Significance
We report that the phytohormones strigolactones promote tomato flowering. Our data suggest that this occurs via transcription of the florigen-encoding SINGLE-FLOWER TRUSS (SFT) gene in the leaves. SFT transcription is linked upstream to transcriptional reprogramming including increased levels of miR319 and decreased transcripts of its LANCEOLATE target, a repressor of SFT transcription, in the leaves and meristems. A higher content of gibberellins is also likely to contribute to the poor reproductive performance of strigolactone-depleted tomato. Our study opens novel opportunities to manage fruiting time and total yield for this crop.
Keywords: flowering, LANCEOLATE, miR319, strigolactones, tomato
Abstract
Strigolactones are a class of phytohormones with various functions in plant development, stress responses, and in the interaction with (micro)organisms in the rhizosphere. While their effects on vegetative development are well studied, little is known about their role in reproduction. We investigated the effects of genetic and chemical modification of strigolactone levels on the timing and intensity of flowering in tomato (Solanum lycopersicum L.) and the molecular mechanisms underlying such effects. Results showed that strigolactone levels in the shoot, whether endogenous or exogenous, correlate inversely with the time of anthesis and directly with the number of flowers and the transcript levels of the florigen-encoding gene SINGLE FLOWER TRUSS (SFT) in the leaves. Transcript quantifications coupled with metabolite analyses demonstrated that strigolactones promote flowering in tomato by inducing the activation of the microRNA319-LANCEOLATE module in leaves. This, in turn, decreases gibberellin content and increases the transcription of SFT. Several other floral markers and morpho-anatomical features of developmental progression are induced in the apical meristems upon treatment with strigolactones, affecting floral transition and, more markedly, flower development. Thus, strigolactones promote meristem maturation and flower development via the induction of SFT both before and after floral transition, and their effects are blocked in plants expressing a miR319-resistant version of LANCEOLATE. Our study positions strigolactones in the context of the flowering regulation network in a model crop species.
The switch from the vegetative to the reproductive phase is called floral transition and is characterized by the production of flowers instead of leaves by the shoot apical meristem. In plants such as the day-neutral Solanum lycopersicum (tomato), shoot apical meristems robustly transition to flowering after producing six to nine leaves, depending on the cultivar. Following transition, flower buds enter flower development, the rate of which contributes to defining the timing and intensity of flowering in the plant. The right timing of this transition and of flowering itself plays a pivotal role in the plant life cycle: It is a prerequisite for successful reproduction and environmental adaptation, upon which plant survival depends. Floral transition and flower development are also crucial variables for productivity in fruit and grain crops, with huge agronomical relevance and direct impact on yield.
Flowering is finely regulated by the interaction of multiple genetic pathways and responds both to endogenous hormonal cues and environmental signals (1). A common feature of all flowering plants is that a mobile and graft-transmissible signal is produced in the leaves and reaches the apical meristem via the phloem stream. Such signal, initially called “florigen,” is now characterized and in tomato is the protein encoded by SFT (SINGLE FLOWER TRUSS), the homologue of FLOWERING LOCUS T (FT) in Arabidopsis thaliana (Arabidopsis) (2). Because of rising SFT levels, the apical meristem undergoes conversion to a transitional meristem and then to inflorescence and floral meristems (3, 4). SFT is also crucial for flower development (5): the tomato sft mutants (6) are not only late flowering but they also produce reduced inflorescences or a few flowers and then revert to vegetative functioning. The floral transition requires multiple players and complex interactions; at least five integrated flowering pathways are known in Arabidopsis, all of which affect the expression of FT (4, 7, 8). Contrarily to Arabidopsis, tomato floral transition is not affected by the photoperiod (3) or by vernalization (9); it is instead strongly influenced by the age-dependent pathway, by the action of gibberellins, and by a recently described pathway encompassing the microRNA miR319 (10).
The age-dependent pathway ensures that flowering takes place when the plant has accumulated enough resources to sustain it. It requires the age-dependent reduction of miR156 levels and the transcript increase of its main targets, the transcription factors SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE (SPLs or SBP-box, later SBPs) (11). In tomato, SBPs activate phase transition by directly inducing SFT in leaves and MADS-box genes in the shoot apical meristem (10–12). In parallel, the phytohormones gibberellins also play a key role in flowering induction, but their effects are species dependent: while they promote floral transition in Arabidopsis (13), they act as inhibitors in tomato (10, 14). Upon gibberellin perception, the DELLA proteins are degraded by the proteasome. DELLAs are key negative regulators of the gibberellin signaling pathway (15, 16) and can either activate or deactivate their targets; they also bear genetically separable roles in controlling vegetative and reproductive development (17).
The age-dependent and gibberellin pathways are integrated by the miR319 pathway. In tomato, miR319 promotes flowering by decreasing the transcripts of the TEOSINTE BRANCHED1/CYCLOIDEA/PCF (TCP) gene LANCEOLATE (LA), as confirmed by the early-flowering phenotype of LA-silenced plants (18) and by the delayed flowering of plants expressing a miR319-resistant version of LA (10). LA also increases the expression of gibberellin biosynthetic genes, decreases the expression of their catabolic genes, and thus induces higher levels of the active phytohormone, which contributes to delayed flowering in tomato (10, 19). Furthermore, LA represses SBP transcription, thus interacting both with the age-dependent and the gibberellin pathways. However, LA also directly inhibits SFT expression in leaves, and thus, miR319 can promote floral transition and flower development without the need for gibberellins or SBPs (10). Which or how other hormones may affect the miR319-LA-SFT module is currently unknown.
Strigolactones were discovered as novel carotenoid-derived phytohormones in 2008. Beyond the initial role as signaling molecules in the rhizosphere, they shape plant architecture by inhibiting axillary bud outgrowth, promoting secondary shoot growth and leaf senescence, and affecting root development (20). They are also involved in the responses to abiotic stress (20). For example, they boost antioxidant responses and modulate stomatal activity, at least partly, via cross talk with abscisic acid (ABA) and the microRNA miR156 in tomato (21–23). Finally, strigolactone mutants show general reproductive defects in several—though not all—species. For example, knocking down the biosynthetic gene CAROTENOID CLEAVAGE DIOXYGENASE 7 (CCD7) makes Lotus japonicus produce fewer flowers, fruits, and seeds (24). Among solanaceous plants, the most severely affected potato lines silenced for CCD8 (encoding the dioxygenase acting downstream of CCD7) do not flower at all (25) and in petunia, delayed flowering time and smaller flowers have been reported for analogous lines (26). In tomato, CCD8 silencing causes fewer and smaller flowers and fruits (27). So far, little effort has been put into investigating the molecular underpinnings of these phenotypes, but for the finding that auxin amounts and distribution are altered during fruit ripening in strigolactone-depleted vs. wild-type (wt) tomato (27). However, auxin levels have been found equal in flowers of wt and CCD7-silenced L. japonicus (24). Thus, the molecular mechanisms explaining strigolactone effects on flowering remain largely unclear.
Our study aimed at investigating the role of strigolactones in the molecular network regulating vegetative to reproductive phase transition and flower development in tomato, focusing mainly on the generation of the florigen signal in leaves. We assessed the developmental and molecular effects of excess or depleted strigolactone levels in the shoot, and we showed that endogenous and exogenous strigolactones promote flowering. Through transcriptomics and targeted expression analysis, we examined which functional gene families and pathways linked to flowering are regulated by these hormones. We demonstrated that strigolactones promote flowering by affecting many flowering-related genes, notably by activating the miR319-LA-SFT module in leaves and meristems, with a likely contribution by a reduction of bioactive gibberellin content.
Results
CCD7 Transcript Levels Correlate with Flower Development and SFT Transcript Accumulation in Leaves.
