Abstract
BACKGROUND:
Adapted boxing can help improve the physical functioning and health-related quality of life (HRQoL) of individuals with Parkinson’s disease (PD). Whether these benefits persist longitudinally is unclear.
OBJECTIVE:
The purpose of this retrospective study was to evaluate the impact of a community-based adapted boxing program on the physical functioning and HRQoL of individuals with PD over 1–1.5 years.
METHODS:
Twenty-six individuals with PD agreed to share their results on tests administered upon enrollment in the program (PRE) and ∼431 days later (POST). The tests included the Fullerton Advanced Balance scale, (FAB), the Timed Up-and-Go test (TUG), the 30-second Sit-to-Stand test (30-STS), and the PD questionnaire-39 (PDQ-39).
RESULTS:
From PRE to POST, performance significantly improved on the TUG and 30-STS tests (both p < 0.001), but not on the FAB (p = 0.79). Over the same period, PDQ-39 scores significantly increased (p = 0.05). No PRE to POST changes surpassed the minimal detectable change threshold.
CONCLUSION:
The results of this study suggest that adapted boxing is at worst non-detrimental and at best potentially beneficial for muscle strength, endurance, and functional mobility in individuals with PD. However, adapted boxing probably cannot fully counteract the HRQoL decrements that accompany PD progression.
Keywords: Community-based adapted boxing, Parkinson’s disease, exercise, functional capacity, health-related quality of life, physical functioning
1. Introduction
Parkinson’s disease (PD) is an incurable neurodegenerative disorder associated with a progressive loss of midbrain dopamine neurons (Poewe et al., 2017). It is accompanied by many motor and non-motor symptoms, including rigidity, bradykinesia, tremors, postural instability, and cognitive decline (Poewe et al., 2017). As it progresses, PD diminishes quality of life, strains caretakers, and contributes a substantial socioeconomic burden (Collaborators, 2018). Perhaps most alarmingly, the global age-adjusted prevalence of PD has increased by ∼22% from 1990 to 2016 (Collaborators, 2018), leading some authors to describe the disease as an emerging pandemic (Bloem et al., 2021; Dorsey et al., 2018). Until effective disease-modifying treatments are available, developing interventions that can help mitigate PD-related symptoms is critical.
Pharmacological treatments such as dopamine therapy are important for patient management and can effectively alleviate motor and non-motor symptoms (Connolly & Lang, 2014). However, PD medications can cause serious side effects (e.g.., dyskinesia) and are known to lose their effectiveness over time (Connolly & Lang, 2014). Among non-pharmacological interventions, exercise may be the most potent. Indeed, studies based on self-reported levels of physical activity in individuals with PD suggest that regular exercise may help slow decrements in motor and cognitive function, mental health, and quality of life (Amara et al., 2019; Combs-Miller & Moore, 2019; Oguh et al., 2014; Paul et al., 2019; Rafferty et al., 2017). Likewise, intervention studies have shown that exercise training can improve the physical capacity (Corcos et al., 2013; Prodoehl et al., 2015; Schenkman et al., 2012; Shulman et al., 2013), the clinical signs (Corcos et al., 2013; Schenkman et al., 2012; Schenkman et al., 2018; Uc et al., 2014), and the quality of life (Corcos et al., 2013; Uc et al., 2014) of individuals with PD. These and other studies form the backbone for the current exercise guidelines for individuals with PD, encouraging the latter to participate in training programs that incorporate aerobic, resistance, balance, and flexibility exercises (Liguori, 2021).
Over the past ∼15–20 years, adapted boxing has gained considerable traction as an exercise modality of interest for individuals with PD. Boxing requires anticipatory and feedback-based postural adjustments, rapid multidirectional limb movements across many ranges of motion, and the execution of precise movement sequences, all of which are impacted by PD (King & Horak, 2009). With over 840 affiliates worldwide, Rock Steady Boxing (RSB) is perhaps the most well-known adapted boxing program for individuals with PD. Founded in 2006 by Scott C. Newman, who was diagnosed with PD at age 40, and boxer Vincent Issac Perez Sr., RSB was created to develop strength, balance, independence, and confidence in individuals with PD. Despite the longstanding popularity of adapted boxing and the RSB program, the body of scientific research supporting its benefits for individuals with PD has only recently started to burgeon (Combs et al., 2013; Combs et al., 2011; Domingos et al., 2022; Ghaffar et al., 2019; Hermanns et al., 2021; Horbinski et al., 2021; Larson et al., 2022; Moore et al., 2021; Patel et al., 2023; Sangarapillai et al., 2021; Shearin et al., 2021). Current evidence suggests that adapted boxing may help improve balance (Combs et al., 2011; Domingos et al., 2022; Hermanns et al., 2021; Horbinski et al., 2021; Moore et al., 2021) and reduce the risk of falls in individuals with PD (Horbinski et al., 2021). It also appears to improve muscle strength and endurance (Horbinski et al., 2021), walking capacity (Combs et al., 2013; Combs et al., 2011; Shearin et al., 2021), and functional mobility (Combs et al., 2013; Combs et al., 2011; Moore et al., 2021) (but see Domingos et al., 2022). Most importantly, adapted boxing may help improve patient quality of life (Combs et al., 2013; Domingos et al., 2022; Ghaffar et al., 2019; Hermanns et al., 2021; Larson et al., 2022) and diminish both motor (Patel et al., 2023) and non-motor (Ghaffar et al., 2019; Patel et al., 2023) signs and symptoms (but see Sangarapillai et al., 2021). Although these studies are encouraging, most investigated the impact of adapted boxing over a few weeks to a few months, making it difficult to determine whether the reported physical functioning and quality of life improvements can be maintained over prolonged periods (i.e., ≥∼1-year). Moreover, most of the studies that investigated the impact of adapted boxing on the physical functioning and health-related quality of life (HRQoL) of individuals with PD used prospective study designs, which may introduce so-called “research participation biases” (McCambridge et al., 2014). Hence, despite the unequivocal value of prospective studies, their results may not always be generalizable to non-research settings. With this study, our goal was to investigate the longitudinal (≥∼ 1-year) impact of a community-based adapted boxing program on the physical functioning and HRQoL of individuals with PD. To minimize potential research participation biases and shed light on the benefits that individuals with PD can accrue from adapted boxing in non-research settings, a retrospective study design was used. Our hope is that the results of this study will provide coaches, trainers, and healthcare professionals with evidence that the long-term participation in community-based adapted boxing programs can help people with PD maintain physical function and HRQoL in spite of disease progression.
