Abstract
Continuous glucose monitoring is valuable for people with diabetes but faces limitations due to enzyme–electrode interactions and biofouling from biological samples that reduce sensor sensitivity and the monitoring performance. We created an enzyme-based electrochemical system with a unique nanocomposite coating that incorporates the redox molecule, aminoferrocene (NH2-Fc). This coating enhances stability via electroactivity and reduces nonspecific binding, as demonstrated through cyclic voltammetry. Our approach enables real-time glucose detection via chronoamperometry with a calculated linear range of 0.5 to 20 mM and a 1 mM detection limit. Validated with plasma and saliva, this platform shows promise for robust metabolite detection in clinical and research contexts. This versatile platform can be applied to accurately monitor a wide range of metabolites in various biological matrices, improving patient outcomes.
Keywords: antifouling, glucose biosensor, enzymatic, continuous monitoring, aminoferrocene
1. Introduction
Diabetes prevalence has drastically increased worldwide, with more than half a billion people affected by this condition [1]. The current prevalence rate of 6.1% places diabetes as one of the leading causes of death globally [1,2], and it is considered a significant contributor to other chronic diseases, including heart attack, stroke, and kidney disease [2,3]. Elevated levels of glucose in the blood, if not recognized early, are extremely life threatening, yet 23% of adults living with diabetes remain undiagnosed in North America [4]. Therefore, the accurate, reliable, and timely monitoring of glucose from human samples (e.g., saliva, plasma, blood, etc.) is essential for the prevention and management of diabetes and its related complications. Although many glucose biosensors based on different transduction principles have been developed, electrochemical-based glucose biosensors utilizing enzymatic sensing currently dominate the market [3,5,6]. These devices harness the inherent electrochemical properties (e.g., reduction or oxidation capabilities) of metabolites, such as glucose, to develop systems that can rapidly quantify the target analyte in complex biological fluids with high reliability. Most glucose biosensors rely on the enzymatic oxidation of glucose mainly due to the high selectivity and stability of glucose oxidase (GluOx) [6,7,8], because this results in better performances in biological fluids and a longer shelf life than those of other technologies.
Currently, the rapid quantification of glucose levels from finger-prick blood samples is achieved using a glucometer, a handheld biosensing electrochemical device that enables regular monitoring at home. However, several factors, such as deteriorated test strips, reader’s imprecision, improper storage, interference from multiple medications, and environmental conditions, may impact the reliability of these blood glucose measurements [9,10,11,12]. In addition, the sample site may become painful, and wounds from finger-pricks are at risk of infection due to the requirement for repeated testing [3,12]. Also, these devices utilize a single-point measurement mode that is not practical for continuous monitoring, which is required for the optimal detection of hypo/hyperglycemic levels and improved glycemic control in diabetes patients [10,11]. This is important because glucose levels in blood can be influenced by factors of intrinsic nature (biochemical reactions) or exogenous origin (diet or medication).
Continuous glucose monitors (CGMs) that relay glucose levels in real-time have been developed to overcome these limitations. CGMs can be life changing as they facilitate more personalized insight into glucose level changes during the day, offering patients the power to make more informed treatment decisions and prevent episodes of hypo/hyperglycemia [11,13]. Although many companies have successfully created and commercialized several CGMs, their application for long-term monitoring, to date, remains restricted due to inaccuracy at low glucose concentrations, high costs, the short duration of single implantation, and biocompatibility issues [13,14].
One of the key challenges to obtaining accurate and sensitive real-time measurements of glucose concentrations in complex biological fluids results from the non-specific binding of various biomolecules found in the sample matrix, which can interfere with specific signal detection. For example, biosensors exposed to physiological environments for extended periods may exhibit a high background current or signal drift that can compromise the detection sensitivity and analytical accuracy [12]. This is because these sensors are constructed using materials that are prone to passivation by fouling agents (i.e., proteins, amino acids, lipids, etc.) that form an impermeable layer on the electrode surface, affecting charge transfer kinetics between the target analyte and the electrode surface [15]. This effect discourages the application of biosensors for continuous monitoring since fouling degrades the sensor surface, resulting in a low signal-to-noise ratio and reduced sensitivity [12,15]. Therefore, it is critical to develop methods to address biofouling-related problems to continuously monitor metabolites, such as glucose, in real time over extended periods.
We have previously addressed the crucial challenge of overcoming signal interference due to biofouling by developing a novel nanocomposite coating based on the glutaraldehyde (GA) crosslinking of conductive nanomaterials, such as reduced graphene oxide (prGOx), dissolved with bovine serum albumin (BSA) in PBS [16]. This nanocomposite formulation not only reduces fouling by creating a more hydrophilic surface at the electrode/electrolyte interface, preventing non-specific adsorption, but also offers enhanced electroconductive properties promoting their electrochemical performance in biological fluids. The prGOx in the nanocomposite formulation serves as a conductive material that facilitates electron transfer across the nanocomposite coating to the underlying electrode; it also provides functional groups required for facile surface modification. We successfully demonstrated the potential of this antifouling coating on an electrochemical sensor for single-point measurements of various biomolecules, including IL-6, PCT, CRP, SARS-CoV NS1, GFAP, and NFL in different biological matrices [17,18,19]. In the present study, we demonstrated that a prGOx/BSA/GA nanocomposite coating can be used to construct a glucose biosensor that addresses the electrode fouling challenges observed in enzyme-based continuous monitoring systems.
