Abstract
Background:
Elevated blood pressure (BP) is a modifiable risk factor associated with cognitive impairment and cerebrovascular diseases. However, the causal effect of BP on white matter brain aging remains unclear.
Methods:
In this study, we focused on N = 228 473 individuals of European ancestry who had genotype data and clinical BP measurements available (103 929 men and 124 544 women, mean age = 56.49, including 16 901 participants with neuroimaging data available) collected from UK Biobank (UKB). We first established a machine learning model to compute the outcome variable brain age gap (BAG) based on white matter microstructure integrity measured by fractional anisotropy derived from diffusion tensor imaging data. We then performed a two-sample Mendelian randomization analysis to estimate the causal effect of BP on white matter BAG in the whole population and subgroups stratified by sex and age brackets using two nonoverlapping data sets.
Results:
The hypertension group is on average 0.31 years (95% CI = 0.13–0.49; P < 0.0001) older in white matter brain age than the nonhypertension group. Women are on average 0.81 years (95% CI = 0.68–0.95; P < 0.0001) younger in white matter brain age than men. The Mendelian randomization analyses showed an overall significant positive causal effect of DBP on white matter BAG (0.37 years/10 mmHg, 95% CI 0.034–0.71, P = 0.0311). In stratified analysis, the causal effect was found most prominent among women aged 50–59 and aged 60–69.
Conclusion:
High BP can accelerate white matter brain aging among late middle-aged women, providing insights on planning effective control of BP for women in this age group.
Keywords: blood pressure, brain aging, causal inference, Mendelian randomization, white matter fractional anisotropy
INTRODUCTION
Elevated blood pressure (BP) is a primary risk factor for cerebrovascular and cardiovascular diseases [1,2] and associated with a higher risk of cognitive impairment and dementia [3,4]. Studies have reported that high BP strongly correlates with alterations of both brain structure and neurobiological functions [5,6]. Previous study also showed a significant genetic correlation between BP and white matter integrity [7]. The microstructure of the human brain is constantly changing with normal aging, reflecting brain shrinkage, and cognitive and memory decline [8]. Thus, it is important to know how increased BP causes accelerated brain aging to reveal the underlying mechanism of BP on the brain and cognitive dysfunction. The prevalence of hypertension increases with age and differs among men and women in different age cycles [9,10], especially for older women after menopause. In this study, we will evaluate the causal effect of BP on white matter brain aging in the general population as well as sex-specific and age-specific groups to understand the benefits of controlling BP in reducing accelerated brain aging.
Brain age predicts chronological age from structural or functional neuroimaging features using machine learning algorithm [11,12]. In our study, we used multiple fractional anisotropy tract measures from diffusion tensor imaging (DTI) data to predict an age-adjusted white matter brain age metric, brain age gap (BAG), which represents the difference between individuals’ brain age and their chronological age and regard it as the main outcome. Increases in BAG demonstrate evidence of accelerated aging and poorer brain health. The scalar BAG measure has been widely used in the literature as an alternative to multivariate imaging models to yield more robust and interpretable results in brain aging studies [13,14].
BAG can be influenced by multiple genetic, biological, environmental, and lifestyle factors [15]. Previous studies on BAG associated with lifestyle risk factors have focused on smoking and alcohol consumption [14,16,17]. For high blood pressure and brain age, one study showed that elevated blood pressure was associated with brain aging but no direct causality was reported [18]. In traditional observational studies, it is challenging to identify the causal relationship solely between high blood pressure and BAG, as other health risk factors related to abnormal brain aging, such as smoking, alcohol use, and chronic diseases, often co-occur with high blood pressure [19,20]. The Mendelian randomization framework has provided an unprecedented chance to overcome this challenge by using the genetic variants as instrumental variables of the risk factor to estimate its causal effect on the outcome, which is less prone to reverse causation and confounding [21–23]. The genetic determinants of BP are increasingly characterized in large-scale genome-wide association study (GWAS) in the literature [24], which made it possible to identify better instruments for Mendelian randomization study. The Mendelian randomization methods have been successfully applied to investigate the causal effects of high blood pressure on myocardial infarction, atrial fibrillation, and other cardiovascular diseases as well as other chronic diseases [25–28], but no Mendelian randomization studies have looked at the causal effect of BP on white matter brain aging in a large-scale observational study.
To fill the gap, we used fractional anisotropy measures of DTI data from the UK Biobank (UKB) cohort to build an machine learning model to estimate white matter BAG and performed a two-sample Mendelian randomization analysis to investigate the causal effect of BP on BAG in the general population as well as stratified by sex and age groups. We hypothesized that elevated BP would accelerate brain aging; such causal effect is more prominent in more vulnerable age and sex groups. Our study highlights the age and sex disparities in the causal effects of modifiable cardiovascular disease-related risk factors on white matter microstructure change, which provides implications for understanding the contributions of the late-life cognitive impairment risk.
METHODS
Study population and variables
The data used in the present study were from the UKB cohort, which is a large-scale population-based study recruiting around 500 000 individuals aged 40–69 years and collecting comprehensive physical, genomic, health, and brain imaging phenotypic data [29]. We focused on nonpregnant, family-unrelated individuals of European ancestry (i.e. primarily British and Irish) who had available genotypes, two nonnull clinical BP measurements from the initial assessment visits (2006–2010) and further excluded individuals with discordant self-reporting vs. genotype determined sex, those with extreme white matter hyperintensities, and those who took antihypertensive medicine (see Supplementary Material section 1.1, http://links.lww.com/HJH/C276). We also performed several sensitivity analyses to ensure there is no selection bias. The final pool for analysis includes N = 228 473 participants, including 16 901 participants with neuroimaging data available (Fig. 1).
FIGURE 1.
