Abstract
Immunotherapies have been established as safe and efficient modalities for numerous tumor treatments. The lymphatic system, which is an important system, can modulate the immune system via a complex network, which includes lymph nodes, vessels, and lymphocytes. With the deepening understanding of tumor immunology, a plethora of immunotherapies, which include vaccines, photothermal therapy, and photodynamic therapy, have been established for antitumor treatments. However, the deleterious off-target effects and nonspecific targeting of therapeutic agents result in low efficacy of immunotherapy. Fortunately, nanoparticle-based approaches for targeting the lymphatic system afford a unique opportunity to manufacture drugs that can simultaneously tackle both aspects, thereby improving tumor treatments. Over the past decades, great strides have been made in the development of DC vaccines and nanomedicine as antitumor treatments in the field of lymphatic therapeutics and diagnosis. In this review, we summarize the current strategies through which nanoparticle technology has been designed to target the lymphatic system and describe applications of lymphatic imaging for the diagnosis and image-guided surgery of tumor metastasis. Moreover, improvements in the tumor specificity of nanovaccines and medicines, which have been realized through targeting or stimulating the lymphatic system, can provide amplified antitumor immune responses and reduce side effects, thereby promoting the paradigm of antitumor treatment into the clinic to benefit patients.
Graphical abstract
Keywords: Lymphatic targeting, Nanoplatform, Tumor metastasis, Image-guided therapy, Lymphoscintigraphy, DC vaccine, Combinational immunotherapy
Introduction
Tumors with a high mortality rate are a major threat to human survival and health worldwide. Currently, the most common antitumor treatments involve surgical removal of the tumor, radiation therapy, chemotherapy, immunotherapy, and combinations of these treatments. Although surgical resection, radiation therapy, and chemotherapy are utilized in the clinic, challenges remain, such as side effects that are caused by X-ray radiation and off-target toxicity of chemotherapy, which hamper the optimal efficiency of developing these therapies. Immunotherapy, which is a safer and more efficient approach, has been extensively exploited and widely applied by many researchers for tumor treatment [1–4].
Given the intricate mechanisms of tumors [5], it should come as no surprise that antitumor treatment cannot be used to realize optimal human health and well-being. Whether immunotherapies require the unleashing of cytotoxic T lymphocyte functions, exhaustion of immunosuppressive cell populations, or alteration of the tumor microenvironment, they share the common goal to modulate the immunity to ultimately overcome malignant growth [6–9]. The lymphatic system is a nonnegligible component of the immune system and is composed of a complex network of lymphoid tissues, nodes, and thin-walled capillaries for draining excess fluids and their solutes (e.g., antigens, cells, and exosomes) [10, 11]. Lymphatics have demonstrated feasibility as efficient delivery channels for immunomodulators (adjuvants, cytokines, and monoclonal antibodies), by which the diverse range of autoimmune diseases that are caused by off-target “immune-related adverse events” are minimized. In recent decades, immunostimulatory agents have been introduced into the tumor microenvironment or surrounding immune-rich tissues with the assistance of lymphatic targeting for immune response reshaping and tumor-associated treatments [12].
The direct administration of therapeutic agents often results in undesirable effects such as suboptimal pharmacokinetics, vulnerability to biodegradation, and compromised targeting. More recently, nanoparticle-based approaches for targeting lymphatic cells have become emerging strategies for improving immunomodulation modalities. These approaches not only can be used to surmount the aforementioned hurdles by fine-tuning the physical properties of nanocarriers, but can also significantly decrease toxicity due to their reduced uptake by untargeted tissues [13]. Moreover, nanomedicine or medical treatment with the assistance of nanotechnology can offer flexible and designable approaches for increasing antitumor immunity [2].
In the clinic, organ dysfunction that is caused by cancer metastasis, namely, the dissemination of tumor cells via the lymphatic or haematogenous pathway, is the main reason for the majority of cancer mortalities. Surgical resection usually serves as the primary treatment. Thus, sentinel lymph-node (SLN) mapping in the preoperative lymphoscintigraphy is especially important for the identification of metastasis. Additionally, for intraoperative localization, surgeons rely heavily on lymphatic imaging techniques. Therefore, a lymphatic-enriched image tracer delivery system that is facilitated by a nanoplatform can facilitate the acquisition of accurate lymphoscintigraphy images and, consequently, provide a better prognostic perspective.
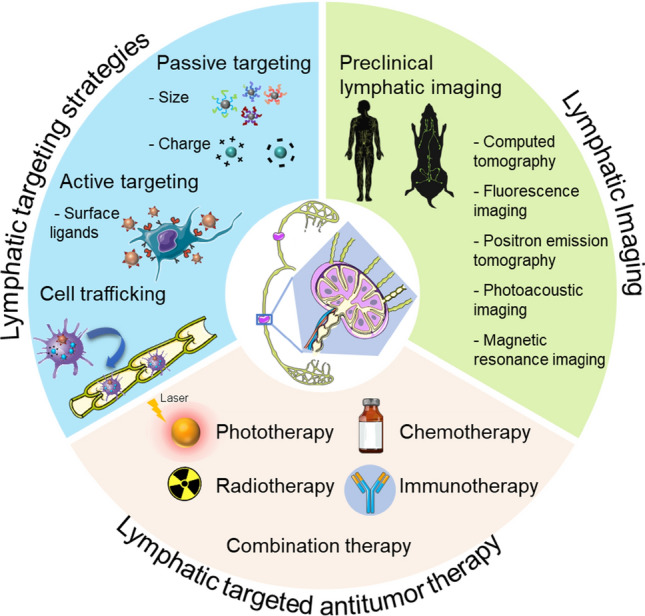
In this review, we outline current strategies through which nanoparticle technology has been devised for targeting the lymphatic system for antitumor therapies. First, nanoparticle-based methods for targeting the lymphatic system are summarized, and the following main topics are considered: the design parameters for effective targeting of the lymphatic system, the strategies for targeting lymphatic vessels and specified cells within the lymph nodes separately, and various administration routes for targeting the lymphatic system. Then, lymphatic imaging, which provides direct visualization of the lymphatic system, is discussed, because it plays a vital role in diagnosis and acts as a guideline for tumor metastasis. Finally, and more importantly, an overview of recent research on nanoparticles that target the lymphatic system for antitumor treatment is presented.
Nanoparticles that target the lymphatic system
Nanoparticles (NPs) provide a superior versatile platform for delivering therapeutic and diagnostic agents for targeting the lymphatic system. In principle, the design of advanced nanoparticles that target the lymphatic system increases the efficiency of lymph-node delivery, thereby optimizing the treatments against cancer and autoimmune diseases by reducing the agent dose required and reducing deleterious off-target effects and toxicities [14, 15].
Various nanoparticle formulations that target the lymphatic system have been undergoing rapid development, such as polymeric micelles, liposomes, dendrimers, metallic NPs, and nature/biomimetic NPs [16–19]. Immunomodulatory agents (adjuvants, secretory cytokines, and antibodies) can be codelivered via either encapsulation inside the core of an NP or by surface modification of the NP [12]. A variety of strategies, such as synthetic chemistry and engineering techniques, can help improve targeting effects and enhance the immunomodulatory outcomes by tuning the physicochemical properties of NPs and adopting various administration routes to facilitate lymphatic system uptake [20]. In addition, nanoparticle self-assembly in vivo has been employed for sustained drug delivery by inducing transitions in nanostructure morphology [21]. Used as a theranostic strategy, nanoparticles are promising because of their improved bioresorptive capacity and modularity that enables their customization, thereby enhancing immune responses.
Influential parameters for the effective targeting of the lymphatic system
Various factors, such as the size, shape, surface charge, molecular weight of carriers and the surface hydrophobicity, rigidity, and surface-conjugated ligand, determine the fate of NPs in the lymphatic system [20, 22].
For effectively reaching lymphatic system targets, small spherical NPs are preferred. Leveraging their permeation abilities, NPs can be used to passively target the lymphatic system. NPs with a diameter of 10–100 nm can be efficiently absorbed into lymphatic vessels [23, 24]. Similarly, it has been demonstrated that compounds of higher molecular weight possess an inferior blood capillary extravasation capability. For massive substances, lymphatic drainage is the primary efflux route initiated at the injection site, and the proportion of the dose that is absorbed by the lymphatics is typically linearly correlated with the molecular weight [22]. In general, the critical molecular weight threshold has been identified as 70 kDa, at which mass, the molecule can cross the conduit barrier that is created by the reticular network [25, 26]. In addition, NP shape can significantly affect the interactions between NPs and antigen presenting cells (APCs), because the contact angle determines the energy required for the formation of the actin cup and ring structure and, subsequently, the efficiency of NP phagocytosis and other types of internalization by macrophages and dendritic cells (DCs). Thus, spherical NPs have higher APC uptake and greater APC activation effects than worm-like particles due to the smaller contact angle [27, 28].
The surface charge, which is a crucial NP feature, contributes to NP stability and the compatibility between NPs and biological systems, and it can be tuned by modulating the cationic and anionic ion-forming materials or modifying the surfaces of NPs with materials with the desired charges. Glycosaminoglycans are the major components that contribute to the negative charge of the interstitial extracellular matrix (ECM), which greatly affects the passage of charged species through the tissue interstitium. Therefore, NPs with a negative charge undergo faster drainage and promote interstitial transfer due to fewer electrostatic interactions with the matrix [26]. As a result, negatively charged NPs accumulate more easily in lymph nodes than neutral and positively charged NPs [29]. In addition, it is posited that hydrophilic NPs move through interstitial water channels more effectively than hydrophobic NPs [30].
In addition to tuning the chemical and physical properties of NPs, the use of surface-conjugated ligands is a powerful strategy for targeting specified cells and tissues in the lymphatic system for inducing the reduction in nonspecific toxicity and the promotion of immune responses to enhance therapeutic efficacy (Table 1). Recently, lymphatic-specific receptors, such as lymphatic vessel endothelial hyaluronic acid receptor 1 (LYVE-1) [31], scavenger receptor class B member 1 (SR-B1) [32], vascular endothelial growth factor receptor 3 (VEGFR-3) [33], peripheral node addressins (PNAds) [10], and podoplanin [34], have been utilized for biomedical engineering; therefore, NPs that are decorated with correlated synthetic ligands are expected to target lymph nodes and promote interaction and uptake by the lymphatic endothelium. Small-molecule ligands are also involved in the surface modification of NPs to target specified lymphatic cells. For example, mannose [35] demonstrates the potential to serve as a ligand for targeting CD206 on macrophages and DCs, and hyaluronic acid (HA) [36] and DEC-205 [37] can be used to induce NP interactions with the CD44 receptor of macrophages and the CD205 receptor of DCs, respectively.
Table 1.
Ligands/antibodies that are used for lymphatic targeting
| Immune cell/tissue targeting | Ligands/antibodies | Delivery system/payload | Target sites | A.R | Application | Ref |
|---|---|---|---|---|---|---|
| LEC | HA | Polymeric NP | LYVE-1 | / | / | [49] |
| TL | LyP-1 | PEG-PLGA NP | P32 | s.c | Lymphatic metastatic tumors | [50] |
| TL | LyP-1 | Liposome | P32 | s.c | Lymphatic metastatic tumors | [51] |
| Macrophage | HA | HA-PEI/miR-125b | CD44 | i.p | Anticancer immunotherapy | [36] |
| Macrophage | HA | HA-PEI/miR-223 | CD44 | / | Re-polarization of peritoneal macrophages | [57] |
| DC | Mannose | Chitosan NPs/TCL | CD206 | s.c | Cancer vaccine | [56] |
| DC | Galactose | GDR nanogel/OVA | MGL | i.p | Cancer vaccine | [59] |
| DC | R4F | HPPS/Ap and CpG | SR-B1 | s.c | Cancer vaccine | [67] |
| DC | Anti-DEC-205 | Ferrous NPs/CpG and OVA | CD205 | i.v | Antitumor immunotherapy | [37] |
| HEV | MECA79 | Polymeric NP/anti-CD3 | PNAd | i.v | Immune therapy in transplantation | [78] |
| HEV | MECA79 | Polymeric NP/tacrolimus | PNAd | i.v | Immune therapy in transplantation | [79] |
| TAM | M2pep-R4F | HPPS/siRNA | M2/SR-B1 | i.v | Antitumor immunotherapy | [89] |
| SLN | HA /R4F | HPPS/DiR-BOA | CD44/SR-B1 | i.d | Diagnosis of LN metastases | [100] |
Endogenous macromolecules are also utilized to develop nanovesicles due to their minimal heterogeneity and distinct targeting features. Protein-based NPs are advantageously designed to interact with APCs to facilitate downstream antigen presentation and immune stimulation. Ovalbumin (OVA) [38] and heat-shock proteins (HSPs) [31] are the typical protein matrices for NP designed for use in cancer immunotherapy. Another emerging class of naturally originating delivery vehicles is cell membrane vesicles, such as white blood cell hybrids [39], red blood cell hybrids [40], and cancer cell hybrids [41], which possess diverse membrane biomolecules and intrinsic biomarkers for specific cell targeting and immune response. Thus, engineered tumor or immune cell membranes that are loaded with exogenous functional moieties can realize specific targeting and enriched accumulation.
Strategies for targeting lymphatic vessels
Lymphatic vessels are essential for immune function, tissue-fluid homeostasis, and the absorption of dietary fat [42]. Lymphatic uptake and transport of exogenous NPs in the tissue interstitium are driven by expansion and compression of the initial lymphatics, which are similar in terms of fluid, endogenous macromolecules, and cells [43]. Since the ECM forms a meshwork with irregular spacings of approximately 100 nm and is composed of glycosaminoglycans with a net negative charge [44], NPs with a 10–100 nm diameter [24, 45], a mass of 20–30 kDa, and neutral or negative charge are prone to travel across the interstitium and access lymphatic vessels [46, 47]. Small NPs, with a diameter of less than 10 nm (or molecular mass of 16–20 kDa [46]), are absorbed primarily via the blood capillaries draining the injection site not lymphatic vessels [48]. However, particles that are larger than 100 nm are poorly transported into lymphatic vessels due to reduced diffusion and convection through the interstitium [48]. This passive strategy is primarily utilized to target therapeutic delivery to lymphatic vessels.
