Abstract
Unpredictable, undesirable, and confusing reactions in the face of psychological or medical interventions make the clinical presentation more complicated and may represent clinically unexplained symptoms and also disturbed the doctor–patients relationship and decrease patients’ benefits of treatment. It seems that negative expectations from the treatment (nocebo phenomenon) can explain such reactions. The aim of the current study is a scoping review and investigate different aspects of the nocebo phenomenon (negative expectations) in clinical interventions. This paper follows a scoping review of the existence, importance, and multidimensions of the nocebo phenomenon in medical and psychological interventions. Data sources include literature databases (ProQuest, PubMed, Google Scholar, and Scopus) reviewed from inception dates to 2023, and the terms negative expectations, nocebo effect, placebo effect, negative placebo, and clinical interventions were searched. The review of the available articles showed that negative expectations play an important role in the process and effectiveness of clinical interventions. Negative expectations (here named nocebo effect) can significantly interfere with rapport and treatment processes. Some underlying components of the nocebo effect include negative expectancies, conditioning, social learning, memory, cognitive distortions, meaning, motivation, somatic focus, negative reinforcements, personality, anxiety, and neurophysiological factors such as CCK, dopamine, and cortisol are proposed for development and presence of nocebo phenomenon in clinical practice. Negative expectations with its biopsychosocial aspects play an important and amazing role in disorganizing medical and psychological interventions. Using appropriate methods to reduce nocebo effects in therapeutic interventions may increase treatment compliance and adherence and increase the effectiveness of interventions.
Keywords: Clinical interventions, negative expectation, nocebo effect, placebo effect
Introduction
Expectations play an important role in the health process in such a way that the expectation of pain reduction can reduce the intensity of pain and create a better feeling.[1] Positive expectations of the treatment increase the effectiveness of the therapeutic intervention, and there are many researches in this regard that patients with physical pains or psychological disorders have reported.[2,3] In the context of therapeutic interventions, such an effect is referred to as the placebo effect. Patients do not always have fixed expectations from the treatment, when a patient has negative expectations of his/her treatment results, it can reduce the effectiveness of the treatment or even cause side effects or adverse results. In such a situation, the term nocebo effect is used.[4] In other words, the concept of expectation is the underlying principle of placebo and nocebo phenomena and the role that positive and negative expectations play in therapeutic interventions can be determined by the placebo and placebo effects. However, the volume of studies conducted on the concepts of nocebo is very small compared to placebo effects. A 2015 study by Weimer et al.[5] compared the number of placebo and nocebo-related articles published in PubMed from 1950 to 2013; out of a total of 2852 articles, 2672 articles related to placebo and only 280 articles related to nocebo have been published. And the culmination of attention to the issue of nocebo has also been in this direction since 2008. Although, the use of the word nocebo is mostly used for the side effects of drugs, today, the use of the word nocebo has been expanded and refers to any adverse effect following medical, psychological interventions, or includes any context in which intervention or advice is made.[6] Negative and pessimistic expectations about treatment effectiveness—so-called the nocebo effect, can result from direct negative experiences with medical or psychological treatments or other sources of information.[7] Negative treatment expectancies can distract the success of psychological interventions.[8] Therefore, paying attention to the patient's expectations in therapeutic interventions is of particular importance. Attempts to identify and reduce negative perceptions of treatment in the literature of therapeutic interventions have not been studied as expected.[9] In clinical settings, the nocebo effect is easily induced by verbal inductions. These negative effects are usually caused unintentionally during the explanation of treatment effects and side effects, and during the clinical consultation. These verbal inductions may be issued from unreliable medical sources, such as family, media, or the internet, so writings or sayings can create negative expectations and nocebo effects.[10] Also, due to the psychological importance of the expectations, other terms besides placebo and nocebo,[11] such as placebo and nocebo responses, active placebo, nocebo side effect[12], lessebo,[13] and precebo,[14] have been raised in the research literature related to expectations. The nocebo effect is basically defined as the harmful effect caused by an ineffective or inert treatment.[15] The nocebo effect is complementary to the placebo effect, i.e., (the placebo effect) is a beneficial effect for health that occurs after a passive treatment due to positive expectation of treatment. Although the placebo effect was a more well-known and deceptive counterpart that has received more research attention, the nocebo effect has demonstrably more important effects on medical care. A large amount of nocebo effect is dependent on medical treatments, which results in a defect in the quality of life of many patients, and in particular, it is considered a factor in the lack of treatment follow-up and instability in performing therapeutic procedures, which in its own way leads to high going to medical expenses.[16] It is clear that the negative effects of treatment lead to a decrease in the effectiveness of future interventions.[17,18] The results of recent research in clinical trials indicate that almost 70–80 percent of the obtained effects cannot be documented as the effects of drugs and intervention.[19] Additionally, approximately 25% of patients randomized to placebo in a clinical trial reported adverse effects[20] and this rate of incidence increases when specifically asked about a specific complication.[6]
However, even though the relationship between th placebo effect (positive expectations) in clinical interventions was widely investigated, there were not enough studies on negative expectations (nocebo effect).[21] Accordingly, we attempted to close this research gap by studying the different aspects of negative expectations in clinical interventions and providing materials to improve. The aim of the present study is to investigate the effects of the negative expectations in therapeutic interventions and how negative expectations or in other words the nocebo responses affect the therapeutic results.
