ABSTRACT
Influenza viruses (IVs) threaten global human health due to the high morbidity, infection, and mortality rates. Currently, the influenza drugs recommended by the Food and Drug Administration are oseltamivir, zanamivir, peramivir, and baloxavir marboxil. These recommended antivirals are currently effective for major subtypes of IVs as the compounds target conserved domains in neuraminidase or polymerase acidic (PA) protein. However, this trend may gradually change due to the selection of antiviral drugs and the natural evolution of IVs. Therefore, there is an urgent need to develop drugs related to the treatment of influenza to deal with the next pandemic. Here, we summarized the cutting-edge research in mechanism of action, inhibitory activity, and clinical efficacy of drugs that have been approved and drugs that are still in clinical trials for influenza treatment. We hope this review will provide up-to-date and comprehensive information on influenza antivirals and generate hypotheses for screens and development of new broad-spectrum influenza drugs in the near future.
KEYWORDS: influenza virus, antiviral drugs, mechanism of action, clinical drugs, adverse event
INTRODUCTION
Influenza is an acute, contagious respiratory illness, caused by influenza viruses (IVs), that presents an increasing public health burden (1, 2). IV is a negative-sense, single-stranded RNA (ssRNA) virus of the Orthomyxovirus genus, characterized with high genetic variability, and has circulated in the human population at least since 1580 (3). IV is classified as A, B, C, and D according to the antigenicity of nucleoproteins (NPs) and matrix (M) proteins in vivo (4). The genome of IV consists of eight ssRNA segments for influenza A and B viruses (IAV and IBV) and seven ssRNA segments for influenza C virus (ICV). These ssRNA segments are each complexed with the viral RNA-dependent RNA polymerase (RdRp) and NP, forming the viral ribonucleoprotein (vRNP) (1), which is packaged into viral particles. IAV and IBV infect humans with high morbidity and mortality, causing severe economic losses (4). Children are the main infection population of ICV, which can cause mild respiratory diseases. There are no reports of influenza D virus (IDV) infection in humans. Seasonal influenza is the main cause of worldwide pandemic flu.
According to the World Health Organization data, about 3–5 million cases of serious illness and 290,000–650,000 deaths from respiratory diseases are related to seasonal influenza in the world every year (4). In addition, highly pathogenic avian influenza, once they acquire the ability to be transmitted from human to human, can also pose a serious threat to human health. It is mainly spread between poultry and wild birds. People could become infected avian IVs when they contact with carrying or diseased poultry (5). Most recently, the main avian IVs that can infect humans include the H5, H7, and H9 subtypes (5). The U.S. Center for Disease Control and Prevention (CDC) recommends oseltamivir, a neuraminidase inhibitor, as the primary treatment drug for avian influenza (6).
CURRENT STATUS OF IV CONTROL
Influenza vaccines have dramatically decreased the number of influenza cases each year (4). However, due to the weak subjective prevention and control awareness, particularly in high-risk populations, and potential inaccurate prediction of the circulating strain, the influenza vaccine alone is not sufficient (7). Influenza infections are further complicated by joint bacterial pneumonia and/or cytokine storm which may further complicate drug treatment. In addition, IV may be overlooked if it occurs simultaneously with other respiratory viruses. Therefore, in the absence of timely prevention and lack of immunity, especially when influenza patients with severe illnesses are infected with seasonal IVs, new influenza strains or pandemics occur; therefore, the treatment and emergency prevention of influenza antiviral drugs are particularly important (7, 8).
From the development of the first anti-influenza drug amantadine to the more recent RNA polymerase inhibitor baloxavir marboxil, currently, only two classes of drugs are Food and Drug Administration (FDA) approved and recommended for use (9). Meanwhile, there are several influenza antiviral drugs in development, some of which have completed clinical trials, with different mechanisms of action and different inhibitory effects (8). These drugs are required to be highly broad-spectrum and safe in order to have a chance of being licensed. As new drugs are approved, the threat of influenza to special high-risk groups will continue to decrease. In this review, we will describe the anti-influenza viral drugs that have been marketed and located in the clinic with reference to the different stages of the IV life cycle, aiming to provide references and suggestions for subsequent drugs development and the emergence of new therapies.
DRUG CANDIDATE TARGETS BASED ON VIRAL STRUCTURE AND LIFE CYCLE
Although the genome of IV is simple, all viral proteins have non-redundant roles in the infection process and complete the entire replication cycle together. Therefore, these specific proteins can be used as targets for drug design or screening (Fig. 1; Tables 1 to 3).
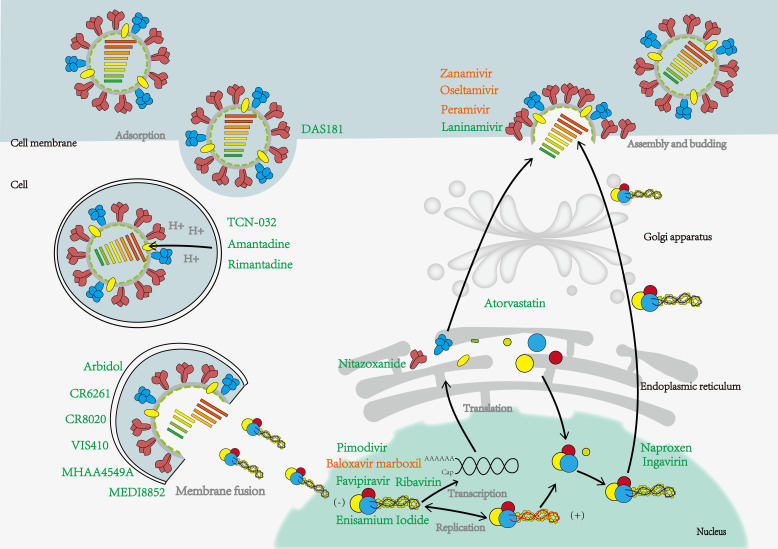
Fig 1.
IV infections life cycle and associated drug action stages contain currently approved and unapproved drugs by the FDA, with approved drugs shown in orange font and others in green. IV contacts sialic acid on host cells through surface HA and encapsulates virus particles in endosomes by endocytosis. In the low pH environment, H+ enters the virus through the M2 ion channel, and under the action of the fusion peptide, the viral membrane fuses with the endosome membrane, releasing the viral genome into the cytoplasm and then into the nucleus. The viral RNA is transcribed, copied, and translated by RNA polymerase. Finally, it is assembled on the surface of the cell membrane and hydrolyzes sialic acid with neuraminidase (NA) to help release the mature progeny virus.
TABLE 1.
FDA-approved influenza drugs (include no longer recommended and currently recommended)
| Name | Target | Usage | Applicable people (treatment/prophylaxis) |
Against | Recommended dosage (adults) | Reference |
|---|---|---|---|---|---|---|
| No longer recommended | ||||||
| Amantadine | M2 ion channel | ral | −a | IAV | − | (10–19) |
| Rimantadine | M2 ion channel | ral | − | IAV | − | |
| Recommended | ||||||
| Zanamivir | NA active site | Inhaled | People 7 years and older/people 5 years and older | IAV and IBV | Treatment: 10 mg twice daily for 5 days; Prophylaxis: 10 mg once daily for 7 days |
(20–26) |
| Oseltamivir | NA active site | ral | People 2 weeks and older/people 1 year and older | IAV and IBV | Treatment: 75 mg twice daily for 5 days; Prophylaxis: 75 mg once daily for 7 days |
(10, 27–35) |
| Peramivir | NA active site | Intravenous | People 2 years and older/- | IAV and IBV | A single dose of 600 mg, >15 min | (10, 20, 36–41) |
| Baloxavir marboxil | PA | ral | People 5 years and older/- | IAV and IBV | <80 kg: a single dose of 40 mg; >80 kg: a single dose of 80 mg |
(42–50) |
"−" indicates that no relevant information is available at the time of publication.
TABLE 2.
Other countries’ approved influenza drugs
| Name | Target | Usage | Country/time | Applicable people (treatment/prophylaxis) |
Against | Recommended dosage (adults) | Reference |
|---|---|---|---|---|---|---|---|
| Arbidol | HA Stalk | ral | Russia, 1993; China, 2006 | People 5 years and older/people 5 years and older | IAV and IBV | Treatment: 200 mg four times daily for 5 days; Prophylaxis: 200 mg twice a week for 3 weeks |
(51–64) |
| Favipiravir | PB1 | ral | Japan 2014 | Adults | IAV, IBV, and ICV | Day 1: 1,600 mg twice daily; Days 2–5: 600 mg twice daily |
(65–73) |
| Laninamivir | NA active site | Inhaled | Japan, 2010 | People 5 years and older/- | IAV and IBV | A single dose of 160 mg | (74–83) |
| Ingavirin | NP | ral | Russia, 2009 | People 13–17 years | IAV and IBV | Treatment: 60 mg once daily for 5 days |
(84–87) |
TABLE 3.
Influenza drugs in clinical trials (peptides and small molecule drugs)
| Name | Mechanism of action | IC50 (μg/mL) |
100% Protection in vivo |
Adverse event | Usage | Clinical studiesa | Reference |
|---|---|---|---|---|---|---|---|
| Target the host | |||||||
| DAS181 | Mimic sialic acid | 0.04–0.9 nM |
Pre: 0.3 U/treat/day Post: 30 U/treat/day |
Elevated alkaline phosphatase | Inhalation | I NCT00527865, October 2007 to January 2009; I NCT01651494, August 2011 to September 2012; II NCT01037205, December 2009 to September 2011 |
(88–90) |
| Atorvastatin | Inhibition of lipid droplet formation in the viral envelope | −b | − | − | II NCT02056340, October 2013 to June 2018 |
(91, 92) | |
| Nitazoxanide | Affects maturation of HA | 0.31–1 μM | 120 mg/kg/day | Headache | ral | II and III NCT01227421, December 2010 to May 2011; II NCT02057757, February 2014 to January 2018 |
(93–102) |
| Target the virus | |||||||
| Ribavirin | Inhibition of RdRp activity | 5.1 | − | Nausea, vomiting, and diarrhea | ral | I and II NCT00867139, March 2009 to January 2010; II NCT01227967, September 2010 to March 2017 |
(8, 103–108) |
| Pimodivir | Inhibition of RdRp activity | 0.13–3.2 nM | 20 mg/kg twice daily | Diarrhea | ral | IIa NCT01561807, March 2012- ; IIb NCT02342249, December 2014 to May 2016; II NCT02532283, December 2015 to March 2017; III NCT03376321, January 2018 to April 2020 |
(109–113) |
| Enisamium iodide | Inhibition of RdRp activity | − | − | − | ral | II and III NCT04682444, April 2009 to May 2010 |
(114, 115) |
| Naproxen | Inhibiting NP binding to RNA | 16.7–19.2 μM | 10 mg/kg/day | No | ral | IIb and III NCT04315194, December 2017 to December 2019 |
(116, 117) |
| XC221 | − | − | − | − | I NCT03459391, May 2017 to September 2017; II NCT03455491, February 2018 to June 2018; III NCT05544916, August 2022 to May 2023 |
− | |
| JNJ4796 | Inhibition of membrane fusion | 0.012–3.24 μM | 10 mg/kg twice daily | ral | − | (118) | |
NCT, clinical trial number.
"−" indicates that no relevant information is available at the time of publication.
Hemagglutinin (HA) is a glycoprotein on the surface of IV, consisting of three identical HA monomers to form a trimer structure (119). According to genetic and antigenic variation, the IAV HA is divided into group 1 (H1, H2, H5, H6, H8, H9, H11, H12, H13, H16, H17, and H18) and group 2 (H3, H4, H7, H10, H14, and H15) (120). HA can be divided into head and stalk parts. In the case of human influenza, the receptor-binding sites located in the head will preferentially target the α2,6-linked sialic acid attached to the galactose of the glycan receptor located on the human respiratory epithelial cell surface, which then encapsulates the virus particle in endosomes through clathrin-mediated or other means of endocytosis, such as micropinocytosis (121, 122). Next, the M2 ion channel opens and guides H+ into the virus core, causing weak acidification of the virus core (123). Under low-pH conditions, fusion peptides located in the HA stalk can mediate the fusion of viral and host membranes (121, 124). In the meantime, the interaction between M1 and vRNP is disrupted, allowing the RNP to be released and transported to the nucleus (123, 125).
Next, through the action of RdRp, Virus RNA (vRNA) undergoes transcription, replication, and translation. RdRp is a protein complex consisting of three subunits, including acidic protein (PA), basic protein 1 (PB1), and basic protein 2 (PB2) (126). The transcription of IV requires primers; the PB2 subunit can recognize the Cap structure (7-methyl GTP) of the host mRNA and then use the endonuclease activity of the PA subunit to cut about 8–14 bases downstream of the Cap structure. The base sequence is used as a primer, and the viral mRNA is synthesized using PB1 with RNA polymerase function (1, 126). In contrast to the transcription process, IV replication does not require primers, and RNA polymerase uses vRNA as a template to synthesize cRNA, followed by the synthesis of vRNA using cRNA (1, 126).
Subsequently, various structural and non-structural viral proteins are synthesized on the ribosomes, and proteins involved in RNP assembly are returned to the nucleus for assembly and transported to the surface of the cell membrane along with other protein structures (116). Finally, in the presence of neuraminidase (NA), the virus leaves the host in a budding manner (74). NA is a tetrameric structure, and in IAV, NA is classified into 11 subtypes, which is an important basis for the classification of IAV subtypes, along with HA (120). It is noteworthy that only the N1–N9 isoforms are active in hydrolyzing sialic acid (127, 128). Compared with HA, NA has less evolutionary pressure and is a good target for drug action. Small molecules and antibody drugs targeting NA have shown excellent broad spectrum and protective effects against different subtypes of IV (27, 129, 130).
APPROVED DRUGS FOR INFLUENZA
At present, several anti-influenza viral drugs have been approved by the FDA or other countries and can be divided into three categories according to their mechanisms of action, including inhibiting viral entry into host cells (HA inhibitor and M2 inhibitors), inhibiting viral replication (RdRp inhibitors), and inhibiting viral particle release from infected cells (NA inhibitors; Fig. 1 Tables 1 and 2) (131). The availability of these drugs has greatly reduced the duration of illness, alleviated the disease, and made an outstanding contribution to suppressing the spread and outbreak of influenza.
