Abstract
Background
Antibiotics are drugs of natural or synthetic origin used to treat various infections. The practice of excessive and inappropriate antibiotics use is the main global cause of bacterial resistance, which is one of the most serious global public health threats. It is estimated that about 50% of global antibiotic prescriptions are inappropriate. This study assesses the prevalence and pattern of inappropriate prescriptions of antibiotics amongst ambulatory care visits in Ethiopia.
Methods
A facility-based, cross-sectional study with a quantitative approach was conducted amongst randomly selected prescriptions issued for outpatients from May to June 2022 at Debre Markos Specialized Comprehensive Hospital, Northwest Ethiopia. Descriptive statistics, such as frequencies and percentages, were computed. For group comparisons, χ2 and independent sample t-tests were computed. The statistical significance of the association was considered at p<0.05.
Results
A total of 2640 antibiotics were prescribed for patients in the outpatient setting with various bacterial infections via 911 prescriptions, of which 49.5% were non-compliant with the national treatment guideline. Guideline non-compliant prescriptions increased remarkably amongst patients in the outpatient setting diagnosed with community-acquired pneumonia (38.8% versus 30.1%; p=0.006) and peptic ulcer disease (14.9% versus 9%; p=0.006). Moreover, inappropriate prescription was significantly higher amongst patients taking amoxicillin/clavulanic acid (33.2% versus 48.2%; p<0.001) and cephalexin (17.8% versus 24.3%; p=0.016).
Conclusion
Large proportions of antibiotic prescriptions for outpatients were non-compliant with the national treatment guideline, suggesting that prescribers need to give special attention to outpatients whilst ordering antibiotics such as amoxicillin/clavulanic acid and cephalexin. Antibiotic stewardship efforts to optimize outpatient antibiotic prescriptions and reduce the use of potentially inappropriate antibiotics are needed in Ethiopia.
Keywords: ambulatory care, antibiotic prescriptions, antibiotics, Ethiopia
Introduction
Bacterial infections are one of the leading causes of morbidity and mortality worldwide.1 Antibiotics represent the most widely prescribed therapeutic agents used to treat bacterial infections.2,3 In the last two decades, antibiotic consumption has been rising quickly, increasing by 65%, mainly in low-income and middle-income countries.4–6 Although ~50% of hospitalized patients receive at least one antibiotic during their hospital stay, up to 90% of antimicrobial use takes place in outpatient settings.7,8
Appropriate use of medications entails prescribing the appropriate medication to the right person at the right time and in the right dosage. The appropriate use of antibiotics is critical to reducing the burden of antimicrobial resistance and establishing a cost-effective healthcare system.9 However, it is estimated that ~50% of antibiotic prescriptions globally are inappropriate, resulting in increased side-effects, high healthcare costs and the promotion of antibiotic resistance.10–13 Inappropriate antibiotic prescribing is detrimental at the societal level because it fosters the spread of antimicrobial resistance and harmful at an individual level because they are associated with adverse drug events, including allergic reactions (e.g. skin rashes or anaphylaxis) and microbiome disruption-related conditions (e.g. infections caused by Clostridium difficile).14 About 6.5% of morbidity, hospital admissions and mortality are related to improper antibiotic prescribing.15,16
A common way to measure the appropriateness of antibiotic use is by evaluating whether they are prescribed in accordance with local guidelines and, if these are unavailable, to national or international guidelines.17 The implementation of appropriate prescription practices is crucial towards mitigating inappropriate and excessive utilization of antibiotics, thus mitigating the rapid growth of bacterial resistance to antibiotics.15 Understanding the extent and pattern of antibiotic use is essential for defining regional intervention programmes to promote rational use of antibiotics and subsequently curb the spread of antibiotic resistance. There is little evidence about the patterns and appropriateness of antibiotic use in outpatient departments in developing nations like Ethiopia, in which there are indications of inappropriate use of antibiotics in the country. Therefore, this study was conducted to explore how antibiotics are prescribed to adult patients attending the outpatient department at Debre Markos Specialized Comprehensive Hospital (DMSCH) — a state-owned tertiary care level hospital located in northwest Ethiopia. The hospital serves approximately five million people in the surrounding area through seven pharmacy units, which include inpatient, chronic care, outpatient, antiretroviral, emergency, gynaecology and ophthalmology pharmacies. Amongst these units, the outpatient pharmacy department (OPD) provides services for ambulatory patients attending the hospital as well as to outpatients with referral papers from all over the region.
Methods
Study design, period and area
A facility-based, cross-sectional study with a quantitative approach was employed amongst randomly selected prescriptions issued for outpatients from 1 May to 1 June 2022 at DMSCH, Northwest Ethiopia. The hospital is located 300 km northwest of Addis Ababa, the capital city of Ethiopia. DMSCH is expected to provide healthcare services to more than 3.5 million people in its catchment area.
