Abstract
Introduction
Metabolic syndrome comprises a collection of metabolic disorders stemming from factors like genetic predisposition, inadequate nutrition, stress, decreased physical activity, aging, and ethnicity. Although traditional pharmaceutical treatments exist for metabolic syndrome, their limited popularity is attributed to high costs and adverse effects. Consequently, natural products with fewer side effects have been explored for managing this condition. This literature review aims to explore the role of natural products including herbs, botanicals, vitamins, minerals, probiotics, and dietary supplements in managing metabolic syndrome.
Methods
This scoping review was conducted in five steps, involving the formulation of a research question, the retrieval and extraction of relevant studies, the selection of pertinent studies, the organization of information into tables, and the reporting of results. Data was collected from various databases including Embase, Science Direct, PubMed, Google Scholar, Scopus, and Web of Science, with a focus on studies published from 2010 to the present, available in English and with full-text accessibility.
Results
We identified 1,259 articles, screened their titles, abstracts, and full texts, ultimately incorporating 169 pertinent articles into this review (comprising 90 review articles, 32 trial articles, 6 in vitro articles, 38 in vivo articles, 1 experimental article and 2 observational articles). The study’s outcomes revealed that natural products, encompassing plants and their derivatives, vitamins and supplements, as well as probiotics, can exert a beneficial influence on metabolic syndrome by regulating blood sugar, blood pressure, lipid profiles, obesity, and abnormal cholesterol and triglyceride levels.
Conclusion
The current study underscores the significance of natural products in addressing metabolic syndrome. Consequently, it is advisable to conduct further extensive research to assess the efficacy of these products, potentially integrating them into treatment regimens for individuals with metabolic syndrome.
Keywords: natural products, plants, complementary and alternative medicine, metabolic syndrome, scoping review
1 Introduction
Metabolic syndrome (MetS) is a cluster of metabolic disorders, including impairments in protein, glucose, lipid, and carbohydrate (Di et al., 2019). It is characterized by a combination of risk factors for atherosclerotic cardiovascular diseases (ASCVD) and type 2 diabetes (Hou et al., 2019; Nna et al., 2023). These risk factors lead to the development of high blood pressure, high blood sugar or diabetes, obesity, excess abdominal fat, and abnormal cholesterol or triglyceride levels (Hou et al., 2019). The diagnosis of metabolic syndrome typically requires the presence of three or more of the following conditions: (Di et al., 2019): waist circumference ≥102 cm in men and ≥88 cm in women, (Nna et al., 2023), triglycerides ≥150 mg/dL or undergoing drug therapy for high triglycerides, (Hou et al., 2019), HDL-C < 40 mg/dL in men and <50 mg/dL in women or undergoing drug therapy for lowering cholesterol, (Jang et al., 2016a), systolic blood pressure ≥130 mmHg, diastolic blood pressure ≥85 mmHg or undergoing antihypertensive treatment for individuals with a history of hypertension, and (van der Pal et al., 2018) fasting glucose ≥100 mg/dL or undergoing treatment to control glucose levels (Jang et al., 2016a; van der Pal et al., 2018). However, the specific measurement for waist size may vary slightly based on country and ethnicity (Jang et al., 2016a). Factors contributing to the development of metabolic syndrome include genetic predisposition, poor dietary habits, stress, sedentary lifestyle, age, and ethnicity (Raja Kumar et al., 2019; Nna et al., 2023). Some of these factors, such as diet and physical activity, can be managed over time (Raja Kumar et al., 2019).
The treatment of metabolic syndrome has traditionally involved dietary modifications and the use of chemical drugs targeting specific biochemical pathways involved in food metabolism (Hameed and Al-Ameri, 2022). Beta-blockers, statins, fibrates and glibenclamide are usually known as the most commonly used drugs for patients with metabolic syndrome (Gharipour et al., 2011). However, these drugs are often costly, poorly tolerated by patients, associated with various side effects and do not have sustainable effectiveness (Nigussie, 2021). Additionally, typically represent monotherapy and address only a limited range of health outcomes associated with metabolic disorders (Tola et al., 2023). Consequently, there is a need to explore and develop alternative and complementary approaches with fewer complications for managing metabolic diseases (Nigussie, 2021).
Complementary and alternative medicine (CAM) has been used globally for centuries and consists of a wide range of therapies (Suganya et al., 2017; Adeniyi et al., 2021; Nasiri et al., 2023). One of these treatments is the use of natural products (Nasiri et al., 2023). Many medicinal plants and natural products are considered by the public as a safe and natural alternative to synthetic drugs (Waltenberger et al., 2016b). It has been shown that natural products or their derivatives are a valuable source of therapeutic agents (Atanasov et al., 2015; Fadhel and Hassan, 2023). Growing evidence indicates that natural products and their bioactive compounds can provide various benefits to the human health (Nainu et al., 2023). Natural products such as herbal drugs in addition to their secondary metabolites act as endless sources of promising drug leads that revealed significant anti-inflammatory as well as anti-obesity potential (Youssef et al., 2022). They revealed higher safety margins, eco-friendly, and less expensive with respect to synthetic chemical entities (Li et al., 2021). Consistent with this approach, researchers have focused on natural products in the field of prevention or treatment of MetS (Dong et al., 2012; Taghipour et al., 2019). In this regard, a study by Jang et al. (2016) demonstrated the effect of herbal medicines on reducing waist circumference, blood glucose, blood lipids, and blood pressure, suggesting their potential for treating metabolic syndrome (Jang et al., 2016a). Sabarathinam et al. (2022) highlighted that certain plant extracts contain natural active components that target multiple biological pathways, offering the opportunity to address various defects associated with metabolic syndrome simultaneously (Sabarathinam et al., 2022). Additionally, a study by Taghipour et al. (2019) revealed that Plant-based natural products improve obesity-associated MetS such as hyperglycemia, hyperlipidemia, and insulin resistance, reduce systolic and diastolic blood pressure, and reduce body weight gain (Taghipour et al., 2019).
Metabolic syndrome is now recognized as a disease with developmental origins, and it has become a significant focus of recent research (Hou et al., 2019). Given the increasing popularity of complementary and alternative medicine (CAM) for managing various health conditions, including metabolic syndrome, a review of the existing literature is crucial to comprehend the scope of CAM usage, its reported effectiveness, and potential safety considerations in relation to metabolic syndrome. While there is evidence suggesting that certain natural products can positively affect components of metabolic syndrome (Jang et al., 2016a; Taghipour et al., 2019; Sabarathinam et al., 2022), a comprehensive review is necessary to evaluate the quality of this evidence. Moreover, there are still gaps in understanding which natural products show the most promising outcomes, their mechanisms of action, and their potential interactions with conventional therapies. A scoping review can help identify these gaps and guide the design of future studies.
Healthcare practitioners and patients require evidence-based information to make informed decisions about the use of natural products for managing metabolic syndrome. Therefore, conducting a scoping review can offer a comprehensive overview of the current state of knowledge and help elucidate the potential role of natural products in this field. This scoping review aimed to investigate natural products in the management of metabolic syndrome.
2 Materials and methods
This scoping review was conducted in five stages: 1) formulating the research question, 2) searching for and extracting relevant studies, 3) selecting related studies, 4) tabulating, summarizing, and synthesizing the information and data, and 5) reporting the results (Mak and Thomas, 2022). Following the formulation of the research question (what is the role of natural products in the management of patients with metabolic syndrome?), a search strategy was devised, inclusion criteria for the selected studies were established, data extraction forms were prepared, and data analysis program was specified.
2.1 Information sources and searches
In the book authored by Pinzon-Perez et al. (2015) (Pinzon-Perez and Pérez, 2016), 6 sub-categories for natural products (herbs, botanicals, vitamins, minerals, probiotics, and dietary supplements) are delineated, and the researchers also employed this keywords to devise an optimal search strategy. For this purpose, researchers conducted searches across several databases, including Embase, Science Direct, PubMed, Google Scholar, Scopus, and Web of Science. They used a range of keywords such as “herbs”, “botanicals”, “vitamins”, “minerals”, “probiotics”, “dietary supplements”, “natural product”, “essential oils” and “metabolic syndrome” to retrieve information from these databases.
2.2 Inclusion and exclusion criteria
The inclusion criteria included reviewing all experimental, quasi-experimental, systematic and Meta-analysis, clinical trial, review, interventional, observational, in vivo and in vitro articles, A full texts articles which were published in reputable journals were included. Additionally, articles related to animal phases were considered. The exclusion criteria covered articles that did not specifically investigate the impact of complementary and alternative medicine on metabolic syndrome, studies published before 2010, and studies published in languages other than English.
2.3 Selection of relevant studies
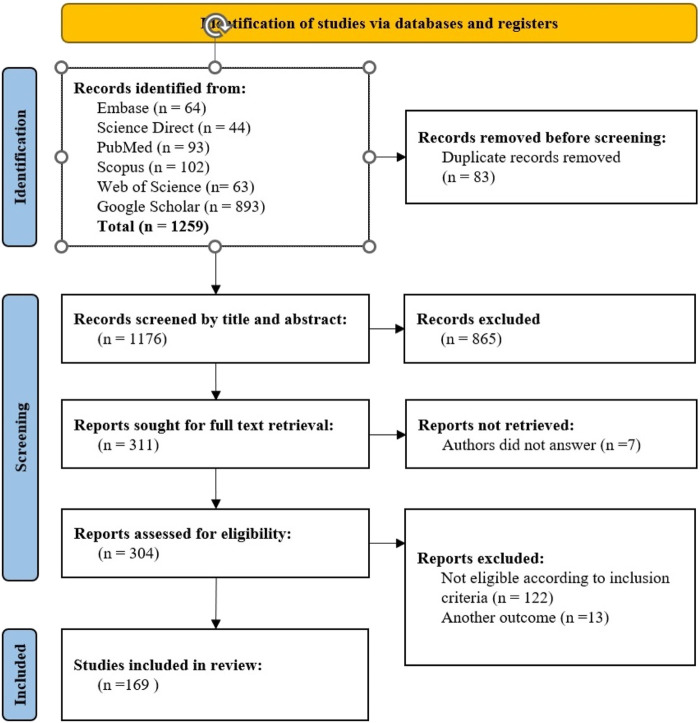
Initially, one of the researchers imported all search results from the databases into the EndNote Desktop program, with duplicates removed. Subsequently, two researchers independently reviewed articles titles and abstracts based on predetermined eligibility criteria. Any discrepancies in study selection between the two researchers were resolved through a full-text evaluation. Efforts were made to obtain inaccessible articles and unpublished data by contacting the corresponding authors of eligible studies. Initially, 1,256 articles were identified through the database search. Following the elimination of 83 duplicates, 1,176 titles and abstracts were screened, leading to the review of 311 full texts. All of them underwent assessment for eligibility criteria culminating in the inclusion of 169 articles in the study (see Figure 1). Furthermore, the reference lists of the extracted articles were examined, but no additional articles meeting the inclusion criteria were identified for this study.
FIGURE 1.
Flowchart of review and selection of articles.
Data extraction and synthesis were carried out using a standardized form, which included categories such as study identifiers (study’s author, year of publication), country and language, study type, study objective, sample size, materials and methods, results, and conclusion(s).
To comprehensively understand the research landscape on natural products for the treatment of metabolic syndrome (MetS), researchers have adopted a systematic approach to categorize studies based on their methodology and focus. This classification includes clinical trials, in vitro and in vivo studies, observational studies, and review studies. Clinical trials provide empirical evidence on the efficacy and safety of interventions in human subjects, while in vitro and in vivo studies offer mechanistic insights into the effects of natural products on metabolic pathways and physiological functions. Observational studies identify associations between product consumption, natural outcomes related to metabolic syndrome, and contributing populations. These studies are referred to as original studies (see Table 1). Additionally, review studies provide a synthesis of the existing literature and highlight the importance of herbs and natural products in addressing MetS; therefore, they are considered complementary studies. Studies are categorized based on the type of natural product investigated, including herbs, vitamins, minerals, probiotics, and dietary supplements.
TABLE 1.
Studies conducted on the effect of different natural products on metabolic syndrome.
