Abstract
Rationale
The disruption of the balance between fatty acid (FA) uptake and oxidation (FAO) leads to cardiac lipotoxicity, serving as the driving force behind diabetic cardiomyopathy (DbCM). Sirtuin 5 (Sirt5), a lysine de-succinylase, could impact diverse metabolic pathways, including FA metabolism. Nevertheless, the precise roles of Sirt5 in cardiac lipotoxicity and DbCM remain unknown.
Objective
This study aims to elucidate the role and underlying mechanism of Sirt5 in the context of cardiac lipotoxicity and DbCM.
Methods and results
The expression of myocardial Sirt5 was found to be modestly elevated in diabetic heart failure patients and mice. Cardiac dysfunction, hypertrophy and lipotoxicity were exacerbated by ablation of Sirt5 but improved by forced expression of Sirt5 in diabetic mice. Notably, Sirt5 deficiency impaired FAO without affecting the capacity of FA uptake in the diabetic heart, leading to accumulation of FA intermediate metabolites, which mainly included medium- and long-chain fatty acyl-carnitines. Mechanistically, succinylomics analyses identified carnitine palmitoyltransferase 2 (CPT2), a crucial enzyme involved in the reconversion of fatty acyl-carnitines to fatty acyl-CoA and facilitating FAO, as the functional succinylated substrate mediator of Sirt5. Succinylation of Lys424 in CPT2 was significantly increased by Sirt5 deficiency, leading to the inactivation of its enzymatic activity and the subsequent accumulation of fatty acyl-carnitines. CPT2 K424R mutation, which mitigated succinylation modification, counteracted the reduction of enzymatic activity in CPT2 mediated by Sirt5 deficiency, thereby attenuating Sirt5 knockout-induced FAO impairment and lipid deposition.
Conclusions
Sirt5 deficiency impairs FAO, leading to cardiac lipotoxicity in the diabetic heart through the succinylation of Lys424 in CPT2. This underscores the potential roles of Sirt5 and CPT2 as therapeutic targets for addressing DbCM.
Keywords: Sirtuin 5, Diabetic cardiomyopathy, Carnitine palmitoyltransferase 2, Lysine succinylation, Fatty acid oxidation
Graphical abstract
Schematic diagram showing the proposed role of Sirt5 deficiency in aggravating DbCM. Sirt5 deficiency promotes Ksuc of CPT2 to impair FAO, resulting in cardiac lipotoxicity and dysfunction during DbCM development. Conversely, Sirt5 forced expression de-succinylates CPT2 and improves FAO to alleviate cardiac lipotoxicity and dysfunction, preventing development of DbCM.
1. Introduction
Diabetic cardiomyopathy (DbCM), which is widely recognized as an important independent cause for heart failure (HF), represents a significant and potentially fatal complication of diabetes-associated cardiac injury [[1], [2], [3]]. In the early stages of DbCM, diastolic dysfunction is the main manifestation, which may progress to systolic dysfunction over time [4]. Even after adjusting for multiple risk factors (e.g., age, hypertension, and coronary artery disease), the incidence of heart failure remains much higher in DbCM patients. Therefore, DbCM is expanded to underscore the heightened susceptibility of the myocardium to dysfunction in diabetic patients [5]. However, clinical trials have revealed that while intensive glucose control may reduce the incidence of myocardial infarction in diabetic patients, it does not decrease the rate of rehospitalization or mortality due to HF [6,7]. Therefore, there is an urgent need to elucidate the underlying mechanisms in order to identify potential therapeutic targets for DbCM.
DbCM is recognized as a cardiac metabolic disorder, characterized by the accumulation of toxic lipid metabolites in the diabetic heart, a phenomenon known as cardiac lipotoxicity [8,9], which could be attributable to the increased fatty acid (FA) uptake by cardiomyocytes and/or the decreased allosteric control of mitochondrial FA uptake and subsequent fully fatty acid oxidation (FAO) [10]. In fact, under DbCM, FAO in cardiomyocytes becomes insufficient to fully metabolize all the ingested FA over time [[11], [12], [13]]. Consequently, excessive FA and FA-derived intermediate metabolites, such as ceramides and diacylglycerol, accumulate in cardiomyocytes, resulting in structural and functional abnormality of the heart and leading to HF [14]. Increased cardiac FAO has long been considered to reduce cardiac glucose utilization and energy expenditure [15]. However, the inhibition of FAO showed sharply conflictive results, ranging from beneficial to unchanged to harmful effects [[16], [17], [18], [19]]. One of the most plausible explanation of these contradictory results could be the different targets and strategies for FAO inhibition or different animal models used. Therefore, it is necessary to explore more targets that are effective in the regulation of FAO and DbCM.
Sirtuins, evolutionarily conserved lysine deacylases that rely on nicotinamide adenine dinucleotide, play a pivotal role in the pathogenesis of cardiometabolic diseases [20]. The sirtuin family comprises seven members, including Sirt1-7, of which Sirt1 [21], Sirt2 [22], Sirt3 [23], and Sirt6 [23] have been demonstrated to be crucial in DbCM. While Sirt4 [24], Sirt5 [25], and Sirt7 [26] have been implicated in the regulation of fatty acid metabolism, their specific roles in DbCM remain unclear. Unlike other sirtuins, Sirt5 mediates lysine de-succinylation, de-malonylation, and de-glutarylation in addition to de-acetylation. Sirt5 is prominently expressed in the heart, and its absence disrupts FAO and diminishes physiological cardiac function through the succinylation of the mitochondrial trifunctional enzyme subunit alpha [25]. Global knockout of Sirt5 has been shown to increase mortality in mice subjected to cardiac stress overload induced by transverse aortic constriction [27]. These findings suggest that Sirt5 regulates cardiac metabolism and function. However, the precise roles of Sirt5 in the pathogenesis of DbCM remains unclear.
In this study, we present convincing evidence supporting the involvement of Sirt5 in cardiac lipotoxicity and DbCM. Our findings revealed a modest increase in myocardial Sirt5 expression in diabetic HF patients and db/db mice. Gain- and loss-of-function experiments identified Sirt5 as an important inhibitor of cardiac dysfunction and hypertrophy in diabetic mice. Ablation of Sirt5 contributed to cardiac FAO dysfunction, exacerbating cardiac lipotoxicity and dysfunction. Mechanistically, we demonstrated that Sirt5 deficiency mediated Lys424 succinyaltion of CPT2 to inactivate its enzyme activity, which led to the cardiac FAO impairment and lipids deposition in cardiomyocytes. Our study unveiled a novel role of Sirt5 and its mediated succinylation of CPT2 in modulating FAO, potentially elucidating the mechanism by which Sirt5 deficiency exacerbates cardiac lipotoxicity and DbCM. These findings suggest that Sirt5 and CPT2 could represent promising therapeutic targets in the context of DbCM.
2. Materials and methods
2.1. DbCM models
The global Sirt5 knockout mouse model (C57BL/6-Sirt5tm1cyagen) was created by CRISPR/Cas-mediated genome engineering. Briefly, Sirt5 gene (NCBI Reference Sequence: NM_178848; Ensembl: ENSMUSG00000054021) is located on mouse chromosome 13. Nine exons are identified, with the ATG start codon in exon 2 and the TAA stop codon in exon 9 (Transcript Sirt5-211: ENSMUST00000223194). Exon 3 was selected as the target site, this region contains 134 bp coding sequence. gRNA1 (matching the forward strand): GCTGAGCCGTCTTTTACTATTGG. gRNA2 (matching the reverse strand): ATTTGCTCCCGTAGTCATCGGGG. Ribonucleoprotein (RNP) was co-injected with the gRNAs into fertilized eggs for KO mouse production. The pups were genotyped by PCR followed by sequencing analysis. The PCR Primers for genotyping: Forward primer: 5′-TGGTGACAATGCCTTTCCTCTGAG-3′, Reverse primer: 5′-GGAGCCAGAGACTACTGAGCATC-3’. Homozygotes: ∼550 bp. Heterozygotes: ∼550 bp/325 bp/973 bp. Wildtype allele: 325 bp/973 bp (Note: If DNA sample is not very pure or enough PCR extension time, the 973 bp PCR product may not be amplified). The heterozygous Sirt5 knockout mice (Sirt5+/−) were obtained from Cyagen Biosciences, and were crossed to generate littermates with the following genotypes: Sirt5−/− (the SIRT5 KO mice), Sirt5+/− (the heterozygous mice) and Sirt5+/+ (the wild-type control mice). C57BL/6-Sirt5tm1cyagen was the strain name of the mice, and all of these mice were on the C57BL/6 N background. Male C57BLKsJ-db/db mice were obtained from GemPharmatech, with age-matched male C57BLKsJ-m/m mice serving as controls. The mice were group-housed in a 12-h light/dark cycle environment with ad libitum access to rodent chow and tap water. All animal procedures adhered to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and were approved by the Institutional Animal Care and Use Committee (IACUC) of Sun Yat-sen University [28]. Euthanasia for terminal organ harvest was conducted by the IACUC approved method. In summary, euthanasia was conducted through inhalant anesthetic overdose followed by exsanguination. A precision vaporizer with an induction chamber and waste gas scavenger was utilized to administer >4.5 % Isoflurane in oxygen, which was continued until respiratory arrest persisted for >60 s. Subsequently, the chamber was flushed with oxygen only, the animals were removed, and rapid exsanguination was carried out via vena cava puncture to ensure euthanasia, followed by prompt organ harvest.
8-week-old WT and Sirt5−/− male mice were randomly divided into HFD/STZ group and control group. Mice in the HFD/STZ group received intraperitoneal injections of low-dose STZ (30 mg/kg, dissolved in citric acid buffer) after a 12-h fast, administered daily for 5 consecutive days, and were simultaneously placed on a HFD for a duration of 24 weeks. Mice in the control group were injected with an equivalent volume of citric acid buffer and were fed a standard chow diet. The HFD consisted of 20 kcal% carbohydrate, 20 kcal% protein, and 60 kcal% fat, while the chow diet comprised 70 kcal% carbohydrate, 20 kcal% protein, and 10 kcal% fat. Both the high-fat diet (D12492) and chow diet (D12450B) were procured from the Research Diets, Inc. Body weight and fasting blood glucose levels of the mice were monitored every 6 weeks. Following the 24-week induction period, cardiac function and left ventricular pressure were assessed using echocardiography and left ventricular catheterization, respectively. Glucose tolerance test (GTT), insulin tolerance test (ITT), and FA uptake were conducted prior to sacrifice. Blood samples were collected for the quantification of cholesterol, triglycerides (TG), and insulin. Heart tissues were excised and weighted (Heart weight, HW) before using for proteomics, metabolomics, TG quantification, and pathological staining. Cardiomyocytes were isolated from the myocardium for FAO detection. Tibial length (TL) was measured.
20-week-old m/m and db/db mice were randomly allocated to receive AAV9-Sirt5 or AAV9-control via tail vein injection. Subsequently, the mice were maintained on a chow diet for a duration of 8 weeks. Baseline and end-of-study assessments included the examination of body weight and fasting blood glucose levels. Echocardiography was conducted to assess the systolic and diastolic function of the mice prior to sacrifice. HW and TL were measured. Blood samples were collected for the quantification of cholesterol, TG, and insulin. Additionally, heart tissues were excised for TG quantification and pathological staining.
2.2. Cardiac sirtuins expression in diabetic HF patients
A diabetic HF-related expression profiling microarray (GSE26887) was obtained from the Gene Expression Omnibus (GEO) repository. The dataset comprised LV cardiac biopsies from five control non-HF patients and seven diabetic HF patients, with gene expression assessed using the Affymetrix GeneChips Human Gene 1.0 ST array. Log2 GC-RMA signals of Sirt1-7 from the dataset were used to compare their gene expressions. All procedures were performed according to the Declaration of Helsinki and were approved by the local medical ethics review committees.
2.3. Cardiac functions
Upon completion of the intervention, 1 % continues inhale isoflurane anesthesia was used to anesthetized the mice. Echocardiograms were acquired and analyzed using a Visual Sonics Echo System (Vevo2100, FUJIFILM VisualSonics, Inc, Toronto, Canada) and MicroScan Transducer (MS-400, 30 MHz, FUJIFILM VisualSonics, Inc) according to our previous publication [28]. Left ventricular (LV) end-diastolic dimension, LV end-systolic dimension, LV mass (LVM), E wave, A wave, and e’ wave were measured utilizing Vevo LAB software (Version 3.1.1, FUJIFILM Visualsonics, Inc). LV ejection fraction (LVEF), LV fractional shortening (LVFS), E/A ratio, and E/e’ ratio were calculated in accordance with previously established methods [29]. Subsequently, a catheter manometer (PowerLab, ADInstruments, Bella Vista, Australia) was employed to monitor LV pressure, inserted via the right carotid artery into the left ventricle. Data were recorded, and dp/dtmax and dp/dtmin were calculated using LabChart (ADInstruments). All the techniques were approved by the institutional IACUC.
