Abstract
In addition to its effects on macrophage function, macrophage-stimulating protein (MSP) is a growth and motility factor for epithelial cells. The growth and survival of epithelial cells generally require two signals, one generated by interaction with extracellular matrix via integrins, the other initiated by a growth factor. Therefore we investigated the effect of MSP on epithelial cell survival. Survival of epithelial cells cultured overnight in serum-free medium was promoted by adhesion, which activated both the phosphatidylinositol 3′-kinase (PI3-K)/AKT and mitogen-activated protein kinase (MAPK) pathways, operating independently of one another. The number of apoptotic cells resulting from inhibition of either pathway alone was approximately doubled by simultaneous inhibition of both pathways. This shows that each pathway made a partial contribution to the prevention of apoptosis. In the presence of an inhibitor of either pathway, MSP increased the activity of the other pathway so that the single uninhibited pathway alone was sufficient to prevent apoptosis. In contrast to the results with adherent cells, although MSP also prevented apoptosis of cells in suspension (anoikis), its effect was mediated only by the PI3-K/AKT pathway. Despite activation of MAPK by MSP, anoikis was not prevented in suspended cells with a blocked PI3-K/AKT pathway. Thus, activation of MAPK alone is not sufficient to mediate MSP antiapoptotic effects. Cell adhesion generates an additional signal, which is essential for MSP to use MAPK in an antiapoptotic pathway. This may involve translocation of MSP-activated MAPK from the cytoplasm into the nucleus, which occurs only in adherent cells. Our results suggest that there is cross talk between cell matrix adhesion and growth factors in the regulation of cell survival via the MAPK pathway. Growth factors induce MAPK activation, and adhesion mediates MAPK translocation from the cytoplasm into the nucleus.
Apoptosis, or programmed cell death, is a mechanism for the regulation of tissue morphogenesis and homeostasis. It controls tissue cell number by elimination of either damaged cells or excess normal cells. Apoptosis can be detected by altered cell morphology and intranucleosomal DNA fragmentation (35, 51). Active suppression of apoptosis via incoming survival signals is necessary for cell survival. In the case of anchorage-dependent cells like epithelia and endothelia, suppression of apoptosis depends on attachment of cells to the extracellular matrix (ECM) and the presence of growth factors (7, 8, 27, 53, 54, 77, 84).
Cell-ECM interaction is mediated via integrins, transmembrane noncovalently linked heterodimeric receptors consisting of α and β subunits (37, 40). When integrin-ECM interaction is inhibited, anchorage-dependent cells undergo apoptosis (27, 28, 66). Cell-ECM disruption-induced apoptosis is called anoikis (homelessness) (27, 28). It occurs in vivo in normal skin (62), the digestive tract (34), the involuting mammary gland (7), and at the cavitation step of embryogenesis (11). The anchorage independence of neoplastic epithelial and endothelial cells is a reflection of their resistance to anoikis, which is one of the factors contributing to metastatic potential (26, 71, 74).
In addition to integrin-ECM interactions, growth factors can also protect cells from various types of apoptosis (22, 76, 87), including anoikis (8, 77). Integrins and growth factors may mediate their antiapoptotic effects via activation of the same pathways (60, 61, 71).
Among the intracellular signal transduction molecules that are involved in suppressing anoikis in anchorage-dependent cells, the roles of focal adhesion kinase (FAK), phosphatidylinositol 3′-kinase (PI3-K), and AKT have received considerable attention. FAK, which is generally believed to be directly associated with β integrins (58, 68), plays a critical role in suppressing anoikis in some experimental systems. Constitutive activation of FAK is sufficient to rescue epithelial cells from anoikis (29), whereas apoptosis occurs if FAK is inhibited by expression of FAK-antisense oligonucleotides (86), by a peptide representing the FAK-binding site of β1 integrin, or by anti-FAK antibodies (36). Anoikis does not occur in cells expressing activated src or ras oncogenes (27, 44, 45, 52) or PI3-K or AKT kinase (44, 45). In v-src-transformed cells, hyperphosphorylation of FAK was observed (27), which suggests that anoikis resistance of v-src-transformed cells may be related to activation of FAK by src.
PI3-K activity is associated with antiapoptotic signaling in many cell types, including neurons, fibroblasts, and epithelial and hematopoietic cells (5, 21, 42, 43, 60, 69). PI3-K transduces the antiapoptotic effect of selected growth factors (6, 20, 73) by activating AKT (25), a serine- and threonine-specific kinase also known as PKB (1, 19). Activation of the PI3-K/AKT pathway is also the basis for protection of epithelial cells from anoikis by transfection of activated ras (44, 45).
Another important regulator of apoptosis is mitogen-activated protein kinase (MAPK) (12, 85). MAPK is activated by both growth factors and integrins (47, 57, 72). MAPK is required for survival signaling in response to FGF (30, 33), EGF (55), and insulin-like growth factor 1 (IGF 1) (56, 59). Growth factor deprivation leads to a dramatic inhibition of MAPK activity (85) that might be directly related to apoptosis induction, since inhibition of MAPK causes apoptosis (5, 85). Although there is no direct evidence that MAPK plays a role in integrin-mediated antiapoptotic signaling, it has been shown that integrins are linked to the MAPK pathway via the adapter protein Shc, and Shc regulates integrin-mediated cell survival (83). These data suggest that MAPK may contribute to integrin-mediated antiapoptotic effects.
Macrophage-stimulating protein (MSP) is an 80-kDa heterodimer belonging to the plasminogen-related protein family (18, 88). MSP was found as a serum protein that regulates the motility of macrophages (49, 50). Recent investigations have shown that MSP is also a growth and motility factor for epithelial cells (79), and it regulates integrin-dependent epithelial cell adhesion and motility (15; A. Danilkovitch and E. J. Leonard, J. Leukoc. Biol. Suppl., p. 19, 1997). MSP mediates its activity via the RON-STK receptor tyrosine kinase (31, 38, 64, 80, 82). It induces tyrosine phosphorylation and catalytic activity of a number of signal transduction proteins, including FAK, c-src, MAPK (13), and PI3-K (81). Since each of these molecules is involved in protection by growth factors from apoptosis in selected cell systems, we hypothesized that MSP may have the capacity to inhibit apoptosis of epithelial cells. Accordingly, we studied the effects of MSP on epithelial cell anoikis, as well as apoptosis of adherent cells under serum-free conditions.
MATERIALS AND METHODS
Cell lines and culture conditions.
Madin-Darby canine kidney (MDCK) cells were from the American Type Culture Collection, Manassas, Va. RE7 cells (MDCK cells transfected with RON cDNA) were as described previously (82). These cell lines were cultured in DME (Gibco BRL, Gaithersburg, Md.) with 10% fetal calf serum (Hyclone, Logan, Utah). The mouse PAM 212 keratinocyte cell line (89) was donated by S. Yuspa (National Cancer Institute), the human NOC keratinocyte cell line was donated by R. Schlegel (Georgetown University), and the human keratinocyte cell line HaCat was donated by N. Fusenig (Heidelberg, Germany). All keratinocyte cell lines were cultured in keratinocyte serum-free medium (Gibco BRL).
Antibodies and other reagents.
Rabbit polyclonal anti-FAK (C-20) and anti-hemagglutinin (HA) (Y-11) antibodies and goat anti-CPP32 (caspase 3) (L-18) and anti-AKT1 (C-20) antibodies were from Santa Cruz (Santa Cruz, Calif.). Mouse monoclonal antiphosphotyrosine (clone 4G10) and anti-Src (clone GD11) were from UBI, Lake Placid, N.Y. Rabbit polyclonal poly(ADP-ribose) polymerase (PARP) antibodies were from Biomol Research Laboratories (Plymouth Meeting, Pa.). Mouse monoclonal anti-phosphoMAPK antibodies were from New England Biolabs (Beverly, Mass.). Mouse monoclonal anti-extracellular signal-regulated kinase 1 (ERK1) antibodies were from Transduction Laboratories (Lexington, Ky.).
