Abstract
Introduction
Micronutrient deficiencies are prevalent in West Africa, particularly among women of reproductive age (WRA) and young children. Bouillon is a promising food fortification vehicle due to its widespread consumption. This study aims to evaluate the impact of multiple micronutrient-fortified bouillon cubes, compared to control bouillon cubes (fortified with iodine only), on micronutrient status and hemoglobin concentrations among lactating and non-lactating WRA and young children in northern Ghana.
Methods
This randomized, controlled doubly-masked trial will be conducted in the Kumbungu and Tolon districts in the Northern Region of Ghana, where prior data indicate multiple micronutrient deficiencies are common. Participants will be: 1) non-pregnant non-lactating WRA (15–49 y), 2) children 2–5 y, and 3) non-pregnant lactating women 4–18 months postpartum. Eligible participants will be randomly assigned to receive household rations of one of two types of bouillon cubes: 1) a multiple micronutrient-fortified bouillon cube containing vitamin A, folic acid, vitamin B12, iron, zinc, and iodine, or 2) a control cube containing iodine only.
Each participant’s household will receive a ration of bouillon cubes every 2 weeks, and households will be advised to prepare meals as usual, using the study-provided cubes. The trial duration will be 9 months for non-pregnant non-lactating WRA and children, and 3 months for lactating women. The primary outcomes will be changes in biomarkers of micronutrient status and hemoglobin among WRA and children and milk micronutrient concentrations among lactating women. Secondary outcomes will include change in prevalence of micronutrient deficiency and anemia; dietary intake of bouillon and micronutrients; inflammation, malaria, and morbidity symptoms; and child growth and development.
Discussion
Evidence from this study will inform discussions about bouillon fortification in Ghana and West Africa.
Trial registration
The trial was registered on ClinicalTrials.gov (NCT05178407) and the Pan-African Clinical Trial Registry (PACTR202206868437931). This manuscript reflects protocol version 4 (August 29, 2022).
Introduction
Micronutrient deficiencies are severe and widespread in West Africa, particularly among young children, pregnant and lactating women, and women of reproductive age [1–3]. These deficiencies have developmental and physical consequences, including increased risk of morbidity and mortality [4], which, in turn, have economic consequences [5]. While various programs to address micronutrient deficiencies have been scaled up, including industrial fortification of cooking oil and wheat flour with micronutrients [6], it is unlikely that a single such program will eliminate all micronutrient deficiencies, since delivery platforms may provide only a subset of nutrients or reach only a subset of the deficient population.
In Ghana, micronutrient deficiencies remain common, particularly among women and children, despite the presence of various decades-long micronutrient intervention programs [7]. For example, in the 2017 Ghana Micronutrient Survey, the percentage of preschool children who had iron deficiency (serum ferritin <12 μg/L, adjusted for inflammation) was 40% for the Northern Belt, 18% for the Middle Belt, and 13% for the Southern Belt [8]. Likewise, the percentage of children with vitamin A deficiency (retinol binding protein <0.70 μmol/L, adjusted for inflammation) was 31%, 18% and 17% for the Northern, Middle, and Southern Belts, respectively. Among non-pregnant women (15–49 y), the prevalence of iron deficiency (serum ferritin <15 μg/L, adjusted for inflammation) followed the same pattern as that for pre-school children, with the Northern Belt (22%) being worst off, compared with the Middle (11%) and Southern (14%) Belts. Approximately half of women (51% in the Northern belt) had serum folate concentrations < 10 nmol/L [8].
Wheat flour fortification (iron, zinc, vitamin A, folic acid, and other B vitamins) and cooking oil fortification (vitamin A) are mandatory in Ghana; however, achieving and sustaining high compliance with target fortification levels is a challenge [9]. The 2017 Ghana Micronutrient Survey found that only 56% of oil samples (36% in the Northern Belt) were adequately fortified with vitamin A (≥10 mg/kg vitamin A), and only 6% of wheat flour samples (6% in the Northern Belt) had adequate iron concentrations (≥ 58.5 mg/kg as mandated by Ghana’s food fortification standards) [10]. Similar challenges with implementation of fortification programs have been observed elsewhere in the region [11]. Further efforts are thus required to address the high prevalence of micronutrient deficiencies in Ghana and elsewhere in West Africa.
Bouillon cubes are widely consumed in West Africa and therefore hold great potential for addressing micronutrient deficiencies in the region [12–14]. Bouillon is particularly attractive as a food vehicle because consumption is high even among subsets of the population typically at risk for micronutrient deficiencies, such as those residing in rural areas and households with low socioeconomic status [12,15]. Estimates of the proportion of households consuming bouillon in the past week have ranged from 79% to 99% in Burkina Faso, Cameroon, Niger, and Senegal [16]. In Ghana, preliminary analyses of household data [17] suggested that 72% of households had acquired bouillon products in the prior 18 days.
In West Africa, some brands of bouillon have been produced with iodized salt since the 1990s, and bouillon products fortified with iron or vitamin A, in addition to iodine, have been introduced by some companies on a voluntary basis [18]. A study in Senegal found that iodine fortification of bouillon could potentially contribute substantially to iodine intake, by providing between 7%-115% of the recommended daily intake, depending on the iodine content of the cubes [19]. In northern Ghana, a study of schoolchildren 6–13 years of age [20] found that around two-thirds (2/3) of dietary iodine was obtained from iodine-fortified bouillon cubes. Other modelling studies have found that bouillon cube fortified with vitamin A could reduce the prevalence of inadequate vitamin A intakes without leading to an increase in excessive intakes among women or children in Cameroon [21], and would complement other intervention programs in reducing inadequate intake of vitamin A [22].
The foregoing evidence suggests that the fortification of bouillon, in combination with other ongoing measures, could contribute to the prevention of micronutrient deficiencies in Ghana. However, the amounts of micronutrients that are currently included in commercial bouillon products on a voluntary basis (e.g., iron) may not be sufficient to meet public health needs [23]. Moreover, no commercial bouillon products have been released that address deficiencies in other key nutrients, such as zinc, folate, and vitamin B-12. Therefore, research is needed to explore the efficacy of fortified bouillon for addressing micronutrient deficiencies. To our knowledge, no studies, in West Africa or elsewhere, have tested the impact of multiple micronutrient-fortified bouillon on micronutrient status.
Study objectives
The primary objectives are to assess the effects of household use of multiple micronutrient-fortified bouillon cubes (containing iodine in addition to vitamin A, folic acid, vitamin B12, iron, and zinc), compared to control bouillon cubes (fortified with iodine only), on micronutrient status and hemoglobin concentrations among non-pregnant, non-lactating women 15–49 years of age (referred to hereafter as women of reproductive age, WRA) and among children 2–5 years of age, and breast milk micronutrient concentrations among non-pregnant, lactating women 15–49 years of age at 4–18 months postpartum (hereafter, lactating women).
Secondary objectives include the effect of the multiple micronutrient-fortified bouillon, compared to the control bouillon, on the prevalence of micronutrient deficiencies and anemia; dietary intake of bouillon and micronutrients; inflammation, malaria, and morbidity symptoms; and children’s anthropometric measures and child development (details of primary and secondary outcomes for each physiological group are listed in supplemental material S1 and S2 Tables). Sub-studies will also assess opinions, attitudes, and acceptance (OAA) of the multiple micronutrient-fortified bouillon and hypothetical willingness-to-pay (WTP) for multiple micronutrient-fortified bouillon.
We generally hypothesize that micronutrient status, hemoglobin concentrations, dietary nutrient intake, child growth, and child development scores will be greater, and that prevalence of each micronutrient deficiency, anemia, and inflammation and morbidity will be lower, among participants who receive the multiple micronutrient-fortified bouillon compared to those who receive the control bouillon. Specific hypotheses will be reported in statistical analysis plans that will be posted prior to data analysis.
Materials and methods
The trial was registered on ClinicalTrials.gov (NCT05178407) and the Pan-African Clinical Trial Registry (PACTR202206868437931). The full study protocol (S1 File) and statistical analysis plans are posted on Open Science Framework (https://osf.io/t3zrn/); S2 File includes information on adherence of this manuscript to SPIRIT recommendations.
Study design
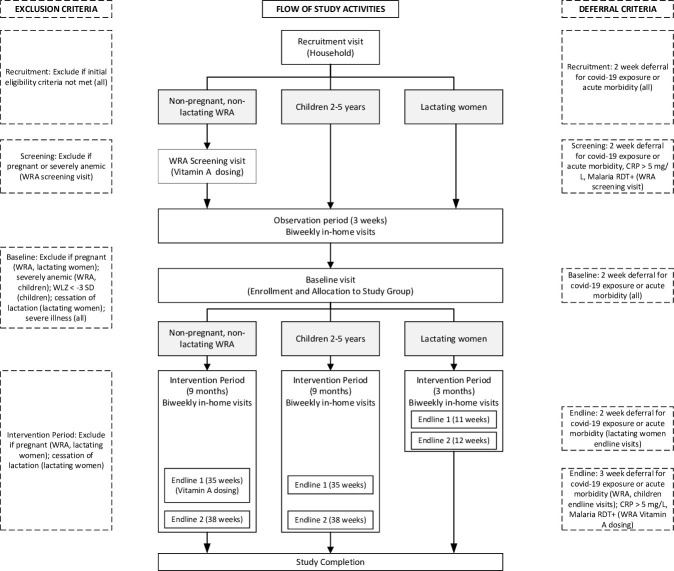
This community-based trial will follow a randomized, controlled doubly-masked design. WRA (n = 1028), children (n = 690), and lactating women (n = 690) will be recruited from households in selected communities within the Kumbungu and Tolon districts, Northern Region, Ghana. The flow of study activities for each of the 3 physiological groups, and summary of exclusion and deferral criteria, are illustrated in Fig 1, while the schedule of data collection is summarized in Table 1. Written informed consent will be obtained from participating women and from caregivers of participating children. Consented WRA will be invited for a screening visit for assessment of total body vitamin A stores using the retinol isotope dilution (RID) technique [24]. Following a 3-week observation period, which includes 2 in-home visits, all participants will undergo baseline assessment, and eligible participants will be enrolled by being randomly assigned to receive household rations of one of two types of bouillon cubes: 1) a multiple micronutrient-fortified bouillon cube containing vitamin A, folic acid, vitamin B12, iron, zinc, and iodine, or 2) a control bouillon cube fortified only with iodine. Households will be advised to prepare their meals as usual, but will be requested to use the study-provided bouillon instead of the commercially available bouillon they typically purchase. Households will then be visited every 2 weeks for delivery of study cubes, monitoring of adherence, and recording of morbidity symptoms. Endline assessments will be conducted after ∼9 months (35 and 38 weeks) for WRA and children, and after ∼3 months (11 and 12 weeks) for lactating women. Details of study procedures and data collection at each time point are summarized in Table 1 and below.
Fig 1. Flow of study activities.
CRP, C-reactive protein; RDT, rapid diagnostic test; WLZ, weight-for-length Z-score; WRA, women of reproductive age.
