Abstract
The present work aimed at differentiating five Amaranthus species from Saudi Arabia according to their morphology and the ability in nanoparticle formulation. Biogenic silver nanoparticles (AgNPs) were synthesized from leaf extracts of the five Amaranthus species and characterized by different techniques. Fourier-transform infrared spectroscopy (FT-IR) was used to identify the phyto-constituents of Amaranthus species. The nanoparticles (NPs) were characterized by UV-visible spectroscopy, dynamic light scattering (DLS), transmission electron microscopy (TEM), and energy-dispersive X-ray spectroscopy (EDX). The antibacterial activity of the synthesized NPs was tested against Staphylococcus aureus, E. coli, Klebsiella pneumoniae and Pseudomonas aeruginosa using the agar well diffusion method. Spherical NPs varying in size and functional groups from the five plant species were demonstrated by TEM, DLS and FTIR analysis, respectively. Variations in NPs characteristics could be related to the phytochemical composition of each Amaranthus species since they play a significant role in the reduction process. EDX confirmed the presence of Ag in plant fabricated AgNPs. Antibacterial activity varied among the species, possibly related to the NPs characteristics. Varied characteristics for the obtained AgNPs may reflect variations in the phytochemical composition type and concentration among Amaranthus species used for their fabrication.
Keywords: Amaranthus spp., Taxonomy, Antimicrobial, Silver nanoparticles
Introduction
The genus Amaranthus (Caryophyllales Berch. & J. Presl, Amaranthaceae Juss.) includes 65–70 species distributed worldwide, half of them are native to America (Hernández-Ledesma et al., 2015; Iamonico, 2015). Several Amaranthus species are used as ornamental plants, as food, or as medicines. They are able to escape from cultivation causing a negative impact on agricultural systems, mainly due to their high seed production and resistance to herbicides, as reported for some species (Das, 2016; Iamonico, 2010; Tranel & Trucco, 2009). Additionally, they are a low-cost source of protein, minerals, and vitamins; they represent a source of nutrition for many centuries in Asia, Africa, Central, and South America. Furthermore, the genus Amaranthus has a diverse genetic pool and has the ability to withstand drought stress, making it an ideal crop for ensuring nutritional security in a climate-changing planet (Jamalluddin et al., 2021). From a medical perspective, the crude extract of Amaranthus plants contains alkaloids (betacyanins and betaxanthin), polyphenols (flavonoids, steroids, catechuic acid, and tannins), terpenoids (cerasinone and norecasantalic acid), and saponins depending on the plant maturity stage, cultivar type, and geographical location (Peter & Gandhi, 2017). Each of these compounds has variable biological activities, including anticancerous, antioxidant, immunomodulatory, and antimicrobial, which inspired researchers all over the world their investigation (Gandhi et al., 2021).
Amaranthus was originally described in the 1st edition of Species Plantarum (Linnaeus, 1753), that showed a difficulty in identification of its various species due to high phenotypic variability and hybridization, which resulted in nomenclature and name misapplications (Assad et al., 2017; Costea, Sanders & Waines, 2001; Iamonico, 2016a; Iamonico, 2016b; Iamonico, 2020a; Iamonico, 2017). In fact, many studies revealed that many names are actually heterotypic synonyms (Iamonico, 2014a; Iamonico, 2014b; Iamonico, 2020b; Iamonico, 2020c; Iamonico & Palmer, 2020).
According to Chaudhary (1999), the flora of Saudi Arabia includes eight Amaranthus species. However, based on our ongoing study (Hassan et al., 2022), they are actually 12 species (16 taxa including varieties), one of them is A. tricolor L., which is only cultivated. But, the presence of another one (A. graecizans. subsp. thellungianus (Nevski) Gusev) is still doubtful.
Several tools based on morphological, numerical and molecular studies have been used for taxonomic identification of plant species (Haider, 2018). However, nanoparticles mediated by plant extracts have tremendous application in many ways of nanotechnology due to its low cost and environmental favorable effect.
Several studies have been undertaken on green synthesis of NPs using plant leaf extracts that are capable of forming silver NPs (AgNPs) with varying sizes and shapes depending on many factors, such as plant type and origin, soil and climate conditions (Mohammed Afrah et al., 2021; Mondal et al., 2011). Due to the significance of employing plant extract to create silver nanoparticles that differs in their characteristics in relation to the plant type, therefore, varied plant species may have different abilities in nanoparticles (NPs) fabrication suggesting varied phytochemicals and varied NPs properties accordingly.
This special capability of green fabrication of NPs inspired our research group to use this modern technique to differentiate among angiosperms, based on the plant’s ability to synthesize NPs of various sizes, shapes, and concentrations. Previous taxonomic study using FTIR technique was based on AgNPs synthesized from the extracts of eight Launaea species (Zareh et al., 2018). The current work represents a pioneer attempt to study AgNPs properties in relation to the Amaranthus sp. applied for their fabrication with reference to their bio-reducing and capping abilities which may offers AgNPs with varied characteristics. Such variation could be expected since varied species may have varied phytoconstituents that highly affect the NPs characteristics (Alharbi, Abaker & Makawi, 2023). The selected species existing in Saudi Arabia were as follows: A. blitoides S. Watson s.str. (naturalized alien in Saudi Arabia), A. blitum L. s.str. (naturalized), A. dubius Mart. ex Thell. (casual), A. graecizans. s.str. (native), and A. viridis L. (naturalized). The fabricated NPs were characterized using different techniques and their antimicrobial activity against some pathogenic bacteria was tested.
Materials and Methods
Morphology
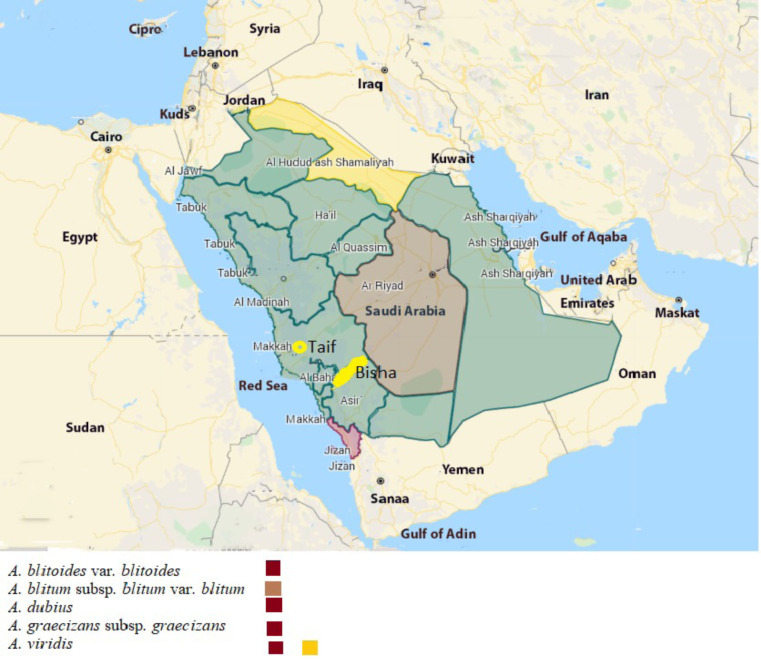
Five taxa of Amaranthus (A. blitoides var. blitoides, A. blitum subsp. blitum var. blitum, A. dubius, A. graecizans subsp. graecizans, and A. viridis) were collected from Saudi Arabia (Fig. 1) during the spring of 2021. They were identified and deposited in the herbaria PNUH and RO (Thiers, 2022). High resolution images of the synflorescences were obtained using the Leica IC80 HD photo camera and Leica Application Suite program, version 4.5.0. (Leica, Wetzlar, Germany). The images were later processed using Helicon Focus, version 6.6.1 Pro where different focus of the same sample were merged together.
