Abstract
Background and Aims
Throughout the Cenozoic, Africa underwent several climatic and geological changes impacting the evolution of tropical rain forests (TRFs). African TRFs are thought to have extended from east to west in a ‘pan-African’ TRF, followed by several events of fragmentation during drier climate periods. During the Miocene, climate cooling and mountain uplift led to the aridification of tropical Africa and open habitats expanded at the expense of TRFs, which probably experienced local extinctions. However, in plants, these drivers were previously inferred using limited taxonomic and molecular data. Here, we tested the impact of climate and geological changes on diversification within the diverse clade Monodoreae (Annonaceae) composed of 90 tree species restricted to African TRFs.
Methods
We reconstructed a near-complete phylogenetic tree, based on 32 nuclear genes, and dated using relaxed clocks and fossil calibrations in a Bayesian framework. We inferred the biogeographical history and the diversification dynamics of the clade using multiple birth–death models.
Key Results
Monodoreae originated in East African TRFs ~25 million years ago (Ma) and expanded toward Central Africa during the Miocene. We inferred range contractions during the middle Miocene and document important connections between East and West African TRFs after 15–13 Ma. Our results indicated a sudden extinction event during the late Miocene, followed by an increase in speciation rates. Birth–death models suggested that African elevation change (orogeny) is positively linked to speciation in this clade.
Conclusion
East Africa is inferred as an important source of Monodoreae species, and possibly for African plant diversity in general. Our results support a ‘sequential scenario of diversification’ in which increased aridification triggered extinction of TRF species in Monodoreae. This was quickly followed by fragmentation of rain forests, subsequently enhancing lagged speciation resulting from vicariance and improved climate conditions. In contrast to previous ideas, the uplift of East Africa is shown to have played a positive role in Monodoreae diversification.
Keywords: Annonaceae, aridification, biogeography, birth–death models, diversification, East Africa, forest fragmentation, macroevolution, tropical rain forests, Madagascar, Dahomey Gap
INTRODUCTION
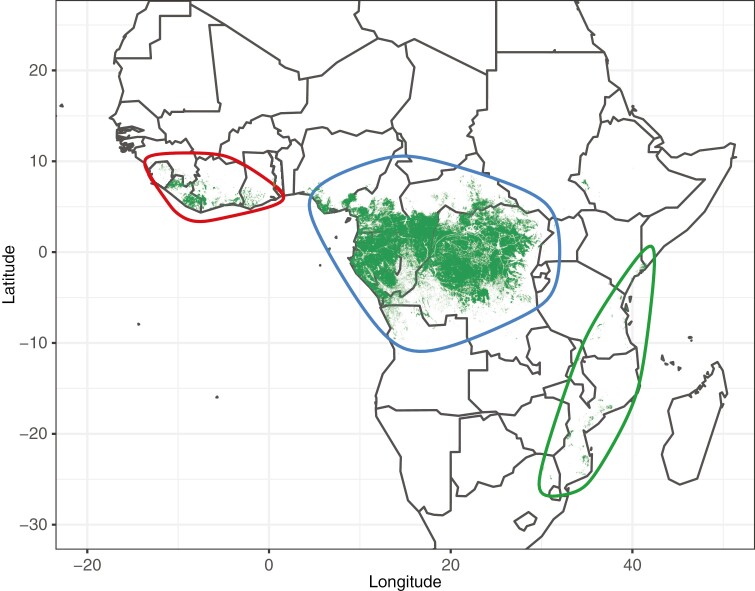
Tropical rain forests (TRFs) are one of the more biodiverse ecosystems on Earth. Covering just 7 % of the land area, they contain over half of the world’s biodiversity (Wilson, 1988; Eiserhardt et al., 2017). After the Amazon basin, the Guineo-Congolian region of Africa contains the second largest continuous extent of TRF in the world (Malhi et al., 2014). The African TRFs are divided in two major forested blocks: West-Central and East TRFs (Fig. 1; Olson et al., 2001; Couvreur et al., 2008; Droissart et al., 2018; Brée et al., 2020). The West-Central block is divided into two smaller blocks separated by the Dahomey gap located in Benin. The current distribution of TRFs across the continent is thought to be the result of various climatic and tectonic events that have shaped the distribution and evolution of African TRFs biodiversity (Morley, 2000; Couvreur et al., 2021). A recent review identified six major geo-climatic periods affecting the evolution of tropical Africa biodiversity (Couvreur et al., 2021). In particular, the Miocene (~23–5.3 million years ago, Ma) is considered crucial in terms of diversification of the African flora (Plana, 2004; Couvreur et al., 2021). For example, temperature increased during the middle Miocene Climatic Optimum (MMCO, ~17–14.7 Ma) and TRFs expanded across tropical Africa due to a warmer and moister climate (Morley, 2000, 2011). Then, from the middle Miocene Climatic Transition (MMCT, ~15–13 Ma), global temperatures and pCO2 dropped (Zachos et al., 2008; Westerhold et al., 2020). These climate changes, as well as the uplift of East Africa, led to the aridification of Africa throughout the Miocene (Sepulchre et al., 2006; Herbert et al., 2016) favouring the expansion of open, grass-dominated habitats (Retallack et al., 1990; Morley, 2000; Jacobs, 2004; Senut et al., 2009). From 10 Ma, palaeo-vegetation records show an increasing percentage of C4 plants until the present (Ségalen et al., 2007; Uno et al., 2016; Polissar et al., 2019) and increases in diversification rates of herbaceous plants such as Poaceae and Asteraceae (Kergoat et al., 2018; Palazzesi et al., 2022), indicating increasing dominance of grasslands across Africa. While open grassland expanded, TRFs contracted across its distribution (Plana, 2004). This contraction is suggested to have led to vicariance events within TRF clades before the MMCT, as detected in several phylogenetic studies (Couvreur et al., 2008; Dimitrov et al., 2012; Menegon et al., 2014; Brée et al., 2020). As drier habitats expanded, some forest-adapted clades experienced habitat shifts towards drier habitats during the Miocene (Davis et al., 2002; Bouchenak-Khelladi et al., 2010; Armstrong et al., 2014; Tosso et al., 2018; Veranso-Libalah et al., 2018). In the early Pliocene (~5.3–3.6 Ma), the climate became warmer and moister (Haywood et al., 2013) and the TRFs re-expanded while savannas contracted (Morley, 2000; Plana, 2004), suggesting reconnection of the West-Central and East TRF blocks (Fer et al., 2017; Joordens et al., 2019).
Fig. 1.
Map of the tropical rain forest of Africa (after Mayaux et al., 2004): Western block (red), Central block (blue), Eastern block (green) and Madagascar (yellow).
These climatic changes and rain forest dynamics gave rise to one of the major hypotheses explaining the distribution of TRF clades in Africa: the fragmentation–refugia mechanism (Couvreur et al., 2008, 2021; Pokorny et al., 2015). This mechanism links multi-million-year climatic fluctuations and continent-wide expansion–contraction of TRF dynamics to numerous vicariant speciation events in TRF-restricted clades (Moritz et al., 2000; Plana, 2004; Loader et al., 2007; Couvreur et al., 2008, 2021). This suggests the existence of a ‘pan-African forest’ extending continuously from Western to Eastern Africa during favourable climatic times, and fragmenting into isolated Western-Central and Eastern blocks during drier climate periods (Morley, 2000, 2011; Plana, 2004; Couvreur et al., 2021). The fragmentation–refugia mechanism was suggested to explain the present-day distribution patterns of some African TRF clades with species restricted to East or West-Central Africa in plants (Davis et al., 2002; Couvreur et al., 2008; Brée et al., 2020), butterflies (Aduse-Poku et al., 2021), birds (Fjeldså et al., 2007; Fjeldså and Bowie, 2008; Voelker et al., 2010; Huntley and Voelker, 2016; Fuchs et al., 2017), chameleons (Tolley et al., 2013; Nkonmeneck et al., 2022), frogs (Bell et al., 2017; Leaché et al., 2019) and mammals (Demos et al., 2014; Bryja et al., 2017; Nicolas et al., 2020). To date, this biogeographical mechanism has been inferred based on few data (Couvreur et al., 2008) at the taxonomic and molecular marker sampling level, especially in plants. In particular, the number of vicariant events used to infer this mechanism was generally low (Davis et al., 2002; Couvreur et al., 2008; Brée et al., 2020).
Another major hypothesis explaining African TRFs diversity is the impact of extinction (Morley and Richards, 1993; Couvreur, 2015). Under the environmental changes, contraction of the did not only triggered speciation via allopatric speciation, but also probably triggered extinction across TRF clades (Morley and Richards, 1993; Plana, 2004; Aduse-Poku et al., 2021). Major extinction events in TRF clades were suggested to have occurred at the Eocene–Oligocene boundary ~33.9 Ma (Pan et al., 2006; Faye et al., 2016; Currano et al., 2021), in the first half of the Miocene between 23 and 13.8 Ma (Morley, 2000; Couvreur, 2015), in the late Miocene around 11.6 and 7.2 Ma (Morley, 2011), and in the late Pliocene ~3.6–2.58 Ma (Morley, 2000; Couvreur, 2015). However, signal of past extinction in African TRF plants has rarely been tested with a comprehensive dated phylogeny (e.g. Brée et al., 2020).
The opposite view to these hypotheses is that speciation and extinction remained relatively constant through time. Indeed, African TRFs have lower extant species richness compared to TRFs from the Americas and from South-East Asia (Parmentier et al., 2007; Couvreur, 2015). One of the hypotheses invoked to explain such a pattern is that diversification was rather constant in African TRFs, whereas American and South-East Asian TRFs experienced bursts of speciation (Gentry, 1982; Couvreur, 2015). This hypothesis has been supported by global-scale molecular studies of TRF clades showing no diversification shifts in the African lineages (Erkens et al., 2012; Kissling et al., 2012; Baker and Couvreur, 2013).
The bursts of speciation in the American TRFs are in part attributed to Andean uplift (Hoorn et al., 2010; Lagomarsino et al., 2016; Boschman and Condamine, 2022). Indeed, mountain uplift is generally linked to increased diversification, through various mechanisms such as the creation of new habitats or population isolation (Dimitrov et al., 2012; Antonelli et al., 2018; Muellner-Riehl, 2019; Rahbek et al., 2019; Couvreur et al., 2021). These topographical uplifts also have indirect effects via the modification of climate (Sepulchre et al., 2006). In Africa, mountains harbour high levels of species diversity and endemism (Fjeldså and Lovett, 1997; Burgess et al., 2007). East Africa, which has the highest topographical complexity compared to the rest of Africa, has been inferred to be rich in both neo- and palaeo-endemics (Dagallier et al., 2020). Several geological uplifts occurred during the Cenozoic (Guillocheau et al., 2018; Couvreur et al., 2021), and the uplift of East Africa was documented to have started during the late Miocene (Griffiths, 1993; Sepulchre et al., 2006; Macgregor, 2015; Couvreur et al., 2021). However, the impact of mountain uplift on TRF clade diversification has never been tested.
The Monodoreae tribe is an African TRF-restricted clade of the plant family Annonaceae. The tribe contains, to date, 11 genera and 90 species (Dagallier et al., in press) restricted to Africa, with one genus also occurring in Madagascar (Isolona). Species in Monodoreae show high levels of endemism across the discrete TRF blocks, occurring either in West-Central or East Africa, with a single species occurring across both blocks (Dagallier, 2021; Dagallier et al., in press). To date, there is little knowledge about the mode of dispersal across the tribe. However, fruit morphology provides some indication about dispersal. Species of this tribe have large fruits (generally >3 cm long), most of them cauliflorous (i.e. growing on the trunk), lack the typical stipe at the base of each individual monocarp composing fruits in Annonaceae (van Setten and Koek-Noorman, 1992) and are generally dull-coloured, varying from brown, to green and orange. This fruit morphology suggests dispersal syndromes targeting mainly ruminant mammals (Gautier-Hion et al., 1985). Indeed, some studies have shown that species of Monodoreae are included in the diet of TRF understorey mammals such as gorillas, chimpanzees and forest elephants (Gautier-Hion et al., 1985; Tutin and Fernandez, 1993; White et al., 1993; Remis et al., 2001; Rogers et al., 2004). Cauliflorous fruits in particular limit plant dispersal because they target understorey dispersers, which are relatively sedentary and habitat-specific (Onstein et al., 2018, 2019). Monodoreae are thus unlikely to disperse over long distances (i.e. hundreds or thousands of kilometres), and especially not across different habitats. Finally, very few species have adapted to dry conditions (e.g. Hexalobus monopetalus, Botermans et al., 2011) Together, these data suggest that tribe Monodoreae is probably TRF-restricted with limited opportunities for long-distance dispersal, and thus is a good model to test hypotheses about TRF dynamics through time, in particular the fragmentation–refugia mechanism (Couvreur et al., 2008).
Here we infer a near complete species-level dated phylogenomic tree of Monodoreae to test: (1) if the ancestral range of the tribe extended across Africa, supporting the existence of a pan-African rain forest as suggested under the fragmentation–refugia mechanism; (2) if multiple discrete and synchronous speciation events occurred between major forest blocks and if they coincided with increased climatic aridity in Africa leading to TRF fragmentation; (3) if diversification was constant, or if TRFs experienced major increases or decreases of diversification, or sudden extinctions; and (4) if diversification was associated with changes in palaeotemperature, proportion of C4 plants or elevation linked to mountain uplift.
MATERIAL AND METHODS
Taxon sampling and species distribution
Species delimitation within Monodoreae was previously investigated and validated using a densely sampled phylogenetic tree and morphology (Dagallier, 2021; Dagallier et al., in press). This led to the description of several new species and the placement of two genera within the new tribe Ophrypetaleae. Here, we sampled 88 of the 90 known species of Monodoreae (97.8 % of the total species richness). Differences in species number with Dagallier (2021) where 92 species of Monodoreae were reported are due to recent changes: the name Uvariopsis sessiliflora is now a synonym of U. dioica, and ‘U. sp. nov. 1 Uganda’ has yet to be formally described due to limited morphological data (Dagallier et al., in press). One specimen per species was sampled, except in the following two cases. In cases of species with described varieties or subspecies, one specimen per variety or subspecies was sampled. Most of the species are geographically restricted to one of the following regions: East Africa, West Africa, Central Africa, or West and Central Africa. In the case of species distributed in more than one region, we included one specimen per region when possible. Our dataset also comprised outgroups within the Annonaceae from different closely related tribes, such as the Ophrypetaleae (see Dagallier, 2021; Dagallier et al., in press) and Uvarieae tribes, and from more distantly related tribes (Malmeoideae, Annonoideae). We also included one specimen of the genus Anaxagorea which is the earliest diverging Annonaceae genus (Couvreur et al., 2019), and one specimen of Eupomatiaceae (Eupomatia), which is the sister family of Annonaceae (Sauquet et al., 2003). Details on the vouchers used in this study can be found in Supplementary DataTable S1. DNA extractions and library preparations were done following the protocols described in Couvreur et al. (2019), Dagallier (2021) and Dagallier et al. (in press). DNA was sequenced on an Illumina HiSeq 2500 with paired-end reads of 150 bp.