Methods and Materials
Data sources
In order to provide the available evidence of the nocebo effect in clinical practice and to guide further research, we started to review the scope.[22] Unlike traditional systematic reviews, the purpose of the present study is to provide a preliminary assessment of existing research or ongoing studies and to identify potentially important research areas. We kept our search broad enough due to the lack of information on the extent of nocebo effect and clinical practice in the literature. We examined the five databases (ProQuest, PubMed, Google Scholar, and Scopus) from their respective inception dates to January 2023 for primary studies.
Search terms
We started a preliminary search of these databases to identify papers and establish terms that may refer to nocebo, negative expectations, placebo, and clinical practice. This search showed that few publications use “nocebo, negative expectations, placebo and clinical practice, clinical interventions” anywhere in the article; some databases had no or very little publications where the term was used. We found no established search strings for identifying nocebo, negative expectations, and clinical practice-related papers and no alternative Medical Subject Headings (MESH) phrases. Several papers used alternative phrases such as “negative placebo effects,” “nocebo side effect,” “adverse effects of placebo,” “side effects of placebo,” and clinical practice. Since the use of the word “placebo” in clinical trials is common, using it will make the subject of study weak and disproportionate. We tried to limit the search terms to “nocebo,” “negative expectations” with clinical practice, clinical interventions, and one specific alternative term—“Negative placebo effects”. Reference lists in included articles were searched for relevant studies. The inclusion and exclusion criteria are presented in Table 1.
Table 1.
Study selection criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| 1 Empirical article | 1 non-empirical articles, including audits, letters, opinions, editorials |
| • The prevalence of nocebo effect and clinical practice | |
| • Demographic features related to nocebo effect | 2 Empirical articles that only emphasize on placebo |
| • The neurophysiological basis of the nocebo effect | 3 Nocebo articles that related to other conditions except clinical practice or interventions. |
| • The psychological basis of the nocebo effect | |
| • Effect of nocebo on clinical presentation | 4 Studies not in English |
| • Influence of healthcare provider/patient relationship on nocebo effect prevalence in patients | 5 Case histories |
| • Effect of nocebo on adherence to therapy |
Selection criteria
Titles and abstracts were reviewed for all studies and the inclusion and exclusion criteria of the study were used. Full texts of all articles that either clearly met or possibly met the inclusion criteria were obtained. All articles were reviewed. The differences in the articles were examined and the data were extracted. The quality of the selected articles was evaluated using Dixon Woods et al.'s[23] (2005) five-step process include: aims and objectives of the research, appropriateness of research design for the aims and objectives, clear account of the process, enough data to support their interpretation and conclusions, and appropriateness analysis method.
In order to extract the data, the method of scoping studies was used, and according to the keywords from all the 294 articles [Figure 1] obtained from the literature databases, duplicate items were discarded. After checking the title and abstract of 93 articles, 57 articles were fully reviewed. And after separating 18 articles due to lack of empirical results, a total of 17 experimental and 22 review articles related to the subject of the present study were analyzed.
Figure 1.
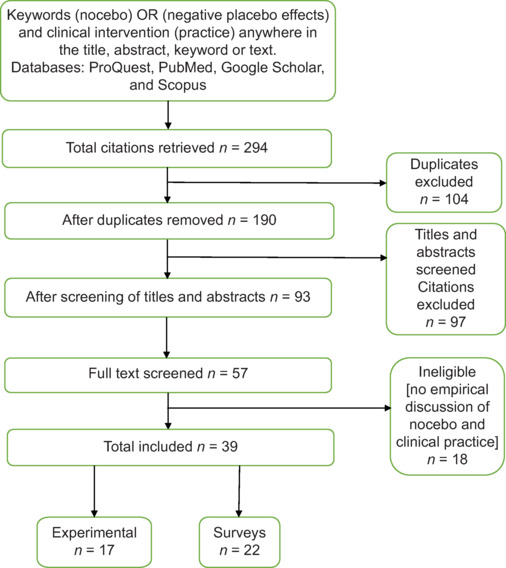
Summary of the review process
Results
A review of the literature showed that expectations of treatment are created through personal experiences (learning), observational learning, books or information received from therapists or treatment staff or information obtained from other sources such as the internet, and conversations of others.[18,24]
The therapist's empathy and other factors related to doctor–patient relationship can decrease or increase the quality of the therapist and patient interaction and affect positive and negative expectations (placebo and nocebo responses). Kaptchuk et al.