Abstract
A large number of neuron-specific genes characterized to date are under the control of negative transcriptional regulation. Many promoter regions of neuron-specific genes possess the repressor element repressor element 1/neuron-restrictive silencing element (RE1/NRSE). Its cognate binding protein, REST/NRSF, is an essential transcription factor; its null mutations result in embryonic lethality, and its dominant negative mutants produce aberrant expression of neuron-specific genes. REST/NRSF acts as a regulator of neuron-specific gene expression in both nonneuronal tissue and developing neurons. Here, we shown that heterologous expression of REST/NRSF in Saccharomyces cerevisiae is able to repress transcription from yeast promoters engineered to contain RE1/NRSEs. Moreover, we have taken advantage of this observation to show that this repression requires both yeast Sin3p and Rpd3p and that REST/NRSF physically interacts with the product of the yeast SIN3 gene in vivo. Furthermore, we show that REST/NRSF binds mammalian SIN3A and HDAC-2 and requires histone deacetylase activity to repress neuronal gene transcription in both nonneuronal and neuronal cell lines. We show that REST/NRSF binding to RE1/NRSE is accompanied by a decrease in the acetylation of histones around RE1/NRSE and that this decrease requires the N-terminal Sin3p binding domain of REST/NRSF. Taken together, these data suggest that REST/NRSF represses neuronal gene transcription by recruiting the SIN3/HDAC complex.
Neuronal differentiation is accompanied by a massive change in cellular phenotype that requires expression of both panneuronal and neuronal cell type-specific genes. However, the transcriptional mechanisms that underlie these changes in gene expression are only beginning to be understood. Numerous experiments have implicated the role of both positive and negative transcriptional regulation in determining the complement of expressed genes in a cell both during the acquisition of neuronal identity and also in its maintenance (3). It is becoming increasingly apparent that negative transcriptional regulation plays a major role in defining neuron-specific gene expression (51, 64). A great many 5′ regulatory regions of neuron-specific genes have been shown to possess negative-acting cis elements. One such element is the RE1/NRSE (repressor element 1/neuron-restrictive silencer element), a 21-bp negative element that has been found in numerous genes (52). Functional characterization of this element has been demonstrated for many promoters, including those of the sodium type II channel gene (33), the SCG 10 gene (35), the M4 gene (34, 63), synapsin I gene (17, 32, 49), and the GluR2 gene (38). In all these cases, the RE1/NRSE silences expression of the reporter gene in nonneuronal cells. However, transgenic analysis of the promoters of the β2 nicotinic acetylcholine receptor subunit (7) and L1 cell adhesion molecule gene (24) has demonstrated that the RE1/NRSE, as well as mediating repression in both neuronal and nonneuronal cells, can mediate activation in subsets of neuronal cells.
The RE1/NRSE is bound by the zinc finger transcription factor REST/NRSF (12, 50), which is expressed in most embryonic and adult nonneuronal tissue. In the nervous system, expression is largely restricted to undifferentiated neuroepithelium, although differentiated neurons express low levels of REST/NRSF splice variants (40). REST/NRSF knockout mice show up-regulation and ectopic expression of the βIII tubulin gene, morphological perturbations in the head mesenchyme and somites, and, ultimately, lethality around embryonic day 9.5 to 10. Ectopic expression of a dominant-negative REST/NRSF mutant in developing chick embryos results in up-regulation of a number of RE1/NRSE-containing genes, e.g., SCG10 and Ng-CAM, within the developing nervous system (11). These observations underscore the importance of REST/NRSF in normal development and in regulating the expression of differentiated neuronal genes.
REST/NRSF has been shown to possess at least two independent repression domains, one encompassed by the 83 N-terminal residues and the other by the C-terminal zinc finger, both of which can function when fused to a heterologous DNA binding domain (57). The C-terminal repression domain has been shown to interact with a novel protein, CoREST (2). CoREST contains two SANT (SWI13/ADA2/N-CoR/TFIIIB) domains (1) separated by 191 amino acids and is a motif found in N-CoR/SMRTe (16, 41).
The N-terminal repression domain of REST/NRSF does not show sequence homology to any other known repression domains. Furthermore, nothing is known about the mechanism by which it represses transcription.
Many repressors interact with components of the TFIID/RNApolII holocomplex or activators to repress transcription (for a review, see reference 21 and references therein). However, it has recently become clear that the transparency of chromatin itself to transcription factors and components of the general transcriptional machinery can be modulated by repressors and activators in order to allow gene-specific expression (29, 39, 46, 60). In mammalian cells, a number of DNA binding repressors such as Mad:Max, Mxi:Max, unliganded nuclear receptors, and Pit-1 recruit the SIN3 complex and associated histone deacetylase activity to mediate the transcriptional repression of genes governing a variety of cellular processes (reviewed in reference 43). In Saccharomyces cerevisiae, Ume6 binds to the URS1 element and recruits the Sin3-Rpd3 complex (23) to repress transcription of a large variety of yeast promoters (55). Indeed it has been shown that the histone deacetylase activity per se of rpd3p is important for Sin3-Rpd3-mediated repression (23).
However, not all repressors require Sin3 to recruit histone deacetylases. Transcriptional repressors can directly interact with histone deacetylases, e.g., YY1 (65) or Rb (9), or recruit histone deacetylases complexed with other proteins (such as Mi2), e.g., the human papillomavirus E7 zinc finger protein (10).
Although the N-terminal tails of histones H2A, H2B, H3, and H4 are targets for acetylation and deacetylation (66), a number of other proteins have been shown to be regulated by this kind of modification, including p53 (15), TFIIEβ (19), HMGI(Y) (36), and GATA-1 (8).
Many aspects of gene regulation are highly conserved between yeast and higher eukaryotes to the point that when introduced into yeast, a number of transcription factors from Drosophila melanogaster and mammals, are able to modulate transcription at yeast promoters (6, 26, 38, 45, 47, 62). Here, we report that upon expression in yeast, the HZ4 fragment of REST/NRSF is able to repress transcription from an RE1/NRSE-bearing GAL1 promoter. This repression has a genetic requirement for the yeast SIN3 and RPD3 genes and REST/NRSF associates with yeast Sin3p in vivo. We also show that these interactions are conserved in mammalian cells and that the N-terminal repression domain interacts with mammalian SIN3A and HDAC-2. Also, the N-terminal repression domain requires deacetylase activity to repress transcription of neuronal genes. Moreover, we show by chromatin immunoprecipitation experiments that REST/NRSF binding coincides with a reduction in the amount of acetylated histone H3 binding around the RE1/NRSE. Our results therefore indicate that the SIN3/HDAC complex is important for REST/NRSF-mediated silencing and provide evidence that this corepressor complex plays a direct role in regulating differentiated neuronal phenotype via chromatin modification.
MATERIALS AND METHODS
Yeast strains.
All yeast experiments were performed in S. cerevisiae strain FM242 (designated YPH925 in reference 54) and derivatives thereof. Details of cloning procedures, oligonucleotide sequence, etc., are available on request. All transformations were performed according to Schiestl and Gietz (48). The SIN3 disruption was generated by cloning a yeast SIN3 fragment into pRS306(URA3 integrative) (53), linearizing it, transforming it into FM242, and selecting for uracil autotrophy. SIN3 disruption was confirmed by PCR. Strain yA2, expressing hemagglutination (HA)-tagged ySin3p, was generated by cloning an in-frame HA tag-bearing PCR product into pRS306, linearizing it, transforming it into FM242, and selecting for uracil autotrophy. Integrants were screened for by PCR. The rpd3 knockout strain yA3 was generated by single-step gene disruption with a PCR fragment bearing the HIS3 gene and RPD3 5′ and 3′ flanking sequences.
Plasmids.
Yeast reporter and expression plasmids are summarized in Table 1. Details of cloning procedures are available on request. The plasmid pBM2389(RE1)3UAS was generated by cloning an RE1/NRSE double-stranded oligonucleotide derived from the M4 promoter (top strand, 5′-GATCCGAGCTGTCCGAGGTGCTGAATCTGGGAGCTGTCCGAGGTGCTGAATCTGCCTA-3′) and Gal4p consensus binding site double-stranded oligonucleotide (top strand, 5′-GATCGCGGACTGTCCTCCGG-3′) upstream of GAL1TATA in pBM2389 (32a). The plasmid pAR1000 was generated by replacing the HIS3 fragment of pBM2389 with the lacZ fragment of pβGAL (Clontech) and recombining the (RE1)3UAS sequence from pBM2389(RE1)3UAS into pBMGAL by gap rescue. The expression plasmid pLEONOV(HX) was generated by cloning a multiple-cloning site oligonucleotide (top strand, 5′-AGCTTGAATTCGCGGCCGCGGATCCCTCGAGT-3′) into HindIII-cut pGAD10. pLEONOV.NRSF(1-600) was generated by cloning a myc epitope-tagged HZ4 sequence (50) (corresponding to NRSF(1-600) from pMT.HZ4 into pLEONOV. pLEONOV.NRSF(142-445) was generated by cloning a myc-tagged DNA binding domain of mREST (11) into pLEONOV. pLEONOV.NRSF(139-600) was derived from pLEONOV.NRSF(1-600) by removing the coding region corresponding to residues 1 to 138 of NRSF. The glutathione S-transferase (GST) fusions of REST/NRSF and mMAD1 fragments were generated in the plasmid pGEX4T3 (Pharmacia). The gal4-NRSF(1-73) expression plasmid was generated by first cloning the gal4 DNA binding domain coding sequence in frame with myc-tagged HZ4, yielding pMT.G4.HZ4. The gal4-NRSF(1-73) coding region was then amplified and cloned into pTarget (Promega). pMT.Gal4 was derived from pMT.G4.HZ4 by removing the NRSF(1-600) coding region. M4 reporter constructs have been described elsewhere (63). The NaII reporter plasmids were generated by cloning a HindIII/PstI fragment of pSDK7 into pBluescript, excising a HindIII fragment, and cloning into HindIII-cut pGL3 (Promega) to generate pGL3.-1051/+177. pGL3.-134/+177 was generated by digesting pGL3.-1051/+177 with BglII and religating. pGL3-5UAS-7TetO.Inr was generated as follows: a double-stranded oligonucleotide encompassing the adenovirus major late promoter-initiator element (44) was cloned into pGL3 to form pGL3.Inr. The XmaI fragment encompassing 5XUAS-TATA from T7G5TATA (44), a gift from L. Lania, IIGB, Naples, Italy, was cloned into pTRE-Luc (Clontech) to generate pTRE-UAS-TATA. The 7XTetO-5XUAS region was cloned into pGL3.Inr.