Figure 1. Map of Saudi Arabia showing the distribution of Amaranthus species used in this study.
The map was prepared using ArcGIS programme (map credit: Esri, Redlands, California, USA).
The map was prepared using ArcGIS programme (Esri, Redlands, California, USA).
Nanoparticles investigation
Preparation of Amaranthus spp leaf extract
Leaves from the five Amaranthus species were collected and washed with double distilled water to remove the adhering substance before being cut into small pieces. The leaf extracts were prepared by adding 100 ml of distilled water to the washed leaves and heating at 80 °C for 10 min. The leaf extracts were filtered through Whatmann No. 1 filter paper. The leaf extract of each species was used separately for AgNPs synthesis and the filtrate was stored in the refrigerator at 4 °C for further use (Muthukumar et al., 2020).
Biosynthesis of silver nanoparticles
Aqueous leaf extracts were added separately to 1 mM AgNO3 at a ratio of 1:10 (v:v) in dark pottles and heated for 15 min at 80 °C. The reaction was kept in the dark at room temperature for 24 h until the developed color was stable. The mixture was centrifuged for 15 min at 14,000 rpm, the supernatant was removed, and the pellet was washed twice with distilled water. The pellet was allowed to dry at room temperature for further analysis, as described before (Mohammed Afrah et al., 2021)
Characterization of Amaranthus species synthesized
Characterization of the biogenic synthesized silver nanoparticles (AgNPs) using Amaranthus species was carried out with the following techniques:
Ultraviolet–visible spectroscopy.
After the dark brown color became stable, ultraviolet–visible (UV–Vis) spectroscopy absorption was determined using a spectrophotometer (UVProbe software, UV-1800; Shimadzu Corporation, Kyoto, Japan). The absorption spectra of the biosynthesized NPs solution were measured in the range of 200–600 nm after 24 h of reaction, where plant extracts were used as a blank (Dasari et al., 2013).
Fourier transform infrared (FTIR) spectroscopy.
The FTIR spectrum was performed to detect the potential of biomolecules in the Amaranthus spp. leaf extracts to reduce AgNO3. The SPECTRUM100 (Perkin-Elmer, Shelton, CT, USA) was used for this purpose and the data scanning was done at a range of 450–3,500 cm−1 using a diffuse reflectance accessory, as described previously (Siddiqi et al., 2018).
Hydrodynamic size and surface potential analysis.
The dynamic light scattering (DLS) method was used to determine the pattern of the size distribution. A Zetasizer (NANO ZSP, ver 7.11, Malvern Instruments Ltd, Malvern, UK) was used for this measurement, as described by Paul, Singh & Sasikumar (2015).
Morphology and size distribution analysis using transmission electron microscopy (TEM).
Determination of the size distribution and morphology of the synthesized AgNPs was performed by the transmission electron microscope (TEM) model JEM-1011 (JEOL, Tokyo, Japan) at 80 kV. A drop of AgNPs solution was applied on the carbon-coated copper grid (200 mesh). The size of particles was calculated using ImageJ 1.45 s software1493, as described previously (Mie et al., 2014).
Energy-dispersive X-ray spectroscopy (EDS).
The JED-2200 series (JEOL) scanning electron microscope (SEM) supplied with energy-dispersive X-ray spectroscopy (EDS) was used to confirm the presence of the silver element by surface analysis of the biosynthesized NPs, as described elsewhere (Paul, Singh & Sasikumar, 2015)
Antimicrobial activity of the Amaranthus spp.-based silver nanoparticles
The antibacterial activity of biosynthesized AgNPs was performed in vitro against one Gram-positive bacteria, Staphylococcus aureus (MW846278) and three Gram-negative bacteria, which were Pseudomonas aeruginosa (MW846270), Escherichia coli (MW846276) and Klebsiella pneumoniae (MW846277) using the agar well diffusion assay, as described by Awwad, Salem & Abdeen (2013). The bacterial strains were obtained from the Bio-house Medical Lab (Riyadh, Saudi Arabia). Briefly, Oxoid nutrient agar medium plates were streaked with the suspension of test bacterial strain (2.5 ×105 cfu/ml) and left to dry before making the wells. To each well on the seeded agar plate, 20 ul of AgNPs, plant aqueous extracts and AgNO3 were added separately, and ampicillin was included as a positive control. The plates were incubated at 37 °C for 24 h and the inhibition zones around wells were measured in millimetres. The assay was performed in triplicate, according to the guidelines of the Clinical and Laboratory Standards Institute (Wayne, 2010).
Statistical analysis
Statistical analysis and the antimicrobial data (means ± standard deviations) beside the figure were produced using GraphPad PRISM 9.1 (San Diego, CA, USA). One-way and two-way ANOVA (Tukey’s multiple comparisons and Fisher’s LSD) were applied to analyse the differences among the antimicrobial activities of all AgNPs against each bacterial strain.
Results
Morphology
The five Amaranthus taxa differed from each other in synflorescence characters (general structure), perianth (number of tepals), and fruit (surface). A. dubius differed from the other four taxa by the number of tepals (five). A. blitoides was characterized by a perianth with four tepals and leaves with a white marginal vein. A. graecizans s.str., A. blitum s.str., and A. viridis had three tepals. A. graecizans displayed synflorescences in axillary glomerules, whereas the other two taxa had synflorescences arranged in spike- or panicle-like structures. The difference between A. viridis and A. blitum s.str. was in the fruit, which was smooth, or slightly rugose on the surface and strongly rugose, respectively (Fig. 2).
Figure 2. The images of the synflorescences of; (A) A. dubius, (B) A. blitoides (C) A. graecizans, (D) A. blitum, (E) A. viridis.
Where red arrows refer to bracts, black arrows refer to tepals and blue arrows refer to the fruit.
