Abstract
Parkinson's disease (PD) is a prevalent neurodegenerative disorder with a poorly understood etiology. An accurate diagnosis of idiopathic PD remains challenging as misdiagnosis is common in routine clinical practice. Moreover, current therapeutics focus on symptomatic management rather than curing or slowing down disease progression. Therefore, identification of potential PD biomarkers and providing a better understanding of the underlying disease pathophysiology are urgent. Herein, hydrophilic interaction liquid chromatography–mass spectrometry (LC-MS/MS) and gas chromatography-mass spectrometry (GC-TOF MS) based metabolomics approaches were used to profile the serum metabolome of 50 patients with different stages of idiopathic PD (early, mid and advanced) and 45 age-matched controls. Levels of 57 metabolites including cysteine-S-sulfate and N-acetyl tryptophan were significantly higher in patients with PD compared to controls, with lower amounts of additional 51 metabolites including vanillic acid, and N-acetylaspartic acid. Xanthines, including caffeine and its downstream metabolites, were lowered in patients with PD relative to controls indicating a potential role caffeine and its metabolites against neuronal damage. Seven metabolites, namely cysteine-S-sulfate, 1-methylxanthine, vanillic acid, N-acetylaspartic acid, 3-N-acetyl tryptophan, 5-methoxytryptophol, and 13-HODE yielded a ROC curve with a high classification accuracy (AUC 0.977). Comparison between different PD stages showed that cysteine-S-sulfate levels were significantly increasing with the advancement of PD stages while LPI 20:4 was significantly decreasing with disease progression. Our findings provide new biomarker candidates to assist in the diagnosis of PD and monitor its progression. Unusual metabolites like cysteine-S-sulfate might point to therapeutic targets that could enhance the development of novel PD treatments, such as NMDA antagonists.
Keywords: Parkinson's disease, Cysteine-S-Sulfate, Biomarker, Diagnosis, Metabolomics, Xanthines, Neurodegenerative
1. Introduction
Parkinson's disease (PD) is the second most frequent neurodegenerative condition and the most prevalent age-related movement disorder worldwide [1,2]. The incidence of PD is increasing at a faster rate compared to other neurological conditions, with projections suggesting that its prevalence will be more than double within the next three decades [3]. Additionally, the economic, social, and emotional burden of PD will increase as the population ages.
PD is a chronic progressive disorder. Pathological features include motor (e.g. bradykinesia, muscular rigidity, resting tremor) and nonmotor (e.g. sleep disorders, depression, and cognitive changes) symptoms that substantially impact patients’ mobility, cognition and behavior over a period of 15–20 years [4,5]. The death of dopaminergic neurons located in the substantia nigra, coupled with the accumulation of intracytoplasmic inclusions known as Lewy Bodies, have been pinpointed as the primary causes behind the motor symptoms of PD [6]. Although most cases of PD are idiopathic, genetic and environmental factors have been linked to the disease pathogenesis [7]. Despite decades of research, there are still no definitive tests to confirm the diagnosis of PD. Current diagnosis depends mainly on medical history, physical examination and response to dopaminergic treatment [1,7]. Accurate diagnosis of PD is challenging. Underlying pathophysiological changes will take place before the onset of motor symptoms, whereas the latter may not be clinically evident until approximately 50 %–80 % of dopaminergic neurons are lost [8]. Moreover, early nonmotor presentations are not PD specific which further complicates an early diagnosis [5,9], particularly in idiopathic PD [3].
To date, PD medications are focused on symptomatic management rather than preventing or slowing down disease progression [10]. Given that the pathogenesis of the disease remains enigmatic, together with the lack of definitive diagnostic test and treatment therapeutics, current research has focused on applying new analytical approaches to unravel the molecular mechanisms behind PD, discover potential diagnostic biomarkers and identify new therapeutic targets for drug development.
Metabolomics is a hypothesis generating analytical approach the enables the identification and the measurements of hundreds of metabolites in an untargeted manner. Metabolomics couples state of the art analytical approaches with bioinformatics to identify unique biomarkers, monitor disease progression, and obtain valuable insights into the underlying pathophysiological mechanisms of diseases. In PD research, untargeted metabolomics have been mainly applied to case-control studies using blood sample (plasma or serum) due to its minimal invasive and easily accessible nature compared to other samples such as brain tissue [[11], [12], [13], [14], [15], [16]]. Moreover, metabolites profiling using other biological fluids such as cerebrospinal fluid (CSF) [[17], [18], [19], [20]], or urine samples have also been reported [21,22]. To increase metabolomic coverage, multiple assays should be combined. Consequently, we here present, for the first time, combination of GC-MS and LC-MS data to profile the serum metabolome of patients with idiopathic PD and age-matched controls and between different stages of PD (early, mid and advanced). This will aid in the identification of potential diagnostic biomarkers and providing a better understanding of PD pathophysiology. Additionally, comparing different stages of PD will detect metabolites that can aid in monitoring disease progression and highlighting promising therapeutic targets.
2. Material and methods
2.1. Ethical considerations
Ethical approval for this study was obtained from the institutional review board of Jordan University of Science and Technology, Irbid, Jordan (Ref.: 26/143/2021, date 07.09.2021). All participants (patients and controls) signed an informed written consent before participation.
2.2. Subjects and selection criteria
Fifty Jordanian patients referred to private and public neurology clinics and diagnosed with idiopathic PD by registered neurologists were enrolled in this study. Patients with PD were at the “on” stage of medication, and without a history of deep brain stimulation surgery. Patients underwent classification into three clinical subgroups according to Hoehn Yahr staging criteria post subjective evaluation by the examining physician: the early group (H–Y stages I and II, n = 28), mid group (H–Y stage III, n = 14), and advanced subgroup (H–Y stages IV and V, n = 8) [23]. The exclusion criteria are detailed in Ref. [24]. Additionally, 45 adult volunteers, with no clinical evidence of PD or other movement or neurodegenerative disorders, were recruited as healthy control from the local community. The healthy controls and patients with PD were matched for age, gender, body mass index (BMI) and ethnic origin. The same cohorts were previously used to investigate serum lipidome in LC-MS lipidomic study [24].
