Abstract
Psoriasis is a common chronic inflammatory skin disease. Abnormal proliferation of keratinocytes plays an important role in the pathogenesis of psoriasis. Long non-coding RNAs (lncRNAs) are involved in the regulation of a variety of cell biological processes. The purpose of this study was to investigate the potential role of lncRNA MIR181A2HG in the proliferation of human keratinocytes. qRT-PCR and Western blotting were performed to measure the expression levels of MIR181A2HG, SRSF1, KRT6, and KRT16 in tissue specimens and HaCaT keratinocytes. The effects of MIR181A2HG on HaCaT keratinocytes proliferation were evaluated using Cell Counting Kit-8 (CCK-8) assays, 5-Ethynyl-2’-deoxyuridine (EdU) incorporation, and cell-cycle assays. RNA pulldown-mass spectrometry (MS) was applied to identify the proteins interacting with MIR181A2HG. RNA pull-down-Western blotting and RNA immunoprecipitation coupled with real-time quantitative reverse transcription-PCR (RIP-qRT-PCR) assays were used to determine the interactions between MIR181A2HG and its RNA-binding proteins (RBPs). MIR181A2HG was down-regulated in psoriasis tissues. MIR181A2HG overexpression induced G0/G1 and G2/M phase cell cycle arrest and decreased the protein levels of KRT6, KRT16, Cyclin D1, CDK4, and Cyclin A2 in HaCaT keratinocytes. MIR181A2HG knockdown showed the opposite effect. By using RNA pulldown-MS, 356 proteins were identified to interact with MIR181A2HG potentially. Bioinformatics analysis showed that NOP56 and SRSF1 may be RNA binding proteins (RBPs) that may be interact with MIR181A2HG. Furthermore, by using RNA pull-down-Western blotting and RIP-qRT-PCR, SRSF1 was determined to interact with MIR181A2HG. Moreover, silencing of SRSF1 inhibited keratinocytes proliferation, which could be reversed with the knockdown of MIR181A2HG. Our findings indicated that MIR181A2HG can negatively regulate HaCaT keratinocytes proliferation by binding SRSF1, suggesting that MIR181A2HG and SRSF1 may serve as potential targets for the treatment of psoriasis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10616-024-00621-6.
Keywords: LncRNAs, MIR181A2HG, Keratinocytes, Proliferation, SRSF1, Psoriasis
Introduction
Psoriasis is one of the most common inflammatory skin diseases in humans, which is characterized by excessive proliferation and abnormal differentiation of keratinocytes in lesions, accompanied by inflammatory cell infiltration (Billi et al. 2019; Mahil and Smith 2019). So far, its pathogenesis has not been fully elucidated. However, it is believed that the disease is an autoimmune disease mediated by skin keratinocytes and immune cells under the influence of internal and external environmental factors. The abnormal crosstalk between keratinocytes and immune cells plays an important role in the pathogenesis of psoriasis(Albanesi et al. 2018; Furue et al. 2020; Ni and Lai 2020). The aberrant proliferation of keratinocytes is considered to be the amplifier of inflammatory response in psoriatic lesions (Afonina et al. 2021; Hawkes et al. 2017; Madonna et al. 2019; Orsmond et al. 2021). The study on the regulation of keratinocyte proliferation is of great significance for understanding the pathogenesis of psoriasis.
Long non-coding RNAs (LncRNAs) represent a group of non-coding RNAs with a length of more than 200 nucleotides. Increasing evidence has shown that lncRNAs are important regulators in almost all physical and pathological events, including the occurrence and development of psoriasis (Song et al. 2020; Statello et al. 2021). LncRNA PRINS was found to be up-regulated in psoriatic lesions, which could regulate the expression of anti-apoptotic gene G1P3 and directly interact with NPM to promote keratinocyte proliferation (Széll et al. 2016). MSX2P1 was found to be involved in the abnormal proliferation of keratinocytes induced by IL-22 as ceRNA (Qiao et al. 2018). RP6-65G23.1 could promote keratinocyte proliferation through the activation of AKT signaling pathway (Duan et al. 2020). MEG3 was reported to regulate the proliferation and apoptosis of keratinocytes by targeting caspase-8 with the absorption of miR-21 (Jia et al. 2019). We also previously found that MIR31HG was up-regulated in psoriatic lesions and could affect keratinocyte proliferation by regulating the cell cycle (Gao et al. 2018).
MIR181A2HG, a 617-nt lncRNA (NR_038975.1), was found to be down-regulated in vascular endothelial cells induced by high glucose and inhibit cell proliferation, suggesting that it may be a potential therapeutic target for HG‑induced endothelial dysfunction (Wang et al. 2021). However, the possible roles and mechanisms of MIR181A2HG in psoriasis are not investigated. In this current study, we found that MIR181A2HG is down-regulated in psoriatic lesions and functions as a proliferation-suppressor in keratinocytes by binding serine and arginine-rich splicing factor 1 (SRSF1). This study highlighted the important roles of MIR181A2HG in regulating the proliferation of keratinocytes, providing insights into the mechanism of abnormal proliferation of keratinocytes in psoriasis.
Methods
Tissue samples collection
Punch biopsies (4 mm) of 10 patients (7 male and 3 female, average age 53.3 years) with psoriasis who had not received any treatment for nearly 4 weeks were collected. Samples of healthy skin were taken from individuals undergoing plastic surgery. All samples were frozen in liquid nitrogen and stored at -80℃ until RNA extraction. Before participating in the study, all individuals provided informed consent. This study had been approved by the Ethics Committee of Guilin Medical University (GYLL2018060, 3/2/2018, Guilin, Guangxi, China).
Cell culture
HaCaT keratinocytes (Bio-73,031) were purchased from Kunming Cell Bank, Kunming Institute of Zoology, Chinese Academy of Sciences (Kunming, China), and cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% Fetal Bovine Serum (FBS) at 37℃ with 5% CO2. Primary normal human epidermal keratinocytes (NHEKs, FC-0007) were purchased from Lifeline Cell Technology (Walkersville, MD USA) and cultured in DermaLife Basal Medium supplemented with DermaLife K LifeFactors Kit (Lifeline Cell Technology) at 37℃ with 5% CO2.