2.4. AAV9 construction and transfection
AAV9 was utilized to facilitate the overexpression of Sirt5 in cardiomyocytes for the in vivo study. The AAV expression vector pAAV-CMV-Sirt5-3FLAG-P2A-mNeonGreen-CW3SL was synthesized and constructed by OBiO Biology (Shanghai, China). AAV packaging was conducted in accordance with our previous protocol [30]. In brief, 293AAV cells were co-transfected with the expression vectors and packaging vectors using a calcium phosphate-mediated protocol. Subsequently, after 72 h, the cells were harvested, and AAVs were purified utilizing cesium chloride (CsCl) gradient centrifugation. AAV9 was administered at a concentration of 1.0 × 1011 vg/mouse via tail vein injection.
2.5. Immunofluorescence staining
Bloods were flushed from the hearts using PBS containing 10 % potassium chloride, following which they were fixed in 4 % paraformaldehyde, embedded in paraffin, and sectioned into 6-μm thick slices. The samples were then blocked with 5 % bovine serum albumin for 1 h at room temperature, and subsequently incubated with primary antibodies against Wheat Germ Agglutinin (abcam, ab178444) overnight at 4 °C. Following this, the samples were exposed to a secondary antibody against rabbit IgG H&L (Alexa Fluor® 488) (abcam, ab150077) for 1 h at room temperature. Nuclei were stained with DAPI, and images were captured using a fluorescence microscope. The cross-sectional area of cardiomyocytes in 5 randomly selected high-power fields (400 × ) within each section was measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.6. Oil red O staining
4 % paraformaldehyde was used to fix frozen sections (10‐μm‐thick) of the myocardial tissues or neonatal mouse cardiomyocytes (NMCMs). Lipids in cardiomyocytes were stained with 0.5 % Oil red O (Sigma‐Aldrich, O0625, St. Louis, MO, USA) in 60 % isopropanol. Oil red O dye that was not bound in cardiomyocytes was removed with 60 % isopropanol [28]. Subsequently, the nuclei were stained with hematoxylin, followed by sealing with Glycerol Jelly Mounting Medium (Beyotime, C0187, Shanghai, China). Images were captured using an optical microscope. In the case of NMCMs, absolute isopropanol was used to extract the stained Oil red O dye, and the optical density of the extracted dye was measured at 540 nm [28]. For tissue sections, the Oil red O area of 5 randomly selected high-power fields (200 × ) within each section was analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.7. Transmission electron microscopy
Upon completion of the intervention, ventricles (1 mm3) of the mice were fixed in 2.5 % glutaraldehyde in 0.1 M cacodylate buffer (Servicebio®, G1102, Hubei, China), followed by incubation in 2 % osmium tetroxide (Sigma-Aldrich) and 1 % aqueous uranyl acetate (Sigma-Aldrich) [28]. Subsequently, the samples underwent dehydration through a series of graded ethanol incubations and were embedded in epoxy resin (Sigma-Aldrich) [28]. The 60–80 nm ultrathin sections were cut using an ultra-microtome (Ultra 45°, Daitome) and double-stained with uranyl acetate (Sigma‐Aldrich) and lead citrate (Sigma‐Aldrich) [28]. The ultrastructure was examined using a transmission electron microscope (HT7700, Hitachi Ltd, Ohta, Japan) at 80 kV [28]. Lipid droplets were identified as electron-translucent structures lacking a surrounding membrane and were quantitatively analyzed in 10 randomly selected fields ( × 6.0k) using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Mitochondrial morphology was estimated using a 5-grade scoring system as described in a previous publication [31]. A score of 4 indicated cristae content >80 %, with well-defined and intact cristae [31]. A score of 3 denoted cristae content ranging from 60 % to 80 %, with slightly irregular cristae [31]. A score of 2 represented cristae content between 30 % and 60 %, characterized by major distortions and discontinuous membranes and cristae [31]. A score of 1 indicated cristae content between 10 % and 30 %, with severely fragmented or swollen cristae and warped membranes [31]. Finally, a score of 0 signified cristae content <10 %, with severely broken membranes and almost absent cristae [31].
2.8. GTT and ITT
Upon completion of the intervention, the mice underwent a fasting period of 12–16 h, followed by intraperitoneal injection with 1 g/kg D-(+)-Glucose (Sigma-Aldrich, G7021) for GTT or 1U/kg insulin (Novo Nordisk A/S, Novolin® R, Copenhagen, Denmark) for ITT. Blood glucose levels of the mice were assessed using a glucometer (OneTouch®, OneTouch Verio Vue®, NJ, USA) at 0, 30, 60, and 120 min post-injection. GraphPad Prism software (Version 9.0.0, San Diego, CA, USA) was utilized to plot the blood glucose curve at different time points and calculate the area under the curve (AUC).
2.9. Serum measurement
Upon completion of the intervention, blood samples were obtained from the eye sockets of the mice following a 12-h fast after sacrifice. These samples were allowed to clot at room temperature and then centrifuged to collect serum. The serum insulin concentrations were determined using a commercial Mouse Insulin (INS) ELISA Kit (CUSABIO, CSB-E05071 m, Hubei, China) in accordance with the manufacturer's protocol. Additionally, the serum concentrations of glucose, TG, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were assessed using commercial kits, including the Glucose Assay Kit (Rayto, S03039, Guangdong, China), TG Assay Kit (Rayto, S03027, Guangdong, China), TC Assay Kit (Rayto, S03042, Guangdong, China), HDL-C Assay Kit (Rayto, S03025, Guangdong, China), and LDL-C Assay Kit (Rayto, S03029, Guangdong, China) respectively. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the equation [Fasting blood glucose (FBG) (mmol/L) * Fasting insulin (FINS) (mIU/L)]/22.5, as per previous reports [32].
2.10. TG content
Upon completion of the intervention, cardiac tissues were excised from the mice and cryopreserved in liquid nitrogen. TG content in the heart tissues and NMCMs was assessed using a commercial TG Assay Kit (Nanjing Jiancheng Bioengineering Institute, A110-1-1, Jiangsu, China) and normalized by the protein concentration in accordance with the manufacturer's protocol.
2.11. FA uptake measurement
For in vivo assessment, upon completion of the intervention, the mice were anesthetized with 80 mg/kg sodium pentobarbital via peritoneal injection. Subsequently, the mice received an injection of 1 μg/g BODIPY™ FL C16 (Invitrogen™, D3821) via their tail vein and were sacrificed 1 h later. Their hearts were excised and rinsed in PBS to swiftly remove residual blood. X-ray and fluorescence imaging were conducted using a small animal living fluorescence imaging system (In-Vivo Xtreme, Bruker, Germany), and the mean photons were analyzed using Bruker MI SE software (Version 7.2, Bruker, Germany). The detection parameters were set as follows: excitation wavelength 460 nm, emission wavelength 535 nm, and exposure time 2 s. For in vitro assessment, following treatment with palmitic acid (PA) or BSA, NMCMs were rinsed with PBS and cultured in 1 % BSA containing 50 μM BODIPY™ FL C16 at 37 °C for 30 min. Subsequently, the NMCMs were rinsed with 1 % BSA in PBS three times and fixed with 4 % paraformaldehyde at room temperature for 15 min. DAPI was utilized to stain the nucleus. Images were captured using a fluorescence microscope and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.12. Cardiac FAO measurement
Upon completion of the intervention, adult mouse cardiomyocytes (AMCMs) were isolated from the mice using Langendorff according to a previously reported protocol. The AMCMs were seeded in a Seahorse XF96 Culture Microplate (Agilent, 101085-004) and cultured with XF Assay Medium Modified DMEM (Seahorse Bioscience, 103680-100) containing 0.5 mM glucose, 1 × GlutaMAX (Gibco™, 35050079), and 0.5 mM Carnitine (Sigma-Aldrich, 8.40092) overnight. The medium was then replaced with FAO assay medium (111 mM NaCl, 4.7 mM KCl, 1.25 mM CaCl2, 2.0 mM MgSO4, 1.2 mM Na2HPO4, 2.5 mM glucose, 0.5 mM carnitine, and 5 mM HEPES) at 37 °C for 30 min prior to the start of the assay. Palmitate-BSA (200 μM, Sigma-Aldrich, P0500) or BSA (34 μM, Sigma-Aldrich, 126575) was added to the medium. The OCR was measured by hydrated probes in the Agilent Seahorse Xfe96 Extracellular Flux Analyzer at baseline and after the injection of oligomycin (1 μM, MCE, HY-N6782, Montclair, NJ, USA), carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP, 1 μM, MCE, HY-100410), and antimycin A (0.5 μM, Sigma-Aldrich, A8674). Total protein was then extracted with Cell Lysis Buffer (CST, #9803) and quantified using the BCA Protein Quantitation Kit (Shenergy Biocolors, K3000, Shanghai, China). The OCR values were normalized by the protein concentration.
2.13. Widely targeted metabolomics
Upon completion of the study, heart tissues from both WT (n = 4) and Sirt5−/− (n = 3) mice, which were treated with HFD/STZ, were excised after sacrifice and sent to PTM Biolabs Inc (Zhejiang, China) for widely targeted metabolomics analysis. In brief, the heart tissues were homogenized and combined with 70 % methanol containing internal standard extract. The mixture was then centrifuged, and the supernatant was stored in a −20 °C refrigerator overnight. Subsequently, the supernatant was centrifuged again and transferred to the liner of the corresponding injection bottle for on-board analysis. The sample extracts were analyzed using a LC-ESI-MS/MS system (UPLC, ExionLC AD, https://sciex.com.cn/; MS, QTRAP® System, https://sciex.com/) [33]. LIT and triple quadrupole scans were acquired on a triple quadrupole-linear ion trap mass spectrometer (QTRAP), QTRAP® LC-MS/MS System, equipped with an ESI Turbo Ion-Spray interface, operating in positive and negative ion mode and controlled by Analyst 1.6.3 software (Sciex) [33]. A specific set of MRM transitions were monitored for each period according to the metabolites eluted within this period.
Unsupervised principal component analysis (PCA) was conducted using the “prcomp” package within R. Prior to the unsupervised PCA, the data was unit variance scaled [34]. The results of the hierarchical cluster analysis (HCA) for both samples and metabolites were visualized as heatmaps with dendrograms [34]. Additionally, Pearson correlation coefficients (PCC) between samples were calculated using the cor function in R and presented as heatmaps [34]. Both HCA and PCC analyses were performed using the R package ComplexHeatmap [34]. For the HCA, normalized signal intensities of metabolites (unit variance scaling) were represented as a color spectrum [34]. Significantly regulated metabolites between groups were identified based on variable importance in projection (VIP) values ≥ 1 and absolute Log2FC ≥ 1 [34]. VIP values were extracted from the orthogonal partial least squares-discriminant analysis (OPLS-DA) result, which also included score plots and permutation plots, and was generated using the R package MetaboAnalystR [34]. The data was log-transformed (log2) and mean-centered prior to OPLS-DA [34]. To prevent overfitting, a permutation test (200 permutations) was conducted [34].
