Abstract
Introduction
We hypothesized that multidisciplinary, proactive electronic consultation (MPE) could overcome barriers to prescribing guideline-directed medical therapies (GDMTs) for patients with type 2 diabetes (T2D) and chronic kidney disease (CKD).
Research design and methods
We conducted an efficacy-implementation pilot study of MPE for T2D and CKD for primary care provider (PCP)–patient dyads at an academic health system. MPE included (1) a dashboard to identify patients without a prescription for sodium-glucose cotransporter-2 inhibitors (SGLT2i) and without a maximum dose prescription for renin–angiotensin–aldosterone system inhibitors (RAASi), (2) a multidisciplinary team of specialists to provide recommendations using e-consult templates, and (3) a workflow to deliver timely e-consult recommendations to PCPs. In-depth interviews were conducted with PCPs and specialists to assess feasibility, acceptability, and appropriateness of MPE and were analyzed using an iterative qualitative analysis approach to identify major themes. Prescription data were extracted from the electronic health record to assess preliminary effectiveness to increase GDMT.
Results
20 PCPs agreed to participate, 18 PCPs received MPEs for one of their patients with T2D and CKD, and 16 PCPs and 2 specialists were interviewed. Major themes were as follows: appropriateness of prioritization of GDMT for T2D and CKD, acceptability of the content of the recommendations, PCP characteristics impact experience with MPE, acceptability and appropriateness of multidisciplinary collaboration, feasibility of MPE to overcome patient-specific barriers to GDMT, and appropriateness of workflow. At 6 months postbaseline, 7/18 (39%) patients were newly prescribed an SGLT2i, and 7/18 (39%) patients were either newly prescribed or had increased dose of RAASi.
Conclusions
MPE was an acceptable and appropriate health system strategy to identify and address gaps in GDMT among patients with T2D and CKD. Adopting MPE could enhance GDMT, though PCPs raised feasibility concerns which could be improved with program enhancements, including follow-up e-consults for reinforcement, and administrative support for navigating system-level barriers.
Keywords: Kidney Diseases; Diabetes Mellitus, Type 2; Quality Improvement; Health Services Research
What is already known on this topic
Proactive e-consults are a novel strategy to implement guideline-directed medical therapy; however, they have not been tested for patients with type 2 diabetes (T2D) and chronic disease, which requires complex medical decision-making and benefits from input from multiple specialists.
What this study adds
Evidence that multidisciplinary, proactive e-consults can be implemented to collaboratively manage a population of patients with complex needs in a way that is acceptable and appropriate for primary care providers and specialists.
How this study might affect research, practice, or policy
Health systems could adopt multidisciplinary proactive e-consults as a population health strategy to close quality gaps for complex chronic disease management such as T2D and chronic kidney disease.
Introduction
In patients with type 2 diabetes (T2D) and chronic kidney disease (CKD), the early use of sodium-glucose cotransporter-2 inhibitors (SGLT2i) and renin–angiotensin–aldosterone system inhibitors (RAASi) reduces progression of CKD, cardiovascular mortality, and healthcare utilization.1–3 Despite international guidelines recommending their use, there are gaps in prescriptions for RAASi and SGLT2i in patients with T2D and CKD particularly in early stages of CKD, with inequities related to race and ethnicity.1 4–9 The majority of patients with early stages of CKD are cared for by primary care providers (PCPs).10 11 Barriers to early initiation of these medications in the primary care setting include competing patient care priorities, lack of identification or prioritization of CKD, complexity of prescribing due to concerns for polypharmacy and side effects, formulary restrictions, and fragmented care coordination between primary and specialty physicians.11–16 We hypothesized that multidisciplinary, proactive electronic consultation (MPE) could overcome many of these barriers to improve guideline-directed medical therapies (GDMTs) for patients with T2D and CKD.17
In collaboration with endocrinologists and nephrologists, we designed an MPE program for patients with T2D and CKD to improve GDMT by PCPs. With conventional e-consults, PCPs with patient care inquiries request advice from a specialist, who then offers the necessary guidance. With proactive e-consults, specialists initiate the process to address gaps in GDMT. This innovative strategy, also termed Targeted Automatic e-Consults (TACo) or reverse e-consults, has been described by other research teams but is not widely adopted.18–20 Relying on individual PCPs to implement GDMT for individual patients is prone to variation due to competing priorities, knowledge, and implicit bias. MPE uses the electronic health record (EHR) to create a dashboard to systematically identify patients meeting specific criteria which enhances institutional capacity for efficient, targeted population health management.18 21 Practical considerations need to be addressed to ensure sustainability and scalability of MPE, including feasibility; acceptability of timing, tone, content of recommendations; and appropriateness of patients and PCPs targeted.
In this pilot, we studied the implementation and preliminary efficacy of MPE for patients with T2D and CKD at a large, academic integrated health system that primarily serves a medically complex and socially vulnerable population. We expected the program to be feasible, accepted, and appropriate for PCPs and specialists while increasing GDMT.
