Abstract
Parkinson’s disease is the world’s fastest growing brain disorder, and exposure to environmental toxicants is the principal reason. In this paper, we consider alternative, but unsatisfactory, explanations for its rise, including improved diagnostic skills, aging populations, and genetic causes. We then detail three environmental toxicants that are likely among the main causes of Parkinson’s disease— certain pesticides, the solvent trichloroethylene, and air pollution. All three environmental toxicants are ubiquitous, many affect mitochondrial functioning, and all can access humans via various routes, including inhalation and ingestion. We reach the hopeful conclusion that most of Parkinson’s disease is thus preventable and that we can help to create a world where Parkinson’s disease is increasingly rare.
Keywords: Parkinson disease, pesticides, solvents, tetrachloroethylene, trichloroethylene, water pollution, indoor air pollution, mitochondria, genetics, primary prevention
THE RISE OF PARKINSON’s DISEASE— AND POSSIBLE EXPLANATIONS
In 1817, Dr. James Parkinson described six individuals with what he considered a novel condition [1]. Two centuries later, over six million people were estimated to have Parkinson’s disease (PD). Moreover, the Global Burden of Disease study found that PD was the world’s fastest growing neurological disorder in terms of prevalence [2], a finding that has largely held in more recent studies [3]. Why is this rise taking place? In this paper, we argue it is principally due to environmental factors.
Improved diagnosis?
A common response to the rise is that awareness of PD has increased and that our ability to diagnose it has improved over time. While these assertions may be true, they would only explain the relative rise of PD if they were more true of PD than other neurological disorders, such as multiple sclerosis and stroke. However, the opposite is likely the case. Advances in neuroimaging have greatly enhanced our ability to diagnose individuals with these two latter conditions, often at an earlier stage of disease [3, 4]. Yet, neither has grown nearly as rapidly as PD [3]. Moreover, PD is often missed and thus, the true burden is underestimated. Door-to-door studies suggest that anywhere from 12% (Rotterdam, The Netherlands) to 48% (Beijing) to 100% (rural Bolivia) of individuals with the disease are undiagnosed [4].
Aging?
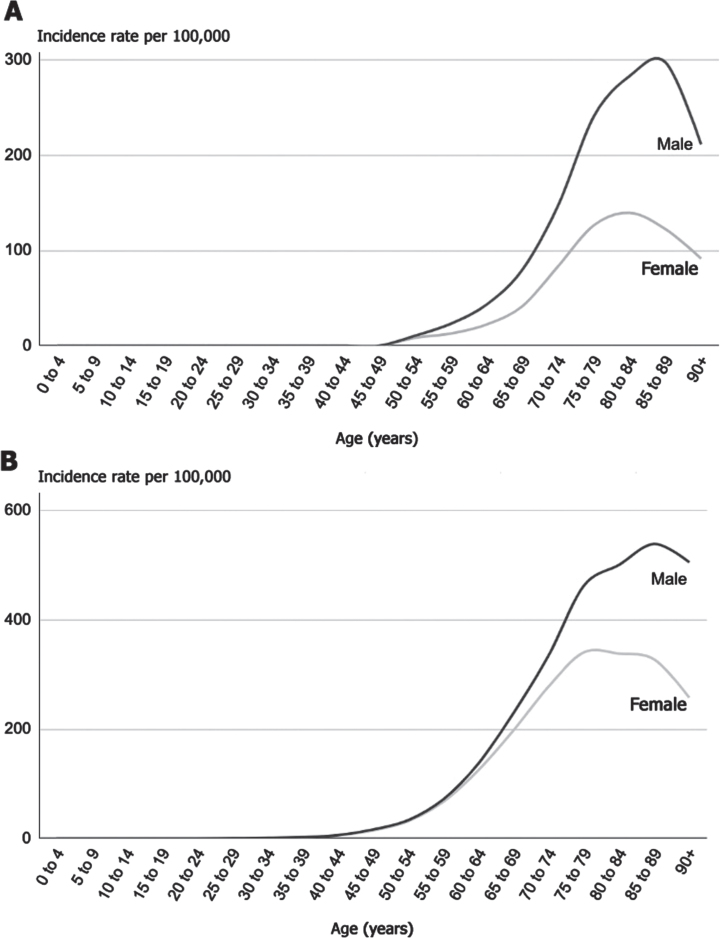
A second explanation commonly offered is that the rise of PD is due to aging. Indeed, the incidence of PD (Fig. 1A) begins to rise in individuals roughly in their 50 s and then doubles or triples every decade thereafter until at least the ninth decade of life. However, the rates of PD have risen in both absolute terms and, when adjusted for age [5]. By contrast, the global burden of Alzheimer’s disease has more than doubled in absolute terms from 1990 to 2016 but, adjusted for age, has changed little [3]. So aging alone cannot explain the rise in PD.
Fig. 1.
A) Incidence of Parkinson’s disease by age and sex in the United Kingdom, 2017 [16]. B) Incidence of lung cancer by age and sex in the United Kingdom, 2016–2018 [17].
More importantly, there is hardly any evidence that aging per se is a cause of PD. Aging, though, comes with a time-related decline in physiological functions [6], and these changes could contribute to PD. For example, aging is associated with reduced protein clearance [7] and lysosomal impairment [8], tasks that may be relevant to PD and other neurodegenerative conditions.
Longevity, the length of life, is critical to PD [9]. PD, like heart disease and many cancers, is an age-related disorder. A comparison to lung cancer is illuminating. The increased incidence of PD with age is almost identical to that of lung cancer (Fig. 1B) [10]. Few would argue that lung cancer is due to aging. Indeed, prior to the advent of cigarettes in the early 20th century, lung cancer was so rare that when confronted with a case, doctors considered it “a once-in-a-lifetime oddity” [10]. However, twenty-five years after the introduction of cigarettes, the rates of lung cancer began to rise, in an age-related manner [10]. Longevity allows for both sufficient exposure to toxicants (e.g., enough pack-years to cause a malignancy) and time to pass for the disease to become manifest. Indeed, a long life may be a prerequisite to a diagnosis of lung cancer and PD as the pathological processes underlying both disorders take years, if not decades, to unfold [11].
PD and lung cancer share another similarity (Fig. 1). In most studies, both are more common in men [12]. Sociology, rather than biology, may be the reason. Historically, men have smoked more often than women; however, as the gender differences in smoking have diminished [13], the incidence of lung cancer in women has risen. In the case of PD, men in many countries are more likely to be engaged in work that exposes them to environmental toxicants, such as pesticides. However, in a few countries, such as Japan, PD is more common in women [14], and there, the majority of farmers historically have been women [15].
Genetics?
The third explanation is that genetic mutations are the principal cause of PD. Genetic factors do contribute to the etiology of PD, as Professors Shen-Yang Lim and Christine Klein detail well in a companion paper in this journal [18]. However, purely genetic causes of PD are rare (Table 1) [19], and its heritability (estimated at 0.34 from twin studies) is low [20]. We have known since Sir William Gowers’s case series of PD in 1903 that only about 15% of individuals with the condition have a family history of the disease [21]. A century later, Tanner and colleagues examined PD in twins and found similar rates in monozygotic and dizygotic twins leading to the conclusion that “genetic factors do not play a major role in causing typical PD” [22]. Compared to other conditions where heritability was also studied in twins, PD is near the bottom in terms of genetic contribution [20].
Table 1.
Genetic causes of Parkinson’s disease, and their interactions with environmental toxicants [19]
| GENES LINKED TO | FREQUENCY IN | ENVIRONMENTAL | |
| PARKINSON’S | PARKINSON’S | INTERACTIONS | |

| |||
| SNCA | < < 1% | Paraquat, rotenone | |
| LRRK2 | ∼2-3% | Paraquat | |
| VPS35 | < 1% | Rotenone | |

| |||
| Parkin | ∼1% | Paraquat, rotenone | |
| PINK1 | < 1% | Manganese | |
| DJ-1 | < < 1% | Rotenone | |
| ATP13A2, PLA2G6, PARK9, FBX07 | < < 1% | Manganese | |

| |||
| GBA | 5–14% | MPTP |
Purely genetic causes of PD account for about 2% of individuals with the disease. Even “common” genetic causes, such as LRRK2 mutations, are present in only 2–3% of individuals and have incomplete penetrance (25 to 43%) [23]. Other factors, including environmental ones, must be necessary for the disease to develop [24]. Indeed, rather than causing the disease, the principal role of genetics is likely to help determine (along with other environmental factors [25]) who among the exposed is likely to develop the disease. The latter contention is supported by experimental work showing that almost all the principal genetic causes and risk factors tied to PD have known interactions with environmental toxicants (Table 1). In other words, the underlying genetic predisposition likely enhances the risk of nigrostriatal damage following exposure to toxic chemicals.