Table 1. Schedule of recruitment, screening, enrolment, intervention, and assessments.
| Type of data collection | Recruitment | Screening (WRA) | Observation period | Baseline |
Intervention Period | Study check-out | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Biweekly | 4-weekly | Endline 1 | Endline 2 | ||||||
| ENROLMENT | ||||||||||
| Household eligibility | x | |||||||||
| Individual eligibility | x | x | x | |||||||
| Informed consent | x | |||||||||
| Retinol isotope dosing (WRA) | x | x | ||||||||
| Enrolment | x | |||||||||
| Study group allocation | x | |||||||||
| INTERVENTION | ||||||||||
| Bouillon provision | ||||||||||
| Adherence monitoring | x | x | ||||||||
| ASSESSMENTS | ||||||||||
| Household data collection | ||||||||||
| Demographics, SES | x | |||||||||
| Household bouillon KAP | x | x | X | x | x | |||||
| FACT | x | X | x | |||||||
| Willingness to pay | x | x | ||||||||
| Opinions, attitudes, acceptance1 | x | x (+ 60 d) | x (-60 d) | |||||||
| Food and water security | x | X | x | |||||||
| Index participant data collection | ||||||||||
| Anthropometry | ||||||||||
| MUAC | x | x | x | |||||||
| Length, weight, waist and hip circumference | x | x | ||||||||
| Morbidity | ||||||||||
| Health history | x | X | ||||||||
| Blood pressure | x | x | x | x | ||||||
| Morbidity surveillance | x | x | x | X | x | x | ||||
| Blood sample collection | ||||||||||
| Hemoglobin | x | x | x | x | ||||||
| Hematocrit | x | x | x | |||||||
| Infection indicators (malaria, CRP, AGP) | x | x | x | x | ||||||
| Vitamin A isotopes (WRA) | x | x | ||||||||
| Micronutrient status indicators (plasma ferritin, sTfR, zinc, RBP, retinol, B12; whole blood folate, plasma/serum folate, ZPP) | x | x | x | |||||||
| Urine sample collection | ||||||||||
| HCG (WRA) | x | x | ||||||||
| Iodine, sodium | x | x | x | |||||||
| Breast milk sample collection | ||||||||||
| Micronutrient status indicators (vitamin A, B12) |
x |
x |
x |
|||||||
| Stool sample collection | ||||||||||
| Inflammation indicators (calprotectin) | x | x | ||||||||
| Microbiome | x | x | ||||||||
| Dietary | ||||||||||
| Food frequency questionnaire | X | x | ||||||||
| Micronutrient supplement use | X | x | ||||||||
| 24 h recalls | x | x (+ 30 d) |
|
|
x (-30 d) | |||||
| Child development | ||||||||||
| Home inventory | x | x (-21 d) | ||||||||
| MDAT | x | x (-21 d) | ||||||||
| EYT | x | x (-21 d) | ||||||||
1 In addition, one round of focus group discussions will be conducted during the intervention period among a subset of participants in the OAA substudy. AGP, alpha-1-acid glycoprotein; CRP, C-reactive protein; EYT, Early Years Toolbox; FACT, Fortification Assessment Coverage Tool; HCG, human chorionic gonadotropin; KAP, knowledge attitudes and practices; MDAT, Malawi Developmental Assessment Tool; MUAC, mid-upper arm circumference; RBP, retinol-binding protein; SES, socioeconomic status; WRA, women of reproductive age; ZPP, zinc protoporphyrin.
Study site
This proposed research will take place in the Tolon and Kumbungu districts in the Northern Region. Micronutrient biomarker data from a pilot survey in these districts (not yet published) confirmed prior research [10] that suggests micronutrient deficiencies are common. For example, pilot data indicated that 28% of WRA and 53% of children 2–5 years of age were iron deficient (serum ferritin concentration <15 or <12 μg/L, respectively, adjusted for inflammation). In addition, 34% of children had evidence of vitamin A deficiency (serum retinol <0.70 μmol/L, adjusted for inflammation) although only ∼1% of WRA had serum retinol <0.70 μmol/L. Serum vitamin B12 concentrations were low (<221 pmol/L) among 12% of women and 19% of children 2–5 y, while low vitamin B12 concentrations in breast milk (<221 pmol/L) were observed among 40% of lactating women. Anemia was also common among both women (31%) and children 2–5 years of age (36%). Consumption of micronutrient supplements of any type was rare, with fewer than 10% of WRA and children interviewed in the pilot survey reporting consumption in the previous 30 days. Similarly, pilot survey data also confirmed inadequate compliance with mandatory target micronutrient fortification levels of industrially fortified foods. Data on micronutrient levels in food samples collected at the study site indicate that oil samples were fortified with amounts equivalent to ∼69% of target vitamin A levels and wheat flour samples were fortified with ∼33–34% of target levels for iron and zinc (not yet published).
Identification and recruitment of participants
Participants in the study will be 1) non-pregnant non-lactating WRA 15–49 years of age, 2) children 2–5 years of age, and 3) non-pregnant, lactating women 15–49 years of age at 4–18 months postpartum. Multiple participants may be recruited from the same household if they represent different physiological groups (for example, a WRA and child 2–5 years of age), but only one index participant (e.g., one WRA) from each physiological group will be recruited from each household. A household is defined as a group of people who recognize the same head of household, live together, and share living expenses and meals (see S3 File for full definition). Sub-study respondents will be 1) OAA: an adult household member (≥ 15 years of age) who is not recruited in the trial, and 2) WTP: the household member who is primarily responsible for making decisions about bouillon purchasing.
Within the Tolon and Kumbungu districts, we will recruit potential participants from 16 recruitment sites composed of 14 “rural” (comprising both rural and semi-urban areas) and 14 urban Ghana Statistical Service designated sampling areas. Recruitment of each physiological group (WRA, children, lactating women) will be balanced within each recruitment site so that the final sample of participants in each physiological group represents a similar geographic area.
Before recruitment, a study team member will carry out sensitization visits to the study communities; these visits will be used to meet with community leaders to introduce the study and answer questions. Households in selected recruitment sites will be identified through a random walk method with door-to-door recruitment [25]. Trained enumerators fluent in English and the local language (Dagbani) will approach households to assess eligibility, administer recruitment questionnaires, and conduct the informed consent process.
Consent
Upon selecting a household to approach, enumerators will first obtain oral consent from the head of household to proceed with the interview and then will administer a series of questionnaires to assess interest in study participation and complete a household roster. The household roster will be used to identify potentially eligible individuals. If more than one potentially eligible individual per physiological group is present in the home (for example, if there are two children between 2–5 years of age), one individual per physiological group will be randomly selected based on a Kish Table [26]. Potentially eligible participants will then complete the written informed consent process, wherein enumerators read aloud the consent form in the preferred language of the participant (English or Dagbani), concluding with an informed consent quiz to confirm the participant’s understanding of the study procedures. Informed consent will be confirmed with a signature or thumbprint; impartial witnesses (community members not affiliated with the study) fluent in English and Dagbani will be present for the consent process for non-literate participants to ensure the information presented orally matches the written informed consent sheet. Caregivers will provide consent for children 2–5 years; participants 15–17 years who have never married and are living with their parents will provide assent and their caregiver will provide consent. After an index participant has consented to participate in the study, respondents for the OAA and WTP sub-studies will be recruited. For the OAA sub-study, enumerators will use the household’s roster and a random number generator to randomly select the OAA participant from all remaining adult household members (e.g., ≥15 years and not recruited into the RCT); one OAA participant will be recruited per household. Upon agreement to participate in the OAA sub-study, the selected adult household member will provide written informed consent. For WTP, the household member with primary responsibility for bouillon purchasing (who may or may not be a participant in the RCT) will be asked to provide oral consent to respond to questions to elicit their hypothetical willingness to pay for a fortified bouillon cube.
Inclusion and exclusion criteria, and deferral criteria
Detailed descriptions of the research inclusion and exclusion criteria by physiological group and at each stage of the study are included in S3 File and summarized in Fig 1. Briefly, households will be eligible for recruitment if the household is willing to use the study-provided bouillon cubes for the duration of the study and no one in the household i) is currently participating in another research trial, ii) has a chronic medical condition requiring frequent blood transfusions, or iii) has an allergy to ingredients in the bouillon cubes (shrimp, wheat, milk, soy, eggs, celery, fish, or mollusc). WRA, lactating women, and children will be eligible for inclusion if they i) do not have a severe acute or chronic illness, ii) are able to provide informed consent, and iii) plan to remain in the study area for the duration of the study.
WRA must have a negative malaria rapid diagnostic test (RDT) result and C-reactive protein (CRP) <5 mg/L at the RID screening visit, and hemoglobin (Hb) ≥80 g/L at the screening and baseline visits. Children must have mid-upper arm circumference (MUAC) of ≥11.5 cm at recruitment, and Hb ≥70 g/L, weight-for-height z-score (WHZ) ≥-3 SD, and no bilateral pitting oedema at baseline.
Throughout the study, WRA and lactating women who report pregnancy, and lactating women who report lactation cessation, will be excluded. Participants who present with COVID-19-related symptoms, or malaria or elevated CRP (WRA screening visit only), will be required to defer study assessments for 2–3 weeks (Fig 1 and S3 File). At the RID screening or baseline visits, if participants do not meet inclusion criteria after the deferral period, they will be excluded.
The following sections describe the sequence of study visits and procedures.
Screening and baseline visits
Following recruitment and consent, WRA will visit the study site to be screened for eligibility for the RID test for assessment of total body stores of vitamin A [24]. Then, during a 3-wk observation period, descriptive information will be collected for all participants at two in-home visits. Following the 3-wk observation period, all participants will visit the study site for baseline assessments; eligible participants will be randomized to a treatment group. Procedures at each visit are summarized below.
RID screening visit for non-pregnant, non-lactating women of reproductive age
WRA will be screened for eligibility for the RID test using procedures described below. Fieldworkers will assess participants for recent morbidity, vitamin A intake (e.g., liver or supplements in the past 24h) and pregnancy (based on human chorionic gonadotropin in urine), measure blood pressure and collect a capillary blood sample. WRA with no recent (past 72h) morbidity or intake of vitamin A-rich foods (e.g., liver) in the past 24h, who have a negative pregnancy test, Hb ≥80 g/L, CRP <5 mg/L, and a negative malaria RDT result will receive an oral dose of d6-labelled vitamin A (2 mg retinol equivalent, sourced from Buchem BV, Apeldorn, Netherlands) in sunflower oil. The labelled vitamin A will be certified as food grade (Food Chemical Codex IV) and approved by the Ghana Food and Drugs Authority. All WRA who received the d6-labelled vitamin A dose will be invited to the biospecimen collection site 21 days after the isotope dose (based on Green et al., 2021 [27]) for a venous blood sample to assess total body vitamin A stores using the RID technique. This 21-day visit will be scheduled to coincide with the trial baseline visit (procedures described below).
Observation period
For all participants, during the ∼3-week period between recruitment and the baseline visit (i.e., the observation period), information on participant and household characteristics, dietary intake, morbidity, and bouillon use will be collected during two household visits conducted 2 weeks apart. Baseline child development assessments will also occur during this period. Additionally, OAA participants will complete a demographic questionnaire and one quantitative survey about opinions, attitude, and acceptance of commercial bouillon.