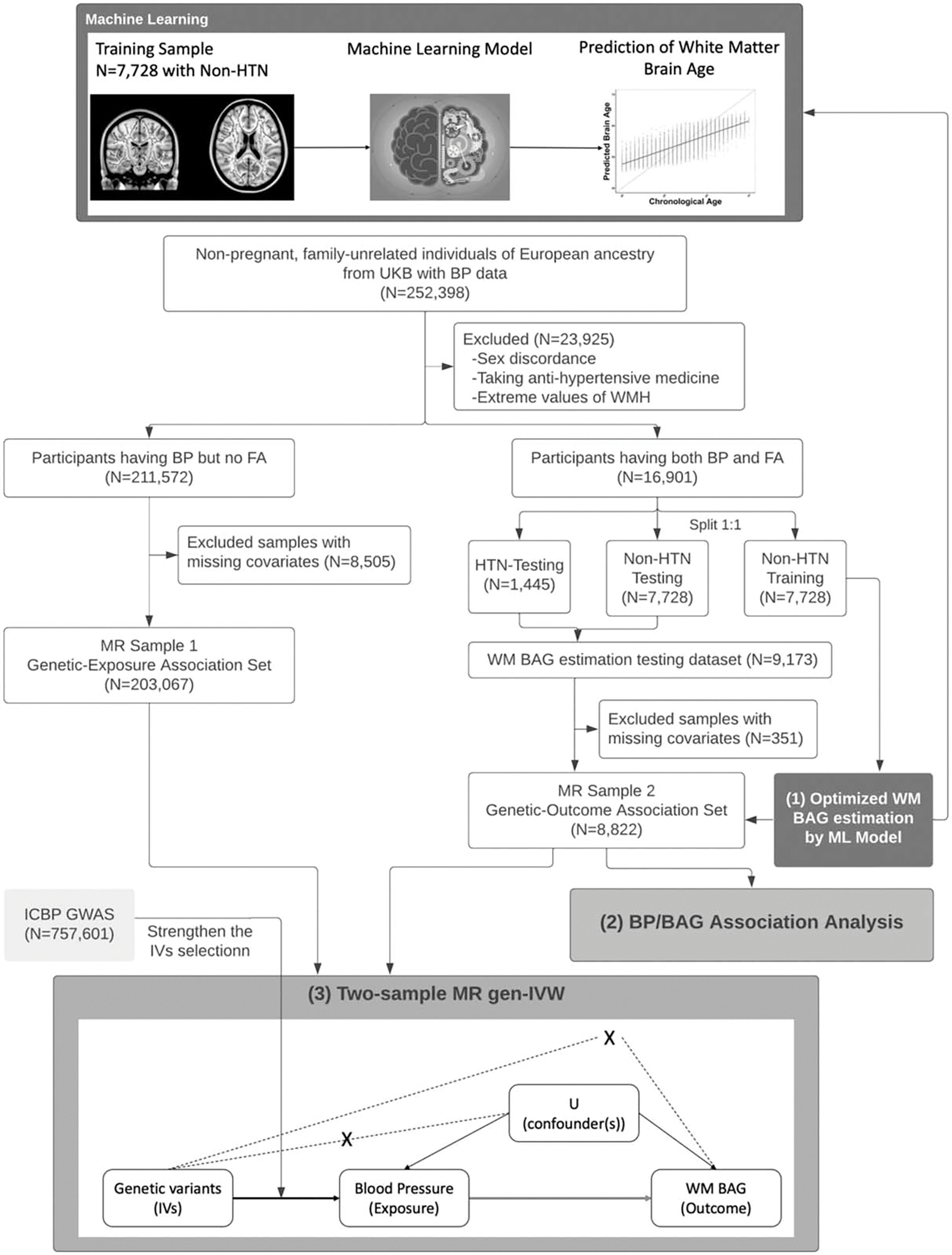
Flowchart of our main analysis procedures and the number of participants included at each step of the analysis. The main analyses are in three parts: estimation of age bias-corrected WM BAG using machine learning model; linear association analysis between BP and BAG; two-sample MR analysis to evaluate causal effect of BP on BAG. In the directed acyclic graph of MR analysis, the solid arrows correspond to the causal relationship, the dashed arrows with cross correspond to the instrumental variables assumptions (ii) and (iii) in MR (see Supplementary Materials section 1.7, http://links.lww.com/HJH/C276), the arrow is the causal relationship of interest in MR model. BAG, brain age gap; BP, blood pressure; MR, Mendelian randomization; WM, white matter.
Genotype data
The genotype data of participants provided by the UKB were assayed using two genotyping arrays, the UK BiLEVE Axiom Array and UK Biobank Axiom Array [30,31]. We further applied quality control to the genotype data (see Supplementary Material section 1.2, http://links.lww.com/HJH/C276).
Blood pressure data
As the exposures of interest, we analyzed the following two BP traits with complete data collected from the initial (2006–2010) assessment visit: SBP and DBP, both calculated as the mean of two nonnull BP measurements using phenotype codes 4079 and 4080 in UKB, respectively. Hypertension (HTN) was defined using International Classification of Diseases edition 10 (ICD-10) codes I10–I15 available in UKB. Hypertension status will be used to split the data for training and testing purposes.
Neuroimaging data
We used regional white matter fractional anisotropy measures collected from DTI data from UKB imaging assessment starting from 2014 to compute the outcome of the study, the BAG. According to ENIGMA protocol, the per-tract mean value of each brain white matter tract was calculated by using a Track-Based Spatial Statistics analysis from the DTI fractional anisotropy images [32]. Fractional anisotropy measure, ranging from 0 to 1, represents the degree of anisotropy of a diffusion process and the integrity of cortical white matter [33]. A lower value of fractional anisotropy indicates less probability of diffusion in one direction (isotropic). This study focuses on fractional anisotropy data for a total of 39 tracts that covered multiple brain regions (see the list of 39 regional white matter fractional anisotropy measures in Table S1, http://links.lww.com/HJH/C275).
Potential confounders
We treated sex, age, BMI, alcohol consumption, smoking status, fruit and vegetable consumption, and sedentary lifestyle as potential confounders for our association analysis and Mendelian randomization analysis based on a previous study [34] (see Supplementary Material section 1.3, http://links.lww.com/HJH/C276). We performed complete case analysis by excluding individuals with any missing covariates. Two-sample t tests were conducted comparing missingness vs. complete case group for each missing covariate to ensure the missing complete at random assumption is not violated (Table S5, http://links.lww.com/HJH/C275). The continuous age variable is categorized into 40–49, 50–59, 60–69 age groups. In this study, we conducted both overall analysis as well as stratified analysis within each sex and age group.
Existing large-scale blood pressure genome-wide association study summary data
To strengthen the instrumental variable selection step in two-sample Mendelian randomization analysis, in addition to UKB data alone, we also collected the largest existing GWAS summary data on BP from a meta-analysis of over 750 000 participants of European ancestry that combined a total of 78 different cohorts [from International Consortium of Blood Pressure (ICBP) and part of UKB cohort] [24]. We used this existing meta-analyzed GWAS summary data to help select stronger and reproducible instrumental variables in our two-sample Mendelian randomization analysis.