Human recombinant MSP was from Toyobo, Osaka, Japan. [γ-32P]ATP was from Dupont, NEN, Boston, Mass. ECL was from Amersham, Arlington Heights, Ill. PI3-K inhibitor LY294002 and MAPK kinase (MEK) inhibitor PD98059 were from Biomol Research Laboratories. Poly(2-hydroxyethyl methacrylate) (poly-Hema) and rabbit enolase were from Sigma. Histone H2B, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL), and DNA fragmentation enzyme-linked immunosorbent assay (ELISA) kits were from Boehringer Mannheim (Indianapolis, Ind.). Other chemicals were from Sigma, Bio-Rad, and Gibco.
Induction of apoptosis by disruption of cell-matrix interactions (anoikis).
Cells from confluent monolayers in 100-mm-diameter tissue culture dishes were collected by limited trypsinization and transferred to poly-Hema-coated 100-mm-diameter dishes prepared in advance as described previously (24). After 18 to 20 h of incubation in suspension (5 × 106 cells/dish) in serum-free medium with or without 5 nM MSP, the cells were harvested and apoptosis was quantified by TUNEL staining or DNA fragmentation ELISA.
Induction of apoptosis by serum starvation.
Cells were grown to 80% confluence on six-well plates, after which serum-containing medium was replaced by medium without serum. The effect of MSP on cell survival was investigated on the background of a blocked PI3-K/AKT and/or MAPK pathway. PI3-K activity was inhibited by the addition of 50 μM LY294002. AKT activity was inhibited via kinase-dead AKT overexpression. MAPK activation was inhibited by 50 μM PD98059. To block both PI3-K/AKT and MAPK pathways simultaneously, a combination of LY294002 and PD98059 was used or PD98059 was added to dominant-negative AKT-expressing cells. After 24 h of cell incubation without serum in the presence of the inhibitors, with or without 5 nM MSP, all cells (adherent and detached) were collected. Apoptosis was quantified by TUNEL staining or DNA fragmentation ELISA.
Detection of apoptosis.
Apoptosis was measured either with a TUNEL staining kit (Boehringer Mannheim) or by a DNA fragmentation ELISA method using the cell death detection ELISA kit (Boehringer Mannheim) according to the manufacturer's instructions. Also, apoptosis was detected in total cell lysates by Western blotting with anti-PARP or with anti-CPP32 antibodies (see “Immunoprecipitation, immunoblotting, and immunocomplex kinase assays” below).
Expression vectors.
The mouse FAK mutant Y397F and K454R cDNAs containing three HA tags in a pcDNA3 plasmid (70) were kindly provided by D. Schlaepfer (Scripps Research Institute, La Jolla, Calif.). The chicken c-Src mutant K295M/Y527F (MF) and Δ251 (with deletion of the kinase domain) cDNAs in a CAIO vector were kindly provided by P. Schwarzberg (National Institutes of Health, Bethesda, Md.). The AKT kinase-dead cDNA mutant K195M in a cytomegalovirus vector (pCMV) (16) was kindly provided by K. Datta (Fox Chase Cancer Center, Philadelphia, Pa.).
Transient transfections and positive selection of transfected cells.
RE7 cells at 70% confluence in 15-cm-diameter dishes were transiently transfected with 20 μg of various cDNA constructs or empty vectors as controls and simultaneously cotransfected with 5 μg of MACS4 (Miltenyi Biotec, Auburn, Calif.) using SuperFect (Qiagen, Santa Clarita, Calif.). After 36 h of incubation, transfected cells were separated with the MACS4 magnetic bead kit and used for experiments.
Cell stimulation and lysis.
Transfected and nontransfected starved cells were stimulated with 5 nM MSP for 15 min. For experiments with inhibitors, the cells were pretreated with 100 nM wortmannin (WTM) or 50 μM LY294002 or PD98059 or with a combination of LY294002 and PD98059 for 15 min and then stimulated with MSP for 15 min. For time course experiments, the cells were stimulated with 5 nM MSP for different times, from 2 to 60 min. After stimulation, the cells were lysed in lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 10% glycerol, 1 mM EDTA, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 100 mM NaF, 1% Triton X-100, 10 μg of leupeptin/ml, 10 U of aprotinin/ml, 1 mM phenylmethylsulfonyl fluoride [PMSF]). Insoluble material was removed by centrifugation. Supernatants were used for immunoprecipitation, immunoblotting, and immunocomplex kinase assays in vitro.
Immunoprecipitation, immunoblotting, and immunocomplex kinase assays.
Expression of FAK, AKT, and c-Src dominant-negative constructs in total cell lysates of transfected cells was detected by immunoblotting with anti-HA, anti-AKT, and anti-Src antibodies, respectively.
For kinase assays, FAK, c-Src, or AKT was immunoprecipitated from cell lysates by anti-FAK, anti-Src, or anti-AKT antibodies. Immunoprecipitates (IPs) were washed twice in HNTG buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 0.1% Triton X-100, 10% glycerol) and twice in kinase buffer (20 mM HEPES [pH 7.4], 10% glycerol, 10 mM MgCl2, 10 mM MnCl2, 150 mM NaCl, 2 mM dithiothreitol [DTT]). To initiate kinase reactions, 15 μCi of [γ-32P]ATP (3,000 Ci/mmol; 10 μCi/ml) was added, and IPs were incubated for 15 to 30 min at 30°C in a 15-μl total volume. For c-Src and AKT kinase assays, exogenous substrate rabbit enolase (10) or histone H2B (16) was added to the reaction mixture at a concentration of 0.5 μg/reaction. The reactions were stopped with 5 μl of 4× sample buffer. Phosphorylated FAK, AKT or its substrate histone H2B and rabbit enolase as a substrate of c-Src were visualized after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by autoradiography.
MAPK activation was determined in total cell lysates (15 μg/lane) by Western blotting analysis using anti-phosphoMAPK (New England BioLabs) or anti-ERK1 (Transduction Laboratories) antibodies.
Cleavage of CPP32 caspase by cells kept in suspension for 8 h in the absence or presence of 5 nM MSP was detected as a decrease in uncleaved CPP32 in total cell lysates by immunoblotting with antibodies to uncleaved CPP32.
Cleavage of PARP by cells kept in suspension overnight in the absence or presence of 5 nM MSP was detected in total cell lysates by immunoblotting with anti-PARP antibodies, which recognize both 116-kDa PARP and the 85-kDa apoptosis-related cleaved PARP fragment.
Detection of MAPK translocation.
Starved RE7 cells (MDCK cells stably expressing RON receptor) were stimulated with 5 nM MSP for 15 min either in suspension or on coverslips. Cell incubation with medium alone served as a negative control. After stimulation, MAPK translocation from the cytoplasm into the nucleus was detected by indirect immunofluorescence analysis and by Western blotting of separated cytoplasmic and nuclear cell fractions.
Indirect immunofluorescence.
Stimulated and nonstimulated cells were fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS) for 30 min and then permeabilized with 0.5% Triton X-100 in PBS for 5 min at room temperature. Nonspecific sites were blocked by incubation with PBS containing 0.1% normal goat serum for 30 min at room temperature. The coverslips were incubated with primary anti-phosphoMAPK antibodies (New England BioLabs; 1:1,000 dilution) for 1 h and then with secondary goat anti-mouse antibodies labeled with fluorescein isothiocyanate (Sigma; 1:100 dilution). After each step the coverslips were extensively washed in PBS, and after a final washing the coverslips were mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, Calif.) and examined under epifluorescent illumination. Parental MDCK cells, which do not respond to MSP, served as a control for MSP effects. Cells stained with secondary antibodies only served as a control for staining specificity.