Table 1 shows subjects’ demographic data in terms of age, gender, and BMI.
Table 1.
Demographic data of recruited control and patients with PD.
| Demographic and clinical characteristics | PD (n = 50) |
Control (n = 45) |
p-valueb | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 64.2 | 13.3 | 59.4 | 10.4 | 0.06 |
| Gender (F/M)a | 19/31 | NA | 23/22 | NA | 0.20 |
| BMI (kg/m2) | 28.3 | 5.2 | 29.9 | 4.4 | 0.10 |
| Systolic blood pressure (mmHg) | 131.6 | 16.4 | NA | NA | |
| Diastolic blood pressure (mmHg) | 84.2 | 6.8 | NA | NA | |
| Duration of PD (Years) | 9.8 | 7.2 | NA | NA | |
| Total Cholesterol (mmol/L) | 5.18 | 0.53 | NA | NA | |
| HDL-Cholesterol (mmol/L) | 1.28 | 0.25 | NA | NA | |
| LDL-Cholesterol (mmol/L) | 3.45 | 0.90 | NA | NA | |
PD: Parkinson's disease; F/M: Female and Male; BMI: Body mass index.
Presented as the number of subjects in each group. Values are presented as mean ± SD.
p-value using Student's t-test for age and BMI and chi-square test for gender.
2.3. Sample collection, processing, and storage
Blood samples were collected from participants after a 6-h fasting period. A volume of 5 mL of blood was collected in plain tubes and then centrifuged at 1500×g for 10 min (Hermle centrifuge, Germany). The obtained serum was transferred into 1.5 mL microtubes (Eppendorf, Hamburg, Germany) and stored at −80 °C until further analysis.
2.4. Metabolite extraction
Metabolite extraction was performed as previously described [24]. All solvents used were LC-MS grade obtained from Fisher Scientific, USA. Briefly, cold methanol and chloroform were added to 35 μL serum followed by the addition of water and then shaking. Equal volumes of chloroform and water were added before centrifugation at 10,000×g for 5 min. From the upper separated phase (moderate-to-highly polar metabolites), 400 μL was taken and divided into two Eppendorf tubes (200 μL each); one for GC-MS analysis and other one for hydrophilic interaction liquid chromatography-mass spectrometry (HILIC-MS) experiment. All samples were dried using vacuum centrifugal evaporator (Eppendorf, Hamburg, Germany) and stored at −80 °C until further analysis. The lower organic phase (non-polar metabolites and lipids) was used in a separate reversed phase LC-MS lipidomic study as detailed in Ref. [24].
2.5. Metabolite profiling using hydrophilic interaction liquid chromatography (HILIC) coupled with quadrupole-time-of-flight mass spectrometry
Dried samples were resuspended in 100 μL of HILIC resuspension solution (80:20 acetonitrile (ACN):H2O) containing 41 in house prepared internal standards as quality control markers. Samples were vortexed, sonicated for 5 min, centrifuged at 16,100×g for 2 min and finally transferred (the upper 60 μL) to autosampler vials with 200 μL inserts. LC-MS based metabolomics was performed using Agilent 1290 UHPLC connected to a Sciex TripleTOF 6600 mass spectrometer. 5 μL samples were injected into a Waters Acquity UPLC Premier BEH Amide column (1.7 μm, 2.1 × 50 mm). Chromatography was performed using a mobile phase composed of 100 % LC-MS grade H2O with 10 mM ammonium formate and 0.125 % formic acid as solvent A, and 95:5 (v/v) ACN:H2O with 10 mM ammonium formate with 0.125 % formic acid as solvent B with a flow rate of 0.8 mL/min. The LC gradient was set at 100 % B for 0.5 min, 70 % B at 1.95 min, 30 % B at 2.55 min, 100 % B at 3.15 min till 3.8 min. Column temperature was kept at 45 °C. Data was acquired using Sciex TripleTOF 6600 mass spectrometer operating in positive and negative ESI in data-dependent acquisition (DDA) mode (5 DDA scans per cycle) using the following MS parameters: mass range, 50–1500 m/z for MS1 and 40–1000 m/z for MS2; ion source gas 1 and 2, 60; curtain gas, 35; ion source temperature, 600 °C; ion source voltage, 4000 V and collision energy, 40. Analyst 1.8 was used for data acquisition.
To evaluate the LC-MS instrument's performance, a combined quality control (QC) sample was prepared by mixing 30 μL from each resuspended individual sample. QC samples were then injected after every 10 individual samples, and the coefficient of variability (CV) was calculated for all mass ions to ensure the system's suitability and stability.
2.6. Metabolomics using gas chromatography-time of flight mass spectrometry
Before GC-time of flight (GC-TOF) analysis, metabolites were derivatized by adding 10 μL of methoxyamine hydrochloride in pyridine (40 mg/mL) to each dried sample followed by shaking at 30 °C for 90 min. A volume of 90 μL of N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA, Sigma-Aldrich, USA) was added for trimethylsilylation followed by the addition of C8–C30 fatty acid methyl esters (FAMEs, Sigma-Aldrich, USA) for retention time correction. Samples were shaken at 37 °C for 30 min. Derivatized samples were analyzed using Agilent 7890A GC coupled to a Leco Pegasus HT TOF mass spectrometer applying the same chromatographic and MS parameters previously described [25].
2.7. Data processing and metabolites identification
Raw GC-TOF MS data was processed and annotated using metabolomics GC-BinBase Database and then manually curated as detailed previously [25]. For HILIC-TTOF MS data, the freely available software MS-DIAL version 4.92 was used for data processing including peak picking, alignment and metabolite annotation [26]. Metabolites detected via HILIC-TTOF MS were annotated based upon precursor m/z paired with retention time compared to previously analyzed reference standards, or by matching of experimental MSMS spectra with NIST/MoNA library spectra, or by a combination of both approaches. HILIC-TTOF MS metabolite annotation confidence levels were assigned per Metabolomics Standards Initiative (MSI) guidelines as described previously [27]. Final processed GC and HILIC MS data were combined for subsequent statistical analyses.