Baseline visit
After completion of the observation period, eligible participants will be invited to the mobile biospecimen collection site for the baseline visit (Fig 1 and Table 1). Following morbidity screening, participants will undergo baseline data collection procedures, including anthropometry, measurement of blood pressure and collection of a spot urine sample (WRA and children only), and collection of a stool sample (children only). Venous blood will be collected from WRA (fasted) and children (nonfasted) for assessment of hemoglobin, malaria, and biomarkers of micronutrient status and inflammation. For lactating women, a full breast milk sample will be collected for micronutrient analysis. Questionnaires will be used to collect individual level information related to the interpretation of biospecimen results.
Following the baseline data collection procedures, households with participants who remain eligible for the study (according to criteria in Fig 1 and Supplemental Methods) will be randomized to a study arm; an enumerator will visit the household on the following day (the initial home visit) to deliver the first ration of bouillon cubes that corresponds to the assigned treatment group.
Randomization and blinding
The unit of randomization will be at the household level, regardless of the number of individual participants (up to 3) eligible within that household. To randomize the households, the trial statistician will create a computer-generated block randomization scheme with randomly selected block lengths of four or eight. Pieces of paper bearing intervention group assignments represented by 4 different 3-digit alphanumeric codes (2 codes per study arm) and numbered 1–2408 (maximum number of households if each participant resides in a different household) will be placed in opaque envelopes and stacked in increasing order by study staff not involved in the randomization process. For each household enrollment, a designated and trained study personnel will select the four topmost envelopes from the stack and ask the participant to choose an envelope to reveal their study group assignment. At this point, the household ID (and the participant IDs for each eligible participant within the randomized household) will be linked to one of the four study codes indicating the intervention the household will receive.
All study bouillon cubes will be individually wrapped in identical packaging and will be identified only by the study code (a 3-digit alphanumeric code). Boxes of cubes will be labelled with the study code and an associated group color. Participants and study personnel will know the code (and for study personnel, the group color) to which each household is assigned but will not know the micronutrient content of the products.
Two code keepers (one at the University of California, Davis, UCD, and one at the University of Ghana) who are not otherwise affiliated with the study will be responsible for assigning the 4 study codes to the intervention products and communicating directly with the manufacturer about the group assignments. In addition, a hard copy of the randomization code will be stored in a sealed envelope in a locked filing cabinet at the University of Ghana. Two individuals, unaffiliated with the study, will have a key to the office and access to the key to the filing cabinet. In the case of a severe adverse reaction pertaining to an individual participant, the blinding code will be broken only to identify the bouillon cube formulation that the individual participant received, if necessary for their medical care. Only individuals directly involved in the care of the study participant will be unblinded on a strict need-to-know basis.
The specific bouillon cube formulation associated with each of the 4 study codes will also be stored in sealed envelopes held by the co-principal investigators and the statistician. The process for unblinding of the study team will be pre-specified in the statistical analysis plan for the primary outcomes (available at https://osf.io/t3zrn).
Intervention period procedures
Enumerators will visit participant households every 2 weeks throughout the study to provide the study bouillon rations and collect information on adherence (e.g., household inventory of study bouillon and commercial bouillon, bouillon wrapper counts, reported bouillon use), morbidity, and micronutrient supplement use. All reasonable efforts will be made to complete follow-up assessments, regardless of adherence. Questionnaires to collect information on household food insecurity, water insecurity, consumption of fortified foods, and dietary patterns (e.g., dietary diversity and child feeding) will also be administered during the initial home visit and every 4 weeks thereafter (Table 1). Cube delivery and data collection will be conducted with “no contact” methods (e.g., phone interview) in the case of COVID-19 exposure or symptoms. Baseline dietary assessment will be conducted by administering two 24-hour dietary recall interviews per participant on non-consecutive days within 30 days after the baseline visit (all participants), and two additional 24-hour dietary recalls will be conducted on non-consecutive days within the 30 days before the second endline visit (WRA and children only).
Within the first two months of the intervention period, OAA participants will complete a similar quantitative survey to that administered in the observation period but focused on the study-supplied bouillon cubes, and again within the last two months of the intervention period. OAA participants in households in which the only trial participant is a lactating woman will complete only one intervention period survey.
Endline visits
At the end of the intervention period, participants will be invited to the mobile biospecimen collection site for the two endline data collection visits (35 weeks and 38 weeks for WRA and children; 11 weeks and 12 weeks for lactating women). The second endline assessment is included to enhance statistical power. A 3-week period between endline visits was chosen based on the desired timing between the isotope dose administration and blood collection for assessment of total body vitamin A stores. Delivery of study bouillon cubes will continue through the last endline assessment for each household. Data collection procedures at the first endline visit will be similar to those described at the baseline visit. Briefly, data collection will include anthropometric measurements and morbidity symptoms (all participants); spot urine samples, blood pressure measurement and venous blood collection (WRA and children); breast milk collection (lactating women); and stool collection (children).
In addition, at the first endline visit (35 wk), WRA will receive an oral dose of 13C10-retinyl acetate (2 mg retinol equivalents, sourced from Buchem BV, Apeldorn, Netherlands) to assess total body vitamin A stores. 13C10-retinyl acetate will be used at endline to avoid any potential interference from the baseline dose of d6-retinyl acetate. To assess eligibility for the endline isotope dose, participants will undergo screening procedures identical to those at the pre-intervention screening visit, including assessment of morbidity symptoms, vitamin A intake, pregnancy (based on measurement of HCG in urine), anemia, malaria infection, and inflammation. Based on results of these assessments, WRA will either be 1) eligible to receive the endline isotope dose and continue with the study; 2), deferred and scheduled to be re-screened for isotope dosing at the 2nd endline visit (recent morbidity, CRP > 5 g/L, positive malaria RDT); 3) excluded from isotope dosing and the second endline blood collection (participants with Hb < 80 g/L), or 4) excluded from the study (positive pregnancy test) (see S3 File for detailed exclusion and deferral criteria).
Data collection procedures at the second endline assessment will include a subset of the outcomes measured at the first endline assessment, measured using identical methods. These will include morbidity symptoms (all groups), venous blood collection (WRA and children) and breast milk collection (lactating women). WRA who are deferred at the first endline visit, and do not receive the vitamin A isotope dose, will be re-screened at the second endline visit using the same procedures for eligibility described above. WRA who are eligible to receive the isotope dose at the second endline will be asked to return for an additional endline visit to collect a follow-up venous blood sample for RID assessment 3 weeks later.
In between the two endline visits (35wk and 38wk), a single endline child development assessment will be completed for each task. After the second endline visit, a final home visit will be conducted to complete the study exit form and collect all remaining study bouillon cubes from the household. In each household, the baseline assessment of hypothetical willingness-to-pay for a fortified bouillon cube will be repeated at endline.
Intervention: Fortified bouillon development
Product formulation
Bouillon cubes for the trial will be produced based on a non-proprietary formulation that is comparable to commercial cubes sold in Ghana and elsewhere in West Africa [12], containing ∼52% salt by weight, along with palm fat, corn starch, spices and flavour enhancers, colouring, and micronutrient premix (Nicholas Archer, CSIRO, personal communication). Shrimp flavour was selected, as it was the primary bouillon flavour used by all households in the pilot survey in the study community [28]. Table 2 presents the micronutrient concentrations, chemical fortificant forms of each nutrient, and equivalent daily target micronutrient doses for women and young children in the active arm of the study. The specific fortificants were selected on the basis of technical compatibility with the food matrix and experience with use as fortificants [29]. In the case of iron, the combination of ferric pyrophosphate (FePP) with a citric acid/trisodium citrate buffer (CA/TSC) is expected to enhance bioavailability compared to FePP alone [30].
Table 2. Target fortification levels, estimated daily dose of micronutrients delivered by fortified bouillon, and percent of the recommended daily allowance for women of reproductive age and young children1.
| Micronutrient (Fortificant) | Amount of micronutrient per gram bouillon2 | Daily amount provided to women3 | Daily amount provided to women, as proportion of the RDA | Daily amount provided to children | Daily amount provided to children as proportion of the RDA |
|---|---|---|---|---|---|
| Vitamin A (retinyl palmitate) | 200 μg | 400–500 μg | 57–71% | 200 μg | 50–67% |
| Folic acid | 80 μg | 160–200 μg | 67–83% | 80 μg | 67–89% |
| Vitamin B12 | 1.2 μg | 2.4–3.0 μg | 100–125% | 1.2 μg | 100–133% |
| Iron (FePP/CA/TSC) | 4 mg | 8–10 mg | 44–56% | 4 mg | 40–57% |
| Zinc (ZnO) | 3 mg | 6–7.5 mg | 75–94% | 3 mg | 60–100% |
| Iodine (KIO3) | 30 μg | 60–75 μg | 40–50% | 30 μg | 33% |
1Recommended Daily Allowance (RDA) values are for women 19–50 years of age and children 1–3 y and 4–8 y [31]. FePP/CA/TSC: Ferric pyrophosphate/citric acid/trisodium citrate. Values do not include overage. Overages based on expert opinion will be added to account for losses during storage and cooking (30% vitamin A, folic acid, and vitamin B12; 20% iodine; 0% iron and zinc).
2Values represent the amount of the active form of the nutrient, e.g. 200 μg retinol equivalents as retinyl palmitate and 4 mg iron as FePP/CA/TSC.
3Range of values reflects assumed bouillon cube intake of 2–2.5 grams per day among WRA. For children, calculations assume 1 gram per day and RDA categories of 1–3 years and 4–8 years of age (representing children 2–5 years of age).
With estimated typical bouillon consumption of 2–2.5 g/d among WRA and 1 g/d among children, these cubes supply a daily dose of micronutrients equivalent to 33% to 133% of the corresponding US Recommended Daily Allowance for the 6 micronutrients added to the cubes (Table 2). The concentrations of micronutrients were selected based on: 1) modelling of national survey data from Ghana and other West African countries to estimate the contribution of hypothetical fortification of bouillon to adequacy of dietary micronutrient intake (to estimate the potential public health benefit) [14]; 2) the estimated amount of bouillon consumed in the communities selected for the study; 3) expected efficacy, based on a literature review of the effect of micronutrient interventions on change in micronutrient status as measured by biomarkers or on clinical outcomes (anemia and neural tube defects) and estimated accumulation of micronutrient stores (vitamin A and iron); and 4) safety considerations, including the likelihood of study participants or their household members exceeding the tolerable upper intake level (UL), and a review of the literature to identify adverse effects of micronutrient interventions at varying daily micronutrient doses. Additional details on the rationale for the selected fortification levels and estimated daily micronutrient doses are provided as a Technical Appendix to the study protocol (available at https://osf.io/t3zrn/).
The control cube formulation contains iodine but no other added micronutrients. This selection of micronutrient for the control cube is consistent with current policy in Ghana, where salt iodization is mandated (15 mg/kg at household level) [32] and commercial bouillon cubes are typically made with iodized salt [18].