Methods
Study design, setting, and participants
We conducted a pilot study of MPE for patients with T2D and CKD as part of a quality improvement initiative at an integrated, academic health system with 20 primary care locations in the northeast serving a predominantly low-income, publicly insured, diverse population disproportionately affected by T2D and CKD.22–26 The study used a Hybrid Type 2 effectiveness-Implementation design and was conducted between July 21, 2022, and March 24, 2023.27
MPE was pilot-tested at five sites: one teaching practice, three community-based practices affiliated with the health system, and one primary care practice for patients with HIV. The program was introduced to PCPs by the study investigators during routine primary care practice meetings and PCPs were recruited to participate through e-mails following the meetings. We aimed to recruit 20 PCPs and deliver MPE to 20 PCP–patient dyads. Each participating PCP received a proactive e-consult for their patient with T2D and CKD with an upcoming appointment. In addition to the PCPs, 1 nephrologist and one endocrinologist were recruited to participate as e-consult providers, based on recommendations from division leaders and prior experience with e-consults. The nephrologist and endocrinologist were asked to complete a proactive e-consult for PCP–patient dyads assigned to them by study staff. Informed consent was obtained from PCPs. Informed consent was waived for patients as they were not study subjects and their data were collected as part of routine care.
Implementation strategies
We held four focus groups with primary care, nephrology, endocrinology, and clinical pharmacy providers to identify barriers to GDMT. Insights from these sessions were used to refine the implementation strategies to overcome known barriers to GDMT by (1) allowing the health system to proactively identify patients with gaps in prescription of RAASi and SGLT2i, (2) prioritizing T2D and CKD management in a primary care setting, (3) increasing knowledge of evidence-based management of T2D and CKD, and (4) increasing multidisciplinary collaboration. The strategies were further refined by incorporating feedback from the nephrology and endocrinology e-consult providers during weekly debriefing sessions during program implementation.
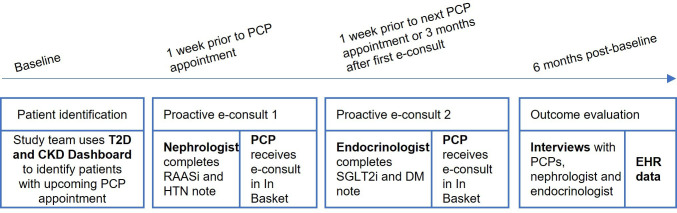
MPE for patients with T2D and CKD consisted of bundle of three implementation strategies. Strategy 1 used EHR data to create a dashboard to identify eligible patients with T2D and CKD who did not have a prescription for SGLT2i and did not have a prescription for maximum tolerated dose of RAASi.7 Criteria for inclusion in the dashboard were (a) diagnosis of T2D, (b) presence of CKD with albuminuria as evidenced by either 2+ on a spot urinalysis or >30 mg/g and <5000 mg/g on a quantified urine sample in the last 18 months; or latest estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 and ≥30 mL/min/1.73 m2 (eGFR caclulated with the CKD Epidemiology Collaboration equation: CKD-EPI 2021),28 or both, and (c) an appointment with a PCP in the last 12 months. The dashboard also included last blood pressure and hemoglobin A1C, quantified urine albumin, eGFR, and current medications. Strategy 2 provided specialist recommendations via a standardized e-consult note template. First, a nephrologist provided recommendations about RAASi intensification, hypertension, and CKD management, then an endocrinologist provided recommendations about SGLT2i and T2D management. The templates included three sections: (a) the purpose of the e-consult to improve GDMT for patients with T2D and CKD, (b) relevant patient information including lab values, vital signs, current medications, and preferred medications for a patient’s insurance plan, and (c) patient-specific recommendations for medication management based on their review of the patient’s history (see online supplemental appendix 1 for e-consult note templates). Strategy 3 developed a workflow for delivering the two e-consults, specifically, timing the delivery of the nephrology e-consult into the PCP’s EHR In Basket the week before an upcoming patient’s PCP appointment, and timing the endocrinology e-consult with the patient’s next primary care appointment or 3 months after the first e-consult, whichever occurred first (figure 1).
Figure 1.
Multidisciplinary, proactive e-consult workflow and study timeline. CKD, chronic kidney disease; DM, diabetes mellitus; EHR, electronic health record; HTN, hypertension; PCP, primary care provider; RAASi, renin–angiotensin–aldosterone system inhibitors; SGLT2i, sodium-glucose cotransporter-2 inhibitors; T2D, type 2 diabetes.
bmjdrc-2024-004155supp001.pdf (145.8KB, pdf)
Outcome measures and data sources
We used The Taxonomy of Implementation Outcomes29 to guide our selection of implementation outcomes, specifically adoption, acceptability, and appropriateness. Adoption was defined as the proportion of PCPs who read the e-consults assessed in the EHR. To test appropriateness, acceptability, and feasibility, in-depth interviews were conducted with participating PCPs, nephrologists, and endocrinologists by a study team member with training in qualitative research. The Consolidated Framework for Implementation Research was used to inform the interview guides.30 For example, appropriateness focused on suitability of patients identified using the T2D and CKD dashboard; acceptability asked about content and tone of the e-consult note; feasibility addressed workflows for sending and receiving the e-consults (see online supplemental appendix 2 for interview guide). We also interviewed the two specialists to assess their perception of workflow feasibility, appropriateness of dashboard, and acceptability of the e-consult program. Interviews were digitally recorded and transcribed using Microsoft Teams. The transcripts were then entered into Dedoose for data management and analysis.31 To assess preliminary efficacy, we used PCP-initiated change in SGLT2i and/or RAASi prescription at 3 and 6 months postbaseline, after the receipt of each of two MPEs.
bmjdrc-2024-004155supp002.pdf (147.1KB, pdf)
Qualitative and statistical analysis
Using an iterative qualitative analysis approach, the interviews were independently coded by three study investigators (SR, LG, and IA) using a priori primary codes that reflected implementation outcomes: acceptability, appropriateness, and feasibility. Secondary codes were added to document themes that emerged from the interviews. The study team created a preliminary set of secondary codes based on a few transcripts that were reviewed as a group. Then these preliminary set of codes were applied to all interview transcripts separately by the three study investigators. After all interviews were reviewed, a secondary codebook was created for the major emergent themes. The interviews were then divided among the three study investigators who independently applied the secondary codes.