TABLE 1.
Stains and plasmids used in this study
| Strain or plasmid | Genotype | Reference or source |
|---|---|---|
| S. cerevisiae strains FM242(YPH177) | MATα ura3-52 lys2-801 ade2-101 his3Δ200 trp1Δ63 leu2Δ1 cyh2 kar1Δ15 | 54 |
| yA1 | FM242(sin3::URA3) | This study |
| yA2 | FM242(sin3::URA3-HASIN3) | This study |
| yA3 | FM242(Δrpd3::HIS3) | This study |
| Plasmids | ||
| pBM2389 | TRP1 CEN GAL1TATA-HIS3 | 32a |
| pBM2389(RE1)3UAS | TRP1 CEN UASGAL1(RE1)3GAL1TATA-HIS3 | This study |
| pBM2389.LacZ | TRP1 CEN GAL1TATA-LacZ | This study |
| pAR1000 | TRP1 CEN UASGAL1(RE1)3GAL1TATA-LacZ | This study |
| pLEONOV | LEU2 2μm ADHpro/ADHterm | This study |
Yeast reporter assays.
His3 growth assays were performed as follows: single yeast colonies were streaked onto appropriate synthetic dropout plates supplemented with either 2% glucose or 2% galactose–2% raffinose and stated concentrations of 3-amino-1,2,4-triazole (3-AT) (Sigma), incubated at 30°C for 3 days, and assessed for growth. β-Galactosidase assays were performed with Lumigal reagent (Clontech). Briefly, 100 μl of saturated cultures in synthetic dropout medium with 2% glucose was pelleted, resuspended in 1 ml of YP medium with an appropriate carbon source, grown to an optical density at 600 nm of 0.5 to 1.0, harvested, freeze-thawed into Z buffer (Clontech) with liquid nitrogen, analyzed on a Turner TD-20e luminometer as per the manufacturer's instructions, and normalized to cell density.
Chromatin immunoprecipitation (ChIP) assays.
ChIP assays in yeast were performed essentially as previously described (13). Yeast strain yA2 (250 ml) expressing either NRSF(1-600) or NRSF(142-445) was grown to an optical density at 600 nm of 0.5, cross-linked with 1% formaldehyde, and treated according to Dedon et al. (13). Supernatant was subjected to PCR with primers GAL1s (5′-GCCACCTGACGTTTAAGAAACC-3′) and GAL1.789a (5′-CTCCTTGACGTTAAAGTATAGAGG-3′) to amplify (RE1)3UAS sequences from the reporter plasmid.
ChIP assays in mammalian cells were performed essentially as described in the Upstate Biotechnology home page (http://www.upstatebiotech.com). Briefly, a 10-cm2 dish of 25 to 50% confluent Neuro-2A cells was transfected with 5 μg of reporter plasmid and 5 μg of pMTGal4 or pMTGal4.NRSF(1-138) plasmid with 21 μl of Tfx-50 (Promega) in 2 ml of OPTImem and fed 4 h posttransfection. Forty-eight hours after feeding, cells were cross-linked and treated as per the protocol. One microgram of each indicated antibody was used (anti-mSIN3A, anti-HDAC2, and anti-HA antibody from Santa Cruz Biotechnology; anti-acetyl histone-H3 from Upstate Biotechnology).
Precipitated DNA was resuspended in 50 μl of water. To detect the precipitated plasmid sequence, 1 μl of DNA was used in a 10-μl, 25-cycle PCR with primers RV3 (5′-CTAGCAAAATAGGCTGTCCC-3′) (Promega) and M4 RE1a (5′-GTACAGGCAGATTCAGCACCTCGGACAGCTCC-3′).
GST pull-down assays.
GST or GST fusion proteins were expressed in Escherichia coli and purified using glutathione-Sepharose beads (Pharmacia) equilibrated with NETN (20 mM Tris [pH 8.0], 100 mM NaCl, 1 mM EDTA, 0.5% NP-40) with 5 mM dithiothreitol and 1 mM PMSF (phenylmethylsulfonyl fluoride). Beads loaded with 10 μg of GST fusion protein were rocked at 4°C with 200 μg of HEK293 whole-cell extract, prepared by freeze-thaw of cells in whole-cell extraction buffer (20 mM HEPES [pH 7.9], 450 mM NaCl, 0.4 mM EDTA, 25% glycerol) and diluted 10-fold in NETN. After overnight incubation, the beads were washed three times with NETN and boiled, and bound protein was subjected to Western blot analysis. Bound mSIN3A was detected by immunoblotting with anti-mSIN3A antibody (Santa Cruz Biotechnology) and ECL detection (Amersham). For the SIN3 domain-mapping experiment, 5 μg of plasmid expressing the indicated fragments of mSIN3A (myc tagged) was transfected into 25 to 50% confluent Neuro-2A cells in 10-cm2 dishes. Protein was harvested 48 h later into 1 ml of NETN Complete Protease Block (Boehringer) and sonicated, and debris was cleared by centrifugation. A total of 5 μg of the indicated antibody and 25 μl of Sepharose G beads were added to 200 μg of protein and rocked overnight at 4°C. Beads were washed five times in NETN supplemented with 10% glycerol and 0.1% sodium dodecyl sulfate (SDS), boiled, subjected to SDS-polyacrylamide gel electrophoresis (PAGE), transferred, and probed with anti-myc antibody.
Gel shift assays.
The two primers, NRSF 1s (5′-GAGACCATGGCCACCCAGGTGATGGG-3′) and NRSF 1040a (5′-GAGATCTAGACTTCATGCTGATTAGAGGCCAC-3′), were used to amplify the coding region of ΔNRSF with cDNA derived from Neuro-2A cells. The resulting PCR product was cloned into pTARGET (Promega) and used as a template for in vitro transcription with Sp6 RNA polymerase. ΔNRSF RNA was then translated in vitro with wheat germ extracts (Promega) according to the manufacturer's instructions. Aliquots of the in vitro-translated protein were used in gel mobility shift assays which were performed as previously described (63). The DNA probe used was a HindIII/BglII fragment from the sodium type II promoter containing the RE1/NRSE (27). Monoclonal anti-REST/NRSF antibody 12C11 was used in supershift experiments.
Cell line maintenance and transfection analysis.
HEK293 and Neuro-2A cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 2 mM glutamine, streptomycin (10 g/liter), and penicillin (10 g/liter). All transfections were performed in 24-well plates, with wells of 1-cm diameter each. For experiments using gal4/NRSF fusions, 0.1 ng of CMV-Renilla (Promega) and 8.3 ng (each) of pUHC13-3 (expressing Tet-VP16), the indicated gal4 fusion plasmid, and a reporter plasmid were combined with 0.056 μl of Tfx-50 (Promega) in 20 μl of OPTImem for 10 min at room temperature, made up to a total volume of 200 μl of OPTImem, and added to cells. Cells were fed after 4 h. For experiments using M4 or NaII reporter plasmids, 27 ng of reporter plasmid and 0.1 ng of CMV-Renilla were combined with 0.056 μl of Tfx-50 and treated as above. Where required, Trichostatin-A (TSA) (Wako Chemical) was added at 100 nM 24 h posttransfection and harvested 24 h later. Firefly luciferase and Renilla luciferase assays were performed in a Turner TD-20e luminometer with the Dual Luciferase kit (Promega) as per the manufacturer's instructions.
