Abstract
Background:
The National Hemophilia Foundation (NHF) conducted extensive all-stakeholder inherited bleeding disorder (BD) community consultations to inform a blueprint for future research. Sustaining and expanding the specialized and comprehensive Hemophilia Treatment Center care model, to better serve all people with inherited BDs (PWIBD), and increasing equitable access to optimal health emerged as top priorities.
Research Design and Methods:
NHF, with the American Thrombosis and Hemostasis Network (ATHN), convened multidisciplinary expert working groups (WG) to distill priority research initiatives from consultation findings. WG5 was charged with prioritizing health services research (HSR); diversity, equity, and inclusion (DEI); and implementation science (IS) research initiatives to advance community-identified priorities.
Results:
WG5 identified multiple priority research themes and initiatives essential to capitalizing on this potential. Formative studies using qualitative and mixed methods approaches should be conducted to characterize issues and meaningfully investigate interventions. Investment in HSR, DEI and IS education, training, and workforce development are vital.
Conclusions:
An enormous amount of work is required in the areas of HSR, DEI, and IS, which have received inadequate attention in inherited BDs. This research has great potential to evolve the experiences of PWIBD, deliver transformational community-based care, and advance health equity.
Keywords: Community-based, participatory research, diversity, equity, inclusion, Health equity, Health services research, Implementation science, Inherited bleeding disorders, National Hemophilia, Foundation, Public health
PLAIN LANGUAGE SUMMARY
Research into how people get their health care, called health services research, is important to understand if care is being delivered equitably and efficiently. This research figures out how to provide the best care at the lowest cost and finds out if everyone gets equally good care. Diversity and inclusion research focuses on whether all marginalized and minoritized populations (such as a given social standing, race, ethnicity, sex, gender identity, sexuality, age, income, disability status, language, culture, faith, geographic location, or country of birth) receive equitable care. This includes checking whether different populations are all getting the care they need and looking for ways to improve the care. Implementation science studies how to make a potential improvement work in the real world. The improvement could be a new way to diagnose or treat a health condition, a better way to deliver health care or do research, or a strategy to remove barriers preventing specific populations from getting the best available care. The National Hemophilia Foundation focuses on improving the lives of all people with bleeding disorders (BD). They brought BDs doctors, nurses, physical therapists, social workers, professors, and government and industry partners together with people and families living with BDs to discuss research in the areas described above. The group came up with important future research questions to address racism and other biases, and other changes to policies, procedures, and practices to make BD care equitable, efficient, and effective.
1. Introduction
1.1. Historic focus in inherited bleeding disorders research
Inherited bleeding disorders (BD) research has historically focused largely on medical therapies to enhance hemostasis in people with hemophilia A or B [1–4]. Clinical research has improved diagnostic precision [5], management of musculoskeletal sequelae of bleeding episodes [6–8], and prevention and treatment of morbidities caused by virally (e.g. HIV and hepatitis) contaminated therapies [9,10]. Recent therapeutic advances may offer many individuals with hemophilia A and B the potential of a full lifespan, a lifestyle unimpaired by disease complications, and even a cure through gene therapy [3]. However, equitable opportunities to optimize health are not ensured for all people with inherited bleeding disorders (PWIBD). Ultra-rare inherited BDs [11], bleeding that particularly impacts women and other people with the potential to menstruate (PPM) [12], and inherited mucocutaneous BDs (e.g. von Willebrand disease [VWD] and platelet defects) [13] have not been researched to the same extent as hemophilia [14] and are less well understood.
Cost, accessibility, and quality of care available to PWIBD are hugely influential on health outcomes yet remain largely unaddressed. Policies and practices can create or dismantle barriers to optimal care, experiences, and outcomes. Successfully implementing research findings and evidence- based practices into routine care is essential to ensuring advances benefit all PWIBD. Investment in appropriately prioritized research in these areas will be fundamental to improving the experiences and outcomes of all PWIBD, and PWIBD must have a voice in that prioritization [15].
1.2. Health services research
Health services research (HSR) is a multidisciplinary scientific field that investigates how social factors, financing systems, organizational structures/processes, health technologies, and personal behaviors affect access to, and quality and cost of, health care and ultimately, health and well-being [16]. HSR seeks in part to illuminate the mechanisms underlying the US health disadvantage, a phenomenon created by the high cost of health care and lack of adequate access to care for many communities [17,18]. PWIBD face unique health care cost and access challenges, necessitating innovative approaches to address their needs within the imperfect US health care system. Most PWIBD in the US receive care at specialized, multidisciplinary Hemophilia Treatment Centers (HTC) [19] which evolved to achieve optimal outcomes for individuals and families [20]. Through regional networks, HTCs facilitate cooperation with public health agencies including the Health Resources and Services Administration (HRSA) and Centers for Disease Control and Prevention (CDC) [21,22]. HTC comprehensive care teams aim to meet the physical, psychosocial, and behavioral health needs of PWIBD through team-based shared decision-making, and achieve HRSA and CDC clinical care and surveillance goals (Table 1) [20,23]. HSR examining the influence of the HTC model on health outcomes, particularly for the most medically underserved populations, is scarce.
Table 1.
| Core HTC team | |
| • Hematologist | |
| • Nurse coordinator | |
| • Physical therapist | |
| • Social worker | |
| • Laboratory personnel | |
| • Pharmacist | |
| • Data manager | |
| Extended HTC team * | |
| • Orthopedic surgeon | |
| • Dentist or dental hygienist | |
| • Psychologist | |
| • Genetic counselor | |
| • Nutritionist | |
| • Obstetrician/gynecologist | |
| • Infectious disease specialist | |
| • Hepatologist | |
| HTC activities and services † | |
| • Integrated and comprehensive diagnosis and treatment of PWIBD | |
| • Education for PWIBD and their families | |
| • Counseling (e.g. genetic, behavioral health, and financial) | |
| • Surveillance | |
| • Outreach clinics (physical and virtual) | |
| • Outpatient pharmacy services | |
| • Standardized specialized coagulation laboratory | |
| • Standardized specialized blood bank | |
| • Advocacy | |
| • HCP education and training | |
| • Research |
The extended HTC team may include any combination of these professionals, and possibly others
Different HTCs feature different subsets of these activities and services
HCP: health care professional, HTC: Hemophilia Treatment Center, PWIBD: person with an inherited bleeding disorder
The US HTC Network (USHTCN) consists of 144 HTCs [19]. Eight regional core centers disburse HRSA and CDC funds and ensure federal requirement compliance [21,22]. They also facilitate communication and education between HTCs, promulgating policies/procedures, convening ad hoc working groups, and engaging with partners and community-based organizations [23]. Evidence-based clinical practice guidelines on hemophilia care models [24] and treatment guidelines and standards established by the Medical and Scientific Advisory Council (MASAC) [25,26] of the National Hemophilia Foundation (NHF), World Federation of Hemophilia (WFH), International Society on Thrombosis and Haemostasis (ISTH), and American Society of Hematology (ASH) [27–29] promote standardized care delivery across HTCs [21]. The USHTCN fosters research and surveillance in partnership with CDC and the American Thrombosis and Hemostasis Network (ATHN) [30].
While PWIBD receiving care at HTCs generally report a high level of satisfaction [31–33], their numbers are increasing [19,34] and the treatment landscape complexity is intensifying [35,36]. HSR examines access to high quality, affordable care, health inequities, and individuals who may encounter more barriers [20,32,37]. Most HTCs rely on revenue generated through the HRSA US 340B Drug Pricing Program to finance essential staff and services [20,38]. Sustainable funding of the HTC model in the changing treatment paradigm and financial barriers encountered by PWIBD related to high treatment costs, insurance coverage, copay assistance program issues, and out-of-pocket costs constitute important current and imminent challenges that could be better understood through HSR [19,20,34,39].
1.3. Diversity, equity, and inclusion
Defining key terms provides a common foundation to foster diversity, equity, and inclusion (DEI) to strengthen health equity for all PWIBD. Diversity is any dimension that can be used to differentiate groups and people from one another. It means respect for and appreciation of differences. Diversity encompasses the range of similarities and differences each individual brings to society, including but not limited to national origin, language, race, color, disability, ethnicity, gender, age, religion, sexual orientation, gender identity, socioeconomic status, veteran status, and family structures [40]. Equality is achieved when each individual or group of people is given the same resources or opportunities [41]. Equity is achieved when it is recognized that each individual or group of people have different circumstances and resources are allocated accordingly to reach an equal outcome acknowledging that these disparities are rooted in historical and contemporary injustices and disadvantages [41,42]. Equity in health means everyone has a fair and just opportunity to attain their highest level of health. Achieving this requires focused and ongoing societal efforts to address historical and contemporary injustices; overcome economic, social, and other obstacles to health and health care; and eliminate preventable health disparities [43,44]. This definition supports operationalization of the right to the highest standard of health attainable by the most socially advantaged, for all [45]. Inclusion is the creation of environments in which any individual or group can be and feel welcomed, respected, supported, and valued to fully participate, where differences are embraced and respect is offered in words and actions for all people [46]. Racism threatens equity and inclusion. Racism is systemic prejudicial and discriminatory actions based on the idea that one race/ethnicity is superior and other racial/ethnic groups are inferior. It includes the systematic subjugation and oppression of members of certain racial/ethnic groups who have traditionally held less socio-political power to uphold the racial/ethnic majority group’s norms and ideals [47–50]. A legacy of colonization and slavery with profound intergenerational effects on health outcomes, racism exists today as a deeply ingrained aspect of life that reflects norms and practices often perceived as ordinary, constant, and chronic [51–53]. Antiracism is a commitment to confronting and dismantling racism and unearned privilege, with institutional, social, attitudinal, and behavioral dimensions [51,54].
Social determinants of health (SDoH) are non-medical factors that influence health outcomes; the conditions in which people are born, grow, work, live, and age; and the wider set of forces and systems shaping the conditions of daily life. These forces (e.g. racism and climate) and systems include economic policies and systems, development agendas, social norms, social policies, and political systems [55,56]. Determinants that can impact health outcomes such as mortality, morbidity, life expectancy, health care expenditures, health status, and functional limitations fall into six categories (Table 2) [57,58].
Table 2.
| Category | Examples |
|---|---|
|
| |
| Economic stability | |
| • Employment and job characteristics | |
| • Income | |
| • Expenses | |
| • Debt | |
| • Medical bills | |
| • Support | |
| Neighborhood and physical environment | |
| • Housing | |
| • Transportation | |
| • Safety | |
| • Parks | |
| • Playgrounds | |
| • Walkability | |
| • Zip code/geography | |
| Education | |
| • Literacy | |
| • Language | |
| • Early childhood education | |
| • Vocational training | |
| • Higher education | |
| Food | |
| • Hunger | |
| • Access to health options | |
| Community and social context | |
| • Social integration | |
| • Support systems | |
| • Community engagement | |
| • Discrimination stress | |
| Health care system | |
| • Health coverage | |
| • Provider availability | |
| • Provider linguistic and cultural competency | |
| • Quality of care | |
Health disparities are systematic, plausibly avoidable health differences adversely affecting socially disadvantaged groups, reflecting social injustice [43]. Eliminating health disparities, achieving health equity, and attaining health literacy to improve the health and well-being of all are among the US government’s Healthy People 2030 objectives [44]. Implementing steps to address SDoH such as combating structural racism or systemic bias are key to achieving health equity [44,59]. Health equity is also limited by greater socio-historical constraints. It cannot be achieved through health-specific projects alone, particularly for individuals who embody cumulative effects of historic and current marginalization, if mechanisms generating inequities remain unaddressed and unmitigated [60].
The root causes of social drivers that impact PWIBD’s health outcomes are not fully understood [37]; such research constitutes an important first step toward mitigating inequities within the community [39]. It is important to center discussions of data and health care on people and their experiences with health care and science, recognizing their societal context [61]. Negative experiences and collective memory of community trauma (e.g. deaths due to poor health care) impact seeking and accessing effective care [61].
A recent systematic review of the impact of SDoH on inherited BD outcomes suggested an association with bleeding frequency, chronic pain, cost, and quality of life (QoL), and a possible influence on clinical progression [39]. This review underscores the need for multidisciplinary comprehensive care and reduction of economic burden with sustainable population health strategies and treatment options. One study found that living rurally was associated with diagnostic delays and suggested that socioeconomic status and race may influence access to care for PWIBD [37]. Sexism in BDs has resulted in inequity for affected females for centuries [4]. The misconception that hemophilia affects only males, with females strictly asymptomatic carriers, led to decades of marginalization for affected women and girls [62]. Inappropriate application of the ‘carrier’ label can hamper diagnosis, clinical care, and research for females affected by X-linked BDs [63]. Defining hematologic laboratory reference intervals (e.g. in anemia and iron deficiency) differently for Black and White individuals is not uncommon [64], though there is no scientific evidence that these variables differ based on the social constructs of race/ethnicity. Establishing separate thresholds or that ‘normal’ values vary by race validates misperceptions of innate physiological differences between Black and White people (i.e. racialization of biology) [65] and may even have downstream effects on diagnostic and treatment decisions, exacerbating racial health disparities [64].
