Key Points
Question
What is the natural history and cardiovascular burden of the V142I variant of the transthyretin (TTR) gene among US Black carriers across mid to late life?
Findings
Across 4 cohort studies, carriers (754/23 338) faced a substantially increased risk for heart failure (by age 63 years) and death (by age 72 years), similarly in men and women, which was estimated to contribute to approximately 1 million years of life lost among US Black individuals aged ≥50 years.
Meaning
These data show the large, age-dependent burden of V142I, which may guide discussions regarding the initiation and results of genetic screening, provide clinicians with risk estimates to share with patients, and inform strategies for early targeted therapy.
Abstract
Importance
Individual cohort studies concur that the amyloidogenic V142I variant of the transthyretin (TTR) gene, present in 3% to 4% of US Black individuals, increases heart failure (HF) and mortality risk. Precisely defining carrier risk across relevant clinical outcomes and estimating population burden of disease are important given established and emerging targeted treatments.
Objectives
To better define the natural history of disease in carriers across mid to late life, assess variant modifiers, and estimate cardiovascular burden to the US population.
Design, Setting, and Participants
A total of 23 338 self-reported Black participants initially free from HF were included in 4 large observational studies across the US (mean [SD], 15.5 [8.2] years of follow-up). Data analysis was performed between May 2023 and February 2024.
Exposure
V142I carrier status (n = 754, 3.2%).
Main Outcomes and Measures
Hospitalizations for HF (including subtypes of reduced and preserved ejection fraction) and all-cause mortality. Outcomes were analyzed by generating 10-year hazard ratios for each age between 50 and 90 years. Using actuarial methods, mean survival by carrier status was estimated and applied to the 2022 US population using US Census data.
Results
Among the 23 338 participants, the mean (SD) age at baseline was 62 (9) years and 76.7% were women. Ten-year carrier risk increased for HF hospitalization by age 63 years, predominantly driven by HF with reduced ejection fraction, and 10-year all-cause mortality risk increased by age 72 years. Only age (but not sex or other select variables) modified risk with the variant, with estimated reductions in longevity ranging from 1.9 years (95% CI, 0.6-3.1) at age 50 to 2.8 years (95% CI, 2.0-3.6) at age 81. Based on these data, 435 851 estimated US Black carriers between ages 50 and 95 years are projected to cumulatively lose 957 505 years of life (95% CI, 534 475-1 380 535) due to the variant.
Conclusions and Relevance
Among self-reported Black individuals, male and female V142I carriers faced similar and substantial risk for HF hospitalization, predominantly with reduced ejection fraction, and death, with steep age-dependent penetrance. Delineating the individual contributions of, and complex interplay among, the V142I variant, ancestry, the social construct of race, and biological or social determinants of health to cardiovascular disease merits further investigation.
This study used data on Black participants in 4 large observational studies to better define the natural history of disease in V142I variant carriers across mid to late life, assess variant modifiers, and estimate cardiovascular burden to the US population.
Introduction
Transthyretin cardiac amyloidosis (ATTR-CA) is an increasingly recognized cardiomyopathy resulting from extracellular cardiac deposition of the misfolded transthyretin protein. ATTR-CA can occur in the presence of a detected genetic variant (ATTRv-CA, variant related) or its absence (ATTRwt-CA, wild-type related). The amyloidogenic V142I (legacy nomenclature V122I) variant of the transthyretin (TTR) gene has been reported to be present in a significant proportion of US Black individuals (3%-4%) (particularly in comparison with other ancestry groups),1,2 is the most common cause of ATTRv-CA in the US,3 and demonstrates age-dependent anatomic penetrance on autopsy analysis.4 The American College of Medical Genetics recently classified the transthyretin variants as clinically actionable reportable secondary findings,5 and individual epidemiologic studies concur that carriers face increased risk for heart failure (HF) and all-cause mortality.1,6,7,8,9,10 The availability of several targeted treatments, which may be more effective earlier in the disease process, has heightened interest in identifying at-risk individuals.11,12
Because testing for the V142I variant is typically performed after a carrier presents with disease, precise data are needed regarding long-term, and modifiers of, risk in unselected carriers. To better define the natural history of disease in V142I carriers, data from Black participants in 4 large observational studies based in the US were combined. Pooling data from geographically diverse cohorts facilitated more precise and generalizable risk estimation across mid to late life, the ability to assess less-frequent outcomes (including subtypes of HF), analysis of effect modifiers (particularly sex, which is thought to influence disease manifestations),2,6 and estimation of years of life lost among V142I carriers.
Methods
We included 23 338 self-reported Black participants, including 754 carriers (3.2%) who were initially free of HF with available genotyping (Figure 1). This study involved self-reported Black participants in keeping with prior cohort studies included in this analysis that studied risk stratification with the variant, although race is a social construct and should not be used as a proxy for ancestry.1,8,10 Participants provided written informed consent, and institutional review board approval was received from all participating institutions. This study followed the reporting guidelines of the Strengthening the Reporting of Genetic Association Studies (STREGA, an extension of the STROBE Statement).
Figure 1. Analysis of Black Participants From 4 Large Cohort Studies.