In addition, variability in the genes encoding for proteins that metabolize some notable toxicants, especially pesticides [26], may be critical in explaining who develops PD and who does not. Indeed, this gene-environment interaction might well explain why some of the older mutations only became relevant when humans began producing certain toxicants. For example, genetic variants in GBA (penetrance 10–20% [27]), encoding the lysosomal enzyme glucocerebrosidase, are common risk factors for PD today. However, the founder effects for these genetic variants are likely thousands of years old [28] with little contribution to PD prior to the 19th century.
Finally, genetic differences also cannot readily explain the near perfect correlation between use of pesticides and PD prevalence [29], the strong association between agricultural activities and its incidence [30], the 10-fold variation in prevalence within countries [31], or the five-fold difference in age-adjusted prevalence between countries [5]. Nor do they account for the finding that the rates of PD are generally highest in industrialized nations like Canada and the U.S., lowest in less industrialized regions, and rising most rapidly in industrializing nations like India and China [5]. In short, while additional (poly- or epi-) genetic causes of the disease remain to be identified, a century of studies suggest that the overall heritability of PD is low [20].
THE PRINCIPAL CAUSES OF PARKINSON’s DISEASE
The main causes of PD are environmental toxicants. Chief among these are certain pesticides, industrial solvents like trichloroethylene (TCE), and air pollution. These are not the only toxicants tied to PD [32], and more may be found. However, these are all linked to PD by both epidemiological and pre-clinical studies, many damage mitochondria (which are known to be impaired in PD [33]), and they all expose humans through multiple routes (including inhalation and ingestion) and various means (occupational and environmental).
Pesticides
Pesticides are the environmental toxicants with the clearest relationship to PD. Numerous epidemiological and animal studies argue for a causal relationship between certain pesticides and PD. An important clue came in the 1980s when seven young adults developed end-stage parkinsonism sub-acutely after intravenous injection of the designer drug 1-methyl-4-phenyl-l,2,3,6-tetrahydropyridine (MPTP) [34]. Damage to the nigrostriatal dopaminergic neurons occurs after MPTP is converted to its metabolite 1-methyl-4-phenylpyridinium (MPP+), which kills dopaminergic neurons through inhibition of mitochondrial complex 1. MPP+ shows a striking structural resemblance to paraquat, one of the world’s most widely used weedkillers which also impairs mitochondrial function [35].
Later epidemiological work further suggested a link between PD and prior exposure to pesticides. For example, Barbeau and colleagues found that the correlation between the prevalence of PD and the use of pesticides in nine areas of Quebec, Canada, was 0.967 [29]. Since then, similar studies have been reported in the U.S. [36], Israel [37], and France [30]. In 2011, Tanner and colleagues found an association between PD and use of the pesticides rotenone (odds ratio 2.5) and paraquat (odds ratio 2.5), both of which cause mitochondrial dysfunction [38].
Importantly, the risk of PD is not confined to those working professionally with pesticides. Those living near their application are also at risk. For example, in France the increased risk of developing PD is not limited to farmers [39, 40] but extends to those living near farmland or vineyards [30]. Pesticide exposure may also lead to faster disease progression after the diagnosis has been established [41, 42].
Numerous laboratory studies support these epidemiological findings. For example, rotenone inhibits complex I of the respiratory chain and causes nigrostriatal dopaminergic degeneration in rats, along with visible signs of bradykinesia and rigidity [43]. Similar findings have been demonstrated with paraquat [44]. Today, several animal models of PD are based on exposure to pesticides and other neurotoxicants [45].
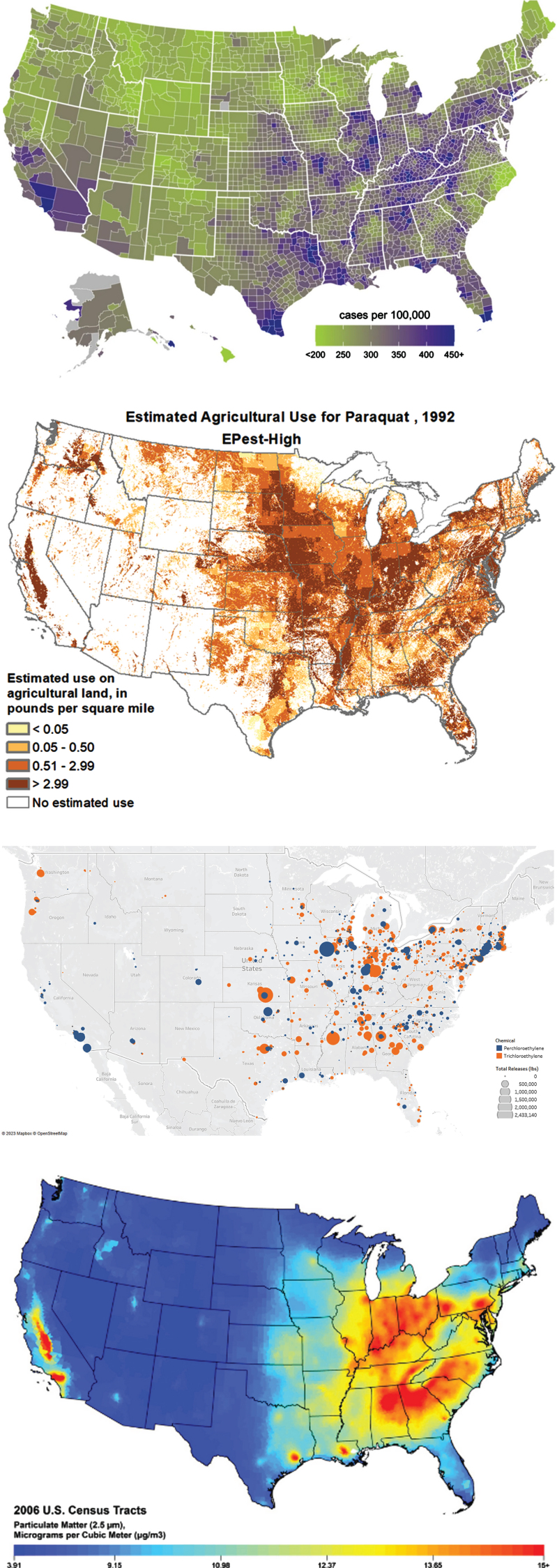
Several pesticides that have been linked to PD (e.g., paraquat, rotenone, heptachlor) are now banned in many countries, but major concerns remain. Some of these toxic pesticides continue to be used in many parts of the world including, as Professor Shen-Yang Lim and colleagues have reported, in the Western Pacific [46]. Paraquat is still marketed in Argentina, Australia, Japan, India and the U.S. [47], where its use has more than doubled from 2013 to 2018 [48]. A visual inspection of the use of paraquat in the U.S. from 1992 [48] and the incidence of PD is concerning (Fig. 2A, B).
Fig. 2.
A) Incidence of Parkinson’s disease in the U.S., 2012 [49]. B) Estimated annual use of paraquat in the U.S., 1992 [50]. Map of release of trichloroethylene (orange) and perchloroethylene (in blue) in the U.S, 1987. Map courtesy of Meghan Pawlik, University Rochester, based on data from the Toxic Release Inventory from the U.S. Environmental Protection Agency. D) Map of particulate matter smaller than 2.5 microns in the U.S., 2006 [51].
In addition, serious deficiencies in current regulatory policies prevent adequate assessment of currently used pesticides when it comes the risk of PD and other neurodegenerative disorders. Our concerns are detailed elsewhere [52], but here we highlight three major drawbacks. First, current screens for neurotoxicity depend on the manifestation of parkinsonism in exposed animals but frequently ignore relevant nigrostriatal neuronal loss which often precedes clinically discernible signs. This limitation had been long acknowledged by Parkinson’s experts and is now recognized by the European Food Safety Authority [53].
Second, many safety studies have been performed by the industry itself, which may not have been fully transparent about the health risks of their products. A recent investigative report by the British newspaper The Guardian reviewed legal documents that paraquat’s manufacturer submitted in response to lawsuits from individuals with PD. According to the reporters, the manufacturer had conducted research showing that rats and mice exposed to very high doses of the pesticide displayed a stiff gait or tremors. The studies were conducted in the 1960s [47]. So toxic is the chemical that regulators in 1974 expressed concern about workers “who might inadvertently lick small quantities of paraquat residue off lips, or inhale paraquat mist” [47]. The company was also concerned in 2009 that the scientific community would “conclude from the laboratory and epidemiology data that paraquat exposure is a causal factor (its emphasis) in Parkinson’s Disease or parkinsonism” [47]. Similarly, the manufacturers of glyphosate, another weed killer, withheld a relevant study from regulators which provided evidence of developmental neurotoxicity in young rats [54]. Despite the evidence linking certain pesticides to PD and other diseases (e.g., amyotrophic lateral sclerosis [55]), their use persists. To limit exposure to these toxicants requires greater awareness of industry practices employed to manufacture doubt and accountability for its actions [56–59].