RESULTS
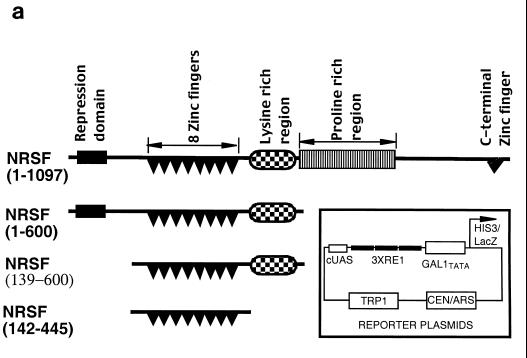
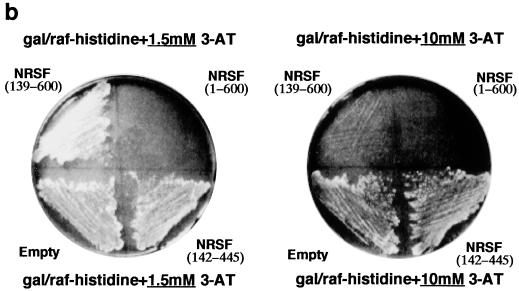
In order to perform genetic screens in yeast to decipher mechanisms by which REST/NRSF regulates gene transcription in mammalian cells, we wished to determine whether REST/NRSF could repress the GAL1 promoter in yeast. A single consensus gal4 binding site, UASgal1, and three RE1/NRSEs from the M4 promoter were placed upstream of GAL1-HIS3 and GAL1-lacZ to generate the centromeric reporter plasmids pBM2389(RE1)3UAS and pAR1000 respectively (Fig. 1a). These were introduced into yeast strain FM242 along with plasmids driving expression of various fragments of REST/NRSF or the empty expression vector (Fig. 1a) and grown on galactose to activate the GAL1 promoter. NRSF(1-600) was able to repress Gal4p-activated GAL1 expression as judged by the inability of the yeast to grow on plates lacking histidine (Fig. 1b) and a >10-fold reduction in lacZ expression than cells not expressing NRSF(1-600) (Fig. 2 compare lanes 1 and 3). Yeast expressing just the DNA binding domain of REST/NRSF [NRSF(142-445)] failed to repress lacZ expression (Fig. 2 compare lanes 1 and 7).
FIG. 1.
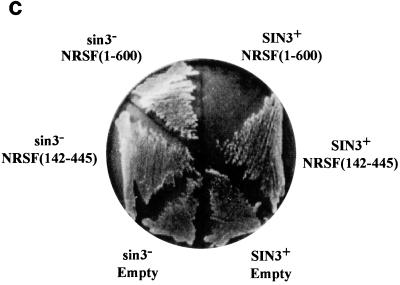
NRSF(1-600) represses GAL1-HIS3 transcription in yeast in a SIN3-dependent manner. (a) Fragments of REST/NRSF used in this study. Inset: yeast reporter plasmid containing three RE1/NRSEs and a single consensus gal4 binding site upstream of GAL1, driving either HIS3 or lacZ. (b) Yeast strain FM242 bearing the reporter pBM2389(RE1)3UAS and expressing the designated REST/NRSF fragments was streaked onto synthetic dropout plates containing 2% galactose–2% raffinose, lacking histidine, with the stated concentrations of 3-AT and incubated at 30°C for 3 days. (c) Wild-type and sin3− (yA1) yeasts expressing the HZ4 fragment of REST/NRSF [NRSF(1-600)] or the DNA binding domain [NRSF(142-445)] or containing empty expression vector were assayed for histidine autotrophy as in Fig. 1b (see Materials and Methods). Synthetic dropout plates contain 2% galactose–2% raffinose and 10 mM 3-AT and lack histidine.
FIG. 2.
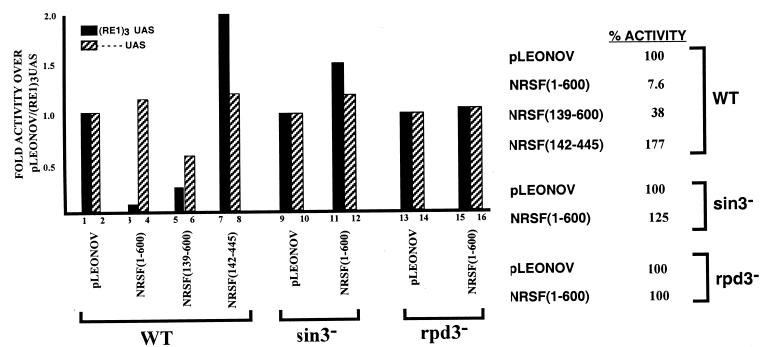
GAL1-lacZ expression in yeast expressing REST/NRSF fragments. REST/NRSF repression of GAL1 transcription was quantified by replacing the HIS3 gene of pBM2389(RE1)3UAS with lacZ. β-Galactosidase assays were performed as described in Materials and Methods. Results are the average of at least seven assays and standard errors were less than 10%. The percent activity is defined as the lacZ value with RE1/NRSEs divided by lacZ values without RE1/NRSEs multiplied by 100.
The 83 N-terminal residues encompass one of two repression domains identified by Tapia-Ramirez et al. (57). To assess whether the repression mechanisms employed by the REST/NRSF fragment in yeast were the same as those used in mammalian cells, we took advantage of the observation that only the N-terminal repression domain was contained within NRSF(1-600). Removal of residues 1 to 138 resulted in significant, though incomplete, loss of repression as judged by the inability of the reporter strain to grow in the presence of 10 mM 3-AT (Fig. 1b). Also, lacZ assays showed that NRSF(139-600) only repressed GAL1 expression to 38% of maximum compared to 7.6% with NRSF(1-600) (Fig. 2, compare lanes 1 and 5). This is consistent with the removal of a repressor domain. However, NRSF(139-600) still retained repressor ability, which could be abolished by deleting residues 446 to 600, the lysine-rich domain (LRD) of REST/NRSF, to give NRSF(142-445). The ability of the LRD to repress transcription in mammalian cells and its physiological relevence to REST/NRSF function are currently under investigation.
Many repressors of transcription work by recruiting corepressor complexes. The Sin3p molecule has been implicated in the repression mechanism of many repressors and is conserved between yeast and mammals. To assess the role of Sin3p in REST/NRSF-mediated repression, we generated a sin3− yeast strain, yA1, in which we performed repression assays. Figures 1c and 2 show that loss of SIN3 results in the abolition of REST/NRSF's ability to repress Gal4p-activated transcription of the GAL1 promoter, as judged by the ability to grow on plates lacking histidine and by maximal activity of lacZ expression (Fig. 2, lanes 9 to 12). Sin3p has been shown to be associated with the product of the RPD3 gene, the deacetylase activity of which is important in mediating repression (22). To determine whether Rpd3p was required for REST/NRSF repression in yeast, we generated the strain yA3, which is deleted for the RPD3 gene in which we performed lacZ assays. As is seen in Fig. 2, abolition of the RPD3 gene results in loss of ability of NRSF(1-600) to repress activated GAL1 expression in yeast (Fig. 2, compare lanes 13 and 15). Thus, REST/NRSF is able to repress transcription in yeast in a manner that requires the products of the yeast SIN3 and RPD3 genes.
As expected, glucose repression of the GAL1 promoter was still intact in strain yA1 (data not shown), indicating that SIN3 disruption had not rendered the GAL1 promoter in pBM2389(RE1)3UAS immune to repressor signals in general. Further, REST/NRSF-mediated repression of GAL1 transcription in a strain disrupted for the SSN6 gene, whose product is part of the Ssn6p/Tup1p corepressor complex involved in glucose repression (59), was not affected (data not shown), suggesting that REST/NRSF has a specific requirement for the Sin3p corepressor complex.
To see if the genetic interaction between REST/NRSF and Sin3p/Rpd3p was due to a physical interaction between the two molecules in vivo, we performed a ChIP assay (Fig. 3). A strain expressing HA epitope-tagged Sin3p was generated (yA2) bearing the reporter pBM2389(RE1)3UAS, which expressed either myc-tagged NRSF(1-600) or myc-tagged NRSF(142-445). Formaldehyde cross-linking and immunoprecipitation with anti-HA antibody precipitated the reporter sequence in the strain expressing NRSF(1-600) but not in the strain expressing NRSF(142-445) (Fig. 3, compare lanes 1 and 2). Anti-myc antibody precipitated the reporter sequence in both strains (Fig. 3, compare lanes 3 and 4). Also, anti-HA antibody precipitated promoter DNA from the IME2 gene, which is known to recruit Sin3p (22), but not SUC2 promoter DNA, which is not regulated by Sin3p (data not shown). The HZ4 fragment of REST/NRSF [NRSF(1-600)] is therefore able to interact with the Sin3p complex and recruit Sin3p to the GAL1 promoter via RE1/NRSE sequences in vivo.
FIG. 3.
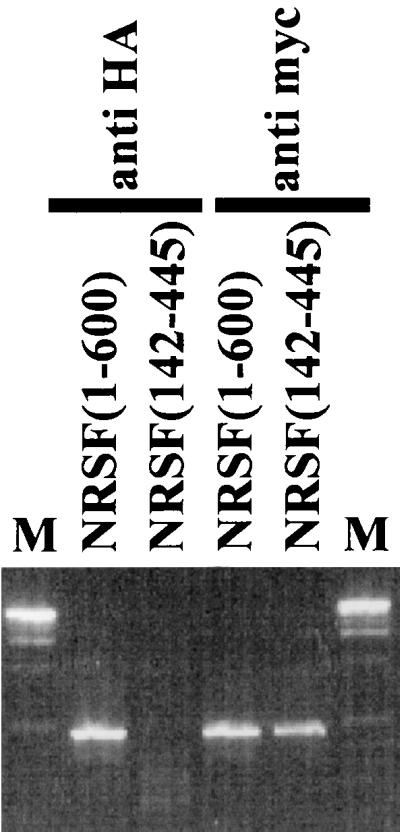
NRSF(1-600) and yeast Sin3p interact in vivo. myc-tagged NRSF(1-600) and NRSF(142-445) were expressed in yeast strain yA2 bearing an HA-tagged SIN3 gene and carrying the reporter pBM2389(RE1)3UAS. Cells were grown to mid-log phase, cross-linked with formaldehyde, and lysed, and chromatin was sheared. Immunoprecipitation with anti-HA or anti-myc antibody was followed by decross-linking, and the resulting precipitate was subjected to PCR with primers to the (RE1)3UAS cassette.