Research to advance health equity for all PWIBD must ask not if, but how, racism is operating in this space; researchers must be prepared to do the work required to bring about transformational change from personal to systems and policy levels. HSR and implementation science (IS) methodologies hold promise for robust studies that advance health equity work for the inherited BD community.
1.4. Implementation science
Research continues to build the knowledge foundation for effective diagnosis and treatment, and payers increasingly demand evidence-based care. However, timely, successful adoption or implementation of research findings in community settings is far from guaranteed [66]. This prevents individuals from benefiting from research (often federally funded) and may prolong suffering. IS is the study of methods to promote systematic uptake of research findings and evidence-based practices into routine practice [67]. Bridging research evidence and real-world service delivery, IS examines the factors that lead to uptake, scale, and sustainability of practices, programs, and policies to improve outcomes [68]. IS investigates the influences on health care provider and organizational behavior, encompassing clinical, community, and policy contexts [67].
IS can play a key role in promoting DEI. Integrating strategies for equitable implementation of programs, practices, therapeutics, and policies improves their chances of success. These strategies include building trusting relationships with specific communities, dismantling power structures (e.g. between funders/research leaders and community members), centering equity in investments and decision-making, operationalizing community-defined evidence, (cultural) adaptation, and critical evaluation from the perspective of equitable outcomes [68,69].
Much research is still required to fully understand inherited BDs and to determine their optimal management [11–14]. However, clinical practice guidelines have been established for the diagnosis and treatment of hemophilia [27], VWD [28,29], and other BDs [70–73] and for the integrated team- based approach to care central to HTCs [24]. These guidelines present important opportunities to measure whether/how recommended practices may benefit a maximum number of PWIBD. Exciting progress in novel therapies research must be accompanied by advances in the therapeutic valuation framework, care delivery infrastructure, and stakeholder education if groundbreaking treatments are to benefit all [74–77]. As foundational diagnostic and therapeutic research progresses, it may be advantageous to consider hybrid trials, which examine both the effectiveness of a particular intervention and factors influencing successful implementation jointly, rather than separately and sequentially [78].
1.5. Community-identified areas of priority research
NHF, the largest US BD patient advocacy organization, espouses a mission of finding cures and addressing and preventing complications of inherited BDs through research, education, and advocacy, enabling people and families to thrive [79]. To articulate the mission, in 2020 NHF asked the inherited BD community what they need to thrive [80,81], including PWIBD and their families as they are the lived experience experts (LEE) of their disorders [15]. NHF also invited input from clinical specialists, allied health care professionals (HCP), researchers, federal agency partners, and industry representatives. NHF sought diversity of gender, age, race, ethnicity, and urban versus rural living and representation of diverse factor deficiencies, VWD, and platelet dysfunctions.
Consultations revealed the community’s primary emphasis: to sustain the specialized and comprehensive care model provided by HTCs and to expand it to better serve all PWIBD [81]. Concerns about disparities in access across underrepresented populations, affordability and insurance coverage of effective and innovative diagnostics and therapies, psychosocial and behavioral health, pain management, development of the future workforce, and evolution of the HTC model were top among priorities spanning inherited BDs [81].
Meeting community needs will require judiciously designed and prioritized research. In May 2020 NHF initiated a collaboration with ATHN to develop a National Research Blueprint for Inherited Bleeding Disorders [80,81], culminating in the NHF State of the Science Research Summit (SOSRS). Steering Committee (SC) members analyzed the consultation data; six areas emerged. SC members then devised six multidisciplinary expert Working Groups (WG), charging each with distilling community priorities into concrete research questions. WG5 determined the top 3–5 research priorities concerning HSR, DEI, and IS (Figure 1).
Figure 1.
Working Group 5 Diversity, Equity, and Inclusion, Health Services Research, and Implementation Science schematic of community-identified areas for priority research framework
*Marginalized and minoritized populations include people who have been traditionally underserved, excluded, and/or oppressed based on a given social standing or some characteristic including but not limited to race, ethnicity, sex, gender identity, sexuality, age, income, disability status, language, culture, faith, geographic location, and country of birth.
† [82]
BD: bleeding disorder, CDC: (United States) Centers for Disease Control and Prevention, PROM: patient-reported outcome measure, QoL: quality of life, SOC: standard of care, VWD: von Willebrand disease.
We report, herein, WG5’s efforts to identify and prioritize HSR, DEI, and IS research questions with the greatest potential to transform the lives of PWIBD. Cochairs presented preliminary conclusions to the community during the NHF SOSRS (September 12–15, 2021) [80,81]. Summit discussions and attendee questions/comments contributed to the refinement of this report, which we offer to the National Research Blueprint for Inherited Bleeding Disorders [83].
2. Methods
2.1. Working Group 5 composition
Three cochairs were recruited, and together with the SOSRS SC, populated WG5 with the needed diverse expertise (Table 3). LEEs who live with, or care for someone with, an inherited BD [15] were central to the group, which also included health care providers, researchers, representatives from federal agencies and industry, NHF staff, and a local NHF chapter executive. Members’ research specializations included, but were not limited to, health equity, inclusion, and diversity; public health; and social epidemiology and gender minority representation.
Table 3.
Members of Working Group 5.
| Member | Community Role | Affiliation |
|---|---|---|
|
| ||
| Judith R. Baker, DrPH, MHSA (cochair) | Public Health researcher; US Hemophilia Treatment Center Network Regional Administrator | Center for Inherited Blood Disorders; Western States Regional Hemophilia Network, Orange, California |
| Tyler W. Buckner, MD, MSc (cochair) | Hematologist | Department of Medicine, Hematology; Hemophilia and Thrombosis Center, University of Colorado School of Medicine, Aurora, Colorado |
| Vanessa R. Byams, DrPH, MPH (cochair) | Health scientist; Federal agency partner | Division of Blood Disorders, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, Georgia |
| Cindy Bailey PT, DPT, OCS, SCS, ATC | Physical therapist | Los Angeles Orthopedic Hemophilia Treatment Center, Los Angeles, California |
| Nathan T. Connell, MD, MPH | Hematologist | Hematology, Boston Hemophilia Center, Brigham and Women’s Hospital; Harvard Medical School, Boston, Massachusetts |
| Melissa S. Creary, PhD, MPH | LEE; Senior Director, Office of Public Health Initiatives, ATHN; | American Thrombosis and Hemostasis Network (ATHN); University of Michigan School of Public Health, Ann |
| Health, equity, inclusion, and diversity researcher | Arbor, Michigan | |
| Randall G. Curtis, MBA | LEE; Researcher | Hematology Utilization Group Study (HUGS), University of Southern California, Los Angeles, California; Patient Reported Outcomes, Burdens and Experiences (PROBE), Washington, DC |
| Alexis Dinno, ScD, MPH, MEM | Social epidemiology and gender minority representation researcher | Oregon Health & Science University-Portland State University School of Public Health, Portland, Oregon |
| Christine J. Guelcher, RN-BC, MS, PPCNP-BC | Advanced practice provider | Children’s National Hospital, Washington, DC |
| Michelle Kim, Esq. | LEE; NHF Chapter Executive Director | Hemophilia Foundation of Southern California, Pasadena, California |
| Roshni Kulkarni, MD | Pediatric Hematologist | Center for Bleeding and Clotting Disorders, College of Human Medicine, Michigan State University, East Lansing, Michigan |
| Susan Lattimore, RN, GCPH | Regional HTC Director, Nurse Researcher | Mountain States Regional Hemophilia Network; Oregon Health & Science University, Portland, Oregon |
| Kathryn M. McLaughlin†, MPH | Federal agency partner | Division of Services for Children with Special Health Needs, Maternal and Child Health Bureau, Health Resources and Services Administration, Washington, DC |
| Keri L. Norris, PhD, JM, MPH, MCHES | NHF Vice President of Health Equity, Diversity, and Inclusion | NHF, New York, NY |
| Lucy Ramirez, MSW, LCSW | Social worker | Rush Hemophilia and Thrombophilia Treatment Center, Rush University Medical Center, Chicago, Illinois |
| Mark W. Skinner, JD | LEE; researcher | Institute for Policy Advancement, Washington, DC; Health Sciences, McMaster University, Hamilton, Ontario, Canada |
| Susan Symington, MPAS, PA-C, DFAAPA | Industry | Genentech, Phoenix, Arizona |
| Patricia Tobase, PT, DPT, OCS | Physical therapist | University of California San Francisco Hemophilia Treatment Center, San Francisco, California |
| Esmeralda Vázquez‡ | LEE | NHF, Chicago, Illinois |
| Beth B. Warren, MD, MSCS | Pediatric Hematologist | Department of Pediatrics, Hemophilia and Thrombosis Center, University of Colorado Anschutz Medical Campus, Aurora, Colorado |
| Emily Wheat, PhD | Psychologist | Department of Pediatrics, Hematology and Oncology, Hemophilia and Thrombosis Center, University of Colorado School of Medicine, Aurora, Colorado |
NHF SOSRS Advisory Committee
NHF SOSRS Steering Committee
ATHN: American Thrombosis and Hemostasis Network, LEE: lived experience expert, NHF: National Hemophilia Foundation, SOSRS: State of the Science Research Summit, US: United States
2.2. Ways of working
The WG met virtually, weekly March–August 2021, using an electronic video conferencing platform, sharing documents, and using a digital interactive whiteboard as a collaborative space for real-time and offline engagement. NHF SOSRS Advisory and Steering Committee members facilitated coordination of ideas between WGs and alignment with overall National Research Blueprint and SOSRS objectives.
The WG’s first month (March 2021) was dedicated to member orientation, foundational reading, and familiarization with the population/patient problem, intervention, control/comparison, outcome, timing, setting (PICOTS) approach to formulating research questions. Cochairs then broke their mandate into three blocks, which the entire WG studied sequentially together, allocating approximately 1 month to each:
Health services research (April 2021)
Diversity, equity, and inclusion (May 2021)
Implementation science (June 2021)
Questions of public health, digital health, health care delivery networks, communication, and patient-reported outcome measures (PROM) for all PWIBD were interwoven throughout.
Importantly, LEEs were fully engaged as equal participants in WG activities. Their lived experiences and perspectives were actively sought by the cochairs. All WG members contributed to fostering an environment of mutual respect and learning. An NHF staff accompanied LEEs throughout the process with individual and group consultations designed to empower and enable participation. The WG continuously adapted its approach based on feedback from members and cochair consensus. Members shared statements of personal positionality, creating a learning community in which they challenged themselves and their assumptions, to frame and address difficult questions. They returned frequently to community input, gathered by the NHF in the consultative stages, to re-center discussions on expressed needs and priorities.
Cochairs recruited experts to deepen WG5’s collectively broad knowledge of HSR, DEI, and IS. Each subject block began with an invited expert presentation to establish a common language and foundational understanding, describing the scope and types of research carried out with illustrative examples (Table 4). Michael Nichol, PhD, delivered the expert presentation on how HSR might be applied to questions of access to health care and services, quality of care, costs, satisfaction, and clinical and health-related QoL (HRQoL) outcomes (e.g. PROMs development) in inherited BDs. A review of foundational resources and the expert presentation of Melissa S. Creary, PhD, MPH initiated discussions regarding DEI in inherited BDs. The expert presentation by Brian Mittman, PhD, established the foundation for WG5’s discussions of how to use IS in the arena of inherited BDs research.
Table 4.
