compared to the control at P6 [49]. The above results demonstrated that pericytes function not only directly in vessel pruning but also in pericyte-EC interactions.
Retinal astrocytes colocalize with the inner layer of retinal blood vessels as oxygen sensors during neonatal development and vascular remodeling [9]. Astrocyte prolyl hydroxylase domain proteins (PHDs) are oxygen sensors, and their deletion results in elevated hypoxia inducible factor (HIF)-2α protein levels and fewer mature astrocytes. Immature astrocytes cause increased retinal vascular density due to defective vessel pruning [50]. Astrocytes express not only VEGF but also β- and γ-crystallins, which function together in regulating vessel regression of the developing eye [51–53]. The expression of Aquaporin 4 (AQP 4), the major water channel in astrocytes, is elevated in astrocytes in human persistent fetal vasculature (PFV) disease. The loss of AQP4 leads to astrocytes ensheathing the hyaloid artery, thus preventing regression of the hyaloid vessels [54]. Conditional knockout of Vhl in retinal astrocytes using floxed alleles in glial fibrillary acidic protein (GFAP)-Cre mice resulted in transient accelerated vessel regression, followed by increased vessel branch points of primary hyaloid vessels [9]. In the process of vessel pruning in the zebrafish brain, microglia act as scavengers to clear apoptotic ECs but are dispensable for vessel pruning [35]. These results demonstrate the contribution of astrocytes during vessel remodeling.
The role of hemodynamics in vessel remodeling
The inner layer of the vascular structure is composed of a monolayer of ECs that directly sense and transduce hemodynamic forces into molecular signaling to regulate vascular development. Endothelial mechanic sensors such as integrins, platelet endothelial cell adhesion molecule-1 (PECAM-1)/vascular endothelial cell (VE-cadherin)/vascular endothelial growth factor receptor 2 (VEGFR2) complex, and notch1 contribute to angiogenesis, vessel integrity and vessel remodeling [55–59]. Although the early stages of vascular development, such as vasculogenesis and angiogenesis, have been well studied, the maturation of vascular networks, such as vessel remodeling, needs to be further studied.
The segment with lower blood flow will be pruned
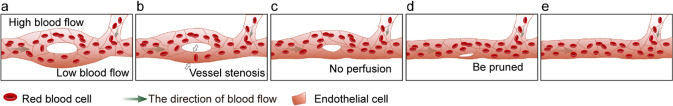
Hemodynamics plays a very important role in vascular development, vessel remodeling, maturation, and vessel quiescence under physiological conditions. The magnitude of flow shear stress (FSS) is coincident with blood flow velocity and inversely correlated with vessel diameter. Correspondingly, arteries, capillaries, and veins present different magnitudes of blood flow to adapt to tissue development. We have previously reported that decreasing blood flow by tnnt2a MO inhibits CVP angiogenesis in zebrafish embryos and leads to an oversimplified CVP vasculature, which is regulated by the ERK5-klf2a-nos2b axis [60]. When zebrafish embryos were exposed to a simulated microgravity (SM) environment from 24 to 36 hpf, the heartbeat of zebrafish was significantly reduced. This SM environment resulted in an increased intercapillary number and a wider CVP in zebrafish embryos [61, 62]. Several groups have identified that vessel pruning preferentially occurs at segments under low blood flow and the stabilization of segments under high blood flow [20, 28, 30]. The average blood flow velocity in unpruned segments is higher than that of pruned segments during vessel pruning of zebrafish midbrain vasculature [30]. To prove this theory in vivo, time-lapse imaging of CV pruning in Tg(flk1:EGFP;gata1:dsRed) transgenic fish was performed to clarify the relationship between the magnitude of blood flow and vessel pruning [28]. The average velocity of red blood cells (RBCs) in the vascular branch was calculated to assess the magnitude of blood flow. The data showed that the diameters of the two branches were almost the same before the initiation of CV pruning in zebrafish (Fig. 4a). However, the blood flow of the lower branch is slower than that of the upper branch at this stage (Fig. 4a). Then, vessel stenosis occurs at the lower branch (Fig. 4b). The difference in blood flow gradually increased between the upper and lower branches until there was no blood perfusion in the lower branch (Fig. 4c). Finally, the lower branch is pruned, while the upper branch remains (Fig. 4d, e). This result revealed that a decrease in blood flow occurred before vessel stenosis in the pruned segment, which may trigger the segment with lower blood flow to prune [28]. Furthermore, we found that klf6a could respond to blood flow to regulate CV pruning [28]. Kochhan et al. found that maintaining blood flow through the dorsal CrDI by laser ablation of the adjacent nasal ciliary artery (NCA) prevents dorsal CrDI pruning [26]. SIV plexus pruning, CV pruning, and CrDI pruning are blocked after slowing blood flow by triciane treatment or tnnt2a MO injection at the single-cell stage in zebrafish embryos [26–28]. CrDI pruning is blocked during heartbeat blocking with tricaine treatment but recovers vessel pruning after drug withdrawal [26]. Lucitti et al. found that vessel remodeling is defective in Mlc2a−/− mouse embryos, in which both plasma and erythroblast flow in the circulation are disrupted [63]. In conclusion, hemodynamics is the key factor in regulating vessel remodeling.
Fig. 4.
The role of blood flow during vessel pruning, in which the segment with lower blood flow is pruned. a The diameter of the upper branch and the lower branch is the same before vessel pruning, while the blood flow velocity of the lower branch is lower than that of the upper branch. b Vessel stenosis occurs at the lower branch with low blood flow. c No blood perfusion in the lower branch. d, e The lower branch is pruned, while the upper branch remains
Shear stress induces ECs to align in their direction and to polarize [64, 65]. Cell polarization in the direction of blood flow plays a vital role in cell migration [65]. Franco et al. found that ECs polarized toward adjacent vessels during vessel pruning [20]. However, there are misaligned or nonpolar ECs at the lower wall shear stress segments relative to adjacent segments with higher wall shear stress [20]. Our results demonstrated that ECs did not exhibit polarity in pruned segment during zebrafish CV pruning [28]. EC polarity is tightly associated with the magnitude of blood flow. However, CV exhibits low blood flow from 48 to 60 hpf compared to arteries, when arterial ECs already become polar [64]. This may be one of the reasons to explain the nonpolarity ECs during CV pruning.