2.14. 4D label-free relative quantitative proteomics and succinylomics
Upon completion of the study, heart tissues were excised from WT (n = 6) and Sirt5−/− (n = 6) mice, all of which were treated with HFD/STZ. These tissues were combined into one sample to ensure sufficient protein content in each group, and subsequently sent to PTM Biolabs Inc (Zhejiang, China) for 4D label-free proteomics and succinylome analyses. In brief, the heart tissues were homogenized in liquid nitrogen and lysed in a lysis buffer (8 M urea, 1 % Protease Inhibitor Cocktail, 3 μM TSA, and 50 mM nicotinamide). The protein concentration was determined using the BCA kit. Equivalent proteins from each group were precipitated by 20 % trichloroacetic acid, washed with acetone, sonicated with 200 mM triethylammonium bicarbonate, and then digested with trypsin. Samples were reduced with 5 mM Dithiothreitol and alkylated with 11 mM Iodoacetamide. Affinity enrichment of succinylated peptides was performed using agarose beads-conjugated pan anti-succinyllysine antibody (PTM BIO, PTM-402). The bound peptides were eluted from the agarose beads by 0.1 % trifluoroacetic acid. The eluted fractions were combined and vacuum-dried, and then desalted with C18 ZipTips (Millipore, ZTC18 M). The peptides were dissolved in solvent A and separated in the NanoElute UPLC system. Subsequently, the peptides were subjected to NSI source followed by tandem mass spectrometry (MS/MS) in timsTOF Pro coupled online to the UPLC. Both the peptide precursor and its secondary fragments were detected and analyzed using TOF. The m/z scan range was 100-1700 for full scan, and the parallel accumulation serial fragmentation (PASEF) mode was set for data acquisition. The resulting MS/MS data were processed using the Maxquant search engine (v1.6.6.0). Tandem mass spectra were searched against the Mouse uniprot database concatenated with a reverse decoy database, and proteins and peptide spectrum matches (PSMs) were identified with a 1 % FDR.
The differentially succinylated proteins encompassed those exhibiting an increase of more than 2-fold in Sirt5−/− + HFD/STZ compared with WT + HFD/STZ, as well as those exclusively detected in Sirt5−/− + HFD/STZ. Subcellular localization of the differentially succinylated proteins was predicted using Wolfpsort software. Furthermore, the differentially succinylated proteins were annotated using KEGG online service tools KAAS and subsequently mapped onto the KEGG pathway database using KEGG online service tools KEGG mapper. Targeted functional proteins of Sirt5 in regulating FAO were identified based on their succinylation ratio, whether they function as rate-limiting enzymes, and through integrated analysis with results from the widely targeted metabolomics.
2.15. Immunoprecipitation (IP) and western blotting
Upon completion of the study, total protein was extracted from the heart tissues of the mice using Pierce™ IP Lysis Buffer (Thermo Scientific, 87788) containing EDTA-free protease inhibitor cocktail (abcam, ab201111), nicotinamide (50 mM; Sigma-Aldrich, N3376), and trichostatin A (3 μM; Selleck, S1045). Protein concentrations were quantified using the BCA Protein Quantitation Kit (Shenergy Biocolors, K3000). For IP, 2 mg of protein was incubated with primary antibodies against CPT2 (Proteintech, 26555-1-AP, Chicago, IL, USA) overnight at 4 °C. The antibody-antigen complex was then immunoprecipitated using Pierce™ Protein A/G Magnetic Beads (Thermo Scientific, 88802) and eluted with 1 × Pierce™ Lane Marker Non-Reducing Sample Buffer (Thermo Scientific, 39001). The eluted samples or 3 % input were resolved on SDS-PAGE gels and subsequently blotted onto 0.2 μm PVDF membranes. The membranes were probed with primary antibodies against Ksuc (PTM BIO, PTM-401), Sirt1 (CST, 2314), Sirt2 (CST, 12650), Sirt3 (CST, 5490), Sirt4 (CST, 24662), Sirt5 (CST, 8782), Sirt6 (CST, 12486), Sirt7 (CST, 5360), CPT2, or TUBA (CST, 2144) overnight at 4 °C, and then incubated with horseradish peroxidase-linked secondary antibodies against rabbit IgG (CST, 7074), mouse IgG (CST, 7076), or conformation-specific rabbit IgG (CST, 5127) for 1 h at room temperature. Immunoblots were detected using an ECL system (Merck Millipore, WBULS0500). The intensity of bands was quantified with Image J (National Institutes of Health, Bethesda, MD, USA) and normalized to the control.
2.16. NMCMs isolation and PA treatment
NMCMs were enzymatically isolated from the ventricles of WT and Sirt5−/− mice aged 1–3 days, following previously established protocols [28]. In brief, ventricles were excised from neonatal mice and digested with 0.1 % trypsin (Gibco™, 15050065) in D-hanks buffer at 4 °C overnight. Subsequently, the ventricles underwent three cycles of digestion with collagenase II (Gibco™, 17101015) in D-hanks buffer at 37 °C for 10 min per cycle. NMCMs were collected from each cycle and purified using a differential-speed adherence method. Cytosine-B-D-arabino-furanoside hydrochloride (Sigma-Aldrich, C6645) was added to the culture medium to inhibit the proliferation of non-cardiomyocytes. The NMCMs were starved with fetal bovine serum-free medium overnight and then treated with BSA (control) or PA (200 μM) for 48 h, followed by harvest for analyses.
2.17. Adenovirus construction and transfection
We utilized adenovirus to overexpress Sirt5, Cpt2-WT, or Cpt2-K424R in NMCMs for in vitro studies and in myocardia for in vivo studies. Ad-Sirt5, Ad-control, Ad-6His-Cpt2-WT, and Ad-6His-Cpt2-K424R were synthesized and constructed by OBiO Biology (Shanghai, China). The AdMax system was employed to package the adenovirus, following our previous protocol [28]. In brief, AD293 cells were transfected with expression vectors and packaging vectors simultaneously using a calcium phosphate-mediated protocol. After 72 h, the cells were harvested, and the adenovirus was purified using CsCl gradient centrifugation. Ad-Sirt5, Ad-control, Ad-6His-Cpt2-WT, and Ad-6His-Cpt2-K424R were used at a MOI of 100 for transfecting NMCMs. Ad-6His-Cpt2-WT and Ad-6His-Cpt2-K424R were administered at a concentration of 1 × 109 vg/mouse via tail vein injection.
2.18. CPT2 activity measurement
CPT2 activity in NMCMs and myocardia from the mice was assessed following established procedures [35]. In brief, NMCMs or myocardia were homogenized with lysis buffer (10 mM HEPES, pH7.4; 0.1 % Triton X-100; 11.5 % (w/v) sucrose; 5 % (v/v) protease inhibitor cocktail) and centrifuged at 1500×g at 4 °C for 5 min. The supernatant was collected and added to the reaction buffer (200 mM HEPES, 10 mM EGTA, 0.2 M sucrose, 400 mM KCl, 2 mM DTNB, 0.13 % (w/v) bovine serum albumin, 20 μM palmityl-CoA, 10 μM malonyl-CoA), and incubated for 1 h at 25 °C. The reaction was initiated by adding 15 mM l-carnitine and measured using a spectrophotometer. Total protein was quantified using a BCA Protein Quantitation Kit (Shenergy Biocolors, K3000). The CPT2 activity was determined as μmol/h/mg prot.
2.19. Statistical analysis
Data were presented as mean ± SEM. Comparisons of two groups were analyzed by Student's t-test. Comparisons of multiple groups were analyzed by one-way ANOVA, followed by Bonferroni post hoc analysis. All statistical analyses were performed using the GraphPad Prism Software (San Diego, CA, USA). P-values less than 0.05 were considered statistically significant.
3. Results
3.1. Sirt5 is the only sirtuin gene altered in myocardia from diabetic HF patients
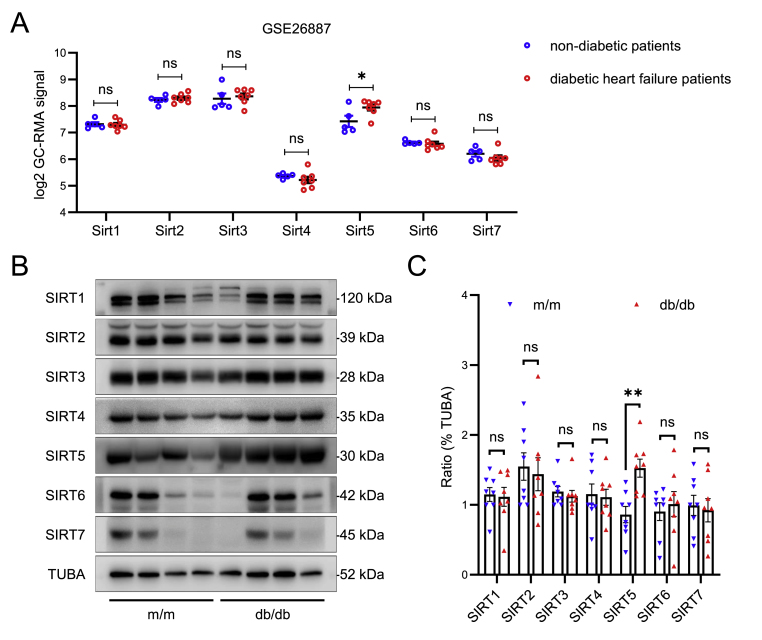
In order to ascertain the changes in sirtuins expressions during the development of DbCM, we obtained a diabetic HF-related expression profiling microarray (GSE26887) from the GEO repository and conducted an analysis using log2 GC-RMA signal. The dataset comprised LV cardiac biopsies from five control non-HF patients and seven diabetic HF patients, with gene expression assessed using the Affymetrix GeneChips Human Gene 1.0 ST array. As depicted in Fig. 1A, only the expression of Sirt5 exhibited a modestly upregulation in diabetic HF patients, while no discernible alteration could be observed in the expression of other sirtuins.
Fig. 1.
Myocardial expression of Sirt5 in diabetic patients and mice. A, Myocardial sirtuins (Sirt1-7) expression in non-diabetic (n = 5) and diabetic HF (n = 7) patients in the GSE26887 from GEO dataset. Raw data of log2 GC-RMA signal were analyzed using Student's t-test. B, Representative Western blot of myocardial sirtuins (Sirt1-7) protein expression in 32-week-old male m/m mice and db/db mice (n = 8/group), TUBA (α-tubulin) was used as the loading control. C, Statistical analysis of the Western blot using Student's t-test. Data are expressed as mean ± SEM. ns, no significant, *P < 0.05, **P < 0.01.
To further validate these results, we used 32-week-old db/db mice as the model for DbCM and assessed cardiac sirtuins. Consistent with the results found in human data, the cardiac protein expression of Sirt5 was indeed upregulated in db/db mice compared to m/m control mice (Fig. 1B and C). Conversely, no significant difference in the protein expression of other sirtuins could be observed between the m/m mice and db/db mice (Fig. 1B and C). These findings showed that Sirt5 was significantly upregulated in diabetic heart.
3.2. Sirt5 is an important regulator of cardiac function and structure in diabetic mice
To investigate the role of Sirt5 in DbCM, both WT mice and Sirt5−/− mice were subjected to low-dose STZ injections for 5 consecutive days and maintained on a HFD for 24 weeks to induce DbCM. The absence of Sirt5 did not result in compensatory expression of other sirtuins in the diabetic heart (Figs. S1A–S1B). Both WT and Sirt5−/− mice exhibited significant increase in body weight and FBG due to HFD/STZ (Figs. S2A–S2B). However, the knockout of Sirt5 did not affect body weight or FBG during HFD/STZ induction (Figs. S2A–S2B). Subsequently, we assessed whether Sirt5 regulates glucose tolerance and insulin resistance. As depicted in Figs. S2C–S2F, no difference was observed in GTT and ITT between WT and Sirt5−/− mice following HFD/STZ induction. Furthermore, the absence of Sirt5 did not appear to influence serum insulin contents or HOMA-IR in the mice (Figs. S2G–S2H). These findings indicated that Sirt5 did not play a regulatory role in glucose metabolism in DbCM.
To further elucidate the role of Sirt5 in DbCM, we conducted echocardiography and histological analyses. Following HFD/STZ induction, Sirt5−/− mice exhibited a more pronounced decrease in E/A ratio, e’, and dp/dtmin, along with a more obvious increase in the E/e’ ratio compared to WT mice (Fig. 2A–D and 2G), indicating a deterioration in diastolic dysfunction. Additionally, while no disparity was observed in LVEF and LVFS between WT and Sirt5−/− mice, dp/dtmax was significantly reduced in Sirt5−/− mice (Fig. 2A, 2E-2F and 2H), suggesting the presence of systolic dysfunction. Compared with the WT mice, more significant increases in HW and HW/TL ratio could be observed in Sirt5−/− mice following HFD/STZ induction (Fig. 2I–J and 2L-2M). Consistent with these findings, WGA staining demonstrated an augmentation in myocyte cross-sectional area in Sirt5−/− mice compared to WT mice (Fig. 2K and N). Collectively, these results indicated that the deficiency of Sirt5 exacerbated cardiac dysfunction and hypertrophy in HFD/STZ-induced DbCM, independent of blood glucose and insulin resistance.
