Abstract
Brugada syndrome (BrS), early repolarization syndrome (ERS), and idiopathic ventricular fibrillation (iVF) have long been considered primary electrical disorders associated with malignant ventricular arrhythmia and sudden cardiac death. However, recent studies have revealed the presence of subtle microstructural abnormalities of the extracellular matrix in some cases of BrS, ERS, and iVF, particularly within right ventricular subepicardial myocardium. Substrate-based ablation within this region has been shown to ameliorate the electrocardiographic phenotype and to reduce arrhythmia frequency in BrS. Patients with ERS and iVF may also exhibit low-voltage and fractionated electrograms in the ventricular subepicardial myocardium, which can be treated with ablation. A significant proportion of patients with BrS and ERS, as well as some iVF survivors, harbor pathogenic variants in the voltage-gated sodium channel gene, SCN5A, but the majority of genetic susceptibility of these disorders is likely to be polygenic. Here, we postulate that BrS, ERS, and iVF may form part of a spectrum of subtle subepicardial cardiomyopathy. We propose that impaired sodium current, along with genetic and environmental susceptibility, precipitates a reduction in epicardial conduction reserve, facilitating current-to-load mismatch at sites of structural discontinuity, giving rise to electrocardiographic changes and the arrhythmogenic substrate.
Keywords: arrhythmogenic cardiomyopathies, Brugada syndrome, ventricular fibrillation
Some arrhythmia syndromes appear to occur in the absence of overt structural abnormalities. The long QT syndrome is one such example.1 The now well-defined pathophysiological basis of long QT syndrome and associated arrhythmias in the absence of structural myocardial abnormalities characterizes the syndrome as a dominant (if not 100% genetic) ion channel disease, a channelopathy. Various other channelopathies have been described, including Brugada syndrome (BrS), catecholaminergic polymorphic ventricular tachycardia, and short QT syndrome. BrS, a leading cause of autopsy-negative sudden death,2 is defined by coved J-point (ST-segment) elevation in the right precordial leads in association with ventricular fibrillation (VF) in the absence of structural abnormalities. J-point elevation is also a requisite feature of the early repolarization syndrome (ERS), which refers to the finding of early repolarization pattern in patients with idiopathic VF (iVF). BrS and ERS therefore constitute a continuous spectrum of J-wave phenotypic expression in the ECG, and thus have been designated J-wave syndromes.3 Other clinical entities can mimic the electrocardiographic pattern observed in BrS, but are etiologically distinct and elicited by other factors, such as myocardial ischemia, metabolic abnormalities, or mechanical compression.4 Early repolarization pattern is also more commonly observed in competitive athletes compared with the general population.5 In the absence of an overt electrical or structural phenotype, iVF exists as a diagnosis of exclusion, referring to the occurrence of VF without a pathophysiological explanation.6
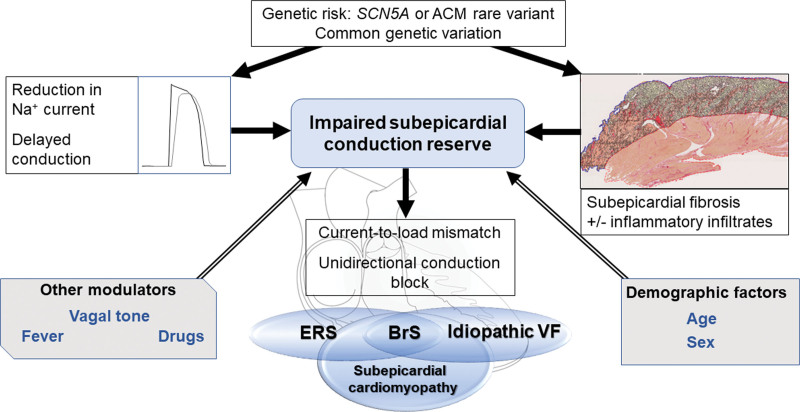
Because the 3 disorders, in at least some patients, share the presence of subtle changes in the extracellular matrix, a common pathophysiological basis for BrS, ERS, and iVF appears plausible (Figure 1). The presence of a vulnerable electrophysiological substrate, in conjunction with triggers commonly arising from the Purkinje system or right ventricular (RV) outflow tract (RVOT), likely plays an important role in arrhythmogenesis, particularly when combined with genetic and environmental modifiers. This is consistent with our view that syndromic descriptions of BrS and ERS point to a different region of the heart and to a different severity of the microstructural changes, whereas iVF may be associated with abnormalities in various regions. In contrast to primary cardiomyopathic disorders, in which the heart muscle appears both structurally and functionally abnormal, alterations in ion channel interfaces and protein architecture have led some to recognize cardiac channelopathies as a subgroup of primary cardiomyopathies rather than purely electrical diseases.7 We therefore propose that most patients with BrS, ERS, or iVF have a common subepicardial cardiomyopathy based on nontransmural, subtle microstructural changes present within the subepicardial myocardium.8 Whether these microstructural changes are also present in patients with other primary electrical diseases is unclear, although a recent study by Pappone et al9 suggests that, in patients with long QT syndrome, cardiac structural abnormalities may be present as well. Here, we discuss the replacement of the syndromic descriptions of BrS, ERS, and iVF with a common unifying pathophysiological definition.
Figure 1.
Proposed mechanisms and modulating factors underlying arrhythmogenesis in the subepicardial cardiomyopathy, giving rise to BrS, ERS, and idiopathic VF. ACM indicates arrhythmogenic cardiomyopathy; BrS, Brugada syndrome; ERS, early repolarization syndrome; and VF, ventricular fibrillation.
Evidence of Structural Disease in These Phenotypes
The presence of an apparently abnormal myocardial substrate in BrS has been widely reported in the literature, but the histological descriptions vary. Cardiomyopathic changes, including myocardial fibrofatty replacement of the RV free wall or the presence of inflammatory infiltrates, have been described in a series of studies.10–13 Microstructural abnormalities have been reported in an earlier case series of 6 individuals with apparent iVF, one of whom fulfilled contemporary electrocardiographic criteria for BrS.14 A recent study by Miles et al15 reported pathological and clinical characteristics in a group of 28 decedents with BrS compared with control subjects without cardiac death. Quantitative analysis of cardiac tissue components demonstrated a 45% increase in collagen content among the BrS group compared with control subjects. Increases in collagen were observed across all sampled regions of RV and left ventricular myocardium, with the highest collagen proportions within the subepicardium of the RVOT. These findings suggest that fibrosis predominates in RV subepicardial myocardium but also appears to represent an adverse remodeling process in both ventricles. However, the syndromic description of BrS classifies patients as having RVOT disease, failing to recognize its true extent.16
Although BrS and ERS are often considered distinct arrhythmia syndromes, overlapping clinical and pathophysiological features are increasingly recognized. It has been proposed that the electrocardiographic J wave, previously characterized in experimental studies by accentuation of the notch in the subepicardial action potential (caused by the transient outward potassium current [Ito]), can also be caused by or related to activation delay,17,18 underpinning a spectrum of BrS and ERS disease phenotypes associated with malignant ventricular arrhythmias and sudden cardiac death.3 The conventional view of ERS as an exclusive disorder of enhanced local early repolarization in the absence of apparent structural cardiac abnormalities has also been challenged.19,20 Boukens et al20 recently documented the presence of fibrosis within regions of inferior RV myocardium colocalizing with electrophysiological J waves observed during epicardial unipolar mapping. Here, transmural myocardial biopsies were obtained from a patient with ERS with recurrent VF undergoing epicardial mapping and ablation. High-resolution activation mapping identified the latest moment of electrical activation within the inferior RV free wall, in which high-amplitude local J waves were present on unipolar recordings occurring after the moment of slurring/notching in the QRS complex of the ECG. Extensive subepicardial fibrosis was observed histologically, along with fragments of surviving myocardium.
In patients with iVF, microstructural changes with potentials similar to those observed in BrS can also occur. More than two-thirds of 50 patients with iVF who underwent comprehensive exclusion of underlying cardiac causes showed evidence of low-amplitude and fractionated electrograms detected during electrophysiological catheter mapping procedures, indicative of abnormal conduction and arrhythmogenicity.21 Abnormal conduction was found predominantly in RV (65%) subepicardial myocardium, whereas Purkinje premature ventricular contractions were the dominant cause in patients with iVF without conduction alterations. Concordant with biventricular fibrotic involvement in BrS,15 microstructural alterations of the left ventricular myocardium with or without the RV myocardium were identified in a significant minority (35%). Localized abnormal electrograms within both ventricles were also commonly reported in a series of patients with iVF subjected to endocardial and epicardial mapping.22 Here, Haïssaguerre et al22 identified sites of abnormal ECGs among 15 of 24 patients with iVF. Furthermore, abnormal areas were found to colocate with VF drivers; clinical recurrences were reduced after substrate-based ablation. The presence of myocardial fibrosis and fatty infiltration in cardiac tissue has also been associated with the distribution of J waves on the 12-lead ECG.23
A Common Underlying Pathophysiology?
