Abstract
Very old critically ill patients pose a growing challenge for intensive care. Critical illness and the burden of treatment in the intensive care unit (ICU) can lead to a long-lasting decline of functional and cognitive abilities, especially in very old patients. Multi-complexity and increased vulnerability to stress in these patients may lead to new and worsening disabilities, requiring careful assessment, prevention and rehabilitation. The potential for rehabilitation, which is crucial for optimal functional outcomes, requires a systematic, multi-disciplinary approach and careful long-term planning during and following ICU care. We describe this process and provide recommendations and checklists for comprehensive and timely assessments in the context of transitioning patients from ICU to post-ICU and acute hospital care, and review the barriers to the provision of good functional outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-024-01306-1.
Keywords: Critical care, Old patients, Geriatric rehabilitation, Comprehensive geriatric assessment, Frailty, Post-ICU syndrome
Introduction
Very old patients (aged 80+) are the fastest growing patient population in intensive care in many countries [1]. Critical illness and the burden of treatment in the intensive care unit (ICU) can lead to a long-lasting decline of functional and cognitive abilities, especially in the very old, frail patient with reduced resilience to stress [2, 3]. The restoration of functional integrity and the associated improvement in overall quality of life are considered to be both the goal and the main patient-centred outcome measure among this age cohort. Thus, rehabilitation that supports this process is a key component of managing critical conditions in very old individuals. Evidence suggests that early mobilization and rehabilitation improves patient-centred outcome measures [4–6]. The process of rehabilitation should start in the ICU and continues far beyond discharge. It requires coordination between intensive care and geriatric medicine at both the in-patient and out-patient settings to employ the full armamentarium of the latter in the most effective way [7, 8]. This narrative review will focus on rehabilitation for very old ICU patients and will discuss steps required in the ICU to prepare these geriatric patients for rehabilitation following discharge.
Geriatric rehabilitation
Very old critically ill patients typically present the greatest clinical and rehabilitation challenges due to complex multi-morbidity and a substantially increased vulnerability to stress [9]. Rehabilitation delivers a range of complementary tailored interventions to attain the goal of optimal physical and cognitive function with minimal disability among people with impairments [10].
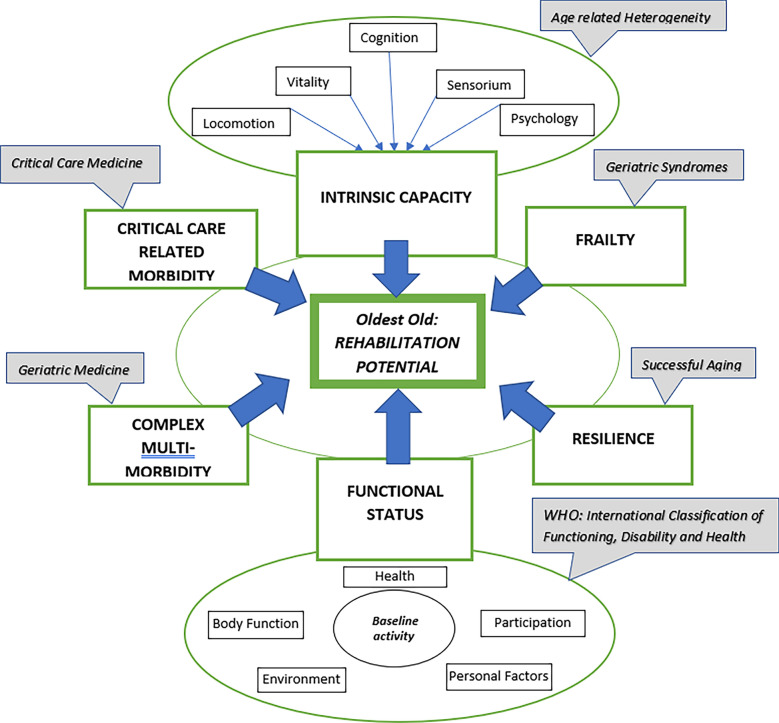
In the last 20 years, the World Health Organization has adopted the International Classification of Functioning, Disability and Health (ICF), which represents a conceptual shift from the classical model of "disease-impairment-disability-handicap" [11]. The ICF model emphasizes a multiplicity of interacting synergistic factors, which lead towards the final common endpoint of disability (Fig. 1). Placed within the ICF concept, rehabilitation must address the five interacting domains of “health conditions”, “body functions and structure”, “participation”, “personal factors” and “environmental barriers”. Geriatric medicine in general, and geriatric rehabilitation in particular, is increasingly recognizing the importance of the emerging concept of "Intrinsic Capacity". Attempting to operationalize and deepen the understanding of this novel entity, contemporary aging theorists consistently return to the geriatric core issues of locomotion, neuromuscular function, sensorium (hearing/vision), and physical vitality (frailty/resilience/homeostasis/reserve), alongside cognitive, psychological, and social function [12–14].
Fig. 1.
A proposed conceptual framework for rehabilitation potential of very old patients in the ICU. The proposed framework recognizes the multiple factors which influence rehabilitation potential of very old patients in the ICU. In addition to specific considerations of both critical and geriatric medical care, the individual's baseline functional status and level of activity is recognized to be an important determinant of subsequent rehabilitation potential. The emerging concept of intrinsic capacity, reflecting vital domains which display a wide heterogeneity among older people, is also introduced into the proposed framework, in addition to the modulating factors of frailty and resilience
The concepts of frailty and resilience among older people are useful tools to help understand and quantify the increasing heterogeneity across multiple biological systems, which typifies the aging process [15–17]. This observation is true for not only trajectories of disease, function and survival, but also for rehabilitation potential. The gap between the concepts of biological and chronological aging among the oldest old emphasizes the importance of accurate personalized assessment, covering the wide range of geriatric, functional, and rehabilitation potential and goals [18, 19]. In the absence of such individualized and multi-dimensional assessment, critical decisions throughout the patients' care concerning ICU admission, continuation of intensive care, as well as post-ICU placement are likely to be based erroneously upon chronological age alone [20, 21].