Isolation of cytoplasmic and nuclear cell fractions.
After stimulation with MSP, suspended cells (2 × 106) were washed three times with Tris-buffered saline (TBS), with subsequent pelleting by spinning in a microcentrifuge, and then the cell pellets were resuspended in 400 μl of cold buffer A (10 mM HEPES [pH 8.0], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF). Adherent cells were washed three times by addition of TBS directly onto 10-cm-diameter dishes, and then 400 μl of cold buffer A was added per dish, and the cells were scraped and transferred into Eppendorf tubes. After that, the collected adherent and suspended cells were allowed to swell on ice for 15 min, after which 25 μl of 10% NP-40 (Sigma) was added, the tubes were vortexed, and the homogenates were centrifuged for 30 s in a minicentrifuge. The supernatants containing cytoplasm and RNA were transferred to fresh tubes and stored frozen at −70°C or immediately used for experiments. The nuclear pellets were washed five times with buffer A and then resuspended in 50 μl of ice-cold buffer B (20 mM HEPES [pH 8.0], 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF) and incubated on ice for 15 min with occasional vortexing. The nuclear extracts were centrifuged for 5 min in a minicentrifuge at 4°C, and supernatants were immediately frozen at −70°C or used for experiments. Protein concentrations in cytoplasmic and nuclear extracts were measured with a Bio-Rad protein assay kit, and samples containing 20 μg were prepared and used for SDS-PAGE and Western blotting (see “Immunoprecipitation, immunoblotting, and immunocomplex kinase assays” above).
RESULTS
MSP prevents epithelial cell anoikis.
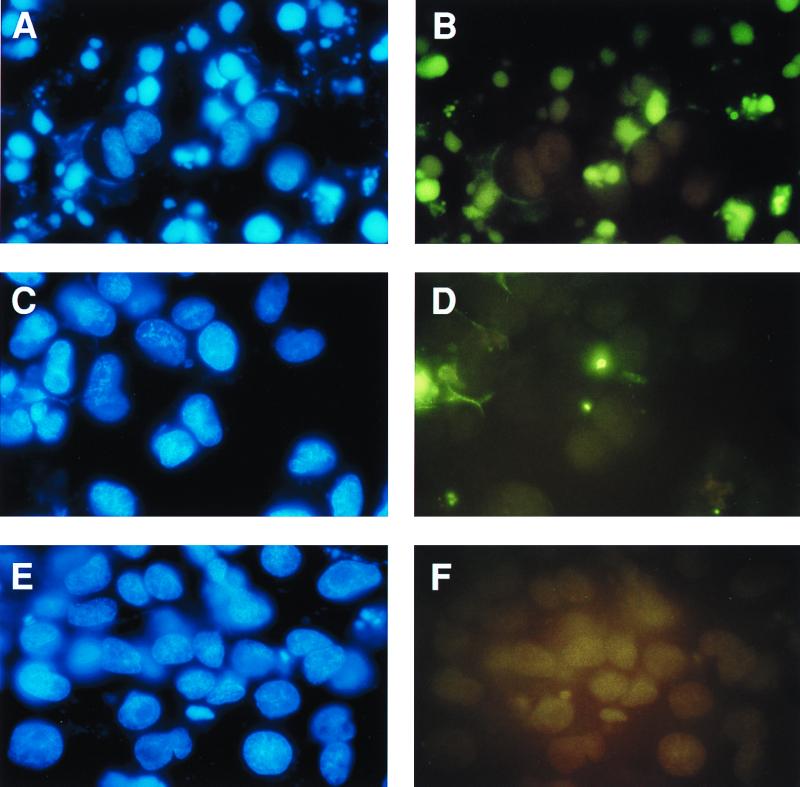
Epithelial cells become apoptotic when they are prevented from adhering to extracellular matrix or to tissue culture plastic. This type of apoptosis is referred to as anoikis (27). We tested the effect of MSP on the survival of epithelial cells under conditions that lead to anoikis. Anoikis was induced by incubation of cells in dishes coated with poly-Hema, which prevents cell attachment (24). We used the MDCK-RE7 epithelial cell line, which stably expresses RON, the receptor for MSP. The parental MDCK cell line with undetectable RON was used as a negative control. After overnight incubation in serum-free medium with or without 5 nM MSP, apoptosis was quantified by TUNEL staining. Figure 1 shows that MSP protected MDCK-RE7 cells from anoikis. There were more than 40% apoptotic cells in medium alone (Fig. 1A and B) and fewer than 10% in cultures with 5 nM MSP (Fig. 1C and D). Unlike with cells in suspension, the percentage of apoptotic cells in serum-free medium attached to the surfaces of uncoated petri dishes (Fig. 1E and F) was less than 5%. These findings were confirmed by an ELISA that quantifies DNA fragmentation (data not shown).
FIG. 1.
MSP prevents anoikis in RE7 cells. For induction of anoikis, epithelial cells from regular petri dishes were transferred to poly-Hema-coated dishes. Poly-Hema prevents the attachment of cells to plastic, and nonattached cells undergo apoptosis, which was quantified by TUNEL staining after overnight incubation in serum-free medium (A and B) or in the presence of 5 nM MSP (C and D). Cells attached to uncoated petri dishes in serum-free medium were also evaluated (E and F). Left panels, 4′,6-diamidino-2-phenylindole (DAPI)-stained blue nuclei demonstrating the total number of cells in the field; right panels, fluorescein isothiocyanate-stained apoptotic cells in the same field.
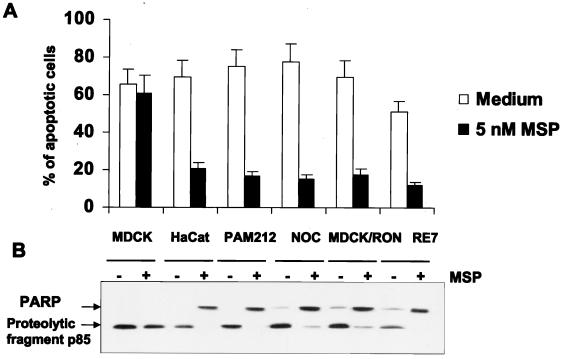
Using TUNEL staining (Fig. 2A) and immunoblot analysis of PARP cleavage (Fig. 2B), we demonstrated that MSP also prevented the anoikis of mouse PAM212 and human HaCat and NOC keratinocyte cell lines that express endogenous MSP receptor as well as the MDCK-RON cell line, which stably transfected and expressed MSP receptor at a level comparable with endogenous expression. MSP did not prevent the anoikis of parental MDCK cells (Fig. 2).
FIG. 2.
MSP prevents anoikis in various RON-expressing keratinocyte and epithelial cell lines. Anoikis was induced as described in the legend to Fig. 1. (A) The number of apoptotic cells was determined by TUNEL staining. Each experimental point represents the mean percentage (+ standard error of the mean) of apoptotic cells for three independent experiments. (B) Detection of apoptosis by immunoblot analysis of PARP cleavage in total cell lysates.
MSP activates FAK, c-Src, and MAPK, but inhibition of one or another of these kinase activities does not interfere with the antianoikis effect of MSP.