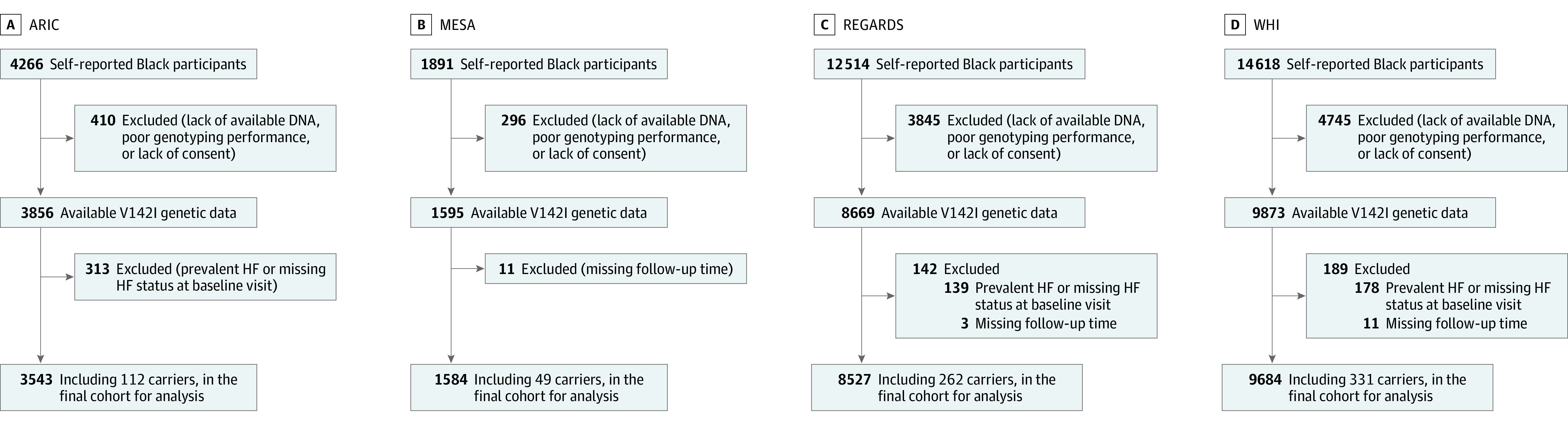
The process for inclusion and exclusion of participants is delineated for each study. A total of 23 338 participants were ultimately analyzed, including 754 carriers of the V142I variant. ARIC indicates Atherosclerosis Risk in Communities; HF, heart failure; MESA, Multi-Ethnic Study of Atherosclerosis; REGARDS, Reasons for Geographic and Racial Differences in Stroke; and WHI, Women’s Health Initiative.
Cohort Studies
Atherosclerosis Risk in Communities Study
The Atherosclerosis Risk in Communities Study is a prospective study in 4 communities across the US composed of 15 792 participants, aged 45 to 64 years, recruited between 1987 and 1989.13 A total of 3543 Black participants (including 112 participants who were heterozygous for V142I) were included for analysis.
Multi-Ethnic Study of Atherosclerosis
The Multi-Ethnic Study of Atherosclerosis is a cohort study of 6 communities and recruited 6814 participants free of clinical cardiovascular disease aged 45 to 84 years between July 2000 and August 2002.14 A total of 1584 Black participants, including 49 participants who were heterozygous for V142I, were included for analysis.
Reasons for Geographic and Racial Differences in Stroke
The Reasons for Geographic and Racial Differences in Stroke Study recruited 30 239 adults at least 45 years old between January 2003 and October 2007.15 Participants were randomly selected from a commercially available list to create a sample balance on race and sex across the stroke buckle, the stroke belt, and the rest of the continental US. A total of 8527 Black participants (including 259 heterozygous and 3 homozygous for V142I) met inclusion criteria for the study.10
Women’s Health Initiative
Women were eligible to participate in the Women’s Health Initiative if they were 50 to 79 years of age, generally healthy, and postmenopausal at the time of enrollment.16 A total of 161 808 participants were enrolled between 1993 and 1998 in the observational or clinical trial groups. A total of 9684 Black women (including 330 heterozygous and 1 homozygous for V142I) were included in this analysis.
The median African genetic ancestry percentage for each cohort was determined. Details regarding individual study cohorts, population descriptors, genotyping, ancestry analysis, comorbidities, and laboratory values are provided in the eMethods in Supplement 1.
Study Outcomes
Longitudinal outcomes included first HF hospitalization, first HF hospitalization subtypes (HF with reduced ejection fraction [HFrEF] and HF with preserved ejection fraction [HFpEF]), all-cause mortality, and a composite of HF hospitalization or all-cause mortality. HFpEF was defined by an EF of 50% or greater or qualitatively normal EF, while HFrEF was defined by an EF less than 50% or a qualitatively low EF (eMethods in Supplement 1). This EF dichotomization facilitated inclusion of qualitative reports of EF.
Statistical Analysis
Baseline characteristics were summarized using descriptive statistics, stratified by carrier status. We performed Cox proportional hazards models, stratifying by age, sex, and genotype platform, while adjusting for interactions between genotyping platform and the first 10 principal components,8,10 using time from the baseline visit as the time scale to describe the relationship between the variant and adverse outcomes. Proportional hazards were assessed via Schoenfeld residuals.
We assessed for interactions between carrier status and several variables for HF hospitalization, all-cause mortality, and the combined outcome (HF hospitalization or all-cause mortality). Given strong effect modification by age, subsequent modeling focused on using sequences of 10-year age windows (eg, beginning age 50 through 59 years), generating 10-year adjusted hazard ratios using Cox regression, stratifying by sex and genotype platform while adjusting for the interactions between genotyping platform and principal components.9 To better visualize these resulting estimates, we applied locally weighted scatterplot smoothing (LOWESS).9,17,18 For LOWESS, tricube weighting was used with bandwidth of 0.8 (80% of observations used at each point).19 This process was repeated for starting years between ages 50 and 90 years for HF hospitalization and death analyses, and between 58 and 90 years for HFpEF and HFrEF hospitalization (given fewer events at the lower age range). We reported age at first nominally statistically significant risk in each outcome. We performed post hoc sensitivity analyses using nominal statistical significance at α = .01 to acknowledge multiple age ranges tested. Participants were censored if they died (for HF hospitalization analyses) or were lost to follow-up, while participants were administratively censored at the time of last follow-up. For incident HFpEF hospitalization analyses, participants experiencing HF hospitalization with undetermined EF or reduced EF were censored (with parallel considerations for incident HFrEF hospitalization).