Construction and transfection of plasmid
The full-length MIR181A2HG cDNA was cloned to pcDNA3.1(Invitrogen, Carlsbad, CA, USA). HaCaT keratinocytes were cultured to 80% confluence and transfected with MIR181A2HG overexpression plasmid (pcDNA3.1-MIR181A2HG) and the corresponding empty vector (pcDNA3.1) according to the instructions of Lipo8000™ transfection reagent (Beyotime Biotechnology, China) for 24–96 h.
siRNAs and transfection
Specific small interfering RNAs (siRNAs) and scramble siRNA (siNC) were designed and synthesized from Gene-Pharma (Shanghai, China) and transfected with siRNA-Mate transfection reagent (Gene-Pharma) according to the instructions. Briefly, HaCaT keratinocytes were cultured to 30–50% confluence and transfected with 50nM siRNA for 24–96 hours. The sequence of siMIR181A2HG was as follows: 5’-AGGUAGAUUCUGCAUCCACTT-3’. The sequence of siSRSF1 was as follows: 5’-GCCCAGAAGUCCAAGUUAUTT-3’.
Cell proliferation assay
CCK-8 kit (Dojindo, Kumamoto, Japan) was used to measure cell viability. HaCaT keratinocytes were seeded into 96-well plates, and transfected with siRNAs for 24–96 h. 10 µl CCK-8 solution was added to each well and incubated for 2 h at 37 °C in the dark. The absorbance value at 450 nm was measured using a Multiskan Spectrum (ThermoFisher).
5-ethynyl-2’-deoxyuridine (EdU) assay
We used the Beyo Click™ EdU-488 assay kit (Beyotime Biotechnology, China) to measure cell proliferation. According to the protocol, HaCaT keratinocytes were seeded into 12-well plates, cultured to 30–50% cell confluence, transfected for 48 h, and then incubated with 50 µM EdU for 2 h at 37 °C. Cells were fixed in 4% paraformaldehyde for 15 min and permeabilized with 0.5% Triton X-100 for 20 min at room temperature. After washing with PBS, stained with Beyo Click EdU solution and Hoechst 33,342. Images of the cells were acquired under a Nikon Eclipse Ti-U microscope (Nikon, Japan). EdU incorporation rate was evaluated using Image J software.
RNA extraction and quantitative RT-PCR assays (qRT-PCR)
Total RNA was isolated from HaCaT keratinocytes using TRIzol™ reagent (Invitrogen). cDNA was synthesized using the RevertAid First Strand cDNA Synthesis Kit (ThermoFisher). The quantitative PCR was carried out with SYBR Green qPCR Master Mix kit (TaKaRa) in CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad). The quantitative PCR reaction procedure was 95 °C for 5 min, followed by 40 cycles at 95 °C for 5 s, 60 °C for 10 s, and 72 °C for 10 s. GAPDH was used as an internal reference. Primers sequences were as follows:
MIR181A2HG, 5′-GTCGTTGCTGCTTTCTCCCA-3′ (forward) and.
5′-ACGGATCGAGAGCCTGTTAC-3′ (reverse);
KRT6, 5′-GGGTTTCAGTGCCAACTCAGCCAGGC-3′ (forward) and.
5′-CCATACAGACTGCGG-3′ (reverse);
KRT16, 5′-TTCCCCAGCTGCATATAAAGGT-3′ (forward) and.
5′-GCAGTTGGCTGAAAGAAGGAAA-3′ (reverse);
SRSF1, 5′-TCAGCCCACATCCTACTGGA-3′ (forward) and.
5′-GATGCACAGACGTGAGCACT-3′ (reverse);
GAPDH, 5′-CACATGGCCTCCAAGGAGTAA-3′ (forward) and.
5′-TGAGGGTCTCTCTCTTCCTCTTGT-3′ (reverse).
The relative expression was calculated by 2−ΔΔCt.
Flow cytometry analysis of cell cycle
The cells were collected after 48 h of transfection and fixed overnight in 70% ethanol at 4 °C. After washing three times with cold phosphate-buffered saline (PBS), sodium propanol iodide (PI) and RNase A were added and incubated for 30 min at room temperature. Finally, cell cycle analysis was performed using a FACS Aria III flow cytometer (BD Biosciences).
Western blotting
Cells were collected 48 h after transfection and lysed with cell lysis buffer (Be-yotime Biotechnol, China) containing 1 mM phenylmethylsulfonyl fluoride (PMSF) for 15 min on the ice. The supernatant protein concentration was measured using a Bradford Protein Concentration Kit (Solarbio, Beijing). Approximately 40 µg of protein was taken for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and later transferred to polyvinyl difluoride (PVDF) membranes, which were closed at room temperature for 2 h in 5% skimmed milk. The primary antibodies [Rabbit anti-KRT16 (ab181055), anti-Cyclin D1 (ab134175), anti-CDK4 (ab108357), anti-Cyclin A2 (ab32386), anti-SRSF1(ab129108)] (purchased from Abcam), [Mouse anti-KRT6 E2250041)] (purchased from EnoGene) and anti-GAPDH (purchased from zsbio) were incubated overnight at 4 °C. After being washed with 0.5% TBS-Tween (TBS-T) three times, the membranes were incubated with HRP-conjugated secondary antibody at room temperature for 2 h. Finally, the membranes were subjected to chemiluminescence substrate (Pierce, USA) and transferred to ChemiDoc™ XRS + System (Bio-Rad, USA) for visualizing protein signals. The intensity of protein bands was measured using ImageJ software. GAPDH is used as an internal reference.
RNA pulldown
MIR181A2HG pulldown probe (MIR181A2HG) and antisense strand negative control probe (Antisense) were designed and synthesized by BersinBio (Guangzhou, China). The RNA pulldown experiment was carried out with an RNA pulldown kit (BersinBio) according to the instructions. The main steps include: RNA secondary structure formation, probe magnetic bead preparation, total protein extraction of NHEKs, nucleic acid removal and pre-washing of protein samples, pulldown, and protein product collection.
RNA pulldown protein products were collected for SDS-PAGE, silver staining, mass spectrometry (MS), and Western blotting analysis. MS was performed on Q Exactive mass spectrometer (ThermoFisher) with a routine protocol.
The same amount of RNA pulldown protein products were separated by 10% SDS-PAGE and transferred onto PVDF membranes (Millipore, Billerica, MA, USA). Primary antibodies anti-SRSF1 (ab129108, Abcam), anti-NOP56 (YT5785, Immunoway), and the secondary antibody were used to detect the interaction protein. Protein bands were visualized in the ChemiDoc™ XRS + System (Bio-Rad).
RNA immunoprecipitation (RIP) assay
The RNA RIP kit (BersinBio) was used to investigate the SRSF1 and MIR181A2HG interaction. The cell lysates were incubated with RIP buffer containing magnetic beads conjugated with human anti-SRSF1 antibody (ab129108, Abcam) or IgG. The magnetic beads were centrifuged and washed with wash buffer, and the RNA was extracted and qRT-PCR was used to measure MIR18A2HG enrichment.