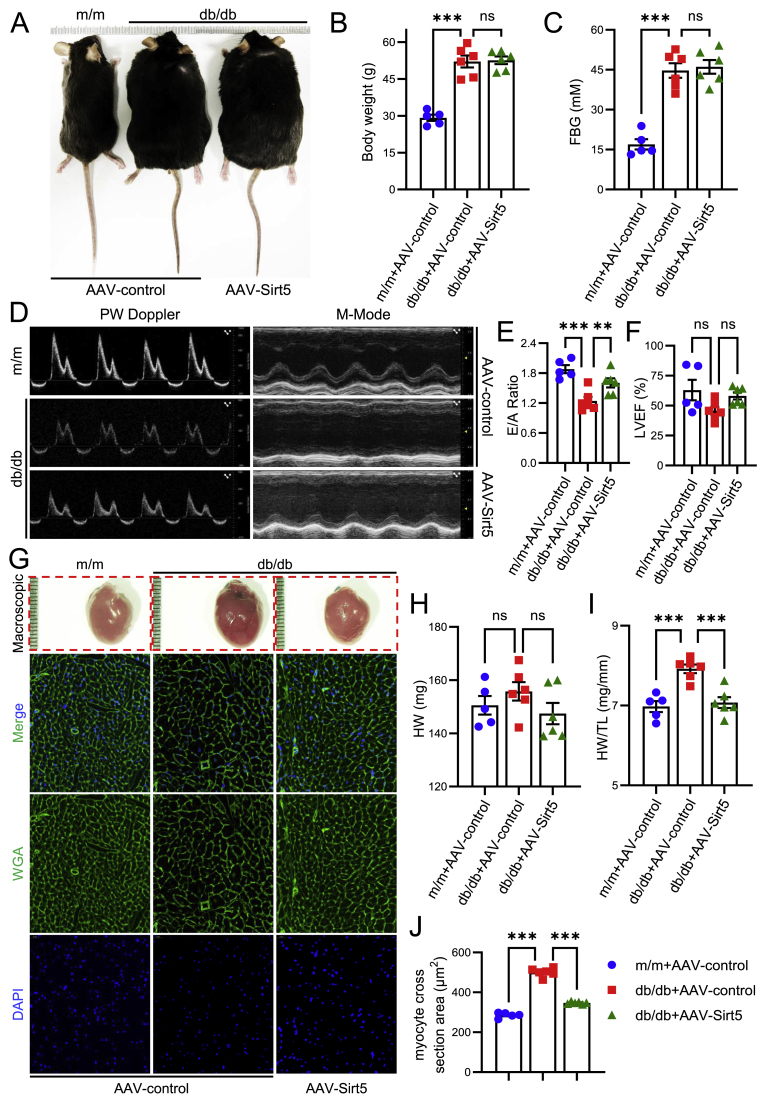
Fig. 2.
Effects of Sirt5 knockout on HFD/STZ-induced cardiac dysfunction and hypertrophy. A, Representative echocardiographic images of PW Doppler (left), LV TDI (Left ventricular tissue doppler imaging) (middle) and M-Mode (right) in WT and Sirt5−/− mice after HFD/STZ induction (n = 6/group). B–F, Statistical analyses of E/A Ratio (B), E/e’ Ratio (C), e’ (D), LVEF (E) and LVFS (F) of the above mice. G-H, Statistical analyses of LV pressure parameters, including dp/dtmin (G) and dp/dtmax (H) of the above mice (n = 6/group). I-J, Representative macroscopic images of the mice (I) and their hearts (J). K, Representative immunofluorescent images of WGA staining of myocardia of the above mice (n = 6/group). Green represents WGA, blue represents DAPI, scale bars represent 20 μm. L-M, Statistical analyses of HW (L) and HW/TL (M) of the above mice (n = 6/group). N, Statistical analysis of myocyte cross section area of the WGA staining of the above mice (n = 6/group). Data are expressed as mean ± SEM. ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data in B, C, D, E and F were analyzed using multiple unpaired t tests. Data in G, H, L, M and N were analyzed using Student's t-test. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Given the significant effect of Sirt5 deficiency on DbCM development, we next sought to verify whether forced expression of Sirt5 could protect against DbCM. AAV9 was used to mediate cardiac-specific forced expression of Sirt5 in db/db mice. In comparison with m/m mice, greater weight gain and higher fasting blood glucose levels were observed in db/db mice, while cardiac Sirt5 overexpression had no significant effect on weight gain and fasting blood glucose levels in db/db mice (Fig. 3A–C). Forced expression of Sirt5 significantly correct the diastolic dysfunction of db/db mice, as showed by improved E/A ratio (Fig. 3D and E). Meanwhile, no significant difference in systolic function was observed among m/m mice, db/db mice, and Sirt5-forced expressed db/db mice (Fig. 3D and F). HW and the HW/TL ratio were also significantly corrected by cardiac Sirt5 expression in db/db mice (Fig. 3G–I). WGA staining showed that the increase in myocyte cross-sectional area in db/db mice was attenuated by cardiac Sirt5 forced expression (Fig. 3G and J). These data indicate that forced cardiac Sirt5 expression alleviates diastolic dysfunction and hypertrophy in DbCM.
Fig. 3.
Effects of Sirt5 forced expression on cardiac function and hypertrophy in db/db mice. 20-week-old male m/m and db/db mice were infected with AAV9-Sirt5 to force express Sirt5 in cardiomyocytes, AAV9-control was used as control virus, cardiac function and hypertrophy were examined 8 weeks after infection. m/m + AAV-control: n = 5, db/db + AAV-control: n = 6, db/db + AAV-Sirt5: n = 6. A, Representative macroscopic images of the above mice. B, Statistical analysis of body weight of the above mice. C, Statistical analysis of FBG of the above mice. D, Representative echocardiographic images of PW Doppler (left) and M-Mode (right) of the above mice. E-F, Statistical analyses of E/A Ratio (E) and LVEF (F) of the above mice. G, Representative macroscopic images of hearts (top row) and immunofluorescent images of WGA staining of myocardia of the above mice. Green represents WGA, blue represents DAPI, scale bars represent 20 μm. H–I, Statistical analyses of HW (L) and HW/TL (M) of the above mice. J, Statistical analysis of myocyte cross section area of the WGA staining of the above mice. Data are expressed as mean ± SEM. ns, not significant. **P < 0.01, ***P < 0.001. Data in B, C, E, F, H, I and J were analyzed using one-way ANOVA followed by Bonferroni post hoc analysis. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Taken together, gain- and loss-of-function evidence clarify Sirt5 as a pivotal determinant of cardiac function and hypertrophy in the development of DbCM.
3.3. Cardiac lipotoxicity is exacerbated by Sirt5 knockout but attenuated by Sirt5 forced expression in DbCM
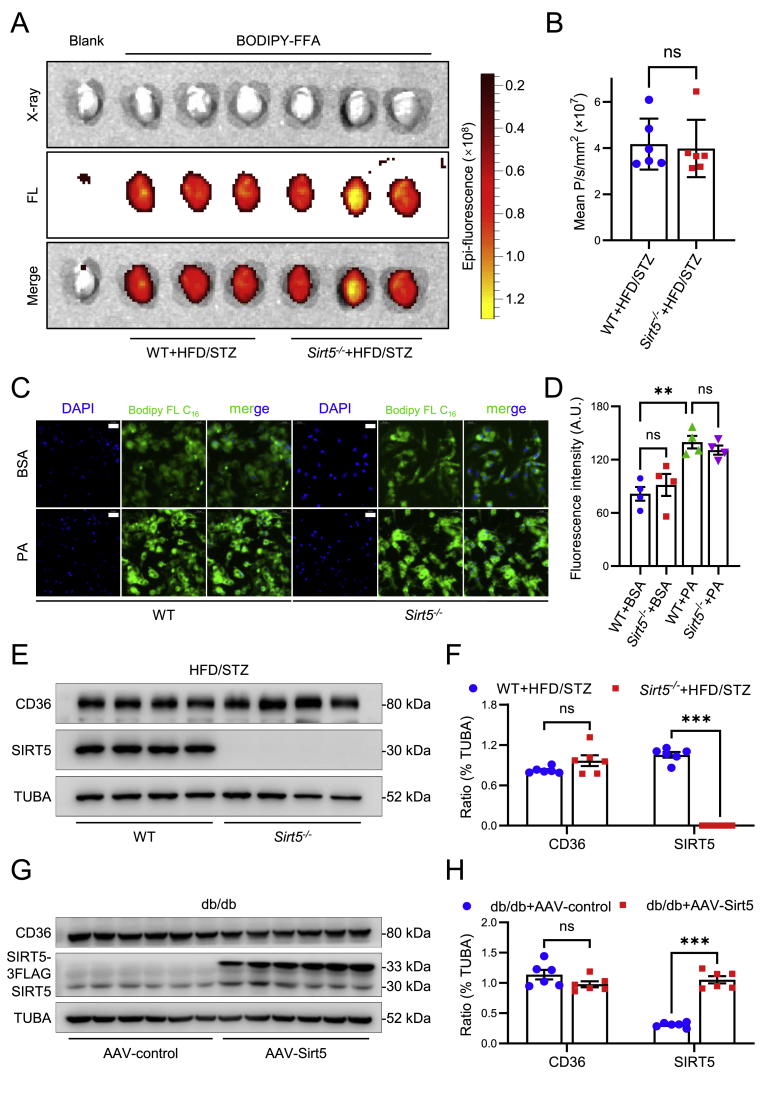
Considering that cardiac lipotoxicity is a pivotal pathogenic factor of DbCM, we therefore further investigate the impact of Sirt5 on ectopic fat deposition in cardiomyocytes. Firstly, FFA uptakes were examined both in vivo and in vitro. As depicted in Fig. 4A and B, no discernible difference was observed in the uptake of BODPY-FA by heart between the WT mice and Sirt5−/− mice after HFD/STZ induction. Similar results were obtained in PA-induced lipotoxicity in cardiomyocytes (Fig. 4C and D). Furthermore, CD36, the FA transporter in cardiomyocytes responsible for mediating cardiac lipid uptake in DbCM, exhibited comparable protein expression levels between WT and Sirt5−/− mice following HFD/STZ (Fig. 4E and F), as well as between control and Sirt5 forced expression db/db mice (Fig. 4G and H). Taken together, these results showed that Sirt5 does not affect FFA uptake in cardiomyocytes.
Fig. 4.
Effects of Sirt5 on FA uptake in diabetic mice and PA-treated NMCMs. A, Representative X-ray and fluorescent images of cardiac BODIPY™ FL C16 uptake in WT and Sirt5−/− mice after HFD/STZ induction (n = 6/group). FL: Fluorescence. B, Statistical analysis of epi-fluorescence of X-ray and fluorescent images of the above mice. C, Representative fluorescent images of Bodipy™ FL C16 uptake in WT and Sirt5−/− NMCMs treated with BSA or 200 μM PA for 48 h (n = 4/group). Green: Bodipy™ FL C16. Blue: DAPI. Scale bars: 20 μm. D, Statistical analysis of fluorescence intensity of the fluorescence images of the above NMCMs. E, Representative Western blot of myocardial FA transporter CD36 protein expression in WT and Sirt5−/− mice after HFD/STZ induction (n = 6/group), TUBA (α-tubulin) was used as the loading control. F, Statistical analysis of the Western blot. G, Representative Western blot of myocardial CD36 protein expression in db/db mice transfected with AAV9-control or AAV9-Sirt5 (n = 6/group), TUBA (α-tubulin) was used as the loading control. H, Statistical analysis of the Western blot. Data are expressed as mean ± SEM. ns, not significant, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data in B was analyzed using Student's t-test. Data in D was analyzed using one-way ANOVA followed by Bonferroni post hoc analysis. Data in F and H was analyzed using multiple unpaired t tests. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Next, we further examine the function of Sirt5 on lipid deposition in cardiomyocytes. In vivo, Oil red O staining revealed a greater accumulation of lipid droplets in the myocardium of Sirt5−/− mice compared to WT mice following HFD/STZ induction (Fig. 5A and B). Similarly, TEM demonstrated that the absence of Sirt5 resulted in increased deposition of lipid droplets in cardiomyocytes compared to WT (Fig. 5A and C). Given that cardiomyocytes are weak in FA synthesis and mainly rely on the uptake it from the blood circulation, we subsequently investigated the effect of Sirt5 on the lipid profiles. As depicted in Fig. 5D, Sirt5 knockout significantly elevated cardiac TG content compared to WT following HFD/STZ induction. However, the serum lipid profiles, including TG, TC, LDL-C, and HDL-C, remained similar between WT mice and Sirt5−/− mice after HFD/STZ induction (Fig. 5E and F). In vitro, PA was utilized to induce cardiomyocytes lipotoxicity to model DbCM. Corroborating the in vivo findings, Oil red O staining demonstrated that the absence of Sirt5 significantly enhanced PA-induced lipid droplet deposition and increased TG contents (Figs. S3A–S3D). Therefore, these data indicated that the deficiency of Sirt5 exacerbated cardiac lipotoxicity in DbCM, potentially contributing to cardiac dysfunction and hypertrophy.