Molecular dataset
For each specimen in our dataset, we retrieved cleaned reads from previous analyses (Dagallier, 2021; Dagallier et al., in press). These reads were then processed and assembled using HybPiper 1.3.1 (Johnson et al., 2016). The reads were aligned to the reference (i.e. the sequences of the 469 nuclear genes targeted) using Burrow–Wheeler Alignment (Li and Durbin, 2009) and sorted according to the targeted gene. Reads were then assembled into contigs using SPAdes (Bankevich et al., 2012). Contigs were then aligned to the reference and intron sequences were then generated partially (or completely in case of short introns). Finally, the contigs and the introns were assembled into supercontigs (later also called ‘genes’ or ‘loci’), i.e. reconstructed genes with multiple exons and partial introns.
The sequences of each gene were then aligned using MAFFT 7.305 (Katoh and Standley, 2013) using the automatic selection of the alignment algorithm (parameter ‘--auto’). Poorly aligned regions were cleaned using Gblocks 0.91b (Talavera and Castresana, 2007), and the alignments were filled with gaps so that all the sequences in the alignment have the same length and so that every species is included in the alignment. We filtered the dataset to retain only genes for which the exon sequences mapped at least at 75 % of the reference gene and that were retrieved in 75 % of the species (75/75 dataset).
HybPiper also includes a tool that flags some of the retrieved genes as potential paralogues where different contigs targeted the same reference gene with similar coverage depth (i.e. meaning that variants of the same gene may occur). We executed an exploratory maximum-likelihood phylogenetic reconstruction of these putative paralogues using RAxML 8.2.9 (Stamatakis, 2014), and examined the reconstructed phylogeny to check whether the variants of the genes formed two clear clusters of species. If so, the alignment for this gene was discarded from the dataset.
Using Bayesian inference (BI) to simultaneously infer phylogenetic relationships and divergence times allows us to explicitly incorporate topological and age uncertainties. However, the use of BI with hundreds of genes is computationally impossible to achieve in a reasonable time. We thus applied a ‘gene shopping’ approach to select a subset of the most clock-like genes to ease convergence and reduce the computational time (Smith et al., 2018). We first reconstructed a maximum-likelihood phylogenetic tree with RAxML for each gene. Each gene tree was then rooted with the outgroup Eupomatia using phyx (Brown et al., 2017) and we calculated the root-to-tip variance of the trees using SortaDate (Smith et al., 2018). We then selected a subset composed of the 32 most clock-like genes, that is the 32 genes with the lowest root-to-tip variance. This gene selection strategy has already been used to infer dated phylogenetic trees in Annonaceae (Brée et al., 2020; Helmstetter et al., 2020). Note that we tried with a higher number of genes, but BEAST could not run. For each of these 32 genes, we inferred the best fitting substitution model using ModelTest-NG based on the Bayesian Information Criterion (Darriba et al., 2020).
Phylogenetic reconstruction and molecular dating
We simultaneously reconstructed and dated the phylogenetic tree of Monodoreae using BEAST 2.6.4 (Bouckaert et al., 2019). The alignment of the 32 selected genes (see above) was first converted from fasta to nexus format using PGDSpider (Lischer and Excoffier, 2012) and was assigned a molecular partition in BEAUTi (Bouckaert et al., 2019). Substitution models were defined for each gene following the best model retrieved by ModelTest-NG (see Supplementary DataTable S2) with no estimation of the substitution rate.
To calibrate the molecular clock, we relied on primary fossil calibrations to provide age priors at specific nodes. First, the fossil Endressinia brasiliana is a leafy shoot dated from the Aptian–Albian boundary (113.4–112.6 Ma), which confers a maximum age for the crown node of Magnoliineae (i.e. including Annonaceae and Eupomatiaceae) as shown by its phylogenetic placement (Massoni et al., 2015b). Second, the fossil Futabanthus asamigawaensis is a flower dated from the early Coniacian (~ 89 Ma) and provides a minimum age for the crown node of Annonaceae (Pirie and Doyle, 2012). We thus constrained the root of the tree (i.e. Annonaceae + Eupomatiaceae) with a hard upper bound at 112.6 Ma and we set a wide uniform prior from 89 to 112.6 Ma on the crown Annonaceae (see nodes marked with an orange triangle in Supplementary DataFig. S1).
We set the tree prior as the Yule (pure birth) model, and the clock model as the uncorrelated log-normal (UCLD) model (Drummond et al., 2006) with estimation of the clock rate. We ran four Markov chain Monte Carlo (MCMC) in parallel, running for 500 million generations each, sampling every 50 000 generations. We evaluated the convergence of the MCMC with the effective sampling size (ESS) using Tracer 1.7 (Rambaut et al., 2018). We then used TreeAnnotator to draw the maximum clade credibility (MCC) tree, and to compute the mean node ages, the 95 % highest posterior density (HPD) of node ages and the posterior probability (PP) for each node, after discarding the burn-in. We then pruned the dated MCC tree to keep only the Monodoreae tribe and perform downstream analyses.
Biogeographical history
To reconstruct the ancestral ranges of the Monodoreae and to identify vicariant events, we relied on the Dispersion–Extinction–Cladogenesis (DEC) model (Ree and Smith, 2008) as implemented in the BioGeoBEARS R package (Matzke, 2014). We did not run the DEC + J model because founder events (or colonization of new ranges via jump dispersal without intermediate widespread dispersion) are very unlikely in Monodoreae due to their low dispersal abilities (see Introduction), and because the DEC + J model inflates the contribution of time-independent cladogenetic events to the likelihood of the model (Ree and Sanmartín, 2018).
First, we assigned each Monodoreae species to a geographical area, corresponding to a TRF region (Fig. 1) in which the species are currently distributed (West Africa, Central Africa, East Africa or Madagascar). Most of the species were assigned to a single region (endemic range), but some of them were assigned to a widespread range (e.g. West-Central Africa). We restricted the allowed ranges to West (W), Centre (C), East (E), Madagascar (M), West-Centre (WC), Centre-East (CE), East-Madagascar (EM) and West-Centre-East (WCE) and constrained the maximum number of areas per ancestral range to three. We also defined an ‘area adjacency’ matrix in which Madagascar is only adjacent to East-Africa, and defined the dispersal probabilities between areas to one, except for the dispersal probabilities from and to Madagascar that were set to zero, and the dispersal from East to Madagascar that was set to 0.1.
The marginal probabilities of each range were then plotted on the ancestral nodes and we considered the most likely ancestral range as the one having the highest marginal probability. We then considered several biogeographical events to occur at the ancestral nodes. A branch starting at an ancestral node with an endemic range (W, C, E or M) leading to at least one daughter node with a widespread range (WC, CE, EM or WCE) was considered as a range expansion (like ‘dispersal’ in BioGeoBEARS). A branch starting at an ancestral node with widespread range (WC, CE, EM or WCE) leading to one daughter node with an endemic range (W, C, E or M) was considered as a range contraction (‘sympatry subset’ in BioGeoBEARS). Finally, an ancestral node with widespread range (WC, CE, EM or WCE) leading to two daughter nodes with a different endemic range each (W, C, E or M) was considered as a vicariance event (‘vicariance narrow’ in BioGeoBEARS). The branches (for range expansion or range contraction events) and nodes (for vicariance events) at which each of these events occurred were then plotted.
Diversification analyses
Testing for branch- and clade-specific diversification rates.
To test for diversification rate shifts across the reconstructed tree, we used three different methods designed to estimate the branch- or clade-specific speciation and extinction rates. The net diversification rate is defined as speciation minus extinction (later referred to as ‘diversification rate’). The use of several different models allows us to cross-check the estimations (Condamine et al., 2018). Nonetheless, it is worth mentioning that each method differs at several points in the way speciation and extinction rates are estimated.
First, we used the cladogenetic diversification rate shift (ClaDS) model to estimate branch-specific diversification rates. ClaDS implements a birth–death model where speciation rates are inherited at a speciation event, but with a shift drawn from a probability distribution parameterized with the parental rates (Maliet et al., 2019). The extinction rate also varies across branches, but the turnover (i.e. speciation divided by extinction) is constant across the branches. The model is then computed using data augmentation in an MCMC with three chains. To assess convergence, the Gelman statistics are then computed every 200 iterations and the chains stop when these statistics drop below 1.05. We used the ClaDS model as implemented in the PANDA Julia package (Maliet and Morlon, 2021).
Second, we used RevBayes to model the estimation of branch-specific diversification (speciation and extinction) rates (BSDR; Höhna et al., 2019). The BSDR model is a birth–death model that breaks time into small intervals and for which speciation and extinction rates can change at each time interval. The new speciation and extinction rates at a new interval are drawn from a lognormal distribution. To ease the computation, the lognormal distribution is approximated using discrete rate categories. We set up the model following the RevBayes tutorial (available at: https://revbayes.github.io/tutorials/divrate/branch_specific.html). The parameters were then estimated using two reverse-jumping MCMC (rjMCMC) of 4000 iterations each, sampling every 200 iterations. We performed several analyses with different sets of priors to check the consistency of the results between the different prior specifications. We set the discrete rate categories prior to 6 or 10, and the initial value of the number of expected rate shift to 1, 4 or 10. The convergence of the rjMCMC was checked with ESS > 200 using Tracer 1.7 (Rambaut et al., 2018).
Third, we used the Bayesian analysis of macroevolutionary mixtures (BAMM) to estimate the variation of speciation and extinction rates through time, but also to detect significant clade-specific shifts in these rates (Rabosky, 2014). BAMM uses rjMCMC to explore the space of parameters and the number of rate shifts. We set prior values using the BAMMtools R package (Rabosky et al., 2014), with an expected number of rate shifts equal to 1. We ran four rjMCMC for 10 000 000 generations, sampling every 10 000 generations. We then calculated the ESS of the log-likelihood and the number of shifts using the coda R package (Plummer et al., 2020) after discarding 10 % of burn-in to assess the convergence of the chains (ESS > 200).
Each of these three models accounts for the sampling fraction in the calculation of the parameters. For ClaDS and BAMM, we set the sampling fraction to be clade-specific, with every genus fully sampled (i.e. a sampling fraction of 1) except for Uvariopsis and Lukea having a sampling fraction of 0.85 and 0.5, respectively. For the BSDR model, we set the sampling fraction for the whole phylogeny at ~0.978.
Testing for sudden extinction events.
To test whether the evolutionary history of the Monodoreae was impacted by one or several sudden extinction events, we used the compound Poisson process on Mass-Extinction Times model (CoMET; May et al., 2016) as implemented in the TESS R package (Höhna et al., 2016). This model estimates the tree-wide speciation and extinction rates, and the probability of several tree-wide events occurring, such as shifts in speciation rate, shifts in extinction rates or sudden extinction events (i.e. when several lineages go extinct with a prior probability). The speciation and extinction rates are constant between two events. The events are estimated under the independent compound Poisson process. Parameters are estimated using a an rjMCMC over various episodic birth–death models. Model confidence is assessed by computing the Bayes factor (BF) between each model (May et al., 2016). For our CoMET analysis, we set the priors on the number of extinction events and number of expected changes to 2. Note that the prior on the number of events does not impact the results as the models are compared using BFs (Höhna et al., 2015). We performed ten different CoMET analyses with prior values on the survival probability to an extinction event ranging every 5 % from 5 to 50 %. The rjMCMC was run until the ESS for each parameter reached at least 500. We considered the support for a rate shift or for a sudden extinction event as substantially significant for 2lnBF > 2, as strongly significant for 2lnBF > 6 and as decisive for 2lnBF > 10 (Kass and Raftery, 1995; Höhna et al., 2015).
Testing the impact of the palaeoenvironment.
To test if the diversification of the Monodoreae was associated with changes in past environmental variables (temperature, elevation variation of the African continent, and proportion of C4 plants in the African palaeoflora), we fitted several birth–death models that estimate the speciation and extinction rates along a continuous period of time in a maximum-likelihood framework. We set the models allowing the speciation and extinction rates to vary exponentially with time (time-dependent models) or exponentially with environmental variables (environment-dependent models) (Condamine et al., 2013). We also fitted models with constant or null speciation and extinction rates. The best fitting model was then evaluated using the corrected Akaike Information Criterion (AICc). The complete list of models fitted is given in Table 1.
Table 1.
Results of the environment-dependent birth–death models applied to the Monodoreae with RPANDA. When the rates are not constant, they vary exponentially with time or with the environmental variables (temperature = Temp, or elevation = Elev, or percentage of C4 plants = PercC4). logL, log-likelihood; λ0, speciation rate; α, rate of variation in speciation according to the palaeo-environmental variable; μ0, extinction rate; β, rate of variation in extinction according to the palaeo-environmental variable.
| Model | Speciation varies with: | Extinction varies with: | logL | AICc | λ0 | α | μ0 | β | ΔAICc |
|---|---|---|---|---|---|---|---|---|---|
| BDPercC4 | C4 proportion | C4 proportion | −224.08 | 456.63 | 1.6829 | −0.0439 | 2.1736 | −0.0656 | −12.66 |
| BElev | Elevation | No extinction | −232.57 | 469.29 | 0.0017 | 0.0066 | − | − | 0 |
| BElevDConst | Elevation | Constant | −232.63 | 471.55 | 0.0029 | 0.0059 | 0.0005 | − | 2.26 |
| BTemp | Temperature | No extinction | −234.17 | 472.48 | 0.1255 | 0.0829 | − | − | 3.19 |
| BConst | Constant | No extinction | −235.75 | 473.55 | 0.1797 | NA | − | − | 4.26 |
| BPercC4 | C4 proportion | No extinction | −234.83 | 473.81 | 0.2396 | −0.0072 | − | − | 4.52 |
| BConstDPercC4 | Constant | C4 proportion | −234.06 | 474.41 | 0.1963 | NA | 0.3638 | −0.0958 | 5.12 |
| BTempDConst | Temperature | Constant | −234.17 | 474.62 | 0.1254 | 0.083 | 0 | − | 5.33 |
| BTime | Time | No extinction | −235.67 | 475.49 | 0.1865 | −0.0072 | − | − | 6.2 |
| BDConst | Constant | Constant | −235.75 | 475.63 | 0.1796 | NA | 0 | − | 6.34 |
| BPercC4DConst | C4 proportion | Constant | −234.83 | 475.96 | 0.2396 | −0.0072 | 0 | − | 6.67 |
| BDTemp | Temperature | Temperature | −234.17 | 476.82 | 0.1255 | 0.0829 | 0 | 0.0156 | 7.53 |
| BTimeDConst | Time | Constant | −235.67 | 477.63 | 0.1864 | −0.0071 | 0 | − | 8.34 |
| BConstDTime | Constant | Time | −235.75 | 477.78 | 0.1797 | NA | 0 | −0.0499 | 8.49 |
| BConstDElev | Constant | Elevation | −235.75 | 477.79 | 0.1797 | NA | 0.0809 | −0.0781 | 8.5 |
| BConstDTemp | Constant | Temperature | −235.75 | 477.79 | 0.1796 | NA | 0 | −0.0016 | 8.5 |
| BDTime | Time | Time | −235.67 | 479.83 | 0.1865 | −0.0072 | 0 | 0.0259 | 10.54 |
| BDElev | Elevation | Elevation | −236.44 | 481.36 | 0.2674 | −0.0005 | 0 | 0.0022 | 12.07 |
The environment-dependent models use environmental data spanning the time interval covered by the phylogenetic tree. Missing values were interpolated using the spline interpolation implemented in the sm.spline function from the pspline R package (Ripley, 2017). Past temperature at global scales was inferred from the widely used oxygen isotope data recovered from benthic foraminifer shells (Zachos et al., 2008; Westerhold et al., 2020). Past elevation change across the African continent was newly computed using the data from Scotese & Wright (2018). First, the geo-referenced occurrences of the Monodoreae species were extracted from the RAINBIO database (Dauby et al., 2016), and the convex hull containing all the point coordinates was computed using the chull function from the grDevices R package. After conversion to SpatialPolygonsDataFrame object with the sp R package (Pebesma et al., 2021), the intersection of the envelope with the African coastline was computed using the gIntersection function in the rgeos R package (Bivand et al., 2020). Within this intersected envelope, we computed the geo-coordinates of every unique point with latitude and longitude values at one degree precision (e.g. points with coordinates 10°N 10°E, 10°N 11°E, 10°N 12°E, etc.), and extracted the elevation value for each of these points every 5 million years (Myr) from the present to 30 Ma, using the reconstruct function in the chronosphere R package (Kocsis and Raja, 2021). We then computed the mean elevation for each time.
