Abstract
Background
Melioidosis is an infection caused by Burkholderia pseudomallei, a Gram-negative bacterium. It is a disease endemic to Southeast Asia and northern Australia although its global incidence has been rising. It most commonly infects people with certain identified risk factors such as diabetes, alcoholism, thalassemia, and underlying chronic disease involving lungs, kidney and liver. This bacterium is capable of producing a wide array of clinical manifestations ranging from asymptomatic disease to localised infections such as in the lung, bone or skin to disseminated infection.
Case description
This is a case, from United Arab Emirates, of a 40-year-old male recently diagnosed with diabetes who presented with multiple abscesses and was eventually diagnosed with disseminated melioidosis. He was treated successfully with antibiotics and drainage of abscesses.
Conclusion
In non-endemic regions, melioidosis can be easily missed in common diagnostic approaches. This gap of awareness could delay the diagnosis and allow further deterioration of the patient due to complications. Thus, case reports like this can enlighten internists about changing incidences and complexity of clinical presentations, thus preparing them to better handle such patients in the future.
LEARNING POINTS
Owing to its considerably rare incidence in non-endemic regions including the United Arab Emirates, melioidosis can easily be overlooked or misdiagnosed.
Moreover, due to similarity with multiple other diseases and infections as well as significant absence from standard medical curricula, melioidosis is rarely on the differential list of an internist.
This report aims to enhance awareness and alertness to aid earlier detection and avoid severe complications.
Keywords: Burkholderia pseudomallei, melioidosis, disseminated, neuromelioidosis, sepsis
INTRODUCTION
During times of exponentially rising globalisation and rapid travel opportunities, it is essential to emphasise the possibility of encountering infections that may previously have been restricted to endemic regions. One such disease is melioidosis, endemic to northern Australia and Southeast Asia[1]. Owing to its considerably rare incidence in the United Arab Emirates (UAE), it can easily be overlooked or misdiagnosed as other infections that tend to resemble it. Thus, its diagnosis may be delayed, allowing the development of serious complications. This report highlights a unique case of disseminated melioidosis detected in UAE with culture confirmation that presented with complications including abscesses, arthritis, and leptomeningeal enhancement.
The mode of transmission of melioidosis includes inhalation, ingestion and inoculation from contaminated soil and water, often affecting farmers who walk barefoot. Certain identified risk factors include diabetes, alcoholism, thalassemia and underlying chronic renal and lung disease[1]. Clinical presentation may be asymptomatic or limited to local infection such as in the lung, skin, or soft tissue. Melioidosis can affect a wide array of organs leading to various complications including abscess formation in the prostate, spleen, kidney, liver and brain[2].
CASE DESCRIPTION
A 40-year-old Filipino male who recently travelled from the Philippines where he had worked on an animal farm presented with a myriad of symptoms. He began experiencing fever and chills for a week, which was followed by pustular lesions that initially emerged on his face before extending to the rest of his body. He also complained of generalised body aches, recurrent abdominal pain, abdominal distension and diarrhoea with loose stool. He had recently been diagnosed with type 2 diabetes and had a history of heavy alcohol use.
In the Emergency Department, the patient appeared toxic and in distress. Physical examination revealed pale and jaundiced skin with erythematous pustular lesions distributed across the body, particularly on the face and arms. Vital signs included a temperature elevated to 38.8 °C, blood pressure of 122/78, respiratory rate of 20, oxygen saturation of 95% and an elevated heart rate of 128 beats per minute. Auscultation revealed bibasilar crepitations. Abdominal examination was positive for distension, tenderness, and a palpable liver edge 4 cm below the costal margin. No neurological deficits were noted.
The initial laboratory work up (shown in Table 1) revealed elevated inflammatory markers, indicative of a severe systemic response. Notable findings included an elevated erythrocyte sedimentation rate (ESR) of 120 mm/1hr, an elevated C-reactive protein (CRP) level of 267.3 mg/l and an elevated procalcitonin level of 12.75 ng/ml. Furthermore, the patient’s total white blood cell count was 18.8 × 109/l, and routine urine tests confirmed the presence of infection. His HbA1C level of 11.3% pointed towards the presence of long-standing uncontrolled diabetes, underscoring the significance of underlying comorbidities in melioidosis cases.
Table 1.
Laboratory results.
| Parameter | Result | Reference Range |
|---|---|---|
| Erythrocyte sedimentation rate (ESR) | 120 mm/1hr | 2–28 mm/1hr |
| C-reactive protein (CRP) | 267.3 mg/l | < 5.0 mg/l |
| Procalcitonin | 12.75 ng/ml | < 0.05 ng/ml |
| Total white blood cells (WBC) | 18.8 × 109/l | 3.6–11.0 × 109/l |
| Haemoglobin A1c (HbA1c) | 11.3% | <5.7% |
| Albumin | 2.5 g/dl | 3.4–4.8 g/dl |
| Aspartate aminotransferase (AST) | 103 U/l | 0–40 U/l |
| Gamma-glutamyl transferase (GGT) | 178 U/l | 5–36 U/l |
| Alkaline phosphatase (ALP) | 266 U/l | 35–104 U/l |
| Amylase | 85 U/l | 28–100 U/l |
| Creatinine | 1.3 mg/dl | 0.50–0.90 mg/dl |
The patient was suspected to have chickenpox and was initially managed as a case of sepsis, receiving empiric treatment with vancomycin, piperacillin-tazobactam and acyclovir. Due to worsening clinical status and the inconclusive rash appearance, he was transferred to the Infectious Diseases department. Blood cultures returned positive for Gram-negative rods subsequently identified as Burkholderia pseudomallei. Antibiotic therapy was adjusted to ceftazidime and later to meropenem upon the patient’s complaints of headache, considering the possibility of central nervous system involvement.
