Abstract
This updated guideline replaces the “Guideline for the application of heart rate and heart rate variability in occupational medicine and occupational health science” first published in 2014. Based on the older version of the guideline, the authors have reviewed and evaluated the findings on the use of heart rate (HR) and heart rate variability (HRV) that have been published in the meantime and incorporated them into a new version of this guideline.
This guideline was developed for application in clinical practice and research purposes in the fields of occupational medicine and occupational science to complement evaluation procedures with respect to exposure and risk assessment at the workplace by the use of objective physiological workload indicators. In addition, HRV is also suitable for assessing the state of health and for monitoring the progress of illnesses and preventive medical measures. It gives an overview of factors influencing the regulation of the HR and HRV at rest and during work. It further illustrates methods for measuring and analyzing these parameters under standardized laboratory and real workload conditions, areas of application as well as the quality control procedures to be followed during the recording and evaluation of HR and HRV.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12995-024-00414-9.
Keywords: Autonomous nervous system, Sympathetic nervous system, Parasympathetic nervous system, Stress, Strain
Preliminary remarks
In addition to individual minor editorial and content-related changes, the updated guideline contains major changes in the following areas:
Statement on the usability of mobile wearables (Section: Technical possibilities and requirements),
Discriminatory power between HRV and pulse wave variability (Section: Technical possibilities and requirements),
Addition of innovations in the field of measurement technology since 2014 (Table 1),
Updating the overview table of HRV parameters (Appendix 1),
Updating the evidence on factors influencing HRV and restructuring (Table 2),
Additions to the usability of the HR under dynamic loads (Section: Heart rate),
Complete revision of the chapter on HRV reference values (Section: Heart rate variability),
Update on the use of HRV in the prognosis of diseases (Section: Application for risk stratification of diseases).
Introduction
The HR provides information about the strain of the cardiovascular system in response to physical and mental workload. The HRV gives additional information regarding the dynamics and mechanisms of cardiovascular regulation [1]. Both physiological parameters have been established for the use in inpatient and outpatient care (e.g. cardiology, intensive care, endocrinology, neurology, occupational medicine, sports medicine, obstetrics) as well as medicine and scientific research (occupational physiology, exercise physiology, occupational science, sport science, psychology and pharmacology) for many years because of their non-invasive data acquisition and comfortable methods of, analysis.
Definitions
In medical assessment, a distinction is required between the HR (measured centrally) and the pulse rate (measured peripherally). A difference can occur, for example, in certain forms of cardiac arrhythmia as a pulse deficit.
HR is defined as the number of beats or contractions of the heart per minute. It can also be calculated as a ratio of 60,000 and the average NN interval1 in milliseconds. The HR is a measure of the individual workload response of the cardiovascular system and is affected by various factors (see Section: Factors influencing the individual HR and HRV). HR should be distinguished from the pulse rate, which is defined as the number of pulsations per minutes palpated at the periphery, e.g. at the wrist or at the neck. A difference between HR and pulse rate may occur in certain types of cardiac arrhythmias where some contractions of the heart do not produce a palpable pulse at the periphery. A difference between the HR and the pulse rate is called pulse deficit.
In adults, the resting HR (HRrest) is usually between 60 and 80 beats/min (bpm). In people with endurance training, values well below 50 bpm may be obtained at rest
The HRrest typically varies between 60 and 80 bpm in adults. It is usually higher in children i.e. up to 120 bpm [2]. In endurance trained adults, the HRrest is often below 50 bpm.
The maximum achievable HR varies greatly between individuals and depends on age and biological sex, among other factors. It is recommended to determine it individually as part of ergometric exercise during general dynamic muscle work. The use of formulae to estimate the maximum heart rate (HRmax) should be used with great caution due to the very wide variation
The HR reaches a maximum during physical exertion. The maximum value differs between individuals and decreases with age. The most commonly used empirical formula for estimating the HRmax is [3]:
However, this formula underestimates the HRmax in individuals > 40 years of age [4]. Based on a meta-analysis and their own examinations, Tanaka et al. [4] calculated a regression formula to estimate age-dependent HRmax by:
in which sex-related differences have not been considered [4, 5]. The high inter-individual heterogeneity of the HRmax as a function of age is confirmed by clinical studies for women [6] and men [7]. The determination of the individual HRmax requires maximum physical exertion under conditions of dynamic muscle activity of a larger muscle mass, e.g. a cardiac stress test using treadmill or bicycle ergometry [8]. Depending on the specificity of the subjects, usually other instruments like the arm crank ergometer may be used as well [9].
The recovery HR (HRrecovery), the working HR (HRwork) and the integral of the HRrecoveryare available for further assessment of the performance of the cardiovascular system
In the field of exercise physiology, the HR following a maximal exercise test is frequently taken as an indicator of the fitness level of a subject. The value is measured one minute2 after the cessation of a maximal exercise test. It reflects the rapid regulative phase of recovery and is called the HRrecovery.
In the fields of occupational medicine and occupational health science, the HR during work 3 (HRwork) is taken into consideration while analysing the respective activity, e.g., evaluation of physical work. The HRwork is defined as the difference between HRrest and the value measured during physical work [10–12]. HRwork is also known as net HR (HRnet) [13]. HRnet correlates better with the physical exertion than the HR, provided a resting phase without physical or emotional stress of at least five minutes (ideally, fifteen minutes) before starting the work is maintained to assess a valid baseline HR. This is a necessary requirement while carrying out tests in the laboratory, whereas in cases of measurements at real workplaces, it could be difficult to achieve these conditions before the working shift. Under circumstances of unreliable and not representative HRrest measurements, absolute HR might better reflect the intensity of the workload during physical exertion than HRwork. Alternatively, the reference HR (HR reference ) can also be determined for light dynamic work (see Section Heart rate at rest).
In addition, the individual physical exertion is also frequently described by calculating the summated recovery HR as a measure of the fatigue and recovery [14]. For this procedure, all heart beats during the recovery phase are summed until HR reaches the baseline level (e.g., HRrest) (Calculation of the integral of the heartbeats above HRrest over the time from end of exercise to normalisation of HR).
HRV is based on a mathematical analysis of a time series of consecutive heart actions - the so-called NN intervals.
The term HRV comprises a number of mathematically calculated parameters, which characterise the variance, rhythm or complexity of a time series of consecutive heart beats – the so-called NN interval. Because of robustness and reliability issues the R-wave is usually used as a sign of electrical heart activation during automatic detection (Fig. 1). A detailed list of the frequently used HRV parameters can be found in Section: Analytical methods and parameters of HRV.
Fig. 1.
Principle of determining the NN intervals from the ECG as a measure of the distance between two R-spikes
Physiological mechanisms
Physiological mechanisms of HR
The autonomic modulation of HR by sympathetic cardiac nerves and the vagus nerve (parasympathetic) is primarily mediated via the sinus node.
During the resting phase, the frequency of the heartbeat is triggered by the primary impulse generating tissue (pacemaker), the sino-atrial node (SA-node). The frequency of the non-innervated sinus node is stated differently in the literature. In the short term after heart transplantation, in which the transplant is denervated by the surgery, the HR is higher than the normal physiological HR, which is 60 to 80 bpm [15]. For a longer period of time after transplantation, it changes back towards normal physiological HR, probably due to partial reinnervation. After transplantation, however, the beta-adrenergic activation of the sinus node cells by circulating adrenaline from the adrenal medulla continues, which may contribute to the increased HRrest. All downstream pacemaker tissues also capable of spontaneous depolarisation (AV node, bundle of His, Purkinje fibres) exhibit lower activation frequencies. The autonomic modulation of HR by the sympathetic and the parasympathetic (on the heart singularly influenced by the vagus nerve) is primarily mediated by the SA-node. This two-way control of the autonomic nervous system (ANS) was demonstrated in blocking experiments of the adrenergic (beta-blockers) and muscarinic-cholinergic (atropine) receptors [16–18].
Physiological mechanisms of HRV
The HR is subject to physiological variability even during constant stress, which reflects the interaction of the sympathetic nervous system (SNS) and vagus nerve (as part of the parasympathetic nervous system [PNS]), among other factors.
Even under constant physical exertion, HR shows a physiological variability, which predominantly reflects the interplay in the ANS between the SNS and the N. vagus (as part of the PNS). The sympathetic part of the ANS leads to a reduced HRV through the release of adrenaline and noradrenaline, while the parasympathetic (vagal) part leads to an increase in HRV through the release of acetylcholine [19].
At rest and during mild exertion, the parasympathetic (vagal) control outweighs the sympathetic effect. This leads to an increased variability of the heartbeats: the difference in the gap between two consecutive heartbeats increases.
The HRV analysis is used particularly for the differential evaluation of the interplay between the sympathetic and the PNS under various conditions. Therefore, the quantification of the autonomic activity is carried out by analysing the periodic fluctuations of the heartbeat. Rapid changes in the HR with a cycle length of about 2–7 s are closely associated with breathing (Respiratory Sinus Arrhythmia [RSA]). These high-frequency fluctuations are modulated almost exclusively by the parasympathetic branch of the ANS (vagus nerve); whereas the slow fluctuations (cycle length of about 10 s) are modulated by the efferent nerve fibers of both parts of the ANS [20]. However, for the interpretation of HR and HRV, it must be taken into consideration that both parameters reflect the net effect of autonomic cardiac efferent nerve activity but also other modulating factors like humoral and mechanical influences during physical exertion, heat and other environmental factors. In the case of temperature changes, the effects are mediated on the one hand via the modulation of the ANS and on the other hand directly via temperature effects on the sinoatrial node cells of the heart.
The vagal resting tone is higher the better the heart is adapted to cope with high physical stress, which is why trained people (e.g. endurance athletes) generally have a higher HRV in addition to a lower HRrest. In addition to changes in the activity of autonomic efferent nerve fibers, endurance training also leads to changes in the expression of ion channel proteins and membrane transport proteins [21], which has an additional lowering effect on HRV.
Determination of the NN intervals for the calculation of HR and HRV
Technical possibilities and requirements
Different measuring systems are available for recording heart actions. Their measurement accuracy for a subsequent HRV analysis varies. It is recommended to use an ECG-based measurement for this purpose. The devices should measure non-invasively at a high sampling rate (ideally 1,000 Hz, minimum 250 Hz), be mechanically robust and non-reactive.
Several methods are available to record the interbeat intervals: stationary ECG instrument – which is more suitable for laboratory studies or intensive care units – and mobile measurement techniques that are convenient in field studies. The mobile measuring systems include 24-hour ECG devices, chest strap systems with direct storage or storage on an external data module (e.g. in a separate heart rate monitor) as well as a number of mobile measuring systems that have become available on the market in recent years (e.g. watches without a chest strap, systems with measurements in the ear canal, etc.). What all measuring systems have in common is that they record the NN intervals with different levels of measurement accuracy and deviations can therefore occur in the subsequent HRV analysis [22, 23].
Pulse oximeters can also determine the distance between two pulse waves. However, these differ from the NN intervals measured on the heart using electrodes. A systematic review [24] showed that only in young, healthy subjects under resting conditions are there acceptable correspondences between NN intervals and pulse NN intervals, but HRV parameters based on the pulse NN intervals are sometimes significantly increased (e.g. in HF). It is therefore important to make a strict distinction between HRV and the so-called pulse rate variability (PRV).
For HRV analysis, a so-called “beat-to-beat recording” with assessment of all cardiac actions and a high sampling rate (ideally 1,000 Hz, minimum 250 Hz [25]) is gold standard in order to record the distances between individual cardiac actions with high temporal accuracy.
In addition, the instruments should fulfill the following requirements:
non-invasive,
mechanically robust (for examinations at workplaces, which involve heavy physical work or difficult environmental factors like heat, cold and wet conditions) and.
non-interfering (the method itself should not influence the results in any way).
The advantages and disadvantages of the different measurement systems are given in Table 1.
Table 1.
Advantages and disadvantages of the different measurement systems
| Advantages | Disadvantages | |
|---|---|---|
| Stationary (24-hour) ECG |
• ECG recording • non-invasive • visual monitoring of R-wave detection • medical device according to the Medical Devices Act |
• not portable, suitable only for laboratory examinations and intensive care units • bothersome cable |
| Portable (24-hour) ECG |
• portable, small devices • suitable for laboratory and field studies • ECG recording • non-invasive • visual monitoring of R-wave detection • medical device according to the Medical Devices Act |
• bothersome cable |
| Chest strap systems with storage in a separate heart rate monitor |
• portable, small devices • high freedom from reaction • non-invasive |
• no ECG recording • interference with data transmission (because of power lines, vehicles, etc.) • not a medical device according to the Medical Devices Act |
| Chest strap systems with storage directly in the chest strap |
• portable, small devices • high freedom from reaction • non-invasive |
• no ECG recording • not a medical device according to the Medical Devices Act |
| Watch systems without a chest strap and with sensors to record the pulse wave |
• portable, small devices • high freedom from reaction • non-invasive |
• no ECG recording • formally no NN interval recording • not suitable for measuring HRV • not a medical device according to the Medical Devices Act |
| Measurement in the ear canal |
• portable, small devices • high freedom from reaction • non-invasive |
• no ECG recording • formally no NN interval recording • not suitable for measuring HRV • not a medical device according to the Medical Devices Act |
| Measurement using ear clips based on a pulse oximeter |
• portable, small devices • high freedom from reaction • non-invasive |
• no ECG recording • formally no NN interval recording • not suitable for measuring HRV • not a medical device according to the Medical Devices Act |
Devices according to the Medical Devices Act are specifically for diagnostic or therapeutic purposes and are intended by the manufacturer for use on humans
Electrodes
It is recommended that the electrodes be adequately prepared for optimal measurement results.
The following should be done to avoid errors during measurement:
adhesive electrodes should be used so that they do not lose contact with the skin even after longer periods of recording (e.g. 24 h) and in cases of sweating,
the electrodes on the chest belt (contact points) should be moistened,
the chest belt should fit firmly and.
a textile strap should be preferred, because it can adapt itself optimally to the individuals upper body.
Preparation of the skin
It is recommended that the skin be adequately prepared for optimal measurement results. This includes, among other things, reducing the oil film on the skin and, if necessary, removing existing (chest) hair.
The skin should be prepared carefully in order to obtain optimal results of measurements, especially if long-term recordings (24 h) are carried out. In cases of skin-electrode contact with high impedance, the quality of the recording decreases and the probability of the appearance of artifacts are high.
The main objective of preparing the skin is to remove the natural oil film of the skin. This reduces the contact resistance between the skin and electrodes and enables a better adherence of the electrodes. The contact points on the skin for the electrodes are first cleaned with a dermatologically safe, degreasing solution (e.g. alcohol solution). However, any damage or injury to the skin has to be avoided. If chest hair grows, it may be necessary to carefully remove the hair from the corresponding adhesive areas before attaching the adhesive electrodes. An additional fixing of the electrodes and the cables is useful for long-term recordings or other conditions.
Lead choice and electrode positioning
It is recommended to select the ECG lead with the largest amplitude of the R wave of the QRS complex.
The ECG leads must be chosen based on the largest amplitude of the R-wave of the QRS complex (see Fig. 1). In principle, recordings from a single lead are sufficient. If possible, multiple leads should be used to enable a reliable correction of artifacts.
During the automatic determination of the NN interval, it should be ensured that R-wave detection is consistently based on the same lead. Changing the lead during the same recording can lead to an artificially generated increase in the HRV. While the point of time at which the QRS complex begins is almost identical in most of the leads, the fiducial point (R-wave), which serves as the basis for determining the NN interval, can significantly vary between the different leads [26, 27].
The positioning of the electrodes influences the quality of the recordings. If electrodes are not positioned appropriately, recording quality might suffer, resulting in an accumulation of artifacts. The intercostal spaces are suitable areas for positioning the electrodes. Within these spaces, flat and even areas of the skin should be selected (e.g. positioning above dermal naevi should be avoided).
Quality assurance while determining the HR
When recording the HR, quality assurance requires the determination of HRrest, artifact control, the highest possible sampling rate and the consideration of possible influencing factors.
The following aspects should be taken into consideration for the purpose of quality assurance:
the determination of HRrest 4 before the beginning of the exertion as physiological baseline for the evaluation (see Section: Heart rate at rest),
checking for artifacts and, if possible, removal of artifacts (e.g. by visually checking the data during analysis, automatic methods for correcting artifacts),
a high sampling rate (see above),
the possible influencing factors depending on the case (see Table 2) and.
the circadian rhythm should be kept in mind for comparable examinations.
Table 2.
