Abstract
Fresh Wolffia globosa, the smallest flowering plant well-known for its favorable nutrient composition and rich content of bioactive compounds, was subjected to boiling, freeze–thawing, and mechanical crushing to reduce its excessive (95–96%) moisture level and consequent drying time. The resultant three wolffia matrixes were filtered through a plankton net to fractionate into the residue and the filtrate. The proximate composition, bioactive metabolites, antioxidant activity, and characterization of bioactive metabolites by LC-ESI-QTOF-MS/MS and Fourier transform infrared spectroscopy were made from oven-dried residues and filtrates. Among residues, crude protein (29.84%), crude lipid (5.77%), total carotenoids (TCC; 722.8 μg/g), and vitamin C (70.02 mg/100 g) were the highest (p < 0.05) for freeze–thawing against higher ash (7.99%), total phenolic content (TPC; 191.47 mg GAE g–1 dry weight), total flavonoid content (TFC; 91.54 mg QE g–1 dry weight), DPPH activity (47.46%), and ferric reducing antioxidant power (FRAP) activity (570.19 μmol FeSO4 equiv/mg) for the crushed counterpart and Chl-b in residues from boiling. No significant variation was evident in the total tannin content (TTC). Among filtrates, higher total phenolic content (773.29 mg GAE g–1 dry weight), TFC (392.77 mg QE g–1 dry weight), TTC (22.51 mg TAE g–1), and antioxidant activity as DPPH activity (66.46%) and FRAP (891.62 μmol FeSO4 equiv/mg) were evident for boiling, while that from crushing exhibited the highest TCC (1997.38 μg/g DM). LC-ESI-QTOF-MS/MS analysis identified 72 phenolic compounds with the maximum in residue (33) and filtrate (33) from freeze–thawing, followed by crushing (18 and 19) and boiling (14 and 13) in order, respectively. The results indicated that the predrying cell rupturing method significantly impacted quantitative, as well as qualitative compositions of residues and filtrates from fresh wolffia.
1. Introduction
Wolffia globosa (wolffia), belonging to the duckweed family Lemnaceae, is a tiny, rootless, free-floating plant with characteristic oval-shaped discs (diameter about 0.5–1.5 mm). It grows throughout the year at high turnover rates (doubling time 2–3 days) under tropical and subtropical conditions. Due to its favorable nutritional attributes, including abundance, high-quality protein and starch, i.e., no known antinutritional factors, it has been traditionally utilized as food by the local people of Southeast Asian countries, including Thailand, Laos, and Myanmar. Further, it is rich in a variety of bioactive metabolites, including flavonoids, antioxidants, polyunsaturated fatty acids, multiple vitamins, and minerals, which have varied industrial applications in human food, animal and fish feed, nutraceuticals, cosmetics, and pharmaceuticals.1−6 We have observed that fresh wolffia is also consumed voraciously by a large number of herbivorous–omnivorous fishes, including carps, barbs, tilapia, perches, and even catfishe starting in the early stages of their lives. Pradhan et al.3 reported that feeding Labeo rohita (rohu) fry (20 dph) exclusively with fresh wolffia resulted in remarkably high survival and growth rates compared to that fed on artificial feed under the semi-intensive culture system.
Considering that feed in intensified aquaculture is the single largest contributor to the feed cost as well as to the carbon footprint, the application of fresh wolffia, a locally producible renewable nutrient resource, has important implications for aquaculture sustainability, climate resilience, and the carbon footprint of fed aquaculture. However, while use in fresh form has merits, its extremely high moisture content (95–96%) makes it very bulky and poses great problems in its transport and storage by facilitating not only microbial spoilage and hydrolytic rancidities but also the degradation of antioxidants.7 Accordingly, it is imperative to dry the biomass to reduce the moisture contents quickly so as to preserve the qualitative as well as quantitative biochemical compositions. However, drying intact fresh biomass of wolffia poses great difficulties in the form of slow heat transfer, high energy requirements, elevated temperatures, and prolonged exposure time for evaporating the associated water.6 For that matter, evaporating 1 L of water kept at normal room temperature, viz., 25 °C, requires approximately 2900 kJ. Further, drying at elevated temperatures and prolonged exposure causes irreversible changes in the cell wall and the polysaccharide matrices, which hold the high moisture content of wolffia and also serve various other structural and physiological functions.10 Further, it also leads to the oxidation of bioactive compounds, including phenolics, vitamin C, and antioxidants, causing changes in their functionality.8 The low-temperature (50 °C) drying of products can reduce the degradation of product quality9 but inherently requires a longer time, hence the low turnover.
The rupturing of the cell walls of fresh wolffia will lead to an instant reduction in the moisture level and an alteration in the tissue structure, which is likely to substantially reduce subsequent drying time and, hence, improve drying efficiency as well as quality.10,11 In addition, pretreatments to rupture the cell walls and membranes may also reduce product quality degradation12 by preventing structural deformation during drying.12,13 On the other hand, however, the instant removal of bound water matrix due to cell wall ruptures may also carry with it macronutrients as well as bioactive compounds such as phenolics, flavonoids, vitamins, and minerals, leading to a loss of nutrients, i.e., a reduction in the quality of the resultant cake-matrix.
Multiple techniques have been used to rupture the cell walls and release intracellular compounds, including blending/mechanical crushing, boiling, freeze–thawing, and ultrasonication, causing osmotic pressure changes, microwaving, and autoclaving.14,15 The most popular pretreatment methods used by manufacturers include boiling, freeze–thawing, and mechanical crushing/blending, as they are among the most effective.16 It has been demonstrated that freeze–thawing pretreatment significantly enhanced the performance of different thermal drying processes (microwave drying, air drying, and microwave-vacuum drying). Recently, Wang et al.17 reported that freeze–thawing pretreatment effectively shortened the vacuum freeze-drying duration by 4–6 h and reduced energy consumption by 10.0–15.1 g/100 g compared with the traditional alkaline-dewaxing method. During the freeze–thaw treatment, ice crystals are pierced into the cell wall, causing water to be better able to exit the plant body since the ice crystals pierce the cell wall.18,19 Dhiman et al.20 described blanching as the result of solubilization of pectic polymers that play an important role in cell–cell adhesion, as well as depolymerization of these polymers.
To the best of our knowledge, no qualitative or quantitative information is available in nutrients of solid and liquid fractions subjected to different predrying cell rupture methods. In the present study, we evaluated the impact of three common low-technology and low-cost predrying methods, namely, boiling, freeze–thawing, and mechanical crushing/blending on proximate composition, select bioactive metabolites, antioxidant activities, and the characterization of bioactive metabolites in the resultant liquid and solid fractions of fresh wolffia.
2. Results and Discussion
2.1. Effect of Cell Wall Rupture Methods on Proximate Composition of Wolffia
The three cell wall rupture strategies, viz., boiling, freeze–thawing, and mechanical crushing, applied to 1 kg of fresh wolffia biomass released 560, 650, and 590 mL of cell-bound water, respectively. The corresponding quantities of obtained residues were 315.9 g (boiling), 325.8 g (freeze–thawing), and 356.9 g (mechanical crushing). The proximate analysis of residues and filtrates of wolffia is presented in Table 1. The moisture content in wolffia residues, obtained from various cell rupture methods, did not exhibit statistically significant variations (p > 0.05). However, the filtrate of mechanical crushing, crushed wolffia filtrate (CWF) exhibited a significantly high moisture content (9.25%) when compared to filtrates of boiling as well as of freeze–thawing. The crude protein content exhibited significant variations (p < 0.05) in the residues as well as in the filtrates, with significantly higher values of 29.84% in the thawed wolffia residue (TWR), followed by 24.35% in the crushed wolffia residue (CWR) and 19.55% in the boiled wolffia residue (BWR), which is the lowest. Among wolffia filtrates, the CWF showed a significantly (p < 0.05) higher crude protein (42.02%) content, followed by the boiled wolffia filtrate (BWF, 28.81%) and the thawed wolffia filtrate (TWF, 16.81%), which was the lowest. The lowest protein in the TWF might be due to partial cell wall rupture, unlike mechanical crushing, which releases less protein in the TWF.21 Sørensen et al.22 stated that breaking cell walls facilitated the release of protein. The highest protein content in the CWF may possibly be due to completer and more aggressive cell rupture in mechanical crushing when compared to freeze–thawing and boiling. The higher crude protein in the residue of freeze–thawing also indicated the lowest degree of release of protein from the cell. Crude lipid was significantly higher (5.77%) in the TWR, while the lowest was reported (3.60%) in the BWR. In the wolffia filtrate, crude lipid was significantly (p < 0.05) higher in the BWF (2.3%), while it was the lowest in the TWF (0.13%). It is reported that different disruption methods based on mechanical or nonmechanical methods may have different effects.23 The CWR (7.99%) had a significantly higher concentration of ash content, followed by the TWR (7.19%) and the BWR (6.46%). The leaching of mineral compounds into boiling water may explain the reduction in the ash content of BWR samples.
Table 1. Proximate Composition of Residues and Filtrates of Wolffia Subjected to Different Cell Rupture Methods (Dry Matter Basis)a.
| parameters | wolffia
residue |
wolffia
filtrate |
||||
|---|---|---|---|---|---|---|
| BWR | TWR | CWR | BWF | TWF | CWF | |
| moisture (%) | 6.63 ± 0.17a | 7.08 ± 0.29a | 6.41 ± 0.22a | 7.47 ± 0.12b | 7.79 ± 0.12b | 9.25 ± 0.07a |
| crude protein (%) | 19.55 ± 0.00c | 29.84 ± 0.59a | 24.35 ± 0.69b | 28.81 ± 0.30b | 16.81 ± 0.84c | 42.02 ± 0.86a |
| crude lipid (%) | 3.60 ± 0.15c | 5.77 ± 0.09a | 4.10 ± 0.00b | 2.30 ± 0.06a | 0.13 ± 0.03c | 1.33 ± 0.03b |
| ash (%) | 6.69 ± 0.05c | 7.19 ± 0.02b | 7.68 ± 0.09a | 8.46 ± 0.21a | 4.28 ± 0.10b | 4.40 ± 0.08b |
Values represented are the means of triplicates ± SE (standard error). Different superscripts for a fraction in the same raw are significantly different (p < 0.05).
2.2. Effect of Cell Wall Rupture Methods on Bioactive Metabolites of Wolffia
The results of the total phenolic content (TPC), total flavonoid content (TFC), total tannin content (TTC), total carotenoid content (TCC), Chl-a, Chl-b, and vitamin C content are presented in Table 2. The phenolic compounds in plants have a high level of redox stability and are therefore capable of imparting antioxidant activity.24 In the current study, CWR exhibited a significantly higher (p < 0.05) value of TPC of 191.47 ± 1.93 mg GAE g–1, compared to the TWR and the BWR, which have lower values of 145.41 ± 0.66 and 138.73 ± 2.65 mg GAE g–1, respectively. The inactivation of oxidative enzymes by thermal pretreatment might lower the degradation of TPC in BWR samples. A reduced percentage of bioactive compounds was recorded by Raghunath et al.25 during boiling. While in wolffia filtrate samples, the BWF had the significantly higher (p < 0.05) TPC of 773.29 ± 4.72 mg GAE g–1, followed by 395.83 ± 4.58 and 312.54 ± 2.59 mg GAE g–1 in the TWF and CWF samples, respectively. Due to the decomposition of the tissue by heat treatments, cellular components and nutrients are able to migrate into the boiling water.26
Table 2. Bioactive Compounds of Wolffia Residues and Filtrates Subjected to Different Cell Rupture Methodsa.
| parameters | wolffia
residue |
wolffia
filtrate |
||||
|---|---|---|---|---|---|---|
| BWR | TWR | CWR | BWF | TWF | CWF | |
| TPC (mg GAE g–1) | 138.73 ± 2.65b | 145.41 ± 0.66b | 191.47 ± 1.93a | 773.29 ± 4.72a | 395.83 ± 4.58b | 312.54 ± 2.59c |
| TFC (mg QE g–1) | 53.58 ± 6.02b | 41.88 ± 2.50b | 91.54 ± 3.95a | 392.77 ± 4.23a | 169.89 ± 3.25b | 157.46 ± 3.07c |
| TTC (mg TAE g–1) | 7.36 ± 0.97a | 8.24 ± 1.61a | 6.81 ± 0.32a | 22.51 ± 0.26a | 14.37 ± 0.40b | 8.73 ± 0.08c |
| TCC (μg/g sample) | 662.37 ± 26.39b | 722.84 ± 4.72a | 352.82 ± 1.24c | 365.82 ± 13.40b | 342.58 ± 0.85b | 1997.38 ± 4.06a |
| Chl-a (μg/g sample) | 16.14 ± 0.36a | 16.71 ± 0.25a | 5.55 ± 0.15b | 3.22 ± 0.16b | 2.64 ± 0.08c | 26.88 ± 0.10a |
| Chl-b (μg/g sample) | 6.05 ± 0.32a | 4.45 ± 0.12b | 3.67 ± 0.14c | 3.81 ± 0.18b | 3.86 ± 0.03b | 18.52 ± 0.39a |
| vitamin C (mg 100 g–1) | 39.57 ± 3.04c | 70.02 ± 3.04a | 57.84 ± 3.04b | ND | ND | ND |
Values represented are the means of triplicates ± SE (standard error). Different superscripts for a fraction in the same raw are significantly different (p < 0.05). ND; not determined.
