Abstract
Background
Alzheimer’s disease (AD) is a progressive, chronic, and neurodegenerative disease, and the most common cause of dementia worldwide. Currently, the mechanisms underlying the disease are far from being elucidated. Thus, the study of proteins involved in its pathogenesis would allow getting further insights into the disease and identifying new markers for AD diagnosis.
Methods
We aimed here to analyze protein dysregulation in AD brain by quantitative proteomics to identify novel proteins associated with the disease. 10-plex TMT (tandem mass tags)-based quantitative proteomics experiments were performed using frozen tissue samples from the left prefrontal cortex of AD patients and healthy individuals and vascular dementia (VD) and frontotemporal dementia (FTD) patients as controls (CT). LC–MS/MS analyses were performed using a Q Exactive mass spectrometer.
Results
In total, 3281 proteins were identified and quantified using MaxQuant. Among them, after statistical analysis with Perseus (p value < 0.05), 16 and 155 proteins were defined as upregulated and downregulated, respectively, in AD compared to CT (Healthy, FTD and VD) with an expression ratio ≥ 1.5 (upregulated) or ≤ 0.67 (downregulated). After bioinformatics analysis, ten dysregulated proteins were selected as more prone to be associated with AD, and their dysregulation in the disease was verified by qPCR, WB, immunohistochemistry (IHC), immunofluorescence (IF), pull-down, and/or ELISA, using tissue and plasma samples of AD patients, patients with other dementias, and healthy individuals.
Conclusions
We identified and validated novel AD-associated proteins in brain tissue that should be of further interest for the study of the disease. Remarkably, PMP2 and SCRN3 were found to bind to amyloid-β (Aβ) fibers in vitro, and PMP2 to associate with Aβ plaques by IF, whereas HECTD1 and SLC12A5 were identified as new potential blood-based biomarkers of the disease.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-023-04791-y.
Keywords: Alzheimer’s disease, Quantitative proteomics, 10-plex TMT, Neurodegeneration, Biomarkers, Amyloid-β interactors, Liquid biopsy
Background
Alzheimer’s disease (AD) is the most common cause of dementia worldwide. It is a chronic and neurodegenerative disease that affects wide areas of the cerebral cortex and hippocampus [1, 2]. The histopathological changes associated with AD progression begin more than 10 years before noticeable symptoms arise, at the preclinical and prodromal stages [3]. Furthermore, most AD patients live between 10–20 years after diagnosis in a state of disability and dependence, which, together with the high prevalence of AD in aging population (older than 65 years old), contributes to increase its socioeconomic impact [3]. Although new technologies for the specific detection of AD in living patients and at early stages are becoming available [4–7], its definite diagnosis still requires post-mortem verification. In addition, most AD patients are currently clinically diagnosed when symptoms appear and treatments are less effective [8].
The main histological hallmarks of AD are extracellular deposits of amyloid plaques, caused by the accumulation of insoluble forms of amyloid-β (Aβ) peptide and intracellular accumulations of neurofibrillary tangles (NFTs) formed by aggregates of hyperphosphorylated Tau protein [9–12]. AD pathology produces synaptic loss, selective neuronal death, a decrease in specific neurotransmitters, neuroinflammation, glial activation, and extracellular deposits of Aβ peptides [8, 13, 14]. However, the molecular mechanisms underlying these neuropathological changes during AD progression are still unclear [15]. Therefore, the identification of novel proteins involved in its pathogenesis would allow getting further insights into the disease and potentially identifying useful markers for its diagnosis at early stages and as potential targets of intervention.
AD progression has been defined according to NFTs expansion into six Braak stages. At Braak stages I and II, NFTs are limited to the transentorhinal region and correspond to the clinically silent phase of the disease. At Braak stages III and IV, NFTs expand to the limbic region (incipient AD or mild cognitive impairment—MCI) and the first clinical symptoms of AD come out, and, finally, at Braak stages V and VI, NFTs reach neocortical regions and the worst clinical symptoms of AD appear. As the other histopathological changes occur together with NFTs expansion, the study of brain tissue samples from AD patients at different Braak stages, which encompasses both neurons and surrounded cellular components, is mandatory for a better understanding of the biology of AD. In this sense, the comparison of multiple samples in a single experiment by quantitative proteomics is posited as an interesting tool for the study of molecular pathways, networks, and changes associated with chronic diseases [16–23].
We aimed here to analyze and further validate protein dysregulation in AD to get a better understanding of the molecular and functional pathways altered in the disease. We focused the analysis on the left prefrontal cortex, a brain region that plays an important role in integrating cognitive and affective behavior, and in regulating autonomic and neuroendocrine functions [24], which is associated with neural reserve in patients with higher education [25, 26]. The left prefrontal cortex is vulnerable to neurodegeneration with healthy aging and AD, being a brain region affected late in AD by Tau pathology [27, 28]. To this end, 10-plex TMT (tandem mass tag)-based quantitative proteomics and frozen brain tissue samples from AD patients at Braak stages IV–VI and healthy individuals and vascular (VD) and frontotemporal (FTD) dementia patients as controls were used. The prefrontal cortex of VD and FTD patients is affected in both pathologies, with these dementias either being the most common after AD or associated with young-onset dementia, respectively [29–35]. Moreover, abnormal protein deposits associated with AD coexist with blood vessel alterations linked with VD [31, 36, 37]. In contrast, FTD is one of the most common dementias associated with NFTs, characteristic of AD patients [29, 37]. Thus, their use as controls would potentially allow to identify AD-associated proteins non-related to VD alterations, and other NFTs associated dementias (FTD), and to get a better understanding of the pathological mechanisms specifically associated with the development and progression of AD. In total, 3281 proteins were identified and quantified using a Q Exactive mass spectrometer. Among them, 171 proteins were defined as dysregulated in the left prefrontal cortex throughout AD progression in comparison to controls (AD/CT ratio ≥ 1.5 or ≤ 0.67, and p value < 0.05). After bioinformatics analysis, we selected ten dysregulated proteins in AD to analyze their role in the pathogenesis of the disease and as potential markers of the disease by real-time quantitative PCR (qPCR), western blot (WB), immunohistochemistry (IHC), immunofluorescence (IF), pull-down, and/or ELISA, using a cell model of AD and tissue and plasma samples from AD patients and from VD and FTD patients and healthy individuals as controls.
Methods
Tissue samples and cells
Tissue samples with indicated pathological conditions were obtained from the CIEN Foundation’s Tissue Bank (BT-CIEN). AD pathology was confirmed post-mortem in all patients [38]. Blood used in the study was collected prior to histopathological assessment when AD patients were in moderate or advanced clinical AD stage. According to the brain bank’s protocols, neuropathological diagnosis and classification of cases was performed on the basis of international consensus criteria [39–41]. Briefly, in all cases, post-mortem examination was limited to the cranial cavity, according to the brain bank protocol [38]. After fixation, a full neuropathological study was performed in the left half brain by obtaining 25 tissue blocks from cortical and subcortical brain regions [42]. Neuropathological classification of cases was based on the examination of hematoxylin−eosin stained paraffin sections of all blocks and immunostaining with a panel of antibodies (Aβ, tau AT100, α-synuclein, ubiquitin, and TDP-43) and myelin stain. Consensus criteria were used for disease diagnosis and Braak, Thal, and CERAD staging of AD patients and controls (Supplementary Table S1). For comparison purposes and stratification of AD patients, Braak stages were used. Alzheimer’s histopathological changes were assessed following NIAAA guidelines [40]. The BT-CIEN develops a brain donation program based on standard operating procedures (SOPs), meeting the ethical and legal requirements established by current legislation regarding the protection of personal data procedures and with regard to the use of samples of human origin for biomedical research. Written informed consent was obtained from all patients. The Institutional Ethical Review Board of the Spanish Research Center for Neurological Diseases Foundation (CIEN) and the Instituto de Salud Carlos III approved this study on proteomics analysis and biomarker discovery of Alzheimer’s disease (CEI PI 49).
For mass spectrometry analysis, a total of 21 frozen tissue samples from the left prefrontal cortex of AD patients (n = 12), healthy individuals (n = 2), and VD (n = 2) and FTD (n = 5) patients were used (Table 1, Supplementary Table S1).
Table 1.
Information of the tissue and plasma samples from AD patients and controls used in the study
| Analysis | Tissue samples | Number (n) | Age average ± SD (years) | Age range (years) | Gender (n) | Stage (n) | |||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | IV | V | VI | |||||
| TMT (LC–MS/MS) | Healthy individuals | 2 | 78.0 ± 28.3 | 58–98 | 0 | 2 | – | – | – |
| VD/FTD patients | 7 | 75.6 ± 14.2 | 55–93 | 3 | 4 | – | – | – | |
| AD patients | 12 | 80.1 ± 4.4 | 77–92 | 6 | 6 | 4 | 4 | 4 | |
| Western blot (WB) | Healthy individuals | 3 | 59.3 ± 15.6 | 43–74 | 1 | 2 | – | – | – |
| VD/FTD patients | 2 | 79.5 ± 19.1 | 66–93 | 0 | 2 | – | – | – | |
| AD patients | 9 | 82.2 ± 8.1 | 68–92 | 4 | 5 | 3 | 3 | 3 | |
| qPCR | Healthy individuals | 4 | 72.8 ± 18.2 | 61–98 | 0 | 4 | – | – | – |
| VD/FTD patients | 4 | 83.8 ± 12.1 | 66–93 | 1 | 3 | – | – | – | |
| AD patients | 9 | 82.2 ± 8.1 | 68–92 | 4 | 5 | 3 | 3 | 3 | |
| Immunofluorescence (IF) | Healthy individuals | 1 | 58 | 58 | 0 | 1 | – | – | – |
| AD patients | 1 | 82 | 82 | 0 | 1 | – | 1 | – | |
| Pull-down | Healthy individuals | 6 | 69.5 ± 19.6 | 58–98 | 1 | 5 | – | – | – |
| AD patients | 12 | 80.8 ± 7.3 | 71–88 | 6 | 6 | – | 6 | 6 | |
| Analysis | Plasma samples | Number (n) | Age average ± SD (years) | Age range (years) | Gender (n) | Stage (n) | |||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | IV | V | VI | |||||
| ELISA | Healthy individuals | 33 | 75.3 ± 8.7 | 51–90 | 13 | 19 | – | – | – |
| AD patients | 47 | 85.7 ± 7.1 | 62–101 | 10 | 37 | 7 | 29 | 11 | |
For WB and qPCR analysis, we used frozen left prefrontal cortex tissue samples of AD patients at Braak IV–VI (n = 9) and healthy individuals (n = 3 for WB and n = 4 for qPCR) and VD (n = 1 for WB and n = 2 for qPCR) and FTD (n = 1 for WB and n = 2 for qPCR) patients as controls (Table 1, Supplementary Table S1). For pull-down assays, frozen left prefrontal cortex tissue samples of AD patients at Braak V (n = 6) and Braak VI (n = 6) and of healthy individuals (n = 6) were used, whereas for IF 4 μm tissue sections of formalin-fixed paraffin-embedded (FFPE) left prefrontal cortex from one AD patient (Braak V) and one healthy individual were used.
For ELISA tests, 80 individual plasma samples from AD patients at Braak stages IV–VI (n = 47) and healthy individuals (n = 33) were used (Table 1, Supplementary Table S2).hNS1 cells, a model of immortalized, non-transformed, human fetal forebrain-derived, and multipotent neural stem cells (hNSCs) [43, 44], overexpressing APP695 (amyloid precursor protein) or control were used in the study [45, 46]. APP overexpression in hNS1 cells was carried out by lentiviral transduction [45, 46]. Two different transduced hNS1 subclones were generated: control Φ-eGFP hNS1 cells overexpressing the green fluorescence protein (GFP) and L.APP-IRES-eGFP hNS1 cells overexpressing both human isoform APP695 and GFP proteins. hNS1 cells were cultured on a chemically defined human stem cell (HSC) medium [DMEM:F12 containing GlutaMAX (Gibco), 0.6% d-glucose (Merck), 1 × N2 supplement (Gibco), 0.26% AlbuMAX (Gibco), 5 mM HEPES (Gibco), 1 × non-essential amino acids (NEAA; Gibco), 1 × penicillin–streptomycin (Lonza)] supplemented with 20 ng/ml epidermal growth factor (EGF; PeproTech), and 20 ng/ml basic fibroblast growth factor (FGF2; PeproTech). Cells were proliferated on poly-L-lysine (10 μg/ml; Sigma)-coated plastic at 37 °C and 5% CO2. Cells were differentiated by withdrawal of growth factors (EGF and FGF2) and addition of 0.5% heat-inactivated fetal bovine serum (FBS; Gibco) at indicated times [45].
Protein extraction and SDS-PAGE
Approximately, 100 mg of frozen left prefrontal brain tissue samples used for the study was cut into small pieces on dry ice and lysed by mechanical disaggregation in 500 µL of lysis buffer (RIPA, Sigma-Aldrich) supplemented with 1 × protease and phosphatase inhibitors (MedChemExpress) 1:100 diluted using 16G and 18G needle syringes until homogeneity was observed. Then, samples were centrifuged at 10,000×g at 4 ℃ during 10 min and protein extracts (supernatants) collected and stored at − 80 ℃ until use.
Protein concentration was determined by the Trp quantification method [47] and confirmed by Coomassie blue staining after 10% SDS-PAGE under reducing conditions.
10-plex TMT labeling and protein fractionation
Dysregulation of proteins in the left prefrontal cortex of AD patients’ brain was studied by 10-plex TMT-based quantitative proteomics. For this experiment, 21 individual brain tissue samples were pooled into six groups: AD patients at Braak IV (n = 4), Braak V (n = 4), and Braak VI (n = 4), healthy individuals (n = 2), and VD (n = 2) and FTD (n = 5) patients. AD Braak V and Braak VI, healthy individuals, and FTD patients’ pools were included in duplicate in the analysis.
In total, 8 μg of each protein pool in 100 μl of RIPA was reduced with 10 μl 100 mM tris(2-carboxyethyl)phosphine (TCEP, Sigma-Aldrich) for 45 min at 37 ℃ and 600 rpm and alkylated with 11 μL of 0.4 M chloroacetamide (Sigma-Aldrich) for 30 min at room temperature (RT) and 600 rpm in darkness. Next, samples were incubated with 100 µL of SeraMag magnetic beads mix (50% hydrophilic beads–50% hydrophobic beads, GE Healthcare) and 200 μL of acetonitrile 100% for 35 min at RT and 600 rpm to allow protein binding to the beads [48]. Then, supernatants were discarded, and magnetic beads were washed twice with ethanol 70% and once with acetonitrile 100%. Finally, supernatants were discarded and proteins digested overnight at 37 ℃ and 600 rpm with 1 μg of porcine trypsin (Thermo Fisher Scientific) in 80 μl 20 mM HEPES pH 8.0. The next day, samples were sonicated and the supernatants were collected. All peptides from the ten samples were separately labeled with ten different tandem mass tags (Thermo Scientific, San Jose, CA) according to manufacturer's instructions. Finally, the content of the ten tubes was pooled and dried under vacuum prior to separation using high pH reversed-phase peptide fractionation kit (Pierce). Desiccated samples were reconstituted in 300 μl H2O Milli-Q, TFA 0.1%, applied to the columns and peptides fractionated [49]. In brief, columns were equilibrated with acetonitrile 100% and H2O Milli-Q, TFA 0.1%. Then, peptides were loaded onto the columns, the columns were washed twice with 300 μl of H2O Milli-Q, and TFA 0.1%, and peptides separated in 12 fractions of 300 µL each in triethylamine 0.1% and acetonitrile 2.5–100% (Fig. 1). The fractions were then mixed in six fractions by pooling the latest fractions with the initial ones (2, 9 and 1; 7 and 3; 10 and 4; 8 and 5; 11 and 6; 12). Finally, the fractions were dried under vacuum and stored at -80ºC until analysis in six LC–MS/MS runs using a Q Exactive mass spectrometer (Thermo Fisher Scientific). For the LC–MS/MS analysis, samples were resuspended in 10 µL of formic acid 0.1%.
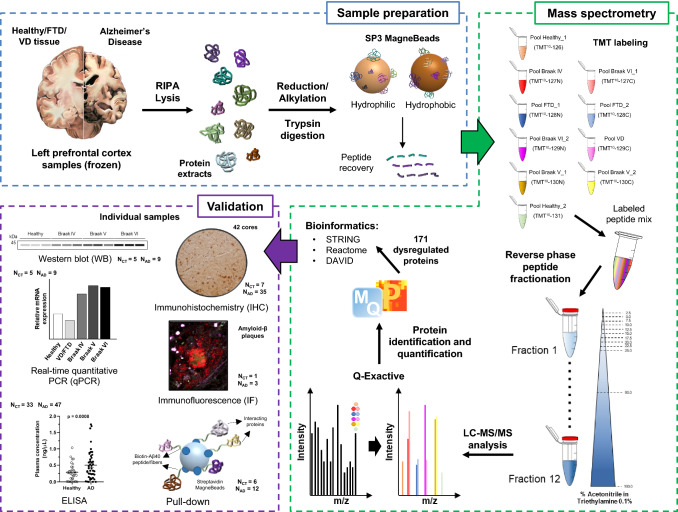
Fig. 1.
