Abstract
Following myocardial ischemic injury, the most effective clinical intervention is timely restoration of blood perfusion to ischemic but viable myocardium to reduce irreversible myocardial necrosis, limit infarct size, and prevent cardiac insufficiency. However, reperfusion itself may exacerbate cell death and myocardial injury, a process commonly referred to as ischemia/reperfusion (I/R) injury, which primarily involves cardiomyocytes and cardiac microvascular endothelial cells (CMECs) and is characterized by myocardial stunning, microvascular damage (MVD), reperfusion arrhythmia, and lethal reperfusion injury. MVD caused by I/R has been a neglected problem compared to myocardial injury. Clinically, the incidence of microvascular angina and/or no-reflow due to ineffective coronary perfusion accounts for 5–50% in patients after acute revascularization. MVD limiting drug diffusion into injured myocardium, is strongly associated with the development of heart failure. CMECs account for > 60% of the cardiac cellular components, and their role in myocardial I/R injury cannot be ignored. There are many studies on microvascular obstruction, but few studies on microvascular leakage, which may be mainly due to the lack of corresponding detection methods. In this review, we summarize the clinical manifestations, related mechanisms of MVD during myocardial I/R, laboratory and clinical examination means, as well as the research progress on potential therapies for MVD in recent years. Better understanding the characteristics and risk factors of MVD in patients after hemodynamic reconstruction is of great significance for managing MVD, preventing heart failure and improving patient prognosis.
Keywords: Microvascular damage, Microvascular obstruction, Microvascular leakage, Microvascular endothelial cells
Introduction
In recent decades, global environmental changes, modification of modern lifestyles, such as the Western diet and physical inactivity, and increased population aging are all closely related to people's health [1, 2]. As a result, the prevalence of metabolic risk factors, including hypertension, dyslipidemia, hyperglycemia/insulin resistance, and overweight/obesity, has increased significantly worldwide [3]. These risk factors lead to the development of a single metabolic disorder into a metabolic syndrome, and eventually to the development of many comorbidities, one of which is cardiovascular disease (CVD) [3]. Each year, more people die from CVD than from any other causes [4]. In 2019, 17.9 million people died from CVD, representing 32% of global deaths [5]. Moreover, according to the World Health Organization report, it is estimated that about 23.6 million people will die from CVD by 2030 [4]. Globally, ischemic heart disease (IHD) has the highest burden and the mortality among all forms of CVD [3, 6], including acute myocardial infarction (AMI), unstable angina, chronic IHD and its associated heart failure [7, 8]. Such stark facts raise an alarm and call all governments and cardiologic professionals around the world working together to seek and establishing effective strategies for the prevention and treatment of IHD.
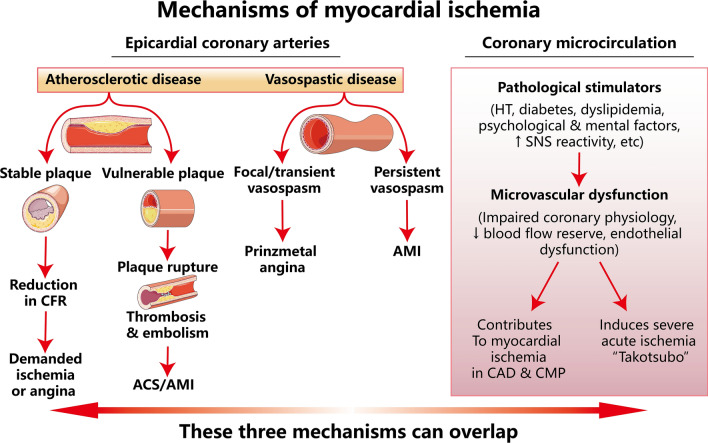
The coronary arteries consist of epicardial coronary arteries (diameter > 400 µm) and coronary microcirculation [9]. The latter are usually present in vessels < 400 μm in diameter and are not visible on coronary angiography. Different from the epicardial coronary arteries, coronary microcirculation is composed of small arteries (diameter < 400 µm), arterioles (diameter < 100 µm), and capillaries (diameter < 10 µm) [10]. Epicardial coronary arteries carry out functions that include delivery of blood, oxygen, and nutrients and removal of waste products from the blood [11]. Coronary microcirculation is responsible for regulating blood flow distribution, modulating vascular resistance, and exchanging oxygen and nutrients in the heart [12]. Figure 1 elucidates the mechanisms of myocardial ischemia induced by a variety of pathophysiological conditions. Atherosclerotic plaque formation and rupture are the primary cause of coronary artery disease (CAD)/myocardial ischemia, according to an intravascular ultrasound substudy of the WISE study, 79% of patients had coronary atherosclerosis [13]. Non-obstructive CAD including epicardial coronary spasm and microvascular dysfunction account for 20–50% of myocardial ischemia [14, 15]. Epicardial CAD is commonly classified into atherosclerotic disease and vasospastic disease. Atherosclerotic lesion includes stable plaque and vulnerable plaque. The former reduces coronary flow reserve (CFR) and eventually leads to demand ischemia/angina, while vulnerable plaques are prone to rupture which results in thrombosis/embolism, the primary cause of acute coronary syndromes (ACS)/permanent MI. Coronary vasospasm can trigger prinzmetal angina by focal/transient vasospasm or permanent MI by persistent vasospasm. Coronary microcirculation dysfunction, mainly caused by pathological stimulations, such as hypertension, diabetes, dyslipidemia, psychological and mental factors as well as increased sympathetic nervous system reactivity etc., can impair coronary physiology, decrease CFR and damage endothelial function. Coronary microcirculation dysfunction not only contributes to myocardial ischemia in CAD and cardiomyopathy, but also induces severe acute ischemia, e.g., ‘Takotsubo’. Moreover, these three mechanisms can overlap, leading to permanent myocardial necrosis [9]. The coronary perfusion territory distal to the site of the occlusion is referred to as the area at risk of infarction. Within a given area at risk, both the duration and the severity of coronary blood flow reduction determine the nature and amount of injury [16]. The most effective clinical intervention currently available is timely restoration of blood perfusion to ischemic but viable myocardium to reduce irreversible myocardial necrosis, limit infarct size and prevent cardiac insufficiency [17–19]. However, reperfusion itself may exacerbate cell death and myocardial injury, a process commonly referred to as myocardial ischemia/reperfusion (I/R) injury [19], which primarily involves cardiomyocytes and cardiac microvascular endothelial cells (CMECs) [20] and is characterized by myocardial stunning, microvascular damage (MVD), reperfusion arrhythmia, and lethal reperfusion injury [21]. MI size is the major determinants of mortality, heart failure and arrhythmias after AMI [22–24]. Sustained damage to myocardial tissue and coronary microvasculature by reperfusion is a complex multifactorial process that is estimated to cause up to 50% of the final infarct size [25]. Therefore, myocardial I/R injury substantially limits the clinical benefit of reperfusion strategies [20, 26].
Fig. 1.
Mechanisms of myocardial ischemia. (modified from [9] with a permission license 5,623,560,707,414) CFR coronary flow reserve, ACS acute coronary syndromes, AMI acute myocardial infarction, HT hypertension, SNS sympathetic nervous system, CMP cardiomyopathy
I/R-induced coronary microcirculatory disturbances have been a neglected problem compared to myocardial injury [26–28]. Clinically, the incidence of microvascular angina and/or no-reflow due to ineffective coronary perfusion accounts for 5–50% in patients after acute revascularization [26]. MVD limiting drug diffusion into the damaged myocardium, is strongly associated with the development of heart failure, and few drugs are currently available for its treatment.
Over the past few decades, scientists have investigated underlying mechanisms and various therapeutic strategies to protect the heart against I/R injury. To date, the translation of cardioprotection from basic research findings to the clinic is still much less than expected. One reason for this is due to the focus of research has been largely on the cardiomyocyte itself, with a considerable degree of neglect in coronary microcirculation which actually plays a critical role in cardiomyocyte survival and maintenance of cardiac function. Therefore, to understand the characteristics and risk factors of coronary MVD owing to I/R injury as well as the pathological changes in CMECs is of great significance for preventing coronary microvascular dysfunction and improving patient prognosis [18, 29].
Classification of microvascular damage after myocardial I/R injury
All human vascular lumens consist of a single layer of ECs, vascular smooth muscle cells (VSMCs) and pericytes [10]. The surface area of ECs on the human surface is > 1000 m2 [30], which plays an important role in vascular tone, fluid balance between the lumen and tissues, macromolecule and cellular permeability, as well as in coagulation and angiogenesis [31]. CMECs account for > 60% of the cardiac cellular components, and their role in myocardial ischemic injury cannot be ignored [32]. A systematic review provides comprehensive analysis on coronary microvascular regulatory mechanisms involving mechanical determinants, direct endothelium effectors, humoral mediators and neuronal mechanisms [33]. The molecular mechanisms of coronary MVD following I/R injury are not fully understood, and its manifestations may include microvascular obstruction (MVO), also known as "no-reflow" phenomenon, and microvascular leakage (MVL).
Microvascular obstruction
According to the latest European Society of Cardiology international guidelines, direct percutaneous coronary intervention (PCI) is the preferred reperfusion strategy in patients with acute ST-segment elevation MI (STEMI) [34], and up to 95% of clinically occluded coronary arteries can be reopened in this manner [35]. However, despite the restoration of infarct-related arterial blood flow, epicardial blood flow is slow or inadequate due to the onset of MVO and consequent inadequate tissue perfusion, a phenomenon known as coronary artery “non-reflow” [18, 36]. I/R induced swelling of CMECs, reduced blood perfusion, and a change in local fluid dynamics from laminar to turbulent flow, which promotes erythrocyte aggregation, platelet plug and thrombosis as well as inflammatory cell embolism to block blood and nutrients diffuse to myocytes (Fig. 2) [37]. In addition, Heusch and coworkers reported that the accumulation of atherosclerotic debris and soluble substances (such as serotonin, thromboxane B2 and TNFα) released from ruptured plaques also plays a key role in microvascular perfusion [38]. Clinical data further support that an increase in the size of the no-reflow zone limits blood flow in the area at risk to about 44% of the baseline flow before the onset of myocardial ischemia [39], and similar correlations have been reported in animal models [40, 41]. Statistically, MVO can affect 11–41% of STEMI patients treated with PCI, significantly reduces the beneficial effects of reperfusion therapy, leads to poorer clinical and functional outcomes, and is an independent predictor of increased infarct extent and 5-year mortality [42–44], while the MI size is not independently associated with adverse events [45, 46]. In addition, clinical studies have shown that advanced age, delayed reperfusion, lower systolic blood pressure (SBP), left ventricular ejection fraction (LVEF) and myocardial blush grade (MBG) at admission, and higher Killip's grade also increase the risk of no-reflow [47].
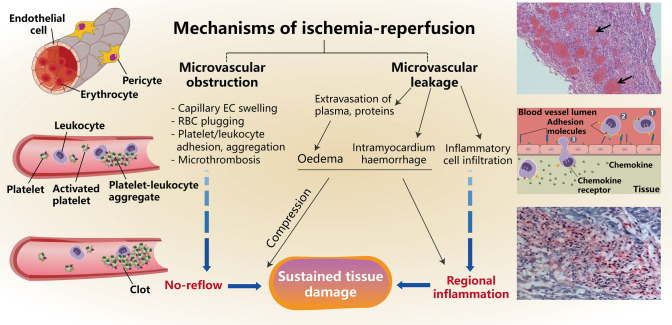
Fig. 2.
Pathological mechanisms of microvascular damage following myocardium ischemia/reperfusion
Microvascular leakage
The inner surface of the coronary microvasculature consists of a single layer of CMECs [10]. The barrier function and permeability of microvasculature to water or other molecules depend on the gap junction structure between CMECs, the degree of encapsulation of the basement membrane and pericytes outside CMECs [48, 49]. Under physiological conditions, water and solute transport across capillaries is mainly carried out through gap junctions between CMECs. The gaps between CMECs account for approximately 0.2% of the cell surface area, and the permeability of the gaps is maintained by an intercellular junction device and adherents, composed of multiple cell adhesion molecules and various connexins of CMECs, which form a tight junction structure [48, 49]. These molecules bind to each other and are maintained with cytoskeletal proteins, which in turn form stable intercellular junctions. Experimental studies have found that myocardial I/R injury damages CMECs and barrier function which led to hyperpermeability of microvasculature. Extravasation of blood components and immune cells through the dysfunctional barrier results in intramyocardial hemorrhage (IMH), interstitial edema and local inflammatory responses, a phenomenon known as MVL (Fig. 2) [50]. In addition, the MVO, namely, the no-reflow phenomenon, will be further aggravated by IMH and the force of external compression owing to interstitial edema caused by MVL. It seems from the histological analysis [51] that no-reflow is always in the area of the infarcted tissue [52], but the size of MVL is significantly larger than the infarcted or no-reflow area [50].
MVD worsens in a time-dependent manner during I/R injury [10], which expands the infarct size and increases perioperative mortality in patients treated with revascularization [53]. Thus, despite the great success of treatments to reduce post-ischemic cardiomyocyte injury, exploration of more mechanistic studies and therapeutic agents are needed to solve the cardiac microvascular dysfunction during myocardial I/R injury. So far, most studies on MVD after myocardial I/R have been limited to single-center studies. Table 1 shows the incidence of MVD after I/R and its corresponding diagnostic method in clinical studies searched on PubMed over the past 10 years. The incidence of MVO varied among diagnostic methods, with 39.5% detected by ECG and 2.1–42.0% by CAG, while cardiac magnetic resonance (CMR) was the most sensitive, detecting 35.2–72.2% of MVO. Currently, MVL is only indirectly reflected in the detection of IMH and edema by CMR. The incidence of IMH was 33.6–50.0% and edema was 30.8–72.1% (refer to Table 1).
Table 1.
Incidence of MVD after myocardial I/R and diagnostic methods in clinical studies over the past 10 years
| Events | No. of patients | Event present | Diagnostic method | Incidence % | References |
|---|---|---|---|---|---|
| MVO | 569 | 225 | ECG | 39.5 | Erkol et al. (2014) [54] |
| 16 | 6 | CAG | 37.5 | Grygier et al. (2014) [55] | |
| 569 | 179 | 31.5 | Erkol et al. (2014) [54] | ||
| 120 | 14 | 11.7 | Li et al. (2015) [56] | ||
| 5997 | 128 | 2.1 | Cenko et al. (2016) [57] | ||
| 105 | 15 | 14.3 | Chen et al. (2016) [58] | ||
| 86 | 31 | 36.0 | Durante et al. (2017) [59] | ||
| 733 | 54 | 16.1 | Liang et al. (2017) [60] | ||
| 203 | 38 | 18.7 | Li et al. (2018) [61] | ||
| 100 | 27 | 2.7 | Mahmoud et al. (2019) [62] | ||
| 1658 | 491 | 42.0 | Yang et al. (2020) [63] | ||
| 173 | 24 | 13.9 | Rossington et al. (2020) [64] | ||
| 118 | 41 | 34.7 | Sadeghian et al. (2022) [65] | ||
| 909 | 101 | 11.1 | d'Entremont et al. (2023) [66] | ||
| 774 | 381 | CMR | 49.2 | Eitel et al. (2013) [67] | |
| 281 | 99 | 35.2 | Hadamitzky et al. (2014) [68] | ||
| 151 | 100 | 66.0 | Kandler et al. (2014) [69] | ||
| 108 | 78 | 72.2 | Ding et al. (2015) [70] | ||
| 55 | 39 | 70.9 | Kim et al. (2015) [71] | ||
| 55 | 35 | 63.6 | Desch et al. (2016) [72] | ||
| 245 | 133 | 54.3 | Carrick et al. (2016) [73] | ||
| 63 | 38 | 60.3 | Nazir et al. (2016) [74] | ||
| 86 | 58 | 67.4 | Durante et al. (2017) [59] | ||
| 1688 | 960 | 56.9 | Kloner et al. (2017) [75] | ||
| 98 | 60 | 61.2 | Broch et al. (2021) [76] | ||
| 234 | 125 | 53.4 | Bulluck et al. (2022) [77] | ||
| 170 | 94 | 55.3 | Tiller et al. (2022) [78] | ||
| 122 | 51 | 41.8 | Li et al. (2022) [79] | ||
| IMH | 699 | 243 | CMR | 34.8 | Eitel et al. (2013) [67] |
| 304 | 102 | 33.6 | Husser et al. (2013) [80] | ||
| 151 | 76 | 50.0 | Kandler et al. (2014) [69] | ||
| 108 | 53 | 49.0 | Ding et al. (2015) [70] | ||
| 38 | 16 | 42.1 | Nazir et al. (2016) [74] | ||
| 30 | 13 | 43.0 | Carrick et al. (2016) [81] | ||
| 245 | 101 | 41.2 | Carrick et al. (2016) [73] | ||
| 170 | 59 | 34.7 | Tiller et al. (2022) [78] | ||
| 234 | 96 | 41.0 | Bulluck et al. (2022) [77] | ||
| Edema | 197 | 109 | CMR | 55.3 | Nazir et al. (2016) [74] |
| 86 | 62 | 72.1 | Durante et al. (2017) [59] | ||
| 800 | 246 | 30.8 | Garg et al. (2017) [82] |
MVD microvascular damage, MVO microvascular obstruction, I/R ischemia/reperfusion, ECG electrocardiogram, CAG coronary artery angiography, CMR cardiac magnetic resonance, IMH intramyocardial hemorrhage
Molecular mechanisms of microvascular damage after myocardial I/R injury
The overall mechanism of coronary MVD including MVO and MVL is not fully understood, and microcirculatory blockage and microvascular hyperpermeability are the possible driven forces involved. At present, MVO following myocardial I/R injury have been extensively studied, but few studies focus on MVL [83]. In the following sections, we will provide an overview on the possible mechanisms based on current knowledge.
Impaired microvascular function
To date, many studies have recognized that dysfunction or death of CMECs is an essential cause of cardiac MVD following I/R injury [37, 84, 85]. When coronary artery obstruction occurs, blood supply and oxygen are drastically reduced and aerobic ATP is decreased, causing dysfunction of the cell membrane Na+–K+ pump, which in turn leads to intracellular water and sodium retention. Thus, during reperfusion the cells in the ischemic area swell and compress the micro-vessels, while the swollen CMECs protrude into the lumen causing luminal narrowing and obstructing blood perfusion. The development of edema during reperfusion is characterized by bi-modal changes, with an early peak after 120 min which coincided with the beginning of the inflammatory response and followed by a near disappearance of edema after 24 h, and a second peak occurred after 7 days, which coincided with the deposition of collagen [86].
The tension of coronary microvasculature is regulated by the balance between vasodilators, e.g., bradykinin and nitric oxide (NO) and vasoconstrictors, e.g., endothelin-1 (ET-1), both are released by CMECs [10]. CMECs promote vascular relaxation by synthesizing and releasing NO via endothelial nitric oxide synthase (eNOS). However, after myocardial I/R injury, mitochondria-derived superoxide induces the oxidation of the eNOS co-factor tetrahydrobiopterin (BH4) to dihydrobiopterin, which prevents BH4 from binding to eNOS, thereby uncoupling eNOS and reducing the production of NO [87]. In addition, nicotinamide adenine dinucleotide phosphate oxidases (NOXs), the main enzymes that generate reactive oxygen species (ROS), are expressed in ECs as four different NOX isoforms, superoxide-generating NOX1, NOX2, and NOX5 as well as hydrogen peroxide-generating NOX4 [88]. NOX-derived ROS can reduce NO bioavailability by oxidizing NO to peroxynitrite, a highly reactive and vasoconstrictive ROS species [10]. Consequently, downregulation of phosphorylated eNOS expression, rapid upregulation of endothelial-type vasoconstrictor ET-1 expression, and decreased NO production and utilization lead to microvascular diastolic dysfunction. Moreover, increased levels of ROS in CMECSs promote angiotensin II type-1 (AT-1) receptor-mediated vasoconstriction [89]. Although, in general, coronary artery constriction during hypoxia is an adaptive response to accelerate blood flow, MVO limits sufficient nutrients to cardiomyocytes. Clinically, Neil and coworkers have observed an elevated levels of vasoconstrictor (thromboxane A2, ET-1, and neuropeptide Y) in coronary sinus blood immediately after PCI, and the level of neuropeptide Y was closely associated with norepinephrine-mediated vasoconstriction and the index of microcirculatory resistance (IMR) elevation, which in turn led to more severe MVO [90].
Besides the dysfunction of CMECs, it was found that pericytes, a type of cell that surrounds endothelial cells in microvasculature and veins [91] are also involved in MVD and repair after I/R through the following approaches (1) monitoring and stabilizing the maturation process of CMECs through cellular communication via physical contact and paracrine signaling [92]; (2) directly contract to reduce microvascular blood flow during I/R [93], which can be reversed by adenosine [93]; (3) participating in the inflammatory response after I/R by releasing inflammatory inhibitory factors, chemokines, and others [94, 95]; (4) attenuating leukocytes recruitment and transendothelial trafficking [10]; (5) promoting angiogenesis by increasing the production of angiogenic factors, such as vascular endothelial growth factor (VEGF) and angiopoietin-1 (Ang-1) [96].
Endothelial barrier function damage and microvascular leakage
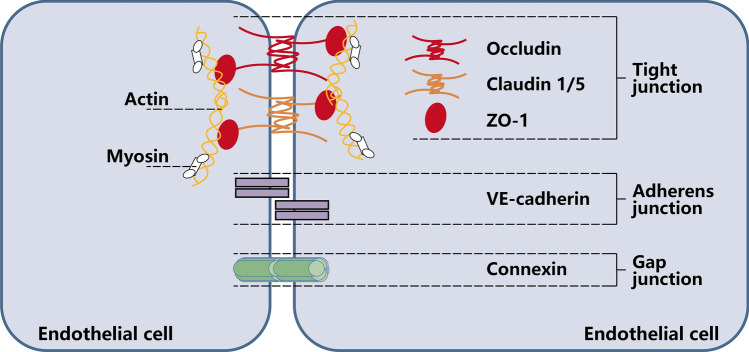
In addition to the impaired vasodilatory function described above, loss of barrier function is another key reason for the occurrence of MVL. The endothelial barrier function is made up by the glycocalyx, endothelial cells, pericytes and junction proteins [16]. Under physiological conditions, junction structures and myofilament–myosin skeleton fibers between CMECs determine the permeability of endothelial barrier [97]. Junction structures are maintained by an intercellular tight junction, adherens junction, and gap junction, including occludin, claudin-1/5, zonula occludens protein-1 (ZO-1), VE–cadherin, and connexin 43 (Fig. 3) [48, 97–100]. After myocardial I/R injury, the production of large amounts of ROS promotes leukocyte adhesion and increased vascular wall permeability. In the presence of C5a-activated neutrophils [101], the cytoskeleton proteins and gap junctions of CMECs are reorganized and resulting in an increased permeability of CMECs, which are essential for the development of MVL [102]. VEGF, a highly specific pro-vascular endothelial cell growth factor, promotes increased vascular permeability, extracellular matrix degeneration, vascular endothelial cell migration, proliferation, and angiogenesis. It has been shown that ischemic/hypoxia could lead to upregulation of VEGF in diabetic mice through activation of hypoxia-inducible factor 1 (HIF-1) [103] and also can increase vascular permeability by inducing phosphorylation of tight junction proteins, such as occludin and ZO-1 [104, 105].