Mechanical sensors of ECs in vessel remodeling
Blood flow coordinates the behavior and function of ECs to form a mature vascular network, but how ECs read and interpret the signals generated by hemodynamics is not clear. The branching structure of the arterial tree is based on the adaptive response of the vessel diameter induced by wall shear stress [66]. ECs are exposed to different magnitudes of FSS, which in turn sense and respond to changes in blood flow to regulate the morphology and function of blood vessels. Our understanding of how ECs recognize the distributions of the different magnitudes of blood flow to regulate gene expression and adjust vascular morphology is limited. Recently, scientists have gained some insights into EC mechanosensors and signal transduction, including integrins, the PECAM-1/VE-cadherin/VEGFR2 complex, and notch1 (Fig. 5).
Fig. 5.
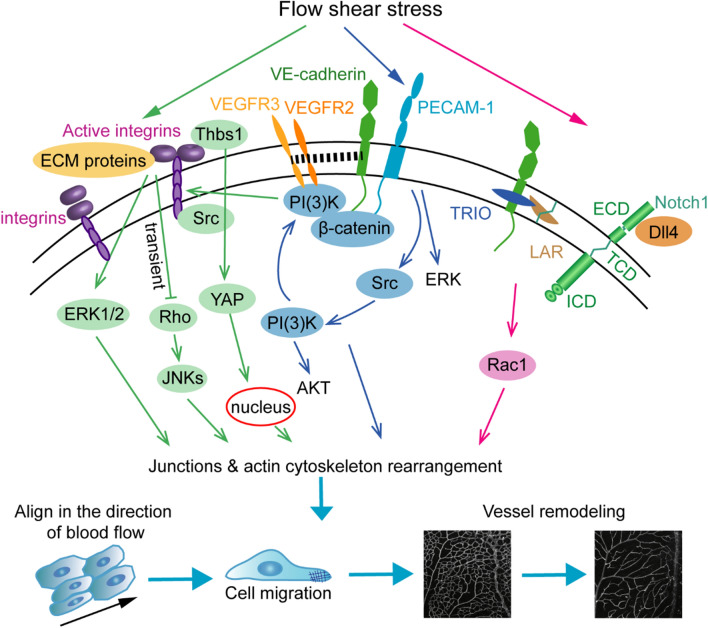
Mechanosensors regulate vessel remodeling, including integrin signaling, VE-cadherin, VEGFR2/3, PECAM-1 complex and Notch1 signaling. Integrins interact with Thbs 1 to promote YAP nuclear translocation and regulate vessel remodeling by promoting cell migration, junctions and actin cytoskeleton rearrangement. Flow shear stress (FSS)-induced ERK1/2 activation and Akt phosphorylation depend on integrin binding to extracellular matrix (ECM) proteins. The combination of integrins and ECM proteins induces a transient inhibition of Rho and the activation of downstream JNKS, which is necessary for cytoskeletal alignment in the direction of flow. The mechanosensory complex, PECAM-1-VE-Cadherin-VEGFRs, activates the PI3K-Akt pathway to promote cell migration. PECAM-1 directly senses mechanical force and then activates Src, and VE-cadherin binds with β-catenin and VEGFR2/3 to activate downstream P13K and integrin. The NOTCH1 mechanosensory complex senses FSS and regulates junctions and actin dynamics, which includes the processes of (i) FSS-induced endocytosis of DLL4; (ii) cleavage of NOTCH1 to expose the transcellular domain (TCD); and (iii) binding to the LAR with VE-cadherin and TRIO to activate the downstream target RAC1
In the event of mechanotransduction, the extracellular matrix (ECM) plays a pivotal role in the interaction of the matrix and cells (Fig. 5). The matricellular protein thrombospondin-1 (Thbs1) binds to integrin αVβ1 to regulate the focal adhesion-actin complex by promoting the nuclear shuttling of YAP. Deletion of Thbs1 in mice disrupted mechanotransduction by inhibiting Thbs1/integrin/YAP signaling, resulting in abnormal vessel remodeling [58]. Integrins mediate FSS-induced AKT phosphorylation by PI3K and ERK1/2 activation [56, 67, 68]. FSS stimulates the activation of integrins and binding to ECM proteins, which induces the transient inactivation of Rho and the activation of downstream JNKs. The transient inactivation of Rho is adequate for cytoskeletal alignment in the direction of flow [57]. Integrins also interact with cadherins to regulate actin cytoskeleton alignment, intracellular forces, endothelial integrity, focal adhesion remodeling and cell contractility, which are critical for cell migration [57, 69–71]. These results indicate that integrins may participate in FSS-involved vessel remodeling by regulating the actin cytoskeleton and junction rearrangement.
The mechanosensory complexes PECAM-1, VE-cadherin and VEGFR2 transmit mechanical force into cell signaling to regulate vessel remodeling, cardiac development and atherogenesis (Fig. 5) [55]. PECAM-1 directly senses mechanical force and then activates Src, whereas VE-cadherin functions as an adaptor with its binding partner β-catenin to bind with VEGFR2 and activate downstream PI3K and integrins [55, 72, 73]. Another study from the same group showed that VEGFR3 is also a component of this complex and is involved in regulating the hemodynamic response of ECs [74]. The shear stress “set point” at which ECs have a preferred level of FSS is related to the level of VEGFR3 in the cells. Increasing the level of VEGFR3 in ECs decreases the “set point”, but this is the opposite of lymphatic ECs [75, 76]. PECAM-1−/− mice exhibited thinner intima-media and adventitia induced by partial carotid ligation, which implied that PECAM-1-dependent signaling is necessary for flow-induced vessel remodeling [77]. The downstream target of low shear stress (LSS), a small fraction of VE-cadherin phosphorylation on Y658, causes the dissociation of P120ctn and binds to polarity protein LGN to mediate FSS sensing [78]. VE-cadherin phosphorylation by treatment with LSS and DAPT (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester, an inhibitor of the Notch pathway, inhibits vessel fusion [79]. However, Src inhibition can prevent VE-cadherin phosphorylation in ECs to rescue hyperfusion [79]. VEGF stimulation promotes the fusion of blood vessels in mouse allantoic-derived vascular spheroids or in avian embryos [15, 18]. Overexpression of VEGF by implanting VEGF-soaked heparin chromatography beads causes an increase in vascularity and an enlarged dorsal aorta in quail embryos because of increased vessel fusion events between the dorsal aorta and lateral capillary plexus [17]. Inhibition of circulating VEGF by the fusion protein Flk1/KDR receptor leads to increased capillary density but no difference in cell apoptosis during the programed regression of the PM [13]. The transmembrane semaphorin6A (Sema6A)-null mice shows a reduction of hyaloid network complexity and branch points due to increased cell death by downregulating VEGFR2 [80]. The deficiency of metabolic enzyme CDP-diacylglycerol synthetase-2 (CDS2) induces the secretion of VEGFA, which in turn promotes vessel remodeling by regulating PIP3 and FOXO1 nuclear accumulation [81]. In conclusion, the mechanosensory complexes PECAM-1, VE-Cadherin and VEGFRs not only perform mechanotransduction but are also important for vessel remodeling.
