Abstract
The burden of cancer exerts a disproportionate impact across different regions and population subsets. Disease-specific attributes, coupled with genetic and socioeconomic factors, significantly influence cancer treatment outcomes. Precision oncology promises the development of safe and effective options for specific ethnic phenotypes and clinicodemographic profiles. Currently, clinical trials are concentrated in resource-rich geographies with younger, healthier, white, educated, and empowered populations. Vulnerable and marginalized people are often deprived of opportunities to participate in clinical trials. Despite consistent endeavors by regulators, industry, and other stakeholders, factors including diversity in trial regulations and patient and provider-related cultural, logistic, and operational barriers limit the inclusiveness of clinical trials. Understanding and addressing these constraints by collaborative actions involving regulatory initiatives, industry, patient advocacy groups, community engagement in a culturally sensitive manner, and designing and promoting decentralized clinical trials are vital to establishing a clinical research ecosystem that promotes equity in the representation of population subgroups.
Key words: diversity, inclusion, equity, clinical trials, regulators
Highlights
-
•
Some races, ethnicities, geographies, genders, ages, abilities, and economic classes are underrepresented in cancer trials.
-
•
There is strong scientific and ethical rationale for inclusiveness, equity, and diversity in cancer trial populations.
-
•
Stakeholders including regulators, sponsors, CROs, patient groups, and oncology journals are advocating for such diversity.
-
•
Cancer trials should answer scientific questions of relevance to the diverse participating subgroups.
Introduction
Cancer remains a leading cause of morbidity and mortality, impacting the physical, mental, and social well-being of patients worldwide. Approximately 19.3 million new cancer cases and 10.0 million cancer deaths were reported globally in 2020.1 As the global population ages, the absolute incidence of cancer cases is estimated to increase significantly. Globally, nearly half of all reported incident cancer cases and 58.3% of cancer deaths occur in Asia, while Europe reports 22.8% of cancer cases and 19.6% of deaths, followed by the Americas, which account for 20.9% of the cancer incidence and 14.2% of the deaths. Compared to other regions, Asia (58.3%) and Africa (7.2%) exhibit a higher proportion of cancer deaths relative to their respective incidence rates (49.3% and 5.7%).1 This geographical heterogeneity is partly explained by the lack of equitable distribution of cancer care resources, oncology workforce, and use of targeted therapies and immunotherapy in resource-rich high-income countries (HICs). In sub-Saharan Africa, cancer incidence trends show an upward trajectory, with incident cases having doubled in the past 30 years, possibly associated with infection, increasing life expectancy, adoption of unhealthy lifestyles, and genetics.2,3 Moreover, limited access to early detection, diagnostic facilities, and comprehensive treatment options, coupled with a low adoption rate of vaccinations, exacerbate the burden of cancer and contribute to the disparities in cancer outcomes across the African continent.4
In-depth inquiry into clinical and disease-specific characteristics is critical; it is also essential to account for inter-individual variability in a drug’s pharmacokinetics, pharmacodynamics, efficacy, and safety. Despite significant progress in cancer treatment with novel therapies and precision medicine, individuals belonging to diverse racial, ethnic, sex, age, and socioeconomic groups are often at a disadvantage in receiving the appropriate standard of care for their specific phenotype because of possible underrepresentation of various sub-populations in oncology clinical trials.
This review presents an overview of inequality in the representation of diverse population groups in oncology trials, amelioration efforts by various stakeholders in the drug development space, and the way forward for conducting more inclusive clinical trials. The search strategy and selection criteria are summarized in Box 1. This article is based on previously conducted studies and does not contain any studies with human participants or animals carried out by any of the authors.
Box 1. Search strategy and selection criteria.
The evidence for this review was identified through a computerized targeted literature search of various electronic databases, including PubMed, Embase, Web of Science, and websites of regulatory agencies, with the search terms ‘diversity’, ‘underrepresented populations’, ‘ethnic minorities’, ‘gender disparities’, ‘regulatory guidelines for enhancing diversity’, ‘barriers to participation’, and ‘strategies to enhance diversity’ in combination with the term ‘in oncology clinical trials’. The studies published after 2010 were considered for this review as the United States Food and Drug Administration (FDA) released its first guidance on diversity, the Affordable Care Act, and established its Office of Minority Health to increase racial and ethnic diversity in clinical trial populations in 2010. Studies and review articles published in languages other than English were excluded. The articles were assessed to identify barriers and facilitators for inclusive oncology clinical trials recommended by the FDA and other regulatory agencies. Citations were included based on relevance to the broad scope of this article. We present a narrative review of initiatives by regulatory agencies, the industry, and clinical research community along with relevant data from real-world practices to promote diversity and inclusion in oncology clinical trials.
Disparity in clinical trial participation
Racial and ethnic disparity
Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103373, reports selected publications representing disparity in oncology clinical trial participation.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24
The Food and Drug Administration (FDA) oncology drug approval database of the past decade (2008-2018) revealed that only one-third of the trials documented participants’ racial information.7 Generally, whites were overrepresented, African Americans and Asians were underrepresented, while Native Americans had consistently poor/no representation in the United States clinical trials.8 Indigenous communities, such as American Indian/Alaska Native populations, have the lowest representation within the racial and ethnic spectrum in the United States.25 Notably, global cancer clinical trials have limited representation in some regions, such as Africa26 and Latin America.27 These observations are disturbing, given distinct genetic and health disparities impacting treatment responses, particularly among African patients. Appropriate representation of diverse racial and ethnic populations in oncology clinical trials can reveal disparities in cancer incidence, prevalence, data collection, reporting, and treatment outcomes. For instance, Asian patients with non-small-cell lung cancer tend to exhibit better survival [median overall survival (OS) 29.1 months, 95% confidence interval (CI) 25.2 months-not reached] than non-Asian patients (median OS 20.6 months, 95% CI 16.1-25.5 months) with gefitinib, potentially due to a higher incidence of somatic activating mutations in the EGFR gene.28
Sociodemographic disparity
Age
Clinical trials consistently exhibit enrollment biases,29 with participants skewed toward younger, healthier, and less diverse individuals than the broader population observed in clinical settings. Elderly individuals are often excluded from oncology clinical trials due to concerns about comorbidities and potential complications of aggressive cancer treatments. Recent research showed that older patients benefit from poly (ADP-ribose) polymerase inhibitor therapy, leading to prolonged progression-free survival similar to younger patients with comparable hematologic safety profiles.30
Gender
Over half of the genes targeted by FDA-approved cancer drugs revealed sex-based variation in molecular signatures, such as differences in somatic mutations, gene expressions, methylation patterns, copy number alterations, and protein abundance.31 An analysis of molecular profiling data for patients treated with immune-checkpoint blockade therapies identified molecular disparities in immunotherapy responsiveness and revealed distinct patterns of sex bias in immune features across multiple cancer types.32 Despite overall improvements in gender-based disparities in clinical studies, women are underrepresented in cancer trials. Interestingly, female reproductive cancers, including ovarian, cervical, and uterus, are significantly underfunded compared to other cancer sites.33 Conversely, males are often excluded from randomized phase III breast cancer (BC) clinical trials, representing only 0.09% of total participants, significantly lower than their proportion in the United States BC population.34