Statistical analysis
Figure 1 shows a flowchart of the main statistical analysis performed and the number of participants included at each step of the analysis. For two-sample Mendelian randomization analysis, we split data into two nonoverlapping sets: Genetic-Exposure Association Set consisting of participants with only BP but no fractional anisotropy data (Mendelian randomization sample 1; N = 203 067) and Genetic-Outcome Association Set consisting of participants with both BP and BAG outcome variables available (Mendelian randomization sample 2; N = 8822) (Fig. 1). The main analyses are in three parts. In the first part, we used machine learning model to estimate the outcome white matter BAG based on 39 regional fractional anisotropy measures and the chronological age, among those participants with both BP and fractional anisotropy data available. In the second part, we applied a multiple linear regression model to test for the association between BP and BAG in Mendelian randomization sample 2. In the last part, we performed a two-sample Mendelian randomization analysis to evaluate the causal effects of BP on BAG treating candidate genetic variants as Instrumental variables. Detailed steps of each part of the analysis can be found in the Supplementary Material section 1.4–1.7, http://links.lww.com/HJH/C276.
All statistical analyses were conducted using R (version 4.0.5) [35]. R packages, including ‘MendelianRandomization’ (version 0.5.1) [36], ‘MRPRESSO’ (version 0.1.0) [37], and ‘MRMix’ (version 0.1.0) [38] were used to perform Mendelian randomization analyses. Except for GWAS analysis and instrumental variables selection, P less than 0.05 was regarded as statistically significant for all other analyses unless otherwise specified. For both association analysis and Mendelian randomization analysis, the Benjamini–Hochberg (BH) method was used to adjust for multiple comparisons [39]. We also performed several sensitivity analyses to ensure our analysis is robust and unbiased (see Supplementary Material section 1.8, http://links.lww.com/HJH/C276).
RESULTS
Descriptive statistics
The Genetic-Exposure Dataset consists of 91 771 men and 111 296 women, mean age of 56.71 (SD = 8.02) years. Non-HTN Training Dataset consists of 3513 men and 4215 women [mean age = 54.23 (SD = 7.35)], Non-HTN Testing Dataset with 4116 men and 3309 women [mean age = 54.31 (SD = 7.36)], and HTN Testing Dataset with 639 men and 758 women [mean age = 58.48 (SD = 6.53)] (Table 1). No systematic difference in the distribution of age, sex, BMI and BP was observed between the non-HTN training group and non-HTN testing group (Table S3, http://links.lww.com/HJH/C275).
TABLE 1.
Characteristics of Participants in Genetic-Exposure Association Set (MR sample 1), Genetic-Outcome Association set (MR sample 2 including non-HTN and HTN testing dataset) and the non-HTN training dataseta from UK Biobank
| Genetic-Outcome dataset |
|||||
|---|---|---|---|---|---|
| Characteristics | Level | Non-HTN training dataset | Non-HTN testing dataset | Non-HTN testing dataset | Genetic-Exposure dataset |
|
| |||||
| N | 7728 | 7425 | 1397 | 203 067 | |
| Age [mean (SD)] | 54.23 (7.35) | 54.31 (7.36) | 58.48 (6.53) | 56.71 (8.02) | |
| Sex (%) | Female | 4215 (54.5) | 4116 (55.4) | 639 (45.7) | 111 296 (54.81) |
| Male | 3513 (45.5) | 3309 (44.6) | 758 (54.3) | 91 771 (45.19) | |
| BMI [mean (SD)] | 26.06 (3.96) | 26.07 (3.93) | 28.54 (4.64) | 27.23 (4.65) | |
| SBP [mean (SD)] | 132.49 (16.48) | 132.38 (16.51) | 145.33 (17.95) | 137.97 (18.58) | |
| DBP [mean (SD)] | 80.26 (9.38) | 80.01 (9.32) | 86.32 (10.64) | 82.25 (10.08) | |
| Smoking status (%) | Never | 4916 (63.71) | 4665 (62.83) | 797 (57.05) | 111 814 (55.06) |
| Previous | 2351 (30.47) | 2332 (31.41) | 536 (38.37) | 70 756 (34.84) | |
| Current | 449 (5.82) | 428 (5.76) | 64 (4.58) | 20 497 (10.09) | |
| Alcohol drinker status (%) | Never | 159 (2.06) | 143 (1.93) | 28 (2.00) | 6036 (2.97) |
| Previous | 153 (1.98) | 122 (1.64) | 38 (2.72) | 6826 (3.36) | |
| Current | 7414 (95.96) | 7160 (96.43) | 1331 (95.28) | 190205 (93.67) | |
| Fruit consumption [mean (SD)] | 3.07 (2.40) | 3.07 (2.28) | 3.13 (2.47) | 2.99 (2.48) | |
| Vegetable consumption [mean (SD)] | 4.72 (2.91) | 4.71 (2.71) | 4.94 (3.17) | 4.81 (3.08) | |
| Sedentary lifestyle [mean (SD)] | 4.16 (2.39) | 4.20 (2.33) | 4.80 (2.45) | 4.53 (2.48) | |
SD, standard deviation.
See Fig. 1.
Estimation of white matter brain age gap
The optimal RF regression model selected 25 fractional anisotropy measures for BAG estimation (Figures S1, http://links.lww.com/HJH/C276). After correcting age-bias on white matter BAG, the adjusted predicted BAG achieved R2 = 0.94 in both HTN test dataset (MAE = 2.64 years, P < 2.2 × 10−16) and non-HTN test dataset (MAE = 2.48 years, P < 2.2 × 10−16) (Fig. 2b), greatly improving the prediction performance in testing data when the bias is not corrected (Fig. 2a). The hypertension group was on average 0.31 years (95%CI = 0.13–0.49; P < 0.0001) older in white matter brain age than the nonhypertension group, and such trend is consistent across age groups (Fig. 2c–d). Furthermore, our data showed that, women on average had 0.81 years (95% CI = 0.68–0.95; P < 0.0001) younger brain age than men and the difference is consistent across all age strata (all P < 0.01, Fig. 2e). The detailed difference between men and women by age strata and by each testing dataset can be found in Table S4, http://links.lww.com/HJH/C275. Our results validated a previous finding that the adult women’s brains are on average a few years younger than the men’s brains in terms of brain metabolism [40].
FIGURE 2.