[25] (2008) studied how empathy affects placebo responses. In this study, patients with irritable bowel syndrome were studied and placed in two experimental and control groups. In one of the groups, there was an empathetic treatment of the therapist with the patients, while in the second group, the interactions were usual and formal and there were no warm interactions. Although both groups improved more than patients on the waiting list, patients who received empathic interventions showed greater improvement. And this represents a positive aspect of the therapist–patient relationship that can enhance the placebo response, in other words, patients had positive expectations of their treatment. Other studies in this direction have also shown similar results. Initial attempts to use placebo mechanisms in improving treatment outcomes indicate that this strategy is well-prepared to improve clinical care. Several factors can be included in treatment measures to improve and improve placebo responses and maximize treatment results. These factors include optimizing the patient's expectations from the treatment, using a positive role model to prove the effectiveness of the treatment, and inducing positive experiences before treatment with similar drugs. Modifying and improving aspects of therapeutic interaction such as empathy and collaborative decision-making and improving the patient's perception of the therapist's adequacy can improve therapeutic outcomes. Although more studies are needed in this field.[18]
Contrary to the effect of negative expectations in therapeutic interventions, positive expectations can create significant effects. Studies have shown that certain personality traits can be associated with more positive expectations. For example, people who are more altruistic and extroverted are more likely to have placebo responses. A person's previous experiences as related to the positive expectations can play a role in the placebo response. Additionally, some neurobiological findings confirm the role of patients’ expectations in this regard, a person's genetic makeup can determine a placebo response. For example, the catecholamine-methyltransferase gene, which regulates the metabolism of a number of neurotransmitters, including dopamine, appears to play a role in predicting placebo response, albeit in a number of individuals in the appropriate context. The nocebo effect is probably more related to the expectations, so that the way in which the treatment exerts its effect can be affected by nocebo effects. For example, if a patient is told that the drug has side effects, that person is more likely to report side effects. In addition, people with type A personality, who are likely to have higher neuroticism and pessimism, will also have more negative expectations and show more nocebo responses.[26] Having negative expectations can also be related to personality traits. Personality factors can significantly influence the patients’ positive and negative expectations in clinical interventions by presenting placebo and nocebo effects.[27,28] Factors such as optimistic mood,[29] hypnotic suggestibility,[30] bodily focus,[29] empathy,[31,32] neuroticism, altruism,[33] propensity for social relationships,[34] dopamine-related personality trait,[35] anxiety,[36] fear of pain,[37] focus on ego resilience,[38] pessimism,[39] pain catastrophizing,[33] harm avoidance,[39] and persistence[28] are related to placebo and nocebo effects.[28] On the other hand, anxiety, harm avoidance, persistence, and pain catastrophizing[33,40] have been associated with negative expectations and the nocebo effects. Anxiety and harm avoidance have a positive correlation with the nocebo effects. While optimism and persistence have a negative correlation with nocebo effects.[39]
Research in the field of nocebo effect shows that negative expectations play an important role both psychologically and neurobiologically in the occurrence of nocebo phenomenon. So that, negative expectations by mediating negative emotions such as stress, fear, and anxiety,[1,41,42,43] and at the neurophysiological level by activating the hypothalamus pituitary adrenal axis (HPA), which acts in response to stress and the accumulation of the stress hormone cortisol.[42,44] Both the nocebo effect and the HPA hyperactivity effect are reduced by anti-anxiety drugs.[42] Anticipatory anxiety facilitates pain transmission at least through cholecystokinin receptors,[45,46] and causes hypersensitivity.[1,47] On the other hand, the effect of negative expectations is associated with a decrease in the activity of dopaminergic and opioid systems.[48,49] Depressed patients may also be at risk for cognitive distortions and frequent catastrophic thoughts, and hence, more likely to form negative expectancies and provide nocebo responses.[50] Therefore, depression can make it difficult for patients to benefit from receiving medical services due to their negative expectations of medical interventions. Maybe this issue can explain some of the negative prognostic factors of depressed patients in treatment or the difficulty of therapeutic interventions in patients with depressive disorder. In total, the results of the present study have shown that, the role of factors such as learning aspects, neurobiological status, verbal and non-verbal behaviors of the therapist,[51,52,53] depression, anxiety, misattribution of the symptoms[8] and social contagion[33,54] are important in the emergence of negative expectations and their negative role in influencing therapeutic interventions. Among other results of the present study, we can point out the importance of preventing the occurrence of negative expectations and the occurrence of nocebo phenomenon, which according to the above-mentioned cases, it is necessary for therapists to consider in their therapeutic interventions.
