Abstract
Iron is an essential micro-element, involved in multiple biological activities in vertebrates. Excess iron accumulation has been identified as an important mediator of lipid deposition. However, the underlying mechanisms remain unknown. In the present study, we found that a high-iron diet significantly increased intestinal iron content and upregulated the mRNA expression of two iron transporters (zip14 and fpn1). Intestinal iron overload increased lipogenesis, reduced lipolysis and promoted oxidative stress and mitochondrial dysfunction. Iron-induced lipid accumulation was mediated by hypoxia-inducible factor-1 α (HIF1α), which was induced in response to mitochondrial oxidative stress following inhibition of prolyl hydroxylase 2 (PHD2). Mechanistically, iron promoted lipid deposition by enhancing the DNA binding capacity of HIF1α to the pparγ and fas promoters. Our results provide experimental evidence that oxidative stress, mitochondrial dysfunction and the HIF1α-PPARγ pathway are critical mediators of iron-induced lipid deposition.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-022-04423-x.
Keywords: Iron overload, Oxidative stress, Mitochondrial dysfunction, Lipid metabolism
Introduction
Iron (Fe) is an essential mineral required for multiple physiological processes in vertebrates, including energy metabolism, oxygen transport and DNA synthesis/repair [1, 2]. However, excess iron is toxic due to its redox reactivity, which promotes oxidative stress, a feature of multiple disorders [3]. Consequently, iron metabolism is tightly controlled. Iron transport and regulation involves several proteins, including divalent metal transporter 1 (DMT1), ZRT-IRE-like protein 14 (ZIP14), transferrin, transferrin receptors (TFR1 and TFR2), ferroportin (FPN1), ceruloplasmin, hephaestin, ferritin and hepcidin [1, 4].
Lipids are essential metabolites, constitute building blocks of cellular membranes and participate in multiple metabolic processes [5, 6]. Lipid metabolism is disrupted in various diseases, such as obesity, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD) [7]. Previous studies have investigated the effects of dietary iron on lipid stores and metabolism in the liver [8–10], but analogous studies in the intestinal tract are currently lacking. Intestinal enterocytes play a crucial role in dietary absorption and metabolism of iron [11] and lipids [12]. However, potential crosstalk between intestinal lipid and iron metabolism has not been explored.
Mitochondria constitute the major intracellular source of reactive oxygen species (ROS) [13]. At physiological concentrations, ROS are essential for normal cellular function and homeostasis. However, excessive ROS accumulation can damage lipids and proteins, leading to mitochondria dysfunction [14]. Oxidative stress and the ensuing damage of mitochondria, but also whole cells and tissues, is further aggravated by catalytic amounts of unshielded iron via Fenton and Haber–Weiss reactions [15, 16]. ROS toxicity is counterbalanced by ROS scavenging systems, which include key antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) [17]. Furthermore, ROS toxicity is ameliorated by tight control of iron homeostasis. There is evidence that oxidative stress regulates lipogenic metabolism by disrupting mitochondrial functions and by reducing β-oxidation of fatty acids [6, 18]. Thus, we hypothesize that mitochondrial oxidative stress is a key mediator of iron-induced effects on lipid metabolism.
Hypoxia-inducible factors (HIF) are heterodimeric transcription factors composed of regulated HIF-α (HIF1α, HIF2α or HIF3α) and a constitutive HIF1β subunit [19–21]. HIF-1 is the most extensively studied isoform [20]. Under a hypoxic environment, the HIF1α subunit is stabilized against oxidative degradation and translocates from the cytoplasm to the nucleus. This allows its interaction with HIF1β to form HIF-1, which in turn binds to hypoxia response elements (HREs; 5′-AGCGTG-3′) in the promoter region of target genes for transcriptional regulation [22]. HIF1α is not only stabilized under hypoxia but also in response to oxidative stress. The mechanism involves inhibition of prolyl hydroxylase 2 (PHD2s), an iron-and oxygen-dependent enzyme that tags HIF1α for degradation via the proteasome pathway [23]. HIF1α is an important regulator of many cellular processes including energy metabolism [24]. However, little is known on how HIF1α affects lipid metabolism.
Fishes constitute the most diverse and widely distributed group of vertebrates in the world. Although fishes are not evolutionarily close to mammals, their metabolic pathways and nutritional sensing systems are conserved [25]. Thus, teleost fishes represent attractive models to explore the novel function of genes and their impact on metabolism and physiology. Luo et al. [10] demonstrated that dietary iron supplementation modulates lipid metabolism in the liver of yellow catfish Pelteobagrus fulvidraco, an omnivorous freshwater aquaculture species widely distributed in several Asian countries including China. However, there are no studies on how dietary iron affects intestinal lipid metabolism. We previously demonstrated that intestinal lipid accumulation is associated with oxidative stress and HIF1α induction[10]. In light of these findings, we sought herein to explore whether iron influenced lipid metabolism via the interplay between oxidative stress and HIF1αin the intestine of the yellow catfish.
Materials and methods
Ethical statement
The experimental procedures performed in animals followed the Institutional Ethics guidelines of Huazhong Agricultural University (HZAU) and were approved by the Ethics Committee of HZAU (identification code: Fish-2019-09-21). Circumstances relating to yellow catfish culture meet the International Guiding Principles for Biomedical Research Involving Animals, as issued by the Council for the International Organizations of Medical Sciences. All efforts were made to minimize suffering in animals.
Chemicals and reagents
The kits for Oil Red O staining and analysis of triglycerides (TG), total protein and activities of T-SOD, CAT, GPX and T-AOC were from Nanjing Jian Cheng Bioengineering Institute (Nanjing, China). MDA content and GSH/GSSG ratio were measured with kits from Beyotime Biotechnology (Shanghai, China). Trizol reagent, radioactive immunoprecipitation assay (RIPA) lysis buffer, PMSF protease, Bodipy 493/503 and Lipofectamine 2000 were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), phosphate buffer saline (PBS), fetal bovine serum (FBS) and 0.25% trypsin–EDTA were from Gibco company (Thermo Fisher Scientific, Waltham, MA, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was supplied by Aladdin industrial Inc. The ATP contents assay kits, BCA Protein Assay Kit, DCFH-DA fluorescence probe and JC-1 probe were obtained from Beyotime Biotechnology (Shanghai, China). The MitoSOX™ fluorescent probe was obtained from Invitrogen (California, USA). Dual-Luciferase and Passive Lysis Buffer were from Promega company (Madison, WI, USA). Other reagents used in the present study were analytical grade and purchased from Sinopharm chemical Reagent Co. Ltd. (Shanghai, China).
Animals feeding and sampling
The experimental protocols for feed formulation, the culture and management of yellow catfish were as described in our recent study [26, 27]. Three experimental diets were formulated with ferrous sulfate monohydrate (FeSO4·7H2O) supplemented at 0 (L-Fe), 200 Fe (M-Fe) and 2000 (H-Fe) mg/kg diet. The dietary Fe content in the three experimental diets was determined to be 43.72 (L-Fe), 84.07 (M-Fe), and 483.96 (H-Fe) mg/kg by inductively coupled plasma optical emission spectrometry (ICP-OES, Optima 8000DV, PerkinElmer, USA), respectively. Based on the study by Luo et al. [10], 55.73 mg Fe/kg diet was the optimal dietary iron requirement for yellow catfish, while M-Fe and H-Fe diets were supplemented with iron in excess and have toxic effects on the yellow catfish. The experimental procedures were described in our previous studies [26, 27]. In brief, 180 uniform-sized yellow catfish (initial body weight: 10.51 ± 0.07 g, mean ± SEM) were randomly stocked in 9 circular tanks (300 L in water volume), 20 fish for each tank. Each experimental diet was assigned to three tanks. During the feeding period, the fish were fed to the satiation twice daily. The measured Fe content in water was 0.0148 ± 0.0001 mg/L throughout the feeding experiment, which lasted for 12 weeks.
At the end of the 12-wk experiment, all animals were fasted for 24 h before sampling to avoid post-prandial effects. Then yellow catfish were euthanized with 100 mg/L MS-222 (Sinopharm Chemical Reagent Co., Ltd, AE1052101) solution. Another six yellow catfish were randomly collected from each tank, and the intestinal samples were obtained, frozen in liquid nitrogen and stored in a − 80 °C freezer for subsequent RNA and protein isolation. In addition, another three yellow catfish were selected from each tank and the intestinal tissues were used for histological and ultrastructural analysis and for immunofluorescence. Another six yellow catfish were collected from each tank, and their intestinal tissues were frozen in liquid nitrogen, and then kept in a − 80 °C freezer for subsequent analysis of intestinal TG, iron and ATP concentrations, and enzymatic activities.
Cell culture and treatments
Intestinal epithelial cells (IECs) were isolated from the intestine of yellow catfish based on the protocols described in our recent publication [28, 29]. They were cultured in Dulbecco’s Modified Eagle’s medium (Thermo Fisher Scientific) media containing 10% fetal bovine serum (FBS) (Thermo Fisher Scientific) in an incubator at 28℃. HEK293T cell lines were from the Cell Resource Center of HZAU and used for the analysis of promoter function. They were cultured in DMEM supplemented with 10% FBS containing 5% CO2 at 37℃. The primary IECs of yellow catfish were incubated in the control or Fe (300 μM) for 24 h in DMEM, or combined with 2-h DFO (100 μM, 138-14-7, MCE) pretreatment to explore whether Fe affected oxidative stress and lipid metabolism. Mito-TEMPO (15 μM, Sigma) was used as a mitochondria-targeted superoxide dismutase mimetic. The concentrations of Fe, Mito-TEMPO and DFO were selected according to our pilot and recent studies [27]. For example, 300 μM iron had no significant adverse effects on cell viability (Fig. S1), and used as the optimal concentration for in vitro studies, in combination with 100 μM DFO or 15 μM Mito-TEMPO.
Sample analysis
Oil red O, Prussian blue staining and transmission electron microscopy (TEM)
Oil red O (ORO) and Prussian blue staining were conducted to determine the amounts of LDs and iron (hemosiderin) deposition in intestinal specimens of yellow catfish, according to the manufacture’s instruction. For the statistical analysis of the relative areas of LDs in ORO staining, we randomly quantified ten fields from each sample by the software Image J (NIH, Bethesda, MD, USA). TEM observation was conducted as the method by Zhao et al. [26].