Fig. 3.
Simplified and incomplete scheme showing the molecular composition of endothelial junction complexes. Occludin, Claudin1/5, and ZO-1 constitute tight junction that are essential for endothelial barrier permeability. The central structural and functional component of adherens junction is VE–cadherin, which is uniquely expressed in ECs. Gap junction consist of connexin and allow the passage of molecules. Decrease in junction proteins and loosening of interendothelial cell junction structures due to ischemia/reperfusion are the main causes of leakage of blood components from microvasculature. ZO-1, zonula occludens protein-1
Angiopoietin (Ang)/Tie-2 is another pathway distinct from VEGF that has been clearly demonstrated to maintain vascular integrity and is important for the regulation of endothelial barrier function [106], especially Ang-1 and Ang-2. The former is a natural ligand of Tie-2 receptor and maintains the integrity of the microvascular endothelial barrier by regulating the phosphorylation of VE–cadherin [107]. In contrast, Ang-2 is an important biomarker of MI [108]. It has been shown to act as an antagonist to Ang-1 at the Tie-2 receptor [107], which can promote MVL by decreasing VE–cadherin [105, 109]. In addition, sphingosine 1 phosphate (S1P), a blood-derived lysophospholipid, stabilizes endothelial cell–cell junctions by interacting with the type 1 receptor (S1P1) in ECs [110]. The main effects are increase of VE–cadherin binding, enhancement of junction proteins, e.g., claudin 5 transport to the cell periphery [111], and promotion of phosphorylation of the gap junction protein connexin43 [112].
Numerous studies have confirmed the importance of interendothelial cell junction structures. Glycocalyx degradation contributes to the reduction of endothelial barrier function and edema formation, and tumor necrosis factor α (TNF-α) is an essential mediator of glycocalyx degradation. Glycocalyx degradation can promote leukocyte and platelet adhesion, which increases microthrombosis and thus aggravates MVO [16]. It is evident that downregulation of various endothelial cell junction proteins and degradation of glycocalyx can lead to loss of barrier function of CMECs, which in turn to promotes extravasation of leukocytes and blood cells and leakage of plasma proteins [48, 49, 52, 98, 105, 109, 113, 114]. In addition, hemorrhage-induced interstitial iron deposition can also induce an inflammatory response to exacerbate ischemia/reperfusion injury [52].
Coronary microembolization and microthrombosis
Microembolization and microthrombosis are also important in the development of MVD after I/R. Spontaneous or iatrogenic (PCI-induced) rupture of epicardial atherosclerotic plaques can release plaque debris into the microvascular along with many soluble substances [52]. These substances, which cannot be captured by filter devices or meshes, directly contribute to the physical obstruction of microvessels (microembolization) and subsequent signaling or inflammatory cascades [115]. In addition, damaged ECs are dislodged from the microvessel wall and enter the circulation during MI. Apoptotic or necrotic circulating ECs can promote microvascular inflammatory responses by interacting with the endothelium [10], play an active role in pro-coagulant and immune processes, and have been suggested to be a biomarker of thrombotic risk [116]. The levels of circulating ECs were 4.5-fold higher in patients with MI than in healthy subjects [117]. Detached ECs after I/R also induce phosphatidylserine exposure and cytoskeletal protein remodeling, and then release endothelial-derived microparticles (EMPs, in size of 100 nm-1 μm) [118, 119]. It was demonstrated that EMPs downregulated the expression of tight junction proteins, such as ZO-1 and claudin 5, and upregulated the expression of transcellular transport-related proteins, such as caveolin-1. Moreover, EMPs promoted the production of platelet-derived microparticles (PMPs), and both synergistically mediated disruption of endothelial integrity and subsequent MVL after I/R [120]. Therefore, the number of circulating ECs and EMPs may serve as potential biomarkers of acute and chronic endothelial dysfunction [10].
After I/R injury, it is worth mentioning that the linear morphology of erythrocytes is concomitantly altered and promotes their aggregation in the microvasculature [37]. The platelet surface contains various biomolecules, such as GPIbIX-V, αIIbβ3, ICAM-1, p-selectin and α2β1 integrin [121]. On one hand, these molecules facilitate platelets to bind to the corresponding receptors or collagen on the surface of CMECs, including von Willebrand Factor (vWF), αVβ3 integrin and p-selectin glycoprotein ligand (PSGL-1). On the other hand, neutrophils can be activated and rapidly influx between CMECs and vascular elastic lamina [122], releasing neutrophil extracellular traps (NETs) [123] after reperfusion. This process leads to increased ROS production and a series of inflammatory cascades by enhancing leukocyte–platelet–CMECs adhesion/aggregation, promoting CMECs to highly express a series of inflammatory molecules, such as intercellular adhesion molecule (ICAM), vascular adhesion molecule (VCAM), p-selectin, thromboxane A2, and ET-1 [124, 125]. Rodrigues et al. specifically described in their study that about 25% of platelets bind directly to CMECs, while the remaining 75% of adherent platelets attach to leukocytes (mainly neutrophils) bound to the vascular wall, resulting in blood stasis [114]. Moreover, neutrophils have been shown to increase vascular permeability and promote MVL by releasing matrix metalloprotein-9 [126]. These findings illustrate that I/R leads to MVO and MVL via microembolization and microthrombosis [126].
Calcium overload in microvascular endothelial cells
Research evidence have showed that calcium overload is an early sign of cell apoptosis and necrosis after myocardial I/R injury [127]. Persistent calcium oscillation triggering mitochondrial dysfunction which leads to oxidative stress and the opening of the mitochondrial permeability transition pore (mPTP), and then activates mitochondrion-dependent apoptosis [128]. In a mouse cardiac I/R model, restriction of CMECs mitochondrial calcium ([Ca2+]m) influx increased microvascular perfusion, reduced MVO and inflammatory cell infiltration and limited infarct size, which associated with mitochondrial morphological and functional protection [85, 129]. The [Ca2+]m influx is primarily mediated by the mitochondrial calcium uniporter (MCU) complex, which consists mainly of four MCU subunits and essential MCU regulators (EMREs) [130, 131] The regulatory proteins that interact with MCU subunits include mitochondrial calcium uptake 1/2 (MICU1/2), which is responsible for mitochondrial calcium uptake [84], while the MCUb is a paralog of MCU, characterized as dominant negative subunit of uniporter complex, and acts as a negative regulator of MCU activity [132]. The four MCU subunits are upregulated in response to I/R injury, causing [Ca2+]m overload and mitochondrial dysfunction [133]. Another critical intracellular Ca2+ regulator, sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), catalyzes Ca2+ transport across membranes in an ATP-dependent manner [134]. Among more than 10 known isoforms of SERCA, the 110 kDa monomeric SERCA2a is the major isoform expressed in adult cardiac muscle, accounting for approximately 40% of the sarcoplasmic reticulum (SR) protein [135]. SERCA2a performs two major functions: it decreases cytoplasmic Ca2+ levels to initiate muscle relaxation and reloads Ca2+ required for muscle contraction into SR [135, 136]. In a recent study, cardiac-specific overexpression of SERCA in mice significantly reduced [Ca2+]m overload and myocyte apoptosis and improved coronary microvascular function following cardiac I/R injury by preventing MCU upregulation [129]. However, the detailed mechanisms by which the MCU complex regulates mitochondrial function and microvascular protection have not been fully explored. It was recently reported that histidine triad nucleotide-binding 2 (HINT2), located in mitochondria and belonging to the HIT superfamily, could improve CMECs mitochondrial dynamics and MCU complex-associated [Ca2+]m homeostasis by regulating the MCU complex [137], and thereby attenuating I/R-induced myocardial MVD [84].
Endoplasmic reticulum (ER) stress
ER is an important organelle for Ca2+ storage, lipid synthesis, protein translation, modification and folding in eukaryotic cells [138]. I/R injury leads to oxidative stress and increased intracellular ROS in ECs, causing unfolded or misfolded proteins to accumulate and aggregate within the ER, known as endoplasmic reticulum stress (ERS) [139]. ERS is generally considered to be the initial response or co-initial pathway that precedes other cellular stresses, such as mitochondrial oxidative stress. ERS is a cellular protective response that reduces the accumulation of unfolded proteins and restores the normal function of the ER. However, persistent ERS can shift its pro-survival mechanism to a pro-apoptotic pathway, leading to apoptosis [139]. Battson et al. provides a systematic review of ERS and the development of endothelial cell dysfunction, including the activation of protein kinase RNA-like endoplasmic reticulum kinase (PERK), inositol requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6), as well as up-regulation of ER chaperon protein 78 kDa glucose-regulated protein (GRP78), which plays a role in regulating multiple aspects of protein synthesis, degradation, oxidative stress, inflammation and apoptosis [140]. It is noteworthy that mitochondria are an important source of ROS generation during ERS. ER Ca2+ released by cells under ERS is taken up by the mitochondria and ultimately results in cyt-c release and ROS generation [141, 142]. This functional relationship is mediated by an anatomical connection between two organelles called the mitochondria-associated ER membrane (MAM). The MAM is essential for the inter-organelle exchange of Ca2+, ATP, and ROS, as well as for coordinating cellular functions [140, 143]. He et al. demonstrated that Ca2+ accumulated in the cytoplasm after I/R transfers along the MAM ultimately leading to the opening of mPTP, which induces endothelial cell death [144].
Mitochondrial dysfunction
Mitochondria are involved in endothelial growth, proliferation and senescence [145]. In addition to CMECs dysfunction owing to [Ca2+]m influx addressed above, a number of studies have identified mitochondrial damage as a central causative factor of MVD caused by cardiac I/R injury [12, 146, 147]. Mitochondria are dynamic organelles with self-regulations via fission and fusion, two diametrical processes, one separating the damaged part from the whole mitochondrion and another merging the two divided bodies into a healthy mitochondrion. The balance of fission and fusion is essential for maintaining mitochondrial and endothelial function [37, 148]. It is now believed that structural disorders of mitochondria precede their dysfunction [26]. When myocardial blood flow was restricted, the energy metabolism of CMECs was reduced, so mitochondrial fission was activated to increase the number of mitochondria to meet cellular metabolic demands, and mitochondrial fusion was inhibited instead [149]. At the molecular level, mitochondrial fission is a process of phosphorylation of dynamin-related protein 1 (Drp1) and subsequent recruitment by mitochondrial receptors, including mitochondrial fission factor (Mff) and mitochondrial fission protein 1 (Fis1) [150]. Studies have shown that I/R injury led to phosphorylation of Drp1 and Fis1, but had no effect on phosphorylation of Mff [26]. Phosphorylation of Drp1 accelerated its transfer from the cytoplasm to the mitochondrial surface [151], and phosphorylation of Fis1 increased its affinity with cytoplasmic Drp1 [152].
This pathological mitochondrial fission also damages mitochondrial DNA (mtDNA) during mitosis, decreases mitochondrial membrane potential (MMP), further exacerbates the accumulation of mitochondrial ROS (mROS) and the subsequently increases in cytoplasmic ROS [84, 153]. It is generally recognized that mitochondrial complexes I and III are the main sources of ROS generation under pathological conditions, and NOXs are the main enzymes for mROS production in ECs. Besides the vasoconstrictor function described above, NOXs-derived ROS promote apoptosis, inflammation, and senescence, which in turn lead to coronary MVD [154]. Its main downstream targets in ECs include NF-κB, activated protein-1 (AP1), HIF-1, p53, p38, c-Jun N-terminal kinase, and Src kinases [155].
Notably, antioxidant molecules such as glutathione (GSH), superoxide dismutase (SOD), and glutathione peroxidase (GPX) are significantly depleted, whereas ROS are unbalancedly increased in CMECs during I/R injury [10]. CMECs suffer from a complete loss of MMP and an increased opening of mPTP [37], which ultimately leads to oxidative phosphorylation uncoupling, ATP depletion or cell death [18, 156]. Moreover, when mitochondrial pro-apoptotic proteins such as cytochrome c (cyt-c) and second mitochondria-derived activator of caspases (Smac) entered the cytosol, they also activate members of the caspase family to promote apoptosis [26]. In this case, mitochondria switches from generators to endothelial apoptosis inducers [157], and CMECs become dysfunctional or died, eventually leading to microvascular swelling and vessel wall collapse, further blocking the blood flow to the ischemic myocardium [158, 159]. Such a pattern of cell death leads to many types of CVD, including I/R injury and heart failure [84, 160].
Impaired mitophagy in microvascular endothelial cells
The benefits of mitophagy in CMECs have been recognized [157, 161, 162]. Compared to aforementioned imbalance of mitochondrial fission/fusion provoked CMECs apoptosis [157, 163–165], mitophagy is an important protective mechanism that restores mitochondrial fission/fusion balance and thus maintains mitochondrial function and structure in CMECs after I/R injury [84, 166]. In general, the enhancement of mitochondrial fission mediated by phosphorylation of Drp1 is accompanied by increased mitophagy and mitochondrial biogenesis. Under normal physiological conditions, PTEN-induced putative kinase 1 (PINK1) is imported into mitochondria, where it is cleaved by proteases [167]. Low membrane potential mitochondrial fragments are stabilized by PINK1 on the outer mitochondrial membrane (OMM) due to inhibitory input, which in turn recruits E3 ubiquitin ligase (Parkin) to mitochondria. Meanwhile, the mitochondrial outer membrane localized mitophagy receptor FUN14 domain-containing protein 1 (FUNDC1) is activated by post-transcriptional phosphorylation. Mitochondria interacts with the autophagy marker microtubule-associated protein 1 light chain 3 (LC3) on lysosomes via Parkin and FUNDC1, forming autophagosomes. Therefore, mitophagy promotes the degradation of mitochondrial debris and maintained the dynamic homeostasis of mitochondria [10]. It has been shown that mitophagy not only attenuated high glucose-induced CMECs injury by inhibiting oxidative responses [168], but also inhibited oxidative LDL-induced [169] and/or oxidative stress-induced CMECs apoptosis [29, 159]. During I/R injury, the expression of pro-inflammatory factors such as IL-8, matrix metalloprotein-9 and monocyte chemotactic protein 1 (MCP-1) was increased in CMECs, which could be reversed by mitophagy [159]. This suggests that under hypoxic conditions, the MMP is reduced and Parkin in CMECs translocates to mitochondria, which can induce mitophagy [170] and may have an anti-inflammatory effect. It is a proposed hypoxic preconditioning mechanism.
Several studies have found that reoxidation after hypoxia inhibits mitophagy, leading to the accumulation of damaged mitochondria and mediating mitochondrial apoptosis in CMECs [171] and cardiomyocytes [172]. NR4A1 is a member of the nuclear receptor subfamily 4 group A (NR4A) family, which exerts transcriptional regulation to participate in life activities, such as energy metabolism and protein interactions [159]; Casein kinase 2α (CK2α), a constitutive Ser/Thr kinase [173], was originally described as an inhibitor of FUNDC1 and is associated with FUNDC1 phosphorylation at Ser13 [174]. In recent studies, significant upregulation of NR4A1 [159] and CK2α [175] has been observed in murine myocardial I/R model. Their expression levels were positively correlated with microvascular collapse, endothelial apoptosis and mitochondrial damage [159], as well as further cardiomyocyte death, infarct area enlargement and cardiac dysfunction [175], whereas deletion of these two genes mitigated I/R induced cardiac injury through maintaining FUNDC1-mediated mitophagy [159, 175]. NR4A1 has the ability to simultaneously promote Mff-induced mitochondrial fission and FUNDC1-required mitophagy, which can disrupt the dynamic homeostasis of mitochondria, promote endothelial cell apoptosis and trigger microvascular dysfunction [159], while the latter enhances the phosphorylation (inactivation) of FUNDC1 through the post-transcriptionally modified Ser13 site, thus effectively inhibiting mitophagy [175]. As a result, impaired mitophagy is unable to clear damaged mitochondrial debris, which leads to a series of pathophysiological changes, such as mitochondrial genome collapse, blocked mitochondrial biogenesis, oxidative stress, mPTP opening and mitochondrial debris accumulation, ultimately to CMECs injury and microvascular dysfunction [159, 175]. Thus, it is generally accepted that ischemia/hypoxia induces mitophagy to attenuate mitochondrial damage, whereas ischemia–reperfusion/hypoxia–reoxygenation inhibits mitophagy, which in turn leads to mitochondrial dysfunction and cell death [10].
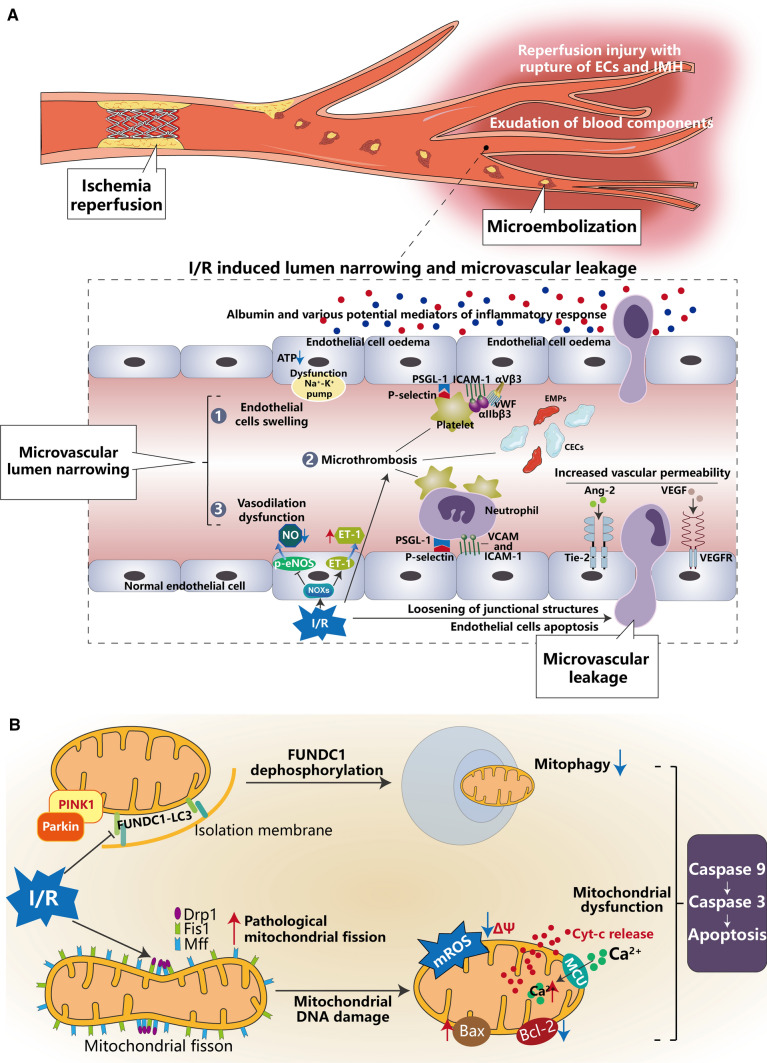
In summary, these studies have demonstrated the latest research findings on the mechanisms of MVD development after myocardial I/R, especially the regulation of the balance between vasodilators and vasoconstrictors; CMECs swelling and loss of vascular barrier function; mitochondrial metabolism and dysfunction within CMECs. Among these mechanisms, microembolization, microthrombosis, inflammatory cell infiltration, energy metabolism and dysfunction of CMECs are relatively well-elucidated; while aspects such as loosening of junctions between CMECs, [Ca2+]m overload, ERS and impaired mitophagy need to be further explored, particularly as the upstream and/or downstream signals of the known signaling pathways are not fully defined. MVD after myocardial I/R is an intricate process involving multifaceted and multifactorial interactions at molecular, cellular and tissue levels (Fig. 4). A better understanding of the pathophysiological mechanisms of MVD may pave a path for more precise management and improvement of the prognosis of myocardial I/R injury and reduction of CVD mortality.
Fig. 4.