Entry inhibitors
The IV entry inhibitors mainly contain HA inhibitors and M2 inhibitors, of which the M2 inhibitor amantadine was also the first anti-influenza drug approved by the FDA. However, the resistance of IVs to these inhibitors increased dramatically during their use, leading to drug failure, and they are no longer recommended for subsequent use. However, they can still be investigated as drug candidates against emerging IVs.
HA inhibitors
Arbidol (Umifenovir) was first reported in 1993, a small indole derivative [Fig. 2(7) and 3(1)] (51). It was subsequently approved for the prevention and treatment of influenza in Russia and China (52). It is a broad-spectrum antiviral drug with activity against a variety of viruses in vitro and in vivo studies, including Zika virus, flaviviruses, Ebola, SARS-CoV-2, etc. (53–56). Arbidol primarily targets IAV and IBV, and its epitope is located in the hydrophobic groove of the HA-Stalk, approximately ~16 Å from and interacting with the fusion peptide (57). It blocks the low pH-mediated membrane fusion process by stabilizing the pre-fusion conformation of HA (58).
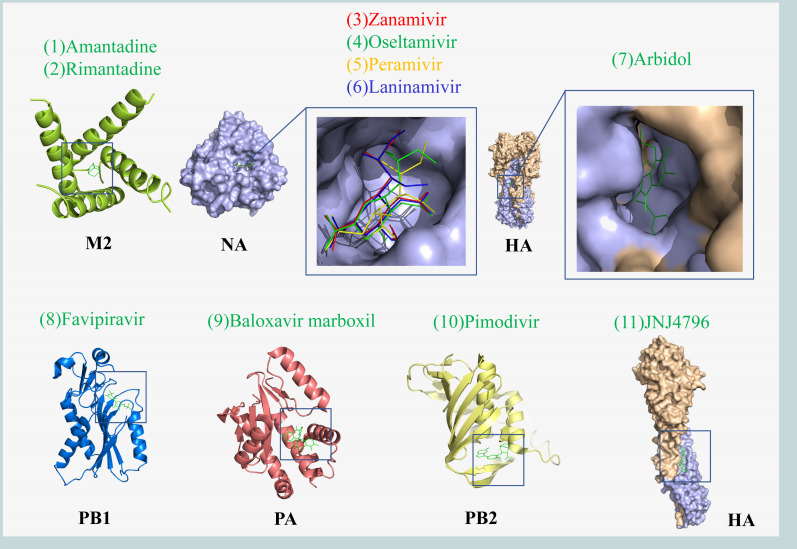
Fig 2.
Crystal structures of small molecule drugs of IV. The binding sites of small molecules and related subunits are shown in the box. M2 protein and Amantadine complex (PDB code: 3C9J), NA and Zanamivir, Oseltamivir, Peramivir, Laninamivir complex (PDB code: 4MWR, 4MWW, 4MX0 and 4MWY), HA and Arbidol, JNJ4796 complex (PDB code: 5T6S and 6CFG), PB1 and Favipiravir complex (PDB code: 4KN6), PA and Baloxavir marboxil complex (PDB code: 6FS6), and PB2 and pimodivir complex (PDB code: 7AS0). The crystal structures were obtained from the Protein Data Bank (PDB) and rendered using PyMol software.
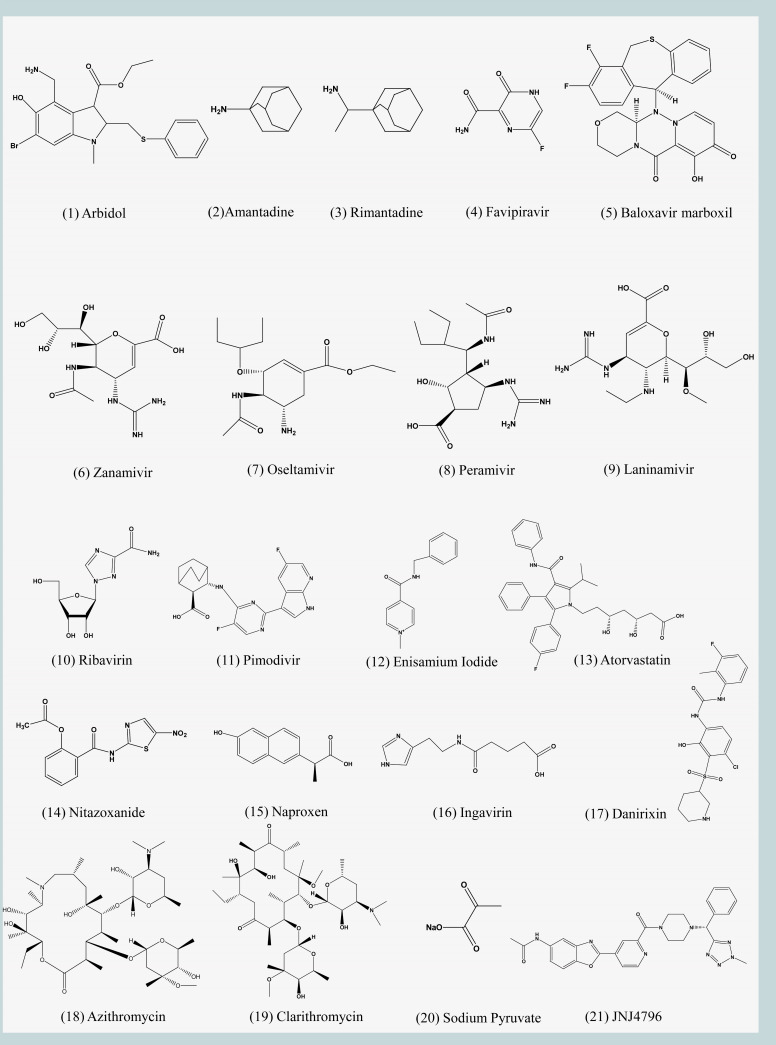
Fig 3.
Chemical structures of IV small molecule drugs. The chemical structures of related small molecule drugs were drawn using Chemdraw software.
In vitro experiments revealed that the 50% inhibitory concentration (IC50) of arbidol on IAV is 4.4–12.1 μM (59). In addition, another study showed that arbidol is equally sensitive and unlikely to develop resistance to 18 clinically isolated strains of IAVs circulating in 2012–2014 seasons (60). In a mouse model, arbidol inhibited weight loss and reduced pulmonary viral load at 25 mg/kg. Also, the treatment of ferrets with arbidol down-regulated several influenza-induced proinflammatory cytokines (IL-10, TNF-α, IL-8, and IL-6) (59). Several clinical studies have shown that arbidol is effective in inhibiting influenza and can lower the patient’s body temperature with minimal side effects (61, 62). Oral administration of arbidol 200 mg four times daily for 5 days to adult influenza patients significantly relieved symptoms after 60 hours, which was able to significantly promote viral shedding (63). Recent data indicated that arbidol is most effective in treating the acute phase of influenza in adults (64).
M2 channel inhibitors
Amantadine (trade name generic) and its methyl derivative rimantadine (Flumadine and generic) are the earliest antiviral drugs approved by the U.S. FDA for the prophylaxis and therapy of IAV [Fig. 2(1)(2) and 3A(2) (10]. Amantadine and rimantadine bind to the M2 protein and inhibit ion channel activity, thereby preventing the acidification of the viral core, a key step for viral entry. In the absence of viral core acidification, vRNP is no longer released from the endosome of infected cells, thus blocking the influenza life cycle (11, 12).
In 12 clinical trials, amantadine proved effective in preventing IAV infection in children, but it required its use in up to 17 children over a period of 14–18 weeks. It is also difficult to distinguish adverse effects caused by the drug, such as nausea and dizziness, from the clinical manifestations of influenza (13, 14). Unlike amantadine, the data are insufficient to determine the preventive effects of rimantadine in children, and it should only be used to assist in reducing fever in children (13). In the elderly group, based on a randomized subgroup of 482 cases, rimantadine was less protective than zanamivir, an NA blocker which we discuss in the subsequent section (13, 15). The efficacy of amantadine in adult patients has been widely recognized, but due to pronounced intestinal adverse reactions, it may produce more serious adverse reactions of the central nervous system, and the treatment dose is close to the dose of side effects, therefore, it is difficult to promote in clinical application (16).
At the same time, during prolonged and frequent treatment with amantadine and rimantadine, corresponding drug-resistant viruses are produced, mainly H1N1 and H3N2 subtypes that constitute seasonal influenza, and the proportion of drug-resistant strains increases year by year (17–19). Therefore, these inhibitors are no longer recommended by the US CDC for the treatment or chemoprophylaxis of IAV currently circulating (10).
Replication inhibitors (RdRp inhibitors)
RdRp is considered to be one of the most promising targets against RNA viruses and is the basis for the current design of the COVID-19 drugs Remdesivir and Molnupiravir (132, 133). RdRp is a key enzyme for the replication and transcription of the genetic material of RNA viruses. RdRp is also highly conserved in different subtypes of IVs (42, 43, 65, 134). Therefore, RdRP inhibitors may be one of the hot spots for future influenza drug development.
Favipiravir (T-705, trade name Avigan) is a purine nucleoside analog that has been shown to inhibit IV transcription and replication by competing with substrates of PB1 (66). The mechanism of action of favipiravir differs from approved influenza antivirals in that antiviral activity against H5N1 and H7N9 avian IVs has been demonstrated in non-clinical data (65, 67). As a result, favipiravir was approved in Japan in 2014 for the treatment of novel or re-emerging IV in view of the outbreak of influenza infection at that time. Subsequently, favipiravir also proved to be a broad-spectrum inhibitor that is against IAV, IBV, and ICV [Fig. 2(8) and 3(4)] (67, 68). Because the structure and function of PB1 are similar in different viruses, it is also used to treat respiratory syncytial virus, Ebola virus, and SARS-CoV-2 infection (69–71). Favipiravir is usually used in combination with oseltamivir in clinical trials of severe influenza (72, 73). The latest research by Bin Cao’s team at China-Japan Friendship Hospital showed that the combination of favipiravir and oseltamivir was more effective in reducing the duration of illness in patients with severe influenza than oseltamivir monotherapy (72). Currently, two clinical trials of favipiravir for the treatment of uncomplicated influenza in adults have been completed (NCT02026349 and NCT02008344). Both trials found that favipiravir significantly reduced viral titers, but the time to symptom relief compared to placebo was different in the two trials, at 14.4 hours and 6.1 hours. While this may be accounted for by a variety of factors including drug dose, greater benefit may be obtained by increasing the dose of favipiravir or by combining it with other drugs (135).
Baloxavir marboxil (S-033188, trade name Xofluza) is developed by Shionogi & Co., Ltd. and Roche for the treatment of IAV and IBV [Fig. 2(9) and 3(5)] (42). It was approved by the FDA in October 2018 for the treatment of acute uncomplicated influenza (influenza) in patients 12 years of age and older who have had symptoms for 48 hours or less (44). In 2022, the population is expanded to include pediatric patients 5–11 years of age who are symptomatic for less than 2 days with influenza (136). As the latest anti-influenza drug, baloxavir marboxil acts differently from its predecessors in that it targets the cap-dependent endonuclease in PA subunit, preventing viral mRNA transcription and thereby inhibiting IV replication (42, 43). PA/I38 is the main binding site for baloxavir marboxil, which can lead to the development of drug resistance when mutations occur (45). Preclinical models showed that compared with oseltamivir and favipiravir, baloxavir marboxil had higher inhibitory activity against IAV and IBV; however, in viruses with PA I38T, the van der Waals force between the drug and the epitope was significantly weakened, and the inhibitory activity was greatly reduced (46). Baloxavir marboxil is safe for the treatment of uncomplicated influenza in adults and adolescents, and it can significantly shorten the duration of influenza symptoms, which is better than oral oseltamivir (47). Notably, baloxavir marboxil and oseltamivir are comparable in high-risk outpatients, but combining the two drugs in routine situations is not recommended (48, 49). Furthermore, pharmacokinetic analysis of three different populations showed that body weight and ethnicity were the most important factors affecting the clearance rate and distribution volume of baloxavir marboxil (50). Currently, studies in China and Korea have shown that baloxavir dosing regimens are appropriate, and the pharmacokinetics and safety profile are consistent in Asian populations (137, 138).
Neuraminidase inhibitors
To date, three NA inhibitors (zanamivir, oseltamivir, and peramivir) have been approved by the FDA. Laninamivir was approved for influenza treatment in Japan in 2010. While no single study directly compared the efficacy of all four drugs, oseltamivir remains the drug of choice with a safer, broader trial population that includes pregnant women and infants.
Zanamivir (trade name Relenza) is the first NA inhibitor to be approved by the FDA. It is a sialic acid and its transition state analog. Based on 2-deoxy-2,3-didehydro-N-acetylneuraminic acid (DANA, which has been shown previously to inhibit bacterial, viral, and mammalian neuraminidases), it replaces 4-hydroxyl into 4-guanidino group, which increases the binding area of zanamivir to the enzyme activity center [Fig. 2(3) and 3(6) ] (20, 21). It is an inhaled powdered drug mainly used for IAV and IBV; however, the powdered nature of the drug is contraindicated in patients with underlying respiratory disease (22). IV replication peaks between 24 and 72 hours after onset, so drugs that act during the replication phase of the virus, such as NA inhibitors, should be used as early as possible (23). Clinical data show that zanamivir reduced the duration of illness by inhalation in adults and had no significant improvement in children (24, 25). However, zanamivir is safe and effective in the intravenous treatment of children infected with influenza, and there are no significant complications (26). Therefore, zanamivir is primarily suitable for adults and children older than 7 years of age who have contracted acute influenza within 2 days.
