Abstract
Objectives
To assess the feasibility and change in clinical outcomes associated with continuous glucose monitoring (CGM) use among a rural population in Malawi living with type 1 diabetes.
Design
A 2:1 open randomised controlled feasibility trial.
Setting
Two Partners In Health-supported Ministry of Health-run first-level district hospitals in Neno, Malawi.
Participants
45 people living with type 1 diabetes (PLWT1D).
Interventions
Participants were randomly assigned to Dexcom G6 CGM (n=30) use or usual care (UC) (n=15) consisting of Safe-Accu glucose monitors and strips. Both arms received diabetes education.
Outcomes
Primary outcomes included fidelity, appropriateness and severe adverse events. Secondary outcomes included change in haemoglobin A1c (HbA1c), acceptability, time in range (CGM arm only) SD of HbA1c and quality of life.
Results
Participants tolerated CGM well but were unable to change their own sensors which resulted in increased clinic visits in the CGM arm. Despite the hot climate, skin rashes were uncommon but cut-out tape overpatches were needed to secure the sensors in place. Participants in the CGM arm had greater numbers of dose adjustments and lifestyle change suggestions than those in the UC arm. Participants in the CGM arm wore their CGM on average 63.8% of the time. Participants in the UC arm brought logbooks to clinic 75% of the time. There were three hospitalisations all in the CGM arm, but none were related to the intervention.
Conclusions
This is the first randomised controlled trial conducted on CGM in a rural region of a low-income country. CGM was feasible and appropriate among PLWT1D and providers, but inability of participants to change their own sensors is a challenge.
Trial registration number
PACTR202102832069874.
Keywords: DIABETES & ENDOCRINOLOGY, General diabetes, International health services
Strengths and limitations of this study.
Randomised controlled trial evaluating feasibility and acceptability of continuous glucose monitoring (CGM) in a rural, low-literacy population in a low-income country.
Study participants were followed for a period of 90 days, allowing for longitudinal data on impact of CGM.
Limited by small sample size.
Introduction
Type 1 diabetes (T1D) is a severe autoimmune condition which leads to hyperglycaemia and a lifelong insulin dependency.1 People living with type 1 diabetes (PLWT1D) require uninterrupted access to insulin, tools for glucose monitoring, adequate and uninterrupted access to needles and syringes, and continuous access to education and healthcare services to reduce the risk of mortality, adverse events and long-term complications. In low-income countries (LICs) and lower-middle-income countries, access to affordable and high-quality care is limited. T1D incidence and mortality in these settings are likely underestimated as misdiagnosis and non-diagnosis are common.2–5 Without adequate care, the life expectancy of a child with newly diagnosed T1D in most LICs might be as short as 1 year.6 7 Evidence suggests that currently, almost 9 million individuals are living with T1D, of which one-fifth (1 665 997 people) are in LICs and middle-income countries.8 In Malawi, 6530 people were estimated to be living with T1D in 2022.8 Given these current estimates, it is imperative to improve diabetes care in these settings with integrated care delivery, education and training.
An intermediate level of care for T1D (defined as multiple daily injections of insulin, self-monitoring of blood glucose (SMBG) two to four times per day, consistent point-of-care haemoglobin A1c (HbA1c), complication screening and a team approach to diabetes education and support) is an achievable goal for resource-limited settings that could decrease complication rates and premature mortality.9
SMBG has improved clinical outcomes and quality of life (QoL) for PLWT1D and was the gold standard of care following the Diabetes Control and Complications Trial.10 Novel technological advances for glucose monitoring are now available, requiring an interstitial patch and a reader for real-time continuous glucose monitoring (CGM) using Bluetooth technology. Products including Dexcom G6 (Dexcom, San Diego, California, USA) have reduced the burden of finger sticks by providing interstitial glucose readings, trends and alerts in real-time with a significant reduction in the frequency of severe hypoglycaemic episodes.11
CGM addresses many limitations related to HbA1c testing and SMBG. HbA1c gives only a point estimate of the mean of blood glucose control. SMBG gives some information on variability but not a complete picture, and neither provides real-time alerts about hypoglycaemia or hyperglycaemia. The uptake of CGM devices in many high-income countries is gradually increasing, with good acceptability and clinical outcomes. A recent international consensus statement on the use of CGM technology concluded that CGM data should be used for therapeutic treatment decisions related to hypoglycaemia and glucose variability.12
Currently, no data exist on the feasibility and effect on clinical outcomes of CGM for PLWT1D in rural areas of LICs especially in areas without electricity and having low literacy and numeracy. To address this lack of evidence, we conducted a randomised trial to evaluate the feasibility of CGM technology and change in clinical outcomes among PLWT1D with limited literacy receiving diabetes care at two district hospitals in rural Malawi. Here, we report quantitative results. While the qualitative results are important to understanding the feasibility of CGM in this setting, we report them in a separate paper to provide greater opportunity for discussion of themes and quotes.13 This study is approved by the National Health Sciences Research Committee of Malawi (IRB Number IR800003905) and the Mass General Brigham (IRB number 2019P003554). The protocol was previously published.14
Objectives
The objectives of this study are to (1) assess the feasibility and appropriateness of CGM use among a rural population of PLWT1D and limited literacy in an LIC; (2) to determine if CGM use can have an effect on diabetes clinical outcomes among PLWT1D in rural regions of LICs and (3) determine the SD of HbA1c across individuals at baseline to inform further studies.