2. Methods
2.1. The local community-based adapted boxing program
This study is based on physical functioning and HRQoL data collected at the local community-based adapted boxing program for individuals with PD. One of the authors of this study (A.T.) is the founder, scientific director, and coordinator of this community-based program. Briefly, the local community-based adapted boxing program consists of 90-minute group training sessions held twice weekly (Mondays and Thursdays) at the local boxing club. Each session is animated and supervised by at least one RSB-certified trainer with a university degree in a health-related field (A.T., nursing; S.B., physical education and kinesiology) and a head-boxing coach certified by the Canadian National Coaching Certification Program. To coach in an RSB affiliate, coaches and trainers need to be certified by RSB to guarantee that they have the competency to 1) ensure the safety of all participants, 2) assist participants during the training sessions, and 3) create and demonstrate the training sessions based on the RSB philosophy, which prioritizes exercises that target balance, speed, and dexterity. If they choose, participants can be accompanied by a caregiver who can assist them and/or participate in the training sessions. The training sessions vary from week to week (and sometimes session to session) but always follow the RSB principles and are chronologically organized as follows: 1) warm-up (20–30 min), 2) adapted boxing (30–40 min), core strengthening (5–10 min), and 4) cool-down (5–10 min). Participants train in one of two group sessions depending on disease severity (HYI-II or HYIII-IV). The general content of HYI-II and HYIII-IV sessions is identical, but the exercises are scaled according to the limitations imposed by disease severity (e.g.., the HYI-II group might complete a given exercise standing up, whereas the HYIII-IV group might complete the same exercise sitting down). Exercises were also scaled on a per-participant basis when necessary.
As part of the program, participants are required to undergo a pre-participation evaluation comprising physical functioning tests and a HRQoL questionnaire (see section 2.3 for details). This evaluation is repeated every 12–16 months if the participants remain with the program. In the present study, we retrospectively analyzed the data obtained from the pre-participation (PRE) and subsequent (POST) evaluation to reveal how physical functioning and HRQoL change with long-term participation in a community-based adapted boxing program. All evaluations were administered by authors of the present study (A.T., ∼80%; S.B., ∼20%) between June 2017 and May 2019.
2.2. Participants
To participate in the local community-based adapted boxing program, individuals are required to 1) have a clinical PD diagnosis, 2) be capable of understanding the exercise-related instructions (i.e., show minimal cognitive impairment) and 3) be free of medical conditions that either preclude physical activity or contraindicate it. When this study was approved by the institutional review board, 26 local community-based adapted boxing program participants had PRE and POST evaluation data. The analyses presented in this study are based on these retrospectively gathered data. Baseline demographics for all participants are presented in Table 1. This study was approved by the CIUSSS de l’Estrie CHUS scientific and ethical review board (IRB #: 2020–3258). All participants gave their informed and written consent before any data were retrieved from their records.
Table 1.
Participant demographics
| Sample size | 26 |
| Sex, Males/Females | 16/10 |
| Hoehn and Yahr stage, I-II/III-IV | 20/6 |
| Age, years | 69 (10) |
| Disease duration, years | 4.87 (4.65) |
2.3. Participant evaluations
Participants were identically evaluated at two time points (PRE and POST) with a battery of tests and questionnaires that have been validated in individuals with PD. The first test was the Fullerton Advanced Balance (FAB) scale, which is used to assess balance and postural control (Klein et al., 2011; Schlenstedt et al., 2015). The FAB assesses 10 balance-based activities, all of which are scored using a 5-point Lickert scale (0–4). On this test, higher scores indicate better balance and a reduced risk of falls. The second test was the Timed Up-and-Go (TUG), which is used to evaluate mobility, dynamic balance, and risk of falls (Balash et al., 2005; Bennie et al., 2003; Brusse et al., 2005; Huang et al., 2011; Mak & Pang, 2009; Morris et al., 2001; Schlenstedt et al., 2015; Steffen & Seney, 2008). To complete the TUG test, participants are timed using a stopwatch as they get up from a standard-height (∼46 cm) chair, walk 3 m, turn, walk back to the chair, and sit down. Shorter TUG times indicate better mobility, dynamic balance, and a lower risk of falls. The third test was the 30 s Sit-to-Stand (30-STS), which is used to evaluate leg strength and endurance (Jones et al., 1999; Petersen et al., 2017). The 30-STS requires participants to stand up from and sit back down on a standard-height chair as many times as possible over 30 s. The test starts with participants sitting down. For this test, a higher score (i.e., more repetitions) indicates better leg strength and endurance. Finally, the PD Questionnaire-39 (PDQ-39) was used to assess HRQoL (Jenkinson et al., 1997). The PDQ-39 is a self-administered 5-point Lickert scale (0–4) composed of eight dimensions that evaluate mobility, activities of daily living, emotional well-being, stigma, social support, cognition, communication, and bodily discomfort (39 items total). The score for each dimension is defined as a percentage of the highest possible score on that dimension. Then, from the individual dimension scores, a summary index (PDQ-39-SI), defined as the average score across all dimensions, is calculated. For the PDQ-39-SI (and each dimension), higher scores imply lower HRQoL.
To interpret the meaningfulness of PRE to POST changes in our study sample, the minimal detectable changes (at a 95% confidence level, MDC95) for the FAB, TUG, 30-STS, and PDQ-39 were determined from reliability studies in individuals with PD. For the TUG, the MDC95 has been reported to range from 1.6 to 11 s (Dal Bello-Haas et al., 2011; Huang et al., 2011; Lim et al., 2005; Steffen & Seney, 2008). For the 30-STS, Petersen et al. (2017) reported an MDC95 of 3 repetitions. For the PDQ-39, Fitzpatrick et al. (2004) reported the MDC95 for the summary index (PDQ-39-SI) and its constituent dimensions as follows: PDQ-39-SI, 10.56%; mobility, 16.75%; activities of daily living, 23.57%; emotional wellness, 20.85%; stigma, 29.57%; social support, 43.34%; cognition, 31.65%; communication, 30.30%; bodily discomfort, 35.68%. To our knowledge, the literature has not directly reported an MDC95 for the FAB in individuals with PD. Therefore, we calculated the MDC95 for this test based on the results of Schlenstedt et al. (2015) using the following equations (Haley & Fragala-Pinkham, 2006):
| (1) |
| (2) |
In equation 1, SEM corresponds to the standard error of measurement, SD corresponds to the standard deviation of the first measurement, and rtest-retest corresponds to the coefficient of the test-retest reliability estimate. In equation 2, 1.96 corresponds to the z-score for the chosen confidence level (i.e., 95%). Using this approach, we calculated an MDC95 of 2.27 points for the FAB.
2.4. Statistical analyses
We did not conduct an a priori power analysis for the present study. Instead, we aimed to recruit as many individuals with PD as possible from the local community-based adapted boxing program. That said, post hoc analyses using G*Power (Faul et al., 2009) revealed that for our main analyses, our sample size (N = 23–26 depending on the outcome variable, see below) was large enough to detect medium effect sizes with 80% power (α= 0.05). We are thus confident that PRE to POST changes characterized by medium or larger effect sizes are likely not the result of type I errors. On the flip side, our study lacks the statistical power to identify smaller than medium effect sizes. However, because small effect sizes generally indicate a low effect magnitude-to-variability ratio (i.e., high effect uncertainty), we contend that PRE to POST changes associated with smaller than medium effect sizes are unlikely to have a meaningful impact in individuals with PD.