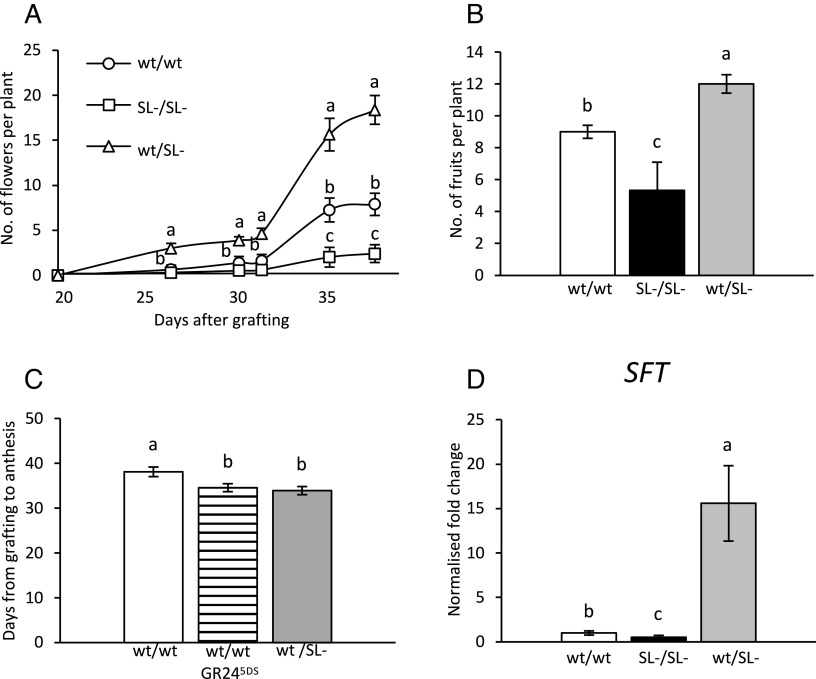
While the vegetative and stress-related phenotype of CCD7-silenced tomato plants (SL- hereafter) has been described (22, 28–32), their reproductive defects have not been investigated to date. To address this point, we contrasted self-grafted SL- plants with the corresponding self-grafted wt and with heterografted plants, in which a wt scion is grafted onto a SL- rootstock (wt/SL-). The latter combination leads to a significant transcriptional activation of strigolactone biosynthetic genes in the leaves, as demonstrated earlier in tomato (32) and other species such as pea (33), and shows no obvious morphological deviation from wt plants during the vegetative phase (SI Appendix, Fig. S1A). Thus, wt/SL-plants were used here to describe the effects of increased endogenous strigolactones in a wt shoot in relation to flowering. The low strigolactone levels in the SL-/SL- self-grafted plants led to a significant decrease in the number of flowers per plant [each counted only once, at the anthesis stage (34)], statistically detectable from 35 d after grafting. Conversely, starting 25 d after grafting, the new flowers on the wt/SL- plants were more than double the number per plant compared to the self-grafted wt/wt plants (Fig. 1A). The number of fruits per plant collected 60 d after the grafting reflected these differences (Fig. 1B), while the cumulative plant yield at the end of the harvesting season did not (SI Appendix, Fig. S2A) because wt/wt plants generally produced bigger fruits. Heterografted plants also showed shorter times to anthesis than wt/wt plants (Fig. 1C), and the number of leaves at anthesis correlates with the timing of flowering (i.e., fewer leaves at anthesis in wt/SL- than wt/wt plants, SI Appendix, Fig. S2B). Note that grafting can hardly be performed before the early reproductive stage, when phase transition has already occurred; thus, our plants had already transitioned at grafting, so this difference can only reflect faster flower development in heterografted plants, and not faster meristem transition. Finally, SFT transcripts 30 d after grafting correlate positively with the transcriptional activity of the strigolactone biosynthetic pathway in leaves, since the heterografted plants displayed higher values than wt/wt, and SL-/SL- plants showed the lowest (Fig. 1D).
Fig. 1.
Effects of different grafting combinations on flowering. (A) Number of new individual flowers at anthesis per plant, counted from 20 to 40 d after homo- or heterografting of wt and strigolactone-depleted (SL-) scions and rootstocks. (B) Number of ripening fruits 60 d after grafting (and no previous harvest). (C) Number of days from grafting to anthesis in homografted wt plants, treated or untreated with 5 µM GR245DS (25 d after grafting), and heterografted plants. (D) Transcript quantification of SFT in leaves of different grafts. Transcript abundance was normalized to endogenous EF1α and ACT and presented as fold-change values over mean values of wt/wt plants, which were set to 1. Data in all panels represent the mean ± SE of n = 5 biological replicates but in panel C, where n = 10. All analyses were run in technical triplicates. Different letters indicate significant differences as determined by a one-way ANOVA test (P < 0.05) and Tukey’s HSD post hoc test.
Treatment with the Synthetic Strigolactone Analogue GR245DS Promotes Flowering and Leads to SFT Induction in Leaves.
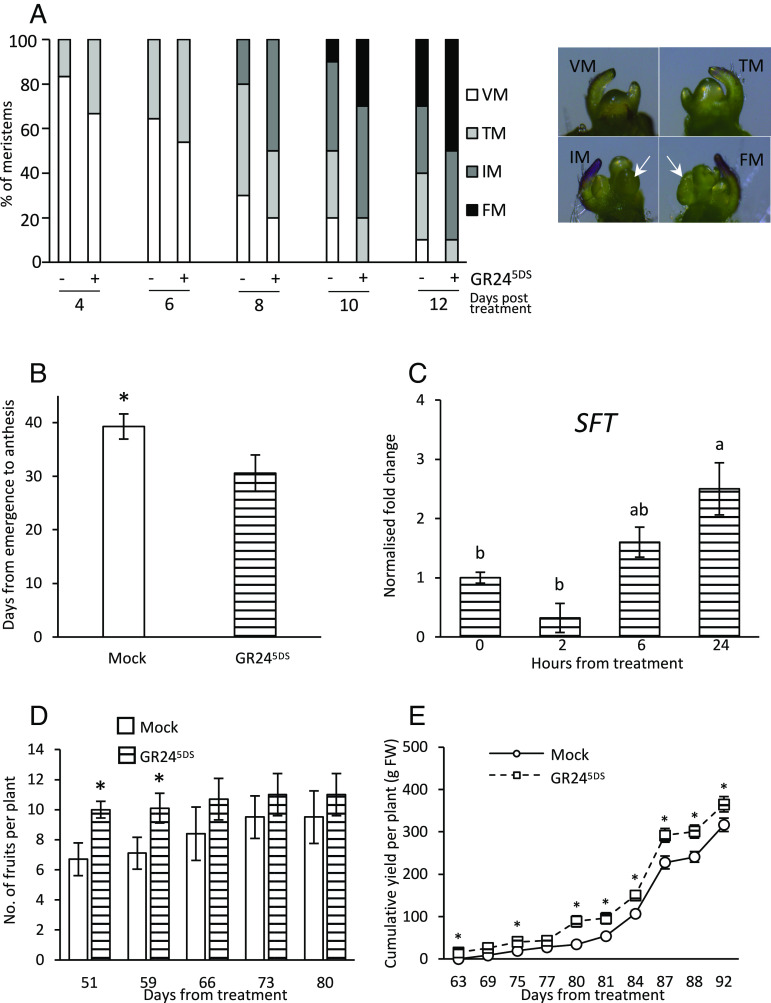
To gain further support for the role of strigolactones in flowering, and to capture the possible modulation of floral transition, we investigated the effect of spraying plant leaves with a 5 µM solution of the synthetic strigolactone analogue GR245DS on meristem development and time to anthesis. Exogenous strigolactones appeared to accelerate the speed of meristem maturation when delivered on juvenile plants (SI Appendix, Fig. S3) and more so when a second treatment was delivered right after transition (Fig. 2A). In addition, when 3-wk-old (beginning of the reproductive phase) wt plants were treated, they brought anthesis significantly forward compared to control plants (Fig. 2B); a similar trend was observed on wt/wt, self-grafted plants (Fig. 1C) treated 25 d after grafting. As for wt/SL- plants, the number of leaves at the time of flowering tended to be lower (albeit not significantly) for GR245DS-treated than mock-treated wt/wt plants (SI Appendix, Fig. S2 B and C). Consistently with the previous observations on grafted plants, SFT transcripts increased in leaves to become significantly higher than the mock-treated control 24 h after GR245DS treatment (Fig. 2C). A consistent trend was apparent in fruit production, with treated plants showing a higher number of fruits [assessed as described earlier (35)] at all time points, compared to mock-treated controls, and more clearly in the early time points (Fig. 2D). Interestingly for its possible agronomic implications, the increase in the number of fruits corresponded to a higher cumulative yield per plant, statistically significant starting 63 d after treatment (Fig. 2E). All these data suggest that treatment with the synthetic strigolactone analogue GR245DS promotes flower development by inducing SFT expression in reproductive tomato plants, while the effects on floral transition, although present, may be more modest.