We used descriptive analyses to report on PCP demographics, site of practice, characteristics, and MPE adoption. Preliminary effectiveness, measured as proportions of patients with prescriptions for RAASi and SGLT2i, was calculated at baseline and 3 and 6 months postbaseline.
Results
A total of 20 PCPs agreed to participate, 18 PCPs received MPEs for one of their patients with T2D and CKD, and 16 PCPs and 2 specialists were interviewed. Two PCPs did not have patients who met inclusion criteria and two PCPs left the institution prior to the completion of the study. PCP characteristics are described in table 1. Participating PCPs practiced in a variety of practice types and had training in general internal medicine (GIM) or family medicine (FM) and a few had additional specialization in infectious diseases (ID). The mean (SD) self-reported year of primary care experience was 2211 and the mean (SD) proportion of patients with T2D and CKD of total patient panel was 23% (10%). One nephrologist and one endocrinologist delivered the MPE for 18 PCP–patient pairs and both were interviewed. Patient baseline characteristics are shown in table 2.
Table 1.
Primary care physician, nephrology, and endocrinology provider characteristics
| Participant | Practice type | Practice specialty | Years in practice | Number of patients per week | Proportion of patients with T2D and CKD |
| Dr A | Teaching | GIM, ID | 13 | 25 | 16 |
| Dr B | Teaching | GIM | – | – | – |
| Dr C | Teaching | GIM | 12 | 14 | 29 |
| Dr D | Teaching | GIM | 30 | 60 | 25 |
| Dr E | Teaching | GIM | 10 | 40 | 38 |
| Dr F | Teaching | FM | 15 | 48 | 19 |
| Dr G | Teaching | GIM | 20 | 30 | 20 |
| Dr H | Teaching | GIM and ID | 10 | 20 | 25 |
| Dr I | Community | FM | 18 | 90 | 44 |
| Dr J | Community | GIM | 40 | 100 | 20 |
| Dr K | Community | GIM | 30 | 75 | 20 |
| Dr L | Community | GIM | – | – | – |
| Dr M | Community | GIM and ID | 2 | 100 | 30 |
| Dr N | Community | GIM | 32 | 90 | 33 |
| Dr O | Community | GIM | – | – | – |
| Dr P | HIV | ID | 30 | 85 | 20 |
| Dr Q | HIV | ID | 35 | 75 | 8 |
| Dr R | HIV | ID | 31 | 18 | 6 |
| Dr S | Specialty | Endocrinology | 5 | 40 | 10 |
| Dr T | Specialty | Nephrology | 2 | 20 | 10 |
Blank cells (–) are for PCPs that were not interviewed.
CKD, chronic kidney disease; FM, family medicine; GIM, general internal medicine; ID, infectious diseases; PCP, primary care provider; T2D, type 2 diabetes.
Table 2.
Primary care patient baseline characteristics
| Variable | Mean, SD |
| Age | 65.4, 10.9 |
| n, % | |
| Sex | |
| Female | 9, 50.0% |
| Male | 9, 50.0% |
| Race | |
| Black or African American | 10, 55.6% |
| White | 0, 0% |
| Asian | 0, 0% |
| Other, unspecified | 5, 27.8% |
| Decline or unavailable | 3, 16.7% |
| Ethnicity | |
| Hispanic or Latino | 6, 33.3% |
| Non-Hispanic | 10, 55.6% |
| Decline or unavailable | 2, 11.1% |
| HbA1c | |
| At goal ≤ 7 | 8, 44.4% |
| Not at goal | 10, 55.6% |
| Blood pressure | |
| At goal ≤130/80 | 5, 27.8% |
| Not at goal | 13, 72.2% |
| eGFR categories | |
| Category 1, eGFR ≥90 | 3, 16.7% |
| Category 2, eGFR 60–89 | 9, 50.0% |
| Category 3, eGFR 30–59 | 5, 26.3% |
| Category 4, eGFR <30 | 1, 5.6% |
| Degree of proteinuria | |
| Moderate proteinuria | 6, 33.3% |
| Severe proteinuria | 12, 66.7% |
| Count of diabetes-related prescriptions* | |
| 0 | 1, 5.6% |
| 1 | 9, 50.0% |
| 2 | 3, 16.7% |
| 3 | 3, 16.7% |
| 4 | 2, 11.1% |
| Count of hypertension-related prescriptions † | |
| 0 | 6, 33.3% |
| 1 | 7, 38.9% |
| 2 | 3, 16.7% |
| 3 | 1, 5.6% |
| 4 | 1, 5.6% |
| Insurance type | |
| Medicaid | 3, 16.7% |
| Medicare | 10, 55.6% |
| Commercial | 5, 27.8% |
| Uninsured | 0, 0% |
*Count of prescriptions in EHR-defined pharmaceutical classes: insulins, biguanides, glucagon-like peptide 1 (GLP-1) agonists, dipeptidyl peptidase 4 (DPP-4) inhibitors, sulfonylureas, thiazolidinediones, and alpha-glucosidase inhibitors; excludes SGLT2i prescription.
