Abstract
The role of gamma interferon (IFN-γ) in the permanent control of infection with a noncytopathic virus was studied by comparing immune responses in wild-type and IFN-γ-deficient (IFN-γ −/−) mice infected with a slowly invasive strain of lymphocytic choriomeningitis virus (LCMV Armstrong). While wild-type mice rapidly cleared the infection, IFN-γ −/− mice became chronically infected. Virus persistence in the latter mice did not reflect failure to generate cytotoxic T-lymphocyte (CTL) effectors, as an unimpaired primary CTL response was observed. Furthermore, while ex vivo CTL activity gradually declined in wild-type mice, long-standing cytolytic activity was demonstrated in IFN-γ −/− mice. The prolonged effector phase in infected IFN-γ −/− mice was associated with elevated numbers of CD8+ T cells. Moreover, a higher proportion of these cells retained an activated phenotype and was actively cycling. However, despite the increased CD8+ T-cell turnover, which might have resulted in depletion of the memory CTL precursor pool, no evidence for exhaustion was observed. In fact, at 3 months postinfection we detected higher numbers of LCMV-specific CTL precursors in IFN-γ −/− mice than in wild-type mice. These findings indicate that in the absence of IFN-γ, CTLs cannot clear the infection and are kept permanently activated by the continuous presence of live virus, resulting in a delicate new balance between viral load and immunity. This interpretation of our findings is supported by mathematical modeling describing the effect of eliminating IFN-γ-mediated antiviral activity on the dynamics between virus replication and CTL activity.
CD8+ effector T cells are central mediators of antiviral immunity. These cells have been found to exert their antiviral functions by at least two distinct mechanisms. First, CD8+ effector T cells can recognize and kill virus-infected cells either via perforin-dependent lysis or through Fas-Fas ligand interaction, leading to apoptosis of the target cell (18, 40, 45). Second, virus-specific CD8+ T cells are potent producers of antiviral cytokines, in particular gamma interferon (IFN-γ), which may attenuate viral replication, e.g., by rendering uninfected cells refractory to viral infection (32, 33). The relative importance of these two different effector mechanisms (cell lysis versus antiviral cytokines) in the elimination of a viral infection is hypothesized to be heavily influenced by the virus and its life cycle. Thus, resolution of cytopathic viruses is thought to be mediated mainly by soluble mediators such as antibodies and interferons, whereas cytotoxicity should be crucial for the clearance of a noncytopathic virus (17). Murine lymphocytic choriomeningitis virus (LCMV) infection is often taken as a protypic example of an infection with a noncytopathic virus; consistent with the above-cited hypothesis, the majority of studies using this model system have revealed that IFN-γ plays only a marginal role in clearance of acute LCMV infection (15, 21, 29, 39, 42, 44). In contrast, this cytokine seems to be important for adoptive immunotherapy of persistent virus carriers (mice infected at birth) (31, 39). However, a recent study by our group has revealed that the importance of IFN-γ during acute LCMV infection is strongly influenced by the invasiveness of the virus strain used to probe the role of cytokines (30). Thus, using mice deficient in IFN-γ (IFN-γ −/− mice), we have found that systemic infection with a rapidly invasive strain of LCMV (the Traub strain) leads to a CD8+ effector T-cell-mediated wasting syndrome and subsequent death in the majority of infected mice. This disease appears to be the result of an imbalance between virus replication in internal organs and the host response, resulting in severe immune-mediated tissue damage. Infection of mice with a lower dose of the same virus strain resulted in reduced mortality and persistent infection in survivors (unpublished observation). In contrast, IFN-γ was not required to protect mice infected with the slowly invasive LCMV Armstrong strain, although higher virus levels were observed in organs of IFN-γ −/− mice evaluated 10 days postinfection (p.i.). However, whether lack of IFN-γ merely causes a delay in virus clearance or fundamentally impairs the ability to control LCMV infection has not been resolved. As this distinction is scientifically important, the purpose of the present study was to investigate whether complete and permanent virus control could be accomplished in the absence of IFN-γ. This question was addressed using IFN-γ −/− mice infected with a moderate dose of LCMV Armstrong (10). Since a persistent infection was induced under these conditions, the cellular immune response was analyzed in order to clarify whether persistence of virus resulted from exhaustion of the capacity to generate virus-specific cytotoxic cell lymphocytes (CTLs) or from a more subtle change in the equilibrium that normally is established between the virus and the host (9, 35, 36, 38, 43). Our results reveal that virus persistence is not due to exhaustion of virus-specific CTLs or to impaired CTL memory. Rather, our results disclose that cytotoxic effectors are insufficient to completely eliminate LCMV but are kept in a permanently activated state by the continuous presence of antigen. This results in a shift in the equilibrium of CTL activity and virus replication, leading to a finely balanced long-standing coexistence of viral persistence and active T-cell immune surveillance. The discovery of this new pattern in LCMV-infected mice has implications for our general understanding of the role of host immunity in chronic viral infections in humans (e.g., infection caused by hepatitis B and C viruses, cytomegalovirus, and human immunodeficiency virus).
MATERIALS AND METHODS
Mice.
C57BL/6 (B6) wild-type mice were obtained either from Bomholtgaard Ltd. (Ry, Denmark) or the Jackson Laboratory (Bar Harbor, Maine). IFN-γ −/− mice (C57BL/6-ifg) were also derived from the Jackson Laboratory either directly or as the progeny of breeder pairs thus obtained. Seven- to 10-week-old mice were used in all experiments, and animals were always allowed to acclimatize to the local environment for at least 1 week before use. All animals were housed under specific-pathogen-free conditions as validated by screening of sentinels. All animal experiments were conducted according to institutional guidelines.
Virus.
LCMV of the Armstrong strain (clone 53b) was used in all experiments (50). Mice to be infected received a dose of 4,800 PFU in an intravenous (i.v.) injection of 0.3 ml.
Virus titration.
Organ virus titers were assayed by intracerebral inoculation of 10-fold dilutions of a 10% organ suspension into young adult Swiss mice. Titration endpoints were calculated by the Kärber method and expressed as mean lethal dose.
In vivo BrdU labeling.