Multidisciplinary approach
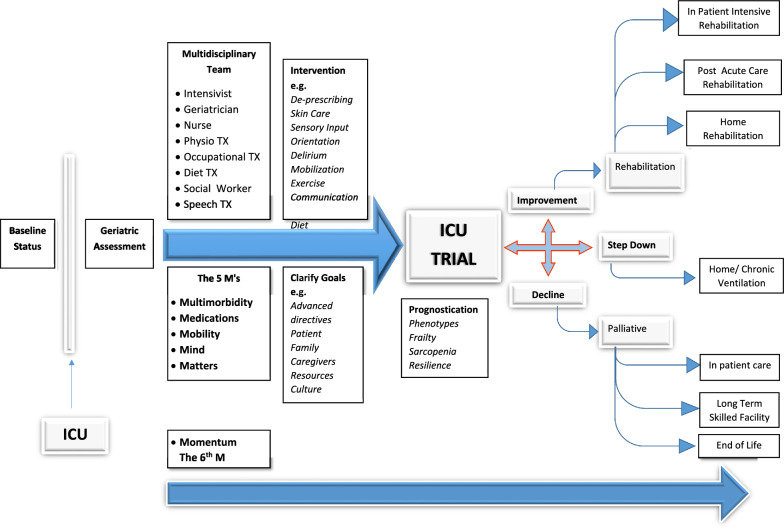
A multidisciplinary team embedded in the ICU is a prerequisite for optimal geriatric rehabilitation of the very old ICU patient. These include physicians (intensivists, geriatricians, and rehabilitation specialists, as well as endocrinologist and palliative care specialists as needed), nurses, physiotherapists, occupational therapists, dieticians, social workers and speech therapists (Fig. 2). Regular assessment as well as ongoing follow-up are essential to accurately determine the short and long-term rehabilitation potential in view of the patient's chronic conditions, acute illness, medications, physical and mental reserve and treatment preferences. Prognostication in this patient population is challenging due to multi-morbidity, complexity and frailty, however identification of different phenotypes has been shown to assist in decision making and in tailoring interventions including early rehabilitation [22]. Multidisciplinary discussions involving the ICU team, members of the rehabilitation team, patients and family members are required to facilitate shared decision-making concerning short-term rehabilitation goals [8], and if necessary, the replacement of invasive and/or intensive treatments by palliative oriented care [23, 24].
Fig. 2.
The trajectory of critically ill very old patients through ICU admission and post discharge. The trajectory of very old critically ill patients includes baseline comorbidity and function, ICU multidisciplinary assessment and treatment, time limited trials and defining goals of care, rehabilitation assessment and planning. Post ICU trajectories include short and long term rehabilitation in acute and subsequently chronic/home care as well as end of life decision making
Geriatric assessment and goal setting in the ICU
Primary goals of care in the treatment of very old patients in intensive care include organ support and recovery of organ function, improved survival rates and prevention of hospital acquired complications. From a geriatric medicine perspective, goals of care also include improved physical and cognitive function, maintenance of a reasonable quality of life and provision of adequate rehabilitation during and following hospital admission. In the geriatric patient population, comprehensive geriatric assessment (CGA) is the standard tool to identify risks for adverse outcomes and prioritise treatment targets in the long term, such as restoration of mobility [25]. Although evidence for the beneficial role of CGA in the ICU is still missing [26], it is known that patients receiving a CGA on hospital admission are more likely to survive and be discharged to their own homes [27]. Even though it appears unrealistic to have a complete CGA in the ICU due to the incapacity of the patient and resource constraints, abbreviated versions of the CGA can be employed to extract pivotal information. For example, the 5Ms framework focuses on core elements of the CGA: 'Mobility', 'Mind', 'Medications', 'Multi-complexity', and what 'Matters most' for older patients and has been recommended for geriatric emergency care [28–30]. The feasibility of conducting core elements of the CGA within the acute care setting is challenging and remains an area to be further studied in order to facilitate implementation. Among the limited research that does exist, evidence supports common tools for the assessment of frailty and cognition [14, 31, 32] (Table 1).
Table 1.
Title core elements for geriatric assessment in and following ICU: the 6M's
| Core domain | Site | Agent of care | Timing | Example of intervention |
|---|---|---|---|---|
| Mobility | ||||
|
Mobilization Passive/active Posturing |
ICU/ Step down |
ICU Nursing staff/ Physiotherapist |
Post admission Stable patient |
Positioning in & out of bed; Passive range of movements |
|
Post admission Stable patient /awake/ cooperative |
Active Range of movements/Transfers/ Strength exercise/Balance/ Dynamic/static/coordination | |||
| Mind | ||||
|
Cognitive assessment Delirium Assessment |
ICU | ICU Nursing staff | Post admission/ Awake/ or RASS > -2 | Delirium (hypo / hyperactive) CAM |
| Step down | GNP | Awake | CGA | |
| Cognition/orientation | ICU | ICU Nursing staff |
Post admission Communicating |
Follow up |
| Step down | GNP | CGA | ||
| Psychological Status/mood/sleep | ICU | ICU Nursing staff | Post admission/ Communicating/ No delirium | Follow up |
| Step down | GNP | CGA | ||
| Competency/capacity | ICU | GNP/ Geriatrician/ Psychiatrist | Post admission/ Communicating/ No delirium/ No narcotics/ sedatives | Kitchen Picture Test [39] |
| Step down/ General ward | ||||
| Communication | ICU | Speech Therapist/ Nursing staff | Post admission/ Communicating/ No delirium/ No narcotics/ sedatives | |
| Step down/ General ward | ||||
| Sensory Integrity/Assistive sensory aids | ICU |
Ophthalmologist Otolaryngologist |
Post admission | Family and patient interview/Past Medical History/Physical assessment/ ENT/ophthalmologist |
| Step down/ General Ward | ||||
| Pain/nausea | ICU |
ICU Nursing staff/ Pain clinic consultants/ GNP for delirious patients |
From day one of admission | BPS for sedated patient/ VAS for alert patient |
| Step down/ General ward | ||||
| Medications | ||||
| Polypharmacy | ICU |
Physician/GNP Clinical Pharmacist |
Continuous | Assess chronic medications/withholding/reducing/ restarting according to patient's condition |
| Step down General Ward | Continuous |
Adjusting medications to patient's condition Preparation for stable dosing and long-term treatment |
||
| De-prescribing: Psychoactive/Central acting Drugs | ICU | Physician/ GNP | From admission, According to patient's condition. Aim for Minimum dose |
BEERS [40] STOPP START [41] |
| Step down/ General Ward | ||||
| Multimorbidity | ||||
| Sphincter Control/ Autonomic function | ICU/ Step down/ General Ward | Nursing staff follow up | From admission, according to patient's condition | |
| Skin care/pressure sores | ICU/ Step down/ General Ward | Nursing staff follow up | Continuous assessment from admission, according to patient's condition | NORTON scale for risk assessment/ Daily skin assessment [42] |
| Oral care | ICU/ Step down/ General Ward | ICU Nursing staff | Daily assessment and standard care | |
| Swallowing assessment | ICU/ Step down/ General Ward |
Condition dependent Not intubated Awake and cooperative Routing feeding-Nursing Staff |
Continuous assessment from admission, according to patient's condition | Staff report swallowing difficulties/ known pathology/ Speech Therapist/ ENT |
| Nutritional Status | ICU/ Step Down/ General Ward | Dietician | Continuous assessment from admission, according to patient's condition | |
| Frailty/Physiological reserve | ICU | Nursing staff | Assessed on admission | History/Screening tools. CFS [43] |
| Step Down/General Ward | Physician/ GNP | Assessed on admission | Diagnostic tools e.g. HANDGRIP/ TUG | |
| What Matters Most | ||||
| Preferences/goals/ cultural background/Integrity/dignity | ICU/ Step down/ General Ward |
Physician/Nursing/ GNP/ Palliative care consultation/ Social Worker (S/W)/ Advanced directives/ Next of Kin (NOK)/ Surrogates |
Conscious and competent patients-Assessed on admission or earliest timing possible | Patient preferences/advance directives/surrogates/custodians/family interview |
| Momentum | ||||
| Prognostics/Trajectory of critical illness | ICU/ Step Down/ General Ward | Physicians | Updating according to available data | Time-limited trial with short term goals/ monitor biomarkers |
| Motivation/Compliance/Resilience | Step Down/General Ward |
Physician/Nursing/GNP/ Physiotherapists/Social workers/ all staff |
Conscious/Competent patient at earliest timing possible | |