Third, farmers, rural residents, and ultimately almost all of us are exposed to mixtures of multiple pesticides. Such mixtures are either used by farmers or arise from environmental contamination. For example, following use on crops, glyphosate is carried across long distances via the air, and in Germany, is found in measurable levels across the entire country, including in the most protected natural areas [60]. The large international SPRINT stud (Sustainable Plant Protection Transition) identified high concentrations of numerous pesticides, particularly in dust in farmhouses [61]. Multiple pesticides were also found in blood and stool samples of both farmers and people living near farmlands. Due to cumulative or possibly even synergistic effects, such cocktails may contribute to a much higher risk of developing PD. A recent study in California suggests that co-exposure to multiple pesticides is associated with much greater damage to dopaminergic neurons than exposure to any single compound [62], yet current regulatory actions only consider the neurotoxicity of isolated compounds.
The result of these shortcomings is that few of the commonly used pesticides have been adequately tested for their risk of PD. Improved neurotoxicity studies that focus specifically on the risk of PD and evaluate different combinations are urgently needed. And such experiments should be performed by independent researchers and not only by the industry itself.
Trichloroethylene
Pesticides may account for PD in rural areas but are unlikely to be a satisfactory explanation for the high rates of the disease in urban areas [38]. Other environmental toxicants are thus likely to be responsible as well. One may be the ubiquitous chemical, trichloroethylene or TCE [63]. However, despite its widespread use and broad contamination, few are aware that they have ever been exposed [64]. The result is that its influence on PD is likely (vastly) underappreciated.
First created in 1864, use of TCE began in the 1920s, and it has since found a myriad of military, industrial and commercial applications including degreasing metals, decaffeinating coffee, and dry cleaning clothes [63]. At one point, one out of every 12 workers in the U.K. and 10 million Americans are estimated to have worked with the volatile solvent [65]. In Italy, a population study found TCE in the urine of 75% of individuals [66]. TCE is a known carcinogen, and its toxic effects have been known since at least 1932 [67]. Like many pesticides, TCE is a mitochondrial toxicant that inhibits complex I of the respiratory chain [63]. In addition, recent research has found that like certain LRRK2 mutations, TCE induces LRRK2 kinase activity in the brains of rats and causes nigrostriatal dopaminergic degeneration [68].
Since 1969, case reports have linked TCE exposure to parkinsonism or PD [65, 69–71]. In 2008, Gash and colleagues found that among 30 factory workers, three developed PD after using TCE for many years to degrease and clean metal gauges. These three individuals were stationed closest to an open vat of TCE, and 14 of 27 individuals who worked further from the source “displayed many features of parkinsonism, including significant motor slowing” [72]. Four years later, Goldman and colleagues [73] found that the risk of PD among individuals who had hobby or work exposure to the solvent was 500% higher than their twins who did not. The lag time between exposure and PD diagnosis was 10 to 40 years [73]. Gash [72] and others [69, 74–76] have found that the lipophilic compound causes selective loss of dopaminergic neurons in animal studies.
Exposure through work and consumer applications (including in carpet cleaners, paint removers, and typewriter correction fluid [77]) may just be the beginning. Environmental exposure to the chemical through outdoor air, contaminated ground water, and polluted indoor air may be responsible for the bulk of exposures, most of which is invisible to individuals [63]. One of the most glaring examples of TCE (and a closely related chemical, perchloroethylene [63], with likely similar toxicity [73]) contamination is the U.S. Marine base Camp Lejeune, where the drinking water was contaminated with the two chemicals for thirty years at up to 3000 times those permitted by safety standards [4, 78]. A recent study by Goldman and colleagues found that individuals who served at Camp Lejeune had a 70% increased risk of PD compared to those who served at a much less polluted base [79].
However, TCE contamination does not end with water. Beginning in the 1970s, researchers began to realize that the volatile TCE could evaporate from contaminated water and enter people’s workplaces, schools, and homes, usually undetected, through a process called vapor intrusion [63]. Today, a school in Shanghai, China [80], and multi-million dollar homes in Newport Beach, California [81], sit on top of contaminated sites, and their occupants may be subject to the adverse health effect of inhaling this toxicant. The number of TCE-contaminated sites globally are too numerous to count; the state of Michigan alone is estimated to have thousands [82]. Figure 2C shows a map of U.S. release of TCE and other chlorinated solvents into the air from 1987 (the first year for which data are available [83]) and the incidence of PD. The U.S. Environmental Protection Agency just recently proposed a ban [84] on the known carcinogen, an action some European countries have already taken. However, global use, especially in China, is increasing [85].
Air pollution
Concerns about the health effects of outdoor air pollution date to at least the time of Hippocrates, around 400 BCE, who linked multiple ailments to poor air quality [86]. The Roman philosopher Seneca noted that escaping from the polluted air of Rome around 64 CE resolved the “sluggishness in [his] brain” [86]. However, industrial air pollution first took hold in London. There, in 1661, air pollution from burning coal to power steam engines captured the attention of the British writer John Evelyn when he wrote that the glorious city wrapped “her stately head in clouds of smoke and sulphur, so full of stink and darkness” [86]. When Dr. James Parkinson wrote An Essay on the Shaking Palsy in 1817, the people he described would have long been exposed to the toxic effects of the infamous London fog [87].
However, the potential link between air pollution and PD is only now beginning to become clear [88]. The source of air pollution varies by time (open fires were the likely concern of Hippocrates) and place (coal burning in London, car exhaust in Los Angeles, wildfires in Canada). In 2008, Calderón-Garcidueñas and colleagues sought to evaluate the effects of air pollution in Mexico City, which had the world’s dirtiest air in the 1990s. To do so, she examined the brains of children and young adults (ages 11 months to 40 years) who died suddenly [89]. She found Lewy neurites in the olfactory bulb of toddlers, which were present in 68% of children in their second decade of life [90] and alpha-synuclein immunoreactivity in their substantia nigra [91]. Since that time, several studies have linked traffic-related air pollution to an increased risk of PD in Denmark [92], Taiwan [93], and South Korea [94]. Given the high content of particulate matter from wildfires, their effects on PD requires further investigation [95–97].
Tied to these pathological findings may be deficits in the sense of smell [98], which is a common feature of PD that typically antedates the onset of the motor syndrome by many years. It is possible that this hyposmia is a remnant of the toxic effects of air pollutants that damage the olfactory system early in the disease process, while on their way to cause further harm to the brain. The aforementioned observations on the individuals with MPTP-induced parkinsonism provide further fascinating evidence to support this idea. Despite having severe and irreversible clinical parkinsonism, the smell in six MPTP patients was normal, noting that the entry route for the neurotoxin had been an intravenous one [99]. However, the chemist who had manufactured this designer drug developed parkinsonism several years later even though he had never used MPTP intravenously (J.W. Langston, personal communication). His parkinsonism was presumably related to exposure to lower MPTP doses via inhalation. Unfortunately, his sense of smell was never formally tested.
The reason for the relationship between air pollution and PD is uncertain, but it may be particulate matter. Suspended in the smog visible in large urban areas are tiny particles of dirt and soot. The smallest of these (measured in microns or even nanometers) can bypass the body’s normal protective mechanisms and enter noses, airways, and lungs [100, 101]. Hitchhiking on these particles are heavy metals from cars (e.g., lead from gasoline, iron from brakes, platinum from catalytic converters) and industry (e.g., arsenic, manganese, mercury) that are toxic to neurons [102]. Recent research has found that fine particulate matter (smaller than 2.5 microns) in transgenic mice causes mitochondrial dysfunction, triggers the fibrillization of alpha-synuclein, and leads to the formation of alpha-synuclein fibrils [103]. Figures 2A and 2D depict the U.S. incidence of PD [49] and a map of particulate air pollution. The relationship remains to be quantified, and numerous factors, including the appropriate time lag between exposure and incidence and effects of migration, need to be addressed. Nonetheless, on the surface, the maps and similar ones [104] are concerning about a possible relationship, which requires further investigation.