Methods
Study setting
The study was conducted at two rural Ministry of Health (MOH)-supported first-level hospitals in Neno district, Malawi, with a population of about 138 000,15 primarily relying on subsistence agriculture. Neno District Hospital is in a mountainous region near the Mozambique border and Lisungwi Community Hospital is in the lower, drier area near the Shire River. Both hospitals are similar in protocol and resources and are overseen by the same district leadership. Since 2007, Partners In Health (PIH), a US-based non-government organisation known locally as Abwenzi Pa Za Umoyo, has partnered with MOH to improve healthcare and socioeconomic development in Neno district. In 2018, two advanced non-communicable disease (NCD) clinics providing high-quality care for complex NCDs, consistent with the package of essential medicines for NCD-plus opened at Upper Neno and Lisungwi.16–18 Patients with T1D enrolled in these clinics receive care from mid-level providers with specialised NCD training. All insulin, syringes and tools for SMBG are provided free of charge to all patients at their routine monthly appointments. PLWT1D typically use human insulin, intermediate-acting (neutral protamine Hagedorn (NPH)) two times per day and fast-acting (regular) two to three times per day. Every household in Neno is visited by a community health worker monthly for education and screening for multiple common conditions, enrolment into maternal and chronic care, and accompaniment to the clinic.19
Study participants
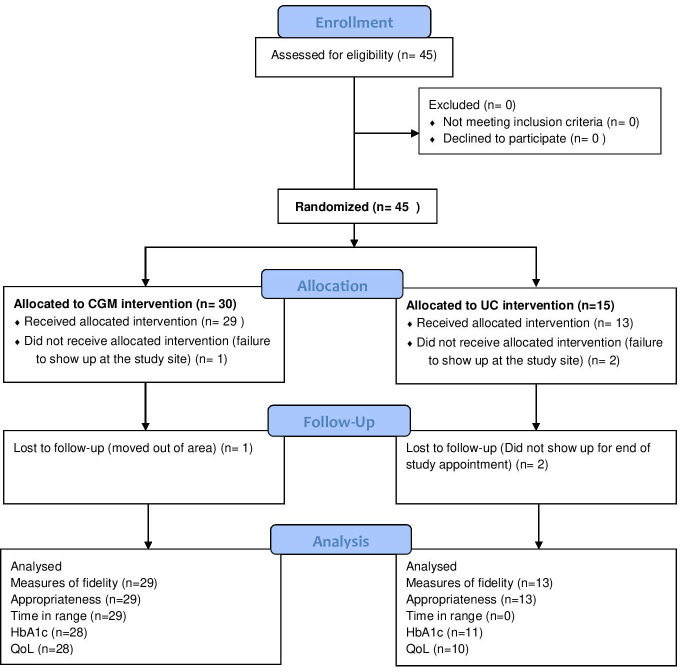
Eligibility criteria for this study included a clinical diagnosis of T1D in PLWT1D, in diabetes care for at least 1 year, and seeking care at either of the PIH-supported MOH hospitals. We did not exclude anyone based on age. Exclusion criteria included pregnancy, mental impairment and the inability of the subject or care provider to use a CGM device. Figure 1 shows the flow diagram of the recruitment process.
Figure 1.
Consort study flow diagram. CGM, continuous glucose monitoring; CONSORT, Consolidated Standards of Reporting Trials; HbA1c, haemoglobin A1c; QoL, quality of life; UC, usual care.
Each participant was required to complete an informed consent/assent (children <18 years of age) form on the day of the enrolment. Study staff were trained to assist patients with limited literacy with the consent process.
Design
Randomisation
All 45 participants known to have T1D and seeking care at hospitals in Neno met the study criteria and were approached for willingness to participate in this study. All agreed and were randomly assigned via a random numbers table to either of the two arms: CGM (Dexcom G6) arm and usual care (UC) arm (using blood glucose meter) in a 2:1 ratio. Study investigators and personnel were masked to the randomisation sequence which was created by a senior researcher.
Provider training
Clinical providers were required to complete 1 month of virtual training on routine diabetes care and understanding CGM in the management of diabetes performed by the study team (including two nurse practitioners and two clinical officers trained in T1D care). Then, providers completed a 2-week in-person hands-on training where they were required to wear a CGM and learn how to use Clarity (Dexcom CGM software). Providers were trained to review data from CGM downloads and SMBG logbook data and make individualised dose adjustments, changes in alarm alerts on the CGM reader, and recommendations for lifestyle and insulin dosing as per usual practice. Clear protocols warranting medical attention were supplied to the providers, and any reported adverse events were immediately assessed and documented. Provider training focused on: glucose targets; goal of time in range (TIR), insulin dosing techniques and principles; basics of insulin therapy and meal planning; understanding signs and strategies for managing hypoglycaemia and hyperglycaemia; understanding sick day management; understanding food insecurity and insulin dose adjustments; and troubleshooting common problems with Dexcom devices.
Intervention
Participants in the CGM arm were provided with a transmitter, a receiver and sensors (Dexcom G6) inserted under the skin using an applicator to wear real-time continuous glucose monitoring technology for 3 months. All CGM equipment was provided free by Dexcom. Each transmitter had a shelf life of 90 days and each sensor had a shelf life of 10 days after which a new sensor needs to be applied. Participants in the CGM arm were instructed to use CGM daily and were advised to either change the sensor on their own or follow up after 10 days for new sensor insertion. Individualised clinical recommendations were made by their providers at each visit using standardised material developed for the study based on Dexcom training materials (online supplemental appendix A). Participants in the CGM arm received a Chichewa-language handout at the beginning of the study to educate them about the features of CGM and readings obtained from the reader.
bmjopen-2023-075554supp001.pdf (2.2MB, pdf)
Comparator
Participants in the UC arm were asked to perform home blood glucose monitoring using Safe-Accu glucose meters and test strips at least once daily and record in the logbooks as per established protocol.20 Providers were encouraged to review retrospective glucose data using SMBG logbook with participants and use the data to adjust insulin and lifestyle recommendations for individualised management.
