Abstract
Objectives
This study aimed to investigate the timing sequence recovery effects of single and repeated Mild Hyperbaric Oxygen Therapy (MHOT) on muscle fatigue induced by cycling exercise through a comprehensive set of parameters.
Methods
This study employed a controlled crossover design involving 12 Chinese secondary national-level male athletes. Each participant completed two identical trials over six days. Each trial consisted of a 90-min cycling exercise followed by either a Control (CON) intervention (1 atm absolute (ATA), 20.9 % oxygen, 60 min) or MHOT intervention (1.25 ATA, 26%–28 % oxygen, 60 min). Various physiological parameters including Rating of Perceived Exertion (RPE), Heart Rate (HR), Peripheral Oxygen Saturation (SpO2), Perfusion Index (PI%), Creatine Kinase (CK), Lactate Dehydrogenase (LDH), Lactic Acid (LA), Blood Urea Nitrogen (BUN), Superoxide Dismutase (SOD), Malondialdehyde (MDA), and Standing Long Jump Distance (SLJ) were measured at six different time points throughout the trials.
Results
RPE revealed that the MHOT group experienced reduced subjective fatigue in comparison to the CON group (P < 0.05). Additionally, MHOT demonstrated quicker recovery in HR and PI% compared to the CON group (P < 0.05). Regarding CK, LA, BUN, SOD, and MDA levels, the MHOT group exhibited accelerated recovery post-6 intervention and at the 24-h mark after six interventions, showing significant improvement over the CON group (P < 0.05). However, no notable disparity was observed between groups concerning SpO2, LDH, and SLJ.
Conclusions
Both single and repeated sessions of MHOT demonstrated efficacy in alleviating subjective fatigue and promoting recovery of heart rate and blood perfusion following muscle fatigue, ensuring parallel structure and consistency in their effects. Repeated MHOT sessions (six times) exhibit a significant reduction in levels of blood markers associated with muscle damage, metabolites, and oxidative stress. However, the impact of a single MHOT intervention was less pronounced.
Keywords: Muscle fatigue, Mild hyperbaric oxygen therapy, Male athletes, Recovery methods
1. Introduction
Fatigue and exhaustion are inevitable outcomes of specific exercise modalities, influenced by variables such as intensity, duration, and type. These factors affect bodily systems, leading to various sensations of fatigue within the athlete's mind and body.1 Muscle fatigue, marked by a decrease in force generation capacity post-exercise, is a common occurrence that impairs performance in sports and other strenuous activities.2 Prolonged activities like cycling can induce cumulative fatigue, which, if left unaddressed, may lead to overtraining, chronic fatigue syndrome, endocrine imbalances, immune dysfunction, and various health complications.2,3
Recovery strategies aim to mitigate the adverse impacts of training or competition, facilitating a swift return to optimal performance levels.4 Such methods have gained particular importance among elite athletes, underscoring the necessity of post-exercise recovery routines.5 Current guidelines by the World Anti-Doping Agency permit the use of supplemental oxygen,6 with hyperbaric oxygen therapy (HBOT)7,8 and hyperoxia6 being notable examples employed to restore physiological balance post-exercise. HBOT is recognized for its efficacy in tissue repair and mitigating fatigue,7 enhancing endurance during exercise,8 and offering relief in chronic pain conditions like fibromyalgia.9 Hyperoxia, on the other hand, has been shown to bolster performance during intense and prolonged activities.6 Despite the benefits, HBOT and hyperoxia carry drawbacks, including potential side effects such as barotrauma and oxygen toxicity,10 as well as the risk of increased oxidative stress with prolonged hyperoxia exposure.6 Additionally, some studies suggest that HBOT has a placebo effect on post-training recovery, and its impact on improving fatigue is not evident.11 Therefore, further research is needed to better understand the effect of oxygen therapy on sports recovery.
Mild Hyperbaric Oxygen Therapy (MHOT), characterized by exposure to slightly elevated atmospheric pressure (1.24–1.29 ATA) and enriched oxygen levels (25%–40 % oxygen), emerges as a promising alternative.12,13 By avoiding the pitfalls of barotrauma and excessive reactive oxygen species production, MHOT is poised to offer therapeutic advantages in metabolic regulation and disease management.12,13 Its role in combating muscle atrophy and enhancing cellular metabolism makes it a compelling option for sports recovery.14,15 Consequently, MHOT has garnered attention and is increasingly considered for improving exercise performance and enhancing fatigue recovery.16,17 Despite the established benefits of hyperoxia and HBOT in this realm, MHOT remains relatively underexplored, with scant data on its effectiveness in athletic recovery.
This study aims to elucidate the restorative effects of both single and repeated MHOT sessions on muscle fatigue induced by prolonged cycling among collegiate male athletes. We hypothesize that MHOT will provide superior recuperative benefits compared to standard recovery interventions, thereby facilitating enhanced recovery from exercise-induced fatigue.
2. Methods
2.1. Study design
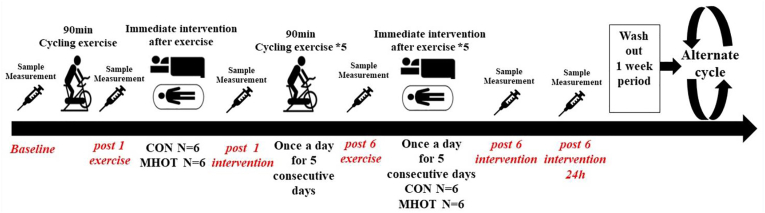
This investigation adopted a controlled crossover design16 (Fig. 1) to examine the impact of various recovery approaches on muscle fatigue induced by a standardized cycling exercise regimen. Over two separate weeks, 12 male athletes underwent identical sessions of a cycling exercise protocol at the China Institute of Sport Science's laboratory, aimed at inducing muscle fatigue, performed daily for six consecutive days. Following each session, participants were divided into two groups: six received a standard control (CON) treatment, while the remaining six were provided with MHOT. These interventions were swapped in the subsequent experimental cycle, ensuring all participants experienced both treatments across the study's duration, which encompassed 12 sessions in total.
Fig. 1.
Study design
CON: Control group; MHOT: Mild Hyperbaric Oxygen Therapy group.
Data collection occurred at six time points during each trial: before the exercise (baseline), after a single exercise session (post-1 exercise), following one intervention session (post-1 intervention), after completing six exercise sessions (post-6 exercise), after six intervention sessions (post-6 intervention), and 24 h after the final intervention session (pos- 6 intervention 24 h). The parameters assessed included physiological measures such as the rating of perceived exertion (RPE), heart rate (HR), peripheral oxygen saturation (SpO2), and finger capillary perfusion index (PI%); biochemical markers in the blood such as creatine kinase (CK), lactate dehydrogenase (LDH), lactic acid (LA), blood urea nitrogen (BUN), superoxide dismutase (SOD), and malondialdehyde (MDA); along with a performance indicator of lower limb strength, specifically the standing long jump distance (SLJ).
A one-week washout period was incorporated between each trial to ensure full muscular recovery.18,19 Participants were advised against engaging in additional exercise training outside of the study protocol to mitigate the onset of further muscle fatigue. All exercise and intervention sessions were conducted under controlled conditions in a laboratory, maintaining a temperature range of 22 °C° to 28 °C and a relative humidity between 45 % and 55 %. To ensure consistency and reduce bias, a single experimenter conducted all measurements within a fixed schedule, thereby minimizing the potential influence of human-related confounding factors and diurnal variations.
2.2. Participants
Drawing from prior published research in exercise and oxygen therapy11,16,17,19,20 and aiming to achieve a medium effect size with 80 % statistical power at a 0.05 significance threshold (as determined by G*Power analysis), this investigation enlisted 12 male athletes. This cohort comprised six secondary national-level middle and long-distance runners (specializing in 800 m, 1500 m, and 3000 m events) and six secondary national-level professional soccer players affiliated with the Capital University of Physical Education and Sports.