A Complex process of multifaceted and multifactorial interactions of microvascular damage in molecules, cellular and tissue levels after myocardial ischemia/reperfusion (I/R). After I/R injury, endothelial cells swelling, vasodilation dysfunction, and microthrombosis cause luminal narrowing, known as microvascular obstruction (MVO), whereas loosening of the interendothelial cell junction structures, massive death of endothelial cells and increased vascular permeability cause microvascular leakage (MVL). B Mitochondrial dysfunction leads to endothelial cells apoptosis. Due to I/R injury, mitochondrial fission/fusion was imbalanced and pathological mitochondrial fission damages mtDNA, promoting cytochrome c (cyt-c) release from mitochondria to the cytoplasm and activating the caspase family to promote endothelial cells apoptosis. Moreover, impaired mitophagy is unable to remove damaged mitochondrial debris, leading to a series of pathophysiological changes that eventually lead to endothelial cells injury and apoptosis. p-eNOS phosphorylated endothelial nitric oxide synthase, ET-1 endothelin-1, vWF von Willebrand Factor, PSGL-1 p-selectin glycoprotein ligand, ICAM intercellular adhesion molecules, CECs circulating endothelial cells, EMPs endothelial-derived microparticles, Ang-2 angiopoietin-2, Tie-2 TEK receptor tyrosine kinase a receptor of Ang-2, VEGF vascular endothelial growth factor, VEGFR vascular endothelial growth factor receptor, PINK1 PTEN-induced putative kinase, Parkin E3 ubiquitin ligase, FUNDC1 FUN14 domain-containing protein 1, LC3 light chain 3, Drp1 dynamin-related protein 1, Mff mitochondrial fission factor, Fis1 mitochondrial fission protein 1, mROS mitochondrial ROS, MCU the mitochondrial calcium uniporter complex
Detections of microvascular damage after myocardial I/R injury
Experimental assays of microvascular damage after myocardial I/R injury
Detection of microvascular obstruction
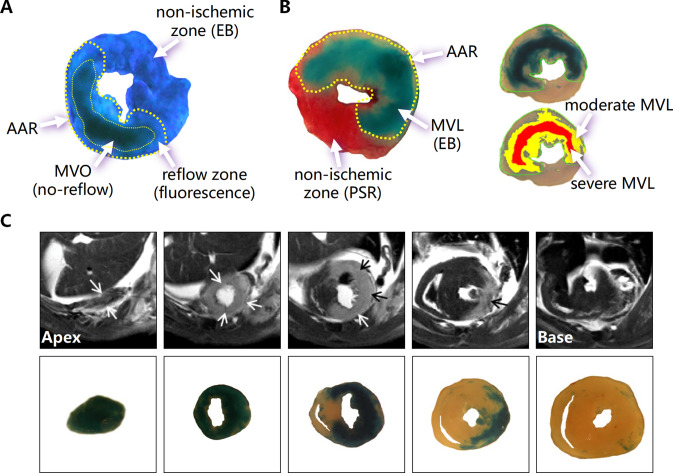
The thioflavin-S and Evans blue dual staining is a common method used in experimental animal studies to determine the extent of MVO after I/R surgery [41, 50, 176]. The brief experimental procedure is as followings: first, after establishment of mouse myocardial I/R model, the thorax is reopened at the end of reperfusion and the left coronary artery is re-ligated at the same position of the occlusion, and then 4% thioflavin-S fluorescent dye via the inferior vena cava injected immediately, and subsequently perfused with 3% Evans blue retrogradely via the ascending aorta to stain the non-ischemic myocardium. Second, the heart is harvested, frozen on dry ice, the LV is serially sectioned at a thickness of 1 mm and then compressed between two slides. Finally, LV sections are exposed to UV light and digital image acquired. The area of ischemic region (area at risk, AAR, Evans blue non-stained region) and MVO region (thioflavin-S fluorescence diminished or absent region) were determined and the area of MVO was expressed as a percentage of AAR [50, 176–179] (Fig. 5A).
Fig. 5.
Experimental assays of microvascular damage after myocardial I/R injury. A Extent of MVO by the absence of thioflavin-S fluorescence (indicated by the thin yellow dotted line) within the AAR (indicated by the thick yellow dotted line) and NIZ by EB. B Left image detects MVL by EB within the AAR (indicated by the yellow dotted line) and NIZ by picrosirus red (PSR). The right image determines moderate MVL (yellow filled area) and severe MVL (red filled area). C Representative serial CMR images from the apex to the base of a mouse heart showing LGE (arrows) and serial cardiac transverse sections showing regions with MVL indicated by EB staining after I/R (1 h/24 h) (modified from [50])
High-precision imaging techniques include non-invasive echocardiograph and CMR imaging are promising ways for detecting MVO in live animals. Ultra-high-frequency (UHF) cardiac ultrasound systems can be used to study myocardial local perfusion by injecting ultrasound microbubbles in combination with a laser device. This technique has been developed for the popular Vevo 3100 high-frequency cardiac ultrasound system. However, the extremely transient presence of microbubbles in the myocardium, the high technical requirements, individual and operational errors, and the immaturity of fast three-dimensional imaging technologies have limited the application of this technique for the detection of MVO. With the development of small animal cardiac MRI technology, it is expected that a method to dynamically detect the extent of MVO in live animals by detecting the size of the "no-reflow" region will be achieved in the future.
Histological measurement of microvascular leakage
In experimental animal studies, MVL is mainly measured by tail vein injection of Evans blue as an MVL tracer to measure the size of myocardial blue-stained areas or by extracting Evans blue from the myocardium for chemical quantification [50, 179]. Briefly, following the establishment of cardiac I/R model in mice, Evans blue is injected via the tail vein to infiltrate through the damaged micro-vessel into the myocardial interstitium. After a period of time (several hours) the thorax is re-opened and the aorta canulated and retrogradely perfused with saline to flush away blood containing Evans blue in the cardiac vasculature. Next the LV is isolated and serially sliced for digital imaging. Total blue-stained areas and dark blue (severe MVL) or light blue-stained areas (light–moderate MVL) can be measured using image analysis software and expressed as a percentage of the blue-stained area to total LV section area [50] (Fig. 5B).
Quantitative detection of microvascular leakage
Quantitative chemical assay can be conducted by extracting Evans blue dye from blue-stained myocardium. Briefly, following the digital imaging, blue-stained portion of the LV sections is separated, minced and digested in 50% trichloroacetic acid (TCA) to extract the blue dye. The absorbance of Evans blue in the supernatant of digested tissue after centrifugation is measured at 620 nm using a spectrophotometer and calculated values are normalized by the wet weight of the myocardial tissue to quantify the Evans blue content in certain amount of myocardium (μg/mg) [50, 177, 178].
CMR detection of microvascular leakage
CMR imaging can be used for detecting MVL. We have previously documented the usefulness of this technique in small size animals [50]. Following the establishment of cardiac I/R model in mice, Evans blue as a tracer and gadolinium-based contrast agents were injected intravenously and CMR imaging for the isolated LV was performed using a 9.4 T Agilent animal scanner to assess myocardial MVL within couple of hours after the euthanasia. Microvascular hyperpermeability increases contrast agent retention within the ischemic tissue, that is, late gadolinium enhancement (LGE) in the acute phase of MI. Results surprisingly demonstrated that the area of MVL, i.e., LGE area was larger than both infarct size and no-reflow areas. Contour analysis of CMR–LGE images and histological MVL images showed a high degree of spatial co-localization (r = 0.959, P < 0.0001), indicating the accuracy of CMR imaging in the detection of MVL [50]. Notably, the size of the MVL detected by CMR imaging was highly correlated with the degree of LV enlargement and reduced ejection fraction, suggesting that MVL size could be used to predict LV function and remodeling [50] (Fig. 5C). Currently, the CMR technique for MVL is only performed in the static heart, the application in a live animal needs to be further developed.
Clinical diagnoses of MVD after myocardial I/R injury
Electrocardiogram (ECG)
ECG is a widely used, convenient, inexpensive tool for indirect assessment of MVD in clinic. Previous studies reported that by comparison the difference of ST-segment elevation from abnormal leads of ECG before and after PCI in patients with STEMI to assess microcirculatory status [180]. ST-segment regression (STR) is one of the highly accurate parameter for assessment of the effect of PCI, and at least 50% of patients with STEMI have a peri-procedural STR < 50%, indicating that microcirculatory perfusion may be impaired [181]. The great advantage of perioperative ECG analysis is that it can be performed in real time during the procedure, thus allowing for immediate and new interventions and so having an important clinical value [181]. Therefore, STR is a useful tool to monitor the response to treatments, such as fibrinolysis or PCI in STEMI patients, both in the acute phase and during follow-up.
Coronary artery angiography (CAG)
Coronary artery angiography (CAG) can be applied to evaluate the opening status of a diseased coronary artery received intervention. The Thrombolysis in Myocardial Infarction (TIMI) flow grade is a direct indicator of coronary flow after intracoronary thrombolysis or intervention in AMI with four grades of 0–3 [182, 183]. Grade 0 indicates no distal reperfusion, i.e., no passage of the distal contrast agents or complete occlusion of the culprit coronary artery. Grade 1 designates that a small amount of contrast agents passes through the obstruction but the distal artery is not visualized. Grade 2 denotes that the culprit coronary artery is completely visualized but the blood flow is slow compared to normal vessels. Grade 3 means that the culprit coronary artery is completely visualized with a normal distal perfusion [184, 185]. There are clinical studies reported a close association between TIMI flow grade and the risk of major adverse cardiovascular events (MACE) [186]. Mortality was significantly higher in patients with TIMI flow grade 0/1 than in patients with TIMI flow grade 2/3 following interventions [187]. Furthermore, the initial TIMI flow grade before intervention was also an independent predictor of infarct size, MVO, myocardial salvage index (MSI) and the risk of clinical events [188]. Although TIMI flow grade can evaluate degree of coronary artery flow, its ability to accurately reflect status of microvascular perfusion has been challenged, as nearly half of patients with TIMI flow grade 3 present MVD detected by CMR [186]. In practice, TIMI flow grade is somewhat subjective, so an alternative and more objective angiographic method has been established. Since the left anterior descending artery is longer than the circumflex and right coronary arteries, a correction factor has been generated for compensation. Usually, the number of frames from beginning of contrast agent coloring to their passage through the left anterior descending branch is divided by 1.7 for a correction of the vessel length, therefore, obtaining a corrected TIMI frame count (CTFC). CTFC expresses the time required for epicardial blood flow to the distal end of the damaged micro-vessel as a number of frames from 0 to 100 (when the angiographic rate is 30 frames/second), allowing for a more accurate depiction of flow rate [189].
Real-time myocardial contrast echocardiography (RTMCE)
Real-time myocardial contrast echocardiography (RTMCE) is a relatively new technique that utilizes microbubbles smaller than the diameter of erythrocyte to simulate erythrocyte rheology within a vessel [190], thereby displaying the entire myocardial microvascular perfusion, RTMCE is expected to quantify in real time the total volume of blood and estimate the velocity of blood flow through a localized region of the myocardium, including small myocardial arteries, capillaries, and small veins [191]. Specifically, by standard echocardiography the ventricular wall motion analysis can be conducted using the American Society of Echocardiography 17-segment LV model. RTMCE is performed in the setting of tissue harmonic imaging in three standard apical views (four-chamber, two-chamber, and three-chamber) with a continuous intravenous infusion of lipid-coated microbubbles as the contrast agent, and real-time images acquired at a mechanical index of 0.15–0.19 [192]. In clinical practice, normal microvascular perfusion (MVP) can be defined if all segments of myocardium supplied by the infarct-related artery are completely replenished within 4 s after a high mechanical index pulse. Delayed microvascular perfusion (dMVP) is defined if a perfusion defect was still observed in the infarct area within 4 s but was completely replenished within 10 s. If the plateau strength is reached and two or more parts of the infarct zone still have persistent defects, it is defined as MVO [192]. A recent RTMCE study reported that MVP, dMVP and persistent MVO were detected in 36%, 29% and 35% STEMI patients after successful PCI, respectively [192]. In fact, more than 60% of STEMI patients received emergency PCI still have different degrees of microvascular perfusion abnormality after 24–48 h, which was associated with poor recovery of LV systolic function at the 6-month follow-up [192]. Analyses from RTMCE studies indicate a superiority of this technique over clinical biomarkers, ECG and coronary angiographic parameters in assessment of microvascular perfusion status and prediction of LV functional recovery after PCI [190, 192, 193].
Cardiac magnetic resonance (CMR) imaging
CMR imaging technology is now commonly used in the diagnosis of CVD. With good soft tissue contrast resolution, a large scanning field of view, and cross-sectional images in all directions and different angles, it allows for unique in vivo assessment of cardiovascular abnormalities in STEMI patients [194, 195]. Currently, CMR is considered the best diagnostic imaging modality to detect MVD, including MVO, myocardial edema, and IMH [196–198].
From a technical point of view, cardiac MRI characterizes different tissues by the evaluation of proton relaxation times T1 and T2 [198]. The T1 value expresses the rapidity of the longitudinal relaxation of the tissue, while the T2 value describes the rapidity of the transverse relaxation of the tissue. Since the main magnetic field cannot reach absolute homogeneity, the transverse magnetization decays more rapidly, yielding a smaller value than T2, the so-called T2* value [198, 199]. Increased myocardial water content raises the signal on T2-weighted images, such as inflammation. Myocardial edema at the acute phase of MI shows up as a bright signal on T2-weighted images [200–202]. The main advantage of this technique is to quantify the proportion of myocardial salvage assessed retrospectively by comparing T2-weighted edema size with late-enhanced images [203]. Gadolinium (Gd) is a metal element with magnetic resonance paramagnetism and is often used as an extracellular contrast agent to improve the quality of images produced by CMR, which exhibits significantly high signal under specific conditions, such as myocardial necrosis or fibrosis, and conversely low signal in surviving myocardium [204, 205]. LGE images are T1-weighted inversion recovery sequences acquired 10 min after intravenous Gd injection, and inversion time is selected to null myocardial signal using “inversion time scout” or “look locker” sequences [206]. In I/R injury, increasing micro-vessel permeability causes myocardial edema and expansion of myocardial interstitial volume, resulting in spread distribution of Gd contrast agent in these areas, which manifests as abnormal enhancement in the infarcted regions. As the disease progresses, the damaged myocardial cells are replaced by extracellular collagen fibers and fibrosis, which leads to an expansion of the interstitial volume and prolonged Gd contrast agent influx/clearance time, so that the contrast agent appears concentrated in the fibrous scar areas, the so-called LGE [207], which has also been validated in animal models [208]. The LGE–CMR technique is now widely used to assess disease severity, risk stratification, prognosis, and the effectiveness of therapeutic interventions [209–212].
MVO can be evaluated using CMR first-pass perfusion and delayed post-contrast sequences [213]. On contrast-enhanced CMR images, MVO appears as a dark hypointense core within the hyper-enhanced areas on either early gadolinium enhancement (referred to as early MVO) or conventional late gadolinium enhancement (referred to as late MVO) [45]. Clinically, early and late MVO are generally assessed at approximately 1 and 15 min after gadolinium injection, respectively. Since the presence and extent of MVO decreases over time, early MVO is more sensitive to less pronounced MVD, which bears a low prognostic value in predicting post-infarction adverse events. Conversely, late MVO reflects severe myocardial arterial circulation disturbances and correlates closely with clinical outcome [45, 214]. In one of the earliest experimental studies, Judd et al. investigated the correlation of MRI findings with pathological changes by applying contrast-enhanced fast MRI to a dog cardiac I/R (90 min/2 days) model, and subsequently removed the heart and delineated the no-reflow zone by thioflavin-S staining. They found that a hypo-enhancement zone within the bright regions of LGE on delayed post-contrast sequences, so-called “dark zones”, were “no-reflow” areas [208]. In humans, Lima et al. performed rapid enhancement CMR on patients with recent MI and observed a well-defined time-intensity profile of the enhancement in different regions of MI, which was described based on the wash-in/wash-out kinetics of the contrast agent [215]. In addition, persistent hypo-enhancement (10–20 min after injection of a contrast agent) on delayed enhancement sequences was also found to appear to be characteristic of persistent MVO [216]. Recently, Li et al. and Alessandro et al. performed LGE–CMR in STEMI patients after 2–7 days of PCI and confirmed that the MVO was defined as a hypo-enhanced region within LGE area, which occurred in 41.8–67% STEMI patients [59, 79].
Except MVO, CMR can only indirectly assess MVL by detecting/measuring degree and extent of myocardial edema and IMH due to lack of proper indicator/tracer for MVL. As stated above, T2-weighted images can be used to assess myocardial edema and increased active water content within the ischemic myocardium, which appears as a bright signal on the images [198, 217, 218]. IMH can be detected by CMR as the hemoglobin catabolites are paramagnetic and IMH may attenuate the T2-weighted signal intensity of the edematous area [16, 52, 219]. Pankaj et al. performed CMR and obtained extracellular volume (ECV)-maps at the acute (24–72 h) and recovery (3 months) phases in STEMI patients who underwent PCI within 12 h. Adapted from the 17 segments of the American Heart Association (AHA) model [199], after excluding the apex, 16 segments of each patient's LV could be classified as normal segments (no edema on T2-weighted imaging and no infarction on LGE imaging); edema segments (predominantly edema on T2-weighted imaging and no infarction on LGE imaging) and infarct segments (presence of any infarction on LGE imaging with or without edema on T2-weighted imaging) [82]. The changes in myocardial extracellular volume and ventricular wall thickening were analyzed in a total of 800 segments from 50 patients during the acute phase. Among them, 325 segments (40.6%) were found to be normal, 246 segments (30.8%) were edematous, and 229 segments (28.6%) were infarcted [82]. At the recovery phase, it was found that normal myocardial extracellular volume mildly expanded and that edematous segments were associated with deterioration of cardiac systolic function through comparison of ECV-maps at baseline and follow-up scans [82].
IMH, owing to endothelial barrier disruption and blood cells leakage, is another indirect parameter can be applied to assess MVL after ischemic insult [69]. Clinical studies have shown that T2* imaging is the preferred CMR method for comprehensive assessment of IMH [69]. The specific imaging for IMH is based on dephasing of T2* relaxation times [81]. The area of IMH after perfusion is presented as a hypointense infarct core with a T2* value < 20 ms [81, 220]. Kandler et al. performed CMR in 151 STEMI patients within 8 days after PCI. LGE sequences were used to visualize MVO and T2*-weighted sequences were used to detect IMH. They found that 50% patients presented with IMH and those with a TIMI flow grade ≤ 1 before PCI were more likely to have IMH, which was associated with impaired LV function and increased myocardial infarct size [69].
With the development of new indicators/tracers, it is expected that the degree of MVL will be accurately reflected by CMR techniques, as shown in our experimental studies [50], which providing novel methods and tools for a direct diagnosis and assessment of MVL in clinic.
In summary, MVD after myocardial I/R injury can be detected clinically by ECG, CAG, RTMCE [126]. CMR allows visualization of MVO, myocardial edema, and IMH with LGE imaging and T2* mapping. Although MVL can be indirectly reflected by edema and IMH, a direct method of measuring MVL has not yet been developed. As mentioned in Sectinon Experimental assays of microvascular damage after myocardial I/R injury, our research team, for the first time, characterized MVL in a mouse model of cardiac I/R through a series of histological, biochemical/semi-chemical quantitative assays and CMR imaging. This is currently the only way to detect MVL straightforwardly in an animal model. However, in clinical practice, factors such as the high technique demand in ultrasound technology and the cost of expensive CMRs have limited the clinical performance of many of these examinations. These reasons have led to the underestimation of MVD and especially MVL in a significant proportion of patients in the clinical setting.
Potential therapeutic approaches for MVD after myocardial I/R injury
There have been numerous clinical studies evaluating the effects of several interventions on infarct size and cardiomyocyte protection, but few preclinical studies have explored potential therapeutic approaches for MVD [45]. Currently, some therapeutic modalities have been shown to ameliorate MVD due to endothelial cell dysfunction after I/R in animal models or at the endothelial cell level, but the mechanisms still need to be further explored and the therapeutic efficacy also requires a clinical validation.
Mechanical means
Transient/repeated myocardial ischemic stimuli before sustained ischemia (ischemic preconditioning, IPC) or at the beginning of reperfusion following ischemia (ischemic postconditioning, IPostC), or even carried on such intervention in the distal region or distal organs, e.g., kidneys, skeletal muscle (remote ischemic conditioning, RIC), can reduce myocardial infarct size [221]. These mechanical interventions augmenting resistance of the heart or other targeted organs to severe ischemic insult is named as ischemic conditioning. Heusch systematically reviewed the role of ischemic conditioning in I/R and related mechanisms, including physical stimuli, chemical stimuli, and intracellular signaling, especially in mitochondria [222].
IPC has been shown to have clinical benefit on perioperative outcomes in 22 clinical trials comprising 933 patients [221]. It has also been confirmed in animal models can activate hypoxic signaling pathways, resist inflammation, prevent oxidative stress, which in turn promotes endogenous neuroprotection [223], attenuates renal I/R injury [224], facilitates flap survival after skin surgery [225], and protects cardiac function [226]. In studies on ECs, IPC has been mainly shown to counteract MVD by regulating NO and ET levels, protecting hepatic microvascular endothelial barrier function and blocking leukocyte adhesion [227]. Lin et al. also found that IPC reduces neurovascular damage after cerebral I/R by inhibiting neuronal and vascular endothelial cell apoptosis [228].
Due to the clinical unpredictability of AMI, the application of IPostC is more suitable for clinical application [229]. IPostC exerts neuroprotective effects by modulating central and peripheral glutamate in a cerebral I/R model [230], and acts by altering autophagy activity and expression of Beclin-1 in a time-dependent manner in a rat cardiac I/R model [231]. Albeit cardioprotection of IPostC has been confirmed in animal studies [229], but its translation to the clinic has not been promising, with contradictory results of " improved [232] and not improved [71]" on the effect of IPostC on endpoint events in different studies.
Compared to the limitation of IPC and IPostC in clinical practice, RIC has the advantage that distal regions such as the extremities are more ischemia-tolerant, easier to operate with lower risk to cause new I/R injury [233]. RIC has been found to protect brain [234], kidney [235] and cardiac [236] following sustained I/R. Its cardioprotective effects have been suggested to be possibly mediated by microparticles [237, 238]. However, microparticles have been shown to be associated with MVD. Boulanger et al. reported that circulating microparticles in rats with AMI caused endothelial dysfunction and vasodilatory dysfunction by selectively inhibiting eNOS expression [239]. A clinical study also demonstrated that the level of microparticles in the coronary arteries was the marker of persistent microthrombosis after I/R and correlation between microparticles and indices of MVD (CAG and ST resolution) implicated a critical role of microparticles in mediating MVD [240]. However, there are still some evidences that RIC can reduce MVO or edema/MVL [241], and such benefits have also been confirmed in clinical investigations [242, 243]. Overall, IPC exerts strongest cardioprotection not only for cardiomyocytes but also for CMECs, while both IPostC and RIC show ambiguous results, further investigations of precise mechanisms of these mechanical means on MVD protection are needed.
Potential pharmacological therapies
Many pharmacological agents currently have been tested in experimental models/or clinical trials, but most focus on myocardial protection rather than microvascular or CMECs. In this part, we focus on medicines or agents possessing actions of microvascular protection including conventional clinical drugs and some potential agents that still need to be explored and developed.
Sodium glucose co-transporter 2 inhibition
Sodium glucose co-transporter 2 (SGLT2) inhibitors, including canagliflozin, dapagliflozin, and empagliflozin, are a class of medicines for type 2 diabetes treatment that reducing glucose reabsorption and increases urinary glucose excretion via inhibition of SGLT2 in the proximal tubules of the kidney, hereby decreasing plasma glucose levels [244, 245]. Recently, numerous clinical studies have demonstrated multiple cardioprotective effects of this type of drugs with reduced risk of nonfatal MI, heart failure and mortality from all causes [246–250], and these effects appear to be independent of glycemic control [251, 252].