The flavonoids (e.g., quercetin, kaempferol, catechins, and anthocyanins) have antioxidant and anti-inflammatory properties.16 In this study, the TFC was reported to be significantly (p < 0.05) higher, 91.54 ± 3.95 mg QE g–1, in the CWR sample. However, no statistically significant difference was observed between the TWR and the BWR, as indicated in Table 2. The reason for the higher flavonoid content in CWR might be the small particle size during mechanical crushing.27 Becker et al.28 stated that grinding Hieracium pilosella L. increases the content of identified flavonoids and phenolics. Moreover, it was also reported that freeze–thawed pretreatment in lotus root contained low bioactive compounds due to the fact that weight loss and that the loose structure after drying was not conducive to the retention of active ingredients during hot drying.29 In the wolffia filtrate, the BWF sample had the highest TFC of 392.77 ± 4.23 mg QE g–1 compared to other samples.
There was no significant difference in the TTC between the samples, as indicated in Table 2. However, in the wolffia filtrate, the BWF showed significantly a higher (p < 0.05) TTC of 22.51 ± 0.26 mg TAE g–1, followed by 14.37 ± 0.40 mg TAE g–1 in the TWF sample. The higher TTC in the BWF might be due to the leaching of tannins into the boiling water.30
Carotenoids, the most commonly used natural antioxidants, have a positive effect on health and prevent chronic diseases.31Table 2 showed that the TCC was reported to be higher in the TWR (722.84 ± 4.72 μg/g sample), followed by the BWR (662.37 ± 26.39 μg/g sample) and the CWR (352.82 ± 1.24 μg/g sample). Jiao et al.32 stated that freeze–thawing increased the extraction of corn carotenoids (lutein and zeaxanthin). In freeze–thawed pretreatment, large ice crystals can easily form, preserving insoluble constituents that bind carotenoids in the freezing process and causing higher levels of carotenoids in the thawed sample.33 Chl-a was reported higher in the TWR, while Chl-b was reported to be higher in the BWR. In the wolffia filtrate, the TCC, Chl-a, and Chl-b were reported to be significantly higher 1997.38 ± 4.06, 26.88 ± 0.10, and 18.52 ± 0.39 μg/g sample, respectively, in CWF samples.
Vitamin C is an important antioxidant that shows several beneficial effects in the human body.34 Unfortunately, it is a highly labile and heat-sensitive compound.34 In this study vitamin C was reported significantly higher (p < 0.05) 70.02 ± 3.04 mg 100–1 in the TWR samples, while the lowest was reported 39.57 ± 3.04 mg 100–1 in the BWR sample (Table 2). The reason for the low vitamin C content in the BWR sample might be the thermal degradation. In previous study also it is reported that thermal treatment has negative impacts on vitamin C content.35 Nonenzymatic browning was observed during thermal treatment, which was correlated with the loss of ascorbic acid.36 Dhiman et al.20 reported that blanching okra results in a higher loss of ascorbic acid. In our study, the most beneficial method in terms of preserving the greatest amount of the vitamin C TWR, which had not undergone thermal treatment.
2.3. Effect of Cell Wall Ruptures Methods on Antioxidant Activities of Wolffia
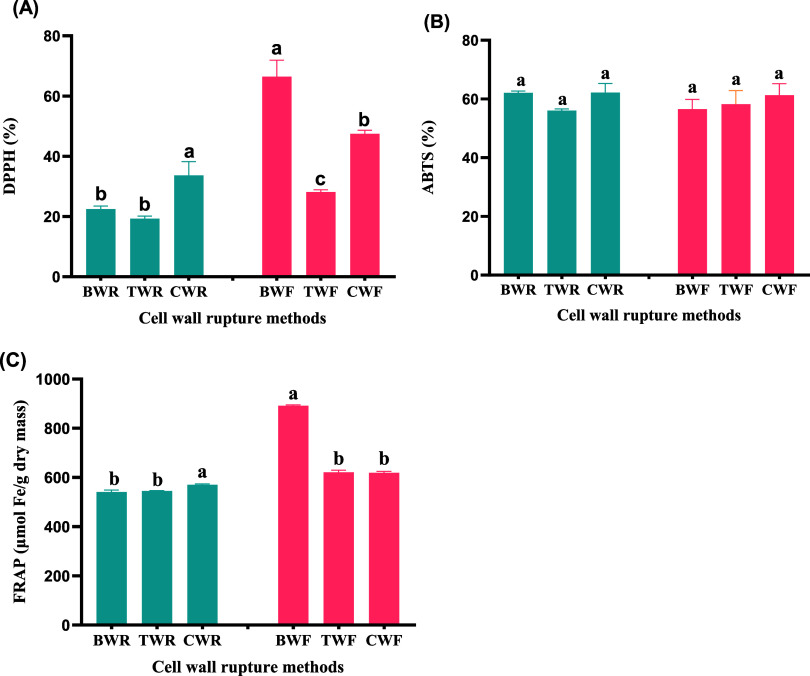
Antioxidant activity is directly proportional to the concentration of ascorbic acid, phenols, and flavonoids.37 The DPPH free radical scavenging activities were significantly higher (p < 0.05) in the CWR, with a value of 33.66%. In contrast, the TWR (19.28%) and the BWR (22.46%) did not exhibit a significant difference, as depicted in Figure 1A. This shows that phenolics (mainly flavonoids) make a very noticeable contribution to the DPPH scavenging activity in the CWR samples. It is known that polyphenolic compounds can enhance strong antioxidant activities against DPPH radicals.38,39 In the wolffia filtrate, the BWF had the highest (66.46%) DPPH scavenging activity, followed by those of the CWF and TWF samples, 47.46 and 28.17%, respectively.
Figure 1.
Effect of cell wall rupture methods on antioxidant activities: (A) DPPH free radical scavenging activity, (B) ABTS free radical scavenging activity, and (C) ferric reducing antioxidant power (FRAP). The values are the means ± SE of triplicate determination. Values with different letters (a, b, c, etc.) indicate significant differences (p < 0.05). (Analyzed using one-way ANOVA; F-statistic).
The ABTS and FRAP antioxidant activities exhibited no significant differences, as illustrated in Figure 1B,C, respectively. However, in the wolffia filtrate, FRAP was significantly (p < 0.05) higher, at 891.62 μmol Fe/g dry mass in the BWF. During pretreatment with bleaching, the covalent bond between insoluble polymeric substances and antioxidants is likely to be destroyed, releasing compounds with antioxidant properties, including phenolic acid, polyphenols, and flavonoids.40
2.4. FT-IR Analysis
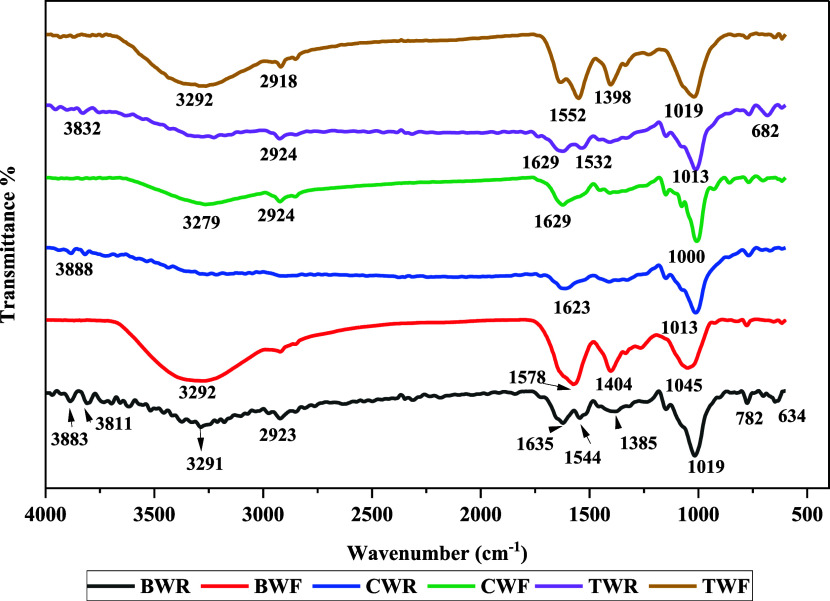
The Fourier transform infrared spectroscopy (FT-IR) spectra of wolffia from 400 to 4000 cm–1 obtained by different cell wall rupture methods are shown in Figure 2. The band identified in the BWR at 3291 cm–1 shows the stretching vibration of the O–H group or the O–H wagging of phenolic compounds.41,42 The BWF, CWF, and TWF samples showed enhanced peaks compared to those of other samples. These enhanced peaks might be due to the higher TPC in the samples. A major contribution of the OH group to antidiabetes, antioxidants, and antibacterial properties is attributed to its presence.43 The absorption bands around 2900–2924 cm–1 indicate C–H stretching of the CH2 groups. Based on these bands, aliphatic CH groups are found in the present compounds.44 A further band in 1715–1630 cm–1 was observed for hydroxybenzoic acids and/or hydroxycinnamic acids. The sharp absorption peaks near 1709–1500 cm–1 reflect the C=O stretching vibrations in carbonyl compounds.45 The sample BWF shows a sharp peak at near 1578 cm–1. This may be attributed to the presence of high flavonoid content in the wolffia sample. Moreover, FT-IR spectra between 1385 and 1450 cm–1 wavelength represent deformation and stretching vibration of C–C bonds in phenolic and carbonyl groups.46 In addition, a sharp absorption band between 1000 and 1045 cm–1 is assigned to the C–O group of molecules.47 A weak absorption near 782 cm–1 indicated that the C–H bend shows alkanes. Similarly, Santhi and Sengottuvel48 also reported a band at 684.08 cm–1, indicating the C–H bends show alkanes. The results of the FT-IR analysis were in good agreement with the results of the LC-ESI-QTOF-MS/MS analysis.
Figure 2.
FT-IR spectra of the filtrate and the residue of fresh wolffia subjected to different cell rupture methods.
2.5. Correlation of the Phenolic Content and the Antioxidants Assay
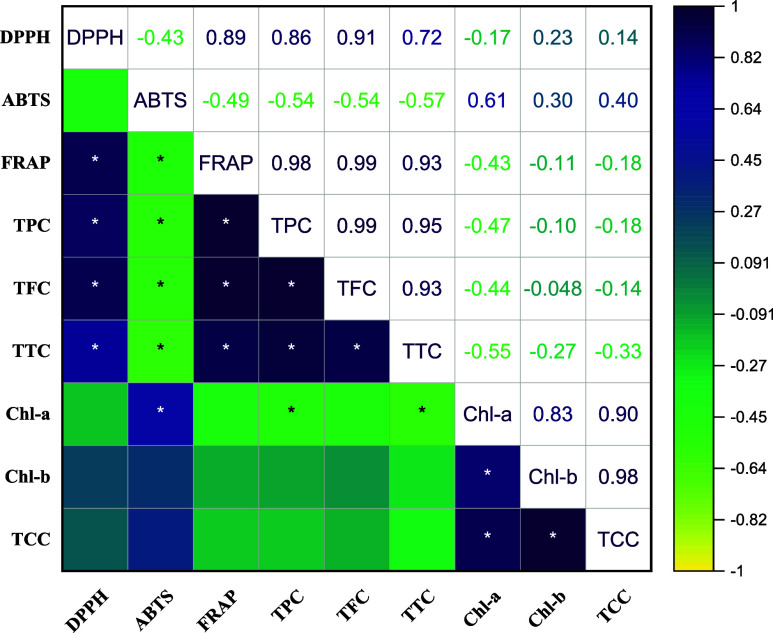
Pearson correlation between the TPC (TFC, TTC, Ca, Cb, and TCC) and three antioxidant activities (DPPH, ABTS, and FRAP) was performed to investigate the relationship between the phenolic contents and antioxidant capacities of wolffia (Figure 3).
Figure 3.
Correlation matrix heatmap shows the values of the Pearson correlation coefficient for all studied parameters: the positive values are in red, and negative values are in blue. It ranges from −1 to 1, where by −1 means a perfect negative linear relationship between variables, 1 indicates a perfect positive linear relationship between variables and 0 indicates that there is no relationship between studied variables. (*significance difference level p < 0.05).