The participants' demographic profile included an age of 20.41 ± 1.31 years (mean ± SD), standing height of 176.75 ± 3.49 cm, body weight of 70.58 ± 6.27 kg, weekly training regimen of 8.33 ± 1.87 h, and a history of athletic training of 6.66 ± 2.10 years. Their maximal oxygen uptake (O2 max) was recorded at 47.30 ± 3.72 ml kg−1.min−1. Initial medical assessments confirmed that all athletes were in excellent health, with no previous involvement in oxygen therapy interventions and no diagnosed cardiovascular, respiratory, endocrine, claustrophobia, or otic disorders. Throughout the study, participants refrained from engaging in regular or sporadic sports training.
Strict lifestyle guidelines were adhered to during the duration of the study; subjects refrained from smoking, taking supplements, and consuming alcohol, caffeinated beverages, and spicy foods. A controlled dietary regimen was implemented, consisting of a simple Chinese diet providing approximately 800–1000 kcal, with water intake set between 1500 and 2000 ml during exercise and 550 ml throughout the intervention phases. Participants were required to document their daily dietary intake for the duration of the study. Written informed consent, outlining the study's benefits and potential risks, was obtained from all participants This study was approved by the Ethical Committee of the China Institute of Sport Science (CISSLA-2020062801), and adhered to the ethical principles of the Declaration of Helsinki.
2.3. Exercise protocol
Before the main experiment commenced, participants visited the laboratory for preliminary baseline assessments. Maximal oxygen uptake (O2max) was measured using a motorized cycle ergometer (VIA sprint 150 P, ergoline GmbH, Germany), and breath-by-breath cardiopulmonary data was recorded with a Cortex Metalyzer 3B (Cortex Biophysik GmbH, Germany). The protocol for O2max testing began at a power setting of 10 W, with incremental increases of 20 W every 2 min until participants reached volitional exhaustion, maintaining a pedaling speed within 60–70 revolutions per minute.21,22 Criteria for O2max included a leveling off of oxygen consumption despite an increase in workload, a respiratory exchange ratio greater than 1.1, a heart rate exceeding 90 % of the estimated maximum, and subjective signs of exhaustion preventing further cycling.
After the initial O2max assessment, all participants received a briefing on the exercise protocol, which was aligned with methodologies from relevant studies on hyperoxia, HBOT, and MHOT in the context of exercise.17,23, 24, 25, 26 The exercise regimen consisted of 90 min of cycling, segmented into three consecutive 30-min intervals at intensities corresponding to 65 %, 70 %, and 75 % of O2max, respectively. Beginning with a 5-min warm-up at 100 W, the session progressed with the ergometer's power adjusted to match the predetermined percentages of each participant's O2max. This cycling exercise consisted of continuous pedaling without any rest intervals, maintaining a pedaling speed range of 60–70 rpm.27,28 The protocol was rigorously followed once daily for six days, culminating in 12 cycles throughout the study (Fig. 2), with the regimen standardized across both participant groups. Due to the demanding nature of the protocol, participants were advised against engaging in additional exercise throughout the study.
Fig. 2.
Participants completed exercise protocol and MHOT intervention.
2.4. Treatment protocol
All study procedures were designed based on prior published studies on HBOT and sports,29, 30, 31, 32, 33 MHOT and sports.16,34,35 Participants assigned to the CON group were positioned comfortably on a sofa bed for 60 min under ambient room conditions (1 atm absolute (ATA), 20.9 % oxygen, with a temperature range of 22–28 °C). No information on the research topic and the recovery method was provided to the participant to limit a potential placebo effect. Conversely, participants in the MHOT group were placed in the MHOT chamber (Beijing Chuangxin Kaida Technology Co., Ltd). The chamber includes a soft, oxygen-equipped cabin measuring 210 cm in length and 72 cm in width, designed to comfortably accommodate one individual in a reclined position. It is accompanied by a compressor control unit (56 cm by 50 cm by 99 cm) housing an oxygen concentrator and air compressor. The control unit, featuring a computer-assisted system, regulates the chamber's atmospheric pressure and oxygen concentration. For this study, MHOT conditions were set to 1.25 ATA with an oxygen concentration between 26 % and 28 % (a preset configuration of the device). MHOT sessions were administered for 60 min immediately following each exercise session, once daily for six days, resulting in a total of six MHOT sessions per trial.12,16
The internal environment of the MHOT chamber was maintained at a stable temperature of approximately 22 ± 8 °C, with relative humidity (RH) kept between 40 % and 60 %. The specific parameters for oxygen pressure, concentration, and exposure duration were selected following methodologies recommended by Takemura16 and Ishihara12 et al., and informed by preliminary outcomes from prior participant experiences. Before entering the oxygen chamber, subjects were advised to dry off sweat and use the restroom to ensure comfort and prevent any disruptions during the session. Throughout the pressurization and depressurization phases, individuals were given the option to perform maneuvers aimed at equalizing eustachian tube pressure, such as the Valsalva maneuver (pinching the nose and gently blowing) and swallowing, to alleviate any discomfort associated with pressure changes (Fig. 2).
2.5. Measurements
The Borg Rating of Perceived Exertion (RPE) scale was employed to evaluate muscle fatigue in both lower and upper extremities, with subjective fatigue assessments recorded at six distinct time points using the RPE scale. Peripheral oxygen saturation (SpO2) and the perfusion index in capillary blood were continuously monitored at these time points using a clip-type finger oximeter (Heal Force Prince-100NW, Likang Biomedical Technology Holdings Co., Ltd., Shanghai, China) placed on the index finger of each participant. Heart rate recovery, indicative of adaptation to training load and physiological adjustments, was measured at the same six intervals using a heart rate monitor (OH1 TEAM Team Heart Rate Monitoring System, POLAR, Finland).
Concurrently, 8 mL of venous blood was drawn from each subject's antecubital vein into a serum separator tube at these time points. The samples were then centrifuged at 3000 rpm at 4 °C for 15 min to separate the serum, which was subsequently stored in aliquots at −80 °C pending biochemical analysis. Serum creatine kinase (CK) and lactate dehydrogenase (LDH) concentrations were quantified using VITROS Chemistry CK and LDH DT slides with kits supplied by the Nanjing Jiancheng Bioengineering Institute, Nanjing, China. Additionally, serum levels of blood urea nitrogen (BUN) and lactic acid (LA) were determined utilizing commercially available kits, following the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Malondialdehyde (MDA) levels were assessed through the Yagi method, with the formation of thiobarbituric acid reactive substances measured spectrophotometrically at 532 nm. Superoxide dismutase (SOD) concentrations were evaluated using ELISA kits as per the provider's guidelines (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
For the standing long jump (SLJ) tests, participants initiated from a bipedal stance and were instructed to execute a forward squat jump, covering the maximal horizontal distance possible. This distance was precisely measured using an electronic jump rangefinder (Beijing Xindonghuateng Sports Equipment Co., Ltd., Beijing, China). Each participant performed two jumps at every time point, with the longest jump distance being recorded.
2.6. Statistical analysis
All presented data are expressed as mean ± standard deviation (±SD). Statistical analyses were performed using SPSS software (version 22.0, IBM SPSS Statistics, Chicago, IL), with a significance threshold set at P < 0.05. The study employed a two-way repeated-measures ANOVA to evaluate the impact of recovery interventions (CON vs. MHOT) across six predetermined time points (baseline, post-1 exercise, post-1 intervention, post-6 exercise, post-6 intervention, post-6 intervention 24 h) on variables such as RPE, HR, SpO2, PI%, as well as serum biomarkers (CK, LDH, LA, BUN, SOD, MDA), and SLJ performance. Assumptions of normality were verified using the Kolmogorov–Smirnov test and sphericity assumption was assessed via Mauchly's test. In instances where sphericity was violated, the Greenhouse-Geisser correction was applied to ensure an accurate interpretation of the ANOVA results. Upon identifying significant interaction effects, we conducted a simple effects analysis to explore within-condition comparisons, aiming to detect differences in the same condition at specific time points. Additionally, between-condition comparisons were performed to identify differences in conditions at the same time point.