2.8. Statistical analysis (Multivaritate and univariate analyses)
Univariate and multivariate statistical analyses were performed using MetaboAnalyst version 5.0 (McGill University, Montreal, QC, Canada) [28,29] and Simca P+14 (Sartorius Stedim Data Analytics AB, Umea, Sweden), respectively as detailed previousely [24]. Group separation and clustering were evaluated using partial least square-discriminative analysis (PLS-DA). High model fitness values (R2Y close to one) and predictive ability (Q2 values ≥ 0.5) reflect robust models. PLS-DA model was also validated by performing a permutation test (999 permutations). Variable importance in the projection (VIP) > 1 was used to extract significantly altered metabolites in PLS-DA model.
Heat maps, volcano plots and receiver operating characteristic (ROC) curve were generated using MetaboAnalyst. After normalization and pareto-scaling of the datasets, student's independent t-test (for binary comparison between PD and control cases) and one-way ANOVA (comparison between PD stages) were used to identify significantly altered metabolites among the compared groups. Correction for multiple comparison was peformed using Benjamini–Hochberg false discovery rate (FDR). Only metabolites with VIP score >1 (in multivarite analysis) and FDR <0.05 (in univariate analysis) were considered potential diagnostic biomarker for PD.
Individual metabolite abundance comparisons between control and PD cases and between PD stages were performed using GraphPad Prism 8 (version 8, San Diego, CA, USA). A P-value less than 0.05 is defined significant.
3. Results
3.1. Demographic and clinical data of participants
Table 1, Table 2 show the characteristics of the study population. No significant differences were detected between control and PD cases in terms of age, BMI and gender, Table 1. Similarly, patients in the different PD stages; early, mid and advanced were matched with regards to age, BMI and gender, Table 2.
Table 2.
Demographic data of recruited patients with PD at early, mild and advanced stages.
| Demographic and clinical characteristics | PD-Early (n = 28) | PD-Mid (n = 14) | PD-Advanced (n = 8) | p-valueb |
|---|---|---|---|---|
| Age (years) | 63.9 ± 12.1 | 68.7 ± 13.3 | 57.5 ± 15.8 | 0.07 |
| Gender (F/M)a | 10/18 | 6/8 | 3/5 | 0.12 |
| BMI (kg/m2) | 28.2 ± 6.4 | 28.5 ± 2.8 | 28.3 ± 3.4 | 0.19 |
PD: Parkinson's disease; F/M: Female and Male; BMI: Body mass index.
Presented as the number of subjects in each group. Values are presented as mean ± SD.
p-value using one-way ANOVA test for age and BMI and chi-square test for gender.
3.2. Metabolite profiling in patients with PD compared to healthy controls
In combination, around 600 metabolites were structurally annotated by GC-TOF MS and HILIC-MS/MS assays. Herein, 446 unique metabolites were detected by HILIC-MS/MS with additional 97 unique metabolites were identified by GC-MS while only 55 compounds were detected by both platforms. PLS-DA revealed a complete separation of the two study groups reflecting significant changes in the metabolic profile in the circulation of patients with PD compared to controls, Fig. 1A. The PLS-DA model had satisfactory R2Y (0.95) and Q2 (0.82) values and passed the validity permutation test (Fig. S1 in Supplementary Information). Among the metabolites responsible for the class separation in the PLS-DA model (VIP >1) are dipeptides (e.g Glu-Ala, Pro-Gln, Pro-Ser), the PD drug treatment l-DOPA and its related metabolite 3-methoxytyrosine (also known as 3-O-methyldopa), 5-methoxytryptophol and sulfated metabolites (e.g. cysteine-S-sulfate, homovanillic sulfate), Fig. 1B and Table S1.
Fig. 1.
Multivariate analysis of patients with PD (n = 50) compared to healthy controls (n = 45) (A) PLS-DA score plot (R2Y = 0.954, Q2 = 0.817) (B) Top significant metabolites and compounds with the highest VIP scores in the PLS-DA model.
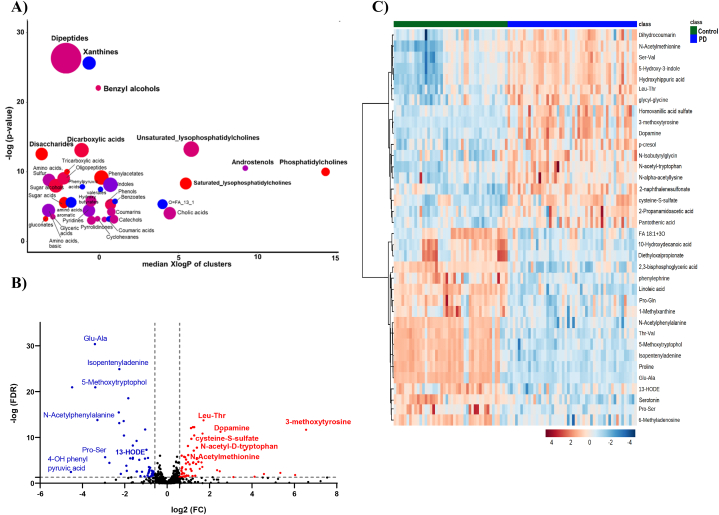
Set enrichment analysis using ChemRICH software [30] showed that several metabolite clusters were impacted in PD cases including dipeptides, xanthines, amino acids (sulfur and aromatic), benzyl alcohol, dicarboxylic acids and disaccharides (Fig. 2A). Additionally, perturbations in lipid clusters in PD were observed in saturated and unsaturated lysophosphatidylcholines (LPC) and phosphatidylcholines. Of note, all metabolites identified in xanthines cluster were down regulated in PD compared to controls. Caffeine and the caffeine metabolites 5-acetylamino-6-formylamino-3-methyluracil, 1,3,7-trimethyluric acid, 1-methylxanthine, and 5-acetylamino-6-amino-3-methyluracil along with tea metabolites theophylline and vanillic acid were observed significantly lower in PD patients relative to healthy controls.
