Graphical abstract
Keywords: Clinical chemistry tests, Estradiol, Gender identity, Medical informatics, Testosterone, Transgender persons
Highlights
-
•
For the population taking gender-affirming hormones, legal sex impacts normal/abnormal flagging of laboratory tests.
-
•
Creatinine and liver enzymes are the most frequently ordered tests potentially impacted by gender-affirming hormones.
-
•
Sexual orientation/gender identity (SOGI) fields can help identify those potentially taking gender-affirming hormones.
-
•
Cardiac biomarkers and iron studies merit further investigation for the impact of gender-affirming hormones.
Abstract
Background
Gender-affirming hormone therapy with either estradiol or testosterone for transgender persons can significantly impact chemistry and hematology laboratory tests. The sex used for assignment of reference intervals (RIs) in the electronic health record (EHR) will influence normal/abnormal flagging of test results.
Objective
To analyze common non-hormonal laboratory tests with sex-specific RIs ordered in patients with sexual orientation/gender identify (SOGI) field differences (one or more differences between legal sex, sex assigned at birth, and gender identity) in the EHR at an academic medical center in midwestern United States.
Methods
We utilized a previously characterized data set of patients at our institution that included chart review information on gender identity and gender-affirming therapy. We focused on the subset of these patients that had orders for 18 common laboratory tests in calendar year 2021.
Results
A total of 1336 patients with SOGI field differences (1218 or 91.2% identifying as gender-expansive; 892 or 66.8% receiving estradiol or testosterone as gender-affirming therapy) had a total of 9374 orders for 18 laboratory tests with sex-specific RIs. Hemoglobin, creatinine, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and high-density lipoprotein were the most frequently ordered tests. For patients taking estradiol, 128 of 970 (13.2%) creatinine and 39 of 193 (20.2%) hemoglobin measurements were within the RI for one sex but not the other. For those taking testosterone, 119 of 531 (22.4%) creatinine and 49 of 120 (40.8%) hemoglobin measurements were within the RI for one sex but not the other. Values above the cisgender female RI but within the cisgender male RI were common for hemoglobin, alkaline phosphatase, alanine aminotransferase, and aspartate aminotransferase in patients taking testosterone.
Conclusions
Clinicians should be aware of the potential impact of gender-affirming therapy on laboratory tests and what sex/gender is being used in the EHR to assign RIs.
Introduction
Gender-affirming hormone therapy is standard of care for transgender and nonbinary people who wish to medically transition [1], [2], [3]. Transgender and nonbinary people experience incongruence between their sex assigned at birth (SAAB; ‘birth sex') and gender identity (GI), the internal sense of being a woman, man or somewhere between [1], [2], [4]. Feminizing gender-affirming therapy typically includes estradiol (oral, transdermal, or intramuscular injection) with or without adjunct agents that may include progesterone and androgen blockers (e.g., cyproterone, finasteride, spironolactone) [5]. Masculinizing gender-affirming therapy utilizes testosterone, commonly either intramuscular or subcutaneous injection or a topical route such as testosterone gel or transdermal patch [6].
A number of common laboratory tests have been shown to be impacted by gender-affirming feminizing or masculinizing therapy [7], [8], [9], [10]. Some of these tests are recommended by expert opinion or clinical practice guidelines (including the Endocrine Society and the The World Professional Association for Transgender Health) in the monitoring of patients receiving gender-affirming hormones [1], [2]. Examples of laboratory tests recommended in at least one guideline include creatinine (CRT), hemoglobin (HB)/hematocrit (HCT), estradiol, prolactin, and testosterone. There is consensus between guidelines in recommending monitoring of HB/HCT in those receiving testosterone as gender-affirming therapy. In addition to monitoring of gender-affirming therapy, transgender and nonbinary patients may have laboratory tests ordered for other clinical indications. Laboratory tests significantly impacted by gender-affirming hormones are predominantly tests that have sex-specific reference intervals (RIs) or target ranges based on SAAB [7], [8], [9], [10]. Prospective studies in those stably taking gender-affirming hormones have determined that some analytes have RIs that are significantly different than those associated with SAAB [11], [12], [13], [14], [15]. For example, empirically determined RIs for HB/HCT in the transgender and nonbinary population taking gender-affirming hormones align with affirmed gender and not SAAB (e.g., transmen taking testosterone have essentially the same RI as cisgender men) [11].
A major informatics challenge is how to utilize functionality within the electronic health record (EHR) to provide RIs or interpretive comments for laboratory tests ordered on transgender and nonbinary patients who may be receiving gender-affirming therapy [16], [17], [18], [19]. In recent years, EHR packages in the United States (US) have incorporated demographic fields for sexual orientation and gender identity (SOGI) [20], [21]. This integration allows patients or members or the healthcare team to list GI, SAAB, and sexual orientation within individual patient medical records, sometimes along with other items such as affirmed name, preferred pronouns, organ inventory, history of gender-affirming therapy, and presence of differences in sexual differentiation (otherwise known as intersex or variation of sex characteristics). Access to this information can help the clinical care team provide better service for gender and sexual minority groups [18], [22].
Studies at large medical centers in the US have shown rates of SOGI field use by adult patients in the 20–30 % range, with much higher use by those identifying within the lesbian, gay, bisexual, transgender, and queer/questioning (LGBTQ) umbrella [17], [23], [24]. Several factors influence rate of SOGI field adoption including collection of information during check-in for encounters, use of patient portals, and healthcare provider encouragement. There are some potential risks to patients providing SOGI information that will be accessible in the EHR [24], [25], [26], [27], [28]. Common patient concerns include discrimination, disapproving comments, bias from the healthcare team, and privacy of adolescent health information.
In the US, legal sex in the EHR will typically match the sex designation on health insurance cards and state identity documents [17]. The process to officially change the sex designation on identity documents varies substantially across US states and territories [29]. Legal sex is typically the sex marker used for assignment of sex-specific RIs for laboratory tests such as HB/HCT, CRT, reproductive hormones, and other laboratory tests. In the majority of patients, legal sex will match SAAB. However, when a patient goes through the process to change legal sex in the EHR, this will change the sex used to assign RI for tests with sex-specific intervals [17].
With current EHR functionality, the clinical laboratory may not be able to easily identify patients who may be taking gender-affirming hormones [17], [18], [19]. The complexity of SOGI field options also presents practical challenges for designing specific rules based on patient SOGI responses [17], [23], [24]. As an alternative, a binary rule based on the presence or absence of any ‘SOGI field difference’ may be used to identify a subset of patients highly likely to identify as gender diverse and possible also be receiving gender-affirming hormones [17]. This is in fact an option for which the EHR vendor for our institution provided rules and guidance using the SOGI field options in our EHR (Table 1). In a previous study, we performed detailed analysis of SOGI field responses for all patients with at least one completed in-person inpatient and/or outpatient encounter within our academic medical center system over an approximately 3-year period [17].
Table 1.
Choices available for gender and sex in the sexual orientation/gender identity (SOGI) data fields.
| Legal Sex1 | Gender Identity1 | Sex Assigned at Birth1 |
|---|---|---|
| Female | Female | Female |
| Male | Male | Male |
| Unknown | Nonbinary | Choose not to disclose |
| Other | Not recorded on birth certificate | |
| Transgender Female /Male-to-Female | Uncertain | |
| Transgender Male /Female-to-Male | Unknown | |
| Choose not to disclose | [Blank] | |
| [Blank] |
1Legal sex cannot be changed directly by patient; any changes in legal sex must be done by authorized users following hospital policy. Gender identity and sex assigned at birth can be left blank, whereas legal sex must be one of the three options. There is option to auto-fill sex gender identity and sex assigned at birth by selecting either cisgender female or cisgender male. This option will match sex assigned at birth and gender identity to the legal sex currently in the system for the patient.
In the present study, we determined which common non-hormonal laboratory tests with sex-specific RIs were ordered most often in the population of patients with SOGI field differences in the EHR. Using our prior dataset of patients well-characterized by chart review for GI and use of gender-affirming hormones [17], we focused on the subset that in calendar year 2021 had orders for 18 frequently ordered non-hormonal laboratory tests that have sex-specific RIs for the general population for at least some ages. Of the laboratory tests analyzed, CRT, alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), HB, and HDL have strong evidence across multiple published studies of being impacted by gender-affirming hormones (either estradiol, testosterone, or both; Table 2) [9], [10], [11], [15], [30], [31], [32], [33], [34], [35], [36], [37], [38]. Our secondary aims were to analyze how often test results for patients were in a range where the difference in legal sex results in discordance between cisgender female and male RIs in terms of normal/abnormal flagging and to assess the impact of changing legal sex on that flagging.
Table 2.