Finally, the proportion of C4 plants in the African palaeoflora was taken from the reconstructions made by Pollisar et al. (2019) based on the composition of the 13C isotope in plant waxes. To represent the proportion of C4 plants in tropical Africa, we selected the data from the sites 959 (Guinean Gulf), 241 and 235 (East Africa). Given the oldest C4 value at 23.38 Ma is very low compared to the values from 15 to 20 Ma (1.66 vs. 10.69–13.05), we replaced it by the lowest value found between 15 and 20 Ma (i.e. 10.69) to avoid distortion of the interpolation toward negative values. Moreover, as 23.38 Ma is younger than the clade age (25 Ma), we artificially duplicated the 10.69 C4 value at 25.1 Ma (Supplementary DataFig. S17).
The scripts for the biogeographical and diversification analyses carried out in this study are available at https://github.com/LPDagallier/Monodoreae_macroevolution.
RESULTS
Gene recovery
We recovered all 369 exons, at least partially, targeted by the Annonaceae bait kit (Couvreur et al., 2019). After filtering and removal of paralogous loci, the 75/75 dataset contained 318 supercontigs. The 32 supercontigs most clock-like used for the phylogenetic reconstruction and dating had a length of between 509 and 4796 bp. Their best substitution models are listed in Supplementary DataTable S2.
Phylogeny
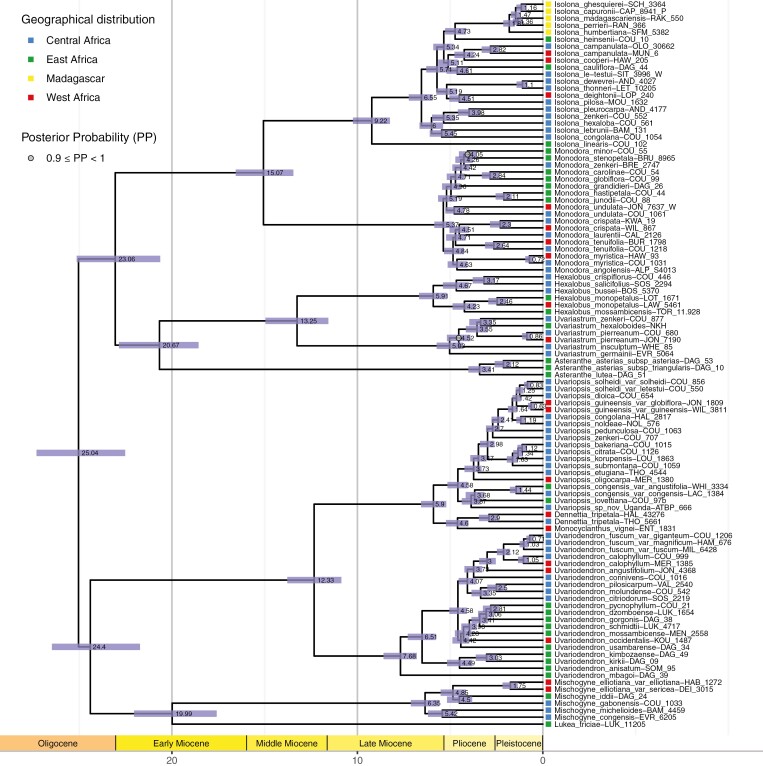
Among the six independent MCMC runs with a Yule tree prior, only one fully converged to a likelihood of −399 351.6164 after discarding 14 % of burn-in (ESS for all the parameters >200 after discarding the burn-in). The other chains were converging toward a slightly higher likelihood value, but the ESS values for several loci, for the prior and for the Yule model were below 150. After discarding the burn-in, the MCC tree was estimated from 8601 posterior trees. All the nodes in the MCC tree show strong support with all the nodes having a PP of 1, except for two nodes having a PP > 0.9 (Fig. 2). All the genera were retrieved as monophyletic.
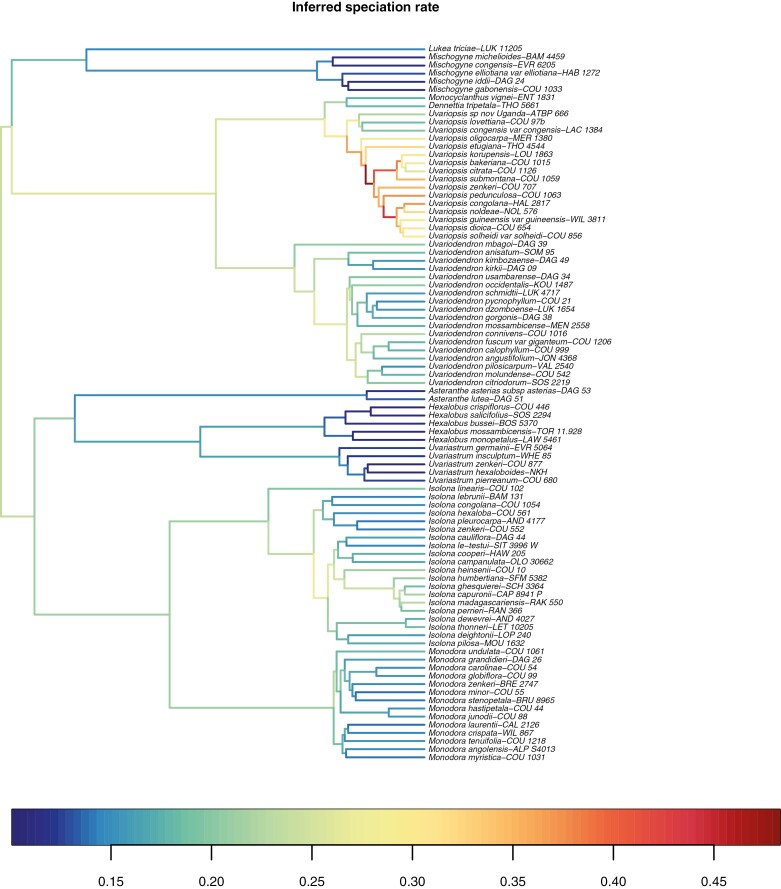
Fig. 2.
Phylogenetic tree of the Monodoreae specimens, as pruned from the maximum credibility clade tree estimated from 8601 posterior trees inferred based on 32 nuclear loci with uncorrelated molecular clock and fossil calibration in BEAST. Values at nodes indicate the mean age from the posterior probability density, and the blue bar indicates the 95 % highest posterior density interval of the age. All nodes received a posterior probability of 1 except for those indicated by a grey circle. The colour square at the tip indicates the geographical origin of the specimen. Colours follow Fig. 1.
The mean age of the divergence between Eupomatia (outgroup) and the Annonaceae (i.e. the crown node of Magnoliineae) is estimated in the Early Cretaceous at 110.68 Ma (95 % HPD: 105.53–112.60 Ma). The crown node of Annonaceae is estimated in the Late Cretaceous at 89.87 Ma (95 % HPD: 89.00–92.55 Ma) (Supplementary DataFig. S1). Although the distribution probabilities of the calibration points set on these nodes were broad uniforms, the age estimates for the crown node of Monodoreae and the crown node of Annonaceae respectively converged to the lower and upper bounds of the prior distribution. This indicates that the data contain enough information to overcome the lack of precise information of such wide priors. The age of the Monodoreae tribe is estimated in the late Oligocene at 25.07 Ma (95 % HPD: 22.52–27.30 Ma) (Fig. 2).
Biogeographical events
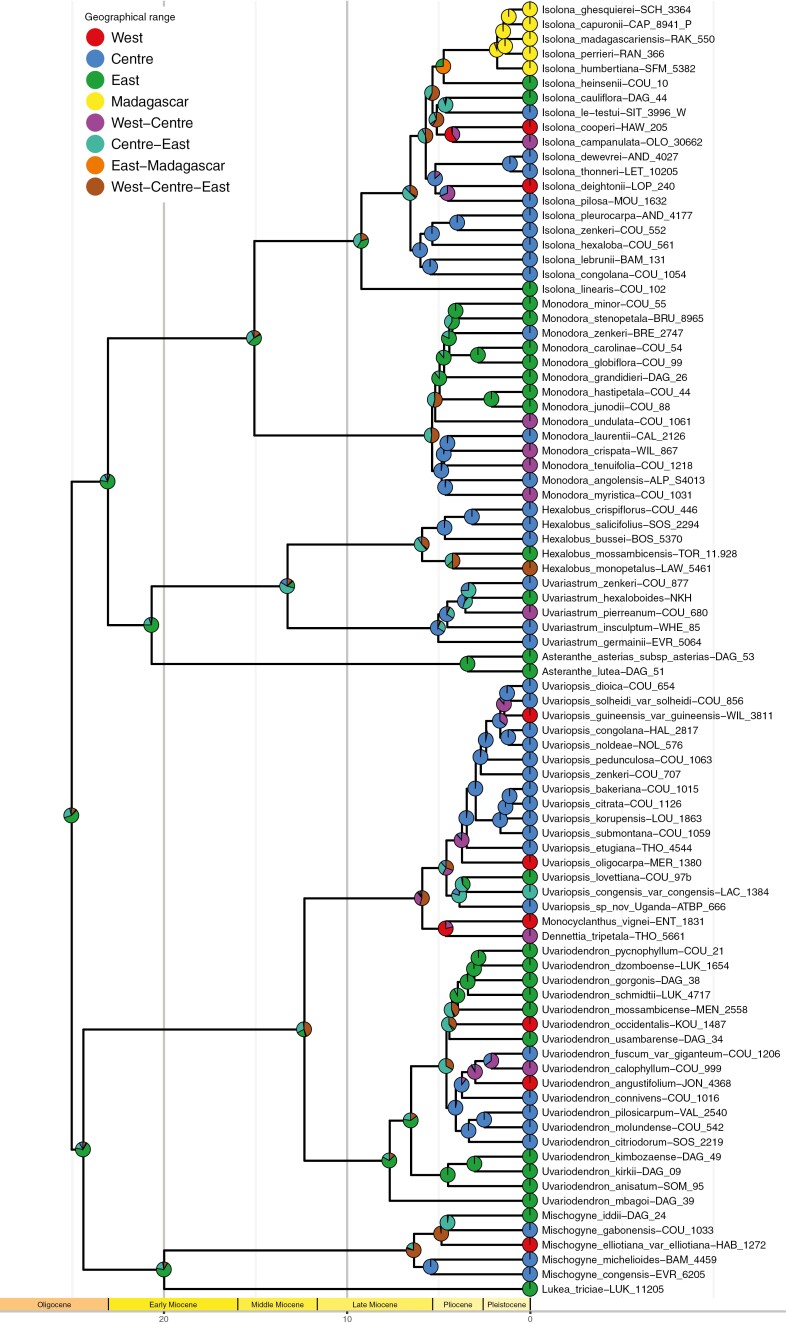
DEC analysis estimated the rate of ‘dispersal’ (range expansion) to 0.039 events/lineage/Myr and the rate of ‘extinction’ (range contraction) to 0.008. East Africa was identified as the most likely geographical origin of the Monodoreae (Fig. 3), as well as for most of the ancestral nodes until ~15 Ma. The ancestral range of Hexalobus, Isolona, Mischogyne, Monodora and Uvariopsis was probably widespread (Centre-East or West-Centre-East), the ancestral range of Asteranthe, Lukea and Uvariodendron was probably East Africa, while the ancestral range of Uvariastrum was probably Central Africa. Between the origin and ~13.5 Ma, the range of the Monodoreae expanded, and from ~13.5 to 6.5 Ma the range of some taxa contracted while the range of some other taxa possibly expanded (Fig. 4). From the late Miocene (~6.5 Ma), the Monodoreae underwent several synchronous range expansion events, range contraction events and vicariance events. Within Monodoreae, a single dispersal event occurred from East Africa to Madagascar in the genus Isolona dated between 5.27 and 4.14 Ma. This led to a founder event followed by a small radiation with five known Malagasy species (Fig. 3). Interestingly, the DEC model retrieved this divergence as a vicariance event (Fig. 3). However, as Madagascar had already separated from mainland Africa, it seems unlikely that the ancestor (node 153, Supplementary DataFig. S1) was distributed in both East Africa and Madagascar. We thus interpret this event as a founder event (Fig. 4).
Fig. 3.
Phylogenetic tree of Monodoreae species, as pruned from the maximum credibility clade tree estimated from 8601 posterior trees inferred based on 32 nuclear loci with uncorrelated molecular clock and fossil calibration in BEAST, with present-day (next to names) and ancestral ranges (pies at the internal nodes) reconstructed using the dispersal–extinction–cladogenesis (DEC) analysis implemented in BioGeoBEARS. The relative proportions of the coloured slices in the pies are proportional to the likelihood of each state.
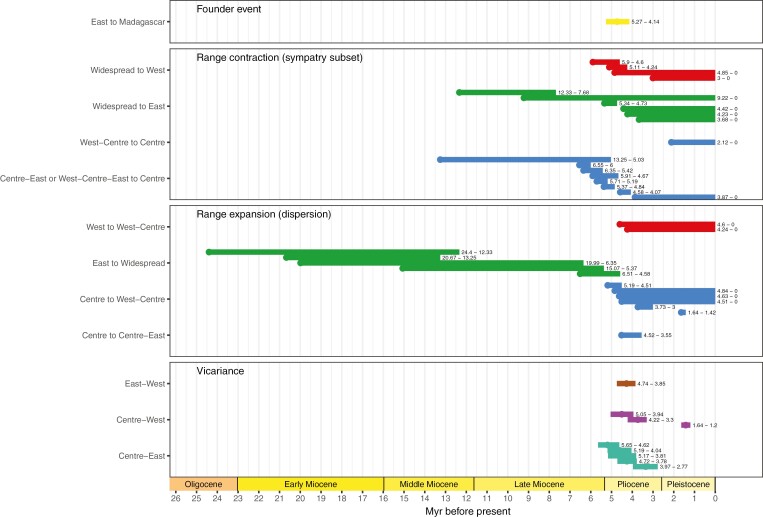
Fig. 4.
Chronological time frame of the biogeographical events as interpreted from the dispersal–extinction–cladogenesis (DEC) model. The founder event and vicariance events occurred at nodes of the tree in Fig. 3: the dot and horizontal bar represent the mean age and 95 % highest posterior density interval of the age of the node, respectively. Range contraction (RC) and range expansion (RE) events occurred along branches connecting two nodes: the dot represents the mean age of the widespread (RC) or endemic (RE) ancestral node, and the horizontal bar extends toward the mean age of the endemic (RC) or widespread (RE) daughter node. Colours follow the DEC ranges (see Fig. 3) and have different meaning depending on the event: for founder events, it is the colour of the range after the founder event; for range contraction, it is the colour of the endemic range after range contraction; for range expansion, it is the colour of the endemic range before range expansion; and for vicariance, it is the colour of the widespread range before the vicariance event. Labels on the y-axis indicate the direction of the event (e.g. range expansion from East to Widespread). ‘Widespread’ includes all the widespread ranges (West-Centre, Centre-East or West-Centre-East). See Material and Methods for the details of the events.