Effect sizes were calculated according to Cohen's guidelines to interpret the magnitude of observed differences. Values ranging from 0.2 to 0.5 were considered indicative of small effects, while those from 0.5 to 0.8 represented medium effects, and values exceeding 0.8 denoted large effects. This methodological approach facilitated a comprehensive assessment of how recovery interventions influenced various metrics over the study duration.
3. Results
3.1. Exercise parameters
Both the CON and MHOT groups engaged in daily exercise sessions lasting 90 min. In the CON group, the RPE scale was 17.75 ± 1.05 and 13.00 ± 1.65, HR was 150.33 ± 8.31 bpm and 118.66 ± 10.72 bpm/min, and power was 220 ± 16.51 W and 226.66 ± 15.56 W after one exercise session and after six exercise sessions, respectively. In the MHOT group, the RPE scale was 17.16 ± 1.26 and 12.66 ± 2.83, HR was 148.83 ± 7.06 bpm and 115.41 ± 10.17 bpm, and power was 224.16 ± 13.11 W and 230.00 ± 13.48 W after one exercise session and after six exercise sessions, respectively.
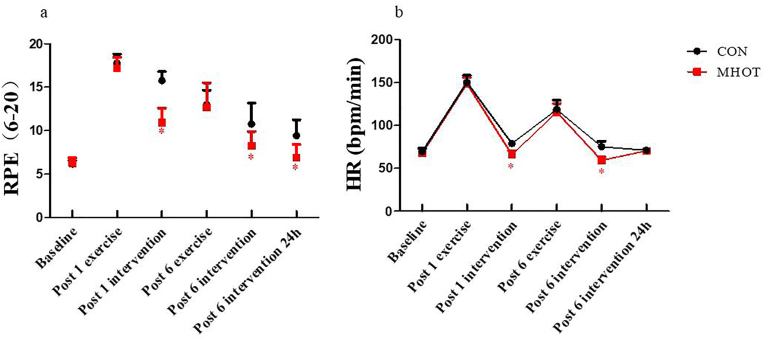
3.2. Rating of perceived exertion and heart rate
The results shown in Fig. 3 revealed a significant time group interaction effect for the RPE score (F(2.953) = 8.179, p < 0.01). A significant main effect of time was also observed (F(2.953, 64.959) = 157.166, p < 0.01). Simple main effects analysis indicated that the RPE score in the MHOT group significantly decreased compared to the CON group at post-1 intervention (p = 0.001, ES = 0.86), post-6 intervention (p = 0.007, ES = 0.51), and post-6 intervention 24 h (p = 0.001, ES = 0.59). The decline in RPE score was fastest in the MHOT group, reaching levels close to baseline at post-6 intervention, followed by a slower decline in the CON group. Furthermore, the results demonstrated a significant time group interaction effect for HR (F(2.738) = 6.217, p < 0.01). In the MHOT group, HR was significantly reduced at post-1 intervention (p = 0.001, ES = 0.85) and post-6 intervention (p = 0.001, ES = 0.82) compared to the CON group. A significant main effect of time was observed for HR (F(2.738, 60.240) = 671.442, p < 0.01), indicating faster recovery in the MHOT group at post-1 intervention and post-6 intervention compared to the CON group.
Fig. 3.
Effects of different interventions on RPE Scale and HR (Six-time points)
CON: Control group; MHOT: Mild Hyperbaric Oxygen Therapy group; (3a): RPE Scale; (3b): HR; All values were presented as mean ± SD, n = 12 for each group. * significantly different from CON group (P < 0.05).
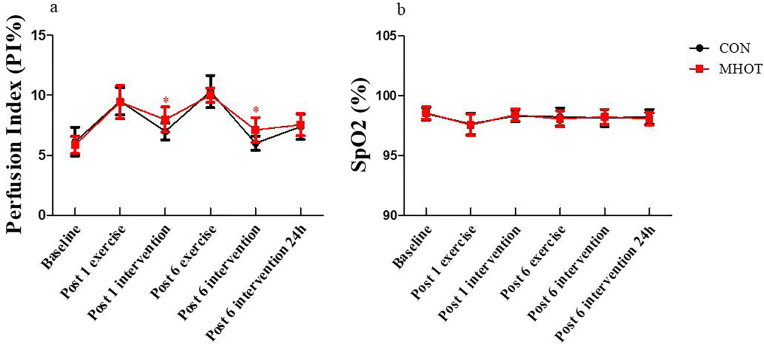
3.3. Perfusion index and peripheral oxygen saturation
The PI% is shown in Fig. 4. Results demonstrated a time group interaction effect (F(4.2) = 2.444, p < 0.05). Simple main effects analysis revealed that at post-1 intervention (p = 0.014, ES = 0.48) and post-6 intervention (p = 0.004, ES = 0.54), the PI% in the MHOT group significantly increased compared to the CON group. Additionally, a significant main effect of time was observed (F(4.2, 92.41) = 66.190, p < 0.01), indicating a faster recovery in the MHOT group at post-1 intervention and post-6 intervention compared to the CON group.
Fig. 4.
Effects of different intervention on PI% and SpO2 (Six time points)
CON: Control group; MHOT: Mild Hyperbaric Oxygen Therapy group; (4a): PI%; (4b): SpO2; All values were presented as mean ± SD, n = 12 for each group. * significantly different from CON group (P < 0.05).
Regarding SpO2, the time group interaction effect was not significant (F(4.086) = 0.218, p > 0.05), but a significant main effect of time was observed (F(4.086, 89.90) = 5.289, p < 0.01). However, no significant difference in SpO2 was found between the two groups at the six-time points.
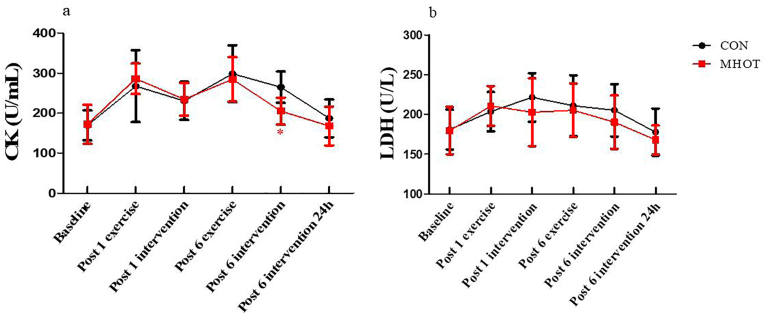
3.4. Creatine kinase and lactate dehydrogenase
The results in Fig. 5 indicated that CK and LDH levels did not show a significant time group interaction effect (F(4.23) = 1.763, p > 0.05; F(3.118) = 0.593, p > 0.05). Simple main effects analysis revealed that the CK levels in the MHOT group were significantly reduced compared to the CON group at post-6 intervention (p = 0.001, ES = 0.63). However, there were no significant differences in LDH levels between the two groups at the six-time points.
Fig. 5.
Effects of different interventions on CK and LDH (Six-time points)
CON: Control group; MHOT: Mild Hyperbaric Oxygen Therapy group; (5a): CK; (5b): LDH; All values were presented as mean ± SD, n = 12 for each group. * significantly different from CON group (P < 0.05).
Furthermore, a significant main effect of time was observed for both CK (F(4.23, 93.052) = 23.205, p < 0.01) and LDH (F(3.118, 68.592) = 7.049, p < 0.01) levels. The CK levels in the MHOT group declined fastest, with values at post-6 intervention close to the baseline. Meanwhile, CK levels in the CON group at post-6 intervention 24 h were close to the baseline. The LDH levels in the MHOT group also declined the fastest, with a relatively higher extent of decline compared to the CON group. Regarding the coefficients of variation, the CK coefficient of variation was 23.33 % in the CON group and 20.60 % in the MHOT group, while the LDH coefficient of variation was 15.08 % in the CON group and 15.69 % in the MHOT group.
