Abstract
Recovery from leukemia induced by Friend virus complex (FV) requires strong CD4+ helper, CD8+ cytotoxic T-lymphocyte, and B-cell responses. The development of these immune responses is dependent on the major histocompatibility complex (MHC) (H-2) genotype of the mouse. In H-2b/b mice, which spontaneously recover from FV-induced erythroleukemia, neutralization of gamma interferon (IFN-γ) in vivo inhibited recovery, which indicated that IFN-γ was a necessary component of the immune response to FV. Furthermore, in H-2b/b mice, high numbers of IFN-γ-producing cells were detected after FV infection, whereas in H-2a/b mice, which have a low-recovery phenotype, only low numbers of IFN-γ-producing cells were detected. Similarly, H-2bm14/b mice, which cannot recover from FV infection due to a point mutation in one allele of the H-2Db gene, also had low numbers of IFN-γ-producing T cells. Surprisingly, this effect was observed for both CD8+ and CD4+ T cells. These findings reveal a novel influence of MHC class I genes on CD4+ T-cell responses to viral infection. Furthermore, the influence of MHC class I genotype on the generation of both IFN-γ-producing CD4+ and CD8+ T cells helps explain the major impact of the H-2D gene on recovery from FV disease.
Retroviruses induce neoplastic and nonneoplastic diseases in humans and many species of animals. Although there are numerous studies on the development of disease following retroviral infections, the types of immune responses required to successfully overcome retroviral infections are not well understood. There are several murine retrovirus models where infection induces neoplasms and/or immunosuppression in mice. However, in most cases, this occurs only following infection of neonatal mice, which lack a mature, competent immune system. In contrast, Friend virus complex (FV) induces erythroleukemia and immunosuppression in immunocompetent adult mice, providing a good model for studying the immune response to retroviral infection.
In susceptible mouse strains, infection with FV induces a rapid polyclonal erythroblast proliferation, which leads to splenomegaly, erythroleukemia, and death (14, 17, 20). However, mice with certain major histocompatibility complex (MHC) haplotypes can spontaneously recover from leukemic splenomegaly (7, 11, 17). For example, after infection with a high dose of FV, (C57BL/10 × A.BY)F1 mice (H-2b/b) initially develop splenomegaly but then recover and generally live without relapse for a normal life span. In contrast, after similar high-dose infection, (B10.A × A.BY)F1 mice (H-2a/b) also develop splenomegaly but do not recover and eventually die (4, 10). The ability of H-2b/b mice to recover is dependent upon the generation of strong immune responses, including the production of neutralizing antibodies, generation of FV-specific cytotoxic T lymphocytes (CTL), and activation of FV-specific CD4+ T cells (17). Ablation of any of these three arms of the immune response either by depletion of CD4+ or CD8+ T-cell subsets (30) or by use of specific H-2b/b strains (A.BY or BALB.B), which are genetically unable to make humoral immune responses to FV (13, 15), results in a failure to recover from FV-induced splenomegaly. The FV-specific neutralizing antibody response appears to control viremia and reduce viral spread, while FV-specific CD8+ T cells provide CTL activity against FV-infected cells (reviewed in reference 17). CD4+ T cells can regulate the immune response through cytokine production and help both the B-cell and CD8+ T-cell responses to FV. Because nonrecovering H-2a/b mice generate a neutralizing antibody response comparable to that of H-2b/b mice (8), the main difference between H-2a/b and H-2b/b mice is most likely in the CD4+ and/or the CD8+ T-cell responses. Previous studies have shown differences between H-2b/b and H-2a/b mice in the frequency and magnitude of the CD8+ T-cell response (30) and in the kinetics of the CD4+ T-cell response to FV (4). Since gamma interferon (IFN-γ) has been shown to be an important component for recovery from several types of viral infections (5, 6, 21, 29, 31), the present experiments were aimed at studying the role of IFN-γ in recovery from FV. The IFN-γ response of FV-specific CD4+ and CD8+ T cells was analyzed in recovering and nonrecovering mice.