In label-based electrochemical sensors, detection is usually achieved through the diffusion of redox molecules, such as potassium ferro/ferricyanide, from electrolyte to electrode surfaces [20]. Although these biosensors are robust and reliable, sometimes redox probes can contaminate electrode systems, giving rise to non-specific signals, which could be overcome by functionalizing a stable redox-active functional molecule, such as a ferrocene derivative [21,22]. Specific recognition between the capture molecule and target analyte will impede the charge transfer between the immobilized redox probe and electrode surface, which can be leveraged to analyze the biosensor performance. Several research groups have developed biosensing platforms modified with redox mediators to detect various analytes including glucose [21,22], enzymes, proteins, and nucleic acids [23]. However, these platforms do not address the challenges related to biofouling that must be eliminated to translate these devices toward successful commercialization.
Here, we explored whether the functionalization of the prGOx/BSA/GA nanocomposite coating with a redox-based molecule that has low redox potential and good electrochemical stability can be used to engineer a multifaceted biosensing platform for the continuous monitoring of glucose in complex biological fluids. Our results describe the successful fabrication of a redox-embedded nanocomposite sensor and its application for glucose detection in two clinically relevant biological matrices: saliva and plasma. To accomplish this, we covalently coupled the redox molecule, aminoferrocene (NH2-Fc; a ferrocene derivative with NH2 substitution) to prGOx/BSA/GA. NH2-Fc undergoes reversible oxidation and reduction reactions (Fc ↔ Fc+) and exhibits good electrochemical behavior that favors a reagentless determination of target analytes. In our platform, the NH2-Fc efficiently mediates the enzymatic oxidation of glucose via immobilized glucose oxidase and retains the activity of an enzyme-immobilized glucose biosensor. Also, the low redox potential (~0.2 V) of NH2-Fc reduces the background signal from other interfering electroactive species present in biological samples. Through rigorous experimental evaluations and comparative analyses, we demonstrate, for the first time, the potential of a NH2-Fc functionalized prGOx/BSA/GA nanocomposite-coated electrochemical sensor for the continuous monitoring of glucose with high sensitivity and selectivity.
2. Materials and Methods
2.1. Nanocomposite Deposition
Screen-printed carbon electrodes (SPCEs) (DropSens, Metrohm, Riverview, FL, USA) were cleaned with ethanol (Sigma Aldrich, Burlington, MA, USA), polished with Kimwipes, and washed with deionized water. The SPCEs were dried, and oxygen plasma-treated at 0.5 mbar and 50% power for 8 min (Zepto Diener Plasma, Diener Electronics, Ebhausen, Germany). An antifouling nanocomposite coating consisting of pentaamine-functionalized reduced graphene oxide (prGOx; Sigma Aldrich, Burlington, MA, USA) and bovine serum albumin (BSA; IgG-Free, Protease-Free; Jackson ImmunoResearch, West Grove, PA, USA) was prepared in 10 mM phosphate-buffered saline (PBS, pH 7.4) as reported in our previous work [16]. Briefly, the prepared solution was subjected to tip sonication with a 1 s ON/OFF pulse at 50% amplitude for 30 min (Q125, QSonica LLC, Newtown, CT, USA), followed by heating at 105 °C for 5 min. The protein-denatured solution was centrifuged (Centrifuge 5418R, Eppendorf, Framingham, MA, USA) at 16.2 rcf for 15 min to remove excess prGOx/BSA aggregates. A mixture of the prGOx/BSA supernatant solution and 70% glutaraldehyde (GA; Sigma Aldrich, Burlington, MA, USA) was prepared in the ratio of 69:1, applied over the plasma-treated SPCEs, and incubated overnight in a humidity chamber. The prGOx/BSA/GA nanocomposite-deposited SPCEs were washed the following day with PBS on a shaker at 500 rpm for 10 min and dried using a slide spinner.