COMMON CHARACTERISTICS
Air pollution, certain pesticides, and TCE share some important characteristics. First, many are mitochondrial toxicants. The brain is a highly energy-dependent organ [105]. While it accounts for only 3% of human body mass, it is responsible for over 20% of oxygen metabolism. Within the brain, neurons consume 75–80% of the energy produced [105], and dopaminergic neurons in the substantia nigra are among the most demanding. Their massive axon arbor is unmyelinated and is at least an order of magnitude greater than other classes of less susceptible dopaminergic neurons [106]. Its axons also give rise to more than one million synapses and have a total length exceeding four meters, factors that may explain its selective vulnerability in PD [106]. Importantly, mitochondrial dysfunction is also central to the pathophysiology for many genetic causes (e.g., LRRK2, Parkin, SNCA) [33, 107, 108], providing support for shared mechanism of action and offering a rational explanation for a gene-environment interaction that converges on the respiratory chain of susceptible dopaminergic neurons.
Second, while exposure to these toxicants can come from various means (e.g., skin, gastrointestinal tract, pulmonary), air pollution, pesticides, and TCE can all be inhaled. Entry through the nose bypasses normal protective barriers in the body (e.g., skin, gastrointestinal tract) and the brain (e.g., blood-brain barrier) and avoids detoxification by the liver. As evidenced from studies in humans, animals or both, air pollution and pesticides like paraquat can all enter the olfactory bulb and beyond [90, 91, 109, 110]. Loss of smell is associated with exposure to small particulate matter in humans [111] and to paraquat in male mice [110]. Hyposmia is an early and common (∼95%) feature of PD [112, 113]. Similarly, synucleinopathy in the olfactory bulb is nearly a universal finding in PD and likely an early feature of the disease [114]. Based on a recent postmortem study, Borghammer and colleagues estimate that two-thirds of individuals with PD may have an olfactory bulb- or brain-first model of the disease [115]. In short, for many cases of PD, the nose may be the front door to the brain [116].
Finally, these environmental toxicants— air pollution, pesticides, and TCE— are generally new. While air pollution has been present for centuries, large-scale pollution is tied to the Industrial Revolution. Some pesticides (e.g., rotenone, pyrethrins [117]) that are linked to PD are produced naturally by plants and, although very rare, ancient descriptions of what could have been PD exist [118–120]. These cases could be tied to pure genetic causes that have been around for millennia or to exposure to naturally occurring toxins (rotenone has been used to kill fish for centuries; Dr. Parkinson’s first case was in a gardener [1]). However, synthetic pesticides are largely products of World War II, and global use has risen sharply since then. Similarly, commercial use of TCE began in the 1920s. All could be contributing to the global rise of PD, a condition that, according to Dr. Parkinson, had not been classified in the medical literature until his classic 1817 essay [1].
HOPE FOR THE FUTURE
To the extent that environmental toxicants are fueling the rise of PD, the disease is preventable. Few studies have examined the incidence of PD over time, but in most countries, it appears to be increasing [121–123]. However, while more work remains to be done, the age-adjusted prevalence of PD appears to be slowing in countries (e.g., in western Europe) that have taken more aggressive means to clean their environment than rapidly industrializing nations [5].
For example, in the Netherlands, the prevalence of PD has risen by 30% in the last decade [124], but this growth is possibly less than in other parts of the world. Prevalence is the net result of survival and incidence. Part of the growth in existing cases may be attributed to increased survival in the Netherlands because of optimal care from the national ParkinsonNet infrastructure. In one study, specialized physiotherapy, given as part of the Dutch ParkinsonNet approach to healthcare [125], was associated with a tendency towards a reduced mortality [126]. ParkinsonNet (which reached full nationwide implementation in the Netherlands in 2010) is also associated with a marked reduction in hip fractures, other orthopedic injuries,and aspiration pneumonias [126–128], which could be tied to survival benefits. Determining whether the incidence is changing is more difficult. In 2016, the Rotterdam study found that the incidence of PD in this Dutch city may be decreasing [129], but two caveats should be considered. First, the diagnosis was not established by experienced movement disorders experts so relevant diagnoses might have been missed. Second, the observed data applied only to this large urban area and not the entire country. Indeed, the latest analyses show that the incidence of PD across all of the Netherlands has generally remained stable during the last decade (A. Talebi and S. Darweesh, unpublished observations).
But let’s assume that the incidence of PD is indeed decreasing in urban parts of the Netherlands. The authors of the Rotterdam study could not identify a satisfactory explanation for their hopeful finding, but said, “Further insight into the factors that caused this shift may open the door to protective strategies at the population level” [129]. While not certain, the drop in incidence could be explained by changes in air pollution, the use of pesticides, and TCE [4]. From 1990 to 2012, emissions of multiple air pollutants in the Netherlands decreased by 50% or more [4, 130]. Use of pesticides linked to PD decreased in the country. The Netherlands was among the first countries in the world to ban paraquat [131], and use of other pesticides linked to PD, such as DDT [132] and dieldrin [133], also fell. Levels of these pesticides and their metabolites in the blood, fat, and breast milk of Dutch citizens declined. For example, from 1968 to 1986, fat levels of dieldrin decreased by 75% and levels of DDT by 90% [134]. Finally, the Netherlands banned TCE, and in 1981 the levels of this toxicant in the air were among the lowest in all of Europe. While encouraging, additional actions are needed to prevent PD.
THE WAY FORWARD
Sixty years ago, Rachel Carson warned us that the indiscriminate use of pesticides would puzzle doctors who would confront “new kinds of sickness appearing among their patients” [135]. The answer to the enigma of PD is likely under our noses. The rise of PD, its epidemiology, and pathophysiology are all consistent with the environmental factors detailed here. In some affected individuals, these toxicants are superimposed upon an underlying genetic predisposition [18].
Since the publication of Silent Spring, engineers have developed safer cars and airplanes. Chemists can do the same for pesticides and solvents. Some companies even promote such alternatives [136]. Improved regulatory actions can enhance screening for neurotoxicity of pesticides and other chemicals including co-exposure to multiple toxicants. The COVID-19 pandemic demonstrated how quickly we can clean our polluted air [137, 138]. These toxicants are not essential to a healthy life, a prospering economy, or a flourishing society. Clean food, water, and air are. The quicker we recognize the truth, the faster we take action, the sooner we will give our generation and generations to come a world largely free of PD.
ACKNOWLEDGMENTS
The Radboudumc Centre of Expertise for Parkinson & Movement Disorders was supported by a center of excellence grant of the Parkinson’s Foundation. We kindly thank Michel Verbruggen for his assistance in drafting the figures, and Franka Goossens for editorial support.
CONFLICT OF INTEREST
Dr. Dorsey has received honoraria for speaking at American Academy of Neurology, American Neurological Association, Excellus BlueCross BlueShield, International Parkinson’s and Movement Disorders Society, National Multiple Sclerosis Society, Northwestern University, Physicians Education Resource, LLC, PRIME Education, LLC, Stanford University, Texas Neurological Society, and Weill Cornell; received compensation for consulting services from Abbott, Abbvie, Acadia, Acorda, Bial-Biotech Investments, Inc., Biogen, Boehringer Ingelheim, California Pacific Medical Center, Caraway Therapeutics, Curasen Therapeutics, Denali Therapeutics, Eli Lilly, Genentech/Roche, Grand Rounds, Huntington Study Group, Informa Pharma Consulting, Karger Publications, LifeSciences Consultants, MCM Education, Mediflix, Medopad, MedRhythms, Merck, Michael J. Fox Foundation, NACCME, Neurocrine, NeuroDerm, NIH, Novartis, Origent Data Sciences, Otsuka, Physician’s Education Resource, Praxis, PRIME Education, Roach, Brown, McCarthy & Gruber, Sanofi, Seminal Healthcare, Spark, Springer Healthcare, Sunovion Pharma, Theravance, Voyager and WebMD; research support from Biogen, Biosensics, Burroughs Wellcome Fund, CuraSen, Greater Rochester Health Foundation, Huntington Study Group, Michael J. Fox Foundation, National Institutes of Health, Patient-Centered Outcomes Research Institute, Pfizer, PhotoPharmics, Safra Foundation, and Wave Life Sciences; editorial services for Karger Publications; stock in Included Health, stock in Mediflix and ownership interests in SemCap.
Prof. Bloem currently serves as co-Editor in Chief for the Journal of Parkinson’s Disease but was not involved in any way in the peer review process of this manuscript. He serves on the editorial board of Practical Neurology and Digital Biomarkers, has received honoraria from serving on the scientific advisory board for Abbvie, Biogen and UCB, has received fees for speaking at conferences from AbbVie, Zambon, Roche, GE Healthcare and Bial, and has received research support from the Netherlands Organization for Scientific Research, the Michael J Fox Foundation, UCB, Not Impossible, the Hersenstichting Nederland, the Parkinson’s Foundation, Verily Life Sciences, Horizon 2020 and the Parkinson Vereniging (all paid to the institute).
