Abstract
Across diverse insect taxa, the behavior and physiology of females dramatically changes after mating—processes largely triggered by the transfer of seminal proteins from their mates. In the vinegar fly Drosophila melanogaster, the seminal protein sex peptide (SP) decreases the likelihood of female flies remating and causes additional behavioral and physiological changes that promote fertility including increasing egg production. Although SP is only found in the Drosophila genus, its receptor, sex peptide receptor (SPR), is the widely conserved myoinhibitory peptide (MIP) receptor. To test the functional role of SPR in mediating postmating responses in a non-Drosophila dipteran, we generated 2 independent Spr-knockout alleles in the yellow fever mosquito, Aedes aegypti. Although SPR is needed for postmating responses in Drosophila and the cotton bollworm Helicoverpa armigera, Spr mutant Ae. aegypti show completely normal postmating decreases in remating propensity and increases in egg laying. In addition, injection of synthetic SP or accessory gland homogenate from D. melanogaster into virgin female mosquitoes did not elicit these postmating responses. Our results demonstrate that Spr is not required for these canonical postmating responses in Ae. aegypti, indicating that other, as yet unknown, signaling pathways are likely responsible for these behavioral switches in this disease vector.
Keywords: Aedes aegypti, sex peptide, mating receptivity, oviposition, reproduction
Introduction
Female insects undergo major changes after mating that alter their behavior and physiology, ultimately contributing to successful reproduction. Much of our understanding of these changes comes from work in Drosophila melanogaster, where postmating changes are primarily initiated by seminal fluid proteins derived from male reproductive organs and transferred to females within the ejaculate (reviewed in Avila et al. 2011; Wigby et al. 2020). Many postmating changes in D. melanogaster are induced by a 36 amino acid seminal “sex peptide” (SP); its most emblematic effects are decreasing the receptivity of females to remating and increasing egg production (Chen et al. 1988; Chapman et al. 2003; Liu and Kubli 2003). SP exerts many of its effects through a G protein-coupled receptor, SPR (the SP receptor) that is expressed in neurons as well as in reproductive tract tissues (Yapici et al. 2008; Hasemeyer et al. 2009; Yang et al. 2009).
Impairment or loss of SPR in D. melanogaster leads to a reduction in postmating egg laying and to refractoriness to remating (Yapici et al. 2008), as well impairing other effects including postmating sleep patterns (Garbe et al. 2016), sperm release (Avila et al. 2015), long-term memory (Scheunemann et al. 2019), and gut growth (White et al. 2021). These responses have been shown to be integrated largely through the Sex Peptide sensory neurons (SPSN) and the Sex Peptide abdominal ganglion (SAG) (Rezával et al. 2012; Feng et al. 2014; Wang et al. 2020; Wang et al. 2021; reviewed in Okamoto and Watanabe 2022). Consistent with the rapid evolution seen for many reproductive proteins (e.g. Civetta and Singh 1998; Swanson and Vacquier 2002; Haerty et al. 2007), SP is only found in some species of Drosophila (Tsuda and Aigaki 2016; Hopkins et al. 2023). However, SPR is conserved across several Drosophila species (Kim et al. 2010) and other insects (Yapici et al. 2008). This widespread sequence conservation likely reflects the role of SPR as a myoinhibitory peptide (MIP) receptor, which has been suggested to be its ancestral function. It has been hypothesized that SPR was co-opted to a reproductive function in lineages where it acquired expression in the female reproductive tract (Kim et al. 2010; Poels et al. 2010; Tsuda and Aigaki 2016). Experiments aimed at investigating a reproductive role for MIPs suggest that they do not induce postmating responses, as mating receptivity was not altered by either pan-neuronal knockdown of MIPs or injection of MIPs in D. melanogaster females (Kim et al. 2010).
SPR has also been found to be important for postmating responses in some insects in addition to Drosophila. RNAi knockdown of SPR expression in olive fruit flies (Bactrocera oleae) and Oriental fruit flies (Bactrocera dorsalis) led to lower oviposition rates (Zheng et al. 2015; Gregoriou and Mathiopoulos 2020). Additionally, mutation of the SPR gene in B. dorsalis resulted in a reduction in egg-laying ability, viability of eggs laid, and underdeveloped ovaries (Chen et al. 2023). Injection of D. melanogaster SP into the cotton bollworm, Helicoverpa armigera (which has no obvious SP gene in its genome) suppressed sex pheromone production leading to decreased calling behaviors; these effects did not occur if SPR was knocked down simultaneous with SP injection, indicating SP functioned through SPR (Hanin et al. 2012). Moreover, H. armigera females with little to no SPR laid significantly fewer eggs after mating and displayed altered pheromone calling behavior and remating rates after 24 and 48 h (Hanin et al. 2012; Liu et al. 2021). A reproductive role for SPR was also reported in the tobacco cutworm (Spodoptera litura): females with SPR knockdown laid fewer eggs than controls after injection of male accessory gland lysate and remained receptive to mating (Li et al. 2014).
Collectively, these studies indicate that SPR plays important postmating roles in several insect taxa. However, the role of SPR in medically important mosquitoes, such as Aedes aegypti, has not been determined. If SPR is involved in inducing postmating responses it could potentially be a target for mosquito reproductive control efforts (Cator et al. 2021). RNAseq analyses of Ae. aegypti have identified SPR transcripts in neuronal and reproductive tissues (Alfonso-Parra et al. 2016; Matthews et al. 2016), consistent with its expression patterns in those Drosophila species where it exerts a reproductive role (Tsuda et al. 2015). Although the Ae. aegypti genome does not contain a recognizable SP gene (Hopkins et al. 2023 and our unpublished genome searches), neither does the genome of H. armigera, where SPR is necessary for inducing postmating responses (Hanin et al. 2012; Liu et al. 2021).
To test the functional role of SPR in mediating Ae. aegypti postmating responses, we employed CRISPR/Cas9 genome editing to generate 2 independent mutant alleles in the SPR gene. We tested female mutants for fertility and survival, as well as for the most salient reproductive phenotypes: postmating egg laying and decreased mating receptivity, both of which are induced by seminal fluid proteins (Leahy and Craig 1965; Craig 1967; Helinski et al. 2012a; Villarreal et al. 2018). Our data indicate that despite the sequence and biochemical conservation of SPR (Yapici et al. 2008; Kim et al. 2010; Lee et al. 2020), it does not play a detectable role in these postmating changes in Ae. aegypti. Consistent with this conclusion, our injection of synthetic Drosophila SP (which can bind to and activate Ae. aegypti SPR in vitro (Yapici et al. 2008; Kim et al. 2010)) or injection of Drosophila male accessory gland (dMAG) extracts into virgin or gravid Ae. aegypti females, gave no effect on these postmating behaviors. Our results contrast with reported SPR activity in other Diptera such as D. melanogaster and in Lepidoptera (H. armigera and S. litura), suggesting that SPR may have been repeatedly co-opted for a reproductive function. Our results indicate that unknown signaling pathways are likely responsible for the postmating switches governing long-term refractoriness to remating and induction of egg laying in Ae. aegypti.
Materials and methods
Our groups generated Spr-knockout alleles and characterized them independently. These mutant alleles, which were unique and in different genetic backgrounds, yielded the same results where compared. We refer to these alleles, generated at Cornell and Johns Hopkins respectively, as SprΔ235 and SprECFP.
Mosquito rearing
SprΔ235: The SprΔ235 NHEJ allele was generated in a Thai background (e.g. Villarreal et al. 2018), and its controls and backcrosses were of that background. Our Thai colony of Ae. aegypti, derived from field-collected mosquitoes (15°72N, 101°75E) maintained since 2009 with annual supplementation, and a homozygous transgenic line with DsRed-labelled sperm (Smith et al. 2007) were used. After vacuum-hatching eggs, larvae were reared under uniform conditions to ensure medium body size adults (Helinski and Harrington 2011). Mosquitoes were maintained at 28°C and 70% relative humidity with a 10 h light:10 h dark cycle that included 2 h of simulated dusk and dawn. Virgin males and females were obtained by separating pupae by sex prior to adult eclosion. All mosquitoes were maintained on 10% w/v sucrose. Biological replicates were derived from independently hatched cohorts.
SprECFP: The SprECFP homology-directed repair (HDR) allele was generated in the LVPib12 strain (Nene et al. 2007), which also served as the genetic background for assays with this mutant allele. Mosquitoes were maintained with a 12 h light:dark photoperiod at 27°C and 80% relative humidity using a standardized rearing protocol (Wohl and McMeniman 2023a). Adult mosquitoes were provided constant access to a 10% w/v sucrose solution and virgins were isolated as pupae and sexed within 12 h of emergence. The Exu-Cas9 strain (marked with Opie2-DsRed) expressing Cas9 under the maternal germline promoter exuperantia (Li et al. 2017) was used for CRISPR/Cas9 mutagenesis. Exu-Cas9 was backcrossed to LVPib12 each generation for stock maintenance.
SPR mutant generation via CRISPR
SprΔ235: We generated a mosquito line harboring a NHEJ-based deletion in exon 2 of Spr (AAEL019881), by CRISPR/Cas9-based editing according to the procedures in Kistler et al. (2015). Two sites for Cas9 cleavage targeting exon 2 were identified using CHOPCHOP (Labun et al. 2016) and validated in vivo. Guide RNAs (gRNA) were synthesized in vitro using the MEGAScript kit (Thermo Fisher Scientific) from DNA generated from a template-free polymerase chain reaction (PCR) consisting of a primer targeting the Cas9 cut site with a T7 promoter and a universal reverse primer (sequences listed in Supplementary Table 1). After reaction purification with MEGAClear (Thermo Fisher Scientific) and size confirmation using a Bioanalyzer 2100 (Agilent Technologies), 40 ng/μL each of the 2 gRNAs together with 333 ng/μL Cas9 (PNA Bio) was injected into Thai strain embryos by the University of Maryland Insect Transformation Facility (https://www.ibbr.umd.edu/facilities/itf). After G0 females were backcrossed to Thai wild type males, blood fed, and allowed to lay eggs, genomic DNA from the G0 mosquitoes was extracted using Puregene reagents (Qiagen) and PCR was used to detect deletions in the Spr gene (Supplementary Table 1 and Fig. 1a). Eggs from deletion-positive females were hatched and backcrossed for 5 generations. Heterozygous males and females were crossed, and progeny screened for genotype using genomic DNA extracted from a single leg (Smith et al. 2018). Males and females homozygous for the Spr deletion were crossed together to generate a stable mutant line (SprΔ235/Δ235). A wild type control line (Spr+/+) derived from a backcross of heterozygous SprΔ235/+ individuals was also maintained. SprΔ235 heterozygotes (SprΔ235/+), derived from a cross between SprΔ235 homozygotes and this wild type line, served as an additional control.