†Count of prescriptions in EHR-defined pharmaceutical classes: thiazide, loop and potassium-sparing diuretics, beta-blockers, calcium channel blockers, combined alpha and beta-blockers, alpha-antagonists, and hydralazine; excludes RAASi prescription.
eGFR, estimated glomerular filtration rate; EHR, electronic health record; GFR, glomerular filtration rate; HbA1C, hemoglobin A1C; RAASi, renin–angiotensin–aldosterone system inhibitors; SGLT2i, sodium-glucose cotransporter-2 inhibitors.
Appropriateness of patient identification and feasibility of using T2D and CKD dashboard
The dashboard initially identified 7055 patients; however, there were challenges balancing sensitivity with specificity of the criteria used to identify CKD longitudinally. The dashboard included patients with transient proteinuria from acute illness, thus criteria were updated to include (1) two GFR lab values <60 at least 3 months apart, the latest of which occurred in the last 18 months or (2) two of any of the following urine protein values at least 3 months apart, the latest of which was in the last 18 months: microalbumin/creatinine ratio >30 mg/g, total protein/creatinine ratio >50 mg/g, or total protein measured in a 24-hour urine collection >50 mg/g. The more specific dashboard initially identified 4178 patients with T2D and CKD. With this dashboard, at 3 months and 6 months, respectively, 26% and 45% of patients no longer met inclusion criteria due to lack of repeat urine protein analysis or lack of PCP follow-up, while patients newly meeting criteria entered the dashboard.
Adoption of MPE
Of the 20 MPE planned, 18 were completed. PCPs read 17/18 (94%) of the nephrology e-consults and 16/18 (89%) of the endocrinology e-consults. Nephrology and endocrinology referrals were placed for two and three patients, respectively.
PCP perceptions of the appropriateness, acceptability, and feasibility of MPE
We identified six major themes, summarized in table 3, that reflected perceptions of the appropriateness, acceptability, and feasibility of MPE for T2D and CKD. These themes included feasibility of program workflow, acceptability of content of recommendation to help with agenda setting for patients with T2D and CKD, and timing of recommendation. Emergent themes from our analysis included how PCP characteristics impacted their perception of whether MPE achieved multidisciplinary collaboration.
Table 3.
Themes identified in qualitative interviews with primary care physicians for implementation of multidisciplinary, proactive e-consults for patients with type 2 diabetes and chronic kidney disease
| Clinical prioritization |
|
| Implementation barriers external to the implementation strategies |
|
| Content of recommendation |
|
| PCP characteristics |
|
| Multidisciplinary collaboration |
|
| Workflow |
|
EHR, electronic health record; GDMT, guideline-directed medical therapy; PCP, primary care provider.
Clinical prioritization
PCPs found the proactive e-consult appropriate and acceptable for prioritizing T2D and CKD management during patient visits. Some appreciated maintaining autonomy in deciding whether to implement the recommendations. Nephrology and endocrinology specialists emphasized the dashboard’s role in early CKD identification.
If I miss anything you know, then it’s a reminder. So, it’s always good to have somebody you know, [to remind and say] ‘hey, you know this patient could benefit from this.’ (Dr J, GIM)
I’m familiar with the data supporting it and… I just didn’t… tie the data to that particular patient. I think it made my care better. (Dr P, ID)
The recommendations were somewhat optional as far as whether to use medication or not, but the pros and cons were presented and so then it was ultimately my decision. (Dr N, GIM)
I think this is a good way of identifying patients with diabetic kidney disease early on because most of the patient that we see are when they already have advanced CKD. (Dr T, Nephrology e-consultant)
Implementation barriers external to the implementation strategies
PCPs reported challenges outside of their control which impacted the feasibility of implementing the recommendations; these included patient missing appointments, out-of-pocket costs of SGLT2i, insurance prior authorization requirements for SGLT2i, and patient hesitation to start or change medications. Multiple PCPs highlighted how clinical pharmacists could assist with medication access.
Sometimes [it is hard] to get access to the medications due to insurance issues and so having someone else there to help us navigate through that [would be helpful]. (Dr I, GIM)
Unfortunately for patients without Medicaid, the copay cost can be so high. So, it’s not an option for many people in that population of patients. (Dr I, GIM)
The primary barrier that we encounter [is] an unwillingness of patients sometimes to add additional medications. (Dr K, GIM)
If they don’t come in for that follow up or the follow up is delayed, then it can take some time to actually action the recommendations. (Dr A, GIM and ID)
Content of recommendation
PCPs reported that the evidence-based content of the notes was appropriate. Most PCPs reported that the length and tone were acceptable. The specialist e-consultants and PCPs both appreciated that the notes could be customized for individual patients with complex needs. Many PCPs indicated that they used the notes as a reference when seeing patients and some incorporated the content of the notes into their own assessment and plans.