A significantly (p < 0.05) positive correlation between TPC and antioxidant activities (DPPH, r = 0.86; FRAP, r = 0.98) was observed. A positive correlation was reported between TPC and DPPH in previous studies as well.49 In view of the results, it appears phenolic compounds were a significant contributor to the activity of antioxidants in wolffia samples, which is a beneficial effect. However, significant (p < 0.05) negative correlation between TPC and ABTS (r = −0.54) was also reported.
The TFC was significantly (p < 0.05) positively correlated with the TPC (r = 0.99), TTC (r = 0.93), DPPH (r = 0.91), and FRAP (r = 0.99). Wolffia’s antioxidant activity is largely attributed to flavonoids, which are the most abundant phenolic compounds. Also, significant positive correlations were observed between the TTC and antioxidant activities DPPH (r = 0.72) and FRAP (r = 0.93). This suggests that TTC also had influence on DPPH and FRAP. A strong significant (p < 0.05) positive correlation between TCC and Ca and Cb (r = 0.90 and 0.98, respectively).
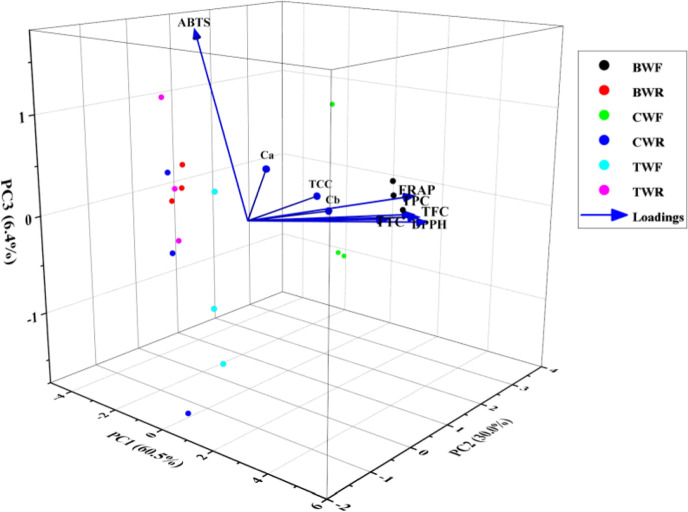
2.6. Principal Component Analysis
A principal component analysis was also conducted to investigate the overall relationship between the phenolic compounds and three antioxidant activities of wolffia. The principal component analysis (PCA) analysis indicates first three principal components accounted for 96.86% variability of the data (Figure 4). The PC1 alone explained 60.52% variation; PC2 explained 29.95% variation, while PC3 explained 6.39% variation. PCA loading plots depicted the relationship among different phenolic compound and antioxidant activities. In the plot, acute angels (<90°) observed between the TPC, TFC, TTC, DPPH, and FRAP showed the close positive relationship between those bioactive compounds and antioxidant activities. In previous study also, PCA revealed a close relationship between total phenolics, tannins, and DPPH antioxidant activity.50 Moreover, in the plot, an obtuse (>90°) angle of ABTS showed negative correlation with the TPC, TFC, TTC, DPPH, and FRAP.
Figure 4.
PCA based on bioactive compounds and antioxidant activity parameters of the wolffia. The samples were divided into two groups along with three principal components (PCs). PC1, PC2, and PC3 explained 60.52, 29.95, and 6.39% of the total variation, respectively. Blue arrows specify the increasing values of each variable.
2.7. LC-ESI-QTOF-MS/MS Characterization
As a part of a qualitative analysis, liquid chromatography-electrospray ionization-quantum time-of-flight mass spectrometry (LC-ESI-QTOF-MS/MS) in both positive and negative ionization modes was used to identify phenolic compounds in different processes of wolffia (Table 3). A total of 72 phenolic compounds were detected, including 18 phenolic acids, 29 flavonoids, and 25 polyphenols. Compounds identified and characterized in the BWR, BWF, TWR, TWF, CWR, and CWF samples were 15, 13, 33, 33, 18, and 19, respectively. The chemical structure and total ion chromatograms are provided in the Supporting Information Figures S1 and S2, respectively.
Table 3. Phenolic Compounds Tentatively Identified in the Wolffia Residue and Filtrate Samples Using LC-ESI-QTOF-MS/MSb.
| s. no. | proposed compounds | molecular formula | RT (min) | mode of ionization (ESI–/ESI+) | molecular weight | observed (m/z) | mass error (ppm) | samples |
|---|---|---|---|---|---|---|---|---|
| Phenolic Acids | ||||||||
| Hydroxycinnamic Acids | ||||||||
| 1 | chlorogenic acid | C18H28O3 | 7.042 | [M – H]− | 354.0961 | 353.0885 | –2.95 | CWR, CWF |
| 2 | caffeic acid | C9H8O4 | 6.252 | [M – H]− | 180.0414 | 179.0341 | 4.91 | CWR, CWF |
| 3 | isoferulic acid | C10H10O4 | 5.768 | [M – H]− | 194.0569 | 193.0495 | 4.96 | CWF |
| 4 | m-coumaric acid | C9H8O3 | 3.418 | [M – H]− | 164.0463 | 163.039 | 6.36 | CWR |
| 5 | sagecoumarin | C27H20O12 | 5.595 | [M – H]− | 536.0998 | 581.098 | –8.09 | BWF |
| 6 | 6-O-p-coumaroyl-d-glucose | C15H18O8 | 4.343 | [M – H]− | 326.105 | 325.0973 | –14.75 | BWF |
| 7 | monotrans-p coumaroylmesotartaric acid | C13H12O8 | 4.715 | [M – H]− | 296.053 | 295.0457 | 0.73 | TWR, TWF |
| 8 | hordatineA | C28H38N8O4 | 13.707 | [M – H]− | 550.2966 | 549.2913 | 9.02 | BWR |
| 9 | esculetin | C9H6O4 | 4.313 | [M – H]− | 178.0251 | 177.0178 | 8.34 | TWF |
| 10 | 6-demethoxycapillarisin | C15H10O6 | 8.046 | [M – H]− | 286.0477 | 285.0403 | 0.27 | TWR |
| Hydroxybenzoic Acids | ||||||||
| 11 | benzoic acid | C7H6O2 | 5.504 | [M – H]− | 122.0362 | 121.0288 | 4.73 | TWF, CWF |
| 12 | ellagic acid | C14H6O8 | 5.623 | [M – H]− | 302.0046 | 300.9973 | 5.67 | TWR, TWF, CWR, CWF |
| 13 | gallicacid3-O-(6-galloylglucoside) | C20H20O14 | 5.247 | a[M – H]− | 484.0757 | 483.0703 | 19.89 | TWF |
| 14 | 2,6-dihydroxybenzoic acid | C7H6O4 | 4.131 | [M – H]− | 154.0259 | 153.0187 | 4.3 | CWR, CWF |
| 15 | resorcinol | C6H6O2 | 4.092 | [M – H]− | 110.0366 | 109.0293 | 1.9 | CWR, CWF |
| 16 | 4-(3,5-diphenylcyclohexyl)phenol | C24H24O | 7.817 | [M + H]+ | 328.1833 | 351.1722 | –1.69 | TWR |
| 17 | [7]-paradol | C18H28O3 | 13.261 | [M – H]− | 292.2032 | 291.196 | 2.07 | TWF |
| 18 | 2,4,6-trihydroxybenzoic acid | C7H6O5 | 1.566 | [M – H]− | 170.0198 | 169.0127 | 9.88 | CWR, TWR |
| Flavonoids | ||||||||
| Flavonols | ||||||||
| 19 | kaempferol 3-rhamnoside 7-xyloside | C26H28O14 | 5.529 | a[M – H]− | 564.1533 | 563.1466 | –10.27 | BWR, BWF, TWR, TWF, CWR, CWF |
| 20 | kaempferol 3-O-β-d-galactoside | C21H20O11 | 5.16 | [M – H]− | 448.0992 | 447.092 | 3.12 | TWR, CWR |
| 21 | kaempferol 3-xylosylglucoside | C26H28O15 | 4.732 | [M – H]− | 580.1405 | 579.1333 | 4.02 | TWR, CWR |
| 22 | kaempferol 7-O-glucoside | C21H20O11 | 5.913 | [M – H]− | 448.1008 | 447.0934 | –0.54 | CWR, CWF, TWF |
| 23 | kaempferol | C15H10O6 | 7.673 | [M – H]− | 286.0479 | 285.0405 | –0.43 | CWF, TWF |
| 24 | quercitrin | C21H20O11 | 5.23 | [M – H]− | 448.106 | 447.0988 | –12.03 | BWR, BWF, CWR, CWF |
| 25 | quercetin | C15H10O7 | 7.654 | [M – H]− | 302.0412 | 301.0341 | 4.88 | TWR |
| 26 | quercetin 3,7-dirhamnoside | C27H30O15 | 4.851 | [M – H]− | 594.1585 | 593.1513 | –0.13 | TWF, TWR, CWF |
| 27 | rustoside | C26H28O15 | 4.816 | [M – H]− | 580.1492 | 579.1413 | –10.01 | BWR, BWF, TWR, CWF |
| 28 | rutin | C27H30O16 | 4.256 | [M – H]− | 610.1534 | 609.1457 | 0.8 | TWF,TWR, CWF, CWR |
| 29 | allivicin | C27H30O16 | 4.812 | [M – H]− | 610.1591 | 609.1519 | –9.43 | BWR, BWF |
| Flavones | ||||||||
| 30 | astragalin 7-rhamnoside | C27H30O15 | 4.639 | [M – H]− | 594.1558 | 593.1486 | 4.56 | CWR, TWR, TWF |
| 31 | (3″-apiosyl-6″-malonyl)astragalin | C29H30O18 | 6.141 | [M – H]− | 666.1307 | 725.144 | 18.72 | BWF |
| 32 | apigenin 7-glucoside | C21H20O10 | 5.628 | a[M – H]− | 432.104 | 431.0968 | 3.79 | TWR, TWF, CWF |
| 33 | luteolin 4′-O-glucoside | C21H20O11 | 5.549 | [M – H]− | 448.1007 | 447.0941 | –1.46 | TWF, CWF |
| 34 | ephedranninA | C30H20O11 | 4.802 | [M – H]− | 556.0936 | 615.1092 | 12.46 | BWF, TWR |
| 35 | cynaroside | C21H20O11 | 5.589 | [M – H]− | 448.1054 | 447.0982 | –10.78 | BWR |
| 36 | 6-C-galactosylluteolin | C21H20O11 | 5.179 | a[M – H]− | 448.0946 | 447.0921 | 2.78 | TWR, TWF, CWR |
| 37 | nicotiflorin | C27H30O15 | 4.683 | [M – H]− | 594.1642 | 593.157 | –9.67 | BWR, BWF |
| 38 | maritimetin | C15H10O6 | 7.79 | [M + H]+ | 286.0438 | 287.0508 | 13.89 | TWF |
| Flavanones | ||||||||
| 39 | 3,3′,5-trihydroxy-4′,7-dimethoxyflavanone | C17H16O7 | 1.229 | [M + H]+ | 332.0899 | 377.0899 | –0.92 | BWR |
| Flavanols | ||||||||
| 40 | ent-fisetinidol-(4β→8)-catechin-(6→4β)-ent-fisetinidol | C45H38O17 | 5.242 | [M – H]− | 850.1954 | 895.1938 | 18.2 | TWF, BWF |
| Isoflavonols | ||||||||
| 41 | genistein 4′,7-O-diglucuronide | C27H26O17 | 5.136 | [M – H]− | 622.1104 | 621.1048 | 10.67 | BWR |
| 42 | genistein 8-C-glucoside | C21H20O10 | 5.658 | [M – H]− | 432.1113 | 431.099 | –1.2 | BWR, BWF, TWF, CWF |
| 43 | 2-hydroxychrysophanol | C15H10O5 | 8.549 | [M – H]− | 270.0525 | 269.0453 | 1.01 | TWF |
| Biflavonoids | ||||||||
| 44 | ent-epiafzelechin(2a→7,4a→8)epiafzelechin 3-(4-hydroxybenzoic acid) | C37H28O12 | 6.561 | [M – H]− | 664.1511 | 709.148 | 12.21 | BWF |
| 45 | sciadopitysin | C33H24O10 | 4.813 | [M + H]+ | 580.1358 | 581.1432 | 2.02 | TWR, TWF |
| Flavans | ||||||||
| 46 | kuwanonZ | C34H26O10 | 4.898 | [M + H]+ | 594.1511 | 595.1584 | 2.56 | TWR, TWF |
| 47 | flavidulolC | C34H42O4 | 13.489 | [M + H]+ | 514.3074 | 537.2969 | 1.83 | TWR, TWF |
| Other Polyphenols | ||||||||
| Pigments | ||||||||
| 48 | pheophorbide a | C35H36N4O5 | 17.592 | a[M + H]+ | 592.2613 | 615.2511 | 11.4 | TWR, TWF, CWR |
| 49 | 3-cis-hydroxy-b,e-caroten-3′-one | C40H54O | 19.537 | [M + H]+ | 550.4103 | 551.4176 | 12.94 | TWR, TWF |
| 50 | 4Z,15E-bilirubin IXa | C33H36N4O6 | 16.527 | [M + H]+ | 584.2595 | 607.2477 | 6.83 | TWR |
| Alkaloids | ||||||||
| 51 | calycanthine | C22H26N4 | 16.436 | [M – H]− | 346.217 | 327.0041 | –7.34 | BWR |
| 52 | ritterazine A | C54H76N2O10 | 20.154 | [M – H]− | 912.5433 | 971.5591 | 7.3 | BWR |
| 53 | (−)-sparteine | C15H26N2 | 8.427 | [M + H]+ | 234.2058 | 235.2131 | 16.04 | TWF |
| 54 | vinpocetine | C22H26N2O2 | 9.489 | [M + H]+ | 350.202 | 351.2093 | –7.39 | TWF |
| 55 | cancentrine | C36H34N2O7 | 16.093 | [M + H]+ | 606.2405 | 607.2476 | –6.39 | TWF, TWR |
| 56 | terminaline | C23H41NO2 | 12.477 | [M + H]+ | 363.3085 | 364.3158 | 14.39 | TWF |
| 57 | embelin | C17H26O4 | 10.944 | [M – H]− | 294.1826 | 293.175 | 1.8 | TWR, CWR |
| 58 | thalidasine | C22H26N2O2 | 12.6 | [M – H]− | 652.3191 | 711.3352 | –6.46 | TWR |
| 59 | 3β,6β-dihydroxynortropane | C7H13NO2 | 1.09 | [M + H]+ | 143.0918 | 144.0993 | 19.63 | TWR, CWR |