Fig. 2.
Effects of GR245DS treatment on flowering and SFT transcription. (A) Meristem maturation of mock- or GR245DS -treated plants. Right panel: representative images of the four sequential developmental stages: vegetative meristem (VM), transition meristem (TM), inflorescence meristem (IM), and floral meristem (FM). Plants were treated with a 5 μM solution 4 and 10 d after seedling emergence, i.e., before floral transition and when about 50% of them were at transition. The meristems were evaluated under the stereomicroscope 4 to 12 d after the first treatment (n = 6 to 13). (B) Comparisons between mock-treated plants and plants treated with 5 µM GR245DS 3 wk after seedling emergence, for the number of days from emergence to anthesis. (C) Quantification of SFT transcript in wt leaves after mock treatment or 2, 6, and 24 h after treatment with 5 µM GR245DS. Transcript abundances were normalized to endogenous EF1α and ACT and presented as fold-change values over mean values of mock-treated plants, which were set to 1. Data represent the mean ± SE of n = 5 biological replicates, each analyzed in technical triplicates. Different letters indicate significant differences as determined by a one-way ANOVA test and Tukey’s HSD post hoc test (P < 0.05). (D) Comparisons between mock-treated plants and plants treated with 5 µM GR245DS 4 wk after seedling emergence, for the number of fruits counted 51 to 80 d from the treatment, and (E) average cumulative yield per plant assessed from 63 to 92 d after the treatment. For all tests in B, D, and E, the data represent the mean ± SE of n = 8 biological replicates, and * indicates significant differences between treated and untreated plants for any given time point, as determined by Student's t test (P < 0.05).
Strigolactone-depleted Plants Show an Altered Expression Pattern in the Flowering-related Gene Ontology (GO) Terms.
To get an overview of the regulation of the main metabolic processes and signaling pathways overrepresented in the two genotypes, we RNA-sequenced the leaves of 3-wk-old, ungrafted wt and SL- plants; main results are summarized here, while a broader overview can be found in SI Appendix, Results. The differentially expressed genes (DEGs) were subjected to GO enrichment analysis, which highlighted over 500 enriched GO terms in the Biological process subcategory (Dataset S1); they were grouped in 40 functional categories that display a very different proportion of up- and down-regulated genes. As shown in SI Appendix, Fig. S4, most of the genes related to Developmental processes (GO:0032502) were found to be down-regulated in the SL- plants (191 vs. 64 up-regulated), especially for genes related to Reproduction (GO: 0000003) (114 vs. 34) (Dataset S2). SI Appendix, Table S1 shows a list of DEGs linked to Photoperiodism (GO:0048573); Flowering (GO:2000028); Regulation of flower development (GO:0009909); Floral meristem determinacy (GO:0010582); Vegetative-to-reproductive phase transition of meristem (GO:0010228); and Floral organ morphogenesis (GO:0048444). It is important to highlight here that the list includes, among down-regulated DEGs in SL- plants, some well-characterized flowering-related genes such as the floral inducer SFT, FT-like genes such as SELF PRUNING 6A (SP6A), and SBP3, whose expression is crucial to control the early stages of flower development (36). In addition, we recorded a slight but significant upregulation of LA [log2 fold change (log2FC) = 0.85] (10, 19). The list also comprises several up- and down-regulated genes related to hormone signaling and biosynthesis, including auxin, gibberellins, ethylene, and brassinosteroids (for a review of their functions, see ref. 37).
Strigolactones Promote Flowering via the miR319-LA-SFT Module.
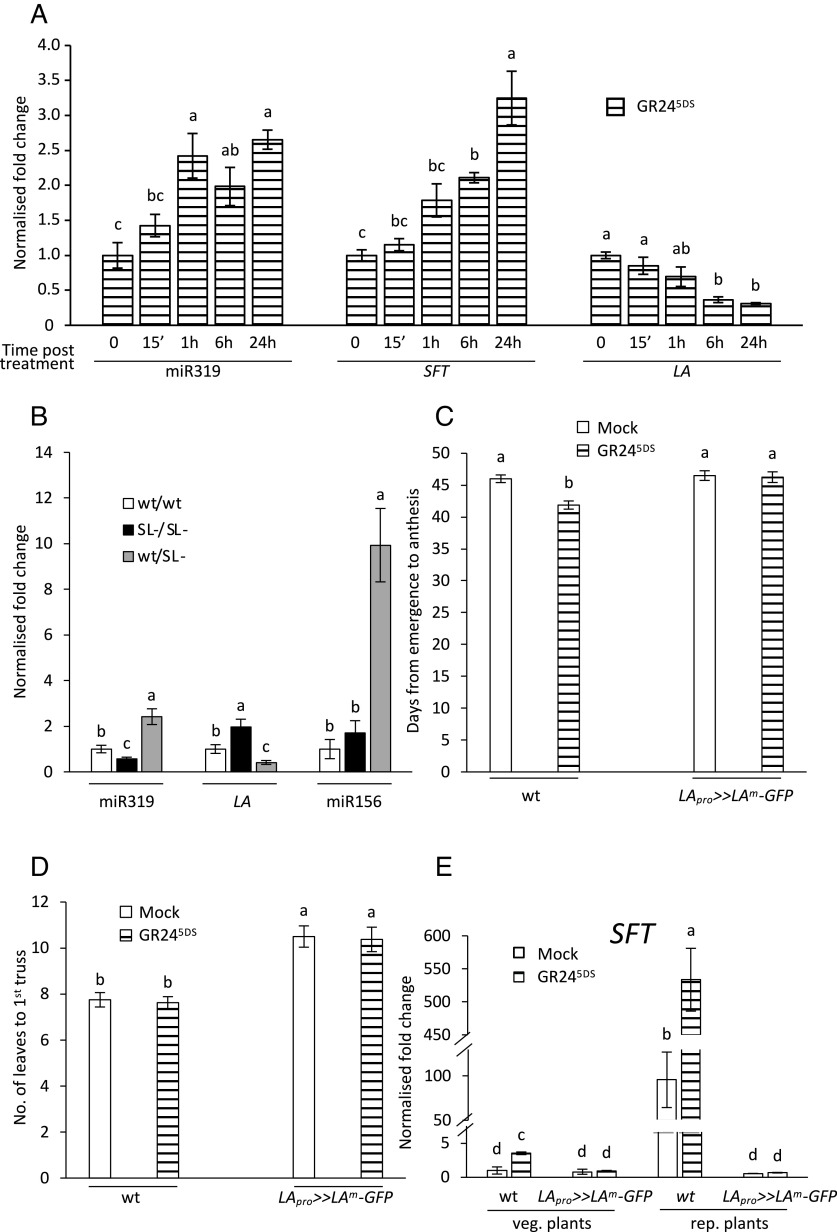
Both LA and the gibberellin biosynthetic and catabolic genes known to be targeted by LA (19) are among the identified DEGs (SI Appendix, Results). To investigate the possibility that strigolactones affect them and flowering via miR319, we quantified the mature miR319 form along with LA and SFT transcripts after spraying wt leaves with GR245DS, and found a rather early induction of mature miR319 followed by the repression of LA and the induction of SFT (Fig. 3A). We also verified that endogenous strigolactones correlate with module activity by quantifying mature miR319 and LA transcripts in the leaves of the wt/wt, SL-/SL- and wt/SL- grafted lines (Fig. 3B). Thus, besides confirming the divergent levels of miR319 and LA transcripts, our results reveal a positive correlation between strigolactone levels and miR319, also reflected by LA (Fig. 3 A and B) and SFT transcript abundance (Figs. 1D, 2C, and 3A).
