Abstract
Rationale
Diffuse alveolar hemorrhage (DAH) is a life-threatening manifestation of antineutrophil cytoplasmic antibody–associated vasculitis (AAV). The PEXIVAS (Plasma Exchange and Glucocorticoids in Severe Antineutrophil Cytoplasmic Antibody–Associated Vasculitis) (NCT00987389) trial was the largest in AAV and the first to enroll participants with DAH requiring mechanical ventilation.
Objectives
Evaluate characteristics, treatment effects, and outcomes for patients with AAV with and without DAH.
Methods
PEXIVAS randomized 704 participants to plasma exchange (PLEX) or no-PLEX and reduced or standard-dose glucocorticoids (GC). DAH status was defined at enrollment as no-DAH, nonsevere, or severe (room air oxygen saturation of ⩽ 85% as measured by pulse oximetry, or use of mechanical ventilation).
Measurements and Main Results
At enrollment, 191 (27.1%) participants had DAH (61 severe, including 29 ventilated) and were younger, more frequently relapsing, PR3 (proteinase 3)-ANCA positive, and had lower serum creatinine but were more frequently dialyzed than participants without DAH (n = 513; 72.9%). Among those with DAH, 8/95 (8.4%) receiving PLEX died within 1 year versus 15/96 (15.6%) with no-PLEX (hazard ratio, 0.52; confidence interval [CI], 0.21–1.24), whereas 13/96 (13.5%) receiving reduced GC died versus 10/95 (10.5%) with standard GC (hazard ratio, 1.33; CI, 0.57–3.13). When ventilated, ventilator-free days were similar with PLEX versus no-PLEX (medians, 25; interquartile range [IQR], 22–26 vs. 22–27) and fewer with reduced GC (median, 23; IQR, 20–25) versus standard GC (median, 26; IQR, 25–28). Treatment effects on mortality did not vary by presence or severity of DAH. Overall, 23/191 (12.0%) with DAH died within 1 year versus 34/513 (6.6%) without DAH. End-stage kidney disease and serious infections did not differ by DAH status or treatments.
Conclusions
Patients with AAV and DAH differ from those without DAH in multiple ways. Further data are required to confirm or refute a benefit of PLEX or GC dosing on mortality.
Original clinical trial registered with www.clinicaltrials.gov (NCT00987389).
Keywords: diffuse alveolar hemorrhage, glucocorticoids, plasma exchange, respiratory failure
At a Glance Commentary
Scientific Knowledge on the Subject
Diffuse alveolar hemorrhage (DAH) is a potentially life-threatening manifestation of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), but there are limited data on the effects of specific treatment approaches for DAH due to AAV. Historically, patients with DAH requiring mechanical ventilation have been excluded from clinical trials.
What This Study Adds to the Field
The PEXIVAS (Plasma Exchange and Glucocorticoids in Severe Antineutrophil Cytoplasmic Antibody–Associated Vasculitis) trial was the largest ever conducted in AAV and the first to allow enrollment of participants with DAH requiring mechanical ventilation. Thus, this trial presents a unique opportunity to examine the characteristics and outcomes of patients with ANCA-associated vasculitis with and without DAH, including severe presentations, and to evaluate the effects of randomized treatments—plasma exchange and two glucocorticoid dosing regimens. Our analysis includes baseline characteristics, mortality, and treatment effects. We found that participants with DAH differed from those without DAH in multiple ways, including baseline characteristics and a significantly increased risk of death at 30 days and 1 year. The randomized treatments did not have clear effects on mortality when looking at presence or severity of DAH.
Diffuse alveolar hemorrhage (DAH) is a severe manifestation of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) characterized by neutrophilic capillaritis with bleeding into the distal airspaces of the lungs, which can cause life-threatening respiratory failure (1–3). The treatment of DAH involves supportive care and treating the underlying AAV with glucocorticoids (GC) and other forms of immunosuppression (e.g., rituximab and/or cyclophosphamide) to reduce inflammation, halt tissue injury, and allow restoration of endothelial integrity (4, 5). Despite aggressive treatment, DAH in AAV can be fatal, with first-year mortality estimated at 15–50% (2, 3, 6–9). Furthermore, current treatment also increases the risk of serious infections, the most common cause of death in the first year in AAV, including in patients with DAH (10–17).
The optimal treatment for DAH in patients with AAV is uncertain. Plasma exchange (PLEX) has been used in organ- or life-threatening AAV, most frequently in patients with DAH and/or glomerulonephritis, with the rationale that swift removal of pathogenic antibodies and other mediators may expedite resolution of active inflammation (18–25). Similarly, high-dose GC are frequently used to control severe manifestations of AAV, but there are limited data to define the relative benefits and risks of dose regimens (4, 5).
The PEXIVAS (Plasma Exchange and Glucocorticoids in Severe Antineutrophil Cytoplasmic Antibody–Associated Vasculitis) trial was a randomized trial comparing the use of PLEX to no-PLEX, and two GC dosing regimens, in addition to cyclophosphamide or rituximab, in participants with severe AAV (NCT00987389) (26). This was the largest trial ever conducted in AAV, the first to evaluate GC dosing in a randomized fashion, and the first to allow enrollment of participants with DAH requiring mechanical ventilation. Importantly, PEXIVAS provides an opportunity to describe the characteristics and outcomes of patients with AAV with and without DAH and to estimate the effects of PLEX and GC dosing.
Study Design and Methods
PEXIVAS Trial
The protocol of the PEXIVAS trial was previously described (26, 27). Briefly, participants with severe AAV (granulomatosis with polyangiitis [formerly called Wegener's granulomatosis (WG)] or microscopic polyangiitis), including glomerulonephritis and/or DAH, were randomized into four treatment groups to evaluate the effects of PLEX and two GC dosing regimens on a composite primary endpoint of end-stage kidney disease or death. The initial immunosuppressive regimen, with either cyclophosphamide or rituximab, was determined by the local investigator, as was the dose of intravenous methylprednisolone (1–3 g over 3 d). Ethics approval was obtained at each site of the PEXIVAS trial. Written informed consent was provided by patients or surrogate decision-makers for trial inclusion and subsequent analyses.
Definitions
DAH due to AAV was defined at trial entry by chest imaging with bilateral pulmonary infiltrates and absence of an alternative explanation, such as volume overload or infection. In addition, participants needed at least one of four other findings: 1) evidence of DAH by bronchoscopy, such as increasingly bloody return on bronchoalveolar lavage; 2) hemoptysis; 3) anemia with hemoglobin <10 g/dl or drop in hemoglobin >1 g/dl that is otherwise unexplained; or 4) an elevated DlCO by pulmonary function testing.
Participants were categorized as having severe DAH if they had an oxygen saturation of ⩽85% on room air or were on mechanical ventilation.
End-stage kidney disease was defined as the need for dialysis for at least 12 consecutive weeks, or kidney transplantation.
Serious infection was defined as an infectious syndrome requiring hospitalization or intravenous antimicrobials (26).
Patient Selection
All participants randomized in PEXIVAS were included in this analysis. Participants who developed DAH after randomization were not considered to have DAH for these analyses. Randomization to treatment groups in PEXIVAS included stratification by severity of DAH (26, 27).
Statistical Analysis
This subgroup analysis was prespecified in the PEXIVAS trial protocol (27). Descriptive statistics were used for baseline characteristics and outcomes. Categorical variables were summarized by frequencies and proportions, then compared using chi-square test. Continuous variables were summarized using mean ± SD or median and interquartile range (IQR) and compared using a two-sample t test or Wilcoxon rank-sum test, respectively. Follow-up time was censored at 1 year. Outcomes were compared using Cox proportional hazards models, adjusted for the trial’s stratification characteristics (chosen for their likely association with mortality and kidney failure) of age (<60 vs. ⩾60 yr old), ANCA subtype (anti–proteinase 3 [anti-PR3] vs. anti-myeloperoxidase [anti-MPO]), serum creatinine (<500 μmol/L [5.6 g/dl] or ⩾500 μmol/L [5.6 g/dl]), and initial treatments (i.v. cyclophosphamide vs. oral cyclophosphamide vs. rituximab), as well as sex (male vs. female).
Among participants on mechanical ventilation, ventilator-free days were defined as days alive and free of ventilation within the first 30 days after randomization (rather than 28 d, to align with results for deaths at 30 d). Duration (in days) that the participant required ventilation were recorded during the trial and subtracted from 30 if they were successfully liberated from ventilation; ventilator-free days = 0 if the participant died within 30 days of mechanical ventilation, or if the participant was ventilated for >30 days. Results were summarized as median and IQR and compared by Kruskal-Wallis test. Sensitivity analysis was performed using the duration of ventilation.
All statistical analyses were performed using Stata version 15. A P value < 0.05 was considered statistically significant for all comparisons.