Factors influencing HR and HRV, sorted according to the four main areas depicted in Fig. 4, sorted alphabetically within the main area
| Influencing factor | Effect on resting heart rate (HRrest) | Effect on heart rate variability (HRV) |
|---|---|---|
| Non-influenceable physiological factors | ||
| Age | HRrest [28] and HRmax [8] normally decrease with increase in age | HRV increases sharply in the first year of life, then increases until the age of 15 [29], is highest in young adulthood and falls non-linearly with age [28–46]. |
| Circadian rhythm/time of the day | The HR follows a circadian rhythm, with a fall of HR at night [47]. | The HR follows a circadian rhythm, but the HRV is decreased at night due to the predominance of the parasympathetic activity and reduced during the day because of the predominance of the sympathetic activity [48]. |
| Genetic | HRV appears to vary between members of different ethnic backgrounds [49]. | |
| Pregnancy | During pregnancy there is usually an increase in HR [50]. | During pregnancy, a reduction in HRV usually occurs as the pregnancy progresses [50] and is lowest in the 2nd trimester [51, 52]. |
| Biological sex | The HR is normally higher in women than in men [53]. |
Most of the studies showed a higher parasympathetic activity in women as compared to men [31, 32, 54–59], which however showed a narrower difference after the age of 50 [33–35]. Some of the studies showed a higher baseline sympathetic activity in women [36, 37, 60, 61]. |
| Diseases | ||
| Cancer diseases | The influence of breast cancer on HRV is unclear [62]. | |
| Cardiovascular diseases | Cardiac insufficiency leads to a raised HR [63] and unrestricted maximum HR. | Cardiac insufficiency generally leads to a reduction in the HRV [5, 64–67]. |
| With hypertension, HRV is usually reduced [68, 69]. | ||
| In patients with previous myocardial infarction, the activation of the sympathetic nervous system often leads to an increase in the HR, which is important for the prognosis [70–75]. | HRV is usually reduced in coronary heart disease (CHD) with and without angina pectoris and after myocardial infarction [76, 77]. | |
| Chronic obstructive pulmonary disease (COPD) | With COPD, HRV is usually reduced [78, 79]. | |
| Chronic renal insufficiency | In chronic kidney failure, HRV is usually reduced [78]. | |
| Duchenne muscular dystrophy | HRV is usually significantly reduced in the early stages of Duchenne muscular dystrophy and in manifest disease [80]. | |
| Headaches, regular | Regular headaches are usually associated with reduced HRV [81, 82]. | |
| Metabolic disorders | Diabetes mellitus is often associated with increased sympathetic activity and hence a raised HR [83]. | The HRV is often reduced in patients with diabetes mellitus [58, 84–87], however, a correlation between the value of the HRV and the duration of the diabetes exists especially in cases of badly controlled diabetes [88]. The reduction is due to peripheral neuropathy due to microcirculation disorders [89]. |
| A metabolic syndrome often leads to a reduction of the HRV [88, 90–96], this is particularly evident in women [97]. | ||
| Pain | In chronic pain, HRV is usually reduced [98]. | |
| Psychiatric disorders | Patients with anxiety disorders and panic attacks usually have an increased HR [99]. | Patients with anxiety disorders [38, 100–103] and panic attacks [99, 101] usually show a reduction in the HRV. |
| Patients with anorexia nervosa usually have a reduced HR [104]. | HRV is usually reduced in patients with anorexia nervosa [102]. | |
| HRV is usually reduced in patients with bulimia nervosa [105]. | ||
| Posttraumatic stress disorder often leads to a reduced HRV [106]. | ||
| A major depression often leads to an increase in HR [107–109]. | A (major) depression often leads to a decrease in HRV [38, 108, 110–114]. | |
| In epilepsy, HRV is usually reduced [115]. | ||
| In borderline personality disorder, HRV is usually reduced [116]. | ||
| In bipolar disorder [38, 113, 117, 118]./ schizophrenia [119], HRV is usually reduced. | ||
| In the case of substance addiction [38], HRV is usually reduced. | ||
| Rheumatoid arthritis | Based on a systematic literature search, HRV does not currently appear to be changed in the presence of rheumatoid arthritis [120]. | |
| Sleep disorders | A reduction in HRV in the presence of sleep disorders is currently not supported by the scientific literature [121]. Something similar can be found in untreated obstructive sleep apnea syndrome. | |
| Stroke | A stroke is usually associated with reduced HRV [122]. | |
| Influenceable lifestyle factors | ||
| Alcohol consumption | With acute alcohol consumption, HRV is usually reduced [123]. Low, constant alcohol consumption with an alcohol content of one standard drink for women or two standard drinks for men usually leads to a short-term but no long-term change in HRV or an increased HRV, while chronic alcohol abuse leads to a reduction of HRV [123, 124]. | |
| Body fat/body weight | Increased body mass index (BMI) generally leads to a raised HR [125], which can be partly explained by the stimulating effect of leptin on central sympathetic neurons [126, 127]. | Increased body mass index (BMI) and increased mass of body fat often cause a fall in the HRV [128]. |
| Fitness activities, performance capacity, sports | High-intensity interval training (HIIT) generally increases HRV, which has been shown particularly in healthy subjects and patients with metabolic syndrome [129]. High-intensity training and competition series, on the other hand, can lead to reduced HRV [130, 131]. | |
| During strength training, there is usually no change in HRV in healthy people, while strength training is usually associated with an increase in HRV in subjects with chronic illnesses [132]. | ||
| Initially, there is a rise in the resting HR due to the increased physical activity; however, regular exercise without symptoms of overtraining leads to a decrease in the HR due to an increase in the parasympathetic activity and an optimisation of the cardiac output [133]. The expression of ion channels for the pacemaker potential is reduced [21]. Therefore, endurance training often results in exercise-induced bradycardia [134–137]. | Initially, there is a fall in the HRV due to increased activity of the sympathetic system as a result of the physical activity [138], but regular physical activity leads to an increase in the parasympathetic activity which in turn causes a rise in HRV [36, 131, 138–140]. Endurance training normally increases the HRV [130, 131, 141–143]. These effects can be also seen in patients with myocardial infarction and patients with cardiac insufficiency [141] or Diabetes mellitus II [144]. | |
| Smoking | Active [145] and passive smoking [146] can lead to an increase in HR. | Smoking can lead to a decrease in HRV [147], this effect is dose dependent [146]. Even in non-smokers, passive smoking e.g. at home or at work leads to a reduction in the HRV [146–148]. |
| Stress/mental tension | Stress (e.g. mental, workplace related) generally leads to an increase in the HR [149–152]. | Stress (e.g. mental, workplace related) generally leads to decreased parasympathetic activity and thus to a reduction in the HRV [150, 152–157]. |
| External factors | ||
| Breathing | During inspiration there is a short-term increase in HR, during expiration there is a short-term decrease in HR [158, 159]. This is essentially due to pulmonary afferents from stretch receptors and interactions from central respiratory neurons to the circulatory center in the medulla oblongata. | The effects of respiration on HRV are reflected in the form of respiratory sinus arrhythmia (RSA) and is seen in the HF band. On the whole, the HRV parameter, RMSSD, does not seem to be affected by respiration [160]. For the rest of the parameters, the present state of knowledge is not conclusive [161–163]. |
| Cold, low temperatures | In men, low ambient temperatures usually lead to a decrease in HR both at rest and during exercise, while in women there is no decrease in HR, but rather a slight increase in HR [164]. | Only few studies about the effects of low temperatures on HRV are currently available: a reduction in the sympathetic activity and thus a raised HRV has been observed [165], while long-term exposure to cold, including in winter months or occupational exposure to cold, no influence on HRV could be shown [166–168]. |
| Hazardous substances |
Neurotoxic substances can lead to a reduction in the HRV: e.g. carbon disulphide [169, 170], however, not in the case of long-term low-dose exposure [171]; acute diesel and biodiesel inhalation [172]; chronic lead [173, 174], acute cadmium [175] or long-term mercury exposures [176] and neurotoxic styrene exposure [177, 178]. The data regarding the effects of chronic solvent exposure is not conclusive, both - a fall in the HRV and no differences - have been described [179–181]. In contrast, there was no evidence of a reduction in HRV through mercury exposure [182]. Only fetal mercury exposure appears to lead to a reduction in HRV [176]. Exposure to particulate matter (PM2.5) appears to reduce HRV [183]. |
|
| Heat, high temperatures | High environmental temperatures lead to an increase in the HR [13, 184, 185] caused on the one hand by direct temperature effects on the sinoatrial node and on the other hand by the increase in sympathetic activity as a result of the activation of warm receptors. | High environmental temperatures lead to an increase in the sympathetic activity and a reduced HRV [166, 186]. |
| Hypoxia | Hypobaric hypoxia usually leads to short-term sympathetic activation [187] and long-term to a reduction in HRV [188]. | |
| Noise | Noise often causes a rise in the HR [189], caused by activation of sympathetic nerves [190]. | Only few studies that give information about the effects of noise on HRV are available; HRV appears to fall in the presence of noise [191–194]. |
| Pharmacological drugs | Pharmacological drugs can have an increasing or decreasing effect on HR [195]. | Pharmacological drugs can have an increasing or decreasing effect on HRV [195]. |
| Shift work including night shift | Shift work with a night shift usually results in an activation of the SNS and a reduction in the PNS and thus a reduction in HRV, whereby there is a correlation between the duration of shift work in years and the reduction in HRV [196–202]. | |
Quality assurance while determining the HRV
For quality assurance when determining HRV, it is recommended to take a resting ECG, check for artifacts and, if necessary, correct them, use recordings with only a few extrasystoles and choose a suitable analysis method and measurement duration, if possible, to use high sampling rates and to take possible influencing factors into account.
The following aspects should be taken into consideration for the purpose of quality assurance:
a resting ECG should be recorded before the HRV is analysed in order to rule out cardiac arrhythmias (e.g. atrial fibrillation),
recordings with more than 1% of ventricular or supraventricular extrasystoles should be evaluated critically because of the apparent increase in the HRV [203],
checking for artifacts and, if possible, removal of artifacts (e.g. by visually checking the data during analysis, automatic methods for correcting artifacts),
the analytical method of choice (e.g. Fast Fourier Transformation, Autoregressive Model, Trigonometric Regressive Spectral Analysis) to enable comparable interpretations (see Section: Analytical methods and parameters of HRV),
the selected duration of recording (subsequent length of the sequence of analysis) or the underlying amount of data depending on the analytical method selected and the research question (see Appendix 1),
a high sampling rate (see above),
the possible influencing factors depending on the analytical method selected and the research question (see Table 2) and.
the circadian rhythm should be considered as a possible confounder if comparing repeated measurements.
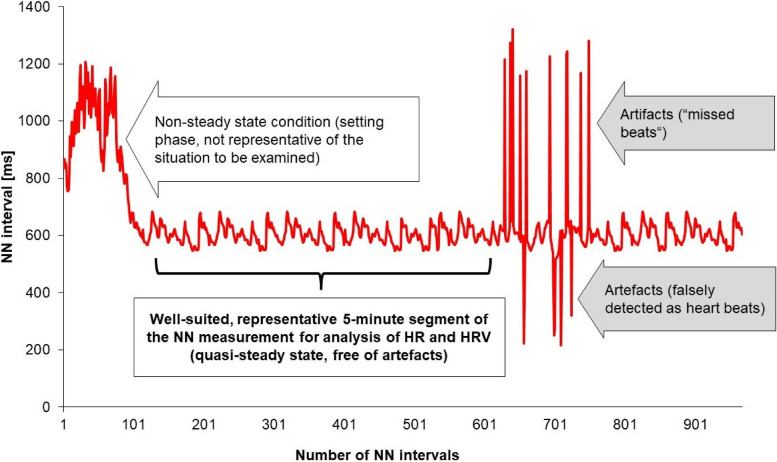
In the case of short-term recordings, the selection of a suitable, representative area of the NN intervals is an important quality criterium for HRV analysis. For this, the non-steady setting phase at the beginning of the examination and the recordings with artifacts should be avoided for the analysis as far as possible (see Fig. 2).
Fig. 2.
Principle of selecting a suitable 5-min range of the NN measurement from an artifact-superimposed recording with an early non-stationary range
Other sources of interference
There are also other sources of interference (such as electromagnetic fields) that can influence the recording of HR and HRV.
When chest belts with wireless data transmission are used in the vicinity of electromagnetic fields from power poles or power supply lines [1] or used in vehicles and their vicinity [204, 205], interferences can occur. Artifacts due to body movements and due to electrical activity of other muscles can occur during physical activity. In the case of an ECG recording, these artifacts should be detected and manually removed at the end of the recording, whereas in cases of gathering data without ECG recording (like in most cases of chest belts systems), it is not always possible to attribute the artifacts to the movements.
Analytical methods and parameters of HRV
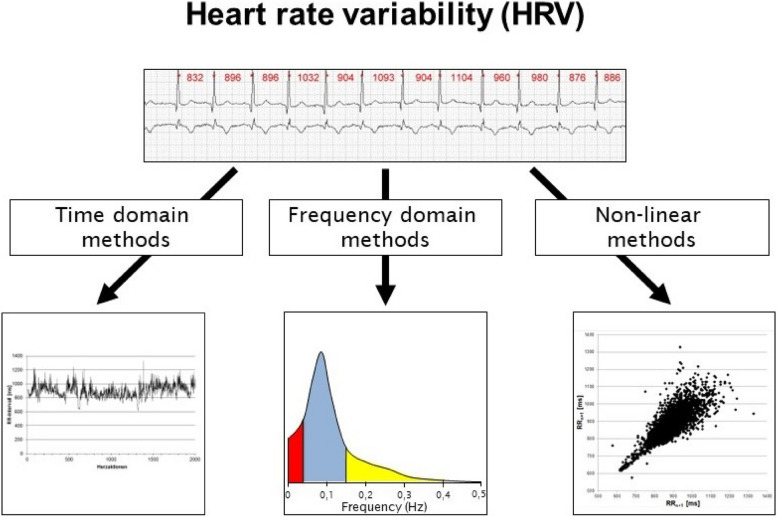
HRV can be quantified using time and frequency domain methods as well as nonlinear analysis. Care must be taken to make the correct selection in relation to the objective and evaluation time.
HRV is quantified using time and frequency domain methods as well as methods of non-linear analysis (see Fig. 3).
Fig. 3.
Overview of the possibilities of HRV analysis with examples of possible graphical representations
Time domain methods are divided into statistical and geometrical methods. In the case of the statistical methods, the NN intervals are evaluated mathematically with respect to its variance and the measurement of the rhythm is tagged with the time dimension or the percentage values, whereas geometrical methods provide an evaluation of HRV based on geometric forms. For these purpose histograms, HRV triangular index and its modifications, triangular interpolation of the NN interval histogram are used [206].
The analyses of the frequency range include, among others, the Fast Fourier transformation and Autoregression methods [207, 208]. Spectral analysis decomposes the periodic oscillation of the NN-signal, into different frequencies and amplitudes. This makes it possible to split the NN interval series and the periodic oscillations of the heartbeat into different frequencies and amplitudes, which in turn represent different physiological processes or different control systems [209].
In some cases, the Lomb algorithm is also used to analyse recordings with varying lengths and non-equidistant sampling. This represents a spectral analysis method of non-equidistantly sampled measured values [210]. The Lomb algorithm is an extremely slow method; but approximation methods have been established to speed up the application of the algorithm [211].
The methods of non-linear dynamics (e.g. Approximate Entropy [ApEn], Sample Entropy [SampEn], Detrended Fluctuation Analysis [DFA]) [30, 212–214] vary from the traditional time and frequency parameters in that they do not reflect the strength of the HRV, but they rather indicate qualitative aspects of the series of NN intervals [212]. These methods often prove to be suitable for long-term as well as short-term recordings and are considered more robust against artifacts.
One form of visualisation of the time series of NN intervals is offered by the so-called Poincaré Plot5 [213] (see Fig. 3). From this plot various indices can be determined and interpreted (e.g. length and width of the scatter-plot). Further, the form can also give hints about certain diseases [215].
A detailed listing of the HRV parameters is given in Appendix 1.
Factors influencing the individual HR and HRV
HR and HRV are influenced by numerous changeable and non-changeable factors (uncontrollable physiological factors, diseases, controllable lifestyle factors and external factors), regardless of the acute stress.
Aside from acute physical exercise/exertion HR and HRV can be affected by several modifiable and non-modifiable factors. In addition to physiological parameters that cannot be influenced (e.g. age), there are a number of changeable influencing factors - e.g. living habits of the test subjects or the resulting consequences or external conditions. Furthermore, a variety of diseases are associated with reduced HRV, although the influence on the ANS can be viewed as a consequence of the disease and only rarely as a potential cause.
The individual influencing factors can be divided into four main areas (physiological factors that cannot be influenced, diseases, lifestyle factors that can be influenced and external factors) (see Fig. 4). The most relevant factors for investigations in the field of occupational medicine and occupational health science are described in Table 2. The consideration of these factors is of importance when HR and HRV are evaluated. In addition, various other factors and conditions (e.g. HRV in patients with sepsis that needs intensive care) have been mentioned in scientific literature. As these cases are normally not relevant in the field of occupational medicine and occupational health science they will not be considered any further in the current guideline.
Fig. 4.
Grouping of the different factors influencing HRV into four main areas (modified taken from [216]). * = HRV reduction as a result of the physiological response to the physical stimulus.