FV-specific production of IFN-γ by in vitro activation of spleen cells from FV-infected mice.
In order to determine if FV infection generated a strong IFN-γ response in recovering H-2b/b mice, spleen cells from FV-infected H-2b/b mice were removed 10 days after intravenous infection with FV-B (11). Spleen cells (5 × 106/ml) were cultured with 2.5 × 106 antigen-presenting cells (APCs) per ml in a 24-well plate. For FV-specific activation, 5 μg of FV per ml, purified from culture supernatant from AA41 FV erythroleukemia cells (3, 22), was added to specific wells. After 72 h culture supernatants were analyzed for IFN-γ by enzyme-linked immunosorbent assay (ELISA).
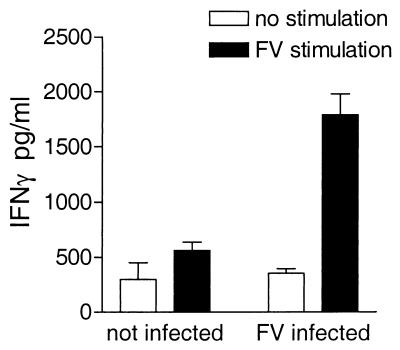
Spleen cells from FV-infected H-2b/b mice produced high levels of IFN-γ after restimulation with purified FV, while unstimulated spleen cells did not produce IFN-γ. Thus, antigen-specific stimulation was required for production of IFN-γ by spleen cells from FV-infected H-2b/b mice (Fig. 1). To test the requirement for prior immunization by FV infection, spleen cells from naive mice were compared with cells from FV-infected mice. Spleen cells from naive mice stimulated in vitro produced only low levels of IFN-γ, while spleen cells from FV-infected mice cultured with FV produced high levels of IFN-γ, demonstrating that infection was necessary for the generation of FV-specific IFN-γ-producing cells (Fig. 1). The requirements for in vitro antigen stimulation and in vivo sensitization suggested that the IFN-γ was mainly produced by FV-specific CD4+ or CD8+ T cells, rather than macrophages or natural killer cells.
FIG. 1.
FV-specific IFN-γ production by spleen cells from FV-infected H-2b/b mice. Spleens were removed from either uninfected H-2b/b mice or H-2b/b mice infected with 1,500 SFFU at 10 days postinfection. Spleen cells from these mice were depleted of red blood cells and then cultured with irradiated syngeneic spleen cells from naive mice as APCs and either 5 μg of purified FV per ml or no antigen. After 72 h, supernatants were harvested and analyzed for IFN-γ using an IFN-γ-specific sandwich ELISA. Rat anti-mouse IFN-γ MAb R4-6A2 (Pharmingen, San Diego, Calif.) was used as the coating antibody, and biotinylated XMG1.2 (Pharmingen) was used as the detection antibody. Data are presented as the means plus the standard errors of the means for three mice per group.
H-2b/b mice have a greater number of FV-specific IFN-γ-producing spleen cells than H-2a/b mice.
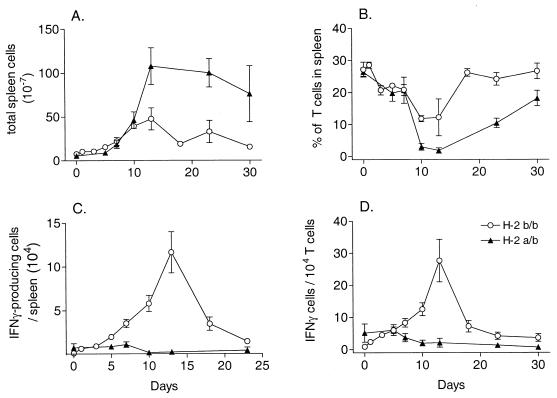
The uncontrolled erythroblast proliferation and splenomegaly in low-recovery H-2a/b mice made it difficult to do direct comparisons with high-recovery H-2b/b mice using the ELISA because the overall cell numbers were higher in the grossly enlarged spleens of H-2a/b mice (Fig. 2A), making the relative percentages of T cells much lower (Fig. 2B). Therefore, to determine if production of IFN-γ correlated with recovery from FV-induced erythroleukemia, the actual numbers of IFN-γ-producing cells in H-2b/b or H-2a/b mice were compared at various times after FV infection using an IFN-γ-specific enzyme-linked immunospot (ELISPOT) assay (26). Spleen cells were serially diluted and added to each well of anti-mouse IFN-γ-monoclonal antibody (MAb) (R4-6A2)-coated filtration plates along with 2.5 × 105 irradiated syngeneic spleen cells and recombinant human interleukin 2 (1.25 ng/ml). Spleen cells were stimulated with irradiated (10 Gy) spleen cells from syngeneic FV-infected (1,500 spleen focus-forming units [SFFU]) mice depleted of CD4+ and CD8+ T cells. After 36 h, the plates were washed and developed using biotinylated XMG1.2 as the secondary antibody and aminoethyl-carbazole as the substrate.