SprECFP: We generated a disruptive insertion in exon 2 of Spr using CRISPR/Cas9-mediated homologous recombination. Guide RNAs were designed targeting exon 2 of Spr using CHOPCHOP (Labun et al. 2016) and validated individually with in vitro cleavage assays using a PCR amplicon spanning the putative cut sites, each in vitro transcribed gRNA (MEGAscript, Invitrogen) and Cas9 protein (PNA bio). A single gRNA with validated cleavage activity was chosen for incorporation into a synthetic gBlock (Integrated DNA Technologies) that contained the Ae. aegypti U6 (AAEL017774) promoter, a modified gRNA scaffold and a terminator (Chen et al. 2021) for subsequent subcloning into the backbone of the following HDR donor plasmid. We first integrated homology arms into the base donor plasmid pSL1180-HR-PUbECFP (Addgene #47917) (McMeniman et al. 2014), in which enhanced cyan fluorescent protein (ECFP) is under the control of the polyubiquitin protmoter. Homology arms 1,425 bp (left) and 1,797 bp (right) in length flanking the gRNA cut site were amplified with CloneAmp (Takara) from a consensus genomic DNA clone covering Spr exon 2 and flanking introns (primers listed in Supplementary Table 2). We PCR-amplified the gBlock using primers with 5′ In-Fusion adaptors (Supplementary Table 2). Next, we integrated the gBlock fragment with the U6 expression cassette into the backbone of this donor plasmid at the NdeI restriction site using In-Fusion cloning (Takara). This yielded a final construct (pMW001) containing a U6 expression cassette and HDR cassette targeting Spr exon 2.
We microinjected 300 ng/μL of pMW001 (endotoxin-free) into the posterior pole of Exu-Cas9 pre-blastoderm stage embryos (LVPib12 strain) using an Eppendorf FemtoJet 4X. Transformed G1 larvae with constitutive ECFP fluorescence were identified by screening for fluorescent bodies (PUb-ECFP) at the L3-L4 stage. Transgenic animals were then outcrossed to LVPib12 for 2 generations before crossing the lines to generate a homozygous viable Spr mutant strain (SprECFP/ECFP). Insertion of the disruptive donor cassette into Spr exon 2 was confirmed by PCR amplification of genomic DNA using a 3 primer genotyping strategy (Supplementary Table 2 and Fig. 1b) where one forward primer was centered on the CRISPR cut site so that it would only anneal to the wild type allele, one forward primer was placed in the polyubiquitin (PUb) sequence in the integrated cassette, and one reverse primer was nested in the right homology arm. This yielded a 342 bp amplicon for the wild type allele with an intact gRNA site, and a 524 bp amplicon for the mutant allele.
Identification and characterization of wild type and mutant Spr transcript isoforms
SprΔ235: Total RNA was isolated from Thai Spr+/+ and SprΔ235/Δ235 female abdomens and thoraces (n = 3) in TRIzol (Invitrogen) that were frozen at −80°C and homogenized with a motorized pestle. Following isopropanol precipitation and pellet washes in 75% ethanol, 500 ng resuspended RNA was treated with DNase and converted to cDNA with the iScript gDNA Clear cDNA Synthesis Kit (BioRad) per manufacturer instructions.
SprECFP: To generate cDNA from LVPib12 Spr+/+ and SprECFP/ECFP, abdomens and thoraces of 3-to-4-day-old virgin Ae. aegypti females were frozen in liquid nitrogen and homogenized with a motorized pestle. Total RNA was extracted using the Purelink RNA Mini Kit (Invitrogen) and cleaned with the Zymo RNA clean and Concentrator kit. Total RNA (3 μg) was used for first strand synthesis with Superscript III (Invitrogen).
The resulting cDNA at a 1:4 dilution for all samples was used as template for PCR reactions (Q5; New England Biolabs) using the primers “Spr RT-PCR 5′UTR Forward” and “Spr RT-PCR 3′UTR Reverse” anchored in the 5′ untranslated region (UTR) and 3′UTR of Spr, respectively (Supplementary Table 3 and Fig. 2). A control 81 bp amplicon from the Ae. aegypti actin-1 gene (Aaeact-1, Genbank U20287) was amplified using the primers “Actin RT-PCR Forward” and “Actin RT-PCR Reverse” (Cook et al. 2006).
Splicing patterns were identified and characterized by sequence analysis of amplicons generated using the primers “Spr RT-PCR Exon 1 Forward” and “Spr RT-PCR Exon 6 Reverse”, anchored at the end of the first exon and the beginning of the sixth exon of Spr, respectively (Supplementary Table 3). For sequence analysis, PCR amplicons were resolved using standard agarose gel electrophoresis, and visible bands that were consistently amplified in SprECFP, SprΔ235, and wild type cDNA were excised and cleaned using the Monarch gel extraction kit (New England Biolabs). Target amplicons were then cloned using the TOPO-TA cloning kit (Invitrogen) and sequenced using Nanopore sequencing (Plasmidsaurus). Identified transcripts are summarized in Supplementary Fig. 3.
To examine the likelihood of downstream start (ATG) codons being competent as in-frame alternative translation start sites, we ran the cDNA sequence through the ATGpr algorithm (https://atgpr.dbcls.jp; Nishikawa et al. 2000). We identified 2 potential ATG sites (M49 and M140, Supplementary Fig. 4d) with limited translational start potential downstream of targeted CRISPR sites, but upstream of the first transmembrane domain.
Cloning of wild type and Spr mutant cDNA target regions for cell-based activity assays
Target wild type and mutant Spr transcript regions were cloned into the transient cDNA expression vector pME18S at XhoI-NotI restriction sites by In-Fusion cloning (Takara). Spr cDNA regions that were inserted into pME18S included 3 isoforms derived from wild type cDNA (Spr A, Spr B, and Spr C); one isoform from SprΔ235 mutant cDNA (SprΔ235) and 5 isoforms from Spr ECFP mutant cDNA (Spr ECFP-B, Spr ECFP-1, Spr ECFP-2, Spr ECFP-3, Spr ECFP-4, and Spr ECFP-5) (Supplementary Fig. 4a–c). Additionally, 2 hypothetical Spr A transcripts (Spr A-M49 and Spr A-M140) that are 5′ truncated and which could employ potential translational start sites downstream of the editing events characterized in Spr ECFP and SprΔ235 mutants, respectively, were constructed by PCR (see Supplementary Table 4 for primers) and inserted into pME18S (Supplementary Fig. 4d). Plasmids for transient transfection were purified using the ZymoPure II Plasmid Maxiprep Kit.
Mating, fertility, and body size of the SprΔ235 allele in Thai background
Mating was examined by mating en masse 2-to-3-day-old homozygous (SprΔ235/Δ235) or heterozygous (SprΔ235/+) females to 3-to-4-day-old heterozygous males. Mating was performed in an 8 L container for 24 h in the absence of 10% sucrose. Mated females were dissected to confirm mating status by scoring the presence of sperm in the spermathecae. Eggs from both homozygous and heterozygous females mated to heterozygous males had similar hatch rates. Two biological replicates were performed from independently hatched cohorts. To determine relative body sizes of the mosquitoes, we used wing-length measurements as a proxy (Nasci 1990).
Postmating receptivity
SprΔ235 allele in Thai background: A single 2-to-3-day-old homozygous (SprΔ235/Δ235) or heterozygous (SprΔ235/+) female was released into an 8 L container with 10 3-to-4-day-old heterozygous (SprΔ235/+) males and observed closely. Once the female mated (maintained a copula for >8 sec), the mating pair was immediately collected and the female was placed in a 0.5 L cup. The mated male was excluded from additional matings and the mating arena was replenished with an additional virgin male. Two 3-to-4-day-old males with DsRed-marked sperm were introduced into each 0.5 L cup either immediately or 24 h after the first female mating and then held together for 24 h. The lower reproductive tract of each female was then dissected and the spermathecae and bursa examined under a fluorescence microscope for the presence of red sperm along with nonfluorescent sperm, indicative of remating. As a control, individual virgin homozygous females or virgin heterozygous females with 2 DsRed-sperm males were placed together in 0.5 L cups for 24 h (n = 10 for each genotype for each replicate) and the dissected reproductive tracts of females were examined to make sure the DsRed-sperm males could mate successfully with SprΔ235 mutant females (17/20 mated for SprΔ235/+ females; 20/20 mated for SprΔ235/Δ235 females). Two biological replicates from independently hatched cohorts were conducted. The genotype order of initial matings was reversed in replicate 2 to avoid bias. Remating rates after 1 h from SprΔ235/+ females were consistent with wild type Thai females mated with wild type Thai males (between 8–30% this study; also Degner and Harrington 2016).
SprECFP in LVPib12 background: To similarly test if SprECFP mutants were refractory to remating, a single 4-to-5-day-old homozygous (SprECFP/ECFP) or heterozygous (SprECFP/+) female was released into a 355 mL insulated solo cup with 10 5-to-6-day-old wild type (Spr+/+) LVPib12 males and observed closely. Once the female mated (maintained a copula for >8 sec), the mating pair was immediately collected. The male was removed and discarded, and the female was placed in a separate solo cup with other newly mated females. This mating scheme was then replicated until the target number of copulated females per genotype was obtained. After 1–1.5 h following their first mating, these females were introduced into a 20.3 × 20.3 × 20.3 cm cage (Bioquip, 1450A) with 5-to-6-day-old males with DsRed-marked sperm (Smith et al. 2007). The female to male ratio was 1:2 and they were held together for 24 h at which point males were removed by aspiration. The lower reproductive tract of each female was then dissected and the spermathecae examined under a fluorescence microscope for the presence of red sperm, indicative of remating.
Control matings were performed between homozygous (SprECFP/ECFP) females (n = 32) and wild type (Spr+/+) LVPib12 males as described above to estimate insemination rates after the first mating (31/32 mated). As a supplemental control to validate mating compatibility in this postmating receptivity assay, homozygous (SprECFP/ECFP) females (n = 20) were placed with 2 times the number DsRed-sperm males in a 20.3 × 20.3 × 20.3 cm cage (Bioquip, 1450A) for 24 h and the dissected reproductive tracts of females were examined for the presence of red sperm (20/20 mated). Finally, twenty virgin females of each genotype (SprECFP/ECFP and SprECFP/+) were dissected to confirm virginity (20/20 for each genotype were unmated).