Pharmacological drugs can have significant impact on the ANS or the electrical conduction system of the heart and thus should be considered when assessing and evaluating HR and HRV. Due to the large number of possible pharmacological interactions, the groups of beta blockers, acetylcholinesterase inhibitors, antiarrhythmics and psychotropic drugs are only mentioned here as examples [195].
Evaluation and interpretation of HR and HRV
In order to be able to correctly evaluate and interpret the results of HR and HRV analyses, it is recommended that the specific question, the data collection method used and the evaluation strategy be coordinated in advance of the examinations.
For a valid and reliable evaluation and interpretation of HR and HRV adequate study designs, data sampling strategies and analysis methods are necessary prerequisites. HR and HRV parameters mirror the individual physiological workload response within a given context of individual, psychophysiological and work-related factors (see Table 2). Thus, measurements of HR and HRV should always be combined with complementary data (e.g., questionnaire about the subjective stress, perception of stress and the state of health). If possible, information about the ambient conditions at the workplace, like noise and temperature, should be collected at the same time.
Heart rate (HR)
The main influencing factors for HR are dynamic loads on large muscle groups, but also static muscle loads, thermal and psychological loads.
Important factors that influence HR are dynamic activity of larger muscles, static muscular load of smaller muscles and thermal stress as well as mental workload [217, 218]. These factors often act together on the cardiovascular system and can induce a corresponding increase of HR during exertion. These effects were studied by e.g. Hettinger and Wobbe [189] in cases of different muscle, temperature and thermal radiation loads. A demarcation of the individual components is possible under controlled conditions only. So, HR during dynamic work of larger muscles can be used for estimating the energy expenditure only if the activity of smaller muscles and the mental workload are negligible and thermal conditions remain neutral [218]. Additional, static muscle strain as well as psychological and thermal strain lead to a decrease in the quality of the correlation between energy expenditure and HR (weak correlation coefficient).
Heart rate at rest (HRrest)
It is recommended to use HRrestas the baseline value for interpreting HR changes under workloads
The HRrest is the preferred baseline value for an individual evaluation of the HR during physical exertion (see Section: Quality assurance while determining the HR). Baseline measurement conditions (e.g. posture of the person, duration of recording) should be standardized to enable within- and between-subject comparisons. Both, an increased or a decreased HRrest can be associated with an apparent cardiac disease [219, 220]. After considering physiological contributors to HRrest (see Section: Factors influencing the individual HR and HRV) persons with unexplained higher (tachycardia) or a lower (bradycardia) HRrest should be subjected to a cardiological examination.
Sometimes the determination of HRrest is difficult in field studies, due to confounding effects on HR (psychological factors, environmental conditions like noise, ambient temperature etc.). Therefore, Hettinger and Wobbe [189] recommended the determination of a HRreference during light dynamic work (e.g. 20 W on a bicycle ergometer for 10 min). Since this workload is typically perceived as a “light exertion”, the effect of psycho-emotional stress (“psychological heart rate“) is largely eliminated. Compared to the resting value in the supine position the HR increases by an average of 18.5 bpm in men during this procedure; while in women, an average increase of 24.5 bpm with relatively narrow limits of agreement can be expected [189].
Maximum heart rate (HRmax)
It is recommended to use the HRmaxas an exercise criterion and to determine it using a standardized exercise protocol.
The HRmax serves as a criterium for maximum physical exertion and can be determined during a standardised exhausting exercise protocol [221]. The most widely used methods for this are the treadmill and the bicycle ergometry. An optimal motivation to bring about the maximum performance and the observance of the stop criteria are the main requirements for the determination of the HRmax. Personal physical exertion can also be measured using the Borg scale as an example [222, 223]. However, one should keep in mind that apart from factors like age, sex and fitness level [224] and certain bradycardia producing drugs [8], the value of the HRmax determined largely depends on the muscle mass that is used.
For an appropriate estimation of cardiac workload, the interpretation of the HR response during a given (occupational) physical task should always be referred to the individual HRrest and HRmax (see Section: Physiological mechanisms). Here, a value of the HR during physical exertion (occupational), which lies closer to the HRmax, indicates a higher degree of stress on the heart. The continuous performance limit can also be referred to for the interpretation (see Section: Continuous performance limit (CPL)).
Recovery heart rate (HRrecovery)
HRrecoverycan be used to estimate the recovery ability of the cardiovascular and metabolic systems.
The HRrecovery can be used to estimate the recovery capacity of the cardiovascular and the metabolic systems. It strongly correlates with the function of the parasympathetic branch of the ANS [225] and typically decreases exponentially after the end of the exertion. The main factors that influence the temporal kinetic of the recovery of the vagus are intensity, duration and method of the physical exertion, initial performance level and the type of recovery [226–228].
Continuous performance limit (CPL)
The CPL for physical work is the maximum physical work that can be maintained over a work shift (approx. 8 h) without progressive symptoms of fatigue.
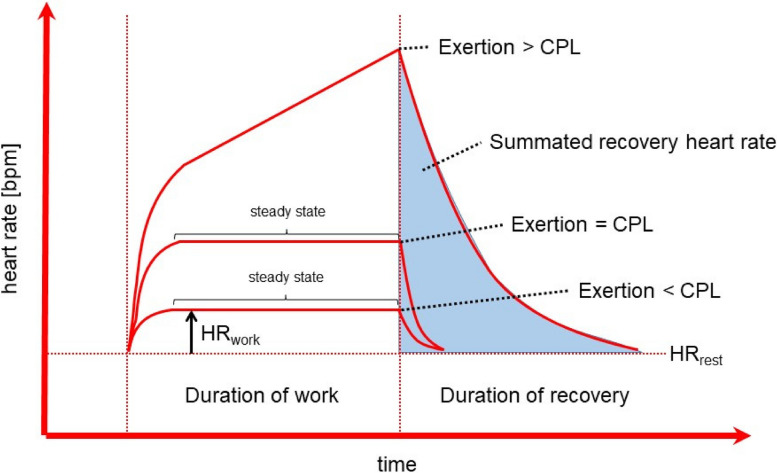
The CPL of physical exertion characterizes the maximum muscular work that can be maintained over a regular working shift (about 8 h) without any progressive symptoms of fatigue and where the measurable physiological parameters return to baseline or fall even below baseline within 15 min after the work cessation [13]. If the CPL is adhered to, overloading and injuries can be avoided and adequate recovery for the next (work) shift is possible. The CPL can be used for the identification of muscular physical exertion without fatigue (below the CPL) and muscular exertion inducing fatigue (above the CPL) with respect to an 8-hour working shift [10, 229, 230]. The value of CPL can be determined using cardiac (e.g., HR) as well as metabolic parameters (e.g., energy turnover, lactate). Spiroergometry can also be used as an alternative method for the determination (e.g. 40% of the maximum oxygen intake). HR in particular is suitable as an easy-to-collect cardiac parameter for recording cardio-pulmonary stress. In the cases of dynamic activity of larger muscle masses, the CPL ranges between 105 and 110 bpm or alternatively between HRrest + 30–35 bpm [13]. It should be noted that HR used for the determination of the CPL, like the individual HRrest and HRmax, also underlies a strong individuality due to e.g., age and the level of physical fitness.
Below the CPL, the HR shows a linear increase along with the intensity of workload. In the case of light work with a constant performance over time, the HR reaches an almost constant deflection (“steady state“) within a short period of time (few minutes). Typically, this “steady state“ can be maintained over the entire 8-hour working shift (see Fig. 5).
Fig. 5.
Heartrate during different loads (below or above the CPL as well as in the range of the CPL) with respective recoveries, schematic representation, modified according to Müller [14], CPL = continuous performance limit
Small, short-term overshoots beyond the CPL (e.g., HR of 130–140 bpm) are common during a work shift and do not pose any health risks, while scheduled breaks during constant physical exertions with a HR > 130 bpm help to overcome muscular fatigue. The more intense the physical strain is and the more the state of exhaustion is reached, i.e., the further the CPL is exceeded, the more these tiring activities must be limited with respect to duration.
If the CPL is being continuously exceeded, this kind of work is classified as heavy physical work or hard labour, in terms of energy [231]. It leads to increasing muscle fatigue (along with anaerobic metabolism), which is generally reversible without any effects on health. There is a continuous rise in HR and a rise in fatigue as well (see Fig. 5). Heavy physical work is also relevant from the motor biomechanical point of view, because the skeletal system (joints, intervertebral discs) might be injured under relevant conditions [230]. However, these aspects are beyond the scope of this guideline.
Apart from the CPL, the integral of HRrecovery (see Fig. 5) is also considered an indicator of the individual physical exertion [14].
It should be noted that an evaluation of workload based on the CPL (as described above) is only valid, if larger muscle masses (> 1/6 of the total muscle mass) are dynamically active. If smaller muscles masses are used for dynamic tasks, the CPL has to be adjusted (e.g., decreased) in proportion to the muscle mass used. Typically, HR, HRwork and oxygen update are lower compared to whole body exertion, despite similar or even shorter time until exhaustion. Sometimes, e.g. in cases of work done by the arm or hand, the CPL is not valid for estimating time to fatigue or exhaustion [232]. Under these conditions local muscle load is limiting for endurance of work. In cases of isometric muscular work or with an increasing portion of isometric muscle activity during dynamic tasks, an evaluation of the workload intensity with respect to the CPL is not valid.
With respect to work structuring, the occupational tasks that are seen as being causal for an increase over the CPL should be considered in detail.
Heart rate variability (HRV)
In order to interpret HRV parameters under external stress, it is recommended that sensible planning and implementation of the measurement of cardiac actions be taken into account, especially with regard to the length of the recording.
Several methods are available for the analysis of HRV. In principle, the time domain parameters of HRV can be calculated using mathematical functions in established spreadsheet programmes. In addition, many manufacturers of mobile ECG instruments provide software programmes that enable the calculation of the time domain and the frequency domain parameters of HRV and also permit a non-linear analysis of the NN interval series. Freely available software packets with good documentation are also available.
For statements about the ANS (see Sections: Application to assess physical exertion and Application to assess mental workload), suitable HRV parameters should be used and recorded or analyzed with a time range suitable for the activity or rest measurement (see Appendix 1). It must be taken into account here whether an influence of the SNS or PNS is to be measured primarily and how long the recording time can reasonably be carried extended.
While assumptions based on specific HRV parameters require long-term recordings (see Appendix 1), or the 24-hour measurement of HRV can be advantageous for gaining an overall impression of the ANS, HRV parameters that are suitable for short-term measurements are primarily used for the evaluation of physical stress at work with rapidly changing requirements. The first minute after a change in load should not be included in the data analysis, because transient adaptation processes of the vegetative control circuits dominate during this phase.
Due to the high inter-individual variability and numerous exogenous and endogenous factors affecting the NN interval, which usually cannot be changed as part of a measurement of the NN intervals, HRV analysis should be used only in combination with a baseline assessment or with repeated measurements during work under the comparable conditions.
Some studies with reference values are available for the comparability of resting measurements. Attention must be paid to the age and biological sex dependence of HRV and to a reference population fitting to the test person.
In recent years, a number of studies has been published that use reference values to differentiate between increased and reduced HRV in individual HRV measurements. Due to the age and biological sex dependence of HRV described above, corresponding HRV reference values should also take this into account, but this has only been done in some of the published studies. An overview of studies with reference values can be found in Appendix 2. Furthermore, due to the possible genetic influence of ethnicity, a study should be used which was composed of test subjects who are similar to the working population.
Even though this provides limit values for HRV parameters, it should be borne in mind that these were calculated purely empirically. Therefore, no general health-related statements are possible. Standardised serial measurements (individual longitudinal studies) of HRV in combination with the medical history, clinical examination and other methods (e.g. questionnaires) can be valuable in explaining the individual health risks and help to evaluate the effectiveness of medical preventive measures.
Application in the fields of occupational medicine and occupational health science
In the field of occupational medicine and occupational health science, HR and HRV can be used to answer various questions.
The methods used for the recording and evaluation of HR and HRV can be used to gain an objective view of the activity of the ANS. The applications in the fields of occupational medicine and occupational health science may be summarized:
complementary examinations for the risk analysis and risk assessment to identify the core areas of work-related stress,
analysis of the individual physical and mental workload and a process-integrated measurement for an objective view of the workload response over the course of the working day,
determination of a health status indicator,
derivation of actions to be recommended for each individual e.g. workplace design,
determination of the fatigue and recovery behaviour and.
evaluation of interventions in medicine and occupational medicine.
Application to assess physical exertion
The use of HRV during physical stress, especially during dynamic muscle work, has long been established. The analysis of HRV also offers added value compared to the linear behaviour of HR due to the often two-phase or multi-phase behaviour under increasing loads.
The HRV parameters SDNN, RMSSD, Total Power, LF-Power and/or HF-Power should be used for this purpose. A minimum recording time of 5 min is recommended
The evaluation of physical exertion using the HR especially during dynamic muscle work has been established for a long time. The knowledge gained through HRV in such cases and under standardised conditions are: (i) a proven correlation between HRV parameters and (ii) the metabolic and respiratory stress indicators, (iii) the multi-phase course during progressively increasing exertion and (iv) the recovery behaviour after varying degrees of exertion [233, 234]. This enables an accurate evaluation of the physical exertion without the use of a time-consuming, cost-intensive recording method that is also partly unavailable in the ambulatory and reactive forms. In addition to the parameters of total variability, e.g. SDNN or Total Power, the HRV parameters RMSSD, LF power and HF power and the non-linear indices are suitable for the determination of the acute physical exertion. These often show a two-phase or multi-phase behaviour under increasing load and, thus, have an added value compared to the linear behaviour of the HR, especially under moderate and high physical loads [235, 236].
Application to assess mental workload
HR and HRV can be used for mental stress assessment. However, the selection of suitable HRV parameters is limited
The HRV parameters RMSSD, LF, HF, LF/HF, DQ and SD1 should be used for this purpose. A minimum recording time of 5 min is recommended
Mental stress is characterised by deflections in HR and HRV, which can therefore be used as mental stress indicators. Since the construct of mental workload is difficult to measure, HR and HRV are taken as parameters of general activation and can be used to reflect the vegetative balance of the organism. These parameters may therefore be used to draw conclusions about mental stress [101, 237–248]. In addition, HRV can also be used as an indicator of both - the psychophysical condition of the organism and the restriction in the adaptability for biopsychosocial problems. The HRV parameters, RMSSD, LF, HF or LFnu and HFnu, LF/HF as well as DQ and SD1 are considered mental workload indicators. However, ULF and VLF are not suitable. Resting HRV may not be a predictor of cognitive capacity in cross-sectional studies.
Application for risk stratification of diseases
A reduced HRV correlates with increased morbidity and mortality in some diseases (e.g. after myocardial infarction, coronary bypass surgery, heart failure, stroke, chronic obstructive pulmonary disease, and high blood pressure)
A prognostic value of HRV has currently only been proven for a few diseases. Large cohort studies have shown, among other things, that mortality was significantly higher in patients after myocardial infarction with a lower HRV compared to patients with higher HRV parameters in the post-infarction phase [77, 249, 250]. According to a meta-analysis of five studies with a total of 3,489 patients, the mortality risk in patients after myocardial infarction and an SDNN < 70 ms was 21.7%, while for n SDNN > 70 ms it was 8.1% [249]. The threeyears mortality rate after myocardial infarction was thus 2–3 times higher in the group with low HRV.
A correlation between reduced HRV (here SDNN < 93 ms) and recurrence of a coronary event was also found in patients after coronary bypass surgery. During an average three-year follow-up period, 13 out of 74 patients with reduced HRV suffered such an event, while this only affected 3 out of 132 patients in the comparison group (SDNN ≥ 93 ms) [251]. A correlation between low HRV and overall mortality or cardiac endpoints was also found for patients with heart failure [252] and in patients with strokes, the individual HRV after the stroke correlated with the long-term outcome [122].
Schmidt et al. [253] were able to show in intensive care patients with multiple organ failure that the logarithmic value of the frequency-related HRV parameter VLF (lnVLF) allows an estimate of mortality in the short-term prognosis (up to 60 days). A systematic review of the relationship between chronic obstructive pulmonary disease and HRV reported that the reduction in HRV correlates with the severity of COPD [78]. Furthermore, there appears to be a correlation between a higher HRV and longer survival in the context of tumor disease [254, 255]. In addition, it has been shown that HRV is reduced in individuals diagnosed with type I diabetes mellitus even before clinical signs of autonomic dysfunction appear [256].
There also appears to exist a correlation between a lower HRV and later manifestation of hy-pertension. In a group of 2,061 subjects controlled for age, biological sex, ethnicity, current smoking status, diabetes mellitus and educational status, subjects in the lowest quartile of HRV showed a 2.44-fold increased risk of new manifestations of hypertension after three years [257]. Based on data from the Framingham Heart Study (2,024 subjects), Singh et al. [258] were able to show an increased risk of developing hypertension with reduced HRV in men, but not in women. Schroeder et al. [259] studying 11,061 subjects, found in those individuals which belonged to the lowest quartile - in relation to the RMSSD value - a 1.36 higher risk of developing high blood pressure compared to individuals of the highest quartile.