Summary of expert presentations and discussion themes for each WG5 subject work block.
| Health services research | |
| Speaker | Michael Nichol, PhD |
| Affiliation | Professor, Health PolicyUniversity of Southern California, Sol Price School of Public PolicyPI, Hematology Utilization Group Studies www.hugsresearch.net |
| Fields of expertise | Health policy, health care cost and resource utilization, health state preference assessment, cost effectiveness |
| Presentation focus | Health care access, costs, quality, outcomes as they pertain to patients, providers, institutions, insurers, policies |
| Discussion prompts | Access, cost effectiveness, outcomes, health care delivery models, global perspectives, pain, mental and behavioral health, etc. |
| # Questions generated | Approx. 85 |
| Diversity, equity, and inclusion | |
| Speaker | Melissa Crearv, PhD, MPH |
| Affiliation | Senior Director, Office of Public Health InitiativesAmerican Thrombosis and Hemostasis Network Assistant Professor, University of Michigan School of Public Health |
| Fields of expertise Presentation focus | Intersection of public health, science and technology studies, medical anthropology Centering DEI, health equity, and antiracism in BDs research |
| Discussion prompts | Application of antiracist lens: How do racism, sexism, phobias operate in data collection, clinical care, community engagement, policies/ systems? What must be challenged? What structural forces contribute to inequities? |
| # Questions generated | 13 |
| Implementation science | |
| Speaker | Brian Mittman, PhD |
| Affiliation | Research Scientist, Kaiser Permanente Division of Health Services Research and Implementation Science U.S. Department of Veterans Affairs, University of Southern California, and the University of California, Los Angeles |
| Fields of expertise Presentation focus | Implementation science, innovative approaches to health care delivery and improvement Applying implementation science in BDs research |
| Discussion prompts | How to apply IS methods to HSR? How does IS intersect with DEI research? How to apply IS to other WG topics and NHF SOSRS patient input? |
| # Questions generated | 27 |
BD: bleeding disorder, DEI: diversity, equity, and inclusion, HSR: health services research, IS: implementation science, NHF: National Hemophilia Foundation, PI: principal investigator, SOSRS: State of the Science Research Summit, WG: working group
WG members shared what they initially learned from the expert presentations and accompanying discussions via the digital whiteboard. An iterative process of in-depth discussion, questioning, and reflection evolved these into draft research questions. Following completion of the three individual work blocks, research questions were further refined and scored (see below).
2.3. Feasibility-impact-risk scoring
NHF tasked each WG with scoring actionable research questions derived from community-identified priorities in their respective fields, on feasibility, impact, and risk, using a predefined matrix of criteria (Supplementary Table S1) [80,81,84]. Totaling scores of the three dimensions yielded a single overall question score, for comparative purposes. A potentially very impactful question with little risk but challenging methodology could score as highly as a question that is easily addressed but might not be expected to have as great an impact on the target population.
Following WG discussions of the feasibility, impact, and risk of each set of questions, all members were given the opportunity to score all questions, with the cochairs scoring all questions on all criteria. The average, minimum, and maximum total score and average score for each dimension were then calculated, and further discussed with the whole WG. Application of the scoring matrix (Suppl Table S1) to the questions was challenging. The most urgent research needs were in some cases broad; the depth or foundational nature of the work required could not always be meaningfully characterized within the scoring criteria provided, especially within the realm of DEI. The WG, therefore, worked from the scored lists to generate consensus lists of five to six questions for each subject reflecting prominent themes, considering feasibility, impact, and risk, but without assigning numerical scores.
3. Results
3.1. Health services research
The WG drafted 85 HSR questions addressing access to health care and services, quality of care, costs, satisfaction, and clinical and HRQoL outcomes, following the PICOTS approach, and further refined them to 42 presented and scored in Suppl Table S2. Through an in-depth discussion and feasibility-impact-risk scoring of these 42 questions, WG5 arrived at 5 priority HSR questions (Table 5).
Table 5.
Priority research questions in the areas of health services research; diversity, equity, and inclusion; and implementation science.
| Priority# | Question |
|---|---|
|
| |
| Health services research | |
| 1 | Among PWIBD and their caregivers, do those who use telehealth visits have the same quality of care, satisfaction, adherence to treatment, and clinical |
| and HRQoL outcomes as patients who are primarily treated in person? | |
| 2 | Do behavioral health support services* beyond psychosocial assessments completed as a part of annual comprehensive care visits improve the patient experience and clinical and HRQoL outcomes? Do these services affect direct and/or indirect costs for inherited BD diagnosis and illness management? |
| 3 | How do the type and characteristics of insurance coverage affect patient access to HTC care, treatment, bleeding, and other clinical and HRQoL outcomes? |
| 4 | In PWIBD, how do care, treatment, and clinical and HRQoL outcomes of those receiving specialized/integrated care at HTCs compare to those outside the HTCs? |
| 5 | How do provision of care components (e.g. access to care, patient satisfaction, and treatment) and clinical and HRQoL outcomes of PWIBD from marginalized and minoritized populations** compare to PWIBD who do not identify as part of these populations? |
| Diversity, equity, and inclusion | |
| 1 | What conscious and unconscious biases are present within HTCs and among the multidisciplinary care team? What interventions can be implemented to mitigate identified biases? |
| 2 | What policies/processes/practices can local and national patient organizations and other stakeholders implement to ensure active partnership with marginalized and minoritized populations in the development and implementation of data collection (e.g. registries, surveillance, clinical trials, research, repositories) and educational initiatives? |
| 3 | What data are viewed as important and meaningful to members of marginalized and minoritized populations that are not currently being collected by clinical trials/surveillance studies? |
| 4 | What are inequities in experiences of care between non-marginalized and marginalized populations in the HTC setting? (Conduct needs assessment to identify the problem.) |
| 5 | What are barriers and facilitators for marginalized and minoritized populations to substantively engage in the conceptualization, design, and conduct of research and to participate in research? |
| 6 | What programs and/or pilot projects should be implemented to mitigate individual provider behaviors, clinical and organizational structures, and other systems in place that hinder equitable delivery of care and services? |
| Implementation science | |
| 1 | For any given evidence-based treatment/intervention, what elements need to be adapted to enhance fit and cultural relevance to a specific subpopulation (e.g. gender, language, marginalized and minoritized populations, etc.) or setting? |
| 2 | In HTCs caring for people with severe hemophilia, what practices, programs, or center characteristics lead to adherence to the prescribed treatment plan? What are factors that lead to concordance between PWIBD and HCP? |
| 3 | What data-driven benchmarks and requirements for implementation of, and adherence to, VWD and hemophilia guidelines in health systems (starting with the USHTCN) could be developed and monitored, which also include markers of outcomes specific to marginalized and minoritized populations? |
| 4 | In HTCs caring for PWIBD, what characteristics increase the adoption of evidence-based behavioral health support services and interventions for PWIBD? |
| 5 | What organizational factors, within HTCs or the institutions with which they are affiliated, promote or hinder health equity and antiracism within an organization/institution (e.g. organizational climate and culture; internal policies supporting equity; employee and leader attitudes and motivations regarding equity)? |
Behavioral health support services are any service that aims to support mental health, well-being/QoL, pain management, and healthy lifestyles
Marginalized and minoritized populations include people who have been traditionally underserved, excluded, and/or oppressed based on a given social standing or some characteristic including but not limited to race, ethnicity, sex, gender identity, sexuality, age, income, disability status, language, culture, faith, geographic location, and country of birth
BD: bleeding disorder, HCP: health care professional, HRQoL: health-related quality of life, HTC: Hemophilia Treatment Center, PWIBD: person with an inherited bleeding disorder, QoL: quality of life, USHTCN: United States Hemophilia Treatment Center Network, VWD: von Willebrand disease
WG5 confirmed the need for HSR investments for the inherited BD community; five priority themes provide direction (Figure 2). First, research is needed to characterize telehealth’s impact on access to care, examining its influence on treatment adherence, patient outcomes, and satisfaction [31,37]. Second, HSR can help measure health disparity trends in marginalized and minoritized populations, investigating the reach of guideline informed care into groups that have been marginalized. The WG defined ‘marginalized and minoritized populations’, as including ‘people who have been traditionally underserved, excluded, and/ or oppressed based on a given social standing or some characteristic including but not limited to race, ethnicity, sex, gender identity, sexuality, age, income, disability status, language, culture, faith, geographic location, and country of birth’. Whether Healthy People 2020 Objective [85–88] attainment in joint disease, school absenteeism, high school graduation, and timely VWD diagnosis is consistent among marginalized and minoritized HTC patient populations constitutes one example of such a study. The value of the HTC specialized care model can be examined via HSR. HSR can illuminate whether management in (versus outside) an HTC improves delivery and postpartum bleeding outcomes for pregnant people with VWD or improves outcomes for transgender PWIBD undergoing gender-affirming surgery.
Figure 2.
Priority research themes identified by WG5 in the area of health services research.
*Marginalized and minoritized populations include people who have been traditionally underserved, excluded, and/or oppressed based on a given social standing or some characteristic including but not limited to race, ethnicity, sex, gender identity, sexuality, age, income, disability status, language, culture, faith, geographic location, and country of birth. **Behavioral health support services are any service that aims to support mental health, well-being/QoL, pain management, and healthy lifestyles.
HSR: health services research, HTC: Hemophilia Treatment Center, QoL: quality of life, WG: working group.
Third, HSR can advance our understanding of the criticality of behavioral, mental health and pain management services for PWIBD, topics prioritized in community consultations [80,81]. WG5 defined ‘behavioral health support services’ as any service that aims to support mental health, well-being /QoL, pain management, and healthy lifestyles. HSR can determine whether behavioral health support services improve experiences and outcomes of PWIBD and/or direct and indirect costs associated with BDs. Next, HSR can examine how insurance characteristics impact access to care and outcomes [89]. For example, examining whether PWIBD who have insurance that restricts access to federally funded HTCs are more likely to have poorer outcomes compared to insurance plans that do not delay, deny, or modify HTC access or treatment plans. HSR not only characterizes problems articulated in the five themes but also tests potential solutions.
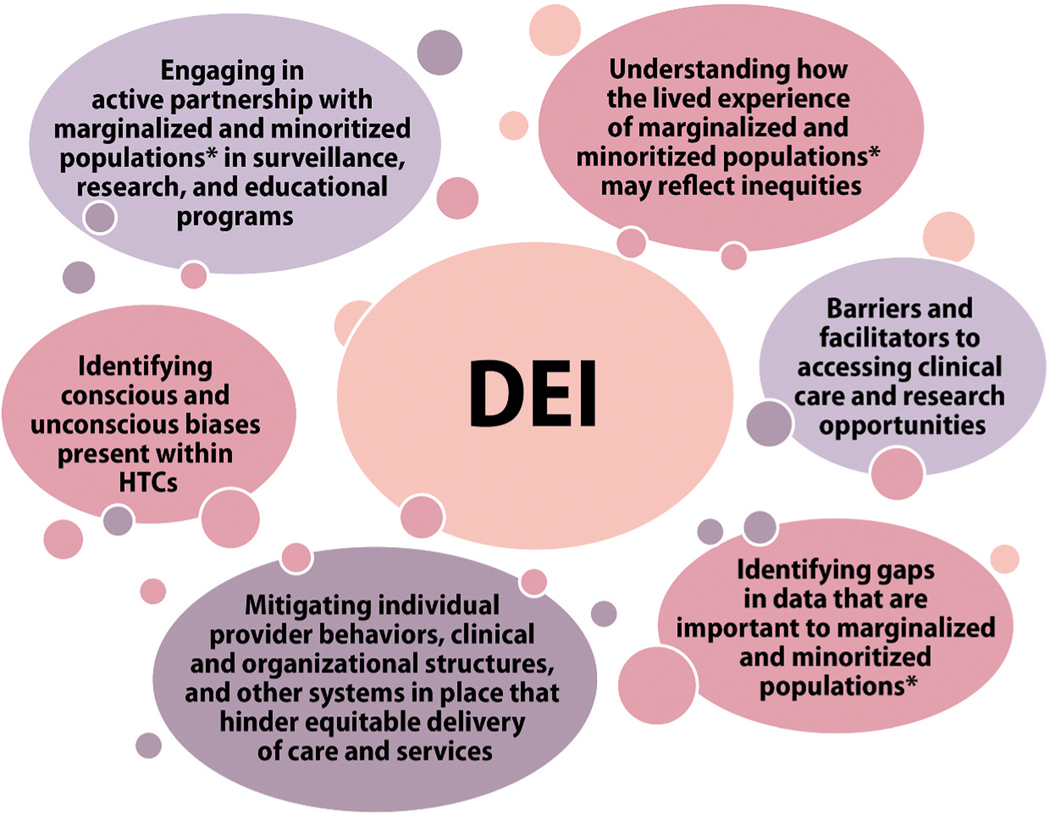
3.2. Diversity, equity, and inclusion
Discussions regarding DEI in inherited BDs research coalesced around several themes (Table 4, Figure 3), yielding 13 scored priority research questions (Suppl Table S3). The importance of recognizing how entrenched racism is in the US and the resulting need to engage in systems-based solutions that encourage everyone to question, unlearn, and relearn how to integrate concepts of DEI and antiracism into research with the inherited BD community were highlighted. WG5 reflections on how racism, sexism, and other phobias operate within the practices of data collection, clinical care, engagement with the community, and at the policy and systems level revealed many examples of issues/forces contributing to inequities. Research investment in these issues can help accelerate their mitigation.
Figure 3.
Priority research themes identified by WG5 in the area of diversity, equity, and inclusion.