Fig. 3.
Strigolactones promote flowering via the miR319-LA-SFT module. (A) treatment with GR245DS 5 μM rapidly induces the accumulation of mature miR319 and SFT transcripts in leaves of 4-wk-old wt plants. (B) Effects of endogenous strigolactones on LA transcripts and mature miR319 and miR156 quantified in leaves of the graft combinations wt/wt, strigolactone-depleted SL-/SL- and wt/SL- (heterografted plants: wt scions on SL- rootstocks), 2 wk after grafting. (C) Exogenous strigolactones must be able to lower LA transcripts by increasing miR319 levels to shorten the time to anthesis in tomato. GR245DS 5 μM was sprayed before floral transition (8 d after seedling emergence) on the leaves of M82 plants (wt) or same-age plants expressing the miR319-resistant La-2 allele under the control of its own promoter in the same genetic background (LApro >> LAm-GFP), with n = 8. (D) In the same experiment as in C, the number of leaves at anthesis was counted. (E) Exogenous strigolactones induce SFT transcription only if LA transcripts are free to decrease in dependence of miR319 increase. SFT transcripts were quantified 24 h after treatment with GR245DS 5 μM on 8-d-old (vegetative) or 4-wk-old (reproductive) plants. In A, B and E, data represent the mean ± SE of n = 5 biological replicates analyzed in technical triplicates. In all panels, letters indicate significant differences as determined by a one-way ANOVA test and Tukey’s HSD post hoc test (P < 0.05). SFT and LA-transcript abundances were normalized to endogenous EF1α and ACT, while mature miR319 and miR156 levels were normalized to EF1α and snRU6 and presented as fold-change values over mean values of untreated wt or wt/wt plants, which were set to 1.
To obtain a causative link between the promotion of flowering by strigolactones and the miR319-LA-SFT module, we treated with GR245DS tomato plants that express the La-2 mutant allele (insensitive to miR319-mediated degradation), under the control of the endogenous LA promoter (LApro >> LAm-GFP) (18). The experiment was conducted before floral transition (on 8-d-old seedlings) and confirmed in the first place that strigolactone treatment shortens the time to anthesis (visible in GR245DS-treated vs. mock-treated wt plants in Figs. 1C and 3C), but seems not to significantly change the number of leaves at the time of anthesis (Fig. 3D), consistently with the observations in SI Appendix, Fig. S2 B and C. Most importantly, no effect of GR245DS treatment could be detected on LApro >> LAm-GFP plants, demonstrating that a miR319-dependent degradation of LA transcripts is necessary for the shortening of flowering time by GR245DS to occur. Consistently, transcript quantification in leaves treated with GR245DS shows that strigolactone-induced SFT activation is completely dependent on the lowering of LA transcripts by miR319 action (Fig. 3E). Such effect is visible in the leaves of both vegetative and reproductive plants, but is more marked in the latter.
The Effects of Exogenous Strigolactones Can Be Seen Also in Meristems.
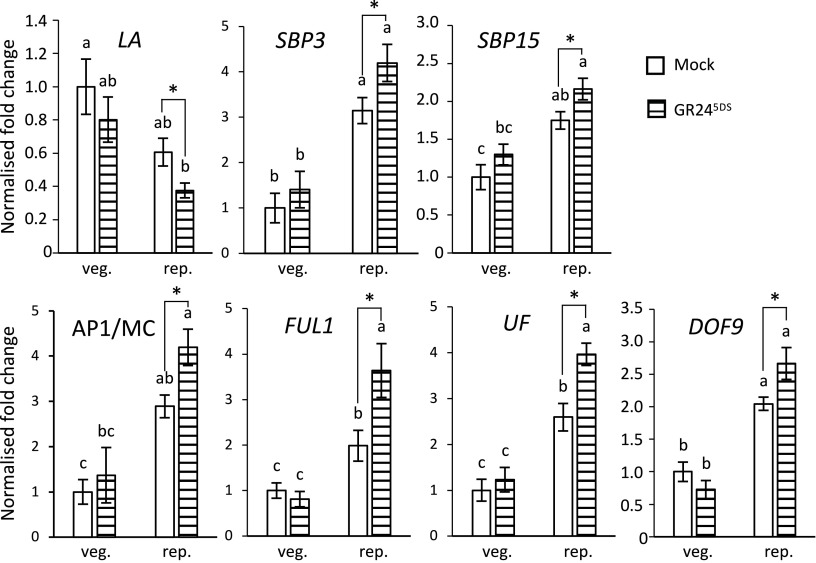
To assess whether strigolactones may not only affect the flowering network in the leaves, we also quantified (in the meristems, and after treatment with GR245DS) the transcripts of several genes related to meristem transition and development (38, 39).The experiment was performed on plants treated 1 wk before sampling, either before floral transition (vegetative plants, sampled 15 d after germination) or after (reproductive plants, sampled at 30 d). It showed that several of them, namely LA, SBP3 and SBP15, FRUITFULL-like1 (FUL1) (40), UNIFLORA (UF) (41), APETALA1/MICROCALYX (AP1/MC) (42), and DNA-binding with one zinc finger9 (DOF9) (43), are affected not only by age but also by exogenous strigolactones (Fig. 4). This effect attained significant levels especially for treatment after floral transition, even though a nonsignificant trend was often visible also in plants treated before transition. Other genes involved in flower development were also tested, showing a similar trend although not reaching the threshold for statistical significance (SI Appendix, Fig. S5). One possible explanation for the lack of significance on genes in SI Appendix, Fig. S5 may be, that their window of regulation by GR245DS may be shifted with respect to the sampling. Alternatively, or additionally—especially for the genes showing a more marked trend (FA, AN, DST, for example)—the possibility exists that the statistical power of our set-up was not sufficient to catch a real difference, or even that strigolactones may impact floral differentiation via a pathway independent of these regulators. Notably, despite the expected auxin-dependent signature in the transcriptome comparison between wt vs SL- leaves (SI Appendix, Table S2), the AUXIN RESPONSE FACTOR5 (ARF5) transcripts were not induced in meristems by GR245DS treatment (SI Appendix, Fig. S5). As a whole, this dataset confirms and reinforces the hypothesis that strigolactones affect flowering by promoting meristem maturation and especially flower development.
Fig. 4.
Effects of exogenous strigolactones on transcripts of marker genes for meristematic development. Vegetative wt plants were treated 8 d after seedling emergence with 5 µM GR245DS and harvested 1 wk later (veg.); another subset was treated also in the reproductive phase, 23 d after germination, and harvested 30 d after germination (rep.). Transcript abundances were normalized to endogenous EF1α and ACT and presented as fold-change value over mean values of meristems in untreated vegetative plants, which were set to 1. Data represent the mean ± SE of n = 6 biological replicates (each the pool of 10 apical meristems), analyzed in technical triplicates. Different letters on top of bars indicate statistically significant differences among all samples as determined with one-way ANOVA followed by Tukey’s post hoc test; asterisks indicate significant differences for pairwise comparisons between treated and untreated samples of the same age, as detected by Student’s t test (P < 0.05).
Strigolactones May Promote Flowering Also by Mitigating Inhibition by Gibberellins.