Mice were given 5-bromo-2′-deoxyuriduine (BrdU; Sigma Chemical Co., St. Louis, Mo.) at 0.4 mg/ml in their drinking water for a period of 7 days. BrdU-containing water was protected from light and changed daily.
Cell preparations.
Spleens from mice were aseptically removed and transferred to Hanks' balanced salt solution. Single-cell suspensions were obtained by pressing the organs through a fine sterile steel mesh, and erythrocytes were lysed by 0.83% NH4Cl treatment. The cells were washed twice with Hanks' balanced salt solution, and cell concentration was adjusted in RPMI 1640 containing 10% fetal calf serum, supplemented with 2-mercaptoethanol, l-glutamine, and penicillin-streptomycin solution.
Limiting dilution analysis (LDA).
CTL precursor (CTLp) frequencies were determined as previously described (11). Threefold dilutions of responder cells were added in 100 μl of medium to round-bottom 96-well microtiter plates. Replicates (24 wells) were plated for each responding cell dilution and cocultured with 100 μl (3 × 105 cells) of γ-irradiated (2,500 R), T-cell-depleted wild-type splenocytes either unpulsed or pulsed with glycoprotein 33-41 (GP33-41) or nucleoprotein 396-404 (NP396-404) (the two immunodominant major histocompatibility complex [MHC] class I-restricted peptides of LCMV in H-2b mice [13, 34, 49]). The medium contained 10 U of human recombinant interleukin-2 per liter. Three identical sets of cultures were initiated with different stimulators and incubated for 7 days at 37°C in a humidified atmosphere. On day 4, 20 μl of medium with interleukin-2 (100 U/ml) was added to the cultures. The contents of individual wells were tested for cytotoxicity at the end of the culture period by incubating each well with 5,000 51Cr-labeled, peptide-pulsed or unpulsed EL-4 cells (H-2b, MHC-I+II−) for 6 h. Wells were considered positive if the cytotoxic activity exceeded the average + 3 standard deviations of the spontaneous release of target cells incubated with medium alone. Minimal estimates of pCTL frequencies were obtained according to the Poisson distribution.
Cytotoxicity assays.
Virus-specific CTL activity was assayed in a standard 51Cr release assay using EL-4 cells pulsed for 1 h at 37°C with LCMV GP33-41 or NP396-404 peptide; unpulsed EL-4 cells served as control targets. Assay time was 5 h, and percent specific release was calculated as described previously (22, 37).
MAbs for flow cytometry.
The following monoclonal antibodies (MAbs) were all purchased from PharMingen as rat anti-mouse antibodies: fluorescein isothiocyanate- and biotin-conjugated anti-CD49d (common α chain of LPAM-1 and VLA-4), phycoerythrin-conjugated anti-CD8a, biotin-conjugated anti-CD62 ligand (CD62L; L-selectin, MEL-14), and biotin-conjugated anti-CD44. Fluorescein isothiocyanate-conjugated anti-BrdU was purchased from Becton Dickinson (San Jose, Calif.).
Flow cytometric analysis.
One million cells were stained with directly labeled MAb in staining buffer (10% rat serum, 1% bovine serum albumin, and 0.1% NaN3 in phosphate-buffered saline [PBS]) for 20 min in the dark at 4°C and subsequently washed. If biotin-conjugated MAb was used, cells were additionally incubated with streptavidin–Tri-color or streptavidin-CyCrome (Caltag Laboratories, San Francisco, Calif.), washed, and fixed with 1% paraformaldehyde (2, 3, 8).
For BrdU staining (4, 12, 41), cells were stained for surface markers as described above, resuspended in PBS–1% NaN3, transferred to ice-cold 0.15 M NaCl solution, and fixed by adding ice-cold 96% ethanol drop by drop. After incubation for 30 min on ice, cells were washed with PBS and resuspended in PBS with 0.01% Tween 20 and 1% paraformaldehyde. After a 1-h incubation at room temperature, cells were pelleted and resuspended in PBS with 0.15 M NaCl–4.2 mM MgCl2 (pH 5) containing DNase I (50 Kunitz U/ml; Sigma). Following incubation for 15 min at 37°C, cells were washed once in PBS before addition of the anti-BrdU MAb. After incubation with antibody for 30 min at room temperature, cells were washed in PBS and analyzed using CellQuest software.
Mathematical modeling.
To analyze the effect of eliminating IFN-γ production on the dynamics between virus replication and the CTL response, we consider a simple mathematical model consisting of three variables: uninfected target cells (x), infected target cells (y), and a CTL response (z). It is given by the following set of differential equations:
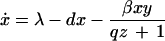 |
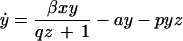 |
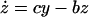 |
Uninfected cells are produced at a constant rate λ, die at a rate dx, and become infected by the virus at a rate βxy. The rate of virus replication is reduced by IFN-γ, secreted from CTLs, at a rate qz + 1. Infected cells die at a rate ay and become lysed by CTLs at a rate pyz. The CTL response expands in response to antigen at a rate cy and decays at a rate bz. The efficacy of IFN-γ-mediated virus inhibition is therefore captured in the parameter q. IFN-γ −/− mice are characterized by a value of q = 0, while wild-type mice have a value of q >> 0. If the basic reproductive ratio of the virus (R0 = βλ/da) is greater than unity, the virus can initially establish an infection, resulting in expansion of the CTL response. The system subsequently converges to a stable equilibrium, described by the following expressions:
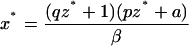 |
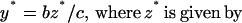 |
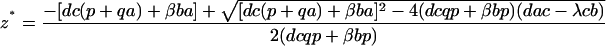 |
RESULTS
Kinetics of virus clearance in IFN-γ −/− mice.