To estimate years of life lost among carriers compared with noncarriers, we used previously validated actuarial (age-based) methods to calculate nonparametric Kaplan-Meier estimates of overall survival and HF hospitalization–free survival at every year of age specified.17,18 This method uses age (at a given starting age and at the time of death) as the time component. The area under the survival curve reflected projected event-free survival and overall survival. For each age between 50 and 95 years, we compared survival estimates of carriers and noncarriers. Estimates of survival gains were smoothed with LOWESS.
Finally, we applied these actuarial estimates of years of life lost at each age to the US population of Black individuals. For each age between 50 and 95 years, we extracted data on the estimated number of living Black individuals from the US Census Bureau’s American Community Survey 5-Year Estimates from 2022, which was then multiplied by the carrier frequency observed in the current analysis to estimate the number of carriers at each age in the US (accounting for decreasing frequency of the variant aging given increasing mortality risk). The years of life lost at each age among carriers was multiplied by the number of estimated carriers in the population to generate total years lost at each age, which was then summed to estimate the numbers of years lost among carriers at least age 50 years. Analyses were performed using Stata version 18 (StataCorp LLC). For interaction testing, a 2-sided P value <.05 divided by 9 (ie, number of subgroups) was considered significant. For other analyses, a 2-sided P value <.05 was considered significant.
Results
Baseline Characteristics
The mean (SD) age at visit 1 was 62 (9) years, 76.7% were women (due to the large sample size included from WHI), 62.9% had hypertension, and 21.9% had diabetes (Table 1). Carrier percentages were similar between men (3.0%) and women (3.3%). Characteristics were generally balanced across V142I carrier status.
Table 1. Clinical Characteristics of the Pooled Study Cohorts by V142I Carrier Status at Baseline Visit.
| V142I noncarriers (n = 22 584) | V142I carriers (n = 754) | |
|---|---|---|
| Study cohort, No. (%) | ||
| Atherosclerosis Risk in Communities | 3431 (15.2) | 112 (14.9) |
| Multi-Ethnic Study of Atherosclerosis | 1535 (6.8) | 49 (6.5) |
| Reasons for Geographic and Racial Differences in Stroke | 8265 (36.6) | 262 (34.7) |
| Women’s Health Initiative | 9353 (41.4) | 331 (43.9) |
| Participant characteristics | ||
| Age, mean (SD), y | 62 (9) | 61 (9) |
| Sex, No. (%) | ||
| Female | 17 308 (76.6) | 591 (78.4) |
| Male | 5276 (23.4) | 163 (21.6) |
| Clinical characteristics | ||
| Physical examination | ||
| Systolic blood pressure, mean (SD), mm Hg [No.] | 131 (18) [22 534] | 131 (19) [752] |
| Diastolic blood pressure, mean (SD), mm Hg [No.] | 78 (10) [22 527] | 78 (11) [753] |
| Heart rate, mean (SD), beats/min [No.] | 69 (19) [22 441] | 68 (13) [746] |
| Body mass index, mean (SD) [No.]a | 30.7 (6.6) [22 435] | 30.2 (6.7) [748] |
| Comorbidities, No./total (%) | ||
| Hypertension | 13 965/22 174 (63.0) | 460/744 (61.8) |
| Obesity | 10 789/22 435 (48.1) | 326/748 (43.6) |
| Diabetes | 4909/22 349 (22.0) | 156/748 (20.9) |
| Coronary heart disease | 1570/22 544 (7.0) | 51/753 (6.8) |
| Laboratory testing | ||
| Glucose, mg/dL | ||
| Mean (SD) [No.] | 109 (45) [20 670] | 108 (45) [684] |
| >125 mg/dL, No. (%) | 3249 (15.7) | 99 (14.5) |
| Creatinine, mg/dL | ||
| Mean (SD) [No.] | 0.93 (0.59) [20 468] | 0.89 (0.37) [677] |
| >1.5 mg/dL, No. (%) | 692 (3.4) | 21 (3.1) |
| Low-density lipoprotein cholesterol, mg/dL | ||
| Mean (SD) [No.] | 131 (41) [20 216] | 131 (40) [669] |
| ≥190 mg/dL, No. (%) | 1691 (8.4) | 49 (7.3) |
SI conversion factors: To convert cholesterol to mmol/L, multiply by 0.0259; creatinine to μmol/L, multiply by 88.4; and glucose to mmol/L, multiply by 0.0555.
Calculated as weight in kilograms divided by height in meters squared.
Carrier Status and Cardiovascular Outcomes
Total events and hazard ratios are shown in Table 2 and Kaplan-Meier event curves are displayed in eFigures 1 and 2 in Supplement 1. With a mean (SD) 15.5 (8.2) years of follow-up, carriers faced an increased risk for all study outcomes, though the association with HFpEF hospitalization did not reach statistical significance. Carrier risks for HF and death were comparable with several traditional cardiovascular risk factors (eTable 1 in Supplement 1). Risk for HF hospitalization or death was significantly increased among the 4 homozygotes compared with heterozygotes (hazard ratio, 7.75 [95% CI, 2.24-26.79]) (eTable 2 in Supplement 1). eTable 3 in Supplement 1 shows interaction P values between carrier status and select variables, including study cohort, age, sex, hypertension, diabetes, systolic blood pressure, heart rate, body mass index, and African ancestry with study outcomes. Of these, only age was identified as a significant modifier of variant risk.