The integrated activities of two core molecular modules, miR156-SBPs and miR319-LA, and of the phytohormone gibberellins act in concert to modulate the transcription of SFT in tomato (10). In a previous work, we demonstrated that mature miR156 levels correlate positively with strigolactones, as defective strigolactone biosynthesis prevents drought-triggered miR156 accumulation in leaves, and the synthetic strigolactone analogue GR245DS induces miR156 (22). As a further confirmation, the heterografted plants (wt/SL-) of this work, in which we see an activation of the strigolactone biosynthetic pathway in leaves (32) and early and profuse flowering along with SFT induction (Figs. 1 and 2C), also show a marked increase in miR156 levels (Fig. 3B). Thus, we reasoned that strigolactones are unlikely to promote flowering by activating the age-related pathway to flowering, in which miR156 should rather decrease to allow SFT induction in leaves and the transition from the vegetative to the reproductive phase, as well as flower development. Rather, strigolactones appear to act despite the positive correlation with mature miR156 in leaf cells.
Therefore, we focused on the possible role played by alternative components of the flowering network as mediators of strigolactone effects on SFT transcription. Gibberellins were assessed, also considering the proven connection between the miR319-LA module and their biosynthesis (10, 19). The KEGG pathway enrichment analysis of 7140 DEGs between wt and SL- plants confirms widespread dysregulation of genes involved in the biosynthesis of secondary metabolites and signal transduction pathways of plant hormones (SI Appendix, Fig. S6). To better understand the role of gibberellins in strigolactone-mediated flowering promotion, we checked the expression of key components of their signaling and biosynthetic pathways (SI Appendix, Table S3). We found a downregulation of core signal transduction genes, including the ones encoding the receptors GA-INSENSITIVE DWARF (GID) 1a and GID1b1, the F-box protein SLEEPY1 (SLY1) and the downstream transcription factor PHYTOCHROME INTERACTING FACTOR3 (PIF3) (SI Appendix, Table S3). In addition, several genes for biosynthetic enzymes were found differentially expressed between the two genotypes. Those coding for the enzymes that catalyze the last biosynthetic steps toward bioactive gibberellin forms (SI Appendix, Fig. S7) were found strongly up-regulated in the SL- line: Le3OH-23b-hydroxylase (GA3ox-2) and GIBBERELLIN 20 oxidase-2 (GA20ox-2). Instead, GIBBERELLIN 2 oxidase (GA2ox) genes, encoding enzymes that lead to inactive gibberellin forms, were either up- (GA2ox2, GA2ox3) or down-regulated (GA2ox4) (SI Appendix, Table S3). These results were confirmed via quantitative RT-PCR (qRT-PCR) on independent samples for GA2ox4, GA20ox2, and GA3ox2 (SI Appendix, Fig. S8). Thus, on balance, the results suggested that more abundant bioactive gibberellins may contribute to the late and reduced flowering in the SL- plants.
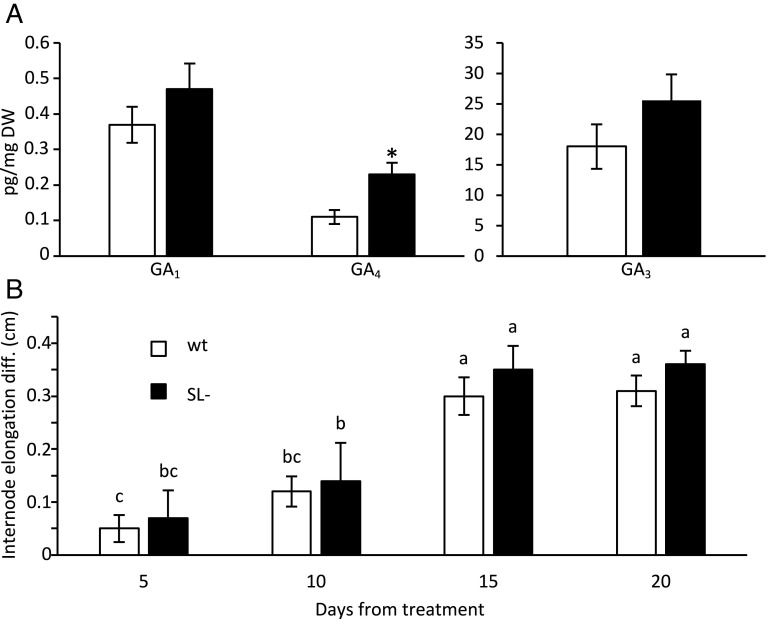
To test this hypothesis, we quantified gibberellins in the leaves of the wt and SL- lines. Fig. 5A shows a trend toward higher amounts of the bioactive forms GA1, GA3, and GA4 in the latter genotype, which is significant for GA4. Such metabolites are produced by the sequential action of the GA20- and GA3-oxidase enzymes, the transcripts of which are strongly up-regulated in these plants. The amounts of other biosynthetic gibberellin intermediates and catabolites (SI Appendix, Figs. S7 and S9) together with the transcription profile of biosynthetic/catabolic genes (SI Appendix, Table S3 and Fig. S8) suggest that gibberellin metabolism is steadily skewed toward more active and less inactive metabolites when strigolactone levels are decreased. Instead, despite the downregulation of genes coding for gibberellin receptors, sensitivity to exogenous gibberellins seemed unaffected in SL- plants, at least in terms of elongation of the first internode upon gibberellin treatment (Fig. 5B).
Fig. 5.
Effect of strigolactone depletion on gibberellin metabolism and sensitivity. (A) Concentration of the active gibberellins GA1, GA4 and GA3 in wt and strigolactone-depleted (SL-) plants, 4 wk after seedling emergence. Data represent the mean ± SE of n = 3 biological replicates analyzed in technical quadruplicates. * indicates significant differences between treated and untreated plants for any given time point, as determined by Student’s t test (P < 0.05). See SI Appendix, Fig. S7 for metabolite positioning in the gibberellin pathway. (B) Gibberellin treatment (10 µM GA3) 2 wk after seedling emergence has no different effect on the length increment of the first internode in wt vs. SL- plants, according to pairwise comparison with a Student’s t test (P < 0.05). Data are the difference between the values of the GA3-treated and mock-treated plants of the same genotype at different time points after treatment and represent the mean ± SE of n = 8 biological replicates. Different letters on top of bars indicate statistically significant differences among all samples as determined with one-way ANOVA followed by Tukey’s post hoc test.
Discussion
Strigolactones Promote Flowering in Tomato.
The reproductive defects of strigolactone mutants have been reported anecdotally, without detailed analysis of the possible underlying mechanisms (24–27). Our results show that in tomato, the numbers of flowers and fruits are strictly linked over time to the levels of strigolactones, be they endogenous or exogenous. Furthermore, strigolactones correlate inversely with the time from germination to anthesis. Thus, they offer a promising, innovative research avenue to manage fruiting time and total yield, two commercially pivotal parameters in tomato cultivation.
It is noteworthy that a defect in reproduction has been shown, besides this work, in strigolactone-related mutants of solanaceous and in one legume species, but not in Arabidopsis, rice or pea, despite the early availability of similar mutants in these species. In rice, it has been even shown that a partial loss-of-function of the CCD7 orthologue increases yield by increasing tillering (44), as does strigolactone insensitivity in Brassica napus (45). This suggests that reproduction is affected species-specifically by strigolactones, and that their action superimposes on the conserved pathways controlling flowering, which may be differently wired to each other in different species. In tomato, an anticipated and more profuse flowering induced by strigolactones may well integrate with lower resource allocation to lateral buds (the first hormonal function assigned to strigolactones), namely in genotypes such as the determinate M82 cultivar where overall vegetative growth is limited (2). Also, given the induction of the strigolactone biosynthetic pathway in leaves under drought (32), the hypothesis that strigolactones may contribute to the drought escape mechanism, whereby flowering is brought forward by a previous stress, is worth further investigation.
Strigolactones Affect the Expression of a Large Number of Flowering-related Loci in Leaves and Meristems.