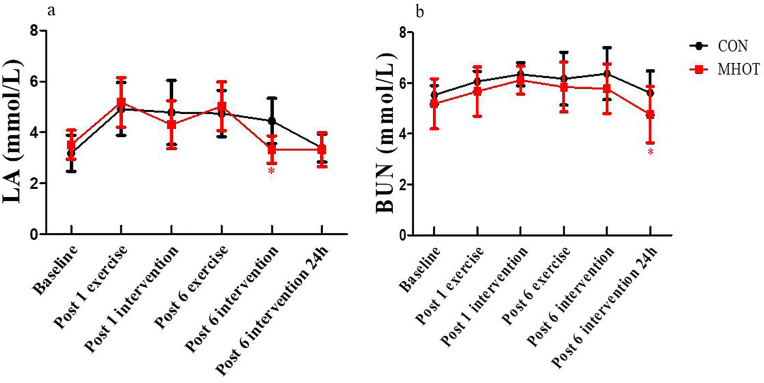
3.5. Lactic acid and blood urea nitrogen
The results in Fig. 6 revealed a significant time group interaction effect for LA levels (F(3.529) = 2.889, p < 0.05). Simple main effects analysis indicated that LA levels in the MHOT group were significantly reduced at the post-6 intervention time point compared to the CON group (p = 0.001, ES = 0.61). Additionally, a significant main effect of time was observed (F(3.529, 77.632) = 19.674, p < 0.01), with LA levels in the MHOT group declining faster. Specifically, the level at post-6 intervention was close to the baseline, while the level in the CON group at post-6 intervention 24 h was close to the baseline. In terms of coefficients of variation, the LA coefficient of variation was 20.74 % in the CON group and 18.53 % in the MHOT group.
Fig. 6.
Effects of different interventions on LA and BUN (Six-time points)
CON: Control group; MHOT: Mild Hyperbaric Oxygen Therapy group; (6a): LA; (6b): BUN; All values were presented as mean ± SD, n = 12 for each group. * significantly different from CON group (P < 0.05).
Regarding BUN levels, the time group interaction effect was not significant (F(2.330) = 0.620, p > 0.05). However, BUN levels in the MHOT group significantly decreased at post-6 intervention 24 h compared to the CON group (p = 0.048, ES = 0.439). A significant main effect of time was observed for BUN levels (F(2.330, 51.266) = 8.482, p < 0.01), with the MHOT group showing the fastest decline, and the extent of the decline was relatively higher than that in the CON group. In terms of coefficients of variation, the BUN coefficient of variation was 11.43 % in the CON group and 16.97 % in the MHOT group.
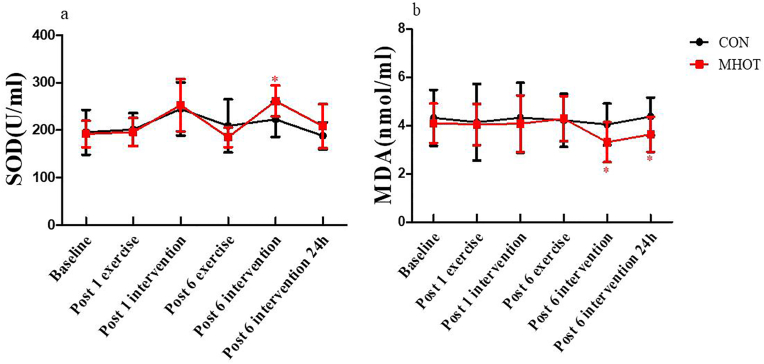
3.6. Superoxide dismutase and malondialdehyde
The results from Fig. 7 indicated that SOD and MDA levels did not exhibit a significant time group interaction effect (F(3.913) = 2.246, p > 0.05; F(3.655) = 0.662, p > 0.05). However, simple main effects analysis revealed that SOD levels in the MHOT group significantly increased at post-6 intervention compared to the CON group (p = 0.012, ES = 0.48). Additionally, at post-6 intervention and post-6 intervention 24 h, the MDA levels in the MHOT group were significantly reduced compared to the CON group (p = 0.045, ES = 0.40; p = 0.027, ES = 0.43, respectively).
Fig. 7.
Effects of different interventions on SOD and MDA (Six-time points)
CON: Control group; MHOT: Mild Hyperbaric Oxygen Therapy group; (7a): SOD; (7b):MDA; All values were presented as mean ± SD, n = 12 for each group. * significantly different from CON group (P < 0.05).
A significant main effect of time was observed for SOD levels (F(3.913, 86.090) = 11.841, p < 0.01), indicating that SOD levels in the MHOT group increased fastest and had a substantial increase in within-group comparisons, while remaining around the baseline in the CON group. Conversely, the main effect of time for MDA levels was not significant (F(3.655, 80.408) = 1.063, p > 0.05).
Regarding coefficients of variation, the SOD coefficient of variation was 20.44 % in the CON group and 16.23 % in the MHOT group, while the MDA coefficient of variation was 27.17 % in the CON group and 22.62 % in the MHOT group.
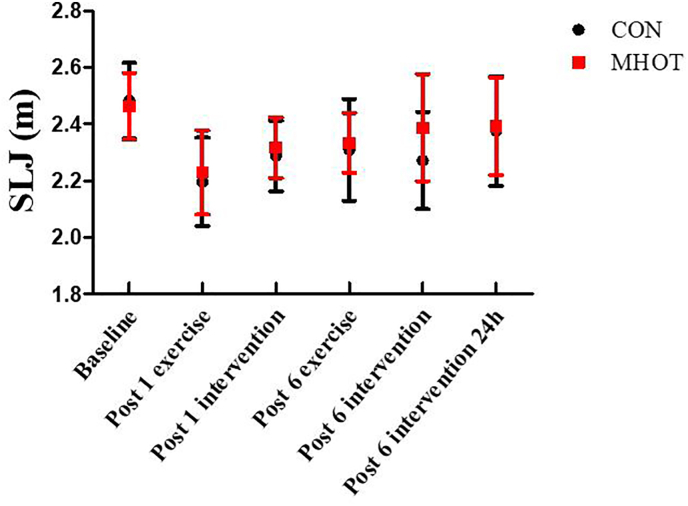
3.7. Standing long jump distance
The results revealed that SLJ levels did not show a significant time group interaction effect (F(3.344) = 1.032, p > 0.05), indicating no significant difference in SLJ between the two groups at the six-time points (Fig. 8). However, a significant main effect of time was observed (F(3.344, 73.576) = 15.174, p < 0.01), suggesting that SLJ results in the MHOT group increased fastest, and the extent of the increase was significant. However, the result was not significantly different within the group.
Fig. 8.
Effects of different interventions on SLJ (Six-time points)
CON: Control group; MHOT: Mild Hyperbaric Oxygen Therapy group; All values were presented as mean ± SD, n = 12 for each group.
4. Discussion
4.1. Muscle fatigue model
The chosen exercise protocol was primarily designed to target the athletes' specific technical prowess, notably their robust endurance capabilities. This approach also aligns with existing literature on the effects of hyperoxia and HBOT during exercise.6,24,36 The regimen predominantly involved prolonged sessions on a cycling ergometer, a method known for its efficacy in endurance training.25,27,28 Moreover, the incremental variation in power during these long-duration cycling sessions predisposes athletes to muscle fatigue, a central focus of this study.
We assessed the impact of this exercise regimen on muscle fatigue through a multidimensional approach, incorporating subjective fatigue assessments, heart rate monitoring, and the analysis of key blood markers.2,37,38 Notably, participants experienced a significant increase in subjective muscle soreness upon completing the exercise protocol, with many unable to sustain the activity despite encouragement. This was evidenced by a marked reduction in movement stability and coordination, alongside RPE scores exceeding 19. Heart rates persistently surpassed 180 beats per minute, accompanied by rapid and irregular breathing patterns. Additionally, participants struggled to maintain the requisite cycling power of 60 rpm for more than 60 s at their initial exercise intensity. An abnormal surge in CK levels post-exercise further indicated muscle damage and fatigue.2,37,38 Nonetheless, the body's inherent adaptive mechanisms became apparent with successive exercise sessions, as observed in the gradual acclimatization to the workout intensity, which was reflected in the progressive decrease in RPE and heart rate measurements.