Product testing
Acceptability of the unfortified, non-proprietary bouillon cube formulation was previously confirmed through consumer testing in northern Ghana; this study included the use of commercial cubes as a reference (Nicholas Archer, CSIRO, personal communication). The effects of micronutrient fortification on micronutrient stability and sensory properties of the multiple micronutrient-fortified bouillon were evaluated in accelerated storage trials. The results confirmed good stability and cube quality after 2 weeks of storage of bouillon cubes (in individual wrappers) under accelerated storage conditions (37°C, 75% relative humidity), and for at least 5 months for storage under optimal conditions (23°C, 50% relative humidity) (Nicholas Archer, CSIRO, personal communication). Finally, acceptability of the multiply-fortified and control cube formulations used in this study was evaluated among a sample of women and their households in the study community [33]; overall, the study results suggested that the bouillon formulations were well-liked and would be suitable for use in further research studies.
Bouillon cube production and management
Bouillon cubes will be produced at industrial scale by GB Foods (Barcelona, Spain). Bouillon cubes will be shipped by air or sea to Ghana and transported to a dedicated storage facility at the study site. Boxes of bouillon will be stored on pallets in a cool (12–16°C) temperature-controlled environment with frequent temperature and humidity monitoring. Micronutrient content of the micronutrient premix and the study bouillon cubes will be analyzed to ensure proper coding and formulation of the bouillon cubes before distribution to participants.
Household bouillon ration
The household bouillon ration will be determined based on household size and estimated bouillon consumption (2.5 g/capita/d, based on the number of individuals in the household). This approach was based on previous research in the study community showing that median (p25, p75) per-household bouillon consumption was 20 (12, 24) g/d and that bouillon consumption was 2.4 g/d among WRA and 1.0 g/d among children, similar to the assumptions in Table 2 [28]. The household bouillon ration may be revised over the course of the study based on observations and questionnaire responses (e.g., changes to household composition) but will not exceed 4 g/capita/d. Bouillon rations will be delivered by enumerators to participants’ homes every 2 weeks and stored in study-provided plastic containers. Households will be encouraged to use the study bouillon (in lieu of commercially purchased bouillon cubes) according to their typical cooking habits. At the initial home visit (first day of bouillon distribution) only, the enumerator will request to collect all commercial bouillon in the household; if the household agrees, they will be compensated according to a table of current brand-specific market prices for bouillon. During the trial, households are free to purchase and use commercial bouillon cubes as they choose.
Data collection methods
Questionnaires
Trained enumerators will administer all questionnaires in the preferred language of the participant (English or Dagbani) as structured oral interviews using electronic tablets. Data on demographic and socio-economic status (household size and composition, assets, housing quality, sanitation and hygiene, and individuals’ education and occupations), food security [Household Food Insecurity Access Scale (HFIAS) [34]] and water security [Household Water Insecurity Experiences (HWISE) [35]] will be collected. Pilot-tested questionnaires will also collect information on household bouillon acquisition, consumption, and stocks; bouillon knowledge, attitudes and practices (KAP); consumption of fortified foods, including bouillon [Fortification Assessment Coverage Tool (FACT)[26]]; consumption of micronutrient supplements; and hypothetical willingness-to-pay (WTP) for a new micronutrient-fortified bouillon cube. Information on health history, non-communicable disease risk [WHO STEPwise approach to NCD risk factors surveillance (STEPS) questionnaire] [36], and breastfeeding and infant and young child feeding practices (IYCF) [37] and dietary diversity [Dietary Quality Questionnaire (DQQ)] [38] will also be collected. Parent perception of child weight and parental beliefs, attitudes and practices toward child feeding will be assessed with a child feeding questionnaire (CFQ) [39]. Structured questionnaires will be used to assess recent morbidity, including a pre-defined list of selected morbidity symptoms (e.g., cough), health care visits, and serious adverse events (e.g., hospitalization).
Adherence
Households will be requested to save wrappers from all bouillon (both study-provided and commercial) used in the household since the last biweekly visit. To monitor adherence, at each biweekly visit during the intervention period, enumerators will count the bouillon wrappers and record an inventory of all bouillon cubes in the household (both study-provided and commercial). In addition, enumerators will administer a questionnaire to gather data about household purchases of bouillon and household bouillon use in the past 2 weeks, including sharing of study bouillon cubes with non-household members. Households with sustained low adherence (i.e., low consumption of study-provided bouillon) will be identified through routine adherence data monitoring; enumerators will be instructed to inquire about the reasons for low adherence and to encourage use of the study bouillon.
24 hour recalls
Quantitative dietary intake data will be collected through 24-hour dietary recall interviews to capture bouillon and nutrient intake both prior to and while receiving the study cubes. All participants (WRA, lactating women, children) will complete one dietary recall during the observation period and two baseline dietary recalls on non-consecutive days within the first 30 days of the intervention period. WRA and children will complete two additional endline recalls on non-consecutive days within the 30 days before the second endline visit. Lactating women will not complete endline dietary recalls due to the shorter study duration.
Dietary recalls will be conducted by enumerators with extensive training and practice on the 24-hour recall technique. Interviews will follow a multiple pass methodology [40] to collect information on all foods and beverages consumed by each participant during a defined 24 hour period. Recall data will be collected using a tablet-based data collection system (an adaptation of OpenDRS [41]) that allows for direct entry of dietary data and recipe collection on the tablet, through a platform that guides interviewers through the dietary recall interview and suggests prompts to aid in the interview process. Dietary recalls will be conducted in participant households to take advantage of the opportunity to use the participants’ typical utensils for portion size estimation where possible. Enumerators will also carry “kits” with common utensils and foods (e.g., salt, sugar, spices, etc.) to obtain weights for portions and ingredients where possible.
Opinions, attitude, and acceptance (OAA) towards the study bouillon among adult household members
The OAA sub-study will employ a mixed methods approach (quantitative survey and focus group discussions). OAA towards commercial bouillon will be evaluated with a questionnaire during the observation period, and towards the study-provided bouillon with a similar questionnaire once within the first 60 days and once within the final 60 days of the intervention period. One round of focus group discussions (FGDs) will also be conducted among a subset of participants who complete the OAA questionnaires to qualitatively assess OAA of the study-supplied bouillon cubes. FGDs will be conducted in Dagbani following a pre-tested FGD guide and audio-recorded.
Anthropometry
Anthropometric data will be collected by trained, standardized anthropometrists, following protocols established by the Food and Nutrition Technical Assistance Project [42]. Children’s MUAC (left arm; UNICEF S0145620), standing height of all participants (SECA 217), and the waist and hip circumference of WRA (SECA 201) will be measured to 0.1 cm precision. Weight of all participants (lightly clothed) will be measured to 50 g precision (SECA 874). All measurements will be collected in duplicate, and the average of the two measurements calculated per participant for each outcome. If the two measurements differ by more than 0.1 kg (weight), or 0.5 cm (MUAC, height, waist or hip circumference), the measurement will be repeated, and the two closest measurements will be retained for data analysis.
Child development
Child development will be assessed with the Malawi Development Assessment Tool (MDAT) [43], which measures four domains of development (gross motor, fine motor, language, and personal-social), and the tablet-based Early Years Toolbox Go-No-Go Task which measures executive function (EYT) [44]. Assessments will take place at a central data collection site or (if necessary) in a low-distraction location at the participant’s home. Characteristics of the home environment and caregiver-child interaction will be assessed at a household visit with the Home Observation Measurement of the Environment (HOME) tool [45]. To assess the test-retest reliability of the EYT a convenience sample of 30 children will be asked to come back 1 week later to repeat the EYT.
Prior to data collection, enumerators trained in child development assessment will be required to take a written and practical assessment on the data collection procedures. Inter-rater reliability of the enumerators will be assessed for the MDAT and the HOME to ensure consistency of data collection. The MDAT and HOME were previously piloted in the study site and found to be feasible for use in this setting.
Biological sample collection and laboratory analyses
Capillary and venous blood collection. At the pre-intervention RID-screening visit, capillary blood samples will be obtained from WRA to measure hemoglobin and CRP concentrations using the QuikRead Go (Aidian Oy, Espoo, Finland); malaria antigenemia will be measured by RDT (BioZEK, B.V., Apeldoorn, Netherlands). At the post-intervention RID-screening visit, these measurements will be done using venous blood that is collected for assessment of endline micronutrient status.
At baseline and endline assessments, venous blood samples will be collected from children and WRA by trained laboratory technicians, following standard biological safety protocols and procedures recommended by the International Zinc Nutrition Consultative Group (IZiNCG) [46]. Samples will be collected at a central location in each community (mobile biospecimen collection site) in the early morning. WRA, but not children, will be asked to fast for at least 8 hours prior to their blood draw appointment; time of blood collection and last meal will be recorded for all participants. At all blood sample events (baseline, endline 1 and endline 2), 7.5 ml of blood will be drawn from the antecubital vein into an evacuated, trace element-free polyethylene blood collection tube containing lithium heparin (Sarstedt AG & Co, Numbrecht, Germany; ref 01.1604.400). For all WRA at baseline and endline 2, as well as a subset of WRA at endline 1, an additional 4.5 ml of blood will be drawn from the antecubital vein into an evacuated serum blood collection tube (Sarstedt AG & Co, Numbrecht, Germany; ref 05.1104.100); this will occur prior to blood collection in the lithium heparin tube. In addition, if WRA receive the vitamin A isotope dose at endline 2 (due to deferral), an additional 4.5 ml of blood will be drawn from the antecubital vein into an evacuated serum blood collection tube (Sarstedt AG & Co, Numbrecht, Germany; ref 05.1104.100) at a third time point. If the phlebotomist is unable to obtain a venous blood sample after two attempts, a capillary sample will be obtained by fingerstick and collected into a 200 μl microvette containing lithium heparin (Sarstedt AG & Co, Numbrecht, Germany; ref 20.1292.100). Following blood collection, all blood collection tubes will be placed into a battery-operated cold box (4–8°C) until processing.
Immediately following blood collection, point-of-care assessments will be performed using whole blood remaining in the butterfly tubing or needle (or from the capillary sample); if necessary, whole blood may be aliquoted from the lithium heparin blood collection tubes for the point-of-care tests. Hemoglobin will be measured using a portable photometer (Hemocue 301, HemoCue AB, Angelholm, Sweden). Malaria antigenemia will be assessed with a RDT. Hematocrit (WRA only) will be measured using the GCH-24 Hematocrit centrifuge (Globe Scientific, Mahwah, New Jersey, USA). In addition, hemoglobin, haematocrit, and zinc protoporphyrin (ZPP) will be measured using the iCheck Anemia point-of-care device (Bioanalyt, Teltow, Germany).