Discussion
Findings of the present study indicated that the consequences of negative expectations or the nocebo effects in clinical practice are always unpleasant and can make therapeutic intervention more painful and reduce therapeutic response and make symptoms more severe and lead to harmful events and cause failure to follow treatment orders or continue treatment.[55] For example, in Varelmann, et al.[56] study in epidural anesthesia, when patients are told that they will feel something like a bee sting, it can be much more painful than when the patient is told that they will experience some kind of numbness. Similarly, using the word “pain” rather than a “cold sensation” in describing this task can make the injection more painful.[57] Similarly, in Luparello, et al.[58] (1970) study, the efficacy of active pharmaceutical ingredients can be reduced by providing conflicting information. In clinical situations, the nocebo effect is easily induced by verbal inductions. These negative effects are usually caused unintentionally during the explanation of treatment effects and side effects and during the clinical consultation. This information may not be stated directly and may be on the written sheet next to the medicines. These verbal inductions may come from medically unreliable sources, such as family, media, or the internet, so writings or sayings can create negative and nocebo effects.[51,52] Nocebo effect may be caused non-verbally through the therapist's body language,[53] observation of symptoms, side effects, or behavior of other patients during the treatment, where it may lead to stopping the treatment.[1,59] In general, the nocebo effect, which is the product of negative expectations from therapeutic intervention, is always negative and unwanted, and it is easily caused by the wrong choice of words or sentences. It can also be learned from other person's adverse experiences or observations of others. And it is caused by the classical or operant conditioning or other forms of learning, and sometimes, patients are not even aware of it. Knowing the nocebo effect is important because it can make the treatment harmful and ineffective. For example, there is no improvement, or the improvement is much less than expected, or the treatment is not tolerated, and patients report too many side effects to the point where they change the treatment. However, patients report non-specific complaints after taking a drug or even after changing treatment, their symptoms worsen. Nocebo effect is also responsible for non-compliance and non-adherence to treatment. When patients expect to get worse or not improve, bad feelings show themselves through treatment follow-up and they do not continue the treatment regularly, and sometimes, it leads to the use of less than the therapeutic dose of drugs. Therefore, any possible nocebo effect should be known and reduced in therapy or clinical trials.[43]
A longitudinal study of patients with rheumatoid arthritis found that patients with greater concerns about their medications were more likely to report side effects within six months of starting treatment.[59] Similar to this finding, women receiving hormones for breast cancer had more expected complications than baseline and reported more non-adherence to treatment.[60] The belief that a person is specifically sensitive to the actions or side effects of drugs is a relatively common belief. About one-fifth of the general population agrees with the statement, “My body is too sensitive to drugs.”[61] Also, concern about personal sensitivity to an environmental factor can activate the nocebo effect and even cause the belief that others who have such symptoms are the result of their encounter with such factors.[18]
Against learning theories, some researchers have found the role of context in the emergence of nocebo effects to be important. For example, some pharmaceutical brands can create nocebo responses, and this is when patients sometimes compare generic vs. brands name of drugs or vice versa. Approximately, 20–30 percent of patients and a similar percentage of pharmacists and doctors have a negative opinion about generic drugs and consider them less useful and evaluate the commercial equivalent of the same drug as more useful.[10] Laboratory research has shown that even when the drugs are placebo, changing the drug label from commercial to generic can increase its reported side effects and reduce its usefulness.[62] During the switch from brand name to generic, there is also an increase in reports of side effects and complaints that new drugs are less effective.[63,64] Limiting the choice of drugs can also reduce the nocebo effect. Many health care systems use this method to reduce costs.[65] However, limiting the brands can also reduce the usefulness of placebo effects.
The results of the present study have pointed out the role of social contagion in the emergence of negative expectations and nocebo phenomenon. Seeing a person reporting side effects after receiving medical treatment can trigger similar nocebo responses in others. In their study, Vögtle et al.[33] (2013) introduced a person as a model for others who uses an ointment to heal his pains, but the use of the ointment caused complications for him, the same pattern also occurred in the observers. There is evidence that women are probably more sensitive to the effects of role modeling than men. But it seems that empathy can be the foundation of this problem. Research has shown that modeling and observing models can increase nocebo responses in observers. Nocebo effects can spread on a larger scale through mass media. Experimental studies have shown that television reports can intensify signs and symptoms and physical experiences and cause symptoms in viewers.[55]
According to the findings of the present study, learning factors play a role in the phenomenon of the nocebo. In classical conditioning, the presence of two stimuli that are frequently accompanied can cause the previously neutral stimulus to produce a physiological response similar to that produced by the original conditioned stimulus (unconditioned stimulus). Numerous studies have shown the role of conditioning learning in creating similar symptoms.[18] Nocebo responses can be created through the operant conditioning model and observational learning model.