2.2. Fabrication of the Redox-Functionalized Glucose Sensor
The surfaces of the nanocomposite-deposited SPCEs were activated using carbodiimide chemistry, wherein 400 mM of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC; Thermo Fisher Scientific, Waltham, MA, USA) and 200 mM of N-Hydroxysuccinimide (NHS; Sigma Aldrich, Burlington, MA, USA) dissolved in 50 mM 2-(N-morpholino)ethanesulfonic acid (MES; Thermo Fisher Scientific, Waltham, MA, USA) buffer at pH 6.2 were added to the sensor and incubated for 30 min at room temperature. The SPCEs were then rinsed with MilliQ water and dried before the addition of redox molecules. Aminoferrocene (NH2-Fc; Sigma Aldrich, Burlington, MA, USA) dissolved in PBS at a 1 mg/mL concentration was added to the working electrode surface and incubated for 45 min, and then rinsed with PBS via agitation at 500 rpm for 5 min and dried. Following the functionalization of NH2-Fc, the working electrodes were selectively spotted with 2 μL of an enzymatic solution obtained by mixing glucose oxidase at 80 mg/mL with GA in a 1:1 v/v ratio. The glucose oxidase-immobilized SPCEs were dried overnight at 4 °C to let the enzyme composition adhere to the prGOx/BSA/GA nanocomposite-coated surfaces. When the electrode surface was completely dry, it was washed gently with PBS and kept stored at 4 °C until use.
2.3. Electrochemical Measurements
All electrochemical measurements were carried out on an Autolab PGSTAT128N potentiostat (Metrohm, Riverview, FL, USA). Electrochemical impedance spectroscopy (EIS) was used to characterize the interfacial properties of the electrode surface modification at each step of the glucose biosensor fabrication. Cyclic voltammetry (CV) was used to electrochemically characterize the NH2-Fc-functionalized prGOx/BSA/GA nanocomposite coating and to determine the oxidation/reduction potential of NH2-Fc. The potential for chronoamperometry measurements used for glucose quantification was obtained from CV measurements. Calibration curves were built for glucose concentrations between 0 mM and 50 mM based on chronoamperometry.
2.4. Sample Collection
In this study, saliva and plasma samples were collected from a volunteer following ethical guidelines and with informed consent. The saliva samples were obtained using passive drool collection, while the plasma samples were collected through venipuncture and processed to obtain the plasma fraction. Both the saliva and plasma samples were stored at −80 °C. Prior to electrochemical analysis, the samples were thawed at room temperature and thoroughly mixed. The samples were tested both in their natural state and spiked with glucose, each undergoing three tests for accuracy and reliability.
3. Results
3.1. Fabrication of the Nanocomposite Interface with Confined Redox Probes
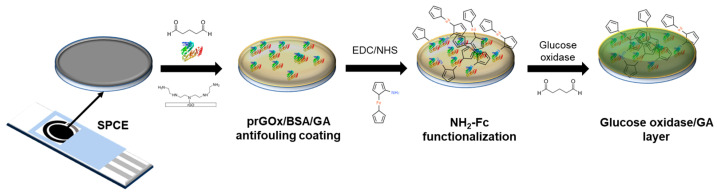
The NH2-Fc-functionalized glucose biosensor interface was fabricated in three steps (Figure 1) with electrochemical characterization performed after each step (Figure S1). First, the prGOx/BSA/GA nanocomposite coating was drop-cast onto the SPCE surface. The conductive material (prGOx) facilitates electron transfer and improves the electroactive surface area, while the BSA cross-linked with GA provides structural stability and prevents biofouling. Second, the redox mediator, NH2-Fc, was covalently functionalized onto the nanocomposite coating through EDC/NHS chemistry. The carbonyl carbon of the NHS ester group undergoes nucleophilic substitution on reaction with the amine group of NH2-Fc derivative, resulting in a stable amide bond. Finally, an enzyme layer was coated onto the NH2-Fc modified SPCE by drop-casting a solution containing glucose oxidase and GA, and then dried. The GA-crosslinked enzyme layer offers selective permeability for glucose and provides long-term stability for glucose oxidase.
Figure 1.
Schematic representing the steps involved in the fabrication of glucose biosensors. The sensor was constructed sequentially with a nanocomposite-based antifouling coating, surface-functionalized redox, and enzyme layers that support glucose detection in biological samples.
3.2. Electrochemical Characterization of the Redox Immobilized Glucose Sensor
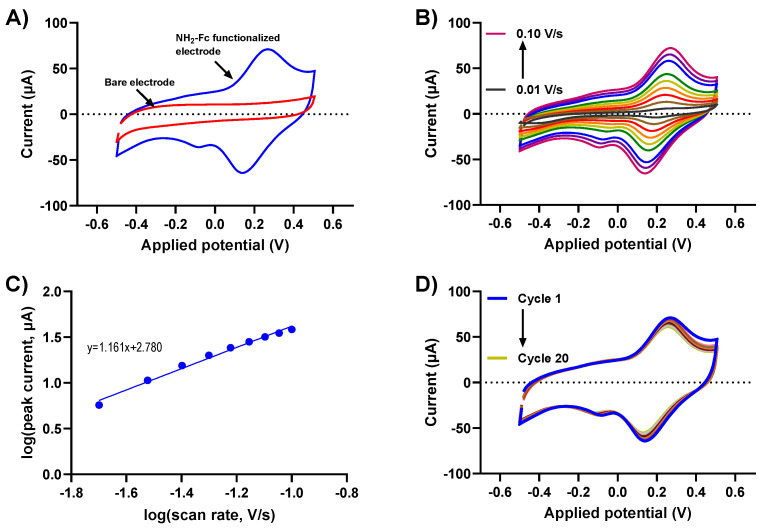
Understanding the charge transfer kinetics of NH2-Fc-functionalized electrode surfaces is crucial to determining their electrochemical performance. Biomolecular binding interactions at the electrode/electrolyte interfaces alter the redox-based charge transfer processes, which can be utilized to design an efficient electrochemical biosensor for various applications. Here, the redox behaviors of the NH2-Fc-modified and bare prGOx/BSA/GA nanocomposite-coated SPCEs were obtained using CV measurements in PBS. The CV of NH2-Fc-modified SPCEs revealed a defined quasi-reversible oxidation and reduction peak at 0.23 V and 0.15 V, respectively, with a peak separation of 80mV. In contrast, the bare nanocomposite-coated SPCE (Figure 2A) showed no well-defined peaks. This is indicative of the oxidation and reduction of an Fc derivative in NH2-Fc during the electrochemical processes, and the obtained peaks were consistent with the redox potential of NH2-Fc, confirming the successful functionalization of NH2-Fc onto the prGOx/BSA/GA nanocomposite coating.