Bioinformatics analysis
The datasets GSE13355, GSE14905, and GSE50790 were obtained from Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). Human Protein Atlas (HPA)(http://www.proteinatlas.org/)and Genotype-Tissue Expression Project (GTEx) (https://gtexportal.org/) were applied to explore the expression level of MIR181A2HG in normal tissues. LncATLAS (http://lncatlas.crg.eu/) was used to query the subcellular localization of MIR181A2HG. To explore MIR181A2HG-related gene functions, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were performed using the cluster profile R package. To explore the function of differentially expressed RBPs, Gene ontology (GO) and KEGG enrichment analyses were performed by Metascape (http://metascape.org/). Search Tool for the Retrieval of Interacting Genes (STRING; http://string-db.org) was applied to predict the protein-protein interaction (PPI) network of MS-identified proteins. Cytoscape (Version 3.6.1, http://www.cytoscape.org) was used to construct a protein interaction network and a degree > 50 was considered the key gene.
Statistical analysis
Data are presented as means ± standard errors of at least three independent experiments. Graphpad Prism 6 software was used for statistical analysis. Statistical significance was analyzed by t-test. P < 0.05 was considered significant.
Results
MIR181A2HG was down-regulated in psoriatic lesions
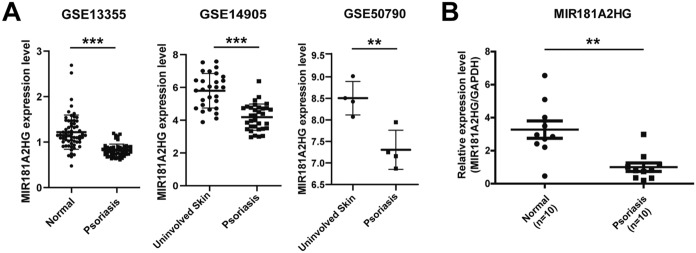
The datasets GSE13355, GSE14905 and GSE50790 were selected to explore the expression of MIR181A2HG in psoriatic lesions and normal skins. MIR181A2HG was found to be down-regulated in psoriatic lesions (Fig. 1A). qRT-PCR was performed to measure the expression level of MIR181A2HG in clinical specimens. Consistently, the results revealed that the RNA expression of MIR181A2HG was reduced in psoriatic lesions, compared with the normal skin tissues (Fig. 1B). HPA and GTEx database results showed that MIR181A2HG was highly expressed in skin tissues (Supplementary Fig. 1). Taken together, these results suggested that MIR181A2HG may be involved in the pathogenesis of psoriasis.
Fig. 1.
The expression of MIR181A2HG in psoriatic lesions and normal tissues. A The expression of MIR181A2HG in normal skin and psoriatic lesions was analyzed using publicly available GEO datasets (GSE13355, GSE14905 and GSE50790). ***P < 0.001, **P < 0.01. B qRT-PCR was applied to measure the expression of MIR181A2HG in the psoriatic lesions and normal skin. GAPDH served as an internal reference. **P < 0.01
MIR181A2HG was predicted to be involved in cell cycle regulation
LncATLAS database analysis showed that the cellular localization of MIR181A2HG in different cells was not identical and showed cellular specificity (Fig. 2A). Nuclear/cytoplasmic separation qRT-PCR result showed that MIR181A2HG transcript was localized both in the nucleus and cytoplasm, and slightly higher in the nucleus (Fig. 2B), implying that MIR181A2HG may play a regulatory role in a pre-transcriptional, transcriptional and post-transcriptional level. To predict the function of MIR181A2HG, KEGG enrichment analysis of MIR181A2HG-related genes was performed in GEO datasets (GSE13355, GSE14905 and GSE50790). Correlation analysis was performed between protein-coding genes and MIR181A2HG in GEO datasets. The protein-coding genes with MIR181A2HG correlation coefficients > 0.3 and P values < 0.05 were considered MIR181A2HG-related genes. KEGG enrichment analysis showed that MIR181A2HG-related genes were enriched in various pathways, including Cell cycle pathway (Fig. 2C and D). These results suggested that MIR181A2HG may play a potential role in the regulation of the cell cycle.
Fig. 2.
MIR181A2HG was predicted to be involved in regulating the cell cycle. A LncATLAS was used to explore the localization of MIR181A2HG. B Nuclear/cytoplasmic separation qRT-PCR was performed to detect the nuclear and cytoplasmic distribution of MIR181A2HG. GAPDH served as extranuclear RNA control. MALAT1 and U3 served as intranuclear RNA controls. C KEGG pathway enrichment analysis of MIR181A2HG-related genes. D The intersection of KEGG analysis results in three GEO datasets
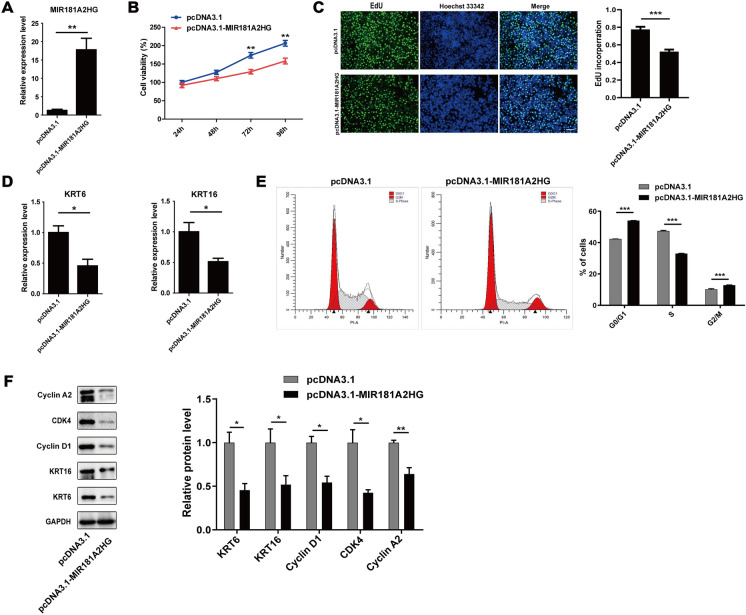
Enhanced expression of MIR181A2HG inhibited HaCaT keratinocytes proliferation
In order to determine the possible effects of MIR181A2HG on HaCaT keratinocyte proliferation, we transfected the overexpression vector of MIR181A2HG into HaCaT keratinocytes. qRT-PCR assay revealed that MIR181A2HG overexpression plasmid transfection significantly increased the MIR181A2HG level (Fig. 3A). CCK-8 assay showed that MIR181A2HG overexpression inhibited HaCaT keratinocyte proliferation (Fig. 3B). EdU assay indicated that the number of HaCaT keratinocytes incorporating EdU in the MIR181A2HG overexpression group was significantly reduced compared with control group (Fig. 3C). Keratin 6 (KRT6) and keratin 16 (KRT16) were thought to be the hall-marks of psoriatic keratinocytes hyperproliferation (Korge et al. 1990; Leigh et al. 1995; Thewes et al. 1991). qRT-PCR assay indicated that MIR181A2HG up-regulation resulted in a significant reduction of the expression of KRT6 and KRT16 (Fig. 3D). Interestingly, overexpression of MIR181A2HG significantly increased the proportion of G0/G1 and G2/M phase cells, whereas the proportion of S phase cells significantly decreased (Fig. 3E), suggesting that MIR181A2HG overexpression can inhibit the proliferation of HaCaT keratinocytes by inducing G0/G1 and G2/M phase arrest. Western blotting results further validated that MIR181A2HG overexpression decreased the expression of KRT6, KRT16, Cyclin D1, CDK4 and Cyclin A2 (Fig. 3F).