The GO enrichment analysis of DEGs obtained from mRNA sequencing of wt and SL- tomato leaves confirmed that, within a wide transcriptional reorganization, the expression of several genes related to the term Reproduction (GO: 0000003) was altered. Perhaps most notably, this occurred for some crucial flowering genes of the SP family. SP factors belong to the CETS (CENTRORADIALIS/TERMINAL FLOWER 1/SP) family, which is shared by all land plants and has been further described in tomato to contain six FT-like proteins (36). Among them, functional analysis confirmed that SP3D/SFT is a major flowering activator that exhibits the same expression in long-day and short-day conditions, and is regulated by the paralogous factors SP5G, SP5G1, SP5G2, and SP5G3 (46, 47). These are flowering repressors with different photoperiodic expression, which are proposed to act via competition with SP3D/SFT for binding in the same functional complex, or for the formation of two different complexes competing for a common target (46). In our analysis, the significant transcript drops for SP3D/SFT can alone justify the flowering defects of SL- plants, which are similar to what observed in the sp3d mutants (36). The transcriptional decrease of SP5G, instead, might be seen as part of an attempted compensation mechanism. Moreover, the zinc-finger transcription factors CONSTANS3 (CO3), and CO-like4a (COL4a) were recently proposed as potential activators of SFT in tomato (48), and we found the corresponding genes to be significantly down-regulated in SL- plants (SI Appendix, Table S1). Consistently, also genes acting downstream of SFT are detectable among our DEGs: The products of FUL2 and MADS BOX PROTEIN20 (MBP20), which are strongly down-regulated in SL- plants, promote flowering probably by interacting with SFT and SBP factors (SI Appendix, Table S1) (40, 49). A peak in the expression of these genes has been detected in the meristem during the vegetative-to-reproductive transition, and is thought to induce tomato flowering additively and to repress inflorescence branching together with FUL1 (also down-regulated in SL- leaves, SI Appendix, Fig. S8, and up-regulated in GR245DS-treated meristems, Fig. 4). In addition, Jointless (J) contributes to maintaining the inflorescence meristem identity and to preventing both the return to a vegetative state and an early conversion to a floral meristem (49). The phenotype observed in the j mutants resembles the one seen in the SL- plants (in which J is down-regulated, SI Appendix, Table S1), at least in terms of delayed flowering. All these DEGs, with others included in Fig. 4 and SI Appendix, Table S1 and Figs. S5 and S8 confirm a role for strigolactones in the flowering process. In fact, all genes in these figures, except DOF9, are positive regulators of reproduction; the induction of the latter may be seen as an attempt to compensate for the shift toward transition and faster flower development triggered by GR245DS. It should be added that even if much of the supporting transcriptional analysis was done in leaves, leaf transcriptomes are indeed very relevant to floral transition and the speed of flower development because they are the organs that generate the reproductive signal. Thus, while gene activities in meristems are mostly inferred in this work, they are also consistent with phenotypes and are indeed validated in meristems, in some specific examples.
Positioning Strigolactones in the Flowering Network of Tomato: A Connection with the miR319-LA Module and Gibberellins.
Looking to define a molecular link between strigolactones and SFT expression, we investigated the three main flowering pathways described in tomato: the age-, the gibberellin-, and the miR319-LA dependent (10).
Considering the positive correlation between strigolactones and mature miR156 levels (22) (this work, Fig. 3B), the age pathway was deprioritized, while we investigated more in depth the gibberellin pathway by RNAseq, metabolite analysis, and sensitivity assays. In our DEGs set, transcripts of the biosynthetic genes GA3ox-2 and GA20ox-2 (50) were much more concentrated in SL- leaves than in the wt. On the other hand, expression of catabolic GA2ox genes (50) followed divergent patterns: GA2ox4 was found to be down-regulated, which would rather push for more bioactive gibberellins. Conversely, the upregulation of GA2ox2 and GA2ox3 in the transgenic line could be seen as an attempt to keep gibberellin homeostasis (SI Appendix, Table S3). The expression changes in gibberellin biosynthetic and catabolic genes are confirmed by hormone quantification (Fig. 5A); in fact, the concentrations of bioactive gibberellins and of their intermediates tended to be higher in SL- plants, while lower for the inactive catabolites. This trend indicates that strigolactones may indeed mitigate gibberellin effects on flowering in tomato by decreasing their biosynthesis without affecting perception (as suggested by the internode elongation test, Fig. 5B). It is tempting to speculate here that the lack of reproductive defects in strigolactone-related mutants of Arabidopsis and other model species may be due to the opposite effect of gibberellins on flowering, as in tomato vs. Arabidopsis (10, 14). In regard to the strigolactone-gibberellin connection, it is also worth noting first that a reverse relationship—gibberellin inhibiting the biosynthesis of strigolactones—has been reported in rice (51). Second, previous work has described the strigolactone-dependent physical interaction between the strigolactone receptor DWARF14 (D14) and the DELLA protein in rice (52). Although later considered not relevant in the context of branching control, it may be worth exploring whether the interaction with D14 is conserved for the only tomato DELLA protein PROCERA and whether it may rather be relevant for flowering. Indeed, the effects of DELLAs in vegetative and reproductive development are genetically separable (17).
The miR319-LA module is the third flowering pathway characterized in tomato (10); we confirmed here its role, and the divergent profile of mature miR319 and of the LA and SFT transcripts. We also added a tight link to strigolactones. In fact, we found significantly more mature miR319 in the wt in comparison to the SL- plants. Moreover, its levels were even higher in leaves treated with GR245DS and in the leaves of wt/SL- plants, where the strigolactone-biosynthetic pathway is overactivated. Finally, we could establish a definitive cause-effect link between the promotion of flowering by exogenous strigolactones, the activation of SFT and the degradation of LA transcripts by miR319. In fact, no induction of SFT transcripts by GR245DS treatment could be observed in vegetative or reproductive tomato plants expressing a miR319-resitant version of LA. GR245DS treatment accelerated meristem maturation, although it did not significantly affect the number of leaves at anthesis. This apparent discrepancy with the phenotype of wt/SL- plants, for which the number of leaves at anthesis was reduced instead, may be due to the persistent action of slightly overactivated synthesis in heterografted plants vs a pulse treatment with GR245DS, and to the inherently lower power of a statistical test on the number of leaves vs the number of days to anthesis. Importantly, earlier anthesis associated with high strigolactone levels is likely due to a promotion of flower development via the miR319-LA-SFT module. This is consistent with the known role of SFT on flower development (6).
Finally, it is worth noting again here that LA has been characterized not only as a direct repressor of flowering genes, SFT included, but also as an inducer of the gibberellin pathway. In fact, miR319 overexpression in tomato leads to lower gibberellin content via downregulation of GA20ox1 and upregulation of GA2ox4. The opposite happens in plants not expressing miR319 or expressing a miR319-resistant form of LA (19), thus coming full circle with the strigolactone-dependent increase of bioactive gibberellins. In our dataset, GA20ox2 (a close paralogue of GA20ox1) is indeed strongly up-regulated in SL- plants, while GA2ox4 is down-regulated; and bioactive gibberellins are higher (SI Appendix, Table S3 and Fig. 5A). Note that in spite of the known role of auxins in reproduction and effects of strigolactones on auxin fluxes (53), and the fact that the expected signature of altered auxin signaling was detected in our leaf transcriptome of SL- plants (SI Appendix, Table S2), the auxin-dependent factor ARF5 (homolog of Arabidopsis MONOPTEROS), which is important for reproduction in tomato (53) was not significantly induced by GR245DS in meristems (SI Appendix, Fig. S5). This is consistent with the fact that similar auxin concentrations were found in flowers of wt and strigolactone-depleted Lotus plants (24). On the other hand, the auxin-dependent repressor of flowering DOF9 is induced by treatment in meristems (Fig. 4), and ARF3 (homolog of Arabidopsis ETTIN) (53) is down-regulated in SL- leaves (SI Appendix, Table S2). Thus, more investigations are necessary to rule out or confirm the possible contribution by auxin to strigolactone-dependent reproductive defects. Fig. 6 summarizes our findings and proposes a draft model of strigolactone interactions within the flowering network in tomato leaves. Future work aimed at describing the transcriptome in meristems of wt vs. SL- vs. heterografted SL-/wt plants, along with full phytohormonal profiling, will help refine the findings in our study and add components and connections to this sketch.