Both arms
The study staff provided guidelines for routine diabetes management and education to participants in both arms. Follow-up visits for both arms occurred monthly on the usual clinic schedule. The CGM group had additional visits for new sensor insertion and data downloads. Study staff had phone calls with participants to review for any severe adverse events during the study. Participants in both groups received financial compensation for travel to the clinic for each study visit. All diabetes and testing materials were provided free to all participants.
Data collection and interviews
QoL and HbA1c were measured at baseline and the end of the study using the WHO Quality of Life Brief Version (WHOQOL-BREF) questionnaire and a point-of -care HbA1c testing device, respectively. At each visit, logbooks for those in the UC arm and Clarity reports for those in the CGM arm were reviewed. Five participants from each arm were interviewed by the study staff at baseline and endline to discuss their satisfaction with content, use, complexity, comfort and challenges of CGM and glucose meter technology in their setting. Five providers were interviewed regarding their opinions on both technologies. The recruitment of study participants began in March 2022 and data collection was completed by July 2022.
Outcomes
While the primary aim of this study is to understand the feasibility of CGM in a low-resource setting, it is also important to ensure that even if the technology is functional it does not have negative effects on clinical outcomes for users. For that reason, we include two clinical outcomes, HbA1c and TIR. Primary outcomes were split into implementation outcomes, defined using the Proctor21 framework, and clinical outcomes.
Implementation outcomes
Fidelity
Fidelity is defined here using variables reflecting patients’ adherence to the technology used.21 In the CGM arm, fidelity was defined by number of sensors worn, the per cent of time sensors were worn (based on Clarity reports) and times that dose or lifestyle adjustments were made. In the UC arm, fidelity was defined as the per cent of expected blood glucose readings logged, the per cent of participants who brought logbooks to the clinic during the study period, per cent of expected times blood glucose test was performed, the number of times insulin adjustments were made, and how often lifestyle adjustments were suggested.
Appropriateness
Appropriateness was defined as the perceived fit and relevance or compatibility of CGM.21 This was based on sensor problems, reporting of technological issues and qualitative interviews.
Clinical outcomes
Severe adverse events
Severe adverse events were measured from patient self-reports, CGM or home glucose meters, or clinician reports.
Secondary outcomes
Implementation outcomes
Acceptability was defined using Proctor’s framework as the perception that CGM was agreeable, palatable or satisfactory.21 This was measured through qualitative interviews with PLWT1D and providers.
Clinical outcomes
We were only able to measure TIR in the CGM arm, which was calculated using downloaded CGM data. We defined ‘in range’ as blood glucose reading between 70 and 180 mg/dL.22 ‘Very high’ was defined as over 250 mg/dL and ‘very low’ as below 54 mg/dL. Because two participants only had fewer than 5 days of CGM readings each, we included a sensitivity analysis removing these participants’ data.
Change in HbA1c
Change in HbA1c was measured as the change from baseline to endline measured using PTS Diagnostics A1CNow+ point of care test kits. Due to lower-than-expected HbA1c measurements, we also included a comparison of endline HbA1c results and the 90-day estimated average glucose values calculated using Clarity reports of patients in the CGM arm of the study.
The SD in HbA1c was calculated using the overall HbA1c SD in the baseline point-of-care tests. QoL was measured using the WHOQOL-BREF both at baseline and at the end of the study. The WHOQOL-BREF includes four domains: physical health, psychological, social relationships and environment. QoL was calculated both by individual domain and overall.
Statistical methods
Statistical analyses were conducted using R V.4.2.2 or Stata V.14. We did not conduct sample size calculations because we recruited all PLWT1D receiving care at two PIH-assisted hospitals where this study was being conducted. Rather, we calculated power to detect the difference in HbA1c with the number of patients who participated (29 in the CGM arm and 13 in the UC arm). Given a pooled SD of 2.05 and an alpha level of 0.05, we had 80% power to detect a 1.96 percentage point difference in HbA1c between the two study arms (SAs) in a two-sample t-test. Initial power calculations relied on a larger number of expected participants.14 We conducted analysis as intention to treat.
HbA1c analysis
To test whether the change in HbA1c differed between the CGM and UC arms, we used the linear regression model specified below, equivalent to longitudinal analysis of covariance, where HbA1c at follow-up (HbA1ct1) is predicted by SA, HbA1c at baseline (HbA1ct0), facility site (Site), age (Age), female gender (Fem), diagnosis year (DY) and Body Mass Index (BMI), with an error term, ε, assumed normally distributed. The coefficient on SA, β1, was the parameter of interest.
We report the point estimate and 95% CI for this parameter estimate from the fully adjusted model above as well as a minimally adjusted model, only including the terms for SA and baseline HbA1c.
To test the relatively low HbA1c levels, we compared the difference between endline HbA1c results and the 90-day estimated average glucose values for participants in the CGM arm. Estimated average glucose (EAG) was calculated in the Clarity application. The standard formula of EAG (mg/dL) = 28.7×A1c−46.7 was used to convert EAG to estimated HbA1c.23 Paired t-test was used to compare the estimated HbA1c to the point-of-care HbA1c.
QoL analysis
To estimate the difference in the change in QoL between SA, we used the same approach as for HbA1c. We conducted a regression for each of the four domains of the WHOQOL-BREF as well as the overall score, reporting the point estimate and 95% CI for the estimated difference in the change between the arms from the fully adjusted model, adjusting for the same variables as in the HbA1c analysis described above except for BMI.