| Social support/Family support | ICU/ Step Down/General Ward | S/W | Continuous assessment from admission, background, acute and chronic conditions changes in patient's condition | Family and other caregivers/Barriers to future care/ Finances |
BEERS—Beers Criteria for Potentially Inappropriate Medication, BPS—Behavioural Pain Scale, CAM—Confusion Assessment Method, CFS—Clinical Frailty Scale, CGA—Complete Geriatric Assessment, ENT—Ear Nose and Throat, GNP—Geriatric Nurse Practitioner, ICU—Intensive Care Unit, NOK—Next of Kin, RASS—Richmond Agitation and Sedation Scale, STOPP-START—Screening Tool of Older People's Prescriptions (STOPP), Screening Tool to Alert to Right Treatment (START), S/W—Social Worker, TUG—Timed Up and Go, VAS—Visual Analog Scale
Rather than a single static geriatric assessment, it is often the older patient's trajectory over time in the ICU that is of critical importance in both prognostication and decision-making [33, 34] (Fig. 2). The patient's changing state is a reflection of their resilience, i.e. their ability to "bounce back". The overall direction (Momentum) of older patients' response over time, reflects not only their response to disease/organ-specific treatment, but is also highly influenced by the core geriatric concepts of frailty/intrinsic capacity/ resilience. Indeed, taken as the sum of these core determinants within the critical care scenario, we would suggest that an assessment of the patient's "Momentum" over time might be seen as the 6th "M" to be considered in addition to the 5Ms framework [35].
The findings of the geriatric assessment have to be integrated with information about the critical illness in order to determine goals and potential care trajectories [22]. In that context, the focus on patient-centered outcome measures means [35]:
to include the patient's individual preferences and beliefs in order to frame choices within the dimensions of benefit as well as harms and burdens of care,
to favour therapies that optimize benefit with regard to quality of life and minimize harm,
to consider feasibility of interventions in the patient's and caregivers' social and cultural context.
The effective and sustainable implementation of the above processes requires a designated coordinator with a medical or advanced nursing background [36].
Early assessment of the potential involvement of significant family members and caregivers is an important prognostic factor for functional outcome [37], and an integral step towards facilitating their participation in aspects of both critical care as well as rehabilitation. An assessment of social support and sensitivity towards cultural background and core values is essential in order to help promote and optimize both patient and family compliance with rehabilitation goals and ensure meaningful communication [38].
Assessment of rehabilitation potential
The overall goal of the initial assessment is to aid in prognosticating the functional outcome of intensive care in very old individuals. Existing literature gathered over the last two decades emphasizes the value of assessment of frailty, sarcopenia, functional and cognitive status, and functional performance measures, in addition to illness severity at the time of ICU admission [14, 31, 32]. Predictions of rehabilitation potential and outcomes are difficult, and very few evidence-based recommendations to operationalize this concept exist. However, among the existing literature, consistent associations are noted between multi-morbidity, functional status, and particularly frailty as predictors of both short and long-term function among ICU older patients [9, 31, 44–47]. Fuest et al. described an intelligent-based algorithm for mobilization protocols in four clusters of patients, aimed at increasing the likelihood of discharging patients to their home [48]. They found that most patients, including the cluster of frail patients and non-frail old patients, benefited from frequent mobilization efforts.
Although screening for delirium as an obstacle for rehabilitation has become an essential component of intensive care, other components of the neurocognitive status, including pre-existing cognitive impairments, as well as brief screening for decision-making capacity and competence, require close attention. The close relationship between pain, agitation, and delirium with generalized neurocognitive status has been recognized in the PAD (Pain, Agitation and Delirium) guidelines [49, 50], which were subsequently updated in 2018 to also include sleep and immobility in the PADIS (Pain, Agitation, Delirium, Immobility, Sleep) guidelines [50, 51]. The importance of family and caregiver involvement is also recognized as a positive prognostic factor [37], and is included in the ABCDEF bundle (Assess-manage pain; Breathing trials; Choice of analgesic and sedation; Delirium; Early mobilization and Exercise; Family engagement and empowerment)- all of which have been found to be associated with good functional outcomes during and post-ICU [52].
Other rate-limiting factors to the rehabilitation process include cardiovascular and pulmonary reserve, in conjunction with severe sarcopenia, ongoing infection, catabolic, and inflammatory status, which may worsen sarcopenia, further complicating the potential for rehabilitation. Functional assessment of performance measures should be performed to aid in assessment of potential: gait, balance, coordination, strength, range of movement, as well as locomotion; assessment of sensory integrity, autonomic and involuntary function, bowel and sphincter control; skin integrity, nutritional status, swallowing and feeding concerns; as well as communication and assistive aids. Unnecessary lines and catheters should be removed as soon as possible. The question arises concerning when is the optimal timing for assessment of more complex motor, sensory and autonomic function. Clearly clinical judgement is necessary, accounting for the acute nature of the patient's condition. Nonetheless, once the patient is stable, early assessment rather than later is likely to lead to earlier intervention, which in turn is likely to prevent further subsequent deterioration of function. According to the patient's status, the more advanced functional measures might be reserved for assessment prior to discharge to step-down post ICU care.
For convenience we have listed several common assessment tools. These were chosen since they are all very common, standardized, well validated clinical scales, widely used in everyday clinical practice, and accepted in current up to date literature (Table 1 and Supplement Table 1).
Rehabilitation in the ICU
Despite methodological difficulties, lack of standardized treatment protocols, lack of age-dependent stratification, differing criteria for inclusion/exclusion, as well as a generalized under-representation of very old patients in the relevant studies, most studies tend to confirm the proven benefits seen from early mobilization and active exercise programs, whilst confirming a high degree of safety [50, 51, 53, 54].
Physiotherapy aims at the restoration and improvement of neuromuscular integrity and function, strength, coordination, locomotion, and mobilization. Accepted physiotherapy techniques aimed at ICU patients tend to be inclusive of all ages, limited by the patients’ level of performance alone. These include positioning, mobilization, manual hyperinflation, percussion, vibrations, suction, cough, and breathing exercises [55]. Nonetheless, it is likely that age-associated premorbid conditions (e.g. musculoskeletal degenerative disease, chronic pain, sarcopenia) are likely to complicate rehabilitation, and further emphasize the need for individualized specific care for the very old ICU patient [56, 57].