Diversification analyses
Branch- and clade-specific diversification rates.
The three methods used in our study identified to various degrees changes in the speciation and extinction rates across Monodoreae and its genera. Nevertheless, BAMM did not detect any significant diversification rate shifts across the phylogeny (Supplementary Data Figs S5 and S6). ClaDS inferred low speciation rates in the species-poor (five or fewer species) genera Asteranthe, Hexalobus, Lukea, Mischogyne and Uvariastrum, and relatively high speciation rates in one of the most diverse genera Uvariopsis (Fig. 5). RevBayes estimated higher speciation rates around the crown of the genera Isolona, Monodora, Uvariodendron and Uvariopsis (Fig. S3a). The variation of the speciation rate estimated by BAMM was very low, from 0.17 events/lineage/Myr around the root of the phylogeny to 0.2 events/lineage/Myr in the genus Uvariopsis (Fig. S4a). These three methods estimated very low extinction rates (<0.03 events/lineage/Myr; Figs S2, S3b and S4b). The three methods have also in common a higher diversification rate in the genus Uvariopsis.
Fig. 5.
Species-specific speciation rates through time of Monodoreae estimated using ClaDS (events per million years).
Tree-wide diversification rates.
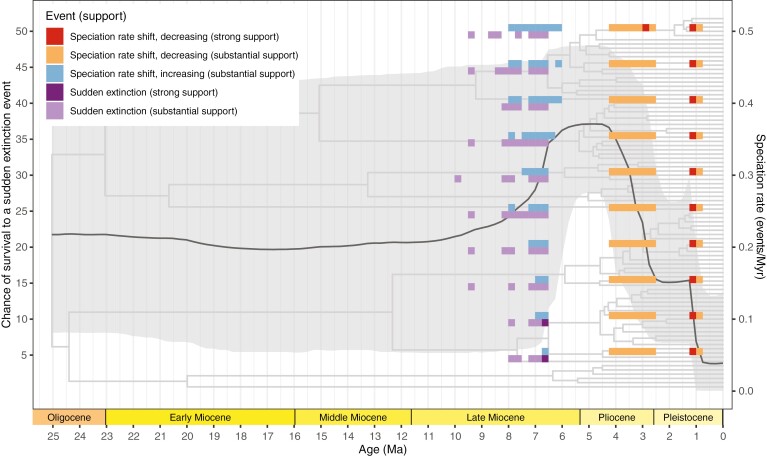
Even when using different priors on the survival probability of a sudden extinction event, the different CoMET analyses return similar results (Supplementary Data Figs S7–S16). CoMET found the speciation rate to vary through time: speciation is rather constant at around ~0.2 events/lineage/Myr until ~7 Ma, then increases to around 0.35 events/lineage/Myr between 6.5 and 4 Ma, and finally drops after 4 Ma to below 0.15 events/lineage/Myr (Fig. 6), which corresponds to the detected shifts (see below). The extinction rate is inferred to be rather low and constant through the history of the tribe (Figs S7–S16). CoMET detected a speciation rate shift increase with substantial (2lnBF > 2) to strong (2lnBF > 6) support between 6.5 and 7 Ma, followed by a speciation rate shift decrease between 2.5 and 4.25 Ma with substantial support, and a final shift towards even lower speciation rates around 1 Ma with strong support (Fig. 6). CoMET also identified several extinction events with substantial support in the late Miocene (between 10 and 6.5 Ma), with strong support between 6 and 6.25 Ma when the survival probability was <10 % (Fig. 6).
Fig. 6.
Summary of speciation rate shifts and sudden extinction (ME) events at each time slice in the CoMET analysis, with the priors on the chance of survival to a sudden extinction event (ME) ranging from 5 to 50 % (Supplementary Data Figs S7–S16). Strong support indicates 2lnBF > 6, and substantial support indicates 2lnBF > 2. Black line represents the speciation rate and grey shading its 95 % credibility interval, both averaged from all the CoMET analyses (Figs S7–S16).
Paleoenvironmental birth–death models.
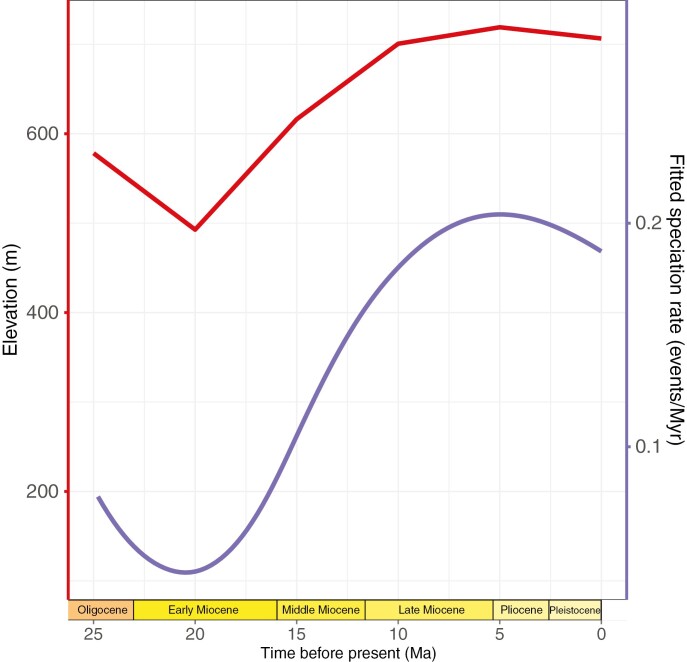
The environmental birth–death model with the lowest AICc score is the BDPerC4 model (four parameters), which is a model with speciation and extinction rates varying exponentially with the proportion of C4 plants through time (Table 1). However, the estimated parameters for this model are unrealistic as the inferred diversification rate is negative (i.e. extinction rate higher than speciation rate) at the beginning of the clade’s history, which is biologically implausible. The second-best model is the BElev model (two parameters), which is a model with speciation rate varying with mean elevation in tropical Africa through time and no extinction (Table 1; Fig. 7). Note that the difference of AICc between BElev and BElevDConst (speciation varying with elevation through time and constant extinction) is low, but these two models are very similar as the constant extinction rate estimated for BElevDConst is 0.005 events/lineage/Myr (Table 1).
Fig. 7.
Clade-independent speciation rate through time of Monodoreae, as estimated in RPANDA with the speciation rate varying exponentially with the mean elevation of tropical Africa through time (BElev model, Table 1).
DISCUSSION
In this study we generated a near complete species-level dated phylogenomic tree for a diverse rain forest-restricted clade of pan-African and Madagascar trees. Using this framework, we tested several hypotheses linked to the biogeography and diversification history of rain forests across the continent throughout the Miocene (last 23 Myr). We used BEAST to co-estimate the phylogeny and the divergence times, based on a subsample of the molecular markers available (32 most clock-like markers). The resulting phylogenetic tree is topologically very similar to the one obtained using the full set of molecular makers except for some nodes (mainly in Isolona, Monodora and Uvariastrum) for which phylogenetic conflict was inferred using both concatenation and gene tree approaches (Dagallier et al., in press). Reticulate processes of evolution might occur at these nodes, and the selection of only 32 loci for the molecular dating might increase the bias towards one or the other topology. One notable difference is the position of Monodora minor, which was inferred as sister to the genus with strong support using the full dataset (Dagallier, 2021; Dagallier et al., in press) but is recovered nested within the genus with moderate support in this 32-marker dataset using BEAST (Fig. 2).
East African origin and biogeography of Monodoreae
Our biogeographical analysis inferred East Africa as the most likely ancestral area for the tribe (Fig. 3). Indeed, the East African restricted genera Asteranthe or Lukea are recovered as sister to the more widely distributed clades. At the genus level, East African endemic species are generally recovered as sister to the rest of the genus (Isolona linearis; Uvariodendron mbagoi; Monodora minor, see above for the last), although not always (Uvariopsis). This scenario contrasts with other TRF groups inferred to have originated in Central Africa before dispersing to East Africa (e.g. Davis et al., 2002; Brée et al., 2020; Aduse-Poku et al., 2021). Interestingly, within Annonaceae, several other East African endemic genera are recovered as sister to different tribes such as Mkilua fragrans, sister to the rest of Bocageeae (Couvreur et al., 2011a), and the two monotypic endemic genera Sanrafaelia and Ophrypetalum, sister to the diverse palaeotropical tribe Uvarieae (Dagallier, 2021; Dagallier et al., in press). East African rain forests are thus not only important in terms of species diversity and endemism (Couvreur et al., 2006), but could also potentially contain a unique and old evolutionary history within Annonaceae, stressing their importance for conservation.
The most recent common ancestor of extant Monodoreae (crown node) is dated to 25 Ma, during the late Oligocene (Fig. 2). Initially, it was suggested that Monodoreae originated just before the Eocene–Oligocene Transition (EOT, 33.9 Ma), but this was based on plastid markers that included the monotypic genera Sanrafaelia and Ophrypetalum in Monodoreae (Couvreur et al., 2008; Chatrou et al., 2012). Based on the analyses of hundreds of nuclear markers both genera are now recovered as sister to tribe Uvarieae and have been transferred to their own tribe: Ophrypetaleae (Couvreur et al., 2019; Dagallier, 2021; Dagallier et al., in press), leading to a 7-Myr younger crown node age for Monodoreae. The late Oligocene (29–24 Ma) was a time of relatively favourable conditions for TRFs, re-expanding after the EOT crisis (Couvreur et al., 2021). Although the interpretation of East African ecosystems at that time is complex (Jacobs et al., 2010; Linder, 2017), TRF-like vegetation is clearly documented in northern Ethiopia (Jacobs et al., 2005, 2010; Bonnefille, 2010; Pan, 2010; Currano et al., 2011) and in northern Kenya as mosaics of TRFs and semi-deciduous forests (Vincens et al., 2006). The inferred age of Monodoreae also fits well with the origin of several other animal TRF clades, underling the importance of the late Oligocene in the origin of African rain forest biodiversity (Couvreur et al., 2021).
From East Africa, our biogeographical analysis suggests several range expansions of Monodoreae into Central Africa becoming widespread during the early to middle Miocene (~24–13.5 Ma) (Figs 3 and 4, range expansion, green). Given the low potential for long-distance dispersal of this tribe, our results support the hypothesis of important TRF East/Central connections possibly via the existence of a continuous pan-African rain forest (Couvreur et al., 2021; but see Linder, 2017 for a counter argument about the existence of a pan-African forest, and below). Indeed, this coincides with the warmer climate of the MMCO (~17–14.7 Ma) during which the TRF was suggested to occur from coast to coast (Morley, 2000, 2011; Couvreur et al., 2021). It is during the early Miocene (~20–21 Ma) that the genera Asteranthe and Lukea originated (Fig. 3). These genera are species-poor (two species each) and are endemic to East African coastal and mountain forests (Verdcourt, 1971; Vollesen, 1980; Cheek et al., 2022). Such long-term persistence of clades originating in the Oligocene–Miocene has also been documented in plants and animals (Tolley et al., 2011; Dimitrov et al., 2012; Loader et al., 2014; Couvreur et al., 2021), and is associated with potential climate stability of the region in the East African mountains region (Lovett et al., 2005; Finch et al., 2009). The divergence between Asteranthe and the clade Hexalobus–Uvariastrum was interpreted as a vicariance eventat ~16.8 Ma by Couvreur et al. (2008). Here, with full taxon sampling of these genera, we rather estimate a range expansion from the East to East-Centre ~20.7 Ma with long-term persistence of the genus Asteranthe in the East. This period still coincides with warmer and wetter climates across Africa, even though the existence of a continuous TRF between East and West Africa remains debated (Jacobs et al., 2010; Linder, 2017; Couvreur et al., 2021).
Just after the MMCO, during the MMCT (~15–13 Ma), global temperatures and pCO2 dropped (Westerhold et al., 2020) leading to the expansion of open grasslands across Africa and contraction of TRF (Jacobs, 2004; Plana, 2004; Linder, 2017; Couvreur et al., 2021). Previous molecular studies postulated vicariance events in TRF-restricted clades before the MMCT, supporting the contraction of the TRF during the MMCT (Couvreur et al., 2008; Dimitrov et al., 2012; Menegon et al., 2014; Brée et al., 2020). However, in Monodoreae, we did not find evidence of vicariance (i.e. splitting of one species into two) before 6 Ma (Fig. 4). Rather, our results support a range contraction scenario (i.e. the disappearance of a species from part of its past range) from ~13.5 to 6.5 Ma (Fig. 4), leading to the origins of Uvariodendron in East Africa, Uvariastrum in Central Africa, and corresponding to the divergence between the East African species Isolona linearis and the rest of Isolona (Fig. 3). Even if these clades evolved in isolation, and would support a contraction of the TRF at that time, their sister clades are inferred as being widespread (i.e. distributed across both East and Central Africa, Fig. 3). Moreover, possible range expansions also continued between ~13.5 and 6.5 Ma (Fig. 4). Overall, these results support the idea that the East and Central African TRFs might have been more connected than previously suggested after the MMCT. Indeed, as noted by Jacobs et al. (2010), numerous palaeofloras of the middle Miocene of East Africa document the presence of taxa sharing affinities with both wet and dry forests of Central and West Africa. A striking example is that of Baringo in the Ngorora Formation in the East African Rift valley (west Kenya; ~12.2 Ma), inferred as being a moist to wet forest containing several tropical plant lineages, including Annonaceae, and several taxa were suggested to have affinities with modern West African species (Jacobs et al., 2010). Also, as the Monodoreae experienced sudden extinction in the late Miocene (see below), we might miss the ancestral ranges of the extinct (and unknown) species, which may overshadow the signal inferred by DEC between two nodes separated by a long branch.
From the late Miocene and during the Pliocene (6.5–2 Ma), the biogeographical history of the Monodoreae increases in complexity. First, most of the extant Monodoreae species diverged during this time interval (Figs 2 and 3) supporting evidence of the importance of this period for the origin of African biodiversity (Voelker et al., 2010; Couvreur et al., 2021). Second, our biogeographical analysis resulted in numerous simultaneous range expansions, contractions and vicariance events (Fig. 4). This suggests that if TRF fragmentation events occurred, they were not strong enough to affect the whole tribe. Instead, the TRF blocks were probably connected during the early Pliocene, as previously suggested based on a palaeo-vegetation record (Morley, 2000), either as a continuous forest block (Fer et al., 2017) or via forested corridors between East and Centre-West Africa (Joordens et al., 2019). Thus, the late Miocene and Pliocene divergences in the Monodoreae might be explained by species-specific or region-specific mechanisms.
Although most species of Monodoreae are restricted to rain forests, a few isolated species have adapted to dry conditions. Two sister species, Hexalobus monopetalus and H. mossambicensis, are more dry-adapted than the other Hexalobus species distributed in Central Africa (Botermans et al., 2011). Hexalobus monopetalus has the typical savanna distribution all around the Guineo-Congolian region (Botermans et al., 2011) like other savanna species (Lehmann et al., 2011; Gonçalves et al., 2021). In addition, Monodora stenopetala (Couvreur, 2009) and Uvariastrum hexaloboides (Couvreur, 2014) are also reported to be dry-adapted, both occurring in woodlands or dense thickets of southern East Africa. These species, shifting from a forested and wetter to a drier and more open habitat, were estimated to have originated during the late Miocene between 6 and 3 Ma (Fig. 2). These three independent habitat shifts followed by little or no speciation contrast with other plant clades for which multiple shifts into drier habitats followed by speciation have been documented during the late Miocene, such as in the African Melatsomataceae (Veranso-Libalah et al., 2018), Cucurbitaceae (Holstein and Renner, 2011), Fabaceae (Bouchenak-Khelladi et al., 2010; Tosso et al., 2018) and Sapotaceae (Armstrong et al., 2014). This supports the view that Annonaceae show little ecological capacity to diversify in drier or arid conditions in Africa in line with previous results (Couvreur et al., 2011b).