| 60 | retronecine | C8H13NO2 | 1.168 | [M + H]+ | 155.0921 | 156.0994 | 16.22 | TWR |
| 61 | ipecac (psychotrine) | C28H36N2O4 | 12.477 | [M + H]+ | 462.2513 | 463.2617 | 4.6 | TWR |
| 62 | cepharanthine | C37H38N2O6 | 19.039 | [M + H]+ | 606.2768 | 607.2841 | –6.26 | TWR |
| 63 | benzosimuline | C20H19NO2 | 2.174 | [M + H]+ | 305.1452 | 328.1343 | –11.86 | TWF |
| Terpenoids | ||||||||
| 64 | ganosporelactoneA | C30H40O7 | 18.552 | [M + H]+ | 512.2746 | 611.1532 | 5.29 | TWR, TWF |
| 65 | 7-O-acetylaustroinulin | C22H36O4 | 18.642 | [M + H]+ | 364.2561 | 387.2453 | 14.57 | TWR, TWF |
| 66 | triterpenoid | C30H48O75 | 18.245 | a[M – H]− | 552.3066 | 551.3062 | –0.95 | BWR, CWF |
| 67 | geranylfarnesyl diphosphate | C25H44O7P2 | 17.514 | a[M + H]+ | 518.2563 | 577.2705 | –0.17 | CWF |
| 68 | tocopheronic acid | C16H22O5 | 11.167 | [M + H]+ | 294.143 | 317.1319 | 12.59 | TWR |
| 69 | tsangane l 3-glucoside | C19H34O7 | 12.945 | [M – H]− | 374.2279 | 433.2418 | 6.92 | BWR |
| 70 | ganoderic acid F | C32H42O9 | 17.896 | [M + H]+ | 570.2791 | 593.2688 | 5.7 | TWR, TWF |
| 71 | 3,3′-bisanigorufone | C38H22O4 | 5.137 | [M + H]+ | 542.1587 | 565.1479 | –12.65 | TWF |
| Tannin | ||||||||
| 72 | 2,6-digalloylglucose | C20H20O14 | 5.287 | [M – H]− | 484.0815 | 483.0765 | 7.15 | BWF |
Compounds were detected in the negative [M – H]− and positive [M + H]+ modes of ionization, while only single mode of data was presented.
RT: retention time.
2.7.1. Phenolic Acids
In this study, a total of 18 phenolic acids were identified from all of the samples. The phenolic acids included were 10 hydroxycinnamic acids and 8 hydroxybenzoic acids.
2.7.1.1. Hydroxycinnamic Acids
The compounds 1 ([M – H]−m/z 353.0885) had been tentatively identified as chlorogenic acid. Earlier, this compound was identified in several microalgae, such as Ankistrodesmus sp., Spirogyra sp., Euglena cantabrica, Caespitella pascheri,51 and brown algae.52 It is an important and biologically active dietary polyphenol with a number of therapeutic properties, including antioxidant activity, anti-inflammatory activity,53 antibacterial activity, hepatoprotection, cardioprotection, antipyretic activity, neuroprotection, weight loss, antiviral activity, antimicrobial activity, and hypertension prevention.54 Compound 2 was observed in ESI– modes and detected ([M – H]−m/z 179.0341) as caffeic acid. In our study, these compounds were identified in the CWR and the CWF. In previous studies, chlorogenic acid and caffeic acid were detected in duckweed Spirodela polyrrhiza, which was consistent with our results.55 Compounds 3, 4, and 5 were identified as isoferulic acid, m-coumaric acid, and sagecoumarin, respectively. Precursor ions of the compounds were m/z 193.0495, m/z 163.039, and m/z 581.098, respectively. The results suggested that isoferulic acid and m-coumaric acid were detected in the CWF and the CWR, respectively, while sagecoumarin was only detected in the BWF. A number of coumaric acid isomers have previously been identified, such as p-coumaric acid and o-coumaric acid, in duckweed (Lemna minor)56 as well as Cystophora sp.57 Compounds 6, 7, 8, 9, and 10 were identified as 6-O-p-coumaroyl-d-glucose, monotrans-p coumaroylmesotartaric acid, hordatine A, esculetin, and 6-demethoxycapillarisin with precursor ions m/z 325.0973, m/z 295.0457, m/z 549.2913, m/z 177.0178, and m/z 285.0403, respectively. All of the compounds were detected only in the negative ion mode.
2.7.1.2. Hydroxybenzoic Acid
Compound 11 ([M – H]−m/z 121.0288) had been tentatively identified as benzoic acid. The compound was detected in the TWF and CWF samples. Compound 12 with [M – H]− with precursor ions at m/z 300.9973 was tentatively identified as ellagic acid in the TWR, TWF, CWR, and CWF samples. Compound 13 was observed in both the ESI– mode and the ESI+ mode, having precursor ions at m/z 483.0703 identified as gallic acid 3-O-(6-galloylglucoside). A number of studies have found that ellagic acid and gallic acid may act as natural antifungal agents.58−60 Compounds 14, 15, 16, 17, and 18 were identified as 2,6-dihydroxybenzoic acid, resorcinol, 4-(3,5-diphenylcyclohexyl)phenol, [7]-paradol, and 2,4,6-trihydroxybenzoic acid. Precursor ions of the compounds were m/z 483.0703, m/z 153.0187, m/z 109.0293, m/z 351.1722, and m/z 291.196.
2.7.2. Flavonoids
A flavonoid is a phenolic compound that acts as an antioxidant and a scavenger of free radicals. In the present study, a total of 29 flavonoids classified into 7 subclasses were characterized, including 11 flavonols, 9 flavones, 1 flavanone, 1 flavanols, 3 isoflavonols, 2 biflavonoids, and 2 flavans. As shown in Table 3.
2.7.2.1. Flavonols Derivatives
Compound 19, 20, 21, 22, and 23 were tentatively identified as kaempferol 3-rhamnoside 7-xyloside, kaempferol 3-O-β-d-galactoside, kaempferol 3-xylosylglucoside, kaempferol 7-O-glucoside, and kaempferol with precursor ions m/z 563.1466, m/z 447.092, m/z 579.1333, and m/z 285.0405, respectively. Compound 19 was observed in both the ESI– mode and the ESI+ mode, while the rest were all observed in the ESI– mode. Kaempferol and its derivatives have been shown to have pharmacological benefits in several pathological conditions, including cardiovascular diseases61 and cancer.62 Compounds 24, 25, and 26 were tentatively identified as quercitrin, quercetin, and quercetin 3,7-dirhamnoside with precursor ions [M – H]−m/z 447.0988, [M – H]−m/z 301.0341, and [M – H]−m/z 593.1513, respectively. Quercetin has been reported to have antidiabetic properties and to protect tissues from oxidative injury induced by diabetes.63 Previously, kaempferol, quercetin, and its derivatives have been detected in duckweed variety (68-red)16 and brown alga, Thalassiophyllum clathrus.64
Rustoside was proposed as compound 27, detected from the BWR, the BWF, the TWR, and the CWF in the negative mode with a precursor ion [M – H]−m/z 579.1413. Compounds 28 and 29 were tentatively identified as rutin and allivicin with precursor ions [M – H]−m/z 609.1457 and [M – H]−m/z 609.1519, respectively. There are many pharmacological properties of rutin, including antibacterial, antiprotozoal, antitumor, anti-inflammatory, antiallergenic, antiviral, cytoprotective, vasoactive, hypolipidaemic, antiplatelet, and antihypertensive.65,66
2.7.2.2. Flavone Derivatives
Compounds 30 and 31 had been tentatively identified as astragalin 7-rhamnoside ([M – H]−m/z 593.1486) and (3″-apiosyl-6″-malonyl)astragalin ([M – H]−m/z 725.144) in the CWR, the TWR, the TWF, and the BWF, respectively, in our study. In previous research, this compound was identified in Hibiscus mutabilis L.67 and Dryopteris sp.,68 which has been proven to be able to inhibit the growth of liver cancer,69 as well as to inhibit the proliferation of lung cancer.70 Compounds 32 and 33 were identified in the TWR, TWF, and CWF samples as apigenin 7-glucoside and luteolin 4′-O-glucoside with the precursor ion [M – H]−m/z 431.0968 and [M – H]−m/z 447.0941, respectively. Compound 32 was observed in the ESI– and ESI+ modes. Previously these compounds were reported in common duckweed, L. minor,71L. minor L.,72 and Landoltia punctata.73 Chen et al.70 reported that apigenin 7-glucoside has been shown in vitro anti-inflammatory activities.
2.7.2.3. Isoflavonols
Three isoflavonols were detected in the negative mode in the BWR, BWF, TWF, and CWF samples. Genistein 4′,7-O-diglucuronide detected in the BWR was assigned for compound (41) based on the observed [M – H]−m/z 621.1048. Compounds 42 and 43 were tentatively identified as genistein 8-C-glucoside and 2-hydroxychrysophanol having a precursor ion [M – H]−m/z 431.099 and [M – H]−m/z 269.0453, respectively.
2.7.2.4. Flavanones, Flavanols, Biflavonoids, and Flavans Derivatives
Compound 39 was only detected in the BWR sample in the positive (ESI+) mode with an observed molecular ion peak [M + H]+m/z at 377.0899. This compound was assigned to 3,3′,5-trihydroxy-4′,7-dimethoxyflavanone a characteristic flavonoid found in Blumea balsamifera.74 Compound 40 having a precursor ion [M – H]−m/z 895.1938 was tentatively identified as ent-fisetinidol-(4β→8)-catechin-(6→4β)-ent-fisetinidol and was present in the TWF and BWF samples.
ent-Epiafzelechin(2a→7,4a→8)epiafzelechin 3-(4-hydroxybenzoic acid) (compound 44 with [M – H]−m/z 709.148) and sciadopitysin (compound 45 with [M + H]+m/z 581.1432) were the only bioflavonoids found in the BWR and TWR and TWF samples, respectively.
Compound 46 having the precursor ion [M + H]+m/z 595.1584 was tentatively characterized as kuwanon Z. It has previously been reported that derivatives of kuwanon Z are abundant in Morus alba and are associated with a variety of health-promoting properties, including neuroprotection and anti-inflammatory activities.75 Compound 47 with the precursor ion [M + H]+m/z 537.2969 was tentatively characterized as flavidulol C. Both the compounds were characterized in the TWR and TWF samples in the positive ESI+ mode.
2.7.3. Other Polyphenols
A total of 25 polyphenolic compounds, including 3 pigments, 13 alkaloids, 8 terpenoids, and 1 tannin were characterized in our study.
Pheophorbide a (compound 48) showing a precursor ion at [M + H]+m/z 615.2511 was detected in the TWR, TWF, and CWR samples. Compound 49, showing a precursor ion at [M + H]+m/z 551.4176, was tentatively characterized as 3-cis-hydroxy-b,e-caroten-3′-one. Compound 50 with the precursor ion at [M + H]+m/z 607.2477 was tentatively characterized as 4Z,15E-bilirubin IXa in TWR samples. Saide et al.76 found that chlorophyll breakdown products generally possess antioxidant and anti-inflammatory properties.