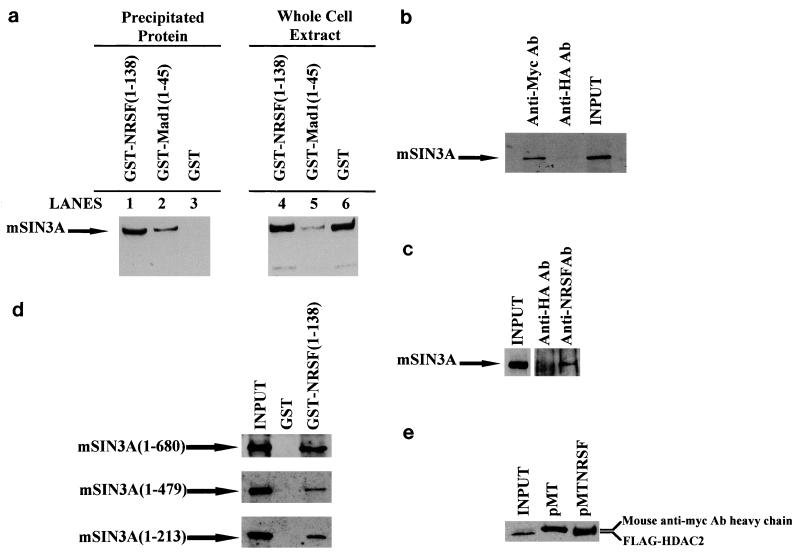
Having established that REST/NRSF is able to repress transcription in yeast by recruiting the Sin3p complex, we sought to determine if REST/NRSF was able to interact with mammalian SIN3. The 138 N-terminal residues of REST/NRSF encompassing the previously characterized N-terminal repression domain (57) were fused to GST and purified from E. coli. The fusion protein was incubated with cell extracts from HEK293 cells, spun down, and washed, and the pellet was assessed for the presence of mSIN3A by Western blot analysis. GST-NRSF(1-138) was able to precipitate mSIN3A under conditions which failed to allow mSIN3A to bind GST alone (Fig. 4a, compare lanes 1 and 3). As expected, GST fused to the SIN3 interaction domain of MAD1 (residues 1 to 45) (4) precipitated mSIN3A (Fig. 4a, compare lanes 2 and 3). This demonstrates that NRSF(1-138), a fragment of REST/NRSF required for full repression in yeast and known to repress autonomously in mammalian cells, interacts with mammalian SIN3A protein in vitro.
FIG. 4.
REST/NRSF N-terminal repression domain interacts with mammalian SIN3A and HDAC2. (a) GST fusions to residues 1 to 138 of REST/NRSF or Mad1 residues (1 to 45) were purified from E. coli, loaded onto glutathione-Sepharose beads, and incubated with extracts from HEK293 cells. Beads were washed with NETN buffer (see Materials and Methods), boiled, and subjected to SDS-PAGE. Gels were blotted and probed with anti-mSIN3A antibody. Lanes 1, 2, and 3 represent the precipitated proteins pulled down with Sepharose beads coupled to the indicated fusion proteins. Lanes 4, 5, and 6 represent the input to the binding reaction (10% of lysate used in lanes 1, 2, and 3). (b) Neuro-2A cells transfected with pMTNRSF were lysed, and the extract was subjected to immunoprecipitation as described in Materials and Methods. Sepharose-G-bound anti-myc or anti-HA antibody was incubated with extract, washed in NETN, and subjected to SDS-PAGE. The input lane represents 10% of lysate used in the immunoprecipitation reactions. (c) Untransfected Neuro-2A cells were lysed, and the extract was subjected to immunoprecipitation as described in Materials and Methods. Sepharose-G-bound anti-HA or anti-NRSF antibody (12c11) was incubated with extract, washed in NETN, and subjected to SDS-PAGE. Input represents 2 to 5% of lysate used in the immunoprecipitation reactions. The input lane was exposed to film for 30 s, the immunoprecipitation lanes were exposed to film for 30 min. (d) HEK cells were transfected with plasmids expressing indicated myc-tagged fragments of mSIN3A, and extracts were prepared as in Materials and Methods. Glutathione-conjugated Sepharose beads loaded with either GST or GST fused to the 138 N-terminal residues of REST/NRSF were incubated with extracts, washed in NETN, subjected to SDS-PAGE, and probed with anti-myc antibody. The input lane represents 10% of lysate used in the pull-down assays. (e) Neuro-2A cells were transfected with plasmid-expressing FLAG-HDAC2 in combination with either pMTNRSF (expressing myc-tagged REST/NRSF) or pMT (expressing just myc epitope). Extracts were prepared, subjected to immunoprecipitation with anti-myc antibody, washed in NETN, run on an SDS gel, transferred, and probed with anti-FLAG antibody. The input lane represents 10% of extract used in the immunoprecipitation reaction. The anti-myc antibody heavy-chain band is visible because both the anti-myc and anti-FLAG antibodies are raised in mouse.
To address whether full-length REST/NRSF could interact with mammalian SIN3A, a plasmid driving myc-tagged full-length REST/NRSF expression was transfected into Neuro-2A cells, and the cell extract was subjected to immunoprecipitation with anti-myc antibody. The presence of mSIN3A was assessed by Western analysis of the immunocomplex with anti-mSIN3A antibody. Figure 4b shows that mSIN3A was precipitated with anti-myc antibody (lane 3) but failed to precipitate when anti-HA antibody was used (lane 2), demonstrating that full-length REST/NRSF can interact with mSIN3A in mammalian cells. Anti-REST/NRSF antibody (12c11) was then used in immunoprecipitation assays to see whether endogenous REST/NRSF was bound to endogenous mSIN3A as an in vivo complex. Figure 4c shows that mSIN3A is precipitated with anti-REST/NRSF antibody (lane 3) but not with anti-HA antibody (lane 2) from Neuro-2A cell extracts. REST/NRSF therefore forms a complex with mSIN3A in vivo.
In order to define more closely the domain of mSIN3A that REST/NRSF binds, we generated a series of myc-tagged mSIN3A deletion mutants: mSIN3A(1-680), mSIN3A(1-479), and mSIN3A(1-272). The fragments were expressed in HEK cells and subjected to GST pull-down assays (Fig. 4d). GST-NRSF(1-138) was able to bind all three mutants (lane 3) under conditions that prevented GST alone from binding (lane 2). This result suggests that mSIN3A residues 1 to 272 are sufficient for REST/NRSF binding.
Because repression by HZ4 in yeast was dependant not only on Sin3p but also on Rpd3p, we wished to determine whether REST/NRSF recruited mammalian Rpd3p (HDAC2) as part of the mammalian SIN3A complex. Flag-tagged HDAC2 and myc-tagged REST/NRSF were expressed in Neuro-2A cells, and protein extracts were subjected to immunoprecipitation with anti-myc antibody. As seen in Fig. 4e, FLAG-HDAC2 is precipitated by anti-myc antibody (lane 3) but not by anti-HA antibody (lane 2). HDAC2 therefore is able to interact with REST/NRSF to form a REST/NRSF-SIN3-HDAC2 complex.
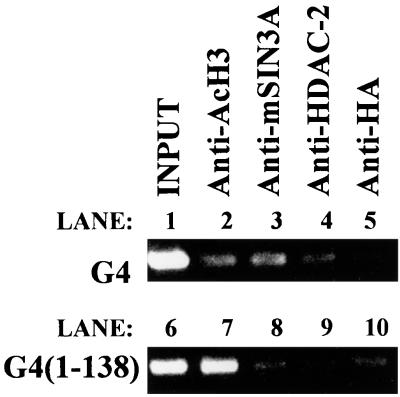
Based on the above genetic and biochemical data, we postulated that REST/NRSF represses RE1/NRSE-containing genes by recruiting the SIN3/HDAC complex to maintain neighboring nucleosomes in a hypoacetylated state. To test this, we performed a ChIP analysis on Neuro-2A/1 cells (Neuro-2A/1 cells express full-length REST/NRSF [data not shown]) transfected with the M4 reporter plasmid pGL3LC.-677/+80 (see below) which possesses a functional RE1/NRSE (63) in combination with a plasmid expressing either the yeast gal4 DNA binding domain or gal4 fused to residues 1 to 138 of human NRSF [G4(1-138)]. We reasoned that overexpressing G4(1-138) would result in competition of the SIN3/HDAC complex away from endogenous REST/NRSF and so should result in (i) a decrease in the occupancy of the RE1/NRSE by mSIN3A and HDAC2, and (ii) an increase in the presence of acetylated histones around REST/NRSF-occupied RE1/NRSEs. After cross-linking with formaldehyde, cells were lysed, and the extract was subjected to immunoprecipitation with antibodies to acetylated histone H3, mSIN3A, HDAC2, or HA epitope (as a negative control). Precipitated DNA was washed, liberated from cross-linked protein, and subjected to PCR with primers around the M4 RE1/NRSE (Fig. 5). Antibodies to mSIN3A and HDAC2 clearly precipitate the reporter sequence in cells expressing just the gal4 DNA binding domain (Fig. 5, lanes 3 and 4 with lane 5). However, in cells overexpressing G4(1-138), the same antibodies fail to precipitate significant reporter DNA compare lane 8 with lanes 3 and 10 and lane 9 with lanes 4 and 10). In contrast, immunoprecipitation with antibody to acetylated histone H3 yielded greater quantities of reporter DNA from cells transfected with G4(1-138) than the gal4 DNA binding domain alone (compare lane 7 with lanes 2 and 10). The ChIP observations show that mSIN3A and HDAC2 are bound to REST/NRSF at the M4 RE1/NRSE via the N terminus of REST/NRSF and that their binding is associated with a reduction of hyperacetylated nucleosomes at this site.