Fig. 5.
Effects of Sirt5 knockout or Sirt5 forced expression on cardiac lipotoxicity in diabetic mice. A, Representative images of myocardial Oil red O staining (left) and TEM (right) in WT and Sirt5−/− mice after HFD/STZ induction (n = 6/group). Scale bar (left): 50 μm (200 × ) or 20 μm (630 × ). Scale bar (right): 10 μm (1500 × ) or 2 μm (6000 × ). B–C, Statistical analyses of lipid droplets area in Oil red O staining (B) and lipid droplets per HPF in TEM (C) in the above mice (n = 6/group). D-F, Statistical analyses of cardiac TG content (D), serum TG concentration (E) and serum cholesterols concentration (including TC, LDL-C and HDL-C) (F) of the above mice (n = 6/group). G, Representative images of myocardial Oil red O staining (left) and TEM (right) in the diabetic mice transfected with AAV9-control or AAV9-Sirt5. Scale bar (left): 50 μm (200 × ) or 20 μm (630 × ). Scale bar (right): 10 μm (1500 × ) or 2 μm (6000 × ). H–I, Statistical analyses of lipid droplets area in Oil red O staining (H) and lipid droplets per HPF in TEM (I) in the above mice. J-L, Statistical analyses of cardiac TG content (J), serum TG concentration (K) and serum cholesterols concentration (including TC, LDL-C and HDL-C) (L) of the above mice. Data are expressed as mean ± SEM. ns, not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data in B-E were analyzed using Student's t-test. Data in F was analyzed using multiple unpaired t tests. Data in H–K was analyzed using one-way ANOVA followed by Bonferroni post hoc analysis. Data in L was analyzed using two-way ANOVA followed by Tukey's post hoc analysis. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
We proceeded to validate the impact of Sirt5 forced expression on cardiac lipids deposition during DbCM. In vivo, Oil red O staining revealed a greater accumulation of lipids in the myocardium of db/db mice compared with m/m mice, which was significantly reduced by cardiac Sirt5 forced expression in the db/db mice (Fig. 5G and H). Similarly, TEM showed that less lipid droplets were deposited in cardiomyocytes in db/db mice transfected with AAV9-Sirt5 compared with the control AAV9 (Fig. 5G and I). Although no significant influence on serum TG, TC, LDL-C, and HDL-C could be observed, cardiac Sirt5 forced expression led to a reduction in cardiac TG levels (Fig. 5J–L). In vitro, adenovirus was used to force express Sirt5 in cardiomyocytes. Oil red O staining showed that Sirt5 forced expression significantly inhibited PA-induced lipid droplet deposition and the PA-induced increase in TG content in NMCMs (Figs. S3E–S3H). Therefore, these data indicated that forced expression of Sirt5 alleviated cardiac lipotoxicity in DbCM.
To further dissect the function of Sirt5 on cardiac lipids deposition, a widely targeted metabolomics analysis of the myocardia from the WT and Sirt5−/− mice after HFD/STZ induction was conducted. In this metabolomics study, a total of 614 metabolites were detected with a VIP≥1 in the myocardia of the mice, among which 17 metabolites were increased and 14 metabolites were decreased by more than 2-fold in Sirt5−/− mice compared with WT mice (Fig. 6A). More than a half (17/31) of the differential contents of metabolites were fatty acyl-carnitines, the majority of which were medium (C8–C12) and long (C14–C18)-chain fatty acyl-carnitines (Fig. 6B and C). Among the fatty acyl-carnitines, 14 were increased while only 3 were decreased by Sirt5 knockout (Fig. 6B and C). Consistently, Pearson correlation plot showed that differential metabolites were significantly clustered in fatty acyl-carnitines (Fig. 6B). These data further indicated that Sirt5 deficiency led to an accumulation of FA-derived intermediate metabolites in the diabetic heart.
Fig. 6.
Effects of Sirt5 on cardiac FAO and mitochondrial integrity in diabetic mice. A-C, Widely targeted metabolomics of myocardia in WT (n = 3) and Sirt5−/− (n = 4) mice after HFD/STZ induction. A, Volcano plot of the metabolomics. B, Pearson correlation plot of the differential metabolites of the metabolomics. Metabolites are ordered according to correlation coefficient. Correlations between each pair of metabolites are displayed in the cells of the heatmap. Cells are color coded with colors ranging from green to red to depict correlations ranging from −1 to 1. C, Classification of the differential metabolites of the metabolomics. D, Seahorse metabolic analysis of FAO of AMCMs, that were isolated from WT and Sirt5−/− mice after HFD/STZ induction (n = 6/groups, 3–4 wells/mouse), at the presence of BSA or PA. E, Seahorse metabolic analysis of FAO of AMCMs, that were isolated from db/db mice transfected with AAV9-control or AAV9-Sirt5 (n = 3/groups, 2–5 wells/mouse), at the presence of BSA or PA. F, Representative images of myocardial TEM in WT and Sirt5−/− mice after HFD/STZ induction (n = 6/group). Scale bar: 2 μm (6000 × ). G, Pie chart shows the prevalence of the individual score grades across the analyzed mitochondrial population in myocardia from WT and Sirt5−/− following HFD/STZ induction. H, Bar graph shows the overall mean scores in each group (n = 6/group). I, Representative images of myocardial TEM in m/m mice and db/db mice transfected with AAV9-control or AAV9-Sirt5. Scale bar: 2 μm (6000 × ). J, Bar graph shows the overall mean scores in each group (n = 5–6/group). Data are expressed as mean ± SEM. ns, not significant, *P < 0.05, ***P < 0.001, ****P < 0.0001. Data in D-E and J was analyzed using one-way ANOVA followed by Bonferroni post hoc analysis. Data in H was analyzed using Student's t-test. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Collectively, the aforementioned data indicated that Sirt5 improves cardiac function and mitigated hypertrophy in DbCM by suppressing cardiac lipotoxicity. The accumulation of FA-derived intermediate metabolites in cardiomyocytes following Sirt5 knockout could be attributed to insufficiency in FAO.
3.4. Sirt5 increases cardiac FAO in DbCM
Considering that FA uptake was not affected by Sirt5, we proceeded to verify the effect of Sirt5 on FAO in cardiomyocytes during DbCM. AMCMs were isolated from the WT and Sirt5−/− mice after HFD/STZ induction, or from the db/db mice that were transfected with AAV9-control or AAV9-Sirt5, followed by FAO measurement immediately after the attachment of AMCMs to the culture microplate. We found that both basal respiration and maximal respiration were significantly attenuated by Sirt5 knockout in AMCMs isolated from the mice after HFD/STZ induction (Fig. 6D). Conversely, maximal respiration was significantly enhanced by Sirt5 overexpression in AMCMs isolated from db/db mice (Fig. 6E). These data indicate a pivotal role of Sirt5 in the regulation of cardiac FAO in DbCM. As mitochondria is the major organelles responsible for FAO in the context of DbCM, the sufficiency of FAO could serve as an indicator of mitochondrial function indirectly, which depends on the morphological integrity of the mitochondria. Therefore, we further investigated the effect of Sirt5 on mitochondrial integrity in cardiomyocytes during DbCM. A five-grade scoring system, as per previous publications [31], was employed to evaluate mitochondrial morphology (Figs. S4A–S4B). As illustrated in Fig. 6F–H, the damage in mitochondrial morphology, characterized by swelling, distorted membranes, irregularities, and loss of cristae, was exacerbated by the deletion of Sirt5 in mice following HFD/STZ induction, in comparison with WT. Conversely, forced expression of Sirt5 significantly ameliorated diabetes-induced damage to mitochondrial morphology (Fig. 6I and J). Collectively, these findings indicated that Sirt5 improved FAO, resulting in lipid accumulation in cardiomyocytes and cardiac lipotoxicity.
3.5. Sirt5 regulates cardiac FAO and exacerbates lipotoxicity through Lys424 succinylation of CPT2
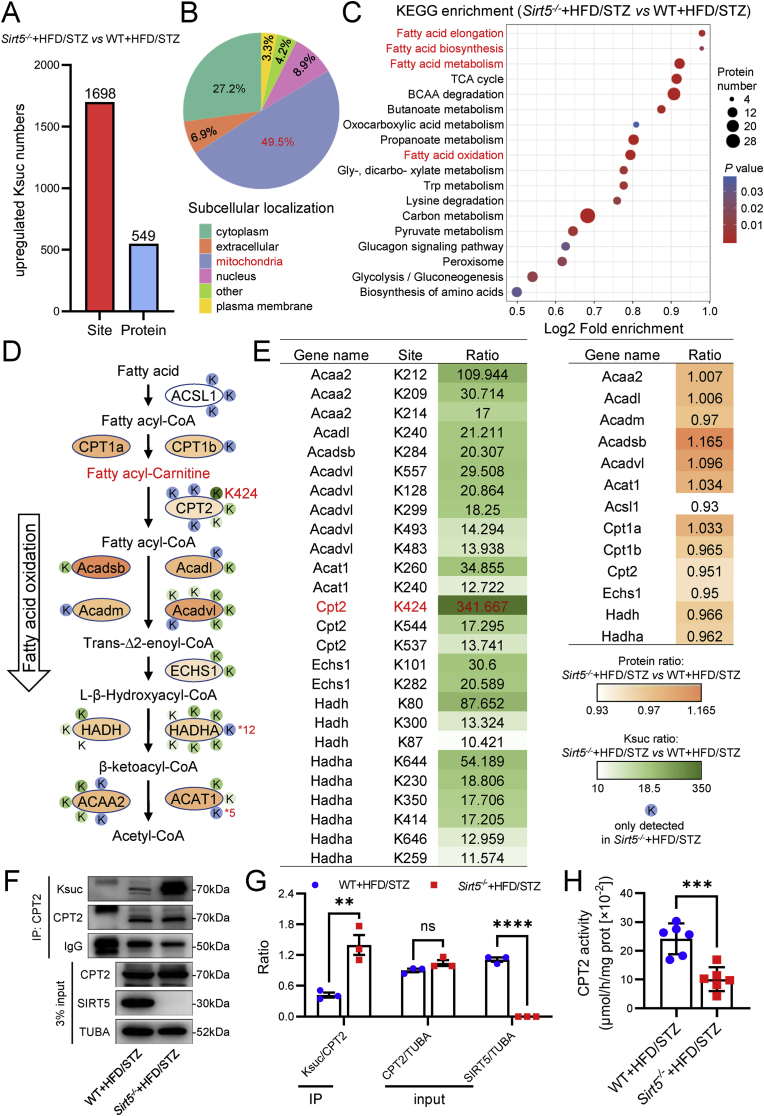
We next sought to elucidate the molecular mechanism of Sirt5 in regulating FAO. Considering that Sirt5 operates through the regulation of de-acylation of substrate proteins, our subsequent investigation involved the assessment of pan-acylation of cardiac proteins in WT and Sirt5−/− mice after HFD/STZ induction. As illustrated in Figs. S5A–S5B, lysine succinylation (Ksuc) exhibited the most significant increase, followed by lysine glutarylation, while lysine malonylation and acetylation showed no marked alterations in cardiac proteins between Sirt5 knockout and WT heart. Building upon these findings, 4D label-free proteomics and succinylome analysis of the myocardia from WT and Sirt5−/− mice after HFD/STZ induction were conducted to identify the substrates of Sirt5 in the regulation of FAO. Ksuc levels of 1698 peptides were found to be increased by more than 2-fold in Sirt5−/− mice compared with WT mice, and these were mapped to 549 unique proteins (Fig. 7A). The differentially succinylated proteins were predominantly located in mitochondria (Fig. 7B) and functionally enriched in fatty acid metabolism, including FAO, fatty acid elongation, and fatty acid biosynthesis (Fig. 7C).
Fig. 7.