Occupational therapy aims to optimize an individual's "occupation" in meaningful activities, aiming to develop, improve or regain mental and physical performance [58, 59]. Occupational therapy in the ICU setting when relevant, aims at maximizing optimal sensory input, assistive technology, cognitive treatment, self-care skills, as well as specific postural aids and splints. Closely coordinated work between dietitians and speech therapists aiming to overcome swallowing difficulty as well as to optimize dietary intake. The growing availability of virtual reality within the rehabilitation scenario is finding its way into the ICU, both for cognitive as well as physical exercise training. Additionally, its use is being examined as a potential preventive tool for delirium, using its potential to induce relaxation and counteract the noxious cognitive and sensory stimuli of the ICU environment [50, 51, 60, 61]. As awareness grows concerning the potential usefulness of early intervention of both existing and novel rehabilitation modalities, further exploration of the feasibility and implementation challenges of these interventions in the ICU may be warranted.
The critically important role of specialized and skilled nursing care is often the final common pathway in patient care, integrating the results of all the multidisciplinary treatment modalities into the day-by-day care. Translating short-term goals of the specific rehabilitation modalities into everyday practice requires mindful nursing care which is actively oriented towards the rehabilitation goals. Thus, for example the primary goals nursing care of the very old ICU patient may be aimed at optimal skin care, pain control, bowel habit, oral hygiene, early mobilization, attention to sensory input (eyeglasses and hearing aids), improved communication and orientation, reduced restraints and environmental stressors, and improved family involvement and support. However, such steps are likely to induce secondary effects and benefits such as reduced delirium and psychomotor agitation, improved sleep and mood disorders, reduction in stress and levels of depression, as well as improved disposition towards their environment and greater compliance with rehabilitation interventions [62, 63]. Successful rehabilitation frequently requires optimal control of disturbing symptoms, and a palliative assessment may be indicated. Furthermore, it may become apparent during the ICU admission that rather than rehabilitation and recovery, the primary goals of care are oriented to the end of life. In such cases the involvement and support by palliation teams may improve patient and family stress and suffering and allow for an easier transition through the ICU stay towards end-of-life care [64, 65]. (Table 1 and Supplement Table 1).
Rehabilitation interventions after discharge from the ICU
The common sequelae of critical illness among the very old survivors of ICU with severe generalized deconditioning and functional decline are described in Table 2, and may be collectively termed "Post Intensive Care Syndrome"—PICS [37, 66–68]. PICS is a complex syndrome of multiple physiological cognitive and psychological impairments following intensive care treatment. Examples include intensive care acquired weakness, dysfunctional swallowing, memory loss, delirium, post-traumatic stress disorder and depression [68]. These long-term effects may also influence family members (PICS-F) who have to cope with these functional changes and necessitate a family-centered rehabilitation approach [69].
Table 2.
Common sequelae following ICU admission among very old people
| Deconditioning and weakness | Cognitive impairment |
|
ICU–acquired weakness Neuropathy Myopathy Sarcopenia Frailty Medication induced |
Dysfunction across multiple domains Impaired decisional capacity/competence |
| Psychological disorders | |
|
Confusion Anxiety Depression Post-traumatic stress Psychosis | |
| Feeding and Nutritional Problems | Behavioural |
|
Oral/dental problems Swallowing disorder Dysphagia Post-intubation damage Reduced intake Anorexia/cachexia Malabsorption Catabolic state |
Psychomotor agitation Sleep disorder Negative disposition/reduced compliance Reduced interaction with environment Withdrawal |
| Sensory impairment | |
|
Hearing Vision Taste Smell | |
| Skin and Wounds | |
|
Breakdown Infections Pressure Sores Delayed Healing | |
| Inflammatory status | |
|
Catabolic state Inflammation Ongoing infections Immunocompromised/suppressed | |
| Reduced Physiological Reserve | |
|
Cardiovascular Hemodynamic Pulmonary Endocrine homeostasis Renal Immunological Bone metabolism | |
| Functional Decline | |
|
Immobility Incontinence Dependence in Basic Activities/Function | |
| Pain | Procedure related morbidity |
|
Musculoskeletal system Contractures/Range of movement Prolonged immobility Invasive procedures |
IV lines Catheters Drains |
| Delirium | |
| Associated with previous impairment | |
| Predicts subsequent impairment |
Despite the common preconception of poor long-term rehabilitation potential and outcomes among very old ICU survivors, little high-quality evidence-based research actually exists to either support or disclaim this view. Findings that do exist are often inconclusive, and conflicting. Thus, for example, a Canadian study of ICU patients aged 80+ showed that 25% of subjects had survived and returned to their baseline function after 12 months [32]. These findings stand in contrast to a Finnish study of people age 80+, which showed that among the 62% surviving to 12 months, 78% actually reached their baseline function [31]. Indeed current NICE clinical guidelines for rehabilitation following critical illness make no distinction based upon chronological age alone [70, 71]. The negative effects of ICU admission on close family members and informal caregivers is also recognized, both short and long-term [72]. These in turn may result in additional subsequent negative repercussions upon the patient’s rehabilitation potential.
As important as initial triage, the “seamless transition of care” at the time of discharge from ICU is a critical step in the patient’s trajectory (Fig. 2). As emphasized by UK guidelines, the assessment of subsequent rehabilitation potential at the time of discharge, is a vital determinant of the subsequent degree of appropriate care, occurring at the critical moment of “stepping-down” [71]. While multidisciplinary assessment at ICU admission may be both ambitious and challenging, it becomes gradually more realistic and feasible to perform increasingly complex core elements of assessment along the trajectory through ICU, as the patient stabilizes and their rehabilitation potential becomes apparent. Thus, the CGA might be perceived as an evolving assessment, culminating in a truly comprehensive picture prior to decision-making at the moment of ICU discharge and step-down. Often reflecting the patient's degree of existing resilience, cognition, neuromuscular and cardiovascular reserves, as well as motivation, compliance and disposition towards rehabilitation, the intensity of different geriatric rehabilitation settings are varied [73, 74]. In general, step down from ICU to general medical wards within the general hospital precedes subsequent transfer to rehabilitation facilities. The UK guidelines remain relevant for these intermediate settings. Nonetheless, there is a major need to encourage the widespread implementation of such guidelines, emphasizing the rehabilitation needs of the patient at the time of transfer out of the ICU so that the accepting ward continues the relevant assessment and planning.