SprECFP oviposition assays
For oviposition assays with virgin or mated wild type (Spr+/+), heterozygous (SprECFP/+) or homozygous (SprECFP/ECFP) females, 3-to-5 day old females were blood fed to repletion using anesthetized Swiss-Webster mice (Johns Hopkins University Animal Care and Use Committee, Approval Number: MO21H373). For assays with mated females, all crosses were made between virgin females of the target genotype and 2-day-old wild type virgin males. Crosses were established with a sex ratio of 1 female: 2 males in a 20.3 × 20.3 × 20.3 cm cage (Bioquip, 1450A) with constant access to 10% sucrose. These small group crosses were left to mate for 48 h before blood feeding the females. Seventy-two hours postblood feeding, females were placed in single oviposition vials, each containing a filter paper cone moistened with 4 mL dH2O (Wohl and McMeniman 2023b). Females were allowed to lay eggs on the filter paper for 48 h at which point they were removed from vials and eggs were counted. Egg papers from females who died before collection were not counted. Counts were analyzed statistically as described below.
Drosophila SP injection
Synthetic SP (3 pmol; CanPeptide) in 69 nl Aedes saline (Hayes 1953) was injected into the thorax of virgin 2-to-5-day-old Thai background Ae. aegypti (n = 18) and Canton S D. melanogaster females (n = 30) with a Nanoject II (Drummond, Broomall, PA). Controls included injection with Aedes saline and a noninjected group. Males were introduced (ratio 1:1) 12 h after injection. Mating events were confirmed by immediate direct observation for Drosophila. The number of eggs laid by females in the treatment groups was recorded. Ae. aegypti females were examined for sperm presence or absence after being held with males for 2 days.
To independently test if D. melanogaster SP (dSP) could induce oviposition behavior in the Ae. aegypti LVPib12 genetic background, we injected synthetic dSP (Aapptec, sequence: WEWPWNRKOTKFOIOSONORDKWCRLNLGPAWGGRC with a disulfide bridge between the 2 cysteines) (Chen et al. 1988) dissolved in phosphate-buffered saline (PBS; Gibco, pH 7.2) into the thorax of virgin females. We injected a concentration series spanning the dynamic range of dSP concentrations known to elicit oviposition responses in D. melanogaster. Specifically, we performed 150 nl injections of 10 μM, 100 μM, and 1 mM dSP which equates to 1.5 pmol, 15 and 150 pmol of dSP respectively. First, 3-to-5-day-old virgin females were blood fed using anesthetized Swiss-Webster mice, and 48 h later, synthetic dSP or D. melanogaster male accessory gland homogenate (dMAG), Ae. aegypti male abdominal tip (aeMAT) homogenate (preparation described in next section) and PBS controls were injected into the thorax with a Nanoject II (Drummond). Oviposition was assayed as described above.
Accessory gland homogenate injection assays
We tested both the effects of injecting Drosophila and Aedes male accessory gland (MAG) homogenates on mating and egg laying:
To first evaluate the effect of Drosophila MAG (dMAG) on Thai Ae. aegypti mating behavior and oviposition, accessory glands were dissected from 38 6-day-old virgin D. melanogaster Canton S males in 38 μl Aedes saline (Hayes 1953). The tissues were ground, sonicated in a water bath for 15 sec, and then spun at 13,400 rpm for 15 min at 0°C. The supernatant was removed and virgin Thai Ae. aegypti females (3-to-6 days old) were injected in the thorax with 0.25 μL of D. melanogaster homogenate (equivalent to 0.25 of an accessory gland). Females injected with Aedes saline were used as controls as well as females injected with Ae. aegypti male accessory gland extract (aeMAG); and females that were mated without injection. Females were divided into 2 cohorts. One group was tested for refractoriness to mating by placing females in individual 0.5 L cups with males (aged 7-to-9 days) 3 days after injection for 2 days (n = 10). Two days later, females were removed and their spermathecae dissected to determine insemination status. The other group was tested for oviposition behavior. Females were blood fed on a human arm (M.E.H.) 4 days after injection. Unfed females were removed and offered blood the following day. Three to four days after feeding, fed females were placed in individual cups (0.5 L) for oviposition. Four days later, females were removed and the number of eggs for each female was counted. Live females with zero deposited eggs were dissected to determine insemination status (only when females had been exposed to males) and for the presence of fully developed eggs in ovaries. Virgin females injected with saline and noninjected females mated with males from the same cohort were used as controls.
In the second independent assessment of the impact of Drosophila and Aedes accessory gland extracts on egg laying, Drosophila MAGs were prepared by dissecting accessory glands from 50 fly abdomens (Canton-S strain) into 50 μL of PBS (Gibco, pH 7.2) and homogenized with a pellet pestle motor (Kimble). This mixture was centrifuged at 14,000×g for 30 min at 4°C. The supernatant was applied to a 0.22 μm filter column (Millipore) and centrifuged at 14,000×g for 10 min at 4°C. The liquid flow through (dMAG) was kept at 4°C and injected within 1 week of preparation. Aedes accessory gland homogenates were prepared using an identical protocol from 3-to-5-day-old LVPib12 virgin males that had been anesthetized on ice with the following modifications: The male abdominal tip (MAT) was dissected into a drop of PBS by grasping the genital claspers with forceps and pulling the terminal abdominal segment away from the rest of the abdomen. This isolated the last segment of the abdomen including the male accessory glands and other reproductive organs. MAT from 200 males were dissected into 200 μL PBS (Gibco, pH 7.2) on ice (Helinski et al. 2012a). The liquid flow through after filtration (aeMAT) was kept at 4°C and injected within 2 weeks of preparation.
To test if SPR is required for the postmating reduction in mating receptivity resulting from injection of Ae. aegypti MAG (aeMAG) homogenate, Thai wild type and SprΔ235 homozygous females were injected with aeMAG as previously described (Amaro et al. 2021). Modified PBS buffer alone (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 3 mM KH2PO4, 2 mM CaCl2 pH 7.0) served as a control. Two days after injection, females were blood fed on a human host (S.P.) for 8 min, fully engorged females were separated individually into 0.5 L cups and 2 wild type Thai males were introduced 5 days postblood feeding. After 2 days female spermathecae were dissected and scored for the presence or absence of sperm.
Cell-based assays
Cell-based assays were performed as in Duvall et al. (2019). Briefly, HEK293T cells (Thermo Fisher Scientific) were maintained using standard protocols in a Thermo Scientific water jacketed carbon dioxide incubator. Cells were transiently transfected with 0.5 μg each of plasmid (pME18S) expressing GCaMP6s (Chen et al. 2013), mouse Gqa15 (Offermanns and Simon 1995), and either wild type SPR (AAEL019881) or other transcript variants (Supplementary Fig. 4) using Lipofectamine 2000 (Invitrogen). Transfected cells were seeded into 96 well plates (Greiner Bio-one) and incubated overnight in Fluorobrite DMEM media (Thermo Fisher Scientific) supplemented with Fetal Bovine Serum (Invitrogen) at 37°C and 5% carbon dioxide. Cells were directly imaged in 40 μL Fluorobrite DMEM media (Thermo Fisher Scientific) using green fluorescent protein-channel fluorescence of a BioTek Synergy Neo plate reader with liquid handling system and automated dispenser. Whole male homogenate was obtained using a similar collection method to the MAT homogenate described above. Briefly, 10 whole LVPib12 males were homogenized in 250 μL PBS on ice, centrifuged at 14,000×g for 30 min at 4°C, supernatant was applied to a 0.22 μm filter column (Millipore) and centrifuged at 14,000×g for 10 min at 4°C. The liquid flow through after filtration was stored at 4°C. Peptides were prepared at 3 × concentration in reading buffer [Hank's Balanced Salt Solution (GIBCO), 20 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; Sigma-Aldrich), pH 7.4], male homogenate was diluted to 0.25 male equivalent in reading buffer and tested within 1 week of collection. Wells were imaged every 0.5 sec for 3 min. 20 μL of peptide or male homogenate was added to each well after 30 sec of baseline fluorescence recording. Normalized responses were calculated as (ΔF/F0)experimental – (ΔF/F0)no receptor control. AstAR (AAEL006076) response to Ast1 was used as a positive control in each plate. D. mel SP (Aapptec) and Ae. aegypti peptides previously detected in Predel et al. (2010) and tested in vitro in Duvall et al. (2019) were synthesized by Bachem and maintained as lyophilized powders or in 100% dimethyl sulfoxide (DMSO) (Sigma-Aldrich) stock solutions at −20°C. All peptides were tested in a solution that contained < 1% DMSO. Eight to fourteen replicates were performed per transfection and each experimental well included matched positive and negative (no receptor transfected) control wells in the same plate. Any well in which the matched positive control response was < 0 was excluded from analysis.
Data analysis. Power analysis using G*power (Faul et al. 2007) was used to determine appropriate sample size prior to experiments based on preliminary data. Statistical analysis was performed using SPSS (IBM Statistics, version 25). A Levene's test was first performed to determine homoscedasticity of male and female body size data, then wing lengths were compared with Kruskal–Wallis and Mann–Whitney tests. Replicate effects were analyzed and replicates were combined when appropriate. Remating was analyzed using a binary generalized linear mixed model to compare differences by time points and genotype (SprΔ235/Δ235 or heterozygous (SprΔ235/+); to compare remating in the SprECFP mutants vs. wild type, a 2 sample proportions test was performed. Egg counts from oviposition assays were analyzed with Graphpad Prism 9.4.1 software with a one-way Analysis of Variance followed by post hoc t-tests between experimental groups. P values were adjusted for multiple comparisons with Dunnett's correction when the comparisons were all against a control group; and adjusted with Tukey's correction when comparisons were made between experimental groups. In cell-based assays fluorescence signal responses were analyzed with Graphpad Prism 9.3.1 software with one sample t and Wilcoxon Signed Rank test or Dunn's multiple comparisons test.
Results
Generation and characterization of Aedes aegypti Spr mutants
To test whether SP receptor (SPR) is required for canonical postmating receptivity and egg-laying behaviors in Ae. aegypti, we generated 2 independent mutant alleles in the wild type Spr gene locus using CRISPR/Cas9 mutagenesis (Fig. 1a).