The FAB, TUG, and 30-STS analyses were carried out on all 26 participants. Due to missing data, the PDQ-39 analyses were carried out on 23 participants (Hoehn and Yahr stage I-II (HYI-II), N = 19; Hoehn and Yahr stage III-IV (HYIII-IV), N = 4). All data were visualized with histograms and evaluated with Shapiro-Wilk tests to determine if they were normally distributed. Given that only the 30-STS data followed a normal distribution, we decided to use non-parametric statistical tests to analyze all the data (parametric analysis of the 30-STS data yielded qualitatively similar results). The main analyses compared PRE vs. POST data across all participants using Wilcoxon signed-rank tests. The secondary analyses compared participants grouped by disease stages (HYI-II vs. HYIII-IV). Specifically, we compared PRE, POST, and changes from PRE to POST between groups using Mann-Whitney U tests. The results from these secondary analyses were Bonferroni-corrected (i.e., p-values were multiplied by 3) to reduce the risk of type 1 errors. Given the lopsided representation of HYI-II (N = 20 for the physical functioning tests and N = 19 for the PDQ-39) vs. HYIII-IV (N = 6 for the physical functioning tests and N = 4 for the PDQ-39) individuals in our study sample, the results of the secondary analyses should be interpreted tentatively. If anything, we suggest that the reader consider these analyses as exploratory or qualitative. For all analyses, we report the p-values (“p” for the main analyses, “pB” for the Bonferroni-corrected secondary analyses) and effect size estimates (r), which can be interpreted as small (0.1), medium (0.3) or large (0.5) (Cohen, 1988; Field, 2009). The two-tailed statistical significance level was set at α= 0.05. The results are presented as medians and interquartile ranges due to their non-normal distributions.
3. Results
3.1. Evaluation timing and attendance
The median time elapsed between the PRE and POST evaluations was 431 (59) days (range = [364, 572]). Biweekly session attendance was 57.81 (31.14)% (range = [19.20, 84.54]).
3.2. Balance
Changes in physical function are shown in Fig. 1. Across all participants, no significant change was observed on the FAB scale from PRE to POST (PRE = 34.5 (9) points, POST = 33.5 (8) points, p = 0.79, r = 0.05, Fig. 1A). Seven participants (3 HYIII-IV) improved their FAB score by at least the MDC95 (2.27 points), while an equal number of participants (2 HYIII-IV) saw their FAB score diminish by at least the same amount. When we compared participants across disease stages, we found that HYI-II had significantly higher FAB scores than HYIII-IV at PRE (36.5 (6) vs. 24 (10) points, pB = 0.018, r = 0.54) and tended to have higher FAB scores at POST (36.5 (7) vs. 26.5 (17) points, pB = 0.084, r = 0.58). PRE to POST changes in FAB scores did not significantly differ between HYI-II and HYIII-IV (0 (4) vs. 1 (11) point, pB = 1.0, r = 0.05).
Fig. 1.
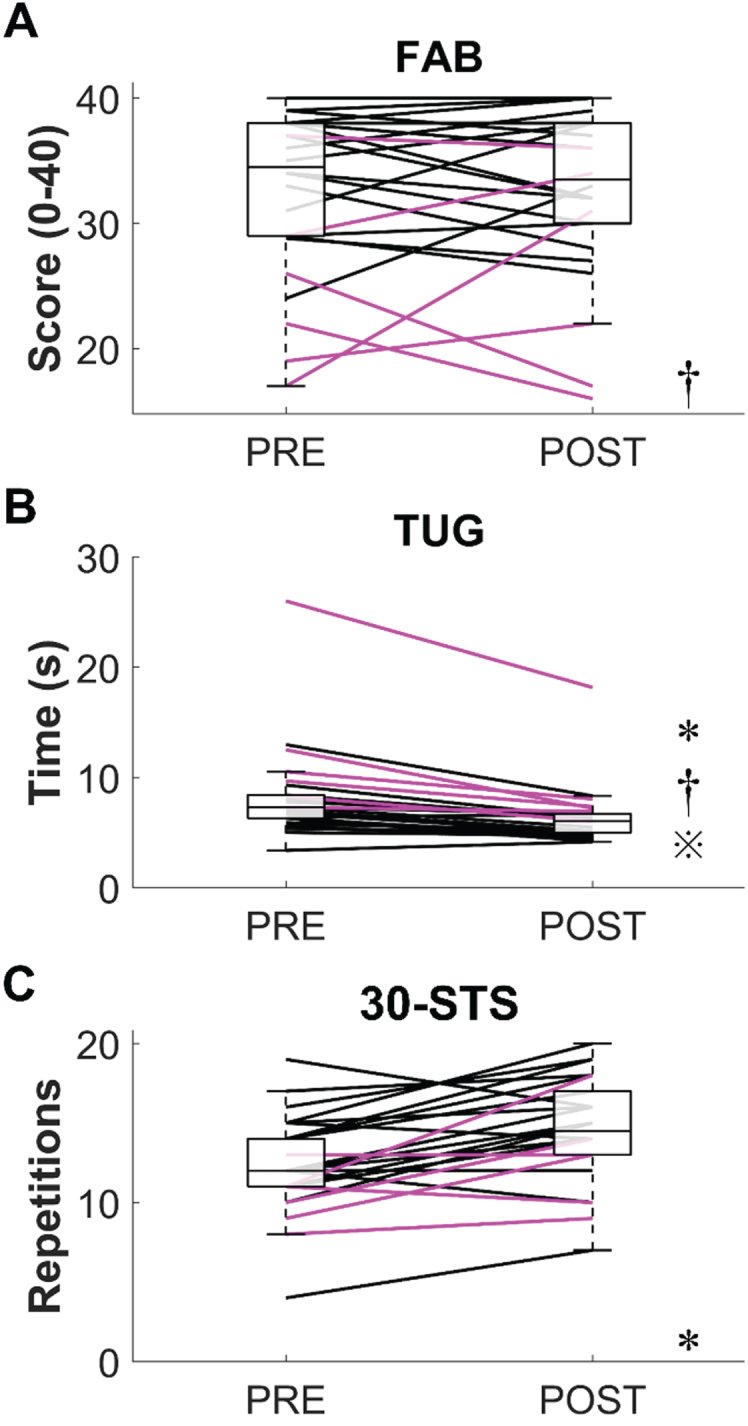
Results from the physical functioning tests. A) Fullerton Advanced Balance (FAB) scale. B) Timed Up-and-Go (TUG) test. C) 30-second Sit-to-Stand (30-STS) test. The boxplots represent the data aggregated from all participants regardless of their Hoehn and Yahr (HY) stage at PRE and POST. The black and magenta lines respectively represent individual data from HYI-II and HYIII-IV participants. *, significant difference between PRE and POST (all participants). †, significant difference between HYI-II and HY III-IV at PRE.  , significant difference between HYI-II and HY III-IV at POST.
, significant difference between HYI-II and HY III-IV at POST.
