Abstract
Obesity has been linked to an increased risk of and a worse prognosis for several types of cancer. A number of interrelated mediators contribute to obesity’s pro-tumor effects, including chronic adipose inflammation and other perturbations of immune cell development and function. Here, we review studies examining the impact of obesity-induced immune dysfunction on cancer risk and progression. While the role of adipose tissue inflammation in obesity-associated cancer risk has been well characterized, the effects of obesity on immune cell infiltration and activity within the tumor microenvironment are not well studied. In this review, we aim to highlight the impact of both adipose-mediated inflammatory signaling and intratumoral immunosuppressive signaling in obesity-induced cancer risk, progression, and metastasis.
Keywords: Immunotherapy, Leptin, Chemoresistance
Introduction
Obesity and cancer
According to the Centers for Diseases Control and Prevention (CDC), approximately 40% of adults in the USA were considered obese in 2017 [1]. In the same year, the Organization for Economic Cooperation and Development predicted that this number will approach 50% by 2030 [2]. The CDC and the World Health Organization (WHO) define obesity as a body mass index (BMI) ≥ 30 kg/m2 and severe obesity as ≥ 40 kg/m2. However, the WHO has recommended defining obesity in Asian adults as BMI ≥ 27.5 kg/m2 due to studies indicating a higher body fat percentage and higher rates of obesity-related health complications at lower BMIs in this population, relative to non-Hispanic whites [3, 4]. In concert with chronic adipose tissue inflammation, obesity increases the risk of several comorbidities such as heart disease, type 2 diabetes, and cancer. Obesity has been specifically associated with an increased risk of developing 13 cancers, including cancers of the esophagus (adenocarcinoma), gastric cardia, colon and rectum, liver, gallbladder, pancreas, postmenopausal breast, uterus, ovaries, kidneys, and thyroid as well as meningioma and multiple myeloma [5, 6]. It has been generally accepted that, with the exception of lung, brain/central nervous system, and malignant melanoma, obesity is also associated with increased mortality in most cancers, particularly liver, uterine, and kidney cancer [7]. However, there is a growing body of literature that supports the existence of an “obesity paradox” in cancer patients treated with immunotherapies, where obesity is associated with a better response to these treatments [8]. The effects of obesity on patient response to various cancer therapies will be described in more detail in the last section of this review.
Menopause, obesity, and associated cancer risks
Among women, menopause is also an important factor in susceptibility to certain cancers, and obesity interacts with this factor. Obesity has been shown to delay menopause, and a delay of 5 years increases postmenopausal breast cancer risk by 17% [9]. For ovarian and endometrial cancer, obesity increases risk regardless of menopausal status [10–12]. However, obesity protects against breast cancer in premenopausal women, particularly estrogen receptor positive disease, while the opposite is true in postmenopausal women [10, 13, 14]. One possible explanation for this contrast is that, while the ovaries produce estrogen prior to menopause, adipose tissue is the primary site of estrogen production in postmenopausal women due to their lack of ovarian aromatase activity and an increase in adipose tissue aromatase activity with age [15, 16]. In addition, increased inflammation due to adipose tissue dysfunction has been associated with increased adipose aromatase expression in obese postmenopausal women, suggesting that this inflammation contributes to estrogen-mediated breast cancer risk [17]. Postmenopausal obese women also have lower availability of sex hormone binding globulin (SHBG) [18], and weight is inversely proportional to SHBG [19, 20]. Reduced SHBG increases the levels of estradiol in circulation and therefore increases the risk of not just breast cancer but also endometrial cancer [21]. Additionally, hormone replacement therapy usage in postmenopausal women can contribute to an increased risk of certain subtypes of breast cancer [22]. However, this increased estrogen exposure has not been shown to significantly increase breast, endometrial, or ovarian cancer risk in obese postmenopausal women [23–27]. Finally, a change in fat distribution is observed in postmenopausal women, likely due to pro-androgen hormonal shifts, causing an accumulation of central fat compared to premenopausal women [28]. This central obesity has been linked to a greater risk of colorectal cancer in women [29].
WAT homeostasis and inflammation
Development of WAT inflammation
Obesity is often described as a state of chronic inflammation, primarily in the white adipose tissue (WAT). The WAT consists of a complex network of cells including adipocytes and immune cells like neutrophils, lymphocytes, and macrophages. One of the hallmarks of obesity-mediated inflammation is the presence of crown-like structures, which are the clusters of macrophages that form around dying/dead adipocytes in the WAT. This pathology is a defining feature of WAT inflammation. While it is commonly observed in obese patients, crown-like structures and WAT inflammation can also be seen in patients with normal BMI [30]. Homeostasis in the WAT of lean individuals is maintained by several anti-inflammatory cell types, such as the T regulatory (Treg) population through the release of cytokines like interleukin (IL)-10 and IL-4. These Tregs are also responsible for maintaining insulin sensitivity in lean adipose tissue [31]. In addition, adipose stromal cells produce IL-33, which helps maintain the type 2 innate lymphoid cells that release type 2 cytokines like IL-4 and IL-5 [32]. Further, this environment supports the M2 macrophage phenotype, which suppresses the pro-inflammatory M1 phenotype [33]. The adipose tissue hypoxia and mechanical stress that results from obesity disrupts this balance and triggers nuclear factor kappa B (NF-κB)-related pathways, inducing inflammatory changes in the WAT [34–37].
Adipocytes secrete the adipokine leptin, which regulates metabolism and satiety through signaling to the hypothalamus [38, 39]. In the obese WAT, healthy expansion of the adipose tissue occurs through adipogenesis, wherein the preadipocytes become mature adipocytes. However, excess calorie intake can result in adipocyte hypertrophy or increased fat accumulation into mature adipocytes, which is marked by hypoxia and subsequent inflammation [40]. Additionally, patient-derived adipocytes show increased leptin and pro-inflammatory cytokine secretion with increased adipocyte size, while anti-inflammatory IL-10 and IL-1ra and adiponectin secretion are decreased [41–43]. Furthermore, hypoxia inhibits the differentiation of preadipocytes to mature adipocytes and promotes the synthesis of leptin and inflammatory cytokines by these preadipocytes [35]. Increased leptin production by the WAT and the stressed environment of the tissue results in the recruitment of pro-inflammatory factors to the region [44]. Inflammation in this region also induces T-cells to break out of the tolerogenic state and become more Th1-like, further exacerbating the inflammation [45, 46]. The increased production of leptin and inflammatory cytokines like tumor necrosis factor alpha (TNF-α) results in a persistent insult to the WAT that also promotes a paradoxical leptin resistance commonly observed in the obese WAT [47, 48]. This resistance plays a key role in promoting obesity, sustaining the stressful WAT environment and chronic inflammation. Although some studies show the presence of metabolically healthy obese individuals who do not present with adipose inflammation, they are still at increased risk for later insulin resistance, type II diabetes, and cardiovascular disease [40].
WAT inflammation and cancer
Chronic inflammation is associated with an increased risk of several cancers [49]. Dysfunctional adipose tissue also results in altered adipokine profiles, increased pro-inflammatory cytokines, and hypoxia, which can all promote tumor progression [50]. Many pathways involved in carcinogenesis are implicated in the inflammation process as well, including STAT3/5 as well as toll-like receptor (TLR) recognition initiated inflammasome activation [51–53]. Additionally, increased aromatase activity in the WAT due to increased adiposity is correlated with WAT inflammation[30]. In vitro, prostaglandin E2 (PGE2) production by breast cancer cells and macrophages was shown to be enhanced by obese patient-derived sera, and this PGE2 was shown to promote aromatase production in preadipocytes [54, 55]. Furthermore, WAT inflammation in obese women has been shown to correlate with metastatic progression in breast cancer [56]. M1 macrophage-derived TNF-α has also been shown to promote ovarian cancer cell metastasis through the NF-κB pathway, and accumulation of these macrophages in the adipose tissue has been observed in obese individuals [57].
Leptin signaling
Leptin receptors and resistance
Leptin is a member of the adipokine family of hormones that work to regulate energy metabolism through interaction with the arcuate nucleus of the hypothalamus. It is primarily produced by adipocytes and signals satiety to the brain. Leptin interacts with cells through the leptin receptor, Ob-R, which is found on several cell types. Through RNA splicing, there are six isoforms of this receptor, with five forms predominating: the long Ob-Rb and four short forms (Ob-Ra, Ob-Rc, Ob-Rd, and Ob-Rf). The sixth form, Ob-Re, is a secretory form that lacks intracellular domains and has been mostly shown to provide a quenching effect on circulating leptin as a regulatory mechanism [58]. The long form, Ob-Rb, is found in the hypothalamus and on B and T lymphocytes where it influences their activity [59]. Ob-Rb differs from the other forms of Ob-R by also expressing suppressor of cytokine signaling (SOCS) motifs, which suppress leptin signaling. The commonly found short form of the leptin receptor, Ob-Ra, is expressed on multiple cell types, like those of the kidney and liver as well as macrophages. Signaling through the leptin receptor can take place via the JAK-STAT3/STAT5, PI3K/Akt, and MAPK (SHP2-dependent and SHP2-independent) pathways [58].
Obese individuals tend to have high levels of circulating leptin and present with “leptin resistance.” While the mechanism of leptin resistance has not been clearly elucidated, some proposed mediators include decreased leptin permeability through the blood brain barrier due to increased leptin concentrations in the brain, downregulation of the Ob-Rb isoform in the presence of excess leptin, and delayed cell surface receptor expression [60, 61]. These mechanisms suggest that leptin resistance primarily affects the hypothalamic neurons, while leptin sensitivity may be retained in other cell types. It has been shown that neuronal deletion of SOCS3 restores leptin sensitivity in diet-induced obese mice [62].
Inflammatory role of leptin
Leptin has been shown to promote the production of IL-2 and interferon gamma (IFN-γ) in stimulated T cells while suppressing Treg proliferation [63–65]. Leptin also skews the monocyte and macrophage populations to the pro-inflammatory type by stimulating the release of TNF-α and IL-6 in vitro [66, 67]. Furthermore, leptin and other adipokines have been shown to regulate the activity of inflammasomes, large multimolecular complexes that are key players in the innate immune system and the development of obesity-associated adipose tissue inflammation [68]. Leptin has specifically been linked to an inflammasome-mediated increase in macrophage production of IL-18 in vitro [69]. Although leptin has been shown to be involved in several pro-inflammatory processes, subphysiological to physiological doses of leptin have an anti-inflammatory effect through the reduction of circulating macrophage chemoattractant protein (MCP)-1 and IL-6, potentially by downregulating their mRNA expression in adipose tissues [70]. This anti-inflammatory effect has also been observed in glial cells as well as models of pancreatitis and acute colitis [71–73].
Leptin and cancer
Leptin is involved in many cancer-promoting mechanisms including proliferation, chemoresistance [74–76], and angiogenesis [77, 78]. In vitro studies have shown that leptin not only supports the proliferation of cancer cells through pathways like the Wnt pathway, but also promotes the expression of epithelial-to-mesenchymal transition-related genes through the PI3K/Akt pathway [79, 80]. Furthermore, leptin promotes the migration and invasion of several cancer types including breast, lung, prostate, and ovarian cancer. In addition to adipocytes and immune cells, cancer stem cells also express the leptin receptor, and in vitro leptin has been shown to promote cancer stem cell enrichment in breast cancer through the induction of stem cell transcription factors NANOG, OCT4, and SOX2 in a STAT3-dependent manner [81, 82]. Leptin from adipose stromal/stem cells in the tumor microenvironment can also promote the metastasis of estrogen receptor positive breast cancer [83].
Hyperinsulinemia and insulin resistance
Insulin is another hormone implicated in obesity-associated cancers. Insulin plays a key role in adipocyte maintenance through various mechanisms, including generation of adipocytes from preadipocytes as well as lipogenesis [84]. Chronic inflammation promotes insulin resistance in many obese individuals, as shown in studies assessing systemic inflammation and insulin resistance [85]. Insulin promotes triglyceride storage into adipocytes, which prevents their degradation by lipases into free fatty acids (FFA). These FFAs are often elevated in the plasma of obese patients and can cause insulin resistance in secondary sites like the skeletal muscles due to the accumulation of lipid products like fatty acyl-CoA, which interfere with the insulin signaling cascade [86]. FFAs can also act in a pro-inflammatory manner by binding the toll-like receptors TLR2 and TLR4, which then activate the NF-κB pathway to induce the release of TNF-α and MCP-1 and therefore further increase pro-inflammatory immune cell infiltrations into the region [87]. The pro-inflammatory cytokine TNF-α has also been shown to induce insulin resistance via activation of inhibitor of nuclear factor kappa B kinase subunit beta (IKK-β) and subsequent serine phosphorylation of the insulin receptor, which interferes with insulin signaling [88].
Conversely, insulin signaling has been linked to adipose tissue inflammation in obese individuals. Some studies report elevated adipose tissue inflammation and macrophage infiltration in diabetic patients receiving insulin [89]. In addition, a reduction in circulating insulin levels in obese mice resulted in a significant decrease in adipose tissue inflammation [90], likely by preventing insulin-induced (via ERK signaling) production of MCP-1 from adipocytes, which can occur even in the context of insulin resistance [91].