Rehabilitation may be provided in different modalities and intensity depending on the patients' potential and capacity. Variously named as geriatric rehabilitation, sub-acute care, post-acute care, and transitional care, there is often a very large variability in definition, standards and intensity of care, and costs (Fig. 2). In the absence of high-quality research, it is largely unclear how different care-settings actually affect patient outcomes. Amongst the oldest old geriatric rehabilitation patient, the entire spectrum of geriatric medicine comes into the forefront. The range of different services among different healthcare systems reflect the ongoing debate concerning the optimal rehabilitation setting for these complex and challenging patients [67].
Home rehabilitation
In addition to intense geriatric rehabilitation, the area of home rehabilitation is becoming increasingly popular. Indeed, home care in general is an area of rapid growth, having received renewed interest following the recent COVID-19 pandemic [75]. The recent growth of innovative technology, enabling for example smart homes, remote monitoring, telemedicine, as well as enormous financial incentives to reduce the burden on in-patient hospital beds: all these together have led to renewed interest and a surge in the provision of home rehabilitation [76]. Whilst contingent upon a high degree of both patient, family, and caregiver compliance, home care is commonly a preferred option by the dyad of patient and family caregivers and seen as a financial win–win for the Healthcare provider in many places.
Like many aspects of rehabilitation among the oldest old ICU patient, there still is very little evidence-based research concerning home rehabilitation. Among existing research, the direction of findings is generally supportive of the positive home rehabilitation outcomes among survivors of ICU, across measures of locomotion, quality of life, safety, respiratory function as well as financial viability of home based care [76, 77]. Furthermore, among patients returning home following ICU despite failure to wean from invasive ventilation, in some countries home hospital is viewed as a preferred option among many prolonged mechanically ventilated patients, among whom quality of life, mood, and measures of caregiver stress consistently support home versus long-term care facilities [78–80].
Common barriers
The common barriers to rehabilitation among ICU patients in and following ICU admission, are more frequent among older people (Table 3). Patient-centered barriers include: fatigue/weakness/pain/polypharmacy/anxiety/poor motivation/confusion/restraints. Common recurring themes to emerge among ICU patients across all ages, emphasize loss of self-autonomy and competence, dehumanization and a need for recalibration of self-identity [81]. It seems likely that these symptoms are more common or pronounced among the very old patients, resonating with pre-existing themes shared in common with aging. It is essential to strive towards early identification of potential barriers which may complicate subsequent discharge and site of care, and to identify potential surrogate decision-makers in the absence of advanced planning directives.
Table 3.
Potential barriers to rehabilitation for the very old critically ill patients
| Patients Centered | Environmental |
| Fatigue | Inadequate availability of rehabilitation therapists |
| Weakness | Inadequate availability of rehabilitation equipment |
| Pain | Negative perception of rehabilitation by staff members |
| Polypharmacy | Inequalities in provision of rehabilitation for very old |
| Anxiety/depression | |
| Confusion | Organizational |
| Agitation | Poor evidence base for this patient population |
| Ongoing delirium | Financial constraints |
| Pressure sores | Attitudes of Stakeholders and Policy makers |
| Lack of Motivation | Local and national health care policy |
| Poor compliance | Ethical and cultural norms |
| Need for restraints | Ageism |
| Family and Caregiver centered | |
| Inadequate social support | |
| Inadequate family support | |
| Caregiver burden and burnout | |
| Lack of consensus concerning goals | |
| Financial constraints |
In addition to patient-centered factors, environmental factors influencing rehabilitation outcomes are numerous [82]. The quantitative lack of resources include low staffing-levels of multidisciplinary healthcare professionals specializing in very old patients; low-frequency or complete lack of geriatric rehabilitation multidisciplinary meetings; lack of specialized rehabilitation equipment; as well as poor availability of subsequent geriatric rehabilitation beds for older people, in and out of acute care. Community based facilities for the rehabilitation of the very old, as well as an adequate infrastructure of knowledgeable, multi-disciplinary teams for home rehabilitation and support are lacking. Thus, there is a need for specialized and mindful planning of potential rehabilitation services and care plans in the community, as well as education programs for the relevant stakeholders, health-care professionals, patients, family members and other caregivers.
No less important are the qualitative barriers to geriatric rehabilitation, especially the lack of acknowledgment and under-appreciation of the necessity and benefits of early geriatric rehabilitation among older critically ill patients. This under appreciation of the benefits, or disinclination to recognize the importance of rehabilitation for the oldest old patient is ultimately responsible for the inadequate delivery of appropriate geriatric rehabilitation, leading to a self-fulfilling prophecy [83].
Inequalities in the provision of geriatric rehabilitation services is perhaps one of the most obstinate barriers to be faced. Limited resources, local and national health policy, stakeholders, as well as financial incentives are but some of the complex factors to be confronted in order to address this pressing issue [71]. Educational steps to revert this imbalance should be aimed not only at ground-level health professionals, but perhaps more importantly, at health-policy and decision-makers. Thus, a multi-tiered approach is necessary to address the current state of affairs whereby the geriatric rehabilitation needs of critically ill old people remain largely unmet [84]. In order to make a more efficient change, further exploration of strategies to both identify and mitigate these numerous barriers may be warranted.
Conclusions
Very old critically ill patients are a rapidly growing population in intensive care units, posing a great challenge in acute care and rehabilitation, due to multi-morbidity, disease complexity and frailty. In parallel to and following treatment for the acute illness, careful geriatric assessment may help evaluate rehabilitation potential, taking into consideration patient heterogeneity in terms of functional capacity, frailty, and resilience. Multi-disciplinary assessment is required for planning optimal rehabilitation, cognitive and sensory function and early mobilization, during and following the ICU stay. A comprehensive multi-system checklist may guide healthcare workers in assessment and planning. Measures to prevent long term sequela of critical illness and to overcome barriers to rehabilitation should be implemented. Family support, not only during but also following ICU care is essential for the continuum of rehabilitation post admission and the provision of good functional outcomes.
Supplementary Information
Author contributions
JMJ, AR, MB and SS wrote the manuscript and prepared the figures and tables. All authors reviewed and approved the final version.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
N/A.
Declarations
Ethics approval and consent to participate
N/A
Consent for publication
N/A.
Competing interests
There are no financial and non-financial competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aliberti MJR, Bailly S, Anstey M. Tailoring treatments to older people in intensive care A way forward. Intensive Care Med. 2022;48(12):1775–1777. doi: 10.1007/s00134-022-06916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herridge MS, Chu LM, Matte A, Tomlinson G, Chan L, Thomas C, et al. The RECOVER Program: disability risk groups and 1-year outcome after 7 or more days of mechanical ventilation. Am J Respir Crit Care Med. 2016;194(7):831–844. doi: 10.1164/rccm.201512-2343OC. [DOI] [PubMed] [Google Scholar]
- 3.Kaushik R, Ferrante LE. Long-term recovery after critical illness in older adults. Curr Opin Crit Care. 2022;28(5):572–580. doi: 10.1097/MCC.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe S, Hirasawa J, Naito Y, Mizutani M, Uemura A, Nishimura S, et al. Association between the early mobilization of mechanically ventilated patients and independence in activities of daily living at hospital discharge. Sci Rep. 2023;13(1):4265. doi: 10.1038/s41598-023-31459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor RS, Dalal HM, Zwisler AD. Cardiac rehabilitation for heart failure: 'Cinderella' or evidence-based pillar of care? Eur Heart J. 2023;44(17):1511–1518. doi: 10.1093/eurheartj/ehad118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perioperative Care of People Living with Frailty. https://cpoc.org.uk/guidelines-resources-guidelines/perioperative-care-people-living-frailty.