Figure 2.
Electrochemical performance of NH2-Fc-functionalized prGOx/BSA/GA in PBS. (A) Comparison of CV responses for bare and NH2-Fc-functionalized nanocomposite-coated SPCEs. (B) CV of NH2-Fc-functionalized SPCEs measured in PBS at different scan rates. (C) Plot demonstrating a linear relationship between logarithm of peak current vs. logarithm of scan rate. (D) Cyclic voltammogram recorded in PBS for 20 cycles showing electrochemical stability of NH2-Fc-functionalized prGOx/BSA/GA coating.
The electrochemical oxidation and reduction of NH2-Fc functionalization were further evaluated with scan rates ranging from 10 mV/s to 100 mV/s. The voltammograms show a characteristic CV curve with increasing redox peak currents for NH2-Fc with an increasing scan rate (Figure 2B). The plot for the log of the scan rate versus the log of the redox peak current exhibited a linear relationship with a slope of 1.1, which is strongly indicative of NH2-Fc functionalization contributing toward surface-controlled electrochemical behavior (the expected slope is 1.0) (Figure 2C).
To further elucidate the stability of NH2-Fc functionalization, the prepared prGOx/BSA/GA nanocomposite-coated sensor was subjected to CV measurements in PBS by cycling the potential between −0.5 V and +0.5 V at 100 mV/s for 20 cycles. No significant change in peak current with increasing scan cycles was observed (Figure 2D), confirming the long-term electrochemical stability of the NH2-Fc-functionalized prGOx/BSA/GA nanocomposite coating.
3.3. Glucose Detection in a Standard Solution
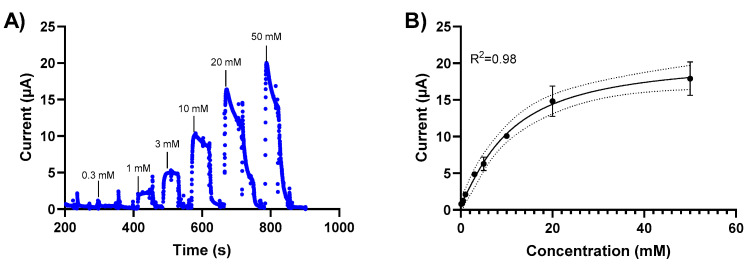
The sensor response to glucose was measured for glucose concentrations between 0.1 mM and 50 mM using chronoamperometry. Initially, we investigated chronoamperometry measurements at different potentials (0, 0.2 V) to determine the signal sensitivity. We subsequently performed glucose detection measurements using chronoamperometry at 0.2V based on these results. Changes in the current response of the NH2-Fc-functionalized prGOx/BSA/GA sensor were measured when exposed to varying glucose concentrations in PBS (Figure 3). A calibration curve was generated by plotting the average of the current response 20 sec after glucose exposure. The sensor exhibits a linear behavior between 0 mM and 20 mM glucose concentrations with a detection limit of 1 mM (Figure 3B) and stable signal response with <10% signal loss over 60 min (Figure S2).
Figure 3.
Glucose level-dependent changes in the current response of the NH2-Fc-functionalized prGOx/BSA/GA sensor. (A) Chronoamperometric response of the NH2-Fc-functionalized prGOx/BSA/GA sensor at 0.2 V in PBS obtained with the successive addition of glucose from 0 to 50 mM. (B) Calibration dose–response curve derived from the amperometric signal response. The graph shows a linear response for glucose concentrations between 0 and 20 mM and the saturation for concentrations higher than 20 mM of glucose. Data represented here are from n = 2 replicates.