*Marginalized and minoritized populations include people who have been traditionally underserved, excluded, and/or oppressed based on a given social standing or some characteristic including but not limited to race, ethnicity, sex, gender identity, sexuality, age, income, disability status, language, culture, faith, geographic location, and country of birth.
DEI: diversity, equity, and inclusion, HTC: Hemophilia Treatment Center, WG: working group.
Research into understanding lived experiences of marginalized and minoritized populations can help devise effective interventions to enhance access to care. National data document that a large majority of PWIBD receiving care at HTCs report a high level of overall satisfaction, with a minority suggesting opportunities for improvement (e.g. sexual health information) [31–33]. The WG identified conducting a needs assessment to hear directly from marginalized and minoritized populations, who have reported more barriers [20,32], regarding their experience of care and services within HTCs as an important first step. Even more challenging is assessing the needs of PWIBD who do not/cannot access care at HTCs. Identifying barriers and facilitators to accessing clinical care and research opportunities for these populations emerged as an important theme. Identifying conscious and unconscious biases present in HTCs, at the level of individual multidisciplinary care team HCPs, and at organizational and institutional levels, emerged as a very high priority. Research into programs and interventions that may best mitigate individual HCP behaviors, clinical and organizational structures, and other systems that hinder equitable delivery of care and services must parallel these identification efforts. Engaging in active partnerships with marginalized and minoritized populations in surveillance, education, and research programs was another recurring theme. All members of the inherited BD community have valuable contributions to make to prioritization of research questions, designing research approaches, and engagement with research participants. Including excluded populations is essential.
WG5 distilled 13 initial questions (Suppl Table S2) into 6 top-priority research questions (Table 5).
3.3. Implementation science
WG5 discussed how IS can foster HSR, how it intersects with DEI research, and how IS might advance research priorities proposed by WGs 1–4 [11–14]. Integrating IS methods and outcomes assessments [90,91] into clinical study designs going forward will be key to building a body of evidence upon which to base, disseminate, and implement best practices. Hybrid qualitative–quantitative study designs, which include implementation outcomes [78] will need to be encouraged, supported, and required to identify factors key to successful uptake and sustained use of interventions, in real-world clinical practice. WG5 formed and scored 27 actionable IS research questions (Suppl Table S4) grouped into 5 themes (Figure 4).
Figure 4.
Priority research themes identified by WG5 in the area of implementation science. HTC: Hemophilia Treatment Center, IS: implementation science, WG: working group.
Detailed discussions of the 27 questions, feasibility-impact-risk scores, and themes (Figure 4) resulted in the prioritization of five research questions (Table 5). As evidence-based guidelines have been developed for the diagnosis and management of VWD [28,29] and hemophilia [27] and for the integrated team-based approach to care [24], IS methods offer structured practices to advance optimal dissemination and adoption of these guidelines in all care environments. Identification of implementation monitoring strategies and milestones specific to marginalized and minoritized populations deserves particular attention. Given the central role of the HTC in the care of many PWIBD, identification of HTC characteristics associated with higher rates of concordance between PWIBD and HCPs on therapeutic goals and evidence-based treatment regimens would be expected to generate useful and translatable learnings. Prioritization of identification of elements that increase adoption of evidence-based behavioral health support services (defined above) and interventions in HTCs reflects the high importance placed on mental health and pain outcomes by LEEs in community consultations [80,81]. Studies that identify how best to adapt any evidence-based treatment or intervention to enhance cultural fit or relevance to a specific population or setting are of particular interest for their potential to expand access to the benefits of research advances. WG5 noted promise in capitalizing on IS research to address health equity within organizations/institutions.
4. Discussion
Research focused on health services; diversity, equity, inclusion, and antiracism; and implementation science for the inherited BD community has received limited support and attention. Investing in and advancing these fields significantly aids the pursuit of health equity for all PWIBD; the potential impact of this work is profound. Without a deep understanding of how resource utilization impacts PWIBD, which health care delivery models enable PWIBD to thrive, and how best to organize an equitable and just approach to putting new therapies into practice, the inherited BD community will struggle to realize its full health potential. Furthermore, sustaining the HTC network, a critically important health care delivery model that is a key community priority, will remain in jeopardy.
To reach these goals, much foundational work is required, including formative studies using qualitative or mixed methods approaches to characterize and understand issues such that meaningful investigations of specific interventions can be designed. Despite the challenges of assigning numerical values to the feasibility, impact, and risk of the research priorities identified in these subject areas, WG5 members were confident that the priorities identified were essential next steps toward achieving the goals set by community members.
4.1. Limitations
The research required to address community-identified priorities is broad. Application of the prescribed feasibility-impact-risk scoring matrix to prioritize HSR, IS, and especially DEI questions proved problematic. The same scoring criteria were provided to WGs 1–5 [80,81,84] and may have been better suited to some of the very precise research questions prioritized by WGs 1–4 [11–14]. Rather than creating individual research questions focused on specific populations or settings, WG5 found that the process of distilling the community input lent itself better to formulating themes to be prioritized for future investigation. The research priorities thus identified were often formative in nature, requiring qualitative or mixed methods approaches, refractive to PICOTS question formulation, and could not be characterized using the predetermined scoring questions (Suppl Table S1, e.g. the availability of resources available to carry out a particular investigation). Nor were the resulting single summed scores appropriate for comparative prioritization. The WG chose, therefore, to report five to six questions that typified the priority research themes yielded by their discussions in each field (Table 5). An alternative, albeit more time consuming, methodology such as a Delphi consensus process might be an interesting approach for any future similar endeavor.
While some WG5 members were experts in the theory and/or practice of HSR, DEI, and/or IS, many were not. This necessitated the dedication of considerable time to foundational education in each subject work block, which all members undertook with great enthusiasm and commitment. WG5’s varied levels of expertise in HSR, DEI, and/or IS, combined with the compressed timeline to complete the charge, leads them to respectfully acknowledge that much work remains in prioritizing research in these three areas for the inherited BD community.
4.2. Education to empower the community
HSR, DEI, and IS research are unfamiliar topics to many in the inherited BD community; educational initiatives are needed to build a foundation for the transformative potential of this research. Much work is needed to educate PWIBD, clinicians, researchers, advocacy organizations, and other stakeholders. Future initiatives should seek out and engage experts in the three fields, including those outside the inherited BD community. Mobilizing partnerships in these new fields will be mutually beneficial: enhancing the visibility of HSR, DEI, and IS research and introducing the unique contributions these methodologies bring to inherited BD population health improvements. Explaining to the inherited BD community the types of questions HSR, DEI, and IS each investigate; how they can answer priority inherited BD research questions; and the precision the three fields can bring to our investigation of health care systems and access to care services can cumulatively transform existing work to improve options for PWIBD. Substantive investments in conducting HSR, DEI, and IS research, including training in research and communication methods for health equity across the workforce, are warranted to improve the quality of, and access to, care for the entire inherited BD community in an efficient, equitable, and just way.
4.3. Health services research
HTC multidisciplinary comprehensive care teams aim to achieve optimal physical, psychosocial, and behavioral health outcomes for PWIBD and their families at the lowest overall cost of care utilizing shared decision-making with LEEs [15] as empowered partners [20]. Community consultations prioritized securing and expanding the HTC care model, with the goal of achieving comparable outcomes for a wide array of inherited BDs beyond hemophilia [3,81]. This will require advances in our understanding of community needs and experiences, in the financing of HTCs and care, and in the workforce. The backdrop of a shifting hemophilia treatment landscape [35,36] which could potentially impact revenue streams such as the 340B Program [38], and increasingly diverse demands on HTCs [19,34], amplifies the urgency of capitalizing on HSR for innovative solutions.
WG5 promotes characterizing the interactions of the whole inherited BD community with HTCs to understand their experiences and their needs. They envision analyzing the number/frequency of comprehensive care visits, the complement of HCPs and services PWIBD need, health outcomes, and total cost of care, against demographic and clinical details to better understand the differential impacts of these factors in marginalized and minoritized populations. Collecting these data begins with identifying the outcome measures that best assess person-centered care for these populations.
Two inherited BD HSR-focused research consortia illuminate national and international initiatives. The Hematology Utilization Group Study (HUGS) established an important set of standards for the characterization of economic burden, health outcomes, and QoL among men with hemophilia at HTCs in the US [92–99], and recently expanded its investigation to VWD and sickle cell disease [100–102]. The patient-led Patient Reported Outcomes, Burdens and Experiences (PROBE) initiative optimized a hemophilia PRO survey, validated it in different populations including cross-cultural contexts and international implementation, used it to characterize various elements of well-being, integrated it with several registries, and operationalized data collection via an online survey and mobile device app [103–110]. The ambitious data collection envisaged by WG5 should build on the solid foundation established by these two initiatives, and benefit from the implementation of the principles for the future of HSR established by the National Academy of Medicine [111].
WG5 is conscious that not all PWIBD can [98] or wish to receive their care at HTCs. Understanding and meeting the needs of these populations is also important and challenging. Here too, the first step is using HSR methods to robustly document their experiences, quality of care, and outcomes and to identify elements most associated with better/worse outcomes and possible ameliorating interventions.
Complete robust data are essential to effectively advocate to change policy to strengthen systems of care for PWIBD, and monitor changes implemented [112]. Securing and evolving the HTC model, a clearly stated community priority [81], is reliant on reducing workforce shortages so HTCs have capacity to deliver tailored care to all PWIBD. HSR methods could evaluate the impact of policy changes aimed at expanding the workforce, such as designating non- neoplastic hematology as a specialty shortage area, thereby opening existing incentives to build the blood disorders workforce [113].
Cost-effectiveness demonstrations may provide evidence that supports equal access to the USHTCN for all PWIBD [20,89] and policies that set optimal reimbursement rates for the HTC team-based model of ambulatory care. The Centers for Medicare and Medicaid Services Innovation Center (CMMI) recently released a Strategic Refresh [114]; its five objectives align closely with WG5’s three foci, potentially opening opportunities for rare disorders research collaborations.
Behavioral and mental health and pain management also often feature atop community priority lists [81], so they must be top priorities of their HTC team, either addressing these topics themselves or connecting PWIBD with the specialist care that they require. Advances in the management of bleeding create an opportunity to shift some focus to integrating advanced behavioral research into HTCs. Adopting gold standard methodology and outcome measures designed specifically to assess elements of psychological and psychosocial health, to study issues of pain management or mental health, and to investigate adherence in the context of novel therapies would advance top community priorities currently largely unexplored, with the potential to impact a growing number of PWIBD in the near future.
4.4. Diversity, equity, and inclusion
In addition to the inherited BD community [26], advancing health equity [43] is a current priority across health care in the United States. Health equity is an objective of the US government’s Healthy People 2030 initiative [44], a pillar of the CMMI Strategy Refresh [114], the focus of CDC’s Core Health Equity Science and Intervention Strategy [115], and one of the critical areas driving the Blueprint for Change for the system of services for children and youth with special health care needs and their families developed by HRSA’s Maternal and Child Health Bureau [116]. Efforts to advance health equity for all PWIBD must partner with these initiatives and capitalize upon the resources they offer.
It is essential – within inherited BD research, diagnosis, and care spaces – to identify how racism, sexism, and other phobias exist, operate, and intersect, at the individual and institutional levels, and how their pervasive presence in society prevents marginalized and minoritized BD populations from achieving their optimal health potential. Multiple intersecting socially disadvantaged identities (based on race, gender identity, sexuality, disability, etc.) must also be considered as the health disparities for these populations are often compounded [117]. Some PWIBD feel a sense of ‘otherness’ or stigma due to their BD [118,119]; socially disadvantaged PWIBD may experience further isolation. The necessary DEI conversations and initiatives require all individuals to think critically and challenge their own assumptions. These conversations and initiatives must be accompanied by concrete actions to mitigate bias, implicit or otherwise, to develop cultural competencies and humility as part of patient-centered care [120], to dismantle policies and practices that propagate inequities, and to integrate antiracist policies and practices [53,121].
Community-based and professional organizations are also strengthening their commitment to health equity. NHF’s recently updated guidelines and recommendations for HTCs outline expectations for the culturally and linguistically appropriate delivery of care and education [26]. The inherited BD community should be able to count on the support of professional associations such as ASH, the American Public Health Association (APHA), and the American Medical Association (AMA), all of whom espouse the operationalization of health equity and elimination of health disparities [122–124]. The national initiatives highlighted above champion a range of data collection, modeling, education, accountability, and outreach strategies and tactics [44,114,116]. These efforts require support from all levels of management and the community.
Social determinants of health (SDoH), such as financial or food insecurity, joblessness and insurance coverage issues, precarious housing, or transportation challenges, all have an enormous impact on the opportunity for an individual to achieve optimal health [57]. HTCs and advocacy organizations, like NHF, must establish and steward relationships with other community organizations, connecting PWIBD to the supports they need to clear these hurdles to thriving with their BD.