FIG. 2.
Kinetics of IFN-γ-producing cells in H-2b/b and H-2a/b mice after FV infection. All results are the means ± the standard errors of the means for three to five mice per group. (A) Total number of spleen cells in spleens of H-2b/b and H-2a/b mice at various time points after infection with 1,500 SFFU of FV. (B) Percentage of T cells in each spleen. Spleen cells were analyzed by flow cytometry for the percentage of CD3+ T cells (fluorescein isothiocyanate-labeled 145-2C11 MAb; Pharmingen) in each spleen. (C) Number of FV-specific IFN-γ-producing cells per spleen as determined by ELISPOT assay. Spleen cells from H-2b/b or H-2a/b mice were serially diluted, activated with FV-infected stimulator cells, and analyzed for the number of IFN-γ-producing cells using an IFN-γ-specific ELISPOT assay. Background dots from wells with stimulator and feeder cells only were subtracted to derive the total number of spots for each well. (D) Ratio of IFN-γ-producing cells to T cells in spleens. The number of IFN-γ-producing cells in each spleen was divided by the actual number of T cells in each spleen to account for the changes in the percentage of T cells.
From 7 to 30 days postinfection, the number of FV-specific IFN-γ-producing cells was higher in spleens from H-2b/b mice than in spleens from H-2a/b mice (Fig. 2C). Thus, high numbers of IFN-γ-producing cells correlated with recovery from FV-induced splenomegaly. To correct for the changes in percentage of T cells in the spleen during FV-induced erythroleukemia (Fig. 2B), the number of IFN-γ-producing cells was also calculated as the number of IFN-γ-positive cells per 104 T cells based on the percentage of CD3+ cells in each spleen (Fig. 2D). This calculation did not affect the overall conclusion that there were significantly higher numbers of FV-specific IFN-γ-producing cells in spleens from H-2b/b mice than in spleens from H-2a/b mice.
IFN-γ is necessary for recovery from FV-induced splenomegaly.
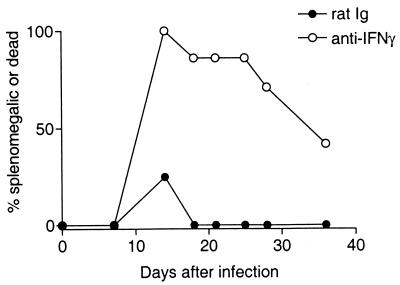
The production of IFN-γ by recovering H-2b/b mice and not by nonrecovering H-2a/b mice suggests that IFN-γ-producing T cells may have an important role in controlling FV infection. To determine if IFN-γ was required for recovery from FV-induced erythroleukemia, H-2b/b mice were treated with anti-IFN-γ (XMG1.2) after infection with FV. Mice were injected with 0.5 ml of either control rat immunoglobulin (Ig) or anti-IFN-γ at a concentration of 0.5 mg/ml every 3 days for 21 days beginning on the day of infection. Anti-IFN-γ treatment suppressed recovery from FV-induced splenomegaly in H-2b/b mice from day 12 to the last observation time, at day 37 (Fig. 3). In contrast, all mice treated with anti-rat Ig recovered from FV-induced splenomegaly by 18 days postinfection. The persistence of splenomegaly in the anti-IFN-γ-treated H-2b/b mice and not in the isotype control group indicated that IFN-γ was a necessary component for recovery from FV-induced splenomegaly.