Whole blood samples from the serum blood collection tubes will be allowed to clot for 30–60 minutes, after which time they will be centrifuged at 1097 x g (3100 RPM) for 10 minutes (PowerSpin Centrifuge Model LX C856E; United Products & Instruments, Inc, Dayton, NJ). Serum will be aliquoted for the measurement of concentrations of [12C]retinol, d6-retinol and [13C10]retinol and folate. From the lithium heparin blood collection tube, 100 μl of whole blood will be aliquoted into a cryovial containing 1 ml of 1% ascorbic acid solution for the measurement of folate concentration (to calculate erythrocyte folate) among WRA. The remaining heparinized blood will be centrifuged within 8 h of collection at 1097 x g (3100 RPM) for 10 minutes (PowerSpin Centrifuge Model LX C856E; United Products & Instruments, Inc, Dayton, NJ). Plasma will be aliquoted into cryovials for the measurement of micronutrient status (i.e., ferritin, soluble transferrin receptor [sTfR], retinol binding protein [RBP], retinol, zinc, folate and B12) and indicators of systemic inflammation (i.e., CRP, and alpha-1-acid glycoprotein, AGP).
Breast milk collection and processing
At baseline and endline assessments, breast milk samples will be collected from lactating women using the “full milk sample” methodology [47] using the Medela Swing single electric breast pump (Barr, Switzerland). Breast milk will be collected from the breast from which the infant has not fed for a longer time (i.e., the breast that is ‘more full’), with a minimum of one hour elapsed between the milk sample collection and previous feeding. Time of day and time since last feed will be recorded. Fat will be measured in fresh milk in triplicate using the creamatocrit method (Creamatocrit Plus Centrifuge; EKF Diagnostics) [48]. The milk sample will be mixed well and ∼6 ml will be aliquoted for the measurement of vitamins A and B12; the remainder of the milk sample will be returned to the lactating woman to feed to her child.
Urine collection and processing
Urine samples will be collected from both WRA and children at baseline and endline 1 assessments. Among WRA only, spot urine samples will be collected on the day of the pre- and post-intervention RID-screening visits (prior to retinol isotope dosing) to assess pregnancy status based on rapid tests for human chorionic gonadotropic (HCG). At all time points, urine will be aliquoted into cryovials for the measurement of iodine, sodium, potassium, and creatinine concentrations.
Stool collection and processing
Stool samples will be collected from children at baseline and endline 1. The child’s caregiver will be provided with stool sample collection kits (self-standing transport tube with screw cap and spoon, absorbent pad and/or diaper, gloves, biohazard bags, soap) and requested to collect the first available fresh stool sample from the child on the morning of the baseline visit, partially filling the transport tube. In a randomly selected subset of participants, caregivers will also be provided with a stool nucleic acid collection and preservation tube (Norgen Biotek; Ontario, Cananda), and requested to collect a small sample of stool into a second tube. Unpreserved stool samples will be aliquoted into cryovials for the measurement of calprotectin; no processing will be necessary for the microbiome analysis samples in the nucleic acid collection and preservation tubes.
Biological sample storage
All biological sample aliquots will be stored in a battery-operated cold box (4–8°C) and transported daily from the biospecimen collection site to the District Hospital at Tolon for storage in a -86°C freezer, with regular temperature monitoring. Samples thus stored will be periodically transported to the University of Ghana for longer-term frozen storage before analysis. Samples to be analysed outside Ghana will be shipped by air on dry ice to the respective labs, as described below.
Laboratory analysis
Plasma ferritin, sTfR, RBP, CRP, and AGP concentrations will be measured in duplicate using a combined sandwich enzyme-linked immunosorbent assay (ELISA) technique at VitMin Lab (Willstaett, Germany) [49]. Serum concentrations of d6-retinol, [12C]retinol and [13C10]retinol will be measured by liquid chromatography with tandem mass spectrometry (LC/MS/MS) at Newcastle University (Newcastle, UK) [50]. Plasma [51] and breast milk [52] vitamin A concentrations will be measured by high performance liquid chromatography (HPLC) at the UCD (Davis, CA). Plasma zinc concentrations will be analyzed by inductively-coupled plasma optical emission spectrophotometry (ICP-OES; Agilent 5100 SVDV, Santa Clara, CA) at the University of California, San Francisco (UCSF) Benioff Children’s Hospital (Oakland, CA) [53]. Whole blood folate and serum/plasma folate will be analyzed by the US Centers for Disease Control and Prevention (Atlanta, GA) and used to calculate erythrocyte folate concentrations [54]. Vitamin B12 concentrations in plasma and breast milk, will be measured by the Western Human Nutrition Research Center (Davis, CA). Urinary iodine concentration will be assessed using a modification of the Sandell-Kolthoff reaction at the University of Ghana [32]. Urinary sodium, potassium and creatinine concentrations will be analyzed at the UCSF Benioff Children’s Hospital (Oakland, CA). Stool calprotectin will be analysed using a commercially available kit and gut microbiota will be characterized by bacterial 16S rRNA sequencing at UCD (Davis, CA). Novel indicators and other indicators of micronutrient status, growth and infection may be assessed if funding becomes available, among participants who consented to the use of their specimen for future research.
Data management and analysis
Data management and confidentiality
Data will be collected directly on tablets via forms developed to include pre-determined logic checks and range validations using the SurveyCTO® program. Selected data collected on paper forms will be double-entered. Data will be monitored daily by field supervisors. Weekly reports for monitoring of key variables, including study enrollment, attrition, missing data/forms, adherence, and selected quality control monitoring will be routinely reviewed by the research team and field supervisors to identify action items (e.g., form corrections, refresher trainings, participant visits to encourage adherence, data corrections, etc.). Study procedures are designed so that all Personally Identifying Information is kept separately from all other data throughout data collection, storage, and analysis and only accessible to a limited number of authorized members of the research team.
Outcome definitions
For WRA and children, primary outcomes are hemoglobin concentrations and biomarkers of status of iron (plasma ferritin and sTfR), zinc (plasma zinc), folate (plasma folate, erythrocyte folate), vitamin A (total body stores of vitamin A [WRA only], serum retinol, plasma RBP), and vitamin B12 (plasma B12) measured in venous blood samples. Pilot data indicated that vitamin A deficiency was common among children but not WRA in the community; therefore, total body vitamin A stores were selected as the vitamin A outcome for WRA because serum retinol is expected to respond to increased vitamin A intake only when vitamin A stores are low [55]. For lactating women, primary outcomes are concentrations of vitamin A (corrected for milk fat) and vitamin B12 in human milk. Secondary outcomes for women and children include anemia and prevalence of micronutrient deficiencies, malaria and inflammation (CRP and AGP), blood pressure and hypertension, and urinary iodine and sodium. Additional secondary outcomes include dietary intake of bouillon and micronutrients, and morbidity symptoms (all participants); low milk micronutrient concentrations (lactating women); and child development and stool calprotectin (children). S1 and S2 Tables include detailed lists of primary and secondary outcomes, and S3 and S4 Tables specify the micronutrient biomarker cutoffs to be used.
Data analysis
Statistical analysis plans (SAPs) will be developed and posted publicly prior to conducting any data analysis. Data analysis will be conducted using SAS 9.4 (SAS Institute, Cary, NC, USA), STATA v16 (StataCorp LLC, College Station, TX, USA), and/or R version 4.1.1 (R Core Team, Vienna, Austria). For each analysis, a flow diagram for participation will be prepared according to CONSORT guidelines. In most cases, separate analyses will be conducted for each physiological group. Because randomization will occur at the household level and only one individual per physiological group will be enrolled per household, the participants will be considered independent with repeated measurements on the same participant being accounted for with robust standard errors [56]. Additionally, a variable representing the recruitment site of each participant will be included as a control covariate.
The primary analysis will be based on a complete-case intention to treat framework and analysed as a pre-post study design, controlling for baseline measure of the outcome [57]. Testing will be two-sided superiority testing. For continuous outcomes, this will follow an ANCOVA approach with non-normal outcomes transformed prior to analysis (typically log transformed). For binary outcomes, we will use either logistic regression to estimate odds ratios or binomial log-link / modified Poisson regression to estimate risk ratios (approach will be pre-specified in each SAP). In addition to the outcome-specific testing, certain analyses may use a multivariate analysis to examine the simultaneous impact of the intervention on multiple outcomes (approach will be pre-specified in each SAP).
In keeping with the randomized design of the study, we will rely on the minimally adjusted analysis (only controlling for baseline measure and recruitment site) as our primary analysis. For selected biomarkers (for example, plasma ferritin), the primary analysis will use inflammation-corrected outcomes and/or a minimally adjusted analysis that includes markers of inflammation (CRP and AGP) at each time point, if biologically relevant and if the outcome is associated with the markers of inflammation in bivariate analyses (details will be pre-specified in each SAP). In a secondary analysis, we will estimate adjusted parameters by including variables that are strongly associated with the outcome to potentially improve the precision of our estimates (i.e., decrease the standard errors). The variable selection approach will be pre-specified in each SAP. Additionally, pre-specified effect modification testing will be conducted by including an interaction term in minimally adjusted models.
Substantial attrition (>20% loss) after randomization will trigger sensitivity analyses to assess the impact of missingness on inference. This will be conducted either by multiple imputation or inverse probability of censoring-weighted analysis (pre-specified in each SAP). We will also conduct a per protocol analysis using data on adherence to identify the subgroup of participants in “high compliance” households.
A qualitative SAP will also be developed for analysis of the OAA focus group data. FGD transcripts will be translated and transcribed to English by two independent parties. A codebook will be developed, and two researchers will independently code the transcripts with Cohen’s Kappa used to assess intercoder reliability (ICR); transcripts with an ICR <0.6 [58] (less than moderate agreement) will trigger discussion and recoding. Selection of qualitative themes will occur through discussions with the wider research team.
Sample size.
Detection of an effect size of 0.26 SDs was selected based on calculations of the change in total body vitamin A stores among WRA and iron stores among women and children over the 9-mo trial duration; details are presented in the Technical Appendix to the study protocol, available at https://osf.io/t3zrn/). Assuming this effect size and a level of significance of α = 0.05 with 80% power we will target an analytic sample size of 234 per physiological group per intervention group. Although this sample size calculation is based on a simple t-test using a single cross-sectional measurement, final regression models will incorporate a baseline measurement and repeated endline measurements to provide additional statistical power.
Attrition will be accounted for at multiple time points due to the multi-staged study design involving recruitment, screening and baseline study visits prior to randomization to study group. Overall attrition rates were estimated using cross-sectional pilot survey data and are set at ∼32% for children 2–5 y of age and lactating women and at 54% for WRA to account for additional participant exclusion due to pregnancy during the intervention period. Therefore, the recruitment targets will be 690 lactating women, 690 children 2–5 y of age, and 1028 WRA; each split evenly by intervention group.
Safety and ethics
Ethical and institutional approvals
This trial, including the investigational product, has been reviewed and approved by the Ghana Food and Drug Authority (FDA, clinical trial certificate number FDA/CT/2213), the Ghana Health Service Ethical Review Committee (GHS- ERC) (GHSERC ID: 024/11/21), and the UCD, USA, Institutional Review Board (IRB) Human Subjects Committee (IRB ID: 1837253). The trial was registered on ClinicalTrials.gov (NCT05178407) and the Pan-African Clinical Trial Registry (PACTR202206868437931). Any protocol modifications will be submitted to the FDA, GHS-ERC, and UCD IRB for review, and the trial registries will be updated with approved modifications.