During the hospital stay, the patient developed reactive arthritis affecting the right knee and left wrist. Radiological investigations, including chest X-ray revealed bilateral pneumonic infiltrates. Computed tomography (CT) scans further unveiled a liver abscess (Fig. 1) and multiple prostatic abscesses (Fig. 2). Trimethoprim/sulfamethoxazole and meropenem were initiated for dual coverage against B. pseudomallei. Magnetic resonance imaging showed leptomeningeal enhancement, and a lumbar puncture revealed elevated protein counts in the cerebrospinal fluid with no growth on culture. Interventional procedures included ultrasound-guided drainage of the liver abscess and transurethral drainage of prostatic abscesses.
Figure 1.
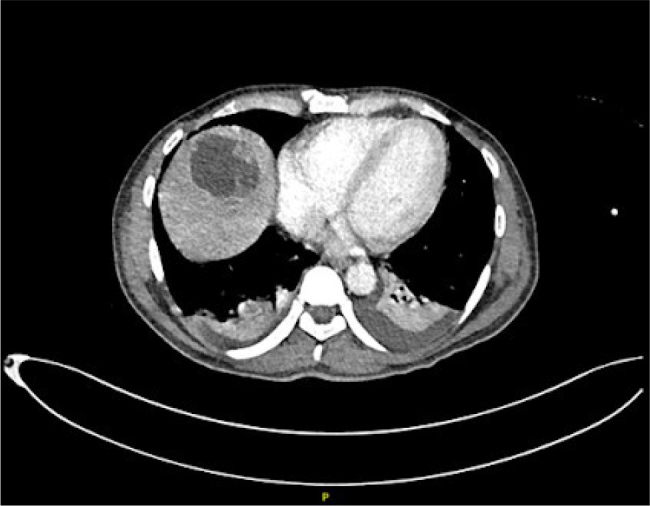
CT scan showing liver abscess.
Figure 2.
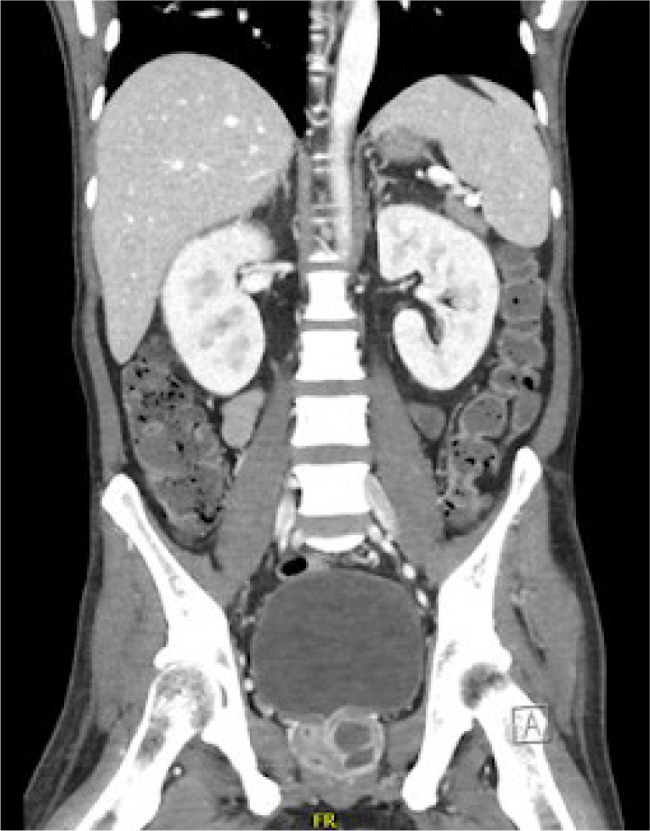
CT scan showing multiple prostatic abscesses.
Upon stabilisation, the patient was discharged on oral antibiotics including cotrimoxazole for 6 months, and fluconazole and linezolid for 4 weeks. The choice of these medications was based on the specific microbiological findings and the need for comprehensive coverage against B. pseudomallei.
DISCUSSION
Melioidosis, a disease caused by the infective agent B. pseudomallei, a Gram-negative bacillus, is potentially lethal. It is frequently found in the soil in endemic regions. Major modes of transmission include percutaneous inoculation or inhalation; incidence rises greatly in the rainy season. Sufferers of chronic diseases such as diabetes, chronic kidney disease, thalassemia or those in immunocompromised states are especially susceptible to this disease[1]. This suggests that functional neutrophil deficiency could play a significant role in the development of melioidosis. Other studies have defined virulence factors that allow evasion of phagocytic killing mechanisms and possibly cell-mediated immunity[1].