FIG. 3.
Anti-IFN-γ inhibits recovery from FV-induced splenomegaly in H-2b/b mice. Following infection with 150 SFFU of FV, H-2b/b mice were given either 0.5 ml of 0.5-mg/ml anti-IFN-γ (XMG1.2) or 0.5 ml of 0.5-mg/ml rat Ig (Sigma, St. Louis, Mo.) intraperitoneally, every 3 days starting on the day of infection and ending at 21 days postinfection. Mice were palpated for splenomegaly as described previously (18). Data are presented as the percentage of splenomegalic or dead mice of 8 to 10 mice per group.
Point mutation in H-2D influences the production of FV-specific IFN-γ-producing cells.
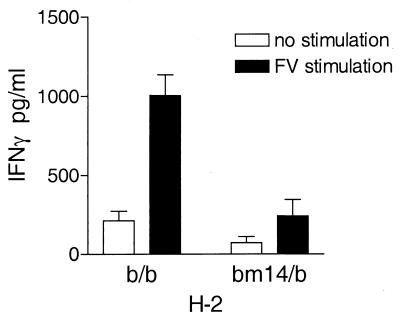
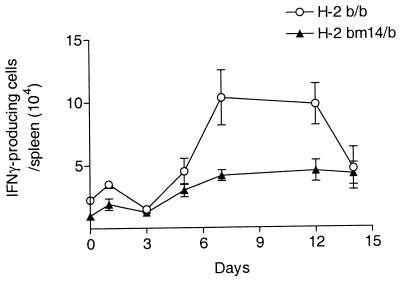
In previous experiments studying recovery from FV infection, a major effect of H-2 was mapped to the D subregion of H-2 (25). Moreover, a point mutation (bm14) that changes a Gln to His at position 70 in the H-2D protein was sufficient to dramatically reduce recovery from FV-infection (19, 24). To determine if this single mutation would also effect the generation of FV-specific IFN-γ-producing cells, spleen cells from H-2bm14/b mice were compared to those from H-2b/b mice for the production of IFN-γ and the generation of IFN-γ-producing spleen cells. Since the bm14 mutation is on the C57BL/6 genetic background, (C57BL/6 × A.BY)F1 mice (H-2b/b) were compared with (bm14 × A.BY)F1 mice (H-2bm14/b). Culture supernatants from FV-stimulated spleen cells from infected H-2bm14/b mice had very low levels of IFN-γ compared to spleen cells from infected H-2b/b mice (Fig. 4), indicating that the bm14 allele significantly influenced the FV-specific production of IFN-γ. By ELISPOT analysis, the number of IFN-γ-producing cells in the spleens of FV-infected H-2b/b mice was also significantly greater than in H-2bm14/b mice during the peak of the immune response, at 7 and 12 days postinfection (Fig. 5). Thus, the number of FV-specific IFN-γ-producing cells generated during FV infection was influenced by the single amino acid change encoded in the bm14 H-2D allele.
FIG. 4.
FV-specific IFN-γ production by spleen cells from FV-infected H-2b/b and H-2bm14/b mice. Seven days after infection with 1,500 SFFU, spleen cells from either H-2b/b or H-2bm14/b mice were stimulated in vitro with 5 μg of purified FV per ml. After 72 h, supernatants were harvested and analyzed for IFN-γ using an IFN-γ-specific sandwich ELISA as described for Fig. 1. Data are presented as the averages plus the standard errors of the means for four mice per group.
FIG. 5.
Kinetics of IFN-γ-producing cells in H-2b/b and H-2bm14/b mice after FV infection. Spleen cells from H-2b/b or H-2a/b mice were serially diluted, activated with stimulator cells, and analyzed for the number of IFN-γ-producing cells using an IFN-γ-specific ELISPOT assay as described for Fig. 2. The number of IFN-γ-producing spleen cells per spleen was determined by multiplying the number of IFN-γ-producing cells per 5 × 105 cells by the number of total cells per spleen. Data are shown as the mean numbers of IFN-γ-producing cells per spleen ± the standard errors of the means for 3 to 10 mice per group.