In conclusion, the exercise protocol effectively induced muscle fatigue in participants, satisfying the study's criteria for modeling exercise-induced muscle fatigue. This protocol's thorough evaluation underscores its potential applicability in examining the nuances of muscle fatigue and recovery dynamics in athletic training scenarios.
4.2. MHOT intervention can alleviate subjective fatigue
High-intensity sports training and competition often expose athletes to stress and potential injuries, leading to physical and psychological strain that can adversely affect their sleep and overall mood.39 This study aimed to explore the effects of singular and repeated MHOT interventions on physiological indicators after exercise-induced fatigue. The goal was to assess MHOT's potential to mitigate fatigue. Our findings indicate that both single and repeated (six times) MHOT sessions can significantly alleviate subjective feelings of fatigue and diminish the intensity of pain and fatigue experienced by athletes. These observations align with existing literature, suggesting a potential effect in MHOT's ability to alleviate subjective symptoms like fatigue, as noted in Kim's study,40 where participants reported a sense of rejuvenation post-MHOT. Similarly, Takemura16 highlighted MHOT's efficacy in reducing post-exercise fatigue-related symptoms, potentially linked to a decrease in heart rate following MHOT. Further research corroborates MHOT's capacity to modulate subjective well-being and emotional states post-exercise.41 Moreover, Izquierdo42 suggested MHOT as a preferable treatment for individuals with fibromyalgia, given its ability to enhance pain thresholds and mitigate fatigue and perceived pain. Additionally, Yagishita's findings43 on Hyperbaric Oxygen Therapy (HBOT) underscore its role in pain reduction and expedited recovery from sports-related injuries. The observed benefits, including enhanced comfort, reduced heart rate, anti-inflammatory responses, and improved cerebral blood flow, may collectively contribute to the observed reduction in subjective fatigue following MHOT.
Some studies challenge the efficacy of HBOT in addressing subjective muscle soreness and its role in the recuperation from exercise-induced muscle injuries and delayed-onset muscle soreness.44 Future research should adopt stricter experimental controls and incorporate specific pain biomarkers, such as neuropeptide Y, for a more comprehensive analysis of MHOT's therapeutic potential in sports recovery.
4.3. MHOT intervention promotes the recovery of heart rate and enhances blood flow after exercise
In this study, both single and repeated MHOT sessions were found to effectively reduce HR. According to Takemura's research,16 MHOT may serve as a viable recovery modality for mood regulation by facilitating HR reduction after high-intensity workouts. Parallel findings indicate superior HR recovery in MHOT-treated individuals, suggesting that MHOT could diminish peripheral fatigue post-maximal exertion.19 Consequently, MHOT appears to upregulate parasympathetic tone, leading to decreased HR and enhancing autonomic nervous system balance.45 SpO2, a crucial respiratory circulation parameter, maintained consistent levels across all study time points, with no notable differences observed between groups. This suggests that hyperoxia supplementation might not significantly influence athletes' recovery quality or blood oxygen saturation, potentially hinting at a psychological placebo effect.46 Previous investigations into single HBOT interventions revealed minimal impact on blood and tissue oxygenation under standard atmospheric conditions.29 However, some reports highlight MHOT's pronounced effect on SpO2 enhancement, attributed to increased dissolved oxygen levels facilitated by the therapy.35,45
MHOT was also shown to significantly elevate the perfusion index and local blood flow, aiding fatigue recovery. This aligns with Ishihara's findings,35 where MHOT notably doubled blood flow relative to baseline measurements, boosting plasma dissolved oxygen and enhancing cellular and tissue metabolism. Hyperoxia during exercise has been noted to improve microvascular oxygenation, maintaining stable oxygen levels and enhancing blood flow.47
Despite these positive outcomes, it is crucial to note that the current investigation focused solely on local tissue perfusion indices, without examining whole-body microcirculation and perfusion.
4.4. Repeated MHOT intervention reduces serum markers of muscle damage and metabolites after exercise-induced muscle fatigue
Exercise-induced muscle damage, resulting from acute and prolonged strain on muscle cells and tissues, can be inferred through the serum levels of enzymes such as CK and LDH. These enzymes are released from muscle tissues into the bloodstream during physical activity, serving as indicators of muscle damage.36 Understanding the influence of both single and repeated MHOT interventions on these biomarkers of post-exercise fatigue can shed light on MHOT's underlying mechanisms for fatigue mitigation. This investigation revealed that multiple MHOT sessions (six times) led to a decrease in serum CK levels, albeit without a significant impact on LDH levels. Conversely, a single MHOT session did not yield noticeable effects. This outcome suggests a dose-dependent, cumulative benefit of MHOT, where the sustained application intensifies the therapeutic impact, unlike the transient effects of a solitary treatment. Similar findings from HBOT research indicate a substantial reduction in CK and LDH levels, highlighting its efficacy in mitigating exercise-induced muscle trauma during the recovery phase.36,39 Such evidence positions MHOT as a potentially analogous intervention, capable of enhancing blood oxygen partial pressure, improving oxygen diffusion from capillaries to adjacent cells, and augmenting tissue and fluid oxygen content, thereby facilitating tissue repair and membrane protection by elevating dissolved oxygen levels.
MHOT's capacity to alleviate tissue hypoxia, expedite metabolite clearance, and bolster cell membrane repair and protection underscores its therapeutic potential. The evaluation of training load and physiological status using blood markers unveiled a noteworthy reduction in BUN levels among MHOT-treated subjects following the final intervention. This suggests MHOT's cumulative effects and its contribution to maintaining internal homeostasis by augmenting dissolved oxygen availability and decreasing blood ammonia production, consequently facilitating BUN clearance. Although some studies report unchanged BUN levels post-HBOT,39,48 the definitive impact of HBOT on serum BUN improvement remains to be further explored. In addition, the present study showed MHOT was associated with reduced LA levels post-intervention, aligning with research indicating MHOT's capacity to alleviate peripheral fatigue post-exhaustive exercise, positioning it as a novel agent for lactate management and fatigue recovery.19 Notably, the lactate clearance rate following intense exercise under MHOT conditions exceeded that observed under normobaric air conditions. This implies MHOT's efficacy at lower atmospheric pressures, which impose less physiological stress compared to higher pressures.7,40 MHOT's advantages extend to enhancing oxygen delivery, mitigating edema, reducing inflammation, alleviating ischemia/reperfusion injury, and promoting collagen synthesis, neovascularization, and angiogenesis.49 While hyperoxia interventions are recognized for their ability to decrease lactate levels post-exercise50 and modulate lactate accumulation,51 the distinct effects of MHOT, particularly in repeated applications, necessitate further investigation. This is crucial given the diverse factors influencing lactate dynamics.29,52 Future research should rigorously control for these variables to elucidate MHOT's precise role in exercise recovery and metabolic regulation.
4.5. Repeated MHOT intervention reduces oxidative stress levels after exercise-induced muscle fatigue
Exercise-induced hypoxia in active muscles may contribute to increased oxidative stress, resulting in a prolonged imbalance between physical training and recovery phases. This disruption can affect the equilibrium within the oxidative and antioxidant systems.53,54 SOD and MDA serve as key biomarkers that reflect shifts in oxidative stress levels. This investigation revealed that multiple MHOT sessions notably diminished serum MDA concentrations while elevating SOD levels, a pattern not distinctly observed following a single MHOT application. Consistent with Ishihara's findings,35 MHOT was shown to augment plasma dissolved oxygen content and bolster cellular and tissue metabolism without exacerbating oxidative stress. Kim's research40 further underscores MHOT's capacity to mitigate oxidative stress and enhance fatigue recovery with minimal risk.