Workflow for the identification of left prefrontal cortex dysregulated proteins in AD patients by proteomics. Pooled protein extracts from AD patients at Braak stages IV, V, or VI, healthy individuals, and VD and FTD patients were trypsin digested, labeled with TMT reagents, and analyzed by LC–MS/MS using a Q Exactive. Differences in protein levels between AD patients at different Braak stages and the control group (healthy individuals and VD and FTD patients) were observed using MaxQuant and Perseus. Bioinformatics analysis of the 171 dysregulated proteins selected as more prone to be involved in AD pathogenesis after a stringent statistical analysis was subsequently performed with STRING, Reactome, and DAVID databases. Finally, ten proteins up- or downregulated in AD were selected to get further insights into their role in the progression of the disease by qPCR, WB, IHC, IF, and pull-down followed by WB analysis, and ELISA. The number of individual samples from the controls groups (CT) or the AD groups (AD) used for validation is indicated for each technique
LC–MS/MS analysis
Peptide separations were carried out on an Easy-nLC 1000 nano system (Thermo Fisher Scientific). For each analysis, samples were loaded into a precolumn Acclaim PepMap 100 (Thermo Scientific) and eluted in an RSLC PepMap C18, 50 cm long, 75 µm inner diameter, and 2 µm particle size (Thermo Scientific). The mobile phase flow rate was 300 nl/min using 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). The gradient profile was set as follows: 3–7% solvent B for 5 min, 7–25% solvent B for 95 min, 25–60% solvent B for 14 min, 60–95% solvent B for 1 min, and 95% solvent B for 5 min. Four microliters of each sample was injected. MS analysis was performed using a Q Exactive mass spectrometer (Thermo Fisher Scientific).
For ionization, 1900 V of liquid junction voltage and 270 ℃ capillary temperature were used. The full scan method employed a m/z 300–1800 mass selection, an Orbitrap resolution of 70,000 (at m/z 200), a target automatic gain control (AGC) value of 3e6, and maximum injection times (IT) of 100 ms. After the survey scan, the 15 most intense precursor ions were selected for MS/MS fragmentation. Fragmentation was performed with a normalized collision energy of 27 and MS/MS scans were acquired with a dynamic first mass, AGC target 1e5, resolution 35,000, intensity threshold 2e4, isolation window 1.6 m/z units, and maximum IT 100 ms. Charge state screening was enabled to reject unassigned, singly charged, and greater than or equal to seven protonated ions. A dynamic exclusion time of 30 s was used to discriminate against previously selected ions.
MS data analysis
MS data were analyzed with MaxQuant (version 1.6.6.0) using standardized workflows. Mass spectra *.raw files were searched against Uniprot UP000005640_9606.fasta Homo sapiens (human) 2019 database (20,962 protein entries, downloaded: May 2019) using Reporter ion MS2 type. Trypsin/P was specified as cleavage enzyme, allowing a mass tolerance of 20 ppm (Orbitrap). Precursor and reporter mass tolerances were set to 4.5 ppm and 0.003 Da, respectively, allowing two missed tryptic cleavages. Carbamidomethylation of cysteines was set as a fixed modification, and methionine oxidation, N-terminal acetylation, and Ser, Thr, and Tyr phosphorylation were set as variable modifications. Reporter ion intensities were bias corrected for the overlapping isotope contributions from the TMT tags according to the manufacturer’s certificate. Unique and razor peptides were considered for quantification. Minimal peptide length and maximal peptide mass were fixed to seven amino acids and 4600 Da, respectively. Identified peptides were filtered by their precursor intensity fraction with an FDR (false discovery rate) threshold of 0.01. Proteins identified with at least one peptide and an ion score above 99% were considered for evaluation, whereas proteins identified as potential contaminants were excluded from the analysis. The protein sequence coverage was estimated for specific proteins by the percentage of matching amino acids from the identified peptides having confidence greater than or equal to 95% divided by the total number of amino acids in the sequence.
As the same amount of protein was labeled in each TMT sample, differences in the total sum of signals of each channel were corrected by computing normalization factors to equal these sums. For data normalization, sample loading (SL) normalization was carried out with R Studio (version 3.6.2) according to established protocols (https://github.com/pwilmart), using the “tidyverse”, “psych”, “gridExtra”, and “scales” packages (Supplementary Fig. 1) [50]. Data were then exported to Microsoft Excel 2019 and Perseus (version 1.6.10) for subsequent analysis. Duplicated samples were obtained and processed separately as individual samples (biological replicates). For the analysis with Perseus, reverse and contaminant proteins were removed before statistical analysis. In addition, only proteins identified in at least three of the ten samples with one or more peptides were considered for the analysis. Samples were classified as Healthy (Healthy_1 and Healthy_2), FTD/VD (FTD_1, FTD_2 and VD), AD (Braak IV, Braak V_1, Braak V_2, Braak VI_1 and Braak VI_2), Braak V (Braak V_1 and Braak V_2), Braak VI (Braak VI_1 and Braak VI_2), or CT (Healthy_1, Healthy_2, FTD_1, FTD_2 and VD) groups and averaged for statistical analysis after confirming by principal component analysis (PCA) that biological replicates behave similarly. For two-sample test analysis of the AD, Braak V, or Braak VI groups against the CT group, a Student’s t test was performed. To compare more than two conditions, multiple-sample test was performed using FDR < 0.2 for truncation. Then, post hoc correction test for one-way ANOVA with an FDR < 0.2 (Tukey’s honestly significant difference, THSD) was performed for all proteins and pairwise comparisons to highlight significant hits. Among significant hits dysregulated in AD, Braak V, and Braak VI in comparison to healthy individuals (post hoc correction), proteins with an expression ratio ≥ 1.5 (upregulated) or ≤ 0.67 (downregulated) and statistically dysregulated in AD patients in comparison with CT (p value < 0.05) were selected as potential proteins dysregulated in AD. The AD/CT expression ratios were used as fold change. FDR and expression ratio cutoffs were selected according to previous reports [21, 49, 51–55]. The less stringent significance FDR cutoff was selected to minimize missing false negative proteins in the dataset, whereas a higher stringent significance expression ratio cutoff was selected to avoid as much as possible the presence of false positives among the dysregulated proteins.
Bioinformatic analysis
Heatmap of the TMT relative abundance of selected proteins was obtained with MultiExperiment Viewer (MeV, version 4.9.0). STRING (version 11.0), Reactome Pathway Database, and DAVID (version 6.8) were used to study protein enrichment and to identify the altered networks and pathways in which proteins were identified as dysregulated in AD patients at different Braak stages in comparison to controls [56–58]. STRING settings were fixed to MCL clustering enrichment 2 and 0.4 confidence score. Heatmap of protein enrichment was obtained with Gitools (version 2.3.1) [59]. Synaptic Gene Ontologies (synGo 1.1) dataset was used to define the role of dysregulated proteins in the synapse.
In silico analysis
Data from Bai et al. 2020 available at the PRIDE database with the accession number PXD007985 were used for comparison purposes and for correlation analysis of the proteomics dataset obtained here and proteomics data from the frontal cortical brain tissue and cerebrospinal fluid (CSF) of AD patients [60]. 90 individual tissue samples from individuals with a low pathology of plaques and tangles (LPC, n = 18), individuals with high Aβ pathology but no detectable cognitive defects (HPC, n = 18), mild cognitive impairment patients with high Aβ pathology and a slight defect in cognition (MCI, n = 18), and large-stage AD patients with high pathology scores of plaques and tangles (AD, n = 18) were used [60]. Samples were divided into ten pools (2 per group) of n = 9 samples per pool. For the re-analysis, TMT relative abundance of each pool was averaged for the statistical analysis based on a Student’s t test two-by-two comparison. p value < 0.05 was considered statistically significant. Regarding CSF, data from proteomics analysis of CSF samples from LCP (n = 5) and AD (n = 8) patients were also used [60]. For the re-analysis, signal intensities were averaged for the statistical analysis based on a Student’s t test two-by-two comparison. p value < 0.05 was considered statistically significant.
RNA extraction and qPCR
For RNA extraction, about 100 mg of frozen left prefrontal tissue samples of AD patients at Braak IV (n = 3), Braak V (n = 3), and Braak VI (n = 3), healthy individuals (n = 4), and VD (n = 2) and FTD (n = 2) patients were cut on dry ice and incubated with 1 mL of NZYol (NZYtech) for 5 min at RT and mechanically disaggregated using the TissueLyser II (Qiagen) (2 cycles of 30 s at 30 Hz). Then, samples were incubated with 200 µL of chloroform (Sigma-Aldrich) for 3 min at RT and centrifuged 15 min at 12000 g and 4 ℃, and the upper phase, containing the RNA, was transferred to a new tube. Finally, RNA samples were purified using the RNeasy Mini Kit (Qiagen) following the manufacturer’s instructions, and purified RNA was quantified with the Nanodrop 2000C (Thermo Fisher). cDNA was obtained from 1 µg of RNA using the NZY First-Strand cDNA Synthesis Kit (NZYtech) following the manufacturer’s instructions and directly used for the quantification of ANLN, NRGN, BIN1, SCRN3, PMP2, PPP1R14A, SLC12A5, EXOC2, HECTD1, and TSC2 mRNA levels in brain tissue samples. qPCRs analyses were performed onto the Light Cycler 480 (Roche) (40 cycles at 65 ℃) using the TB Green Premix Ex Taq II (Takara) and the corresponding specific oligonucleotides (Supplementary Table S3). The mRNA levels of HPRT were used for normalization.
Western blot
5–10 µg of each protein extract from AD patients (n = 9), healthy individuals (n = 3), and VD (n = 1) and FTD (n = 1) patients were alternatively separated on 10% or 15% SDS-PAGE under reducing conditions and transferred to nitrocellulose membranes at 100 V for 90 min to study the dysregulation of proteins by WB. Then, membranes were blocked with 0.1% Tween PBS 1 × supplemented with 3% skimmed milk (blocking buffer) for 1 h at RT and incubated with primary antibodies at optimized dilutions (Supplementary Table S4) in blocking buffer overnight (O/N) at 4 ℃. Then, membranes were washed three times with 0.1% Tween PBS 1 × and incubated with the appropriate indicated HRP-conjugated secondary antibodies (Supplementary Table S4) diluted in blocking buffer for 1 h at RT. Next, membranes were washed three times with 0.1% Tween PBS, and, finally, signal was developed using the ECL Pico Plus chemiluminescent reagent (Thermo Fisher Scientific) and detected on an Amersham Imager 680 (GE Healthcare). RhoGDi and tubulin were used as controls. Protein band intensities were quantified using ImageJ software and normalized according to the total protein content of each line as observed using Ponceau red staining.
Immunohistochemistry
Immunohistochemical staining was conducted in 4 μm FFPE brain tissue sections from a tissue microarray (TMA) containing 44 cores from AD patients at Braak stages IV–VI and VD, FTD, and healthy individuals as controls [61]. Slides were deparaffinized by incubation at 60 ℃. Biopsies were cut and incubated with PT-Link (Dako) for 20 min at 95 ℃ in a high pH buffered solution. To block endogenous peroxidase, holders were incubated with peroxidase blocking reagent (Dako). Biopsies were stained for 20 min with the corresponding antibody at optimized dilution (Supplementary Table S4), followed by incubation with the corresponding HRP-conjugated secondary antibody. Sections were then visualized with 3,3′-diaminobenzidine for 5 min and counterstained with hematoxylin. Immunoreactivity was graded as 0, absent; 1, mild staining; 2, moderate staining; or 3, intense staining. Cases were classified according to total staining (intensity of the staining per percentage of areas showing reaction). In all cases, an external negative control was included. In addition, TMA incubation with the HRP-conjugated secondary antibody was performed as negative control, which confirmed the absence of background signal. 40× images were obtained with the NanoZoomer scanner (Hamamatsu photonics) and processed with the NDP.view Software.
Immunocytochemistry
At the specified time points (proliferation and differentiation), cells were rinsed with PBS and fixed for 10 min with 4% PFA (Sigma). Cultures were blocked for 30 min in 5% normal horse serum (NHS; Gibco) with 0.25% Triton X-100 in PBS and incubated overnight at 4 ℃ with rabbit antibodies against PMP2 and SCRN3 at optimized dilutions (Supplementary Table S4). Cells were then incubated with Alexa 555-conjugated donkey anti-rabbit antibody (Invitrogen) for 1 h at RT. Cell nuclei were counterstained with Hoechst 33,258 (Molecular Probes) at 0.2 μg/ml in PBS for 10 min at RT. As negative control, cells were incubated with the Alexa 555-conjugated donkey anti-rabbit antibody, which confirmed the absence of background signal.
Analysis and photographs of fluorescent-immunostained cultures were carried out in an inverted DM IL LED microscope (Leica, Nussloch, Germany) equipped with digital camera DFC345FX using LAS V4.0 software (Leica). Image analysis was performed using Photoshop CS6 after randomly capturing at least seven separate fields per well, with a minimum of three wells per experimental group, to quantify the number of positive cells for PMP2 and SCRN3 compared to the total number of cells (Hoechst). The percentage of positive cells for PMP2 or SCRN3 normalized to total cells (Hoechst) was calculated and the statistical analysis performed using two-way ANOVA (multiple comparison).
Immunofluorescence
IF staining was performed using 4 μm FFPE brain tissue sections from AD patients at Braak stage IV (n = 1), V (n = 1), and VI (n = 1) and healthy individuals (n = 1) (Supplementary Table S1). First, slides were deparaffinized in an oven at 60 ℃ for 30 min, and washed twice in xylene, twice in 100% ethanol, twice in 96% ethanol, once in 75% ethanol, and once in Milli-Q water (H2Omq), 5 min per wash and at RT. Then, slices were incubated with Antigen Retrieval solution (10 mM citrate, pH 6.0) and warmed 5 min at 120 ℃ and 5 min at 90 ℃. Slices were washed 5 min with PBS 1x, 30 min with 1% Triton X-100 PBS 1x, and, finally, 5 min with PBS 1 × in rotation.
Deparaffinized tissues were blocked in 10% FBS, 0.1% Triton X-100, and 0.05% BSA PBS 1x (blocking buffer 2) 1 h at RT, and incubated with both a mouse monoclonal amyloid-β antibody and a rabbit polyclonal antibody against the protein of interest (Supplementary Table S4), 1:200 and 1:100 diluted in blocking buffer 2, respectively, O/N and at 4ºC. Then, tissues were washed three times with PBS 1x, incubated, subsequently, with an anti-mouse IgG-AF555 and an anti-rabbit IgG-AF647, 1:200 diluted in blocking buffer 2 for 1 h at RT (Supplementary Table S4), washing three times prior to each incubation with PBS 1x. Then, tissues were washed three times with PBS 1 × and incubated with 1 µg/mL Hoechst (Hoechst 33342 20 mM Solution, Thermo Fisher Scientific) 1:1000 diluted in blocking buffer 2 for 15 min at RT. Finally, slices were washed three times with PBS 1x, and the staining was preserved with Prolong (Thermo Fisher Scientific). Negative control was obtained by incubating the FFPE AD tissue at Braak V with mouse monoclonal amyloid-β antibody, followed by the IgG-AF555 and IgG-AF647 secondary antibodies to assess the unspecific staining of IgG-AF647 antibody. Tissue samples were acquired with an HCX PL APO 63 × oil immersion objective (N.A. 1.4) in a Leica Stellaris 8 (STED-Falcon) confocal microscope (Leica Microsystems).
All images were acquired using the Leica Confocal Software (Las X) and processed with ImageJ software. To ensure comparison among images, acquisitions were performed with the same settings (pixel size, excitation laser power, and detector sensitivity). Staining specificity was additionally checked by acquiring lifetime images of SCRN3 and PMP2 staining. In addition, λ spectra of Alexa Fluor 647 channel were obtained from tissue regions with a clear lipofuscin autofluorescence mixed with Alexa Fluor 647 and from just specific Alexa Fluor 647 tissue regions.