FIG. 5.
The N terminus of REST/NRSF recruits SIN3A and HDAC2 to the RE1/NRSE to mediate nucleosome deacetylation. Neuro-2A/1 cells were transfected with the plasmid pGL3LC.-677/+80 in combination with either pMTG4 (expressing the gal4 DNA binding domain) or pMTG4(1-138) (expressing gal4 DNA binding domain fused to the 138 N-terminal residues of REST/NRSF). Cells were cross-linked and immunoprecipitated with antibodies to acetylated histone H3, mSIN3A, HDAC2, or HA as described in Materials and Methods. Precipitated DNA was then PCR amplified with primers to the reporter plasmid around the M4 RE1/NRSE and resolved on an agarose gel.
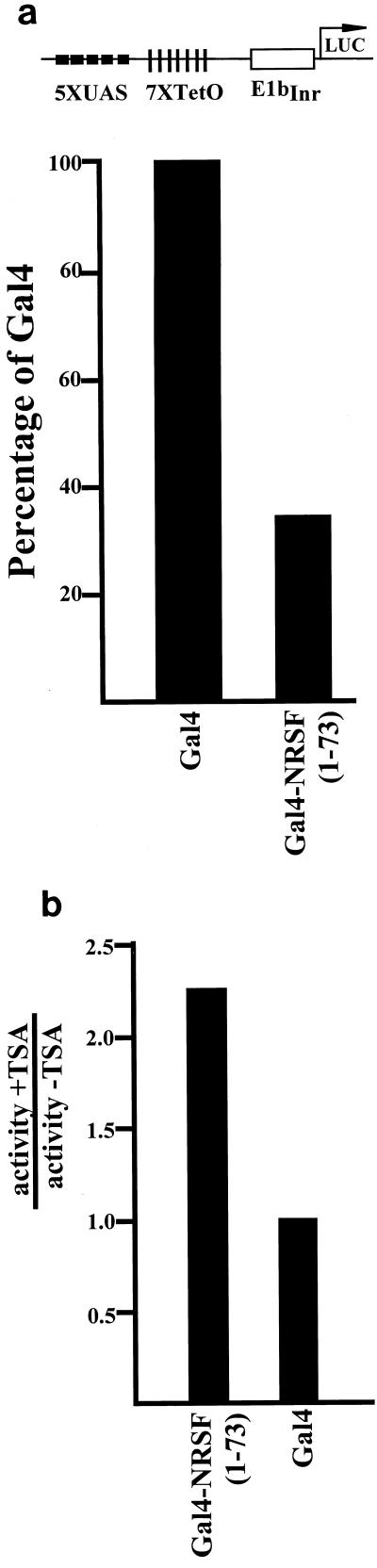
We next wanted to see whether the nucleosomal hypoacetylation observed when mSIN3A and HDAC2 were bound to the N terminus of REST/NRSF resulted in the repressed transcription of a reporter gene. To test this, we performed reporter assays on transfected cells treated with the histone deacetylase inhibitor TSA. HEK293 cells were cotransfected with reporter plasmid pGL3.UAS.TRE.Inr (five GAL1 upstream activation sequence elements and seven tetO elements driving the initiator from the adenovirus major late promoter), pTETOFF (expressing tet-VP16), and fusions of the gal4 DNA binding domain with fragments of REST/NRSF. Figure 6a shows that the 73 N-terminal residues, when fused to gal4, can repress VP16-activated Inr-derived transcription. Further, gal4NRSF(1-73)-mediated repression is sensitive to TSA (Fig. 6b). These data show that the N-terminal repression domain of REST/NRSF can repress heterologous promoters via a mechanism that requires histone deacetylase activity in mammalian cells.
FIG. 6.
The N-terminal repression domain represses transcription from a viral promoter in a TSA-sensitive manner. (a) Vectors expressing gal4 DNA binding domain alone or fused to NRSF(1-73) were transfected into HEK293 cells along with vectors expressing TET-VP16 and the reporter plasmid shown (top). Values (bottom) are the averages of three independent transfections, each performed in triplicate with Tfx-50, normalized to cotransfected CMV-Renilla (see Materials and Methods), and expressed as a percentage of the value obtained with Gal4 alone. Standard errors were less than 10%. (b) Cells transfected as described were treated with 100 nM TSA for 24 h preharvest and analyzed as in panel a. Values are normalized to cotransfected CMV-Renilla and expressed as activity plus TSA relative to GAL4 alone divided by activity minus TSA relative to GAL4 alone.
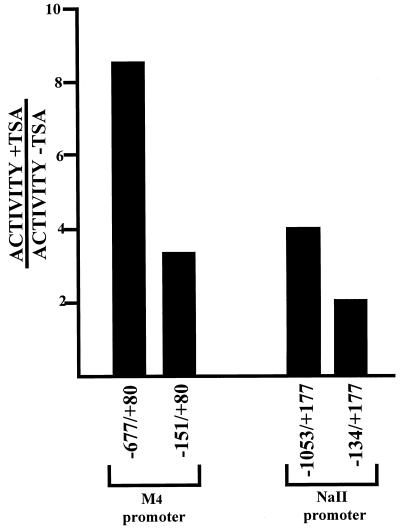
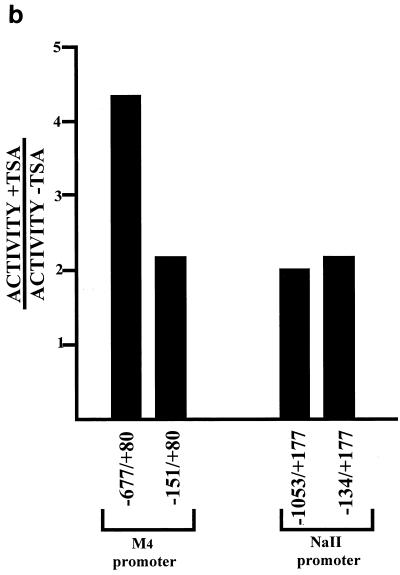
Functional characterization of RE1/NRSE has been demonstrated for a number of promoters of neuronal specific genes (20, 32, 35, 42), including those of the sodium type II channel gene (NaII) (33) and the type 4 muscarinic acetylcholine receptor gene (M4) (34, 63). To determine whether REST/NRSF repressed neuronal-specific genes via a histone deacetylase-dependent mechanism, we performed transient transfection assays in 3T3 fibroblasts with reporter constructs bearing 5′ regions of the NaII and M4 genes with or without their respective RE1/NRSEs. Figure 7 shows that there is a 2.4-fold increase in reporter activity of the M4 promoter construct pGL3LC.-677/+80 (which possesses an RE1/NRSE) over pGL3LC.-151/+80 (which lacks an RE1/NRSE) in the presence of 100 nM TSA. There is a 2.0-fold increase in reporter activity of the NaII promoter construct pGL3.-1053/+177 (which possesses an RE1/NRSE) over pGL3.-134/+177 (which lacks an RE1/NRSE) in the presence of 100 nM TSA. These data are consistent with REST/NRSF repressing neuronal-specific genes via a mechanism that requires histone deacetylase activity.
FIG. 7.
M4 and NaII transcriptional repression by the RE1/NRSE is TSA sensitive. NIH 3T3 cells were transfected with the stated promoter-luciferase reporter constructs and treated with 100 nM TSA for 24 h. Luciferase values were determined and normalized to CMV-Renilla in the absence of TSA. Values are the average of three independent experiments performed in triplicate. Standard errors were less than 20%.