It was very thorough. The note that the endocrinologist wrote, you know, had different scenarios, different possibilities… They did give me some pointers on other medicines to use for her diabetes that would also preserve her kidney function. So, I thought that was helpful. (Dr R, ID)
Straight to the point. It wasn’t a long message… It was just bullet points. It was easy, very good actually. … so I put it as a bullet point in my plan for the patient. (Dr M, GIM and ID)
If I had any concerns or felt that it may not have been the right [medications or felt] we need more information. I would just lay it out there. The note [template] is really for the guidelines. So, there was a little finessing in some instances. (Dr S, Endocrinology e-consultant)
PCP characteristics
PCP’s individual characteristics influenced whether they found the proactive e-consults acceptable, appropriate, and feasible. PCPs who specialized in GIM or FM expressed baseline confidence in managing T2D and CKD. In contrast, PCPs with subspecialization in infectious diseases said that the proactive e-consults increased their confidence and awareness of T2D and CKD management. PCPs in all practice settings mentioned feeling overburdened with clinical responsibilities with a simultaneous willingness to implement new GDMT.
I think that it helps because not everybody has time to know what all of the newest recommendations are and then to implement them. And sometimes it involves medical knowledge that we just don't have yet. (Dr H, GIM and ID)
I mean another potential advantage of going through this is that not only will I manage one patient better, but I could sort of learn how to manage other patients better. And maybe you know, access the specialists with more expertise too. (Dr Q, ID)
I think that could be a challenge for a lot of providers it’s just we're so overwhelmed with hundreds of messages every day. And once you fall behind, it’s impossible to see everything. So I think that might be a challenge with the workflow. (Dr C, GIM)
Multidisciplinary collaboration
Most PCPs and the nephrology and endocrinology specialty e-consultants believed the proactive e-consult increased multidisciplinary collaboration. A few PCPs reported that being able to tell patients that the recommendations came from a specialist improved their patient’s acceptance of medication changes. Many appreciated a “second set of eyes.”
[This helps] us as we educate the patients as to why we may need to make some of these changes and it reassures the patient that the intervention is recommended by not just primary care, but you know by nephrology or endocrinology as well, which sometimes we need to reassure the patients. (Dr K, GIM)
I think the idea behind it is excellent… The intervention itself is a nice way to get specialists to help the primary care out. There’s a huge delay to get appointments just because of the volume of patients and the number of providers. (Dr S, Endocrinology e-consultant)
I think it was excellent. You know, I think PCPs are really used to having to…go out and seek out people to get whatever recommendations or help, and sometimes that could be frustrating because you can't get to them or the appointment is 8 months away or something. So, this was nice that it was more proactive and in some cases might address something that you didn’t even know and needed to be addressed. (Dr H, GIM and ID)
However, some PCPs reported that the proactive e-consult provided unidirectional communication and was not collaborative. These PCPs felt that the burden of caring for patients with T2D and CKD remained with the PCP and the proactive e-consult added to their workload. Some suggested a follow-up workflow with the specialists for additional assistance in GDMT implementation.
It doesn’t feel multidisciplinary to me. It feels more like it’s another thing that I am supposed to be doing and I feel bad when I don’t. (Dr C, GIM)
You can’t really see any collaboration. You’re just getting 2 notes from two different people, so it doesn’t necessarily feel very collaborative. It’s more prescriptive to a certain extent. (Dr A, GIM and ID)
Workflow
Most PCPs found the proactive e-consult workflow acceptable. However, concerns arose about overlooked recommendations if the patient missed their appointment and if PCPs were overwhelmed with the volume of messages. To address this, PCPs suggested follow-up e-consults for reinforcement in cases of missed appointments or initial implementation challenges.
If the patient is in the office and they mentioned something, then I’ll…have the note there and see what they recommend in real time. That way I can discuss it with the patient. (Dr J, GIM and ID)
I think the big challenge with primary care providers is that we get is we are hundreds of messages every day that I am responsible for. (Dr C, GIM)
Follow up would be, would be great. You know, saying, you know, how’s the patient doing? Have you made these changes? …I think a follow up would be good. (Dr R, ID)
I do wonder now about the follow up for this. I think because it wasn’t in the protocol for how to check on the follow up and it’s not like I had these patients saved on my own radar to follow them. We see this a lot: the PCP prescribes it, the patient never gets it. (Dr S, Endocrinology e-consultant
Preliminary efficacy
At baseline, 0/18 patients were prescribed an SGLT2i, and 1/18 patient was prescribed a maximum dose of RAASi. At 3 months postbaseline (approximately 3 months after the nephrology, proactive e-consult), 3/18 (17%) patients were prescribed an SGLT2i, and 7/18 (39%) patients were either newly prescribed or had increased dose of RAASi compared with baseline. At 6 months postbaseline (approximately 3 months after the endocrinology, proactive e-consult), 7/18 (39%) patients were prescribed an SGLT2i, and 7/18 (39%) patients were either newly prescribed or had increased dose of RAASi compared with baseline.
Discussion
We successfully implemented a pilot of MPE as a population health strategy to identify and address care gaps among vulnerable patients with T2D and CKD suggesting scalability and sustainability of this approach. Adopting MPEs could enhance patient care, though PCPs raised feasibility concerns. To address these, PCPs proposed program enhancements, including follow-up e-consults for reinforcement, and administrative support for navigating system-level barriers to care. Preliminary evidence showed increased prescribing of GDMT by PCPs, suggesting effectiveness of MPE to improve GDMT.