Table 2. Events and Hazard Ratios for Adverse Cardiovascular Outcomes by V142I Carrier Status.
| Outcomes | No./total (%) | Absolute difference, % (95% CI) | Hazard ratio (95% CI)a,b | P value | |
|---|---|---|---|---|---|
| V142I noncarrier events | V142I carrier events | ||||
| HF hospitalization | 2373/22 584 (10.5) | 134/754 (17.8) | 7.3 (4.5 to 10.0) | 1.84 (1.54 to 2.20) | <.001 |
| All-cause death | 8744/22 584 (38.7) | 329/754 (43.6) | 4.9 (1.3 to 8.5) | 1.27 (1.13 to 1.42) | <.001 |
| HF hospitalization or all-cause death | 9290/22 584 (41.1) | 355/754 (47.1) | 5.9 (2.3 to 9.6) | 1.31 (1.18 to 1.46) | <.001 |
| By HF typec | |||||
| HFrEF hospitalization | 740/21 599 (3.4) | 57/721 (7.9) | 4.5 (2.5 to 6.5) | 2.47 (1.87 to 3.26) | <.001 |
| HFpEF hospitalization | 722/21 599 (3.3) | 32/721 (4.4) | 1.1 (−0.4 to 2.6) | 1.43 (0.99 to 2.05) | .054 |
Abbreviations: HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Models adjusted for interactions between study genotyping platform and the first 10 principal components and stratified by age, sex, and the genotyping platform.
No proportional hazards violations were detected.
ARIC participants did not have HF events adjudicated (including ejection fraction) until 2005, leading to different participant numbers for HFrEF and HFpEF events compared with other events.
To further accommodate the age-related effects on outcomes, we used 10-year rolling hazard ratios (Figure 2; and eFigure 3 in Supplement 1). The risk for all outcomes generally increased between middle to late life. Statistically significant increased 10-year risk was first detected at age 63 years for HF, 65 for HF or death, and 72 for death. The increased HF risk was driven predominantly by an increased risk of HFrEF, with an elevated 10-year risk detected at age 65 years that strongly increased over time, in contrast to a later modest signal for HFpEF hospitalization emerging at age 76 years. Risk for HF hospitalization or death was similar between men and women (eFigure 4 in Supplement 1), in keeping with the lack of interaction reported by sex (eTable 3 in Supplement 1). Sensitivity analyses using 99% CIs were largely similar, noting modestly longer time to statistical significance (eFigures 5-6 in Supplement 1). Within analyses using 10-year intervals, there were still some indications of proportional hazards violations with respect to age.
Figure 2. V142I Hazard Ratios for Adverse Cardiovascular Events by Age.
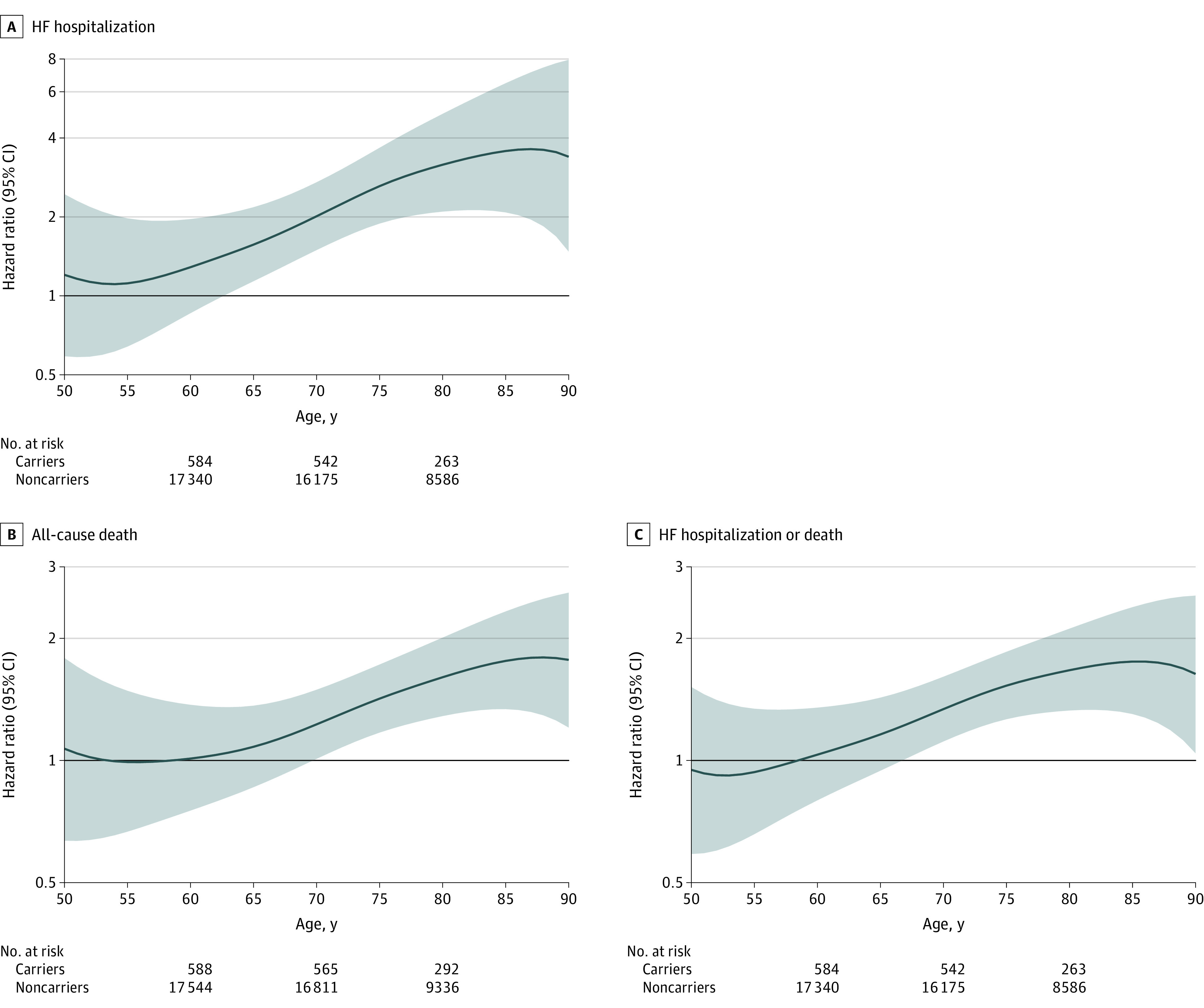
Ten-year hazard ratios and 95% CIs (shaded areas) are estimated at each age between 50 and 90 years for carriers vs noncarriers. Analyses are adjusted for interactions between study genotyping platform and the first 10 principal components, while stratified by sex and study genotyping platform. HF indicates heart failure.