REFERENCES
- [1]. Parkinson J (2002) An essay on the shaking palsy.1817. J Neuropsychiatry Clin Neurosci 14, 223–236; discussion 222. [DOI] [PubMed] [Google Scholar]
- [2]. GBD 2015 Neurological Disorders Collaborator Group (2017) Global, regional, and national burden of neurological disorders during 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 16, 877–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. GBD 2016 Neurology Collaborators (2019) Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18, 459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Dorsey R, Sherer T, Okun MS, Bloem BR(2020) Ending Parkinson’s Disease: A Prescription for Action, Public Affairs.
- [5]. GBD 2016 Parkinson’s Disease Collaborators (2018)Global, regional, and national burden of Parkinson’s disease,1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. .Lancet Neurol 17, 939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Gilbert SF (2000) Aging: The biology of senescence. In Developmental Biology, 6th Edition. Sinauer Associates,Sunderland (MA).
- [7]. Vilchez D, Saez I, Dillin A (2014) The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat Commun 5, 5659. [DOI] [PubMed] [Google Scholar]
- [8]. Collier TJ, Kanaan NM, Kordower JH (2017) Aging and Parkinson’s disease: Different sides of the same coin? Mov Disord 32, 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. McDonald RB, Ruhe RC (2011) Aging and longevity: Why knowing the difference is important to nutrition research. Nutrients 3, 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Proctor RN (2012) The history of the discovery of the cigarette-lung cancer link: Evidentiary traditions, corporate denial, global toll. Tob Control 21, 87–91. [DOI] [PubMed] [Google Scholar]
- [11]. Savica R, Rocca WA, Ahlskog JE (2010) When does Parkinson disease start? Arch Neurol 67, 798–801. [DOI] [PubMed] [Google Scholar]
- [12]. de Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5, 525–535. [DOI] [PubMed] [Google Scholar]
- [13]. Graham H (1996) Smoking prevalence among women in the European Community 1950–1990. Soc Sci Med 43, 243–254. [DOI] [PubMed] [Google Scholar]
- [14]. Kimura H, Kurimura M, Wada M, Kawanami T, Kurita K, Suzuki Y, Katagiri T, Daimon M, Kayama T, Kato T (2002) Female preponderance of Parkinson’s disease in Japan. Neuroepidemiology 21, 292–296. [DOI] [PubMed] [Google Scholar]
- [15]. Satake A(2020) Number of Women Farmers in Japan Continues to Decline. United States Department of Agriculture Foreign Agricultural Service, Global Agricultural Information Networkhttps://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Number%20of%20Women%20Farmers%20in%20Japan%20Continues%20to%20Decline_Tokyo_Japan_05-19-2020.
- [16]. Parkinson’s UK(2017) The incidence and prevalence of Parkinson’s in the UK. Results from the Clinical Practice Research Datalink Reference Report. Parkinson’s UKhttps://www.parkinsons.org.uk/sites/default/files/2018-01/Prevalence%20%20Incidence%20Report%20Latest_Public_2.pdf.
- [17]. Cancer Research UK, Cancer Statistics for the UKhttps://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk, Accessed February 1,2023.
- [18]. Lim S-Y, Klein C(2023) Parkinson’s disease is predominantly a genetic disease. J Parkinsons Dis, in press. [DOI] [PMC free article] [PubMed]
- [19]. Klein C, Westenberger A (2012) Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med 2, a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. van Dongen J, Slagboom PE, Draisma HH, Martin NG, Boomsma DI (2012) The continuing value of twin studies in the omics era. Nat Rev Genet 13, 640–653. [DOI] [PubMed] [Google Scholar]
- [21]. Duvoisin RC (1984) Is Parkinson’s disease acquired or inherited? Can J Neurol Sci 11, 151–155. [DOI] [PubMed] [Google Scholar]
- [22]. Tanner CM, Ottman R, Goldman SM, Ellenberg J, Chan P, Mayeux R, Langston JW (1999) Parkinson disease in twins: An etiologic study. JAMA 281, 341–346. [DOI] [PubMed] [Google Scholar]
- [23]. Lee AJ, Wang Y, Alcalay RN, Mejia-Santana H, Saunders-Pullman R, Bressman S, Corvol JC, Brice A, Lesage S, Mangone G, Tolosa E, Pont-Sunyer C, Vilas D, Schüle B, Kausar F, Foroud T, Berg D, Brockmann K, Goldwurm S, Siri C, Asselta R, Ruiz-Martinez J, Mondragón E, Marras C, Ghate T, Giladi N, Mirelman A, Marder K (2017) Penetrance estimate of LRRK2 p.G2019S mutation in individuals of non-Ashkenazi Jewish ancestry. Mov Disord 32, 1432–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Lee JW, Cannon JR (2015) LRRK2 mutations and neurotoxicant susceptibility. Exp Biol Med (Maywood) 240, 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Lee PC, Bordelon Y, Bronstein J, Ritz B (2012) Traumatic brain injury, paraquat exposure, and their relationship to Parkinson disease. Neurology 79, 2061–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Cannon JR, Greenamyre JT (2013) Gene-environment interactions in Parkinson’s disease: Specific evidence in humans and mammalian models. Neurobiol Dis 57, 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Balestrino R, Tunesi S, Tesei S, Lopiano L, Zecchinelli AL, Goldwurm S (2020) Penetrance of glucocerebrosidase (GBA) mutations in Parkinson’s disease: A kin cohort study. Mov Disord 35, 2111–2114. [DOI] [PubMed] [Google Scholar]
- [28]. Bogers JS, Bloem BR, Den Heijer JM (2023) The aetiology of Parkinson’s disease –new perspectives from gene-environment interactions. J Parkinsons Dis, in press. [DOI] [PMC free article] [PubMed]
- [29]. Barbeau A, Roy M, Bernier G, Campanella G, Paris S (1987) Ecogenetics of Parkinson’s disease: Prevalence and environmental aspects in rural areas. Can J Neurol Sci 14, 36–41. [DOI] [PubMed] [Google Scholar]
- [30]. Kab S, Spinosi J, Chaperon L, Dugravot A, Singh-Manoux A, Moisan F, Elbaz A (2017) Agricultural activities and the incidence of Parkinson’s disease in the general French population. Eur J Epidemiol 32, 203–216. [DOI] [PubMed] [Google Scholar]
- [31]. Wright Willis A, Evanoff BA, Lian M, Criswell SR, Racette BA (2010) Geographic and ethnic variation in Parkinson disease: A population-based study of US Medicare beneficiaries. Neuroepidemiology 34, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Goldman SM (2014) Environmental toxins and Parkinson’s disease. Annu Rev Pharmacol Toxicol 54, 141–164. [DOI] [PubMed] [Google Scholar]
- [33]. Bose A, Beal MF (2016) Mitochondrial dysfunction in Parkinson’s disease. J Neurochem 139(Suppl 1), 216–231. [DOI] [PubMed] [Google Scholar]
- [34]. Nonnekes J, Post B, Tetrud JW, Langston JW, Bloem BR (2018) MPTP-induced parkinsonism: An historical case series. Lancet Neurol 17, 300–301. [DOI] [PubMed] [Google Scholar]
- [35]. Barbeau A, Dallaire L, Buu NT, Poirier J, Rucinska E (1985) Comparative behavioral, biochemical and pigmentary effects of MPTP, MPP+ and paraquat in Rana pipiens. Life Sci 37, 1529–1538. [DOI] [PubMed] [Google Scholar]
- [36]. James KA, Hall DA (2015) Groundwater pesticide levels and the association with Parkinson disease. Int J Toxicol 34, 266–273. [DOI] [PubMed] [Google Scholar]
- [37]. Yitshak Sade M, Zlotnik Y, Kloog I, Novack V, Peretz C, Ifergane G (2015) Parkinson’s disease prevalence and proximity to agricultural cultivated fields. Parkinsons Dis 2015, 576564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW (2011) Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect 119, 866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Moisan F, Spinosi J, Delabre L, Gourlet V, Mazurie JL, Bénatru I, Goldberg M, Weisskopf MG, Imbernon E, Tzourio C, Elbaz A (2015) Association of Parkinson’s disease and its subtypes with agricultural pesticide exposures in men: A case-control study in France. Environ Health Perspect 123, 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Perrin L, Spinosi J, Chaperon L, Kab S, Moisan F, Ebaz A (2021) Pesticides expenditures by farming type and incidence of Parkinson disease in farmers: A French nationwide study. Environ Res 197, 111161. [DOI] [PubMed] [Google Scholar]
- [41]. Li S, Ritz B, Gong Y, Cockburn M, Folle AD, Del Rosario I, Yu Y, Zhang K, Castro E, Keener AM, Bronstein J, Paul KC (2023) Proximity to residential and workplace pesticides application and the risk of progression of Parkinson’s diseases in Central California. Sci Total Environ 864, 160851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Schneider Medeiros M, S PR, M PS, Schumacher-Schuh AF, Mello Rieder CR (2020) Occupational pesticide exposure and the risk of death in patients with Parkinson’s disease: An observational study in southern Brazil. Environ Health 19, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3, 1301–1306. [DOI] [PubMed] [Google Scholar]
- [44]. McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA (2002) Environmental risk factors and Parkinson’s disease: Selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis 10, 119–127. [DOI] [PubMed] [Google Scholar]
- [45]. Jackson-Lewis V, Blesa J, Przedborski S (2012) Animal models of Parkinson’s disease. Parkinsonism Relat Disord 18,Suppl 1,S183–185. [DOI] [PubMed] [Google Scholar]
- [46]. Lim S-Y, Tan AH, Ahmad-Annuar A, Klein C, Tan LCS, Rosales RL, Bhidayasiri R, Wu Y-R, Shang H-F, Evans AH, Pal PK, Hattori N, Tan CT, Jeon B, Tan E-K, Lang AE (2019) Parkinson’s disease in the Western Pacific Region. Lancet Neurol 18, 865–879. [DOI] [PubMed] [Google Scholar]
- [47]. Carey Gillam AU, Secret files suggest chemical giant feared Weedkiller’s link to Parkinson’s disease,https://www.theguardian.com/us-news/2022/oct/20/syngenta-weedkiller-pesticide-parkinsons-disease-paraquat-documents.