Per cent of time-worn and TIR analyses
This CGM device measures glucose levels roughly every 5 min. We summarised the measurements in several ways. First, we calculated the proportion of expected observations that were missing values. We did this by dividing time into 5-minute increments. If no observation was present for a period longer than 5.06 min, we considered each 5-minute increment between the previous and subsequent observations as missing. Then, we calculated the proportion of all observations that were missing. We calculated the proportion of non-missing observations within the desired blood glucose range (70–180 mg/dL) to estimate TIR, as well as the proportion that was very low (under 54 mg/dL), low (54–69 mg/dL), high (181–250 mg/dL) and very high (over 250 mg/dL). We additionally calculated the mean and IQR of the non-missing observations.
The CGM sensors lasted 10 days, but many patients returned to the clinic every 14 days to obtain replacement sensors. Therefore, a substantial proportion of the missingness was related to timing of sensor replacement. We estimated this proportion by assuming that any missingness on the day of a sensor replacement (recorded by study clinicians) was related to the replacement, and any missingness contiguous with (ie, no non-missing observations between) and prior to (including in previous days) that period of missingness was categorised as related to the sensor replacement. We then tabulated the proportion of missing observations related to sensor replacement. Not all individuals experienced long periods missing a sensor, as some felt comfortable replacing sensors at home and were given extra sensors by study staff.
Qualitative methods
We conducted a series of semistructured interviews with 10 patients (5 in each arm) at the beginning and end of the study. We also interviewed five providers (two nurses and three clinicians) who provided care to the patients during the study period. Trained members of the study team conducted all interviews. Provider interviews were conducted in English. Patient interviews were conducted in Chichewa and translated by a bilingual researcher. All interviews were audio recorded and transcribed by a trained researcher. Interviews were coded in Dedoose and analysed using a thematic framework using a priori themes.
Deviations from protocol
We initially planned a 2-day training for participants, with 1 day devoted to comprehensive T1D education. However, due to long distances needed to travel for participants and resulting missed school and work, 2 consecutive days was not feasible. Instead, for 2 months before the start of the study, providers gave enhanced diabetes education to all participants. In the protocol outcomes, we had stated the per cent of expected times CGM and SMBG information was used to inform lifestyle-adjusted interventions, and we were unable to determine the per cent so we used number of times instead. We had initially included change in HbA1c as a primary outcome, but due to lack of power we changed this to a secondary outcome.
Patient and public involvement
PLWT1D were engaged throughout the study. Three of the outcomes of this research were feasibility, acceptability and appropriateness, so much of the study involved gaining perspectives, experiences and views of the technology by PLWT1D. Two of the study coauthors (GF and AG) are living with T1D and were involved throughout the design of the protocol, tools, training and implementation of the study.
Results
Participants
There were 45 individuals with T1D meeting the inclusion criteria at the two eligible hospitals. When approached by phone, all agreed to be included and were randomised, 30 to the CGM arm and 15 to the UC arm. On the day of trial initiation, one from the CGM arm and two from the UC arm did not present and therefore did not participate. At the end of the study, one participant in the CGM arm and two from the UC arm were not present for their final evaluations and were considered lost to follow-up (figure 1). The trial was initiated on 11 April 2022 in Lisungwi District Hospital and 14 April 14th 2022 in Upper Neno District Hospital and ran for 90 days. Table 1 shows baseline characteristics of trial participants in both arms.
Table 1.
Characteristics of participants at baseline
| Study arm | All participants (N=42) | ||
| CGM (n=29) | Usual care (n=13) | ||
| Location (% Upper Neno) | 48.0 | 46.0 | 47.6 |
| Age (years) (mean (range)) | 30.9 (8–51) | 29.6 (8–46) | 30.5 (8–51) |
| Age (years) (median) | 32 | 30 | 31 |
| Sex (%) | |||
| Female | 48.0 | 38.0 | 45.2 |
| Male | 52.0 | 62.0 | 54.8 |
| Age at diagnosis (mean (SD)) | 25 (10.1) | 26.3 (9.9) | 25.4 (10.4) |
| Age of diagnosis (median) | 26 | 26 | 26 |
| Years since diagnosis (mean (SD)) | 6.2 (6.2) | 3.7 (1.7) | 5.4 (5.3) |
| Years since diagnosis (median) | 4 | 4 | 4 |
| BMI (mean (SD)) | 21.4 (3.6) | 24.5 (5.6) | 22.4 (4.6) |
| Baseline HbA1c (%) (mean (SD)) | 8.5 (2.2) | 7.9 (2.1) | 8.3 (2.1) |
| Baseline total daily insulin dose (units/day) | 53.59 | 49.23 | 52.24 |
BMI, Body Mass Index; CGM, continuous glucose monitoring; HbA1c, haemoglobin A1c.
Primary outcomes
Implementation outcomes
Fidelity
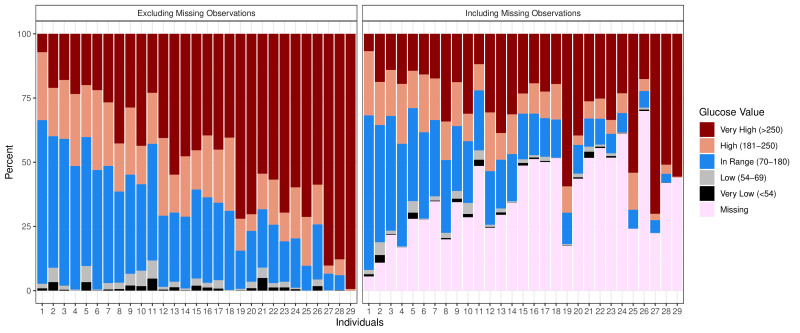
Major fidelity outcomes are seen in table 2 and figure 2. There was a higher rate of consultations in the CGM arm (mean 8.3) compared with the UC arm (1.3). In the CGM arm, participants used a mean of 6.8 sensors over the study period, with a range of 2–9 sensors. The average participant had recordings taken by their sensors for 63.8% of the time (median: 65.5%; IQR: 49.9%–75.6%). A sensitivity analysis done dropping two individuals with only 2 days of observation made little change to the result (average 63.5% median: 65.5%; IQR: 49.3%–75.7%). As many participants were unable to change the sensor on their own and clinic days were only once a week, there was, on average, a 4-day lag between one sensor ending and the next sensor being applied. We estimated the amount of each individual’s missingness due to this 4-day lag and found that, on average, 72.7% of the missingness was due to lags between sensor changes (median: 83.4%; IQR: 63.7%–92.6%). Sensitivity analysis showed only minimal change to the result (mean 74.4%; median 83.4%; IQR: 65.1%–92.6%). Among the time we did not classify as ‘missing due to sensor change’ because of missingness adjacent to documented sensor changes, participants had sensor recordings an average of 87.0% of the time (sensitivity analysis 86.9%).