Fig. 3.
Effect of MIR181A2HG overexpression on proliferation of HaCaT keratinocytes. A HaCaT keratinocytes were seeded in 12-well plates and transfected with pcDNA3.1 or pcDNA3.1-MIR181A2HG for 48 h. qRT-PCR was applied to measure the expression of MIR181A2HG. **P < 0.01. B CCK-8 assays were carried out to record the growth curves of HaCaT keratinocytes transfected with pcDNA3.1 or pcDNA3.1-MIR181A2HG for 24–96 h. **P < 0.01. C EdU assays were used to detect the proliferation rate of HaCaT keratinocytes transfected with pcDNA3.1 or pcDNA3.1-MIR181A2HG for 48 h. Scale bars, 100 μm. ***P < 0.001. D KRT6 and KRT16 expression were measured by qRT-PCR in HaCaT keratinocytes transfected with pcDNA3.1 or pcDNA3.1-MIR181A2HG for 72 h. *P < 0.05. E The cell cycle distribution of HaCaT keratinocytes at 48 h transfected with pcDNA3.1 or pcDNA3.1-MIR181A2HG was analyzed by flow cytometry. ***P < 0.001. F Cells were collected and total proteins were extracted for Western blotting to detect KRT6, KRT16, Cyclin D1, CDK4 and Cyclin A2 protein levels. GAPDH served as an internal reference. **P < 0.01, *P < 0.05
Knockdown of MIR181A2HG promoted HaCaT keratinocytes proliferation
We further explored the impact of MIR181A2HG knockdown on the proliferation of HaCaT keratinocytes. qRT-PCR assay showed that MIR181A2HG expression was significantly suppressed by specific siRNA (Fig. 4A). CCK-8 assay revealed that MIR181A2HG knockdown could promote HaCaT keratinocytes growth (Fig. 4B). Consistently, the EdU incorporation was obviously increased after siRNA transfection (Fig. 4C). qRT-PCR assay showed that MIR181A2HG knockdown resulted in significant up-regulation of KRT6 and KRT16 expression in HaCaT keratinocytes (Fig. 4D). Flow cytometry assay results indicated that MIR181A2HG knockdown significantly decreased the percentage of G0/G1 phase cells, while the percentage of S phase cells was remarkably increased (Fig. 4E). Furthermore, Western blotting results showed that KRT6, KRT16, Cyclin D1, CDK4 and Cyclin A2 expression were markedly up-regulated with MIR181A2HG knockdown (Fig. 4F).
Fig. 4.
Effect of MIR181A2HG knockdown on the proliferation of HaCaT keratinocytes. A HaCaT keratinocytes were seeded in 12-well plates and transfected with 50 nM scramble siRNA (siNC) or MI181A2HG siRNA (siMIR181A2HG) for 48 h. qRT-PCR was performed to measure the expression of MIR181A2HG. *P < 0.05. B CCK-8 assays were carried out to record the growth curves of HaCaT keratinocyte transfected with 50 nM siNC or siMIR181A2HG for 24–96 h. *P < 0.05. C EdU assays were used to detect the proliferation rate of HaCaT keratinocytes transfected with siNC or siMIR181A2HG for 48 h. Scale bars, 100 μm. **P < 0.01. D KRT6 and KRT16 expression were measured by qRT-PCR in HaCaT keratinocytes transfected with siNC or si-MIR181A2HG for 72 h. *P < 0.05. E The cell cycle distribution of HaCaT keratinocytes at 48 h transfected with 50nM siNC or siMIR181A2HG was analyzed by flow cytometry. ***P < 0.001, **P < 0.01. F Cells were collected and total proteins were extracted for Western blotting to detect KRT6, KRT16, Cyclin D1, CDK4 and Cyclin A2 protein levels. GAPDH served as an internal reference. *P < 0.05
MIR181A2HG could interact with SRSF1 in keratinocytes
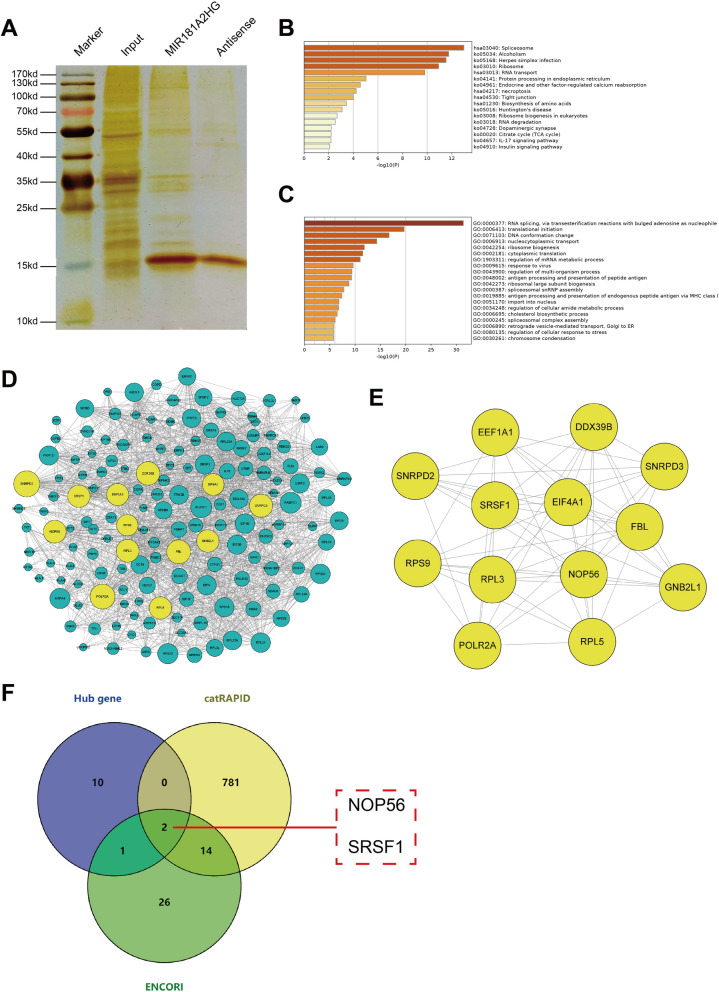
As important regulatory RNAs, lncRNAs can play a variety of regulatory roles by binding to proteins. In order to screen the proteins interacted with MIR181A2HG, RNA pulldown-MS was used to identify the proteins that may bind to MIR181A2HG. Compared with the antisense strand probe, MIR181A2HG probe could pull down multiple proteins. 356 proteins were finally identified by MS (Fig. 5A). KEGG pathway enrichment analysis showed that these proteins were mainly related to spliceosome, RNA transport, protein processing (Fig. 5B). Gene ontology (GO) biological process analysis showed that these proteins were mainly involved in RNA processing and transport, mRNA metabolism (Fig. 5C). These results suggested that MIR181A2HG may interact with RNA-binding proteins (RBPs) to regulate RNA processing process, thereby affecting cell proliferation. We used Cytoscape software to construct the interaction network of these proteins (Fig. 5D). Among them, 13 genes were considered as key genes (degree > 50) (Fig. 5E). The detailed information of these genes was shown in Supplemental Table 1. In order to explore the possible RBPs interacting with MIR181A2HG, we took the intersection of these 13 key proteins with the interaction proteins predicted in catRAPID and ENCORI databases. The result showed that NOP56 and SRSF1 may be RBPs interacting with MIR181A2HG (Fig. 5F).