The design of oseltamivir (trade name Tamiflu) is also based on DANA, which was approved by the FDA in 1999 as an oral treatment for uncomplicated influenza [Fig. 2(4) and 3(7)] (27–29). It inhibits the activity of NA and prevents the emergence, replication, and infection of the virus, mainly responsible for the treatment and prevention of infection with IAV and IBV (27). Numerous clinical trials have shown that oseltamivir is sufficiently safe for influenza in adults (30, 31). Early treatment with oseltamivir in adult hospitalized patients can shorten fever and hospitalization and reduce the risk of complications but may cause nausea and vomiting (31). Oseltamivir is also recommended for early use in hospitalized children, and in an evaluation of 55,799 hospitalized pediatric patients, it was shown to be effective in reducing clinical symptoms produced by influenza when administered early in the course of illness (139). The CDC reported that antiviral treatment within 48 hours of the onset of influenza provides the greatest clinical benefit (10).
After the 2009 influenza pandemic, oseltamivir was also approved for treatment of infants aged >2 weeks, but there is still insufficient clinical data to demonstrate the safety and efficacy of oseltamivir (32). Kimberlin et al. determined the dose of oseltamivir, in children aged <2 years, twice daily is 3.0 mg/kg for infants aged 8 months and 3.5 mg/kg for infants aged 9–11 months. In clinical trials of oseltamivir in the treatment of influenza in children, it has been shown that oseltamivir can significantly shorten the duration of illness and reduce the incidence of otitis media complications (28, 33). Current seasonal influenza has very low resistance to NA inhibitors compared to amantadine, but the CDC suggests that this may change (10). Studies have shown that oseltamivir A(H1N1) resistant strains generally include the H275Y mutation (34, 35).
Peramivir (BCX-1812, RWJ-270201, trade name Rapivab) is a novel cyclopentane sialic acid analog that has been designed to target NA with inhibitory activity against IAV and IBV [Fig. 2(5) and 3(8)] (20, 36). Unlike oseltamivir, peramivir is less effective when taken orally and is therefore administered intravenously, but it is also the best option for young infants unable to swallow pills (10). Early treatment with peramivir can effectively reduce mortality (37). It was approved by the FDA in 2017 for the treatment of uncomplicated influenza in adults and children from the age of 2 years (38). The clinical efficacy of peramivir has been demonstrated to be safe and effective in several studies (37, 39, 40). Recently, a preliminary randomized controlled trial showed that intravenous peramivir and oral oseltamivir were equally effective in the treatment of emergency uncomplicated influenza patients with similar complications (37, 41). Oseltamivir was significantly more effective than paramivir in clinical studies for the treatment of hospitalized children infected with IAV, while studies in the treatment of infected IBV have shown similar results for both (140).
Laninamivir (R-125489) is an NA inhibitor obtained by replacing the 7-methoxy of zanamivir with 7-hydroxyl [Fig. 2(6) and 3(9)] (74). Laninamivir Octanoate (CS-8958) is a prodrug of laninamivir, which is inhaled and converted into laninamivir through the respiratory tract (75, 76). Both drugs were approved in Japan in 2010 and are currently undergoing clinical trials in other countries (75, 77, 78). Laninamivir is a long-acting inhibitor with a half-life of up to 3 days and no significant adverse events (79). Based on recently updated data, a single inhalation of 160 mg has been recommended for the treatment of patients over 5 years of age in Japan (80). Double-blind, multicenter, randomized, placebo-controlled studies show that a single inhaled dose of 20 mg laninamivir octanoate is effective and well-tolerated in the treatment of influenza in adults and children (78, 81). Other studies have reported that laninamivir octanoate is comparable to oseltamivir and zanamivir in the treatment of influenza and significantly reduces the duration of treatment in children infected with oseltamivir-resistant A(H1N1) viruses carrying H274Y NA substitution (82, 83).
DRUGS IN THE CLINICAL TRIALS
Polypeptide or small molecule drugs
Entry inhibitors
DAS181 (Fludase) is an inhaled recombinant protein, which is composed of Actinomyces viscosus sialidase catalytic domain and a cell surface anchored sequence. It accurately removes sialic acid from the epithelial cells of the respiratory tract, a receptor for parainfluenza, IV and other respiratory viruses (Fig. 1; Table 3) (88). In this way, multiple subtypes of seasonal and avian influenza were effectively inhibited with 50% effective concentration (EC50) values ranging from 0.04 nM to 0.9 nM. DAS181 showed positive preventive and protective effects in mice and ferrets (88). In addition to acting on the upper respiratory tract, it also prevents the H5N1 IV from infecting human lung tissue. Data from an in vitro study showed that DAS181, administered at 100 U/mL every 12 hours, produced the most effective inhibitory activity (88).
A double-blind, placebo-controlled phase II clinical trial (NCT01037205) evaluated the efficacy and safety of DAS181 in patients primarily infected with seasonal influenza. Compared to the placebo group, viral load was significantly lower in both the multi-dose group (10 mg daily for 3 days) and the single-dose group (10 mg) pharyngeal washout, but testing at 3 or 5 days later found that only the multi-dose group significantly reduced patients’ viral load (89). The study showed that the major side effect of DAS181 was the elevation of alkaline phosphatase in the blood (89). Other studies (NCT00527865 and NCT01651494) found that DAS181 was not suitable for continuous treatment beyond 7 days because of adverse effects, such as dyspnea, inducing specific neutralizing IgG antibodies and rendering the drug ineffective (90).
Replication inhibitors
Ribavirin (Virazole), a synthetic purine nucleoside analog, is a broad-spectrum viral inhibitor approved for the treatment of hepatitis C and respiratory syncytial virus [Fig. 3(10)] (141, 142). It also has inhibitory activity against IV, acting on the PB1 subunit of RNA polymerase and blocking the synthesis of viral mRNA (8). Ribavirin and several drugs have shown synergistic effects against IV both in vivo and in vitro, including baicalein, favipiravir, oseltamivir, and amantadine (103–106). Preclinical data showed that the combination of ribavirin, oseltamivir, and amantadine exhibited high inhibitory activity against multiple IV, including drug-resistant strains, and was superior to oseltamivir alone. The pharmacokinetics (NCT00867139) of these three drugs (200 mg, 50 mg, and 75 mg, respectively) are similar to monotherapy and are safe for use in immunocompromised patients (107). Results from a randomized, double-blind, multicenter phase II clinical trial (NCT01227967) showed that despite a significant reduction in viral output at 72 hours compared with monotherapy, this difference was not associated with improved clinical benefits (108). Therefore, more data are needed for analysis and discussion to identify the effectiveness of Ribavirin.
Pimodivir (JNJ-63623872, VX-787) is an indole analog that interacts with the cap-binding domain of the PB2 subunit to block viral mRNA transcription [Fig. 2(10) and 3(11)] (109). Pimodivir has excellent inhibitory activity against IAV, including H1N1 pdm09, H3N2, and H5N1 subtypes, as well as viruses resistant to oseltamivir and amantadine., with EC50 values of 0.13–3.2 nM (110). In vivo, 20 mg/kg twice daily doses of pimodivir completely prevented H3N2 and H1N1 infection in mice. Therefore, it shows more effectiveness than the same dose of oseltamivir (111). The elderly are always the most vulnerable group to influenza. In a phase II clinical study (NCT02532283) evaluating pimodivir combination treatment with oseltamivir IAV pharmacokinetic in different groups, the result showed that pimodivir pharmacokinetic parameters were similar in non-elderly and elderly patients, and combined treatment can reduce complications and sick time, better than that of oseltamivir monotherapy (112). In a double-blind IIb study (NCT02342249) evaluating the efficacy of pimodivir in the treatment of uncomplicated acute influenza in adults, viral load was lower with pimodivir (300 mg and 600 mg) or the combination of pimodivir and oseltamivir (600 mg + 75 mg) compared to the placebo group (113). However, the Phase III data (NCT03376321) did not demonstrate an additional benefit of pimodivir monotherapy in hospitalized patients with IAV, and the clinical development trial was subsequently terminated.
Enisamium iodide is an anti-respiratory viral (including IAV and IBV) drug that acts on viral RNA polymerase via its metabolite VR17-04 [Fig. 3(12)] (114). In the primary normal human bronchial epithelial cells, 500 µg/mL of the drug significantly inhibited H1N1 replication, and 200 mg/kg enisamium iodide daily could cure ferrets infected with 105 TCID50 of H3N2 and reduced respiratory viral load (115). A phase II trial evaluated the efficacy of enisamium iodide in patients aged 18–60 years with confirmed influenza and showed a reduction the viral load and reduced duration of illness on day 3 of treatment compared to placebo (114). Currently, enisamium iodide is used to treat influenza in countries such as the Commonwealth of Independent States and Mongolia (115).
Atorvastatin is a 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor that effectively reduces plasma cholesterol levels [Fig. 3(13)] (91). The envelope of IV contains a large amount of cholesterol, and Atorvastatin can effectively inhibit the synthesis of cholesterol in lipid droplets caused by infection stress in cells, thereby affecting IV replication (92). A phase II trial (NCT02056340) is currently being completed in Israel to evaluate Atorvastatin in patients hospitalized for acute influenza. However, follow-up results have not yet been published.
Assembly inhibitors
Nitazoxanide is the only FDA-approved drug for the treatment of Cryptosporidium infection and also has inhibitory effects against intestinal parasites such as helminths and tapeworms [Fig. 3(14)] (93). Later, Nitazoxanide was found to have broad-spectrum antiviral activity against influenza, SARS-CoV-2, MERS-CoV, paramyxovirus, and norovirus (94–97). The IV progeny viruses require complete assembly of the progeny virions at the cell membrane surface, but nitazoxanide blocks HA terminal glycosylation and affects HA transport between the endoplasmic reticulum and the Golgi complex, thereby inhibiting the maturation of HA glycoproteins and the assembly of the IV (98). Significant inhibitory activity of nitazoxanide against H1N1, H3N2, and IBVs prevalent between March 2014 and August 2016 was demonstrated using plaque reduction assays (99). Preclinical studies have shown that nitazoxanide in combination with oseltamivir is more effective than monotherapy in the prevention of H1N1 infection, but substantial clinical data are needed for nitazoxanide in combination with other drugs (100). In a Phase IIb/ III trial (NCT01227421), 600 mg twice daily nitazoxanide treatment for 5 days reduced symptom duration in patients with acute uncomplicated influenza, and headache was the main and common adverse event reported in different groups (101). However, a recent clinical trial (NCT02057757) in patients with severe acute influenza reflected that the effect was not satisfactory (102).
Naproxen is a non-steroidal anti-inflammatory drug agent that inhibits IAV and IBV in vitro and in vivo. Related studies have shown that 10 mg/kg daily of Naproxen is more effective than the equivalent dose of oseltamivir in treating mice infected with IBV [Fig. 3(15)] (116). The host export protein CRM1 binds to the NP and mediates the export of the RNP complex from the nucleus. The mechanism of action suggests that Naproxen recognizes NP and inhibits the binding of CRM1 to NP protein through antagonism, thus inhibiting the NP output of IV. The critical residues on IAV NP and IBV NP were different, Y148 and F209, respectively (116). A recent clinical trial (NCT04315194) conducted in adult hospitalized patients with H3N2 infection revealed that combined therapy of naproxen (200 mg)-oseltamivir (75 mg)-clarithromycin (500 mg), compared with the control arm of oseltamivir (75 mg) monotherapy, reduced 30-day mortality by 7.3%, supporting the use of multi-drug-combined therapy (117).
Other
The following drugs are currently in the late clinical stage or have been approved for the treatment of influenza in Russia. Despite the lack of relevant clinical data and literature, they are still shown for reference only. See www.clinicaltrials.gov for details. XC221 is an innovative drug, and a phase II clinical trial (NCT03455491) has been completed in Russia to evaluate the efficacy and safety of XC221 in the treatment of IV or other acute respiratory virus infections (SARS-CoV-2). Ingavirin (Imidazolyl ethanamide pentandioic acid), a drug developed by Valenta, a Russian pharmaceutical company, inhibits IV by blocking the transport of newly synthesized NP from the cytoplasm to the nucleus [Fig. 3(16)] (84). Literature has shown that Ingavirin can effectively inhibit influenza subtypes such as H1N1, H3N2, H5N1, and B in vivo or in vitro (85–87). Currently, a phase IV clinical trial (NCT03154515) has been completed.
Monoclonal antibody drugs
Monoclonal antibody drugs against IV have higher affinity and inhibitory activity than small molecule drugs (143). The current clinical antibody epitopes are different from the small molecule drugs in the market, which has application value in the development of broad-spectrum influenza vaccine and drug combination (144). Currently, monoclonal antibody drugs involved in clinical studies are mostly derived from vaccinated sera of healthy volunteers or recovered patients, except TCN-032, all of which are directed against the stalk domain of the HA. Importantly, these antibodies can inhibit multiple IV subtypes in vitro and in vivo (145). Due to its functional specificity, the stalk domain has a significantly lower mutation rate than the Head, which leads to the antibodies or compounds targeting the Stalk are usually highly broad-spectrum (146). Therefore, the antibodies targeting the Stalk are expected to become the basis for the research on universal vaccines (147, 148). However, subsequent clinical results have shown that the efficacy of these antibodies as monotherapy is generally unsatisfactory, and the direction of adjuvant combination therapy should be considered in the future (Fig. 1; Table 3).
CR6261 is a human broad-spectrum neutralizing antibody screened by phage display technology (Fig. 4; Table 4). It neutralizes H1, H2, H5, H6, H8, H9, H13, and H16 subtypes in vitro with IC50 values of 0.12–14.87 μg/mL (149, 150). Meanwhile, CR6261 showed a cross-protective effect in both prevention and treatment of H5N1 and H1N1 infection in mice (149). The heavy chain variable region of CR6261 is encoded by the IGHV1-69 gene, which can recognize the conserved hydrophobic pocket on HA-Stalk and prevents low pH-mediated HA conformational changes, thus inhibiting the fusion of viral and host membranes (151). Although preclinical trials have shown promising prophylactic and therapeutic efficacy, in a subsequent randomized, double-blind, placebo-controlled phase II trial (NCT02371668) in which 50 mg/kg of CR6261 was administered to healthy volunteers at 24 hours after H1N1 infection, no significant prophylactic or therapeutic effects were reported for monotherapy CR6261 (152, 153).