Pull-down and amyloid-β (Aβ) fibers
For the detection of Aβ fibrils interacting proteins, biotinylated-amyloidβ40 (1–40, Anaspec, AS-23512; Aβ40) and scrambled-β-amyloid (1–40, Anaspec, AS-24626) peptides were used. First, 0.48 mM scramble-amyloid peptide was incubated with 10 mM EZ-Link NHS-Biotin (Thermo Scientific, 20,217) for 30 min at RT and in rotation for biotinylation. Then, for the formation of Aβ fibers, 10 µM of each biotinylated peptide (scrambled-amyloidβ as control and Aβ40 peptides) were incubated in PBS 1x, in a final volume of 100 µL, at 37 ℃ and 400 rpm for 7 days. Then, the peptide contents were fixed with 1% paraformaldehyde for 30 min at 37 ℃, and samples were stored at − 20ºC until use. Verification of Aβ fibers formation was performed by transmission electron microscopy (TEM). For the pull-down assay with Aβ fibers, 0.32 nmol (32 µL) of biotinylated-Aβ40 fibers or biotinylated-scramble amyloidβ (treated as biotinylated-Aβ40 for fiber formation, but unable to form them) were incubated with 10 µL of Streptavidin Mag Sepharose beads (MBs, Merck, Cytiva 28-9857-38) per protein extract in separate tubes, for 30 min at RT and 25 rpm in a final volume of 300 µL with binding buffer (50 mM Tris, 30 mM NaCl, pH 7.5). Streptavidin magnetic beads were previously incubated twice with 500 µL of binding buffer for 5 min at RT and 25 rpm for equilibration. After anchoring, beads were washed three times with 500 µL of binding buffer, 5 min at RT and in rotation, and protein extracts from the left prefrontal cortex brain tissue samples from AD patients at Braak V and VI and healthy individuals (Table 1, Supplementary Table S1) were pooled into three groups (n = 6). Then, 2 mg of each pool was preincubated with 20 µL of MBs for 1 h at RT and 25 rpm in a final volume of 300 µL with binding buffer, and then incubated with the Aβ fibrils bound to the MBs for 2 h at RT and 25 rpm. MBs were washed three times with 500 µL of binding buffer for 5 min at RT in rotation, and interacting proteins were eluted in 50 µL of elution buffer (188 mM Tris pH 6.8, 30% glycerol, 3% SDS, 0.01% bromophenol blue, and 1.5% β-mercaptoethanol), 5 min at 95ºC and 600 rpm. Finally, 2 µg of biotinylated peptides as purity control and 20 µL of eluted proteins were separated using 15% SDS-PAGE gels for staining with Coomassie blue or to perform WB analysis using appropriate optimized antibody dilutions (Supplementary Table S4).
Transmission electron microscopy
Samples were incubated over glow-discharged carbon-coated grids for 5 min and negatively stained with 2% aqueous uranyl acetate. Then, an FEI Tecnai 12 electron microscope equipped with a LaB6 filament operated at 120 kV was used for sample analysis, and images were recorded with the FEI Ceta digital camera.
Sandwich ELISA
Sandwich ELISA for the quantification of NRGN, ANLN, HECTD1, and SLC12A5 levels in human sera were performed following manufacturer’s instructions (MyBiosource). Briefly, 96-well plates coated with the corresponding antibody were incubated with individual plasma samples (1:10 diluted) from AD patients at Braak IV (n = 7), Braak V (n = 29), and Braak VI (n = 11) and healthy individuals (n = 33). Then, plates were incubated with the HRP-conjugated reagent and color was developed according to the manufacturer’s instructions. Finally, colorimetric signal was read at 450 nm using the Spark multimode microplate reader (Tecan Trading AG).
Statistical analysis
Plots, mean, and standard error of the mean (SEM) of WBs, qPCRs, ELISAs, and IHC were performed with Microsoft Excel 2019 and GraphPad Prims (version 8.0.1) programs. Student’s t test values were calculated with Perseus (version 1.6.10) and Microsoft Word 2019. p values < 0.05 were considered statistically significant. ROC curves (receiver operating characteristic curves) of each individual protein or in combination were constructed with R (version 3.6.2), using the “ModelGood” and “Epi” packages. Venn diagram was obtained with R (version 3.6.2), using “Venn” package or the jvenn website (http://bioinfo.genotoul.fr/jvenn) [62]. Statistical analysis was performed with Student’s t test. p values < 0.05 were considered statistically significant.
Results
In the present study, we performed the profiling of protein alterations in the left prefrontal cortex of AD patients in comparison to healthy individuals and patients with other dementias as controls by TMT 10-plex based quantitative proteomics to identify dysregulated proteins specifically associated with AD and non-related to other dementias (Fig. 1). Here, we used a non-targeted proteomics approach, in contrast to our previous work using antibody microarrays [61], to increase the knowledge in the pathology and to get significant, consistent, and validated data related to AD.
TMT proteomics analysis was performed with frozen left prefrontal cortex tissue samples from AD patients at different Braak stages and healthy individuals and VD and FTD patients as controls. Pooled samples were analyzed by proteomics instead of individual samples to avoid biological variations and potential aberrations appearing in individual patients, which might increase the depth into the proteome and the capacity to identify the most significant and consistent changes associated with AD. Then, a higher number of well-characterized individual samples were used for the verification and validation of ten dysregulated proteins not previously associated with AD by WB, IHC, qPCR, ELISA, or IF. Moreover, besides healthy samples, VD and FTD samples were also used as controls for the identification of proteins specifically altered in AD and non-related to VD alterations and/or NFTs associated dementias (FTD). Besides their use as controls regarding the most incident dementia after AD (VD), and young-onset dementia (FTD) [29–35], they should also allow, in combination with healthy individuals as controls, to get further insights into AD pathology. In addition, different AD Braak stage samples were used to identify dysregulated proteins during the course of the disease. A schematic workflow of the study is depicted in Fig. 1.
Profiling of protein dysregulation in AD by LC–MS/MS
Tissue samples from AD patients at Braak stages IV to VI were analyzed by LC–MS/MS in comparison to healthy individuals and other dementias to identify proteins dysregulated in AD. First, individual protein extracts from the left prefrontal cortex of AD patients at Braak IV (n = 4), Braak V (n = 4), and Braak VI (n = 4), and healthy individuals (n = 2) and VD (n = 2) and FTD (n = 5) patients as controls were separately pooled and analyzed by Coomassie blue staining to evaluate the quality of the samples prior to the TMT analysis (Fig. 2a). Healthy and FTD controls and AD at Braak V and VI duplicate samples were separately prepared and analyzed as biological replicates.
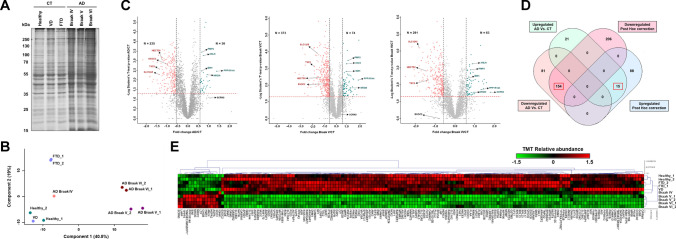
Fig. 2.
Statistics of the 10-plex TMT analysis of the brain protein content from AD patients and controls. A Coomassie blue staining of the SDS-PAGE performed for quality control of the pooled tissue extracts from AD patients and controls used for LC–MS/MS analysis. B Principal component analysis (PCA) of the proteome data in a 2D graph of PC1 and PC2 showed a clear separation between healthy, VD, FTD, and AD at Braak IV, V, and VI stages. C Volcano plots of proteins identified as altered in AD, Braak V, or Braak VI in comparison to controls (CT). The x-axis is log2 fold change of protein expression differences between two groups; the y-axis is p value based on − log10. The colored dots represent differentially expressed proteins upregulated (green) and downregulated (red) in AD with p value < 0.05 (p value = 0.05 represented by a red dashed horizontal line) and 1.5-fold expression difference (represented by two black dashed vertical lines—upregulated proteins ≥ 1.5 fold change, in the figure denoted as log2fold change ≥ 0.58; downregulated proteins ≤ 0.67 fold change, in the figure denoted as log2fold change ≤ -0.58). D Venn diagram analysis of the significantly dysregulated proteins as obtained by ANOVA and post hoc correction and t test comparison between AD and controls. In total, 154 downregulated and 15 upregulated proteins were observed in common in both statistical analysis and selected for further analysis. E Heatmap of the TMT protein relative abundance obtained for the 171 proteins (169 proteins of panel D together with EXOC2 and SCRN3) selected to investigate their role in AD, with statistically significant (p value < 0.05) differences between groups and ≥ 1.5 or ≤ 0.67 expression ratio in AD in comparison to controls. Proteins were hierarchically clustered according to Pearson correlation metrics into upregulated (red) or downregulated (green) in AD patients (Braak IV to VI) in comparison to controls (healthy individuals, and VD and FTD patients)
Then, protein pools were trypsin digested and separately labeled with TMT reagents. Labeled peptides were pooled together, separated into 12 fractions, and analyzed by LC–MS/MS using a Q Exactive mass spectrometer. Following normalization, a total of 3281 proteins were quantified (Supplementary Table S5). Among them, we found APP, amyloidβ peptide (LVFFAEDVGSNK), and MAPT (microtubule-associated protein Tau), and their relative abundance was monitored (Supplementary Fig. 2a). As a non-previous enrichment was performed to solubilize amyloidβ peptide, and Tau from amyloidβ fibers and NFTs, we could not observe significant differences between AD patients and controls, which is in concordance with previous proteomics reports focusing on the analysis of different localizations of AD brain proteome [60].
Next, as a surrogate of the quality of the obtained proteomics data, and prior to data analysis, PCA allowed not only to confirm the good reproducibility of the replicates (Fig. 2b), but also to observe how analyzed samples behave among them. PCA analysis showed separate clusters for AD, healthy, VD, and FTD samples, suggesting that a differential protein content among the clustered samples could be obtained. Then, we focused the analysis on those proteins with a fold change ≥ 1.5 (upregulated) or ≤ 0.67 (downregulated) in AD in comparison to controls (CT; healthy individuals and VD and FTD patients) regarding all AD Braak stages, Braak V or Braak VI (Fig. 2c). Depending on the comparison, up to 373 significantly downregulated proteins and 74 significantly upregulated proteins were observed (p value < 0.05). Next, with the aim of identifying proteins statistically dysregulated in any of the AD Braak stages in comparison to the healthy group, or in comparison with healthy and FTD/VD groups, ANOVA multiple test, followed by post hoc correction statistical analysis, was performed. From these analyses, 212 and 474 proteins were identified as upregulated and downregulated, respectively, in AD in comparison with the healthy and VD/FTD groups. Regarding the statistical analysis according to the Braak stage of AD patient’s vs healthy group, at Braak V, 228 and 738 proteins were upregulated and downregulated, respectively, whereas at Braak VI, 219 and 742 proteins were upregulated and downregulated, respectively. Taking together these results, it was observed that 103 upregulated proteins and 360 downregulated proteins were commonly found to be significantly dysregulated in AD, Braak V, and Braak VI in comparison to the healthy and FTD/VD groups (Supplementary Fig. 2b, Supplementary Table S6). Remarkably, some of the identified dysregulated proteins have been previously reported as altered in AD, such as CDK5, PRKCG, or PRKCB, supporting the effectiveness of the assay and the statistical analysis for the identification of proteins dysregulated in AD [63–66].
In addition, from the total number of proteins we identified 34 and 15 proteins specifically dysregulated at Braak V and Braak VI, respectively, when comparing AD samples with the CT and the healthy group (Supplementary Table 7 and Supplementary Fig. 4a). Interestingly, proteins specifically altered at Braak V were related to GTPase signaling pathways, previously described as altered at early stages of AD [67], whereas proteins altered at Braak VI were related to the Notch and EPH-ephrin signaling, previously described as aberrant in AD (Supplementary Fig. 4b) [68–70].
Finally, these 463 dysregulated proteins were filtered in comparison with the analysis of the AD/CT expression ratio by Student’s t test to identify proteins significantly altered in AD samples not associated with vascular and frontotemporal dementias, new mechanisms, or altered pathways underlying Alzheimer’s disease and potential AD-specific biomarkers (Fig. 2c-d, Supplementary Table S6). In total, 15 upregulated (AD/CT ratio ≥ 1.5) proteins and 154 downregulated (AD/CT ratio ≤ 0.67) proteins were observed as statistically significant (p value < 0.05) dysregulated proteins more prone to be involved in AD and not associated with other dementias (Fig. 2d, Supplementary Table S6). In addition, SCRN3 and EXOC2 were also included in the dataset (Supplementary Table S6). SCRN3 was found among the top upregulated proteins in AD and significantly upregulated in Braak VI samples in comparison to CT. Besides, another protein of the secernin family of proteins (SCRN1) was previously associated with AD [71]. In contrast, EXOC2 was found to be statistically downregulated in AD and Braak V samples in comparison to CT. EXOC2 has been described as critical to neuronal function, and thus it was also considered as an interesting target that could be relevant for the study of AD pathology.
Collectively, the dysregulation of these proteins at different Braak stages in comparison with the CT group confirmed that the defined 171 dysregulated proteins were also altered at Braak V and/or Braak VI (Fig. 2c–e, Supplementary Table S6). A representative heatmap of the TMT relative abundance in each sample for the 171 selected proteins is shown in Fig. 2e.
Bioinformatics analysis of proteins altered in AD
Next, the 171 dysregulated proteins in AD of our proteomics dataset were investigated by bioinformatics using STRING, Reactome, and DAVID databases to identify dysregulated protein networks and pathways in which these proteins are involved, and to determine its association with AD pathogenesis.
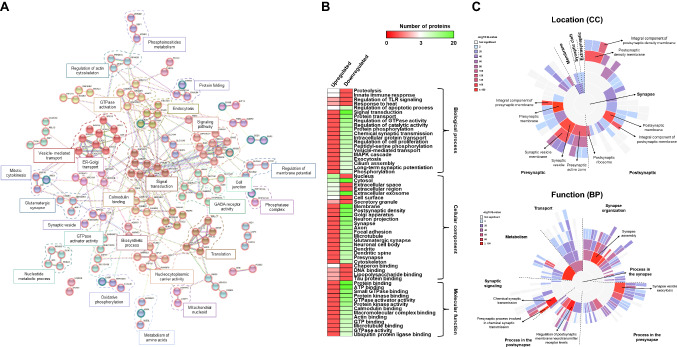
With STRING, 37 different clusters of direct and indirect protein interactions were observed (Fig. 3a). These protein clusters were significantly (FDR < 0.05) related to metabolic processes, translation, synapse, actin-cytoskeleton regulation, signal transduction, and protein transport, which are pathways closely related to AD. With Reactome, we found significantly dysregulated pathways involving most of these dysregulated proteins, highlighting postsynaptic signal transmission (13/208 proteins, p value = 1.27e-05), chemical synapses (15/273, p value = 1.72e-05), membrane trafficking (24/636, p value = 2.86e-05), insulin processing (5/27, p value 6.00e-05), synaptic plasticity (5/31, p value = 1.14e-04), vesicle-mediated transport (25/762, p value = 1.78e-04), signaling by Rho GTPases (22/678, p value = 5.19e-04), neuronal system (16/419, p value = 5.86e-04), post-NMDA receptor activation events (6/84 proteins, p value = 0.002), axon guidance (18/558, p value = 0.002), calmodulin-induced events (4/35, p value = 0.002), or trafficking of GluR2-containing AMPA receptors (3/17 proteins, p value = 0.002) (Supplementary Fig. 2c). As these proteins are dysregulated in AD, these pathways might be altered during the ongoing course of the pathology or might be implicated in the development and pathogenesis of the disease, and thus would be of interest for the study of AD. In addition, the biological processes and molecular functions in which these proteins are involved, which could be important for the development of the pathological changes observed during AD progression, were mapped with DAVID (Fig. 3b). Among them, the most important biological processes related to AD involving upregulated proteins were proteolysis (3 proteins, p value = 0.05), innate immune response (3 proteins, p value = 0.048), and regulation of apoptotic process (3 proteins, p value = 0.038), whereas the most important biological processes involving ten or more downregulated proteins were related to signal transduction (22 proteins, p value = 4.00e-04), GTPase activity (13 proteins, p value = 5.20e-06), protein phosphorylation (11 proteins, 6.10e-03), protein transport (15 proteins, 6.50e-06), and synapse (10 proteins, p value = 2.60e-04). Regarding their cellular localization, upregulated proteins were mainly nuclear (11 proteins, p value = 2.8e-03), cytoplasmic (9 proteins, p value = 0.026), and extracellular proteins (5 proteins, p value = 0.047), whereas most of the downregulated proteins were localized on exosomes (26 proteins, p value = 0.028), plasma membrane (51 proteins, p value = 0.014), or neurons (more than 10 proteins, p value < 2.6e-03). Furthermore, we observed an increasing number of molecular functions significantly altered during the progression of the disease, such as protein binding, ATP binding, or GTPase binding (more than 15 proteins, p value < 9.5e-04). We finally studied the synapse function and localization of dysregulated proteins using SynGo dataset (Fig. 3c). Interestingly, most of these proteins (> 50) were significantly found in the pre- and postsynaptic processes (q-value < 0.01). Regarding synapse functions, metabolism, organization, signaling, and pre- and postsynaptic processes were significantly enriched (q value < 0.01).