3.4. Specificity and Selectivity
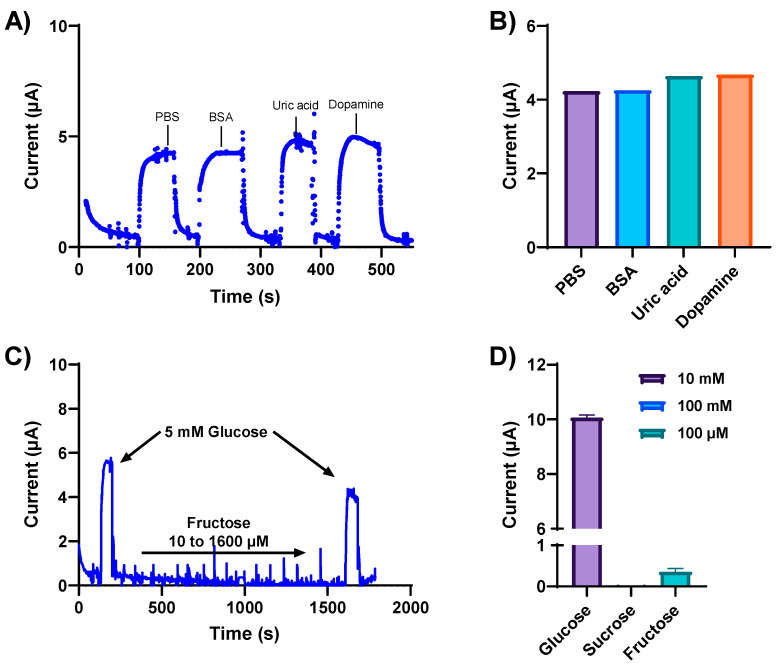
Biological fluids contain a plethora of metabolites and electrolytes that could potentially interfere with glucose measurements, giving rise to non-specific signal responses. Therefore, the specificity and selectivity of our developed glucose sensor were determined in the presence of various interfering electroactive constituents. The NH2-Fc-functionalized glucose sensor was first exposed to physiologically relevant concentrations of potentially interfering substances, such as BSA (2.5 mg/mL), uric acid (1 mM), and dopamine (10 μM), separately spiked in a 5 mM glucose solution. The NH2-Fc-functionalized glucose sensor responded selectively toward glucose detection compared to other interferents (Figure 4A). Metabolites such as uric acid and dopamine have positive redox potentials; however, no change in the electrochemical signal response was observed with these potential interferents (Figure 4A,B), and the current response remained almost the same as the test solution spiked with only glucose. This indicates that there is no electrochemical interference from these metabolites, and the electrochemical signal we obtained is from the oxidation/reduction of an Fc derivative NH2-Fc immobilized on the antifouling coating.
Figure 4.
Specificity and selectivity of the glucose sensor were determined in the presence of various interfering electroactive constituents. (A) Chronoamperometric response obtained at 0.2 V from the NH2-Fc-functionalized glucose biosensor for 5 mM glucose in PBS spiked with the electroactive interferants BSA, uric acid, and dopamine. (B) Box plot representation of current response obtained with 2.5 mg/mL BSA, 1 mM uric acid, and 10 μM dopamine spiked separately in a solution containing 5 mM glucose in PBS. (C) Amperometry response of the NH2-Fc-functionalized glucose sensor with varying concentrations of fructose demonstrating sensor specificity. (D) Plot showing the specificity of the developed glucose biosensor for glucose detection compared with other saccharides, sucrose and fructose. Data represented here are from n = 2 replicates.
The specificity of our glucose sensor was further evaluated with varying concentrations of other sugar molecules, such as fructose and sucrose (Figure 4D). The current increased with the addition of 5 mM glucose (Figure 4C), confirming the functional sensitivity of the sensor for glucose detection. Moreover, when subjected to varying fructose concentrations, the same sensor showed no changes in the observed current response, followed by an increase in current response to 5 mM glucose. These results confirm the high specificity of the NH2-Fc-functionalized nanocomposite coated sensor for glucose detection.
3.5. Glucose Detection in Complex Biological Fluids
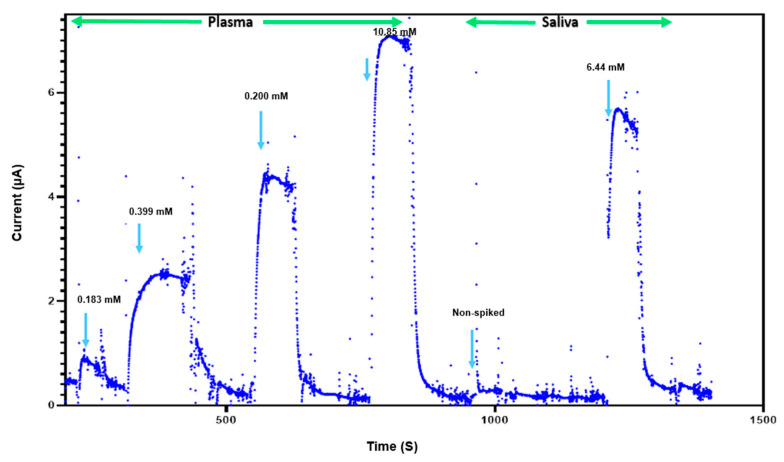
The feasibility of glucose detection in true biological samples is essential for determining the usefulness of this sensor for clinical applications. The performance of our NH2-Fc-functionalized glucose sensor was investigated using plasma and saliva samples containing different glucose concentrations (Figure 5). The sensor consistently exhibited current responses in the detectable range, indicating that its reliability and robustness are appropriate for potential future clinical use. However, a sensor performance evaluation with real samples will need to be carried out further to evaluate the commercial viability of this glucose sensor. Furthermore, the glucose biosensor shows a linear detection range of 1–10 mM that is well within the expected values for normal blood glucose levels in humans (4–6 mM) compared to other state-of-the-art glucose biosensors reported in prior published studies (Table 1).