SGLT2 inhibition yields conspicuous effects in microvascular protection in various pathological conditions. In a mouse cardiac I/R injury model, empagliflozin [26] and dapagliflozin [253] were administered within 7 days before surgery. Both empagliflozin and dapagliflozin treatment showed the beneficial effects in maintaining microvascular architecture, reducing luminal stenosis, preventing microthrombosis and attenuating the inflammatory response resulted from the endothelial hyperpermeability [26, 253]. In a rat model of diabetic cardiomyopathy, administration of dapagliflozin through drinking water for 8 consecutive weeks improved myocardial fibrosis by blocking endothelial-to-mesenchymal transition [254]. Moreover, empagliflozin treatment in heart failure patient with preserved ejection fraction improved endothelial and cardiomyocyte function thereby protect the heart [255].
Endothelial protection by SGLT2 inhibition has also been documented in ex vivo cell experiments [26, 253]. In human coronary artery endothelial cell (HCAECs) hypoxia and reoxygenation (H/R) culture model, it was found that addition of empagliflozin before H/R could inhibit Drp1 phosphorylation and DNA–PKCs-induced Fis1 phosphorylation, thereby attenuating mitochondrial fission and normalizing mitochondrial fission/fusion balance [26]. Moreover, addition of dapagliflozin before hypoxia improved endothelial barrier function, eNOS activity and angiogenic capacity and reduced H/R-induced apoptosis by preventing cofilin-dependent F-actin depolymerization and cytoskeletal degradation [253]. Furthermore, dapagliflozin pre-treatment also down-regulated xanthine oxidase activity induced by H/R, inhibited the oxidation and inactivation of SERCA2, and suppressed intracellular calcium overload, thereby effectively protecting cytoskeletal integrity and endothelial cell activity [253]. These findings suggest that SGLT2 inhibitors could be a potentially effective drug to improve MVD and deserve further experimental and clinical investigations.
Relaxin
Relaxin is a natural peptide hormone and is considered to be one of the most pleiotropic hormones in the human body, capable of inducing a wider range of biological effects in addition to its role in reproductive function [256–258]. Relaxin-like family peptide receptor 1 (RXFP1) is the primary receptor for relaxin [257, 259, 260]. Since major cardiac cells, including cardiomyocytes, fibroblasts and CMECs all express RXFP1, so the heart is recognized as an effector organ of relaxin [257, 260]. Relaxin has been proposed as a therapeutic strategy for a number of CVD, such as heart failure, atrial fibrillation (AF), acute MI, ischemic heart disease, and hypertension [261].
In a guinea pig model of smoking-induced cardiovascular damage, administration of recombinant human relaxin-2 (serelaxin) through the entire induction period reversed smoking-induced abnormalities, such as aortic vessel wall thickening and massive loss of endothelial cells [262]. In a model in which guinea pig aortic endothelial cells were exposed to cigarette smoke extract solution, serelaxin pretreatment could maintain ECs viability and eNOS expression, thereby reducing smoking-mediated vascular injury and dysfunction [262]. Moreover, in a mouse cardiac I/R model, serelaxin treatment immediately after coronary artery occlusion alleviated MVD including both MVO and MVL measured at the end of reperfusion with maintenance of capillary opening and inhibition of local inflammatory responses, such protection led to a smaller infarct size and improved cardiac function and remodeling [176]. Further cell experiment documented that serelaxin treatment significantly reduced permeability of microvascular endothelial cell monolayers induced by H/R intervention with the underlying mechanism of preservation of VE–cadherin expression. These findings suggest that relaxin may be as a microvessel protective agent for MVD treatment.
Traditional Chinese medicine
Salvia miltiorrhiza (named Danshen in Chinese) is an important natural medicine (Chinese herb) which improves circulation and blood stasis [263]. Due to the effect of coronary artery dilatation [264], it is widely used in the treatment of CVDs in Asian countries to prevent myocardial ischemia [265], myocardial infarction [266], improve microcirculation [267] and reduce myocardial oxygen consumption [268, 269]. Tanshinone IIA (Tan IIA) is mainly isolated from salvia miltiorrhiza and is a representative of the lipolytic components. In a mouse cardiac I/R model, Tan IIA possesses following beneficial effects: (1) reduced endothelial cell apoptosis; (2) preserved the phosphorylation of eNOS, reduced ET-1 levels and mitigated vasoconstriction; (3) inhibited ICAM1 expression; (4) downregulated transcriptional expression of inflammatory mediators, including matrix metalloprotein-9, TNFα, IL-6 and MCP-1, thereby maintaining microvascular structure, decreasing susceptibility to thrombosis; attenuating regional inflammatory response and protecting microvascular barrier function [12]. Such protection is essential to prevent leakage of blood components into myocardial interstitium. Similar results were also observed by Chen et al. in a rat model of chronic intermittent hypoxia by continuous 8-week intraperitoneal injection of Tan IIA (20 mg/kg/d) [270]. Moreover, in CMECs H/R challenge model, Tan IIA intervention exerted anti-oxidative action to prevent mitochondrial damage evidenced by preserving expression of Sirtunin 1 and PGC1α, scavenging mitochondrial ROS, maintaining MMP and reducing mPTP opening [12]. Tan IIA also inhibited pro-apoptotic enzyme, caspase-9 activity and limited mitochondrial cytochrome C release [12]. Thus, maintenance of mitochondrial homeostasis is an important protective mechanism of Tan IIA.
Scutellarin is a source of an herb called scutellaria altissima, which is commonly used clinically for the treatment of paralysis after cerebrovascular disease. In 2018, Mo et al. reported that scutellarin could antioxidize and prevent atherosclerosis through up-regulation of SOD and down-regulation of NOX4 [271]. In addition, scutellarin protects vascular ECs from hyperglycemia-induced injury by upregulating mitophagy through the PINK1/Parkin signaling pathway [272]. Another herbal medicine called tetrahydrocurcumin is the main bioactive component of curcuma longa. It has been shown to ameliorate mitochondrial dysfunction in brain cells and thereby reduce brain damage and MVL in vivo [273] and ex vivo cell experiments [274] in mice. Quercetin and procyanidin B2 are both flavonoids widely distributed in plants. The former showed endothelial protective effects in both HUVECs [275] and cerebrovascular ECs [276], and the main mechanisms included promoting endothelial cell proliferation, migration and angiogenesis, maintaining MMP, inhibiting ERS, and inhibiting apoptosis. Nie et al. found that procyanidin B2 attenuated endothelial cell ERS through PPARδ-dependent pathway [139].
FX-06
FX-06 (fibrin-derived peptide Bβ15-42) is a fibrin Bβ chain-derived peptide and is constitutively liberated by the plasmin degradation of cross-linked fibrin [277]. It has been shown to reduce infarct size, maintain the endothelial barrier, and improve hemodynamic changes and survival in rodent and porcine I/R models [278]. FX-06 was found to bind to VE–cadherin and inhibit leukocyte migration, thereby initiating VE–cadherin-mediated signaling. The mechanisms of stabilizing endothelial cell connectivity and barrier function include the following: (1) counteracting stress fiber formation by preventing myosin light chain phosphorylation, and thereby rebalancing the activation state of RhoA/Rac 1 to inhibit dissociation between endothelial cells and VE–cadherin; (2) competing with the binding of the fibrin E1 fragment to VE–cadherin and preventing the dissociation of intercellular VE–cadherin complexes and preventing leukocyte migration [278]; and (3) binding to the VLDL receptor separates Fyn from VE–cadherin and prevents endothelial cell contraction [31].
CU06-1004
CU06-1004 (formerly Sac-1004), a pseudosaccharide derivative of cholesterol, is also known as an endothelial dysfunction blocker. CU06-1004 has been shown to improve prognosis by inhibiting the reduction of MVL in a variety of diseases [279]. In a rat I/R model, CU06-1004 enhances the endothelial barrier through the cAMP/Rac/cortical actin pathway and increases the formation of cortical actin rings, thereby preventing VEGF-induced destabilization of actin filaments and ultimately decreasing I/R-induced disruption of the blood–brain barrier [280]. A recent study found that CU06-1004 also significantly inhibited astrocyte end-feet swelling after I/R by decreasing the levels of connexin 43 and aquaporin 4. In addition, CU06-1004 inhibited the degradation of β1-integrin and β-dystroglycan anchored to the cortical actin ring of ECs, as well as suppressed the loss of laminin and type IV collagen [279]. In a mouse model of diabetic retinopathy, it similarly prevented retinal vascular leakage [281]. These findings illustrate the multiple MVL-targeted protective functions of CU06-1004, including maintaining vascular integrity, inducing vascular normalization [282], improving cardiac remodeling, and inhibiting edema and inflammation.
Potential agents targeting angiopoietin/Tie-2 pathway
Section “Impaired microvascular function” has described the important role of the angiopoietin/Tie-2 pathway in maintaining vascular integrity, and recent studies have identified a number of negative regulators of this pathway as potential therapeutic targets for MVL. The overall activity of the Ang-1/Ang-2/Tie-2 system can be variably controlled by the dephosphorylating activity of vascular endothelial–protein tyrosine phosphatase (VE–PTP) [283]. Razuprotafib (AKB-9778) is a potent and selective inhibitor of VE–PTP that activates Tie-2, enhances Ang-1-induced Tie-2 activation, and stimulates phosphorylation of signaling molecules in the Tie-2 pathway, including Akt, eNOS, and ERK. AKB-9778-induced Tie-2 phosphorylation strongly inhibits neovascularization in a mouse model [283]. Another way to target the angiopoietin/Tie-2 system is through the application of vasculotide, a short synthetic peptide (HHHRHSF), which binds with high affinity to Tie-2 and activates Ang-1-like receptors. Vasculotide prevents I/R injury-induced renal vascular leakage, improves renal function and reduces mortality in mice [31]. In addition, there are drugs, such as Ang-1 variant (COMP-Ang-1), which have been shown to have protective effects on peritoneal vascular permeability stabilization and peritoneal transport function in a rat sepsis model [284]. The mechanism is mainly increased phosphorylation of Tie-2 receptors, enhanced pericyte coverage and expression of endothelial cell junction proteins.
S1P1 agonist
SAR247799 is a g-protein-selective S1P1 agonist. SAR247799 activates S1P1 on ECs without inducing receptor desensitization and is endothelium-protective in rats and pigs. In a rat model of renal I/R injury, SAR247799 was endothelium-protective and preserved renal structure and function at doses that did not induce S1P1 desensitization. Conversely, clinically used functional antagonists of S1P1 provide only minimal renal protection and desensitize S1P1 resulting in side effects (endothelial injury) [285]. Thus, pharmacologically S1P1 can be sustainably activated with no effect on the immune response, which provides a novel approach for the treatment of diseases associated with endothelial dysfunction and vascular hyperpermeability. The product is currently in Phase 2 clinical trials [31].
As summarized in Table 2, different drugs have multifaceted protective effects on the microvasculature in different models. Considering the crucial role of endothelial barrier and subcellular organelles, e.g., mitochondrion and ER in the prevention of MVD, especially MVL, targeting interventions by increase mitophagy flux, coping with ERS in CMECs and maintaining endothelial cell connectivity may have beneficial effect on MVD protection after I/R. Table 3 lists some of the targeted therapeutic strategies to improve mitochondrial/ER/endothelial barrier function and the corresponding underlying mechanisms. Nevertheless, these interventions are still poorly studied in ECs and MVD. In the future, further clarification of the synergistic roles of mitochondrial fission/fusion, mitophagy, ERS and endothelial dysfunction in MVD pathogenesis is required. In addition, how to rapidly and effectively detect subcellular organelle damage after reperfusion and select the appropriate time node to use targeted drugs are also key challenges to be solved in the clinical setting. There are a number of clinical trials tested currently used drugs with improvement of ischemic myocardial salvage or acute and chronic outcomes accompanied by mitigation of MVO, including β-blockers; statins; adenosine, a potent vasodilator of coronary microvasculature [45]; atrial natriuretic peptide which suppressing ET-1 production, exenatide, a glucagon-like peptide-1 agonist and antiplatelet therapy [286]. Physical interventions such as therapeutic hyperoxaemia/super-saturated oxygen (SSO2) and therapeutic hypothermia have also been shown to be cardioprotective and attenuate MVD after I/R [18]. As inflammation is the primary driving force for further tissue damage following ischemia–reperfusion, many clinical trials also evaluate the protective effects of agents targeting on a number of established inflammatory mediators, including IL-1β, IL-, IL-6, CRP at ligand or receptor level [286, 287]. Most these clinical trials end up with partial protection or improvement in infarct size, cardiac function or acute and chronic prognosis and in MVO reduction, but none of evaluated agents and therapeutic targets achieved complete protection. Thus, identification of novel targets, proper model selection, comprehensive large scale and multicenter clinical trial implementation are the future directions of research.
Table 2.
Multifaceted protective effects of the drugs mentioned in the section of Potential pharmacological therapies on the microvasculature of different models
| Model | Species/ cell type |
Drug (dose) |
Vascular wall thickening/ lumen narrowing |
Inflammation | Thrombosis | Vasodilation/ eNOS activity |
Pathological mitochondrial fission | Mitophagy | Endothelial cells death | Ref No. |
|---|---|---|---|---|---|---|---|---|---|---|
| I/R (45 min/2 h) |
Mice HCAECs |
Empagliflozin (10 mg/kg/d) (10 µM) | ↓ | ↓ | ↓ | ↑ | ↓ | N | ↓ | [26] |
| I/R (45 min/2 h) | Mice | Empagliflozin (10 mg/kg/d) | ↓ | ↓ | ↓ | ↑ | ↓ | ↑ | ↓ | [37] |
| HFpEF | ZDF rats | Empagliflozin (0.25 mg/kg) | N | ↓ | N | ↑ | ↓ | N | N | [255] |
| I/R (45 min/2 h) |
Mice HCAECs |
Dapagliflozin (40 mg/kg/d) (10 µM) | ↓ | ↓ | N | ↑ | ↓ | N | ↓ | [253] |
| DCM |
Rats HUVECs |
Dapagliflozin (1 mg/kg/d) (1 μM) | N | N | N | N | N | N | ↓ | [254] |
| Smoking- induced CVD |
guinea pigs GPAECs |
Serelaxin (10 μg/day) (100 ng/ml) | ↓ | ↓ | N | ↑ | N | N | ↓ | [262] |
| I/R (1 h/24 h) (6 h/6 h) |
Mice H5V |
Serelaxin (50 μg/kg) (100 ng/ml) | ↓ | ↓ | N | ↑ | N | N | N | [176] |
| I/R (45 min/4 h) | Mice | Tan IIA (25 mg/kg) | N | ↓ | ↓ | ↑ | N | N | ↓ | [12] |
| CIH | Rats | Tan IIA (20 mg/kg/d) | ↓ | N | N | ↑ | N | N | ↓ | [270] |
I/R ischemia/reperfusion, HCAECs human coronary artery endothelial cells, HUVECs human umbilical vein endothelial cells, GPAECs guinea pig aortic endothelial cells H5V, mouse heart immortalized endothelial cells line, HFpEF heart failure with preserved ejection fraction, ZDF rats Zucker Diabetic Fatty rats, DCM Diabetic cardiomyopathy, CIH chronic intermittent hypoxia, Tan IIA Tanshinone IIA, ↑ increased or upregulated, ↓ decreased or downregulated, N information not available/mentioned
Table 3.
Targeted therapeutic strategies to improve mitochondrial/endoplasmic reticulum/barrier function in endothelial cells
| Model | Species/ cell type |
Drug (dose) | Target point | Mechanism | Ref No. |
|---|---|---|---|---|---|
| Cardiac I/R (45 min/2 h) | Mice | Empagliflozin (10 mg/kg/d) |
Mitochondrial function |
Empagliflozin attenuates cardiac microvascular ischemia/reperfusion through activating the AMPKα1/ULK1/FUNDC1/mitophagy pathway in cardiac microvascular endothelial cells | [37] |
| Cardiac I/R (45 min/2 h) |
Mice HCAECs |
Dapagliflozin (40 mg/kg/d) (10 µM) | Dapagliflozin reduces pathological mitochondrial division during cardiac I/R injury by normalizing the XO–SERCA2–CaMKII–coffilin pathway, thereby protecting endothelial and microvascular function | [253] | |
| Hyperglycemia | HUVECs | Liraglutide (10–80 nM) | Liraglutide prevents high glucose-induced dysfunction of endothelial cells by inhibiting PINK1/Parkin-dependent mitophagy | [288] | |
| Oxidative stress |
Rats HUVECs |
Melatonin (40 mg/kg) (100 µM) | Melatonin increases Parkin-dependent mitophagy through activating the AMPK–TFEB signaling pathway, attenuating oxidative stress and endothelial cell apoptosis, which in turn promotes random pattern skin flaps survival | [289] | |
| Hyperglycaemia Hyperlipidaemia | RAECs | Hydrogen sulphide | Hydrogen sulphide reduces endothelial cell apoptosis in a high-glucose and high-palmitate environment by inhibiting oxidative stress, reducing mitochondrial fragmentation and promoting mitophagy | [290] | |
| Hyperhomocysteinemia | HUVECs | Tan IIA (100 µM) | Tan IIA protects endothelial cells from hyperhomocysteine-induced vascular endothelial damage by inhibiting intracellular oxidative stress and mitochondrial dysfunction through activation of the NNMT/SIRT 1-mediated NRF2/HO-1 and AKT/MAPKs signaling pathways | [291] | |
| Hyperglycemia | HUVECs | Scutellarin (30 μM) | Scutellarin protects vascular endothelial cells from hyperglycemia-induced injury by upregulating mitophagy through the PINK1/Parkin signaling pathway | [272] | |
|
Type 2 diabetic and lung I/R (1.5 h/4 h) |
Rats | Adiponectin (100 μg/kg) | Adiponectin attenuates I/R injury-induced oxidative stress, inflammation, endothelial cell apoptosis, and mitochondrial dysfunction by activating mitophagy mediated by the SIRT1–PINK1 signaling pathway | [292] | |
| Hyperhomocysteinemia | bEnd3 | Tetrahydrocurcumin (15 μM) | Pretreatment of endothelial cells with tetrahydrocurcumin ameliorated Homocysteine-induced oxidative damage, mitochondrial fission/fusion and mitophagy | [274] | |
| High glucose and palmitate | HAECs | Ginseng-Sanqi-Chuanxiong extracts (GSC) | GSC extracts reverse mitophagy damage caused by high glucose and palmitate via AMPK pathway, regulates mitophagy, and thus ameliorates diabetes-induced endothelial cell senescence | [293] | |
| Cerebral I/R | Mice | Tetrahydrocurcumin (25 mg/kg) | Tetrahydrocurcumin eliminates cerebral edema and MVL in the brain parenchyma by improving mitochondrial function in cerebrovascular endothelial cells during ischemic stroke | [273] | |
| H/R (8 h/2 h) | HUVECs | Acetylcholine (10 μM) |
MAM function |
Acetylcholine inhibits H/R-induced intracellular Ca2+ overload in endothelial cells; inhibits ER Ca2 + depletion and mitochondrial Ca2+ overload during H/R; suppresses mitochondria–ER-dependent apoptosis by inhibiting M3AChR; and protects against I/R injury by inducing disruption of MAM in a NO-dependent manner | [144] |
| Sepsis | HUVECs | Adiponectin (10–20 μg/mL) |
ER function |
Adiponectin treatment reduces sepsis-induced endothelial cell apoptosis by attenuating oxidative stress and activating ERS pathways | [294] |
| H/R (12 h/8 h) | HBMECs | Quercetin (1 μM) | Quercetin has protective effects on HBMECs, including inhibiting apoptosis by promoting endothelial cell proliferation, migration and angiogenesis, maintaining MMP, and inhibiting ERS | [276] | |
| Hyperglycemia | HUVECs | Procyanidin B2 (10 μM) | Procyanidin B2 attenuates endothelial cell ERS through a PPARδ-dependent mechanism | [139] | |
| Hyperglycemia | HCAECs | Liraglutide (1 μM) | Liraglutide protects endothelial cells by attenuating ERS by inhibiting the phosphorylation of IRE1α and PERK and the expression of ATF6 and GRP78 | [295] | |
| Tunicamycin induced ERS | HCAECs | Dapagliflozin (1 μM) | Dapagliflozin protects endothelial cells by attenuating ERS by inhibiting the phosphorylation of IRE1α and PERK and the expression of ATF6 and GRP78 | [295] | |
| Hyperglycemia | HCAECs | Metformin (2.5 mM) | Metformin protects endothelial cells by attenuating ERS by inhibiting the phosphorylation of IRE1α and PERK and the expression of ATF6 and GRP78 | [295] | |
| ox-LDL-induced ERS | HUVECs | Melatonin (5 μM) | Melatonin attenuates ox-LDL-induced endothelial dysfunction by inhibiting the upregulation of CHOP and GRP78 to reduce ERS, which in turn inhibits JNK/Mff signaling | [296] | |
| ZDF rats | Rats | Tongxinluo 50 mg/kg |
Barrier function |
Tongxinluo protects the integrity of the endothelial barrier by activating Angptl, significantly reducing endothelial cell apoptosis, and enhancing tight junction protein and VE–cadherin expression, thereby preventing MVL and IMH | [297] |
| Cardiac I/R (20 min/2 h) | Rats | FX-06 (2.4 mg/kg) | FX-06 inhibits dissociation between endothelial cells and VE–cadherin by rebalancing the activation state of RhoA/Rac 1; prevents migration of leukocytes by blocking dissociation of the intercellular VE–cadherin complex and prevents leukocyte migration; and prevents contraction of endothelial cells by binding to the VLDL receptor | [277] | |
|
Cerebral I/R (1.5 h) OGD/R (6 h/16 h) |
Mice HBMECs |
CU06-1004 (10 mg/kg) | CU06-1004 not only enhances the endothelial barrier via the cAMP/Rac/cortical actin pathway, but also reduces I/R-induced disruption of the blood–brain barrier by decreasing the levels of connexin43 and aquaporin 4 | [279] | |
| H/R (12 h/8 h) | HBMECs | Quercetin (0.1–10 μM) | Quercetin can increase the level of endothelial intercellular junction proteins such as Claudin 5 to maintain the integrity of the blood–brain barrier | [275] | |
|
Ischemic retinopathy Ang2 induced injury |
Mice HUVECs |
Razuprotafib (20 mg/kg) (0.17–50 μM) | Razuprotafib (AKB-9778) selectively inhibits VE–PTP, activates Tie-2, and stimulates phosphorylation of signaling molecules in the Tie-2 pathway, including Akt, eNOS, and ERK, which strongly inhibits neovascularization and protects the endothelial barrier | [283] | |
| Renal I/R (35 min/24 h) | Mice | HHHRHSF (200 ng) | HHHRHSF activates Tie-2 signaling and blocks the vascular endothelial barrier in I/R ischemic kidneys without damaging renal tubules, thereby improving renal function and reducing mortality in mice | [298] | |
| Subtotal nephrectomy induced uremia | Rats | COMP-Ang-1 (1 × 1010 pfu/ml per) | COMP-Ang-1 protects peritoneal vascular permeability stability and peritoneal transport function by increasing Tie-2 receptor phosphorylation, enhancing pericyte coverage and endothelial cell junction protein expression | [284] | |
| Renal I/R (25 min/24 h) | Rats | SAR247799 (5 mg) | In a rat model of renal I/R injury, SAR247799 is endothelium-protective and preserves renal structure and function at doses that do not induce S1P1 desensitization | [285] |
I/R ischemia/reperfusion, HCAECs human coronary artery endothelial cells, RAECs rat aortic endothelial cells, HUVECs human umbilical vein endothelial cells, bEnd3 mouse brain endothelial cells, HAECs human aortic endothelial cells HBMECs human brain microvascular endothelial cells, ox-LDL- oxidized low-density lipoprotein, H/R hypoxia/reoxygenation, Tan IIA Tanshinone IIA, MAM mitochondria-associated ER membrane, OGD/R oxygen–glucose deprivation/reoxygenation, rats Zucker Diabetic Fatty rats
The role and impact of microvascular damage in other diseases
Cerebrovascular disease
Currently, cerebrovascular disease is also the major leading cause of death globally, behind IHD [299]. There were 6.55 million people died of stroke in 2019, accounting for 11.6% of all deaths [300]. Cerebrovascular disease mainly includes ischemic stroke, hemorrhagic stroke, and subarachnoid hemorrhage (SAH). Acute ischemic stroke (AIS) is an infarction of brain tissue due to occlusion of cerebral arteries, accompanied by neuronal damage with high morbidity and mortality [301, 302]. Similar to myocardial I/R injury, oxidative stress, inflammatory response and microvascular endothelial cell dysfunction occur during cerebral ischemia and hypoxia [303–305], while oxygen supply is rapidly restored during reperfusion with a surge of ROS and secondary mitochondrial dysfunction [306–308]. These series of pathological changes eventually lead to massive microvascular endothelial cells death, cerebrovascular dysfunction, and more severe brain tissue infarction.