Results
Clinical Characteristics
Of 704 participants enrolled in PEXIVAS, 191 (27.1%) had DAH, with 61 (31.9% of DAH group) severe, including 29 (47.5% of the severe subgroup) on mechanical ventilation (Figure 1). No participants without DAH were on mechanical ventilation.
Figure 1.
Presence and severity of DAH among the 704 participants in PEXIVAS (Plasma Exchange and Glucocorticoids in Severe Antineutrophil Cytoplasmic Antibody–Associated Vasculitis) and distribution among treatment groups. DAH = diffuse alveolar hemorrhage; GC = glucocorticoids; PLEX = plasma exchange.
The characteristics of participants with and without DAH at enrollment are outlined in Table 1. Participants with DAH were younger, more frequently positive for anti-PR3 antibodies rather than anti-myeloperoxidase antibodies, more often had relapsing disease at enrollment, and had higher baseline scores on the Birmingham Vasculitis Activity Score/Wegener’s Granulomatosis (28). Participants with DAH had lower baseline creatinine concentrations but were more frequently started on hemodialysis. There were no differences in the proportions of participants randomized to PLEX or the reduced-dose GC regimen, but those with DAH more commonly received intravenous versus oral cyclophosphamide.
Table 1.
Clinical Characteristics of Participants in the PLEX and GCs in Severe Antineutrophil Cytoplasmic Antibody–Associated Vasculitis Trial by Presence of DAH
| Any DAH (n = 191) | No DAH (n = 513) | P Value | |
|---|---|---|---|
| Age, yr, mean (SD) | 61.1 (15.4) | 63.9 (13.4) | 0.018 |
| Female | 78 (40.8) | 229 (44.6) | 0.37 |
| ANCA | <0.001 | ||
| Anti-PR3 | 99 (51.8) | 187 (36.5) | |
| Anti-MPO | 92 (48.2) | 326 (63.6) | |
| New diagnosis of AAV | 164 (85.8) | 477 (93.0) | 0.004 |
| BVAS/WG | 11 (9–13) | 7 (6–9) | <0.001 |
| Creatinine, μmol/L | 184 (115–320) | 304 (212–442) | <0.001 |
| Dialysis at baseline | 48 (25.3) | 92 (18.0) | 0.033 |
| Randomized to PLEX | 95 (49.7) | 257 (50.1) | 0.93 |
| Randomized to reduced GC | 96 (50.3) | 257 (50.1) | 0.97 |
| Immunosuppression | <0.001 | ||
| Oral cyclophosphamide | 44 (23.0) | 197 (38.4) | |
| Intravenous cyclophosphamide | 107 (56.0) | 247 (48.2) | |
| Rituximab | 40 (20.9) | 69 (13.4) |
Definition of abbreviations: AAV = ANCA-associated vasculitis; ANCA = antineutrophil cytoplasmic antibody; BVAS/WG = Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis; DAH = diffuse alveolar hemorrhage; GC = glucocorticoid; MPO = myeloperoxidase; PLEX = plasma exchange; PR3 = proteinase 3.
Data are presented as n (%) or median (25th–75th interquartile range) unless otherwise noted.
Looking specifically at participants with DAH at enrollment, baseline characteristics of participants with severe DAH compared with those with nonsevere DAH are presented in Table 2. A high proportion of participants in both groups had hemoptysis at presentation. The median oxygen saturation on room air for those with severe DAH was 81%, versus 93% in those with nonsevere DAH. Among the 29 participants with DAH on mechanical ventilation, 15 were randomized to receive PLEX (95 total with any DAH) and 14 to the no-PLEX group (96 total with any DAH); 13 were randomized to standard GC regimen (95 total with any DAH) versus 16 to reduced GC regimen (96 total with any DAH).
Table 2.
Clinical Characteristics of Participants in the PLEX and GCs in Severe Antineutrophil Cytoplasmic Antibody–Associated Vasculitis Trial by Severity of DAH
| Nonsevere DAH (n = 130) | Severe DAH (n = 61) | P Value | |
|---|---|---|---|
| Lowest SpO2 | 93 (91–95) | 81 (78–85) | NA |
| Ventilation | 0 (0) | 29 (47.5) | NA |
| Hemoptysis | 92 (70.7) | 50 (82.0) | 0.10 |
| Creatinine, μmol/L | 275 (141–456) | 222 (137–391) | 0.43 |
| Dialysis at baseline | 31 (23.9) | 17 (28.3) | 0.51 |
Definition of abbreviations: DAH = diffuse alveolar hemorrhage; NA = not applicable because these variables were used to define the nonsevere DAH and severe DAH groups; SpO2 = oxygen saturation on room air as measured by pulse oximetry.
Data are presented as n (%) or median (25th–75th interquartile range) unless otherwise noted.
Treatment Effects
The effect of PLEX on survival in participants with versus without DAH, adjusted for age, sex, ANCA subtype, kidney function, and initial treatments, is presented in Figure 2. For those with DAH, death occurred in 6 in the PLEX group and 12 in the no-PLEX group at 3 months and 8 versus 15 at 1 year (hazard ratio [HR], 0.52; 95% confidence interval [CI], 0.21–1.24) (P-interaction = 0.37 for any DAH vs. no DAH and P-interaction = 0.42 for nonsevere DAH vs. no DAH and 0.44 for severe DAH vs. no DAH) (Table 3).
Figure 2.

One-year survival by plasma exchange and severity of DAH. Diamond represents effect estimate, and horizontal line represents the 95% CI based on a Cox proportional hazards model adjusted for age, sex, antineutrophil cytoplasmic antibody subtype, kidney function, and initial treatments. CI = confidence interval; DAH = diffuse alveolar hemorrhage; HR = hazard ratio; PLEX = plasma exchange.
Table 3.
Risk of Death at 3 Months and 1 Year in PLEX and GCs in Severe Antineutrophil Cytoplasmic Antibody–Associated Vasculitis Trial Participants with or without DAH, and by Severity of DAH, by PLEX Treatment Allocation
| Died 3 Mo |
Died 1 Yr |
Effect of PLEX |
||||
|---|---|---|---|---|---|---|
| PLEX | No PLEX | PLEX | No PLEX | HR (95% CI) | P-interaction | |
| Overall | 18 (5.1) | 21 (6.0) | 25 (7.1) | 32 (9.1) | 0.74 (0.44–1.26) | |
| No DAH | 12 (4.7) | 9 (3.5) | 17 (6.6) | 17 (6.6) | 0.86 (0.43–1.71) | |
| Any DAH | 6 (6.3) | 12 (12.5) | 8 (8.4) | 15 (15.6) | 0.52 (0.21–1.24) | 0.37 |
| Nonsevere DAH | 1 (1.6) | 3 (4.6) | 2 (3.1) | 5 (7.6) | 0.43 (0.08–2.31) | 0.42 |
| Severe DAH | 5 (16.1) | 9 (30.0) | 6 (19.4) | 10 (33.3) | 0.45 (0.14–1.40) | 0.44 |
Definition of abbreviations: CI = confidence interval; DAH = diffuse alveolar hemorrhage; HR = hazard ratio; PLEX = plasma exchange.
Effects of PLEX are expressed as HR over the first year and are adjusted for age, sex, antineutrophil cytoplasmic antibody subtype, baseline kidney function, and initial treatments.
The effect of GC dosing regimen on survival in participants with versus without DAH, adjusted for age, sex, ANCA subtype, kidney function, and initial treatments, is presented in Figure 3. For those with DAH, death occurred in 9 in the reduced GC group and 9 in the standard GC group at 3 months and in 13 and 10 at 1 year (HR, 1.33; 95% CI, 0.57–3.13) (P-interaction = 0.10 for any DAH vs. no DAH, 0.65 for nonsevere DAH vs. no DAH, and 0.08 for severe DAH vs. no DAH) (Table 4).
Figure 3.