Characterization of the nanoparticles prepared by Amaranthus species
Ultraviolet–visible spectroscopy
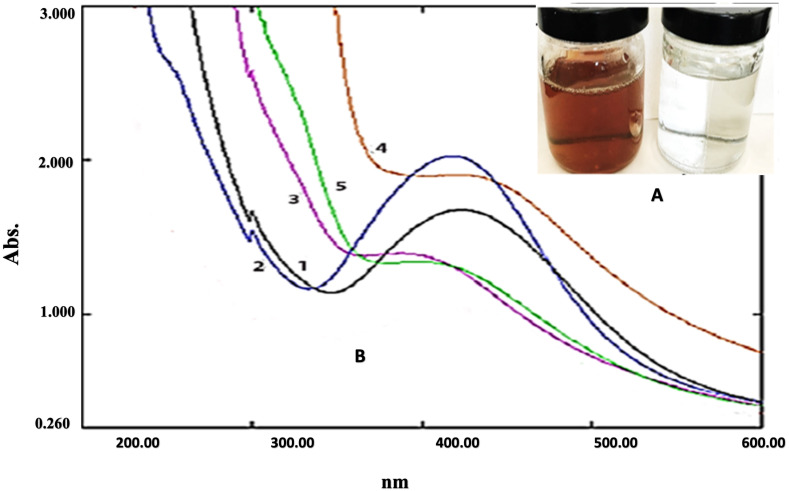
The fabrication of Ag ions into AgNPs was achieved by the five species of Amaranthus providing B-AgNPs, G-AgNPs, D-AgNPs, L-AgNPs, and V-AgNPs for those prepared by A. blitum subsp. blitum var. blitum, A. graecizans. subsp. graecizans, A. dubius, A. blitoides var. blitoides, and A. viridis, respectively. AgNPs formation was first detected by gradual change in the mixture color from colorless to stable brown after 24 h for all tested plant species (Fig. 3A), which was further confirmed by UV spectra that indicated wavelengths ranging from 400 to 450 nm (Fig. 3B). A slight variation in the time needed for color change among the tested species was noted.
Figure 3. Ultraviolet–visible spectroscopy results of biosynthesis of AgNPs using leaf extracts of five Amaranthus species.
(A) shows the change in color of AgNO 3 from colorless to brown after addition of leaf extracts. (B) the UV/vis spectrum for: (1) B-AgNPs, (2) G-AgNPs, (3) D-AgNPs, (4) L-AgNPs (4), and (5) V-AgNPs.
Fourier transform infrared spectroscopy
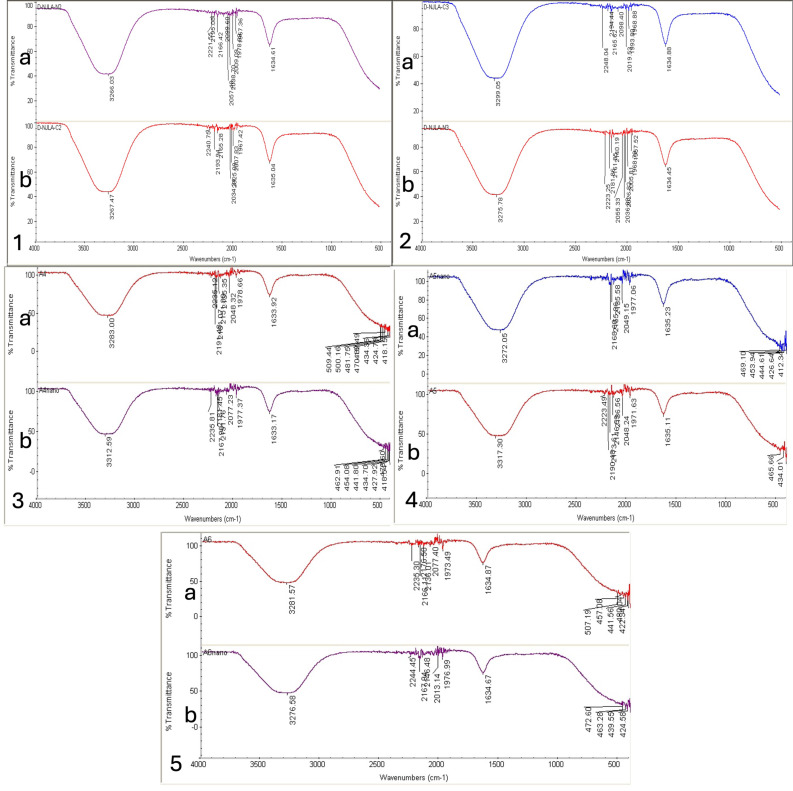
The Fourier transform infrared (FTIR) spectroscopy was applied to demonstrate the functional groups involved in AgNPs fabrication from the five Amaranthus species in comparison with the aqueous plant extracts utilized for their synthesis (Fig. 4). Variations in the FTIR peak values were noted between those of the plant extracts and those of plant extract-based NPs for the five Amaranthus species, indicating slight differences in the functional groups. Peaks of strong absorbance for A. blitum subsp. blitum var. blitum extract was recorded at 1,635.04 and 3,267.47 cm−1, while those for B-AgNPs were 1,634.61 and 3,266.03 cm−1. A. graecizans. subsp. graecizans extract recorded two strong peaks at 1,634.88 and 3,299.05 cm−1, while 1,634.45 and 3,275.78 cm−1 were recorded for G-AgNPs. The signals of A. dubius extract were at 1,633.92 and 3,283 cm−1, but moved to 1,633.27 and 3,312.59 cm−1 for D-AgNPs. A. blitoides var. blitoides extract signals were at 1,635.11 and 3,317.30 cm−1 that changed with L-AgNPs to 1,634.23 and 3,272.05 cm−1. The signals at 1,634.87 and 3,281.57 cm−1 were noted for A. viridis extract, which changed to 1,634.67 and 3,276.58 cm−1 in V-AgNPs.
Figure 4. FTIR absorbance peaks of aqueous extracts the Amaranthus species (A) and the corresponding fabricated AgNPs (B).
A. blitum subsp. blitum var. blitum (1), A. graecizans L. subsp. graecizans (2), A. dubius (3), A. blitoides var. blitoides (4), and A. viridis (5).
Hydrodynamic size and surface potential analysis
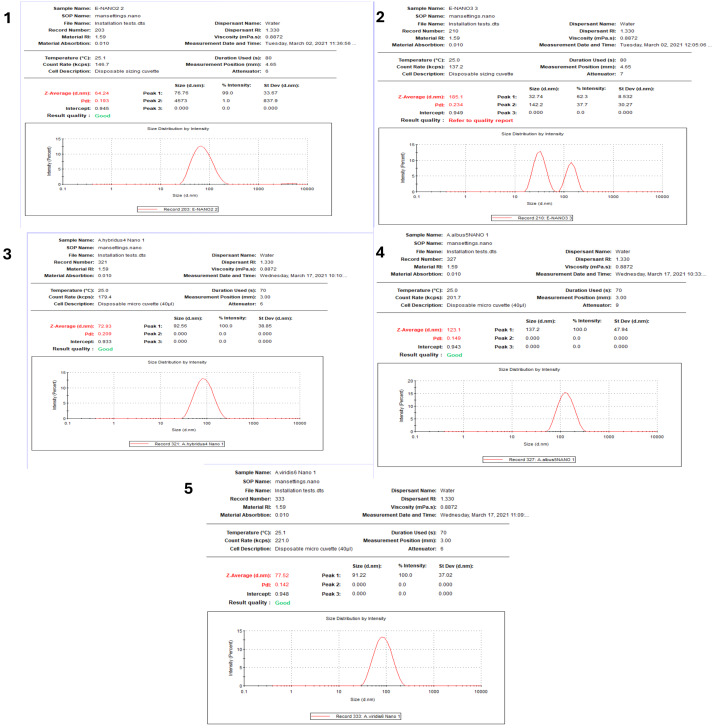
The DLS system results showed variation in AgNPs sizes of the NPs produced by the five Amarathus species. Sizes of 64.24, 185.1, 72.93, 123.1, and 77.52 nm and polydispersity indexes (PDI) of 0.19, 0.23, 0.20, 0.14, and 0.14 were recorded for B-AgNPs, G-AgNPs, D-AgNps, L-AgNPs and V-AgNPs, respectively (Fig. 5). Following the same sequences of the AgNPs type, the zeta potential values were −33, −23.7, −18.3, −28.5, and −14, respectively (Fig. 6). Generally, the AgNPs prepared by the five Amaranthus species showed variations in NPs characteristics, as shown in Table 1.