Compound 51 with the precursor ion [M – H]−m/z 327.0041 was tentatively characterized as calycanthine in the BWR sample. Previously, this compound was identified in Meratia praecox.77 Embelin (compound 57) was the important alkaloid tentatively characterized with a precursor ion [M – H]−m/z 293.175 in the TWR and CWR samples. Previous studies have identified embelin in marine macroalgae, such as Turbinaria species.78 The presence of alkaloids protects against chronic diseases such as inflammatory and neurogenerative diseases,79 hypertension, hyperglycemia, and hyperlipidemia,80 and cholesterol reduction.81
Plant terpenes metabolites have been shown to have a wide range of biological activities, including cancer chemoprevention, antimicrobial, antifungal, antiviral, antihyperglycemic, anti-inflammatory, and antiparasitic effects.82,83 Ganoderic acid F (compound 70) is an important triterpenoid characterized with a precursor ion ([M + H]+m/z 593.2688) in the positive mode in the TWR and TWF samples. Wolffia is a highly potential source of phenolic compounds that may find a wide range of applications in the pharmacy, feed, cosmetics, and food industries, as well as many other fields.
2.8. Venn Graphing of the Distribution of Phenolic Compounds in Wolffia
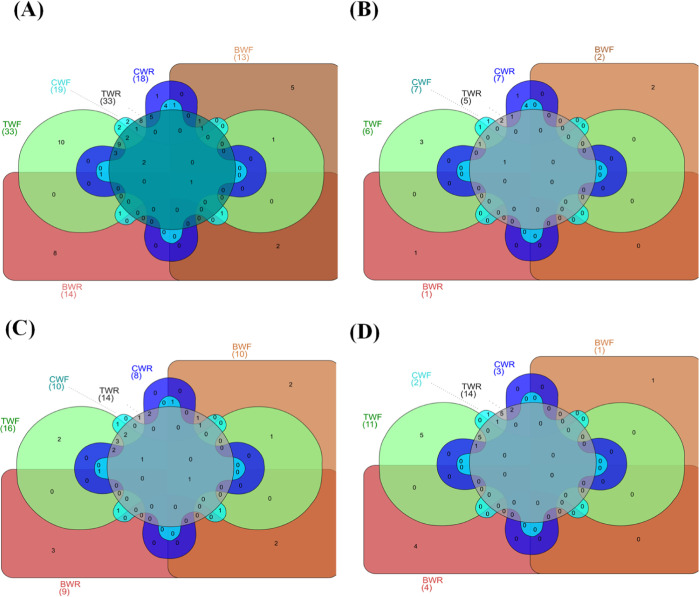
An extensive range of phenolic compounds is contained in wolffia species. The distribution of phenolic compounds in the wolffia was analyzed using Venn graphs in order to determine the most effective method of removing moisture prior to predrying (Figure 5).
Figure 5.
Venn diagram of phenolic compounds present in different predrying cell rupture methodologies. (A) Relation of total phenolic contents among the different cell rupture methodologies. (B) Relation of phenolic acids among the different cell rupture methodologies. (C) Relation of flavonoids among the different cell rupture methodologies. (D) Relation between the other polyphenols in different cell rupture methodologies.
Figure 5A shows the total phenolic compounds in differently processed samples. A total of 10, 8, 8, 5, 2, and 1 were recognized as unique compounds in the TWF, the TWR, the BWR, the BWF, the CWF, and the CWR, respectively. While only one compound was shared among all the samples. The freeze–thawed samples, TWR (33) and TWF (33), had higher phenolic content compared to other samples.
The numbers of unique compounds in phenolic acids were 3, 2, 2, 1, 1, and 1 in the TWF, the TWR, the BWF, the CWF, the CWR, and the BWR, respectively (Figure 5B). In the flavonoid (Figure 5C), only 1 compound (kaempferol 3-rhamnoside 7-xyloside) was shared by all of the samples. The numbers of unique compounds belonging to the BWR, the BWF, the TWR, and the TWF were 3, 2, 2, and 2 respectively.
Figure 5D shows the distribution of other polyphenolic compounds in wolffia. A total of 5, 5, 4, 1, and 1 were recognized as unique compounds in the TWR, the TWF, the BWR, the BWF, and the CWF, respectively. Five compounds were shared between the TWR and the TWF, while two compounds were shared between the TWR and the CWR.
3. Materials and Methods
3.1. Collection of Plant Material and Preprocessing for Cell Rupture
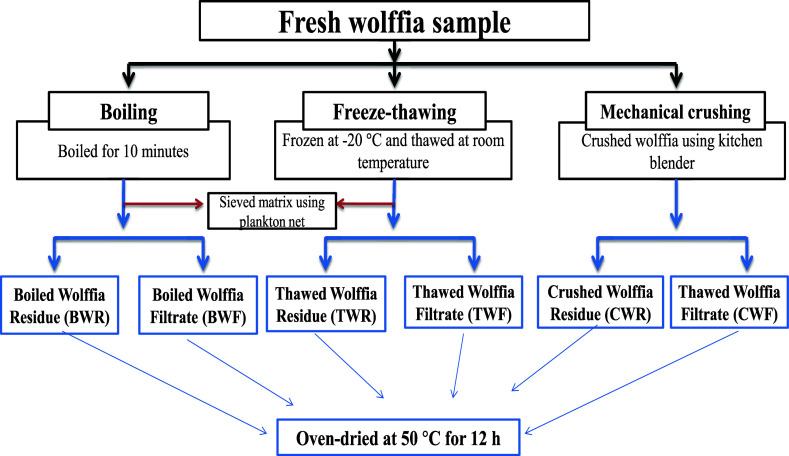
W. globosa was collected from a pilot-scale culture in the earthen pond of the College of Fisheries pond at Lembucherra, Tripura. The holdfasts and conspicuous epiphytes were meticulously removed by picked out by being hand and washed copiously with clean isotonic water from the same pond collected after splashing the floating wolffia biomass away. Subsequently, the harvested biomass was carried to laboratories within 10–15 min and made into batches of 1000 g, one batch for each predrying treatment for cell rupture of fresh wolffia, as detailed below:
-
(a)
Boiling: boiling fresh wolffia biomass for 10 min in a pressure cooker without the addition of any water.
-
(b)
Freeze–thawing: fresh wolffia was placed into polythene bags provided with airtight zippers (25.5 cm × 18 cm × 2 cm) and frozen at −20 °C for 12 h, followed by thawing in tap water at room temperature by placing the bags in the tap water in a tray for 2–3 h.
-
(c)
Mechanical crushing/blending: fresh wolffia biomass was grounded/blended into batches of 500 g in a kitchen blender (Bajaj GX-1, Bajaj Electricals Ltd., India; blended at 1120 rcf).
3.2. Fractionation and Sample Preparation
The schematic representation of the sample preparation procedure is depicted in Figure 6. The resultant semiliquid matrix, after each of the three above-mentioned cell-rupture methods, was passed through a plankton/nylon mesh net (pore size not more than 30–35 μm) to fractionate them into respective residues and filtrates. The recovery rates of residues and filtrates from each were weighed and then dried at 50 °C in a hot air oven for 24 h, separately. The dried samples were ground into a fine powder in a mortar and pestle.
Figure 6.
Schematic representation of the sample preparation procedure.
3.3. Proximate Composition Analysis
The proximate composition of the obtained residues and filtrates of wolffia after the three cell-rupture methods was carried out by using standard procedures of the Association of Official Analytical Collaboration (AOAC 2005).84 The moisture content was determined by overdrying weighted grounded samples in large Petri-plates (Borosil S-line; diameter 200 mm) at 50 °C for 24 h. The protein percentage in both residues and filtrates was determined after digesting the samples in concentrated H2SO4 and catalyst (mix 7 parts of K2SO4 with 1 part of CuSO4) @ 410 °C, for 1 h 45 min (Kel Plus Kes 12b E, Pelican Equipment, Chennai, India) and distillating in an autodistillation unit (Kjeltec 8400, FOSS, Denmark). The crude lipid was determined by using the Soxtec system (ST 243 Soxtec, FOSS Denmark).
3.4. Determination of Bioactive Compounds and Antioxidant Activity in Wolffia Residues and Filtrates
3.4.1. Preparation of Sample Extraction
Samples were extracted using the method described by Firoozi et al.85 with slight modifications. A total of 10 mg of ground powder of the wolffia residue and filtrate was dissolved/suspended in 10 mL of distilled water (DW) (1 mg mL–1) in a volumetric flask and kept in a cooling incubator shaker for 12 h (4 °C at 120 rpm); following that, the samples were centrifuged at 4 °C at 3438 rcf for 15 min using an Eppendorf centrifuge (Eppendorf 5910 Ri, Germany).
3.4.2. Total Phenolic Content
The TPC was determined, as described by Gurung.86 20 μg of samples were taken and added to 1 mL of DW in 15 mL falcon tubes. Following that, 2.5 mL of 20% sodium carbonate and 500 μL of diluted phenol-filian reagent (1:1 with water) were added. The mixture was thoroughly mixed and allowed to develop its color for 40 min in the dark. The absorbance was measured using a Multiskan GO microplate spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) at 725 nm. The results were expressed in mg of gallic acid equivalent (mg GAE g–1 sample).
3.4.3. Total Flavonoid Content
The total flavonoid content was calculated by a colorimetric assay, as described by Chang et al.87 A 0.5 mL sample was taken in a tube. Followed by 1.5 mL of methanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M potassium acetate, and 2.8 mL of DW were added, and this mixture was then left at room temperature for 30 min. The absorbance was recorded at 415 nm and compared with a calibration curve of quercetin. The results were expressed as mg QE–1 (quercetin equivalent).
3.4.4. Total Tannin Content
Tannin content was measured utilizing the Folin–Denis technique, as described by Polshettiwar et al.88 A 0.1 mL sample was mixed with 7.5 mL of DW in a falcon tube. After that, 0.5 mL of Folin–Denis reagent and 1 mL of 35% sodium carbonate solution were added and made up to 10 mL using DW. The mixture was shaken well and kept at room temperature for 30 min until the development of a blue color. The absorbance was measured at 700 nm. Tannic acid was used to make a standard curve, and the results were expressed as equivalent to tannic acid (mg TAE/g of sample).
3.4.5. Total Carotenoid Content
Total carotenoid content was estimated, according to Wellburn.89 A 0.2 g portion of dried wolffia powder was taken and homogenized with 10 mL of 100% acetone. After that, the homogenate was filtered with Whatman no. 1 filter paper and centrifuged for 10 min at 873.4 rcf. The supernatant was separated, and the absorbance was measured using a spectrophotometer at 470, 662, and 645 for the carotenoids and chlorophyll a and b respectively. The total chlorophyll a (Ca), chlorophyll b (Cb), and total carotenoids (Cx+c) were calculated using equations as follows
| 1 |
| 2 |
| 3 |
3.4.6. Vitamin C Content
The volumetric method described by Thimmaiah90 was used to estimate vitamin C concentration in the sample. A conical flask containing 10 mL of 4% oxalic acid and 5 mL of standard ascorbic acid was used to titrate against the 2,6-dichlorophenol indophenols dye. The pink color appearance and persistence were used as the end point. Ascorbic acid is equivalent to the amount of dye that was absorbed (V1 mL). The sample was prepared by taking 0.5 g of powder in 10 mL of 4% oxalic acid and centrifuged. After that, 5 mL of supernatant pipet was mixed with 10 mL of 4% oxalic acid and titrated against the dye in a conical flask (V2 mL). The following formula was used to determine the ascorbic acid content
| 4 |
3.5. Antioxidant Activities
Three types of antioxidant activities, namely DPPH, ABTS, and FRAP assay, were measured in differently dried wolffia to have a comprehensive understanding of antioxidant activities.
3.5.1. DPPH Assay
The antioxidants activity percent of the sample was determined by DPPH free radical scavenging assay according to the method described by Brand-Williams et al.91 The reaction mixture was prepared using 10 to 150 μL of sample, 3 mL of absolute ethanol, and 2 mL of a 0.06 mM (in ethanol) DPPH radical solution. The free radical scavenging of the sample was observed as a change in color from deep violet to light yellow. After 30 min of reaction, the absorbance of the mixture was measured spectrophotometrically at 517 nm. Solution of ethanol and DPPH radical solution were used as the control. The scavenging activity percentage was calculated using the following formula.
| 5 |
3.5.2. ABTS Assay
The ABTS assay (2,2-azino-bis-3-ethyl-benzothiazoline-6-sulfonic acid) was estimated by the method described by Re et al.92 ABTS working solution was prepared by mixing 7.4 mM ABTS solution with 2.6 mM potassium persulfate solution in an equal amount and allowing the mixture to react for 12 h in the dark. The mixture was diluted with methanol (14.5 mL of methanol for 1 mL of the mixed solution). An aliquot (100 μL) of the sample was mixed with 1900 μL of the ABTS working solution, which was then left in the dark for 2 h. Methanol was used for the blank, and the absorbance was measured at 734 nm. The ABTS % was calculated using the following formula.
| 6 |
where A0 = OD value of control; A1 = OD value of sample.