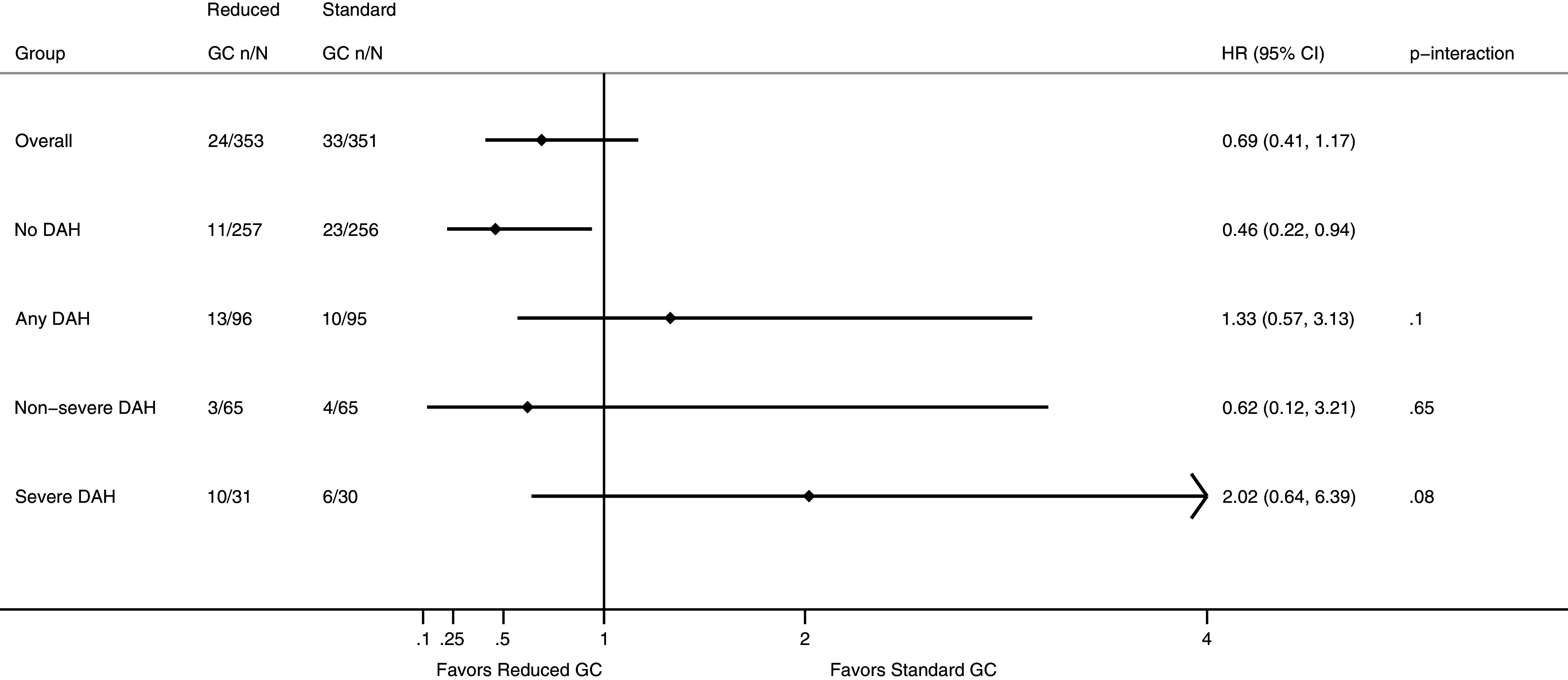
One-year survival by GC regimen and severity of DAH. Diamond represents effect estimate, and horizontal line represents the 95% CI based on a Cox proportional hazards model adjusted for age, sex, antineutrophil cytoplasmic antibody subtype, kidney function, and initial treatments. CI = confidence interval; DAH = diffuse alveolar hemorrhage; GC = glucocorticoids; HR = hazard ratio.
Table 4.
Risk of Death at 3 Months and 1 Year in Patients with or without DAH, and by Severity of DAH, by GC Regimen Treatment Allocation
| Died 3 Mo |
Died 1 Yr |
Effect of Reduced GC |
||||
|---|---|---|---|---|---|---|
| Reduced GC | Standard GC | Reduced GC | Standard GC | HR (95% CI) | P-interaction | |
| Overall | 16 (4.5) | 23 (6.6) | 24 (6.8) | 33 (9.4) | 0.69 (0.41–1.17) | |
| No DAH | 7 (2.7) | 14 (5.5) | 11 (4.3) | 23 (9.0) | 0.46 (0.22–0.94) | |
| Any DAH | 9 (9.4) | 9 (9.5) | 13 (13.5) | 10 (10.5) | 1.33 (0.57–3.13) | 0.10 |
| Nonsevere DAH | 1 (1.5) | 3 (4.6) | 3 (4.6) | 4 (6.2) | 0.62 (0.12–3.21) | 0.65 |
| Severe DAH | 8 (25.8) | 6 (20.0) | 10 (32.3) | 6 (20.0) | 2.02 (0.64–6.39) | 0.08 |
Definition of abbreviations: CI = confidence interval; DAH = diffuse alveolar hemorrhage; GC = glucocorticoid; HR = hazard ratio.
Effects of GC are expressed as HR over the first year and are adjusted for age, sex, antineutrophil cytoplasmic antibody subtype, baseline kidney function, and initial treatments.
Initial immunosuppression was specified by the treating clinicians at the time of randomization, before learning the allocated PLEX or GC regimen, rather than assigned by the trial. Given the differences in agent selected between those with versus without DAH, as presented in Table 1, the effects of these on death at 1 year among those with DAH were evaluated and no differences were found. Using intravenous cyclophosphamide as the referent, oral cyclophosphamide had a HR of 1.00 (95% CI, 0.57–1.78) and rituximab an HR of 0.79 (95% CI, 0.32–1.94).
Among participants with DAH requiring mechanical ventilation, there was no difference in ventilator-free days between the PLEX (median, 25; IQR, 22–26) and no-PLEX groups (median, 25; IQR, 22–27; P = 0.83). Participants receiving the reduced GC had fewer ventilator-free days (median, 23; IQR, 20–25) than those receiving the standard GC regimen (median, 26; IQR, 25–28; P = 0.009) (Figure 4). Sensitivity analysis comparing duration of mechanical ventilation yielded similar results (data not shown).
Figure 4.

Ventilator-free days within 30 days of randomization among participants with diffuse alveolar hemorrhage requiring mechanical ventilation. Box plots representing median ventilator-free days and 25th–75th interquartile range, represented by (A) PLEX treatment allocation and (B) GC regimen. GC = glucocorticoids; PLEX = plasma exchange.
The effects of PLEX or GC regimen on serious infections in the first year did not vary based on the presence or absence of DAH. Serious infections in the DAH group occurred in 40 versus 39 (PLEX vs. no-PLEX; odds ratio, 1.13; 95% CI, 0.61–2.10), and 38 versus 41 (reduced versus standard GC; odds ratio, 0.89; 95% CI, 0.48–1.65) (P-interaction = 0.85 for PLEX and 0.91 for GC regimen). This was also not impacted by the severity of DAH (P-interaction = 0.28 to 0.33 for PLEX and P-interaction = 0.92 for GC regimen) (Table 5).
Table 5.
Effects of PLEX and GC Dosing on Number of Patients with One or More Serious Infections within 1 Year
| PLEX [n (%)] | No PLEX [n (%)] |
OR (95% CI) | P-interaction | Standard GC [n (%)] | Reduced GC [n (%)] | OR (95% CI) | P-interaction | |
|---|---|---|---|---|---|---|---|---|
| Overall | 149 (53) | 131 (37) | 1.25 (0.92–1.70) | 146 (42) | 134 (38) | 0.85 (0.63–1.16) | ||
| No DAH | 109 (42) | 92 (36) | 1.26 (0.88–1.81) | 105 (41) | 96 (37) | 0.87 (0.61–1.25) | ||
| Any DAH | 40 (42) | 39 (41) | 1.13 (0.61–2.10) | 0.85 | 41 (43) | 38 (40) | 0.89 (0.48–1.65) | 0.91 |
| Nonsevere DAH | 22 (34) | 26 (39) | 0.84 (0.39–1.84) | 0.33 | 41 (43) | 38 (40) | 0.88 (0.40–1.91) | 0.92 |
| Severe DAH | 18 (58) | 13 (43) | 1.68 (0.50–5.60) | 0.28 | 16 (53) | 15 (48) | 0.92 (0.30–2.81) | 0.92 |
Definition of abbreviations: CI = confidence interval; DAH = diffuse alveolar hemorrhage; GC = glucocorticoid; OR = odds ratio; PLEX = plasma exchange.
Overall Outcomes at 1 Year: Survival
Survival analysis by presence or absence of DAH, and by severity of DAH, is presented in Figure 5. Participants with DAH at enrollment had reduced survival compared with those without DAH (adjusted HR, 2.22; 95% CI, 1.29–3.81; P = 0.004). Among the 191 participants with DAH, 23 (12.0%) died within the first year, versus 34 of 513 (6.6%) among those without DAH (P = 0.019). The majority of deaths among participants with DAH occurred within the first 30 days after randomization (14/23; 60.9%) versus 11/34 (32.4%) for those without DAH (P = 0.033). In both groups, most deaths occurred within the first 3 months after randomization (18/23 [78.3%] with DAH and 21/34 [61.8%] without DAH). The group with severe DAH predominantly accounted for the reduced survival among those with DAH (adjusted HR, 5.17; 95% CI, 2.80–9.56; P < 0.001), with 16 of the 23 (69.6%) deaths in the DAH group occurring in those with severe DAH.