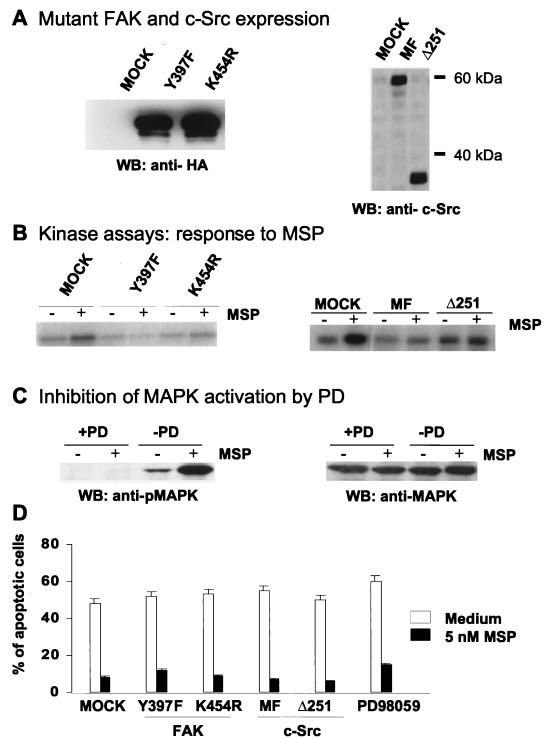
Interaction of epithelial cells with ECM proteins occurs via integrin receptors (9), engagement of which leads to activation of diverse intracellular signals, including FAK and c-Src. Cells transfected with either activated FAK or c-Src are protected from anoikis (27, 29, 45). MSP, which protected cells from anoikis (Fig. 1 and 2), has also been shown to activate FAK and c-Src (13). Therefore, it was relevant to study anoikis in cells in which FAK or c-Src was inhibited. We transiently overexpressed dominant-negative kinase-dead K454R or tyrosine autophosphorylation site Y397F FAK mutants or kinase-inactive c-Src MF or Δ251 mutants in RE7 cells. Expression of FAK or c-Src was detected in total cell lysates of successfully transfected cells after selection with a MACS4 select kit (Fig. 3A). Transfected cells were used for detection of MSP-induced FAK and c-Src kinase activity (Fig. 3B) and for study of MSP antianoikis activity (Fig. 3D). The data show that despite blockage of the catalytic activity of either FAK (Fig. 3B, left) or c-Src (Fig. 3B, right), MSP could still protect cells from anoikis (Fig. 3D). As an additional control, we tested the response of cells expressing FAK mutants to being plated on collagen-coated dishes. Expression of either the Y397F or K454R FAK mutant blocked collagen-induced FAK phosphorylation (data not shown).
FIG. 3.
Inhibition of MSP-induced FAK, c-Src, or MAPK activation does not prevent the antianoikis effect of MSP. (A) RE7 cells were transiently transfected with dominant-negative FAK Y397F or K454R or c-Src MF or Δ251 or empty vector (mock). Lysates (15 μl/lane) from transfected and MACS4-selected cells were analyzed by SDS-PAGE followed by Western blotting with anti-HA antibodies for detection of FAK expression (left) and with anti-c-Src antibodies for detection of c-Src expression (right) (B) A suspension of transiently transfected RE7 cells (2 × 106/ml) was stimulated with 5 nM MSP for 10 min. FAK and c-Src kinases were then immunoprecipitated from cell lysates with anti-FAK or anti-c-Src antibodies. The kinase activities of FAK and c-Src were measured with [32P]ATP as the capacity of the FAK IP to autophosphorylate FAK (left) or the c-Src IP to phosphorylate enolase (right), detected by SDS-PAGE and autoradiography. (C) A suspension of RE7 cells was pretreated with 50 μM MEK inhibitor PD98059 for 15 min, and then the cells were stimulated with 5 nM MSP for an additional 15 min. Activation of MAPK was determined by Western blotting with anti-phosphoMAPK (pMAPK) (left) or anti-MAPK (right) after SDS-PAGE of total cell lysates. (D) Anoikis was induced in mutant FAK or c-Src-transfected RE7 cells as described in the legend to Fig. 1. After overnight incubation in medium with or without 5 nM MSP, the apoptotic cells were quantified by TUNEL staining. Each experimental point represents the mean percentage (+ standard error of the mean) of apoptotic cells for three independent experiments.
MSP also activates MAPK, a kinase that may be in a pathway that supports cell survival (85). To block activation of MAPK, cells were treated with 50 μM PD98059 inhibitor (2), which prevented MSP-induced phosphorylation of MAPK (Fig. 3C). For experiments with anoikis, cells were incubated overnight with PD98059 in the presence or absence of 5 nM MSP. Although PD98059 inhibited MSP-induced MAPK phosphorylation (Fig. 3C), it did not block the antianoikis effect of MSP (Fig. 3D).
The data on FAK, cSrc, and MAPK show that although MSP activates each of these components, and any one of them might be contributing to the antianoikis effect of MSP, blocking any one of them alone does not prevent MSP action. This is in contrast to AKT, as described below.
MSP activates AKT kinase by a PI3-K-dependent mechanism.
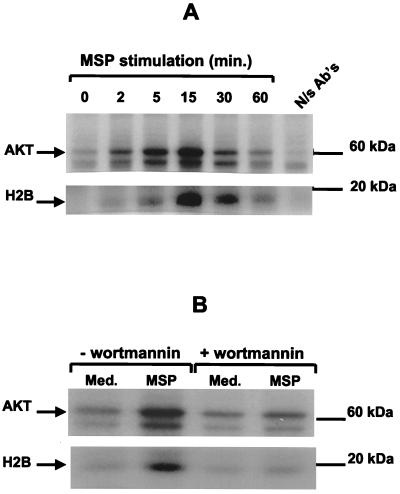
We next tested the possible role of AKT kinase as a component of the signal cascade mediating the antiapoptosis action of MSP because (i) several growth factors and cytokines mediate their antiapoptotic effects via AKT, (ii) AKT is a downstream component of PI3-K in the case of several growth factor receptors, and (iii) MSP causes activation of PI3-K (81) and protects cells from apoptosis (see data above). Stimulation of cells with MSP led to AKT activation that began within 5 min and returned to baseline by 1 h (Fig. 4A). To determine whether AKT is downstream of PI3-K, we treated the cells with WTM, a specific inhibitor of PI3-K when used in nanomolar concentrations (63, 75). WTM prevented activation of AKT by MSP, which shows that MSP-induced AKT activation is PI3-K dependent (Fig. 4B), and that AKT is in the MSP/RON signaling pathway, downstream of PI3-K.
FIG. 4.
MSP stimulates AKT enzymatic activity by a PI3-K-dependent mechanism. (A) After stimulation of RE7 cells with 5 nM MSP, the cells were lysed at the indicated times. AKT was immunoprecipitated and tested for kinase activity by incubation with [32P]ATP and exogenous substrate histone H2B. Incorporation of 32P into AKT (top) and histone H2B (bottom) was detected by SDS-PAGE and autoradiography. N/s Ab's, antibodies isolated from nonimmune rabbit serum. (B) RE7 cells were pretreated with 100 nM WTM for 15 min and then stimulated with 5 nM MSP for an additional 15 min. AKT kinase activity was detected as described for panel A.
Expression of kinase-dead AKT in epithelial cells inhibits the antianoikis effect of MSP.
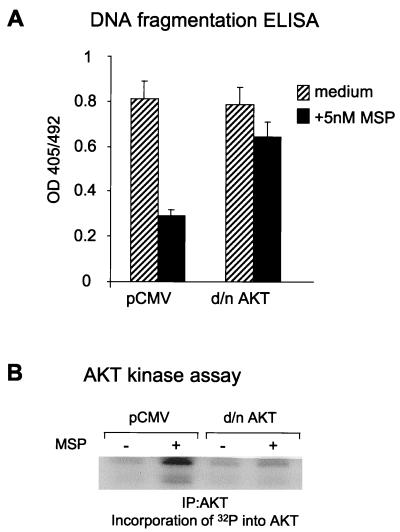
To clarify the role of AKT in MSP signaling, we used a kinase-dead AKT construct with substitution of Met for a Lys residue in the ATP-binding site of the kinase domain (K179M) (16). We transiently transfected cells with this mutant AKT cDNA or pCMV as a vector control and tested the effect of MSP on epithelial cell anoikis. Kinase-dead AKT blocked MSP antianoikis action (Fig. 5A), as detected by a DNA fragmentation ELISA. Overexpression of kinase-dead AKT inhibited MSP-dependent endogenous AKT activation (Fig. 5B).