The bm14 mutation affects the generation of IFN-γ-producing FV-specific CD4+ T cells and CD8+ T cells.
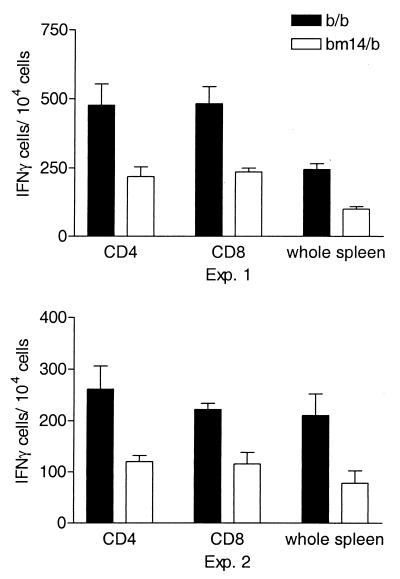
Since CD8+ T cells are stimulated by viral peptides bound to MHC class I, the influence of the bm14 MHC class I mutation on the number of IFN-γ-producing cells suggested that the majority of these cells might be CD8+. However, during viral infections both virus-specific CD4+ Th1 cells and CD8+ CTL have been shown to produce IFN-γ after activation (21). To determine which types of FV-specific T cells produced IFN-γ, CD4+ and CD8+ T cells from H-2b/b or H-2bm14/b mice were isolated by positive selection from spleen cells at 7 days postinfection and were then analyzed for IFN-γ production by ELISPOT assay. Positively selected CD4+ and CD8+ T-cell populations were greater than 90% positive for their specific cell type as determined by flow cytometry. The number of IFN-γ-producing cells was calculated as the number of positive cells per 104 cells of each cell type.
IFN-γ-producing cells were detected in both CD4+ and CD8+ populations, and a significantly higher number of IFN-γ-producing cells was observed for both CD4+ T cells and CD8+ T cells from H-2b/b mice than from H-2bm14/b mice (Fig. 6). Thus, the presence of the bm14 mutation in only one allele of H-2D was sufficient to influence the number of FV-specific IFN-γ-producing cells in both CD8+ and CD4+ T-cell populations. This effect of bm14 on CD4+ T cells was surprising and indicated that the H-2D genotype was critical for the FV-specific responses of both major T-cell subsets.
FIG. 6.
FV-specific IFN-γ production by T-cell subsets. CD4+ T cells and CD8+ T cells from spleens of FV-infected H-2b/b and H-2bm14/b mice were purified by positive selection using the MidiMACS separation system (Miltenyi Biotech, Bergisch Gladbach, Germany) and analyzed for the number of IFN-γ-producing cells by ELISPOT analysis as described for Fig. 5. Cell purity of each population was measured using fluorescein isothiocyanate-labeled anti-CD3 (145-2C11), anti-CD4 (GK1.5), anti-CD8 (169.4), or Thy1.2 (all purchased from Pharmingen). Data are presented as the number of IFN-γ-producing cells per 106 cells of each specific cell type for each mouse strain. In experiment 1, data are the means plus the standard errors of the means of six points for each mouse strain, whereas in experiment 2, data are the means plus the standard errors of the means of two points per strain.
The results presented here demonstrate for the first time that recovery from FV-induced splenomegaly is dependent upon the ability of mice to generate IFN-γ-producing cells. Furthermore, the ability to produce IFN-γ was influenced by the MHC class I gene H-2D, which affected the generation of both IFN-γ-producing CD4+ and CD8+ T cells after FV infection. The production of IFN-γ by both T-cell subsets may enhance the immune response against FV by upregulating MHC class I and class II surface expression, by activating macrophages and dendritic cells, and/or by providing direct antiviral activity (1, 21). FV-specific, IFN-γ-producing CD4+ T cells may control FV infections by directing the immune system towards a Th1 response and by providing help for the maintenance of FV-specific CTL (27), while the CD8+ T cells detected by the production of IFN-γ may provide this CTL activity (31). The ability of the MHC class I loci to regulate the level of both IFN-γ-producing CD4+ and CD8+ T cells in response to FV may explain why the MHC class I H-2D locus has such a major impact on recovery from FV-induced erythroleukemia.