| Author | Country | Study type | Study aim | Sample size | Materials and methods | Results | Article’s quality |
|---|---|---|---|---|---|---|---|
| Herbs | |||||||
| Nikaein et al. (2016) (Nikaein et al., 2016) | Iran | Clinical trial | The Effects of Satureja hortensis L. Dried Leaves on Metabolic Syndrome Patients | N = 47 | Daily intake 1 capsule (450 mg) for 10 weeks | Reducing in total cholesterol, low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), diastolic blood pressure (DBP), and hs-CRP as well as an elevation in high-density lipoprotein cholesterol (HDL-C) | High |
| Seong et al. (2021) (Seong et al., 2021) | Korea | Clinical trial | The effect of Korean red Panax ginseng on metabolic syndrome | N = 60 | Receiving KRG capsules (Korean red ginseng powder 100%, 6,000 mg/day) or placebo capsules (4,200 mg/day) for 8 weeks | Decreasing systolic blood pressure (SBP) | High |
| Klupp et al. (2016) (Klupp et al., 2016) | Australia | Clinical trial | The effect of Ganoderma lucidum on metabolic syndrome | N = 84 | Daily intake of 3 g for 16 weeks | No effect of Ganoderma on hyperglycemia and cardiovascular risk factors | High |
| Amin et al. (2015) (Amin et al., 2015) | Pakistan | Clinical trial | The effect of simultaneous consumption of Curcuma longa and Nigella on metabolic syndrome | N = 250 | Using black seeds (1.5 g per day), turmeric (2.4 g per day), and their combination (900 mg of black seeds and 1.5 g of turmeric) in different groups for 8 weeks | Reducing waist circumference, lipid profile, BMI, cholesterol, fasting blood glucose and C-reactive protein | High |
| Belcaro et al. (2013) (Belcaro et al., 2013) | Italy | Clinical trial | The effect of green phytosome on metabolic syndrome | N = 100 | Use of 2 tablets (total equivalent of 300 mg per day) for 24 weeks | Improvement of weight, lipid profile and blood pressure | High |
| Devaraj et al. (2013) (Devaraj et al., 2013) | The U. S | Clinical trial | The effect of Aloe vera on people with metabolic syndrome | N = 45 | Daily intake of 2 capsules of 500 mg for 8 weeks | Reduction of LDL-C level, glucose and fructosamine, reduction of HbA1c, fasting glucose and insulin | High |
| Najmi et al. (2013) (Najmi et al., 2013) | India | Clinical trial | Effect of herbal product Nigella sativa on metabolic syndrome | N = 90 | Receiving 500 mg/day capsule of Nigella sativa for 8 weeks | Antihypertensive, reducing LDL-c | High |
| Mansouri et al. (2012) (Mansouri et al., 2012) | Iran | Clinical trial | Effect of Anethum graveolens (dill) on metabolic syndrome | N = 24 | Receiving 600 mg per day for 3 months | Reducing triglyceride | High |
| Shah et al. (2012) (Shah et al., 2012) | Pakistan | Clinical trial | Effect of Nigella sativa on metabolic syndrome | N = 159 | Receiving 250 mg twice daily for 6 weeks | Beneficial effects in fasting blood sugar, low density lipoproteins and high density lipoproteins, blood pressure, circumference of waist and serum triglyceride | Moderate |
| Mohtashami (2019) (Mohtashami, 2019) | Iran | Clinical trial | Effects of Bread with Nigella Sativa on Metabolic Syndrome | N = 51 | Receiving daily a bread with N. sativa for 2 months | No effect on FBG, BP, weight, WC, and BMI. | High |
| Basu et al. (2010) (Basu et al., 2010) | Oklahoma | Clinical trial | Effect of Green Tea Supplementation on Metabolic Syndrome | N = 35 | Receiving green tea (4 cups/d) or green tea extracts (2 capsules, 4 cups water/d) for 8 weeks | Reducing body weight, BMI, LDL-cholesterol and LDL/high-density lipoprotein (HDL) | High |
| (Each cup of green tea provided approximately 110 mg of EGCG1/Each capsule provided approximately 230 mg EGCG) | |||||||
| Jain et al. (2017) (Gupta Jain et al., 2017) | India | Clinical trial | The effect of oral Cinnamomum zeylanicum on metabolic syndrome | N = 116 | Daily intake of cinnamon [6 capsules (3 g)] or wheat flour [6 capsules (2.5 g)] for 16 weeks | Reducing waist-hip ratio, blood pressure, serum total cholesterol, low-density lipoprotein cholesterol, serum triglycerides, and high-density lipoprotein cholesterol | High |
| Morovati et al. (2019) (Morovati et al., 2019) | Iran | Clinical trial | Effects of cumin (Cuminum cyminum L.) essential oil supplementation on metabolic syndrome | N = 56 | Receiving 75 mg CuEO or placebo soft gel thrice daily for 8 weeks | Reducing diastolic blood pressure | High |
| No effect on the others parameters | |||||||
| Ghitea et al. (2021) (Ghitea et al., 2021) | The U.S | Clinical Trials | The effect of Origanum vulgare L. on Metabolic Syndrome | N = 106 | Receiving 0.8 mL (2 drops) twice a day for 10 days | Antibacterial effect | High |
| Lopes Galeno et al. (2014) (Lopes et al., 2014) | Brazil | In vitro | The effect of extract from Eugenia punicifolia on Metabolic Syndrome | - | - | Antioxidant, reducing carbohydrate absorption rate | Moderate |
| Mopuri et al. (2018) (Mopuri et al., 2018) | India | In vitro | The effects of Ficus carica on metabolic syndrome | - | Using extract of Ficus carica | Antidiabetic and antiobesogenic | Moderate |
| Jakubczyk et al. (2021) (Jakubczyk et al., 2021) | Poland | In vitro | The Influence of Hypericum perforatum L. on Metabolic Syndrome | - | Preparation of cookies with the St. John’s wort (SJW) herb at 0.5 and 1 g/100 g | Higher content of bioactive compounds and antioxidant and anti-metabolic syndrome effects | Moderate |
| Kulabas et al. (2018) (Kulabas et al., 2018) | Turkey | In vitro | The effect of Lavandula stoechas on metabolic syndrome | - | Aqueous extract of Lavandula stoechas was extracted and analyzed | Preventing of insulin resistance and inflammation | Moderate |
| Cicolari et al. (2020) (Cicolari et al., 2020) | Italy | In vitro | The effect of Hydromethanolic Extracts from Adansonia digitata L. on Metabolic Syndrome | - | Using extracts of A. digitata L | Antihypertensive and antidyslipidemic | Moderate |
| Chae et al. (2022) (Chae et al., 2022) | The U.S | In vitro | Evaluate the Efficacy of Aquilaria sinensis Flower Extract against Metabolic Syndrome | - | Using extracts of Aquilaria sinensis Flower | Hyperlipidemia and hyperglycemia | Moderate |
| Dobhal et al. (2022) (Dobhal et al., 2022) | India | In vivo | The effect of Lemongrass (Cymbopogon citratus) on metabolic syndrome | N = 42 | Lemongrass ethanolic extract and aqueous extract were prepared and lemongrass oil were given to the animals for 42 days | Reducing body weight, BMI, and fasting blood sugar normalizing serum insulin, insulin resistance, leptin and lipid profile | Moderate |
| Mayer et al. (2019) (Mayer et al., 2019) | France | In vivo | Preventive Effects of the Marine Microalga Phaeodactylum tricornutum Metabolic Syndrome | N = 18 | Receiving diet supplemented with 12% of freeze-dried microalga P. tricornutum | Decreasing Body weight, fat mass, inflammatory markers, insulinemia, Plasma total cholesterol, triacylglycerols | Moderate |
| Jakovljevic et al. (2018) (Jakovljevic et al., 2018) | Serbia | In vivo | The effect of Aronia melanocarpa extract on metabolic syndrome in mice | N = 60 | Daily intake of 0.45 mL/kg extract for 4 weeks | Lowering blood pressure, improving heart function and glucose tolerance, reducing liver pathological changes and oxidative stress | Moderate |
| Preez et al. (2021) (du Preez et al., 2021) | Australia | In vivo | The effect of Nannochloropsis oceanica as a Microalgal Food Intervention on Metabolic Syndrome | N = 48 | Receiving a diet containing 5% N. oceanica for 8 weeks | Decreasing fat mass, source of essential amino acids and prebiotics | Moderate |
| Owis et al. (2017) (Owis et al., 2017) | Egypt | In vivo | The effect of Leaves of Cordia boissieri on metabolic syndrome | N = 100 | Receiving 5 mg/kg/day Cordia boissieri for 4 weeks | Improving insulin sensitivity, glucose tolerance, kidney function, lipid profiles, and reduced oxidative stress and inflammation | Moderate |
| Reshidan et al. (2019) (Reshidan et al., 2019) | Malysia | In vivo | The effect of Pandanus amaryllifolius extract on metabolic syndrome in rats | N = 30 | The administration of 10% plant leaf juice extract in the diets of rats within various groups over an 8-week period | Decreased BMI, fat cell size, blood pressure, fasting glucose, triglycerides, and high-density lipoprotein | Moderate |
| Singh et al. (2017) (Singh et al., 2017) | Canada | In vivo | The alcohol extract of North American ginseng (Panax quinquefolius) on metabolic syndrome | - | Receiving 200 mg/kg/100 μL of NAGE daily | Balance between glucose and fatty acid metabolism, lipoprotein secretion, and energy homeostasis | Moderate |
| Thomaz et al. (2022) (Thomaz et al., 2022) | Australia | In vivo | The effect of Wasabi (Eutrema japonicum) on Metabolic Syndrome | N = 48 | Intake 5% wasabi powder for 16 weeks | Reducing blood pressure, obesity, inflammation and lipid deposition | Moderate |
| Jamshidi et al. (2018) (Jamshidi et al., 2018) | Iran | In vivo | The effect of Wild pistachio (Pistacia atlantica mutica) oil on metabolic syndrome | N = 72 | Intake hull and kernel oils of WP (2 mL/kg/day) for 10 weeks | Preventing hyperglycemia, hypertriglyceridemia, hypercholesterolemia, inflammation and pancreatic secretory disorders | High |
| Wang et al. (2020) (Wang et al., 2020) | Taiwan | In vivo | The effect Cinnamomum zeylanicum and Taiwanofungus camphoratus on Metabolic | N = 48 | Receiving different doses of CO and TC for 24 weeks | Improving abnormal blood glucose regulation and restore the balance of gut microbiota | Moderate |
| Syndrome | |||||||
| Vílchez et al. (2022) (Vílchez et al., 2022) | The United Kingdom | In vivo | The effect of GlucoMedix (Stevia rebaudiana and Uncaria tomentosa) on metabolic syndrome in rats | N = 40 | Different groups used doses of 250, 1,000 and 2000 mg for 28 days | Reduced hyperglycemia, reduced lipid profile and blood pressure | Moderate |
| Alauddin et al. (2016) (Alauddin et al., 2016) | Japan | In vivo | Effect of fermented rice bran on metabolic syndrome in rats | N = 12 | Use of 2 g/kg fermented and nonfermented rice bran in different groups | Lowering blood pressure, improving leptin disorder and increasing serum adiponectin levels, improving glucose tolerance and lipid profile | Moderate |
| Bhaswant et al. (2015) (Bhaswant et al., 2015) | Japan | In vivo | The effect of Green and Black Elettaria cardamomum on Metabolic Syndrome | N = 72 | Receiving diet supplemented with 3% green or black cardamom | Black cardamom attenuated the signs of metabolic syndrome while green cardamom exacerbated adiposity, decreased liver function and worsened cardiovascular structure and function | Moderate |
| Chul Kho et al. (2016) (Kho et al., 2016) | South Korea | In vivo | The effect of red ginseng and Fallopia multiflora on metabolic syndrome in rats | N = 50 | Daily intake of 100 and 300 mg by different groups for 8 weeks | Improving blood pressure, obesity, high lipid profile, vascular inflammation and insulin resistance | Moderate |
| Kuate et al. (2015) (Kuate et al., 2015) | Malysia | In vivo | The effect of Tetrapleura tetraptera spice on metabolic syndrome indicators | - | Using different oral doses of HET (200 and 400 mg/kg) in rats for 28 days | Anti-insulin, anti-lipid, anti-obesity, reduced blood pressure and anti-inflammatory effect | Moderate |
| Tan et al. (2011) (Tan et al., 2011) | China | In vivo | The effect of Chinese herbal extracts on metabolic syndrome | N = 36 | Intake of 4 g/kg for 4 weeks | Reduction of visceral fat, cholesterol and triglycerides | Moderate |
| Chen et al. (2011) (Chen et al., 2011) | The U.S | In vivo | Effects of Green Tea on Metabolic Syndrome | N = 118 | Receiving 10 cups of green tea (2 g tea leaves per cup) per day for 17 weeks | Reducing lipid absorption and reduced levels of inflammatory cytokines | Moderate |
| Chen et al. (2021) (Chen et al., 2021) | China | In vivo | Effect of Ligustrum robustum (Roxb.) blume extract on Metabolic Syndrome | - | Receiving 200 mg/kg/d for 16 weeks | Preventing gut microbiota dysbiosis | Moderate |
| Kasabri et al. (2014) (Kasabri et al., 2014) | Jordan | In vivo | Effect of Salvia officinalis on Metabolic Syndrome | - | 125, 250, 500, 1,000 and 2000 μg/mL | Antidiabesity | Low |
| Mayer et al. (2022) (Mayer et al., 2022) | France | In vivo | The effect of the Marine Microalga Diacronema lutheri on Metabolic Syndrome | N = 18 | Daily intake of 24 mg | Preventing of weight gain and improving in lipid and glucose homeostasis | Moderate |
| De Martin et al. (2018) (De Martin et al., 2018) | Italy | Experimental | The effect of two types of Phaeophyceae on metabolic syndrome | N = 50 | Daily administration of 3 capsules containing algae (237.5 mg Ascophyllum nodosum, 12.5 mg Fucus vesiculosus and 7.5 μg chromium) for 6 months | Reduction of waist circumference, glucose level, blood insulin | Moderate |
| Hermans et al. (2020) (Hermans et al., 2020) | Belgium | Observational | Effect of a Combination of Olive (Olea europea L.) Leaf and Fruit Extracts on Metabolic Syndrome | N = 145 | Receiving 2 capsules/daily (each capsule contains 167 mg of Olea europea leaf dry extract and 53 mg of Olea europea L. fruit dry extract) | Reducing triglycerides, fasting blood glucose, waist circumference, hypertension and increasing high-density lipoprotein cholesterol (HDL-C) | High |
| Li et al. (2020) (Li et al., 2020) | China | In vivo | Effect of Eriobotrya japonica leaf on metabolic syndrome | - | Receiving 200 mg/kg for 12 weeks | Reducing body weight, relative liver weight, relative visceral adipose weight, cholesterol, triglycerides, low-density lipoprotein cholesterol, hepatic total cholesterol, and hepatic triglycerides | Moderate |
| Benkhaled et al. (2020) (Benkhaled et al., 2022) | Algeria | In vivo | effects of aqueous leaf extract of Limoniastrum guyonianum on metabolic syndrome | N = 42 | Receiving 100, 200 and 300 mg/kg b.w./day for 9 weeks | Hypoglycemic, hypolipidemic, antioxidant and renoprotective | Moderate |
| Basu et al. (2013) (Basu et al., 2013) | The U. S | Clinical trial | The effect of green tea on metabolic syndrome | N = 35 | Daily intake of 4 cups of green tea or 2 capsules of green tea extract for 8 weeks | Antioxidant activity | High |
| Nagata et al. (2012) (Nagata et al., 2012) | Japan | Clinical trial | The effect of KBG2 on endothelial function in patients with metabolic syndrome | N = 92 | Use of 2 g of KBG, three times a day after meals for 4 weeks | Improving endothelial function in patients with metabolic syndrome factors | High |
| Gurrola-Di´az (2010) (Gurrola-Dlaz et al., 2010) | Mexico | Clinical trial | The effect of Hibiscus sabdariffa extract on metabolic syndrome | N = 124 | Daily intake of 100 mg of plant extract for 1 month | Reducing glucose and total cholesterol levels, improving insulin resistance, reducing triglycerides, blood pressure, and dyslipidemia | Moderate |