The major facilitator for implementing proactive e-consults is the acceptability of using EHR data to identify and deliver timely recommendations that may otherwise not be prioritized during a PCP visit. Other multilevel initiatives that included a platform to proactively identify patients with T2D and gaps in prescriptions for GDMT resulted in an increase in GDMT.32 33 Through small-scale testing, we iteratively refined the T2D and CKD dashboard criteria and identified a future target for intervention: timely repetition of urine protein testing for patients with CKD. Dashboard refinement further provided insights into implementation strategies from key stakeholders for better management at the early stages of T2D and CKD. Other studies testing this approach emphasized buy-in from both the specialists and primary teams for targeted conditions and cautioned that the strategy may not be effective if overused.18 19 MPE is particularly important for CKD which is often not recognized at early stages but has the potential to be controlled with the optimization of GDMT.34–37 A recent publication described the protocol for the Kidney Coordinated HeAlth Management Partnership trial which will test the effectiveness of proactive e-consults sent from nephrologists and clinical pharmacists to PCPs for primary care patients with CKD.38 Preliminary data from our study and others suggest that this strategy can be scaled to implement other high-impact guidelines at institutional and national levels to close practice and care gaps.18 21
We found that PCPs and specialists valued multidisciplinary collaboration; however, some PCPs felt that MPE felt more prescriptive than collaborative. Like traditional e-consults, MPE increases access to specialty expertise which is critical given challenges such as limited supply of specialists and specialty appointments.39 40 Negotiating the balance between the opportunity for improved population health management and the impact on PCP workload and autonomy is important to consider as health systems test proactive e-consults.19 Multidisciplinary care models, particularly those that include clinical pharmacists, have shown to delay the progression of CKD in adults, and reduce hospitalizations and cardiovascular events.41–43 Ensuring that the content and tone of the e-consult notes are professional and not condescending is important for acceptability. An alternative population health strategy that has been described is to proactively identify patients with T2D and CKD who would benefit from an MPE while maintaining PCP autonomy would give PCPs an option to order the e-consult.44 However, this approach requires an additional step which may limit feasibility and acceptability. To improve multidisciplinary collaboration, an adjunct strategy is for the specialists to pend orders for the medications and/or laboratory tests for the PCPs to review and sign if appropriate.19 This may improve the perception of multidisciplinary task sharing and enhance the effectiveness of MPE.
This study had important limitations. The small scale of the study limits the generalizability of the implementation data and inference from the preliminary effectiveness data. The observed increasing trend in use of these agents with time may be a reflection of penetration of guideline recommendations into practice independent of the proactive e-consult recommendation. Future studies are needed to evaluate the efficacy of MPE compared with usual care for increasing GDMT. Scalability and financial sustainability of the approach are uncertain as current insurance reimbursements require a request for consultation.18 E-consult billing codes could be used; however, the patient would need to be made aware of the e-consult. Value-based care initiatives that capitate payments, but incentivize coordination and efficient collaboration, may provide sustainability options for proactive e-consults. An alternative and more cost-effective population health strategy, which has been shown to be effective in increasing GDMT for heart failure, is to use real-time, targeted, and tailored EHR-based alerting systems.45 46 However, the complexity of comorbidity management, alert fatigue, and lack of specialist collaboration may limit the utility of this approach among persons with T2D and CKD.
MPE for T2D and CKD is an acceptable and appropriate way for nephrology and endocrinology specialists to share their expertise with PCPs who currently manage most patients with T2D and early CKD. Based on lessons learned from this implementation and preliminary efficacy study, we will refine and enhance multidisciplinary proactive e-consult program for a future trial designed to evaluate patient outcomes including progression of CKD and surrogate outcomes for cardiovascular disease such as hypertension and diabetes control. Proactive e-consults are a novel strategy for implementing evidence-based care, this study adds to the literature supporting their use for population health management and generalizability at US institutions experiencing gaps in GDMT.
Footnotes
Contributors: SR was responsible for the overall content as guarantor. SR, LG, and LB were involved in the study conception and design, conduct, analyses, interpretation of data, and writing. IA and YD were involved in project conduct, acquisition of data and analysis, and review and editing. KD, NS, and SG were involved in the study conception and design, conduct, and review and editing.
Funding: This research was supported by the Agency for Healthcare Research and Quality (R03HS028877-01) and the Einstein-Mount Sinai Diabetes Research Center, Albert Einstein College of Medicine (P30DK020541-47).
Competing interests: KD and LG report grant funding from Astra Zeneca and the American College of Clinical Pharmacy for research related to optimizing medical therapy for patients with chronic kidney disease. The other authors have no disclosures.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Deidentified participant data are available upon request from the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Albert Einstein College of Medicine Institutional Review Board, reference number 106473, ID number 2021-13017. Participants gave informed consent to participate in the study before taking part.