Immunocompromised state
Recent epidemiological studies have indicated that individuals with compromised immune systems due to human immunodeficiency virus (HIV) infection, organ transplant recipients, or those with genetic immunodeficiency represent a distinct patient population with unique challenges and responses to cancer therapies; however, they are often excluded from participating in oncology clinical trials. Analyzing their response to cancer treatment may improve our understanding and care for this vulnerable patient population. However, a few cancer clinical trials implement stringent criteria for certain conditions, such as excluding patients with stable hepatitis B virus (HBV) infection with lower reactivation risk. Over time, regulatory agencies have implemented significant changes in eligibility criteria for clinical trial enrollment, fostering inclusivity. The FDA recommends expanding the eligibility criteria to be more inclusive of patients with HBV, hepatitis C virus, and HIV in cancer trials.35 A recent phase I study evaluating the safety of pembrolizumab in patients with HIV who have relapsed or refractory cancer demonstrated positive outcomes with mild treatment-related adverse events comparable to those observed in patients without HIV infection.36
Social determinants
Disparities in the global distribution of clinical trials are evident, with the majority being conducted in HICs, very few in lower-middle-income countries, and almost none in low-income countries. Significant geographical disparities also exist within countries; <10% of newly diagnosed cancer patients in India could access therapeutic trials even within their state or region. Disparities were even more marked based on the cancer site, with a greater focus on specific sites.37 Although rural populations experience poorer health outcomes, they remain inadequately represented in clinical trials. Further, socioeconomic factors are also known to influence trial participation, as higher income correlated with greater trial involvement.38 Among patients with uniform access to protocol-driven cancer care, socioeconomically deprived individuals exhibited worse OS [hazard ratio (HR) 1.28, 95% CI 1.20-1.37, P < 0.001], progression-free survival (HR 1.20, 95% CI 1.13-1.28, P < 0.001), and cancer-specific survival (HR 1.27, 95% CI 1.18-1.37, P < 0.001), compared with trial participants residing in affluent regions.39
Evolving landscape for inclusive clinical trials
Regulatory authority guidance
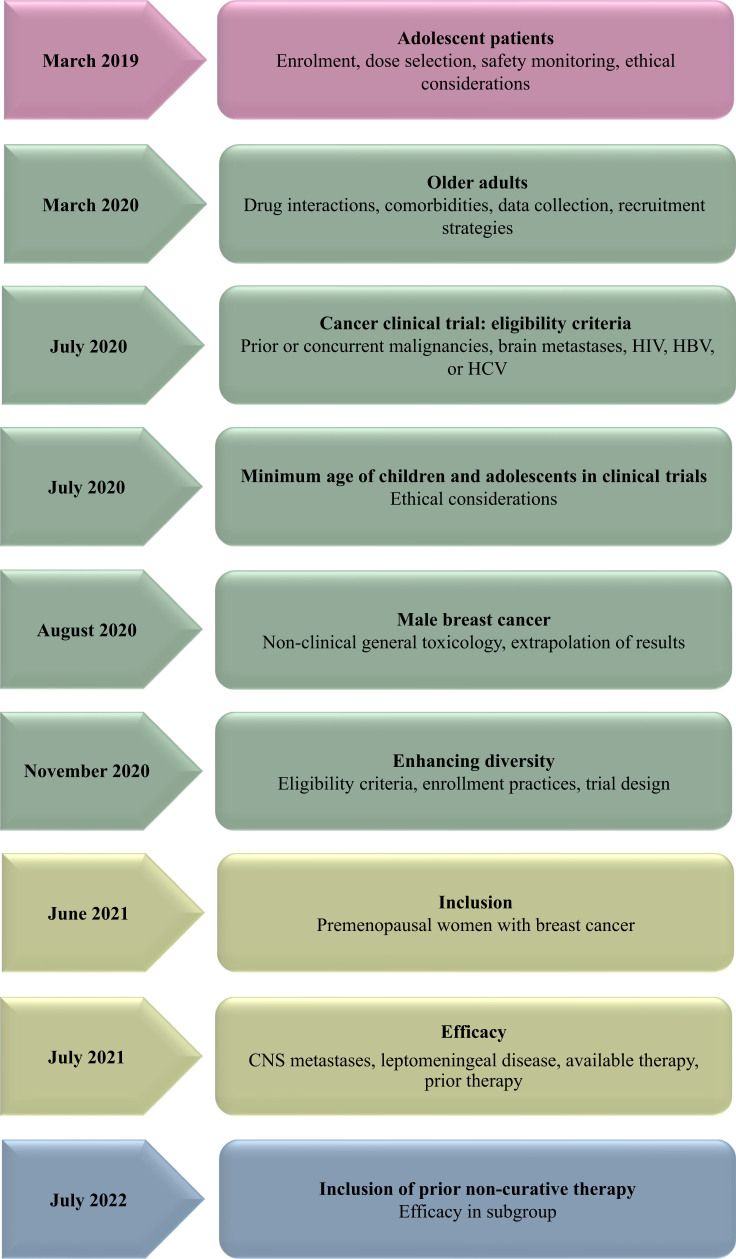
The United States FDA has been instrumental in providing several oncology-specific guidance aimed at enhancing the inclusion of representative populations across all the phases of drug development (Figure 1).40, 41, 42, 43, 44, 45, 46, 47, 48, 49 In 2020, the United States FDA released a landmark guidance entitled “Enhancing the Diversity of Clinical Trial Populations” outlining various strategies, including broadening eligibility criteria, decentralized study sites, virtual consultations, flexibility in visit windows, and leveraging electronic communication and digital health technology tools for remote data collection.50 Subsequent 2022 FDA guidance mandates sponsors submit a race and ethnicity diversity plan early in clinical development.43 The FDA’s Office of Minority Health and Health Equity has established the Diversity in Clinical Trials Initiative to promote health and safety communication among minority populations and to raise awareness about their increased participation in clinical trials.51 Recently, the United States administration launched an initiative to improve cancer outcomes in low-income areas through collaborating with various stakeholders to promote and increase equitable access to cancer care, irrespective of socioeconomic status.52 Further, as part of transparency efforts, the FDA snapshots offer concise summaries of their product evaluations and decisions. The FDA oncology has mandated post-marketing commitments for sponsors to characterize the safety and efficacy of oncology drugs in a diverse population.53 Similarly, in 2022, the European Union introduced a program to improve the study initiation process and design to provide a comprehensive approach to address patient requirements and support transparency.54 The recommendations put forth by the regulatory authorities across various countries emphasized the significance of diversity and inclusivity in clinical research (Table 1).43,50,55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69
Figure 1.
Oncology trial-specific diversity and inclusion guidance documents issued by the FDA. CNS, central nervous system; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; US FDA, United States Food and Drug Administration.
Table 1.
Guidelines/recommendations by regulatory authorities to promote diversity and inclusion in clinical trials
| Guidelines | Month, year | Recommendations |
|---|---|---|
| Australia | ||
| Australian Clinical Trial Alliance56 | June 2020 |
|
| Canada | ||
| Tri-Agency Statement on Equity, Diversity, and Inclusion55 |
|
|
| Tri-Agency Equity, Diversity, and Inclusion Action Plan for 2018-202557 |
|
|
| New Frontiers in Research Fund Best Practices in Equity, Diversity, and Inclusion | 2020 |
|
| Considerations for Inclusion of Women in Clinical Trials and Analysis of Sex Differences58 | May 2013 |
|
| Europe | ||
| EU Clinical Trial Regulation No. 536/201459 | January 2022 |
|
| ICH guideline E8 (R1) on general considerations for clinical studies60 | April 2022 |
|
| ICH E6 (R3) Guideline on good clinical practice61 | May 2023 |
|
| India | ||
| The Functioning of the Central Drugs Standard Control Organization62 | May 2012 |
|
| Ministry Of Health and Family Welfare (Department of Health and Family Welfare) The Gazette of India, Extraordinary, PART II—Section 3—Sub-section (i) REGD. NO. D. L.-33004/99 New Delhi63 | March 2019 |
|
| Japan | ||
| Basic Principles on Global Clinical Trials64 | September 2012 |
|
| UK | ||
| NHS Guidance to Increase Diversity in Research Participation65 | February 2023 |
|
| Guidance from the NIHR INCLUDE project66 | July 2022 |
|
| NIHR Toolkit: Increasing Participation of Black Asian and Minority Ethnic Groups in Health and Social Care Research67 | December 2018 |
|
| United States | ||
| NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research68 | November 2017 |
|
| FDA: Enhancing the Diversity of Clinical Trial Populations—Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry50 | November 2020 |
|
| Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Subgroups in Clinical Trials43 | April 2022 |
|
| Decentralized Clinical Trials for Drugs, Biological Products, and Devices69 | May 2023 |
|
CALD, culturally and linguistically diverse; EU, European Union; FDA, Food and Drug Administration; ICH, International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; INCLUDE, Innovations in Clinical Trial Design and Delivery for the Under-served; NHS, National Health Service; NIH, National Institutes of Health; NIHR, National Institute for Health and Care Research; PMDA, Pharmaceuticals and Medical Devices Agency.
Industry and academic efforts for inclusive trials
The industry has made notable efforts to address clinical trial diversity. The Society for Clinical Research Sites (SCRS) developed a Diversity Site Assessment Tool, a validated instrument that evaluates a site’s capability to recruit diverse patient populations.70 The Multi-Regional Clinical Trials (MRCT) Center of Brigham and Women’s Hospital and Harvard formed an MRCT Center Diversity Workgroup that published a guidance document and toolkit that provides practical guidance on promoting diversity in clinical trial design and implementation.71 In 2020, the Pharmaceutical Research and Manufacturers of America and its member companies released industry-wide principles to address the topic of diversity in clinical research, including site selection, broadening of eligibility criteria, and following patient-centric approaches. Recently, under the Clinical Treatment Act, Medicaid covers clinical trial-related expenses, reducing financial barriers and promoting equitable access for marginalized patients in the United States.72 However, there has been slow progress in states adopting and funding the standard-of-care costs for Medicaid beneficiaries.