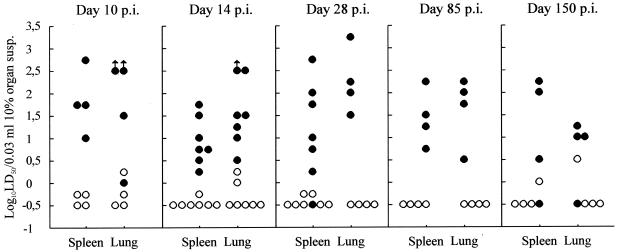
While wild-type mice exhibit few symptoms, i.v. infection with the rapidly invasive LCMV Traub strain induces severe, mostly fatal wasting disease in IFN-γ-deficient mice (30). Previous analysis has revealed that this outcome is the result of augmented immunopathology associated with exacerbated virus replication in the viscera. In contrast, IFN-γ −/− mice infected with even higher doses of LCMV Armstrong strain exhibit only mild and transient disease. To determine whether the latter clinical outcome reflects complete and permanent virus control in this case, the kinetics of virus clearance were followed in IFN-γ −/− mice infected with a relatively low dose (4,800 PFU) of the slowly invasive LCMV Armstrong strain. At different time points after infection, groups of gene knockout mice and similarly infected wild-type animals were sacrificed, and spleens and lungs were assayed for virus contents. As shown in Fig. 1, infected IFN-γ −/− mice developed a persistent infection with substantial levels of virus in spleens and lungs for as long as 5 months after infection. In contrast, most wild-type mice cleared the infection in less than 2 weeks. Thus, IFN-γ is mandatory for control of LCMV Armstrong replication even though this virus strain spreads slowly in the host and has little potential for causing persistent infection in fully immunocompetent mice even at high doses (26, 38).
FIG. 1.
Organ virus titers in IFN-γ −/− (●) and wild-type (○) B6 mice infected i.v. with 4,800 PFU of LCMV Armstrong. Points represent individual mice. (Parallel analysis of organ virus levels in mice deficient in both IFN-γ and perforin revealed titers roughly 3 log10 higher [not shown].) LD50, mean lethal dose.
Kinetics of ex vivo virus-specific CTL activity.
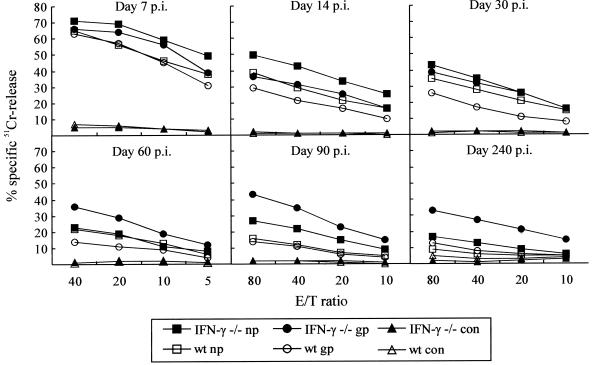
LCMV infection is primarily controlled through CTL-mediated lysis of infected cells (18, 45). Therefore, to investigate whether the impaired capacity to control the infection in IFN-γ −/− mice resulted from a failure to generate virus-specific CTL effectors, we assayed ex vivo splenic CTL activity to GP33-41 and NP396-404 at several time points (7, 14, 30, 60, and 90 days) after infection. As evident from Fig. 2, CTL activity during the acute phase of the infection, i.e., day 7 p.i., was of similar magnitude in the two strains, confirming previous findings indicating that IFN-γ is not essential for effector cell differentiation. However, a significant difference in long-term maintenance of ex vivo cytotoxicity was noted between the strains. Specifically, substantial CTL activity was maintained in IFN-γ −/− mice, and ex vivo lysis at 3 months p.i. was of the same magnitude as that measured after 2 weeks. In contrast, CTL activity in wild-type mice decreased with time and was almost undetectable by 3 months p.i. (Fig. 2). Interestingly, the relative strength of the CTL responses to GP33-41 and NP396-404 shifted with time in most infected IFN-γ −/− mice. Thus, NP396-404-pulsed target cells tended to be killed more efficiently than GP33-41-pulsed targets early in the infection (days 7 and 14 p.i.; eight mice individually analyzed). However, with time a gradual shift was noted, such that at 90 days p.i., seven out of eight mice had higher cytolytic activity against GP33-41-pulsed targets; a similar shift was not observed in wild-type mice, in which equivalent killing of both types of targets was consistently observed. Only two IFN-γ −/− mice were analyzed at 240 days p.i., but the pattern was similar to that observed on days 60 and 90 p.i. Together, the above findings indicate that virus persistence in IFN-γ-deficient mice is not due to failure of effector T-cell generation. Rather, a chronic coexistence of infectious virus and virus-specific CTL effectors is noted.
FIG. 2.
Time course of ex vivo T-cell-mediated cytotoxicity in IFN-γ −/− and wild-type (wt) B6 mice infected i.v. with 4,800 PFU of LCMV Armstrong. Cytotoxicity toward LCMV GP33-41 (gp)- and NP396-404 (np)-pulsed, histocompatible EL-4 cells was analyzed; unpulsed EL-4 cells served as control (con) targets. CTL activity in individual mice was assayed; medians of two to eight mice per time point are depicted. On day 60 p.i., effector/target ratios were 40:1, 20:1, 10:1, and 5:1; at all other time points, the ratios were 80:1, 40:1, 20:1, and 10:1.
Chronic activation of CD8+ T cells in IFN-γ −/− mice.