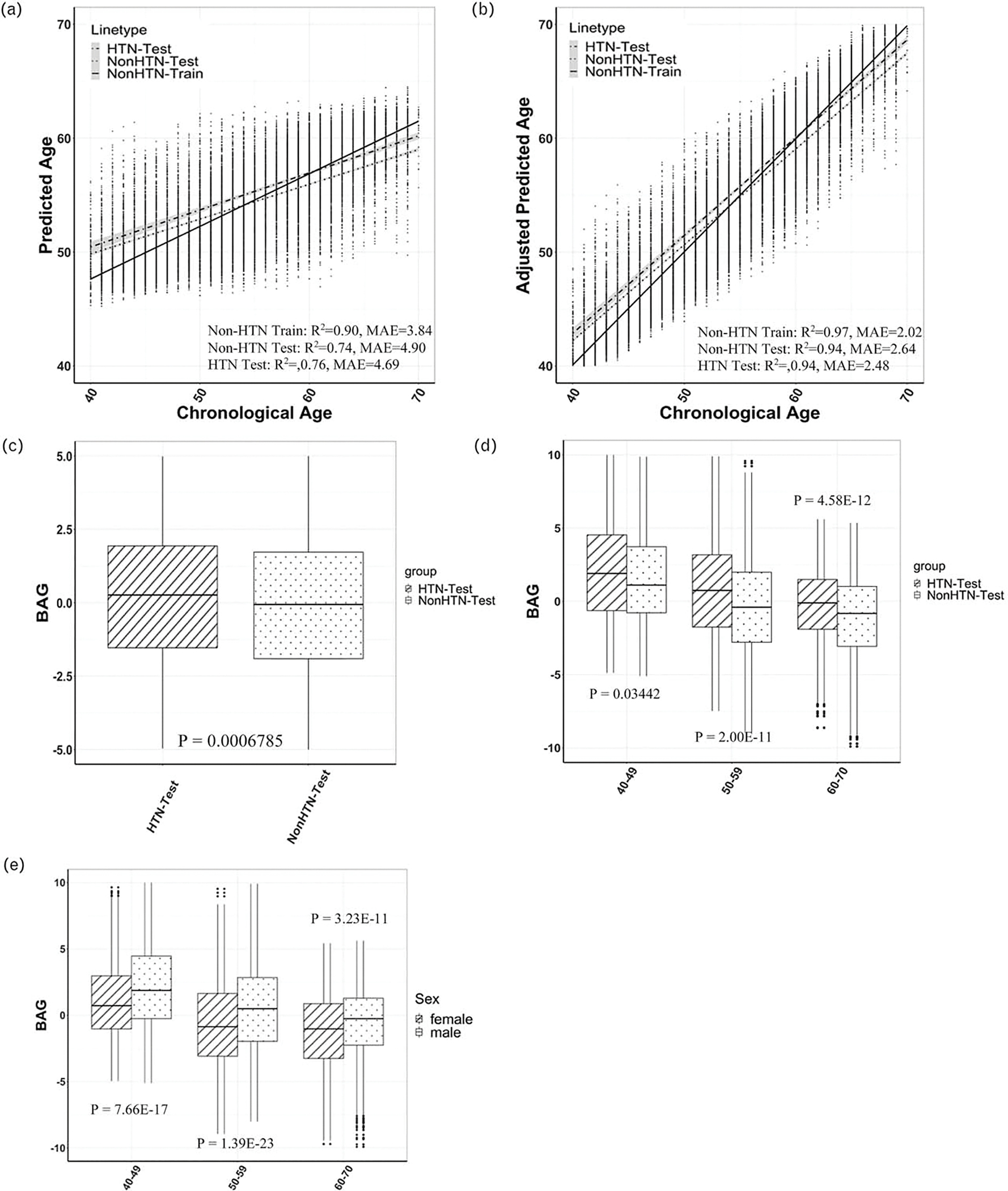
(a) Predicted brain age vs. chronological without age bias correction. (b) Predicted brain age vs. chronological with age bias correction. (c) Box plots of estimated WM BAG in HTN vs. non-HTN test datasets. (d) Box plots of estimated WM BAG in HTN vs. non-HTN test datasets in different age groups. (e) Box plots of estimated WM BAG between women and men in different age groups. BAG, brain age gap; HTN, hypertension; WM, white matter.
Linear association analysis
Both SBP and DBP were found positively associated with white matter BAG (Table 2: βSBP = 0.012, 95% CI = 0.0083–0.011, P = 4.30 × 10−10; βDBP = 0.018, 95% CI = 0.011–0.024, P = 1.31 × 10−7). With age and sex stratification, we found that both SBP and DBP were associated with white matter BAG specifically in 50–59 (βSBP = 0.016, P= 3.74 × 10−7; βDBP = 0.026, P = 5.54 × 10−6) and 60–69 (βSBP = 0.0086, P = 4.25 × 10−3; βDBP = 0.015, P = 7.11 × 10−3) age groups, and more significant association was found among women (βSBP = 0.015, P = 7.38 × 10−9; βDBP = 0.023, P = 3.59 × 10−7). Further stratification by both sex and age groups showed that BP was most significantly associated with white matter BAG among older women, but no association was found between BP and white matter BAG in 40–49 age group for both sexes (Table 2 and Fig. 3). These results remained largely consistent in several sensitivity analyses (Table S8–S10, http://links.lww.com/HJH/C275).
TABLE 2.
Linear association (left) and two-sample MR analysis (right) results
| Linear association | MR analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| BP | Group | Beta estimate | Lower 95% CI | Upper 95% CI | BH-adjusted P value | Standardized estimate | Lower 95% CI | Upper 95% CI | P value |
|
| |||||||||
| Without stratification | |||||||||
| SBP | Whole | 0.0120 | 0.0083 | 0.0156 | 4.30E-10*** | −0.0048 | −0.0234 | 0.0137 | 6.09E-01 |
| DBP | 0.0175 | 0.0111 | 0.0239 | 1.31E-07 *** | 0.0371 | 0.0034 | 0.0709 | 3.11E-2 * | |
| With age stratification | |||||||||
| SBP | 40–49 | 0.0070 | −0.0016 | 0.0156 | 1.64E-01 | −0.0460 | −0.0860 | −0.0060 | 2.44E-2 * |
| DBP | 0.0117 | −0.0013 | 0.0246 | 1.18E-01 | 0.0069 | −0.0539 | 0.0676 | 8.25E-01 | |
| SBP | 50–59 | 0.0163 | 0.0103 | 0.0223 | 3.74E-07 *** | −0.0002 | −0.0290 | 0.0287 | 9.91E-01 |