Impact of gender-affirming hormones on selected analytes from published studies.
| Analyte | Estradiol1 | Testosterone2 | Most Likely Clinical Impact3 |
|---|---|---|---|
| Alkaline phosphatase | No change (or minimal decrease) | Increase | Testosterone may shift values slightly above cisgender female RI |
| Alanine aminotransferase | No change (or minimal decrease) | Increase | Testosterone may shift values slightly above cisgender female RI |
| Aspartate aminotransferase | No change (or minimal decrease) | Increase | Testosterone may shift values slightly above cisgender female RI |
| Creatinine | No change (or minimal decrease) | Increase | Choice of sex will impact eGFR and potentially category of chronic kidney disease |
| Hemoglobin/hematocrit | Decrease | Increase | Decrease due to estradiol may be misinterpreted as anemia; increase due to testosterone may be misinterpreted as erythrocytosis |
| High-density lipoprotein | Variable (no change or increase) | Decrease | Testosterone may shift values below cisgender female RI |
1Estradiol gender-affirming therapy shifts reference interval for hemoglobin and hematocrit to lower values, aligning with cisgender female reference intervals. For the other four tests, estradiol has minimal effect, either slight decrease or no change depending on published study.
2In general for these five laboratory tests, testosterone gender-affirming therapy shifts reference interval to higher values, aligning with cisgender male reference intervals.
Materials and methods
Setting and electronic health record design
The study was conducted at an 860 bed tertiary/quaternary care academic medical center that includes a 190 bed children's hospital. The medical center includes an emergency department, inpatient units, and outpatient clinics at a central campus location. Adult and pediatric clinics are also located throughout the state. The EHR for the medical center since 2009 has been Epic (Epic Systems, Inc., Madison, WI, USA), with Epic Beaker Anatomic Pathology and Clinical Pathology for the institutional laboratory information system [39], [40]. The institution adopted SOGI field capability for all patients in December 2018 [17], [18]. This included fields for GI, SAAB, sexual orientation, preferred name, and preferred pronoun as basic SOGI functionality for which patients could volunteer information during registration, pre-visit questionnaires, or via the patient portal (Epic MyChart). Fields for organ inventory, use of gender-affirming hormones, and history of gender-affirming gonadectomy are available but have not been utilized much to date at our institution [17].
Study design and data retrieval
The data in this study was collected as part of a retrospective study approved by the Institutional Review Board (protocol # 202202388) covering the timeframe from December 1, 2018 to February 17, 2022. During the retrospective analysis timeframe, the EHR allowed for 3 options for Legal Sex (Male, Female, Unknown), 8 options for GI, and 7 options for SAAB (Table 1). Legal Sex is a mandatory field, while GI and SAAB are voluntary and can be left blank. The total number of possible combinations for Legal Sex, GI, and SAAB (including GI and/or SAAB being left blank) is 168 [17].
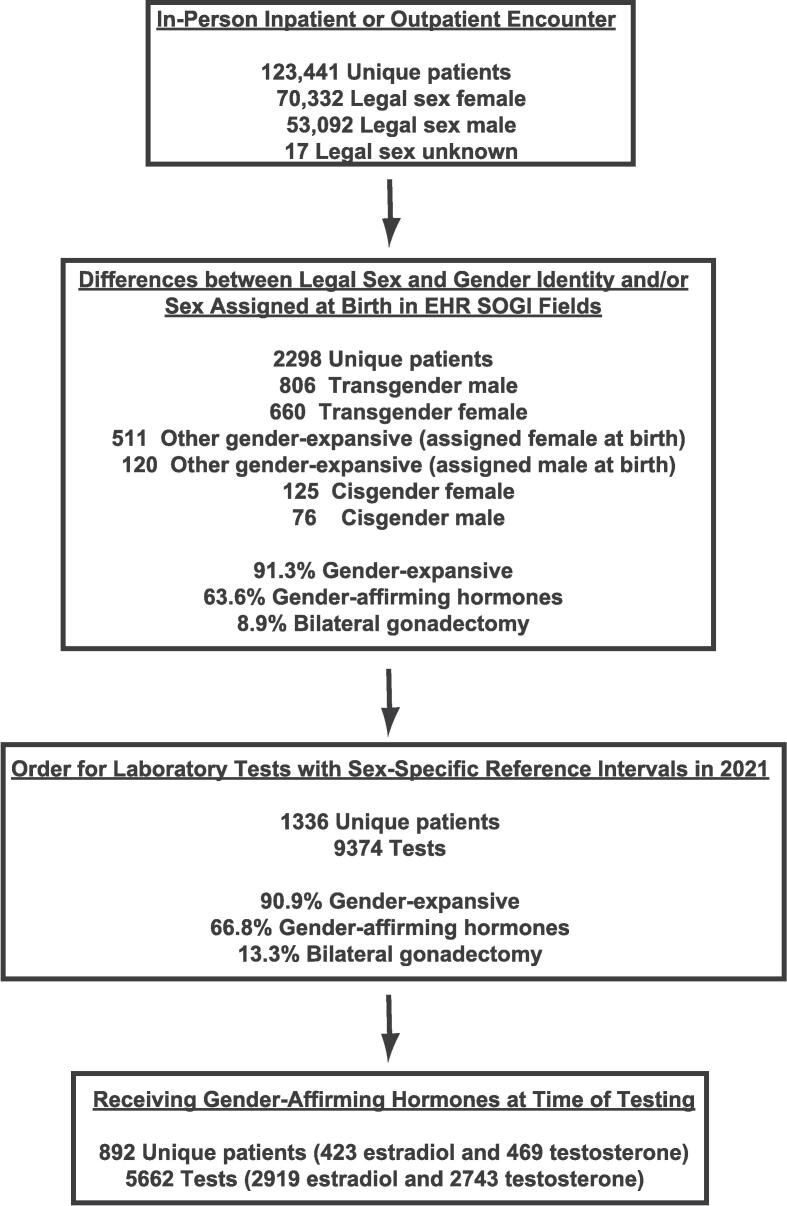
In a previous study [17], out of a total of 123,441 unique patients, 2298 patients had SOGI field differences, defined as a non-blank response for GI and/or SAAB that differed from Legal Sex (e.g., Legal Sex of “Female” and GI of “Transgender Male” or Legal Sex of “Male” and SAAB as “Female”). Detailed chart review was performed for all of the 2298 patients, showing 91.3 % identified within the broad umbrella of gender-expansive (includes transgender, nonbinary, gender fluid, gender queer, transfeminine, and transmasculine), and 63.6 % were actively taking gender-affirming hormones.
The present study focuses on the subset that had frequently ordered, non-hormonal laboratory tests with sex-specific RIs. These encompassed the following 18 tests: CRT, ALP, ALT, AST, gamma-glutamyltransferase (GGT), lactate dehydrogenase (LDH), creatine kinase (CK), HB, ferritin, high-density lipoprotein (HDL), iron, iron percent saturation, N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity (generation 5) troponin T (hs-TnT), total calcium, phosphorus, erythrocyte sedimentation rate, and uric acid. Details on the laboratory assays (vendor, assay name, RIs at our institution) are summarized in Supplemental Tables 1 and 2. The flow diagram for data retrieval and analysis is shown in Fig. 1. Chart review for all patients is described in a previous publication [17].
Fig. 1.
Flow diagram for the study. Using analysis from a previous study [17], we utilized a data set of 2298 unique patients with SOGI field differences whose charts had been reviewed for gender identity and use of gender-affirming hormones. From these 2298 unique patients, 1336 patients had one or more orders in calendar year 2021 for 18 laboratory tests that have sex-specific reference intervals for the patient age at time of specimen collection. Of these 1336 unique patients, 892 patients (66.8%) were receiving gender-affirming hormones (either estradiol or testosterone) at the time of laboratory testing.
An Epic Reporting Workbench report retrieved all patients with orders within calendar year 2021 for the 18 laboratory tests listed above [41]. This report captures laboratory-only encounters as well as laboratory tests ordered in conjunction with an in-person inpatient, outpatient, or emergency department encounter. This dataset was used to cross-reference orders for these laboratory tests to the 2298 patients with SOGI field differences. We focused only on patient age/laboratory test combinations that have sex-specific RIs and thus excluded age/test combinations for which our institution has a single RI across both sexes (Supplemental Table 2). This analysis included tests ordered individually and also within panels (e.g., HB within complete blood count; CRT within basic metabolic, comprehensive metabolic, or renal panels). Laboratory test RIs at our institution are applied based on legal sex.
Potential impact of change of legal sex in the EHR
To estimate the potential impact of changes in legal sex, we focused the analysis on ALP, ALT, AST, CRT, and HB (five of the most frequently ordered tests with sex-specific RIs in the population with SOGI field changes). The two scenarios were: (1) legal sex equaled SAAB for everyone and (2) legal sex was instead GI for the patient population taking gender-affirming hormones. This analysis used our institutional sex-specific RIs and not any RIs empirically derived from published studies describing specifics RIs for the transgender/nonbinary population receiving gender-affirming hormones. For data with CRT, we analyzed how the use of either female or male for the estimated glomerular filtration rate (eGFR) calculation impacts the assignment of eGFR categories for chronic kidney disease (CKD) stages from the Kidney Disease: Improving Global Outcomes (KDIGO) 2013 working group recommendations [42] for the cohorts taking estradiol or testosterone as gender-affirming therapy. The agreement between CKD stages was assessed using the weighted Cohen’s kappa (κ) statistic.