References
- 1. Tuttle KR, Alicic RZ, Duru OK, et al. Clinical characteristics of and risk factors for chronic kidney disease among adults and children: an analysis of the CURE-CKD registry. JAMA Netw Open 2019;2:e1918169. 10.1001/jamanetworkopen.2019.18169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tuttle KR, Brosius FC, Cavender MA, et al. Sglt2 inhibition for CKD and cardiovascular disease in type 2 diabetes: report of a scientific workshop sponsored by the national kidney foundation. Am J Kidney Dis 2021;77:94–109. 10.1053/j.ajkd.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 3. de Boer IH, Caramori ML, Chan JCN, et al. Executive summary of the 2020 KDIGO diabetes management in CKD guideline: evidence-based advances in monitoring and treatment. Kidney Int 2020;98:839–48. 10.1016/j.kint.2020.06.024 [DOI] [PubMed] [Google Scholar]
- 4. Murphy DP, Drawz PE, Foley RN. Trends in angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use among those with impaired kidney function in the United States. J Am Soc Nephrol 2019;30:1314–21. 10.1681/ASN.2018100971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schernthaner G, Shehadeh N, Ametov AS, et al. Worldwide inertia to the use of cardiorenal protective glucose-lowering drugs (Sglt2I and GLP-1 RA) in high-risk patients with type 2 diabetes. Cardiovasc Diabetol 2020;19:185. 10.1186/s12933-020-01154-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patakfalvi L, Brazeau AS, Dasgupta K. Physician experiences with sodium-glucose cotransporter (Sglt2) inhibitors, a new class of medications in type 2 diabetes, and adverse effects. Prim Health Care Res Dev 2018;20:e50. 10.1017/S1463423618000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rikin S, Deccy S, Zhang C, et al. Care gaps in sodium-glucose cotransporter-2 inhibitor and renin angiotensin system inhibitor prescriptions for patients with diabetic kidney disease. J Gen Intern Med 2023;38:1599–605. 10.1007/s11606-022-07863-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eberly LA, Yang L, Eneanya ND, et al. Association of race/Ethnicity, gender, and socioeconomic status with sodium-glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open 2021;4:e216139. 10.1001/jamanetworkopen.2021.6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lamprea-Montealegre JA, Madden E, Tummalapalli SL, et al. Association of race and ethnicity with prescription of Sglt2 inhibitors and Glp1 receptor agonists among patients with type 2 diabetes in the veterans health administration system. JAMA 2022;328:861–71. 10.1001/jama.2022.13885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brady M, O’Donoghue D. The role of primary care in managing chronic kidney disease. Br J Gen Pract 2010;60:396–7. 10.3399/bjgp10X502065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saunders M, Laiteerapong N. Clinical practice guideline update for diabetes management of chronic kidney disease: an important first step, more work to do. Ann Intern Med 2022;176:417–8. [DOI] [PubMed] [Google Scholar]
- 12. Neumiller JJ, Alicic RZ, Tuttle KR. Overcoming barriers to implementing new therapies for diabetic kidney disease: lessons learned. Adv Chronic Kidney Dis 2021;28:318–27. 10.1053/j.ackd.2021.02.001 [DOI] [PubMed] [Google Scholar]
- 13. Ziemer DC, Doyle JP, Barnes CS, et al. An intervention to overcome clinical inertia and improve diabetes mellitus control in a primary care setting. Arch Intern Med 2006;166:507. 10.1001/archinte.166.5.507 [DOI] [PubMed] [Google Scholar]
- 14. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med 2001;135:825–34. 10.7326/0003-4819-135-9-200111060-00012 [DOI] [PubMed] [Google Scholar]
- 15. Okemah J, Peng J, Quiñones M. Addressing clinical inertia in type 2 diabetes mellitus: a review. Adv Ther 2018;35:1735–45. 10.1007/s12325-018-0819-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reach G, Pechtner V, Gentilella R, et al. Clinical inertia and its impact on treatment intensification in people with type 2 diabetes mellitus. Diabetes Metab 2017;43:501–11. 10.1016/j.diabet.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 17. Schmittdiel JA, Gopalan A, Lin MW, et al. Population health management for diabetes: health care system-level approaches for improving quality and addressing disparities. Curr Diab Rep 2017;17:31. 10.1007/s11892-017-0858-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Judson TJ, Mourad M, Wachter RM. Building a targeted automatic e-consult (taco) program. Jt Comm J Qual Patient Saf 2022;48:114–9. 10.1016/j.jcjq.2021.10.007 [DOI] [PubMed] [Google Scholar]
- 19. Spece LJ, Weppner WG, Weiner BJ, et al. Primary care provider experience with proactive e-consults to improve COPD outcomes and access to specialty care. Chronic Obstr Pulm Dis 2023;10:46–54. 10.15326/jcopdf.2022.0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wachter RM, Judson TJ, Mourad M. Reimagining specialty consultation in the digital age: the potential role of targeted automatic electronic consultations. JAMA 2019;322:399–400. 10.1001/jama.2019.6607 [DOI] [PubMed] [Google Scholar]
- 21. Au DH, Collins MP, Berger DB, et al. Health system approach to improve chronic obstructive pulmonary disease care after hospital discharge: stepped-wedge clinical trial. Am J Respir Crit Care Med 2022;205:1281–9. 10.1164/rccm.202107-1707OC [DOI] [PubMed] [Google Scholar]