| DBP | 0.0259 | 0.0154 | 0.0364 | 5.54E-06 *** | 0.0151 | −0.0357 | 0.0659 | 5.60E-01 | |
| SBP | 60–69 | 0.0086 | 0.0032 | 0.0140 | 4.25E-03 ** | 0.0230 | −0.0099 | 0.0559 | 1.70E-01 |
| DBP | 0.0147 | 0.0048 | 0.0246 | 7.11E-03 ** | 0.0991 | 0.0414 | 0.1568 | 7.68E-04 *** | |
| With sex stratification | |||||||||
| SBP | Female | 0.0149 | 0.0100 | 0.0197 | 7.38E-09 *** | 0.0112 | −0.0130 | 0.0355 | 3.64E-01 |
| DBP | 0.0233 | 0.0146 | 0.0319 | 3.59E-07 *** | 0.0484 | 0.0062 | 0.0905 | 2.45E-02 * | |
| SBP | Male | 0.0074 | 0.0017 | 0.0131 | 2.05E-02 * | −0.0281 | −0.0635 | 0.0073 | 1.20E-01 |
| DBP | 0.0094 | −0.0001 | 0.0189 | 9.54E-02 ~. | 0.0297 | −0.0293 | 0.0887 | 3.24E-01 | |
| With age by sex stratification | |||||||||
| SBP | 40–49 female | 0.0053 | −0.0056 | 0.0161 | 3.44E-01 | −0.0529 | −0.0983 | −0.0075 | 2.23E-02 * |
| DBP | 0.0095 | −0.0075 | 0.0265 | 2.75E-01 | −0.0139 | −0.0893 | 0.0615 | 7.17E-01 | |
| SBP | 40–49 male | −0.0004 | −0.0150 | 0.0141 | 9.54E-01 | −0.0495 | −0.1222 | 0.0231 | 1.81E-01 |
| DBP | 0.0072 | −0.0139 | 0.0283 | 5.04E-01 | 0.0201 | −0.0781 | 0.1184 | 6.88E-01 | |
| SBP | 50–59 female | 0.0153 | 0.0077 | 0.0228 | 7.36E-05 *** | 0.0329 | −0.0029 | 0.0688 | 7.18E-02 ~. |
| DBP | 0.0352 | 0.0212 | 0.0492 | 8.91E-07 *** | 0.0686 | 0.0054 | 0.1318 | 3.35E-02 * | |
| SBP | 50–59 male | 0.0125 | 0.0027 | 0.0223 | 1.24E-02 * | −0.0438 | −0.0986 | 0.0109 | 1.17E-01 |
| DBP | 0.0127 | −0.0035 | 0.0288 | 1.24E-01 | −0.0441 | −0.1245 | 0.0363 | 2.83E-01 | |
| SBP | 60–69 female | 0.0114 | 0.0038 | 0.0190 | 3.46E-03 ** | 0.0137 | −0.0263 | 0.0537 | 5.01E-01 |
| DBP | 0.0177 | 0.0034 | 0.0319 | 1.50E-02 * | 0.0962 | 0.0209 | 0.1714 | 1.22E-02* | |
| SBP | 60–69 male | 0.0007 | −0.0070 | 0.0085 | 8.51E-01 | 0.0289 | −0.0214 | 0.0793 | 0.2602 |
| DBP | 0.0104 | −0.0037 | 0.0246 | 1.47E-01 | 0.0949 | −0.0029 | 0.1926 | 5.71E-02 ~. | |
Multiple potential confounders are adjusted in both models. Results for both overall analysis (i.e. without stratification) and stratified analysis by age and sex are shown.
P < 0.1.
P < 0.05.
P < 0.01.
P < 0.001.
FIGURE 3.
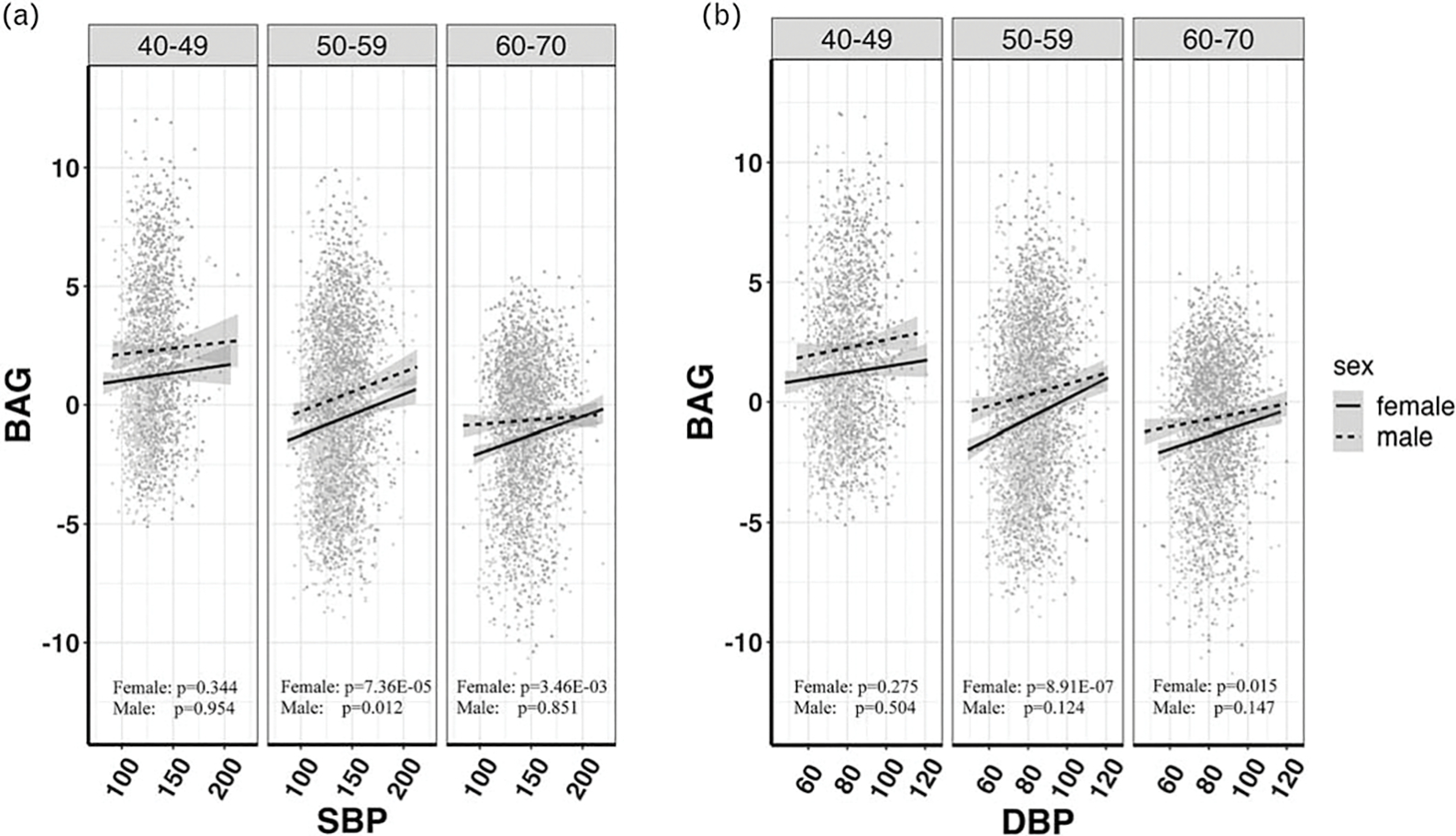
Scatterplots and linear relationships between brain age gap and blood pressure [(a) SBP and (b) DBP] in different sex and age subgroups.