The Just-in-Time (JIT) trial model facilitates expanded access to clinical trials, particularly representing patients from diverse racial, ethnic, and socioeconomic backgrounds. JIT trials unveil latent prospects by leveraging patient registries, real-world data patient-centered technologies, and streamlined processes. Unlike the conventional approach, JIT trials pre-identify potential participants (through patient registries and prescreening) before site activation, thus allowing sponsors to select sites with the greatest capacity for diverse enrollment. Subsequently, contract research organizations must harness this diverse range of well-characterized patient data and boost the inclusion of underrepresented populations in clinical trials.73 The Clinical Study Diversity Score (CSDS) allows quantitative diversity measurement for individual research studies and facilitates site selection based on intricate diversity profiles spanning numerous dimensions. The CSDS scores can be calculated, monitored, and managed from the initial planning stage, progressing through patient enrollment and study implementation.74
A recent collaborative report, built upon a roundtable discussion involving key stakeholders in pharmaceuticals, life sciences, and health sectors, called for concerted actions across all levels of the research ecosystem (funders, regulators, companies, research teams, and communities).75 Allied to this, several tools and resources have been developed to support researchers in embedding equity, diversity, and inclusion into clinical research in the UK (Table 2).76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87 Additionally, government-funded health research bodies from Australia,88 Canada,89 and New Zealand90 have distinct policies for involving indigenous populations in research and securing funding.
Table 2.
Supporting resources and tools for researchers in integrating diversity and inclusivity in UK clinical trials
| Resources | Important points of recommendations |
|---|---|
| Improving Diversity in Health Research and Trials76 |
|
| Expectations for EDI–UKRI77 | Expectations are categorized into six areas and supported by toolkits, articles, webinars, samples of existing grants, policies, current practices, and other resources. |
| FOR Equity78 | This website offers resources and tools to make research evidence more applicable for policymaking to eliminate social and health disparities. |
| Language interpreting and translation: migrant health guide79 | Health care practitioners can get advice and direction on the medical requirements for immigrant patients, including how to cater to their language needs for communication. |
| Equity and Inclusion Guiding Engagement Principles80 | This document outlines guiding principles to incorporate diversity, equity, and inclusion at the core of health research partnerships. |
| Six-Step Stakeholder Engagement Framework81 | This document by Cochrane outlines a six-step engagement framework to support research groups with stakeholder engagement experience. |
| Writing about ethnicity82 | This website is a resource for inclusive writing about ethnicity, including words and phrases. |
| NIHR Race Equality Framework83 | Co-created by the NIHR and REPAG, this framework is designed to help organizations make the changes needed to eliminate inequities in health research and care. |
| EqIA Toolkit85 | Provides comprehensive training, helpful resources, and guidance to improve equality analysis, practice, and results. |
| NIHR Research Design Service EDI Toolkit84 | This toolkit resource enables researchers to better understand methods to integrate EDI into research design and to meet NIHR EDI requirements. |
| Addressing barriers to engagement, involvement, and participation in research by ethnic minorities86 | Highlights the work carried out by NIHR partners in the Southeast of England to examine barriers to the participation of ethnic minority groups in research. |
| NHS Accelerated Access Collaborative—increasing diversity in research participation: a good practice guide for engaging with underrepresented groups87 | This document provides researchers with practical information on how to engage more diverse participants in health research. |
EDI, equality, diversity, and inclusion; EqIA, Equality Impact Assessment; FOR, Focus on Research; NHS, National Health Services; NIHR, National Institute for Health and Care Research; REPAG, Research’s Race Equality Public Action Group; UKRI, United Kingdom Research and Innovation.
Decentralized clinical trials (DCTs) are playing an increasingly important role in oncology.91 Elements of DCT can be harnessed to recruit a more diverse population into cancer trials.92
Innovations in Clinical Trial Design and Delivery for the Under-served (INCLUDE) is an initiative from the UK National Health Service (NHS) that aims to identify historically underrepresented groups and the barriers they face, and incorporate innovations in trial design that would facilitate their participation in oncology clinical trials.14,93
Collaborative academic research groups have an important role to play in championing the cause of diversity, equity, and inclusion in oncology drug development.27 More research needs to be conducted on how innovative clinical trial design, including the use of synthetic control arms and real-world data, could optimize the use of clinico-genomic data from a more diverse and representative population in the drug development process.
Biomedical journals: reporting on diversity and inclusiveness in clinical trials
Medical societies and scientific journals have developed recommendations to enhance diversity in clinical trials, emphasizing the importance of inclusive trial design, patient engagement, and solutions to address barriers to participation in oncology trials. The American Society of Clinical Oncology (ASCO) and the Association of Community Cancer Centers (ACCC) have recommended systematic, standardized, and automated ways to capture the patients’ data during each step including enrollment that enabled sites to identify specific strategies to increase racial/ethnic diversity in oncology trials.94 The Sex and Gender Equity in Research (SAGER) guidelines are a tool to standardize sex and gender reporting in scientific publications. The International Committee of Medical Journal Editors (ICMJE) recommends that authors provide detailed information on participant demographics, including sex, age, and race or ethnicity, to better understand the study outcomes across different patient populations.
Recently, progress has been noted in incorporating sex-disaggregated data in manuscripts. An informal pilot study was conducted to monitor the adherence to SAGER guidelines in Lancet journals, which revealed that 82% of the research articles published between June 2021 and April 2022 included sex and gender data in the abstracts, compared to none in the previous issues of 2021. Similarly, there was an increase in reporting of adverse events based on sex- or gender-disaggregated data (0%-28%) and subgroup analysis by sex or gender (5%-41%) during the same period. Integrating the SAGER checklist into manuscript assessment and peer-review process may enable journal editors to promote best reporting practices for considering sex and gender variables.95
In line with the growing emphasis on diversity and inclusivity in scientific research, the New England Journal of Medicine (NEJM) now requires supplementary tables providing background information on the representativeness of study groups relative to real-world patient populations.96 Similarly, the Cell Press journals have implemented an inclusion and diversity form for authors which covers not only the diversity in scientific content but also encompasses the diversity of authorship, allowing authors to include an inclusion and diversity statement in their published papers.97 The objective is to enhance transparency in clinical research, conduct, and reporting while promoting awareness of diversity and inclusion in academia.
Barriers to inclusive participation
Stringent regulatory approval processes help promote and maintain patient well-being and lawful conduct of clinical trials; however, they cause significant delays, hindering the timely inclusion of diverse population subgroup. Barriers to participation in multinational clinical trials vary from country to country and may involve legal, ethical, administrative, and logistical considerations. Regulatory obligations, documentation, ethical review process, consent procedures, and insurance coverage differ extensively across countries, adding administrative complexity. Table 3 outlines the potential regulatory challenges in enrolling diverse clinical participants across multiple countries.
Table 3.
Potential regulatory barriers in clinical trial conduct and enrollment of a more diverse population
| Country | Clinical trial application language | In-country sponsor presence/representation required | Specimen export regulations | Insurance and compensation | Key considerations while including vulnerable populations in clinical trials |
||
|---|---|---|---|---|---|---|---|
| Children/minors | Neuro-divergent | Pregnant and nursing women | |||||
| Australia | Unspecified | Yes | Yes | Sponsor should provide insurance and also explain to participants the compensation and/or treatment available to them in the event of trial-related injuries |
|
|
|
| Brazil | Portuguese | No | Yes | Sponsor is responsible for providing insurance coverage for any unforeseen injury to research participants as well as to provide compensation to research participants and/or their legal heirs in the event of trial-related injuries or death |
|
|
|
| Canada | English or French | Yes | Yes | Does not require the sponsor to provide insurance coverage or compensation for trial participants in the event of trial-related injuries or death |
|
|
|
| China | Chinese | Yes | Yes (in collaboration with Chinese research institutions) | Sponsor is responsible for providing the investigator and clinical trial institution with legal and economic insurance or a guarantee related to the clinical trial, as well as to provide compensation to research participants in the event of trial-related injuries or death |
|
|
|
| India | English | Yes | Yes | Sponsor should provide insurance coverage or a provision in the budget for possible compensation for trial-related injuries as well as responsible for providing compensation to research participants and/or their legal heir(s) in the event of trial-related injuries |
|
|
|
| UK | English | Yes | Yes | Sponsor and investigator are responsible for covering insurance and for providing compensation to research participants and/or their legal heirs in the event of trial-related injuries or death |
|
|
|
| United States | English | No | Yes | Insurance: not required Compensation: sponsor’s policies and procedures should address the costs of treatment of trial subjects in the event of trial-related injuries |
|
|
|
| South Africa | English | Yes | Yes | Sponsor should provide insurance coverage or a provision in the budget for possible compensation for trial-related injuries as well as responsible to provide compensation to research participants and/or their legal heir(s) in the event of trial-related injuries |
|
|
|
FDA, Food and Drug Administration; IC, informed consent; ICF, informed consent forms; ICH, International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; LAR, legally authorized representative.