Figure 5.
One-year survival based on presence or absence of DAH at enrollment. Cox proportional hazards model adjusted for age, sex, antineutrophil cytoplasmic antibody subtype, kidney function, and initial treatments. DAH = diffuse alveolar hemorrhage.
Overall Outcomes at 1 Year: End-Stage Kidney Disease, Serious Infections
Twenty-five participants with DAH (13.1%) developed end-stage kidney disease within the first year, as did 22 of 130 (16.9%) of those with nonsevere DAH and 3 of 61 (4.9%) of those with severe DAH. This was similar to those without DAH (82/513; 16.0%; P = 0.34).
Serious infections occurred in 79/191 (41.4%) participants with DAH and 201/513 (39.2%) without DAH (P = 0.60). Among those with DAH, serious infections were less common in participants with nonsevere DAH (48/130; 36.9%) than those with severe DAH (31/61; 50.8%).
Discussion
There are limited published data on the outcomes of patients with DAH due to AAV. The PEXIVAS trial, the largest trial in AAV and the first to include participants with DAH requiring mechanical ventilation, presented a unique opportunity to examine characteristics and outcomes of patients with DAH due to AAV.
There are also few studies informing the characteristics of patients who develop DAH in AAV. Participants in PEXIVAS with DAH were younger, more commonly anti-PR3 positive, and more frequently had relapsing AAV rather than a new diagnosis at enrollment. Although participants with DAH had lower median creatinine at enrollment, they more commonly received hemodialysis. This may reflect the acuity of their presentation with active multiorgan disease, the diagnostic challenge of differentiating pulmonary edema from DAH, an increased propensity to pulmonary edema in patients with DAH and/or kidney injury, or other factors. Notably, approximately three-quarters of PEXIVAS participants with DAH had hemoptysis. This frequency, similar to that in other reports, highlights the severity of this patient cohort, while also serving as a reminder that lack of hemoptysis should not diminish clinical suspicion for DAH (2, 3, 6, 8, 9). Importantly, participants with DAH, especially those with severe DAH, were at increased risk of death, particularly within the first 30 days and 3 months.
The role of plasma exchange in DAH due to AAV remains an area of interest and debate. The numerical differences in mortality at 3 and 12 months for those with DAH between the PLEX and no-PLEX groups are of unclear clinical significance because they are based on small numbers of events and not statistically significant. Given that DAH can be acutely fatal both through flooding of alveoli with blood and concomitant ventilator-acquired lung injury, high inspired oxygen concentrations, and acute respiratory distress syndrome, ventilator-free days may be a more sensitive measure of treatment effects in the most severe DAH. There was no change in ventilator-free days with PLEX compared with no-PLEX, but there was a difference between GC dosing regimens, with fewer ventilator-free days among participants receiving the reduced-dose regimen. Although there was also a numerical difference in deaths between GC dosing regimens, this was again based on a small number of events and not statistically significant. Thus, the clinical significance of the difference in ventilator-free days is unclear. Whether longer duration of mechanical ventilation or higher dose and/or slower GC taper carries more risk of infection and other downstream sequelae should be considered on an individual basis. More research is necessary to understand optimal dosing of GCs in this population.
The strengths of this study include use of data from a large, well-defined group of patients with DAH due to AAV who received randomized treatments and that all data were collected prospectively. However, there are also several limitations to consider for this report. Although this is one of the largest cohorts of patients with DAH in AAV, it is still small to study relevant treatment effects, particularly when considering the severe DAH group, resulting in imprecise estimates. Bronchoscopy was not required to define DAH. This pragmatic decision avoided delays in enrollment and treatment of patients with this severe manifestation of AAV, recognizing that procedural availability may differ across the 95 participating clinical sites. The remaining three findings that could meet the definition of DAH (hemoptysis, otherwise unexplained anemia, or elevated diffusing capacity), in the context of appropriate radiographic appearance and clinical picture, are consistent with the presence of erythrocytes in the lower respiratory tract, through visualization or functionally. Nevertheless, it remains possible that some participants were misclassified as having DAH who in fact did not, which would bias the association between DAH and mortality to the null, particularly in the nonsevere category. The definition of severe DAH was set at an oxygen saturation of 85% on room air or the use of mechanical ventilation. The threshold to use invasive mechanical ventilation may have differed across the 95 centers. Similarly, the duration of ventilation before randomization may have differed across participants and sites, a limitation of starting the calculation of ventilator-free days at randomization. However, randomization attenuated differences between treatment groups, and sensitivity analysis using duration of ventilation yielded similar results to those for ventilator-free days. Finally, the initial immunosuppressive regimens, including the doses of pulse methylprednisolone, were left to the local investigators and could have changed after randomization, mitigating differences in the effects of the randomized therapies. For future trials to detect treatment effects in severe DAH, a rare, severe manifestation of a rare disease, a larger sample size driven by engagement of an even broader network would be necessary, together with consideration of alternative statistical approaches. Protocol restrictions intended to address the limitations outlined above may ultimately result in more difficulty recruiting and enrolling participants with severe DAH.
In conclusion, severe DAH represents an acute manifestation of AAV that is potentially life-threatening, particularly early during the disease course. These findings highlight the need for development of more effective, safer therapies for patients with severe DAH in AAV.
Acknowledgments
Acknowledgment
Terumo BCT, Terumo BCT Mexico, Fresenius Medical Care Australia, Baxter Healthcare (Australia), and Asahi Kasei Medical provided in-kind plasma-exchange disposables for the PEXIVAS trial.
PEXIVAS Collaborators