3.5.3. FRAP Assay
The ferric-reducing antioxidant capabilities (FRAP) were determined according to the method described by Benzie and Strain.93 The working reagent was prepared by mixing 0.1 M acetate buffer (pH 3.6), 10 mM TPTZ, and 20 mM ferric chloride and in the proportions: 10:1:1 (v/v/v). The prepared reagent was kept in the water bath at 37 °C for 30 min. After that, the sample was evaluated by adding 100 μL of samples with 0.3 mL of DW and 3 mL of freshly prepared FRAP reagent. The absorbance was then measured at 593 nm. Results were expressed as micromole FeSO4 equiv/g dry weight samples based on an aqueous FeSO4 solution used as the standard.
3.6. FT-IR Spectroscopy
The dried powdered samples were subjected to FT-IR analysis (ALPHA-FT-IR, Bruker, Bremen, Germany) to identify and characterize functional groups in the sample. An organic compound’s infrared spectrum offers a distinctive fingerprint that can be easily differentiated from the absorption patterns of all other compounds. Infrared spectra were recorded between 4000 and 400 cm–1. Spectral data were collected and baseline normalized using OriginPro version 9.8.0.200 (Northampton, MA, USA).
3.7. Characterization of Phenolic Compounds through LC-ESI-QTOF-MS/MS Analysis
Characterization of individual phenolic compounds from the samples was according to the method of Priyadarshini et al.94 The equipment used was an Agilent 1290 Infinity HPLC coupled to jet-speed dual electrospray ionization quadrupole time-of-flight (G 6550A) mass spectrometry, HPLC-dual AJS-ESI-QTOF-MS/MS (Agilent Technologies, Palo Alto, CA, USA). The chromatographic separation was performed on a Zorbax Eclipse Plus C18 column (2.1 mm × 150 mm, 5 μm). The injection volume was 3 μL of each sample, and the flow rate was set at 0.3 mL/min. The mobile phases were acidified water (0.1% formic acid, v/v) and acetonitrile, respectively. The column temperature and auto sampler compartment were set at 35 and 4 °C, respectively. Peaks were identified in both the positive and negative ionization modes over a mass spectrum m/z range of 150 to 1000 m/z, and the detection window was set to 100 ppm. All operations were processed through Mass Hunter Qualitative Analysis B.05.01 (Agilent Technologies).
3.8. Statistical Analysis
The experimental data was sorted by Excel and subjected to a one-way ANOVA utilizing software (IBM SPSS Statistics version 21.0 for Windows) to ascertain whether there were any significant differences between the different treatment groups (p < 0.05). A posthoc, Duncan’s options and descriptive comparisons test was performed after the one-way ANOVA analysis when the substantial differences were discovered. Graphs were plotted using GraphPad Prism 9.5.1 software (GraphPad software, California, USA). Venny (version 2.1) software was used to draw Venn diagrams. PCA and correlation matrix heatmap were performed using OriginPro version 9.8.0.200 software (Northampton, MA, USA).
4. Conclusions
The excessive moisture content of fresh wolffia is a serious impediment to the development of its value chain and full realization of its potential applications in human food, animal or aquaculture, feed, nutraceuticals, cosmetics, and allied industries. The present study evaluated three strategies to cause cell rupture and reduce the predrying moisture of fresh wolffia. The cell rupture released 560–650 mL of water from 1 kg of fresh wolffia, leading to a substantial reduction in the predrying moisture level and consequent drying time and cost. The screening of nutrients and bioactive metabolites in residues and filtrates of fresh wolffia subjected to different cell wall rupture methods aided not only in understanding the loss of nutrients in general and bioactive compounds in particular but also in identifying the preferable cell rupture methods for developing two standalone product lines of the wolffia residue and the wolffia filtrate. It became evident that boiling led to the highest and most prominent leaching of crude lipid, mineral (ash), DPPH and FRAP antioxidant activities, TPC, TTC, and TFC in the filtrate, while mechanical crushing led to the lowest leaching of TPC, TFC, and TTC but the highest leaching of the crude protein, TCC, Chl-a, and Chl-b. The filtrate of freeze–thawing led to the lowest leaching of crude protein, crude lipid, Chl-a, and Chl-b into the filtrates. On the other hand, the highest numbers of phenolic compounds (33) were captured by LC-ESI-QTOF-MS/MS, followed by the filtrate of freeze–thawing against 19 by mechanical crushing, and 13 by boiling. Similar trends were evident for flavonoids and polyphenols. Accordingly, boiling may be a suitable cell-rupturing strategy for the development of a liquid product with rich TPC, TFC, and TTC and high antioxidant activity (DPPH and FRAP). However, it comes with trade-offs in the form of substantially reduced vitamin C and a diversity of phenolic compounds.
On the other hand, freeze–thawing appeared to conserve the highest contents of crude protein, crude lipid, TCC and vitamin C, Chl-a, and varieties of phenolic compounds (33), flavonoids (14), and other polyphenols (14) in the precipitate residues of fresh wolffia against the highest TPC and TFC in that of mechanical crushing. In general, a majority of the measured parameters had intermediate values among the three for mechanical crushing and the lowest in the precipitate residue of boiling.
Considering the energy cost, turnover rate, and ease of performing with respect to the minimal requirement of instrumentations, mechanical crushing followed by screening from the desired mesh size appeared to be the most cost-effective as well as efficient method of cell rupturing for predrying moisture removal. Even if the utility of filtrate as a distinct product is kept aside, mechanical crushing may save energy costs and reduce drying time substantially for developing wolffia residue-based products. A future study should be planned to optimize mechanical crushing and filtration by ascertaining the impact dynamics on nutrients, phenolic compounds, composition, and antioxidant activities.
Acknowledgments
The authors would like to express their gratitude to the Vice Chancellor, Central Agricultural University, Imphal, and the Dean of the College of Fisheries, CAU, Tripura, for providing all the infrastructure facilities needed to complete the study. The authors would also like to recognize the financial help received from the Department of Biotechnology (DBT), Govt. of India (grant no. BT/PR41748/NER/95/1862/2021; Scheme: I&ED; 0155).
Glossary
Abbreviations of Samples
- BWR
boiled wolffia residue
- BWF
boiled wolffia filtrate
- TWR
thawed wolffia residue
- TWF
thawed wolffia filtrate
- CWR
crushed wolffia residue
- CWF
crushed wolffia filtrate
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c09674.
Chemical structures and assigned mass spectra of phenolic compounds tentatively identified in wolffia residue and filtrate samples using LC-ESI-QTOF-MS/MS (PDF)
Author Contributions
NKY: methodology, formal analysis, and writing–original draft. ABP: conceptualization, supervision, formal analysis, and writing–review and editing. SD: methodology and formal analysis. MBP: formal analysis and writing–review and editing. HP: writing–review and editing.
The authors declare no competing financial interest.
Supplementary Material
References
- Kaplan A.; Zelicha H.; Tsaban G.; Yaskolka Meir A.; Rinott E.; Kovsan J.; Novack L.; Thiery J.; Ceglarek U.; Burkhardt R.; et al. Protein bioavailability of Wolffia globosa duckweed, a novel aquatic plant-A randomized controlled trial. Clin. Nutr. 2019, 38, 2576–2582. 10.1016/j.clnu.2018.12.009. [DOI] [PubMed] [Google Scholar]
- Patel A. B.; Kumar G.; Debbarma S.; Mutum D.; Debnath S.; Yadav N. K.. Live Duckweed Based Circular Aquaculture for Climate Resilience and Carbon Footprint Reduction of Fed Aquaculture. In Outlook of Climate Change and Fish Nutrition; Sinha A., Kumari S. K., Eds.; Springer Nature: Singapore, 2022; pp 337–351. [Google Scholar]
- Pradhan A.; Patel A. B.; Singh S. K. Evaluation of live duckweed, Wolffia globosa as an allochthonous feed for Labeo rohita fry during nursery rearing. Aquacult. Res. 2019, 50, 1557–1563. 10.1111/are.14025. [DOI] [Google Scholar]
- Appenroth K. J.; Sree K. S.; Böhm V.; Hammann S.; Vetter W.; Leiterer M.; Jahreis G. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 2017, 217, 266–273. 10.1016/j.foodchem.2016.08.116. [DOI] [PubMed] [Google Scholar]
- Shen N.; Wang Q.; Zhu J.; Qin Y.; Liao S.; Li Y.; Zhu Q.; Jin Y.; Du L.; Huang R. Succinic acid production from duckweed (Landoltia punctata) hydrolysate by batch fermentation of Actinobacillussuccinogenes GXAS137. Bioresour. Technol. 2016, 211, 307–312. 10.1016/j.biortech.2016.03.036. [DOI] [PubMed] [Google Scholar]
- Ifie I.; Olatunde S.; Ogbon O.; Umukoro J. E. Processing techniques on phytochemical content, proximate composition, and toxic components in duckweed. Int. J. Veg. Sci. 2021, 27, 294–302. 10.1080/19315260.2020.1781320. [DOI] [Google Scholar]
- Zhao S. Y.; An N. N.; Zhang K. Y.; Li D.; Wang L. J.; Wang Y. Evaluation of drying kinetics, physical properties, bioactive compounds, antioxidant activity and microstructure of Acanthopanax sessiliflorus fruits dried by microwave-assisted hot air drying method. J. Food Eng. 2023, 357, 111642. 10.1016/j.jfoodeng.2023.111642. [DOI] [Google Scholar]
- Mannozzi C.; Tylewicz U.; Tappi S.; Dalla Rosa M.; Rocculi P.; Romani S. The influence of different pre-treatments on the quality and nutritional characteristics in dried undersized yellow Kiwifruit. Appl. Sci. 2020, 10 (23), 8432. 10.3390/app10238432. [DOI] [Google Scholar]
- Shewale S. R.; Hebbar H. U. Low humidity air and radiofrequency wave based sequential drying of Rosmarinus officinalis for improvement of quality. Ind. Crops Prod. 2021, 162, 113303. 10.1016/j.indcrop.2021.113303. [DOI] [Google Scholar]
- Srimagal A.; Mishra S.; Pradhan R. C. Effects of ethyl oleate and microwave blanching on drying kinetics of bitter gourd. J. Food Sci. Technol. 2017, 54 (5), 1192–1198. 10.1007/s13197-017-2518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.; Wu M.; Zhang X.; Wang B.; Wang S.; Zheng Z.; Li D.; Wang F. Application of blanching pretreatment in herbaceous peony (Paeonialactiflora Pall.) flower processing: Improved drying efficiency, enriched volatile profile and increased phytochemical content. Ind. Crops Prod. 2022, 188, 115663. 10.1016/j.indcrop.2022.115663. [DOI] [Google Scholar]
- Huang D.; Men K.; Li D.; Wen T.; Gong Z.; Sunden B.; Wu Z. Application of ultrasound technology in the drying of food products. Ultrason. Sonochem. 2020, 63, 104950. 10.1016/j.ultsonch.2019.104950. [DOI] [PubMed] [Google Scholar]
- Ando Y.; Maeda Y.; Mizutani K.; Wakatsuki N.; Hagiwara S.; Nabetani H. Impact of blanching and freeze-thaw pretreatment on drying rate of carrot roots in relation to changes in cell membrane function and cell wall structure. Lebensm.-Wiss. Technol. 2016, 71, 40–46. 10.1016/j.lwt.2016.03.019. [DOI] [Google Scholar]