The transmembrane receptor Notch1, as an important signal pathway in the process of angiogenesis and vessel remodeling, has been proven to be a mechanosensor [39, 59, 82, 83]. The transmembrane domain of Notch1, together with VE-cadherin, the transmembrane protein tyrosine phosphatase LAR and the RAC1 guanidine-exchange factor TRIO, forms a receptor complex that senses mechanical force and contributes to vascular barrier function by promoting the assembly of adherens junctions (Fig. 5) [59]. During ISV differentiation in zebrafish, venous blood flow or weak pulsatility induces upstream migration of ECs to replace arterial ECs and transformation of the vISV. However, arterial blood flow or strong pulsatility of the two adjacent ISVs prevents venous EC migration by activating Notch signaling in ECs [84]. Therefore, blood flow magnitude-induced Notch signaling activation is necessary for the differentiation of aISVs and vISVs. Notch signaling is only responsible for postnatal vein and perivenous capillary plexus remodeling but not for artery remodeling in a mouse retina model [85]. Lobov et al. demonstrated that the Delta-like ligand 4 (Dll4)/Notch pathway is involved in vessel remodeling and regression in oxygen-induced retinopathy (OIR) or the maturation of the neoretina [86]. Dll4/Notch inhibits the perfusion of microvessels by reducing the expression of the vasodilator adrenomedullin and promoting the expression of the vasoconstrictor angiotensinogen to regulate vessel remodeling [86]. Loss of Notch-regulated ankyrin repeat protein (Nrarp) leads to excessive vessel pruning events regulated by Notch-dependent cell cycle arrest and Cyclin D1-induced Lef1/ctnnb1/Wnt signaling in retinal vessel segments [87]. Overall, Notch1 functions as a mechanosensor and plays an indispensable role in angiogenesis and vessel remodeling.
Wnt signaling regulates vessel remodeling
The Wnt family consists of 19 highly conserved glycoproteins, which are classified into canonical (β-catenin-dependent) and non-canonical (β-catenin-independent) signaling. Wnt signaling plays a pivotal role in vascular angiogenesis, vessel remodeling and vascular regeneration during vascular development and disease [88–90]. In this section, we will illustrate the role of Wnt signaling in vessel remodeling (Fig. 6).
Fig. 6.
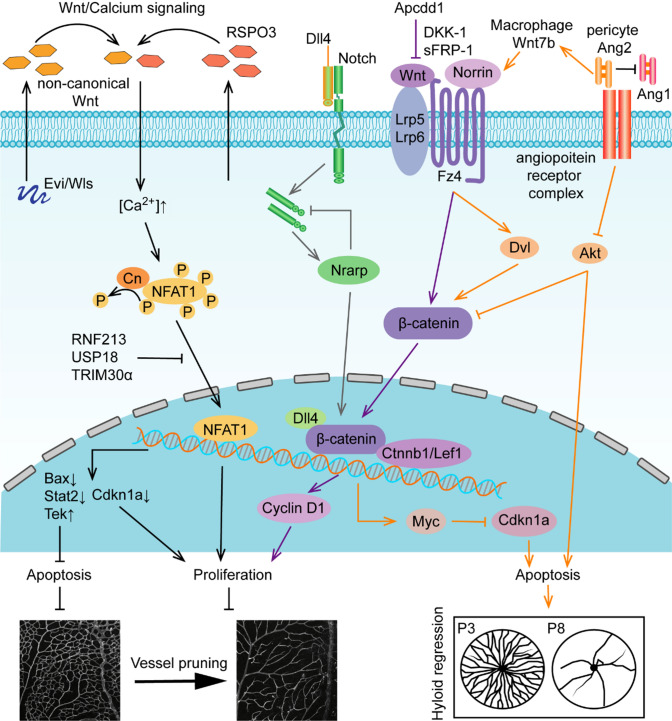
Role of Wnt signaling in vessel remodeling. Non-canonical Wnt ligands activate Wnt/Ca2+ signaling and regulate vessel remodeling at the transcriptional level of apoptosis- and proliferation-related genes. Evi/Wls/R-spondin3 (Rspo3) activate non-canonical WNT/calcium signaling at the level of NFAT1 by downregulating Rnf213, Usp18, and Trim30α, which balance the level of cell survival genes to regulate vessel pruning in the retina. Canonical Wnt signaling receptors, coreceptors and ligands cooperate with Dll4/Notch signaling, and pericytes secrete Ang II to balance the progress of vessel remodeling. Canonical Norrin/Fz4/Lrp5/6 accelerate β-catenin nuclear translocation and control the transcription of Cyclin D1 or Myc-Cdkn1a to regulate cell survival. The negative regulatory factor Apcdd1 controls vessel density transiently in retina during P10-12. Dll4/Notch signaling stimulates expression of Nrarp and contributes to canonical Wnt signaling by interacting with Lef1/Ctnnb1. Ang2 produced by pericytes has a dual identity in the regulation of cell death. On the one hand, Ang2 suppresses Akt to permit cell death. On the other hand, Ang2 promotes the secretion of Wnt7b by macrophages to activate the Wnt/β-catenin pathway, which inhibits cell death by promoting cell cycle entry to regulate hyaloid regression
Conditional knockout of Evi, the Wnt secretion factor, causes decreased microvessel density in the mouse retina and tumor angiogenesis [91]. Costaining of IB4 and Col. IV revealed increased empty basement membrane sleeves in Evi-ECKO mouse retinas, indicating that the decreased microvessel density was caused by increased vessel pruning. Further studies have shown that downregulation of the apoptosis-related gene Tek and upregulation of the proliferation-related gene Cdkn1a and apoptosis-related genes Bax and Stat2 account for increased vessel pruning [91]. Wnt signaling enhancer R-spondins 3 (Rspo3) mutant mice die at approximately E10, causing the primary capillary plexus of the placenta to fail to undergo proliferation and remodeling [92]. Scholz et al. found that the phenotype of Rspo3-ECKO was consistent with that in Evi-ECKO mouse retinas, showing decreased vascular density, excessive vessel pruning, and increased EC apoptosis [91, 93]. Rnf213, Usp8, and Trim30α expression is increased in Rspo3-ECKO ECs, which inactive non-canonical WNT/calcium signaling at the level of NFAT, thus causing excessive vessel pruning. During the development of the mouse retina, the loss of the non-canonical Wnt ligands Wls, Wnt5a or Wnt11 leads to an increase in the sensitivity of ECs to shear stress, resulting in endothelial polarization and EC migration against blood flow under LSS, thus aggravating vessel pruning [94]. APCDD1 is a negative regulatory protein of Wnt/β-catenin signaling. Apcdd1 knockout mice show a transient increase in vascular density during P10–P12 due to decreased vessel pruning in the retinal vasculature. However, there was no difference between Apcdd1−/− and WT mice in retinal vascular density at P14 [95]. Treatment with the canonical Wnt inhibitor DKK-1 or sFRP-1 prevents microvessel pruning and increases vascular density in a rat mesentery model [96]. Deletion of Frizzzed (Fz)4, the coreceptor low-density lipoprotein receptor-related protein (Lrp)5 or Ndp results in decreased vascular density during postnatal retinal vascular development in mice [97, 98]. However, deficiency of fzd4 in adult zebrafish increases the vascular density of the ventral retina, not the dorsal retina [99]. Endothelial-specific deletion of β-catenin leads to embryonic death, causing abnormal vascular morphogenesis and an inability to remodel to a tree-like structure, such as enlarged or irregular lumen, abnormal branching of umbilical vessels, lacunae-like