Fig. 3.
Protein interactome and enrichment analysis of the dysregulated proteins in AD. A STRING revealed 37 significant (FDR < 0.05) different clusters of interaction closely affected during AD pathogenesis. Upregulated proteins are circled in green, and downregulated proteins in red. Disconnected proteins were not included in the network. B Heatmap of the biological processes, molecular function, and cellular component more significantly represented regarding the identified as upregulated or downregulated proteins in AD (p value < 0.05). Data were retrieved from DAVID database. C SynGo analysis of the synapse location (top) and function (bottom) of dysregulated proteins. Significant process related to synapse, presynaptic, and postsynaptic processes where dysregulated proteins were involved are depicted (q value < 0.01)
After bioinformatics analysis, proteins were selected for validation by orthogonal techniques based on (i) proteins showing the highest dysregulation in AD (6 proteins among the top 10 upregulated proteins, and 3 proteins among the top 20 downregulated proteins), (ii) proteins belonging to different protein clusters as observed by STRING that could be of interest for a deeper study and knowledge of the disease, and (iii) existing information about the association with AD of proteins of the same family (SCRN3) or proteins having a role in the maintenance of neuronal functions (EXOC2). Selected proteins—ANLN, BIN1, EXOC2, HECTD1, NRGN, PMP2, PPP1R14A, SCRN3, SLC12A5, and TSC2—were further investigated by in silico analysis, qPCR, WB, IHC, IF, pull-down, and ELISA for the validation of their association with AD pathogenesis (Table 2, Fig. 4).
Table 2.
Proteins dysregulated in AD selected for validation
| Protein IDs | Protein names | Gene names | Dysregulation |
|---|---|---|---|
| O00499 | Myc box-dependent-interacting protein 1 | BIN1 | Upregulated |
| P02689 | Myelin P2 protein | PMP2 | Upregulated |
| P49815 | Tuberin | TSC2 | Downregulated |
| Q0VDG4 | Secernin-3 | SCRN3 | Upregulated |
| Q92686 | Neurogranin;NEUG(55–78) | NRGN | Upregulated |
| Q96A00 | Protein phosphatase 1 regulatory subunit 14A | PPP1R14A | Upregulated |
| Q96KP1 | Exocyst complex component 2 | EXOC2 | Downregulated |
| Q9H2X9 | Solute carrier family 12 member 5 | SLC12A5 | Downregulated |
| Q9NQW6 | Actin-binding protein anillin | ANLN | Upregulated |
| Q9ULT8 | E3 ubiquitin-protein ligase HECTD1 | HECTD1 | Downregulated |
Fig. 4.
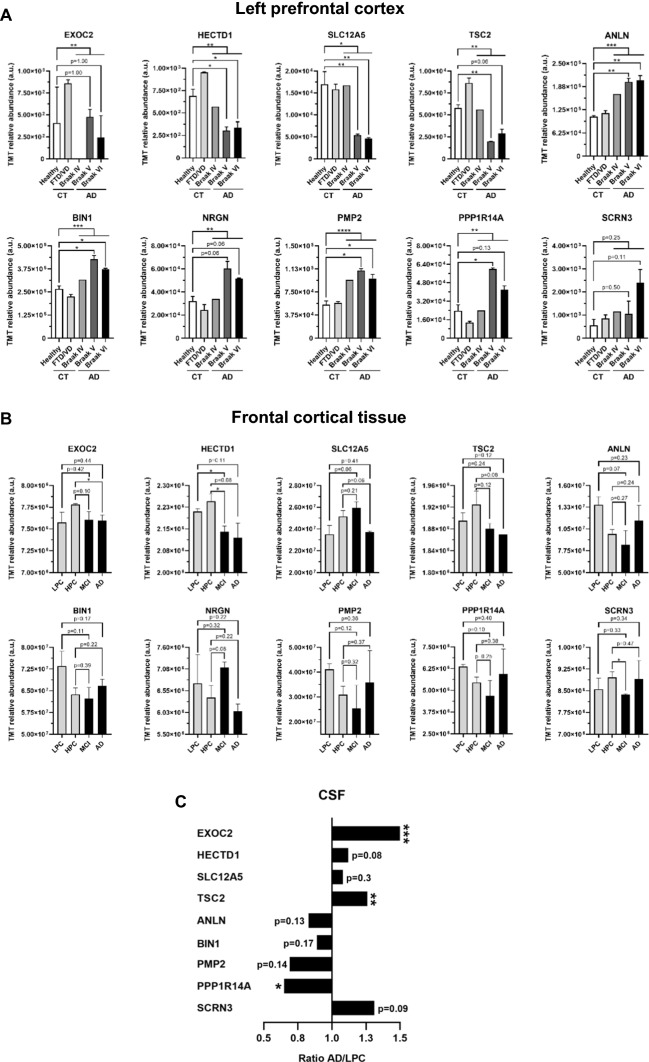
In silico analysis of the selected dysregulated proteins for validation in an alternative brain tissue localization and CSF. A Graph bar representing the mean and SEM of the TMT relative protein abundance of the 10 dysregulated proteins selected for further analysis in healthy individuals, VD and FTD patients, and Braak IV, Braak V, and Braak VI groups as obtained from mass spectrometry analysis. Error bars for Braak IV are not represented because one pooled sample was only analyzed. B Graph bar representing the TMT relative abundance of proteins in the frontal cortical tissue of AD patients (MCI and AD) and controls (LPC and HPC) [60]. C Representation of the AD/LPC ratio of the TMT relative abundance of selected proteins in the CSF of AD patients (AD) and controls (LPC) [60]. LPC, low pathology controls; HPC, high pathology controls; MCI, mild cognitive impairment patients; AD, late-stage AD patients. *p value < 0.05; **p value < 0.01; ***p value < 0.001. a.u, arbitrary units
Confirmation by in silico analysis of protein dysregulation in AD
First, we performed an in silico analysis of the ten selected proteins using published data to obtain information about the correlation of our proteomics dataset (Fig. 4a) with previously reported proteomics data on different brain regions (Fig. 4b, c) [60].
We focused on a large study exploring protein dysregulation in the frontal cortical tissue and cerebrospinal fluid (CSF) of AD patients by TMT quantitative proteomics analysis (Fig. 4b, c) [60], using 90 frontal cortical tissue samples from individuals with a low pathology of plaques and tangles (LPC, n = 18), individuals with high Aβ pathology but no detectable cognitive defects (HPC, n = 18), mild cognitive impairment patients with high Aβ pathology and a slight defect in cognition (MCI, n = 18), and large-stage AD patients with high pathology scores of plaques and tangles (AD, n = 18) (Fig. 4b), and LPC (n = 5) and AD (n = 8) CSF samples (Fig. 4c). Dysregulation found in the frontal cortical tissue by TMT proteomics analysis was in agreement with our prefrontal cortex TMT proteomics analysis for HECTD1, TSC2, and EXOC2 when comparing MCI and/or AD patients with LPC (HECTD1 and TSC2) or HPC (EXOC2, HECTD1 and TSC2) controls. In addition, the expression of SLC12A5, NRGN, and ANLN showed protein dysregulation during the progression of the disease in the frontal cortical tissue, with a partial concordance with their dysregulation observed by proteomics in the left prefrontal cortex. Finally, an opposite dysregulation was found for BIN1, PMP2, PPP1R14A, and SCRN3 in the frontal cortical tissue of AD patients in comparison with the dysregulation observed in the left prefrontal cortex in this study (Fig. 4b). These results highlight that these proteins are dysregulated at different brain regions in the pathogenesis of AD.
Interestingly, all candidate proteins but NRGN were identified in the CSF of AD patients. Protein levels of EXOC2, HECTD1, SLC12A5, and TSC2, identified as downregulated in the left prefrontal and frontal cortex of AD patients, and SCRN3, identified as upregulated in the left prefrontal cortex of AD patients, were higher in the CSF of AD patients than controls. In contrast, PMP2, PPP1R14A, ANLN, and BIN1, identified as upregulated in the prefrontal cortex of AD patients, were observed downregulated in the CSF of AD patients (Fig. 4c).
Dysregulation of candidate proteins during AD progression
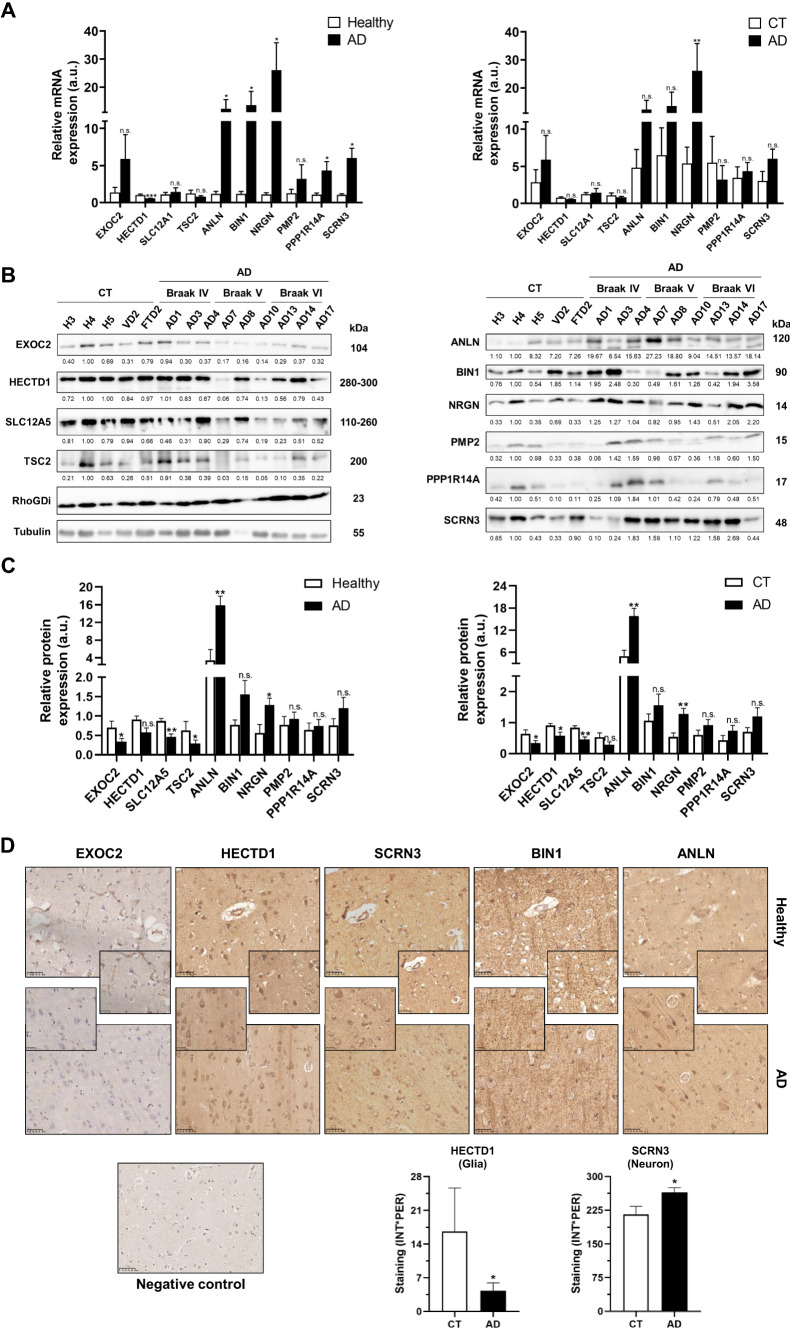
To confirm the implication of the ten dysregulated proteins in the pathogenesis of AD, we first studied alterations of these proteins in the left prefrontal cortex of AD patients at mRNA level. mRNA extracts from AD patients at Braak IV–VI (n = 9), healthy individuals (n = 4), and VD (n = 2) and FTD (n = 2) patients were retrotranscribed to cDNA and differences in mRNA expression levels analyzed by qPCR. We found statistically significant differences between AD patients and healthy individuals for all of them but EXOC2, SLC12A5, TSC2, and PMP2 (Fig. 5a), with HECTD1 downregulated and ANLN, BIN1, NRGN, PPP1R14A, and SCRN3 upregulated in AD patients. Similar differences in mRNA expression were observed in AD patients in comparison with controls (healthy individuals and FTD/VD patients), but for PMP2 (Fig. 5a). When comparing mRNA levels between healthy individuals, FTD/VD patients and AD patients at different stages of the disease, we found reduced levels of HECTD1 and TSC2 at Braak IV, V and VI, whereas mRNA levels of EXOC2 and SLC12A5 were mainly reduced at intermediate stages (Braak IV). On the contrary, mRNA levels of ANLN, BIN1, NRGN, and SCRN3 were statistically upregulated at Braak IV, V and VI, while mRNA levels of PPP1R14A were upregulated mainly at Braak V and VI, and levels of PMP2 were mainly upregulated at Braak IV (Supplementary Fig. 3a). However, similar mRNA levels at these Braak stages were obtained for HECTD1, PMP2, and PPP1R14A in comparison with FTD/VD patients, highlighting that changes at mRNA level for these proteins are not specific of AD. These results are in concordance with alterations at protein level in the left prefrontal cortex (here observed) and, partially, with cortical regions of AD patients observed by proteomics [60], which would suggest an important role of these proteins in AD pathogenesis.
Fig. 5.
Validation of the dysregulation of the ten selected proteins at mRNA and protein level. A Graph bar of the relative mRNA expression of HECTD1, EXOC2, SLC12A5, TSC2, PPP1R14A, SCRN3, PMP2, ANLN, BIN1, and NRGN in healthy individuals, VD, FTD, and AD patients at Braak IV, V and VI, regarding the comparisons of AD vs healthy individuals and AD versus controls. B, C Western blotting analysis of prefrontal cortex brain tissue samples of AD patients at stages IV, V and VI, and healthy individuals (H) and VD and FTD as controls. Comparisons between AD vs healthy individuals and AD vs controls are shown. Significant dysregulation of proteins in AD was observed for most of the comparisons. RhoGDI and tubulin were used as controls of the assay. Protein band intensities were quantified by densitometry with ImageJ software and normalized according to the total protein content of each line as observed by Ponceau red staining. D Tissue microarrays, composed of 44 cores from AD patients at Braak stages IV, V, and VI and VD, FTD and healthy individuals as controls, confirmed the significant dysregulation for HECTD1 and SCRN3 when comparing AD patients’ samples with controls at glia or neuron, respectively. Statistically significant dysregulation for ANLN, BIN1, SCRN3, HECTD, and EXOC2 at different Braak stages was also observed (see Supplementary Fig. 3). Besides the specific staining of the antibodies where significant differences between AD and controls were detected, an antibody-dependent fibrillar background was also observed. Representative 40 × magnification images for the staining of the left prefrontal cortex core of a healthy individual and an AD patient are depicted with specific antibodies against EXOC2, HECTD1, SCRN3, BIN1, and ANLN, besides the negative control (staining of a section with an HRP-conjugated secondary antibody). Insets at 80 × magnification are shown for a better appreciation of the differences in staining between AD and healthy individuals. *p value < 0.05, **p value < 0.01, ***p value < 0.001, n.s. not significant, a.u arbitrary units, CT controls, AD AD patients
Next, protein dysregulation was investigated by WB and IHC for confirmation of the association between protein expression levels and the progression of the disease. For WB analysis, we used protein extracts from frozen left prefrontal cortex tissue samples of AD patients at Braak IV–VI (n = 9), healthy individuals (n = 3), and VD and FTD patients (n = 2) (Supplementary Fig. 3b). As expected, we validated the dysregulation at protein level for all these proteins in the left prefrontal cortex of AD patients in agreement with the dysregulation observed by proteomics (Fig. 5b, c, Supplementary Fig. 3). EXOC2, SLC12A5, and TSC2 showed significant (p value < 0.05) downregulation in AD patients in parallel to the progression of the disease in comparison to healthy individuals. Moreover, ANLN and NRGN showed significant (p value < 0.05) upregulation in AD patients in comparison to healthy individuals and controls (healthy individuals and VD/FTD patients). In addition, EXOC2, HECTD1, and SLC12A5 were found to be significantly downregulated in AD patients in comparison to controls (Fig. 5c). On the other hand, EXOC2, HECTD1, TSC2, and SCRN3 were found to be dysregulated mainly at advanced stages of the disease (Braak V and VI), whereas PPP1R14A was upregulated mainly at intermediate stages of AD (Braak IV), and SLC12A5, ANLN, BIN1, NRGN, and PMP2 were dysregulated during the ongoing course of the disease (Supplementary Fig. 3c), suggesting a potential role of these proteins in the development of AD. Noticeable, the other dysregulated proteins identified in the study showed similar non-significant dysregulation trends as observed by proteomics (p values ranging 0.07–0.18). Similar protein levels were observed between healthy individuals and FTD/VD patients for all proteins but TSC2, BIN1, PMP2, and PPP1R14A. While PMP2 and PPP1R14A showed an opposite dysregulation in AD patients and VD/FTD patients, TSC2 and BIN1 were dysregulated in the same sense in VD/FTD patients, but at a lower extent than AD patients (Fig. 5b, c, Supplementary Fig. 3c).