Figure 5. Size distribution of AgNPs synthesized from five Amaranthus species.
B-AgNPs (1), G-AgNPs (2), D-AgNPs (3), L-AgNPs (4), and V-AgNPs (5).
Figure 6. Zeta potential of AgNPs synthesized from five Amaranthus species.
B-AgNPs (1), G-AgNPs (2), D-AgNPs (3), L-AgNPs (4), and V-AgNPs (5).
Table 1. Size, potential and polydispersity index (PDI) for the AgNPs prepared by the five Amaranthus species.
| NPs typea | Size | Potential | PDI | |
|---|---|---|---|---|
| 1 | B-AgNPs | 64.24 | −33 | 0.19 |
| 2 | G-AgNPs | 185.1 | −23.7 | 0.23 |
| 3 | D-AgNPs | 72.93 | −18.3 | 0.20 |
| 4 | L-AgNPs | 123.1 | −28.5 | 0.14 |
| 5 | V-AgNps | 77.52 | −14 | 0.14 |
Notes.
Amaranthus spp. based AgNPs. B-AgNPs, G-AgNPs, D-AgNPs, L-AgNPs and V-AgNPs were the AgNPs prepared from A. blitum subsp. blitum var. blitum, A. graecizans L. subsp. graecizans, A. dubius, A. blitoides var. blitoides, and A. viridis, respectively.
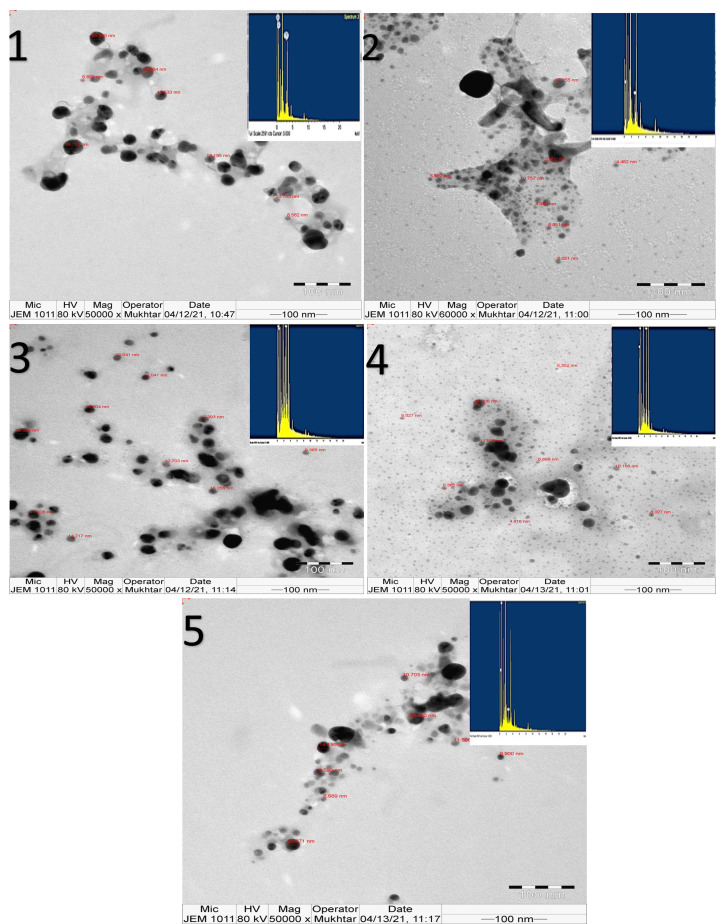
Morphology and size distribution analysis using transmission electron microscopy and energy-dispersive X-ray spectroscopy
The transmission electron microscopy (TEM) imaging for synthesized AgNPs from the five Amaranthus species showed mostly spherical NPs. Areas around AgNPs represent clear capping agents from the plant extract (Fig. 7).
Figure 7. TEM image and EDX presenting morphological features of NPs synthesized from five Amaranthus species showing surface morphology and quantitative analysis of silver atoms, carbon and oxygen for B-AgNPs (1), G-AgNps (2), D-AgNPs (3), L-AgNPs (4), and V-AgNPs (5).
The EDX analysis confirmed the presence of the silver element in all synthesized NPs, besides the carbon and oxygen that originated from the plant extract (Fig. 7). The results demonstrated silver signals at 3 keV, along with carbon and oxygen peaks for all synthesized AgNPs.
Determination of the antibacterial activity of AgNPs
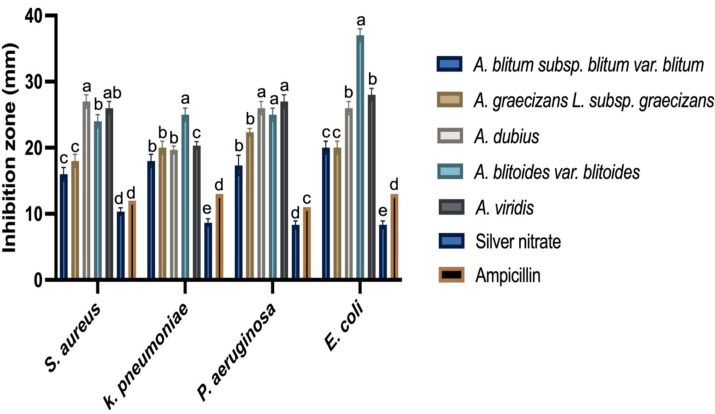
Inhibition of the bacterial growth by the AgNPs synthesized by the five Amaranthus spp. was evaluated and analysed in comparison with the controls (ampicillin disc, AgNO3 and the tested Amaranthus spp. aqueous extracts) against four pathogenic bacterial strains: S. aureus, P. aeruginosa, E. coli, and K. pneumoniae.
No antibacterial activity was detected for all the Amaranthus spp. aqueous extracts and low activity was noted for AgNO3. However, Amaranthus spp. based AgNPs showed significantly larger inhibition zones for both tested Gram-negative and Gram-positive bacteria in relation to AgNO3 (P <0.0001). The highest antibacterial activity was observed for L-AgNPs against E. coli with 37 mm inhibition zone, whereas the lowest antibacterial activity was observed for B-AgNPs against S. aureus with 16 mm inhibition zone. E. coli showed the highest sensitivity to B-AgNPs, L-AgNPs, V-AgNPs, but its sensitivity to G-AgNPs and D-AgNPs was also high. No specific trend of observation was noted regarding the response of Gram-negative and Gram-positive to the biofabricated AgNPs. Generally, the antibacterial activity of the tested agents against all bacterial strains showed significant variations, as shown in Fig. 8 and Table S1. The source of variations, agents, bacterial strain, and their interaction were highly significant P < 0.0001 (Table S2). Detailed descriptions of the data analysis by two-way ANOVA are presented as supporting materials as Table S3 (Tukey’s test) and S4 (Fisher’s LSD).