Study population and inclusion criteria
All prescriptions issued for outpatients at DMSCH were the data source, whilst the study object was prescriptions issued for adult outpatients (18 years and older) for the management of bacterial infections during the time of data collection at DMSCH. Prescriptions that contained multiple or unspecified diagnoses, prescriptions with incomplete information and illegible prescriptions were excluded from the study.
Study variables
The dependent variable was inappropriate antibiotic prescriptions. Whereas sociodemographic variables, including age and sex, type of bacterial infection, and antibiotic-related characteristics, such as type, frequency and dose of the antibiotics prescribed, were the independent variables.
Operational definition
Inappropriate antibiotic prescription is defined as a prescription that is non-compliant by considering dose, duration, route, frequency and indication with pharmacotherapy recommendations of clinical guidelines and existing literature.1,18
Data collection tools and procedure
After examining the current literature, the authors developed a structured questionnaire that was utilized for collecting data. The questionnaire was divided into three major sections, including (1) the baseline characteristics of outpatients with antibiotic prescriptions and the types of bacterial diagnosis for which antibiotics were prescribed; (2) the list of antibiotics and their dosage and duration of therapy; and (3) the types of inappropriate antibiotic prescriptions. After assessing the eligibility criteria, prescriptions containing antibiotics for outpatients aged 18 or older who attended the hospital from 1 May to 1 June 2022 were included in the study. The patient’s age, sex, diagnosis, type and dosage of antibiotics, duration of therapy and route of administration were recorded from the prescriptions. Finally, the evaluation of antibiotic prescription appropriateness was determined using the recommendations of the 2021 Ethiopian national standard treatment guideline as there is no local treatment guideline in the study setting.19 Each antibiotic in the prescriptions selected for a specific diagnosis was checked for both the appropriateness of drug selection and the appropriateness of dosage and duration of therapy; the first group contains those antibiotics that were selected inappropriately for the diagnosis mentioned on the prescriptions, whilst the second group contains those antibiotics that were selected appropriately, but the duration/dose of the antibiotics was inappropriate for the respective diagnosis the antibiotics prescribed for. A multidisciplinary team consisting of one senior Internist, one General practitioner and one Pharmacist performed the evaluation by comparing it to the 2021 Ethiopian standard treatment guideline.20 Each prescription of medication was evaluated for appropriateness in dose, frequency, duration and indication for each specific patient based on the underlying diagnosis. Antibiotic regimen appropriateness was independently reviewed by each team member, and disagreement was always solved in short discussions between the specialists.
Data processing and analysis
The collected data were checked for completeness, accuracy and clarity before analysis. The data were coded, entered into Epi-data 4.6, and transferred to SPSS version 26 for statistical analysis. Descriptive statistics, including mean and standard deviation for continuous variables and frequency and percentage for categorical variables, were used to summarize the sociodemographic and relevant clinical characteristics of the study participants and the prescribing pattern of antibiotics. For group comparisons, χ2 and independent sample t-tests were computed to assess the correlation between inappropriate antibiotic prescriptions with sociodemographic or relevant clinical characteristics of the study participants. The statistical significance of the association was considered at a p value of <0.05.
Informed consent statement
Patient consent was waived due to the use of anonymized routine data.
Ethical approval
This study was approved by Ethical Review Committee of Debre Markos University, College of Health Sciences. The information obtained from the study was not disclosed to any third party, and code numbers were used to identify prescriptions.
Results
Baseline characteristics and type of diagnosis amongst outpatient department visitors
There were a total of 2217 prescriptions provided for 1879 OPD patients during the 1-month study period, of which 1226 (55.3%) contained at least one oral antibiotic. Of these, only 911 prescriptions for 804 patients met the inclusion criteria and were therefore subject to review. The reasons the remaining prescriptions (approximately 25%) were omitted following the exclusion criteria are described in Figure 1. The mean age of participants was 38±13.85 years whilst 48% of the study participants were women. Respiratory tract infections (30.6%), gastrointestinal tract infections (28.8%) and urinary tract infections (27.6%) were the top three diagnoses amongst outpatients that were treated with antibiotic prescriptions (Table 1).
Figure 1.
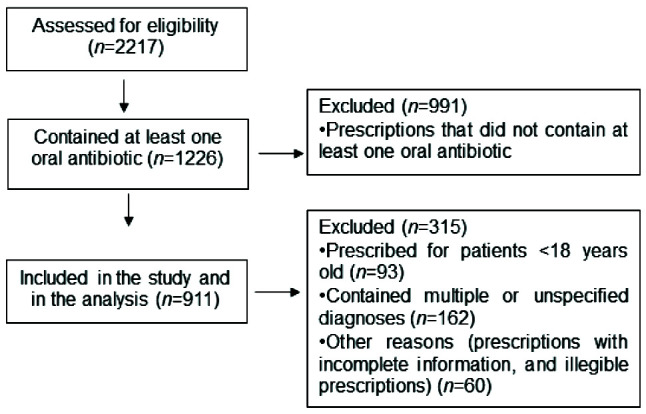
CONSORT diagram showing prescription eligibility assessment steps amongst outpatients attending Debre Markos Specialized Comprehensive Hospital.