Kathy Paizis, Giles Walters, Meg Jardine, Caroline Milton, Abu Ibraham, Brian Siva, Michael Desmond, Vlado Perkovic, Jadadeesh Kurtkoti, Eswari Vilayur, Alan Cass, Shaun Summers, Fiona Brown, Jessica Ryan, Peter Kerr, Euan Noble, Grant Luxton, David W. Mudge, Carmel Hawley, David W. Johnson, Chen Au Peh, Randall J. Faull, Dwarakanathan Ranganathan, Lisa Jeffs, Kathy Nicholls, Peter Hughes, Bruce Cooper, Neil Boudville, Sharon Ford, Robyn Langham, Donna Reidlinger, Alicia Morrish, Sunil V. Badve, Elaine Pascoe, Peta-Anne Paul-Brent, Laura Robison, Andrea Valks, Daniel Blockmans, Liesbet Henckaerts, Ben Sprangers, Rita Suri, Soumeya Brachemi, William Clark, Amit Garg, Simon Carette, Christian Pagnoux, Heather Reich, David Barth, Michael Walsh, Nader Khalidi, Gerry Cox, Andrea Mazzetti, Diane Robins, Ron Wald, Jeffrey Perl, Katerina Pavenski, Niki Dacouris, Adeera Levin, Michael Copland, Todd Fairhead, Neesh Pannu, Muhammad Uwais Qarni, Syed Habib, Louis Girard, Braden Manns, Vladimir Tesar, Zdenka Hruskova, Zdenka Chocova, Johan Povlsen, Jon Gregersen, Per Ivarsen, Henrik Birn, Elizabeth Krarup, Erling B. Pedersen, Ingrid Thomsen, Jesper Nørgaard Bech, Wladmir Szpirt, Martin Egfjord, Rafik Mesbah, Pierre Bataille, Isabelle Rey, François Chantrel, Philipe Vanhille, Thomas Quémeneur, Pierre-Louis Carron, Philippe Zaoui, Claire de Moreuil, Morgane Gosselin, Aurélien Delluc, Catherine Hanrotel-Saliou, Mathilde Le Jeune, Maxence Ficheux, Julien Aniort, Christian Lavigne, Jean Francois Augusto, Dominique Chauveau, Joëlle Guitard, Antoine Huart, David Ribes, Philippe Gatault, Camille Becmeur, Sandrine Muller, Valérie Betz, Alexandre Klein, Gilles Blaison, Raphaele Seror, Hélène Francois, Xavier Mariette, Aurore Aubrun, Baptiste Coustet, Elisabeth Palazzo, Sébastien Ottaviani, Tiphaine Goulenok, Eric Daugas, Philipe Dieudé, Thomas Papo, Céline Lebas, Arnaud Lionet, Loïc Guillevin, Luc Mouthon, Xavier Puéchal, Noémie Jourde-Chiche, Marc Ruivard, Alexandre Karras, Nicolas Limal, Thomas Kofman, Alain Le Quellec, François Maurier, Aude Gibelin, Antoine Parrot, Claude Bachmeyer, Bruno Gombert, Mathilde Nouvier, Jean-Christophe Lega, Olivier Fain, Emmanuel Andrès, Rachel Cottet, Gina Gregorini, Guido Jeannin, Stefano Possenti, Carlo Buzio, Augusto Vaglio, Elena Oliva, Hirofumi Makino, Eri Muso, Tomomi Endo, Hiroko Kakita, Hiroyuki Suzuki, Takaya Handa, Youngna Kang, Yuki Ariyasu, Tatsuo Tsukamoto, Shuichiro Endo, Hitomi Miyata, Hiroyuki Yamada, Toshiko Ito-Ihara, Shunya Uchida, Hajime Kono, Yoshihide Fujigaki, Hirotoshi Kikuchi, Toshihiro Nanki, Hideki Kato, Akiko Okamoto, Kurumi Asako, Kazuo Suzuki, Yoshitomo Hamano, Kunihiro Yamagata, Joichi Usui, Shouichi Fujimoto, Yuji Sato, Masao Kikuchi, Luis Felipe Flores-Suárez, Sergio A. Sánchez-Guerrero, Michael Collins, John Schollum, Janak de Zoysa, Vicki Quincy, Peter Sizeland, Knut Aasarod, Marit Solbu, Trude Jannecke Bruun, Wenche Koldingsnes, Anna Wludarczyk, Ilona Nowak, Jacek Gorka, Jan Sznajd, Agnieszka Padjas, Milosz Jankowski, Agnieszka Widawska, Wojciech Szczeklik, Jose Ballarin, Annette Bruchfeld, Mats Efvergren, Per Eriksson, Kerstin Westman, Daina Selga, Caroline Heijl, Sophie Ohlsson, Marten Segelmark, Neil Basu, Dana Kidder, Nicholas Fluck, David R. W. Jayne, Rona Smith, Lisa Wilcocks, Mark McClure, Rachel Jones, Sapna Trivedi, Seerapani Gopaluni, Elizabeth Brettell, Paul Crump, Annika Feilbach, Catherine Hewitt, Nick Hilken, Andrew Howman, Terry Hughes, Natalie Ives, Hugh Jarrett, Samir Mehta, Rebecca Record, Gemma Ryan, Chaka Sidile, Keith Wheatley, Sheerin, Alison Brown, Laura Anne Baines, Jim Lordan, Charles Pusey, Anisha Tanna, Stephen McAdoo, Jeremy Levy, Megan Griffith, Bernhard Klebe, Timothy Doulton, Graham Warwick, James Burton, Jonathon Barratt, Peter Topham, Richard Baines, Nigel Brunskill, Reem Al-Jayyousi, Patrick Hamilton, Mumtaz Patel, Sandip Mitra, Nina Brown, Edward Sharples, Raashid Luqmani, Lorraine Harper, Benjamin Rhodes, Dimitrios Chanouzas, Matthew Morgan, Peter Hewins, Oliver Floßmann, Nitin Bhandary, Julie Foxton, Linda Jones, Jenny King, Lucy Smyth, Richard D’Souza, Richard Haigh, Maxine Hough, Alan Salama, Aine Burns, Mark Little, Neeraj Dhaun, Ajay Dhaygude, Kolitha Basnayake, Neil Iggo, Daniel Jones, David Oliveira, Iain A. M. MacPhee, Emma Dunn, Andrew J. P. Lewington, Stanley Linsun Fan, Ravindra Rajakariar, Magdi Yaqoob, Andrew Short, Colin Geddes, Bruce Mackinnon, Alan G. Jardine, Paul Monach, Peter A. Merkel, Naomi Amudala, Karen Quillen, Michael Weisman, Daniel Wallace, Lindsy Forbess, Swamy Venuturupalli, Carol Langford, Rula Hajj-Ali, Anna Koo, Gary Hoffman, Ulrich Specks, Karina Keogh, Steven Ytterberg, Jeff Winters, Kenneth Warrington, Rodrigo Cartin-Ceba, Tobias Peikert, Fernando Fervenza, Misbah Baqir, Patrick Nachman, Randy Detwiler, Amy Mottl, Vimal Derebail, JulieAnne McGregor, Peter A. Merkel, Antoine Sreih, Rennie Rhee, Carol McAlear, Nicole Aqui, Larry Moreland, Joseph Kiss, Kimberly Liang, Niveditha Mohan, Rasheed Balogun, Tingting Li, Richard Brasington, Andrew Rees, David Scott, Paul Roderick, Martin Landray, Richard Watts, Jonathan Emberson
PEXIVAS Collaborators by Site
Australia – Austin Hospital: Kathy Paizis; Canberra Hospital: Giles Walters; Concord Repatriation General Hospital: Meg Jardine; Flinders Medical Centre: Caroline Milton; Fremantle/Fiona Stanley Hospitals: Abu Ibraham and Brian Siva; Geelong Hospital: Michael Desmond; George Institute for Global Health: Vlado Perkovic; Gold Coast University Hospital: Jadadeesh Kurtkoti; John Hunter Hospital: Eswari Vilayur; Menzies School of Health Research: Alan Cass; Monash Medical Centre: Shaun Summers, Fiona Brown, Jessica Ryan, and Peter Kerr; Nambour General Hospital: Euan Noble; Prince of Wales Hospital: Grant Luxton; Princess Alexandra Hospital: David W. Mudge, Carmel Hawley, and David W. Johnson; Royal Adelaide Hospital: Chen Au Peh and Randall J. Faull; Royal Brisbane and Women’s Hospital: Dwarakanathan Ranganathan; Royal Hobart Hospital: Lisa Jeffs; Royal Melbourne Hospital: Kathy Nicholls and Peter Hughes; Royal North Shore Hospital: Bruce Cooper; Sir Charles Gairdner Hospital: Neil Boudville; St. Vincent’s Hospital: Sharon Ford and Robyn Langham; Australasian Kidney Trials Network, University of Queensland: Donna Reidlinger, Alicia Morrish, Sunil V. Badve, Elaine Pascoe, Peta-Anne Paul-Brent, Laura Robison, and Andrea Valks.
Belgium – University Hospitals Leuven: Daniel Blockmans, Liesbet Henckaerts, and Ben Sprangers.
Canada – Hôpital Saint-Luc: Rita Suri and Soumeya Brachemi; London Health Sciences Centre: William Clark and Amit Garg; Mount Sinai Hospital/Toronto General Hospital: Simon Carette, Christian Pagnoux, Heather Reich, and David Barth; St. Joseph’s Healthcare Hamilton: Michael Walsh, Nader Khalidi, Gerry Cox, Andrea Mazzetti, and Diane Robins; St. Michael’s Hospital: Ron Wald, Jeffrey Perl, Katerina Pavenski, and Niki Dacouris; St. Paul’s Hospital: Adeera Levin and Michael Copland; The Ottawa Hospital: Todd Fairhead; University of Alberta: Neesh Pannu, Muhammad Uwais Qarni, and Syed Habib; University of Calgary: Louis Girard and Braden Manns.
Czech Republic – General Faculty Hospital: Vladimir Tesar, Zdenka Hruskova, and Zdenka Chocova.
Denmark – Aarhus University Hospital: Johan Povlsen, Jon Gregersen, Per Ivarsen, and Henrik Birn; Herlev Hospital: Elizabeth Krarup; Holstebro Hospital, Aarhus University: Erling B. Pedersen, Ingrid Thomsen, and Jesper Nørgaard Bech; Rigshospitalet Nephrology, Copenhagen University Hospital: Wladmir Szpirt and Martin Egfjord.