- Larrosa A. P.; Camara A. S.; Moura J. M.; Pinto L. A. Spirulina sp. biomass dried/disrupted by different methods and their application in biofilms production. Food Sci. Biotechnol. 2018, 27, 1659–1665. 10.1007/s10068-018-0397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umair M.; Sultana T.; Xiaoyu Z.; Senan A. M.; Jabbar S.; Khan L.; Abid M.; Murtaza M. A.; Kuldeep D.; Al-Areqi N. A. S.; et al. LC-ESI-QTOF/MS characterization of antimicrobial compounds with their action mode extracted from vine tea (Ampelopsis grossedentata) leaves. Food Sci. Nutr. 2022, 10 (2), 422–435. 10.1002/fsn3.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Li C.; Yan R.; Yu R.; Ji M.; Chen F.; Fan S.; Meng J.; Liu F.; Zhou G.; et al. Metabolome and transcriptome analyses of the flavonoid biosynthetic pathway for the efficient accumulation of anthocyanins and other flavonoids in a new duckweed variety (68-red). J. Plant Physiol. 2022, 275, 153753. 10.1016/j.jplph.2022.153753. [DOI] [PubMed] [Google Scholar]
- Wang M.; Gao Y.; Hu B.; Wang J.; Si X.; Zhu B.; Fan J.; Zhang B. Freeze-thaw pretreatment improves the vacuum freeze-drying efficiency and storage stability of goji berry (Lyciumbarbarum. L.). Lebensm.-Wiss. Technol. 2023, 189, 115439. 10.1016/j.lwt.2023.115439. [DOI] [Google Scholar]
- Wu J.; Wang X.; Fei B.; Xu X.; Lian C.; Chen H. The mechanical properties and thermal conductivity of bamboo with freeze-thaw treatment. J. Wood Sci. 2021, 67 (1), 66. 10.1186/s10086-021-01998-0. [DOI] [Google Scholar]
- Feng Y.; Ping Tan C.; Zhou C.; Yagoub A. E. A.; Xu B.; Sun Y.; Ma H.; Xu X.; Yu X. Effect of freeze-thaw cycles pretreatment on the vacuum freeze-drying process and physicochemical properties of the dried garlic slices. Food Chem. 2020, 324, 126883. 10.1016/j.foodchem.2020.126883. [DOI] [PubMed] [Google Scholar]
- Dhiman A.; Kaur R.; Chandel R.; Kumar S.; Singh B.; Kumar D.; Kumar V.; Suhag R. Effect of blanching and dehydration on rheological, structural, functional and physicochemical properties of okra. J. Food Meas. Char. 2023, 17, 4236–4248. 10.1007/s11694-023-01952-2. [DOI] [Google Scholar]
- Lin Y.; Lin L.; Lin Y.; Lin M.; Ritenour M. A.; Lin H. Comparison between two cultivars of longan fruit cv.‘Dongbi’and ‘Fuyan’in the metabolisms of lipid and energy and its relation to pulp breakdown. Food Chem. 2023, 398, 133885. 10.1016/j.foodchem.2022.133885. [DOI] [PubMed] [Google Scholar]
- Sørensen M.; Kousoulaki K.; Hammerø R.; Kokkali M.; Kleinegris D.; Marti-Quijal F. J.; Barba F. J.; Palihawadana A. M.; Egeland E. S.; Johnsen C. A.; et al. Mechanical processing of Phaeodactylum tricornutum and Tetraselmis chui biomass affects phenolic and antioxidant compound availability, nutrient digestibility and deposition of carotenoids in Atlantic salmon. Aquaculture 2023, 569, 739395. 10.1016/j.aquaculture.2023.739395. [DOI] [Google Scholar]
- Lee S. Y.; Cho J. M.; Chang Y. K.; Oh Y. K. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresour. Technol. 2017, 244, 1317–1328. 10.1016/j.biortech.2017.06.038. [DOI] [PubMed] [Google Scholar]
- Ayele D. T.; Akele M. L.; Melese A. T. Analysis of total phenolic contents, flavonoids, antioxidant and antibacterial activities of Croton macrostachyus root extracts. BMC Chem. 2022, 16, 30. 10.1186/s13065-022-00822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunath S.; Budaraju S.; Gharibzahedi S. M. T.; Koubaa M.; Roohinejad S.; Mallikarjunan K. Processing technologies for the extraction of value-added bioactive compounds from tea. Food Eng. Rev. 2023, 15, 276–308. 10.1007/s12393-023-09338-2. [DOI] [Google Scholar]
- Obajemihi O. I.; Esua O. J.; Cheng J. H.; Sun D. W. Effects of pretreatments using plasma functionalized water, osmodehydration and their combination on hot air drying efficiency and quality of tomato (Solanum lycopersicum L.) slices. Food Chem. 2023, 406, 134995. 10.1016/j.foodchem.2022.134995. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Q.; Chen S. C.; Wang Q. L.; Liu C. Q.; Xiao J. H.; Huang D. W. Effects of traditional grinding and superfine grinding technologies on the properties and volatile components of Protaetia brevitarsis larvae powder. Lebensm.-Wiss. Technol. 2023, 173, 114307. 10.1016/j.lwt.2022.114307. [DOI] [Google Scholar]
- Becker L.; Zaiter A.; Petit J.; Karam M. C.; Sudol M.; Baudelaire E.; Scher J.; Dicko A. How do grinding and sieving impact on physicochemical properties, polyphenol content and antioxidant activity of Hieracium pilosella L. powders?. J. Funct. Foods 2017, 35, 666–672. 10.1016/j.jff.2017.06.043. [DOI] [Google Scholar]
- Zhang L.; Yu X.; Arun S M.; Zhou C. Effect of freeze-thaw pretreatment combined with variable temperature on infrared and convection drying of lotus root. Lebensm.-Wiss. Technol. 2022, 154, 112804. 10.1016/j.lwt.2021.112804. [DOI] [Google Scholar]
- Managa M. G.; Remize F.; Garcia C.; Sivakumar D. Effect of moist cooking blanching on colour, phenolic metabolites and glucosinolate content in Chinese cabbage (Brassica rapa L. subsp. chinensis). Foods 2019, 8 (9), 399. 10.3390/foods8090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir M. T.; Rahman M. H.; Shah M.; Jamiruddin M. R.; Basak D.; Al-Harrasi A.; Bhatia S.; Ashraf G. M.; Najda A.; El-kott A. F.; et al. Therapeutic promise of carotenoids as antioxidants and anti-inflammatory agents in neurodegenerative disorders. Biomed. Pharmacother. 2022, 146, 112610. 10.1016/j.biopha.2021.112610. [DOI] [PubMed] [Google Scholar]
- Jiao Y.; Li D.; Chang Y.; Xiao Y. Effect of freeze-thaw pretreatment on extraction yield and antioxidant bioactivity of corn carotenoids (lutein and zeaxanthin). J. Food Qual. 2018, 2018, 1–8. 10.1155/2018/9843503. [DOI] [Google Scholar]
- Zhao S.; Baik O. D.; Choi Y. J.; Kim S. M. Pretreatments for the efficient extraction of bioactive compounds from plant-based biomaterials. Crit. Rev. Food Sci. Nutr. 2014, 54 (10), 1283–1297. 10.1080/10408398.2011.632698. [DOI] [PubMed] [Google Scholar]
- Zhao C. N.; Li Y.; Meng X.; Li S.; Liu Q.; Tang G. Y.; Gan R. Y.; Li H. B. Insight into the roles of vitamins C and D against cancer: myth or truth?. Cancer Lett. 2018, 431, 161–170. 10.1016/j.canlet.2018.05.039. [DOI] [PubMed] [Google Scholar]
- Grobelna A.; Kalisz S.; Kieliszek M. Effect of processing methods and storage time on the content of bioactive compounds in blue honeysuckle berry purees. Agronomy 2019, 9 (12), 860. 10.3390/agronomy9120860. [DOI] [Google Scholar]
- Lu Q.; Peng Y.; Zhu C.; Pan S. Effect of thermal treatment on carotenoids, flavonoids and ascorbic acid in juice of orange cv. Cara Cara. Food Chem. 2018, 265, 39–48. 10.1016/j.foodchem.2018.05.072. [DOI] [PubMed] [Google Scholar]
- Rama H.; Ndaba B.; Maaza M.; Dhlamini M. S.; Cochrane N.; Roopnarain A. Effect of extraction methods on phytochemical constituents and antioxidant activity of de-kernelled Sclerocaryabirrea seeds. J. Sci. Food Agric. 2023, 103, 7757–7763. 10.1002/jsfa.12865. [DOI] [PubMed] [Google Scholar]
- Meng W.; Sun H.; Mu T.; Garcia-Vaquero M. Extraction, purification, chemical characterization and antioxidant properties in vitro of polyphenols from the brown macroalga Ascophyllum nodosum. Algal Res. 2023, 70, 102989. 10.1016/j.algal.2023.102989. [DOI] [Google Scholar]
- Baiseitova A.; Shah A. B.; Khan A. M.; Idrees M.; Kim J. H.; Lee Y. H.; Kong I. K.; Park K. H. Antioxidant potentials of furanodihydrobenzoxanthones from Artocarpus elasticus and their protection against oxLDL induced injury in SH-SY5Y cells. Biomed. Pharmacother. 2023, 165, 115278. 10.1016/j.biopha.2023.115278. [DOI] [PubMed] [Google Scholar]
- Nayak B.; Liu R. H.; Tang J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—a review. Crit. Rev. Food Sci. Nutr. 2015, 55 (7), 887–918. 10.1080/10408398.2011.654142. [DOI] [PubMed] [Google Scholar]
- Baltacıoğlu H.; Baltacıoğlu C.; Okur I.; Tanrıvermiş A.; Yalıç M. Optimization of microwave-assisted extraction of phenolic compounds from tomato: Characterization by FTIR and HPLC and comparison with conventional solvent extraction. Vib. Spectrosc. 2021, 113, 103204. 10.1016/j.vibspec.2020.103204. [DOI] [Google Scholar]
- Wang J.; Vanga S. K.; Raghavan V. High-intensity ultrasound processing of kiwifruit juice: Effects on the ascorbic acid, total phenolics, flavonoids and antioxidant capacity. Lebensm.-Wiss. Technol. 2019, 107, 299–307. 10.1016/j.lwt.2019.03.024. [DOI] [Google Scholar]
- Sabandar C. W.; Jalil J.; Ahmat N.; Aladdin N. A. Medicinal uses, chemistry and pharmacology of Dillenia species (Dilleniaceae). Phytochemistry 2017, 134, 6–25. 10.1016/j.phytochem.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Joshi R.; Sathasivam R.; Jayapal P. K.; Patel A. K.; Nguyen B. V.; Faqeerzada M. A.; Park S. U.; Lee S. H.; Kim M. S.; Baek I.; Cho B. K. Comparative Determination of Phenolic Compounds in Arabidopsis thaliana Leaf Powder under Distinct Stress Conditions Using Fourier-Transform Infrared (FT-IR) and Near-Infrared (FT-NIR) Spectroscopy. Plants 2022, 11, 836. 10.3390/plants11070836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur B.; Panesar P. S.; Anal A. K. Standardization of ultrasound assisted extraction for the recovery of phenolic compounds from mango peels. J. Food Sci. Technol. 2022, 59, 2813–2820. 10.1007/s13197-021-05304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmood A.; Ishaq M.; Zhao L.; Yaqoob S.; Safdar B.; Nadeem M.; Munir M.; Wang C. Impact of ultrasound and conventional extraction techniques on bioactive compounds and biological activities of blue butterfly pea flower (Clitoriaternatea L.). Ultrason. Sonochem. 2019, 51, 12–19. 10.1016/j.ultsonch.2018.10.013. [DOI] [PubMed] [Google Scholar]
- Patle T. K.; Shrivas K.; Kurrey R.; Upadhyay S.; Jangde R.; Chauhan R. Phytochemical screening and determination of phenolics and flavonoids in Dilleniapentagyna using UV-vis and FTIR spectroscopy. Spectrochim. Acta, Part A 2020, 242, 118717. 10.1016/j.saa.2020.118717. [DOI] [PubMed] [Google Scholar]
- Santhi K.; Sengottuvel R. Functional group analysis of Moringa concanensisNimmo (Moringaceae) by FTIR spectrum. IOSR J. Pharm. 2017, 07, 28–33. 10.9790/3013-0701012833. [DOI] [Google Scholar]
- Zhu Z.; Zhong B.; Yang Z.; Zhao W.; Shi L.; Aziz A.; Rauf A.; Aljohani A. S.; Alhumaydhi F. A.; Suleria H. A. R. Lc-esi-qtof-ms/ms characterization and estimation of the antioxidant potential of phenolic compounds from different parts of the lotus (nelumbonucifera) seed and rhizome. ACS Omega 2022, 7, 14630–14642. 10.1021/acsomega.1c07018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oualcadi Y.; Aityoub A.; Berrekhis F. Investigation of different antioxidant capacity measurements suitable for bioactive compounds applied to medicinal plants. J. Food Meas. Char. 2021, 15, 71–83. 10.1007/s11694-020-00613-y. [DOI] [Google Scholar]
- Jerez-Martel I.; García-Poza S.; Rodríguez-Martel G.; Rico M.; Afonso-Olivares C.; Gómez-Pinchetti J. L. Phenolic profile and antioxidant activity of crude extracts from microalgae and cyanobacteria strains. J. Food Qual. 2017, 2017, 2924508. 10.1155/2017/2924508. [DOI] [Google Scholar]