FIG. 5.
Dominant-negative AKT blocks the antianoikis effect of MSP. (A) RE7 cells were transiently transfected with kinase-dead AKT (d/n AKT) or with the pCMV vector as a control. Anoikis was induced as described in the legend to Fig. 1, and DNA fragmentation in apoptotic cells was quantified by cell death ELISA. Three independent experiments were performed. The results of a representative experiment are shown. The error bars indicate the standard deviations for triplicate ELISA wells. (B) Expression of dominant-negative AKT blocks MSP-induced endogenous AKT enzymatic activity. RE7 cells, transiently transfected with the empty vector (pCMV) or dominant-negative AKT cDNA, were stimulated with 5 nM MSP for 15 min. The cells were then lysed, and AKT was immunoprecipitated and tested for kinase activity in vitro. The kinase assay was performed as described in the legend to Fig. 4A. Incorporation of 32P into AKT was detected by SDS-PAGE and autoradiography.
In addition, we detected an effect of dominant-negative AKT expression on CPP32 (caspase 3) cleavage. Cleavage of CPP32 correlates with activation of this enzyme and induction of apoptosis (15, 23). Treatment of suspended cells with MSP prevented cleavage of CPP32 to its active form, whereas cleavage occurred in cells with kinase-dead AKT (data not shown).
Apoptosis due to serum deprivation in adherent cell cultures is prevented by MSP via either an AKT or MAPK pathway.
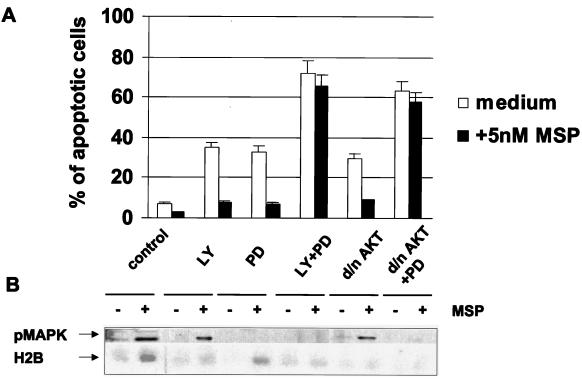
Adherent epithelial cells can survive in vitro in the absence of serum, due in part to the fact that adherence leads to low-level, but prolonged, activation of the PI3-K/AKT pathway (45). Interference with this pathway causes apoptosis of adherent cells (45, 46, 87). As shown in Fig. 5A, the PI3-K/AKT pathway is involved in the protection of suspended epithelial cells from anoikis by MSP. To extend this finding, we investigated the MSP antiapoptotic effect on adherent epithelial cells cultured in the absence of serum. We blocked the PI3-K/AKT pathway in RE7 epithelial cells attached to the surfaces of plastic petri dishes in serum-free medium by overexpression of kinase-inactive AKT or by treatment of cells with the PI3-K inhibitor LY294002 (67, 78). Under these conditions, we found more than 30% apoptotic cells after 24 h of culture (Fig. 6A). We conclude that apoptosis in serum-free medium was prevented, at least in part, by activation of a PI3-K/AKT pathway. However, whereas MSP did not protect suspended cells from anoikis if AKT was inhibited (Fig. 5A), MSP prevented adherent epithelial cell apoptosis, despite inhibition of AKT activity by either LY294002 or overexpressed kinase-dead AKT (Fig. 6B, bottom). These results indicate that there is more than one pathway that mediates antiapoptotic effects on adherent epithelial cells. The following experiments identified an additional pathway. Inhibition of the MAPK pathway with PD98059, an inhibitor of MEK1 and -2 (Fig. 6B, top), caused the appearance of more than 30% apoptotic cells within 24 h, and MSP protected these cells from apoptosis, presumably via the PI3-K/AKT pathway (Fig. 6A). When both PI3-K and MAPK pathways were blocked simultaneously, the number of apoptotic cells increased to 70%, and MSP had no protective effect (Fig. 6A). The following biochemical data showed that AKT and MAPK are located in different MSP-activated pathways. Treatment of cells with the PI3-K inhibitor LY294002 or overexpression of kinase-dead AKT inhibited MSP-induced activation of AKT without affecting MAPK, and conversely, the MAPK inhibitor PD98059 blocked activation of MAPK by MSP without affecting AKT (Fig. 6B). The data show that MSP-induced activation of either the PI3-K or MAPK pathway is sufficient to rescue adherent epithelial cells from apoptosis. This is in contrast to the anoikis conditions, in which PI3-K/AKT is essential for mediating the antiapoptotic effect of MSP (Fig. 5A) and activation of the MAPK (Fig. 3C) pathway apparently cannot prevent anoikis.
FIG. 6.
MSP rescues epithelial cells from serum deprivation-induced apoptosis via PI3-K/AKT and MAPK pathways. (A) The effect of MSP on RE7 cells or RE7 cells with transiently expressed dominant-negative AKT was studied in serum-free medium in the presence of inhibitor LY294002 or PD98059 or both combined. After 24 h of incubation, the percentage of apoptotic cells was determined by TUNEL staining. Each experimental point represents the mean percentage (+ standard deviation) of apoptotic cells in three independent experiments. (B) AKT kinase activity was determined as phosphorylation of histone H2B in vitro as described in the legend to Fig. 3. MAPK activity was determined in total cell lysates by Western blotting with antibodies specific for phosphorylated MAPK.
Translocation of MSP-activated MAPK from the cytoplasm into the nucleus occurs in adherent cells but not in suspended cells.
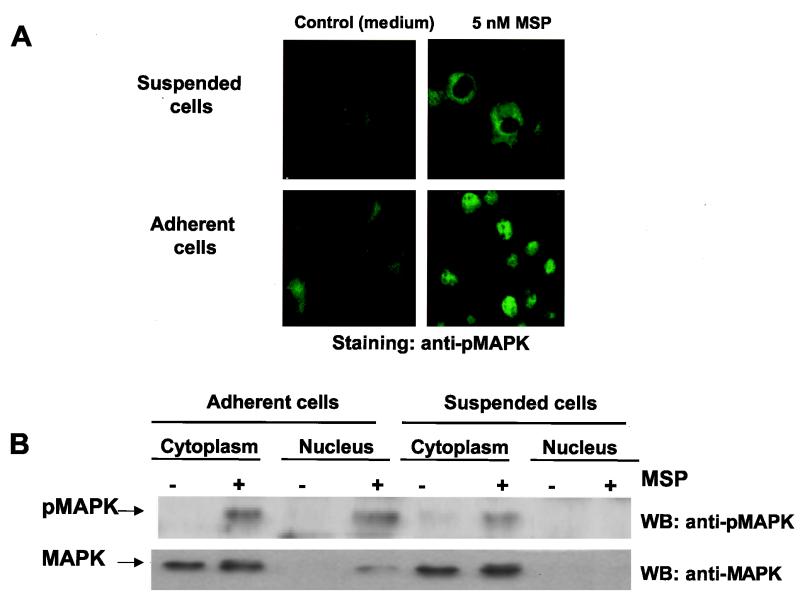
The above-mentioned experiments show that adhesion is required for MSP to mediate an antiapoptotic effect via the MAPK pathway. Comparison of the cellular localization of MSP-activated MAPK (phosphoMAPK) in suspended and adherent cells showed by two independent methods that MSP induced translocation of phosphoMAPK from the cytoplasm into the nuclei of adherent cells but not of cells in suspension (Fig. 7). Although the absence of detectable phosphoMAPK in the nuclei of suspended cells might be attributed to insufficient sensitivity, this is unlikely in view of the intense fluorescence in the suspended-cell cytoplasm and its absence from the nuclei. This difference was found despite similar levels of MSP-induced activated MAPK in the isolated cytoplasmic fraction of adherent and suspended cells (Fig. 7B). Addition of the MEK inhibitor PD98059 to RON-expressing RE7 cells blocked both MSP-induced MAPK activation (Fig. 3C) and adhesion-dependent MAPK translocation (data not shown). Thus, there are two requirements for MSP-induced MAPK translocation to the nucleus, MAPK activation and epithelial cell adhesion.