Table 2.
Measures of fidelity in participants
| Study arm | ||
| CGM (n=29) |
UC (n=13) |
|
| Consultations attended (mean) | 8.3 | 1.3 |
| Individuals with insulin adjustments (n (%)) | 20.0 (69.0) | 2.0 (15.0) |
| Insulin adjustments made (n) | 35.0 | 2.0 |
| Insulin adjustments per individual (mean) | 1.2 | 0.2 |
| Lifestyle change suggestions (n) | 13.0 | 3.0 |
| Lifestyle change suggestions per individual (mean) | 0.4 | 0.2 |
| CGM arm | ||
| Sensors worn (mean (range)) | 6.8 (2,9) | |
| Per cent of time-worn (mean (SD)) | 63.8 (16.1) | |
| UC arm | ||
| Consultations with logbook brought to clinic (%) | 75.0 | |
| Readings logged (%) | 51.3 | |
CGM, continuous glucose monitoring; UC, usual care.
Figure 2.
Time in range for each participant with missing data included and not included. Individuals 27 and 29 used continuous glucose monitoring devices for less than 1 week.
In the UC arm, participants brought logbooks to consultations 75% of the time. However, readings in logbooks corresponded to glucose meters readings only 51.3% of the time.
Of the 29 individuals in the CGM arm, 20 (69%) had an insulin adjustment made, compared with only 2 individuals (15%) in the UC arm. There were a total of 35 insulin adjustments in the CGM arm, which came to an average of 1.2 per individual, compared with 0.2 per individual in the UC arm. There were roughly double the amount of suggested lifestyle changes in the CGM arm (0.4 per person) compared with the UC arm (0.2) (table 2).
Appropriateness
Over the course of the trial, only one participant in the trial arm was able to change the sensors himself. Two others felt confident to physically change the sensor but were unable to enter the code, so they still needed to come into the clinic to change the sensor. Clinicians reported that after multiple CGM insertions, patients felt confident with the application process and were able to self-apply with guidance; however, they were unable to correctly input the sensor codes. In total, there were 28 cases of sensor failure over the 3-month trial period. During the first sensor use, three individuals complained of discomfort but worked with providers to find a more comfortable way of wearing them. In the first month, three participants accidentally removed the sensors, but there were no reported cases after the first month. Rashes and skin irritation were not a commonly encountered complaint in the CGM arm. The hot weather caused a few participants difficulty with keeping the sensor attached. We overcame this using skin Tac adhesive and cut-out tape overpatches to secure the sensors in place and prevent removal. No sensor-related bleeding or potential skin reaction around or under the sensor was observed. There were no reported problems with the solar chargers, and participants were able to use the solar chargers for light in their houses.
Clinical outcomes
During the study, there were three hospitalisations in the CGM arm and none in the UC arm. None of the hospitalisations were attributed to the intervention. One was due to a long-standing non-healing diabetic foot issue, one was due to low blood sugar due to the participant having no food, and one was due to high blood glucose levels.
Mean endline point-of-care HbA1c was 7.4% (95% CI 6.6% to 8.1%). Mean estimated HbA1c was significantly higher at 10.1% (95% CI 9.3% to 10.8%) and a mean difference of 2.7% (95% CI 2.2% to 3.2%; p<0.05). Online supplemental figure 1 shows point-of-care HbA1c and estimated HbA1c for each participant in the CGM arm.
Secondary outcomes
Overall, participants and providers found the CGM devices acceptable. The main reported complaints concerned the length of time that sensors lasted, and the alarms on the CGM monitors, and some participants reported not liking the visual aspect of the sensor. We go further into qualitative outcomes in our companion piece.13
The average per cent of TIR in recorded readings (not including missing data) was 30.6% (SD 16.1%) (figure 2). Among the 27 CGM arm participants with more than 1 week of recorded data, the average TIR was 32.6% (SD 14.7%). Over the course of the study, there was an increase in the time in the range starting in week 6 (online supplemental figure 2). The average TIR was 30.8% in week 1 and 38.7% in week 10. To examine whether this increase in TIR was due to drop off of non-compliant participants, we conducted a sensitivity analysis looking at only participants whom we had data for at 10 weeks. Among the 20 participants with greater than 5% non-missing data in week 10, the average TIR in week 1 was 34.5%, and the average in week 10 was 37.5% (online supplemental figure 2).
After 3 months, we observed an increase of 0.2 percentage points in HbA1c in the UC arm (n=11 as follow-up HbA1cs missing for two participants) and a reduction of 1.2 percentage points in the CGM arm (n=28) compared with baseline. After adjusting for baseline HbA1c levels and other covariates, participation in CGM compared with UC was associated with a 1.1 percentage point lower HbA1c; CIs were compatible with a moderate to null reduction in the CGM arm relative to the UC arm (95% CI 2.4 percentage point reduction to 0.3 percentage point increase, table 3).