Surface coating of chaperones that are complementary to highly expressed receptors on lymphatic endothelial cells (LECs) has also been utilized advantageously for boosting NP interactions with LECs and enhancing the uptake of NPs by the lymphatic endothelium. HA [49] and LyP-1 [50, 51] have been applied to endow chaperones with the capacity for targeting LYVE-1 and p32 on the surface of LECs, respectively. Other receptors, namely, podoplanin and prox-1, along with secreted cytokines (e.g., IFN-γ and TGF-β [52]) to promote the adhesion and trafficking of immune cells, are also intriguing for the utilization in lymphatic vessel targeting. In addition, the VEGFC-VEGFR3 and VEGFD-VEGFR3 axes make substantial contributions to the lymphangiogenic signaling pathway [33, 53]. VEGFR-3 can be employed as a specific marker for engineered NPs, and has been utilized to restrict angiogenesis and lymph-node metastasis [54]. Therefore, NPs that constitute or respond to these components can provide additional routes for specific lymphatic-specific delivery (Table 1).
Alternatively, cell-mediated trafficking of NPs may be another promising approach for lymphatic-specific delivery. Exogenous materials are phagocytosed by interstitial APCs such as dendritic cells and subsequently induced to mature, enter the lymphatic system, and migrate to lymph nodes, where they present antigens to lymphocytes [55]. Therefore, designed NPs that are subcutaneously administered and internalized by APCs can be trafficked to draining lymph nodes in association with the APC homing process. It was found that the mannose receptor (CD206) [35, 56], HA receptor (CD44) [57, 58], and lectin receptor (CD205) [35, 59] are exclusively expressed on the surfaces of APCs. Thus, improved targeting of APCs may be realized via the modification of NPs with mannosylation, hyaluronic acidification, and galactosylation.
Strategies for targeting immunocytes in LNs
The lymph nodes are the primary lymphoid organs and serve as depots where lymphocytes (e.g., NK, B, and T lymphocytes) elicit an efficient antitumor response. Therefore, LN-resident lymphocyte targeting is beneficial for regulating immune functions and anticancer immunotherapy.
LN-resident B cells are primarily distributed in lymph-node follicles, and the access of B cells to antigens is tightly managed by the subcapsular sinus. Therefore, B-cell targeting requires antigen transit by barrier capsule cells, typically subcapsular sinus macrophages [60]. Conduits serve as another pathway for distributing antigens to the follicular cells. Only soluble antigens (< 70 kDa) can be solely sampled by B cells in the lymph-node conduits. The lack of effective targeting strategies for B cells remains a challenge to date [61]. In contrast to conventional macrophages, subcapsular sinus macrophages have poor phagocytic ability, and their low capacity for processing particles makes them prone to serve as cellular barriers that direct antigen exposure to other lymphocytes. Thus, compared to phagocytosis, endocytosis is more reliable for the subcapsular sinus macrophages, since the scavenging is down-regulated [62]. In this case, liposomes are the preferred vehicles due to their amphipathic composition and enhanced internalization by subcapsular sinus macrophages. A typical example of subcapsular sinus macrophage targeting is the delivery of clodronate for macrophage depletion on demand [63, 64], which can be further utilized for deeper delivery into the lymph nodes.
DCs are critical for presenting antigens to LN-resident T cells and inducing the production of antigen-specific cytotoxic CD8+ T cells. LN-resident DCs can be sorted into immature DCs (imDCs) and mature DCs (mDCs), which demonstrate different phagocytic capacities [65, 66]. In contrast to enhanced antigen processing and presentation, the nonspecific uptake capacity of DCs is dramatically down-regulated during maturation; hence, their potential as vaccine targets has been substantially overestimated. In one of our recent studies [67], a peptide-based vaccine was avidly delivered to LN-resident mDCs via the SR-B1 pathway. The self-assembled α-peptide controlled the size of the vehicles to approximately 30 nm and provided mDC affinity. By encapsulating CpG oligodeoxynucleotides, the obtained nanovaccine demonstrated substantially enhanced antitumor efficacy in prophylactic and therapeutic tumor models. The excellent outcome demonstrated the potential of LN-resident mDC targeted vaccination for clinical cancer immunotherapy (Table 1).
LN-resident T lymphocytes play crucial roles in the adaptive immune response, and they are critical for metastatic colonization and various immune system diseases. Nanoplatforms that target T lymphocyte for drug/gene delivery have shown substantial promise in cancer immunotherapy. One of the most extensively used strategies is use of the HEV, since T cells are widely distributed in the paracortex near blood capillaries [10]. Due to the high level of PNAd expressed in the draining lymph nodes with chronic inflammation, the surface ligand MECA-79 enables nanoplatforms to target the PNAd [68, 69] (Table 1). Therefore, therapeutic agents can be selectively delivered to draining LN-resident T-cell populations, which can also be applied to direct cancer immunomodulation.
Administration routes for targeting the lymphatic system
The lymphatic system is engaged in immune surveillance and the generation of immune responses. In addition, it plays a nonnegligible role in initial cancer metastasis. Therefore, the delivery of exogenous therapeutic agents into the lymphatic system is important for anticancer treatment. Facilitated by nanoparticles, cargos can be administered transdermally (intradermally or subcutaneously) in peripheral tissues or injected intravenously to provide low but sustained levels of lymphatic delivery and to regulate the immune system systematically, respectively. For antigen-delivering nanovehicles with a ~ 300–900 nm diameter, limited accumulation in lymph nodes can be addressed via direct lymph-node injection [70, 71]. The invasive intra-lymph-node approach, which has been explored in vaccination studies for decades, can lead to uptake of the required drug levels in the lymph node for local release and bypass the systemically deleterious effects that originate from superfluous dosage used to overcome insufficient blood transport.
Regarding patient compliance and the potential generation of a systemic immune response, oral delivery to the target lymphatic system that is mediated by intestinal APCs has been utilized for decades in clinical vaccination [72]. To be transported to mesenteric lymphatic nodes efficiently, drug delivery systems must overcome several barriers, such as the mucosa and gut barriers [73]. Once intestinal APCs capture nanoparticles, they are activated, migrate along mesenteric lymphatics, and accumulate in lymph nodes. Many physiochemical properties of a delivery system, such as the size, charge, and surface decoration, may influence this process pleiotropically and contextually. To date, the pathways and mechanisms of nanoparticle-mucosal APC interactions and subsequent immune responses remain to be elucidated. Nevertheless, oral delivery remains crucial for the intestinal lymphatic transport of highly lipophilic drugs due to its high efficiency.
A prominent challenge in oral drug delivery is the poor bioavailability that stems from the first-pass metabolism [74]. Intestinal lymphatic transport can circumvent this issue, but relatively limited studies have described its benefits for lymphatic targeting of the orally administered therapeutic agents used for cancer treatment [55, 72, 75, 76]. The major barrier that hampers research progress is related to the elusive underlying mechanisms of mucosal uptake. Interestingly, lymphatic entrance and accumulation in the sites of lymphatic cancer metastasis after subcutaneous or intravenous administration offer other possibilities. Numerous designs have been developed for the delivery of therapeutic agents with various clinical objectives, such as the utilization of microneedle patches to overcome interstitial efflux [77] and the surface functionalization of nanoparticles with anti-PNAd antibodies for HEV targeting via intravenous injection [78, 79]. Hence, the route of administration helps define the distribution and bioavailability of the delivered therapeutic agents, and it should be comprehensively considered for efficient lymphatic targeting based on disease models.
In vivo lymphatic imaging
The lymphatic system plays crucial roles in collecting circulating fluid, maintaining the tissue-fluid balance, and regulating immune responses [80, 81]. Various pathological processes (e.g., lymphoedema and cancer metastases) and local immunocompromise can lead to significant morbidity and trigger structural and pathophysiological changes in the lymphatic system, thereby generating diagnostic information [82]. Thus, the visualization of lymphatic anatomy and function has presented intriguing opportunities for lymph-directed cancer therapies.
Due to the invisibility and fragility of the lymphatic vessels, direct injection into the lymphatic vessels is a delicate task for surgeons and researchers [83]. To date, the vast majority of lymphatic visualization is based on the interstitial administration of a contrast agent and its subsequent transportation and accumulation, which enables detection by various imaging modalities. An overview of preclinical contrast agents for lymphatic imaging is presented in Table 2, and further clinical applications, such as image-guided photothermal treatment and surgery, are discussed below (Fig. 1).
Table 2.
Contrast media for multimodality lymphatic imaging
| Nanoplatform | Imaging methodology | Lymphatic targeting system | Specific targets | Encapsulation | Disease models | Features | Ref |
|---|---|---|---|---|---|---|---|
| HPPS | FI | Active targeting via ligands | SR-B1 targeting ligand | DiR-BOA | Lymphoma (E.G7-OVA) | Targeting of mDC and fluorescence trafficking | [67] |
| Aluminum hydroxide-polymer NPs | FI | Passive targeting | CpG; OVA | Melanoma (B16F10) | Aluminum-based nanoparticles for LN-targeted delivery | [84] | |
| Albumin/MEB nanocomplex | PET-FI | Passive targeting via a self-assembled nanocomplex | CpG; molecular vaccine | Lung metastatic-like tumor via i.v. injection of MC38 cells | Self-assembled nanovaccine with the advantages of facilitated preparation and high lymphatic-targeting efficiency | [86] | |
| Liposome | CT-FI | Active targeting | Negative charge | Lipiodol; DiR-BOA | Murine mammary carcinoma | Direct injection of the lipiodol system for the long-distance mapping of the lymphatic system | [90] |
| Gold NPs | Bioluminescence Imaging | Cell-mediated trafficking via DCs | CpG; OVA | Adenovirus; lentivirus lung infection | Optimal size determination for lymphatic uptake | [91] | |
| Gold NPs | CT-FI | Cell-mediated trafficking via APCs | CpG ODN as TLR9 ligand | RFP; CpG; | Melanoma | Noninvasive tracking of the nanovaccine with CT | [92] |
| Fe3O4 NPs | MRI | Cell-mediated trafficking via DCs | ZnO shell | Carcinoembryonic antigen | Colon carcinoma | Noninvasive tracking of the nanovaccine with MRI | [93] |
| SWCNT | PAI-FI | Passive targeting | 4T1 breast tumor model | Imaging-guided PTT | [95] | ||
| Gd-doped Au@Prussian blue NPs | MRI-SERS | Cell-mediated trafficking via DCs | OVA | Background-free SERS with insusceptibility and detection sensitivity | [106] | ||
| -AP-fmNPs | MRI-FI | Cell-mediated trafficking via DCs | SR-B1 targeting ligand | Antigen peptide; ICG | Lymphoma (E.G7-OVA) | Enhanced migration of DCs via a magnetic pull force | [107] |
| [64Cu]-labeled PDI NPs | PET- PAI | Passive targeting | U87MG tumor xenograft model | A PET-PAI dual-modality imaging tracer with self-assembly and size-tunable properties | [111] |
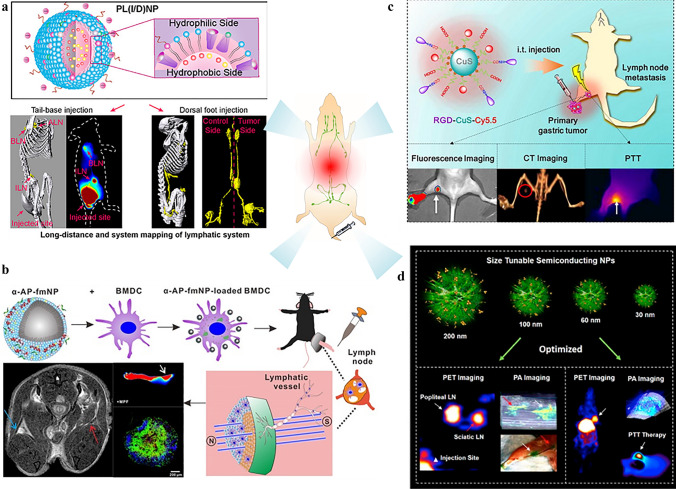
Fig. 1.
Preclinical applications of lymphatic imaging: a system mapping of the lymphatic system [90]. Copyright 2019, American Chemical Society. b In vivo tracking of DC vaccines with multimodality imaging [107]. c Image-guided photothermal therapy [113]. Copyright 2018, Elsevier Ltd. d Image-guided surgery [24]. Copyright 2017, American Chemical Society
Imaging techniques that are applied in lymphatic mapping
As tracers for imaging the lymphatic system are typically administered via intradermal or subcutaneous injection, the main obstacle is gaining efficient entry into the lymphatic system after passing the interstitial matrix. The physicochemical properties of nanoparticles can substantially influence the fate of an interstitially administered contrast agent [84]. A study on the optimal sized of subcutaneously injected particles for lymphatic uptake showed that particles that ranged in size from 10 to 80 nm tended to be taken up by APCs, while smaller particles drained into the systemic circulation [85]. Zhu et al. developed nanocomplexes that were self-assembled in vivo by utilizing the well-known interaction between Evans blue and endogenous albumin [86]. The resulting hybrid complexes, which exceed the scale threshold for systemic dissemination, can be endocytosed efficiently by APCs and subsequently homed to the lymphatic nodes for fluorescence tracking and immunomodulation.
Similarly, molecular markers that are highly expressed in the lymphatic system serve as additional crucial parameters that have long been correlated with the development of lymphatic imaging. Recently, it was demonstrated that LECs that are characterized by a high level of SR-B1 facilitate high-density lipoprotein (HDL)-mediated reverse cholesterol transport via active transcytosis [32]. Zhang and Zheng et al. developed a phospholipid scaffold (HPPS) with an amphipathic α-helical peptide in which the size of the scaffold was structurally and functionally controlled, which demonstrated increased draining efficiency and SR-B1 targeting capability [87–89].