Measurement of TG, TC, NEFA, total lipid, iron and ATP content in the intestine and isolated IECs of yellow catfish
TG, TC and NEFA contents were analyzed with commercial kits (Nanjing Jian Cheng Bioengineering Institute, Nanjing, China), based on the manufacturer’s instructions. Intestinal total lipid contents were measured using the Dyer and Bligh procedure as previously described [30]. Intestinal iron content was determined by ICP-AES based on Chen et al. [27]. ATP content was measured using an ATP Assay Kit (Beyotime, Haimen, China). Soluble protein concentrations were determined with a Bradford protein assay kit (A045-2-2; Nanjing Jiancheng Bioengineering Institute).
Detection of ADP/ATP ratio in the intestine and isolated IECs of yellow catfish
ADP/ATP ratio assay was carried out using ADP/ATP Ratio Assay Kit (Abnova Corporation, China) based on the manufacturer’s instructions. The assay involved two steps. In the first step, the working reagent lysed cells to release ATP and ADP. In the presence of luciferase, ATP immediately reacted with the substrate D-luciferin to produce light. Then, the concentration of intracellular ATP was directly measured according to the brightness. In the second step, the ADP was converted to ATP through an enzyme reaction. This newly formed ATP reacted with D-luciferin as mentioned in the first step.
Determination of enzymatic activities, ferritin concentration and indices of oxidative stress in the intestine and isolated IECs of yellow catfish
To investigate the effects of Fe on lipid metabolism, the activities of several enzymes related to lipogenesis, such as glucose 6-phosphate dehydrogenase (G6PD), 6-phosphogluconate dehydrogenase (6PGD), malic enzyme (ME), isocitrate dehydrogenase (ICDH) and fatty acid synthase (FAS), and the activity of carnitine palmitoyltransferase 1 (CPT1) involved in fatty acid β-oxidation were performed according to the methods described in our previous studies [17, 28]. The concentration of FTL (YJ150129) and FTH (YJ103695) was measured using a fish Ferritin ELISA kit (Shanghai Enzyme-linked Biotechnology Co., Ltd; Shanghai, China), according to the manufacturer’s instructions. Intestine and isolated IECs samples were diluted per manufacturer instructions. Absorbance measurements were recorded at 450 nm using a spectrophotometer. The activity of total superoxide dismutase (total-SOD, A001-3), catalase (CAT, A007-1-1) and glutathione peroxidase (GPX, A005-1-1), total antioxidant capacity (T-AOC, A015-1) were analyzed by using kits from Nanjing Jiancheng Institute of Bioengineering (Nanjing, China). The malondialdehyde (MDA, S0131S, Beyotime, China) content, and GSH/GSSG (S0053, Beyotime, China) ration were determined with kits from Beyotime Biotechnology.
RNA isolation and quantitative real-time PCR (qPCR) assay in the intestine and isolated IECs of yellow catfish
We used quantitative PCR to measure the mRNA expression of genes related to iron metabolism (dmt1, zip14, fpn1, hephaestin, transferrin, tfr1, tfr2, hepcidin, mtf-1, irp1 and irp2), lipogenesis (g6pd, 6pgd, acca, fas, lxr, pparγ and srebp1), lipolysis (hsl, atgl, cpt1α, pparα), β-oxidation (acads, acad8, acadsb, acadm, acadl, acox1, acox3 and hadhb), oxidative stress (sod1, sod2, gpx1, cat, keap1, nrf2, phd2, hif1α and hif2α), ferroptosis (acsl4, slc7a11, gpx4, lpcat3, ptgs2, lox and nox1), and glycolysis (gk, hk1, hk2, pfk, pgk1, pk, ldha and glut1). Total RNA was isolated with the Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA) and transcribed into cDNA by using a Reverse Transcription Kit (TaKaRa, Tokyo, Japan). qPCR assays were performed based on our published protocols [26]. Eight housekeeping genes (β-actin, beta2-microglobulin (b2m), translation elongation factor (elfa), glyceraldehyde-3-phosphate dehydrogenase (gapdh), hypoxanthine–guanine phosphoribosyltransferase (hprt), ribosomal protein L7 (rpl7), 18S ribosomal RNA and ubiquitin-conjugating enzyme (ubce)) were used to analyze their expression stability. The geNorm software (https://genorm.cmgg.be/) was used to determine the most stable two genes as the control. The 2−ΔΔCt method was used to calculate the mRNA abundances of genes. The primers for qPCR were given in Table S1.
Western blot analysis of protein expression in the intestine and isolated IECs of yellow catfish
We used Western blotting to analyze protein expression, based on the protocols described in our studies [26]. Briefly, the total protein of intestinal tissues and IECs were extracted by the RIPA lysis buffer (Thermo Fisher Scientific), including the PMSF protease inhibitor (Thermo Fisher Scientific). Then, we used the BCA Protein Assay Kit (Beyotime Biotechnology, Shanghai, China) to determine the protein concentration. Equal amount of protein extracts (20 mg) was separated by 10 or 12% SDS PAGE and then transferred to PVDF membranes. The membranes were blocked with the TBST with 8% skim milk powder for 1.5 h. Then the membranes were incubated overnight at 4 °C with antibodies against DMT1 (1:1000, A10231, ABclonal, Wuhan, China), Fpn1 (1:1000, A14884, Abclonal, Wuhan, China), GAPDH (1:10000, 10494-1-AP; Proteintech Group, Wuhan, China), HIF1α (1:1000, 20,960–1-AP, Proteintech Group, Wuhan, China), HIF2α (1:1000, 26422-1-AP, Proteintech Group, Wuhan, China), PHD2 (1:1000, 19886-1-AP, Proteintech Group, Wuhan, China), PPARγ (1:1000, 16643-1-AP, Proteintech Group, Wuhan, China), SREBP1 (1:1000, 14088-1-AP, Proteintech Group, Wuhan, China) and ZIP14 (1:1000, A10413, Abclonal, Wuhan, China). Next day, the membranes were incubated with corresponding secondary antibodies HRP-conjugated anti-rabbit IgG antibody (7074; Cell Signaling Technology, Danvers, MA, USA). After washing, the Image-Pro Plus 6.0 software (Media Cybernetics) was used to quantify the western blot results. All experiments were repeated at least three times.
Immunofluorescence analysis of intestinal tissue of yellow catfish
We used immunofluorescence to measure the distribution and expression of HIF1α protein in the intestine as described in our precious study [26]. Generally, samples were fixed with 4% paraformaldehyde, permeabilized with PBS containing 0.1% Triton X-100 (PBS-T), and blocked with normal goat serum. Slides were blocked for 30 min with normal goat serum and incubated overnight at 4 °C with the anti-HIF1α antibody (1:250, 20960-1-AP, Proteintech Group, Wuhan, China). After a wash step, slides were incubated with IgG H&L (Alexa Fluor® 647, 1:500, ab150079; Abcam, Cambridge, MA, USA) for 1 h at room temperature in the dark, and then nuclei were stained with DAPI (1 μg/mL, ab228549; Abcam, Cambridge, MA, USA). The images of the section were captured with laser scanning confocal microscope (Leica Microsystems, Wetzlar, Germany).
Cell viability
Cell viability was tested by the 3-(4,5-dimethyl-2-thiazolyl) -2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay, based on the methods described in our recent publication [28].
Bodipy 493/503 staining of the isolated IECs of yellow catfish
Bodipy 493/503 staining were conducted as described by Zhao et al. [26]. The IECs were cultured in twelve-well plates, incubated with the corresponding treatments for 24 h, washed twice with PBS and incubated with 5 μg/ml Bodipy 493/503 (D3922; Thermo Fisher Scientific) for 30 min, followed by thrice PBS washes. The IECs were observed using a laser scanning confocal microscope (Leica, Germany) to visualize the intensity of the fluorescence. The green dots were defined as lipid droplets, which were quantified with a CytoFlex flow cytometer (Beckman Coulter).
Detection of cellular Fe2+, cytosolic ROS and mitochondrial ROS contents in the isolated IECs of yellow catfish
Labile iron concentrations in cytoplasm were assessed by using FerroOrange probes (10 μM, Dojindo Molecular Technology, Japan) through a confocal microscope, respectively. Cytosolic and mitochondrial ROS contents were detected by 2,7-dichlorodihydro-fluorescein diacetate (DCFH-DA, 287810, Sigma) or MitoSOX™ Red mitochondrial superoxide indicator (M36008, Thermo Fisher Scientific, USA), according to the manufacturer’s protocols. In brief, the IECs were seeded in 6-well plates and harvested after the corresponding treatment. After the treatment for 24 h, cells were collected and washed twice with PBS, and then covered with 10 μM DCFH-DA or MitoSOX reagent stock solution (5 μM, diluted with PBS) for 30 min at 37 °C. After washing three times with PBS, the IECs were stained with 5 μg/mL DAPI (1 μg/mL, ab228549; Abcam, Cambridge, MA, USA) at 37 ℃ for 15 min in the dark and then washed three times with PBS. Finally, the IECs were observed using a laser scanning confocal microscope (Leica, Germany) to visualize the intensity of the fluorescence. We quantified the fluorescence of the stained cells on a CytoFlex Flow Cytometer (Beckman Coulter) and performed data analysis with FlowJo v.10 software.
Determination of mitochondrial membrane potential (MMP) of isolated IECs
The mitochondrial membrane potentials (MMP) of the IECs were monitored using 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolcarbocyanine (JC-1) (5 μg/mL, C2006, Beyotime, China), according to the protocols in our publications [17]. Firstly, the IECs were seeded on slides. After treatment, the IECs were incubated with JC-1 staining solution (5 μg/mL) for 30 min at 37 °C in the dark. Then the cells were washed with PBS, and the fluorescence was observed with a laser scanning confocal microscope (TCS, SP8, Leica Microsystems, Wetzlar, Germany). JC-1 monomer (green) fluorescence was excited at the wavelength of 485 nm and the emission was detected at 530 nm. JC-1 aggregate form (red) fluorescence was excited at 485 nm and the emission was detected at 590 nm. The change in MMP of the IECs was calculated as the fluorescence ratio of red to green.