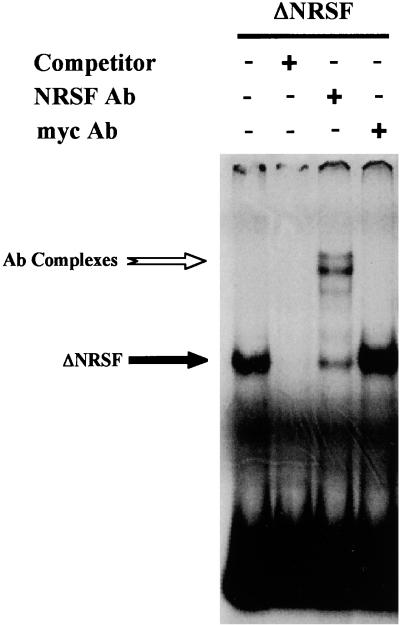
However, REST/NRSF is known to possess at least two repression domains other than the 73 N-terminal residues, i.e., the C-terminal Zinc finger (57) and the LRD (unpublished data). To be sure that the TSA sensitivity observed in cells expressing full-length REST/NRSF is due to the recruitment of a SIN3/HDAC complex by the N-terminal repression domain, we took advantage of the following observations: (i) REST/NRSF exists in multiple spliced isoforms (40); (ii) the cell line Neuro-2A expresses only the short isoform (ΔNRSF) which encompasses residues 1 to 327 of full-length REST/NRSF and thus contains only the N-terminal repression domain and five of eight putative zinc fingers (40); and (iii) Neuro-2A cells do not express the M4 gene but do express the NaII gene (A. Roopra and I. C. Wood, unpublished observations). To show that ΔNRSF could bind DNA despite a shortened DNA binding domain, we performed gel mobility shift assays with in vitro-translated ΔNRSF (Fig. 8). In vitro-translated ΔNRSF produced a shifted band when incubated with the RE1/NRSE from the NaII gene (albeit less robust than the shift obtained with in vitro-translated full-length REST/NRSF; data not shown) as seen in Fig. 8, lane 1, which was able to be competed with excess cold competitor (lane 2). The shifted band could be supershifted with anti-REST/NRSF antibody (lane 3) but not the unrelated antibody, anti-myc (lane 4). Identical results were obtained with the M4 gene promoter (data not shown).
FIG. 8.
In vitro-translated ΔNRSF can bind the NaII RE1/NRSE. In vitro-translated ΔNRSF was incubated with probe alone (lane 1, counting from the left), excess cold probe (lane 2), anti-REST/NRSF monoclonal antibody, 12C11 (lane 3), or unrelated anti-myc antibody (lane 4). Shifted bands due to ΔNRSF binding the RE1/NRSE (ΔNRSF) and supershifted bands (Ab Complexes) are labelled.
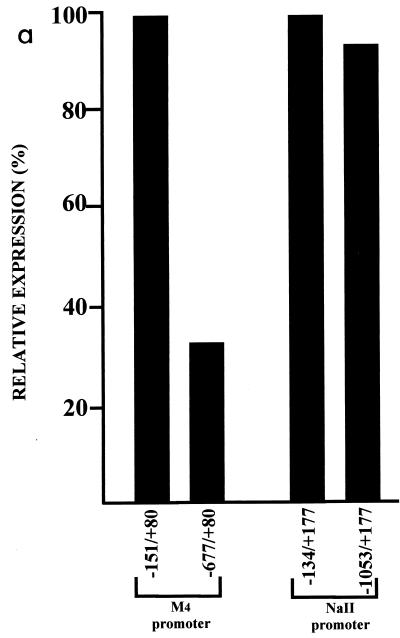
Having shown that ΔNRSF is able to bind the RE1/NRSE, we tested whether ΔNRSF-mediated repression of neuronal genes was TSA sensitive. Neuro-2A cells were transfected with reporter plasmids bearing the 5′ region of the M4 and NaII genes with or without an RE1/NRSE. As is shown in Fig. 9a, promoter-luciferase reporter construct −677/+80 was repressed to 36% of -151/+80. However, -1053/+177 expressed the reporter gene to the same extent as -134/+177. We therefore conclude that ΔNRSF can repress M4 gene expression in transient transfection assays but fails to repress NaII expression. Figure 9b shows that while TSA has little effect on the NaII promoter, TSA causes a 2.4-fold derepression of the M4 promoter in the presence of the M4 RE1/NRSE.
FIG. 9.
The M4 and NaII promoters are differentially regulated by ΔNRSF in Neuro-2A cells in a TSA-sensitive manner. (a) Neuro-2A cells were transfected with the stated promoter-luciferase reporter constructs. Values are the averages of three independent transfections, each performed in triplicate, normalized to cotransfected CMV-Renilla (see Materials and Methods), and expressed as a percentage of the value obtained without an RE1/NRSE. Standard errors were less than 10%. (b) Cells were transfected with the stated promoter-luciferase reporter constructs and treated with 100 nM TSA for 24 h. Luciferase values were determined and normalized to CMV-Renilla. Values are the average of three independent experiments performed in triplicate. Standard errors were less than 15%.
Therefore, we conclude that REST/NRSF represses neuronal promoters via their RE1/NRSEs by recruiting the mSIN3A/HDAC2 complex. Because the repression seen is sensitive to TSA, we conclude that the deacetylase activity of the mSIN3A/HDAC2 complex is important for REST/NRSF-mediated repression.
DISCUSSION
REST/NRSF is an essential transcription factor which regulates the expression of numerous genes associated with neuronal differentiation. Using a functional assay in yeast, we have identified the SIN3/HDAC complex as a corepressor of REST/NRSF. We show that REST/NRSF interacts with yeast and mammalian SIN3 in vivo. Repression of neuronal promoters by REST/NRSF requires deacetylase activity and occurs concommitantly with core histone deacetylation.
The ability of REST/NRSF to repress the yeast GAL1 promoter suggested that REST/NRSF was repressing via a mechanism conserved between yeast and mammals. The corepressor SIN3 is highly conserved across species from yeast to mammals and has been found to associate with many transcription factors regulating a variety of cellular processes (see references 14, 22, and 43 and references therein). The genetic requirement and physical interaction with yeast Sin3p and mammalian SIN3A suggests that REST/NRSF recruits the SIN3 complex to repress transcription at RE1/NRSE-containing promoters. The N-terminal repression domain of REST/NRSF is able to interact with the 213 N-terminal residues of mSIN3A. Whether this interaction is direct or not is not known. However mSIN3A, mSIN3B, and yeast SIN3 possess four paired amphipathic helices, motifs that mediate protein-protein interactions (5). Like MAD, which possesses an N-terminal amphipathic helix with which it interacts with PAH2 of SIN3 (5), REST/NRSF residues 1 to 73 model well to an ampipathic helix (data not shown) and so may interact with PAH1 (residues 140 to 187 in mSIN3A).
Yeast Sin3p and mSIN3A are associated with histone deacetylases (Rpd3p and HDAC1/HDAC2/HDAC3, respectively) (22, 56) which are the catalytic cores of the SIN3 complex. The observation that the deacetylase activity of histone deacetylases is required for repression (23) and that repression of genes is associated with histone deacetylation around repression elements (reviewed in reference 14) suggests that REST/NRSF represses transcription by recruiting histone deacetylase activity to RE1/NRSE-containing genes. Although we show that REST/NRSF interacts with HDAC2, as judged by immunoprecipitation (Fig. 4e) and ChIP (Fig. 5) assays, it is unlikely that REST/NRSF binds directly to HDAC2; REST/NRSF repression in yeast is lost in a sin3− RPD3+ strain, and so it is likely that REST/NRSF requires Sin3p to mediate the interaction.
The ChIP assays (Fig. 5) show that mSIN3A and HDAC2 form a complex with REST/NRSF bound at the RE1/NRSE. Overexpression of the N terminus of REST/NRSF results in quenching of the amount of SIN3/HDAC bound to the RE1/NRSE but also a large increase in the amount of acetylated histone H3 associated around the RE1/NRSE. Overexpression of the REST/NRSF N terminus also results in diminished repression of reporter gene expression due to quenching (31 and our unpublished observations). These observations are entirely consistent with REST/NRSF recruiting histone deacetylase activity to generate a region of hypoacetylated core histone around the RE1/NRSE to mediate repression of RE1/NRSE-containing genes.
Because deacetylation occurs in the vicinity of the repressor binding site (14), it is tempting to speculate that REST/NRSF-induced hypoacetylation around the RE1/NRSE causes reduced access to the M4 initiator element by the TFIID/RNAP holocomplex. Indeed, the RE1/NRSE at the M4 locus is only 540 bp upstream of the transcription start site (63), and so local changes to nucleosomal structure may have a direct effect on TFIID/RNAP holocomplex access to the promoter. However, RE1/NRSEs are found at various distances from the promoters of other neuron-specific genes, e.g., the L1 gene has a functional RE1/NRSE 18 kb downstream from the transcription start site. It is unclear how the effects of local nucleosomal hypoacetylation induced by REST/NRSF binding at such a distant RE1/NRSE will be transmitted to the promoter of such genes.
Although REST/NRSF binding is associated with hypoacetylation and reduced reporter gene expression, many other molecular targets of acetyltransferases have recently been identified which may also be legitimate substrates for the REST/NRSF-recruited SIN3 complex (8, 15, 19, 36), and so core histone deacetylation may not be the only mechanism by which REST/NRSF-recruited deacetylases mediate repression.