Insulin and cancer
Increased circulating insulin can contribute to carcinogenesis as in vitro studies show chronic exposure to insulin and glucose promotes proliferation and aberrant chromosomal alterations in melanocytes [92]. In colon cancer cells, chronic insulin exposure also results in increased chemoresistance to the drugs oxaliplatin and SN38 in vitro [93]. In addition, overexpression of insulin receptor substrate-1 (IRS-1) aids in cell proliferation and inhibits autophagy in fibroblasts [92, 94]. Hyperinsulinemia-induced increases in IRS-1, as seen in patients, also promote colon and endometrial cancer progression [95, 96]. Further, in humans and mouse models, hyperinsulinemia results in increased expression of insulin-like growth factor-1 [97], which has been shown to promote metastasis [98, 99]. Additionally, insulin resistance has been linked to an increased risk of breast, pancreatic, endometrial, colorectal, and prostate cancer [99–103].
Obesity-mediated immune cell dysfunction in the adipose tissue
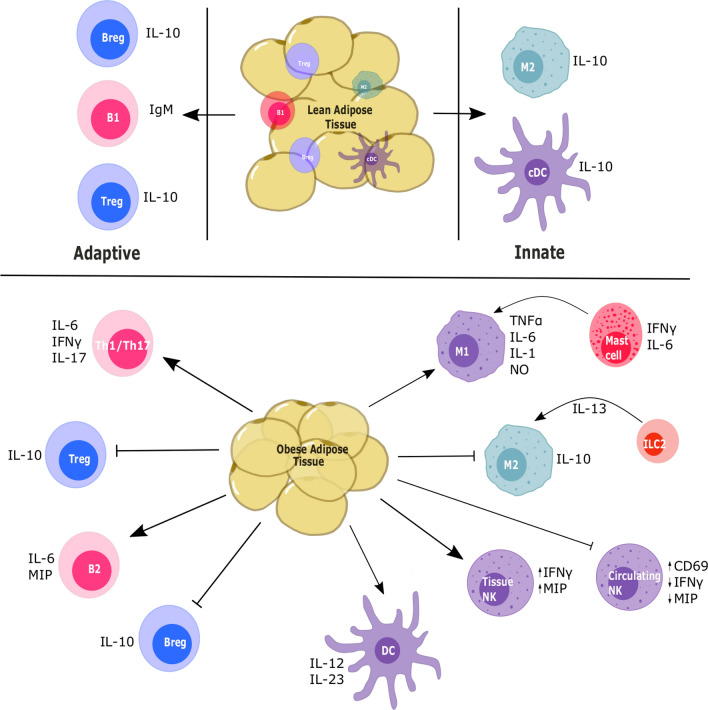
Adipose tissue homeostasis maintained by suppressive immune cells like Treg cells, regulatory B cells (Bregs), and M2-like macrophages is disturbed in obese conditions, with increased TNF-α and MCP-1 production by the adipocytes resulting in a pro-inflammatory immune profile [104]. In obese individuals, this chronic inflammation alters the immune profile to not only an inflammatory type but also a dysfunctional one as shown in Fig. 1. Chronic inflammation has been associated with an increased risk of several cancers like colon, pancreatic, and ovarian cancer [105], and pro-inflammatory alterations in the immune profile of WAT are also associated with higher cancer risk [106].
Fig. 1.
Schematic showing the immune profile of the lean and obese adipose tissue. The immune profile of the obese adipose tissue is generally skewed toward the inflammatory phenotype due to the presence of cytokines like IL-6 and TNF-α secreted by the adipose tissue as a result of mechanical stress, hypoxia, and necrosis
Adaptive immune cells
T cells
Obesity modulates the antigen specific adaptive population of the immune system. It accelerates T cell senescence in the visceral adipose tissue (VAT) of HFD-fed mice, resulting in T cells that resemble those typically found in aging VAT [107]. In human and murine adipose tissue, increased adiposity results in the activation of NF-κb pathways, which induce the expression of inflammatory cytokines that recruit Th1-like cells to the region [45, 108]. Th1 cells secrete additional IL-6 and IFN-γ in the WAT microenvironment, exacerbating the inflammation [46]. Increased leptin expression also aids in induction of pro-inflammatory cytokines from the T cells, which express the long form of the leptin receptor (Ob-Rb) [109]. In addition, leptin appears to be important in lymphocyte generation and development since leptin-deficient ob/ob [39] and leptin receptor-deficient db/db mice [110, 111] exhibit a significant reduction in functional T cells [112].
Obesity in mice has also been shown to predispose the T-cell type to Th17 due to increased IL-6 presence [113]. Th17 cells are a helper T cell type characterized by the production of the pro-inflammatory cytokine IL-17 and the transcription factor RAR-related orphan receptor gamma t (RORγt). T cells in obese mice selectively promote Th17 cells and these cells produce a greater amount of IL-17, which was abrogated in IL6−/− mice [113]. In addition to IL-6, Th17 cells are also induced through the IL-21 pathway. Obese mice show increased expression of IL-21 in the adipose tissue, which has been shown to promote IL-17 expression in Th17 cells in an autocrine process that is STAT3-dependent [114]. Furthermore, this population is maintained and supported by IL-23 secreted by adipose tissue dendritic cells (DCs) [115, 116]. In the adipose tissue, obesity results in increased fatty acid metabolism and acetyl CoA carboxylase 1 expression. This enzyme modulates RORγt function in Th17 cells, promoting their growth in obese individuals [117].
As the major T cell population in lean adipose tissue, Tregs are important for homeostasis through their anti-inflammatory actions. The VAT in mice contains more Tregs than the spleen, lung, and liver, but these numbers decline in obese mice, highlighting their importance in the adipose tissue [46]. The increased circulating leptin in obese mice can bind leptin receptors expressed on Treg cells to suppress their proliferation and negatively regulate their anti-inflammatory activity [65]. Furthermore, these cells have decreased forkhead box P3 (FOXP3) expression and are in an anergic state, which can be reversed with a leptin monoclonal antibody. Monoclonal antibody blockade of leptin signaling also results in increased ERK1/2 phosphorylation, which restores Treg cell responsiveness [65]. Adipocyte-derived IL-21 also suppresses Treg accumulation in the VAT, and IL-21 knockout improved Treg numbers, reduced body weight, and improved insulin sensitivity in HFD-fed mice [118].
B cells
Obesity also affects B-lymphocyte development and function. B cells express the leptin receptor (Ob-Rb) [59] and respond to leptin by upregulating expression of the pro-inflammatory cytokines IL-6 and TNF-α as well as anti-inflammatory IL-10 [119]. Leptin plays an important role in not only T cell development but hematopoiesis in general, and this has been shown through reduced B cell numbers in both db/db and ob/ob mice [120, 121].
B cells promote inflammation by modulating T-cell function through release of pro-inflammatory cytokines like IL-6 and macrophage inflammatory protein 2 (MIP-2, also known as CXCL2) [122]. While the role of B cells in adipose tissue is not clearly understood, obesity-associated inflammation has been linked with suppressed B cell responses through leptin-mediated STAT3 induction of TNF-α [123], which in unstimulated B cells results in preactivation and lack of response to further antigenic stimulation [124]. Further, B cells derived from obese individuals showed impaired IL-6 secretion and increased immunoglobulin IgM levels upon stimulation, and an impaired response was also observed in B cells from HFD-fed mice [125]. However, others have shown that B cells accumulate in the VAT of obese individuals and are responsible for the activation of inflammatory macrophages and T cells here as well production of IgG autoantibodies, promoting insulin resistance [126]. Additionally, depletion of B cells in HFD-fed mice led to a decreased inflammatory profile, improved insulin sensitivity, and alterations in the IgG profile [126].
An anti-inflammatory regulatory B-cell type (Bregs), similar to Tregs, can also be found in the adipose tissue. These Bregs constitutively produce IL-10, which inhibits adipose tissue inflammation. In diet-induced obese mice, deletion of IL-10 from B cells results in increased CD8+ T cell and M1 macrophage infiltration in the adipose tissue and reduced insulin sensitivity [127]. Further, decreased frequencies of Bregs were observed in the adipose tissue and peripheral blood of overweight and obese individuals compared to normal weight individuals [128].
Innate immune cells
Macrophages
In the adipose tissue, resident macrophages are found in the M2-like state, maintaining homeostasis [129]. Obesity affects this homeostasis, as seen with the WAT inflammation mentioned previously. Important to this process is the formation of crown-like structures (CLS) in the adipose tissue where, due to mechanical stress and hypoxia in the adipocytes, M1-like macrophages surround dead or dying adipocytes [85, 130]. In mice and human subjects, CLS are responsible for the release of several types of damage-associated molecular patterns, which bind with nod-like receptors on the macrophages, activating NF-κb-mediated release of MCP-1 [131] and TNF-α [132] and thereby promoting regional inflammation. Although it is a marker for DCs, CD11c expression is observed on M1-like macrophages that accumulate in the adipose tissue of obese mice but not in lean mice [133, 134]. In addition, obesity-induced leptin resistance enhances the release of FFAs, which bind TLRs on the macrophages and therefore promote the expression of pro-inflammatory genes, further contributing to WAT inflammation [135, 136]. Macrophages and monocytes also express the leptin receptor and produce pro-inflammatory cytokines like TNF-α and IL-6 in response to leptin [66, 137, 138]. Macrophage production of several other pro-inflammatory factors, such as IL-1β, nitric oxide (NO) and IFN-γ, can also be induced by leptin in vitro [138, 139]. Furthermore, obesity-associated TNF-α release in mice has been associated with an increase in circulating monocytes, which contribute to insulin resistance and subsequent hyperinsulinemia [140]. Studies have also shown that higher plasma levels of MCP-1 in the leptin receptor-deficient db/db mouse model of obesity or adipocyte specific-MCP-1 overexpressing mice results in increased macrophage recruitment into adipose tissues, which promotes insulin resistance [141, 142].
Dendritic cells
DCs are critical antigen presenting cells responsible for T cell activation. In lean mice, conventional DCs in the adipose tissue promote a tolerogenic state through IL-10 production as well as upregulating Wnt and PPARγ pathways, which regulate adipocyte differentiation and limit T cell activation [143, 144]. These lean adipose tissue anti-inflammatory DCs have not been identified in humans, but adipose tissue resident DCs have been found to contribute to inflammation and insulin resistance through the induction of Th17 cells in both mice and humans [145]. In humans, a positive correlation was observed between BMI and CD11c + CD1c + DCs, which induced Th17 responses ex vivo. CD11chighF4/80low DCs from mice also induced Th17 cells [146]. DCs also express the leptin receptor, and leptin promotes a pro-inflammatory DC phenotype [147]. In contrast, DCs obtained from leptin-deficient ob/ob and leptin receptor-deficient db/db mice have impaired functioning due to reduced expression of co-stimulatory molecules and inability to stimulate allogeneic T cells [148, 149]. In vitro, leptin has been shown to stimulate increased DC production of IL-12, but not IL-10 or transforming growth factor beta (TGF-β). IL-12 favors the polarization of CD4 + T cells to the pro-inflammatory Th1 and Th17 type [150].
Natural killer cells
Natural killer (NK) cells, while part of the innate system, exhibit lymphoid-like features due to shared lineage with T and B lymphocytes. Obese patients have been shown to have significantly lower levels of circulating NK cells compared to lean controls [151]. Further, circulating NK cells from obese patients show increased activation through CD69 upregulation, but they are functionally impaired with decreased IFN-γ and macrophage inflammatory protein (MIP)-1β production [152]. Furthermore, obesity reduces circulating NK cell degranulation, despite increased CD69 levels, suggesting greater activation and exhaustion of these cells in comparison to lean controls [153]. NK cells from obese individuals also have decreased oxidative phosphorylation and glycolytic activity upon cytokine stimulation [154]. This metabolic dysfunction results in decreased cytotoxic function in terms of perforin and granzyme activity as well as decreased anti-tumor activity. However, a subsequent study found no difference in the total NK cell population between obese and normal weight humans, but did note that the low cytotoxic CD56bright NK cell population was increased in obese individuals compared to the cytotoxic CD56dim phenotype [155].
Other cell types
Additionally, other innate cells are involved in maintaining adipose tissue homeostasis. Innate lymphoid cells have been shown to promote an anti-inflammatory Th2 environment by sustaining the alternatively activated macrophage population [156]. Neutrophils express the short form of the leptin receptor (Ob-Ra). However, leptin only plays an indirect role in neutrophil migration and activation in vivo, which is directly mediated by the release of TNF-α from monocytes and chemokine (C-X-C motif) ligand 1 (CXCL1) from peritoneal cells [157, 158]. In pancreatic cancer, tumor-associated neutrophils are recruited by adipocyte-derived IL-1β and activated pancreatic stellate cells, which release additional IL-1β to further increase neutrophil recruitment and therefore promote cancer progression and chemoresistance [159].
Another immune population, the mast cell, also expresses the leptin receptor [160] and is increased in the WAT of obese individuals. These cells have been shown to promote obesity and glucose intolerance, as well as adipose tissue apoptosis and angiogenesis, through release of IL-6 and IFN-γ [161]. Further, leptin-deficient (ob/ob) mast cells were shown to play a protective role when administered to wild-type diet-induced obese mice, mitigating obesity and diabetes by aiding in polarization of WAT macrophages to the anti-inflammatory M2-type [162].