Fig. 5.
Screening of MIR181A2HG interaction protein. A The silver staining result of RNA pulldown proteins. B Pathway analysis of MIR181A2HG interacting proteins. C GO biological process analysis of MIR181A2HG interacting protein. D The construction of protein interaction network of MIR181A2HG interaction protein. E The interaction network of 13 key genes. F The intersection of 13 key proteins with the proteins predicted by catRAPID and ENCORI
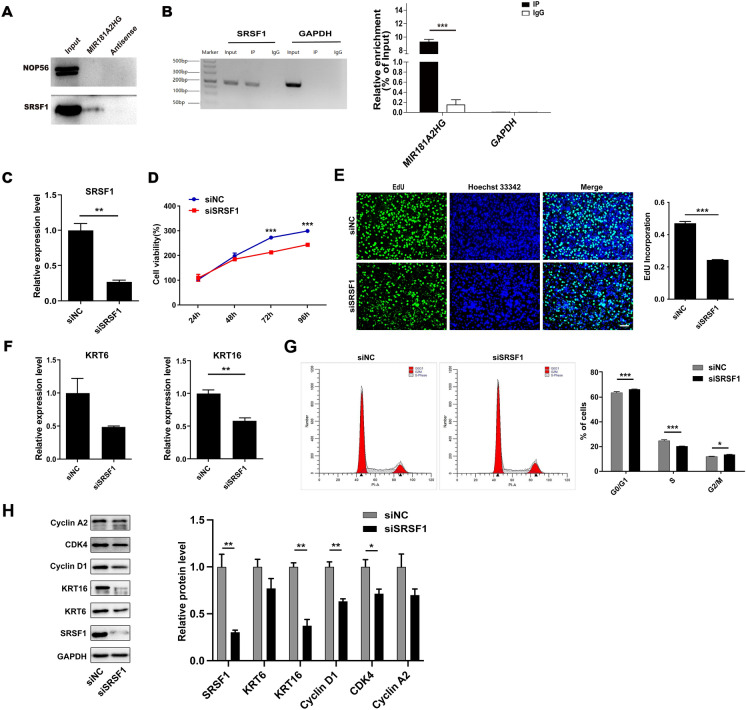
In order to confirm whether MIR181A2HG interacts with NOP56 and SRSF1, RNA pulldown-Western blotting assay was performed. The results indicated that MIR181A2HG probe could bind to SRSF1, but not to NOP56 (Fig. 6A). RIP-qRT-PCR was carried out to further confirm the interaction between MIR181A2HG and SRSF1. The results showed that MIR181A2HG was enriched in SRSF1 but not GAPDH immunoprecipitates in NHEKs (Fig. 6B). These results suggested that MIR181A2HG may function through binding SRSF1.
Fig. 6.
MIR181A2HG could bind to SRSF1. A RNA pulldown-Western blotting assay was used to verify the interaction of MIR181A2HG and NOP56/SRSF1. B RIP-qRT-PCR assay was performed to confirm the interaction between MIR181A2HG and SRSF1. C HaCaT keratinocytes were seeded in 12-well plates and transfected with 50 nM scramble siRNA (siNC) or SRSF1 siRNA (siSRSF1) for 48 h. qRT-PCR was applied to measure the expression of SRSF1. **P < 0.01. D CCK-8 assays were carried out to record the growth curves of HaCaT keratinocytes transfected with 50nM siNC or siSRSF1 for 24–96 h. ***P < 0.001. E EdU assays were used to detect the proliferation rate of HaCaT keratinocytes transfected with 50nM siNC or siSRSF1 for 48 h. Scale bars, 100 μm. ***P < 0.001. F KRT6 and KRT16 expression were measured by qRT-PCR in HaCaT keratinocytes transfected with 50nM siNC or siSRSF1 for 72 h. **P < 0.01. G The cell cycle distribution of keratinocytes at 48 h transfected with 50nM siNC or siSRSF1 was analyzed by flow cytometry. ***P < 0.001, *P < 0.05. H Cells were collected and total proteins were extracted for Western blotting to detect KRT6, KRT16, Cyclin D1, CDK4 and Cyclin A2 protein levels. GAPDH served as an internal reference. **P < 0.01, *P < 0.05
To further explore the role of SRSF1 in keratinocytes proliferation, siRNA was used to knockdown the expression of SRSF1 in HaCaT keratinocytes. qRT-PCR assay showed that the specific siRNA targeting SRSF1 remarkably reduced its expression (Fig. 6C). The results of CCK-8 assay showed that SRSF1 knockdown significantly decreased cell proliferation compared with siNC group (Fig. 6D). Consistent with the CCK-8 results, SRSF1 knockdown resulted in a decrease in the proportion of EdU-incorporated cells (Fig. 6E). qRT-PCR revealed that knockdown SRSF1 induced down-regulation of KRT6 and KRT16 expression (Fig. 6F). Flow cytometry assay results indicated that interference with SRSF1 increased the percentage of G0/G1 and G2/M phase cells, while the percentage of S phase cells significantly decreased (Fig. 6G). Moreover, Western blotting results showed that SRSF1 knockdown could inhibit KRT6, KRT16, Cyclin D1, CDK4 and Cyclin A2 expression (Fig. 6H). These data indicated that SRSF1 could be involve in regulating HaCaT keratinocytes proliferation.