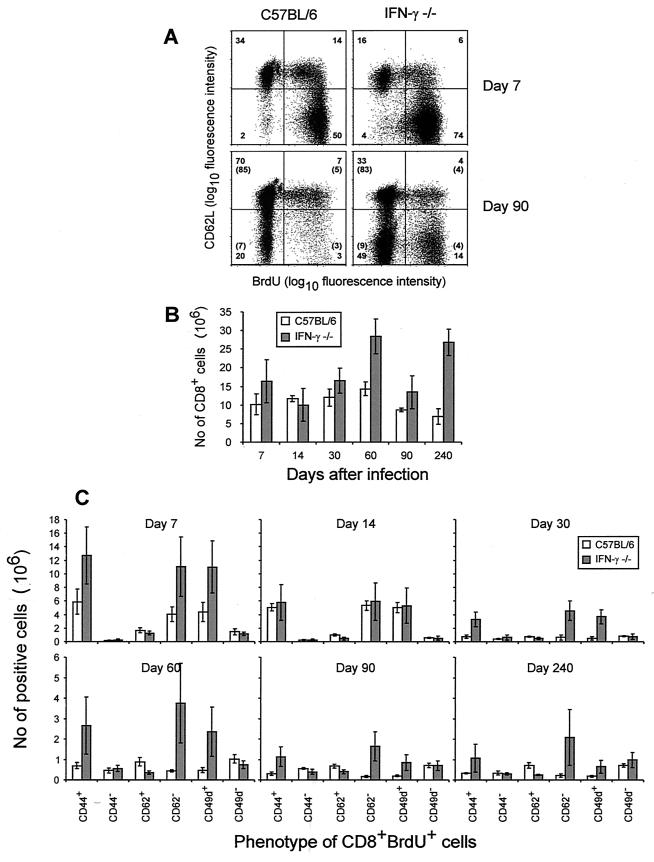
The continuously elevated ex vivo CTL activity observed in LCMV-infected IFN-γ −/− mice suggested that the number of activated CD8+ T cells as well as CD8+ cell cycling might be increased in these mice. Phenotypic characterization (2) of splenocytes from infected mice (Fig. 3) revealed that activated CD8+ T cells initially were generated at almost equal numbers in both mouse strains. However, at 2 to 3 months p.i., most splenic CD8+ T cells in wild-type mice had a naive phenotype (CD44low VLA-4low L-selhigh) as expected once the infection was controlled (2, 3). In contrast, infected IFN-γ −/− mice had substantially more splenic CD8+ T cells than wild-type mice (Fig. 3B), and a high proportion of these cells had an activated phenotype (CD44high VLA-4high L-sellow) (Fig. 3A shows a typical example). Furthermore, analysis of CD8+ T-cell proliferation by use of BrdU labeling (Fig. 3c) revealed that more splenic CD8+ T cells from knockout mice were actively cycling at 2 and 3 months p.i. Notably, the majority of cycling cells in IFN-γ −/− mice had retained the activated L-sellow phenotype (Fig. 3A and C). This is in contrast to the situation in wild-type mice, in which this pattern was seen only in the acute phase of infection. It should be added that only marginal differences between wild-type and IFN-γ −/− mice were detected in uninfected controls. Altogether, these results suggest that generation of CTL effectors is insufficient to mediate virus clearance in the absence of IFN-γ, but LCMV-specific CD8+ effectors are maintained in an activated state by the continuous presence of high levels of viral antigen.
FIG. 3.
Phenotypic analysis of splenic CD8+ T cells in B6 and IFN-γ −/− mice infected i.v. with 4,800 PFU of LCMV Armstrong. (A) CD62L and BrdU expression on CD8+ T cells. Numbers in parentheses refer to mean sizes of the same subsets in uninfected controls. (B) Total number of splenic CD8+ T cells as a function of time after infection. Values shown are averages ± standard deviations from groups of four to five mice. (C) Phenotype of CD8+ BrdU+ splenocytes as a function of time after infection. Values shown are averages ± standard deviations from groups of four to five mice. Note that on day 240 p.i., only two IFN-γ −/− mice were analyzed.
pCTL levels in IFN-γ −/− mice.
Since the persistent infection in IFN-γ −/− mice was associated with long-standing CD8+ T-cell activation, it was pertinent to ask whether this resulted in depletion of the CTL memory pool (persisting antigen might drive the specific T cells toward terminal differentiation, thereby reducing their proliferative potential). Functionally the most basic memory T cells are characterized by the capacity to undergo rapid and extensive expansion, thus leading to a marked clonal burst of differentiated effector cells (25). Since LDA is the only means to quantitate this functionally defined subset, groups of IFN-γ −/− and wild-type mice were infected with 4,800 PFU of LCMV Armstrong, and the frequencies of virus-specific pCTLs were measured by LDA at 3 months p.i.; virus-specific CTL memory was evaluated using two immunodominant MHC class I-restricted peptides (GP33-41 and NP396-404) (13, 34, 49). As evident from Table 1, the total number of LCMV-specific pCTL was not lower in infected IFN-γ −/− mice than in wild-type mice; rather, precursor levels were consistently higher in IFN-γ −/− mice. Thus, in two independent experiments the knockout mice contained three to six times more LCMV-specific pCTLs. Persistent infection thus did not drive the CTL memory pool toward extinction.
TABLE 1.
Number of LCMV-specific CD8+ T cells in B6 and IFN-γ −/− micea
| Expt | Specificity | No. of LCMV-specific CD8+ T cells
|
|
|---|---|---|---|
| B6 | IFN-γ −/− | ||
| 1 | NP | 1.3 × 104 ± 4.3 × 103 | 5.2 × 104 ± 2.5 × 104 |
| GP | 8.6 × 103 ± 3.8 × 103 | 2.5 × 104 ± 1.2 × 104 | |
| 2 | NP | 1.1 × 105 ± 5.1 × 104 | 3.6 × 105 ± 1.4 × 105 |
| GP | 7.5 × 104 ± 1.9 × 104 | 4.6 × 105 ± 2.9 × 105 | |
In two independent experiments, B6 and IFN-γ −/− mice were infected with 4,800 PFU of LCMV Armstrong. Ninety days later, individual pCTL frequencies toward the two dominant LCMV epitopes were determined as described in Materials and Methods. Results are averages ± standard deviations of three to five mice per group.
Mathematical modeling of virus control and CTL activity in LCMV-infected IFN-γ −/− and wild-type mice.