| Verhoeven et al. (2015) (Verhoeven et al., 2015) | Belgium | Clinical trial | The effect of red yeast rice and Olea europaea (olive) extract on metabolic syndrome | N = 50 | Daily intake of a product containing 10.82 mg of monaculin and 9.32 mg of hydroxytyrosol, for 8 weeks | Lowering cholesterol (LDL) and blood pressure | High |
| Vitamins | |||||||
| Ferreira et al. (2020) (Ferreira et al., 2020) | Brazil | Clinical trial | The effect of vitamin D3 on metabolic syndrome in postmenopausal women | N = 160 | Intake of 1,000 units of vitamin D3 per day for 9 months | Decrease in triglycerides and insulin resistance and reduction in the risk of hypertriglyceridemia and hyperglycemia | High |
| Farag et al. (2019) (Farag et al., 2019) | Iraq | Clinical trial | The effect of vitamin C on metabolic syndrome | N = 120 | Daily intake of 500 mg for 12 weeks | Reduced BMI | High |
| Wenclewska et al. (2019) (Wenclewska et al., 2019) | Poland | Clinical trial | The effect of vitamin D on metabolic syndrome | N = 92 | Daily intake of 2000 units of vitamin D for 3 months | Reduced DNA damage, oxidative stress, and HbA1c | High |
| Ahmadi et al. (2014) (Ahmadi et al., 2014) | Iran | Clinical trial | The effects of vitamin E and omega-3 on metabolic syndrome | N = 90 | Daily intake of 400 units of vitamin E or 2.4 g of omega-3 for 8 weeks | Improving endothelial function using omega-3, lack of effect of vitamin E on endothelial function | High |
| Farag et al. (2018) (Farag et al., 2018) | Iran | Clinical trial | effects of vitamin D and vitamin C supplementations on metabolic syndrome | N = 180 | Receiving 500 mg/day vitamin C or 2000 IU/day vitamin D for 12 weeks | Improvements fasting plasma glucose, total cholesterol, low-density lipoprotein cholesterol and blood pressure, waist circumference, triglyceride, and high-density lipoprotein | High |
| Salekzamani et al. (2016) (Salekzamani et al., 2016) | Iran | Clinical trial | Effect of high-dose vitamin D supplementation on metabolic syndrome | N = 80 | Receiving 50,000 IU vitamin D weekly for 16 weeks | Decreasing triglyceride, but did not have any beneficial effects on other cardiometabolic risk factors | High |
| Salekzamani et al. (2017) (Salekzamani et al., 2017) | Iran | Clinical trial | Effect of vitamin D supplementation on metabolic syndrome | N = 80 | Receiving 50,000 IU vitamin D weekly for 16 weeks | Improvement some proatherogenic inflammatory markers, but no changes of high sensitivity C-reactive protein and carotid intima media thickness | High |
| Erbas et al. (2014) (Erbaş et al., 2014) | Turkey | In vivo | The effect of vitamin D on metabolic syndrome | N = 24 | Intake of 0.3 μg/kg/day for 2 weeks | Anti-inflammatory, antioxidant activities | Moderate |
| Bilbis et al. (2012) (Bilbis et al., 2012) | Nigeria | In vivo | The effect of vitamins A, C and E on the treatment of metabolic syndrome in mice | N = 30 | Daily intake of 100 mg/kg of vitamin C | Reducing blood pressure, serum total cholesterol, triglycerides, low and very low-density lipoprotein cholesterol, and increasing high-density lipoprotein cholesterol and antioxidant activity | Moderate |
| 6 mg/kg vitamin A, 60 mg/kg vitamin E for 4 weeks | |||||||
| Mazur-Bialy & Poche´c (2016) (Mazur-Bialy and Pocheć, 2016) | Poland | In vivo | The effect of vitamin B2 on metabolic syndrome | - | Receiving 10.4–1,000 nM | Reducing the conditions associated with the mild inflammation linked to obesity | Moderate |
| Mostafa et al. (2016) (Mostafa et al., 2016) | Egypt | In vivo | The effect of vitamin D on metabolic syndrome | N = 50 | Receiving 6 ng/kg SC vitamin D daily for 8 weeks | Improve hypertension, metabolic and structural abnormalities | Moderate |
| Manning et al. (2013) (Manning et al., 2013) | Australia | Clinical trial | The effect of lipoic acid and vitamin E on metabolic syndrome | N = 151 | Receiving 100 IU/day vitamin E or 600 mg/day lipoic acid for 12 months | Reducing plasma NEFA3, But does not change insulin or glucose levels | High |
| Matsumoto et al. (2011) (Matsumoto et al., 2011) | Japan | In vivo | Effects of Zn(II) complex with vitamins C and U, and carnitine on metabolic syndrome | N = 30 | Receiving 40 mg zinc kg1 body weight over the period of 9–13 weeks | Reducing the visceral adipose tissues content and/or improvement in blood fluidity | Moderate |
| Minerals | |||||||
| Kelishadi et al. (2010) (Kelishadi et al., 2010) | Iran | Clinical trial | The effect of zinc on metabolic syndrome | N = 60 | Daily intake of 20 mg of zinc for 8 weeks | Reduction of total cholesterol and LDL, insulin resistance, and BMI | High |
| Dietary Supplements | |||||||
| Usui et al. (2013) (Usui et al., 2013) | Japan | Clinical trial | The effect of natural supplement of S-equol on metabolic syndrome | N = 54 | Daily intake of 10 mg of S-equol for 12 weeks | Reduction of HbA1c, serum low-density lipoprotein cholesterol level and CAVI4 score | High |
| McPherson et al. (2016) (McPherson et al., 2016) | Australia | In vivo | Effect of micronutrient supplementation on metabolic syndrome | N = 20 | Receiving a diet containing vitamin C, vitamin E, folate, lycopene, zinc, selenium and green tea extract for 17 weeks | Preventing early growth restriction, fat accumulation and dyslipidaemia | Low |
| Akrami et al. (2018) (Ak et al., 2018) | Iran | Clinical trial | effects of flaxseed (Inum usitatissimum) oil and sunflower seed oil on metabolic syndrome | N = 60 | Receiving 25 mL/d SO or 25 mL/d FO for 7 weeks | Reducing total cholesterol, low-density lipoprotein cholesterol, and triglyceride levels, systolic and diastolic BP, weight, waist circumference | High |
| Sanchez-Rodriguez et al. (2018) (Sanchez-Rodriguez et al., 2018) | Spain | Clinical Trials | Effects of Virgin Olive Oils on Metabolic Syndrome | N = 58 | Daily intake 30 mL for 3 weeks | Improving high density lipoprotein cholesterol (HDLc) | High |
| Pedersen et al. (2010) (Pedersen et al., 2010) | Denmark | Clinical Trials | Effects of fish oil supplementation on metabolic syndrome | N = 78 | Receiving 1.5 g/day fish oil for 16 weeks | Improving blood pressure | High |
| Ruyvaran et al. (2022) (Ruyvaran et al., 2022) | Iran | Clinical Trials | The effect of Safflower (Carthamus tinctorius L.) oil on metabolic syndrome | N = 67 | Daily intake 8 gr Safflower oil for 12 weeks | Improving abdominal obesity, blood pressure, and insulin resistance | High |
| Nimrouzi et al. (2020) (Nimrouzi et al., 2020) | Iran | In vivo | The effect of Oil and extract of safflower seed on metabolic syndrome | N = 80 | Receiving different doses of oil and extract daily for 16 weeks | Antioxidant and anti-inflammatory | Moderate |
| Pilar et al. (2017) (Pilar et al., 2017) | Brazil | In vivo | The effect of Flaxseed Oil and Flaxseed Lignan Secoisolariciresinol Diglucoside on Metabolic Syndrome | N = 48 | Receiving 1 mL/kg body weight FO or 20 mg/kg body weight SDG for 30 days | Reducing oxidative stress | Moderate |
| Olid et al. (2023) (Olid et al., 2023) | Spain | In vivo | Effect of extra virgin olive oil compared to butter on metabolic syndrome | N = 35 | Receiving olive oil or butter for 12 weeks | Antimicrobial activity to keep blood pressure low | Moderate |
| Ram´rez-Higuera et al. (2019) (Ramírez-Higuera et al., 2020) | Mexico | In vivo | Sterculic Oil on Metabolic Syndrome | N = 24 | Receiving 0.06 g of SO emulsified with 3% Tween 20 in water for 8 weeks | Improvement blood pressure, insulin resistance, serum glucose and triglycerides, steatosis, and adiposity | Moderate |
| Shen et al. (2020) (Shen et al., 2020) | The U.S | In vivo | Effect of Milk thistle (Silybum marianum) seed cold press oil on metabolic syndrome | N = 15 | Receiving 0.1% oil/50 gm body weight/day for 8 weeks | Reducing obesity, fasting hyperglycemia, hypertension, and induced markers of mitochondrial fusion and browning of white adipose | Moderate |
| Barrios-Ramos et al. (2012) (Barrios-Ramos et al., 2012) | Mexico | In vivo | Effect of cocoa, soy, oats and fish oil on metabolic syndrome | N = 96 | Receiving the mix of cocoa + soy + oats + fish oil for 14 weeks | Reducing levels of triglycerides, glucose, blood pressure and total cholesterol | Moderate |
| Mert et al. (2022) (Mert et al., 2022) | Turkey | In vivo | The effect of evening primrose oil (Oenothera biennis) on metabolic syndrome | N = 40 | Daily Intake 0.1 mL primrose oil for 57 days | Reducing oxidative stress, increasing adiponectin levels and insulin sensitivity, anti-inflammatory, regulating dyslipidemia and systolic blood pressure | High |
| Padiya et al. (2011) (Padiya et al., 2011) | India | In vivo | Effect of Garlic on metabolic syndromes | N = 21 | Receiving 250 mg/kg/day for 8 weeks | Reducing serum glucose, insulin, triglyceride and uric acid levels, insulin resistance | Moderate |
| Pérez-Torres et al. (2016) (Pérez-Torres et al., 2016) | Mexico | In vivo | The effect of garlic on metabolic syndrome in rats | N = 16 | Intake of 125 mg of extract every 12 h | Reducing cholesterol, improving heart function and reducing coronary artery resistance | Moderate |
| Hi and Endang (2020) (Hi and Endang, 2020) | Indonesia | Observational | The Effect of Black Seed Oil on metabolic syndrome | N = 62 | Intake BSO with a dose of 3 mL/day for 20 days | No significant difference in both groups test in the BMI, blood serum glucose, blood pressure and cholesterol levels | High |
| Al-Okbi et al. (2014) (Al-Okbi et al., 2014) | Egypt | In vivo | Effect of Clove Oil and Eugenol Microemulsions on Metabolic Syndrome | N = 30 | Receiving 40 mg CO/kg rat body weight, or 31 mg eugenol/kg rat body weight | Improvement on inflammatory fatty liver (steatohepatitis) and dyslipidemia | Moderate |
epiogallocatechin-3-gallate.
- keishibukuryogan (KBG; Guizhi-Fuling-Wan) (Dried herbal powder consisting of Amomi Cortex, Paeoniae Radix, Moutan Cortex, Persicae Semen and Hoelen mixed with honey.).
nonesterified fatty acid.
- Cardio-ankle vascular index.
2.4 Quality assessment of articles
The Mixed Methods Appraisal Tool (MMAT) was used to evaluate the quality of the studies (Pace et al., 2012). Each section of the tool is categorized based on the research design employed. This tool is valuable for assessing the appropriateness of a study’s objective, methods, study design, data collection, study selection, data analysis, presentation of findings, discussion, and conclusion(s). The quality of the articles and their inclusion after data extraction are determined by reviewing these aspects. Articles in each domain are assessed for quality using a percentage scale ranging from 25% (indicating that only one criterion is met) to 100% (indicating that all criteria are met). In this study, articles scoring below 25% are considered low quality, while those scoring above 80% are considered high quality (Pace et al., 2012; Madlabana et al., 2020). Based on the findings of the current study, the evaluation of article quality yielded an average score of 67.5%.
3 Results
The analysis of Table 1 data presents a comprehensive view of the impact of natural products on metabolic syndrome, reflecting a broad spectrum of research methodologies including clinical trials, in vitro and in vivo studies, and observational analyses. This diversity underscores the multifaceted approach in investigating the potential of natural interventions. Categorization by type of natural product reveals a predominant focus on plant-based interventions, signalling a notable scientific interest in exploring their efficacy in addressing metabolic syndrome. However, amidst the majority of studies demonstrating high to moderate quality, one in vivo article stands out for its notably low quality. Nonetheless, the collective outcomes suggest promising benefits associated with natural products, spanning various metabolic parameters such as blood pressure, glucose levels, lipid profile, and markers of obesity. These findings underscore the potential utility of natural interventions in managing the complexities of metabolic syndrome.
The predominance of clinical trials and in vivo studies in Table 1 underscores a comprehensive approach to investigating the therapeutic potential of natural products for managing metabolic syndrome. Clinical trials, being directly relevant to human health outcomes, provide crucial evidence regarding the efficacy and safety of interventions in real-world settings, reflecting a commitment to evidence-based medicine. Additionally, the inclusion of in vivo studies offers valuable insights into the underlying biological mechanisms of natural interventions, informing the design and interpretation of clinical trials. This diversity of research approaches not only contributes to a nuanced understanding of the efficacy and safety profiles of natural products but also highlights their translational potential from preclinical research to clinical practice.
3.1 Herbs
Upon evaluating the results of the studies, it becomes evident that herbs play a significant role in addressing various aspects of metabolic syndrome. A wide array of herbs have demonstrated the capacity to directly influence key metabolic parameters such as blood pressure, blood glucose levels, lipid profiles, obesity, and cholesterol and triglyceride levels (Nikaein et al., 2016; Seong et al., 2021; Amin et al., 2015; Belcaro et al., 2013; Devaraj et al., 2013; Najmi et al., 2013; Mansouri et al., 2012; Shah et al., 2012; Basu et al., 2010; Gupta Jain et al., 2017; Mopuri et al., 2018; Cicolari et al., 2020; Chae et al., 2022; Dobhal et al., 2022; Jakovljevic et al., 2018; Reshidan et al., 2019; Verhoeven et al., 2015; Gurrola-Dlaz et al., 2010; Benkhaled et al., 2022; Li et al., 2020; Hermans et al., 2020; De Martin et al., 2018; Mayer et al., 2022; Kasabri et al., 2014; Tan et al., 2011; Kuate et al., 2015; Kho et al., 2016). However, while the breadth of herbs studied is extensive, evaluations reveal variations in their efficacy and mechanisms of action.
Several plants exhibit promising effects through mechanisms such as antibacterial activity (Ghitea et al., 2021), antioxidant properties (Basu et al., 2013; Lopes et al., 2014), and the presence of bioactive compounds (Jakubczyk et al., 2021). These mechanisms contribute to the prevention of inflammation and insulin resistance (Kulabas et al., 2018), reduction of fat mass, and even serve as sources of essential amino acids and prebiotics (du Preez et al., 2021). Additionally, certain plants show potential in improving kidney function (Owis et al., 2017), regulating glucose and fatty acid metabolism (Reshidan et al., 2019), and balancing lipoprotein secretion and energy homeostasis (Singh et al., 2017). Furthermore, some plants demonstrate the ability to restore abnormal blood glucose regulation and rebalance intestinal microbiota (Wang et al., 2020), thus presenting multifaceted approaches to addressing metabolic syndrome.