Table 3.
Change in HbA1c at 3 months
| Arm | Mean difference (95% CI) | P value | ||
| CGM (n=28) Mean (SD) |
UC (n=11) Mean (SD) |
|||
| HbA1c at follow-up | 7.4 (1.9) | 7.9 (2.0) | ||
| Crude change from baseline | −1.2 (1.9) | 0.2 (2.7) | −1.38 (−2.92 to 0.17) | 0.08 |
| Model 1 | −0.88 (−2.15 to 0.40) | 0.17 | ||
| Model 2 | −1.07 (−2.39 to 0.26) | 0.11 | ||
Model 1 adjusted for baseline HbA1c only; Model 2 adjusted for baseline HbA1c, facility site, age, sex, diagnosis year and BMI. Note: 28 of the original 29 were included from the CGM arm because 1 person did not have a follow-up HbA1c measurement, and 11 of 13 were included in the UC arm because of missing follow-up measures.
CGM, continuous glucose monitoring; HbA1c, haemoglobin A1c; UC, usual care;
Pretrial, there was an SD of 2.1 in HbA1c pooled across two arms, although baseline HbA1c was low overall compared with what is generally expected in this type of setting.24–26
Over the course of the study, QoL (n=28 in CGM and n=10 in UC) was assessed using WHO-BREF increased across all domains (online supplemental table 1). Though unadjusted QoL increased slightly more in the UC arm (9.0) than the CGM arm (6.7), CIs for differences in the change in QoL between groups were large, and we did not find any strong evidence of differences.
Discussion
Summary of main results
This is the first randomised control trial (RCT) to be carried out on the feasibility of CGM use in a rural area of an LIC. While participants wore their sensors just under two-thirds of the time, much of the missingness (over 70% on average) was attributable to their inability to change their sensors. The most pervasive barrier to CGM use among patients was the reported limited digital literacy and confidence with the sensor application process, which required patients in the CGM arm to come more frequently into the clinic than the UC arm. However, with time and multiple CGM insertions, patients felt confident with the application process and could self-apply under the guidance of the clinicians but still needed help with numerically entering sensor codes to activate them. Skin rashes were not a notable complaint, although due to the hot weather there was some difficulty with sensor adhesion that was rectified by using skin Tac adhesive and cut-out tape overpatches to secure the sensors in place. After the first few weeks, participants tolerated the CGM well, and clinicians were far more likely to make dose adjustments in the CGM arm than in the UC arm. There was a trend towards greater reduction in HbA1c in the CGM arm than in the UC arm. However, there were many more consultations in the CGM arm, so it is difficult to attribute the improvement to the CGM or the greater number of consultations. Given the 4-day lag between sensor end and replacement, the reduction may have been greater without this lag. The intervention was deemed acceptable by participants with the greatest complaint being around sensor beeping.
Comparisons with other studies
This is the first RCT conducted in a rural setting in an LIC to assess the feasibility of CGM and its effect on clinical outcomes and QoL among people living with T1D. To date, there are less than a handful of studies on CGM use in the African continent, none of which are RCTs. One of these studies evaluated the glycaemic profile—glucose exposure, variability, stability and risk of hypoglycaemia—of people living with T1D and type 2 diabetes (T2D) in South Africa, across 16 different clinics.27 In Uganda, Niwaha and colleagues conducted a study to assess the risk of hypoglycaemia for people living with T2D being treated with sulfonylureas or insulin and did not include PLWT1D.28 While the study in South Africa mentioned that some sensors failed to record data, neither this study nor that of Niwaha looked specifically at fidelity, appropriateness or acceptability. A short observational study by McClure Yauch et al was conducted at national referral hospitals in urban areas in Kenya and Uganda to assess feasibility of CGM use and the glycaemic profile of children and young adults affected by T1D using CGM technology.29 They found the use of this technology was tolerated by patients and expressed hope for wider use in the future. This urban study reported an average HbA1c of 10.9% with an SD of 2.7 compared with our average baseline HbA1c of 8.3% and endline HbA1c of 7.5% with an SD of 2.1. Their TIR was 31% compared with the TIR in our study of over 37% by week 10 (32.6% across the whole study period among the 27 participants in the CGM arm with more a few days of data). All three of these studies used the Freestyle Libre Pro, and users were blinded to their glucose data and had CGM use of 14 days. In our study, we used the Dexcom G6 CGM for 90 days, which provides real-time glucose data to the user and can be used to make treatment decisions. None of these studies examined any association between CGM use and QoL.
Comparison of endline point-of-care HbA1c to estimated HbA1c based on CGM values showed that point-of-care HbA1c may be overestimating glycaemic control. A few theories for the discrepancy between HbA1c and mean blood glucose levels have been proposed, including the presence of haemoglobinopathies, individual variations in the lifespan of red blood cells, renal impairment and nutritional deficiencies (eg, iron-deficiency anaemia, Kwashiorkor, Marasmus).30 31 No haemoglobinopathies are present in this patient population. Additionally, numerous assays for point-of-care HbA1c testing have become available over the last decade of possibly varying quality. These findings reinforce that HbA1c alone may not be adequate to evaluate glycaemic control in PLWT1D, adding to current literature highlighting the importance of availability for additional ways to evaluate glycaemic control, such as SMBG or CGM.
Despite challenges participants experienced with changing sensors and data missingness, the amount of glucose data recorded from sensor readings in this study—63.8% of the time (median: 65.5%; IQR: 49.9%–75.6%; sensitivity analysis is mean: 63.5%; median: 65.5%; IQR: 49.3%–75.7%) and 87% when excluding missingness due to lag in sensor change —is higher than data from sensor readings (mean of 51.14 days (60.9%) (SD=20.86), range 20–81 days) in a 90-day pretest and post-test pre-experimental study with children, adolescents and young adults with poorly controlled diabetes living in the USA.32 This underscores the importance, benefits and potential for high impact of ensuring access to glucose monitoring devices for PLWT1D in low-resource settings.