Obesity-mediated immune cell dysfunction in the tumor microenvironment
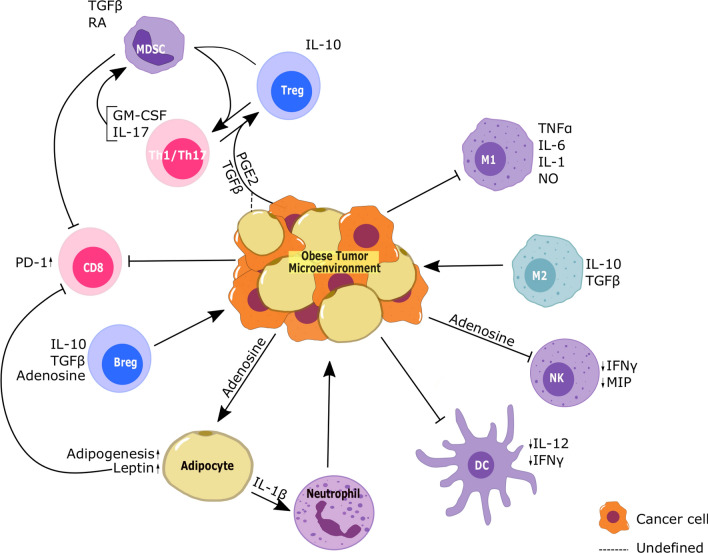
The tumor microenvironment is generally considered to be immunosuppressive due to the presence of IL-10 and TGF-β and the suppressive properties of various cell types like myeloid-derived suppressor cells (MDSCs), M2 macrophages and Tregs in addition to the cancer cells as shown in Fig. 2. In the obese tumor microenvironment, the involvement of the adipose tissue and adipokines like leptin and adiponectin can influence the immune profiles as well.
Fig. 2.
Schematic showing the immune profile of the obese tumor microenvironment. Unlike the adipose tissue, due to the immunosuppressive factors from the cancer cells, the obese tumor microenvironment is infiltrated by mostly suppressive populations such as M2 macrophages, MDSCs, and Tregs
Adaptive immune cells
T cells
Obesity has been shown to promote immune exhaustion through upregulation of PD-1 on CD8 + T cells, as seen in the tumors of HFD-fed mice [163]. This effect has been beneficial in mouse models of breast cancer, lung cancer, and melanoma as well as colorectal cancer patients in their response to PD-1/PD-L1 blockade [164]. Further, treatment with anti-PD-1 antibodies in esophageal cancer not only improved the killing capacity of the T cells but also reduced the number of PD-1 + T cells in the tumors [165]. Increased T cell PD-1 expression has been linked to leptin signaling through an indirect mechanism involving the STAT3 pathway as leptin resistant db/db T cells had decreased PD-1 expression compared to wild-type T cells after adoptive transfer into tumor bearing diet-induced obese Rag2−/− mice [164].
Th17 and Treg: the inflammatory environment in obesity favors the generation of Th17 cells through the release of not only IL-23 from the adipose DCs but also through IL-1β released from adipose macrophages [166]. While Th17 is generally an inflammatory T cell type, Th17 cells in the tumor microenvironment often promote tumor progression by inducing infiltration of pro-tumor immunosuppressive MDSCs [167] as well as angiogenesis and chemoresistance through granulocyte–macrophage colony stimulation factor (GM-CSF) and IL-17 secretion and stimulation of vascular endothelial growth factor (VEGF) production [168–170].
In colorectal cancer patients, Foxp3 + IL-17 + T cells are present in the microenvironment that not only express high levels of TGF‐β and RORγt, but can also promote the induction of cancer initiating cells ex vivo [171]. These T cells have immunosuppressive effects in colon cancer patients despite releasing high levels of IL-17 [172]. Further, Foxp3 + RORγt + regulatory T cells also have immunosuppressive activity as seen in pancreatic cancer patient samples through suppression of T cell proliferation. However, they can also be pro-inflammatory through release of TGF-β and IL-6, which promote IL-17 production from T cells, and through their own production of IL-17 [173]. MDSCs have been shown to promote the induction of Tregs and the transdifferentiation of Th17 to Tregs through TGF-β and retinoic acid [174]. In the tumor microenvironment of an ovarian cancer mouse model, tumor-derived TGF-β and PGE2 were also shown to promote the transdifferentiation of Th17 cells to suppressive Tregs, and these Th17-Treg subsets have been identified in patient samples [175]. The enzyme cyclooxygenase-2 (COX2), which is responsible for the production of prostaglandins like PGE2, is highly expressed in adipose tissue [176], and COX2 signaling is also upregulated in the subcutaneous adipose tissue of obese individuals [177]. Hence, adipocyte-derived PGE2 could contribute to the generation of an immunosuppressive T cell population. Further, adipocyte-derived TGF-β, which has been shown to be increased in the adipose tissue of ob/ob and db/db mice [178], can also contribute to this Th17-Treg subset.
B cells
Tumor-infiltrating B cells are generally associated with a positive outcome in anti-tumor immunity [179] and are comprised of activated B cells [180, 181]. B cells are recruited to the tumor microenvironment through the action of CXCL13, produced by the tumor cells. This chemokine is also produced by mature adipocytes [182].
However, several studies in mice have shown that Breg infiltration into tumors causes immunosuppression through IL-10 production and therefore promotes tumor growth [183]. Breg cells have also been shown to promote tumor growth in head and neck cancer patients through an increase in the production of adenosine seen ex vivo [184], which suppresses the cytotoxicity of NK cells. However, the role of tumor-infiltrating B cells in the obese TME has not been fully characterized.
Innate immune cells
Macrophages
Obesity results in the increased recruitment of macrophages to the tumor microenvironment, which are then polarized to the M2-like tumor-associated macrophages (TAMs) that promote an immunosuppressive tumorigenic environment [185]. Although M2 to M1 polarization does occur in the adipose tissue, this skewing has not been observed in tumors, where M2-like TAM cells predominate [135, 163]. TAMs contribute to cancer progression through the release of TGF-β, promoting cancer growth and suppressing immune response [186, 187]. Angiogenesis is an important factor in breast cancer progression, and macrophages have also been implicated via in vitro studies in the promotion of stromal vascularization and angiogenesis through CXCL12 when recruited and activated by adipocyte-derived MCP-1 and IL-1β [188]. Of note, exosomes derived from melanoma cells were shown to be able to generate a hybrid M1/M2 macrophage type in vitro with upregulation of both types of macrophage markers. These macrophages were associated with pro-tumor activity [189].
Dendritic cells
DCs play an important role in antigen presentation and T cell education. In the tumor microenvironment of diet-induced obese mice, there is an increase in the inhibitory type of DC, which suppresses T cell proliferation [190]. Further, these cells also produce less IL-12 and TNF-α than DCs in normal weight mice, which contributes to their impaired functionality and results in reduced T cell function [190].
Natural killer cells
NK cells express both long and short forms of the leptin receptor [191]. Leptin plays an important role in the development of NK cells, as leptin receptor deficiency in db/db mice decreases NK cell numbers in the liver, spleen, lung, and peripheral blood and also impairs the activation of these cells [192]. Interestingly, leptin treatment in a hepatocellular carcinoma mouse model suppressed tumor progression through the increased proliferation and activation of NK cells [193]. However, obesity in humans results in significantly reduced hepatic NK cell numbers as well as Ob-Rb expressing NK cells [194]. Resistance to leptin signaling through suppressed downstream JAK/STAT signaling has also been implicated in the NK cell dysfunction in diet-induced obese rat models [195]. This suggests that while leptin promotes NK cell activity, obesity results in dysfunctional NK cells, leading to impaired tumor immunity. Dysfunctional NK cells have also been implicated in promoting metastasis in db/db and ob/ob mice due to their lack of leptin signaling [196].
In addition to increased leptin and hyperinsulinemia, obesity also results in increased circulating levels of adenosine [197]. Adenosine is implicated in dampening immune responses and, like adipocytes and Breg cells, cancer cells produce adenosine through CD73 and CD39 activity. NK cells recruited to the tumor microenvironment express the A2a receptor, which binds adenosine, and this results in decreased cytotoxic functionality [198].
Metastatic microenvironment
The metastatic potential of tumors is influenced by several factors, including mutational burden of the tumor, expression of genes promoting metastasis, and the cancer type, as some cancers are more susceptible to metastasis [199, 200]. However, obesity has also been shown to contribute to increased metastasis in several cancers such as breast, prostate, and colorectal cancer [201].
The innate immune population of myeloid-derived cells are seen as key players in metastasis due to their pro-angiogenic (VEGF) actions and pro-inflammatory actions (TNF-α) [202]. Comparison of the primary tumors and metastatic lesions in murine breast cancer models show an increase in polymorphonuclear neutrophils in the latter, while the primary tumors show increased inflammatory monocytes [203]. In the breast cancer 4T1 mouse model, HFD diet-fed mice showed decreased survival through increased tumor volumes as well as lung and liver metastasis [204]. Further, in vitro studies show increased proliferation, migration, and invasion capacity of the cancer cells when treated with cytokines elevated specifically in the HFD-fed mice, such as macrophage colony-stimulating factor (M-CSF) [204]. In breast cancer, obesity also results in increased neutrophil count causing neutrophilia, which has been shown to promote lung metastasis through the release of GM-CSF and IL-5 by the lung neutrophils [205]. In obesity resistant mice, HFD was sufficient to promote tumor growth and lung metastasis in colon cancer through expression of genes regulating inflammation and proliferation [206]. These mice also show increased macrophage infiltration into the adipose tissue [206].
The tumor metabolite sphingosine-1 phosphate (S1P), formed by the phosphorylation of sphingosine by sphingosine kinase 1, is upregulated by HFD in both syngeneic and spontaneous tumor models of breast cancer [207]. S1P results in increased macrophage recruitment to the lungs, a common site of breast cancer metastasis, and IL-6 produced by the infiltrating cells helps promote lung metastasis. The role of IL-6 in promoting breast cancer metastasis has also been shown in other studies [208, 209]. HFD also increases circulating cholesterol, and its derivative 27-hydroxycholesterol has been shown to increase the neutrophil count (IL-17 responders) as well as γδ-T cells (IL-17 producers) in metastatic sites while decreasing the CD8 + T cell count [210].
Obesity’s implications for current therapies
Chemotherapy and adipose tissue-mediated chemoresistance
Several studies have also shown that increased adiposity results in poorer response to chemotherapy. In a retrospective study of breast cancer patients enrolled in the BIG 2–98 adjuvant clinical trial comparing docetaxel-based and non-docetaxel-based chemotherapy, BMI > 30 kg/m2 was associated with lower overall and disease-free survival rates in those receiving docetaxel-based therapies [211]. Excess visceral adipose tissue in postmenopausal breast cancer patients has been linked to decreased chemosensitivity [212–214]. In vitro studies have shown that adipose stromal cells can promote chemoresistance in prostate cancer cells by inducing the expression of epithelial-to-mesenchymal transition-related genes like Cdh2, fibronectin, vimentin, Zeb2, and Slug1 [215]. In ovarian cancer, adipocytes have been shown to promote chemoresistance through upregulation of genes involved in apoptosis like Bclxl [216]. Adipocyte-derived leptin can also promote resistance to the taxane chemotherapy paclitaxel in breast cancer cells via increased fatty acid oxidation [217]. Further, adipocytes can metabolize chemotherapy drugs, rendering them inactive, as seen in the conversion of daunorubicin to inactive daunorubicinol [218]. Prevention of doxorubicin uptake through its accumulation into cytoplasmic vesicles and eventual expulsion via upregulation of transport proteins like major vault protein has also been observed in co-cultures of adipocytes and breast cancer cells [219]. Fatty acid synthase (FASN) is an important enzyme in de novo lipogenesis and is upregulated in several cancers. Melanoma tumors from HFD-fed mice had increased FASN expression compared to normal diet mice, while conditioned media from obese patient-derived adipose macrophages (but not monocyte-derived macrophages) stimulates FASN expression in breast cancer and colon cancer cells [220, 221]. This FASN overexpression promotes resistance to the chemotherapy drug cisplatin in breast and ovarian cancers, which can be overcome by treatment with the FASN inhibitor C75 [222, 223]. However, FASN expression in renal cancer was shown to be inversely correlated with obesity, and high BMI was determined to be a prognostic factor for longer survival and response to targeted therapy. These findings highlight the importance of varying BMI considerations for different cancers [224]. Overall, excess adipose tissue surrounding solid tumors like breast cancer can make it challenging to effectively target the cancer cells, which are being affected by the fatty acids, adipokines, and multiple other factors produced by that adipose tissue.
Another major challenge to effective chemotherapy treatment in obese cancer patients is under-dosing. Previously, up to 40% of obese patients were under-dosed because clinicians did not use patients’ actual body weights to calculate doses, resulting in a variation of up to 25% in calculated doses for patients at the same body weight. The under-dosing was largely due to concerns about increased susceptibility to chemotherapy toxicity in obese patients, though there was no evidence of elevated short- or long-term toxicity with chemotherapy doses based on their full weight. The American Society of Clinical Oncology published practice guidelines in 2012 indicating that obese patients’ full weight should be used for dose calculations since toxicity concerns are limited and under-dosing results in greater risks [225]. Further, several clinical trials are currently being conducted to assess obese patients’ response to chemotherapy drugs for various cancers, which will help shed light on the efficacy of these drugs in the context of obesity (Table 1).
Table 1.