HEK293 cells express full-length REST/NRSF which, in addition to the N-terminal repression domain, possesses a further two repression domains: the C-terminal zinc finger (57) and the LRD (unpublished data). We therefore turned to the neuroblastoma cell line Neuro-2A which exclusively expresses a splice variant of REST/NRSF, ΔNRSF possessing only the N-terminal repression domain and a truncated DNA binding domain (residues 1 to 327) (40). Despite a truncated DNA binding domain, ΔNRSF can still bind RE1/NRSEs from both the NaII and M4 genes, albeit less efficiently than full-length REST/NRSF (Fig. 7 and data not shown). The RE1/NRSE-containing M4 promoter was repressed in this cell line, and importantly, this repression was sensitive to TSA, consistent with the N terminus recruiting the SIN3 complex to repress M4 transcription. The NaII promoter however was not repressed via the RE1/NRSE. It is not clear at present whether this lack of repression is due to a lower affinity of ΔNRSF with the NaII RE1 or if the factors that drive NaII expression in Neuro-2A cells render the NaII promoter immune to ΔNRSF-mediated repression. The observation that ΔNRSF differentially regulates M4 and NaII transcription in Neuro-2A cells opens up the possibility that REST/NRSF splicing may allow differential gene regulation of RE1/NRSE-containing genes within the nervous system. Indeed, both yeast and mammalian SIN3 are associated with a number of other peptides, at least one of which, SAP30, can confer transcription factor selectivity on the complex (28). Also, N-CoR (originally isolated in a screen to identify corepressors of unliganded nuclear receptors [16]) can confer histone deacetylation-independent repressor activity upon the SIN3 complex by directly interacting with the general transcription factors (37). Whether REST/NRSF can use these other factors and associated modes of repression is not yet known.
The regulation of histone deacetylase binding to CBF1 by Notch has been implicated in the acquisition of neuronal cell fate via the Notch/Delta pathway (18, 25). Here, we provide evidence suggesting that REST/NRSF, a gene required to maintain appropriate expression of neuronal genes within the nervous system (7, 11, 24, 58) and silence expression outside the nervous system (11) utilizes the SIN3-HDAC complex to silence transcription. HDACs therefore seem to play a direct role in both acquisition of neuronal cell fate and neuronal differentiation.
ACKNOWLEDGMENTS
We are indebted to the lab of Mark Johnston, Washington University, St. Louis, Mo., for plasmid pBM2389 and invaluable guidance throughout the course of the yeast work. We thank Luigi Lania, IIGB, Naples, Italy, for the T7G5TATA reporter plasmid. Plasmid pSKD7 containing the NaII promoter region was a generous gift from Gail Mandel, Stony Brook, N.Y. We thank Mireia Garriga-Canut for construction of pGL3.Inr, Helene Marie for construction of pLEONOV.NRSF(139-600), Francesca Caccuci and Richard Rowe for their contributions, and Martin Raff, University College London, London, United Kingdom, for critical reading of the manuscript.
This work was supported by the Wellcome Trust and by NIH grant NS23476 to David Anderson, Howard Hughes Medical Institute, California Institute of Technology. A.R. is the recipient of a Wellcome Prize Fellowship, and A.J.P. is supported by an NIH predoctoral training grant.
REFERENCES
- 1.Aasland R, Stewart A F, Gibson T. The SANT domain: a putative DNA-binding domain in the SWI-SNF and ADA complexes, the transcriptional co-repressor N-CoR and TFIIIB. Trends Biochem Sci. 1996;21:87–88. [PubMed] [Google Scholar]
- 2.Andres M E, Burger C, Peral-Rubio M J, Battaglioli E, Anderson M E, Grimes J, Dallman J, Ballas N, Mandel G. CoREST: A functional corepressor required for regulation of neural-specific gene expression. Proc Natl Acad Sci USA. 1999;96:9873–9878. doi: 10.1073/pnas.96.17.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnone M I, Davidson E H. The hardwiring of development: organization and function of genomic regulatory systems. Development. 1997;124:1851–1864. doi: 10.1242/dev.124.10.1851. [DOI] [PubMed] [Google Scholar]
- 4.Ayer D E, Laherty C D, Lawrence Q A, Armstrong A P, Eisenman R N. Mad proteins contain a dominant transcription repression domain. Mol Cell Biol. 1996;16:5772–5781. doi: 10.1128/mcb.16.10.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayer D E, Lawrence Q A, Eisenman R N. Mad-Max transcriptional repression is mediated by ternary complex formation with mammalian homologs of yeast repressor Sin3. Cell. 1995;80:767–776. doi: 10.1016/0092-8674(95)90355-0. [DOI] [PubMed] [Google Scholar]
- 6.Baur E V, Harbers M, Um S-J, Benecke A, Chambon P, Losson R. The yeast Ada complex mediates the ligand-dependent activation function AF-2 of retinoid X and estrogen receptors. Genes Dev. 1998;12:1278–1289. doi: 10.1101/gad.12.9.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bessis A, Champtiaux N, Chatelin L, Changeux J P. The neuron-restrictive silencer element: a dual enhancer/silencer crucial for patterned expression of a nicotinic receptor gene in the brain. Proc Natl Acad Sci USA. 1997;94:5906–5911. doi: 10.1073/pnas.94.11.5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396:594–598. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- 9.Brehm A, Miska E A, McCance D J, Reid J L, Bannister A J, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- 10.Brehm A, Nielsen S J, Miska E A, McCance D J, Reid J L, Bannister A J, Kouzarides T. The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. EMBO J. 1999;18:2449–2458. doi: 10.1093/emboj/18.9.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z F, Paquette A J, Anderson D J. NRSF/REST is required in vivo for repression of multiple neuronal target genes in both neuronal and non-neuronal tissues during embryogenesis. Nat Genet. 1998;20:136–142. doi: 10.1038/2431. [DOI] [PubMed] [Google Scholar]
- 12.Chong J A, et al. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell. 1995;80:949–957. doi: 10.1016/0092-8674(95)90298-8. [DOI] [PubMed] [Google Scholar]
- 13.Dedon P C, Soults J A, Allis C D, Gorovsky M A. A simplified formaldehyde fixation and immunoprecipitation technique for studying protein-DNA interactions. Anal Biochem. 1991;197:83–90. doi: 10.1016/0003-2697(91)90359-2. [DOI] [PubMed] [Google Scholar]
- 14.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;398:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 15.Gu W, Roeder R G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–605. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 16.Horlein A J, et al. Ligand-dependent repression by the thyroid hormone receptor mediated by a nuclear co-repressor. Nature. 1995;377:387–388. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 17.Howland D S, Hemmendinger L M, Carroll P D, Estes P S, Melloni R H, Jr, DeGennaro L J. Positive- and negative-acting promoter sequences regulate cell type-specific expression of the rat synapsin I gene. Brain Res Mol Brain Res. 1991;11:345–353. doi: 10.1016/0169-328x(91)90044-x. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh J J, Zhou S, Chen L, Young D B, Hayward S D. CIR, a corepressor linking the DNA binding factor CBF1 to the histone deacetylase complex. Proc Natl Acad Sci USA. 1998;96:23–28. doi: 10.1073/pnas.96.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imhof A, Yang X J, Ogryzko V V, Nakatani Y, Wolffe A P, Ge H. Acetylation of general transcription factors by histone acetyltransferases. Curr Biol. 1997;7:689–692. doi: 10.1016/s0960-9822(06)00296-x. [DOI] [PubMed] [Google Scholar]
- 20.Ishiguro H, Kim K T, Joh T H, Kim K S. Neuron-specific expression of the human dopamine beta-hydroxylase gene requires both the cAMP-response element and a silencer region. J Biol Chem. 1993;268:17987–17994. [PubMed] [Google Scholar]
- 21.Johnson A. The price of repression. Cell. 1995;81:655–658. doi: 10.1016/0092-8674(95)90524-3. [DOI] [PubMed] [Google Scholar]
- 22.Kadosh D, Struhl K. Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell. 1997;89:365–71. doi: 10.1016/s0092-8674(00)80217-2. [DOI] [PubMed] [Google Scholar]
- 23.Kadosh D, Struhl K. Histone deacetylase activity of Rpd3 is important for transcriptional repression in vivo. Genes Dev. 1998;12:797–805. doi: 10.1101/gad.12.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallunki P, Edelman G M, Jones F S. Tissue-specific expression of the L1 cell adhesion molecule is modulated by the neural restrictive silence element. J Cell Biol. 1997;138:1343–1354. doi: 10.1083/jcb.138.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kao H-Y, Ordentlich P, Koyano-Nakagawa N, Tang Z, Downes M, Kintner C R, Evans R, Kadesch T. A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev. 1998;12:2269–2277. doi: 10.1101/gad.12.15.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S, Na J, Hampsey M, Reinberg D. The Dr1/DRAP1 heterodimer is a global repressor of transcription in vivo. Proc Natl Acad Sci USA. 1997;94:820–825. doi: 10.1073/pnas.94.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraner S D, Chong J A, Tsay H J, Mandel G. Silencing the type II sodium channel gene: a model for neural-specific gene regulation. Neuron. 1992;9:37–44. doi: 10.1016/0896-6273(92)90218-3. [DOI] [PubMed] [Google Scholar]