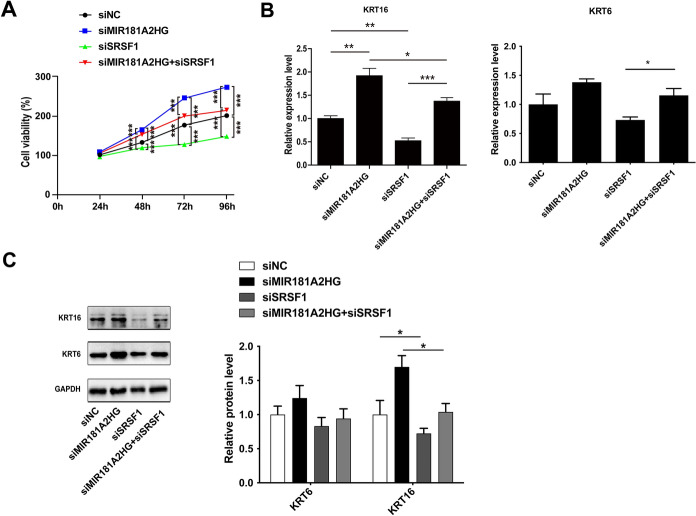
In order to investigate whether MIR181A2HG regulates keratinocytes proliferation is dependent on SRSF1, we performed rescue experiments. Interestingly, CCK-8 assay showed that SRSF1 knockdown inhibited the HaCaT keratinocytes proliferation, which could be restored by MIR181A2HG knockdown (Fig. 7A). This conclusion was also confirmed by qRT-PCR and Western blotting (Fig. 7B and C).
Fig. 7.
The knockdown of MIR181A2HG could partially restore the inhibitory effect of cell proliferation caused by SRSF1 knockdown. A CCK-8 assay for cell viability in HaCaT keratinocytes transfected with 50 nM siRNA (siNC), MIR181A2HG siRNA (siMIR181A2HG) or SRSF1 siRNA (siSRSF1) for 24–96 h. ***P < 0.001. B KRT6 and KRT16 expression were measured by qRT-PCR in HaCaT keratinocytes transfected with 100nM siNC, siMIR181A2HG or siSRSF1. ***P < 0.001, **P < 0.01, *P < 0.05. C Cells were collected and total proteins were extracted for Western blotting to detect KRT6 and KRT16 protein levels. GAPDH served as an internal reference. *P < 0.05
Discussion
LncRNAs are a kind of important regulatory RNA. A large number of researches have shown that lncRNAs play important regulatory roles in epigenetic, transcriptional and post-transcriptional levels, thereby affecting a variety of biological processes including growth, proliferation and differentiation, and its abnormal function can lead to a variety of diseases (Beermann et al. 2016; Esteller 2011; Shi et al. 2013). In recent years, transcriptome studies have shown that the expression profile of lncRNAs differs between the lesions of patients with psoriasis and normal individuals’ skin tissues (Tsoi et al. 2015), suggesting that lncRNAs may play a potential role in the pathogenesis of psoriasis. In this study, we found that lncRNA MIR181A2HG was expressed at a high level in skin tissues among 53 normal human tissues but was expressed at a low level in psoriatic lesions (Fig. 1, Supplementary Fig. 1), suggesting that it may be involved in the pathogenesis of psoriasis by regulating skin homeostasis. Previous studies have shown that MIR181A2HG is down-regulated in vascular endothelial cells induced by high glucose and acts as a ceRNA to target AKT2 and inhibit its expression, thereby inhibiting the proliferation and migration of vascular endothelial cells (Wang et al. 2021). However, we found that the expression of MIR181A2HG is negatively associated with the proliferative capability of keratinocytes, exhibiting anti-proliferative effects. These results suggested that MIR181A2HG may serve as different proliferation modulators to regulate cell fates in different cells.
The intracellular localization of lncRNAs provides an important clue for the study of their function (Statello et al. 2021). In this study, we found that MIR181A2HG was distributed both inside and outside the nucleus, suggesting that MIR181A2HG may play a regulatory role inside or outside the nucleus. KEGG enrichment revealed that MIR181A2HG may play a role in cell cycle regulation (Fig. 2). The cell cycle is divided into four phases, which are coordinated by many protein factors, closely related to cell proliferation, and control the normal growth and metabolism of cells (Sun et al. 2022). Cell cycle progression was positively regulated by cell cycle proteins and CDKs (Wang 2021). Cyclin A/D has been reported to be a key cell cycle protein in S and G2/M phases and is widely recognized as a marker of cell proliferation (Liu et al. 2012; Montalto and De Amicis 2020). The results showed that MIR181A2HG could modulate keratinocyte proliferation through regulating cell cycle (Figs. 3 and 4). LncRNAs often exert their functions by interacting with proteins. In this study, 356 proteins were identified as potential targets interacting with MIR181A2HG by RNA pulldown-MS analysis (Fig. 5). Enrichment analysis results indicated that these proteins mainly enrich the RNA processing process, implying that MIR181A2HG may interact with RBPs to regulate the RNA processing process. Recruiting RBPs appears to be a common mechanism among lncRNAs (Zhang et al. 2017). Lnc 10 binds to QKI-5 and regulates germ cell apoptosis via the p38 MAPK signaling pathway (Li et al. 2019). LncRNA CCDC26 interacts with CELF2 to enhance myeloid leukemia cell proliferation and invasion (Li et al. 2021). In the current study, MIR181A2HG was found to directly interact with SRSF1 to affect the proliferation of keratinocytes (Fig. 6). SRSF1 is a classic member of the selective splicing factor SR protein family, which is involved in a variety of physiological processes of precursor mRNA (Das and Krainer 2014). By regulating the splicing of genes, SRSF1 affects the expression and function of downstream related proteins, and then regulates cell proliferation, autophagy, and apoptosis (Chang and Lin 2019; Lv et al. 2021; Sheng et al. 2018). SRSF1 seems to be an important binding target for lncRNAs. It has been found that several lncRNAs including PFI, MIR155HG, GASAL1, AGAP2-AS1, and HOXA11-AS could interact with SRSF1, thus participating in the regulation of cell proliferation and apoptosis (Li et al. 2020; Liu et al. 2019, 2020; Shen et al. 2020; Sun et al. 2021). SRSF1 was found to be up-regulated and function as an oncogene in various cancers, such as hepatocellular carcinoma (Xu et al. 2020), breast cancer (Anczuków et al. 2015) and gastric cancer (Wu et al. 2019). Here, we found that inhibition of SRSF1 attenuated the proliferation of keratinocytes, exhibiting a positive proliferative regulatory effect, which could be reversed by the knockdown of MIR181A2HG (Fig. 7). These results suggested that MIR181A2HG can bind to SRSF1 and inhibit its pro-proliferative effect in keratinocytes. LncRNA PFI was found protect against pulmonary fibrosis by interacting with SRSF1 and block its function (Sun et al. 2021). In the current study, we found similar regulatory roles of MIR181A2HG. The down-regulation of MIR181A2HG mediated hyper-proliferation of keratinocytes might be derived from insufficient blockade of SRSF1, suggesting that combining with SRSF1 and interfering with its function seems to be an important way for lncRNA to function. However, identification of downstream genes and further investigation of specific mechanisms involved in psoriatic keratinocytes hyper-proliferation should be elucidated.