The underlying pathophysiology of BrS has been a matter of much debate.24 The 2 main electrophysiological hypotheses are the repolarization and depolarization theories.25 The repolarization theory is based on experiments in perfused canine RV wedge preparations and refers to transmural dispersion of repolarization in the absence of structural abnormalities.26 According to this hypothesis, the notch of the action potential is accentuated due to reduction of net inward Na+ current (NaV1.5), along with nonuniform increases in the Ito within the subepicardial myocardium. In the depolarization theory, ST-segment elevation observed in the right precordial leads is explained by severely compromised conduction, including slow or asynchronous conduction, localized block, and absence of activation within the RVOT, creating a large potential difference with respect to the body of the RV.27–30 Reduction of sodium current by sodium channel blockade, the presence of a SCN5A pathogenic variant, high-rate pacing, or extrasystoles can unmask the substrate.27,29 Experimental models also provide a mechanistic basis for the association between fibrosis and BrS. For example, a study using a haploinsufficient SCN5A+/− mouse model demonstrated fibrotic changes within both ventricles; epicardial activation analysis also showed increased late conducting components.31 Conduction deficits and myocardial fibrosis have been elegantly described in a porcine model of SCN5A deficiency, underscoring the pleotropic nature of sodium channel disease.32
It has been proposed that a reduction of sodium current is caused by current-to-load mismatch and localized conduction block, resulting in excitation failure within fibrotic myocardium in the RV epicardium.28 In a porcine model and in computer simulations, Hoogendijk et al29 showed that localized excitation failure by current-to-load mismatch can cause ST-segment elevation modulated by the balance of sodium current, Ito, and calcium current. In the presence of structural discontinuities, a decrease in depolarizing (or an increase in the repolarizing) current may result in unidirectional conduction block. These findings are concordant with clinical data demonstrating excitation failure and localized RV epicardial activation delay in BrS myocardium.33,34
The most compelling data in favor of the depolarization hypothesis were put forward by Nademanee et al,30 who studied 9 patients with BrS with recurrent VF and frequent implantable cardioverter defibrillator discharges. Electroanatomic mapping showed low-voltage fragmented electrograms of prolonged duration over the epicardial aspect of the RVOT. Catheter ablation resulted in normalization of the type 1 Brugada electrocardiographic pattern, and no further arrhythmia was inducible, a phenomenon also observed by others.35 In our view, these findings suggested that localized J-point and ST-segment elevation is a consequence of delayed depolarization of the RVOT with current-to-load mismatch at areas of cardiac tissue discontinuity. This was made plausible by recording of delayed monophasic unipolar electrograms after sodium channel blocker administration in patients with BrS with or without early repolarization pattern.36 A monophasic morphology of a unipolar electrogram is commonly accepted as a sign of absence of local activation.37,38 These monophasic potentials are visible in lead V1 as a J-point elevation.39
Similar observations have been made in patients with ERS.19,20 In an electroanatomic study of 58 patients with inferolateral J waves/ERS, 2 distinct electrical subtypes were identified. The majority was made up of those with depolarization abnormalities located predominantly at the inferior part of RV epicardium. A smaller group included individuals with no apparent depolarization abnormality but early repolarization unipolar signals (pure ERS) in which Purkinje-related VF triggers likely predominate.19 Mechanisms underlying iVF often relate to the presence of premature ventricular contraction triggers arising from the distal Purkinje system, and this classification should be considered after careful exclusion of covert structural or molecular cardiac causes.
Others have questioned the role of delayed conduction in BrS. The canine wedge model demonstrated fractionated electrograms and late potentials as a consequence of perturbations in epicardial repolarization (reactivation of calcium current) and action potential duration,40 although these electrograms show different characteristics in timing and continuity compared with those in human patients with BrS.39 Radiofrequency ablation of myocardium showing fractionated potentials mitigated the BrS electrocardiographic phenotype.41 Furthermore, important differences have also been described in the electrocardiographic response of BrS and ERS to sodium channel blockade,42 which appears to accentuate J-wave amplitude in BrS while causing a reduction in ERS. Although this suggests that distinct mechanisms may underlie both conditions, computer simulation data indicate that differences in J-wave manifestation occur due to regional patterns of delayed activation and reduction in sodium current. Additional conduction slowing in the entire heart (eg, by sodium channel blockade) may attenuate J waves and J-point elevations on the ECG because of masking due to global QRS widening.18 In humans with BrS, the presence of late potentials on the signal-averaged ECG has been associated with a positive response to the sodium channel blocker provocation test.43
Underlying Genetic Causes
Since the landmark discovery of pathogenic variants in the first gene linked to BrS,44 SCN5A remains the only gene consistently associated with the clinical phenotype. To date, >300 mutations in SCN5A have been associated with BrS that underlie ≈20% of patients meeting diagnostic criteria.45 Pathogenic SCN5A variants in BrS cause loss of function due to reduction in the amplitude of the sodium channel current by reduced expression or altered voltage-gating properties. SCN5A variants have been described in various other cardiac pathologies, including long QT syndrome, premature cardiac conduction defect, and dilated cardiomyopathy. However, it should be noted that not all SCN5A variants are pathogenic, according to the Koch or Bradford Hill criteria.46,47 In fact, Probst et al48 found that within families with hereditary BrS and a pathogenic SCN5A variant, the genetic variant can be absent in symptomatic patients who comply with the syndromic criteria. Furthermore, given the relatively modest monogenic contribution of SCN5A to the BrS phenotype, it is clear that inheritance patterns are more complex than previously thought.49
Bezzina and et al50 provided initial support for this idea through a genome-wide association study exploring the role of common genetic variation in BrS. They identified 3 loci associated with BrS: rs10428132 and rs11708996, both at SCN5A/SCN10A, and rs9388451 near HEY2. These common variants were thought to account for ≈7% of variance in BrS susceptibility. Furthermore, disease risk increased consistently with increasing numbers of carried risk alleles. A follow-up study suggested that the weighted contribution of these variants may allow an individualized approach to diagnosis along with established clinical factors.51 A strong polygenic susceptibility was underscored by a further, much larger genome-wide association study implicating 21 common variants at 12 loci in BrS.52
The presence of SCN5A variants has also been reported in ERS, albeit at a lower diagnostic yield.53,54 In a study of 262 probands with BrS and 104 with ERS, Zhang et al54 identified a 10% yield of pathogenic SCN5A variants in the ERS group compared with 23% for BrS. This is further supported by patients with ERS undergoing ablation being more likely to harbor an SCN5A variant.19 This suggests that overlapping genetic features may underlie a significant minority of J-wave syndromes, ultimately contributing to reduced conduction reserve within RV epicardium.55 Furthermore, previous studies have shown that NaV1.5 may also have a role in the maintenance of normal cardiac structural integrity. Loss of NaV1.5 in heterozygous SCN5A+/− murine models has resulted in conduction defects, in keeping with premature cardiac conduction defect, and the occurrence of age-dependent fibrotic cardiac remodeling, which appears to be triggered by activation of a transforming growth factor-β signaling pathway.31,56,57
Evidence for Genetic Overlap With Arrhythmogenic Cardiomyopathy
Genetic variants in the desmosomal gene PKP2 have been associated with clinically affected patients with BrS, and, conversely, SCN5A has been implicated in the pathogenesis of arrhythmogenic cardiomyopathy (ACM).58,59 The majority of annotated ACM pathogenic PKP2 variants are radical alternations (frameshift or nonsense mutations), but nonsynonymous variants have also been associated with additional cardiac phenotypes such as BrS.60 This suggests a pleotropic role of the plakophilin-2 protein, which may have additional functions besides linking cadherins to intermediate filaments in the cytoskeleton.61 These findings are emphasized by experimental models detailing a molecular interaction between desmosomal proteins and the sodium channel, suggesting that both disease states may exist on a continuum, manifesting variable degrees of electrical and structural dysfunction.62,63 For example, biochemical, patch clamp, and optical mapping experiments have reported important associations between plakophilin-2 and NaV1.5 at a cellular level while also demonstrating adverse effects of PKP2 knockdown on sodium current function.64 Similarly, PKP2 variants were functionally detrimental to sodium channel current in a series of patients with SCN5A-negative BrS, all of whom failed to exhibit structural features of cardiomyopathy.65 Functional in vitro evaluation resulted in decreased sodium current at sites of cell-to-cell contact. This was reversed after transfection of wild-type PKP2 in cellular models but not in mutant forms associated with BrS.