Research into SDoH and their impact on the health outcomes of PWIBD, currently poorly understood, is essential to mitigating inequities within the community and enabling all PWIBD to thrive [37,39]. This requires defining and collecting data that measure SDoH and meaningful markers of health equity from populations that represent the true diversity of the inherited BD community. A recent hemophilia model integrates PROM and medical milestones in a path to health equity that it posits is achievable [3].
Expansion of this model to account for SDoH and extend it to other inherited BDs would be a substantial advance. Analysis of the cohort of women and girls in the ATHNdataset (data volunteered by LEEs and collected via ATHN affiliates) found a lack of women/girls-specific data elements, including underreporting of reproductive tract bleeding and its treatment [125]. Efforts to create more women/girls-specific data entry elements are ongoing. CDC’s Community Counts Public Health Surveillance of Bleeding Disorders project includes data collection on some female-specific outcomes such as the use of prophylaxis treatment for menstrual bleeding and history of hysterectomy [126]. Community Counts data collection also includes sex, race/ethnicity, 3-digit zip code, and insurance status [127]. A more complete collection and analysis of SDoH data could benefit from applying the National Institutes of Health (NIH) National Institute on Minority Health and Health Disparities recommendations for the standardization of measures for studying health disparities [128], combined with a minority oversampling technique [129]. This approach may improve predictive accuracy when analyzing a rare outcome in health disparity studies [130].
Participation of a truly representative diversity of PWIBD throughout the research process will improve its rigor and foster more accurate results. While marginalized and minoritized populations express medical distrust, some as a result of remembered historical grave mistreatment exacerbated by current day negative experiences [53,131,132], there is evidence that race concordance and emphasizing researchers’ respect and value of participants may encourage enrollment in research despite this mistrust [133]. Initiatives such as pre-trial multistakeholder engagement, training diverse HCPs in recruitment, and providing multilingual study materials may improve retention of these populations [134]. Many PWIBD from a marginalized and minoritized population accessing care in HTCs likely know other members of their community who do not. Building a relationship of trust and respect may encourage them to open doors to the community for HCPs and researchers and to invite members of their community to explore opportunities with the HTC.
Community-based participatory research builds upon a foundation of respectful and trusting inclusive relationships between community and researchers, to design and conduct studies as equal partners [135,136]. Recognizing the strength of each partner, they collaborate on all aspects such as needs assessment, planning, research intervention design, implementation, evaluation, and dissemination of community-level interventions [137]. These studies may not adhere to a typical timeline, budget, or protocol envisaged for a traditional research study. Flexibility and adaptability from potential partners are needed to support the integration of this community-centered research methodology.
Equitable hiring and career progression practices constitute an important opportunity to develop an inherited BD workforce that reflects the community it serves. Initiatives such as annual mandatory implicit bias mitigation training of decision makers can positively impact the diversity of the workforce [121]. Fellowships and training programs targeting HCPs from marginalized and minoritized populations [138,139], including several in the fields of hematology and inherited BDs (e.g. offered by NHF and ASH), tend to focus on clinicians and medical students [140,141]. These initiatives should be expanded to include all comprehensive care team professionals at all career stages, and their numbers increased.
HSR and IS offer important methodologies and frameworks with which to assess and address the context in which inherited BDs care and research are carried out. DEI and health equity for PWIBD can only be improved by harnessing both of these areas of research.
4.5. Implementation science
Exciting innovations in inherited BDs are being pursued in research laboratories and increasingly making their way to the clinic [142–144]. Translating clinical trial evidence of the efficacy and safety of an intervention into clinical practice and widespread benefit, however, will only succeed if it is informed by the real-world context of PWIBD and the systems in which they receive care [145]. This requires observational and clinical studies that examine the barriers and facilitators to uptake and implementation, designed and executed in collaboration with a diverse and inclusive community. From elucidating and amplifying the characteristics of HTCs associated with greater concordance between HCPs and PWIBD on guideline-recommended management plans and the adoption of evidence-based behavioral health support services, to the identification and dismantling of barriers to antiracist practices and equitable access to evidence-based care, IS offers powerful tools and insights.
Tailoring and adaptation of implementation initiatives in complex care, with its multicomponent multidisciplinary coordinated nature, must be the expectation, not the exception [146], without compromising the elements essential to its effectiveness such that all populations may benefit maximally. This requires the identification of the core functions of an intervention and the forms or strategies of its implementation that can be adapted to specific contexts and need not be incompatible with study designs powered to return significant results including randomized controlled trials [147,148]. Study designs must also recognize that knowledge generation is not the sole purvey of bench, laboratory, or clinical trial research but coproduced in practice contexts and by all stakeholders involved in the implementation of an intervention [149].
Integrating an equity lens from the earliest planning stages can enrich the selection of implementation strategies most likely to be effective and reduce disparities across marginalized and minoritized populations. Pre-implementation planning should include [150]:
Identifying important stakeholders related to equity and establishing roles for partners throughout the implementation process
Including equity-related considerations when deciding which interventions to implement and/or de-implement
Characterizing the outcome and performance gaps related to the intervention in diverse marginalized and minoritized populations
Identifying and prioritizing barriers faced by marginalized and minoritized populations including structural racism and power dynamics
The equity capacity of implementation initiatives can be bolstered by assessing the internal climate and culture of the research/deployment team with an equity focus, building equity into all policies, equitable hiring and training, and sharing power with new equity partners [151], for example through a community-based participatory approach [137]. Characterizing how end users experience implementation should inform its tailoring with an explicit focus on equity. For example, while telehealth and online tools have important merits, fewer than two in three US adults who identify as Hispanic or African American have broadband internet at home, with similar patterns for those with lower income or education levels [152,153]. Implementation strategies consciously rooted in these realities may avoid perpetuating or exacerbating disparities and health inequity. Collecting data on equity relevant outcomes and building equity into models will help to grow the evidence base linking social determinants to health outcomes [151]. Disorder- agnostic interventions may also be necessary in recognition of the need for justice across societal sectors and the limitations imposed upon health equity by larger socio-historical constraints [60]. Equity must also be built into dissemination, for example, ensuring that the tools used resonate with key stakeholders [151].
A strong commitment to health equity from funders, researchers, practitioners, advocates, evaluators, and policy- makers is required to ensure that resources invested in health research contribute to the elimination of disparities and advancement of health equity [151].
5. Conclusions
Optimization of research that incorporates HSR, DEI and antiracism, and IS has the potential to evolve the experiences of PWIBD and to deliver transformational community-based health care. Achieving this potential will require advances on many fronts. WG5 offers the health services research; diversity, equity, and inclusion; and implementation science research priorities (Table 5) reported herein as contributions to the development of a National Research Blueprint for Inherited Bleeding Disorders [83] and entreats funding agencies, researchers, institutions, and the community as a whole to invest in them. They also identified a lack of expertise in these three fields in the inherited BD community as an important obstacle to immediate progress. They call upon NHF and other organizations to invest significantly in the recruitment, education, and training of professionals to do this work, including actively seeking out and engaging expertise from outside the inherited BD community to ensure its success.
WG5 was struck by the enormity of the work to be done in inherited BDs in these three fields. The respectful and difficult conversations in which they engaged, in an atmosphere of commitment, honesty, and partnership inspired a shared sense of purpose. They detailed the extent and urgency of this work at individual, institutional, and systems levels to empower each community member in their efforts to bring the benefits of all advances in research and care to all PWIBD. It is critical that this work continues across all inherited BDs. The work reported herein, and the priorities proposed, are small steps on a long path, but they constitute an important commitment to change.
Supplementary Material
LEE Perspective.
Patients are typically only included in research after the areas of interest have been formulated. In this groundbreaking project we, PWIBD and/or caregivers from diverse communities, were acknowledged as lived experience experts (LEE) and invited to contribute as full WG members to the identification of research priorities. Acknowledging our various privileges such as advanced degrees, citizenship, English language fluency, and research experience; we engaged in candid and safe conversations with our colleagues about the many barriers to equal access to treatment and ancillary support for PWIBD. Our voice, and sometimes simply our presence, was a reminder that any decision being made will have real impacts on the lives of real individuals. We emphasized issues whose importance we have lived personally including systemic racism in the health care system, inequities facing low-income neighborhoods and marginalized and minoritized populations, stereotyping these populations as uniform, barriers to accessing HTCs imposed by some health insurance policies, challenges to undocumented individuals seeking care (e.g. terror of deportation), struggling with English as a second language, gender and race inequalities in the power dynamic of rare disorders’ care, and the need to collect quality data on patient-important outcomes and share it with other countries. This project is vital for our community, and other rare disorder groups who may choose to replicate our efforts. It is only through centering health equity, diversity, and inclusion in the prioritization and design of data driven research and implementation science that we can truly advocate for change for all PWIBD in the US.
Acknowledgments
The authors would like to thank Kathryn M. McLaughlin, MPH from the Health Resources and Services Administration and Lena Volland PT, DPT from the National Hemophilia Foundation for contributing their time, expertise, and insights to the working group. The authors wish to thank subject matter experts Michael Nichol, PhD; Melissa S. Creary, PhD, MPH; and Brian Mittman, PhD, for contributing their expertise to the working group, setting foundational knowledge about health services research; diversity, equity and inclusion; and implementation science.
The Executive Committee of the National Hemophilia Foundation (NHF) National Research Blueprint initiative was actively engaged in the conception, design, preparation, and oversight of each of the State of the Science manuscripts in this supplement. Maria E. Santaella actively engaged with the lived experience expert (LEE) WG members throughout the process, empowering their inclusion and participation. The Executive Committee consisted of Kevin Mills, Michael Recht, Michelle L. Witkop, Maria E. Santaella, Donna DiMichele, Keri L. Norris, Esmeralda Vázquez, and Brett Spitale. The authors thank Keri L. Norris, PhD, JM, MPH, MCHES for her review of the manuscript.
The authors acknowledge Fiona Robinson, PhD, for providing professional medical writing support during manuscript development, paid for by NHF, and Matt Evans for creating professional illustrations, paid for by NHF.
Funding
The entire State of the Science Research Summit and this manuscript were funded by the National Hemophilia Foundation (NHF).
Declaration of interests
The authors are integrated members of the inherited bleeding disorders community: people with inherited bleeding disorders, their family members, health care providers and researchers (including physicians, nurses, physical therapists, pharmacists, social workers/psychologists, geneticists/ genetic counselors, etc.), industry partners, government officials/regulators, local community organization representatives and others.
Tyler W. Buckner discloses: All support for the present manuscript: NHF: Medical writing support for this paper; Grants or contracts from any entity: ATHN, NIH, NHLBI; Consulting fees: Tremeau Pharmaceuticals, BioMarin; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events: Partners Physicians Academy, Octapharma; Support for attending meetings and/or travel: Octapharma; Participation on a Data Safety Monitoring Board or Advisory Board: Advisory board participation: CSL Behring, Genzyme, Novo Nordisk, Spark, Takeda, Tremeau Pharmaceuticals, and uniQure; Leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid: ATHN Board member, NHF MASAC member. Alexis Dinno discloses: Grants or contracts from any entity: (2021-2022) OHSU-PSU Anti-Racist Faculty Fellowship (2021-2023) Oregon Health Authority: GRA funding for SOGI data in minors (2021-2022) Oregon Health Authority: GRA funding for REALD & SOGI tool evaluation; Consulting fees: (2022) Oregon AIDS Education & Training Center SOGI data Community of Practice; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events: (2022) Eastern Oregon Community Care Organization Annual Summit speaker fee (2021) Southeast Regional Hemophilia Technical Assistance Meeting speaker fee; Leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid: (2022) Oregon AIDS Education & Training Center SOGI data Community of Practice: Co-Organizer. Beth B. Warren discloses Support for attending meetings and/or travel: Binaytara Foundation; Participation on a Data Safety Monitoring Board or Advisory Board: Novo Nordisk, Genentech. Christine J Guelcher discloses Stock or stock options: Genentech; Other financial or non-financial interests: Employee of Genentech. Cindy Bailey discloses Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events: Speaker’s Bureau for Sanofi, UC San Diego Musculoskeletal Ultrasound faculty and speaker; Leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid: Board - Partners Program, Indiana, WFH MSK executive committee, NHF PTWG. Keri L. Norris discloses Employee of NHF. Mark W. Skinner discloses Grants or contracts from any entity: Various - Institution has received research funding for the PROBE study; Consulting fees: NHF. Melissa S. Creary discloses Consulting fees: ATHN; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events: FWGBD, Partners Physician Academy; Support for attending meetings and/or travel: NHF; Leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid: SCDAA. Michelle Kim discloses Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events: Speaker, Takeda La Voz Group; Leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid: BioMarin North American Patient Advocacy Forum, BioMarin US Patient Advocacy Panel. Nathan T. Connell discloses Consulting fees: Takeda; Participation on a Data Safety Monitoring Board or Advisory Board: Takeda, Genentech; Stock or stock options: Doximity. Randall Curtis discloses All support for the present manuscript: University of Southern California, Institute for Policy Advancement, Ltd.; Consulting fees: HUGS, PROBE; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events: Global Blood Disorders Foundation; Support for attending meetings and/or travel: National Hemophilia Foundation (NHF), Hematology Utilization Group Study (HUGS), Patient Reported Outcomes, Burdens and Experiences (PROBE); Leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid: Hemophilia Foundation of Northern California, Center for Inherited Blood Disorders. Roshni Kulkarni discloses Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events: CSL Behring; Participation on a Data Safety Monitoring Board or Advisory Board: Sanofi Genzyme, Novo Nordisk, Pfizer. Susan Symington discloses Other financial or non-financial interests: Full-time employee at Genentech. The remaining authors have no relevant disclosures. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by CDC/HHS, or the US Government.