3.3. Mobility, dynamic balance, walking ability and risk of fall
TUG times significantly diminished from PRE to POST (PRE = 7.29 (2.12) s, POST = 6.04 (1.7) s, p < 0.001, r = 0.83, Fig. 1B), indicating improved mobility, balance, and walking ability. Only two participants performed worse on the TUG test in POST compared to PRE (+0.3 s and +0.78 s, respectively). If we assume an MDC95 of 1.6 s (Lim et al., 2005), 14 participants (5 HYIII-IV) improved their TUG time by at least the MDC95. However, if we assume an MDC95 of 3.5 s (Huang et al., 2011), only 3 participants (2 HYIII-IV) improved their TUG time by at least the MDC95 (range = [4.64, 7.84] s). When we compared participants across disease stages, we found that HYI-II had significantly shorter TUG times than HYIII-IV at both PRE (6.56 (2.04) vs. 10.11 (4.22) s, pB = 0.018, r = 0.53) and POST (5.12 (1.67) vs. 7.29 (1.12) s, pB = 0.009, r = 0.58). PRE to POST reductions in TUG time tended to be smaller in HYI-II compared to HYIII-IV (–1.55 (1.40) vs. –2.41 (3.05) s, pB = 0.078, r = 0.44).
3.4. Leg strength and endurance
Concerning the 30-STS, scores significantly increased from PRE to POST (PRE = 12 (3) reps, POST = 14.5 (4) reps, p < 0.001, r = 0.67, Fig. 1C), indicating improved leg strength and endurance. Performance improvements exceeded or equalled the MDC95 (3 reps) in 15 participants (3 HYIII-IV), while only one participant (HYI-II) showed a commensurate performance decrement. When we compared participants across disease stages, we found that HYI-II tended to have higher 30-STS scores compared to HYIII-IV at PRE (12 (3.5) vs. 10.5 (2) reps, pB = 0.075, r = 0.44) but not at POST (15 (3.5) vs. 13 (4) reps, pB = 0.30, r = 0.32). PRE to POST changes in 30-STS scores did not significantly differ between HYI-II and HYIII-IV (3 (3.5) vs. 2.5 (4) reps, pB = 1.0, r = 0.03).
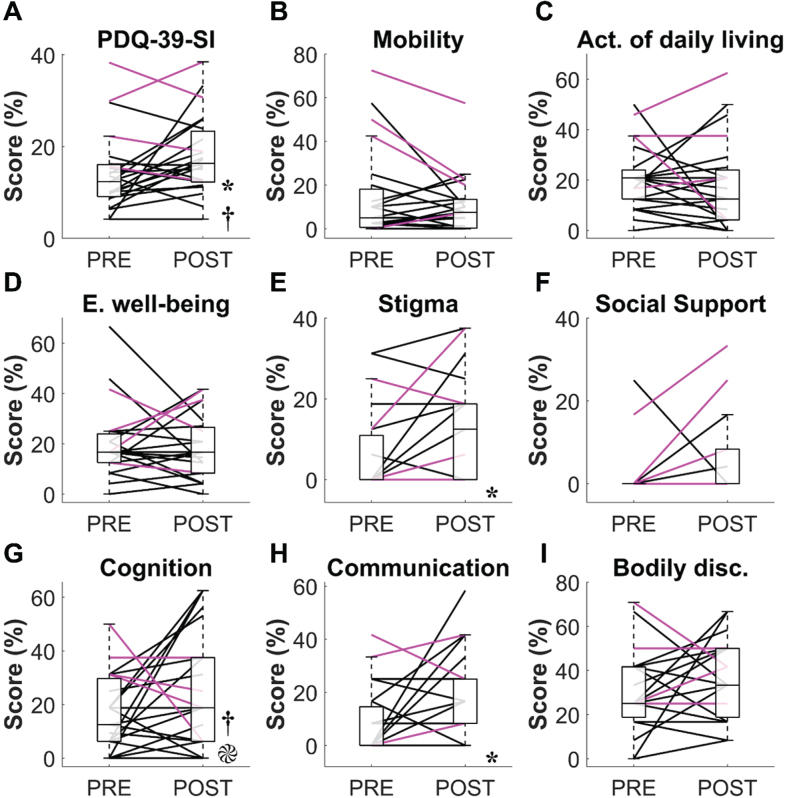
3.5. Health-related quality of life (HRQoL)
All PDQ-39-related variables are depicted in Fig. 2. The PDQ-39-SI significantly increased from PRE to POST (PRE = 12.34 (6.95)%, POST = 16.35 (11.06)%, p = 0.05, r = 0.41, Fig. 2A), which suggests that HRQoL diminished longitudinally in our study sample. However, it should be noted that the median increase in PDQ-39-SI was well below the scale’s MDC95 (10.54%). In fact, only 5 participants (all HYI-II) showed a PDQ-39-SI increase greater than the MDC95. The only PDQ-39 dimensions that significantly increased from PRE to POST were “Stigma” (PRE = 0 (10.93)%, POST = 12.5 (18.75)%, p < 0.006, r = 0.58, Fig. 2E) and “Communication” (PRE = 0 (14.59)%, POST = 8.33 (16.67)%, p < 0.016, r = 0.50; other PDQ-39 dimensions: p≥0.083, r≤0.36). Much like the PDQ-39-SI, the median increases in “Stigma” and “Communication” scores were much smaller than their estimated MDC95 (i.e., “stigma” = 29.57%; “communication” = 30.30%) and increased beyond these thresholds in only 1 and 3 participants (all HYI-II), respectively.
Fig. 2.
Results from the Parkinson’s disease questionnaire (PDQ-39). A) PDQ-39 summary index (PDQ-39-SI). B) Mobility. C) Activities of daily living. D) Emotional well-being. E) Stigma. F) Social support. G) Cognition. H) Communication. I) Bodily discomfort. The boxplots represent the data aggregated from all participants regardless of their Hoehn and Yahr (HY) stage at PRE and POST. The black and magenta lines respectively represent individual data from HYI-II and HYIII-IV participants. *, significant difference between PRE and POST (all participants). †, significant difference between HYI-II and HY III-IV at PRE.  , significant difference in PRE to POST change between HYI-II and HY III-IV.
, significant difference in PRE to POST change between HYI-II and HY III-IV.
As expected, we found that HYI-II had a lower PDQ-39-SI compared to HYIII-IV at PRE (10.16 (5.57)% vs. 26.07 (15.15)%, pB = 0.021, r = 0.57, Fig. 2A). This difference was primarily driven by the “Cognition” dimension (HYI-II = 12.50 (12.50)%, HYIII-IV = 34.375 (12.50)%, pB = 0.012, r = 0.61, Fig. 2G), because no other dimension significantly differed at PRE (pB≥0.144, r≤0.41). At POST, neither the PDQ-39-SI (HYI-II = 15.89 (9.13)%, HYIII-IV = 24.80 (18.91)%, pB = 0.40, r = 0.31) nor any of the eight dimensions (pB≥0.096, r≤0.45) significantly differed between groups. Longitudinally, PRE to POST changes in PDQ-39-SI were not significantly different between groups (HYI-II = 3.39 (10.05)%, HYIII-IV = –3.28 (8.12)%, pB = 0.47, r = 0.30). That said, PRE to POST changes in the “Cognition” dimension significantly differed between groups, with HYI-II showing a 6.25 (28.91)% increase and HYIII-IV showing a 9.38 (25)% decrease (pB = 0.048, r = 0.5). PRE to POST changes in the other PDQ-39 dimensions did not significantly differ between HYI-II and HYIII-IV (pB≥0.159, r≤0.40).