MHOT utilizes moderate oxygen pressures and concentrations to augment systemic oxygen supply, expand the effective oxygen diffusion radius, and broaden the range of oxygen distribution. This leads to an increase in the body's dissolved oxygen content. There were no significant side effects or adverse reactions reported by the participants, suggesting that repeated MHOT sessions could beneficially modulate tissue hypoxia, potentially stimulating increased SOD production and fostering more efficient free radical scavenging. Moreover, repeated exposures to MHOT may modify microcirculation and the intracellular environment following muscle fatigue. This can effectively mitigate free radical-induced tissue damage, reduce lipid peroxidation, and maintain the oxidative-antioxidant balance. MHOT's impact on the body's oxidative-antioxidative dynamics suggests its potential to promote a more stable internal environment, thereby aiding in oxidative stress management. Nuclear factor erythroid 2-related factor 2 (NRF2) is believed to play a crucial role in MHOT's cellular defense mechanism against oxidative stress.55 It is suggested that MHOT interventions may aid in the removal of free radicals, thereby preventing potential apoptotic pathways triggered by oxygen-derived free radicals and therefore, exerting a protective effect. Typically administered daily for 1 to 2 h at pressures ranging from 2.0 to 2.8 ATA, HBOT is commonly utilized over 3 to 10 sessions for sports-related injuries.49 In this context, a single MHOT session only appears to exert a transient effect on oxidative stress markers, whereas multiple sessions enhance antioxidant capacity, indicating a beneficial and cumulative impact of MHOT on oxidative stress mitigation.
4.6. MHOT intervention did not affect jumping performance
SLJ serves as a straightforward, field-based evaluation of lower-body explosive strength, where the jump distance is utilized as an indirect indicator of lower-body muscular power. Within the scope of this investigation, neither single nor repeated sessions of MHOT demonstrated a significant impact on SLJ performance, suggesting a negligible effect on lower-body explosive strength. Contrastingly, literature on hyperoxia and HBOT highlights potential benefits on athletic performance, notably with hyperoxia gas supplementation enhancing average cycling power. Utilization of hyperoxia during training could potentially augment performance in repetitive sprint cycling activities by diminishing muscle fatigue, predominantly through peripheral mechanisms.56 Further research suggests that hyperoxia can restore maximal voluntary isometric contractions in muscles experiencing fatigue from high-intensity, intermittent exercises. Both peripheral and central fatigue demonstrate sensitivity to ambient oxygen concentrations.57 The performance in lower extremity exercises is influenced by a multitude of factors, including the specific exercise protocol, testing methodologies employed, the timing of measurements, and the body's adaptive responses. The precise mechanisms by which MHOT influences athletic performance remain to be elucidated, warranting further research.
4.7. MHOT intervention and the placebo effect
The Society for Interdisciplinary placebo studies defines placebo effects as changes specifically attributable to placebo mechanisms (e.g., neurobiological and psychological mechanisms of expectations).58 In other words, the placebo effect is a psychobiological response after a sham treatment.58 Some studies have pointed out that, the placebo effect can influence sports performance.59 Although the precise mechanism of treatment is not fully understood, the individual's belief in the efficacy of the treatment will most likely elicit positive changes and manifest the desired physiological and/or psychological benefit.59,60 Therefore, the involvement of psychological mechanisms in the effectiveness of MHOT should not be overlooked. Indeed, considering the widespread acceptance of MHOT as an effective recovery strategy, its efficacy may not solely hinge on oxygen pressure and concentration, potentially leaving room for a placebo effect. In this study, the absence of a sham control group precludes a comparison of the potential placebo effect of MHOT. Future studies should include a sham control group to enable a more comprehensive examination of the placebo effect associated with MHOT.
5. Conclusion
This study investigated the effects of both single and multiple sessions of MHOT on muscle fatigue induced by prolonged cycling in athletes, while also assessing the recovery timeline. The findings suggest that MHOT holds promise as a viable approach for alleviating muscle fatigue in athletes. The study demonstrates that both a single MHOT session and repeated applications (six times) effectively mitigate subjective fatigue and enhance recovery in heart rate and blood perfusion post-muscle fatigue. Notably, multiple MHOT sessions are associated with reduced serum markers indicative of muscle damage, metabolic byproducts, and oxidative stress, leading to decreased metabolite accumulation, diminished oxidative stress levels, and expedited muscle fatigue clearance. This cumulative benefit of MHOT appears to be more pronounced with repeated sessions, while the influence of a single MHOT session is comparatively less prominent. However, MHOT's effects on lower limb power did not exhibit significant changes, highlighting the necessity for further investigations into its broader impact on athletic performance metrics. The study suggests MHOT as an effective and practical modality for muscle fatigue recovery following prolonged exercise duration, with repeated sessions showing greater efficacy and potential for implementation in sports training and competitive settings. Additionally, the potential placebo effect associated with MHOT intervention warrants further examination to better understand its role and implications.
It is important to describe the current study's limitations. Without a strictly controlled placebo intervention for comparison, understanding the true effects of MHOT on athletes' muscle recovery after fatigue is challenging. The absence of a sham control group prevents a thorough analysis of MHOT's potential placebo effect. Additionally, the small sample size (n = 12 per group) consisting only of male participants may restrict the generalizability of the findings. Although dietary and training regimens were regulated during the study, daily lifestyle factors could still influence the outcomes related to MHOT's effectiveness. Moreover, the inherent variability in individual responses to the exercise protocol and recovery interventions suggests the need for caution in interpreting the results.
Future research endeavors should aim to address these limitations by incorporating larger and more diverse participant cohorts, including female athletes, to enhance the generalizability of the findings. Moreover, efforts should be made to control extraneous variables more rigorously to validate the preliminary results. Subsequent studies ought to expand beyond lower limb performance assessments to provide a comprehensive evaluation of MHOT's placebo effects and the underlying mechanisms. Employing cellular, omics, and biophysiological evaluations, along with assessments of aerobic and anaerobic capacities, will enrich our understanding and optimization of MHOT protocols. Finally, exploring the dose-response relationship and identifying the optimal method for MHOT administration can enhance muscle recovery and performance.
Author contributions
Conceptualization: CY Qu, JX Zhao. Data curation: MX Xu, ZJ Rao. Formal analysis: CY Qu, P Huang. Funding acquisition: JX Zhao. Investigation: CY Qu, X Geng. Methodology: CY Qu. Writing – original draft: CY Qu, JX Zhao. Writing – review & editing: CY Qu, Santiago Lorenzo, JX Zhao.
Data availability statement
The corresponding author is available to share the primary data with those interested.
Funding
This work was supported by the General Research of Natural Science Foundation of Beijing (5212020) and the Doctoral Research Initiation Fund Project of Hebei Normal University (L2024B35).
Declaration of competing interest
All authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the Laboratory of China Institute of Sport Science and Hebei Normal University Physical Education College. The authors thank all the participants for their efforts and participation in this study. The authors would like to express their appreciation to Professor Craig G. Crandall and Dongzhe Wu for editing and improving the manuscript.