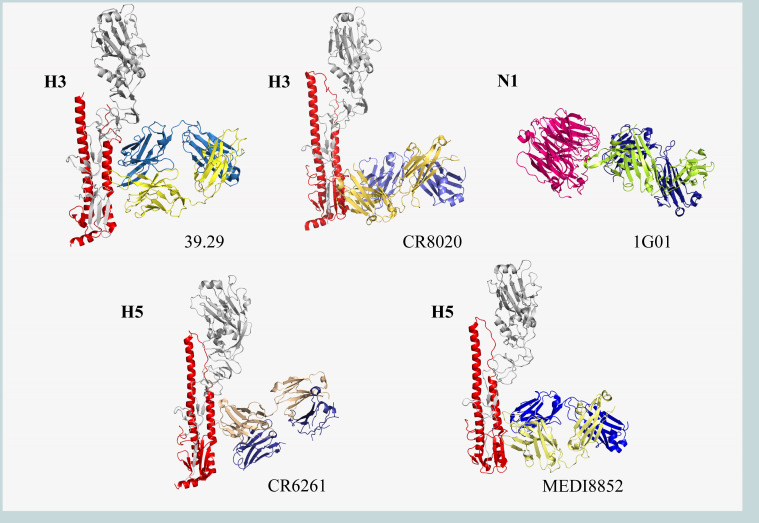
Fig 4.
Crystal structures of antibodies against IV glycoprotein. The HA monomers (gray and red) bind to the heavy (blue) and light (yellow) chains of the antibodies. MHAA4549A (39.29; PDB code: 4KVN), CR8020 (PDB code: 3SDY), CR6261 (PDB code: 3GBM), MEDI8852 (PDB code: 5JW4), and 1G01 (PDB code: 6Q23). Graphics acquisition and rendering methods are the same as Fig. 2.
TABLE 4.
Influenza drugs in clinical trials (monoclonal antibody drugs)
| Name | Binding to | IC50 (μg/mL) |
100% protection in vivo |
Adverse event |
Usage | Clinical studies | Reference |
|---|---|---|---|---|---|---|---|
| CR6261 | HA-stalk (H1, H2, H5, H6, H9, H13, and H16) |
0.12–14.87 | Pre: 5 mg/kg Post: 15 mg/kg |
−a | Intravenous | I NCT01406418, February 2013 to November 2013; II NCT02371668, February 2015 to November 2018 |
(149–153) |
| CR8020 | HA-stalk (H3, H4, H7, H10, H14, and H15) | 1.1–13.1 | Pre: 3 mg/kg Post: 15 mg/kg |
− | Intravenous | I NCT01756950, January 2013 to November 2013; II NCT01938352, October 2013 to January 2014 |
(150) |
| VIS410 | HA-stalk (H1, H2, H3, H5, H6, H7, and H9) | 0.3–11 | Post: 2.5 mg/kg | Mild diarrhea | Intravenous | I NCT02045472, September 2014 to May 2015; II NCT02989194, January 2017 to October 2017 |
(154–157) |
| MHAA4549A | HA-stalk (H1, H2, H3, H5, and H7) |
1.3–45.1 | Post: 100 and 900 µg | Headache | Intravenous | I NCT01877785, July 2013 to November 2013; I NCT02284607, November 2014 to March 2015; IIa NCT01980966, November 2013 to June 2014; IIb NCT02293863, January 2015 to May 2017 |
(158–161) |
| MEDI8852 | All HA-stalk | 0.064 | Pre: 1 mg/kg Post: 10 mg/kg |
Headache, hypoglycemia, and bronchitis | Monotherapy | I II NCT02350751, December 2015 to December 2016 |
(162, 163) |
| TCN-032 | M2e (H1, H2, H3, H5, H7, andH9) | − | − | − | Monotherapy | I NCT01390025, September 2011 to March 2012; II NCT01719874, August 2012 to March 2013 |
(145, 164) |
| 1G01 | N1-N9 NA and IBV NA |
0.01–2 | Pre: 0.3 mg/kg Post: 5 mg/kg |
− | − | − | (129) |
"−" indicates that no relevant information is available at this time of publication.
The same approach was used to screen the H3-HA responding monoclonal antibody CR8020, which has now completed a phase II clinical trial (NCT01938352) evaluating intravenous therapy, but no results have been published (Fig. 4; Table 4). The initial studies showed that CR8020 neutralized the H3, H7, and H10 subtypes in vitro with IC50 values of 1.1–13.1 μg/mL and complementation with CR6261 to neutralize almost all subtypes of IAV. In vivo, 3 mg/kg of CR8020 could effectively prevent and protect mice from infection with lethal doses of H3N2 and H7N7 (150). Both CR8020 and CR6261 target HA-Stalk; the difference is that CR8020 acts near the proximal end of the viral membrane and inhibits the conformational changes of HA and the membrane fusion process by stabilizing the structure of the fusion peptide (150). VIS410 targets HA-Stalk and broadly neutralizes the H1, H3, and H5 subtypes of IAV in vitro with EC50 of 0.3–11 μg/mL (Table 4). A single therapeutic dose of VIS410 protected BALB/c and DBA mice infected with H3N2 and H7N9, respectively (154). Moreover, a synergistic effect was demonstrated in combination with oseltamivir. VIS410 inhibited the viral membrane fusion process, targeted the elimination of infected cells through antigen-dependent cell-mediated cytotoxicity, and controlled IV-induced acute respiratory distress symptoms (154, 155). Given the good preclinical trial data support, VIS410 was quickly followed up with relevant studies. Phase I clinical trial (NCT02045472) has demonstrated that VIS410 at 50 mg/kg is safe, well tolerated, with no significant adverse reactions, and has good therapeutic benefits for the hospitalized population over 65 years of age (156). In a Phase II trial (NCT02989194), a single intravenous dose of 2,000 mg or 4,000 mg was effective in reducing viral load in the nasopharynx of patients with uncomplicated influenza compared with placebo (157). The main adverse reaction of treatment was mild diarrhea, which was dose-dependent (157). This provides strong support for the development of monoclonal antibodies to treat and prevent severe influenza infections.
MHAA4549A (39.29) can neutralize the H1 and H3 subtypes with IC50 of 1.3–45.1 nM (Figure, Table 4). MHAA4549A was more effective than oseltamivir in mice and ferrets infected with H1N1 and H3N2 (158). Furthermore, a synergistic effect was noted when used in combination with oseltamivir. The crystal structure shows that MHAA4549A binds to the top of highly conserved stalk helix A (158). Subsequently, two phase I randomized, double-blind, and placebo-controlled clinical trials (NCT01877785 and NCT02284607) confirmed the safety and tolerability of MHAA4549A in healthy volunteers, with no serious adverse events and no occurrence of immunogenicity (159). Although MHAA4549A also proved to be well tolerated and significantly reduced influenza-related symptoms in phase II clinical trial (NCT01980966), MHAA4549A did not significantly shorten the time to normal respiratory function and was less clinically effective than oseltamivir alone in an interim trial combining MHAA4549A and oseltamivir in patients hospitalized with severe influenza (NCT02293863), and the trial was subsequently discontinued (160, 161).
MEDI8852 is a broad-spectrum human mAb effective against H1, H2, H3, H5, H6, H7, and H9 subtypes with mean EC50 of 0.064 µg/mL (Fig. 4; Table 4) (162). It has higher antibody neutralization breadth and potency than other monoclonal antibodies, and in mouse and ferret models, MEDI8852 acts in a dose-dependent manner, showing a strong therapeutic effect that was superior to oseltamivir (162). MEDI8852 binds to the hydrophobic groove and most of the fusion peptides by coordinating the movement of its own heavy- and light-chain complementary variable regions (162). However, in the Phase IIa trial (NCT02603952), MEDI8852 produced slightly more adverse events than the oseltamivir group and did not demonstrate clinical significance due to the small number of participants in the studies of treatment alone and in combination with oseltamivir. Therefore, the company decided to discontinue the follow-up trial (163).
Most of the influenza antibody drugs currently in clinical use target HA, while TCN-032 is the only monoclonal antibody that targets the M2 extracellular region (highly conserved in IAV) (Fig. 4; Table 4) (145). Although M2-targeting antibodies have no neutralizing effect, they inhibit the emergence and assembly of the virus by binding to M2 (165). The antibody was obtained from B cells of healthy volunteers, and in vivo experiments showed that TCN-032 ensured the survival of mice infected with lethal doses of H5N1 or H1N1 and helped them regain weight rapidly (145). TCN-032 has been shown to be well tolerated and pharmacokinetic (NCT01719874) in healthy people without immunogenicity or other significant side effects, but in a phase II intravenous evaluation (NCT01719874), TCN-032 was not able to significantly reduce influenza-related symptoms (164).
POTENTIAL FUTURE DIRECTION
Combination therapy is commonly used in clinical trials, where a combination of two or three drugs with different mechanisms of action at a safe and effective dose can provide a more comprehensive inhibition. For example, the combination of antibodies CR6261 and CR8020 neutralizes all subtypes of IAV and oseltamivir in combination with anti-inflammatory drugs reduces the duration of influenza symptoms. Paxlovid, a COVID-19-specific drug consisting of Nirmatrelvir-Ritonavir, uses Ritonavir (a protease inhibitor and cytochrome P450 3A inhibitor) to inhibit key drug metabolism enzyme cytochrome P450 and increase the plasma concentration of Nirmatrelvir (a main protease inhibitor), preventing viral replication (166). In addition, frequent single dosing may lead to the development of drug-resistant strains of influenza, whereas combination therapy with influenza drugs with different mechanisms of action would greatly reduce the selection pressure of drug-resistant viruses (167, 168).
Treatment of uncomplicated influenza can be treated with drugs that target the virus itself, while serious acute IV infection can lead to pneumonia and acute respiratory failure, causing concurrent bacterial infection (169). Therefore, relevant anti-inflammatory drugs are needed to reduce the occurrence of influenza-related complications in order to shorten the time of illness of influenza patients. Severe acute influenza has been shown to trigger an overreaction of neutrophils, resulting in alveolar damage and increased viral load (170). Danirixin (GSK1325756) is a reversible and selective CXC chemokine receptor 2 (CXCR2, the key receptor for neutrophils to chemotaxis to inflammatory sites) antagonist that blocks neutrophils’ chemotaxis in inflammation, thereby reducing excessive inflammatory response [Fig. 3(17)] (171). Macrolides are a class of antimicrobial antibiotics that act on bacterial ribosomes and inhibit protein synthesis. They also regulate the body’s immunity function and eliminate excessive inflammatory response [Fig. 3(18)(19)] (172). Sodium pyruvate (the exogenous form of pyruvate) has been shown to have powerful anti-inflammatory and antioxidant effects [Fig. 3(20)]. It can reduce the immune response by inhibiting the production of reactive oxygen species in mitochondria and effectively reduce the related inflammatory cytokines caused by IV (173). These drugs have been clinically involved in the adjuvant treatment of patients with influenza infection.
The emergence of COVID-19 has once again demonstrated that it is difficult to develop specific drugs against outbreak viruses in a timely manner, and only extensive screening of existing drugs can shorten the time to clinical use. Due to the particularity of the mechanism of action, multiple drugs possess cross-inhibitory activity against viruses, making them candidates for the inhibition of multiple viruses. Ribavirin, an RNA polymerase inhibitor approved for the treatment of hepatitis C and respiratory syncytial virus, has also been reported to have inhibitory activity against the IV, SARS-CoV-2 (174). Baloxavir marboxil, the newly approved influenza drug, and pimodivir, developed by Johnson & Johnson, are also RNA polymerase inhibitors with potential cross-inhibiting activity. In addition, Molnupiravir, a novel coronavirus drug developed by Merck Sharp & Dohme, also acts on RNA polymerase and leads to the accumulation of errors in the genome of SARS-CoV-2 through its metabolites, thereby inhibiting replication. This mechanism of action may be applicable to various viral polymerases (175). Thus, the RNA polymerase of IV may become a major choice for future-targeted drugs.
Influenza drugs targeting other sites are also under development, including full-human antibody 1G01 targeting NA [Fig. 3(19)(20) and 4], and oral small-molecule drug JNJ4796 [Fig. 2(11) and 3(21)], which was screened with CR6261 target as a reference, showed strong and extensive binding inhibitory activity in vitro and in vivo experiments (118, 129). These drugs are ideal clinical candidates. Ignoring the virus will not cause it to disappear. Under the premise of the decline of the threat to human health by SARS-CoV-2, IV has returned to the public view, causing various concerns, including the current widespread seasonal influenza, frequent outbreaks in poultry and occasional cross-species transmission of H5 subtype avian influenza outbreaks. We all need to respond promptly and effectively.
CONCLUDING REMARKS
IV has coexisted with humans for millennia. While significant efforts have been made on influenza antiviral drug development, influenza infection still poses a severe public health burden due to drug resistance problems, such as with amantadine and rimantadine. As mentioned above, selecting combinations of drugs with different mechanisms of action in response to drug failure due to viral mutations may be the main way to combat IVs in the future. Another way to mitigate drug resistance issue is by designing drugs that target core structural components or enzymatic active sites of viral proteins, as mutations in these residues likely produce structurally unstable or inactive protein. In addition, there are many druggable nodes in the IV life cycle that are necessary for virus-host interactions. To ensure the structural stability and function of related proteins, even if resistance mutations occur, there are still a large number of other non-mutated sites as alternatives that would be good drug targets and would minimize the problem of drug resistance. It is worth mentioning that drugs that have developed serious resistance problems may be used in future emerging infectious diseases.
In summary, the four influenza pandemics are constant reminders that negligence will produce incalculable damage that will continue to affect a generation or even generations. Constant discovery of novel influenza antiviral drug for the prevention of influenza pandemics is indeed essential.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (81974302), the Natural Science Foundation of Hebei Province (H2021204001), the program for “333 talents project” of Hebei Province (A202002003), the Science and Technology Project of Hebei Education Department (QN2021071), Hebei Province’s Program for Talents Returning from Studying Overseas (C20220363), and Baoding Science and Technology Plan Project (2272P006, 2111F003 and 2263P005).