bifurcations, and blind ending [100]. β-Catenin gain-of-function (GOF) embryos show the absence of a perineural vascular plexus due to a lack of correct remodeling of small vessels. Dll4/Notch signaling and downstream effectors are significantly increased in β-catenin GOF mutants, which impair EC migration and vessel pruning in the yolk sac and head [101]. Nrarp acts as a downstream target of Dll4/Notch signaling in ECs to regulate Notch- and Lef1-dependent Wnt signaling, thus contributing to vessel stability during angiogenesis. Therefore, the balance of Notch signaling is the key factor for vessel pruning in this event. Nrarp is specifically expressed at newly formed branch points, and its deletion leads to excessive vessel pruning with reduced vessel density and branch points. Loss of Lef1 and endothelial-specific deletion of Ctnnb1 phenocopy the deficient retinas of Nrarp mice. The expression of the Notch target Hey2 is increased, and the expression of Cyclin D1 is decreased in Nrarp−/− mice. Dll4/Notch signaling induces the expression of Nrarp, which negatively regulates Notch signaling and interferes with p21CIP-dependent cell cycle arrest. Nrarp could significantly induce Lef1/Ctnnb complex-regulated cell cycle arrest by transcriptional activation of Cyclin D1 [87]. In conclusion, Nrarp-mediated cell proliferation by balancing Notch and Wnt signaling may be a main mechanism accounting for vascular stabilization.
In addition, Wnt signaling also regulates hyaloid vessel regression (Fig. 6). Lrp5 or Lrp6 mutant mice show a defect in hyaloid regression and delayed retinal vascular growth caused by a halted cell cycle and decreased cell apoptosis [102]. Knockout of either Ndp or Fz4 leads to delayed hyaloid regression. Norrin activates canonical Wnt signaling by interacting with the Fz4 receptor and Lrp coreceptor to regulate hyaloid regression [97, 103]. Myc, Wnt/β-catenin pathway target gene and the gene regulates cell cycle and cell death, deletion of it increases the expression of Cdkn1a and resulting in the persistence of hyaloid vessels. Myc/Cdkn1a is required for cell cycle entry and proper levels of cell apoptosis to promote hyaloid regression [4]. Lobov et al. found that the expression of Wnt7b is increased in hyaloid macrophages from P1 to P5 [7]. Deficiency of Lrp5, Lef1 and Wnt7b results in persistent hyaloid vessels, causing reduced EC apoptosis [7]. Macrophage Wnt7b activates the WNT pathway in adjacent ECs through cell–cell contact and then regulates EC cell cycle entry, apoptosis and programmed capillary regression [7]. Wnt7b in macrophages is stimulated by the suppression of PI3K-Akt survival signaling in ECs through angiopoietin (Ang)2, which triggers ECs to enter the cell cycle and die in the G1 phase of the cell cycle as a result of reduced VEC apoptosis [104].
Conclusions and perspectives
To ensure functional vascular network formation, vessel remodeling is indispensable for the process of vascular development. In this review, we described the differences and characteristics of vessel pruning, vessel regression and vessel fusion. Our previous studies showed that hemodynamic foci are required for EC junctions and actin cytoskeleton rearrangement, EC migration, cell proliferation and cell apoptosis, which are necessary for angiogenesis and vessel remodeling [28, 60, 62, 105]. Studies have found that segments with low blood flow tend to be pruned, while segments with high blood flow are maintained [20, 28]. In addition, the segment that is longer than others also likes to be pruned, which is accompanied by EC apoptosis [35]. The mechanosensory complexes PECAM-1/VE-Cadherin/VEGFRs and Notch1 play an important role in vessel remodeling. PECAM-1 directly senses FSS and transduces the focus to cell signaling. VE-cadherin is an adapter for the mechanic sensor [55]. Furthermore, VE-cadherin and actin cytoskeleton rearrangement contribute to cell migration during vessel pruning. In addition, blood flow connects with Notch signaling, the Wnt pathway and VEGFR to regulate vessel remodeling.
The study of biomechanics and vessel remodeling mechanisms aims to explore scientific issues and principles and to serve clinical medicine. Vessel remodeling is not only indispensable during physiology vasculature development, but essential in pathogenic process of various cardiovascular disorders, including atherosclerosis, hypertension, stroke, tumors metastasis [106]. Vessel remodeling is characterized by EC morphological structure and phenotype changes, such as endothelial-to-mesenchymal transition (EndoMT), under pathological conditions. ECs generally function in migration, hypertrophy, proliferation and apoptosis [16, 23]. Pathological vessel remodeling is also influenced by hemodynamic forces. We aim to elucidate how mechanical factors produce biological effects and regulate cardiovascular development under physiological conditions. In addition, we determined the mechanically sensitive genes to explore their effect on vascular development and the molecular mechanism. Overall, insight into the mechanism of vessel remodeling under physiology may discovery new disease-related genes and cell signaling, which may be the entry point for vessel remodeling-associated disease treatment.
The department of “Mechano-Developmental Biology” was established at Chongqing University in 2010. A series of hemodynamic-vascular developmental biology studies have been carried out, and a number of research papers have been published. Our lab used transgenic zebrafish (Tg(flk1:EGFP), Tg(kdrl:mCherry), Tg(gata1a:dsRed), Tg(fli1a:nEGFP), Tg(UAS:EGFP), Tg(fli1a:B4GALT1-mCherry), Ki(cdh5-mRFP)) to explore the effect of blood flow on angiogenesis and vessel remodeling during development and arterial stenosis under physiological and pathological conditions. The combination of confocal microscopy and an in vivo microcirculation real-time tracing system makes zebrafish a powerful tool for studying blood flow-associated vascular development. We found that the reduction in blood flow velocity affects the angiogenesis and pruning process of CVP in zebrafish embryos [28, 60].