Fig. 2.
Significantly altered metabolites clusters and metabolites in patients with PD compared to healthy controls (A) ChemRICH set enrichment result for significantly impacted metabolite clusters. The plot y-axis shows the most significantly altered clusters on the top. Cluster colors give the proportion of increased or decreased compounds (red = increased, blue = decreased). (B) Volcano plots of up (red) and down (blue) regulated metabolites using false discovery rate (FDR) and fold change (FC) cutoffs of <0.05 and 1.5, respectively. (C) Heatmap of the top altered metabolites. The higher values (red) reflect higher metabolite abundance, and lower values (blue) reflect lower abundance. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Volcano plot revealed that the levels of 57 and 51 metabolites were significantly (FDR<0.05) higher and lower, respectively, in PD patients compared to controls, Fig. 2B. The level of cysteine-S-sulfate, 3-methoxytyrosin, and N-acetyl tryptophan was significantly increased, while the level of 5-methoxytryptophol, N-acetylphenyl alanine, vanillic acid, N-acetylaspartic acid and 13-Hydroxyoctadecadienoic acid (13-HODE) was significantly decreased in PD. Visualization of the top perturbed metabolites between the two groups is presented in the heatmap in Fig. 2C.
Out of the univariate and multivariate analyses, 187 metabolites and chemicals were found to be significant in both (using VIP>1 and FDR<0.05) (Table S1 in Supplementary). These included mainly amino acids, dipeptides, hippuric acids, xanthines and fatty acids (Fig. S2 in Supplementary). Seven of the significantly altered and clinically relevant metabolites namely: cysteine-S-sulfate, 3-N-acetyl tryptophan, 5-methoxytryptophol, 1-methylxanthine, vanillic acid, N-acetylaspartic acid and 13-HODE were further evaluated as potential biomarkers to discriminate between controls and PD cases. Fig. 3A shows the levels of the seven selected metabolites in PD patients and age-matched controls. These metabolites yielded a satisfactory ROC curve with Area Under the Curve (AUC) value of 0.977 (Fig. 3B).
Fig. 3.
Receiver operating characteristics (ROC) curve (A) generated from seven significantly altered metabolites between control and PD (B). Significance between the two groups is expressed as ***P-value ≤0.001, ****P-value ≤0.0001 (Student's independent t-test).
3.3. Metabolite profiling of the three different stages of PD; early, mid and advanced
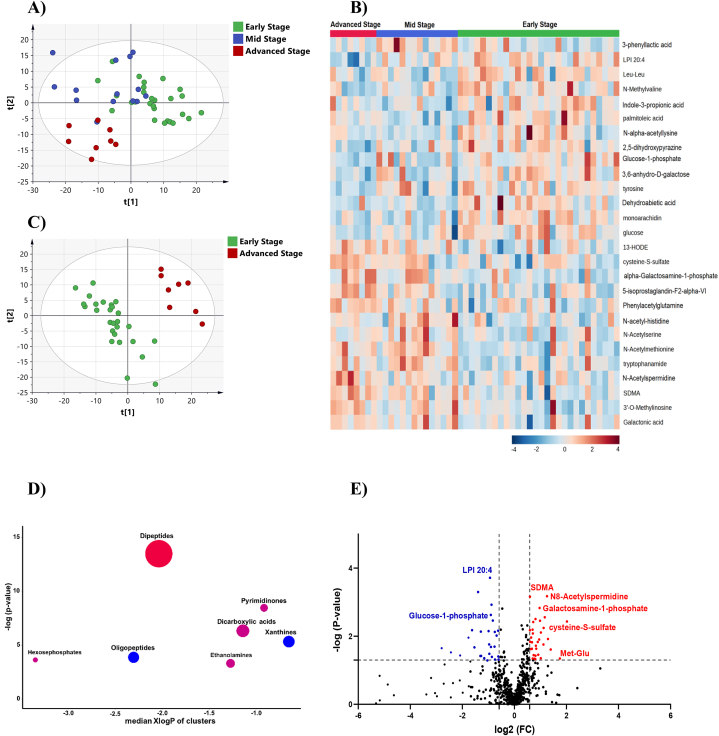
Complete separation between the three PD stages could not be acheived in the PLS-DA score plot (Fig. 4A). The early and mid stage cases partially overlaped but were separated from the advanced stage. Univariate analysis revealed that 70 metabolites were disturbed between PD stages (Table S2). The differential metabolites can be mainly classified as amino acids, dipeptides, saturated fatty acids, TCA acids, dicarboxylic acids, and xanthines (Fig. S3 in supplemental information). Heatmap of the top altered metabolites between the three groups is shown in Fig. 4B.
Fig. 4.
Metabolite profiling of different stages of PD; early, mid and advanced (A) PLS-DA score plot (R2Y = 0.742, Q2 = 0.34) of different PD stages; early (n = 28), mid (n = 14) and advanced (n = 8) (B) Heatmap of the top altered metabolites. The higher values (red) reflect higher metabolite abundance, and lower values (blue) reflect lower abundance. (C) PLS-DA score plot (R2Y = 0.94, Q2 = 0.42) for class separation between early and advanced stages of PD (D) ChemRICH set enrichment result for significantly impacted metabolite clusters in advanced stage PD compared with early stage. The plot y-axis shows the most significantly altered clusters on the top. Cluster colors give the proportion of increased or decreased compounds (red = increased, blue = decreased). (E) Volcano plot of up (red) and down (blue) regulated metabolites in advanced PD compared with early stage using p-value and fold change (FC) cutoffs of <0.05 and 1.5, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Expectedly, most significant metabolic changes were detected between early and advanced stages of PD. PLS-DA score plot revealed complate separation between the two groups (Fig. 4C), while enrichemnt analysis indicated increaed level of dipeptides and decreased level of xanthines and oligopeptides in advanced stage PD (Fig. 4D). Compared to early stage, the level of cysteine-S-sulfate, N8-acetylspermidine and galactosamine-1-phosphate was significantly increased in adavnced stage while the level of lysophosphatidylinositol 20:4 (LPI 20:4) and glucose-1-phosphate was significantly decreased (Fig. 4E).