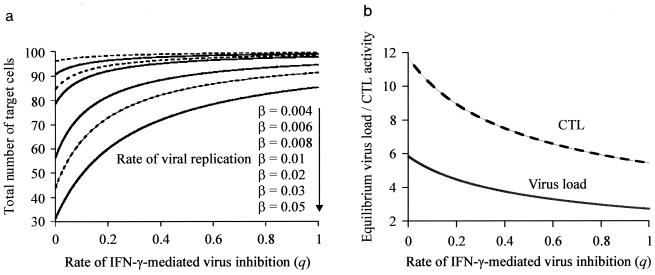
Finally, to investigate whether the above findings could be explained in simple quantitative terms, we used the mathematical model described in Materials and Methods to analyze the effect of eliminating IFN-γ production on the dynamics between virus replication and the CTL response. We looked at the effect of the rate of IFN-γ-mediated virus inhibition on (i) the degree of CTL-induced pathology and (ii) the correlation between CTL and virus load at equilibrium. The rate of IFN-γ-mediated virus inhibition is described by the parameter q. IFN-γ −/− mice are described by a value of q = 0, while wild-type mice are characterized by a value of q >> 0. Figure 4a shows the effect of IFN-γ production on the degree of CTL-induced pathology, measured by the total number of target cells (uninfected and infected) seen at equilibrium. The degree of CTL-induced pathology observed in IFN-γ −/− mice (q = 0) depends on the rate of viral replication. If the virus replicates at a high rate, strong depletion of target cells is observed in IFN-γ knockout mice, and the host is likely to die, as we have previously observed following infection with LCMV Traub. On the other hand, if the virus replicates slowly, there is minimal target cell depletion, and the host is likely to survive with virus and CTLs coexisting. Figure 4b shows the effect of IFN-γ production on the level of virus load and CTLs at equilibrium. The lower the rate of IFN-γ-mediated virus inhibition, the higher the virus load and the higher the number of CTLs seen at equilibrium. Figure 5 illustrates a typical time course for CTL activity and virus load in knockout mice and wild-type animals. Thus, the model predicts that IFN-γ −/− mice show both a higher LCMV load and a higher number of LCMV-specific CTLs. If CTLs lose the ability to secrete IFN-γ, they become less efficient at inhibiting viral replication. The less efficient the CTLs, the more that can persist due to the higher availability of antigen. This result is similar to predator-prey dynamics in ecology: if each predator is very efficient at killing the prey, a given prey population will not be able to sustain a large number of predators. In contrast, if each predator is inefficient and consumes only a small fraction of the prey population, a large number of predators may coexist at equilibrium. It should be noted that the above model assumes that IFN-γ acts by reducing the rate of viral replication. An alternative, indirect mechanism would be that IFN-γ augments the efficacy of in vivo CTL-mediated killing. However, if this possibility is explicitly included into the model, the results concerning the effect of IFN-γ knockout remain identical. Mathematical models of lytic and nonlytic immunity have recently been analyzed by Wodarz and Nowak (47).
FIG. 4.
Effect of IFN-γ-mediated virus inhibition (q) on the outcome of LCMV infection, as predicted by the mathematical model. IFN-γ −/− mice are characterized by a value of q = 0. (a) Effect of IFN-γ-mediated virus inhibition on the context of CTL-induced pathology, described by the total number of target cells (uninfected plus infected) at equilibrium. Reducing IFN-γ activity results in increased levels of target cell depletion. However, the exact extent of CTL-mediated pathology strongly depends on the replication rate of the virus, β. The faster the replication kinetics of the virus, the stronger the degree of CTL-induced pathology, especially in IFN-γ −/− mice. If viral replication is slow, there is no significant pathology even in the absence of IFN-γ. (b) Effect of IFN-γ-mediated virus inhibition on the level of virus load and CTL activity at equilibrium. Decreasing the rate of IFN-γ activity results in an increase in both virus load and virus-specific CTL activity. Baseline parameters were chosen as follows: λ = 10; d = 0.1; β = 0.05; a = 0.1; p = 0.1; c = 0.2; b = 0.1.
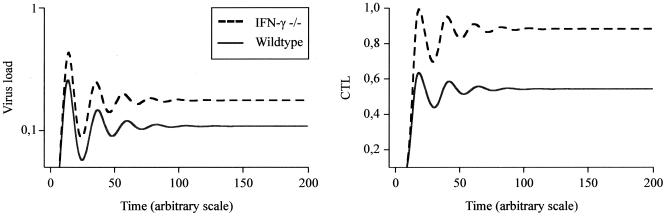
FIG. 5.
Time series of virus load and CTL activity in wild-type (q = 1) and IFN-γ −/− (q = 0) mice as predicted by the mathematical model. In IFN-γ −/− mice, virus load and CTL activity are higher than in wild-type mice, both during the acute phase of the infection and at equilibrium. Parameters were chosen as follows: λ = 10l; d = 0.1; β = 0.01; a = 0.1; p = 1; c = 0.5; b = 0.1.
DISCUSSION
It has been difficult to obtain a clear impression of the relative importance of antiviral cytokines versus cell contact-dependent lysis as effector mechanisms in antiviral immunity. A prevailing hypothesis predicts that soluble mediators such as IFN-γ and antibodies are important in controlling cytopathic viruses, whereas T-cell-mediated cytotoxicity is crucial for elimination of noncytopathic viruses (17). However, although cytotoxicity is obviously required for clearance of a noncytopathic virus such as LCMV (18, 45), it is clearly not sufficient (19, 31, 36), and IFN-γ may be an additional required factor (33). Thus, although some studies have suggested that IFN-γ is redundant in the resolution of infection with noncytopathic virus (15, 21, 29, 39, 42, 44), we have recently found this cytokine to be mandatory for control of infection with noncytopathic but rapidly invasive LCMV Traub (30). In contrast, IFN-γ seemed less important for the acute control of the slowly invasive LCMV Armstrong strain. However, whether complete and permanent virus control could be accomplished in the absence of IFN-γ was not addressed at the time. The primary aim of the present study was therefore to resolve that important question. This was done by assessing the role of IFN-γ in the long-term control of infection with the latter LCMV strain. Our results disclose that systemic infection with LCMV Armstrong in mice deficient in IFN-γ with few exceptions evolves into a chronic persistent infection, despite the fact that this virus strain has a limited potential for causing persistent infection in immunocompetent wild-type mice even at very high doses (26, 27). To determine what happens with regard to the cell-mediated immune response in persistently infected IFN-γ −/− mice, we have analyzed different parameters of the LCMV-specific CD8+ T-cell response. Most importantly, we found significantly higher ex vivo virus-specific CTL activity in IFN-γ −/− mice than in wild-type mice, especially in the late phase of infection (3 months p.i.). Thus, the failure to eliminate the virus in LCMV Armstrong-infected IFN-γ −/− mice is not caused by failure of effector cell generation. This observation confirms our recent interpretation that the collapse of the LCMV-specific CTL response noted in LCMV Traub-infected, IFN-γ −/− mice is an immunopathological event and not the result of a requirement for IFN-γ in effector cell differentiation as suggested by others (20, 46). At 3 months p.i. we also found an expanded population of LCMV-specific pCTLs in IFN-γ −/− mice. Although