HEK293T cells transfection and Dual-luciferase reporter assay
HEK293T cells were first seeded in plates for 24 h (80–90% confluency in each 24-well plate), and then plasmids were transfected as described in our previous studies [31]. The 400 ng reporter plasmids and 20 ng pRL-TK were co-transfected into HEK293T cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, US) following the manufacturer’s protocols and our previous study [31]. After 4 h, DMEM (10% FBS) or DMEM (10% FBS) + 300 μM Fe was used to replace the transfection medium. Then, after 24-h treatment, the cells were collected for the determination of the relative luciferase activities by the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
The HIF1α binding sites of pparγ and fas promoters from yellow catfish were predicted, based on the protocols in our recent study [18]. The site mutation of the HIF1α binding sites was conducted via the QuickChange II Site-Directed Mutagenesis Kit (Vazyme, Piscataway, NJ, US) based on the manufacturer’s instructions. pGl3-pparγ-1575 and pGl3-fas -1525 were used as the templates. The mutagenesis primers were presented in Table S2. The mutant plasmids were named as HRE1-pparγ, HRE2-pparγ, HRE1- pparγ, HRE2-fas, and HRE3-fas, respectively. The activities of promoters were determined by the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
Electrophoretic mobility-shift assay (EMSA)
The EMSA was conducted to determine the direct binding of HIF1α to pparγ and fas promoters, based on our published protocols [31]. We incubated each oligonucleotide duplex of HIF1α binding sites with 10 µg nuclear extracts via the LightShift Chemiluminescent EMSA Kit (Invitrogen, Carlsbad, CA, USA). At first, we pre-incubated each unlabeled probe for 10 min. Then, the biotin-labeled probe was added and reacted at room temperature for 30 min. Finally, we resolved the main complexes in 6% native polyacrylamide gels electrophoresis using 0.5 × TBE buffer for 1 h. In this study, 100-fold unlabeled double stranded oligonucleotides were used for the competition analyses with or without the mutation. The oligonucleotide sequences of EMSA were presented in Table S2.
Statistical analysis
All statistical analysis was undertaken with SPSS 19.0 software (Armonk, NY, USA). Each experiment was performed independently at least three times and data were presented as means ± standard errors of means (SEM). To compare the differences among three treatments (low Fe, middle Fe and high Fe), we used one-way analysis of variance and post hoc Duncan’s multiple range test. Data analysis between two groups was performed using Student’s T-test for independent samples. Differences were considered significant at P < 0.05.
Results
In vivo studies
High dietary iron accumulates in the intestine of yellow catfish and modulates lipid metabolism
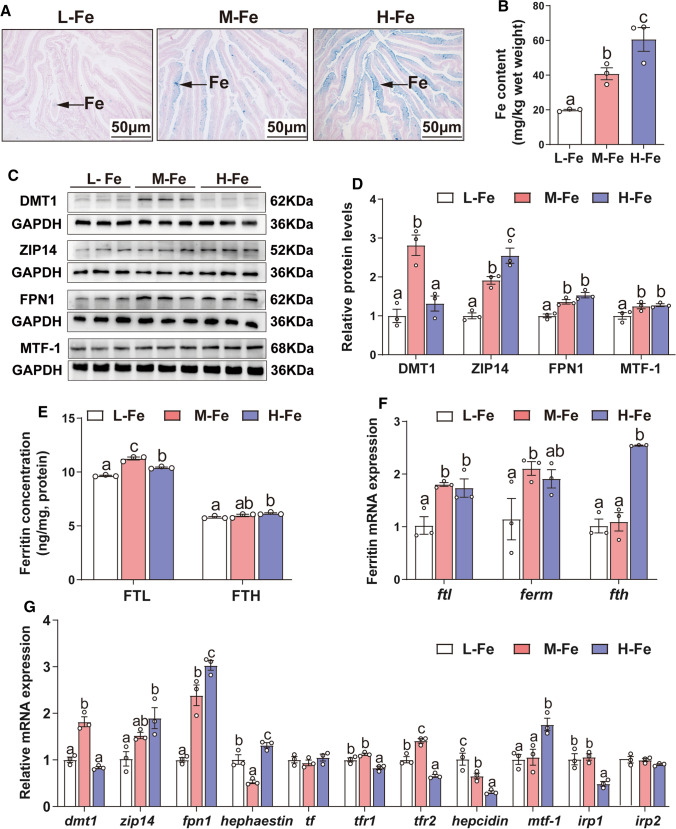
We aimed to investigate the effects of dietary iron supplementation on intestinal iron deposition and lipid metabolism in yellow catfish. Thus, following exposure of the animals to diets with variable iron content, we determined intestinal tissue iron concentration, as well as the expression of genes of iron and lipid metabolism at the protein and mRNA levels. Prussian blue staining and ICP-AES assay showed that intestinal iron content was proportional to the dietary iron concentration (Fig. 1A, B). Expression of DMT1, the apical iron transporter in mammalian enterocytes, was higher in the control M-Fe group compared to the others. By contrast, the expression of the zinc and iron transporter Zip14 increased with increasing dietary iron levels. Finally, levels of FPN1, the basolateral iron transporter in mammalian enterocytes, and the metal regulatory transcription factor MTF-1 were decreased in the L-Fe group and showed no significant differences between the other two groups (Fig. 1C, D). Compared with the L-Fe group, H-Fe diet increased the concentration of FTL and FTH (Fig. 1E). The mRNA expression of ftl and ferm were lowest in L-Fe group, and shown no significant differences between the other two groups. Compared to the L- and M-Fe group, H-Fe increased the mRNA abundance of fth (Fig. 1F). The increased ferritin expression during iron overload reflects the need to store excess iron, minimizing free iron toxicity. Among three dietary groups, dmt1 and tfr2 mRNA expression were highest, and hephaestin mRNA expression was lowest for fish fed the M-Fe diet. Expression of zip14, fpn1 and mtf-1 mRNAs increased, but tfr1, hepcidin and irp1 mRNAs decreased in response to high dietary iron. The transferrin and irp2 mRNA levels showed no significant differences among the three groups (Fig. 1G). These data suggest that high dietary iron leads to intestinal iron overload and modulates the expression of iron proteins.
Fig. 1.
Effects of dietary Fe levels on Fe metabolism in the intestine tissues of yellow catfish. A Prussian blue staining. B Fe content. C, D Protein expression of Fe transport-related genes (DMT1, ZIP14, FPN1 and MTF-1). E The ferritin concentration. F The relative mRNA expression of Ferritin (ftl, ferm and fth). G The relative mRNA expression of genes related to Fe metabolism. Values are shown as means ± SEM (n = 3). Bars that share different letters within the same gene indicate significant differences among the three groups (P < 0.05)
Dietary iron addition increased the number of intestinal lipid droplets (Fig. 2A, B), TG (Fig. 2C), lipid and TC content (Fig. S2A-B) in the intestine. Compared with the L-Fe group, H-Fe diet significantly increased the activities of lipogenic enzymes (G6PD, 6PGD, ME, ICDH and FAS) (Fig. 2D), and decreased the activity of lipolytic enzyme CPT1 (Fig. S2C). SREBP1 and PPARγ protein levels were the highest in fish fed the H-Fe diet and showed no significant differences between other groups (Fig. 2E–G). Furthermore, we also examined the expression of glycolysis-related genes. The mRNA expression of hk1, pfk and gltu1 were lowest in the L-Fe group, and showed no significant differences between the other two groups. Compared to the L- and M-Fe group, H-Fe increased the mRNA abundance of gk and pk. Among the three dietary groups, ldha mRNA expression was highest, and pgk1 mRNA expression was lowest for fish fed the M-Fe diet. The hk2 mRNA expression did not significantly differ among three treatments (Fig. S2D). Compared to the L-Fe group, adequate or high dietary iron up-regulated mRNA expression of lipogenic genes (g6pd, 6pgd, acca and fas), and the lipogenic transcriptional factors (lxr, pparγ and srebp1), and down-regulated the mRNA expression of lipolytic genes (hsl, cpt1 and pparα) (Fig. 2H). Thus, dietary iron addition promoted glycolysis to increase lipogenesis, inhibited lipolysis and increased TG deposition.
Fig. 2.
Effects of dietary Fe levels on lipid metabolism in the intestine tissues of yellow catfish. A Representative microphotograph of oil red O staining (200 × magnification; bars, 50 μm); Ld, lipid droplet. B Relative areas for lipid droplets in oil red O staining. C TG content. D Activities of lipogenic enzymes (G6PD, 6PGD, ME, ICDH and FAS). E–G Western blot and protein expression of lipogenic genes. H The relative mRNA expression of lipid metabolism-related genes. Values are shown as means ± SEM (n = 3. Bars that share different letters within the same gene indicate significant differences among the three groups (P < 0.05)
High dietary iron induces oxidative stress and increases expression of HIF1α in the intestine of yellow catfish
Prompted by the evidence that oxidative stress modulates lipid metabolism [18, 32], we investigated the effects of dietary Fe supplementation on intestinal redox status. Dietary iron significantly decreased activities of the antioxidant enzymes GPX, T-SOD and CAT, as well as the GSH/GSSG ratio, and increased MDA content (Fig. 3A–C). Compare with the L-Fe group, high dietary iron also decreased sod2 and gpx1, and increased keap1 and nrf2 mRNAs. Expression of sod1 mRNA was the lowest in the M-Fe group and showed no significant differences between the other two groups (Fig. 3D). Expression of cat mRNA did not respond to dietary iron manipulations. In addition, we also examined the expression of genes involved in ferroptosis. Compared to the L-Fe group, H-Fe increased the mRNA abundance of acsl4, ptgs2, lox and nox1, decreased the mRNA expression of slc7a11 and gpx4, but did not significantly affect lpcat3 mRNA expression (Fig. S3). Taken together, these data indicate that high dietary iron disrupts antioxidant balance, leads to oxidative stress and induces ferroptosis.
Fig. 3.
Effects of dietary Fe levels on oxidative stress and the expression of HIF1α and PHD2 in the intestine tissues of yellow catfish. A Activity of antioxidant enzymes. B MDA content. C The ratio of GSH/GSSG. D The relative mRNA expression of genes involved in antioxidant. E Western blot analysis of PHD2 and HIF1α protein expression. F Quantification of PHD2, HIF1α and HIF2α relative protein level. G mRNA expression of phd2, hif1α and hif2α. H Immunofluorescent analysis of HIF1α stained with anti-HIF1α antibody (red) or DAPI nuclear stain (blue). Bars represent 250 μm. Values are shown as means ± SEM (n = 3). Bars that share different letters within the same gene indicate significant differences among the three groups (P < 0.05)
It is well established that oxidative stress triggers upregulation of HIF1α by inhibiting the activity of PHD2, an Fe (II)-dependent dioxygenase [23]. Notably, Du et al. [33] demonstrated that HIF is involved in the regulation of lipid metabolism. Therefore, we investigated the effects of dietary iron on the expression of PHD2, HIF1α and HIF2α. Our data show that high dietary iron addition decreased PHD2 but increased HIF1α and HIF2α protein expression (Fig. 3E and F). The mRNA levels of phd2 were significantly lower in the H-Fe group, while hif1α and hif2α mRNA expression declined in the L-Fe group (Fig. 3G). Immunofluorescence staining corroborates the immunoblot data showing iron-dependent upregulation of hif1α (Fig. 3H). Taken together, these data are consistent with iron-induced oxidative stress that inhibits PHD2 and increases HIF1α and HIF2α expression.