Several patient-level barriers encompassing cultural misconceptions, apprehension toward investigational drugs or a placebo, lack of confidence in the quality of care provided in the trials, concerns about experimental procedures, and a sense of exploitation can deter trial participation. The apprehension is particularly pronounced among minority groups as they grapple with trust issues regarding equitable treatment. Language barriers98 and limited health literacy can further hinder effective communication and comprehension of study information, impacting willingness to participate in the trials. This is particularly evident in linguistically diverse societies like South Africa, with 11 official languages.
The influence of clinicians on patient referral and enrollment in clinical trials is significant, as most patients are referred to trials by their oncologists. These clinicians may harbor biases, such as the belief that individuals from specific racial and ethnic minorities may be unwilling or unable to adhere to trial protocols, which could impact their referral patterns and limit diversity. Additionally, a lack of awareness about available trials, limited time, and resources to search for trials contribute to clinicians failing to refer patients. These challenges may be extensive at health care facilities that serve individuals from racial and ethnic minority populations and for community oncologists who are not affiliated with research networks. Study staff may be apprehensive and use their discretion while enrolling people with low education levels, those from remote areas, and vulnerable populations due to safety or concerns related to trial retention and providing timely emergency care. Inadequate representation of women in trials is driven by limited awareness, perceived burdens, and misconceptions among sponsors regarding recruitment challenges. Furthermore, pregnant women are commonly excluded from trials to ensure homogeneity of treatment effect and safeguard maternal and fetal health.
Lack of accessibility to trial sites may prevent potential participants, especially the elderly, physically restricted, pregnant women, and those in rural and remote areas, from participating in trials. Geographical barriers can further exacerbate the logistic challenges as clinical trial sites are often concentrated in urban or centralized areas. In a prospective study, most patients with cancer declined trial participation due to distance from the trial site, despite meeting eligibility criteria.99 Scheduling conflicts and the associated costs can create financial burden and discourage individuals from participating in research activities. Further, the complexity of clinical trial designs, characterized by strict eligibility criteria based on comorbid conditions and functional status, reduces the pool of eligible participants.
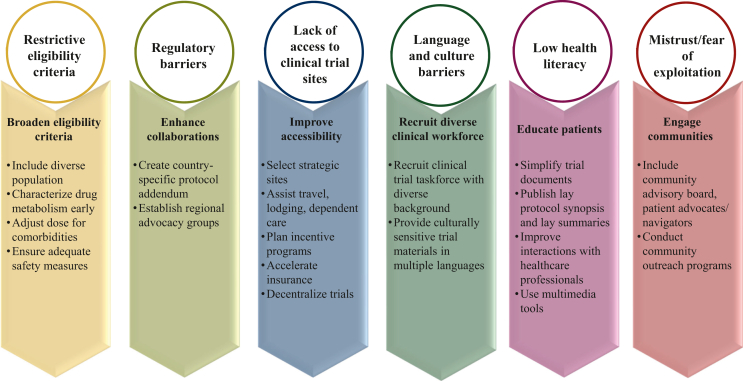
Figure 2 depicts barriers to participating in a clinical trial and strategies to enhance the inclusion and diversity of participants.
Figure 2.
Barriers to inclusive participation in cancer clinical trials and facilitators of diversity and inclusion.
Facilitators of diversity, equity, and inclusion
Broadening eligibility criteria
The ASCO and the Friends of Cancer Research (ASCO–Friends) proposed recommendations to modernize eligibility criteria to make cancer clinical trials more accessible, and represent accurate cancer patient population.100 These recommendations include: (i) laboratory evaluations can be used in place of wash-out periods for concomitant medications for inclusion in clinical trials; (ii) patients with reduced performance status should be included unless there is a scientific and/or clinical rationale for exclusion; (iii) laboratory reference values for exclusion should account for potential normal variations due to race, ethnicity, age, sex, and gender identity. The LUNGevity Working Group adapted these recommendations to make lung cancer clinical trials more inclusive.101 Analysis of 122 protocols based on the modernization of seven eligibility criteria as recommended by the Cancer Therapy Evaluation Program approved in the years 2018-2020 found that modernization has been implemented to a relatively high degree, ranging from a low of 54.1% for prior and/or concurrent malignancies to a high of 93.4% for eligibility criteria related to HIV infection.102
Enrichment of trial design is another strategy to demonstrate the drug’s efficacy by expanding the target population. These strategies may include biomarker-based selections, disease-specific subtypes, or risk-based criteria for inclusion.50 In 2019, the FDA proposed Enrichment Strategies for Clinical Trials to Support the Determination of the Effectiveness of Human Drugs and Biological Products, wherein design options and interpretation of the results for enrichment strategies studies have been discussed.103 Few of the strategies proposed in this guidance are for adjuvant therapies that can be assessed in the high-risk groups, e.g. prostate-specific antigen velocity group in prostate cancer, women with a deleterious BRCA1 or BRCA2 mutation in trials assessing prevention or delay in mortality.
An interim data analysis can be proposed in the protocol to identify treatment-sensitive patient subgroups. This creates an opportunity for revising the entry criteria accounting for patient heterogeneity and corresponding treatment outcomes; the safety monitoring committee may consider such decisions.
Efforts are ongoing to expand the inclusion criteria to achieve greater representation of children and adolescents in clinical trials involving adults, when appropriate.49 As per FDA guidance, inclusion of pregnant women in clinical trials is recommended with due ethical considerations and safety measures because of the following reasons: (i) women need safe and effective treatment during pregnancy; (ii) failure to establish the dose/dosing regimen, safety, and efficacy of treatments during pregnancy may compromise the health of women and their fetuses; and (iii) in some settings, enrollment of pregnant women in clinical trials may offer the possibility of direct benefit to the woman and/or fetus that is unavailable outside the research setting.104
Community engagement
The disengagement between researchers and participants can be bridged by active and continued community involvement in planning, implementing, and disseminating clinical trials through the community advisory board, patient advocates/navigators, and community members. Outreach programs can engage the community in a culturally and linguistically sensitive manner through trusted voices, community congregations, and civil organizations by building trust and awareness about the importance of clinical research, fostering transparency, and promoting a sense of ownership among potential participants (Figure 2). The impact of one such outreach program was studied in Ireland, wherein participants reported a greater understanding of cancer signs and symptoms (62.2%-81.6%) and risk factors (49%-61.2%) after outreach events.105
The active involvement of patient advocates for trial oversight and patient participation in clinical trial grants can facilitate the designing of patient-centric clinical trials.106 Patient advocates are collaborating with scientists from the inception of research ideas. In January 2019, a group of basic, translational, and clinical investigators and patient advocates assembled in Miami, FL, to discuss the current state of the low-grade serous carcinoma of the ovary or peritoneum to review current knowledge, discuss ongoing research by established researchers, and frame critical questions or issues for future directions.107
Several non-profit and academic research organizations have emerged to conduct multi-institutional clinical and translational cancer research. The NRG Oncology has outlined the strategies to enhance the participation of minorities and under-served populations in clinical trials, including actively involving these communities in disease site committees, considering their input in the protocol development, analyzing the educational workshops, developing mentoring programs, and disseminating information and research.108 The ANGEL Advocacy Program, by the Tigerlily Foundation, has been instrumental in recruiting, training, and mobilizing the community, fostering trust and developing embedded solutions before commencing the trials. This approach offers a genuine and meaningful community engagement experience rather than just a performative step taken by sponsors during trial recruitment.109 A recent study examined community-based interventions related to triple-negative BC over the past 10 years. It revealed that most prospective studies primarily focused on screening, diagnosis, and treatment, with a minimum focus on addressing social determinants and incorporating participatory research principles. The research group recommended increased funding for community-engaged approaches, enhancing public awareness, and fostering multi-stakeholder collaboration to improve outcomes.110
Patient education
Engaging patients at every stage of the trial supports the integration of their perspectives, needs, and preferences into the research process. Improving patient centricity includes simplifying trial-related documents, developing a plain language protocol synopsis, and enabling personal interactions with health care professionals. Additionally, by leveraging multimedia tools, such as videos, websites, and social media campaigns, researchers can reach broader, diverse, and underrepresented population groups in a culturally sensitive manner. Patient organizations advocate a paradigm shift in the perception of patients and advocacy organizations in the health care sector. They propose the integration of patients and advocates as active contributors alongside sponsors and stakeholders in driving the progress. Moreover, such groups actively contribute to developing clinical trial content with significant input from patients.