However, evaluations also reveal complexities and contradictions within the findings. For instance, while the majority of plants studied exhibit beneficial effects, the use of green Elettaria cardamomum in one particular in vivo study led to unexpected outcomes. Contrary to expectations, green cardamom was found to exacerbate obesity, impair liver function, and adversely affect cardiovascular structure and function (Bhaswant et al., 2015). On the other hand, one clinical trial concluded that Ganoderma lucidum had no significant impact on hyperglycemia and cardiovascular risk factors (Klupp et al., 2016). Similarly, another clinical trial found that Cuminum cyminum L only decreased diastolic blood pressure without affecting other parameters of metabolic syndrome (Morovati et al., 2019). Additionally, the results of a clinical trial study indicated that Nigella Sativa failed to yield no significant differences between the two study groups regarding BMI, blood serum glucose, blood pressure, weight, WC and cholesterol levels (Mohtashami, 2019).
3.2 Vitamins and minerals
The results gleaned from studies investigating the impact of vitamins and minerals on metabolic syndrome and its components shed light on the potential benefits and limitations of various vitamin interventions. Specifically, vitamins C, D, E, A, B2, and U and zinc have demonstrated efficacy in influencing key parameters associated with metabolic syndrome, including blood pressure, blood glucose levels, lipid profiles, obesity, cholesterol, and triglycerides (Kelishadi et al., 2010; Farag et al., 2018; Farag et al., 2019; Ferreira et al., 2020). Additionally, these vitamins have shown promise in reducing DNA damage and oxidative stress (Wenclewska et al., 2019), as well as inflammatory markers (Erbaş et al., 2014; Mazur-Bialy and Pocheć, 2016; Salekzamani et al., 2017), while also decreasing plasma non-esterified fatty acids (NEFA), thereby contributing to the improvement of metabolic syndrome. However, critical evaluation of the findings reveals inconsistencies and challenges in translating these results into clinical practice. For instance, two clinical trial studies reported no significant effect of vitamin E on endothelial function, insulin levels, or glucose levels, highlighting a discrepancy between theoretical benefits and observed outcomes (Manning et al., 2013; Ahmadi et al., 2014). Similarly, two other clinical trials found that vitamin D supplementation failed to yield beneficial effects on cardiometabolic risk factors, high-sensitivity C-reactive protein levels, and carotid intima-media thickness (Salekzamani et al., 2016; Salekzamani et al., 2017).
3.3 Dietary supplements
Dietary supplements represent another category of natural products that have shown promise in addressing various aspects of metabolic syndrome, as indicated by the findings of studies included in this review. These supplements have demonstrated the ability to mitigate early growth restriction and inhibit fat accumulation by effectively modulating key metabolic parameters such as blood pressure, blood glucose levels, dyslipidemia, obesity, cholesterol, and triglycerides (Pedersen et al., 2010; Barrios-Ramos et al., 2012; Usui et al., 2013; McPherson et al., 2016; Ak et al., 2018; Sanchez-Rodriguez et al., 2018; Ramírez-Higuera et al., 2020; Shen et al., 2020; Ruyvaran et al., 2022). Additionally, they have been associated with improvements in insulin resistance (Mert et al., 2022), exerting antioxidant and anti-inflammatory effects (Nimrouzi et al., 2020), reducing oxidative stress (Pilar et al., 2017), decreasing coronary artery resistance (Pérez-Torres et al., 2016), and ameliorating inflammatory fatty liver conditions such as steatohepatitis (Al-Okbi et al., 2014). Noteworthy among these supplements are nutrients, garlic, as well as vegetable and animal oils. Despite various findings on the effectiveness of nutritional supplements for metabolic syndrome, an observational study yielded inconclusive results. It showed that black seed exhibited no significant difference between the two studied groups in terms of BMI, serum glucose, blood pressure, weight, waist circumference, and cholesterol levels (Hi and Endang, 2020).
4 Discussion
This scoping review was conducted to investigate the influence of natural products on metabolic syndrome. Based on the findings from the reviewed studies, we obtained a wide range of natural products, including herbs, vitamins, minerals and dietary supplements that affected the treatment and prevention of metabolic syndrome. These products have effects on blood pressure, blood sugar, obesity, waist circumference fat, and abnormal cholesterol or triglyceride levels.
The findings of this research show that plants play a role in improving metabolic syndrome by influencing blood glucose levels, lipid profile, obesity, cholesterol, and triglyceride levels. Various studies have identified a wide range of plants for this purpose. For instance, a review study by Pérez-Muñoz et al. (2022) (Pérez-Muñoz et al., 2022) demonstrated that the Eryngium plant reduces LDL and HDL levels, improves glucose metabolism, and controls dyslipidemia and blood glucose. In another study, Akber Aisa et al. (2019) (Aisa et al., 2019) found that Cicer arietinum L modulates glycemic response and regulates dyslipidemia. A systematic review and meta-analysis by García-Muñoz et al. (2023) (García-Muñoz et al., 2023) reported the effectiveness of Hibiscus sabdariffa in weight loss, reduction of fat tissue, lowering blood pressure, and improving lipid profiles. Apart from studies directly emphasizing the effects of plants on the main components of metabolic syndrome, some studies have also shown that plants can improve metabolic syndrome through antibacterial activities, antioxidant properties, anti-inflammatory activities, and reduction of insulin resistance. One such plant is spirulina, which possesses anti-inflammatory properties. By reducing inflammation, this herb can help improve vascular function and blood pressure, thereby positively impacting metabolic health (Yousefi et al., 2018).
Green tea and turmeric also possess antioxidant and anti-inflammatory properties. In metabolic syndrome, chronic low-grade inflammation and oxidative stress can contribute to insulin resistance and impaired glucose metabolism. Therefore, these herbs can help mitigate these effects, leading to improved insulin sensitivity and lower blood sugar levels (Basu et al., 2013; Amin et al., 2015). Additionally, green tea contains polyphenols such as epigallocatechin gallate (EGCG), which have demonstrated the potential to increase fat oxidation and thermogenesis, aiding in weight management (Basu et al., 2013; Wong et al., 2020). Another herb that can assist in regulating blood sugar levels is cinnamon, which can impact fat storage and metabolism by regulating blood sugar levels (Mollazadeh and Hosseinzadeh, 2016).
Contrary to the aforementioned findings, some studies present conflicting results and suggest that herbs may not have a significant impact on metabolic syndrome and its components. For instance, one study revealed that G. lucidum does not exhibit a significant effect on blood sugar and cardiovascular risk factors (Klupp et al., 2016). Similarly, another study found that Nigella Sativa did not yield statistically significant differences between the two study groups regarding BMI, blood serum glucose, blood pressure, weight, waist circumference, and cholesterol levels (Mohtashami, 2019). Additionally, according to the results of another study, E. cardamomum was associated with obesity, liver dysfunction, and adverse effects on cardiovascular structure and function (Bhaswant et al., 2015). Several factors could contribute to these discrepancies in results. Variations in study design, such as sample size and duration, as well as the diversity of bioactive compounds in plants, may be among the reasons. Demographic differences, dosage variations, and confounding variables such as diet and lifestyle could also play a role. Furthermore, publication bias, study quality, and the inherent complexity of metabolic syndrome itself contribute to variability in outcomes. Ultimately, these findings underscore the necessity for further research, particularly clinical trials, to elucidate the precise mechanisms and potential adverse effects associated with specific herbal interventions for metabolic syndrome.
It is important to note that the mechanisms of action for natural products can vary widely, and not all natural products have been extensively studied in the context of metabolic syndrome. Additionally, individual responses to natural products can vary, and their effects may depend on factors such as dosage, duration of use, and specific characteristics of a person with metabolic syndrome. Therefore, it is necessary to conduct research, particularly clinical studies, on the effect of natural products on the components of metabolic syndrome.
Another finding of the present study suggests that vitamins and minerals can play an effective role in managing metabolic syndrome and its components. For instance, the study by Melguizo-Rodríguez et al. (2021) (Melguizo-Rodríguez et al., 2021) demonstrated that vitamin D supplementation improves lipid profile, insulin resistance, hyperglycemia, obesity, and high blood pressure. Similarly, Moukayed & Grant et al. (2019) (Moukayed and Grant, 2019)discovered that vitamin D possesses anti-inflammatory properties, inhibits primary adipogenesis, enhances glucose absorption, mitigates hyperleptinemia, ameliorates insulin resistance, and reduces high blood pressure. Wong et al. (2020) (Wong et al., 2020) confirmed the antioxidant and anti-inflammatory properties of vitamin C.
However, there are also several studies that contradict the positive effects of vitamins and nutrients on metabolic syndrome. Ahmadi et al. (2014) (Ahmadi et al., 2014) and Manning et al. (2015) (Manning et al., 2013) reported no significant effects of vitamin E on endothelial function, insulin levels, or glucose levels. Similarly, studies by Salekzamani et al. (2017) (Salekzamani et al., 2017) and Salekzamani et al. (2016) (Salekzamani et al., 2016) indicated that vitamin D supplementation failed to yield beneficial effects on cardiometabolic risk factors, high-sensitivity C-reactive protein levels, and carotid intima-media thickness. AlAnouti et al.’s study (2020) (AlAnouti et al., 2020)also demonstrated the limited impact of vitamin D on improving dyslipidemia. Furthermore, Panchal et al. (2017) (Panchal et al., 2017) suggested conflicting results regarding the effect of these micronutrients on metabolic syndrome.
The inconsistency in findings regarding the effect of vitamins on metabolic syndrome can be attributed to several factors. Variations in study design, such as sample size, duration of intervention, and methods used to measure outcomes, can lead to conflicting results. Studies with small sample sizes or short durations may fail to capture the full effects of vitamins and nutrients on components of the metabolic syndrome. Additionally, differences in study population characteristics, including age, gender, ethnicity, and baseline health status, can influence the response to vitamin and nutrient supplementation. What proves effective in one population may not yield the same results in another.
Furthermore, vitamins may interact with other nutrients or medications, potentially influencing their effects on metabolic syndrome, and these interactions can differ between studies. Conversely, the dose and duration of vitamin and nutrient supplementation can significantly impact their effectiveness. Therefore, it is essential to design studies with optimal doses and longer durations to observe the significant effects of vitamins and nutrients on the components of metabolic syndrome.
The results of the present study also confirmed the effect of food supplements, including nutrients and vegetable and animal oils, on the components of metabolic syndrome. Vegetable and animal oils exert their effect on metabolic syndrome through a combination of factors. The composition of their fatty acids plays an essential role. Oils rich in unsaturated fats, such as olive oil and certain vegetable oils, as well as those containing omega-3 fatty acids, such as flaxseed and fish oil, help improve lipid profiles and reduce inflammation, and are beneficial for people with metabolic syndrome. The results of the studies by Nimrouzi et al. (2020) (Nimrouzi et al., 2020), Mert et al. (2022) (Mert et al., 2022), and Al-Okbi et al. (2014) (Al-Okbi et al., 2014) confirmed these findings. Additionally, Olid et al. (2023) (Olid et al., 2023) stated that olive oil has antimicrobial activity to keep blood pressure low.
The results of a study showed that omega-3 fatty acids, which are found in some natural products such as flax seeds, have demonstrated the ability to reduce the levels of triglycerides and cholesterol by decreasing the production of triglycerides in the liver and increasing the clearance of triglycerides from the bloodstream (Yang et al., 2020). Additionally, flaxseed oil exhibited the ability to reduce oxidative stress in a study (Mohammadifard et al., 2021).
Garlic is another food supplement that contains allicin, and by increasing the production of nitric oxide in the blood vessels, it induces vasodilation. This widening of blood vessels can lead to a decrease in resistance to blood flow and subsequently result in a decrease in blood pressure (Al Disi et al., 2016). However, the results of Hi et al.’s study (2020) (Hi and Endang, 2020) showed that black seed oil did not cause significant differences in BMI, blood glucose, blood pressure, and cholesterol levels between the two groups. Furthermore, the results of Tamtaji et al. (2020) (Tamtaji et al., 2020) indicated that flaxseed oil had no effect on certain inflammatory factors and other biomarkers of oxidative stress. Similarly, Pastor et al. (2021) (Pastor et al., 2021) demonstrated that olive oil has no effect on metabolic syndrome.
Biological variability among participants, genetic factors, publication bias, different study designs, varied doses, and the overlap of some foods with dietary supplements may have influenced the study results, highlighting the importance of well-designed randomized controlled trials. Systematic reviews are essential to elucidate the true effects of dietary supplements on metabolic syndrome and to provide evidence-based recommendations.
The strength of the present study lies in comprehensive exploration of natural products in relation to their potential impact on metabolic syndrome and its components. This broad perspective adds to the existing body of knowledge and provides valuable insights into the potential benefits of these products for people with metabolic syndrome. However, this study also had limitations. For instance, despite the researchers’ efforts to obtain the full text of the articles, this was not achieved in the case of some articles, and as a result, some related articles were excluded from the research. Additionally, while eligible articles were identified and reviewed, it is possible that some unpublished studies may have been missed. Utilizing specific entry criteria, such as excluding articles published before 2010 in this study, may result in the omission of relevant articles. However, the researchers in this particular case adopted this criterion to enhancing the organization of the articles. Also, Utilizing a particular search strategy in articles may result in the omission of related articles.
5 Conclusion
This study underscores the considerable potential of natural products, including herbs, vitamins, minerals, and dietary supplements, in effectively managing metabolic syndrome. These interventions offer promising avenues for addressing various aspects of metabolic syndrome through diverse mechanisms of action. Among natural products, the study found that plants have been the subject of more research compared to other categories. While both positive and negative effects have been observed across all categories of natural products, there appears to be more uncertainty surrounding the effects of vitamins. This suggests that the effects of vitamins are often unclear and may differ depending on the specific circumstances. However, the study acknowledges the need for further research to validate these findings and determine the optimal dosage and duration of supplementation. Long-term randomized controlled trials with larger sample sizes are particularly necessary to provide more conclusive evidence regarding the efficacy and safety of natural products in managing metabolic syndrome.