France – Centre Hospitalier de Boulogne: Rafik Mesbah and Pierre Bataille; Centre Hospitalier de la Région d’Annecy: Isabelle Rey; Centre Hospitalier de Mulhouse: François Chantrel; Centre Hospitalier de Valenciennes: Philipe Vanhille and Thomas Quémeneur; Centre Hospitalier Universitaire de Grenoble: Pierre-Louis Carron and Philippe Zaoui; CHRU Brest-Hôpital La Cavale Blanche: Claire de Moreuil, Morgane Gosselin, Aurélien Delluc, Catherine Hanrotel-Saliou, and Mathilde Le Jeune; CHU de Caen: Maxence Ficheux; CHU de Clermont-Ferrand: Julien Aniort; CHU d’Angers: Christian Lavigne and Jean Francois Augusto; CHU de Toulouse-Hôtel Dieu Saint Jacques: Dominique Chauveau, Joëlle Guitard, Antoine Huart, and David Ribes; CHU de Tours-Hôpital Bretonneau: Philippe Gatault; Hôpital Civil de Colmar: Camille Becmeur, Sandrine Muller, Valérie Betz, Alexandre Klein, and Gilles Blaison; Hôpital Bicêtre, Kremlin-Bicêtre: Raphaele Seror, Hélène Francois, and Xavier Mariette; Hôpital Bichat Claude Bernard, Paris: Aurore Aubrun, Baptiste Coustet, Elisabeth Palazzo, Sébastien Ottaviani, Tiphaine Goulenok, Eric Daugas, Philipe Dieudé, and Thomas Papo; Hôpital Claude Huriez – CHRU Lille: Céline Lebas and Arnaud Lionet; Hôpital Cochin, Paris: Loïc Guillevin, Luc Mouthon, and Xavier Puéchal; Hôpital de la Conception, Marseille: Noémie Jourde-Chiche; Hôpital Estaing, Clermont-Ferrand: Marc Ruivard; Hôpital Européen Georges-Pompidou, Paris: Alexandre Karras; Hôpital Henri Mondor, Créteil: Nicolas Limal and Thomas Kofman; Hôpital Saint Eloi, Montpellier: Alain Le Quellec; Hôpital Belle Isle, Metz: François Maurier; Hôpital Tenon, Paris: Aude Gibelin, Antoine Parrot, and Claude Bachmeyer; Centre Hospitalier de La Rochelle: Bruno Gombert; Hôpital Lyon Sud: Mathilde Nouvier and Jean-Christophe Lega; Hôpital Saint Antoine, Paris: Olivier Fain; Hôpital Civil de Strasbourg: Emmanuel Andrès and Rachel Cottet.
Italy – Azienda Ospedaliera Spedali Civili di Brescia: Gina Gregorini, Guido Jeannin, and Stefano Possenti; Azienda Ospedaliera Universitaria di Parma: Carlo Buzio, Augusto Vaglio, and Elena Oliva.
Japan – Okayama University: Hirofumi Makino; Kitano Hospital: Eri Muso, Tomomi Endo, Hiroko Kakita, Hiroyuki Suzuki, Takaya Handa, Youngna Kang, and Yuki Ariyasu; Kyoto University Hospital: Tatsuo Tsukamoto, Shuichiro Endo, Hitomi Miyata, Hiroyuki Yamada, and Toshiko Ito-Ihara; Teikyo University Hospital: Shunya Uchida, Hajime Kono, Yoshihide Fujigaki, Hirotoshi Kikuchi, Toshihiro Nanki, Hideki Kato, Akiko Okamoto, Kurumi Asako, and Kazuo Suzuki; Tokyo Metropolitan Geriatric Hospital: Yoshitomo Hamano; University of Tsukuba: Kunihiro Yamagata and Joichi Usui; University of Miyazaki: Shouichi Fujimoto, Yuji Sato, and Masao Kikuchi.
Mexico – Instituto Nacional de Enfermedades Respiratorias: Luis Felipe Flores-Suárez; Instituto Nacional de Cancerologia, Mexico City: Sergio A. Sánchez-Guerrero.
New Zealand – Auckland City Hospital: Michael Collins; Dunedin Hospital: John Schollum; North Shore Hospital: Janak de Zoysa; Waikato Hospital: Vicki Quincy and Peter Sizeland.
Norway – St. Olav’s Hospital: Knut Aasarod; University Hospital North Norway: Marit Solbu, Trude Jannecke Bruun, and Wenche Koldingsnes.
Poland – Jagiellonian University: Anna Wludarczyk, Ilona Nowak, Jacek Gorka, Jan Sznajd, Agnieszka Padjas, Milosz Jankowski, Agnieszka Widawska, and Wojciech Szczeklik.
Spain – Fundacio Puigvert: Jose Ballarin.
Sweden – Karolinska Institute: Annette Bruchfeld and Mats Efvergren; Linkoping University Hospital: Per Eriksson; Skane University Hospital: Kerstin Westman, Daina Selga, Caroline Heijl, Sophie Ohlsson, and Marten Segelmark.
United Kingdom – Aberdeen Royal Infirmary: Neil Basu, Dana Kidder, and Nicholas Fluck; Addenbrooke’s Hospital: David R. W. Jayne, Rona Smith, Lisa Wilcocks, Mark McClure, Rachel Jones, Sapna Trivedi, and Seerapani Gopaluni; Birmingham Clinical Trials Unit: Elizabeth Brettell, Paul Crump, Annika Feilbach, Catherine Hewitt, Nick Hilken, Andrew Howman, Terry Hughes, Natalie Ives, Hugh Jarrett, Samir Mehta, Rebecca Record, Gemma Ryan, and Chaka Sidile; Cancer Research UK Clinical Trials Unit: Keith Wheatley; Freeman Hospital: Sheerin, Alison Brown, Laura Anne Baines, and Jim Lordan; Hammersmith Hospital: Charles Pusey, Anisha Tanna, Stephen McAdoo, Jeremy Levy, and Megan Griffith; Kent and Canterbury Hospital: Bernhard Klebe and Timothy Doulton; Leicester General Hospital: Graham Warwick, James Burton, Jonathon Barratt, Peter Topham, Richard Baines, Nigel Brunskill, and Reem Al-Jayyousi; Manchester Royal Infirmary: Patrick Hamilton, Mumtaz Patel, Sandip Mitra, and Nina Brown; Oxford University Hospitals NHS Trust: Edward Sharples and Raashid Luqmani; Queen Elizabeth Hospital: Lorraine Harper, Benjamin Rhodes, Dimitrios Chanouzas, Matthew Morgan, and Peter Hewins; Royal Berkshire Hospital: Oliver Floßmann, Nitin Bhandary, Julie Foxton, Linda Jones, and Jenny King; Royal Devon and Exeter Hospital: Lucy Smyth, Richard D’Souza, Richard Haigh, and Maxine Hough; Royal Free Hospital: Alan Salama, Aine Burns, and Mark Little; Royal Infirmary of Edinburgh: Neeraj Dhaun; Royal Preston Hospital: Ajay Dhaygude; Royal Sussex County Hospital: Kolitha Basnayake and Neil Iggo; St. George’s Hospital: Daniel Jones, David Oliveira, and Iain A. M. MacPhee; St. James’ University Hospital: Emma Dunn and Andrew J. P. Lewington; The Royal London Hospital: Stanley Linsun Fan, Ravindra Rajakariar, and Magdi Yaqoob; University of Coventry and Warwickshire: Andrew Short; Western Infirmary: Colin Geddes, Bruce Mackinnon, and Alan G. Jardine.
United States – Boston University School of Medicine: Paul Monach, Peter A. Merkel, Naomi Amudala, and Karen Quillen; Cedars-Sinai Medical Center: Michael Weisman, Daniel Wallace, Lindsy Forbess, andSwamy Venuturupalli; Cleveland Clinic: Carol Langford, Rula Hajj-Ali, Anna Koo, and Gary Hoffman; Mayo Clinic: Ulrich Specks, Karina Keogh, Steven Ytterberg, Jeff Winters, Kenneth Warrington, Rodrigo Cartin-Ceba, Tobias Peikert, Fernando Fervenza, and Misbah Baqir; University of North Carolina: Patrick Nachman, Randy Detwiler, Amy Mottl, Vimal Derebail, and JulieAnne McGregor; University of Pennsylvania: Peter A. Merkel, Antoine Sreih, Rennie Rhee, Carol McAlear, and Nicole Aqui; University of Pittsburgh Medical Center: Larry Moreland, Joseph Kiss, Kimberly Liang, and Niveditha Mohan; University of Virginia: Rasheed Balogun; Washington University School of Medicine in St. Louis: Tingting Li and Richard Brasington.
Trial Steering Committee: Andrew Rees (Medical University of Vienna), David Scott (Norfolk and Norwich University Hospital), and Paul Roderick (University of Southampton).
Data Safety and Monitoring Committee: Martin Landray (University of Oxford), Richard Watts (University of East Anglia), and Jonathan Emberson (University of Oxford).
Footnotes
A complete list of PEXIVAS collaborators may be found before the beginning of the References.
The PEXIVAS trial was supported by National Institute for Health Research, United Kingdom grant HTA 08/56/04; U.S. Food and Drug Administration grant FDA R01 FD003516; National Institute of Arthritis and Musculoskeletal and Skin Diseases grant U54 AR0573319; the NIH of the U.S. Department of Health and Human Services; National Health and Medical Research Council, Australia grants 626939, APP1086192, 631731, and APP1092957; Canadian Institutes of Health Research grants 211079 and 159662; French Ministry of Health, Programme Hospitalier de Recherche Clinique grant AOM11142; the Research Committee on Intractable Vasculitides of the Ministry of Health, Labor, and Welfare of Japan; and the Japan Agency for Medical Research and Development: the Strategic Study Group to Establish the Evidence for Intractable Vasculitis Guideline. Sponsors of the PEXIVAS trial provided study therapeutics and equipment. No sponsor had a role in study design, data collection, analysis or interpretation of data, writing of the report, or the decision to submit the original trial or this subgroup analysis for publication.