Results
Characteristics of the population studied
Many laboratory tests have sex-specific RIs, either for all ages or specific age ranges. We focused on 18 commonly ordered non-hormonal chemistry and hematology tests with sex-specific RIs for at least some age ranges at our institution. Out of a previously published dataset of 2298 patients with SOGI field differences in our institutional EHR, we identified that 1336 of the 2298 patients had 9374 orders for these 18 laboratory tests within the calendar year 2021 at an age for which our institution has sex-specific RIs (Supplemental Table 2). Of the 1336 patients with laboratory orders, 1218 (91.2 %) were classified as gender-expansive based on previous chart review [17], with 892 (66.8 %) receiving gender-affirming hormones at the time of laboratory testing. The present study focuses mainly on these 892 patients. Of the 892 patients receiving gender-affirming hormones, 118 (13.2 %) also had history of gender-affirming bilateral gonadectomy (i.e., ovariectomy or orchiectomy).
Supplemental Table 3 provides a summary of demographics, estradiol or testosterone medication formulation at time of laboratory testing, change of legal sex in the EHR, and gender identity from chart review (transgender, nonbinary, or other). Supplemental Table 4 provides the number of orders, number of unique patients, and number of unique patients with one or more values outside at least one sex-specific RI for all the laboratory tests analyzed in the present study for the population taking gender-affirming estradiol or testosterone. A flow diagram of the study is in Fig. 1.
Distribution of laboratory test ordering and results in the population with SOGI differences
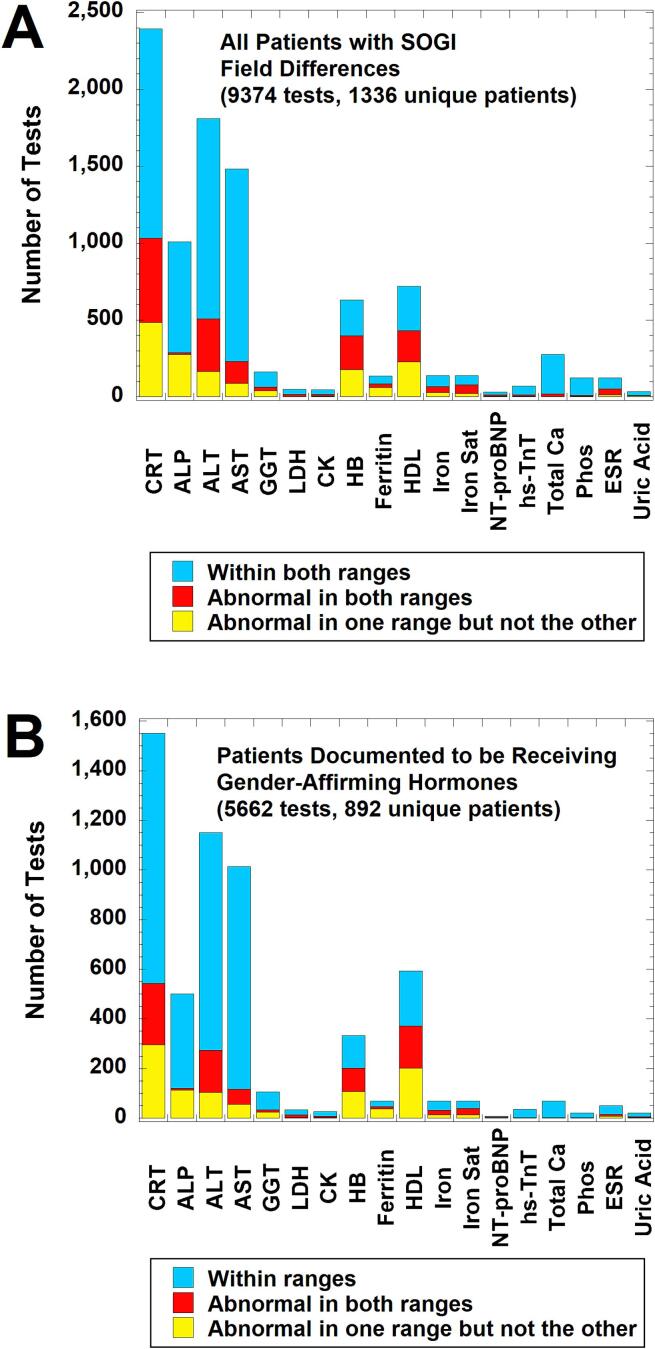
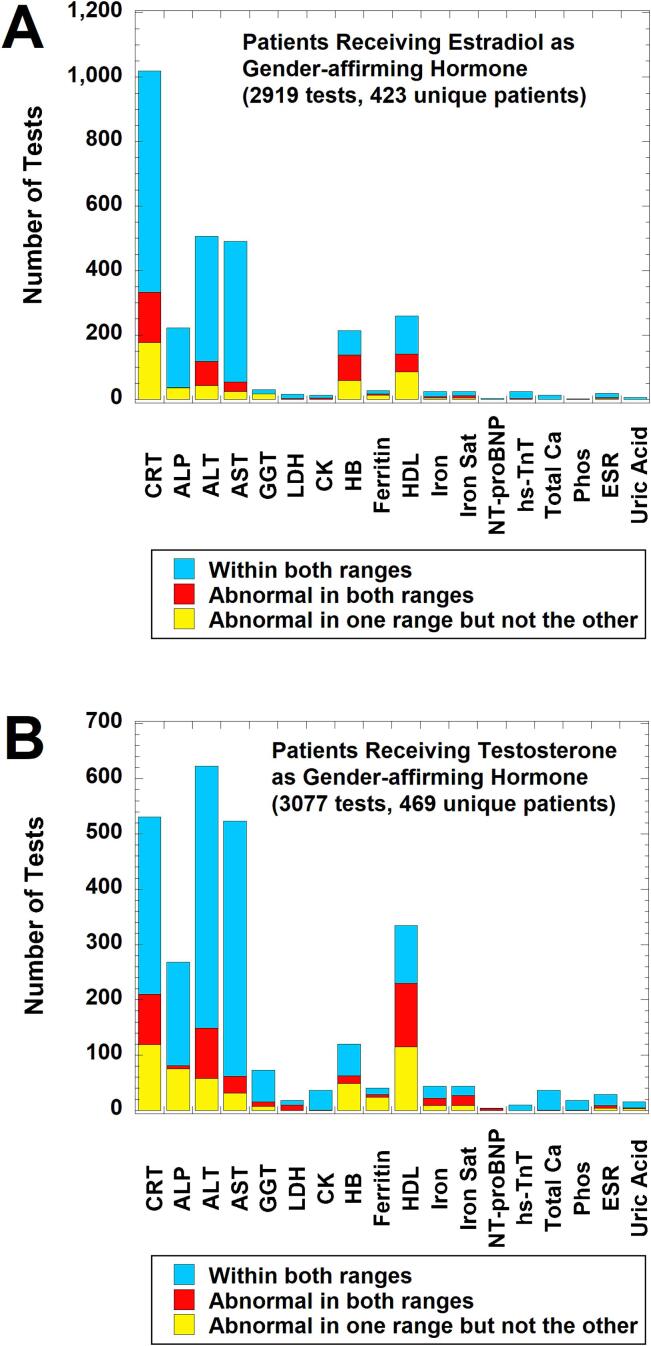
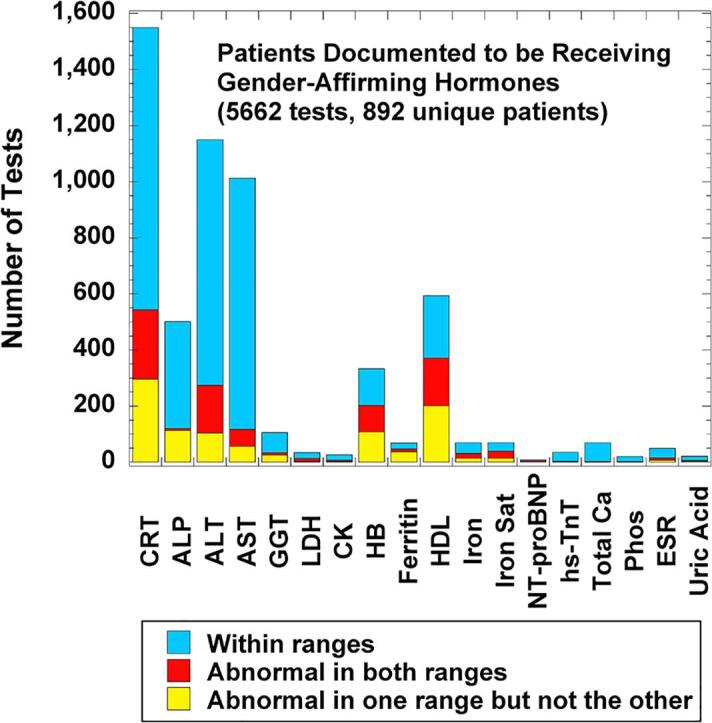
Fig. 2 shows a breakdown of whether the results for a laboratory test performed on patients with SOGI field differences were within both cisgender male and female RIs, abnormal in one range but not the other, or outside both male and female RIs for the patient age at the time of specimen collection. With some minor differences, the profile for all 1336 unique patients with SOGI field differences (Fig. 2A) is very similar to the subset known to be receiving gender-affirming hormones (either feminizing therapy with estradiol with or without adjunct agents or masculinizing therapy with testosterone) as verified by chart review (5662 tests on 892 unique patients; Fig. 2B). The top six most frequently ordered tests in descending order were CRT, ALT, AST, ALP, HDL, and HB. Within these six tests, CRT, HDL, ALT, and HB had the greatest number of results that were outside one or both age-matched sex-specific RIs for that test at our institution (yellow or red bars in Fig. 2). For the subset of patients known to be receiving gender-affirming hormones, the overall patterns for the estradiol cohort (2919 tests on 423 unique patients; Fig. 3A) was very similar to the overall patterns seen in Fig. 2. For the testosterone cohort (2743 tests on 469 unique patients; Fig. 3B), the pattern was also similar except ALT was ordered slightly more often at ages with sex-specific RIs than CRT.