Additional studies have shown deleterious interactions between pathogenic variants in other desmosomal genes and sodium current, as in the cases of DSG2,66 DSP,67 and JUP.68 The clinical phenotype of BrS has also been observed in a patient with a pathogenic LMNA variant.69 However, such genes currently have insufficient evidence for their inclusion in genetic testing panels for BrS.70 Survivors of unexplained cardiac arrest, including patients with iVF, may also harbor pathogenic variants in SCN5A in a small proportion, but more significantly, disease-causing variants in cardiomyopathy-related genes, including ACM, have been implicated in 10%.71 Indeed, interaction between pathogenic desmosomal variants and calcium current may also represent an important arrhythmia mechanism in the absence of overt structural defects. The concept of a desmosome-dyad axis has been proposed whereby disruption of the desmosome can lead to downregulation of the calcium handling protein integrin β1D, which precipitates hyperphosphorylation of RYR2 (Ser-2030) and predisposes to catecholaminergic polymorphic ventricular tachycardia–like ventricular arrhythmias.72,73 Moreover, disruption of calcium current homeostasis has also been reported in PKP2-deficient mice, in which an RV-predominant arrhythmogenic substrate was observed in advance of any overt cardiomyopathic changes.74 However, we presume that much of the remaining heritability in ERS and iVF could also have a polygenic basis.
Clinical Overlap With Cardiomyopathy
Over recent years, several studies have reported overlapping clinical features between ACM and BrS in some patients, suggesting that a common disease pathway may underlie such cases.75,76 Several investigators have postulated that such changes may relate to changes in the connexome,77–79 a network at the intercalated disk that integrates mechanical junctions, gap junctions, and the voltage-gated sodium channel.
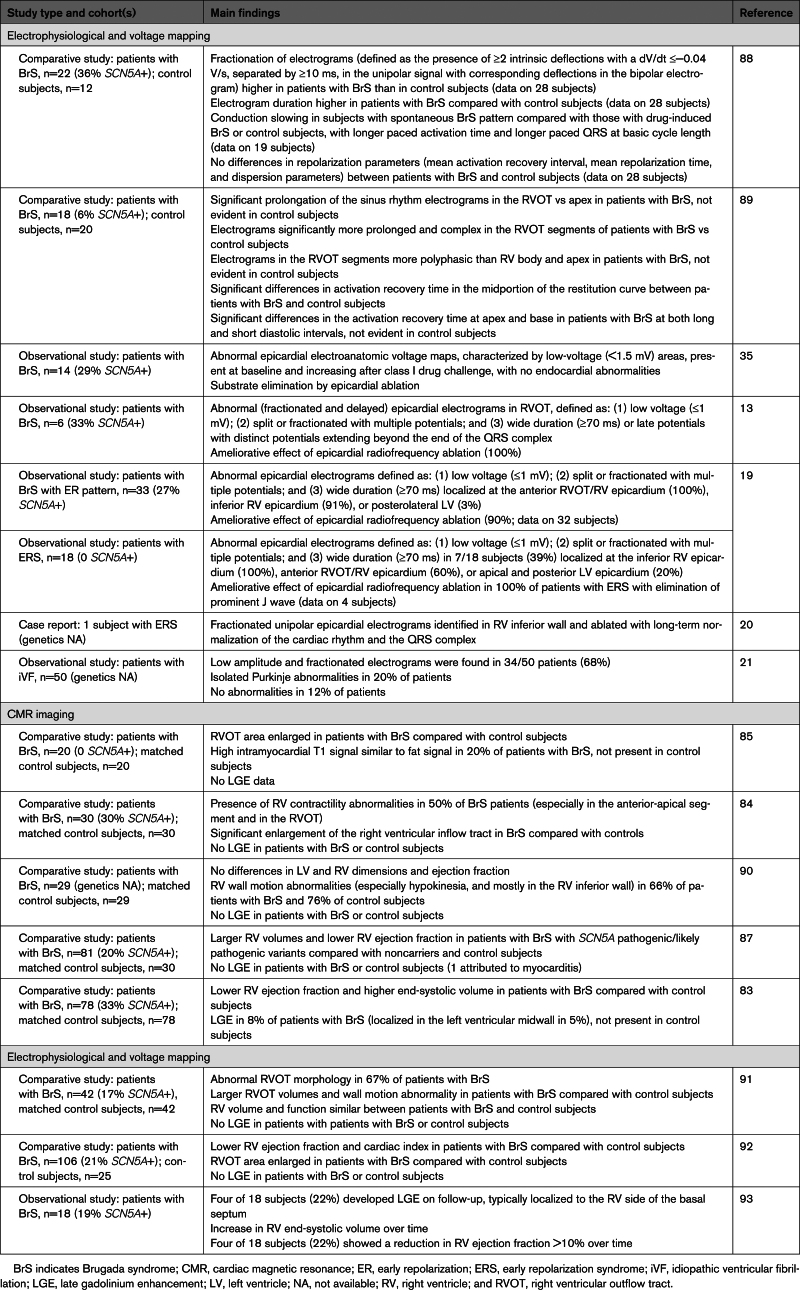
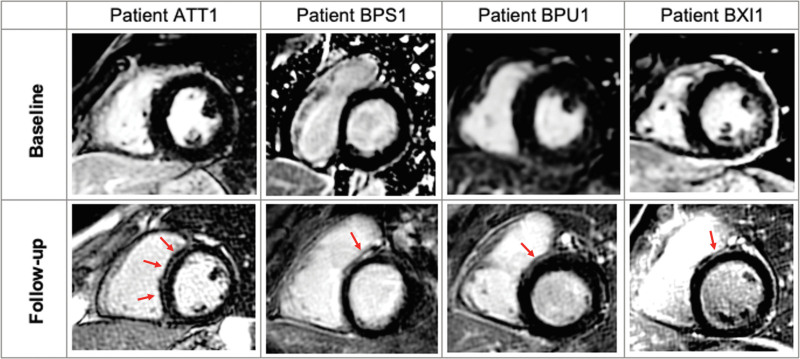
Case series have documented RV electromechanical abnormalities (including epsilon waves) in BrS and the presence of a provocable type 1 Brugada ECG in patients meeting task force criteria for ACM.76,80–82 Furthermore, the association between BrS and morphological abnormalities of the RV has been explored83–87 (Table 1). Gray et al91 compared cardiac magnetic resonance (CMR) imaging data among patients with BrS, patients with ACM, and control subjects. The BrS cohort was characterized by increased volumes and abnormal function of the RVOT compared with controls, but, unlike the ACM group, the BrS group did not show global RV dilatation or systolic impairment. Some patients with BrS also demonstrate left ventricular late gadolinium enhancement on CMR,83 suggesting a degree of phenotypic overlap with cardiomyopathies such as ACM. One CMR study showed emergence of focal septal late gadolinium enhancement in 4 patients with BrS during follow-up, suggesting that a progressive evolution of imaging abnormalities occurs in some patients (Figure 2).93 Despite these reports, not all CMR studies have shown such changes.90 Moreover, there are a lack of data indicating evolving myocardial impairment in patients with BrS. This is in line with our view that microstructural changes are minor, undetectable by conventional imaging, and unlikely to cause overt myocardial dysfunction (Figure 3). Nonetheless, in one study, structural RVOT abnormalities appeared to confer a worse prognosis in BrS, representing a potential marker for arrhythmic risk.95 The Table 1 summarizes previously published electrophysiological and imaging data on the apparent microstructural substrate present in BrS, ERS, and iVF. To the best of our knowledge, CMR imaging has not been studied in ERS or iVF.
Table 1.
Studies Detailing Electrophysiological and CMR Features of Subjects With BrS, ERS, and iVF
Figure 2.
Development of LGE on CMR imaging during follow-up in patients with BrS. Four (22%) patients (ATT1, BPS1, BPU1, and BXI1) developed focal septal late gadolinium enhancement (LGE) during assessment with serial cardiac magnetic resonance (CMR). Average time between follow-up imaging was 5.0±1.7 years. BrS indicates Brugada syndrome. Reproduced with permission from Isbister et al.93 Copyright © 2023 Elsevier.