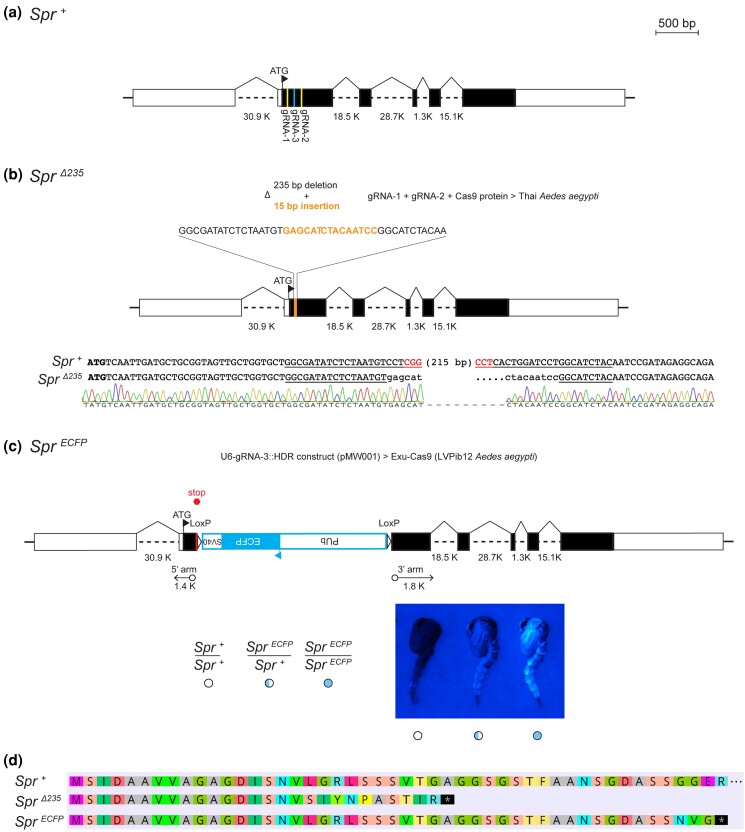
Fig. 1.
Generation of Aedes aegypti SP receptor mutants. a) Diagram of the wild type Ae. aegypti SP receptor (Spr+) genomic locus. Boxes denote exons which are open for untranslated regions and filled for the open reading frame. Guide RNA sites used for generating mutants are shown. b) Diagram of the SprΔ235 mutant allele generated by deletion of 235 bp and insertion of 15 bp using gRNA1 and gRNA2. Sanger trace (below) validates the altered sequence of this allele relative to wild type. Translational start site is bold, gRNA target site underlined, and protospacer adjacent motif sequence highlighted in red. c) Diagram of Spr ECFP mutant allele generated by insertion of a disruptive ECFP expression cassette via homology directed repair. Representative images of wild type (open circle), heterozygous (half cyan circle) and homozygous (full cyan circle) SprECFP mutant pupae are shown below. d) Amino acid alignments of wild type and truncated SprΔ235 and SprECFP mutant alleles. Premature stop codons are indicated by the star.
We generated a NHEJ allele (SprΔ235) in the Thai Ae. aegypti genetic background by injecting 2 gRNAs targeting exon 2 of Spr (the first coding exon of this gene) along with Cas9 protein into pre-blastoderm stage embryos (Fig. 1b). Subsequently using PCR genotyping (Supplementary Fig. 1a), we successfully recovered the SprΔ235 mutant allele consisting of a 235 bp deletion (bp 50–285 in the open reading frame) with an insertion of 15 bp in this exon.
We also generated an independent HDR allele (SprECFP) in the LVPib12 Ae. aegypti genetic background by injecting a donor construct with a gRNA expression cassette in its backbone into pre-blastoderm stage embryos from the germline Exu-Cas9 Ae. aegypti strain (Fig. 1c). In this allele, a 2,617 bp cassette was inserted by homology-dependent repair into exon 2 of Spr. This disruptive insertion included a stop cassette and marks mutants visibly with ECFP under the control of the polyubiquitin promoter (Fig. 1c) and can be detected in the Spr locus by a custom genotyping assay using PCR (Supplementary Fig. 1b).
Predicted protein structure based on amino acid alignments indicate that relative to the wild type Spr+ allele, both SprΔ235 and SprECFP mutant alleles are likely truncated before the first transmembrane domain of SPR (Fig. 1d). Specifically, the SprΔ235 allele has a frameshift mutation in the Spr coding sequence that yields a premature stop codon at amino acid 28, while precise insertion of a stop codon in SprECFP directly after amino acid 47 is similarly predicted to prematurely terminate translation.
We then examined the Spr transcripts in wild type and mutant lines by reverse transcription (RT)-PCR to determine whether these early nonsense mutations in the open reading frame resulted in nonsense-mediated decay of the transcripts (Lykke-Andersen and Jensen 2015). However, we observed transcript levels in mutant lines from both backgrounds that were comparable with wild type levels (Supplementary Fig. 2). To determine whether other mechanisms might yield functional SPR in our mutants, we searched for potential in-frame alternative translational start sites (Nishikawa et al. 2000) and also identified and characterized splice variants in the LVPib12 mosquito background (Supplementary Fig. 3). To test for the possibility that SprΔ235 and SprECFP mutant alleles could generate isoforms that function like full-length SPR, we cloned cDNA sequences observed in wild type and both mutant backgrounds, including observed splice variants, into an expression vector (Supplementary Fig. 4a–c).
We next tested for activity with known SPR ligands in a cell-based assay using HEK293T cells cotransfected with each of these vectors and a promiscuous G protein (Gqa15) (Offermanns and Simon 1995) and the genetically encoded calcium sensor GCaMP6s (Chen et al. 2013). Activation of a given receptor is read out by calcium-induced increase in fluorescence of GCaMP6s. We confirmed that the Ae. aegypti SPR receptor is activated by homogenate extracted from whole male Ae. aegypti mosquitoes, MIP peptides, and D. melanogaster SP (dSP) consistent with previous studies (Yapici et al. 2008; Kim et al. 2010) (Supplementary Fig. 5a and b). To determine whether the mutant or alternate transcript products are functional we tested their responses to 15 μM MIP1, which reliably activates full-length SPR. Although full-length SPR responds to this stimulus, responses from all other isoforms detected in mutant or wild type backgrounds were significantly reduced (Supplementary Fig. 5c). Among the cDNA constructs, we also engineered ones based on the possibility of cryptic translational start sites downstream of our CRISPR mutations, but upstream of the first transmembrane domain (M49 and M140; Supplementary Fig. 4d). Similar to the other SPR constructs that are not full-length, responses of these amino-terminally truncated receptors to MIP1 were significantly reduced in the cell-based assay (Supplementary Fig. 5c). Hence, we are confident that both Spr mutations that we generated in Ae. aegypti are very strong loss-of-function, indeed likely null, alleles.
Spr-knockout mutants are viable and fertile
We determined that both of our loss-of-function Spr mutant alleles (SprΔ235 and SprECFP) were homozygous viable. We have successfully maintained these homozygous mutant lines for more than 6 years and 1 year, respectively since their establishment, using standard protocols.
Detailed morphometric and fitness characterization of the SprΔ235 allele, revealed that homozygous mutant males were smaller (average = 1.99 to 2.16 mm wing length) than wild type (Spr+/+) or heterozygous (SprΔ235/+) males reared under same conditions (2.13 to 2.23 mm) across replicates and remating experiments; Z = −3.79, P < 0.00 (wild type), Z = −4.05, P < 0.00 (heterozygous). Mutant females were smaller in body size compared to wild type and SprΔ235/+ females in replicate 1: SprΔ235/Δ235 female = 2.53 ± 0.15 mm; Spr+/+ female = 2.78 ± 0.20 mm (Z = −4.039, P < 0.01), but not significantly different in Replicate 2: SprΔ235/+ female = 2.9 ± 0.06 mm; SprΔ235/Δ235 female = 2.88 ± 0.08; Spr+/+ female = 2.91 ± 0.05. Mutant females mated successfully with males at the same percentage as controls, as demonstrated by the presence of sperm in their spermathecae after 24 h [96% SprΔ235/Δ235 (87/90) and 99% SprΔ235/+ (91/92) females mated with SprΔ235/+ males].
SPR in vitro response screen
The smaller body size of our Spr mutants prompted us to further investigate the nature of the ligand of SPR. Work from Kim et al. (2010) suggests that Aedes SPR can be potently activated by non-SP, non-MIP ligands found in whole mosquito extracts. We performed in vitro assays to profile Ae. aegypti SPR responses to various classes of neuropeptides using the cell-based assay described above. SPR responses were profiled to a 10 μM dose of 50 known neuropeptides. Although modest responses were noted to several neuropeptides (Ast3, AT, sNPF1, sNPF2 + 4, TKRP2, TKRP3), these showed low efficacy compared to positive controls (AstAR activation by Ast1 peptide) (Supplementary Fig. 5d). We did not observe activation by Head Peptide-1 (HP-I) (Supplementary Fig. 5a and d), a peptide known to play a role in short-term receptivity suppression (Duvall et al. 2017). We thus did not identify additional high efficacy agonists of SPR among our neuropeptide panel at this ligand concentration.
Spr-knockout females show normal changes in postmating refractoriness
After mating, Ae. aegypti females quickly become refractory to mating with a subsequent male (Craig 1967; Helinski et al. 2012b; Degner and Harrington 2016). While initial changes are induced by a seminal peptide HP-1 (Duvall et al. 2017), this effect is temporary, acting only within 1 h of mating. The nature of the molecule(s) that induce(s) lifelong refractoriness in these females is unknown.
By assessing the presence of second-male sperm in spermathecae, we initially observed that SprΔ235/Δ235 homozygous mutant females are as likely to mate with a second male as are SprΔ235/+ control females, regardless of the time after the first mating (Fig. 2a; P = 0.88 and P = 0.18 for tests immediately, and 24 h, after mating, respectively). To confirm this result, we injected male accessory gland homogenate (MAG), which is known to induce mating refractoriness (Helinski et al. 2012a), from wild type Thai males into both SprΔ235/Δ235 and wild type Thai females and found no difference in mating receptivity when males were presented to females 5 days after injection [SprΔ235/Δ235, 1.37% females remated (n = 74); wild type, 1.16% females remated (n = 86)]. This further demonstrates that SPR is not required for the postmating reduction in mating receptivity resulting from injection of Ae. aegypti MAG homogenate.
Fig. 2.