However, some issues remain to be studied: (1) The molecular mechanism of the interaction between ECs and periendothelial cells in regulating vessel remodeling; (2) How ECs sense different intensities of FSS to conduct vascular remodeling; and (3) The signaling pathway should be studied to better understand vessel remodeling under both physiological conditions and pathological conditions. In most cases, the vascular system is simplified and simulated, or only a small part of the blood vessels are separated from the whole vascular network to be studied, which lacks the integrity of the vascular network. In fact, the vascular network is a tree-like structure with complex vascular branches. Previous studies have shown that the differentiation of arteries/veins is regulated by Notch signaling [107]. Arterial, venous or capillary ECs show different polarities in the same period during development, which is regulated by apelin receptor signaling [64]. These results demonstrated that arteries, veins and capillaries are regulated by different signaling pathways. Further studies are needed to explore the molecular mechanisms of blood vessel formation mediated by blood flow. Only by better reconstruction of a full-scale model of circulation can we better analyze its molecular mechanisms. Fortunately, using zebrafish, we can monitor blood flow distribution in the whole blood vessel network in real time, which allows us to investigate in vivo the pathways that modulate flow sensing and response in different types of vessels. Our ultimate aim is to draw a hemodynamics-sensitive gene map covering the vascular network. In addition, this model can be used to explore the initiation and early development of diseases and their molecular mechanisms, including hypertension, atherosclerosis and other pathologic conditions. In general, we hope that our research will contribute to the treatment of numerous pathological conditions in the clinic.
Acknowledgements
This work was supported in part by JinFeng Laboratory, Chongqing, China (jfkyjf202203001). The author would like to thank all the other members of the Public Experimental Center of the National Bioindustry Base (Chongqing) in charge of Professor Guixue Wang for their constructive discussion and support.
Author contributions
LW design, investigation and writing of the original draft. LW, WHY and LZ literature search, analysis and discussion. GXW and CJT conceptualization, critical revisions of the manuscript, supervision. All authors contributed to final approval of the manuscript.
Funding
This work was supported in part by grants from the National Natural Science Foundation of China (12032007, 31971242) and JinFeng Laboratory, Chongqing, China (jfkyjf202203001).
Availability of data and materials
All data generated during this study are included in this published article.
Declarations
Conflict of interest
The authors have no relevant financial interests to disclose.
Ethics approval and consent to participate
This is a review article, which did not need ethical approval.
Consent for publication
Human experiments are not involved in this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chaojun Tang, Email: zjtang@suda.edu.cn.
Guixue Wang, Email: wanggx@cqu.edu.cn.
References
- 1.Suzanne H, Huijun Y, Tailoi C-L. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 2000;41(5):1217–1228. [PubMed] [Google Scholar]
- 2.Andreas Z, Secomb TW, Pries AR. Angioadaptation: keeping the vascular system in shape. News Physiol Sci. 2002;17:197–201. doi: 10.1152/nips.01395.2001. [DOI] [PubMed] [Google Scholar]
- 3.Pries AR, Secomb TW. Making microvascular networks work: angiogenesis, remodeling, and pruning. Physiology. 2014;29(6):446–455. doi: 10.1152/physiol.00012.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gowri N, Yoshinobu O, Vikram P, et al. Developmental vascular regression is regulated by a Wnt/β-catenin, MYC and CDKN1A pathway that controls cell proliferation and cell death. Development. 2018;145(12):dev154898. doi: 10.1242/dev.154898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claudia K, Augustin HG. Mechanisms of vessel pruning and regression. Dev Cell. 2015;34(1):5–17. doi: 10.1016/j.devcel.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Udan Ryan S, Vadakkan Tegy J, Dickinson Mary E. Dynamic responses of endothelial cells to changes in blood flow during vascular remodeling of the mouse yolk sac. Development. 2013;140(19):4041–4050. doi: 10.1242/dev.096255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lobov IB, Sujata R, Carroll TJ, et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437(7057):417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang Richard A, Michael BJ. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74(3):453–462. doi: 10.1016/0092-8674(93)80047-i. [DOI] [PubMed] [Google Scholar]
- 9.Toshihide K, Westenskow PD, Krohne TU, et al. Astrocyte pVHL and HIF-α isoforms are required for embryonic-to-adult vascular transition in the eye. J Cell Biol. 2011;195(4):689–701. doi: 10.1083/jcb.201107029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiroyasu T, Takashi K, Huaqing G, Tsugio A. Apoptosis of the hyaloid artery in the rat eye. Ann Anat. 1999;181(6):555–560. doi: 10.1016/S0940-9602(99)80061-2. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Ismail SZ, Ryan PJ, et al. Bim expression in endothelial cells and pericytes is essential for regression of the fetal ocular vasculature. PLoS ONE. 2017;12(5):e0178198. doi: 10.1371/journal.pone.0178198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitsuru A, Shigeo Y, Takahito N, et al. Involvement of periostin in regression of hyaloidvascular system during ocular development. Invest Ophthalmol Vis Sci. 2012;53(10):6495–6503. doi: 10.1167/iovs.12-9684. [DOI] [PubMed] [Google Scholar]
- 13.Meeson AP, Michael A, Kyung Ko, et al. VEGF deprivation-induced apoptosis is a component of programmed capillary regression. Development. 1999;126(7):1407–1415. doi: 10.1242/dev.126.7.1407. [DOI] [PubMed] [Google Scholar]
- 14.Annette M, Michelle P, Marcella C, Richard L. A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development. 1996;122(12):3929–3938. doi: 10.1242/dev.122.12.3929. [DOI] [PubMed] [Google Scholar]
- 15.Drake CJ, Little CD. VEGF and vascular fusion: implications for normal and pathological vessels. J Histochem Cytochem. 1999;47(11):1351–1356. doi: 10.1177/002215549904701101. [DOI] [PubMed] [Google Scholar]
- 16.Gifre-Renom L, Elizabeth AVJ. Vessel enlargement in development and pathophysiology. Front Physiol. 2021;12:639645. doi: 10.3389/fphys.2021.639645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finkelstein EB, Poole TJ. Vascular endothelial growth factor: a regulator of vascular morphogenesis in the Japanese quail embryo. Anat Rec A Discov Mol Cell Evol Biol. 2003;272(1):403–414. doi: 10.1002/ar.a.10047. [DOI] [PubMed] [Google Scholar]