We will seek permission from the Regional (Northern Region) and District (Tolon and Kumbungu) Directors of Health of Services as well as community leaders before recruiting participants into the study. With the assistance of the local health personnel, we will then hold general informational meetings with community leaders of each of the communities in the catchment area about the objectives, methods and possible benefits of the study for the study participants and communities. Additional information regarding the ethical, cultural, and scientific considerations specific to inclusivity in global research is included in the Supporting Information (S4 File).
Safety monitoring
A Data and Safety Monitoring Board (DSMB) will be established for this project. The board will consist of 5 members (3 in Ghana, 2 international) not otherwise involved in this study, without competing interests, and who are experts in child health and nutrition research (with one or more members with expertise in medicine, infectious disease, and/or biostatistics). The DSMB will meet at predefined intervals (with intermediate meetings added if deemed necessary by the committee or investigators) to review study progress (including recruitment, loss to follow up, and protocol compliance) and safety monitoring data. The DSMB may recommend actions or modifications, which will be addressed by the study investigators, who will then notify and/or seek approval from the relevant ethical review committees, as appropriate. Blinded interim analysis of severe adverse advents (SAE) and selected adverse events (AE, such as diarrhea and malaria) will be conducted for safety considerations and reviewed by the DSMB. No interim analyses related to intervention efficacy are pre-planned.
The GHS-ERC, Ghana FDA (which regulates clinicals trials in Ghana), UCD IRB, or the DSMB may request unblinded safety data to discuss in closed sessions. Unblinding of the intervention group may be requested if there is a pattern of SAEs which may be related to study treatment where the DSMB or other regulatory agency feels there is “potential for harm”. In this case, the randomization code will be provided by one of the two code keepers.
Suspected SAEs, such as deaths or hospitalization of study participants, will be reported immediately to the principal investigators, who will notify the GHS-ERC, the Ghana FDA, the UCD IRB, and the DSMB in a timely manner as required by their policies; these agencies and committees will then determine whether modifications are required to ensure participant safety and welfare.
To minimize chance of exposure to COVID-19, we have developed Standard Operating Procedures for our field staff and study participants based on recommendations by the Government of Ghana and the GHS-ERC. A physician assistant will refer participants for medical advice and treatment if they are found to have severe malnutrition (children), severe anemia (children or WRA), malaria infection (children or WRA), hypertension (WRA), or other signs of severe illness. Morbidity symptoms will be monitored at biweekly home visits and participants will be referred for follow up treatment based on symptoms reported during these visits. The cost of the national health insurance annual premium will be covered for all randomized participants.
Participants who become pregnant will be excluded from the study for scientific reasons (effects of pregnancy on micronutrient biomarker concentrations, and lack of validation of the RID method for pregnant women). Although exclusion of study participants due to pregnancy is not based on any safety concerns, we will follow all index participants who become pregnant until the resolution of their pregnancy (miscarriage, still birth, live birth, etc.) via telephone interview to monitor pregnancy outcomes.
Trial management
The trial will be implemented by investigators at the University of Ghana and UCD, who jointly developed the trial protocol and all data collection procedures and instruments. Data collection staff will be hired by the University of Ghana, including supervisors, anthropometrists, phlebotomist and laboratory staff, nurse, medical assistant, enumerators, an information technology manager, data entry clerks, and an inventory manager. Staff will participate in intensive training on research ethics, the study protocol, and data collection procedures, including didactic sessions with accompanying quizzes, demonstrations, and pilot-testing. UCD staff will support the study implementation through in-person supervision and remote data monitoring. Investigators from both universities meet weekly to discuss study progress. As the trial is subject to audit at the discretion of the Ghana FDA, the study investigators have contracted with a Local Monitor who is responsible for conducting Good Clinical Practices training of field staff, assessing and approving site readiness to begin data collection, and conducting regular visits to the study site to monitor study implementation. The Regional (Northern Region) and District (Tolon and Kumbungu Districts) Health Administrations were informed about the project on a regular basis throughout pilot research conducted in 2020–2022, and these interactions will continue through trial implementation and results dissemination.
This study is part of a larger initiative supported by the Bill & Melinda Gates Foundation that is focused on generating evidence to inform discussions of bouillon fortification policy among countries in West Africa. Helen Keller International received support from the Bill & Melinda Gates Foundation for research and policy engagement activities related to bouillon fortification in West Africa (INV-007916), that includes supporting the activities of multi-stakeholder bouillon Country Working Groups in Nigeria, Senegal, and Burkina Faso. Helen Keller International also administers a subaward to UCD for conduct of this trial as well as a portfolio of modelling research focused on estimating the impacts, costs, and cost-effectiveness of micronutrient-fortified bouillon. The larger initiative includes research on technical aspects of bouillon fortification by the Commonwealth Scientific and Industrial Research Organisation (CSIRO) and Research Institutes of Sweden AB (RISE), in collaboration with a consortium of representatives of companies involved in production of bouillon or micronutrient fortificants. These consortium partners developed the non-proprietary unfortified bouillon cube which served as the base formulation for the multiple micronutrient-fortified cubes tested in this trial. All members of this large initiative were given the opportunity to review the study protocol and provide comments. One consortium member (GB Foods) will produce the bouillon cubes used in the trial (with production and shipping costs covered by the research project). The trial investigators selected the micronutrient levels in the investigational bouillon cubes and retain all final decision-making authority related to all aspects of study design, implementation, and dissemination (subject to requirements of the Ghana FDA and ethical committees). Regular updates on study progress will be provided to consortium partners through email and virtual meetings.
Dissemination
The results from this study will be disseminated in national and international conferences and published as Open Access publications in peer-reviewed journals. Authorship will be determined based on international criteria [59]. Dissemination workshops will be held with national stakeholders in Ghana, and with regional and district stakeholders in the study area. Study results will also be disseminated through the network of bouillon-related partners and stakeholders associated with this project, with particular focus on the West Africa region. Specific dissemination activities will be informed by discussions of the trial Dissemination Advisory Group, convened by University of Ghana, UCD, and Helen Keller International, and consisting of representatives from academia and the public sector in Ghana, Burkina Faso, Senegal, and Nigeria. Publications, Statistical Analysis Plans, and other study resources will be posted on Open Science Framework (https://osf.io/t3zrn/).
Trial status
Recruitment began on January 16, 2023. Data collection is expected to be completed in mid-2024.
Discussion
The burden of micronutrient deficiencies remains unacceptably high in many parts of the world. In West Africa, bouillon fortification has the potential to reduce this burden. This study will generate the first information on the effects of micronutrient-fortified bouillon cube on the micronutrient content of human milk and the micronutrient status of women and young children, populations that are vulnerable to micronutrient deficiency. As such, the study serves as “proof of concept” regarding the potential efficacy of micronutrient-fortified bouillon to achieve adequate micronutrient status and reduce the consequences of deficiency. This information will be useful to national, regional and global stakeholders involved in assessing the potential for fortified bouillon cubes to contribute to addressing deficiencies in one or more of the micronutrients examined.
Research and policy discussions related to bouillon fortification must consider the generalizability of study results, with specific attention to the 1) study population diets and other characteristics, 2) micronutrient concentrations in the fortified bouillon, and 3) differences between research and programmatic/commercial contexts for bouillon. The trial is being conducted in a community with frequent bouillon intake and high prevalence of micronutrient deficiency, and the micronutrient concentrations of the cubes tested in this trial were selected as those likely to be both efficacious (i.e., increasing micronutrient status) and safe (i.e. not causing adverse effects) in this context. While these characteristics also apply to many communities throughout West Africa, there is great variation within and across countries in bouillon consumption and micronutrient deficiency [14,16]. Discussions on bouillon fortification standards must consider the impacts on population subgroups with both adequate and inadequate micronutrient intake, and populations with varying bouillon consumption.
Where countries move ahead with bouillon fortification standards (or at the regional level, as cooking oil and wheat flour fortification are implemented in West Africa), decisions around selection of the desired micronutrient concentrations in the standards should use population-specific data on dietary intake as a starting point [29] rather than the levels tested in this trial. Micronutrient concentrations in standards may be different than those tested in this study, and standards may also include micronutrients that were not tested in this study. Modelling of dietary intake or household consumption surveys data can help inform what micronutrients and what concentrations will meet dietary gaps for target population subgroups while minimizing intakes above the UL [60]. Standards must also consider technical and commercial constraints, such as required changes to product formulas and manufacturing processes, premix cost, and the ability to pass along some or all of these costs to bouillon consumers. Moreover, it is important to note that in the trial context the bouillon cubes will be maintained in climate-controlled storage conditions (up to the point of delivery to the household) to ensure acceptability and micronutrient stability of the cubes; these characteristics will have to be confirmed under commercial conditions. Finally, discussions on bouillon fortification policy must also address stakeholder concerns about product formulation, such as the content of sodium or monosodium glutamate [12,61].
In this trial, bouillon is regularly delivered to participant households, free of charge. The household bouillon ration is capped to avoid extra-household sharing and artificially inflating the impact of fortified bouillon if bouillon consumption were to increase due to providing bouillon free of charge to participating households. Nevertheless, in a program setting the effects of fortified bouillon may be diluted if less-expensive, unfortified bouillon products are available or if households reduce their bouillon consumption in response to price increases due to fortification. Moreover, program compliance with standards is a challenge with other types of large-scale food fortification programs [11], and especially for easily imported products, therefore highlighting the need for effective monitoring and evaluation programs to secure the effectiveness of any future bouillon fortification programs.
Given the wide and equitable reach of bouillon in West Africa, including among populations at risk of micronutrient deficiencies who do not consume other fortified foods, addition of micronutrients to bouillon holds great potential to reduce deficiencies. This study will provide evidence regarding the efficacy of bouillon fortification in a specific West African context for improving micronutrient status. This new evidence will inform ongoing discussions on large-scale food and condiment fortification and other strategies to reduce the burden of micronutrient deficiencies and their consequences in the region.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(PDF)
(DOC)
(DOCX)
(DOCX)
Acknowledgments
We gratefully acknowledge the contributions of the local medical monitor Dr. Mohammed Hafiz Kanamu (Tamale Teaching Hospital) and the local study implementation monitors Michael Ntiri and Morrison Asiamah. We thank Dr. Bess Caswell (USDA Western Human Nutrition Research Center) and Boateng Bannerman (University of Ghana, Legon) for assistance with study blinding and Professor Sam Newton (Kwame Nkrumah University of Science and Technology (KNUST)), Dr. Justice Moses K. Aheto (University of Ghana, Legon), Dr. Edward Frongillo (University of South Carolina), Dr. Agartha Ohemeng (University of Ghana, Legon), and Professor Andrew Prendergast (Queen Mary University of London) for serving on the Data and Safety Monitoring Board.
We thank GB foods for producing the trial-specific micronutrient-fortified bouillon cubes for this study, particularly Félix Sancho, Juan Carlos Velasco, and Patricia Lopez. We appreciate the contributions by colleagues at the Commonwealth Scientific and Industrial Research Organisation (CSIRO) and Research Institutes of Sweden (RISE), who, along with members of a consortium, created the model bouillon formulation used in this study and advised on technical issues related to bouillon storage and management.