Limitations
This was a feasibility trial with only data from 42 individuals, as such it was not powered for seeing differences between SAs in outcomes like HbA1c and QoL. Due to the inability of patients in the CGM arm to change their device sensor, many patients ended up seeing providers twice a month compared with once a month in the UC arm, making it difficult to separate effects of technology versus the effect of the increased frequency of visits. Additionally, providers were excited about the new technology and may have paid greater attention to patients in the CGM arm. All participants in the study had a diagnosis of T1D; however, limited resources and a lack of pancreatic antibody and C-peptide testing may mean some patients were misdiagnosed. This study was conducted for 3 months. While this is far longer than other studies, reduction in HbA1c levels and behaviour change can take longer than 3 months, so a longer study may have found greater effects. Conversely, we do not know what adherence would look like after 3 months.
Implications for future research and practice
Our study suggests that CGM is feasible, appropriate and acceptable in rural Malawi and may show greater effectiveness in lowering HbA1c than SMBG. We highlight the need to include practical digital literacy and numeracy training for patients when considering CGM as a viable clinical option in diabetes management in such settings, and future studies and practice should explore ways participants with low literacy can learn to change sensors independently. Newer models of CGM (Dexcom G7, Freestyle Libre 2 and Freestyle Libre 3) do not require sensor codes to be inputted for activation, so may be better suited to this setting. As devices were donated by Dexcom, this study did not examine costs, but continued global advocacy is necessary to ensure equitable access to intermediate T1D care for PLWT1D in LICs. Other studies may examine if short periods of intensive CGM use are equally effective as a training tool for both patients and providers allowing a more granular assessment of glycaemic control than previously possible with glucose meters. In contrast, other studies looking at longer lengths of time using CGM may be able to explore if this is a tool that can enhance PLWT1D’s understanding of their condition, improve diabetes self-management, decrease adverse events and diabetes-related complications, advance providers’ skills and knowledge and assist with decision-making around insulin initiation for people living with T2D. Further, examining if there is added benefit and cost-effectiveness of real-time CGM compared with flash glucose monitoring and un-blinded CGM compared with blinded in this setting is warranted.
Conclusion
This is the first RCT conducted on CGM in a rural region of an LIC. Overall, this small feasibility study conducted in one Malawian district found CGM to be feasible and appropriate among PLWT1D and their healthcare providers. Inability of participants to change their own sensor is the biggest challenge, though could be addressed with use of newer sensor models. Although not statistically significant, the downward trend in HbA1c in SA is promising and worth investigating over a longer period, especially in light of increased TIR from baseline to endline. The current model of care needs to be strengthened and TIR continues to be low—posing higher risk for acute and chronic complications among this population.
Supplementary Material
Acknowledgments
Roy Beck for advice on trial design.
Footnotes
@ApoorvaGomber
Contributors: Study and tool design: AJA, TR, FV, CT, GF, AM, EBW, CK, GB and PHP. Training: AG, CT and GF. Data analysis: MMC, FV, AG, AJA, AT and LD. Guarantor: AJA. All authors contributed to the final manuscript.
Funding: This work was supported by the Leona M. and Harry B. Helmsley Charitable Trust grant number 2105-04638. Dexcom generously donated CGM Dexcom 6 glucose meters and sensors for the study free of charge.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Deidentified data are available upon reasonable request from the corresponding author (AJA) at aadler2@bwh.harvard.edu.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the National Health Sciences Research Committee of Malawi (IRB Number IR800003905) and the Mass General Brigham (IRB number 2019P003554). Participants gave informed consent to participate in the study before taking part.
References
- 1. WHO . Diabetes distress assessment & resource center, Contract no.: September 25; 2017.
- 2. Bahendeka SK. Diabetes in sub-Saharan Africa: let us not forget type 1. Lancet Diabetes Endocrinol 2017;5:575–7. 10.1016/S2213-8587(17)30223-1 [DOI] [PubMed] [Google Scholar]
- 3. Sandy JL, Besançon S, Sidibé AT, et al. Rapid increases in observed incidence and prevalence of type 1 diabetes in children and youth in Mali, 2007-2016. Pediatr Diabetes 2021;22:545–51. 10.1111/pedi.13191 [DOI] [PubMed] [Google Scholar]
- 4. Bhutta ZA, Ul Haq Z, Basit A. Diabetes in Pakistan: addressing the crisis. Lancet Diabetes Endocrinol 2022;10:309–10. 10.1016/S2213-8587(22)00102-4 [DOI] [PubMed] [Google Scholar]
- 5. Marshall SL, Edidin DV, Arena VC, et al. Mortality and natural progression of type 1 diabetes patients enrolled in the Rwanda LFAC program from 2004 to 2012. Int J Diabetes Dev Ctries 2017;37:507–15. 10.1007/s13410-016-0536-z [DOI] [Google Scholar]
- 6. Beran D, Yudkin JS. Diabetes care in sub-Saharan Africa. Lancet 2006;368:1689–95. 10.1016/S0140-6736(06)69704-3 [DOI] [PubMed] [Google Scholar]
- 7. Castle WM, Wicks AC. A follow-up of 93 newly diagnosed African diabetics for 6 years. Diabetologia 1980;18:121–3. 10.1007/BF00290487 [DOI] [PubMed] [Google Scholar]
- 8. Index TD. Type 1 diabetes around the world. n.d. Available: https://www.t1dindex.org
- 9. Ogle GD, von Oettingen JE, Middlehurst AC, et al. Levels of type 1 diabetes care in children and adolescents for countries at varying resource levels. Pediatr Diabetes 2019;20:93–8. 10.1111/pedi.12801 [DOI] [PubMed] [Google Scholar]
- 10. Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 11. Akturk HK, Dowd R, Shankar K, et al. Real-world evidence and glycemic improvement using Dexcom G6 features. Diabetes Technol Ther 2021;23:S21–6. 10.1089/dia.2020.0654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Battelino T, Alexander CM, Amiel SA, et al. Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol 2023;11:42–57. 10.1016/S2213-8587(22)00319-9 [DOI] [PubMed] [Google Scholar]
- 13. Thapa A, Chibvunde S, Schwartz L, et al. n.d. A qualitative study of appropriateness and acceptability of continuous glucose monitoring in people with type 1 diabetes at rural first-level hospitals in Malawi. Submitted to BMJ Open [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adler AJ, Ruderman T, Valeta F, et al. Protocol for a feasibility randomised control trial for continuous glucose monitoring in patients with type 1 diabetes at first-level hospitals in rural Malawi. BMJ Open 2022;12:e052134. 10.1136/bmjopen-2021-052134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Statistical Office . 2018 Malawi population and housing census main report; 2018.