Two-sample Mendelian randomization analysis
We identified 4813 and 5646 genetic variants with GWAS P value less than 5 × 10−8 for SBP and DBP, respectively. A majority of these single nucleotide polymorphisms (SNPs) were mapped to blood pressure-related genes (e.g. CACNB2, ATP2B1, and ARHGAP42) that have been reported in previous studies [41,42] (full gene annotations were summarized in Table S6, http://links.lww.com/HJH/C275). The final number of variants selected as valid instrumental variables are 153 for both SBP and DBP (the number of variants passing each instrumental variables selection step is summarized in Table S7, http://links.lww.com/HJH/C275). Using these instrumental variables, we found an overall significant causal effect of DBP (β = 0.037, 95% CI = 0.0034–0.071, P = 0.0311) on white matter BAG (Table 2), that is, increment of 10 mmHg of DBP increases brain age by an additional 0.37 years. In stratified analysis, DBP had most significant causal effect on white matter BAG in 60–69 age group (β = 0.0991, 95% CI = 0.041–0.16, P = 7.68 × 10−4) and among women (β = 0.0484, 95% CI = 0.0062–0.091, P = 2.45 × 10−2). Late middle-aged women are most vulnerable to blood pressure change: women in 50–59 age group (β = 0.069, 95% CI = 0.0054–0.13, P = 0.0335) and 60–69 age group (β = 0.096, 95% CI = 0.021–0.17, P = 0.0122) had a significant causal effect of DBP on white matter BAG, that is, increasing 10 mmHg DBP increases brain age by additional 0.69–0.96 years in women aged 50–69. Those significant results were confirmed by implementing several sensitivity analyses using different Mendelian randomization methods and leave-one-out approach (see Table S11, http://links.lww.com/HJH/C275). On the other hand, a weak positive causal effect of DBP in age 60–69 (P = 0.0571) was presented in the men’s group, and a weak causal effect for SBP in the 50–59 aged women’s group (P = 0.0718). No consensus results for the causal effects of DBP on white matter BAG were found among the men’s groups by reviewing all the Mendelian randomization analyses, thus we could not draw any statistically valid causal conclusion for the men’s group (Table 2 and Tables S7, and S8, http://links.lww.com/HJH/C275). We also performed another Mendelian randomization analysis by switching the outcome and the exposure, no significant causal relationship was observed thus no reverse causality existed (Table S12, http://links.lww.com/HJH/C275).
DISCUSSION
In this study, we used the fractional anisotropy data and chronological age to train an optimal RF model in a healthy reference population without a diagnosis of hypertension to calculate white matter BAG with age-related bias corrected. We found significant increases in white matter BAG in HTN individuals and men compared with non-HTN individuals and women, respectively. Our linear association analysis between BP and BAG confirmed that high blood pressure is highly related to elevated brain aging. Furthermore, the two-sample Mendelian randomization analysis identified age-dependent and sex-dependent causal effect of BP on white matter BAG. Specifically, we found positive causal effects of DBP on white matter BAG among 50–69 age-group women.
In our investigation, we found that women have younger brains (neoteny) than men, consistent with the findings by Goyal et al. [40] that the adult women’s brains are a few years younger than men’s over the entire adult life span. Interestingly, even though women exhibited younger brains, we observed a more robust causal relationship between DBP and brain aging in women starting at the age of 50 years, compared with men. This causal effect became even more pronounced among women aged 60–69, compared with those aged 50–59. On the other hand, studies have revealed that the prevalence of HTN and brain aging increases with age and differs among men and women in different age cycles [9,10], especially for older adults and women after menopause. This may be because of the sex difference in sex hormones and the vascular functions at molecular, cellular, and tissue levels [43]. Sex hormones produced among women of reproductive age may protect them from hypertension and related organ function (cardio vasculature, kidneys, and brain) [44–46]. We provided novel insights on how BP causes increasing brain aging in humans, especially in late middle-aged women, and affects differently in an age-dependent and sex-dependent manner, but the causal effects warrant further investigations. Our study focused on the causal effect of BP measure at baseline on white matter brain aging measured at the imaging visit and its age-specific and sex-specific pattern comparison. Since the imaging visit (instance 2) is 4–12 years apart from baseline, we can regard the baseline BP as past BP measurement. As the prior history of elevated BP before baseline is not available in UKB, we do not further distinguish the past elevated BP based on years of exposure of elevated BP without medications. Several longitudinal studies found that both concurrent and past elevated blood pressure were associated with white matter hyperintensities, but concurrent BP had weaker effect than past BP measurements taken several years ago WMH [47–51]. However, the evidence is still limited, and we will leave it to future studies.
Our study identified that DBP, not SBP, had a causal impact on white matter BAG in the entire population aged between 40 and 69. As individuals age, SBP typically increases, while DBP may decrease because of changes in the cardiovascular system or other factors [52,53]. Our findings are consistent with previous research, which suggests that a history of high blood pressure in midlife may contribute to brain changes that can lead to white matter lesions [54]. However, there have been more debates and uncertainties surrounding the effect of DBP on brain structures and cognitive function compared with SBP [55,56]. Nevertheless, both genetically proxied and regular measurements of SBP and DBP have shown diverse causal effects or significant associations with various targets and regional effects related to white matter fractional anisotropy tracks, brain structures, white matter lesions, cortical volume, and cerebral blood flow [56–59]. The relationship between blood pressure and brain aging is complex, multifactorial [60], time-dependent, or even nonlinear [61]. Other factors, such as genetics, lifestyle, and comorbidities, may also contribute to brain changes and cognitive decline in advanced age. Further research is required to fully understand the complexities of how these blood pressure measures influence the brain and its functions.
The study has several strengths. First, the two-sample Mendelian randomization approach represents a robust method for inferring causality between a modifiable exposure and an outcome in observational studies using genetic variants as instrumental variables. Mendelian randomization methods reduced the influence from confounding factors and reverse causation, thus enhancing the validity of the causal inference. Secondly, we used large-sample high-quality genotype and phenotype data from UKB and conducted both linear association and a two-sample Mendelian randomization analysis in overall and age-stratified and sex-stratified groups facilitating a more comprehensive understanding of the causal relationship between blood pressure and white matter BAG. Thirdly, we calculated white matter BAG by training an optimal machine learning model and adjusted for age bias, which resulted in an excellent predictive performance of the chronological age. Lastly, we conducted several sensitivity analyses in both association tests and two-sample Mendelian randomization to provide a more valid and robust estimation.