All authors listed have made a substantial contribution to the work and approved it for publication. Y.L., S.H., Z.Y., Z.T., and F.H. wrote the paper. P.L., Y.L., and F.Y. revised the paper.
Contributor Information
Peng Liu, Email: liupeng@cwbz.ac.cn.
Yue Liu, Email: yue.liu2@ucsf.edu.
Fei Yu, Email: shmyf@hebau.edu.cn.
Vinayaka R. Prasad, Albert Einstein College of Medicine, Bronx, New York, USA
EDITOR'S NOTE
The American Society for Microbiology and mBio would like to inform the readers that this article is a republished version of https://doi.org/10.1128/mbio.01273-23, which was retracted (https://doi.org/10.1128/mbio.00106-24). As stated in the retraction notice, after publication, "A number of factual errors were found in the paper" by the authors. Additionally, "there were some spelling errors and language presentation in the article which may lead to misinterpretation of the content by readers." This led to a number of inaccuracies in the minireview, too many to be addressed through a correction. Therefore, the authors were allowed to submit an entire, corrected manuscript for consideration to the journal. The manuscript was editorially reviewed and subsequently accepted.
REFERENCES
- 1. Te Velthuis AJW, Fodor E. 2016. Influenza virus RNA polymerase: insights into the mechanisms of viral RNA synthesis. Nat Rev Microbiol 14:479–493. doi: 10.1038/nrmicro.2016.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Long JS, Mistry B, Haslam SM, Barclay WS. 2019. Host and viral determinants of influenza A virus species specificity. Nat Rev Microbiol 17:67–81. doi: 10.1038/s41579-018-0115-z [DOI] [PubMed] [Google Scholar]
- 3. Saunders-Hastings PR, Krewski D. 2016. Reviewing the history of pandemic influenza: understanding patterns of emergence and transmission. Pathogens 5:66. doi: 10.3390/pathogens5040066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO . 2023. Influenza (seasonal). Available from: https://www.who.int/health-topics/influenza-seasonal
- 5. CDC . 2023. Specific avian flu viruses. Available from: https://www.cdc.gov/flu/avianflu/specific-flu-viruses.htm
- 6. CDC . 2023. Interim guidance on the use of antiviral medications for treatment of human infections with novel influenza a viruses associated with severe human disease. Available from: https://www.cdc.gov/flu/avianflu/novel-av-treatment-guidance.htm
- 7. Koszalka P, Tilmanis D, Hurt AC. 2017. Influenza antivirals currently in late-phase clinical trial. Influenza Other Respir Viruses 11:240–246. doi: 10.1111/irv.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hayden FG, Shindo N. 2019. Influenza virus polymerase inhibitors in clinical development. Curr Opin Infect Dis 32:176–186. doi: 10.1097/QCO.0000000000000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Hanlon R, Shaw ML. 2019. Baloxavir marboxil: the new influenza drug on the market. Curr Opin Virol 35:14–18. doi: 10.1016/j.coviro.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 10. CDC . 2023. Influenza antiviral medications: summary for clinicians. Available from: https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm
- 11. Jing X, Ma C, Ohigashi Y, Oliveira FA, Jardetzky TS, Pinto LH, Lamb RA. 2008. Functional studies indicate amantadine binds to the pore of the influenza A virus M2 proton-selective ion channel. Proc Natl Acad Sci U S A 105:10967–10972. doi: 10.1073/pnas.0804958105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang C, Takeuchi K, Pinto LH, Lamb RA. 1993. Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block. J Virol 67:5585–5594. doi: 10.1128/JVI.67.9.5585-5594.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alves Galvão MG, Rocha Crispino Santos MA, Alves da Cunha AJL. 2014. Amantadine and rimantadine for influenza A in children and the elderly. Cochrane Database Syst Rev 2014:CD002745. doi: 10.1002/14651858.CD002745.pub4 [DOI] [PubMed] [Google Scholar]
- 14. Bryson YJ, Monahan C, Pollack M, Shields WD. 1980. A prospective double-blind study of side effects associated with the administration of amantadine for influenza A virus prophylaxis. J Infect Dis 141:543–547. doi: 10.1093/infdis/141.5.543 [DOI] [PubMed] [Google Scholar]
- 15. Gravenstein S, Drinka P, Osterweil D, Schilling M, Krause P, Elliott M, Shult P, Ambrozaitis A, Kandel R, Binder E, Hammond J, McElhaney J, Flack N, Daly J, Keene O. 2005. Inhaled zanamivir versus rimantadine for the control of influenza in a highly vaccinated long-term care population. J Am Med Dir Assoc 6:359–366. doi: 10.1016/j.jamda.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 16. Jefferson T, Demicheli V, Di Pietrantonj C, Rivetti D. 2006. Amantadine and rimantadine for influenza A in adults. Cochrane Database Syst Rev 2006:CD001169. doi: 10.1002/14651858.CD001169.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bright RA, Medina M, Xu X, Perez-Oronoz G, Wallis TR, Davis XM, Povinelli L, Cox NJ, Klimov AI. 2005. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet 366:1175–1181. doi: 10.1016/S0140-6736(05)67338-2 [DOI] [PubMed] [Google Scholar]
- 18. Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. 2006. Adamantane resistance among influenza A viruses isolated early during the 2005-2006 influenza season in the United States. JAMA 295:891–894. doi: 10.1001/jama.295.8.joc60020 [DOI] [PubMed] [Google Scholar]
- 19. Deyde VM, Xu X, Bright RA, Shaw M, Smith CB, Zhang Y, Shu Y, Gubareva LV, Cox NJ, Klimov AI. 2007. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J Infect Dis 196:249–257. doi: 10.1086/518936 [DOI] [PubMed] [Google Scholar]
- 20. Babu YS, Chand P, Bantia S, Kotian P, Dehghani A, El-Kattan Y, Lin TH, Hutchison TL, Elliott AJ, Parker CD, Ananth SL, Horn LL, Laver GW, Montgomery JA. 2000. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem 43:3482–3486. doi: 10.1021/jm0002679 [DOI] [PubMed] [Google Scholar]
- 21. Meindl P, Bodo G, Palese P, Schulman J, Tuppy H. 1974. Inhibition of neuraminidase activity by derivatives of 2-deoxy-2,3-dehydro-N-acetylneuraminic acid. Virology 58:457–463. doi: 10.1016/0042-6822(74)90080-4 [DOI] [PubMed] [Google Scholar]
- 22. von Itzstein M, Wu W-Y, Kok GB, Pegg MS, Dyason JC, Jin B, Van Phan T, Smythe ML, White HF, Oliver SW, Colman PM, Varghese JN, Ryan DM, Woods JM, Bethell RC, Hotham VJ, Cameron JM, Penn CR. 1993. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 363:418–423. doi: 10.1038/363418a0 [DOI] [PubMed] [Google Scholar]
- 23. Moscona A. 2005. Neuraminidase inhibitors for influenza. N Engl J Med 353:1363–1373. doi: 10.1056/NEJMra050740 [DOI] [PubMed] [Google Scholar]
- 24. Heneghan CJ, Onakpoya I, Thompson M, Spencer EA, Jones M, Jefferson T. 2014. Zanamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ 348:g2547. doi: 10.1136/bmj.g2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jefferson T, Jones M, Doshi P, Del Mar C. 2009. Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis. BMJ 339:b5106. doi: 10.1136/bmj.b5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bradley JS, Blumer JL, Romero JR, Michaels MG, Munoz FM, Kimberlin DW, Pahud B, DeBiasi RL, Yamamoto G, Roberts G, Hossain M, Shortino D, Yates PJ, Adams B, Peppercorn A. 2017. Intravenous zanamivir in hospitalized patients with influenza. Pediatrics 140:e20162727. doi: 10.1542/peds.2016-2727 [DOI] [PubMed] [Google Scholar]
- 27. Kim CU, Lew W, Williams MA, Liu H, Zhang L, Swaminathan S, Bischofberger N, Chen MS, Mendel DB, Tai CY, Laver WG, Stevens RC. 1997. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J Am Chem Soc 119:681–690. doi: 10.1021/ja963036t [DOI] [PubMed] [Google Scholar]
- 28. Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. 2018. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 66:1492–1500. doi: 10.1093/cid/cix1040 [DOI] [PubMed] [Google Scholar]
- 29. Nicholson K, Aoki F, Osterhaus A, Trottier S, Carewicz O, Mercier C, Rode A, Kinnersley N, Ward P. 2000. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet 355:1845–1850. doi: 10.1016/S0140-6736(00)02288-1 [DOI] [PubMed] [Google Scholar]
- 30. Schirmer P, Holodniy M. 2009. Oseltamivir for treatment and prophylaxis of influenza infection. Expert Opin Drug Saf 8:357–371. doi: 10.1517/14740330902840519 [DOI] [PubMed] [Google Scholar]
- 31. Dobson J, Whitley RJ, Pocock S, Monto AS. 2015. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet 385:1729–1737. doi: 10.1016/S0140-6736(14)62449-1 [DOI] [PubMed] [Google Scholar]
- 32. Kimberlin DW, Acosta EP, Prichard MN, Sánchez PJ, Ampofo K, Lang D, Ashouri N, Vanchiere JA, Abzug MJ, Abughali N, Caserta MT, Englund JA, Sood SK, Spigarelli MG, Bradley JS, Lew J, Michaels MG, Wan W, Cloud G, Jester P, Lakeman FD, Whitley RJ, National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group . 2013. Oseltamivir pharmacokinetics, dosing, and resistance among children aged <2 years with influenza. J Infect Dis 207:709–720. doi: 10.1093/infdis/jis765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bueno M, Calvo C, Méndez-Echevarría A, de José MI, Santos M, Carrasco J, Tovizi M, Guillén S, de Blas A, Llorente M, Tarrago A, Escosa L, Cilleruelo MJ, Tomatis C, Blazquez D, Otheo E, Mazagatos D, García-García ML. 2013. Oseltamivir treatment for influenza in hospitalized children without underlying diseases. Pediatr Infect Dis J 32:1066–1069. doi: 10.1097/INF.0b013e31829be4bc [DOI] [PubMed] [Google Scholar]
- 34. Webster D, Li Y, Bastien N, Garceau R, Hatchette TF. 2011. Oseltamivir-resistant pandemic H1N1 influenza. CMAJ 183:E420–2. doi: 10.1503/cmaj.100313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee N, Hurt AC. 2018. Neuraminidase inhibitor resistance in influenza: a clinical perspective. Curr Opin Infect Dis 31:520–526. doi: 10.1097/QCO.0000000000000498 [DOI] [PubMed] [Google Scholar]
- 36. Bantia S, Arnold CS, Parker CD, Upshaw R, Chand P. 2006. Anti-influenza virus activity of peramivir in mice with single Intramuscular injection. Antiviral Res 69:39–45. doi: 10.1016/j.antiviral.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 37. Hata A, Akashi-Ueda R, Takamatsu K, Matsumura T. 2014. Safety and efficacy of peramivir for influenza treatment. Drug Des Devel Ther 8:2017–2038. doi: 10.2147/DDDT.S46654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scott LJ. 2018. Peramivir: a review in uncomplicated influenza. Drugs 78:1363–1370. doi: 10.1007/s40265-018-0981-8 [DOI] [PubMed] [Google Scholar]
- 39. Kohno S, Kida H, Mizuguchi M, Shimada J, S-021812 Clinical Study Group . 2010. Efficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infection. Antimicrob Agents Chemother 54:4568–4574. doi: 10.1128/AAC.00474-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen J-Y, Wei S-K, Lai C-C, Weng T-S, Wang H-H. 2020. A meta-analysis comparing the efficacy and safety of peramivir with other neuraminidase inhibitors for influenza treatment. Medicina (Kaunas) 56:63. doi: 10.3390/medicina56020063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hsieh Y, Dugas AF, LoVecchio F, McBryde B, Ricketts EP, Saliba‐Shaw K, Rothman RE. 2021. Intravenous peramivir vs oral oseltamivir in high-risk emergency department patients with influenza: results from a pilot randomized controlled study. Influenza Resp Viruses 15:121–131. doi: 10.1111/irv.12794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heo Y-A. 2018. Baloxavir: first global approval. Drugs 78:693–697. doi: 10.1007/s40265-018-0899-1 [DOI] [PubMed] [Google Scholar]
- 43. O’Hanlon R, Shaw ML. 2019. Baloxavir marboxil: the new influenza drug on the market. Curr Opin Virol 35:14–18. doi: 10.1016/j.coviro.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 44. FDA . 2023. FDA approves new drug to treat influenza. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treat-influenza
- 45. Uehara T, Hayden FG, Kawaguchi K, Omoto S, Hurt AC, De Jong MD, Hirotsu N, Sugaya N, Lee N, Baba K, Shishido T, Tsuchiya K, Portsmouth S, Kida H. 2020. Treatment-emergent influenza variant viruses with reduced baloxavir susceptibility: impact on clinical and virologic outcomes in uncomplicated influenza. J Infect Dis 221:346–355. doi: 10.1093/infdis/jiz244 [DOI] [PubMed] [Google Scholar]
- 46. Omoto S, Speranzini V, Hashimoto T, Noshi T, Yamaguchi H, Kawai M, Kawaguchi K, Uehara T, Shishido T, Naito A, Cusack S. 2018. Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci Rep 8:9633. doi: 10.1038/s41598-018-27890-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hayden FG, Sugaya N, Hirotsu N, Lee N, de Jong MD, Hurt AC, Ishida T, Sekino H, Yamada K, Portsmouth S, Kawaguchi K, Shishido T, Arai M, Tsuchiya K, Uehara T, Watanabe A, Baloxavir Marboxil Investigators Group . 2018. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 379:913–923. doi: 10.1056/NEJMoa1716197 [DOI] [PubMed] [Google Scholar]