Conclusions
In conclusion, the results of present work demonstrated that lncRNA MIR181A2HG was down-regulated in psoriatic lesions and negatively regulated keratinocyte proliferation by binding SRSF1. This study will provide evidence that MIR181A2HG plays an important role in regulation of keratinocytes hyper-proliferation in psoriasis and may be an attractive therapeutic target.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary material 1 (DOCX 254.4 kb)
Acknowledgements
Not applicable.
Author contributions
J.G. conceived and designed the study. X.F., M.L., M.N., F.C., Z.M., P.Y., and M.W. performed experiments. Q.L., and B.L. performed bioinformatics analysis. X.F. drafted manuscript. S.G. and C.W. analyzed data and reviewed manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by National Natural Science Foundation of China (32360167 and 31860314), Natural Science Foundation of Guangxi Zhuang Autonomous Region (2018GXNSFAA281041 and 2016GXNSFBA380146), Innovation Project of Guangxi Graduate Education (YCSW2023420) and Graduate Research Program of Guilin Medical University (GYYK2021003).
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
This study had been approved by the Ethics Committee of Guilin Medical University (GYLL2018060, 3/2/2018, Guilin, Guangxi, China). Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaomei Fan and Mingzhao Li contributed equally to this work.
References
- Afonina IS, Van Nuffel E, Beyaert R. Immune responses and therapeutic options in psoriasis. Cell Mol Life Sci. 2021;78:2709–2727. doi: 10.1007/s00018-020-03726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanesi C, Madonna S, Gisondi P, Girolomoni G. The interplay between keratinocytes and Immune cells in the pathogenesis of psoriasis. Front Immunol. 2018;9:1549. doi: 10.3389/fimmu.2018.01549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anczuków O, Akerman M, Cléry A, Wu J, Shen C, Shirole NH, Krainer AR. SRSF1-regulated alternative splicing in breast cancer. Mol Cell. 2015;60:105–117. doi: 10.1016/j.molcel.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev. 2016;96:1297–1325. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- Billi AC, Gudjonsson JE, Voorhees JJ. Psoriasis: past, present, and future. J Invest Dermatol. 2019;139:e133–e142. doi: 10.1016/j.jid.2019.08.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HL, Lin JC. SRSF1 and RBM4 differentially modulate the oncogenic effect of HIF-1α in lung cancer cells through alternative splicing mechanism. Biochim Biophys Acta Mol Cell Res. 2019;1866:118550. doi: 10.1016/j.bbamcr.2019.118550. [DOI] [PubMed] [Google Scholar]
- Das S, Krainer AR. Emerging functions of SRSF1, splicing factor and oncoprotein, in RNA metabolism and cancer. Mol Cancer Res. 2014;12:1195–1204. doi: 10.1158/1541-7786.Mcr-14-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q, Wang G, Wang M, Chen C, Zhang M, Liu M, Zheng Y. LncRNA RP6-65G23.1 accelerates proliferation and inhibits apoptosis via p-ERK1/2/p-AKT signaling pathway on keratinocytes. J Cell Biochem. 2020;121:4580–4589. doi: 10.1002/jcb.29685. [DOI] [PubMed] [Google Scholar]
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- Furue M, Furue K, Tsuji G, Nakahara T. Interleukin-17A and Keratinocytes in psoriasis. Int J Mol Sci. 2020;21:1275. doi: 10.3390/ijms21041275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Chen F, Hua M, Guo J, Nong Y, Tang Q, Qin L. Knockdown of lncRNA MIR31HG inhibits cell proliferation in human HaCaT keratinocytes. Biol Res. 2018;51:30. doi: 10.1186/s40659-018-0181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140:645–653. doi: 10.1016/j.jaci.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HY, Zhang K, Lu WJ, Xu GW, Zhang JF, Tang ZL. LncRNA MEG3 influences the proliferation and apoptosis of psoriasis epidermal cells by targeting miR-21/caspase-8. BMC Mol Cell Biol. 2019;20:46. doi: 10.1186/s12860-019-0229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge B, Stadler R, Mischke D. Effect of retinoids on hyperproliferation-associated keratins K6 and K16 in cultured human keratinocytes: a quantitative analysis. J Invest Dermatol. 1990;95:450–455. doi: 10.1111/1523-1747.ep12555613. [DOI] [PubMed] [Google Scholar]
- Leigh IM, Navsaria H, Purkis PE, McKay IA, Bowden PE, Riddle PN. Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br J Dermatol. 1995;133:501–511. doi: 10.1111/j.1365-2133.1995.tb02696.x. [DOI] [PubMed] [Google Scholar]
- Li K, Zhong S, Luo Y, Zou D, Li M, Li Y, Song W. A long noncoding RNA binding to QKI-5 regulates germ cell apoptosis via p38 MAPK signaling pathway. Cell Death Dis. 2019;10:699. doi: 10.1038/s41419-019-1941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Guo S, Zhang M, Li L, Wang F, Song B. Long non-coding RNA AGAP2-AS1 accelerates cell proliferation, migration, invasion and the EMT process in colorectal cancer via regulating the miR-4,668-3p/SRSF1 axis. J Gene Med. 2020;22:e3250. doi: 10.1002/jgm.3250. [DOI] [PubMed] [Google Scholar]