Expected Overall and Event-Free Survival by Age
To describe the effect of the variant on years of life lost without relying on modeling assumptions, we provided several model-free estimates. Figure 3A shows expected overall survival at each age between 50 and 95 years by carrier status (additional details in eTable 4 in Supplement 1). Carriers lived 2 to 2.5 fewer years compared with noncarriers until approximately age 85 years, when differences in longevity began to attenuate (Figure 3B). Because the survival loss difference between carriers and noncarriers remained relatively constant across ages 50 to 85 years, while expected survival decreased, the relative (percentage) loss in expected remaining carrier longevity generally increased over time (eFigure 7 in Supplement 1). Specifically, carriers at age 50 years subsequently lived 1.9 fewer years (95% CI, 0.6-3.1) than noncarriers, representing a 6% reduction (95% CI, 2%-10%) in expected longevity. This longevity disparity increased to a maximum of 2.8 years (95% CI, 2.0-3.6) at age 81 years. The maximum relative reduction in longevity was 34% (95% CI, 11%-56%), achieved at age 90 years. Parallel findings were demonstrated using event-free survival (eTable 5 and eFigures 8-9 in Supplement 1).
Figure 3. Effect of V142I on Overall Survival of Carriers Compared With Noncarriers.
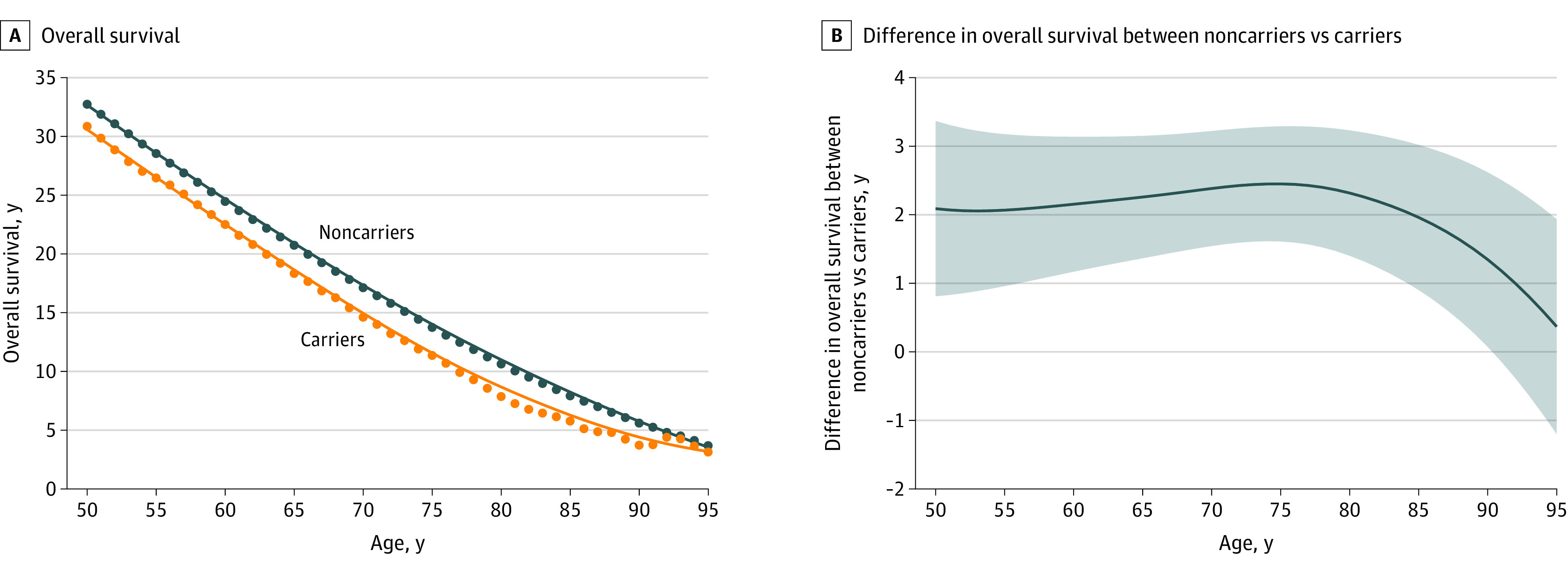
A, Estimated mean overall survival in carriers and noncarriers for every year between ages 50 and 95 years. B, The difference in mean years lost among carriers compared with noncarriers shown with the smoothed estimate (blue line), and 95% CI of the smoothed estimate (shaded area) after application of a locally weighted scatterplot smoothing procedure.
Extrapolation of Years Lost Based on Carrier Prevalence in the US Population
We applied carrier rates derived from this analysis to US population data to estimate the burden of disease in the US Black population. Among the 13 284 819 estimated Black adults aged 50 to 95 years, there are an estimated 435 851 carriers (eTable 4 in Supplement 1). Based on the expected loss of longevity at each age among carriers compared with noncarriers, US Black individuals at least 50 years old are projected to cumulatively lose 957 505 years of life (95% CI, 534 475-1 380 535) compared with noncarriers.
Discussion
Leveraging individual-level participant data from 4 large observational cohort studies facilitated several analyses highlighting risk encountered by carriers of the amyloidogenic V142I variant of the TTR gene. First, this study provided more precise estimates of cardiovascular risk across mid to late life (>90 years old) than were previously available. These risks strongly related to age, but were not modified by sex, suggesting underdiagnosis of ATTRv-CA in women, as some clinical studies have reported male predominance of phenotypic penetration. Second, the earliest statistically significant increase in 10-year risk was identified at earlier ages than previously shown (age 63 years for HF hospitalization and age 72 years for mortality), likely due to the inclusion of a larger sample size.9 Third, the risk for HF hospitalization was driven by HFrEF, in contrast with its typical HFpEF association, a more modest signal that emerged in later life. Fourth, at each age from mid to late life, carriers died 2 to 2.5 years earlier than noncarriers until age 85 years (when differences began to attenuate), leading to an overall significant decrease in percentage expected longevity with increasing age. Fifth, accounting for the age-dependent frequency of the variant, Black carriers at least 50 years old are projected to live nearly 1 million fewer years than noncarriers. Given established and emerging treatment strategies, which may be more effective earlier in disease course,11,12 early identification and treatment of V142I carriers with ATTRv-CA may have significant public health impact.