SP receptor is not required for refractoriness to remating over varied timescales or induction of egg laying in Aedes aegypti. a) Percent of SprΔ235 heterozygous (half black circle) and homozygous mutant (full black circle) females that remated (gray shading) immediately (<1 h) or 24 h after their first mating. Raw number of females in each remating category is denoted within each bar. Cumulative sample size (n) from 2 independent biological replicates indicated. b) Percent of SprECFP heterozygous (half cyan circle) and homozygous (full cyan circle) mutant females that remated (gray shading) 1 to 1.5 h after their first mating. Raw number of females in each remating category are denoted within each bar. Sample size indicated below genotypes. c) Eggs laid per female for wild type (open circle), SprECFP heterozygous (half cyan circle) and SprECFP homozygous (full cyan circle) mutant genotypes after a bloodmeal that were either virgin (−) or mated (+). n.s. = P > 0.05 (Dunnett's multiple comparisons test). Box plots show median, interquartile range, and 5–95 percentile.
Similarly, we observed in an independent postmating receptivity assay, that SprECFP/ECFP homozygous mutant females are as likely to mate with a second male as are SprEFCP/+ control females (Fig. 2b; Z = 0.88, P = 0.37). In this assay, females were tested for their propensity to remate within 1–1.5 h after initial mating and we similarly detected no significant differences in postmating receptivity.
Thus, Spr does not seem to be necessary for induction of postmating refractoriness, including short, medium or long-term refractoriness, in Ae. aegypti females.
Spr is not required for egg laying in Aedes aegypti
Mating stimulates oviposition in gravid Aedes aegypti females (Lang 1956; Judson 1967), and this effect is mediated by unknown protein/s transferred to females in male accessory gland fluid (Leahy and Craig 1965; Hiss and Fuchs 1972; Villarreal et al. 2018). To test whether SPR is required for postmating oviposition behavior in Ae. aegypti, we next tested whether egg laying was impacted using the SprECFP mutant allele.
We determined that gravid wild type Spr+/+, heterozygous SprECFP/+ and homozygous SprECFP/ECFP mutant females laid very few eggs if they were not mated: 6.5 ± 6.5, 8.8 ± 4.9 and 17.5 ± 8.8 eggs, respectively (mean ± S.E.M., Fig. 2c). We hypothesized that if SPR was required for postmating oviposition behavior, homozygous SprECFP/ECFP females would not lay eggs even after mating. However, the number of eggs laid by mated homozygous Spr mutant females (90.4 ± 10.0 eggs) was not significantly different from that laid by heterozygous (92.9 ± 3.1 eggs, comparison: P = 1.000) or wild type females (mean: 105.3 ± 3.5, comparison: P = 0.774) (mean eggs ± S.E.M., Fig. 2c).
We conclude that SPR is not required for postmating oviposition in Ae. aegypti.
Neither D. melanogaster SP nor D. melanogaster male accessory gland homogenate induces mating refractoriness in Ae. aegypti females
We next tested whether injection of synthetic D. melanogaster SP (dSP) could induce mating refractoriness in Ae. aegypti, as demonstrated for D. melanogaster and H. armigera (Chen et al. 1988; Schmidt et al. 1993; Fan et al. 1999; Fan et al. 2000).
We first confirmed activity of synthetic dSP by injecting 3 pmol (or saline) into 30 virgin D. melanogaster and assessing activity. Mating refractoriness assays revealed that only 6.6% of SP-injected D. melanogaster female flies mated, compared with 63% of those injected with saline. Similarly in egg laying assays, 46.7% of SP-injected D. melanogaster virgin female flies laid eggs compared to 0% of D. melanogaster females injected with saline. These data are all consistent with previous reports of the effects of dSP on mating refractoriness and egg laying in D. melanogaster (Chen et al. 1988; Schmidt et al. 1993; Liu and Kubli 2003).
Having demonstrated activity of the synthetic dSP, we then examined the effect of injecting synthetic 3 pmol of dSP, or saline vehicle, on mating refractoriness in the Thai-strain Ae. aegypti virgin females. In these assays, at 12 h postinjection, injected females were allowed to mate for 48 h and afterwards, females were dissected and examined for sperm in their spermathecae. We observed that sperm were present in all dSP-injected and all saline-injected females, indicating that dSP had no effect on Ae. aegypti mating receptivity in this context (Fig. 3a).
Fig. 3.
Drosophila melanogaster SP and accessory gland extracts do not induce postmating behaviors in Aedes aegypti females. a) Percentage of virgin Ae. aegypti females injected with saline vehicle or synthetic Drosophila SP (dSP) that mated (gray shading). b) Percentage of virgin Ae. aegypti females injected with saline vehicle or dMAG homogenate that mated (gray shading). c) Eggs laid per virgin gravid Thai Ae. aegypti female after injection with saline vehicle or male accessory gland homogenate from Ae. aegypti (aeMAG) or Drosophila melanogaster (dMAG), or normal mating. n.s. = P > 0.05, ****P < 0.0001 (Dunnett's multiple comparisons test). d) Eggs laid per virgin gravid LVPib12 Ae. aegypti female after injection with saline vehicle (PBS), male abdominal tip homogenate from Ae. aegypti (aeMAT), dMAG, or different dosages of dSP. n.s. = P > 0.05, ****P < 0.0001 (Dunnett's multiple comparisons test). Box plots show median, interquartile range, and 5–95 percentile.
Consistent with our results with synthetic SP, when 0.25 male D. melanogaster male accessory gland (dMAG) equivalents were injected into virgin Thai Ae. aegypti females (0.25 μL homogenate), we also found no effect of dMAG on mating receptivity (Fig. 3b; dMAG = 89% (n = 9); Saline = 100% (n = 10); Z-test, Z = −1.08, P = 0.14) in contrast to when aeMAG is injected into virgin females as previously shown [wild type Thai, 1.16% aeMAG-injected females remated (n = 86)] (Craig 1967; Helinski et al. 2012a). We conclude that intrathoracic injection of synthetic dSP as well as homogenate from dMAG, the source of endogenous SP, is insufficient to induce mating refractoriness in Ae. aegypti.
Neither D. melanogaster SP nor D. melanogaster male accessory gland homogenate induces egg laying in Ae. aegypti females
Intrathoracic injection of Ae. aegypti male accessory gland homogenate has previously been shown to be sufficient to stimulate oviposition behavior in virgin gravid Ae. aegypti females (Leahy and Craig 1965; Judson 1967; Hiss and Fuchs 1972). Independently, our 2 groups next assessed whether intrathoracic injection of D. melanogaster homogenates putatively representing the complement of seminal fluid proteins from this species, inclusive of dSP, could similarly act to induce oviposition in virgin Ae. aegypti females.
In our assay series in the Thai Ae. aegypti strain, we confirmed that injection of Ae. aegypti accessory gland homogenate (aeMAG) triggers oviposition from virgin gravid females (66.0 ± 8.0), approaching levels of egg laying observed from mated females (62.1 ± 8.1) (mean eggs ± S.E.M, Fig. 3c). In contrast, virgin gravid females injected with dMAG laid very few eggs (5.3 ± 4.2) not significantly different from saline-injected controls (5.4 ± 5.0).
Our assay series in the LVPib12 strain similarly revealed a crude homogenate prepared from the terminal abdominal segment of virgin males (Ae. aegypti male abdominal tip: aeMAT) containing the accessory glands and all other male reproductive glands was sufficient to induce oviposition behavior when injected into the thorax of virgin gravid females. Females injected with only PBS solvent laid on average 4.2 ± 3.3 eggs whereas those injected with aeMAT laid on average 60.7 ± 13.0 eggs (mean eggs ± S.E.M. aeMAT vs. PBS control, P < 0.0001, Fig. 3d). In contrast, dMAG did not stimulate egg laying from virgin gravid Ae. aegypti females (9.2 ± 8.6 eggs), yielding a similar level of oviposition to those injected with the PBS solvent control (mean eggs ± S.E.M., dMAG vs PBS control: P = 0.992, Fig. 3d).
Finally, to probe whether Drosophila SP (dSP) is capable of stimulating oviposition behavior in Ae. aegypti, we injected synthetic D. melanogaster SP (dSP) over a concentration series ranging from 1.5 to 150 pmol (Fig. 3d) into virgin gravid females from the LVPib12 strain. We determined that dSP injection did not have an effect on egg-laying in this context. The number of eggs (mean eggs ± S.E.M.) laid by females injected with 1.5 pmol dSP (0.3 ± 0.2 vs. PBS control: P = 0.997), 15 pmol dSP (15.3 ± 10.0 vs. PBS control: P = 0.787) and 150 pmol dSP (11.0 ± 6.7 vs. PBS control: P = 0.961) did not differ significantly relative to the PBS solvent control.
We conclude that intrathoracic injection of dMAG as well as synthetic dSP are insufficient to stimulate oviposition behavior in virgin gravid Ae. aegypti females. However, aeMAT and aeMAG is sufficient to stimulate oviposition behavior within this context.
Discussion
Elucidating the molecular pathways involved in establishing postmating responses in Ae. aegypti from both males and females is important to our understanding of their reproductive biology and has implications for strategies for vector control. Here, we explored the role of SPR, a critical female receptor in some other insects, including the dipteran D. melanogaster, in mediating multiple postmating responses. The primary D. melanogaster SPR ligand responsible for controlling reproductive outcomes is SP, a seminal peptide that is not found in insects outside Drosophila genus (Tsuda and Aigaki 2016; McGeary and Findlay 2020; Hopkins et al. 2023). Interestingly, injecting D. melanogaster SP into unmated female H. armigera moths reduces egg laying, suppresses pheromone synthesis, and reduces calling behaviors (Fan et al. 1999, 2000; Hanin et al. 2012), thus mimicking postmating responses analogous to those induced by SP in Drosophila. However, roles of SPR, and effects of SP were not consistent across insects. For example, injection of SP21–36 into the tarnished plant bug Lygus herperus had no effect on mating receptivity and although this region of SP binds in vitro to SPR from D. melanogaster and H. armigera it was unable to bind to SPR from L. herperus (Hull and Brent 2014). Although Ae. aegypti oviposition was reported to be induced by surgical implantation of whole D. melanogaster accessory glands (Leahy 1967) or intrathoracic injection of whole Drosophila male body lysates in the thorax of blood fed, virgin females (Hiss and Fuchs 1972), it is not known what male molecule(s) had this effect nor what receptor they bound to in the female. It should be noted that the stimulation of oviposition with intrathoracic injection of dMAG homogenate into gravid virgin Ae. aegypti females was not observed when independently tested by both the Cornell and the Johns Hopkins groups. In contrast, we found that Ae. aegypti accessory gland homogenate preparations were fully sufficient to evoke the postmating receptivity and egg-laying phenotypes that we characterized in this study, consistent with previous reports (Hiss and Fuchs 1972; Helinski et al. 2012a; Villarreal et al. 2018).