Several Monodoreae species span both West and Centre TRF blocks (Figs 2 and 3). The Dahomey Gap (DG) located in Benin and Togo mediates the connection between the West and the Central African forests blocks (Droissart et al., 2018). The DG was suggested to be dominated by open vegetation during most of the late Quaternary, from ~1.05 Ma (Demenou et al., 2018), and became forested during the mid-Holocene (~8000–4000 years before the present), before being dominated again by open vegetation until the present due to climatic variations (Salzmann and Hoelzmann, 2005). These vegetation changes had an impact in the intraspecific genetic structure of many forest tree species (Duminil et al., 2015; Demenou et al., 2018, 2020; Lompo et al., 2018). In this study, we dated the intraspecific divergence times of eight species co-occurring west and east of the DG (one individual per side sampled). For three of these species (Monodora myristica, Uvariastrum pierreanum and Uvariodendron calophyllum), divergence times were estimated to the late Quaternary (i.e. after 1.05 Ma) (Fig. 2). In addition, no interspecific vicariance events between West and Centre species were inferred during this period (Figs 3 and 4), suggesting that the presence of the DG acted as a barrier only for genetic differentiation within species as is generally reported (Couvreur et al., 2021). We also inferred infraspecific divergence times prior to 1.05 Ma spanning 6.57–1.20 Ma associated with inter- and intraspecific vicariance and range expansion events between the West and Central TRF blocks (Figs 2–4). This result agrees well with the scenario of multiple expansion and retractions of savannah vegetation in West Africa, connecting and disconnecting rain forests between West and Central Africa throughout the last 7 Myr (Bonnefille, 2010; Jacobs et al., 2010). In particular, the climatic variations in the Pliocene and the early Pleistocene (mainly glacial and interglacial cycles, Couvreur et al., 2021) triggered strong isolations in some populations, leading to splits between West and Central African species (e.g. origin of Isolona deightonii, Uvariopsis guineensis or Uvariopsis oligocarpa), but also seem to have allowed the re-connection of the populations of some species distributed in both West and Central Africa (e.g. Monodora undulata, Dennettia tripetala). A similar pattern with both inter- and intraspecific divergence between West and Centre Forest blocks has been observed during the Pliocene and Pleistocene in the bird genera Bleda and Criniger (Huntley and Voelker, 2016; Huntley et al., 2018).
Madagascar broke apart from Africa in the Early Cretaceous (more than 100 Ma; Kocsis & Scotese, 2021) and the shortest distance today separating the African coast to Madagascar is ~400 km (Rabinowitz and Woods, 2006). Similar recent splits between Malagasy clades and continental relatives have been inferred in animals and plants (Renner, 2004; Rabinowitz and Woods, 2006; Yoder and Nowak, 2006; Tolley et al., 2013), including other Annonaceae (Thomas et al., 2015; Chen et al., 2019), and have generally been explained by long-distance dispersal (LDD). Small vertebrates and invertebrates are hypothesized to have drifted on vegetation rafts from continental African rivers with the help of eastwards oceanic currents (Rabinowitz and Woods, 2006; Ali and Huber, 2010). LDD from the continent to Madagascar is likely in wind-dispersed plant species and is also documented in bird-dispersed plant species (Renner, 2004; Yao et al., 2016). Isolona is the only Monodoreae genus that reached Madagascar. We estimated that this event happened during the early Pliocene between 5.27 and 4.14 Ma followed by a small radiation of five species (Figs 3 and 4). The timing of this dispersal occurs on the more recent timeline of endemic plant lineage origins in Madagascar which span the middle Eocene/Oligocene to the Pleistocene (Buerki et al., 2013; Antonelli et al., 2022). The fruits of Isolona are large syncarps probably dispersed by large to medium-bodied primates (Couvreur, 2009), and terrestrial LDD is considered unlikely in this genus (Couvreur et al., 2008). To explain the presence of Isolona in Madagascar, we speculate that some propagules (fruits, seeds or seedlings) derived from mainland rivers in East Africa to the ocean and reached Madagascar by drifting via an eastward oceanic current. This is supported by the fact that the sister species to the Malagasy clade is Isolona heinsenii endemic to the East African rain forests in Tanzania (Verdcourt, 1971; Couvreur, 2009; Dagallier, 2021; Dagallier et al., in press). Dispersal by drifting to Madagascar is suggested as the main hypothesis for the origin of Malagasy biodiversity (Yoder and Nowak, 2006; Buerki et al., 2013; Antonelli et al., 2022), and has also been suggested in the palaeotropical Annonaceae genus Uvaria, for which the African species are also unlikely to disperse across the ocean (Zhou et al., 2012). Although seed survival of Isolona (and Monodoreae in general) in salt water remains unknown, it is possible that some propagules survived during the crossing, especially if they drifted on rafts of vegetation detached from East Africa (Ali and Huber, 2010). An alternative hypothesis could be that the propagules of an ancestral Isolona species reached Madagascar via land bridges linking Africa and Madagascar (e.g. Masters et al., 2021) and allowing different animal migrations, notably at three distinct moments during the Cenozoic, including the Miocene (12—5 Ma). However, viable land bridges across the Mozambique Channel were shown to be very unlikely (Ali and Huber, 2010; see Ali and Hedges, 2022 for a review). In addition, crossing the channel even when the sea level was low is also improbable, given that the bathymetry is deep, and even a drop of 200 m would only reduce the distance between the continent and Madagascar to 360 km (Rabinowitz and Woods, 2006). Finally, the presence of Isolona in Madagascar was used as a counter argument to our assumption of low potential for LDD of Monodoreae species (Linder, 2017). However, this successful dispersal event occurred only once throughout the evolutionary history of Monodoreae and should thus be viewed as additional proof of the low LDD capacity of the species in this tribe, not the opposite.
Diversification of the Monodoreae
Speciation rates for Monodoreae, as inferred with CoMET, were relatively stable until the late Miocene some 10 Ma (Fig. 6). The first event detectable with our data was a sudden extinction occurring during the late Miocene between 10 and 6.5 Ma (Fig. 6; Supplementary DataFigs S7–S16). Similarly, Brée et al. (2020) found substantial support in favour of a sudden extinction around the same time, between 8 and 6.5 Ma in the Piptostigmateae, another TRF African Annonaceae tribe, although they do not consider it significant (see fig. 4 in Brée et al., 2020). This pattern has also been retrieved in the African butterfly genus Bicyclus for which the forest clade underwent a drop in diversification rate from ~15 Ma (Aduse-Poku et al., 2021). These results are consistent with the late Miocene cooling suggested to have increased aridification across Africa and leading to the expansion of open grasslands (Jacobs, 2004; Uno et al., 2016; Couvreur et al., 2021), although this trend was not homogenous through time or space (Jacobs, 2004; Bonnefille, 2010). This supports growing evidence that the open grasslands in the late Miocene occurred at the expense of the TRFs, which might have experienced drastic and sudden extinction due to the reduction of areas having a suitable warm and wet climate (Morley, 2000, 2011).
Interestingly, using two different methods (CoMET and the environment-dependent diversification model) our results indicate that this sudden extinction event was immediately succeeded by a potential increase in speciation rates dated to have taken place between 6.5 and 4.25 Ma (Figs 6 and 7; Supplementary DataFigs S7–S16). This increase coincided with the early Pliocene which was marked by a renewed warmed climate across Africa (Jacobs et al., 2010; Couvreur et al., 2021). Our biogeographical analysis identifies numerous vicariance events between major regions also dating to the early Pliocene (Fig. 4).
In addition, the model with change of elevation through time best explained the speciation rate with no extinction in Monodoreae (BElev model; Fig. 7; Table 1, but see Results). This is, to our knowledge, the first time African elevation change (orogeny) is positively linked to speciation in an African rain forest tree clade. The timing of the orogeny of the East African Rift System is complex (Chorowicz, 2005; Ring et al., 2018; Couvreur et al., 2021), but our results would fit with an active phase of uplift suggested to have occurred along the Western branch around 15–10 Ma (Chorowicz, 2005; Wichura et al., 2015; Ring et al., 2018). The rise of the East African Rift has generally been interpreted as having a negative impact on African rain forest biodiversity leading to precipitation reduction and the drying of the Congo basin for example (Sepulchre et al., 2006; Sommerfeld et al., 2016). However, rifting in East Africa could have led to the fragmentation or complete genetic isolation of both East and West/Central Forest blocks favouring vicariance (Fig. 4).
Taken together, our results suggest that both aridification and rifting initially had a negative impact on TRF diversity but this was quickly followed by an increase in speciation linked to vicariance. We suggest here a ‘sequential scenario of diversification’ starting with an increased aridification triggering extinction of TRF taxa followed by the fragmentation of rain forests resulting from a reduction in rainfall that subsequently enhanced lagged speciation events resulting from vicariance and improved climate conditions.
Uvariopsis, an outlier in the diversification of Monodoreae?
At the clade level, our BAMM analyses failed to detect any significant shifts in diversification rates across Monodoreae (Supplementary DataFigs S5 and S6). This is in line with previous studies that failed to detect significant diversification shifts either across Annonaceae as a whole (Couvreur et al., 2011a; Erkens et al., 2012) or within the Annonoideae subfamily (which includes Monodoreae) (Massoni et al., 2015a), suggesting a rather constant rate of diversification at least at higher taxonomic levels in Annonaceae. Nevertheless, diversification rates in Monodoreae are not homogeneous across genera (Fig. 5; Figs S3 and S4), and BAMM has been shown in some cases not to detect shifts even though they exist (Rabosky, 2014; Mitchell et al., 2019). All analyses found higher diversification rates in the genus Uvariopsis when compared to the other genera. With 20 species (17 described and three undescribed, see Dagallier et al., in press), Uvariopsis is one of the most species-rich and youngest genera of the tribe (crown node: median 5.90 Ma, 95 % HPD 5.21–6.57 Ma). Using the methods of moments (Magallón and Sanderson, 2001), that is using species richness and age of the stem node, Uvariopsis was identified as one of the fastest diversifying Annonaceae genera under a no extinction hypothesis (Couvreur et al., 2011a). Indeed, all extant species diverged between ~4.5 and 1 Ma, whereas most of the species in the other rich genera [Isolona (20 species), Monodora (14 species), Uvariodendron (18 species)] diverged between 10 and 4 Ma (Fig. 2). Morphologically, Uvariopsis has several features differentiating it from other Monodoreae. All but one species are monoecious, that is have separated male (staminate) and female (pistillate) flowers on the same individual (except U. bisexualis, not included in this study) and they all present a reduction of the perianth parts (two sepals and three or four petals) when compared to the typical Annonaceae flower (three sepals and 6 petals) (Dagallier, 2021; Dagallier et al., in press). Such an association between monoecy and reduction of perianth parts has been suggested to be an optimization of resource allocation to sex (Jong et al., 2008). Monoecy could be evolutionary advantageous to avoid self-pollination (Pang et al., 2013), but the flowers of most Annonaceae species generally avoid self-pollination with temporal variation in the maturation of stamens and carpels (Pang and Saunders, 2014). Another evolutionary advantage of monoecy is probably conferred by the flexibility in resource allocation to male and female functions (Bertin, 1993). However, recent analyses found unisexual flowers associated with lower diversification rates than in bisexual flowers and androdioecous flowers at the population level (Xue et al., 2020). Three species of Uvariopsis are pollinated by Diptera (Gottsberger et al., 2011; Mertens et al., 2018), which is quite uncommon in Annonaceae (Saunders, 2012). Shifts in pollination syndromes have been suggested to be responsible for shifts in diversification (Valente et al., 2012; Breitkopf et al., 2015; Lagomarsino et al., 2016; Serrano-Serrano et al., 2017). One would be tempted to relate the peculiarities of Uvariopsis (monoecy and Diptera pollination) to its higher diversification rates relative to the other Monodoreae genera. However, given these characters appeared only once in Monodoreae, testing for their influence on diversification rates (e.g. with state-dependent speciation and extinction models; Maddison et al., 2007) could provide spurious inferences (Maddison and FitzJohn, 2015).
CONCLUSION
Our study brings new and more detailed insights into the evolution of TRFs in Africa during the Cenozoic. By focusing on Monodoreae, we found that this clade originated in East African TRFs during the late Oligocene (~25 Ma) after the EOT and expanded toward Central/West Africa during the early to middle Miocene, supporting the existence of a pan-African rain forest until ~13.5 Ma. There is no evidence for clear discrete and synchronous vicariance events between forest blocks during the middle Miocene as previously suggested, but instead range contractions that led to speciation support the hypothesis of TRF fragmentation due to aridification in Africa. However, we also inferred clades spanning several forest blocks during the middle Miocene, suggesting that the TRF blocks were more connected than previously thought. The Monodoreae probably experienced sudden extinction during the late Miocene, supporting the drastic reduction of the TRFs linked to aridification. This was quickly followed by high speciation rates, suggesting that both the fragmentation and the following re-connection of the TRFs in the early Pliocene stimulated diversification. Such a high diversification was probably associated with the increasing African palaeo-elevation during the late Miocene and Pliocene, supporting the idea that the uplift of East Africa was a factor that promoted diversification.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following. Fig. S1: Phylogenetic inference of the Monodoreae and outgroups. Fig. S2: Extinction rates of Monodoreae estimated using ClaDS. Fig. S3: Speciation, extinction and diversification of Monodoreae estimated using RevBayes. Fig. S4: Clade-specific speciation, extinction and diversification rates through time of Monodoreae estimated using BAMM. Fig. S5: Traces of the MCMC run for the BAMM analysis. Fig. S6: Posterior probabilities of the number of diversification rate shifts in the Monodoreae phylogeny according to BAMM. Figs S7–S16: Results of the CoMET analysis, with different priors on the chance of survival to a mass extinction event. Fig. S17: Estimated proportion of C4 plants in tropical Africa interpolated from Polissar et al. (2019). Table S1: Information on the vouchers used in this study. Table S2: Length and best substitution model of the 32 loci used in this study.
ACKNOWLEDGEMENTS
We thank Lars Chatrou, Susanne Renner, Muthama Muasya, Sidonie Bellot, Myriam Gaudeul and Gael Kergoat for discussions on an earlier version of the manuscript. The authors acknowledge the ISO 9001 certified IRD i-Trop HPC (member of the South Green Platform) at IRD Montpellier for providing HPC resources that have contributed to the research results reported within this paper: https://bioinfo.ird.fr/-http://www.southgreen.fr
Contributor Information
Léo-Paul M J Dagallier, DIADE, Université de Montpellier, IRD, CIRAD, Montpellier, France; Institute of Systematic Botany, The New York Botanical Garden, Bronx, NY 10458, USA.
Fabien L Condamine, CNRS, Institut des Sciences de l’Evolution de Montpellier (Université de Montpellier), Place Eugène Bataillon, 34095 Montpellier, France.
Thomas L P Couvreur, DIADE, Université de Montpellier, IRD, CIRAD, Montpellier, France.