Currently, tissue-type plasminogen activator (tPA) is the only approved thrombolytic drug for the treatment of AIS [309], with significant clinical benefits when administered within 4.5 h after stroke onset [310–312]. However, tPA administration, especially delayed administration, may also increase cerebral edema, intracranial hemorrhage (ICH), and mortality [311, 313]. In addition to its own neurotoxicity, tPA, more importantly, can activate matrix metalloproteinase that degrade extracellular matrix integrity and increase risks of neurovascular cell death, blood–brain barrier leakage, edema, and hemorrhage [314–316], ultimately limiting the therapeutic effect in approximately 50% of patients in clinical practice [317]. In a model of lacunar infarction induced by injecting ET-1 into the rat internal capsule, transplantation of healthy endothelial cells into the internal capsule of the brain produced beneficial effect on the outcome of ischemic brain injury [318]. This result indicates the significance of maintenance of normal endothelial cell numbers and function in AIS treatment.
Diabetes
Diabetes mellitus (DM) is a metabolic disorder characterized by hyperglycemia, with an estimated global prevalence of 10.5% (536.6 million people) aged 20–79 years in 2021, rising to 12.2% (783.2 million people) in 2045 [319, 320]. Currently, approximately 90% of adult diabetics are type 2 diabetes [321]. Asia has become the main region, where DM is rapidly increasing [321]. The most common damage of DM is the diabetic microvascular complication (DMC), which significantly affects the quality of life, prognosis and life expectancy of diabetic patients [322, 323]. DMC represents a “syndrome”, occurring in multiple organs and tissues, such as retina, kidney, heart, nerve tissue, skin and extremities [324, 325]. It manifests as blindness due to diabetic retinopathy, end-stage renal failure due to diabetic nephropathy, numbness and pain in the extremities due to diabetic peripheral neuropathy, and finger/toe-end necrosis and long-term incurable skin ulcers due to MVD [322–324, 326]. DMC is characterized by vascular endothelial damage, increased blood viscosity, erythrocyte aggregation, platelet adhesion, micro-thrombosis and MVO [324, 327]. The mechanism is similar to that of ischemic cardiac MVD. Microvascular ECs mainly utilize glycolysis to produce energy [328]; however, excess glucose can damage microvascular ECs by generating large amounts of ROS via the polyol pathway and large amounts of advanced glycosylation end products (AGEs) via the sorbitol pathway [328, 329]. Moreover, chronic hyperglycemia also inhibits NO production by endothelial cells, stimulates the release of vasoconstrictive factors and accelerates apoptosis of microvascular ECs, which leads to abnormal constriction of micro-vessels [330]. Besides, degradation of the vascular basement membrane is another mechanism of DMC. Matrix metalloproteinases play an important role in the regulation of cellular homeostasis by degrading the extracellular matrix [331]. Excessive ROS stimulates the synthesis and secretion of matrix metalloproteinases, accelerating the degradation of vascular basement membranes and the development of DMC. In a cell model of mouse microvascular endothelial cell damage caused by a high glucose (30 mM) stimulation for 72 h, inhibition of ROS levels and matrix metalloproteinase activity by ropivacaine could attenuate high glucose-induced endothelial cell injury [332].
Kidney disease
Acute kidney injury (AKI) is a major risk factor for progressive renal insufficiency. Currently, more than 20% of hospitalized adults worldwide experience certain degree of AKI, most commonly due to kidney I/R injury and sepsis, AKI can lead to irreversible renal function loss as well as end-stage renal fibrosis [333, 334]. Damage to the renal microcirculation, particularly to the peritubular capillary (PTC) network, has been reported to be a key factor in the development of interstitial fibrosis in patients with kidney disease [335]. There are substantial evidences that PTC injury, sparing and interstitial fibrosis are typical pathological changes whatever the etiology of the kidney disease [335, 336]. PTC endothelial damage after renal I/R is similar to MVD of myocardial I/R injury, which manifests as early and progressive endothelial swelling, subendothelial edema, endothelial thickening and increased capillary permeability [333, 337]. After kidney I/R injury, damaged renal parenchymal cells and various inflammatory mediators upregulate the expression of adhesion molecules and mediate the migration of various types of inflammatory cells to the site of kidney injury, and the progressively increasing inflammatory response and excessive ROS production can lead to renal MECs dysfunction [338]. In addition to causing MVD, hypoxia can also cause an overexpression of HIF-1α in tubular epithelial cells, which stimulates proliferation of inflammatory cells and their recruitment to the kidney, induces maladaptive expression of matrix-modifying factors in hypoxic tubular epithelial cells and promotes epithelial-to-mesenchymal transition, eventually resulting in renal fibrosis [335, 339].
Conclusion
In industrialized countries, due to the spread of preventive measures in society and advances in medical technology, the majority of patients survive AMI and the mortality rate continues to decline [340]. However, the prevalence of heart failure after MI is on the rise, and the main reason for this lies in the weakened myocardium [341] due to complications after revascularization, such as MVD, including MVO and MVL. MVD has a significant impact on the severity, development, and prognosis of IHD [342, 343]. Clinically, most patients are of advanced age and have a long history of comorbidities, such as hypertension, dyslipidemia, diabetes mellitus and chronic inflammatory diseases. It is difficult for clinical studies to avoid many influential and confounding factors. Moreover, MVD itself involves a variety of clinical manifestations and molecular mechanisms, and the interaction between them is a complex signal transduction cascade. Experimental studies usually focus on “single” mechanism/intervention in young, male and healthy animals, ignoring the reciprocal or synergistic roles of other pathways. Therefore, the current array of clinical tests and intervention strategies for MVD after myocardial I/R injury has not yielded satisfactory clinical results. In addition, majority of previous studies have focused on the cardiomyocyte injury, but few studies on CMECs, the most abundant cardiac cellular components. The protection of the microvasculature has continued to receive less attention despite the overwhelming pathophysiologic evidence of the significance of MVD, especially MVL. Limited experimental benefits in MVL intervention still require clinical confirmation. In the future, targeting CMECs protection may represent a new direction in the battle against cardiac I/R injury, rather than focusing on cardiomyocyte only, which call for more comprehensive studies to explore molecular mechanisms and novel therapeutic interventions to prevent MVD.
Author contributions
BHZ, AR, LZ and QLL drafted the manuscript, JT, NX and AXD generated the images, MTG and XFS edited the manuscript and XMG revised the manuscript and responsible for funding support. All of the authors have read and approved the content of the manuscript.
Funding
This work was supported by grants of the Natural Science Foundation of China (No. U1903212, 81870272), opening project of the Xinjiang Key Laboratory (2021D04020), the State Key Laboratory of Pathogenesis, Prevention and Treatment of Central Asia High Incidence Diseases fund (SKL-HIDCA-2021-XXG1, SKL-HIDCA-2022-XXG1) and the key project of the Natural Science Foundation of Xinjiang Uygur Autonomous Region (2023).
Availability of data and materials
Not applicable.
Declarations
Conflict of interest
There are no conflicts of interest to declare.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li Y, Schoufour J, Wang DD, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ. 2020;368:l6669. doi: 10.1136/bmj.l6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatnagar A. Environmental determinants of cardiovascular disease. Circ Res. 2017;121(2):162–180. doi: 10.1161/CIRCRESAHA.117.306458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W, Hu M, Liu H, et al. Global Burden of Disease Study 2019 suggests that metabolic risk factors are the leading drivers of the burden of ischemic heart disease. Cell Metab. 2021;33(10):1943–1956e1942. doi: 10.1016/j.cmet.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Balakumar P, Maung UK, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res. 2016;113(Pt A):600–609. doi: 10.1016/j.phrs.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 5.Organization WH (2021) Cardiovascular diseases (CVDs). Retrieved from https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed 14 Feb 2023
- 6.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smit M, Coetzee AR, Lochner A. The pathophysiology of myocardial ischemia and perioperative myocardial infarction. J Cardiothorac Vasc Anesth. 2020;34(9):2501–2512. doi: 10.1053/j.jvca.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 9.Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35(17):1101–1111. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang X, Lochner A, Wang HH, et al. Coronary microvascular injury in myocardial infarction: perception and knowledge for mitochondrial quality control. Theranostics. 2021;11(14):6766–6785. doi: 10.7150/thno.60143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleinbongard P, Heusch G. A fresh look at coronary microembolization. Nat Rev Cardiol. 2022;19(4):265–280. doi: 10.1038/s41569-021-00632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong J, Ouyang H, Sun M, et al. Tanshinone IIA attenuates cardiac microvascular ischemia-reperfusion injury via regulating the SIRT1-PGC1alpha-mitochondrial apoptosis pathway. Cell Stress Chaperones. 2019;24(5):991–1003. doi: 10.1007/s12192-019-01027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khuddus MA, Pepine CJ, Handberg EM, et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) J Interv Cardiol. 2010;23(6):511–519. doi: 10.1111/j.1540-8183.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds HR, Diaz A, Cyr DD, et al. Ischemia with nonobstructive coronary arteries: insights from the ISCHEMIA Trial. JACC Cardiovasc Imaging. 2023;16(1):63–74. doi: 10.1016/j.jcmg.2022.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356(8):830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 16.Heusch G. The coronary circulation as a target of cardioprotection. Circ Res. 2016;118(10):1643–1658. doi: 10.1161/CIRCRESAHA.116.308640. [DOI] [PubMed] [Google Scholar]
- 17.Lambert L, Brown K, Segal E, et al. Association between timeliness of reperfusion therapy and clinical outcomes in ST-elevation myocardial infarction. JAMA. 2010;303(21):2148–2155. doi: 10.1001/jama.2010.712. [DOI] [PubMed] [Google Scholar]
- 18.He J, Bellenger NG, Ludman AJ, et al. Treatment of myocardial ischaemia-reperfusion injury in patients with ST-segment elevation myocardial infarction: promise, disappointment, and hope. Rev Cardiovasc Med. 2022;23(1):23. doi: 10.31083/j.rcm2301023. [DOI] [PubMed] [Google Scholar]
- 19.Wu S, Chang G, Gao L, et al. Trimetazidine protects against myocardial ischemia/reperfusion injury by inhibiting excessive autophagy. J Mol Med (Berl) 2018;96(8):791–806. doi: 10.1007/s00109-018-1664-3. [DOI] [PubMed] [Google Scholar]
- 20.Davidson SM, Ferdinandy P, Andreadou I, et al. Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J Am Coll Cardiol. 2019;73(1):89–99. doi: 10.1016/j.jacc.2018.09.086. [DOI] [PubMed] [Google Scholar]
- 21.Yang CF. Clinical manifestations and basic mechanisms of myocardial ischemia/reperfusion injury. Ci Ji Yi Xue Za Zhi. 2018;30(4):209–215. doi: 10.4103/tcmj.tcmj_33_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu E, Ortiz JT, Tejedor P, et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart. 2008;94(6):730–736. doi: 10.1136/hrt.2007.122622. [DOI] [PubMed] [Google Scholar]
- 23.Majidi M, Kosinski AS, Al-Khatib SM, et al. Reperfusion ventricular arrhythmia 'bursts' predict larger infarct size despite TIMI 3 flow restoration with primary angioplasty for anterior ST-elevation myocardial infarction. Eur Heart J. 2009;30(7):757–764. doi: 10.1093/eurheartj/ehp005. [DOI] [PubMed] [Google Scholar]
- 24.Saia F, Grigioni F, Marzocchi A, et al. Management of acute left ventricular dysfunction after primary percutaneous coronary intervention for ST elevation acute myocardial infarction. Am Heart J. 2010;160(6 Suppl):S16–21. doi: 10.1016/j.ahj.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 26.Zou R, Shi W, Qiu J, et al. Empagliflozin attenuates cardiac microvascular ischemia/reperfusion injury through improving mitochondrial homeostasis. Cardiovasc Diabetol. 2022;21(1):106. doi: 10.1186/s12933-022-01532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Toan S, Zhou H. New insights into the role of mitochondria in cardiac microvascular ischemia/reperfusion injury. Angiogenesis. 2020;23(3):299–314. doi: 10.1007/s10456-020-09720-2. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Toan S, Zhou H. Mitochondrial quality control in cardiac microvascular ischemia-reperfusion injury: new insights into the mechanisms and therapeutic potentials. Pharmacol Res. 2020;156:104771. doi: 10.1016/j.phrs.2020.104771. [DOI] [PubMed] [Google Scholar]
- 29.Wu D, Ji H, Du W, et al. Mitophagy alleviates ischemia/reperfusion-induced microvascular damage through improving mitochondrial quality control. Bioengineered. 2022;13(2):3596–3607. doi: 10.1080/21655979.2022.2027065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chia PY, Teo A, Yeo TW. Overview of the assessment of endothelial function in humans. Front Med (Lausanne) 2020;7:542567. doi: 10.3389/fmed.2020.542567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kloka JA, Friedrichson B, Wulfroth P, et al. Microvascular leakage as therapeutic target for ischemia and reperfusion injury. Cells. 2023 doi: 10.3390/cells12101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinto AR, Ilinykh A, Ivey MJ, et al. Revisiting cardiac cellular composition. Circ Res. 2016;118(3):400–409. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassenge E, Heusch G. Endothelial and neuro-humoral control of coronary blood flow in health and disease. Rev Physiol Biochem Pharmacol. 1990;116:77–165. doi: 10.1007/3540528806_4. [DOI] [PubMed] [Google Scholar]
- 34.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol (Engl Ed) 2017;70(12):1082. doi: 10.1016/j.rec.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 36.Annibali G, Scrocca I, Aranzulla TC, et al. "No-Reflow" phenomenon: a contemporary review. J Clin Med. 2022 doi: 10.3390/jcm11082233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai C, Guo Z, Chang X, et al. Empagliflozin attenuates cardiac microvascular ischemia/reperfusion through activating the AMPKalpha1/ULK1/FUNDC1/mitophagy pathway. Redox Biol. 2022;52:102288. doi: 10.1016/j.redox.2022.102288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heusch G, Kleinbongard P, Bose D, et al. Coronary microembolization: from bedside to bench and back to bedside. Circulation. 2009;120(18):1822–1836. doi: 10.1161/CIRCULATIONAHA.109.888784. [DOI] [PubMed] [Google Scholar]
- 39.Alidoosti M, Lotfi R, Lotfi-Tokaldany M et al (2018) Correlates of the "No-Reflow" or "Slow-Flow" phenomenon in patients undergoing primary percutaneous coronary intervention. J Tehran Heart Cent, 13(3), 108–114. https://www.ncbi.nlm.nih.gov/pubmed/30745923. Accessed 15 Aug 2023 [PMC free article] [PubMed]
- 40.Ambrosio G, Weisman HF, Mannisi JA, et al. Progressive impairment of regional myocardial perfusion after initial restoration of postischemic blood flow. Circulation. 1989;80(6):1846–1861. doi: 10.1161/01.cir.80.6.1846. [DOI] [PubMed] [Google Scholar]
- 41.Reffelmann T, Kloner RA. Microvascular reperfusion injury: rapid expansion of anatomic no reflow during reperfusion in the rabbit. Am J Physiol Heart Circ Physiol. 2002;283(3):H1099–1107. doi: 10.1152/ajpheart.00270.2002. [DOI] [PubMed] [Google Scholar]
- 42.Harrison RW, Aggarwal A, Ou FS, et al. Incidence and outcomes of no-reflow phenomenon during percutaneous coronary intervention among patients with acute myocardial infarction. Am J Cardiol. 2013;111(2):178–184. doi: 10.1016/j.amjcard.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz BG, Kloner RA. Coronary no reflow. J Mol Cell Cardiol. 2012;52(4):873–882. doi: 10.1016/j.yjmcc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Ndrepepa G, Tiroch K, Fusaro M, et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol. 2010;55(21):2383–2389. doi: 10.1016/j.jacc.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 45.Niccoli G, Montone RA, Ibanez B, et al. Optimized treatment of ST-elevation myocardial infarction. Circ Res. 2019;125(2):245–258. doi: 10.1161/CIRCRESAHA.119.315344. [DOI] [PubMed] [Google Scholar]
- 46.van Kranenburg M, Magro M, Thiele H, et al. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging. 2014;7(9):930–939. doi: 10.1016/j.jcmg.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Aggarwal P, Rekwal L, Sinha SK, et al. Predictors of no-reflow phenomenon following percutaneous coronary intervention for ST-segment elevation myocardial infarction. Ann Cardiol Angeiol (Paris) 2021;70(3):136–142. doi: 10.1016/j.ancard.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Ramadan R, Vromans E, Anang DC, et al. Connexin43 hemichannel targeting with TAT-Gap19 alleviates radiation-induced endothelial cell damage. Front Pharmacol. 2020;11:212. doi: 10.3389/fphar.2020.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L, Ge C, Zhang X. Sufentanil ameliorates oxygen-glucose deprivation/reoxygenation-induced endothelial barrier dysfunction in HCMECs via the PI3K/Akt signaling pathway. Exp Ther Med. 2022;24(1):437. doi: 10.3892/etm.2022.11364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao XM, Wu QZ, Kiriazis H, et al. Microvascular leakage in acute myocardial infarction: characterization by histology, biochemistry, and magnetic resonance imaging. Am J Physiol Heart Circ Physiol. 2017;312(5):H1068–H1075. doi: 10.1152/ajpheart.00073.2017. [DOI] [PubMed] [Google Scholar]
- 51.Kloner RA, Ganote CE, Jennings RB. The "no-reflow" phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54(6):1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heusch G. Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res Cardiol. 2019;114(6):45. doi: 10.1007/s00395-019-0756-8. [DOI] [PubMed] [Google Scholar]
- 53.Kang S, Yang Y. Coronary microvascular reperfusion injury and no-reflow in acute myocardial infarction. Clin Invest Med. 2007;30(3):E133–145. doi: 10.25011/cim.v30i3.1082. [DOI] [PubMed] [Google Scholar]
- 54.Erkol A, Oduncu V, Turan B, et al. The value of plasma D-dimer level on admission in predicting no-reflow after primary percutaneous coronary intervention and long-term prognosis in patients with acute ST segment elevation myocardial infarction. J Thromb Thrombolysis. 2014;38(3):339–347. doi: 10.1007/s11239-013-1044-3. [DOI] [PubMed] [Google Scholar]
- 55.Grygier M, Araszkiewicz A, Lesiak M, et al. Intracoronary adenosine administered during aortocoronary vein graft interventions may reduce the incidence of no-reflow phenomenon. A pilot randomised trial. Kardiol Pol. 2014;72(2):126–133. doi: 10.5603/KP.a2013.0213. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Wu L, Tian X, et al. Intravascular ultrasound observation of the mechanism of no-reflow phenomenon in acute myocardial infarction. PLoS ONE. 2015;10(6):e0119223. doi: 10.1371/journal.pone.0119223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cenko E, Ricci B, Kedev S, et al. The no-reflow phenomenon in the young and in the elderly. Int J Cardiol. 2016;222:1122–1128. doi: 10.1016/j.ijcard.2016.07.209. [DOI] [PubMed] [Google Scholar]
- 58.Chen WR, Tian F, Chen YD, et al. Effects of liraglutide on no-reflow in patients with acute ST-segment elevation myocardial infarction. Int J Cardiol. 2016;208:109–114. doi: 10.1016/j.ijcard.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Durante A, Laricchia A, Benedetti G, et al. Identification of high-risk patients after ST-segment-elevation myocardial infarction: comparison between angiographic and magnetic resonance parameters. Circ Cardiovasc Imaging. 2017;10(6):e005841. doi: 10.1161/CIRCIMAGING.116.005841. [DOI] [PubMed] [Google Scholar]
- 60.Liang T, Liu M, Wu C, et al. Risk factors for no-reflow phenomenon after percutaneous coronary intervention in patients with acute coronary syndrome. Rev Invest Clin. 2017;69(3):139–145. doi: 10.24875/ric.17002190. [DOI] [PubMed] [Google Scholar]
- 61.Li H, Fu DG, Liu FY, et al. Evaluation of related factors, prediction and treatment drugs of no-reflow phenomenon in patients with acute ST-segment elevation myocardial infarction after direct PCI. Exp Ther Med. 2018;15(4):3940–3946. doi: 10.3892/etm.2018.5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahmoud AH, Taha NM, Baraka K, et al. Clinical and procedural predictors of suboptimal myocardial reperfusion in primary percutaneous coronary intervention. Int J Cardiol Heart Vasc. 2019;23:100357. doi: 10.1016/j.ijcha.2019.100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang L, Cong H, Lu Y, et al. Prediction of no-reflow phenomenon in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Medicine (Baltimore) 2020;99(26):e20152. doi: 10.1097/MD.0000000000020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rossington JA, Sol E, Masoura K, et al. No-reflow phenomenon and comparison to the normal-flow population postprimary percutaneous coronary intervention for ST elevation myocardial infarction: case-control study (NORM PPCI) Open Heart. 2020 doi: 10.1136/openhrt-2019-001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sadeghian M, Mousavi SH, Aamaraee Z, et al. Administration of intracoronary adenosine before stenting for the prevention of no-reflow in patients with ST-elevation myocardial infarction. Scand Cardiovasc J. 2022;56(1):23–27. doi: 10.1080/14017431.2022.2035807. [DOI] [PubMed] [Google Scholar]