Enhancing patient-centered care involves clinicians collaborating with patients and caregivers to align with their preferences and needs. To improve equitable and effective care for all patients and caregivers, learnings from marginalized populations like transgender, ethnic minorities, and pediatric patients can be implemented at a direct patient and systems level across oncology care more broadly. Recommendations included consulting with transgender patients for respectful terminology, providing culturally sensitive care, creating a welcoming atmosphere to involve caregivers in discussions, and actively partnering with community outreach groups. For pediatric cancer care, it is essential to balance family dynamics and provide information in the context of the child’s developmental and chronological age, validate patient-reported symptom scale, and bridge gaps between the child and caregivers.111
Optimizing patient accessibility to clinical trials
A critical step to enhancing health equity is enabling equitable access to participate in oncology research trials. The potential approaches to address accessibility issues are selecting trial sites that are easily reachable by public transportation, providing transportation assistance, or even adopting decentralized or hybrid trial models. Trial site selection and site development decisions should be based on strategic and data-driven frameworks that include a rigorous assessment of epidemiological data, site historical enrollment data, and diversity data such as race/ethnicity, age, gender, and other social determinants of health measures. The coronavirus disease 2019 pandemic induced larger-scale experience with DCTs. However, oncology trials are the least impacted, mainly because patients require high-acuity care due to the burden of cancer-related symptoms. Nevertheless, advances in drug delivery, digital technologies, and hybrid care models have increased the feasibility of decentralized trials in oncology. The technology solutions facilitating decentralized and/or hybrid clinical trials encompass various components such as telemedicine, eConsent, electronic clinical outcome assessment, electronic patient-reported outcome (ePRO), and eSource or electronic source data that assist in reducing the number of on-site visits during the conduct of a trial.112 Effective implementation of ePROs may improve care for all patients, including racial and ethnic minority populations, older patients, and patients with less education. Resources to aid practices in implementing ePROs are available from the PROTEUS Consortium (Patient-Reported Outcomes Tools: Engaging Users & Stakeholders).113
Newer digital technologies, such as biomarkers (such as portable, implantable, and wearable devices) and diagnostics, enable continued data collection, reduce patient travel, maintain patient engagement, and allow real-time insights to clinicians on treatment outcomes. A recent survey revealed that less than half of the physicians are acquainted with remote, wearable, and DCT solutions, and almost 50% of them raised concerns about oversight and data quality. However, a high proportion of physicians (64%) favored implementing them and had a positive outlook on their potential to enhance patient retention, enrollment, and accessibility for patients residing in remote areas and those with limited mobility.91
Incentive programs may enhance inclusivity, clinical outcomes, and the accessibility to novel therapies. Federal policies should guide regulators to enforce existing accountability measures and establish a task force to evaluate incentives for diverse participation in drug and device trials. Possible incentives include tax credits for research and development, expedited reviews, exemption from regulatory fees, extended market exclusivity for compliant sponsors, and rejection of applications lacking demographic representation. Additionally, the Resources and AssIstance for Support and Empowerment (RAISE) platform can be utilized to improve trial diversity, adherence, and accrual by eliminating financial or logistic barriers faced by patients.
Use of multilingual material and recruitment of a diverse clinical workforce
The FDA recommends providing trial resources and documents in multiple languages to encourage the participation of individuals with limited English comprehension.50 Researchers can effectively communicate the trial’s purpose, procedures, and potential risks and benefits to a broader range of individuals by translating trial-related documents like informed consent forms, participant information sheets, and questionnaires into local languages. In addition to translators, recruiting the clinical trial workforce (including principal investigators, clinical research coordinators, clinical research associates, and others) with diverse backgrounds can further assist participants with clear and effective communication during in-person visits and discussions, thereby meaningfully engaging the participants in the trial process.
Collecting data on social determinants of health
Collecting data on social determinants of health is crucial to understanding the factors influencing health outcomes. The FDA has issued guidance on using a standardized approach for collecting and reporting race and ethnicity data to accurately characterize the participating populations and identify gaps in representation.114 The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines emphasize the importance of identifying both these exogenous and endogenous factors specific to each new chemical or biological entity early in the clinical development process.115
Conclusion
The outcome of cancer care is heavily influenced by the complex interplay of ethnic, genetic, clinical, environmental, and sociodemographic factors. Therefore, diversity, equity, and inclusion must be prioritized in cancer research. Patient-centric recruitment, adaptive trial designs, culturally sensitive patient education programs, decentralized trials, and digital technology are enhancing the inclusion of marginalized and vulnerable populations in oncology trials. However, the disparity in cancer care persists despite consistent efforts from regulators and sponsors. The health care industry needs to periodically assess the disparity in clinical trial representation and adapt the international regulatory recommendations to regional, sociodemographic, and cultural settings to pave the way for each eligible participant to receive equitable cancer care.
Acknowledgments
Funding
None declared.
Disclosure
KSS reports consulting fees from the European Commission, and stock and/or other ownership interests in Labcorp Inc., Fortrea Inc., and Quantum Health Analytics (UK) Ltd., outside the submitted work. RAP is an employee of Labcorp; declares payment or honorarium as an advisory board member of Myriad Genetics and Natera; and holds stocks in Labcorp. HAA is an employee of Emergence Therapeutics. CO is an employee of Daiichi Sankyo and has shares with the company. All other authors have declared no conflicts of interest.