Fig. 2.
Laboratory tests with sex-specific reference intervals (RIs) ordered in calendar year 2021 on patients with sexual orientation/gender identity (SOGI) field differences for legal sex, sex assigned at birth, and gender identity. The bar graphs indicate how often laboratory tests were within sex-specific RIs for both sexes (light blue), abnormal in both intervals (red), or abnormal in only one sex-specific interval (yellow) using the patient age at time of laboratory testing. The sex-specific RIs were the ones used by the laboratory for all patients (see Supplemental Table 2), not any RIs empirically derived for the population on gender-affirming therapy. (A) Analysis for all patients (9374 tests on 1336 unique patients). (B) Analysis for the subset of patients documented to be receiving gender-affirming hormones (either estradiol or testosterone) at the time of laboratory testing (5662 tests on 892 unique patients). Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; CK, creatine kinase; CRT, creatinine; ESR, erythrocyte sedimentation rate; GGT, gamma-glutamyltransferase; HB, hemoglobin; HDL, high-density lipoprotein; hs-TnT, high-sensitivity troponin T; Iron Sat, iron saturation %; LDH, lactate dehydrogenase; NT-proBNP, NT-Pro B-type natriuretic peptide; Phos, inorganic phosphorus; SOGI, sexual orientation/gender identity. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Laboratory tests with sex-specific RIs ordered in calendar year 2021 on patients documented to be on either estradiol or testosterone gender-affirming therapy at the time of laboratory testing. The bar graphs indicate how often these tests were within sex-specific RIs for both sexes (light blue), abnormal in both intervals (red), or abnormal in only one sex-specific interval (yellow) using the patient age at time of laboratory testing. (A) Analysis for patients receiving estradiol as gender-affirming therapy at the time of laboratory testing (2919 laboratory tests on 423 unique patients). (B) Analysis for patients receiving testosterone as gender-affirming therapy at the time of laboratory testing (2743 laboratory tests on 469 unique patients). Abbreviations and reference intervals are the same as described in Fig. 2. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Potential impact of change of legal sex in the population receiving gender-affirming hormones
For ALP, ALT, AST, CRT, and HB, we analyzed data involving the population documented to be taking gender-affirming hormones considering the following two scenarios: (1) legal sex was always the same as SAAB and (2) legal sex was instead affirmed gender. This analysis used our institutional sex-specific RIs and not any RIs empirically derived for the transgender/nonbinary population receiving gender-affirming hormones. We then assessed whether the flagging for normal/abnormal for the RI actually matches the known impacts of gender-affirming hormones on these five tests (Table 2). This assumes estradiol as gender-affirming hormone does not significantly impact ALP, ALT, AST, and CRT, while testosterone therapy shifts ALP, ALT, AST, CRT, and HB values to essentially match cisgender male RIs. For HB, gender-affirming feminizing hormones also shift values to match cisgender female RIs.
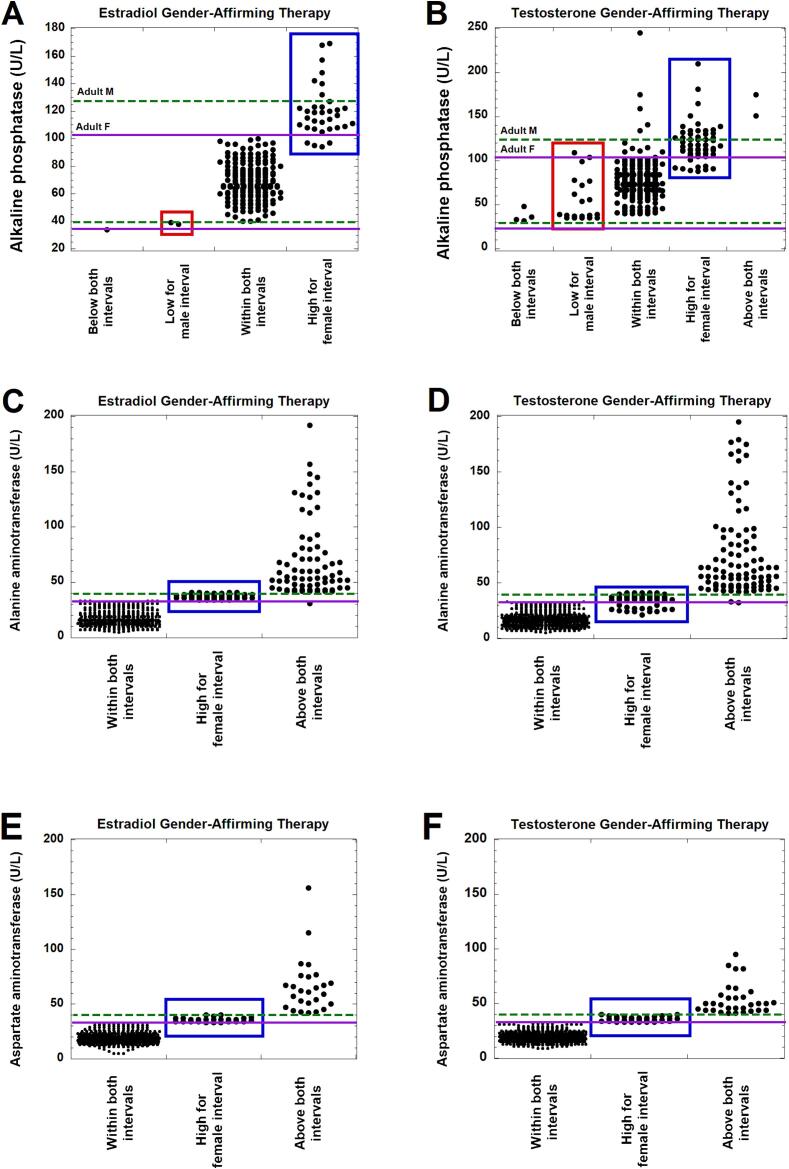
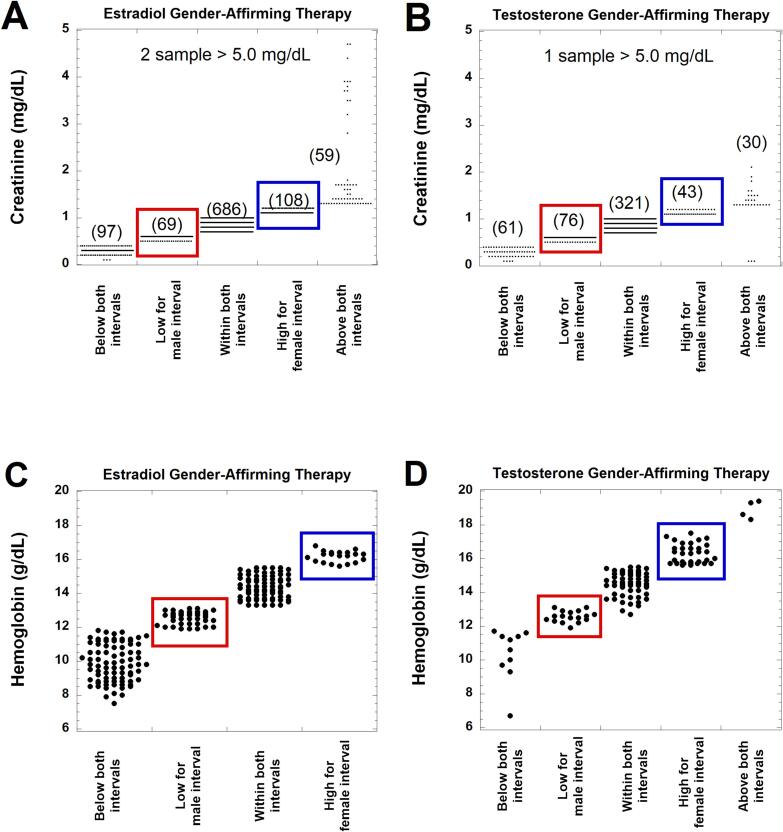
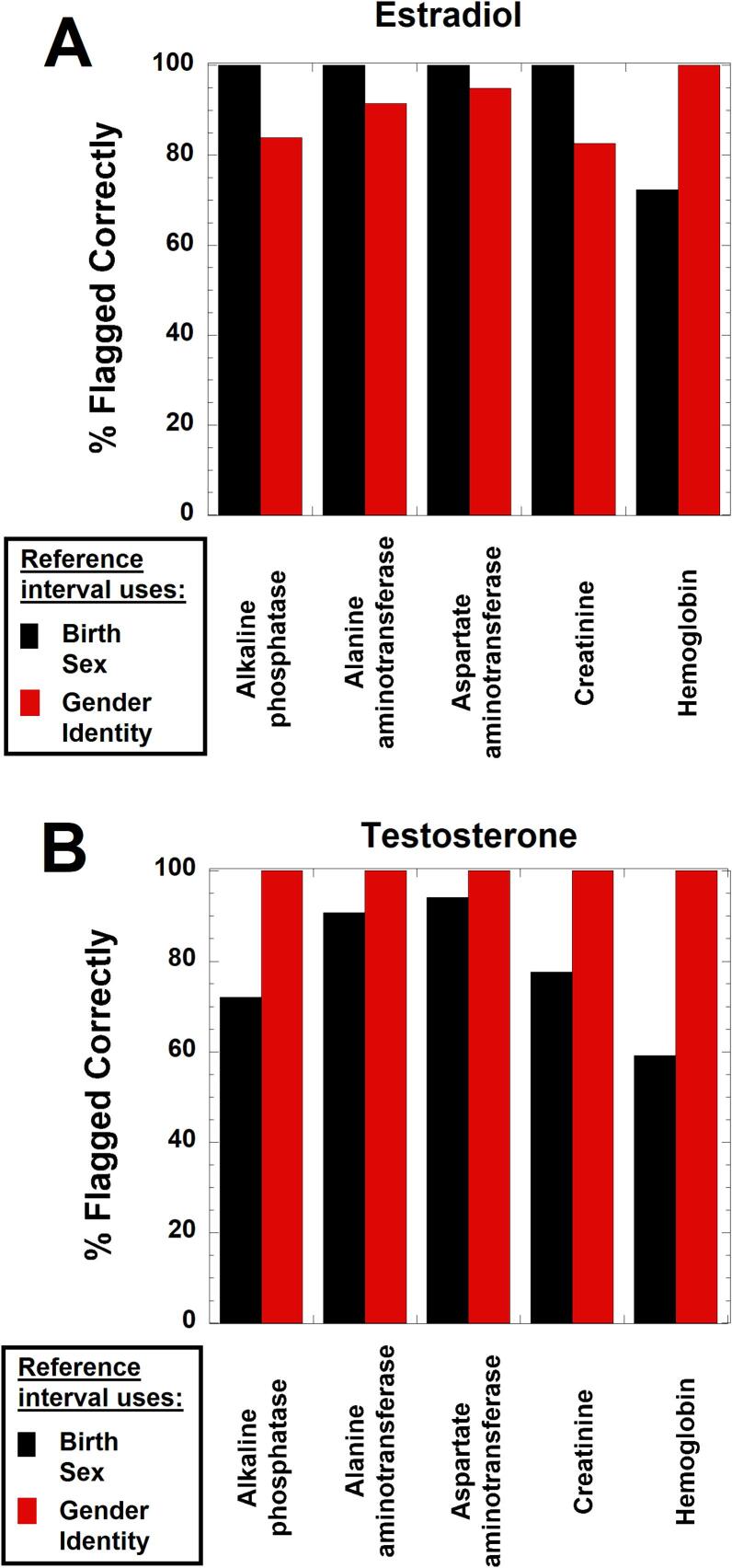
Fig. 4, Fig. 5 shows dot plots for where observed values for ALP, ALT, AST, CRT, and HB fall with regard to cisgender male and female RIs for the population taking gender-affirming hormones. In these two figures, values within the red boxes were below cisgender male RI but within the cisgender female RI, while blue boxes indicate values that were within the cisgender male RI but above the cisgender female RI. Fig. 6 summarizes the data for the population taking estradiol (Fig. 6A) or testosterone (Fig. 6B) in terms of how many values would have been flagged correctly for ALP, ALT, AST, CRT, and HB (given known effects of gender-affirming hormones) if using either SAAB (black boxes) or affirmed gender (red boxes) as the sex for the RI.
Fig. 4.
Dot plots of laboratory testing for alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) in patients receiving either estradiol (A, C, E) or testosterone (B, D, F) as gender-affirming hormone at the time of laboratory testing. The values are subdivided into those that are below the lower limits for both cisgender female and male reference intervals (RIs), lower than the male RI but within the female RI (highlighted by red boxes), within both RIs, higher than the female RI but within the male RI (highlighted by blue boxes), and above both the female and male RIs. ALT and AST have identical lower limits for both females and males. The RIs are indicated by green dashed lines for the adult male RI and solid purple lines for the adult female RI. The ALP data does include some teenage patients whose values would be within adult RIs but not within the specific sex-specific RI by age. The ALT and AST RIs just indicate the upper limit of the range (lower limit for female and male is 0 U/L for both tests). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