Reduced HRV correlates with prognosis scores for the occurrence of cardiovascular events
A significant correlation between the reduction of the HRV parameter RMSSD and various prognosis scores for the occurrence of coronary heart disease or stroke was demonstrated in a collective of 11,994 subjects from the Mannheim industrial cohort [260]. This could be shown for the PROCAM score according to Assmann et al. [261], for the Coronary Heart Disease Framingham Score [262] and for the cardiovascular prognosis indicators SCORE, related to the risk of coronary heart disease (SCORE-CHD) and cardiovascular disease (SCORE-CVD) according to Conroy et al. [263].
Application to evaluate preventive measures
HRV can be used as part of the evaluation of prevention measures in compliance with the quality criteria. Repeated HRV measurements should be used for this purpose
HRV has been established particularly as a useful parameter for the evaluation of preventive measures like stress reduction courses, dietary changes, judicious use of stimulants, changes in eating behaviour, sport activities including the preventive monitoring of overtraining syndromes [264, 265] and measures to reduce weight in order to evaluate the success of the corresponding preventive or interventional measures in longitudinal comparisons [266]. For example, a change in the sympathetic-parasympathetic balance and a higher parasympathetic baseline activity (e.g. raised SDNN or RMSSD, reduced LF/HF ratio) indicate efficacy of the preventive measures.
Application in biofeedback
HR and HRV parameters can be used to objectify relaxation effects in the context of biofeedback. Long-term effects could not be shown with HRV-based biofeedback methods
HR and HRV have been used for biofeedback in cases of stress recovery and recently also in the treatment of posttraumatic stress disorder e.g. for an objective view on the effects of stress relaxation [156, 267–270]. However, until now, only short-term effects of such interventions have been observed. It has not yet been possible to demonstrate a long-term effect [270]. In addition, suitable and validated methods must be used to utilise the short-term effects of HRV biofeedback methods.
With reference to the determination and the evaluation of HRV (see Chap. 4), it is inevitable that the biofeedback methods, which determine the HRV with the help of pulsoximeter or respiratory activity, cannot be seen as valid measurement methods to assess HRV and therefore cannot be recommended for HRV-based biofeedback.
Conclusions
The practicability of the HR and HRV analysis on a daily basis for field studies at workplaces has been proven. These analytical methods can be used with a goal-oriented approach for various tasks when the methodological requirements are met. Under these conditions, HR and HRV can be recommended for the use not only in research institutes, but also for practising by occupational physicians and company doctors. This might help to improve diagnostic efficiency and to elucidate heart and health related mechanisms in the field of modern occupational medicine facing an ever-changing working environment and a demographic change in general. For a practical use a checklist is attached in the appendix to support scientists and users.
Supplementary Information
Abbreviations
- ANS
Autonomic nervous system
- ApEn
Approximate Entropy
- BMI
Body weight index
- bpm
beats/min
- COPD
Chronic obstructive pulmonary disease
- CPL
Continuous performance limit
- DFA
Detrended Fluctuation Analysis
- HIIT
High-intensity interval training
- HR
Heart rate
- HRmax
Maximum heart rate
- HRnet
Net heart rate
- HRrecovery
Recovery heart rate
- HRreference
Reference heart rate
- HRrest
Resting HR
- HRV
Heart rate variability
- HRwork
Working heart rate
- MV
Mean value
- n.o
No unit assigned
- n/a
Not available
- PNS
Parasympathetic nervous system
- RSA
Respiratory Sinus Arrhythmia
- SampEn
Sample Entropy
- SD
Standard deviation
- SNS
Sympathetic nervous system
Appendix 1: parameters of HRV
| Method | Measure of variability | Other names | Unit of measurement | Definition and explanation | Indicator of … | Activity as part of the autonomic nervous system | Recommendation for evaluation time |
|---|---|---|---|---|---|---|---|
| Time domain methods | |||||||
| Statistical | SDNN | RRSD, SD, SDRR | ms | Standard deviation of NN intervals within the measurement area | Short-term and long-term variability [43] | Sympathetic and parasympathetic [44] | |
| CVRR | CV | n.o. | Coefficient of variation of NN intervals, equal to the standard deviation of NN intervals divided by the mean of NN intervals | No clear assignment | No clear assignment | ||
| SDANN | ms | Standard deviation of the average of all consecutive 5-minute NN intervals for estimation of HRV for long-term measurements | Long-term variability [44] | No clear assignment | Long-term recording, ideally 24 h | ||
| RMSSD | R-MSSD, rMSSD | ms | Root Mean Square of successive differences: Square root of the arithmetic mean of the squared differences between adjacent NN intervals | Short-term variability [43] | Parasympathetic | ||
| SDNN-Index | SDANN-Index, SDNNi | ms | Standard deviation of the average of all normal NN intervals of 5-min segments from the 24-hour ECG | Long-term variability, short-term variability [43] | No clear assignment | Long-term recording, ideally 24 h | |
| NN 50 | n.o. | The number of pairs of neighbouring NN intervals that deviate from one another by more than 50 ms | Spontaneous variability, long-term variability | Parasympathetic [43] | |||
| pNN 50 | % | Percentage of consecutive NN intervals that deviate from one another by more than 50 ms | Spontaneous variability, long-term variability | Parasympathetic [44] | |||
| SAa | ms | Absolute sinus arrhythmia: sum of the differences of the consecutive NN intervals, divided by their number | No clear assignment | No clear assignment | |||
| geometric | HRV triangular Index | RR triangular index | n.o. | The integral of the density distribution (number of all NN intervals divided by the maximum (height) of the density distribution) or ratio of the absolute number of all NN intervals to the number of all modal NN intervals | Total variability | No clear assignment | At least 20 min |
| TINN | ms | Triangular interpolation of NN interval histogram: is the baseline width of the minimum square difference of the triangular interpolation for the highest value of the histogram of all the NN intervals | No clear assignment | No clear assignment | At least 20 min | ||
| Frequency domain methods | |||||||
| FFT (Fast Fourier Transformation) and Autoregressive Model (AR) | TP | ms2 | Total power: total performance or total spectrum; corresponds to energy density between 0.00001 to 0.4 Hz | Total variability | No clear assignment | ||
| ULF | ms2 | Ultra very low frequency: power density spectrum below 0.003 Hz | No clear assignment | No clear assignment | |||
| ULF% | % | Percentage of ULF in the total spectrum | No clear assignment | No clear assignment | |||
| VLF | ms2 | Very low frequency power: power density spectrum in the frequency range of 0.003 to 0.04 Hz | No clear assignment | Parasympathetic [45] | |||
| VLF% | % | Percentage of VLF in the total spectrum | No clear assignment | Parasympathetic [45] | |||
| LF | B Band | ms2 | Low frequency power: power density spectrum in the frequency range of 0.04 to 0.15 Hz | No clear assignment | Sympathetic and parasympathetic | At least 5 min [46] | |
| LF% | relative B Band | % | Percentage of LF in the total spectrum | No clear assignment | Sympathetic and parasympathetic | At least 5 min [46] | |
| HF | C Band, respiratory sinus arrhythmia, Respiratory | ms2 | High frequency power: power density spectrum in the frequency range of 0.15 to 0.40 Hz | No clear assignment | Parasympathetic [47] | At least 5 min [46] | |
| HF% | C Band, respiratory sinus arrhythmia, Respiratory band | % | Percentage of HF in the total spectrum | No clear assignment | Parasympathetic | At least 5 min [46] | |
| LF nu | LF n.U., LF norm | nu | Low frequency normalised unit: standardized power or power of the LF in standardized units; corresponds with LF/(TP-VLF) x 1001 | No clear assignment | Sympathetic and parasympathetic | At least 5 min [46] | |
| HF nu | HF n.U., HF norm | nu | High frequency normalized unit: standardized power or power of the HF in standardized units; corresponds with HF/(Total Power – VLF) x 1001 | No clear assignment | Parasympathetic [47] | At least 5 min [46] | |
| LF/HF | Quotient of LF and HF; LF/HF Ratio | n.o. | Quotient of the spectrum in LF and the spectrum in HF | No clear assignment | Ratio or coefficient or ratio between LF and HF band power [47] | At least 5 min [46] | |
| VLF Peak | Hz | Very low frequency peak: frequency peak in the VLF band; thermoregulation peak | No clear assignment | No clear assignment | |||
| LF Peak | Hz | Low frequency peak: frequency peak in the LF band; baroreflex peak | No clear assignment | No clear assignment | At least 5 min [46] | ||
| HF Peak | Hz | High frequency peak: frequency peak HF band; respiratory peak | No clear assignment | No clear assignment | At least 5 min [46] | ||
| Non-linear methods | |||||||
| Poincaré-Plot | DL | DL, Lorenz length | ms | Length of the major axis of the ellipse (95% confidence region) | Long-term variability | No clear assignment | |
| DQ | Dq, DW, Lorenz width | ms | Length of the minor axis of the ellipse (95% confidence region) | Short-term variability | No clear assignment | ||
| SD1 | SDQ, SDw, stdb, SOQ, SD-horizontal, SOW | ms | Standard deviation of the distances of the points from the minor axis2 | Short-term variability [40, 48] | Parasympathetic [49] | ||
| SD2 | SDL, SD-vertical, stda, SOL | ms | Standard deviation of the distances of the points from the major axis2 | Long-term variability [40, 48] | Sympathetic and parasympathetic [49] | ||
| Detrended fluctuation analysis (DFA) or trend-correcting fluctuation analysis | DFA1 | alpha 1 | n.o. | The degree of coincidence /correlation; ranges from 0.5 (coincidental) to 1.5 (correlated) with normal value of 1.0; is often used as a non-linear parameter for short NN interval data | Short-term variability [40, 50, 51] | No clear assignment | |
| DFA2 | alpha 2 | n.o. | Is often used as a non-linear parameter for RR intervals of longer durations of recording, reduced values are associated with a bad prognosis | Long-term variability [40, 50, 51] | No clear assignment | ||
| D2 | n.o. | Correlation dimension | No clear assignment | No clear assignment | |||
| Recurrence plot | Lmean | beats | Mean line length | No clear assignment | No clear assignment | ||
| Lmax | beats | Max line length | No clear assignment | No clear assignment | |||
| REC | % | Recurrence rate | No clear assignment | No clear assignment | |||
| DET | % | Determinism | No clear assignment | No clear assignment | |||
| ShanEn | beats | Shannon Entropy | No clear assignment | No clear assignment | |||
| Others | ApEn | n.o. | Approximate entropy, High values correspond to high variability, values independent of the recording length [52] | Short-term variability [40] | No clear assignment | ||
| SampEn | n.o. | Sample entropy: High values correspond to high variability, values independent of the recording length [52] | Short-term variability [40] | No clear assignment | |||
n.o no unit assigned.
1 LF nu and HF nu behave reciprocally to each other, the sum of both parameters equals 100%.
2 SD1 and SD2 correlate directly with the HRV parameters SDNN and the SDNN index and are therefore not classified by some authors as non-linear parameters, but rather as time-related HRV parameters [53].
Appendix 2: overview of the various studies with details of the standardised values
| Study | Probands/ age group | N | Duration of HRV-analysis | Age-related | Biological sex-related | Specification of the standard values as… |
|---|---|---|---|---|---|---|
| Bigger et al. 1995 [245]/ ESC/NASPE 1996 [47] | adults | 274 | 24 h | no | no | MV ± SD |
| ESC/NASPE 1996 [47] | adults | n/a | 5 min | no | no | MV ± SD |
| Nunan et al. 2010 [246] | systematic review | 1–36 studies | 5 min | no | yes | MV ± SD |
| Kim & Woo 2011 [247] | adult participants in check-up examinations in Korea | 3,396 | 5 min | yes | yes | MV ± SD |
| Voss et al. 2012 [81] | representative adult population from Germany | 1,906 | 5 min | yes | no | percentile |
| Zeng et al. 2014 [248] | probands from China | 371 | 15 min | yes | yes, but no difference detected | percentile |
| Voss et al. 2015 [242] | representative adult population from Germany | 1,906 | 5 min | yes | yes | MV ± SD |
| Sammito & Böckelmann 2017 [243] | adult population from Germany (20–60 years) | 695 | 24 h | yes | yes | percentile |
| Sammito & Böckelmann 2017 [244, 244] | adult population from Germany (20–60 years) | 673 | 5 min | yes | yes | percentile |
MV Mean value, SD Standard deviation, n/a not available
Authors' contributions
All authors have written this guideline in a consensues process. All authors read and ap-proved the final manuscript.
Funding
There was no additional funding.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
SAM declared that he is an active Bundeswehr (Medical Service) officer and work for the Federal Ministry of Defence. This paper reflects the opinion of the authors and not necessarily the opinion of the German Department of Defense or the Surgeon General of the Air Force. AK got payment from the IG Metall, Germany for a presentation and got study support from the BG Verkehr, Germany. AD got payment for advising the Dr. Franz Köhler Chemie GmbH, Germany, Else Kröner-Fresenius-Stiftung, Germany and the European Space Agency, Netherland. IB declared that she was paid from the Grüntenthal GmbH, Germany for a presentation. BT and KMB declared that they have no competing interests.
Footnotes
NN interval = normal-to-normal interval, used synonymously with the terms RR interval, IBI (interbeat interval), cycle length variability, heart period variability.
In some load protocols, the HR is also analysed after 3–5 min after the end of the load.
Older publications often use the term work pulse rather than work HR.
The following procedure is recommended: The measurement should preferably be taken in a sitting position, after at least five, ideally 15 min of rest and should be determined by two consecutive measurements. The measurement duration should be at least 30 s. The person should have avoided smoking, eating, caffeine or physical exertion half an hour before the measurement [28, 29].
The terms, Lorenz plot or Scatter plot, are used as synonyms.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hottenrott K. Trainingskontrolle mit Herzfrequenz-Messgeräten. Aachen, Germany: Meyer & Meyer Verlag; 2007. [Google Scholar]
- 2.Piper HM. Herzerregung. In: Schmidt RF, Lang F, Heckmann M, editors. Physiologie des Menschen mit Pathophysiologie. 31. Heidelberg, Germany: Springer Medizin Verlag; 2011. pp. 517–538. [Google Scholar]
- 3.Fox SM, Skinner JS. Uber die Beziehung zwischen körperlicher Aktivität und Koronarer Herzkrankheit. [Interrelation of physical activity and coronary heart disease] Verh Dtsch Ges Kreislaufforsch. 1971;37:82–93. [PubMed] [Google Scholar]
- 4.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 5.Guzzetti S, Magatelli R, Borroni E, Mezzetti S. Heart rate variability in chronic heart failure. Auton Neurosci. 2001;90:102–105. doi: 10.1016/S1566-0702(01)00274-0. [DOI] [PubMed] [Google Scholar]
- 6.Gulati M, Black HR, Shaw LJ, Arnsdorf MF, Merz CNB, Lauer MS, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353:468–475. doi: 10.1056/NEJMoa044154. [DOI] [PubMed] [Google Scholar]
- 7.Morris CK, Myers J, Froelicher VF, Kawaguchi T, Ueshima K, Hideg A. Nomogram based on metabolic equivalents and age for assessing aerobic exercise capacity in men. J Am Coll Cardiol. 1993;22:175–182. doi: 10.1016/0735-1097(93)90832-l. [DOI] [PubMed] [Google Scholar]
- 8.Such U, Meyer T. Die maximale Herzfrequenz. Dtsch Z Sportmed. 2010;61:310–311. [Google Scholar]
- 9.Bulthuis Y, Drossaers-Bakker W, Oosterveld F, van der Palen J, van de Laar M. Arm crank ergometer is reliable and valid for measuring aerobic capacity during submaximal exercise. J Strength Cond Res. 2010;24:2809–2815. doi: 10.1519/JSC.0b013e3181e31242. [DOI] [PubMed] [Google Scholar]
- 10.Grandjean E. Physiologische Arbeitsgestaltung. Landsberg, Germany: ecomed; 1991. [Google Scholar]
- 11.Hettinger T, Müller BH. Ergonomie. In: Reichel G, editor. Grundlagen der Arbeitsmedizin. Stuttgart, Berlin, Köln, Mainz, Germany: Kohlhammer; 1985. pp. 427–472. [Google Scholar]
- 12.Hettinger T. Klimawirkungen auf den Menschen. In: Konietzko J, Dupuis H, editors. Handbuch der Arbeitsmedizin. Landsberg, Germany: ecomed; 1989. pp. 1–16. [Google Scholar]
- 13.Triebig G, Kenter M, Schiele R, editors. Arbeitsmedizin – Handbuch für Theorie und Praxis. 4th ed. Stuttgart: Gentner Verlag; 2014.