Acknowledgments
The researchers thank the officials of Baghdad University and all those who helped them in the implementation of this research.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Author contributions
MA: Writing–original draft, Writing–review and editing. SA-F: Writing–original draft, Writing–review and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Adeniyi O., Washington L., Glenn C. J., Franklin S. G., Scott A., Aung M., et al. (2021). The use of complementary and alternative medicine among hypertensive and type 2 diabetic patients in Western Jamaica: a mixed methods study. PLOS ONE 16 (2), e0245163. 10.1371/journal.pone.0245163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahamad J., Toufeeq I., Khan M. A., Ameen M. S. M., Anwer E. T., Uthirapathy S., et al. (2019). Oleuropein: a natural antioxidant molecule in the treatment of metabolic syndrome. Phytother. Res. 33 (12), 3112–3128. 10.1002/ptr.6511 [DOI] [PubMed] [Google Scholar]
- Ahmadi A., Gharipour M., Arabzadeh G., Moin P., Hashemipour M., Kelishadi R. (2014). The effects of vitamin E and omega-3 PUFAs on endothelial function among adolescents with metabolic syndrome. Biomed. Res. Int. 2014, 906019. 10.1155/2014/906019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisa H. A., Gao Y., Yili A., Ma Q., Cheng Z. (2019). “Beneficial role of chickpea (cicer arietinum L.) functional factors in the intervention of metabolic syndrome and diabetes mellitus,” in Bioactive food as dietary interventions for diabetes. Editors Watson R. R., Preedy V. R. (Cambridge, Massachusetts, United States: Academic Press; ), 615–627. 10.1016/B978-0-12-813822-9.00039-4 [DOI] [Google Scholar]
- Akrami A., Nikaein F., Babajafari S., Faghih S., Yarmohammadi H. (2018). Comparison of the effects of flaxseed oil and sunflower seed oil consumption on serum glucose, lipid profile, blood pressure, and lipid peroxidation in patients with metabolic syndrome. J. Clin. Lipidol. 12 (1), 70–77. 10.1016/j.jacl.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Alam M., Uddin R., Subhan N., Rahman M., Jain P., Mahmud Reza H. (2015). Beneficial role of bitter melon supplementation in obesity and related complications in metabolic syndrome. J. Lipids. 10.1155/2015/496169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlAnouti F., Abboud M., Papandreou D., Mahboub N., Haidar S., Rizk R. (2020). Effects of vitamin D supplementation on lipid profile in adults with the metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Nutrients 12 (11), 3352. 10.3390/nu12113352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alauddin M., Shirakawa H., Koseki T., Kijima N., et al. (2016). Fermented rice bran supplementation mitigates metabolic syndrome in strokeprone spontaneously hypertensive rat. BMC Complementary Altern. Med. 16, 442. 10.1186/s12906-016-1427-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Disi S. S., Anwar M. A., Eid A. H. (2016). Anti-hypertensive herbs and their mechanisms of action: Part I. Front. Pharmacol. 6, 323. 10.3389/fphar.2015.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alembagheri A., Hajimehdipoor H., Choopani R., Esmaeili S. (2023). The role of selected medicinal plants from Iranian traditional medicine for the treatment of fatigue in metabolic syndrome. Tradit. Med. Res. 8 (4), 23. 10.53388/TMR20220706001 [DOI] [Google Scholar]
- Alkhatib D. H., Jaleel A., Tariq M. N. M., Feehan J., Apostolopoulos V., Cheikh Ismail L., et al. (2021). The role of bioactive compounds from dietary spices in the management of metabolic syndrome: an overview. Nutrients 14 (1), 175. 10.3390/nu14010175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Okbi S. Y., Mohamed D. A., Hamed T. E., Edris A. E. (2014). Protective effect of clove oil and eugenol microemulsions on fatty liver and dyslipidemia as components of metabolic syndrome. J. Med. Food 17 (7), 764–771. 10.1089/jmf.2013.0033 [DOI] [PubMed] [Google Scholar]
- Amin F., Islam N., Anila N., Gilani A. H. (2015). Clinical efficacy of the co-administration of Turmeric and Black seeds (Kalongi) in metabolic syndrome — a double blind randomized controlled trial — TAK-MetS trial. Complement. Ther. Med. 23 (2), 165–74. 10.1016/j.ctim.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Atanasov A. G., Waltenberger B., Pferschy-Wenzig E.-M., Linder T., Wawrosch C., Uhrin P., et al. (2015). Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol. Adv. 33 (8), 1582–1614. 10.1016/j.biotechadv.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awwad A., Poucheret P., Idres A. Y., Bidel L., Tousch D. (2020). The bitter Asteraceae: an interesting approach to delay the metabolic syndrome progression. NFS J. 18, 29–38. 10.1016/j.nfs.2020.01.001 [DOI] [Google Scholar]
- Babajafari S., Nikaein F., Mazloomi S., Zibaeenejad M., Zargaran A. (2015). A review of the benefits of satureja species on metabolic syndrome and their possible mechanisms of action. J. Evidence-Based Complementary Altern. Med., 1–12. 10.1177/21565872145641 [DOI] [PubMed] [Google Scholar]
- Barrios-Ramos J. P., Garduño-Siciliano L., Loredo M., Chamorro-Cevallos G., Jaramillo-Flores M. E. (2012). The effect of cocoa, soy, oats and fish oil on metabolic syndrome in rats. J. Sci. Food Agric. 92 (11), 2349–2357. 10.1002/jsfa.5637 [DOI] [PubMed] [Google Scholar]
- Basu A., Betts N. M., Mulugeta A., Tong C., Newman E., Lyons T. J. (2013). Green tea supplementation increases glutathione and plasma antioxidant capacity in adults with the metabolic syndrome. Nutr. Res. 33 (3), 180–187. 10.1016/j.nutres.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A., Sanchez K., Leyva M. J., Wu M., Betts N. M., Aston C. E., et al. (2010). Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 29 (1), 31–40. 10.1080/07315724.2010.10719814 [DOI] [PubMed] [Google Scholar]
- Bayliak M. M., Dmytriv T. R., Melnychuk A. V., Strilets N. V., Storey K. B., Lushchak V. I. (2021). Chamomile as a potential remedy for obesity and metabolic syndrome. EXCLI J. 20, 1261–1286. 10.17179/excli2021-4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcaro G., Ledda A., Hu S., Cesarone M., Feragalli B., Dugall M. (2013). Greenselect phytosome for borderline metabolic syndrome. Evidence-Based Complementary Altern. Med., 10.1155/2013/869061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkhaled A., Réggami Y., Boudjelal A., Senator A., Bouriche H., Demirtaş I., et al. (2022). Chemical characterisation, hypoglycaemic and renoprotective effects of aqueous leaf extract of Limoniastrum guyonianum on fructose-induced metabolic syndrome in rats. Arch. Physiol. Biochem. 128 (4), 914–923. 10.1080/13813455.2020.1739715 [DOI] [PubMed] [Google Scholar]
- Bhaswant M., Poudyal H., Mathai M. L., Ward L. C., Mouatt P., Brown L. (2015). Green and black cardamom in a diet-induced rat model of metabolic syndrome. Nutrients 7 (9), 7691–7707. 10.3390/nu7095360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbis L. S., Muhammad S. A., Saidu Y., Adamu Y. (2012). Effect of vitamins a, C, and e supplementation in the treatment of metabolic syndrome in albino rats. Biochem. Res. Int. 2012, 678582. 10.1155/2012/678582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobescu E., Bălan A., Moga M. A., Teodorescu A., Mitrică M., Dima L. (2020). Are there any beneficial effects of spirulina supplementation for metabolic syndrome components in postmenopausal women? Mar. Drugs 18 (12), 651. 10.3390/md18120651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera-Lanestosa A., Moguel-Ordóñez Y., Segura-Campos M. (2017). Stevia rebaudiana bertoni: a natural alternative for treating diseases associated with metabolic syndrome. J. Med. Food 20 (10), 933–943. 10.1089/jmf.2016.0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carresi C., Gliozzi M., Musolino V., Scicchitano M., Scarano F., Bosco F., et al. (2020). The effect of natural antioxidants in the development of metabolic syndrome: focus on bergamot polyphenolic fraction. Nutrients 12 (5), 1504. 10.3390/nu12051504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae H.-S., Dale O., Mir T. M., Avula B., Zhao J., Khan I. A., et al. A multitarget approach to evaluate the efficacy of aquilaria sinensis flower extract against metabolic syndrome. Molecules 2022, 27, 629. 10.3390/molecules27030629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez-Delgado E. L., Jacobo-Velázquez D. A., Essential O. (2023). Recent advances on their dual role as food preservatives and nutraceuticals against the metabolic syndrome. Foods 12, 1079. 10.3390/foods12051079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. K., Cheung C., Reuhl K. R., Liu A. B., Lee M. J., Lu Y. P., et al. (2011). Effects of green tea polyphenol (-)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice. J. Agric. Food Chem. 59 (21), 11862–11871. 10.1021/jf2029016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Zheng J., Zou X., Ye C., Xia H., Yang M., et al. (2021). Ligustrum robustum (Roxb.) blume extract modulates gut microbiota and prevents metabolic syndrome in high-fat diet-fed mice. J. Ethnopharmacol. 268, 113695. 10.1016/j.jep.2020.113695 [DOI] [PubMed] [Google Scholar]
- Choi E., Jang E., Lee J. (2019). Pharmacological activities of alisma orientale against nonalcoholic fatty liver disease and metabolic syndrome: literature review. Evidence-Based Complementary Altern. Med., 10.1155/2019/2943162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicolari S., Dacrema M., Tsetegho Sokeng A. J., Xiao J., Atchan Nwakiban A. P., Di Giovanni C., et al. (2020). Hydromethanolic extracts from Adansonia digitata L. Edible parts positively modulate pathophysiological mechanisms related to the metabolic syndrome. Molecules 25 (12), 2858. 10.3390/molecules25122858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakshinamurti K. (2015). Vitamins and their derivatives in the prevention and treatment of metabolic syndrome diseases (diabetes). Can. J. Physiol. Pharmacol. 93 (5), 355–362. 10.1139/cjpp-2014-0479 [DOI] [PubMed] [Google Scholar]
- Davatgaran Taghipour Y., Hajialyani M., Naseri R., Hesari M., Mohammadi P., et al. (2019). Nanoformulations of natural products for management of metabolic syndrome. Int. J. Nanomedicine 14, 5303–5321. 10.2147/IJN.S213831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davì G., Santilli F., Patrono C. (2010). Nutraceuticals in diabetes and metabolic syndrome. Cardiovasc Ther. 28 (4), 216–226. 10.1111/j.1755-5922.2010.00179.x [DOI] [PubMed] [Google Scholar]
- De Martin S., Gabbia D., Carrara M., Ferri N. (2018). The Brown algae fucus vesiculosus and ascophyllum nodosum reduce metabolic syndrome risk factors: a clinical study. Nat. Product. Commun. 13 (12). 10.1177/1934578X1801301228 [DOI] [Google Scholar]
- Devaraj S., Yimam M., Brownell L. A., Jialal I., Singh S., Jia Q. (2013). Effects of Aloe vera supplementation in subjects with prediabetes/metabolic syndrome. Metab. Syndr. Relat. Disord. 11 (1), 35–40. 10.1089/met.2012.0066 [DOI] [PubMed] [Google Scholar]
- Di S., Wang Y., Han L., Bao Q., Gao Z., Wang Q., et al. (2019). The intervention effect of traditional Chinese medicine on the intestinal flora and its metabolites in glycolipid metabolic disorders. Evidence-Based Complementary Altern. Med. 2019, 2958920. 10.1155/2019/2958920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo C., Dell’Agli M., Colombo E., Sangiovanni E., Restani P. (2013). Metabolic syndrome and inflammation: a critical review of in vitro and clinical approaches for benefit assessment of plant food supplements. Evidence-Based Complementary Altern. Med., 10.1155/2013/782461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobhal S., Singh M. F., Setya S., Bisht S. (2022). Comparative assessment of the effect of lemongrass (cymbopogon citratus) ethanolic extract, aqueous extract and essential oil in high fat diet and fructose induced metabolic syndrome in rats. Indian J Pharm. Educ. Res. 56 (2s), s281–s293. 10.5530/ijper.56.2s.99 [DOI] [Google Scholar]
- Dong H., Lu F. E., Zhao L. (2012). Chinese herbal medicine in the treatment of nonalcoholic fatty liver disease. Chin. J. Integr. Med. 18 (2), 152–160. 10.1007/s11655-012-0993-2 [DOI] [PubMed] [Google Scholar]
- Dong Y., Xu M., Chen L., Bhochhibhoya A. (2019). Probiotic foods and supplements interventions for metabolic syndromes: a systematic review and meta-analysis of recent clinical trials. Ann. Nutr. Metab. 74 (3), 224–241. 10.1159/000499028 [DOI] [PubMed] [Google Scholar]
- du Preez R., Majzoub M. E., Thomas T., Panchal S. K., Brown L. (2021). Nannochloropsis oceanica as a microalgal food intervention in diet-induced metabolic syndrome in rats. Nutrients 13 (11), 3991. 10.3390/nu13113991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekeuku S. O., Nur Azlina M. F., Chin K.-Y. (2021). Effects of piper sarmentosum on metabolic syndrome and its related complications: a review of preclinical evidence. Appl. Sci. 11 (21), 9860. 10.3390/app11219860 [DOI] [Google Scholar]
- Erbaş O., Solmaz V., Aksoy D., Yavaşoğlu A., Sağcan M., Taşkıran D. (2014). Cholecalciferol (vitamin D 3) improves cognitive dysfunction and reduces inflammation in a rat fatty liver model of metabolic syndrome. Life Sci. 103 (2), 68–72. 10.1016/j.lfs.2014.03.035 [DOI] [PubMed] [Google Scholar]
- Fadhel M., Hassan A. (2023). Protective effect of omega-7 against doxorubicin-induced cardiotoxicity in male rats. Iraqi J. Pharm. Sci. 32 (3). 10.31351/vol32iss3pp35-40 [DOI] [Google Scholar]