Population studies, including several included here, individually have shown significantly higher risk among V142I carriers for HF hospitalization and death, though the risk of homozygotes in these studies has remained unclear.1,7,8,9,10,20 The small group of homozygotes (n = 4) in this study was at substantially elevated risk even in comparison with heterozygotes (demonstrating allele dose dependence), albeit with wide confidence intervals. These findings were overall consistent with data from clinical populations.21 Pooling data importantly allowed for greater power to evaluate for interactions between clinical characteristics and variant status that might account for incomplete phenotypic penetrance. Only age was consistently identified as a strong modifier of risk. Clinical studies have demonstrated a greater risk for ATTRv-CA among male carriers,2,6 which has been postulated to relate to less-aggressive disease trajectory in women.22 However, sex did not significantly modify risk in this study with very similar estimates of HF hospitalization or death in men and women, suggesting that previous reports may be explained by underdiagnosis of ATTRv-CA in women.2,6,8,23,24 Women with ATTRv-CA present with thinner left ventricular walls compared with men, and not accounting for differences in body size may engender underdiagnosis.23 Without clear alternative modifiers of risk, age may currently be the most reliable clinical marker to identify risk for progressive disease.
Additionally, this larger study enabled more precise risk estimation and earlier detection of phenotypic penetrance. Previous research in the Atherosclerosis Risk in Communities Study alone identified inflection points in 10-year risk around age 70 years for HF hospitalization and 75 years for mortality.9 The greater sample size in the current study showed that risk increased at even earlier ages (63 and 72 years, respectively). Defining these ages is clinically impactful and relevant when considering initiation of treatments such as stabilizers, silencers, or even in vivo gene editing, where treatment prior to the onset of symptoms might be most effective, though further data are needed to clarify the impact of early treatment.11,25,26
Notably, the risk for HF hospitalization in this study was driven by incident HFrEF hospitalization. While ATTR-CA has traditionally been associated with HFpEF, V142I carriers are more likely to experience HFrEF events. Indeed, V142I-associated ATTRv-CA presents with greater disease severity (including lower left ventricular EF) and faster disease progression compared with both ATTRwt-CA and non-V142I ATTRv-CA.27,28 Recent data from a clinical trial in ATTR-CA supported the presence of reduced EF in a significant proportion of patients with a tendency to progress in ATTR-CA.29 Therefore, among Black hospitalized patients with HFrEF, suspicion for ATTR-CA should be heightened and not restricted to patients with preserved EF.30,31,32,33 It is important to note that not all carriers with HFrEF have ATTRv-CA because carriers are at risk for other causes of cardiomyopathy as well (such as coronary artery disease), emphasizing the importance of further cardiovascular evaluation for etiology. Identification of ATTR-CA in the HFrEF population is also important because implementation of some guideline-directed medical therapies may not be well tolerated in ATTR-CA.34 The more modest association with increased HFpEF hospitalization in late life may represent a survivor bias, whereby those carriers who survive to late life may have an inherently more benign form of the disease.
Our actuarial analyses demonstrated that carriers die approximately 2 to 2.5 years earlier than noncarriers. Because these findings were consistent until approximately 85 years of age, the relative reduction in longevity of carriers compared with noncarriers significantly increased with aging, further reflecting the steep age-dependent penetrance of the variant. Because the variant is relatively common (3%-4% among US Black individuals), these years of life lost at the individual level translate to a substantial burden at the population level. The approximately 435 000 living carriers between ages 50 and 95 years will collectively lose nearly 1 million years of life. Because targeted therapies are either available, emerging, or promising in ATTR-CA,11,25,26,35 these results suggest that identification of disease and implementation of efficacious therapies might extend longevity in this large population.11 These results may be increasingly relevant with greater access to genetic testing in the population, whether accomplished through biobanks, direct-to-consumer testing, or broad population assessments (including the All of Us Research Program36). Additionally, these results support the inclusion of the variant as a clinically actionable secondary finding.5
Limitations
There are several limitations of this study. First, specific phenotypic markers or diagnoses of cardiac amyloidosis were not broadly available in these studies, and therefore understanding which individual carriers have ATTR-CA (as opposed to other causes of HF) is limited. However, at a population level, the absolute difference estimates of HF events provide understanding of the burden of ATTRv-CA. Second, despite pooling studies, interaction analyses may still be underpowered to detect modifiers of variant risk. Third, extrapolation of the current estimates to the US population assumes that the cohorts studied here are broadly representative, and these data may not accurately inform estimates in other parts of the world where variant prevalence and event rates may vary.2 Fourth, participants who self-reported as Black were included, although race does not capture the significant genetic diversity within Black individuals in the US and worldwide. Delineating the individual contributions of, and complex interplay between, the V142I variant, ancestry, race as a social construct, and biological or social determinants of health to cardiovascular disease merits further investigation. Fifth, adjudication of HFrEF and HFpEF events varied by study and were obtained through available medical record review.