- 14.Müller EA, editor. Handbuch der gesamten Arbeitsmedizin: Band 1. Berlin: Urban und Schwarzenberg; 1961.
- 15.Awad M, Czer LSC, Hou M, Golshani SS, Goltche M, de Robertis M, et al. Early denervation and later reinnervation of the heart following cardiac transplantation: a review. J Am Heart Assoc. 2016. 10.1161/JAHA.116.004070. [DOI] [PMC free article] [PubMed]
- 16.Eckoldt K. Untersuchungen über die Wirkungen der vegetativen Herznerven mit Hilfe von unblutigen Meßverfahren. Germany: Humboldt-Universität Berlin; 1975.
- 17.Jose AD, Taylor RR. Autonomic blockade by propranolol and atropine to study intrinsic myocardial function in man. J Clin Invest. 1969;48:2019–2031. doi: 10.1172/JCI106167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jose AD. Effect of combined sympathetic and parasympathetic blockade on heart rate and cardiac function in man. Am J Cardiol. 1966;18:476–478. doi: 10.1016/0002-9149(66)90073-7. [DOI] [PubMed] [Google Scholar]
- 19.Griebenow R, Gülker H, editors. Autonomes Nervensystem und Herzrhythmusstörungen. Stuttgart, New York: Thieme Verlag; 1990.
- 20.Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–48. 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed]
- 21.D’Souza A, Bucchi A, Johnsen AB, Logantha SJRJ, Monfredi O, Yanni J, et al. Exercise training reduces resting heart rate via downregulation of the funny channel HCN4. Nat Commun. 2014;5:3775. 10.1038/ncomms4775. [DOI] [PMC free article] [PubMed]
- 22.Dobbs WC, Fedewa MV, MacDonald HV, Holmes CJ, Cicone ZS, Plews DJ, Esco MR. The accuracy of acquiring heart rate variability from portable devices: a systematic review and meta-analysis. Sports Med. 2019;49:417–435. doi: 10.1007/s40279-019-01061-5. [DOI] [PubMed] [Google Scholar]
- 23.Sammito S, Bockelmann I. Options and limitations of heart rate measurement and analysis of heart rate variability by mobile devices: a systematic review. [Möglichkeiten und Einschränkungen der Herzfrequenzmessung und der Analyse der Herzfrequenzvariabilitat mittels mobiler Messgerate: eine systematische Literaturübersicht] Herzschrittmacherther Elektrophysiol. 2016;27:38–45. doi: 10.1007/s00399-016-0419-5. [DOI] [PubMed] [Google Scholar]
- 24.Schäfer A, Vagedes J. How accurate is pulse rate variability as an estimate of heart rate variability? A review on studies comparing photoplethysmographic technology with an electrocardiogram. Int J Cardiol. 2013;166:15–29. doi: 10.1016/j.ijcard.2012.03.119. [DOI] [PubMed] [Google Scholar]
- 25.Kwon O, Jeong J, Kim HB, Kwon IH, Park SY, Kim JE, Choi Y. Electrocardiogram sampling frequency Range acceptable for heart rate variability analysis. Healthc Inf Res. 2018;24:198. doi: 10.4258/hir.2018.24.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-González MA, Ramos-Castro J, Fernández-Chimeno M. The effect of electrocardiographic lead choice on RR time series. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:1933–1936. doi: 10.1109/IEMBS.2011.6090546. [DOI] [PubMed] [Google Scholar]
- 27.Weippert M, Rieger A, Stoll R. Individuell unterschiedliche Vergleichbarkeit von R-R-Detektionen mit verschiedenen Messgeräten. In: Hottenrott K, Hoos O, Esperer HD, editors. Herzfrequenzvariabilität: Gesundheitsförderung, Trainingsteuerung, Biofeedback. Hamburg, Germany: Czwalina; 2011. pp. 212–220. [Google Scholar]
- 28.Britton A, Shipley M, Malik M, Hnatkova K, Hemingway H, Marmot M. Changes in heart rate and heart rate variability over time in middle-aged men and women in the general population (from the Whitehall II Cohort Study) Am J Cardiol. 2007;100:524–527. doi: 10.1016/j.amjcard.2007.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eyre ELJ, Duncan MJ, Birch SL, Fisher JP. The influence of age and weight status on cardiac autonomic control in healthy children: a review. Auton Neurosci. 2014;186:8–21. doi: 10.1016/j.autneu.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Esperer HD. Nichtlineare HRV-Analyse im sport: Grundlagen, Anwendungen und Limitationen. In: Hottenrott K, editor. Herzfrequenzvariabilität: Methoden und Anwendungen in Sport und Medizin. Hamburg, Germany: Czwalina; 2006. pp. 64–97. [Google Scholar]
- 31.Abhishekh HA, Nisarga P, Kisan R, Meghana A, Chandran S, Trichur R, Sathyaprabha TN. Influence of age and gender on autonomic regulation of heart. J Clin Monit Comput. 2013;27:259–264. doi: 10.1007/s10877-012-9424-3. [DOI] [PubMed] [Google Scholar]
- 32.Barantke M, Krauss T, Ortak J, Lieb W, Reppel M, Burgdorf C, et al. Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J Cardiovasc Electrophysiol. 2008;19:1296–1303. doi: 10.1111/j.1540-8167.2008.01257.x. [DOI] [PubMed] [Google Scholar]
- 33.Fagard RH, Pardaens K, Staessen JA. Influence of demographic, anthropometric and lifestyle characteristics on heart rate and its variability in the population. J Hypertens. 1999;17:1589–1599. doi: 10.1097/00004872-199917110-00013. [DOI] [PubMed] [Google Scholar]
- 34.Fagard RH. A population-based study on the determinants of heart rate and heart rate variability in the frequency domain. Verh K Acad Geneeskd Belg. 2001;63:57–89; discussion 90 − 1. [PubMed] [Google Scholar]
- 35.Kuo TB, Lin T, Yang CC, Li CL, Chen CF, Chou P. Effect of aging on gender differences in neural control of heart rate. Am J Physiol. 1999;277:H2233–2239. doi: 10.1152/ajpheart.1999.277.6.H2233. [DOI] [PubMed] [Google Scholar]
- 36.Felber Dietrich D, Schindler C, Schwartz J, Barthélémy J-C, Tschopp J-M, Roche F, et al. Heart rate variability in an ageing population and its association with lifestyle and cardiovascular risk factors: results of the SAPALDIA study. Europace. 2006;8:521–529. doi: 10.1093/europace/eul063. [DOI] [PubMed] [Google Scholar]
- 37.Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31:593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- 38.Alvares GA, Quintana DS, Hickie IB, Guastella AJ. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: a systematic review and meta-analysis. J Psychiatry Neurosci. 2016;41:89–104. doi: 10.1503/jpn.140217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrari AU. Modifications of the cardiovascular system with aging. Am J Geriatr Cardiol. 2002;11:30–33. doi: 10.1111/1467-8446.00044-i1. [DOI] [PubMed] [Google Scholar]
- 40.Fukusaki C, Kawakubo K, Yamamoto Y. Assessment of the primary effect of aging on heart rate variability in humans. Clin Auton Res. 2000;10:123–130. doi: 10.1007/BF02278016. [DOI] [PubMed] [Google Scholar]
- 41.Greiser KH, Kluttig A, Schumann B, Swenne CA, Kors JA, Kuss O, et al. Cardiovascular diseases, risk factors and short-term heart rate variability in an elderly general population: the CARLA study 2002–2006. Eur J Epidemiol. 2009;24:123–142. doi: 10.1007/s10654-009-9317-z. [DOI] [PubMed] [Google Scholar]
- 42.Haerting J, Kluttig A, Greiser KH, Nuding S, Werdan K. A cohort study investigating risk factors for cardiovascular disease in an urban elderly East-German population (CARLA study). [Kohortenstudie zu Risikofaktoren für Herz-Kreislauf-Krankheiten in einer urbanen älteren Ostdeutschen Allgemeinbevölkerung (CARLA-Studie)] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55:795–800. doi: 10.1007/s00103-012-1493-4. [DOI] [PubMed] [Google Scholar]
- 43.Shiogai Y, Stefanovska A, McClintock PVE. Nonlinear dynamics of cardiovascular ageing. Phys Rep. 2010;488:51–110. doi: 10.1016/j.physrep.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stein PK, Barzilay JI, Chaves PHM, Domitrovich PP, Gottdiener JS. Heart rate variability and its changes over 5 years in older adults. Age Ageing. 2009;38:212–218. doi: 10.1093/ageing/afn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voss A, Heitmann A, Schroeder R, Peters A, Perz S. Short-term heart rate variability–age dependence in healthy subjects. Physiol Meas. 2012;33:1289–1311. doi: 10.1088/0967-3334/33/8/1289. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J. Effect of age and sex on heart rate variability in healthy subjects. J Manipulative Physiol Ther. 2007;30:374–379. doi: 10.1016/j.jmpt.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Boudreau P, Yeh W-H, Dumont GA, Boivin DB. Circadian variation of heart rate variability across sleep stages. Sleep. 2013;36:1919–1928. doi: 10.5665/sleep.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sammito S, Sammito W, Böckelmann I. The circadian rhythm of heart rate variability: a systematic review of the literature. Biol Rhythm Res. 2016;47:717–730. doi: 10.1080/09291016.2016.1183887. [DOI] [Google Scholar]
- 49.Hill LK, Hu DD, Koenig J, Sollers JJ, Kapuku G, Wang X, et al. Ethnic differences in resting heart rate variability: a systematic review and meta-analysis. Psychosom Med. 2015;77:16–25. doi: 10.1097/PSY.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bett GCL. Hormones and sex differences: changes in cardiac electrophysiology with pregnancy. Clin Sci. 2016;130:747–759. doi: 10.1042/CS20150710. [DOI] [PubMed] [Google Scholar]
- 51.Ekholm EM, Erkkola RU. Autonomic cardiovascular control in pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:29–36. doi: 10.1016/0301-2115(95)02255-4. [DOI] [PubMed] [Google Scholar]
- 52.Lucini D, Strappazzon P, Dalla Vecchia L, Maggioni C, Pagani M. Cardiac autonomic adjustments to normal human pregnancy: insight from spectral analysis of R-R interval and systolic arterial pressure variability. J Hypertens. 1999;17:1899–1904. doi: 10.1097/00004872-199917121-00019. [DOI] [PubMed] [Google Scholar]
- 53.Lutfi MF, Sukkar MY. The effect of gender on heart rate variability in asthmatic and normal healthy adults. Int J Health Sci (Qassim) 2011;5:146–154. [PMC free article] [PubMed] [Google Scholar]
- 54.Agelink MW, Malessa R, Baumann B, Majewski T, Akila F, Zeit T, Ziegler D. Standardized tests of heart rate variability: normal ranges obtained from 309 healthy humans, and effects of age, gender, and heart rate. Clin Auton Res. 2001;11:99–108. doi: 10.1007/BF02322053. [DOI] [PubMed] [Google Scholar]
- 55.Jensen-Urstad K, Storck N, Bouvier F, Ericson M, Lindblad LE, Jensen-Urstad M. Heart rate variability in healthy subjects is related to age and gender. Acta Physiol Scand. 1997;160:235–241. doi: 10.1046/j.1365-201X.1997.00142.x. [DOI] [PubMed] [Google Scholar]
- 56.Snieder H, van Doornen, Lorenz JP, Boomsma DI, Thayer JF. Sex differences and heritability of two indices of heart rate dynamics: a twin study. Twin Res Hum Genet. 2007;10:364–372. doi: 10.1375/twin.10.2.364. [DOI] [PubMed] [Google Scholar]
- 57.Sookan T, McKune AJ. Heart rate variability in physically active individuals: reliability and gender characteristics. Cardiovasc J Afr. 2012;23:67–72. doi: 10.5830/CVJA-2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsuji H, Venditti FJ, Manders JR, Evans ES, Larson JC, Feldman MG, Levy CL. Determinants of heart rate variability. J Am Coll Cardiol. 1996;28:1539–1546. doi: 10.1016/s0735-1097(96)00342-7. [DOI] [PubMed] [Google Scholar]
- 59.Koenig J, Thayer JF. Sex differences in healthy human heart rate variability: a meta-analysis. Neurosci Biobehav Rev. 2016;64:288–310. doi: 10.1016/j.neubiorev.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 60.Huang W, Zhu T, Pan X, Hu M, Lu S-E, Lin Y, et al. Air pollution and autonomic and vascular dysfunction in patients with cardiovascular disease: interactions of systemic inflammation, overweight, and gender. Am J Epidemiol. 2012;176:117–126. doi: 10.1093/aje/kwr511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramaekers D, Ector H, Demyttenaere K, Rubens A, van de Werf F. Association between cardiac autonomic function and coping style in healthy subjects. Pacing Clin Electrophysiol. 1998;21:1546–1552. doi: 10.1111/j.1540-8159.1998.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 62.Arab C, Dias DPM, Barbosa RTA, de Carvalho TD, Valenti VE, Crocetta TB, et al. Heart rate variability measure in breast cancer patients and survivors: a systematic review. Psychoneuroendocrinology. 2016;68:57–68. doi: 10.1016/j.psyneuen.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 63.Bouvy ML, Heerdink ER, Leufkens HGM, Hoes AW. Predicting mortality in patients with heart failure: a pragmatic approach. Heart. 2003;89:605–609. doi: 10.1136/heart.89.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biswas PK, Basu S, Mitra KK, Chowdhury SP, Chatterjee BP, Das Biswas A, et al. Heart rate variability in dilated cardiomyopathy. Indian Heart J. 2000;52:187–191. [PubMed] [Google Scholar]
- 65.Davies LC, Colhoun H, Coats AJS, Piepoli M, Francis DP. A noninvasive measure of baroreflex sensitivity without blood pressure measurement. Am Heart J. 2002;143:441–447. doi: 10.1067/mhj.2002.121263. [DOI] [PubMed] [Google Scholar]
- 66.Lasisi GT, Adebola AP, Ogah OS, Daniel FA. Prevalence of ventricular arrhythmias and heart rate variability pattern in chronic heart failure. Niger Postgrad Med J. 2012;19:157–162. [PubMed] [Google Scholar]
- 67.Scalvini S, Volterrani M, Zanelli E, Pagani M, Mazzuero G, Coats AJ, Giordano A. Is heart rate variability a reliable method to assess autonomic modulation in left ventricular dysfunction and heart failure? Assessment of autonomic modulation with heart rate variability. Int J Cardiol. 1998;67:9–17. doi: 10.1016/s0167-5273(98)00252-6. [DOI] [PubMed] [Google Scholar]
- 68.Carthy ER. Autonomic dysfunction in essential hypertension: a systematic review. Ann Med Surg (Lond) 2014;3:2–7. doi: 10.1016/j.amsu.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Silvetti MS, Drago F, Ragonese P. Heart rate variability in healthy children and adolescents is partially related to age and gender. Int J Cardiol. 2001;81:169–174. doi: 10.1016/s0167-5273(01)00537-x. [DOI] [PubMed] [Google Scholar]
- 70.Bigger JT, Kleiger RE, Fleiss JL, Rolnitzky LM, Steinman RC, Miller JP. Components of heart rate variability measured during healing of acute myocardial infarction. Am J Cardiol. 1988;61:208–215. doi: 10.1016/0002-9149(88)90917-4. [DOI] [PubMed] [Google Scholar]