- [48]. Pesticide National Synthesis Project, Estimated Annual Agricultural Pesticide Use,https://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=2018&map=PARAQUAT&hilo=HAccessed October 25, 2023.
- [49]. Willis AW, Roberts E, Beck JC, Fiske B, Ross W, Savica R, Van Den Eeden SK, Tanner CM, Marras C (2022) Incidence of Parkinson disease in North America. NPJ Parkinsons Dis 8, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Pesticide National Synthesis Project, Estimated Annual Agricultural Pesticide Use,https://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=1992&map=PARAQUAT&hilo=H.Accessed October 25,2023.
- [51]. USC Contextual Data Resource, Air Pollution: O2 and PM2.5,https://gero.usc.edu/cbph/cdr/air-pollution-o2-and-pm2-5/.
- [52]. Bloem BR, Boonstra TA (2023) The inadequacy of current pesticide regulations for protecting brain health: The case of glyphosate and Parkinson’s disease. Lancet Planet Health 7, e948–e949. [DOI] [PubMed] [Google Scholar]
- [53]. European Food Safety Authority (2022) Workshop on the EFSA NAMs project on environmental neurotoxicants, https://www.pan-europe.info/sites/pan-europe.info/files/public/resources/other/Agreed%20minutes%207-8%20September%202022.pdf.
- [54]. Mie A, Rudén C (2023) Non-disclosure of developmental neurotoxicity studies obstructs the safety assessment of pesticides in the European Union. Environ Health 22, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Su F-C, Goutman SA, Chernyak S, Mukherjee B, Callaghan BC, Batterman S, Feldman EL (2016) Association of environmental toxins with amyotrophic lateral sclerosis. JAMA Neurol 73, 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Dorsey ER, Ray A (2023) Paraquat, Parkinson’s disease, and agnotology. Mov Disord 38, 949–952. [DOI] [PubMed] [Google Scholar]
- [57]. Michaels D(2020) The Triumph of Doubt: Dark Money and the Science of Deception Oxford University Press.
- [58]. Schiebinger L, Proctor RN (2008) Agnotology: The Making and Unmaking of IgnoranceStanford University Press,Standford.
- [59]. Naomi Oreskes EMC (2011),Merchants of Doubt: How a Handful of Scientists Obscured the Truth on Issues from Tobacco Smoke to Climate Change,Bloomsbury Publishing.
- [60]. Kruse-Plaß M, Hofmann F, Wosniok W, Schlechtriemen U, Kohlschütter N (2021) Pesticides and pesticide-related products in ambient air in Germany. Environ Sci Eur 33, 114. [Google Scholar]
- [61]. Navarro I, de la Torre A, Sanz P, Baldi I, Harkes P, Huerta-Lwanga E, Nørgaard T, Glavan M, Pasković I, Pasković MP, Abrantes N, Campos I, Alcon F, Contreras J, Alaoui A, Hofman J, Vested A, Bureau M, Aparicio V, Mandrioli D, Sgargi D, Mol H, Geissen V, Silva V, Martínez M (2023) Occurrence of pesticide residues in indoor dust of farmworker households across Europe and Argentina. Sci Total Environ 905, 167797. [DOI] [PubMed] [Google Scholar]
- [62]. Paul KC, Krolewski RC, Lucumi Moreno E, Blank J, Holton KM, Ahfeldt T, Furlong M, Yu Y, Cockburn M, Thompson LK, Kreymerman A, Ricci-Blair EM, Li YJ, Patel HB, Lee RT, Bronstein J, Rubin LL, Khurana V, Ritz B (2023) A pesticide and iPSC dopaminergic neuron screen identifies and classifies Parkinson-relevant pesticides. Nat Commun 14, 2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. De Miranda BR, Greenamyre JT (2020) Trichloroethylene, a ubiquitous environmental contaminant in the risk for Parkinson’s disease. Environ Sci Process Impacts 22, 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Dorsey ER, Zafar M, Lettenberger SE, Pawlik ME, Kinel D, Frissen M, Schneider RB, Kieburtz K, Tanner CM, De Miranda BR, Goldman SM, Bloem BR (2023) Trichloroethylene: An invisible cause of Parkinson’s disease? J Parkinsons Dis 13, 203–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. Reis J, Benbrick E, Bonneterre V, Spencer PS (2016) Parkinson’s disease and solvents: Is there a causal link? Rev Neurol (Paris) 172, 761–765. [DOI] [PubMed] [Google Scholar]
- [66]. Brugnone F, Perbellini L, Giuliari C, Cerpelloni M, Soave M (1994) Blood and urine concentrations of chemical pollutants in the general population. Med Lav 85, 370–389. [PubMed] [Google Scholar]
- [67]. McCord CP (1932) Toxicity of trichloroethylene. JAMA 99, 409–409. [Google Scholar]
- [68]. De Miranda BR, Castro SL, Rocha EM, Bodle CR, Johnson KE, Greenamyre JT (2021) The industrial solvent trichloroethylene induces LRRK2 kinase activity and dopaminergic neurodegeneration in a rat model of Parkinson’s disease. Neurobiol Dis 153, 105312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Guehl D, Bezard E, Dovero S, Boraud T, Bioulac B, Gross C (1999) Trichloroethylene and parkinsonism: A human and experimental observation. Eur J Neurol 6, 609–611. [DOI] [PubMed] [Google Scholar]
- [70]. Huber F (1969) [Clinical aspects and neuropathology of trichloroethylene poisoning]. Z Unfallmed Berufskr 62, 226–267. [PubMed] [Google Scholar]
- [71]. Kochen W, Kohlmüller D, De Biasi P, Ramsay R (2003) The endogeneous formation of highly chlorinated tetrahydro-beta-carbolines as a possible causative mechanism in idiopathic Parkinson’s disease. Adv Exp Med Biol 527, 253–263. [DOI] [PubMed] [Google Scholar]
- [72]. Gash DM, Rutland K, Hudson NL, Sullivan PG, Bing G, Cass WA, Pandya JD, Liu M, Choi D-Y, Hunter RL, Gerhardt GA, Smith CD, Slevin JT, Prince TS (2008) Trichloroethylene: Parkinsonism and complex 1 mitochondrial neurotoxicity. Ann Neurol 63, 184–192. [DOI] [PubMed] [Google Scholar]
- [73]. Goldman SM, Quinlan PJ, Ross GW, Marras C, Meng C, Bhudhikanok GS, Comyns K, Korell M, Chade AR, Kasten M, Priestley B, Chou KL, Fernandez HH, Cambi F, Langston JW, Tanner CM (2012) Solvent exposures and Parkinson disease risk in twins. Ann Neurol 71, 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74]. Keane PC, Hanson PS, Patterson L, Blain PG, Hepplewhite P, Khundakar AA, Judge SJ, Kahle PJ, LeBeau FEN, Morris CM (2019) Trichloroethylene and its metabolite TaClo lead to degeneration of substantia nigra dopaminergic neurones: Effects in wild type and human A30P mutant α-synuclein mice. Neurosci Lett 711, 134437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Liu M, Choi DY, Hunter RL, Pandya JD, Cass WA, Sullivan PG, Kim HC, Gash DM, Bing G (2010) Trichloroethylene induces dopaminergic neurodegeneration in Fisher 344 rats. J Neurochem 112, 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76]. Liu M, Shin EJ, Dang DK, Jin CH, Lee PH, Jeong JH, Park SJ, Kim YS, Xing B, Xin T, Bing G, Kim HC (2018) Trichloroethylene and Parkinson’s disease: Risk assessment. Mol Neurobiol 55, 6201–6214. [DOI] [PubMed] [Google Scholar]
- [77]. Agency for Toxic Substances and Disease Registry (2019) Toxicological profile for trichloroethylene.Agency for Toxic Substances and Disease Registry.https://www.atsdr.cdc.gov/toxprofiles/tp19.pdf. [PubMed]
- [78]. Agency for Toxic Substances and Disease Registry, ATSDR’s Position on the Water Contamination at Camp Lejeune,Accessed October 25,2023https://www.atsdr.cdc.gov/sites/lejeune/background.html.