Table 1.
Patient demographics and type of diagnosis amongst patients with bacterial infections attending outpatient department of Debre Markos Specialized Comprehensive Hospital.
| Variable | Frequency (n) | Percentage (%) | ||
|---|---|---|---|---|
| Sex | Male | 474 | 52.0 | |
| Female | 437 | 48.0 | ||
| Age (mean ± standard deviation) | 38±13.85 | |||
| Diagnosis | Respiratory tract infection | Community-acquired pneumonia | 311 | 30.6 |
| Urogenital tract infections | Uncomplicated urinary tract infection | 238 | 23.4 | |
| Recurrent urinary tract infection | 23 | 2.3 | ||
| Complicated urinary tract infection | 18 | 1.9 | ||
| Gastrointestinal tract infections | Typhoid fever | 102 | 10.1 | |
| Amoebiasis | 51 | 5.0 | ||
| Giardiasis | 33 | 3.2 | ||
| Peptic ulcer diseasea | 107 | 10.5 | ||
| Skin and soft tissue infections | Osteomyelitis | 61 | 6.0 | |
| Cellulitis | 17 | 1.6 | ||
| Sexually transmitted diseases | Vaginal discharge | 15 | 1.3 | |
| Othersb | 41 | 4.1 | ||
Peptic ulcer disease caused by Helicobacter pylori.
Bronchitis, lung abscess, oral abscess, otitis media, rheumatic fever.
Patterns of antibiotic prescriptions amongst outpatients with bacterial infection
A total of 2640 antibiotics were prescribed for outpatients with various bacterial infections via 911 prescriptions. Penicillins were the most commonly prescribed class of antibiotics, accounting for 41.46% of all prescriptions, followed by fluoroquinolones (15.83%) and tetracyclines (10.45%). From the individual drugs, amoxicillin was the most frequently prescribed antibiotic (19.73%), followed by ciprofloxacin (15.83%) and amoxicillin/clavulanic acid (13.86%) (Table 2).
Table 2.
Patterns of antibiotic prescriptions amongst patients with bacterial infections attending outpatient department of Debre Markos Specialized Comprehensive Hospital.
| Class of antibiotics | Specific antibiotics | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Cephalosporin | Cephalexin | 190 | 7.19 |
| Fluoroquinolone | Ciprofloxacin | 418 | 15.83 |
| Imidazole derivative | Metronidazole | 234 | 8.86 |
| Tinidazole | 26 | 1.04 | |
| Macrolide | Azithromycin | 179 | 6.78 |
| Clarithromycin | 92 | 3.48 | |
| Penicillin | Amoxicillin | 521 | 19.73 |
| Amoxicillin/clavulanic acid | 366 | 13.86 | |
| Cloxacillin | 208 | 7.87 | |
| Sulfonamide | Sulfamethoxazole/trimethoprim | 87 | 3.29 |
| Tetracycline | Doxycycline | 276 | 10.45 |
| Othersa | 43 | 1.62 |
Benzyl penicillin, ceftriaxone, nitrofurantoin, norfloxacin, tetracycline.
Appropriateness of antibiotic prescriptions amongst outpatients with bacterial infection
From the total of 2640 antibiotics prescribed, 1308 (49.54%) were non-compliant with the national treatment guideline in 46% of patients. Amoxicillin (19.2%) and ciprofloxacin (15.9%) made up the largest proportion of guideline non-compliant prescriptions (Table 3).
Table 3.
Frequency of inappropriate antibiotic prescriptions amongst patients with bacterial infections attending outpatient department of Debre Markos Specialized Comprehensive Hospital.
| Class of prescribed antibiotics | Name of prescribed antibiotics | Frequency of non-compliance | Percentage (%) |
|---|---|---|---|
| Penicillin | Amoxicillin | 252 | 19.2 |
| Amoxicillin/clavulanic acid | 204 | 15.6 | |
| Cloxacillin | 108 | 8.3 | |
| Fluoroquinolones | Ciprofloxacin | 208 | 15.9 |
| Imidazole derivative | Metronidazole | 109 | 8.4 |
| Tinidazole | 10 | 0.8 | |
| Sulfonamide | Sulfamethoxazole/trimethoprim | 39 | 2.9 |
| Macrolides | Azithromycin | 83 | 6.3 |
| Clarithromycin | 40 | 3.0 | |
| Tetracycline | Doxycycline | 133 | 10.2 |
| Cephalosporin | Cephalexin | 103 | 7.9 |
| Othersa | 19 | 1.5 |
Norfloxacin, nitrofurantoin, ceftriaxone.