On the other hand, an important factor underlying the nocebo effect is the frequency of reported physical symptoms in the general population. Symptoms usually appear when people seem to be suffering from a disease. A review study of the general population has shown that the main symptoms and signs of the previous week were five symptoms and 23% of the sample population studied showed 10 or more symptoms. Only 11% of the study population had no symptoms in the past week. The main reported symptoms included back pain 38%, fatigue 36%, headache 35%, nasal symptoms 34%, joint pain 34%, insomnia 29%, cough 28%, muscle pain 23%.[18] It should be noted that at the heart of nocebo's answers lies the process of misattribution. However, misattribution is a complex process due to the fact that existing symptoms and new symptoms can be misinterpreted as the effect of treatment or other stimuli. So that a large part of the symptoms that people report after receiving the drug can be the same symptoms that the person has already experienced. But now, it is attributed to the treatment effect,[62] and what affects the misinterpretation of signs the most is negative emotion.[18]
Despite the existing research, there is little research on how to reduce negative expectations.[9] One of these cases is providing treatment suggestions appropriate to the context so that the therapists can convey their information to the patients and this can reduce the effects of nocebo.[66] The second way is to provide less information about the side effects of the treatment, which can be effective in reducing the nocebo effect.[54] However, they have also criticized it,[54] and consider it to be against professional ethics and cause the patient's autonomy to be questioned.[67] The third way is to optimize the information and provide it according to the patient's needs. On the other hand, the side effects of the treatment method are framed and information is presented to the patients in a positive way, for example: “95% of patients tolerate this treatment without problems.” On the other hand, before starting the treatment, the patient's anxiety should be checked and then information should be provided.[68,69] The fourth method is different from the previous three methods. In this method, instead of reducing the information and reframing the information, the working method is to familiarize the patients with the effects of nocebo and then start the treatment. Explaining the nocebo effect is a method that can reduce side effects and facilitate treatment.[70] Lembo (2020) in response to the question, what strategies are there to reduce the effect of nocebo in patients with irritable bowel syndrome? responds that “I try to minimize potential nocebo effects in working with patients with irritable bowel syndrome by managing their expectations.[26] In other words, I emphasize the rare chance that complications may occur.” At the same time emphasize the patients who received placebo in the clinical trial and reported symptoms of side effects. For example, instead of saying that 10% of the patients in the clinical trial reported headache, I prefer to state that “Only 2% of patients in clinical trials reported headaches. Of course, many therapists don't emphasize side effects.” Complex expression reduces readability and creates a negative effect on the information presentation process.[71] The type of communication and lack of information can play a key role in the creation of nocebo effects and factors related to information transfer and ultimately negatively affect the effectiveness of treatment.[72,73]
Conclusion
Nocebo phenomenon as one of the new topics, which rooted in the negative expectations is very important both in the field of clinical trials and therapeutic interventions. A significant part of disproportionate responses to treatment, such as non-specific side effects, intolerance to treatment, as well as non- adherence, and overall unwanted reactions to treatment interventions, can be seen in patients’ negative expectations from treatment. Therefore, paying attention to the issue of nocebo phenomenon is of great clinical importance. From a research point of view, to determine the effectiveness of a treatment, researchers seek to eliminate placebo and nocebo effects in RCTs so that they can find the real effect of their intervention, while in therapeutic interventions we seek to increase the placebo effect and decrease the nocebo effect so that we can have any kind of therapeutic outcomes. The present study showed that not paying attention to the nocebo effect in clinical practice cause problems in the treatment outcomes and affect the effectiveness of the treatment. It is suggested that the topic of nocebo effect in clinical trials should be considered in future studies.
Ethics approval
Ethical approval was obtained through the Tehran Islamic Azad University of Medical Sciences Institutional Research Ethics Research Committee (IR.IAU.TMU.REC.1399.107), and the approval date was 2020.06.07.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are thankful to the participants of the SEPAHAN project and the authorities of Isfahan University of Medical Sciences for their excellent cooperation.
References