- Farag H. A. M., Hosseinzadeh-Attar M. J., Muhammad B. A., Esmaillzadeh A., Bilbeisi A. H. E. (2018). Comparative effects of vitamin D and vitamin C supplementations with and without endurance physical activity on metabolic syndrome patients: a randomized controlled trial. Diabetol. Metab. Syndr. 10, 80. 10.1186/s13098-018-0384-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag H. A. M., Hosseinzadeh-Attar M. J., Muhammad B. A., Esmaillzadeh A., El Bilbeisi A. H. (2019). Effects of vitamin C supplementation with and without endurance physical activity on components of metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. Exp. 26, 23–33. 10.1016/j.yclnex.2019.05.003 [DOI] [Google Scholar]
- Fernando S., Ryu B., Ahn G., Yeo I.-K., Jeon Y.-J. (2020). Therapeutic potential of algal natural products against metabolic syndrome: a review of recent developments. Trends Food Sci. Technol. 97. 10.1016/j.tifs.2020.01.020 [DOI] [Google Scholar]
- Ferreira P. P., Cangussu L., Bueloni-Dias F. N., Orsatti C. L., Schmitt E. B., Nahas-Neto J., et al. (2020). Vitamin D supplementation improves the metabolic syndrome risk profile in postmenopausal women. Climacteric 23 (1), 24–31. 10.1080/13697137.2019.1611761 [DOI] [PubMed] [Google Scholar]
- Firouzi S., Malekahmadi M., Ghayour-Mobarhan M., Ferns G., Rahimi H. (2018). Barberry in the treatment of obesity and metabolic syndrome: possible mechanisms of action. Diabetes, Metabolic Syndrome and Obesity. Targets Ther. 11, 699–705. 10.2147/DMSO.S181572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentin M., Elisaf M. S., Mikhailidis D. P., Liberopoulos E. N. (2010). Vitamin D and metabolic syndrome: is there a link? Curr. Pharm. Des. 16 (30), 3417–3434. 10.2174/138161210793563509 [DOI] [PubMed] [Google Scholar]
- Gabbia D., De Martin S. (2020). Brown seaweeds for the management of metabolic syndrome and associated diseases. Molecules 25 (18), 4182. 10.3390/molecules25184182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galavi A., Hosseinzadeh H., Razavi B. M. (2021). The effects of Allium cepa L. (onion) and its active constituents on metabolic syndrome: a review. Iran. J. Basic Med. Sci. 24 (1), 3–16. 10.22038/ijbms.2020.46956.10843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Muñoz A. M., García-Guillén A. I., Victoria-Montesinos D., Abellán-Ruiz M. S., Alburquerque-González B., Cánovas F. (2023). Effect of the combination of Hibiscus sabdariffa in combination with other plant extracts in the prevention of metabolic syndrome: a systematic review and meta-analysis. Foods 12, 2269. 10.3390/foods12112269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandi Ibrahim K., Ahmad Adeshina K., Bashir Bello M., Malami I., Abubakar B., Bello Abubakar M., et al. (2021). Prophylactic use of natural products against developmentally programmed metabolic syndrome. Planta Med. 88 (8), 650–663. 10.1055/a-482-2343 [DOI] [PubMed] [Google Scholar]
- Gharipour M., Akhavan Tabib A., Toghianifar N., Tavassoli A., Gharipour A., Sarrafzadegan N. (2011). The pattern of pharmacological treatment in subjects with metabolic syndrome: findings from isfahan healthy heart program. J. Isfahan Med. Sch. 29 (2). [Google Scholar]
- Ghitea T. C., El-Kharoubi A., Ganea M., Bimbo-Szuhai E., Nemeth T. S., Ciavoi G., et al. (2021). The antimicrobial activity of origanum vulgare L. Correlated with the gastrointestinal perturbation in patients with metabolic syndrome. Molecules 26 (2), 283. 10.3390/molecules26020283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf B., Raskin I., Cefalu W., Ribnicky D. (2010). Plant-derived therapeutics for the treatment of metabolic syndrome. Curr. Opin. Investig. Drugs 11 (10), 1107–1115. PMID: 20872313; PMCID: PMC3755736. [PMC free article] [PubMed] [Google Scholar]
- Gumbarewicz E., Jarząb A., Stepulak A., Kukula-Koch W. (2022). Zingiber officinale rosc. In the treatment of metabolic syndrome disorders-A review of in vivo studies. Int. J. Mol. Sci. 23 (24), 15545. 10.3390/ijms232415545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta Jain S., Puri S., Misra A., Gulati S., Mani K. (2017). Effect of oral cinnamon intervention on metabolic profile and body composition of Asian Indians with metabolic syndrome: a randomized double -blind control trial. Lipids Health Dis. 16 (1), 113. 10.1186/s12944-017-0504-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurrola-Dlaz C., Garcı´a-Lo´pez P., Sa´ nchez-E. ´quez S., Troyo-Sanroma´n R., Andrade-Gonza´lez I., Go´mez-Leyva J. (2010). Effects of Hibiscus sabdariffa extract powder and preventive treatment (diet) on the lipid profiles of patients with metabolic syndrome (MeSy). Phytomedicine 17, 500–505. 10.1016/j.phymed.2009.10.014 [DOI] [PubMed] [Google Scholar]
- Hameed E., Al-Ameri L. (2022). Surrogates markers of insulin resistance. Al-Kindy Coll. Med. J. 18, 1–2. 10.47723/kcmj.v18i1.837 [DOI] [Google Scholar]
- Hashim K. N., Chin K. Y., Ahmad F. (2021). The mechanism of honey in reversing metabolic syndrome. Molecules 26 (4), 808. 10.3390/molecules26040808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani F. V., Shirani K., Hosseinzadeh H. (2016). Rosemary (Rosmarinus officinalis) as a potential therapeutic plant in metabolic syndrome: a review. Naunyn Schmiedeb. Arch. Pharmacol. 389 (9), 931–949. 10.1007/s00210-016-1256-0 [DOI] [PubMed] [Google Scholar]
- Hedayati N., Bemani Naeini M., Mohammadinejad A., Mohajeri S. A. (2019). Beneficial effects of celery (Apium graveolens) on metabolic syndrome: a review of the existing evidences. Phytother. Res. 33 (12), 3040–3053. 10.1002/ptr.6492 [DOI] [PubMed] [Google Scholar]
- Hermans M. P., Lempereur P., Salembier J. P., Maes N., Albert A., Jansen O., et al. (2020). Supplementation effect of a combination of olive (olea europea L.) leaf and fruit extracts in the clinical management of hypertension and metabolic syndrome. Antioxidants (Basel) 9 (9), 872. 10.3390/antiox9090872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hi W., Endang D. (2020). The effect of black seed oil as adjuvant therapy on nuclear factor erythroid 2-related factor 2 levels in patients with metabolic syndrome risk. Iran. J. Pharm. Sci. 16 (1), 9–18. 10.22037/ijps.v16.40441 [DOI] [Google Scholar]
- Hosseini A., Hosseinzadeh H. (2015). A review on the effects of Allium sativum (Garlic) in metabolic syndrome. J. Endocrinol. Invest. 38 (11), 1147–1157. 10.1007/s40618-015-0313-8 [DOI] [PubMed] [Google Scholar]
- Hou C. Y., Tain Y. L., Yu H. R., Huang L. T. (2019). The effects of resveratrol in the treatment of metabolic syndrome. Int. J. Mol. Sci. 20 (3), 535. 10.3390/ijms20030535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim K. G., Mukonowenzou N. C., Usman D., Adeshina K. A., Erlwanger K. H. (2023). The potential of Artemisia species for use as broad-spectrum agents in the management of metabolic syndrome: a review. Arch. Physiol. Biochem. 129 (3), 752–770. 10.1080/13813455.2021.1871761 [DOI] [PubMed] [Google Scholar]
- Jakovljevic V., Milic P., Bradic J., Jeremic J., Zivkovic V., Srejovic I., et al. (2018). Standardized aronia melanocarpa extract as novel supplement against metabolic syndrome: a rat model. Int. J. Mol. Sci. 20 (1), 6. 10.3390/ijms20010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubczyk A., Kiersnowska K., Ömero˘ glu B., Gawlik-Dziki U., Tutaj K., Rybczy´ nska-Tkaczyk K., et al. The influence of Hypericum perforatum L. Addition to wheat cookies on their antioxidant, anti-metabolic syndrome, and antimicrobial properties. Foods 2021,10, 1379. 10.3390/.foods10061379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi S., Hejazi N., Golmakani M. T., Tanideh N. (2018). Wild pistachio (Pistacia atlantica mutica) oil improve metabolic syndrome features in rats with high fructose ingestion. Iran. J. Basic Med. Sci. 21 (12), 1255–1261. 10.22038/ijbms.2018.30511.7351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S., Jang B. H., Ko Y., Sasaki Y., Park J. S., Hwang E. H., et al. (2016a). Herbal medicines for treating metabolic syndrome: a systematic review of randomized controlled trials. Evid. Based Complement. Altern. Med. 2016, 5936402. 10.1155/2016/5936402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S., Jang B.-H., Ko Y., Sasaki Y., Park J.-S., Hwang E.-H., et al. (2016b). Herbal medicines for treating metabolic syndrome: a systematic review of randomized controlled trials. Evidence-Based Complementary Altern. Med., 5936402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo Flores M. E. (2019). Cocoa flavanols: natural agents with attenuating effects on metabolic syndrome risk factors. Nutrients 11 (4), 751. 10.3390/nu11040751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungbauer A., Medjakovic S. (2012). Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas 71 (3), 227–239. 10.1016/j.maturitas.2011.12.009 [DOI] [PubMed] [Google Scholar]
- Kania-Dobrowolska M., Baraniak J. D. (2022). Taraxacum officinale L. As a source of biologically active compounds supporting the therapy of Co-existing diseases in metabolic syndrome. Foods 11, 2858. 10.3390/foods11182858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasabri V., Afifi F., Abu-Dahab R., Mhaidat N., Bustanji Y., Abaza I., et al. (2014). In vitro modulation of metabolic syndrome enzymes and proliferation of obesity related-colorectal cancer cell line panel by Salvia species from Jordan. Rev. Roum. Chim. 59, 693–705. [Google Scholar]
- Kaur G., Mukundan S., Wani V., Kumar M. (2015). Nutraceuticals in the management and prevention of metabolic syndrome. Austin J. Pharmacol. Ther. 3 (1), 1063. [Google Scholar]
- Kelishadi R., Hashemipour M., Adeli K., Tavakoli N., Movahedian-Attar A., Shapouri J., et al. (2010). Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. Metab. Syndr. Relat. Disord. 8 (6), 505–510. 10.1089/met.2010.0020 [DOI] [PubMed] [Google Scholar]
- Kern H. J., Mitmesser S. H. (2018). Role of nutrients in metabolic syndrome: a 2017 update. Nutr. Diet. Suppl. 10, 13–26. 10.2147/NDS.S148987 [DOI] [Google Scholar]
- Kho M., Lee Y., Park J., Cha J., et al. (2016). Combination with Red ginseng and Polygoni Multiflori ameliorates highfructose diet induced metabolic syndrome. BMC Complementary Altern. Med. 16, 98. 10.1186/s12906-016-1063-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klupp N. L., Kiat H., Bensoussan A., Steiner G. Z., Chang D. H. (2016). A double-blind, randomised, placebo-controlled trial of Ganoderma lucidum for the treatment of cardiovascular risk factors of metabolic syndrome. Sci. Rep. 6, 29540. 10.1038/srep29540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuate D., Nouemsi Kengne N., Nya Biapa C., Kingue Azantsa B., Manan Bin Wan Muda W. (2015). Tetrapleura tetraptera spice attenuates high-carbohydrate, high-fat diet-induced obese and type 2 diabetic rats with metabolic syndrome features. Lipids Health Dis. 14, 50. 10.1186/s12944-015-0051-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulabas S. S., Ipek H., Tufekci A. R., Arslan S., Demirtas I., Ekren R., et al. (2018). Ameliorative potential of Lavandula stoechas in metabolic syndrome via multitarget interactions. J. Ethnopharmacol. 223, 88–98. 10.1016/j.jep.2018.04.043 [DOI] [PubMed] [Google Scholar]
- Li D., Zhang T., Lu J., Peng C., Lin L. (2021). Natural constituents from food sources as therapeutic agents for obesity and metabolic diseases targeting adipose tissue inflammation. Crit. Rev. Food Sci. Nutr. 61, 1947–1965. 10.1080/10408398.2020.1768044 [DOI] [PubMed] [Google Scholar]
- Li F., Li Y., Li Q., Shi X. (2020). Eriobotrya japonica leaf triterpenoid acids ameliorate metabolic syndrome in C57BL/6J mice fed with high-fat diet. Biomed. Pharmacother. 132, 110866. 10.1016/j.biopha.2020.110866 [DOI] [PubMed] [Google Scholar]
- Liu W., Wan C., Huang Y., Li M. (2020). Effects of tea consumption on metabolic syndrome: a systematic review and meta-analysis of randomized clinical trials. Phytotherapy Res., 1–10. 10.1002/ptr.6731 [DOI] [PubMed] [Google Scholar]
- Lopes G. D. M., Carvalho R. P., Boleti A. P., Lima A. S., Oliveira de Almeida P. D., Pacheco C. C., et al. (2014). Extract from Eugenia punicifolia is an antioxidant and inhibits enzymes related to metabolic syndrome. Appl. Biochem. Biotechnol. 172 (1), 311–324. 10.1007/s12010-013-0520-8 [DOI] [PubMed] [Google Scholar]
- Madlabana C., Mashamba-Thompson T., Petersen I. (2020). Performance management methods and practices among nurses in primary health care settings: a systematic scoping review protocol. Syst. Rev. 9, 40. 10.1186/s13643-020-01294-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdian D., Abbaszadeh-Goudarzi K., Raoofi A., Dadashizadeh G., Abroudi M., Zarepour E., et al. (2020). Effect of Boswellia species on the metabolic syndrome: a review. Iran. J. Basic Med. Sci. 23 (11), 1374–1381. 10.22038/ijbms.2020.42115.9957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak S., Thomas A. (2022). Steps for conducting a scoping review. J. Grad. Med. Educ. 14 (5), 565–567. 10.4300/JGME-D-22-00621.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallappa R. H., Rokana N., Duary R. K., Panwar H., Batish V. K., Grover S. (2012). Management of metabolic syndrome through probiotic and prebiotic interventions. Indian J. Endocr. Metab. 16, 20–27. 10.4103/2230-8210.91178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. J., Sutherland W. H., Williams S. M., Walker R. J., Berry E. A., De Jong S. A., et al. (2013). The effect of lipoic acid and vitamin E therapies in individuals with the metabolic syndrome. Nutr. Metab. Cardiovasc Dis. 23 (6), 543–549. 10.1016/j.numecd.2011.11.006 [DOI] [PubMed] [Google Scholar]