FUNDING
This work was supported by the Agence Nationale de la Recherche (AFRODYN Grant No.: ANR-15-CE02-0002-01) and the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement No. 865787) both to T.L.P.C.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
LITERATURE CITED
- Aduse-Poku K, van Bergen E, Sáfián S, et al. 2021. Miocene climate and habitat change drove diversification in Bicyclus, Africa’s largest radiation of satyrine butterflies. Systematic Biology 71: 570–588. doi: 10.1093/sysbio/syab066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali JR, Hedges SB.. 2022. A review of geological evidence bearing on proposed Cenozoic land connections between Madagascar and Africa and its relevance to biogeography. Earth-Science Reviews 232: 104103. doi: 10.1016/j.earscirev.2022.104103. [DOI] [Google Scholar]
- Ali JR, Huber M.. 2010. Mammalian biodiversity on Madagascar controlled by ocean currents. Nature 463: 653–656. doi: 10.1038/nature08706. [DOI] [PubMed] [Google Scholar]
- Antonelli A, Kissling WD, Flantua SGA, et al. 2018. Geological and climatic influences on mountain biodiversity. Nature Geoscience 11: 718–725. doi: 10.1038/s41561-018-0236-z. [DOI] [Google Scholar]
- Antonelli A, Smith RJ, Perrigo AL, et al. 2022. Madagascar’s extraordinary biodiversity: evolution, distribution, and use. Science 378: eabf0869. doi: 10.1126/science.abf0869. [DOI] [PubMed] [Google Scholar]
- Armstrong KE, Stone GN, Nicholls JA, et al. 2014. Patterns of diversification amongst tropical regions compared: a case study in Sapotaceae. Frontiers in Genetics 5: 362. doi: 10.3389/fgene.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker WJ, Couvreur TLP.. 2013. Global biogeography and diversification of palms sheds light on the evolution of tropical lineages. I. Historical biogeography. Journal of Biogeography 40: 274–285. [Google Scholar]
- Bankevich A, Nurk S, Antipov D, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of Computational Biology 19: 455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RC, Parra JL, Badjedjea G, et al. 2017. Idiosyncratic responses to climate-driven forest fragmentation and marine incursions in reed frogs from Central Africa and the Gulf of Guinea Islands. Molecular Ecology 26: 5223–5244. doi: 10.1111/mec.14260. [DOI] [PubMed] [Google Scholar]
- Bertin RI. 1993. Incidence of monoecy and dichogamy in relation to self-fertilization in angiosperms. American Journal of Botany 80: 557–560. doi: 10.1002/j.1537-2197.1993.tb13840.x. [DOI] [PubMed] [Google Scholar]
- Bivand R, Rundel C, Pebesma E, et al. 2020. rgeos: interface to Geometry Engine - Open Source (‘GEOS’) https://CRAN.R-project.org/package=rgeos.
- Bonnefille R. 2010. Cenozoic vegetation, climate changes and hominid evolution in tropical Africa. Global and Planetary Change 72: 390–411. doi: 10.1016/j.gloplacha.2010.01.015. [DOI] [Google Scholar]
- Boschman LM, Condamine FL.. 2022. Mountain radiations are not only rapid and recent: ancient diversification of South American frog and lizard families related to Paleogene Andean orogeny and Cenozoic climate variations. Global and Planetary Change 208: 103704. doi: 10.1016/j.gloplacha.2021.103704. [DOI] [Google Scholar]
- Botermans M, Sosef MSM, Chatrou LW, Couvreur TLP.. 2011. Revision of the African genus Hexalobus (Annonaceae). Systematic Botany 36: 33–48. doi: 10.1600/036364411x553108. [DOI] [Google Scholar]
- Bouchenak-Khelladi Y, Maurin O, Hurter J, van der Bank M.. 2010. The evolutionary history and biogeography of Mimosoideae (Leguminosae): an emphasis on African acacias. Molecular Phylogenetics and Evolution 57: 495–508. doi: 10.1016/j.ympev.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Bouckaert R, Vaughan TG, Barido-Sottani J, et al. 2019. BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Computational Biology 15: e1006650. doi: 10.1371/journal.pcbi.1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brée B, Helmstetter AJ, Bethune K, Ghogue J-P, Sonké B, Couvreur TLP.. 2020. Diversification of African rainforest restricted clades: Piptostigmateae and Annickieae (Annonaceae). Diversity 12: 227. doi: 10.3390/d12060227. [DOI] [Google Scholar]
- Breitkopf H, Onstein RE, Cafasso D, Schlüter PM, Cozzolino S.. 2015. Multiple shifts to different pollinators fuelled rapid diversification in sexually deceptive Ophrys orchids. New Phytologist 207: 377–389. doi: 10.1111/nph.13219. [DOI] [PubMed] [Google Scholar]
- Brown JW, Walker JF, Smith SA.. 2017. Phyx: phylogenetic tools for unix. Bioinformatics 33: 1886–1888. doi: 10.1093/bioinformatics/btx063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja J, Šumbera R, Peterhans JCK, et al. 2017. Evolutionary history of the thicket rats (genus Grammomys) mirrors the evolution of African forests since late Miocene. Journal of Biogeography 44: 182–194. [Google Scholar]
- Buerki S, Devey DS, Callmander MW, Phillipson PB, Forest F.. 2013. Spatio-temporal history of the endemic genera of Madagascar. Botanical Journal of the Linnean Society 171: 304–329. doi: 10.1111/boj.12008. [DOI] [Google Scholar]
- Burgess ND, Butynski TM, Cordeiro NJ, et al. 2007. The biological importance of the Eastern Arc Mountains of Tanzania and Kenya. Biological Conservation 134: 209–231. doi: 10.1016/j.biocon.2006.08.015. [DOI] [Google Scholar]
- Chatrou LW, Pirie MD, Erkens RHJ, et al. 2012. A new subfamilial and tribal classification of the pantropical flowering plant family Annonaceae informed by molecular phylogenetics. Botanical Journal of the Linnean Society 169: 5–40. doi: 10.1111/j.1095-8339.2012.01235.x. [DOI] [Google Scholar]
- Cheek M, Luke WRQ, Gosline G.. 2022. Lukea gen. nov. (Monodoreae-Annonaceae) with two new threatened species of shrub from the forests of the Udzungwas, Tanzania and Kaya Ribe, Kenya. Kew Bulletin 77: 647–664. doi: 10.1007/s12225-022-10039-y. [DOI] [Google Scholar]
- Chen J, Thomas DC, Saunders RMK.. 2019. Geographic range and habitat reconstructions shed light on palaeotropical intercontinental disjunction and regional diversification patterns in Artabotrys (Annonaceae). Journal of Biogeography 46: 2690–2705. doi: 10.1111/jbi.13703. [DOI] [Google Scholar]
- Chorowicz J. 2005. The East African rift system. Journal of African Earth Sciences 43: 379–410. doi: 10.1016/j.jafrearsci.2005.07.019. [DOI] [Google Scholar]
- Condamine FL, Rolland J, Morlon H.. 2013. Macroevolutionary perspectives to environmental change. Ecology Letters 16: 72–85. doi: 10.1111/ele.12062. [DOI] [PubMed] [Google Scholar]
- Condamine FL, Rolland J, Höhna S, Sperling FAH, Sanmartín I.. 2018. Testing the role of the Red Queen and Court Jester as drivers of the macroevolution of Apollo butterflies. Systematic Biology 67: 940–964. doi: 10.1093/sysbio/syy009. [DOI] [PubMed] [Google Scholar]
- Couvreur TLP. 2009. Monograph of the syncarpous African genera Isolona and Monodora (Annonaceae). Systematic Botany Monographs 87: 1–150. [Google Scholar]
- Couvreur TLP. 2014. Revision of the African genus Uvariastrum (Annonaceae). PhytoKeys 33: 1–40. doi: 10.3897/phytokeys.33.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvreur TLP. 2015. Odd man out: why are there fewer plant species in African rain forests? Plant Systematics and Evolution 301: 1299–1313. doi: 10.1007/s00606-014-1180-z. [DOI] [Google Scholar]
- Couvreur TLP, Gereau RE, Wieringa JJ, Richardson JE.. 2006. Description of four new species of Monodora and Isolona (Annonaceae) from Tanzania and an overview of Tanzanian Annonaceae diversity. Adansonia 28: 243–266. [Google Scholar]
- Couvreur TLP, Chatrou LW, Sosef MSM, Richardson JE.. 2008. Molecular phylogenetics reveal multiple Tertiary vicariance origins of the African rain forest trees. BMC Biology 6: 54. doi: 10.1186/1741-7007-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvreur TLP, Pirie MD, Chatrou LW, et al. 2011a. Early evolutionary history of the flowering plant family Annonaceae: steady diversification and boreotropical geodispersal. Journal of Biogeography 38: 664–680. doi: 10.1111/j.1365-2699.2010.02434.x. [DOI] [Google Scholar]
- Couvreur TLP, Porter-Morgan H, Wieringa JJ, Chatrou LW.. 2011b. Little ecological divergence associated with speciation in two African rain forest tree genera. BMC Evolutionary Biology 11. Article no.: 296. doi: 10.1186/1471-2148-11-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvreur TLP, Helmstetter AJ, Koenen EJM, et al. 2019. Phylogenomics of the major tropical plant family Annonaceae using targeted enrichment of nuclear genes. Frontiers in Plant Science 9. Article no.: 1941. doi: 10.3389/fpls.2018.01941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvreur TLP, Dauby G, Blach‐Overgaard A, et al. 2021. Tectonics, climate and the diversification of the tropical African terrestrial flora and fauna. Biological Reviews 96: 16–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currano ED, Jacobs BF, Pan AD, Tabor NJ.. 2011. Inferring ecological disturbance in the fossil record: a case study from the late Oligocene of Ethiopia. Palaeogeography, Palaeoclimatology, Palaeoecology 309: 242–252. doi: 10.1016/j.palaeo.2011.06.007. [DOI] [Google Scholar]
- Currano ED, Jacobs BF, Pan AD.. 2021. Is Africa really an ‘odd man out?’ Evidence for diversity decline across the Oligocene–Miocene boundary. International Journal of Plant Sciences 182: 551–563. doi: 10.1086/714308. [DOI] [Google Scholar]
- Dagallier LPMJ. 2021. Diversification of the tropical African flora: spatial and temporal approaches. Thesis, University of Montpellier, France. [Google Scholar]
- Dagallier L-PMJ, Janssens SB, Dauby G, et al. 2020. Cradles and museums of generic plant diversity across tropical Africa. New Phytologist 225: 2196–2213. doi: 10.1111/nph.16293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagallier LPMJ, Mbago FM, Couderc M, et al. 2023. Phylogenomic inference of the African tribe Monodoreae (Annonaceae) and taxonomic revision of Dennettia, Uvariodendron and Uvariopsis. PhytoKeys 233: 1–200. doi: 10.3897/phytokeys.233.103096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D, Posada D, Kozlov AM, Stamatakis A, Morel B, Flouri T.. 2020. ModelTest-NG: a new and scalable tool for the selection of dna and protein evolutionary models. Molecular Biology and Evolution 37: 291–294. doi: 10.1093/molbev/msz189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauby G, Zaiss R, Overgaard A-B, et al. 2016. RAINBIO: a mega-database of tropical African vascular plants distributions. PhytoKeys 74: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CC, Bell CD, Fritsch PW, Mathews S.. 2002. Phylogeny of Acridocarpus-Brachylophon (Malpighiaceae): implications for Tertiary tropical floras and Afroasian biogeography. Evolution 56: 2395–2405. doi: 10.1111/j.0014-3820.2002.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Demenou BB, Doucet J-L, Hardy OJ.. 2018. History of the fragmentation of the African rain forest in the Dahomey Gap: insight from the demographic history of Terminalia superba. Heredity 120: 547–561. doi: 10.1038/s41437-017-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demenou BB, Migliore J, Heuertz M, et al. 2020. Plastome phylogeography in two African rain forest legume trees reveals that Dahomey Gap populations originate from the Cameroon volcanic line. Molecular Phylogenetics and Evolution 150: 106854. doi: 10.1016/j.ympev.2020.106854. [DOI] [PubMed] [Google Scholar]
- Demos TC, Kerbis Peterhans JC, Agwanda B, Hickerson MJ.. 2014. Uncovering cryptic diversity and refugial persistence among small mammal lineages across the Eastern Afromontane biodiversity hotspot. Molecular Phylogenetics and Evolution 71: 41–54. doi: 10.1016/j.ympev.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Dimitrov D, Nogués-Bravo D, Scharff N.. 2012. Why do tropical mountains support exceptionally high biodiversity? The Eastern Arc Mountains and the drivers of Saintpaulia diversity. PLoS One 7: e48908. doi: 10.1371/journal.pone.0048908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droissart V, Dauby G, Hardy OJ, et al. 2018. Beyond trees: biogeographical regionalization of tropical Africa. Journal of Biogeography 45: 1153–1167. doi: 10.1111/jbi.13190. [DOI] [Google Scholar]
- Drummond AJ, Ho SYW, Phillips MJ, Rambaut A.. 2006. Relaxed phylogenetics and dating with confidence. PLoS Biology 4: e88. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duminil J, Mona S, Mardulyn P, et al. 2015. Late Pleistocene molecular dating of past population fragmentation and demographic changes in African rain forest tree species supports the forest refuge hypothesis. Journal of Biogeography 42: 1443–1454. doi: 10.1111/jbi.12510. [DOI] [Google Scholar]
- Eiserhardt WL, Couvreur TLP, Baker WJ.. 2017. Plant phylogeny as a window on the evolution of hyperdiversity in the tropical rainforest biome. New Phytologist 214: 1408–1422. doi: 10.1111/nph.14516. [DOI] [PubMed] [Google Scholar]
- Erkens RHJ, Chatrou LW, Couvreur TLP.. 2012. Radiations and key innovations in an early branching angiosperm lineage (Annonaceae; Magnoliales). Botanical Journal of the Linnean Society 169: 117–134. doi: 10.1111/j.1095-8339.2012.01223.x. [DOI] [Google Scholar]
- Faye A, Pintaud J-C, Baker WJ, Vigouroux Y, Sonke B, Couvreur TLP.. 2016. Phylogenetics and diversification history of African rattans (Calamoideae, Ancistrophyllinae). Botanical Journal of the Linnean Society 182: 256–271. doi: 10.1111/boj.12454. [DOI] [Google Scholar]
- Fer I, Tietjen B, Jeltsch F, Trauth MH.. 2017. Modelling vegetation change during Late Cenozoic uplift of the East African plateaus. Palaeogeography, Palaeoclimatology, Palaeoecology 467: 120–130. doi: 10.1016/j.palaeo.2016.04.007. [DOI] [Google Scholar]
- Finch J, Leng MJ, Marchant R.. 2009. Late Quaternary vegetation dynamics in a biodiversity hotspot, the Uluguru Mountains of Tanzania. Quaternary Research 72: 111–122. doi: 10.1016/j.yqres.2009.02.005. [DOI] [Google Scholar]
- Fjeldså J, Bowie RCK.. 2008. New perspectives on the origin and diversification of Africa’s forest avifauna. African Journal of Ecology 46: 235–247. doi: 10.1111/j.1365-2028.2008.00992.x. [DOI] [Google Scholar]
- Fjeldså J, Lovett JC.. 1997. Geographical patterns of old and young species in African forest biota: the significance of specific montane areas as evolutionary centres. Biodiversity & Conservation 6: 325–346. [Google Scholar]
- Fjeldså J, Johansson US, Lokugalappatti LGS, Bowie RCK.. 2007. Diversification of African greenbuls in space and time: linking ecological and historical processes. Journal of Ornithology 148: 359–367. doi: 10.1007/s10336-007-0179-4. [DOI] [Google Scholar]
- Fuchs J, Pons J-M, Bowie RCK.. 2017. Biogeography and diversification dynamics of the African woodpeckers. Molecular Phylogenetics and Evolution 108: 88–100. doi: 10.1016/j.ympev.2017.01.007. [DOI] [PubMed] [Google Scholar]
- Gautier-Hion A, Duplantier J-M, Quris R, et al. 1985. Fruit characters as a basis of fruit choice and seed dispersal in a tropical forest vertebrate community. Oecologia 65: 324–337. doi: 10.1007/BF00378906. [DOI] [PubMed] [Google Scholar]
- Gentry AH. 1982. Neotropical floristic diversity: phytogeographical connections between Central and South America, Pleistocene climatic flutuations, or an accident of the Andean orogeny? Annals of the Missouri Botanical Garden 69: 557–593. doi: 10.2307/2399084. [DOI] [Google Scholar]
- Gonçalves M, Siegismund HR, van Vuuren BJ, Ferrand N, Godinho R.. 2021. Evolutionary history of the roan antelope across its African range. Journal of Biogeography 48: 2812–2827. doi: 10.1111/jbi.14241. [DOI] [Google Scholar]
- Gottsberger G, Meinke S, Porembski S.. 2011. First records of flower biology and pollination in African Annonaceae: Isolona, Piptostigma, Uvariodendron, Monodora and Uvariopsis. Flora - Morphology, Distribution, Functional Ecology of Plants 206: 498–510. doi: 10.1016/j.flora.2010.08.005. [DOI] [Google Scholar]
- Griffiths CJ. 1993. The geological evolution of East Africa. In: Lovett JC, Wasser SK, eds. Biogeography and ecology of the rain forests of Eastern Africa. Cambridge: Cambridge University Press, 9–22. [Google Scholar]
- Guillocheau F, Simon B, Baby G, Bessin P, Robin C, Dauteuil O.. 2018. Planation surfaces as a record of mantle dynamics: the case example of Africa. Gondwana Research 53: 82–98. doi: 10.1016/j.gr.2017.05.015. [DOI] [Google Scholar]
- Haywood AM, Hill DJ, Dolan AM, et al. 2013. Large-scale features of Pliocene climate: results from the Pliocene Model Intercomparison Project. Climate of the Past 9: 191–209. doi: 10.5194/cp-9-191-2013. [DOI] [Google Scholar]
- Helmstetter AJ, Béthune K, Kamdem NG, Sonké B, Couvreur TLP.. 2020. Individualistic evolutionary responses of Central African rain forest plants to Pleistocene climatic fluctuations. Proceedings of the National Academy of Sciences 117: 32509–32518. doi: 10.1073/pnas.2001018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert TD, Lawrence KT, Tzanova A, Peterson LC, Caballero-Gill R, Kelly CS.. 2016. Late Miocene global cooling and the rise of modern ecosystems. Nature Geoscience 9: 843–847. doi: 10.1038/ngeo2813. [DOI] [Google Scholar]
- Höhna S, May MR, Moore BR.. 2015. Phylogeny simulation and diversification rate analysis with TESS.