In the tumor lymphatic metastasis model, diagnostic imaging for the identification of LNs with lymphatic metastases demonstrated the feasibility of quantification. Conventional clinical imaging techniques (e.g., MRI, CT, and PET) have demonstrated unique functionalities [85]; for example, MRI provides high resolution in soft tissue, CT provides deep tissue penetration, and PET provides excellent spatial resolution for the identification of early tumor foci. However, long-distance mapping of the lymphatic system remains challenging due to the absence of a suitable image contrast system. On the basis of liposomes that consist of lipids with spatial complementary structures, a PL(I/D) nanoparticle was designed by our group to encapsulate lipiodol and DiR-BOA, which are a CT tracer and a near-infrared (NIR) dye, respectively [90]. CT can provide high spatial resolution, desirable imaging depth, and precise anatomic information. Meanwhile, hydrophobic lipiodol exhibits excellent lymphatic retention, not the natural diffusion out of lymphatic vessels (LVs) that is observed with the water-soluble contrast agents. The utilization of lipid nanovehicles facilitates the resolution of the technical difficulties and adverse responses (pulmonary oil embolization) during the clinical application of lipiodol. Dual-modality lymphatic imaging produces a long-distance map of the lymphatic system with a remarkable length of ~ 68 mm and provides accurate information on the size of the lymphatic nodes and the lengths of the LVs.
Optical imaging approaches, which include fluorescence and PAI, demonstrate superior advantages for surgeons over conventional techniques due to the real-time LN mapping and 3D tumor foci localization. Fluorescent imaging using indocyanine green (ICG) is a typical example of preoperative tumor imaging and LN mapping for numerous cancers in the clinic [93, 94]. However, translation barriers, namely, high dosage demands and confined span of the clinical operation, impede the clinical application of NIR fluorescent imaging. NIR-II fluorophores (900–1700 nm) demonstrate reduced tissue scattering compared to NIR-I fluorophores (650–900 nm); thus, the tissue penetration and spatial resolution of NIR-II fluorophores are much better and have recently attracted substantial attention. Chao et al. utilized SWCNTs for NIR-II fluorescent imaging [95]. Photothermal ablation was also conducted ~ 90 min post-injection when the accumulation of the SWNTs reached its peak level, and metastatic spread was substantially inhibited. Nonnegligible challenges that are encountered during the clinical translation of NIR-II fluorophores are the low extinction coefficients and quantum yields.
PA provides the high soft-tissue contrast that is desirable for LN mapping and represents another emerging clinical technique that demonstrates substantial potential for visualization of the lymphatic system. Intensive studies have been conducted on the development of photoacoustic contrast agents, such as gold and perfluorocarbon nanoparticles, which aid in SLN mapping procedures [96–99]. A tumor-node-metastasis staging study using the PA technique was performed by our group to discriminate inflamed LNs (Inf-LNs) and normal LNs (N-LNs) from tumor-metastatic LNs (T-MLNs). The NIR-PA contrast agent (DiR-BOA) was loaded in HPPS, and hyaluronic acid was utilized for surface decoration. The obtained dual receptor-targeted nanoparticles demonstrated specific selectivity to breast cancer cells in vitro and in vivo. The biodistribution of the PA signal in LN aided in distinguishing T-MLN from Inf-LN and N-LN. The distinct distribution of the PA signal was demonstrated to be originate from the characteristic distribution patterns of nanoparticles, which are mainly in the tumor cells of the T-MLN and in the subcapsular sinus macrophages of the Inf-LN [100]. However, for clinical implementation, safer organic contrast agents and more cost-effective instrumentation must be developed.
Lymphatic imaging for the in vivo tracking of DC vaccines
Antigen presentation by DCs occurs primarily in the lymph nodes. Thus, lymph-node homing of DC is essential for the curative effect of DC vaccines, and monitoring of the lymph-node homing of DC vaccines is a paramount challenge. Engineered DCs can be efficiently labeled ex vivo via the coincubation with numerous nanoparticles as imaging tracers due to their superior phagocytosis capability of the DCs [101]. Cho et al. designed an iron-oxide-zinc oxide core–shell nanoparticle for the delivery of the antigen to DCs that simultaneously functioned as an MRI agent, which enabled the monitoring of DC migration in vivo [102]. In another study, quantum dots (QDs), which are NIR-emitting fluorescent semiconductor nanocrystals, were used by Noh et al. to identify the movement of injected DCs to a target tissue [103]. Using QDs as a contrast agent, QD-labeled DCs were tracked from injected footpads into the popliteal and inguinal lymph nodes by NIR fluorescence. The results demonstrated negligible influences of the QDs on the DC phenotype and maturation potential. Limited by sophisticated physiological environments, suitable visualization and quantitative information are acquired only by the multimodality biomedical imaging, even though tracing of the lymphatic architecture has been realized via single imaging strategies at superficial sites.
The combination of magnetic resonance and near-infrared fluorescence imaging has produced superior results for in vivo DC tracking due to its capacity to provide both anatomical background information and high-sensitivity detection [104, 105]. Nanoparticles that are loaded with antigens, namely, nanovaccines, can also deliver adjuvants and imaging reagents for immunomodulation and tracking of the biodistribution of endogenous DCs in vivo. Zhang et al. demonstrated a multifunctional DC-targeted nanoplatform for immunotherapy [106]. The Au nanoparticles that were modified with Prussian blue as the core were coated with ovalbumin (APG@OVA) to form a DC-targeted nanoplatform with high sensitivity to magnetic fields and inelastic photon scattering. The activation of DCs and the real-time tracking of the DC migration process were displayed by MRI. In addition, the distribution of colonized DCs in the lymph nodes was demonstrated by background-free Raman mapping at the single-cell level via the SERS technique.
Image-guided surgery and photothermal therapy of cancer metastasis
Lymphatic metastasis is among the most common forms of cancer metastasis. Studies have shown that tumor-associated lymphatic vessels are essential for tumor metastasis, because they spread tumors to draining lymph nodes and adjacent tissues [108], and lymphangiogenesis within tumor-draining LNs might be the earliest sign of metastasis [109, 110]. Lymph-node metastasis and prognosis are closely related to the expression of lymphangiogenic factors and the density of lymphatic vessels. Thus, to obtain a clinical diagnosis, it is crucial to acquire a systemic, precise and quantitative in vivo map of the lymphatic system. In addition, lymphoscintigraphy can be of substantial assistance to surgeons.
Lymphoscintigraphy used for surgery, that is, image-guided surgery, is typically classified into preoperative, intraoperative, and postoperative imaging. Accurate preoperative identification of LN metastasis can offer physiological information (tumor distribution and anatomical position), and consequently, more reasonable treatment schedules can be established for the patient. In addition, SLN mapping demonstrates the potential to be used to detect low-volume metastasis, which facilitates tumor staging and refined regional lymphadenectomy [111]. Intraoperative imaging has emerged as a valuable tool for tumor margin delineation for surgical resection by which tumor recurrence that is caused by incomplete tumor resection is minimized. Chen’s group implemented dual-modal PET and intraoperative photoacoustic imaging for LN mapping, which provides deep penetration for LN imaging and high spatial and temporal resolution, respectively [24].
Although the primary tumor can be removed during surgery, in-transit tumor cells in the lymphatic vessels can still cause tumor metastasis. It has been reported that photodynamic therapy (PDT) and liposomal verteporfin can effectively destroy lymphatic vessels, which not only eliminates tumor cells in the lymphatic system but also prevents the metastasis of cancer cells and subsequent recurrence [112]. Recently, image-guided PTT was utilized against metastatic gastric cancer cells via αvβ3 integrin-mediated endocytosis, which enabled rapid self-monitoring of PTT efficacy in vivo [113]. Multimodal imaging guidance has contributed to the identification of tumor metastasis and efficient photothermal ablation. The therapeutic regimens of PTT are optimized, and further treatment for distant metastasis deep in organs remains to be explored.
As demonstrated in the study that is discussed above, visualization of the lymphatic system may indeed facilitate pathophysiological diagnosis with multiple imaging modalities. The pathological investigation of the lymphatic system also improves the molecular understanding of lymphatic-based immunomodulation. In addition, the tracer delivery strategies that have been developed for lymphatic imaging can be directly transferred to the transport of nanoscale theranostic agents, which may open up new avenues for lymphatic therapy. In light of lymphatic metastasis, mapping, visualization, and further surgical removal of tumor metastases substantially rely on lymphatic imaging [114]. Imaging-guided surgery holds great potential for the clinical dissection of cancer lesions and remains to be developed for clinical trials. In addition, antigen trafficking with a sophisticated platform in the lymphatic system has been investigated for the next generation of vaccines [115]. One highlighted strategy for vaccination is the cascade of delivering antigens and adjuvants to lymph-node-resident dendritic cells via interstitial-to-lymphatic flow, which triggers the desirable immune responses smartly and precisely [116].
Nanoparticle-based approaches for targeting the lymphatic system for antitumor treatment
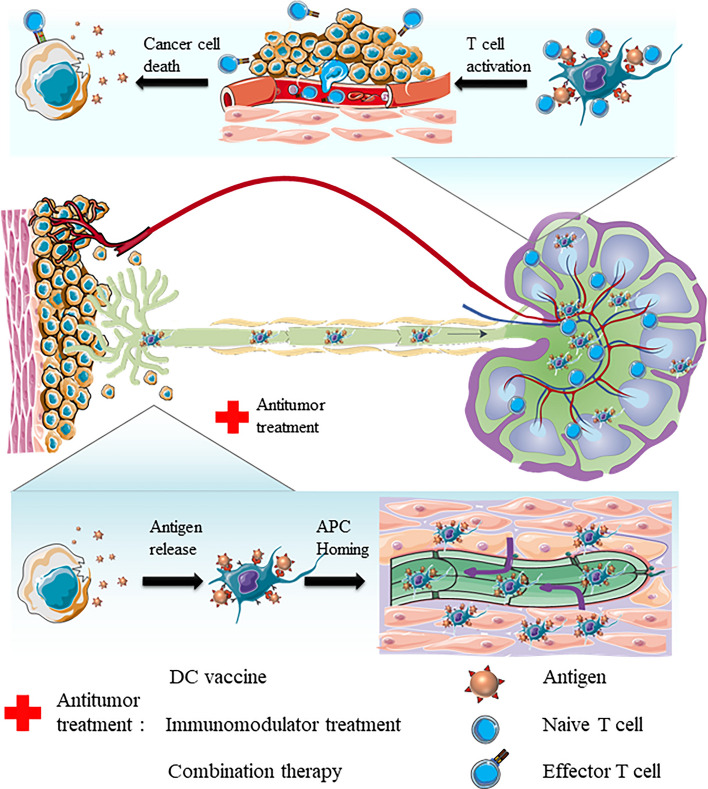
The eradication of tumor foci by the immune system and the elicitation of systemic antitumor responses are involved in the following stepwise processes, which can be referred to as the cancer-immunity cycle (Fig. 2) [117]. Tumor-associated antigens and neoantigens are released from various antitumor treatments and transported into LNs by draining or APC trafficking. Subsequently, the activation of LN-resident T cells is elicited with costimulatory agents. The following antitumor immune responses are realized by the migration and infiltration of effector T cells from LN to tumor foci via blood circulation with the stimulation of various chemokines and integrins. Finally, tumor cells are recognized by effector T cells and killed via cellular apoptosis or lysis. Collectively, the lymphatic system is the key junction of multimodal antitumor treatment.
Fig. 2.
Schematic illustration of the cancer-immunity cycle
Currently, immunotherapy, which includes DC vaccines and numerous immunomodulatory therapies, has been one of the most apparent motivations for targeting the lymphatic system. For immunotherapy, the quality and quantity of the immune response are finely manipulated by adjusting the activation state of APCs. Therefore, many studies are being conducted on the regulation of the functions of lymphocytes via various approaches, such as costimulation [118], antigen presentation [119], and targeting [120]. In addition, the nanoparticle-based lymphatic system targeting approaches can dramatically influence the pharmacokinetics and biodistribution of delivered cargos, and various conventional and emerging cancer therapies are now used in combination with immunotherapies, thereby offering the possibility of amplifying antitumor immune responses and sensitizing tumors to immunotherapies safely and effectively [121–124]. In this section, we focus on the progress of vaccine and immune therapeutic agent treatment for antitumor therapy with the assistance of nanoparticle strategies, following the introduction of the emerging trend of combinational therapies.
DC vaccine approaches to cancer immunotherapy
Currently, emerging cancer immunotherapy repertoires include the checkpoint blockade antibody treatment for modulating the cancer immune microenvironment and chimeric antigen receptor T-cell therapy for patient-specific adoptive immunotherapy [125]. However, there remains a substantial population for whom newly developed immunotherapy agents or combination treatments are ineffective and/or induce significant toxicity. To enhance immunotherapy efficacy and reduce deleterious effects, extensive scientific efforts have been directed towards designing smart-biomaterials-based drug delivery systems in which antibodies are encapsulated and targeted for delivery [126].