- Mansouri M., Nayebi N., Keshtkar A., Hasani-Ranjbar S., Taheri E., Larijani B. (2012). The effect of 12 weeks Anethum graveolens (dill) on metabolic markers in patients with metabolic syndrome; a randomized double blind controlled trial. Daru 20 (1), 47. 10.1186/2008-2231-20-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Motoyasu N., Sera K., Fujii T., Yoshikawa Y., Yasui H., et al. (2011). Effects of Zn(II) complex with vitamins C and U, and carnitine on metabolic syndrome model rats. Metallomics 3 (7), 683–685. 10.1039/c1mt00018g [DOI] [PubMed] [Google Scholar]
- Mayer C., Côme M., Ulmann L., Chini Zittelli G., Faraloni C., Nazih H., et al. (2019). Preventive effects of the marine microalga phaeodactylum tricornutum, used as a food supplement, on risk factors associated with metabolic syndrome in wistar rats. Nutrients 11 (5), 1069. 10.3390/nu11051069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C., Côme M., Ulmann L., Martin I., Zittelli G. C., Faraloni C., et al. (2022). The potential of the marine microalga diacronema lutheri in the prevention of obesity and metabolic syndrome in high-fat-fed wistar rats. Molecules 27 (13), 4246. 10.3390/molecules27134246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur-Bialy A. I., Pocheć E. (2016). Riboflavin reduces pro-inflammatory activation of adipocyte-macrophage Co-culture. Potential application of vitamin B2 enrichment for attenuation of insulin resistance and metabolic syndrome development. Molecules 21 (12), 1724. 10.3390/molecules21121724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson N. O., Fullston T., Kang W. X., Sandeman L. Y., Corbett M. A., Owens J. A., et al. (2016). Paternal under-nutrition programs metabolic syndrome in offspring which can be reversed by antioxidant/vitamin food fortification in fathers. Sci. Rep. 6, 27010. 10.1038/srep27010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melguizo-Rodríguez L., Costela-Ruiz V. J., García-Recio E., De Luna-Bertos E., Ruiz C., Illescas-Montes R. (2021). Role of vitamin D in the metabolic syndrome. Nutrients 13 (3), 830. 10.3390/nu13030830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mert H., İrak K., Çibuk S., Yıldırım S., Mert N. (2022). The effect of evening primrose oil (Oenothera biennis) on the level of adiponectin and some biochemical parameters in rats with fructose induced metabolic syndrome. Arch. Physiol. Biochem. 128 (6), 1539–1547. 10.1080/13813455.2020.1781900 [DOI] [PubMed] [Google Scholar]
- Mirza R., Chi N., Chi Y. (2013). Therapeutic potential of the natural product mangiferin in metabolic syndrome. J. Nutr. Ther. 2 (2), 74–79. 10.6000/1929-5634.2013.02.02.2 [DOI] [Google Scholar]
- Mizuno H., Taketomi A., Nakabayashi T. (2018). Potentially beneficial effects of st. John’s wort (Hypericum perforatum) in patients with metabolic syndrome. OBM Integr. Complementary Med. 3 (3). 10.21926/obm.icm.1803021 [DOI] [Google Scholar]
- Mohammadifard N., Sajjadi F., Haghighatdoost F. (2021). Effects of soy consumption on metabolic parameters in patients with metabolic syndrome: a systematic review and meta-analysis. EXCLI J. 20, 665–685. 10.17179/excli2021-3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohtashami A. (2019). Effects of bread with Nigella sativa on blood glucose, blood pressure and anthropometric indices in patients with metabolic syndrome. Clin. Nutr. Res. 8 (2), 138–147. 10.7762/cnr.2019.8.2.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollazadeh H., Hosseinzadeh H. (2016). Cinnamon effects on metabolic syndrome: a review based on its mechanisms. Iran. J. Basic Med. Sci. 19 (12), 1258–1270. 10.22038/ijbms.2016.7906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mopuri R., Ganjayi M., Meriga B., Koorbanally N. A., Islam M. S. (2018). The effects of Ficus carica on the activity of enzymes related to metabolic syndrome. J. Food Drug Anal. 26 (1), 201–210. 10.1016/j.jfda.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morovati A., Pourghassem Gargari B., Sarbakhsh P. (2019). Effects of cumin (Cuminum cyminum L.) essential oil supplementation on metabolic syndrome components: a randomized, triple-blind, placebo-controlled clinical trial. Phytother. Res. 33 (12), 3261–3269. 10.1002/ptr.6500 [DOI] [PubMed] [Google Scholar]
- Mostafa D. K., Nasra R. A., Zahran N., Ghoneim M. T. (2016). Pleiotropic protective effects of Vitamin D against high fat diet-induced metabolic syndrome in rats: one for all. Eur. J. Pharmacol. 792, 38–47. 10.1016/j.ejphar.2016.10.031 [DOI] [PubMed] [Google Scholar]
- Moukayed M., Grant W. B. (2019). Linking the metabolic syndrome and obesity with vitamin D status: risks and opportunities for improving cardiometabolic health and well-being. Diabetes Metab. Syndr. Obes. 12, 1437–1447. 10.2147/DMSO.S176933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y., Goto H., Hikiami H., Nogami T., et al. (2012). Effect of keishibukuryogan on endothelial function in patients with at least one component of the diagnostic criteria forMetabolic syndrome: a controlled clinical trial with crossover design. Evidence-Based Complementary Altern. Med., 10.1155/2012/359282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nainu F., Frediansyah A., Mamada S., Permana A., Salampe M., Chandran D., et al. (2023). Natural products targeting inflammation-related metabolic disorders. A Compr. Rev. 9 (6), e16919. 10.1016/j.heliyon.2023.e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm W., Lie D. (2010). Herbals used for diabetes, obesity, and metabolic syndrome. Prim. Care 37 (2), 237–254. 10.1016/j.pop.2010.02.008 [DOI] [PubMed] [Google Scholar]
- Najmi A., Nasiruddin M., Khan R., Haque S. F. (2013). Indigenous herbal product Nigella sativa proved effective as an antihypertensive in metabolic syndrome. Asian J. Pharm. Clin. Res. 6, 61–64. [Google Scholar]
- Nasiri E., Jafari F., Nasiri R. (2023). Evaluation of attitude and use of complementary and alternative medicine in doctoral graduates of mazandaran university of medical science. Complement. Med. J. 13 (1), 52–59. 10.52547/cmja-13018 [DOI] [Google Scholar]
- Nigussie G. (2021). A review on traditionally used medicinal plants for scabies therapy in Ethiopia. Adv. Traditional Med. 21 (2), 199–208. 10.1007/s13596-020-00453-7 [DOI] [Google Scholar]
- Nikaein F., Babajafari S., Mazloomi S., Zibaeenezhad M., Zargaran A. (2016). The effects of satureja hortensis L. Dried leaves on serum sugar, lipid profiles, hs-CRP, and blood pressure in metabolic syndrome patients: a double-blind randomized clinical trial. Iran. Red. Crescent Med. J., e34931. 10.5812/ircmj.34931 [DOI] [Google Scholar]
- Nimrouzi M., Ruyvaran M., Zamani A., Nasiri K., Akbari A. (2020). Oil and extract of safflower seed improve fructose induced metabolic syndrome through modulating the homeostasis of trace elements, TNF-α and fatty acids metabolism. J. Ethnopharmacol. 254, 112721. 10.1016/j.jep.2020.112721 [DOI] [PubMed] [Google Scholar]
- Nna V. U., McGrowder D., Nwokocha C. (2023). Nutraceutical management of metabolic syndrome as a palliative and a therapeutic to coronavirus disease (COVID) crisis. Arch. Physiol. Biochem. 129 (5), 1123–1142. 10.1080/13813455.2021.1903041 [DOI] [PubMed] [Google Scholar]
- Nyakudya T., Tshabalala T., Dangarembizi R., Erlwanger K., Ndhlala A., The potential therapeutic value of medicinal plants in the management of metabolic disorders. 2020;25:2669. 10.3390/molecules25112669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura N., Atsumi G., Uehara S., Ohta M., Taniguchi M. (2019). Ashitaba (angelica keiskei) exerts possible beneficial effects on metabolic syndrome. OBM Integr. Complementary Med. 4 (1). 10.21926/obm.icm.1901005 [DOI] [Google Scholar]
- Olid M. C., Hidalgo M., Prieto I., Cobo A., Martínez-Rodríguez A. M., Segarra A. B., et al. (2023). Evidence supporting the involvement of the minority compounds of extra virgin olive oil, through gut microbiota modulation, in some of the dietary benefits related to metabolic syndrome in comparison to butter. Molecules 28 (5), 2265. 10.3390/molecules28052265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owis A. I., Abo-Youssef A. M., Osman A. H. (2017). Leaves of Cordia boissieri A. DC. as a potential source of bioactive secondary metabolites for protection against metabolic syndrome-induced in rats. Z Naturforsch C J. Biosci. 72 (3-4), 107–118. 10.1515/znc-2016-0073 [DOI] [PubMed] [Google Scholar]
- Pace R., Pluye P., Bartlett G., Macaulay A. C., Salsberg J., Jagosh J., et al. (2012). Testing the reliability and efficiency of the pilot Mixed Methods Appraisal Tool (MMAT) for systematic mixed studies review. Int. J. Nurs. Stud. 49 (1), 47–53. 10.1016/j.ijnurstu.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Padiya R., Khatua T. N., Bagul P. K., Kuncha M., Banerjee S. K. (2011). Garlic improves insulin sensitivity and associated metabolic syndromes in fructose fed rats. Nutr. Metab. (Lond). 8, 53. 10.1186/1743-7075-8-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palla A. H., Amin F., Fatima B., Shafiq A., Rehman N. U., Haq I., et al. (2021). Systematic review of polyherbal combinations used in metabolic syndrome. Front. Pharmacol. 12, 752926. 10.3389/fphar.2021.752926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal S. K., Wanyonyi S., Brown L. (2017). Selenium, vanadium, and chromium as micronutrients to improve metabolic syndrome. Curr. Hypertens. Rep. 19 (3), 10. 10.1007/s11906-017-0701-x [DOI] [PubMed] [Google Scholar]
- Pandey N., Mishra P., Tripathi Y. (2021). Cyperus rotundus in the management of metabolic syndrome – benefit in the treatment of metabolic syndrome. Life Sci. Inf. Publ. 7 (3), 20. 10.26479/2021.0703.03 [DOI] [Google Scholar]
- Papathanasopoulos A., Camilleri M. (2010). Dietary fiber supplements: effects in obesity and metabolic syndrome and relationship to gastrointestinal functions. Gastroenterology 138 (1), 65–72. 10.1053/j.gastro.2009.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor R., Bouzas C., Tur J. A. (2021). Beneficial effects of dietary supplementation with olive oil, oleic acid, or hydroxytyrosol in metabolic syndrome: systematic review and meta-analysis. Free Radic. Biol. Med. 172, 372–385. 10.1016/j.freeradbiomed.2021.06.017 [DOI] [PubMed] [Google Scholar]
- Patil A. S., Mahajan U. B., Agrawal Y. O., Patil K. R., Patil C. R., Ojha S., et al. (2020). Plant-derived natural therapeutics targeting cannabinoid receptors in metabolic syndrome and its complications: a review. Biomed. Pharmacother. 132, 110889. 10.1016/j.biopha.2020.110889 [DOI] [PubMed] [Google Scholar]
- Payab M., Hasani-Ranjbar S., Shahbal N., Qorbani M., Aletaha A., Haghi-Aminjan H., et al. (2020). Effect of the herbal medicines in obesity and metabolic syndrome: a systematic review and meta-analysis of clinical trials. Phytother. Res. 34 (3), 526–545. 10.1002/ptr.6547 [DOI] [PubMed] [Google Scholar]
- Pedersen M. H., Mølgaard C., Hellgren L. I., Lauritzen L. (2010). Effects of fish oil supplementation on markers of the metabolic syndrome. J. Pediatr. 157 (3), 395–400. 10.1016/j.jpeds.2010.04.001 [DOI] [PubMed] [Google Scholar]
- Pérez-Muñoz E. P., Antunes-Ricardo M., Martínez-Ávila M., Guajardo-Flores D. (2022). Eryngium species as a potential ally for treating metabolic syndrome and diabetes. Front. Nutr. 9, 878306. 10.3389/fnut.2022.878306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Torres I., Torres-Narváez J. C., Pedraza-Chaverri J., Rubio-Ruiz M. E., Díaz-Díaz E., Del Valle-Mondragón L., et al. (2016). Effect of the aged garlic extract on cardiovascular function in metabolic syndrome rats. Molecules 21 (11), 1425. 10.3390/molecules21111425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona J. S. (2017). Membrane lipid alterations in the metabolic syndrome and the role of dietary oils. Biochim. Biophys. Acta Biomembr. 1859 (9 Pt B), 1690–1703. 10.1016/j.bbamem.2017.04.015 [DOI] [PubMed] [Google Scholar]
- Pilar B., Güllich A., Oliveira P., Ströher D., Piccoli J., Manfredini V. (2017). Protective role of flaxseed oil and flaxseed lignan secoisolariciresinol diglucoside against oxidative stress in rats with metabolic syndrome. J. Food Sci. 82 (12), 3029–3036. 10.1111/1750-3841.13964 [DOI] [PubMed] [Google Scholar]
- Pinzon-Perez H., Pérez M. (2016). Complementary, alternative, and integrative health: a multicultural perspective. Hoboken, New Jersey, United States: Wiley. ISBN: 978-1-118-88042-5. [Google Scholar]
- Ponticelli M., Russo D., Faraone I., Sinisgalli C., Labanca F., Lela L., et al. (2021). The promising ability of humulus lupulus L. Iso-acids vs. Diabetes, inflammation, and metabolic syndrome: a systematic review. Molecules 26, 954. 10.3390/molecules26040954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja Kumar S., Mohd Ramli E. S., Abdul Nasir N. A., Ismail N. H. M., Mohd Fahami N. A. (2019). Preventive effect of Naringin on metabolic syndrome and its mechanism of action: a systematic review. Evidence-Based Complementary Altern. Med., 10.1155/2019/9752826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Higuera A., Peña-Montes C., Herrera-Meza S., Mendoza-López R., Valerio-Alfaro G., Oliart-Ros R. M. (2020). Preventive action of sterculic oil on metabolic syndrome development on a fructose-induced rat model. J. Med. Food 23 (3), 305–311. 10.1089/jmf.2019.0177 [DOI] [PubMed] [Google Scholar]
- Ramli N. Z., Chin K. Y., Zarkasi K. A., Ahmad F. (2018). A review on the protective effects of honey against metabolic syndrome. Nutrients 10 (8), 1009. 10.3390/nu10081009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi B., Hosseinzadeh H. (2017). Saffron: a promising natural medicine in the treatment of metabolic syndrome. J. Sci. Food Agric. 97, 1679–1685. 10.002/jsfa.8134 [DOI] [PubMed] [Google Scholar]