FIG. 7.
Adhesion promotes translocation of MSP-activated MAPK from the cytoplasm into the nucleus. Starved RE7 epithelial cells were stimulated with 5 nM MSP for 15 min in suspension or on coverslips, and then translocation of MAPK was detected by indirect immunofluorescence analysis (A) or by Western blotting of cytoplasmic and nuclear cell extracts (B) with antibodies to phosphoMAPK (pMAPK) (top) or total MAPK (bottom).
DISCUSSION
The present work provides the first evidence that MSP is a survival factor for epithelial cells. The molecular mechanism of MSP-mediated antiapoptotic action in serum-free medium depends on whether epithelial cells are suspended or adherent. MSP inhibited the anoikis that developed when cells were cultured overnight under conditions that prevented adherence to substrate (Fig. 1C and D, 2, 3D, and 5A). Anoikis has been inhibited by other growth factors, including hepatocyte growth factor (HGF) (27), IGF-1 (77), insulin, and NGF (8), but intracellular signaling pathways have not been determined. Inasmuch as MSP causes activation of numerous intracellular mediators (13), the problem was to identify which ones mediated inhibition of anoikis. It was previously reported that MSP caused binding of PI3-K to the RON receptor. Stimulation of motility by MSP was dependent on the concomitant phosphorylation and activation of PI3-K (81). We now show that prevention of anoikis by MSP involves PI3-K-dependent activation of AKT (Fig. 4). This pathway is essential, since dominant-negative AKT prevented the antianoikis action of MSP (Fig. 5A). The role of activation of AKT in MSP-dependent prevention of anoikis is a new finding. In a different model, transfection with an activated ras oncogene made MDCK cells resistant to anoikis via the PI3-K/AKT pathway, which was necessary and sufficient to mediate the protective effect (45). Thus, PI3-K/AKT is a common pathway for anoikis prevention, in which PI3-K is activated by direct interaction with a growth factor receptor in one case and by ras in the other.
Despite activation by MSP of FAK, c-Src, and MAPK, these signals were not involved in the antianoikis action of MSP (Fig. 3). However, FAK—and possibly all three of these mediators—is involved in protection from apoptosis when normal epithelial cells are attached to ECM via integrins (28). Taking protection via the PI3-K/AKT pathway as an example, there are two routes leading to its activation, one via a growth factor like MSP and the other via integrin ligation. In the case of MSP, activation of PI3-K is direct, since this enzyme is bound to ligated RON and concomitantly activated. Activation of PI3-K subsequent to integrin ligation also occurs. Candidate pathways are initiated either by FAK phosphorylation or by association of the adapter protein Shc with ligated integrin, which can lead to PI3-K activation via Ras.
In addition to studying anoikis, we studied apoptosis of adherent epithelial cells under serum-free conditions. In the absence of added growth factors, two independent pathways operate to prevent apoptosis of adherent, serum-free epithelial cells. One pathway involves PI3-K/AKT and the other includes MAPK. The number of apoptotic cells resulting from inhibition of either pathway alone was approximately doubled by simultaneous inhibition of both pathways (Fig. 6A). This shows that each pathway makes a partial contribution to the prevention of apoptosis. In the presence of an inhibitor of either pathway, MSP increased the activity of the other pathway so that the single pathway alone was sufficient to prevent apoptosis. Although sufficiently high concentrations of LY294002 may inhibit kinases in addition to PI-3K (39, 65), the similarity of the effects of dominant-negative AKT and low concentrations of LY294002 in these experiments is consistent with specific inhibition of PI3-K.
The presence of independent PI3-K and MAPK antiapoptotic pathways has recently been shown for HeLa cells. Addition of a MAPK inhibitor to cells in serum-free medium resulted in phosphorylation and activation of the proapoptotic kinase p38. This was prevented by serum, and the protective effect of serum was mediated by the PI3-K/AKT pathway (5). Other recent publications suggesting more than one pathway for prevention of apoptosis include data on the response to IGF-1 of UV-irradiated fibroblasts (46), the protection of neurons from apoptosis by NGF (60), and IGF-1-mediated survival of brown adipocytes (56).
MSP activates MAPK in both suspended and adherent cells. Despite activation of MAPK in MSP-stimulated suspended cells (Fig. 3C), MAPK mediates MSP antiapoptotic effects only on adherent cells (Fig. 3D and 6). This suggests that adhesion to uncoated plastic induces a signal that enables activated MAPK to protect against apoptosis. Our view is that cell adhesion initiates the required cooperating signals, which can be adherence to tissue culture plastic or in vivo adherence to ECM. Disruption of the cortical actin network that maintains the spread morphology of cells interfered with adherence-dependent enhancement of growth factor signaling to MAPK (3, 41).
Just as we have shown that a cell adhesion signal is required for prevention of apoptosis via the MAPK pathway, Le Gall et al. reported that an anchorage-dependent signal in addition to MAPK was required for cell cycle progression (48). It is becoming evident that signals from adhesion receptors can affect both proliferative and apoptotic responses. Both growth factors and anchorage are required for transition from the G0 to S phase of the cell cycle by controlling D1 cyclin induction (4, 32, 48). Prevention of cell adhesion leads to a decrease of cyclin-dependent kinase activity and accumulation of the active form of retinoblastoma (Rb) protein. Active Rb suppresses the transcription factor E2F, which leads to apoptosis of selected cell lines (17). Thus, our data together with data in the literature show that growth factors and adhesion-activated pathways cooperate in the control not only of cell growth and differentiation but also of cell survival. Adhesion-dependent pathways that promote MAPK translocation remain to be identified.
ACKNOWLEDGMENTS
We thank S. Yuspa (National Cancer Institute), R. Schlegel (Georgetown University), and N. Fusenig (Heidelberg, Germany) for donations of keratinocyte cell lines; D. Schlaepfer (Scripps Research Institute, La Jolla, Calif.) for donation of the mouse FAK mutant cDNAs; P. Schwarzberg (NIH, Bethesda, Md.) for the chicken c-Src mutant cDNAs; K. Datta (Fox Chase Cancer Center, Philadelphia, Pa.) for the AKT kinase-dead cDNA mutant; and A. Miagkov (Johns Hopkins University, Baltimore, Md.) for his help in figure preparation.