Data sharing
All data and references mentioned in this manuscript are from publicly available sources. Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ngwa W., Addai B.W., Adewole I., et al. Cancer in sub-Saharan Africa: a Lancet Oncology Commission. Lancet Oncol. 2022;23(6):e251–e312. doi: 10.1016/S1470-2045(21)00720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dlamini Z., Molefi T., Khanyile R., et al. From incidence to intervention: a comprehensive look at breast cancer in South Africa. Oncol Ther. 2023;12:1–11. doi: 10.1007/s40487-023-00248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamdi Y., Abdeljaoued-Tej I., Zatchi A.A., et al. Cancer in Africa: the untold story. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.650117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zullig L.L., Fortune-Britt A.G., Rao S., Tyree S.D., Godley P.A., Carpenter W.R. Enrollment and racial disparities in National Cancer Institute cancer treatment clinical trials in North Carolina. N C Med J. 2016;77(1):52–58. doi: 10.18043/ncm.77.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duma N., Vera Aguilera J., Paludo J., et al. Representation of minorities and women in oncology clinical trials: review of the past 14 years. J Oncol Pract. 2018;14(1):e1–e10. doi: 10.1200/JOP.2017.025288. [DOI] [PubMed] [Google Scholar]
- 7.Loree J.M., Anand S., Dasari A., et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol. 2019;5(10) doi: 10.1001/jamaoncol.2019.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behring M., Hale K., Ozaydin B., Grizzle W.E., Sodeke S.O., Manne U. Inclusiveness and ethical considerations for observational, translational, and clinical cancer health disparity research. Cancer. 2019;125(24):4452–4461. doi: 10.1002/cncr.32495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration . U.S. Food and Drug Administration; 2022. Drug trial snapshots summary report 2021.https://www.fda.gov/media/158482/download . 2022. Available at. [Google Scholar]
- 10.Jan J., Osho A., Murphy C.C., Mazure C.M., Singal A.G., Rich N.E. Gender, age, racial and ethnic disparities in clinical trial enrollment for primary liver cancer. Gastroenterology. 2022;163(1):14–20.e2. doi: 10.1053/j.gastro.2022.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Candelario N.M., Major J., Dreyfus B., et al. Diversity in clinical trials in Europe and the USA: a review of a pharmaceutical company’s data collection, reporting, and interpretation of race and ethnicity. Ann Oncol. 2023;34(12):1194–1197. doi: 10.1016/j.annonc.2023.09.3107. [DOI] [PubMed] [Google Scholar]
- 12.Molina-Aguilar C., Robles-Espinoza C.D. Tackling the lack of diversity in cancer research. Dis Model Mech. 2023;16(9) doi: 10.1242/dmm.050275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedrak M.S., Ji J., Tiwari A., Mohile S.G., Dale W., Le-Rademacher J.G. Clinical trial enrollment, ineligibility, and reasons for decline in older vs younger patients with cancer in the National Cancer Institute Community Oncology Research Program. JAMA Netw Open. 2022;5(10) doi: 10.1001/jamanetworkopen.2022.35714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel D., Kilburn L., Fox L., Hall E., Bliss J., Lewis R. Equality, diversity, and inclusion in oncology clinical trials: an audit of essential documents and data collection against INCLUDE under-served groups in a UK academic trial setting. BMC Med Ethics. 2023;24(1):105. doi: 10.1186/s12910-023-00987-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodcock J., Anagnostiadis E., Lolic M. U.S. Food and Drug Administration; 2019. Drug trials snapshots summary report 2018.https://www.fda.gov/media/120253/download . 2019. Available at. [Google Scholar]
- 16.Lee E., Wen P. Gender and sex disparity in cancer trials. ESMO Open. 2020;5(suppl 4) doi: 10.1136/esmoopen-2020-000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dymanus K.A., Butaney M., Magee D.E., et al. Assessment of gender representation in clinical trials leading to FDA approval for oncology therapeutics between 2014 and 2019: a systematic review-based cohort study. Cancer. 2021;127(17):3156–3162. doi: 10.1002/cncr.33533. [DOI] [PubMed] [Google Scholar]
- 18.Ramaswami R., Paulino E., Barrichello A., et al. Disparities in breast, lung, and cervical cancer trials worldwide. J Glob Oncol. 2018;4:1–11. doi: 10.1200/JGO.17.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells J.C., Sharma S., Del Paggio J.C., et al. An analysis of contemporary oncology randomized clinical trials from low/middle-income vs high-income countries. JAMA Oncol. 2021;7(3):379–385. doi: 10.1001/jamaoncol.2020.7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson B.E., Pearson S.A., Barton M.B., Amir E. Regional variations in clinical trial outcomes in oncology. J Natl Compr Canc Netw. 2022;20(8):879–886.e2. doi: 10.6004/jnccn.2022.7029. [DOI] [PubMed] [Google Scholar]
- 21.Rubagumya F., Hopman W.M., Gyawali B., et al. Participation of lower and upper middle–income countries in oncology clinical trials led by high-income countries. JAMA Network Open. 2022;5(8) doi: 10.1001/jamanetworkopen.2022.27252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Galaly T.C., Gaidzik V.I., Gaman M.A., et al. A lack of diversity, equity, and inclusion in clinical research has direct impact on patient care. Hemasphere. 2023;7(3):e842. doi: 10.1097/HS9.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson T.M., Le H., Campbell E., et al. Clinical trial deserts: US urban vs rural patient enrollment among patients with advanced cancer in phase 1 clinical trials at a major cancer center. J Clin Oncol. 2023;41(suppl 16) [Google Scholar]
- 24.Habr D., Singh M., Uehara R. Diversity in oncology clinical trials: current landscape for industry-sponsored clinical trials in Asia. Oncol Ther. 2024;12(1):115–129. doi: 10.1007/s40487-023-00254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vigil D., Sinaii N., Karp B. American Indian and Alaska Native enrollment in clinical studies in the National Institutes of Health’s Intramural Research Program. Ethics Hum Res. 2021;43(3):2–9. doi: 10.1002/eahr.500090. [DOI] [PubMed] [Google Scholar]
- 26.Kizub D., Manner C.K., Graef K., et al. Action for increasing diversity, market access, and capacity in oncology registration trials—is Africa the answer? Report from a satellite session of the Accelerating Anti-Cancer Agent Development and Validation Workshop. JCO Glob Oncol. 2022;(8) doi: 10.1200/GO.22.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gössling G., Rebelatto T.F., Villarreal-Garza C., et al. Current scenario of clinical cancer research in Latin America and the Caribbean. Curr Oncol. 2023;30(1):653–662. doi: 10.3390/curroncol30010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mok T.S., Cheng Y., Zhou X., et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244–2250. doi: 10.1200/JCO.2018.78.7994. [DOI] [PubMed] [Google Scholar]
- 29.Huang H., Tang Y., Wu D., et al. Unfair older patients restriction in cancer drug trials in mainland China and corresponding solution. BMC Geriatr. 2023;23:199. doi: 10.1186/s12877-023-03886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maiorano B.A., Maiorano M.F.P., Lorusso D., Maio M.D., Maiello E. Efficacy and safety of PARP inhibitors in elderly patients with advanced ovarian cancer: a systematic review and meta-analysis. Int J Gynecol Cancer. 2022;32(11):1410–1418. doi: 10.1136/ijgc-2022-003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan Y., Liu L., Chen H., et al. Comprehensive characterization of molecular differences in cancer between male and female patients. Cancer Cell. 2016;29(5):711–722. doi: 10.1016/j.ccell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye Y., Jing Y., Li L., et al. Sex-associated molecular differences for cancer immunotherapy. Nat Commun. 2020;11(1):1779. doi: 10.1038/s41467-020-15679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spencer R.J., Rice L.W., Ye C., Woo K., Uppal S. Disparities in the allocation of research funding to gynecologic cancers by Funding to Lethality scores. Gynecol Oncol. 2019;152(1):106–111. doi: 10.1016/j.ygyno.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corrigan K.L., Mainwaring W., Miller A.B., et al. Exclusion of men from randomized phase III breast cancer clinical trials. Oncologist. 2020;25(6):e990–e992. doi: 10.1634/theoncologist.2019-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cancer clinical trial eligibility criteria: patients with HIV, hepatitis B virus, or hepatitis C virus infections. FDA guidance document; 2020. https://www.fda.gov/media/121319/download . 2020. Available at. [Google Scholar]
- 36.Uldrick T.S., Gonçalves P.H., Abdul-Hay M., et al. Assessment of the safety of pembrolizumab in patients with HIV and advanced cancer—a phase 1 study. JAMA Oncol. 2019;5(9):1332–1339. doi: 10.1001/jamaoncol.2019.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chakraborty S., Mallick I., Luu H.N., et al. Geographic disparities in access to cancer clinical trials in India. Ecancermedicalscience. 2021;15:1161. doi: 10.3332/ecancer.2021.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer S., Woldu H.G., Sheets L.R. Sociodemographic diversity in cancer clinical trials: new findings on the effect of race and ethnicity. Contemp Clin Trials Commun. 2021;21 doi: 10.1016/j.conctc.2021.100718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unger J.M., Moseley A.B., Cheung C.K., et al. Persistent disparity: socioeconomic deprivation and cancer outcomes in patients treated in clinical trials. J Clin Oncol. 2021;39(12):1339–1348. doi: 10.1200/JCO.20.02602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cancer clinical trial eligibility criteria: available therapy in non-curative settings. FDA guidance document; 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cancer-clinical-trial-eligibility-criteria-available-therapy-non-curative-settings . 2022. Available at. [Google Scholar]
- 41.Evaluating cancer drugs in patients with central nervous system metastases. FDA guidance document; 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/evaluating-cancer-drugs-patients-central-nervous-system-metastases . 2021. Available at. [DOI] [PubMed] [Google Scholar]
- 42.Premenopausal women with breast cancer: developing drugs for treatment. FDA guidance document; 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/premenopausal-women-breast-cancer-developing-drugs-treatment . 2021. Available at. [Google Scholar]
- 43.Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials guidance for industry. FDA guidance document; 2022. https://www.fda.gov/media/157635/download . 2022. Available at. [Google Scholar]
- 44.Male breast cancer: developing drugs for treatment. FDA guidance document; 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/male-breast-cancer-developing-drugs-treatment . 2020. Available at. [Google Scholar]
- 45.Cancer clinical trial eligibility criteria: patients with HIV, hepatitis B virus, or hepatitis C virus infections. FDA guidance document; 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cancer-clinical-trial-eligibility-criteria-patients-hiv-hepatitis-b-virus-or-hepatitis-c-virus . 2020. Available at. [Google Scholar]
- 46.Cancer clinical trial eligibility criteria: patients with organ dysfunction or prior or concurrent malignancies. FDA guidance document. 2020 https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cancer-clinical-trial-eligibility-criteria-patients-organ-dysfunction-or-prior-or-concurrent Available at. [Google Scholar]
- 47.Cancer clinical trial eligibility criteria: brain metastases. FDA guidance document; 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cancer-clinical-trial-eligibility-criteria-brain-metastases . 2020. Available at. [Google Scholar]
- 48.Cancer clinical trial eligibility criteria: minimum age considerations for inclusion of pediatric patients. FDA guidance document; 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cancer-clinical-trial-eligibility-criteria-minimum-age-considerations-inclusion-pediatric-patients . 2020. Available at. [Google Scholar]
- 49.Considerations for the inclusion of adolescent patients in adult oncology clinical trials. Food and Drug Administration. FDA guidance document; 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-inclusion-adolescent-patients-adult-oncology-clinical-trials . 2020. Available at. [Google Scholar]
- 50.Enhancing the diversity of clinical trial populations—eligibility criteria, enrollment practices, and trial designs guidance for industry. U.S. FDA guidance document; 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enhancing-diversity-clinical-trial-populations-eligibility-criteria-enrollment-practices-and-trial . 2020. Available at. [Google Scholar]
- 51.Food and Drug Administration Office of Minority Health and Health Equity. 2023. https://www.fda.gov/about-fda/office-commissioner/office-minority-health-and-health-equity Available at.