List of Abbreviations
- AMA
American Medical Association
- APHA
American Public Health Association
- ASH
American Society of Hematology
- ATHN
American Thrombosis and Hemostasis Network
- BD
Bleeding disorder
- CDC
U.S. Centers for Disease Control and Prevention
- CMMI
Centers for Medicare and Medicaid Services Innovation Center
- DEI
Diversity, equity, and inclusion
- F
Factor
- HCP
Health care professional
- HIV
Human immunodeficiency virus
- HRQoL
Health-related quality of life
- HRSA
Health Resources and Services Administration
- HSR
Health services research
- HTC
Hemophilia Treatment Center
- HUGS
Hematology Utilization Group Study
- IS
Implementation science
- ISTH
International Society on Thrombosis and Haemostasis
- LEE
Lived experience expert
- MASAC
Medical and Scientific Advisory Council
- NHF
National Hemophilia Foundation
- NIH
National Institutes of Health
- PI
Principal investigator
- PICOTS
Population/patient problem, intervention, control/comparison, outcome, timing, setting
- PPM
Person who has or had the potential to menstruate
- PROBE
Patient Reported Outcomes, Burdens and Experiences
- PROM
Patient-reported outcome measure
- PWIBD
Person with an inherited bleeding disorder
- QoL
Quality of life
- SC
Steering Committee
- SDoH
Social determinants of health
- SOC
Standard of care
- SOSRS
State of the Science Research Summit
- US
United States
- USHTCN
US Hemophilia Treatment Center Network
- VWD
Von Willebrand disease
- WFH
World Federation of Hemophilia
- WG
Working Group
Footnotes
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Presentation of content
VRB, JRB, and TWB presented highlights of the deliberations of Working Group 5 at the National Hemophilia Foundation State of the Science (virtual) Research Summit (SOSRS), September 12–15, 2021. Summit discussions informed the Discussion and Conclusions of this paper.
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17474086.2023.2183836
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Mahlangu J, Young G, Hermans C, et al. Defining extended half-life rFVIII-A critical review of the evidence. Haemophilia. 2018;24 (3):348–358. [DOI] [PubMed] [Google Scholar]
- 2.Mahlangu JN, Blanchette V, Klamroth R. Redefining prophylaxis in the modern era. Haemophilia. 2021;27(Suppl 3):21–27. [DOI] [PubMed] [Google Scholar]
- 3.Skinner MW, Nugent D, Wilton P, et al. Achieving the unimaginable: health equity in haemophilia. Haemophilia. 2020;26(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weyand AC, James PD. Sexism in the management of bleeding disorders. Res Pract Thromb Haemost. 2021;5(1):51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meijer K, van Heerde W, Gomez K. Diagnosis of rare bleeding disorders. Haemophilia. 2022;28(Suppl 4):119–124. [DOI] [PubMed] [Google Scholar]
- 6.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. [DOI] [PubMed] [Google Scholar]
- 7.Manco-Johnson MJ, Lundin B, Funk S, et al. Effect of late prophylaxis in hemophilia on joint status: a randomized trial. J Thromb Haemost. 2017;15(11):2115–2124. [DOI] [PubMed] [Google Scholar]
- 8.Warren BB, Thornhill D, Stein J, et al. Young adult outcomes of childhood prophylaxis for severe hemophilia A: results of the joint outcome continuation study. Blood Adv. 2020;4(11):2451–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evatt BL. The tragic history of AIDS in the hemophilia population, 1982–1984. J Thromb Haemost. 2006;4(11):2295–2301. [DOI] [PubMed] [Google Scholar]
- 10.Farrugia A, Liumbruno GM, Candura F, et al. Factors affecting the quality, safety and marketing approval of clotting factor concentrates for haemophilia. Blood Transfus. 2018;16(6):525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nugent D, Acharya SS, Baumann KJ, et al. Building the foundation for a community-generated national research blueprint for inherited bleeding disorders: research priorities for ultra-rare inherited bleeding disorders. Expert Rev Hematol. 2023;16 (S1):53–67. DOI: 10.1080/17474086.2023.2175661. • Report of NHF SOS RS WG3
- 12. Baldwin MK, Ahmadzia HK, Bartlett DL, et al. Building the foundation for a community-generated national research blueprint for inherited bleeding disorders: research to advance the health of people with inherited bleeding disorders with the potential to menstruate. Expert Rev Hematol. 2023;16(S1):71–86. DOI: 10.1080/17474086.2023.2175660. • Report of NHF SOS RS WG4
- 13. Sidonio RF Jr., Bryant PC, Di Paola J, et al. Building the foundation for a community-generated national research blueprint for inherited bleeding disorders: research priorities for mucocutaneous bleeding disorders. Expert Rev Hematol. 2023;16(S1):39–54. DOI: 10.1080/17474086.2023.2171983. • Report of NHF SOS RS WG2
- 14. Tran DQ, Benson CC, Boice JA, et al. Building the foundation for a community-generated national research blueprint for inherited bleeding disorders: research priorities to transform the care of people with hemophilia. Expert Rev Hematol. 2023;16(S1):19–37. DOI: 10.1080/17474086.2023.2171981. • Report of NHF SOS RS WG1
- 15. Vázquez E, Kim M, Santaella ME. Lived Experience Experts: a name created by us for us. Expert Rev Hematol. 2023;16(S1):7–11. DOI: 10.1080/17474086.2023.2178410. • Perspective of people with inherited bleeding disorders and their families in the NHF State of the Science Research Summit and National Research Blueprint initiatives on the recognition of their expertise
- 16. Agency for Healthcare Research and Quality. An organizational guide to building health services research capacity [cited 2022 May 15]. Available from: https://www.ahrq.gov/funding/training-grants/hsrguide/hsrguide.html. •• Describes how organizations can develop and enhance abilitities to plan, conduct, and fund health services research
- 17.Centers for Disease Control and Prevention. U.S. health disadvantage: causes and potential solutions U.S. health disadvantage: causes and potential solutions[cited 2023 Feb 14]. Available from: https://www.cdc.gov/policy/chep/health/index.html. [Google Scholar]
- 18.U.S. Department of Health and Human Services. Community Health and Economic Prosperity: Engaging Businesses as Stewards and Stakeholders—A Report of the Surgeon General 2021. [cited 2023 Feb 14]. Available from: https://www.hhs.gov/sites/default/files/chep-sgr-full-report.pdf. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. HTC population profile patient characteristics march 2022 - technical notes 2022. [cited 2022 Nov 4. Available from: https://www.cdc.gov/ncbddd/hemophilia/communitycounts/data-reports/2022-03/index.html. [Google Scholar]
- 20. Valentino L, Baker J, Butler R, et al. Integrated hemophilia patient care via a national network of care centers in the United States: a model for rare coagulation disorders. J Blood Med. 2021;12:897–911. • Overview of the Hemophilia Treatment Center Network in the US
- 21.Centers for Disease Control and Prevention. Hemophila Treatment Centers [cited 2022 Aug 17]. Available from: https://www.cdc.gov/ncbddd/hemophilia/documents/a-simmons_htc-brochure-508c.pdf. [Google Scholar]
- 22.Skinner MW, Soucie JM, McLaughlin K. The national haemophilia program standards, evaluation and oversight systems in the United States of America. Blood Transfus. 2014;12(Suppl 3): e542–8.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker JR, Crudder SO, Riske B, et al. A model for a regional system of care to promote the health and well-being of people with rare chronic genetic disorders. Am J Public Health. 2005;95 (11):1910–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pai M, Key NS, Skinner M, et al. NHF-McMaster guideline on care models for haemophilia management. Haemophilia. 2016;22(Suppl 3):6–16. [DOI] [PubMed] [Google Scholar]
- 25. National Hemophilia Foundation Medical and Scientific Advisory Committee. MASAC Documents [cited 2022 Aug 17]. Available from: https://www.hemophilia.org/healthcare-professionals/guidelines-on-care/masac-documents • Expert consensus-based recommendations for clinical care of persons with bleeding disorders
- 26.National Hemophilia Foundation Medical and Scientific Advisory Committee. MASAC document 269- standards and criteria for the care of persons with congenital bleeding disorders [cited 2022 May 19. Available from: https://www.hemophilia.org/healthcare-professionals/guidelines-on-care/masac-documents/masac-document-269-standards-and-criteria-for-the-care-of-persons-with-congenital-bleeding-disorders. [Google Scholar]
- 27. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26 (Suppl 6):1–158. • Evidence-based recommendations for comprehensive diagnosis, treatment and management of hemophilia
- 28. Connell NT, Flood VH, Brignardello-Petersen R, et al. ASH ISTH NHF WFH 2021 guidelines on the management of von Willebrand disease. Blood Adv. 2021;5(1):301–325. •Evidence-based recommendations for treatment of VWD in the setting of major and minor surgery, testing during invasive procedures, use of desmopressin, and use of prophylaxis
- 29. James PD, Connell NT, Ameer B, et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021;5(1):280–300. • Evidence-based guidelines to improve accurate diagnosis of VWD, minimize inappropriate testing and avoid harms from over-diagnosis
- 30.American Thrombosis and Hemostasis Network. What We Do Overview [cited 2022 Jan 5. Available from: https://athn.org/what-we-do/overview.html. [Google Scholar]
- 31. Riske B, Shearer R, Baker JR. Patient satisfaction with US hemophilia treatment center care, teams and services: the first national survey. Haemophilia. 2020;26(6):991–998. • Report of >5000 people with inherited bleeding disorders and families on satisfaction with US Hemophilia Treatment Centers.
- 32.Butler RB, Cheadle A, Aschman DJ, et al. National needs assessment of patients treated at the United States federally-funded hemophilia treatment centers. Haemophilia. 2016;22(1):e11–7. [DOI] [PubMed] [Google Scholar]
- 33.National HTC Patient Satisfaction Survey. Third National Survey of US hemophilia treatment centers – over 5300 patients report on care obtained in 2020: national HTC patient satisfaction survey; 2022. [cited 2022 Aug 21]. Available from: https://www.htcsurvey.com/results. [Google Scholar]
- 34.Baker JR, Riske B, Drake JH, et al. US hemophilia treatment center population trends 1990–2010: patient diagnoses, demographics, health services utilization. Haemophilia. 2013;19(1):21–26. [DOI] [PubMed] [Google Scholar]
- 35.Regling K, Callaghan MU, Sidonio R Jr. Managing severe hemophilia a in children: pharmacotherapeutic options. Pediatric Health Med Ther. 2022;13:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierce GF. Uncertainty in an era of transformative therapy for haemophilia: addressing the unknowns. Haemophilia. 2021;27 (Suppl 3):103–113. [DOI] [PubMed] [Google Scholar]
- 37.Arya S, Wilton P, Page D, et al. Healthcare provider perspectives on inequities in access to care for patients with inherited bleeding disorders. PLoS One. 2020;15(2):e0229099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Health Resources & Services Administration. 340B drug pricing program [cited 2022 Aug 18]. Available from: https://www.hrsa.gov/opa. [Google Scholar]
- 39.Lopez K, Norris K, Hardy M, et al. Defining the Impact of social drivers on health outcomes for people with inherited bleeding disorders. J Clin Med. 2022;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Department of Housing and Human Development (HUD). Diversity and inclusion definitions 2021. [cited 2023 Jan 30]. Available from: https://www.hud.gov/program_offices/administration/admabout/diversity_inclusion/definitions. [Google Scholar]
- 41.George Washington University. Equity vs. equality: what’s the difference? 2020. [cited 2023 Jan 30]. Available from: https://onlinepublichealth.gwu.edu/resources/equity-vs-equality/. [Google Scholar]
- 42.University of Toronto Division of the Vice President Research & Innovation. Equity, Diversity & Inclusion [cited 2022 Aug 18]. Available from: https://research.utoronto.ca/equity-diversity-inclusion/equity-diversity-inclusion. [Google Scholar]
- 43.Braveman P, Arkin E, Orleans T, et al. What is health equity? And what difference does a definition make? Princeton, NJ: Robert Wood Johnson foundation; 2017. [cited 2023 Jan 31]. Available from: https://healthforward.org/wp-content/uploads/2017/11/rwjf-Health-Equity-Full-Report-437393.pdf. [Google Scholar]
- 44.U.S. Department of health and human services office of disease prevention and health promotion. health equity in healthy people 2030. [cited 2022 Aug 18]. Available from: https://health.gov/healthypeople/priority-areas/health-equity-healthy-people-2030. [Google Scholar]
- 45. Braveman P, Gruskin S. Defining equity in health. J Epidemiol Community Health. 2003;57(4):254–258. •• Discussion of defining, operationalizing, and measuring health equity.