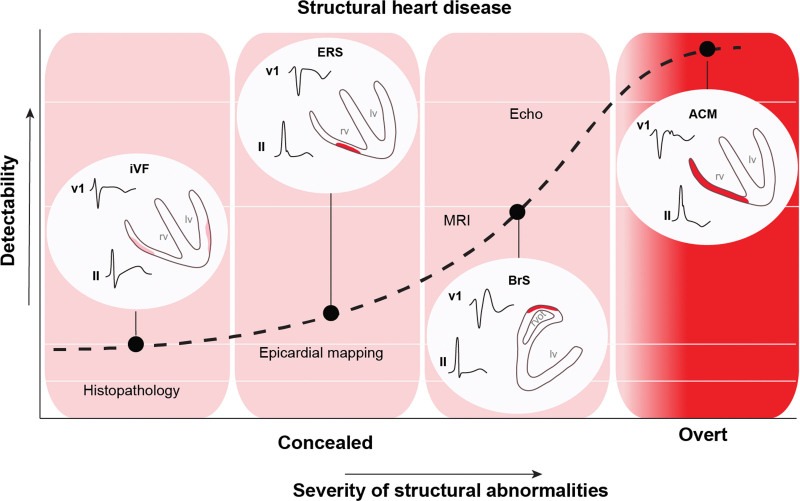
Figure 3.
Detectability and severity of structural abnormalities in BrS, ERS, and ACM with respect to cardiac diagnostic modalities. ACM indicates arrhythmogenic cardiomyopathy; BrS, Brugada syndrome; ERS, early repolarization syndrome; iVF, idiopathic ventricular fibrillation; and MRI, magnetic resonance imaging. Adapted from Boukens et al94 with permission. Copyright © 2020 Elsevier.
Figure 3 shows the relationship between these disorders and the severity of the microstructural changes with respect to their detectability by various diagnostic methods. Depending on the resolution of the imaging or diagnostic method used, the syndromes are defined as structural heart disease or progressive or not. However, gadolinium enhancement lacks sensitivity for diffuse patterns of interstitial fibrosis, which may be better served by novel imaging techniques such as T1 mapping.
Predilection of Location
There is a potentially shared predilection of location of the microstructural abnormalities and VF origin in the subepicardial (right) ventricular myocardium in at least a subset of patients with 1 of the 3 syndromes. This raises the question of the cause for this predilection. A possible explanation may be found in cardiac development. The progenitor cells of the left ventricular and RV compartments have a different developmental history and have been exposed to different signals and gene programs before their differentiation.96 Studies in chickens have revealed that the RVOT is derived from the outflow tract (OFT) of the embryonic and fetal heart.97 The electrophysiological properties and gene expression in the cardiomyocytes of the prenatal OFT differ from those of the ventricles. The prenatal OFT conducts the cardiac impulse slowly, and the main protein responsible for intercellular electrical communication, connexin43, is not expressed in the OFT. Some remnants of these phenotypic differences may be maintained in the OFT, when it matures to form the RVOT. This may explain why the RVOT may form the basis for reentrant arrhythmias that are facilitated by slow conduction and uncoupling.98
A role for cardiac development in disease susceptibility is further supported by the genome-wide association study that associated BrS with several common variants in or near genes encoding transcription factors crucial for electrophysiological patterning of the ventricular myocardium during development, such as TBX5, HEY2, IRX3, and IRX5.52 These transcription factors directly or indirectly modulate the expression of SCN5A and could be causally related to reentry by slowing conduction.50,99,100 TBX5 and IRX3 are expressed predominantly in the ventricular conduction system and have been associated with atrioventricular conduction disturbance and iVF, respectively.101,102 HEY2 and IRX5 are expressed in the ventricular myocardium and dictate the transmural gradient in Ito.103,104 In mice, Irx5 is expressed in an endocardial-to-epicardial gradient and represses the expression of Kcnd2, a potassium channel carrying Ito, leading to low Ito magnitude in the subendocardium. On the other hand, Hey2 is expressed in an epicardial-to-endocardial gradient. Mice heterozygous for Hey2 show reduced Kcnd2 expression and lower Ito magnitude in the subepicardium compared with controls, indicating that Hey2 is required for high magnitude of Ito in the subepicardium.104 Computer simulation experiments have shown that large Ito reduces sodium current, contributes to a slower conduction in the subepicardium than the subendocardium (especially in the presence of sodium channel blockers), and, in the presence of subtle structural discontinuities, facilitates conduction block.29,105
Clinical Implications
Microstructural defects within the cardiac architecture of patients with BrS, ERS, and iVF or their electrophysiological manifestations are increasingly recognized. From our previous hypothesis of impaired epicardial conduction reserve in the RVOT underlying the BrS,55 we postulate that impaired conduction, along with genetic and environmental susceptibility, within sites of microstructural discontinuity in patients with BrS and a large proportion of patients with ERS and iVF, precipitates a reduction in epicardial conduction reserve, which, in turn, leads to the arrhythmogenic substrate and can give rise to the electrocardiographic phenotype in BrS and inferolateral J waves in ERS (Figure 1).
There are currently major deficiencies in our ability to diagnose the underlying cause in cases of initially unexplained cardiac arrest with no apparent structural cardiac abnormality. The rapidly expanding use of high-density electroanatomic mapping and digital analysis software may facilitate digital quantification of tissue and cellular components in which histological changes are subtle or localize to particular regions of myocardium. When combined with machine learning algorithms, this may allow artificial intelligence–led diagnostics and a reduced reliance on current qualitative and descriptive techniques used in cardiac pathology. Future studies may also consider mRNA sequencing of tissue specimens to enable transcriptome-wide analysis of molecular pathways implicated in collagen synthesis, which could enable the development of metabolically targeted therapies. Furthermore, modern imaging modalities such as CMR T1 mapping or photon-counting computed tomography have provided an invaluable opportunity to visualize fibrosis patterns in vivo. This could potentially facilitate objective comparisons of such phenotypes and may have implications for clinical practice, particularly early detection of these pathologies. For example, high-resolution imaging techniques used to detect and longitudinally assess myocardial fibrosis could form the basis of future investigations into its role in arrhythmic risk stratification and local therapy. Understanding the histological and electrophysiological substrate may also help in developing morphometric diagnostic criteria for a subepicardial cardiomyopathy.
Conclusions
BrS, ERS, and iVF potentially form part of a spectrum of a common disease defined by subtle subepicardial microstructural abnormalities: a subepicardial cardiomyopathy. Although genetic susceptibility is uncertain and variable, these microstructural abnormalities are consistent with the electrocardiographic characteristics of each of the syndromes, the mechanism of arrhythmogenesis, and the relationship with modulating genetic and environmental factors.
Article Information
Sources of Funding
Dr Miles is the recipient of a British Heart Foundation clinical research training fellowship (FS/18/28/33549). Drs Behr and Miles received research funding from the Robert Lancaster Memorial Fund, sponsored by McColl’s Retail Group Ltd, UK. Dr Wilde receives research funding from CVON (Predict-2). Dr Behr and the GenUCA investigators received research funding from the British Heart Foundation.
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- ACM
- arrhythmogenic cardiomyopathy
- BrS
- Brugada syndrome
- CMR
- cardiac magnetic resonance
- ERS
- early repolarization syndrome
- iVF
- idiopathic ventricular fibrillation
- OFT
- outflow tract
- RV
- right ventricular
- RVOT
- right ventricular outflow tract
- VF
- ventricular fibrillation
R. Coronel and E.R. Behr contributed equally.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Sources of Funding and Disclosures, see page 1630.
Circulation is available at www.ahajournals.org/journal/circ
Contributor Information
Chris Miles, Email: cmiles@sgul.ac.uk.
Bastiaan J. Boukens, Email: b.boukens@maastrichtuniversity.nl.
Chiara Scrocco, Email: cscrocco@sgul.ac.uk.
Arthur A.M. Wilde, Email: a.a.wilde@amc.uva.nl.
Koonlawee Nademanee, Email: wee@pacificrimep.com.
Michel Haissaguerre, Email: michel.haissaguerre@chu-bordeaux.fr.
Ruben Coronel, Email: rubencoronel@gmail.com.