- 52.National Institutes of Health Biden-Harris Administration launches initiative to improve cancer outcomes in low-income areas. 2023. https://www.nih.gov/news-events/news-releases/biden-harris-administration-launches-initiative-improve-cancer-outcomes-low-income-areas Available at.
- 53.Postmarketing requirements and commitments: introduction. FDA guidance document; 2018. https://www.fda.gov/drugs/guidance-compliance-regulatory-information/postmarket-requirements-and-commitments Available at. [Google Scholar]
- 54.European Medicines Agency (EMA) Accelerating clinical trials in the EU (ACT EU) 2022. https://www.ema.europa.eu/en/human-regulatory/research-development/clinical-trials/accelerating-clinical-trials-eu-act-eu Available at.
- 55.Government of Canada Equity, diversity and inclusion. 2022. https://www.nserc-crsng.gc.ca/InterAgency-Interorganismes/EDI-EDI/index_eng.asp Available at.
- 56.Australian Clinical Trial Alliance Recommendations to improve cultural and linguistic diversity in clinical trials 2023. https://clinicaltrialsalliance.org.au/latest-news/recommendations-to-improve-cultural-diversity-in-clinical-trials/ Available at.
- 57.Government of Canada Action plan. 2022. https://www.nserc-crsng.gc.ca/InterAgency-Interorganismes/EDI-EDI/Action-Plan_Plan-dAction_eng.asp Available at.
- 58.Government of Canada Guidance document: considerations for inclusion of women in clinical trials and analysis of sex differences. 2013. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/clinical-trials/considerations-inclusion-women-clinical-trials-analysis-data-sex-differences.html Available at.
- 59.European Commision Clinical trials - Regulation EU No 536/2014 | FAMHP. https://www.famhp.be/en/eu_regulation_5362014 Available at.
- 60.European Medicines Agency (EMA) European Medicines Agency; 2018. ICH E8 General considerations for clinical studies - scientific guideline.https://www.ema.europa.eu/en/ich-e8-general-considerations-clinical-studies-scientific-guideline Available at. [Google Scholar]
- 61.European Medicines Agency (EMA) ICH E6 (R3) Guideline on good clinical practice (GCP) Step 2b. 2023. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-ich-e6-r3-guideline-good-clinical-practice-gcp-step-2b_en.pdf Available at.
- 62.Government of India . Rajya Sabha Secretariat; New Delhi: 2012. Department-related Parliamentary Standing Committee on Health and Family Welfare. Fifty-Ninth Report on the Functioning of the Central Drugs Standard Control Organisation (CDSCO)https://casemindia.org/wp-content/uploads/2020/05/59th-Report-of-the-Parliamentary-Standing-Committee-on-Health-on-the-Functioning-of-the-CDSCO-2012.pdf Available at. [Google Scholar]
- 63.Government of India New drugs and clinical trials rules. 2019. https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/NewDrugs_CTRules_2019.pdf Available at.
- 64.Pharmaceuticals and Medical Devices Agency, Japan Basic principles on global clinical trials (reference cases) https://www.pmda.go.jp/files/000208185.pdf Available at.
- 65.NHS England Increasing diversity in research participation: a good practice guide for engaging with underrepresented groups. 2023. https://www.england.nhs.uk/aac/wp-content/uploads/sites/50/2023/02/B1905-increasing-diversity-in-research-participation-v2.pdf.pdf Available at.
- 66.National Institute for Health and Care Research Improving inclusion of under-served groups in clinical research: Guidance from INCLUDE project. 2020. https://www.nihr.ac.uk/documents/improving-inclusion-of-under-served-groups-in-clinical-research-guidance-from-include-project/25435 Available at.
- 67.NIHR | Applied Research Collaboration East Midlands Increasing participation of Black Asian and Minority Ethnic Groups in health and social care research. https://arc-em.nihr.ac.uk/clahrcs-store/increasing-participation-black-asian-and-minority-ethnic-groups-health-and-social-care Available at.
- 68.Online Survey Software Qualtrics Survey Solutions. https://nihodoercomm.az1.qualtrics.com/jfe/form/SV_eypqaXlx2j1IY9T?Q_CHL=si&Q_CanScreenCapture=1 Available at.
- 69.Decentralized clinical trials for drugs, biological products, and devices. FDA guidance document; 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/decentralized-clinical-trials-drugs-biological-products-and-devices . 2023. Available at. [Google Scholar]
- 70.Foster D. The Diversity Site Assessment Tool (DSAT), reliability and validity of the industry gold standard for establishing investigator site ranking. Int J Med Sci. 2020;7:1–13. [Google Scholar]
- 71.Diversity, inclusion, and equity in clinical research. FDA guidance document. https://mrctcenter.org/diversity-in-clinical-research/guidance/guidance-document/ Available at.
- 72.Takvorian S.U., Guerra C.E., Schpero W.L. A hidden opportunity—Medicaid’s role in supporting equitable access to clinical trials. N Engl J Med. 2021;384(21):1975–1978. doi: 10.1056/NEJMp2101627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patel K., Mukhi H., Touloukian E., Brown B. Can just-in-time clinical trials reduce disparities in participation? Targeted Ther Oncol. 2022;11:23. 23. [Google Scholar]
- 74.Moultrie A. From “brave spaces” to battling bias in patient engagement. Appl Clin Trials. 2023;32(6):6–8. [Google Scholar]
- 75.Why diversity in clinical trials is essential to the future of UK life sciences. 2023. https://www.imperial.nhs.uk/about-us/news/why-diversity-in-clinical-trials-is-essential-to-the-future-of-uk-life-sciences Available at.
- 76.Egality diversity in research equality in health. Improving diversity in health research and trials, a conversation with medical research charities. 2021. https://slginvolvement.org.uk/wp-content/uploads/2021/03/Egality-Improving-diversity-in-health-research-and-trials-public.pdf Available at.
- 77.UK Research and Innovation Expectations for equality, diversity and inclusion – UKRI. https://www.ukri.org/what-we-do/supporting-healthy-research-and-innovation-culture/equality-diversity-and-inclusion/epsrc/expectations-for-equality-diversity-and-inclusion/ Available at.
- 78.NIHR | Applied Research Collaboration North East and North Cumbria. FOR-EQUITY – resources to help make research evidence more relevant to reduce social and health inequalities. https://arc-nenc.nihr.ac.uk/resources/for-equity-resources-to-help-make-research-evidence-more-relevant-to-reduce-social-and-health-inequalities/ Available at.
- 79.GOV.UK Office for Health Improvement and Disparities. Language interpreting and translation: migrant health guide. 2021. https://www.gov.uk/guidance/language-interpretation-migrant-health-guide Available at.
- 80.Patient-Centered Outcomes Research Institute Equity and inclusion guiding engagement principles | PCORI. 2021. https://www.pcori.org/about/pcoris-advisory-panels/advisory-panel-patient-engagement/equity-and-inclusion-guiding-engagement-principles Available at.