Next, for further confirmation of the dysregulation of these proteins in the brain of AD patients in comparison to controls, we performed an IHC analysis using TMAs containing 44 cores from AD patients at Braak stages IV-VI and VD, FTD, and healthy individuals as controls. We observed dysregulation for EXOC2, HECTD1, ANLN, BIN1, and SCRN3 when comparing AD patients’ samples with controls in agreement with proteomics and WB data (Fig. 5d). Specifically, SCRN3 was found to be significantly (p value < 0.05) upregulated in neurons from AD patients, and HECTD1 and EXOC2 significantly downregulated in AD patients’ glia. In addition, these proteins together with ANLN and BIN1 were also observed significantly dysregulated at AD patients’ glia or neurons at different Braak stages (Supplementary Fig. 3d).
Dysregulation validation in cellular models of AD and association to Aβ aggregates
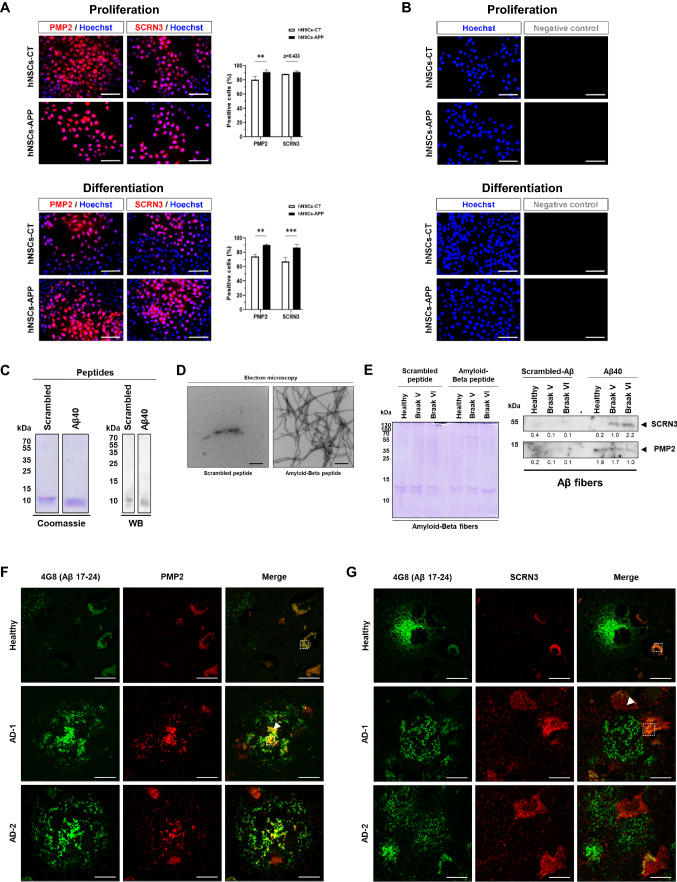
Next, we investigated if these proteins were also dysregulated in established cellular models of AD. hNS1 cells overexpressing APP were used as proliferative or differentiated neural cells, in comparison with control cells. This neural model is based on APP stimulated proliferation and differentiation of NSCs, and thus proteins found dysregulated in this model might be involved in AD-associated Aβ pathology. SCRN3 and PMP2 proteins were also found to be upregulated in neural cells overexpressing APP (Fig. 6a, b).
Fig. 6.
Association of dysregulated proteins with Aβ aggregates. A Immunofluorescence analysis of PMP2 and SCRN3 in control hNS1 cells (hNSCs-CT) and APP overexpressing hNS1 cells (hNSCs-APP) at proliferation and differentiation conditions. Scale bar: 50 µm. B Immunofluorescence negative control (no primary antibody) in control hNS1 cells (hNSCs-CT) and APP overexpressing hNS1 cells (hNSCs-APP) at proliferation (left) and differentiation (right) conditions. Scale bar: 50 µm. C Coomassie blue staining (left) and western blot (right) with streptavidin–HRP of the biotinylated scrambled and amyloid-beta peptides revealed the quality of the peptides used in the pull-down assay. D Representative TEM images of scrambled peptide and Aβ40 fibrils after the separate incubation of biotinylated scrambled and Aβ40 peptide. Scale bar: 200 nm. E Coomassie blue staining (top) and western blot (bottom) of proteins eluted from the pull-down with Aβ40 fibrils, where SCRN3 and PMP2 were found to interact with Aβ40 fibrils. F, G Immunofluorescence analysis of paraffin-embedded brain tissue samples from one AD patient at Braak V and one healthy individual. AD-1 and AD-2 denote two different plaques from the same FFPE tissue. F An association (co-localization) between PMP2 and Aβ plaques (4G8 antibody, aa 17–24 Aβ peptide), not observed in surrounded non-plaque tissue or in healthy individuals, was observed. AD-1, a plaque containing additionally lipofuscin-like staining is depicted in AD-1. AD-2, a plaque non-containing lipofuscin-like staining is shown. G In contrast, although SCRN3 showed no co-localization with Aβ plaques, cellular components in the surrounding of Aβ plaques showed a higher SCRN3 staining in comparison to healthy individuals, controls, and surrounding non-plaque tissue. See Supplementary Fig. 5 for lifetime images of PMP2 and SCRN3 staining that show the differential specific staining of both proteins in the tissue samples of AD patients from the unspecific autofluorescence staining of tissues (background) and lipofuscin-like structures. White dashed squares denote regions containing lipofuscin-like staining, whereas white arrows denote regions of specific staining of PMP2 or SCRN3, as observed in Supplementary Fig. 5. Scale bar: 20 µm. **p value < 0.01; ***p value < 0.001
Then, we wanted to address whether SCRN3 or PMP2 proteins could be associated with Aβ pathology, and thus, two subsequent analyses were performed. First, we investigated the role of SCRN3 and PMP2 as potential interactors of Aβ peptide aggregates. To this end, a pull-down assay was performed using pooled protein extracts from AD patients at Braak V and VI, where SCRN3 and PMP2 were accumulated, and extracts from healthy individuals. Prior to the pull-down assays, the quality of the biotinylated scrambled (negative control) and Aβ40 peptides was assessed by Coomassie blue staining and WB (Fig. 6c). TEM analysis confirmed the formation of Aβ40 fibrils from the biotinylated Aβ40 peptide in contrast to biotinylated scrambled-Aβ peptide (Fig. 6d). Then, biotinylated peptides incubated with the three pooled left prefrontal cortex brain tissue protein extracts were used for pull-down of Aβ40 fibrils interacting proteins (Fig. 6e). WB analysis of eluted interacting proteins with Aβ40 fibrils revealed the presence of SCRN3 and PMP2 among the proteins of the pull-down assays in comparison with the pull-down performed with the scrambled peptide. PMP2 from healthy, Braak V, and Braak VI protein extracts was observed to bind to Aβ40 fibrils and aggregates, in contrast to the scramble. Moreover, SCRN3 was observed to bind Aβ40 fibrils and aggregates at higher extents with Braak VI and Braak V. These results suggest that PMP2 probably possess higher ability than SCRN3 to interact with Aβ40 fibrils as depicted from the fact that PMP2 was observed in the pull-down assays with either healthy or AD protein samples in contrast to SCRN3 that was only observed with AD Braak V and VI samples, where SCRN3 was highly accumulated as observed by WB and proteomics analyses in comparison to healthy samples.
Second, we investigated whether PMP2 and SCRN3 could be interacting with Aβ plaques by IF using paraffin-embedded tissue samples from AD patients (Braak V) and healthy individuals. Tissue samples were simultaneously stained using 4G8 antibody (recognizing Aβ amyloid peptide amino acids 17-24) and SCRN3 or PMP2 antibodies. Interestingly, an enrichment in PMP2 could be observed in a diffuse pattern in the plaques. This enrichment was not observed in surrounding non-plaque tissue or in healthy individuals, where Aβ plaques were not observed (Fig. 6f). In contrast, we could not observe any co-localization of SCRN3 with Aβ plaques. However, an accumulation of SCRN3 was observed in cellular components within or close to Aβ plaques that was neither observed in the surrounding non-plaque tissue nor in healthy individuals (Fig. 6g and Supplementary Fig. 5). In addition, the absence of staining for IgG-AF647 secondary antibody in the negative controls confirmed the specificity of the staining observed for PMP2 and SCRN3 in AD FFPE tissue (Supplementary Fig. 5). Finally, to further assess the presence of specific IgG-AF647 signal over autofluorescence staining, lifetime images and λ spectra of the IgG-AF647 channel were obtained (Supplementary Fig. 5). These data allowed to discriminate between tissue autofluorescence and lipofuscin-like staining from the specific PMP2 or SCRN3 IgG-AF647 signal, confirming the previous IF results. In this sense, lipofuscin-like staining was observed in healthy individuals, whereas in AD patient’s lipofuscin-like staining and specific PMP2 or SCRN3 staining were observed (Fig. 6f, g and Supplementary Fig. 5c), confirming the IF specific staining of PMP2 and SCRN3 results. Collectively, our results suggest that although SCRN3 and PMP2 could interact with Aβ aggregates and fibrils in vitro, only PMP2 was observed to co-localize with Aβ plaques, suggesting PMP2 might be key in amyloid-β pathology and in the formation of amyloid-β plaques.
Diagnostic ability of the measurement of HECTD1 and SLC12A5 in plasma of AD patients
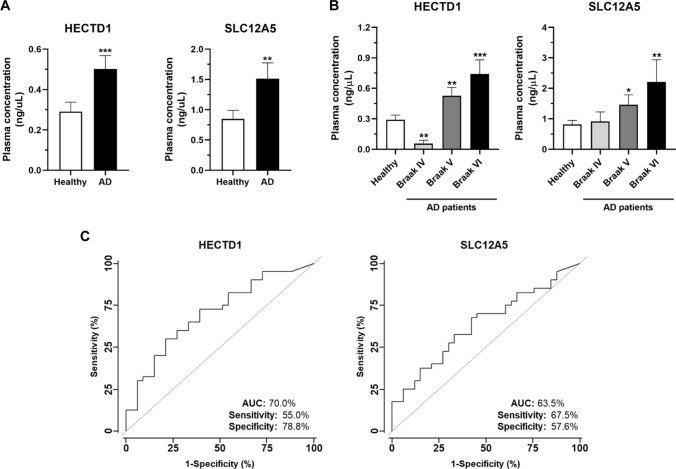
As an opposite dysregulation of EXOC2, HECTD1, TSC2, ANLN, BIN1, SLC12A5, PMP2, and PPP1R14A protein levels were found in the left prefrontal cortex and CSF of AD patients, we finally investigated if their protein dysregulation observed in CSF could also be observed in plasma samples, and thus some of these proteins could potentially possess diagnostic ability of the disease. We focused the analysis on HECTD1, SLC12A5, ANLN, and NRGN according to reagents’ availability.
Then, the potential accumulation of these four proteins in plasma of AD patients (n = 47) at Braak IV (MCI), and Braak V and VI (Clinical AD) was analyzed by ELISA in comparison to plasma samples from healthy individuals (n = 33). We found higher levels of HECTD1 and SLC12A5 in AD patients’ plasma samples than controls’ (Fig. 7a). Interestingly, the accumulation of both proteins in plasma increased in parallel to the progression of the disease (Fig. 7b). These results indicate a direct correlation between their low protein accumulation in brain of AD patients and a high clearance throughout CSF and blood. No differences in protein levels in plasma were found for ANLN or NRGN in AD patients in comparison to controls.
Fig. 7.
ELISA for HECTD1 and SLC12A5 quantification in plasma of AD patients and healthy individuals. A Graph bar of the protein concentration of HECTD1 and SLC12A5 in plasma samples of AD patients and healthy individuals. Both proteins, identified as downregulated in the left prefrontal cortex of AD patients, were found at higher levels in plasma of AD patients in comparison to healthy individuals. B Graph bar of HECTD1 and SLC12A5 concentration in plasma according to the progression of the disease. Plasma levels of both proteins were observed to increase in parallel to the progression of AD. C ROC curves analysis of the diagnostic value of HECTD1 and SLC12A5. Both proteins showed individual AUC values higher than 63%, and sensitivity and specificity values higher than 55%
Finally, ROC curves were obtained to determine the potential diagnostic ability of the quantification of HECTD1 and SLC12A5 protein levels in plasma. Both proteins showed AUC values above 60%. SLC12A5 reached an AUC of 63.5% and sensitivity and specificity of 67.5% and 57.6%, respectively, for discriminating AD patients from controls using plasma, whereas AUC, sensitivity, and specificity values of 70, 55, and 78.8% were obtained for HECTD1.
Collectively, the analysis by different orthogonal techniques of brain tissue samples from the left prefrontal cortex of AD patients and controls confirmed the dysregulation of NRGN, PMP2, PPP1R14A, ANLN, BIN1, SCRN3, EXOC2, SLC12A5, HECTD1, and TSC2 at mRNA and/or protein level in AD, the association of SCRN3 and PMP2 to Aβ fibers in vitro and PMP2 to Aβ plaques, and the potential AD diagnostic ability of HECTD1 and SLC12A5 as blood-based biomarkers.
Discussion
Alzheimer’s disease’s prevalence is increasing in developed countries as the life expectancy rises. Currently, there is no cure for AD or efficient treatments against the clinical symptoms associated with the pathology or the pathological changes that occurs during the progression of the disease. A better understanding of the mechanisms involved in the onset and development of AD might help to elucidate the molecular and functional pathways altered in the disease and to identify new therapeutic targets and markers for its early diagnosis.
Quantitative proteomics approaches are valuable tools for the identification of protein biomarkers associated with different pathologies with a high sensitivity and specificity, supporting the identification and study of biological pathways and networks in which those proteins are involved and that would be of interest. Microarrays, such as protein, antibody, or phage microarrays, are targeted analyses that allow the study of thousands of proteins at the same time using low sample volumes, and the identification of proteins differentially expressed as well as autoantigens specific of a disease, with the limitation of missing proteins, isoforms, or post-translational modifications (PTMs) non-printed onto the array [5, 61, 72]. In contrast, mass spectrometry analyses are non-targeted approaches that allow for the identification and quantification of thousands of proteins at the same time, including PTMs, only limited by the knowledge of the human proteome [21, 23].
Here, we performed a quantitative 10-plex TMT analysis comparing the protein profile of the left prefrontal cortex of AD patients at Braak IV, V, and VI, and controls, including healthy individuals and FTD and VD patients, to identify proteins specifically dysregulated in AD that could be involved in the onset or the progression of the disease, and to gain further insights into the pathways and functions altered in the pathology. In contrast to other mass spectrometry-based studies related to AD exploring this brain localization [60, 73], pooled samples instead of individual samples were preferred to try to find the dominant differences between AD patients and controls, and avoid as much as possible the identification of dysregulated proteins associated with individual samples because of the heterogeneity of the disease. The pooling strategy averaged biological variations associated with uncontrollable factors as diet, lifestyle, environmental factors, ante-mortem and pharmacological treatments, or end-of-life illnesses [60]. This pooling strategy has also been used in other different successful proteomics studies with validated data related to the pathology [60, 61, 73, 74]. Here, we followed a previously used pooling strategy using antibody microarrays to identify dysregulated proteins in the left prefrontal cortex of AD patients [61]. Despite the identification of interesting dysregulated proteins associated with AD with the pooling strategy that were further validated with individual samples to confirm their actual value and their association to the disease, the pooling strategy used in our Braak-dependent proteomics study might also present some disadvantages as (i) loss of information on the biological inter-individual variation, which we tried to minimize using homogeneous groups of controls and AD patients according to the Braak stage, or (ii) missed information regarding small changes between groups because of a dilution effect, and thus, providing information mostly related to major changes between AD and controls. Importantly, the survey of the levels of amyloidβ peptide (LVFFAEDVGSNK), APP, and MAPT in our proteomics dataset did not show significant differences among AD patients and controls, and hyperphosphorylated Tau peptides were not quantified in our proteomics dataset because of the absence of previous enrichment in phosphorylated peptides enrichment. These issues related to APP and MAPT have been previously observed in other proteomics studies focusing on the study of AD brain localizations different from the left prefrontal cortex [60]. Moreover, as amyloidβ peptide (LVFFAEDVGSNK) is also present in APP, a bias toward the quantification of trypsin-digested APP in these results, instead of the peptide derived from Aβ aggregates or plaques, could not be discarded. Furthermore, it has been described that although amyloid plaques (Aβ) and cerebrovascular amyloid (Aβ) and neurofibrillary tangles (Tau) can be purified away from other insoluble components and separated from each other under specific conditions such as sequential differential ultracentrifugation and solubilization [75–78], they resist solubilization with the detergents used in this work (Triton X-100 and SDS). Moreover, it has also been reported that Triton X-100 and SDS enhance aggregation of Aβ fibers and NFTs [77], and thus in our proteomics approach a broad solubilization and digestion of Aβ fibers and/or plaques and hyperphosphorylated Tau aggregates could have not occurred. These issues might have mitigated the observed differences for amyloidβ peptide, APP, and MAPT between AD and controls, which could have been overcome by using differential fractionation and solubilization, or laser microcapture and harsh solubilization of Aβ plaques and hyperphosphorylated Tau NFTs [79, 80].