Figure 8. Anti-bacterial activity of AgNPs prepared by the five Amaranthus species as inhibition zones (mm) on nutrient agar inoculated with different bacterial strains.
Ampicillin, and AgNO 3 were included as controls. The data presented are mean values from three replicates and different letters indicated the significant variations among the AgNPs tested against each bacterial species.
Discussion
Taxonomic impression of the morphometric features
Studies on the genus Amaranthus face a plenty of challenges with regard to identification and taxonomic divisions (Assad et al., 2017; Iamonico, 2020a). Starting from the study on the Linnaean name (Iamonico, 2014a; Iamonico, 2014b), it was revealed that most names are considered as heterotypic synonyms (Iamonico, 2016a; Iamonico, 2016b; Iamonico, 2020c; Iamonico & Palmer, 2020). Results of the present study indicated an interesting morphological variability, especially in A. blitoides and high phenotypic variability (vegetative and generative characters) in A. graecizans and A. blitum. These results are in accordance with those of Das & Iamonico, 2014; Iamonico (2014b). The main characteristic features that differentiate between the tested species were the number of the tepals, structure of the synflorescence, dehiscence/indehiscence of the fruit, and ornamentation of the fruit surface. These characteristics are valid for the description of some Amaranthus species (Cappers & Bekker, 2022; Iamonico, 2015).
Nano-materials fabrication in relation to Amaranthus species
Conversion of metal ions to metal NPs using biological means could be mainly attributed to phytomolecules, such as alcohols or proteins and hydrocarbons that act as agents for metal ions reduction and capping the resultant metal NPs, respectively. Successful fabrication of AgNPs using Amaranthus species was first observed by the brown colour obtained after mixing plant extracts with AgNO3 as typical attributes of the plasmon vibration excitation in AgNPs surface (Tripathy et al., 2010). NPs chracterestics could be explained by Amaranthus species biomolecules (type and concentration) that helped in the reduction process of NPs (Rajeshkumar & Bharath, 2017). All prepared AgNPs were noted at 400–450 nm surface plasmon resonance (SPR) bands by UV, which is the known range for well dispersed AgNPs in solution with unique features (Korbekandi & Iravani, 2012; Yamada et al., 2018). Uniformity of AgNPs in size and shape could be predicted from the shape of SPR, where spherical NPs is expected due to single band (Shinde et al., 2013). A. dubius was previously used for AgNPs fabrication and showed SPR band occuring at 420 nm (Firdhouse & Lalitha, 2012; Sigamoney et al., 2016), which is comparable to the current findings for the five Amaranthus species.
Fourier transform infrared spectroscopy
In comparison with the five extracts and NPs tested, it was clear that all spectra showed similar main troughs of 3,250–3,320 and 1,630–1,640 cm−1, which are mostly assigned to functional groups like −NH or −OH and carbonyl stretching in proteins, respectively. Therefore, it could be assumed that the NPs were capped with polyphenolic compounds and protein from the plant extracts, which could be responsible for the reduction process of Ag ions to AgNPs. FTIR spectra values shifted slightly between the plant extract and NPs for each species indicating that the same functional groups were consumed for NPs fabrication as reducing and capping agents. Further, the FTIR peaks displayed similar functional groups for all tested AgNPs suggesting that the phytochemicals that capped the surface of the biosynthesized AgNPs were similar, but they differed only in their concentrations. Slight variations between the species in the FTIR data of NPs could be related to phytomolecules types and concentrations in each species that might affect the NPs size, potential and activity. Therefore, variations in AgNPs properties could be mainly affected by the plant metabolites capping the NPs that were noted in the FTIR spectrum. Further, EDX indicated the presence of C and O beside Ag ions, thus confirming the FTIR data (Ali et al., 2015).
Hydrodynamic size and surface area analysis
Significantly smaller mean size particle diamater of B-AgNPs, D-AgNPs and V-AgNPs compared with that of G-AgNPs and L-AgNPs could possibly be related to the biomolecules from each extract that might be species- specific, since various phyto molecules may influence the AgNPs synthesis, size, shape and yield, as explained by Sigamoney et al. (2016), who investigated different plant parts of A. dubius. The relationship between phytoconstituents and AgNPs size was well documented by Nguyen et al. (2020), who suggested that higher concentrations of alkaloids and saponins in Momordica charantia when compared with Psidium guajava could be the reason for M. charantia in providing smaller NPs.
The size of B-AgNPs, D-AgNPs and V-AgNPs were lower than 80 nm compared to G-AgNPs and L-AgNPs that indicated more than 120 nm size which might be related to the nature of the capping agent since it has great role in AgNPs production. Accordingly, it might be suggested that higher concentration of the phtochemicals leading to higher reducing and capping ability that would have suppressed AgNPs agglomeration leading to the small size NPs. Therefore, A. blitum subsp. blitum var. blitum, A. dubius and A. viridis could be categorized as one group and A. graecizans. subsp. graecizans and A. blitoides var. Blitoides is an another group since each group has its own potential to provide specific NPs size.
Furthermore, all plant species provided NPs with negative zeta potential, which could be a good indication for contribution of phyto-molecules that have negatively charged functional groups around AgNPs. Such phenomenon indicates good stability of AgNPs due to electrostatic repulsion between the NPs (Sujitha & Kannan, 2013). This study synthesized NPs that varied in size and potential. These characteristics are affected by many factors including NPs prepartion conditions; but, the conditions in synthesizing the NPs in this study were the same, therefore, these variations could be mainly attributed to the plant constituents types and concentrations due to their role in reduction and stabilization processes.
Morphology and size distribution analysis using transmission electron microscopy with energy-dispersive X-ray spectroscopy
In the current investigation, TEM analysis indicated the spherical shape for the synthesized AgNPs, regardless of the plant species, which was previously observed for AgNPs prepared by A. dubius (Firdhouse & Lalitha, 2012). Regarding Ag weight (%) detected by EDX-and the AgNPs size, a negative correlation is expected since larger particles size is supposed to have more Ag ions. However, it was not the case in the current findings; this might be due to the significant variations among the species tested, as higher AgNPs yield is phytochemically dependent (Hemath Naveen et al., 2010).
Antibacterial activity of the biosynthesized AgNPs
Higher plants are capable of producing a large number of natural products, which are known as secondary metabolites (Bonanomi, Vinale & Scala, 2009). For example, Thymus vulgaris, Origanum vulgare, Rosmarinus officinalis, and Cinnamon comprise compounds, such as thymol, carvacrol, camphor, and cinnamaldehyde, that exhibited antimicrobial activity against many pathogenic bacterial species, such as Salmonella enterica Typhimurium, Staphylococcus aureus, and Listeria monocytogenes (Guo et al., 2020; Pesavento et al., 2015; Rao, Chen & McClements, 2019). Also, plants exhibit diverse organic compounds like phenols and oxygenated terpenoids, which could enhance their antimicrobial activity (Rao, Chen & McClements, 2019). Plant terpenoids may increase membrane permeability by disturbing the fatty acid composition of the bacterial cell membrane, leading to leakage of cellular contents (Trombetta et al., 2005). Phenols may also lead to structural and functional damages of bacterial cell membranes (Rao, Chen & McClements, 2019). However, the currently tested Amaranthus spp. had no direct antibacterial effect against the tested strains. This suggests that the concentrations of the plant extracts used was not enough for bacterial suppression. But, the biofabricated NPs by these extracts inhibited the growth of all tested bacterial strains, thus indicating the higher efficiency of the fabricated AgNPs in bacterial suppression in relation to their individual constituents (AgNO3 and plant extracts), which might be explained by the fact that small size particles are better in penetrating the bacterial cells.