Figure 5.
Glucose sensing in complex biological fluids. Response of biosensor to presence and absence of plasma (0.183 mM, 0.399 mM, 0.200 mM, 10.85 mM) and saliva (less than 0.111 mM and 6.44 mM).
Table 1.
Comparison with reported state-of-the-art glucose biosensors.
| Platform | Redox | Detection Method | Linear Range | Detection Limit | Comments |
|---|---|---|---|---|---|
| Pyrolytic carbon [20] | Potassium ferro/ferricyanide | Amperometry | 0.001–1 | 0.4 µM | Redox species in solution |
| Gold [24] | GOx modified with ferrocencecarboxaldehyde | Cyclic voltammetry |
1.0–5.0 mM | 5.2–210 µM | Variable response due to high oxygen levels |
| Glassy carbon electrode [25] | Poly(glycidyl methacrylateco-vinylferrocene) redox copolymer | Amperometry | 0.5 to 6 mM | 3 µM | Requires FAD as a cofactor |
| Multiwalled carbon nanotubes [26] | Osmium redox polymer | Voltammetry /amperometry |
- | - | Moderate stability |
| ZnO Nanorods [27] | - | Cyclic voltammetry |
1–13.8 mM | 1 mM | Non-enzymatic sensing may have selectivity issues |
| prGOx/GA/BSA (This work) | NH2-Fc | Amperometry | 0.0–10 mM | 1 mM | Scalable and cost effective |
4. Discussion
Enzyme-catalyzed glucose oxidation has been the standard for developing electrochemical-based glucose biosensors. The general chemical principle involves glucose oxidase, an enzyme that selectively oxidizes glucose to form gluconic acid and hydrogen peroxide (H2O2) in the presence of oxygen, as indicated by the following reaction:
H2O2 is further oxidized at the working electrode, leading to the release of two free electrons, constituting an electrochemical signal response proportional to the glucose concentration. However, detection approaches based on O2 consumption or H2O2 production may not be suited for continuous monitoring due to oxygen limitations, especially for sensor systems employed in vivo. An alternative strategy is to replace O2 with molecules that can serve as electron mediators, which forms the basis of our work.
The development of an enzymatic electrochemical readout based on a prGOx/BSA/GA nanocomposite-based antifouling coating with a functionalized redox molecule addresses many challenges that need to be overcome to significantly advance metabolomic and diagnostic applications. This new approach reduces cross-reactivity and signal instability in traditional diagnostic devices, which should enable the continuous monitoring of metabolites, such as glucose, over extended periods.
In this study, the charge transport between the redox center of glucose oxidase and nanocomposite-coated SPCE was achieved using NH2-Fc based on the following reaction:
The electrochemical responses we measured to establish that NH2-Fc mediates the sensing response by tunneling electrons between the electrode and enzyme redox center, which is crucial for the development of glucose biosensors for continuous monitoring. We also showed that the covalent coupling of NH2-Fc to the nanocomposite-modified SPCE prevents the leaching of NH2-Fc, which is required for building a sensitive detection system. The linear relationship between the glucose concentration and current response demonstrates adequate glucose oxidase loading in the enzyme layer formed via GA crosslinking. Furthermore, the reproducible current response in the presence of various interfering species and the biological sample matrix demonstrates the potential of developing low-cost, rapid, sensitive, and portable electrochemical detection devices for monitoring multiple metabolites, as well as, potentially, for multiplexing these sensors. The simple fabrication methods used and flexibility of modifying the enzyme layer allow for the quick optimization of the developed biosensor platform to different target analytes, opening new avenues for continuous monitoring for various research and clinical applications. In addition, a multivariate analysis can be effectively performed by leveraging advancements in machine learning, resulting in a more accurate and rapid biosensor.
5. Conclusions
In summary, we reported the development of electrochemical sensors that address critical challenges in glucose detection and that can potentially offer a new approach for continuous glucose monitoring. The high sensitivity and selectivity of this glucose sensor is based on the use of a nanocomposite-based antifouling coating, a functionalized (Fc derivatized) ferrocene-based redox mediator, and a redox enzyme. The NH2-Fc exhibits reversible redox behavior, facilitating electron transport with the redox center of glucose oxidase and supporting the regeneration of enzyme activity for the continuous determination of glucose. A clinical application of the developed platform was demonstrated in plasma and saliva samples, and it showed no interference from other electroactive species. The demonstration that glucose detection can be carried out in complex biological fluids and in the presence of potential interferents expands the application scope of the continuous sensing of glucose as well as other small metabolites. This versatility broadens its potential applications in clinical diagnostics, metabolic profiling, and drug development, in addition to personalized medicine, and, hence, potentially impacts a broad range of healthcare practices.