- 28.Laherty C D, et al. SAP30, a component of the mSin3 corepressor complex involved in N-CoR-mediated repression by specific transcription factors. Mol Cell. 1998;2:33–42. doi: 10.1016/s1097-2765(00)80111-2. [DOI] [PubMed] [Google Scholar]
- 29.Lee D Y, Hayes J J, Pruss D, Wolffe A P. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993;72:73–84. doi: 10.1016/0092-8674(93)90051-q. [DOI] [PubMed] [Google Scholar]
- 30.Lehming N, Thanos D, Brickman J M, Ma J, Maniatis T, Ptashne M. An HMG-like protein that can switch a transcriptional activator to a repressor. Nature. 1994;371:175–179. doi: 10.1038/371175a0. [DOI] [PubMed] [Google Scholar]
- 31.Leichter M, Thiel G. Transcriptional repression by the zinc finger protein REST is mediated by titratable nuclear factors. Eur J Neurosci. 1999;11:1937–1946. doi: 10.1046/j.1460-9568.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Suzuki T, Mori N, Greengard P. Identification of a functional silencer element involved in neuron-specific expression of the synapsin I gene. Proc Natl Acad Sci USA. 1993;90:1460–1464. doi: 10.1073/pnas.90.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Liu J, Wilson T E, Milbrandt J, Johnston M. Methods Companion. Methods Enzymol. 1993;5:125–137. [Google Scholar]
- 33.Maue R A, Kraner S D, Goodman R H, Mandel G. Neuron-specific expression of the rat brain type II sodium channel is directed by upstream regulatory elements. Neuron. 1990;4:223–231. doi: 10.1016/0896-6273(90)90097-y. [DOI] [PubMed] [Google Scholar]
- 34.Mieda M, Haga T, Saffen D W. Expression of the rat m4 muscarinic acetylcholine receptor is regulated by the neuron-restrictive silencer element/repressor element 1. J Biol Chem. 1997;272:5854–5860. doi: 10.1074/jbc.272.9.5854. [DOI] [PubMed] [Google Scholar]
- 35.Mori N, Stein R, Sigmund D, Anderson D J. A cell type-preferred silencer element that controls the neural specific expression of the SCG10 gene. Neuron. 1990;4:583–594. doi: 10.1016/0896-6273(90)90116-w. [DOI] [PubMed] [Google Scholar]
- 36.Munshi N, Merika M, Yie J, Senger K, Chen G, Thanos D. Acetylation of HMGI(Y) by CBP turns off IFN beta expression by disrupting the enhanceosome. Mol Cell. 1998;2:457–467. doi: 10.1016/s1097-2765(00)80145-8. [DOI] [PubMed] [Google Scholar]
- 37.Muscat G E O, Burke L J, Downes M. The corepressor N-CoR and its variants RIP13a and RIP13Delta1 directly interact with the basal transcription factors TFIIB, TAFII32 and TAFII70. Nucleic Acids Res. 1998;26:2899–2907. doi: 10.1093/nar/26.12.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myers S J, Peters J, Huang Y, Comer M B, Barthel F, Dingledine R. Transcriptional regulation of the GluR2 gene: neural-specific expression, multiple promoters and regulatory Elements. J Neurosci. 1998;18:6723–6739. doi: 10.1523/JNEUROSCI.18-17-06723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nightingale K P, Wellinger R E, Sogo J M, Becker P B. Histone acetylation facilitates RNA polymerase II transcription of the Drosophila hsp26 gene in chromatin. EMBO J. 1998;17:2865–2876. doi: 10.1093/emboj/17.10.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palm K, Belluardo N, Metsis M, Timmusk T. Neuronal expression of zinc finger transcription factor REST/NRSF/XBR gene. J Neurosci. 1998;18:1280–1296. doi: 10.1523/JNEUROSCI.18-04-01280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park E J, Schroen D J, Yang M, Li H, Li L, Chen J D. SMRTe, a silencing mediator for retinoid and thyroid hormone receptors-extended isoform that is more related to the nuclear receptor corepressor. Proc Natl Acad Sci USA. 1999;96:3519–3524. doi: 10.1073/pnas.96.7.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pathak B G, Nuemann J C, Croyle M L, Lingrel J B. The presence of both negative and positive elements in the 5′-flanking sequence of the rat Na,K-ATPase alpha3 subunit gene are required for brain expression in transgenic mice. Nucleic Acids Res. 1994;22:4748–4755. doi: 10.1093/nar/22.22.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pazin M J, Kadonaga J T. What's up and down with histone deacetylation and transcription? Cell. 1997;89:325–328. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- 44.Pengue G, Lania L. Kruppel-associated box-mediated repression of RNA polymerase II promoters is influenced by the arrangement of basal promoter elements. Proc Natl Acad Sci USA. 1996;93:1015–1020. doi: 10.1073/pnas.93.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterson C L, Herskowitz I. Characterization of the yeast SWI1, SWI2, and SWI3 genes, which encode a global activator of transcription. Cell. 1992;68:573–583. doi: 10.1016/0092-8674(92)90192-f. [DOI] [PubMed] [Google Scholar]
- 46.Rundlett S E, Carmen A A, Suka N, Turner B M, Grunstein M. Transcriptional repression by UME6 involves deacetylation of lysine 5 of histone H4 by RPD3. Nature. 1998;392:831–835. doi: 10.1038/33952. [DOI] [PubMed] [Google Scholar]
- 47.Saha S, Brickman J M, Lehming N, Ptashne M. New eukaryotic transcriptional repressors. Nature. 1993;363:648–652. doi: 10.1038/363648a0. [DOI] [PubMed] [Google Scholar]
- 48.Schiestl R H, Gietz R D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 49.Schoch S, Cibelli G, Thiel G. Neuron-specific gene expression of synapsin I. J Biol Chem. 1996;271:3317–3323. doi: 10.1074/jbc.271.6.3317. [DOI] [PubMed] [Google Scholar]
- 50.Schoenherr C J, Anderson D J. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science. 1995;267:1360–1363. doi: 10.1126/science.7871435. [DOI] [PubMed] [Google Scholar]
- 51.Schoenherr C J, Anderson D J. Silencing is golden: negative regulation in the control of neuronal gene transcription. Curr Opin Neurobiol. 1995;5:566–571. doi: 10.1016/0959-4388(95)80060-3. [DOI] [PubMed] [Google Scholar]
- 52.Schoenherr C J, Paquette A J, Anderson D J. Identification of potential target genes for the neuron-restrictive silencer factor. Proc Natl Acad Sci USA. 1996;93:9881–9886. doi: 10.1073/pnas.93.18.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sikorski R S, Heiter P. A system of shuttle vectors and yeast host strains designed for the efficient manipulation of DNA in Saccaromyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spencer F, Hugerat Y, Simchen G, Hurko O, Conelly C, Hieter P. Yeast kar1 mutants provide an effective method for YAC transfer to new hosts. Genomics. 1994;22:118–126. doi: 10.1006/geno.1994.1352. [DOI] [PubMed] [Google Scholar]
- 55.Strich R, Surosky R T, Steber C, Dubois E, Messenguy F, Esposito R E. UME6 is a key regulator of nitrogen repression and meiotic development. Genes Dev. 1994;8:796–810. doi: 10.1101/gad.8.7.796. [DOI] [PubMed] [Google Scholar]
- 56.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 57.Tapia-Ramirez J, Eggen B, Peral-Rubio M, Toledo-Aral J, Mandel G. A single zinc finger motif in the silencing factor REST represses the neural-specific type II sodium channel promoter. Proc Natl Acad Sci USA. 1997;94:1177–1182. doi: 10.1073/pnas.94.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Timmusk T, Palm K, Lendahl U, Metsis M. Brain-derived neurotrophic factor expression in vivo is under the control of neuron-restrictive silencer element. J Biol Chem. 1999;274:1078–1084. [PubMed] [Google Scholar]
- 59.Treital M A, Carlson M. Repression by SSN6-TUP1 is directed by MIG1, a repressor/activator protein. Proc Natl Acad Sci USA. 1995;92:3132–3136. doi: 10.1073/pnas.92.8.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ura K, Kurumizaka H, Dimitrov S, Almouzni G, Wolffe A P. Histone acetylation: influence on transcription, nucleosome mobility and positioning, and linker histone-dependent transcriptional repression. EMBO J. 1997;16:2096–2107. doi: 10.1093/emboj/16.8.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wade P A, Jones P L, Vermaak D, Wolffe A P. A multiple subunit Mi-2 histone deacetylase from Xenopus laevis cofractionates with an associated Snf2 superfamily ATPase. Curr Biol. 1998;8:843–846. doi: 10.1016/s0960-9822(98)70328-8. [DOI] [PubMed] [Google Scholar]
- 62.Winston F, Carlson M. Yeast SNF/SWI transcriptional activators and the SPT/SIN chromatin connection. Trends Genet. 1992;8:387–391. doi: 10.1016/0168-9525(92)90300-s. [DOI] [PubMed] [Google Scholar]
- 63.Wood I C, Roopra A, Buckley N J. Neural specific expression of the m4 muscarinic acetylcholine receptor gene is mediated by a RE1/NRSE-type silencing element. J Biol Chem. 1996;271:14221–14225. doi: 10.1074/jbc.271.24.14221. [DOI] [PubMed] [Google Scholar]
- 64.Wuenschell C W, Mori N, Anderson D J. Analysis of SCG10 gene expression in transgenic mice reveals that neural specificity is achieved through selective derepression. Neuron. 1990;4:595–602. doi: 10.1016/0896-6273(90)90117-x. [DOI] [PubMed] [Google Scholar]
- 65.Yang W, Inouye C, Zeng Y, Bearss D, Seto D. Transcriptional repression by YY1 is mediated by interaction with a mammalian homolog of the yeast global regulator RPD3. Proc Natl Acad Sci USA. 1996;93:12845–12850. doi: 10.1073/pnas.93.23.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang W, Bone J R, Edmondson D G, Turner B M, Roth S Y. Essential and redundant functions of histone acetylation revealed by mutation of target lysines and loss of the Gcn5p acetyltransferase. EMBO J. 1998;17:3155–3167. doi: 10.1093/emboj/17.11.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]