- 7.Reid A, Young P. What intensivists can learn from geriatric medicine. ICU Manag Pract. 2020;20(3):195–197. [Google Scholar]
- 8.Woodbridge HR, Norton C, Jones M, Brett SJ, Alexander CM, Gordon AC. Clinician and patient perspectives on the barriers and facilitators to physical rehabilitation in intensive care: a qualitative interview study. BMJ Open. 2023;13(11):e073061. doi: 10.1136/bmjopen-2023-073061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beil M, Flaatten H, Guidet B, Sviri S, Jung C, de Lange D, et al. The management of multi-morbidity in elderly patients: ready yet for precision medicine in intensive care? Crit Care. 2021;25(1):330. doi: 10.1186/s13054-021-03750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . Rehabilitation: key for health in the 21st century. Geneva: Switzerland, World Health Organization; 2017. [Google Scholar]
- 11.World Health Organization . The international classification of functioning, disability and health. Geneva: World Health Organization; 2001. [Google Scholar]
- 12.Beard JR, Jotheeswaran AT, Cesari M, Araujo de Carvalho I. The structure and predictive value of intrinsic capacity in a longitudinal study of ageing. BMJ Open. 2019;9(11):e026119. doi: 10.1136/bmjopen-2018-026119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitnitski AB, Rutenberg AD, Farrell S, Rockwood K. Aging, frailty and complex networks. Biogerontology. 2017;18(4):433–446. doi: 10.1007/s10522-017-9684-x. [DOI] [PubMed] [Google Scholar]
- 14.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howlett SE, Rutenberg AD, Rockwood K. The degree of frailty as a translational measure of health in aging. Nat Aging. 2021;1(8):651–665. doi: 10.1038/s43587-021-00099-3. [DOI] [PubMed] [Google Scholar]
- 16.Mitnitski A, Howlett SE, Rockwood K. Heterogeneity of human aging and its assessment. J Gerontol A Biol Sci Med Sci. 2017;72(7):877–884. doi: 10.1093/gerona/glw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wernly B, Bruno RR, Beil M, Flaatten H, Kelm M, Sigal S, et al. Frailty's influence on 30-day mortality in old critically ill ICU patients: a Bayesian analysis evaluating the clinical frailty scale. Ann Intensive Care. 2023;13(1):126. doi: 10.1186/s13613-023-01223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beil M, van Heerden PV, de Lange DW, Szczeklik W, Leaver S, Guidet B, et al. Contribution of information about acute and geriatric characteristics to decisions about life-sustaining treatment for old patients in intensive care. BMC Med Inform Decis Mak. 2023;23(1):1. doi: 10.1186/s12911-022-02094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung C, Guidet B, Flaatten H. Frailty in intensive care medicine must be measured, interpreted and taken into account! Intensive Care Med. 2023;49(1):87–90. doi: 10.1007/s00134-022-06887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassford C, Griffiths F, Svantesson M, Ryan M, Krucien N, Dale J, et al. Developing an intervention around referral and admissions to intensive care: a mixed-methods study. Health Serv Deliv Res. 2019;7(39):1–284. doi: 10.3310/hsdr07390. [DOI] [PubMed] [Google Scholar]
- 21.Bruno RR, Wernly B, Bagshaw SM, van den Boogaard M, Darvall JN, De Geer L, et al. The Clinical Frailty Scale for mortality prediction of old acutely admitted intensive care patients: a meta-analysis of individual patient-level data. Ann Intensive Care. 2023;13(1):37. doi: 10.1186/s13613-023-01132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mousai O, Tafoureau L, Yovell T, Flaatten H, Guidet B, Jung C, et al. Clustering analysis of geriatric and acute characteristics in a cohort of very old patients on admission to ICU. Intensive Care Med. 2022;48(12):1726–1735. doi: 10.1007/s00134-022-06868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beil M, van Heerden PV, Joynt GM, Lapinsky S, Flaatten H, Guidet B, et al. Limiting life-sustaining treatment for very old ICU patients: cultural challenges and diverse practices. Ann Intensive Care. 2023;13(1):107. doi: 10.1186/s13613-023-01189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vink EE, Azoulay E, Caplan A, Kompanje EJO, Bakker J. Time-limited trial of intensive care treatment: an overview of current literature. Intensive Care Med. 2018;44(9):1369–1377. doi: 10.1007/s00134-018-5339-x. [DOI] [PubMed] [Google Scholar]
- 25.Parker SG, McCue P, Phelps K, McCleod A, Arora S, Nockels K, et al. What is Comprehensive Geriatric Assessment (CGA)? An umbrella review. Age Ageing. 2018;47(1):149–155. doi: 10.1093/ageing/afx166. [DOI] [PubMed] [Google Scholar]
- 26.Wissanji T, Forget MF, Muscedere J, Beaudin D, Coveney R, Wang HT. Models of care in geriatric intensive care-a scoping review on the optimal structure of care for critically ill older adults admitted in an ICU. Crit Care Explor. 2022;4(4):e0661. doi: 10.1097/CCE.0000000000000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis G, Gardner M, Tsiachristas A, Langhorne P, Burke O, Harwood RH, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2017;9(9):Cd006211. doi: 10.1002/14651858.CD006211.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tinetti M, Huang A, Molnar F. The Geriatrics 5M's: a new way of communicating what we do. J Am Geriatr Soc. 2017;65(9):2115. doi: 10.1111/jgs.14979. [DOI] [PubMed] [Google Scholar]
- 29.Lucke JA, Mooijaart SP, Heeren P, Singler K, McNamara R, Gilbert T, et al. Providing care for older adults in the Emergency Department: expert clinical recommendations from the European Task Force on Geriatric Emergency Medicine. Eur Geriatr Med. 2022;13(2):309–317. doi: 10.1007/s41999-021-00578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yogaparan T, Burrell A, Talbot-Hamon C, Sadowski CA, Grief C, MacDonald E, et al. The aging care 5Ms competencies: a modified Delphi study to revise medical student competencies for the care of older adults. Acad Med. 2023;99:198–207. doi: 10.1097/ACM.0000000000005475. [DOI] [PubMed] [Google Scholar]
- 31.Pietiläinen L, Hästbacka J, Bäcklund M, Parviainen I, Pettilä V, Reinikainen M. Premorbid functional status as a predictor of 1-year mortality and functional status in intensive care patients aged 80 years or older. Intensive Care Med. 2018;44(8):1221–1229. doi: 10.1007/s00134-018-5273-y. [DOI] [PubMed] [Google Scholar]
- 32.Heyland DK, Stelfox HT, Garland A, Cook D, Dodek P, Kutsogiannis J, et al. Predicting performance status 1 year after critical illness in patients 80 years or older: development of a multivariable clinical prediction model. Crit Care Med. 2016;44(9):1718–1726. doi: 10.1097/CCM.0000000000001762. [DOI] [PubMed] [Google Scholar]