The vaccine, which has created the paradigm for modulating the immune system since its discovery, is now playing a vital role in antitumor immunotherapy. Various types of cancer vaccines, including tumor cell lysates and dendritic cells [127], have been developed, and the lymphoid system is strategically positioned to be targeted. DCs, which are a diverse group of specialized APCs, constitute a rare immune cell population within peripheral tissues and lymph nodes but play essential roles in immune activation and link between innate and adaptive immunity [128]. Immune responses are ineffective in many tumors due to the immunosuppressive environment and the promoted exhaustion of effector T cells [129–131]. The absence or malfunction of endogenous DCs can be addressed by DC vaccines through the manipulation of autologous DCs to enhance T-cell responses that are directed against the tumor. To date, DC-based cancer vaccination has made significant progress by exploiting autologous DCs that have been incubated with specific tumor-associated antigens or whole-tumor lysates ex vivo to elicit the cancer elimination immune response. The first therapeutic DC vaccine (PROVENGE), which is a type of autologous cellular immunotherapy, was approved by the US Food and Drug Administration (FDA) for the clinical treatment of prostate cancer in 2010 [132]. PROVENGE was less clinically effective than anticipated. The median survival time of patients was prolonged by only 4.1 months. It is anticipated that the low efficiency can contribute to the inefficient migration of DCs to the lymph nodes, where DCs activate antigen-specific T cells [133]. To improve DC migration, proinflammatory cytokines (such as tumor necrosis factors) are preinjected to produce an inflammatory microenvironment for the migration of DCs through lymphatic vessels [134]. The quantity of injected DCs and the degree of DC maturation may also affect the migration efficiencies of the injected DCs [99]. In addition to these efforts, magnetic nanoparticles can be exploited to facilitate the migration and selective accumulation of in vitro cultured DCs in lymph nodes under a locally applied magnetic field. In a recent study by our group [107], iron oxide nanoparticles with a α-helix peptide-functionalized phospholipid shell (α-AP-fmNPs) were developed in combination with a magnetic pull force (MPF) to manipulate the migration of DC vaccines in vitro and in vivo. α-AP-fmNPs endowed DC vaccines with MPF sensitivity, and their migration efficiency was dramatically augmented, thereby resulting in significant potentiation of antitumor efficacy. DC-swallowed α-AP-fmNPs can be further used for migration tracking and lymph-node imaging by multimodality imaging.
Despite these promising results, the successful translation of ex vivo DC vaccines into clinical applications has been restrained by variable yields and quality. The generation of DC vaccines ex vivo is labor-intensive and involves the use of specialized equipment for the differentiation of peripheral blood monocytes and the pulsing of DCs with antigens [135, 136]. Moreover, the yield and quality of DCs that present MHC-peptide complexes vary by batch depending on the condition of the patient’s peripheral blood monocytes. Furthermore, only a low rate (< 4%) of lymphoid tissue homing is typically realized upon the administration of DCs after intradermal injection [137]. To address these limitations, an alternative approach is proposed: the direct delivery of antigens that are complexed with antibodies that target DC receptors to DCs in vivo with facilitation by nanovehicles. To date, various surface proteins (e.g., CD205 [138], DCIR [139], DC-SIGN [140], Dectin 1 [141], CLEC9a [142], and Langerin [143]) that are specifically expressed in DC subsets have been leveraged for the preparation of DC vaccines in vivo. However, the expression levels of the ligands are inconsistent among DC subsets; hence, DC targeting strategies must be based on their physiological functionality. For instance, lymph-node-resident mature DCs (mDCs) can mediate immune responses by presenting antigens to lymph-node-resident T cells. Their nonspecific uptake capability is dramatically down-regulated during maturation. To deliver the antigen peptide directly to LN-resident mDCs for DC vaccination, we designed a nanoparticle for delivering the peptide antigen via the SR-B1 pathway [67]. The small size (~ 30 nm) facilitated passive targeting of the lymphatic system, and SR-B1 targeting substantially enhanced antigen uptake by mDCs, thereby leading to the activation of a specific immune response and significantly retarded tumor growth. In addition to the aforementioned efforts, newly emerging engineering approaches can also improve DC vaccines. For example, in situ vaccination with an injectable hydrogel can recruit immature DCs from local tissues and prime the antitumor immune response without the need for ex vivo manipulation of DCs [144]. The utilization of exosomes to culture DCs can also promote antigen presentation that results from membrane exchange between tumor antigen-containing exosomes and DCs [145].
For cancer vaccines, antigens are regarded as a critical component. However, the quality of tumor-associated antigens is not well defined, thereby resulting in the empirical development of an efficient vaccine in most cases. Synthetic peptides represent a new generation of the explicit subunit vaccine concept. Compared to full-length-protein-based vaccines, peptide-based vaccines often demonstrate the advantages of general safety, ease of large-scale production, and high purity [146]. The main limitation of synthetic peptide-based vaccines is their rarely sufficient immunogenicity when used alone. Similarly, the newly generated neoantigens, which originate from tumor-specific mutations and have been proven to be highly relevant to tumor control, are restrained by the sophisticated prognosis of individuals [147–149]. In contrast to the well-defined specific tumor antigens, whole-cell tumor antigens can provide a broad spectrum of patient-specific tumor antigens that facilitate the elicitation of a strong antitumor immune response, thereby leading to the diminishment of tumor escape and recurrence [150]. Recently, talimogene laherparepvec (T-VEC, which is also known as OncoVEXGM−CSF), an oncolytic viral treatment, was approved for melanoma immunotherapy by the FDA in 2015 [151]. Intratumorally injected oncolytic viruses result in the release of various endogenous tumor-associated antigens and damage-associated molecular patterns (e.g., ATP and calreticulin), thereby increasing the immunogenicity of the tumor microenvironment [152, 153]. In another study, Shi et al. developed a chitosan-based nanoplatform for loading whole-tumor antigens from tumor cell lysates for LN-resident DC targeting [56]. The mannose derivative-based nanovehicle enhanced DC-mediated cell trafficking to draining lymph nodes and subsequently enhanced a cytotoxic T lymphocyte response. Since the preparation of the tumor lysate antigens requires a substantial time commitment and is inconvenient, our group proposed a strategy for utilizing melittin for the induction of tumor antigen release in situ by promoting tumor necrosis or apoptosis [154]. As a traditional medicine, melittin has demonstrated a wide variety of immunomodulatory capabilities as a cationic host defense peptide. However, the immunomodulatory effects of melittin on the antitumor response are mild due to the narrow safe dose range and hemolytic side effects. Moreover, melittin can be rapidly metabolized after parenteral administration via passive fusion into the blood circulation. The reduced cytotoxicity to red blood cells encouraged further application of the obtained melittin-lipid nanoparticles (α-melittin-NPs) to antitumor treatment. Due to their ultrasmall size (10–20 nm), α-melittin-NPs have an optimal capacity for LN-targeting for direct drainage into lymphatic capillaries and lymph nodes, where melittin can exert its full immunomodulatory effect via the activation of APCs, namely, DCs and macrophages. After intratumoral injection of α-melittin-NPs in a bilateral flank B16F10 tumor model, the in situ vaccination elicited prominent systemic humoral and cellular immune responses and led to the elimination of 70% of primary tumors and 50% of distant tumors. The advantages of α-melittin-NP, such as simple preparation, excellent stability, and inherent oncolytic properties, render them ideal LN-targeted nanovaccines and endow them with substantial potential for clinical translation because of their remarkable immunoregulatory efficiency.
In addition to the DC vaccine delivered ex vivo or in vivo with numerous antigens (e.g., synthetic peptide, neoantigens, or whole-cell tumor antigens), recent studies have suggested that antitumor immunity can also triggered in tumor foci in situ, especially in organs with tissue-resident APCs, such as the liver [155, 156]. Due to the intrinsic immune suppression environment of the liver organ, immunotherapies, such as checkpoint inhibitors, chimeric antigen receptor cell therapies, and cancer vaccines, produce unsatisfactory outcomes in preventing liver metastasis compared with other antitumor treatments. The most attractive strategy for preventing liver metastasis that has been developed thus far involves reversing the tolerogenicity of the liver by either eliminating suppressor lymphocytes (e.g., Tregs and MDSCs) or activating hepatic effector cells (e.g., NK and T cells) [157, 158]. Furthermore, nonprofessional APCs were recently found to be involved in the modulation of immune responses in the liver, in which they can be activated via special immunomodulators (e.g., melittin) and subsequently reverse the immunosuppressive environment of the liver and minimize liver metastasis, which is discussed below in detail.
As demonstrated in the aforementioned studies, novel professional/nonprofessional APC-targeting nanovaccines or immunomodulators may indeed provide valuable strategies in anticancer treatment. Nevertheless, antitumor immunity is a stepwise physiological process that involves a variety of lymphocytes, and in which the lymphatic system plays a vital role. Therefore, this strategy may be a particular helpful addition to surgery. Further cancer vaccination developments remain to be evaluated in future clinical trials.
Lymphatic-targeted nanotherapeutic agents for antitumor treatment
Lymphatic-targeted nanotherapeutic agents, namely, nanoparticles, serve as delivery tools for immunostimulatory agents (e.g., adjuvants, cytokines, and monoclonal antibodies) to specifically target lymphatic cells and have substantial potential to increase antitumor immunity by leveraging proper nanoformulation designs compared to free formulations [12].
Adjuvants, which include small molecules and biomolecules, can stimulate pathogen recognition receptors (PRRs) on APCs and, thus, induce strong proinflammatory responses [159]. They are commonly codelivered with antigens to elicit potent tumor-specific responses. Adjuvants also demonstrate potency as monotherapies due to the nonspecific stimulation of immune activity. As a typical nucleic acid-based adjuvant, cytosine-phosphate-guanine (CpG) is known to modulate the immune response for antitumor treatments via TLR9 signaling [160, 161]. Synthetic high-density lipoprotein (sHDL) nanodiscs that are loaded with Adpgk neoplastic antigens and CpG adjuvants can be targeted for delivery to lymph nodes, thereby creating an individualized vaccine response [162]. Recently, virus-like particles (VLPs) have garnered tremendous attention, since they can be readily employed to induce immune responses. For example, VLPs covalently bind tumor-associated antigens gp100 and CpG and significantly inhibit the growth of melanoma [163]. Additionally, the modulation of tolerogenic APCs in the liver can be a promising alternative for the activation of a specific antitumor immune response due to their immunomodulatory properties. It was reported that peptides that contain the RXR or RXXR sequences can demonstrate the capacity to target LSECs [164]. Thus, α-melittin-NPs have the potential to target liver sinusoidal endothelial cells (LSECs) in the liver and modulate immune responses to suppress liver metastasis. In our recent study [165], α-melittin-NPs were intravenously injected into multiple experimental liver metastasis models. Intravital imaging showed fast targeting of LSECs within 20 s after i.v. injection. Upon exposure to the treatment of α-melittin-NPs, the cytokine/chemokine milieu in the liver dramatically switched from being a hepatic immunosuppressive environment to an activated state environment. After ingesting the antigen in situ, tissue-resident APCs do not migrate to the tumor-draining lymph node but present antigens directly to local infiltrating T cells, thereby initiating an antitumor immune response with high efficiency. Moreover, the survival rate strikingly reached 80% in spontaneous liver metastatic tumor models, and the median survival time exceeded 100 days, compared to less than 40 days in the control groups. This novel immunomodulator may have substantial potential for clinical translation by controlling liver metastasis through the immunomodulation of LSECs.
In addition to adjuvants, a different category of cytokines is engaged in the signaling and communication of various immune cells, thereby exerting the function of immunomodulation in complex ways [8, 166]. On the basis of physiological features, cytokines can be classified into two types: proinflammatory cytokines (e.g., IL-12, IL-2, IL-18, and IFN-γ) and functional inhibitory cytokines (e.g., TGF-β and IL-10). Proinflammatory cytokines can directly stimulate immune effector cells at the tumor site and enhance tumor cell susceptibility to immune attack. For example, the encapsulation of cytokine IL-2 into a nanogel enables the sustained release of IL-2 and retards the growth of tumors by significantly reducing systemic side effects, thereby resulting in enhanced therapeutic efficacy compared to that of the free formulation [167]. Functional inhibitory cytokines are involved in the induction of an immunosuppressive tumor microenvironment. The coencapsulation of inhibitory cytokines and proinflammatory cytokines produces enhanced antitumor responses by modulating multiple immune pathways. Park et al. designed a nanoscale liposome polymer gel (nLGs) that synchronically delivered TGF-β inhibitors and IL-2 to subcutaneous and lung metastasis foci, thereby resulting in increasing performance due to the activation of both innate and adaptive immune responses by the nLGs [168].
Agonists can also serve as candidates for promoting immune stimulation or antagonizing immunosuppressive interactions [169]. CD40, OX40, STING, and ICOS are among the most commonly used immune targets for agonists [170]. However, the clinical sequelae of agonists are substantially neutralized by deleterious effects that originate from their deficient bioavailability. Nanoparticle-mediated targeted delivery of agonists can provide an opportunity to address issues of bioavailability and diminished effects. Luo et al. developed luminescent mesoporous silica nanoparticles (LPSiNPs) that were loaded with a CD40 agonist (FGK45) [171]. The LPSiNPs remarkably enhanced the local immune responses and generated activated B cells in an amount equal to that induced by the application of 30- to 40-fold free FGK45.
In clinical trials, immune checkpoint blockade (ICB) therapy based on checkpoint inhibitors (e.g., PD-1/PD-L1 and CTLA4) has produced exciting results for treating various tumors and has been approved by the FDA [172, 173]. PD-1 belongs to a family of CD28 proteins that are overexpressed in multiple immune cells, such as activated T cells, B cells, NKs, and DCs. In addition, PD-L1 is overexpressed on various tumor cells and binds with PD-1 on immune cells to inhibit immune killing functions [174, 175]. To further amplify the efficacy of ICB therapy and decrease off-target effects on healthy tissue, refined nanoformulations that are based on a platelet-derived PD-1-containing membrane were proposed. These NPs accumulate at the tumor site postresection and boost the activity of CD8+ T cells to inhibit tumor recurrence [176].