- Li C, Mu J, Shi Y, Xin H. LncRNA CCDC26 interacts with CELF2 protein to enhance myeloid leukemia cell proliferation and invasion via the circRNA_ANKIB1/miR-195-5p/PRR11 Axis. Cell Transpl. 2021;30:963689720986080. doi: 10.1177/0963689720986080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liu Y, Ma C, Zhang Q, Yu L, Ma J, Zhang L, Zhu D. The key role of transforming growth factor-beta receptor I and 15-lipoxygenase in hypoxia-induced proliferation of pulmonary artery smooth muscle cells. Int J Biochem Cell Biol. 2012;44:1184–1202. doi: 10.1016/j.biocel.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang YM, Ma FB, Pan SR, Liu BZ. Long noncoding RNA HOXA11-AS promotes gastric cancer cell proliferation and invasion via SRSF1 and functions as a biomarker in gastric cancer. World J Gastroenterol. 2019;25:2763–2775. doi: 10.3748/wjg.v25.i22.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang Q, Ma N. LncRNA GASAL1 interacts with SRSF1 to regulate trophoblast cell proliferation, invasion, and apoptosis via the mTOR signaling pathway. Cell Transpl. 2020;29:963689720965182. doi: 10.1177/0963689720965182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y, Zhang W, Zhao J, Sun B, Qi Y, Ji H, Wang Y. SRSF1 inhibits autophagy through regulating Bcl-x splicing and interacting with PIK3C3 in lung cancer. Signal Transduct Target Ther. 2021;6:108. doi: 10.1038/s41392-021-00495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madonna S, Girolomoni G, Dinarello CA, Albanesi C. The significance of IL-36 hyperactivation and IL-36R targeting in psoriasis. Int J Mol Sci. 2019;20:3318. doi: 10.3390/ijms20133318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahil SK, Smith CH. Psoriasis biologics: a new era of choice. Lancet. 2019;394:807–808. doi: 10.1016/s0140-6736(19)31772-6. [DOI] [PubMed] [Google Scholar]
- Montalto FI, De Amicis F. Cyclin D1 in cancer: a molecular connection for cell cycle control, adhesion and invasion in tumor and stroma. Cells. 2020;9:2648. doi: 10.3390/cells9122648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X, Lai Y. Keratinocyte: a trigger or an executor of psoriasis? J Leukoc Biol. 2020;108:485–491. doi: 10.1002/jlb.5mr0120-439r. [DOI] [PubMed] [Google Scholar]
- Orsmond A, Bereza-Malcolm L, Lynch T, March L, Xue M. Skin barrier dysregulation in psoriasis. Int J Mol Sci. 2021;22:10841. doi: 10.3390/ijms221910841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao M, Li R, Zhao X, Yan J, Sun Q. Up-regulated lncRNA-MSX2P1 promotes the growth of IL-22-stimulated keratinocytes by inhibiting mir-6731-5p and activating S100A7. Exp Cell Res. 2018;363:243–254. doi: 10.1016/j.yexcr.2018.01.014. [DOI] [PubMed] [Google Scholar]
- Shen L, Li Y, Hu G, Huang Y, Song X, Yu S, Xu X. MIR155HG Knockdown inhibited the progression of cervical cancer by binding SRSF1. Onco Targets Ther. 2020;13:12043–12054. doi: 10.2147/ott.S267594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng J, Zhao Q, Zhao J, Zhang W, Sun Y, Qin P, Wang Y. SRSF1 modulates PTPMT1 alternative splicing to regulate lung cancer cell radioresistance. EBioMedicine. 2018;38:113–126. doi: 10.1016/j.ebiom.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Sun M, Liu H, Yao Y, Song Y. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339:159–166. doi: 10.1016/j.canlet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Song JK, Yin SY, Li W, Li XD, Luo Y, Luo Y, Kuai L. An update on the role of long non-coding RNAs in psoriasis. Chin Med J. 2020;134:379–389. doi: 10.1097/cm9.0000000000001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Jin T, Su W, Guo Y, Niu Z, Guo J, Liang H. The long non-coding RNA PFI protects against pulmonary fibrosis by interacting with splicing regulator SRSF1. Cell Death Differ. 2021;28:2916–2930. doi: 10.1038/s41418-021-00792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Tian Y, He J, Tian Y, Zhang G, Zhao R, Gao P. Linc01133 contributes to gastric cancer growth by enhancing YES1-dependent YAP1 nuclear translocation via sponging miR-145-5p. Cell Death Dis. 2022;13:51. doi: 10.1038/s41419-022-04500-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Széll M, Danis J, Bata-Csörgő Z, Kemény L. PRINS, a primate-specific long non-coding RNA, plays a role in the keratinocyte stress response and psoriasis pathogenesis. Pflugers Arch. 2016;468:935–943. doi: 10.1007/s00424-016-1803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thewes M, Stadler R, Korge B, Mischke D. Normal psoriatic epidermis expression of hyperproliferation-associated keratins. Arch Dermatol Res. 1991;283:465–471. doi: 10.1007/bf00371784. [DOI] [PubMed] [Google Scholar]
- Tsoi LC, Iyer MK, Stuart PE, Swindell WR, Gudjonsson JE, Tejasvi T, Elder JT. Analysis of long non-coding RNAs highlights tissue-specific expression patterns and epigenetic profiles in normal and psoriatic skin. Genome Biol. 2015;16:24. doi: 10.1186/s13059-014-0570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Regulation of cell cycle progression by growth factor-induced cell signaling. Cells. 2021;10:3327. doi: 10.3390/cells10123327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zheng B, Zhao H, Li Y, Zhang X, Wen J. Downregulation of lncRNA MIR181A2HG by high glucose impairs vascular endothelial cell proliferation and migration through the dysregulation of the miRNAs/AKT2 axis. Int J Mol Med. 2021;47:1. doi: 10.3892/ijmm.2021.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZH, Liu CC, Zhou YQ, Hu LN, Guo WJ. OnclncRNA-626 promotes malignancy of gastric cancer via inactivated the p53 pathway through interacting with SRSF1. Am J Cancer Res. 2019;9:2249–2263. [PMC free article] [PubMed] [Google Scholar]
- Xu L, Wang Z, Yin C, Pan F, Shi T, Tian Y. Long noncoding RNA LINC02580 suppresses the invasion-metastasis cascade in hepatocellular carcinoma by targeting SRSF1. Biochem Biophys Res Commun. 2020;533:685–691. doi: 10.1016/j.bbrc.2020.10.061. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wei Y, Yan Z, Wu C, Chang Z, Zhu Y, Xu Y. The characteristic landscape of lncRNAs classified by RBP-lncRNA interactions across 10 cancers. Mol Biosyst. 2017;13:1142–1151. doi: 10.1039/c7mb00144d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (DOCX 254.4 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article.