- 48. Ison MG, Portsmouth S, Yoshida Y, Shishido T, Mitchener M, Tsuchiya K, Uehara T, Hayden FG. 2020. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis 20:1204–1214. doi: 10.1016/S1473-3099(20)30004-9 [DOI] [PubMed] [Google Scholar]
- 49. Kumar D, Ison MG, Mira J-P, Welte T, Hwan Ha J, Hui DS, Zhong N, Saito T, Katugampola L, Collinson N, Williams S, Wildum S, Ackrill A, Clinch B, Lee N. 2022. Combining baloxavir marboxil with standard-of-care neuraminidase inhibitor in patients hospitalised with severe influenza (FLAGSTONE): a randomised, parallel-group, double-blind, placebo-controlled, superiority trial. Lancet Infect Dis 22:718–730. doi: 10.1016/S1473-3099(21)00469-2 [DOI] [PubMed] [Google Scholar]
- 50. Koshimichi H, Retout S, Cosson V, Duval V, De Buck S, Tsuda Y, Ishibashi T, Wajima T. 2020. Population pharmacokinetics and exposure-response relationships of baloxavir marboxil in influenza patients at high risk of complications. Antimicrob Agents Chemother 64:e00119–00120. doi: 10.1128/AAC.00119-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gagarinova VM, Ignat’eva GS, Sinitskaia LV, Ivanova AM, Rodina MA, Tur’eva AV. 1993. [The new chemical preparation arbidol: its prophylactic efficacy during influenza epidemics]. Zh Mikrobiol Epidemiol Immunobiol:40–43. [PubMed] [Google Scholar]
- 52. Blaising J, Polyak SJ, Pécheur E-I. 2014. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res 107:84–94. doi: 10.1016/j.antiviral.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fink SL, Vojtech L, Wagoner J, Slivinski NSJ, Jackson KJ, Wang R, Khadka S, Luthra P, Basler CF, Polyak SJ. 2018. The antiviral drug arbidol inhibits Zika virus. Sci Rep 8:8989. doi: 10.1038/s41598-018-27224-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang X, Cao R, Zhang H, Liu J, Xu M, Hu H, Li Y, Zhao L, Li W, Sun X, Yang X, Shi Z, Deng F, Hu Z, Zhong W, Wang M. 2020. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov 6:28. doi: 10.1038/s41421-020-0169-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hulseberg CE, Fénéant L, Szymańska-de Wijs KM, Kessler NP, Nelson EA, Shoemaker CJ, Schmaljohn CS, Polyak SJ, White JM. 2019. Arbidol and other low-molecular-weight drugs that inhibit Lassa and Ebola viruses. J Virol 93:e02185–02118. doi: 10.1128/JVI.02185-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haviernik J, Štefánik M, Fojtíková M, Kali S, Tordo N, Rudolf I, Hubálek Z, Eyer L, Ruzek D. 2018. Arbidol (Umifenovir): a broad-spectrum antiviral drug that inhibits medically important arthropod-borne flaviviruses. Viruses 10:184. doi: 10.3390/v10040184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kadam RU, Wilson IA. 2017. Structural basis of influenza virus fusion inhibition by the antiviral drug arbidol. Proc Natl Acad Sci U S A 114:206–214. doi: 10.1073/pnas.1617020114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zeng L-Y, Yang J, Liu S. 2017. Investigational hemagglutinin-targeted influenza virus inhibitors. Expert Opin Investig Drugs 26:63–73. doi: 10.1080/13543784.2017.1269170 [DOI] [PubMed] [Google Scholar]
- 59. Wang Y, Ding Y, Yang C, Li R, Du Q, Hao Y, Li Z, Jiang H, Zhao J, Chen Q, Yang Z, He Z. 2017. Inhibition of the infectivity and inflammatory response of influenza virus by arbidol hydrochloride in vitro and in vivo (mice and ferret). Biomed Pharmacother 91:393–401. doi: 10.1016/j.biopha.2017.04.091 [DOI] [PubMed] [Google Scholar]
- 60. Leneva IA, Falynskova IN, Makhmudova NR, Poromov AA, Yatsyshina SB, Maleev VV. 2019. Umifenovir susceptibility monitoring and characterization of influenza viruses isolated during ARBITR clinical study. J Med Virol 91:588–597. doi: 10.1002/jmv.25358 [DOI] [PubMed] [Google Scholar]
- 61. Wang MZ, Cai BQ, Li LY, Lin JT, Su N, Yu HX, Gao H, Zhao JZ, Liu L. 2004. [Efficacy and safety of Arbidol in treatment of naturally acquired influenza]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 26:289–293. [PubMed] [Google Scholar]
- 62. Kolobukhina LV, Malinovskaia VV, Gatich RZ, Merkulova LN, Burtseva EI, Isaeva EI, Parshina OV, Guseva TS, Orlova TG, Voronina FV. 2008. [Evaluation of the efficacy of wiferon and arbidol in adult influenza]. Vopr Virusol 53:31–33. [PubMed] [Google Scholar]
- 63. Kiselev OI, Maleev VV, Deeva EG, Leneva IA, Selkova EP, Osipova EA, Obukhov AA, Nadorov SA, Kulikova EV. 2015. [Clinical efficacy of arbidol (umifenovir) in the therapy of influenza in adults: preliminary results of the multicenter double-blind randomized placebo-controlled study ARBITR]. Ter Arkh 87:88–96. doi: 10.17116/terarkh201587188-96 [DOI] [PubMed] [Google Scholar]
- 64. Pshenichnaya NY, Bulgakova VA, Lvov NI, Poromov AA, Selkova EP, Grekova AI, Shestakova IV, Maleev VV, Leneva IA. 2019. Clinical efficacy of umifenovir in influenza and ARVI (study ARBITR). Terapevticheskii arkhiv 91:56–63. doi: 10.26442/00403660.2019.03.000127 [DOI] [PubMed] [Google Scholar]
- 65. Furuta Y, Komeno T, Nakamura T. 2017. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci 93:449–463. doi: 10.2183/pjab.93.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Furuta Y, Takahashi K, Kuno-Maekawa M, Sangawa H, Uehara S, Kozaki K, Nomura N, Egawa H, Shiraki K. 2005. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother 49:981–986. doi: 10.1128/AAC.49.3.981-986.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. 2013. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 100:446–454. doi: 10.1016/j.antiviral.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shiraki K, Daikoku T. 2020. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther 209:107512. doi: 10.1016/j.pharmthera.2020.107512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Brendish NJ, Clark TW. 2017. Antiviral treatment of severe non-influenza respiratory virus infection. Curr Opin Infect Dis 30:573–578. doi: 10.1097/QCO.0000000000000410 [DOI] [PubMed] [Google Scholar]
- 70. Sissoko D, Laouenan C, Folkesson E, M’Lebing A-B, Beavogui A-H, Baize S, Camara A-M, Maes P, Shepherd S, Danel C, et al. 2016. Experimental treatment with favipiravir for Ebola virus disease (the JIKI trial): a historically controlled, single-arm proof-of-concept trial in guinea. PLoS Med 13:e1001967. doi: 10.1371/journal.pmed.1001967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Costanzo M, De Giglio MAR, Roviello GN. 2020. SARS-CoV-2: recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr Med Chem 27:4536–4541. doi: 10.2174/0929867327666200416131117 [DOI] [PubMed] [Google Scholar]
- 72. Wang Y, Fan G, Salam A, Horby P, Hayden FG, Chen C, Pan J, Zheng J, Lu B, Guo L, Wang C, Cao B. 2020. Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection. J Infect Dis 221:1688–1698. doi: 10.1093/infdis/jiz656 [DOI] [PubMed] [Google Scholar]
- 73. Wang Y, Zhong W, Salam A, Tarning J, Zhan Q, Huang J, Weng H, Bai C, Ren Y, Yamada K, et al. 2020. Phase 2a, open-label, dose-escalating, multi-center pharmacokinetic study of favipiravir (T-705) in combination with oseltamivir in patients with severe influenza. eBioMedicine 62:103125. doi: 10.1016/j.ebiom.2020.103125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McKimm-Breschkin JL. 2013. Influenza neuraminidase inhibitors: antiviral action and mechanisms of resistance. Influenza Other Respir Viruses 7 Suppl 1:25–36. doi: 10.1111/irv.12047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vavricka CJ, Li Q, Wu Y, Qi J, Wang M, Liu Y, Gao F, Liu J, Feng E, He J, Wang J, Liu H, Jiang H, Gao GF. 2011. Structural and functional analysis of laninamivir and its octanoate prodrug reveals group specific mechanisms for influenza NA inhibition. PLoS Pathog 7:e1002249. doi: 10.1371/journal.ppat.1002249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Koyama K, Takahashi M, Nakai N, Takakusa H, Murai T, Hoshi M, Yamamura N, Kobayashi N, Okazaki O. 2010. Pharmacokinetics and disposition of CS-8958, a long-acting prodrug of the novel neuraminidase inhibitor laninamivir in rats. Xenobiotica 40:207–216. doi: 10.3109/00498250903447691 [DOI] [PubMed] [Google Scholar]
- 77. Adams SE, Lugovtsev VY, Kan A, Bovin NV, Donnelly RP, Ilyushina NA. 2020. Laninamivir-interferon Lambda 1 combination treatment promotes resistance by influenza A virus more rapidly than laninamivir alone. Antimicrob Agents Chemother 64:e00301–00320. doi: 10.1128/AAC.00301-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nakano T, Ishiwada N, Sumitani T, Uemori M, Isobe K, for the Laninamivir Prophylaxis Study Group . 2016. Inhaled laninamivir octanoate as prophylaxis for influenza in children. Pediatrics 138:e20160109. doi: 10.1542/peds.2016-0109 [DOI] [PubMed] [Google Scholar]
- 79. Ishizuka H, Yoshiba S, Okabe H, Yoshihara K. 2010. Clinical pharmacokinetics of laninamivir, a novel long-acting neuraminidase inhibitor, after single and multiple inhaled doses of its prodrug, CS-8958, in healthy male volunteers. J Clin Pharmacol 50:1319–1329. doi: 10.1177/0091270009356297 [DOI] [PubMed] [Google Scholar]
- 80. PMDA . 2021. Laninamivir octanoate hydrate (partial change approval). Available from: https://www.pmda.go.jp/files/000239716.pdf
- 81. Watanabe A, Chang S-C, Kim MJ, Chu DW-S, Ohashi Y, MARVEL Study Group . 2010. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis 51:1167–1175. doi: 10.1086/656802 [DOI] [PubMed] [Google Scholar]
- 82. Kawai N, Ikematsu H, Iwaki N, Kondou K, Hirotsu N, Kawashima T, Maeda T, Tanaka O, Doniwa K-I, Kashiwagi S. 2009. Clinical effectiveness of oseltamivir for influenza A(H1N1) virus with H274Y neuraminidase mutation. J Infect 59:207–212. doi: 10.1016/j.jinf.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 83. Higashiguchi M, Matsumoto T, Fujii T. 2018. A meta-analysis of laninamivir octanoate for treatment and prophylaxis of influenza. Antivir Ther 23:157–165. doi: 10.3851/IMP3189 [DOI] [PubMed] [Google Scholar]
- 84. Federation, M.o.H.o.t.R . 2021. Information about ingavirin. Available from: https://grls.rosminzdrav.ru/Grls_View_v2.aspx?routingGuid=02df9760-85ad-42e5-a6a2-323d1bcd5d35&t=
- 85. Leneva IA, Fediakina IT, Eropkin MI, Gudova NV, Romanovskaia AA, Danilenko DM, Vinogradova SM, Lepeshkin AI, Shestopalov AM. 2010. [Study of the antiviral activity of Russian anti-influenza agents in cell culture and animal models]. Vopr Virusol 55:19–27. [PubMed] [Google Scholar]
- 86. Shishkina LN, Nebol’sin VE, Kabanov AS, Skarnovich MO, Mazurkova NA, Sergeev AA, Serova OA, Stavskiĭ EA, Drozdov IG. 2011. [In vitro and in vivo efficacy of Ingavirin against strains of pandemic influenza virus A(H1N1/09)v]. Zh Mikrobiol Epidemiol Immunobiol:93–96. [PubMed] [Google Scholar]
- 87. Zarubaev VV, Garshinina AV, Kalinina NA, Shtro AA, Nebol’sin VE, Kiselev OI. 2010. [Antiviral activity of Ingavirin in experimental lethal influenza due to influenza virus B in albino mice]. Antibiot Khimioter 55:8–11. [PubMed] [Google Scholar]
- 88. Malakhov MP, Aschenbrenner LM, Smee DF, Wandersee MK, Sidwell RW, Gubareva LV, Mishin VP, Hayden FG, Kim DH, Ing A, Campbell ER, Yu M, Fang F. 2006. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob Agents Chemother 50:1470–1479. doi: 10.1128/AAC.50.4.1470-1479.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Moss RB, Hansen C, Sanders RL, Hawley S, Li T, Steigbigel RT. 2012. A phase II study of DAS181, a novel host directed antiviral for the treatment of influenza infection. Journal of Infectious Diseases 206:1844–1851. doi: 10.1093/infdis/jis622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zenilman JM, Fuchs EJ, Hendrix CW, Radebaugh C, Jurao R, Nayak SU, Hamilton RG, McLeod Griffiss J. 2015. Phase 1 clinical trials of Das181, an inhaled sialidase, in healthy adults. Antiviral Research 123:114–119. doi: 10.1016/j.antiviral.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nawrocki JW, Weiss SR, Davidson MH, Sprecher DL, Schwartz SL, Lupien PJ, Jones PH, Haber HE, Black DM. 1995. Reduction of LDL cholesterol by 25% to 60% in patients with primary hypercholesterolemia by atorvastatin, a new HMG-CoA reductase inhibitor. Arterioscler Thromb Vasc Biol 15:678–682. doi: 10.1161/01.atv.15.5.678 [DOI] [PubMed] [Google Scholar]
- 92. Episcopio D, Aminov S, Benjamin S, Germain G, Datan E, Landazuri J, Lockshin RA, Zakeri Z. 2019. Atorvastatin restricts the ability of influenza virus to generate lipid droplets and severely suppresses the replication of the virus. FASEB J 33:9516–9525. doi: 10.1096/fj.201900428RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. White Jr AC. 2004. Nitazoxanide: a new broad spectrum antiparasitic agent. Expert Rev Anti-Infect Ther 2:43–49. doi: 10.1586/14787210.2.1.43 [DOI] [PubMed] [Google Scholar]