It is surprising that changes in the MHC class I H-2D allele affected the generation of FV-specific CD4+ T cells, since CD4+ T cells are signaled through the MHC class II-peptide complex and not through MHC class I. Possibly the generation of the CD4+ T-cell response is itself altered by the CD8+ T-cell response, which does require signaling through MHC class I. In the experimental model of respiratory syncytial virus, virus-specific CD8+ T cells were able to downregulate the production of Th2-like CD4+ T cells, indicating that CD8+ T cells can influence the development of CD4+ cells (32). FV-specific CD8+ T cells may influence the generation of FV-specific CD4+ T cells by producing IFN-γ, which may help direct the CD4+ T cells towards a Th1 phenotype. CD8+ T cells may also affect the CD4+ T-cell response indirectly by controlling the initial spread of FV and allowing the CD4+ T-cell response to develop successfully. In previous studies CD8+ T cells appeared to initiate the process of recovery from FV-induced splenomegaly, while CD4+ T cells were required to maintain the recovered status (16, 30). By controlling the levels of viremia and erythroleukemia, CD8+ T cells may help control the environment for favorable stimulation of CD4+ T cells. The failure of CD8+ to control the levels of viremia and erythroblast proliferation in H-2a/b and H-2bm14/b mice could alter the microanatomy of the spleen or dilute out APCs and therefore prevent FV-specific CD4+ T cells from receiving the appropriate signals necessary for full activation.
The bm14 mutation results in a residue pointing inwards into the peptide binding grove (19), which appears to prevent proper binding of H-2Db-restricted peptides to this MHC molecule (2). Mice homozygous for the bm14 mutation are unable to produce H-2Db-restricted CTL responses to Moloney leukemia virus or H-Y antigen (12, 33), demonstrating that this mutation interferes with priming of CD8+ T cells. However, mice heterozygous for this mutation do produce H-2Db-restricted CTL responses, indicating that one H-2Db allele is sufficient for generation of these CTL responses (12). This contrasts with our results, which showed that both H-2Db alleles are needed for optimal generation of IFN-γ-producing cells (Fig. 2, 4, and 5) in response to FV infection. Furthermore, both H-2Db alleles are also required for recovery from FV-induced erythroleukemia (24) and optimal development of FV-specific CTL (9). Previous results suggested that the effect of this H-2D gene dose on recovery was not due to the level of surface expression of H-2Db (18). Instead, the influence of the H-2D gene may be at the level of the T-cell repertoire. If the H-2Dbm14 or -Dd gene affects the selection of the T-cell repertoire, it could alter the overall number and/or specificity of FV-reactive, H-2Db-restricted T cells present in H-2Dbm14/b and H-2Dd/b mice. The lower number of IFN-γ-producing responder cells in H-2bm14/b and H-2a/b mice than in H-2b/b mice could be due to a lower level of initial responder T cells present in H-2a/b and H-2bm14/b mice. However, when H-2a/b mice are given a low dose of FV, they can spontaneously recover from FV-induced erythroleukemia (10), indicating that H-2a/b mice do have at least some of the appropriate T cells necessary to develop a protective response against FV.
H-2a/b mice differ from H-2b/b mice at both the class I and class II loci. Although the MHC class I H-2D locus strongly influences the response to FV, the MHC class II loci also influence recovery from FV-induced erythroleukemia (22, 23, 28). In contrast to the recessive influence of the MHC class I allele on recovery from FV-induced erythroleukemia, the influence of MHC class II on recovery from FV is dominant, with only one b allele being necessary to provide protection. In the present study, all strains of mice had a least one b allele at H-2A, providing the dominant gene at the MHC class II locus required for recovery from FV-induced erythroleukemia. However, the present data clearly demonstrated that this class II MHC gene was not sufficient for development of either the CD4+ or CD8+ T-cell IFN-γ response to FV. For full responsiveness, both b alleles in MHC class I (H-2D) were required.