Combinational cancer immunotherapy
The major limitation of immunotherapy in antitumor treatment is its limited efficacy when applied to large tumors. In addition to the prioritized surgical removal of the primary tumor, noninvasive trials are occasionally needed when tumor resection is infeasible. In this context, combinational antitumor treatments offer alternatives. Chemotherapy, which is the standard-of-care cancer treatment, has been extensively studied. Various chemotherapeutic agents, such as checkpoint blockades and kinase inhibitors, can modulate immune responses and alter the tumor microenvironment [177, 178]. Others, such as doxorubicin (DOX), mitoxantrone, and oxaliplatin, demonstrate the capacity to directly kill tumor cells and trigger immunogenic cell death and systemic immune activation [179, 180]. Lymph-targeted chemotherapy optimizes the efficiency of therapeutic agent delivery to lymphatics-resident cancers (such as lymphomas and lymphatic metastases) and reduces off-target toxicity, especially for combination therapies that include immunotherapeutic agents, which are dose-limited due to concerns that they can induce systemic autoimmune disease. Improved lymph-node targeting has been realized using a wide range of nanoparticles platforms, such as liposomes [181], inorganic matrices [182], and polymer–drug conjugates [30]. Radiotherapy is another standard antitumor therapy, and over 50% of patients with cancer receive radiation during the course of their disease [183]. Similar to chemotherapy, radiotherapy can also trigger immunogenic cell death (ICD) of tumor cells and, subsequently, release a variety of soluble danger signals, namely, calreticulin, ATP, CXC-chemokine ligand 10 (CXCL10), and high-mobility group box 1 (HMGB1). In contrast to cancer vaccines that rely on a defined set of tumor antigens, ICD-inducing damage-associated molecular patterns can elicit antitumor immune responses against a broad spectrum of tumor antigens that are found on dying tumor cells. Thus, the administration of radiotherapy has been regarded as a key clinical approach for improving the abscopal effect of checkpoint inhibitor-based immunotherapy. Min et al. developed an antigen-capturing nanoplatform for enhancing antigen delivery to APCs after radiotherapy [184]. By utilizing a set of engineered antigen-capturing nanoparticles with various surface properties, the efficacy of anti-programmed cell death 1 (anti-PD 1) treatment demonstrated a significant improvement in the B16F10 melanoma model, and a cure rate as high as 20% was realized. Mechanistic studies elucidated the expansion of CD8+ T cells and increased ratios of both CD4+T/Treg and CD8+T/Treg cells. These synergic therapeutic outcomes can also be realized by the combination of photothermal therapy and immunotherapy. Nanoparticles have been designed to interact with external energy resources such as lasers and to carry immunostimulatory drugs. For example, neutral PEGylated polymeric gold nanorods can be delivered and accumulated in lymph-node-resident tumors through lymphatics in an attempt to enable local photothermal therapy [185]. Gold nanorods demonstrated robust efficacy against lymph-node metastasis when combined with photothermal therapy and immunotherapy through noninvasive techniques, thereby opening an alternative avenue for the treatment of lymph-node-resident tumors. Despite the promising efficacy of the combined therapy of local laser irradiation and immunostimulants, the correlated immunological mechanism, which is crucial for understanding the process of antitumor immunity initiation, remains to be elucidated. In one of our recent studies [186], a 980 nm laser and a novel immunostimulant (N-dihydrogalactochitosan, GC) were applied to investigate the mechanism of laser immunotherapy spatially and temporally. Intravital imaging and immunological assays showed that tumor infiltration increased along with the motility of lymphocytes. Tumor-infiltrating lymphocytes were also shown to have long-term interactions with tumor cells, which was attributed to long-term T-cell immune memory.
Finally, various nanoparticle-based antitumor treatment modalities have been developed. In contrast to surgical resection, which is the primary clinical approach and often results in tumor recurrence due to residual tumors, combinational immunotherapies have demonstrated the ability to ablate tumors and induce humoral and cellular immune responses by triggering the release of tumor antigens and intracellular danger signals. Preclinical and clinical studies can provide a substantial basis for clinical translation.
Conclusions and outlook
Over the past decade, nanoparticle-based approaches for targeting the lymphatic system have made tremendous contributions to efficient tumor treatment. Compared to traditional therapy approaches, tailored nanoformulations show many unique and excellent advantages, such as sustained release, targeted delivery, and improved pharmaceutical properties (e.g., half-life, stability, and solubility) of cargos. The convergence of nanotechnology and tumor immunology, which targets nanoparticles to the lymphatic system, can realize superior immune therapy and reduce systemic and long-term toxicity. By rationally tuning the physicochemical features of NPs such as size, shape, surface charge, and targeting ligand, and by utilization of various administration routes, both approaches have made excellent contributions to LN-targeting, antigen release, APC interaction, and immune response in vivo [15, 20, 187]. Due to the utilization of elaborately designed NPs for targeting the lymphatic system, lymphatic imaging can be used to visualize the lymphatic vessels, track cargoes, and provide holistic information on the lymphatic system, which vitally influences the clinical diagnosis and image-guided approach of surgeons [84]. Moreover, nanovaccines and/or nanotherapeutic agents, in combination with other treatment modalities, such as radiation, laser ablation, and resection, can increase lymphatics-enriched immunomodulation efficiency and further improve antitumor nanotherapeutic agents.
Despite the impressive experimental results that have been generated using nanoparticle-based approaches for antitumor treatments, these approaches have seemingly failed in clinical translation [188]. The heterogeneity of tumors and the limited understanding of lymphatic remodeling during tumor progression hinder the clinical transformation of lymphotropic targeted therapies. Emerging techniques such as lymphoid organoids cultures provide validated models and facilitate the study of the immune response and the prediction of in vivo distribution of therapeutics [189]. Cascade delivery in which the size of the well-designed vehicle is tunable upon the stimulations from the environment, can also be an efficient strategy for enhancing vaccine utility by leveraging the size barriers of the lymphatic structure. Further improvement and innovation of lymphatic-targeted cancer therapy will be associated with the identification and regulation of immune targets and reduced systemic and long-term toxicity. Interdisciplinarity, among materials science, immunology, and oncology and other fields, will create enormous research opportunities for filling knowledge gaps and expediting potential clinical translation.
Furthermore, many studies are being conducted on the lymphatic system, in addition to cerebral tissue. The blood–brain barrier (BBB) restricts therapeutic delivery into disease foci. In addition, breaching the integrity of the BBB to optimize the delivery efficiency subsequently hampers the ability to maintain an isolated environment, which is required by brain tissues for their unique functionalities. This tug-of-war difficulty must be addressed deep in cervical lymph nodes, which link the cervical lymph nodes and cerebral spinal fluid for mass exchange [190]. A new strategy in which nanoparticle-mediated transport through the brain lymphatic vasculature is utilized bypasses the BBB and yields 44-fold higher drug uptake than conventional drug administration [191]. This strategy paves a new avenue for the treatment of various brain diseases.
We expect that various nanoparticle-based strategies, such as immunomodulation via the lymphatic system, reconstitution of the tumor-associated microenvironment, and the combination thereof, will eventually shift the paradigm of tumor treatment and dramatically increase the patient survival in the foreseeable future.
Acknowledgements
This work was supported by the Hainan University Scientific Research Foundation (KYQD(ZR)20078, KYQD(ZR)19107) and the Innovation Fund of WNLO.
Abbreviations
- A.R.
Administration route
- Anti-PD 1
Anti-programmed cell death 1
- Ap
Antigen
- APC
Antigen presenting cell
- BBB
Blood–brain barrier
- CpG
Cytosine-phosphate-guanine
- CT
Computed tomography
- CXCL10
CXC-chemokine ligand 10
- DC
Dendritic cell
- DiR-BOA
1,1′-Dioctadecyl-3,3,3′,3′-tetra-methylindotricarbocyanine iodide bis-oleate
- DOX
Doxorubicin
- ECM
Interstitial extracellular matrix
- FDA
Food and Drug Administration
- FI
Fluorescence imaging
- GC
N-dihydrogalactochitosan
- GDR
Galactosyl-dextran-retinal
- HA
Hyaluronic acid
- HDL
High-density lipoprotein
- HEV
High endothelial venules
- HMGB1
High-mobility groups box 1
- HPPS
High-density lipoprotein-mimicking peptide-phospholipid scaffold
- HSP
Heat-shock protein
- i.d.
Intradermal
- i.p.
Intraperitoneal
- i.v.
Intravenous
- ICB
Immune checkpoint blockade
- ICD
Immunogenic cell death
- ICG
Indocyanine green
- Inf-LN
Inflamed LN
- LEC
Lymphatic endothelial cell
- LSEC
Liver sinusoidal endothelial cell
- LV
Lymphatic vessel
- LYVE-1
Lymphatic vessel endothelial hyaluronic acid receptor 1
- MEB
Maleimide-functionalized Evans blue derivative
- MGL
Galactose C-type lectin
- MPF
Magnetic pull force
- MRI
Magnetic resonance imaging
- M2pep
M2 macrophage binding peptide
- NIR
Near-infrared
- N-LN
Normal LNs
- NP
Nanoparticle
- OVA
Ovalbumin
- PAI
Photoacoustic imaging
- PDI
Perylene diimide
- PDT
Photodynamic therapy
- PEG
Polyethylene glycol
- PEI
Polyethylene imine
- PET
Positron emission tomography
- PLGA
Poly(lactic-co-glycolic acid)
- PNAd
Peripheral node addressin
- PRR
Pathogen recognition receptor
- PTT
Photothermal therapy
- QD
Quantum dot
- RFA
Red fluorescent protein
- R4F
ApoA-1 mimetic peptide
- s.c.
Subcutaneous
- SERS
Surface-enhanced Raman scattering
- SLN
Sentinel lymph node
- SR-B1
Scavenger receptor class B member 1
- SWCNT
Single-walled carbon nanotube
- TAM
Tumor-associated macrophages
- TCL
Tumor cell lysates
- TL
Tumor lymphatic
- T-MLN
Tumor-metastasis LN
- VLP
Virus-like particle
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xingzhou Peng and Junjie Wang have contributed equally to this work.
Contributor Information
Qian Liu, Email: qliu@hainanu.edu.cn.
Zhihong Zhang, Email: czyzzh@hust.edu.cn.
References
- 1.Klevorn LE, Teague RM. Adapting cancer immunotherapy models for the real world. Trends Immunol. 2016;37(6):354–363. doi: 10.1016/j.it.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchbinder EI, Hodi FS. Immune-checkpoint blockade—durable cancer control. Nat Rev Clin Oncol. 2016;13(2):77–78. doi: 10.1038/nrclinonc.2015.237. [DOI] [PubMed] [Google Scholar]
- 4.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5(9):915. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4(1):11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 9.Waldmann TA. Cytokines in cancer immunotherapy. CSH Perspect Biol. 2018;10(12):a028472. doi: 10.1101/cshperspect.a028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girard J-P, Moussion C, Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12(11):762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 11.Xie Y, Bagby TR, Cohen MS, Forrest ML. Drug delivery to the lymphatic system: importance in future cancer diagnosis and therapies. Expert Opin Drug Deliv. 2009;6(8):785–792. doi: 10.1517/17425240903085128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuang J, Holay M, Park JH, Fang RH, Zhang J, Zhang L. Nanoparticle delivery of immunostimulatory agents for cancer immunotherapy. Theranostics. 2019;9(25):7826. doi: 10.7150/thno.37216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huo D, Jiang X, Hu Y. Recent advances in nanostrategies capable of overcoming biological barriers for tumor management. Adv Mater. 2019;32(27):1904337. doi: 10.1002/adma.201904337. [DOI] [PubMed] [Google Scholar]
- 14.Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, Santamaria P. Nanoparticle-based immunotherapy for cancer. ACS Nano. 2015;9(1):16–30. doi: 10.1021/nn5062029. [DOI] [PubMed] [Google Scholar]
- 15.Schudel A, Francis DM, Thomas SN. Material design for lymph node drug delivery. Nat Rev Mater. 2019;4(6):415–428. doi: 10.1038/s41578-019-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo J-W, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10(7):521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 17.Craparo EF, Bondì ML. Application of polymeric nanoparticles in immunotherapy. Curr Opin Allergy Clin Immunol. 2012 doi: 10.1097/ACI.0b013e3283588c57. [DOI] [PubMed] [Google Scholar]
- 18.Torres-Sangiao E, Holban AM, Gestal MC. Advanced nanobiomaterials: vaccines, diagnosis and treatment of infectious diseases. Molecules. 2016 doi: 10.3390/molecules21070867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang RH, Kroll AV, Gao W, Zhang L. Cell membrane coating nanotechnology. Adv Mater. 2018;30(23):1706759. doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ke X, Howard GP, Tang H, Cheng B, Saung MT, Santos JL, Mao H-Q. Physical and chemical profiles of nanoparticles for lymphatic targeting. Adv Drug Del Rev. 2019;151–152:72–93. doi: 10.1016/j.addr.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Karabin NB, Allen S, Kwon H-K, Bobbala S, Firlar E, Shokuhfar T, Shull KR, Scott EA. Sustained micellar delivery via inducible transitions in nanostructure morphology. Nat Commun. 2018;9(1):624. doi: 10.1038/s41467-018-03001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh I, Swami R, Khan W, Sistla R. Lymphatic system: a prospective area for advanced targeting of particulate drug carriers. Expert Opin Drug Deli. 2014;11(2):211–229. doi: 10.1517/17425247.2014.866088. [DOI] [PubMed] [Google Scholar]