References
- 1.Ament W., Verkerke G.J. Exercise and fatigue. Sports Med. 2009;39(5):389–422. doi: 10.2165/00007256-200939050-00005. [DOI] [PubMed] [Google Scholar]
- 2.Wan J.J., Qin Z., Wang P.Y., Sun Y., Liu X. Muscle fatigue: general understanding and treatment. Exp Mol Med. 2017;49(10) doi: 10.1038/emm.2017.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbiss C.R., Laursen P.B. Models to explain fatigue during prolonged endurance cycling. Sports Med. 2005;35(10):865–898. doi: 10.2165/00007256-200535100-00004. [DOI] [PubMed] [Google Scholar]
- 4.Cullen M.L., Casazza G.A., Davis B.A. Passive recovery strategies after exercise: a narrative literature review of the current evidence. Curr Sports Med Rep. 2021;20(7):351–358. doi: 10.1249/JSR.0000000000000859. [DOI] [PubMed] [Google Scholar]
- 5.Bezuglov E., Lazarev A., Khaitin V., et al. The prevalence of use of various post-exercise recovery methods after training among elite endurance athletes. Int J Environ Res Public Health. 2021;18(21) doi: 10.3390/ijerph182111698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperlich B., Zinner C., Hauser A., Holmberg H.C., Wegrzyk J. The impact of hyperoxia on human performance and recovery. Sports Med. 2017;47(3):429–438. doi: 10.1007/s40279-0160590-1. [DOI] [PubMed] [Google Scholar]
- 7.Ishii Y., Deie M., Adachi N., et al. Hyperbaric oxygen as an adjuvant for athletes. Sports Med. 2005;35(9):739–746. doi: 10.2165/00007256-200535090-00001. [DOI] [PubMed] [Google Scholar]
- 8.Huang X., Wang R., Zhang Z., Wang G., Gao B. Effects of pre-, post- and intra-exercise hyperbaric oxygen therapy on performance and recovery: a systematic review and meta-analysis. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.791872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Shewy K.M., Kunbaz A., Gad M.M., et al. Hyperbaric oxygen and aerobic exercise in the long-term treatment of fibromyalgia: a narrative review. Biomed Pharmacother. 2019;109:629–638. doi: 10.1016/j.biopha.2018.10.157. [DOI] [PubMed] [Google Scholar]
- 10.Heyboer M 3rd, Sharma D., Santiago W., McCulloch N. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care. 2017;6(6):210–224. doi: 10.1089/wound.2016.0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branco B.H., Fukuda D.H., Andreato L.V., Santos J.F., Esteves J.V., Franchini E. The effects of hyperbaric oxygen therapy on post-training recovery in Jiu-Jitsu athletes. PLoS One. 2016;1(3) doi: 10.1371/journal.pone.0150517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishihara A. Mild hyperbaric oxygen: mechanisms and effects. J Physiol Sci. 2019;69(4):573–580. doi: 10.1007/s12576-019-00678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishihara A. Mild hyperbaric oxygen and oxygenation under normobaric conditions: response to Dr. Andel's letter submitted to the editor. J Physiol Sci. 2019;69(6):1105–1106. doi: 10.1007/s12576-019-00700-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takemura A., Roy R.R., Yoshihara I., Ishihara A. Unloading-induced atrophy and decreased oxidative capacity of the soleus muscle in rats are reversed by pre- and postconditioning with mild hyperbaric oxygen. Physiol Rep. 2017;5(14) doi: 10.14814/phy2.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishihara A. Effects of exposure to mild hyperbaric oxygen during unloading on muscle properties in rats. J Muscle Res Cell Motil. 2019;40(3-4):365–372. doi: 10.1007/s10974-019-09530-0. [DOI] [PubMed] [Google Scholar]
- 16.Takemura A., Eda N., Saito T., Shimizu K. Mild hyperbaric oxygen for the early improvement of mood disturbance induced by high-intensity exercise. J Sports Med Phys Fitness. 2022;62(2):250–257. doi: 10.23736/S0022-4707.21.11971-1. [DOI] [PubMed] [Google Scholar]
- 17.Mihailovic T., Bouzigon R., Bouillod A., Grevillot J., Ravier G. Post-exercise hyperbaric oxygenation improves recovery for subsequent performance. Res Q Exerc Sport. 2023;94(2):427–434. doi: 10.1080/02701367.2021.2002797. [DOI] [PubMed] [Google Scholar]
- 18.White J., Dawson B., Landers G., Croft K., Peeling P. Effect of supplemental oxygen on post-exercise inflammatory response and oxidative stress. Eur J Appl Physiol. 2013;113(4):1059–1067. doi: 10.1007/s00421-012-2521-7. [DOI] [PubMed] [Google Scholar]
- 19.Park S.H., Park S.J., Shin M.S., Kim C.K. The effects of low-pressure hyperbaric oxygen treatment before and after maximal exercise on lactate concentration, heart rate recovery, and antioxidant capacity. J Exerc Rehabil. 2018;14(6):980–984. doi: 10.12965/jer.1836468.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tucker R., Kayser B., Rae E., Raunch L., Bosch A., Noakes T. Hyperoxia improves 20 km cycling time trial performance by increasing muscle activation levels while perceived exertion stays the same. Eur J Appl Physiol. 2007;101(6):771–781. doi: 10.1007/s00421-007-0458-z. [DOI] [PubMed] [Google Scholar]
- 21.Prieur F., Benoit H., Busso T., Castells J., Geyssant A., Denis C. Effects of moderate hyperoxia on oxygen consumption during submaximal and maximal exercise. Eur J Appl Physiol. 2002;88(3):235–242. doi: 10.1007/s00421-002-0707-0. [DOI] [PubMed] [Google Scholar]
- 22.Lovering A.T., Stickland M.K., Amann M., et al. Hyperoxia prevents exercise-induced intrapulmonary arteriovenous shunt in healthy humans. J Physiol. 2008;586(18):4559–4565. doi: 10.1113/jphysiol.2008.159350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallette M.M., Stewart D.G., Cheung S.S. The effects of hyperoxia on sea-level exercise performance, training, and recovery: a meta-analysis. Sports Med. 2018;48(1):153–175. doi: 10.1007/s40279-017-0791-2. [DOI] [PubMed] [Google Scholar]
- 24.Burgos C., Henríquez-Olguín C., Andrade D.C., et al. Effects of exercise training under hyperbaric oxygen on oxidative stress markers and endurance performance in young soccer players: a pilot study. J Nutr Metab. 2016;2016 doi: 10.1155/2016/5647407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amann M., Eldridge M.W., Lovering A.T., Stickland M.K., Pegelow D.F., Dempsey J.A. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol. 2006;575(Pt 3):937–952. doi: 10.1113/jphysiol.2006.113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linossier M.T., Dormois D., Arsac L., et al. Effect of hyperoxia on aerobic and anaerobic performances and muscle metabolism during maximal cycling exercise. Acta Physiol Scand. 2000;68(3):403–411. doi: 10.1046/j.1365-201x.2000.00648.x. [DOI] [PubMed] [Google Scholar]
- 27.Choi S.K., Baek S.H., Choi S.W. The effects of endurance training and thiamine supplementation on anti-fatigue during exercise. J Exerc Nutrition Biochem. 2013;17(4):189–198. doi: 10.5717/jenb.2013.17.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulrich S., Hasler E.D., Müller-Mottet S., et al. Mechanisms of improved exercise performance under hyperoxia. Respiration. 2017;93(2):90–98. doi: 10.1159/000453620. [DOI] [PubMed] [Google Scholar]
- 29.Hodges A.N., Delaney S., Lecomte J.M., Lacroix V.J., Montgomery D.L. Effect of hyperbaric oxygen on oxygen uptake and measurements in the blood and tissues in a normobaric environment. Br J Sports Med. 2003;37(6):516–520. doi: 10.1136/bjsm.37.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawada S., Fukaya K., Ohtani M., Kobayashi K., Fukusaki C. Effects of pre-exposure to hyperbaric hyperoxia on high-intensity exercise performance. J Strength Cond Res. 2008;22(1):66–74. doi: 10.1519/JSC.0b013e31815eefa2. [DOI] [PubMed] [Google Scholar]