Fig. 6.
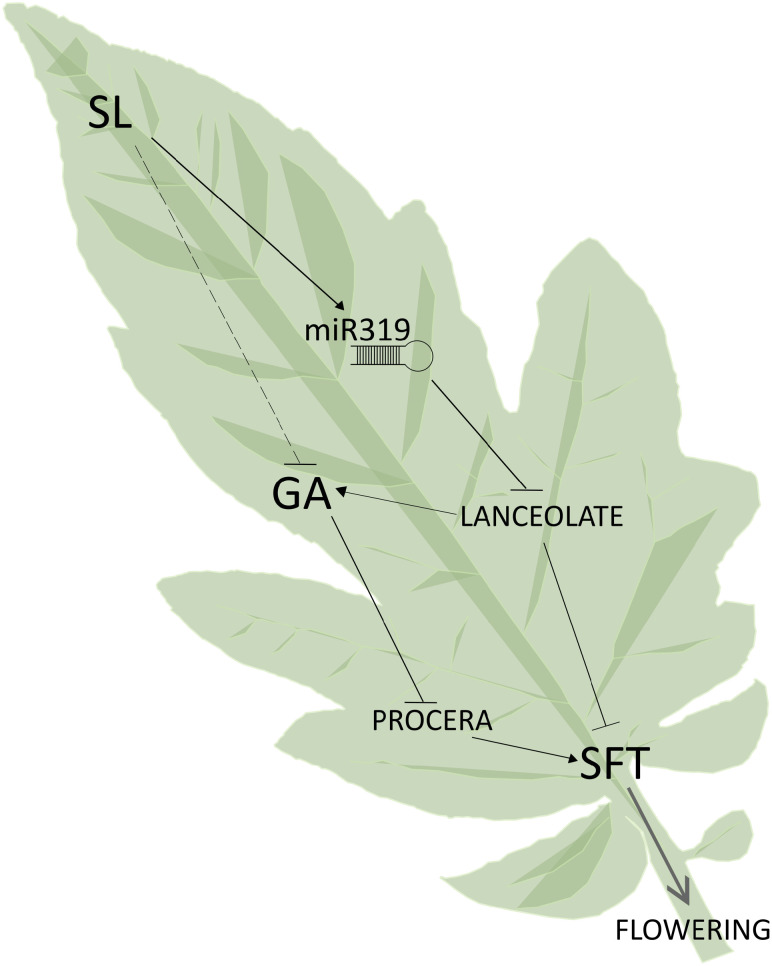
Role of strigolactones in SINGLE FLOWER TRUSS (SFT) induction in leaves. Strigolactones (SL) induce the accumulation of mature miR319, leading to a drop in the concentration of LANCEOLATE transcripts and thus, to an increase in SFT expression. The LANCEOLATE decrease would also mitigate the gibberellin (GA) pathway and enhance the activity of the only DELLA protein of tomato, PROCERA (10). Whether the effect of strigolactones on gibberellin content may also partly be independent of LANCEOLATE is still to be assessed.
Conclusions
This study aimed to establish the effect of strigolactones on flowering in tomato and justify the reproductive phenotype of strigolactone-related mutants in this species. We show that strigolactones accelerate floral transition to a certain extent, and especially flower development; and that their levels correlate with the number of flowers and fruits in tomato. Furthermore, we demonstrate that impaired strigolactone synthesis causes a dysregulation of several pathways involved in flowering and propose the miR319-LA module as a key link between strigolactones, SFT transcription, and gibberellin content in leaves. Our study positions strigolactones in the flowering regulation network of a model crop species and opens to applicative impacts in the management of tomato fruiting time and total yield.
Materials and Methods
Details on the materials and methods used in our manuscript are provided in SI Appendix, Materials and Methods on the PNAS website, including on:
Plant Material, Observations, and Treatments.
The tomato SlCCD7-silenced line 6936 and its wt genotype M82 were a kind gift by H. J. Klee (University of Florida) (28); the LApro >> Lam-GFP genotype was published earlier (18) along with procedures for meristem observations and determination of floral transition. Grafting and treatment with GR245DS were performed as reported (32).
Molecular Procedures.
Library construction, sequencing and processing of mRNA data, functional analysis of tomato DEGs, gene transcript quantification by qRT-PCR, gibberellin quantification by ultrahigh performance liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS), and statistical analysis were conducted according to established procedures for which details are published as SI Appendix.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (CSV)
Dataset S02 (XLS)
Dataset S03 (XLSX)
Acknowledgments
I.V., G.R., P.K.K., C.C., F.G., E.D., A.S. and F.C. have received funding from the European Union’s Horizon 2020 research and innovation program under the Grant Agreements No. 727929 (TOMRES); I.V., G.R., P.K.K., C.C., F.G., A.S. and F.C., also under No. 101000622 (RADIANT) as well as from Horizon Europe under innovation action no. 101081858 (ECONUTRI) and from PRIMA (project VEG-ADAPT), a program supported by the European Union. F.T.S.N. and L.F.F. have received funding from the Sao Paulo Research Foundation (FAPESP) under grant no. 18/17441–3. The work was also supported by the European Regional Development Fund Project No. CZ.02.01.01/00/22_008/0004581 (toward next generation of crops) to D.T. C.C. contributed to this publication while attending the PhD program in Scientific, Technological and Social Methods Enabling Circular Economy at the University of Padova (IT), Cycle XXXVIII, with the support of a scholarship financed by the Ministerial Decree no. 351 of 9th April 2022, based on the NRRP (funded by the European Union–NextGenerationEU–Mission 4, Component 1, Investment 4.1). None of these funding sources were involved in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. We wish to thank Dr. Klee (University of Florida, USA) for the CCD7-silenced line and Mrs. D. Valentino (University of Turin, IT) for technical help.
Author contributions
I.V., F.T.S.N., A.S., and F.C. designed research; I.V., L.F.F., C.C., D.T., F.G., and E.D. performed research; F.T.S.N. contributed new reagents/analytic tools; I.V., L.F.F., G.R., P.K.K., D.T., F.T.S.N., and F.C. analyzed data; I.V., L.F.F., G.R., C.C., F.G., E.D., F.T.S.N., and A.S. read and approved the final version; F.C. provided financial support; and I.V. and F.C. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
Raw sequencing data can be found at the Gene Expression Omnibus (GEO) under the series record GSE264066 (accession numbers GSM8209523, GSM8209524 and GSM8209525 for the wt genotype; WWGSM8209505, GSM8209506 and GSM8209507 for the SL- plants) (54).