- 23.Ryan GM, Kaminskas LM, Porter CJH. Nano-chemotherapeutics: maximising lymphatic drug exposure to improve the treatment of lymph-metastatic cancers. J Control Release. 2014;193:241–256. doi: 10.1016/j.jconrel.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Tian R, Wu J, Fan Q, Yung BC, Niu G, Jacobson O, Wang Z, Liu G, Yu G, Huang W, Song J, Chen X. Impact of semiconducting perylene diimide nanoparticle size on lymph node mapping and cancer imaging. ACS Nano. 2017;11(4):4247–4255. doi: 10.1021/acsnano.7b01261. [DOI] [PubMed] [Google Scholar]
- 25.Stylianopoulos T, Poh M-Z, Insin N, Bawendi MG, Fukumura D, Munn Lance L, Jain RK. Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic interactions. Biophys J. 2010;99(5):1342–1349. doi: 10.1016/j.bpj.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swartz MA. The physiology of the lymphatic system. Adv Drug Del Rev. 2001;50(1):3–20. doi: 10.1016/S0169-409X(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 27.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci of USA. 2006;103(13):4930. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinnear C, Moore TL, Rodriguez-Lorenzo L, Rothen-Rutishauser B, Petri-Fink A. Form follows function: nanoparticle shape and its implications for nanomedicine. Chem Rev. 2017;117(17):11476–11521. doi: 10.1021/acs.chemrev.7b00194. [DOI] [PubMed] [Google Scholar]
- 29.Tseng Y-C, Xu Z, Guley K, Yuan H, Huang L. Lipid–calcium phosphate nanoparticles for delivery to the lymphatic system and SPECT/CT imaging of lymph node metastases. Biomaterials. 2014;35(16):4688–4698. doi: 10.1016/j.biomaterials.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao DA, Forrest ML, Alani AWG, Kwon GS, Robinson JR. Biodegradable PLGA based nanoparticles for sustained regional lymphatic drug delivery. J Pharm Sci. 2010;99(4):2018–2031. doi: 10.1002/jps.21970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10(2):86–103. doi: 10.1379/csc-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim Hwee Y, Thiam Chung H, Yeo Kim P, Bisoendial R, Hii Chung S, McGrath Kristine CY, Tan Kar W, Heather A, Alexander J, Steven J, Angeli V. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab. 2013;17(5):671–684. doi: 10.1016/j.cmet.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, Kubo H, Thurston G, McDonald DM, Achen MG, Stacker SA, Alitalo K. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20(6):1223–1231. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahn HJ, Marks A. A new monoclonal antibody, D2–40, for detection of lymphatic invasion in primary tumors. Lab Invest. 2002;82(9):1255–1257. doi: 10.1097/01.LAB.0000028824.03032.AB. [DOI] [PubMed] [Google Scholar]
- 35.Hamdy S, Haddadi A, Hung RW, Lavasanifar A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv Drug Del Rev. 2011;63(10):943–955. doi: 10.1016/j.addr.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 36.Parayath NN, Parikh A, Amiji MM. Repolarization of tumor-associated macrophages in a genetically engineered nonsmall cell lung cancer model by intraperitoneal administration of hyaluronic acid-based nanoparticles encapsulating microRNA-125b. Nano Lett. 2018;18(6):3571–3579. doi: 10.1021/acs.nanolett.8b00689. [DOI] [PubMed] [Google Scholar]
- 37.Shen L, Krauthäuser S, Fischer K, Hobernik D, Abassi Y, Dzionek A, Nikolaev A, Voltz N, Diken M, Krummen M, Montermann E, Tubbe I, Lorenz S, Strand D, Schild H, Grabbe S, Bros M. Vaccination with trifunctional nanoparticles that address CD8+ dendritic cells inhibits growth of established melanoma. Nanomedicine. 2016;11(20):2647–2662. doi: 10.2217/nnm-2016-0174. [DOI] [PubMed] [Google Scholar]
- 38.Li P, Luo Z, Liu P, Gao N, Zhang Y, Pan H, Liu L, Wang C, Cai L, Ma Y. Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. J Controlled Release. 2013;168(3):271–279. doi: 10.1016/j.jconrel.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 39.Deng G, Sun Z, Li S, Peng X, Li W, Zhou L, Ma Y, Gong P, Cai L. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano. 2018;12(12):12096–12108. doi: 10.1021/acsnano.8b05292. [DOI] [PubMed] [Google Scholar]
- 40.Wei X, Beltrán-Gastélum M, Karshalev E, Esteban-Fernández de Ávila B, Zhou J, Ran D, Angsantikul P, Fang RH, Wang J, Zhang L. Biomimetic micromotor enables active delivery of antigens for oral vaccination. Nano Lett. 2019;19(3):1914–1921. doi: 10.1021/acs.nanolett.8b05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fontana F, Shahbazi M-A, Liu D, Zhang H, Mäkilä E, Salonen J, Hirvonen JT, Santos HA. Multistaged nanovaccines based on porous silicon@acetalated dextran@cancer cell membrane for cancer immunotherapy. Adv Mater. 2017;29(7):1603239. doi: 10.1002/adma.201603239. [DOI] [PubMed] [Google Scholar]
- 42.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14(3):159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 43.Willard-Mack CL. Normal structure, function, and histology of lymph nodes. Toxicol Pathol. 2006;34(5):409–424. doi: 10.1080/01926230600867727. [DOI] [PubMed] [Google Scholar]
- 44.Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92(3):1005–1060. doi: 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- 45.Oussoren C, Zuidema J, Crommelin DJA, Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection: II. Influence of liposomal size, lipid composition and lipid dose. Biochim Biophys Acta. 1997;1328(2):261–272. doi: 10.1016/S0005-2736(97)00122-3. [DOI] [PubMed] [Google Scholar]
- 46.Supersaxo A, Hein WR, Steffen H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm Res. 1990;7(2):167–169. doi: 10.1023/A:1015880819328. [DOI] [PubMed] [Google Scholar]
- 47.McLennan DN, Porter CJH, Edwards GA, Heatherington AC, Martin SW, Charman SA. The absorption of darbepoetin alfa occurs predominantly via the lymphatics following subcutaneous administration to sheep. Pharm Res. 2006;23(9):2060–2066. doi: 10.1007/s11095-006-9064-8. [DOI] [PubMed] [Google Scholar]
- 48.Ryan GM, Kaminskas LM, Porter CJH. Nano-chemotherapeutics: maximising lymphatic drug exposure to improve the treatment of lymph-metastatic cancers. J Controlled Release. 2014;193:241–256. doi: 10.1016/j.jconrel.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 49.Jiang G, Park K, Kim J, Kim KS, Oh EJ, Kang H, Han S-E, Oh Y-K, Park TG, Kwang Hahn S. Hyaluronic acid–polyethyleneimine conjugate for target specific intracellular delivery of siRNA. Biopolymers. 2008;89(7):635–642. doi: 10.1002/bip.20978. [DOI] [PubMed] [Google Scholar]
- 50.Luo G, Yu X, Jin C, Yang F, Fu D, Long J, Xu J, Zhan C, Lu W. LyP-1-conjugated nanoparticles for targeting drug delivery to lymphatic metastatic tumors. Int J Pharm. 2010;385(1):150–156. doi: 10.1016/j.ijpharm.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 51.Yan Z, Wang F, Wen Z, Zhan C, Feng L, Liu Y, Wei X, Xie C, Lu W. LyP-1-conjugated PEGylated liposomes: a carrier system for targeted therapy of lymphatic metastatic tumor. J Controlled Release. 2012;157(1):118–125. doi: 10.1016/j.jconrel.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 52.Card CM, Yu SS, Swartz MA. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J Clin Invest. 2014;124(3):943–952. doi: 10.1172/JCI73316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petrova TV, Makinen T, Alitalo K. Signaling via vascular endothelial growth factor receptors. Exp Cell Res. 1999;253(1):117–130. doi: 10.1006/excr.1999.4707. [DOI] [PubMed] [Google Scholar]
- 54.He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T, Alitalo K. Vascular endothelial cell growth factor receptor 3–mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65(11):4739. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- 55.Trevaskis NL, Kaminskas LM, Porter CJH. From sewer to saviour—targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov. 2015;14(11):781–803. doi: 10.1038/nrd4608. [DOI] [PubMed] [Google Scholar]
- 56.Shi G-N, Zhang C-N, Xu R, Niu J-F, Song H-J, Zhang X-Y, Wang W-W, Wang Y-M, Li C, Wei X-Q, Kong D-L. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials. 2017;113:191–202. doi: 10.1016/j.biomaterials.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 57.Tran T-H, Krishnan S, Amiji MM. MicroRNA-223 induced repolarization of peritoneal macrophages using CD44 targeting hyaluronic acid nanoparticles for anti-inflammatory effects. PLoS ONE. 2016;11(5):e0152024. doi: 10.1371/journal.pone.0152024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beldman TJ, Senders ML, Alaarg A, Pérez-Medina C, Tang J, Zhao Y, Fay F, Deichmöller J, Born B, Desclos E, van der Wel NN, Hoebe RA, Kohen F, Kartvelishvily E, Neeman M, Reiner T, Calcagno C, Fayad ZA, de Winther MPJ, Lutgens E, Mulder WJM, Kluza E. Hyaluronan nanoparticles selectively target plaque-associated macrophages and improve plaque stability in atherosclerosis. ACS Nano. 2017;11(6):5785–5799. doi: 10.1021/acsnano.7b01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang C, Li P, Liu L, Pan H, Li H, Cai L, Ma Y. Self-adjuvanted nanovaccine for cancer immunotherapy: role of lysosomal rupture-induced ROS in MHC class I antigen presentation. Biomaterials. 2016;79:88–100. doi: 10.1016/j.biomaterials.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 60.McClure M, Gopaluni S, Jayne D, Jones R. B cell therapy in ANCA-associated vasculitis: current and emerging treatment options. Nat Rev Rheumatol. 2018;14(10):580–591. doi: 10.1038/s41584-018-0065-x. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez SF, Pitcher LA, Mempel T, Schuerpf F, Carroll MC. B cell acquisition of antigen in vivo. Curr Opin Immunol. 2009;21(3):251–257. doi: 10.1016/j.coi.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol. 2009;10(7):786–793. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27(1):160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 64.Pucci F, Garris C, Lai CP, Newton A, Pfirschke C, Engblom C, Alvarez D, Sprachman M, Evavold C, Magnuson A, von Andrian UH, Glatz K, Breakefield XO, Mempel TR, Weissleder R, Pittet MJ. SCS macrophages suppress melanoma by restricting tumor-derived vesicle–B cell interactions. Science. 2016;352(6282):242. doi: 10.1126/science.aaf1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henri S, Vremec D, Kamath A, Waithman J, Williams S, Benoist C, Burnham K, Saeland S, Handman E, Shortman K. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167(2):741. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 66.Platt CD, Ma JK, Chalouni C, Ebersold M, Bou-Reslan H, Carano RAD, Mellman I, Delamarre L. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc Natl Acad Sci U S A. 2010;107(9):4287. doi: 10.1073/pnas.0910609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qian Y, Jin H, Qiao S, Dai Y, Huang C, Lu L, Luo Q, Zhang Z. Targeting dendritic cells in lymph node with an antigen peptide-based nanovaccine for cancer immunotherapy. Biomaterials. 2016;98:171–183. doi: 10.1016/j.biomaterials.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 68.Arata-Kawai H, Singer MS, Bistrup A, Av Z, Wang Y-Q, Ito Y, Bao X, Hemmerich S, Fukuda M, Rosen SD. Functional contributions of N- and O-glycans to L-selectin ligands in murine and human lymphoid organs. Am J Pathol. 2011;178(1):423–433. doi: 10.1016/j.ajpath.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirakawa J, Tsuboi K, Sato K, Kobayashi M, Watanabe S, Takakura A, Imai Y, Ito Y, Fukuda M, Kawashima H. Novel anti-carbohydrate antibodies reveal the cooperative function of sulfated N- and O-glycans in lymphocyte homing. J Biol Chem. 2010;285(52):40864–40878. doi: 10.1074/jbc.m110.167296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith KA, Tam VL, Wong RM, Pagarigan RR, Meisenburg BL, Joea DK, Liu X, Sanders C, Diamond D, Kündig TM, Qiu Z, Bot A. Enhancing DNA vaccination by sequential injection of lymph nodes with plasmid vectors and peptides. Vaccine. 2009;27(19):2603–2615. doi: 10.1016/j.vaccine.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 71.Ribas A, Weber JS, Chmielowski B, Comin-Anduix B, Lu D, Douek M, Ragavendra N, Raman S, Seja E, Rosario D, Miles S, Diamond DC, Qiu Z, Obrocea M, Bot A. Intra–lymph node prime-boost vaccination against melan A and tyrosinase for the treatment of metastatic melanoma: results of a phase 1 clinical trial. Clin Cancer Res. 2011;17(9):2987. doi: 10.1158/1078-0432.CCR-10-3272. [DOI] [PubMed] [Google Scholar]
- 72.Lavelle EC, O’Hagan DT. Delivery systems and adjuvants for oral vaccines. Expert Opin Drug Deliv. 2006;3(6):747–762. doi: 10.1517/17425247.3.6.747. [DOI] [PubMed] [Google Scholar]
- 73.Thomas SN, Schudel A. Overcoming transport barriers for interstitial-, lymphatic-, and lymph node-targeted drug delivery. Curr Opin Chem Eng. 2015;7:65–74. doi: 10.1016/j.coche.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu L, Quach T, Han S, Lim SF, Yadav P, Senyschyn D, Trevaskis NL, Simpson JS, Porter CJH. Glyceride-mimetic prodrugs incorporating self-immolative spacers promote lymphatic transport, avoid first-pass metabolism, and enhance oral bioavailability. Angew Chem Int. 2016;55(44):13700–13705. doi: 10.1002/anie.201604207. [DOI] [PubMed] [Google Scholar]
- 75.Sabin AB. Oral poliovirus vaccine: history of its development and use and current challenge to eliminate poliomyelitis from the world. J Infect Dis. 1985;151(3):420–436. doi: 10.1093/infdis/151.3.420. [DOI] [PubMed] [Google Scholar]