Acknowledgments
We thank G. Merry for her editing assistance.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nano14090796/s1, Figure S1: EIS characterization of sensor fabrication. Nyquist plot showing the changes in Rct for each step involved in the glucose sensor fabrication. Z’ and Z” represent the real and imaginary part of measured impedance; Figure S2: Stability of glucose detection over the span of 60 min in 1 mM glucose. Bar plot and the dotted line represents average current response obtained every 10 and over 60 min, respectively; Figure S3: The calibration curve of glucose standard solutions using colorimetric assay. Linear regression analysis: y = 9.1417x with an R2 of 0.9967, demonstrating a strong correlation between the variables.
Author Contributions
Conceptualization, S.J., P.J. and D.E.I.; methodology, S.J., N.R.S. and P.J.; investigation, S.J., N.R.S. and P.J.; data curation and analysis, S.J., N.R.S. and P.J.; resources, D.W. and D.E.I.; writing—original draft preparation, S.J. and N.R.S.; writing—review and editing, N.R.S., P.J., U.S., P.K., S.M. and D.E.I.; visualization, S.J., N.R.S. and P.J.; supervision, P.J. and D.E.I.; project administration, P.J. and D.E.I.; funding acquisition, P.J. and D.E.I. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
S.J., N.R.S., P.J. and D.I. are listed as inventors on patents describing this technology. The remaining authors declare no conflicts of interest.
Funding Statement
This work was supported by the Biomedical Advanced Research and Development Authority (BARDA) Award 75A50121C00075, Wyss Institute for Biologically Inspired Engineering at Harvard University, and Mitacs Globalink Research Award (IT27139).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ong K.L., Stafford L.K., McLaughlin S.A., Boyko E.J., Vollset S.E., Smith A.E., Dalton B.E., Duprey J., Cruz J.A., Hagins H., et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203–234. doi: 10.1016/S0140-6736(23)01301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leon B.M., Maddox T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes. 2015;6:1246–1258. doi: 10.4239/wjd.v6.i13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng Z., Xie X., Tan Q., Kang H., Cui J., Zhang X., Li W., Feng G. Blood glucose sensors and recent advances: A review. J. Innov. Opt. Health Sci. 2022;15:2230003. doi: 10.1142/s1793545822300038. [DOI] [Google Scholar]
- 4.CDC National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. [(accessed on 1 June 2023)];2023 Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fdiabetes%2Fdata%2Fstatistics-report%2Fdiagnosed-undiagnosed-diabetes.html#.
- 5.Mohamad Nor N., Ridhuan N.S., Abdul Razak K. Progress of Enzymatic and Non-Enzymatic Electrochemical Glucose Biosensor Based on Nanomaterial-Modified Electrode. Biosensors. 2022;12:1136. doi: 10.3390/bios12121136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pullano S.A., Greco M., Bianco M.G., Foti D., Brunetti A., Fiorillo A.S. Glucose biosensors in clinical practice: Principles, limits and perspectives of currently used devices. Theranostics. 2022;12:493–511. doi: 10.7150/thno.64035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olejnik A., Karczewski J., Dołęga A., Siuzdak K., Grochowska K. Novel approach to interference analysis of glucose sensing materials coated with Nafion. Bioelectrochemistry. 2020;135:107575. doi: 10.1016/j.bioelechem.2020.107575. [DOI] [PubMed] [Google Scholar]
- 8.Witkowska Nery E., Kundys M., Jeleń P.S., Jönsson-Niedziółka M. Electrochemical Glucose Sensing: Is There Still Room for Improvement? Anal. Chem. 2016;88:11271–11282. doi: 10.1021/acs.analchem.6b03151. [DOI] [PubMed] [Google Scholar]
- 9.Erbach M., Freckmann G., Hinzmann R., Kulzer B., Ziegler R., Heinemann L., Schnell O. Interferences and Limitations in Blood Glucose Self-Testing: An Overview of the Current Knowledge. J. Diabetes Sci. Technol. 2016;10:1161–1168. doi: 10.1177/1932296816641433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majewski J., Risler Z., Gupta K. Erroneous Causes of Point-of-Care Glucose Readings. Cureus. 2023;15:e36356. doi: 10.7759/cureus.36356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klonoff D.C. Continuous glucose monitoring: Roadmap for 21st century diabetes therapy. Diabetes Care. 2005;28:1231–1239. doi: 10.2337/diacare.28.5.1231. [DOI] [PubMed] [Google Scholar]