- 66.d'Entremont MA, Alazzoni A, Dzavik V, et al. No-reflow after primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction: an angiographic core laboratory analysis of the TOTAL Trial. EuroIntervention. 2023;19(5):e394–e401. doi: 10.4244/EIJ-D-23-00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eitel I, Wohrle J, Suenkel H, et al. Intracoronary compared with intravenous bolus abciximab application during primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: cardiac magnetic resonance substudy of the AIDA STEMI trial. J Am Coll Cardiol. 2013;61(13):1447–1454. doi: 10.1016/j.jacc.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 68.Hadamitzky M, Langhans B, Hausleiter J, et al. Prognostic value of late gadolinium enhancement in cardiovascular magnetic resonance imaging after acute ST-elevation myocardial infarction in comparison with single-photon emission tomography using Tc99m-Sestamibi. Eur Heart J Cardiovasc Imaging. 2014;15(2):216–225. doi: 10.1093/ehjci/jet176. [DOI] [PubMed] [Google Scholar]
- 69.Kandler D, Lucke C, Grothoff M, et al. The relation between hypointense core, microvascular obstruction and intramyocardial haemorrhage in acute reperfused myocardial infarction assessed by cardiac magnetic resonance imaging. Eur Radiol. 2014;24(12):3277–3288. doi: 10.1007/s00330-014-3318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ding S, Li Z, Ge H, et al. Impact of early ST-segment changes on cardiac magnetic resonance-verified intramyocardial haemorrhage and microvascular obstruction in ST-elevation myocardial infarction patients. Medicine (Baltimore) 2015;94(35):e1438. doi: 10.1097/MD.0000000000001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim EK, Hahn JY, Song YB, et al. Effect of ischemic postconditioning on myocardial salvage in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: cardiac magnetic resonance substudy of the POST randomized trial. Int J Cardiovasc Imaging. 2015;31(3):629–637. doi: 10.1007/s10554-015-0589-y. [DOI] [PubMed] [Google Scholar]
- 72.Desch S, Stiermaier T, de Waha S, et al. Thrombus aspiration in patients with ST-segment elevation myocardial infarction presenting late after symptom onset. JACC Cardiovasc Interv. 2016;9(2):113–122. doi: 10.1016/j.jcin.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 73.Carrick D, Haig C, Ahmed N, et al. Myocardial Hemorrhage after acute reperfused ST-segment-elevation myocardial infarction: relation to microvascular obstruction and prognostic significance. Circ Cardiovasc Imaging. 2016;9(1):e004148. doi: 10.1161/CIRCIMAGING.115.004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nazir SA, McCann GP, Greenwood JP, et al. Strategies to attenuate micro-vascular obstruction during P-PCI: the randomized reperfusion facilitated by local adjunctive therapy in ST-elevation myocardial infarction trial. Eur Heart J. 2016;37(24):1910–1919. doi: 10.1093/eurheartj/ehw136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kloner RA. The importance of no-reflow/microvascular obstruction in the STEMI patient. Eur Heart J. 2017;38(47):3511–3513. doi: 10.1093/eurheartj/ehx288. [DOI] [PubMed] [Google Scholar]
- 76.Broch K, Anstensrud AK, Woxholt S, et al. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2021;77(15):1845–1855. doi: 10.1016/j.jacc.2021.02.049. [DOI] [PubMed] [Google Scholar]
- 77.Bulluck H, Carberry J, Carrick D, et al. A noncontrast CMR risk score for long-term risk stratification in reperfused ST-segment elevation myocardial infarction. JACC Cardiovasc Imaging. 2022;15(3):431–440. doi: 10.1016/j.jcmg.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 78.Tiller C, Reindl M, Holzknecht M, et al. Association of plasma interleukin-6 with infarct size, reperfusion injury, and adverse remodelling after ST-elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2022;11(2):113–123. doi: 10.1093/ehjacc/zuab110. [DOI] [PubMed] [Google Scholar]
- 79.Li Z, Yin H, Wang D, et al. Prediction of microvascular obstruction by coronary artery angiography score after acute ST-segment elevation myocardial infarction: a single-center retrospective observational study. BMC Cardiovasc Disord. 2022;22(1):410. doi: 10.1186/s12872-022-02836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Husser O, Monmeneu JV, Sanchis J, et al. Cardiovascular magnetic resonance-derived intramyocardial hemorrhage after STEMI: Influence on long-term prognosis, adverse left ventricular remodeling and relationship with microvascular obstruction. Int J Cardiol. 2013;167(5):2047–2054. doi: 10.1016/j.ijcard.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 81.Carrick D, Haig C, Ahmed N, et al. Temporal evolution of myocardial hemorrhage and edema in patients after acute ST-segment elevation myocardial infarction: pathophysiological insights and clinical implications. J Am Heart Assoc. 2016 doi: 10.1161/JAHA.115.002834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garg P, Broadbent DA, Swoboda PP, et al. Extra-cellular expansion in the normal, non-infarcted myocardium is associated with worsening of regional myocardial function after acute myocardial infarction. J Cardiovasc Magn Reson. 2017;19(1):73. doi: 10.1186/s12968-017-0384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Homme RP, George AK, Singh M, et al. Mechanism of blood-heart-barrier leakage: implications for COVID-19 induced cardiovascular injury. Int J Mol Sci. 2021 doi: 10.3390/ijms222413546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li S, Chen J, Liu M, et al. Protective effect of HINT2 on mitochondrial function via repressing MCU complex activation attenuates cardiac microvascular ischemia-reperfusion injury. Basic Res Cardiol. 2021;116(1):65. doi: 10.1007/s00395-021-00905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tian F, Zhang Y. Overexpression of SERCA2a alleviates cardiac microvascular ischemic injury by suppressing Mfn2-mediated ER/mitochondrial calcium tethering. Front Cell Dev Biol. 2021;9:636553. doi: 10.3389/fcell.2021.636553. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Fernandez-Jimenez R, Garcia-Prieto J, Sanchez-Gonzalez J, et al. Pathophysiology underlying the bimodal edema phenomenon after myocardial ischemia/reperfusion. J Am Coll Cardiol. 2015;66(7):816–828. doi: 10.1016/j.jacc.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 87.Kar S, Kavdia M. Modeling of biopterin-dependent pathways of eNOS for nitric oxide and superoxide production. Free Radic Biol Med. 2011;51(7):1411–1427. doi: 10.1016/j.freeradbiomed.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jones SA, O'Donnell VB, Wood JD, et al. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol. 1996;271(4 Pt 2):H1626–1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- 89.Schroder K, Zhang M, Benkhoff S, et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res. 2012;110(9):1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 90.Herring N, Tapoulal N, Kalla M, et al. Neuropeptide-Y causes coronary microvascular constriction and is associated with reduced ejection fraction following ST-elevation myocardial infarction. Eur Heart J. 2019;40(24):1920–1929. doi: 10.1093/eurheartj/ehz115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alex L, Frangogiannis NG. Pericytes in the infarcted heart. Vasc Biol. 2019;1(1):H23–H31. doi: 10.1530/VB-19-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Simonavicius N, Ashenden M, van Weverwijk A, et al. Pericytes promote selective vessel regression to regulate vascular patterning. Blood. 2012;120(7):1516–1527. doi: 10.1182/blood-2011-01-332338. [DOI] [PubMed] [Google Scholar]
- 93.O'Farrell FM, Mastitskaya S, Hammond-Haley M, et al. Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. Elife. 2017 doi: 10.7554/eLife.29280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shi Y, Hu G, Su J, et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20(5):510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 95.Stark K, Eckart A, Haidari S, et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and 'instruct' them with pattern-recognition and motility programs. Nat Immunol. 2013;14(1):41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 96.Deckelbaum RA, Lobov IB, Cheung E, et al. The potassium channel Kcne3 is a VEGFA-inducible gene selectively expressed by vascular endothelial tip cells. Angiogenesis. 2020;23(2):179–192. doi: 10.1007/s10456-019-09696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84(3):869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 98.Pohl U. Connexins: key players in the control of vascular plasticity and function. Physiol Rev. 2020;100(2):525–572. doi: 10.1152/physrev.00010.2019. [DOI] [PubMed] [Google Scholar]
- 99.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 100.Strauss RE, Gourdie RG. Cx43 and the actin cytoskeleton: novel roles and implications for cell-cell junction-based barrier function regulation. Biomolecules. 2020 doi: 10.3390/biom10121656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yuan SY, Wu MH, Ustinova EE, et al. Myosin light chain phosphorylation in neutrophil-stimulated coronary microvascular leakage. Circ Res. 2002;90(11):1214–1221. doi: 10.1161/01.res.0000020402.73609.f1. [DOI] [PubMed] [Google Scholar]
- 102.Shen Q, Rigor RR, Pivetti CD, et al. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc Res. 2010;87(2):272–280. doi: 10.1093/cvr/cvq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang H, He J, Johnson D, et al. Deletion of placental growth factor prevents diabetic retinopathy and is associated with Akt activation and HIF1alpha-VEGF pathway inhibition. Diabetes. 2015;64(1):200–212. doi: 10.2337/db14-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Antonetti DA, Barber AJ, Hollinger LA, et al. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274(33):23463–23467. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- 105.Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018 doi: 10.3390/ijms19061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hilbert T, Klaschik S. The angiopoietin/TIE receptor system: focusing its role for ischemia-reperfusion injury. Cytokine Growth Factor Rev. 2015;26(3):281–291. doi: 10.1016/j.cytogfr.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y, Kontos CD, Annex BH, et al. Angiopoietin-tie signaling pathway in endothelial cells: a computational model. iScience. 2019;20:497–511. doi: 10.1016/j.isci.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Patel JV, Lim HS, Varughese GI, et al. Angiopoietin-2 levels as a biomarker of cardiovascular risk in patients with hypertension. Ann Med. 2008;40(3):215–222. doi: 10.1080/07853890701779586. [DOI] [PubMed] [Google Scholar]
- 109.Rangasamy S, Srinivasan R, Maestas J, et al. A potential role for angiopoietin 2 in the regulation of the blood-retinal barrier in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52(6):3784–3791. doi: 10.1167/iovs.10-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raza Z, Saleem U, Naureen Z. Sphingosine 1-phosphate signaling in ischemia and reperfusion injury. Prostaglandins Other Lipid Mediat. 2020;149:106436. doi: 10.1016/j.prostaglandins.2020.106436. [DOI] [PubMed] [Google Scholar]
- 111.Wilkerson BA. The role of sphingosine-1-phosphate in endothelial barrier function. Biochim Biophys Acta. 2014;1841(10):1403–1412. doi: 10.1016/j.bbalip.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morel S, Christoffersen C, Axelsen LN, et al. Sphingosine-1-phosphate reduces ischaemia-reperfusion injury by phosphorylating the gap junction protein Connexin43. Cardiovasc Res. 2016;109(3):385–396. doi: 10.1093/cvr/cvw004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alarcon-Martinez L, Villafranca-Baughman D, Quintero H, et al. Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature. 2020;585(7823):91–95. doi: 10.1038/s41586-020-2589-x. [DOI] [PubMed] [Google Scholar]
- 114.Rodrigues SF, Granger DN. Role of blood cells in ischaemia-reperfusion induced endothelial barrier failure. Cardiovasc Res. 2010;87(2):291–299. doi: 10.1093/cvr/cvq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dorge H, Neumann T, Behrends M, et al. Perfusion-contraction mismatch with coronary microvascular obstruction: role of inflammation. Am J Physiol Heart Circ Physiol. 2000;279(6):H2587–2592. doi: 10.1152/ajpheart.2000.279.6.H2587. [DOI] [PubMed] [Google Scholar]
- 116.Giordano G, Napolitano M, Cellurale M, et al. Circulating endothelial cell levels correlate with treatment outcomes of splanchnic vein thrombosis in patients with chronic myeloproliferative neoplasms. J Pers Med. 2022 doi: 10.3390/jpm12030364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Damani S, Bacconi A, Libiger O, et al. Characterization of circulating endothelial cells in acute myocardial infarction. Sci Transl Med. 2012;4(126):126ea133. doi: 10.1126/scitranslmed.3003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen CW, Wang LL, Zaman S, et al. Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovasc Res. 2018;114(7):1029–1040. doi: 10.1093/cvr/cvy067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ridger VC, Boulanger CM, Angelillo-Scherrer A, et al. Microvesicles in vascular homeostasis and diseases. Position Paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb Haemost. 2017;117(7):1296–1316. doi: 10.1160/TH16-12-0943. [DOI] [PubMed] [Google Scholar]
- 120.Zhang J, Zhu Y, Wu Y, et al. Synergistic effects of EMPs and PMPs on pulmonary vascular leakage and lung injury after ischemia/reperfusion. Cell Commun Signal. 2020;18(1):184. doi: 10.1186/s12964-020-00672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li F, Liu D, Liu M, et al. Tregs biomimetic nanoparticle to reprogram inflammatory and redox microenvironment in infarct tissue to treat myocardial ischemia reperfusion injury in mice. J Nanobiotechnology. 2022;20(1):251. doi: 10.1186/s12951-022-01445-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kloner RA, Giacomelli F, Alker KJ, et al. Influx of neutrophils into the walls of large epicardial coronary arteries in response to ischemia/reperfusion. Circulation. 1991;84(4):1758–1772. doi: 10.1161/01.cir.84.4.1758. [DOI] [PubMed] [Google Scholar]
- 123.Ge L, Zhou X, Ji WJ, et al. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: therapeutic potential of DNase-based reperfusion strategy. Am J Physiol Heart Circ Physiol. 2015;308(5):H500–509. doi: 10.1152/ajpheart.00381.2014. [DOI] [PubMed] [Google Scholar]
- 124.Coenen DM, Mastenbroek TG, Cosemans J. Platelet interaction with activated endothelium: mechanistic insights from microfluidics. Blood. 2017;130(26):2819–2828. doi: 10.1182/blood-2017-04-780825. [DOI] [PubMed] [Google Scholar]
- 125.Schanze N, Bode C, Duerschmied D. Platelet contributions to myocardial ischemia/reperfusion injury. Front Immunol. 2019;10:1260. doi: 10.3389/fimmu.2019.01260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Konijnenberg LSF, Damman P, Duncker DJ, et al. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc Res. 2020;116(4):787–805. doi: 10.1093/cvr/cvz301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rasola A, Bernardi P. Mitochondrial permeability transition in Ca(2+)-dependent apoptosis and necrosis. Cell Calcium. 2011;50(3):222–233. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 128.Orrenius S, Gogvadze V, Zhivotovsky B. Calcium and mitochondria in the regulation of cell death. Biochem Biophys Res Commun. 2015;460(1):72–81. doi: 10.1016/j.bbrc.2015.01.137. [DOI] [PubMed] [Google Scholar]
- 129.Li C, Ma Q, Toan S, et al. SERCA overexpression reduces reperfusion-mediated cardiac microvascular damage through inhibition of the calcium/MCU/mPTP/necroptosis signaling pathways. Redox Biol. 2020;36:101659. doi: 10.1016/j.redox.2020.101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alevriadou BR, Patel A, Noble M, et al. Molecular nature and physiological role of the mitochondrial calcium uniporter channel. Am J Physiol Cell Physiol. 2021;320(4):C465–C482. doi: 10.1152/ajpcell.00502.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tanwar J, Singh JB, Motiani RK. Molecular machinery regulating mitochondrial calcium levels: the nuts and bolts of mitochondrial calcium dynamics. Mitochondrion. 2021;57:9–22. doi: 10.1016/j.mito.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Raffaello A, De Stefani D, Sabbadin D, et al. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013;32(17):2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu L, Tan JL, Chen ZY, et al. Cardioprotection of post-ischemic moderate ROS against ischemia/reperfusion via STAT3-induced the inhibition of MCU opening. Basic Res Cardiol. 2019;114(5):39. doi: 10.1007/s00395-019-0747-9. [DOI] [PubMed] [Google Scholar]
- 134.Gambardella J, Trimarco B, Iaccarino G, et al. New insights in cardiac calcium handling and excitation-contraction coupling. Adv Exp Med Biol. 2018;1067:373–385. doi: 10.1007/5584_2017_106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gwathmey JK, Yerevanian AI, Hajjar RJ. Cardiac gene therapy with SERCA2a: from bench to bedside. J Mol Cell Cardiol. 2011;50(5):803–812. doi: 10.1016/j.yjmcc.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35(4):430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- 137.Chen L, Sun Q, Zhou D, et al. HINT2 triggers mitochondrial Ca(2+) influx by regulating the mitochondrial Ca(2+) uniporter (MCU) complex and enhances gemcitabine apoptotic effect in pancreatic cancer. Cancer Lett. 2017;411:106–116. doi: 10.1016/j.canlet.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 138.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 139.Nie X, Tang W, Zhang Z, et al. Procyanidin B2 mitigates endothelial endoplasmic reticulum stress through a PPARdelta-Dependent mechanism. Redox Biol. 2020;37:101728. doi: 10.1016/j.redox.2020.101728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Battson ML, Lee DM, Gentile CL. Endoplasmic reticulum stress and the development of endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2017;312(3):H355–H367. doi: 10.1152/ajpheart.00437.2016. [DOI] [PubMed] [Google Scholar]
- 141.Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21(3):396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rizzuto R, De Stefani D, Raffaello A, et al. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13(9):566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 143.Rainbolt TK, Saunders JM, Wiseman RL. Stress-responsive regulation of mitochondria through the ER unfolded protein response. Trends Endocrinol Metab. 2014;25(10):528–537. doi: 10.1016/j.tem.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 144.He X, Bi XY, Lu XZ, et al. Reduction of mitochondria-endoplasmic reticulum interactions by acetylcholine protects human umbilical vein endothelial cells from hypoxia/reoxygenation injury. Arterioscler Thromb Vasc Biol. 2015;35(7):1623–1634. doi: 10.1161/ATVBAHA.115.305469. [DOI] [PubMed] [Google Scholar]
- 145.Kirkman DL, Robinson AT, Rossman MJ, et al. Mitochondrial contributions to vascular endothelial dysfunction, arterial stiffness, and cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2021;320(5):H2080–H2100. doi: 10.1152/ajpheart.00917.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Daiber A, Andreadou I, Oelze M, et al. Discovery of new therapeutic redox targets for cardioprotection against ischemia/reperfusion injury and heart failure. Free Radic Biol Med. 2021;163:325–343. doi: 10.1016/j.freeradbiomed.2020.12.026. [DOI] [PubMed] [Google Scholar]
- 147.Jiang X, Wu D, Jiang Z, et al. Protective effect of nicorandil on cardiac microvascular injury: role of mitochondrial integrity. Oxid Med Cell Longev. 2021;2021:4665632. doi: 10.1155/2021/4665632. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 148.Maier-Begandt D, Comstra HS, Molina SA, et al. A venous-specific purinergic signaling cascade initiated by Pannexin 1 regulates TNFalpha-induced increases in endothelial permeability. Sci Signal. 2021 doi: 10.1126/scisignal.aba2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhou H, Hu S, Jin Q, et al. Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening. J Am Heart Assoc. 2017 doi: 10.1161/JAHA.116.005328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kalia R, Wang RY, Yusuf A, et al. Structural basis of mitochondrial receptor binding and constriction by DRP1. Nature. 2018;558(7710):401–405. doi: 10.1038/s41586-018-0211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kraus F, Roy K, Pucadyil TJ, et al. Function and regulation of the divisome for mitochondrial fission. Nature. 2021;590(7844):57–66. doi: 10.1038/s41586-021-03214-x. [DOI] [PubMed] [Google Scholar]
- 152.Yu Y, Peng XD, Qian XJ, et al. Fis1 phosphorylation by Met promotes mitochondrial fission and hepatocellular carcinoma metastasis. Signal Transduct Target Ther. 2021;6(1):401. doi: 10.1038/s41392-021-00790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen Y, Li S, Zhang Y, et al. The lncRNA Malat1 regulates microvascular function after myocardial infarction in mice via miR-26b-5p/Mfn1 axis-mediated mitochondrial dynamics. Redox Biol. 2021;41:101910. doi: 10.1016/j.redox.2021.101910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Depoix CL, Colson A, Hubinont C, et al. Impaired vascular endothelial growth factor expression and secretion during in vitro differentiation of human primary term cytotrophoblasts. Angiogenesis. 2020;23(2):221–230. doi: 10.1007/s10456-019-09702-z. [DOI] [PubMed] [Google Scholar]
- 155.Damico R, Zulueta JJ, Hassoun PM. Pulmonary endothelial cell NOX. Am J Respir Cell Mol Biol. 2012;47(2):129–139. doi: 10.1165/rcmb.2010-0331RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol. 2010;105(2):151–154. doi: 10.1007/s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 157.Zhou H, Shi C, Hu S, et al. BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk-Nox2-Drp1-mitochondrial fission pathways. Angiogenesis. 2018;21(3):599–615. doi: 10.1007/s10456-018-9611-z. [DOI] [PubMed] [Google Scholar]