- Höhna S, May MR, Moore BR.. 2016. TESS: an R package for efficiently simulating phylogenetic trees and performing Bayesian inference of lineage diversification rates. Bioinformatics 32: 789–791. doi: 10.1093/bioinformatics/btv651. [DOI] [PubMed] [Google Scholar]
- Höhna S, Freyman WA, Nolen Z, Huelsenbeck JP, May MR, Moore BR.. 2019. A Bayesian approach for estimating branch-specific speciation and extinction rates. bioRxiv 2019 02 20. [Google Scholar]
- Holstein N, Renner SS.. 2011. A dated phylogeny and collection records reveal repeated biome shifts in the African genus Coccinia (Cucurbitaceae). BMC Evolutionary Biology 11: 28. doi: 10.1186/1471-2148-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoorn C, Wesselingh FP, Steege H, et al. 2010. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330: 927–931. [DOI] [PubMed] [Google Scholar]
- Huntley JW, Voelker G.. 2016. Cryptic diversity in Afro-tropical lowland forests: the systematics and biogeography of the avian genus Bleda. Molecular Phylogenetics and Evolution 99: 297–308. doi: 10.1016/j.ympev.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Huntley JW, Harvey JA, Pavia M, Boano G, Voelker G.. 2018. The systematics and biogeography of the Bearded Greenbuls (Aves: Criniger) reveals the impact of Plio-Pleistocene forest fragmentation on Afro-tropical avian diversity. Zoological Journal of the Linnean Society 183: 672–686. doi: 10.1093/zoolinnean/zlx086. [DOI] [Google Scholar]
- Jacobs BF. 2004. Palaeobotanical studies from tropical Africa: relevance to the evolution of forest, woodland and savannah biomes. Philosophical Transactions of the Royal Society B: Biological Sciences 359: 1573–1583. doi: 10.1098/rstb.2004.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BF, Tabor N, Feseha M, et al. 2005. Oligocene terrestrial strata of northwestern Ethiopia: a preliminary report on paleoenvironments and paleontology. Palaeontologia Electronica [Electronic Resource]. 8: 19. [Google Scholar]
- Jacobs BF, Pan AD, Scotese CR.. 2010. A review of the Cenozoic vegetation history of Africa. In: Werdelin L, Sanders WJ, eds.. Cenozoic mammals of Africa. Berkeley: University of California Press, 57–72. [Google Scholar]
- Johnson MG, Gardner EM, Liu Y, et al. 2016. HybPiper: extracting coding sequence and introns for phylogenetics from high-throughput sequencing reads using target enrichment. Applications in Plant Sciences 4: 1600016. doi: 10.3732/apps.1600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong TJ, Shmida A, Thuijsman F.. 2008. Sex allocation in plants and the evolution of monoecy. Evolutionary Ecology Research 10: 1087–1109. [Google Scholar]
- Joordens JCA, Feibel CS, Vonhof HB, Schulp AS, Kroon D.. 2019. Relevance of the eastern African coastal forest for early hominin biogeography. Journal of Human Evolution 131: 176–202. doi: 10.1016/j.jhevol.2019.03.012. [DOI] [PubMed] [Google Scholar]
- Kass RE, Raftery AE.. 1995. Bayes Factors. Journal of the American Statistical Association 90: 773–795. doi: 10.1080/01621459.1995.10476572. [DOI] [Google Scholar]
- Katoh K, Standley DM.. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kergoat GJ, Condamine FL, Toussaint EFA, et al. 2018. Opposite macroevolutionary responses to environmental changes in grasses and insects during the Neogene grassland expansion. Nature Communications 9: 5089. doi: 10.1038/s41467-018-07537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissling WD, Eiserhardt WL, Baker WJ, et al. 2012. Cenozoic imprints on the phylogenetic structure of palm species assemblages worldwide. Proceedings of the National Academy of Sciences 109: 7379–7384. doi: 10.1073/pnas.1120467109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis AT, Raja NB.. 2021. chronosphere: Earth system history variables. https://CRAN.R-project.org/package=chronosphere.
- Lagomarsino LP, Condamine FL, Antonelli A, Mulch A, Davis CC.. 2016. The abiotic and biotic drivers of rapid diversification in Andean bellflowers (Campanulaceae). New Phytologist 210: 1430–1442. doi: 10.1111/nph.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaché AD, Portik DM, Rivera D, et al. 2019. Exploring rain forest diversification using demographic model testing in the African foam‐nest treefrog Chiromantis rufescens. Journal of Biogeography 46: 2706–2721. doi: 10.1111/jbi.13716. [DOI] [Google Scholar]
- Lehmann CER, Archibald SA, Hoffmann WA, Bond WJ.. 2011. Deciphering the distribution of the savanna biome. New Phytologist 191: 197–209. doi: 10.1111/j.1469-8137.2011.03689.x. [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R.. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder HP. 2017. East African Cenozoic vegetation history. Evolutionary Anthropology: Issues, News, and Reviews 26: 300–312. doi: 10.1002/evan.21570. [DOI] [PubMed] [Google Scholar]
- Lischer HEL, Excoffier L.. 2012. PGDSpider: an automated data conversion tool for connecting population genetics and genomics programs. Bioinformatics 28: 298–299. doi: 10.1093/bioinformatics/btr642. [DOI] [PubMed] [Google Scholar]
- Loader SP, Pisani D, Cotton JA, Gower DJ, Day JJ, Wilkinson M.. 2007. Relative time scales reveal multiple origins of parallel disjunct distributions of African caecilian amphibians. Biology Letters 3: 505–508. doi: 10.1098/rsbl.2007.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loader SP, Ceccarelli FS, Menegon M, et al. 2014. Persistence and stability of Eastern Afromontane forests: evidence from brevicipitid frogs. Journal of Biogeography 41: 1781–1792. [Google Scholar]
- Lompo D, Vinceti B, Konrad H, Gaisberger H, Geburek T.. 2018. Phylogeography of African locust bean (Parkia biglobosa) reveals genetic divergence and spatially structured populations in West and Central Africa. Journal of Heredity 109: 811–824. doi: 10.1093/jhered/esy047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett JC, Marchant R, Taplin J, Küper W.. 2005. The oldest rainforests in Africa: stability or resilience for survival and diversity? In: Purvis A, Gittleman JL, Brooks T, eds. Phylogeny and conservation. Cambridge: Cambridge University Press, 198–229. [Google Scholar]
- Macgregor D. 2015. History of the development of the East African Rift System: a series of interpreted maps through time. Journal of African Earth Sciences 101: 232–252. doi: 10.1016/j.jafrearsci.2014.09.016. [DOI] [Google Scholar]
- Maddison WP, FitzJohn RG.. 2015. The unsolved challenge to phylogenetic correlation tests for categorical characters. Systematic Biology 64: 127–136. doi: 10.1093/sysbio/syu070. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Midford PE, Otto SP.. 2007. Estimating a binary character’s effect on speciation and extinction. Systematic Biology 56: 701–710. doi: 10.1080/10635150701607033. [DOI] [PubMed] [Google Scholar]
- Magallón S, Sanderson MJ.. 2001. Absolute diversification rates in angiosperm clades. Evolution 55: 1762–1780. doi: 10.1111/j.0014-3820.2001.tb00826.x. [DOI] [PubMed] [Google Scholar]
- Malhi Y, Gardner TA, Goldsmith GR, Silman MR, Zelazowski P.. 2014. Tropical forests in the Anthropocene. Annual Review of Environment and Resources 39: 125–159. doi: 10.1146/annurev-environ-030713-155141. [DOI] [Google Scholar]
- Maliet O, Hartig F, Morlon H.. 2019. A model with many small shifts for estimating species-specific diversification rates. Nature Ecology & Evolution 3: 1086–1092. doi: 10.1038/s41559-019-0908-0. [DOI] [PubMed] [Google Scholar]
- Maliet O, Morlon H.. 2021. Fast and accurate estimation of species-specific diversification rates using data augmentation. Systematic Biology 71: 353–366. doi: 10.1093/sysbio/syab055. [DOI] [PubMed] [Google Scholar]
- Massoni J, Couvreur TLP, Sauquet H.. 2015a. Five major shifts of diversification through the long evolutionary history of Magnoliidae (Angiosperms). BMC Evolutionary Biology 15: 49. doi: 10.1186/s12862-015-0320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoni J, Doyle J, Sauquet H.. 2015b. Fossil calibration of Magnoliidae, an ancient lineage of Angiosperms. Palaeontologia Electronica 18: 1–25. [Google Scholar]
- Masters JC, Génin F, Zhang Y, et al. 2021. Biogeographic mechanisms involved in the colonization of Madagascar by African vertebrates: rifting, rafting and runways. Journal of Biogeography 48: 492–510. doi: 10.1111/jbi.14032. [DOI] [Google Scholar]
- Matzke NJ. 2014. Model selection in historical biogeography reveals that founder-event speciation is a crucial process in island clades. Systematic Biology 63: 951–970. doi: 10.1093/sysbio/syu056. [DOI] [PubMed] [Google Scholar]
- May MR, Höhna S, Moore BR.. 2016. A Bayesian approach for detecting the impact of mass-extinction events on molecular phylogenies when rates of lineage diversification may vary. Methods in Ecology and Evolution 7: 947–959. doi: 10.1111/2041-210x.12563. [DOI] [Google Scholar]
- Mayaux P, Bartholomé E, Fritz S, Belward A.. 2004. A new land-cover map of Africa for the year 2000. Journal of Biogeography 31: 861–877. doi: 10.1111/j.1365-2699.2004.01073.x. [DOI] [Google Scholar]
- Menegon M, Loader SP, Marsden SJ, Branch WR, Davenport TRB, Ursenbacher S.. 2014. The genus Atheris (Serpentes: Viperidae) in East Africa: phylogeny and the role of rifting and climate in shaping the current pattern of species diversity. Molecular Phylogenetics and Evolution 79: 12–22. doi: 10.1016/j.ympev.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Mertens JEJ, Tropek R, Dzekashu FF, Maicher V, Fokam EB, Janeček S.. 2018. Communities of flower visitors of Uvariopsis dioica (Annonaceae) in lowland forests of Mt. Cameroon, with notes on its potential pollinators. African Journal of Ecology 56: 146–152. [Google Scholar]
- Mitchell JS, Etienne RS, Rabosky DL.. 2019. Inferring diversification rate variation from phylogenies with fossils. Systematic Biology 68: 1–18. doi: 10.1093/sysbio/syy035. [DOI] [PubMed] [Google Scholar]
- Moritz C, Patton JL, Schneider CJ, Smith TB.. 2000. Diversification of rainforest faunas: an integrated molecular approach. Annual Review of Ecology and Systematics 31: 533–563. doi: 10.1146/annurev.ecolsys.31.1.533. [DOI] [Google Scholar]
- Morley RJ. 2000. Origin and evolution of tropical rain forests. Chichester: John Wiley & Sons Ltd. [Google Scholar]
- Morley RJ. 2011. Cretaceous and Tertiary climate change and the past distribution of megathermal rainforests. In: Bush M, Flenley J, Gosling W, eds. Tropical rainforest responses to climatic change. Berlin: Springer, 1–34. [Google Scholar]
- Morley RJ, Richards K.. 1993. Gramineae cuticle: a key indicator of Late Cenozoic climatic change in the Niger Delta. Review of Palaeobotany and Palynology 77: 119–127. doi: 10.1016/0034-6667(93)90060-8. [DOI] [Google Scholar]
- Muellner-Riehl AN. 2019. Mountains as evolutionary arenas: patterns, emerging approaches, paradigm shifts, and their implications for plant phylogeographical research in the Tibeto-Himalayan region. Frontiers in Plant Science 10: 195. doi: 10.3389/fpls.2019.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas V, Fabre P-H, Bryja J, et al. 2020. The phylogeny of the African wood mice (Muridae, Hylomyscus) based on complete mitochondrial genomes and five nuclear genes reveals their evolutionary history and undescribed diversity. Molecular Phylogenetics and Evolution 144: 106703. doi: 10.1016/j.ympev.2019.106703. [DOI] [PubMed] [Google Scholar]
- Nkonmeneck WPT, Allen KE, Hime PM, et al. 2022. Diversification and historical demography of Rhampholeon spectrum in West-Central Africa. PLoS One 17: e0277107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson DM, Dinerstein E, Wikramanayake ED, et al. 2001. Terrestrial ecoregions of the world: a new map of life on Earth. BioScience 51: 933. doi: 10.1641/0006-3568(2001)051[0933:teotwa]2.0.co;2. [DOI] [Google Scholar]
- Onstein RE, Baker WJ, Couvreur TLP, et al. 2018. To adapt or go extinct? The fate of megafaunal palm fruits under past global change. Proceedings of the Royal Society B: Biological Sciences 285: 20180882. doi: 10.1098/rspb.2018.0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onstein RE, Kissling WD, Chatrou LW, Couvreur TLP, Morlon H, Sauquet H.. 2019. Which frugivory-related traits facilitated historical long-distance dispersal in the custard apple family (Annonaceae)? Journal of Biogeography 46: 1874–1888. doi: 10.1111/jbi.13552. [DOI] [Google Scholar]
- Palazzesi L, Hidalgo O, Barreda VD, Forest F, Höhna S.. 2022. The rise of grasslands is linked to atmospheric CO2 decline in the late Palaeogene. Nature Communications 13: 293. doi: 10.1038/s41467-021-27897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan AD. 2010. Rutaceae leaf fossils from the Late Oligocene (27.23Ma) Guang River flora of northwestern Ethiopia. Review of Palaeobotany and Palynology 159: 188–194. doi: 10.1016/j.revpalbo.2009.12.005. [DOI] [Google Scholar]
- Pan AD, Jacobs BF, Dransfield J, Baker WJ.. 2006. The fossil history of palms (Arecaceae) in Africa and new records from the Late Oligocene (28–27 Mya) of north-western Ethiopia. Botanical Journal of the Linnean Society 151: 69–81. doi: 10.1111/j.1095-8339.2006.00523.x. [DOI] [Google Scholar]
- Pang C-C, Scharaschkin T, Su YCF, Saunders RMK.. 2013. Functional monoecy due to delayed anther dehiscence: a novel mechanism in Pseuduvaria mulgraveana (Annonaceae). PLoS One 8: e59951. doi: 10.1371/journal.pone.0059951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang C-C, Saunders RMK.. 2014. The evolution of alternative mechanisms that promote outcrossing in Annonaceae, a self-compatible family of early-divergent angiosperms. Botanical Journal of the Linnean Society 174: 93–109. [Google Scholar]
- Parmentier I, Malhi Y, Senterre B, et al. 2007. The odd man out? Might climate explain the lower tree α-diversity of African rain forests relative to Amazonian rain forests? Journal of Ecology 95: 1058–1071. doi: 10.1111/j.1365-2745.2007.01273.x. [DOI] [Google Scholar]
- Pebesma E, Bivand R, Rowlingson B, et al. 2021. sp: classes and methods for spatial data.