To the best of our knowledge, the current study is the first comparative investigation that sought out the characteristics and biological activities of AgNPs fabricated using five Amaranthus spp. in Saudi Arabia. From the current findings, E. coli was the most sensitive tested pathogen by most of the AgNPs prepared by Amaranthus spp., especially L–AgNPs. In a previous study, AgNPs and alcoholic extract of Amaranthus retroflexus had higher effect against the Gram-negative bacteria (P. aeruginosa) than the Gram-positive bacteria, S. aureus (Rasi-Bonab et al., 2018). Moreover, the green synthesis of AgNPs using A. gangeticus Linn leaf extract revealed NPs with antimicrobial properties against Bacillus subtilis, and Shigella flexineri (Kolya et al., 2015). The antibacterial properties of AgNPs could rely on their ability to enter the microbial cells, damage the DNA, and lose cell contents (Manivasagan et al., 2013). Furthermore, it could also be related to the development of reactive oxygen species that interact with glycoproteins on the bacterial cell wall, which facilitate their entry into the cytoplasm (Osonga et al., 2020). Additionally, the small NPs size increases their contact surface with the outer membrane of the bacterial cells, thus enhancing their antibacterial effect (Nakagawa et al., 1999). NPs characteristics, such as antimicrobial activity, are also shape- dependent since triangular NPs are better than spherical and both are better than rod shape NPs (Pal, Tak & Song, 2007).
Generally, the investigated Amaranthus species revealed AgNPs with various characteristics that were noted via UV-absorption, DLS data, TEM and EDX analysis, FTIR as well as antibacterial activity. No special trend of observations was noted regarding the antibacterial effect of the prepared NPs, which might be explained by the fact that NPs chracterestics slightly varied, especially in size and potential, however, the NPs effect could also be species- specific.
Conclusion
The current study indicated that different Amaranthus species fabricated NPs that have varied characteristics specifically size, in addition to their morphological evidence. Bio fabricated NPs indicated variations in properties in terms of size, potential, production level, as well as antibacterial effect. Size and potential variability of the NPs might be explained by types of plant constituents and concentrations that helped in the reduction, capping and stabilization processes of NPs. Slight variations were noted in the FTIR spectra, which suggest that the phyto-molecules detected in each species could be slightly different in terms of type and concentration, which in turn could be the main effective factors in phytofabrication of NPs.
Supplemental Information
Acknowledgments
Dr. Yahya S. Masrahi and Dr. M. Remesh, Herbarium, Department of Biology, Faculty of Science, Jazan University, helped in samples collection.
Funding Statement
This research has been supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R187), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Duilio Iamonico is an employee of Garden of the Apennine Flora of Capracotta, Italy.
Author Contributions
Walaa A. Hassan conceived and designed the experiments, performed the experiments, prepared figures and/or tables, and approved the final draft.
Afrah E. Mohammed performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Najla A. AlShaye performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Hana Sonbol performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Salma A. Alghamdi performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Duilio Iamonico performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Shereen M. Korany conceived and designed the experiments, performed the experiments, prepared figures and/or tables, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are available in the Supplemental Files.
References
- Alharbi, Abaker & Makawi (2023).Alharbi FN, Abaker ZM, Makawi SZA. Phytochemical substances—mediated synthesis of zinc oxide nanoparticles (ZnO NPS) Inorganics. 2023;11(8):328. doi: 10.3390/inorganics11080328. [DOI] [Google Scholar]
- Ali et al. (2015).Ali K, Ahmed B, Dwivedi S, Saquib Q, Al-Khedhairy AA, Musarrat J. Microwave accelerated green synthesis of stable silver nanoparticles with Eucalyptus globulus l eaf extract and their antibacterial and antibiofilm activity on clinical isolates. PLOS ONE. 2015;10(7):1–20. doi: 10.1371/journal.pone.0131178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad et al. (2017).Assad R, Reshi ZA, Jan S, Rashid I. Biology of amaranths. The Botanical Review. 2017;83:382–436. doi: 10.1007/s12229-017-9194-1. [DOI] [Google Scholar]
- Awwad, Salem & Abdeen (2013).Awwad AM, Salem NM, Abdeen AO. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. International Journal of Industrial Chemistry. 2013;4:1–6. doi: 10.1186/2228-5547-4-1. [DOI] [Google Scholar]
- Bonanomi, Vinale & Scala (2009).Bonanomi G, Vinale F, Scala F. Plant-derived natural products. Cham: Springer; 2009. The role of natural products in plant-microbe interactions; pp. 301–320. [Google Scholar]
- Cappers & Bekker (2022).Cappers RTJ, Bekker RM. A manual for the identification of plant seeds and fruits. Barkhuis, 2013. JSTOR. 2022 doi: 10.2307/j.ctt20p56j9. [DOI] [Google Scholar]
- Chaudhary (1999).Chaudhary SA. Flora of the Kingdom of Saudi Arabia, illustrated. Ministry of Agriculture & Water, National Herbarium; 1999. [Google Scholar]
- Costea, Sanders & Waines (2001).Costea M, Sanders A, Waines G. Preliminary results toward a revision of the Amaranthus hybridus species complex (Amaranthaceae) SIDA, Contributions to Botany. 2001;19:931–974. [Google Scholar]
- Das (2016).Das S. Amaranthus: a promising crop of future. Cham: Springer; 2016. [Google Scholar]
- Das & Iamonico (2014).Das S, Iamonico D. Amaranthus bengalense (Amaranthaceae) a new species from India, with taxonomical notes on A. blitum aggregate. Phytotaxa. 2014;181:293–300. doi: 10.11646/phytotaxa.181.5.4. [DOI] [Google Scholar]
- Dasari et al. (2013).Dasari S, Suresh KA, Rajesh M, Reddy S, Samba C, Hemalatha CS, Wudayagiri R, Valluru L. Biosynthesis, characterization, antibacterial and antioxidant activity of silver nanoparticles produced by lichens. Journal of Bionanoscience. 2013;7:237–244. doi: 10.1166/jbns.2013.1140. [DOI] [Google Scholar]