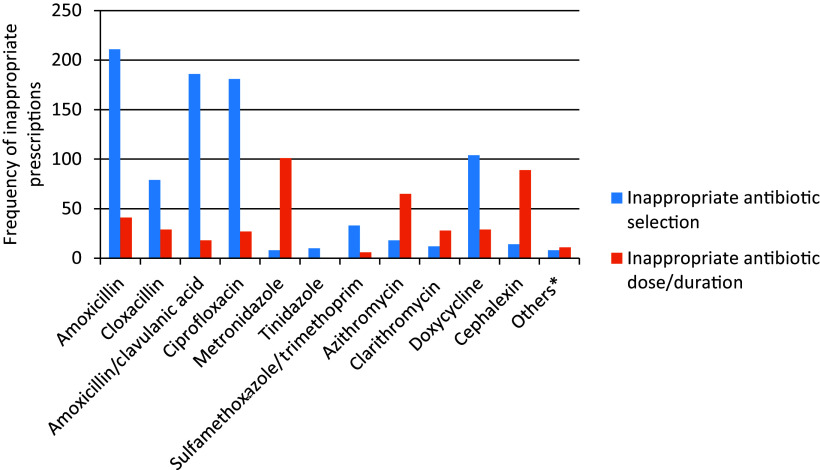
Regarding the types of inappropriate antibiotic prescriptions there were two sub-divisions: the first containing those antibiotics that were selected inappropriately for the diagnosis mentioned on the prescriptions and the second containing those antibiotics that were selected appropriately but the duration/dose of the antibiotics was inappropriate for their respective diagnosis. Inappropriate selection of antibiotics was observed in 397 (43.6%) of the prescriptions with 776 of the antibiotics prescribed, in which amoxicillin was the most inappropriately selected medication whilst metronidazole was the most commonly prescribed medication with inappropriate duration/dose followed by cephalexin (Figure 2).
Figure 2.
Types of inappropriate antibiotic prescriptions amongst patients with bacterial infections attending outpatient department of Debre Markos Specialized Comprehensive Hospital.
*Norfloxacin, nitrofurantoin, ceftriaxone.
Distribution of inappropriate antibiotic prescriptions for various bacterial infections
There was no significant difference between patients in the group with compliance to the guidelines and that without compliance regarding age (39.10±14.004 versus 38.36±13.702 years, respectively; p=0.605) or sex (52.9% versus 51.1% men, respectively; p=0.587). Furthermore, the proportion of patients with urogenital tract infections, skin and soft tissue infections, and sexually transmitted diseases did not differ significantly between the two groups. On the other hand, the presence of community-acquired pneumonia as a diagnosis increased remarkably in the guideline non-compliance group (38.8% versus 30.1%; p=0.006). Moreover, cases of peptic ulcer disease were significantly more in the guideline non-compliance group (14.9% versus 9%; p=0.006) (Table 4).
Table 4.
Distribution of inappropriate antibiotic prescriptions for various bacterial infection diagnosis amongst patients attending outpatient department of Debre Markos Specialized Comprehensive Hospital.
| Variable | Compliance to guideline (n=488) | Non-compliance to guideline (n=423) | χ2 test | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | ||||||
| Sex of outpatients with antibiotic prescription | Male | 258 | 52.9 | 216 | 51.1 | 0.296 | 0.587 | ||
| Female | 230 | 47.1 | 207 | 48.9 | |||||
| Age of outpatients (mean ± SD)a | 39.10±14.004 | 38.36±13.702 | 0.517 | 0.605 | |||||
| Diagnosis | Respiratory tract infections | CAP | Yes | 147 | 30.1 | 164 | 38.8 | 7.537 | 0.006b |
| Urogenital tract infections | Uncomplicated UTI | Yes | 121 | 24.8 | 117 | 27.7 | 0.963 | 0.326 | |
| Recurrent UTI | Yes | 12 | 2.5 | 11 | 2.6 | 0.018 | 0.892 | ||
| Complicated UTI | Yes | 11 | 2.3 | 7 | 1.7 | 0.420 | 0.517 | ||
| Gastrointestinal tract infections | Typhoid fever | Yes | 50 | 10.2 | 52 | 12.3 | 0.955 | 0.328 | |
| Amoebiasis | Yes | 33 | 6.8 | 18 | 4.3 | 2.695 | 0.101 | ||
| Giardiasis | Yes | 20 | 4.1 | 13 | 3.1 | 0.682 | 0.409 | ||
| Peptic ulcer disease | Yes | 44 | 9.0 | 63 | 14.9 | 7.551 | 0.006b | ||
| Skin and soft tissue infections | Osteomyelitis | Yes | 31 | 6.4 | 30 | 7.1 | 0.198 | 0.656 | |
| Cellulites | Yes | 9 | 1.8 | 8 | 1.9 | 0.003 | 0.958 | ||
| Sexual transmitted diseases | Vaginal discharge | Yes | 7 | 1.4 | 8 | 1.9 | 0.292 | 0.589 | |
| Othersc | Yes | 23 | 4.7 | 18 | 4.3 | 0.110 | 0.740 | ||
Age is a continuous variable expressed as mean ±SD and p value was computed with an independent t-test.