- Pirie MD, Doyle JA.. 2012. Dating clades with fossils and molecules: the case of Annonaceae. Botanical Journal of the Linnean Society 169: 84–116. doi: 10.1111/j.1095-8339.2012.01234.x. [DOI] [Google Scholar]
- Plana V. 2004. Mechanisms and tempo of evolution in the African Guineo-Congolian rainforest. Philosophical Transactions of the Royal Society B: Biological Sciences 359: 1585–1594. doi: 10.1098/rstb.2004.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M, Best N, Cowles K, et al. 2020. coda: output analysis and diagnostics for MCMC. https://CRAN.R-project.org/package=coda.
- Pokorny L, Riina R, Mairal M, et al. 2015. Living on the edge: timing of Rand Flora disjunctions congruent with ongoing aridification in Africa. Frontiers in Genetics 6: 154. doi: 10.3389/fgene.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polissar PJ, Rose C, Uno KT, Phelps SR, deMenocal P.. 2019. Synchronous rise of African C4 ecosystems 10 million years ago in the absence of aridification. Nature Geoscience 12: 657–660. doi: 10.1038/s41561-019-0399-2. [DOI] [Google Scholar]
- Rabinowitz PD, Woods S.. 2006. The Africa–Madagascar connection and mammalian migrations. Journal of African Earth Sciences 44: 270–276. doi: 10.1016/j.jafrearsci.2005.12.005. [DOI] [Google Scholar]
- Rabosky DL. 2014. Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PLoS One 9: e89543. doi: 10.1371/journal.pone.0089543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabosky DL, Grundler M, Anderson C, et al. 2014. BAMMtools: an R package for the analysis of evolutionary dynamics on phylogenetic trees. Methods in Ecology and Evolution 5: 701–707. doi: 10.1111/2041-210x.12199. [DOI] [Google Scholar]
- Rahbek C, Borregaard MK, Colwell RK, et al. 2019. Humboldt’s enigma: what causes global patterns of mountain biodiversity? Science 365: 1108–1113. doi: 10.1126/science.aax0149. [DOI] [PubMed] [Google Scholar]
- Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA.. 2018. Posterior summarization in bayesian phylogenetics using Tracer 1.7. Systematic Biology 67: 901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ree RH, Sanmartín I.. 2018. Conceptual and statistical problems with the DEC+J model of founder-event speciation and its comparison with DEC via model selection. Journal of Biogeography 45: 741–749. doi: 10.1111/jbi.13173. [DOI] [Google Scholar]
- Ree RH, Smith SA.. 2008. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology 57: 4–14. doi: 10.1080/10635150701883881. [DOI] [PubMed] [Google Scholar]
- Remis MJ, Dierenfeld ES, Mowry CB, Carroll RW.. 2001. Nutritional aspects of western lowland gorilla (Gorilla gorilla gorilla) diet during seasons of fruit scarcity at Bai Hokou, Central African Republic. International Journal of Primatology 22: 807–836. [Google Scholar]
- Renner SS. 2004. Multiple Miocene Melastomataceae dispersal between Madagascar, Africa and India. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences 359: 1485–1494. doi: 10.1098/rstb.2004.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retallack GJ, Dugas DP, Bestland EA.. 1990. Fossil soils and grasses of a Middle Miocene East African grassland. Science 247: 1325–1328. doi: 10.1126/science.247.4948.1325. [DOI] [PubMed] [Google Scholar]
- Ring U, Albrecht C, Schrenk F.. 2018. The east African rift system: tectonics, climate and biodiversity. In: Hoorn C, Perrigo A, Antonelli A, eds.. Mountains, climate and biodiversity. Chichester: John Wiley & Sons, 391–406. [Google Scholar]
- Ripley B. 2017. pspline: penalized smoothing splines. https://CRAN.R-project.org/package=pspline.
- Rogers ME, Abernethy K, Bermejo M, et al. 2004. Western gorilla diet: a synthesis from six sites. American Journal of Primatology 64: 173–192. doi: 10.1002/ajp.20071. [DOI] [PubMed] [Google Scholar]
- Salzmann U, Hoelzmann P.. 2005. The Dahomey Gap: an abrupt climatically induced rain forest fragmentation in West Africa during the late Holocene. The Holocene 15: 190–199. doi: 10.1191/0959683605hl799rp. [DOI] [Google Scholar]
- Saunders RMK. 2012. The diversity and evolution of pollination systems in Annonaceae. Botanical Journal of the Linnean Society 169: 222–244. doi: 10.1111/j.1095-8339.2011.01208.x. [DOI] [Google Scholar]
- Sauquet H, Doyle JA, Scharaschkin T, et al. 2003. Phylogenetic analysis of Magnoliales and Myristicaceae based on multiple data sets: implications for character evolution. Botanical Journal of the Linnean Society 142: 125–186. doi: 10.1046/j.1095-8339.2003.00171.x. [DOI] [Google Scholar]
- Scotese CR, Wright N.. 2018. PALEOMAP Paleodigital Elevation Models (PaleoDEMS) for the Phanerozoic. PALEOMAP Project.
- Ségalen L, Lee-Thorp JA, Cerling T.. 2007. Timing of C4 grass expansion across sub-Saharan Africa. Journal of Human Evolution 53: 549–559. doi: 10.1016/j.jhevol.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Senut B, Pickford M, Ségalen L.. 2009. Neogene desertification of Africa. Comptes Rendus Geoscience 341: 591–602. doi: 10.1016/j.crte.2009.03.008. [DOI] [Google Scholar]
- Sepulchre P, Ramstein G, Fluteau F, Schuster M, Tiercelin J-J, Brunet M.. 2006. Tectonic uplift and eastern Africa aridification. Science 313: 1419–1423. doi: 10.1126/science.1129158. [DOI] [PubMed] [Google Scholar]
- Serrano-Serrano ML, Rolland J, Clark JL, Salamin N, Perret M.. 2017. Hummingbird pollination and the diversification of angiosperms: an old and successful association in Gesneriaceae. Proceedings of the Royal Society B: Biological Sciences 284: 20162816. doi: 10.1098/rspb.2016.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Setten AK, Koek-Noorman J.. 1992. Fruits and seeds of Annonaceae: morphology and its significance for classification. Stuttgart: Schweizerbart. [Google Scholar]
- Smith SA, Brown JW, Walker JF.. 2018. So many genes, so little time: a practical approach to divergence-time estimation in the genomic era. PLoS One 13: e0197433. doi: 10.1371/journal.pone.0197433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld A, Prömmel K, Cubasch U.. 2016. The East African Rift System and the impact of orographic changes on regional climate and the resulting aridification. International Journal of Earth Sciences 105: 1779–1794. doi: 10.1007/s00531-014-1102-x. [DOI] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera G, Castresana J.. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology 56: 564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- Thomas DC, Chatrou LW, Stull GW, et al. 2015. The historical origins of palaeotropical intercontinental disjunctions in the pantropical flowering plant family Annonaceae. Perspectives in Plant Ecology, Evolution and Systematics 17: 1–16. doi: 10.1016/j.ppees.2014.11.001. [DOI] [Google Scholar]
- Tolley KA, Tilbury CR, Measey GJ, Menegon M, Branch WR, Matthee CA.. 2011. Ancient forest fragmentation or recent radiation? Testing refugial speciation models in chameleons within an African biodiversity hotspot: Palaeoendemic chameleon lineages in East Africa. Journal of Biogeography 38: 1748–1760. doi: 10.1111/j.1365-2699.2011.02529.x. [DOI] [Google Scholar]
- Tolley KA, Townsend TM, Vences M.. 2013. Large-scale phylogeny of chameleons suggests African origins and Eocene diversification. Proceedings of the Royal Society B: Biological Sciences 280: 20130184. doi: 10.1098/rspb.2013.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosso F, Hardy OJ, Doucet J-L, Daïnou K, Kaymak E, Migliore J.. 2018. Evolution in the Amphi-Atlantic tropical genus Guibourtia (Fabaceae, Detarioideae), combining NGS phylogeny and morphology. Molecular Phylogenetics and Evolution 120: 83–93. doi: 10.1016/j.ympev.2017.11.026. [DOI] [PubMed] [Google Scholar]
- Tutin CEG, Fernandez M.. 1993. Composition of the diet of chimpanzees and comparisons with that of sympatric lowland gorillas in the Lopé Reserve, Gabon. American Journal of Primatology 30: 195–211. doi: 10.1002/ajp.1350300305. [DOI] [PubMed] [Google Scholar]
- Uno KT, Polissar PJ, Jackson KE, deMenocal PB.. 2016. Neogene biomarker record of vegetation change in eastern Africa. Proceedings of the National Academy of Sciences 113: 6355–6363. doi: 10.1073/pnas.1521267113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente LM, Manning JC, Goldblatt P, Vargas P.. 2012. Did pollination shifts drive diversification in southern African Gladiolus? Evaluating the model of pollinator-driven speciation. The American Naturalist 180: 83–98. doi: 10.1086/666003. [DOI] [PubMed] [Google Scholar]
- Veranso-Libalah MC, Kadereit G, Stone RD, Couvreur TLP.. 2018. Multiple shifts to open habitats in Melastomateae (Melastomataceae) congruent with the increase of African Neogene climatic aridity. Journal of Biogeography 45: 1420–1431. doi: 10.1111/jbi.13210. [DOI] [Google Scholar]
- Verdcourt B. 1971. Flora of Tropical East Africa: Annonaceae. Richmond, UK: Royal Botanic Gardens, Kew. [Google Scholar]
- Vincens A, Tiercelin J-J, Buchet G.. 2006. New Oligocene–early Miocene microflora from the southwestern Turkana Basin. Palaeogeography, Palaeoclimatology, Palaeoecology 239: 470–486. doi: 10.1016/j.palaeo.2006.02.007. [DOI] [Google Scholar]
- Voelker G, Outlaw RK, Bowie RCK.. 2010. Pliocene forest dynamics as a primary driver of African bird speciation. Global Ecology and Biogeography 19: 111–121. doi: 10.1111/j.1466-8238.2009.00500.x. [DOI] [Google Scholar]
- Vollesen K. 1980. Notes on Annonaceae from Tanzania. Botaniska Notiser 133: 53–62. [Google Scholar]
- Westerhold T, Marwan N, Drury AJ, et al. 2020. An astronomically dated record of Earth’s climate and its predictability over the last 66 million years. Science 369: 1383–1387. doi: 10.1126/science.aba6853. [DOI] [PubMed] [Google Scholar]
- White LJT, Tutin CEG, Fernandez M.. 1993. Group composition and diet of forest elephants, Loxodonta africana cyclotis Matschie 1900, in the Lopé Reserve, Gabon. African Journal of Ecology 31: 181–199. doi: 10.1111/j.1365-2028.1993.tb00532.x. [DOI] [Google Scholar]
- Wichura H, Jacobs LL, Lin A, et al. 2015. A 17-My-old whale constrains onset of uplift and climate change in east Africa. Proceedings of the National Academy of Sciences 112: 3910–3915. doi: 10.1073/pnas.1421502112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EO. 1988. Biodiversity, National Academy of Sciences/Smithsonian Institution, Division on Earth and Life Studies, Commission on Life Sciences, eds. Washington, DC: National Academies Press. [Google Scholar]
- Xue B, Guo X, Landis JB, et al. 2020. Accelerated diversification correlated with functional traits shapes extant diversity of the early divergent angiosperm family Annonaceae. Molecular Phylogenetics and Evolution 142: 106659. doi: 10.1016/j.ympev.2019.106659. [DOI] [PubMed] [Google Scholar]
- Yao G, Drew BT, Yi T-S, Yan H-F, Yuan Y-M, Ge X-J.. 2016. Phylogenetic relationships, character evolution and biogeographic diversification of Pogostemon s.l. (Lamiaceae). Molecular Phylogenetics and Evolution 98: 184–200. doi: 10.1016/j.ympev.2016.01.020. [DOI] [PubMed] [Google Scholar]
- Yoder AD, Nowak MD.. 2006. Has vicariance or dispersal been the predominant biogeographic force in Madagascar? Only time will tell. Annual Review of Ecology, Evolution, and Systematics 37: 405–431. doi: 10.1146/annurev.ecolsys.37.091305.110239. [DOI] [Google Scholar]
- Zachos JC, Dickens GR, Zeebe RE.. 2008. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451: 279–283. doi: 10.1038/nature06588. [DOI] [PubMed] [Google Scholar]
- Zhou L, Su YCF, Thomas DC, Saunders RMK.. 2012. ‘Out-of-Africa’ dispersal of tropical floras during the Miocene climatic optimum: evidence from Uvaria (Annonaceae). Journal of Biogeography 39: 322–335. doi: 10.1111/j.1365-2699.2011.02598.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.