- 12.Vaddiraju S., Burgess D.J., Tomazos I., Jain F.C., Papadimitrakopoulos F. Technologies for continuous glucose monitoring: Current problems and future promises. J. Diabetes Sci. Technol. 2010;4:1540–1562. doi: 10.1177/193229681000400632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston L., Wang G., Hu K., Qian C., Liu G. Advances in Biosensors for Continuous Glucose Monitoring towards Wearables. Front. Bioeng. Biotechnol. 2021;9:733810. doi: 10.3389/fbioe.2021.733810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janapala R.N., Jayaraj J.S., Fathima N., Kashif T., Usman N., Dasari A., Jahan N., Sachmechi I. Continuous Glucose Monitoring Versus Self-monitoring of Blood Glucose in Type 2 Diabetes Mellitus: A Systematic Review with Meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma H., Kalita D., Naskar U., Mishra B.K., Kumar P., Mirza K.B. Prediction of Glucose Sensor Sensitivity in the Presence of Biofouling Using Machine Learning and Electrochemical Impedance Spectroscopy. IEEE Sens. J. 2023;23:18785–18797. doi: 10.1109/JSEN.2023.3289619. [DOI] [Google Scholar]
- 16.Sabaté del Río J., Henry O.Y., Jolly P., Ingber D.E. An antifouling coating that enables affinity-based electrochemical biosensing in complex biological fluids. Nat. Nanotechnol. 2019;14:1143–1149. doi: 10.1038/s41565-019-0566-z. [DOI] [PubMed] [Google Scholar]
- 17.Zupančič U., Jolly P., Estrela P., Moschou D., Ingber D.E. Graphene Enabled Low-Noise Surface Chemistry for Multiplexed Sepsis Biomarker Detection in Whole Blood. Adv. Funct. Mater. 2021;31:2010638. doi: 10.1002/adfm.202010638. [DOI] [Google Scholar]
- 18.Timilsina S.S., Ramasamy M., Durr N., Ahmad R., Jolly P., Ingber D.E. Biofabrication of multiplexed electrochemical immunosensors for simultaneous detection of clinical biomarkers in complex fluids. Adv. Healthc. Mater. 2022;11:2200589. doi: 10.1002/adhm.202200589. [DOI] [PubMed] [Google Scholar]
- 19.Najjar D., Rainbow J., Sharma Timilsina S., Jolly P., de Puig H., Yafia M., Durr N., Sallum H., Alter G., Li J.Z. A lab-on-a-chip for the concurrent electrochemical detection of SARS-CoV-2 RNA and anti-SARS-CoV-2 antibodies in saliva and plasma. Nat. Biomed. Eng. 2022;6:968–978. doi: 10.1038/s41551-022-00919-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma D., Lim Y., Lee Y., Shin H. Glucose sensor based on redox-cycling between selectively modified and unmodified combs of carbon interdigitated array nanoelectrodes. Anal. Chim. Acta. 2015;889:194–202. doi: 10.1016/j.aca.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 21.Dey R.S., Raj C.R. Redox-Functionalized Graphene Oxide Architecture for the Development of Amperometric Biosensing Platform. ACS Appl. Mater. Interfaces. 2013;5:4791–4798. doi: 10.1021/am400280u. [DOI] [PubMed] [Google Scholar]
- 22.Bartlett P.N., Bradford V.Q., Whitaker R.G. Enzyme electrode studies of glucose oxidase modified with a redox mediator. Talanta. 1991;38:57–63. doi: 10.1016/0039-9140(91)80009-O. [DOI] [PubMed] [Google Scholar]
- 23.Kang D., Zuo X., Yang R., Xia F., Plaxco K.W., White R.J. Comparing the Properties of Electrochemical-Based DNA Sensors Employing Different Redox Tags. Anal. Chem. 2009;81:9109–9113. doi: 10.1021/ac901811n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudkaitė V., Bagdžiūnas G. Functionalization of Glucose Oxidase in Organic Solvent: Towards Direct Electrical Communication across Enzyme-Electrode Interface. Biosensors. 2022;12:335. doi: 10.3390/bios12050335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Şenel M., Abasıyanık M.F. Construction of a Novel Glucose Biosensor Based on Covalent Immobilization of Glucose Oxidase on Poly(glycidyl methacrylate-co-vinylferrocene) Electroanalysis. 2010;22:1765–1771. doi: 10.1002/elan.200900644. [DOI] [Google Scholar]
- 26.Jayakumar K., Bennett R., Leech D. Electrochemical glucose biosensor based on an osmium redox polymer and glucose oxidase grafted to carbon nanotubes: A design-of-experiments optimisation of current density and stability. Electrochim. Acta. 2021;371:137845. doi: 10.1016/j.electacta.2021.137845. [DOI] [Google Scholar]
- 27.Chung R.J., Wang A.N., Liao Q.L., Chuang K.Y. Non-Enzymatic Glucose Sensor Composed of Carbon-Coated Nano-Zinc Oxide. Nanomaterials. 2017;7:36. doi: 10.3390/nano7020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article.