In these observations, several results pointed out to a good performance of the non-targeted proteomics approach followed in the study. First, biological replicates behaved similarly, as observed by PCA. Second, the depth into the proteome was similar to that achieved with individual samples in other AD-related proteomics studies [60, 81]. Third, validation of the data was in agreement with proteomics results. In this regard, and as one of the goals of the study was to generate validated data on the here identified dysregulated proteins involved in the pathology, the verification of the proteomics results by using individual AD samples and controls and orthogonal techniques, such as IF, IHC, WB, ELISA, or qPCR, represent one of the major achievements of the study. In this sense, ten proteins were selected for further investigation as more prone to be involved in AD pathology. By in silico analysis, it was observed that the ten candidate proteins showed dysregulation at different extents in the frontal cortical tissue of AD patients [60], showing full or partial concordance of HECTD1, TSC2, EXOC2, SLC12A5, NRGN, and ANLN with our proteomics dataset of the left prefrontal cortex. In addition, recent proteomics studies also revealed downregulation of HECTD1, SLC12A5, and TSC2 in the frontal cortex of AD patients [73, 82, 83], whereas ANLN was found to be upregulated and SLC12A5 and EXOC2 downregulated in the dorsolateral prefrontal cortex (DLPFC) of patients [84], as here observed by proteomics and western blot analysis in the left prefrontal cortex of AD patients. These results suggest that these proteins showing concordant or opposite protein dysregulation at different brain regions might be highly involved in the development of the disease. Besides, all the proteins here identified and validated as dysregulated in AD, but TSC2 and BIN1, were found to be specifically dysregulated in AD patients, not associated with VD or FTD dementias, suggesting that these proteins might have a potential role in AD development. In addition, we also found dysregulation at mRNA level for all of them but PMP2 at different Braak stages of the disease, with EXOC2 and SLC12A5 levels downregulated at Braak IV or V, and PPP1R14A mRNA levels upregulated at late stages Braak V and VI of the disease. Interestingly, we found similar mRNA levels for HECTD1, TSC2, ANLN, NRGN, and PMP2 between VD and Braak IV AD patients. As many AD patients also present vascular lesions, mRNA dysregulations might be common to both pathologies even though these similarities were not observed at protein level [85, 86].
Interestingly, some of the proteins here identified and validated as altered in AD have been previously associated with AD or other brain diseases, or as involved in different processes closely related to the disease. Anillin (ANLN) has been described as upregulated in cerebral atherosclerosis [36]. In addition, ANLN is also described as overexpressed in different cancers, such as colorectal, breast, or lung cancer, where it is associated with a higher metastatic ability and poor prognosis [87–89]. Single nucleotide polymorphisms (SNPs) in the Myc box-dependent-interacting protein 1 (BIN1 or Amphiphysin 2) gene have been associated with a lower survival of AD patients and as genetic risk factors for the development of AD. In addition, BIN1 was previously described as dysregulated in AD and associated with Tau and Aβ plaques neuropathology, favoring the internalization of Tau aggregates and endocytosis of APP, precursor of Aβ peptide, through clathrin-mediated endocytosis [90–92]. Neurogranin (NRGN) has been described as a postsynaptic marker upregulated in both AD affected tissues and CSF of AD patients [93–95]. E3 ubiquitin-protein ligase HECTD1 plays a role in base excision repair processes in response to DNA damage through its interaction with DNA-damage binding protein 1 (DDB1), with the latter being described to interact with APP [96, 97]. Exocyst complex component 2 (EXOC2 or Sec5) is critical to neuronal function, and it is involved in the tethering and fusion of vesicles to the plasma membrane. Mutations in this protein have been associated with defects in human brain development [98]. Solute carrier family 12 member 5 (SLC12A5) is a potassium chloride cotransporter located in neurons’ membranes and required for neuronal Cl− homeostasis [99]. Variations in solute carriers have been associated with neurological and neuropsychiatric disorders. Specifically, SLC12A5 overexpression has been associated with Huntington disease, and, due to its effect on GABAergic neurotransmission, SLC12A5 downregulation might be implicated in AD [100–103]. Tuberous sclerosis 2 protein (TSC2) regulates cell metabolic activity and growth through the regulation of mTOR, PTEN–AKT pathway, and is downregulated in the prefrontal cortex of AD patients, although their role in the pathogenesis of AD remains unclear [104–106]. On the contrary, peripheral myelin protein 2 (PMP2), protein phosphatase 1 regulatory subunit 14A (PPP1R14A or CPI-17), and secernin-3 (SCRN3) have not been previously associated with any neuropathological disorder, although PMP2 and PPP1R14A are involved in myelination regulation and thus could play a major role in the disease [107, 108]. In addition, SCRN3 has been found in intracellular vesicles, and therefore it could be involved in cell–cell brain communication.
Remarkably, by WB and pull-down assays, we found interaction between aggregates of Aβ peptides in vitro and PMP2 and SCRN3 proteins, and ex vivo by IF using FFPE tissues for PMP2 with Aβ plaques. These results suggest an association of SCRN3 and PMP2 with Aβ peptide aggregation and potentially with the Aβ pathology during the progression of AD. Additionally, it can be hypothesized that PMP2 might be involved in the accumulation of Aβ aggregates, either as a cause or a consequence of amyloid plaque pathology and the cognitive impairment associated with the disease [109, 110]. In addition, using cell models of AD pathology by overexpressing APP, we found similar protein expression changes for SCRN3 and PMP2 to that observed in AD brain tissue samples of patients, which confirmed an important role of SCRN3 and PMP2 in AD. Moreover, it could also be speculated that the observed localized expression of SCRN3 in cellular components close or within the plaques by IF, without co-localization with amyloid-β plaques, could be somehow modulated by any component of Aβ plaques. In contrast, another secernin—SCRN1—was recently identified as an amyloid plaque-associated protein using localized proteomics [111, 112]. SCRN1 was shown to associate with neurofibrillary tangles in AD, co-localize with pTau, and accumulate in AD and not in other tauopathies [71]. Therefore, these results, together with the observation that SCRN3 binds to Aβ aggregates in vitro and accumulates in AD, suggest a role of the secernin family of proteins in AD that deserve to be further investigated.
Finally, we found statistically significant higher levels of HECTD1 and SLC12A5 proteins in plasma of AD patients in comparison to healthy individuals. These proteins were described here as downregulated in the brain of patients, suggesting a higher clearance of these proteins through the CSF, mainly due to neural death or brain injury. Therefore, the measurement of the protein concentration of HECTD1 and SLC12A5 in plasma might help in combination with other markers in the early detection of the disease [4, 5, 113, 114]. However, further research involving a higher cohort of plasma samples from AD patients at early stages of the disease (Braak I-III) is needed.
Despite exciting observations on dysregulated proteins identified and quantified as novel proteins associated with AD, our study has some limitations. As indicated, in our AD TMT-based Braak-dependent proteomics study, we used pooled samples, and accordingly, conclusions might be biased toward major proteomic changes between AD and controls with missed information regarding small changes between groups because of a dilution effect. Other limitations of the study are related to (i) the use of VD and FTD patients as controls, which could produce the identification in our dataset of some falsely down- or upregulated proteins in AD out of the validated dysregulated proteins, because some proteins or pathways may be specifically altered in VD and FTD patients inducing any bias in the control group, (ii) the lack of AD samples from patients at initial stages of the disease (Braak I-III), which could allow to confirm the dysregulation of these novel proteins in the development and progression of the disease, and (iii) the number of samples used for validation of HECTD1 and SLC12A5 as plasma biomarkers, where additional analyses with a larger number of samples from different repositories, hospitals, and biobanks would allow to further demonstrate their usefulness as diagnostic biomarkers of AD.
Conclusions
In summary, in this study, we identified by quantitative proteomics and validated by orthogonal techniques the dysregulation in AD at mRNA and/or protein level by different techniques of ANLN, BIN1, EXOC2, NRGN, PMP2, PPP1R14A, SCRN3, SLC12A5, and TSC2. Thus, further functional analyses of these highly dysregulated proteins in AD are ensured to determine whether these proteins might be interesting targets of intervention of the disease. Specifically, EXOC2, HECTD1, SLC12A5, and TSC2 were downregulated, whereas ANLN, BIN1, NRGN, PMP2, PPP1R14A, and SCRN3 showed upregulation in tissue samples. Among them, it was remarkable to demonstrate that HECTD1 and SLC12A5 proteins were found increased in the blood of AD patients, showing their quantification in plasma statistically significant ability in discriminating AD from controls. Besides, the association of SCRN3 and PMP2 with Aβ aggregates, and PMP2 with Aβ plaques indicates interesting new proteins associated with AD and amyloid-β pathology.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Supplementary Fig. 1. MaxQuant data normalization. Left, box plots of the mean of log2 protein intensities before (top) and after (bottom) data normalization of each TMT reporter. Right, histogram of the log2 protein intensities for each TMT reporter before (top) and after (bottom) data normalization. After SL normalization, box plots and density distributions of each TMT reporter were aligned. file1 (PPTX 465 KB)
Supplementary Supplementary Fig. 2. Bioinformatics analysis of APP, MAPT, and dysregulated proteins in AD. A Graph bar representing the TMT relative protein abundance of APP, MAPT or amyloidβ peptide in the individual analyzed samples including duplicates of healthy individuals, VD, FTD patients, Braak IV, Braak V and Braak VI, as obtained from mass spectrometry analysis, confirmed higher protein levels of APP and MAPT in AD samples. B Venn Diagram analysis of the significantly downregulated (left) or upregulated (right) proteins as obtained by ANOVA and Post Hoc correction regarding AD vs healthy and DV/FTD, Braak V vs healthy and Braak VI vs healthy comparisons. C Genome-wide overview of reactome pathways. Significant pathways in which the 171 significantly dysregulated proteins are involved were represented in yellow (p value < 0.05). Light grey represents pathways with no significant representation. file2 (PPTX 1251 KB)
Supplementary Supplementary Fig. 3. Validation of mRNA and protein dysregulation of the 10 candidate proteins. A Differences at mRNA expression levels in healthy individuals, VD and FTD patients, Braak IV, Braak V and Braak VI AD groups were separately found for the 10 selected proteins, with HECTD1 significantly downregulated in AD patients and PPP1R14A, SCRN3, ANLN, BIN1 and NRGN statistically significant upregulated in patients. B Coomassie-blue staining (upper panel) and Ponceau red staining (lower panel) for quality control of individual protein extracts of the left prefrontal cortex brain tissue samples of AD patients and controls and for normalization of the total protein content per sample used for WB validation, respectively. C Statistically significant differences at protein level as observed by WB were separately found among healthy individuals and VD and FTD patients, and AD patients at Braak IV, Braak V and Braak VI for HECTD1, EXOC2, SLC12A5, TSC2, ANLN, NRGN and SCRN3. D Immunohistochemistry analysis revealed dysregulation of ANLN, BIN1, SCRN3, EXOC2 and HECTD1 protein levels at each Braak stage in comparison to healthy individuals. *: p value < 0.05; **: p value < 0.01. ***: p value < 0.001; ****: p value < 0.0001; n.s.: not significant; CT: controls; AD: AD patients file3 (PPTX 3532 KB)
Supplementary Supplementary Fig. 4. Analysis of the proteins specifically dysregulated at Braak V or Braak VI in comparison with Healthy and VD and FTD patients. A Venn diagram analysis of the significantly dysregulated proteins at Braak V (left) or Braak VI (right) stages obtained by ANOVA and Post Hoc correction and t-test comparison between AD and controls or healthy individuals. A total of 34 and 15 proteins were identified as dysregulated at Braak V or Braak VI, respectively. B Genome-wide overview of reactome pathways significantly altered at Braak V or Braak VI AD stages. Significant pathways were represented in yellow (p value < 0.05). Light grey represents pathways with no significant representation file4 (PPTX 1034 KB)
Supplementary Supplementary Fig. 5. Immunofluorescence staining of human Aβ plaques. A Negative control of immunofluorescence experiment. AD Braak V tissue was incubated with 4G8 and its corresponding secondary antibody (Alexa Fluor 555) and with Alexa Fluor 647 to confirm the specific staining of PMP2 and SCRN3 in AD tissue samples. B Immunofluorescence images of SCRN3 staining showing the nuclear staining with Hoechst for the confirmation of the presence of SCRN3 within the cells. C Lifetime images of SCRN3 and PMP2 staining showed the differential specific staining of both proteins in the tissue samples of AD patients and healthy individuals from the autofluorescence of tissue and lipofuscin-like structures. Shorter lifetime: positive Alexa Fluor 647 staining; Longer lifetime: lipofuscin-like structures; Scale bar: 20 µm. D λ spectra of Alexa-Fluor 647 from lipofuscin-like regions (AF) and specific 647 signals (PMP2 or SCRN3) regions as indicated in Fig. 6f,g. AF: autofluorescence. file5 (PDF 260 KB)
Acknowledgements
The protein identification by nLC–MS/MS was carried out in the Proteomics and Genomics Facility (CIB-CSIC), a member of ProteoRed-ISCIII network.
Abbreviations
- AD
Alzheimer’s disease
- ANLN
Anillin
- Aβ
Amyloid-β
- BIN1
Myc box-dependent-interacting protein 1
- CSF
Cerebrospinal fluid
- DLPFC
Dorsolateral prefrontal cortex
- EXOC2
Exocyst complex component 2
- FTD
Frontotemporal dementia
- GFP
Green fluorescence protein
- HECTD1
E3 ubiquitin-protein ligase
- hNSCs
Multipotent neural stem cells
- HPC
High pathology controls
- HSC
Human stem cell
- IF
Immunofluorescence
- IHC
Immunohistochemistry
- LC–MS/MS
Liquid chromatography coupled with tandem mass spectrometry
- LPC
Low-pathology controls
- MBs
Streptavidin magnetic beads
- MCI
Mild cognitive impairment
- NFT
Neurofibrillary tangles
- NRGN
Neurogranin
- PMP2
Peripheral myelin protein 2
- PPP1R14A
Protein phosphatase 1 regulatory subunit 14A
- qPCR
Real-time quantitative PCR
- ROC
Receiver operating characteristic curve
- SCRN3
Secernin-3
- SLC12A5
Solute carrier family 12 member 5
- TMT
Tandem mass tags
- TSC2
Tuberous sclerosis 2 protein
- VD
Vascular dementia
- WB
Western blot
Authors’ contributions
Conceptualization: AM-C and RB. Methodology: AM-C, G.S-F, RC, AR, VR, AP-G, IL, and RB. Investigation: AM-C, RC, MG-A, GS-F, VR, MJF-A, MM, JM-U, DM, MTM-C, and AP-G. Writing—original draft: AM-C and RB. Writing—review and editing: AM-C, GS-F, RC, MG-A, AR, VR, MJF-A, MM, JM-U, DM, MTM-C, AP-G; IL, and RB. Resources: AR, VR, MM, IL, and RB. Supervision: AM-C, MJF-A, AP-G, IL, and RB. Funding acquisition: IL, and RB. All authors read and approved the manuscript.
Funding
This work was supported by the financial support of the PI17CIII/00045 and PI20CIII/00019 grants from the AES-ISCIII program to R.B. The FPU predoctoral contract to A.M-C. is supported by the Spanish Ministerio de Educación, Cultura y Deporte. G.S-F. is recipient of a predoctoral contract (grant number 1193818N) supported by The Flanders Research Foundation (FWO). M. G-A. is recipient of a Margarita Salas postdoctoral grant for the requalification of the Spanish university system.
Availability of data and materials
The mass spectrometry proteomics data have been deposited at the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD030751.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
The Institutional Ethical Review Board of the Spanish Research Center for Neurological Diseases Foundation (CIEN) and the Instituto de Salud Carlos III approved this study on proteomics analysis and biomarker discovery of Alzheimer’s disease (CEI PI 49).