Dot plots of laboratory testing for creatinine and hemoglobin in patients receiving either estradiol (A, C) or testosterone (B, D) as gender-affirming hormone at the time of laboratory testing. The values are subdivided into those that are below the lower limits for both cisgender female and male reference intervals (RIs), lower than the male RI but within the female RI (highlighted by red boxes), within both RIs, higher than the female RI but within the male RI (highlighted by blue boxes), and above both the female and male RIs. The number of laboratory tests in each category is provided in parentheses for creatinine. In the estradiol cohort for creatinine, there were two samples from two unique patients that exceeded 5.0 mg/dL for creatinine (5.4 and 7.2 mg/dL, respectively). In the testosterone cohort for creatinine, there was a single sample that exceeded 5.0 mg/dL for creatinine (8.4 mg/dL). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
Estimate of correct normal/abnormal flagging using reference intervals (RIs) related to either sex assigned at birth (black boxes) or gender identity (red boxes) for patients either taking estradiol (A) or testosterone (B) as gender-affirming hormones. This analysis assumes that estradiol as gender-affirming therapy does not significantly alter RIs for alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatinine; estradiol does, however, lead to downward shift of hemoglobin RI to essentially match that for cisgender females. The analysis assumes that RIs for ALP, ALT, AST, creatinine, and hemoglobin align with cisgender male RIs for those taking testosterone as gender-affirming hormone. For example, a hemoglobin value of 16.0 g/dL in a transgender man taking testosterone would be above the cisgender female RI but within the cisgender male RI. Use of the sex assigned at birth (female) for RI would lead to incorrect interpretation (hemoglobin concentration too high) compared to using gender identity (male) for the RI (hemoglobin within RI). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For ALP, ALT, and AST, the main category that is discordant with respect to cisgender male and female RIs are those where the observed values are within the cisgender male RI but above the cisgender female RI (Fig. 4). For those taking estradiol, these could be misclassified as abnormally high if using affirmed gender (female) for the RI, as estradiol does not significantly impact ALP, ALT, and AST (Fig. 4A, C, E). In contrast, testosterone has been shown to significantly increase ALP, ALT, and AST, so the affirmed gender (male) would be the appropriate RI in those taking masculinizing hormones (Fig. 4B, D, F).
CRT and HB were more complicated as there were similar number of patients for both tests that are in two categories: below cisgender male RI but within cisgender female RI, or within cisgender male RI but above the cisgender female RI (Fig. 5A, 6A). For the estradiol cohort, the biggest clinical impact of using affirmed gender (female) for the CRT RI would likely be the 10.6 % of patients that are within the cisgender male RI but above the cisgender female RI. In this range, values may be incorrectly interpreted as indicative of diminished renal function based on the cisgender female RI, because estradiol has minimal impact on CRT. However, the issue of eGFR is also complicated and needs futher investigation in the population taking gender-affirming hormones [43].
Equations for eGFR commonly incorporate sex (female or male) into the calculation. Utilizing the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2021 equation without race [44], we calculated eGFR for the patients who were taking either estradiol or testosterone as gender-affirming therapy and who were also 18 years or older at the time CRT was measured (CKD-EPI equation is not recommended for those younger than 18 years old). This resulted in a data set of 992 CRT measurements on 406 unique patients taking estradiol and 477 CRT measurements on 214 unique patients taking testosterone. Table 3 shows how the use of either female or male for the eGFR calculation impacts the assignment of eGFR categories for CKD stages from the KDIGO 2013 working group recommendations [42]. For those taking estradiol, 403 of 992 (40.6 %) CRT measurements were in different eGFR categories for female compared to male (most commonly category 2 vs. 1). For those taking testosterone, 130 of 477 (27.3 %) CRT measurements were in different eGFR categories for female compared to male.
Table 3.