High dietary iron causes mitochondrial dysfunction in the intestine of yellow catfish
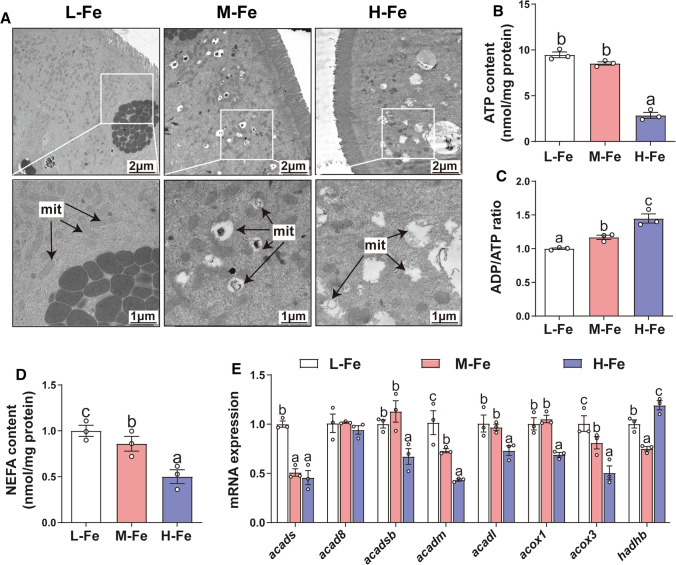
Considering that iron and mitochondrial dysfunction influence lipid metabolism [6, 9], we investigated the effect of dietary iron addition on mitochondrial structure and function in the intestine of yellow catfish. TEM analysis shows normal ultrastructure of mitochondria in the L-Fe group; however, in the M-Fe and H-Fe groups the mitochondria were reduced in numbers and markedly swollen with a severe degeneration of cristae, which suggests intestinal mitochondrial injury (Fig. 4A). ATP is an important indicator of mitochondrial metabolism and function [13]. We found that the intestinal ATP (Fig. 4B), and NEFA content (Fig. 4D) decreased, while ADP/ATP ratio (Fig. 4C) increased with dietary Fe addition. We also determined the expression of several fatty acid β-oxidation genes, and found that dietary iron addition reduced mRNA expression of acads, acadsb, acadm, acadl, acox1 and acox3 (Fig. 4E). Moreover, hadhb expression was the lowest for fish fed the M-Fe diet and the highest for fish fed the H-Fe diet. By contrast, acad8 mRNA expression showed no significant differences among the three groups. Taken together, these results suggest that dietary iron induces mitochondrial dysfunction and inhibits fatty acid β -oxidation in the intestine of yellow catfish.
Fig. 4.
Effects of dietary Fe levels on mitochondrial dysfunction in the intestine tissues of yellow catfish. A Representative TEM images (1700 × magnification, 2 μm; 3500 × magnification,1 μm); mit, mitochondria. B ATP content. C ADP/ATP ratio. D NEFA content. E Relative mRNA expression of β-oxidation-related genes. Values are shown as means ± SEM (n = 3). Bars that share different letters within the same gene indicate significant differences among the three groups (P < 0.05)
In vitro studies
Intracellular iron deposition, ROS production and lipid accumulation are directly related to the incubation of IECs with iron
To validate whether iron directly promoted oxidative stress and lipogenesis, we used the iron chelator DFO. The MTT assay demonstrated that 300 μM iron (Figs. S1, S4) and 100 μM DFO (Fig. S4) had no adverse effect on the viability of primary IECs. By using the FerroOrange probe, we found that the treatment of IECs with 300 μM iron increased cytosolic labile iron content, and this was abrogated by DFO pre-treatment (Fig. 5A, B). Iron incubation also significantly up-regulated ROS levels, as assessed by flow cytometry and laser confocal microscopy; again, DFO pre-treatment alleviated these effects (Fig. 5C–E). Moreover, the co-localization of iron and ROS showed that DFO pretreatment not only alleviated Fe-induced increase in ROS, but also reduced the co-localization of iron with ROS (Fig. S5). Iron incubation reduced DMT1 protein expression but increased the protein levels of ZIP14 and FPN1 in the IECs, and DFO pretreatment alleviated these iron-induced changes (Fig. 5F, G). In addition, DFO pre-treatment alleviated the increase of FTL and FTH concentration induced by Fe (Fig. S6A). Compared to the control, iron incubation upregulated the mRNA abundance of ftl, fth, zip14, fpn1, hephaestin, tfr1 and mtf-1, but downregulated the expression of dmt1, transferrin, tfr2 and hepcidin mRNAs. These changes were reversed by DFO pre-treatment (Fig. S6B-C). Collectively, the above results suggest that treatment of IECs with iron leads to intracellular iron accumulation, which in turn triggers responses to inhibit iron uptake and promote iron exclusion.
Fig. 5.
DFO attenuated Fe overload-induced Fe2+ accumulation and ROS generation in the primary intestinal epithelial cells (IECs) of yellow catfish. The primary IECs from P. fulvidraco were incubated in the control or Fe (300 μM) for 24 h in DMEM medium with or without 2-h pre-treatment with 100 μM DFO (iron chelator). A Representative fluorescent images of intracellular iron level in IECs were identified by using FerroOrange (orange). Scale bars: 10 μm; B The Fe.2+ ions were quantified by flow cytometric analysis with FerroOrange staining. C Representative confocal microscopic image of ROS stained with DCFH-DA in hepatocytes. D, E The ROS was quantified by flow cytometric analysis with DCFH-DA staining. F, G Western blot and protein level of DMT1, ZIP14 and FPN1. GAPDH was used as internal standard. H MDA content. I Total-AOC. (J) Activity of total-SOD, CAT and GPX. Values are shown as means ± SEM (n = 3). P value was calculated by Student’s t test. *P < 0.05
DFO pre-treatment markedly attenuated the iron-induced increase of MDA levels (Fig. 5H) and reversed the iron-induced down-regulation of T-AOC, T-SOD, CAT and GPX activities (Fig. 5I, J).
We also explored whether iron-induced lipid deposition was directly related to iron incubation. We found that DFO pre-treatment significantly alleviated the iron-induced increase in TG and TC content, as well as the Bodipy 493/503 fluorescence intensity and lipid droplet number (Fig. S7A-E). Meanwhile, DFO pre-treatment attenuated the iron-induced increase in the mRNA expression of lipogenic genes and related transcription factors (fas, pparγ and srebp1). DFO pre-treatment also blunted the iron-induced suppression of cpt1a and hsl mRNAs (Fig. S7F). Taken together, these data indicate that exogenous iron directly induces oxidative stress and stimulates ROS production in IECs, which up-regulates lipogenic metabolism and increases TG deposition.
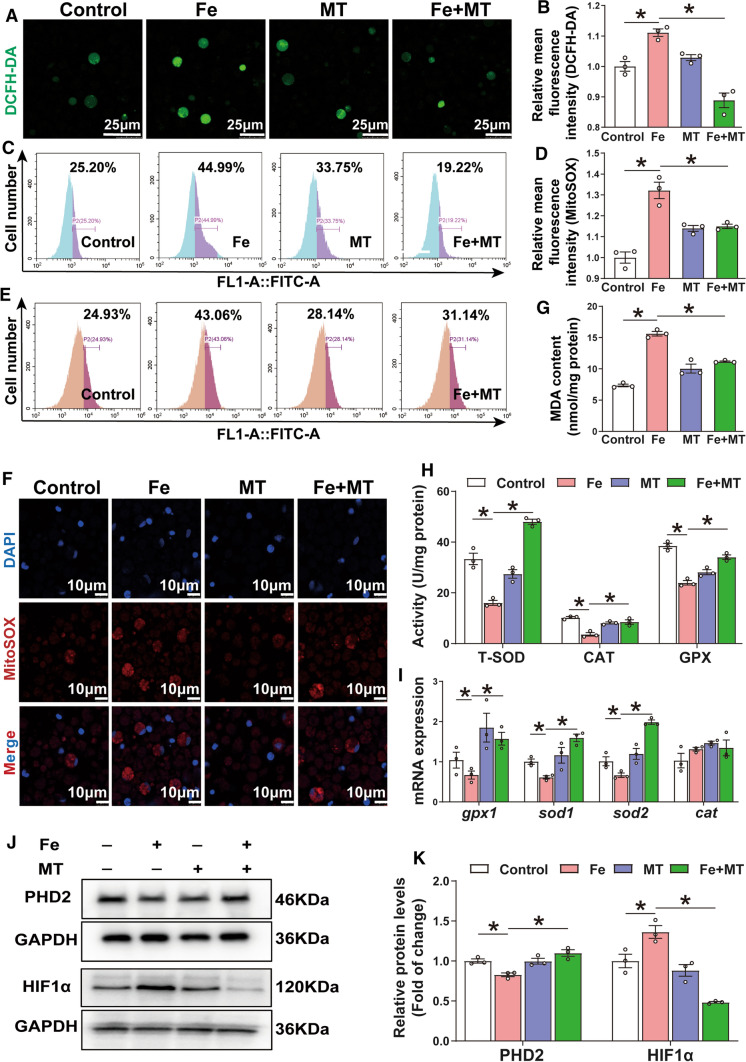
Mitochondrial oxidative stress (mtROS) up-regulates HIF1α protein expression and mediates iron-induced lipid deposition in IECs of yellow catfish
Further experiments were conducted in IECs to illuminate whether mitochondrial oxidative stress is involved in iron-induced lipid accumulation. Cell viability was not affected by 15 µM Mito-TEMPO pre-treatment (Fig. S8). By using the DCFH-DA probe, we found that iron significantly induced intracellular ROS production, and Mito-TEMPO pre-treatment significantly attenuated this effect (Fig. 6A–C). To further explore the site of ROS production, we utilized the Mito-SOX red probe. Mito-TEMPO pre-treatment significantly antagonized the iron-induced increase of mitochondrial ROS (Fig. 6D–F) and MDA content (Fig. 6G), the iron-induced reduction in T-SOD, CAT and GPX activities (Fig. 6H), and the mRNA levels of gpx1, sod1 and sod2 (Fig. 6I). Since excessive ROS production can directly induce HIF1α by inhibiting PHD2 activity [23], we further explored whether iron triggers these responses in IECs. We found that pre-incubation with Mito-TEMPO attenuated the iron-induced up-regulation of HIF1α and down-regulation of PHD2 protein expression (Fig. 6J–K). These results suggest that iron-induced mtROS generation results in inhibition of PHD2, which allows HIF1α stabilization and activation in IECs from yellow catfish.