- 18.Carmine G, Fleming Paul A, Vladimir M, et al. VEGF-mediated fusion in the generation of uniluminal vascular spheroids. Dev Dyn. 2008;237(10):2918–2925. doi: 10.1007/978-3-642-16483-5_5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming PA, Scott AW, Carmine G, et al. Fusion of uniluminal vascular spheroids: a model for assembly of blood vessels. Dev Dyn. 2010;239(2):398–406. doi: 10.1002/dvdy.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claudio AF, Martin LJ, Miguel OB, et al. Dynamic endothelial cell rearrangements drive developmental vessel regression. PLoS Biol. 2015;13(5):e1002163. doi: 10.1371/journal.pbio.1002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzanne H, Tailoi C-L. Roles of endothelial cell migration and apoptosis in vascular remodeling during development of the central nervous system. Microcirculation. 2000;7(5):317–333. doi: 10.1111/j.1549-8719.2000.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 22.Andreas S, Connor KM, Przemyslaw S, et al. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010;51(6):2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senthil S, Tejas K, Marcus F. Retinal vasculature development in health and disease. Prog Retin Eye Res. 2018;63:1–19. doi: 10.1016/j.preteyeres.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Ruslan R, Lisa G, Berre D, Schwab Martin E. A revised view on growth and remodeling in the retinal vasculature. Sci Rep. 2019;9(1):3263. doi: 10.1038/s41598-019-40135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jinxuan W, Jianxiong X, Guangchao Z et al (2022) Trans-2-enoyl-CoA reductase Tecr-driven lipid metabolism in endothelial cells protects against transcytosis to maintain blood-brain barrier homeostasis. Research 2022:9839368. 10.34133/2022/9839368 [DOI] [PMC free article] [PubMed]
- 26.Eva K, Anna L, Elin E, et al. Blood flow changes coincide with cellular rearrangements during blood vessel pruning in zebrafish embryos. PLoS ONE. 2013;8(10):e75060. doi: 10.1371/journal.pone.0075060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anna L, Stephan D, Charles B, et al. Endothelial cell self-fusion during vascular pruning. PLoS Biol. 2015;13(4):e1002126. doi: 10.1371/journal.pbio.1002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin W, Tao Z, Jinxuan W, et al. The blood flow-klf6a-tagln2 axis drives vessel pruning in zebrafish by regulating endothelial cell rearrangement and actin cytoskeleton dynamics. PLoS Genet. 2021;17(7):e1009690. doi: 10.1371/journal.pgen.1009690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yannick B, Georg BH, Elin E, et al. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol. 2008;316(2):312–322. doi: 10.1016/j.ydbio.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 30.Qi C, Luan J, Chun L, et al. Haemodynamics-driven developmental pruning of brain vasculature in zebrafish. PLoS Biol. 2012;10(8):e1001374. doi: 10.1371/journal.pbio.1001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukas H, Yannick B, Alice K, et al. Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr Biol. 2011;21(22):1942–1948. doi: 10.1016/j.cub.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Pedro B, Carvalho JR, Franco CA. Endothelial cell dynamics in vascular remodeling. Clin Hemorheol Microcirc. 2016;64(4):557–563. doi: 10.3233/CH-168006. [DOI] [PubMed] [Google Scholar]
- 33.Lowell TE, Claudio AF, Holger G, Miguel OB. On the preservation of vessel bifurcations during flow-mediated angiogenic remodeling. PLoS Comput Biol. 2021;17(2):e1007715. doi: 10.1371/journal.pcbi.1007715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charles B, Anna L, Georg BH, Markus A. Cell behaviors and dynamics during angiogenesis. Development. 2016;143(13):2249–2260. doi: 10.1242/dev.135616. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Yu, Bing Xu, Qi C, et al. Apoptosis of endothelial cells contributes to brain vessel pruning of zebrafish during development. Front Mol Neurosci. 2018;11:222. doi: 10.3389/fnmol.2018.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nathalie T, Carmen R. Contribution of cell death signaling to blood vessel formation. Cell Mol Life Sci. 2021;78(7):3247–3264. doi: 10.1007/s00018-020-03738-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nathalie T, Aida F-V, Rosario Y, et al. Caspase-8 modulates physiological and pathological angiogenesis during retina development. J Clin Invest. 2019;129(12):5092–5107. doi: 10.1172/JCI122767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson EC, Koenig MN, Grant ZL, et al. Apoptosis regulates endothelial cell number and capillary vessel diameter but not vessel regression during retinal angiogenesis. Development. 2016;143(16):2973–2982. doi: 10.1242/dev.137513. [DOI] [PubMed] [Google Scholar]
- 39.Nicolas R, Michael S. When it is better to regress: dynamics of vascular pruning. PLoS Biol. 2015;13(5):e1002148. doi: 10.1371/journal.pbio.1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuki W, Shigetomo F, Koji A, et al. Cdc42 mediates Bmp-Induced sprouting angiogenesis through Fmnl3-driven assembly of endothelial filopodia in zebrafish. Dev Cell. 2015;32(1):109–122. doi: 10.1016/j.devcel.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 41.Kun PL, Véronique G, Katie B, et al. Formin-mediated actin polymerization at endothelial junctions is required for vessel lumen formation and stabilization. Dev Cell. 2015;32(1):123–132. doi: 10.1016/j.devcel.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Hans S, Muna T, Odenthal SM, et al. Actin filament dynamics and endothelial cell junctions: The Ying and Yang between stabilization and motion. Cell Tissue Res. 2014;355(3):529–543. doi: 10.1007/s00441-014-1856-2. [DOI] [PubMed] [Google Scholar]
- 43.Girard PR, Nerem RM. Shear stress modulates endothelial cell morphology and F-actin organization through the regulation of focal adhesion-associated proteins. J Cell Physiol. 1995;163(1):179–193. doi: 10.1002/jcp.1041630121. [DOI] [PubMed] [Google Scholar]