Impact of sex used for estimated glomerular filtration calculation in the population taking gender-affirming hormones.
| Estradiol cohort1 | eGFR category for CKD if male used for eGFR calculation2,3 | ||||||
|---|---|---|---|---|---|---|---|
|
eGFR category for CKD if female used for eGFR calculation2,3 |
1 | 2 | 3a | 3b | 4 | 5 | |
| 1 | 530 | ||||||
| 2 | 318 | 53 | |||||
| 3a | 61 | ||||||
| 3b | 15 | 1 | |||||
| 4 | 1 | ||||||
| 5 | 9 | 4 | |||||
| Testosterone cohort1 | eGFR category for CKD if male used for eGFR calculation2,3 | ||||||
| eGFR category for CKD if female used for eGFR calculation2,3 | 1 | 2 | 3a | 3b | 4 | 5 | |
| 1 | 345 | ||||||
| 2 | 99 | 20 | |||||
| 3a | 25 | ||||||
| 3b | 5 | 1 | |||||
| 4 | 2 | 1 | |||||
| 5 | 1 | ||||||
1The estradiol data consisted of 992 creatinine measurements on 406 unique patients 18 years and older taking estradiol as gender-affirming therapy. Observed agreement 0.59, weighted κ 0.40 (95 % CI 0.35–0.46).
2Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
3eGFR category for CKD: stage 1, normal or high (eGFR > 90 ml/min/173 m2); stage 2, mildly decreased (eGFR 60–89); stage 3a, mildly to moderately decreased (eGFR 45–59); stage 3b, moderately to severely decreased (eGFR 30–44); stage 4, severely decreased (eGFR 15–29); stage 5, kidney failure (eGFR < 15). eGFR was calculated using the CKD-EPI (2021) eGFR equation without race refit.
4The testosterone data consisted of 477 creatinine measurements on 214 unique patients 18 years and older taking testosterone as gender-affirming therapy. Observed agreement 0.74, weighted κ 0.46 (95 % CI 0.38–0.54).
The largest discordant population for HB (18.3 %) in the estradiol cohort are those that have values that are below the cisgender male RI but within the cisgender female RI; these could be incorrectly classified as anemia if using SAAB (male) for the RI when in fact gender-affirming therapy can explain the decrease in HB (Fig. 5C, 6A). It is also worth noting that HB values below both cisgender male and female ranges were common (80 of 193, 41.5 %) in the estradiol cohort. This likely reflects anemia regardless of SAAB.
For the subgroup taking testosterone as the gender-affirming hormone, use of affirmed gender (male) would result in correct categorization for CRT and HB (Fig. 5B, D; Fig. 6B). For CRT, 8.1 % of the values are in a range that is above the cisgender female RI but within the cisgender male RI (Fig. 5B and 6B). These values may be interpreted as abnormally high if using SAAB (female) for the RI. For HB, over 40 % of the values are in categories that are discordant between the cisgender male and female RIs (Fig. 5D and 6B). Of these, 26.7 % of the HB values are above the cisgender female RI but within the cisgender male RI (Fig. 5D); these may be incorrectly labeled as erythrocytosis if using SAAB (female) as the RI. In contrast, 14.2 % of the HB values are within the cisgender female RI but below the cisgender male RI (Fig. 5D); these may be incorrectly labeled as normal if using SAAB for the RI but may in fact indicate anemia in the context of someone using testosterone as gender-affirming hormone.
Fig. 6 summarizes the overall impact of using affirmed gender or SAAB as the sex for the RI for ALP, ALT, AST, CRT, and HB in the population taking gender-affirming hormones. In our dataset, use of affirmed gender for the RI for these five tests will overall assign the correct normal/abnormal flagging more often based on current knowledge of gender-affirming tests. Use of affirmed gender for the RI has a particularly large impact on HB normal/abnormal flagging for both the estradiol and testosterone groups (Fig. 6). Supplemental Tables 5 and 6 summarize the data for ALP, ALT, AST, CRT, and HB, breaking down the impact of using SAAB or affirmed gender for the RIs for these five tests.
Potential clinical impact of gender-affirming hormones on less commonly ordered laboratory tests
There were other less frequently ordered laboratory tests with sex-specific RIs ordered in patients documented to be taking gender-affirming hormones (Fig. 2, Fig. 3). Some of these tests were rarely ordered in our data set at ages that have sex-specific RIs, and differences between RIs seem unlikely to have significant clinical impact in the population taking gender-affirming hormones (CK, total calcium, serum phosphorus, erythrocyte sedimentation rate, and uric acid). GGT showed a pattern similar to ALT and AST, with the most common discordant pattern in terms of normal/abnormal flagging being values that were above the cisgender female RI but within the cisgender male RI (33 of 85 or 38.8 % of GGT results were in this category). LDH was only ordered 34 times in the patient population taking gender-affirming hormones, with only a single result (2.9 %) above the cisgender female RI but within the cisgender male RI. All other results for LDH were either within both cisgender RIs or abnormal in both RIs.
Ferritin and iron studies were ordered 68 and 69 times, respectively, in patients taking gender-affirming hormones. While serum iron and percent iron saturation have fairly narrow differences between cisgender female and male RIs at our institution, ferritin has wider gaps between the lower and upper limits of normal for female and male RIs (Supplemental Table 2). As result, 13 of 28 (46.4 %) ferritin values for those taking estradiol and 24 of 40 (60.0 %) ferritin values for those taking testosterone were discordant between cisgender female and male RIs. To our knowledge, there is currently no published literature on the impact of gender-affirming hormones on ferritin and iron studies; thus, the clinical impact of this is not clear.
Lastly, the cardiac markers hs-TnT (35 orders on 25 unique patients) and NT-proBNP (8 orders on 6 unique patients) were ordered infrequently in the population taking gender-affirming hormones (Fig. 2, Fig. 3). For hs-TnT, only 3 values (2 unique patients) were outside cisgender female and/or male RIs and were explainable by myocardial infarction in both patients. For NT-proBNP, 3 values on 1 unique patient were outside both cisgender female and male RIs and were explainable by heart failure later managed by cardiac transplant.
Discussion
In the present study, we used a previously well-characterized dataset of patients with respect to GI and use of gender-affirming therapy to focus on laboratory test ordering and the potential impact of gender-affirming hormones [17]. The most frequently ordered non-hormonal laboratory tests were ALP, ALT, AST, CRT, HB, and HDL. In patients taking testosterone as gender-affirming hormone, ALP, ALT, AST, CRT, and HB essentially align with cisgender male RIs based on current published data, while published data for HDL and other lipids have been more variable [9], [10], [11], [15], [31], [33], [34], [35], [36], [37], [38]. Thus, use of the cisgender female RI (corresponding to SAAB) can lead to discordant normal/abnormal flagging for ALP, ALT, AST, CRT, and HB compared to use of the cisgender male RI. In contrast, ALP, ALT, AST, and CRT are not significantly impacted by estradiol as gender-affirming therapy [9], [10], [15], [31], [33], [45], although there has been some variability across studies for some of these analytes [32], [34], [36], [38], [46], particularly for CRT. Estradiol gender-affirming therapy does, however, result in HB values that essentially align with cisgender female RIs [10], [11], [47]. We observed that HB values below both cisgender female and male RIs were common in the estradiol cohort, comprising 41.5 % of total measurements. This finding warrants future investigation. Unfortunately, iron studies were not commonly co-ordered with HB in this cohort so we did not have laboratory evidence to help discern type of anemia.
For ALP, ALT, AST, CRT, and HB, the sex used for assignment of RIs has implications for whether values flag normal or abnormal and for clinical decision-making. Use of affirmed gender will provide the appropriate RI for these five tests in those taking testosterone and for HB in those taking estradiol, but can lead to misinterpretation for ALP, ALT, AST, and CRT in those taking estradiol. There is a possibility to offer “transgender-specific” test codes for some laboratory tests for the population taking gender-affirming hormones; however, this is logistically complicated (especially with these tests utilized in different testing panels) and depends on providers reliably using such order codes. For HB, use of SAAB for the RI could lead to inaccurate assignment of erythrocytosis in transmen taking testosterone or anemia in transwomen taking estradiol. For CRT, use of SAAB for the RI in transmen could lead to erroneous conclusion of declining renal function, even though increases in CRT are on average expected from testosterone gender-affirming therapy. The sex used in eGFR calculations can also impact clinical classifications in those taking gender-affirming hormones [48], [49], [50]; along these lines, we found that approximately 40 % of CRT measurements in the estradiol and testosterone cohorts would be classified in different eGFR categories using the CKD-EPI 2021 equation.
In the US, legal sex is typically what is used for assignment of RIs in the EHR or laboratory information system. The process to change legal sex in the US varies considerably [29]. Some states/territories have a simple process, while others either do not allow change of sex designation or require history of gender-affirming treatment (some state laws reference language such as “gender reassignment surgery”) together with documentation from healthcare provider. Healthcare organizations also have varying processes for a patient to change legal sex within the EHR. Nonetheless, healthcare providers should be aware that some patients change their legal sex, which can impact a variety of processes that may have sex-specific nomenclature and logic, including laboratory test RIs, billing rules, and radiologic imaging codes [18], [19].