Fig. 6.
Oxidative stress mediated Fe-induced up-regulation of HIF1α protein in the primary IECs of yellow catfish. The primary IECs of yellow catfish were incubated in the control or Fe (300 μM) for 24 h in DMEM medium, or combined with 2-h MT (15 μM) pretreatment. A The confocal microscopy image of ROS generation. B, C The ROS was measured by DCFH-DA fluorescence staining. D, E The mitochondria ROS was measured by Mito-SOX fluorescence staining. F The confocal microscopy image of mitochondria ROS generation. G MDA content. H Activity of total-SOD, CAT and GPX. I The relative mRNA expression of genes involved in antioxidant responses. J, K Western blot and protein level of PHD2 and HIF1α. Values are shown as means ± SEM (n = 3). P value was calculated by Student’s t test. *P < 0.05
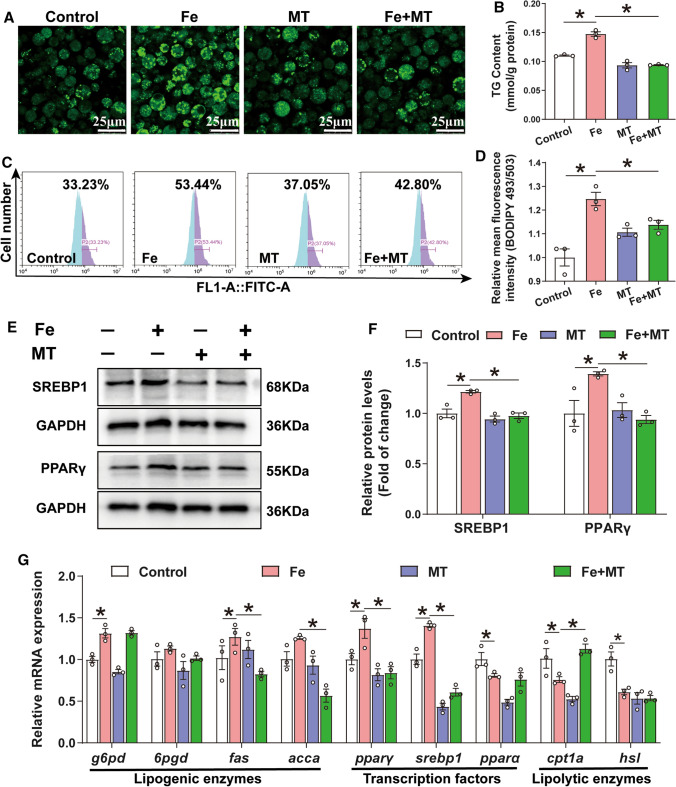
To further explore the key role of mitochondrial oxidative stress in iron-induced lipid deposition, we utilized lipid metabolism-related indicators. Mito-TEMPO pre-treatment significantly alleviated the iron-induced increase in TG content and Bodipy 493/503 fluorescence intensity, as well as the number and size of lipid droplets (Fig. 7A–D). Moreover, Mito-TEMPO pre-treatment abrogated the iron-induced increase in SREBP1 and PPARγ protein expression (Fig. 7E, F) and activities of the lipogenic enzymes G6PD, ME, ICDH and FAS (Fig. S9). In addition, pre-treatment with Mito-TEMPO also attenuated the iron-induced increase of the mRNA expression of genes (fas, pparγ and srebp1), and attenuated the iron-induced reduction of cpt1a mRNA (Fig. 7G). Overall, these results demonstrate that iron overload induce ROS production, activates HIF1α and promotes lipid deposition in IECs from yellow catfish.
Fig. 7.
Oxidative stress mediated Fe-induced lipid accumulation in the primary IECs of yellow catfish. The primary IECs of yellow catfish were incubated in the control or Fe (300 μM) for 24 h in DMEM medium, or combined with 2-h MT (15 μM) pretreatment. A Representative confocal microscopic image of the IECs stained with Bodipy 493/503. B TG content. C, D Bodipy 493/503 fluorescence staining was used to quantify the relative lipid content. E, F Expressions of SREBP1 and PPARγ were measured by Western blot analysis (n = 3). GAPDH was used as internal standard. G The relative mRNA expression of lipid metabolism-related genes. Values are shown as means ± SEM (n = 3). P value was calculated by Student’s t test. *P < 0.05. MT: Mito-TEMPO (a mitochondria-targeted superoxide dismutase mimetic)
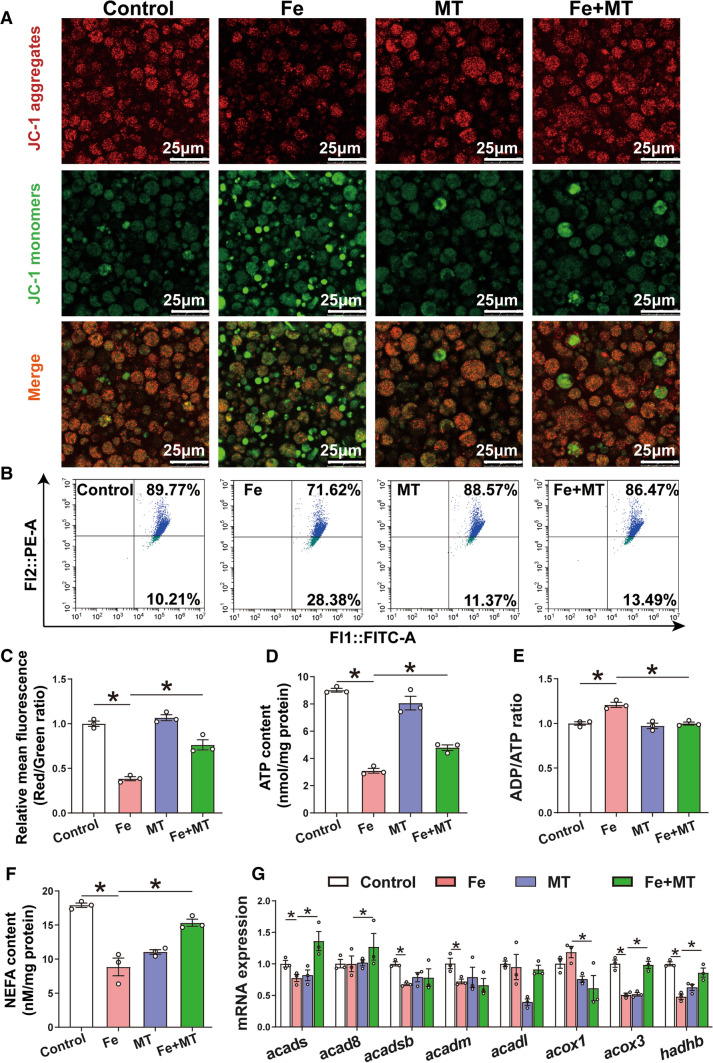
Oxidative stress-mediated mitochondrial dysfunction contributes to lipid deposition in IECs of yellow catfish
Accumulating evidence indicates that mitochondrial dysfunction caused by oxidative stress is one of the common molecular mechanisms underlying lipid accumulation [6, 18]. Therefore, we investigated whether oxidative stress-mediated mitochondrial dysfunction contributed to lipid deposition in IECs of yellow catfish. JC-1 is an ideal fluorescent probe that is widely used to detect the MMP, and the transition of JC-1 from red fluorescence (JC-1 aggregates) to green fluorescence (JC-1 monomers) can be used as an indicator of mitochondrial dysfunction [17]. JC-1 staining showed weak green fluorescence and bright red fluorescence in the control and the opposite in iron-treated group, indicating that iron incubation reduced the MMP. Mito-TEMPO pretreatment alleviated the iron-induced changes of the MMP and mitochondrial dysfunction (Fig. 8A). These data were further validated by a flow cytometry assay (Fig. 8B). Compared to the control, iron incubation reduced the ratio of the red to green fluorescence intensity, and Mito-TEMPO preincubation attenuated the reduction in MMP induced by iron (Fig. 8C). Mito-TEMPO pre-incubation abrogated the iron-induced reduction of ATP contents, and the increase of ADP/ATP ratio (Fig. 8D, E). Taken together, these data uncover the iron-induced mitochondrial dysfunction in the IECs of yellow catfish.
Fig. 8.
Oxidative stress mediated Fe-induced mitochondrial dysfunction in the IECs of yellow catfish. The primary IECs of yellow catfish were incubated in the control or Fe (300 μM) for 24 h in DMEM medium, or combined with 2-h MT (15 μM) pretreatment. A Representative confocal microscopic image of IECs stained with JC-1 (green for monomers, red for aggregates); Bars represent 25 μm. B The presence of mitochondrial potentials with JC-1 staining were demonstrated by flow cytometry. C Quantitative analysis of the ratio of red fluorescence intensity over green fluorescence. D ATP content. E ADP/ATP ratio. F NEFA content. G Relative mRNA expression of β-oxidation genes in IECs cells. Values are shown as means ± SEM (n = 3). P value was calculated by Student’s t test. *P < 0.05. MT: Mito-TEMPO (a mitochondria-targeted superoxide dismutase mimetic)
Next, we monitored changes in mRNA expression of genes relevant to fatty acid β-oxidation. Mito-TEMPO pre-incubation antagonized the iron-induced reduction of NEFA content (Fig. 8F), and acads, acox3 and hadhb mRNA expression (Fig. 8G). Thus, these data demonstrate that mitochondrial dysfunction plays an important role in lipid accumulation induced by iron.
Increased HIF1α contributes to lipogenesis by regulating the transcription of pparγ and fas
To explore whether iron-induced lipid accumulation was mediated by HIF1α targeting the pparγ and fas genes, we performed promoter analysis of these genes in HEK293T cells. By analyzing the pparγ and fas promoters obtained in our laboratory [34], we predicted HIF1α binding sites consisting of the evolutionarily conserved core sequence ACGTG (Fig. 9A, B); the predicted HIF1α binding sites for pparγ and fas promoters are presented in Figs. S10 and S11.