- 158.Zhou H, Wang J, Zhu P, et al. Ripk3 regulates cardiac microvascular reperfusion injury: The role of IP3R-dependent calcium overload, XO-mediated oxidative stress and F-action/filopodia-based cellular migration. Cell Signal. 2018;45:12–22. doi: 10.1016/j.cellsig.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 159.Zhou H, Wang J, Zhu P, et al. NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2alpha. Basic Res Cardiol. 2018;113(4):23. doi: 10.1007/s00395-018-0682-1. [DOI] [PubMed] [Google Scholar]
- 160.Kwong JQ, Davis J, Baines CP, et al. Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ. 2014;21(8):1209–1217. doi: 10.1038/cdd.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhu H, Toan S, Mui D, et al. Mitochondrial quality surveillance as a therapeutic target in myocardial infarction. Acta Physiol (Oxf) 2021;231(3):e13590. doi: 10.1111/apha.13590. [DOI] [PubMed] [Google Scholar]
- 162.Zhou H, Ren J, Toan S, et al. Role of mitochondrial quality surveillance in myocardial infarction: From bench to bedside. Ageing Res Rev. 2021;66:101250. doi: 10.1016/j.arr.2020.101250. [DOI] [PubMed] [Google Scholar]
- 163.Song Y, Xing H, He Y, et al. Inhibition of mitochondrial reactive oxygen species improves coronary endothelial function after cardioplegic hypoxia/reoxygenation. J Thorac Cardiovasc Surg. 2021 doi: 10.1016/j.jtcvs.2021.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen Y, Liu C, Zhou P, et al. Coronary Endothelium No-Reflow Injury Is Associated with ROS-Modified Mitochondrial Fission through the JNK-Drp1 Signaling Pathway. Oxid Med Cell Longev. 2021;2021:6699516. doi: 10.1155/2021/6699516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ribeiro LF, Catarino T, Carvalho M, et al. Ligand-independent activity of the ghrelin receptor modulates AMPA receptor trafficking and supports memory formation. Sci Signal. 2021 doi: 10.1126/scisignal.abb1953. [DOI] [PubMed] [Google Scholar]
- 166.Skyschally A, Amanakis G, Neuhauser M, et al. Impact of electrical defibrillation on infarct size and no-reflow in pigs subjected to myocardial ischemia-reperfusion without and with ischemic conditioning. Am J Physiol Heart Circ Physiol. 2017;313(5):H871–H878. doi: 10.1152/ajpheart.00293.2017. [DOI] [PubMed] [Google Scholar]
- 167.Chang X, Li Y, Cai C, et al. Mitochondrial quality control mechanisms as molecular targets in diabetic heart. Metabolism. 2022;137:155313. doi: 10.1016/j.metabol.2022.155313. [DOI] [PubMed] [Google Scholar]
- 168.Jin H, Zhu Y, Li Y, et al. BDNF-mediated mitophagy alleviates high-glucose-induced brain microvascular endothelial cell injury. Apoptosis. 2019;24(5–6):511–528. doi: 10.1007/s10495-019-01535-x. [DOI] [PubMed] [Google Scholar]
- 169.Zheng J, Lu C. Oxidized LDL Causes Endothelial Apoptosis by Inhibiting Mitochondrial Fusion and Mitochondria Autophagy. Front Cell Dev Biol. 2020;8:600950. doi: 10.3389/fcell.2020.600950. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 170.Sun L, Hao Y, An R, et al. Overexpression of Rcan1–1L inhibits hypoxia-induced cell apoptosis through induction of mitophagy. Mol Cells. 2014;37(11):785–794. doi: 10.14348/molcells.2014.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhou H, Zhang Y, Hu S, et al. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res. 2017 doi: 10.1111/jpi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bravo-San Pedro JM, Kroemer G, Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circ Res. 2017;120(11):1812–1824. doi: 10.1161/CIRCRESAHA.117.311082. [DOI] [PubMed] [Google Scholar]
- 173.Battistutta R, Lolli G. Structural and functional determinants of protein kinase CK2alpha: facts and open questions. Mol Cell Biochem. 2011;356(1–2):67–73. doi: 10.1007/s11010-011-0939-6. [DOI] [PubMed] [Google Scholar]
- 174.Chen G, Han Z, Feng D, et al. A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol Cell. 2014;54(3):362–377. doi: 10.1016/j.molcel.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 175.Zhou H, Zhu P, Wang J, et al. Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. 2018;25(6):1080–1093. doi: 10.1038/s41418-018-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gao XM, Su Y, Moore S, et al. Relaxin mitigates microvascular damage and inflammation following cardiac ischemia-reperfusion. Basic Res Cardiol. 2019;114(4):30. doi: 10.1007/s00395-019-0739-9. [DOI] [PubMed] [Google Scholar]
- 177.Gao XM, Wang BH, Woodcock E, et al. Expression of active alpha(1B)-adrenergic receptors in the heart does not alleviate ischemic reperfusion injury. J Mol Cell Cardiol. 2000;32(9):1679–1686. doi: 10.1006/jmcc.2000.1201. [DOI] [PubMed] [Google Scholar]
- 178.Gao XM, Liu Y, White D, et al. Deletion of macrophage migration inhibitory factor protects the heart from severe ischemia-reperfusion injury: a predominant role of anti-inflammation. J Mol Cell Cardiol. 2011;50(6):991–999. doi: 10.1016/j.yjmcc.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 179.Gao XM, Moore XL, Liu Y, et al. Splenic release of platelets contributes to increased circulating platelet size and inflammation after myocardial infarction. Clin Sci (Lond) 2016;130(13):1089–1104. doi: 10.1042/CS20160234. [DOI] [PubMed] [Google Scholar]
- 180.McLaughlin MG, Stone GW, Aymong E, et al. Prognostic utility of comparative methods for assessment of ST-segment resolution after primary angioplasty for acute myocardial infarction: the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. J Am Coll Cardiol. 2004;44(6):1215–1223. doi: 10.1016/j.jacc.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 181.Karamasis GV, Russhard P, Al Janabi F, et al. Peri-procedural ST segment resolution during Primary Percutaneous Coronary Intervention (PPCI) for acute myocardial infarction: predictors and clinical consequences. J Electrocardiol. 2018;51(2):224–229. doi: 10.1016/j.jelectrocard.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 182.Bethke A, Halvorsen S, Bohmer E, et al. Myocardial perfusion grade predicts final infarct size and left ventricular function in patients with ST-elevation myocardial infarction treated with a pharmaco-invasive strategy (thrombolysis and early angioplasty) EuroIntervention. 2015;11(5):518–524. doi: 10.4244/EIJY15M04_02. [DOI] [PubMed] [Google Scholar]
- 183.Roubille F, Mewton N, Elbaz M, et al. No post-conditioning in the human heart with thrombolysis in myocardial infarction flow 2–3 on admission. Eur Heart J. 2014;35(25):1675–1682. doi: 10.1093/eurheartj/ehu054. [DOI] [PubMed] [Google Scholar]
- 184.Moroni F, Azzalini L, Caixeta A, et al. TIMI flow and myocardial blushing after rescue PCI and cardiac magnetic resonance: results from the Myocardial Salvage After Rescue Angioplasty: Evaluation by Magnetic Resonance (SAVE-ME) study. Int J Cardiol. 2022 doi: 10.1016/j.ijcard.2022.08.014. [DOI] [PubMed] [Google Scholar]
- 185.Ellis SG, Topol EJ, Gallison L, et al. Predictors of success for coronary angioplasty performed for acute myocardial infarction. J Am Coll Cardiol. 1988;12(6):1407–1415. doi: 10.1016/s0735-1097(88)80003-2. [DOI] [PubMed] [Google Scholar]
- 186.Ge H, Ding S, An D, et al. Frame counting improves the assessment of post-reperfusion microvascular patency by TIMI myocardial perfusion grade: evidence from cardiac magnetic resonance imaging. Int J Cardiol. 2016;203:360–366. doi: 10.1016/j.ijcard.2015.10.194. [DOI] [PubMed] [Google Scholar]
- 187.Bailleul C, Aissaoui N, Cayla G, et al. Prognostic impact of prepercutaneous coronary intervention TIMI flow in patients with ST-segment and non-ST-segment elevation myocardial infarction: Results from the FAST-MI 2010 registry. Arch Cardiovasc Dis. 2018;111(2):101–108. doi: 10.1016/j.acvd.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 188.Joost A, Stiermaier T, Eitel C, et al. Impact of initial culprit vessel flow on infarct size, microvascular obstruction, and myocardial salvage in acute reperfused ST-elevation myocardial infarction. Am J Cardiol. 2016;118(9):1316–1322. doi: 10.1016/j.amjcard.2016.07.056. [DOI] [PubMed] [Google Scholar]
- 189.Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93(5):879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 190.Jiang L, Yao H, Liang ZG. Postoperative assessment of myocardial function and microcirculation in patients with acute coronary syndrome by myocardial contrast echocardiography. Med Sci Monit. 2017;23:2324–2332. doi: 10.12659/msm.901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Verkaik M, van Poelgeest EM, Kwekkeboom RFJ, et al. Myocardial contrast echocardiography in mice: technical and physiological aspects. Am J Physiol Heart Circ Physiol. 2018;314(3):H381–H391. doi: 10.1152/ajpheart.00242.2017. [DOI] [PubMed] [Google Scholar]
- 192.Aggarwal S, Xie F, High R, et al. Prevalence and predictive value of microvascular flow abnormalities after successful contemporary percutaneous coronary intervention in acute ST-segment elevation myocardial infarction. J Am Soc Echocardiogr. 2018;31(6):674–682. doi: 10.1016/j.echo.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 193.Greaves K, Dixon SR, Fejka M, et al. Myocardial contrast echocardiography is superior to other known modalities for assessing myocardial reperfusion after acute myocardial infarction. Heart. 2003;89(2):139–144. doi: 10.1136/heart.89.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Reinstadler SJ, Thiele H, Eitel I. Risk stratification by cardiac magnetic resonance imaging after ST-elevation myocardial infarction. Curr Opin Cardiol. 2015;30(6):681–689. doi: 10.1097/HCO.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 195.Stiermaier T, Jobs A, de Waha S, et al. Optimized prognosis assessment in ST-segment-elevation myocardial infarction using a cardiac magnetic resonance imaging risk score. Circ Cardiovasc Imaging. 2017 doi: 10.1161/CIRCIMAGING.117.006774. [DOI] [PubMed] [Google Scholar]
- 196.Eitel I, de Waha S, Wohrle J, et al. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64(12):1217–1226. doi: 10.1016/j.jacc.2014.06.1194. [DOI] [PubMed] [Google Scholar]
- 197.Zhou D, Xu J, Zhao S, et al. CMR publications from China of the last more than 30 years. Int J Cardiovasc Imaging. 2020;36(9):1737–1747. doi: 10.1007/s10554-020-01873-x. [DOI] [PubMed] [Google Scholar]
- 198.Perazzolo Marra M, Lima JA, Iliceto S. MRI in acute myocardial infarction. Eur Heart J. 2011;32(3):284–293. doi: 10.1093/eurheartj/ehq409. [DOI] [PubMed] [Google Scholar]
- 199.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 200.Higgins CB, Herfkens R, Lipton MJ, et al. Nuclear magnetic resonance imaging of acute myocardial infarction in dogs: alterations in magnetic relaxation times. Am J Cardiol. 1983;52(1):184–188. doi: 10.1016/0002-9149(83)90093-0. [DOI] [PubMed] [Google Scholar]
- 201.Aletras AH, Tilak GS, Natanzon A, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113(15):1865–1870. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 202.Simonetti OP, Finn JP, White RD, et al. "Black blood" T2-weighted inversion-recovery MR imaging of the heart. Radiology. 1996;199(1):49–57. doi: 10.1148/radiology.199.1.8633172. [DOI] [PubMed] [Google Scholar]
- 203.Friedrich MG, Abdel-Aty H, Taylor A, et al. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51(16):1581–1587. doi: 10.1016/j.jacc.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 204.Reinstadler SJ, Stiermaier T, Fuernau G, et al. The challenges and impact of microvascular injury in ST-elevation myocardial infarction. Expert Rev Cardiovasc Ther. 2016;14(4):431–443. doi: 10.1586/14779072.2016.1135055. [DOI] [PubMed] [Google Scholar]
- 205.Shin JM, Choi EY, Park CH, et al. Quantitative T1 mapping for detecting microvascular obstruction in reperfused acute myocardial infarction: comparison with late gadolinium enhancement imaging. Korean J Radiol. 2020;21(8):978–986. doi: 10.3348/kjr.2019.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218(1):215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 207.Dastidar AG, Harries I, Pontecorboli G, et al. Native T1 mapping to detect extent of acute and chronic myocardial infarction: comparison with late gadolinium enhancement technique. Int J Cardiovasc Imaging. 2019;35(3):517–527. doi: 10.1007/s10554-018-1467-1. [DOI] [PubMed] [Google Scholar]
- 208.Judd RM, Lugo-Olivieri CH, Arai M, et al. Physiological basis of myocardial contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation. 1995;92(7):1902–1910. doi: 10.1161/01.cir.92.7.1902. [DOI] [PubMed] [Google Scholar]
- 209.Ibrahim T, Nekolla SG, Hornke M, et al. Quantitative measurement of infarct size by contrast-enhanced magnetic resonance imaging early after acute myocardial infarction: comparison with single-photon emission tomography using Tc99m-sestamibi. J Am Coll Cardiol. 2005;45(4):544–552. doi: 10.1016/j.jacc.2004.10.058. [DOI] [PubMed] [Google Scholar]
- 210.Kirschner R, Varga-Szemes A, Brott BC, et al. Quantification of myocardial viability distribution with Gd(DTPA) bolus-enhanced, signal intensity-based percent infarct mapping. Magn Reson Imaging. 2011;29(5):650–658. doi: 10.1016/j.mri.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.McAlindon E, Pufulete M, Lawton C, et al. Quantification of infarct size and myocardium at risk: evaluation of different techniques and its implications. Eur Heart J Cardiovasc Imaging. 2015;16(7):738–746. doi: 10.1093/ehjci/jev001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Stone GW, Selker HP, Thiele H, et al. Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials. J Am Coll Cardiol. 2016;67(14):1674–1683. doi: 10.1016/j.jacc.2016.01.069. [DOI] [PubMed] [Google Scholar]
- 213.Wu KC, Kim RJ, Bluemke DA, et al. Quantification and time course of microvascular obstruction by contrast-enhanced echocardiography and magnetic resonance imaging following acute myocardial infarction and reperfusion. J Am Coll Cardiol. 1998;32(6):1756–1764. doi: 10.1016/s0735-1097(98)00429-x. [DOI] [PubMed] [Google Scholar]
- 214.Bulluck H, Dharmakumar R, Arai AE, et al. Cardiovascular magnetic resonance in acute ST-segment-elevation myocardial infarction: recent advances, controversies, and future directions. Circulation. 2018;137(18):1949–1964. doi: 10.1161/CIRCULATIONAHA.117.030693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lima JA, Judd RM, Bazille A, et al. Regional heterogeneity of human myocardial infarcts demonstrated by contrast-enhanced MRI. Potential mechanisms. Circulation. 1995;92(5):1117–1125. doi: 10.1161/01.cir.92.5.1117. [DOI] [PubMed] [Google Scholar]
- 216.Hombach V, Grebe O, Merkle N, et al. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J. 2005;26(6):549–557. doi: 10.1093/eurheartj/ehi147. [DOI] [PubMed] [Google Scholar]
- 217.Garcia-Dorado D, Oliveras J, Gili J, et al. Analysis of myocardial oedema by magnetic resonance imaging early after coronary artery occlusion with or without reperfusion. Cardiovasc Res. 1993;27(8):1462–1469. doi: 10.1093/cvr/27.8.1462. [DOI] [PubMed] [Google Scholar]
- 218.Boxt LM, Hsu D, Katz J, et al. Estimation of myocardial water content using transverse relaxation time from dual spin-echo magnetic resonance imaging. Magn Reson Imaging. 1993;11(3):375–383. doi: 10.1016/0730-725x(93)90070-t. [DOI] [PubMed] [Google Scholar]
- 219.Robbers LF, Eerenberg ES, Teunissen PF, et al. Magnetic resonance imaging-defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur Heart J. 2013;34(30):2346–2353. doi: 10.1093/eurheartj/eht100. [DOI] [PubMed] [Google Scholar]
- 220.O'Regan DP, Ahmed R, Karunanithy N, et al. Reperfusion hemorrhage following acute myocardial infarction: assessment with T2* mapping and effect on measuring the area at risk. Radiology. 2009;250(3):916–922. doi: 10.1148/radiol.2503081154. [DOI] [PubMed] [Google Scholar]
- 221.Walsh SR, Tang TY, Kullar P, et al. Ischaemic preconditioning during cardiac surgery: systematic review and meta-analysis of perioperative outcomes in randomised clinical trials. Eur J Cardiothorac Surg. 2008;34(5):985–994. doi: 10.1016/j.ejcts.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 222.Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116(4):674–699. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 223.Liu J, Gu Y, Guo M, et al. Neuroprotective effects and mechanisms of ischemic/hypoxic preconditioning on neurological diseases. CNS Neurosci Ther. 2021;27(8):869–882. doi: 10.1111/cns.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wever KE, Menting TP, Rovers M, et al. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS ONE. 2012;7(2):e32296. doi: 10.1371/journal.pone.0032296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lee JH, You HJ, Lee TY, et al. Current status of experimental animal skin flap models: ischemic preconditioning and molecular factors. Int J Mol Sci. 2022 doi: 10.3390/ijms23095234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ruze A, Chen BD, Liu F, et al. Macrophage migration inhibitory factor plays an essential role in ischemic preconditioning-mediated cardioprotection. Clin Sci (Lond) 2019;133(5):665–680. doi: 10.1042/CS20181013. [DOI] [PubMed] [Google Scholar]
- 227.Cutrn JC, Perrelli MG, Cavalieri B, et al. Microvascular dysfunction induced by reperfusion injury and protective effect of ischemic preconditioning. Free Radic Biol Med. 2002;33(9):1200–1208. doi: 10.1016/s0891-5849(02)01017-1. [DOI] [PubMed] [Google Scholar]
- 228.Lin WY, Chang YC, Ho CJ, et al. Ischemic preconditioning reduces neurovascular damage after hypoxia-ischemia via the cellular inhibitor of apoptosis 1 in neonatal brain. Stroke. 2013;44(1):162–169. doi: 10.1161/STROKEAHA.112.677617. [DOI] [PubMed] [Google Scholar]
- 229.Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579–588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 230.You J, Feng L, Xin M, et al. Cerebral ischemic postconditioning plays a neuroprotective role through regulation of central and peripheral glutamate. Biomed Res Int. 2018;2018:6316059. doi: 10.1155/2018/6316059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Guo L, Xu JM, Mo XY. Ischemic postconditioning regulates cardiomyocyte autophagic activity following ischemia/reperfusion injury. Mol Med Rep. 2015;12(1):1169–1176. doi: 10.3892/mmr.2015.3533. [DOI] [PubMed] [Google Scholar]
- 232.Dong M, Mu N, Guo F, et al. The beneficial effects of postconditioning on no-reflow phenomenon after percutaneous coronary intervention in patients with ST-elevation acute myocardial infarction. J Thromb Thrombolysis. 2014;38(2):208–214. doi: 10.1007/s11239-013-1010-0. [DOI] [PubMed] [Google Scholar]
- 233.Li CY, Ma W, Liu KP, et al. Advances in intervention methods and brain protection mechanisms of in situ and remote ischemic postconditioning. Metab Brain Dis. 2021;36(1):53–65. doi: 10.1007/s11011-020-00562-x. [DOI] [PubMed] [Google Scholar]
- 234.Liu C, Yang J, Zhang C, et al. Remote ischemic conditioning reduced cerebral ischemic injury by modulating inflammatory responses and ERK activity in type 2 diabetic mice. Neurochem Int. 2020;135:104690. doi: 10.1016/j.neuint.2020.104690. [DOI] [PubMed] [Google Scholar]
- 235.Zhou CC, Yao WT, Ge YZ, et al. Remote ischemic conditioning for the prevention of contrast-induced acute kidney injury in patients undergoing intravascular contrast administration: a meta-analysis and trial sequential analysis of 16 randomized controlled trials. Oncotarget. 2017;8(45):79323–79336. doi: 10.18632/oncotarget.18106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Min F, Jia XJ, Gao Q, et al. Remote ischemic post-conditioning protects against myocardial ischemia/reperfusion injury by inhibiting the Rho-kinase signaling pathway. Exp Ther Med. 2020;19(1):99–106. doi: 10.3892/etm.2019.8176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sluijter JPG, Davidson SM, Boulanger CM, et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: position paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2018;114(1):19–34. doi: 10.1093/cvr/cvx211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Loyer X, Vion AC, Tedgui A, et al. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ Res. 2014;114(2):345–353. doi: 10.1161/CIRCRESAHA.113.300858. [DOI] [PubMed] [Google Scholar]
- 239.Boulanger CM, Scoazec A, Ebrahimian T, et al. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation. 2001;104(22):2649–2652. doi: 10.1161/hc4701.100516. [DOI] [PubMed] [Google Scholar]
- 240.Porto I, Biasucci LM, De Maria GL, et al. Intracoronary microparticles and microvascular obstruction in patients with ST elevation myocardial infarction undergoing primary percutaneous intervention. Eur Heart J. 2012;33(23):2928–2938. doi: 10.1093/eurheartj/ehs065. [DOI] [PubMed] [Google Scholar]
- 241.Baranyai T, Giricz Z, Varga ZV, et al. In vivo MRI and ex vivo histological assessment of the cardioprotection induced by ischemic preconditioning, postconditioning and remote conditioning in a closed-chest porcine model of reperfused acute myocardial infarction: importance of microvasculature. J Transl Med. 2017;15(1):67. doi: 10.1186/s12967-017-1166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.White SK, Frohlich GM, Sado DM, et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2015;8(1 Pt B):178–188. doi: 10.1016/j.jcin.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 243.Eitel I, Stiermaier T, Rommel KP, et al. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Heart J. 2015;36(44):3049–3057. doi: 10.1093/eurheartj/ehv463. [DOI] [PubMed] [Google Scholar]