- Aminina N. M.; Karaulova E. P.; Vishnevskaya T. I.; Yakush E. V.; Kim Y. K.; Nam K. H.; Son K. T. Characteristics of polyphenolic content in brown algae of the Pacific Coast of Russia. Molecules 2020, 25 (17), 3909. 10.3390/molecules25173909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwafor E. O.; Lu P.; Zhang Y.; Liu R.; Peng H.; Xing B.; Liu Y.; Li Z.; Zhang K.; Zhang Y.; Liu Z. Chlorogenic acid: Potential source of natural drugs for the therapeutics of fibrosis and cancer. Transl. Oncol. 2022, 15 (1), 101294. 10.1016/j.tranon.2021.101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveed M.; Hejazi V.; Abbas M.; Kamboh A. A.; Khan G. J.; Shumzaid M.; Ahmad F.; Babazadeh D.; FangFang X.; Modarresi-Ghazani F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- Pagliuso D.; Palacios Jara C. E.; Grandis A.; Lam E.; Pena Ferreira M. J.; Buckeridge M. S. Flavonoids from duckweeds: Potential applications in the human diet. RSC Adv. 2020, 10 (73), 44981–44988. 10.1039/d0ra06741e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.; Moates G. K.; Wellner N.; Collins S. R. A.; Coleman M. J.; Waldron K. W. Chemical characterisation and analysis of the cell wall polysaccharides of duckweed (Lemna minor). Carbohydr. Polym. 2014, 111, 410–418. 10.1016/j.carbpol.2014.04.079. [DOI] [PubMed] [Google Scholar]
- Subbiah V.; Duan X.; Agar O. T.; Dunshea F. R.; Barrow C. J.; Suleria H. A. Comparative study on the effect of different drying techniques on phenolic compounds in Australian beach-cast brown seaweeds. Algal Res. 2023, 72, 103140. 10.1016/j.algal.2023.103140. [DOI] [Google Scholar]
- Torgbo S.; Sukatta U.; Kamonpatana P.; Sukyai P. Ohmic heating extraction and characterization of rambutan (Nephelium lappaceum L.) peel extract with enhanced antioxidant and antifungal activity as a bioactive and functional ingredient in white bread preparation. Food Chem. 2022, 382, 132332. 10.1016/j.foodchem.2022.132332. [DOI] [PubMed] [Google Scholar]
- Mekam P. N.; Martini S.; Nguefack J.; Tagliazucchi D.; Stefani E. Phenolic compounds profile of water and ethanol extracts of Euphorbia hirta L. leaves showing antioxidant and antifungal properties. J. S. Afr. Bot. 2019, 127, 319–332. 10.1016/j.sajb.2019.11.001. [DOI] [Google Scholar]
- El-Bilawy E. H.; Al-Mansori A. N. A.; Soliman S. A.; Alotibi F. O.; Al-Askar A. A.; Arishi A. A.; Sabry A. E. N.; Elsharkawy M. M.; Heflish A. A.; Behiry S. I.; et al. Antifungal, antiviral, and HPLC analysis of phenolic and flavonoid compounds of Amphiroaanceps extract. Sustainability 2022, 14 (19), 12253. 10.3390/su141912253. [DOI] [Google Scholar]
- Chen X.; Qian J.; Wang L.; Li J.; Zhao Y.; Han J.; Khan Z.; Chen X.; Wang J.; Liang G. Kaempferol attenuates hyperglycemia-induced cardiac injuries by inhibiting inflammatory responses and oxidative stress. Endocrine 2018, 60, 83–94. 10.1007/s12020-018-1525-4. [DOI] [PubMed] [Google Scholar]
- Wang X.; Yang Y.; An Y.; Fang G. The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomed. Pharmacother. 2019, 117, 109086. 10.1016/j.biopha.2019.109086. [DOI] [PubMed] [Google Scholar]
- Azeem M.; Hanif M.; Mahmood K.; Ameer N.; Chughtai F. R. S.; Abid U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2023, 80 (1), 241–262. 10.1007/s00289-022-04091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaulova E. P.; Aminina N. M.; Vishnevskaya T. I.; Yakush E. V. A Study of Polyphenol Fractions in an Extract from the Brown Alga Thalassiophyllum clathrus (Postels&Ruprecht, 1840) and Their Antioxidant Activities. Russ. J. Mar. Biol. 2021, 47 (2), 134–142. 10.1134/S1063074021020048. [DOI] [Google Scholar]
- Kaushik D.; Kumar M.; Kaushik R.; Kumar A.. Role of Bioactive Compounds of Bauhinia variegata and their Benefits. In Harvesting Food from Weeds; John Wiley & Sons, 2023; pp 217–266. [Google Scholar]
- Patel K.; Patel D. K.. The beneficial role of rutin, a naturally occurring flavonoid in health promotion and disease prevention: A systematic review and update. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Watson R. S., Preedy V. R., Eds.; Academic Press, 2019; pp 457–479. [Google Scholar]
- Wu C. Y.; Guo Y. Y.; Zhou J.; Long F.; Zhang W.; Shen H.; Xu J. D.; Zhou S. S.; Li S. L. Holistic quality evaluation of HibisciMutabilis Folium by integrating UPLC-QTOF-MS/MS chemical profiling and UPLC-TQ-MS/MS quantification approaches. J. Pharm. Biomed. Anal. 2022, 218, 114869. 10.1016/j.jpba.2022.114869. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Lim D. J.; Song J. S.; Kim J. A.; Lee B. H.; Son Y. K. Identification and Comparison of Bioactive Components of Two Dryopteris sp. Extract Using LC-QTOF-MS. Plants 2022, 11 (23), 3233. 10.3390/plants11233233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Hao J.; Zhang L.; Cheng Z.; Deng X.; Shu G. Astragalin reduces hexokinase 2 through increasing miR-125b to inhibit the proliferation of hepatocellular carcinoma cells in vitro and in vivo. J. Agric. Food Chem. 2017, 65 (29), 5961–5972. 10.1021/acs.jafc.7b02120. [DOI] [PubMed] [Google Scholar]
- Chen M.; Cai F.; Zha D.; Wang X.; Zhang W.; He Y.; Huang Q.; Zhuang H.; Hua Z. C. Astragalin-induced cell death is caspase-dependent and enhances the susceptibility of lung cancer cells to tumor necrosis factor by inhibiting the NF-κB pathway. Oncotarget 2017, 8 (16), 26941–26958. 10.18632/oncotarget.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimirova I. N.; Georgiyants V. A. Biologically active compounds from Lemna minor SF Gray. Pharm. Chem. J. 2014, 47, 599–601. 10.1007/s11094-014-1016-8. [DOI] [Google Scholar]
- Zhang M.; Li Q.; Yu K.; Li J.; Wu J.; Li S.; Chen Y.; Cai W.; Ma J. Systemic chemical characterization of Lemna minor by UHPLC-Q-ExactiveOrbitrap MS coupled with parallel reaction monitoring. J. Mass Spectrom. 2023, 58, e4923 10.1002/jms.4923. [DOI] [PubMed] [Google Scholar]
- Su H.; Zhao Y.; Jiang J.; Lu Q.; Li Q.; Luo Y.; Zhao H.; Wang M. Use of duckweed (Landoltia punctata) as a fermentation substrate for the production of higher alcohols as biofuels. Energy Fuels 2014, 28 (5), 3206–3216. 10.1021/ef500335h. [DOI] [Google Scholar]
- Pang Y.; Wang D.; Fan Z.; Chen X.; Yu F.; Hu X.; Wang K.; Yuan L. Blumeabalsamifera—A phytochemical and pharmacological review. Molecules 2014, 19 (7), 9453–9477. 10.3390/molecules19079453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J.; Thakur A.; Pan G.; Yan J.; Gaurav I.; Thakur S.; Yang Z.; Cili A.; Zhang K. Morus alba derived Kuwanon-A combined with 5-fluorouracil reduce tumor progression via synergistic activation of GADD153 in gastric cancer. MedComm: Oncol. 2023, 2 (1), 24. 10.1002/mog2.24. [DOI] [Google Scholar]
- Saide A.; Lauritano C.; Ianora A. Pheophorbide a: State of the Art. Mar. Drugs 2020, 18 (5), 257. 10.3390/md18050257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manske R. H. Calycanthine. I. The Isolation of Calycanthine from Meratia praecox. J. Am. Chem. Soc. 1929, 51 (6), 1836–1839. 10.1021/ja01381a034. [DOI] [Google Scholar]
- Stranska-Zachariasova M.; Kurniatanty I.; Gbelcova H.; Jiru M.; Rubert J.; Nindhia T. G. T.; D’Acunto C. W.; Sumarsono S. H.; Tan M. I.; Hajslova J.; et al. Bioprospecting of Turbinaria macroalgae as a potential source of health protective compounds. Chem. Biodivers. 2017, 14 (2), 1600192. 10.1002/cbdv.201600192. [DOI] [PubMed] [Google Scholar]
- Aryal B.; Raut B. K.; Bhattarai S.; Bhandari S.; Tandan P.; Gyawali K.; Sharma K.; Ranabhat D.; Thapa R.; Aryal D.; Ojha A.; et al. Potential therapeutic applications of plant-derived alkaloids against inflammatory and neurodegenerative diseases. eCAM 2022, 2022, 7299778. 10.1155/2022/7299778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y. M.; Liang X. A.; Kong Y.; Jia B. Structural diversity and biological activities of indolediketopiperazine alkaloids from fungi. J. Agric. Food Chem. 2016, 64 (35), 6659–6671. 10.1021/acs.jafc.6b01772. [DOI] [PubMed] [Google Scholar]
- Maharjan B.; Payne D. T.; Ferrarese I.; Giovanna Lupo M.; Kumar Shrestha L.; Hill J. P.; Ariga K.; Rossi I.; Sharan Shrestha S.; Panighel G.; Lal Swagat Shrestha R.; et al. Evaluation of the effects of natural isoquinoline alkaloids on low density lipoprotein receptor (LDLR) and proproteinconvertasesubtilisin/kexin type 9 (PCSK9) in hepatocytes, as new potential hypocholesterolemic agents. Bioorg. Chem. 2022, 121, 105686. 10.1016/j.bioorg.2022.105686. [DOI] [PubMed] [Google Scholar]
- Maphetu N.; Unuofin J. O.; Masuku N. P.; Olisah C.; Lebelo S. L. Medicinal uses, pharmacological activities, phytochemistry, and the molecular mechanisms of Punicagranatum L. (pomegranate) plant extracts: A review. Biomed. Pharmacother. 2022, 153, 113256. 10.1016/j.biopha.2022.113256. [DOI] [PubMed] [Google Scholar]
- Singh K.; Bano A.; Sharma R.; Sharma S.. Terpenoids in Nanomaterials: Synthesis, Characterization, and Their Application. In Secondary Metabolites Based Green Synthesis of Nanomaterials and Their Applications; Springer Nature Singapore: Singapore, 2023; pp 91–118. [Google Scholar]
- AOAC . Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists International: Maryland, USA, 2005. [Google Scholar]
- Firoozi S.; Jamzad M.; Yari M. Biologically synthesized silver nanoparticles by aqueous extract of Saturejaintermedia CA Mey and the evaluation of total phenolic and flavonoid contents and antioxidant activity. J. Nanostruct. Chem. 2016, 6, 357–364. 10.1007/s40097-016-0207-0. [DOI] [Google Scholar]
- Gurung R. Preliminary phytochemical screening, total phenol and flavonoid content of Mimosa rubicaulis and Reinwarditaindica. Int. J. Pharm. Pharmaceut. Sci. 2020, 12, 54–58. 10.22159/ijpps.2020v12i1.35914. [DOI] [Google Scholar]
- Chang C.-C.; Yang M. H.; Wen H. M.; Chern J. C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 3. 10.38212/2224-6614.2748. [DOI] [Google Scholar]
- Ganjiwale R.; Wadher S.; Yeole P.; Polshettiwar S. Spectrophotometric estimation of total tannins in some ayurvedic eye drops. Indian J. Pharm. Sci. 2007, 69, 574. 10.4103/0250-474X.36949. [DOI] [Google Scholar]
- Wellburn A. R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. 10.1016/S0176-1617(11)81192-2. [DOI] [Google Scholar]
- Thimmaiah S. K. In Standard Method of Biochemical Analysis, 3rd ed.; Kalyani Publisher: New Delhi, 1999; p 278. [Google Scholar]
- Brand-Williams W.; Cuvelier M. E.; Berset C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. 10.1016/s0023-6438(95)80008-5. [DOI] [Google Scholar]
- Re R.; Pellegrini N.; Proteggente A.; Pannala A.; Yang M.; Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26 (9–10), 1231–1237. 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Benzie I.; Strain J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Priyadarshini M. B.; Balange A. K.; Xavier K. A. M.; Reddy R.; Nayak B. B.; Sanath Kumar H. The Effect of Lyophilized Coconut Mesocarp—Aqueous and Ethanol Phenolic Extracts on the Gel Quality of Tilapia Surimi. J. Aquat. Food Prod. Technol. 2021, 30, 1330–1343. 10.1080/10498850.2021.1989100. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.