there is a continuing debate concerning this issue, pCTLs as detected by LDA are likely to represent the true memory cells (25), i.e., the cells critical for long-term maintenance of CTL responsiveness. Thus, it may be concluded that virus-specific CTL memory is induced and maintained in IFN-γ −/− mice at a level which is not lower than observed in wild-type mice. This finding excludes that IFN-γ is required for the generation of LCMV-specific memory pCTLs, a mechanism recently proposed to limit the responsiveness of LCMV-infected CD40 ligand-deficient mice (6). More important, it is evident that the antiviral CD8+ T-cell response is not pushed toward exhaustion in persistently infected IFN-γ −/− mice. Virus persistence due to exhaustion of CTL memory has previously been documented in mice lacking either CD4+ T cells or B cells as well as in wild-type mice infected with very high doses of virus and in mice infected with certain rapidly invasive LCMV strains (1, 5, 7, 14, 24, 26, 28, 36). Thus, the chronic infection of IFN-γ −/− mice represents a previously undescribed outcome of LCMV infection characterized by long-term coexistence of virus-specific effector cells and substantial viral infection. Precisely how IFN-γ exerts its antiviral role is not known. One possibility is that this cytokine directly impairs virus spreading by reducing the susceptibility of uninfected cells to infection. Another possibility is that IFN-γ works by augmenting antigen processing and presentation and thus indirectly enhances the effector capability of available CTL effectors. While the present data do not allow a distinction between these possibilities, it is evident that the effect is found in the efferent phase, not in the afferent or central phase, of the T-cell response.
Interestingly, ex vivo CTL activity against the two major viral epitopes, GP33-41 and NP396-404, tends to change over time in IFN-γ −/− mice. Thus, responses of about equal magnitude were seen during the acute phase of infection. With time, however, CTL activity against GP33-41 gradually becomes more prominent, and at 3 months p.i. CTL activity against this epitope is found to be significantly higher. This finding indicates that CD8+ T cells directed toward the two immunodominant epitopes are differentially affected by the chronic infection in IFN-γ −/− mice. A couple of studies have previously revealed significant changes in the T-cell repertoire during persistent viral infection. One study on CD4-deficient mice chronically infected with LCMV revealed a selective deletion of NP396-404-specific CTLs, whereas most GP33-41-specific CTLs became anergic. In another study, selection of CD8+ T cells with highly focused specificity was found in mice chronically infected with JHM strain of mice hepatitis virus in the central nervous system (23). Whether selection of certain memory or persistent CTLs takes place during the apoptotic phase of acutely activated CTLs or via antigen-delivered restimulation of distinct memory cell subsets is still a matter of debate (25). It is also unclear whether high-avidity CTLs are more prone to apoptosis or selectively maintained during persistence by low-level antigen stimulation (51). In this context, it is of interest that a recent study indicate that NP396-404-specific CTLs are more efficient as antiviral effectors in vivo (14). Moreover, this difference appeared to be even more pronounced in mice lacking IFN-γ functions. Thus, interestingly, preferential maintenance of a seemingly less efficient CTL subset seems to take place in persistently infected IFN-γ −/− mice.
In agreement with the prolonged effector response observed in infected IFN-γ −/− mice, flow cytometric analysis revealed the persistence of an activated (L-sellow VLA-4high CD44high [2, 3]) and cycling (BrdU+) CD8+ T-cell subset in these mice. Although the specificity of the activated CD8+ T cells has not been directly demonstrated, the presence of these cells is clearly related to the ongoing infection, as a similar pattern is not observed in uninfected controls. Therefore, our results indicate that in the absence of IFN-γ, CTLs are insufficient to clear the virus but are kept in an activated state (as evidenced functionally and, probably, also phenotypically) by the continuous presence of live virus. This results in a delicate new balance between viral load and host immunity. This interpretation of the experimental findings is indeed supported by theoretical modeling describing the effects elimination of IFN-γ-mediated antiviral activity would have on the steady-state levels of virus and CTL activity provided that IFN-γ deficiency is not associated with impairment of CTL expansion and differentiation. The model predicts that the steady-state levels of both virus replication and CTL activity are markedly influenced by the absence of IFN-γ if this cytokine contributes significantly to virus control. In the absence of IFN-γ, the equilibrium levels of both populations are increased, which is in total agreement with our experimental observations. This result also agrees with data showing a positive correlation between CTL and provirus load among asymptomatic human T-cell leukemia virus type 1 (HTLV-1) carriers (48). There is evidence that in HTLV-1 infection, a higher provirus load is the result of a weaker CTL response, and this is associated with higher numbers of CTLs at equilibrium (16).
In a previous study, we found that the role of IFN-γ in controlling LCMV infection is critically dependent on the invasiveness of the virus strain under investigation (30). Thus, infection with the rapidly invasive LCMV Traub was found to result in severe tissue damage and subsequent death in the absence of IFN-γ. In the present report, we show that IFN-γ −/− mice infected with LCMV Armstrong survive but never completely control the infection. This is in agreement with the theoretical prediction that a persistent infection coexisting with CTLs in the absence of substantial immunopathology is possible only for slowly replicating virus strains. Persistence of virus is not due to exhaustion of CTL effector capacity or impaired CTL memory. The present study therefore demonstrates that IFN-γ is absolutely instrumental for complete and permanent control of infection with this noncytopathic virus even in the face of a permanently hypercompensated CTL response. Thus, the capacity to produce IFN-γ critically regulates the virus-host balance during what is normally perceived as the memory phase of this infection, and in IFN-γ-deficient mice a new equilibrium, characterized by long-term stable coexistence of virus-specific CTL effectors and widespread virus replication, is established. This is in contrast to previous studies which found that chronic LCMV infection normally terminates either by immune exhaustion and complete collapse of virus control or by elimination below the level of detection.