References
- 1.Modell SM, Lehmann MH. The long QT syndrome family of cardiac ion channelopathies: a HuGE review. Genet Med. 2006;8:143–155. doi: 10.1097/01.gim.0000204468.85308.86 [DOI] [PubMed] [Google Scholar]
- 2.Papadakis M, Papatheodorou E, Mellor G, Raju H, Bastiaenen R, Wijeyeratne Y, Wasim S, Ensam B, Finocchiaro G, Gray B, et al. The diagnostic yield of Brugada syndrome after sudden death with normal autopsy. J Am Coll Cardiol. 2018;71:1204–1214. doi: 10.1016/j.jacc.2018.01.031 [DOI] [PubMed] [Google Scholar]
- 3.Antzelevitch C, Yan GX, Ackerman MJ, Borggrefe M, Corrado D, Guo J, Gussak I, Hasdemir C, Horie M, Huikuri H, et al. J-wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. Europace. 2017;19:665–694. doi: 10.1093/europace/euw235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anselm DD, Evans JM, Baranchuk A. Brugada phenocopy: a new electrocardiogram phenomenon. World J Cardiol. 2014;6:81–86. doi: 10.4330/wjc.v6.i3.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noseworthy PA, Weiner R, Kim J, Keelara V, Wang F, Berkstresser B, Wood MJ, Wang TJ, Picard MH, Hutter AM, et al. Early repolarization pattern in competitive athletes. Circ Arrhythm Electrophysiol. 2011;4:432–440. doi: 10.1161/CIRCEP.111.962852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conte G, Giudicessi JR, Ackerman MJ. Idiopathic ventricular fibrillation: the ongoing quest for diagnostic refinement. Europace. 2021;23:4–10. doi: 10.1093/europace/euaa211 [DOI] [PubMed] [Google Scholar]
- 7.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB; American Heart Association. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287 [DOI] [PubMed] [Google Scholar]
- 8.Behr ER. J-wave syndromes, SCN5A, and cardiac conduction reserve: two sides of the same coin? J Am Coll Cardiol. 2021;78:1618–1620. doi: 10.1016/j.jacc.2021.09.003 [DOI] [PubMed] [Google Scholar]
- 9.Pappone C, Ciconte G, Anastasia L, Gaita F, Grant E, Micaglio E, Locati ET, Calovic Z, Vicedomini G, Santinelli V. Right ventricular epicardial arrhythmogenic substrate in long-QT syndrome patients at risk of sudden death. EP Europace. 2023;25:948–955. doi: 10.1093/europace/euac264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corrado D, Nava A, Buja G, Martini B, Fasoli G, Oselladore L, Turrini P, Thiene G. Familial cardiomyopathy underlies syndrome of right bundle branch block, ST segment elevation and sudden death. J Am Coll Cardiol. 1996;27:443–448. doi: 10.1016/0735-1097(95)00485-8 [DOI] [PubMed] [Google Scholar]
- 11.Frustaci A, Priori SG, Pieroni M, Chimenti C, Napolitano C, Rivolta I, Sanna T, Bellocci F, Russo MA. Cardiac histological substrate in patients with clinical phenotype of Brugada syndrome. Circulation. 2005;112:3680–3687. doi: 10.1161/CIRCULATIONAHA.105.520999 [DOI] [PubMed] [Google Scholar]
- 12.Coronel R, Casini S, Koopmann TT, Wilms-Schopman FJG, Verkerk AO, de Groot JR, Bhuiyan Z, Bezzina CR, Veldkamp MW, Linnenbank AC, et al. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome: a combined electrophysiological, genetic, histopathologic, and computational study. Circulation. 2005;112:2769–2777. doi: 10.1161/CIRCULATIONAHA.105.532614 [DOI] [PubMed] [Google Scholar]
- 13.Nademanee K, Raju H, De Noronha SV, Papadakis M, Robinson L, Rothery S, Makita N, Kowase S, Boonmee N, Vitayakritsirikul V, et al. Fibrosis, connexin-43, and conduction abnormalities in the Brugada syndrome. J Am Coll Cardiol. 2015;66:1976–1986. doi: 10.1016/j.jacc.2015.08.862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martini B, Nava A, Thiene G, Buja GF, Canciani B, Scognamiglio R, Daliento L, Dalla Volta S. Ventricular fibrillation without apparent heart disease: description of six cases. Am Heart J. 1989;118:1203–1209. doi: 10.1016/0002-8703(89)90011-2 [DOI] [PubMed] [Google Scholar]
- 15.Miles C, Asimaki A, Ster IC, Papadakis M, Gray B, Westaby J, Finocchiaro G, Bueno-Beti C, Ensam B, Basu J, et al. Biventricular myocardial fibrosis and sudden death in patients with Brugada syndrome. J Am Coll Cardiol. 2021;78:1511–1521. doi: 10.1016/j.jacc.2021.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coronel R, Berecki G, Opthof T. Why the Brugada syndrome is not yet a disease: syndromes, diseases, and genetic causality. Cardiovasc Res. 2006;72:361–363. doi: 10.1016/j.cardiores.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 17.Boukens BJ, Opthof T, Coronel R. J-waves in epicardial electrograms can guide ablation of arrhythmogenic substrates. Circ Res. 2019;124:205–207. doi: 10.1161/CIRCRESAHA.118.314414 [DOI] [PubMed] [Google Scholar]
- 18.Meijborg VMF, Potse M, Conrath CE, Belterman CNW, de Bakker JMT, Coronel R. Reduced sodium current in the lateral ventricular wall induces inferolateral J-waves. Front Physiol. 2016;7:365. doi: 10.3389/fphys.2016.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nademanee K, Haissaguerre M, Hocini M, Nogami A, Cheniti G, Duchateau J, Behr ER, Saba M, Bokan R, Lou Q, et al. Mapping and ablation of ventricular fibrillation associated with early repolarization syndrome. Circulation. 2019;140:1477–1490. doi: 10.1161/CIRCULATIONAHA.118.039022 [DOI] [PubMed] [Google Scholar]
- 20.Boukens BJ, Benjacholamas V, van Amersfoort S, Meijborg VM, Schumacher C, Jensen B, Haissaguerre M, Wilde A, Prechawat S, Huntrakul A, et al. Structurally abnormal myocardium underlies ventricular fibrillation storms in a patient diagnosed with the early repolarization pattern. JACC Clin Electrophysiol. 2020;6:1395–1404. doi: 10.1016/j.jacep.2020.06.027 [DOI] [PubMed] [Google Scholar]
- 21.Haïssaguerre M, Duchateau J, Dubois R, Hocini M, Cheniti G, Sacher F, Lavergne T, Probst V, Surget E, Vigmond E, et al. Idiopathic ventricular fibrillation: role of Purkinje system and microstructural myocardial abnormalities. JACC Clin Electrophysiol. 2020;6:591–608. doi: 10.1016/j.jacep.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haïssaguerre M, Hocini M, Cheniti G, Duchateau J, Sacher F, Puyo S, Cochet H, Takigawa M, Denis A, Martin R, et al. Localized structural alterations underlying a subset of unexplained sudden cardiac death. Circ Arrhythm Electrophysiol. 2018;11:e006120. doi: 10.1161/CIRCEP.117.006120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funabashi N, Kobayashi Y. J waves reaching to equal or more than 2 of 3 LV inferior wall leads may predict the presence of organized myocardial fibrotic or fat change in survivors of ventricular fibrillation. Eur Heart J. 2022;43:ehab849.037. doi: 10.1093/eurheartj/ehab849.037 [Google Scholar]
- 24.Wilde AAM, Postema PG, Di Diego JM, Viskin S, Morita H, Fish JM, Antzelevitch C. The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J Mol Cell Cardiol. 2010;49:543–553. doi: 10.1016/j.yjmcc.2010.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blok M, Boukens BJ. Mechanisms of arrhythmias in the Brugada syndrome. Int J Mol Sci. 2020;21:7051. doi: 10.3390/ijms21197051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litovsky SH, Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res. 1988;62:116–126. doi: 10.1161/01.res.62.1.116 [DOI] [PubMed] [Google Scholar]
- 27.Boukens BJ, Potse M, Coronel R. Fibrosis and conduction abnormalities as basis for overlap of Brugada syndrome and early repolarization syndrome. Int J Mol Sci. 2021;22:1570. doi: 10.3390/ijms22041570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoogendijk MG, Potse M, Linnenbank AC, Verkerk AO, den Ruijter HM, van Amersfoorth SCM, Klaver EC, Beekman L, Bezzina CR, Postema PG, et al. Mechanism of right precordial ST-segment elevation in structural heart disease: excitation failure by current-to-load mismatch. Heart Rhythm. 2010;7:238–248. doi: 10.1016/j.hrthm.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 29.Hoogendijk MG, Potse M, Vinet A, de Bakker JMT, Coronel R. ST segment elevation by current-to-load mismatch: an experimental and computational study. Heart Rhythm. 2011;8:111–118. doi: 10.1016/j.hrthm.2010.09.066 [DOI] [PubMed] [Google Scholar]