- 81.Eve C., Parker R., NIHR Network Support Fellows Six-step stakeholder engagement framework. 2021. https://training.cochrane.org/sites/training.cochrane.org/files/public/uploads/Six%20Step%20Stakeholder%20Engagement%20Framework.pdf Available at.
- 82.GOV.UK Writing about ethnicity. https://www.ethnicity-facts-figures.service.gov.uk/style-guide/writing-about-ethnicity Available at.
- 83.NIHR Race Equality Framework. 2022. https://www.nihr.ac.uk/documents/nihr-race-equality-framework/30388 Available at.
- 84.NIHR. EDI Toolkit. https://www.rdsresources.org.uk/edi-toolkit Available at.
- 85.NIHR | Applied Research Collaboration East Midlands Equality Impact Assessment (EqIA) toolkit. https://arc-em.nihr.ac.uk/clahrcs-store/equality-impact-assessment-eqia-toolkit Available at.
- 86.NIHR | Applied Research Collaboration Kent, Surrey and Sussex Addressing barriers to engagement, involvement & participation in research by ethnic minorities. 2022. https://arc-kss.nihr.ac.uk/news/kss-nihr-partners-working-together-with-communities-to-address-barriers-to-engagement-involvement-participation-in-research-by-ethnic-minorities Available at.
- 87.NHS England Accelerated Access Collaborative. Increasing diversity in research participation: a good practice guide for engaging with underrepresented groups. 2023. https://www.england.nhs.uk/aac/publication/increasing-diversity-in-research-participation/ Available at.
- 88.Funding rules involving Aboriginal and Torres Strait Islander peoples | NHMRC. https://www.nhmrc.gov.au/health-advice/aboriginal-and-torres-strait-islander-health/funding-rules-involving-aboriginal-and-torres-strait-islander-people Available at.
- 89.Government of Canada Institute of Indigenous Peoples’ Health - CIHR. 2003. https://cihr-irsc.gc.ca/e/8668.html Available at.
- 90.The New Zealand Health Research Prioritisation Framework | Health Research Council of New Zealand. https://www.hrc.govt.nz/resources/new-zealand-health-research-prioritisation-framework Available at.
- 91.de las Heras B., Daehnke A., Saini K.S., et al. Role of decentralized clinical trials in cancer drug development: results from a survey of oncologists and patients. Digit Health. 2022;8 doi: 10.1177/20552076221099997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adesoye T., Katz M.H.G., Offodile A.C. Meeting trial participants where they are: decentralized clinical trials as a patient-centered paradigm for enhancing accrual and diversity in surgical and multidisciplinary trials in oncology. JCO Oncol Pract. 2023;19(6):317–321. doi: 10.1200/OP.22.00702. [DOI] [PubMed] [Google Scholar]
- 93.Witham M.D., Anderson E., Carroll C., et al. Developing a roadmap to improve trial delivery for under-served groups: results from a UK multi-stakeholder process. Trials. 2020;21(1):694. doi: 10.1186/s13063-020-04613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guerra C., Pressman A., Hurley P., et al. Increasing racial and ethnic equity, diversity, and inclusion in cancer treatment trials: evaluation of an ASCO-Association of Community Cancer Centers site self-assessment. JCO Oncol Pract. 2023;19(4):e581–e588. doi: 10.1200/OP.22.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Epps H.V., Astudillo O., Martin Y.D.P., Marsh J. The sex and gender equity in research (SAGER) guidelines: implementation and checklist development. Eur Sci Ed. 2022;48 [Google Scholar]
- 96.Rubin E. Striving for diversity in research studies. N Engl J Med. 2021;385(15):1429–1430. doi: 10.1056/NEJMe2114651. [DOI] [PubMed] [Google Scholar]
- 97.Sweet D.J. New at Cell Press: the inclusion and diversity statement. Cell. 2021;184(1):1–2. doi: 10.1016/j.cell.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 98.Hughson J.-A., Woodward-Kron R., Parker A., et al. A review of approaches to improve participation of culturally and linguistically diverse populations in clinical trials. Trials. 2016;17(1):263. doi: 10.1186/s13063-016-1384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kumar G., Chaudhary P., Quinn A., Su D. Barriers for cancer clinical trial enrollment: a qualitative study of the perspectives of healthcare providers. Contemp Clin Trials Commun. 2022;28 [Google Scholar]
- 100.Kim E.S., Uldrick T.S., Schenkel C., et al. Continuing to broaden eligibility criteria to make clinical trials more representative and inclusive: ASCO-Friends of Cancer Research joint research statement. Clin Cancer Res. 2021;27(9):2394–2399. doi: 10.1158/1078-0432.CCR-20-3852. [DOI] [PubMed] [Google Scholar]
- 101.Bonomi P., Blumenthal G., Ferris A.S., et al. Making lung cancer clinical trials more inclusive: recommendations for expanding eligibility criteria. J Thorac Oncol. 2018;13(6):748–751. doi: 10.1016/j.jtho.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 102.Denicoff A.M., Ivy S.P., Tamashiro T.T., et al. Implementing modernized eligibility criteria in US National Cancer Institute clinical trials. J Natl Cancer Inst. 2022;114(11):1437–1440. doi: 10.1093/jnci/djac152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clampet J. Enrichment strategies for clinical trials to support determination of effectiveness of human drugs and biological products guidance for industry. 2019. https://www.govinfo.gov/content/pkg/FR-2019-03-15/pdf/2019-04815.pdf Available at.
- 104.Pregnant women: scientific and ethical considerations for inclusion in clinical trials. FDA guidance document. 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pregnant-women-scientific-and-ethical-considerations-inclusion-clinical-trials Available at.
- 105.Niranjan V., Fitzpatrick P., Morrogh R., O’Hagan K. Impact of community outreach programme on improving cancer related preventive health behaviour. Eur J Public Health. 2022;32(suppl 3) doi: 10.1016/S0140-6736(22)02277-2. ckac131.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saini K.S., Heras B., Plummer R., et al. Reimagining global oncology clinical trials for the postpandemic era: a call to arms. JCO Glob Oncol. 2020;6:1357–1362. doi: 10.1200/GO.20.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Slomovitz B., Gourley C., Carey M.S., et al. Low-grade serous ovarian cancer: state of the science. Gynecol Oncol. 2020;156(3):715–725. doi: 10.1016/j.ygyno.2019.12.033. [DOI] [PubMed] [Google Scholar]
- 108.Brooks S.E., Muller C.Y., Robinson W., et al. Increasing minority enrollment onto clinical trials: practical strategies and challenges emerge from the NRG oncology accrual workshop. J Oncol Pract. 2015;11(6):486–490. doi: 10.1200/JOP.2015.005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karmo M., Pierre A. Pull up a seat: engaging patients as empowered partners in health equity transformation. J Adv Pract Oncol. 2022;13(3):202–204. doi: 10.6004/jadpro.2022.13.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Regnante J., Pratt-Chapman M.L., Bodiford K., et al. The case for acceleration of prospective multi-stakeholder led community-based research in young Black women with triple negative breast cancer (TNBC) J Clin Oncol. 2023;41:1089. [Google Scholar]
- 111.Katz N.T., Alpert A.B., Aristizabal M.P., et al. Partnering with patients and caregivers in cancer care: lessons from experiences with transgender, Hispanic, and pediatric populations. Am Soc Clin Oncol Educ Book. 2023;43 doi: 10.1200/EDBK_397264. [DOI] [PubMed] [Google Scholar]
- 112.Rosa C., Campbell A.N.C., Miele G.M., Brunner M., Winstanley E.L. Using e-technologies in clinical trials. Contemp Clin Trials. 2015;45:41–54. doi: 10.1016/j.cct.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pritchett J.C., Patt D., Thanarajasingam G., Schuster A., Snyder C. Patient-reported outcomes, digital health, and the quest to improve health equity. Am Soc Clin Oncol Educ Book. 2023;43 doi: 10.1200/EDBK_390678. [DOI] [PubMed] [Google Scholar]
- 114.Kahn J.M., Gray D.M.I.I., Oliveri J.M., Washington C.M., DeGraffinreid C.R., Paskett E.D. Strategies to improve diversity, equity, and inclusion in clinical trials. Cancer. 2022;128(2):216–221. doi: 10.1002/cncr.33905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.International Conference of Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Final concept paper E7(R1): studies in support of special populations: geriatrics (Revision of the ICH E7 guideline) 2008. https://database.ich.org/sites/default/files/E7_Q%26As_Concept_Paper.pdf Available at.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.