- 76.Florence AT. Nanoparticle uptake by the oral route: fulfilling its potential? Drug Discov Today Technol. 2005;2(1):75–81. doi: 10.1016/j.ddtec.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 77.Zaric M, Lyubomska O, Touzelet O, Poux C, Al-Zahrani S, Fay F, Wallace L, Terhorst D, Malissen B, Henri S, Power UF, Scott CJ, Donnelly RF, Kissenpfennig A. Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-D, L-lactide-co-glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano. 2013;7(3):2042–2055. doi: 10.1021/nn304235j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bahmani B, Uehara M, Jiang L, Ordikhani F, Banouni N, Ichimura T, Solhjou Z, Furtmüller GJ, Brandacher G, Alvarez D, von Andrian UH, Uchimura K, Xu Q, Vohra I, Yilmam OA, Haik Y, Azzi J, Kasinath V, Bromberg JS, McGrath MM, Abdi R. Targeted delivery of immune therapeutics to lymph nodes prolongs cardiac allograft survival. J Clin Invest. 2018;128(11):4770–4786. doi: 10.1172/JCI120923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Azzi J, Yin Q, Uehara M, Ohori S, Tang L, Cai K, Ichimura T, McGrath M, Maarouf O, Kefaloyianni E, Loughhead S, Petr J, Sun Q, Kwon M, Tullius S, von Andrian UH, Cheng J, Abdi R. Targeted delivery of immunomodulators to lymph nodes. Cell Rep. 2016;15(6):1202–1213. doi: 10.1016/j.celrep.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bouta EM, Bell RD, Rahimi H, Xing L, Wood RW, Bingham CO, Ritchlin CT, Schwarz EM. Targeting lymphatic function as a novel therapeutic intervention for rheumatoid arthritis. Nat Rev Rheumatol. 2018;14(2):94–106. doi: 10.1038/nrrheum.2017.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernier-Latmani J, Petrova TV. Intestinal lymphatic vasculature: structure, mechanisms and functions. Nat Rev Gastroenterol Hepatol. 2017;14(9):510–526. doi: 10.1038/nrgastro.2017.79. [DOI] [PubMed] [Google Scholar]
- 82.Jiang X, Nicolls MR, Tian W, Rockson SG. Lymphatic dysfunction, leukotrienes, and lymphedema. Annu Rev Physiol. 2018;80(1):49–70. doi: 10.1146/annurev-physiol-022516-034008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Proulx ST, Luciani P, Dieterich LC, Karaman S, Leroux J-C, Detmar M. Expansion of the lymphatic vasculature in cancer and inflammation: new opportunities for in vivo imaging and drug delivery. J Controlled Release. 2013;172(2):550–557. doi: 10.1016/j.jconrel.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 84.Jiang H, Wang Q, Li L, Zeng Q, Li H, Gong T, Zhang Z, Sun X. Turning the old adjuvant from gel to nanoparticles to amplify CD8+ T cell responses. Adv Sci. 2018;5(1):1700426. doi: 10.1002/advs.201700426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nune SK, Gunda P, Majeti BK, Thallapally PK, Forrest ML. Advances in lymphatic imaging and drug delivery. Adv Drug Del Rev. 2011;63(10):876–885. doi: 10.1016/j.addr.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu G, Lynn GM, Jacobson O, Chen K, Liu Y, Zhang H, Ma Y, Zhang F, Tian R, Ni Q, Cheng S, Wang Z, Lu N, Yung BC, Wang Z, Lang L, Fu X, Jin A, Weiss ID, Vishwasrao H, Niu G, Shroff H, Klinman DM, Seder RA, Chen X. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat Commun. 2017;8(1):1954. doi: 10.1038/s41467-017-02191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Z, Cao W, Jin H, Lovell JF, Yang M, Ding L, Chen J, Corbin I, Luo Q, Zheng G. Biomimetic nanocarrier for direct cytosolic drug delivery. Angew Chem Int. 2009;48(48):9171–9175. doi: 10.1002/anie.200903112. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Z, Chen J, Ding L, Jin H, Lovell JF, Corbin IR, Cao W, Lo PC, Yang M, Tsao MS, Luo Q, Zheng G. HDL-mimicking peptide-lipid nanoparticles with improved tumor targeting. Small. 2010;6(3):430–437. doi: 10.1002/smll.200901515. [DOI] [PubMed] [Google Scholar]
- 89.Qian Y, Qiao S, Dai Y, Xu G, Dai B, Lu L, Yu X, Luo Q, Zhang Z. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophages. ACS Nano. 2017;11(9):9536–9549. doi: 10.1021/acsnano.7b05465. [DOI] [PubMed] [Google Scholar]
- 90.Xu G, Qian Y, Zheng H, Qiao S, Yan D, Lu L, Wu L, Yang X, Luo Q, Zhang Z. Long-distance tracing of the lymphatic system with a computed tomography/fluorescence dual-modality nanoprobe for surveying tumor lymphatic metastasis. Bioconj Chem. 2019;30(4):1199–1209. doi: 10.1021/acs.bioconjchem.9b00144. [DOI] [PubMed] [Google Scholar]
- 91.Zhou Q, Zhang Y, Du J, Li Y, Zhou Y, Fu Q, Zhang J, Wang X, Zhan L. Different-sized gold nanoparticle activator/antigen increases dendritic cells accumulation in liver-draining lymph nodes and CD8+ T cell responses. ACS Nano. 2016;10(2):2678–2692. doi: 10.1021/acsnano.5b07716. [DOI] [PubMed] [Google Scholar]
- 92.Lee I-H, Kwon H-K, An S, Kim D, Kim S, Yu MK, Lee J-H, Lee T-S, Im S-H, Jon S. Imageable antigen-presenting gold nanoparticle vaccines for effective cancer immunotherapy in vivo. Angew Chem Int. 2012;51(35):8800–8805. doi: 10.1002/anie.201203193. [DOI] [PubMed] [Google Scholar]
- 93.Paredes P, Vidal-Sicart S, Campos F, Tapias A, Sánchez N, Martínez S, Carballo L, Pahisa J, Torné A, Ordi J, Carmona F, Lomeña F. Role of ICG-99mTc-nanocolloid for sentinel lymph node detection in cervical cancer: a pilot study. Eur J Nucl Med Mol Imag. 2017;44(11):1853–1861. doi: 10.1007/s00259-017-3706-4. [DOI] [PubMed] [Google Scholar]
- 94.Ankersmit M, Hoekstra OS, van Lingen A, Bloemena E, Jacobs MAJM, Vugts DJ, Bonjer HJ, van Dongen GAMS, Meijerink WJHJ. Perioperative PET/CT lymphoscintigraphy and fluorescent real-time imaging for sentinel lymph node mapping in early staged colon cancer. Eur J Nucl Med Mol Imag. 2019;46(7):1495–1505. doi: 10.1007/s00259-019-04284-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liang C, Diao S, Wang C, Gong H, Liu T, Hong G, Shi X, Dai H, Liu Z. Tumor metastasis inhibition by imaging-guided photothermal therapy with single-walled carbon nanotubes. Adv Mater. 2014;26(32):5646–5652. doi: 10.1002/adma.201401825. [DOI] [PubMed] [Google Scholar]
- 96.Pu K, Shuhendler AJ, Jokerst JV, Mei J, Gambhir SS, Bao Z, Rao J. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat Nanotechnol. 2014;9(3):233–239. doi: 10.1038/nnano.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Y, Bhattarai P, Dai Z, Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem Soc Rev. 2019;48(7):2053–2108. doi: 10.1039/C8CS00618K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheng Y, Cheng H, Jiang C, Qiu X, Wang K, Huan W, Yuan A, Wu J, Hu Y. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat Commun. 2015;6(1):8785. doi: 10.1038/ncomms9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hannah A, Luke G, Wilson K, Homan K, Emelianov S. Indocyanine green-loaded photoacoustic nanodroplets: dual contrast nanoconstructs for enhanced photoacoustic and ultrasound imaging. ACS Nano. 2014;8(1):250–259. doi: 10.1021/nn403527r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dai Y, Yu X, Wei J, Zeng F, Li Y, Yang X, Luo Q, Zhang Z. Metastatic status of sentinel lymph nodes in breast cancer determined with photoacoustic microscopy via dual-targeting nanoparticles. Light Sci Appl. 2020;9(1):164. doi: 10.1038/s41377-020-00399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu H, Cao X. Dendritic cell vaccines in cancer immunotherapy: from biology to translational medicine. Front Med. 2011;5(4):323–332. doi: 10.1007/s11684-011-0172-4. [DOI] [PubMed] [Google Scholar]
- 102.Cho N-H, Cheong T-C, Min JH, Wu JH, Lee SJ, Kim D, Yang J-S, Kim S, Kim YK, Seong S-Y. A multifunctional core–shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat Nanotechnol. 2011;6(10):675–682. doi: 10.1038/nnano.2011.149. [DOI] [PubMed] [Google Scholar]
- 103.Noh Y-W, Lim YT, Chung BH. Noninvasive imaging of dendritic cell migration into lymph nodes using near-infrared fluorescent semiconductor nanocrystals. FASEB J. 2008;22(11):3908–3918. doi: 10.1096/fj.08-112896. [DOI] [PubMed] [Google Scholar]
- 104.Lim YT, Noh Y-W, Han JH, Cai Q-Y, Yoon K-H, Chung BH. Biocompatible polymer-nanoparticle-based bimodal imaging contrast agents for the labeling and tracking of dendritic cells. Small. 2008;4(10):1640–1645. doi: 10.1002/smll.200800582. [DOI] [PubMed] [Google Scholar]
- 105.Noh Y-W, Jang Y-S, Ahn K-J, Lim YT, Chung BH. Simultaneous in vivo tracking of dendritic cells and priming of an antigen-specific immune response. Biomaterials. 2011;32(26):6254–6263. doi: 10.1016/j.biomaterials.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 106.Zhang C, Xu Z, Di H, Zeng E, Jiang Y, Liu D. Gadolinium-doped Au@prussian blue nanoparticles as MR/SERS bimodal agents for dendritic cell activating and tracking. Theranostics. 2020;10(13):6061–6071. doi: 10.7150/thno.42114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jin H, Qian Y, Dai Y, Qiao S, Huang C, Lu L, Luo Q, Chen J, Zhang Z. Magnetic enrichment of dendritic cell vaccine in lymph node with fluorescent-magnetic nanoparticles enhanced cancer immunotherapy. Theranostics. 2016;6(11):2000–2014. doi: 10.7150/thno.15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene. 2012;31(42):4499–4508. doi: 10.1038/onc.2011.602. [DOI] [PubMed] [Google Scholar]
- 109.Brown M, Assen FP, Leithner A, Abe J, Schachner H, Asfour G, Bago-Horvath Z, Stein JV, Uhrin P, Sixt M, Kerjaschki D. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science. 2018;359(6382):1408. doi: 10.1126/science.aal3662. [DOI] [PubMed] [Google Scholar]
- 110.Naxerova K, Reiter JG, Brachtel E, Lennerz JK, van de Wetering M, Rowan A, Cai T, Clevers H, Swanton C, Nowak MA, Elledge SJ, Jain RK. Origins of lymphatic and distant metastases in human colorectal cancer. Science. 2017;357(6346):55. doi: 10.1126/science.aai8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Buda A, Passoni P, Corrado G, Bussi B, Cutillo G, Magni S, Vizza E. Near-infrared fluorescence-guided sentinel node mapping of the ovary with indocyanine green in a minimally invasive setting: a feasible study. J Minim Invasive Gynecol. 2017;24(1):165–170. doi: 10.1016/j.jmig.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 112.Tammela T, Saaristo A, Holopainen T, Ylä-Herttuala S, Andersson LC, Virolainen S, Immonen I, Alitalo K. Photodynamic ablation of lymphatic vessels and intralymphatic cancer cells prevents metastasis. Sci Transl Med. 2011;3(69):69ra11. doi: 10.1126/scitranslmed.3001699. [DOI] [PubMed] [Google Scholar]
- 113.Shi H, Yan R, Wu L, Sun Y, Liu S, Zhou Z, He J, Ye D. Tumor-targeting CuS nanoparticles for multimodal imaging and guided photothermal therapy of lymph node metastasis. Acta Biomater. 2018;72:256–265. doi: 10.1016/j.actbio.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 114.Yang C-T, Ghosh KK, Padmanabhan P, Langer O, Liu J, Eng DNC, Halldin C, Gulyás B. PET-MR and SPECT-MR multimodality probes: development and challenges. Theranostics. 2018;8(22):6210–6232. doi: 10.7150/thno.26610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang C, Fan W, Zhang Z, Wen Y, Xiong L, Chen X. Advanced nanotechnology leading the way to multimodal imaging-guided precision surgical therapy. Adv Mater. 2019;31(49):1904329. doi: 10.1002/adma.201904329. [DOI] [PubMed] [Google Scholar]
- 116.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25(10):1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 117.Chen Daniel S, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 118.Ma L, Dichwalkar T, Chang JYH, Cossette B, Garafola D, Zhang AQ, Fichter M, Wang C, Liang S, Silva M, Kumari S, Mehta NK, Abraham W, Thai N, Li N, Wittrup KD, Irvine DJ. Enhanced CAR–T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science. 2019;365(6449):162. doi: 10.1126/science.aav8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.June CH, Sadelain M. Chimeric antigen receptor therapy. New Engl J Med. 2018;379(1):64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Torres Andón F, Alonso MJ. Nanomedicine and cancer immunotherapy—targeting immunosuppressive cells. J Drug Target. 2015;23(7–8):656–671. doi: 10.3109/1061186X.2015.1073295. [DOI] [PubMed] [Google Scholar]
- 121.Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol. 2003;3(12):973–983. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- 122.Duan X, Chan C, Guo N, Han W, Weichselbaum RR, Lin W. Photodynamic therapy mediated by nontoxic core–shell nanoparticles synergizes with immune checkpoint blockade to elicit antitumor immunity and antimetastatic effect on breast cancer. J Am Chem Soc. 2016;138(51):16686–16695. doi: 10.1021/jacs.6b09538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lu K, He C, Guo N, Chan C, Ni K, Weichselbaum RR, Lin W. Chlorin-based nanoscale metal–organic framework systemically rejects olorectal cancers via synergistic photodynamic therapy and checkpoint blockade immunotherapy. J Am Chem Soc. 2016;138(38):12502–12510. doi: 10.1021/jacs.6b06663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ngiow Shin F, McArthur Grant A, Smyth Mark J. Radiotherapy complements immune checkpoint blockade. Cancer Cell. 2015;27(4):437–438. doi: 10.1016/j.ccell.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 125.Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts IM, Trebska-McGowan K, Wunderlich JR, Yang JC, Rosenberg SA. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016;22(4):433–438. doi: 10.1038/nm.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chao Y, Chen Q, Liu Z. Smart injectable hydrogels for cancer immunotherapy. Adv Func Mater. 2020;30(2):1902785. doi: 10.1002/adfm.201902785. [DOI] [Google Scholar]