The Bill & Melinda Gates Foundation Design Analyze Communicate (DAC) team provided helpful comments on an early version of the study protocol and facilitated trial design simulations by Mediana which resulted in the decision to include repeated endline assessments. We further appreciate the technical and overall guidance of Dipika Matthias (PATH), Ann Tarini (independent consultant), and colleagues at Helen Keller International. We acknowledge the UCSF MLK Cores Research Facility for support with sample analysis plans. We also thank Yakubu Adams and Felix Kyereh (University of Ghana) and Dr. Lindsay Allen (USDA) for contributions to study preparations, Lauren Thompson (UC Davis) for assistance with stool collection protocols, and Dr. Michael Green (The Pennsylvania State University), Dr. Anthony Oxley, and Dr. Alice Goddard (Newcastle University) for support with RID assessment. We are deeply grateful to the late Dr. Elizabeth Prado for her guidance and inspiration, especially on the child development assessment, which could not have been undertaken without her.
Abbreviations
- AGP
alpha-1-acid glycoprotein
- BIS
Body iron stores
- BMI
Body mass index
- BP
Blood pressure
- CA/TSC
Citric acid/trisodium citrate
- CoMIT
Condiment Micronutrient Innovation Trial
- CRP
C-reactive protein
- CSIRO
Commonwealth Scientific and Industrial Research Organization
- DSMB
Data and safety monitoring board
- DFE
Dietary folate equivalents
- ERC
Ethical review committee
- EYT
Early Years Toolbox
- FACT
Fortification Assessment Coverage Tool
- FDA
Food and Drugs Authority
- FGD
Focus group discussion
- FFQ
Food frequency questionnaire
- FePP
Ferric pyrophosphate
- GHS
Ghana Health Service
- Hb
Hemoglobin
- HCG
Human chorionic gonadotropin
- Hct
Hematocrit
- HFIAS
Household Food Insecurity Access Scale
- HH
Household
- HOME
Home Observation Measurement of the Environment
- HPLC
High performance liquid chromatography
- HWISE
Household Water Insecurity Experiences
- ID
Identification
- IRB
Institutional Review Board
- KAP
Knowledge Attitudes and Practices
- MDAT
Malawi Development Assessment Tool
- MDD-W
Minimum Dietary Diversity- Women
- MUAC
Mid-upper arm circumference
- OAA
Opinions, attitudes, and acceptance
- PI
Personal identifying information
- RAE
Retinol Activity Equivalents
- RBC
Red blood cell
- RBP
Retinol binding protein 4
- RDA
Recommended dietary allowance
- RDT
Rapid diagnostic test
- RID
Retinol isotope dilution
- RISE
Research Institutes of Sweden Ab
- SAE
Serious Adverse Event
- SAP
Statistical analysis plan
- SES
Socioeconomic status
- SOP
Standard operating procedure
- STEPS
WHO STEPwise Approach to NCD Risk Factor Surveillance
- sTfR
Soluble transferrin receptor
- WRA
Women of Reproductive Age
- UL
Tolerable Upper Intake level
- ZPP
Zinc protoporphyrin.
Data Availability
Consistent with policy of the Bill & Melinda Gates Foundation, data and forms will be made available online at osf.io/t3zrn/ three years after the completion of data collection.
Funding Statement
This work was supported by a grant from Helen Keller International (66504-UCD-01; RES and SAV), through support from the Bill & Melinda Gates Foundation (INV-007916), to the University of California, Davis. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. The funders were given the opportunity to review the study protocol and provide comments. However, the trial investigators made final decisions regarding all aspects of study design and implementation. The funders did not have a role in the decision to publish or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.Stevens GA, Beal T, Mbuya MNN, Luo H, Neufeld LM. Micronutrient deficiencies among preschool-aged children and women of reproductive age worldwide: a pooled analysis of individual-level data from population-representative surveys. Lancet Glob Health. 2022;10(11):e1590–e9. Epub 2022/10/15. doi: 10.1016/S2214-109X(22)00367-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens G, Bennett J, Hennocq Q, Lu Y, De-Regil L, Rogers L, et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. Lancet Glob Health. 2015;3:e528–36. doi: 10.1016/S2214-109X(15)00039-X [DOI] [PubMed] [Google Scholar]
- 3.Rogers LM, Cordero AM, Pfeiffer CM, Hausman DB, Tsang BL, De-Regil LM, et al. Global folate status in women of reproductive age: a systematic review with emphasis on methodological issues. Ann N Y Acad Sci. 2018;1431(1):35–57. Epub 2018/09/22. doi: 10.1111/nyas.13963 ; PubMed Central PMCID: PMC6282622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey RL, West KP Jr., Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66 Suppl 2:22–33. Epub 2015/06/06. doi: 10.1159/000371618 . [DOI] [PubMed] [Google Scholar]
- 5.Alderman H. The economic cost of a poor start to life. Journal of developmental origins of health and disease. 2010;1(1):19–25. Epub 2010/02/01. doi: 10.1017/S2040174409990158 . [DOI] [PubMed] [Google Scholar]
- 6.Global Fortification Data Exchange [16/05/2023]. Available from: http://www.fortificationdata.org.
- 7.Wegmüller R, Bentil H, Wirth JP, Petry N, Tanumihardjo SA, Allen L, et al. Anemia, micronutrient deficiencies, malaria, hemoglobinopathies and malnutrition in young children and non-pregnant women in Ghana: Findings from a national survey. PLoS One. 2020;15(1):e0228258. Epub 2020/01/31. doi: 10.1371/journal.pone.0228258 ; PubMed Central PMCID: PMC6991996 are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of UNICEF. This does not alter our adherence to PLOS ONE policies on sharing data and materials.’. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.University of Ghana, GroundWork, University of Wisconsin-Madison, KEMRI-Wellcome Trust, UNICEF. Ghana Micronutrient Survey 2017. Accra, Ghana: 2017.
- 9.Nyumuah RO, Hoang TC, Amoaful EF, Agble R, Meyer M, Wirth JP, et al. Implementing large-scale food fortification in Ghana: lessons learned. Food Nutr Bull. 2012;33(4 Suppl):S293–300. Epub 2013/03/01. doi: 10.1177/15648265120334S305 . [DOI] [PubMed] [Google Scholar]
- 10.Univ Ghana/GroundWork/Univ Wisconsin-Madison/KEMRI/UNICEF. Ghana Micronutrient Survey [Internet]. UNICEF-Accra, Accra, Ghana [cited 2019-05-21].Available from: http://groundworkhealth.org/wp-content/uploads/2018/06/UoG-GroundWork_2017-GHANA-MICRONUTRIENT-SURVEY_Final_180607.pdf. 2017.
- 11.Osendarp SJM, Martinez H, Garrett GS, Neufeld LM, De-Regil LM, Vossenaar M, et al. Large-Scale Food Fortification and Biofortification in Low- and Middle-Income Countries: A Review of Programs, Trends, Challenges, and Evidence Gaps. Food Nutr Bull. 2018;39(2):315–31. Epub 2018/05/26. doi: 10.1177/0379572118774229 ; PubMed Central PMCID: PMC7473077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthias D, McDonald CM, Archer N, Engle-Stone R. The Role of Multiply-Fortified Table Salt and Bouillon in Food Systems Transformation. Nutrients. 2022;14(5). Epub 2022/03/11. doi: 10.3390/nu14050989 ; PubMed Central PMCID: PMC8912775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Casal MN, Peña-Rosas JP, McLean M, De-Regil LM, Zamora G. Fortification of condiments with micronutrients in public health: from proof of concept to scaling up. Ann N Y Acad Sci. 2016;1379(1):38–47. Epub 2016/08/11. doi: 10.1111/nyas.13185 . [DOI] [PubMed] [Google Scholar]
- 14.Engle-Stone R, Adams KP, Kumordzie SM, Luo H, Wessells K, Adu-Afarwuah S, et al., editors. Analyses using national survey data from Cameroon, Haiti, and Ghana indicate the potential for bouillon fortification to help fill dietary gaps for 5 nutrients2021: Current Developments in Nutrition. [Google Scholar]
- 15.Engle-Stone R, Nankap M, Ndjebayi AO, Brown KH. Simulations based on representative 24-h recall data predict region-specific differences in adequacy of vitamin A intake among Cameroonian women and young children following large-scale fortification of vegetable oil and other potential food vehicles. J Nutr. 2014;144(11):1826–34. doi: 10.3945/jn.114.195354 [DOI] [PubMed] [Google Scholar]
- 16.Hess SY, Brown KH, Sablah M, Engle-Stone R, Aaron GJ, Baker SK. Results of Fortification Rapid Assessment Tool (FRAT) surveys in sub-Saharan Africa and suggestions for future modifications of the survey instrument. Food Nutr Bull. 2013;34(1):21–38. Epub 2013/06/19. doi: 10.1177/156482651303400104 . [DOI] [PubMed] [Google Scholar]
- 17.Kumordzie SK, Adams KP, Arnold CD, Tan X, Vosti SA, Engle-Stone R. Assessment of the potential Impact of bouillon fortification on micronutrient Intakes in Ghana using a household expenditure survey. Submission to Micronutrient Forum 2020 conference:. 2019. [Google Scholar]
- 18.Klassen-Wigger P, Geraets M, Messier MC, Detzel P, Lenoble HP, Barclay DV. Chapter 39 ‐ Micronutrient fortification of bouillon cubes in Central and West Africa. Food Fortification in a Globalized World: Academic Press; 2018. p. 363–72. [Google Scholar]
- 19.Spohrer R, Knowles J, Jallier V, Ndiaye B, Indorf C, Guinot P, et al. Estimation of population iodine intake from iodized salt consumed through bouillon seasoning in Senegal. Ann N Y Acad Sci. 2015;1357:43–52. Epub 2016/01/16. doi: 10.1111/nyas.12963 . [DOI] [PubMed] [Google Scholar]
- 20.Abizari AR, Dold S, Kupka R, Zimmermann MB. More than two-thirds of dietary iodine in children in northern Ghana is obtained from bouillon cubes containing iodized salt. Public Health Nutr. 2017;20(6):1107–13. Epub 2016/12/03. doi: 10.1017/S1368980016003098 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engle-Stone R, Nankap M, Ndjebayi AO, Brown KH. Simulations based on representative 24-h recall data predict region-specific differences in adequacy of vitamin A intake among Cameroonian women and young children following large-scale fortification of vegetable oil and other potential food vehicles. J Nutr. 2014;144(11):1826–34. Epub 2014/10/22. doi: 10.3945/jn.114.195354 . [DOI] [PubMed] [Google Scholar]
- 22.Vosti SA, Kagin J, Engle-Stone R, Luo H, Tarini A, Clermont A, et al. Strategies to achieve adequate vitamin A intake for young children: Options for Cameroon. Ann N Y Acad Sci. 2020;1465(1):161–80. doi: 10.1111/nyas.14275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo H, Stewart CP, Brown KH, Engle-Stone R. Predicted effects of current and potential micronutrient intervention programs on adequacy of iron intake in a national sample of women and young children in Cameroon. Micronutrient Forum; Cancun, Mexico: 2016. [Google Scholar]
- 24.Green MH, Ford JL, Green JB, Berry P, Boddy AV, Oxley A, et al. A Retinol Isotope Dilution Equation Predicts Both Group and Individual Total Body Vitamin A Stores in Adults Based on Data from an Early Postdosing Blood Sample. J Nutr. 2016;146(10):2137–42. Epub 2016/08/12. doi: 10.3945/jn.116.233676 ; PubMed Central PMCID: PMC5037874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Affairs DoEaS. Designing household survey samples: Practical guidelines. New York: United Nations, 2005 Contract No.: ST/ESA/STAT/SER.F/98.