Consent for publication
All authors of this article give consent for publication of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377(9770):1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 2.Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer's disease. Nat Rev Dis Primers. 2015;1:15056. doi: 10.1038/nrdp.2015.56. [DOI] [PubMed] [Google Scholar]
- 3.(2020) 2020 Alzheimer's disease facts and figures. Alzheimers Dement 16(3):391–460 [DOI] [PubMed]
- 4.Montero-Calle A, San Segundo-Acosta P, Garranzo-Asensio M, Rabano A, Barderas R. The molecular misreading of APP and UBB induces a humoral immune response in Alzheimer's disease patients with diagnostic ability. Mol Neurobiol. 2020;57(2):1009–1020. doi: 10.1007/s12035-019-01809-0. [DOI] [PubMed] [Google Scholar]
- 5.San Segundo-Acosta P, Montero-Calle A, Fuentes M, Rabano A, Villalba M, Barderas R. Identification of Alzheimer's disease autoantibodies and their target biomarkers by phage microarrays. J Proteome Res. 2019;18(7):2940–2953. doi: 10.1021/acs.jproteome.9b00258. [DOI] [PubMed] [Google Scholar]
- 6.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorsell A, Bjerke M, Gobom J, Brunhage E, Vanmechelen E, Andreasen N, Hansson O, Minthon L, Zetterberg H, Blennow K. Neurogranin in cerebrospinal fluid as a marker of synaptic degeneration in Alzheimer's disease. Brain Res. 2010;1362:13–22. doi: 10.1016/j.brainres.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 8.Lane CA, Hardy J, Schott JM. Alzheimer's disease. Eur J Neurol. 2018;25(1):59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 9.Murphy MP, LeVine H., 3rd Alzheimer's disease and the amyloid-beta peptide. J Alzheimers Dis. 2010;19(1):311–323. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hampel H, Hardy J, Blennow K, Chen C, Perry G, Kim SH, Villemagne VL, Aisen P, Vendruscolo M, Iwatsubo T, et al. The amyloid-beta pathway in Alzheimer's disease. Mol Psychiatry. 2021 doi: 10.1038/s41380-021-01249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ono M, Watanabe H, Kitada A, Matsumura K, Ihara M, Saji H. Highly selective Tau-SPECT imaging probes for detection of neurofibrillary tangles in Alzheimer's disease. Sci Rep. 2016;6:34197. doi: 10.1038/srep34197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer's disease. Mol Neurodegener. 2019;14(1):32. doi: 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goedert M, Spillantini MG. A century of Alzheimer's disease. Science. 2006;314(5800):777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 14.Tonnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer's disease. J Alzheimers Dis. 2017;57(4):1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sery O, Povova J, Misek I, Pesak L, Janout V. Molecular mechanisms of neuropathological changes in Alzheimer's disease: a review. Folia Neuropathol. 2013;51(1):1–9. doi: 10.5114/fn.2013.34190. [DOI] [PubMed] [Google Scholar]
- 16.Domon B, Aebersold R. Mass spectrometry and protein analysis. Science. 2006;312(5771):212–217. doi: 10.1126/science.1124619. [DOI] [PubMed] [Google Scholar]
- 17.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422(6928):198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Aguilar J, Chik J, Nicholson J, Semaan C, McKay MJ, Molloy MP. Quantitative mass spectrometry for colorectal cancer proteomics. Proteom Clin Appl. 2013;7(1–2):42–54. doi: 10.1002/prca.201200080. [DOI] [PubMed] [Google Scholar]
- 19.Babel I, Barderas R, Diaz-Uriarte R, Martinez-Torrecuadrada JL, Sanchez-Carbayo M, Casal JI. Identification of tumor-associated autoantigens for the diagnosis of colorectal cancer in serum using high density protein microarrays. Mol Cell Proteom. 2009;8(10):2382–2395. doi: 10.1074/mcp.M800596-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barderas R, Babel I, Casal JI. Colorectal cancer proteomics, molecular characterization and biomarker discovery. Proteom Clin Appl. 2010;4(2):159–178. doi: 10.1002/prca.200900131. [DOI] [PubMed] [Google Scholar]
- 21.Barderas R, Mendes M, Torres S, Bartolome RA, Lopez-Lucendo M, Villar-Vazquez R, Pelaez-Garcia A, Fuente E, Bonilla F, Casal JI. In-depth characterization of the secretome of colorectal cancer metastatic cells identifies key proteins in cell adhesion, migration, and invasion. Mol Cell Proteom. 2013;12(6):1602–1620. doi: 10.1074/mcp.M112.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garranzo-Asensio M, San Segundo-Acosta P, Poves C, Fernandez-Acenero MJ, Martinez-Useros J, Montero-Calle A, Solis-Fernandez G, Sanchez-Martinez M, Rodriguez N, Ceron MA, et al. Identification of tumor-associated antigens with diagnostic ability of colorectal cancer by in-depth immunomic and seroproteomic analysis. J Proteom. 2020;214:103635. doi: 10.1016/j.jprot.2020.103635. [DOI] [PubMed] [Google Scholar]
- 23.Mendes M, Pelaez-Garcia A, Lopez-Lucendo M, Bartolome RA, Calvino E, Barderas R, Casal JI. Mapping the spatial proteome of metastatic cells in colorectal cancer. Proteomics. 2017;17(19):1700094. doi: 10.1002/pmic.201700094. [DOI] [PubMed] [Google Scholar]
- 24.Salat DH, Kaye JA, Janowsky JS. Selective preservation and degeneration within the prefrontal cortex in aging and Alzheimer disease. Arch Neurol. 2001;58(9):1403–1408. doi: 10.1001/archneur.58.9.1403. [DOI] [PubMed] [Google Scholar]
- 25.Franzmeier N, Hartmann J, Taylor ANW, Araque-Caballero MA, Simon-Vermot L, Kambeitz-Ilankovic L, Burger K, Catak C, Janowitz D, Muller C, et al. The left frontal cortex supports reserve in aging by enhancing functional network efficiency. Alzheimers Res Ther. 2018;10(1):28. doi: 10.1186/s13195-018-0358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franzmeier N, Duering M, Weiner M, Dichgans M, Ewers M. Alzheimer's disease neuroimaging I: Left frontal cortex connectivity underlies cognitive reserve in prodromal Alzheimer disease. Neurology. 2017;88(11):1054–1061. doi: 10.1212/WNL.0000000000003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 28.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 29.Goedert M, Ghetti B, Spillantini MG. Frontotemporal dementia: implications for understanding Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(2):a006254. doi: 10.1101/cshperspect.a006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jobson DD, Hase Y, Clarkson AN, Kalaria RN. The role of the medial prefrontal cortex in cognition, ageing and dementia. Brain Commun. 2021;3(3):fcab125. doi: 10.1093/braincomms/fcab125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vieira RT, Caixeta L, Machado S, Silva AC, Nardi AE, Arias-Carrion O, Carta MG. Epidemiology of early-onset dementia: a review of the literature. Clin Pract Epidemiol Ment Health. 2013;9:88–95. doi: 10.2174/1745017901309010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogan DB, Jette N, Fiest KM, Roberts JI, Pearson D, Smith EE, Roach P, Kirk A, Pringsheim T, Maxwell CJ. The prevalence and incidence of frontotemporal dementia: a systematic review. Can J Neurol Sci. 2016;43(Suppl 1):S96–S109. doi: 10.1017/cjn.2016.25. [DOI] [PubMed] [Google Scholar]
- 34.Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci. 2017;131(11):1059–1068. doi: 10.1042/CS20160607. [DOI] [PubMed] [Google Scholar]
- 35.Wolters FJ, Ikram MA. Epidemiology of vascular dementia. Arterioscler Thromb Vasc Biol. 2019;39(8):1542–1549. doi: 10.1161/ATVBAHA.119.311908. [DOI] [PubMed] [Google Scholar]
- 36.Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Perez JM, Evans AC. Alzheimer's disease neuroimaging I: early role of vascular dysregulation on late-onset Alzheimer's disease based on multifactorial data-driven analysis. Nat Commun. 2016;7:11934. doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noor A, Zafar S, Zerr I. Neurodegenerative proteinopathies in the proteoform spectrum-tools and challenges. Int J Mol Sci. 2021;22(3):1085. doi: 10.3390/ijms22031085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez-Martin P, Avila J, Investigators ADRU. Alzheimer Center Reina Sofia Foundation: fighting the disease and providing overall solutions. J Alzheimers Dis. 2010;21(2):337–348. doi: 10.3233/JAD-2010-101149. [DOI] [PubMed] [Google Scholar]
- 39.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8(1):1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123(1):1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirra SS, Hart MN, Terry RD. Making the diagnosis of Alzheimer's disease. A primer for practicing pathologists. Arch Pathol Lab Med. 1993;117(2):132–144. [PubMed] [Google Scholar]
- 42.Zea-Sevilla MA, Fernandez-Blazquez MA, Calero M, Bermejo-Velasco P, Rabano A. Combined Alzheimer's disease and cerebrovascular staging explains advanced dementia cognition. Alzheimers Dement. 2015;11(11):1358–1366. doi: 10.1016/j.jalz.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Liste I, Garcia-Garcia E, Bueno C, Martinez-Serrano A. Bcl-XL modulates the differentiation of immortalized human neural stem cells. Cell Death Differ. 2007;14(11):1880–1892. doi: 10.1038/sj.cdd.4402205. [DOI] [PubMed] [Google Scholar]
- 44.Liste I, Garcia-Garcia E, Martinez-Serrano A. The generation of dopaminergic neurons by human neural stem cells is enhanced by Bcl-XL, both in vitro and in vivo. J Neurosci. 2004;24(48):10786–10795. doi: 10.1523/JNEUROSCI.3208-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coronel R, Lachgar M, Bernabeu-Zornoza A, Palmer C, Dominguez-Alvaro M, Revilla A, Ocana I, Fernandez A, Martinez-Serrano A, Cano E, et al. Neuronal and glial differentiation of human neural stem cells is regulated by amyloid precursor protein (APP) Levels. Mol Neurobiol. 2019;56(2):1248–1261. doi: 10.1007/s12035-018-1167-9. [DOI] [PubMed] [Google Scholar]
- 46.Coronel R, Palmer C, Bernabeu-Zornoza A, Monteagudo M, Rosca A, Zambrano A, Liste I. Physiological effects of amyloid precursor protein and its derivatives on neural stem cell biology and signaling pathways involved. Neural Regen Res. 2019;14(10):1661–1671. doi: 10.4103/1673-5374.257511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wisniewski JR, Gaugaz FZ. Fast and sensitive total protein and peptide assays for proteomic analysis. Anal Chem. 2015;87(8):4110–4116. doi: 10.1021/ac504689z. [DOI] [PubMed] [Google Scholar]
- 48.Lopez-Janeiro A, Ruz-Caracuel I, Ramon-Patino JL, De Los RV, Villalba Esparza M, Berjon A, Yebenes L, Hernandez A, Masetto I, Kadioglu E, et al. Proteomic analysis of low-grade, early-stage endometrial carcinoma reveals new dysregulated pathways associated with cell death and cell signaling. Cancers. 2021;13(4):794. doi: 10.3390/cancers13040794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garranzo-Asensio M, Rodriguez-Cobos J, San Millan C, Poves C, Fernandez-Acenero MJ, Pastor-Morate D, Vinal D, Montero-Calle A, Solis-Fernandez G, Ceron MA, et al. In-depth proteomics characterization of Np73 effectors identifies key proteins with diagnostic potential implicated in lymphangiogenesis, vasculogenesis and metastasis in colorectal cancer. Mol Oncol. 2022;16(14):2672–2692. doi: 10.1002/1878-0261.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Exploring data normalization and analysis in large TMT experimental designs [https://pwilmart.github.io/IRS_normalization/understanding_IRS.html]. Accessed 13 Oct 2019
- 51.Tan H, Yang K, Li Y, Shaw TI, Wang Y, Blanco DB, Wang X, Cho JH, Wang H, Rankin S, et al. integrative proteomics and phosphoproteomics profiling reveals dynamic signaling networks and bioenergetics pathways underlying T cell activation. Immunity. 2017;46(3):488–503. doi: 10.1016/j.immuni.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Borel C, Li L, Muller T, Williams EG, Germain PL, Buljan M, Sajic T, Boersema PJ, Shao W, et al. Systematic proteome and proteostasis profiling in human Trisomy 21 fibroblast cells. Nat Commun. 2017;8(1):1212. doi: 10.1038/s41467-017-01422-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diz AP, Carvajal-Rodriguez A, Skibinski DO. Multiple hypothesis testing in proteomics: a strategy for experimental work. Mol Cell Proteom. 2011;10(3):M110.004374. doi: 10.1074/mcp.M110.004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jimenez A, Lu D, Kalocsay M, Berberich MJ, Balbi P, Jambhekar A, Lahav G. Time-series transcriptomics and proteomics reveal alternative modes to decode p53 oscillations. Mol Syst Biol. 2022;18(3):e10588. doi: 10.15252/msb.202110588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pelaez-Garcia A, Barderas R, Batlle R, Vinas-Castells R, Bartolome RA, Torres S, Mendes M, Lopez-Lucendo M, Mazzolini R, Bonilla F, et al. A proteomic analysis reveals that Snail regulates the expression of the nuclear orphan receptor Nuclear Receptor Subfamily 2 Group F Member 6 (Nr2f6) and interleukin 17 (IL-17) to inhibit adipocyte differentiation. Mol Cell Proteom. 2015;14(2):303–315. doi: 10.1074/mcp.M114.045328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC, et al. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35:169–175. doi: 10.1093/nar/gkm415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4(5):P3. [PubMed] [Google Scholar]
- 59.Perez-Llamas C, Lopez-Bigas N. Gitools: analysis and visualisation of genomic data using interactive heat-maps. PLoS ONE. 2011;6(5):e19541. doi: 10.1371/journal.pone.0019541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bai B, Wang X, Li Y, Chen PC, Yu K, Dey KK, Yarbro JM, Han X, Lutz BM, Rao S, et al. Deep multilayer brain proteomics identifies molecular networks in Alzheimer's disease progression. Neuron. 2020;105(6):975–991 e977. doi: 10.1016/j.neuron.2019.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garranzo-Asensio M, San Segundo-Acosta P, Martinez-Useros J, Montero-Calle A, Fernandez-Acenero MJ, Haggmark-Manberg A, Pelaez-Garcia A, Villalba M, Rabano A, Nilsson P, et al. Identification of prefrontal cortex protein alterations in Alzheimer's disease. Oncotarget. 2018;9(13):10847–10867. doi: 10.18632/oncotarget.24303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bardou P, Mariette J, Escudie F, Djemiel C, Klopp C. jvenn: an interactive Venn diagram viewer. BMC Bioinform. 2014;15:293. doi: 10.1186/1471-2105-15-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson ECB, Dammer EB, Duong DM, Ping L, Zhou M, Yin L, Higginbotham LA, Guajardo A, White B, Troncoso JC, et al. Large-scale proteomic analysis of Alzheimer's disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat Med. 2020;26(5):769–780. doi: 10.1038/s41591-020-0815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shukla V, Skuntz S, Pant HC. Deregulated Cdk5 activity is involved in inducing Alzheimer's disease. Arch Med Res. 2012;43(8):655–662. doi: 10.1016/j.arcmed.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adav SS, Park JE, Sze SK. Quantitative profiling brain proteomes revealed mitochondrial dysfunction in Alzheimer's disease. Mol Brain. 2019;12(1):8. doi: 10.1186/s13041-019-0430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jayapalan S, Subramanian D, Natarajan J. Computational identification and analysis of neurodegenerative disease associated protein kinases in hominid genomes. Genes Dis. 2016;3(3):228–237. doi: 10.1016/j.gendis.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bolognin S, Lorenzetto E, Diana G, Buffelli M. The potential role of rho GTPases in Alzheimer's disease pathogenesis. Mol Neurobiol. 2014;50(2):406–422. doi: 10.1007/s12035-014-8637-5. [DOI] [PubMed] [Google Scholar]
- 68.Kapoor A, Nation DA. Role of Notch signaling in neurovascular aging and Alzheimer's disease. Semin Cell Dev Biol. 2021;116:90–97. doi: 10.1016/j.semcdb.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woo HN, Park JS, Gwon AR, Arumugam TV, Jo DG. Alzheimer's disease and Notch signaling. Biochem Biophys Res Commun. 2009;390(4):1093–1097. doi: 10.1016/j.bbrc.2009.10.093. [DOI] [PubMed] [Google Scholar]
- 70.Buhl E, Kim YA, Parsons T, Zhu B, Santa-Maria I, Lefort R, Hodge JJL. Effects of Eph/ephrin signalling and human Alzheimer's disease-associated EphA1 on Drosophila behaviour and neurophysiology. Neurobiol Dis. 2022;170:105752. doi: 10.1016/j.nbd.2022.105752. [DOI] [PubMed] [Google Scholar]