- 71.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 72.Haas J, Liebrich A, Himmrich E, Treese N. Kurzzeitmessung der Herzfrequenzvariabilität bei Postinfarktpatienten. Herzschrittmachertherapie und Elektrophysiologie. 2000;11:102–109. doi: 10.1007/s003990070044. [DOI] [Google Scholar]
- 73.Klingenheben T, Ptaszynski P, Schimpf R, Schuchert A. Synkope. [Syncope] Herzschrittmacherther Elektrophysiol. 2013;24:70–1. doi: 10.1007/s00399-013-0256-8. [DOI] [PubMed] [Google Scholar]
- 74.Kovar D, Cannon CP, Bentley JH, Charlesworth A, Rogers WJ. Does initial and delayed heart rate predict mortality in patients with acute coronary syndromes? Clin Cardiol. 2004;27:80–86. doi: 10.1002/clc.4960270207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lombardi F, Sandrone G, Mortara A, La Rovere MT, Colombo E, Guzzetti S, Malliani A. Circadian variation of spectral indices of heart rate variability after myocardial infarction. Am Heart J. 1992;123:1521–1529. doi: 10.1016/0002-8703(92)90804-5. [DOI] [PubMed] [Google Scholar]
- 76.Huikuri HV, Mäkikallio TH. Heart rate variability in ischemic heart disease. Auton Neurosci. 2001;90:95–101. doi: 10.1016/S1566-0702(01)00273-9. [DOI] [PubMed] [Google Scholar]
- 77.Huikuri HV, Stein PK. Heart rate variability in risk stratification of cardiac patients. Prog Cardiovasc Dis. 2013;56:153–159. doi: 10.1016/j.pcad.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Roque AL, Valenti VE, Massetti T, da Silva TD, Monteiro CBM, Oliveira FR, et al. Chronic obstructive pulmonary disease and heart rate variability: a literature update. Int Arch Med. 2014;7:43. doi: 10.1186/1755-7682-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mohammed J, Meeus M, Derom E, Da Silva H, Calders P. Evidence for autonomic function and its influencing factors in subjects with COPD: a systematic review. Respir Care. 2015;60:1841–1851. doi: 10.4187/respcare.04174. [DOI] [PubMed] [Google Scholar]
- 80.da Silva TD, Massetti T, Crocetta TB, de Mello Monteiro CB, Carll A, Vanderlei LCM, et al. Heart rate variability and cardiopulmonary dysfunction in patients with duchenne muscular dystrophy: a systematic review. Pediatr Cardiol. 2018;39:869–883. doi: 10.1007/s00246-018-1881-0. [DOI] [PubMed] [Google Scholar]
- 81.Koenig J, Williams DP, Kemp AH, Thayer JF. Vagally mediated heart rate variability in headache patients–a systematic review and meta-analysis. Cephalalgia. 2016;36:265–278. doi: 10.1177/0333102415583989. [DOI] [PubMed] [Google Scholar]
- 82.Barloese MCJ. A review of cardiovascular autonomic control in cluster headache. Headache. 2016;56:225–239. doi: 10.1111/head.12730. [DOI] [PubMed] [Google Scholar]
- 83.Fakhrzadeh H, Yamini-Sharif A, Sharifi F, Tajalizadekhoob Y, Mirarefin M, Mohammadzadeh M, et al. Cardiac autonomic neuropathy measured by heart rate variability and markers of subclinical atherosclerosis in early type 2 diabetes. ISRN Endocrinol. 2012;2012:168264. doi: 10.5402/2012/168264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karayannis G, Giamouzis G, Cokkinos DV, Skoularigis J, Triposkiadis F. Diabetic cardiovascular autonomic neuropathy: clinical implications. Expert Rev Cardiovasc Ther. 2012;10:747–765. doi: 10.1586/erc.12.53. [DOI] [PubMed] [Google Scholar]
- 85.Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol. 2012;8:405–416. doi: 10.1038/nrendo.2012.21. [DOI] [PubMed] [Google Scholar]
- 86.Singh JP, Larson MG, O’Donnell CJ, Wilson PF, Tsuji H, Lloyd-Jones DM, Levy D. Association of hyperglycemia with reduced heart rate variability (the Framingham Heart Study) Am J Cardiol. 2000;86:309–312. doi: 10.1016/s0002-9149(00)00920-6. [DOI] [PubMed] [Google Scholar]
- 87.Benichou T, Pereira B, Mermillod M, Tauveron I, Pfabigan D, Maqdasy S, Dutheil F. Heart rate variability in type 2 diabetes mellitus: a systematic review and meta-analysis. PLoS One. 2018;13:e0195166. doi: 10.1371/journal.pone.0195166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stein PK, Barzilay JI, Domitrovich PP, Chaves PM, Gottdiener JS, Heckbert SR, Kronmal RA. The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome: the cardiovascular health study. Diabet Med. 2007;24:855–863. doi: 10.1111/j.1464-5491.2007.02163.x. [DOI] [PubMed] [Google Scholar]
- 89.Barrett EJ, Liu Z, Khamaisi M, King GL, Klein R, Klein BEK, et al. Diabetic microvascular disease: an endocrine society scientific statement. J Clin Endocrinol Metab. 2017;102:4343–4410. doi: 10.1210/jc.2017-01922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gehi AK, Lampert R, Veledar E, Lee F, Goldberg J, Jones L, et al. A twin study of metabolic syndrome and autonomic tone. J Cardiovasc Electrophysiol. 2009;20:422–428. doi: 10.1111/j.1540-8167.2008.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Assoumou HGN, Pichot V, Barthelemy JC, Dauphinot V, Celle S, Gosse P, et al. Metabolic syndrome and short-term and long-term heart rate variability in elderly free of clinical cardiovascular disease: the PROOF study. Rejuvenation Res. 2010;13:653–663. doi: 10.1089/rej.2010.1019. [DOI] [PubMed] [Google Scholar]
- 92.Chang Y-W, Lin J-D, Chen W-L, Yen C-F, Loh C-H, Fang W-H, Wu L-W. Metabolic syndrome and short-term heart rate variability in adults with intellectual disabilities. Res Dev Disabil. 2012;33:1701–1707. doi: 10.1016/j.ridd.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 93.Hemingway H, Shipley M, Brunner E, Britton A, Malik M, Marmot M. Does autonomic function link social position to coronary risk? The Whitehall II study. Circulation. 2005;111:3071–3077. doi: 10.1161/CIRCULATIONAHA.104.497347. [DOI] [PubMed] [Google Scholar]
- 94.Koskinen T, Kähönen M, Jula A, Mattsson N, Laitinen T, Keltikangas-Järvinen L, et al. Metabolic syndrome and short-term heart rate variability in young adults. The cardiovascular risk in young finns study. Diabet Med. 2009;26:354–361. doi: 10.1111/j.1464-5491.2009.02686.x. [DOI] [PubMed] [Google Scholar]
- 95.Liao D, Sloan RP, Cascio WE, Folsom AR, Liese AD, Evans GW, et al. Multiple metabolic syndrome is associated with lower heart rate variability. The atherosclerosis risk in communities study. Diabetes Care. 1998;21:2116–2122. doi: 10.2337/diacare.21.12.2116. [DOI] [PubMed] [Google Scholar]
- 96.Min KB, Min JY, Paek D, Cho SI. The impact of the components of metabolic syndrome on heart rate variability: using the NCEP-ATP III and IDF definitions. Pacing Clin Electrophysiol. 2008;31:584–591. doi: 10.1111/j.1540-8159.2008.01045.x. [DOI] [PubMed] [Google Scholar]
- 97.Stuckey MI, Tulppo MP, Kiviniemi AM, Petrella RJ. Heart rate variability and the metabolic syndrome: a systematic review of the literature. Diabetes Metab Res Rev. 2014;30:784–793. doi: 10.1002/dmrr.2555. [DOI] [PubMed] [Google Scholar]
- 98.Koenig J, Falvay D, Clamor A, Wagner J, Jarczok MN, Ellis RJ, et al. Pneumogastric (Vagus) nerve activity indexed by heart rate variability in chronic pain patients compared to healthy controls: a systematic review and meta-analysis. Pain Physician. 2016;19:E55–78. [PubMed] [Google Scholar]
- 99.Friedman BH, Thayer JF. Autonomic balance revisited: panic anxiety and heart rate variability. J Psychosom Res. 1998;44:133–151. doi: 10.1016/s0022-3999(97)00202-x. [DOI] [PubMed] [Google Scholar]
- 100.Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol. 2007;74:185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 101.Aasman J, Mulder G, Mulder LJ. Operator effort and the measurement of heart-rate variability. Hum Factors. 1987;29:161–170. doi: 10.1177/001872088702900204. [DOI] [PubMed] [Google Scholar]
- 102.Chalmers JA, Quintana DS, Abbott MJ-A, Kemp AH. Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front Psychiatry. 2014;5:80. doi: 10.3389/fpsyt.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paniccia M, Paniccia D, Thomas S, Taha T, Reed N. Clinical and non-clinical depression and anxiety in young people: a scoping review on heart rate variability. Auton Neurosci. 2017;208:1–14. doi: 10.1016/j.autneu.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 104.Mazurak N, Enck P, Muth E, Teufel M, Zipfel S. Heart rate variability as a measure of cardiac autonomic function in anorexia nervosa: a review of the literature. Eur Eat Disord Rev. 2011;19:87–99. doi: 10.1002/erv.1081. [DOI] [PubMed] [Google Scholar]
- 105.Peschel SKV, Feeling NR, Vögele C, Kaess M, Thayer JF, Koenig J. A meta-analysis on resting state high-frequency heart rate variability in bulimia nervosa. Eur Eat Disord Rev. 2016;24:355–365. doi: 10.1002/erv.2454. [DOI] [PubMed] [Google Scholar]
- 106.Sammito S, Thielmann B, Zimmermann P, Böckelmann I. Einfluss einer Posttraumatischen Belastungsstörung auf die Herzfrequenzvariabilität als Marker des autonomen Nervensystems - eine systematische Literaturübersicht. [Influence of post-traumatic stress disorder on heart rate variability as marker of the autonomic nervous system - a systematic review] Fortschr Neurol Psychiatr. 2015;83:30–37. doi: 10.1055/s-0034-1398779. [DOI] [PubMed] [Google Scholar]
- 107.Berger S, Kliem A, Yeragani V, Bär K-J. Cardio-respiratory coupling in untreated patients with major depression. J Affect Disord. 2012;139:166–171. doi: 10.1016/j.jad.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 108.Birkhofer A, Schmidt G, Förstl H. Herz und Hirn - Die Auswirkungen psychischer Erkrankungen und ihrer Therapie auf die Herzfrequenzvariabilität. [Heart and brain - the influence of psychiatric disorders and their therapy on the heart rate variability] Fortschr Neurol Psychiatr. 2005;73:192–205. doi: 10.1055/s-2004-830109. [DOI] [PubMed] [Google Scholar]
- 109.Kim CK, McGorray SP, Bartholomew BA, Marsh M, Dicken T, Wassertheil-Smoller S, et al. Depressive symptoms and heart rate variability in postmenopausal women. Arch Intern Med. 2005;165:1239–1244. doi: 10.1001/archinte.165.11.1239. [DOI] [PubMed] [Google Scholar]
- 110.Kapfhammer HP. Der Zusammenhang von Depression, Angst und Herzerkrankung - Eine psychosomatische Herausforderung. [The relationship between depression, anxiety and heart disease - a psychosomatic challenge] Psychiatr Danub. 2011;23:412–424. [PubMed] [Google Scholar]
- 111.Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 112.Stapelberg NJ, Hamilton-Craig I, Neumann DL, Shum DHK, McConnell H. Mind and heart: heart rate variability in major depressive disorder and coronary heart disease - a review and recommendations. Aust N Z J Psychiatry. 2012;46:946–957. doi: 10.1177/0004867412444624. [DOI] [PubMed] [Google Scholar]
- 113.Bassett D. A literature review of heart rate variability in depressive and bipolar disorders. Aust N Z J Psychiatry. 2016;50:511–519. doi: 10.1177/0004867415622689. [DOI] [PubMed] [Google Scholar]
- 114.Brown L, Karmakar C, Gray R, Jindal R, Lim T, Bryant C. Heart rate variability alterations in late life depression: a meta-analysis. J Affect Disord. 2018;235:456–466. doi: 10.1016/j.jad.2018.04.071. [DOI] [PubMed] [Google Scholar]
- 115.Lotufo PA, Valiengo L, Benseñor IM, Brunoni AR. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia. 2012;53:272–282. doi: 10.1111/j.1528-1167.2011.03361.x. [DOI] [PubMed] [Google Scholar]
- 116.Koenig J, Kemp AH, Feeling NR, Thayer JF, Kaess M. Resting state vagal tone in borderline personality disorder: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:18–26. doi: 10.1016/j.pnpbp.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 117.Faurholt-Jepsen M, Kessing LV, Munkholm K. Heart rate variability in bipolar disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;73:68–80. doi: 10.1016/j.neubiorev.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 118.Carr O, de Vos M, Saunders KEA. Heart rate variability in bipolar disorder and borderline personality disorder: a clinical review. Evid Based Ment Health. 2018;21:23–30. doi: 10.1136/eb-2017-102760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Clamor A, Lincoln TM, Thayer JF, Koenig J. Resting vagal activity in schizophrenia: meta-analysis of heart rate variability as a potential endophenotype. Br J Psychiatry. 2016;208:9–16. doi: 10.1192/bjp.bp.114.160762. [DOI] [PubMed] [Google Scholar]
- 120.Adlan AM, Lip GYH, Paton JFR, Kitas GD, Fisher JP. Autonomic function and rheumatoid arthritis: a systematic review. Semin Arthritis Rheum. 2014;44:283–304. doi: 10.1016/j.semarthrit.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 121.Dodds KL, Miller CB, Kyle SD, Marshall NS, Gordon CJ. Heart rate variability in insomnia patients: a critical review of the literature. Sleep Med Rev. 2017;33:88–100. doi: 10.1016/j.smrv.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 122.Yperzeele L, van Hooff R-J, Nagels G, de Smedt A, de Keyser J, Brouns R. Heart rate variability and baroreceptor sensitivity in acute stroke: a systematic review. Int J Stroke. 2015;10:796–800. doi: 10.1111/ijs.12573. [DOI] [PubMed] [Google Scholar]
- 123.Ralevski E, Petrakis I, Altemus M. Heart rate variability in alcohol use: a review. Pharmacol Biochem Behav. 2019;176:83–92. doi: 10.1016/j.pbb.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 124.Karpyak VM, Romanowicz M, Schmidt JE, Lewis KA, Bostwick JM. Characteristics of heart rate variability in alcohol-dependent subjects and nondependent chronic alcohol users. Alcohol Clin Exp Res. 2014;38:9–26. doi: 10.1111/acer.12270. [DOI] [PubMed] [Google Scholar]
- 125.Dehghan H, Mortazavi SB, Jafari MJ, Maracy MR. Cardiac strain between normal weight and overweight workers in hot/humid weather in the Persian Gulf. Int J Prev Med. 2013;4:1147–1153. [PMC free article] [PubMed] [Google Scholar]
- 126.Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol. 2013;305:R566–581. doi: 10.1152/ajpregu.00180.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fraley MA, Birchem JA, Senkottaiyan N, Alpert MA. Obesity and the electrocardiogram. Obes Rev. 2005;6:275–281. doi: 10.1111/j.1467-789X.2005.00199.x. [DOI] [PubMed] [Google Scholar]