Supporting Information
References
- 1.Cho L.-H., Yoon J., An G., The control of flowering time by environmental factors. Plant J. 90, 708–719 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Krieger U., Lippman Z. B., Zamir D., The flowering gene SINGLE FLOWER TRUSS drives heterosis for yield in tomato. Nat. Genet. 42, 459–463 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Jarillo J. A., Piñeiro M., Timing is everything in plant development. The central role of floral repressors. Plant Sci. 181, 364–378 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Ó’Maoiléidigh D. S., Graciet E., Wellmer F., Gene networks controlling Arabidopsis thaliana flower development. New Phytol. 201, 16–30 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Molinero-Rosales N., Latorre A., Jamilena M., Lozano R., SINGLE FLOWER TRUSS regulates the transition and maintenance of flowering in tomato. Planta 218, 427–434 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Quinet M., et al. , Genetic interactions in the control of flowering time and reproductive structure development in tomato (Solanum lycopersicum). New Phytol. 170, 701–710 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Srikanth A., Schmid M., Regulation of flowering time: All roads lead to Rome. Cell. Mol. Life Sci. 68, 2013–2037 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turck F., Fornara F., Coupland G., Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu. Rev. Plant Biol. 59, 573–594 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Lozano R., Giménez E., Cara B., Capel J., Angosto T., Genetic analysis of reproductive development in tomato. Int. J. Dev. Biol. 53, 1635–1648 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Silva G. F. F., et al. , Tomato floral induction and flower development are orchestrated by the interplay between gibberellin and two unrelated microRNA-controlled modules. New Phytol. 221, 1328–1344 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Wang H., The miR156/SPL module, a regulatory hub and versatile toolbox, gears up crops for enhanced agronomic traits. Mol. Plant 8, 677–688 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Cui L., et al. , miR156a-targeted SBP-Box transcription factor SlSPL13 regulates inflorescence morphogenesis by directly activating SFT in tomato. Plant Biotechnol. J. 18, 1670–1682 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mutasa-Göttgens E., Hedden P., Gibberellin as a factor in floral regulatory networks. J. Exp. Bot. 60, 1979–1989 (2009). [DOI] [PubMed] [Google Scholar]
- 14.García-Hurtado N., et al. , The characterization of transgenic tomato overexpressing Gibberellin 20-oxidase reveals induction of parthenocarpic fruit growth, higher yield, and alteration of the gibberellin biosynthetic pathway. J. Exp. Bot. 63, 5803–5813 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Hauvermale A. L., Ariizumi T., Steber C. M., Gibberellin signaling: A theme and variations on DELLA repression. Plant Physiol. 160, 83–92 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porri A., Torti S., Romera-Branchat M., Coupland G., Spatially distinct regulatory roles for gibberellins in the promotion of flowering of Arabidopsis under long photoperiods. Development 139, 2198–2209 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Serrano-Mislata A., et al. , DELLA genes restrict inflorescence meristem function independently of plant height. Nat. Plants 9, 749–754 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burko Y., et al. , A role for APETALA1/fruitfull transcription factors in tomato leaf development. Plant Cell 25, 2070–2083 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanai O., Shani E., Russ D., Ori N., Gibberellin partly mediates LANCEOLATE activity in tomato. Plant J. 68, 571–582 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Aquino B., Bradley J. M., Lumba S., On the outside looking in: Roles of endogenous and exogenous strigolactones. Plant J. 105, 322–334 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Cardinale F., Korwin Krukowski P., Schubert A., Visentin I., Strigolactones: Mediators of osmotic stress responses with a potential for agrochemical manipulation of crop resilience. J. Exp. Bot. 69, 2291–2303 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Visentin I., et al. , A novel strigolactone-miR156 module controls stomatal behaviour during drought recovery. Plant Cell Environ. 43, 1613–1624 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Trasoletti M., Visentin I., Campo E., Schubert A., Cardinale F., Strigolactones as a hormonal hub for the acclimation and priming to environmental stress in plants. Plant Cell Environ. 45, 3611–3630 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J., et al. , Carotenoid cleavage dioxygenase 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume Lotus japonicus. J. Exp. Bot. 64, 1967–1981 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasare S. A., et al. , The role of the potato (Solanum tuberosum) CCD8 gene in stolon and tuber development. New Phytol. 198, 1108–1120 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Snowden K. C., et al. , The Decreased apical dominance1/Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. Plant Cell 17, 746–759 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohlen W., et al. , The tomato CAROTENOID CLEAVAGE DIOXYGENASE8 (SlCCD8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytol. 196, 535–547 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Vogel J. T., et al. , SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. Plant J. 61, 300–311 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Santoro V., et al. , Strigolactones control root system architecture and tip anatomy in Solanum lycopersicum L. plants under P starvation. Plants 9, 612 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santoro V., et al. , Strigolactones affect phosphorus acquisition strategies in tomato plants. Plant Cell Environ. 44, 3628–3642 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santoro V., et al. , Tomato plant responses induced by sparingly available inorganic and organic phosphorus forms are modulated by strigolactones. Plant Soil 474, 355–372 (2022). [Google Scholar]
- 32.Visentin I., et al. , Low levels of strigolactones in roots as a component of the systemic signal of drought stress in tomato. New Phytol. 212, 954–963 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Johnson X., et al. , Branching genes are conserved across species. Genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiol. 142, 1014–1026 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazzucato A., Taddei A., Soressi G., The parthenocarpic fruit (pat) mutant of tomato (Lycopersicon esculentum Mill.) sets seedless fruits and has aberrant anther and ovule development. Development 125, 107–114 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Pesaresi P., Mizzotti C., Colombo M., Masiero M., Genetic regulation and structural changes during tomato fruit development and ripening. Front. Plant Sci. 5, 124 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao K., et al. , Four tomato FLOWERING LOCUS T-like proteins act antagonistically to regulate floral initiation. Front. Plant Sci. 6, 1213 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campos-Rivero G., et al. , Plant hormone signaling in flowering: An epigenetic point of view. J. Plant Physiol. 214, 16–27 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Meir Z., et al. , Dissection of floral transition by single-meristem transcriptomes at high temporal resolution. Nat. Plants 7, 800–813 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Park S. J., Jiang K., Schatz M. C., Lippman Z. B., Rate of meristem maturation determines inflorescence architecture in tomato. Proc. Natl. Acad. Sci. U.S.A. 109, 639–644 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang X., et al. , FRUITFULL-like genes regulate flowering time and inflorescence architecture in tomato. Plant Cell 34, 1002–1019 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dielen V., et al. , UNIFLORA, a pivotal gene that regulates floral transition and meristem identity in tomato (Lycopersicon esculentum). New Phytol. 161, 393–400 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Ferreira e Silva G. F., et al. , MicroRNA156-targeted SPL/SBP box transcription factors regulate tomato ovary and fruit development. Plant J. 78, 604–618 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Hu G., et al. , The auxin-responsive transcription factor SlDOF9 regulates inflorescence and flower development in tomato. Nat. Plants 8, 419–433 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Wang Y., et al. , A strigolactone biosynthesis gene contributed to the green revolution in rice. Mol. Plant 13, 923–932 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Stanic M., Hickerson N. M. N., Arunraj R., Samuel M. A., Gene-editing of the strigolactone receptor BnD14 confers promising shoot architectural changes in Brassica napus (canola). Plant J. 19, 639–641 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lifschitz E., et al. , The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc. Natl. Acad. Sci. U.S.A. 103, 6398–6403 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shalit A., et al. , The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proc. Natl. Acad. Sci. U.S.A. 106, 8392–8397 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang T., He Y., Niu S., Yan S., Zhang Y., Identification and characterization of the CONSTANS (CO)/CONSTANS-like (COL) genes related to photoperiodic signaling and flowering in tomato. Plant Sci. 301, 110653. (2020). [DOI] [PubMed] [Google Scholar]
- 49.Périlleux C., Lobet G., Tocquin P., Inflorescence development in tomato: Gene functions within a zigzag model. Front. Plant Sci. 5, 121 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hedden P., Thomas S. G., Gibberellin biosynthesis and its regulation. Biochem. J. 444, 11–25 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Ito S., et al. , Regulation of strigolactone biosynthesis by gibberellin signaling. Plant Physiol. 174, 1250–1259 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura H., et al. , Molecular mechanism of strigolactone perception by DWARF14. Nat. Comm. 4, 2613 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Cucinotta M., Cavalleri A., Chandler J. W., Colombo L., Auxin and flower development: A blossoming field. Cold Spring Harb. Perspect. Biol. 13, a039974 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Visentin I., et al., Effects of strigolactones on tomato leaf transcriptome under irrigated and repeated water stress conditions. GSE264066. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE264066. Deposited 16 April 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (CSV)
Dataset S02 (XLS)
Dataset S03 (XLSX)
Data Availability Statement
Raw sequencing data can be found at the Gene Expression Omnibus (GEO) under the series record GSE264066 (accession numbers GSM8209523, GSM8209524 and GSM8209525 for the wt genotype; WWGSM8209505, GSM8209506 and GSM8209507 for the SL- plants) (54).