- 1.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, et al. The effect of treatment expectation on drug efficacy: Imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 2.Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M, et al. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat Neurosci. 2004;7:587–8. doi: 10.1038/nn1250. [DOI] [PubMed] [Google Scholar]
- 3.Rief W, Nestoriuc Y, von Lilienfeld-Toal A, Dogan I, Schreiber F, Hofmann SG, et al. Differences in adverse effect reporting in placebo groups in SSRI and tricyclic antidepressant trials: A systematic review and meta-analysis. Drug Saf. 2009;32:1041–56. doi: 10.2165/11316580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Cocco G. Erectile dysfunction after therapy with metoprolol: The Hawthorne effect. Cardiology. 2009;112:174–7. doi: 10.1159/000147951. [DOI] [PubMed] [Google Scholar]
- 5.Weimer K, Colloca L, Enck P. Placebo effects in psychiatry: Mediators and moderators. Lancet Psychiatry. 2015;2:246–57. doi: 10.1016/S2215-0366(14)00092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barsky AJ, Saintfort R, Rogers MP, Borus JF. Nonspecific medication side effects and the nocebo phenomenon. JAMA. 2002;287:622–7. doi: 10.1001/jama.287.5.622. [DOI] [PubMed] [Google Scholar]
- 7.Ladwig I, Rief W, Nestoriuc Y. What are the risks and side effects of psychotherapy?-development of an inventory for the assessment of negative effects of psychotherapy (INEP) Verhaltenstherapie. 2014 Jan 1;24(4):252–63. [Google Scholar]
- 8.Constantino MJ, Arnkoff DB, Glass CR, Ametrano RM, Smith JZ. Expectations. J Clin Psychol. 2011;67:184–92. doi: 10.1002/jclp.20754. [DOI] [PubMed] [Google Scholar]
- 9.Seewald A, Rief W. How to change negative outcome expectations in psychotherapy? The role of the therapist's warmth and competence. Clin Psychol Sci. 2023;11:149–63. [Google Scholar]
- 10.Benedetti F, Lanotte M, Lopiano L, Colloca L. When words are painful: Unraveling the mechanisms of the nocebo effect. Neuroscience. 2007;147:260–71. doi: 10.1016/j.neuroscience.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Schedlowski M, Enck P, Rief W, Bingel U. Neuro-bio-behavioral mechanisms of placebo and nocebo responses: Implications for clinical trials and clinical practice. Pharmacol Rev. 2015;67:697–730. doi: 10.1124/pr.114.009423. [DOI] [PubMed] [Google Scholar]
- 12.Faasse K, Huynh A, Pearson S, Geers AL, Helfer SG, Colagiuri B. The influence of side effect information framing on nocebo effects. Ann Behav Med. 2019;53:621–9. doi: 10.1093/abm/kay071. [DOI] [PubMed] [Google Scholar]
- 13.Mestre TA. Nocebo and lessebo effects. Int Rev Neurobiol. 2020;153:121–46. doi: 10.1016/bs.irn.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Shah E, Pimentel M. Placebo effect in clinical trial design for irritable bowel syndrome. J Neurogastroenterol Motil. 2014;20:163–70. doi: 10.5056/jnm.2014.20.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy WP. The nocebo reaction. Med World. 1961;95:203–5. [PubMed] [Google Scholar]
- 16.Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: A review of systematic reviews. Front Pharmacol. 2013;4:91. doi: 10.3389/fphar.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kessner S, Wiech K, Forkmann K, Ploner M, Bingel U. The effect of treatment history on therapeutic outcome: An experimental approach. JAMA Intern Med. 2013;173:1468–9. doi: 10.1001/jamainternmed.2013.6705. [DOI] [PubMed] [Google Scholar]
- 18.Petrie KJ, Rief W. Psychobiological mechanisms of placebo and nocebo effects: Pathways to improve treatments and reduce side effects. Ann Rev Psychol. 2019;70:599–625. doi: 10.1146/annurev-psych-010418-102907. [DOI] [PubMed] [Google Scholar]
- 19.Mahr A, Golmard C, Pham E, Iordache L, Deville L, Faure P. Types, frequencies, and burden of nonspecific adverse events of drugs: Analysis of randomized placebo‐controlled clinical trials. Pharmacoepidemiol Drug Saf. 2017;26:731–41. doi: 10.1002/pds.4169. [DOI] [PubMed] [Google Scholar]
- 20.Clark PI, Leaverton PE. Scientific and ethical issues in the use of placebo controls in clinical trials. Ann Rev Public Health. 1994;15:19–38. doi: 10.1146/annurev.pu.15.050194.000315. [DOI] [PubMed] [Google Scholar]
- 21.Tu Y, Zhang L, Kong J. Placebo and nocebo effects: From observation to harnessing and clinical application. Transl Psychiatry. 2022;12:524. doi: 10.1038/s41398-022-02293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arksey H, O’Malley L. Scoping studies: Towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. [Google Scholar]
- 23.Woods MD, Kirk MD, Agarwal MS, Annandale E, Arthur T, Harvey J, et al. Vulnerable groups and access to health care: A critical interpretive review. National coordinating centre NHS service delivery organ RD (NCCSDO) 2005;27:2012. [Google Scholar]
- 24.Nasiri-Dehsorkhi H, Vaziri S, Esmaillzadeh A, Adibi-Sedeh P. Nocebo and psychological factors in irritable bowel syndrome: A scoping review. Int J Body Mind Culture. 2022;9:271–84. [Google Scholar]
- 25.Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobson EE, et al. Components of placebo effect: Randomised controlled trial in patients with irritable bowel syndrome. Bmj. 2008;336:999–1003. doi: 10.1136/bmj.39524.439618.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lembo AJ. Understanding the placebo and nocebo effects in patients with irritable bowel syndrome. Gastroenterol Hepatol. 2020;16:374–6. [PMC free article] [PubMed] [Google Scholar]