- 94. Siddiq DM, Koo HL, Adachi JA, Viola GM. 2011. Norovirus gastroenteritis successfully treated with nitazoxanide. J Infect 63:394–397. doi: 10.1016/j.jinf.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rossignol J-F. 2016. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J Infect Public Health 9:227–230. doi: 10.1016/j.jiph.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rocco PRM, Silva PL, Cruz FF, Melo-Junior MAC, Tierno PFGMM, Moura MA, De Oliveira LFG, Lima CC, Dos Santos EA, Junior WF, et al. 2021. Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial. Eur Respir J 58:2003725. doi: 10.1183/13993003.03725-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Piacentini S, La Frazia S, Riccio A, Pedersen JZ, Topai A, Nicolotti O, Rossignol J-F, Santoro MG. 2018. Nitazoxanide inhibits paramyxovirus replication by targeting the Fusion protein folding: role of glycoprotein-specific thiol oxidoreductase ERp57. Sci Rep 8:10425. doi: 10.1038/s41598-018-28172-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rossignol JF, La Frazia S, Chiappa L, Ciucci A, Santoro MG. 2009. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. J Biol Chem 284:29798–29808. doi: 10.1074/jbc.M109.029470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tilmanis D, van Baalen C, Oh DY, Rossignol J-F, Hurt AC. 2017. The susceptibility of circulating human influenza viruses to tizoxanide, the active metabolite of nitazoxanide. Antiviral Res 147:142–148. doi: 10.1016/j.antiviral.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 100. Mifsud EJ, Tilmanis D, Oh DY, Ming-Kay Tai C, Rossignol J-F, Hurt AC. 2020. Prophylaxis of ferrets with nitazoxanide and oseltamivir combinations is more effective at reducing the impact of influenza a virus infection compared to oseltamivir monotherapy. Antiviral Res 176:104751. doi: 10.1016/j.antiviral.2020.104751 [DOI] [PubMed] [Google Scholar]
- 101. Haffizulla J, Hartman A, Hoppers M, Resnick H, Samudrala S, Ginocchio C, Bardin M, Rossignol J-F, US Nitazoxanide Influenza Clinical Study Group . 2014. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis 14:609–618. doi: 10.1016/S1473-3099(14)70717-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gamiño-Arroyo AE, Guerrero ML, McCarthy S, Ramírez-Venegas A, Llamosas-Gallardo B, Galindo-Fraga A, Moreno-Espinosa S, Roldán-Aragón Y, Araujo-Meléndez J, Hunsberger S, Ibarra-González V, Martínez-López J, García-Andrade LA, Kapushoc H, Holley HP, Smolskis MC, Ruiz-Palacios GM, Beigel JH, Mexico Emerging Infectious Diseases Clinical Research Network (LaRed) . 2019. Efficacy and safety of nitazoxanide in addition to standard of care for the treatment of severe acute respiratory illness. Clin Infect Dis 69:1903–1911. doi: 10.1093/cid/ciz100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Nguyen JT, Hoopes JD, Smee DF, Prichard MN, Driebe EM, Engelthaler DM, Le MH, Keim PS, Spence RP, Went GT. 2009. Triple combination of oseltamivir, amantadine, and ribavirin displays synergistic activity against multiple influenza virus strains in vitro. Antimicrob Agents Chemother 53:4115–4126. doi: 10.1128/AAC.00476-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nguyen JT, Smee DF, Barnard DL, Julander JG, Gross M, de Jong MD, Went GT. 2012. Efficacy of combined therapy with amantadine, oseltamivir, and ribavirin in vivo against susceptible and amantadine-resistant influenza A viruses. PLoS ONE 7:e31006. doi: 10.1371/journal.pone.0031006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chen L, Dou J, Su Z, Zhou H, Wang H, Zhou W, Guo Q, Zhou C. 2011. Synergistic activity of baicalein with ribavirin against influenza A (H1N1) virus infections in cell culture and in mice. Antiviral Res 91:314–320. doi: 10.1016/j.antiviral.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 106. Vanderlinden E, Vrancken B, Van Houdt J, Rajwanshi VK, Gillemot S, Andrei G, Lemey P, Naesens L. 2016. Distinct effects of T-705 (favipiravir) and ribavirin on influenza virus replication and viral RNA synthesis. Antimicrob Agents Chemother 60:6679–6691. doi: 10.1128/AAC.01156-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Seo S, Englund JA, Nguyen JT, Pukrittayakamee S, Lindegardh N, Tarning J, Tambyah PA, Renaud C, Went GT, de Jong MD, Boeckh MJ. 2013. Combination therapy with amantadine, oseltamivir and ribavirin for influenza A infection: safety and pharmacokinetics. Antivir Ther 18:377–386. doi: 10.3851/IMP2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Beigel JH, Bao Y, Beeler J, Manosuthi W, Slandzicki A, Dar SM, Panuto J, Beasley RL, Perez-Patrigeon S, Suwanpimolkul G, Losso MH, McClure N, Bozzolo DR, Myers C, Holley HP Jr, Hoopes J, Lane HC, Hughes MD, Davey RT, IRC003 Study Team . 2017. Oseltamivir, amantadine, and ribavirin combination antiviral therapy versus oseltamivir monotherapy for the treatment of influenza: a multicentre, double-blind, randomised phase 2 trial. Lancet Infect Dis 17:1255–1265. doi: 10.1016/S1473-3099(17)30476-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Clark MP, Ledeboer MW, Davies I, Byrn RA, Jones SM, Perola E, Tsai A, Jacobs M, Nti-Addae K, Bandarage UK, et al. 2014. Discovery of a novel, first-in-class, orally bioavailable azaindole inhibitor (VX-787) of influenza PB2. J Med Chem 57:6668–6678. doi: 10.1021/jm5007275 [DOI] [PubMed] [Google Scholar]
- 110. Byrn RA, Jones SM, Bennett HB, Bral C, Clark MP, Jacobs MD, Kwong AD, Ledeboer MW, Leeman JR, McNeil CF, Murcko MA, Nezami A, Perola E, Rijnbrand R, Saxena K, Tsai AW, Zhou Y, Charifson PS. 2015. Preclinical activity of VX-787, a first-in-class, orally bioavailable inhibitor of the influenza virus polymerase PB2 subunit. Antimicrob Agents Chemother 59:1569–1582. doi: 10.1128/AAC.04623-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Smee DF, Barnard DL, Jones SM. 2016. Activities of JNJ63623872 and oseltamivir against influenza A H1N1pdm and H3N2 virus infections in mice. Antiviral Research 136:45–50. doi: 10.1016/j.antiviral.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 112. O’Neil B, Ison MG, Hallouin-Bernard MC, Nilsson AC, Torres A, Wilburn JM, van Duijnhoven W, Van Dromme I, Anderson D, Deleu S, Kosoglou T, Vingerhoets J, Rossenu S, Leopold L. 2022. A phase 2 study of pimodivir (JNJ-63623872) in combination with oseltamivir in elderly and nonelderly adults hospitalized with influenza A infection: OPAL study. J Infect Dis 226:109–118. doi: 10.1093/infdis/jiaa376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Finberg RW, Lanno R, Anderson D, Fleischhackl R, van Duijnhoven W, Kauffman RS, Kosoglou T, Vingerhoets J, Leopold L. 2019. Phase 2B study of pimodivir (JNJ-63623872) as monotherapy or in combination with oseltamivir for treatment of acute uncomplicated seasonal influenza A. J Infect Dis 219:1026–1034. doi: 10.1093/infdis/jiy547 [DOI] [PubMed] [Google Scholar]
- 114. Te Velthuis AJW, Zubkova TG, Shaw M, Mehle A, Boltz D, Gmeinwieser N, Stammer H, Milde J, Müller L, Margitich V. 2021. Enisamium reduces influenza virus shedding and improves patient recovery by inhibiting viral RNA polymerase activity. Antimicrob Agents Chemother 65:e02605–02620. doi: 10.1128/AAC.02605-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Cocking D, Cinatl J, Boltz DA, Peng X, Johnson W, Muzzio M, Syarkevych O, Kostyuk G, Goy A, Mueller L, Margitich VI. 2018. Antiviral effect of a derivative of isonicotinic acid enisamium iodide (FAV00A) against influenza virus. Acta virologica 62:191–195. doi: 10.4149/av_2018_211 [DOI] [PubMed] [Google Scholar]
- 116. Zheng W, Fan W, Zhang S, Jiao P, Shang Y, Cui L, Mahesutihan M, Li J, Wang D, Gao GF, Sun L, Liu W. 2019. Naproxen exhibits broad anti-influenza virus activity in mice by impeding viral nucleoprotein nuclear export. Cell Rep 27:1875–1885. doi: 10.1016/j.celrep.2019.04.053 [DOI] [PubMed] [Google Scholar]
- 117. Hung IFN, To KKW, Chan JFW, Cheng VCC, Liu KSH, Tam A, Chan T-C, Zhang AJ, Li P, Wong T-L, Zhang R, Cheung MKS, Leung W, Lau JYN, Fok M, Chen H, Chan K-H, Yuen K-Y. 2017. Efficacy of clarithromycin-naproxen-oseltamivir combination in the treatment of patients hospitalized for influenza A(H3N2) infection: an open-label randomized, controlled. Chest 151:1069–1080. doi: 10.1016/j.chest.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 118. van Dongen MJP, Kadam RU, Juraszek J, Lawson E, Brandenburg B, Schmitz F, Schepens WBG, Stoops B, van Diepen HA, Jongeneelen M, et al. 2019. A small-molecule fusion inhibitor of influenza virus is orally active in mice. Science 363:eaar6221. doi: 10.1126/science.aar6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Byrd-Leotis L, Cummings RD, Steinhauer DA. 2017. The interplay between the host receptor and influenza virus hemagglutinin and neuraminidase. Int J Mol Sci 18:1541. doi: 10.3390/ijms18071541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, et al. 2013. New world bats harbor diverse influenza A viruses. PLoS Pathog 9:e1003657. doi: 10.1371/journal.ppat.1003657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wiley DC, Skehel JJ. 1987. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem 56:365–394. doi: 10.1146/annurev.bi.56.070187.002053 [DOI] [PubMed] [Google Scholar]
- 122. Lee PS, Yoshida R, Ekiert DC, Sakai N, Suzuki Y, Takada A, Wilson IA. 2012. Heterosubtypic antibody recognition of the influenza virus hemagglutinin receptor binding site enhanced by avidity. Proc Natl Acad Sci U S A 109:17040–17045. doi: 10.1073/pnas.1212371109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Stauffer S, Feng Y, Nebioglu F, Heilig R, Picotti P, Helenius A. 2014. Stepwise priming by acidic pH and A high K+ concentration is required for efficient uncoating of influenza A virus cores after penetration. J Virol 88:13029–13046. doi: 10.1128/JVI.01430-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Anderson CS, Ortega S, Chaves FA, Clark AM, Yang H, Topham DJ, DeDiego ML. 2017. Natural and directed antigenic drift of the H1 influenza virus hemagglutinin stalk domain. Sci Rep 7:14614. doi: 10.1038/s41598-017-14931-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ye Z, Liu T, Offringa DP, McInnis J, Levandowski RA. 1999. Association of influenza virus matrix protein with ribonucleoproteins. J Virol 73:7467–7473. doi: 10.1128/JVI.73.9.7467-7473.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Fodor E. 2013. The RNA polymerase of influenza a virus: mechanisms of viral transcription and replication. Acta Virol 57:113–122. doi: 10.4149/av_2013_02_113 [DOI] [PubMed] [Google Scholar]
- 127. Li Q, Sun X, Li Z, Liu Y, Vavricka CJ, Qi J, Gao GF. 2012. Structural and functional characterization of neuraminidase-like molecule N10 derived from bat influenza A virus. Proc Natl Acad Sci U S A 109:18897–18902. doi: 10.1073/pnas.1211037109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ciminski K, Ran W, Gorka M, Lee J, Malmlov A, Schinköthe J, Eckley M, Murrieta RA, Aboellail TA, Campbell CL, Ebel GD, Ma J, Pohlmann A, Franzke K, Ulrich R, Hoffmann D, García-Sastre A, Ma W, Schountz T, Beer M, Schwemmle M. 2019. Bat influenza viruses transmit among bats but are poorly adapted to non-bat species. Nat Microbiol 4:2298–2309. doi: 10.1038/s41564-019-0556-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Stadlbauer D, Zhu X, McMahon M, Turner JS, Wohlbold TJ, Schmitz AJ, Strohmeier S, Yu W, Nachbagauer R, Mudd PA, Wilson IA, Ellebedy AH, Krammer F. 2019. Broadly protective human antibodies that target the active site of influenza virus neuraminidase. Science 366:499–504. doi: 10.1126/science.aay0678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chen Y-Q, Wohlbold TJ, Zheng N-Y, Huang M, Huang Y, Neu KE, Lee J, Wan H, Rojas KT, Kirkpatrick E, Henry C, Palm A-KE, Stamper CT, Lan LY-L, Topham DJ, Treanor J, Wrammert J, Ahmed R, Eichelberger MC, Georgiou G, Krammer F, Wilson PC. 2018. Influenza infection in humans induces broadly cross-reactive and protective neuraminidase-reactive antibodies. Cell 173:417–429. doi: 10.1016/j.cell.2018.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. FDA . 2023. FDA approved drugs for influenza. Available from: https://www.fda.gov/drugs/information-drug-class/influenza-flu-antiviral-drugs-and-related-information#ApprovedDrugs
- 132. Lamb YN. 2020. Remdesivir: first approval. Drugs 80:1355–1363. doi: 10.1007/s40265-020-01378-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Syed YY. 2022. Molnupiravir: first approval. Drugs 82:455–460. doi: 10.1007/s40265-022-01684-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Brown AJ, Won JJ, Graham RL, Dinnon KH 3rd, Sims AC, Feng JY, Cihlar T, Denison MR, Baric RS, Sheahan TP. 2019. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res 169:104541. doi: 10.1016/j.antiviral.2019.104541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hayden FG, Lenk RP, Stonis L, Oldham-Creamer C, Kang LL, Epstein C. 2022. Favipiravir treatment of uncomplicated influenza in adults: results of two phase 3, randomized, double-blind, placebo-controlled trials. J Infect Dis 226:1790–1799. doi: 10.1093/infdis/jiac135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. FDA . 2022. New drug therapy approvals 2022