4. Discussion
This study retrospectively assessed≥1-year changes in physical functioning and HRQoL in individuals with PD enrolled in a community-based adapted boxing program. Physical functioning was evaluated with the FAB scale, the TUG test, and the 30-STS test, whereas HRQoL was assessed with the PDQ-39. From PRE to POST, participants displayed significant improvements in TUG and 30-STS performance, but no change on the FAB scale. Unsurprisingly, participants rated as HYI-II generally performed better on all physical functioning tests compared to those rated as HYIII-IV. From PRE to POST, our sample also displayed slight, albeit statistically significant, increases in their PDQ-39 scores, with these being significantly higher in HYI-II compared to HYIII-IV at PRE. Overall, these results could be interpreted to suggest that adapted boxing improves functional mobility, muscle strength, and muscle endurance, but is not sufficient to improve balance or counter PD progression-related HRQoL decrements. Upon scrutinizing the data, however, a more nuanced interpretation is required.
On average, our participants displayed statistically significant improvements in TUG and 30-STS performance from PRE to POST, with over half of the participants improving their performance beyond the smallest MDC95 reported for these tests (Lim et al., 2005; Petersen et al., 2017). These results are in line with the results of previous adapted boxing studies in individuals with PD that reported significant improvements on the TUG test (Combs et al., 2013; Moore et al., 2021) and a 15-second variant of the 30-STS test (Horbinski et al., 2021). However, the median improvements on the TUG and 30-STS tests did not surpass their respective MDC95. Regarding the TUG test, a PD-specific ceiling effect may explain the lack of meaningful performance gains. Indeed, the average PRE and POST TUG times in our sample were above the 75th percentile reported for a comparable sample of individuals with PD (Schenkman et al., 2011), which may have left little room for improvement. Regarding the 30-STS test, it should be noted that performance at POST, but not PRE, reached the criterion for maintaining physical independence in community-dwelling older adults (Rikli & Jones, 2013). Hence, despite coming half a repetition short of surpassing the MDC95, it is reasonable to believe that the muscle strength and endurance gained from adapted boxing could help individuals with PD achieve and maintain physical independence. The only physical function variable that did not show a statistically significant improvement with training was balance, which we assessed with the FAB scale. In contrast to our results, Moore et al. (2021) reported that 6 months of adapted boxing both significantly and meaningfully improved FAB scores in their sample of individuals with PD (see also Hermanns et al., 2021). Although the higher weekly frequency of training (3x/week) in their study could explain these discordant findings, it is also possible that meaningful performance gains were difficult to achieve in our study sample due to the relatively high FAB scores at PRE. In fact, our sample’s median FAB score was ∼4 points above that of a comparable sample of individuals with PD (Schlenstedt et al., 2015), with three of our participants achieving the maximal score of 40 at PRE, POST, or both. The idea that a ceiling effect could explain the lack of notable balance improvements echoes the results of Horbinski et al. (2021). In their longitudinal study on the impact of adapted boxing in 98 individuals with PD, these authors could not identify significant improvements on a 30-second balance test unless they removed the data from participants who were able to maintain their balance for the duration of the test. Overall, our results suggest that adaptive boxing is at worse non-detrimental and at best probably beneficial for muscle strength, endurance, and functional mobility in individuals with PD. Whether adapted boxing can meaningfully improve balance may depend on the initial level of balance impairment.
From PRE to POST, we observed a significant ∼4% increase in PDQ-39-SI, suggesting a slight decrease in HRQoL. This appeared to be driven by the “stigma” and “communication” dimensions since these were the only ones to significantly increase from POST to PRE. Much like the changes in TUG and 30-STS performance, the median increase in PDQ-39-SI from PRE to POST fell short of the MDC95, as did the increases in the “stigma” and “communication” dimensions. Consequently, the idea that the HRQoL decreased from PRE to POST in our sample must be taken with a grain of salt. The significant increase in PDQ-39-SI scores is nevertheless surprising because most of the previous studies that investigated HRQoL in individuals with PD partaking in adapted boxing reported significant improvements (or at least a trend for improvement) with training (Combs et al., 2013; Combs et al., 2011; Domingos et al., 2022; Larson et al., 2022; Patel et al., 2023; Sangarapillai et al., 2021). We speculate that our discordant results could be a by-product of the length of our study, coupled with the fact that there were only two, lengthily separated assessment points (i.e., PRE and POST). Indeed, the lack of intermediary assessments precludes us from determining how HRQoL temporally evolved throughout the study. It is entirely plausible, for instance, that HRQoL improved early in training and then slowly dwindled with disease progression, reaching a low point at POST. In this hypothetical scenario, rather than concluding that adapted boxing does not improve HRQoL, we might conclude that adapted boxing helps maintain HRQoL but cannot nullify the impact of disease progression. We had little control over the frequency of assessments in this retrospective study, but future investigators may want to plan multiple assessments, especially in studies where participants are followed over a year or more.
This study has some weaknesses. First, there was no control group, which limits the extent to which we can interpret the results. Take the mild deterioration of HRQoL that we observed from PRE to POST, for instance. A control group of individuals with PD could have displayed a significantly greater worsening of HRQoL over the same period. Had this been the case, the mild deterioration observed in our adaptive boxing sample would have been interpreted as a net positive. Second, the battery of tests did not include the Movement Disorders Society’s Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), the gold standard for the clinical assessment of PD severity (Goetz et al., 2008). The MDS-UPDRS would have provided valuable information regarding disease progression and for data interpretation (e.g., were TUG and 30-STS improvements associated with a reduction of PD-specific clinical signs?). To our knowledge, only two adapted boxing studies used the MDS-UPDRS or UPDRS to evaluate their participants (Combs et al., 2011; Ghaffar et al., 2019; Patel et al., 2023), with two showing statistically significant improvements in motor or non-motor symptoms (Ghaffar et al., 2019; Patel et al., 2023). Although the lack of MDS-UPDRS assessments in this retrospective study constitutes a missed opportunity, it would have been too time-consuming to administer in addition to the physical functioning tests and HRQoL assessments. Third, no intermediary assessments were carried out between PRE and POST. As mentioned above, this precluded us from determining how physical functioning and HRQoL temporally evolved throughout the study. Fourth, this study included only 26 individuals with PD, which limits its inferential power. However, when compared to other studies that investigated the impact of adapted boxing on physical function and HRQoL in individuals with PD (Combs et al., 2011, 2013; Domingos et al., 2022; Ghaffar et al., 2019; Hermanns et al., 2021; Horbinski et al., 2021; Moore et al., 2021; Patel et al., 2023; Sangarapillai et al., 2021; Shearin et al., 2021), the size of our sample is above average on a per-group basis (range: [6, 98], median: 17.5). Fifth, there are a few weaknesses related to the retrospective nature of this study. Retrospective study designs, although valuable, can be plagued by missing data and both recall and selection biases, all of which can confound results (Talari & Goyal, 2020). In the present study, the only outcome variable with missing data was the PDQ-39. Although unfortunate, the PDQ-39 results are still based on the data from 23/26 individuals, which provides ample statistical power to detect effect sizes in the medium range. Given the lack of recall-based outcome measures, recall bias was not an issue in the present study. However, it is possible that our results encompassed some sort of selection bias. Indeed, it must be remembered that all participants willingly enrolled in the local community-based adapted boxing program before the study started. Hence, our sample might have been biased towards individuals who perceived that integrating an exercise program was feasible, perhaps due to relatively high levels of baseline physical function and/or HRQoL. We surmise that this could explain the modest longitudinal changes observed in this study, as individuals with fairly high levels of physical functioning and HRQoL may be less likely to benefit from exercise compared to individuals with low baseline levels of physical functioning and HRQoL. That said, our sample is probably representative of the participants most likely to take part in community-based exercise programs, and thus the results of this study are arguably highly generalizable to other community-based adapted boxing programs. Finally, because our sample attended only ∼58% of bi-weekly sessions, our results may underestimate the true potential of adapted boxing for improving physical functioning and HRQoL in individuals with PD.