Six metabolites demonstrated a pattern in the change of their level with the progression of PD from early to advanced stage, Fig. 5A–F. The level of cysteine-S-Sulfate, N8-acetyl spermidine, 13-HODE, galactosamine-1-phosphate was significantly increasing with the progression of PD while the level of LPI 20:4 was significantly decreasing.
Fig. 5.
Metabolites significantly altered during the progression of PD. One-way ANOVA analysis using Turkey's post hoc test was used to indicate significance. * P-value ≤0.05, **P-value ≤0.01, ***P-value ≤0.001, ****P-value ≤0.0001.
4. Discussion
Parkinson's disease is a neurodegenerative disorder with recently increasing incidence due to aging population. Loss of dopaminergic-neurons in the substantia nigra, misfolding and accumulation of proteins, oxidative stress, and neuroinflammation are the hallmarks of the disease. Although age, nutrition, environmental and genetic factors are described as risk factors for PD, the etiology of the disease remains poorly understood, particularly for idiopathic PD [31]. In the current work, serum samples from patients with different stages of PD (early, mid and advanced) and age-matched controls were analyzed for the first time using two metabolomics assays; HILIC-MS/MS and GC-TOF MS to identify metabolites significantly altered between PD and control cases and between the different stages of the disease. These metabolites might act as potential biomarkers to aid in disease diagnosis and monitoring, provide new insights into the underlying biochemical mechanisms associated with the pathophysiology of PD and identify new therapeutic targets that could enhance the development of novel treatment strategies.
Lower level of xanthines, including caffeine and its downstream metabolites, was observed herein in PD patients relative to healthy controls (Fig. 6). Several studies investigated the potential beneficial role of xanthines (i.e caffeine and theobromine) in improving cognitive decline in neurodegenerative diseases. Due to their antioxidant properties and their ability to act as histone deacetylase activators and adenosine receptor (AR) antagonists, xanthines are believed to modulate mechanisms related to PD pathophysiology such as accumulation of misfolded proteins, oxidative stress and neuroinflammation [32,33]. Multiple epidemiological studies could already demonstrate an inverse association between coffee drinking and PD [33,34]. Moreover, lower circulating levels of caffeine and its metabolites have been reported in PD patients compared to control groups [[35], [36], [37], [38]]. Our finding is in line with previous results which further supports the protective effect of caffeine and its metabolites against neuronal damage. Additionally, we observed lower xanthines in advanced stage PD compared to early stage indicating that caffeine and its related metabolites might play a role in decelerating PD progression among early-stage PD individuals.
Fig. 6.
Significantly altered metabolites in PD compared to control and how they might be related to the pathogenesis of PD. Red and blue arrows refer to metabolites increased and decreased in PD compared to control, respectively. N8-acetylspermidine was increased in advanced stage PD compared to early stage PD. ODC refers to ornithine decarboxylase. Figure was created with BioRender.com. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Evidence showed that N-acetylaspartic acid (NAA), an abundant amino acid in the brain localized primarily to neurons, Fig. 6 [39], has important roles in the osmoregulation of neuronal cells as well as maintaining nitrogen balance in the brain and energy metabolism in neuronal mitochondria [40,41]. Recently, NAA has also been described as a potent protein aggregation inhibitor [42]. Lower brain level of NAA has been reported with various neurodegenerative conditions associated with neuronal loss and damage such as PD and Alzheimer's disease using magnetic resonance spectroscopy [43,44]. NAA released by neurons to the extracellular space is taken up by astrocytes and then excreted to the circulation [45]. Herein, a significant decrease in the level of circulating NAA was observed for the first time in PD patients compared to controls which might be a consequence of its lower level in the brain. Our finding suggests that circulating level of NAA might be an indicator of the functional and structural integrity of neurons in the brain.
Among the metabolites that were significantly increased between PD stages is the polyamine N8-acetylspermidine. Polyamines, including spermine and spermidine, are polycations with important roles in modulating cell growth and proliferation and interacting with DNA and RNA [46]. Acetylation is a crucial mechanism to modulate polyamines level and functions. Acetylation of the intracellular spermidine at the N8 position by spermidine N8‐acetyltransferase will lead to the formation of N8‐acetylspermidine which is then excreted from the cell to the circulation [47], Fig. 6. Since N8‐acetylspermidine is an excretion product, its plasma level has been suggested as an indicator of intracellular polyamine activity where an increase in its excretion is linked to brain injury, neuroinflammation and neuronal cell damage [48,49]. Elevated N8-acetylspermidine levels were detected in the plasma and the CSF of patients with PD compared to control [50,51]. Additionally, N8-acetylspermidine was increased in fast progressing PD patients compared to slow progressing ones [52]. Herein, the circulating level of N8-acetylspermidine was significantly increased between early and advanced stage PD cases which is plausibly a cellular response to neuroinflammation exacerbation as a consequence of the progression of the disease. Our finding reinforces previous work that measuring circulating level of N8-acetylspermidine is a promising approach to aid in monitoring motor symptom progression in PD [52].