Clinical trials assessing drug efficacy in obese (BMI ≥ 30 kg/m2) cancer patients
| Cancer | Drug | Study summary | Sex | Trial number |
|---|---|---|---|---|
| Multiple, advanced | Xeloda (Capecitabine) | Capecitabine pharmacokinetics in patients dosed as per ideal vs actual body weight | A | NCT01828554 |
| Colorectal | Metformin | Assessment of metformin in reduction of pS6Serine235 expression on mucosal tissue | A | NCT01312467 |
| Breast cancer | Doxorubicin, Cyclophosphamide | Assessment of drug plasma clearance between normal weight and obese patients | F | NCT01537029 |
| Endometrial cancer | Metformin | Assessment of effects of metformin administration prior to hysterectomy | F | NCT01877564 |
Endocrine therapy
In prostate and breast cancer, endocrine therapies such as androgen agonists and aromatase inhibitors (AI) are commonly administered to control tumor growth. AIs like anastrozole, letrozole, and exemestane are used in breast cancer to decrease estrogen production. However, increased adiposity results in a greater concentration of circulating estradiol in obese postmenopausal patients due to greater aromatase activity in their adipose tissue, leading to concerns regarding the effectiveness of AIs in obese breast cancer patients [226]. Trials involving AIs versus tamoxifen (ATAC, BIG 1–98, TEAM, ABCSG-12) have in some cases suggested that AIs can be more beneficial than tamoxifen in women with higher BMI, but results are inconsistent [226, 227].
Immunotherapies
In more recent years, immune-based targeted approaches to cancer therapy have emerged. Some examples include chimeric antigen receptor (CAR), monoclonal antibody, and cytokine therapies.
In CAR-based therapies, patients’ immune cells are genetically modified to express a cancer antigen specific receptor, enhancing their anti-tumor ability. However, the increased cytotoxicity of CAR-based therapy, particularly in CAR-T cells, can produce a severe immune reaction in which too many cytokines are released too quickly, known as a cytokine storm. Given the underlying chronic inflammation present in most obese patients, these individuals may be at particularly high risk of adverse side effects from such CAR-based therapies. In addition, monoclonal antibody treatment in aged mice with increased adiposity and preexisting inflammation has been shown to further increase inflammation as well as morbidity, relative to young lean mice [228, 229].
While monoclonal antibody therapies such as epidermal growth factor receptor (EGFR) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) blockade (panitumumab and ipilimumab, respectively) have recommended doses based on weight, it has been suggested that fixed dosing be used for cost-effectiveness due to the potency of these therapies [230, 231]. The safety of αPD-1 treatment in obese patients has been prospectively assessed in esophageal cancer, where it was shown to be safe due to a lack of extratumoral pro-inflammatory T cell phenotype rescue in the αPD-1-treated patients [165]. In addition, recent retrospective studies of patients treated with αPD-1/PD-L1 and/or CTLA-4 checkpoint inhibitors have shown improved clinical outcomes in patients with BMI ≥ 25, compared to those with BMI < 25. These studies, which included patients with non-small cell lung cancer, melanoma, and several other cancer types, also found no issues with elevated toxicity in the overweight/obese patients [232–234]. As previously mentioned, obesity has been shown to increase PD-1 expression on cytotoxic CD8 T cells, which promotes tumor progression but also greatly aids in αPD-1/PD-L1 therapy effectiveness in mice and patients [164]. This enhanced efficacy was demonstrated in male patients with metastatic melanoma that received combinations of immunotherapy (trametnib, vemurafenid, nivolumab, cobimetinib, ipilimumab, pembrolizumab, and atezolizumab), as improved survival was observed in the obese men compared to lean men, but this effect was not seen in female patients [235]. For additional information, Deshpande et al. [236] recently published a review extensively detailing the effects of obesity, as well as lifestyle factors and diabetes, on response to checkpoint inhibitors.
Cytokines such as IL-2, IL-7, and IL-15 have also been used as immunotherapeutic agents. Combination therapy of IL-2 with αCD40 in aging obese mice has been shown to exacerbate inflammation, leading to death [228, 229]. However, more studies are needed to determine the safety of interleukin-based cancer therapies in the context of obesity.
Concluding remarks
Obesity-associated chronic adipose inflammation and dysfunction in the development and activity of several types of tumor-infiltrating immune cells have been shown to promote cancer growth as well as metastasis. Immunotherapy-based rescue of the dysfunctional immune cells, alone or in combination with other therapies, could aid in the treatment of obesity-associated cancers. However, additional studies will be needed to evaluate the efficacy and safety of these therapies for specific obesity-associated cancers. Further, obese status needs to be considered during administration for effective dosage to prevent under-dosing as well as overactivation of the immune system. More in vivo model data are also required to properly evaluate the impact of obesity on the immune microenvironment of tumors, as the role of some tumor-infiltrating immune cell types remains poorly characterized. As the rate of obesity continues to increase across the globe, greater understanding of the impact of obesity on the tumor microenvironment will help inform the development of improved treatment regimens, which are urgently needed to reduce the burden of obesity on cancer mortality.
Author contributions
AK performed the literature review and wrote the manuscript; LB supervised the review and writing process and edited the manuscript.
Funding
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest or competing interests.
Ethical approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data. 2020;360:1–8. [PubMed] [Google Scholar]
- 2.Obesity Update - OECD. https://www.oecd.org/health/obesity-update.htm. Accessed 6 Jun 2020
- 3.Jih J, Mukherjea A, Vittinghoff E, Nguyen TT, Tsoh JY, Fukuoka Y, Bender MS, Tseng W, Kanaya AM. Using appropriate body mass index cut points for overweight and obesity among Asian Americans. Prev Med. 2014;65:1–6. doi: 10.1016/j.ypmed.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 5.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer: viewpoint of the IARC working group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colditz GA, Peterson LL. Obesity and cancer: evidence, impact, and future directions. Clin Chem. 2018;64:154–162. doi: 10.1373/clinchem.2017.277376. [DOI] [PubMed] [Google Scholar]
- 7.Bhaskaran K, Dos-santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6:944–953. doi: 10.1016/S2213-8587(18)30288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallo M, Adinolfi V, Barucca V, Prinzi N, Renzelli V, Barrea L, Di Giacinto P, Ruggeri RM, Sesti F, Arvat E, Baldelli R, Arvat E, Colao A, Isidori A, Lenzi A, Baldell R, Albertelli M, Attala D, Bianchi A, Di Sarno A, Feola T, Mazziotti G, Nervo A, Pozza C, Puliani G, Razzore P, Ramponi S, Ricciardi S, Rizza L, Rota F, Sbardella E, Zatelli MC, EOLO Group Expected and paradoxical effects of obesity on cancer treatment response. Rev Endocr Metab Disord. 2020 doi: 10.1007/s11154-020-09597-y. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh CC, Trichopoulos D, Katsouyanni K, Yuasa S. Age at menarche, age at menopause, height and obesity as risk factors for breast cancer: associations and interactions in an international case-control study. Int J Cancer. 1990;46:796–800. doi: 10.1002/ijc.2910460508. [DOI] [PubMed] [Google Scholar]
- 10.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, Gabra H, Paraskevaidis E, Martin-Hirsch P, Tsilidis KK, Kyrgiou M. Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer. 2019;145:1719–1730. doi: 10.1002/ijc.31961. [DOI] [PubMed] [Google Scholar]
- 12.Fortner RT, Poole EM, Wentzensen NA, Trabert B, White E, Arslan AA, Patel AV, Setiawan VW, Visvanathan K, Weiderpass E, Adami H-O, Black A, Bernstein L, Brinton LA, Buring J, Clendenen TV, Fournier A, Fraser G, Gapstur SM, Gaudet MM, Giles GG, Gram IT, Hartge P, Hoffman-Bolton J, Idahl A, Kaaks R, Kirsh VA, Knutsen S, Koh W-P, Lacey JV, Lee I-M, Lundin E, Merritt MA, Milne RL, Onland-Moret NC, Peters U, Poynter JN, Rinaldi S, Robien K, Rohan T, Sánchez M-J, Schairer C, Schouten LJ, Tjonneland A, Townsend MK, Travis RC, Trichopoulou A, van den Brandt PA, Vineis P, Wilkens L, Wolk A, Yang HP, Zeleniuch-Jacquotte A, Tworoger SS. Ovarian cancer risk factors by tumor aggressiveness: an analysis from the ovarian cancer cohort consortium. Int J Cancer. 2019;145:58–69. doi: 10.1002/ijc.32075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonnenschein E, Toniolo P, Terry MB, Bruning PF, Kato I, Koenig KL, Shore RE. Body fat distribution and obesity in pre- and postmenopausal breast cancer. Int J Epidemiol. 1999;28:1026–1031. doi: 10.1093/ije/28.6.1026. [DOI] [PubMed] [Google Scholar]
- 14.Pichard C, Plu-Bureau G, Neves-e Castro M, Gompel A. Insulin resistance, obesity and breast cancer risk. Maturitas. 2008;60:19–30. doi: 10.1016/j.maturitas.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Cleland WH, Mendelson CR, Simpson ER. Effects of aging and obesity on aromatase activity of human adipose cells. J Clin Endocrinol Metab. 1985;60:174–177. doi: 10.1210/jcem-60-1-174. [DOI] [PubMed] [Google Scholar]
- 16.Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225–230. doi: 10.1016/S0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 17.Brown KA, Iyengar NM, Zhou XK, Gucalp A, Subbaramaiah K, Wang H, Giri DD, Morrow M, Falcone DJ, Wendel NK, Winston LA, Pollak M, Dierickx A, Hudis CA, Dannenberg AJ. Menopause is a determinant of breast aromatase expression and its associations with BMI, inflammation, and systemic markers. J Clin Endocrinol Metab. 2017;102:1692–1701. doi: 10.1210/jc.2016-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enriori CL, Orsini W, del Carmen CM, Etkin AE, Cardillo LR, Reforzo-Membrives J. Decrease of circulating level of SHBG in postmenopausal obese women as a risk factor in breast cancer: reversible effect of weight loss. Gynecol Oncol. 1986;23:77–86. doi: 10.1016/0090-8258(86)90118-6. [DOI] [PubMed] [Google Scholar]
- 19.Wu F, Ames R, Evans MC, France JT, Reid IR. Determinants of sex hormone-binding globulin in normal postmenopausal women. Clin Endocrinol (Oxf) 2001;54:81–87. doi: 10.1046/j.1365-2265.2001.01183.x. [DOI] [PubMed] [Google Scholar]
- 20.Goldštajn MŠ, Toljan K, Grgić F, Jurković I, Baldani DP. Sex hormone binding globulin (SHBG) as a marker of clinical disorders. Coll Antropol. 2016;40:211–218. [PubMed] [Google Scholar]
- 21.Lukanova A, Lundin E, Micheli A, Arslan A, Ferrari P, Rinaldi S, Krogh V, Lenner P, Shore RE, Biessy C, Muti P, Riboli E, Koenig KL, Levitz M, Stattin P, Berrino F, Hallmans G, Kaaks R, Toniolo P, Zeleniuch-Jacquotte A. Circulating levels of sex steroid hormones and risk of endometrial cancer in postmenopausal women. Int J Cancer. 2004;108:425–432. doi: 10.1002/ijc.11529. [DOI] [PubMed] [Google Scholar]
- 22.Li CI, Malone KE, Porter PL, Weiss NS, Tang M-TC, Cushing-Haugen KL, Daling JR. Relationship between long durations and different regimens of hormone therapy and risk of breast cancer. JAMA. 2003;289:3254–3263. doi: 10.1001/jama.289.24.3254. [DOI] [PubMed] [Google Scholar]
- 23.Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, Hennekens CH, Rosner B, Speizer FE, Willett WC. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278:1407–1411. doi: 10.1001/jama.1997.03550170037029. [DOI] [PubMed] [Google Scholar]
- 24.Beral V, Reeves G, Bull D, Green J. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103:296–305. doi: 10.1093/jnci/djq527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reeves GK, Beral V, Green J, Gathani T, Bull D. Hormonal therapy for menopause and breast-cancer risk by histological type: a cohort study and meta-analysis. Lancet Oncol. 2006;7:910–918. doi: 10.1016/S1470-2045(06)70911-1. [DOI] [PubMed] [Google Scholar]
- 26.McCullough ML, Patel AV, Patel R, Rodriguez C, Feigelson HS, Bandera EV, Gansler T, Thun MJ, Calle EE. Body mass and endometrial cancer risk by hormone replacement therapy and cancer subtype. Cancer Epidemiol Biomarkers Prev. 2008;17:73–79. doi: 10.1158/1055-9965.EPI-07-2567. [DOI] [PubMed] [Google Scholar]