- 71.Pires G, McElligott S, Drusinsky S, Halliday G, Potier MC, Wisniewski T, Drummond E. Secernin-1 is a novel phosphorylated tau binding protein that accumulates in Alzheimer's disease and not in other tauopathies. Acta Neuropathol Commun. 2019;7(1):195. doi: 10.1186/s40478-019-0848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montero-Calle A, Aranguren-Abeigon I, Garranzo-Asensio M, Poves C, Fernández-Aceñero MJ, Martínez-Useros J, Sanz R, Dziaková J, Rodriguez-Cobos J, Solís-Fernández G, et al. Multiplexed biosensing diagnostic platforms detecting autoantibodies to tumor-associated antigens from exosomes released by CRC cells and tissue samples showed high diagnostic ability for colorectal cancer. Engineering. 2021;7(10):1393. doi: 10.1016/j.eng.2021.04.026. [DOI] [Google Scholar]
- 73.Bai B, Vanderwall D, Li Y, Wang X, Poudel S, Wang H, Dey KK, Chen PC, Yang K, Peng J. Proteomic landscape of Alzheimer's Disease: novel insights into pathogenesis and biomarker discovery. Mol Neurodegener. 2021;16(1):55. doi: 10.1186/s13024-021-00474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li KW, Ganz AB, Smit AB. Proteomics of neurodegenerative diseases: analysis of human post-mortem brain. J Neurochem. 2019;151(4):435–445. doi: 10.1111/jnc.14603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rudrabhatla P, Jaffe H, Pant HC. Direct evidence of phosphorylated neuronal intermediate filament proteins in neurofibrillary tangles (NFTs): phosphoproteomics of Alzheimer's NFTs. FASEB J. 2011;25(11):3896–3905. doi: 10.1096/fj.11-181297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roher AE, Chaney MO, Kuo YM, Webster SD, Stine WB, Haverkamp LJ, Woods AS, Cotter RJ, Tuohy JM, Krafft GA, et al. Morphology and toxicity of Abeta-(1–42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer's disease. J Biol Chem. 1996;271(34):20631–20635. doi: 10.1074/jbc.271.34.20631. [DOI] [PubMed] [Google Scholar]
- 77.Esparza TJ, Wildburger NC, Jiang H, Gangolli M, Cairns NJ, Bateman RJ, Brody DL. Soluble amyloid-beta aggregates from human Alzheimer's disease brains. Sci Rep. 2016;6:38187. doi: 10.1038/srep38187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lutz BM, Peng J. Deep Profiling of the Aggregated Proteome in Alzheimer's Disease: From Pathology to Disease Mechanisms. Proteomes. 2018;6(4):46. doi: 10.3390/proteomes6040046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fasman GD, Perczel A, Moore CD. Solubilization of beta-amyloid-(1–42)-peptide: reversing the beta-sheet conformation induced by aluminum with silicates. Proc Natl Acad Sci U S A. 1995;92(2):369–371. doi: 10.1073/pnas.92.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rayaprolu S, Higginbotham L, Bagchi P, Watson CM, Zhang T, Levey AI, Rangaraju S, Seyfried NT. Systems-based proteomics to resolve the biology of Alzheimer's disease beyond amyloid and tau. Neuropsychopharmacology. 2021;46(1):98–115. doi: 10.1038/s41386-020-00840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Higginbotham L, Ping L, Dammer EB, Duong DM, Zhou M, Gearing M, Hurst C, Glass JD, Factor SA, Johnson ECB, et al. Integrated proteomics reveals brain-based cerebrospinal fluid biomarkers in asymptomatic and symptomatic Alzheimer's disease. Sci Adv. 2020 doi: 10.1126/sciadv.aaz9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sathe G, Na CH, Renuse S, Madugundu AK, Albert M, Moghekar A, Pandey A. Quantitative proteomic profiling of cerebrospinal fluid to identify candidate biomarkers for Alzheimer's disease. Proteom Clin Appl. 2019;13(4):e1800105. doi: 10.1002/prca.201800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Z, Yu K, Tan H, Wu Z, Cho JH, Han X, Sun H, Beach TG, Peng J. 27-Plex tandem mass tag mass spectrometry for profiling brain proteome in Alzheimer's disease. Anal Chem. 2020;92(10):7162–7170. doi: 10.1021/acs.analchem.0c00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 1999;13(Suppl 3):S115–123. doi: 10.1097/00002093-199912003-00017. [DOI] [PubMed] [Google Scholar]
- 86.Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer's disease–lessons from pathology. BMC Med. 2014;12:206. doi: 10.1186/s12916-014-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou W, Wang Z, Shen N, Pi W, Jiang W, Huang J, Hu Y, Li X, Sun L. Knockdown of ANLN by lentivirus inhibits cell growth and migration in human breast cancer. Mol Cell Biochem. 2015;398(1–2):11–19. doi: 10.1007/s11010-014-2200-6. [DOI] [PubMed] [Google Scholar]
- 88.Wang G, Shen W, Cui L, Chen W, Hu X, Fu J. Overexpression of Anillin (ANLN) is correlated with colorectal cancer progression and poor prognosis. Cancer Biomark. 2016;16(3):459–465. doi: 10.3233/CBM-160585. [DOI] [PubMed] [Google Scholar]
- 89.Xu J, Zheng H, Yuan S, Zhou B, Zhao W, Pan Y, Qi D. Overexpression of ANLN in lung adenocarcinoma is associated with metastasis. Thorac Cancer. 2019;10(8):1702–1709. doi: 10.1111/1759-7714.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karch CM, Goate AM. Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015;77(1):43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tan MS, Yu JT, Tan L. Bridging integrator 1 (BIN1): form, function, and Alzheimer's disease. Trends Mol Med. 2013;19(10):594–603. doi: 10.1016/j.molmed.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 92.Shen R, Zhao X, He L, Ding Y, Xu W, Lin S, Fang S, Yang W, Sung K, Spencer B, et al. Upregulation of RIN3 induces endosomal dysfunction in Alzheimer's disease. Transl Neurodegener. 2020;9(1):26. doi: 10.1186/s40035-020-00206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Portelius E, Zetterberg H, Skillback T, Tornqvist U, Andreasson U, Trojanowski JQ, Weiner MW, Shaw LM, Mattsson N, Blennow K, et al. Cerebrospinal fluid neurogranin: relation to cognition and neurodegeneration in Alzheimer's disease. Brain. 2015;138(Pt 11):3373–3385. doi: 10.1093/brain/awv267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Remnestal J, Just D, Mitsios N, Fredolini C, Mulder J, Schwenk JM, Uhlen M, Kultima K, Ingelsson M, Kilander L, et al. CSF profiling of the human brain enriched proteome reveals associations of neuromodulin and neurogranin to Alzheimer's disease. Proteomics Clin Appl. 2016;10(12):1242–1253. doi: 10.1002/prca.201500150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pereira JB, Janelidze S, Ossenkoppele R, Kvartsberg H, Brinkmalm A, Mattsson-Carlgren N, Stomrud E, Smith R, Zetterberg H, Blennow K, et al. Untangling the association of amyloid-beta and tau with synaptic and axonal loss in Alzheimer's disease. Brain. 2021;144(1):310–324. doi: 10.1093/brain/awaa395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmidt MF, Gan ZY, Komander D, Dewson G. Ubiquitin signalling in neurodegeneration: mechanisms and therapeutic opportunities. Cell Death Differ. 2021;28(2):570–590. doi: 10.1038/s41418-020-00706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bennett L, Madders E, Parsons JL. HECTD1 promotes base excision repair in nucleosomes through chromatin remodelling. Nucleic Acids Res. 2020;48(3):1301–1313. doi: 10.1093/nar/gkz1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Bergen NJ, Ahmed SM, Collins F, Cowley M, Vetro A, Dale RC, Hock DH, de Caestecker C, Menezes M, Massey S, et al. Mutations in the exocyst component EXOC2 cause severe defects in human brain development. J Exp Med. 2020 doi: 10.1084/jem.20192040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arroyo JP, Kahle KT, Gamba G. The SLC12 family of electroneutral cation-coupled chloride cotransporters. Mol Aspects Med. 2013;34(2–3):288–298. doi: 10.1016/j.mam.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 100.Hu C, Tao L, Cao X, Chen L. The solute carrier transporters and the brain: physiological and pharmacological implications. Asian J Pharm Sci. 2020;15(2):131–144. doi: 10.1016/j.ajps.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ayka A, Sehirli AO. The role of the SLC transporters protein in the neurodegenerative disorders. Clin Psychopharmacol Neurosci. 2020;18(2):174–187. doi: 10.9758/cpn.2020.18.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Savas JN, Wang YZ, DeNardo LA, Martinez-Bartolome S, McClatchy DB, Hark TJ, Shanks NF, Cozzolino KA, Lavallee-Adam M, Smukowski SN, et al. Amyloid accumulation drives proteome-wide alterations in mouse models of Alzheimer's disease-like pathology. Cell Rep. 2017;21(9):2614–2627. doi: 10.1016/j.celrep.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Doshina A, Gourgue F, Onizuka M, Opsomer R, Wang P, Ando K, Tasiaux B, Dewachter I, Kienlen-Campard P, Brion JP, et al. Cortical cells reveal APP as a new player in the regulation of GABAergic neurotransmission. Sci Rep. 2017;7(1):370. doi: 10.1038/s41598-017-00325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ferrando-Miguel R, Rosner M, Freilinger A, Lubec G, Hengstschlager M. Tuberin–a new molecular target in Alzheimer's disease? Neurochem Res. 2005;30(11):1413–1419. doi: 10.1007/s11064-005-8511-y. [DOI] [PubMed] [Google Scholar]
- 105.Habib SL, Michel D, Masliah E, Thomas B, Ko HS, Dawson TM, Abboud H, Clark RA, Imam SZ. Role of tuberin in neuronal degeneration. Neurochem Res. 2008;33(6):1113–1116. doi: 10.1007/s11064-007-9558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan E, Tsai PT, Greene-Colozzi E, Sahin M, Kwiatkowski DJ, Malinowska IA. Graded loss of tuberin in an allelic series of brain models of TSC correlates with survival, and biochemical, histological and behavioral features. Hum Mol Genet. 2012;21(19):4286–4300. doi: 10.1093/hmg/dds262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stettner M, Zenker J, Klingler F, Szepanowski F, Hartung HP, Mausberg AK, Kleinschnitz C, Chrast R, Kieseier BC. The role of peripheral myelin protein 2 in remyelination. Cell Mol Neurobiol. 2018;38(2):487–496. doi: 10.1007/s10571-017-0494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takizawa N, Koga Y, Ikebe M. Phosphorylation of CPI17 and myosin binding subunit of type 1 protein phosphatase by p21-activated kinase. Biochem Biophys Res Commun. 2002;297(4):773–778. doi: 10.1016/s0006-291x(02)02302-1. [DOI] [PubMed] [Google Scholar]
- 109.Drummond E, Kavanagh T, Pires G, Marta-Ariza M, Kanshin E, Nayak S, Faustin A, Berdah V, Ueberheide B, Wisniewski T. The amyloid plaque proteome in early onset Alzheimer's disease and down syndrome. Acta Neuropathol Commun. 2022;10(1):53. doi: 10.1186/s40478-022-01356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Malek-Ahmadi M, Perez SE, Chen K, Mufson EJ. Neuritic and diffuse plaque associations with memory in non-cognitively impaired elderly. J Alzheimers Dis. 2016;53(4):1641–1652. doi: 10.3233/JAD-160365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Drummond E, Wisniewski T. The use of localized proteomics to identify the drivers of Alzheimer's disease pathogenesis. Neural Regen Res. 2017;12(6):912–913. doi: 10.4103/1673-5374.208570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Drummond E, Nayak S, Faustin A, Pires G, Hickman RA, Askenazi M, Cohen M, Haldiman T, Kim C, Han X, et al. Proteomic differences in amyloid plaques in rapidly progressive and sporadic Alzheimer's disease. Acta Neuropathol. 2017;133(6):933–954. doi: 10.1007/s00401-017-1691-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.San Segundo-Acosta P, Montero-Calle A, Jernbom-Falk A, Alonso-Navarro M, Pin E, Andersson E, Hellstrom C, Sanchez-Martinez M, Rabano A, Solis-Fernandez G, et al. Multiomics profiling of alzheimer's disease serum for the identification of autoantibody biomarkers. J Proteom Res. 2021;20(11):5115–5130. doi: 10.1021/acs.jproteome.1c00630. [DOI] [PubMed] [Google Scholar]
- 114.Valverde A, Montero-Calle A, Arévalo B, San Segundo-Acosta P, Serafín V, Alonso-Navarro M, Solís-Fernández G, Pingarron J, Campuzano S, Barderas R. Phage-derived and aberrant HaloTag peptides immobilized on magnetic microbeads for amperometric biosensing of serum autoantibodies and Alzheimer's disease diagnosis. Anal Sens. 2021;1(4):161–165. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Supplementary Fig. 1. MaxQuant data normalization. Left, box plots of the mean of log2 protein intensities before (top) and after (bottom) data normalization of each TMT reporter. Right, histogram of the log2 protein intensities for each TMT reporter before (top) and after (bottom) data normalization. After SL normalization, box plots and density distributions of each TMT reporter were aligned. file1 (PPTX 465 KB)
Supplementary Supplementary Fig. 2. Bioinformatics analysis of APP, MAPT, and dysregulated proteins in AD. A Graph bar representing the TMT relative protein abundance of APP, MAPT or amyloidβ peptide in the individual analyzed samples including duplicates of healthy individuals, VD, FTD patients, Braak IV, Braak V and Braak VI, as obtained from mass spectrometry analysis, confirmed higher protein levels of APP and MAPT in AD samples. B Venn Diagram analysis of the significantly downregulated (left) or upregulated (right) proteins as obtained by ANOVA and Post Hoc correction regarding AD vs healthy and DV/FTD, Braak V vs healthy and Braak VI vs healthy comparisons. C Genome-wide overview of reactome pathways. Significant pathways in which the 171 significantly dysregulated proteins are involved were represented in yellow (p value < 0.05). Light grey represents pathways with no significant representation. file2 (PPTX 1251 KB)
Supplementary Supplementary Fig. 3. Validation of mRNA and protein dysregulation of the 10 candidate proteins. A Differences at mRNA expression levels in healthy individuals, VD and FTD patients, Braak IV, Braak V and Braak VI AD groups were separately found for the 10 selected proteins, with HECTD1 significantly downregulated in AD patients and PPP1R14A, SCRN3, ANLN, BIN1 and NRGN statistically significant upregulated in patients. B Coomassie-blue staining (upper panel) and Ponceau red staining (lower panel) for quality control of individual protein extracts of the left prefrontal cortex brain tissue samples of AD patients and controls and for normalization of the total protein content per sample used for WB validation, respectively. C Statistically significant differences at protein level as observed by WB were separately found among healthy individuals and VD and FTD patients, and AD patients at Braak IV, Braak V and Braak VI for HECTD1, EXOC2, SLC12A5, TSC2, ANLN, NRGN and SCRN3. D Immunohistochemistry analysis revealed dysregulation of ANLN, BIN1, SCRN3, EXOC2 and HECTD1 protein levels at each Braak stage in comparison to healthy individuals. *: p value < 0.05; **: p value < 0.01. ***: p value < 0.001; ****: p value < 0.0001; n.s.: not significant; CT: controls; AD: AD patients file3 (PPTX 3532 KB)
Supplementary Supplementary Fig. 4. Analysis of the proteins specifically dysregulated at Braak V or Braak VI in comparison with Healthy and VD and FTD patients. A Venn diagram analysis of the significantly dysregulated proteins at Braak V (left) or Braak VI (right) stages obtained by ANOVA and Post Hoc correction and t-test comparison between AD and controls or healthy individuals. A total of 34 and 15 proteins were identified as dysregulated at Braak V or Braak VI, respectively. B Genome-wide overview of reactome pathways significantly altered at Braak V or Braak VI AD stages. Significant pathways were represented in yellow (p value < 0.05). Light grey represents pathways with no significant representation file4 (PPTX 1034 KB)
Supplementary Supplementary Fig. 5. Immunofluorescence staining of human Aβ plaques. A Negative control of immunofluorescence experiment. AD Braak V tissue was incubated with 4G8 and its corresponding secondary antibody (Alexa Fluor 555) and with Alexa Fluor 647 to confirm the specific staining of PMP2 and SCRN3 in AD tissue samples. B Immunofluorescence images of SCRN3 staining showing the nuclear staining with Hoechst for the confirmation of the presence of SCRN3 within the cells. C Lifetime images of SCRN3 and PMP2 staining showed the differential specific staining of both proteins in the tissue samples of AD patients and healthy individuals from the autofluorescence of tissue and lipofuscin-like structures. Shorter lifetime: positive Alexa Fluor 647 staining; Longer lifetime: lipofuscin-like structures; Scale bar: 20 µm. D λ spectra of Alexa-Fluor 647 from lipofuscin-like regions (AF) and specific 647 signals (PMP2 or SCRN3) regions as indicated in Fig. 6f,g. AF: autofluorescence. file5 (PDF 260 KB)
Data Availability Statement
The mass spectrometry proteomics data have been deposited at the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD030751.