- [79]. Goldman SM, Weaver FM, Stroupe KT, Cao L, Gonzalez B, Colletta K, Brown EG, Tanner CM (2023) Risk of Parkinson disease among service members at Marine Corps Base Camp Lejeune. JAMA Neurol 80, 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80]. Geng C, Luo Q, Chen M, Li Z, Zhang C (2010) Quantitative risk assessment of trichloroethylene for a former chemical works in Shanghai, China. Hum Ecol Risk Assess 16, 429–443. [Google Scholar]
- [81]. Law J (2022) Community Fact Sheet No. 9, Former Ford Aeronutronics Facility –Newport Beach, CA. Santa Ana Regional Water Quality Control Board and Ford Motor Company https://www.waterboards.ca.gov/santaana/water_issues/programs/scp/docs/Ford/2022/cfs9.pdf.
- [82]. Malewitz J Michigan has 7,300 toxic sites. Money for cleanups is almost gone https://www.bridgemi.com/michigan-environment-watch/michigan-has-7300-toxic-sites-money-cleanups-almost-gone17-01-2018.
- [83]. EPA United States Environmental Protection Agency, TRI Data Files: Calendar Years 1987-Present, https://www.epa.gov/toxics-release-inventory-tri-program/tri-basic-data-files-calendar-years-1987-present.
- [84]. EPA United States Environmental Protection Agency, Biden-Harris Administration Proposes Ban on Trichloroethylene to Protect Public from Toxic Chemical Known to Cause Serious Health Risks,https://www.epa.gov/newsreleases/biden-harris-administration-proposes-ban-trichloroethylene-protect-public-toxic.Accessed November 24,2023.
- [85]. Watts ME, Pocock R, Claudianos C (2018) Brain energy and oxygen metabolism: Emerging role in normal function and disease. Front Mol Neurosci 11, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86]. Fowler D, Brimblecombe P, Burrows J, Heal MR, Grennfelt P, Stevenson DS, Jowett A, Nemitz E, Coyle M, Liu X, Chang Y, Fuller GW, Sutton MA, Klimont Z, Unsworth MH, Vieno M (2020) A chronology of global air quality. Philos Trans A Math Phys Eng Sci 378, 20190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87]. Corton CL(2015) London Fog: The Biography. Harvard University Press, Cambridge.
- [88]. Dorsey ER, Okun MS, Tanner CM (2021) Bad air and Parkinson disease-the fog may be lifting. JAMA Neurol 78, 793–795. [DOI] [PubMed] [Google Scholar]
- [89]. Calderón-Garcidueñas L, Gónzalez-Maciel A, Reynoso-Robles R, Delgado-Chávez R, Mukherjee PS, Kulesza RJ, Torres-Jardón R, Ávila-Ramírez J, Villarreal-Ríos R (2018) Hallmarks of Alzheimer disease are evolving relentlessly in Metropolitan Mexico City infants, children and young adults. APOE4 carriers have higher suicide risk and higher odds of reaching NFT stage V at ≤40 years of age. Environ Res 164, 475–487. [DOI] [PubMed] [Google Scholar]
- [90]. Calderón-Garcidueñas L, González-Maciel A, Reynoso-Robles R, Kulesza RJ, Mukherjee PS, Torres-Jardón R, Rönkkö T, Doty RL (2018) Alzheimer’s disease and alpha-synuclein pathology in the olfactory bulbs of infants, children, teens and adults ≤40 years in Metropolitan Mexico City. APOE4 carriers at higher risk of suicide accelerate their olfactory bulb pathology. Environ Res 166, 348–362. [DOI] [PubMed] [Google Scholar]
- [91]. Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C, Torres-Jardón R, Nuse B, Herritt L, Villarreal-Calderón R, Osnaya N, Stone I, García R, Brooks DM, González-Maciel A, Reynoso-Robles R, Delgado-Chávez R, Reed W (2008) Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid β-42 and α-synuclein in children and young adults. Toxicol Pathol 36, 289–310. [DOI] [PubMed] [Google Scholar]
- [92]. Ritz B, Lee PC, Hansen J, Lassen CF, Ketzel M, Sørensen M, Raaschou-Nielsen O (2016) Traffic-related air pollution and Parkinson’s disease in Denmark: A case-control study. Environ Health Perspect 124, 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93]. Lee PC, Liu LL, Sun Y, Chen YA, Liu CC, Li CY, Yu HL, Ritz B (2016) Traffic-related air pollution increased the risk of Parkinson’s disease in Taiwan: A nationwide study. Environ Int 96, 75–81. [DOI] [PubMed] [Google Scholar]
- [94]. Jo S, Kim YJ, Park KW, Hwang YS, Lee SH, Kim BJ, Chung SJ (2021) Association of NO2 and other air pollution exposures with the risk of Parkinson Ddsease. JAMA Neurol 78, 800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95]. Cleland SE, Wyatt LH, Wei L, Paul N, Serre ML, West JJ, Henderson SB, Rappold AG (2022) Short-term exposure to wildfire smoke and PM2.5 and cognitive performance in a brain-training game: A longitudinal study of U.S. adults. Environ Health Perspect 130, 067005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96]. Liu JC, Wilson A, Mickley LJ, Dominici F, Ebisu K, Wang Y, Sulprizio MP, Peng RD, Yue X, Son JY, Anderson GB, Bell ML (2017) Wildfire-specific fine particulate matter and risk of hospital admissions in urban and rural counties. Epidemiol 28, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97]. Calderón-Garcidueñas L, Stommel EW, Rajkumar RP, Mukherjee PS, Ayala A (2021) Particulate air pollution and risk of neuropsychiatric outcomes. What we breathe, swallow, and put on our skin matters. Int J Environ Res Public Health 18, 11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98]. Calderón-Garcidueñas L, Mora-Tiscareño A, Ontiveros E, Gómez-Garza G, Barragán-Mejía G, Broadway J, Chapman S, Valencia-Salazar G, Jewells V, Maronpot RR, Henríquez-Roldán C, Pérez-Guillé B, Torres-Jardón R, Herrit L, Brooks D, Osnaya-Brizuela N, Monroy ME, González-Maciel A, Reynoso-Robles R, Villarreal-Calderon R, Solt AC, Engle RW (2008) Air pollution, cognitive deficits and brain abnormalities: A pilot study with children and dogs. Brain Cogn 68, 117–127. [DOI] [PubMed] [Google Scholar]
- [99]. Doty RL, Singh A, Tetrud J, Langston JW (1992) Lack of major olfactory dysfunction in MPTP-induced parkinsonism. Ann Neurol 32, 97–100. [DOI] [PubMed] [Google Scholar]
- [100]. Cheng H, Saffari A, Sioutas C, Forman HJ, Morgan TE, Finch CE (2016) Nanoscale particulate matter from urban traffic rapidly induces oxidative stress and inflammation in olfactory epithelium with concomitant effects on brain. Environ Health Perspect 124, 1537–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101]. Lucchini RG, Dorman DC, Elder A, Veronesi B (2012) Neurological impacts from inhalation of pollutants and the nose–brain connection. Neurotoxicology 33, 838–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102]. Li B, Xia M, Zorec R, Parpura V, Verkhratsky A (2021) Astrocytes in heavy metal neurotoxicity and neurodegeneration. Brain Res 1752, 147234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103]. Yuan X, Yang Y, Liu C, Tian Y, Xia D, Liu Z, Pan L, Xiong M, Xiong J, Meng L, Zhang Z, Ye K, Jiang H, Zhang Z (2022) Fine particulate matter triggers α-synuclein fibrillization and Parkinson-like neurodegeneration. Mov Disord 37, 1817–1830. [DOI] [PubMed] [Google Scholar]
- [104]. Krzyzanowski B, Nielsen SS, Turner JR, Racette BA (2023) Fine particulate matter and Parkinson disease risk among Medicare beneficiaries.. Neurology 101, e2058–e2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105]. Pissadaki EK, Bolam JP (2013) The energy cost of action potential propagation in dopamine neurons: Clues to susceptibility in Parkinson’s disease. Front Comput Neurosci 7, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106]. Park JS, Davis RL, Sue CM (2018) Mitochondrial dysfunction in Parkinson’s disease: New mechanistic insights and therapeutic perspectives. Curr Neurol Neurosci Rep 18, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107]. Abou-Sleiman PM, Muqit MM, Wood NW (2006) Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci 7, 207–219. [DOI] [PubMed] [Google Scholar]