Fig. 9.
Fe induced the upregulation of PPARγ and FAS expression via promoting the DNA binding HIF1α to the pparγ and fas promoters. A HIF1α binding sequence (HRE) located at − 526 bp to − 522 bp of pparγ promoter of yellow catfish. B HIF1α binding sequence (HRE) located at − 451 bp to − 447 bp of fas promoter of yellow catfish. C Site-mutation analysis of HRE binding sites on pGl3- PPARγ-1575/ + 63 vectors. D Site-mutation analysis of HRE binding sites on pGl3- FAS-1525/ + 39 vectors. E EMSA of putative HIF1α binding sequences (HRE) on the paprγ promoter. F EMSA of putative HIF1α binding sequences (HRE) on the fas promoter. The 5′-biotin labeled double-stranded oligomers were incubated with nuclear protein. A 200 -fold excess of the competitor and mutative competitor oligomers was added to the competition and mutant competition assay, respectively. Values are shown as means ± SEM (n = 3). Asterisk (*) and hash sign (#) denote significance at P < 0.05 between the two groups (Student t-test)
For the pparγ promoter, mutation of the -526/-522 bp HRE site (HRE2), but not the − 1370/ − 1363 bp HRE sequence (HRE1), decreased the luciferase activity in the Fe-treated group, indicating that the HRE2 binding site positively regulates pparγ transcription in response to iron (Fig. 9C). For the fas promoter, the mutation of the -451/-447 bp HRE site (HRE2), but not the -550/-534 bp and -418/-402 bp sites (HRE1 and HRE3), suppressed fas promoter activity following iron treatment (Fig. 9D), indicating that HIF1α can bind to the HRE2 site and control fas transcription.
Finally, we conducted EMSA to investigate whether HIF1α can directly bind to the pparγ and fas promoters (Fig. 9E, F). We found that the -526/-522 bp HIF1α binding site (HRE2) of pparγ promoter and the -451/-447 bp HIF1α binding site (HRE2) of fas promoter could bind with nuclear extracts. The binding is blocked by excess unlabeled competitor, and not affected by mutant competitor. Importantly, iron incubation significantly enhanced the binding activity of HIF1α to HREs (Fig. 9E, F, lane 5), indicating the HRE2 of pparγ promoter and the HRE2 of fas promoter are functional sites for the regulation. Taken together, our study confirms that iron-induced lipogenesis occurs via enhanced HIF1α binding to the pparγ and fas promoters.
Discussion
In the present study, we found that exposure of yellow catfish to high dietary iron increased intestinal iron content and promoted iron absorption and transport. Moreover, excessive iron increased ROS formation, induced mitochondrial dysfunction, reduced fatty acid β-oxidation and inhibited lipolysis in intestinal tissue and IECs of yellow catfish. Mechanistically, oxidative stress activated HIF1, which in turn stimulated transcription of the key lipogenic factors pparγ and fas, promoting lipogenesis. These results reveal a new mechanism of iron-induced fat deposition and highlight novel potential targets to prevent iron overload-induced lipotoxicity.
Intestinal iron content increased with dietary iron administration, in agreement with several studies [10, 35]. Iron homeostasis is regulated by several mechanisms accounting for cellular uptake, intracellular transport, storage and export of iron. To gain insights into these mechanisms, we investigated the expression of iron regulatory genes, including the iron transporters dmt1, zip14 and fpn1. Previous studies [36] reported that DMT1 in the rat liver [36] or mouse duodenum [40] was down-regulated by iron overload and up-regulated by iron deficiency, consistent with our results. ZIP14 is a zinc transporter that can also transport iron into cells [36, 37], and accounts for hepatocellular iron overload in hemochromatosis mouse models [38]. We found that dietary iron administration increased the mRNA and protein levels of zip14 in the intestine and in IECs from the yellow catfish. Similarly, recent studies demonstrated that the protein levels of ZIP14 were increased in the liver of rats fed a high iron diet and in iron-loaded human hepatoma cells [36, 39]. We also observed that iron induced the basolateral iron exporter fpn1 at the mRNA and protein levels, which is in contrast with fpn1 regulation in the mouse duodenum [40]. However, Iyengar et al. [41] found that the expression of FPN1 increased upon supplementation with iron, which probably led to maintenance of cellular iron homeostasis in Caco-2 cells. Ferritin is an iron storage protein and functions in the maintenance of iron balance in organisms. In the present study, our date imply that iron drastically induced expression of ferritin, which is consistent with data obtained in the mouse duodenum [40]. Similarly, Wang et al. [1] pointed out iron overload could promote the expression of ferritin, which was mediated by the iron-regulatory protein (IRP)/iron-responsive element (IRE) system. Furthermore, we also found that iron increased the mRNA levels of hephaestin and mtf-1, but decreased the mRNA levels of tf, tfr1 hepcidin and irp1, consistent with other reports [36, 39]. These results suggest that a protective mechanism exists to maintain iron homeostasis by regulating iron absorption and exclusion.
Lipids are the major source of energy and are involved in fundamental functions such as cellular homeostasis, and regulation of immunity and inflammation [42]. However, imbalance in lipid homeostasis may contribute to lipotoxicity. This leads to organelle dysfunction and cell damage and death, which is intimately associated with NAFLD and NASH [29, 43]. Dietary iron overload is known to affect liver lipid metabolism [8, 9], but possible changes in intestinal lipid metabolism have not been investigated thus far. Herein, we demonstrate that dietary iron increases lipid deposition and TG accumulation via upregulation of lipogenesis and the downregulation of lipolysis in the intestine of yellow catfish.
Lipid metabolism is regulated by a variety of biological processes. Studies have shown that oxidative stress plays an important role in regulating lipid homeostasis and participates in the pathogenesis of NAFLD [26, 44]. Oxidative stress is caused by an imbalance between antioxidant defenses and pro-oxidative loads. Excessive unshielded iron is known to stimulate the production of ROS via Fenton reaction [15, 45]. Along these lines, the present study suggests that high dietary iron decreases intestinal antioxidant enzymes activities and gene expression and increases ROS levels and lipid peroxidation. Thus, iron overload disrupts intestinal antioxidant defenses system and promotes ROS production. Our in vitro study showed that pretreatment with DFO attenuated Fe-induced Fe2+ accumulation, ROS generation, lipid peroxidation, indicating that free Fe ions were responsible for the generation of ROS, consistent with other studies [16]. Maintaining ROS at appropriate levels is important in many biological functions, and excessive ROS generation leads to oxidative stress, which disrupts lipid metabolism, resulting in fat deposition [18, 31, 44]. Therefore, our data suggest that the iron-induced lipid deposition in the intestine of yellow catfish is attributed to iron-dependent generation of mitochondrial ROS. Inhibition of mtROS generation by Mito-Tempo significantly attenuated iron-induced increase in TG concentration and lipogenesis, indicated ROS involvement in lipid accumulation. Similarly, Sun et al. [44] demonstrated the activation of NRF2 in response to oxidative stress promoted lipid deposition by activating SREBP-1-mediated lipogenesis and inhibiting lipolysis. Moreover, in recent studies we also found that oxidative stress mediated the regulation of lipid deposition by damaging the mitochondrial functions and reducing fatty acids β-oxidation [6, 17, 18]. Taken together, these data demonstrate that mitochondrial oxidative stress accounts for Fe-induced lipid accumulation.
Mitochondria are regarded as the major sites of ROS production, ATP synthesis and fatty acid oxidation, and also the center of cellular energy production [13, 46]. Excessive ROS can lead to mitochondrial dysfunction. Disruption of the mitochondrial membrane potential (MMP) leads to defective mitochondrial electron transport chain, decreased oxygen consumption, and ATP depletion, which are major signs of mitochondrial dysfunction [46]. In the present study, iron overload-induced ROS production damaged mitochondrial structure, and led to mitochondrial dysfunction, in agreement with other reports [15]. Maintaining structural and functional integrity of mitochondria is essential for normal cellular physiology and energy production [46]. ROS induced mitochondrial dysfunction, inhibits lipolysis through decreased mitochondrial fatty acid β-oxidation, and result in increased intracellular TG content [6, 17, 18, 31]. Our experiments show that iron significantly down-regulated NEFA content and the mRNA expression of acads, acadsb, acadm and acox3, which play a critical role in mitochondrial β-oxidation [47]. We conclude that oxidative stress due to iron-induced mitochondrial dysfunction decreases mitochondrial fatty acid oxidation, which in turn induced by oxidative stress inhibits lipolysis and aggravates lipid accumulation.
HIF1α is a heterodimeric helix-loop-helix transcription factor that mediates cellular adaptation to hypoxia and also plays an important role in oxidative stress [48]. In our study, excess of dietary Fe increased the expression of HIF1α in the yellow catfish. Similarly, Hu et al. [48] demonstrated that iron overload can promote the expression of HIF1α in mesenchymal stromal cells. Furthermore, excessive iron-induced ROS increased HIF1α expression by down-regulating the mRNA and protein levels of PHD2, consistent with previous data [49]. Previous studies have reported that ascorbic acid, as a cofactor of PHD2 enzyme activity, loses its ability to reduce ferric to ferric under conditions of oxidative stress, which inhibits PHD2 activity and leads to HIF1α activation [50]. Although our study indicates that oxidative stress is a critical mediator in iron-induced changes of lipid metabolism, mechanistic details remain unclear. Belanger et al. [51] confirmed that HIF1α overexpression led to TG accumulation and reduced fatty acid oxidation. In addition, other studies showed that HIF1α inhibited pparα and cpt1 expression at the transcriptional level [32, 52]. By analyzing the structure of promoter regions of lipogenic genes in yellow catfish, we found two functional hypoxia response elements (HRE) sites within the promoters of pparγ and fas. Dual-luciferase reporter assays and EMSA showed that Fe significantly promoted the binding activity of HIF1α to these HREs. Our data corroborate previous studies showing that PPARγ is a direct target gene of HIF1α [19, 53]. Furuta et al. [54] also suggested that HIF1α is involved in the up-regulation of FAS. Taken together, our data and the previous findings show that HIF1α is required for oxidative stress-induced TG accumulation via regulating FAS and PPARγ-driven lipogenesis.