Conclusions
Among self-reported Black individuals, male and female V142I carriers faced similar and substantial risk for HF hospitalization, predominantly with reduced ejection fraction, and all-cause death later in life, with steep age-dependent penetrance. Delineating the individual contributions of, and complex interplay among, the V142I variant, ancestry, the social construct of race, and biological or social determinants of health to cardiovascular disease merits further investigation.
eMethods
eFigure 1. Kaplan-Meier Curves for Heart Failure and Mortality Events by V142I Carrier Status
eFigure 2. Kaplan-Meier Curves for Heart Failure Events by V142I Carrier Status
eFigure 3. V142I Hazard Ratio for Heart Failure Events by Age
eFigure 4. V142I Hazard Ratio for Heart Failure Hospitalization or Death by Age and Sex
eFigure 5. V142I Hazard Ratio for Adverse Cardiovascular Events by Age Using 99%
eFigure 6. V142I Hazard Ratio for Heart Failure Events by Age Using 99% Confidence
eFigure 7. Relative Effect of V142I on Remaining Years of Life Compared With Noncarriers
eFigure 8. Effect of V142I on HFH-free Survival Compared With Noncarriers
eFigure 9. Relative Effect of V142I on Remaining Years of Life Free From HFH Compared With Noncarriers
eTable 1. Hazard Ratios for Heart Failure Hospitalization and Death by Carrier Status and Cardiovascular Risk Factors
eTable 2. Events and Hazard Ratios for Adverse Cardiovascular Outcomes by Number of Alleles
eTable 3. Interaction Analysis of the Variant With Select Variables for Study Outcomes
eTable 4. Estimated Survival Time Comparing Carriers and Noncarriers by Age for All-Cause Mortality With Extrapolation to the United State Population
eTable 5. Estimated Survival Time Comparing Carriers and Noncarriers by Age for Heart Failure Hospitalization or Death
eReferences
Data Sharing Statement
References
- 1.Quarta CC, Buxbaum JN, Shah AM, et al. The amyloidogenic V122I transthyretin variant in elderly Black Americans. N Engl J Med. 2015;372(1):21-29. doi: 10.1056/NEJMoa1404852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandrashekar P, Alhuneafat L, Mannello M, et al. Prevalence and outcomes of p.Val142Ile TTR amyloidosis cardiomyopathy: a systematic review. Circ Genom Precis Med. 2021;14(5):e003356. doi: 10.1161/CIRCGEN.121.003356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maurer MS, Hanna M, Grogan M, et al. ; THAOS Investigators . Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol. 2016;68(2):161-172. doi: 10.1016/j.jacc.2016.03.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in Black Americans. N Engl J Med. 1997;336(7):466-473. doi: 10.1056/NEJM199702133360703 [DOI] [PubMed] [Google Scholar]
- 5.Miller DT, Lee K, Abul-Husn NS, et al. ; ACMG Secondary Findings Working Group . ACMG SF v3.2 list for reporting of secondary findings in clinical exome and genome sequencing: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2023;25(8):100866. doi: 10.1016/j.gim.2023.100866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damrauer SM, Chaudhary K, Cho JH, et al. Association of the V122I hereditary transthyretin amyloidosis genetic variant with heart failure among individuals of African or Hispanic/Latino ancestry. JAMA. 2019;322(22):2191-2202. doi: 10.1001/jama.2019.17935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coniglio AC, Segar MW, Loungani RS, et al. Transthyretin V142I genetic variant and cardiac remodeling, injury, and heart failure risk in Black adults. JACC Heart Fail. 2022;10(2):129-138. doi: 10.1016/j.jchf.2021.09.006 [DOI] [PubMed] [Google Scholar]
- 8.Haring B, Hunt RP, Shadyab AH, et al. Cardiovascular disease and mortality in Black women carrying the amyloidogenic V122I transthyretin gene variant. JACC Heart Fail. 2023;11(9):1189-1199. doi: 10.1016/j.jchf.2023.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selvaraj S, Claggett BL, Quarta CC, et al. Age dependency of cardiovascular outcomes with the amyloidogenic pV142I transthyretin variant among Black individuals in the US. JAMA Cardiol. 2023;8(8):784-788. doi: 10.1001/jamacardio.2023.1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parcha V, Malla G, Irvin MR, et al. Association of transthyretin Val122Ile variant With incident heart failure among Black individuals. JAMA. 2022;327(14):1368-1378. doi: 10.1001/jama.2022.2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurer MS, Schwartz JH, Gundapaneni B, et al. ; ATTR-ACT Study Investigators . Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. doi: 10.1056/NEJMoa1805689 [DOI] [PubMed] [Google Scholar]
- 12.Elliott P, Drachman BM, Gottlieb SS, et al. Long-term survival with tafamidis in patients with transthyretin amyloid cardiomyopathy. Circ Heart Fail. 2022;15(1):e008193. doi: 10.1161/CIRCHEARTFAILURE.120.008193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The ARIC investigators . The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687-702. doi: 10.1093/oxfordjournals.aje.a115184 [DOI] [PubMed] [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871-881. doi: 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 15.Howard VJ, Cushman M, Pulley L, et al. The Reasons for Geographic and Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2005;25(3):135-143. doi: 10.1159/000086678 [DOI] [PubMed] [Google Scholar]
- 16.The Women’s Health Initiative Study Group . Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61-109. doi: 10.1016/S0197-2456(97)00078-0 [DOI] [PubMed] [Google Scholar]
- 17.Claggett B, Packer M, McMurray JJ, et al. ; PARADIGM-HF Investigators . Estimating the long-term treatment benefits of sacubitril-valsartan. N Engl J Med. 2015;373(23):2289-2290. doi: 10.1056/NEJMc1509753 [DOI] [PubMed] [Google Scholar]
- 18.Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396(10244):121-128. doi: 10.1016/S0140-6736(20)30748-0 [DOI] [PubMed] [Google Scholar]