Melioidosis has a variegated presentation ranging from asymptomatic infections to localised infection or disseminated infection, while the rest present with fulminant sepsis or pneumonia commonly mistaken for smear-negative tuberculosis[3]. However, the classical picture involves multiple soft tissue abscesses and respiratory symptoms[1]. In this patient’s case, along with the culture confirmation which is a gold standard for the diagnosis of melioidosis, his presentation was consistent with the diagnosis. This included multiple soft tissue abscesses, respiratory distress, and pustular lesions along with history of risk factors such as long-term undiagnosed diabetes, alcoholism and occupation as a livestock farm worker (indicating contact with soil in known endemic area).
With a reported case fatality rate of 10–50% and data showing high recurrence rates[4], the need to effectively treat the infection at initial presentation is paramount. Treatment guidelines recommend intravenous administration of ceftazidime or meropenem for a minimum of 14 days initially, followed by 3–6 months of oral dual combination therapy typically comprising co-trimoxazole or co-amoxiclav. However, B. pseudomallei resistance to each of these antibiotics has been reported[5].
It is recommended that those who survive the initial illness continue the oral antibiotic trimethoprim and sulfamethoxazole for an additional three months at least after discharge, to eradicate the infection. The most severe chronic complication is that of persistent neurologic deficit in those patients who developed encephalomyelitis, with children being most affected, and deformities or limitations in range of motion resulting from musculoskeletal involvement[4]. Recurrent illness is a major cause of mortality, and many patients may be re-hospitalised within weeks of initial discharge. This may represent a sequela of melioidosis but may also be a consequence of frailty following prolonged hospital stay, or pre-existing risk factors. The risk of death is highest in those patients who progress to septic shock or require mechanical ventilation. The need for follow-up is paramount as studies in endemic regions have demonstrated that nearly a quarter of patients die in the first year following hospitalisation. However many patients are routinely lost to follow-up[6]. This patient, however, continued with follow-up and has been doing well ever since without any relapse, reinfection or development of any chronic complications.
In non-endemic regions, melioidosis can be a challenging diagnosis to make, owing to its status as the “great masquerader” due to unspecified clinical presentations and ability to mimic a variety of diseases[7]. For many a clinician, melioidosis is hardly ever a differential diagnosis due to its conspicuous absence from standard medical curricula. It is also not yet known whether vaccines can prevent infection and serious illness caused by B. pseudomallei. This report gives a detailed account of a unique case of disseminated melioidosis in the United Arab Emirates with culture confirmation[8]. This can aid medical providers in early detection and management to prevent life-threatening complications.
CONCLUSION
Although melioidosis can be asymptomatic or mild, it can also develop multiple abscesses, dissemination and sepsis as in this patient. Thus, it may require a multidisciplinary approach, antibiotic coverage, and necessary interventions. Although it may be under-reported in non-endemic regions, it is imperative to incorporate it as a differential diagnosis in similar cases early on to avoid severe complications.
Footnotes
Conflicts of Interests: The Authors declare that there are no competing interests.
Patient Consent: Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
REFERENCES
- 1.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maluda MCM, Goroh MM, Tan ECH, Syed Abdul Rahim SS, Avoi R, Jeffree MS, et al. Complications of melioidosis: a systematic review. Borneo Epid J. 2020;1:5–15. [Google Scholar]
- 3.Currie BJ. Burkholderia pseudomallei and Burkholderia mallei: melioidosis and glanders. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 28th edition. Amsterdam: Elsevier; 2015. pp. 2541–2551. [Google Scholar]
- 4.Wiersinga WJ, Virk HS, Torres AG, Currie BJ, Peacock SJ, Dance DAB, et al. Melioidosis. Nat Rev Dis Primers. 2018;4:17107. doi: 10.1038/nrdp.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes KB, Richards MI, Burgess G, Armstrong SJ, Bentley C, Maishman TC, et al. Investigation of a combination therapy approach for the treatment of melioidosis. Front Microbiol. 2022;13:934312. doi: 10.3389/fmicb.2022.934312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chantratita N, Phunpang R, Yarasai A, Dulsuk A, Yimthin T, Onofrey LA, et al. Characteristics and one year outcomes of melioidosis patients in Northeastern Thailand: A prospective, multicenter cohort study. Lancet Reg Health Southeast Asia. 2023;9:100118. doi: 10.1016/j.lansea.2022.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yee KC, Lee MK, Chua CT, Puthucheary SD. Melioidosis, the great mimicker: a report of 10 cases from Malaysia. J Trop Med Hyg. 1988;91:249–254. [PubMed] [Google Scholar]
- 8.Dance DA. Melioidosis as an emerging global problem. Acta Trop. 2000;74:115–119. doi: 10.1016/s0001-706x(99)00059-5. [DOI] [PubMed] [Google Scholar]