- 127.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3(11):867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 128.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4(12):941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 129.Baitsch L, Baumgaertner P, Devêvre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, Rufer N, Speiser DE. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lu J-C, Zeng H-Y, Sun Q-M, Meng Q-N, Huang X-Y, Zhang P-F, Yang X, Peng R, Gao C, Wei C-Y, Shen Y-H, Cai J-B, Dong R-Z, Shi Y-H, Sun H-C, Shi YG, Zhou J, Fan J, Ke A-W, Yang L-X, Shi G-M. Distinct PD-L1/PD1 profiles and clinical implications in intrahepatic cholangiocarcinoma patients with different risk factors. Theranostics. 2019;9(16):4678–4687. doi: 10.7150/thno.36276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Davoodzadeh Gholami M, Kardar GA, Saeedi Y, Heydari S, Garssen J, Falak R. Exhaustion of T lymphocytes in the tumor microenvironment: significance and effective mechanisms. Cell Immunol. 2017;322:1–14. doi: 10.1016/j.cellimm.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 132.Longo DL. New therapies for castration-resistant prostate cancer. New Engl J Med. 2010;363(5):479–481. doi: 10.1056/NEJMe1006300. [DOI] [PubMed] [Google Scholar]
- 133.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17(11):3520. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 134.Aarntzen EHJG, Srinivas M, Schreibelt G, Heerschap A, Punt CJA, Figdor CG, Oyen WJ, de Vries IJM. Reducing cell number improves the homing of dendritic cells to lymph nodes upon intradermal vaccination. OncoImmunology. 2013;2(7):e24661. doi: 10.4161/onci.24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kugler A, Stuhler G, Walden P, Zöller G, Zobywalski A, Brossart P, Trefzer U, Ullrich S, Müller CA, Becker V, Gross AJ, Hemmerlein B, Kanz L, Müller GA, Ringert R-H. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell–dendritic cell hybrids. Nat Med. 2000;6(3):332–336. doi: 10.1038/73193. [DOI] [PubMed] [Google Scholar]
- 136.Panelli MC, Wunderlich J, Jeffries J, Wang E, Mixon A, Rosenberg SA, Marincola FM. Phase 1 study in patients with metastatic melanoma of immunization with dendritic cells presenting epitopes derived from the melanoma-associated antigens MART-1 and gp100. J Immunother. 2000 doi: 10.1097/00002371-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 137.Srinivas M, Aarntzen EHJG, Bulte JWM, Oyen WJ, Heerschap A, de Vries IJM, Figdor CG. Imaging of cellular therapies. Adv Drug Del Rev. 2010;62(11):1080–1093. doi: 10.1016/j.addr.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 138.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196(12):1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Klechevsky E, Flamar A-L, Cao Y, Blanck J-P, Liu M, O'Bar A, Agouna-Deciat O, Klucar P, Thompson-Snipes L, Zurawski S, Reiter Y, Palucka AK, Zurawski G, Banchereau J. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010;116(10):1685–1697. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dakappagari N, Maruyama T, Renshaw M, Tacken P, Figdor C, Torensma R, Wild MA, Wu D, Bowdish K, Kretz-Rommel A. Internalizing antibodies to the C-type lectins, L-SIGN and DC-SIGN, inhibit viral glycoprotein binding and deliver antigen to human dendritic cells for the induction of T cell responses. J Immunol. 2006;176(1):426. doi: 10.4049/jimmunol.176.1.426. [DOI] [PubMed] [Google Scholar]
- 141.Ni L, Gayet I, Zurawski S, Duluc D, Flamar A-L, Li X-H, O’Bar A, Clayton S, Palucka AK, Zurawski G, Banchereau J, Oh S. Concomitant activation and antigen uptake via human dectin-1 results in potent antigen-specific CD8+ T cell responses. J Immunol. 2010;185(6):3504. doi: 10.4049/jimmunol.1000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sancho D, Mourão-Sá D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, Carlyle JR, Reis e Sousa C. Tumor therapy in mice via antigen targeting to a novel. DC-restricted C-type lectin J Clin Invest. 2008;118(6):2098–2110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Flacher V, Sparber F, Tripp CH, Romani N, Stoitzner P. Targeting of epidermal Langerhans cells with antigenic proteins: attempts to harness their properties for immunotherapy. Cancer Immunol, Immunother. 2009;58(7):1137–1147. doi: 10.1007/s00262-008-0563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Verbeke CS, Mooney DJ. Injectable, pore-forming hydrogels for In vivo enrichment of immature dendritic cells. Adv Healthcare Mater. 2015;4(17):2677–2687. doi: 10.1002/adhm.201500618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wahlund CJE, Güclüler G, Hiltbrunner S, Veerman RE, Näslund TI, Gabrielsson S. Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci Rep. 2017;7(1):17095. doi: 10.1038/s41598-017-16609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.De Temmerman M-L, Rejman J, Demeester J, Irvine DJ, Gander B, De Smedt SC. Particulate vaccines: on the quest for optimal delivery and immune response. Drug Discov Today. 2011;16(13):569–582. doi: 10.1016/j.drudis.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 147.Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, Oliveira G, Giobbie-Hurder A, Felt K, Gjini E, Shukla SA, Hu Z, Li L, Le PM, Allesøe RL, Richman AR, Kowalczyk MS, Abdelrahman S, Geduldig JE, Charbonneau S, Pelton K, Iorgulescu JB, Elagina L, Zhang W, Olive O, McCluskey C, Olsen LR, Stevens J, Lane WJ, Salazar AM, Daley H, Wen PY, Chiocca EA, Harden M, Lennon NJ, Gabriel S, Getz G, Lander ES, Regev A, Ritz J, Neuberg D, Rodig SJ, Ligon KL, Suvà ML, Wucherpfennig KW, Hacohen N, Fritsch EF, Livak KJ, Ott PA, Wu CJ, Reardon DA. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 149.Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359(6382):1355. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- 150.Kuai R, Yuan W, Son S, Nam J, Xu Y, Fan Y, Schwendeman A, Moon JJ. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci Adv. 2018;4(4):eaao1736. doi: 10.1126/sciadv.aao1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pol J, Kroemer G, Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. OncoImmunology. 2016;5(1):e1115641. doi: 10.1080/2162402X.2015.1115641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu Z, Ravindranathan R, Kalinski P, Guo ZS, Bartlett DL. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat Commun. 2017;8(1):14754. doi: 10.1038/ncomms14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, Kirkwood JM, Gajewski TF, Chen L, Gorski KS, Anderson AA, Diede SJ, Lassman ME, Gansert J, Hodi FS, Long GV. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109–1119. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yu X, Dai Y, Zhao Y, Qi S, Liu L, Lu L, Luo Q, Zhang Z. Melittin-lipid nanoparticles target to lymph nodes and elicit a systemic anti-tumor immune response. Nat Commun. 2020;11(1):1–14. doi: 10.1038/s41467-020-14906-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(S1):S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 156.Bertolino P, Bowen DG, McCaughan GW, de St F, Groth B. Antigen-specific primary activation of CD8+T cells within the liver. J Immunol. 2001;166(9):5430. doi: 10.4049/jimmunol.166.9.5430. [DOI] [PubMed] [Google Scholar]
- 157.Goodwin TJ, Zhou Y, Musetti SN, Liu R, Huang L. Local and transient gene expression primes the liver to resist cancer metastasis. Sci Transl Med. 2016;8(364):364ra153. doi: 10.1126/scitranslmed.aag2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Burdelya LG, Brackett CM, Kojouharov B, Gitlin II, Leonova KI, Gleiberman AS, Aygun-Sunar S, Veith J, Johnson C, Haderski GJ, Stanhope-Baker P, Allamaneni S, Skitzki J, Zeng M, Martsen E, Medvedev A, Scheblyakov D, Artemicheva NM, Logunov DY, Gintsburg AL, Naroditsky BS, Makarov SS, Gudkov AV. Central role of liver in anticancer and radioprotective activities of Toll-like receptor 5 agonist. Pro Natl Acad Sci USA. 2013;110(20):E1857–1866. doi: 10.1073/pnas.1222805110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dubensky TW, Reed SG. Adjuvants for cancer vaccines. Semin Immunol. 2010;22(3):155–161. doi: 10.1016/j.smim.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 160.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 161.Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev. 2011;239(1):178–196. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2017;16(4):489–496. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Molino NM, Neek M, Tucker JA, Nelson EL, Wang S-W. Viral-mimicking protein nanoparticle vaccine for eliciting anti-tumor responses. Biomaterials. 2016;86:83–91. doi: 10.1016/j.biomaterials.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Akhter A, Hayashi Y, Sakurai Y, Ohga N, Hida K, Harashima H. A liposomal delivery system that targets liver endothelial cells based on a new peptide motif present in the ApoB-100 sequence. Int J Pharm. 2013;456(1):195–201. doi: 10.1016/j.ijpharm.2013.07.068. [DOI] [PubMed] [Google Scholar]
- 165.Yu X, Chen L, Liu J, Dai B, Xu G, Shen G, Luo Q, Zhang Z. Immune modulation of liver sinusoidal endothelial cells by melittin nanoparticles suppresses liver metastasis. Nat Commun. 2019;10(1):574. doi: 10.1038/s41467-019-08538-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers. 2011;3(4):3856–3893. doi: 10.3390/cancers3043856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shimizu T, Kishida T, Hasegawa U, Ueda Y, Imanishi J, Yamagishi H, Akiyoshi K, Otsuji E, Mazda O. Nanogel DDS enables sustained release of IL-12 for tumor immunotherapy. Biochem Biophys Res Commun. 2008;367(2):330–335. doi: 10.1016/j.bbrc.2007.12.112. [DOI] [PubMed] [Google Scholar]
- 168.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Licona Limon P, Ferrandino AF, Gonzalez D, Habermann A, Flavell RA, Fahmy TM. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11(10):895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Aranda F, Vacchelli E, Eggermont A, Galon J, Fridman WH, Zitvogel L, Kroemer G, Galluzzi L. Trial watch. OncoImmunology. 2014;3(2):e27297. doi: 10.4161/onci.27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19(5):1035. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gu L, Ruff LE, Qin Z, Corr M, Hedrick SM, Sailor MJ. Multivalent porous silicon nanoparticles enhance the immune activation potency of agonistic CD40 antibody. Adv Mater. 2012;24(29):3981–3987. doi: 10.1002/adma.201200776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A. Pembrolizumab versus Ipilimumab in advanced melanoma. New Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 173.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 174.Zou W, Wolchok JD, Chen L. PD-L1 (B7–H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016;16(4):2334–2340. doi: 10.1021/acs.nanolett.5b05030. [DOI] [PubMed] [Google Scholar]
- 176.Wang C, Sun W, Ye Y, Hu Q, Bomba HN, Gu Z. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat Biomed Eng. 2017;1(2):0011. doi: 10.1038/s41551-016-0011. [DOI] [Google Scholar]
- 177.Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, Molina J, Kim J-H, Arvis CD, Ahn M-J, Majem M, Fidler MJ, de Castro G, Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. The Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 178.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SYS, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. New Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 179.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31(1):51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 180.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 181.Wang C, Liu P, Zhuang Y, Li P, Jiang B, Pan H, Liu L, Cai L, Ma Y. Lymphatic-targeted cationic liposomes: a robust vaccine adjuvant for promoting long-term immunological memory. Vaccine. 2014;32(42):5475–5483. doi: 10.1016/j.vaccine.2014.07.081. [DOI] [PubMed] [Google Scholar]
- 182.An M, Li M, Xi J, Liu H. Silica nanoparticle as a lymph node targeting platform for vaccine delivery. ACS Appl Mater Inter. 2017;9(28):23466–23475. doi: 10.1021/acsami.7b06024. [DOI] [PubMed] [Google Scholar]
- 183.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11(4):239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 184.Min Y, Roche KC, Tian S, Eblan MJ, McKinnon KP, Caster JM, Chai S, Herring LE, Zhang L, Zhang T, DeSimone JM, Tepper JE, Vincent BG, Serody JS, Wang AZ. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017;12(9):877–882. doi: 10.1038/nnano.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Oladipo AO, Oluwafemi OS, Songca SP, Sukhbaatar A, Mori S, Okajima J, Komiya A, Maruyama S, Kodama T. A novel treatment for metastatic lymph nodes using lymphatic delivery and photothermal therapy. Sci Rep. 2017;7(1):45459. doi: 10.1038/srep45459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Qi S, Lu L, Zhou F, Chen Y, Xu M, Chen L, Yu X, Chen WR, Zhang Z. Neutrophil infiltration and whole-cell vaccine elicited by N-dihydrogalactochitosan combined with NIR phototherapy to enhance antitumor immune response and T cell immune memory. Theranostics. 2020;10(4):1814–1832. doi: 10.7150/thno.38515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chem Rev. 2015;115(19):11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yan L, Zhao F, Wang J, Zu Y, Gu Z, Zhao Y. A safe-by-design strategy towards safer nanomaterials in nanomedicines. Adv Mater. 2019;31(45):1805391. doi: 10.1002/adma.201805391. [DOI] [PubMed] [Google Scholar]
- 189.Yuki K, Cheng N, Nakano M, Kuo CJ. Organoid models of tumor immunology. Trends Immunol. 2020;41(8):652–664. doi: 10.1016/j.it.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Da Mesquita S, Fu Z, Kipnis J. The meningeal lymphatic system: a new player in neurophysiology. Neuron. 2018;100(2):375–388. doi: 10.1016/j.neuron.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhao P, Le Z, Liu L, Chen Y. Therapeutic delivery to the brain via the lymphatic vasculature. Nano Lett. 2020;20(7):5415–5420. doi: 10.1021/acs.nanolett.0c01806. [DOI] [PubMed] [Google Scholar]