Author Contributions: All authors had substantial contributions to the conception of this work; acquisition, analysis, and interpretation of the data; writing and reviewing; approval of final version submitted; and agree to be accountable for all aspects of the work. L.A.F.: conceptualization, methodology, investigation, data curation and analysis, visualization, writing – original draft, writing – reviewing and editing. L.F.F.-S., R.C.-C., and P.G.C.: conceptualization, investigation, data curation, writing – reviewing and editing. U.S.: conceptualization, methodology, investigation, data curation and analysis, writing – reviewing and editing. D.R.W.J. and P.A.M.: conceptualization, methodology, investigation, data curation and analysis, writing – reviewing and editing, funding acquisition. M.W.: conceptualization, methodology, investigation, data curation and analysis, visualization, writing – original draft, writing – reviewing and editing, funding acquisition.
Originally Published in Press as DOI: 10.1164/rccm.202308-1426OC on February 12, 2024
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
the PEXIVAS Investigators:
Kathy Paizis, Giles Walters, Meg Jardine, Caroline Milton, Abu Ibraham, Brian Siva, Michael Desmond, Vlado Perkovic, Jadadeesh Kurtkoti, Eswari Vilayur, Alan Cass, Shaun Summers, Fiona Brown, Jessica Ryan, Peter Kerr, Euan Noble, Grant Luxton, David W. Mudge, Carmel Hawley, David W. Johnson, Chen Au Peh, Randall J. Faull, Dwarakanathan Ranganathan, Lisa Jeffs, Kathy Nicholls, Peter Hughes, Bruce Cooper, Neil Boudville, Sharon Ford, Robyn Langham, Donna Reidlinger, Alicia Morrish, Sunil V. Badve, Elaine Pascoe, Peta-Anne Paul-Brent, Laura Robison, Andrea Valks, Daniel Blockmans, Liesbet Henckaerts, Ben Sprangers, Rita Suri, Soumeya Brachemi, William Clark, Amit Garg, Simon Carette, Christian Pagnoux, Heather Reich, David Barth, Michael Walsh, Nader Khalidi, Gerry Cox, Andrea Mazzetti, Diane Robins, Ron Wald, Jeffrey Perl, Katerina Pavenski, Niki Dacouris, Adeera Levin, Michael Copland, Todd Fairhead, Neesh Pannu, Muhammad Uwais Qarni, Syed Habib, Louis Girard, Braden Manns, Vladimir Tesar, Zdenka Hruskova, Zdenka Chocova, Johan Povlsen, Jon Gregersen, Per Ivarsen, Henrik Birn, Elizabeth Krarup, Erling B. Pedersen, Ingrid Thomsen, Jesper Nørgaard Bech, Wladmir Szpirt, Martin Egfjord, Rafik Mesbah, Pierre Bataille, Isabelle Rey, François Chantrel, Philipe Vanhille, Thomas Quémeneur, Pierre-Louis Carron, Philippe Zaoui, Claire de Moreuil, Morgane Gosselin, Aurélien Delluc, Catherine Hanrotel-Saliou, Mathilde Le Jeune, Maxence Ficheux, Julien Aniort, Christian Lavigne, Jean Francois Augusto, Dominique Chauveau, Joëlle Guitard, Antoine Huart, David Ribes, Philippe Gatault, Camille Becmeur, Sandrine Muller, Valérie Betz, Alexandre Klein, Gilles Blaison, Raphaele Seror, Hélène Francois, Xavier Mariette, Aurore Aubrun, Baptiste Coustet, Elisabeth Palazzo, Sébastien Ottaviani, Tiphaine Goulenok, Eric Daugas, Philipe Dieudé, Thomas Papo, Céline Lebas, Arnaud Lionet, Loïc Guillevin, Luc Mouthon, Xavier Puéchal, Noémie Jourde-Chiche, Marc Ruivard, Alexandre Karras, Nicolas Limal, Thomas Kofman, Alain Le Quellec, François Maurier, Aude Gibelin, Antoine Parrot, Claude Bachmeyer, Bruno Gombert, Mathilde Nouvier, Jean-Christophe Lega, Olivier Fain, Emmanuel Andrès, Rachel Cottet, Gina Gregorini, Guido Jeannin, Stefano Possenti, Carlo Buzio, Augusto Vaglio, Elena Oliva, Hirofumi Makino, Eri Muso, Tomomi Endo, Hiroko Kakita, Hiroyuki Suzuki, Takaya Handa, Youngna Kang, Yuki Ariyasu, Tatsuo Tsukamoto, Shuichiro Endo, Hitomi Miyata, Hiroyuki Yamada, Toshiko Ito-Ihara, Shunya Uchida, Hajime Kono, Yoshihide Fujigaki, Hirotoshi Kikuchi, Toshihiro Nanki, Hideki Kato, Akiko Okamoto, Kurumi Asako, Kazuo Suzuki, Yoshitomo Hamano, Kunihiro Yamagata, Joichi Usui, Shouichi Fujimoto, Yuji Sato, Masao Kikuchi, Luis Felipe Flores-Suárez, Sergio A. Sánchez-Guerrero, Michael Collins, John Schollum, Janak de Zoysa, Vicki Quincy, Peter Sizeland, Knut Aasarod, Marit Solbu, Trude Jannecke Bruun, Wenche Koldingsnes, Anna Wludarczyk, Ilona Nowak, Jacek Gorka, Jan Sznajd, Agnieszka Padjas, Milosz Jankowski, Agnieszka Widawska, Wojciech Szczeklik, Jose Ballarin, Annette Bruchfeld, Mats Efvergren, Per Eriksson, Kerstin Westman, Daina Selga, Caroline Heijl, Sophie Ohlsson, Marten Segelmark, Neil Basu, Dana Kidder, Nicholas Fluck, David R. W. Jayne, Rona Smith, Lisa Wilcocks, Mark McClure, Rachel Jones, Sapna Trivedi, Seerapani Gopaluni, Elizabeth Brettell, Paul Crump, Annika Feilbach, Catherine Hewitt, Nick Hilken, Andrew Howman, Terry Hughes, Natalie Ives, Hugh Jarrett, Samir Mehta, Rebecca Record, Gemma Ryan, Chaka Sidile, Keith Wheatley, Sheerin, Alison Brown, Laura Anne Baines, Jim Lordan, Charles Pusey, Anisha Tanna, Stephen McAdoo, Jeremy Levy, Megan Griffith, Bernhard Klebe, Timothy Doulton, Graham Warwick, James Burton, Jonathon Barratt, Peter Topham, Richard Baines, Nigel Brunskill, Reem Al-Jayyousi, Patrick Hamilton, Mumtaz Patel, Sandip Mitra, Nina Brown, Edward Sharples, Raashid Luqmani, Lorraine Harper, Benjamin Rhodes, Dimitrios Chanouzas, Matthew Morgan, Peter Hewins, Oliver Floßmann, Nitin Bhandary, Julie Foxton, Linda Jones, Jenny King, Lucy Smyth, Richard D’Souza, Richard Haigh, Maxine Hough, Alan Salama, Aine Burns, Mark Little, Neeraj Dhaun, Ajay Dhaygude, Kolitha Basnayake, Neil Iggo, Daniel Jones, David Oliveira, Iain A. M. MacPhee, Emma Dunn, Andrew J. P. Lewington, Stanley Linsun Fan, Ravindra Rajakariar, Magdi Yaqoob, Andrew Short, Colin Geddes, Bruce Mackinnon, Alan G. Jardine, Paul Monach, Peter A. Merkel, Naomi Amudala, Karen Quillen, Michael Weisman, Daniel Wallace, Lindsy Forbess, Swamy Venuturupalli, Carol Langford, Rula Hajj-Ali, Anna Koo, Gary Hoffman, Ulrich Specks, Karina Keogh, Steven Ytterberg, Jeff Winters, Kenneth Warrington, Rodrigo Cartin-Ceba, Tobias Peikert, Fernando Fervenza, Misbah Baqir, Patrick Nachman, Randy Detwiler, Amy Mottl, Vimal Derebail, JulieAnne McGregor, Peter A. Merkel, Antoine Sreih, Rennie Rhee, Carol McAlear, Nicole Aqui, Larry Moreland, Joseph Kiss, Kimberly Liang, Niveditha Mohan, Rasheed Balogun, Tingting Li, Richard Brasington, Andrew Rees, David Scott, Paul Roderick, Martin Landray, Richard Watts, and Jonathan Emberson
References
- 1. Travis WD, Hoffman GS, Leavitt RY, Pass HI, Fauci AS. Surgical pathology of the lung in Wegener’s granulomatosis: review of 87 open lung biopsies from 67 patients. Am J Surg Pathol . 1991;15:315–333. doi: 10.1097/00000478-199104000-00001. [DOI] [PubMed] [Google Scholar]
- 2. Haworth SJ, Savage CO, Carr D, Hughes JM, Rees AJ. Pulmonary haemorrhage complicating Wegener’s granulomatosis and microscopic polyarteritis. Br Med J (Clin Res Ed) . 1985;290:1775–1778. doi: 10.1136/bmj.290.6484.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cartin-Ceba R, Diaz-Caballero L, Al-Qadi MO, Tryfon S, Fervenza FC, Ytterberg SR, et al. Diffuse alveolar hemorrhage secondary to antineutrophil cytoplasmic antibody-associated vasculitis: predictors of respiratory failure and clinical outcomes. Arthritis Rheumatol . 2016;68:1467–1476. doi: 10.1002/art.39562. [DOI] [PubMed] [Google Scholar]