Statistically significant.
Other diagnosis includes oral abscess, otitis media and rheumatic fever.
CAP, community-acquired pneumonia; UTI, urinary tract infection.
Distribution of inappropriate antibiotic prescriptions amongst various prescribed antibiotics
Even though there was no statistically significant difference between prescriptions in the compliance and the non-compliance groups regarding the antibiotic prescription appropriateness of amoxicillin (55.1% versus 59.6%; p=0.176), cloxacillin (20.5% versus 25.5%; p=0.071), ciprofloxacin (43% versus 49.2%; p=0.064), metronidazole (25.6% versus 25.8%; p=0.958), tinidazole (3.3% versus 2.4%; p=0.408), sulfamethoxazole/trimethoprim (9.8% versus 9.2%; p=0.758), azithromycin (19.7% versus 19.6%; p=0.985), clarithromycin (10.7% versus 9.5%; p=0.549) and doxycycline (29.3% versus 31.7%; p=0.432), based on the 2021 Ethiopian standard treatment guideline, the guideline non-compliant inappropriate prescriptions were significantly more for outpatients prescribed with amoxicillin/clavulanic acid (33.2% versus 48.2%; p<0.001) and cephalexin (17.8% versus 24.3%; p=0.016) when compared with other antibiotics (Table 5).
Table 5.
Distribution of inappropriate antibiotic prescriptions by considering indication, dose, route, frequency and duration for patients attending outpatient department of Debre Markos Specialized Comprehensive Hospital.
| Variables | Compliance to guideline (n=488) | Non-compliance to guideline (n=423) | χ2 test | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | ||||||
| Utilized antibiotics | Penicillins | Amoxicillin | Yes | 269 | 55.1 | 252 | 59.6 | 1.834 | 0.176 |
| Amoxicillin/clavulanic acid | Yes | 162 | 33.2 | 204 | 48.2 | 21.298 | <0.001a | ||
| Cloxacillin | Yes | 100 | 20.5 | 108 | 25.5 | 3.267 | 0.071 | ||
| Fluoroquinolone | Ciprofloxacin | Yes | 210 | 43.0 | 208 | 49.2 | 3.440 | 0.064 | |
| Imidazole derivative | Metronidazole | Yes | 125 | 25.6 | 109 | 25.8 | 0.003 | 0.958 | |
| Tinidazole | Yes | 16 | 3.3 | 10 | 2.4 | 0.684 | 0.408 | ||
| Sulfonamide | Sulfamethoxazole/trimethoprim | Yes | 48 | 9.8 | 39 | 9.2 | 0.100 | 0.752 | |
| Macrolide | Azithromycin | Yes | 96 | 19.7 | 83 | 19.6 | 0.001 | 0.985 | |
| Clarithromycin | Yes | 52 | 10.7 | 40 | 9.5 | 0.359 | 0.549 | ||
| Tetracycline | Doxycycline | Yes | 143 | 29.3 | 133 | 31.7 | 1.679 | 0.432 | |
| Cephalosporin | Cephalexin | Yes | 87 | 17.8 | 103 | 24.3 | 5.839 | 0.016a | |
| Othersb | Yes | 24 | 4.9 | 19 | 4.5 | 0.092 | 0.762 | ||
Statistically significant.
Other antibiotics include benzyl penicillin, norfloxacin, nitrofurantoin, tetracycline, ceftriaxone.
Discussion
The overall antibiotic prescribing rate was found to be 55.3%, which was high and comparable with studies conducted in Ghana, Nigeria and China.21,22 Compared with results from Congo, India and Pakistan,23–25 a lower use of antibiotics was reported by the current study; this discrepancy might be due to the fact that these studies involved the assessment of antibiotic use in hospitalized patients, taking both oral and parenteral antibiotics. Moreover, our finding was much lower than that of the Netherlands in outpatient clinics.26 The possible reason for this variation might be that their study included all antimicrobials, thus broadening the medication category, whilst our study focused solely on antibiotics.