- 27.Leitzmann MF, Koebnick C, Danforth KN, Brinton LA, Moore SC, Hollenbeck AR, Schatzkin A, Lacey JV. Body mass index and risk of ovarian cancer. Cancer. 2009;115:812–822. doi: 10.1002/cncr.24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ambikairajah A, Walsh E, Tabatabaei-Jafari H, Cherbuin N. Fat mass changes during menopause: a metaanalysis. Am J Obstet Gynecol. 2019;221:393–409.e50. doi: 10.1016/j.ajog.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 29.Chang JW, Shin DW, Han KD, Jeon KH, Yoo JE, Cho IY, Choi YJ, Hong JY. Obesity has a stronger relationship with colorectal cancer in postmenopausal women than premenopausal women. Cancer Epidemiol Biomarkers Prev. 2020;29:2277–2288. doi: 10.1158/1055-9965.EPI-20-0594. [DOI] [PubMed] [Google Scholar]
- 30.Iyengar NM, Brown KA, Zhou XK, Gucalp A, Subbaramaiah K, Giri DD, Zahid H, Bhardwaj P, Wendel NK, Falcone DJ, Wang H, Williams S, Pollak M, Morrow M, Hudis CA, Dannenberg AJ. Metabolic obesity, adipose inflammation and elevated breast aromatase in women with normal body mass index. Cancer Prev Res. 2017;10:235–243. doi: 10.1158/1940-6207.CAPR-16-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahlakõiv T, Flamar A-L, Johnston LK, Moriyama S, Putzel GG, Bryce PJ, Artis D. Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci Immunol. 2019;4(35):eaax0416. doi: 10.1126/sciimmunol.aax0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith TD, Tse MJ, Read EL, Liu WF. Regulation of macrophage polarization and plasticity by complex activation signals. Integr Biol (Camb) 2016;8:946–955. doi: 10.1039/c6ib00105j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Hata A, Kosugi C, Kataoka N, Funaki M. The density of extracellular matrix proteins regulates inflammation and insulin signaling in adipocytes. FEBS Lett. 2010;584:4145–4150. doi: 10.1016/j.febslet.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 35.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 36.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 37.Hara Y, Wakino S, Tanabe Y, Saito M, Tokuyama H, Washida N, Tatematsu S, Yoshioka K, Homma K, Hasegawa K, Minakuchi H, Fujimura K, Hosoya K, Hayashi K, Nakayama K, Itoh H. Rho and Rho-kinase activity in adipocytes contributes to a vicious cycle in obesity that may involve mechanical stretch. Sci Signal. 2011;4:ra3. doi: 10.1126/scisignal.2001227. [DOI] [PubMed] [Google Scholar]
- 38.Golden PL, Maccagnan TJ, Pardridge WM. Human blood-brain barrier leptin receptor. Binding and endocytosis in isolated human brain microvessels. J Clin Invest. 1997;99:14–18. doi: 10.1172/JCI119125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 40.Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, Beguinot F, Miele C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. 2019 doi: 10.3390/ijms20092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tandon P, Wafer R, Minchin JEN. Adipose morphology and metabolic disease. J Exp Biol. 2018;221(Pt Suppl 1):jeb164970. doi: 10.1242/jeb.164970. [DOI] [PubMed] [Google Scholar]
- 42.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 43.Gustafson B, Gogg S, Hedjazifar S, Jenndahl L, Hammarstedt A, Smith U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am J Physiol Endocrinol Metab. 2009;297:E999–E1003. doi: 10.1152/ajpendo.00377.2009. [DOI] [PubMed] [Google Scholar]
- 44.Iikuni N, Lam QLK, Lu L, Matarese G, La Cava A. Leptin and Inflammatio. Curr Immunol Rev. 2008;4:70–79. doi: 10.2174/157339508784325046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nyambuya TM, Dludla PV, Mxinwa V, Nkambule BB. Obesity-induced inflammation and insulin resistance: a mini-review on T-cells. Metabol Open. 2019;3:100015. doi: 10.1016/j.metop.2019.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu R, Nikolajczyk BS. Tissue immune cells fuel obesity-associated inflammation in adipose tissue and beyond. Front Immunol. 2019;10:1587. doi: 10.3389/fimmu.2019.01587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gruzdeva O, Borodkina D, Uchasova E, Dyleva Y, Barbarash O. Leptin resistance: underlying mechanisms and diagnosis. Diabetes Metab Syndr Obes. 2019;12:191–198. doi: 10.2147/DMSO.S182406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi: 10.3389/fimmu.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pérez-Hernández AI, Catalán V, Gómez-Ambrosi J, Rodríguez A, Frühbeck G. Mechanisms linking excess adiposity and carcinogenesis promotion. Front Endocrinol (Lausanne) 2014;5:65. doi: 10.3389/fendo.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moossavi M, Parsamanesh N, Bahrami A, Atkin SL, Sahebkar A. Role of the NLRP3 inflammasome in cancer. Mol Cancer. 2018;17:158. doi: 10.1186/s12943-018-0900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee H, Jeong AJ, Ye S-K. Highlighted STAT3 as a potential drug target for cancer therapy. BMB Rep. 2019;52:415–423. doi: 10.5483/BMBRep.2019.52.7.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koppikar P, Lui VWY, Man D, Xi S, Chai RL, Nelson E, Tobey ABJ, Grandis JR. Constitutive activation of signal transducer and activator of transcription 5 contributes to tumor growth, epithelial-mesenchymal transition, and resistance to epidermal growth factor receptor targeting. Clin Cancer Res. 2008;14:7682–7690. doi: 10.1158/1078-0432.CCR-08-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bowers LW, Maximo IXF, Brenner AJ, Beeram M, Hursting SD, Price RS, Tekmal RR, Jolly CA, deGraffenried LA. NSAID use reduces breast cancer recurrence in overweight and obese women: role of prostaglandin-aromatase interactions. Cancer Res. 2014;74:4446–4457. doi: 10.1158/0008-5472.CAN-13-3603. [DOI] [PubMed] [Google Scholar]
- 55.Bowers LW, Brenner AJ, Hursting SD, Tekmal RR, deGraffenried LA. Obesity-associated systemic interleukin-6 promotes pre-adipocyte aromatase expression via increased breast cancer cell prostaglandin E2 production. Breast Cancer Res Treat. 2015;149:49–57. doi: 10.1007/s10549-014-3223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iyengar NM, Zhou XK, Gucalp A, Morris PG, Howe LR, Giri DD, Morrow M, Wang H, Pollak M, Jones LW, Hudis CA, Dannenberg AJ. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin Cancer Res. 2016;22:2283–2289. doi: 10.1158/1078-0432.CCR-15-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho U, Kim B, Kim S, Han Y, Song YS. Pro-inflammatory M1 macrophage enhances metastatic potential of ovarian cancer cells through NF-κB activation. Mol Carcinog. 2018;57:235–242. doi: 10.1002/mc.22750. [DOI] [PubMed] [Google Scholar]
- 58.Gorska E, Popko K, Stelmaszczyk-Emmel A, Ciepiela O, Kucharska A, Wasik M. Leptin receptors. Eur J Med Res. 2010;15:50–54. doi: 10.1186/2047-783X-15-S2-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Busso N, So A, Chobaz-Péclat V, Morard C, Martinez-Soria E, Talabot-Ayer D, Gabay C. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol. 2002;168:875–882. doi: 10.4049/jimmunol.168.2.875. [DOI] [PubMed] [Google Scholar]
- 60.Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 61.Münzberg H, Myers MG. Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 62.Münzberg H, Flier JS, Bjørbæk C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 63.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 64.Martín-Romero C, Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 65.De Rosa V, Procaccini C, Calì G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 66.Lee S-M, Choi H-J, Oh C-H, Oh J-W, Han J-S. Leptin increases TNF-α expression and production through phospholipase D1 in Raw 264.7 cells. PLoS ONE. 2014;9(7):e102373. doi: 10.1371/journal.pone.0102373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. doi: 10.1096/fsb2fasebj.12.1.57. [DOI] [PubMed] [Google Scholar]
- 68.Pham DV, Park PH. Recent insights on modulation of inflammasomes by adipokines: a critical event for the pathogenesis of obesity and metabolism-associated diseases. Arch Pharm Res. 2020;43:997–1016. doi: 10.1007/s12272-020-01274-7. [DOI] [PubMed] [Google Scholar]
- 69.Fu S, Liu L, Han L, Yu Y. Leptin promotes IL-18 secretion by activating the NLRP3 inflammasome in RAW 264.7 cells. Mol Med Rep. 2017;16:9770–9776. doi: 10.3892/mmr.2017.7797. [DOI] [PubMed] [Google Scholar]
- 70.Hoffmann A, Ebert T, Klöting N, Kolb M, Gericke M, Jeromin F, Jessnitzer B, Lössner U, Burkhardt R, Stumvoll M, Fasshauer M, Kralisch S. Leptin decreases circulating inflammatory IL-6 and MCP-1 in mice. BioFactors. 2019;45:43–48. doi: 10.1002/biof.1457. [DOI] [PubMed] [Google Scholar]
- 71.Patraca I, Martínez N, Busquets O, Martí A, Pedrós I, Beas-Zarate C, Marin M, Ettcheto M, Sureda F, Auladell C, Camins A, Folch J. Anti-inflammatory role of leptin in glial cells through p38 MAPK pathway inhibition. Pharmacol Rep. 2017;69:409–418. doi: 10.1016/j.pharep.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 72.Konturek PC, Jaworek J, Maniatoglou A, Bonior J, Meixner H, Konturek SJ, Hahn EG. Leptin modulates the inflammatory response in acute pancreatitis. Digestion. 2002;65:149–160. doi: 10.1159/000064935. [DOI] [PubMed] [Google Scholar]
- 73.Çakır B, Bozkurt A, Ercan F, Yeğen BÇ. The anti-inflammatory effect of leptin on experimental colitis: involvement of endogenous glucocorticoids. Peptides. 2004;25:95–104. doi: 10.1016/j.peptides.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 74.Daley-Brown D, Harbuzariu A, Kurian AA, Oprea-Ilies G, Gonzalez-Perez RR. Leptin-induced Notch and IL-1 signaling crosstalk in endometrial adenocarcinoma is associated with invasiveness and chemoresistance. World J Clin Oncol. 2019;10:222–233. doi: 10.5306/wjco.v10.i6.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gu F, Zhang H, Yao L, Jiang S, Lu H, Xing X, Zhang C, Jiang P, Zhang R. Leptin contributes to the taxol chemoresistance in epithelial ovarian cancer. Oncol Lett. 2019;18:561–570. doi: 10.3892/ol.2019.10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feng H, Zhang Q, Zhao Y, Zhao L, Shan B. Leptin acts on mesenchymal stem cells to promote chemoresistance in osteosarcoma cells. Aging (Albany NY) 2020;12:6340–6351. doi: 10.18632/aging.103027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yadav NVS, Barcikowski A, Uehana Y, Jacobs AT, Connelly L. Breast adipocyte co-culture increases the expression of pro-angiogenic factors in macrophages. Front Oncol. 2020;10:454. doi: 10.3389/fonc.2020.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu F, Fu R, Liu L, Wang X, Wu T, Shen W, Gui Z, Mo X, Fang B, Xia L. Leptin-induced angiogenesis of Ea.hy926 endothelial cells via the Akt and Wnt signaling pathways in vitro and in vivo. Front Pharmacol. 2019;10:1275. doi: 10.3389/fphar.2019.01275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang X, Wang S, Wang X, Zhang L, Zhao H, Zhang L. Leptin promotes the growth of breast cancer by upregulating the Wnt/β-catenin pathway. Exp Ther Med. 2018;16:767–771. doi: 10.3892/etm.2018.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan D, Avtanski D, Saxena NK, Sharma D. Leptin-induced epithelial-mesenchymal transition in breast cancer cells requires β-catenin activation via Akt/GSK3- and MTA1/Wnt1 protein-dependent pathways. J Biol Chem. 2012;287:8598–8612. doi: 10.1074/jbc.M111.322800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thiagarajan PS, Zheng Q, Bhagrath M, Mulkearns-Hubert EE, Myers MG, Lathia JD, Reizes O. STAT3 activation by leptin receptor is essential for TNBC stem cell maintenance. Endocr Relat Cancer. 2017;24:415–426. doi: 10.1530/ERC-16-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng Q, Banaszak L, Fracci S, Basali D, Dunlap SM, Hursting SD, Rich JN, Hjlemeland AB, Vasanji A, Berger NA, Lathia JD, Reizes O. Leptin receptor maintains cancer stem-like properties in triple negative breast cancer cells. Endocr Relat Cancer. 2013;20:797–808. doi: 10.1530/ERC-13-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strong AL, Ohlstein JF, Biagas BA, Rhodes LV, Pei DT, Tucker HA, Llamas C, Bowles AC, Dutreil MF, Zhang S, Gimble JM, Burow ME, Bunnell BA. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015;17:112. doi: 10.1186/s13058-015-0622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith U, Kahn BB. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J Intern Med. 2016;280:465–475. doi: 10.1111/joim.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zatterale F, Longo M, Naderi J, Raciti GA, Desiderio A, Miele C, Beguinot F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol. 2020 doi: 10.3389/fphys.2019.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol (Lausanne) 2013;4:52. doi: 10.3389/fendo.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 88.de Alvaro C, Teruel T, Hernandez R, Lorenzo M. Tumor necrosis factor α produces insulin resistance in skeletal muscle by activation of inhibitor κB kinase in a p38 MAPK-dependent manner. J Biol Chem. 2004;279:17070–17078. doi: 10.1074/jbc.M312021200. [DOI] [PubMed] [Google Scholar]