- 46.George Washington University. Diversity and inclusion defined [cited 2023 Jan 31]. Available from: https://diversity.gwu.edu/diversity-and-inclusion-defined. [Google Scholar]
- 47.Rethinking Bonilla-Silva E. Racism: Toward a Structural Interpretation. Am Sociol Rev. 1997;62(3):465–480. [Google Scholar]
- 48.James M, Burgos A. Race. in Stanford Encyclopedia of Philosophy 2020. [cited 2023 Jan 31]. Available from: https://plato.stanford.edu/entries/race/. [Google Scholar]
- 49.Operario D, Fiske ST. Racism equals power plus prejudice: a social psychological equation for racial oppression. Confronting racism: the problem and the response. Thousand Oaks CA US: Sage Publications, Inc; 1998. p. 33–53. [Google Scholar]
- 50.Schaefer RT. Racial and Ethnic Groups. 15th ed. New York (NY): Pearson; 2019. [Google Scholar]
- 51.Came H, Griffith D. Tackling racism as a “wicked” public health problem: enabling allies in anti-racism praxis. Soc Sci Med. 2018;199:181–188. [DOI] [PubMed] [Google Scholar]
- 52. Ford CL, Airhihenbuwa CO. The public health critical race methodology: praxis for antiracism research. Soc Sci Med. 2010;71 (8):1390–1398. •• Description of Public Health Critical Race framework to conduct health equity research, better understand health inequities, and challenge power hierarchies that perpetuate inequities.
- 53.Racism: Science and Tools for the Public Health Professional. Washington (DC): APHPA Press; 2019. [cited 2022 Aug 23]. Available from: https://ajph.aphapublications.org/doi/book/10.2105/9780875533049 [Google Scholar]
- 54.Bacong AM, Dillard AS, Bradford NJ. Appendix D: glossary. In: Ford CL, Griffith DM, Bruce MA, et al. , editors. Racism: science & tools for the public health professional. Washington (DC): APHA Press; 2019. p. 519–543. [cited 2022 Aug 23]. Available from: https://ajph.aphapublications.org/doi/abs/10.2105/9780875533049appD [Google Scholar]
- 55.Centers for Disease Control and Prevention. Social Determinants of Health at CDC [cited 2023 Jan 31]. Available from: https://www.cdc.gov/about/sdoh/index.html. [Google Scholar]
- 56.World Health Organization. Social determinants of health 2021. [cited 2023 Jan 31]. Available from: https://www.who.int/teams/social-determinants-ofhealth#:~:text=The%20social%20determinants%20of%20health%20%28SDH%29%20are%20the,agendas%2C%20social%20norms%2C%20social%20policies%20and%20political%20systems. [Google Scholar]
- 57.Artiga S, Hinton E. Beyond health care: the role of social determinants in promoting health and health equity 2018. [cited 2022 Aug 19]. Available from: https://www.kff.org/racial-equity-and-health-policy/issue-brief/beyond-health-care-the-role-of-social-determinants-in-promoting-health-and-health-equity/. [Google Scholar]
- 58.The Community Guide Staff. Guide to community preventive services. advancing health equity 2022. [cited 2022 Oct 27]. Available from: https://www.thecommunityguide.org/pages/advancing-health-equity.html. [Google Scholar]
- 59.U.S. Department of Health and Human Services Office of Disease Prevention and Health Promotion. Social Determinants of Health [cited 2022 Aug 19]. Available from: https://health.gov/healthypeople/priority-areas/social-determinants-health. [Google Scholar]
- 60.Creary MS. Bounded justice and the limits of health equity. Journal of Law, Medicine & Ethics. 2021;49(2):241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuhlberg JA, Headen I, Ballard EA, et al. Advancing community engaged approaches to identifying structural drivers of racial bias in health diagnostic algorithms: data for black lives; 2020. [cited 2022 Aug 20]. Available from: https://www.filepicker.io/api/file/VC60nHDsSnOxtFXvnxw2. [Google Scholar]
- 62.Weyand AC, Sidonio RF Jr., Sholzberg M. Health issues in women and girls affected by haemophilia with a focus on nomenclature, heavy menstrual bleeding, and musculoskeletal issues. Haemophilia. 2022;28(Suppl 4):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Galen KPM, d’Oiron R, James P, et al. A new hemophilia carrier nomenclature to define hemophilia in women and girls: communication from the SSC of the ISTH. J Thromb Haemost. 2021;19 (8):1883–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weyand AC, McGann PT. Eliminating race-based reference ranges in haematology: a call to action. Lancet Haematol. 2021;8(6):e462–e6. [DOI] [PubMed] [Google Scholar]
- 65.Refiguring Krieger N. “race”: epidemiology, racialized biology, and biological expressions of race relations. Int J Health Serv. 2000;30 (1):211–216. [DOI] [PubMed] [Google Scholar]
- 66.Proctor EK, Landsverk J, Aarons G, et al. Implementation research in mental health services: an emerging science with conceptual, methodological, and training challenges. Adm Policy Ment Health. 2009;36(1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Eccles MP, Mittman BS. Welcome to Implementation Science. Implementation Sci. 2006;1(1):1–3. •• Description of implementation science.
- 68.Loper A, Woo B, Metz A. Equity Is Fundamental to ImplementationScience 2021. [cited 2022 Aug 21]. Available from: https://ssir.org/articles/entry/equity_is_fundamental_to_implementation_science. [Google Scholar]
- 69.Mouchantaf R, Auth D, Moride Y, et al. Risk management for the 21st century: current status and future needs. Drug Saf. 2021;44 (4):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bolton-Maggs PH, Chalmers EA, Collins PW, et al. A review of inherited platelet disorders with guidelines for their management on behalf of the UKHCDO. Br J Haematol. 2006;135(5):603–633. [DOI] [PubMed] [Google Scholar]
- 71.Faughnan ME, Mager JJ, Hetts SW, et al. Second international guidelines for the diagnosis and management of hereditary hemorrhagic telangiectasia. Ann Intern Med. 2020;173(12):989–1001. [DOI] [PubMed] [Google Scholar]
- 72.Gresele P Subcommittee on platelet physiology of the international society on thrombosis and Hemostasis. diagnosis of inherited platelet function disorders: guidance from the SSC of the ISTH. J Thromb Haemost. 2015;13(2):314–322. [DOI] [PubMed] [Google Scholar]
- 73.Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162(3):205–213. [DOI] [PubMed] [Google Scholar]
- 74.Garrison LP, Kleinermans D. Is the world ready for gene therapy? Haemophilia. 2022;28(Suppl 2):5–8. [DOI] [PubMed] [Google Scholar]
- 75.Kaczmarek R Gene therapy - are we ready now? Haemophilia. 2022;28(Suppl 4):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miesbach W, Chowdary P, Coppens M, et al. Delivery of AAV-based gene therapy through haemophilia centres-A need for re-evaluation of infrastructure and comprehensive care: a Joint publication of EAHAD and EHC. Haemophilia. 2021;27(6):967–973. [DOI] [PubMed] [Google Scholar]
- 77.Sidonio RF Jr., Pipe SW, Callaghan MU, et al. Discussing investigational AAV gene therapy with hemophilia patients: a guide. Blood Rev. 2021;47:100759. [DOI] [PubMed] [Google Scholar]
- 78.Curran GM, Bauer M, Mittman B, et al. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.National Hemophilia Foundation. Mission & History [cited 2022 Jan 5]. Available from: https://www.hemophilia.org/who-we-are/our-story/mission-history. [Google Scholar]
- 80. Rauch A, Valentino LA, Mills K, et al. Big picture initiatives in bleeding disorders. Haemophilia. 2022;28(Suppl 4):53–60. • Provides principles for NHF national research, specifically to impact lives, have relevance and achieve health equity for those with bleeding disorders
- 81. Valentino LA, Witkop ML, Santaella ME, et al. Building the blueprint: formulating a community-generated national plan for future research in inherited bleeding disorders. Haemophilia. 2022;28 (5):760–768. • Details the NHF State of the Science Research Summit process and methods and the inclusive extensive community consultations underpinning it
- 82.Centers for Disease Control and Prevention. 10 essential public health services [cited 2022 May 25]. Available from: https://www.cdc.gov/publichealthgateway/publichealthservices/essentialhealthservices.html. [Google Scholar]
- 83. Valentino LA, Witkop ML, Santaella ME, et al. The National Hemophilia Foundation State of the Science Research Summit initiative: executive summary. Expert Rev Hematol. 2023;16 (S1):127–132. DOI: 10.1080/17474086.2023.2181782. •• Executive summary of NHF State of the Science Research Summit as the foundation of a National Research Blueprint for inherited bleeding disorders
- 84. Valentino LA, Witkop ML, Santaella ME, et al. The National Hemophilia Foundation’s State of the Science Research Summit initiative: the foundation of an inherited bleeding disorders national research blueprint. Expert Rev Hematol. 2023;16(S1):1–5. DOI: 10.1080/17474086.2023.2178412. • Introductory foreword to this special supplement detailing the feasiblity-impact-risk scoring approach and the work of the NHF SOS RS
- 85.U.S. Department of Health and Human Services. Healthy People 2020: sex-specific objectives [cited 2022 May 5]. Available from: https://www.healthypeople.gov/2020/data-search/Search-the-Data?sex=543. [Google Scholar]
- 86.U.S. Department of Health and Human Services. Search the data | Healthy People 2020: BDBS-14 (Developmental) Increase the proportion of providers who refer women with symptoms suggestive of inherited bleeding disorders for diagnosis and treatment [cited 2022 May 5]. Available from: https://www.healthypeople.gov/2020/data-search/Search-the-Data?nid=4026. [Google Scholar]
- 87.U.S. Department of Health and Human Services. Search the data | Healthy People 2020: BDBS-15 Increase the proportion of persons with von Willebrand disease (VWD) seen in specialty care centers who were diagnosed by 21 years of age [cited 2022 May 5]. Available from: https://www.healthypeople.gov/2020/data-search/Search-the-Data?nid=4027. [Google Scholar]
- 88.U.S. Department of Health and Human Services. Healthy People 2020: Topics & objectives [cited 2022 Sept 24]. Available from: https://wayback.archive-it.org/5774/20220413162703/https://www.healthypeople.gov/2020/topics-objectives. [Google Scholar]
- 89.Baker JR, Riske B, Voutsis M, et al. Insurance, home therapy, and prophylaxis in U.S. youth with severe hemophilia. Am J Prev Med. 2011;41(6 Suppl 4):S338–45. [DOI] [PubMed] [Google Scholar]
- 90.Huynh AK, Hamilton AB, Farmer MM, et al. A pragmatic approach to guide implementation evaluation research: strategy mapping for complex interventions. Front Public Health. 2018;6:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen CX, Baker JR, Nichol MB. Economic burden of illness among persons with hemophilia B from HUGS Vb: examining the association of severity and treatment regimens with costs and annual bleed rates. Value Health. 2017;20(8):1074–1082. [DOI] [PubMed] [Google Scholar]
- 93.Curtis R, Manco-Johnson M, Konkle BA, et al. Comorbidities, health-related quality of life, health-care utilization in older persons with hemophilia-hematology utilization group study part VII (HUGS VII). J Blood Med. 2022;13:229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Globe DR, Cunningham WE, Andersen R, et al. Haemophilia utilization group study: assessment of functional health status in haemophilia. Haemophilia. 2002;8(2):121–128. [DOI] [PubMed] [Google Scholar]
- 95.Globe DR, Cunningham WE, Andersen R, et al. The hemophilia utilization group study (HUGS): determinants of costs of care in persons with haemophilia A. Haemophilia. 2003;9(3):325–331. [DOI] [PubMed] [Google Scholar]
- 96.Niu X, Poon JL, Riske B, et al. Physical activity and health outcomes in persons with haemophilia B. Haemophilia. 2014;20(6):814–821. [DOI] [PubMed] [Google Scholar]
- 97.Poon JL, Zhou ZY, Doctor JN, et al. Quality of life in haemophilia A: hemophilia utilization group study va (HUGS-Va). Haemophilia. 2012;18(5):699–707. [DOI] [PubMed] [Google Scholar]
- 98.Zhou ZY, Riske B, Forsberg AD, et al. Self-reported barriers to hemophilia care in people with factor VIII deficiency. Am J Prev Med. 2011;41(6 Suppl 4):S346–53. [DOI] [PubMed] [Google Scholar]
- 99.Zhou ZY, Wu J, Baker J, et al. Haemophilia utilization group study - Part Va (HUGS Va): design, methods and baseline data. Haemophilia. 2011;17(5):729–736. [DOI] [PubMed] [Google Scholar]