Interestingly, to the best of our knowledge this is the first study to report the significant change in the levels of the two metabolites; cysteine-S-sulfate and LPI 20:4, between patients with PD and controls and between the different stages of PD. The level of LPI 20:4, also known as 1-arachidonoyl-phosphatidylinositol, was significantly lower in PD compared to controls and its level continued to decrease as the disease progress (Fig. 5). LPI is a subspecies of lysophospholipid with inositol in its head group generated by the action of phospholipase A1. Although LPIs are much less explored compared to lysophosphatidic acid (LPA), over the years they have come to be considered as bioactive lipids, and their role as endogenous agonists for GPR55 is now widely accepted [53,54]. GPR55 is implicated in many physiological processes and its activation has been found beneficial in combating PD [55]. Moreover, a neuroprotective effect of LPI against glutamate-induced neuronal cell death and altered neurotransmission in PD has been reported [56]. Lower levels of LPI 20:4 seems to boost PD progression, nevertheless, further research is warranted to investigate the role of LPI in the pathogenesis of PD. On the other hand, cysteine-S-sulfate was found to be increased in PD cases compared to controls and also upon the progression of the disease. Cysteine-S-sulfate is an abnormal sulfate derivative of cysteine produced by the reaction of inorganic sulfite and cystine, Fig. 6 [57]. This reaction is described as the first scavenging mechanism for sulfite in the plasma [58]. Accumulation of cysteine-S-sulfate might lead to low cystine reservoir, and subsequently glutathione (GSH), resulting in disturbances in the redox homeostasis. High level of cysteine-S-sulfate was associated with brain damage and mental retardation in sulfite oxidase deficiency specifying that neurons are sensitive towards these metabolic changes [59]. Therefore, high level of cysteine-S-sulfate in PD, particularly advanced stage, might underline the severe homeostatic dysregulation caused by cysteine-S-sulfate accumulation. Moreover, cysteine-S-sulfate is structurally related to glutamate and is considered a potent N-methyl-d-aspartate (NMDA) glutamatergic receptors agonist [60]. NMDA receptors are widely expressed in the basal ganglia and have a critical role in the regulation of excitatory synaptic transmission. Excessive stimulation of NMDA receptors by agonists will activate a cascade of signaling pathway that eventually will result in neuronal damage and death [61]. Therefore, high level of cysteine-S-sulfate might be a major contributor to neurodegeneration progression seen herein in PD by triggering excitotoxicity and exacerbating motor functions decline. Antagonists of NMDA receptors have displayed promising effects on slowing PD progression and reversing motor symptoms. Our result supports previous studies that highlighted the role of NMDA receptors as new therapeutic targets for treating PD and slowing down its progression.
PD is a multifactorial disorder that involves several pathophysiological mechanisms and pathways. Hence, it is of high importance to have a diverse set of biomarkers that possesses sufficient specificity and sensitivity to accurately diagnose PD. Therefore, seven significantly altered metabolites linked to different mechanisms underlying PD were evaluated. Cysteine-S-sulfate, 1-methylxanthine, vanillic acid, N-acetylaspartic acid, 3-N-acetyl tryptophan, 5-methoxytryptophol, and 13-HODE yielded a ROC curve with a high classification accuracy. This novel panel of potential biomarkers could serve as a diagnostic biomarker set for idiopathic PD. Nevertheless, validation in a larger cohort remains urgent.
5. Conclusion
The current study used for the first time HILIC-MS/MS and GC-TOF MS based metabolomics to identify biomarkers that can assist in the diagnosis of PD and monitor its progression. Altered metabolites shed light on perturbed processes in PD including neuroinflammation, neuronal loss and damage, polyamine activity, lipid metabolism, and redox homeostasis. Several metabolite clusters were impacted in PD, including xanthines which highlights the protective role of caffeine and its metabolites against neuronal damage and in slowing down disease development. Additionally, a novel panel of seven significantly altered metabolites linked to different mechanisms underlying PD yielded a ROC curve with a high classification accuracy suggesting that they could serve as potential diagnostic biomarkers for idiopathic PD. Due to the small sample size in this study, validation for the identified potential biomarkers in a larger cohort of patients with PD is necessary. Future validation can be employed using more sensitive targeted methods for absolute quantification (using LC-triple quadrupole MS) to assess the applicability of the discovered markers and their potential to be used in clinical settings for the diagnosis of PD and monitoring its progression.
Our findings indicate that circulating cysteine-S-sulfate not only could serve as a potential diagnostic biomarker for PD, together with other metabolites, but also has a promising role to monitor the stages of PD advancement. Lower levels of LPI 20:4 seems to boost PD progression However, additional research remains urgent to investigate the role of bioactive lipids particularly LPI in the pathogenesis of PD.
Current treatment of PD is limited to symptomatic relief. The significantly altered metabolites pointed to therapeutic targets that could enhance the development of novel treatment strategies including GPR55 agonists and NMDA antagonists. Notably, NMDA antagonism is increasingly viewed as an important target for the development of new drugs to prevent or treat PD. Further studies exploring the function of these targets in neurodegenerative disorders, particularly PD, are essential. Despite standardizing samples through fasting, the potential variability in diet and medication history among participants remains a limitation of our study. It is possible that some of the variations in metabolites levels identified in this study could be attributed to the medication for PD. This cannot be determined as the control group in our study did not take any PD medication. Despite the difficulty, future research should incorporate prospective trials with subjects who were not on PD medication but later diagnosed with PD, compared to corresponding control subjects.