Genes corresponding to a SP homolog are not readily identified in insects outside Drosophila, but sequences homologous to SPR can be identified in various insect groups, including Ae. aegypti; the mosquito SPR even binds Drosophila SP in vitro, albeit weakly (Kim et al. 2010; Lee et al. 2020). Given the presence of SPR transcripts in Ae. aegypti reproductive tracts (Alfonso-Parra et al. 2016; Matthews et al. 2016), the parallels between postmating responses in Ae. aegypti and D. melanogaster, and the importance of SP in inducing postmating response in H. armigera, we tested here whether SPR is required for postmating responses in Ae. aegypti. We generated 2 independent SPR null mutations by CRISPR/Cas9 gene editing and compared mutant and control females for a variety of postmating responses.
First, we tested whether SPR is needed for the postmating drop in female receptivity. Female Ae. aegypti that have mated are refractory to subsequent mating (Craig 1967; Helinski et al. 2012b); ∼25% remate immediately after mating in laboratory settings, and all the mosquitoes eventually establish strong refractoriness 16–20 h after the initial mating (Degner and Harrington 2016; Duvall et al. 2017). In both of our Spr mutants (SprΔ235 and SprECFP), functional knockout of SPR does not affect the establishment of remating refractoriness, regardless of the interval between mating opportunities.
Stimulation of egg development and oviposition is another postmating response by Ae. aegypti females (Lang 1956; Judson 1967). SprECFP knockout females oviposited a similar number of eggs as heterozygous females after mating and blood feeding, demonstrating that SPR is not required for this postmating process. Knockdown or knockout of SPR in various insect systems have noted a reduced number of eggs laid (Li et al. 2014; Zheng et al. 2015; Gregoriou and Mathiopoulos 2020; Liu et al. 2021), although the degree of reduction varies and it often is not a complete lack of egg laying. Consistent with the lack of effect of SPR knockout on mating receptivity and on oviposition, no phenotypes were observed when 2 commercial sources of synthetic dSP (at or above the biologically effective dose for D. melanogaster) were injected independently into unmated Ae. aegypti females. The lack of any observable impact of intrathoracic microinjection of synthetic SP on Ae. aegypti egg laying thus mirrored that of injecting dMAG homogenate which had no effect as described above.
SPR was reported to bind MIPs, often with stronger affinities than SP, leading to the hypothesis that SPR is an ancestral MIP receptor (Kim et al. 2010; Poels et al. 2010). MIPs in insects are involved in control of hindgut and oviduct muscle contraction (Schoofs et al. 1991; Blackburn et al. 1995, 2001; Paluzzi et al. 2015; Lubawy et al. 2020), modulating nutritional preferences (Hussain et al. 2016; Min et al. 2016), maintaining sleep states in D. melanogaster (Oh et al. 2014), as well as involvement in ecdysis (Davis et al. 2003; Kim et al. 2006a, 2006b; Santos et al. 2007). MIPs have been shown to have allostatic activity by inhibiting Juvenile Hormone synthesis in the cricket Gryllus bimaculatus (Lorenz et al. 1995) and brown-winged green bug Plautia stali (Matsumoto et al. 2017). The B. mori peptide PTSP inhibits ecdysone synthesis from the prothoracic gland (Hua et al. 1999). There are 5 identified MIPs in Ae. aegypti (Predel et al. 2010; Siju et al. 2014) with positive staining in the central nervous system with an anti-MIP antibody (Kim et al. 2010).
In an HEK-based in vitro assay we found that Aedes SPR responded to homogenate from whole male mosquitoes, dSP and MIP peptides at micromolar doses and showed low amplitude responses to several other Ae. aegypti peptides (Ast3, AT, sNPF1, sNPF 2 + 4, TKRP2, and TKRP3). We do not observe significant responses of SPR to HP-I, a peptide from the male accessory gland that is known to be transferred to females during mating and to inhibit initial postmating receptivity (Naccarati et al. 2012; Duvall et al. 2017). We note that SPR was among a group of receptors activated by substances in whole male homogenate, a group that also includes NPYLR1, which responds to HP-I. Intriguingly a non-MIP ligand for Ae. aegypti SPR was partially purified (Kim et al. 2010). These ligands are unlikely to mediate postmating changes based on lack of phenotypes that we observed when SPR was disrupted. Given no strong phenotype in the SPR knockout mosquitoes characterized in the assays presented here, it is still possible that there could be a subtle effect with other known functions of MIPs, such as those described above. It is also possible there are pathways where ligands other than MIPs bind SPR that were not included in our peptide panel or belong to a different class of ligands.
As highlighted above, SPR disruptions in different insects can have varying effects on reproductive traits. By characterizing the effects of loss of SPR function in Ae. aegypti, our work contributes to the evolutionary understanding of this key receptor. The SPR is flexible to evolve binding to similar ligands for different functions, some of which have been used in reproduction. As yet unknown signaling pathways operating during transfer of Ae. aegypti seminal fluid thus likely underlie long-term refractoriness to remating and induction of egg laying in this important disease vector.
Supplementary Material
Acknowledgments
We thank Dr. Ben Matthews for advice on CRISPR strategies for generation of the SprΔ235 mutant allele at Cornell and assistance with sequence interpretation, the University of Maryland Insect Transformation Facility for injecting the constructs, Dr. Erika Mudrak of the Cornell Statistical Consulting Unit for assistance with statistical analysis, the Biotechnology Resource Center at the Cornell Institute of Biotechnology for Sanger sequencing and fragment analysis (RRID:SCR_021727), Dr. Nilay Yapici for valuable discussions, reviewers for helpful suggestions, and Dr. Garrett League, Lindsay Baxter, Elizabeth Martin, Jake Angelico, Natalie Bailey, Brady Dolan, Nicole Blattman, and Sean Lee for mosquito rearing and data collection assistance at Cornell. CJM thanks Bloomberg Philanthropies and Johns Hopkins Malaria Research Institute for generous supplemental funding and supporting infrastructure.
Contributor Information
I Alexandra Amaro, Department of Entomology, Cornell University, Ithaca, NY 14853, USA.
Margot P Wohl, W. Harry Feinstone Department of Molecular Microbiology and Immunology, Johns Hopkins Malaria Research Institute, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD 21205, USA.
Sylvie Pitcher, Department of Entomology, Cornell University, Ithaca, NY 14853, USA.
Catalina Alfonso-Parra, Department of Entomology, Cornell University, Ithaca, NY 14853, USA.
Frank W Avila, Department of Molecular Biology and Genetics, Cornell University, Ithaca, NY 14853, USA.
Andrew S Paige, Department of Biological Sciences, Columbia University, New York, NY 10027, USA.
Michelle E H Helinski, Department of Entomology, Cornell University, Ithaca, NY 14853, USA.
Laura B Duvall, Department of Biological Sciences, Columbia University, New York, NY 10027, USA.
Laura C Harrington, Department of Entomology, Cornell University, Ithaca, NY 14853, USA.
Mariana F Wolfner, Department of Molecular Biology and Genetics, Cornell University, Ithaca, NY 14853, USA.
Conor J McMeniman, W. Harry Feinstone Department of Molecular Microbiology and Immunology, Johns Hopkins Malaria Research Institute, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD 21205, USA; The Solomon H. Snyder Department of Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
Data availability
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.
Supplemental material available at GENETICS online.
Funding
Funding provided by National Institutes of Health (NIH) grant R01-AI095491 to L.C.H. and M.F.W., NIH grant R21AI176101 to C.J.M., and NIH grant R35-GM137888 and a Beckman Young Investigator award provided by the Arnold and Mabel Beckman Foundation to L.B.D. for supporting this research. M.P.W. was supported by postdoctoral fellowships from the NIH (T32AI007417) and a 2022 L’Oreal USA for Women in Science Fellowship. A.S.P. was supported by an National Science Foundation Graduate Research Fellowship Program award.