Amongst all antibiotic prescriptions, penicillins were the most commonly prescribed antibiotics, followed by fluoroquinolones, as observed in studies conducted in Europe and Nepal,27,28 whereas macrolides were the most prescribed antibiotics in studies from China, Malaysia and the USA.22,29,30 This finding illustrated that there is a difference in antibiotic utilization patterns and selection between communities/countries due to factors such as patient pressure, time constraints, medication availability and diagnosis uncertainty.31 Regarding specific medication prescriptions, amoxicillin was the most frequently prescribed antibiotic, similarly to a study conducted in India.32
Regarding the common infectious diseases encountered, respiratory tract infections were the most common indication for visiting the OPD followed by diseases of the gastrointestinal tract and urogenital system. The common individual diseases were community-acquired pneumonia, uncomplicated urinary tract infection and typhoid fever, commonly referred to as diseases of poverty and may be indicative of low socioeconomic development; worm infestation and respiratory diseases are common in poor developing countries.33,34 A similar study conducted in Malaysia reported upper respiratory tract infections followed by skin and soft tissue infections as the most common indications.29
Findings from various studies about inappropriate antibiotic prescription vary widely. A study conducted in the USA amongst ambulatory care visitors reported that approximately 30% of antibiotic prescriptions for outpatients were not appropriate.18 Another study conducted between 2017 and 2019 in primary healthcare facilities in China showed that 70.5% of antibiotic prescriptions were inappropriate.35 A systematic review including studies from six countries in the Gulf region revealed that inappropriate antibiotic prescriptions reach up to 80%.36 The possible reason for the observed difference might be a variation in the guidelines used to evaluate the appropriateness of antibiotic prescribing. In the current study, 49.54% of prescriptions did not adhere to the guideline, in line with a study conducted in Malaysia.29
Penicillins and fluoroquinolones made up the largest proportion of guideline non-compliant prescriptions mainly due to inappropriate selection of antibiotic agent and/or inappropriate dose/duration, and prescriptions for respiratory tract infection were the most frequently inappropriate. In line with other findings,18,37,38 19.2% of amoxicillin and 15.9% of ciprofloxacin use for unjustified indications were noted. Thus, ciprofloxacin and amoxicillin prescriptions have mainly deviated from standard treatment guidelines, facilitating the development of resistance strains to these medications. Besides inappropriate indications, wrong dosage and duration were also the causes for inappropriateness of prescriptions, which is concerning because dose and duration are critical not to exceed the minimum inhibitory concentration of a drug in the organism.39–41 Inappropriate dose/duration was common particularly in the treatment of gastrointestinal infections and urinary tract infections, where by metronidazole and cephalexin were given at a lower and higher daily dose, respectively, than the recommended doses.
Strengths and limitations
To our knowledge, this is the first study to review antibiotic prescription appropriateness focusing primarily on the OPD in Ethiopia with a broad range of antibiotics used. However, some limitations need to be addressed. First, the diagnoses of patients were determined based solely on what was written on the prescriptions. Second, factors needed for dose adjustments and selection of medications, such as monitoring parameters, clinical condition of patients or clinical and laboratory response of patients to the treatment, were not available because medical records were not accessed. Therefore, we recommend future studies with prospective follow-up designs to address the gaps of the current study.
Conclusions
The present study demonstrated that large proportions of antibiotic prescriptions for outpatients were non-compliant with the standard treatment guidelines. The study suggests that prescribers need to give special attention to outpatients whilst ordering antibiotics such as amoxicillin and ciprofloxacin. Antibiotic stewardship efforts to optimize outpatient antibiotic prescriptions and reduce the use of potentially inappropriate antibiotics are needed in Ethiopia. Additional studies are needed to explore antibiotic prescriptions in outpatient care settings and determine potential influencing factors associated with guideline non-compliant antibiotic prescriptions.
Acknowledgements
The authors would like to express their deepest sincere gratitude for data collectors and outpatient hospital pharmacy staff of Debre Markos specialized referral hospital for their efforts exerted in the realization of this study.
Footnotes
Contributions: Conceptualization, RBA; Data curation, TKZ and BMA; Formal analysis, MAA and RBA; Methodology, RBA, TKZ, MAA and BMA; Project administration, BMA and RBA; Supervision, TKZ, RBA,MAA and BMA; Writing original draft, RBA; Writing review and editing, RBA, TKZ, MAA and BMA. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2024/04/dic.2023-12-2-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2024 Abebe RB, Ayal BM, Alemu MA, Zeleke TK. https://doi.org/10.7573/dic.2023-12-2. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Submitted, fee waiver; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights, and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Availability of data and materials
All relevant data are in the manuscript. Additional data used to support the findings of this study are available from the corresponding author upon request.