Funding statement
This work was supported by the Deanship of Scientific Research at The University of Jordan (Grant number 2451), Jordan.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Lina A. Dahabiyeh: Writing – review & editing, Writing – original draft, Supervision, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Refat M. Nimer: Writing – review & editing, Resources, Methodology, Investigation, Conceptualization. Jeremiah D. Wells: Writing – review & editing, Methodology, Investigation, Formal analysis. Eman Y. Abu-rish: Writing – review & editing, Methodology. Oliver Fiehn: Writing – review & editing, Resources, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e30452.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Armstrong M.J., Okun M.S. Diagnosis and treatment of Parkinson disease A review. JAMA, J. Am. Med. Assoc. 2020;323(6):548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 2.Dorsey E.R., et al. Global, regional, and national burden of Parkinson's disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tolosa E., et al. Challenges in the diagnosis of Parkinson's disease. Lancet Neurol. 2021;20(5):385–397. doi: 10.1016/S1474-4422(21)00030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varadi C. Clinical features of Parkinson's disease: the Evolution of critical symptoms. Biology-Basel. 2020;9(5) doi: 10.3390/biology9050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrag A., et al. Prediagnostic presentations of Parkinson's disease in primary care: a case-control study. Lancet Neurol. 2015;14(1):57–64. doi: 10.1016/S1474-4422(14)70287-X. [DOI] [PubMed] [Google Scholar]
- 6.Braak H., et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 7.DeMaagd G., Philip A. Parkinson's disease and its management: Part 1: disease Entity, risk factors, pathophysiology, clinical presentation, and diagnosis. Pharmacy and therapeutics. 2015;40(8):504–532. [PMC free article] [PubMed] [Google Scholar]
- 8.Meissner W.G. When does Parkinson's disease begin? From prodromal disease to motor signs. Rev. Neurol. 2012;168(11):809–814. doi: 10.1016/j.neurol.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Berg D., et al. MDS research criteria for prodromal Parkinson's disease. Movement Disorders. 2015;30(12):1600–1609. doi: 10.1002/mds.26431. [DOI] [PubMed] [Google Scholar]
- 10.Lee T.K., Yankee E.L. A review on Parkinson's disease treatment. Neuroimmunol. Neuroinflammation. 2021;8:222–244. [Google Scholar]
- 11.Yakhine-Diop S.M.S., et al. Metabolic alterations in plasma from patients with familial and idiopathic Parkinson's disease. Aging-Us. 2020;12(17):16690–16708. doi: 10.18632/aging.103992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han W., et al. Profiling novel metabolic biomarkers for Parkinson's disease using in-depth metabolomic analysis. Movement Disorders. 2017;32(12):1720–1728. doi: 10.1002/mds.27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saiki S., et al. Decreased long-chain acylcarnitines from insufficient beta-oxidation as potential early diagnostic markers for Parkinson's disease. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-06767-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatano T., et al. Identification of novel biomarkers for Parkinson's disease by metabolomic technologies. J. Neurol. Neurosurg. Psychiatry. 2016;87(3):295–301. doi: 10.1136/jnnp-2014-309676. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed S., et al. Metabolic profiling of Parkinson's disease: evidence of biomarker from gene expression analysis and rapid neural network detection. J. Biomed. Sci. 2009;16 doi: 10.1186/1423-0127-16-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao Y.P., et al. Comprehensive metabolic profiling of Parkinson's disease by liquid chromatography-mass spectrometry. Mol. Neurodegener. 2021;16(1) doi: 10.1186/s13024-021-00425-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trupp M., et al. Metabolite and Peptide levels in plasma and CSF Differentiating healthy controls from patients with newly diagnosed Parkinson's disease. J. Parkinsons Dis. 2014;4(3):549–560. doi: 10.3233/JPD-140389. [DOI] [PubMed] [Google Scholar]
- 18.Ohman A., Forsgren L. NMR metabonomics of cerebrospinal fluid distinguishes between Parkinson's disease and controls. Neurosci. Lett. 2015;594:36–39. doi: 10.1016/j.neulet.2015.03.051. [DOI] [PubMed] [Google Scholar]
- 19.Stoessel D., et al. Promising metabolite profiles in the plasma and CSF of early clinical Parkinson's disease. Front. Aging Neurosci. 2018;10 doi: 10.3389/fnagi.2018.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon D., et al. Cerebrospinal fluid metabolome in Parkinson's disease and multiple system atrophy. Int. J. Mol. Sci. 2022;23(3) doi: 10.3390/ijms23031879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luan H.M., et al. LC MS-based urinary metabolite Signatures in idiopathic Parkinson's disease. J. Proteome Res. 2015;14(1):467–478. doi: 10.1021/pr500807t. [DOI] [PubMed] [Google Scholar]
- 22.Luan H., et al. Elevated excretion of biopyrrin as a new marker for idiopathic Parkinson's disease. Park. Relat. Disord. 2015;21(11):1371–1372. doi: 10.1016/j.parkreldis.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression, and mortality (Reprinted from Neurology. Neurology. 2001;57(10):S11–S26. vol 17, 1967. [PubMed] [Google Scholar]
- 24.Dahabiyeh L.A., et al. Serum-based lipid panels for diagnosis of IdiopathicParkinson's disease. Metabolites. 2023;13(9):1–14. doi: 10.3390/metabo13090990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barupal D.K., et al. A comprehensive plasma metabolomics dataset for a cohort of Mouse knockouts within the international mouse phenotyping consortium. Metabolites. 2019;9(5) doi: 10.3390/metabo9050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsugawa H., et al. MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods. 2015;12(6) doi: 10.1038/nmeth.3393. 523-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blazenovic I., et al. Structure annotation of all mass spectra in untargeted metabolomics. Anal. Chem. 2019;91(3):2155–2162. doi: 10.1021/acs.analchem.8b04698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pang Z., et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021;49(W1):W388–W396. doi: 10.1093/nar/gkab382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia J., Wishart D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011;6(6):743–760. doi: 10.1038/nprot.2011.319. [DOI] [PubMed] [Google Scholar]
- 30.Barupal D.K., Fiehn O. Chemical Similarity Enrichment Analysis (ChemRICH) as alternative to biochemical pathway mapping for metabolomic datasets. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-15231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balestrino R., Schapira A.H.V. Parkinson disease. Eur. J. Neurol. 2020;27(1):27–42. doi: 10.1111/ene.14108. [DOI] [PubMed] [Google Scholar]