- 89.Jansen HJ, Stienstra R, van Diepen JA, Hijmans A, van der Laak JA, Vervoort GMM, Tack CJ. Start of insulin therapy in patients with type 2 diabetes mellitus promotes the influx of macrophages into subcutaneous adipose tissue. Diabetologia. 2013;56:2573–2581. doi: 10.1007/s00125-013-3018-6. [DOI] [PubMed] [Google Scholar]
- 90.Pedersen DJ, Guilherme A, Danai LV, Heyda L, Matevossian A, Cohen J, Nicoloro SM, Straubhaar J, Noh HL, Jung D, Kim JK, Czech MP. A major role of insulin in promoting obesity-associated adipose tissue inflammation. Mol Metab. 2015;4:507–518. doi: 10.1016/j.molmet.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morvan D, Steyaert JM, Schwartz L, Israel M, Demidem A. Normal human melanocytes exposed to chronic insulin and glucose supplementation undergo oncogenic changes and methyl group metabolism cellular redistribution. Am J Physiol Endocrinol Metab. 2012;302:E1407–1418. doi: 10.1152/ajpendo.00594.2011. [DOI] [PubMed] [Google Scholar]
- 93.Baricevic I, Roberts DL, Renehan AG. Chronic insulin exposure does not cause insulin resistance but is associated with chemo-resistance in colon cancer cells. Horm Metab Res. 2014;46:85–93. doi: 10.1055/s-0033-1354414. [DOI] [PubMed] [Google Scholar]
- 94.Chan S-H, Kikkawa U, Matsuzaki H, Chen J-H, Chang W-C. Insulin receptor substrate-1 prevents autophagy-dependent cell death caused by oxidative stress in mouse NIH/3T3 cells. J Biomed Sci. 2012;19:64. doi: 10.1186/1423-0127-19-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y, Hua S, Tian W, Zhang L, Zhao J, Zhang H, Zhang W, Xue F. Mitogenic and anti-apoptotic effects of insulin in endometrial cancer are phosphatidylinositol 3-kinase/Akt dependent. Gynecol Oncol. 2012;125:734–741. doi: 10.1016/j.ygyno.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 96.Esposito DL, Aru F, Lattanzio R, Morgano A, Abbondanza M, Malekzadeh R, Bishehsari F, Valanzano R, Russo A, Piantelli M, Moschetta A, Lotti LV, Mariani-Costantini R. The insulin receptor substrate 1 (IRS1) in intestinal epithelial differentiation and in colorectal cancer. PLoS ONE. 2012;7:e36190. doi: 10.1371/journal.pone.0036190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gallagher EJ, LeRoith D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol Metab. 2010;21:610–618. doi: 10.1016/j.tem.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu Y, Brodt P, Sun H, Mejia W, Novosyadlyy R, Nunez N, Chen X, Mendoza A, Hong S-H, Khanna C, Yakar S. Insulin-like growth factor-i regulates the liver microenvironment in obese mice and promotes liver metastasis. Cancer Res. 2010;70:57–67. doi: 10.1158/0008-5472.CAN-09-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bruning PF, Bonfrèr JMG, van Noord PAH, Hart AAM, de Jong-Bakker M, Nooijen WJ. Insulin resistance and breast-cancer risk. Int J Cancer. 1992;52:511–516. doi: 10.1002/ijc.2910520402. [DOI] [PubMed] [Google Scholar]
- 100.Stolzenberg-Solomon RZ, Graubard BI, Chari S, Limburg P, Taylor PR, Virtamo J, Albanes D. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294:2872–2878. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 101.Soliman PT, Wu D, Tortolero-Luna G, Schmeler KM, Slomovitz BM, Bray MS, Gershenson DM, Lu KH. Association between adiponectin, insulin resistance, and endometrial cancer. Cancer. 2006;106:2376–2381. doi: 10.1002/cncr.21866. [DOI] [PubMed] [Google Scholar]
- 102.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:385–391. [PubMed] [Google Scholar]
- 103.Hsing AW, Gao Y-T, Chua S, Deng J, Stanczyk FZ. Insulin resistance and prostate cancer risk. J Natl Cancer Inst. 2003;95:67–71. doi: 10.1093/jnci/95.1.67. [DOI] [PubMed] [Google Scholar]
- 104.Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018;48:e12997. doi: 10.1111/eci.12997. [DOI] [PubMed] [Google Scholar]
- 105.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol. 2019;15:139–154. doi: 10.1038/s41574-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shirakawa K, Yan X, Shinmura K, Endo J, Kataoka M, Katsumata Y, Yamamoto T, Anzai A, Isobe S, Yoshida N, Itoh H, Manabe I, Sekai M, Hamazaki Y, Fukuda K, Minato N, Sano M. Obesity accelerates T cell senescence in murine visceral adipose tissue. J Clin Invest. 2016;126:4626–4639. doi: 10.1172/JCI88606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Catrysse L, van Loo G. Inflammation and the metabolic syndrome: the tissue-specific functions of NF-κB. Trends Cell Biol. 2017;27:417–429. doi: 10.1016/j.tcb.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 109.Francisco V, Pino J, Campos-Cabaleiro V, Ruiz-Fernández C, Mera A, Gonzalez-Gay MA, Gómez R, Gualillo O. Obesity, fat mass and immune system: role for leptin. Front Physiol. 2018;9:640. doi: 10.3389/fphys.2018.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci USA. 1996;93:6231–6235. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee G-H, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 112.Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Winer S, Paltser G, Chan Y, Tsui H, Engleman E, Winer D, Dosch H-M. Obesity predisposes to Th17 bias. Eur J Immunol. 2009;39:2629–2635. doi: 10.1002/eji.200838893. [DOI] [PubMed] [Google Scholar]
- 114.Wei L, Laurence A, Elias KM, O’Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Y, Tian J, Tian X, Tang X, Rui K, Tong J, Lu L, Xu H, Wang S. Adipose tissue dendritic cells enhances inflammation by prompting the generation of Th17 cells. PLoS ONE. 2014;9(3):e92450. doi: 10.1371/journal.pone.0092450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol. 2008;181:5948–5955. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Endo Y, Asou HK, Matsugae N, Hirahara K, Shinoda K, Tumes DJ, Tokuyama H, Yokote K, Nakayama T. Obesity drives Th17 cell differentiation by inducing the lipid metabolic kinase, acc1. Cell Rep. 2015;12:1042–1055. doi: 10.1016/j.celrep.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 118.Fabrizi M, Marchetti V, Mavilio M, Marino A, Casagrande V, Cavalera M, Moreno-Navarrete JM, Mezza T, Sorice GP, Fiorentino L, Menghini R, Lauro R, Monteleone G, Giaccari A, Fernandez Real JM, Federici M. IL-21 is a major negative regulator of IRF4-dependent lipolysis affecting Tregs in adipose tissue and systemic insulin sensitivity. Diabetes. 2014;63:2086–2096. doi: 10.2337/db13-0939. [DOI] [PubMed] [Google Scholar]
- 119.Agrawal S, Gollapudi S, Su H, Gupta S. Leptin activates human B cells to secrete TNF-α, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J Clin Immunol. 2011;31:472–478. doi: 10.1007/s10875-010-9507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lam QLK, Wang S, Ko OKH, Kincade PW, Lu L. Leptin signaling maintains B-cell homeostasis via induction of Bcl-2 and cyclin D1. Proc Natl Acad Sci USA. 2010;107:13812–13817. doi: 10.1073/pnas.1004185107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Claycombe K, King LE, Fraker PJ. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc Natl Acad Sci USA. 2008;105:2017–2021. doi: 10.1073/pnas.0712053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, Markham D, Strissel KJ, Watkins AA, Zhu M, Allen J, Bouchard J, Toraldo G, Jasuja R, Obin MS, McDonnell ME, Apovian C, Denis GV, Nikolajczyk BS. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci USA. 2013;110:5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Frasca D, Ferracci F, Diaz A, Romero M, Lechner S, Blomberg BB. Obesity decreases B cell responses in young and elderly individuals. Obesity (Silver Spring) 2016;24:615–625. doi: 10.1002/oby.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Frasca D, Romero M, Diaz A, Alter-Wolf S, Ratliff M, Landin AM, Riley RL, Blomberg BB. A molecular mechanism for TNF-α-mediated downregulation of B cell responses. J Immunol. 2012;188:279–286. doi: 10.4049/jimmunol.1003964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kosaraju R, Guesdon W, Crouch MJ, Teague HL, Sullivan EM, Karlsson EA, Schultz-Cherry S, Gowdy K, Bridges LC, Reese LR, Neufer PD, Armstrong M, Reisdorph N, Milner JJ, Beck M, Shaikh SR. B cell activity is impaired in human and mouse obesity and is responsive to an essential fatty acid upon murine influenza infection. J Immunol. 2017;198:4738–4752. doi: 10.4049/jimmunol.1601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong H, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch H-M, Engleman EG. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nishimura S, Manabe I, Takaki S, Nagasaki M, Otsu M, Yamashita H, Sugita J, Yoshimura K, Eto K, Komuro I, Kadowaki T, Nagai R. Adipose natural regulatory B cells negatively control adipose tissue inflammation. Cell Metab. 2013;18:759–766. doi: 10.1016/j.cmet.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 128.García-Hernández MH, Rodríguez-Varela E, García-Jacobo RE, Hernández-De la Torre M, Uresti-Rivera EE, González-Amaro R, Portales-Pérez DP. Frequency of regulatory B cells in adipose tissue and peripheral blood from individuals with overweight, obesity and normal-weight. Obes Res Clin Pract. 2018;12:513–519. doi: 10.1016/j.orcp.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 129.Hill AA, Bolus WR, Hasty AH. A decade of progress in adipose tissue macrophage biology. Immunol Rev. 2014;262:134–152. doi: 10.1111/imr.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Patel PS, Buras ED, Balasubramanyam A. The role of the immune system in obesity and insulin resistance. J Obes. 2013;2013:616193. doi: 10.1155/2013/616193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I, van den Berg S, Romijn J, Rensen PCN, Joosten LAB, Netea MG, Kanneganti T-D. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci USA. 2011;108:15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wree A, McGeough MD, Inzaugarat ME, Eguchi A, Schuster S, Johnson CD, Peña CA, Geisler LJ, Papouchado BG, Hoffman HM, Feldstein AE. NLRP3 inflammasome driven liver injury and fibrosis: Roles of IL-17 and TNF in mice. Hepatology. 2018;67:736–749. doi: 10.1002/hep.29523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li P, Lu M, Nguyen MTA, Bae EJ, Chapman J, Feng D, Hawkins M, Pessin JE, Sears DD, Nguyen A-K, Amidi A, Watkins SM, Nguyen U, Olefsky JM. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem. 2010;285:15333–15345. doi: 10.1074/jbc.M110.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Suganami T, Ogawa Y. Adipose tissue macrophages: their role in adipose tissue remodeling. J Leukoc Biol. 2010;88:33–39. doi: 10.1189/jlb.0210072. [DOI] [PubMed] [Google Scholar]
- 136.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 137.Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 138.Acedo SC, Gambero S, Cunha FGP, Lorand-Metze I, Gambero A. Participation of leptin in the determination of the macrophage phenotype: an additional role in adipocyte and macrophage crosstalk. Vitro Cell Dev Biol Anim. 2013;49:473–478. doi: 10.1007/s11626-013-9629-x. [DOI] [PubMed] [Google Scholar]
- 139.Zarkesh-Esfahani H, Pockley G, Metcalfe RA, Bidlingmaier M, Wu Z, Ajami A, Weetman AP, Strasburger CJ, Ross RJM. High-dose leptin activates human leukocytes via receptor expression on monocytes. J Immunol. 2001;167:4593–4599. doi: 10.4049/jimmunol.167.8.4593. [DOI] [PubMed] [Google Scholar]
- 140.Breznik JA, Naidoo A, Foley KP, Schulz C, Lau TC, Loukov D, Sloboda DM, Bowdish DME, Schertzer JD. TNF, but not hyperinsulinemia or hyperglycemia, is a key driver of obesity-induced monocytosis revealing that inflammatory monocytes correlate with insulin in obese male mice. Physiol Rep. 2018;6:e13937. doi: 10.14814/phy2.13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 143.LaMarche NM, Lynch L. Adipose dendritic cells come out of hiding. Cell Metab. 2018;27:485–486. doi: 10.1016/j.cmet.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 144.Macdougall CE, Wood EG, Loschko J, Scagliotti V, Cassidy FC, Robinson ME, Feldhahn N, Castellano L, Voisin M-B, Marelli-Berg F, Gaston-Massuet C, Charalambous M, Longhi MP. Visceral adipose tissue immune homeostasis is regulated by the crosstalk between adipocytes and dendritic cell subsets. Cell Metab. 2018;27:588–601.e4. doi: 10.1016/j.cmet.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cho KW, Zamarron BF, Muir LA, Singer K, Porsche CE, DelProposto JB, Geletka L, Meyer KA, O’Rourke RW, Lumeng CN. Adipose tissue dendritic cells are independent contributors to obesity-induced inflammation and insulin resistance. J Immunol. 2016;197:3650–3661. doi: 10.4049/jimmunol.1600820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S, Blin-Wakkach C, Anty R, Iannelli A, Gugenheim J, Tran A, Bouloumié A, Gual P, Wakkach A. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61:2238–2247. doi: 10.2337/db11-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–6828. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- 148.Moraes-Vieira PMM, Larocca RA, Bassi EJ, Peron JPS, Andrade-Oliveira V, Wasinski F, Araujo R, Thornley T, Quintana FJ, Basso AS, Strom TB, Câmara NOS. Leptin deficiency impairs maturation of dendritic cells and enhances induction of regulatory T and Th17 cells. Eur J Immunol. 2014;44:794–806. doi: 10.1002/eji.201343592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lam QLK, Liu S, Cao X, Lu L. Involvement of leptin signaling in the survival and maturation of bone marrow-derived dendritic cells. Eur J Immunol. 2006;36:3118–3130. doi: 10.1002/eji.200636602. [DOI] [PubMed] [Google Scholar]