- Firdhouse & Lalitha (2012).Firdhouse MJ, Lalitha P. Phyto-assisted synthesis and characterization of silver nanoparticles from amaranthus dubius. International Journal of Applied Biotechnology and Pharmaceutical Technology. 2012;2(4):96–101. [Google Scholar]
- Gandhi et al. (2021).Gandhi AD, Kaviyarasu K, Supraja N, Velmurugan R, Suriyakala G, Babujanarthanam R, Zang Y, Soontarapa K, Almaary KS, Elshikh MS, Chen T-W. Annealing dependent synthesis of cyto-compatible nano-silver/calcium hydroxyapatite composite for antimicrobial activities. Arabian Journal of Chemistry. 2021;14:103404. doi: 10.1016/j.arabjc.2021.103404. [DOI] [Google Scholar]
- Guo et al. (2020).Guo L, Wang Y, Bi X, Duo K, Sun Q, Yun X, Zhang Y, Fei P, Han J. Antimicrobial activity and mechanism of action of the Amaranthus tricolor crude extract against Staphylococcus aureus and potential application in cooked meat. Foods. 2020;9:359. doi: 10.3390/foods9030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider (2018).Haider N. A brief review on plant taxonomy and its components. The Journal of Plant Science Research. 2018;34:277–292. doi: 10.32381/JPSR.2018.34.02.17. [DOI] [Google Scholar]
- Hassan et al. (2022).Hassan W, Iamonico D, Al-Shaye N, Alghamdi S. Taxonomic revision of the genus Amaranthus (Amaranthaceae) in Saudi Arabia. Phytotaxa. 2022;576(2):135–157. doi: 10.11646/phytotaxa.576.2.1. [DOI] [Google Scholar]
- Hemath Naveen et al. (2010).Hemath Naveen KS, Kumar G, Karthik L, Bhaskara Rao KV. Extracellular biosynthesis of silver nanoparticles using the filamentous fungus Penicillium sp. Archives of Applied Science Research. 2010;2:161–167. [Google Scholar]
- Hernández-Ledesma et al. (2015).Hernández-Ledesma P, Berendsohn WG, Borsch T, Mering S. Von, Akhani-H, Arias S, Castañeda Noa I, Eggli U, Eriksson R, Flores-Olvera H, Fuentes-Bazán S, Kadereit G, Klak C, Korotkova N, Nyffeler R, Ocampo G, Ochoterena H, Oxelman B, Rabeler RK, Sanchez A, Schlumpberger BO, Uotila P. A taxonomic backbone for the global synthesis of species diversity in the angiosperm order Caryophyllales. Willdenowia. 2015;45:281–383. doi: 10.3372/wi.45.45301. [DOI] [Google Scholar]
- Iamonico (2010).Iamonico D. Biology, life-strategy and invasiveness of Amaranthus retroflexus L. (Amaranthaceae) in central Italy: preliminary remarks. Botanica Serbica. 2010;34:137–145. [Google Scholar]
- Iamonico (2014a).Iamonico D. Lectotypification of Linnaean names in the genus Amaranthus L.(Amaranthaceae) Taxon. 2014a;63:146–150. doi: 10.12705/631.34. [DOI] [Google Scholar]
- Iamonico (2014b).Iamonico D. Amaranthus graecizans sl (Amaranthaceae) in Italy: note tasso- nomically and distributively. Informatore Botanico Italiano. 2014b;46:39–46. (In Italian) [Google Scholar]
- Iamonico (2015).Iamonico D. Taxonomic revision of the genus Amaranthus (Amaranthaceae) in Italy. Phytotaxa. 2015;199:1–84. doi: 10.11646/phytotaxa.199.1.1. [DOI] [Google Scholar]
- Iamonico (2016a).Iamonico D. Nomenclature survey of the genus Amaranthus (Amaranthaceae), 5. Moquin-Tandon’s names. Phytotaxa. 2016a;273:81–114. doi: 10.11646/phytotaxa.273.2.1. [DOI] [Google Scholar]
- Iamonico (2016b).Iamonico D. Nomenclature survey of the genus Amaranthus (Amaranthaceae) 3. Names linked to the Italian flora. Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology. 2016b;150:519–531. doi: 10.1080/11263504.2014.987188. [DOI] [Google Scholar]
- Iamonico (2017).Iamonico D. Amaranthus × romanus (Amaranthaceae), a new hybrid from Italy. Phytotaxa. 2017;295:89–91. doi: 10.11646/phytotaxa.295.1.9. [DOI] [Google Scholar]
- Iamonico (2020a).Iamonico D. A nomenclatural survey of the genus Amaranthus (Amaranthaceae) 9: names published by Roxburgh. Taiwania. 2020a;65:559–566. [Google Scholar]
- Iamonico (2020b).Iamonico D. A nomenclatural survey of the genus Amaranthus (Amaranthaceae) 7: names published by Willdenow. Willdenowia. 2020b;50:147–155. doi: 10.3372/wi.50.50114. [DOI] [Google Scholar]
- Iamonico (2020c).Iamonico D. Nomenclatural survey of the genus Amaranthus (Amaranthaceae), 11. Dioecious Amaranthus species belonging to the sect. Saueranthus. Darwiniana, Nueva Serie. 2020c;8:567–575. doi: 10.14522/darwiniana.2020.82.898. [DOI] [Google Scholar]
- Iamonico & Palmer (2020).Iamonico D, Palmer J. Nomenclature survey of the genus Amaranthus (Amaranthaceae), 6. Names linked to the Australian flora. Australian Systematic Botany. 2020;33:169–173. doi: 10.1071/SB18062. [DOI] [Google Scholar]
- Jamalluddin et al. (2021).Jamalluddin N, Symonds RC, Mayes S, Ho WK, Massawe F. Food security and nutrition. Switzerland: Academic Press; 2021. Diversifying crops for food and nutrition security: a case of vegetable amaranth, an ancient climate-smart crop; pp. 125–146. [Google Scholar]
- Kolya et al. (2015).Kolya H, Maiti P, Pandey A, Tripathy T. Green synthesis of silver nanoparticles with antimicrobial and azo dye (Congo red) degradation properties using Amaranthus gangeticus Linn leaf extract. Journal of Analytical Science and Technology. 2015;6:1–7. doi: 10.1186/s40543-014-0041-2. [DOI] [Google Scholar]
- Korbekandi & Iravani (2012).Korbekandi H, Iravani S. The delivery of nanoparticles. IntechOpen; Rijeka: 2012. Silver nanoparticles. [DOI] [Google Scholar]
- Linnaeus (1753).Linnaeus C. Amaranthus. Species Plant. 1753;2:989–991. [Google Scholar]
- Manivasagan et al. (2013).Manivasagan P, Venkatesan J, Senthilkumar K, Sivakumar K, Kim S-K. Biosynthesis, antimicrobial and cytotoxic effect of silver nanoparticles using a novel Nocardiopsis sp. MBRC-1. BioMed Research International. 2013;2013 doi: 10.1155/2013/287638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mie et al. (2014).Mie R, Samsudin MW, Din LB, Ahmad A, Ibrahim N, Adnan SNA. Synthesis of silver nanoparticles with antibacterial activity using the lichen Parmotrema praesorediosum. International Journal of Nanomedicine. 2014;9:121–127. doi: 10.2147/IJN.S52306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed Afrah et al. (2021).Mohammed Afrah E, Al-Keridis LA, Rahman I, Alotaibi MO, Suliman RS, Alrajhi AM, Elobeid MM, Alothman MR, Alhomaidi EA, Korany SM. Silver nanoparticles formation by jatropha integerrima and LC/MS-QTOF-based metabolite profiling. Nanomaterials. 2021;11:2400. doi: 10.3390/NANO11092400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal et al. (2011).Mondal AK, Mondal S, Samanta S, Mallick S. Synthesis of ecofriendly silver nanoparticle from plant latex used as an important taxonomic tool for phylogenetic interrelationship advances in bioresearch 2. Synthesis. 2011;31:33. [Google Scholar]