- 33.Beil M, Flaatten H, Guidet B, Joskowicz L, Jung C, de Lange D, et al. Time-dependent uncertainty of critical care transitions in very old patients - lessons for time-limited trials. J Crit Care. 2022;71:154067. doi: 10.1016/j.jcrc.2022.154067. [DOI] [PubMed] [Google Scholar]
- 34.Guidet B, Vallet H, Flaatten H, Joynt G, Bagshaw SM, Leaver SK, et al. The trajectory of very old critically ill patients. Intensive Care Med. 2024;50:181–194. doi: 10.1007/s00134-023-07298-z. [DOI] [PubMed] [Google Scholar]
- 35.Boyd C, Smith CD, Masoudi FA, Blaum CS, Dodson JA, Green AR, et al. Decision making for older adults with multiple chronic conditions: executive summary for the American Geriatrics Society guiding principles on the care of older adults with multimorbidity. J Am Geriatr Soc. 2019;67(4):665–673. doi: 10.1111/jgs.15809. [DOI] [PubMed] [Google Scholar]
- 36.Sloane PD. The geriatric-focused emergency department: opportunities and challenges. J Am Med Dir Assoc. 2022;23(8):1288–1290. doi: 10.1016/j.jamda.2022.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Herridge MS, Azoulay É. Outcomes after critical illness. N Engl J Med. 2023;388(10):913–924. doi: 10.1056/NEJMra2104669. [DOI] [PubMed] [Google Scholar]
- 38.Jain S, Murphy TE, O'Leary JR, Leo-Summers L, Ferrante LE. Association between socioeconomic disadvantage and decline in function, cognition, and mental health after critical illness among older adults: a cohort study. Ann Intern Med. 2022;175(5):644–655. doi: 10.7326/M21-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mansbach WE, MacDougall EE, Clark KM, Mace RA. Preliminary investigation of the Kitchen Picture Test (KPT): a new screening test of practical judgment for older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2014;21(6):674–692. doi: 10.1080/13825585.2013.865698. [DOI] [PubMed] [Google Scholar]
- 40.American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052–81. [DOI] [PubMed]
- 41.O'Mahony D, Cherubini A, Guiteras AR, Denkinger M, Beuscart JB, Onder G, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023;14(4):625–632. doi: 10.1007/s41999-023-00777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lospitao-Gómez S, Sebastián-Viana T, González-Ruíz JM, Álvarez-Rodríguez J. Validity of the current risk assessment scale for pressure ulcers in intensive care (EVARUCI) and the Norton-MI scale in critically ill patients. Appl Nurs Res. 2017;38:76–82. doi: 10.1016/j.apnr.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Rockwood K, Theou O. Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J. 2020;23(3):210–215. doi: 10.5770/cgj.23.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brummel NE, Bell SP, Girard TD, Pandharipande PP, Jackson JC, Morandi A, et al. Frailty and subsequent disability and mortality among patients with critical illness. Am J Respir Crit Care Med. 2017;196(1):64–72. doi: 10.1164/rccm.201605-0939OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flaatten H, De Lange DW, Morandi A, Andersen FH, Artigas A, Bertolini G, et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥ 80 years) Intensive Care Med. 2017;43(12):1820–1828. doi: 10.1007/s00134-017-4940-8. [DOI] [PubMed] [Google Scholar]
- 46.Guidet B, de Lange DW, Boumendil A, Leaver S, Watson X, Boulanger C, et al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study. Intensive Care Med. 2020;46(1):57–69. doi: 10.1007/s00134-019-05853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haas LEM, Boumendil A, Flaatten H, Guidet B, Ibarz M, Jung C, et al. Frailty is associated with long-term outcome in patients with sepsis who are over 80 years old: results from an observational study in 241 European ICUs. Age Ageing. 2021;50(5):1719–1727. doi: 10.1093/ageing/afab036. [DOI] [PubMed] [Google Scholar]
- 48.Fuest KE, Ulm B, Daum N, Lindholz M, Lorenz M, Blobner K, et al. Clustering of critically ill patients using an individualized learning approach enables dose optimization of mobilization in the ICU. Crit Care. 2023;27(1):1. doi: 10.1186/s13054-022-04291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 50.Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Executive summary: clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):1532–1548. doi: 10.1097/CCM.0000000000003259. [DOI] [PubMed] [Google Scholar]
- 51.Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 52.Pun BT, Balas MC, Barnes-Daly MA, Thompson JL, Aldrich JM, Barr J, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med. 2019;47(1):3–14. doi: 10.1097/CCM.0000000000003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldfarb M, Semsar-Kazerooni K, Morais JA, Dima D. Early mobilization in older adults with acute cardiovascular disease. Age Ageing. 2021;50(4):1166–1172. doi: 10.1093/ageing/afaa253. [DOI] [PubMed] [Google Scholar]
- 54.Parco C, Kreuels V, Kelm M, Jung C, Wolff G. Robotic-assisted early mobilization and virtual reality: a perspective on innovative support strategies for critically ill patients. Intensive Care Med Exp. 2023;11(1):86. doi: 10.1186/s40635-023-00571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stiller K. Physiotherapy in intensive care: towards an evidence-based practice. Chest. 2000;118(6):1801–1813. doi: 10.1378/chest.118.6.1801. [DOI] [PubMed] [Google Scholar]
- 56.Twose P, Jones U, Cornell G. Minimum standards of clinical practice for physiotherapists working in critical care settings in the United Kingdom: a modified Delphi technique. J Intensive Care Soc. 2019;20(2):118–131. doi: 10.1177/1751143718807019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi T, Kato M, Obata K, Kozu R, Fujimoto T, Yamashita K, et al. Minimum standards of clinical practice for physical therapists working in intensive care units in Japan. Phys Ther Res. 2021;24(1):52–68. doi: 10.1298/ptr.E10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jenkins AS, Isha S, Hanson AJ, Kunze KL, Johnson PW, Sura L, et al. Rehabilitation in the intensive care unit: how amount of physical and occupational therapy impacts patients' functionality and length of hospital stay. Pm R. 2023;16(219):225. doi: 10.1002/pmrj.13116. [DOI] [PubMed] [Google Scholar]
- 59.Costigan FA, Duffett M, Harris JE, Baptiste S, Kho ME. Occupational therapy in the ICU: a scoping review of 221 documents. Crit Care Med. 2019;47(12):e1014–e1021. doi: 10.1097/CCM.0000000000003999. [DOI] [PubMed] [Google Scholar]