ACKNOWLEDGMENTS
We thank Kris Branum and Grethe Thørner Andersen for expert technical assistance.
This study was supported in part by the Danish Medical Research Council, Biotechnology Center for Cellular Communication, Novo Nordisk Foundation, and American Lebanese-Syrian Associated Charities. J.P.C. is the recipient of a fellowship from the Alfred Benzon Foundation, Denmark.
REFERENCES
- 1.Ahmed R, Salmi A, Butler L D, Chiller J M, Oldstone M B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson E C, Christensen J P, Marker O, Thomsen A R. Changes in cell adhesion molecule expression on T cells associated with systemic virus infection. J Immunol. 1994;152:1237–1245. [PubMed] [Google Scholar]
- 3.Andersson E C, Christensen J P, Scheynius A, Marker O, Thomsen A R. Lymphocytic choriomeningitis virus infection is associated with long-standing perturbation of LFA-1 expression on CD8+ T cells. Scand J Immunol. 1995;42:110–118. doi: 10.1111/j.1365-3083.1995.tb03633.x. [DOI] [PubMed] [Google Scholar]
- 4.Andreasen S O, Christensen J P, Marker O, Thomsen A R. Virus-induced non-specific signals cause cell cycle progression of primed CD8(+) T cells but do not induce cell differentiation. Int Immunol. 1999;11:1463–1473. doi: 10.1093/intimm/11.9.1463. [DOI] [PubMed] [Google Scholar]
- 5.Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Zinkernagel R M, Mak T W. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrow P, Tough D F, Eto D, Tishon A, Grewal I S, Sprent J, Flavell R A, Oldstone M B. CD40 ligand-mediated interactions are involved in the generation of memory CD8+ cytotoxic T lymphocytes (CTL) but are not required for the maintenance of CTL memory following virus infection. J Virol. 1998;72:7440–7449. doi: 10.1128/jvi.72.9.7440-7449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen J P, Marker O, Thomsen A R. The role of CD4+ T cells in cell-mediated immunity to LCMV: studies in MHC class I and class II deficient mice. Scand J Immunol. 1994;40:373–382. doi: 10.1111/j.1365-3083.1994.tb03477.x. [DOI] [PubMed] [Google Scholar]
- 8.Christensen J P, Marker O, Thomsen A R. T-cell-mediated immunity to lymphocytic choriomeningitis virus in β2-integrin (CD18)- and ICAM-1 (CD54)-deficient mice. J Virol. 1996;70:8997–9002. doi: 10.1128/jvi.70.12.8997-9002.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciurea A, Klenerman P, Hunziker L, Horvath E, Odermatt B, Ochsenbein A F, Hengartner H, Zinkernagel R M. Persistence of lymphocytic choriomeningitis virus at very low levels in immune mice. Proc Natl Acad Sci USA. 1999;96:11964–11969. doi: 10.1073/pnas.96.21.11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 11.Doherty P C, Topham D J, Tripp R A. Establishment and persistence of virus-specific CD4+ and CD8+ T cell memory. Immunol Rev. 1996;150:23–44. doi: 10.1111/j.1600-065x.1996.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 12.Flynn K J, Riberdy J M, Christensen J P, Altman J D, Doherty P C. In vivo proliferation of naive and memory influenza-specific CD8(+) T cells. Proc Natl Acad Sci USA. 1999;96:8597–8602. doi: 10.1073/pnas.96.15.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gairin J E, Mazarguil H, Hudrisier D, Oldstone M B. Optimal lymphocytic choriomeningitis virus sequences restricted by H-2Db major histocompatibility complex class I molecules and presented to cytotoxic T lymphocytes. J Virol. 1995;69:2297–2305. doi: 10.1128/jvi.69.4.2297-2305.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallimore A, Dumrese T, Hengartner H, Zinkernagel R M, Rammensee H G. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J Exp Med. 1998;187:1647–1657. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R V J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 16.Jeffery K J, Usuku K, Hall S E, Matsumoto W, Taylor G P, Procter J, Bunce M, Ogg G S, Welsh K I, Weber J N, Lloyd A L, Nowak M A, Nagai M, Kodama D, Izumo S, Osame M, Bangham C R. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc Natl Acad Sci USA. 1999;96:3848–3853. doi: 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kagi D, Hengartner H. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr Opin Immunol. 1996;8:472–477. doi: 10.1016/s0952-7915(96)80033-1. [DOI] [PubMed] [Google Scholar]
- 18.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 19.Klavinskis L S, Geckeler R, Oldstone M B. Cytotoxic T lymphocyte control of acute lymphocytic choriomeningitis virus infection: interferon gamma, but not tumour necrosis factor alpha, displays antiviral activity in vivo. J Gen Virol. 1989;70:3317–3325. doi: 10.1099/0022-1317-70-12-3317. [DOI] [PubMed] [Google Scholar]
- 20.Landolfo S, Gariglio M, Gribaudo G, Jemma C, Giovarelli M, Cavallo G. Interferon-gamma is not an antiviral, but a growth-promoting factor for T lymphocytes. Eur J Immunol. 1988;18:503–509. doi: 10.1002/eji.1830180403. [DOI] [PubMed] [Google Scholar]
- 21.Lohman B L, Welsh R M. Apoptotic regulation of T cells and absence of immune deficiency in virus-infected gamma interferon receptor knockout mice. J Virol. 1998;72:7815–7821. doi: 10.1128/jvi.72.10.7815-7821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marker O, Volkert M. Studies on cell-mediated immunity to lymphocytic choriomeningitis virus in mice. J Exp Med. 1973;137:1511–1525. doi: 10.1084/jem.137.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marten N W, Stohlman S A, Smith-Begolka W, Miller S D, Dimacali E, Yao Q, Stohl S, Goverman J, Bergmann C C. Selection of CD8+ T cells with highly focused specificity during viral persistence in the central nervous system. J Immunol. 1999;162:3905–3914. [PubMed] [Google Scholar]