- 30.Nademanee K, Veerakul G, Chandanamattha P, Chaothawee L, Ariyachaipanich A, Jirasirirojanakorn K, Likittanasombat K, Bhuripanyo K, Ngarmukos T. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270–1279. doi: 10.1161/CIRCULATIONAHA.110.972612 [DOI] [PubMed] [Google Scholar]
- 31.Jeevaratnam K, Poh Tee S, Zhang Y, Rewbury R, Guzadhur L, Duehmke R, Grace AA, Lei M, Huang CLH. Delayed conduction and its implications in murine Scn5a+/- hearts: independent and interacting effects of genotype, age, and sex. Pflugers Arch. 2011;461:29–44. doi: 10.1007/s00424-010-0906-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park DS, Cerrone M, Morley G, Vasquez C, Fowler S, Liu N, Bernstein SA, Liu FY, Zhang J, Rogers CS, et al. Genetically engineered SCN5A mutant pig hearts exhibit conduction defects and arrhythmias. J Clin Investig. 2015;125:403–412. doi: 10.1172/JCI76919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ten Sande JN, Coronel R, Conrath CE, Driessen AHG, De Groot JR, Tan HL, Nademanee K, Wilde AAM, De Bakker JMT, Van Dessel PFHM. ST-segment elevation and fractionated electrograms in Brugada syndrome patients arise from the same structurally abnormal subepicardial RVOT area but have a different mechanism. Circ Arrhythm Electrophysiol. 2015;8:1382–1392. doi: 10.1161/CIRCEP.115.003366 [DOI] [PubMed] [Google Scholar]
- 34.Vigmond EJ, Efimov IR, Rentschler SL, Coronel R, Boukens BJ. Fractionated electrograms with ST-segment elevation recorded from the human right ventricular outflow tract. HeartRhythm Case Rep. 2017;3:546–550. doi: 10.1016/j.hrcr.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brugada J, Pappone C, Berruezo A, Vicedomini G, Manguso F, Ciconte G, Giannelli L, Santinelli V. Brugada syndrome phenotype elimination by epicardial substrate ablation. Circ Arrhythm Electrophysiol. 2015;8:1373–1381. doi: 10.1161/CIRCEP.115.003220 [DOI] [PubMed] [Google Scholar]
- 36.Nademanee K, Veerakul G, Nogami A, Lou Q, Hocini M, Coronel R, Behr ER, Wilde A, Boukens BJ, Haissaguerre M. Mechanism of the effects of sodium channel blockade on the arrhythmogenic substrate of Brugada syndrome. Heart Rhythm. 2022;19:407–416. doi: 10.1016/j.hrthm.2021.10.031 [DOI] [PubMed] [Google Scholar]
- 37.Franz MR. Current status of monophasic action potential recording: theories, measurements and interpretations. Cardiovasc Res. 1999;41:25–40. doi: 10.1016/s0008-6363(98)00268-5 [DOI] [PubMed] [Google Scholar]
- 38.Janse MJ, Kleber AG. Electrophysiological changes and ventricular arrhythmias in the early phase of regional myocardial ischemia. Circ Res. 1981;49:1069–1081. doi: 10.1161/01.res.49.5.1069 [DOI] [PubMed] [Google Scholar]
- 39.Haïssaguerre M, Nademanee K, Sacher F, Cheniti G, Hocini M, Surget E, Dubois R, Vigmond E, Bernus O. Multisite conduction block in the epicardial substrate of Brugada syndrome. Heart Rhythm. 2022;19:417–426. doi: 10.1016/j.hrthm.2021.10.030 [DOI] [PubMed] [Google Scholar]
- 40.Szél T, Antzelevitch C. Abnormal repolarization as the basis for late potentials and fractionated electrograms recorded from epicardium in experimental models of Brugada syndrome. J Am Coll Cardiol. 2014;63:2037–2045. doi: 10.1016/j.jacc.2014.01.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patocskai B, Yoon N, Antzelevitch C. Mechanisms underlying epicardial radiofrequency ablation to suppress arrhythmogenesis in experimental models of Brugada syndrome. JACC Clin Electrophysiol. 2017;3:353–363. doi: 10.1016/j.jacep.2016.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawata H, Noda T, Yamada Y, Okamura H, Satomi K, Aiba T, Takaki H, Aihara N, Isobe M, Kamakura S, et al. Effect of sodium-channel blockade on early repolarization in inferior/lateral leads in patients with idiopathic ventricular fibrillation and Brugada syndrome. Heart Rhythm. 2012;9:77–83. doi: 10.1016/j.hrthm.2011.08.017 [DOI] [PubMed] [Google Scholar]
- 43.Cheung CC, Mellor G, Deyell MW, Ensam B, Batchvarov V, Papadakis M, Roberts JD, Leather R, Sanatani S, Healey JS, et al. Comparison of ajmaline and procainamide provocation tests in the diagnosis of Brugada syndrome. JACC Clin Electrophysiol. 2019;5:504–512. doi: 10.1016/j.jacep.2019.01.026 [DOI] [PubMed] [Google Scholar]
- 44.Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. doi: 10.1038/32675 [DOI] [PubMed] [Google Scholar]
- 45.Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, Brugada P, Fressart V, Guerchicoff A, Harris-Kerr C, et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33–46. doi: 10.1016/j.hrthm.2009.09.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falkow S. Molecular Koch’s postulates applied to microbial pathogenicity. Rev Infect Dis. 1988;10(suppl 2):S274–S276. [DOI] [PubMed] [Google Scholar]
- 47.Hill AB. The environment and disease: association or causation? J R Soc Med. 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Probst V, Wilde AAM, Barc J, Sacher F, Babuty D, Mabo P, Mansourati J, Le Scouarnec S, Kyndt F, Le Caignec C, et al. SCN5A mutations and the role of genetic background in the pathophysiology of Brugada syndrome. Circ Cardiovasc Genet. 2009;2:552–557. doi: 10.1161/CIRCGENETICS.109.853374 [DOI] [PubMed] [Google Scholar]
- 49.Arking DE, Pulit SL, Crotti L, Van Der Harst P, Munroe PB, Koopmann TT, Sotoodehnia N, Rossin EJ, Morley M, Wang X, et al. ; CARe Consortium. Genetic association study of QT interval highlights role for calcium signaling pathways in myocardial repolarization. Nat Genet. 2014;46:826–836. doi: 10.1038/ng.3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bezzina CR, Barc J, Mizusawa Y, Remme CA, Gourraud JB, Simonet F, Verkerk AO, Schwartz PJ, Crotti L, Dagradi F, et al. Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat Genet. 2013;45:1044–1049. doi: 10.1038/ng.2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tadros R, Tan HL, El Mathari S, Kors JA, Postema PG, Lahrouchi N, Beekman L, Radivojkov-Blagojevic M, Amin AS, Meitinger T, et al. ; ESCAPE-NET Investigators. Predicting cardiac electrical response to sodium-channel blockade and Brugada syndrome using polygenic risk scores. Eur Heart J. 2019;40:3097–3107. doi: 10.1093/eurheartj/ehz435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barc J, Tadros R, Glinge C, Chiang DY, Jouni M, Simonet F, Jurgens SJ, Baudic M, Nicastro M, Potet F, et al. ; KORA-Study Group. Genome-wide association analyses identify new Brugada syndrome risk loci and highlight a new mechanism of sodium channel regulation in disease susceptibility. Nat Genet. 2022;54:232–239. doi: 10.1038/s41588-021-01007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe H, Nogami A, Ohkubo K, Kawata H, Hayashi Y, Ishikawa T, Makiyama T, Nagao S, Yagihara N, Takehara N, et al. Electrocardiographic characteristics and SCN5A mutations in idiopathic ventricular fibrillation associated with early repolarization. Circ Arrhythm Electrophysiol. 2011;4:874–881. doi: 10.1161/CIRCEP.111.963983 [DOI] [PubMed] [Google Scholar]