- [108]. Simón-Sánchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Krüger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T (2009) Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet 41, 1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109]. Ajmani GS, Suh HH, Pinto JM (2016) Effects of ambient air pollution exposure on olfaction: A review. Environ Health Perspect 124, 1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110]. Anderson T, Merrill AK, Eckard ML, Marvin E, Conrad K, Welle K, Oberdörster G, Sobolewski M, Cory-Slechta DA (2021) Paraquat inhalation, a translationally relevant route of exposure: Disposition to the brain and male-specific olfactory impairment in mice. Toxicol Sci 180, 175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111]. Zhang Z, Rowan NR, Pinto JM, London NR, Lane AP, Biswal S, Ramanathan M, Jr. (2021) Exposure to particulate matter air pollution and anosmia. JAMA Netw Open 4, e2111606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112]. Doty RL (2012) Olfaction in Parkinson’s disease and related disorders. Neurobiol Dis 46, 527–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113]. Haehner A, Hummel T, Reichmann H (2011) Olfactory loss in Parkinson’s disease. Parkinsons Dis 2011, 450939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114]. Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL, 3rd, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG (2009) Unified staging system for Lewy body disorders: Correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117, 613–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115]. Borghammer P, Just MK, Horsager J, Skjærbæk C, Raunio A, Kok EH, Savola S, Murayama S, Saito Y, Myllykangas L, Van Den Berge N (2022) A postmortem study suggests a revision of the dual-hit hypothesis of Parkinson’s disease. NPJ Parkinsons Dis 8, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116]. Borghammer P (2018) How does Parkinson’s disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov Disord 33, 48–57. [DOI] [PubMed] [Google Scholar]
- [117]. Wan F, Yu T, Hu J, Yin S, Li Y, Kou L, Chi X, Wu J, Sun Y, Zhou Q, Zou W, Zhang Z, Wang T (2022) The pyrethroids metabolite 3-phenoxybenzoic acid induces dopaminergic degeneration. Sci Total Environ 838, 156027. [DOI] [PubMed] [Google Scholar]
- [118]. Goetz CG (2011) The history of Parkinson’s disease: Early clinical descriptions and neurological therapies.. Cold Spring Harb Perspect Med 1, a008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119]. Ovallath S, Deepa P (2013) The history of parkinsonism: Descriptions in ancient Indian medical literature. Mov Disord 28, 566–568. [DOI] [PubMed] [Google Scholar]
- [120]. Zhang ZX, Dong ZH, Román GC (2006) Early descriptions of Parkinson disease in ancient China. Arch Neurol 63, 782–784. [DOI] [PubMed] [Google Scholar]
- [121]. Ou Z, Pan J, Tang S, Duan D, Yu D, Nong H, Wang Z (2021) Global trends in the incidence, prevalence, and years lived with disability of Parkinson’s disease in 204 countries/territories from 1990 to 2019. Front Public Health 9, 776847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122]. Park JH, Kim DH, Kwon DY, Choi M, Kim S, Jung JH, Han K, Park YG (2019) Trends in the incidence and prevalence of Parkinson’s disease in Korea: A nationwide, population-based study. BMC Geriatr 19, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123]. Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA (2016) Time trends in the incidence of Parkinson disease. JAMA Neurol 73, 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124]. van der Gaag BL, Hepp DH, Hoff JI, van Hilten JJB, Darweesh SKL, Bloem BR, van de Berg WDJ (2023) [Risk factors for Parkinson’s disease: Possibilities for prevention and intervention]. Ned Tijdschr Geneeskd 167, D6655. [PubMed] [Google Scholar]
- [125]. Bloem BR, Munneke M (2014) Revolutionising management of chronic disease: The ParkinsonNet approach.BMJ 348, g1838. [DOI] [PubMed] [Google Scholar]
- [126]. Ypinga JHL, de Vries NM, Boonen L, Koolman X, Munneke M, Zwinderman AH, Bloem BR (2018) Effectiveness and costs of specialised physiotherapy given via ParkinsonNet: A retrospective analysis of medical claims data. Lancet Neurol 17, 153–161. [DOI] [PubMed] [Google Scholar]
- [127]. Bloem BR, Rompen L, de Vries NM, Klink A, Munneke M, Jeurissen P (2017) ParkinsonNet: A low-cost health care innovation with a systems approach from the Netherlands. Health Affairs 36, 1987–1996. [DOI] [PubMed] [Google Scholar]
- [128]. Talebi AH, Ypinga JHL, De Vries NM, Nonnekes J, Munneke M, Bloem BR, Heskes T, Ben-Shlomo Y, Darweesh SKL (2023) Specialized versus generic allied health therapy and the risk of Parkinson’s disease complications. Mov Disord 38, 223–231. [DOI] [PubMed] [Google Scholar]
- [129]. Darweesh SK, Koudstaal PJ, Stricker BH, Hofman A, Ikram MA (2016) Trends in the incidence of Parkinson disease in the general population: The Rotterdam Study. Am J Epidemiol 183, 1018–1026. [DOI] [PubMed] [Google Scholar]
- [130]. Jimmink BA CP, Droge R, Geilenkirchen GP, Leekstra AJ, van der Maas CWM, te Molder RAB, Peek CJ, Vonk J, Wever D (2012) Emissions of transboundary air pollutants in the Netherlands 1990-2010: Informative Inventory Report 2012. Rijksinstituut voor Volksgezondheid en Milieu,https://www.rivm.nl/bibliotheek/rapporten/680355008.html.
- [131]. Wesseling C, van Wendel de Joode B, Ruepert C, León C, Monge P, Hermosillo H, Partanen TJ (2001) Paraquat in developing countries. Int J Occup Environ Health 7, 275–286. [DOI] [PubMed] [Google Scholar]
- [132]. Rossi M, Scarselli M, Fasciani I, Maggio R, Giorgi F (2017) Dichlorodiphenyltrichloroethane (DDT) induced extracellular vesicle formation: A potential role in organochlorine increased risk of Parkinson’s disease. Acta Neurobiol Exp (Wars) 77, 113–117. [DOI] [PubMed] [Google Scholar]
- [133]. Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V (2005) Dieldrin-induced neurotoxicity: Relevance to Parkinson’s disease pathogenesis. Neurotoxicology 26, 701–719. [DOI] [PubMed] [Google Scholar]
- [134]. Greve PA, Van Zoonen P (1990) Organochlorine pesticides and PCBs in tissues from Dutch citizens (1968–1986). Int J Environ Anal Chem 38, 265–277. [Google Scholar]
- [135]. Carson R (2002) Fawcett Publications. Silent Spring [Google Scholar]
- [136]. 3M Science. Applied to Life, 3M Specialty Fluidshttps://www.3m.com/3M/en_US/novec-us/applications/solvent-cleaning-vapor-degreasing/npb-tce-replacement/,Accessed October 25,2023.
- [137]. Ellis-Petersen H, Ratcliffe R, Cowie S, Parkin Daniels J, Kuo L(2020)‘It’s positively alpine!’: Disbelief in big cities as air pollution falls.The Guardian Issue Date: 08-04-2020
- [138]. Rodríguez-Urrego D, Rodríguez-Urrego L (2020) Air quality during the COVID-19: PM(2.5) analysis in the 50 most polluted capital cities in the world. Environ Pollut 266, 115042. [DOI] [PMC free article] [PubMed] [Google Scholar]