Integrated analysis of the metabolomics and succinylomics identified CPT2 as the functional Ksuc substrate protein of Sirt5 in regulating FAO. A-C, Bioinformatics analyses of the succinylomics in myocardia of the WT and Sirt5−/− mice after HFD/STZ induction. A, Number of succinylated peptides and proteins upregulated in myocardia of Sirt5−/− mice compared with WT mice after HFD/STZ induction. B, Subcellular localization of the differentially succinylated proteins. C, KEGG enrichment of the differentially succinylated proteins. D, Flow chart of integrated analysis of the differential Ksuc sites, Ksuc ratios and protein expression of metabolic enzymes and the differential metabolites in the FAO pathway. E, Tables of the differential Ksuc sites, Ksuc ratios (left) and protein expression (Right) of metabolic enzymes in the FAO pathway. F, Representative immunoprecipitation (IP) and Western blot of Ksuc and proteins expression levels of myocardial CPT2 in WT and Sirt5−/− mice after HFD/STZ induction (n = 3/group). TUBA was used as the loading control. Rabbit IgG was used as the IP control. G, Statistical analysis of the IP and Western blot. H, Statistical analysis of myocardial CPT2 activity in WT and Sirt5−/− mice after HFD/STZ induction (n = 6/group). Data are expressed as mean ± SEM. ns, not significant. **P < 0.01, ***P < 0.001, ****P < 0.0001. Data in G and H was analyzed using Student's t-test.
An integrated analysis of widely targeted metabolomics, 4D label-free proteomics, and succinylomics were conducted to identify the potential substrate protein of Sirt5 that regulates fatty acyl carnitine metabolism in the FAO pathway. As depicted in Fig. 7D and E, the Ksuc level in Lys424 residue of CPT2 was found to be increased nearly 340-fold in Sirt5−/− mice compared with WT mice after HFD/STZ induction, and this site was also identified as the top succinylated site in the succinylomics analysis. To validate this finding, myocardial CPT2 was immunoprecipitated from the mice and detected using a pan-succinylation antibody. Consistent with the succinylomics result, the Ksuc level of CPT2 was significantly increased in Sirt5−/− mice compared with WT mice after HFD/STZ induction (Fig. 7F and G). Consequently, the enzyme activity of CPT2 was found to be lower in the myocardia from Sirt5−/− mice compared with WT mice (Fig. 7H). Conversely, the Ksuc level of cardiac CPT2 was significantly reduced in db/db mice transfected with AAV9-Sirt5 compared to the control AAV9 (Fig. S6A), resulting in an increase in the enzyme activity of CPT2 (Fig. S6B). Considering that CPT2 is critical for FAO, these data suggested that Sirt5 deficiency might impair FAO and lead to the accumulation of fatty acyl-carnitines in diabetic mice by succinylating and inactivating CPT2.
Having identified Lys424 as the top succinylated site in the succinylomics analysis, our next objective was to ascertain whether Lys424 succinylation impacts the enzyme activity and molecular function of CPT2. The mass spectrum of succinylated Lys424 of CPT2 is presented in Fig. 8A, and further homology analysis revealed that Lys424 of CPT2 is highly conserved across different species (Fig. 8B). To investigate the effect of Lys424 de-succinylation on its enzyme activity, WT and Sirt5−/− mice were transfected with adenovirus via tail vein injection to overexpress His-tagged WT or K-to-R mutant (mimicking the de-Ksuc state) CPT2. One week after transfection, exogenous CPT2 was immunoprecipitated from the myocardia of the mice. As a result, the K424R mutant led to a significant reduction in the succinylation level of CPT2 compared with WT CPT2 in Sirt5−/− mice (Fig. 8C and D). Consistent with the alteration in succinylation level, the K424R mutant restored the enzyme activity of CPT2 that was inhibited by Sirt5 deficiency (Fig. 8E). The K424R mutant also significantly increased maximal respiration compared with WT CPT2 in AMCMs from the Sirt5−/− mice (Fig. 8F), indicating that cardiac FAO was restored by Lys424 de-succinylation of CPT2 in Sirt5−/− mice. To further verify the effect of Lys424 de-succinylation on cardiac lipotoxicity, Sirt5−/− NMCMs were transfected with adenovirus to overexpress WT or K424R CPT2. As shown in Fig. 8G and H, forced expression of WT CPT2 was unable to inhibit PA-induced lipid deposition in Sirt5−/− NMCMs. In contrast, forced expression of the K424R mutant CPT2 significantly reduced lipid deposition in Sirt5−/− NMCMs that were treated with PA (Fig. 8G and H). In conclusion, Sirt5 deficiency impaired cardiac FAO and exacerbated cardiac lipotoxicity in DbCM through Lys424 de-succinylation of CPT2.
Fig. 8.
The role of CPT2 Lys424 Ksuc in Sirt5 regulating cardiac FAO and lipotoxicity. A, Mass spectrum of succinylated Lys424 of CPT2 in the succinylomics. B, Homology analysis of Lys424 of CPT2 across different species. C, Representative IP and Western blot of Ksuc and proteins expression levels of myocardial exogenous His-tagged CPT2 in WT and Sirt5−/− mice that were transfected with adenovirus expressing His-tagged WT CPT2 or K424R CPT2 (n = 3/groups). TUBA was used as the loading control. Rabbit IgG was used as the IP control. D, Statistical analysis of the IP and Western blot. E, Statistical analysis of myocardial CPT2 activity in Sirt5−/− mice that were transfected with adenovirus expressing His-tagged WT or K424R CPT2 (n = 3/groups). F, Seahorse metabolic analysis of FAO of AMCMs, that were isolated from Sirt5−/− mice transfected with adenovirus expressing WT or K424R CPT2 (n = 3/groups, 3–4 wells/mouse), at the presence of BSA or PA. G, Representative images of Oil red O staining in Sirt5−/− NMCMs that were transfected with control adenovirus or adenovirus expressing WT or K424R CPT2, at the presence of BSA or PA. Scale bar: 100 μm. H, Statistical analysis of optical density at 540 nm of stained Oil red O dye that was extracted by absolute isopropanol of the above NMCMs (n = 6/group) (left). Statistical analysis of TG concentration in the above NMCMs (n = 3/group) (right). Data are expressed as mean ± SEM. ns, not significant, *P < 0.05, **P < 0.01, ***P < 0.001. Data in D and E were analyzed using Student's t-test. Data in F and H was analyzed using one-way ANOVA followed by Bonferroni post hoc analysis. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Excessive FA and FA-derived metabolites inside cardiomyocytes are the primary pathogenic factor contributing to cardiac lipotoxicity in DbCM, but the way to reduce them and thus mitigate DbCM are not well elucidated. In this study, we unveiled a previously unrecognized role of Sirt5 and its mediated succinylation of CPT2 in the context of cardiac lipotoxicity and DbCM. We observed an upregulation of Sirt5 in the myocardium of diabetic patients and mice. Sirt5 deficiency impaired FAO, thereby contributing to myocardial lipid deposition and cardiac dysfunction in DbCM. Furthermore, a comprehensive analysis combining widely targeted metabolomics with 4D label-free proteomics and succinylomics identified CPT2 as the functional Ksuc substrate mediator of Sirt5, which disrupted the reconversion from fatty acyl-carnitines to fatty acyl-CoA, resulting in the accumulation of fatty acyl-carnitines and impairment of FAO. The K424R mutant inhibited Sirt5 deficiency-mediated CPT2 succinylation and inactivation, thereby rescuing cardiac FAO and mitigating lipotoxicity. De-succinylation of CPT2 by cardiac Sirt5 overexpression increased cardiac FAO and restored mitochondrial integrity, thereby alleviating cardiac lipotoxicity and dysfunction in DbCM.
As a severe complication of diabetes mellitus, DbCM could be evident even in patients with type 2 diabetes mellitus despite with intensive glucose control [6,7]. In DbCM, the excessive accumulation of FA and their intermediate metabolites in cardiomyocytes results in structural and functional damage to the heart [14]. Therefore, strategies aimed at mitigating the accumulation of excessive FA metabolites in cardiomyocytes prevent the development of DbCM and could potentially serve as an adjunctive treatment. However, the mechanisms governing lipid accumulation during the development of DbCM remain elusive. In this study, we have unveiled a novel role of Sirt5/CPT2 in mitigating cardiac lipotoxicity and DbCM for the first time, which represents the most significant finding of our research. Importantly, the observed upregulation of myocardial Sirt5 in db/db mice could potentially have compensatory significance, akin to other established mechanisms (e.g., elevation of brain natriuretic peptide in HF). The failure to correct DbCM despite the increase in Sirt5 levels could be attributed to the modest degree of upregulation (<1.5-fold compared to control). This view point is supported by a recent study reporting the beneficial effect of Sirt5 in improving glucose metabolism in DbCM [36]. Based on our findings, Sirt5 could represent a promising target for the treatment of DbCM and may have potential applications in other diseases related to ectopic fat deposition. However, it is worthy to notice that the compensatory effects of Sirt5 are inferred from the db/db mice, and it should be interpreted with caution in other DbCM model.
Cardiomyocytes are weak in synthesizing or storing FA independently, and the uptake and metabolism of FA are tightly regulated and dynamically balanced [14,37]. FAO is the main pathway for cardiomyocytes to metabolize FA. Therefore, the immediate utilization of FA uptake for FAO is crucial, and any disruption in this process can lead to metabolic inflexibility in cardiomyocytes [14,37]. Impaired FAO can result in the incomplete oxidation of fatty acids, leading to the production of toxic intermediate metabolites and the formation of lipid droplets in cardiomyocytes [14,37]. Therefore, it seems to be reasonable to speculate that increased FAO could protect the heart from dysfunction in the context of DbCM. However, the roles of FAO in the development of DbCM have been under sharply debating. Increased cardiac FAO has long been implicated as the culprit in the generation of reactive oxygen species, resulting in oxidative damage and mitochondrial dysfunction [38]. However, this notion has been challenged by a number of studies during recent years. For one hand, decrease of FAO do not necessary benefit cardiac function. It has been reported that decrease of FAO, either by depletion of CPT1 to directly reduce FAO, or by over-expression of glucose transporter 1 to increase glucose metabolism that reduce FAO in an indirect manner, exacerbate cardiac lipotoxicity and cardiac dysfunctio [39,40]. In line with these evidences, there are studies showing that increasing cardiac FAO do not lead to mitochondrial or cardiac dysfunction either in non-obese mice [39] or HFD-induced mice [41]. For the other hand, Shao et al. showed that increasing FAO through cardiac-specific acetyl coenzyme A carboxylase 2 deletion prevented HFD-induced cardiomyopathy by preserving mitochondrial function [42]. The underlying reasons for these conflict results were unknown and animal model could not be the point since most of them are using the similar models [17,[38], [39], [40]] and the targets for regulating FAO are the main difference among them. Therefore, the targets for FAO regulation may be crucial in determining FAO changes and cardiac results.
All of these studies underscore the pivotal role of targeting FAO to alleviate DbCM and our study propose a novel clue. We identified that CPT2 is a novel functional substrate mediator of Sirt5 in the regulation of FAO. CPT2, which facilitates the translocation of FA intermediate metabolites into mitochondrial matrix [43], is critical for FAO. A systematically pharmacological inhibition of CPT2 exacerbated cardiac dysfunction in endotoxaemia [44] but its role in DbCM has not been reported previously. Among most of the critical proteins involved in FAO, we demonstrated that CPT2 is the only substrate for Sirt5. Firstly, it is reasonable to exclude CPT1a and CTP1b as functional substrate proteins of Sirt5, as no succinylated site or a site with low intensity was detected in the succinylomics. Furthermore, other FAO key enzymes, namely acyl-CoA synthetase, acyl-CoA dehydrogenase, enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and 3-aceyl-CoA acetyltransferase were also excluded, as no significant change in fatty acyl-CoA levels could be observed in the metabolomics, despite being succinylated at multiple lysine sites following Sirt5 deficiency in the succinylomics. Therefore, with robust evidence presented in the current study, we identified that CPT2 was the most important downstream substrate for Sirt5-dependent FAO enhancement. Besides, we provided evidence, for the first time, that succinylation regulation of CPT2 was critical for its function and FAO. Despite the presence of lysine residues (Lys424) that could be susceptible to succinylation by Sirt5, there is currently no experimental evidence supporting this hypothesis in previous studies. In line with our findings that the succinylation of Lys424 inactivated the enzyme activity of CPT2 and impaired cardiac FAO, it is previously reported that the lysine 79 acetylation of CPT2 leads to the accumulation of fatty acyl-carnitines [45], indicating that the PTM of a single amino acid is sufficient to disrupt CPT2 activity. Therefore, CPT2 represents a novel functional succinyl substrate mediator of Sirt5 in the regulation of cardiac FAO in the context of DbCM.