In the present study, we utilized logic within our institutional laboratory information system that identifies SOGI field differences as a binary rule. One major advantage of using SOGI field differences is to considerably narrow the patient population for which interpretive comments might be posted to select laboratory results. While SOGI field differences are not perfect for identifying those who may be taking gender-affirming hormones, alternative approaches to identify this sub-population are currently quite difficult. For example, information in medication/pharmacy records or diagnosis codes are often not accessible to the clinical laboratory for automated interpretation. In theory, individual SOGI field responses could identify those who are transgender or nonbinary. However, our previous study revealed that a wide diversity of SOGI field responses were used out of 168 possible combinations from the SOGI field options in our EHR build for legal sex, SAAB, and GI [17]. The large number of combinations creates substantial technical informatics challenges if attempting to build logic for RIs and interpretive comments based on specific SOGI field combinations.
Of the widely ordered laboratory tests analyzed in our study, ferritin and iron studies are both lacking any published literature on the impacts of gender-affirming hormones. This would certainly be of interest as a future study as our data showed that 46.4 % of those taking estradiol and 60.0 % of those taking testosterone were abnormal for ferritin in one cisgender RI but not the other. The impact of gender-affirming hormones on cardiac markers are also of interest given well-documented cardiovascular health disparities described in the gender-expansive population [51]. Our dataset had few orders for hs-TnT and NT-proBNP, likely reflecting a gender-expansive population on average younger than the overall patient population in our institution. There have been some publications on variation of hs-Tn and NT-proBNP in the transgender population [12], [52], and future investigations would be welcome.
Limitations of our study include analysis at a single academic medical center that serves as a regional center for LGBTQ health care. A small proportion of those receiving gender-affirming hormones also had a history of bilateral gonadectomy, a variable that may have impacted some laboratory tests. Our study did not analyze factors that may have influenced testing ordering and laboratory values including duration of hormone therapy, comorbidities, concomitant medications, and clinical indications for testing. This is a potential area of future investigation. There is also the possibility that some laboratory values were outside RIs by chance and not due to any abnormality or other factor. The efficacy of SOGI fields at our institution was influenced by institutional focus that include workflows that promote use of the SOGI fields in the LGBTQ clinics and other medical center sites [17], [18]. Institutions with low rates of SOGI field promotion and adoption will likely find less benefit with use of SOGI fields. Lastly, the analysis of RIs in the present study focused on normal/abnormal flagging; however, more subtle clinical-decision-making would often take into account trends in values and clinical context, such as changes from baseline measurements obtained prior to starting gender-affirming therapy.
Conclusions
In our study at an academic medical center, ALP, ALT, AST, CRT, and HB were the most frequently ordered non-hormonal laboratory tests with sex-specific RIs in the patient population taking gender-affirming hormone therapy. Values within the RI for one sex but not the other were common in these tests, meaning that changing the legal sex for assignment of RIs impact normal/abnormal flagging. Clinicians should be aware of the potential impact of gender-affirming therapy on laboratory tests and what sex/gender is being used in the EHR to assign RIs. Future studies on renal function (including eGFR), ferritin, iron studies, and cardiovascular markers in the transgender and non-binary population taking gender-affirming hormones would be of interest.
Funding
There was no external funding for this study.
Ethical approval
This study was conducted with ethical approval from the University of Iowa Institutional Review Board as a retrospective study with waiver of informed consent with the approval number 202202388.
Disclosure statement
The authors have nothing to disclose
CRediT authorship contribution statement
Matthew D. Krasowski: Writing – review & editing, Writing – original draft, Project administration, Investigation, Formal analysis, Conceptualization. Nicole G. Hines: Writing – review & editing, Investigation, Conceptualization. Katherine L. Imborek: Writing – review & editing, Project administration, Formal analysis, Conceptualization. Dina N. Greene: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would particularly like to thanks Nick Dreyer and Linda Kleinmeyer from the University of Iowa Health Care Information Systems for assistance on retrieving sexual orientation/gender identity from electronic health record databases.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcte.2024.100350.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data associated with this study has been deposited in Mendeley Data, V1, doi: 10.17632/gy8v292647.1, https://data.mendeley.com/datasets/gy8v292647/1.
References
- 1.Coleman E., Radix A.E., Bouman W.P., Brown G.R., de Vries A.L.C., Deutsch M.B., et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. 2022;23:S1–S259. doi: 10.1080/26895269.2022.2100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hembree W.C., Cohen-Kettenis P.T., Gooren L., Hannema S.E., Meyer W.J., Murad M.H., et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869–3903. doi: 10.1210/jc.2017-01658. [DOI] [PubMed] [Google Scholar]
- 3.Irwig M.S. Transgender care by endocrinologists in the united states. Endocr Pract. 2016;22:832–836. doi: 10.4158/EP151185.OR. [DOI] [PubMed] [Google Scholar]
- 4.Jha S., Bouman W.P. Introduction to healthcare for transgender and gender-diverse people. Best Pract Res Clin Obstet Gynaecol. 2023;87 doi: 10.1016/j.bpobgyn.2022.102299. [DOI] [PubMed] [Google Scholar]
- 5.Tangpricha V., den Heijer M. Oestrogen and anti-androgen therapy for transgender women. Lancet Diabetes Endocrinol. 2017;5:291–300. doi: 10.1016/S2213-8587(16)30319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irwig M.S. Testosterone therapy for transgender men. Lancet Diabetes Endocrinol. 2017;5:301–311. doi: 10.1016/S2213-8587(16)00036-X. [DOI] [PubMed] [Google Scholar]
- 7.Cheung A.S., Lim H.Y., Cook T., Zwickl S., Ginger A., Chiang C., et al. Approach to interpreting common laboratory pathology tests in transgender individuals. J Clin Endocrinol Metab. 2021;106:893–901. doi: 10.1210/clinem/dgaa546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein Z., Corneil T.A., Greene D.N. When gender identity doesn't equal sex recorded at birth: the role of the laboratory in providing effective healthcare to the transgender community. Clin Chem. 2017;63:1342–1352. doi: 10.1373/clinchem.2016.258780. [DOI] [PubMed] [Google Scholar]
- 9.Humble R.M., Imborek K.L., Nisly N., Greene D.N., Krasowski M.D. Common hormone therapies used to care for transgender patients influence laboratory results. J Appl Lab Med. 2019;3:799–814. doi: 10.1373/jalm.2018.027078. [DOI] [PubMed] [Google Scholar]
- 10.SoRelle J.A., Jiao R., Gao E., Veazey J., Frame I., Quinn A.M., et al. Impact of hormone therapy on laboratory values in transgender patients. Clin Chem. 2019;65:170–179. doi: 10.1373/clinchem.2018.292730. [DOI] [PubMed] [Google Scholar]
- 11.Greene D.N., McPherson G.W., Rongitsch J., Imborek K.L., Schmidt R.L., Humble R.M., et al. Hematology reference intervals for transgender adults on stable hormone therapy. Clin Chim Acta. 2019;492:84–90. doi: 10.1016/j.cca.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Greene D.N., Schmidt R.L., Christenson R.H., Rongitsch J., Imborek K.L., Rebuck H., et al. Distribution of high-sensitivity cardiac troponin and N-terminal pro-brain natriuretic peptide in healthy transgender people. JAMA Cardiol. 2022;7:1170–1174. doi: 10.1001/jamacardio.2022.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene D.N., Schmidt R.L., Winston M.G., Rongitsch J., Imborek K.L., Dickerson J.A., et al. Reproductive endocrinology reference intervals for transgender women on stable hormone therapy. J Appl Lab Med. 2021;6:15–26. doi: 10.1093/jalm/jfaa028. [DOI] [PubMed] [Google Scholar]
- 14.Greene D.N., Schmidt R.L., Winston-McPherson G., Rongitsch J., Imborek K.L., Dickerson J.A., et al. Reproductive endocrinology reference intervals for transgender men on stable hormone therapy. J Appl Lab Med. 2021;6:41–50. doi: 10.1093/jalm/jfaa169. [DOI] [PubMed] [Google Scholar]
- 15.Humble R.M., Greene D.N., Schmidt R.L., Winston M.G., Rongitsch J., Imborek K.L., et al. Reference intervals for clinical chemistry analytes for transgender men and women on stable hormone therapy. J Appl Lab Med. 2022;7:1131–1144. doi: 10.1093/jalm/jfac025. [DOI] [PubMed] [Google Scholar]
- 16.Costelloe S.J., Hepburn S. Management of transgender patients in laboratory information management systems - moving on from binary and ternary logic. Ann Clin Biochem. 2021;58:264–266. doi: 10.1177/0004563220984825. [DOI] [PubMed] [Google Scholar]