- 150.Orlova EG, Shirshev SV, Loginova OA. Leptin and ghrelin regulate dendritic cell maturation and dendritic cell induction of regulatory T-cells. Dokl Biol Sci. 2015;462:171–174. doi: 10.1134/S001249661503014X. [DOI] [PubMed] [Google Scholar]
- 151.Lynch LA, O’Connell JM, Kwasnik AK, Cawood TJ, O’Farrelly C, O’Shea DB. Are natural killer cells protecting the metabolically healthy obese patient? Obesity (Silver Spring) 2009;17:601–605. doi: 10.1038/oby.2008.565. [DOI] [PubMed] [Google Scholar]
- 152.Viel S, Besson L, Charrier E, Marçais A, Disse E, Bienvenu J, Walzer T, Dumontet C. Alteration of natural killer cell phenotype and function in obese individuals. Clin Immunol. 2017;177:12–17. doi: 10.1016/j.clim.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 153.Viel S, Besson L, Charrier E, Bienvenu J, Disse E, Walzer T, Dumontet C. Natural killer cells display an activated phenotype but reduced effector functions in obese patients. Blood. 2015;126:3430–3430. doi: 10.1182/blood.V126.23.3430.3430. [DOI] [Google Scholar]
- 154.Michelet X, Dyck L, Hogan A, Loftus RM, Duquette D, Wei K, Beyaz S, Tavakkoli A, Foley C, Donnelly R, O’Farrelly C, Raverdeau M, Vernon A, Pettee W, O’Shea D, Nikolajczyk BS, Mills KHG, Brenner MB, Finlay D, Lynch L. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol. 2018;19:1330–1340. doi: 10.1038/s41590-018-0251-7. [DOI] [PubMed] [Google Scholar]
- 155.Bähr I, Jahn J, Zipprich A, Pahlow I, Spielmann J, Kielstein H. Impaired natural killer cell subset phenotypes in human obesity. Immunol Res. 2018;66:234–244. doi: 10.1007/s12026-018-8989-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Molofsky AB, Nussbaum JC, Liang H-E, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Souza-Almeida G, D’Avila H, Almeida PE, Luna-Gomes T, Liechocki S, Walzog B, Hepper I, Castro-Faria-Neto HC, Bozza PT, Bandeira-Melo C, Maya-Monteiro CM. Leptin mediates in vivo neutrophil migration: involvement of tumor necrosis factor-alpha and CXCL1. Front Immunol. 2018;9:111. doi: 10.3389/fimmu.2018.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zarkesh-Esfahani H, Pockley AG, Wu Z, Hellewell PG, Weetman AP, Ross RJM. Leptin indirectly activates human neutrophils via induction of TNF-alpha. J Immunol. 2004;172:1809–1814. doi: 10.4049/jimmunol.172.3.1809. [DOI] [PubMed] [Google Scholar]
- 159.Incio J, Liu H, Suboj P, Chin SM, Chen IX, Pinter M, Ng MR, Nia HT, Grahovac J, Kao S, Babykutty S, Huang Y, Jung K, Rahbari NN, Han X, Chauhan VP, Martin JD, Kahn J, Huang P, Desphande V, Michaelson J, Michelakos TP, Ferrone CR, Soares R, Boucher Y, Fukumura D, Jain RK. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 2016;6:852–869. doi: 10.1158/2159-8290.CD-15-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Taildeman J, Pérez-Novo CA, Rottiers I, Ferdinande L, Waeytens A, De Colvenaer V, Bachert C, Demetter P, Waelput W, Braet K, Cuvelier CA. Human mast cells express leptin and leptin receptors. Histochem Cell Biol. 2009;131:703–711. doi: 10.1007/s00418-009-0575-3. [DOI] [PubMed] [Google Scholar]
- 161.Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi G-P. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou Y, Yu X, Chen H, Sjöberg S, Roux J, Zhang L, Ivoulsou A-H, Bensaid F, Liu C-L, Liu J, Tordjman J, Clement K, Lee C-H, Hotamisligil GS, Libby P, Shi G-P. Leptin deficiency shifts mast cells toward anti-inflammatory actions and protects mice from obesity and diabetes by polarizing M2 macrophages. Cell Metab. 2015;22:1045–1058. doi: 10.1016/j.cmet.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kado T, Nawaz A, Takikawa A, Usui I, Tobe K. Linkage of CD8 + T cell exhaustion with high-fat diet-induced tumourigenesis. Sci Rep. 2019;9:1–8. doi: 10.1038/s41598-019-48678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, Mirsoian A, Minnar CM, Stoffel KM, Sturgill IR, Grossenbacher SK, Withers SS, Rebhun RB, Hartigan-O’Connor DJ, Méndez-Lagares G, Tarantal AF, Isseroff RR, Griffith TS, Schalper KA, Merleev A, Saha A, Maverakis E, Kelly K, Aljumaily R, Ibrahimi S, Mukherjee S, Machiorlatti M, Vesely SK, Longo DL, Blazar BR, Canter RJ, Murphy WJ, Monjazeb AM. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25:141–151. doi: 10.1038/s41591-018-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Galvin KC, Conroy MJ, Doyle SL, Dunne MR, Fahey R, Foley E, O’Sullivan KE, Doherty DG, Geoghegan JG, Ravi N, O’Farrelly C, Reynolds JV, Lysaght J. Extratumoral PD-1 blockade does not perpetuate obesity-associated inflammation in esophageal adenocarcinoma. Cancer Lett. 2018;418:230–238. doi: 10.1016/j.canlet.2018.01.039. [DOI] [PubMed] [Google Scholar]
- 166.Bing C. Is interleukin-1β a culprit in macrophage-adipocyte crosstalk in obesity? Adipocyte. 2015;4:149–152. doi: 10.4161/21623945.2014.979661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Limagne E, Euvrard R, Thibaudin M, Rébé C, Derangère V, Chevriaux A, Boidot R, Végran F, Bonnefoy N, Vincent J, Bengrine-Lefevre L, Ladoire S, Delmas D, Apetoh L, Ghiringhelli F. Accumulation of MDSC and Th17 Cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX-Bevacizumab drug treatment regimen. Cancer Res. 2016;76:5241–5252. doi: 10.1158/0008-5472.CAN-15-3164. [DOI] [PubMed] [Google Scholar]
- 169.Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407:348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 170.Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes J-M, Jiang Z, Meng YG, Peale FV, Ouyang W, Ferrara N. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19:1114–1123. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 171.Yang S, Wang B, Guan C, Wu B, Cai C, Wang M, Zhang B, Liu T, Yang P. Foxp3+IL-17+ T cells promote development of cancer-initiating cells in colorectal cancer. J Leukoc Biol. 2011;89:85–91. doi: 10.1189/jlb.0910506. [DOI] [PubMed] [Google Scholar]
- 172.Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Roliński J, Radwan P, Fang J, Wang G, Zou W. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol. 2011;186:4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]
- 173.Chellappa S, Hugenschmidt H, Hagness M, Line PD, Labori KJ, Wiedswang G, Taskén K, Aandahl EM. Regulatory T cells that co-express RORγt and FOXP3 are pro-inflammatory and immunosuppressive and expand in human pancreatic cancer. Oncoimmunology. 2016;5:e1102828. doi: 10.1080/2162402X.2015.1102828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011;117:6532–6541. doi: 10.1182/blood-2010-11-317321. [DOI] [PubMed] [Google Scholar]
- 175.Downs-Canner S, Berkey S, Delgoffe GM, Edwards RP, Curiel T, Odunsi K, Bartlett DL, Obermajer N. Suppressive IL-17A+Foxp3+ and ex-Th17 IL-17AnegFoxp3+ Treg cells are a source of tumour-associated Treg cells. Nat Commun. 2017;8:14649. doi: 10.1038/ncomms14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hsieh P-S, Jin J-S, Chiang C-F, Chan P-C, Chen C-H, Shih K-C. COX-2-mediated inflammation in fat is crucial for obesity-linked insulin resistance and fatty liver. Obesity. 2009;17:1150–1157. doi: 10.1038/oby.2008.674. [DOI] [PubMed] [Google Scholar]
- 177.Fain JN, Leffler CW, Cowan GS, Buffington C, Pouncey L, Bahouth SW. Stimulation of leptin release by arachidonic acid and prostaglandin E(2) in adipose tissue from obese humans. Metabolism. 2001;50:921–928. doi: 10.1053/meta.2001.24927. [DOI] [PubMed] [Google Scholar]
- 178.Samad F, Yamamoto K, Pandey M, Loskutoff DJ. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol Med. 1997;3:37–48. doi: 10.1007/BF03401666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wouters MCA, Nelson BH. Prognostic significance of tumor-infiltrating B cells and plasma cells in human cancer. Clin Cancer Res. 2018;24:6125–6135. doi: 10.1158/1078-0432.CCR-18-1481. [DOI] [PubMed] [Google Scholar]
- 180.Nielsen JS, Nelson BH. Tumor-infiltrating B cells and T cells. Oncoimmunology. 2012;1:1623–1625. doi: 10.4161/onci.21650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Garaud S, Buisseret L, Solinas C, Gu-Trantien C, de Wind A, den Eynden GV, Naveaux C, Lodewyckx J-N, Boisson A, Duvillier H, Craciun L, Ameye L, Veys I, Paesmans M, Larsimont D, Piccart-Gebhart M, Willard-Gallo K. Tumor-infiltrating B cells signal functional humoral immune responses in breast cancer. JCI Insight. 2019;4(18):e129641. doi: 10.1172/jci.insight.129641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kabir SM, Lee E-S, Son D-S. Chemokine network during adipogenesis in 3T3-L1 cells. Adipocyte. 2014;3:97–106. doi: 10.4161/adip.28110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schwartz M, Zhang Y, Rosenblatt JD. B cell regulation of the anti-tumor response and role in carcinogenesis. J Immunother Cancer. 2016;4:40. doi: 10.1186/s40425-016-0145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jeske SS, Brand M, Ziebart A, Laban S, Doescher J, Greve J, Jackson EK, Hoffmann TK, Brunner C, Schuler PJ. Adenosine-producing regulatory B cells in head and neck cancer. Cancer Immunol Immunother. 2020;69:1205–1216. doi: 10.1007/s00262-020-02535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Galván GC, Johnson CB, Price RS, Liss MA, Jolly CA, deGraffenried LA. Effects of obesity on the regulation of macrophage population in the prostate tumor microenvironment. Nutr Cancer. 2017;69:996–1002. doi: 10.1080/01635581.2017.1359320. [DOI] [PubMed] [Google Scholar]
- 186.Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Onco. 2019;12:76. doi: 10.1186/s13045-019-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang S, Che D, Yang F, Chi C, Meng H, Shen J, Qi L, Liu F, Lv L, Li Y, Meng Q, Liu J, Shang L, Yu Y. Tumor-associated macrophages promote tumor metastasis via the TGF-β/SOX9 axis in non-small cell lung cancer. Oncotarget. 2017;8:99801–99815. doi: 10.18632/oncotarget.21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Arendt LM, McCready J, Keller PJ, Baker DD, Naber SP, Seewaldt V, Kuperwasser C. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res. 2013;73:6080–6093. doi: 10.1158/0008-5472.CAN-13-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bardi GT, Ann Smith M, Hood JL. Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine. 2018;105:63–72. doi: 10.1016/j.cyto.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.James BR, Tomanek-Chalkley A, Askeland EJ, Kucaba T, Griffith TS, Norian LA. Diet-induced obesity alters dendritic cell function in the presence and absence of tumor growth. J Immunol. 2012;189:1311–1321. doi: 10.4049/jimmunol.1100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhao Y, Sun R, You L, Gao C, Tian Z. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem Biophys Res Commun. 2003;300:247–252. doi: 10.1016/s0006-291x(02)02838-3. [DOI] [PubMed] [Google Scholar]
- 192.Tian Z, Sun R, Wei H, Gao B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem Biophys Res Commun. 2002;298:297–302. doi: 10.1016/S0006-291X(02)02462-2. [DOI] [PubMed] [Google Scholar]
- 193.Elinav E, Abd-Elnabi A, Pappo O, Bernstein I, Klein A, Engelhardt D, Rabbani E, Ilan Y. Suppression of hepatocellular carcinoma growth in mice via leptin, is associated with inhibition of tumor cell growth and natural killer cell activation. J Hepatol. 2006;44:529–536. doi: 10.1016/j.jhep.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 194.Lautenbach A, Breitmeier D, Kuhlmann S, Nave H. Human obesity reduces the number of hepatic leptin receptor (ob-R) expressing NK cells. Endocr Res. 2011;36:158–166. doi: 10.3109/07435800.2011.580442. [DOI] [PubMed] [Google Scholar]
- 195.Nave H, Mueller G, Siegmund B, Jacobs R, Stroh T, Schueler U, Hopfe M, Behrendt P, Buchenauer T, Pabst R, Brabant G. Resistance of Janus kinase-2 dependent leptin signaling in natural killer (NK) cells: a novel mechanism of NK cell dysfunction in diet-induced obesity. Endocrinology. 2008;149:3370–3378. doi: 10.1210/en.2007-1516. [DOI] [PubMed] [Google Scholar]