- 22. Laster M, Shen JI, Norris KC. Kidney disease among African Americans: a population perspective. Am J Kidney Dis 2018;72:S3–7. 10.1053/j.ajkd.2018.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Norris KC, Agodoa LY. Race and kidney disease: the scope of the problem. J Natl Med Assoc 2002;94:1S–6S. [PMC free article] [PubMed] [Google Scholar]
- 24. Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int 2005;68:914–24. 10.1111/j.1523-1755.2005.00485.x [DOI] [PubMed] [Google Scholar]
- 25. Kim MBD, Matte T. Diabetes in New York City: Public Health Burden and Disparities. New York: New York City Department of Health and Mental Hygiene, 2006. [Google Scholar]
- 26. Wu WY, Jiang Q, Di Lonardo SS. Poorly controlled diabetes in New York city: mapping high-density neighborhoods. J Public Health Manag Pract 2018;24:69–74. 10.1097/PHH.0000000000000544 [DOI] [PubMed] [Google Scholar]
- 27. Curran GM, Bauer M, Mittman B, et al. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50:217–26. 10.1097/MLR.0b013e3182408812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate [published correction appears in Ann intern MED. Ann Intern Med 2011;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011;38:65–76. 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Consolidated Framework for Implementation Research, Available: https://cfirguide.org/guide/app
- 31. Dedoose 9.0.17 ed . P. Cloud Application for Managing, Analyzing, and Presenting Qualitative and Mixed Method Research Data. Los Angeles, CA: SocioCultural Research Consultants, LLC, 2021. [Google Scholar]
- 32. Hirsh BJ, Hirsch JS, Hmoud H, et al. “A system approach to improving guideline-directed therapy for cardio-renal-metabolic conditions: the "beyond diabetes" initiative”. Am J Prev Cardiol 2023;16:100608. 10.1016/j.ajpc.2023.100608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pagidipati NJ, Nelson AJ, Kaltenbach LA, et al. Coordinated care to optimize cardiovascular preventive therapies in type 2 diabetes: a randomized clinical trial. JAMA 2023;329:1261–70. 10.1001/jama.2023.2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. The EMPA-KIDNEY Collaborative Group . Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023;388:117–27. 10.1056/NEJMoa2204233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Diamantidis CJ, Zepel L, Wang V, et al. Disparities in chronic kidney disease progression by medicare advantage enrollees. Am J Nephrol 2021;52:949–57. 10.1159/000519758 [DOI] [PubMed] [Google Scholar]
- 36. McGrath K, Edi R. Diabetic kidney disease: diagnosis, treatment, and prevention. Am Fam Physician 2019;99:751–9. [PubMed] [Google Scholar]
- 37. American Diabetes Association Professional Practice Committee . Chapter 9. pharmacologic approaches to glycemic treatment: standards of medical care in Diabetes—2022. Diabetes Care 2022;45:S125–43. 10.2337/dc22-S009 [DOI] [PubMed] [Google Scholar]
- 38. Jhamb M, Weltman MR, Yabes JG, et al. Electronic health record based population health management to optimize care in CKD: design of the kidney coordinated health management partnership (K-CHAMP) trial. Contemp Clin Trials 2023;131:107269. 10.1016/j.cct.2023.107269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gaye M, Mehrotra A, Byrnes-Enoch H, et al. Association of eConsult implementation with access to specialist care in a large urban safety-Net system. JAMA Health Forum 2021;2:e210456. 10.1001/jamahealthforum.2021.0456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vimalananda VG, Gupte G, Seraj SM, et al. Electronic consultations (e-consults) to improve access to specialty care: a systematic review and narrative synthesis. J Telemed Telecare 2015;21:323–30. 10.1177/1357633X15582108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Strand H, Parker D. Effects of Multidisciplinary models of care for adult pre-dialysis patients with chronic kidney disease: a systematic review. Int J Evid Based Healthc 2012;10:53–9. 10.1111/j.1744-1609.2012.00253.x [DOI] [PubMed] [Google Scholar]
- 42. Shi Y, Xiong J, Chen Y, et al. The effectiveness of multidisciplinary care models for patients with chronic kidney disease: a systematic review and meta-analysis. Int Urol Nephrol 2018;50:301–12. 10.1007/s11255-017-1679-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Collister D, Pyne L, Cunningham J, et al. Multidisciplinary chronic kidney disease clinic practices: a scoping review. Can J Kidney Health Dis 2019;6:205435811988266. 10.1177/2054358119882667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith ZG, McNicoll L, Clark TL, et al. Medical neighborhood model for the care of chronic kidney disease patients. Am J Nephrol 2016;44:308–15. 10.1159/000448294 [DOI] [PubMed] [Google Scholar]
- 45. Ghazi L, Yamamoto Y, Riello RJ, et al. Electronic alerts to improve heart failure therapy in outpatient practice: a cluster randomized trial. J Am Coll Cardiol 2022;79:2203–13. 10.1016/j.jacc.2022.03.338 [DOI] [PubMed] [Google Scholar]
- 46. Allen LA, Venechuk G, McIlvennan CK, et al. An electronically delivered patient-activation tool for intensification of medications for chronic heart failure with reduced ejection fraction: the EPIC-HF trial. Circulation 2021;143:427–37. 10.1161/CIRCULATIONAHA.120.051863 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2024-004155supp001.pdf (145.8KB, pdf)
bmjdrc-2024-004155supp002.pdf (147.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Deidentified participant data are available upon request from the corresponding author.