REFERENCES
- 1.Alessi D R, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 2.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 3.Aplin A E, Juliano R L. Integrin and cytoskeletal regulation of growth factor signaling to the MAP kinase pathway. J Cell Sci. 1999;112:695–706. doi: 10.1242/jcs.112.5.695. [DOI] [PubMed] [Google Scholar]
- 4.Assoian R K, Zhu X. Cell anchorage and the cytoskeleton as partners in growth factor dependent cell cycle progression. Curr Opin Cell Biol. 1997;9:93–98. doi: 10.1016/s0955-0674(97)80157-3. [DOI] [PubMed] [Google Scholar]
- 5.Berra E, Diaz-Meco M T, Moscat J. The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J Biol Chem. 1998;273:10792–10797. doi: 10.1074/jbc.273.17.10792. . (Erratum, 273:16630.) [DOI] [PubMed] [Google Scholar]
- 6.Blume-Jensen P, Janknecht R, Hunter T. The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr Biol. 1998;8:779–782. doi: 10.1016/s0960-9822(98)70302-1. [DOI] [PubMed] [Google Scholar]
- 7.Boudreau N, Sympson C J, Werb Z, Bissell M J. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozzo C, Bellomo G, Silengo L, Tarone G, Altruda F. Soluble integrin ligands and growth factors independently rescue neuroblastoma cells from apoptosis under nonadherent conditions. Exp Cell Res. 1997;237:326–337. doi: 10.1006/excr.1997.3777. [DOI] [PubMed] [Google Scholar]
- 9.Clark E A, Brugge J S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 10.Cooper J A, Esch F S, Taylor S S, Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984;259:7835–7841. [PubMed] [Google Scholar]
- 11.Coucouvanis E, Martin G R. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 12.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 13.Danilkovitch A, Leonard E J. Kinases involved in MSP/RON signaling. J Leukoc Biol. 1999;65:345–348. doi: 10.1002/jlb.65.3.345. [DOI] [PubMed] [Google Scholar]
- 14.Danilkovitch A, Skeel A, Leonard E J. Macrophage stimulating protein-induced epithelial cell adhesion is mediated by a PI3-K dependent but FAK independent mechanism. Exp Cell Res. 1999;248:575–582. doi: 10.1006/excr.1999.4429. [DOI] [PubMed] [Google Scholar]
- 15.Darmon A J, Nicholson D W, Bleackley R C. Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature. 1995;377:446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]
- 16.Datta K, Franke T F, Chan T O, Makris A, Yang S I, Kaplan D R, Morrison D K, Golemis E A, Tsichlis P N. AH/PH domain-mediated interaction between Akt molecules and its potential role in Akt regulation. Mol Cell Biol. 1995;15:2304–2310. doi: 10.1128/mcb.15.4.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day M L, Foster R G, Day K C, Zhao X, Humphrey P, Swanson P, Postigo A A, Zhang S H, Dean D C. Cell anchorage regulates apoptosis through the retinoblastoma tumor suppressor/E2F pathway. J Biol Chem. 1997;272:8125–8128. doi: 10.1074/jbc.272.13.8125. [DOI] [PubMed] [Google Scholar]
- 18.Degen S J, Stuart L A, Han S, Jamison C S. Characterization of the mouse cDNA and gene coding for a hepatocyte growth factor-like protein: expression during development. Biochemistry. 1991;30:9781–9791. doi: 10.1021/bi00104a030. [DOI] [PubMed] [Google Scholar]
- 19.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 20.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 21.Eves E M, Xiong W, Bellacosa A, Kennedy S G, Tsichlis P N, Rosner M R, Hay N. Akt, a target of phosphatidylinositol 3-kinase, inhibits apoptosis in a differentiating neuronal cell line. Mol Cell Biol. 1998;18:2143–2152. doi: 10.1128/mcb.18.4.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan S, Wang J A, Yuan R Q, Rockwell S, Andres J, Zlatapolskiy A, Goldberg I D, Rosen E M. Scatter factor protects epithelial and carcinoma cells against apoptosis induced by DNA-damaging agents. Oncogene. 1998;17:131–141. doi: 10.1038/sj.onc.1201943. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes-Alnemri T, Litwack G, Alnemri E S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994;269:30761–30764. [PubMed] [Google Scholar]
- 24.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 25.Franke T F, Kaplan D R, Cantley L C. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 26.Freedman V H, Shin S I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974;3:355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- 27.Frisch S M, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frisch S M, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 29.Frisch S M, Vuori K, Ruoslahti E, Chan-Hui P Y. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner A M, Johnson G L. Fibroblast growth factor-2 suppression of tumor necrosis factor alpha-mediated apoptosis requires Ras and the activation of mitogen-activated protein kinase. J Biol Chem. 1996;271:14560–14566. doi: 10.1074/jbc.271.24.14560. [DOI] [PubMed] [Google Scholar]
- 31.Gaudino G, Follenzi A, Naldini L, Collesi C, Santoro M, Gallo K A, Godowski P J, Comoglio P M. Ron is a heterodimeric tyrosine kinase receptor activated by the HGF homologue MSP. EMBO J. 1994;13:3524–3532. doi: 10.1002/j.1460-2075.1994.tb06659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giancotti F G. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- 33.Guillonneau X, Bryckaert M, Launay-Longo C, Courtois Y, Mascarelli F. Endogenous FGF1-induced activation and synthesis of extracellular signal-regulated kinase 2 reduce cell apoptosis in retinal-pigmented epithelial cells. J Biol Chem. 1998;273:22367–22373. doi: 10.1074/jbc.273.35.22367. [DOI] [PubMed] [Google Scholar]
- 34.Hall P A, Coates P J, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 35.Hockenbery D. Defining apoptosis. Am J Pathol. 1995;146:16–19. [PMC free article] [PubMed] [Google Scholar]
- 36.Hungerford J E, Compton M T, Matter M L, Hoffstrom B G, Otey C A. Inhibition of pp125FAK in cultured fibroblasts results in apoptosis. J Cell Biol. 1996;135:1383–1390. doi: 10.1083/jcb.135.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 38.Iwama A, Okano K, Sudo T, Matsuda Y, Suda T. Molecular cloning of a novel receptor tyrosine kinase gene, STK, derived from enriched hematopoietic stem cells. Blood. 1994;83:3160–3169. [PubMed] [Google Scholar]
- 39.Izzard R A, Jackson S P, Smith G C. Competitive and noncompetitive inhibition of the DNA-dependent protein kinase. Cancer Res. 1999;59:2581–2586. [PubMed] [Google Scholar]
- 40.Joseph-Silverstein J, Silverstein R L. Cell adhesion molecules: an overview. Cancer Investig. 1998;16:176–182. doi: 10.3109/07357909809050034. [DOI] [PubMed] [Google Scholar]
- 41.Juliano R. Cooperation between soluble factors and integrin-mediated cell anchorage in the control of cell growth and differentiation. Bioessays. 1996;18:911–917. doi: 10.1002/bies.950181110. [DOI] [PubMed] [Google Scholar]
- 42.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 44.Khwaja A, Downward J. Lack of correlation between activation of Jun-NH2-terminal kinase and induction of apoptosis after detachment of epithelial cells. J Cell Biol. 1997;139:1017–1023. doi: 10.1083/jcb.139.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulik G, Weber M J. Akt-dependent and -independent survival signaling pathways utilized by insulin-like growth factor I. Mol Cell Biol. 1998;18:6711–6718. doi: 10.1128/mcb.18.11.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar C C. Signaling by integrin receptors. Oncogene. 1998;17:1365–1373. doi: 10.1038/sj.onc.1202172. [DOI] [PubMed] [Google Scholar]
- 48.Le Gall M, Grall D, Chambard J C, Pouyssegur J, Van Obberghen-Schilling E. An anchorage-dependent signal distinct from p42/44 MAP kinase activation is required for cell cycle progression. Oncogene. 1998;17:1271–1277. doi: 10.1038/sj.onc.1202057. [DOI] [PubMed] [Google Scholar]