- 129.de Abreu RM, Rehder-Santos P, Simões RP, Catai AM. Can high-intensity interval training change cardiac autonomic control? A systematic review. Braz J Phys Ther. 2019;23:279–289. doi: 10.1016/j.bjpt.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aubert AE, Seps B, Beckers F. Heart rate variability in athletes. Sports Med. 2003;33:889–919. doi: 10.2165/00007256-200333120-00003. [DOI] [PubMed] [Google Scholar]
- 131.Hottenrott K, Hoos O, Esperer HD. Heart rate variability and physical exercise. Current status. [Herzfrequenzvariabilität und Sport] Herz. 2006;31:544–552. doi: 10.1007/s00059-006-2855-1. [DOI] [PubMed] [Google Scholar]
- 132.Bhati P, Moiz JA, Menon GR, Hussain ME. Does resistance training modulate cardiac autonomic control? A systematic review and meta-analysis. Clin Auton Res. 2019;29:75–103. doi: 10.1007/s10286-018-0558-3. [DOI] [PubMed] [Google Scholar]
- 133.Hollmann W, Strüder HK. Sportmedizin: Grundlagen von körperlicher Aktivität, Training und Präventivmedizin. 5. Stuttgart, Germany: Schattauer; 2009. [Google Scholar]
- 134.Link MS, Homoud MK, Wang PJ, Estes NA. Cardiac arrhythmias in the athlete. Cardiol Rev. 2001;9:21–30. doi: 10.1097/00045415-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 135.Melanson EL. Resting heart rate variability in men varying in habitual physical activity. Med Sci Sports Exerc. 2000;32:1894–1901. doi: 10.1097/00005768-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 136.Shin K, Minamitani H, Onishi S, Yamazaki H, Lee M. Autonomic differences between athletes and nonathletes: spectral analysis approach. Med Sci Sports Exerc. 1997;29:1482–1490. doi: 10.1097/00005768-199711000-00015. [DOI] [PubMed] [Google Scholar]
- 137.Soares-Miranda L, Sandercock G, Vale S, Santos R, Abreu S, Moreira C, Mota J. Metabolic syndrome, physical activity and cardiac autonomic function. Diabetes Metab Res Rev. 2012;28:363–369. doi: 10.1002/dmrr.2281. [DOI] [PubMed] [Google Scholar]
- 138.Bernardi L, Piepoli MF. Autonomic nervous system adaptation during physical exercise. [Adattamenti del sistema nervoso autonomo durante esercizio fisico] Ital Heart J Suppl. 2001;2:831–839. [PubMed] [Google Scholar]
- 139.Braith RW, Edwards DG. Neurohormonal abnormalities in heart failure: impact of exercise training. Congest Heart Fail. 2003;9:70–76. doi: 10.1111/j.1527-5299.2003.00277.x. [DOI] [PubMed] [Google Scholar]
- 140.Rennie KL, Hemingway H, Kumari M, Brunner E, Malik M, Marmot M. Effects of moderate and vigorous physical activity on heart rate variability in a British study of civil servants. Am J Epidemiol. 2003;158:135–143. doi: 10.1093/aje/kwg120. [DOI] [PubMed] [Google Scholar]
- 141.Routledge FS, Campbell TS, McFetridge-Durdle JA, Bacon SL. Improvements in heart rate variability with exercise therapy. Can J Cardiol. 2010;26:303–312. doi: 10.1016/s0828-282x(10)70395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sandercock GRH, Bromley PD, Brodie DA. Effects of exercise on heart rate variability: inferences from meta-analysis. Med Sci Sports Exerc. 2005;37:433–439. doi: 10.1249/01.mss.0000155388.39002.9d. [DOI] [PubMed] [Google Scholar]
- 143.Bellenger CR, Fuller JT, Thomson RL, Davison K, Robertson EY, Buckley JD. Monitoring athletic training status through autonomic heart rate regulation: a systematic review and meta-analysis. Sports Med. 2016;46:1461–1486. doi: 10.1007/s40279-016-0484-2. [DOI] [PubMed] [Google Scholar]
- 144.Bhati P, Shenoy S, Hussain ME. Exercise training and cardiac autonomic function in type 2 diabetes mellitus: a systematic review. Diabetes Metab Syndr. 2018;12(1):69–78. 10.1016/j.dsx.2017.08.015. [DOI] [PubMed]
- 145.Valenti VE, Vanderlei LCM, Ferreira C, Fonseca FLA, Oliveira FR, Sousa FH, et al. Sidestream cigarette smoke and cardiac autonomic regulation. Int Arch Med. 2013;6:11. doi: 10.1186/1755-7682-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Felber Dietrich D, Schwartz J, Schindler C, Gaspoz J-M, Barthélémy J-C, Tschopp J-M, et al. Effects of passive smoking on heart rate variability, heart rate and blood pressure: an observational study. Int J Epidemiol. 2007;36:834–840. doi: 10.1093/ije/dym031. [DOI] [PubMed] [Google Scholar]
- 147.Dinas PC, Koutedakis Y, Flouris AD. Effects of active and passive tobacco cigarette smoking on heart rate variability. Int J Cardiol. 2013;163:109–115. doi: 10.1016/j.ijcard.2011.10.140. [DOI] [PubMed] [Google Scholar]
- 148.Wilson MD, McGlothlin JD, Rosenthal FS, Black DR, Zimmerman NJ, Bridges CD. Ergonomics. The effect of occupational exposure to environmental tobacco smoke on the heart rate variability of bar and restaurant workers. J Occup Environ Hyg. 2010;7:D44–49. doi: 10.1080/15459624.2010.483980. [DOI] [PubMed] [Google Scholar]
- 149.Benschop RJ, Geenen R, Mills PJ, Naliboff BD, Kiecolt-Glaser JK, Herbert TB, et al. Cardiovascular and immune responses to acute psychological stress in young and old women: a meta-analysis. Psychosom Med. 1998;60:290–296. doi: 10.1097/00006842-199805000-00015. [DOI] [PubMed] [Google Scholar]
- 150.Dishman RK, Nakamura Y, Garcia ME, Thompson RW, Dunn AL, Blair SN. Heart rate variability, trait anxiety, and perceived stress among physically fit men and women. Int J Psychophysiol. 2000;37:121–133. doi: 10.1016/s0167-8760(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 151.Huang C-J, Webb HE, Zourdos MC, Acevedo EO. Cardiovascular reactivity, stress, and physical activity. Front Physiol. 2013;4:314. doi: 10.3389/fphys.2013.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Looser RR, Metzenthin P, Helfricht S, Kudielka BM, Loerbroks A, Thayer JF, Fischer JE. Cortisol is significantly correlated with cardiovascular responses during high levels of stress in critical care personnel. Psychosom Med. 2010;72:281–289. doi: 10.1097/PSY.0b013e3181d35065. [DOI] [PubMed] [Google Scholar]
- 153.Chandola T, Britton A, Brunner E, Hemingway H, Malik M, Kumari M, et al. Work stress and coronary heart disease: what are the mechanisms? Eur Heart J. 2008;29:640–648. doi: 10.1093/eurheartj/ehm584. [DOI] [PubMed] [Google Scholar]
- 154.Chandola T, Heraclides A, Kumari M. Psychophysiological biomarkers of workplace stressors. Neurosci Biobehav Rev. 2010;35:51–57. doi: 10.1016/j.neubiorev.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Clays E, de Bacquer D, Crasset V, Kittel F, de Smet P, Kornitzer M, et al. The perception of work stressors is related to reduced parasympathetic activity. Int Arch Occup Environ Health. 2011;84:185–191. doi: 10.1007/s00420-010-0537-z. [DOI] [PubMed] [Google Scholar]
- 156.Lehrer P. Applied psychophysiology: beyond the boundaries of biofeedback (mending a wall, a brief history of our field, and applications to control of the muscles and cardiorespiratory systems) Appl Psychophysiol Biofeedback. 2003;28:291–304. doi: 10.1023/a:1027330909265. [DOI] [PubMed] [Google Scholar]
- 157.Järvelin-Pasanen S, Sinikallio S, Tarvainen MP. Heart rate variability and occupational stress-systematic review. Ind Health. 2018;56:500–511. doi: 10.2486/indhealth.2017-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Badra LJ, Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Respiratory modulation of human autonomic rhythms. Am J Physiol Heart Circ Physiol. 2001;280:H2674–2688. doi: 10.1152/ajpheart.2001.280.6.H2674. [DOI] [PubMed] [Google Scholar]
- 159.Eckberg DL. The human respiratory gate. J Physiol (Lond) 2003;548:339–352. doi: 10.1113/jphysiol.2002.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hill LK, Siebenbrock A. Are all measures created equal? Heart rate variability and respiration - biomed 2009. Biomed Sci Instrum. 2009;45:71–76. [PubMed] [Google Scholar]
- 161.Jennings JR, Mack ME. Does aging differentially reduce heart rate variability related to respiration? Exp Aging Res. 1984;10:19–23. doi: 10.1080/03610738408258536. [DOI] [PubMed] [Google Scholar]
- 162.Kanters JK, Højgaard MV, Agner E, Holstein-Rathlou NH. Influence of forced respiration on nonlinear dynamics in heart rate variability. Am J Physiol. 1997;272:R1149–1154. doi: 10.1152/ajpregu.1997.272.4.R1149. [DOI] [PubMed] [Google Scholar]
- 163.Schaffer T, Hensel B, Weigand C, Schüttler J, Jeleazcov C. Evaluation of techniques for estimating the power spectral density of RR-intervals under paced respiration conditions. J Clin Monit Comput. 2014;28:481–486. doi: 10.1007/s10877-013-9447-4. [DOI] [PubMed] [Google Scholar]
- 164.Graham TE. Thermal, metabolic, and cardiovascular changes in men and women during cold stress. Med Sci Sports Exerc. 1988;20:S185–192. doi: 10.1249/00005768-198810001-00017. [DOI] [PubMed] [Google Scholar]
- 165.Huang CM, Chang HC, Kao ST, Li TC, Wei CC, Chen C, et al. Radial pressure pulse and heart rate variability in heat- and cold-stressed humans. Evid Based Complement Alternat Med. 2011;2011:751317. doi: 10.1155/2011/751317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ren C, O’Neill MS, Park SK, Sparrow D, Vokonas P, Schwartz J. Ambient temperature, air pollution, and heart rate variability in an aging population. Am J Epidemiol. 2011;173:1013–1021. doi: 10.1093/aje/kwq477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bortkiewicz A, Gadzicka E, Szymczak W, Szyjkowska A, Koszada-Włodarczyk W, Makowiec-Dabrowska T. Physiological reaction to work in cold microclimate. Int J Occup Med Environ Health. 2006;19:123–131. doi: 10.2478/v10001-006-0020-y. [DOI] [PubMed] [Google Scholar]
- 168.Harinath K, Malhotra AS, Pal K, Prasad R, Kumar R, Sawhney RC. Autonomic nervous system and adrenal response to cold in man at Antarctica. Wilderness Environ Med. 2005;16:81–91. doi: 10.1580/pr30-04.1. [DOI] [PubMed] [Google Scholar]
- 169.Bortkiewicz A, Gadzicka E, Szymczak W. Heart rate variability in workers exposed to carbon disulfide. J Auton Nerv Syst. 1997;66:62–68. doi: 10.1016/s0165-1838(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 170.Jhun H-J, Yim S-H, Kim R, Paek D. Heart-rate variability of carbon disulfide-poisoned subjects in Korea. Int Arch Occup Environ Health. 2003;76:156–160. doi: 10.1007/s00420-002-0391-8. [DOI] [PubMed] [Google Scholar]
- 171.Reinhardt F, Drexler H, Bickel A, Claus D, Ulm K, Angerer J, et al. Electrophysiological investigation of central, peripheral and autonomic nerve function in workers with long-term low-level exposure to carbon disulphide in the viscose industry. Int Arch Occup Environ Health. 1997;70:249–256. doi: 10.1007/s004200050215. [DOI] [PubMed] [Google Scholar]
- 172.Brito JM, Belotti L, Toledo AC, Antonangelo L, Silva FS, Alvim DS, et al. Acute cardiovascular and inflammatory toxicity induced by inhalation of diesel and biodiesel exhaust particles. Toxicol Sci. 2010;116:67–78. doi: 10.1093/toxsci/kfq107. [DOI] [PubMed] [Google Scholar]
- 173.Böckelmann I, Pfister EA, McGauran N, Robra BP. Assessing the suitability of cross-sectional and longitudinal cardiac rhythm tests with regard to identifying effects of occupational chronic lead exposure. J Occup Environ Med. 2002;44:59–65. doi: 10.1097/00043764-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 174.Murata K, Araki S, Yokoyama K, Nomiyama K, Nomiyama H, Tao YX, Liu SJ. Autonomic and central nervous system effects of lead in female glass workers in China. Am J Ind Med. 1995;28:233–244. doi: 10.1002/ajim.4700280208. [DOI] [PubMed] [Google Scholar]
- 175.Feng W, He X, Chen M, Deng S, Qiu G, Li X, et al. Urinary metals and heart rate variability: a cross-sectional study of urban adults in Wuhan, China. Environ Health Perspect. 2015;123:217–222. doi: 10.1289/ehp.1307563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Grandjean P, Murata K, Budtz-Jørgensen E, Weihe P. Cardiac autonomic activity in methylmercury neurotoxicity: 14-year follow-up of a Faroese birth cohort. J Pediatr. 2004;144:169–176. doi: 10.1016/j.jpeds.2003.10.058. [DOI] [PubMed] [Google Scholar]
- 177.Murata K, Araki S, Yokoyama K, Maeda K. Autonomic and peripheral nervous system dysfunction in workers exposed to mixed organic solvents. Int Arch Occup Environ Health. 1991;63:335–340. doi: 10.1007/BF00381584. [DOI] [PubMed] [Google Scholar]
- 178.Murata K, Araki S, Yokoyama K. Assessment of the peripheral, central, and autonomic nervous system function in styrene workers. Am J Ind Med. 1991;20:775–784. doi: 10.1002/ajim.4700200609. [DOI] [PubMed] [Google Scholar]
- 179.Juntunen J, Matikainen E, Antti-Poika M, Suoranta H, Valle M. Nervous system effects of long-term occupational exposure to toluene. Acta Neurol Scand. 1985;72:512–517. doi: 10.1111/j.1600-0404.1985.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 180.Araki S, Murata K, Yokoyama K. Application of neurophysiological methods in occupational medicine in relation to psychological performance. Ann Acad Med Singap. 1994;23:710–718. [PubMed] [Google Scholar]
- 181.Matikainen E, Juntunen J, Koskenvuo M, Antti-Poika M, Kaprio J. Cardiovascular reflexes in monozygotic twins discordant for exposure to organic solvents. Acta Genet Med Gemellol (Roma) 1987;36:503–507. doi: 10.1017/s0001566000006875. [DOI] [PubMed] [Google Scholar]
- 182.Gribble MO, Cheng A, Berger RD, Rosman L, Guallar E. Mercury exposure and heart rate variability: a systematic review. Curr Environ Health Rep. 2015;2:304–314. doi: 10.1007/s40572-015-0053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Buteau S, Goldberg MS. A structured review of panel studies used to investigate associations between ambient air pollution and heart rate variability. Environ Res. 2016;148:207–247. doi: 10.1016/j.envres.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 184.Rowell LB. Chapter 27 „Cardiovascular adjustments to thermal stress“. In: Shepherd JT, Abboud FM, editors. Handbook of physiology: section 2: the cardiovascular system, volume III, peripheral circulation and organ blood flow, part 2. 1983. p. 967–1023.