- 26.Friesen VM, Jungjohann S, Mbuya MNN, Harb J, Visram A, Hug J, et al. Fortification Assessment Coverage Toolkit (FACT) Manual. Global Alliance for Improved Nutrition (Geneva) and Oxford Policy Management (Oxford), 2019. [Google Scholar]
- 27.Green MH, Green JB. Use of Model-Based Compartmental Analysis and Theoretical Data to Further Explore Choice of Sampling Time for Assessing Vitamin A Status in Groups and Individual Human Subjects by the Retinol Isotope Dilution Method. J Nutr. 2021;151(7):2068–74. Epub 2021/04/10. doi: 10.1093/jn/nxab061 ; PubMed Central PMCID: PMC8245873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumordzie SM, Davis JN, Adams KP, Tan X, Adu-Afarwuah S, Wessells K, et al. Understanding patterns and drivers of bouillon use in Northern Ghana to inform fortification planning. Curr Dev Nutr. 2021;5:655. [Google Scholar]
- 29.Allen L, de Benoist B, Dary O, Hurrell R, editors. Guidelines on food fortification with micronutrients. Geneva: World Health Organization; 2006. [Google Scholar]
- 30.Hackl L, Cercamondi CI, Zeder C, Wild D, Adelmann H, Zimmermann MB, et al. Cofortification of ferric pyrophosphate and citric acid/trisodium citrate into extruded rice grains doubles iron bioavailability through in situ generation of soluble ferric pyrophosphate citrate complexes. The American journal of clinical nutrition. 2016;103(5):1252–9. Epub 2016/04/08. doi: 10.3945/ajcn.115.128173 . [DOI] [PubMed] [Google Scholar]
- 31.Food and Nutrition Board (FNB) Institute of Medicine (IOM). Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington D.C.: National Academy Press, 2001. [PubMed]
- 32.Global Alliance for Improved Nutrition (GAIN), Ghana Health Service, UNICEF. National Iodine Survey Report Ghana 2015. 2017.
- 33.Wessells K, Kumordzie SM, Becher E, Davis JN, Nyaaba KW, Zyba S, et al. Acceptability of multiple micronutrient-fortified bouillon cubes among women and their households in 2 districts in the Northern Region of Ghana. Curr Dev Nutr. 2023;8(1). doi: 10.1016/j.cdnut.2023.102056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Household Food Access: Indicator Guide (v. 3). Washington, D.C.: FHI 360/FANTA, 2007. [Google Scholar]
- 35.Young SL, Boateng GO, Jamaluddine Z, Miller JD, Frongillo EA, Neilands TB, et al. The Household Water InSecurity Experiences (HWISE) Scale: development and validation of a household water insecurity measure for low-income and middle-income countries. BMJ Glob Health. 2019;4(5):e001750. Epub 2019/10/23. doi: 10.1136/bmjgh-2019-001750 ; PubMed Central PMCID: PMC6768340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Organization WH. STEPS Instrument version 3.2 [Internet]. STEPwise Approach to NCD Risk Factor Surveillance (STEPS) [cited 2022 April 29]. Available from: https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/steps.
- 37.Indicators for assessing infant and young child feeding practices: definitions and measurement methods. Geneva: World Health Organization and United Nations Children’s Fund (UNICEF), 2021.
- 38.Herforth AW, Wiesmann D, Martínez-Steele E, Andrade G, Monteiro CA. Introducing a Suite of Low-Burden Diet Quality Indicators That Reflect Healthy Diet Patterns at Population Level. Curr Dev Nutr. 2020;4(12):nzaa168. Epub 2020/12/22. doi: 10.1093/cdn/nzaa168 ; PubMed Central PMCID: PMC7723758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birch LL, Fisher JO, Grimm-Thomas K, Markey CN, Sawyer R, Johnson SL. Confirmatory factor analysis of the Child Feeding Questionnaire: a measure of parental attitudes, beliefs and practices about child feeding and obesity proneness. Appetite. 2001;36(3):201–10. Epub 2001/05/19. doi: 10.1006/appe.2001.0398 . [DOI] [PubMed] [Google Scholar]
- 40.Gibson RS, Ferguson EL. An interactive 24-hour recall for assessing the adequacy of iron and zinc intakes in developing countries: Washington, DC and Cali, Colombia: International Food Policy Research Institute (IFPRI) and International Center for Tropical Agriculture (CIAT); 2008. [Google Scholar]
- 41.Caswell BL, Arnold CD, Engle-Stone R, Davis JN, Olney DK, Stewart CP. Open Dietary Recall System (OpenDRS): An open-access, tablet-based 24-hour dietary recall tool. in review. 2023. [Google Scholar]
- 42.Cashin K, Oot L. Guide to Anthropometry. A practical tool for program planners, managers, and implementers. Washington, D.C.: Food and Nutrition Technical Assistance III Project (FANTA)/FHI; 360, 2018. [Google Scholar]
- 43.Gladstone M, Lancaster G, Umar E, Nyirenda M, Kayira E, van den Broek N, et al. The Malawi Developmental Assessment Tool (MDAT): The creation, validation, and reliability of a tool to assess child development in rural African settings. PloS Med. 2010;7:e1000273. doi: 10.1371/journal.pmed.1000273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howard SJ, Melhuish E. An Early Years Toolbox for Assessing Early Executive Function, Language, Self-Regulation, and Social Development: Validity, Reliability, and Preliminary Norms. J Psychoeduc Assess. 2017;35(3):255–75. Epub 2017/05/16. doi: 10.1177/0734282916633009 ; PubMed Central PMCID: PMC5424850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caldwell BM, Bradley RH. Home observation for measurement of the environment: administration manual. Family & Human Dynamics Research Institute, Arizona State University, 2003. [Google Scholar]
- 46.International Zinc Nutrition Consultative Group (IZiNCG). Assessment of the risk of zinc deficiency in populations and options for its control. Hotz C, Brown KH, eds. Food and Nutrition Bulletin. 2004;25 (suppl 2):S91–204. [PubMed] [Google Scholar]
- 47.Stoltzfus R, Underwood B. Breast-milk vitamin A as an indicator of vitamin A status of women and infants. Bull World Health Organ. 1995;73:703–11. [PMC free article] [PubMed] [Google Scholar]
- 48.Lucas A, Gibbs JA, Lyster RL, Baum JD. Creamatocrit: simple clinical technique for estimating fat concentration and energy value of human milk. Br Med J. 1978;1(6119):1018–20. Epub 1978/04/22. PubMed Central PMCID: PMC1604026. doi: 10.1136/bmj.1.6119.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr. 2004;134(11):3127–32. Epub 2004/10/30. doi: 10.1093/jn/134.11.3127 . [DOI] [PubMed] [Google Scholar]
- 50.Engle-Stone R, Miller JC, Reario MFD, Arnold CD, Stormer A, Lafuente E, et al. Filipino Children with High Usual Vitamin A Intakes and Exposure to Multiple Sources of Vitamin A Have Elevated Total Body Stores of Vitamin A But Do Not Show Clear Evidence of Vitamin A Toxicity. Curr Dev Nutr. 2022;6(8):nzac115. Epub 2022/09/06. doi: 10.1093/cdn/nzac115 ; PubMed Central PMCID: PMC9429969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bieri J, Tolliver T, Catignani G. Simultaneous determination of alpha-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. The American journal of clinical nutrition. 1979;32(10):2143–9. doi: 10.1093/ajcn/32.10.2143 [DOI] [PubMed] [Google Scholar]
- 52.Turner T, Burri BJ, Jamil KM, Jamil M. The effects of daily consumption of beta-cryptoxanthin-rich tangerines and beta-carotene-rich sweet potatoes on vitamin A and carotenoid concentrations in plasma and breast milk of Bangladeshi women with low vitamin A status in a randomized controlled trial. The American journal of clinical nutrition. 2013;98(5):1200–8. Epub 2013/09/06. doi: 10.3945/ajcn.113.058180 . [DOI] [PubMed] [Google Scholar]
- 53.Killilea DW, Schultz K. Pre-analytical variables influence zinc measurement in blood samples. PLOS ONE. 2023. doi: 10.1371/journal.pone.0286073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfeiffer CM, Zhang M, Lacher DA, Molloy AM, Tamura T, Yetley EA, et al. Comparison of serum and red blood cell folate microbiologic assays for national population surveys. J Nutr. 2011;141(7):1402–9. Epub 2011/05/27. doi: 10.3945/jn.111.141515 ; PubMed Central PMCID: PMC3113292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, et al. Biomarkers of Nutrition for Development (BOND)-Vitamin A Review. J Nutr. 2016;146(9):1816s–48s. Epub 2016/08/12. doi: 10.3945/jn.115.229708 ; PubMed Central PMCID: PMC4997277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–6. doi: 10.1111/j.0006-341x.2000.00645.x [DOI] [PubMed] [Google Scholar]
- 57.Bland JM, Altman DG. Best (but oft forgotten) practices: testing for treatment effects in randomized trials by separate analyses of changes from baseline in each group is a misleading approach. The American journal of clinical nutrition. 2015;102(5):991–4. Epub 2015/09/12. doi: 10.3945/ajcn.115.119768 . [DOI] [PubMed] [Google Scholar]
- 58.O’Connor C, Joffe H. Intercoder reliability in qualitative research: Debates and practical guidelines. International Journal of Qualitative Methods. 2020. doi: 10.1177/1609406919899220 [DOI] [Google Scholar]
- 59.International Committee of Medical Journal Editors. Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals 2022 [cited 2022 May 16]. Available from: https://www.icmje.org/icmje-recommendations.pdf. [PubMed]
- 60.Engle-Stone R, Vosti SA, Luo H, Kagin J, Tarini A, Adams KP, et al. Weighing the risks of high intakes of selected micronutrients compared with the risks of deficiencies. Ann N Y Acad Sci. 2019;1446(81–101). doi: 10.1111/nyas.14128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Archer NS, Cochet-Broch M, Mihnea M, Garrido-Bañuelos G, Lopez-Sanchez P, Lundin L, et al. Sodium reduction in bouillon: Targeting a food staple to reduce hypertension in sub-Saharan africa. Frontiers in Nutrition. 2022;9:746018. doi: 10.3389/fnut.2022.746018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(PDF)
(DOC)
(DOCX)
(DOCX)
Data Availability Statement
Consistent with policy of the Bill & Melinda Gates Foundation, data and forms will be made available online at osf.io/t3zrn/ three years after the completion of data collection.