- 54.Zhang ZH, Barajas-Martínez H, Xia H, Li B, Capra JA, Clatot J, Chen GX, Chen X, Yang B, Jiang H, et al. Distinct features of probands with early repolarization and Brugada syndromes carrying SCN5A pathogenic variants. J Am Coll Cardiol. 2021;78:1603–1617. doi: 10.1016/j.jacc.2021.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Behr ER, Ben-Haim Y, Ackerman MJ, Krahn AD, Wilde AAM. Brugada syndrome and reduced right ventricular outflow tract conduction reserve: A final common pathway? Eur Heart J. 2021;42:1073–1081. doi: 10.1093/eurheartj/ehaa1051 [DOI] [PubMed] [Google Scholar]
- 56.Derangeon M, Montnach J, Cerpa CO, Jagu B, Patin J, Toumaniantz G, Girardeau A, Huang CLH, Colledge WH, Grace AA, et al. Transforming growth factor β receptor inhibition prevents ventricular fibrosis in a mouse model of progressive cardiac conduction disease. Cardiovasc Res. 2017;113:464–474. doi: 10.1093/cvr/cvx026 [DOI] [PubMed] [Google Scholar]
- 57.Royer A, Van Veen TAB, Le Bouter S, Marionneau C, Griol-Charhbili V, Léoni AL, Steenman M, Van Rijen HVM, Demolombe S, Goddard CA, et al. Mouse model of SCN5A-linked hereditary Lenègre’s: disease age-related conduction slowing and myocardial fibrosis. Circulation. 2005;111:1738–1746. doi: 10.1161/01.CIR.0000160853.19867.61 [DOI] [PubMed] [Google Scholar]
- 58.Campuzano O, Fernández-Falgueras A, Iglesias A, Brugada R. Brugada syndrome and PKP2: evidences and uncertainties. Int J Cardiol. 2016;214:403–405. doi: 10.1016/j.ijcard.2016.03.194 [DOI] [PubMed] [Google Scholar]
- 59.Te Riele ASJM, Agullo-Pascual E, James CA, Leo-Macias A, Cerrone M, Zhang M, Lin X, Lin B, Rothenberg E, Sobreira NL, et al. Multilevel analyses of SCN5A mutations in arrhythmogenic right ventricular dysplasia/cardiomyopathy suggest non-canonical mechanisms for disease pathogenesis. Cardiovasc Res. 2017;113:102–111. doi: 10.1093/cvr/cvw234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Novelli V, Malkani K, Cerrone M. Pleiotropic phenotypes associated with PKP2 variants. Front Cardiovasc Med. 2018;5:184. doi: 10.3389/fcvm.2018.00184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cerrone M, Remme CA, Tadros R, Bezzina CR, Delmar M. Beyond the one gene-one disease paradigm complex genetics and pleiotropy in inheritable cardiac disorders. Circulation. 2019;140:595–610. doi: 10.1161/CIRCULATIONAHA.118.035954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cerrone M, Delmar M. Desmosomes and the sodium channel complex: implications for arrhythmogenic cardiomyopathy and Brugada syndrome. Trends Cardiovasc Med. 2014;24:184–190. doi: 10.1016/j.tcm.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rivaud MR, Delmar M, Remme CA. Heritable arrhythmia syndromes associated with abnormal cardiac sodium channel function: ionic and non-ionic mechanisms. Cardiovasc Res. 2020;116:1557–1570. doi: 10.1093/cvr/cvaa082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato PY, Musa H, Coombs W, Guerrero-Serna G, Patiño GA, Taffet SM, Isom LL, Delmar M. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ Res. 2009;105:523–526. doi: 10.1161/CIRCRESAHA.109.201418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cerrone M, Lin X, Zhang M, Agullo-Pascual E, Pfenniger A, Chkourko Gusky H, Novelli V, Kim C, Tirasawadichai T, Judge DP, et al. Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation. 2014;129:1092–1103. doi: 10.1161/CIRCULATIONAHA.113.003077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rizzo S, Lodder EM, Verkerk AO, Wolswinkel R, Beekman L, Pilichou K, Basso C, Remme CA, Thiene G, Bezzina CR. Intercalated disc abnormalities, reduced Na+ current density, and conduction slowing in desmoglein-2 mutant mice prior to cardiomyopathic changes. Cardiovasc Res. 2012;95:409–418. doi: 10.1093/cvr/cvs219 [DOI] [PubMed] [Google Scholar]
- 67.Gomes J, Finlay M, Ahmed AK, Ciaccio EJ, Asimaki A, Saffitz JE, Quarta G, Nobles M, Syrris P, Chaubey S, et al. Electrophysiological abnormalities precede overt structural changes in arrhythmogenic right ventricular cardiomyopathy due to mutations in desmoplakin-A combined murine and human study. Eur Heart J. 2012;33:1942–1953. doi: 10.1093/eurheartj/ehr472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Asimaki A, Kapoor S, Plovie E, Arndt AK, Adams E, Liu ZZ, James CA, Judge DP, Calkins H, Churko J, et al. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med. 2014;6:240ra74. doi: 10.1126/scitranslmed.3008008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Armaroli A, Balla C, Trabanelli C, Selvatici R, Brieda A, Sette E, Bertini M, Mele D, Biffi M, Campo GC, et al. Lamin A/C missense mutation R216C pinpoints overlapping features between Brugada syndrome and laminopathies. Circ Genom Precis Med. 2020;13:e002751. doi: 10.1161/CIRCGEN.119.002751 [DOI] [PubMed] [Google Scholar]
- 70.Hosseini SM, Kim R, Udupa S, Costain G, Jobling R, Liston E, Jamal SM, Szybowska M, Morel CF, Bowdin S, et al. ; National Institutes of Health Clinical Genome Resource Consortium. Reappraisal of reported genes for sudden arrhythmic death: evidence-based evaluation of gene validity for Brugada syndrome. Circulation. 2018;138:1195–1205. doi: 10.1161/CIRCULATIONAHA.118.035070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grondin S, Davies B, Cadrin-Tourigny J, Steinberg C, Cheung CC, Jorda P, Healey JS, Green MS, Sanatani S, Alqarawi W, et al. Importance of genetic testing in unexplained cardiac arrest. Eur Heart J. 2022;43:3071–3081. doi: 10.1093/eurheartj/ehac145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Li C, Shi L, Chen X, Cui C, Huang J, Chen B, Hall DD, Pan Z, Lu M, et al. Integrin β1D deficiency-mediated RyR2 dysfunction contributes to catecholamine-sensitive ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy. Circulation. 2020;141:1477–1493. doi: 10.1161/CIRCULATIONAHA.119.043504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delmar M, Alvarado FJ, Valdivia HH. Desmosome-dyad crosstalk: an arrhythmogenic axis in arrhythmogenic right ventricular cardiomyopathy. Circulation. 2020;141:1494–1497. doi: 10.1161/CIRCULATIONAHA.120.046020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim JC, Pérez-Hernández M, Alvarado FJ, Maurya SR, Montnach J, Yin Y, Zhang M, Lin X, Vasquez C, Heguy A, et al. Disruption of Ca2+i homeostasis and connexin 43 hemichannel function in the right ventricle precedes overt arrhythmogenic cardiomyopathy in plakophilin-2-deficient mice. Circulation. 2019;140:1015–1030. doi: 10.1161/CIRCULATIONAHA.119.039710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Corrado D, Zorzi A, Cerrone M, Rigato I, Mongillo M, Bauce B, Delmar M. Relationship between arrhythmogenic right ventricular cardiomyopathy and Brugada syndrome. Circ Arrhythm Electrophysiol. 2016;9:e003631. doi: 10.1161/CIRCEP.115.003631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kataoka S, Serizawa N, Kitamura K, Suzuki A, Suzuki T, Shiga T, Shoda M, Hagiwara N. An overlap of Brugada syndrome and arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Arrhythm. 2016;32:70–73. doi: 10.1016/j.joa.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289–1300. doi: 10.1016/s0140-6736(09)60256-7 [DOI] [PubMed] [Google Scholar]
- 78.Leo-Macias A, Agullo-Pascual E, Delmar M. The cardiac connexome: non-canonical functions of connexin43 and their role in cardiac arrhythmias. Semin Cell Dev Biol. 2016;50:13–21. doi: 10.1016/j.semcdb.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vermij SH, Abriel H, van Veen TAB. Refining the molecular organization of the cardiac intercalated disc. Cardiovasc Res. 2017;113:259–275. doi: 10.1093/cvr/cvw259 [DOI] [PubMed] [Google Scholar]
- 80.Ben-Haim Y, Asimaki A, Behr ER. Brugada syndrome and arrhythmogenic cardiomyopathy: overlapping disorders of the connexome? Europace. 2021;23:653–664. doi: 10.1093/europace/euaa277 [DOI] [PubMed] [Google Scholar]