- 19.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829-836. doi: 10.1080/01621459.1979.10481038 [DOI] [Google Scholar]
- 20.Kozlitina J, Garg S, Drazner MH, et al. Clinical implications of the amyloidogenic V122I transthyretin variant in the general population. J Card Fail. 2022;28(3):403-414. doi: 10.1016/j.cardfail.2021.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddi HV, Jenkins S, Theis J, et al. Homozygosity for the V122I mutation in transthyretin is associated with earlier onset of cardiac amyloidosis in the African American population in the seventh decade of life. J Mol Diagn. 2014;16(1):68-74. doi: 10.1016/j.jmoldx.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 22.Batra J, Rosenblum H, Defilippis EM, et al. Sex differences in the phenotype of transthyretin cardiac amyloidosis due to Val122Ile mutation: insights from noninvasive pressure-volume analysis. J Card Fail. 2021;27(1):67-74. doi: 10.1016/j.cardfail.2020.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruno M, Castaño A, Burton A, Grodin JL. Transthyretin amyloid cardiomyopathy in women: frequency, characteristics, and diagnostic challenges. Heart Fail Rev. 2021;26(1):35-45. doi: 10.1007/s10741-020-10010-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel RK, Ioannou A, Razvi Y, et al. Sex differences among patients with transthyretin amyloid cardiomyopathy: from diagnosis to prognosis. Eur J Heart Fail. 2022;24(12):2355-2363. doi: 10.1002/ejhf.2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillmore JD, Gane E, Taubel J, et al. CRISPR-Cas9 In vivo gene editing for transthyretin amyloidosis. N Engl J Med. 2021;385(6):493-502. doi: 10.1056/NEJMoa2107454 [DOI] [PubMed] [Google Scholar]
- 26.Maurer MS, Kale P, Fontana M, et al. ; APOLLO-B Trial Investigators . Patisiran treatment in patients with transthyretin cardiac amyloidosis. N Engl J Med. 2023;389(17):1553-1565. doi: 10.1056/NEJMoa2300757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane T, Fontana M, Martinez-Naharro A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140(1):16-26. doi: 10.1161/CIRCULATIONAHA.118.038169 [DOI] [PubMed] [Google Scholar]
- 28.Razvi Y, Ioannou A, Patel RK, et al. Deep phenotyping of p.(V142I)-associated variant ATTR amyloid cardiomyopathy: distinct from wild-type ATTR amyloidosis? Eur J Heart Fail. 2024;26(2):383-393. doi: 10.1002/ejhf.3088 [DOI] [PubMed] [Google Scholar]
- 29.Shah SJ, Fine N, Garcia-Pavia P, et al. Effect of tafamidis on cardiac function in patients with transthyretin amyloid cardiomyopathy: a post hoc analysis of the ATTR-ACT randomized clinical trial. JAMA Cardiol. 2024;9(1):25-34. doi: 10.1001/jamacardio.2023.4147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahn VS, Yanek LR, Vaishnav J, et al. Endomyocardial biopsy characterization of heart failure with preserved ejection fraction and prevalence of cardiac amyloidosis. JACC Heart Fail. 2020;8(9):712-724. doi: 10.1016/j.jchf.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.AbouEzzeddine OF, Davies DR, Scott CG, et al. Prevalence of transthyretin amyloid cardiomyopathy in heart failure with preserved ejection fraction. JAMA Cardiol. 2021;6(11):1267-1274. doi: 10.1001/jamacardio.2021.3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sher T, Velarde GP, Gertz MA. V122I transthyretin cardiomyopathy: an opportunity to build trust and resolve disparities. J Am Coll Cardiol. 2020;76(1):93-95. doi: 10.1016/j.jacc.2020.04.074 [DOI] [PubMed] [Google Scholar]
- 33.Griffin JM, Rosenthal JL, Grodin JL, Maurer MS, Grogan M, Cheng RK. ATTR amyloidosis: current and emerging management strategies: JACC: CardioOncology state-of-the-art review. JACC CardioOncol. 2021;3(4):488-505. doi: 10.1016/j.jaccao.2021.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ioannou A, Massa P, Patel RK, et al. Conventional heart failure therapy in cardiac ATTR amyloidosis. Eur Heart J. 2023;44(31):2893-2907. doi: 10.1093/eurheartj/ehad347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Pavia P, Aus dem Siepen F, Donal E, et al. Phase 1 trial of antibody NI006 for depletion of cardiac transthyretin amyloid. N Engl J Med. 2023;389(3):239-250. doi: 10.1056/NEJMoa2303765 [DOI] [PubMed] [Google Scholar]
- 36.The All of Us Research Program Genomics Investigators . Genomic data in the All of Us Research Program. Nature. February 19, 2024. Accessed April 29, 2024. https://www.nature.com/articles/s41586-023-06957-x
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure 1. Kaplan-Meier Curves for Heart Failure and Mortality Events by V142I Carrier Status
eFigure 2. Kaplan-Meier Curves for Heart Failure Events by V142I Carrier Status
eFigure 3. V142I Hazard Ratio for Heart Failure Events by Age
eFigure 4. V142I Hazard Ratio for Heart Failure Hospitalization or Death by Age and Sex
eFigure 5. V142I Hazard Ratio for Adverse Cardiovascular Events by Age Using 99%
eFigure 6. V142I Hazard Ratio for Heart Failure Events by Age Using 99% Confidence
eFigure 7. Relative Effect of V142I on Remaining Years of Life Compared With Noncarriers
eFigure 8. Effect of V142I on HFH-free Survival Compared With Noncarriers
eFigure 9. Relative Effect of V142I on Remaining Years of Life Free From HFH Compared With Noncarriers
eTable 1. Hazard Ratios for Heart Failure Hospitalization and Death by Carrier Status and Cardiovascular Risk Factors
eTable 2. Events and Hazard Ratios for Adverse Cardiovascular Outcomes by Number of Alleles
eTable 3. Interaction Analysis of the Variant With Select Variables for Study Outcomes
eTable 4. Estimated Survival Time Comparing Carriers and Noncarriers by Age for All-Cause Mortality With Extrapolation to the United State Population
eTable 5. Estimated Survival Time Comparing Carriers and Noncarriers by Age for Heart Failure Hospitalization or Death
eReferences
Data Sharing Statement