Conclusions
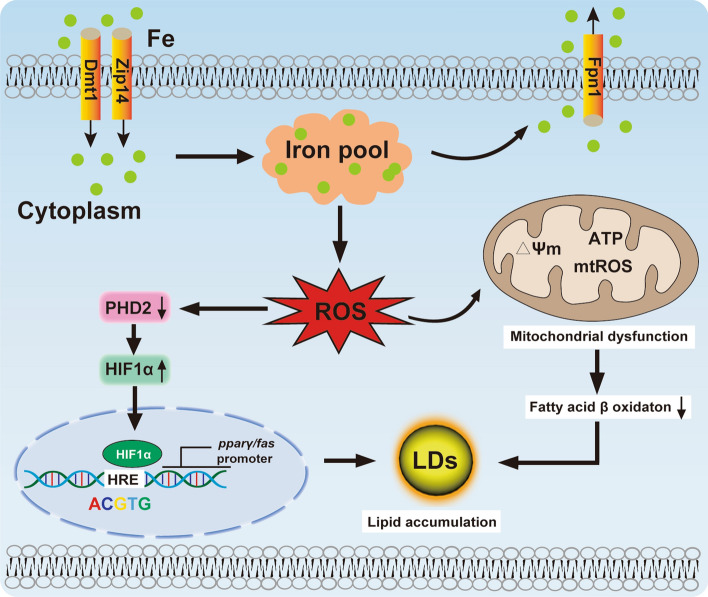
We propose a mechanistic model underlying iron-overload induced intestinal lipid deposition (Fig. 10). According to this, excess dietary iron addition promotes intestinal iron accumulation and triggers mitochondrial oxidative stress. The ensuing mitochondrial dysfunction inhibits lipolysis. Oxidative stress-induced HIF1α expression promotes transcriptional activation of pparγ and fas by binding of HIF1α to the HRE sites in their promoters, which ultimately increases lipogenesis. Thus, HIF1α connects oxidative stress and lipogenic metabolism via iron and oxidative stress. Our data may pave the way to develop new therapeutic strategies for the prevention and treatment of the iron-induced intestinal steatosis.
Fig. 10.
A working model of oxidative stress-mediated mitochondrial dysfunction and HIF1α-PPARγ /FAS pathway participated in Fe-induced lipid deposition
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Key R&D Program of China (grant No. 2018YFD0900400).
Abbreviations
- 6PGD
6-Phosphogluconate dehydrogenase
- ACCα
Acetyl-CoA carboxylase α
- ACSL4
Acyl-CoA synthetase long-chain family member 4
- ATP
Adenosine triphosphate
- B2M
Beta-2 microglobulin
- CAT
Catalase
- DFO
Deferoxamine mesylate
- DMEM
Dulbecco's Modified Eagles Medium
- DMT1
Divalent metal transporter 1
- ELFA
Translation elongation factor
- EMSA
Electrophoretic mobility-shift assay
- FAS
Fatty acid synthase
- Fe
Iron
- FBS
Fetal bovine serum
- FPN1
Ferroportin
- FerM
Ferritin middle chain
- FTH
Ferritin heavy chain
- FTL
Ferritin light chain
- G6PD
Glucose 6-phosphate dehydrogenase
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GLUT1
Solute carrier family 2 member 1
- HK1
Hexokinase 1
- HK2
Hexokinase 2
- GK
Glucokinase
- GPx
Glutathione peroxidase
- GSH
Glutathione
- GSSG
Glutathione disulfide
- HIF1α
Hypoxia-inducible factors-1α
- HIF2α
Hypoxia-inducible factor-2α
- HPRT
Hypoxanthine-guanine phosphoribosyltransferase
- HRE
Hypoxia response elements
- HSL
Hormone-sensitive lipase
- LDH
Lactate dehydrogenase
- ICDH
Isocitrate dehydrogenase
- ICP-OES
Inductively coupled plasma optical emission spectrometry
- JC-1
5,5ʹ,6,6ʹ-Tetrachloro-1,1ʹ,3,3ʹ-tetraethyl-imidacarbocyanine iodide
- LOX
Lipoxygenase
- LPCAT3
Lysophosphatidylcholine acyltransferase 3
- MDA
Malondialdehyde
- ME
Malic enzyme
- MMP
Mitochondrial membrane potential
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide
- mtROS
Mitochondrial ROS
- NEFA
Nonesterified fatty acid
- NOX1
NADPH oxidases 1
- NRF2
Nuclear factor E2-related factor 2
- ORO
Oil red O
- PFK
Phosphofructokinase
- PGK1
Phosphoglycerate kinase 1
- PHD2
Prolyl hydroxylases 2
- PK
Pyruvate kinase
- PPARγ
Peroxisome proliferator-activated receptor γ
- PTGS2
Prostaglandin endoperoxide synthase 2
- ROS
Reactive oxygen species
- RPL7
Ribosomal protein L7
- SEM
Standard error of means
- SLC7A11
Cystine-glutamate antiporter
- SREBP1
Sterol regulatory element-binding proteins 1
- TEM
Transmission electron microscopy
- TF
Transferrin
- TFR1
Transferrin receptor 1
- TFR2
Transferrin receptor 2
- TG
Triglyceride
- T-SOD
Total superoxide dismutase
- TUBA
Tubulin alpha chain
- UBCE
Ubiquitin-conjugating enzyme
- UTR
Untranslated region
- ZIP14
ZRT-IRE-like protein 14
Authors contribution
The authors’ responsibilities were as follows: CCS and ZL designed the experiments; CCS conducted the experiments and sample analyses with the help of GHC, CCZ, TZ, and DGZ; KP provided many critical suggestions for the experimental designs and data analysis; CCS analyzed the data and drafted the manuscript; KP and ZL revised the manuscript; all authors read and approved the final manuscript.
Funding
This work was supported by the National Key R&D Program of China (grant No. 2018YFD0900400).
Data availability
All materials and data supporting this study are available from the corresponding author (luozhi99@mail.hzau.edu.cn) upon reasonable request.
Declarations
Conflict of interest
All the authors disclosed no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J. 2011;434:365–381. doi: 10.1042/BJ20101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93:1721–1741. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- 3.Kim CH, Leitch HA. Iron overload-induced oxidative stress in myelodysplastic syndromes and its cellular sequelae. Crit Rev in Oncol Hematol. 2021;163:103367. doi: 10.1016/j.critrevonc.2021.103367. [DOI] [PubMed] [Google Scholar]
- 4.Katsarou A, Pantopoulos K. Basics and principles of cellular and systemic iron homeostasis. Mol Aspects Med. 2020;75:100866. doi: 10.1016/j.mam.2020.100866. [DOI] [PubMed] [Google Scholar]
- 5.Olivares-Rubio HF, Vega-López A. Fatty acid metabolism in fish species as a biomarker for environmental monitoring. Environ Pollut. 2016;218:297–312. doi: 10.1016/j.envpol.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Xu YH, Hogstrand C, Xu YC, Zhao T, Zheng H, Luo Z. Environmentally relevant concentrations of oxytetracycline and copper increased liver lipid deposition through inducing oxidative stress and mitochondria dysfunction in grass carp Ctenopharyngodon idella. Environ Pollut. 2021;283:117079. doi: 10.1016/j.envpol.2021.117079. [DOI] [PubMed] [Google Scholar]
- 7.Ko SH, Kim HS. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients. 2020;12:202. doi: 10.3390/nu12010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed U, Latham PS, Oates PS. Interactions between hepatic iron and lipid metabolism with possible relevance to steatohepatitis. World J Gastroenterol. 2012;18:4651–4658. doi: 10.3748/wjg.v18.i34.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi JS, Koh IU, Lee HJ, Kim WH, Song J. Effects of excess dietary iron and fat on glucose and lipid metabolism. J Nutr Biochem. 2013;24:1634–1644. doi: 10.1016/j.jnutbio.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Luo Z, Zou GY, Gao Y, Ye HM, Xi WQ, Liu X. Effect of dietary iron (Fe) levels on growth performance, hepatic lipid metabolism and antioxidant responses in juvenile yellow catfish Pelteobagrus fulvidraco. Aquac Nutr. 2017;23:1475–1482. doi: 10.1111/anu.12523. [DOI] [Google Scholar]
- 11.Fuqua BK, Vulpe CD, Anderson GJ. Intestinal iron absorption. J Trace Elem Med Biol. 2012;26:115–119. doi: 10.1016/j.jtemb.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Ko CW, Qu J, Black DD, Tso P. Regulation of intestinal lipid metabolism: current concepts and relevance to disease. Nat Rev Gastroenterol Hepatol. 2020;17:169–183. doi: 10.1038/s41575-019-0250-7. [DOI] [PubMed] [Google Scholar]
- 13.Mansouri A, Gattolliat CH, Asselah T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology. 2018;155:629–647. doi: 10.1053/j.gastro.2018.06.083. [DOI] [PubMed] [Google Scholar]
- 14.Tataranni T, Agriesti F, Mazzoccoli C, Ruggieri V, Scrima R, Laurenzana I, D'Auria F, Falzetti F, Di Ianni M, Musto P, Capitanio N, Piccoli C. The iron chelator deferasirox affects redox signalling in haematopoietic stem/progenitor cells. Br J Haematol. 2015;170:236–246. doi: 10.1111/bjh.13381. [DOI] [PubMed] [Google Scholar]
- 15.Sumneang N, Siri-Angkul N, Kumfu S, Chattipakorn SC, Chattipakorn N. The effects of iron overload on mitochondrial function, mitochondrial dynamics, and ferroptosis in cardiomyocytes. Arch Biochem Biophys. 2020;680:108241. doi: 10.1016/j.abb.2019.108241. [DOI] [PubMed] [Google Scholar]
- 16.Feng Z, Min L, Chen H, Deng W, Tan M, Liu H, Hou J. Iron overload in the motor cortex induces neuronal ferroptosis following spinal cord injury. Redox Biol. 2021;43:101984. doi: 10.1016/j.redox.2021.101984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan YX, Zhuo MQ, Wei CC, Chen GH, Song YF, Luo Z. Oxidative stress and mitochondrial dysfunction mediated Cd-induced hepatic lipid accumulation in zebrafish Danio rerio. Aquat Toxicol. 2018;199:12–20. doi: 10.1016/j.aquatox.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Zhang DG, Zhao T, Hogstrand C, Ye HM, Xu XJ, Luo Z. Oxidized fish oils increased lipid deposition via oxidative stress-mediated mitochondrial dysfunction and the CREB1-Bcl2-Beclin1 pathway in the liver tissues and hepatocytes of yellow catfish. Food Chem. 2021;360:129814. doi: 10.1016/j.foodchem.2021.129814. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan J, Suter M, Windak R, Krebs T, Felley A, Montessuit C, Tokarska-Schlattner M, Aasum E, Bogdanova A, Perriard E, Perriard JC, Larsen T, Pedrazzini T, Krek W. Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab. 2009;9:512–524. doi: 10.1016/j.cmet.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Marin J, Lozano E, Perez MJ. Lack of mitochondrial DNA impairs chemical hypoxia-induced autophagy in liver tumor cells through ROS-AMPK-ULK1 signaling dysregulation independently of HIF-1α. Free Radic Biol Med. 2016;101:71–84. doi: 10.1016/j.freeradbiomed.