Acknowledgments
The first two authors contributed equally to this work.
REFERENCES
- 1.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 2.Boog C J, Boes J, Melief C J. Role of dendritic cells in the regulation of class I restricted cytotoxic T lymphocyte responses. J Immunol. 1988;140:3331–3337. [PubMed] [Google Scholar]
- 3.Britt W J, Chesebro B. H-2D (Rfv-1) gene influence on recovery from Friend virus leukemia is mediated by nonleukemic cells of the spleen and bone marrow. J Exp Med. 1980;152:1795–1804. doi: 10.1084/jem.152.6.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britt W J, Chesebro B. H-2D control of recovery from Friend virus leukemia: H-2D region influences the kinetics of the T lymphocyte response to Friend virus. J Exp Med. 1983;157:1736–1745. doi: 10.1084/jem.157.6.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantin E, Tanamachi B, Openshaw H. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J Virol. 1999;73:3418–3423. doi: 10.1128/jvi.73.4.3418-3423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantin E, Tanamachi B, Openshaw H, Mann J, Clarke K. Gamma interferon (IFN-γ) receptor null-mutant mice are more susceptible to herpes simplex virus type 1 infection than IFN-γ ligand null-mutant mice. J Virol. 1999;73:5196–5200. doi: 10.1128/jvi.73.6.5196-5200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesebro B, Miyazawa M, Britt W J. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu Rev Immunol. 1990;8:477–499. doi: 10.1146/annurev.iy.08.040190.002401. [DOI] [PubMed] [Google Scholar]
- 8.Chesebro B, Wehrly K. Studies on the role of the host immune response in recovery from Friend virus leukemia. I. Antiviral and antileukemia cell antibodies. J Exp Med. 1976;143:73–84. doi: 10.1084/jem.143.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesebro B, Wehrly K. Studies on the role of the host immune response in recovery from Friend virus leukemia. II. Cell-mediated immunity. J Exp Med. 1976;143:85–99. doi: 10.1084/jem.143.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chesebro B, Wehrly K. Rfv-1 and Rfv-2, two H-2-associated genes that influence recovery from Friend leukemia virus-induced splenomegaly. J Immunol. 1978;120:1081–1085. [PubMed] [Google Scholar]
- 11.Chesebro B, Wehrly K, Stimpfling J. Host genetic control of recovery from Friend leukemia virus-induced splenomegaly: mapping of a gene within the major histocompatibility complex. J Exp Med. 1974;140:1457–1467. doi: 10.1084/jem.140.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Waal L P, Melvold R W, Melief C J. Cytotoxic T lymphocyte nonresponsiveness to the male antigen H-Y in the H-2Db mutants bm13 and bm14. Complementation of the response in F1 crosses with the I-Ab mutant bm12 nonresponder and failure of B6 or Db mutant mice tolerant of each other to respond to allogeneic male cells. J Exp Med. 1983;158:1537–1546. doi: 10.1084/jem.158.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doig D, Chesebro B. Anti-Friend virus antibody is associated with recovery from viremia and loss of viral leukemia cell-surface antigens in leukemic mice. Identification of Rfv-3 as a gene locus influencing antibody production. J Exp Med. 1979;150:10–19. doi: 10.1084/jem.150.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friend C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp Med. 1957;105:307. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasenkrug K J, Brooks D M, Chesebro B. Passive immunotherapy for retroviral disease: influence of major histocompatibility complex type and T-cell responsiveness. Proc Natl Acad Sci USA. 1995;92:10492–10495. doi: 10.1073/pnas.92.23.10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasenkrug K J, Brooks D M, Dittmer U. Critical role for CD4+ T cells in controlling retrovirus replication and spread in persistently infected mice. J Virol. 