- 24.Matloubian M, Concepcion R J, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMichael A J, O'Callaghan C A. A new look at T cells. J Exp Med. 1998;187:1367–1371. doi: 10.1084/jem.187.9.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskophidis D, Battegay M, van den Broek M, Laine E, Hoffmann-Rohrer U, Zinkernagel R M. Role of virus and host variables in virus persistence or immunopathological disease caused by a non-cytolytic virus. J Gen Virol. 1995;76:381–391. doi: 10.1099/0022-1317-76-2-381. [DOI] [PubMed] [Google Scholar]
- 27.Moskophidis D, Lechner F, Hengartner H, Zinkernagel R M. MHC class I and non-MHC-linked capacity for generating an anti-viral CTL response determines susceptibility to CTL exhaustion and establishment of virus persistence in mice. J Immunol. 1994;152:4976–4983. [PubMed] [Google Scholar]
- 28.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 29.Muller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 30.Nansen A, Jensen T, Christensen J P, Andreasen S O, Ropke C, Marker O, Thomsen A R. Compromised virus control and augmented perforin-mediated immunopathology in IFN-gamma-deficient mice infected with lymphocytic choriomeningitis virus. J Immunol. 1999;163:6114–6122. [PubMed] [Google Scholar]
- 31.Planz O, Ehl S, Furrer E, Horvath E, Brundler M A, Zinkernagel R M, Hengartner H. A critical role for neutralizing-antibody-producing B cells, CD4(+) T cells, and interferons in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers. Proc Natl Acad Sci USA. 1997;94:6874–6879. doi: 10.1073/pnas.94.13.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramsay A J, Ruby J, Ramshaw I A. A case for cytokines as effector molecules in the resolution of virus infection. Immunol Today. 1993;14:155–157. doi: 10.1016/0167-5699(93)90277-R. [DOI] [PubMed] [Google Scholar]
- 33.Ramshaw I A, Ramsay A J, Karupiah G, Rolph M S, Mahalingam S, Ruby J C. Cytokines and immunity to viral infections. Immunol Rev. 1997;159:119–135. doi: 10.1111/j.1600-065x.1997.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 34.Schulz M, Aichele P, Vollenweider M, Bobe F W, Cardinaux F, Hengartner H, Zinkernagel R M. Major histocompatibility complex-dependent T cell epitopes of lymphocytic choriomeningitis virus nucleoprotein and their protective capacity against viral disease. Eur J Immunol. 1989;19:1657–1667. doi: 10.1002/eji.1830190921. [DOI] [PubMed] [Google Scholar]
- 35.Selin L K, Welsh R M. Cytolytically active memory CTL present in lymphocytic choriomeningitis virus-immune mice after clearance of virus infection. J Immunol. 1997;158:5366–5373. [PubMed] [Google Scholar]
- 36.Thomsen A R, Johansen J, Marker O, Christensen J P. Exhaustion of CTL memory and recrudescence of viremia in lymphocytic choriomeningitis virus-infected MHC class II-deficient mice and B cell-deficient mice. J Immunol. 1996;157:3074–3080. [PubMed] [Google Scholar]
- 37.Thomsen A R, Marker O. MHC and non-MHC genes regulate elimination of lymphocytic choriomeningitis virus and antiviral cytotoxic T lymphocyte and delayed-type hypersensitivity mediating T lymphocyte activity in parallel. J Immunol. 1989;142:1333–1341. [PubMed] [Google Scholar]
- 38.Thomsen A R, Nansen A, Christensen J P, Andreasen S O, Marker O. CD40 ligand is pivotal to efficient control of virus replication in mice infected with lymphocytic choriomeningitis virus. J Immunol. 1998;161:4583–4590. [PubMed] [Google Scholar]
- 39.Tishon A, Lewicki H, Rall G, von Herrath M, Oldstone M B. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology. 1995;212:244–250. doi: 10.1006/viro.1995.1477. [DOI] [PubMed] [Google Scholar]
- 40.Topham D J, Tripp R A, Doherty P C. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 41.Tough D F, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 42.van den Broek M F, Muller U, Huang S, Aguet M, Zinkernagel R M. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J Virol. 1995;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volkert M, Lundstedt C. The provocation of latent lymphocytic choriomeningitis virus infections in mice by treatment with antilymphocytic serum. J Exp Med. 1968;127:327–339. doi: 10.1084/jem.127.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Von Herrath M G, Coon B, Oldstone M B. Low-affinity cytotoxic T-lymphocytes require IFN-gamma to clear an acute viral infection. Virology. 1997;229:349–359. doi: 10.1006/viro.1997.8442. [DOI] [PubMed] [Google Scholar]
- 45.Walsh C M, Matloubian M, Liu C C, Ueda R, Kurahara C G, Christensen J L H M, Young J D, Ahmed R, Clark W R. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wille A, Gessner A, Lother H, Lehmann-Grube F. Mechanism of recovery from acute virus infection. VIII. Treatment of lymphocytic choriomeningitis virus-infected mice with anti-interferon-gamma monoclonal antibody blocks generation of virus-specific cytotoxic T lymphocytes and virus elimination. Eur J Immunol. 1989;19:1283–1288. doi: 10.1002/eji.1830190720. [DOI] [PubMed] [Google Scholar]
- 47.Wodarz D, Nowak M A. Immune responses and viral phenotype: do replication rate and cytopathogenicity influence viral load? J Theor Med. 2000;2:113–127. [Google Scholar]
- 48.Wodarz D, Nowak M A, Bangham C R. The dynamics of HTLV-I and the CTL response. Immunol Today. 1999;20:220–227. doi: 10.1016/s0167-5699(99)01446-2. [DOI] [PubMed] [Google Scholar]
- 49.Yanagi Y, Tishon A, Lewicki H, Cubitt B A, Oldstone M B. Diversity of T-cell receptors in virus-specific cytotoxic T lymphocytes recognizing three distinct viral epitopes restricted by a single major histocompatibility complex molecule. J Virol. 1992;66:2527–2531. doi: 10.1128/jvi.66.4.2527-2531.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young L H, Klavinskis L S, Oldstone M B, Young J D. In vivo expression of perforin by CD8+ lymphocytes during an acute viral infection. J Exp Med. 1989;169:2159–2171. doi: 10.1084/jem.169.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zajac A J, Blattman J N, Murali-Krishna K, Sourdive D J, Suresh M A J, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]