- 4. Chung SA, Langford CA, Maz M, Abril A, Gorelik M, Guyatt G, et al. 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol . 2021;73:1366–1383. doi: 10.1002/art.41773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yates M, Watts RA, Bajema IM, Cid MC, Crestani B, Hauser T, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis . 2016;75:1583–1594. doi: 10.1136/annrheumdis-2016-209133. [DOI] [PubMed] [Google Scholar]
- 6. Hruskova Z, Casian AL, Konopasek P, Svobodova B, Frausova D, Lanska V, et al. Long-term outcome of severe alveolar haemorrhage in ANCA-associated vasculitis: a retrospective cohort study. Scand J Rheumatol . 2013;42:211–214. doi: 10.3109/03009742.2012.754939. [DOI] [PubMed] [Google Scholar]
- 7. Zhou P, Li Z, Gao L, Que C, Li H, Ma J, et al. Pulmonary involvement of ANCA-associated vasculitis in adult Chinese patients. BMC Pulm Med . 2022;22:35–49. doi: 10.1186/s12890-022-01829-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lauque D, Cadranel J, Lazor R, Pourrat J, Ronco P, Guillevin L, et al. Microscopic polyangiitis with alveolar hemorrhage: a study of 29 cases and review of the literature. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM“O”P) Medicine (Baltimore) . 2000;79:222–233. doi: 10.1097/00005792-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 9. Gallagher H, Kwan JT, Jayne DR. Pulmonary renal syndrome: a 4-year, single-center experience. Am J Kidney Dis . 2002;39:42–47. doi: 10.1053/ajkd.2002.29876. [DOI] [PubMed] [Google Scholar]
- 10. Dagostin MA, Nunes SLO, Shinjo SK, Pereira RMR. Mortality predictors in ANCA-associated vasculitis: experience of a Brazilian monocentric cohort of a rheumatology center. Medicine (Baltimore) . 2021;100:e28305. doi: 10.1097/MD.0000000000028305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flossmann O, Berden A, de Groot K, Hagen C, Harper L, Heijl C, et al. European Vasculitis Study Group Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis . 2011;70:488–494. doi: 10.1136/ard.2010.137778. [DOI] [PubMed] [Google Scholar]
- 12. Lai QY, Ma TT, Li ZY, Chang DY, Zhao MH, Chen M. Predictors for mortality in patients with antineutrophil cytoplasmic autoantibody-associated vasculitis: a study of 398 Chinese patients. J Rheumatol . 2014;41:1849–1855. doi: 10.3899/jrheum.131426. [DOI] [PubMed] [Google Scholar]
- 13. Pu L, Li GS, Zou YR, Zhang P, Wang L. Clinical predictors of outcome in patients with anti-neutrophil cytoplasmic autoantibody-related renal vasculitis: experiences from a single-center. Chin Med J (Engl) . 2017;130:899–905. doi: 10.4103/0366-6999.204099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pearce FA, McGrath C, Sandhu R, Packham J, Watts RA, Rhodes B, et al. Outcomes and compliance with standards of care in anti-neutrophil cytoplasmic antibody-associated vasculitis: insights from a large multiregion audit. Rheumatol Adv Pract . 2018;2:rky025. doi: 10.1093/rap/rky025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Solans-Laqué R, Fraile G, Rodriguez-Carballeira M, Caminal L, Castillo MJ, Martínez-Valle F, et al. Spanish Registry of systemic vasculitis (REVAS) from the Autoimmune Diseases Study Group (GEAS) of the Spanish Society of Internal Medicine (SEMI) Clinical characteristics and outcome of Spanish patients with ANCA-associated vasculitides: impact of the vasculitis type, ANCA specificity, and treatment on mortality and morbidity. Medicine (Baltimore) . 2017;96:e6083. doi: 10.1097/MD.0000000000006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charlier C, Henegar C, Launay O, Pagnoux C, Berezné A, Bienvenu B, et al. Risk factors for major infections in Wegener granulomatosis: analysis of 113 patients. Ann Rheum Dis . 2009;68:658–663. doi: 10.1136/ard.2008.088302. [DOI] [PubMed] [Google Scholar]
- 17. Garcia-Vives E, Segarra-Medrano A, Martinez-Valle F, Agraz I, Solans-Laque R. Prevalence and risk factors for major infections in patients with antineutrophil cytoplasmic antibody-associated vasculitis: influence on the disease outcome. J Rheumatol . 2020;47:407–414. doi: 10.3899/jrheum.190065. [DOI] [PubMed] [Google Scholar]
- 18. Glöckner WM, Sieberth HG, Wichmann HE, Backes E, Bambauer R, Boesken WH, et al. Plasma exchange and immunosuppression in rapidly progressive glomerulonephritis: a controlled, multi-center study. Clin Nephrol . 1988;29:1–8. [PubMed] [Google Scholar]
- 19. Pusey CD, Rees AJ, Evans DJ, Peters DK, Lockwood CM. Plasma exchange in focal necrotizing glomerulonephritis without anti-GBM antibodies. Kidney Int . 1991;40:757–763. doi: 10.1038/ki.1991.272. [DOI] [PubMed] [Google Scholar]
- 20. Aasarød K, Iversen BM, Hammerstrøm J, Bostad L, Jørstad S. Clinical outcome of patients with Wegener’s granulomatosis treated with plasma exchange. Blood Purif . 2002;20:167–173. doi: 10.1159/000047004. [DOI] [PubMed] [Google Scholar]
- 21. Klemmer PJ, Chalermskulrat W, Reif MS, Hogan SL, Henke DC, Falk RJ. Plasmapheresis therapy for diffuse alveolar hemorrhage in patients with small-vessel vasculitis. Am J Kidney Dis . 2003;42:1149–1153. doi: 10.1053/j.ajkd.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 22. Frascà GM, Soverini ML, Falaschini A, Tampieri E, Vangelista A, Stefoni S. Plasma exchange treatment improves prognosis of antineutrophil cytoplasmic antibody-associated crescentic glomerulonephritis: a case-control study in 26 patients from a single center. Ther Apher Dial . 2003;7:540–546. doi: 10.1046/j.1526-0968.2003.00089.x. [DOI] [PubMed] [Google Scholar]
- 23. Nakamura T, Matsuda T, Kawagoe Y, Ueda Y, Ebihara I, Koide H. Plasmapheresis with immunosuppressive therapy vs immunosuppressive therapy alone for rapidly progressive anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis. Nephrol Dial Transplant . 2004;19:1935–1937. doi: 10.1093/ndt/gfh293. [DOI] [PubMed] [Google Scholar]
- 24. Jayne DRW, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, et al. European Vasculitis Study Group Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol . 2007;18:2180–2188. doi: 10.1681/ASN.2007010090. [DOI] [PubMed] [Google Scholar]
- 25. Szpirt WM, Heaf JG, Petersen J. Plasma exchange for induction and cyclosporine A for maintenance of remission in Wegener’s granulomatosis: a clinical randomized controlled trial. Nephrol Dial Transplant . 2011;26:206–213. doi: 10.1093/ndt/gfq360. [DOI] [PubMed] [Google Scholar]
- 26. Walsh M, Merkel PA, Peh CA, Szpirt WM, Puéchal X, Fujimoto S, et al. PEXIVAS Investigators Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med . 2020;382:622–631. doi: 10.1056/NEJMoa1803537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walsh M, Merkel PA, Peh CA, Szpirt W, Guillevin L, Pusey CD, et al. PEXIVAS Investigators Plasma exchange and glucocorticoid dosing in the treatment of anti-neutrophil cytoplasm antibody associated vasculitis (PEXIVAS): protocol for a randomized controlled trial. Trials . 2013;14:73–79. doi: 10.1186/1745-6215-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al. International Network for the Study of the Systemic Vasculitides (INSSYS) A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. Arthritis Rheum . 2001;44:912–920. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]