- 27.Colloca L, Grillon C. Understanding placebo and nocebo responses for pain management. Curr Pain Headache Rep. 2014;18:1–7. doi: 10.1007/s11916-014-0419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corsi N, Colloca L. Placebo and nocebo effects: The advantage of measuring expectations and psychological factors. Front Psychol. 2017;8:308. doi: 10.3389/fpsyg.2017.00308. doi: 10.3389/fpsyg.2017.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geers AL, Wellman JA, Fowler SL, Helfer SG, France CR. Dispositional optimism predicts placebo analgesia. J Pain. 2010;11:1165–71. doi: 10.1016/j.jpain.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Pascalis V, Chiaradia C, Carotenuto E. The contribution of suggestibility and expectation to placebo analgesia phenomenon in an experimental setting. Pain. 2002;96:393–402. doi: 10.1016/S0304-3959(01)00485-7. [DOI] [PubMed] [Google Scholar]
- 31.Rütgen M, Seidel EM, Riečanský I, Lamm C. Reduction of empathy for pain by placebo analgesia suggests functional equivalence of empathy and first-hand emotion experience. J Neurosci. 2015;35:8938–47. doi: 10.1523/JNEUROSCI.3936-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rütgen M, Seidel EM, Silani G, Riečanský I, Hummer A, Windischberger C, et al. Placebo analgesia and its opioidergic regulation suggest that empathy for pain is grounded in self-pain. Proc Natl Acad Sci. 2015;112:E5638–46. doi: 10.1073/pnas.1511269112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vögtle E, Barke A, Kröner-Herwig B. Nocebo hyperalgesia induced by social observational learning. Pain. 2013;154:1427–33. doi: 10.1016/j.pain.2013.04.041. [DOI] [PubMed] [Google Scholar]
- 34.Gelfand DM, Gelfand S, Rardin MW. Some personality factors associated with placebo responsivity. Psychol Rep. 1965;17:555–62. doi: 10.2466/pr0.1965.17.2.555. [DOI] [PubMed] [Google Scholar]
- 35.Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. The anatomy of the mesolimbic reward system: A link between personality and the placebo analgesic response. J Neurosci. 2009;29:4882–7. doi: 10.1523/JNEUROSCI.5634-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staats PS, Staats A, Hekmat H. The additive impact of anxiety and a placebo on pain. Pain Med. 2001;2:267–79. doi: 10.1046/j.1526-4637.2001.01046.x. [DOI] [PubMed] [Google Scholar]
- 37.Lyby PS, Aslaksen PM, Flaten MA. Is fear of pain related to placebo analgesia? J Psychosom Res. 2010;68:369–77. doi: 10.1016/j.jpsychores.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Peciña M, Azhar H, Love TM, Lu T, Fredrickson BL, Stohler CS, et al. Personality trait predictors of placebo analgesia and neurobiological correlates. Neuropsychopharmacology. 2013;38:639–46. doi: 10.1038/npp.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corsi N, Emadi Andani M, Tinazzi M, Fiorio M. Changes in perception of treatment efficacy are associated to the magnitude of the nocebo effect and to personality traits. Sci Rep. 2016;6:30671. doi: 10.1038/srep30671. doi: 10.1038/srep30671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Świder K, Bąbel P. The effect of the sex of a model on nocebo hyperalgesia induced by social observational learning. Pain. 2013;154:1312–7. doi: 10.1016/j.pain.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Aslaksen PM, Lyby PS. Fear of pain potentiates nocebo hyperalgesia. J Pain Res. 2015;8:703–10. doi: 10.2147/JPR.S91923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. 2006;26:12014–22. doi: 10.1523/JNEUROSCI.2947-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wartolowska K. The nocebo effect as a source of bias in the assessment of treatment effects. F1000Res. 2019;8:5. doi: 10.12688/f1000research.17611.1. doi: 10.12688/f1000research.17611.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansen O, Brox J, Flaten MA. Placebo and nocebo responses, cortisol, and circulating beta-endorphin. Psychosom Med. 2003;65:786–90. doi: 10.1097/01.psy.0000082626.56217.cf. [DOI] [PubMed] [Google Scholar]
- 45.Benedetti F, Amanzio M, Maggi G. Potentiation of placebo analgesia by proglumide. Lancet. 1995;346:1231. doi: 10.1016/s0140-6736(95)92938-x. [DOI] [PubMed] [Google Scholar]
- 46.Lovick TA. Pro-nociceptive action of cholecystokinin in the periaqueductal grey: A role in neuropathic and anxiety-induced hyperalgesic states. Neurosci Biobehav Rev. 2008;32:852–62. doi: 10.1016/j.neubiorev.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL. Isolating the modulatory effect of expectation on pain transmission: A functional magnetic resonance imaging study. J Neurosci. 2006;26:4437–43. doi: 10.1523/JNEUROSCI.4463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–31. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 49.Svedman P, Ingvar M, Gordh T. “Anxiebo”, placebo, and postoperative pain. BMC Anesthesiol. 2005;5:1–6. doi: 10.1186/1471-2253-5-9. doi: 10.1186/1471-2253-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roderigo T, Benson S, Schöls M, Hetkamp M, Schedlowski M, Enck P, Elsenbruch S. Effects of acute psychological stress on placebo and nocebo responses in a clinically relevant model of visceroception. Pain. 2017;158:1489–98. doi: 10.1097/j.pain.0000000000000940. [DOI] [PubMed] [Google Scholar]