- 49.Leonard E J, Skeel A. A serum protein that stimulates macrophage movement, chemotaxis and spreading. Exp Cell Res. 1976;102:434–438. doi: 10.1016/0014-4827(76)90065-3. [DOI] [PubMed] [Google Scholar]
- 50.Leonard E J, Skeel A H. Isolation of macrophage stimulating protein (MSP) from human serum. Exp Cell Res. 1978;114:117–126. doi: 10.1016/0014-4827(78)90043-5. [DOI] [PubMed] [Google Scholar]
- 51.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 52.McGill G, Shimamura A, Bates R C, Savage R E, Fisher D E. Loss of matrix adhesion triggers rapid transformation-selective apoptosis in fibroblasts. J Cell Biol. 1997;138:901–911. doi: 10.1083/jcb.138.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meredith J E J, Fazeli B, Schwartz M A. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meredith J E J, Schwartz M A. Integrins, adhesion and apoptosis. Trends Cell Biol. 1997;7:146–150. doi: 10.1016/S0962-8924(97)01002-7. [DOI] [PubMed] [Google Scholar]
- 55.Moro L, Venturino M, Bozzo C, Silengo L, Altruda F, Beguinot L, Tarone G, Defilippi P. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 1998;17:6622–6632. doi: 10.1093/emboj/17.22.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Navarro P, Valverde A M, Benito M, Lorenzo M. Insulin/IGF-I rescues immortalized brown adipocytes from apoptosis down-regulating Bcl-xS expression, in a PI 3-kinase- and map kinase-dependent manner. Exp Cell Res. 1998;243:213–221. doi: 10.1006/excr.1998.4168. [DOI] [PubMed] [Google Scholar]
- 57.Nunez G, del Peso L. Linking extracellular survival signals and the apoptotic machinery. Curr Opin Neurobiol. 1998;8:613–618. doi: 10.1016/s0959-4388(98)80089-5. [DOI] [PubMed] [Google Scholar]
- 58.Otey C A. pp125FAK in the focal adhesion. Int Rev Cytol. 1996;167:161–183. doi: 10.1016/s0074-7696(08)61347-9. [DOI] [PubMed] [Google Scholar]
- 59.Parrizas M, Saltiel A R, LeRoith D. Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. J Biol Chem. 1997;272:154–161. doi: 10.1074/jbc.272.1.154. [DOI] [PubMed] [Google Scholar]
- 60.Philpott K L, McCarthy M J, Klippel A, Rubin L L. Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J Cell Biol. 1997;139:809–815. doi: 10.1083/jcb.139.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plopper G E, McNamee H P, Dike L E, Bojanowski K, Ingber D E. Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell. 1995;6:1349–1365. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Polakowska R R, Piacentini M, Bartlett R, Goldsmith L A, Haake A R. Apoptosis in human skin development: morphogenesis, periderm, and stem cells. Dev Dyn. 1994;199:176–188. doi: 10.1002/aja.1001990303. [DOI] [PubMed] [Google Scholar]
- 63.Powis G, Bonjouklian R, Berggren M M, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter W F, Dodge J, Grindey G. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- 64.Ronsin C, Muscatelli F, Mattei M G, Breathnach R. A novel putative receptor protein tyrosine kinase of the met family. Oncogene. 1993;8:1195–1202. [PubMed] [Google Scholar]
- 65.Rosenzweig K E, Youmell M B, Palayoor S T, Price B D. Radiosensitization of human tumor cells by the phosphatidylinositol3-kinase inhibitors wortmannin and LY294002 correlates with inhibition of DNA-dependent protein kinase and prolonged G2-M delay. Clin Cancer Res. 1997;3:1149–1156. [PubMed] [Google Scholar]
- 66.Ruoslahti E, Reed J C. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 67.Sanchez-Margalet V, Goldfine I D, Vlahos C J, Sung C K. Role of phosphatidylinositol-3-kinase in insulin receptor signaling: studies with inhibitor, LY294002. Biochem Biophys Res Commun. 1994;204:446–452. doi: 10.1006/bbrc.1994.2480. [DOI] [PubMed] [Google Scholar]
- 68.Schaller M D, Otey C A, Hildebrand J D, Parsons J T. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scheid M P, Duronio V. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/akt: involvement of MEK upstream of Bad phosphorylation. Proc Natl Acad Sci USA. 1998;95:7439–7444. doi: 10.1073/pnas.95.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schlaepfer D D, Hunter T. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem. 1997;272:13189–13195. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- 71.Schwartz M A. Integrins, oncogenes, and anchorage independence. J Cell Biol. 1997;139:575–578. doi: 10.1083/jcb.139.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seger R, Krebs E G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 73.Songyang Z, Baltimore D, Cantley L C, Kaplan D R, Franke T F. Interleukin 3-dependent survival by the Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:11345–11350. doi: 10.1073/pnas.94.21.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stoker M, O'Neill C, Berryman S, Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer. 1968;3:683–693. doi: 10.1002/ijc.2910030517. [DOI] [PubMed] [Google Scholar]
- 75.Ui M, Okada T, Hazeki K, Hazeki O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem Sci. 1995;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- 76.Ulrich E, Duwel A, Kauffmann-Zeh A, Gilbert C, Lyon D, Rudkin B, Evan G, Martin-Zanca D. Specific TrkA survival signals interfere with different apoptotic pathways. Oncogene. 1998;16:825–832. doi: 10.1038/sj.onc.1201842. [DOI] [PubMed] [Google Scholar]
- 77.Valentinis B, Reiss K, Baserga R. Insulin-like growth factor-I-mediated survival from anoikis: role of cell aggregation and focal adhesion kinase. J Cell Physiol. 1998;176:648–657. doi: 10.1002/(SICI)1097-4652(199809)176:3<648::AID-JCP22>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 78.Vlahos C J, Matter W F, Hui K Y, Brown R F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 79.Wang M-H, Iwama A, Dlugosz A A, Sun Y, Skeel A, Yuspa S H, Suda T, Leonard E J. Macrophage stimulating protein induces proliferation and migration of murine keratinocytes. Exp Cell Res. 1996;226:39–46. doi: 10.1006/excr.1996.0200. [DOI] [PubMed] [Google Scholar]
- 80.Wang M-H, Iwama A, Skeel A, Suda T, Leonard E J. The murine stk gene product, a transmembrane protein tyrosine kinase, is a receptor for macrophage stimulating protein. Proc Natl Acad Sci USA. 1995;92:3933–3937. doi: 10.1073/pnas.92.9.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang M-H, Montero-Julian F A, Dauny I, Leonard E J. Requirement of phosphatidylinositol-3 kinase for epithelial cell migration activated by human macrophage stimulating protein. Oncogene. 1996;13:2167–2175. [PubMed] [Google Scholar]
- 82.Wang M-H, Ronsin C, Gesnel M-C, Coupey L, Skeel A, Leonard E J, Breathnach R. Identification of the ron gene product as the receptor for the human macrophage stimulating protein. Science. 1994;266:117–119. doi: 10.1126/science.7939629. [DOI] [PubMed] [Google Scholar]
- 83.Wary K K, Mainiero F, Isakoff S J, Marcantonio E E, Giancotti F G. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- 84.Werner H, Le Roith D. The insulin-like growth factor-I receptor signaling pathways are important for tumorigenesis and inhibition of apoptosis. Crit Rev Oncog. 1997;8:71–92. doi: 10.1615/critrevoncog.v8.i1.40. [DOI] [PubMed] [Google Scholar]
- 85.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 86.Xu L H, Owens L V, Sturge G C, Yang X, Liu E T, Craven R J, Cance W G. Attenuation of the expression of the focal adhesion kinase induces apoptosis in tumor cells. Cell Growth Differ. 1996;7:413–418. [PubMed] [Google Scholar]
- 87.Yao R, Cooper G M. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 88.Yoshimura T, Yuhki N, Wang M-H, Skeel A, Leonard E J. Cloning, sequencing and expression of human macrophage stimulating protein (MSP) confirms MSP as a kringle protein, and locates the gene on chromosome 3. J Biol Chem. 1993;268:15461–15468. [PubMed] [Google Scholar]
- 89.Yuspa S H, Hawley-Nelson P, Koehler B, Stanley J R. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 1980;40:4694–4703. [PubMed] [Google Scholar]