Literature cited
- Alfonso-Parra C, Ahmed-Braimah YH, Degner EC, Avila FW, Villarreal SM, Pleiss JA, Wolfner MF, Harrington LC. 2016. Mating-induced transcriptome changes in the reproductive tract of female Aedes aegypti. PLoS Negl Trop Dis. 10(2):e0004451. doi: 10.1371/journal.pntd.0004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaro IA, Ahmed-Braimah YH, League GP, Pitcher SA, Avila FW, Cruz PC, Harrington LC, Wolfner MF. 2021. Seminal fluid proteins induce transcriptome changes in the Aedes aegypti female lower reproductive tract. BMC Genomics. 22(1):896. doi: 10.1186/s12864-021-08201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Mattei AL, Wolfner MF. 2015. Sex peptide receptor is required for the release of stored sperm by mated Drosophila melanogaster females. J Insect Physiol. 76:1–6. doi: 10.1016/j.jinsphys.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. 2011. Insect seminal fluid proteins: identification and function. Annu Rev Entomol. 56(1):21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn MB, Jaffe H, Kochansky J, Raina AK. 2001. Identification of four additional myoinhibitory peptides (MIPs) from the ventral nerve cord of Manduca sexta. Arch Insect Biochem Physiol. 48(3):121–128. doi: 10.1002/arch.1064. [DOI] [PubMed] [Google Scholar]
- Blackburn MB, Wagner RM, Kochansky JP, Harrison DJ, Thomas-Laemont P, Raina AK. 1995. The identification of two myoinhibitory peptides, with sequence similarities to the galanins, isolated from the ventral nerve cord of Manduca sexta. Regul Pept. 57(3):213–219. doi: 10.1016/0167-0115(95)00034-9. [DOI] [PubMed] [Google Scholar]
- Cator LJ, Wyer CAS, Harrington LC. 2021. Mosquito sexual selection and reproductive control programs. Trends Parasitol. 37(4):330–339. doi: 10.1016/j.pt.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, Smith HK, Partridge L. 2003. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci U S A. 100(17):9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Sun H, Xie J, Yao Z, Zheng W, Li Z, Deng Z, Li X, Zhang H. 2023. CRISPR/Cas9-induced mutation of sex peptide receptor gene Bdspr affects ovary, egg laying, and female fecundity in Bactrocera dorsalis (Hendel) (Diptera: tephritidae). J Insect Sci. 23(1):2. doi: 10.1093/jisesa/ieac078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Luo J, Wang Y, Gurav AS, Li M, Akbari OS, Montell C. 2021. Suppression of female fertility in Aedes aegypti with a CRISPR-targeted male-sterile mutation. Proc Natl Acad Sci U S A. 118(22):e2105075118. doi: 10.1073/pnas.2105075118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PS, Stumm-Zollinger E, Aigaki T, Balmer J, Bienz M, Böhlen P. 1988. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell. 54(3):291–298. doi: 10.1016/0092-8674(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. 2013. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 499(7458):295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civetta A, Singh RS. 1998. Sex-related genes, directional sexual selection, and speciation. Mol Biol Evol. 15(7):901–909. doi: 10.1093/oxfordjournals.molbev.a025994. [DOI] [PubMed] [Google Scholar]
- Cook PE, Hugo LE, Iturbe-Ormaetxe I, Williams CR, Chenoweth SF, Ritchie SA, Ryan PA, Kay BH, Blows MW, O'Neill SL. 2006. The use of transcriptional profiles to predict adult mosquito age under field conditions. Proc Natl Acad Sci U S A. 103(48):18060–18065. doi: 10.1073/pnas.0604875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig GB Jr. 1967. Mosquitoes: female monogamy induced by male accessory gland substance. Science. 156(3781):1499–1501. doi: 10.1126/science.156.3781.1499. [DOI] [PubMed] [Google Scholar]
- Davis NT, Blackburn MB, Golubeva EG, Hildebrand JG. 2003. Localization of myoinhibitory peptide immunoreactivity in Manduca sexta and Bombyx mori, with indications that the peptide has a role in molting and ecdysis. J Exp Biol. 206(9):1449–1460. doi: 10.1242/jeb.00234. [DOI] [PubMed] [Google Scholar]
- Degner EC, Harrington LC. 2016. Polyandry depends on postmating time interval in the dengue vector Aedes aegypti. Am J Trop Med Hyg. 94(4):780–785. doi: 10.4269/ajtmh.15-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall LB, Basrur NS, Molina H, McMeniman CJ, Vosshall LB. 2017. A peptide signaling system that rapidly enforces paternity in the Aedes aegypti mosquito. Curr Biol. 27(23):3734–3742.e3735. doi: 10.1016/j.cub.2017.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall LB, Ramos-Espiritu L, Barsoum KE, Glickman JF, Vosshall LB. 2019. Small-molecule agonists of Ae. aegypti neuropeptide Y receptor block mosquito biting. Cell. 176(4):687–701.e685. doi: 10.1016/j.cell.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Rafaeli A, Gileadi C, Kubli E, Applebaum SW. 1999. Drosophila melanogaster sex peptide stimulates juvenile hormone synthesis and depresses sex pheromone production in Helicoverpa armigera. J Insect Physiol. 45(2):127–133. doi: 10.1016/S0022-1910(98)00106-1. [DOI] [PubMed] [Google Scholar]
- Fan Y, Rafaeli A, Moshitzky P, Kubli E, Choffat Y, Applebaum SW. 2000. Common functional elements of Drosophila melanogaster seminal peptides involved in reproduction of Drosophila melanogaster and Helicoverpa armigera females. Insect Biochem Mol Biol. 30(8–9):805–812. doi: 10.1016/S0965-1748(00)00052-7. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. 2007. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 39(2):175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Feng K, Palfreyman MT, Hasemeyer M, Talsma A, Dickson BJ. 2014. Ascending SAG neurons control sexual receptivity of Drosophila females. Neuron. 83(1):135–148. doi: 10.1016/j.neuron.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Garbe DS, Vigderman AS, Moscato E, Dove AE, Vecsey CG, Kayser MS, Sehgal A. 2016. Changes in female Drosophila sleep following mating are mediated by SPSN-SAG neurons. J Biol Rhythms. 31(6):551–567. doi: 10.1177/0748730416668048. [DOI] [PubMed] [Google Scholar]
- Gregoriou ME, Mathiopoulos KD. 2020. Knocking down the sex peptide receptor by dsRNA feeding results in reduced oviposition rate in olive fruit flies. Arch Insect Biochem Physiol. 104(2):e21665. doi: 10.1002/arch.21665. [DOI] [PubMed] [Google Scholar]
- Haerty W, Jagadeeshan S, Kulathinal RJ, Wong A, Ravi Ram K, Sirot LK, Levesque L, Artieri CG, Wolfner MF, Civetta A, et al. 2007. Evolution in the fast lane: rapidly evolving sex-related genes in Drosophila. Genetics. 177(3):1321–1335. doi: 10.1534/genetics.107.078865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanin O, Azrielli A, Applebaum SW, Rafaeli A. 2012. Functional impact of silencing the Helicoverpa armigera sex-peptide receptor on female reproductive behaviour. Insect Mol Biol. 21(2):161–167. doi: 10.1111/j.1365-2583.2011.01122.x. [DOI] [PubMed] [Google Scholar]
- Hasemeyer M, Yapici N, Heberlein U, Dickson BJ. 2009. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron. 61(4):511–518. doi: 10.1016/j.neuron.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Hayes RO. 1953. Determination of a physiological saline solution for Aedes aegypti (L.). J Econ Entomol. 46(4):624–627. doi: 10.1093/jee/46.4.624. [DOI] [Google Scholar]
- Helinski ME, Deewatthanawong P, Sirot LK, Wolfner MF, Harrington LC. 2012a. Duration and dose-dependency of female sexual receptivity responses to seminal fluid proteins in Aedes albopictus and Ae. aegypti mosquitoes. J Insect Physiol. 58(10):1307–1313. doi: 10.1016/j.jinsphys.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helinski ME, Harrington LC. 2011. Male mating history and body size influence female fecundity and longevity of the dengue vector Aedes aegypti. J Med Entomol. 48(2):202–211. doi: 10.1603/ME10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helinski ME, Valerio L, Facchinelli L, Scott TW, Ramsey J, Harrington LC. 2012b. Evidence of polyandry for Aedes aegypti in semifield enclosures. Am J Trop Med Hyg. 86(4):635–641. doi: 10.4269/ajtmh.2012.11-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiss EA, Fuchs MS. 1972. The effect of matrone on oviposition in the mosquito, Aedes Aegypti. J Insect Physiol. 18(11):2217–2227. doi: 10.1016/0022-1910(72)90250-8. [DOI] [PubMed] [Google Scholar]
- Hopkins BR, Angus-Henry A, Kim BY, Carlisle JA, Thompson A, Kopp A. 2023. Decoupled evolution of the sex peptide gene family and sex peptide receptor in drosophilidae. Proc Natl Acad Sci U S A. 121(3):e2312380120. doi: 10.1073/pnas.2312380120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua YJ, Tanaka Y, Nakamura K, Sakakibara M, Nagata S, Kataoka H. 1999. Identification of a prothoracicostatic peptide in the larval brain of the silkworm, Bombyx mori. J Biol Chem. 274(44):31169–31173. doi: 10.1074/jbc.274.44.31169. [DOI] [PubMed] [Google Scholar]
- Hull JJ, Brent CS. 2014. Identification and characterization of a sex peptide receptor-like transcript from the western tarnished plant bug Lygus hesperus. Insect Mol Biol. 23(3):301–319. doi: 10.1111/imb.12082. [DOI] [PubMed] [Google Scholar]
- Hussain A, Ucpunar HK, Zhang M, Loschek LF, Grunwald Kadow IC. 2016. Neuropeptides modulate female chemosensory processing upon mating in Drosophila. PLoS Biol. 14(5):e1002455. doi: 10.1371/journal.pbio.1002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson CL. 1967. Feeding and oviposition behavior in the mosquito Aedes aegypti (L.). I. Preliminary studies of physiological control mechanisms. Biol Bull. 133(2):369–378. doi: 10.2307/1539832. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Bartalska K, Audsley N, Yamanaka N, Yapici N, Lee JY, Kim YC, Markovic M, Isaac E, Tanaka Y, et al. 2010. MIPs are ancestral ligands for the sex peptide receptor. Proc Natl Acad Sci U S A. 107(14):6520–6525. doi: 10.1073/pnas.0914764107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Zitnan D, Cho KH, Schooley DA, Mizoguchi A, Adams ME. 2006a. Central peptidergic ensembles associated with organization of an innate behavior. Proc Natl Acad Sci U S A. 103(38):14211–14216. doi: 10.1073/pnas.0603459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Zitnan D, Galizia CG, Cho KH, Adams ME. 2006b. A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles. Curr Biol. 16(14):1395–1407. doi: 10.1016/j.cub.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Kistler KE, Vosshall LB, Matthews BJ. 2015. Genome engineering with CRISPR-Cas9 in the mosquito Aedes aegypti. Cell Rep. 11(1):51–60. doi: 10.1016/j.celrep.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labun K, Montague TG, Gagnon JA, Thyme SB, Valen E. 2016. CHOPCHOP v2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res. 44(W1):W272–W276. doi: 10.1093/nar/gkw398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CA. 1956. The influence of mating on egg production by Aedes aegypti. Am J Trop Med Hyg. 5(5):909–914. doi: 10.4269/ajtmh.1956.5.909. [DOI] [PubMed] [Google Scholar]
- Leahy MG. 1967. Non-specificity of the male factor enhancing egg-laying in Diptera. J Insect Physiol. 13(8):1283–1292. doi: 10.1016/0022-1910(67)90100-X. [DOI] [Google Scholar]