Our study also has some limitations. First, our study only included the participants not taking antihypertensive medicine, which aims to include a better genetically predicted BP data, and hence the ascertainment bias may have been introduced. Reassuringly, our sensitivity analysis by including the participants who took antihypertensive medicine had consistent findings, so the bias is unlikely. Second, because of the lack of data for younger individuals (i.e. age < 40), the potential impact of BP on white matter brain aging over a lifetime is not investigated in our current study. Third, although we demonstrated significant causal effects of BP on white matter BAG, the molecular mechanisms of selected valid instrumental variables remain unclear. It is critical to integrate other advanced methods (i.e. transcriptome-wide association analysis) and/or to use multiple omics data (i.e. proteins and RNA) that would allow us to obtain a better understanding of the biological system underlying the causal pathway between BP and white matter BAG.
Perspectives
Our study has shown that the nonhypertension group and women had younger brains (white matter BAG) across all the mid-to-older year cycles. Hypertension and genetic predisposition to higher BP (i.e. SBP/DBP) can accelerate brain white matter aging in an age-dependent and sex-dependent manner. These findings shed insight on understanding the abnormal white matter brain changes because of high blood pressure and further provide evidence to reduce the incidence of late-life cognitive impairment and dementia, reflecting the effective control of BP in age and sex awareness would be essential for rational planning of health services.
Supplementary Material
ACKNOWLEDGEMENTS
Author contributions: L.F., Z.Y., and S.L. played a major role in the acquisition of data. L.F. and Z.Y. performed the analysis and wrote the manuscript. T.M. and S.C. supervised the project and took the lead in editing the manuscript. C. M., J.W., S.L., S.G., H.K., T.A.C., Y.P., M.M.J.V.G., A.J.H.M. H., K.W., K.S.H., Y.M., D.K.Y.L., C.C., B.D.M., L.E.H. and P. K. contributed to manuscript writing and polishing. All authors provided critical feedback and helped to shape the research, analysis, and manuscript.
Source of funding:
this work was supported by the National Institute on Drug Abuse (NIDA) of National Institutes of Health under award number 1DP1DA048968-01, University of Maryland Grand Challenge grant, University of Maryland MPower Brain Health and Human Performance seed grant, and National Institutes of Health under award numbers R01MH123163, R01EB015611, and S10OD023696.
Abbreviations:
- BAG
brain age gap
- BH
Benjamini–Hochberg
- BP
blood pressure
- DTI
diffusion tensor imaging
- GWAS
genome-wide association study
- HTN
hypertension
- ICBP
International Consortium of Blood Pressure
- ICD-10
International Classification of Diseases edition 10
- MR
Mendelian randomization
- UKB
UK Biobank
Footnotes
Conflicts of interest
There are no conflicts of interest.
Data availability statement:
the raw genetic and phenotypic data used for this study can be found in the UK Biobank (http://www.ukbiobank.ac.uk/).
REFERENCES
- 1.Petitti DB, Crooks VC, Buckwalter JG, Chiu V. Blood pressure levels before dementia. Arch Neurol 2005; 62:112–116. [DOI] [PubMed] [Google Scholar]
- 2.Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol 2010; 67:564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology 1998; 51:986–993. [DOI] [PubMed] [Google Scholar]
- 4.Qiu C, Winblad B, Viitanen M, Fratiglioni L. Pulse pressure and risk of Alzheimer disease in persons aged 75 years and older: a community-based, longitudinal study. Stroke 2003; 34:594–599. [DOI] [PubMed] [Google Scholar]
- 5.Gons RA, de Laat KF, van Norden AG, van Oudheusden LJ, van Uden IW, Norris DG, et al. Hypertension and cerebral diffusion tensor imaging in small vessel disease. Stroke 2010; 41:2801–2806. [DOI] [PubMed] [Google Scholar]
- 6.Power MC, Schneider AL, Wruck L, Griswold M, Coker LH, Alonso A, et al. Life-course blood pressure in relation to brain volumes. Alzheimers Dement 2016; 12:890–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochunov P, Glahn DC, Lancaster J, Winkler A, Karlsgodt K, Olvera RL, et al. Blood pressure and cerebral white matter share common genetic factors in Mexican Americans. Hypertension 2011; 57:330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coelho A, Fernandes HM, Magalhaes R, Moreira PS, Marques P, Soares JM, et al. Signatures of white-matter microstructure degradation during aging and its association with cognitive status. Sci Rep 2021; 11:4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, Csiszar A. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol 2021; 17:639–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez LA, Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens 2018; 31:1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole JH, Franke K. Predicting age using neuroimaging: innovative brain ageing biomarkers. Trends Neurosci 2017; 40:681–690. [DOI] [PubMed] [Google Scholar]
- 12.Cole JH, Marioni RE, Harris SE, Deary IJ. Brain age and other bodily ‘ages’: implications for neuropsychiatry. Mol Psychiatry 2019; 24:266–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole JH, Poudel RPK, Tsagkrasoulis D, Caan MWA, Steves C, Spector TD, Montana G. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. Neuroimage 2017; 163:115–124. [DOI] [PubMed] [Google Scholar]
- 14.Smith SM, Vidaurre D, Alfaro-Almagro F, Nichols TE, Miller KL. Estimation of brain age delta from brain imaging. Neuroimage 2019; 200:528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wrigglesworth J, Ward P, Harding IH, Nilaweera D, Wu Z, Woods RL, Ryan J. Factors associated with brain ageing - a systematic review. BMC Neurol 2021; 21:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole JH. Multimodality neuroimaging brain-age in UK biobank: relationship to biomedical, lifestyle, and cognitive factors. Neurobiol Aging 2020; 92:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo C, Wang J, Ye Z, Ke H, Liu S, Hatch K, et al. Evaluating the causal effect of tobacco smoking on white matter brain aging: a two-sample Mendelian randomization analysis in UK Biobank. Addiction 2023; 118:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherbuin N, Walsh EI, Shaw M, Luders E, Anstey KJ, Sachdev PS, et al. Optimal blood pressure keeps our brains younger. Front Aging Neurosci 2021; 13:694982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ringen PA, Engh JA, Birkenaes AB, Dieset I, Andreassen OA. Increased mortality in schizophrenia due to cardiovascular disease - a nonsystematic review of epidemiology, possible causes, and interventions. Front Psychiatry 2014; 5:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet 2019; 51:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 2013; 37:658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 2014; 23:R89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanderWeele TJ, Tchetgen Tchetgen EJ, Cornelis M, Kraft P. Methodological challenges in mendelian randomization. Epidemiology 2014; 25:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, et al. , Million Veteran Program. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet 2018; 50:1412–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyman MC, Levin MG, Gill D, Walker VM, Georgakis MK, Davies NM, et al. Genetically predicted blood pressure and risk of atrial fibrillation. Hypertension 2021; 77:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malik R, Georgakis MK, Vujkovic M, Damrauer SM, Elliott P, Karhunen V, et al. Relationship between blood pressure and incident cardiovascular disease: linear and nonlinear mendelian randomization analyses. Hypertension 2021; 77:2004–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill D, Pan Y, Lash JP, Kelly TN. Revisiting the effects of blood pressure on kidney function: new insights from a mendelian randomization analysis. Hypertension 2022; 79:2682–2684. [DOI] [PubMed] [Google Scholar]
- 28.Clarke R, Wright N, Walters R, Gan W, Guo Y, Millwood IY, et al. , China Kadoorie Biobank Collaborative Group. Genetically predicted differences in systolic blood pressure and risk of cardiovascular and non-cardiovascular diseases: a Mendelian Randomization Study in Chinese adults. Hypertension 2023; 80:566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015; 12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wain LV, Shrine N, Miller S, Jackson VE, Ntalla I, Artigas MS, et al. Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. Lancet Respir Med 2015; 3:769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018; 562:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multisubject diffusion data. Neuroimage 2006; 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- 33.Timpe JC, Rowe KC, Matsui J, Magnotta VA, Denburg NL. White matter integrity, as measured by diffusion tensor imaging, distinguishes between impaired and unimpaired older adult decision-makers: a preliminary investigation. J Cogn Psychol (Hove) 2011; 23:760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pazoki R, Dehghan A, Evangelou E, Warren H, Gao H, Caulfield M, et al. Genetic predisposition to high blood pressure and lifestyle factors: associations with midlife blood pressure levels and cardiovascular events. Circulation 2018; 137:653–661. [DOI] [PubMed] [Google Scholar]
- 35.R.C. T R: a language and environment for statistical computing. 2013.