- 32.Ikram M., et al. Antioxidant and neuroprotective effects of caffeine against alzheimer's and Parkinson's disease: insight into the role of nrf-2 and A2AR signaling. Antioxidants. 2020;9(9) doi: 10.3390/antiox9090902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ascherio A., et al. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann. Neurol. 2001;50(1):56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka K., et al. Intake of Japanese and Chinese teas reduces risk of Parkinson's disease. Park. Relat. Disord. 2011;17(6):446–450. doi: 10.1016/j.parkreldis.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Bakshi R., et al. Associations of lower caffeine intake and plasma urate levels with idiopathic Parkinson?s disease in the harvard biomarkers study. J. Parkinsons Dis. 2020;10(2):505–510. doi: 10.3233/JPD-191882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujimaki M., et al. Serum caffeine and metabolites are reliable biomarkers of early Parkinson disease. Neurology. 2018;90(5) doi: 10.1212/WNL.0000000000004888. E404-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohmichi T., et al. Biomarker repurposing: therapeutic drug monitoring of serum theophylline offers a potential diagnostic biomarker of Parkinson's disease. PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0201260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crotty G.F., et al. Association of caffeine and related analytes with resistance to Parkinson disease among LRRK2 mutation carriers A metabolomic study. Neurology. 2020;95(24):E3428–E3437. doi: 10.1212/WNL.0000000000010863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai G.C., Coyle J.T. N-ACETYLASPARTATE in neuropsychiatric disorders. Prog. Neurobiol. 1995;46(5):531–540. doi: 10.1016/0301-0082(95)00014-m. [DOI] [PubMed] [Google Scholar]
- 40.Baslow M.H., et al. Brain damage results in down-regulation of N-acetylaspartate as a neuronal osmolyte. NeuroMolecular Med. 2003;3(2):95–103. doi: 10.1385/NMM:3:2:95. [DOI] [PubMed] [Google Scholar]
- 41.Madhavarao C.N., et al. Proceedings of the National Academy of Sciences of the United States of America. 2005. Defective N-acetylaspartate catabolism reduces brain acetate levels and myelin lipid synthesis in Canavan's disease; pp. 5221–5226. 102(14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warepam M., et al. Brain metabolite, N-acetylaspartate is a potent protein aggregation inhibitor. Front. Cell. Neurosci. 2021;15 doi: 10.3389/fncel.2021.617308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moffett J.R., et al. N-acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog. Neurobiol. 2007;81(2):89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nie K., et al. Marked N-acetylaspartate and choline metabolite changes in Parkinson's disease patients with mild cognitive impairment. Park. Relat. Disord. 2013;19(3):329–334. doi: 10.1016/j.parkreldis.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Ruggieri M., et al. Age-related changes of serum N-acetyl-aspartate in healthy controls. Age Ageing. 2011;40(3):391–395. doi: 10.1093/ageing/afr021. [DOI] [PubMed] [Google Scholar]
- 46.Moinard C., Cynober L., de Bandt J.P. Polyamines: metabolism and implications in human diseases. Clin. Nutr. 2005;24(2):184–197. doi: 10.1016/j.clnu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Seiler N. Catabolism of polyamines. Amino Acids. 2004;26(3):217–233. doi: 10.1007/s00726-004-0070-z. [DOI] [PubMed] [Google Scholar]
- 48.Nayak A., et al. N8-Acetylspermidine: a polyamine biomarker in ischemic cardiomyopathy with reduced ejection fraction. J. Am. Heart Assoc. 2020;9(11) doi: 10.1161/JAHA.120.016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zahedi K., et al. Polyamine catabolism is enhanced after traumatic brain injury. J. Neurotrauma. 2010;27(3):515–525. doi: 10.1089/neu.2009.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saiki S., et al. A metabolic profile of polyamines in Parkinson disease: a promising biomarker. Ann. Neurol. 2019;86(2):251–263. doi: 10.1002/ana.25516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paik M.J., et al. Polyamine patterns in the cerebrospinal fluid of patients with Parkinson's disease and multiple system atrophy. Clin. Chim. Acta. 2010;411(19–20):1532–1535. doi: 10.1016/j.cca.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 52.Roede J.R., et al. Serum metabolomics of slow vs. Rapid motor progression Parkinson's disease: a pilot study. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0077629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alhouayek M., Masquelier J., Muccioli G.G. Lysophosphatidylinositols, from cell membrane constituents to GPR55 ligands. Trends Pharmacol. Sci. 2018;39(6):586–604. doi: 10.1016/j.tips.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Birgbauer E., Chun J. New developments in the biological functions of lysophospholipids. Cell. Mol. Life Sci. 2006;63(23):2695–2701. doi: 10.1007/s00018-006-6155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Celorrio M., et al. GPR55: a therapeutic target for Parkinson's disease? Neuropharmacology. 2017;125:319–332. doi: 10.1016/j.neuropharm.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 56.Blondeau N., et al. A potent protective role of lysophospholipids against global cerebral ischemia and glutamate excitotoxicity in neuronal cultures. J. Cerebr. Blood Flow Metabol. 2002;22(7):821–834. doi: 10.1097/00004647-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Zecchini M., Lucas R., Le Gresley A. New insights into the cystine-sulfite reaction. Molecules. 2019;24(13) doi: 10.3390/molecules24132377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kohl J.B., Mellis A.T., Schwarz G. Homeostatic impact of sulfite and hydrogen sulfide on cysteine catabolism. Br. J. Pharmacol. 2019;176(4):554–570. doi: 10.1111/bph.14464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olney J.W., Misra C.H., Degubareff T. CYSTEINE-S-SULFATE - brain damaging metabolite in sulfite oxidase deficiency. JNEN (J. Neuropathol. Exp. Neurol.) 1975;34(2):167–177. doi: 10.1097/00005072-197503000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Kumar A., et al. S-sulfocysteine/NMDA receptor-dependent signaling underlies neurodegeneration in molybdenum cofactor deficiency. J. Clin. Invest. 2017;127(12):4365–4378. doi: 10.1172/JCI89885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Z., et al. Roles of glutamate receptors in Parkinson's disease. Int. J. Mol. Sci. 2019;20(18) doi: 10.3390/ijms20184391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.