- Muthukumar et al. (2020).Muthukumar H, Palanirajan SK, Shanmugam MK, Gummadi SN. Plant extract mediated synthesis enhanced the functional properties of silver ferrite nanoparticles over chemical mediated synthesis. Biotechnology Reports. 2020;26:e00469. doi: 10.1016/j.btre.2020.e00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa et al. (1999).Nakagawa Y, Shimazu K, Ebihara M, Nakagawa K. Aspergillus niger pneumonia with fatal pulmonary oxalosis. Journal of Infection and Chemotherapy. 1999;5:97–100. doi: 10.1007/s101560050016. [DOI] [PubMed] [Google Scholar]
- Nguyen et al. (2020).Nguyen DH, Vo TNN, Nguyen NT, Ching YC, Thi TTH. Comparison of biogenic silver nanoparticles formed by Momordica charantia and Psidium guajava leaf extract and antifungal evaluation. PLOS ONE. 2020;15(9):1–13. doi: 10.1371/JOURNAL.PONE.0239360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osonga et al. (2020).Osonga FJ, Akgul A, Yazgan I, Akgul A, Eshun GB, Sakhaee L, Sadik OA. Size and shape-dependent antimicrobial activities of silver and gold nanoparticles: a model study as potential fungicides. Molecules. 2020;25:2682. doi: 10.3390/molecules25112682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal, Tak & Song (2007).Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Applied and Environmental Microbiology. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, Singh & Sasikumar (2015).Paul S, Singh ARJ, Sasikumar CS. Green synthesis of bio-silver nanoparticles by Parmelia perlata, Ganoderma lucidum and Phellinus igniarius & their fields of application. Indian Journal of Research in Pharmacy and Biotechnology. 2015;3:100. [Google Scholar]
- Pesavento et al. (2015).Pesavento G, Calonico C, Bilia AR, Barnabei M, Calesini F, Addona R, Mencarelli L, Carmagnini L, Di Martino MC, Nostro ALO. Antibacterial activity of Oregano, Rosmarinus and Thymus essential oils against Staphylococcus aureus and Listeria monocytogenes in beef meatballs. Food Control. 2015;54:188–199. doi: 10.1016/j.foodcont.2015.01.045. [DOI] [Google Scholar]
- Peter & Gandhi (2017).Peter K, Gandhi P. Rediscovering the therapeutic potential of Amaranthus species: a review. Egyptian Journal of Basic and Applied Sciences. 2017;4:196–205. doi: 10.1016/j.ejbas.2017.05.001. [DOI] [Google Scholar]
- Rajeshkumar & Bharath (2017).Rajeshkumar S, Bharath LV. Mechanism of plant-mediated synthesis of silver nanoparticles–a review on biomolecules involved. Chemico-Biological Interactions. 2017;273:219–227. doi: 10.1016/j.cbi.2017.06.019. [DOI] [PubMed] [Google Scholar]
- Rao, Chen & McClements (2019).Rao J, Chen B, McClements DJ. Improving the efficacy of essential oils as antimicrobials in foods: mechanisms of action. Annual Review of Food Science and Technology. 2019;10:365–387. doi: 10.1146/annurev-food-032818-121727. [DOI] [PubMed] [Google Scholar]
- Rasi-Bonab et al. (2018).Rasi-Bonab F, Afaghi-Gharamaleki A, Feizi H, Alipoor-Dolatabad S, Alizadeh S, Sadat-Amini M. Evaluation of silver nanoparticles and alcoholic extract of Amaranthus retroflexus on pathogenic bacteria. Health Biotechnology and Biopharma. 2018;2(3):12–23. [Google Scholar]
- Shinde et al. (2013).Shinde VV, Jadhav PR, Kim JH, Patil PS. One-step synthesis and characterization of anisotropic silver nanoparticles: application for enhanced antibacterial activity of natural fabric. Journal of Materials Science. 2013;48:8393–8401. doi: 10.1007/s10853-013-7651-8. [DOI] [Google Scholar]
- Siddiqi et al. (2018).Siddiqi KS, Rashid M, Rahman A, Husen A, Rehman S. Biogenic fabrication and characterization of silver nanoparticles using aqueous-ethanolic extract of lichen (Usnea longissima) and their antimicrobial activity. Biomaterials Research. 2018;22:1–9. doi: 10.1186/s40824-017-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigamoney et al. (2016).Sigamoney M, Shaik S, Govender P, Krishna SBN. African leafy vegetables as bio-factories for silver nanoparticles: a case study on Amaranthus dubius C Mart. Ex Thell. South African Journal of Botany. 2016;103:230–240. doi: 10.1016/j.sajb.2015.08.022. [DOI] [Google Scholar]
- Sujitha & Kannan (2013).Sujitha MV, Kannan S. Green synthesis of gold nanoparticles using Citrus fruits (Citrus limon. Citrus reticulata and Citrus sinensis) aqueous extract and its characterization. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2013;102:15–23. doi: 10.1016/j.saa.2012.09.042. [DOI] [PubMed] [Google Scholar]
- Thiers (2022).Thiers BM. New York Botanical Garden’s Virtual Herbarium. 2022. [15 May 2022]. (continously updated) Index Herbariorum: a global directory of public herbaria and associated staff. [Google Scholar]
- Tranel & Trucco (2009).Tranel PJ, Trucco F. 21st-century weed science: a call for Amaranthus genomics. Weedy and Invasive Plant Genomics. 2009;53:81. [Google Scholar]
- Tripathy et al. (2010).Tripathy A, Raichur AM, Chandrasekaran N, Prathna TC, Mukherjee A. Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. Journal of Nanoparticle Research. 2010;12:237–246. doi: 10.1007/s11051-009-9602-5. [DOI] [Google Scholar]
- Trombetta et al. (2005).Trombetta D, Castelli F, Sarpietro MG, Venuti V, Cristani M, Daniele C, Saija A, Mazzanti G, Bisignano G. Mechanisms of antibacterial action of three monoterpenes. Antimicrobial Agents and Chemotherapy. 2005;49:2474–2478. doi: 10.1128/AAC.49.6.2474-2478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne (2010).Wayne PA. Clinical and Laboratory Standards Institute: performance standards for antimicrobial susceptibility testing: 20th informational supplement. CLSI Document. 2010:M100–S20.
- Yamada et al. (2018).Yamada M, Takahashi S, Takada N, Kanda H, Goto M. Synthesis of silver nanoparticles by atmospheric-pressure pulsed discharge plasma in a slug flow system. Japanese Journal of Applied Physics. 2018;58:16001. [Google Scholar]
- Zareh et al. (2018).Zareh M, Nafady N, Faried A, Mohamed M. Green synthesis of silver nanoparticles from capitula extract of some Launaea (Asteraceae) with notes on their taxonomic significance. Egyptian Journal of Botany. 2018;58:185–194. doi: 10.21608/ejbo.2018.1375.1111. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available in the Supplemental Files.