- Leahy MG, Craig GB Jr. 1965. Accessory gland substance as a stimulant for oviposition in Aedes aegypti and A. albopictus. Mosq News. 25:448–452. [Google Scholar]
- Lee JH, Lee NR, Kim DH, Kim YJ. 2020. Molecular characterization of ligand selectivity of the sex peptide receptors of Drosophila melanogaster and Aedes aegypti. Insect Biochem Mol Biol. 127:103472. doi: 10.1016/j.ibmb.2020.103472. [DOI] [PubMed] [Google Scholar]
- Li C, Yu JF, Lu Q, Xu J, Liu JH, Ye H. 2014. Molecular characterization and functional analysis of a putative sex-peptide receptor in the tobacco cutworm Spodoptera litura(fabricius, 1775) (lepidoptera: noctuidae). Austral Entomol. 53(4):424–431. doi: 10.1111/aen.12088. [DOI] [Google Scholar]
- Li M, Bui M, Yang T, Bowman CS, White BJ, Akbari OS. 2017. Germline Cas9 expression yields highly efficient genome engineering in a major worldwide disease vector, Aedes aegypti. Proc Natl Acad Sci U S A. 114(49):E10540–E10549. doi: 10.1073/pnas.1711538114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kubli E. 2003. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci U S A. 100(17):9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Li B, Liu W, Liu Y, Ren B, Wang G. 2021. Sex peptide receptor mediates the post-mating switch in Helicoverpa armigera (lepidoptera: noctuidae) female reproductive behavior. Pest Manag Sci. 77(7):3427–3435. doi: 10.1002/ps.6391. [DOI] [PubMed] [Google Scholar]
- Lorenz MW, Kellner R, Hoffmann KH. 1995. A family of neuropeptides that inhibit juvenile hormone biosynthesis in the cricket, Gryllus bimaculatus. J Biol Chem. 270(36):21103–21108. doi: 10.1074/jbc.270.36.21103. [DOI] [PubMed] [Google Scholar]
- Lubawy J, Marciniak P, Rosinski G. 2020. Identification, localization in the central nervous system and novel myostimulatory effect of allatostatins in Tenebrio molitor beetle. Int J Mol Sci. 21(10):3510. doi: 10.3390/ijms21103510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke-Andersen S, Jensen TH. 2015. Nonsense-mediated mRNA decay: an intricate machinery that shapes transcriptomes. Nat Rev Mol Cell Biol. 16(11):665–677. doi: 10.1038/nrm4063. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Suetsugu Y, Tanaka Y, Kotaki T, Goto SG, Shinoda T, Shiga S. 2017. Identification of allatostatins in the brown-winged green bug Plautia stali. J Insect Physiol. 96:21–28. doi: 10.1016/j.jinsphys.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Matthews BJ, McBride CS, DeGennaro M, Despo O, Vosshall LB. 2016. The neurotranscriptome of the Aedes aegypti mosquito. BMC Genomics. 17(1):32. doi: 10.1186/s12864-015-2239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeary MK, Findlay GD. 2020. Molecular evolution of the sex peptide network in Drosophila. J Evol Biol. 33(5):629–641. doi: 10.1111/jeb.13597. [DOI] [PubMed] [Google Scholar]
- McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, Vosshall LB. 2014. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell. 156(5):1060–1071. doi: 10.1016/j.cell.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S, Chae HS, Jang YH, Choi S, Lee S, Jeong YT, Jones WD, Moon SJ, Kim YJ, Chung J. 2016. Identification of a peptidergic pathway critical to satiety responses in Drosophila. Curr Biol. 26(6):814–820. doi: 10.1016/j.cub.2016.01.029. [DOI] [PubMed] [Google Scholar]
- Naccarati C, Audsley N, Keen JN, Kim JH, Howell GJ, Kim YJ, Isaac RE. 2012. The host-seeking inhibitory peptide, Aea-HP-1, is made in the male accessory gland and transferred to the female during copulation. Peptides. 34(1):150–157. doi: 10.1016/j.peptides.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasci RS. 1990. Relationship of wing length to adult dry weight in several mosquito species (Diptera: culicidae). J Med Entomol. 27(4):716–719. doi: 10.1093/jmedent/27.4.716. [DOI] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, et al. 2007. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 316(5832):1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Ota T, Isogai T. 2000. Prediction whether a human cDNA sequence contains initiation codon by combining statistical information and similarity with protein sequences. Bioinformatics. 16(11):960–967. doi: 10.1093/bioinformatics/16.11.960. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Simon MI. 1995. Gα15 and Gα16 couple a wide variety of receptors to phospholipase C (∗). J Biol Chem. 270(25):15175–15180. doi: 10.1074/jbc.270.25.15175. [DOI] [PubMed] [Google Scholar]
- Oh Y, Yoon SE, Zhang Q, Chae HS, Daubnerová I, Shafer OT, Choe J, Kim YJ. 2014. A homeostatic sleep-stabilizing pathway in Drosophila composed of the sex peptide receptor and its ligand, the myoinhibitory peptide. PLoS Biol. 12(10):e1001974. doi: 10.1371/journal.pbio.1001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto N, Watanabe A. 2022. Interorgan communication through peripherally derived peptide hormones in Drosophila. Fly (Austin). 16(1):152–176. doi: 10.1080/19336934.2022.2061834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluzzi JP, Haddad AS, Sedra L, Orchard I, Lange AB. 2015. Functional characterization and expression analysis of the myoinhibiting peptide receptor in the Chagas disease vector, Rhodnius prolixus. Mol Cell Endocrinol. 399:143–153. doi: 10.1016/j.mce.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Poels J, Van Loy T, Vandersmissen HP, Van Hiel B, Van Soest S, Nachman RJ, Vanden Broeck J. 2010. Myoinhibiting peptides are the ancestral ligands of the promiscuous Drosophila sex peptide receptor. Cell Mol Life Sci. 67(20):3511–3522. doi: 10.1007/s00018-010-0393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predel R, Neupert S, Garczynski SF, Crim JW, Brown MR, Russell WK, Kahnt J, Russell DH, Nachman RJ. 2010. Neuropeptidomics of the mosquito Aedes aegypti. J Proteome Res. 9(4):2006–2015. doi: 10.1021/pr901187p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezával C, Pavlou HJ, Dornan AJ, Chan YB, Kravitz EA, Goodwin SF. 2012. Neural circuitry underlying Drosophila female postmating behavioral responses. Curr Biol. 22(13):1155–1165. doi: 10.1016/j.cub.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JG, Vömel M, Struck R, Homberg U, Nässel DR, Wegener C. 2007. Neuroarchitecture of peptidergic systems in the larval ventral ganglion of Drosophila melanogaster. PLoS One. 2(8):e695. doi: 10.1371/journal.pone.0000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheunemann L, Lampin-Saint-Amaux A, Schor J, Preat T. 2019. A sperm peptide enhances long-term memory in female Drosophila. Sci Adv. 5(11):eaax3432. doi: 10.1126/sciadv.aax3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T, Choffat Y, Klauser S, Kubli E. 1993. The Drosophila melanogaster sex-peptide: a molecular analysis of structure-function relationships. J Insect Physiol. 39(5):361–368. doi: 10.1016/0022-1910(93)90023-K. [DOI] [Google Scholar]
- Schoofs L, Holman GM, Hayes TK, Nachman RJ, De Loof A. 1991. Isolation, primary structure, and synthesis of locustapyrokinin: a myotropic peptide of Locusta migratoria. Gen Comp Endocrinol. 81(1):97–104. doi: 10.1016/0016-6480(91)90129-T. [DOI] [PubMed] [Google Scholar]
- Siju KP, Reifenrath A, Scheiblich H, Neupert S, Predel R, Hansson BS, Schachtner J, Ignell R. 2014. Neuropeptides in the antennal lobe of the yellow fever mosquito, Aedes aegypti. J Comp Neurol. 522(3):592–608. doi: 10.1002/cne.23434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LB, Kasai S, Scott JG. 2018. Voltage-sensitive sodium channel mutations S989P + V1016G in Aedes aegypti confer variable resistance to pyrethroids, DDT and oxadiazines. Pest Manag Sci. 74(3):737–745. doi: 10.1002/ps.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Walter MF, Hice RH, O'Brochta DA, Atkinson PW. 2007. Testis-specific expression of the beta2 tubulin promoter of Aedes aegypti and its application as a genetic sex-separation marker. Insect Mol Biol. 16(1):61–71. doi: 10.1111/j.1365-2583.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- Swanson WJ, Vacquier VD. 2002. The rapid evolution of reproductive proteins. Nat Rev Genet. 3(2):137–144. doi: 10.1038/nrg733. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Aigaki T. 2016. Evolution of sex-peptide in Drosophila. Fly (Austin). 10(4):172–177. doi: 10.1080/19336934.2016.1193655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Peyre JB, Asano T, Aigaki T. 2015. Visualizing molecular functions and cross-species activity of sex-peptide in Drosophila. Genetics. 200(4):1161–1169. doi: 10.1534/genetics.115.177550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal SM, Pitcher S, Helinski MEH, Johnson L, Wolfner MF, Harrington LC. 2018. Male contributions during mating increase female survival in the disease vector mosquito Aedes aegypti. J Insect Physiol. 108:1–9. doi: 10.1016/j.jinsphys.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wang K, Forknall N, Patrick C, Yang T, Parekh R, Bock D, Dickson BJ. 2020. Neural circuitry linking mating and egg laying in Drosophila females. Nature. 579(7797):101–105. doi: 10.1038/s41586-020-2055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Wang F, Forknall N, Yang T, Patrick C, Parekh R, Dickson BJ. 2021. Neural circuit mechanisms of sexual receptivity in Drosophila females. Nature. 589(7843):577–581. doi: 10.1038/s41586-020-2972-7. [DOI] [PubMed] [Google Scholar]
- White MA, Bonfini A, Wolfner MF, Buchon N. 2021. Drosophila melanogaster sex peptide regulates mated female midgut morphology and physiology. Proc Natl Acad Sci U S A. 118(1):e2018112118. doi: 10.1073/pnas.2018112118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigby S, Brown NC, Allen SE, Misra S, Sitnik JL, Sepil I, Clark AG, Wolfner MF. 2020. The Drosophila seminal proteome and its role in postcopulatory sexual selection. Philos Trans R Soc Lond B Biol Sci. 375(1813):20200072. doi: 10.1098/rstb.2020.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohl MP, McMeniman CJ. 2023a. Batch rearing Aedes aegypti. Cold Spring Harb Protoc. 2023(3): pdb prot108017. doi: 10.1101/pdb.prot108017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohl MP, McMeniman CJ. 2023b. Single-Pair and small-group crosses of Aedes aegypti. Cold Spring Harb Protoc. 2023(3):pdb prot108018. doi: 10.1101/pdb.prot108018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Rumpf S, Xiang Y, Gordon MD, Song W, Jan LY, Jan YN. 2009. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron. 61(4):519–526. doi: 10.1016/j.neuron.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapici N, Kim YJ, Ribeiro C, Dickson BJ. 2008. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 451(7174):33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- Zheng W, Liu Y, Zheng W, Xiao Y, Zhang H. 2015. Influence of the silencing sex-peptide receptor on Bactrocera dorsalis adults and offspring by feeding with ds-spr. J Asia-Pacific Entomol. 18(3):477–481. doi: 10.1016/j.aspen.2015.05.004. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.
Supplemental material available at GENETICS online.