- Reshidan N., Abd Muid S., Mamikutty N. (2019). The effects of Pandanus amaryllifolius (Roxb.) leaf water extracts on fructoseinduced metabolic syndrome rat model. BMC Complementary Altern. Med. 19, 232. 10.1186/s12906-019-2627-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi M. K., Rabail R., Munir S., Inam-Ur-Raheem M., Qayyum M. M. N., Kieliszek M., et al. (2022). Astounding health benefits of jamun (syzygium cumini) toward metabolic syndrome. Molecules 27 (21), 7184. 10.3390/molecules27217184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlani Y., Pothineni N. V., Kovelamudi S., Mehta J. L. (2017). Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc Dis. 11 (8), 215–225. 10.1177/1753944717711379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyvaran M., Zamani A., Mohamadian A., Zarshenas M. M., Eftekhari M. H., Pourahmad S., et al. (2022). Safflower (Carthamus tinctorius L.) oil could improve abdominal obesity, blood pressure, and insulin resistance in patients with metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. J. Ethnopharmacol. 282, 114590. 10.1016/j.jep.2021.114590 [DOI] [PubMed] [Google Scholar]
- Sabarathinam S., Chandra S. K. R., Mahalingam Vijayakumar T. (2022). “Necessity of herbal medicine in the management of metabolic syndrome,” in Lifestyle-related diseases and metabolic syndrome (London, UK: IntechOpen; ). 10.5772/intechopen.105199 [DOI] [Google Scholar]
- Saha S., Pal D. (2022). Impact of flax on metabolic syndrome and related environmental factors. Int. J. Pharm. Sci. Res. 13, 531–542. 10.13040/IJPSR.0975-8232 [DOI] [Google Scholar]
- Salaramoli S., Mehri S., Yarmohammadi F., Hashemy S. I., Hosseinzadeh H. (2022). The effects of ginger and its constituents in the prevention of metabolic syndrome: a review. Iran. J. Basic Med. Sci. 25 (6), 664–674. 10.22038/IJBMS.2022.59627.13231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salekzamani S., Bavil A. S., Mehralizadeh H., Jafarabadi M. A., Ghezel A., Gargari B. P. (2017). The effects of vitamin D supplementation on proatherogenic inflammatory markers and carotid intima media thickness in subjects with metabolic syndrome: a randomized double-blind placebo-controlled clinical trial. Endocrine 57 (1), 51–59. 10.1007/s12020-017-1317-2 [DOI] [PubMed] [Google Scholar]
- Salekzamani S., Mehralizadeh H., Ghezel A., Salekzamani Y., Jafarabadi M. A., Bavil A. S., et al. (2016). Effect of high-dose vitamin D supplementation on cardiometabolic risk factors in subjects with metabolic syndrome: a randomized controlled double-blind clinical trial. J. Endocrinol. Invest. 39 (11), 1303–1313. 10.1007/s40618-016-0507-8 [DOI] [PubMed] [Google Scholar]
- Samakar B., Mehri S., Hosseinzadeh H. (2022). A review of the effects of Urtica dioica (nettle) in metabolic syndrome. Iran. J. Basic Med. Sci. 25 (5), 543–553. 10.22038/IJBMS.2022.58892.13079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Rodriguez E., Lima-Cabello E., Biel-Glesson S., Fernandez-Navarro J. R., Calleja M. A., Roca M., et al. (2018). Effects of virgin olive oils differing in their bioactive compound contents on metabolic syndrome and endothelial functional risk biomarkers in healthy adults: a randomized double-blind controlled trial. Nutrients 10 (5), 626. 10.3390/nu10050626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandar S., Zadra C., Marcotullio M. C. (2023). Coriander (coriandrum sativum) polyphenols and their nutraceutical value against obesity and metabolic syndrome. Molecules 28 (10), 4187. 10.3390/molecules28104187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong E., Bose S., Han S. Y., Song E. J., Lee M., Nam Y. D., et al. (2021). Positive influence of gut microbiota on the effects of Korean red ginseng in metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. EPMA J. 12 (2), 177–197. 10.1007/s13167-021-00243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiee M., Aghili Moghaddam N. S., Nosrati M., Tousi M., Avan A., Ryzhikov M., et al. (2017). Saffron against components of metabolic syndrome: current status and prospective. J. Agric. Food Chem. 65 (50), 10837–10843. 10.1021/acs.jafc.7b03762 [DOI] [PubMed] [Google Scholar]
- Shah A. S., Khan G. M., Badshah A., Shah S. U., Shah K. U., Mirza S. A., et al. (2012). Nigella sativa provides protection against metabolic syndrome. Afr. J. Biotechnol. 11 (48), 10919–10925. 10.5897/AJB12.890 [DOI] [Google Scholar]
- Shakib Z., Shahraki N., Razavi B. M., Hosseinzadeh H. (2019). Aloe vera as an herbal medicine in the treatment of metabolic syndrome: a review. Phytotherapy Res. 33, 2649–2660. 10.1002/ptr.6465 [DOI] [PubMed] [Google Scholar]
- Shen H. H., Alex R., Bellner L., Raffaele M., Licari M., Vanella L., et al. (2020). Milk thistle seed cold press oil attenuates markers of the metabolic syndrome in a mouse model of dietary-induced obesity. J. Food Biochem. 44 (12), e13522. 10.1111/jfbc.13522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Jia L. N., Honma N., Hosono T., Ariga T., Seki T. (2012). Beneficial effects of cinnamon on the metabolic syndrome, inflammation, and pain, and mechanisms underlying these effects - a review. J. Tradit. Complement. Med. 2 (1), 27–32. 10.1016/s2225-4110(16)30067-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. K., Lui E., Wright D., Taylor A., Bakovic M. (2017). Alcohol extract of North American ginseng (Panax quinquefolius) reduces fatty liver, dyslipidemia, and other complications of metabolic syndrome in a mouse model. Can. J. Physiol. Pharmacol. 95 (9), 1046–1057. 10.1139/cjpp-2016-0510 [DOI] [PubMed] [Google Scholar]
- Strange R. C., Shipman K. E., Ramachandran S. (2015). Metabolic syndrome: a review of the role of vitamin D in mediating susceptibility and outcome. World J. Diabetes 6 (7), 896–911. 10.4239/wjd.v6.i7.896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganya M., Vikneshan M., Swathy U. (2017). Usage of complementary and alternative medicine: a survey among Indian dental professionals. Complement. Ther. Clin. Pract. 26, 26–29. 10.1016/j.ctcp.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Taghipour Y. D., Hajialyani M., Naseri R., Hesari M., Mohammadi P., Stefanucci A., et al. (2019). Nanoformulations of natural products for management of metabolic syndrome. Int. J. Nanomedicine 14, 5303–5321. 10.2147/IJN.S213831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamel Selvan K., Goon J. A., Makpol S., Tan J. K. (2023). Effects of microalgae on metabolic syndrome. Antioxidants (Basel) 12 (2), 449. 10.3390/antiox12020449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamtaji O. R., Milajerdi A., Reiner Ž., Dadgostar E., Amirani E., Asemi Z., et al. (2020). Effects of flaxseed oil supplementation on biomarkers of inflammation and oxidative stress in patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 40, 27–33. 10.1016/j.clnesp.2020.09.017 [DOI] [PubMed] [Google Scholar]
- Tan Y., Kamal M. A., Wang Z. Z., Xiao W., Seale J. P., Qu X. (2011). Chinese herbal extracts (SK0506) as a potential candidate for the therapy of the metabolic syndrome. Clin. Sci. (Lond). 120 (7), 297–305. 10.1042/CS20100441 [DOI] [PubMed] [Google Scholar]
- Tenorio-Jiménez C., Martínez-Ramírez M. J., Gil Á., Gómez-Llorente C. (2020). Effects of probiotics on metabolic syndrome: a systematic review of randomized clinical trials. Nutrients 12 (1), 124. 10.3390/nu12010124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theik N. W. Y., Raji O. E., Shenwai P., Shah R., Kalluri S. R., Bhutta T. H., et al. (2021). Relationship and effects of vitamin D on metabolic syndrome: a systematic review. Cureus 13 (8), e17419. 10.7759/cureus [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaz F. S., Tan Y. P., Williams C. M., Ward L. C., Worrall S., Panchal S. K. Wasabi (eutrema japonicum) reduces obesity and blood pressure in diet-induced metabolic syndrome in rats. Foods 2022, 11, 3435. 10.3390/foods11213435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tola M. A., Ibrahim F., Melak H., Tafesse T., Alemayehu M., Nigussie G. (2023). Traditional herbal remedies in the management of metabolic disorders in Ethiopia: a systematic review of ethnobotanical studies and pharmacological activities. Evidence-Based Complementary Altern. Med., 1413038. 10.1155/2023/1413038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H., De Filippis A., Khan H., Xiao J., Daglia M. (2020). An overview of the health benefits of Prunus species with special reference to metabolic syndrome risk factors. Food Chem. Toxicol. 144, 111574. 10.1016/j.fct.2020.111574 [DOI] [PubMed] [Google Scholar]
- Usui T., Tochiya M., Sasaki Y., Muranaka K., Yamakage H., Himeno A., et al. (2013). Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin. Endocrinol. (Oxf) 78 (3), 365–372. 10.1111/j.1365-2265.2012.04400.x [DOI] [PubMed] [Google Scholar]
- van der Pal K. C., Koopman A. D. M., Lakerveld J., van der Heijden A. A., Elders P. J., Beulens J. W., et al. (2018). The association between multiple sleep-related characteristics and the metabolic syndrome in the general population: the New Hoorn study. Sleep. Med. 52, 51–57. 10.1016/j.sleep.2018.07.022 [DOI] [PubMed] [Google Scholar]
- Verhoeven V., Van der Auwera A., Van Gaal L., Remmen R., Apers S., Stalpaert M., et al. (2015). Can red yeast rice and olive extract improve lipid profile and cardiovascular risk in metabolic syndrome? a double blind, placebo controlled randomized trial. BMC Complementary Altern. Med. 15, 52. 10.1186/s12906-015-0576-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vílchez L., Ascencios J., Dooley T. (2022). GlucoMedix®, an extract of Stevia rebaudiana and Uncaria tomentosa, reduces hyperglycemia, hyperlipidemia, and hypertension in rat models without toxicity: a treatment for metabolic syndrome. BMC Complementary Med. Ther. 22, 62. 10.1186/s12906-022-03538-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel-Vicente C., Gutiérrez-Palomo S., Ferri J., Cortes D., Cabedo N. (2021). Natural products and analogs as preventive agents for metabolic syndrome via peroxisome proliferator-activated receptors: an overview. Eur. J. Med. Chem. 221, 113535. 10.1016/j.ejmech.2021.113535 [DOI] [PubMed] [Google Scholar]
- Waltenberger B., Mocan A., Šmejkal K., Heiss E., Atanasov A. (2016a). Natural products to counteract the epidemic of cardiovascular and metabolic disorders. Molecules 21, 807. 10.3390/molecules21060807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltenberger B., Mocan A., Šmejkal K., Heiss E. H., Atanasov A. G. (2016b). Natural products to counteract the epidemic of cardiovascular and metabolic disorders. Molecules 21, 6. 10.3390/molecules21060807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Ke W., Bao R., Hu X., Chen F. (2017). Beneficial effects of ginger Zingiber officinale Roscoe on obesity and metabolic syndrome: a review. Ann. N. Y. Acad. Sci. 1398 (1), 83–98. 10.1111/nyas.13375 [DOI] [PubMed] [Google Scholar]
- Wang Y. Y., Hsieh Y. H., Kumar K. J. S., Hsieh H. W., Lin C. C., Wang S. Y. (2020). The regulatory effects of a formulation of cinnamomum osmophloeum kaneh and taiwanofungus camphoratus on metabolic syndrome and the gut microbiome. Plants (Basel) 9 (3), 383. 10.3390/plants9030383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenclewska S., Szymczak-Pajor I., Drzewoski J., Bunk M., Śliwińska A. (2019). Vitamin D supplementation reduces both oxidative DNA damage and insulin resistance in the elderly with metabolic disorders. Int. J. Mol. Sci. 20 (12), 2891. 10.3390/ijms20122891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimalawansa S. J. (2018). Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J. Steroid Biochem. Mol. Biol. 175, 177–189. 10.1016/j.jsbmb.2016.09.017 [DOI] [PubMed] [Google Scholar]
- Wong S. K., Chin K. Y., Ima-Nirwana S. (2020). Vitamin C: a review on its role in the management of metabolic syndrome. Int. J. Med. Sci. 17 (11), 1625–1638. 10.7150/ijms.47103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier-Santos D., Bedani R., Lima E., Isay S. S. (2019). Impact of probiotics and prebiotics targeting metabolic syndrome. J. Funct. Foods. 10.1016/j.jff.2019.103666 [DOI] [Google Scholar]
- Xia X., Weng J. (2010). Targeting metabolic syndrome: candidate natural agents. J. Diabetes 2 (4), 243–249. 10.1111/j.1753-0407.2010.00090.x [DOI] [PubMed] [Google Scholar]
- Yahyazadeh R., Ghasemzadeh Rahbardar M., Razavi B. M., Karimi G., Hosseinzadeh H. (2021). The effect of Elettaria cardamomum (cardamom) on the metabolic syndrome: narrative review. Iran. J. Basic Med. Sci. 24 (11), 1462–1469. 10.22038/IJBMS.2021.54417.12228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Xu Z., Deng Q., Huang Q., Wang X., Huang F. (2020). Beneficial effects of flaxseed polysaccharides on metabolic syndrome via gut microbiota in high-fat diet fed mice. Food Res. Int. 131, 108994. 10.1016/j.foodres.2020 [DOI] [PubMed] [Google Scholar]
- Yousefi R., Saidpour A., Mottaghi A., The effects of Spirulina supplementation on metabolic syndrome components, its liver manifestation and related inflammatory markers: a Systematic Review, Complementary Ther. Med. (2018), 10.1016/j.ctim.2018.11.013 [DOI] [PubMed] [Google Scholar]
- Youssef F. S., Ovidi E., Rai M., Editorial: natural product based drugs that control obesity and other disorders that trigger and provoke inflammation. Front. Pharmacol. 2022;13:891496. 10.3389/fphar.2022.891496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Chen H., Song Z., Wang X., Sun Z. (2018). Effects of ginger (zingiber officinale roscoe) on type 2 diabetes mellitus and components of the metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Evidence-Based Complementary Altern. Med., 10.1155/2018/5692962 [DOI] [PMC free article] [PubMed] [Google Scholar]