This study also has strengths. One is that the retrospective study design allowed us to take full advantage of the longitudinal data (≥1 year) collected at the local at the local community-based adapted boxing program since 2017. To our knowledge, only Horbinski et al. (2021) have investigated the impact of adapted boxing on the physical functioning of individuals with PD over a comparable timespan. Our results generally support those of Horbinski et al. (2021) (i.e., that bi-weekly adapted boxing sessions can likely help patients develop muscular strength and endurance but only trivially improve balance). We also extend their work by showing that adapted boxing may only trivially improve functional mobility and cannot fully counteract the HRQoL decrements that accompany PD progression. Another strength of this study is its ecological validity. Indeed, this study took place in a real-world community-based setting and its retrospective nature ensured that so-called observer expectancy effects were minimized. In this light, we find it alarming that the median attendance rate in our sample was a meagre ∼58% (i.e., ∼1 adapted boxing session/week). This is puzzling given that current evidence indicates that adapted boxing is both feasible and generally appreciated by individuals with PD (Brunet et al., 2022; Domingos et al., 2022; Domingos et al., 2019). We can only speculate as to why attendance was so low in our sample. However, if this is the turnout that can be expected in similar community-based exercise programs, additional efforts are needed to promote physical activity for individuals with PD and improve the accessibility of community-based exercise programs. More longitudinal studies with larger, independent cohorts are needed to firmly establish the impact that community-based adapted boxing can have on individuals with PD.
5. Conclusion
To conclude, our results suggest that adapted boxing may help individuals with PD improve muscular strength, endurance, and functional mobility but not balance. They also suggest that adapted boxing probably cannot fully counteract the HRQoL decrements that accompany PD progression. Notwithstanding the limitations of this study, our findings support the idea that the long-term practice of adapted boxing can help people with PD maintain physical function and HRQoL despite disease progression. Long-term randomized controlled trials are needed to determine the impact of adapted boxing on PD-related signs and symptoms using validated clinical tools such as the MDS-UPDRS.
Acknowledgments
We would like to thank the participants who agreed to share their data for this study.
Conflict of interest
The authors declare no competing interests.
Ethics statement
This study was approved by the CIUSSS de l’Estrie CHUS scientific and ethical review board (IRB #: 2020-3258).
Informed consent
All participants gave their informed and written consent before any data were retrieved from their records.
Funding
This study was financially supported by La Fondation Vitae of the CUISSS de l’Estrie-CHUS.
References
- Amara, A. W., Chahine, L., Seedorff, N., Caspell-Garcia, C. J., Coffey, C., Simuni, T., Parkinson’s Progression Markers, I. (2019) Self-reported physical activity levels and clinical progression in early Parkinson’s disease, Parkinsonism & Related Disorders, 61, 118–125. 10.1016/j.parkreldis.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Balash, Y., Peretz, C., Leibovich, G., Herman, T., Hausdorff, J. M., Giladi, N. (2005) Falls in outpatients with Parkinson’s disease: Frequency, impact and identifying factors, Journal of Neurology, 252(11), 1310–1315. 10.1007/s00415-005-0855-3. [DOI] [PubMed] [Google Scholar]
- Bennie, A., Bruner, K., Dizon, A., Fritz, H., Goodman, B., Petersen, S. (2003) Measurements of Balance: Comparison of the Timed “Up and Go” Test and Functional Reach Test with the Berg Balance Scale, J Phys Ther Sci, 15(2), 93–97. 10.1589/jpts.15.93. [DOI] [Google Scholar]
- Bloem, B. R., Okun, M. S., Klein, C. (2021) Parkinson’s disease, Lancet, 397(10291), 2284–2303. 10.1016/s0140-6736(21)00218-x. [DOI] [PubMed] [Google Scholar]
- Brunet, J., Price, J., Wurz, A., McDonough, M., Nantel, J. (2022) Boxing with Parkinson’s Disease: Findings from a qualitative study using self-determination theory, Disability and Rehabilitation, 44(15), 3880–3889. 10.1080/09638288.2021.1891465. [DOI] [PubMed] [Google Scholar]
- Brusse, K. J., Zimdars, S., Zalewski, K. R., Steffen, T. M. (2005) Testing functional performance in people with Parkinson disease, Physical Therapy, 85(2), 134–141. https://www.ncbi.nlm.nih.gov/pubmed/15679464. [PubMed] [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). L. Erlbaum Associates. Publisher descriptionhttp://www.loc.gov/catdir/enhancements/fy0731/88012110-d.html.