- 60.Gomes TT, Schujmann DS, Fu C. Rehabilitation through virtual reality: physical activity of patients admitted to the intensive care unit. Rev Bras Ter Intensiva. 2019;31(4):456–463. doi: 10.5935/0103-507X.20190078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naef AC, Jeitziner MM, Gerber SM, Jenni-Moser B, Müri RM, Jakob SM, et al. Virtual reality stimulation to reduce the incidence of delirium in critically ill patients: study protocol for a randomized clinical trial. Trials. 2021;22(1):174. doi: 10.1186/s13063-021-05090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dirkes SM, Kozlowski C. Early mobility in the intensive care unit: evidence, barriers, and future directions. Crit Care Nurse. 2019;39(3):33–42. doi: 10.4037/ccn2019654. [DOI] [PubMed] [Google Scholar]
- 63.Nydahl P, Jeitziner MM, Vater V, Sivarajah S, Howroyd F, McWilliams D, et al. Early mobilisation for prevention and treatment of delirium in critically ill patients: systematic review and meta-analysis. Intensive Crit Care Nurs. 2023;74:103334. doi: 10.1016/j.iccn.2022.103334. [DOI] [PubMed] [Google Scholar]
- 64.Doherty C, Feder S, Gillespie-Heyman S, Akgün KM. Easing suffering for ICU patients and their families: evidence and opportunities for primary and specialty palliative care in the ICU. J Intensive Care Med. 2023 doi: 10.1177/08850666231204305. [DOI] [PubMed] [Google Scholar]
- 65.Puntillo K, Nelson JE, Weissman D, Curtis R, Weiss S, Frontera J, et al. Palliative care in the ICU: relief of pain, dyspnea, and thirst–a report from the IPAL-ICU Advisory Board. Intensive Care Med. 2014;40(2):235–248. doi: 10.1007/s00134-013-3153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Voiriot G, Oualha M, Pierre A, Salmon-Gandonnière C, Gaudet A, Jouan Y, et al. Chronic critical illness and post-intensive care syndrome: from pathophysiology to clinical challenges. Ann Intensive Care. 2022;12(1):58. doi: 10.1186/s13613-022-01038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakanishi N, Liu K, Hatakeyama J, Kawauchi A, Yoshida M, Sumita H, et al. Post-intensive care syndrome follow-up system after hospital discharge: a narrative review. J Intensive Care. 2024;12(1):2. doi: 10.1186/s40560-023-00716-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Renner C, Jeitziner MM, Albert M, Brinkmann S, Diserens K, Dzialowski I, et al. Guideline on multimodal rehabilitation for patients with post-intensive care syndrome. Crit Care. 2023;27(1):301. doi: 10.1186/s13054-023-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vester LB, Holm A, Dreyer P. Patients' and relatives' experiences of post-ICU everyday life: a qualitative study. Nurs Crit Care. 2022;27(3):392–400. doi: 10.1111/nicc.12682. [DOI] [PubMed] [Google Scholar]
- 70.Centre for Clinical Practice at NICE (UK). Rehabilitation After Critical Illness. London: National Institute for Health and Clinical Excellence (UK); PMID: 20704055. https://www.nice.org.uk/guidance/cg83. Accessed Mar 2009 [PubMed]
- 71.Centre for Clinical Practice at NICE (UK). Rehabilitation after critical illness in adults. Quality standard [QS158]: https://www.nice.org.uk/guidance/qs158. Accessed 07 Sep 2017
- 72.Azoulay E, Resche-Rigon M, Megarbane B, Reuter D, Labbé V, Cariou A, et al. Association of COVID-19 acute respiratory distress syndrome with symptoms of posttraumatic stress disorder in family members after ICU discharge. JAMA. 2022;327(11):1042–1050. doi: 10.1001/jama.2022.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Connolly B, Denehy L, Hart N, Pattison N, Williamson P, Blackwood B. Physical Rehabilitation Core Outcomes In Critical illness (PRACTICE): protocol for development of a core outcome set. Trials. 2018;19(1):294. doi: 10.1186/s13063-018-2678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turner-Stokes L, Corner EJ, Siegert RJ, Brown C, Wallace S, Highfield J, et al. The post-ICU presentation screen (PICUPS) and rehabilitation prescription (RP) for intensive care survivors part I: Development and preliminary clinimetric evaluation. J Intensive Care Soc. 2022;23(3):253–263. doi: 10.1177/1751143720988715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Biase S, Cook L, Skelton DA, Witham M, Ten Hove R. The COVID-19 rehabilitation pandemic. Age Ageing. 2020;49(5):696–700. doi: 10.1093/ageing/afaa118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vitacca M, Barbano L, Vanoglio F, Luisa A, Bernocchi P, Giordano A, et al. Does 6-month home caregiver-supervised physiotherapy improve post-critical care outcomes?: A randomized controlled trial. Am J Phys Med Rehabil. 2016;95(8):571–579. doi: 10.1097/PHM.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 77.Werner RM, Coe NB, Qi M, Konetzka RT. Patient outcomes after hospital discharge to home with home health care vs to a skilled nursing facility. JAMA Intern Med. 2019;179(5):617–623. doi: 10.1001/jamainternmed.2018.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jacobs JM, Marcus EL, Stessman J. Prolonged mechanical ventilation: symptomatology, well-being, and attitudes to life. J Am Med Dir Assoc. 2021;22(6):1242–1247. doi: 10.1016/j.jamda.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marcus EL, Jacobs JM, Stessman J. Prolonged mechanical ventilation and caregiver strain: Home vs. long-term care facility. Palliat Support Care. 2023;21(3):429–437. doi: 10.1017/S147895152200027X. [DOI] [PubMed] [Google Scholar]
- 80.Jacobs JM, Marcus EL, Stessman J. Prolonged mechanical ventilation: a comparison of patients treated at home compared with hospital long-term care. J Am Med Dir Assoc. 2021;22(2):418–424. doi: 10.1016/j.jamda.2020.06.038. [DOI] [PubMed] [Google Scholar]
- 81.Corner EJ, Murray EJ, Brett SJ. Qualitative, grounded theory exploration of patients' experience of early mobilisation, rehabilitation and recovery after critical illness. BMJ Open. 2019;9(2):e026348. doi: 10.1136/bmjopen-2018-026348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Potter K, Miller S, Newman S. Environmental factors affecting early mobilization and physical disability post-intensive care: an integrative review through the lens of the world health organization international classification of functioning, disability, and health. Dimens Crit Care Nurs. 2021;40(2):92–117. doi: 10.1097/DCC.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 83.White C, Connolly B, Rowland MJ. Rehabilitation after critical illness. BMJ. 2021;373:n910. doi: 10.1136/bmj.n910. [DOI] [PubMed] [Google Scholar]
- 84.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40(2):502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
N/A.