References
- 1.Ardillon A, Ramblière L, Kermorvant-Duchemin E, et al. Inappropriate antibiotic prescribing and its determinants among outpatient children in 3 low-and middle-income countries: a multicentric community-based cohort study. PLoS Med. 2023;20(6):e1004211. doi: 10.1371/journal.pmed.1004211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clavenna A, Bonati M. Differences in antibiotic prescribing in paediatric outpatients. Arch Dis Child. 2011;96(6):590–595. doi: 10.1136/adc.2010.183541. [DOI] [PubMed] [Google Scholar]
- 3.Demoz GT, Kasahun GG, Hagazy K, et al. Prescribing pattern of antibiotics using WHO prescribing indicators among inpatients in Ethiopia: a need for antibiotic stewardship program. Infect Drug Resist. 2020;13:2783–2794. doi: 10.2147/IDR.S262104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA. 2018;115(15):E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14(8):742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 6.Bortone B, Jackson C, Hsia Y, Bielicki J, Magrini N, Sharland M. High global consumption of potentially inappropriate fixed dose combination antibiotics: analysis of data from 75 countries. PLoS One. 2021;16(1):e0241899. doi: 10.1371/journal.pone.0241899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308–1316. doi: 10.1093/cid/civ076. [DOI] [PubMed] [Google Scholar]
- 8.Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308–1315. doi: 10.1001/jamainternmed.2017.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saleem Z, Saeed H, Hassali MA, et al. Pattern of inappropriate antibiotic use among hospitalized patients in Pakistan: a longitudinal surveillance and implications. Antimicrob Resist Infect Control. 2019;8(1):188. doi: 10.1186/s13756-019-0649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wushouer H, Zhou Y, Zhang X, et al. Secular trend analysis of antibiotic utilisation in China’s hospitals 2011–2018, a retrospective analysis of procurement data. Antimicrob Resist Infect Control. 2020;9(1):53. doi: 10.1186/s13756-020-00709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinman MA, Gonzales R, Linder JA, Landefeld CS. Changing use of antibiotics in community-based outpatient practice, 1991–1999. Ann Intern Med. 2003;138(7):525–533. doi: 10.7326/0003-4819-138-7-200304010-00008. [DOI] [PubMed] [Google Scholar]
- 12.Angebault C, Andremont A. Antimicrobial agent exposure and the emergence and spread of resistant microorganisms: issues associated with study design. Eur J Clin Microbiol Infect Dis. 2013;32:581–595. doi: 10.1007/s10096-012-1795-3. [DOI] [PubMed] [Google Scholar]
- 13.Dellit TH, Owens RC, McGowan JE, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 14.Butler AM, Brown DS, Durkin MJ, et al. Association of inappropriate outpatient pediatric antibiotic prescriptions with adverse drug events and health care expenditures. JAMA Netw Open. 2022;5(5):e2214153. doi: 10.1001/jamanetworkopen.2022.14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xavier SP, Victor A, Cumaquela G, Vasco MD, Rodrigues OAS. Inappropriate use of antibiotics and its predictors in pediatric patients admitted at the Central Hospital of Nampula, Mozambique. Antimicrob Resist Infect Control. 2022;11(1):79. doi: 10.1186/s13756-022-01115-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735–743. doi: 10.1086/591126. [DOI] [PubMed] [Google Scholar]
- 17.van den Bosch CM, Geerlings SE, Natsch S, Prins JM, Hulscher ME. Quality indicators to measure appropriate antibiotic use in hospitalized adults. Clin Infect Dis. 2015;60(2):281–291. doi: 10.1093/cid/ciu747. [DOI] [PubMed] [Google Scholar]
- 18.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA. 2016;315(17):1864–1873. doi: 10.1001/jama.2016.4151. [DOI] [PubMed] [Google Scholar]
- 19.Arega B, Agunie A, Minda A, et al. Guideline recommendations for empirical antimicrobial therapy: an appraisal of research evidence for clinical decision-making in Ethiopia. Infect Dis Ther. 2020;9:451–465. doi: 10.1007/s40121-020-00308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ethiopian Ministry of Health. Standard Treatment Guidelines For General Hospitals. Fourth Edition. Ethiopia: Ethiopian Ministry of Health; 2021. [Accessed May 1, 2024]. https://doctorsonlinee.com/wp-content/uploads/2022/04/STG-Final-4th-edtion-2021-3.pdf . [Google Scholar]
- 21.Prah J, Kizzie-Hayford J, Walker E, Ampofo-Asiama A. Antibiotic prescription pattern in a Ghanaian primary health care facility. Pan Afr Med J. 2017;28:214. doi: 10.11604/pamj.2017.28.214.13940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wushouer H, Du K, Chen S, et al. Outpatient antibiotic prescribing patterns and appropriateness for children in primary healthcare settings in Beijing City, China, 2017–2019. Antibiotics. 2021;10(10):1248. doi: 10.3390/antibiotics10101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.José MW, Jean-Marie LI, Divine MM, Sabine KK. Point prevalence study of antibiotic use in hospitals in Butembo. Int J Med Med Sci. 2016;8(12):133–139. doi: 10.5897/IJMMS2016.1249. [DOI] [Google Scholar]