- Collaborators, G. B. D. P. s. D. (2018) Global, regional, and national burden of Parkinson’s disease,: A systematic analysis for the Global Burden of Disease Study 2016, Lancet Neurology, 17(11), 939–953. 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs-Miller, S. A., Moore, E. S. (2019) Predictors of outcomes in exercisers with Parkinson disease: A two-year longitudinal cohort study, NeuroRehabilitation, 44(3), 425–432. 10.3233/NRE-182641. [DOI] [PubMed] [Google Scholar]
- Combs, S. A., Diehl, M. D., Chrzastowski, C., Didrick, N., McCoin, B., Mox, N., Staples, W. H., Wayman, J. (2013) Community-based group exercise for persons with Parkinson disease: A randomized controlled trial, NeuroRehabilitation, 32(1), 117–124. 10.3233/NRE-130828. [DOI] [PubMed] [Google Scholar]
- Combs, S. A., Diehl, M. D., Staples, W. H., Conn, L., Davis, K., Lewis, N., Schaneman, K. (2011) Boxing training for patients with Parkinson disease: A case series, Physical Therapy, 91(1), 132–142. 10.2522/ptj.20100142. [DOI] [PubMed] [Google Scholar]
- Connolly, B. S., Lang, A. E. (2014) Pharmacological treatment of Parkinson disease: A review, JAMA, 311(16), 1670–1683. 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- Corcos, D. M., Robichaud, J. A., David, F. J., Leurgans, S. E., Vaillancourt, D. E., Poon, C., Rafferty, M. R., Kohrt, W. M., Comella, C. L. (2013) A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease, Movement Disorders, 28(9), 1230–1240. 10.1002/mds.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Bello-Haas, V., Klassen, L., Sheppard, M. S., Metcalfe, A. (2011) Psychometric Properties of Activity, Self-Efficacy, and Quality-of-Life Measures in Individuals with Parkinson Disease, Physiotherapy Canada, 63(1), 47–57. 10.3138/ptc.2009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingos, J., de Lima, A. L. S., Steenbakkers-van der Pol, T., Godinho, C., Bloem, B. R., de Vries, N. M. (2022) Boxing with and without Kicking Techniques for People with Parkinson’s Disease: An Explorative Pilot Randomized Controlled Trial, Journal of Parkinson’s Disease, 12(8), 2585–2593. 10.3233/JPD-223447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingos, J., Radder, D., Riggare, S., Godinho, C., Dean, J., Graziano, M., de Vries, N. M., Ferreira, J., Bloem, B. R. (2019) Implementation of a Community-Based Exercise Program for Parkinson Patients: Using Boxing as an Example, Journal of Parkinson’s Disease, 9(3), 615–623. 10.3233/JPD-191616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey, E. R., Sherer, T., Okun, M. S., Bloem, B. R. (2018) The Emerging Evidence of the Parkinson Pandemic. S-S, Journal of Parkinson’s Disease, 8(s1), 8. 10.3233/JPD-181474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul, F., Erdfelder, E., Buchner, A., Lang, A. G. (2009) Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses, Behavior Research Methods, 41(4), 1149–1160. 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Field, A. P. (2009) Discovering statistics using SPSS: (and sex, drugs and rock ‘n’ roll) (3rd ed.) SAGE Publications.
- Fitzpatrick, R., Norquist, J. M., Jenkinson, C. (2004) Distribution-based criteria for change in health-related quality of life in Parkinson’s disease, Journal of Clinical Epidemiology, 57(1), 40–44. 10.1016/j.jclinepi.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Ghaffar, A., Gallagher, R., Rubin, L., Dharia, S., Ketigian, L., Whalen, J., Scheid, Z., Patel, K., Zhu, J., Michaelides, C., Leder, A. (2019) Effect of non-contact boxing on non-motor symptoms in Parkinson’s disease, Movement Disorders, 34. https://www.mdsabstracts.org/abstract/effect-of-non-contact-boxing-on-non-motor-symptoms-in-parkinsons-disease/.Accessed October 18, 2023. [Google Scholar]
- Goetz, C. G., Tilley, B. C., Shaftman, S. R., Stebbins, G. T., Fahn, S., Martinez-Martin, P., Poewe, W., Sampaio, C., Stern, M. B., Dodel, R., Dubois, B., Holloway, R., Jankovic, J., Kulisevsky, J., Lang, A. E., Lees, A., Leurgans, S., LeWitt, P. A., Nyenhuis, D., Movement Disorder Society, U. R. T. F. (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results, Movement Disorders, 23(15), 2129–2170. 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Haley, S. M., Fragala-Pinkham, M. A. (2006) Interpreting change scores of tests and measures used in physical therapy, Physical Therapy, 86(5), 735–743. https://www.ncbi.nlm.nih.gov/pubmed/16649896. [PubMed] [Google Scholar]
- Hermanns, M., Mastel-Smith, B., Donnell, R., Quarles, A., Rodriguez, M., Wang, T. (2021) Counterpunching to improve the health of people with Parkinson’s disease, Journal of the American Association of Nurse Practitioners, 33(12), 1230–1239. 10.1097/JXX.0000000000000598. [DOI] [PubMed] [Google Scholar]
- Horbinski, C., Zumpf, K. B., McCortney, K., Eoannou, D. (2021) Longitudinal observational study of boxing therapy in Parkinson’s disease, including adverse impacts of the COVID-19 lockdown, BMC Neurology, 21(1), 326. 10.1186/s12883-021-02359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S. L., Hsieh, C. L., Wu, R. M., Tai, C. H., Lin, C. H., Lu, W. S. (2011) Minimal detectable change of the timed “up & go” test and the dynamic gait index in people with Parkinson disease, Physical Therapy, 91(1), 114–121. 10.2522/ptj.20090126. [DOI] [PubMed] [Google Scholar]
- Jenkinson, C., Fitzpatrick, R., Peto, V., Greenhall, R., Hyman, N. (1997) The Parkinson’s Disease Questionnaire (PDQ-39): Development and validation of a Parkinson’s disease summary index score, Age and Ageing, 26(5), 353–357. 10.1093/ageing/26.5.353. [DOI] [PubMed] [Google Scholar]
- Jones, C. J., Rikli, R. E., Beam, W. C. (1999) A 30-s chair-stand test as a measure of lower body strength in community-residing older adults, Research Quarterly for Exercise and Sport, 70(2), 113–119. 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- King, L. A., Horak, F. B. (2009) Delaying mobility disability in people with Parkinson disease using a sensorimotor agility exercise program, Physical Therapy, 89(4), 384–393. 10.2522/ptj.20080214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, P. J., Fiedler, R. C., Rose, D. J. (2011) Rasch Analysis of the Fullerton Advanced Balance (FAB) Scale, Physiotherapy Canada, 63(1), 115–125. 10.3138/ptc.2009-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, D., Yeh, C., Rafferty, M., Bega, D. (2022) High satisfaction and improved quality of life with Rock Steady Boxing in Parkinson’s disease: Results of a large-scale survey, Disability and Rehabilitation, 44(20), 6034–6041. 10.1080/09638288.2021.1963854. [DOI] [PubMed] [Google Scholar]
- Liguori, G. (2021). American College of Sports Medicine’s Guidelines for Exercise Testing and Prescription (G. Liguori, Ed. 11th ed.). Lippincott Williams & Wilkins | Wolters Kluwer.