- 81.Marras E, Basso C, Sciarra L, Delise P. Unexplained syncope, Brugada-like ECG and minimal structural right ventricular abnormalities: which is the right diagnosis? J Cardiovasc Med. 2009;10:273–275. doi: 10.2459/jcm.0b013e328322fc09 [DOI] [PubMed] [Google Scholar]
- 82.Letsas KP, Efremidis M, Weber R, Korantzopoulos P, Protonotarios N, Prappa E, Kounas SP, Evagelidou EN, Xydonas S, Kalusche D, et al. Epsilon-like waves and ventricular conduction abnormalities in subjects with type 1 ECG pattern of Brugada syndrome. Heart Rhythm. 2011;8:874–878. doi: 10.1016/j.hrthm.2011.01.043 [DOI] [PubMed] [Google Scholar]
- 83.Bastiaenen R, Cox AT, Castelletti S, Wijeyeratne YD, Colbeck N, Pakroo N, Ahmed H, Bunce N, Anderson L, Moon JC, et al. Late gadolinium enhancement in Brugada syndrome: a marker for subtle underlying cardiomyopathy? Heart Rhythm. 2017;14:583–589. doi: 10.1016/j.hrthm.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 84.Catalano O, Antonaci S, Moro G, Mussida M, Frascaroli M, Baldi M, Cobelli F, Baiardi P, Nastoli J, Bloise R, et al. Magnetic resonance investigations in Brugada syndrome reveal unexpectedly high rate of structural abnormalities. Eur Heart J. 2009;30:2241–2248. doi: 10.1093/eurheartj/ehp252 [DOI] [PubMed] [Google Scholar]
- 85.Papavassiliu T, Wolpert C, Flüchter S, Schimpf R, Neff W, Haase KK, Düber C, Borggrefe M. Magnetic resonance imaging findings in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2004;15:1133–1138. doi: 10.1046/j.1540-8167.2004.03681.x [DOI] [PubMed] [Google Scholar]
- 86.Papavassiliu T, Veltmann C, Doesch C, Haghi D, Germans T, Schoenberg SO, Van Rossum AC, Schimpf R, Brade J, Wolpert C, et al. Spontaneous type 1 electrocardiographic pattern is associated with cardiovascular magnetic resonance imaging changes in Brugada syndrome. Heart Rhythm. 2010;7:1790–1796. doi: 10.1016/j.hrthm.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 87.Rudic B, Schimpf R, Veltmann C, Doesch C, Tülümen E, Schoenberg SO, Borggrefe M, Papavassiliu T. Brugada syndrome: clinical presentation and genotype-correlation with magnetic resonance imaging parameters. Europace. 2016;18:1411–1419. doi: 10.1093/europace/euv300 [DOI] [PubMed] [Google Scholar]
- 88.Postema PG, van Dessel PFHM, de Bakker JMT, Dekker LRC, Linnenbank AC, Hoogendijk MG, Coronel R, Tijssen JGP, Wilde AAM, Tan HL. Slow and discontinuous conduction conspire in Brugada syndrome: a right ventricular mapping and stimulation study. Circ Arrhythm Electrophysiol. 2008;1:379–386. doi: 10.1161/CIRCEP.108.790543 [DOI] [PubMed] [Google Scholar]
- 89.Lambiase PD, Ahmed AK, Ciaccio EJ, Brugada R, Lizotte E, Chaubey S, Ben-Simon R, Chow AW, Lowe MD, McKenna WJ. High-density substrate mapping in Brugada syndrome: combined role of conduction and repolarization heterogeneities in arrhythmogenesis. Circulation. 2009;120:106–117, 1–4. doi: 10.1161/CIRCULATIONAHA.108.771401 [DOI] [PubMed] [Google Scholar]
- 90.Tessa C, Del Meglio J, Ottonelli AG, Diciotti S, Salvatori L, Magnacca M, Chioccioli M, Lera J, Vignali C, Casolo G. Evaluation of Brugada syndrome by cardiac magnetic resonance. Int J Cardiovasc Imaging. 2012;28:1961–1970. doi: 10.1007/s10554-012-0009-5 [DOI] [PubMed] [Google Scholar]
- 91.Gray B, Gnanappa GK, Bagnall RD, Femia G, Yeates L, Ingles J, Burns C, Puranik R, Grieve SM, Semsarian C, et al. Relations between right ventricular morphology and clinical, electrical and genetic parameters in Brugada Syndrome. PLoS One. 2018;13:e0195594. doi: 10.1371/journal.pone.0195594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hohneck A, Overhoff D, Rutsch M, Rudic B, Tülümen E, Wolpert C, Hetjens S, Akin I, Borggrefe M, Papavassiliu T. Risk stratification of patients with Brugada syndrome: the impact of myocardial strain analysis using cardiac magnetic resonance feature tracking. Hellenic J Cardiol. 2021;62:329–338. doi: 10.1016/j.hjc.2021.05.003 [DOI] [PubMed] [Google Scholar]
- 93.Isbister JC, Gray B, Offen S, Yeates L, Naoum C, Medi C, Raju H, Semsarian C, Puranik R, Sy RW. Longitudinal assessment of structural phenotype in Brugada syndrome using cardiac magnetic resonance imaging. Heart Rhythm O2. 2023;4:34–41. doi: 10.1016/j.hroo.2022.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boukens BJ, Nademanee K, Coronel R. Reply: J-wave syndromes: where’s the scar? JACC Clin Electrophysiol. 2020;6:1863–1864. doi: 10.1016/j.jacep.2020.10.003 [DOI] [PubMed] [Google Scholar]
- 95.Scheirlynck E, Chivulescu M, Lie OH, Motoc A, Koulalis J, de Asmundis C, Sieira J, Chierchia GB, Brugada P, Cosyns B, et al. Worse prognosis in Brugada syndrome patients with arrhythmogenic cardiomyopathy features. JACC Clin Electrophysiol. 2020;6:1353–1363. doi: 10.1016/j.jacep.2020.05.026 [DOI] [PubMed] [Google Scholar]
- 96.Zaffran S, Kelly RG, Meilhac SM, Buckingham ME, Brown NA. Right ventricular myocardium derives from the anterior heart field. Circ Res. 2004;95:261–268. doi: 10.1161/01.RES.0000136815.73623.BE [DOI] [PubMed] [Google Scholar]
- 97.de la Cruz M, Sánchez Gómez C, Arteaga MM, Argüello C. Experimental study of the development of the truncus and the conus in the chick embryo. J Anat. 1977;123:661–686. [PMC free article] [PubMed] [Google Scholar]
- 98.Boukens BJ, Sylva M, de Gier-De Vries C, Remme CA, Bezzina CR, Christoffels VM, Coronel R. Reduced sodium channel function unmasks residual embryonic slow conduction in the adult right ventricular outflow tract. Circ Res. 2013;113:137–141. doi: 10.1161/CIRCRESAHA.113.301565 [DOI] [PubMed] [Google Scholar]
- 99.Kimura Y, Aiba T, Sasano T, Furukawa T, Kusano K, Shimizu W. IRX3 variant as a modifier of Brugada syndrome with frequent ventricular fibrillation. HeartRhythm Case Rep. 2016;2:465–468. doi: 10.1016/j.hrcr.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.al Sayed ZR, Canac R, Cimarosti B, Bonnard C, Gourraud JB, Hamamy H, Kayserili H, Girardeau A, Jouni M, Jacob N, et al. Human model of IRX5 mutations reveals key role for this transcription factor in ventricular conduction. Cardiovasc Res. 2021;117:2092–2107. doi: 10.1093/cvr/cvaa259 [DOI] [PubMed] [Google Scholar]
- 101.van Ouwerkerk AF, Bosada FM, van Duijvenboden K, Houweling AC, Scholman KT, Wakker V, Allaart CP, Uhm JS, Mathijssen IB, Baartscheer T, et al. Patient-specific TBX5-G125R variant induces profound transcriptional deregulation and atrial dysfunction. Circulation. 2022;145:606–619. doi: 10.1161/CIRCULATIONAHA.121.054347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koizumi A, Sasano T, Kimura W, Miyamoto Y, Aiba T, Ishikawa T, Nogami A, Fukamizu S, Sakurada H, Takahashi Y, et al. Genetic defects in a His-Purkinje system transcription factor, IRX3, cause lethal cardiac arrhythmias. Eur Heart J. 2016;37:1469–1475. doi: 10.1093/eurheartj/ehv449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim KH, Rosen A, Bruneau BG, Hui CC, Backx PH. Iroquois homeodomain transcription factors in heart development and function. Circ Res. 2012;110:1513–1524. doi: 10.1161/CIRCRESAHA.112.265041 [DOI] [PubMed] [Google Scholar]
- 104.Veerman CC, Podliesna S, Tadros R, Lodder EM, Mengarelli I, de Jonge B, Beekman L, Barc J, Wilders R, Wilde AAM, et al. The Brugada syndrome susceptibility gene HEY2 modulates cardiac transmural ion channel patterning and electrical heterogeneity. Circ Res. 2017;121:537–548. doi: 10.1161/CIRCRESAHA.117.310959 [DOI] [PubMed] [Google Scholar]
- 105.Portero V, Wilders R, Casini S, Charpentier F, Verkerk AO, Remme CA. KV4.3 expression modulates NaV1.5 sodium current. Front Physiol. 2018;9:178. doi: 10.3389/fphys.2018.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]