- 44.Mehdi I, Laura L, Daria T, et al. The effect of shear stress reduction on endothelial cells: a microfluidic study of the actin cytoskeleton. Biomicrofluidics. 2020;14(2):024115. doi: 10.1063/1.5143391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Hu. Pericyte-endothelial interactions in the retinal microvasculature. Int J Mol Sci. 2020;21(19):7413. doi: 10.3390/ijms21197413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benjamin LE, Itzhak H, Eli K. A plasticity window for blood vessel remodeling is defined by pericyte coverage of the performed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125(9):1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 47.Benjamin LE, Dragan G, Ahuva I, et al. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103(2):159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bodnar RJ, Rodgers ME, Chen William CW, Alan W. Pericyte regulation of vascular remodeling through the CXC receptor 3. Arterioscler Thromb Vasc Biol. 2013;33(12):2818–2829. doi: 10.1161/ATVBAHA.113.302012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Figueiredo AM, Pilar V, Rodrigo D-H, et al. Phosphoinositide 3-kinase-regulated pericyte maturation governs vascular remodeling. Circulation. 2020;142(7):688–704. doi: 10.1161/CIRCULATIONAHA.119.042354. [DOI] [PubMed] [Google Scholar]
- 50.Juan DL, Hua FG. Developmental vascular pruning in neonatal mouse retinas is programmed by the astrocytic oxygen-sensing mechanism. Development. 2019;146(8):dev175117. doi: 10.1242/dev.175117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng Z, Peter G, Celine G, et al. A potential role for β- and γ-crystallins in the vascular remodeling of the eye. Dev Dyn. 2005;234(1):36–47. doi: 10.1002/dvdy.20494. [DOI] [PubMed] [Google Scholar]
- 52.Debasish S, Mallika V, Imran B, et al. βA3/A1-crystallin is required for proper astrocyte template formation and vascular remodeling in the retina. Transgenic Res. 2012;21(5):1033–1042. doi: 10.1007/s11248-012-9608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Debasish S, Andrew K, Yuri S, et al. βA3/A1-crystallin in astroglial cells regulates retinal vascular remodeling during development. Mol Cell Neurosci. 2008;37(1):85–95. doi: 10.1016/j.mcn.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng Z, Laura A, Celine G, et al. A developmental defect in astrocytes inhibits programmed regression of the hyaloid vasculature in the mammalian eye. Eur J Cell Biol. 2011;90(5):440–448. doi: 10.1016/j.ejcb.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eleni T, Mohamed I-T, Kiosses WB, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 56.Carmen U, Walter DH, Zeiher AM, Stefanie D. Laminar shear stress upregulates integrin expression role in endothelial cell adhesion and apoptosis. Circ Res. 2000;87(8):683–689. doi: 10.1161/01.res.87.8.683. [DOI] [PubMed] [Google Scholar]
- 57.Tzima E, Miguel AP, Sanford JS, et al. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20(17):4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshito Y, Quoc TB, Karina R, et al. Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP in the vascular remodeling. Proc Natl Acad Sci USA. 2020;117(18):9896–9905. doi: 10.1073/pnas.1919702117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polacheck WJ, Kutys ML, Jinling Y, et al. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature. 2017;552(7684):258–262. doi: 10.1038/nature24998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie X, Zhou T, Wang Y, et al. Blood flow regulates zebrafish caudal vein plexus angiogenesis by ERK5-klf2a-nos2b signaling. Curr Mol Med. 2018;18(1):3–14. doi: 10.2174/1566524018666180322153432. [DOI] [PubMed] [Google Scholar]
- 61.Ting S, Xiang X, Jianqing Z, et al. Effect of horizontal rotary culture on zebrafish vascular development (in Chinese) Hereditas. 2013;35(4):502–510. doi: 10.3724/SP.J.1005.2013.00502. [DOI] [PubMed] [Google Scholar]
- 62.Xiang X, Daoxi L, Qian Z, et al. Effect of simulated microgravity induced PI3K-nos2b signalling on zebrafish cardiovascular plexus network formation. J Biomech. 2019;87:83–92. doi: 10.1016/j.jbiomech.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 63.Jennifer LL, Elizabeth AVJ, Chengqun H, et al. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134(18):3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bum KH, Shengpeng W, Helker Christian SM, et al. In vivo modulation of endothelial polarization by Apelin receptor signalling. Nat Commun. 2016;7:11805. doi: 10.1038/ncomms11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beata W-S, Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol. 2003;161(2):429–439. doi: 10.1083/jcb.200210135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akira K, Razaq B, Tatsuo T. Adaptive regulation of wall shear stress optimizing vascular tree function. Bull Math Biol. 1984;46(1):127–137. doi: 10.1007/BF02463726. [DOI] [PubMed] [Google Scholar]
- 67.Stefanie D, Birgit A, Corinna H, et al. Fluid shear stress stimulates phosphorylation of akt in human endothelial cells: involvement in suppression of apoptosis. Circ Res. 1998;83(3):334–341. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- 68.Masafumi T, Berk BC. Mitogen-activated protein kinase (ERK1/2) activation by shear stress and adhesion in endothelial cells: Essential role for a herbimycin-sensitive kinase. J Clin Invest. 1996;98(11):2623–2631. doi: 10.1172/JCI119083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eguiluz RCA, Kaylan KB, Underhil GH, Leckband DE. Substrate stiffness and VE-cadherin mechano-transduction coordinate to regulate endothelial monolayer integrity. Biomaterials. 2017;140:45–57. doi: 10.1016/j.biomaterials.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mui KL, Chen CS, Assoian RK. The mechanical regulation of integrin-cadherin crosstalk organizes cells, signaling and forces. J Cell Sci. 2016;129(6):1093–1100. doi: 10.1242/jcs.183699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramasri S, Paulina B-G, Müller CE, et al. P2Y2 receptor modulates shear stress-induced cell alignment and actin stress fibers in human umbilical vein endothelial cells. Cell Mol Life Sci. 2017;74(4):731–746. doi: 10.1007/s00018-016-2365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conway DE, Breckenridge MT, Elizabeth H, et al. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol. 2013;23(11):1024–1030. doi: 10.1016/j.cub.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masaki O, Michitaka M, Ichi KK, Keigi F. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol. 2002;158(4):773–785. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coon BG, Nicolas B, Jinah H, et al. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J Cell Biol. 2015;208(7):975–986. doi: 10.1083/jcb.201408103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nicolas B, Schwartz MA. Biomechanics of vascular mechanosensation and remodeling. Mol Biol Cell. 2016;27(1):7–11. doi: 10.1091/mbc.E14-11-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baeyens N, Nicoli S, Coon BG, et al. Vascular remodeling is governed by a vegfr3-dependent fluid shear stress set point. Elife. 2015;4:e04645. doi: 10.7554/eLife.04645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhongming C, Ellie T. PECAM-1 is necessary for flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29(7):1067–1073. doi: 10.1161/ATVBAHA.109.186692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conway DE, Coon BG, Madhusuthan B, et al. VE-cadherin phosphorylation regulates endothelial fluid shear stress responses through the polarity protein LGN. Curr Biol. 2017;27(14):2219–2225.e5. doi: 10.1016/j.cub.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vincenza C, Peacock HM, Bahar K, et al. Shear stress and VE-cadherin the molecular mechanism of vascular fusion. Arterioscler Thromb Vasc Biol. 2018;38(9):2174–2183. doi: 10.1161/ATVBAHA.118.310823. [DOI] [PubMed] [Google Scholar]