1998;72:6559–6564. doi: 10.1128/jvi.72.8.6559-6564.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasenkrug K J, Chesebro B. Immunity to retroviral infection: the Friend virus model. Proc Natl Acad Sci USA. 1997;94:7811–7816. doi: 10.1073/pnas.94.15.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasenkrug K J, Sprangrude G J, Nishio J, Brooks D M, Chesebro B. Recovery from Friend disease in mice with reduced major histocompatibility complex class I expression. J Virol. 1994;68:2059–2064. doi: 10.1128/jvi.68.4.2059-2064.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemmi S, Geliebter J, Zeff R A, Melvold R W, Nathenson S G. Three spontaneous H-2Db mutants are generated by genetic microrecombination (gene conversion) events. Impact on the H-2-restricted immune responsiveness. J Exp Med. 1988;168:2319–2335. doi: 10.1084/jem.168.6.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabat D. Molecular biology of Friend viral erythroleukemia. Curr Top Microbiol Immunol. 1989;148:1–42. doi: 10.1007/978-3-642-74700-7_1. [DOI] [PubMed] [Google Scholar]
- 21.Kerr I M, Stark G R. The antiviral effects of the interferons and their inhibition. J Interferon Res. 1992;12:237–240. doi: 10.1089/jir.1992.12.237. [DOI] [PubMed] [Google Scholar]
- 22.Miyazawa M, Nishio J, Chesebro B. Genetic control of T cell responsiveness to the Friend murine leukemia virus envelope antigen. Identification of class II loci of the H-2 as immune response genes. J Exp Med. 1988;168:1587–1605. doi: 10.1084/jem.168.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyazawa M, Nishio J, Wehrly K, Chesebro B. Influence of MHC genes on spontaneous recovery from Friend retrovirus-induced leukemia. J Immunol. 1992;148:644–647. [PubMed] [Google Scholar]
- 24.Miyazawa M, Nishio J, Wehrly K, Jay G, Melvold R W, Chesebro B. Detailed mapping of the Rfv-1 gene that influences spontaneous recovery from Friend retrovirus-induced leukaemia. Eur J Immunogenet. 1992;19:159–164. doi: 10.1111/j.1744-313x.1992.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 25.Morrison R P, Earl P L, Nishio J, Lodmell D L, Moss B, Chesebro B. Different H-2 subregions influence immunization against retrovirus and immunosuppression. Nature. 1987;329:729–732. doi: 10.1038/329729a0. [DOI] [PubMed] [Google Scholar]
- 26.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 27.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 28.Perry L L, Miyazawa M, Hasenkrug K, Wehrly K, David C S, Chesebro B. Contrasting effects from a single major histocompatibility complex class II molecule (H-2E) in recovery from Friend virus leukemia. J Virol. 1994;68:4921–4926. doi: 10.1128/jvi.68.8.4921-4926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramshaw I A, Ramsay A J, Karupiah G, Rolph M S, Mahalingam S, Ruby J C. Cytokines and immunity to viral infections. Immunol Rev. 1997;159:119–135. doi: 10.1111/j.1600-065x.1997.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 30.Robertson M N, Spangrude G J, Hasenkrug K, Perry L, Nishio J, Wehrly K, Chesebro B. Role and specificity of T-cell subsets in spontaneous recovery from Friend virus-induced leukemia in mice. J Virol. 1992;66:3271–3277. doi: 10.1128/jvi.66.6.3271-3277.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruby J, Ramshaw I. The antiviral activity of immune CD8+ T cells is dependent on interferon-gamma. Lymphokine Cytokine Res. 1991;10:353–358. [PubMed] [Google Scholar]
- 32.Srikiatkhachorn A, Braciale T J. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stukart M J, Vos A, Boes J, Melvold R W, Bailey D W, Melief C J. A crucial role of the H-2 D locus in the regulation of both the D- and K-associated cytotoxic T-lymphocyte response against Moloney virus, demonstrated with two Db mutants. J Immunol. 1982;128:1360–1364. [PubMed] [Google Scholar]