- 100.Roberts JC, Kulkarni R, Kouides PA, et al. Depression and anxiety in persons with von Willebrand disease. Blood. 2021;138(Suppl Supplement 1):4052. [DOI] [PubMed] [Google Scholar]
- 101.Hematology Utilization Group Study. Research collected from across the U.S. shared all over the world [cited 2022 Sept 25]. Available from: https://hugsresearch.net/research. [Google Scholar]
- 102.Lanzkron S, Crook N, Wu J, et al. PRO66 Health-Related quality of life in persons with sickle cell disease. Value Health. 2021;24(Suppl 5):S209–S210. [Google Scholar]
- 103.Chai-Adisaksopha C, Noone D, Curtis R, et al. Non-severe haemophilia: is it benign? - Insights from the PROBE study. Haemophilia. 2021;27(Suppl 1):17–24. [DOI] [PubMed] [Google Scholar]
- 104.Chai-Adisaksopha C, Skinner MW, Curtis R, et al. Psychometric properties of the patient reported outcomes, burdens and experiences (PROBE) questionnaire. BMJ Open. 2018;8(8):e021900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chai-Adisaksopha C, Skinner MW, Curtis R, et al. Exploring regional variations in the cross-cultural, international implementation of the Patient reported outcomes burdens and experience (PROBE) study. Haemophilia. 2019;25(3):365–372. [DOI] [PubMed] [Google Scholar]
- 106.Chai-Adisaksopha C, Skinner MW, Curtis R, et al. Test-retest properties of the patient reported outcomes, burdens and experiences (PROBE) questionnaire and its constituent domains. Haemophilia. 2019;25(1):75–83. [DOI] [PubMed] [Google Scholar]
- 107.Germini F, Borg Debono V, Page D, et al. User-centered development and testing of the online patient-reported outcomes, burdens, and experiences (PROBE) survey and the myPROBE app and integration with the Canadian bleeding disorder registry: mixed methods study. JMIR Hum Factors. 2022;9(1):e30797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Germini F, Chai-Adisaksopha C, Pete D, et al. Evaluation of the sexual health in people living with hemophilia. Haemophilia. 2021;27(6):993–1001. [DOI] [PubMed] [Google Scholar]
- 109.Hay CRM, Shima M, Makris M, et al. Challenges and key lessons from the design and implementation of an international haemophilia registry supported by a pharmaceutical company. Haemophilia. 2020;26(6):966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Skinner MW, Chai-Adisaksopha C, Curtis R, et al. The patient reported outcomes, burdens and experiences (PROBE) project: development and evaluation of a questionnaire assessing patient reported outcomes in people with haemophilia. Pilot Feasibility Stud. 2018;4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.National Academy of Medicine. The Future of Health Services Research: Advancing Health Systems Research and Practice in the United States. Washington (DC); 2018. [cited 2023 Jan 13]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30640418 [PubMed] [Google Scholar]
- 112.Stonebraker JS, Bolton-Maggs PHB, Brooker M, et al. The World Federation of Hemophilia annual global survey 1999–2018. Haemophilia. 2020;26(4):591–600. [DOI] [PubMed] [Google Scholar]
- 113.Resources Health & Administration Services. What is shortage designation? [cited 2022 Sept 25]. Available from: https://bhw.hrsa.gov/workforce-shortage-areas/shortage-designation. [Google Scholar]
- 114.Centers for Medicare & Medicaid Services. Innovation Center Strategy Refresh [cited 2022 Aug 23]. Available from: https://innovation.cms.gov/strategic-direction-whitepaper. [Google Scholar]
- 115.Centers for Disease Control and Prevention. CDC Core Health Equity Science and Intervention Strategy [cited 2022 Sept 25]. Available from: https://www.cdc.gov/healthequity/core/index.html. [Google Scholar]
- 116.McLellan SE, Mann MY, Scott JA, et al. A blueprint for change: guiding principles for a system of services for children and youth with special health care Needs and their families. Pediatrics. 2022;149(Suppl):7. [DOI] [PubMed] [Google Scholar]
- 117.Bowleg L The problem with the phrase women and minorities: intersectionality-an important theoretical framework for public health. Am J Public Health. 2012;102(7):1267–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Parker M, Hannah M, Zia A. “If I wasn’t a girl”: experiences of adolescent girls with heavy menstrual bleeding and inherited bleeding disorders. Res Pract Thromb Haemost. 2022;6(4):e12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Witkop M, Guelcher C, Forsyth A, et al. Treatment outcomes, quality of life, and impact of hemophilia on young adults (aged 18–30 years) with hemophilia. Am J Hematol. 2015;90(Suppl 2):S3–10. [DOI] [PubMed] [Google Scholar]
- 120.Saha S, Beach MC, Cooper LA. Patient centeredness, cultural competence and healthcare quality. J Natl Med Assoc. 2008;100 (11):1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Capers Q How clinicians and educators can mitigate implicit bias in patient care and candidate selection in medical education. ATS Sch. 2020;1(3):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.American Medical Association Center for Health Equity. Organizational strategic plan to embed racial justice and advance health equity: American medical association; [cited 2022 Aug 23]. Available from: https://www.ama-assn.org/system/files/2021-05/ama-equity-strategic-plan.pdf. [Google Scholar]
- 123.American Society of Hematology. Message from the ASH president on diversity, equity, and inclusion 2020. [cited 2022 Aug 23]. Available from: https://www.hematology.org/newsroom/press-releases/2020/message-from-the-ash-president-on-diversity-equity-and-inclusion. [Google Scholar]
- 124.American Public Health Association. Health Equity [cited 2022 Aug 23]. Available from: https://www.apha.org/topics-and-issues/health-equity. [DOI] [PubMed] [Google Scholar]
- 125.Haley KM, Sidonio RF Jr., Abraham S, et al. A cross-sectional study of women and girls with congenital bleeding disorders: the American thrombosis and hemostasis network cohort. J Womens Health (Larchmt). 2020;29(5):670–676. [DOI] [PubMed] [Google Scholar]
- 126.Byams VR, Miller CH, Bethea FM, et al. Bleeding disorders in women and girls: state of the science and CDC collaborative programs. J Womens Health (Larchmt). 2022;31(3):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Centers for Disease Control and Prevention. About Community Counts [cited 2022 Aug 23]. Available from: https://www.cdc.gov/ncbddd/hemophilia/communitycounts/about.html. [Google Scholar]
- 128.National Institute on Minority Health and Health Disparities. NIMHD Science Visioning Research Strategies [cited 2022 Aug 24]. Available from: https://nimhd.nih.gov/about/overview/science-visioning/visioning-strategies.html#methods. [Google Scholar]
- 129.Chawla NV, Bowyer KW, Hall LO, et al. SMOTE: synthetic minority over-sampling technique. J artif intell res. 2002;16:321–357. [Google Scholar]
- 130.Cascino TM, Somanchi S, Colvin M, et al. Racial and sex inequities in the use of and outcomes after left ventricular assist device implantation among medicare beneficiaries. JAMA Network Open. 2022;5 (7):e2223080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Witt RE, Colvin BN, Lenze SN, et al. Lived experiences of stress of black and Hispanic mothers during hospitalization of preterm infants in neonatal intensive care units. J Perinatol. 2022;42(2):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Witt RE, Malcolm M, Colvin BN, etv al. Racism and Quality of Neonatal Intensive Care: Voices of Black Mothers. Pediatrics. 2022;150(3). DOI: 10.1542/peds.2022-056971. https://www.ncbi.nlm.nih.gov/pubmed/35965288 [DOI] [PubMed] [Google Scholar]
- 133.Statler MC, Wall BM, Richardson JW, et al. African American perceptions of participating in health research despite historical mistrust. ANS Adv Nurs Sci. 2022. [DOI] [PubMed] [Google Scholar]
- 134.O’Donoghue J, Luther J, Hoque S, et al. Strategies to improve the recruitment and retention of underserved children and families in clinical trials: a case example of a school-supervised asthma therapy pilot. Contemp Clin Trials. 2022;128:106884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee LH, Whisenton LH, Benger J, et al. A community-centered approach to sickle cell disease and clinical trial participation: an evaluation of perceptions, facilitators, and barriers. Blood Adv. 2021;5(23):5323–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ricks JM, Arthur EK, Stryker SD, et al. A systematic literature review of community-based participatory health research with sexual and gender minority communities. Health Equity. 2022;6 (1):640–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.National Institute on Minority Health and Health Disparities. Community-Based Participatory Research Program (CBPR) [cited 2022 May 19]. Available from: https://www.nimhd.nih.gov/programs/extramural/community-based-participatory.html. [Google Scholar]
- 138.Winn RA. Diversity in clinical trials award program. Robert A. Winn Diversity in Clinical Trials Award Program [cited 2022 Aug 23]. Available from: https://diversityinclinicaltrials.org/. [Google Scholar]
- 139.National Medical Fellowships. Programs portfolio overview [cited 2022 Aug 23]. Available from: https://nmfonline.org/scholarships-programs/programs-portfolio-overview/. [Google Scholar]
- 140.American Society of Hematology. ASH Minority Recruitment Initiative [cited 2022 Aug 23]. Available from: https://www.hematology.org/awards/minority-recruitment. [Google Scholar]
- 141.National Hemophilia Foundation. Jeanne Marie Lusher diversity fellowship [cited 2022 Aug 23]. Available from: https://www.hemophilia.org/research/fund-your-research/jeanne-marie-lusher-diversity-fellowship. [Google Scholar]
- 142.Pipe SW, Reddy KR, Chowdary P. Gene therapy: practical aspects of implementation. Haemophilia. 2022;28(Suppl 4):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.European Medicines Agency. First gene therapy to treat severe haemophilia A [cited 2022 Jul 20]. Available from: https://www.ema.europa.eu/en/news/first-gene-therapy-treat-severe-haemophilia. [Google Scholar]
- 144.U.S. Food and Drug Administration. FDA approves first gene therapy to treat adults with hemophilia B 2022. [cited 2022 Nov 23]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-treat-adults-hemophilia-b. [Google Scholar]
- 145.Reiss UM, Mahlangu J, Ohmori T, et al. Haemophilia gene therapy-update on new country initiatives. Haemophilia. 2022;28 (Suppl 4):61–67. [DOI] [PubMed] [Google Scholar]
- 146.Skivington K, Matthews L, Simpson SA, et al. Framework for the development and evaluation of complex interventions: gap analysis, workshop and consultation-informed update. Health Technol Assess. 2021;25(57):1–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Perez Jolles M, Lengnick-Hall R, Mittman BS. Core Functions and. Forms of complex health interventions: a patient-centered medical home illustration. J Gen Intern Med. 2019;34(6):1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hawe P, Shiell A, Riley T. Complex interventions: how “out of control” can a randomised controlled trial be? BMJ. 2004;328 (7455):1561–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hawe P Lessons from complex interventions to improve health. Annu Rev Public Health. 2015;36:307–323. [DOI] [PubMed] [Google Scholar]
- 150.Kerkhoff AD, Farrand E, Marquez C, et al. Addressing health disparities through implementation science-a need to integrate an equity lens from the outset. Implement Sci. 2022;17(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brownson RC, Kumanyika SK, Kreuter MW, et al. Implementation science should give higher priority to health equity. Implement Sci. 2021;16(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin JL, Huber B, Amir O, et al. Barriers and facilitators to the implementation of family-centered technology in complex care: feasibility study. J Med Internet Res. 2022;24(8):e30902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pew Research Center. Internet/broadband fact sheet [cited 2022 Aug 26]. Available from: https://www.pewresearch.org/internet/fact-sheet/internet-broadband/?menuItem=3109350c-8dba-4b7f-ad52-a3e976ab8c8f. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.