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21:268–283. doi: 10.1038/s41580-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia Y, Guo Y, Jin Q, Qu H, Qi D, Song P, Zhang X, Wang X, Xu W, Dong Y, Liang Y, Quan C. A SUMOylation-dependent HIF-1α/CLDN6 negative feedback mitigates hypoxia-induced breast cancer metastasis. J Exp Clin Cancer Res. 2020;39:42. doi: 10.1186/s13046-020-01547-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo J, Zhang X, He S, Lou Q, Zhai G, Shi C, Yin Z, Zheng F. Deletion of narfl leads to increased oxidative stress mediated abnormal angiogenesis and digestive organ defects in zebrafish. Redox Biol. 2020;28:101355. doi: 10.1016/j.redox.2019.101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rankin EB, Giaccia AJ. Hypoxic Control of Metastasis. Science. 2016;352:175–180. doi: 10.1126/science.aaf4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang W, Chen Q, Cui K, Chen Q, Li X, Xu N, Mai K, Ai Q. Lipid overload impairs hepatic VLDL secretion via oxidative stress-mediated PKCδ-HNF4α-MTP pathway in large yellow croaker (Larimichthys crocea) Free Radic Biol Med. 2021;172:213–225. doi: 10.1016/j.freeradbiomed.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Zhao T, Wu K, Hogstrand C, Xu YH, Chen GH, Wei CC, Luo Z. Lipophagy mediated carbohydrate-induced changes of lipid metabolism via oxidative stress, endoplasmic reticulum (ER) stress and ChREBP/PPARγ pathways. Cell Mol Life Sci. 2020;77:1987–2003. doi: 10.1007/s00018-019-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen GH, Song CC, Pantopoulos K, Wei XL, Zheng H, Luo Z. Mitochondrial oxidative stress mediated Fe-induced ferroptosis via the NRF2-ARE pathway. Free Radic Biol Med. 2022;180:95–107. doi: 10.1016/j.freeradbiomed.2022.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Wu K, Hogstrand C, Chen GH, Wei CC, Li DD, Luo Z. Zn stimulates the phospholipids biosynthesis via the pathways of oxidative and endoplasmic reticulum stress in the intestine of freshwater teleost yellow catfish. Environ Sci Technol. 2018;52:9206–9214. doi: 10.1021/acs.est.8b02967. [DOI] [PubMed] [Google Scholar]
- 29.Ling SC, Wu K, Zhang DG, Luo Z. Endoplasmic reticulum stress-mediated autophagy and apoptosis alleviate dietary fat-induced triglyceride accumulation in the intestine and in isolated intestinal epithelial cells of yellow catfish. J Nutr. 2019;149:1732–1741. doi: 10.1093/jn/nxz135. [DOI] [PubMed] [Google Scholar]
- 30.Dyer WJ, Bligh EG. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- 31.Song CC, Chen GH, Zhong CC, Chen F, Chen SW, Luo Z. Transcriptional responses of four slc30a/znt family members and their roles in Zn homeostatic modulation in yellow catfish Pelteobagrus fulvidraco. Biochim Biophys Acta Gene Regul Mech. 2021;1864:194723. doi: 10.1016/j.bbagrm.2021.194723. [DOI] [PubMed] [Google Scholar]
- 32.Zhong CC, Zhao T, Hogstrand C, Chen F, Song CC, Luo Z. Copper (Cu) induced changes of lipid metabolism through oxidative stress-mediated autophagy and Nrf2/PPARγ pathways. J Nutr Biochem. 2021;100:108883. doi: 10.1016/j.jnutbio.2021.108883. [DOI] [PubMed] [Google Scholar]
- 33.Du W, Zhang L, Brett-Morris A, Aguila B, Kerner J, Hoppel CL, Puchowicz M, Serra D, Herrero L, Rini BI, Campbell S, Welford SM. HIF drives lipid deposition and cancer in ccRCC via repression of fatty acid metabolism. Nat Commun. 2017;8:1769. doi: 10.1038/s41467-017-01965-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu K, Tan XY, Xu YH, Chen GH, Zhuo MQ. Functional analysis of promoters of genes in lipid metabolism and their transcriptional response to STAT3 under leptin signals. Genes. 2018;9:334. doi: 10.3390/genes9070334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen GH, Luo Z, Chen F, Shi X, Song YF, You WJ, Liu X. PPARα, PPARγ and SREBP-1 pathways mediated waterborne iron (Fe)-induced reduction in hepatic lipid deposition of javelin goby Synechogobius hasta. Comp Biochem Physiol C. 2017;197:8–18. doi: 10.1016/j.cbpc.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Nam H, Wang CY, Zhang L, Zhang W, Hojyo S, Fukada T, Knutson MD. ZIP14 and DMT1 in the liver, pancreas, and heart are differentially regulated by iron deficiency and overload: implications for tissue iron uptake in iron-related disorders. Haematologica. 2013;98:1049–1057. doi: 10.3324/haematol.2012.072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenkitkasemwong S, Wang CY, Coffey R, Zhang W, Chan A, Biel T, Kim JS, Hojyo S, Fukada T, Knutson MD. SLC39A14 is required for the development of hepatocellular iron overload in murine models of hereditary hemochromatosis. Cell Metab. 2015;22:138–150. doi: 10.1016/j.cmet.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao N, Zhang AS, Worthen C, Knutson MD, Enns CA. An iron-regulated and glycosylation-dependent proteasomal degradation pathway for the plasma membrane metal transporter ZIP14. Proc Natl Acad Sci USA. 2014;111:9175–9180. doi: 10.1073/pnas.1405355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katsarou A, Gkouvatsos K, Fillebeen C, Pantopoulos K. Tissue-specific regulation of ferroportin in wild-type and Hjv-/- mice following dietary iron manipulations. Hepatol Commun. 2021;5:2139–2150. doi: 10.1002/hep4.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iyengar V, Pullakhandam R, Nair KM. Coordinate expression and localization of iron and zinc transporters explain iron-zinc interactions during uptake in Caco-2 cells: implications for iron uptake at the enterocyte. J Nutr Biochem. 2012;23:1146–1154. doi: 10.1016/j.jnutbio.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68:280–295. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Ertunc ME, Hotamisligil GS. Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. J Lipid Res. 2016;57:2099–2114. doi: 10.1194/jlr.R066514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun X, Li X, Jia H, Wang H, Shui G, Qin Y, Shu X, Wang Y, Dong J, Liu G, Li X. Nuclear factor E2-related factor 2 mediates oxidative stress-induced lipid accumulation in adipocytes by increasing adipogenesis and decreasing lipolysis. Antioxid Redox Signal. 2020;32:173–192. doi: 10.1089/ars.2019.7769. [DOI] [PubMed] [Google Scholar]
- 45.Galaris D, Barbouti A, Pantopoulos K. Iron homeostasis and oxidative stress: an intimate relationship. Biochim Et Biophys Acta Mol Cell Res. 2019;1866:118535. doi: 10.1016/j.bbamcr.2019.118535. [DOI] [PubMed] [Google Scholar]
- 46.Kim SH, Kim H. Inhibitory effect of astaxanthin on oxidative stress-induced mitochondrial dysfunction-a mini-review. Nutrients. 2018;10:1137. doi: 10.3390/nu10091137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khamseekaew J, Kumfu S, Wongjaikam S, Kerdphoo S, Jaiwongkam T, Srichairatanakool S, Fucharoen S, Chattipakorn SC, Chattipakorn N. Effects of iron overload, an iron chelator and a T-Type calcium channel blocker on cardiac mitochondrial biogenesis and mitochondrial dynamics in thalassemic mice. Eur J Pharmacol. 2017;799:118–127. doi: 10.1016/j.ejphar.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 48.Watts ER, Walmsley SR. Inflammation and Hypoxia: HIF and PHD isoform selectivity. Trends Mol Med. 2019;25:33–46. doi: 10.1016/j.molmed.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Hu J, Meng F, Hu X, Huang L, Liu H, Liu Z, Li L. Iron overload regulate the cytokine of mesenchymal stromal cells through ROS/HIF-1α pathway in Myelodysplastic syndromes. Leuk Res. 2020;93:106354. doi: 10.1016/j.leukres.2020.106354. [DOI] [PubMed] [Google Scholar]
- 50.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Belanger AJ, Luo Z, Vincent KA, Akita GY, Cheng SH, Gregory RJ, Jiang C. Hypoxia-inducible factor 1 mediates hypoxia-induced cardiomyocyte lipid accumulation by reducing the DNA binding activity of peroxisome proliferator-activated receptor alpha/retinoid X receptor. Biochem Biophys Res Commun. 2007;364:567–572. doi: 10.1016/j.bbrc.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 52.Li D, Du Y, Yuan X, Han X, Dong Z, Chen X, Wu H, Zhang J, Xu L, Han C, Zhang M, Xia Q. Hepatic hypoxia-inducible factors inhibit PPARα expression to exacerbate acetaminophen induced oxidative stress and hepatotoxicity. Free Radic Biol Med. 2017;110:102–116. doi: 10.1016/j.freeradbiomed.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Zhao YZ, Liu XL, Shen GM, Ma YN, Zhang FL, Chen MT, Zhao HL, Yu J, Zhang JW. Hypoxia induces peroxisome proliferator-activated receptor γ expression via HIF-1-dependent mechanisms in HepG2 cell line. Arch Biochem Biophys. 2014;543:40–47. doi: 10.1016/j.abb.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 54.Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T, Miura K, Kamada S, Saito K, Iiizumi M, Liu W, Ericsson J, Watabe K. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68:1003–1011. doi: 10.1158/0008-5472.CAN-07-2489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All materials and data supporting this study are available from the corresponding author (luozhi99@mail.hzau.edu.cn) upon reasonable request.