- Lim, L. I., van Wegen, E. E., de Goede, C. J., Jones, D., Rochester, L., Hetherington, V., Nieuwboer, A., Willems, A. M., Kwakkel, G. (2005) Measuring gait and gait-related activities in Parkinson’s patients own home environment: A reliability, responsiveness and feasibility study, Parkinsonism & Related Disorders, 11(1), 19–24. 10.1016/j.parkreldis.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Mak, M. K., Pang, M. Y. (2009) Balance confidence and functional mobility are independently associated with falls in people with Parkinson’s disease, Journal of Neurology, 256(5), 742–749. 10.1007/s00415-009-5007-8. [DOI] [PubMed] [Google Scholar]
- McCambridge, J., Kypri, K., Elbourne, D. (2014) Research participation effects: A skeleton in the methodological cupboard, Journal of Clinical Epidemiology, 67(8), 845–849. 10.1016/j.jclinepi.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, A., Yee, E., Willis, B. W., Prost, E. L., Gray, A. D., Mann, J. B. (2021) A Community-based Boxing Program is Associated with Improved Balance in Individuals with Parkinson’s Disease, Int J Exerc Sci, 14(3), 876–884. https://www.ncbi.nlm.nih.gov/pubmed/35096235. [PMC free article] [PubMed] [Google Scholar]
- Morris, S., Morris, M. E., Iansek, R. (2001) Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease, Physical Therapy, 81(2), 810–818. 10.1093/ptj/81.2.810. [DOI] [PubMed] [Google Scholar]
- Oguh, O., Eisenstein, A., Kwasny, M., Simuni, T. (2014) Back to the basics: Regular exercise matters in parkinson’s disease: Results from the National Parkinson Foundation QII registry study, Parkinsonism & Related Disorders, 20(11), 1221–1225. 10.1016/j.parkreldis.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Patel, R. A., Blasucci, L., Mahajan, A. (2023) A pilot study of a 12-week community-based boxing program for Parkinson’s disease, Journal of Clinical Neuroscience, 107, 64–67. 10.1016/j.jocn.2022.12.006. [DOI] [PubMed] [Google Scholar]
- Paul, K. C., Chuang, Y. H., Shih, I. F., Keener, A., Bordelon, Y., Bronstein, J. M., Ritz, B. (2019) The association between lifestyle factors and Parkinson’s disease progression and mortality, Movement Disorders, 34(1), 58–66. 10.1002/mds.27577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, C., Steffen, T., Paly, E., Dvorak, L., Nelson, R. (2017) Reliability and Minimal Detectable Change for Sit-to-Stand Tests and the Functional Gait Assessment for Individuals With Parkinson Disease, Journal of Geriatric Physical Therapy, 40(4), 223–226. 10.1519/JPT.0000000000000102. [DOI] [PubMed] [Google Scholar]
- Poewe, W., Seppi, K., Tanner, C. M., Halliday, G. M., Brundin, P., Volkmann, J., Schrag, A. E., Lang, A. E. (2017) Parkinson disease, Nat Rev Dis Primers, 3, 17013. 10.1038/nrd2017.13. [DOI] [PubMed] [Google Scholar]
- Prodoehl, J., Rafferty, M. R., David, F. J., Poon, C., Vaillancourt, D. E., Comella, C. L., Leurgans, S. E., Kohrt, W. M., Corcos, D. M., Robichaud, J. A. (2015) Two-year exercise program improves physical function in Parkinson’s disease: The PRET-PD randomized clinical trial, Neurorehabilitation and Neural Repair, 29(2), 112–122. 10.1177/1545968314539732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafferty, M. R., Schmidt, P. N., Luo, S. T., Li, K., Marras, C., Davis, T. L., Guttman, M., Cubillos, F., Simuni, T., all, N. P. F. Q. I. I. I. (2017) Regular Exercise, Quality of Life, and Mobility in Parkinson’s Disease: A Longitudinal Analysis of National Parkinson Foundation Quality Improvement Initiative Data, Journal of Parkinson’s Disease, 7(1), 193–202. 10.3233/JPD-160912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikli, R. E., Jones, C. J. (2013) Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years, Gerontologist, 53(2), 255–267. 10.1093/geront/gns071. [DOI] [PubMed] [Google Scholar]
- Sangarapillai, K., Norman, B. M., Almeida, Q. J. (2021) Boxing vs Sensory Exercise for Parkinson’s Disease: A Double-Blinded Randomized Controlled Trial, Neurorehabilitation and Neural Repair, 35(9), 769–777. 10.1177/15459683211023197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman, M., Ellis, T., Christiansen, C., Baron, A. E., Tickle-Degnen, L., Hall, D. A., Wagenaar, R. (2011) Profile of functional limitations and task performance among people with early- and middle-stage Parkinson disease, Physical Therapy, 91(9), 1339–1354. 10.2522/ptj.20100236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman, M., Hall, D. A., Baron, A. E., Schwartz, R. S., Mettler, P., Kohrt, W. M. (2012) Exercise for people in early- or mid-stage Parkinson disease: A 16-month randomized controlled trial, Physical Therapy, 92(11), 1395–1410. 10.2522/ptj.20110472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman, M., Moore, C. G., Kohrt, W. M., Hall, D. A., Delitto, A., Comella, C. L., Josbeno, D. A., Christiansen, C. L., Berman, B. D., Kluger, B. M., Melanson, E. L., Jain, S., Robichaud, J. A., Poon, C., Corcos, D. M. (2018) Effect of High-Intensity Treadmill Exercise on Motor Symptoms in Patients With De Novo Parkinson Disease: A Phase 2 Randomized Clinical Trial, JAMA Neurology, 75(2), 219–226. 10.1001/jamaneurol.2017.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenstedt, C., Brombacher, S., Hartwigsen, G., Weisser, B., Moller, B., Deuschl, G. (2015) Comparing the Fullerton Advanced Balance Scale with the Mini-BESTest and Berg Balance Scale to assess postural control in patients with Parkinson disease, Archives of Physical Medicine and Rehabilitation, 96(2), 218–225. 10.1016/j.apmr.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Shearin, S., Braitsch, M., Querry, R. (2021) The effect of a multi-modal boxing exercise program on cognitive locomotor tasks and gait in persons with Parkinson disease, NeuroRehabilitation, 49(4), 619–627. 10.3233/NRE-210218. [DOI] [PubMed] [Google Scholar]
- Shulman, L. M., Katzel, L. I., Ivey, F. M., Sorkin, J. D., Favors, K., Anderson, K. E., Smith, B. A., Reich, S. G., Weiner, W. J., Macko, R. F. (2013) Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease, JAMA Neurology, 70(2), 183–190. 10.1001/jamaneurol.2013.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen, T., Seney, M. (2008) Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism, Physical Therapy, 88(6), 733–746. 10.2522/ptj.20070214. [DOI] [PubMed] [Google Scholar]
- Talari, K., Goyal, M. (2020) Retrospective studies - utility and caveats, Journal of the Royal College of Physicians of Edinburgh, 50(4), 398–402. 10.4997/JRCPE.2020.409. [DOI] [PubMed] [Google Scholar]
- Uc, E. Y., Doerschug, K. C., Magnotta, V., Dawson, J. D., Thomsen, T. R., Kline, J. N., Rizzo, M., Newman, S. R., Mehta, S., Grabowski, T. J., Bruss, J., Blanchette, D. R., Anderson, S. W., Voss, M. W., Kramer, A. F., Darling, W. G. (2014) Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting, Neurology, 83(5), 413–425. 10.1212/WNL.0000000000000644. [DOI] [PMC free article] [PubMed] [Google Scholar]