- 185.Wilson TE, Crandall CG. Effect of thermal stress on cardiac function. Exerc Sport Sci Rev. 2011;39:12–17. doi: 10.1097/JES.0b013e318201eed6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wu S, Deng F, Liu Y, Shima M, Niu J, Huang Q, Guo X. Temperature, traffic-related air pollution, and heart rate variability in a panel of healthy adults. Environ Res. 2013;120:82–89. doi: 10.1016/j.envres.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 187.Bhaumik G, Dass D, Bhattacharyya D, Sharma YK, Singh SB. Heart rate variabilty changes during first week of acclimatization to 3500 m altitude in Indian military personnel. Indian J Physiol Pharmacol. 2013;57:16–22. [PubMed] [Google Scholar]
- 188.Dhar P, Sharma VK, Hota KB, Das SK, Hota SK, Srivastava RB, Singh SB. Autonomic cardiovascular responses in acclimatized lowlanders on prolonged stay at high altitude: a longitudinal follow up study. PLoS One. 2014;9:e84274. doi: 10.1371/journal.pone.0084274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hettinger T, Wobbe G. Kompendium der Arbeitswissenschaft. Ludwigshafen, Germany: Kiehl-Verlag; 1993. [Google Scholar]
- 190.Gross R, Kirchheim H. Effects of bilateral carotid and auditory stimulation on renal blood flow and sympathetic nerve activity in the conscious dog. Pflugers Arch. 1980;383:233–239. doi: 10.1007/BF00587524. [DOI] [PubMed] [Google Scholar]
- 191.Kraus U, Schneider A, Breitner S, Hampel R, Rückerl R, Pitz M, et al. Individual daytime noise exposure during routine activities and heart rate variability in adults: a repeated measures study. Environ Health Perspect. 2013;121:607–612. doi: 10.1289/ehp.1205606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lee G-S, Chen M-L, Wang G-Y. Evoked response of heart rate variability using short-duration white noise. Auton Neurosci. 2010;155:94–97. doi: 10.1016/j.autneu.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 193.Schnell I, Potchter O, Epstein Y, Yaakov Y, Hermesh H, Brenner S, Tirosh E. The effects of exposure to environmental factors on heart rate variability: an ecological perspective. Environ Pollut. 2013;183:7–13. doi: 10.1016/j.envpol.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 194.Veternik M, Tonhajzerova I, Misek J, Jakusova V, Hudeckova H, Jakus J. The impact of sound exposure on heart rate variability in adolescent students. Physiol Res. 2018;67:695–702. doi: 10.33549/physiolres.933882. [DOI] [PubMed] [Google Scholar]
- 195.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 196.Ha M, Kim J, Park J, Chung HK. Blood pressure and heart rate variability in workers of 8-hour shifts. J Hum Ergol (Tokyo) 2001;30:229–233. [PubMed] [Google Scholar]
- 197.Lindholm H, Sinisalo J, Ahlberg J, Hirvonen A, Hublin C, Partinen M, Savolainen A. Attenuation of vagal recovery during sleep and reduction of cortisol/melatonin ratio in late afternoon associate with prolonged daytime sleepiness among media workers with irregular shift work. Am J Ind Med. 2012;55:643–649. doi: 10.1002/ajim.22042. [DOI] [PubMed] [Google Scholar]
- 198.Wehrens SMT, Hampton SM, Skene DJ. Heart rate variability and endothelial function after sleep deprivation and recovery sleep among male shift and non-shift workers. Scand J Work Environ Health. 2012;38:171–181. doi: 10.5271/sjweh.3197. [DOI] [PubMed] [Google Scholar]
- 199.Chung MH, Kuo TBJ, Hsu N, Chu H, Chou KR, Yang CCH. Sleep and autonomic nervous system changes - enhanced cardiac sympathetic modulations during sleep in permanent night shift nurses. Scand J Work Environ Health. 2009;35:180–187. doi: 10.5271/sjweh.1324. [DOI] [PubMed] [Google Scholar]
- 200.Järvelin-Pasanen S, Ropponen A, Tarvainen MP, Karjalainen PA, Louhevaara V. Differences in heart rate variability of female nurses between and within normal and extended work shifts. Ind Health. 2013;51:154–164. doi: 10.2486/indhealth.ms1368. [DOI] [PubMed] [Google Scholar]
- 201.Amirian I, Toftegård Andersen L, Rosenberg J, Gögenur I. Decreased heart rate variability in surgeons during night shifts. Can J Surg. 2014;57:300–304. doi: 10.1503/cjs.028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jensen MA, Garde AH, Kristiansen J, Nabe-Nielsen K, Hansen ÅM. The effect of the number of consecutive night shifts on diurnal rhythms in cortisol, melatonin and heart rate variability (HRV): a systematic review of field studies. Int Arch Occup Environ Health. 2016;89:531–545. doi: 10.1007/s00420-015-1093-3. [DOI] [PubMed] [Google Scholar]
- 203.Sammito S, Böckelmann I. Einfluss von Extrasystolen auf die Herzfrequenzvariabilitätsmessungen im Rahmen von 24h-Messungen. In: Hottenrott K, Gronwald T, Schmidt H, editors. Herzfrequenzvariabilität: Grundlagen - Methoden - Anwendungen. Hamburg, Germany: Feldhaus Verlag Edition Czwalina; 2014. pp. 82–86. [Google Scholar]
- 204.Sammito S, Böckelmann I. Validierung von drei verschiedenen Systemen zur Erfassung der Herzschlagfrequenz in Sanitätsfahrzeugen. ErgoMed/Prakt Arbmed. 2012;36:38–45. [Google Scholar]
- 205.Sammito S, Darius S, Böckelmann I. Validierungsstudie zum Einsatz eines funklosen Brustgurtsystems zur Messung der Herzratenvariabilität unter Ruhebedingungen und in Fahrzeugen. Arbeitsmed Sozialmed Umweltmed. 2011;46:60–65. [Google Scholar]
- 206.Billman GE. Heart rate variability - a historical perspective. Front Physiol. 2011;2:86. doi: 10.3389/fphys.2011.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hoos O. Spektralanalyse der Herzfrequenzvariabilität im Sport – Methoden und Anwendungen, Möglichkeiten und Grenzen. In: Hottenrott K, editor. Herzfrequenzvariabilität: methoden und anwendungen in sport und medizin. Hamburg, Germany: Czwalina; 2006. [Google Scholar]
- 208.Burr RL, Cowan MJ. Autoregressive spectral models of heart rate variability. Practical issues. J Electrocardiol. 1992;25 Suppl:224–233. doi: 10.1016/0022-0736(92)90108-c. [DOI] [PubMed] [Google Scholar]
- 209.Rüdiger H, Klinghammer L, Scheuch K. The trigonometric regressive spectral analysis–a method for mapping of beat-to-beat recorded cardiovascular parameters on to frequency domain in comparison with fourier transformation. Comput Methods Programs Biomed. 1999;58:1–15. doi: 10.1016/s0169-2607(98)00070-4. [DOI] [PubMed] [Google Scholar]
- 210.Lomb NR. Least squares frequency algorithmus of unequally sampled data. Astrophys Space Sci. 1976;39:447–462. [Google Scholar]
- 211.Radtke T, Kriemler S, Eser P, Saner H, Wilhelm M. Physical activity intensity and surrogate markers for cardiovascular health in adolescents. Eur J Appl Physiol. 2013;113:1213–1222. doi: 10.1007/s00421-012-2542-2. [DOI] [PubMed] [Google Scholar]
- 212.Mäkikallio TH, Tapanainen JM, Tulppo MP, Huikuri HV. Clinical applicability of heart rate variability analysis by methods based on nonlinear dynamics. Card Electrophysiol Rev. 2002;6:250–255. doi: 10.1023/a:1016381025759. [DOI] [PubMed] [Google Scholar]
- 213.Stein PK, Domitrovich PP, Huikuri HV, Kleiger RE. Traditional and nonlinear heart rate variability are each independently associated with mortality after myocardial infarction. J Cardiovasc Electrophysiol. 2005;16:13–20. doi: 10.1046/j.1540-8167.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- 214.Voss A, Schulz S, Schroeder R, Baumert M, Caminal P. Methods derived from nonlinear dynamics for analysing heart rate variability. Philos Trans Math Phys Eng Sci. 2009;367:277–296. doi: 10.1098/rsta.2008.0232. [DOI] [PubMed] [Google Scholar]
- 215.Schmidt G, Morfill GE. Nonlinear methods for heart rate variability assessment. In: Malik M, Camm AJ, editors. Heart rate variability. Armonk, NY, USA: Futora; 1995. pp. 87–98. [Google Scholar]
- 216.Sammito S, Böckelmann I. Factors influencing heart rate variability. Int J Cardio Forum. 2016 doi: 10.17987/icfj.v6i0.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.DIN 9886:2004. Ergonomie. Ermittlung der thermischen Beanspruchung durch physiologische Messungen (ISO 9886:2004) Mai 2004.
- 218.DIN 8996:2004. Ergonomie der thermischen Umgebung. Bestimmung des körpereigenen Energieumsatzes (ISO 8996:2004) Januar 2005.
- 219.Cooney MT, Vartiainen E, Laatikainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010;159:612–619.e3. doi: 10.1016/j.ahj.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 220.Williams BA, Merhige ME. The prognostic association between resting heart rate and cardiac death–myocardial perfusion defects as a potential mechanism. Atherosclerosis. 2012;221:445–450. doi: 10.1016/j.atherosclerosis.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 221.Beltz NM, Gibson AL, Janot JM, Kravitz L, Mermier CM, Dalleck LC. Graded exercise testing protocols for the determination of VO2max: historical perspectives, progress, and future considerations. J Sports Med (Hindawi Publ Corp) 2016;2016:3968393. doi: 10.1155/2016/3968393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Borg G. Anstrengungsempfinden und körperliche Aktivität. Dtsch Arztebl. 2004;101:A–1016. [Google Scholar]
- 223.Scherr J, Wolfarth B, Christle JW, Pressler A, Wagenpfeil S, Halle M. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur J Appl Physiol. 2013;113:147–155. doi: 10.1007/s00421-012-2421-x. [DOI] [PubMed] [Google Scholar]
- 224.Gulati M, Shaw LJ, Thisted RA, Black HR, Bairey Merz CN, Arnsdorf MF. Heart rate response to exercise stress testing in asymptomatic women: the st. James women take heart project. Circulation. 2010;122:130–137. doi: 10.1161/CIRCULATIONAHA.110.939249. [DOI] [PubMed] [Google Scholar]
- 225.Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24:1529–1535. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 226.Sugawara J, Murakami H, Maeda S, Kuno S, Matsuda M. Change in post-exercise vagal reactivation with exercise training and detraining in young men. Eur J Appl Physiol. 2001;85:259–263. doi: 10.1007/s004210100443. [DOI] [PubMed] [Google Scholar]
- 227.Takahashi T, Miyamoto Y. Influence of light physical activity on cardiac responses during recovery from exercise in humans. Eur J Appl Physiol Occup Physiol. 1998;77:305–311. doi: 10.1007/s004210050338. [DOI] [PubMed] [Google Scholar]
- 228.Takahashi T, Okada A, Saitoh T, Hayano J, Miyamoto Y. Difference in human cardiovascular response between upright and supine recovery from upright cycle exercise. Eur J Appl Physiol. 2000;81:233–239. doi: 10.1007/s004210050036. [DOI] [PubMed] [Google Scholar]
- 229.Rohmert W, Rutenfranz J. Praktische Arbeitsphysiologie. Stuttgart, Germany and New York, USA: Thieme; 1983. [Google Scholar]
- 230.Scheuch K. Arbeitsphysiologie. In: Triebig G, Kenter M, Schiele R, editors. Arbeitsmedizin – Handbuch für Theorie und Praxis. 3. Stuttgart, Germany: Gentner Verlag; 2011. pp. 413–458. [Google Scholar]
- 231.Boutellier U. Sport- und Arbeitsphysiologie. In: Schmidt RF, Lang F, Heckmann M, editors. Physiologie des Menschen mit Pathophysiologie. 31. Heidelberg, Germany: Springer Medizin Verlag; 2011. pp. 854–876. [Google Scholar]
- 232.Frauendorf H, Kobryn U, Gelbrich W. Blutdruck- und Herzschlagfrequenzverhalten bei fünf verschiedenen Formen dynamischer Muskelarbeit. Z Arbwiss. 1990;44:214–216. [Google Scholar]
- 233.Cottin F, Médigue C, Lopes P, Leprêtre P-M, Heubert R, Billat V. Ventilatory thresholds assessment from heart rate variability during an incremental exhaustive running test. Int J Sports Med. 2007;28:287–294. doi: 10.1055/s-2006-924355. [DOI] [PubMed] [Google Scholar]
- 234.Kaikkonen P, Hynynen E, Mann T, Rusko H, Nummela A. Can HRV be used to evaluate training load in constant load exercises? Eur J Appl Physiol. 2010;108:435–442. doi: 10.1007/s00421-009-1240-1. [DOI] [PubMed] [Google Scholar]
- 235.Sandercock GRH, Brodie DA. The use of heart rate variability measures to assess autonomic control during exercise. Scand J Med Sci Sports. 2006;16:302–313. doi: 10.1111/j.1600-0838.2006.00556.x. [DOI] [PubMed] [Google Scholar]
- 236.Gronwald T, Hoos O. Correlation properties of heart rate variability during endurance exercise: a systematic review. Ann Noninvasive Electrocardiol. 2020;25:e12697. doi: 10.1111/anec.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Backs RW, Ryan. Psychological measures of workload during continuous performance. Hum Factors. 1994;36:514–31. [DOI] [PubMed]
- 238.Egelund N. Heart-rate and heart-rate-variability as indicators of driver workload in traffic situations. In: Orlebeke J, Mulder G, van Doornen L, editors. Psychophysiology of cardiovascular control: models, methods, and data. New York, USA: Plenum Press; 1985. pp. 855–863. [Google Scholar]
- 239.Egelund N. Spectral analysis of heart rate variability as an indicator of driver fatigue. Ergonomics. 1982;25:663–672. doi: 10.1080/00140138208925026. [DOI] [PubMed] [Google Scholar]
- 240.Jenkins JG, Mitchell RH, McClure BG. Heart rate variability in the newborn infants. Automedica. 1983;4:263–270. [Google Scholar]
- 241.Jorna PG. Spectral analysis of heart rate and psychological state: a review of its validity as a workload index. Biol Psychol. 1992;34:237–257. doi: 10.1016/0301-0511(92)90017-o. [DOI] [PubMed] [Google Scholar]
- 242.Kalsbeek J, Ettema J. Continuous recording of heart rate and the measurement of perceptual load. Ergonomics. 1963;6:306–307. [Google Scholar]
- 243.Luczak H, Laurig W. An analysis of heart rate variability. Ergonomics. 1973;16:85–97. doi: 10.1080/00140137308924484. [DOI] [PubMed] [Google Scholar]
- 244.Manzey D. Psychophysiologie mentaler Beanspruchung. In: Rösler F, editor. Ergebnisse und Anwendungen der Psychophysiologie: Enzyklopädie der Psychologie. Göttingen, Germany: Hogrefe; 1998. pp. 799–864. [Google Scholar]
- 245.Opmeer CH. The information content of succissive RR-interval times in the ECG. Preliminary results using factor analysis and frequency analysis. Ergonomics. 1973;16:105–112. doi: 10.1080/00140137308924486. [DOI] [PubMed] [Google Scholar]
- 246.Paas FG, van Merriënboer JJ, Adam JJ. Measurement of cognitive load in instructional research. Percept Mot Skills. 1994;79:419–430. doi: 10.2466/pms.1994.79.1.419. [DOI] [PubMed] [Google Scholar]
- 247.van Amelsvoort LG, Schouten EG, Maan AC, Swenne CA, Kok FJ. Occupational determinants of heart rate variability. Int Arch Occup Environ Health. 2000;73:255–262. doi: 10.1007/s004200050425. [DOI] [PubMed] [Google Scholar]
- 248.Voss A, Esperer HD. Herzfrequenzvariabilität - Definition, Analyse und Klinische Bedeutung, Teil I. HerzRhythmus. 1994;6:1–8. [Google Scholar]
- 249.Buccelletti E, Gilardi E, Scaini E, Galiuto L, Persiani R, Biondi A, et al. Heart rate variability and myocardial infarction: systematic literature review and metanalysis. Eur Rev Med Pharmacol Sci. 2009;13(4):299–307. [PubMed]
- 250.Song T, Qu XF, Zhang YT, Cao W, Han BH, Li Y, et al. Usefulness of the heart-rate variability complex for predicting cardiac mortality after acute myocardial infarction. BMC Cardiovasc Disord. 2014;14:59. doi: 10.1186/1471-2261-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lakusic N, Mahovic D, Sonicki Z, Slivnjak V, Baborski F. Outcome of patients with normal and decreased heart rate variability after coronary artery bypass grafting surgery. Int J Cardiol. 2013;166:516–518. doi: 10.1016/j.ijcard.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 252.Sandercock GRH, Brodie DA. The role of heart rate variability in prognosis for different modes of death in chronic heart failure. Pacing Clin Electrophysiol. 2006;29:892–904. doi: 10.1111/j.1540-8159.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- 253.Schmidt H, Hoyer D, Hennen R, Heinroth K, Rauchhaus M, Prondzinsky R, et al. Autonomic dysfunction predicts both 1- and 2-month mortality in middle-aged patients with multiple organ dysfunction syndrome. Crit Care Med. 2008;36:967–970. doi: 10.1097/CCM.0B013E3181653263. [DOI] [PubMed] [Google Scholar]
- 254.Kloter E, Barrueto K, Klein SD, Scholkmann F, Wolf U. Heart rate variability as a prognostic factor for cancer survival - a systematic review. Front Physiol. 2018;9:623. doi: 10.3389/fphys.2018.00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhou X, Ma Z, Zhang L, Zhou S, Wang J, Wang B, Fu W. Heart rate variability in the prediction of survival in patients with cancer: a systematic review and meta-analysis. J Psychosom Res. 2016;89:20–25. doi: 10.1016/j.jpsychores.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 256.França da Silva AK, Penachini da Costa de Rezende Barbosa M, Marques Vanderlei F, Destro Christofaro DG, Marques Vanderlei LC. Application of heart rate variability in diagnosis and prognosis of individuals with diabetes mellitus: systematic review. Ann Noninvasive Electrocardiol. 2016;21:223–35. doi: 10.1111/anec.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Liao D, Cai J, Barnes RW, Tyroler HA, Rautaharju P, Holme I, Heiss G. Association of cardiac autonomic function and the development of hypertension: the ARIC study. Am J Hypertens. 1996;9:1147–1156. doi: 10.1016/s0895-7061(96)00249-x. [DOI] [PubMed] [Google Scholar]
- 258.Singh JP, Larson MG, Tsuji H, Evans JC, O’Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension. 1998;32:293–297. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- 259.Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability: the atherosclerosis risk in communities (ARIC) study. Hypertension. 2003;42:1106–1111. doi: 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- 260.Schuster AK, Fischer JE, Thayer JF, Mauss D, Jarczok MN. Decreased heart rate variability correlates to increased cardiovascular risk. Int J Cardiol. 2016;203:728–730. doi: 10.1016/j.ijcard.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 261.Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) study. Circulation. 2002;105:310–315. doi: 10.1161/hc0302.102575. [DOI] [PubMed] [Google Scholar]
- 262.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 263.Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, de Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 264.Hoos O. Herzfrequenzvariabilität und Physiotherapie: Grundlagen, Methoden und Anwendungen. Z für Physiotherapeuten. 2009;61:277–282. [Google Scholar]
- 265.Djaoui L, Haddad M, Chamari K, Dellal A. Monitoring training load and fatigue in soccer players with physiological markers. Physiol Behav. 2017;181:86–94. doi: 10.1016/j.physbeh.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 266.Tapanainen JM, Thomsen PEB, Køber L, Torp-Pedersen C, Mäkikallio TH, Still A-M, et al. Fractal analysis of heart rate variability and mortality after an acute myocardial infarction. Am J Cardiol. 2002;90:347–352. doi: 10.1016/s0002-9149(02)02488-8. [DOI] [PubMed] [Google Scholar]
- 267.Del Pozo JM, Gevirtz RN, Scher B, Guarneri E. Biofeedback treatment increases heart rate variability in patients with known coronary artery disease. Am Heart J. 2004;147:E11. doi: 10.1016/j.ahj.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 268.Lehrer P, Vaschillo E, Lu S-E, Eckberg D, Vaschillo B, Scardella A, Habib R. Heart rate variability biofeedback: effects of age on heart rate variability, baroreflex gain, and asthma. Chest. 2006;129:278–284. doi: 10.1378/chest.129.2.278. [DOI] [PubMed] [Google Scholar]
- 269.Lehrer PM, Vaschillo E, Vaschillo B, Lu S-E, Eckberg DL, Edelberg R, et al. Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosom Med. 2003;65:796–805. doi: 10.1097/01.psy.0000089200.81962.19. [DOI] [PubMed] [Google Scholar]
- 270.Peira N, Pourtois G, Fredrikson M. Learned cardiac control with heart rate biofeedback transfers to emotional reactions. PLoS One. 2013;8:e70004. doi: 10.1371/journal.pone.0070004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.