- 16. WHO . WHO PEN and integrated outpatient care for severe, chronic NCDs at first referral hospitals in the African region (PEN-plus): report on regional consultation. Brazzaville, Congo: World Health Organization Regional Office for Africa; 2020. [Google Scholar]
- 17. Ruderman T, Chibwe E, Boudreaux C, et al. Training mid-level providers to treat severe non-communicable diseases in Neno, Malawi through PEN-plus strategies. Ann Glob Health 2022;88:69. 10.5334/aogh.3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bukhman G, Mocumbi A, Wroe E, et al. The PEN-plus partnership: addressing severe chronic non- communicable diseases among the poorest billion. Lancet Diabetes Endocrinol 2023;11:384–6. 10.1016/S2213-8587(23)00118-3 [DOI] [PubMed] [Google Scholar]
- 19. Wroe EB, Nhlema B, Dunbar EL, et al. A household-based community health worker programme for non-communicable disease, malnutrition, tuberculosis, HIV and maternal health: a stepped-wedge cluster randomised controlled trial in Neno district, Malawi. BMJ Glob Health 2021;6:e006535. 10.1136/bmjgh-2021-006535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruderman T, Ferrari G, Valeta F, et al. 2023 implementation of self-monitoring of blood glucose for insulin dependent diabetes patients in a rural non-communicable disease clinic in Neno. S Afr Med J 2023;113:84–90. 10.7196/SAMJ.2023.v113i2.16643 [DOI] [PubMed] [Google Scholar]
- 21. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011;38:65–76. 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–40. 10.2337/dc17-1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nathan DM, Kuenen J, Borg R, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–8. 10.2337/dc08-0545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bahendeka S, Mutungi G, Tugumisirize F, et al. Healthcare delivery for paediatric and adolescent diabetes in low resource settings: type 1 diabetes clinics in Uganda. Glob Public Health 2019;14:1869–83. 10.1080/17441692.2019.1611897 [DOI] [PubMed] [Google Scholar]
- 25. Saiyed M, Hasnani D, Alonso GT, et al. Worldwide differences in childhood type 1 diabetes: the SWEET experience. Pediatr Diabetes 2021;22:207–14. 10.1111/pedi.13137 [DOI] [PubMed] [Google Scholar]
- 26. Amos M, Gladys C, Sandeni C, et al. Complications and glycaemic control of type 1 diabetes mellitus amongst children aged 5 to 19 years attending diabetic clinic at Kamuzu central hospital in Malawi. Int J Diabetes Clin Res 2020;7. 10.23937/2377-3634/1410117 [DOI] [Google Scholar]
- 27. Distiller LA, Cranston I, Mazze R. First clinical experience with retrospective flash glucose monitoring (FGM) analysis in South Africa: characterizing glycemic control with ambulatory glucose profile. J Diabetes Sci Technol 2016;10:1294–302. 10.1177/1932296816648165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Niwaha AJ, Rodgers LR, Carr ALJ, et al. Continuous glucose monitoring demonstrates low risk of clinically significant hypoglycemia associated with sulphonylurea treatment in an African type 2 diabetes population: results from the OPTIMAL observational multicenter study. BMJ Open Diabetes Res Care 2022;10:e002714. 10.1136/bmjdrc-2021-002714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McClure Yauch L, Velazquez E, Piloya-Were T, et al. Continuous glucose monitoring assessment of metabolic control in East African children and young adults with type 1 diabetes: a pilot and feasibility study. Endocrinol Diabetes Metab 2020;3:e00135. 10.1002/edm2.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malka R, Nathan DM, Higgins JM. Mechanistic modeling of hemoglobin glycation and red blood cell kinetics enables personalized diabetes monitoring. Sci Transl Med 2016;8:359ra130. 10.1126/scitranslmed.aaf9304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Little RR, Rohlfing CL, Hanson SE, et al. The effect of increased fetal hemoglobin on 7 common HB A1C assay methods. Clin Chem 2012;58:945–7. 10.1373/clinchem.2012.181933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lewis KR, McCrone S, Deiriggi P, et al. Effectiveness of continuous glucose monitoring in children, adolescents, and young adults with poorly controlled type 1 diabetes. J Spec Pediatr Nurs 2017;22. 10.1111/jspn.12162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-075554supp001.pdf (2.2MB, pdf)
Data Availability Statement
Data are available upon reasonable request. Deidentified data are available upon reasonable request from the corresponding author (AJA) at aadler2@bwh.harvard.edu.