- 80.Marta S, Hidetaka O, Dragan M, et al. Semaphorin 6A regulates angiogenesis by modulating VEGF signaling. Blood. 2012;120(19):4104–4115. doi: 10.1182/blood-2012-02-410076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wencao Z, Le C, Hanru Y, et al. Endothelial CDS2 deficiency causes VEGFA-mediated vascular regression and tumor inhibition. Cell Res. 2019;29(11):895–910. doi: 10.1038/s41422-019-0229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ivan L, Natalia M. The role of Dll4/Notch signaling in normal and pathological ocular angiogenesis: Dll4 controls blood vessel sprouting and vessel remodeling in normal and pathological conditions. J Ophthalmol. 2018;2018:3565292. doi: 10.1155/2018/3565292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rostama B, Peterson Sarah M, Vary Calvin PH, Lucy L. Notch signal integration in the vasculature during remodeling. Vascul Pharmacol. 2014;63(2):97–104. doi: 10.1016/j.vph.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bart W, Edgar G, Saikin SK, et al. Blood flow-induced Notch activation and endothelial migration enable vascular remodeling in zebrafish embryos. Nat Commun. 2018;9(1):5314. doi: 10.1038/s41467-018-07732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Manuel E, Susanne A, Rui B, Adams RH. Notch controls retinal blood vessel maturation and quiescence. Development. 2013;140(14):3051–3061. doi: 10.1242/dev.093351. [DOI] [PubMed] [Google Scholar]
- 86.Lobov IB, Eunice C, Rajeev W, et al. The DII4/Notch pathway controls postangiogenic blood vessel remodeling and regression by modulating vasoconstriction and blood flow. Blood. 2011;117(24):6728–6737. doi: 10.1182/blood-2010-08-302067. [DOI] [PubMed] [Google Scholar]
- 87.Kun PL, Michael P, Leslie JD, et al. Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis. Dev Cell. 2009;16(1):70–82. doi: 10.1016/j.devcel.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhongxiao W, Chi-Hsiu L, Shuo H, Jing C. Wnt signaling in vascular eye diseases. Prog Retin Eye Res. 2019;70:110–133. doi: 10.1016/j.preteyeres.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hans C, Roel N. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 90.Elisabetta D. The role of wnt signaling in physiological and pathological angiogenesis. Circ Res. 2010;107(8):943–952. doi: 10.1161/CIRCRESAHA.110.223750. [DOI] [PubMed] [Google Scholar]
- 91.Claudia K, Beate S, Junhao H, et al. Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis. Development. 2014;141(8):1757–1766. doi: 10.1242/dev.104422. [DOI] [PubMed] [Google Scholar]
- 92.Olga K, Bisei O, Melanie H, et al. The Wnt signaling regulator R-spondin 3 promotes angioblast and vascular development. Development. 2008;135(22):3655–3664. doi: 10.1242/dev.027284. [DOI] [PubMed] [Google Scholar]
- 93.Beate S, Claudia K, Jessica W, et al. Endothelial RSPO3 controls vascular stability and pruning through non-canonical WNT/Ca2+/NFAT signaling. Dev Cell. 2016;36(1):79–93. doi: 10.1016/j.devcel.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 94.Franco CA, Jones ML, Bernabeu MO, et al. Non-canonical wnt signalling modulates the endothelial shear stress flow sensor in vascular remodeling. Elife. 2016;5:e07727. doi: 10.7554/eLife.07727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jenna M, Smith JR, Sanjid S, et al. The Wnt inhibitor apcdd1 coordinates vascular remodeling and barrier maturation of retinal blood vessels. Neuron. 2017;96(5):1055–1069.e6. doi: 10.1016/j.neuron.2017.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Glaw JT, Skalak TC, Peirce SM. Inhibition of canonical Wnt signaling increases microvascular hemorrhaging and venular remodeling in adult rats. Microcirculation. 2010;17(5):348–357. doi: 10.1111/j.1549-8719.2010.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qiang Xu, Yanshu W, Alain D, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116(6):883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 98.Xin Ye, Yanshu W, Hugh C, et al. Norrin, Frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139(2):285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lucia C, Prykhozhij SV, Elizabeth C, et al. Frizzled 4 regulates ventral blood vessel remodeling in the zebrafish retina. Dev Dyn. 2019;248(12):1243–1256. doi: 10.1002/dvdy.117. [DOI] [PubMed] [Google Scholar]
- 100.Anna C, Stefan L, Radiosa G, et al. The conditional inactivation of the β-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162(6):1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Monica C, Daniel N, Fabrizio O, et al. The Wnt/β-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/notch signaling. Dev Cell. 2010;18(6):938–949. doi: 10.1016/j.devcel.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen J, Stahl A, Krah NM, et al. Retinal expression of Wnt-pathway mediated genes in low-density lipoprotein receptor-related protein 5 (Lrp5) knockout mice. PLoS ONE. 2012;7(1):e30203. doi: 10.1371/journal.pone.0030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Luhmann Ulrich FO, Jihong L, Niyazi A, et al. Role of the Norrie disease pseudoglioma gene in sprouting angiogenesis during development of the retinal vasculature. Invest Ophthalmol Vis Sci. 2005;46(9):3372–3382. doi: 10.1167/iovs.05-0174. [DOI] [PubMed] [Google Scholar]
- 104.Sujata R, Lobov IB, Vallance JE, et al. Obligatory participation of macrophages in an angiopoietin 2-mediated cell death switch. Development. 2007;134(24):4449–4458. doi: 10.1242/dev.012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xie Xiang Hu, Jianjun WG. Advance in biomechanical study of embryonic vascular system development (in Chinese) Hereditas. 2012;34(9):1123–1132. doi: 10.3724/SP.J.1005.2012.01123. [DOI] [PubMed] [Google Scholar]
- 106.Wang G, Long T, Ying D. Progress and prospect in research of effect of hemodynamic factors on vascular remodeling (in Chinese) J Chongqing Univ. 2003;26(8):102–105. doi: 10.11835/j.issn.1000-582X.2003.08.027. [DOI] [Google Scholar]
- 107.Thomas G. Notch signaling in the vasculature. Curr Top Dev Biol. 2010;92:277–309. doi: 10.1016/S0070-2153(10)92009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated during this study are included in this published article.