- 51.Häuser W, Hansen E, Enck P. Nocebo phenomena in medicine: Their relevance in everyday clinical practice. Dtsches Ärztebl Int. 2012;109:459–65. doi: 10.3238/arztebl.2012.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nestoriuc Y, Orav EJ, Liang MH, Horne R, Barsky AJ. Prediction of nonspecific side effects in rheumatoid arthritis patients by beliefs about medicines. Arthritis Care Res. 2010;62:791–9. doi: 10.1002/acr.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nestoriuc Y, Von Blanckenburg P, Schuricht F, Barsky AJ, Hadji P, Albert US, et al. Is it best to expect the worst? Influence of patients’ side-effect expectations on endocrine treatment outcome in a 2-year prospective clinical cohort study. Ann Oncol. 2016;27:1909–15. doi: 10.1093/annonc/mdw266. [DOI] [PubMed] [Google Scholar]
- 54.Miller FG. Clarifying the nocebo effect and its ethical implications. Am J Bioeth. 2012;12:30–1. doi: 10.1080/15265161.2011.652799. [DOI] [PubMed] [Google Scholar]
- 55.Blasini M, Corsi N, Klinger R, Colloca L. Nocebo and pain: An overview of the psychoneurobiological mechanisms. Pain Rep. 2017;2:e585. doi: 10.1097/PR9.0000000000000585. doi: 10.1097/PR9.0000000000000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varelmann D, Pancaro C, Cappiello EC, Camann WR. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110:868–70. doi: 10.1213/ANE.0b013e3181cc5727. [DOI] [PubMed] [Google Scholar]
- 57.Lang EV, Hatsiopoulou O, Koch T, Berbaum K, Lutgendorf S, Kettenmann E, et al. Can words hurt? Patient–provider interactions during invasive procedures. Pain. 2005;114:303–9. doi: 10.1016/j.pain.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 58.Luparello TJ, Leist N, Lourie CH, Sweet P. The interaction of psychologic stimuli and pharmacologic agents on airway reactivity in asthmatic subjects. Psychosom Med. 1970;32:509–14. doi: 10.1097/00006842-197009000-00009. [DOI] [PubMed] [Google Scholar]
- 59.Faasse K, Grey A, Horne R, Petrie KJ. High perceived sensitivity to medicines is associated with higher medical care utilisation, increased symptom reporting and greater information‐seeking about medication. Pharmacoepidemiol Drug Saf. 2015;24:592–9. doi: 10.1002/pds.3751. [DOI] [PubMed] [Google Scholar]
- 60.Colgan S, Faasse K, Martin LR, Stephens MH, Grey A, Petrie KJ. Perceptions of generic medication in the general population, doctors and pharmacists: A systematic review. BMJ Open. 2015;5:e008915. doi: 10.1136/bmjopen-2015-008915. doi: 10.1136/bmjopen-2015-008915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faasse K, Petrie KJ. The nocebo effect: Patient expectations and medication side effects. Postgrad Med J. 2013;89:540–6. doi: 10.1136/postgradmedj-2012-131730. [DOI] [PubMed] [Google Scholar]
- 62.Crichton F, Petrie KJ. Accentuate the positive: Counteracting psychogenic responses to media health messages in the age of the Internet. J Psychosom Res. 2015;79:185–9. doi: 10.1016/j.jpsychores.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 63.Bartley H, Faasse K, Horne R, Petrie KJ. You can't always get what you want: The influence of choice on nocebo and placebo responding. Ann Behav Med. 2016;50:445–51. doi: 10.1007/s12160-016-9772-1. [DOI] [PubMed] [Google Scholar]
- 64.Faasse K, Petrie KJ. From me to you: The effect of social modeling on treatment outcomes. Curr Dir Psychol Sci. 2016;25:438–43. [Google Scholar]
- 65.Witthöft M, Freitag I, Nußbaum C, Bräscher AK, Jasper F, Bailer J, et al. On the origin of worries about modern health hazards: Experimental evidence for a conjoint influence of media reports and personality traits. Psychol Health. 2018;33:361–80. doi: 10.1080/08870446.2017.1357814. [DOI] [PubMed] [Google Scholar]
- 66.Wells RE, Kaptchuk TJ. To tell the truth, the whole truth, may do patients harm: The problem of the nocebo effect for informed consent. Am J Bioeth. 2012;12:22–9. doi: 10.1080/15265161.2011.652798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bromwich D. Plenty to worry about: Consent, control, and anxiety. Am J Bioeth. 2012;12:35–6. doi: 10.1080/15265161.2012.656810. [DOI] [PubMed] [Google Scholar]
- 68.Bingel U. Avoiding nocebo effects to optimize treatment outcome. JAMA. 2014;312:693–4. doi: 10.1001/jama.2014.8342. [DOI] [PubMed] [Google Scholar]
- 69.Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: Minimize, maximize or personalize? Nat Rev Drug Discov. 2013;12:191–204. doi: 10.1038/nrd3923. [DOI] [PubMed] [Google Scholar]
- 70.Crichton F, Petrie KJ. Health complaints and wind turbines: The efficacy of explaining the nocebo response to reduce symptom reporting. Environ Res. 2015;140:449–55. doi: 10.1016/j.envres.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 71.Taylor HE, Bramley DE. An analysis of the readability of patient information and consent forms used in research studies in anaesthesia in Australia and New Zealand. Anaesth Intensive Care. 2012;40:995–8. doi: 10.1177/0310057X1204000610. [DOI] [PubMed] [Google Scholar]
- 72.Klinger R, Blasini M, Schmitz J, Colloca L. Nocebo effects in clinical studies: Hints for pain therapy. Pain Rep. 2017;2:e586. doi: 10.1097/PR9.0000000000000586. doi: 10.1097/PR9.0000000000000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kirby N, Shepherd V, Howick J, Betteridge S, Hood K. Nocebo effects and participant information leaflets: Evaluating information provided on adverse effects in UK clinical trials. Trials. 2020;21:1–8. doi: 10.1186/s13063-020-04591-w. doi: 10.1186/s13063-020-04591-w. [DOI] [PMC free article] [PubMed] [Google Scholar]