- 137. Liu Y, Retout S, Duval V, Jia J, Zou Y, Wang Y, Cosson V, Jolivet S, De Buck S. 2022. Pharmacokinetics, safety, and simulated efficacy of an influenza treatment, baloxavir marboxil, in Chinese individuals. Clin Transl Sci 15:1196–1203. doi: 10.1111/cts.13237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kim Y, Lee S, Kim Y, Jang I-J, Lee S. 2022. Pharmacokinetics and safety of a novel influenza treatment (baloxavir marboxil) in Korean subjects compared with Japanese subjects. Clin Transl Sci 15:422–432. doi: 10.1111/cts.13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Walsh PS, Schnadower D, Zhang Y, Ramgopal S, Shah SS, Wilson PM. 2022. Association of early oseltamivir with improved outcomes in hospitalized children with influenza, 2007-2020. JAMA Pediatr 176:e223261. doi: 10.1001/jamapediatrics.2022.3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Xu M, Cai T, Yue T, Zhang P, Huang J, Liu Q, Wang Y, Luo R, Li Z, Luo L, Ji C, Tan X, Zheng Y, Whitley R, De Clercq E, Yin Q, Li G. 2023. Comparative effectiveness of oseltamivir versus peramivir for hospitalized children (aged 0-5 years) with influenza infection. Int J Infect Dis. 128:157–165. doi: 10.1016/j.ijid.2022.12.043 [DOI] [PubMed] [Google Scholar]
- 141. Chan-Tack KM, Murray JS, Birnkrant DB. 2009. Use of ribavirin to treat influenza. N Engl J Med 361:1713–1714. doi: 10.1056/NEJMc0905290 [DOI] [PubMed] [Google Scholar]
- 142. Sidwell RW, Huffman JH, Khare GP, Allen LB, Witkowski JT, Robins RK. 1972. Broad-spectrum antiviral activity of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 177:705–706. doi: 10.1126/science.177.4050.705 [DOI] [PubMed] [Google Scholar]
- 143. Wu Q, Qian W, Sun X, Jiang S. 2022. Small-molecule inhibitors, immune checkpoint inhibitors, and more: FDA-approved novel therapeutic drugs for solid tumors from 1991 to 2021. J Hematol Oncol 15:143. doi: 10.1186/s13045-022-01362-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zhang A, Chaudhari H, Agung Y, D’Agostino MR, Ang JC, Tugg Y, Miller MS. 2022. Hemagglutinin stalk-binding antibodies enhance effectiveness of neuraminidase inhibitors against influenza via Fc-dependent effector functions. Cell Rep Med 3:100718. doi: 10.1016/j.xcrm.2022.100718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Grandea AG, Olsen OA, Cox TC, Renshaw M, Hammond PW, Chan-Hui P-Y, Mitcham JL, Cieplak W, Stewart SM, Grantham ML, Pekosz A, Kiso M, Shinya K, Hatta M, Kawaoka Y, Moyle M. 2010. Human antibodies reveal A protective EPITOPE that is highly conserved among human and nonhuman influenza A viruses. Proc Natl Acad Sci U S A 107:12658–12663. doi: 10.1073/pnas.0911806107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kirkpatrick E, Qiu X, Wilson PC, Bahl J, Krammer F. 2018. The influenza virus hemagglutinin head evolves faster than the stalk domain. Sci Rep 8:10432. doi: 10.1038/s41598-018-28706-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Guthmiller JJ, Han J, Utset HA, Li L, Lan LY-L, Henry C, Stamper CT, McMahon M, O’Dell G, Fernández-Quintero ML, et al. 2022. Broadly neutralizing antibodies target a haemagglutinin anchor epitope.. Nature 602:314–320. doi: 10.1038/s41586-021-04356-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Chen Y-Q, Lan LY-L, Huang M, Henry C, Wilson PC. 2019. Hemagglutinin stalk-reactive antibodies interfere with influenza virus neuraminidase activity by steric hindrance. J Virol 93:e01526–01518. doi: 10.1128/JVI.01526-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Throsby M, van den Brink E, Jongeneelen M, Poon LLM, Alard P, Cornelissen L, Bakker A, Cox F, van Deventer E, Guan Y, Cinatl J, Meulen J ter, Lasters I, Carsetti R, Peiris M, de Kruif J, Goudsmit J. 2008. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One 3:e3942. doi: 10.1371/journal.pone.0003942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ekiert DC, Friesen RHE, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJWM, Brandenburg B, Vogels R, Brakenhoff JPJ, Kompier R, Koldijk MH, Cornelissen LAHM, Poon LLM, Peiris M, Koudstaal W, Wilson IA, Goudsmit J. 2011. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333:843–850. doi: 10.1126/science.1204839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ekiert DC, Bhabha G, Elsliger M-A, Friesen RHE, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. 2009. Antibody recognition of a highly conserved influenza virus epitope. Science 324:246–251. doi: 10.1126/science.1171491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Han A, Czajkowski L, Rosas LA, Cervantes-Medina A, Xiao Y, Gouzoulis M, Lumbard K, Hunsberger S, Reed S, Athota R, Baus HA, Lwin A, Sadoff J, Taubenberger JK, Memoli MJ. 2021. Safety and efficacy of CR6261 in an influenza A H1N1 healthy human challenge model. Clin Infect Dis 73:e4260–e4268. doi: 10.1093/cid/ciaa1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Huang C, Venturi M, Majeed S, Moore MJ, Phogat S, Zhang M-Y, Dimitrov DS, Hendrickson WA, Robinson J, Sodroski J, Wyatt R, Choe H, Farzan M, Kwong PD. 2004. Structural basis of tyrosine sulfation and VH-gene usage in antibodies that recognize the HIV type 1 coreceptor-binding site on gp120. Proc Natl Acad Sci U S A 101:2706–2711. doi: 10.1073/pnas.0308527100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Tharakaraman K, Subramanian V, Viswanathan K, Sloan S, Yen H-L, Barnard DL, Leung YHC, Szretter KJ, Koch TJ, Delaney JC, Babcock GJ, Wogan GN, Sasisekharan R, Shriver Z. 2015. A broadly neutralizing human monoclonal antibody is effective against H7N9. Proc Natl Acad Sci U S A 112:10890–10895. doi: 10.1073/pnas.1502374112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Baranovich T, Jones JC, Russier M, Vogel P, Szretter KJ, Sloan SE, Seiler P, Trevejo JM, Webby RJ, Govorkova EA. 2016. The hemagglutinin stem-binding monoclonal antibody VIS410 controls influenza virus-induced acute respiratory distress syndrome. Antimicrob Agents Chemother 60:2118–2131. doi: 10.1128/AAC.02457-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Wollacott AM, Boni MF, Szretter KJ, Sloan SE, Yousofshahi M, Viswanathan K, Bedard S, Hay CA, Smith PF, Shriver Z, Trevejo JM. 2016. Safety and upper respiratory pharmacokinetics of the hemagglutinin stalk-binding antibody VIS410 support treatment and prophylaxis based on population modeling of seasonal influenza A outbreaks. EBioMedicine 5:147–155. doi: 10.1016/j.ebiom.2016.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Hershberger E, Sloan S, Narayan K, Hay CA, Smith P, Engler F, Jeeninga R, Smits S, Trevejo J, Shriver Z, Oldach D. 2019. Safety and efficacy of monoclonal antibody VIS410 in adults with uncomplicated influenza A infection: Results from a randomized, double-blind, phase-2, placebo-controlled study. EBioMedicine 40:574–582. doi: 10.1016/j.ebiom.2018.12.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Nakamura G, Chai N, Park S, Chiang N, Lin Z, Chiu H, Fong R, Yan D, Kim J, Zhang J, Lee WP, Estevez A, Coons M, Xu M, Lupardus P, Balazs M, Swem LR. 2013. An in vivo human-plasmablast enrichment technique allows rapid identification of therapeutic influenza A antibodies. Cell Host Microbe 14:93–103. doi: 10.1016/j.chom.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 159. Lim JJ, Deng R, Derby MA, Larouche R, Horn P, Anderson M, Maia M, Carrier S, Pelletier I, Burgess T, Kulkarni P, Newton E, Tavel JA. 2016. Two phase 1, randomized, double-blind, placebo-controlled, single-ascending-dose studies to investigate the safety, tolerability, and pharmacokinetics of an anti-influenza A virus monoclonal antibody, MHAA4549A, in healthy volunteers. Antimicrob Agents Chemother 60:5437–5444. doi: 10.1128/AAC.00607-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. McBride JM, Lim JJ, Burgess T, Deng R, Derby MA, Maia M, Horn P, Siddiqui O, Sheinson D, Chen-Harris H, Newton EM, Fillos D, Nazzal D, Rosenberger CM, Ohlson MB, Lambkin-Williams R, Fathi H, Harris JM, Tavel JA. 2017. Phase 2 randomized trial of the safety and efficacy of MHAA4549A, a broadly neutralizing monoclonal antibody, in a human influenza A virus challenge model. Antimicrob Agents Chemother 61:e01154–01117. doi: 10.1128/AAC.01154-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Lim JJ, Nilsson AC, Silverman M, Assy N, Kulkarni P, McBride JM, Deng R, Li C, Yang X, Nguyen A, Horn P, Maia M, Castro A, Peck MC, Galanter J, Chu T, Newton EM, Tavel JA. 2020. A phase 2 randomized, double-blind, placebo-controlled trial of MHAA4549A, a monoclonal antibody, plus oseltamivir in patients hospitalized with severe influenza A virus infection. Antimicrob Agents Chemother 64:e00352–00320. doi: 10.1128/AAC.00352-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kallewaard NL, Corti D, Collins PJ, Neu U, McAuliffe JM, Benjamin E, Wachter-Rosati L, Palmer-Hill FJ, Yuan AQ, Walker PA, et al. 2016. Structure and function analysis of an antibody recognizing all influenza A subtypes. Cell 166:596–608. doi: 10.1016/j.cell.2016.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ali SO, Takas T, Nyborg A, Shoemaker K, Kallewaard NL, Chiong R, Dubovsky F, Mallory RM. 2018. Evaluation of MEDI8852, an anti-influenza A monoclonal antibody, in treating acute uncomplicated influenza. Antimicrob Agents Chemother 62:e00694–00618. doi: 10.1128/AAC.00694-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Ramos EL, Mitcham JL, Koller TD, Bonavia A, Usner DW, Balaratnam G, Fredlund P, Swiderek KM. 2015. Efficacy and safety of treatment with an anti-m2e monoclonal antibody in experimental human influenza. J Infect Dis 211:1038–1044. doi: 10.1093/infdis/jiu539 [DOI] [PubMed] [Google Scholar]
- 165. Zebedee SL, Lamb RA. 1988. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol 62:2762–2772. doi: 10.1128/JVI.62.8.2762-2772.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Saravolatz LD, Depcinski S, Sharma M. 2023. Molnupiravir and nirmatrelvir-ritonavir: oral coronavirus disease 2019 antiviral drugs. Clin Infect Dis 76:165–171. doi: 10.1093/cid/ciac180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Scott C, Kankanala J, Foster TL, Goldhill DH, Bao P, Simmons K, Pingen M, Bentham M, Atkins E, Loundras E, Elderfield R, Claridge JK, Thompson J, Stilwell PR, Tathineni R, McKimmie CS, Targett-Adams P, Schnell JR, Cook GP, Evans S, Barclay WS, Foster R, Griffin S. 2020. Site-directed M2 proton channel inhibitors enable synergistic combination therapy for rimantadine-resistant pandemic influenza. PLoS Pathog 16:e1008716. doi: 10.1371/journal.ppat.1008716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Koszalka P, George A, Dhanasekaran V, Hurt AC, Subbarao K. 2022. Effect of baloxavir and oseltamivir in combination on infection with influenza viruses with PA/I38T or PA/E23K substitutions in the ferret model. mBio 13:e0105622. doi: 10.1128/mbio.01056-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Uchide N, Toyoda H. 2011. Antioxidant therapy as a potential approach to severe influenza-associated complications. Molecules 16:2032–2052. doi: 10.3390/molecules16032032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew A-A, Phoon MC, van Rooijen N, Chow VT. 2011. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol 179:199–210. doi: 10.1016/j.ajpath.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Busch-Petersen J, Carpenter DC, Burman M, Foley J, Hunsberger GE, Kilian DJ, Salmon M, Mayer RJ, Yonchuk JG, Tal-Singer R. 2017. Danirixin: a reversible and selective antagonist of the CXC chemokine receptor 2. J Pharmacol Exp Ther 362:338–346. doi: 10.1124/jpet.117.240705 [DOI] [PubMed] [Google Scholar]
- 172. Reijnders TDY, Saris A, Schultz MJ, van der Poll T. 2020. Immunomodulation by macrolides: therapeutic potential for critical care. Lancet Respir Med 8:619–630. doi: 10.1016/S2213-2600(20)30080-1 [DOI] [PubMed] [Google Scholar]
- 173. Abusalamah H, Reel JM, Lupfer CR. 2020. Pyruvate affects inflammatory responses of macrophages during influenza A virus infection. Virus Res 286:198088. doi: 10.1016/j.virusres.2020.198088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Khalili JS, Zhu H, Mak NSA, Yan Y, Zhu Y. 2020. Novel coronavirus treatment with ribavirin: groundwork for an evaluation concerning COVID-19. J Med Virol 92:740–746. doi: 10.1002/jmv.25798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Kabinger F, Stiller C, Schmitzová J, Dienemann C, Kokic G, Hillen HS, Höbartner C, Cramer P. 2021. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol 28:740–746. doi: 10.1038/s41594-021-00651-0 [DOI] [PMC free article] [PubMed] [Google Scholar]