- 24.Landstedt K, Sharma A, Johansson F, Lundborg CS, Sharma M. Antibiotic prescriptions for inpatients having non-bacterial diagnosis at medicine departments of two private sector hospitals in Madhya Pradesh, India: a cross-sectional study. BMJ Open. 2017;7(4):e012974. doi: 10.1136/bmjopen-2016-012974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atif M, Azeem M, Saqib A, Scahill S. Investigation of antimicrobial use at a tertiary care hospital in Southern Punjab, Pakistan using WHO methodology. Antimicrob Resist Infect Control. 2017;6:41. doi: 10.1186/s13756-017-0199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Broek AK, van Hest RM, Lettinga KD, et al. The appropriateness of antimicrobial use in the outpatient clinics of three hospitals in the Netherlands. Antimicrob Resist Infect Control. 2020;9:40. doi: 10.1186/s13756-020-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2015. [DOI]
- 28.Lamichhane D, Giri BR, Pathak OK, Panta OB, Shankar PR. Morbidity profile and prescribing patterns among outpatients in a teaching hospital in Western Nepal. McGill J Med. 2006;9(2):126. [PMC free article] [PubMed] [Google Scholar]
- 29.Tan G-H, Low Q-W, Lim H-C, Seah H-K, Chan HK. Inappropriate antibiotic utilization: outpatient prescription review of a regional secondary hospital in Kedah, Malaysia. J Pharm Pract Community Med. 2017;3(4) doi: 10.5530/jppcm.2017.4.62. [DOI] [Google Scholar]
- 30.Durkin MJ, Jafarzadeh SR, Hsueh K, et al. Outpatient antibiotic prescription trends in the United States: a national cohort study. Infect Control Hosp Epidemiol. 2018;39(5):584–589. doi: 10.1017/ice.2018.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zetts RM, Stoesz A, Smith BA, Hyun DY. Outpatient antibiotic use and the need for increased antibiotic stewardship efforts. Pediatrics. 2018;141(6):e20174124. doi: 10.1542/peds.2017-4124. [DOI] [PubMed] [Google Scholar]
- 32.Kotwani A, Holloway K. Trends in antibiotic use among outpatients in New Delhi, India. BMC Infect Dis. 2011;11:99. doi: 10.1186/1471-2334-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Scaling Up the Response to Infectious Diseases: A Way Out of Poverty: Report on Infectious Diseases 2002. World Health Organization; 2002. [Accessed April 24, 2024]. https://iris.who.int/bitstream/handle/10665/67248/WHO_CDS_2002.7.pdf . [Google Scholar]
- 34.Weatherhead JE, Porter P, Coffey A, et al. Ascaris larval infection and lung invasion directly induce severe allergic airway disease in mice. Infect Immun. 2018;86(12):e00533–18. doi: 10.1128/iai.00533-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu M, Gong Z, Zhu Y, et al. Inappropriate antibiotic prescribing in primary healthcare facilities in China: a nationwide survey, 2017–2019. Clin Microbiol Infect. 2023;29(5):602–609. doi: 10.1016/j.cmi.2022.11.015. [DOI] [PubMed] [Google Scholar]
- 36.Mahmood RK, Gillani SW, Saeed MW, Hafeez MU, Gulam SM. Systematic review: study of the prescribing pattern of antibiotics in outpatients and emergency departments in the Gulf Region. Front Pharmacol. 2020;11:585051. doi: 10.3389/fphar.2020.585051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King LM, Fleming-Dutra KE, Hicks LA. Advances in optimizing the prescription of antibiotics in outpatient settings. BMJ. 2018;363:k3047. doi: 10.1136/bmj.k3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dobson EL, Klepser ME, Pogue JM, et al. Outpatient antibiotic stewardship: interventions and opportunities. J Am Pharm Assoc. 2017;57(4):464–473. doi: 10.1016/j.japh.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 39.Lu Y, Dou Y, Ti J, et al. The effect of Tembusu virus infection in different week-old Cherry Valley breeding ducks. Vet Microbiol. 2016;192:167–174. doi: 10.1016/j.vetmic.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto K, Kijima M, Yoshimura H, Takahashi T. Antimicrobial susceptibilities of Erysipelothrix rhusiopathiae isolated from pigs with swine erysipelas in Japan, 1988–1998. J Vet Med B. 2001;48(2):115–126. doi: 10.1111/j.1439-0450.2001.00427.x. [DOI] [PubMed] [Google Scholar]
- 41.Gashaw T, Sisay M, Tesfa T, Baye Y, Amare F. Amoxicillin utilization pattern at governmental hospitals in Eastern Ethiopia. Infect Drug Resist. 2021;14:193–203. doi: 10.2147/IDR.S288387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are in the manuscript. Additional data used to support the findings of this study are available from the corresponding author upon request.