In conclusion, we have demonstrated a novel role of Sirt5 in preventing DbCM and uncovered a novel functional Ksuc substrate mediator CPT2 of Sirt5 in regulating FAO. Sirt5 deficiency impairs FAO by succinylating CPT2 at Lys424, which exacerbates cardiac lipotoxicity and DbCM. These findings highlight Sirt5 and CPT2 as promising novel targets for the treatment of DbCM.
FUNDING
This work was supported by the National Natural Science Foundation of China to Maoxiong Wu (Grant No. 82100369), Yangxin Chen (Grant No. U23A20397, 81970200 and 82271609), Jingfeng Wang (Grant No. 82070237 and 82270254), Haifeng Zhang (Grant No. 82371573), and Jing Tan (Grant No. 82200289); the Guangdong Basic and Applied Basic Research Foundation to Maoxiong Wu (Grant No. 2019A1515110129, 2023A1515010310, and 2024A1515030240), Jing Tan (Grant No. 2021A1515110233), Haifeng Zhang (Grant No. 2020A1515011237) and Zhiteng Chen (Grant No. 2021A1515111092); the Guangzhou Science and Technology Projects to Maoxiong Wu (Grant No. 2024A04J4767); the Guangzhou Science and Technology Plan Project to Yangxin Chen (Grant No. 2023B01J1011); the Guangzhou Key Laboratory of Molecular Mechanism and Translation in Major Cardiovascular Disease to Jingfeng Wang (Grant No. 202102010007); the Guangzhou Regenerative Medicine and Health Guangdong Laboratory to Jingfeng Wang (Grant No. 2019GZR110406004), the Fundamental Research Funds for the Central Universities, Sun Yat-sen University to Maoxiong Wu (Grant No. 23qnpy140).
CRediT authorship contribution statement
Maoxiong Wu: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Jing Tan: Methodology, Investigation, Funding acquisition. Zhengyu Cao: Methodology, Investigation. Yangwei Cai: Methodology, Investigation. Zhaoqi Huang: Methodology, Investigation. Zhiteng Chen: Methodology, Investigation. Wanbing He: Methodology, Investigation. Xiao Liu: Methodology, Investigation. Yuan Jiang: Methodology, Investigation. Qingyuan Gao: Methodology, Investigation. Bingqing Deng: Methodology, Investigation. Jingfeng Wang: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization. Woliang Yuan: Writing – review & editing, Supervision, Funding acquisition. Haifeng Zhang: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization. Yangxin Chen: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103184.
Contributor Information
Jingfeng Wang, Email: wjingf@mail.sysu.edu.cn.
Woliang Yuan, Email: yuanwl@mail.sysu.edu.cn.
Haifeng Zhang, Email: zhanghf9@mail.sysu.edu.cn.
Yangxin Chen, Email: chenyx39@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Effects of Sirt5 knockout on other sirtuins protein expression in HFD/STZ-induced diabetic mice. A, Representative western blot of myocardial sirtuins (Sirt1-7) protein expression in WT and Sirt5−/− mice after HFD/STZ induction (n = 4/group). TUBA was used as the loading control. B, Statistical analysis of the western blot. Data are expressed as mean ± SEM was analyzed using Student's t-test. ns, not significant, ***P < 0.001.
Effects of Sirt5 knockout on FBG and insulin resistance in HFD/STZ-induced diabetic mice. A, Statistical analysis of body weight of the mice (n = 6/group). B, Statistical analysis of FBG of the above mice (n = 6/group). C, Statistical analysis of GTT of the above mice (n = 6/group). D, Statistical analysis of AUC of the GTT result of the mice. E, Statistical analysis of ITT of the above mice (n = 6/group). F, Statistical analysis of AUC of the ITT result of the above mice. G, Statistical analysis of serum insulin concentration of the above mice (n = 6/group). H, Statistical analysis of HOMA-IR of the above mice (n = 6). Data are expressed as mean ± SEM. ns, not significant. Data in D, E, G and H was analyzed using Student's t-test.
Effect of Sirt5 knockout or forced expression on lipids deposition in PA-treated NMCMs. A, Representative images of Oil red O staining in WT and Sirt5−/− NMCMs treated with BSA or 200 μM PA for 48 h. Scale bar: 20 μm. B, Statistical analysis of optical density at 540 nm of stained Oil red O dye that was extracted by absolute isopropanol of the above NMCMs (n = 4/group). C, Statistical analysis of TG concentration in WT and Sirt5−/− NMCMs treated with BSA or 200 μM PA for 48 h (n = 3/group). D, Representative western blot of Sirt5 protein expression in WT and Sirt5−/− NMCMs treated with BSA or 200 μM PA for 48 h (n = 3/group). Tuba was used as a loading control. E, Representative images of Oil red O staining in NMCMs infected with Ad-null or Ad-Sirt5 at the presence of BSA or 200 μM PA for 48 h. Scale bar: 100 μm. F, Statistical analysis of optical density at 540 nm of stained Oil red O dye that was extracted by absolute isopropanol of the above NMCMs (n = 4/group). G, Statistical analysis of TG concentration in the above NMCMs (n = 3/group). H, Representative western blot of Sirt5 protein expression in NMCMs infected with Ad-null or Ad-Sirt5 at the presence of BSA or 200 μM PA for 48 h (n = 3/group). Tuba was used as a loading control. Data are expressed as mean ± SEM. ns, not significant, *P < 0.05, **P < 0.01, ***P < 0.001. Data in B was analyzed using Student's t-test. Data in C and F-G was analyzed using one-way ANOVA followed by Bonferroni post hoc analysis.
Definition of five-grade scoring system for mitochondrial morphology. A, Representative mitochondrial morphology in TEM using a 5-grade scoring system. B, Morphological appearance of the 5-grade scoring system.
Effect of Sirt5 knockout on pan-acylation of myocardial total proteins in HFD mice. A, Representative western blot images of pan Ksuc, Kglu, Kmal, and Kace of myocardial total proteins in WT and Sirt5−/− mice after HFD/STZ induction. B, Representative image of Coomassie blue staining of myocardial total proteins in the above mice.
Effect of Sirt5 forced expression on succinylation of CPT2 in diabetic mice. A, Representative IP and western blot of Ksuc and proteins expression levels of myocardial CPT2 in db/db mice infected with AAV9-control or AAV9-Sirt5. TUBA was used as the loading control. Rabbit IgG was used as the IP control. B, Statistical analysis of myocardial CPT2 activity in db/db mice infected with AAV9-control or AAV9-Sirt5 (n = 6/group). Data are expressed as mean ± SEM. ****P < 0.0001. Data in B was analyzed using Student's t-test.
Data availability
Data will be made available on request.
References
- 1.Low Wang C.C., Hess C.N., Hiatt W.R., Goldfine A.B. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus - mechanisms, management, and clinical considerations. Circulation. 2016;133(24):2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zou R., Tao J., He J., Wang C., Tan S., Xia Y., Chang X., Li R., Wang G., Zhou H., Fan X. PGAM5-Mediated PHB2 dephosphorylation contributes to diabetic cardiomyopathy by disrupting mitochondrial quality surveillance. Research. 2022 doi: 10.34133/research.0001. 2022:Article 0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee M.M.Y., McMurray J.J.V., Lorenzo-Almoros A., Kristensen S.L., Sattar N., Jhund P.S., Petrie M.C. Diabetic cardiomyopathy. Heart. 2019;105(4):337–345. doi: 10.1136/heartjnl-2016-310342. [DOI] [PubMed] [Google Scholar]
- 4.Tan Y., Zhang Z., Zheng C., Wintergerst K.A., Keller B.B., Cai L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat. Rev. Cardiol. 2020;17(9):585–607. doi: 10.1038/s41569-020-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenny H.C., Abel E.D. Heart failure in type 2 diabetes mellitus. Circ. Res. 2019;124(1):121–141. doi: 10.1161/CIRCRESAHA.118.311371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Control G., Turnbull F.M., Abraira C., Anderson R.J., Byington R.P., Chalmers J.P., Duckworth W.C., Evans G.W., Gerstein H.C., Holman R.R., Moritz T.E., Neal B.C., Ninomiya T., Patel A.A., Paul S.K., Travert F., Woodward M. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52(11):2288–2298. doi: 10.1007/s00125-009-1470-0. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert R.E., Krum H. Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet. 2015;385(9982):2107–2117. doi: 10.1016/S0140-6736(14)61402-1. [DOI] [PubMed] [Google Scholar]
- 8.Jia G., Hill M.A., Sowers J.R. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ. Res. 2018;122(4):624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marfella R., Amarelli C., Cacciatore F., Balestrieri M.L., Mansueto G., D'Onofrio N., Esposito S., Mattucci I., Salerno G., De Feo M., D'Amico M., Golino P., Maiello C., Paolisso G., Napoli C. Lipid accumulation in hearts transplanted from nondiabetic donors to diabetic recipients. J. Am. Coll. Cardiol. 2020;75(11):1249–1262. doi: 10.1016/j.jacc.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Fukushima A., Lopaschuk G.D. Cardiac fatty acid oxidation in heart failure associated with obesity and diabetes. Biochim. Biophys. Acta. 2016;1861(10):1525–1534. doi: 10.1016/j.bbalip.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Carpentier A.C. Abnormal myocardial dietary fatty acid metabolism and diabetic cardiomyopathy. Can. J. Cardiol. 2018;34(5):605–614. doi: 10.1016/j.cjca.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Karwi Q.G., Sun Q., Lopaschuk G.D. The contribution of cardiac fatty acid oxidation to diabetic cardiomyopathy severity. Cells. 2021;10(11) doi: 10.3390/cells10113259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu T., Qu Y., Xu S., Wang Y., Liu X., Ma D. SIRT6: a potential therapeutic target for diabetic cardiomyopathy. Faseb. J. 2023;37(8) doi: 10.1096/fj.202301012R. [DOI] [PubMed] [Google Scholar]
- 14.Ritchie R.H., Abel E.D. Basic mechanisms of diabetic heart disease. Circ. Res. 2020;126(11):1501–1525. doi: 10.1161/CIRCRESAHA.120.315913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopaschuk G.D., Karwi Q.G., Tian R., Wende A.R., Abel E.D. Cardiac energy metabolism in heart failure. Circ. Res. 2021;128(10):1487–1513. doi: 10.1161/CIRCRESAHA.121.318241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lionetti V., Linke A., Chandler M.P., Young M.E., Penn M.S., Gupte S., d'Agostino C., Hintze T.H., Stanley W.C., Recchia F.A. Carnitine palmitoyl transferase-I inhibition prevents ventricular remodeling and delays decompensation in pacing-induced heart failure. Cardiovasc. Res. 2005;66(3):454–461. doi: 10.1016/j.cardiores.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Schwarzer M., Faerber G., Rueckauer T., Blum D., Pytel G., Mohr F.W., Doenst T. The metabolic modulators, Etomoxir and NVP-LAB121, fail to reverse pressure overload induced heart failure in vivo. Basic Res. Cardiol. 2009;104(5):547–557. doi: 10.1007/s00395-009-0015-5. [DOI] [PubMed] [Google Scholar]
- 18.Turcani M., Rupp H. Modification of left ventricular hypertrophy by chronic etomixir treatment. Br. J. Pharmacol. 1999;126(2):501–507. doi: 10.1038/sj.bjp.0702312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolkowicz P.E., Urthaler F., Forrest C., Shen H., Durand J., Wei C.C., Oparil S., Dell'Italia L.J. 2-Tetradecylglycidic acid, an inhibitor of carnitine palmitoyltransferase-1, induces myocardial hypertrophy via the AT1 receptor. J. Mol. Cell. Cardiol. 1999;31(8):1405–1412. doi: 10.1006/jmcc.1999.0977. [DOI] [PubMed] [Google Scholar]
- 20.Kane A.E., Sinclair D.A. Sirtuins and NAD(+) in the development and treatment of metabolic and cardiovascular diseases. Circ. Res. 2018;123(7):868–885. doi: 10.1161/CIRCRESAHA.118.312498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karbasforooshan H., Karimi G. The role of SIRT1 in diabetic cardiomyopathy. Biomed. Pharmacother. 2017;90:386–392. doi: 10.1016/j.biopha.2017.03.056. [DOI] [PubMed] [Google Scholar]