- 196.Mori A, Sakurai H, Choo M-K, Obi R, Koizumi K, Yoshida C, Shimada Y, Saiki I. Severe pulmonary metastasis in obese and diabetic mice. Int J Cancer. 2006;119:2760–2767. doi: 10.1002/ijc.22248. [DOI] [PubMed] [Google Scholar]
- 197.Meriño M, Briones L, Palma V, Herlitz K, Escudero C. Role of adenosine receptors in the adipocyte–macrophage interaction during obesity. Endocrinol Diabetes Nutr. 2017;64:317–327. doi: 10.1016/j.endien.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 198.Chambers AM, Lupo KB, Matosevic S. Tumor microenvironment-induced immunometabolic reprogramming of natural killer cells. Front Immunol. 2018;9:2517. doi: 10.3389/fimmu.2018.02517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yokota J. Tumor progression and metastasis. Carcinogenesis. 2000;21:497–503. doi: 10.1093/carcin/21.3.497. [DOI] [PubMed] [Google Scholar]
- 200.diSibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med. 2008;132:931–939. doi: 10.1043/1543-2165(2008)132[931:MPOCRF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 201.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109:1192–1202. doi: 10.1002/cncr.22534. [DOI] [PubMed] [Google Scholar]
- 202.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27:11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 203.Yu Y-RA, O’Koren EG, Hotten DF, Kan MJ, Kopin D, Nelson ER, Que L, Gunn MD. A protocol for the comprehensive flow cytometric analysis of immune cells in normal and inflamed murine non-lymphoid tissues. PLoS ONE. 2016;11:e0150606. doi: 10.1371/journal.pone.0150606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kim EJ, Choi M-R, Park H, Kim M, Hong JE, Lee J-Y, Chun HS, Lee KW, Yoon Park JH. Dietary fat increases solid tumor growth and metastasis of 4T1 murine mammary carcinoma cells and mortality in obesity-resistant BALB/c mice. Breast Cancer Res. 2011;13:R78. doi: 10.1186/bcr2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Quail DF, Olson OC, Bhardwaj P, Walsh LA, Akkari L, Quick ML, Chen I-C, Wendel N, Ben-Chetrit N, Walker J, Holt PR, Dannenberg AJ, Joyce JA. Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nat Cell Biol. 2017;19:974–987. doi: 10.1038/ncb3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Park H, Kim M, Kwon GT, Lim DY, Yu R, Sung M-K, Lee KW, Daily JW, Park JHY. A high-fat diet increases angiogenesis, solid tumor growth, and lung metastasis of CT26 colon cancer cells in obesity-resistant BALB/c mice. Mol Carcinog. 2012;51:869–880. doi: 10.1002/mc.20856. [DOI] [PubMed] [Google Scholar]
- 207.Nagahashi M, Yamada A, Katsuta E, Aoyagi T, Huang W-C, Terracina KP, Hait NC, Allegood JC, Tsuchida J, Yuza K, Nakajima M, Abe M, Sakimura K, Milstien S, Wakai T, Spiegel S, Takabe K. Targeting the SphK1/S1P/S1PR1 axis that links obesity, chronic inflammation, and breast cancer metastasis. Cancer Res. 2018;78:1713–1725. doi: 10.1158/0008-5472.CAN-17-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Oh K, Lee O-Y, Shon SY, Nam O, Ryu PM, Seo MW, Lee D-S. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res. 2013;15:R79. doi: 10.1186/bcr3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, Cotari J, Alpaugh ML, de Stanchina E, Manova K, Li M, Bonafe M, Ceccarelli C, Taffurelli M, Santini D, Altan-Bonnet G, Kaplan R, Norton L, Nishimoto N, Huszar D, Lyden D, Bromberg J. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2013;15:848–862. doi: 10.1593/neo.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Baek AE, Yu Y-RA, He S, Wardell SE, Chang C-Y, Kwon S, Pillai RV, McDowell HB, Thompson JW, Dubois LG, Sullivan PM, Kemper JK, Gunn MD, McDonnell DP, Nelson ER. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat Commun. 2017;8:864. doi: 10.1038/s41467-017-00910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Desmedt C, Fornili M, Clatot F, Demicheli R, De Bortoli D, Di Leo A, Viale G, de Azambuja E, Crown J, Francis PA, Sotiriou C, Piccart M, Biganzoli E. Differential benefit of adjuvant docetaxel-based chemotherapy in patients with early breast cancer according to baseline body mass index. J Clin Oncol. 2020;38:2883–2891. doi: 10.1200/JCO.19.01771. [DOI] [PubMed] [Google Scholar]
- 212.Vaysse C, Muller C, Fallone F. Obesity: an heavyweight player in breast cancer’s chemoresistance. Oncotarget. 2019;10:3207–3208. doi: 10.18632/oncotarget.26905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Omarini C, Palumbo P, Pecchi A, Draisci S, Balduzzi S, Nasso C, Barbolini M, Isca C, Bocconi A, Moscetti L, Galetti S, Tazzioli G, Torricelli P, Cascinu S, Piacentini F. Predictive role of body composition parameters in operable breast cancer patients treated with neoadjuvant chemotherapy. Cancer Manag Res. 2019;11:9563–9569. doi: 10.2147/CMAR.S216034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Iwase T, Sangai T, Fujimoto H, Sawabe Y, Matsushita K, Nagashima K, Sato Y, Nakagawa A, Masuda T, Nagashima T, Ohtsuka M. Quality and quantity of visceral fat tissue are associated with insulin resistance and survival outcomes after chemotherapy in patients with breast cancer. Breast Cancer Res Treat. 2020;179:435–443. doi: 10.1007/s10549-019-05467-7. [DOI] [PubMed] [Google Scholar]
- 215.Su F, Ahn S, Saha A, DiGiovanni J, Kolonin MG. Adipose stromal cell targeting suppresses prostate cancer epithelial-mesenchymal transition and chemoresistance. Oncogene. 2019;38:1979–1988. doi: 10.1038/s41388-018-0558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cardenas C, Montagna MK, Pitruzzello M, Lima E, Mor G, Alvero AB. Adipocyte microenvironment promotes Bclxl expression and confers chemoresistance in ovarian cancer cells. Apoptosis. 2017;22:558–569. doi: 10.1007/s10495-016-1339-x. [DOI] [PubMed] [Google Scholar]
- 217.Wang T, Fahrmann JF, Lee H, Li Y-J, Tripathi SC, Yue C, Zhang C, Lifshitz V, Song J, Yuan Y, Somlo G, Jandial R, Ann D, Hanash S, Jove R, Yu H. JAK/STAT3-regulated fatty acid β-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 2018;27:136–150.e5. doi: 10.1016/j.cmet.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sheng X, Parmentier J-H, Tucci J, Pei H, Cortez-Toledo O, Dieli-Conwright CM, Oberley MJ, Neely M, Orgel E, Louie SG, Mittelman SD. Adipocytes sequester and metabolize the chemotherapeutic daunorubicin. Mol Cancer Res. 2017;15:1704–1713. doi: 10.1158/1541-7786.MCR-17-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lehuédé C, Li X, Dauvillier S, Vaysse C, Franchet C, Clement E, Esteve D, Longué M, Chaltiel L, Le Gonidec S, Lazar I, Geneste A, Dumontet C, Valet P, Nieto L, Fallone F, Muller C. Adipocytes promote breast cancer resistance to chemotherapy, a process amplified by obesity: role of the major vault protein (MVP) Breast Cancer Res. 2019;21:7. doi: 10.1186/s13058-018-1088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Pandey V, Vijayakumar MV, Ajay AK, Malvi P, Bhat MK. Diet-induced obesity increases melanoma progression: involvement of Cav-1 and FASN. Int J Cancer. 2012;130:497–508. doi: 10.1002/ijc.26048. [DOI] [PubMed] [Google Scholar]
- 221.Mayi TH, Daoudi M, Derudas B, Gross B, Bories G, Wouters K, Brozek J, Caiazzo R, Raverdi V, Pigeyre M, Allavena P, Mantovani A, Pattou F, Staels B, Chinetti-Gbaguidi G. Human adipose tissue macrophages display activation of cancer-related pathways. J Biol Chem. 2012;287:21904–21913. doi: 10.1074/jbc.M111.315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Al-Bahlani S, Al-Lawati H, Al-Adawi M, Al-Abri N, Al-Dhahli B, Al-Adawi K. Fatty acid synthase regulates the chemosensitivity of breast cancer cells to cisplatin-induced apoptosis. Apoptosis. 2017;22:865–876. doi: 10.1007/s10495-017-1366-2. [DOI] [PubMed] [Google Scholar]
- 223.Bauerschlag DO, Maass N, Leonhardt P, Verburg FA, Pecks U, Zeppernick F, Morgenroth A, Mottaghy FM, Tolba R, Meinhold-Heerlein I, Bräutigam K. Fatty acid synthase overexpression: target for therapy and reversal of chemoresistance in ovarian cancer. J Transl Med. 2015;13:146. doi: 10.1186/s12967-015-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Albiges L, Hakimi AA, Xie W, McKay RR, Simantov R, Lin X, Lee J-L, Rini BI, Srinivas S, Bjarnason GA, Ernst S, Wood LA, Vaishamayan UN, Rha S-Y, Agarwal N, Yuasa T, Pal SK, Bamias A, Zabor EC, Skanderup AJ, Furberg H, Fay AP, de Velasco G, Preston MA, Wilson KM, Cho E, McDermott DF, Signoretti S, Heng DYC, Choueiri TK. Body mass index and metastatic renal cell carcinoma: clinical and biological correlations. J Clin Oncol. 2016;34:3655–3663. doi: 10.1200/JCO.2016.66.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Griggs JJ, Mangu PB, Anderson H, Balaban EP, Dignam JJ, Hryniuk WM, Morrison VA, Pini TM, Runowicz CD, Rosner GL, Shayne M, Sparreboom A, Sucheston LE, Lyman GH. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30:1553–1561. doi: 10.1200/JCO.2011.39.9436. [DOI] [PubMed] [Google Scholar]
- 226.Goodwin PJ. Obesity and endocrine therapy: Host factors and breast cancer outcome. Breast. 2013;22:S44–S47. doi: 10.1016/j.breast.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 227.Heetun A, Cutress RI, Copson ER. Early breast cancer: why does obesity affect prognosis? Proc Nutr Soc. 2018;77:369–381. doi: 10.1017/S0029665118000447. [DOI] [PubMed] [Google Scholar]
- 228.Bouchlaka MN, Sckisel GD, Chen M, Mirsoian A, Zamora AE, Maverakis E, Wilkins DEC, Alderson KL, Hsiao H-H, Weiss JM, Monjazeb AM, Hesdorffer C, Ferrucci L, Longo DL, Blazar BR, Wiltrout RH, Redelman D, Taub DD, Murphy WJ. Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J Exp Med. 2013;210:2223–2237. doi: 10.1084/jem.20131219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mirsoian A, Bouchlaka MN, Sckisel GD, Chen M, Pai C-CS, Maverakis E, Spencer RG, Fishbein KW, Siddiqui S, Monjazeb AM, Martin B, Maudsley S, Hesdorffer C, Ferrucci L, Longo DL, Blazar BR, Wiltrout RH, Taub DD, Murphy WJ. Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. J Exp Med. 2014;211:2373–2383. doi: 10.1084/jem.20140116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hendrikx JJMA, Haanen JBAG, Voest EE, Schellens JHM, Huitema ADR, Beijnen JH. Fixed dosing of monoclonal antibodies in oncology. Oncologist. 2017;22:1212–1221. doi: 10.1634/theoncologist.2017-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Heinhuis KM, Beijnen JH, Hendrikx JJMA. Follow up survey for implementation of fixed-dosing of monoclonal antibodies. Int J Clin Pharm. 2020;42:3–6. doi: 10.1007/s11096-020-00971-z. [DOI] [PubMed] [Google Scholar]
- 232.Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, Giusti R, Tiseo M, Michiara M, Di Marino P, Tinari N, De Tursi M, Zoratto F, Veltri E, Marconcini R, Malorgio F, Russano M, Anesi C, Zeppola T, Filetti M, Marchetti P, Botticelli A, Antonini Cappellini GC, De Galitiis F, Vitale MG, Rastelli F, Pergolesi F, Berardi R, Rinaldi S, Tudini M, Silva RR, Pireddu A, Atzori F, Chiari R, Ricciuti B, De Giglio A, Iacono D, Gelibter A, Occhipinti MA, Parisi A, Porzio G, Fargnoli MC, Ascierto PA, Ficorella C, Natoli C. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7:57. doi: 10.1186/s40425-019-0527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rogado J, Romero-Laorden N, Sanchez-Torres JM, Ramos-Levi AM, Pacheco-Barcia V, Ballesteros AI, Arranz R, Lorenzo A, Gullon P, Garrido A, Serra López-Matencio JM, Donnay O, Adrados M, Costas P, Aspa J, Alfranca A, Mondejar R, Colomer R. Effect of excess weight and immune-related adverse events on the efficacy of cancer immunotherapy with anti-PD-1 antibodies. Oncoimmunology. 2020;9:1751548. doi: 10.1080/2162402X.2020.1751548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Xu H, Cao D, He A, Ge W. The prognostic role of obesity is independent of sex in cancer patients treated with immune checkpoint inhibitors: a pooled analysis of 4090 cancer patients. Int Immunopharmacol. 2019;74:105745. doi: 10.1016/j.intimp.2019.105745. [DOI] [PubMed] [Google Scholar]
- 235.McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, Lane S, Lee D-Y, Kaper M, McKean M, Beckermann KE, Rubinstein SM, Rooney I, Musib L, Budha N, Hsu J, Nowicki TS, Avila A, Haas T, Puligandla M, Lee S, Fang S, Wargo JA, Gershenwald JE, Lee JE, Hwu P, Chapman PB, Sosman JA, Schadendorf D, Grob J-J, Flaherty KT, Walker D, Yan Y, McKenna E, Legos JJ, Carlino MS, Ribas A, Kirkwood JM, Long GV, Johnson DB, Menzies AM, Davies MA. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19:310–322. doi: 10.1016/S1470-2045(18)30078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Deshpande RP, Sharma S, Watabe K. The confounders of cancer immunotherapy: roles of lifestyle, metabolic disorders and sociological factors. Cancers (Basel) 2020;12(10):2983. doi: 10.3390/cancers12102983. [DOI] [PMC free article] [PubMed] [Google Scholar]