- 244.Goerg J, Sommerfeld M, Greiner B, et al. Low-Dose empagliflozin improves systolic heart function after myocardial infarction in rats: regulation of MMP9, NHE1, and SERCA2a. Int J Mol Sci. 2021 doi: 10.3390/ijms22115437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91(2):733–794. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 246.Sinha B, Ghosal S. Sodium-glucose cotransporter-2 inhibitors (SGLT-2i) reduce hospitalization for heart failure only and have no effect on atherosclerotic cardiovascular events: a meta-analysis. Diabetes Ther. 2019;10(3):891–899. doi: 10.1007/s13300-019-0597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS Program (Canagliflozin Cardiovascular Assessment Study) Circulation. 2018;137(4):323–334. doi: 10.1161/CIRCULATIONAHA.117.032038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 249.Norhammar A, Bodegard J, Nystrom T, et al. Dapagliflozin and cardiovascular mortality and disease outcomes in a population with type 2 diabetes similar to that of the DECLARE-TIMI 58 trial: a nationwide observational study. Diabetes Obes Metab. 2019;21(5):1136–1145. doi: 10.1111/dom.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Fathi A, Vickneson K, Singh JS. SGLT2-inhibitors; more than just glycosuria and diuresis. Heart Fail Rev. 2021;26(3):623–642. doi: 10.1007/s10741-020-10038-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ceriello A, Ofstad AP, Zwiener I, et al. Empagliflozin reduced long-term HbA1c variability and cardiovascular death: insights from the EMPA-REG OUTCOME trial. Cardiovasc Diabetol. 2020;19(1):176. doi: 10.1186/s12933-020-01147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Park SH, Belcastro E, Hasan H, et al. Angiotensin II-induced upregulation of SGLT1 and 2 contributes to human microparticle-stimulated endothelial senescence and dysfunction: protective effect of gliflozins. Cardiovasc Diabetol. 2021;20(1):65. doi: 10.1186/s12933-021-01252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ma L, Zou R, Shi W, et al. SGLT2 inhibitor dapagliflozin reduces endothelial dysfunction and microvascular damage during cardiac ischemia/reperfusion injury through normalizing the XO-SERCA2-CaMKII-coffilin pathways. Theranostics. 2022;12(11):5034–5050. doi: 10.7150/thno.75121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Tian J, Zhang M, Suo M, et al. Dapagliflozin alleviates cardiac fibrosis through suppressing EndMT and fibroblast activation via AMPKalpha/TGF-beta/Smad signalling in type 2 diabetic rats. J Cell Mol Med. 2021;25(16):7642–7659. doi: 10.1111/jcmm.16601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kolijn D, Pabel S, Tian Y, et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Galpha oxidation. Cardiovasc Res. 2021;117(2):495–507. doi: 10.1093/cvr/cvaa123. [DOI] [PubMed] [Google Scholar]
- 256.Aragon-Herrera A, Feijoo-Bandin S, Anido-Varela L, et al. Relaxin-2 as a potential biomarker in cardiovascular diseases. J Pers Med. 2022 doi: 10.3390/jpm12071021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Du XJ, Bathgate RA, Samuel CS, et al. Cardiovascular effects of relaxin: from basic science to clinical therapy. Nat Rev Cardiol. 2010;7(1):48–58. doi: 10.1038/nrcardio.2009.198. [DOI] [PubMed] [Google Scholar]
- 258.Samuel CS, Du XJ, Bathgate RA, et al. 'Relaxin' the stiffened heart and arteries: the therapeutic potential for relaxin in the treatment of cardiovascular disease. Pharmacol Ther. 2006;112(2):529–552. doi: 10.1016/j.pharmthera.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 259.Bathgate RA, Halls ML, van der Westhuizen ET, et al. Relaxin family peptides and their receptors. Physiol Rev. 2013;93(1):405–480. doi: 10.1152/physrev.00001.2012. [DOI] [PubMed] [Google Scholar]
- 260.Hsu SY, Nakabayashi K, Nishi S, et al. Activation of orphan receptors by the hormone relaxin. Science. 2002;295(5555):671–674. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- 261.Martin B, Romero G, Salama G. Cardioprotective actions of relaxin. Mol Cell Endocrinol. 2019;487:45–53. doi: 10.1016/j.mce.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 262.Pini A, Boccalini G, Baccari MC, et al. Protection from cigarette smoke-induced vascular injury by recombinant human relaxin-2 (serelaxin) J Cell Mol Med. 2016;20(5):891–902. doi: 10.1111/jcmm.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Gao S, Li L, Li L, et al. Effects of the combination of tanshinone IIA and puerarin on cardiac function and inflammatory response in myocardial ischemia mice. J Mol Cell Cardiol. 2019;137:59–70. doi: 10.1016/j.yjmcc.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 264.Uto T, Tung NH, Ohta T, et al. Antiproliferative activity and apoptosis induction by trijuganone C isolated from the root of Salvia miltiorrhiza Bunge (Danshen) Phytother Res. 2018;32(4):657–666. doi: 10.1002/ptr.6013. [DOI] [PubMed] [Google Scholar]
- 265.Zhou R, He LF, Li YJ, et al. Cardioprotective effect of water and ethanol extract of Salvia miltiorrhiza in an experimental model of myocardial infarction. J Ethnopharmacol. 2012;139(2):440–446. doi: 10.1016/j.jep.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 266.Irmak F, Kurt Yazar S, Sirvan SS, et al. Beneficial effects of Salvia miltiorrhiza in the healing of burn wounds: an experimental study in rats. J Plast Surg Hand Surg. 2018;52(4):229–233. doi: 10.1080/2000656X.2018.1461631. [DOI] [PubMed] [Google Scholar]
- 267.Fei YX, Wang SQ, Yang LJ, et al. Salvia miltiorrhiza Bunge (Danshen) extract attenuates permanent cerebral ischemia through inhibiting platelet activation in rats. J Ethnopharmacol. 2017;207:57–66. doi: 10.1016/j.jep.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 268.Guo R, Li L, Su J, et al. Pharmacological activity and mechanism of tanshinone IIA in related diseases. Drug Des Devel Ther. 2020;14:4735–4748. doi: 10.2147/DDDT.S266911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hung YC, Pan TL, Hu WL. Roles of reactive oxygen species in anticancer therapy with Salvia miltiorrhiza bunge. Oxid Med Cell Longev. 2016;2016:5293284. doi: 10.1155/2016/5293284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Chen L, Guo QH, Chang Y, et al. Tanshinone IIA ameliorated endothelial dysfunction in rats with chronic intermittent hypoxia. Cardiovasc Pathol. 2017;31:47–53. doi: 10.1016/j.carpath.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 271.Mo J, Yang R, Li F, et al. Scutellarin protects against vascular endothelial dysfunction and prevents atherosclerosis via antioxidation. Phytomedicine. 2018;42:66–74. doi: 10.1016/j.phymed.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 272.Xi J, Rong Y, Zhao Z, et al. Scutellarin ameliorates high glucose-induced vascular endothelial cells injury by activating PINK1/Parkin-mediated mitophagy. J Ethnopharmacol. 2021;271:113855. doi: 10.1016/j.jep.2021.113855. [DOI] [PubMed] [Google Scholar]
- 273.Mondal NK, Behera J, Kelly KE, et al. Tetrahydrocurcumin epigenetically mitigates mitochondrial dysfunction in brain vasculature during ischemic stroke. Neurochem Int. 2019;122:120–138. doi: 10.1016/j.neuint.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Vacek JC, Behera J, George AK, et al. Tetrahydrocurcumin ameliorates homocysteine-mediated mitochondrial remodeling in brain endothelial cells. J Cell Physiol. 2018;233(4):3080–3092. doi: 10.1002/jcp.26145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Duan H, Zhang Q, Liu J, et al. Suppression of apoptosis in vascular endothelial cell, the promising way for natural medicines to treat atherosclerosis. Pharmacol Res. 2021;168:105599. doi: 10.1016/j.phrs.2021.105599. [DOI] [PubMed] [Google Scholar]
- 276.Li MT, Ke J, Guo SF, et al. The protective effect of quercetin on endothelial cells injured by hypoxia and reoxygenation. Front Pharmacol. 2021;12:732874. doi: 10.3389/fphar.2021.732874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ahrens I, Peter K (2009) FX-06, a fibrin-derived Bbeta15-42 peptide for the potential treatment of reperfusion injury following myocardial infarction. Curr Opin Investig Drugs 10(9): 997–1003. https://www.ncbi.nlm.nih.gov/pubmed/19705343. Accessed 17 Aug 2023 [PubMed]
- 278.Petzelbauer P, Zacharowski PA, Miyazaki Y, et al. The fibrin-derived peptide Bbeta15-42 protects the myocardium against ischemia-reperfusion injury. Nat Med. 2005;11(3):298–304. doi: 10.1038/nm1198. [DOI] [PubMed] [Google Scholar]
- 279.Kim DY, Zhang H, Park S, et al. CU06-1004 (endothelial dysfunction blocker) ameliorates astrocyte end-feet swelling by stabilizing endothelial cell junctions in cerebral ischemia/reperfusion injury. J Mol Med (Berl) 2020;98(6):875–886. doi: 10.1007/s00109-020-01920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zhang H, Park JH, Maharjan S, et al. Sac-1004, a vascular leakage blocker, reduces cerebral ischemia-reperfusion injury by suppressing blood-brain barrier disruption and inflammation. J Neuroinflammation. 2017;14(1):122. doi: 10.1186/s12974-017-0897-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Noh M, Kim Y, Zhang H, et al. Oral administration of CU06-1004 attenuates vascular permeability and stabilizes neovascularization in retinal vascular diseases. Eur J Pharmacol. 2023;939:175427. doi: 10.1016/j.ejphar.2022.175427. [DOI] [PubMed] [Google Scholar]
- 282.Park S, Oh JH, Park DJ, et al. CU06-1004-induced vascular normalization improves immunotherapy by modulating tumor microenvironment via cytotoxic T cells. Front Immunol. 2020;11:620166. doi: 10.3389/fimmu.2020.620166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Shen J, Frye M, Lee BL, et al. Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. J Clin Invest. 2014;124(10):4564–4576. doi: 10.1172/JCI74527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Shi Y, Xiong Y, Lei Y et al (2019) Protective effect of COMP-angiopoietin-1 on peritoneal vascular permeability and peritoneal transport function in uremic peritoneal dialysis rats. Am J Transl Res 11(9): 5932–5943. https://www.ncbi.nlm.nih.gov/pubmed/31632561. Accessed 19 Aug 2023 [PMC free article] [PubMed]
- 285.Poirier B, Briand V, Kadereit D, et al. A G protein-biased S1P(1) agonist, SAR247799, protects endothelial cells without affecting lymphocyte numbers. Sci Signal. 2020 doi: 10.1126/scisignal.aax8050. [DOI] [PubMed] [Google Scholar]
- 286.Vaidya K, Tucker B, Patel S, et al. Acute Coronary Syndromes (ACS)-unravelling biology to identify new therapies-the microcirculation as a frontier for new therapies in ACS. Cells. 2021 doi: 10.3390/cells10092188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Matter MA, Paneni F, Libby P, et al. Inflammation in acute myocardial infarction: the good, the bad and the ugly. Eur Heart J. 2023 doi: 10.1093/eurheartj/ehad486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhang Y, Wang S, Chen X, et al. Liraglutide prevents high glucose induced HUVECs dysfunction via inhibition of PINK1/Parkin-dependent mitophagy. Mol Cell Endocrinol. 2022;545:111560. doi: 10.1016/j.mce.2022.111560. [DOI] [PubMed] [Google Scholar]
- 289.Chen Z, Wu H, Yang J, et al. Activating Parkin-dependent mitophagy alleviates oxidative stress, apoptosis, and promotes random-pattern skin flaps survival. Commun Biol. 2022;5(1):616. doi: 10.1038/s42003-022-03556-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Liu N, Wu J, Zhang L, et al. Hydrogen Sulphide modulating mitochondrial morphology to promote mitophagy in endothelial cells under high-glucose and high-palmitate. J Cell Mol Med. 2017;21(12):3190–3203. doi: 10.1111/jcmm.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zhou ZY, Shi WT, Zhang J, et al. Sodium tanshinone IIA sulfonate protects against hyperhomocysteine-induced vascular endothelial injury via activation of NNMT/SIRT1-mediated NRF2/HO-1 and AKT/MAPKs signaling in human umbilical vascular endothelial cells. Biomed Pharmacother. 2023;158:114137. doi: 10.1016/j.biopha.2022.114137. [DOI] [PubMed] [Google Scholar]
- 292.Jiang T, Liu T, Deng X, et al. Adiponectin ameliorates lung ischemia-reperfusion injury through SIRT1-PINK1 signaling-mediated mitophagy in type 2 diabetic rats. Respir Res. 2021;22(1):258. doi: 10.1186/s12931-021-01855-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wang X, Zhang JQ, Xiu CK, et al. Ginseng-Sanqi-Chuanxiong (GSC) extracts ameliorate diabetes-induced endothelial cell senescence through regulating mitophagy via the AMPK pathway. Oxid Med Cell Longev. 2020;2020:7151946. doi: 10.1155/2020/7151946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Hou Y, Wang XF, Lang ZQ, et al. Adiponectin is protective against endoplasmic reticulum stress-induced apoptosis of endothelial cells in sepsis. Braz J Med Biol Res. 2018;51(12):e7747. doi: 10.1590/1414-431X20187747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kapadia P, Bikkina P, Landicho MA, et al. Effect of anti-hyperglycemic drugs on endoplasmic reticulum (ER) stress in human coronary artery endothelial cells. Eur J Pharmacol. 2021;907:174249. doi: 10.1016/j.ejphar.2021.174249. [DOI] [PubMed] [Google Scholar]
- 296.Li P, Xie C, Zhong J, et al. Melatonin attenuates ox-LDL-induced endothelial dysfunction by reducing ER stress and inhibiting JNK/Mff SIgnaling. Oxid Med Cell Longev. 2021;2021:5589612. doi: 10.1155/2021/5589612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Qi K, Li X, Geng Y, et al. Tongxinluo attenuates reperfusion injury in diabetic hearts by angiopoietin-like 4-mediated protection of endothelial barrier integrity via PPAR-alpha pathway. PLoS ONE. 2018;13(6):e0198403. doi: 10.1371/journal.pone.0198403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Rubig E, Stypmann J, Van Slyke P, et al. The synthetic Tie2 agonist peptide vasculotide protects renal vascular barrier function in experimental acute kidney injury. Sci Rep. 2016;6:22111. doi: 10.1038/srep22111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Shah R, Wilkins E, Nichols M, et al. Epidemiology report: trends in sex-specific cerebrovascular disease mortality in Europe based on WHO mortality data. Eur Heart J. 2019;40(9):755–764. doi: 10.1093/eurheartj/ehy378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Pohl M, Hesszenberger D, Kapus K, et al. Ischemic stroke mimics: a comprehensive review. J Clin Neurosci. 2021;93:174–182. doi: 10.1016/j.jocn.2021.09.025. [DOI] [PubMed] [Google Scholar]
- 302.Collaborators GBDCoD, Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.White BC, Sullivan JM, DeGracia DJ, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179(S1–2):1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- 304.Puig B, Brenna S, Magnus T. Molecular Communication of a Dying Neuron in Stroke. Int J Mol Sci. 2018 doi: 10.3390/ijms19092834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Yoon JS, Jo D, Lee HS, et al. Spatiotemporal protein atlas of cell death-related molecules in the rat MCAO stroke model. Exp Neurobiol. 2018;27(4):287–298. doi: 10.5607/en.2018.27.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Pan J, Konstas AA, Bateman B, et al. Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology. 2007;49(2):93–102. doi: 10.1007/s00234-006-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Sun MS, Jin H, Sun X, et al. Free radical damage in ischemia-reperfusion injury: an obstacle in acute ischemic stroke after revascularization therapy. Oxid Med Cell Longev. 2018;2018:3804979. doi: 10.1155/2018/3804979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Imai T, Matsubara H, Nakamura S, et al. The mitochondria-targeted peptide, bendavia, attenuated ischemia/reperfusion-induced stroke damage. Neuroscience. 2020;443:110–119. doi: 10.1016/j.neuroscience.2020.07.044. [DOI] [PubMed] [Google Scholar]
- 309.Yang D, Li Z, Gao G, et al. Combined analysis of surface protein profile and microRNA expression profile of exosomes derived from brain microvascular endothelial cells in early cerebral ischemia. ACS Omega. 2021;6(34):22410–22421. doi: 10.1021/acsomega.1c03248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 311.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Lees KR, Emberson J, Blackwell L, et al. Effects of Alteplase for acute stroke on the distribution of functional outcomes: a pooled analysis of 9 trials. Stroke. 2016;47(9):2373–2379. doi: 10.1161/STROKEAHA.116.013644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 314.Kim JS. tPA helpers in the treatment of acute ischemic stroke: are they ready for clinical use? J Stroke. 2019;21(2):160–174. doi: 10.5853/jos.2019.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Tsuji K, Aoki T, Tejima E, et al. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005;36(9):1954–1959. doi: 10.1161/01.STR.0000177517.01203.eb. [DOI] [PubMed] [Google Scholar]
- 316.Montaner J, Molina CA, Monasterio J, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107(4):598–603. doi: 10.1161/01.cir.0000046451.38849.90. [DOI] [PubMed] [Google Scholar]
- 317.Mueller-Kronast NH, Zaidat OO, Froehler MT, et al. Systematic evaluation of patients treated with neurothrombectomy devices for acute ischemic stroke: primary results of the STRATIS Registry. Stroke. 2017;48(10):2760–2768. doi: 10.1161/STROKEAHA.117.016456. [DOI] [PubMed] [Google Scholar]
- 318.Puentes S, Kurachi M, Shibasaki K, et al. Brain microvascular endothelial cell transplantation ameliorates ischemic white matter damage. Brain Res. 2012;1469:43–53. doi: 10.1016/j.brainres.2012.06.042. [DOI] [PubMed] [Google Scholar]
- 319.Zimmet P, Alberti KG, Magliano DJ, et al. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol. 2016;12(10):616–622. doi: 10.1038/nrendo.2016.105. [DOI] [PubMed] [Google Scholar]
- 320.Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 322.Kocak MZ, Aktas G, Atak BM, et al. Is Neuregulin-4 a predictive marker of microvascular complications in type 2 diabetes mellitus? Eur J Clin Invest. 2020;50(3):e13206. doi: 10.1111/eci.13206. [DOI] [PubMed] [Google Scholar]
- 323.Ozgumus T, Sulaieva O, Jessen LE, et al. Reduced expression of OXPHOS and DNA damage genes is linked to protection from microvascular complications in long-term type 1 diabetes: the PROLONG study. Sci Rep. 2021;11(1):20735. doi: 10.1038/s41598-021-00183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Xue P, Covassin N, Ran X, et al. Association of parameters of nocturnal hypoxemia with diabetic microvascular complications: a cross-sectional study. Diabetes Res Clin Pract. 2020;170:108484. doi: 10.1016/j.diabres.2020.108484. [DOI] [PubMed] [Google Scholar]
- 325.Vincent AM, Callaghan BC, Smith AL, et al. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7(10):573–583. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- 326.Imamura Y, Suzuki K, Saijo H, et al. Longitudinal physiological remoulding of lower limb skin as a cause of diabetic foot ulcer: a histopathological examination. J Wound Care. 2022;31(Sup8):S29–S35. doi: 10.12968/jowc.2022.31.Sup8.S29. [DOI] [PubMed] [Google Scholar]
- 327.Yang S, Ma C, Wu H, et al. Tectorigenin attenuates diabetic nephropathy by improving vascular endothelium dysfunction through activating AdipoR1/2 pathway. Pharmacol Res. 2020;153:104678. doi: 10.1016/j.phrs.2020.104678. [DOI] [PubMed] [Google Scholar]
- 328.Zhang Z, Yang Z, Zhu B, et al. Increasing glucose 6-phosphate dehydrogenase activity restores redox balance in vascular endothelial cells exposed to high glucose. PLoS ONE. 2012;7(11):e49128. doi: 10.1371/journal.pone.0049128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Toth E, Racz A, Toth J, et al. Contribution of polyol pathway to arteriolar dysfunction in hyperglycemia. Role of oxidative stress, reduced NO, and enhanced PGH(2)/TXA(2) mediation. Am J Physiol Heart Circ Physiol. 2007;293(5):H3096–3104. doi: 10.1152/ajpheart.01335.2006. [DOI] [PubMed] [Google Scholar]
- 330.Kielbik M, Szulc-Kielbik I, Klink M. The potential role of iNOS in ovarian cancer progression and chemoresistance. Int J Mol Sci. 2019 doi: 10.3390/ijms20071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Pei H, Yang Y, Cui L, et al. Bisdemethoxycurcumin inhibits ovarian cancer via reducing oxidative stress mediated MMPs expressions. Sci Rep. 2016;6:28773. doi: 10.1038/srep28773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Lv H, Cheng Q, Li Y, et al. The protective effects of ropivacaine against high glucose-induced brain microvascular endothelial injury by reducing mmps and alleviating oxidative stress. Neurotox Res. 2021;39(3):851–859. doi: 10.1007/s12640-020-00324-8. [DOI] [PubMed] [Google Scholar]
- 333.Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Invest. 2014;124(6):2355–2363. doi: 10.1172/JCI72269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Yang B, Lan S, Dieude M, et al. Caspase-3 is a pivotal regulator of microvascular rarefaction and renal fibrosis after ischemia-reperfusion injury. J Am Soc Nephrol. 2018;29(7):1900–1916. doi: 10.1681/ASN.2017050581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Doreille A, Dieude M, Cardinal H. The determinants, biomarkers, and consequences of microvascular injury in kidney transplant recipients. Am J Physiol Renal Physiol. 2019;316(1):F9–F19. doi: 10.1152/ajprenal.00163.2018. [DOI] [PubMed] [Google Scholar]
- 336.Babickova J, Klinkhammer BM, Buhl EM, et al. Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int. 2017;91(1):70–85. doi: 10.1016/j.kint.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 337.Farris AB, Taheri D, Kawai T, et al. Acute renal endothelial injury during marrow recovery in a cohort of combined kidney and bone marrow allografts. Am J Transplant. 2011;11(7):1464–1477. doi: 10.1111/j.1600-6143.2011.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Furuichi K, Wada T, Kaneko S, et al. Roles of chemokines in renal ischemia/reperfusion injury. Front Biosci. 2008;13:4021–4028. doi: 10.2741/2990. [DOI] [PubMed] [Google Scholar]
- 339.Higgins DF, Kimura K, Bernhardt WM, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117(12):3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Camacho X, Nedkoff L, Wright FL, et al. Relative contribution of trends in myocardial infarction event rates and case fatality to declines in mortality: an international comparative study of 1.95 million events in 80.4 million people in four countries. Lancet Public Health. 2022;7(3):e229–e239. doi: 10.1016/S2468-2667(22)00006-8. [DOI] [PubMed] [Google Scholar]
- 341.Frantz S, Hundertmark MJ, Schulz-Menger J, et al. Left ventricular remodelling post-myocardial infarction: pathophysiology, imaging, and novel therapies. Eur Heart J. 2022;43(27):2549–2561. doi: 10.1093/eurheartj/ehac223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Zhou H, Wang S, Zhu P, et al. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018;15:335–346. doi: 10.1016/j.redox.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Tan Y, Mui D, Toan S, et al. SERCA overexpression improves mitochondrial quality control and attenuates cardiac microvascular ischemia-reperfusion injury. Mol Ther Nucleic Acids. 2020;22:696–707. doi: 10.1016/j.omtn.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.