- 31.Casey D.P., Joyner M.J., Claus P.L., Curry T.B. Hyperbaric hyperoxia reduces exercising forearm blood flow in humans. Am J Physiol Heart Circ Physiol. 2011;300(5):H1892–H1897. doi: 10.1152/ajpheart.00165.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casey D.P., Joyner M.J., Claus P.L., Curry T.B. Vasoconstrictor responsiveness during hyperbaric hyperoxia in contracting human muscle. J Appl Physiol. 2013;114(2):217–224. doi: 10.1152/japplphysiol.01197.2012. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takezawa T. Performance and biological response of middle-power under hyperbaric hyperoxic conditions in judo athletes—pilot studies. Arch Budo. 2021;17:43–50. [Google Scholar]
- 34.Nisa B.U., Hirabayashi T., Maeshige N., Kondo H., Fujino H. Beneficial effects of mild hyperbaric oxygen exposure on microcirculation in peripheral tissues in healthy subjects: a pilot study. J Sports Med Phys Fitness. 2022;62(12):1600–1604. doi: 10.23736/S0022-4707.22.13363-3. [DOI] [PubMed] [Google Scholar]
- 35.Ishihara A., Nagatomo F., Fujino H., Kondo H. Exposure to mild hyperbaric oxygen increases blood flow and resting energy expenditure but not oxidative stress. J Sci Res Rep. 2014;3(14):1886–1896. [Google Scholar]
- 36.Woo J., Min J.H., Lee Y.H., Roh H.T. Effects of hyperbaric oxygen therapy on inflammation, oxidative/antioxidant balance, and muscle damage after acute exercise in normobaric, normoxic and Hypobaric, hypoxic environments: a pilot study. Int J Environ Res Public Health. 2020;17(20):7377. doi: 10.3390/ijerph17207377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nosaka K., Newton M., Sacco P. Muscle damage and soreness after endurance exercise of the elbow flexors. Med Sci Sports Exerc. 2002;34(6):920–927. doi: 10.1097/00005768-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Su Q.S., Tian Y., Zhang J.G., Zhang H. Effects of allicin supplementation on plasma markers of exercise-induced muscle damage, IL-6 and antioxidant capacity. Eur J Appl Physiol. 2008;103(3):275–283. doi: 10.1007/s00421-008-0699-5. [DOI] [PubMed] [Google Scholar]
- 39.Chen C.Y., Chou W.Y., Ko J.Y., Lee M.S., Wu R.W. Early recovery of exercise-related muscular injury by HBOT. BioMed Res Int. 2019;2019 doi: 10.1155/2019/6289380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S., Yukishita T., Lee K. The effect of mild-pressure hyperbaric therapy (oasis O2) on fatigue and oxidative stress. Health. 2011;3(7):432–436. doi: 10.4236/health.2011.37071. [DOI] [Google Scholar]
- 41.Ma J., Hong G., Ha E., et al. Hippocampal cerebral blood flow increased following low-pressure hyperbaric oxygenation in firefighters with mild traumatic brain injury and emotional distress. Neurol Sci. 2021;42(10):4131–4138. doi: 10.1007/s10072-02105094-5. [DOI] [PubMed] [Google Scholar]
- 42.Izquierdo-Alventosa R., Inglés M., Cortés-Amador S., et al. Comparative study of the effectiveness of a low-pressure hyperbaric oxygen treatment and physical exercise in women with fibromyalgia: randomized clinical trial. Ther Adv Musculoskelet Dis. 2020;12 doi: 10.1177/1759720X20930493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yagishita K., Enomoto M., Takazawa Y., Fukuda J., Koga H. Effects of hyperbaric oxygen therapy on recovery acceleration in Japanese professional or semi-professional rugby players with grade 2 medial collateral ligament injury of the knee: a comparative non-randomized study. Undersea Hyperb Med. 2019;46(5):647–654. PMID: 31683363. [PubMed] [Google Scholar]
- 44.Harrison B.C., Robinson D., Davison B.J., Foley B., Seda E., Byrnes W.C. Treatment of exercise-induced muscle injury via hyperbaric oxygen therapy. Med Sci Sports Exerc. 2001;33(1):36–42. doi: 10.1097/00005768-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Takemura A. Exposure to a mild hyperbaric oxygen environment elevates blood pressure. J Phys Ther Sci. 2022;34(5):360–364. doi: 10.1589/jpts.34.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peeling P., Andersson R. Effect of hyperoxia during the rest periods of interval training on perceptual recovery and oxygen re-saturation time. J Sports Sci. 2011;29(2):147–150. doi: 10.1080/02640414.2010.526133. [DOI] [PubMed] [Google Scholar]
- 47.Goulding R.P., Roche D.M., Marwood S. Effect of hyperoxia on critical power and V˙O2 kinetics during upright cycling. Med Sci Sports Exerc. 2020;52(5):1041–1049. doi: 10.1249/MSS.0000000000002234. [DOI] [PubMed] [Google Scholar]
- 48.Chang J.S., Chang E., Lee Y., et al. Hyperbaric oxygen exposure attenuates circulating stress biomarkers: a pilot interventional study. Int J Environ Res Public Health. 2020;17(21):7853. doi: 10.3390/ijerph17217853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moghadam N., Hieda M., Ramey L., Levine B.D., Guilliod R. Hyperbaric oxygen therapy in sports Musculoskeletal injuries. Med Sci Sports Exerc. 2020;52(6):1420–1426. doi: 10.1249/MSS.0000000000002257. [DOI] [PubMed] [Google Scholar]
- 50.Przyklenk A., Gutmann B., Schiffer T., et al. Endurance exercise in hypoxia, hyperoxia and normoxia: mitochondrial and global adaptations. Int J Sports Med. 2017;38(8):588–596. doi: 10.1055/s-0043-106740. [DOI] [PubMed] [Google Scholar]
- 51.Silva T.C., Aidar F.J., Zanona A.F., et al. The acute effect of hyperoxia on onset of blood lactate accumulation (OBLA) and performance in female runners during the maximal treadmill test. Int J Environ Res Public Health. 2021;18(9):4546. doi: 10.3390/ijerph18094546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kon M., Ebi Y., Nakagaki K. Hormonal, metabolic, and angiogenic responses to all-out sprint interval exercise under systemic hyperoxia. Growth Horm IGF Res. 2022;63 doi: 10.1016/j.ghir.2022.101445. [DOI] [PubMed] [Google Scholar]
- 53.Kon M., Taniguchi K., Ebi Y., Nakagaki K. Effects of high-intensity interval exercise under hyperoxia on HSP27 and oxidative stress responses. Respir Physiol Neurobiol. 2021;283 doi: 10.1016/j.resp.2020.103544. [DOI] [PubMed] [Google Scholar]
- 54.Canals-Garzón C., Guisado-Barrilao R., Martínez-García D., Chirosa-Ríos I.J., Jerez-Mayorga D., Guisado-Requena I.M. Effect of antioxidant supplementation on markers of oxidative stress and muscle damage after strength exercise: a systematic review. Int J Environ Res Public Health. 2022;19(3):1803. doi: 10.3390/ijerph19031803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishibashi M., Hayashi A., Akiyoshi H., Ohashi F. The influences of hyperbaric oxygen therapy with a lower pressure and oxygen concentration than previous methods on physiological mechanisms in dogs. J Vet Med Sci. 2015;77(3):297–304. doi: 10.1292/jvms.14-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Porter M.S., Fenton J., Reed K.E. The effects of hyperoxia on repeated sprint cycling performance & muscle fatigue. J Sci Med Sport. 2019;22(12):1344–1348. doi: 10.1016/j.jsams.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Yokoi Y., Yanagihashi R., Morishita K., Goto N., Fujiwara T., Abe K. Recovery effects of repeated exposures to normobaric hyperoxia on local muscle fatigue. J Strength Cond Res. 2014;28(8):2173–2179. doi: 10.1519/JSC.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 58.Hurst P., Foad A., Coleman D., Beedie C. Athletes intending to use sports supplements are more likely to respond to a placebo. Med Sci Sports Exerc. 2017;49(9):1877–1883. doi: 10.1249/MSS.0000000000001297. [DOI] [PubMed] [Google Scholar]
- 59.Beedie C., Benedetti F., Barbiani D., et al. Consensus statement on placebo effects in sports and exercise: the need for conceptual clarity, methodological rigour, and the elucidation of neurobiological mechanisms. Eur J Sport Sci. 2018;18(10):1383–1389. doi: 10.1080/17461391.2018.1496144. [DOI] [PubMed] [Google Scholar]
- 60.Hurst P., Schipof-Godart L., Szabo A., et al. The Placebo and Nocebo effect on sports performance: a systematic review. Eur J Sport Sci. 2020;20(3):279–292. doi: 10.1080/17461391.2019.1655098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author is available to share the primary data with those interested.