- 22.Yuan Q., Zhan L., Zhou Q.Y., Zhang L.L., Chen X.M., Hu X.M., Yuan X.C. SIRT2 regulates microtubule stabilization in diabetic cardiomyopathy. Eur. J. Pharmacol. 2015;764:554–561. doi: 10.1016/j.ejphar.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 23.Kanwal A., Pillai V.B., Samant S., Gupta M., Gupta M.P. The nuclear and mitochondrial sirtuins, Sirt6 and Sirt3, regulate each other's activity and protect the heart from developing obesity-mediated diabetic cardiomyopathy. Faseb. J. 2019;33(10):10872–10888. doi: 10.1096/fj.201900767R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasrin N., Wu X., Fortier E., Feng Y., Bare O.C., Chen S., Ren X., Wu Z., Streeper R.S., Bordone L. SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J. Biol. Chem. 2010;285(42):31995–32002. doi: 10.1074/jbc.M110.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadhukhan S., Liu X., Ryu D., Nelson O.D., Stupinski J.A., Li Z., Chen W., Zhang S., Weiss R.S., Locasale J.W., Auwerx J., Lin H. Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function. Proc. Natl. Acad. Sci. U. S. A. 2016;113(16):4320–4325. doi: 10.1073/pnas.1519858113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshizawa T., Karim M.F., Sato Y., Senokuchi T., Miyata K., Fukuda T., Go C., Tasaki M., Uchimura K., Kadomatsu T., Tian Z., Smolka C., Sawa T., Takeya M., Tomizawa K., Ando Y., Araki E., Akaike T., Braun T., Oike Y., Bober E., Yamagata K. SIRT7 controls hepatic lipid metabolism by regulating the ubiquitin-proteasome pathway. Cell Metabol. 2014;19(4):712–721. doi: 10.1016/j.cmet.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Hershberger K.A., Abraham D.M., Martin A.S., Mao L., Liu J., Gu H., Locasale J.W., Hirschey M.D. Sirtuin 5 is required for mouse survival in response to cardiac pressure overload. J. Biol. Chem. 2017;292(48):19767–19781. doi: 10.1074/jbc.M117.809897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu M.X., Wang S.H., Xie Y., Chen Z.T., Guo Q., Yuan W.L., Guan C., Xu C.Z., Huang Y.N., Wang J.F., Zhang H.F., Chen Y.X. Interleukin-33 alleviates diabetic cardiomyopathy through regulation of endoplasmic reticulum stress and autophagy via insulin-like growth factor-binding protein 3. J. Cell. Physiol. 2021;236(6):4403–4419. doi: 10.1002/jcp.30158. [DOI] [PubMed] [Google Scholar]
- 29.Fu H.Y., Sanada S., Matsuzaki T., Liao Y., Okuda K., Yamato M., Tsuchida S., Araki R., Asano Y., Asanuma H., Asakura M., French B.A., Sakata Y., Kitakaze M., Minamino T. Chemical endoplasmic reticulum chaperone alleviates doxorubicin-induced cardiac dysfunction. Circ. Res. 2016;118(5):798–809. doi: 10.1161/CIRCRESAHA.115.307604. [DOI] [PubMed] [Google Scholar]
- 30.Wang S.H., Zhu X.L., Wang F., Chen S.X., Chen Z.T., Qiu Q., Liu W.H., Wu M.X., Deng B.Q., Xie Y., Mai J.T., Yang Y., Wang J.F., Zhang H.F., Chen Y.X. LncRNA H19 governs mitophagy and restores mitochondrial respiration in the heart through Pink1/Parkin signaling during obesity. Cell Death Dis. 2021;12(6):557. doi: 10.1038/s41419-021-03821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin L., Geng L., Ying L., Shu L., Ye K., Yang R., Liu Y., Wang Y., Cai Y., Jiang X., Wang Q., Yan X., Liao B., Liu J., Duan F., Sweeney G., Woo C.W.H., Wang Y., Xia Z., Lian Q., Xu A. FGF21-Sirtuin 3 Axis confers the protective effects of exercise against diabetic cardiomyopathy by governing mitochondrial integrity. Circulation. 2022;146(20):1537–1557. doi: 10.1161/CIRCULATIONAHA.122.059631. [DOI] [PubMed] [Google Scholar]
- 32.Li X., Wu Y., Zhao J., Wang H., Tan J., Yang M., Li Y., Deng S., Gao S., Li H., Yang Z., Yang F., Ma J., Cheng J., Cai W. Distinct cardiac energy metabolism and oxidative stress adaptations between obese and non-obese type 2 diabetes mellitus. Theranostics. 2020;10(6):2675–2695. doi: 10.7150/thno.40735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang L.H., Dong R.J., Lu Y.W., Wang H.M., Kuang Y.Q., Wang R.R., Li Y.Y. Integration of metabolomics and transcriptomics analyses reveals sphingosine-1-phosphate-mediated S1PR2/PI3K/Akt pathway involved in Talaromyces marneffei infection of macrophages. Microb. Pathog. 2023;175 doi: 10.1016/j.micpath.2023.105985. [DOI] [PubMed] [Google Scholar]
- 34.Song Y., Sun L., Wang H., Zhang S., Fan K., Mao Y., Zhang J., Han X., Chen H., Xu Y., Sun K., Ding Z., Wang Y. Enzymatic fermentation of rapeseed cake significantly improved the soil environment of tea rhizosphere. BMC Microbiol. 2023;23(1):250. doi: 10.1186/s12866-023-02995-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki J. Effects of hyperbaric environment on endurance and metabolism are exposure time-dependent in well-trained mice. Phys. Rep. 2021;9(5) doi: 10.14814/phy2.14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei C., Shi M., Dong S., Li Z., Zhao B., Liu D., Li G., Cen J., Yu L., Liang X., Shi L. SIRT5-related lysine demalonylation of GSTP1 contributes to cardiomyocyte pyroptosis suppression in diabetic cardiomyopathy. Int. J. Biol. Sci. 2024;20(2):585–605. doi: 10.7150/ijbs.83306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seferovic P.M., Paulus W.J. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur. Heart J. 2015;36(27):1718–1727. doi: 10.1093/eurheartj/ehv134. 1727a-1727. [DOI] [PubMed] [Google Scholar]
- 38.Mazumder P.K., O'Neill B.T., Roberts M.W., Buchanan J., Yun U.J., Cooksey R.C., Boudina S., Abel E.D. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53(9):2366–2374. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 39.Kolwicz S.C., Jr., Olson D.P., Marney L.C., Garcia-Menendez L., Synovec R.E., Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ. Res. 2012;111(6):728–738. doi: 10.1161/CIRCRESAHA.112.268128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan J., Young M.E., Cui L., Lopaschuk G.D., Liao R., Tian R. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation. 2009;119(21):2818–2828. doi: 10.1161/CIRCULATIONAHA.108.832915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Z., Ding J., McMillen T.S., Villet O., Tian R., Shao D. Enhancing fatty acid oxidation negatively regulates PPARs signaling in the heart. J. Mol. Cell. Cardiol. 2020;146:1–11. doi: 10.1016/j.yjmcc.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shao D., Kolwicz S.C., Jr., Wang P., Roe N.D., Villet O., Nishi K., Hsu Y.A., Flint G.V., Caudal A., Wang W., Regnier M., Tian R. Increasing fatty acid oxidation prevents high-fat diet-induced cardiomyopathy through regulating parkin-mediated mitophagy. Circulation. 2020;142(10):983–997. doi: 10.1161/CIRCULATIONAHA.119.043319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zlobine I., Gopal K., Ussher J.R. Lipotoxicity in obesity and diabetes-related cardiac dysfunction. Biochim. Biophys. Acta. 2016;1861(10):1555–1568. doi: 10.1016/j.bbalip.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Makrecka-Kuka M., Korzh S., Videja M., Vilskersts R., Sevostjanovs E., Zharkova-Malkova O., Arsenyan P., Kuka J., Dambrova M., Liepinsh E. Inhibition of CPT2 exacerbates cardiac dysfunction and inflammation in experimental endotoxaemia. J. Cell Mol. Med. 2020;24(20):11903–11911. doi: 10.1111/jcmm.15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan X., Wang Y., Cai X., Shen Y., Xu T., Xu Y., Cheng J., Wang X., Zhang L., Dai J., Lin S., Liu J. CPT2 K79 acetylation regulates platelet life span. Blood Adv. 2022;6(17):4924–4935. doi: 10.1182/bloodadvances.2021006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of Sirt5 knockout on other sirtuins protein expression in HFD/STZ-induced diabetic mice. A, Representative western blot of myocardial sirtuins (Sirt1-7) protein expression in WT and Sirt5−/− mice after HFD/STZ induction (n = 4/group). TUBA was used as the loading control. B, Statistical analysis of the western blot. Data are expressed as mean ± SEM was analyzed using Student's t-test. ns, not significant, ***P < 0.001.
Effects of Sirt5 knockout on FBG and insulin resistance in HFD/STZ-induced diabetic mice. A, Statistical analysis of body weight of the mice (n = 6/group). B, Statistical analysis of FBG of the above mice (n = 6/group). C, Statistical analysis of GTT of the above mice (n = 6/group). D, Statistical analysis of AUC of the GTT result of the mice. E, Statistical analysis of ITT of the above mice (n = 6/group). F, Statistical analysis of AUC of the ITT result of the above mice. G, Statistical analysis of serum insulin concentration of the above mice (n = 6/group). H, Statistical analysis of HOMA-IR of the above mice (n = 6). Data are expressed as mean ± SEM. ns, not significant. Data in D, E, G and H was analyzed using Student's t-test.
Effect of Sirt5 knockout or forced expression on lipids deposition in PA-treated NMCMs. A, Representative images of Oil red O staining in WT and Sirt5−/− NMCMs treated with BSA or 200 μM PA for 48 h. Scale bar: 20 μm. B, Statistical analysis of optical density at 540 nm of stained Oil red O dye that was extracted by absolute isopropanol of the above NMCMs (n = 4/group). C, Statistical analysis of TG concentration in WT and Sirt5−/− NMCMs treated with BSA or 200 μM PA for 48 h (n = 3/group). D, Representative western blot of Sirt5 protein expression in WT and Sirt5−/− NMCMs treated with BSA or 200 μM PA for 48 h (n = 3/group). Tuba was used as a loading control. E, Representative images of Oil red O staining in NMCMs infected with Ad-null or Ad-Sirt5 at the presence of BSA or 200 μM PA for 48 h. Scale bar: 100 μm. F, Statistical analysis of optical density at 540 nm of stained Oil red O dye that was extracted by absolute isopropanol of the above NMCMs (n = 4/group). G, Statistical analysis of TG concentration in the above NMCMs (n = 3/group). H, Representative western blot of Sirt5 protein expression in NMCMs infected with Ad-null or Ad-Sirt5 at the presence of BSA or 200 μM PA for 48 h (n = 3/group). Tuba was used as a loading control. Data are expressed as mean ± SEM. ns, not significant, *P < 0.05, **P < 0.01, ***P < 0.001. Data in B was analyzed using Student's t-test. Data in C and F-G was analyzed using one-way ANOVA followed by Bonferroni post hoc analysis.
Definition of five-grade scoring system for mitochondrial morphology. A, Representative mitochondrial morphology in TEM using a 5-grade scoring system. B, Morphological appearance of the 5-grade scoring system.
Effect of Sirt5 knockout on pan-acylation of myocardial total proteins in HFD mice. A, Representative western blot images of pan Ksuc, Kglu, Kmal, and Kace of myocardial total proteins in WT and Sirt5−/− mice after HFD/STZ induction. B, Representative image of Coomassie blue staining of myocardial total proteins in the above mice.
Effect of Sirt5 forced expression on succinylation of CPT2 in diabetic mice. A, Representative IP and western blot of Ksuc and proteins expression levels of myocardial CPT2 in db/db mice infected with AAV9-control or AAV9-Sirt5. TUBA was used as the loading control. Rabbit IgG was used as the IP control. B, Statistical analysis of myocardial CPT2 activity in db/db mice infected with AAV9-control or AAV9-Sirt5 (n = 6/group). Data are expressed as mean ± SEM. ****P < 0.0001. Data in B was analyzed using Student's t-test.
Data Availability Statement
Data will be made available on request.