- 36.Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol 2017; 46:1734–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verbanck M, Chen C-y. Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 2018; 50:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qi G, Chatterjee N. Mendelian randomization analysis using mixture models for robust and efficient estimation of causal effects. Nat Commun 2019; 10:1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 1995; 57:289–300. [Google Scholar]
- 40.Goyal MS, Blazey TM, Su Y, Couture LE, Durbin TJ, Bateman RJ, et al. Persistent metabolic youth in the aging female brain. Proc Natl AcadSci U S A 2019; 116:3251–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet 2009; 41:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehret GB, Caulfield MJ. Genes for blood pressure: an opportunity to understand hypertension. Eur Heart J 2013; 34:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colafella KMM, Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol 2018; 14:185–201. [DOI] [PubMed] [Google Scholar]
- 44.Rabi DM, Khan N, Vallee M, Hladunewich MA, Tobe SW, Pilote L. Reporting on sex-based analysis in clinical trials of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker efficacy. Can J Cardiol 2008; 24:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall JE. Renal dysfunction, rather than nonrenal vascular dysfunction, mediates salt-induced hypertension. Circulation 2016; 133:894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marrocco J, McEwen BS. Sex in the brain: hormones and sex differences. Dialogues Clin Neurosci 2016; 18:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lane CA, Barnes J, Nicholas JM, Sudre CH, Cash DM, Parker TD, et al. Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (Insight 46): an epidemiological study. Lancet Neurol 2019; 18:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aribisala BS, Morris Z, Eadie E, Thomas A, Gow A, Valdés Hernández MC, et al. Blood pressure, internal carotid artery flow parameters, and age-related white matter hyperintensities. Hypertension 2014; 63:1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marcus J, Gardener H, Rundek T, Elkind MS, Sacco RL, DeCarli C, Wright CB. Baseline and longitudinal increases in diastolic blood pressure are associated with greater white matter hyperintensity volume: the northern Manhattan study. Stroke 2011; 42:2639–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lau KK, Li L, Simoni M, Mehta Z, Küker W, Rothwell PM, et al. Long-term premorbid blood pressure and cerebral small vessel disease burden on imaging in transient ischemic attack and ischemic stroke: population-based study. Stroke 2018; 49:2053–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo X, Pantoni L, Simoni M, Bengtsson C, Björkelund C, Lissner L, et al. Blood pressure components and changes in relation to white matter lesions: a 32-year prospective population study. Hypertension 2009; 54:57–62. [DOI] [PubMed] [Google Scholar]
- 52.Franklin SS, Gustin W, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997; 96:308–315. [DOI] [PubMed] [Google Scholar]
- 53.Gurven M, Blackwell AD, Rodriguez DE, Stieglitz J, Kaplan H. Does blood pressure inevitably rise with age?: longitudinal evidence among forager-horticulturalists. Hypertension 2012; 60:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muller M, Sigurdsson S, Kjartansson O, Aspelund T, Lopez OL, Jonnson PV, et al. , Age, Gene/Environment Susceptibility-Reykjavik Study Investigators. Joint effect of mid- and late-life blood pressure on the brain: the AGES-Reykjavik study. Neurology 2014; 82:2187–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reitz C, Luchsinger JA. Relation of blood pressure to cognitive impairment and dementia. Curr Hypertens Rev 2007; 3:166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alateeq K, Walsh EI, Cherbuin N. Higher blood pressure is associated with greater white matter lesions and brain atrophy: a systematic review with meta-analysis. J Clin Med 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siedlinski M, Carnevale L, Xu X, Carnevale D, Evangelou E, Caulfield MJ, et al. Genetic analyses identify brain structures related to cognitive impairment associated with elevated blood pressure. Eur Heart J 2023; 44:2114–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ye Z, Mo C, Liu S, Gao S, Feng L, Zhao B, et al. Deciphering the causal relationship between blood pressure and regional white matter integrity: a two-sample Mendelian randomization study. J Neurosci Res 2023; 101:1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caunca MR, Simonetto M, Cheung YK, Alperin N, Lee SH, Elkind MSV, et al. Diastolic blood pressure is associated with regional white matter lesion load: the Northern Manhattan Study. Stroke 2020; 51:372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hughes TM, Sink KM. Hypertension and its role in cognitive function: current evidence and challenges for the future. Am J Hypertens 2016; 29:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Dijk EJ, Breteler MMB, Schmidt R, Berger K, Nilsson LG, Oudkerk M, et al. The association between blood pressure, hypertension, and cerebral white matter lesions - cardiovascular determinants of dementia study. Hypertension 2004; 44:625–630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
the raw genetic and phenotypic data used for this study can be found in the UK Biobank (http://www.ukbiobank.ac.uk/).