- 17.Hines N.G., Greene D.N., Imborek K.L., Krasowski M.D. Patterns of gender identity data within electronic health record databases can be used as a tool for identifying and estimating the prevalence of gender-expansive people. JAMIA Open. 2023;6:ooad042. doi: 10.1093/jamiaopen/ooad042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imborek K.L., Nisly N.L., Hesseltine M.J., Grienke J., Zikmund T.A., Dreyer N.R., et al. Preferred names, preferred pronouns, and gender identity in the electronic medical record and laboratory information system: is pathology ready? J Pathol Inform. 2017;8:42. doi: 10.4103/jpi.jpi_52_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel K., Lyon M.E., Luu H.S. Providing inclusive care for transgender patients: capturing sex and gender in the electronic medical record. J Appl Lab Med. 2021;6:210–218. doi: 10.1093/jalm/jfaa214. [DOI] [PubMed] [Google Scholar]
- 20.Cahill S.R., Baker K., Deutsch M.B., Keatley J., Makadon H.J. Inclusion of sexual orientation and gender identity in stage 3 meaningful use guidelines: a huge step forward for LGBT health. LGBT Health. 2016;3:100–102. doi: 10.1089/lgbt.2015.0136. [DOI] [PubMed] [Google Scholar]
- 21.Grasso C., Goldhammer H., Brown R.J., Furness B.W. Using sexual orientation and gender identity data in electronic health records to assess for disparities in preventive health screening services. Int J Med Inform. 2020;142 doi: 10.1016/j.ijmedinf.2020.104245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deutsch M.B., Buchholz D. Electronic health records and transgender patients–practical recommendations for the collection of gender identity data. J Gen Intern Med. 2015;30:843–847. doi: 10.1007/s11606-014-3148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubin S., Cook T., Liss A., Doty G., Moore K., Greene R., et al. Comparing electronic health record domains' utility to identify transgender patients. Transgend Health. 2022;7:78–84. doi: 10.1089/trgh.2020.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson H.M., Kronk C.A., Feasley K., Pachwicewicz P., Karnik N.S. Implementation of gender identity and assigned sex at birth data collection in electronic health records: where are we now? Int J Environ Res Public Health. 2021;18:pp. doi: 10.3390/ijerph18126599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldhammer H., Grasso C., Katz-Wise S.L., Thomson K., Gordon A.R., Keuroghlian A.S. Pediatric sexual orientation and gender identity data collection in the electronic health record. J Am Med Inform Assoc. 2022:pp. doi: 10.1093/jamia/ocac048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraschel K.L., Chen A., Turban J.L., Cohen I.G. Legislation restricting gender-affirming care for transgender youth: politics eclipse healthcare. Cell Rep Med. 2022;3 doi: 10.1016/j.xcrm.2022.100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maragh-Bass A.C., Torain M., Adler R., Schneider E., Ranjit A., Kodadek L.M., et al. Risks, benefits, and importance of collecting sexual orientation and gender identity data in healthcare settings: a multi-method analysis of patient and provider perspectives. LGBT Health. 2017;4:141–152. doi: 10.1089/lgbt.2016.0107. [DOI] [PubMed] [Google Scholar]
- 28.Grasso C., Goldhammer H., Thompson J., Keuroghlian A.S. Optimizing gender-affirming medical care through anatomical inventories, clinical decision support, and population health management in electronic health record systems. J Am Med Inform Assoc. 2021;28:2531–2535. doi: 10.1093/jamia/ocab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Identity Document Laws and Policies [https://www.lgbtmap.org/equality-maps/identity_document_laws].
- 30.Adeli K., Higgins V., Nieuwesteeg M., Raizman J.E., Chen Y., Wong S.L., et al. Biochemical marker reference values across pediatric, adult, and geriatric ages: establishment of robust pediatric and adult reference intervals on the basis of the Canadian Health Measures Survey. Clin Chem. 2015;61:1049–1062. doi: 10.1373/clinchem.2015.240515. [DOI] [PubMed] [Google Scholar]
- 31.Allen A.N., Jiao R., Day P., Pagels P., Gimpel N., SoRelle J.A. Dynamic impact of hormone therapy on laboratory values in transgender patients over time. J Appl Lab Med. 2021;6:27–40. doi: 10.1093/jalm/jfaa192. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez J.D., Tannock L.R. Metabolic effects of hormone therapy in transgender patients. Endocr Pract. 2016;22:383–388. doi: 10.4158/EP15950.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashemi L., Zhang Q., Getahun D., Jasuja G.K., McCracken C., Pisegna J., et al. Longitudinal changes in liver enzyme levels among transgender people receiving gender affirming hormone therapy. J Sex Med. 2021;18:1662–1675. doi: 10.1016/j.jsxm.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maheshwari A., Dines V., Saul D., Nippoldt T., Kattah A., Davidge-Pitts C. The effect of gender-affirming hormone therapy on serum creatinine in transgender individuals. Endocr Pract. 2021:pp. doi: 10.1016/j.eprac.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Mueller A., Haeberle L., Zollver H., Claassen T., Kronawitter D., Oppelt P.G., et al. Effects of intramuscular testosterone undecanoate on body composition and bone mineral density in female-to-male transsexuals. J Sex Med. 2010;7:3190–3198. doi: 10.1111/j.1743-6109.2010.01912.x. [DOI] [PubMed] [Google Scholar]
- 36.Stangl T.A., Wiepjes C.M., Defreyne J., Conemans E., Fisher A.D., Schreiner T., et al. Is there a need for liver enzyme monitoring in people using gender-affirming hormone therapy? Eur J Endocrinol. 2021;184:513–520. doi: 10.1530/EJE-20-1064. [DOI] [PubMed] [Google Scholar]
- 37.Wierckx K., Mueller S., Weyers S., Van Caenegem E., Roef G., Heylens G., et al. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med. 2012;9:2641–2651. doi: 10.1111/j.1743-6109.2012.02876.x. [DOI] [PubMed] [Google Scholar]
- 38.Wierckx K., Van Caenegem E., Schreiner T., Haraldsen I., Fisher A.D., Toye K., et al. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European network for the investigation of gender incongruence. J Sex Med. 2014;11:1999–2011. doi: 10.1111/jsm.12571. [DOI] [PubMed] [Google Scholar]
- 39.Blau J.L., Wilford J.D., Dane S.K., Karandikar N.J., Fuller E.S., Jacobsmeier D.J., et al. Implementation of epic beaker anatomic pathology at an academic medical center. J Pathol Inform. 2017;8:47. doi: 10.4103/jpi.jpi_31_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krasowski M.D., Wilford J.D., Howard W., Dane S.K., Davis S.R., Karandikar N.J., et al. Implementation of epic beaker clinical pathology at an academic medical center. J Pathol Inform. 2016;7:7. doi: 10.4103/2153-3539.175798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krasowski M.D., Grieme C.V., Cassady B., Dreyer N.R., Wanat K.A., Hightower M., et al. Variation in results release and patient portal access to diagnostic test results at an academic medical center. J Pathol Inform. 2017;8:45. doi: 10.4103/jpi.jpi_53_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.KDIGO Summary of recommendation statements. Kidney Int Suppl (2011) 2013;3:5–14. doi: 10.1038/kisup.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierre C.C., Marzinke M.A., Ahmed S.B., Collister D., Colon-Franco J.M., Hoenig M.P., et al. AACC/NKF guidance document on improving equity in chronic kidney disease care. J Appl Lab Med. 2023;8:789–816. doi: 10.1093/jalm/jfad022. [DOI] [PubMed] [Google Scholar]
- 44.Delgado C., Baweja M., Crews D.C., Eneanya N.D., Gadegbeku C.A., Inker L.A., et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. 2022;79:268–288 e261. doi: 10.1053/j.ajkd.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Roberts T.K., Kraft C.S., French D., Ji W., Wu A.H., Tangpricha V., et al. Interpreting laboratory results in transgender patients on hormone therapy. Am J Med. 2014;127:159–162. doi: 10.1016/j.amjmed.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Roberts T.K., Fantz C.R. Barriers to quality health care for the transgender population. Clin Biochem. 2014;47:983–987. doi: 10.1016/j.clinbiochem.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Antun A., Zhang Q., Bhasin S., Bradlyn A., Flanders W.D., Getahun D., et al. Longitudinal changes in hematologic parameters among transgender people receiving hormone therapy. J Endocr Soc. 2020;4:bvaa119. doi: 10.1210/jendso/bvaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coig R., Grieve V.L.B., Cirrincione L.R. Clinical pharmacological considerations in transgender medicine. Handb Exp Pharmacol. 2023;282:41–55. doi: 10.1007/164_2023_665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fadich S.K., Kalayjian A., Greene D.N., Cirrincione L.R. A Retrospective analysis of creatinine-based kidney function with and without sex assigned at birth among transgender adults. Ann Pharmacother. 2021 doi: 10.1177/10600280211050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krupka E., Curtis S., Ferguson T., Whitlock R., Askin N., Millar A.C., et al. The effect of gender-affirming hormone therapy on measures of kidney function: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2022;17:1305–1315. doi: 10.2215/CJN.01890222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howerton I., Harris J.K. Transgender identity and cardiovascular disease. Transgend Health. 2022;7:407–415. doi: 10.1089/trgh.2020.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J., Taur A., Chen A., Wu Y.L., Lee M.S. Sex-specific cardiac troponin thresholds in transgender patients with suspected acute coronary syndrome. JAMA Netw Open. 2023;6:e2337345. doi: 10.1001/jamanetworkopen.2023.37345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study has been deposited in Mendeley Data, V1, doi: 10.17632/gy8v292647.1, https://data.mendeley.com/datasets/gy8v292647/1.